User login
Potential Utility of Liposome Bupivacaine in Orthopedic Surgery
Approximately 5.5 million patients undergo orthopedic surgery in the United States each year, and more than 1 million of the procedures are total knee arthroplasty (TKA) or total hip arthroplasty.1 From its 2010 level, demand for joint arthroplasty is expected to double by 2020 and quadruple by 2030.2
About half the patients who have major joint arthroplasty experience severe postsurgical pain.3 Because postsurgical pain may persist for days or weeks, and inadequate treatment is associated with negative outcomes, achieving effective postsurgical analgesia is an important consideration.4-7 Complications of inadequate postsurgical pain management include thromboembolic or pulmonary complications, development of chronic pain, and decrements in health-related quality of life.4,8
In patients who have orthopedic surgery, the inability to adequately control postsurgical pain has been associated with increased hospital length of stay (LOS), delayed time to ambulation, and reduced capacity for exercise.9-12 A recent study involving 4709 patients who had hip or knee arthroplasty found that postsurgical pain relief was the second most highly correlated factor with respect to overall patient satisfaction (how well surgery met patient expectations was the most highly correlated factor),13 suggesting that postsurgical analgesia should be a focus of surgical practice.
A prolonged-release liposomal formulation of the local anesthetic bupivacaine is now available. Bupivacaine liposome injectable suspension (Exparel; Pacira Pharmaceuticals, Inc., Parsippany, New Jersey) is indicated for administration into the surgical site to produce postsurgical analgesia.14 In this article, we review evidence from clinical studies regarding the potential contribution of liposome bupivacaine to improving postsurgical pain management when used as part of a multimodal analgesic regimen in patients undergoing orthopedic surgery.
Postsurgical Pain Management in Orthopedic Surgery
Frequently Used Modalities
Analgesic modalities commonly used for perioperative pain management include central (eg, epidural),4,10,15,16 central regional (eg, neuraxial),4 peripheral regional (eg, peripheral nerve blocks, local/regional surgical site infiltration, intra-articular administration),4,10,15,17-25 and intravenous (IV) patient-controlled analgesia.4,10,25 These pharmacologic interventions may be augmented by nonpharmacologic modalities (eg, transcutaneous electrical nerve stimulation).26
Pharmacologic treatment options for perioperative pain management include opioids, local anesthetics, clonidine, ketamine, nonsteroidal anti-inflammatory drugs, acetaminophen, and calcium-channel blockers.4,26-28 In TKA, “drug cocktails” (eg, combinations of ropivacaine, ketorolac, epinephrine, and clonidine) for regional or intra-articular injection can also provide effective immediate postsurgical analgesia.25 Although opioids are the most commonly used analgesics for management of orthopedic perioperative pain,25 their use is often associated with adverse effects (AEs), including constipation or ileus, nausea, sedation, dizziness, pruritus, urinary retention, and respiratory depression.6
Multimodal Analgesic Regimens for Postsurgical Pain Management
Current American Society of Anesthesiologists guidelines endorse use of multimodal analgesia, whenever possible, to provide effective management of acute perioperative pain.4 Multimodal analgesia involves applying 2 or more agents with different mechanisms of action to achieve a synergistic effect, which allows each agent to be reduced in dose4,28 and thereby may limit the risk and severity of dose-related AEs.4,25,28
Multimodal analgesia aims to reduce the risk for opioid-related AEs (ORAEs) and the impact of opioids on postsurgical milestones (eg, ambulation, discharge) and may reduce opioid consumption, with attendant reductions in ORAE risk.29,30 Health economics studies have shown that postsurgical ORAEs are associated with increased hospital costs and LOS.6 In a study using a national hospital database, development of an ORAE (vs no ORAE) in postsurgical patients was associated with mean increases of about $4700 in hospital costs and 3.3 days in LOS.7 Reducing postsurgical opioid use may also help reduce the risk for opioid abuse, addiction, and diversion.31-33
One approach to reducing opioid use involves continuous or intermittent administration of local anesthetics by elastomeric pumps to extend duration of postsurgical analgesia.34-36 However, use of elastomeric pumps has been associated with risk for AEs, including tissue necrosis, sloughing, wound infection, and chondrolysis.37-40 In addition, AEs related to “dose dumping” (accidental delivery of excessive doses) have been reported.40-44 Key issues that may negatively affect rehabilitation after orthopedic surgery include consistency and accuracy of analgesic delivery and the potential for motor block–induced muscle weakness, which may lead to falls and constrain ambulation.45-47
Liposome Bupivacaine
Description
Drug Delivery Technology. Liposome bupivacaine incorporates DepoFoam drug delivery technology (Pacira Pharmaceuticals, Inc.) to facilitate prolonged release of bupivacaine. This technology is based on creation of multivesicular liposome particles (diameter, 10-30 µm) with multiple aqueous chambers.30,48 After administration into the surgical site, bupivacaine diffuses from chambers in the liposomal particles over time, providing analgesia and reduced opioid requirements for up to 72 hours.29,30
Indication, Mechanism of Action, Pharmacokinetics, and Dose/Administration. Liposome bupivacaine is indicated for single-dose administration into the surgical site to produce postsurgical analgesia in patients at least 18 years old.14 Like other local anesthetics, liposome bupivacaine is thought to exert its pharmacologic effects by interacting with voltage-gated Na+ channels on neural membranes to raise the threshold for electrical excitability, to slow nerve impulse propagation, and to reduce the rate of rise of the action potential.14,49
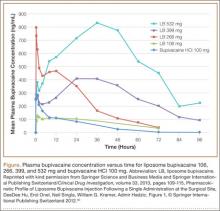
Liposome bupivacaine has dose-proportional pharmacokinetics.50 Presence of a small amount of extra-liposomal bupivacaine in the formulation leads to a bimodal pharmacokinetic profile, with an initial peak serum concentration about 1 hour after administration, followed by a second peak within 12 to 36 hours (Figure).50
Maximum amount of liposome bupivacaine approved for single administration is 266 mg (packaged as 20 mL of a 1.3% solution). However, product labeling includes safety data associated with doses of 532 mg or less.14 The appropriate volume to be used should be based on the amount required to cover the surgical area. Liposome bupivacaine may be expanded with preservative-free normal (0.9%) sterile saline to a total volume of 300 mL: 20 mL liposome bupivacaine plus 280 mL or less diluent, with final concentration of 0.89 mg/mL (1:14 by volume).14
A 25-gauge or larger bore needle should be used to slowly inject liposome bupivacaine into soft tissues of the surgical site, with frequent aspiration to check for blood to minimize risk for intravascular injection.14 Total volume used and fraction injected in specific regions of the surgical site depend on the procedure. For example, a TKA study used 266 mg diluted to a total volume of 60 mL, with 8 mL infiltrated to the area around the medial capsule, 8 mL around the lateral capsule, 12 mL around the posterior capsule, 8 mL around the peripatellar area, 12 mL into the capsulotomy incision, and 12 mL into the subcutaneous tissue on each side of the incision.51
Efficacy
Multiple Surgical Settings. The efficacy of liposome bupivacaine, either alone or as a component of a multimodal analgesic regimen, has been evaluated in a series of 10 phase 2 and 3 studies (8 active-controlled, 2 placebo-controlled) involving 823 patients undergoing TKA, bunionectomy, hemorrhoidectomy, inguinal hernia repair, or mammoplasty.52 Patients received a single liposome bupivacaine dose ranging from 66 to 532 mg.52
Combined analyses of efficacy data from these studies found that liposome bupivacaine–based multimodal analgesic regimens produced postsurgical analgesia for up to 72 hours, increased time to first use of opioid rescue medication after surgery, and reduced total amount of postsurgical opioid consumption versus placebo.52
Compared with standard of care, liposome bupivacaine has been shown to provide effective analgesia in open-label studies in patients undergoing open colectomy,53 laparoscopic colectomy,54 and ileostomy reversal,55,56 as reflected in assessments of postsurgical opioid consumption, LOS, and hospital costs. It has also been studied when administered by infiltration into the transversus abdominis plane (TAP) in patients having laparoscopic prostatectomy and open abdominal hernia repair.57,58
Orthopedic Surgery. In a phase 2 randomized, double-blind, dose-ranging study, TKA patients (N = 138) received bupivacaine HCl 150 mg or liposome bupivacaine 133, 266, 399, or 532 mg administered by local infiltration into the capsulotomy incision and on either side of the incision before wound closure.51 Postsurgical rescue analgesia was available to all patients. Cumulative pain intensity scores with activity (primary efficacy measure) were not statistically different between liposome bupivacaine groups and the bupivacaine HCl group through postoperative day 4. Mean scores in the liposome bupivacaine 266-, 399-, and 532-mg groups were numerically lower than for those treated with bupivacaine HCl on postoperative days 2 to 5, with all doses of liposome bupivacaine having a statistically significant lower pain score at rest on day 5. There were no statistically significant differences across treatment groups with respect to total amount of postsurgical opioids used.
In a phase 3 randomized, double-blind study of TKA patients (N = 245), liposome bupivacaine 532 mg administered into the surgical site was compared with bupivacaine HCl 200 mg for postsurgical analgesia.52 Rescue analgesia was available to all patients. No statistically significant between-group differences were found with respect to postsurgical cumulative pain scores through 72 hours (primary efficacy endpoint).
In a single-center retrospective TKA study, postsurgical outcomes in a patient cohort that received intraoperative periarticular infiltration with liposome bupivacaine 266 mg (n = 65) were compared with a cohort that received infiltration with a combination of ropivacaine 400 mg, morphine 5 mg, and epinephrine 0.4 mg (n = 85).59 Patient-reported postsurgical pain scores were similar in the 2 treatment groups during the first 24 hours after surgery and at discharge. Mean (SD) pain scores during hospitalization after the first 24 hours until discharge were significantly (P = .04) higher in the liposome bupivacaine group, 4.9 (1.4), than in the periarticular group, 4.4 (1.6). There was no significant difference between the 2 treatment groups in postsurgical opioid use. The study demonstrated no advantage to using liposome bupivacaine injections with respect to pain relief, but it was a retrospective review in which pain scores were obtained from electronic medical records. It is essential that liposome bupivacaine be compared with intra-articular injections in well-designed randomized trials.
Another single-center, matched-cohort TKA study (N = 200) compared a liposome bupivacaine regimen with femoral nerve block.60 Compared with patients who received femoral nerve block, patients who received liposome bupivacaine reported lower pain intensity scores after surgery and had shorter LOS, reduced costs, and improved knee flexion at follow-up.60
Results from 2 other studies were presented at the 2014 meeting of the American Academy of Orthopaedic Surgeons (AAOS). One was a single-center, matched-cohort TKA study (N = 72) comparing infiltration of a single dose of liposome bupivacaine into the surgical site with continuous femoral nerve block.61 The 2 treatment groups had similar mean postsurgical pain intensity scores on a 0-to-10 visual analog scale, 1.8 for liposome bupivacaine and 2.3 for continuous nerve block (P = NS), but total amount of postsurgical opioids (hydrocodone-equivalent milligrams) was significantly (P < .0001) less in the liposome bupivacaine group (82 vs 177 mg).
The other study presented at the AAOS meeting was a larger, prospective case–control study comparing outcomes between 1000 patients who had total joint arthroplasty (TJA) with liposome bupivacaine and 1000 control patients who had TJA without liposome bupivacaine.62 For the control and liposome bupivacaine cohorts, respectively, mean postsurgical pain intensity scores were 2.41 and 1.98 (P < .0001), mean LOS was 2.83 days and 2.66 days (P < .02), and incidence of falls was 1.0% and 0.2% (P = .02). Average per-patient costs were $1246 lower in the liposome bupivacaine cohort.
A pivotal phase 3 placebo-controlled study compared liposome bupivacaine 106 mg with placebo in patients undergoing bunionectomy (N = 193).5 Rescue medication was available to all patients. Cumulative pain scores were significantly (P = .0005) lower in the liposome bupivacaine group (125) than in the placebo group (146) through 24 hours after surgery (primary efficacy measure) and significantly (P = .0229) lower (197 vs 220) through 36 hours. Median time to first use of rescue opioids was delayed in favor of the liposome bupivacaine group (7.2 vs 4.3 hours; P < .0001). Mean total number of opioid tablets used within 24 hours after surgery was also significantly lower (3.8 vs 4.7; P = .008), and a larger percentage of patients in the liposome bupivacaine group avoided opioid use altogether through 24 hours (7% vs 1%; P = .04).
Efficacy data for liposome bupivacaine appear promising for relief of pain after joint arthroplasty and other orthopedic procedures but have their limitations. First, no randomized trials have compared liposome bupivacaine with locally injected pain medications (intra-articular injections in TKA or hip arthroplasty). As these injections are quite common now, such analyses are essential. Second, cost-effectiveness studies are needed for orthopedic procedures. Third, most of the published studies were sponsored by the manufacturer of liposome bupivacaine—a situation that raises questions about potential bias. Non-industry-sponsored randomized trials assessing efficacy, safety, and cost-effectiveness are needed.
