User login
Isolated third nerve palsy: Lessons from the literature and 4 case studies
Of all the cranial nerve (CN) palsies that affect the eye, the third (oculomotor) nerve palsy (TNP) requires the most urgent evaluation.1 Third nerve dysfunction may signal an underlying neurologic emergency, such as ruptured cerebral aneurysm or giant cell arteritis. Early recognition and prompt treatment choices are key to reversing clinical and visual defects. The classic presentation of isolated TNP is a “down and out eye” deviation and ptosis with or without pupillary involvement.1
Recognize varying clinical presentations. TNPs, isolated or not, may be partial or complete, congenital or acquired, pupil involving or pupil sparing. In many cases, patients may have additional constitutional, ocular, or neurologic symptoms or signs, such as ataxia or hemiplegia.2 Recognition of these clinical findings, which at times can be subtle, is crucial. Appropriate clinical diagnosis and management rely on distinguishing isolated TNP from TNP that involves other CNs.2
Further clues to underlying pathology. Disruption of the third nerve can occur anywhere along its course from the oculomotor nucleus in the brain to its terminus at the extraocular muscles in the orbit.2 TNP’s effect on the pupil can often aid in diagnosis.3 Pupil-sparing TNP is usually due to microvascular ischemia, as may occur with diabetes or hypertension. Pupil involvement, though, may be the first sign of a compressive lesion.
Influence of age. Among individuals older than 60 years, the annual incidence of isolated TNP has been shown to be 12.5 per 100,000, compared with 1.7 per 100,000 in those younger than 60 years.4 In those older than 50 years, microvascular ischemia tends to be the dominant cause.4 Other possible causes include aneurysm, trauma, and neoplasm, particularly pituitary adenoma and metastatic tumor. In childhood and young adulthood, the most common cause of TNP is trauma.5
Use of vascular imaging is influenced by an individual’s age and clinical risk for an aneurysm. Isolated partial TNP or TNP with pupil involvement suggest compression of the third nerve and the need for immediate imaging. Given the dire implications of intracranial aneurysm, most physicians will focus their initial evaluation on vascular imaging, if available.2 If clinical findings instead suggest underlying microvascular ischemia, a delay of imaging may be possible.
In the text that follows, we present 4 patient cases describing the clinical investigative process and treatment determinations based on an individual’s history, clinical presentation, and neurologic findings.
CASE 1
Herpes zoster ophthalmicus
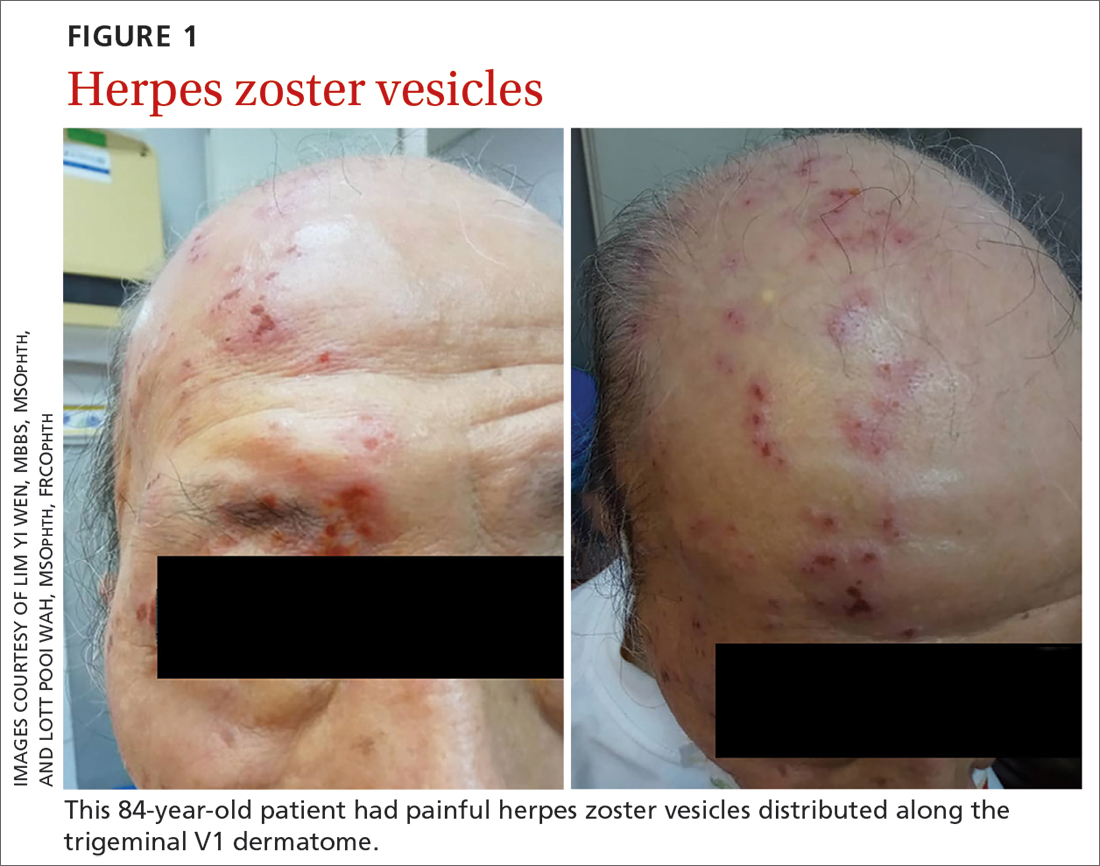
An 84-year-old man with no known medical illness presented to the emergency department (ED) with vesicular skin lesions that had appeared 4 days earlier over his scalp, right forehead, and periorbital region. The vesicles followed the distribution of the ophthalmic division of the trigeminal nerve (FIGURE 1). The patient was given a diagnosis of shingles. The only notable ocular features were the swollen right upper eyelid, injected conjunctiva, and reduced corneal sensation with otherwise normal right eye vision at 6/6. For right eye herpes zoster ophthalmicus (HZO), he was prescribed oral acyclovir 800 mg 5 times per day for 2 weeks.
Continue to: Two days later...
Two days later, he returned after experiencing a sudden onset of binocular diplopia and ptosis of the right eye. Partial ptosis was noted, with restricted adduction and elevation. Pupils were reactive and equal bilaterally. Hutchinson sign, which would indicate an impaired nasociliary nerve and increased risk for corneal and ocular sequelae,6 was absent. Relative afferent pupillary defect also was absent. All other CN functions were intact, with no systemic neurologic deficit. Contrast CT of the brain and orbit showed no radiologic evidence of meningitis, space-occupying lesion, or cerebral aneurysm.
Given the unremarkable imaging findings and lack of symptoms of meningism (eg, headache, vomiting, neck stiffness, or fever), we diagnosed right eye pupil-sparing partial TNP secondary to HZO. The patient continued taking oral acyclovir, which was tapered over 6 weeks. After 4 weeks of antiviral treatment, he recovered full extraocular movement and the ptosis subsided.
