User login
Does taking BP medicine at night (vs morning) result in fewer cardiovascular events?
Evidence summary
Recent UK study shows no difference by timing
A 2022 UK prospective, randomized, multicenter trial assigned 21,104 predominantly White adults (58% men) with hypertension to take their usual antihypertensive medication either in the morning (6
All patient baseline characteristics were equivalent between groups. If troubled by nocturia, patients in the evening group taking diuretics were told to take only the diuretic earlier (6
The median follow-up was 5.2 years. Data were collected at regular intervals through patient completion of online questionnaires and researcher analysis of National Health Service data on hospitalization and death. The intention-to-treat analysis showed no difference in the primary outcome (a composite of vascular death, nonfatal myocardial infarction, or nonfatal stroke) between the evening and morning administration groups (0.69 events vs 0.72 events per 100 person-years; hazard ratio [HR] = 0.95; 95% CI, 0.83-1.10; P = .53).
The controversial Hygia Project favored evening
Prior to the UK study was the Hygia Chronotherapy Trial, a prospective, controlled, multicenter study conducted within the primary care setting in Spain. Caucasian Spanish adults (N = 19,168; mean age, 61 years; 56% men) with hypertension were randomly assigned to take all prescribed antihypertensive medication either at bedtime or upon waking.2
The Hygia Project initially sought to establish the value of ambulatory blood pressure monitoring (ABPM) compared to office blood pressure (BP) monitoring and to explore the prognostic value of sleeping BP.3 The study objectives evolved over time. The randomization process was not clearly described,2,3 but multiple randomizations were alluded to. The authors stated that “for any of these chronotherapy trials” randomizations were done separately for “each participating center” and “randomization of participants to treatment-time regimen is done separately for each hypertension medication or combination being tested.”
The baseline characteristics of patients in the evening and morning administration groups were similar, but statistically significant differences existed in BMI (29.6 vs 29.7; P = .030) and sleep-time systolic BP percent decline (9.3 vs 9.0; P < .001). Mean baseline 48-hour BP was 132/77 mm Hg. Hypertension was defined as an awake systolic BP ≥
Prescribers were free to prescribe medicines from 5 classes (diuretic, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or beta-blocker) as they thought appropriate, were encouraged to use fixed-dose combination pills, and were told not to use split (eg, twice per day) dosing. Annual 48-hour ABPM was completed, and patients’ electronic health records were analyzed by blinded investigators. Median follow-up was 6.3 years, and only 84 participants failed to complete the minimum 1-year participation requirement.
Continue to: The primary outcome...
The primary outcome—a composite of cardiovascular death, myocardial infarction, coronary revascularization, heart failure, or stroke—occurred in 1752 patients, favoring the bedtime group (HR = 0.55; 95% CI, 0.50-0.61; P < .001). The calculated number of events was 1130 in the morning administration group and 622 in the evening administration group; the authors did not explicitly report the event numbers in each group. Each component of the composite outcome also favored evening administration (P < .001 for all): cardiovascular death (HR = 0.44; 95% CI, 0.34-0.56), myocardial infarction (HR = 0.66; 95% CI, 0.52-0.84), coronary revascularization (HR = 0.60; 95% CI, 0.47-0.75), heart failure (HR = 0.58; 95% CI, 0.49-0.70), and stroke (HR = 0.51; 95% CI, 0.41-0.63).
The complicated, layered study design and randomization methods limit the ability to critically appraise the study.
Smaller Spanish study also supported evening administration
A prior, smaller, prospective randomized trial conducted by the same researchers as the Hygia Project found even greater benefits to evening BP medication administration.4 The 2156 Spanish patients (52% men; average age, 55 years) from multiple primary care offices were randomized 1:1 to BP medication administration either upon awakening or at bedtime. Dozens of baseline characteristics were evenly distributed except for age (55.0 vs 56.3; P = .021) and creatinine (0.96 vs 0.98; P = .028), both of which were lower in the evening group.
After a median follow-up of 5.6 years, the bedtime group had significantly lower total events (187 events in the morning group vs 68 in the evening group; relative risk [RR] = 0.39; 95% CI, 0.29-0.51; P < .001). Individual cardiovascular outcomes also dramatically favored the evening group: total deaths (12 vs 28; P = .008), cardiovascular deaths (3 vs 14; P = .006), cardiovascular disease events (30 vs 74; P < .001), stroke (7 vs 24; P = .001), and heart failure (8 vs 33; P < .001).
Limits of both the UK trial and the Hygia Project trial included single countries of study with a lack of racial and ethnic diversity, and greater nonadherence to the evening administration of the medications.
Recommendations from others
A 2022 consensus statement from the International Society of Hypertension, published before the UK trial, recommended against bedtime dosing until more high-quality data became available. They pointed to evidence showing higher medication adherence with morning dosing, risk for asleep BP dropping, and worsening daytime BP control as reasons to continue morning administration.5 Other reviewers have questioned the Hygia Project results due to their reported implausibly large effects on cardiovascular outcomes, noting that independent attempts to verify the methods and the data have proven challenging and are not completed.6
Editor’s takeaway
I confess that I was swayed by the results of the Hygia Project; for a year or so, I advised my patients to take at least 1 BP pill at night. But after the UK study came out, I needed to reconsider. I began to worry that the great outcomes of nocturnal therapy may have been a mirage. I have returned to counseling patients to take their BP medications in whichever way fosters consistency while minimizing adverse effects for them.
1. Mackenzie IS, Rogers A, Poulter NR, et al; TIME Study Group. Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual antihypertensives in the UK (TIME study): a prospective, randomised, open-label, blinded-endpoint clinical trial. Lancet. 2022;400:1417-1425. doi: 10.1016/S0140-6736(22)01786-X
2. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al; Hygia Project Investigators. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J. 2020;41:4565-4576. doi: 10.1093/eurheartj/ehz754
3. Hermida RC. Sleep-time ambulatory blood pressure as a prognostic marker of vascular and other risks and therapeutic target for prevention by hypertension chronotherapy: rationale and design of the Hygia Project. Chronobiol Int. 2016;33:906-936. doi: 10.1080/07420528.2016.1181078
4. Hermida RC, Ayala DE, Mojón A, et al. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651. doi: 10.3109/07420528.2010.510230
5. Stergiou G, Brunström M, MacDonald T, et al. Bedtime dosing of antihypertensive medications: systematic review and consensus statement: International Society of Hypertension position paper endorsed by World Hypertension League and European Society of Hypertension. J Hypertens. 2022;40:1847-1858. doi: 10.1097/HJH.0000000000003240
6. Brunström M, Kjeldsen SE, Kreutz R, et al. Missing verification of source data in hypertension research: The HYGIA PROJECT in Perspective. Hypertension. 2021;78:555-558. doi: 10.1161/HYPERTENSIONAHA.121.17356
Evidence summary
Recent UK study shows no difference by timing
A 2022 UK prospective, randomized, multicenter trial assigned 21,104 predominantly White adults (58% men) with hypertension to take their usual antihypertensive medication either in the morning (6
All patient baseline characteristics were equivalent between groups. If troubled by nocturia, patients in the evening group taking diuretics were told to take only the diuretic earlier (6
The median follow-up was 5.2 years. Data were collected at regular intervals through patient completion of online questionnaires and researcher analysis of National Health Service data on hospitalization and death. The intention-to-treat analysis showed no difference in the primary outcome (a composite of vascular death, nonfatal myocardial infarction, or nonfatal stroke) between the evening and morning administration groups (0.69 events vs 0.72 events per 100 person-years; hazard ratio [HR] = 0.95; 95% CI, 0.83-1.10; P = .53).
The controversial Hygia Project favored evening
Prior to the UK study was the Hygia Chronotherapy Trial, a prospective, controlled, multicenter study conducted within the primary care setting in Spain. Caucasian Spanish adults (N = 19,168; mean age, 61 years; 56% men) with hypertension were randomly assigned to take all prescribed antihypertensive medication either at bedtime or upon waking.2
The Hygia Project initially sought to establish the value of ambulatory blood pressure monitoring (ABPM) compared to office blood pressure (BP) monitoring and to explore the prognostic value of sleeping BP.3 The study objectives evolved over time. The randomization process was not clearly described,2,3 but multiple randomizations were alluded to. The authors stated that “for any of these chronotherapy trials” randomizations were done separately for “each participating center” and “randomization of participants to treatment-time regimen is done separately for each hypertension medication or combination being tested.”
The baseline characteristics of patients in the evening and morning administration groups were similar, but statistically significant differences existed in BMI (29.6 vs 29.7; P = .030) and sleep-time systolic BP percent decline (9.3 vs 9.0; P < .001). Mean baseline 48-hour BP was 132/77 mm Hg. Hypertension was defined as an awake systolic BP ≥
Prescribers were free to prescribe medicines from 5 classes (diuretic, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or beta-blocker) as they thought appropriate, were encouraged to use fixed-dose combination pills, and were told not to use split (eg, twice per day) dosing. Annual 48-hour ABPM was completed, and patients’ electronic health records were analyzed by blinded investigators. Median follow-up was 6.3 years, and only 84 participants failed to complete the minimum 1-year participation requirement.
Continue to: The primary outcome...
The primary outcome—a composite of cardiovascular death, myocardial infarction, coronary revascularization, heart failure, or stroke—occurred in 1752 patients, favoring the bedtime group (HR = 0.55; 95% CI, 0.50-0.61; P < .001). The calculated number of events was 1130 in the morning administration group and 622 in the evening administration group; the authors did not explicitly report the event numbers in each group. Each component of the composite outcome also favored evening administration (P < .001 for all): cardiovascular death (HR = 0.44; 95% CI, 0.34-0.56), myocardial infarction (HR = 0.66; 95% CI, 0.52-0.84), coronary revascularization (HR = 0.60; 95% CI, 0.47-0.75), heart failure (HR = 0.58; 95% CI, 0.49-0.70), and stroke (HR = 0.51; 95% CI, 0.41-0.63).
The complicated, layered study design and randomization methods limit the ability to critically appraise the study.
Smaller Spanish study also supported evening administration
A prior, smaller, prospective randomized trial conducted by the same researchers as the Hygia Project found even greater benefits to evening BP medication administration.4 The 2156 Spanish patients (52% men; average age, 55 years) from multiple primary care offices were randomized 1:1 to BP medication administration either upon awakening or at bedtime. Dozens of baseline characteristics were evenly distributed except for age (55.0 vs 56.3; P = .021) and creatinine (0.96 vs 0.98; P = .028), both of which were lower in the evening group.
After a median follow-up of 5.6 years, the bedtime group had significantly lower total events (187 events in the morning group vs 68 in the evening group; relative risk [RR] = 0.39; 95% CI, 0.29-0.51; P < .001). Individual cardiovascular outcomes also dramatically favored the evening group: total deaths (12 vs 28; P = .008), cardiovascular deaths (3 vs 14; P = .006), cardiovascular disease events (30 vs 74; P < .001), stroke (7 vs 24; P = .001), and heart failure (8 vs 33; P < .001).
Limits of both the UK trial and the Hygia Project trial included single countries of study with a lack of racial and ethnic diversity, and greater nonadherence to the evening administration of the medications.
Recommendations from others
A 2022 consensus statement from the International Society of Hypertension, published before the UK trial, recommended against bedtime dosing until more high-quality data became available. They pointed to evidence showing higher medication adherence with morning dosing, risk for asleep BP dropping, and worsening daytime BP control as reasons to continue morning administration.5 Other reviewers have questioned the Hygia Project results due to their reported implausibly large effects on cardiovascular outcomes, noting that independent attempts to verify the methods and the data have proven challenging and are not completed.6
Editor’s takeaway
I confess that I was swayed by the results of the Hygia Project; for a year or so, I advised my patients to take at least 1 BP pill at night. But after the UK study came out, I needed to reconsider. I began to worry that the great outcomes of nocturnal therapy may have been a mirage. I have returned to counseling patients to take their BP medications in whichever way fosters consistency while minimizing adverse effects for them.
Evidence summary
Recent UK study shows no difference by timing
A 2022 UK prospective, randomized, multicenter trial assigned 21,104 predominantly White adults (58% men) with hypertension to take their usual antihypertensive medication either in the morning (6
All patient baseline characteristics were equivalent between groups. If troubled by nocturia, patients in the evening group taking diuretics were told to take only the diuretic earlier (6
The median follow-up was 5.2 years. Data were collected at regular intervals through patient completion of online questionnaires and researcher analysis of National Health Service data on hospitalization and death. The intention-to-treat analysis showed no difference in the primary outcome (a composite of vascular death, nonfatal myocardial infarction, or nonfatal stroke) between the evening and morning administration groups (0.69 events vs 0.72 events per 100 person-years; hazard ratio [HR] = 0.95; 95% CI, 0.83-1.10; P = .53).
The controversial Hygia Project favored evening
Prior to the UK study was the Hygia Chronotherapy Trial, a prospective, controlled, multicenter study conducted within the primary care setting in Spain. Caucasian Spanish adults (N = 19,168; mean age, 61 years; 56% men) with hypertension were randomly assigned to take all prescribed antihypertensive medication either at bedtime or upon waking.2
The Hygia Project initially sought to establish the value of ambulatory blood pressure monitoring (ABPM) compared to office blood pressure (BP) monitoring and to explore the prognostic value of sleeping BP.3 The study objectives evolved over time. The randomization process was not clearly described,2,3 but multiple randomizations were alluded to. The authors stated that “for any of these chronotherapy trials” randomizations were done separately for “each participating center” and “randomization of participants to treatment-time regimen is done separately for each hypertension medication or combination being tested.”
The baseline characteristics of patients in the evening and morning administration groups were similar, but statistically significant differences existed in BMI (29.6 vs 29.7; P = .030) and sleep-time systolic BP percent decline (9.3 vs 9.0; P < .001). Mean baseline 48-hour BP was 132/77 mm Hg. Hypertension was defined as an awake systolic BP ≥
Prescribers were free to prescribe medicines from 5 classes (diuretic, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or beta-blocker) as they thought appropriate, were encouraged to use fixed-dose combination pills, and were told not to use split (eg, twice per day) dosing. Annual 48-hour ABPM was completed, and patients’ electronic health records were analyzed by blinded investigators. Median follow-up was 6.3 years, and only 84 participants failed to complete the minimum 1-year participation requirement.