Safety
Local anesthetics, including liposome bupivacaine, have the potential for central nervous system (CNS) or cardiac toxicity resulting from excessive systemic absorption or inadvertent IV administration.63 However, reported serious CNS or cardiac-related AEs are rare.63,64
AE Profile. Safety data from 10 phase 2 and 3 studies involving 823 patients who received liposome bupivacaine were evaluated.65 Of these patients, 545 received a dose of 266 mg or less (maximum dose approved by the US Food and Drug Administration [FDA]). Liposome bupivacaine was generally well tolerated. Reported AE incidence was 62% (liposome bupivacaine), 75% (bupivacaine HCl), and 43% (placebo). More than 90% of reported AEs were mild or moderate. The most frequently reported AEs were nausea, constipation, and vomiting (liposome bupivacaine, bupivacaine HCl) and nausea, dizziness, and vomiting (placebo).
Serious AEs were reported in 22 (2.7%) of the 823 patients in the liposome bupivacaine group, 24 (5.4%) of the 446 in the bupivacaine HCl group, and 2 (1.1%) of the 190 in the placebo group.65 None of the serious AEs in the liposome bupivacaine and placebo groups were considered treatment-related. Six patients in the bupivacaine HCl group had treatment-related serious AEs (hypoglycemia, arthrofibrosis, hemarthrosis, joint swelling, scar, knee arthroplasty).
Cardiac Safety. Possible cardiac effects associated with liposome bupivacaine were evaluated with data from studies conducted during the clinical development program.66 One hundred thirty-eight patients participated in the phase 2 safety and efficacy study in TKA. In these patients, a consistent change in mean heart rate (range, +12.2 to +16.5 beats per minute) was found across all liposome bupivacaine doses and with bupivacaine HCl. No clinically relevant changes from baseline in mean electrocardiographic parameters, including QTcF interval (QT interval adjusted using Fridericia’s correction formula), were found. In another analysis,67 liposome bupivacaine administered in a single subcutaneous dose (266, 399, 532, or 665 mg) to healthy volunteers did not prolong (vs placebo) QTc interval.
Wound Healing. The potential effects of liposome bupivacaine on wound healing were evaluated with results from 10 phase 2 and 3 studies.68 The assessments, which varied across studies, included clinicians’ overall satisfaction with patient wound healing, wound status assessment (categories included erythema, drainage, edema, and induration), and wound scarring (categories included pigmentation, height, pliability, and vascularity). Clinician-assessed scores reflected high satisfaction with wound healing overall. There were few statistically significant differences in wound status assessments between liposome bupivacaine and the comparators and no statistically significant differences in scarring between liposome bupivacaine and bupivacaine HCl.
The potential of liposome bupivacaine to have adverse intra-articular effects was assessed with drainage samples from patients (n = 23) who had TKA and received liposome bupivacaine (133, 266, 399, or 532 mg) or bupivacaine HCl (150 mg) by wound infiltration near the intra-articular space.51,65 Only small amounts of bupivacaine were present in drainage fluid collected for 12 hours after liposome bupivacaine administration, comparable to bupivacaine HCl administration.65 Currently, the product is not approved for intra-articular use.
Compatibility With Diluents, Other Medications, and Implant Materials
Liposome bupivacaine may be expanded up to a ratio of 1:14 by volume (to a final total volume of 300 mL or a concentration of 0.89 mg/mL) using preservative-free normal (0.9%) sterile saline for injection.14 It has also been shown in vitro to be compatible with lactated Ringer solution as a diluent.69
Liposome bupivacaine should not be admixed with other medications before administration.14 No formal drug–drug interaction studies have been conducted with liposome bupivacaine, but it has been shown in vitro to be compatible with epinephrine solutions, with certain anti-infective medications (eg, bacitracin, gentamicin, cefazolin, cefuroxime), with certain analgesics (eg, ketorolac, morphine), with an antihypertensive medication (clonidine), with an antihemorrhagic medication (tranexamic acid), and with certain corticosteroids (eg, methylprednisolone, triamcinolone acetonide). These medications may be coadministered in the same location as liposome bupivacaine.69
Topical antiseptics (eg, povidone iodine) may be used in surgical procedures involving liposome bupivacaine as long as they are not directly mixed with liposome bupivacaine and are allowed to dry before it is administered. If a topical antiseptic is used for wound irrigation, the wound should be rinsed clear before liposome bupivacaine administration.14,69
Liposome bupivacaine may be coadministered into the same surgical site immediately after bupivacaine HCl as long as the dose ratio of liposome bupivacaine to bupivacaine HCl is 2:1 or higher. Because of the prolonged-release pharmacokinetic profile of liposome bupivacaine and the potential for increased bupivacaine exposure, bupivacaine HCl should not be administered within 96 hours after administration of liposome bupivacaine.14,69
In vitro coincubation studies of liposome bupivacaine and other local anesthetics, including ropivacaine, lidocaine, and mepivacaine, have found rapid release of free bupivacaine from the liposome matrix. Therefore, after giving any of these other local anesthetics, surgeons should wait at least 20 minutes before administering liposome bupivacaine into the same area.14,69
In vitro studies have shown that liposome bupivacaine is compatible with a wide range of commonly used implant materials, including polypropylene, expanded polytetrafluoroethylene, stainless steel, titanium, and smooth- and textured-type silicone.69
Investigational Use and Ongoing Studies
A phase 2 randomized, double-masked, dose-escalating/deescalating study was conducted to evaluate the efficacy, safety, and pharmacokinetics of liposome bupivacaine (155, 199, or 310 mg) in comparison with bupivacaine HCl 125 mg for ankle nerve block in patients undergoing bunionectomy (N = 58).70 The study medication was injected into 3 sites to reach the posterior tibial, sural, deep peroneal, superficial peroneal, and saphenous nerves. Pharmacokinetic exposure was higher for liposome bupivacaine than for bupivacaine HCl, as reflected by a significantly greater area under the curve, lower Cmax (maximum serum concentration), and longer mean half-life. Mean pain intensity scores were lower in the bupivacaine HCl group than in each liposome bupivacaine group the first 12 hours after surgery. However, the liposome bupivacaine 310-mg group had similar or lower scores than the bupivacaine HCl group from 12 to 96 hours after surgery. The most common AEs in the liposome bupivacaine group were gastrointestinal and not treatment-related.70
The efficacy and safety of liposome bupivacaine, administered as a femoral nerve block for postsurgical analgesia, were assessed in a phase 2/3 manufacturer-sponsored, placebo-controlled, multicenter, randomized, double-blind 2-part study (NCT01683071)71 in 280 TKA patients.71,72 Part 2 of the study, comparing liposome bupivacaine 266 mg (n = 116) and placebo (n = 116), met its primary endpoint, demonstrating statistical significance in favor of liposome bupivacaine for cumulative pain scores over 72 hours (P < .0001), with decreased opioid use (P < .05) and a safety profile similar to that of placebo.72
Other ongoing investigator-sponsored studies in orthopedic populations include comparisons of liposome bupivacaine and bupivacaine HCl for ultrasound-guided periarticular hip infiltration in hip arthroplasty (NTC01917191),73 as femoral nerve block in TKA (NCT01977339),74 and as interscalene brachial plexus block in arthroscopic shoulder surgery (NCT01977352).75 The primary efficacy outcome measure in these studies was postsurgical opioid use.73-75
Health Economics
A series of phase 4 health economics studies was conducted for gastrointestinal surgeries, including open colectomy, laparoscopic colectomy, and ileostomy reversal.53-56,76 These studies, of similar design, showed that a liposome bupivacaine–based multimodal analgesic regimen was associated with reduced opioid use, shorter hospital LOS, and lower hospitalization costs in comparison with a traditional opioid-based regimen.53-56 Although pooled analysis of these studies showed a cost savings of more than $2000 per patient and an LOS decrease of 1.4 days,76 all were conducted in the gastrointestinal surgery setting. Studies are needed to fully assess the economic benefits associated with liposome bupivacaine in the orthopedic surgery setting.
Conclusion
Liposome bupivacaine represents a potentially important contributor to multimodal analgesic regimens used to manage postsurgical pain. Liposome bupivacaine has demonstrated efficacy in providing prolonged postsurgical analgesia and reducing postsurgical opioid use in most surgical settings studied. Additional data from health economics studies in gastrointestinal surgery suggest liposome bupivacaine–based multimodal analgesic regimens may also contribute to reductions in hospital LOS and hospitalization costs. Non-industry-sponsored trials are needed to answer these crucial questions in orthopedic surgery settings. Nevertheless, data on the safety and efficacy of liposome bupivacaine for postsurgical analgesia continue to accumulate, and liposome bupivacaine appears to be a feasible therapeutic option for managing postsurgical pain in orthopedic surgery.
1. Centers for Disease Control and Prevention. Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. http://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf. Accessed January 30, 2015.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Bonica JJ. Postoperative pain. In: Bonica JJ, ed. The Management of Pain. Malvern, PA: Lea & Febiger; 1990:461-480.
4. Apfelbaum JL, Ashburn MA, Connis RT, et al; American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273.
5. Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam® bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther. 2011;28(9):776-788.
6. Oderda G. Challenges in the management of acute postsurgical pain. Pharmacotherapy. 2012;32(9 pt 2):6S-11S.
7. Oderda GM, Gan TJ, Johnson BH, Robinson SB. Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother. 2013;27(1):62-70.
8. Wu CL, Naqibuddin M, Rowlingson AJ, Lietman SA, Jermyn RM, Fleisher LA. The effect of pain on health-related quality of life in the immediate postoperative period. Anesth Analg. 2003;97(4):1078-1085.
9. Morrison RS, Magaziner J, McLaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103(3):303-311.
10. Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d’Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91(1):8-15.
11. Capdevila X, Dadure C, Bringuier S, et al. Effect of patient-controlled perineural analgesia on rehabilitation and pain after ambulatory orthopedic surgery: a multicenter randomized trial. Anesthesiology. 2006;105(3):566-573.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 Suppl 3):12-15.
13. Hamilton DF, Lane JV, Gaston P, et al. What determines patient satisfaction with surgery? A prospective cohort study of 4709 patients following total joint replacement. BMJ Open. 2013;3(4):e002525.
14. Exparel [prescribing information]. Parsippany, NJ: Pacira Pharmaceuticals, Inc.; 2014.
15. DeWeese FT, Akbari Z, Carline E. Pain control after knee arthroplasty: intraarticular versus epidural anesthesia. Clin Orthop Relat Res. 2001;392(11):226-231.
16. Pati AB, Perme D, Trail M, Henry PK, Bryan WJ. Rehabilitation parameters in total knee replacement patients undergoing epidural vs. conventional analgesia. J Orthop Sports Phys Ther. 1994;19(2):88-92.
17. Browne C, Copp S, Reden L, Pulido P, Colwell C Jr. Bupivacaine bolus injection versus placebo for pain management following total knee arthroplasty. J Arthroplasty. 2004;19(3):377-380.
18. Nechleba J, Rogers V, Cortina G, Cooney T. Continuous intra-articular infusion of bupivacaine for postoperative pain following total knee arthroplasty. J Knee Surg. 2005;18(3):197-202.
19. Campbell A, McCormick M, McKinlay K, Scott NB. Epidural vs. lumbar plexus infusions following total knee arthroplasty: randomized controlled trial. Eur J Anaesthesiol. 2008;25(6):502-507.
20. Serpell MG, Millar FA, Thomson MF. Comparison of lumbar plexus block versus conventional opioid analgesia after total knee replacement. Anaesthesia. 1991;46(4):275-277.
21. Lareau JM, Robbins CE, Talmo CT, Mehio AK, Puri L, Bono JV. Complications of femoral nerve blockade in total knee arthroplasty and strategies to reduce patient risk. J Arthroplasty. 2012;27(4):564-568.
22. Charous MT, Madison SJ, Suresh PJ, et al. Continuous femoral nerve blocks: varying local anesthetic delivery method (bolus versus basal) to minimize quadriceps motor block while maintaining sensory block. Anesthesiology. 2011;115(4):774-781.
23. Gottschalk A, Burmeister MA, Radtke P, et al. Continuous wound infiltration with ropivacaine reduces pain and analgesic requirement after shoulder surgery. Anesth Analg. 2003;97(4):1086-1091.
24. Kerr DR, Kohan L. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery: a case study of 325 patients. Acta Orthop. 2008;79(2):174-183.
25. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am. 2011;93(20):1938-1943.
26. White PF. The role of non-opioid analgesic techniques in the management of pain after ambulatory surgery. Anesth Analg. 2002;94(3):577-585.
27. Dahl JB, Kehlet H. Non-steroidal anti-inflammatory drugs: rationale for use in severe postoperative pain. Br J Anaesth. 1991;66(6):703-712.
28. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048-1056.
29. Candiotti K. Liposomal bupivacaine: an innovative nonopioid local analgesic for the management of postsurgical pain. Pharmacotherapy. 2012;32(9 Pt 2):19S-26S.
30. Bergese SD, Onel E, Portillo J. Evaluation of DepoFoam® bupivacaine for the treatment of postsurgical pain. Pain Manag. 2011;1(6):539-547.
31. Joranson DE, Ryan KM, Gilson AM, Dahl JL. Trends in medical use and abuse of opioid analgesics. JAMA. 2000;283(13):1710-1714.
32. Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297(3):249-251.
33. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
34. Ilfeld BM, Morey TE, Enneking FK. Delivery rate accuracy of portable, bolus-capable infusion pumps used for patient-controlled continuous regional analgesia. Reg Anesth Pain Med. 2003;28(1):17-23.
35. Ganapathy S, Amendola A, Lichfield R, Fowler PJ, Ling E. Elastomeric pumps for ambulatory patient controlled regional analgesia. Can J Anaesth. 2000;47(9):897-902.
36. Bray DA Jr, Nguyen J, Craig J, Cohen BE, Collins DR Jr. Efficacy of a local anesthetic pain pump in abdominoplasty. Plast Reconstr Surg. 2007;119(3):1054-1059.
37. Brown SL, Morrison AE. Local anesthetic infusion pump systems adverse events reported to the Food and Drug Administration. Anesthesiology. 2004;100(5):1305-1307.
38. Noyes FR, Fleckenstein CM, Barber-Westin SD. The development of postoperative knee chondrolysis after intra-articular pain pump infusion of an anesthetic medication: a series of twenty-one cases. J Bone Joint Surg Am. 2012;94(16):1448-1457.
39. Rapley JH, Beavis RC, Barber FA. Glenohumeral chondrolysis after shoulder arthroscopy associated with continuous bupivacaine infusion. Arthroscopy. 2009;25(12):1367-1373.
40. Institute for Safe Medication Practices. Process for handling elastomeric pain relief balls (ON-Q PainBuster and others) requires safety improvements. ISMP Medication Safety Alert. http://www.ismp.org/Newsletters/acutecare/articles/20090716.asp. Accessed January 30, 2015.
41. Pepin JL, Dasta JF, New M. Ensuring safe and economical use of elastomeric infusion devices. Am J Health Syst Pharm. 2011;68(24):2330-2331.
42. Birrer KL, Anderson RL, Liu-DeRyke X, Patel KR. Measures to improve safety of an elastomeric infusion system for pain management. Am J Health Syst Pharm. 2011;68(13):1251-1255.
43. Ilfeld BM, Enneking FK. Continuous peripheral nerve blocks at home: a review. Anesth Analg. 2005;100(6):1822-1833.
44. US Food and Drug Administration. Medical device recalls: I-Flow ON-Q Pump with ONDEMAND Bolus Button. http://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm317826.htm. Accessed July 15, 2014.
45. Ilfeld BM, Morey TE, Enneking FK. Portable infusion pumps used for continuous regional analgesia: delivery rate accuracy and consistency. Reg Anesth Pain Med. 2003;28(5):424-432.
46. Ganapathy S. Wound/intra-articular infiltration or peripheral nerve blocks for orthopedic joint surgery: efficacy and safety issues. Curr Opin Anaesthesiol. 2012;25(5):615-620.
47. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554.
48. Angst MS, Drover DR. Pharmacology of drugs formulated with DepoFoam™: a sustained release drug delivery system for parenteral administration using multivesicular liposome technology. Clin Pharmacokinet. 2006;45(12):1153-1176.
49. Catterall WA, Mackie K. Local anesthetics. In: Gutstein HB, Akil H, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011:565-582.
50. Hu D, Onel E, Singla N, Kramer WG, Hadzic A. Pharmacokinetic profile of liposome bupivacaine injection following a single administration at the surgical site. Clin Drug Investig. 2013;33(2):109-115.
51. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536.
52. Bergese SD, Ramamoorthy S, Patou G, Bramlett K, Gorfine SR, Candiotti KA. Efficacy profile of liposome bupivacaine, a novel formulation of bupivacaine for postsurgical analgesia. J Pain Res. 2012;5:107-116.
53. Cohen SM. Extended pain relief trial utilizing infiltration of Exparel®, a long-acting multivesicular liposome formulation of bupivacaine: a phase IV health economic trial in adult patients undergoing open colectomy. J Pain Res. 2012;5:567-572.
54. Candiotti KA, Sands LR, Lee E, et al. Liposome bupivacaine for postsurgical analgesia in adult patients undergoing laparoscopic colectomy: results from prospective phase IV sequential cohort studies assessing health economic outcomes. Curr Ther Res. 2014;76:1-6.
55. Marcet JE, Nfonsam VN, Larach S. An extended paIn relief trial utilizing the infiltration of a long-acting Multivesicular liPosome foRmulation Of bupiVacaine, EXPAREL (IMPROVE): a Phase IV health economic trial in adult patients undergoing ileostomy reversal. J Pain Res. 2013;6:549-555.
56. Vogel JD. Liposome bupivacaine (EXPAREL®) for extended pain relief in patients undergoing ileostomy reversal at a single institution with a fast-track discharge protocol: an IMPROVE phase IV health economics trial. J Pain Res. 2013;6:605-610.
57. Sternlicht A, Shapiro M, Robelen G, Vellayappan U, Tuerk IA. Initial findings using EXPAREL® (bupivacaine liposome injectable suspension) via infiltration into the transversus abdominis plane (TAP) for postsurgical analgesia in robotic prostatectomy (RP). Abstract presented at: Annual Fall Pain Meeting and Workshops of the American Society of Regional Anesthesia and Pain Medicine; November 15-18, 2012; Miami Beach, FL.
58. Feierman DE, Kronenfeld M, Gupta PM, Younger N, Logvinskiy E. Evaluation of Exparel® use via infiltration into the transversus abdominis plane for prolonged postoperative analgesia in subjects undergoing open abdominal hernia repair. Poster presented at: Annual Meeting of the International Anesthesia Research Society; May 4-7, 2013; San Diego, CA.
59. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
60. Broome B, Backlund I. Rapid recovery pain pathway for total knee arthroplasty results in improved pain management, decreased length of stay, and significant cost savings. Poster presented at: Annual Orthopedic and Spine Summit; September 18-20, 2013; San Antonio, TX.
61. Emerson RH, Barrington JW. Comparison of infiltration with long-acting bupivacaine to a femoral nerve catheter for total knee replacement. Abstract presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11-15, 2014; New Orleans, LA. Abstract P124.
62. Barrington JW. Emerging data in the use of liposome bupivacaine: comparative review in 2,000 TJA patients. Oral presentation presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11-15, 2014; New Orleans, LA.
63. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35(2):152-161.
64. D’Angelo R. Are the new local anesthetics worth their cost? Acta Anaesthesiol Scand. 2000;44(6):639-641.
65. Viscusi ER, Sinatra R, Onel E, Ramamoorthy SL. The safety of liposome bupivacaine, a novel local analgesic formulation. Clin J Pain. 2014;30(2):102-110.
66. Bergese SD, Onel E, Morren M, Morganroth J. Bupivacaine extended-release liposome injection exhibits a favorable cardiac safety profile. Reg Anesth Pain Med. 2012;37(2):145-151.
67. Naseem A, Harada T, Wang D, et al. Bupivacaine extended release liposome injection does not prolong QTc interval in a thorough QT/QTc study in healthy volunteers. J Clin Pharmacol. 2012;52(9):1441-1447.
68. Baxter R, Bramlett K, Onel E, Daniels S. Impact of local administration of liposome bupivacaine for postsurgical analgesia on wound healing: a review of data from ten prospective, controlled clinical studies. Clin Ther. 2013;35(3):312-320.
69. Kharitonov V. A review of the compatibility of liposome bupivacaine with other drug products and commonly used implant materials. Postgrad Med. 2014;126(1):129-138.
70. Ilfeld BM. Liposome bupivacaine in peripheral nerve blocks and epidural injections to manage postoperative pain. Expert Opin Pharmacother. 2013;14(17):2421-2431.
71. Femoral nerve block with liposome bupivacaine for postsurgical analgesia following total knee arthroplasty [NCT01683071]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01683071?term=NCT01683071%5C&rank=1. Accessed January 30, 2015.
72. Minkowitz H, Matthews A, Puckett C, Melson T. Liposome bupivacaine in femoral nerve block: initial results from a phase 2/3 pivotal study. Poster presented at: Annual Meeting of the American Society of Regional Anesthesia and Pain Medicine; April 3-6, 2014; Chicago, IL.
73. Ultrasound guided local infiltration analgesia for hip arthroscopy [NCT01907191]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01907191?term=NCT01907191&rank=1. Accessed January 30, 2015.
74. Efficacy of single injection femoral nerve block with liposomal bupivacaine for total knee arthroplasty [NCT01977339]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01977339?term=NCT01977339&rank=1. Accessed January 30, 2015.
75. Efficacy of interscalene brachial plexus block with liposomal bupivacaine for arthroscopic shoulder surgery [NCT01977352]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01977352?term=NCT01977352&rank=1. Accessed January 30, 2015.
76. Cohen SM, Vogel JD, Marcet JE, Candiotti K. Liposome bupivacaine for improvement in economic outcomes and opioid burden in GI surgery: IMPROVE study pooled analysis. J Pain Res. 2014;7:359-366.
Approximately 5.5 million patients undergo orthopedic surgery in the United States each year, and more than 1 million of the procedures are total knee arthroplasty (TKA) or total hip arthroplasty.1 From its 2010 level, demand for joint arthroplasty is expected to double by 2020 and quadruple by 2030.2
About half the patients who have major joint arthroplasty experience severe postsurgical pain.3 Because postsurgical pain may persist for days or weeks, and inadequate treatment is associated with negative outcomes, achieving effective postsurgical analgesia is an important consideration.4-7 Complications of inadequate postsurgical pain management include thromboembolic or pulmonary complications, development of chronic pain, and decrements in health-related quality of life.4,8
In patients who have orthopedic surgery, the inability to adequately control postsurgical pain has been associated with increased hospital length of stay (LOS), delayed time to ambulation, and reduced capacity for exercise.9-12 A recent study involving 4709 patients who had hip or knee arthroplasty found that postsurgical pain relief was the second most highly correlated factor with respect to overall patient satisfaction (how well surgery met patient expectations was the most highly correlated factor),13 suggesting that postsurgical analgesia should be a focus of surgical practice.
A prolonged-release liposomal formulation of the local anesthetic bupivacaine is now available. Bupivacaine liposome injectable suspension (Exparel; Pacira Pharmaceuticals, Inc., Parsippany, New Jersey) is indicated for administration into the surgical site to produce postsurgical analgesia.14 In this article, we review evidence from clinical studies regarding the potential contribution of liposome bupivacaine to improving postsurgical pain management when used as part of a multimodal analgesic regimen in patients undergoing orthopedic surgery.
