User login
What therapies alleviate symptoms of polycystic ovary syndrome?
Treatment of polycystic ovary syndrome (PCOS) in women not actively seeking to become pregnant is symptom-specific. Lifestyle modification (LSM) reduces body weight by 3.5 kg (strength of recommendation [SOR]: A, meta-analysis) and metformin reduces it by 3 kg (SOR B, cohort trial).
LSM may be better tolerated; adding metformin to LSM doesn’t lead to additional weight loss (SOR: B, randomized controlled trial [RCT]).
Spironolactone improves hirsutism scores by an absolute 8% to 22% (SOR: A, multiple RCTs); adding metformin to spironolactone improves Ferriman-Gallwey (FG) hirsutism scores an additional absolute 1.4% (SOR: B, RCT). Oral contraceptive pills (OCPs) are 12 times more likely to result in complete menstrual regularity than metformin (SOR: A, meta-analysis). Combining OCPs with metformin improves hirsutism scores by 8% over using an OCP alone (SOR: A, meta-analysis).
Statin medications don’t alter weight, hirsutism, or menstruation (SOR: B, small meta-analysis).
EVIDENCE SUMMARY
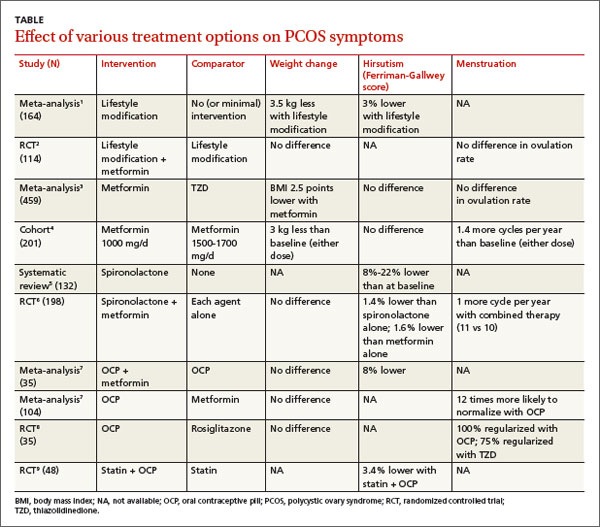
Women with PCOS who are not seeking pregnancy commonly have symptoms such as excessive weight, hirsutism, and menstrual irregularities. This review focuses on interventions to manage those symptoms. The TABLE summarizes the results of the interventions.
Lifestyle modification improves symptoms; no benefit to adding metformin
A Cochrane meta-analysis of 6 RCTs with 164 patients compared LSM (with diet and exercise) and no or minimal intervention. LSM reduced weight more than minimal intervention (mean difference [MD]=-3.5 kg; 95% confidence interval [CI], -4.5 to -2.0).1 It also improved hirsutism, assessed with the 36-point FG score, where a lower score corresponds to less hirsutism (MD=-1.2 points, 95% CI, -2.4 to -0.1). No data were available on menstrual regularity.
A double-blind RCT comparing LSM alone with LSM plus metformin in 114 patients with PCOS found no difference in mean weight reduction (-2- to -3 kg, data from graph), ovulation rate, or androgen levels at 6 months.2 Six patients dropped out of the LSM-with-metformin group, whereas no patients dropped out of the LSM-alone group.
Metformin decreases BMI more than thiazolidinediones
In a meta-analysis of 10 RCTs (459 patients) comparing the effects of metformin and thiazolidinediones (TZDs), metformin reduced body mass index (BMI) more than TZDs at 3 months (weighted mean difference [WMD]=-2.5 kg/m2; 95% CI, -3.3 to -.6) and 6 months (WMD=-0.70 kg/m2; 95% CI, -0.76 to -0.65).3
In a prospective cohort dose-comparison study, 201 women with PCOS received either metformin 1000 mg or 1500 to 1700 mg daily for 6 months. Patients were asked not to modify their diet or exercise routines. In both dosage groups, patients lost weight from baseline (-3 kg; P<.01), and the number of menstrual cycles increased (0.7 per 6 months; P<.001).4 No clear dose-response relationship was observed.
