User login
Are we doing enough to screen for colorectal cancer? Findings from the 1999 Behavioral Risk Factor Surveillance System
OBJECTIVES: To estimate current rates of use of fecal occult blood testing (FOBT) and sigmoidoscopy or colonoscopy; to determine whether test use varies by demographic factors; and to compare 1999 rates of use with 1997 rates.
STUDY DESIGN: The Behavioral Risk Factor Surveillance System is an ongoing, state-based random-digit-dialed telephone survey of the US population that collects various health behavior information, including the use of colorectal cancer (CRC) screening tests.
POPULATION: In 1999, 63,555 persons 50 years of age or older responded to questions regarding FOBT and sigmoidoscopy or colonoscopy.
OUTCOMES MEASURED: The proportion of survey respondents reporting having had FOBT and sigmoidoscopy/colonoscopy at any time; and the proportion reporting having had FOBT and sigmoidoscopy/colonoscopy within recommended time intervals. Data were recorded for the years 1997 and 1999, and analyzed according to various demographic factors.
RESULTS: In 1999, 40.3% of respondents reported having had an FOBT at some time, and 43.8% reported having had a sigmoidoscopy or colonoscopy. Regarding recent test use, 20.6% of respondents reported having had an FOBT within the year, and 33.6% reported having had a sigmoidoscopy or colonoscopy within the past 5 years. Some demographic variation was noted. In 1997, 19.6% reported having had an FOBT within the year, and 30.3% reported having had a sigmoidoscopy or proctoscopy within the past 5 years.
CONCLUSIONS: Use of CRC screening tests increased only slightly from 1997 to 1999. Usage remains low, despite consensus that screening for CRC reduces mortality from the disease. Efforts to promote awareness of, and screening for, CRC must intensify.
- Strong scientific evidence shows that regular colorectal cancer (CRC) screening effectively reduces CRC incidence and mortality.
- Despite this evidence, use of CRC screening tests remains low.
- Clinicians can use available physician-education tools (www.cdc.gov/cancer/colorctl/ calltoaction) to review current screening tests and guidelines and should begin offering regular CRC screening tests to their patients, if they are not already doing so.
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the United States for men and women combined; for women alone, it follows lung and breast cancers, and for men, it follows lung and prostate cancers.1 Strong scientific evidence indicates that regular screening is effective in reducing CRC incidence and mortality.2-8 Randomized controlled trials have demonstrated a reduction in CRC incidence and mortality with annual and biennial fecal occult blood testing (FOBT), and case-control studies have shown a reduction in CRC mortality associated with the use of sigmoidoscopy. Based on this evidence, 3 sets of national guidelines were developed recommending that average-risk persons undergo regular CRC screening with 1 or more of the following tests: FOBT annually, sigmoidoscopy periodically (usually every 5 years), colonoscopy every 10 years, or double-contrast barium enema every 5–10 years.9-11
To estimate current use of CRC screening tests, to evaluate variation in test use by demographic factors, and to compare current test use with previously published rates of use,12 we analyzed data from the 1999 Behavioral Risk Factor Surveillance System (BRFSS) on the use of a home blood stool test (FOBT) and on having had sigmoidoscopy or colonoscopy. Results from the 1999 survey were compared with results from the 1997 survey.
Methods
In 1999, 50 states, the District of Columbia, and Puerto Rico participated in the BRFSS, a state-based, random-digit-dialed telephone survey of the US non-institutionalized, adult (aged 18 years or older) civilian population. The BRFSS collects a wide variety of health behavior information, including the use of CRC screening tests.
During the survey, 63,555 respondents aged 50 years or older were asked 4 questions regarding their use of the FOBT and their having undergone sigmoidoscopy or colonoscopy (Table 1). Variables not measured in this dataset include use of sigmoidoscopy separately from colonoscopy, test indication, or physician specialty. We analyzed CRC tests used at any time and used recently (FOBT within the past year and sigmoidoscopy or colonoscopy within the past five years).
Aggregated rates, standard errors, and 95% confidence intervals were calculated using SAS13 and SUDAAN software.14 Respondents who refused to answer or did not know the answer to a question were excluded from analysis of the specific question. The total number of respondent refusals or unknowns was 1007 (1.6%) for the FOBT questions and 1217 (1.9%) for the sigmoidoscopy questions. Data were weighted, using intercensal estimates, to the sex, racial, ethnic, and age distribution of each state’s adult population, and were age-standardized to the 1999 BRFSS population. To compare 1997 and 1999 estimates, the 1997 data were also age-standardized to the 1999 BRFSS population. The median state response rate for the entire survey was 56.7%, calculated using the cooperation rate formula.15
The 1999 BRFSS questions regarding use of sigmoidoscopy were modified from previous questionnaires. As the scientific evidence supporting CRC screening tests has grown, BRFSS CRC survey questions have changed. The 1997 survey, described previously,12 was the first survey to collect information regarding the use of home-administered FOBT and sigmoidoscopy from all 50 states, the District of Columbia, and Puerto Rico. In 1997, respondents were asked if they had received a sigmoidoscopy or proctoscopy. Proctoscopy, performed with a shorter instrument than a sigmoidoscope, is not recommended as a CRC screening test. In 1999, the term “sigmoidoscopy/proctoscopy” was replaced with “sigmoidoscopy/colonoscopy.” Colonoscopy evaluates the entire colon and is recommended once every 10 years in some guidelines.10,11 For this report, the terms “sigmoidoscopy/proctoscopy” and “sigmoidoscopy/colonoscopy” will each be referred to as “sigmoidoscopy” unless otherwise specified.
TABLE 1
Questions used in the 1999 Behavioral Risk Factor Surveillance System to assess usage of colorectal cancer screening tests
|
Results
The age-adjusted proportion of overall respondents who reported ever receiving CRC screening tests in 1999 was 40.3% for FOBT and 43.8% for sigmoidoscopy (data not shown).
The 1999 age-adjusted CRC screening test rates are presented by demographic subgroups for reported use within recommended time intervals: FOBT within the year preceding the survey, sigmoidoscopy within the past five years, or at least one of the two tests (Table 2). Less than half of the population surveyed reported having either FOBT or sigmoidoscopy within the recommended time interval. In 1999, 20.6% of respondents reported having had FOBT within the previous year; 33.6% reported having had a sigmoidoscopy within the previous 5 years; 44.0% reported having had either FOBT within the previous year or a sigmoidoscopy within the previous 5 years. There was little difference in test use between blacks and whites. Rates of use by Asian/Pacific Islanders and American Indian/Alaska Natives were calculated from small respondent samples and should be interpreted cautiously. Respondents of Spanish or Hispanic origin reported lower rates of FOBT and sigmoidoscopy than respondents who were not of Hispanic origin. Reported test use rose with increasing age of the respondents, up to age 70–79, and then declined for those over 80 years of age. Reported test use increased with education and with annual house hold income. Respondents who had health care coverage were almost twice as likely to have had CRC screening tests as respondents without health care coverage.
CRC screening test rates increased slightly from 1997 to 1999. In 1997, 19.6% of respondents reported having had an FOBT within the previous year and 30.3% reported having had a sigmoidoscopy within the previous 5 years.