Postsurgical Pain Management in Orthopedic Surgery
Frequently Used Modalities
Analgesic modalities commonly used for perioperative pain management include central (eg, epidural),4,10,15,16 central regional (eg, neuraxial),4 peripheral regional (eg, peripheral nerve blocks, local/regional surgical site infiltration, intra-articular administration),4,10,15,17-25 and intravenous (IV) patient-controlled analgesia.4,10,25 These pharmacologic interventions may be augmented by nonpharmacologic modalities (eg, transcutaneous electrical nerve stimulation).26
Pharmacologic treatment options for perioperative pain management include opioids, local anesthetics, clonidine, ketamine, nonsteroidal anti-inflammatory drugs, acetaminophen, and calcium-channel blockers.4,26-28 In TKA, “drug cocktails” (eg, combinations of ropivacaine, ketorolac, epinephrine, and clonidine) for regional or intra-articular injection can also provide effective immediate postsurgical analgesia.25 Although opioids are the most commonly used analgesics for management of orthopedic perioperative pain,25 their use is often associated with adverse effects (AEs), including constipation or ileus, nausea, sedation, dizziness, pruritus, urinary retention, and respiratory depression.6
Multimodal Analgesic Regimens for Postsurgical Pain Management
Current American Society of Anesthesiologists guidelines endorse use of multimodal analgesia, whenever possible, to provide effective management of acute perioperative pain.4 Multimodal analgesia involves applying 2 or more agents with different mechanisms of action to achieve a synergistic effect, which allows each agent to be reduced in dose4,28 and thereby may limit the risk and severity of dose-related AEs.4,25,28
Multimodal analgesia aims to reduce the risk for opioid-related AEs (ORAEs) and the impact of opioids on postsurgical milestones (eg, ambulation, discharge) and may reduce opioid consumption, with attendant reductions in ORAE risk.29,30 Health economics studies have shown that postsurgical ORAEs are associated with increased hospital costs and LOS.6 In a study using a national hospital database, development of an ORAE (vs no ORAE) in postsurgical patients was associated with mean increases of about $4700 in hospital costs and 3.3 days in LOS.7 Reducing postsurgical opioid use may also help reduce the risk for opioid abuse, addiction, and diversion.31-33
One approach to reducing opioid use involves continuous or intermittent administration of local anesthetics by elastomeric pumps to extend duration of postsurgical analgesia.34-36 However, use of elastomeric pumps has been associated with risk for AEs, including tissue necrosis, sloughing, wound infection, and chondrolysis.37-40 In addition, AEs related to “dose dumping” (accidental delivery of excessive doses) have been reported.40-44 Key issues that may negatively affect rehabilitation after orthopedic surgery include consistency and accuracy of analgesic delivery and the potential for motor block–induced muscle weakness, which may lead to falls and constrain ambulation.45-47
Liposome Bupivacaine
Description
Drug Delivery Technology. Liposome bupivacaine incorporates DepoFoam drug delivery technology (Pacira Pharmaceuticals, Inc.) to facilitate prolonged release of bupivacaine. This technology is based on creation of multivesicular liposome particles (diameter, 10-30 µm) with multiple aqueous chambers.30,48 After administration into the surgical site, bupivacaine diffuses from chambers in the liposomal particles over time, providing analgesia and reduced opioid requirements for up to 72 hours.29,30
Indication, Mechanism of Action, Pharmacokinetics, and Dose/Administration. Liposome bupivacaine is indicated for single-dose administration into the surgical site to produce postsurgical analgesia in patients at least 18 years old.14 Like other local anesthetics, liposome bupivacaine is thought to exert its pharmacologic effects by interacting with voltage-gated Na+ channels on neural membranes to raise the threshold for electrical excitability, to slow nerve impulse propagation, and to reduce the rate of rise of the action potential.14,49
Liposome bupivacaine has dose-proportional pharmacokinetics.50 Presence of a small amount of extra-liposomal bupivacaine in the formulation leads to a bimodal pharmacokinetic profile, with an initial peak serum concentration about 1 hour after administration, followed by a second peak within 12 to 36 hours (Figure).50
Maximum amount of liposome bupivacaine approved for single administration is 266 mg (packaged as 20 mL of a 1.3% solution). However, product labeling includes safety data associated with doses of 532 mg or less.14 The appropriate volume to be used should be based on the amount required to cover the surgical area. Liposome bupivacaine may be expanded with preservative-free normal (0.9%) sterile saline to a total volume of 300 mL: 20 mL liposome bupivacaine plus 280 mL or less diluent, with final concentration of 0.89 mg/mL (1:14 by volume).14
A 25-gauge or larger bore needle should be used to slowly inject liposome bupivacaine into soft tissues of the surgical site, with frequent aspiration to check for blood to minimize risk for intravascular injection.14 Total volume used and fraction injected in specific regions of the surgical site depend on the procedure. For example, a TKA study used 266 mg diluted to a total volume of 60 mL, with 8 mL infiltrated to the area around the medial capsule, 8 mL around the lateral capsule, 12 mL around the posterior capsule, 8 mL around the peripatellar area, 12 mL into the capsulotomy incision, and 12 mL into the subcutaneous tissue on each side of the incision.51
Efficacy
Multiple Surgical Settings. The efficacy of liposome bupivacaine, either alone or as a component of a multimodal analgesic regimen, has been evaluated in a series of 10 phase 2 and 3 studies (8 active-controlled, 2 placebo-controlled) involving 823 patients undergoing TKA, bunionectomy, hemorrhoidectomy, inguinal hernia repair, or mammoplasty.52 Patients received a single liposome bupivacaine dose ranging from 66 to 532 mg.52
Combined analyses of efficacy data from these studies found that liposome bupivacaine–based multimodal analgesic regimens produced postsurgical analgesia for up to 72 hours, increased time to first use of opioid rescue medication after surgery, and reduced total amount of postsurgical opioid consumption versus placebo.52
Compared with standard of care, liposome bupivacaine has been shown to provide effective analgesia in open-label studies in patients undergoing open colectomy,53 laparoscopic colectomy,54 and ileostomy reversal,55,56 as reflected in assessments of postsurgical opioid consumption, LOS, and hospital costs. It has also been studied when administered by infiltration into the transversus abdominis plane (TAP) in patients having laparoscopic prostatectomy and open abdominal hernia repair.57,58
Orthopedic Surgery. In a phase 2 randomized, double-blind, dose-ranging study, TKA patients (N = 138) received bupivacaine HCl 150 mg or liposome bupivacaine 133, 266, 399, or 532 mg administered by local infiltration into the capsulotomy incision and on either side of the incision before wound closure.51 Postsurgical rescue analgesia was available to all patients. Cumulative pain intensity scores with activity (primary efficacy measure) were not statistically different between liposome bupivacaine groups and the bupivacaine HCl group through postoperative day 4. Mean scores in the liposome bupivacaine 266-, 399-, and 532-mg groups were numerically lower than for those treated with bupivacaine HCl on postoperative days 2 to 5, with all doses of liposome bupivacaine having a statistically significant lower pain score at rest on day 5. There were no statistically significant differences across treatment groups with respect to total amount of postsurgical opioids used.
In a phase 3 randomized, double-blind study of TKA patients (N = 245), liposome bupivacaine 532 mg administered into the surgical site was compared with bupivacaine HCl 200 mg for postsurgical analgesia.52 Rescue analgesia was available to all patients. No statistically significant between-group differences were found with respect to postsurgical cumulative pain scores through 72 hours (primary efficacy endpoint).
In a single-center retrospective TKA study, postsurgical outcomes in a patient cohort that received intraoperative periarticular infiltration with liposome bupivacaine 266 mg (n = 65) were compared with a cohort that received infiltration with a combination of ropivacaine 400 mg, morphine 5 mg, and epinephrine 0.4 mg (n = 85).59 Patient-reported postsurgical pain scores were similar in the 2 treatment groups during the first 24 hours after surgery and at discharge. Mean (SD) pain scores during hospitalization after the first 24 hours until discharge were significantly (P = .04) higher in the liposome bupivacaine group, 4.9 (1.4), than in the periarticular group, 4.4 (1.6). There was no significant difference between the 2 treatment groups in postsurgical opioid use. The study demonstrated no advantage to using liposome bupivacaine injections with respect to pain relief, but it was a retrospective review in which pain scores were obtained from electronic medical records. It is essential that liposome bupivacaine be compared with intra-articular injections in well-designed randomized trials.
Another single-center, matched-cohort TKA study (N = 200) compared a liposome bupivacaine regimen with femoral nerve block.60 Compared with patients who received femoral nerve block, patients who received liposome bupivacaine reported lower pain intensity scores after surgery and had shorter LOS, reduced costs, and improved knee flexion at follow-up.60
Results from 2 other studies were presented at the 2014 meeting of the American Academy of Orthopaedic Surgeons (AAOS). One was a single-center, matched-cohort TKA study (N = 72) comparing infiltration of a single dose of liposome bupivacaine into the surgical site with continuous femoral nerve block.61 The 2 treatment groups had similar mean postsurgical pain intensity scores on a 0-to-10 visual analog scale, 1.8 for liposome bupivacaine and 2.3 for continuous nerve block (P = NS), but total amount of postsurgical opioids (hydrocodone-equivalent milligrams) was significantly (P < .0001) less in the liposome bupivacaine group (82 vs 177 mg).
The other study presented at the AAOS meeting was a larger, prospective case–control study comparing outcomes between 1000 patients who had total joint arthroplasty (TJA) with liposome bupivacaine and 1000 control patients who had TJA without liposome bupivacaine.62 For the control and liposome bupivacaine cohorts, respectively, mean postsurgical pain intensity scores were 2.41 and 1.98 (P < .0001), mean LOS was 2.83 days and 2.66 days (P < .02), and incidence of falls was 1.0% and 0.2% (P = .02). Average per-patient costs were $1246 lower in the liposome bupivacaine cohort.
A pivotal phase 3 placebo-controlled study compared liposome bupivacaine 106 mg with placebo in patients undergoing bunionectomy (N = 193).5 Rescue medication was available to all patients. Cumulative pain scores were significantly (P = .0005) lower in the liposome bupivacaine group (125) than in the placebo group (146) through 24 hours after surgery (primary efficacy measure) and significantly (P = .0229) lower (197 vs 220) through 36 hours. Median time to first use of rescue opioids was delayed in favor of the liposome bupivacaine group (7.2 vs 4.3 hours; P < .0001). Mean total number of opioid tablets used within 24 hours after surgery was also significantly lower (3.8 vs 4.7; P = .008), and a larger percentage of patients in the liposome bupivacaine group avoided opioid use altogether through 24 hours (7% vs 1%; P = .04).
Efficacy data for liposome bupivacaine appear promising for relief of pain after joint arthroplasty and other orthopedic procedures but have their limitations. First, no randomized trials have compared liposome bupivacaine with locally injected pain medications (intra-articular injections in TKA or hip arthroplasty). As these injections are quite common now, such analyses are essential. Second, cost-effectiveness studies are needed for orthopedic procedures. Third, most of the published studies were sponsored by the manufacturer of liposome bupivacaine—a situation that raises questions about potential bias. Non-industry-sponsored randomized trials assessing efficacy, safety, and cost-effectiveness are needed.
Safety
Local anesthetics, including liposome bupivacaine, have the potential for central nervous system (CNS) or cardiac toxicity resulting from excessive systemic absorption or inadvertent IV administration.63 However, reported serious CNS or cardiac-related AEs are rare.63,64
AE Profile. Safety data from 10 phase 2 and 3 studies involving 823 patients who received liposome bupivacaine were evaluated.65 Of these patients, 545 received a dose of 266 mg or less (maximum dose approved by the US Food and Drug Administration [FDA]). Liposome bupivacaine was generally well tolerated. Reported AE incidence was 62% (liposome bupivacaine), 75% (bupivacaine HCl), and 43% (placebo). More than 90% of reported AEs were mild or moderate. The most frequently reported AEs were nausea, constipation, and vomiting (liposome bupivacaine, bupivacaine HCl) and nausea, dizziness, and vomiting (placebo).
Serious AEs were reported in 22 (2.7%) of the 823 patients in the liposome bupivacaine group, 24 (5.4%) of the 446 in the bupivacaine HCl group, and 2 (1.1%) of the 190 in the placebo group.65 None of the serious AEs in the liposome bupivacaine and placebo groups were considered treatment-related. Six patients in the bupivacaine HCl group had treatment-related serious AEs (hypoglycemia, arthrofibrosis, hemarthrosis, joint swelling, scar, knee arthroplasty).
Cardiac Safety. Possible cardiac effects associated with liposome bupivacaine were evaluated with data from studies conducted during the clinical development program.66 One hundred thirty-eight patients participated in the phase 2 safety and efficacy study in TKA. In these patients, a consistent change in mean heart rate (range, +12.2 to +16.5 beats per minute) was found across all liposome bupivacaine doses and with bupivacaine HCl. No clinically relevant changes from baseline in mean electrocardiographic parameters, including QTcF interval (QT interval adjusted using Fridericia’s correction formula), were found. In another analysis,67 liposome bupivacaine administered in a single subcutaneous dose (266, 399, 532, or 665 mg) to healthy volunteers did not prolong (vs placebo) QTc interval.
Wound Healing. The potential effects of liposome bupivacaine on wound healing were evaluated with results from 10 phase 2 and 3 studies.68 The assessments, which varied across studies, included clinicians’ overall satisfaction with patient wound healing, wound status assessment (categories included erythema, drainage, edema, and induration), and wound scarring (categories included pigmentation, height, pliability, and vascularity). Clinician-assessed scores reflected high satisfaction with wound healing overall. There were few statistically significant differences in wound status assessments between liposome bupivacaine and the comparators and no statistically significant differences in scarring between liposome bupivacaine and bupivacaine HCl.
The potential of liposome bupivacaine to have adverse intra-articular effects was assessed with drainage samples from patients (n = 23) who had TKA and received liposome bupivacaine (133, 266, 399, or 532 mg) or bupivacaine HCl (150 mg) by wound infiltration near the intra-articular space.51,65 Only small amounts of bupivacaine were present in drainage fluid collected for 12 hours after liposome bupivacaine administration, comparable to bupivacaine HCl administration.65 Currently, the product is not approved for intra-articular use.