Continue to: The primary outcome...
The primary outcome—a composite of cardiovascular death, myocardial infarction, coronary revascularization, heart failure, or stroke—occurred in 1752 patients, favoring the bedtime group (HR = 0.55; 95% CI, 0.50-0.61; P < .001). The calculated number of events was 1130 in the morning administration group and 622 in the evening administration group; the authors did not explicitly report the event numbers in each group. Each component of the composite outcome also favored evening administration (P < .001 for all): cardiovascular death (HR = 0.44; 95% CI, 0.34-0.56), myocardial infarction (HR = 0.66; 95% CI, 0.52-0.84), coronary revascularization (HR = 0.60; 95% CI, 0.47-0.75), heart failure (HR = 0.58; 95% CI, 0.49-0.70), and stroke (HR = 0.51; 95% CI, 0.41-0.63).
The complicated, layered study design and randomization methods limit the ability to critically appraise the study.
Smaller Spanish study also supported evening administration
A prior, smaller, prospective randomized trial conducted by the same researchers as the Hygia Project found even greater benefits to evening BP medication administration.4 The 2156 Spanish patients (52% men; average age, 55 years) from multiple primary care offices were randomized 1:1 to BP medication administration either upon awakening or at bedtime. Dozens of baseline characteristics were evenly distributed except for age (55.0 vs 56.3; P = .021) and creatinine (0.96 vs 0.98; P = .028), both of which were lower in the evening group.
After a median follow-up of 5.6 years, the bedtime group had significantly lower total events (187 events in the morning group vs 68 in the evening group; relative risk [RR] = 0.39; 95% CI, 0.29-0.51; P < .001). Individual cardiovascular outcomes also dramatically favored the evening group: total deaths (12 vs 28; P = .008), cardiovascular deaths (3 vs 14; P = .006), cardiovascular disease events (30 vs 74; P < .001), stroke (7 vs 24; P = .001), and heart failure (8 vs 33; P < .001).
Limits of both the UK trial and the Hygia Project trial included single countries of study with a lack of racial and ethnic diversity, and greater nonadherence to the evening administration of the medications.
Recommendations from others
A 2022 consensus statement from the International Society of Hypertension, published before the UK trial, recommended against bedtime dosing until more high-quality data became available. They pointed to evidence showing higher medication adherence with morning dosing, risk for asleep BP dropping, and worsening daytime BP control as reasons to continue morning administration.5 Other reviewers have questioned the Hygia Project results due to their reported implausibly large effects on cardiovascular outcomes, noting that independent attempts to verify the methods and the data have proven challenging and are not completed.6
Editor’s takeaway
I confess that I was swayed by the results of the Hygia Project; for a year or so, I advised my patients to take at least 1 BP pill at night. But after the UK study came out, I needed to reconsider. I began to worry that the great outcomes of nocturnal therapy may have been a mirage. I have returned to counseling patients to take their BP medications in whichever way fosters consistency while minimizing adverse effects for them.
1. Mackenzie IS, Rogers A, Poulter NR, et al; TIME Study Group. Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual antihypertensives in the UK (TIME study): a prospective, randomised, open-label, blinded-endpoint clinical trial. Lancet. 2022;400:1417-1425. doi: 10.1016/S0140-6736(22)01786-X
2. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al; Hygia Project Investigators. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J. 2020;41:4565-4576. doi: 10.1093/eurheartj/ehz754
3. Hermida RC. Sleep-time ambulatory blood pressure as a prognostic marker of vascular and other risks and therapeutic target for prevention by hypertension chronotherapy: rationale and design of the Hygia Project. Chronobiol Int. 2016;33:906-936. doi: 10.1080/07420528.2016.1181078
4. Hermida RC, Ayala DE, Mojón A, et al. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651. doi: 10.3109/07420528.2010.510230
5. Stergiou G, Brunström M, MacDonald T, et al. Bedtime dosing of antihypertensive medications: systematic review and consensus statement: International Society of Hypertension position paper endorsed by World Hypertension League and European Society of Hypertension. J Hypertens. 2022;40:1847-1858. doi: 10.1097/HJH.0000000000003240
6. Brunström M, Kjeldsen SE, Kreutz R, et al. Missing verification of source data in hypertension research: The HYGIA PROJECT in Perspective. Hypertension. 2021;78:555-558. doi: 10.1161/HYPERTENSIONAHA.121.17356
1. Mackenzie IS, Rogers A, Poulter NR, et al; TIME Study Group. Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual antihypertensives in the UK (TIME study): a prospective, randomised, open-label, blinded-endpoint clinical trial. Lancet. 2022;400:1417-1425. doi: 10.1016/S0140-6736(22)01786-X
2. Hermida RC, Crespo JJ, Domínguez-Sardiña M, et al; Hygia Project Investigators. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J. 2020;41:4565-4576. doi: 10.1093/eurheartj/ehz754
3. Hermida RC. Sleep-time ambulatory blood pressure as a prognostic marker of vascular and other risks and therapeutic target for prevention by hypertension chronotherapy: rationale and design of the Hygia Project. Chronobiol Int. 2016;33:906-936. doi: 10.1080/07420528.2016.1181078
4. Hermida RC, Ayala DE, Mojón A, et al. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629-1651. doi: 10.3109/07420528.2010.510230
5. Stergiou G, Brunström M, MacDonald T, et al. Bedtime dosing of antihypertensive medications: systematic review and consensus statement: International Society of Hypertension position paper endorsed by World Hypertension League and European Society of Hypertension. J Hypertens. 2022;40:1847-1858. doi: 10.1097/HJH.0000000000003240
6. Brunström M, Kjeldsen SE, Kreutz R, et al. Missing verification of source data in hypertension research: The HYGIA PROJECT in Perspective. Hypertension. 2021;78:555-558. doi: 10.1161/HYPERTENSIONAHA.121.17356
EVIDENCE-BASED ANSWER:
Does vaginal estrogen use increase the risk for adverse cardiovascular outcomes?
Evidence summary
Cohort studies demonstrate no adverse CV outcomes
A 2020 systematic review and meta-analysis evaluated randomized controlled trials (RCTs) and observational studies to examine the association between menopausal hormone therapy and CV disease.1 The 26 RCTs primarily evaluated oral hormone administration. The observational studies comprised 30 cohort studies, 13 case-control studies, and 5 nested case-control studies, primarily in Europe and North America; 21 reported the route of administration. The trials evaluated women ages 49 to 77 years (mean, 61 years), and follow-up ranged from 1 to 21.5 years (mean, 7 years). In subgroup analyses of the observational studies, nonoral hormone therapy was associated with a lower risk for stroke and MI compared to oral administration (see TABLE1). Study limitations included enrollment of patients with few comorbidities, from limited geographic regions. Results in the meta-analysis were not stratified by the type of nonoral hormone therapy; only 4 studies evaluated vaginal estrogen use.
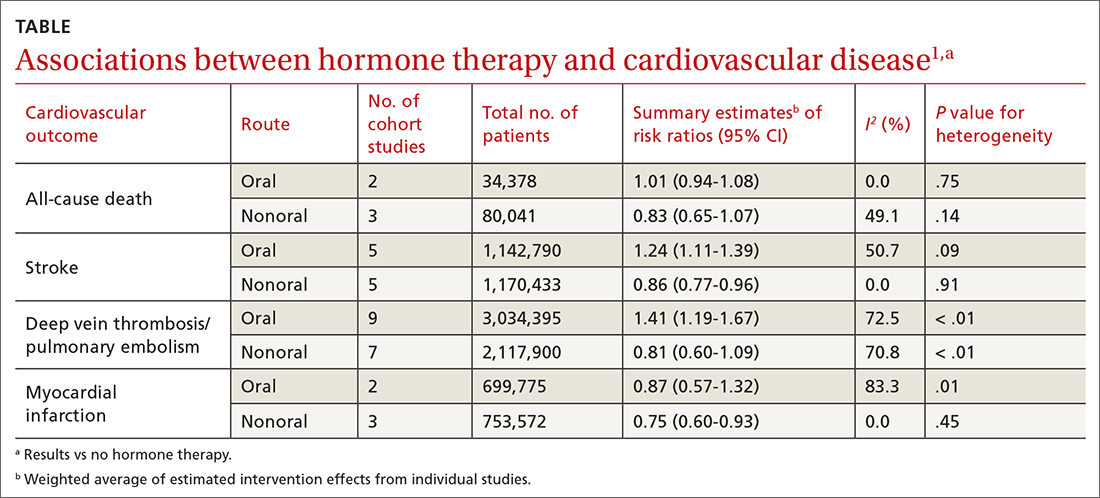
Two large cohort studies included in the systematic review provided more specific data on vaginal estrogens. The first used data from the Women’s Health Initiative in a subset of women ages 50 to 79 years (n = 46,566) who were not already on systemic hormone therapy and who did not have prior history of breast, endometrial, or ovarian cancer.2 Data were collected from self-assessment questionnaires and medical record reviews. The median duration of vaginal estrogen use was 2 years, and median follow-up duration was 7.2 years. Vaginal estrogen users had a 48% lower risk for CHD (adjusted hazard ratio [aHR] = 0.52; 95% CI, 0.31-0.85) than nonusers. Rates for all-cause mortality (aHR = 0.78; 95% CI, 0.58-1.04), stroke (aHR = 0.78; 95% CI, 0.49-1.24), and DVT/PE (aHR = 0.68; 95% CI, 0.36-1.28) were similar. In this and the other cohort studies to be discussed, outcome data for all vaginal estrogen preparations (eg, cream, ring, tablet) were combined.
The other large cohort study in the systematic review evaluated data on postmenopausal women from the Nurses’ Health Study.3 The authors evaluated health reports on 53,797 women as they transitioned through menopause. Patients with systemic hormone therapy use, history of cancer, and self-reported CV disease were excluded. After adjusting for covariates, the authors found no statistically significant difference between users and nonusers of vaginal estrogen and risk for total MI (aHR = 0.73; 95% CI, 0.47-1.13), stroke (aHR = 0.85; 95% CI, 0.56-1.29), or DVT/PE (aHR = 1.06; 95% CI, 0.58-1.93). Study limitations included low prevalence of vaginal estrogen use (< 3%), short duration of use (mean, 37.5 months), and lack of data on the type or dose of vaginal estrogen used. The study only included health professionals, which limits generalizability.
A Finnish cohort study (excluded from the systematic review because it used historical controls) compared rates of CHD and stroke in postmenopausal women who used vaginal estrogen against an age-matched background population. Researchers collected data from a nationwide prescription registry for women at least 50 years old who had purchased vaginal estrogens between 1994 and 2009 (n = 195,756).4 Women who purchased systemic hormone therapy at any point were excluded. After 3 to 5 years of exposure, use of vaginal estrogen was associated with a decreased risk for mortality from CHD (relative risk [RR] = 0.64; 95% CI, 0.57-0.70) and stroke (RR = 0.79; 95% CI, 0.69-0.91). However, after 10 years, these benefits were not seen (CHD: RR = 0.95; 95% CI, 0.90-1.00; stroke: RR = 0.93; 95% CI, 0.85-1.01). All confidence interval data were presented graphically. Key weaknesses of this study included use of both vaginal and systemic estrogen in the comparator background population, and the failure to collect data for other CV risk variables such as weight, tobacco exposure, and blood pressure.
Recommendations from others
In 2022, the North American Menopause Society issued a Hormone Therapy Position Statement that acknowledged the lack of clinical trials directly comparing risk for adverse CV endpoints with different estrogen administration routes.5 They stated nonoral routes of administration might offer advantages by bypassing first-pass hepatic metabolism.
Similarly, the 2015 Endocrine Society Clinical Practice Guideline on the Treatment of Symptoms of the Menopause also stated that the effects of low-dose vaginal estrogen therapy on CV disease or DVT/PE risk had not been adequately studied.6
A 2013 opinion by the American College of Obstetricians and Gynecologists stated that topical estrogen vaginal creams, tablets, and rings had low levels of systemic absorption and were not associated with an increased risk for DVT/PE.7
Editor’s takeaway
The available evidence on vaginal estrogen replacement reassures us of its safety. After decades spent studying hormone replacement therapy with vacillating conclusions and opinions, these cohorts—the best evidence we may ever get—along with a consensus of expert opinions, consistently demonstrate no adverse CV outcomes.
1. Kim JE, Chang JH, Jeong MJ, et al. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci Rep. 2020;10:20631. doi: 10.1038/s41598-020-77534-9
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the WHI Observational Study. Menopause. 2018;25:11-20. doi: 10.1097/GME.0000000000000956
3. Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610. doi: 10.1097/GME.0000000000001284
4. Mikkola TS, Tuomikoski P, Lyytinen H, et al. Vaginal estrogen use and the risk for cardiovascular mortality. Human Reproduction. 2016;31:804-809. doi: 10.1093/humrep/dew014
5. North American Menopause Society. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
6. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236
7. American College of Obstetricians and Gynecologists. Committee Opinion No 565: hormone therapy and heart disease. Obstet Gynecol. 2013;121:1407-1410. doi: 10.1097/01.AOG.0000431053.33593.2d
Evidence summary
Cohort studies demonstrate no adverse CV outcomes
A 2020 systematic review and meta-analysis evaluated randomized controlled trials (RCTs) and observational studies to examine the association between menopausal hormone therapy and CV disease.1 The 26 RCTs primarily evaluated oral hormone administration. The observational studies comprised 30 cohort studies, 13 case-control studies, and 5 nested case-control studies, primarily in Europe and North America; 21 reported the route of administration. The trials evaluated women ages 49 to 77 years (mean, 61 years), and follow-up ranged from 1 to 21.5 years (mean, 7 years). In subgroup analyses of the observational studies, nonoral hormone therapy was associated with a lower risk for stroke and MI compared to oral administration (see TABLE1). Study limitations included enrollment of patients with few comorbidities, from limited geographic regions. Results in the meta-analysis were not stratified by the type of nonoral hormone therapy; only 4 studies evaluated vaginal estrogen use.