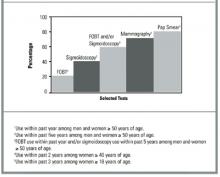
We compared 1999 BRFSS usage rates for FOBT and sigmoidoscopy or colonoscopy with those for mammography and Papanicolaou (Pap) smear (Figure). These are not direct comparisons but, rather, comparisons of the rates of testing within recommended time intervals among appropriate demographic groups. The proportion of persons who used CRC screening tests within recommended time intervals was lower than those for other cancer screening tests.
TABLE 2
Respondents aged 50 years or older who reported colorectal cancer screening tests within recommended time intervals, by demographic variables1
| Fecal occult blood test within previous year | Sigmoidoscopy/colonoscopy within previous 5 years | Either test within recommended time interval | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n2 | % | (95% CI) | n | % | (95% CI) | N | % | (95% CI) | |
| Total | 61,952 | 20.6 | (20.1–21.2) | 61,953 | 33.6 | (33.0–34.2) | 61,537 | 44.0 | (43.3–44.6) |
| Gender | |||||||||
| Male | 23,919 | 19.1 | (18.2–19.9) | 23,850 | 37.9 | (36.8–38.9) | 23,724 | 45.9 | (44.9–47.0) |
| Female | 38,033 | 22.0 | (21.3–22.7) | 38,103 | 30.4 | (29.6–31.1) | 37,813 | 42.6 | (41.7–43.4) |
| Race4 | |||||||||
| White | 55,139 | 21.0 | (20.5–21.6) | 55,170 | 33.6 | (33.0–34.3) | 54,804 | 44.2 | (3.5–44.9) |
| Black | 4,075 | 20.7 | (18.8–22.6) | 4,046 | 32.6 | (30.3–34.9) | 4,020 | 43.3 | (40.9–45.7) |
| Asian/Pacific Islander | 739 | 10.3 | (6.9–13.6) | 739 | 35.4 | (28.4–42.5) | 735 | 40.1 | (33.3–46.9) |
| American Indian/ Alaska Native | 725 | 18.2 | (12.7–23.7) | 725 | 36.0 | (29.4–42.5) | 723 | 43.0 | (36.5–49.6 |
| Spanish or Hispanic origin | |||||||||
| Yes | 3,664 | 11.2 | (9.4–12.9) | 3,667 | 28.6 | (25.6–31.5) | 3,635 | 33.9 | (30.9–37.0) |
| No | 57,993 | 21.4 | (20.9–21.9) | 57,999 | 34.0 | (33.4–34.6) | 57,620 | 44.8 | (44.1–45.5) |
| Age (group) | |||||||||
| 50–59 years | 23,758 | 15.5 | (14.7–16.2) | 23,803 | 26.1 | (25.1–27.0) | 23,667 | 34.7 | (33.7–35.7) |
| 60–69 years | 17,680 | 23.0 | (22.0–24.0) | 17,651 | 36.9 | (35.7–38.1) | 17,574 | 48.1 | (46.9–49.3) |
| 70–79 years | 14,427 | 25.8 | (24.6–27.0) | 14,412 | 40.7 | (39.3–42.1) | 14,306 | 52.7 | (51.4–54.1) |
| ≤80 years | 6,087 | 21.6 | (19.8–23.4) | 6,087 | 36.1 | (34.0–38.2) | 5,990 | 46.9 | (44.7–49.1) |
| Education | |||||||||
| < 12 years | 11,928 | 15.0 | (13.8–16.1) | 11,889 | 27.5 | (25.9–29.1) | 11,756 | 35.6 | (33.9–37.3) |
| High school graduate | 21,183 | 19.7 | (18.8–20.6) | 21,176 | 30.6 | (29.6–31.6) | 21,049 | 41.2 | (40.1–42.3) |
| Some college/ technical school | 14,167 | 23.5 | (22.3–24.7) | 14,162 | 35.9 | (34.6–37.3) | 14,102 | 47.9 | (46.5–49.2) |
| College graduate | 14,503 | 24.3 | (23.2–25.5) | 14,560 | 41.1 | (39.8–42.5) | 14,466 | 51.4 | (50.1–52.7) |
| Income (annual household) | |||||||||
| <$20,000 | 15,204 | 15.3 | (14.3–16.3) | 15,154 | 29.1 | (27.7–30.5) | 15,029 | 37.0 | (35.5–38.4) |
| $20,000–34,999 | 14,354 | 20.8 | (19.7–21.9) | 14,362 | 32.5 | (31.2–33.8) | 14,288 | 43.0 | (41.7–44.4) |
| $35,000–49,999 | 7,721 | 23.3 | (21.6–24.9) | 7,718 | 37.0 | (35.1–39.0) | 7,703 | 48.2 | (46.3–50.1) |
| ≥$50,000 | 11,967 | 24.2 | (22.7–25.6) | 12,002 | 41.7 | (39.9–43.4) | 11,949 | 51.7 | (50.0–53.5) |
| Health care coverage | |||||||||
| Yes | 57,551 | 21.3 | (20.8–21.9) | 57,561 | 34.7 | (34.1–35.4) | 57,169 | 45.4 | (44.7–46.1) |
| No | 4,331 | 12.1 | (9.8–14.5) | 4,326 | 18.7 | (15.6–21.7) | 4,304 | 25.7 | (22.2–29.2) |
| 1From the Behavioral Risk Factor Surveillance System (BRFSS), 1999; estimates are age-adjusted. | |||||||||
| 2Sample size for each question; sample sizes may not sum to totals because of missing data. | |||||||||
| 3Confidence interval. | |||||||||
| 4Sample sizes for racial categories do not add up to column totals. “Other” racial category not presented here. | |||||||||
FIGURE
Comparison of use of colorectal cancer screening tests with other screening tests, BRFSS 1999
Discussion
Currently, 4 widely accepted tests are available for CRC screening, and several new tests are under investigation.16,17 Not enough evidence exists to determine which of the available tests is most appropriate when efficacy, cost-effectiveness, availability, patient acceptability, and safety are taken into consideration. The 1999 BRFSS monitored the use of 3 of these tests: FOBT and sigmoidoscopy or colonoscopy. Our results show that less than half of the US population aged 50 years and older is being screened for CRC with these methods. Persons with health care coverage and with higher education and income levels were more likely to have had CRC tests. Since 1997, the proportion of the U.S. population being screened for CRC has increased slightly, but it remains low and lags far behind the use of other recommended cancer screening tests (Figure). While use of barium enema, one of the recommended colorectal cancer screening tests, is not monitored in the 1999 BRFSS, data from a recent national primary care physician survey suggest that barium enemas are infrequently recommended for colorectal cancer screening (Carrie Klabunde, National Cancer Institute, personal/written communication, 2002).
The 1999 BRFSS was the first BRFSS survey to collect data on the use of colonoscopy. Because BRFSS colonoscopy data have not previously been collected, we do not know whether the reported increase in the use of endoscopy from 1997 to 1999 represents a true increase in sigmoidoscopy usage or previously unmeasured colonoscopy usage. Furthermore, it is likely that some of the tests reported as sigmoidoscopies or proctoscopies in the 1997 BRFSS survey were actually colonoscopies, since some respondents may be unable to clearly distinguish between the endoscopic tests.