CASE 2
Posterior communicating artery aneurysm
A 71-year-old woman with hypercholesterolemia, hypertension, and ischemic heart disease presented to the ED with a 4-day history of headache, vomiting, and neck pain and a 2-day history of a drooping left eyelid. When asked if she had double vision, she said “No.” She had no other neurologic symptoms. Her blood pressure (BP) was 199/88 mm Hg. An initial plain CT of the brain ruled out ischemia, intracranial hemorrhage, and space-occupying lesion.
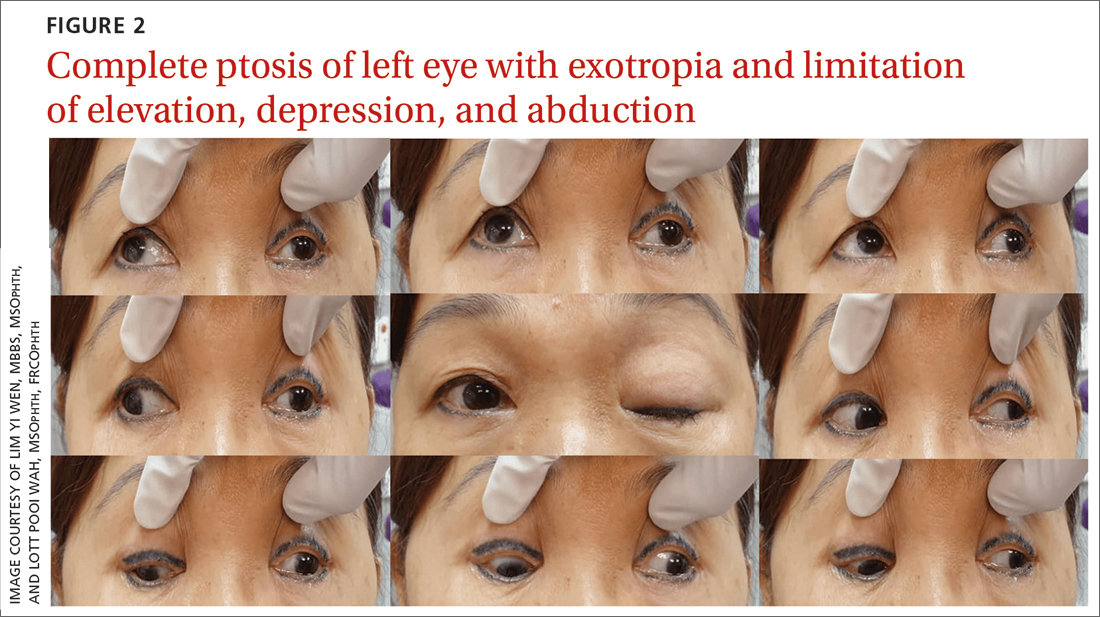
Once her BP was stabilized, she was referred to us for detailed eye assessment. Her best corrected visual acuity was 6/12 bilaterally. In contrast to her right eye pupil, which was 4 mm in diameter and reactive, her left eye pupil was 7 mm and poorly reactive to light. Optic nerve functions were preserved. There was complete ptosis of the left eye, with exotropia and total limitation of elevation, depression, and abduction (FIGURE 2). There was no proptosis; intraocular pressure was normal. Fundus examination of the left eye was unremarkable. All other CN and neurologic examinations were normal. We diagnosed left eye pupil-involving TNP.
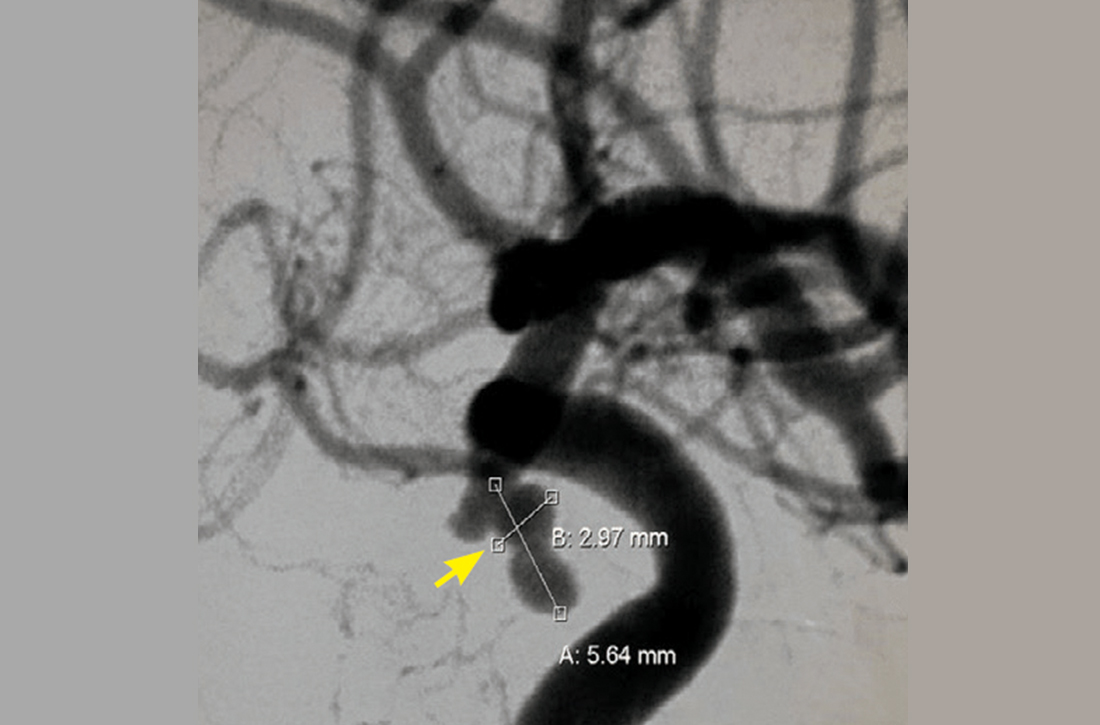
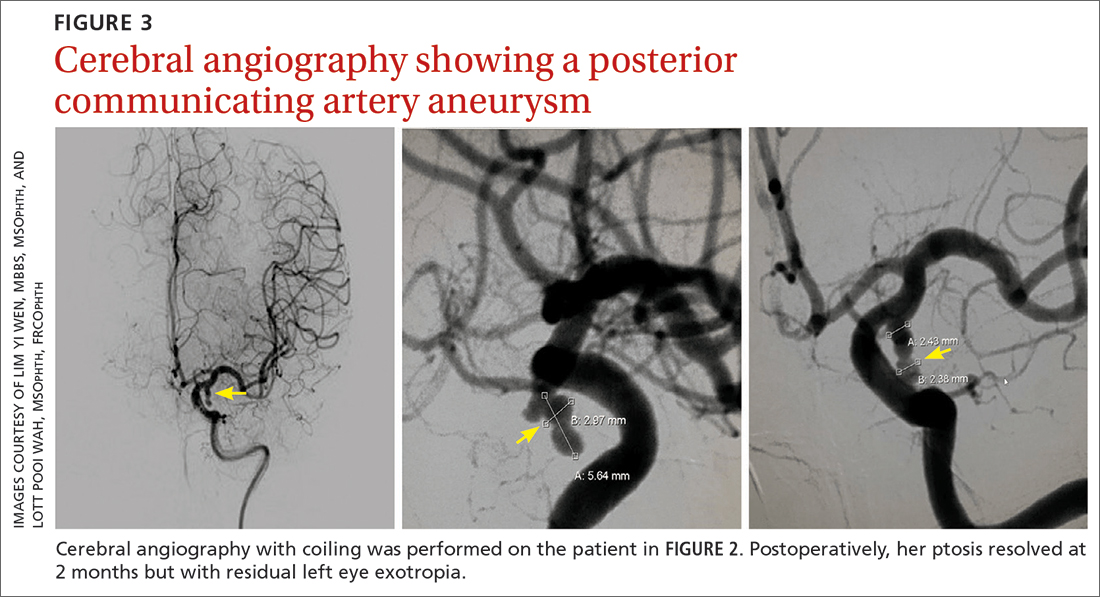
Further assessment of the brain with magnetic resonance imaging (MRI) revealed a left posterior communicating artery aneurysm. We performed cerebral angiography (FIGURE 3) with coiling. Postoperatively, her ptosis resolved at 2 months but with residual left eye exotropia.