Compatibility With Diluents, Other Medications, and Implant Materials
Liposome bupivacaine may be expanded up to a ratio of 1:14 by volume (to a final total volume of 300 mL or a concentration of 0.89 mg/mL) using preservative-free normal (0.9%) sterile saline for injection.14 It has also been shown in vitro to be compatible with lactated Ringer solution as a diluent.69
Liposome bupivacaine should not be admixed with other medications before administration.14 No formal drug–drug interaction studies have been conducted with liposome bupivacaine, but it has been shown in vitro to be compatible with epinephrine solutions, with certain anti-infective medications (eg, bacitracin, gentamicin, cefazolin, cefuroxime), with certain analgesics (eg, ketorolac, morphine), with an antihypertensive medication (clonidine), with an antihemorrhagic medication (tranexamic acid), and with certain corticosteroids (eg, methylprednisolone, triamcinolone acetonide). These medications may be coadministered in the same location as liposome bupivacaine.69
Topical antiseptics (eg, povidone iodine) may be used in surgical procedures involving liposome bupivacaine as long as they are not directly mixed with liposome bupivacaine and are allowed to dry before it is administered. If a topical antiseptic is used for wound irrigation, the wound should be rinsed clear before liposome bupivacaine administration.14,69
Liposome bupivacaine may be coadministered into the same surgical site immediately after bupivacaine HCl as long as the dose ratio of liposome bupivacaine to bupivacaine HCl is 2:1 or higher. Because of the prolonged-release pharmacokinetic profile of liposome bupivacaine and the potential for increased bupivacaine exposure, bupivacaine HCl should not be administered within 96 hours after administration of liposome bupivacaine.14,69
In vitro coincubation studies of liposome bupivacaine and other local anesthetics, including ropivacaine, lidocaine, and mepivacaine, have found rapid release of free bupivacaine from the liposome matrix. Therefore, after giving any of these other local anesthetics, surgeons should wait at least 20 minutes before administering liposome bupivacaine into the same area.14,69
In vitro studies have shown that liposome bupivacaine is compatible with a wide range of commonly used implant materials, including polypropylene, expanded polytetrafluoroethylene, stainless steel, titanium, and smooth- and textured-type silicone.69
Investigational Use and Ongoing Studies
A phase 2 randomized, double-masked, dose-escalating/deescalating study was conducted to evaluate the efficacy, safety, and pharmacokinetics of liposome bupivacaine (155, 199, or 310 mg) in comparison with bupivacaine HCl 125 mg for ankle nerve block in patients undergoing bunionectomy (N = 58).70 The study medication was injected into 3 sites to reach the posterior tibial, sural, deep peroneal, superficial peroneal, and saphenous nerves. Pharmacokinetic exposure was higher for liposome bupivacaine than for bupivacaine HCl, as reflected by a significantly greater area under the curve, lower Cmax (maximum serum concentration), and longer mean half-life. Mean pain intensity scores were lower in the bupivacaine HCl group than in each liposome bupivacaine group the first 12 hours after surgery. However, the liposome bupivacaine 310-mg group had similar or lower scores than the bupivacaine HCl group from 12 to 96 hours after surgery. The most common AEs in the liposome bupivacaine group were gastrointestinal and not treatment-related.70
The efficacy and safety of liposome bupivacaine, administered as a femoral nerve block for postsurgical analgesia, were assessed in a phase 2/3 manufacturer-sponsored, placebo-controlled, multicenter, randomized, double-blind 2-part study (NCT01683071)71 in 280 TKA patients.71,72 Part 2 of the study, comparing liposome bupivacaine 266 mg (n = 116) and placebo (n = 116), met its primary endpoint, demonstrating statistical significance in favor of liposome bupivacaine for cumulative pain scores over 72 hours (P < .0001), with decreased opioid use (P < .05) and a safety profile similar to that of placebo.72
Other ongoing investigator-sponsored studies in orthopedic populations include comparisons of liposome bupivacaine and bupivacaine HCl for ultrasound-guided periarticular hip infiltration in hip arthroplasty (NTC01917191),73 as femoral nerve block in TKA (NCT01977339),74 and as interscalene brachial plexus block in arthroscopic shoulder surgery (NCT01977352).75 The primary efficacy outcome measure in these studies was postsurgical opioid use.73-75
Health Economics
A series of phase 4 health economics studies was conducted for gastrointestinal surgeries, including open colectomy, laparoscopic colectomy, and ileostomy reversal.53-56,76 These studies, of similar design, showed that a liposome bupivacaine–based multimodal analgesic regimen was associated with reduced opioid use, shorter hospital LOS, and lower hospitalization costs in comparison with a traditional opioid-based regimen.53-56 Although pooled analysis of these studies showed a cost savings of more than $2000 per patient and an LOS decrease of 1.4 days,76 all were conducted in the gastrointestinal surgery setting. Studies are needed to fully assess the economic benefits associated with liposome bupivacaine in the orthopedic surgery setting.
Conclusion
Liposome bupivacaine represents a potentially important contributor to multimodal analgesic regimens used to manage postsurgical pain. Liposome bupivacaine has demonstrated efficacy in providing prolonged postsurgical analgesia and reducing postsurgical opioid use in most surgical settings studied. Additional data from health economics studies in gastrointestinal surgery suggest liposome bupivacaine–based multimodal analgesic regimens may also contribute to reductions in hospital LOS and hospitalization costs. Non-industry-sponsored trials are needed to answer these crucial questions in orthopedic surgery settings. Nevertheless, data on the safety and efficacy of liposome bupivacaine for postsurgical analgesia continue to accumulate, and liposome bupivacaine appears to be a feasible therapeutic option for managing postsurgical pain in orthopedic surgery.
Approximately 5.5 million patients undergo orthopedic surgery in the United States each year, and more than 1 million of the procedures are total knee arthroplasty (TKA) or total hip arthroplasty.1 From its 2010 level, demand for joint arthroplasty is expected to double by 2020 and quadruple by 2030.2
About half the patients who have major joint arthroplasty experience severe postsurgical pain.3 Because postsurgical pain may persist for days or weeks, and inadequate treatment is associated with negative outcomes, achieving effective postsurgical analgesia is an important consideration.4-7 Complications of inadequate postsurgical pain management include thromboembolic or pulmonary complications, development of chronic pain, and decrements in health-related quality of life.4,8
In patients who have orthopedic surgery, the inability to adequately control postsurgical pain has been associated with increased hospital length of stay (LOS), delayed time to ambulation, and reduced capacity for exercise.9-12 A recent study involving 4709 patients who had hip or knee arthroplasty found that postsurgical pain relief was the second most highly correlated factor with respect to overall patient satisfaction (how well surgery met patient expectations was the most highly correlated factor),13 suggesting that postsurgical analgesia should be a focus of surgical practice.
A prolonged-release liposomal formulation of the local anesthetic bupivacaine is now available. Bupivacaine liposome injectable suspension (Exparel; Pacira Pharmaceuticals, Inc., Parsippany, New Jersey) is indicated for administration into the surgical site to produce postsurgical analgesia.14 In this article, we review evidence from clinical studies regarding the potential contribution of liposome bupivacaine to improving postsurgical pain management when used as part of a multimodal analgesic regimen in patients undergoing orthopedic surgery.
Postsurgical Pain Management in Orthopedic Surgery
Frequently Used Modalities
Analgesic modalities commonly used for perioperative pain management include central (eg, epidural),4,10,15,16 central regional (eg, neuraxial),4 peripheral regional (eg, peripheral nerve blocks, local/regional surgical site infiltration, intra-articular administration),4,10,15,17-25 and intravenous (IV) patient-controlled analgesia.4,10,25 These pharmacologic interventions may be augmented by nonpharmacologic modalities (eg, transcutaneous electrical nerve stimulation).26
Pharmacologic treatment options for perioperative pain management include opioids, local anesthetics, clonidine, ketamine, nonsteroidal anti-inflammatory drugs, acetaminophen, and calcium-channel blockers.4,26-28 In TKA, “drug cocktails” (eg, combinations of ropivacaine, ketorolac, epinephrine, and clonidine) for regional or intra-articular injection can also provide effective immediate postsurgical analgesia.25 Although opioids are the most commonly used analgesics for management of orthopedic perioperative pain,25 their use is often associated with adverse effects (AEs), including constipation or ileus, nausea, sedation, dizziness, pruritus, urinary retention, and respiratory depression.6
Multimodal Analgesic Regimens for Postsurgical Pain Management
Current American Society of Anesthesiologists guidelines endorse use of multimodal analgesia, whenever possible, to provide effective management of acute perioperative pain.4 Multimodal analgesia involves applying 2 or more agents with different mechanisms of action to achieve a synergistic effect, which allows each agent to be reduced in dose4,28 and thereby may limit the risk and severity of dose-related AEs.4,25,28
Multimodal analgesia aims to reduce the risk for opioid-related AEs (ORAEs) and the impact of opioids on postsurgical milestones (eg, ambulation, discharge) and may reduce opioid consumption, with attendant reductions in ORAE risk.29,30 Health economics studies have shown that postsurgical ORAEs are associated with increased hospital costs and LOS.6 In a study using a national hospital database, development of an ORAE (vs no ORAE) in postsurgical patients was associated with mean increases of about $4700 in hospital costs and 3.3 days in LOS.7 Reducing postsurgical opioid use may also help reduce the risk for opioid abuse, addiction, and diversion.31-33
One approach to reducing opioid use involves continuous or intermittent administration of local anesthetics by elastomeric pumps to extend duration of postsurgical analgesia.34-36 However, use of elastomeric pumps has been associated with risk for AEs, including tissue necrosis, sloughing, wound infection, and chondrolysis.37-40 In addition, AEs related to “dose dumping” (accidental delivery of excessive doses) have been reported.40-44 Key issues that may negatively affect rehabilitation after orthopedic surgery include consistency and accuracy of analgesic delivery and the potential for motor block–induced muscle weakness, which may lead to falls and constrain ambulation.45-47
Liposome Bupivacaine
Description
Drug Delivery Technology. Liposome bupivacaine incorporates DepoFoam drug delivery technology (Pacira Pharmaceuticals, Inc.) to facilitate prolonged release of bupivacaine. This technology is based on creation of multivesicular liposome particles (diameter, 10-30 µm) with multiple aqueous chambers.30,48 After administration into the surgical site, bupivacaine diffuses from chambers in the liposomal particles over time, providing analgesia and reduced opioid requirements for up to 72 hours.29,30
Indication, Mechanism of Action, Pharmacokinetics, and Dose/Administration. Liposome bupivacaine is indicated for single-dose administration into the surgical site to produce postsurgical analgesia in patients at least 18 years old.14 Like other local anesthetics, liposome bupivacaine is thought to exert its pharmacologic effects by interacting with voltage-gated Na+ channels on neural membranes to raise the threshold for electrical excitability, to slow nerve impulse propagation, and to reduce the rate of rise of the action potential.14,49
Liposome bupivacaine has dose-proportional pharmacokinetics.50 Presence of a small amount of extra-liposomal bupivacaine in the formulation leads to a bimodal pharmacokinetic profile, with an initial peak serum concentration about 1 hour after administration, followed by a second peak within 12 to 36 hours (Figure).50
Maximum amount of liposome bupivacaine approved for single administration is 266 mg (packaged as 20 mL of a 1.3% solution). However, product labeling includes safety data associated with doses of 532 mg or less.14 The appropriate volume to be used should be based on the amount required to cover the surgical area. Liposome bupivacaine may be expanded with preservative-free normal (0.9%) sterile saline to a total volume of 300 mL: 20 mL liposome bupivacaine plus 280 mL or less diluent, with final concentration of 0.89 mg/mL (1:14 by volume).14
A 25-gauge or larger bore needle should be used to slowly inject liposome bupivacaine into soft tissues of the surgical site, with frequent aspiration to check for blood to minimize risk for intravascular injection.14 Total volume used and fraction injected in specific regions of the surgical site depend on the procedure. For example, a TKA study used 266 mg diluted to a total volume of 60 mL, with 8 mL infiltrated to the area around the medial capsule, 8 mL around the lateral capsule, 12 mL around the posterior capsule, 8 mL around the peripatellar area, 12 mL into the capsulotomy incision, and 12 mL into the subcutaneous tissue on each side of the incision.51
Efficacy
Multiple Surgical Settings. The efficacy of liposome bupivacaine, either alone or as a component of a multimodal analgesic regimen, has been evaluated in a series of 10 phase 2 and 3 studies (8 active-controlled, 2 placebo-controlled) involving 823 patients undergoing TKA, bunionectomy, hemorrhoidectomy, inguinal hernia repair, or mammoplasty.52 Patients received a single liposome bupivacaine dose ranging from 66 to 532 mg.52
Combined analyses of efficacy data from these studies found that liposome bupivacaine–based multimodal analgesic regimens produced postsurgical analgesia for up to 72 hours, increased time to first use of opioid rescue medication after surgery, and reduced total amount of postsurgical opioid consumption versus placebo.52
Compared with standard of care, liposome bupivacaine has been shown to provide effective analgesia in open-label studies in patients undergoing open colectomy,53 laparoscopic colectomy,54 and ileostomy reversal,55,56 as reflected in assessments of postsurgical opioid consumption, LOS, and hospital costs. It has also been studied when administered by infiltration into the transversus abdominis plane (TAP) in patients having laparoscopic prostatectomy and open abdominal hernia repair.57,58
Orthopedic Surgery. In a phase 2 randomized, double-blind, dose-ranging study, TKA patients (N = 138) received bupivacaine HCl 150 mg or liposome bupivacaine 133, 266, 399, or 532 mg administered by local infiltration into the capsulotomy incision and on either side of the incision before wound closure.51 Postsurgical rescue analgesia was available to all patients. Cumulative pain intensity scores with activity (primary efficacy measure) were not statistically different between liposome bupivacaine groups and the bupivacaine HCl group through postoperative day 4. Mean scores in the liposome bupivacaine 266-, 399-, and 532-mg groups were numerically lower than for those treated with bupivacaine HCl on postoperative days 2 to 5, with all doses of liposome bupivacaine having a statistically significant lower pain score at rest on day 5. There were no statistically significant differences across treatment groups with respect to total amount of postsurgical opioids used.
In a phase 3 randomized, double-blind study of TKA patients (N = 245), liposome bupivacaine 532 mg administered into the surgical site was compared with bupivacaine HCl 200 mg for postsurgical analgesia.52 Rescue analgesia was available to all patients. No statistically significant between-group differences were found with respect to postsurgical cumulative pain scores through 72 hours (primary efficacy endpoint).