Both patient-related and physician-related factors likely contribute to continued underuse of these tests. Patient-related factors include lack of awareness of screening guidelines, embarrassment, and lack of physician recommendation.7,18-20 Physician-related factors include lack of knowledge of the effectiveness of screening, lack of skills in endoscopy, and low reimbursement rates for screening tests.7,18-21
Several factors limit the interpretation of this analysis. First, as this is a telephone survey, only people who have access to telephones are represented in this analysis. However, approximately 95% of households in the United States have telephones.22 Second, 43.3% of the eligible respondents who were successfully contacted did not complete the telephone interview. Third, responses are self-reported and not validated through medical record review. However, a comparison of self-report and record review has found good concordance between results.23 Fourth, in the 1999 BRFSS, sigmoidoscopy use cannot be measured separately from colonoscopy, and screening tests cannot be distinguished from diagnostic tests. The results reported here may therefore be overestimates of use of these tests for screening. Lastly, the specialty of the physicians ordering the tests is unknown, limiting the ability to target interventions towards specific physician specialists. Despite these limitations, the BRFSS provides an excellent data source for routine surveillance of CRC testing.
Conclusion
This report demonstrates that CRC screening tests remain underused, despite their recognized efficacy in reducing CRC incidence and mortality.2-8 Coordinated efforts by clinicians and policy makers to raise awareness about this important disease and promote use of available screening tests must continue.
CORRESPONDENCE
Epidemiology and Health Services Research Branch, Division of Cancer Prevention and Control, Centers for Disease Control and Prevention, Atlanta, GA. Data from this paper were presented at CDC’s 15th National Conference on Chronic Disease Prevention and Control on November 30, 2000. Send correspondence and reprint requests to: Laura C. Seeff, MD; Centers for Disease Control and Prevention, DCPC; 4770 Buford Highway NE; Mailstop K-55; Atlanta, Georgia 30341-3717. E-mail: [email protected].
1. American Cancer Society. Cancer facts and figures, 2002. Atlanta: American Cancer Society, Inc., 2002. Publication 02-250M-No. 5008.02.
2. Mandel JS, Church TR, Bond JH, et al. The effect of fecal occultblood screening on the incidence of colorectal cancer. N Engl J Med 2000;343:1603-7.
3. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from col-orectal cancer by screening for fecal occult blood. N Engl J Med 1993;328:1365-71.
4. Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. A case-con-trol study of screening sigmoidoscopy and mortality from colorec-tal cancer. N Engl J Med 1992;326:653-7.
5. Newcomb PA, Norfleet RG, Storer BE, Surawicz TS, Marcus PM. Screening sigmoidoscopy and colorectal cancer mortality. J Natl Cancer Inst 1992;84:1572-5.
6. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348:1472-1477.
7. Kronborg O, Fenger C, Olsen J, Jorgensen OD. Sondergaard. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996;348:1467-1.
8. Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: Effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst 1999;91:434-7.
9. U.S.Preventive Services Task Force. Guide to clinical preventive services. 2nd ed. Baltimore: Williams and Wilkins, 1996.
10. Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology 1997;112:594-642.
11. Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. CA Cancer J Clin 2001;51:38-75.
12. Shapiro JA, Seeff LC, Nadel MR. Colorectal cancer-screening tests and associated health behaviors. Am J Prev Med 2001;21:132-7.
13. SAS statistical analysis software. Cary, NC: SAS Institute, 1996.
14. SUDAAN software for the statistical analysis of correlated data. Research Triangle Park, NC: Research Triangle Institute, 1997.
15. Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System, 1999. BRFSS Summary Quality Control Report. Atlanta: National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, 1999.
16. Fenlon HM, Nunes DP, Schroy PC, III, Barish MA, Clarke PD, Ferrucci JT. A comparison of virtual and conventional colonoscopy for the detection of colorectal polyps. N Engl J Med 1999;341:1496-503.
17. Ahlquist DA, Skoletsky JE, Boynton KA, et al. Colorectal cancer screening by detection of altered DNA in stool: feasibility of a mul-titarget assay panel. Gastroenterology 2000;119:1219-27.
18. Vernon SW. Participation in colorectal cancer screening: A review. J Natl Cancer Inst 1997;89:1406-22.
19. Peterson SK, Vernon SW. A review of patient and physician adherence to colorectal cancer screening guidelines. Semin Colon Rectal Surg 2000;11:58-72.
20. McCarthy BD, Moskowitz MA. Screening flexible sigmoidoscopy: patient attitudes and compliance. J Gen Intern Med. 1993;8:120-5.
21. Lewis JD, Asch DA. Barriers to office-based screening sigmoi-doscopy: does reimbursement cover costs? Ann Intern Med 1999;130:525-30.
22. Anderson JE, Nelson DE, Wilson RW. Telephone coverage and measurement of health risk indicators: data from the National Health Interview Survey. Am J Public Health 1998;88:1392-5.
23. Baier M, Calonge N, Cutter G, et al. Validity of self-reported col-orectal cancer screening behavior. Cancer Epidemiol Biomarkers Prev 2000;9:229-32.
OBJECTIVES: To estimate current rates of use of fecal occult blood testing (FOBT) and sigmoidoscopy or colonoscopy; to determine whether test use varies by demographic factors; and to compare 1999 rates of use with 1997 rates.
STUDY DESIGN: The Behavioral Risk Factor Surveillance System is an ongoing, state-based random-digit-dialed telephone survey of the US population that collects various health behavior information, including the use of colorectal cancer (CRC) screening tests.
POPULATION: In 1999, 63,555 persons 50 years of age or older responded to questions regarding FOBT and sigmoidoscopy or colonoscopy.
OUTCOMES MEASURED: The proportion of survey respondents reporting having had FOBT and sigmoidoscopy/colonoscopy at any time; and the proportion reporting having had FOBT and sigmoidoscopy/colonoscopy within recommended time intervals. Data were recorded for the years 1997 and 1999, and analyzed according to various demographic factors.
RESULTS: In 1999, 40.3% of respondents reported having had an FOBT at some time, and 43.8% reported having had a sigmoidoscopy or colonoscopy. Regarding recent test use, 20.6% of respondents reported having had an FOBT within the year, and 33.6% reported having had a sigmoidoscopy or colonoscopy within the past 5 years. Some demographic variation was noted. In 1997, 19.6% reported having had an FOBT within the year, and 30.3% reported having had a sigmoidoscopy or proctoscopy within the past 5 years.
CONCLUSIONS: Use of CRC screening tests increased only slightly from 1997 to 1999. Usage remains low, despite consensus that screening for CRC reduces mortality from the disease. Efforts to promote awareness of, and screening for, CRC must intensify.
- Strong scientific evidence shows that regular colorectal cancer (CRC) screening effectively reduces CRC incidence and mortality.
- Despite this evidence, use of CRC screening tests remains low.
- Clinicians can use available physician-education tools (www.cdc.gov/cancer/colorctl/ calltoaction) to review current screening tests and guidelines and should begin offering regular CRC screening tests to their patients, if they are not already doing so.