Spironolactone can significantly reduce hirsutism
A systematic review identified 4 studies (132 patients) of antiandrogen therapy for hirsutism in PCOS. The 3 studies that used the FG score as an outcome all showed significant reductions in hirsutism after 6 to 12 months of treatment with spironolactone.5
A 6-month RCT of 198 patients with PCOS compared outcomes for spironolactone (50 mg/d), metformin (1000 mg/d), or both. Combined therapy was marginally better than either agent alone for reducing the FG score (end score for combined therapy 9.1 vs 9.6 for spironolactone and 9.7 for metformin, an absolute difference for combined therapy vs spironolactone of -0.5 FG points or -1.4%; P<.05).6
OCPs normalize menstrual cycles and reduce hirsutism
A Cochrane review evaluating the effects of OCPs on patients with PCOS included 4 RCTs (104 patients) that compared OCPs with metformin (1500-2000 mg/d) and 2 RCTs (70 patients) that compared the combination of an OCP and metformin with the OCP alone. Use of an OCP was much more likely to normalize menstrual cycling than metformin alone (2 trials, N=35; odds ratio [OR]=12; 95% CI, 2.2-100). Combining an OCP with metformin resulted in slightly better FG scores than an OCP alone (1 trial, N=40; WMD=-2.8 points; 95% CI, -5.4 to -0.17).7 There was no difference in the final BMI between patients taking an OCP alone, metformin alone, or both.
An RCT of 35 patients compared the effect on insulin levels of an OCP with rosiglitazone 4 mg/d and also looked at menstrual cycling as a secondary outcome. The study found no difference in effect on insulin levels in the 2 groups. All patients taking the OCP reported regular menstrual cycles at the end of the study compared with 75% of the patients taking rosiglitazone (P=.7).8 The study was underpowered to find a difference, however.
Statins alone don’t affect hirsutism, menstruation, or BMI
A Cochrane review identified 4 RCTs (244 women, ages 18-39 years) that compared a statin alone with placebo, another agent, or another agent plus a statin.9 One RCT of 48 patients found that a statin combined with an OCP improved hirsutism compared with a statin alone. Two RCTs (85 patients) found that statins didn’t lead to resumption of regular menstrual cycles. Statins also didn’t alter BMI in 3 studies of 105 patients.
Trials report no adverse effects, but VTE may be a concern with OCPs
A meta-analysis evaluated the safety of metformin, OCPs, and antiandrogens in 22 clinical trials with 1335 patients, primarily PCOS patients. The trials reported no cases of lactic acidosis with metformin, no drug-induced liver injury with antiandrogens, and no venous thromboembolism (VTE) with OCPs. The meta-analysis authors noted, however, that in a cohort trial of 1.6 million Danish women followed for 15 years, OCPs were associated with a 2- to 3-fold increase in risk of VTE, with higher risks linked to higher ethinyl estradiol content.10
RECOMMENDATIONS
A 2009 practice bulletin from The American College of Obestetrics and Gynecology (ACOG) recommends OCPs, progestin, metformin, and TZDs for anovulation and amenorrhea in patients with PCOS. OCPs, antiandrogens, metformin, eflornithine, and mechanical hair removal are recommended for hirsutism. ACOG advocates LSM, insulin-sensitizing agents (such as metformin), and statins to prevent cardiovascular disease and diabetes.11
1. Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;(2):CD007506.
2. Ladson G, Dodson WC, Sweet SD, et al. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril. 2011;95:1059-1066.
3. Li XJ, Yu YX, Liu CQ, et al. Metformin vs thiazolidinediones for treatment of clinical, hormonal and metabolic characteristics of polycystic ovary syndrome: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:332-339.
4. Fulghesu AM, Romualdi D, Di Florio C, et al. Is there a doseresponse relationship of metformin treatment in patients with polycystic ovary syndrome? Results from a multicentric study. Hum Reprod. 2012; 27:3057-3066.