CASE 3
Viral infection
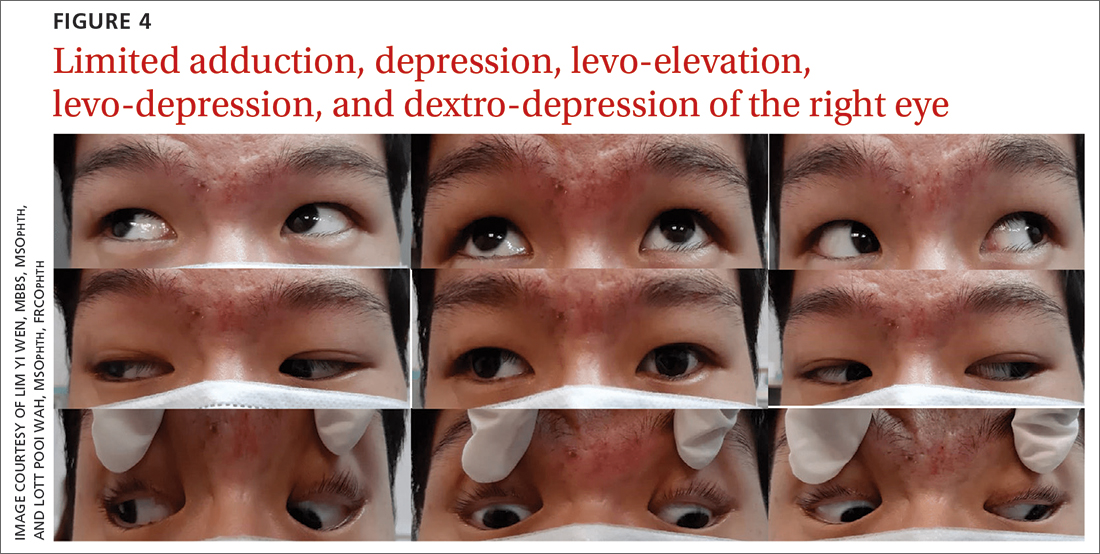
A 20-year-old male student presented to the ED for evaluation of acute-onset diplopia that was present upon awakening from sleep 4 days earlier. There was no ptosis or other neurologic symptoms. He had no history of trauma or viral illness. Examination revealed limited adduction, depression, levo-elevation, levo-depression, and dextro-depression in the right eye (FIGURE 4). Both pupils were reactive. There was no sign of aberrant third nerve regeneration. The optic nerve and other CN functions were intact. A systemic neurologic examination was unremarkable, and the fundus was normal, with no optic disc swelling. All blood work was negative for diabetes, hypercoagulability, and hyperlipidemia.
CT angiography (CTA) and MR angiography (MRA) did not reveal any vascular abnormalities such as intracranial aneurysms, arteriovenous malformations, or berry aneurysm. We treated the patient for right eye partial TNP secondary to presumed prior viral infection that led to an immune-mediated palsy of the third nerve. He was given a short course of low-dose oral prednisolone (30 mg/d for 5 days). He achieved full recovery of his ocular motility after 2 weeks.
Continue to: CASE 4
CASE 4
Trauma
A 33-year-old woman was brought to the ED after she was knocked off her motorbike by a car. A passerby found her unconscious and still wearing her helmet. En route to the hospital, the patient regained consciousness but had retrograde amnesia.
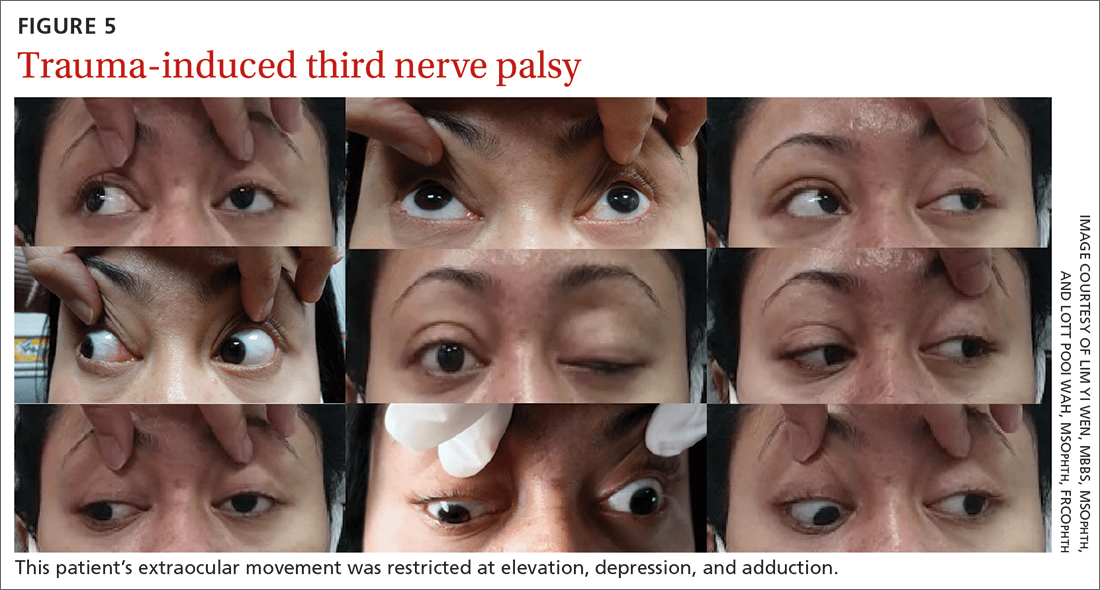
She was referred to us for evaluation of complete ptosis of her left eye. She was fully conscious during the examination. Her left eye vision was 6/9. Complete ptosis with exotropia was noted. Pupillary examination revealed a sluggish dilated left eye pupil of 7 mm with no reverse relative afferent pupillary defect. Extraocular movement was restricted at elevation, depression, and adduction with diplopia (FIGURE 5). All other CN functions were preserved.