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the United States for men and women combined; for women alone, it follows lung and breast cancers, and for men, it follows lung and prostate cancers.1 Strong scientific evidence indicates that regular screening is effective in reducing CRC incidence and mortality.2-8 Randomized controlled trials have demonstrated a reduction in CRC incidence and mortality with annual and biennial fecal occult blood testing (FOBT), and case-control studies have shown a reduction in CRC mortality associated with the use of sigmoidoscopy. Based on this evidence, 3 sets of national guidelines were developed recommending that average-risk persons undergo regular CRC screening with 1 or more of the following tests: FOBT annually, sigmoidoscopy periodically (usually every 5 years), colonoscopy every 10 years, or double-contrast barium enema every 5–10 years.9-11
To estimate current use of CRC screening tests, to evaluate variation in test use by demographic factors, and to compare current test use with previously published rates of use,12 we analyzed data from the 1999 Behavioral Risk Factor Surveillance System (BRFSS) on the use of a home blood stool test (FOBT) and on having had sigmoidoscopy or colonoscopy. Results from the 1999 survey were compared with results from the 1997 survey.
Methods
In 1999, 50 states, the District of Columbia, and Puerto Rico participated in the BRFSS, a state-based, random-digit-dialed telephone survey of the US non-institutionalized, adult (aged 18 years or older) civilian population. The BRFSS collects a wide variety of health behavior information, including the use of CRC screening tests.
During the survey, 63,555 respondents aged 50 years or older were asked 4 questions regarding their use of the FOBT and their having undergone sigmoidoscopy or colonoscopy (Table 1). Variables not measured in this dataset include use of sigmoidoscopy separately from colonoscopy, test indication, or physician specialty. We analyzed CRC tests used at any time and used recently (FOBT within the past year and sigmoidoscopy or colonoscopy within the past five years).
Aggregated rates, standard errors, and 95% confidence intervals were calculated using SAS13 and SUDAAN software.14 Respondents who refused to answer or did not know the answer to a question were excluded from analysis of the specific question. The total number of respondent refusals or unknowns was 1007 (1.6%) for the FOBT questions and 1217 (1.9%) for the sigmoidoscopy questions. Data were weighted, using intercensal estimates, to the sex, racial, ethnic, and age distribution of each state’s adult population, and were age-standardized to the 1999 BRFSS population. To compare 1997 and 1999 estimates, the 1997 data were also age-standardized to the 1999 BRFSS population. The median state response rate for the entire survey was 56.7%, calculated using the cooperation rate formula.15
The 1999 BRFSS questions regarding use of sigmoidoscopy were modified from previous questionnaires. As the scientific evidence supporting CRC screening tests has grown, BRFSS CRC survey questions have changed. The 1997 survey, described previously,12 was the first survey to collect information regarding the use of home-administered FOBT and sigmoidoscopy from all 50 states, the District of Columbia, and Puerto Rico. In 1997, respondents were asked if they had received a sigmoidoscopy or proctoscopy. Proctoscopy, performed with a shorter instrument than a sigmoidoscope, is not recommended as a CRC screening test. In 1999, the term “sigmoidoscopy/proctoscopy” was replaced with “sigmoidoscopy/colonoscopy.” Colonoscopy evaluates the entire colon and is recommended once every 10 years in some guidelines.10,11 For this report, the terms “sigmoidoscopy/proctoscopy” and “sigmoidoscopy/colonoscopy” will each be referred to as “sigmoidoscopy” unless otherwise specified.
TABLE 1
Questions used in the 1999 Behavioral Risk Factor Surveillance System to assess usage of colorectal cancer screening tests
|
Results
The age-adjusted proportion of overall respondents who reported ever receiving CRC screening tests in 1999 was 40.3% for FOBT and 43.8% for sigmoidoscopy (data not shown).
The 1999 age-adjusted CRC screening test rates are presented by demographic subgroups for reported use within recommended time intervals: FOBT within the year preceding the survey, sigmoidoscopy within the past five years, or at least one of the two tests (Table 2). Less than half of the population surveyed reported having either FOBT or sigmoidoscopy within the recommended time interval. In 1999, 20.6% of respondents reported having had FOBT within the previous year; 33.6% reported having had a sigmoidoscopy within the previous 5 years; 44.0% reported having had either FOBT within the previous year or a sigmoidoscopy within the previous 5 years. There was little difference in test use between blacks and whites. Rates of use by Asian/Pacific Islanders and American Indian/Alaska Natives were calculated from small respondent samples and should be interpreted cautiously. Respondents of Spanish or Hispanic origin reported lower rates of FOBT and sigmoidoscopy than respondents who were not of Hispanic origin. Reported test use rose with increasing age of the respondents, up to age 70–79, and then declined for those over 80 years of age. Reported test use increased with education and with annual house hold income. Respondents who had health care coverage were almost twice as likely to have had CRC screening tests as respondents without health care coverage.
CRC screening test rates increased slightly from 1997 to 1999. In 1997, 19.6% of respondents reported having had an FOBT within the previous year and 30.3% reported having had a sigmoidoscopy within the previous 5 years.
We compared 1999 BRFSS usage rates for FOBT and sigmoidoscopy or colonoscopy with those for mammography and Papanicolaou (Pap) smear (Figure). These are not direct comparisons but, rather, comparisons of the rates of testing within recommended time intervals among appropriate demographic groups. The proportion of persons who used CRC screening tests within recommended time intervals was lower than those for other cancer screening tests.