In a single-center retrospective TKA study, postsurgical outcomes in a patient cohort that received intraoperative periarticular infiltration with liposome bupivacaine 266 mg (n = 65) were compared with a cohort that received infiltration with a combination of ropivacaine 400 mg, morphine 5 mg, and epinephrine 0.4 mg (n = 85).59 Patient-reported postsurgical pain scores were similar in the 2 treatment groups during the first 24 hours after surgery and at discharge. Mean (SD) pain scores during hospitalization after the first 24 hours until discharge were significantly (P = .04) higher in the liposome bupivacaine group, 4.9 (1.4), than in the periarticular group, 4.4 (1.6). There was no significant difference between the 2 treatment groups in postsurgical opioid use. The study demonstrated no advantage to using liposome bupivacaine injections with respect to pain relief, but it was a retrospective review in which pain scores were obtained from electronic medical records. It is essential that liposome bupivacaine be compared with intra-articular injections in well-designed randomized trials.
Another single-center, matched-cohort TKA study (N = 200) compared a liposome bupivacaine regimen with femoral nerve block.60 Compared with patients who received femoral nerve block, patients who received liposome bupivacaine reported lower pain intensity scores after surgery and had shorter LOS, reduced costs, and improved knee flexion at follow-up.60
Results from 2 other studies were presented at the 2014 meeting of the American Academy of Orthopaedic Surgeons (AAOS). One was a single-center, matched-cohort TKA study (N = 72) comparing infiltration of a single dose of liposome bupivacaine into the surgical site with continuous femoral nerve block.61 The 2 treatment groups had similar mean postsurgical pain intensity scores on a 0-to-10 visual analog scale, 1.8 for liposome bupivacaine and 2.3 for continuous nerve block (P = NS), but total amount of postsurgical opioids (hydrocodone-equivalent milligrams) was significantly (P < .0001) less in the liposome bupivacaine group (82 vs 177 mg).
The other study presented at the AAOS meeting was a larger, prospective case–control study comparing outcomes between 1000 patients who had total joint arthroplasty (TJA) with liposome bupivacaine and 1000 control patients who had TJA without liposome bupivacaine.62 For the control and liposome bupivacaine cohorts, respectively, mean postsurgical pain intensity scores were 2.41 and 1.98 (P < .0001), mean LOS was 2.83 days and 2.66 days (P < .02), and incidence of falls was 1.0% and 0.2% (P = .02). Average per-patient costs were $1246 lower in the liposome bupivacaine cohort.
A pivotal phase 3 placebo-controlled study compared liposome bupivacaine 106 mg with placebo in patients undergoing bunionectomy (N = 193).5 Rescue medication was available to all patients. Cumulative pain scores were significantly (P = .0005) lower in the liposome bupivacaine group (125) than in the placebo group (146) through 24 hours after surgery (primary efficacy measure) and significantly (P = .0229) lower (197 vs 220) through 36 hours. Median time to first use of rescue opioids was delayed in favor of the liposome bupivacaine group (7.2 vs 4.3 hours; P < .0001). Mean total number of opioid tablets used within 24 hours after surgery was also significantly lower (3.8 vs 4.7; P = .008), and a larger percentage of patients in the liposome bupivacaine group avoided opioid use altogether through 24 hours (7% vs 1%; P = .04).
Efficacy data for liposome bupivacaine appear promising for relief of pain after joint arthroplasty and other orthopedic procedures but have their limitations. First, no randomized trials have compared liposome bupivacaine with locally injected pain medications (intra-articular injections in TKA or hip arthroplasty). As these injections are quite common now, such analyses are essential. Second, cost-effectiveness studies are needed for orthopedic procedures. Third, most of the published studies were sponsored by the manufacturer of liposome bupivacaine—a situation that raises questions about potential bias. Non-industry-sponsored randomized trials assessing efficacy, safety, and cost-effectiveness are needed.
Safety
Local anesthetics, including liposome bupivacaine, have the potential for central nervous system (CNS) or cardiac toxicity resulting from excessive systemic absorption or inadvertent IV administration.63 However, reported serious CNS or cardiac-related AEs are rare.63,64
AE Profile. Safety data from 10 phase 2 and 3 studies involving 823 patients who received liposome bupivacaine were evaluated.65 Of these patients, 545 received a dose of 266 mg or less (maximum dose approved by the US Food and Drug Administration [FDA]). Liposome bupivacaine was generally well tolerated. Reported AE incidence was 62% (liposome bupivacaine), 75% (bupivacaine HCl), and 43% (placebo). More than 90% of reported AEs were mild or moderate. The most frequently reported AEs were nausea, constipation, and vomiting (liposome bupivacaine, bupivacaine HCl) and nausea, dizziness, and vomiting (placebo).
Serious AEs were reported in 22 (2.7%) of the 823 patients in the liposome bupivacaine group, 24 (5.4%) of the 446 in the bupivacaine HCl group, and 2 (1.1%) of the 190 in the placebo group.65 None of the serious AEs in the liposome bupivacaine and placebo groups were considered treatment-related. Six patients in the bupivacaine HCl group had treatment-related serious AEs (hypoglycemia, arthrofibrosis, hemarthrosis, joint swelling, scar, knee arthroplasty).
Cardiac Safety. Possible cardiac effects associated with liposome bupivacaine were evaluated with data from studies conducted during the clinical development program.66 One hundred thirty-eight patients participated in the phase 2 safety and efficacy study in TKA. In these patients, a consistent change in mean heart rate (range, +12.2 to +16.5 beats per minute) was found across all liposome bupivacaine doses and with bupivacaine HCl. No clinically relevant changes from baseline in mean electrocardiographic parameters, including QTcF interval (QT interval adjusted using Fridericia’s correction formula), were found. In another analysis,67 liposome bupivacaine administered in a single subcutaneous dose (266, 399, 532, or 665 mg) to healthy volunteers did not prolong (vs placebo) QTc interval.
Wound Healing. The potential effects of liposome bupivacaine on wound healing were evaluated with results from 10 phase 2 and 3 studies.68 The assessments, which varied across studies, included clinicians’ overall satisfaction with patient wound healing, wound status assessment (categories included erythema, drainage, edema, and induration), and wound scarring (categories included pigmentation, height, pliability, and vascularity). Clinician-assessed scores reflected high satisfaction with wound healing overall. There were few statistically significant differences in wound status assessments between liposome bupivacaine and the comparators and no statistically significant differences in scarring between liposome bupivacaine and bupivacaine HCl.
The potential of liposome bupivacaine to have adverse intra-articular effects was assessed with drainage samples from patients (n = 23) who had TKA and received liposome bupivacaine (133, 266, 399, or 532 mg) or bupivacaine HCl (150 mg) by wound infiltration near the intra-articular space.51,65 Only small amounts of bupivacaine were present in drainage fluid collected for 12 hours after liposome bupivacaine administration, comparable to bupivacaine HCl administration.65 Currently, the product is not approved for intra-articular use.
Compatibility With Diluents, Other Medications, and Implant Materials
Liposome bupivacaine may be expanded up to a ratio of 1:14 by volume (to a final total volume of 300 mL or a concentration of 0.89 mg/mL) using preservative-free normal (0.9%) sterile saline for injection.14 It has also been shown in vitro to be compatible with lactated Ringer solution as a diluent.69
Liposome bupivacaine should not be admixed with other medications before administration.14 No formal drug–drug interaction studies have been conducted with liposome bupivacaine, but it has been shown in vitro to be compatible with epinephrine solutions, with certain anti-infective medications (eg, bacitracin, gentamicin, cefazolin, cefuroxime), with certain analgesics (eg, ketorolac, morphine), with an antihypertensive medication (clonidine), with an antihemorrhagic medication (tranexamic acid), and with certain corticosteroids (eg, methylprednisolone, triamcinolone acetonide). These medications may be coadministered in the same location as liposome bupivacaine.69
Topical antiseptics (eg, povidone iodine) may be used in surgical procedures involving liposome bupivacaine as long as they are not directly mixed with liposome bupivacaine and are allowed to dry before it is administered. If a topical antiseptic is used for wound irrigation, the wound should be rinsed clear before liposome bupivacaine administration.14,69
Liposome bupivacaine may be coadministered into the same surgical site immediately after bupivacaine HCl as long as the dose ratio of liposome bupivacaine to bupivacaine HCl is 2:1 or higher. Because of the prolonged-release pharmacokinetic profile of liposome bupivacaine and the potential for increased bupivacaine exposure, bupivacaine HCl should not be administered within 96 hours after administration of liposome bupivacaine.14,69
In vitro coincubation studies of liposome bupivacaine and other local anesthetics, including ropivacaine, lidocaine, and mepivacaine, have found rapid release of free bupivacaine from the liposome matrix. Therefore, after giving any of these other local anesthetics, surgeons should wait at least 20 minutes before administering liposome bupivacaine into the same area.14,69
In vitro studies have shown that liposome bupivacaine is compatible with a wide range of commonly used implant materials, including polypropylene, expanded polytetrafluoroethylene, stainless steel, titanium, and smooth- and textured-type silicone.69
Investigational Use and Ongoing Studies
A phase 2 randomized, double-masked, dose-escalating/deescalating study was conducted to evaluate the efficacy, safety, and pharmacokinetics of liposome bupivacaine (155, 199, or 310 mg) in comparison with bupivacaine HCl 125 mg for ankle nerve block in patients undergoing bunionectomy (N = 58).70 The study medication was injected into 3 sites to reach the posterior tibial, sural, deep peroneal, superficial peroneal, and saphenous nerves. Pharmacokinetic exposure was higher for liposome bupivacaine than for bupivacaine HCl, as reflected by a significantly greater area under the curve, lower Cmax (maximum serum concentration), and longer mean half-life. Mean pain intensity scores were lower in the bupivacaine HCl group than in each liposome bupivacaine group the first 12 hours after surgery. However, the liposome bupivacaine 310-mg group had similar or lower scores than the bupivacaine HCl group from 12 to 96 hours after surgery. The most common AEs in the liposome bupivacaine group were gastrointestinal and not treatment-related.70
The efficacy and safety of liposome bupivacaine, administered as a femoral nerve block for postsurgical analgesia, were assessed in a phase 2/3 manufacturer-sponsored, placebo-controlled, multicenter, randomized, double-blind 2-part study (NCT01683071)71 in 280 TKA patients.71,72 Part 2 of the study, comparing liposome bupivacaine 266 mg (n = 116) and placebo (n = 116), met its primary endpoint, demonstrating statistical significance in favor of liposome bupivacaine for cumulative pain scores over 72 hours (P < .0001), with decreased opioid use (P < .05) and a safety profile similar to that of placebo.72
Other ongoing investigator-sponsored studies in orthopedic populations include comparisons of liposome bupivacaine and bupivacaine HCl for ultrasound-guided periarticular hip infiltration in hip arthroplasty (NTC01917191),73 as femoral nerve block in TKA (NCT01977339),74 and as interscalene brachial plexus block in arthroscopic shoulder surgery (NCT01977352).75 The primary efficacy outcome measure in these studies was postsurgical opioid use.73-75
Health Economics
A series of phase 4 health economics studies was conducted for gastrointestinal surgeries, including open colectomy, laparoscopic colectomy, and ileostomy reversal.53-56,76 These studies, of similar design, showed that a liposome bupivacaine–based multimodal analgesic regimen was associated with reduced opioid use, shorter hospital LOS, and lower hospitalization costs in comparison with a traditional opioid-based regimen.53-56 Although pooled analysis of these studies showed a cost savings of more than $2000 per patient and an LOS decrease of 1.4 days,76 all were conducted in the gastrointestinal surgery setting. Studies are needed to fully assess the economic benefits associated with liposome bupivacaine in the orthopedic surgery setting.
Conclusion
Liposome bupivacaine represents a potentially important contributor to multimodal analgesic regimens used to manage postsurgical pain. Liposome bupivacaine has demonstrated efficacy in providing prolonged postsurgical analgesia and reducing postsurgical opioid use in most surgical settings studied. Additional data from health economics studies in gastrointestinal surgery suggest liposome bupivacaine–based multimodal analgesic regimens may also contribute to reductions in hospital LOS and hospitalization costs. Non-industry-sponsored trials are needed to answer these crucial questions in orthopedic surgery settings. Nevertheless, data on the safety and efficacy of liposome bupivacaine for postsurgical analgesia continue to accumulate, and liposome bupivacaine appears to be a feasible therapeutic option for managing postsurgical pain in orthopedic surgery.
1. Centers for Disease Control and Prevention. Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. http://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf. Accessed January 30, 2015.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Bonica JJ. Postoperative pain. In: Bonica JJ, ed. The Management of Pain. Malvern, PA: Lea & Febiger; 1990:461-480.
4. Apfelbaum JL, Ashburn MA, Connis RT, et al; American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273.
5. Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam® bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther. 2011;28(9):776-788.
6. Oderda G. Challenges in the management of acute postsurgical pain. Pharmacotherapy. 2012;32(9 pt 2):6S-11S.
7. Oderda GM, Gan TJ, Johnson BH, Robinson SB. Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother. 2013;27(1):62-70.