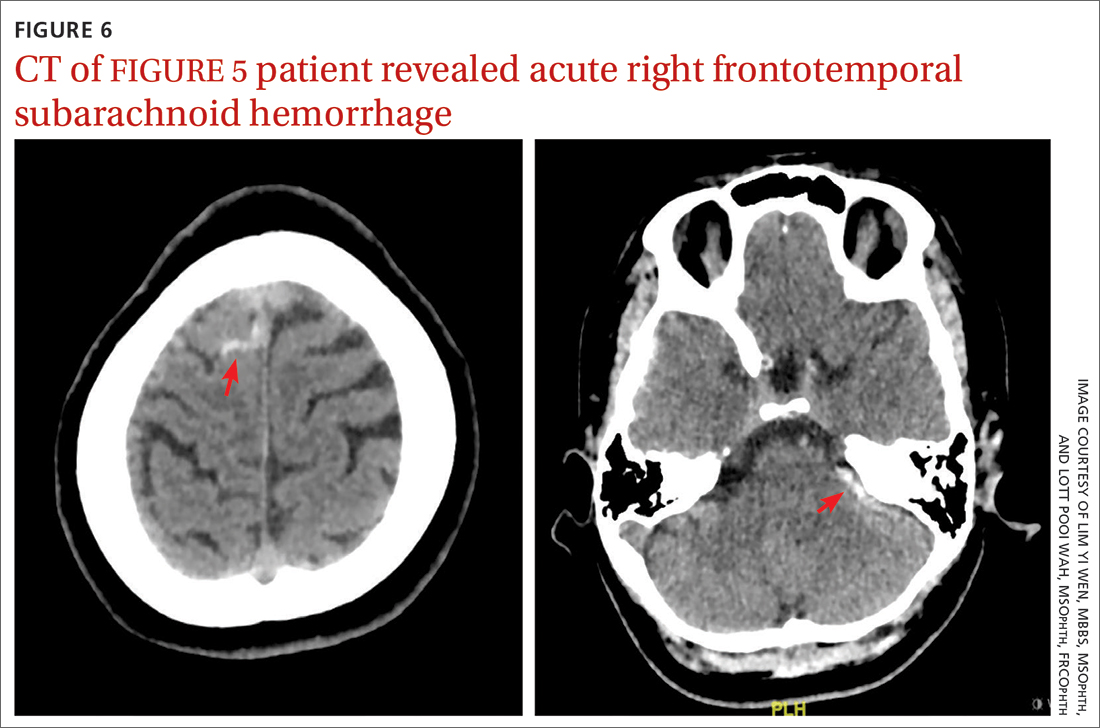
CT of the brain and orbit revealed acute right frontotemporal subarachnoid hemorrhage (FIGURE 6). There was no radiologic evidence of orbital wall fractures or extraocular muscle entrapment. She remained stable during the first 24 hours of monitoring and was given a diagnosis of
Repeat CT of the brain 5 days later revealed complete resolution of the subarachnoid hemorrhage. The patient's clinical condition improved 2 weeks later and included resolution of ptosis and recovery of ocular motility.
Key takeaways from the cases
Case 1: Herpes zoster ophthalmicus
Clinical diagnosis of HZO is straightforward, with painful vesicular lesions occurring along the trigeminal nerve (V1) dermatome, as was seen in this case. The oculomotor nerve is the CN most commonly involved; the trochlear nerve is the least-often affected.6 In a report from the Mayo Clinic, 3 of 86 patients with HZO had oculomotor nerve palsies (3.4%).7 A separate review from an eye hospital study stated that 9.8% (n = 133) of 1356 patients with HZO had extraocular muscle palsy, with TNP in 4 of the patients.8
Ocular complications such as blepharitis, keratoconjunctivits, or iritis occur in 20% to 70% of HZO cases.9 Ophthalmoplegia, which most often involves the oculomotor nerve, is seen in 7% to 31% of HZO cases (mostly in the elderly) and usually occurs within 1 to 3 weeks of the onset of rash.6 Our patient immediately underwent contrast CT of the brain to rule out meningitis and nerve compression.
Treatment with a systemic antiviral agent is crucial. Acyclovir, valaciclovir, and famciclovir are available treatment options, used for treating the skin lesions, reducing the viral load, and reducing the risk for ocular involvement or its progression. Our patient started a 2-week course of oral acyclovir 800 mg 5 times per day. Ophthalmoplegia is usually self-limiting and has a good prognosis. Time to resolution varies from 2 to 18 months. Diplopia, if present, resolves within 1 year.6 Our patient achieved full recovery of his extraocular movement after completing 4 weeks of antiviral treatment.
Continue to: Case 2
Case 2: Posterior communicating artery aneurysm
Given the patient’s high BP, ruling out a hypertensive emergency with CT was the first priority. TNP caused by microvascular ischemia is not uncommon in the elderly. However, her pupil involvement and persistent headache called for an MRI to better evaluate the soft tissues and to rule out possible vascular pathologies. Left posterior communicating artery aneurysm was discovered with MRI, and urgent cerebral angiography and coiling was performed successfully.
Incidence. One report of 1400 patients with TNP confirmed that aneurysm was the cause in 10% of cases, with posterior communicating artery aneurysm accounting for the greatest number, 119 (25.7%).10 Of these cases of posterior communicating artery aneurysm, pupillary involvement was detected in 108 (90.8%). The oculomotor nerve lies adjacent to the posterior communicating artery as it passes through the subarachnoid space of the basal cisterns, where it is susceptible to compression.3
A high index of suspicion for posterior communicating artery aneurysm is crucial for early detection and lifesaving treatment. The patient in this case did well after the coiling. Her ptosis resolved at 2 months, although she had residual left eye exotropia.
Case 3: Viral infection
We chose CTA of the brain instead of contrast CT to rule out the possibility of intracranial aneurysm. CTA has been shown to be an adequate first-line study to detect aneurysms, particularly those greater than 4 mm in diameter.2,11 One study demonstrated an 81.8% sensitivity for aneurysms smaller than 3 mm when performed on a 320-slice CT.12
Additional imaging selection. We also selected MRA to rule out berry aneurysm, which is often asymptomatic. We decided against MRI because of its higher cost and longer acquisition time. It is usually reserved for patients with a negative initial work-up with CT or cerebral angiography if suspicion of a possible aneurysm remains.11 The MRA finding in this case was negative, and we made a presumptive diagnosis of TNP secondary to viral infection.