TABLE 2
Respondents aged 50 years or older who reported colorectal cancer screening tests within recommended time intervals, by demographic variables1
| Fecal occult blood test within previous year | Sigmoidoscopy/colonoscopy within previous 5 years | Either test within recommended time interval | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n2 | % | (95% CI) | n | % | (95% CI) | N | % | (95% CI) | |
| Total | 61,952 | 20.6 | (20.1–21.2) | 61,953 | 33.6 | (33.0–34.2) | 61,537 | 44.0 | (43.3–44.6) |
| Gender | |||||||||
| Male | 23,919 | 19.1 | (18.2–19.9) | 23,850 | 37.9 | (36.8–38.9) | 23,724 | 45.9 | (44.9–47.0) |
| Female | 38,033 | 22.0 | (21.3–22.7) | 38,103 | 30.4 | (29.6–31.1) | 37,813 | 42.6 | (41.7–43.4) |
| Race4 | |||||||||
| White | 55,139 | 21.0 | (20.5–21.6) | 55,170 | 33.6 | (33.0–34.3) | 54,804 | 44.2 | (3.5–44.9) |
| Black | 4,075 | 20.7 | (18.8–22.6) | 4,046 | 32.6 | (30.3–34.9) | 4,020 | 43.3 | (40.9–45.7) |
| Asian/Pacific Islander | 739 | 10.3 | (6.9–13.6) | 739 | 35.4 | (28.4–42.5) | 735 | 40.1 | (33.3–46.9) |
| American Indian/ Alaska Native | 725 | 18.2 | (12.7–23.7) | 725 | 36.0 | (29.4–42.5) | 723 | 43.0 | (36.5–49.6 |
| Spanish or Hispanic origin | |||||||||
| Yes | 3,664 | 11.2 | (9.4–12.9) | 3,667 | 28.6 | (25.6–31.5) | 3,635 | 33.9 | (30.9–37.0) |
| No | 57,993 | 21.4 | (20.9–21.9) | 57,999 | 34.0 | (33.4–34.6) | 57,620 | 44.8 | (44.1–45.5) |
| Age (group) | |||||||||
| 50–59 years | 23,758 | 15.5 | (14.7–16.2) | 23,803 | 26.1 | (25.1–27.0) | 23,667 | 34.7 | (33.7–35.7) |
| 60–69 years | 17,680 | 23.0 | (22.0–24.0) | 17,651 | 36.9 | (35.7–38.1) | 17,574 | 48.1 | (46.9–49.3) |
| 70–79 years | 14,427 | 25.8 | (24.6–27.0) | 14,412 | 40.7 | (39.3–42.1) | 14,306 | 52.7 | (51.4–54.1) |
| ≤80 years | 6,087 | 21.6 | (19.8–23.4) | 6,087 | 36.1 | (34.0–38.2) | 5,990 | 46.9 | (44.7–49.1) |
| Education | |||||||||
| < 12 years | 11,928 | 15.0 | (13.8–16.1) | 11,889 | 27.5 | (25.9–29.1) | 11,756 | 35.6 | (33.9–37.3) |
| High school graduate | 21,183 | 19.7 | (18.8–20.6) | 21,176 | 30.6 | (29.6–31.6) | 21,049 | 41.2 | (40.1–42.3) |
| Some college/ technical school | 14,167 | 23.5 | (22.3–24.7) | 14,162 | 35.9 | (34.6–37.3) | 14,102 | 47.9 | (46.5–49.2) |
| College graduate | 14,503 | 24.3 | (23.2–25.5) | 14,560 | 41.1 | (39.8–42.5) | 14,466 | 51.4 | (50.1–52.7) |
| Income (annual household) | |||||||||
| <$20,000 | 15,204 | 15.3 | (14.3–16.3) | 15,154 | 29.1 | (27.7–30.5) | 15,029 | 37.0 | (35.5–38.4) |
| $20,000–34,999 | 14,354 | 20.8 | (19.7–21.9) | 14,362 | 32.5 | (31.2–33.8) | 14,288 | 43.0 | (41.7–44.4) |
| $35,000–49,999 | 7,721 | 23.3 | (21.6–24.9) | 7,718 | 37.0 | (35.1–39.0) | 7,703 | 48.2 | (46.3–50.1) |
| ≥$50,000 | 11,967 | 24.2 | (22.7–25.6) | 12,002 | 41.7 | (39.9–43.4) | 11,949 | 51.7 | (50.0–53.5) |
| Health care coverage | |||||||||
| Yes | 57,551 | 21.3 | (20.8–21.9) | 57,561 | 34.7 | (34.1–35.4) | 57,169 | 45.4 | (44.7–46.1) |
| No | 4,331 | 12.1 | (9.8–14.5) | 4,326 | 18.7 | (15.6–21.7) | 4,304 | 25.7 | (22.2–29.2) |
| 1From the Behavioral Risk Factor Surveillance System (BRFSS), 1999; estimates are age-adjusted. | |||||||||
| 2Sample size for each question; sample sizes may not sum to totals because of missing data. | |||||||||
| 3Confidence interval. | |||||||||
| 4Sample sizes for racial categories do not add up to column totals. “Other” racial category not presented here. | |||||||||
FIGURE
Comparison of use of colorectal cancer screening tests with other screening tests, BRFSS 1999
Discussion
Currently, 4 widely accepted tests are available for CRC screening, and several new tests are under investigation.16,17 Not enough evidence exists to determine which of the available tests is most appropriate when efficacy, cost-effectiveness, availability, patient acceptability, and safety are taken into consideration. The 1999 BRFSS monitored the use of 3 of these tests: FOBT and sigmoidoscopy or colonoscopy. Our results show that less than half of the US population aged 50 years and older is being screened for CRC with these methods. Persons with health care coverage and with higher education and income levels were more likely to have had CRC tests. Since 1997, the proportion of the U.S. population being screened for CRC has increased slightly, but it remains low and lags far behind the use of other recommended cancer screening tests (Figure). While use of barium enema, one of the recommended colorectal cancer screening tests, is not monitored in the 1999 BRFSS, data from a recent national primary care physician survey suggest that barium enemas are infrequently recommended for colorectal cancer screening (Carrie Klabunde, National Cancer Institute, personal/written communication, 2002).
The 1999 BRFSS was the first BRFSS survey to collect data on the use of colonoscopy. Because BRFSS colonoscopy data have not previously been collected, we do not know whether the reported increase in the use of endoscopy from 1997 to 1999 represents a true increase in sigmoidoscopy usage or previously unmeasured colonoscopy usage. Furthermore, it is likely that some of the tests reported as sigmoidoscopies or proctoscopies in the 1997 BRFSS survey were actually colonoscopies, since some respondents may be unable to clearly distinguish between the endoscopic tests.
Both patient-related and physician-related factors likely contribute to continued underuse of these tests. Patient-related factors include lack of awareness of screening guidelines, embarrassment, and lack of physician recommendation.7,18-20 Physician-related factors include lack of knowledge of the effectiveness of screening, lack of skills in endoscopy, and low reimbursement rates for screening tests.7,18-21
Several factors limit the interpretation of this analysis. First, as this is a telephone survey, only people who have access to telephones are represented in this analysis. However, approximately 95% of households in the United States have telephones.22 Second, 43.3% of the eligible respondents who were successfully contacted did not complete the telephone interview. Third, responses are self-reported and not validated through medical record review. However, a comparison of self-report and record review has found good concordance between results.23 Fourth, in the 1999 BRFSS, sigmoidoscopy use cannot be measured separately from colonoscopy, and screening tests cannot be distinguished from diagnostic tests. The results reported here may therefore be overestimates of use of these tests for screening. Lastly, the specialty of the physicians ordering the tests is unknown, limiting the ability to target interventions towards specific physician specialists. Despite these limitations, the BRFSS provides an excellent data source for routine surveillance of CRC testing.
Conclusion
This report demonstrates that CRC screening tests remain underused, despite their recognized efficacy in reducing CRC incidence and mortality.2-8 Coordinated efforts by clinicians and policy makers to raise awareness about this important disease and promote use of available screening tests must continue.
CORRESPONDENCE
Epidemiology and Health Services Research Branch, Division of Cancer Prevention and Control, Centers for Disease Control and Prevention, Atlanta, GA. Data from this paper were presented at CDC’s 15th National Conference on Chronic Disease Prevention and Control on November 30, 2000. Send correspondence and reprint requests to: Laura C. Seeff, MD; Centers for Disease Control and Prevention, DCPC; 4770 Buford Highway NE; Mailstop K-55; Atlanta, Georgia 30341-3717. E-mail: [email protected].
OBJECTIVES: To estimate current rates of use of fecal occult blood testing (FOBT) and sigmoidoscopy or colonoscopy; to determine whether test use varies by demographic factors; and to compare 1999 rates of use with 1997 rates.
STUDY DESIGN: The Behavioral Risk Factor Surveillance System is an ongoing, state-based random-digit-dialed telephone survey of the US population that collects various health behavior information, including the use of colorectal cancer (CRC) screening tests.
POPULATION: In 1999, 63,555 persons 50 years of age or older responded to questions regarding FOBT and sigmoidoscopy or colonoscopy.