8. Wu CL, Naqibuddin M, Rowlingson AJ, Lietman SA, Jermyn RM, Fleisher LA. The effect of pain on health-related quality of life in the immediate postoperative period. Anesth Analg. 2003;97(4):1078-1085.
9. Morrison RS, Magaziner J, McLaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103(3):303-311.
10. Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d’Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91(1):8-15.
11. Capdevila X, Dadure C, Bringuier S, et al. Effect of patient-controlled perineural analgesia on rehabilitation and pain after ambulatory orthopedic surgery: a multicenter randomized trial. Anesthesiology. 2006;105(3):566-573.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 Suppl 3):12-15.
13. Hamilton DF, Lane JV, Gaston P, et al. What determines patient satisfaction with surgery? A prospective cohort study of 4709 patients following total joint replacement. BMJ Open. 2013;3(4):e002525.
14. Exparel [prescribing information]. Parsippany, NJ: Pacira Pharmaceuticals, Inc.; 2014.
15. DeWeese FT, Akbari Z, Carline E. Pain control after knee arthroplasty: intraarticular versus epidural anesthesia. Clin Orthop Relat Res. 2001;392(11):226-231.
16. Pati AB, Perme D, Trail M, Henry PK, Bryan WJ. Rehabilitation parameters in total knee replacement patients undergoing epidural vs. conventional analgesia. J Orthop Sports Phys Ther. 1994;19(2):88-92.
17. Browne C, Copp S, Reden L, Pulido P, Colwell C Jr. Bupivacaine bolus injection versus placebo for pain management following total knee arthroplasty. J Arthroplasty. 2004;19(3):377-380.
18. Nechleba J, Rogers V, Cortina G, Cooney T. Continuous intra-articular infusion of bupivacaine for postoperative pain following total knee arthroplasty. J Knee Surg. 2005;18(3):197-202.
19. Campbell A, McCormick M, McKinlay K, Scott NB. Epidural vs. lumbar plexus infusions following total knee arthroplasty: randomized controlled trial. Eur J Anaesthesiol. 2008;25(6):502-507.
20. Serpell MG, Millar FA, Thomson MF. Comparison of lumbar plexus block versus conventional opioid analgesia after total knee replacement. Anaesthesia. 1991;46(4):275-277.
21. Lareau JM, Robbins CE, Talmo CT, Mehio AK, Puri L, Bono JV. Complications of femoral nerve blockade in total knee arthroplasty and strategies to reduce patient risk. J Arthroplasty. 2012;27(4):564-568.
22. Charous MT, Madison SJ, Suresh PJ, et al. Continuous femoral nerve blocks: varying local anesthetic delivery method (bolus versus basal) to minimize quadriceps motor block while maintaining sensory block. Anesthesiology. 2011;115(4):774-781.
23. Gottschalk A, Burmeister MA, Radtke P, et al. Continuous wound infiltration with ropivacaine reduces pain and analgesic requirement after shoulder surgery. Anesth Analg. 2003;97(4):1086-1091.
24. Kerr DR, Kohan L. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery: a case study of 325 patients. Acta Orthop. 2008;79(2):174-183.
25. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am. 2011;93(20):1938-1943.
26. White PF. The role of non-opioid analgesic techniques in the management of pain after ambulatory surgery. Anesth Analg. 2002;94(3):577-585.
27. Dahl JB, Kehlet H. Non-steroidal anti-inflammatory drugs: rationale for use in severe postoperative pain. Br J Anaesth. 1991;66(6):703-712.
28. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048-1056.
29. Candiotti K. Liposomal bupivacaine: an innovative nonopioid local analgesic for the management of postsurgical pain. Pharmacotherapy. 2012;32(9 Pt 2):19S-26S.
30. Bergese SD, Onel E, Portillo J. Evaluation of DepoFoam® bupivacaine for the treatment of postsurgical pain. Pain Manag. 2011;1(6):539-547.
31. Joranson DE, Ryan KM, Gilson AM, Dahl JL. Trends in medical use and abuse of opioid analgesics. JAMA. 2000;283(13):1710-1714.
32. Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297(3):249-251.
33. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
34. Ilfeld BM, Morey TE, Enneking FK. Delivery rate accuracy of portable, bolus-capable infusion pumps used for patient-controlled continuous regional analgesia. Reg Anesth Pain Med. 2003;28(1):17-23.
35. Ganapathy S, Amendola A, Lichfield R, Fowler PJ, Ling E. Elastomeric pumps for ambulatory patient controlled regional analgesia. Can J Anaesth. 2000;47(9):897-902.
36. Bray DA Jr, Nguyen J, Craig J, Cohen BE, Collins DR Jr. Efficacy of a local anesthetic pain pump in abdominoplasty. Plast Reconstr Surg. 2007;119(3):1054-1059.
37. Brown SL, Morrison AE. Local anesthetic infusion pump systems adverse events reported to the Food and Drug Administration. Anesthesiology. 2004;100(5):1305-1307.
38. Noyes FR, Fleckenstein CM, Barber-Westin SD. The development of postoperative knee chondrolysis after intra-articular pain pump infusion of an anesthetic medication: a series of twenty-one cases. J Bone Joint Surg Am. 2012;94(16):1448-1457.
39. Rapley JH, Beavis RC, Barber FA. Glenohumeral chondrolysis after shoulder arthroscopy associated with continuous bupivacaine infusion. Arthroscopy. 2009;25(12):1367-1373.
40. Institute for Safe Medication Practices. Process for handling elastomeric pain relief balls (ON-Q PainBuster and others) requires safety improvements. ISMP Medication Safety Alert. http://www.ismp.org/Newsletters/acutecare/articles/20090716.asp. Accessed January 30, 2015.
41. Pepin JL, Dasta JF, New M. Ensuring safe and economical use of elastomeric infusion devices. Am J Health Syst Pharm. 2011;68(24):2330-2331.
42. Birrer KL, Anderson RL, Liu-DeRyke X, Patel KR. Measures to improve safety of an elastomeric infusion system for pain management. Am J Health Syst Pharm. 2011;68(13):1251-1255.
43. Ilfeld BM, Enneking FK. Continuous peripheral nerve blocks at home: a review. Anesth Analg. 2005;100(6):1822-1833.
44. US Food and Drug Administration. Medical device recalls: I-Flow ON-Q Pump with ONDEMAND Bolus Button. http://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm317826.htm. Accessed July 15, 2014.
45. Ilfeld BM, Morey TE, Enneking FK. Portable infusion pumps used for continuous regional analgesia: delivery rate accuracy and consistency. Reg Anesth Pain Med. 2003;28(5):424-432.
46. Ganapathy S. Wound/intra-articular infiltration or peripheral nerve blocks for orthopedic joint surgery: efficacy and safety issues. Curr Opin Anaesthesiol. 2012;25(5):615-620.
47. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554.
48. Angst MS, Drover DR. Pharmacology of drugs formulated with DepoFoam™: a sustained release drug delivery system for parenteral administration using multivesicular liposome technology. Clin Pharmacokinet. 2006;45(12):1153-1176.
49. Catterall WA, Mackie K. Local anesthetics. In: Gutstein HB, Akil H, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011:565-582.
50. Hu D, Onel E, Singla N, Kramer WG, Hadzic A. Pharmacokinetic profile of liposome bupivacaine injection following a single administration at the surgical site. Clin Drug Investig. 2013;33(2):109-115.
51. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536.
52. Bergese SD, Ramamoorthy S, Patou G, Bramlett K, Gorfine SR, Candiotti KA. Efficacy profile of liposome bupivacaine, a novel formulation of bupivacaine for postsurgical analgesia. J Pain Res. 2012;5:107-116.
53. Cohen SM. Extended pain relief trial utilizing infiltration of Exparel®, a long-acting multivesicular liposome formulation of bupivacaine: a phase IV health economic trial in adult patients undergoing open colectomy. J Pain Res. 2012;5:567-572.
54. Candiotti KA, Sands LR, Lee E, et al. Liposome bupivacaine for postsurgical analgesia in adult patients undergoing laparoscopic colectomy: results from prospective phase IV sequential cohort studies assessing health economic outcomes. Curr Ther Res. 2014;76:1-6.
55. Marcet JE, Nfonsam VN, Larach S. An extended paIn relief trial utilizing the infiltration of a long-acting Multivesicular liPosome foRmulation Of bupiVacaine, EXPAREL (IMPROVE): a Phase IV health economic trial in adult patients undergoing ileostomy reversal. J Pain Res. 2013;6:549-555.
56. Vogel JD. Liposome bupivacaine (EXPAREL®) for extended pain relief in patients undergoing ileostomy reversal at a single institution with a fast-track discharge protocol: an IMPROVE phase IV health economics trial. J Pain Res. 2013;6:605-610.
57. Sternlicht A, Shapiro M, Robelen G, Vellayappan U, Tuerk IA. Initial findings using EXPAREL® (bupivacaine liposome injectable suspension) via infiltration into the transversus abdominis plane (TAP) for postsurgical analgesia in robotic prostatectomy (RP). Abstract presented at: Annual Fall Pain Meeting and Workshops of the American Society of Regional Anesthesia and Pain Medicine; November 15-18, 2012; Miami Beach, FL.
58. Feierman DE, Kronenfeld M, Gupta PM, Younger N, Logvinskiy E. Evaluation of Exparel® use via infiltration into the transversus abdominis plane for prolonged postoperative analgesia in subjects undergoing open abdominal hernia repair. Poster presented at: Annual Meeting of the International Anesthesia Research Society; May 4-7, 2013; San Diego, CA.
59. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
60. Broome B, Backlund I. Rapid recovery pain pathway for total knee arthroplasty results in improved pain management, decreased length of stay, and significant cost savings. Poster presented at: Annual Orthopedic and Spine Summit; September 18-20, 2013; San Antonio, TX.
61. Emerson RH, Barrington JW. Comparison of infiltration with long-acting bupivacaine to a femoral nerve catheter for total knee replacement. Abstract presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11-15, 2014; New Orleans, LA. Abstract P124.
62. Barrington JW. Emerging data in the use of liposome bupivacaine: comparative review in 2,000 TJA patients. Oral presentation presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11-15, 2014; New Orleans, LA.
63. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35(2):152-161.
64. D’Angelo R. Are the new local anesthetics worth their cost? Acta Anaesthesiol Scand. 2000;44(6):639-641.
65. Viscusi ER, Sinatra R, Onel E, Ramamoorthy SL. The safety of liposome bupivacaine, a novel local analgesic formulation. Clin J Pain. 2014;30(2):102-110.
66. Bergese SD, Onel E, Morren M, Morganroth J. Bupivacaine extended-release liposome injection exhibits a favorable cardiac safety profile. Reg Anesth Pain Med. 2012;37(2):145-151.
67. Naseem A, Harada T, Wang D, et al. Bupivacaine extended release liposome injection does not prolong QTc interval in a thorough QT/QTc study in healthy volunteers. J Clin Pharmacol. 2012;52(9):1441-1447.
68. Baxter R, Bramlett K, Onel E, Daniels S. Impact of local administration of liposome bupivacaine for postsurgical analgesia on wound healing: a review of data from ten prospective, controlled clinical studies. Clin Ther. 2013;35(3):312-320.
69. Kharitonov V. A review of the compatibility of liposome bupivacaine with other drug products and commonly used implant materials. Postgrad Med. 2014;126(1):129-138.
70. Ilfeld BM. Liposome bupivacaine in peripheral nerve blocks and epidural injections to manage postoperative pain. Expert Opin Pharmacother. 2013;14(17):2421-2431.
71. Femoral nerve block with liposome bupivacaine for postsurgical analgesia following total knee arthroplasty [NCT01683071]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01683071?term=NCT01683071%5C&rank=1. Accessed January 30, 2015.
72. Minkowitz H, Matthews A, Puckett C, Melson T. Liposome bupivacaine in femoral nerve block: initial results from a phase 2/3 pivotal study. Poster presented at: Annual Meeting of the American Society of Regional Anesthesia and Pain Medicine; April 3-6, 2014; Chicago, IL.
73. Ultrasound guided local infiltration analgesia for hip arthroscopy [NCT01907191]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01907191?term=NCT01907191&rank=1. Accessed January 30, 2015.
74. Efficacy of single injection femoral nerve block with liposomal bupivacaine for total knee arthroplasty [NCT01977339]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01977339?term=NCT01977339&rank=1. Accessed January 30, 2015.
75. Efficacy of interscalene brachial plexus block with liposomal bupivacaine for arthroscopic shoulder surgery [NCT01977352]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01977352?term=NCT01977352&rank=1. Accessed January 30, 2015.
76. Cohen SM, Vogel JD, Marcet JE, Candiotti K. Liposome bupivacaine for improvement in economic outcomes and opioid burden in GI surgery: IMPROVE study pooled analysis. J Pain Res. 2014;7:359-366.
1. Centers for Disease Control and Prevention. Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. http://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf. Accessed January 30, 2015.
2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
3. Bonica JJ. Postoperative pain. In: Bonica JJ, ed. The Management of Pain. Malvern, PA: Lea & Febiger; 1990:461-480.
4. Apfelbaum JL, Ashburn MA, Connis RT, et al; American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273.
5. Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam® bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther. 2011;28(9):776-788.