Isolated TNP following viral infection is a clinical diagnosis of exclusion. In 1 reported case, a 39-year-old man developed a superior division palsy after a common cold without fever, underwent no serologic study, and recovered spontaneously 6 weeks later.13 A 5-year-old boy who experienced a superior division palsy immediately after a common cold with fever was found on serologic examination to have an increased titre of influenza A virus. His palsy resolved in 4 months.14
The exact mechanism of viral-induced palsy is unknown. The possibility of postinfectious cranial neuropathy has been postulated, as most reported cases following a flu-like illness resolved within a few months.15 Although the pathogenesis remains speculative, an autoimmune process might have been involved.16 Our patient recovered fully in 1 month following a short course of oral prednisolone 30 mg/d for 5 days.
Case 4: Trauma
Trauma accounts for approximately 12% of all TNP cases.17 Traumatic TNPs are usually sustained in severe, high-speed, closed-head injuries, and are often associated with other CN injuries and neurologic deficits. The damage may be caused indirectly by compression, hemorrhage, or ischemia, or directly at certain vulnerable points including the nerve’s exit from the brainstem and the point at which it crosses the petroclinoid ligament.17 In our case, despite the patient having complete TNP, there was no sign of localized orbital trauma on the CT other than the presence of subarachnoid hemorrhage at the right frontotemporal region.
In a similar reported case, the patient had a right traumatic isolated TNP and was found to have left frontal subarachnoid hemorrhage with no sign of orbital trauma.18 However, the mechanisms of isolated TNP caused by traumatic brain injury are not clear. Possible causes include rootlet avulsion, distal fascicular damage, stretching of the nerve (including the parasellar segment), and decreased blood supply.18
It has been suggested that TNP is more frequently observed in cases of frontal region injury. As orbitofrontal regions are predominantly affected by cortical contusions, the risk for ocular involvement increases.19
Keep these fundamentals in mind
The diagnosis and management of isolated TNP are guided by the patient’s age, by the degree to which each of the oculomotor nerve’s 2 major functions—pupillomotor and oculomotor—are affected, and by the circumstances preceding the onset of TNP.2 Cases 1 and 3 in our series presented with partial TNP, while Cases 2 and 4 exhibited complete TNP. Pupillary involvement was detected only in Case 2. Nevertheless, radiologic imaging was ordered for all 4 cases after the diagnosis of TNP was made, to exclude the most worrying neurologic emergencies. The choice of imaging modality depends on not only the availability of the services but also the clinical signs and symptoms and presumptive clinical diagnosis. A tailored and thoughtful approach with consideration of the anatomy and varied pathologies help clinicians to skillfully discern emergencies from nonurgent cases.
CORRESPONDENCE
Lott Pooi Wah, MSOphth, FRCOphth, Department of Ophthalmology, Faculty of Medicine, Universiti Malaya, 50603 Kuala Lumpur, Malaysia; [email protected] Orcid no: 0000-0001-8746-1528
1. Radia M, Stahl M, Arunakirinathan M, et al. Examination of a third nerve palsy. Brit J Hosp Med. 2017;78:188-192. doi: 10.12968/hmed.2017.78.12.C188
2. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257-268. doi: 10.1055/s-2007-979681
3. Motoyama Y, Nonaka J, Hironaka Y, et al. Pupil-sparing oculomotor nerve palsy caused by upward compression of a large posterior communicating artery aneurysm. Case report. Neurol Med Chir (Tokyo). 2012;52:202-205. doi: 10.2176/nmc.52.202
4. Fang C, Leavitt JA, Hodge DO, et al. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23-28. doi: 10.1001/jamaophthal mol.2016.4456
5. Wyatt K. Three common ophthalmic emergencies. JAAPA. 2014;27:32-37. doi: 10.1097/01.JAA.0000447004.96714.34
6. Daswani M, Bhosale N, Shah VM. Rare case of herpes zoster ophthalmicus with orbital myositis, oculomotor nerve palsy and anterior uveitis. Indian J Dermatol Venereol Leprol. 2017;83:365-367. doi: 10.4103/0378-6323.199582
7. Womack LW, Liesegang TJ. Complications of herpes zoster ophthalmicus. Arch Ophthalmol. 1983;101:42-45. doi: 10.1001/archopht.1983.01040010044004
8. Marsh RJ, Dulley B, Kelly V. External ocular motor palsies in ophthalmic zoster: a review. Br J Ophthalmol. 1977;61:667-682. doi: 10.1136/bjo.61.11.677
9. Lim JJ, Ong YM, Zalina MCW, et al. Herpes zoster ophthalmicus with orbital apex syndrome – difference in outcomes and literature review. Ocul Immunol Inflamm. 2017;26:187-193. doi: 10.1080/09273948.2017.1327604
10. Keane JR. Third nerve palsy: analysis of 1400 personally-examined patients. Can J Neurol Sci. 2010;37:662-670. doi: 10.1017/s0317167100010866
11. Yoon NK, McNally S, Taussky P, et al. Imaging of cerebral aneurysms: a clinical perspective. Neurovasc Imaging. 2016;2:6. doi: 10.1186/s40809-016-0016-3
12. Wang H, Li W, He H, et al. 320-detector row CT angiography for detection and evaluation of intracranial aneurysms: comparison with conventional digital subtraction angiography. Clin Radiol. 2013;68:e15-20. doi: 10.1016/j.crad.2012.09.001
13. Derakhshan I. Superior branch palsy with spontaneous recovery. Ann Neurol. 1978;4:478-479. doi: 10.1002/ana.410040519
14. Engelhardt A, Credzich C, Kompf D. Isolated superior branch palsy of the oculomotor nerve in influenza A. Neuroophthalmol. 1989;9:233-235. doi: 10.3109/01658108908997359