OUTCOMES MEASURED: The proportion of survey respondents reporting having had FOBT and sigmoidoscopy/colonoscopy at any time; and the proportion reporting having had FOBT and sigmoidoscopy/colonoscopy within recommended time intervals. Data were recorded for the years 1997 and 1999, and analyzed according to various demographic factors.
RESULTS: In 1999, 40.3% of respondents reported having had an FOBT at some time, and 43.8% reported having had a sigmoidoscopy or colonoscopy. Regarding recent test use, 20.6% of respondents reported having had an FOBT within the year, and 33.6% reported having had a sigmoidoscopy or colonoscopy within the past 5 years. Some demographic variation was noted. In 1997, 19.6% reported having had an FOBT within the year, and 30.3% reported having had a sigmoidoscopy or proctoscopy within the past 5 years.
CONCLUSIONS: Use of CRC screening tests increased only slightly from 1997 to 1999. Usage remains low, despite consensus that screening for CRC reduces mortality from the disease. Efforts to promote awareness of, and screening for, CRC must intensify.
- Strong scientific evidence shows that regular colorectal cancer (CRC) screening effectively reduces CRC incidence and mortality.
- Despite this evidence, use of CRC screening tests remains low.
- Clinicians can use available physician-education tools (www.cdc.gov/cancer/colorctl/ calltoaction) to review current screening tests and guidelines and should begin offering regular CRC screening tests to their patients, if they are not already doing so.
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the United States for men and women combined; for women alone, it follows lung and breast cancers, and for men, it follows lung and prostate cancers.1 Strong scientific evidence indicates that regular screening is effective in reducing CRC incidence and mortality.2-8 Randomized controlled trials have demonstrated a reduction in CRC incidence and mortality with annual and biennial fecal occult blood testing (FOBT), and case-control studies have shown a reduction in CRC mortality associated with the use of sigmoidoscopy. Based on this evidence, 3 sets of national guidelines were developed recommending that average-risk persons undergo regular CRC screening with 1 or more of the following tests: FOBT annually, sigmoidoscopy periodically (usually every 5 years), colonoscopy every 10 years, or double-contrast barium enema every 5–10 years.9-11
To estimate current use of CRC screening tests, to evaluate variation in test use by demographic factors, and to compare current test use with previously published rates of use,12 we analyzed data from the 1999 Behavioral Risk Factor Surveillance System (BRFSS) on the use of a home blood stool test (FOBT) and on having had sigmoidoscopy or colonoscopy. Results from the 1999 survey were compared with results from the 1997 survey.
Methods
In 1999, 50 states, the District of Columbia, and Puerto Rico participated in the BRFSS, a state-based, random-digit-dialed telephone survey of the US non-institutionalized, adult (aged 18 years or older) civilian population. The BRFSS collects a wide variety of health behavior information, including the use of CRC screening tests.
During the survey, 63,555 respondents aged 50 years or older were asked 4 questions regarding their use of the FOBT and their having undergone sigmoidoscopy or colonoscopy (Table 1). Variables not measured in this dataset include use of sigmoidoscopy separately from colonoscopy, test indication, or physician specialty. We analyzed CRC tests used at any time and used recently (FOBT within the past year and sigmoidoscopy or colonoscopy within the past five years).
Aggregated rates, standard errors, and 95% confidence intervals were calculated using SAS13 and SUDAAN software.14 Respondents who refused to answer or did not know the answer to a question were excluded from analysis of the specific question. The total number of respondent refusals or unknowns was 1007 (1.6%) for the FOBT questions and 1217 (1.9%) for the sigmoidoscopy questions. Data were weighted, using intercensal estimates, to the sex, racial, ethnic, and age distribution of each state’s adult population, and were age-standardized to the 1999 BRFSS population. To compare 1997 and 1999 estimates, the 1997 data were also age-standardized to the 1999 BRFSS population. The median state response rate for the entire survey was 56.7%, calculated using the cooperation rate formula.15
The 1999 BRFSS questions regarding use of sigmoidoscopy were modified from previous questionnaires. As the scientific evidence supporting CRC screening tests has grown, BRFSS CRC survey questions have changed. The 1997 survey, described previously,12 was the first survey to collect information regarding the use of home-administered FOBT and sigmoidoscopy from all 50 states, the District of Columbia, and Puerto Rico. In 1997, respondents were asked if they had received a sigmoidoscopy or proctoscopy. Proctoscopy, performed with a shorter instrument than a sigmoidoscope, is not recommended as a CRC screening test. In 1999, the term “sigmoidoscopy/proctoscopy” was replaced with “sigmoidoscopy/colonoscopy.” Colonoscopy evaluates the entire colon and is recommended once every 10 years in some guidelines.10,11 For this report, the terms “sigmoidoscopy/proctoscopy” and “sigmoidoscopy/colonoscopy” will each be referred to as “sigmoidoscopy” unless otherwise specified.
TABLE 1
Questions used in the 1999 Behavioral Risk Factor Surveillance System to assess usage of colorectal cancer screening tests
|
Results
The age-adjusted proportion of overall respondents who reported ever receiving CRC screening tests in 1999 was 40.3% for FOBT and 43.8% for sigmoidoscopy (data not shown).
The 1999 age-adjusted CRC screening test rates are presented by demographic subgroups for reported use within recommended time intervals: FOBT within the year preceding the survey, sigmoidoscopy within the past five years, or at least one of the two tests (Table 2). Less than half of the population surveyed reported having either FOBT or sigmoidoscopy within the recommended time interval. In 1999, 20.6% of respondents reported having had FOBT within the previous year; 33.6% reported having had a sigmoidoscopy within the previous 5 years; 44.0% reported having had either FOBT within the previous year or a sigmoidoscopy within the previous 5 years. There was little difference in test use between blacks and whites. Rates of use by Asian/Pacific Islanders and American Indian/Alaska Natives were calculated from small respondent samples and should be interpreted cautiously. Respondents of Spanish or Hispanic origin reported lower rates of FOBT and sigmoidoscopy than respondents who were not of Hispanic origin. Reported test use rose with increasing age of the respondents, up to age 70–79, and then declined for those over 80 years of age. Reported test use increased with education and with annual house hold income. Respondents who had health care coverage were almost twice as likely to have had CRC screening tests as respondents without health care coverage.
CRC screening test rates increased slightly from 1997 to 1999. In 1997, 19.6% of respondents reported having had an FOBT within the previous year and 30.3% reported having had a sigmoidoscopy within the previous 5 years.
We compared 1999 BRFSS usage rates for FOBT and sigmoidoscopy or colonoscopy with those for mammography and Papanicolaou (Pap) smear (Figure). These are not direct comparisons but, rather, comparisons of the rates of testing within recommended time intervals among appropriate demographic groups. The proportion of persons who used CRC screening tests within recommended time intervals was lower than those for other cancer screening tests.