Two large cohort studies included in the systematic review provided more specific data on vaginal estrogens. The first used data from the Women’s Health Initiative in a subset of women ages 50 to 79 years (n = 46,566) who were not already on systemic hormone therapy and who did not have prior history of breast, endometrial, or ovarian cancer.2 Data were collected from self-assessment questionnaires and medical record reviews. The median duration of vaginal estrogen use was 2 years, and median follow-up duration was 7.2 years. Vaginal estrogen users had a 48% lower risk for CHD (adjusted hazard ratio [aHR] = 0.52; 95% CI, 0.31-0.85) than nonusers. Rates for all-cause mortality (aHR = 0.78; 95% CI, 0.58-1.04), stroke (aHR = 0.78; 95% CI, 0.49-1.24), and DVT/PE (aHR = 0.68; 95% CI, 0.36-1.28) were similar. In this and the other cohort studies to be discussed, outcome data for all vaginal estrogen preparations (eg, cream, ring, tablet) were combined.
The other large cohort study in the systematic review evaluated data on postmenopausal women from the Nurses’ Health Study.3 The authors evaluated health reports on 53,797 women as they transitioned through menopause. Patients with systemic hormone therapy use, history of cancer, and self-reported CV disease were excluded. After adjusting for covariates, the authors found no statistically significant difference between users and nonusers of vaginal estrogen and risk for total MI (aHR = 0.73; 95% CI, 0.47-1.13), stroke (aHR = 0.85; 95% CI, 0.56-1.29), or DVT/PE (aHR = 1.06; 95% CI, 0.58-1.93). Study limitations included low prevalence of vaginal estrogen use (< 3%), short duration of use (mean, 37.5 months), and lack of data on the type or dose of vaginal estrogen used. The study only included health professionals, which limits generalizability.
A Finnish cohort study (excluded from the systematic review because it used historical controls) compared rates of CHD and stroke in postmenopausal women who used vaginal estrogen against an age-matched background population. Researchers collected data from a nationwide prescription registry for women at least 50 years old who had purchased vaginal estrogens between 1994 and 2009 (n = 195,756).4 Women who purchased systemic hormone therapy at any point were excluded. After 3 to 5 years of exposure, use of vaginal estrogen was associated with a decreased risk for mortality from CHD (relative risk [RR] = 0.64; 95% CI, 0.57-0.70) and stroke (RR = 0.79; 95% CI, 0.69-0.91). However, after 10 years, these benefits were not seen (CHD: RR = 0.95; 95% CI, 0.90-1.00; stroke: RR = 0.93; 95% CI, 0.85-1.01). All confidence interval data were presented graphically. Key weaknesses of this study included use of both vaginal and systemic estrogen in the comparator background population, and the failure to collect data for other CV risk variables such as weight, tobacco exposure, and blood pressure.
Recommendations from others
In 2022, the North American Menopause Society issued a Hormone Therapy Position Statement that acknowledged the lack of clinical trials directly comparing risk for adverse CV endpoints with different estrogen administration routes.5 They stated nonoral routes of administration might offer advantages by bypassing first-pass hepatic metabolism.
Similarly, the 2015 Endocrine Society Clinical Practice Guideline on the Treatment of Symptoms of the Menopause also stated that the effects of low-dose vaginal estrogen therapy on CV disease or DVT/PE risk had not been adequately studied.6
A 2013 opinion by the American College of Obstetricians and Gynecologists stated that topical estrogen vaginal creams, tablets, and rings had low levels of systemic absorption and were not associated with an increased risk for DVT/PE.7
Editor’s takeaway
The available evidence on vaginal estrogen replacement reassures us of its safety. After decades spent studying hormone replacement therapy with vacillating conclusions and opinions, these cohorts—the best evidence we may ever get—along with a consensus of expert opinions, consistently demonstrate no adverse CV outcomes.
Evidence summary
Cohort studies demonstrate no adverse CV outcomes
A 2020 systematic review and meta-analysis evaluated randomized controlled trials (RCTs) and observational studies to examine the association between menopausal hormone therapy and CV disease.1 The 26 RCTs primarily evaluated oral hormone administration. The observational studies comprised 30 cohort studies, 13 case-control studies, and 5 nested case-control studies, primarily in Europe and North America; 21 reported the route of administration. The trials evaluated women ages 49 to 77 years (mean, 61 years), and follow-up ranged from 1 to 21.5 years (mean, 7 years). In subgroup analyses of the observational studies, nonoral hormone therapy was associated with a lower risk for stroke and MI compared to oral administration (see TABLE1). Study limitations included enrollment of patients with few comorbidities, from limited geographic regions. Results in the meta-analysis were not stratified by the type of nonoral hormone therapy; only 4 studies evaluated vaginal estrogen use.

Two large cohort studies included in the systematic review provided more specific data on vaginal estrogens. The first used data from the Women’s Health Initiative in a subset of women ages 50 to 79 years (n = 46,566) who were not already on systemic hormone therapy and who did not have prior history of breast, endometrial, or ovarian cancer.2 Data were collected from self-assessment questionnaires and medical record reviews. The median duration of vaginal estrogen use was 2 years, and median follow-up duration was 7.2 years. Vaginal estrogen users had a 48% lower risk for CHD (adjusted hazard ratio [aHR] = 0.52; 95% CI, 0.31-0.85) than nonusers. Rates for all-cause mortality (aHR = 0.78; 95% CI, 0.58-1.04), stroke (aHR = 0.78; 95% CI, 0.49-1.24), and DVT/PE (aHR = 0.68; 95% CI, 0.36-1.28) were similar. In this and the other cohort studies to be discussed, outcome data for all vaginal estrogen preparations (eg, cream, ring, tablet) were combined.
The other large cohort study in the systematic review evaluated data on postmenopausal women from the Nurses’ Health Study.3 The authors evaluated health reports on 53,797 women as they transitioned through menopause. Patients with systemic hormone therapy use, history of cancer, and self-reported CV disease were excluded. After adjusting for covariates, the authors found no statistically significant difference between users and nonusers of vaginal estrogen and risk for total MI (aHR = 0.73; 95% CI, 0.47-1.13), stroke (aHR = 0.85; 95% CI, 0.56-1.29), or DVT/PE (aHR = 1.06; 95% CI, 0.58-1.93). Study limitations included low prevalence of vaginal estrogen use (< 3%), short duration of use (mean, 37.5 months), and lack of data on the type or dose of vaginal estrogen used. The study only included health professionals, which limits generalizability.
A Finnish cohort study (excluded from the systematic review because it used historical controls) compared rates of CHD and stroke in postmenopausal women who used vaginal estrogen against an age-matched background population. Researchers collected data from a nationwide prescription registry for women at least 50 years old who had purchased vaginal estrogens between 1994 and 2009 (n = 195,756).4 Women who purchased systemic hormone therapy at any point were excluded. After 3 to 5 years of exposure, use of vaginal estrogen was associated with a decreased risk for mortality from CHD (relative risk [RR] = 0.64; 95% CI, 0.57-0.70) and stroke (RR = 0.79; 95% CI, 0.69-0.91). However, after 10 years, these benefits were not seen (CHD: RR = 0.95; 95% CI, 0.90-1.00; stroke: RR = 0.93; 95% CI, 0.85-1.01). All confidence interval data were presented graphically. Key weaknesses of this study included use of both vaginal and systemic estrogen in the comparator background population, and the failure to collect data for other CV risk variables such as weight, tobacco exposure, and blood pressure.
Recommendations from others
In 2022, the North American Menopause Society issued a Hormone Therapy Position Statement that acknowledged the lack of clinical trials directly comparing risk for adverse CV endpoints with different estrogen administration routes.5 They stated nonoral routes of administration might offer advantages by bypassing first-pass hepatic metabolism.
Similarly, the 2015 Endocrine Society Clinical Practice Guideline on the Treatment of Symptoms of the Menopause also stated that the effects of low-dose vaginal estrogen therapy on CV disease or DVT/PE risk had not been adequately studied.6
A 2013 opinion by the American College of Obstetricians and Gynecologists stated that topical estrogen vaginal creams, tablets, and rings had low levels of systemic absorption and were not associated with an increased risk for DVT/PE.7
Editor’s takeaway
The available evidence on vaginal estrogen replacement reassures us of its safety. After decades spent studying hormone replacement therapy with vacillating conclusions and opinions, these cohorts—the best evidence we may ever get—along with a consensus of expert opinions, consistently demonstrate no adverse CV outcomes.
1. Kim JE, Chang JH, Jeong MJ, et al. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci Rep. 2020;10:20631. doi: 10.1038/s41598-020-77534-9
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the WHI Observational Study. Menopause. 2018;25:11-20. doi: 10.1097/GME.0000000000000956
3. Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610. doi: 10.1097/GME.0000000000001284
4. Mikkola TS, Tuomikoski P, Lyytinen H, et al. Vaginal estrogen use and the risk for cardiovascular mortality. Human Reproduction. 2016;31:804-809. doi: 10.1093/humrep/dew014
5. North American Menopause Society. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
6. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236
7. American College of Obstetricians and Gynecologists. Committee Opinion No 565: hormone therapy and heart disease. Obstet Gynecol. 2013;121:1407-1410. doi: 10.1097/01.AOG.0000431053.33593.2d
1. Kim JE, Chang JH, Jeong MJ, et al. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci Rep. 2020;10:20631. doi: 10.1038/s41598-020-77534-9
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the WHI Observational Study. Menopause. 2018;25:11-20. doi: 10.1097/GME.0000000000000956
3. Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610. doi: 10.1097/GME.0000000000001284
4. Mikkola TS, Tuomikoski P, Lyytinen H, et al. Vaginal estrogen use and the risk for cardiovascular mortality. Human Reproduction. 2016;31:804-809. doi: 10.1093/humrep/dew014
5. North American Menopause Society. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
6. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236
7. American College of Obstetricians and Gynecologists. Committee Opinion No 565: hormone therapy and heart disease. Obstet Gynecol. 2013;121:1407-1410. doi: 10.1097/01.AOG.0000431053.33593.2d
EVIDENCE-BASED ANSWER:
NO. In general, nonoral estrogen use for menopausal symptoms is associated with a lower cardiovascular (CV) risk profile than oral estrogen use (strength of recommendation [SOR], B; meta-analysis of cohort studies). Vaginal estrogen use is associated with lower risk for coronary heart disease (CHD) and similar risk for myocardial infarction (MI), stroke, and deep vein thrombosis/pulmonary embolism (DVT/PE) compared with nonuse (SOR, B; cohort studies). Vaginal estrogen therapy also is associated with lower CV-related mortality for 3 to 5 years compared with nonuse (SOR, B; cohort study). No high-quality randomized trials address this topic.
Are manual therapies effective at reducing chronic tension headache frequency in adults?
Evidence summary
Small studies offer mixed evidence of benefit
Seven RCTs using manual therapies to treat chronic tension headaches have reported the change in headache frequency (TABLE1-7). Most, but not all, manual therapies significantly improved headache frequency.