5. Christy NA, Franks AS, Cross LB. Spironolactone for hirsutism in polycystic ovary syndrome. Ann Pharmacother. 2005;39:1517-1521.
6. Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
7. Costello M, Shrestha B, Eden J, et al. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;(1):CD005552.
8. Tfayli H, Ulnach JW, Lee S, et al. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab. 2011;96:1311-1319.
9. Raval AD, Hunter T, Stuckey B, et al. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011;(10):CD008565.
10. Domecq JP, Prutsky G, Mullan RJ, et al. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4646-4654.
11. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 108: Polycystic ovary syndrome. Obstet Gynecol. 2009;114:936-949.
Treatment of polycystic ovary syndrome (PCOS) in women not actively seeking to become pregnant is symptom-specific. Lifestyle modification (LSM) reduces body weight by 3.5 kg (strength of recommendation [SOR]: A, meta-analysis) and metformin reduces it by 3 kg (SOR B, cohort trial).
LSM may be better tolerated; adding metformin to LSM doesn’t lead to additional weight loss (SOR: B, randomized controlled trial [RCT]).
Spironolactone improves hirsutism scores by an absolute 8% to 22% (SOR: A, multiple RCTs); adding metformin to spironolactone improves Ferriman-Gallwey (FG) hirsutism scores an additional absolute 1.4% (SOR: B, RCT). Oral contraceptive pills (OCPs) are 12 times more likely to result in complete menstrual regularity than metformin (SOR: A, meta-analysis). Combining OCPs with metformin improves hirsutism scores by 8% over using an OCP alone (SOR: A, meta-analysis).
Statin medications don’t alter weight, hirsutism, or menstruation (SOR: B, small meta-analysis).
EVIDENCE SUMMARY
Women with PCOS who are not seeking pregnancy commonly have symptoms such as excessive weight, hirsutism, and menstrual irregularities. This review focuses on interventions to manage those symptoms. The TABLE summarizes the results of the interventions.
Lifestyle modification improves symptoms; no benefit to adding metformin
A Cochrane meta-analysis of 6 RCTs with 164 patients compared LSM (with diet and exercise) and no or minimal intervention. LSM reduced weight more than minimal intervention (mean difference [MD]=-3.5 kg; 95% confidence interval [CI], -4.5 to -2.0).1 It also improved hirsutism, assessed with the 36-point FG score, where a lower score corresponds to less hirsutism (MD=-1.2 points, 95% CI, -2.4 to -0.1). No data were available on menstrual regularity.
A double-blind RCT comparing LSM alone with LSM plus metformin in 114 patients with PCOS found no difference in mean weight reduction (-2- to -3 kg, data from graph), ovulation rate, or androgen levels at 6 months.2 Six patients dropped out of the LSM-with-metformin group, whereas no patients dropped out of the LSM-alone group.

Metformin decreases BMI more than thiazolidinediones
In a meta-analysis of 10 RCTs (459 patients) comparing the effects of metformin and thiazolidinediones (TZDs), metformin reduced body mass index (BMI) more than TZDs at 3 months (weighted mean difference [WMD]=-2.5 kg/m2; 95% CI, -3.3 to -.6) and 6 months (WMD=-0.70 kg/m2; 95% CI, -0.76 to -0.65).3
In a prospective cohort dose-comparison study, 201 women with PCOS received either metformin 1000 mg or 1500 to 1700 mg daily for 6 months. Patients were asked not to modify their diet or exercise routines. In both dosage groups, patients lost weight from baseline (-3 kg; P<.01), and the number of menstrual cycles increased (0.7 per 6 months; P<.001).4 No clear dose-response relationship was observed.