TABLE 2
Respondents aged 50 years or older who reported colorectal cancer screening tests within recommended time intervals, by demographic variables1
| Fecal occult blood test within previous year | Sigmoidoscopy/colonoscopy within previous 5 years | Either test within recommended time interval | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n2 | % | (95% CI) | n | % | (95% CI) | N | % | (95% CI) | |
| Total | 61,952 | 20.6 | (20.1–21.2) | 61,953 | 33.6 | (33.0–34.2) | 61,537 | 44.0 | (43.3–44.6) |
| Gender | |||||||||
| Male | 23,919 | 19.1 | (18.2–19.9) | 23,850 | 37.9 | (36.8–38.9) | 23,724 | 45.9 | (44.9–47.0) |
| Female | 38,033 | 22.0 | (21.3–22.7) | 38,103 | 30.4 | (29.6–31.1) | 37,813 | 42.6 | (41.7–43.4) |
| Race4 | |||||||||
| White | 55,139 | 21.0 | (20.5–21.6) | 55,170 | 33.6 | (33.0–34.3) | 54,804 | 44.2 | (3.5–44.9) |
| Black | 4,075 | 20.7 | (18.8–22.6) | 4,046 | 32.6 | (30.3–34.9) | 4,020 | 43.3 | (40.9–45.7) |
| Asian/Pacific Islander | 739 | 10.3 | (6.9–13.6) | 739 | 35.4 | (28.4–42.5) | 735 | 40.1 | (33.3–46.9) |
| American Indian/ Alaska Native | 725 | 18.2 | (12.7–23.7) | 725 | 36.0 | (29.4–42.5) | 723 | 43.0 | (36.5–49.6 |
| Spanish or Hispanic origin | |||||||||
| Yes | 3,664 | 11.2 | (9.4–12.9) | 3,667 | 28.6 | (25.6–31.5) | 3,635 | 33.9 | (30.9–37.0) |
| No | 57,993 | 21.4 | (20.9–21.9) | 57,999 | 34.0 | (33.4–34.6) | 57,620 | 44.8 | (44.1–45.5) |
| Age (group) | |||||||||
| 50–59 years | 23,758 | 15.5 | (14.7–16.2) | 23,803 | 26.1 | (25.1–27.0) | 23,667 | 34.7 | (33.7–35.7) |
| 60–69 years | 17,680 | 23.0 | (22.0–24.0) | 17,651 | 36.9 | (35.7–38.1) | 17,574 | 48.1 | (46.9–49.3) |
| 70–79 years | 14,427 | 25.8 | (24.6–27.0) | 14,412 | 40.7 | (39.3–42.1) | 14,306 | 52.7 | (51.4–54.1) |
| ≤80 years | 6,087 | 21.6 | (19.8–23.4) | 6,087 | 36.1 | (34.0–38.2) | 5,990 | 46.9 | (44.7–49.1) |
| Education | |||||||||
| < 12 years | 11,928 | 15.0 | (13.8–16.1) | 11,889 | 27.5 | (25.9–29.1) | 11,756 | 35.6 | (33.9–37.3) |
| High school graduate | 21,183 | 19.7 | (18.8–20.6) | 21,176 | 30.6 | (29.6–31.6) | 21,049 | 41.2 | (40.1–42.3) |
| Some college/ technical school | 14,167 | 23.5 | (22.3–24.7) | 14,162 | 35.9 | (34.6–37.3) | 14,102 | 47.9 | (46.5–49.2) |
| College graduate | 14,503 | 24.3 | (23.2–25.5) | 14,560 | 41.1 | (39.8–42.5) | 14,466 | 51.4 | (50.1–52.7) |
| Income (annual household) | |||||||||
| <$20,000 | 15,204 | 15.3 | (14.3–16.3) | 15,154 | 29.1 | (27.7–30.5) | 15,029 | 37.0 | (35.5–38.4) |
| $20,000–34,999 | 14,354 | 20.8 | (19.7–21.9) | 14,362 | 32.5 | (31.2–33.8) | 14,288 | 43.0 | (41.7–44.4) |
| $35,000–49,999 | 7,721 | 23.3 | (21.6–24.9) | 7,718 | 37.0 | (35.1–39.0) | 7,703 | 48.2 | (46.3–50.1) |
| ≥$50,000 | 11,967 | 24.2 | (22.7–25.6) | 12,002 | 41.7 | (39.9–43.4) | 11,949 | 51.7 | (50.0–53.5) |
| Health care coverage | |||||||||
| Yes | 57,551 | 21.3 | (20.8–21.9) | 57,561 | 34.7 | (34.1–35.4) | 57,169 | 45.4 | (44.7–46.1) |
| No | 4,331 | 12.1 | (9.8–14.5) | 4,326 | 18.7 | (15.6–21.7) | 4,304 | 25.7 | (22.2–29.2) |
| 1From the Behavioral Risk Factor Surveillance System (BRFSS), 1999; estimates are age-adjusted. | |||||||||
| 2Sample size for each question; sample sizes may not sum to totals because of missing data. | |||||||||
| 3Confidence interval. | |||||||||
| 4Sample sizes for racial categories do not add up to column totals. “Other” racial category not presented here. | |||||||||
FIGURE
Comparison of use of colorectal cancer screening tests with other screening tests, BRFSS 1999
Discussion
Currently, 4 widely accepted tests are available for CRC screening, and several new tests are under investigation.16,17 Not enough evidence exists to determine which of the available tests is most appropriate when efficacy, cost-effectiveness, availability, patient acceptability, and safety are taken into consideration. The 1999 BRFSS monitored the use of 3 of these tests: FOBT and sigmoidoscopy or colonoscopy. Our results show that less than half of the US population aged 50 years and older is being screened for CRC with these methods. Persons with health care coverage and with higher education and income levels were more likely to have had CRC tests. Since 1997, the proportion of the U.S. population being screened for CRC has increased slightly, but it remains low and lags far behind the use of other recommended cancer screening tests (Figure). While use of barium enema, one of the recommended colorectal cancer screening tests, is not monitored in the 1999 BRFSS, data from a recent national primary care physician survey suggest that barium enemas are infrequently recommended for colorectal cancer screening (Carrie Klabunde, National Cancer Institute, personal/written communication, 2002).
The 1999 BRFSS was the first BRFSS survey to collect data on the use of colonoscopy. Because BRFSS colonoscopy data have not previously been collected, we do not know whether the reported increase in the use of endoscopy from 1997 to 1999 represents a true increase in sigmoidoscopy usage or previously unmeasured colonoscopy usage. Furthermore, it is likely that some of the tests reported as sigmoidoscopies or proctoscopies in the 1997 BRFSS survey were actually colonoscopies, since some respondents may be unable to clearly distinguish between the endoscopic tests.
Both patient-related and physician-related factors likely contribute to continued underuse of these tests. Patient-related factors include lack of awareness of screening guidelines, embarrassment, and lack of physician recommendation.7,18-20 Physician-related factors include lack of knowledge of the effectiveness of screening, lack of skills in endoscopy, and low reimbursement rates for screening tests.7,18-21
Several factors limit the interpretation of this analysis. First, as this is a telephone survey, only people who have access to telephones are represented in this analysis. However, approximately 95% of households in the United States have telephones.22 Second, 43.3% of the eligible respondents who were successfully contacted did not complete the telephone interview. Third, responses are self-reported and not validated through medical record review. However, a comparison of self-report and record review has found good concordance between results.23 Fourth, in the 1999 BRFSS, sigmoidoscopy use cannot be measured separately from colonoscopy, and screening tests cannot be distinguished from diagnostic tests. The results reported here may therefore be overestimates of use of these tests for screening. Lastly, the specialty of the physicians ordering the tests is unknown, limiting the ability to target interventions towards specific physician specialists. Despite these limitations, the BRFSS provides an excellent data source for routine surveillance of CRC testing.