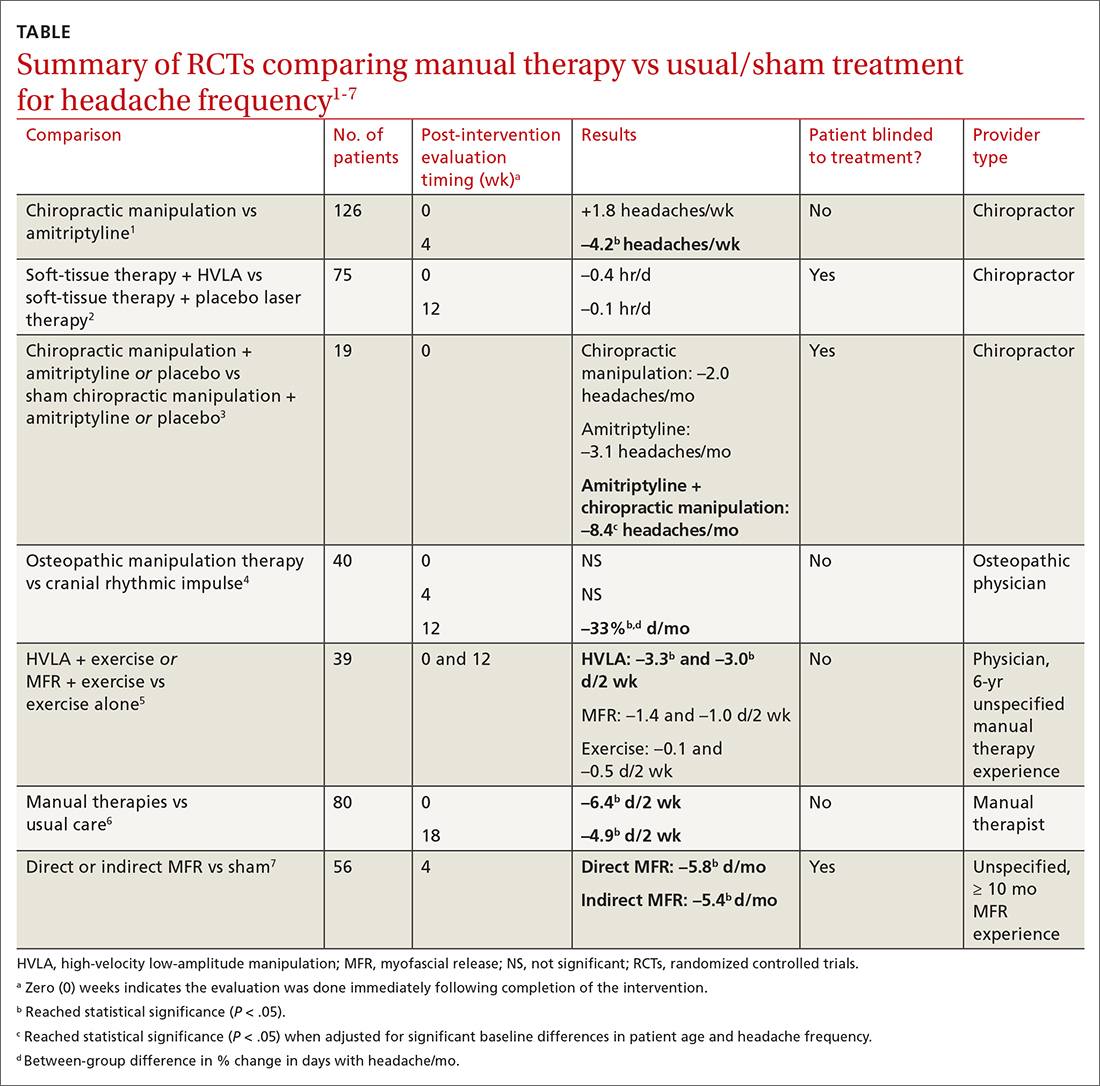
Participants ranged in age from 18 to 65 years, with mean age ranges of 33 to 42 years in each study. At baseline, patients had 10 or more tension-type headaches per month. The manual therapies varied in techniques, duration, and the training of the person performing the intervention:
- Twice-weekly chiropractic spinal manipulation for 6 weeks1
- Soft-tissue therapy plus spinal manipulation (8 treatments over 4 weeks)2
- Chiropractic spinal manipulation with or without amitriptyline for 14 weeks3
- Corrective osteopathic manipulation treatment (OMT) techniques tailored for each patient for 1 month4
- High-velocity low-amplitude manipulation (HVLA) plus exercise or myofascial release plus exercise twice weekly for 8 weeks5
- Manual therapy treatment consisting of a combination of mobilizations of the cervical and thoracic spine, exercises, and postural correction for up to 9 sessions of 30 minutes each6
- One hour of direct or indirect myofascial release treatment twice weekly for 12 weeks.7
Three studies involved chiropractic providers.1-3 One study (n = 19) found a positive effect, in which chiropractic manipulation augmented with amitriptyline performed better than chiropractic manipulation alone.3 Another chiropractic study did not find an immediate posttreatment benefit but did report significant headache reduction at the 4-week follow-up interval.1 The third chiropractic study did not show additional benefit from HVLA manipulation.2
One small study involving osteopathic physicians using OMT found reduced headache frequency after 12 weeks but not at 4 weeks.4 Another study, comparing HVLA or myofascial release with exercise to exercise alone, found benefit for the HVLA group but not for myofascial release; interventions in this study were performed by a physician with at least 6 years of unspecified manual therapy experience.5 A small study of manual therapists found improvement at the end of manual therapy but not at 18 months.6 Another small study using providers with 10 months’ experience with myofascial release found reduced headache frequency 4 weeks after a course of direct and indirect myofascial release (compared with sham release).7
Editor’s takeaway
It isn’t hard to imagine why muscle tension headaches might respond to certain forms of manual therapy. However, all available studies of these modalities have been small (< 100 patients) or lacked blinding, introducing the potential for significant bias. Nevertheless, for now it appears reasonable to refer interested patients with tension headache to an osteopathic physician for OMT or myofascial release to reduce headache frequency.
1. Boline PD, Kassak K, Bronfort G, et al. Spinal manipulation vs amitriptyline for the treatment of chronic tension-type headaches—a randomized clinical-trial. J Manipulative Physiol Ther. 1995;18:148-254.
2. Bove G. Spinal manipulation in the treatment of episodic tension-type headache: a randomized controlled trial. JAMA. 1998;280:1576-1579.
3. Vernon H, Jansz G, Goldsmith CH, et al. A randomized, placebo-controlled clinical trial of chiropractic and medical prophylactic treatment of adults with tension-type headache: results from a stopped trial. J Manipulative Physiol Ther. 2009;32:344-351.
4. Rolle G, Tremolizzo L, Somalvico F, et al. Pilot trial of osteopathic manipulative therapy for patients with frequent episodic tension-type headache. J Am Osteopath Assoc. 2014;114:678-685. doi: 10.7556/jaoa.2014.136
5. Corum M, Aydin T, Ceylan CM, et al. The comparative effects of spinal manipulation, myofascial release and exercise in tension-type headache patients with neck pain: a randomized controlled trial. Complement Ther Clin Pract. 2021;43:101319. doi: 0.1016/j.ctcp.2021.101319
6. Castien RF, van der Windt DAWM, Grooten A, et al. Effectiveness of manual therapy compared to usual care by the general practitioner for chronic tension-type headache: a pragmatic, randomised, clinical trial. Cephalalgia. 2009;31:133-143.
7. Ajimsha MS. Effectiveness of direct vs indirect technique myofascial release in the management of tension-type headache. J Bodyw Mov Ther. 2011;15:431-435. doi: 10.1016/j.jbmt.2011.01.021
Evidence summary
Small studies offer mixed evidence of benefit
Seven RCTs using manual therapies to treat chronic tension headaches have reported the change in headache frequency (TABLE1-7). Most, but not all, manual therapies significantly improved headache frequency.

Participants ranged in age from 18 to 65 years, with mean age ranges of 33 to 42 years in each study. At baseline, patients had 10 or more tension-type headaches per month. The manual therapies varied in techniques, duration, and the training of the person performing the intervention:
- Twice-weekly chiropractic spinal manipulation for 6 weeks1
- Soft-tissue therapy plus spinal manipulation (8 treatments over 4 weeks)2
- Chiropractic spinal manipulation with or without amitriptyline for 14 weeks3
- Corrective osteopathic manipulation treatment (OMT) techniques tailored for each patient for 1 month4
- High-velocity low-amplitude manipulation (HVLA) plus exercise or myofascial release plus exercise twice weekly for 8 weeks5
- Manual therapy treatment consisting of a combination of mobilizations of the cervical and thoracic spine, exercises, and postural correction for up to 9 sessions of 30 minutes each6
- One hour of direct or indirect myofascial release treatment twice weekly for 12 weeks.7
Three studies involved chiropractic providers.1-3 One study (n = 19) found a positive effect, in which chiropractic manipulation augmented with amitriptyline performed better than chiropractic manipulation alone.3 Another chiropractic study did not find an immediate posttreatment benefit but did report significant headache reduction at the 4-week follow-up interval.1 The third chiropractic study did not show additional benefit from HVLA manipulation.2
One small study involving osteopathic physicians using OMT found reduced headache frequency after 12 weeks but not at 4 weeks.4 Another study, comparing HVLA or myofascial release with exercise to exercise alone, found benefit for the HVLA group but not for myofascial release; interventions in this study were performed by a physician with at least 6 years of unspecified manual therapy experience.5 A small study of manual therapists found improvement at the end of manual therapy but not at 18 months.6 Another small study using providers with 10 months’ experience with myofascial release found reduced headache frequency 4 weeks after a course of direct and indirect myofascial release (compared with sham release).7
Editor’s takeaway
It isn’t hard to imagine why muscle tension headaches might respond to certain forms of manual therapy. However, all available studies of these modalities have been small (< 100 patients) or lacked blinding, introducing the potential for significant bias. Nevertheless, for now it appears reasonable to refer interested patients with tension headache to an osteopathic physician for OMT or myofascial release to reduce headache frequency.
Evidence summary
Small studies offer mixed evidence of benefit
Seven RCTs using manual therapies to treat chronic tension headaches have reported the change in headache frequency (TABLE1-7). Most, but not all, manual therapies significantly improved headache frequency.

Participants ranged in age from 18 to 65 years, with mean age ranges of 33 to 42 years in each study. At baseline, patients had 10 or more tension-type headaches per month. The manual therapies varied in techniques, duration, and the training of the person performing the intervention:
- Twice-weekly chiropractic spinal manipulation for 6 weeks1
- Soft-tissue therapy plus spinal manipulation (8 treatments over 4 weeks)2
- Chiropractic spinal manipulation with or without amitriptyline for 14 weeks3
- Corrective osteopathic manipulation treatment (OMT) techniques tailored for each patient for 1 month4
- High-velocity low-amplitude manipulation (HVLA) plus exercise or myofascial release plus exercise twice weekly for 8 weeks5
- Manual therapy treatment consisting of a combination of mobilizations of the cervical and thoracic spine, exercises, and postural correction for up to 9 sessions of 30 minutes each6
- One hour of direct or indirect myofascial release treatment twice weekly for 12 weeks.7
Three studies involved chiropractic providers.1-3 One study (n = 19) found a positive effect, in which chiropractic manipulation augmented with amitriptyline performed better than chiropractic manipulation alone.3 Another chiropractic study did not find an immediate posttreatment benefit but did report significant headache reduction at the 4-week follow-up interval.1 The third chiropractic study did not show additional benefit from HVLA manipulation.2
One small study involving osteopathic physicians using OMT found reduced headache frequency after 12 weeks but not at 4 weeks.4 Another study, comparing HVLA or myofascial release with exercise to exercise alone, found benefit for the HVLA group but not for myofascial release; interventions in this study were performed by a physician with at least 6 years of unspecified manual therapy experience.5 A small study of manual therapists found improvement at the end of manual therapy but not at 18 months.6 Another small study using providers with 10 months’ experience with myofascial release found reduced headache frequency 4 weeks after a course of direct and indirect myofascial release (compared with sham release).7
Editor’s takeaway
It isn’t hard to imagine why muscle tension headaches might respond to certain forms of manual therapy. However, all available studies of these modalities have been small (< 100 patients) or lacked blinding, introducing the potential for significant bias. Nevertheless, for now it appears reasonable to refer interested patients with tension headache to an osteopathic physician for OMT or myofascial release to reduce headache frequency.
1. Boline PD, Kassak K, Bronfort G, et al. Spinal manipulation vs amitriptyline for the treatment of chronic tension-type headaches—a randomized clinical-trial. J Manipulative Physiol Ther. 1995;18:148-254.
2. Bove G. Spinal manipulation in the treatment of episodic tension-type headache: a randomized controlled trial. JAMA. 1998;280:1576-1579.
3. Vernon H, Jansz G, Goldsmith CH, et al. A randomized, placebo-controlled clinical trial of chiropractic and medical prophylactic treatment of adults with tension-type headache: results from a stopped trial. J Manipulative Physiol Ther. 2009;32:344-351.
4. Rolle G, Tremolizzo L, Somalvico F, et al. Pilot trial of osteopathic manipulative therapy for patients with frequent episodic tension-type headache. J Am Osteopath Assoc. 2014;114:678-685. doi: 10.7556/jaoa.2014.136
5. Corum M, Aydin T, Ceylan CM, et al. The comparative effects of spinal manipulation, myofascial release and exercise in tension-type headache patients with neck pain: a randomized controlled trial. Complement Ther Clin Pract. 2021;43:101319. doi: 0.1016/j.ctcp.2021.101319
6. Castien RF, van der Windt DAWM, Grooten A, et al. Effectiveness of manual therapy compared to usual care by the general practitioner for chronic tension-type headache: a pragmatic, randomised, clinical trial. Cephalalgia. 2009;31:133-143.
7. Ajimsha MS. Effectiveness of direct vs indirect technique myofascial release in the management of tension-type headache. J Bodyw Mov Ther. 2011;15:431-435. doi: 10.1016/j.jbmt.2011.01.021
1. Boline PD, Kassak K, Bronfort G, et al. Spinal manipulation vs amitriptyline for the treatment of chronic tension-type headaches—a randomized clinical-trial. J Manipulative Physiol Ther. 1995;18:148-254.
2. Bove G. Spinal manipulation in the treatment of episodic tension-type headache: a randomized controlled trial. JAMA. 1998;280:1576-1579.
3. Vernon H, Jansz G, Goldsmith CH, et al. A randomized, placebo-controlled clinical trial of chiropractic and medical prophylactic treatment of adults with tension-type headache: results from a stopped trial. J Manipulative Physiol Ther. 2009;32:344-351.
4. Rolle G, Tremolizzo L, Somalvico F, et al. Pilot trial of osteopathic manipulative therapy for patients with frequent episodic tension-type headache. J Am Osteopath Assoc. 2014;114:678-685. doi: 10.7556/jaoa.2014.136
5. Corum M, Aydin T, Ceylan CM, et al. The comparative effects of spinal manipulation, myofascial release and exercise in tension-type headache patients with neck pain: a randomized controlled trial. Complement Ther Clin Pract. 2021;43:101319. doi: 0.1016/j.ctcp.2021.101319
6. Castien RF, van der Windt DAWM, Grooten A, et al. Effectiveness of manual therapy compared to usual care by the general practitioner for chronic tension-type headache: a pragmatic, randomised, clinical trial. Cephalalgia. 2009;31:133-143.
7. Ajimsha MS. Effectiveness of direct vs indirect technique myofascial release in the management of tension-type headache. J Bodyw Mov Ther. 2011;15:431-435. doi: 10.1016/j.jbmt.2011.01.021
EVIDENCE-BASED ANSWER:
MAYBE. Among patients with chronic tension headaches, manual therapies may reduce headache frequency more than sham manual therapy, usual care, or exercise treatments—by 1.5 to 4.2 headaches or days with headache per week (strength of recommendation, B; preponderance of evidence from primarily small, heterogeneous randomized controlled trials [RCTs]).
Is low-dose naltrexone effective in chronic pain management?