15. Knox DL, Clark DB, Schuster FF. Benign VI nerve palsies in children. Pediatrics. 1967;40:560-564.
16. Saeki N, Yotsukura J, Adachi E, et al. Isolated superior division oculomotor palsy in a child with spontaneous recovery. J Clin Neurosci. 2000;7:62-64. doi: 10.1054/jocn.1998.0152
17. Nagendran ST, Lee V, Perry M. Traumatic orbital third nerve palsy. Brit J Oral Maxillofac Surg. 2019;57:578-581. doi: 10.1016/j.bjoms.2019.01.029
18. Kim T, Nam K, Kwon BS. Isolated oculomotor nerve palsy in mild traumatic brain injury: a literature review. Am J Phys Med Rehabil. 2020;99:430-435. doi: 10.1097/PHM.0000000000001316
19. Sharma B, Gupta R, Anand R, et al. Ocular manifestations of head injury and incidence of post-traumatic ocular motor nerve involvement in cases of head injury: a clinical review. Int Ophthalmol. 2014;34:893-900. doi: 10.1007/s10792-014-9898-8
Of all the cranial nerve (CN) palsies that affect the eye, the third (oculomotor) nerve palsy (TNP) requires the most urgent evaluation.1 Third nerve dysfunction may signal an underlying neurologic emergency, such as ruptured cerebral aneurysm or giant cell arteritis. Early recognition and prompt treatment choices are key to reversing clinical and visual defects. The classic presentation of isolated TNP is a “down and out eye” deviation and ptosis with or without pupillary involvement.1
Recognize varying clinical presentations. TNPs, isolated or not, may be partial or complete, congenital or acquired, pupil involving or pupil sparing. In many cases, patients may have additional constitutional, ocular, or neurologic symptoms or signs, such as ataxia or hemiplegia.2 Recognition of these clinical findings, which at times can be subtle, is crucial. Appropriate clinical diagnosis and management rely on distinguishing isolated TNP from TNP that involves other CNs.2
Further clues to underlying pathology. Disruption of the third nerve can occur anywhere along its course from the oculomotor nucleus in the brain to its terminus at the extraocular muscles in the orbit.2 TNP’s effect on the pupil can often aid in diagnosis.3 Pupil-sparing TNP is usually due to microvascular ischemia, as may occur with diabetes or hypertension. Pupil involvement, though, may be the first sign of a compressive lesion.
Influence of age. Among individuals older than 60 years, the annual incidence of isolated TNP has been shown to be 12.5 per 100,000, compared with 1.7 per 100,000 in those younger than 60 years.4 In those older than 50 years, microvascular ischemia tends to be the dominant cause.4 Other possible causes include aneurysm, trauma, and neoplasm, particularly pituitary adenoma and metastatic tumor. In childhood and young adulthood, the most common cause of TNP is trauma.5
Use of vascular imaging is influenced by an individual’s age and clinical risk for an aneurysm. Isolated partial TNP or TNP with pupil involvement suggest compression of the third nerve and the need for immediate imaging. Given the dire implications of intracranial aneurysm, most physicians will focus their initial evaluation on vascular imaging, if available.2 If clinical findings instead suggest underlying microvascular ischemia, a delay of imaging may be possible.
In the text that follows, we present 4 patient cases describing the clinical investigative process and treatment determinations based on an individual’s history, clinical presentation, and neurologic findings.
CASE 1
Herpes zoster ophthalmicus
An 84-year-old man with no known medical illness presented to the emergency department (ED) with vesicular skin lesions that had appeared 4 days earlier over his scalp, right forehead, and periorbital region. The vesicles followed the distribution of the ophthalmic division of the trigeminal nerve (FIGURE 1). The patient was given a diagnosis of shingles. The only notable ocular features were the swollen right upper eyelid, injected conjunctiva, and reduced corneal sensation with otherwise normal right eye vision at 6/6. For right eye herpes zoster ophthalmicus (HZO), he was prescribed oral acyclovir 800 mg 5 times per day for 2 weeks.

Continue to: Two days later...
Two days later, he returned after experiencing a sudden onset of binocular diplopia and ptosis of the right eye. Partial ptosis was noted, with restricted adduction and elevation. Pupils were reactive and equal bilaterally. Hutchinson sign, which would indicate an impaired nasociliary nerve and increased risk for corneal and ocular sequelae,6 was absent. Relative afferent pupillary defect also was absent. All other CN functions were intact, with no systemic neurologic deficit. Contrast CT of the brain and orbit showed no radiologic evidence of meningitis, space-occupying lesion, or cerebral aneurysm.
Given the unremarkable imaging findings and lack of symptoms of meningism (eg, headache, vomiting, neck stiffness, or fever), we diagnosed right eye pupil-sparing partial TNP secondary to HZO. The patient continued taking oral acyclovir, which was tapered over 6 weeks. After 4 weeks of antiviral treatment, he recovered full extraocular movement and the ptosis subsided.
CASE 2
Posterior communicating artery aneurysm
A 71-year-old woman with hypercholesterolemia, hypertension, and ischemic heart disease presented to the ED with a 4-day history of headache, vomiting, and neck pain and a 2-day history of a drooping left eyelid. When asked if she had double vision, she said “No.” She had no other neurologic symptoms. Her blood pressure (BP) was 199/88 mm Hg. An initial plain CT of the brain ruled out ischemia, intracranial hemorrhage, and space-occupying lesion.
Once her BP was stabilized, she was referred to us for detailed eye assessment. Her best corrected visual acuity was 6/12 bilaterally. In contrast to her right eye pupil, which was 4 mm in diameter and reactive, her left eye pupil was 7 mm and poorly reactive to light. Optic nerve functions were preserved. There was complete ptosis of the left eye, with exotropia and total limitation of elevation, depression, and abduction (FIGURE 2). There was no proptosis; intraocular pressure was normal. Fundus examination of the left eye was unremarkable. All other CN and neurologic examinations were normal. We diagnosed left eye pupil-involving TNP.

Further assessment of the brain with magnetic resonance imaging (MRI) revealed a left posterior communicating artery aneurysm. We performed cerebral angiography (FIGURE 3) with coiling. Postoperatively, her ptosis resolved at 2 months but with residual left eye exotropia.

CASE 3
Viral infection
A 20-year-old male student presented to the ED for evaluation of acute-onset diplopia that was present upon awakening from sleep 4 days earlier. There was no ptosis or other neurologic symptoms. He had no history of trauma or viral illness. Examination revealed limited adduction, depression, levo-elevation, levo-depression, and dextro-depression in the right eye (FIGURE 4). Both pupils were reactive. There was no sign of aberrant third nerve regeneration. The optic nerve and other CN functions were intact. A systemic neurologic examination was unremarkable, and the fundus was normal, with no optic disc swelling. All blood work was negative for diabetes, hypercoagulability, and hyperlipidemia.

CT angiography (CTA) and MR angiography (MRA) did not reveal any vascular abnormalities such as intracranial aneurysms, arteriovenous malformations, or berry aneurysm. We treated the patient for right eye partial TNP secondary to presumed prior viral infection that led to an immune-mediated palsy of the third nerve. He was given a short course of low-dose oral prednisolone (30 mg/d for 5 days). He achieved full recovery of his ocular motility after 2 weeks.
Continue to: CASE 4
CASE 4
Trauma
A 33-year-old woman was brought to the ED after she was knocked off her motorbike by a car. A passerby found her unconscious and still wearing her helmet. En route to the hospital, the patient regained consciousness but had retrograde amnesia.
She was referred to us for evaluation of complete ptosis of her left eye. She was fully conscious during the examination. Her left eye vision was 6/9. Complete ptosis with exotropia was noted. Pupillary examination revealed a sluggish dilated left eye pupil of 7 mm with no reverse relative afferent pupillary defect. Extraocular movement was restricted at elevation, depression, and adduction with diplopia (FIGURE 5). All other CN functions were preserved.