Conclusion
This report demonstrates that CRC screening tests remain underused, despite their recognized efficacy in reducing CRC incidence and mortality.2-8 Coordinated efforts by clinicians and policy makers to raise awareness about this important disease and promote use of available screening tests must continue.
CORRESPONDENCE
Epidemiology and Health Services Research Branch, Division of Cancer Prevention and Control, Centers for Disease Control and Prevention, Atlanta, GA. Data from this paper were presented at CDC’s 15th National Conference on Chronic Disease Prevention and Control on November 30, 2000. Send correspondence and reprint requests to: Laura C. Seeff, MD; Centers for Disease Control and Prevention, DCPC; 4770 Buford Highway NE; Mailstop K-55; Atlanta, Georgia 30341-3717. E-mail: [email protected].
1. American Cancer Society. Cancer facts and figures, 2002. Atlanta: American Cancer Society, Inc., 2002. Publication 02-250M-No. 5008.02.
2. Mandel JS, Church TR, Bond JH, et al. The effect of fecal occultblood screening on the incidence of colorectal cancer. N Engl J Med 2000;343:1603-7.
3. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from col-orectal cancer by screening for fecal occult blood. N Engl J Med 1993;328:1365-71.
4. Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. A case-con-trol study of screening sigmoidoscopy and mortality from colorec-tal cancer. N Engl J Med 1992;326:653-7.
5. Newcomb PA, Norfleet RG, Storer BE, Surawicz TS, Marcus PM. Screening sigmoidoscopy and colorectal cancer mortality. J Natl Cancer Inst 1992;84:1572-5.
6. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348:1472-1477.
7. Kronborg O, Fenger C, Olsen J, Jorgensen OD. Sondergaard. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996;348:1467-1.
8. Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: Effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst 1999;91:434-7.
9. U.S.Preventive Services Task Force. Guide to clinical preventive services. 2nd ed. Baltimore: Williams and Wilkins, 1996.
10. Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology 1997;112:594-642.
11. Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. CA Cancer J Clin 2001;51:38-75.
12. Shapiro JA, Seeff LC, Nadel MR. Colorectal cancer-screening tests and associated health behaviors. Am J Prev Med 2001;21:132-7.
13. SAS statistical analysis software. Cary, NC: SAS Institute, 1996.
14. SUDAAN software for the statistical analysis of correlated data. Research Triangle Park, NC: Research Triangle Institute, 1997.
15. Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System, 1999. BRFSS Summary Quality Control Report. Atlanta: National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, 1999.
16. Fenlon HM, Nunes DP, Schroy PC, III, Barish MA, Clarke PD, Ferrucci JT. A comparison of virtual and conventional colonoscopy for the detection of colorectal polyps. N Engl J Med 1999;341:1496-503.
17. Ahlquist DA, Skoletsky JE, Boynton KA, et al. Colorectal cancer screening by detection of altered DNA in stool: feasibility of a mul-titarget assay panel. Gastroenterology 2000;119:1219-27.
18. Vernon SW. Participation in colorectal cancer screening: A review. J Natl Cancer Inst 1997;89:1406-22.
19. Peterson SK, Vernon SW. A review of patient and physician adherence to colorectal cancer screening guidelines. Semin Colon Rectal Surg 2000;11:58-72.
20. McCarthy BD, Moskowitz MA. Screening flexible sigmoidoscopy: patient attitudes and compliance. J Gen Intern Med. 1993;8:120-5.
21. Lewis JD, Asch DA. Barriers to office-based screening sigmoi-doscopy: does reimbursement cover costs? Ann Intern Med 1999;130:525-30.
22. Anderson JE, Nelson DE, Wilson RW. Telephone coverage and measurement of health risk indicators: data from the National Health Interview Survey. Am J Public Health 1998;88:1392-5.
23. Baier M, Calonge N, Cutter G, et al. Validity of self-reported col-orectal cancer screening behavior. Cancer Epidemiol Biomarkers Prev 2000;9:229-32.
1. American Cancer Society. Cancer facts and figures, 2002. Atlanta: American Cancer Society, Inc., 2002. Publication 02-250M-No. 5008.02.
2. Mandel JS, Church TR, Bond JH, et al. The effect of fecal occultblood screening on the incidence of colorectal cancer. N Engl J Med 2000;343:1603-7.
3. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from col-orectal cancer by screening for fecal occult blood. N Engl J Med 1993;328:1365-71.
4. Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. A case-con-trol study of screening sigmoidoscopy and mortality from colorec-tal cancer. N Engl J Med 1992;326:653-7.
5. Newcomb PA, Norfleet RG, Storer BE, Surawicz TS, Marcus PM. Screening sigmoidoscopy and colorectal cancer mortality. J Natl Cancer Inst 1992;84:1572-5.
6. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348:1472-1477.
7. Kronborg O, Fenger C, Olsen J, Jorgensen OD. Sondergaard. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996;348:1467-1.
8. Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: Effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst 1999;91:434-7.
9. U.S.Preventive Services Task Force. Guide to clinical preventive services. 2nd ed. Baltimore: Williams and Wilkins, 1996.
10. Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology 1997;112:594-642.
11. Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. CA Cancer J Clin 2001;51:38-75.
12. Shapiro JA, Seeff LC, Nadel MR. Colorectal cancer-screening tests and associated health behaviors. Am J Prev Med 2001;21:132-7.
13. SAS statistical analysis software. Cary, NC: SAS Institute, 1996.
14. SUDAAN software for the statistical analysis of correlated data. Research Triangle Park, NC: Research Triangle Institute, 1997.
15. Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System, 1999. BRFSS Summary Quality Control Report. Atlanta: National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, 1999.
16. Fenlon HM, Nunes DP, Schroy PC, III, Barish MA, Clarke PD, Ferrucci JT. A comparison of virtual and conventional colonoscopy for the detection of colorectal polyps. N Engl J Med 1999;341:1496-503.
17. Ahlquist DA, Skoletsky JE, Boynton KA, et al. Colorectal cancer screening by detection of altered DNA in stool: feasibility of a mul-titarget assay panel. Gastroenterology 2000;119:1219-27.
18. Vernon SW. Participation in colorectal cancer screening: A review. J Natl Cancer Inst 1997;89:1406-22.
19. Peterson SK, Vernon SW. A review of patient and physician adherence to colorectal cancer screening guidelines. Semin Colon Rectal Surg 2000;11:58-72.
20. McCarthy BD, Moskowitz MA. Screening flexible sigmoidoscopy: patient attitudes and compliance. J Gen Intern Med. 1993;8:120-5.
21. Lewis JD, Asch DA. Barriers to office-based screening sigmoi-doscopy: does reimbursement cover costs? Ann Intern Med 1999;130:525-30.
22. Anderson JE, Nelson DE, Wilson RW. Telephone coverage and measurement of health risk indicators: data from the National Health Interview Survey. Am J Public Health 1998;88:1392-5.
23. Baier M, Calonge N, Cutter G, et al. Validity of self-reported col-orectal cancer screening behavior. Cancer Epidemiol Biomarkers Prev 2000;9:229-32.