Evidence summary
Naltrexone is comparable to amitriptyline for diabetic neuropathy pain
A 2021 randomized, double-blind, active-comparator, crossover clinical trial conducted in India examined the efficacy of low-dose naltrexone vs standard-of-care amitriptyline in patients (N = 67) with painful diabetic neuropathy. Participants were adults (ages 18 to 75 years) with painful diabetic neuropathy who had been on a stable dose of nonopioid pain medication for at least 1 month.1
Patients were randomly assigned to start receiving naltrexone 2 mg (n = 33) or amitriptyline 10 mg (n = 34). They received their starting medication for 6 weeks (with follow-up every 2 weeks), then completed a 2-week washout period, and then switched to the other study medication for 6 weeks (same follow-up schedule). If patients reported < 20% pain reduction on the Visual Analog Scale (VAS; 0-100 scoring system with 0 = no pain and 100 = worst pain) at a follow-up visit, their medication dose was titrated up, to a maximum of 4 mg of naltrexone or 25 to 50 mg of amitriptyline.1
The primary outcome of interest was the mean change in VAS pain score following 6 weeks of treatment. There was no statistically different change from baseline VAS pain score between the amitriptyline and naltrexone groups (mean difference [MD] = 1.6; 95% CI, –0.9 to 4.2; P = 0.21). These findings were consistent across the secondary endpoints (Likert 5-point pain scale and McGill Pain Questionnaire scores). There was no statistically significant difference in Hamilton Depression Rating Scale scores (13 in the naltrexone group vs 11 in the amitriptyline group; P = .81), no reports of decreased sleep quality in either group, and no significant difference in Patients’ Global Impression of Change scores at 6-week evaluation.1
The naltrexone cohort experienced 8 adverse events (most commonly, mild diarrhea), while the amitriptyline cohort experienced 52 adverse events (most commonly, somnolence) (P < .001). The limitations of the study include the lack of a placebo arm and a relatively small sample size.1
Greater reduction in pain scores with naltrexone
A 2022 retrospective cohort study evaluated the effectiveness of naltrexone for patients treated at a single outpatient integrative pain management practice in Alaska between 2014 and 2019. The exposure group (n = 36) included patients who had completed at least a 2-month continuous regimen of oral naltrexone 4.5 mg. Controls (n = 42) were selected from the remaining practice population receiving standard care and were primarily matched by diagnosis code, followed by gender, then age +/– 5 years. Patients were divided into subgroups for inflammatory and neuropathic pain.2
The primary outcome measured was the mean change in VAS score or numeric rating score (NRS; both used a 1-10 rating system), which was assessed during a patient’s appointment from initiation of treatment to the most recent visit or at the termination of therapy (intervention interquartile range, 12-14 months). There was no statistically significant difference in VAS/NRS between the low-dose naltrexone and control groups at baseline (6.09 vs 6.38; P = .454). The low-dose naltrexone group experienced a greater reduction in VAS/NRS pain scores compared to the control group (–37.8% vs –4.3%; P < .001).2
Compared with control patients in each group, patients in the inflammatory pain subgroup and the neuropathic pain subgroup who received low-dose naltrexone reported reductions in pain scores of 32% (P < .001) and 44% (P = .048), respectively. There was no statistically significant difference in mean change in VAS/NRS scores between the inflammatory and neuropathic subgroups (P = .763). A multivariate linear regression analysis did not identify significant variables other than low-dose naltrexone that correlated with pain improvement. The number needed to treat to observe a ≥ 50% reduction in pain scores was 3.2.2
Continue to: Limitations for this study...
Limitations for this study include its small sample size and open-label design.2
Low-dose naltrexone is effective for fibromyalgia pain
A 2020 single-blind prospective dose-response study utilized the up-and-down method to identify effective naltrexone dose for patients in a Danish university hospital pain clinic. Patients were White women ages 18 to 60 years (N = 25) who had a diagnosis of fibromyalgia unresponsive to traditional pharmacologic treatment. All patients received treatment with low-dose naltrexone (ranging from 0.75 mg to 6.0 mg) but were blinded to dose.3
Patients were evaluated for improvement in fibromyalgia symptoms using the Patient Global Impression of Improvement (PGI-I) scale—which ranges from 1 (very much improved) to 7 (very much worse), with 4 being “no change”—at baseline and after 2 to 3 weeks of treatment with low-dose naltrexone. A patient was considered a responder if they scored 1 to 3 on the follow-up PGI-I scale or if they experienced a > 30% pain reduction on the VAS. If a patient did not respond to their dose, the next patient began treatment at a dose 0.75 mg higher than the previous patient’s ending dose. If a patient did respond to low-dose naltrexone treatment, the next patient’s starting dose was 0.75 mg less than the previous patient’s. Eleven of 25 patients were considered responders.3
The primary outcomes were effective dose for 50% of fibromyalgia patients (3.88 mg; 95% CI, 3.39-4.35) and effective dose for 95% of fibromyalgia patients (5.4 mg; 95% CI, 4.66-6.13). Secondary outcomes were fibromyalgia symptoms as evaluated on the Fibromyalgia Impact Questionnaire Revised. Five of the 11 responders reported a > 30% improvement in tenderness and 8 of the 11 responders reported a > 30% decrease in waking unrefreshed.3
Limitations of the study include the short time period of treatment before response was assessed and the decision to use low test doses, which may have hindered detection of effective doses > 6 mg in fibromyalgia.3
Editor’s takeaway
Low-dose naltrexone, a less-often-used form of pain management, is a welcome option. Studies show some effectiveness in a variety of pain conditions with few adverse effects. The small number of studies, the small sample sizes, and the limited follow-up duration should encourage more investigation into how to best use this intervention.
1. Srinivasan A, Dutta P, Bansal D, et al. Efficacy and safety of low-dose naltrexone in painful diabetic neuropathy: a randomized, double-blind, active-control, crossover clinical trial. J Diabetes. 2021;13:770-778. doi: 10.1111/1753-0407.13202
2. Martin SJ, McAnally HB, Okediji P, et al. Low-dose naltrexone, an opioid-receptor antagonist, is a broad-spectrum analgesic: a retrospective cohort study. Pain Management. 2022;12:699-709. doi: 10.2217/pmt-2021-0122
3. Bruun-Plesner K, Blichfeldt-Eckhardt MR, Vaegter HB, et al. Low-dose naltrexone for the treatment of fibromyalgia: investigation of dose-response relationships. Pain Med. 2020;21:2253-2261. doi: 10.1093/pm/pnaa001
Evidence summary
Naltrexone is comparable to amitriptyline for diabetic neuropathy pain
A 2021 randomized, double-blind, active-comparator, crossover clinical trial conducted in India examined the efficacy of low-dose naltrexone vs standard-of-care amitriptyline in patients (N = 67) with painful diabetic neuropathy. Participants were adults (ages 18 to 75 years) with painful diabetic neuropathy who had been on a stable dose of nonopioid pain medication for at least 1 month.1
Patients were randomly assigned to start receiving naltrexone 2 mg (n = 33) or amitriptyline 10 mg (n = 34). They received their starting medication for 6 weeks (with follow-up every 2 weeks), then completed a 2-week washout period, and then switched to the other study medication for 6 weeks (same follow-up schedule). If patients reported < 20% pain reduction on the Visual Analog Scale (VAS; 0-100 scoring system with 0 = no pain and 100 = worst pain) at a follow-up visit, their medication dose was titrated up, to a maximum of 4 mg of naltrexone or 25 to 50 mg of amitriptyline.1
The primary outcome of interest was the mean change in VAS pain score following 6 weeks of treatment. There was no statistically different change from baseline VAS pain score between the amitriptyline and naltrexone groups (mean difference [MD] = 1.6; 95% CI, –0.9 to 4.2; P = 0.21). These findings were consistent across the secondary endpoints (Likert 5-point pain scale and McGill Pain Questionnaire scores). There was no statistically significant difference in Hamilton Depression Rating Scale scores (13 in the naltrexone group vs 11 in the amitriptyline group; P = .81), no reports of decreased sleep quality in either group, and no significant difference in Patients’ Global Impression of Change scores at 6-week evaluation.1
The naltrexone cohort experienced 8 adverse events (most commonly, mild diarrhea), while the amitriptyline cohort experienced 52 adverse events (most commonly, somnolence) (P < .001). The limitations of the study include the lack of a placebo arm and a relatively small sample size.1
Greater reduction in pain scores with naltrexone
A 2022 retrospective cohort study evaluated the effectiveness of naltrexone for patients treated at a single outpatient integrative pain management practice in Alaska between 2014 and 2019. The exposure group (n = 36) included patients who had completed at least a 2-month continuous regimen of oral naltrexone 4.5 mg. Controls (n = 42) were selected from the remaining practice population receiving standard care and were primarily matched by diagnosis code, followed by gender, then age +/– 5 years. Patients were divided into subgroups for inflammatory and neuropathic pain.2
The primary outcome measured was the mean change in VAS score or numeric rating score (NRS; both used a 1-10 rating system), which was assessed during a patient’s appointment from initiation of treatment to the most recent visit or at the termination of therapy (intervention interquartile range, 12-14 months). There was no statistically significant difference in VAS/NRS between the low-dose naltrexone and control groups at baseline (6.09 vs 6.38; P = .454). The low-dose naltrexone group experienced a greater reduction in VAS/NRS pain scores compared to the control group (–37.8% vs –4.3%; P < .001).2
Compared with control patients in each group, patients in the inflammatory pain subgroup and the neuropathic pain subgroup who received low-dose naltrexone reported reductions in pain scores of 32% (P < .001) and 44% (P = .048), respectively. There was no statistically significant difference in mean change in VAS/NRS scores between the inflammatory and neuropathic subgroups (P = .763). A multivariate linear regression analysis did not identify significant variables other than low-dose naltrexone that correlated with pain improvement. The number needed to treat to observe a ≥ 50% reduction in pain scores was 3.2.2
Continue to: Limitations for this study...
Limitations for this study include its small sample size and open-label design.2
Low-dose naltrexone is effective for fibromyalgia pain
A 2020 single-blind prospective dose-response study utilized the up-and-down method to identify effective naltrexone dose for patients in a Danish university hospital pain clinic. Patients were White women ages 18 to 60 years (N = 25) who had a diagnosis of fibromyalgia unresponsive to traditional pharmacologic treatment. All patients received treatment with low-dose naltrexone (ranging from 0.75 mg to 6.0 mg) but were blinded to dose.3
Patients were evaluated for improvement in fibromyalgia symptoms using the Patient Global Impression of Improvement (PGI-I) scale—which ranges from 1 (very much improved) to 7 (very much worse), with 4 being “no change”—at baseline and after 2 to 3 weeks of treatment with low-dose naltrexone. A patient was considered a responder if they scored 1 to 3 on the follow-up PGI-I scale or if they experienced a > 30% pain reduction on the VAS. If a patient did not respond to their dose, the next patient began treatment at a dose 0.75 mg higher than the previous patient’s ending dose. If a patient did respond to low-dose naltrexone treatment, the next patient’s starting dose was 0.75 mg less than the previous patient’s. Eleven of 25 patients were considered responders.3
The primary outcomes were effective dose for 50% of fibromyalgia patients (3.88 mg; 95% CI, 3.39-4.35) and effective dose for 95% of fibromyalgia patients (5.4 mg; 95% CI, 4.66-6.13). Secondary outcomes were fibromyalgia symptoms as evaluated on the Fibromyalgia Impact Questionnaire Revised. Five of the 11 responders reported a > 30% improvement in tenderness and 8 of the 11 responders reported a > 30% decrease in waking unrefreshed.3
Limitations of the study include the short time period of treatment before response was assessed and the decision to use low test doses, which may have hindered detection of effective doses > 6 mg in fibromyalgia.3
Editor’s takeaway
Low-dose naltrexone, a less-often-used form of pain management, is a welcome option. Studies show some effectiveness in a variety of pain conditions with few adverse effects. The small number of studies, the small sample sizes, and the limited follow-up duration should encourage more investigation into how to best use this intervention.
Evidence summary
Naltrexone is comparable to amitriptyline for diabetic neuropathy pain
A 2021 randomized, double-blind, active-comparator, crossover clinical trial conducted in India examined the efficacy of low-dose naltrexone vs standard-of-care amitriptyline in patients (N = 67) with painful diabetic neuropathy. Participants were adults (ages 18 to 75 years) with painful diabetic neuropathy who had been on a stable dose of nonopioid pain medication for at least 1 month.1
Patients were randomly assigned to start receiving naltrexone 2 mg (n = 33) or amitriptyline 10 mg (n = 34). They received their starting medication for 6 weeks (with follow-up every 2 weeks), then completed a 2-week washout period, and then switched to the other study medication for 6 weeks (same follow-up schedule). If patients reported < 20% pain reduction on the Visual Analog Scale (VAS; 0-100 scoring system with 0 = no pain and 100 = worst pain) at a follow-up visit, their medication dose was titrated up, to a maximum of 4 mg of naltrexone or 25 to 50 mg of amitriptyline.1
The primary outcome of interest was the mean change in VAS pain score following 6 weeks of treatment. There was no statistically different change from baseline VAS pain score between the amitriptyline and naltrexone groups (mean difference [MD] = 1.6; 95% CI, –0.9 to 4.2; P = 0.21). These findings were consistent across the secondary endpoints (Likert 5-point pain scale and McGill Pain Questionnaire scores). There was no statistically significant difference in Hamilton Depression Rating Scale scores (13 in the naltrexone group vs 11 in the amitriptyline group; P = .81), no reports of decreased sleep quality in either group, and no significant difference in Patients’ Global Impression of Change scores at 6-week evaluation.1
The naltrexone cohort experienced 8 adverse events (most commonly, mild diarrhea), while the amitriptyline cohort experienced 52 adverse events (most commonly, somnolence) (P < .001). The limitations of the study include the lack of a placebo arm and a relatively small sample size.1
Greater reduction in pain scores with naltrexone
A 2022 retrospective cohort study evaluated the effectiveness of naltrexone for patients treated at a single outpatient integrative pain management practice in Alaska between 2014 and 2019. The exposure group (n = 36) included patients who had completed at least a 2-month continuous regimen of oral naltrexone 4.5 mg. Controls (n = 42) were selected from the remaining practice population receiving standard care and were primarily matched by diagnosis code, followed by gender, then age +/– 5 years. Patients were divided into subgroups for inflammatory and neuropathic pain.2
The primary outcome measured was the mean change in VAS score or numeric rating score (NRS; both used a 1-10 rating system), which was assessed during a patient’s appointment from initiation of treatment to the most recent visit or at the termination of therapy (intervention interquartile range, 12-14 months). There was no statistically significant difference in VAS/NRS between the low-dose naltrexone and control groups at baseline (6.09 vs 6.38; P = .454). The low-dose naltrexone group experienced a greater reduction in VAS/NRS pain scores compared to the control group (–37.8% vs –4.3%; P < .001).2
Compared with control patients in each group, patients in the inflammatory pain subgroup and the neuropathic pain subgroup who received low-dose naltrexone reported reductions in pain scores of 32% (P < .001) and 44% (P = .048), respectively. There was no statistically significant difference in mean change in VAS/NRS scores between the inflammatory and neuropathic subgroups (P = .763). A multivariate linear regression analysis did not identify significant variables other than low-dose naltrexone that correlated with pain improvement. The number needed to treat to observe a ≥ 50% reduction in pain scores was 3.2.2
Continue to: Limitations for this study...