Spironolactone can significantly reduce hirsutism
A systematic review identified 4 studies (132 patients) of antiandrogen therapy for hirsutism in PCOS. The 3 studies that used the FG score as an outcome all showed significant reductions in hirsutism after 6 to 12 months of treatment with spironolactone.5
A 6-month RCT of 198 patients with PCOS compared outcomes for spironolactone (50 mg/d), metformin (1000 mg/d), or both. Combined therapy was marginally better than either agent alone for reducing the FG score (end score for combined therapy 9.1 vs 9.6 for spironolactone and 9.7 for metformin, an absolute difference for combined therapy vs spironolactone of -0.5 FG points or -1.4%; P<.05).6
OCPs normalize menstrual cycles and reduce hirsutism
A Cochrane review evaluating the effects of OCPs on patients with PCOS included 4 RCTs (104 patients) that compared OCPs with metformin (1500-2000 mg/d) and 2 RCTs (70 patients) that compared the combination of an OCP and metformin with the OCP alone. Use of an OCP was much more likely to normalize menstrual cycling than metformin alone (2 trials, N=35; odds ratio [OR]=12; 95% CI, 2.2-100). Combining an OCP with metformin resulted in slightly better FG scores than an OCP alone (1 trial, N=40; WMD=-2.8 points; 95% CI, -5.4 to -0.17).7 There was no difference in the final BMI between patients taking an OCP alone, metformin alone, or both.
An RCT of 35 patients compared the effect on insulin levels of an OCP with rosiglitazone 4 mg/d and also looked at menstrual cycling as a secondary outcome. The study found no difference in effect on insulin levels in the 2 groups. All patients taking the OCP reported regular menstrual cycles at the end of the study compared with 75% of the patients taking rosiglitazone (P=.7).8 The study was underpowered to find a difference, however.
Statins alone don’t affect hirsutism, menstruation, or BMI
A Cochrane review identified 4 RCTs (244 women, ages 18-39 years) that compared a statin alone with placebo, another agent, or another agent plus a statin.9 One RCT of 48 patients found that a statin combined with an OCP improved hirsutism compared with a statin alone. Two RCTs (85 patients) found that statins didn’t lead to resumption of regular menstrual cycles. Statins also didn’t alter BMI in 3 studies of 105 patients.
Trials report no adverse effects, but VTE may be a concern with OCPs
A meta-analysis evaluated the safety of metformin, OCPs, and antiandrogens in 22 clinical trials with 1335 patients, primarily PCOS patients. The trials reported no cases of lactic acidosis with metformin, no drug-induced liver injury with antiandrogens, and no venous thromboembolism (VTE) with OCPs. The meta-analysis authors noted, however, that in a cohort trial of 1.6 million Danish women followed for 15 years, OCPs were associated with a 2- to 3-fold increase in risk of VTE, with higher risks linked to higher ethinyl estradiol content.10
RECOMMENDATIONS
A 2009 practice bulletin from The American College of Obestetrics and Gynecology (ACOG) recommends OCPs, progestin, metformin, and TZDs for anovulation and amenorrhea in patients with PCOS. OCPs, antiandrogens, metformin, eflornithine, and mechanical hair removal are recommended for hirsutism. ACOG advocates LSM, insulin-sensitizing agents (such as metformin), and statins to prevent cardiovascular disease and diabetes.11
Treatment of polycystic ovary syndrome (PCOS) in women not actively seeking to become pregnant is symptom-specific. Lifestyle modification (LSM) reduces body weight by 3.5 kg (strength of recommendation [SOR]: A, meta-analysis) and metformin reduces it by 3 kg (SOR B, cohort trial).
LSM may be better tolerated; adding metformin to LSM doesn’t lead to additional weight loss (SOR: B, randomized controlled trial [RCT]).
Spironolactone improves hirsutism scores by an absolute 8% to 22% (SOR: A, multiple RCTs); adding metformin to spironolactone improves Ferriman-Gallwey (FG) hirsutism scores an additional absolute 1.4% (SOR: B, RCT). Oral contraceptive pills (OCPs) are 12 times more likely to result in complete menstrual regularity than metformin (SOR: A, meta-analysis). Combining OCPs with metformin improves hirsutism scores by 8% over using an OCP alone (SOR: A, meta-analysis).