CT of the brain and orbit revealed acute right frontotemporal subarachnoid hemorrhage (FIGURE 6). There was no radiologic evidence of orbital wall fractures or extraocular muscle entrapment. She remained stable during the first 24 hours of monitoring and was given a diagnosis of

Repeat CT of the brain 5 days later revealed complete resolution of the subarachnoid hemorrhage. The patient's clinical condition improved 2 weeks later and included resolution of ptosis and recovery of ocular motility.
Key takeaways from the cases
Case 1: Herpes zoster ophthalmicus
Clinical diagnosis of HZO is straightforward, with painful vesicular lesions occurring along the trigeminal nerve (V1) dermatome, as was seen in this case. The oculomotor nerve is the CN most commonly involved; the trochlear nerve is the least-often affected.6 In a report from the Mayo Clinic, 3 of 86 patients with HZO had oculomotor nerve palsies (3.4%).7 A separate review from an eye hospital study stated that 9.8% (n = 133) of 1356 patients with HZO had extraocular muscle palsy, with TNP in 4 of the patients.8
Ocular complications such as blepharitis, keratoconjunctivits, or iritis occur in 20% to 70% of HZO cases.9 Ophthalmoplegia, which most often involves the oculomotor nerve, is seen in 7% to 31% of HZO cases (mostly in the elderly) and usually occurs within 1 to 3 weeks of the onset of rash.6 Our patient immediately underwent contrast CT of the brain to rule out meningitis and nerve compression.
Treatment with a systemic antiviral agent is crucial. Acyclovir, valaciclovir, and famciclovir are available treatment options, used for treating the skin lesions, reducing the viral load, and reducing the risk for ocular involvement or its progression. Our patient started a 2-week course of oral acyclovir 800 mg 5 times per day. Ophthalmoplegia is usually self-limiting and has a good prognosis. Time to resolution varies from 2 to 18 months. Diplopia, if present, resolves within 1 year.6 Our patient achieved full recovery of his extraocular movement after completing 4 weeks of antiviral treatment.
Continue to: Case 2
Case 2: Posterior communicating artery aneurysm
Given the patient’s high BP, ruling out a hypertensive emergency with CT was the first priority. TNP caused by microvascular ischemia is not uncommon in the elderly. However, her pupil involvement and persistent headache called for an MRI to better evaluate the soft tissues and to rule out possible vascular pathologies. Left posterior communicating artery aneurysm was discovered with MRI, and urgent cerebral angiography and coiling was performed successfully.
Incidence. One report of 1400 patients with TNP confirmed that aneurysm was the cause in 10% of cases, with posterior communicating artery aneurysm accounting for the greatest number, 119 (25.7%).10 Of these cases of posterior communicating artery aneurysm, pupillary involvement was detected in 108 (90.8%). The oculomotor nerve lies adjacent to the posterior communicating artery as it passes through the subarachnoid space of the basal cisterns, where it is susceptible to compression.3
A high index of suspicion for posterior communicating artery aneurysm is crucial for early detection and lifesaving treatment. The patient in this case did well after the coiling. Her ptosis resolved at 2 months, although she had residual left eye exotropia.
Case 3: Viral infection
We chose CTA of the brain instead of contrast CT to rule out the possibility of intracranial aneurysm. CTA has been shown to be an adequate first-line study to detect aneurysms, particularly those greater than 4 mm in diameter.2,11 One study demonstrated an 81.8% sensitivity for aneurysms smaller than 3 mm when performed on a 320-slice CT.12
Additional imaging selection. We also selected MRA to rule out berry aneurysm, which is often asymptomatic. We decided against MRI because of its higher cost and longer acquisition time. It is usually reserved for patients with a negative initial work-up with CT or cerebral angiography if suspicion of a possible aneurysm remains.11 The MRA finding in this case was negative, and we made a presumptive diagnosis of TNP secondary to viral infection.
Isolated TNP following viral infection is a clinical diagnosis of exclusion. In 1 reported case, a 39-year-old man developed a superior division palsy after a common cold without fever, underwent no serologic study, and recovered spontaneously 6 weeks later.13 A 5-year-old boy who experienced a superior division palsy immediately after a common cold with fever was found on serologic examination to have an increased titre of influenza A virus. His palsy resolved in 4 months.14
The exact mechanism of viral-induced palsy is unknown. The possibility of postinfectious cranial neuropathy has been postulated, as most reported cases following a flu-like illness resolved within a few months.15 Although the pathogenesis remains speculative, an autoimmune process might have been involved.16 Our patient recovered fully in 1 month following a short course of oral prednisolone 30 mg/d for 5 days.
Case 4: Trauma
Trauma accounts for approximately 12% of all TNP cases.17 Traumatic TNPs are usually sustained in severe, high-speed, closed-head injuries, and are often associated with other CN injuries and neurologic deficits. The damage may be caused indirectly by compression, hemorrhage, or ischemia, or directly at certain vulnerable points including the nerve’s exit from the brainstem and the point at which it crosses the petroclinoid ligament.17 In our case, despite the patient having complete TNP, there was no sign of localized orbital trauma on the CT other than the presence of subarachnoid hemorrhage at the right frontotemporal region.
In a similar reported case, the patient had a right traumatic isolated TNP and was found to have left frontal subarachnoid hemorrhage with no sign of orbital trauma.18 However, the mechanisms of isolated TNP caused by traumatic brain injury are not clear. Possible causes include rootlet avulsion, distal fascicular damage, stretching of the nerve (including the parasellar segment), and decreased blood supply.18
It has been suggested that TNP is more frequently observed in cases of frontal region injury. As orbitofrontal regions are predominantly affected by cortical contusions, the risk for ocular involvement increases.19
Keep these fundamentals in mind
The diagnosis and management of isolated TNP are guided by the patient’s age, by the degree to which each of the oculomotor nerve’s 2 major functions—pupillomotor and oculomotor—are affected, and by the circumstances preceding the onset of TNP.2 Cases 1 and 3 in our series presented with partial TNP, while Cases 2 and 4 exhibited complete TNP. Pupillary involvement was detected only in Case 2. Nevertheless, radiologic imaging was ordered for all 4 cases after the diagnosis of TNP was made, to exclude the most worrying neurologic emergencies. The choice of imaging modality depends on not only the availability of the services but also the clinical signs and symptoms and presumptive clinical diagnosis. A tailored and thoughtful approach with consideration of the anatomy and varied pathologies help clinicians to skillfully discern emergencies from nonurgent cases.