Limitations for this study include its small sample size and open-label design.2
Low-dose naltrexone is effective for fibromyalgia pain
A 2020 single-blind prospective dose-response study utilized the up-and-down method to identify effective naltrexone dose for patients in a Danish university hospital pain clinic. Patients were White women ages 18 to 60 years (N = 25) who had a diagnosis of fibromyalgia unresponsive to traditional pharmacologic treatment. All patients received treatment with low-dose naltrexone (ranging from 0.75 mg to 6.0 mg) but were blinded to dose.3
Patients were evaluated for improvement in fibromyalgia symptoms using the Patient Global Impression of Improvement (PGI-I) scale—which ranges from 1 (very much improved) to 7 (very much worse), with 4 being “no change”—at baseline and after 2 to 3 weeks of treatment with low-dose naltrexone. A patient was considered a responder if they scored 1 to 3 on the follow-up PGI-I scale or if they experienced a > 30% pain reduction on the VAS. If a patient did not respond to their dose, the next patient began treatment at a dose 0.75 mg higher than the previous patient’s ending dose. If a patient did respond to low-dose naltrexone treatment, the next patient’s starting dose was 0.75 mg less than the previous patient’s. Eleven of 25 patients were considered responders.3
The primary outcomes were effective dose for 50% of fibromyalgia patients (3.88 mg; 95% CI, 3.39-4.35) and effective dose for 95% of fibromyalgia patients (5.4 mg; 95% CI, 4.66-6.13). Secondary outcomes were fibromyalgia symptoms as evaluated on the Fibromyalgia Impact Questionnaire Revised. Five of the 11 responders reported a > 30% improvement in tenderness and 8 of the 11 responders reported a > 30% decrease in waking unrefreshed.3
Limitations of the study include the short time period of treatment before response was assessed and the decision to use low test doses, which may have hindered detection of effective doses > 6 mg in fibromyalgia.3
Editor’s takeaway
Low-dose naltrexone, a less-often-used form of pain management, is a welcome option. Studies show some effectiveness in a variety of pain conditions with few adverse effects. The small number of studies, the small sample sizes, and the limited follow-up duration should encourage more investigation into how to best use this intervention.
1. Srinivasan A, Dutta P, Bansal D, et al. Efficacy and safety of low-dose naltrexone in painful diabetic neuropathy: a randomized, double-blind, active-control, crossover clinical trial. J Diabetes. 2021;13:770-778. doi: 10.1111/1753-0407.13202
2. Martin SJ, McAnally HB, Okediji P, et al. Low-dose naltrexone, an opioid-receptor antagonist, is a broad-spectrum analgesic: a retrospective cohort study. Pain Management. 2022;12:699-709. doi: 10.2217/pmt-2021-0122
3. Bruun-Plesner K, Blichfeldt-Eckhardt MR, Vaegter HB, et al. Low-dose naltrexone for the treatment of fibromyalgia: investigation of dose-response relationships. Pain Med. 2020;21:2253-2261. doi: 10.1093/pm/pnaa001
1. Srinivasan A, Dutta P, Bansal D, et al. Efficacy and safety of low-dose naltrexone in painful diabetic neuropathy: a randomized, double-blind, active-control, crossover clinical trial. J Diabetes. 2021;13:770-778. doi: 10.1111/1753-0407.13202
2. Martin SJ, McAnally HB, Okediji P, et al. Low-dose naltrexone, an opioid-receptor antagonist, is a broad-spectrum analgesic: a retrospective cohort study. Pain Management. 2022;12:699-709. doi: 10.2217/pmt-2021-0122
3. Bruun-Plesner K, Blichfeldt-Eckhardt MR, Vaegter HB, et al. Low-dose naltrexone for the treatment of fibromyalgia: investigation of dose-response relationships. Pain Med. 2020;21:2253-2261. doi: 10.1093/pm/pnaa001
EVIDENCE-BASED ANSWER:
YES. Low-dose naltrexone is as effective as amitriptyline in the treatment of painful diabetic neuropathy and has a superior safety profile (strength of recommendation [SOR], B; single randomized controlled trial [RCT]).
Low-dose naltrexone significantly reduced pain by 32% in inflammatory conditions and 44% in neuropathic conditions (SOR, B; single retrospective cohort study).
Doses as low as 5.4 mg were found to reduce pain in 95% of patients with fibromyalgia (SOR, B; single prospective dose-response study).
Does use of continuous or flash glucose monitors decrease hypoglycemia episodes in T2D?
Evidence summary
Continuous glucose monitoring: Nonsignificant reductions in event rates
A 2021 multicenter RCT (N = 175) evaluated CGM effectiveness in patients with basal insulin–treated T2D.1 Patients (mean age, 57 years; mean A1C, 9.1%) wore a blinded CGM device for baseline glucose measurement (minimum of 168 hours) before being randomly assigned to either CGM (n = 116) or traditional blood glucose monitoring (BGM; n = 59). At 8-month follow-up, patients in the BGM group again had blinded sensors placed. A significant reduction in hypoglycemia duration was observed for the CGM group vs the BGM group at 8 months for glucose values < 70 mg/mL (adjusted mean difference [aMD] = –0.24%; 95% CI, –0.42 to –0.05) and < 54 mg/dL (aMD = –0.10%; 95% CI, –0.15 to –0.04). A nonsignificant decrease in severe hypoglycemic events requiring resuscitative assistance occurred for BGM (2%) vs CGM (1%) patients. Study limitations included virtual visits due to COVID-19 and a short follow-up period.
A 2022 multicenter prospective study (N = 174) examined CGM effects on hypoglycemia frequency and severity in adults with T2D.2 Patients with insulin-requiring T2D (mean age, 61 years; mean A1C, 8.0%) participated in a 12-month study with 6 months of self-monitored blood glucose (SMBG) followed by 6 months of CGM use. The primary outcome was the rate of severe hypoglycemic events. A nonsignificant decrease was observed in the CGM group compared to the SMBG group for hypoglycemic event rate, per participant per 6-month period (relative risk [RR] = 0.43; 95% CI, 0.07-2.64). Four moderate hypoglycemic adverse events occurred in the SMBG phase vs 2 in the CGM phase. Financial support by the study sponsor decreases the study’s validity.
A 2021 prospective study (N = 90) evaluated the use of CGM to improve glycemic control.3 Patients younger than 66 years with insulin-treated T2D and an A1C > 7.5% participated in a 7-day blinded CGM cycle every 4 months for 1 year. A nonsignificant decrease in hypoglycemia duration was observed for glucose values < 70 mg/dL and < 54 mg/dL at 12 months. No change in hypoglycemic event rate was seen with the use of CGM. Funding by the device manufacturer was a limitation of this study.
Flash glucose monitoring: Mixed results on hypoglycemia events
A 2019 open-label RCT (N = 82) assessed the effectiveness of FGM on diabetes control.4 Patients with insulin-treated T2D were randomly assigned to the intervention or standard-care groups. The intervention group (n = 46; mean age, 66 years; mean A1C, 8.3%) used the FGM system for 10 weeks, while the standard-care group (n = 36; mean age, 70 years; mean A1C, 8.9%) maintained use of their glucometers. Both groups received similar types and duration of counseling. Treatment satisfaction was the primary outcome; total hypoglycemic events was a secondary outcome. No significant difference in the number of hypoglycemic episodes was observed between the intervention and control groups at 55 to 70 mg/dL (RR = 0.79; 95% CI, 0.44-1.4) or < 54 mg/dL (RR = 1.27; 95% CI, 0.38-4.2). No adverse events of severe hypoglycemia occurred during the study. Funding by the device manufacturer was a limitation of this study.
A 2017 open-label, multicenter RCT (N = 224) assessed FGM efficacy.5 Adults (mean age, 59 years; mean A1C, 8.8%) with T2D on intensive insulin therapy were randomized to FGM (n = 149) or SMBG (n = 75) after a 14-day masked baseline period. The 6-month treatment phase was unblinded. The duration of hypoglycemic events (glucose values < 70 mg/dL and < 55 mg/dL) was obtained from the sensors. Compared to the SMBG group, the FGM group spent 43% less time at < 70 mg/dL (aMD = –0.47 ± 0.13 h/d; P = .0006) and 53% less time at < 55 mg/dL (aMD = –0.22 ± 0.068 h/d; P = .0014). Hypoglycemic event rates significantly decreased by 28% (aMD = –0.16 ± 0.065; P = 0.016) and 44% (aMD = –0.12 ± 0.037; P = .0017) for glucose levels < 70 mg/dL and < 55 mg/dL, respectively. A nonsignificant difference occurred in severe hypoglycemic events requiring third-party assistance for the FGM (2%) vs control (1%) groups. Involvement of the device manufacturer and unblinded group allocations are study limitations.
A 2021 single-arm, multicenter prospective study looked at the impact of FGM on glycemic control in adults with insulin-treated T2D (N = 90; mean age, 64 years; mean A1C, 7.5%).6 After a 14-day baseline period consisting of masked sensor readings paired with self-monitored fingerstick tests, participants were followed for 11 weeks using the sensor to monitor glucose levels. The primary outcome was amount of time spent in hypoglycemia (< 70 mg/dL), with secondary outcomes including time and events in hypoglycemia (< 70, < 55, or < 45 mg/dL). No significant decrease in hypoglycemia duration or hypoglycemic event rates at < 70, < 55, or < 45 mg/dL was observed for FGM compared to baseline. Adverse events were observed in 64% of participants; 94% of the events were hypoglycemia related. Serious adverse events were reported for 5.3% of participants. The single-arm study format, lack of generalizability due to the single-race study population, and sponsor support were study limitations.
Editor’s takeaway
This reasonably good evidence shows a decrease in measured or monitored hypoglycemia, a disease-oriented outcome, but it did not reach statistical significance for symptomatic hypoglycemia (1% vs 2%), a patient-oriented outcome. Nevertheless, in patients reporting symptomatic hypoglycemia, a continuous or flash glucose monitor may allow for more aggressive glucose control.
1. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
2. Beck SE, Kelly C, Price DA. Non-adjunctive continuous glucose monitoring for control of hypoglycaemia (COACH): results of a post-approval observational study. Diabet Med. 2022;39:e14739. doi: 10.1111/dme.14739
3. Ribeiro RT, Andrade R, Nascimento do O D, et al. Impact of blinded retrospective continuous glucose monitoring on clinical decision making and glycemic control in persons with type 2 diabetes on insulin therapy. Nutr Metab Cardiovasc Dis. 2021;31:1267-1275. doi: 10.1016/j.numecd.2020.12.024
4. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
5. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
6. Ogawa W, Hirota Y, Osonoi T, et al. Effect of the FreeStyle Libre™ flash glucose monitoring system on glycemic control in individuals with type 2 diabetes treated with basal-bolus insulin therapy: an open label, prospective, multicenter trial in Japan. J Diabetes Investig. 2021;12:82-90. doi: 10.1111/jdi.13327
Evidence summary
Continuous glucose monitoring: Nonsignificant reductions in event rates
A 2021 multicenter RCT (N = 175) evaluated CGM effectiveness in patients with basal insulin–treated T2D.1 Patients (mean age, 57 years; mean A1C, 9.1%) wore a blinded CGM device for baseline glucose measurement (minimum of 168 hours) before being randomly assigned to either CGM (n = 116) or traditional blood glucose monitoring (BGM; n = 59). At 8-month follow-up, patients in the BGM group again had blinded sensors placed. A significant reduction in hypoglycemia duration was observed for the CGM group vs the BGM group at 8 months for glucose values < 70 mg/mL (adjusted mean difference [aMD] = –0.24%; 95% CI, –0.42 to –0.05) and < 54 mg/dL (aMD = –0.10%; 95% CI, –0.15 to –0.04). A nonsignificant decrease in severe hypoglycemic events requiring resuscitative assistance occurred for BGM (2%) vs CGM (1%) patients. Study limitations included virtual visits due to COVID-19 and a short follow-up period.
A 2022 multicenter prospective study (N = 174) examined CGM effects on hypoglycemia frequency and severity in adults with T2D.2 Patients with insulin-requiring T2D (mean age, 61 years; mean A1C, 8.0%) participated in a 12-month study with 6 months of self-monitored blood glucose (SMBG) followed by 6 months of CGM use. The primary outcome was the rate of severe hypoglycemic events. A nonsignificant decrease was observed in the CGM group compared to the SMBG group for hypoglycemic event rate, per participant per 6-month period (relative risk [RR] = 0.43; 95% CI, 0.07-2.64). Four moderate hypoglycemic adverse events occurred in the SMBG phase vs 2 in the CGM phase. Financial support by the study sponsor decreases the study’s validity.
A 2021 prospective study (N = 90) evaluated the use of CGM to improve glycemic control.3 Patients younger than 66 years with insulin-treated T2D and an A1C > 7.5% participated in a 7-day blinded CGM cycle every 4 months for 1 year. A nonsignificant decrease in hypoglycemia duration was observed for glucose values < 70 mg/dL and < 54 mg/dL at 12 months. No change in hypoglycemic event rate was seen with the use of CGM. Funding by the device manufacturer was a limitation of this study.