Statin medications don’t alter weight, hirsutism, or menstruation (SOR: B, small meta-analysis).
EVIDENCE SUMMARY
Women with PCOS who are not seeking pregnancy commonly have symptoms such as excessive weight, hirsutism, and menstrual irregularities. This review focuses on interventions to manage those symptoms. The TABLE summarizes the results of the interventions.
Lifestyle modification improves symptoms; no benefit to adding metformin
A Cochrane meta-analysis of 6 RCTs with 164 patients compared LSM (with diet and exercise) and no or minimal intervention. LSM reduced weight more than minimal intervention (mean difference [MD]=-3.5 kg; 95% confidence interval [CI], -4.5 to -2.0).1 It also improved hirsutism, assessed with the 36-point FG score, where a lower score corresponds to less hirsutism (MD=-1.2 points, 95% CI, -2.4 to -0.1). No data were available on menstrual regularity.
A double-blind RCT comparing LSM alone with LSM plus metformin in 114 patients with PCOS found no difference in mean weight reduction (-2- to -3 kg, data from graph), ovulation rate, or androgen levels at 6 months.2 Six patients dropped out of the LSM-with-metformin group, whereas no patients dropped out of the LSM-alone group.

Metformin decreases BMI more than thiazolidinediones
In a meta-analysis of 10 RCTs (459 patients) comparing the effects of metformin and thiazolidinediones (TZDs), metformin reduced body mass index (BMI) more than TZDs at 3 months (weighted mean difference [WMD]=-2.5 kg/m2; 95% CI, -3.3 to -.6) and 6 months (WMD=-0.70 kg/m2; 95% CI, -0.76 to -0.65).3
In a prospective cohort dose-comparison study, 201 women with PCOS received either metformin 1000 mg or 1500 to 1700 mg daily for 6 months. Patients were asked not to modify their diet or exercise routines. In both dosage groups, patients lost weight from baseline (-3 kg; P<.01), and the number of menstrual cycles increased (0.7 per 6 months; P<.001).4 No clear dose-response relationship was observed.
Spironolactone can significantly reduce hirsutism
A systematic review identified 4 studies (132 patients) of antiandrogen therapy for hirsutism in PCOS. The 3 studies that used the FG score as an outcome all showed significant reductions in hirsutism after 6 to 12 months of treatment with spironolactone.5
A 6-month RCT of 198 patients with PCOS compared outcomes for spironolactone (50 mg/d), metformin (1000 mg/d), or both. Combined therapy was marginally better than either agent alone for reducing the FG score (end score for combined therapy 9.1 vs 9.6 for spironolactone and 9.7 for metformin, an absolute difference for combined therapy vs spironolactone of -0.5 FG points or -1.4%; P<.05).6
OCPs normalize menstrual cycles and reduce hirsutism
A Cochrane review evaluating the effects of OCPs on patients with PCOS included 4 RCTs (104 patients) that compared OCPs with metformin (1500-2000 mg/d) and 2 RCTs (70 patients) that compared the combination of an OCP and metformin with the OCP alone. Use of an OCP was much more likely to normalize menstrual cycling than metformin alone (2 trials, N=35; odds ratio [OR]=12; 95% CI, 2.2-100). Combining an OCP with metformin resulted in slightly better FG scores than an OCP alone (1 trial, N=40; WMD=-2.8 points; 95% CI, -5.4 to -0.17).7 There was no difference in the final BMI between patients taking an OCP alone, metformin alone, or both.
An RCT of 35 patients compared the effect on insulin levels of an OCP with rosiglitazone 4 mg/d and also looked at menstrual cycling as a secondary outcome. The study found no difference in effect on insulin levels in the 2 groups. All patients taking the OCP reported regular menstrual cycles at the end of the study compared with 75% of the patients taking rosiglitazone (P=.7).8 The study was underpowered to find a difference, however.