6. Oderda G. Challenges in the management of acute postsurgical pain. Pharmacotherapy. 2012;32(9 pt 2):6S-11S.
7. Oderda GM, Gan TJ, Johnson BH, Robinson SB. Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother. 2013;27(1):62-70.
8. Wu CL, Naqibuddin M, Rowlingson AJ, Lietman SA, Jermyn RM, Fleisher LA. The effect of pain on health-related quality of life in the immediate postoperative period. Anesth Analg. 2003;97(4):1078-1085.
9. Morrison RS, Magaziner J, McLaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103(3):303-311.
10. Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d’Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91(1):8-15.
11. Capdevila X, Dadure C, Bringuier S, et al. Effect of patient-controlled perineural analgesia on rehabilitation and pain after ambulatory orthopedic surgery: a multicenter randomized trial. Anesthesiology. 2006;105(3):566-573.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 Suppl 3):12-15.
13. Hamilton DF, Lane JV, Gaston P, et al. What determines patient satisfaction with surgery? A prospective cohort study of 4709 patients following total joint replacement. BMJ Open. 2013;3(4):e002525.
14. Exparel [prescribing information]. Parsippany, NJ: Pacira Pharmaceuticals, Inc.; 2014.
15. DeWeese FT, Akbari Z, Carline E. Pain control after knee arthroplasty: intraarticular versus epidural anesthesia. Clin Orthop Relat Res. 2001;392(11):226-231.
16. Pati AB, Perme D, Trail M, Henry PK, Bryan WJ. Rehabilitation parameters in total knee replacement patients undergoing epidural vs. conventional analgesia. J Orthop Sports Phys Ther. 1994;19(2):88-92.
17. Browne C, Copp S, Reden L, Pulido P, Colwell C Jr. Bupivacaine bolus injection versus placebo for pain management following total knee arthroplasty. J Arthroplasty. 2004;19(3):377-380.
18. Nechleba J, Rogers V, Cortina G, Cooney T. Continuous intra-articular infusion of bupivacaine for postoperative pain following total knee arthroplasty. J Knee Surg. 2005;18(3):197-202.
19. Campbell A, McCormick M, McKinlay K, Scott NB. Epidural vs. lumbar plexus infusions following total knee arthroplasty: randomized controlled trial. Eur J Anaesthesiol. 2008;25(6):502-507.
20. Serpell MG, Millar FA, Thomson MF. Comparison of lumbar plexus block versus conventional opioid analgesia after total knee replacement. Anaesthesia. 1991;46(4):275-277.
21. Lareau JM, Robbins CE, Talmo CT, Mehio AK, Puri L, Bono JV. Complications of femoral nerve blockade in total knee arthroplasty and strategies to reduce patient risk. J Arthroplasty. 2012;27(4):564-568.
22. Charous MT, Madison SJ, Suresh PJ, et al. Continuous femoral nerve blocks: varying local anesthetic delivery method (bolus versus basal) to minimize quadriceps motor block while maintaining sensory block. Anesthesiology. 2011;115(4):774-781.
23. Gottschalk A, Burmeister MA, Radtke P, et al. Continuous wound infiltration with ropivacaine reduces pain and analgesic requirement after shoulder surgery. Anesth Analg. 2003;97(4):1086-1091.
24. Kerr DR, Kohan L. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery: a case study of 325 patients. Acta Orthop. 2008;79(2):174-183.
25. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am. 2011;93(20):1938-1943.
26. White PF. The role of non-opioid analgesic techniques in the management of pain after ambulatory surgery. Anesth Analg. 2002;94(3):577-585.
27. Dahl JB, Kehlet H. Non-steroidal anti-inflammatory drugs: rationale for use in severe postoperative pain. Br J Anaesth. 1991;66(6):703-712.
28. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048-1056.
29. Candiotti K. Liposomal bupivacaine: an innovative nonopioid local analgesic for the management of postsurgical pain. Pharmacotherapy. 2012;32(9 Pt 2):19S-26S.
30. Bergese SD, Onel E, Portillo J. Evaluation of DepoFoam® bupivacaine for the treatment of postsurgical pain. Pain Manag. 2011;1(6):539-547.
31. Joranson DE, Ryan KM, Gilson AM, Dahl JL. Trends in medical use and abuse of opioid analgesics. JAMA. 2000;283(13):1710-1714.
32. Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297(3):249-251.
33. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
34. Ilfeld BM, Morey TE, Enneking FK. Delivery rate accuracy of portable, bolus-capable infusion pumps used for patient-controlled continuous regional analgesia. Reg Anesth Pain Med. 2003;28(1):17-23.
35. Ganapathy S, Amendola A, Lichfield R, Fowler PJ, Ling E. Elastomeric pumps for ambulatory patient controlled regional analgesia. Can J Anaesth. 2000;47(9):897-902.
36. Bray DA Jr, Nguyen J, Craig J, Cohen BE, Collins DR Jr. Efficacy of a local anesthetic pain pump in abdominoplasty. Plast Reconstr Surg. 2007;119(3):1054-1059.
37. Brown SL, Morrison AE. Local anesthetic infusion pump systems adverse events reported to the Food and Drug Administration. Anesthesiology. 2004;100(5):1305-1307.
38. Noyes FR, Fleckenstein CM, Barber-Westin SD. The development of postoperative knee chondrolysis after intra-articular pain pump infusion of an anesthetic medication: a series of twenty-one cases. J Bone Joint Surg Am. 2012;94(16):1448-1457.
39. Rapley JH, Beavis RC, Barber FA. Glenohumeral chondrolysis after shoulder arthroscopy associated with continuous bupivacaine infusion. Arthroscopy. 2009;25(12):1367-1373.
40. Institute for Safe Medication Practices. Process for handling elastomeric pain relief balls (ON-Q PainBuster and others) requires safety improvements. ISMP Medication Safety Alert. http://www.ismp.org/Newsletters/acutecare/articles/20090716.asp. Accessed January 30, 2015.
41. Pepin JL, Dasta JF, New M. Ensuring safe and economical use of elastomeric infusion devices. Am J Health Syst Pharm. 2011;68(24):2330-2331.
42. Birrer KL, Anderson RL, Liu-DeRyke X, Patel KR. Measures to improve safety of an elastomeric infusion system for pain management. Am J Health Syst Pharm. 2011;68(13):1251-1255.
43. Ilfeld BM, Enneking FK. Continuous peripheral nerve blocks at home: a review. Anesth Analg. 2005;100(6):1822-1833.
44. US Food and Drug Administration. Medical device recalls: I-Flow ON-Q Pump with ONDEMAND Bolus Button. http://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm317826.htm. Accessed July 15, 2014.
45. Ilfeld BM, Morey TE, Enneking FK. Portable infusion pumps used for continuous regional analgesia: delivery rate accuracy and consistency. Reg Anesth Pain Med. 2003;28(5):424-432.
46. Ganapathy S. Wound/intra-articular infiltration or peripheral nerve blocks for orthopedic joint surgery: efficacy and safety issues. Curr Opin Anaesthesiol. 2012;25(5):615-620.
47. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554.
48. Angst MS, Drover DR. Pharmacology of drugs formulated with DepoFoam™: a sustained release drug delivery system for parenteral administration using multivesicular liposome technology. Clin Pharmacokinet. 2006;45(12):1153-1176.
49. Catterall WA, Mackie K. Local anesthetics. In: Gutstein HB, Akil H, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011:565-582.
50. Hu D, Onel E, Singla N, Kramer WG, Hadzic A. Pharmacokinetic profile of liposome bupivacaine injection following a single administration at the surgical site. Clin Drug Investig. 2013;33(2):109-115.
51. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536.
52. Bergese SD, Ramamoorthy S, Patou G, Bramlett K, Gorfine SR, Candiotti KA. Efficacy profile of liposome bupivacaine, a novel formulation of bupivacaine for postsurgical analgesia. J Pain Res. 2012;5:107-116.
53. Cohen SM. Extended pain relief trial utilizing infiltration of Exparel®, a long-acting multivesicular liposome formulation of bupivacaine: a phase IV health economic trial in adult patients undergoing open colectomy. J Pain Res. 2012;5:567-572.
54. Candiotti KA, Sands LR, Lee E, et al. Liposome bupivacaine for postsurgical analgesia in adult patients undergoing laparoscopic colectomy: results from prospective phase IV sequential cohort studies assessing health economic outcomes. Curr Ther Res. 2014;76:1-6.
55. Marcet JE, Nfonsam VN, Larach S. An extended paIn relief trial utilizing the infiltration of a long-acting Multivesicular liPosome foRmulation Of bupiVacaine, EXPAREL (IMPROVE): a Phase IV health economic trial in adult patients undergoing ileostomy reversal. J Pain Res. 2013;6:549-555.
56. Vogel JD. Liposome bupivacaine (EXPAREL®) for extended pain relief in patients undergoing ileostomy reversal at a single institution with a fast-track discharge protocol: an IMPROVE phase IV health economics trial. J Pain Res. 2013;6:605-610.
57. Sternlicht A, Shapiro M, Robelen G, Vellayappan U, Tuerk IA. Initial findings using EXPAREL® (bupivacaine liposome injectable suspension) via infiltration into the transversus abdominis plane (TAP) for postsurgical analgesia in robotic prostatectomy (RP). Abstract presented at: Annual Fall Pain Meeting and Workshops of the American Society of Regional Anesthesia and Pain Medicine; November 15-18, 2012; Miami Beach, FL.
58. Feierman DE, Kronenfeld M, Gupta PM, Younger N, Logvinskiy E. Evaluation of Exparel® use via infiltration into the transversus abdominis plane for prolonged postoperative analgesia in subjects undergoing open abdominal hernia repair. Poster presented at: Annual Meeting of the International Anesthesia Research Society; May 4-7, 2013; San Diego, CA.
59. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
60. Broome B, Backlund I. Rapid recovery pain pathway for total knee arthroplasty results in improved pain management, decreased length of stay, and significant cost savings. Poster presented at: Annual Orthopedic and Spine Summit; September 18-20, 2013; San Antonio, TX.
61. Emerson RH, Barrington JW. Comparison of infiltration with long-acting bupivacaine to a femoral nerve catheter for total knee replacement. Abstract presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11-15, 2014; New Orleans, LA. Abstract P124.
62. Barrington JW. Emerging data in the use of liposome bupivacaine: comparative review in 2,000 TJA patients. Oral presentation presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11-15, 2014; New Orleans, LA.
63. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35(2):152-161.
64. D’Angelo R. Are the new local anesthetics worth their cost? Acta Anaesthesiol Scand. 2000;44(6):639-641.
65. Viscusi ER, Sinatra R, Onel E, Ramamoorthy SL. The safety of liposome bupivacaine, a novel local analgesic formulation. Clin J Pain. 2014;30(2):102-110.
66. Bergese SD, Onel E, Morren M, Morganroth J. Bupivacaine extended-release liposome injection exhibits a favorable cardiac safety profile. Reg Anesth Pain Med. 2012;37(2):145-151.
67. Naseem A, Harada T, Wang D, et al. Bupivacaine extended release liposome injection does not prolong QTc interval in a thorough QT/QTc study in healthy volunteers. J Clin Pharmacol. 2012;52(9):1441-1447.
68. Baxter R, Bramlett K, Onel E, Daniels S. Impact of local administration of liposome bupivacaine for postsurgical analgesia on wound healing: a review of data from ten prospective, controlled clinical studies. Clin Ther. 2013;35(3):312-320.
69. Kharitonov V. A review of the compatibility of liposome bupivacaine with other drug products and commonly used implant materials. Postgrad Med. 2014;126(1):129-138.
70. Ilfeld BM. Liposome bupivacaine in peripheral nerve blocks and epidural injections to manage postoperative pain. Expert Opin Pharmacother. 2013;14(17):2421-2431.
71. Femoral nerve block with liposome bupivacaine for postsurgical analgesia following total knee arthroplasty [NCT01683071]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01683071?term=NCT01683071%5C&rank=1. Accessed January 30, 2015.
72. Minkowitz H, Matthews A, Puckett C, Melson T. Liposome bupivacaine in femoral nerve block: initial results from a phase 2/3 pivotal study. Poster presented at: Annual Meeting of the American Society of Regional Anesthesia and Pain Medicine; April 3-6, 2014; Chicago, IL.
73. Ultrasound guided local infiltration analgesia for hip arthroscopy [NCT01907191]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01907191?term=NCT01907191&rank=1. Accessed January 30, 2015.
74. Efficacy of single injection femoral nerve block with liposomal bupivacaine for total knee arthroplasty [NCT01977339]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01977339?term=NCT01977339&rank=1. Accessed January 30, 2015.
75. Efficacy of interscalene brachial plexus block with liposomal bupivacaine for arthroscopic shoulder surgery [NCT01977352]. ClinicalTrials.gov website. http://clinicaltrials.gov/ct2/show/NCT01977352?term=NCT01977352&rank=1. Accessed January 30, 2015.
76. Cohen SM, Vogel JD, Marcet JE, Candiotti K. Liposome bupivacaine for improvement in economic outcomes and opioid burden in GI surgery: IMPROVE study pooled analysis. J Pain Res. 2014;7:359-366.