Flash glucose monitoring: Mixed results on hypoglycemia events
A 2019 open-label RCT (N = 82) assessed the effectiveness of FGM on diabetes control.4 Patients with insulin-treated T2D were randomly assigned to the intervention or standard-care groups. The intervention group (n = 46; mean age, 66 years; mean A1C, 8.3%) used the FGM system for 10 weeks, while the standard-care group (n = 36; mean age, 70 years; mean A1C, 8.9%) maintained use of their glucometers. Both groups received similar types and duration of counseling. Treatment satisfaction was the primary outcome; total hypoglycemic events was a secondary outcome. No significant difference in the number of hypoglycemic episodes was observed between the intervention and control groups at 55 to 70 mg/dL (RR = 0.79; 95% CI, 0.44-1.4) or < 54 mg/dL (RR = 1.27; 95% CI, 0.38-4.2). No adverse events of severe hypoglycemia occurred during the study. Funding by the device manufacturer was a limitation of this study.
A 2017 open-label, multicenter RCT (N = 224) assessed FGM efficacy.5 Adults (mean age, 59 years; mean A1C, 8.8%) with T2D on intensive insulin therapy were randomized to FGM (n = 149) or SMBG (n = 75) after a 14-day masked baseline period. The 6-month treatment phase was unblinded. The duration of hypoglycemic events (glucose values < 70 mg/dL and < 55 mg/dL) was obtained from the sensors. Compared to the SMBG group, the FGM group spent 43% less time at < 70 mg/dL (aMD = –0.47 ± 0.13 h/d; P = .0006) and 53% less time at < 55 mg/dL (aMD = –0.22 ± 0.068 h/d; P = .0014). Hypoglycemic event rates significantly decreased by 28% (aMD = –0.16 ± 0.065; P = 0.016) and 44% (aMD = –0.12 ± 0.037; P = .0017) for glucose levels < 70 mg/dL and < 55 mg/dL, respectively. A nonsignificant difference occurred in severe hypoglycemic events requiring third-party assistance for the FGM (2%) vs control (1%) groups. Involvement of the device manufacturer and unblinded group allocations are study limitations.
A 2021 single-arm, multicenter prospective study looked at the impact of FGM on glycemic control in adults with insulin-treated T2D (N = 90; mean age, 64 years; mean A1C, 7.5%).6 After a 14-day baseline period consisting of masked sensor readings paired with self-monitored fingerstick tests, participants were followed for 11 weeks using the sensor to monitor glucose levels. The primary outcome was amount of time spent in hypoglycemia (< 70 mg/dL), with secondary outcomes including time and events in hypoglycemia (< 70, < 55, or < 45 mg/dL). No significant decrease in hypoglycemia duration or hypoglycemic event rates at < 70, < 55, or < 45 mg/dL was observed for FGM compared to baseline. Adverse events were observed in 64% of participants; 94% of the events were hypoglycemia related. Serious adverse events were reported for 5.3% of participants. The single-arm study format, lack of generalizability due to the single-race study population, and sponsor support were study limitations.
Editor’s takeaway
This reasonably good evidence shows a decrease in measured or monitored hypoglycemia, a disease-oriented outcome, but it did not reach statistical significance for symptomatic hypoglycemia (1% vs 2%), a patient-oriented outcome. Nevertheless, in patients reporting symptomatic hypoglycemia, a continuous or flash glucose monitor may allow for more aggressive glucose control.
Evidence summary
Continuous glucose monitoring: Nonsignificant reductions in event rates
A 2021 multicenter RCT (N = 175) evaluated CGM effectiveness in patients with basal insulin–treated T2D.1 Patients (mean age, 57 years; mean A1C, 9.1%) wore a blinded CGM device for baseline glucose measurement (minimum of 168 hours) before being randomly assigned to either CGM (n = 116) or traditional blood glucose monitoring (BGM; n = 59). At 8-month follow-up, patients in the BGM group again had blinded sensors placed. A significant reduction in hypoglycemia duration was observed for the CGM group vs the BGM group at 8 months for glucose values < 70 mg/mL (adjusted mean difference [aMD] = –0.24%; 95% CI, –0.42 to –0.05) and < 54 mg/dL (aMD = –0.10%; 95% CI, –0.15 to –0.04). A nonsignificant decrease in severe hypoglycemic events requiring resuscitative assistance occurred for BGM (2%) vs CGM (1%) patients. Study limitations included virtual visits due to COVID-19 and a short follow-up period.
A 2022 multicenter prospective study (N = 174) examined CGM effects on hypoglycemia frequency and severity in adults with T2D.2 Patients with insulin-requiring T2D (mean age, 61 years; mean A1C, 8.0%) participated in a 12-month study with 6 months of self-monitored blood glucose (SMBG) followed by 6 months of CGM use. The primary outcome was the rate of severe hypoglycemic events. A nonsignificant decrease was observed in the CGM group compared to the SMBG group for hypoglycemic event rate, per participant per 6-month period (relative risk [RR] = 0.43; 95% CI, 0.07-2.64). Four moderate hypoglycemic adverse events occurred in the SMBG phase vs 2 in the CGM phase. Financial support by the study sponsor decreases the study’s validity.
A 2021 prospective study (N = 90) evaluated the use of CGM to improve glycemic control.3 Patients younger than 66 years with insulin-treated T2D and an A1C > 7.5% participated in a 7-day blinded CGM cycle every 4 months for 1 year. A nonsignificant decrease in hypoglycemia duration was observed for glucose values < 70 mg/dL and < 54 mg/dL at 12 months. No change in hypoglycemic event rate was seen with the use of CGM. Funding by the device manufacturer was a limitation of this study.
Flash glucose monitoring: Mixed results on hypoglycemia events
A 2019 open-label RCT (N = 82) assessed the effectiveness of FGM on diabetes control.4 Patients with insulin-treated T2D were randomly assigned to the intervention or standard-care groups. The intervention group (n = 46; mean age, 66 years; mean A1C, 8.3%) used the FGM system for 10 weeks, while the standard-care group (n = 36; mean age, 70 years; mean A1C, 8.9%) maintained use of their glucometers. Both groups received similar types and duration of counseling. Treatment satisfaction was the primary outcome; total hypoglycemic events was a secondary outcome. No significant difference in the number of hypoglycemic episodes was observed between the intervention and control groups at 55 to 70 mg/dL (RR = 0.79; 95% CI, 0.44-1.4) or < 54 mg/dL (RR = 1.27; 95% CI, 0.38-4.2). No adverse events of severe hypoglycemia occurred during the study. Funding by the device manufacturer was a limitation of this study.
A 2017 open-label, multicenter RCT (N = 224) assessed FGM efficacy.5 Adults (mean age, 59 years; mean A1C, 8.8%) with T2D on intensive insulin therapy were randomized to FGM (n = 149) or SMBG (n = 75) after a 14-day masked baseline period. The 6-month treatment phase was unblinded. The duration of hypoglycemic events (glucose values < 70 mg/dL and < 55 mg/dL) was obtained from the sensors. Compared to the SMBG group, the FGM group spent 43% less time at < 70 mg/dL (aMD = –0.47 ± 0.13 h/d; P = .0006) and 53% less time at < 55 mg/dL (aMD = –0.22 ± 0.068 h/d; P = .0014). Hypoglycemic event rates significantly decreased by 28% (aMD = –0.16 ± 0.065; P = 0.016) and 44% (aMD = –0.12 ± 0.037; P = .0017) for glucose levels < 70 mg/dL and < 55 mg/dL, respectively. A nonsignificant difference occurred in severe hypoglycemic events requiring third-party assistance for the FGM (2%) vs control (1%) groups. Involvement of the device manufacturer and unblinded group allocations are study limitations.
A 2021 single-arm, multicenter prospective study looked at the impact of FGM on glycemic control in adults with insulin-treated T2D (N = 90; mean age, 64 years; mean A1C, 7.5%).6 After a 14-day baseline period consisting of masked sensor readings paired with self-monitored fingerstick tests, participants were followed for 11 weeks using the sensor to monitor glucose levels. The primary outcome was amount of time spent in hypoglycemia (< 70 mg/dL), with secondary outcomes including time and events in hypoglycemia (< 70, < 55, or < 45 mg/dL). No significant decrease in hypoglycemia duration or hypoglycemic event rates at < 70, < 55, or < 45 mg/dL was observed for FGM compared to baseline. Adverse events were observed in 64% of participants; 94% of the events were hypoglycemia related. Serious adverse events were reported for 5.3% of participants. The single-arm study format, lack of generalizability due to the single-race study population, and sponsor support were study limitations.
Editor’s takeaway
This reasonably good evidence shows a decrease in measured or monitored hypoglycemia, a disease-oriented outcome, but it did not reach statistical significance for symptomatic hypoglycemia (1% vs 2%), a patient-oriented outcome. Nevertheless, in patients reporting symptomatic hypoglycemia, a continuous or flash glucose monitor may allow for more aggressive glucose control.
1. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
2. Beck SE, Kelly C, Price DA. Non-adjunctive continuous glucose monitoring for control of hypoglycaemia (COACH): results of a post-approval observational study. Diabet Med. 2022;39:e14739. doi: 10.1111/dme.14739
3. Ribeiro RT, Andrade R, Nascimento do O D, et al. Impact of blinded retrospective continuous glucose monitoring on clinical decision making and glycemic control in persons with type 2 diabetes on insulin therapy. Nutr Metab Cardiovasc Dis. 2021;31:1267-1275. doi: 10.1016/j.numecd.2020.12.024
4. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
5. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
6. Ogawa W, Hirota Y, Osonoi T, et al. Effect of the FreeStyle Libre™ flash glucose monitoring system on glycemic control in individuals with type 2 diabetes treated with basal-bolus insulin therapy: an open label, prospective, multicenter trial in Japan. J Diabetes Investig. 2021;12:82-90. doi: 10.1111/jdi.13327
1. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
2. Beck SE, Kelly C, Price DA. Non-adjunctive continuous glucose monitoring for control of hypoglycaemia (COACH): results of a post-approval observational study. Diabet Med. 2022;39:e14739. doi: 10.1111/dme.14739
3. Ribeiro RT, Andrade R, Nascimento do O D, et al. Impact of blinded retrospective continuous glucose monitoring on clinical decision making and glycemic control in persons with type 2 diabetes on insulin therapy. Nutr Metab Cardiovasc Dis. 2021;31:1267-1275. doi: 10.1016/j.numecd.2020.12.024
4. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
5. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
6. Ogawa W, Hirota Y, Osonoi T, et al. Effect of the FreeStyle Libre™ flash glucose monitoring system on glycemic control in individuals with type 2 diabetes treated with basal-bolus insulin therapy: an open label, prospective, multicenter trial in Japan. J Diabetes Investig. 2021;12:82-90. doi: 10.1111/jdi.13327
EVIDENCE-BASED REVIEW:
NO. In adults with insulin-treated type 2 diabetes (T2D), continuous glucose monitoring (CGM) and flash glucose monitoring (FGM) do not decrease symptomatic hypoglycemia episodes (strength of recommendation [SOR], B) but do lower time in hypoglycemia (SOR, C; disease-oriented evidence).
CGM, in which glucose levels are sent automatically in numeric and graphic format to a patient’s smart device for their potential action, did not change the hypoglycemic event rate (SOR, B; 2 prospective studies). CGM significantly reduced hypoglycemia duration in an 8-month randomized controlled trial (RCT; SOR, C) but not in a 1-year prospective study (SOR, C).
FGM, in which glucose levels are sent on demand to a device, did not significantly reduce hypoglycemic episodes (SOR, B; 1 small RCT and 1 prospective study). Hypoglycemia duration was reduced significantly with FGM in a 6-month RCT (SOR, B) but not in a 1-year prospective study (SOR, B).
Is there benefit to adding ezetimibe to a statin for the secondary prevention of CVD?
Evidence summary
Adding ezetimibe reduces nonfatal events but does not improve mortality
A 2018 Cochrane meta-analysis included 10 RCTs (N = 21,919 patients) that evaluated the efficacy and safety of ezetimibe plus a statin (dual therapy) vs a statin alone or plus placebo (monotherapy) for the secondary prevention of CVD. Mean age of patients ranged from 55 to 84 years. Almost all of the patients (> 99%) included in the analyses had existing ASCVD. The dose of ezetimibe was 10 mg; statins used included atorvastatin 10 to 80 mg, pitavastatin 2 to 4 mg, rosuvastatin 10 mg, and simvastatin 20 to 80 mg.1
The primary outcomes were MACE and all-cause mortality. MACE is defined as a composite of CVD, nonfatal myocardial infarction (MI), nonfatal stroke, hospitalization for unstable angina, or coronary revascularization procedures. The TABLE1 provides a detailed breakdown of each of the outcomes.