Statins alone don’t affect hirsutism, menstruation, or BMI
A Cochrane review identified 4 RCTs (244 women, ages 18-39 years) that compared a statin alone with placebo, another agent, or another agent plus a statin.9 One RCT of 48 patients found that a statin combined with an OCP improved hirsutism compared with a statin alone. Two RCTs (85 patients) found that statins didn’t lead to resumption of regular menstrual cycles. Statins also didn’t alter BMI in 3 studies of 105 patients.
Trials report no adverse effects, but VTE may be a concern with OCPs
A meta-analysis evaluated the safety of metformin, OCPs, and antiandrogens in 22 clinical trials with 1335 patients, primarily PCOS patients. The trials reported no cases of lactic acidosis with metformin, no drug-induced liver injury with antiandrogens, and no venous thromboembolism (VTE) with OCPs. The meta-analysis authors noted, however, that in a cohort trial of 1.6 million Danish women followed for 15 years, OCPs were associated with a 2- to 3-fold increase in risk of VTE, with higher risks linked to higher ethinyl estradiol content.10
RECOMMENDATIONS
A 2009 practice bulletin from The American College of Obestetrics and Gynecology (ACOG) recommends OCPs, progestin, metformin, and TZDs for anovulation and amenorrhea in patients with PCOS. OCPs, antiandrogens, metformin, eflornithine, and mechanical hair removal are recommended for hirsutism. ACOG advocates LSM, insulin-sensitizing agents (such as metformin), and statins to prevent cardiovascular disease and diabetes.11
1. Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;(2):CD007506.
2. Ladson G, Dodson WC, Sweet SD, et al. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril. 2011;95:1059-1066.
3. Li XJ, Yu YX, Liu CQ, et al. Metformin vs thiazolidinediones for treatment of clinical, hormonal and metabolic characteristics of polycystic ovary syndrome: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:332-339.
4. Fulghesu AM, Romualdi D, Di Florio C, et al. Is there a doseresponse relationship of metformin treatment in patients with polycystic ovary syndrome? Results from a multicentric study. Hum Reprod. 2012; 27:3057-3066.
5. Christy NA, Franks AS, Cross LB. Spironolactone for hirsutism in polycystic ovary syndrome. Ann Pharmacother. 2005;39:1517-1521.
6. Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
7. Costello M, Shrestha B, Eden J, et al. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;(1):CD005552.
8. Tfayli H, Ulnach JW, Lee S, et al. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab. 2011;96:1311-1319.
9. Raval AD, Hunter T, Stuckey B, et al. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011;(10):CD008565.
10. Domecq JP, Prutsky G, Mullan RJ, et al. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4646-4654.
11. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 108: Polycystic ovary syndrome. Obstet Gynecol. 2009;114:936-949.
1. Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;(2):CD007506.
2. Ladson G, Dodson WC, Sweet SD, et al. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril. 2011;95:1059-1066.
3. Li XJ, Yu YX, Liu CQ, et al. Metformin vs thiazolidinediones for treatment of clinical, hormonal and metabolic characteristics of polycystic ovary syndrome: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:332-339.
4. Fulghesu AM, Romualdi D, Di Florio C, et al. Is there a doseresponse relationship of metformin treatment in patients with polycystic ovary syndrome? Results from a multicentric study. Hum Reprod. 2012; 27:3057-3066.
5. Christy NA, Franks AS, Cross LB. Spironolactone for hirsutism in polycystic ovary syndrome. Ann Pharmacother. 2005;39:1517-1521.
6. Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
7. Costello M, Shrestha B, Eden J, et al. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;(1):CD005552.
8. Tfayli H, Ulnach JW, Lee S, et al. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab. 2011;96:1311-1319.
9. Raval AD, Hunter T, Stuckey B, et al. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011;(10):CD008565.
10. Domecq JP, Prutsky G, Mullan RJ, et al. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4646-4654.
11. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 108: Polycystic ovary syndrome. Obstet Gynecol. 2009;114:936-949.
Evidence-based answers from the Family Physicians Inquiries Network