The dual-therapy group compared to the monotherapy group had a lower risk for MACE (26.6% vs 28.3%; 1.7% absolute risk reduction; 6% relative risk reduction; NNT = 59) and little or no difference in the reduction of all-cause mortality. For secondary outcomes, the dual-therapy group had a lower risk for nonfatal MI, nonfatal stroke, and coronary revascularization. There was no difference in cardiovascular mortality or adverse events between the 2 groups. The quality of evidence was high for all-cause mortality and moderate for cardiovascular mortality, MACE, MI, and stroke.1
The 2015 IMPROVE-IT study, the largest included in the Cochrane review, was a double-blind RCT (N = 18,144) conducted at 1147 sites in 39 countries comparing simvastatin 40 mg/d plus ezetimibe 10 mg/d (dual therapy) vs simvastatin 40 mg/d plus placebo (monotherapy). Patients were at least 50 years old (average age, 64 years) and had been hospitalized for acute coronary syndrome (ACS) within the previous 10 days; 76% were male and 84% were White. The average low-density lipoprotein (LDL) concentration at baseline was 94 mg/dL in both groups.2
The primary endpoint was a composite of cardiovascular death, a major coronary event (nonfatal MI, unstable angina requiring hospitalization, coronary revascularization at least 30 days after randomization), or nonfatal stroke, with a median follow-up of 6 years. The simvastatin plus ezetimibe group compared to the simvastatin-only group had a lower risk for the primary end point (HR = 0.94; 95% CI, 0.89-0.99; NNT = 50), but no differences in cardiovascular or all-cause mortality. Since the study only recruited patients with recent ACS, results are only applicable to that specific population.2
The 2022 RACING study was a multicenter, open-label, randomized, noninferiority trial that evaluated the combination of ezetimibe 10 mg and a moderate-intensity statin (rosuvastatin 10 mg) compared to a high-intensity statin alone (rosuvastatin 20 mg) in adults (N = 3780) with ASCVD. Included patients were ages 19 to 80 years (mean, 64 years) and had a baseline LDL concentration of 80 mg/dL (standard deviation, 64-100 mg/dL) with known ASCVD (defined by prior MI, ACS, history of coronary or other arterial revascularization, ischemic stroke, or peripheral artery disease); 75% were male.3
The primary outcome was a composite of cardiovascular death, major cardiovascular events, or nonfatal stroke. At 3 years, an intention-to-treat analysis found no significant difference between the combination and monotherapy groups (9% vs 9.9%; absolute difference, –0.78%; 95% CI, –2.39% to 0.83%). Dose reduction or discontinuation of the study drug(s) due to intolerance was lower in the combination group than in the monotherapy group (4.8% vs 8.2%; P < 0.0001). The study may be limited by the fact that it was nonblinded and all participants were South Korean, which limits generalizability.3
Recommendations from others
A 2022 evidence-based clinical practice guideline published in BMJ recommends adding ezetimibe to a statin to decrease all-cause mortality, cardiovascular mortality, nonfatal stroke, and nonfatal MI in patients with known CVD, regardless of their LDL concentration (weak recommendation based on a systematic review and network meta-analysis).4
In 2019, the American Heart Association and the American College of Cardiology recommended ezetimibe for patients with clinical ASCVD who are on maximally tolerated statin therapy and have an LDL concentration of 70 mg/dL or higher (Class 2b recommendation [meaning it can be considered] based on a meta-analysis of moderate-quality RCTs).5
Editor’s takeaway
The data on this important and well-studied question have inched closer to firm and clear answers. First, adding ezetimibe to a lower-intensity statin when a higher-intensity statin is not tolerated is an effective treatment. Second, adding ezetimibe to a statin improves nonfatal ASCVD outcomes but not fatal ones. What has not yet been made clear, because a noninferiority trial does not answer this question, is whether the highest intensity statin plus ezetimibe is superior to that high-intensity statin alone, regardless of LDL concentration.
1. Zhan S, Tang M, Liu F, et al. Ezetimibe for the prevention of cardiovascular disease and all‐cause mortality events. Cochrane Database System Rev. 2018;11:CD012502. doi: 10.1002/14651858.CD012502.pub2
2. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489 pmid:26039521
3. Kim BK, Hong SJ, Lee YJ, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400:380-390. doi: 10.1016/S0140-6736(22)00916-3
4. Hao Q, Aertgeerts B, Guyatt G, et al. PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations. BMJ. 2022;377:e069066. doi: 10.1136/bmj-2021-069066
5. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
Evidence summary
Adding ezetimibe reduces nonfatal events but does not improve mortality
A 2018 Cochrane meta-analysis included 10 RCTs (N = 21,919 patients) that evaluated the efficacy and safety of ezetimibe plus a statin (dual therapy) vs a statin alone or plus placebo (monotherapy) for the secondary prevention of CVD. Mean age of patients ranged from 55 to 84 years. Almost all of the patients (> 99%) included in the analyses had existing ASCVD. The dose of ezetimibe was 10 mg; statins used included atorvastatin 10 to 80 mg, pitavastatin 2 to 4 mg, rosuvastatin 10 mg, and simvastatin 20 to 80 mg.1
The primary outcomes were MACE and all-cause mortality. MACE is defined as a composite of CVD, nonfatal myocardial infarction (MI), nonfatal stroke, hospitalization for unstable angina, or coronary revascularization procedures. The TABLE1 provides a detailed breakdown of each of the outcomes.

The dual-therapy group compared to the monotherapy group had a lower risk for MACE (26.6% vs 28.3%; 1.7% absolute risk reduction; 6% relative risk reduction; NNT = 59) and little or no difference in the reduction of all-cause mortality. For secondary outcomes, the dual-therapy group had a lower risk for nonfatal MI, nonfatal stroke, and coronary revascularization. There was no difference in cardiovascular mortality or adverse events between the 2 groups. The quality of evidence was high for all-cause mortality and moderate for cardiovascular mortality, MACE, MI, and stroke.1
The 2015 IMPROVE-IT study, the largest included in the Cochrane review, was a double-blind RCT (N = 18,144) conducted at 1147 sites in 39 countries comparing simvastatin 40 mg/d plus ezetimibe 10 mg/d (dual therapy) vs simvastatin 40 mg/d plus placebo (monotherapy). Patients were at least 50 years old (average age, 64 years) and had been hospitalized for acute coronary syndrome (ACS) within the previous 10 days; 76% were male and 84% were White. The average low-density lipoprotein (LDL) concentration at baseline was 94 mg/dL in both groups.2
The primary endpoint was a composite of cardiovascular death, a major coronary event (nonfatal MI, unstable angina requiring hospitalization, coronary revascularization at least 30 days after randomization), or nonfatal stroke, with a median follow-up of 6 years. The simvastatin plus ezetimibe group compared to the simvastatin-only group had a lower risk for the primary end point (HR = 0.94; 95% CI, 0.89-0.99; NNT = 50), but no differences in cardiovascular or all-cause mortality. Since the study only recruited patients with recent ACS, results are only applicable to that specific population.2
The 2022 RACING study was a multicenter, open-label, randomized, noninferiority trial that evaluated the combination of ezetimibe 10 mg and a moderate-intensity statin (rosuvastatin 10 mg) compared to a high-intensity statin alone (rosuvastatin 20 mg) in adults (N = 3780) with ASCVD. Included patients were ages 19 to 80 years (mean, 64 years) and had a baseline LDL concentration of 80 mg/dL (standard deviation, 64-100 mg/dL) with known ASCVD (defined by prior MI, ACS, history of coronary or other arterial revascularization, ischemic stroke, or peripheral artery disease); 75% were male.3
The primary outcome was a composite of cardiovascular death, major cardiovascular events, or nonfatal stroke. At 3 years, an intention-to-treat analysis found no significant difference between the combination and monotherapy groups (9% vs 9.9%; absolute difference, –0.78%; 95% CI, –2.39% to 0.83%). Dose reduction or discontinuation of the study drug(s) due to intolerance was lower in the combination group than in the monotherapy group (4.8% vs 8.2%; P < 0.0001). The study may be limited by the fact that it was nonblinded and all participants were South Korean, which limits generalizability.3
Recommendations from others
A 2022 evidence-based clinical practice guideline published in BMJ recommends adding ezetimibe to a statin to decrease all-cause mortality, cardiovascular mortality, nonfatal stroke, and nonfatal MI in patients with known CVD, regardless of their LDL concentration (weak recommendation based on a systematic review and network meta-analysis).4
In 2019, the American Heart Association and the American College of Cardiology recommended ezetimibe for patients with clinical ASCVD who are on maximally tolerated statin therapy and have an LDL concentration of 70 mg/dL or higher (Class 2b recommendation [meaning it can be considered] based on a meta-analysis of moderate-quality RCTs).5
Editor’s takeaway
The data on this important and well-studied question have inched closer to firm and clear answers. First, adding ezetimibe to a lower-intensity statin when a higher-intensity statin is not tolerated is an effective treatment. Second, adding ezetimibe to a statin improves nonfatal ASCVD outcomes but not fatal ones. What has not yet been made clear, because a noninferiority trial does not answer this question, is whether the highest intensity statin plus ezetimibe is superior to that high-intensity statin alone, regardless of LDL concentration.
Evidence summary
Adding ezetimibe reduces nonfatal events but does not improve mortality
A 2018 Cochrane meta-analysis included 10 RCTs (N = 21,919 patients) that evaluated the efficacy and safety of ezetimibe plus a statin (dual therapy) vs a statin alone or plus placebo (monotherapy) for the secondary prevention of CVD. Mean age of patients ranged from 55 to 84 years. Almost all of the patients (> 99%) included in the analyses had existing ASCVD. The dose of ezetimibe was 10 mg; statins used included atorvastatin 10 to 80 mg, pitavastatin 2 to 4 mg, rosuvastatin 10 mg, and simvastatin 20 to 80 mg.1
The primary outcomes were MACE and all-cause mortality. MACE is defined as a composite of CVD, nonfatal myocardial infarction (MI), nonfatal stroke, hospitalization for unstable angina, or coronary revascularization procedures. The TABLE1 provides a detailed breakdown of each of the outcomes.

The dual-therapy group compared to the monotherapy group had a lower risk for MACE (26.6% vs 28.3%; 1.7% absolute risk reduction; 6% relative risk reduction; NNT = 59) and little or no difference in the reduction of all-cause mortality. For secondary outcomes, the dual-therapy group had a lower risk for nonfatal MI, nonfatal stroke, and coronary revascularization. There was no difference in cardiovascular mortality or adverse events between the 2 groups. The quality of evidence was high for all-cause mortality and moderate for cardiovascular mortality, MACE, MI, and stroke.1
The 2015 IMPROVE-IT study, the largest included in the Cochrane review, was a double-blind RCT (N = 18,144) conducted at 1147 sites in 39 countries comparing simvastatin 40 mg/d plus ezetimibe 10 mg/d (dual therapy) vs simvastatin 40 mg/d plus placebo (monotherapy). Patients were at least 50 years old (average age, 64 years) and had been hospitalized for acute coronary syndrome (ACS) within the previous 10 days; 76% were male and 84% were White. The average low-density lipoprotein (LDL) concentration at baseline was 94 mg/dL in both groups.2
The primary endpoint was a composite of cardiovascular death, a major coronary event (nonfatal MI, unstable angina requiring hospitalization, coronary revascularization at least 30 days after randomization), or nonfatal stroke, with a median follow-up of 6 years. The simvastatin plus ezetimibe group compared to the simvastatin-only group had a lower risk for the primary end point (HR = 0.94; 95% CI, 0.89-0.99; NNT = 50), but no differences in cardiovascular or all-cause mortality. Since the study only recruited patients with recent ACS, results are only applicable to that specific population.2
The 2022 RACING study was a multicenter, open-label, randomized, noninferiority trial that evaluated the combination of ezetimibe 10 mg and a moderate-intensity statin (rosuvastatin 10 mg) compared to a high-intensity statin alone (rosuvastatin 20 mg) in adults (N = 3780) with ASCVD. Included patients were ages 19 to 80 years (mean, 64 years) and had a baseline LDL concentration of 80 mg/dL (standard deviation, 64-100 mg/dL) with known ASCVD (defined by prior MI, ACS, history of coronary or other arterial revascularization, ischemic stroke, or peripheral artery disease); 75% were male.3
The primary outcome was a composite of cardiovascular death, major cardiovascular events, or nonfatal stroke. At 3 years, an intention-to-treat analysis found no significant difference between the combination and monotherapy groups (9% vs 9.9%; absolute difference, –0.78%; 95% CI, –2.39% to 0.83%). Dose reduction or discontinuation of the study drug(s) due to intolerance was lower in the combination group than in the monotherapy group (4.8% vs 8.2%; P < 0.0001). The study may be limited by the fact that it was nonblinded and all participants were South Korean, which limits generalizability.3
Recommendations from others
A 2022 evidence-based clinical practice guideline published in BMJ recommends adding ezetimibe to a statin to decrease all-cause mortality, cardiovascular mortality, nonfatal stroke, and nonfatal MI in patients with known CVD, regardless of their LDL concentration (weak recommendation based on a systematic review and network meta-analysis).4
In 2019, the American Heart Association and the American College of Cardiology recommended ezetimibe for patients with clinical ASCVD who are on maximally tolerated statin therapy and have an LDL concentration of 70 mg/dL or higher (Class 2b recommendation [meaning it can be considered] based on a meta-analysis of moderate-quality RCTs).5
Editor’s takeaway
The data on this important and well-studied question have inched closer to firm and clear answers. First, adding ezetimibe to a lower-intensity statin when a higher-intensity statin is not tolerated is an effective treatment. Second, adding ezetimibe to a statin improves nonfatal ASCVD outcomes but not fatal ones. What has not yet been made clear, because a noninferiority trial does not answer this question, is whether the highest intensity statin plus ezetimibe is superior to that high-intensity statin alone, regardless of LDL concentration.
1. Zhan S, Tang M, Liu F, et al. Ezetimibe for the prevention of cardiovascular disease and all‐cause mortality events. Cochrane Database System Rev. 2018;11:CD012502. doi: 10.1002/14651858.CD012502.pub2
2. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489 pmid:26039521
3. Kim BK, Hong SJ, Lee YJ, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400:380-390. doi: 10.1016/S0140-6736(22)00916-3
4. Hao Q, Aertgeerts B, Guyatt G, et al. PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations. BMJ. 2022;377:e069066. doi: 10.1136/bmj-2021-069066
5. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
1. Zhan S, Tang M, Liu F, et al. Ezetimibe for the prevention of cardiovascular disease and all‐cause mortality events. Cochrane Database System Rev. 2018;11:CD012502. doi: 10.1002/14651858.CD012502.pub2
2. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489 pmid:26039521
3. Kim BK, Hong SJ, Lee YJ, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400:380-390. doi: 10.1016/S0140-6736(22)00916-3
4. Hao Q, Aertgeerts B, Guyatt G, et al. PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations. BMJ. 2022;377:e069066. doi: 10.1136/bmj-2021-069066
5. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
EVIDENCE-BASED REVIEW:
YES. In patients with known cardio- vascular disease (CVD), ezetimibe with a statin decreases
In adults with atherosclerotic CVD (ASCVD), the combination of ezetimibe and a moderate-intensity statin (rosuvastatin 10 mg) was noninferior at decreasing cardiovascular death, major cardiovascular events, and nonfatal stroke, but was more tolerable, compared to a high-intensity statin (rosuvastatin 20 mg) alone (SOR, B; 1 RCT).