CORRESPONDENCE
Lott Pooi Wah, MSOphth, FRCOphth, Department of Ophthalmology, Faculty of Medicine, Universiti Malaya, 50603 Kuala Lumpur, Malaysia; [email protected] Orcid no: 0000-0001-8746-1528
Of all the cranial nerve (CN) palsies that affect the eye, the third (oculomotor) nerve palsy (TNP) requires the most urgent evaluation.1 Third nerve dysfunction may signal an underlying neurologic emergency, such as ruptured cerebral aneurysm or giant cell arteritis. Early recognition and prompt treatment choices are key to reversing clinical and visual defects. The classic presentation of isolated TNP is a “down and out eye” deviation and ptosis with or without pupillary involvement.1
Recognize varying clinical presentations. TNPs, isolated or not, may be partial or complete, congenital or acquired, pupil involving or pupil sparing. In many cases, patients may have additional constitutional, ocular, or neurologic symptoms or signs, such as ataxia or hemiplegia.2 Recognition of these clinical findings, which at times can be subtle, is crucial. Appropriate clinical diagnosis and management rely on distinguishing isolated TNP from TNP that involves other CNs.2
Further clues to underlying pathology. Disruption of the third nerve can occur anywhere along its course from the oculomotor nucleus in the brain to its terminus at the extraocular muscles in the orbit.2 TNP’s effect on the pupil can often aid in diagnosis.3 Pupil-sparing TNP is usually due to microvascular ischemia, as may occur with diabetes or hypertension. Pupil involvement, though, may be the first sign of a compressive lesion.
Influence of age. Among individuals older than 60 years, the annual incidence of isolated TNP has been shown to be 12.5 per 100,000, compared with 1.7 per 100,000 in those younger than 60 years.4 In those older than 50 years, microvascular ischemia tends to be the dominant cause.4 Other possible causes include aneurysm, trauma, and neoplasm, particularly pituitary adenoma and metastatic tumor. In childhood and young adulthood, the most common cause of TNP is trauma.5
Use of vascular imaging is influenced by an individual’s age and clinical risk for an aneurysm. Isolated partial TNP or TNP with pupil involvement suggest compression of the third nerve and the need for immediate imaging. Given the dire implications of intracranial aneurysm, most physicians will focus their initial evaluation on vascular imaging, if available.2 If clinical findings instead suggest underlying microvascular ischemia, a delay of imaging may be possible.
In the text that follows, we present 4 patient cases describing the clinical investigative process and treatment determinations based on an individual’s history, clinical presentation, and neurologic findings.
CASE 1
Herpes zoster ophthalmicus
An 84-year-old man with no known medical illness presented to the emergency department (ED) with vesicular skin lesions that had appeared 4 days earlier over his scalp, right forehead, and periorbital region. The vesicles followed the distribution of the ophthalmic division of the trigeminal nerve (FIGURE 1). The patient was given a diagnosis of shingles. The only notable ocular features were the swollen right upper eyelid, injected conjunctiva, and reduced corneal sensation with otherwise normal right eye vision at 6/6. For right eye herpes zoster ophthalmicus (HZO), he was prescribed oral acyclovir 800 mg 5 times per day for 2 weeks.

Continue to: Two days later...
Two days later, he returned after experiencing a sudden onset of binocular diplopia and ptosis of the right eye. Partial ptosis was noted, with restricted adduction and elevation. Pupils were reactive and equal bilaterally. Hutchinson sign, which would indicate an impaired nasociliary nerve and increased risk for corneal and ocular sequelae,6 was absent. Relative afferent pupillary defect also was absent. All other CN functions were intact, with no systemic neurologic deficit. Contrast CT of the brain and orbit showed no radiologic evidence of meningitis, space-occupying lesion, or cerebral aneurysm.
Given the unremarkable imaging findings and lack of symptoms of meningism (eg, headache, vomiting, neck stiffness, or fever), we diagnosed right eye pupil-sparing partial TNP secondary to HZO. The patient continued taking oral acyclovir, which was tapered over 6 weeks. After 4 weeks of antiviral treatment, he recovered full extraocular movement and the ptosis subsided.
CASE 2
Posterior communicating artery aneurysm
A 71-year-old woman with hypercholesterolemia, hypertension, and ischemic heart disease presented to the ED with a 4-day history of headache, vomiting, and neck pain and a 2-day history of a drooping left eyelid. When asked if she had double vision, she said “No.” She had no other neurologic symptoms. Her blood pressure (BP) was 199/88 mm Hg. An initial plain CT of the brain ruled out ischemia, intracranial hemorrhage, and space-occupying lesion.
Once her BP was stabilized, she was referred to us for detailed eye assessment. Her best corrected visual acuity was 6/12 bilaterally. In contrast to her right eye pupil, which was 4 mm in diameter and reactive, her left eye pupil was 7 mm and poorly reactive to light. Optic nerve functions were preserved. There was complete ptosis of the left eye, with exotropia and total limitation of elevation, depression, and abduction (FIGURE 2). There was no proptosis; intraocular pressure was normal. Fundus examination of the left eye was unremarkable. All other CN and neurologic examinations were normal. We diagnosed left eye pupil-involving TNP.

Further assessment of the brain with magnetic resonance imaging (MRI) revealed a left posterior communicating artery aneurysm. We performed cerebral angiography (FIGURE 3) with coiling. Postoperatively, her ptosis resolved at 2 months but with residual left eye exotropia.

CASE 3
Viral infection
A 20-year-old male student presented to the ED for evaluation of acute-onset diplopia that was present upon awakening from sleep 4 days earlier. There was no ptosis or other neurologic symptoms. He had no history of trauma or viral illness. Examination revealed limited adduction, depression, levo-elevation, levo-depression, and dextro-depression in the right eye (FIGURE 4). Both pupils were reactive. There was no sign of aberrant third nerve regeneration. The optic nerve and other CN functions were intact. A systemic neurologic examination was unremarkable, and the fundus was normal, with no optic disc swelling. All blood work was negative for diabetes, hypercoagulability, and hyperlipidemia.

CT angiography (CTA) and MR angiography (MRA) did not reveal any vascular abnormalities such as intracranial aneurysms, arteriovenous malformations, or berry aneurysm. We treated the patient for right eye partial TNP secondary to presumed prior viral infection that led to an immune-mediated palsy of the third nerve. He was given a short course of low-dose oral prednisolone (30 mg/d for 5 days). He achieved full recovery of his ocular motility after 2 weeks.
Continue to: CASE 4
CASE 4
Trauma
A 33-year-old woman was brought to the ED after she was knocked off her motorbike by a car. A passerby found her unconscious and still wearing her helmet. En route to the hospital, the patient regained consciousness but had retrograde amnesia.
She was referred to us for evaluation of complete ptosis of her left eye. She was fully conscious during the examination. Her left eye vision was 6/9. Complete ptosis with exotropia was noted. Pupillary examination revealed a sluggish dilated left eye pupil of 7 mm with no reverse relative afferent pupillary defect. Extraocular movement was restricted at elevation, depression, and adduction with diplopia (FIGURE 5). All other CN functions were preserved.

CT of the brain and orbit revealed acute right frontotemporal subarachnoid hemorrhage (FIGURE 6). There was no radiologic evidence of orbital wall fractures or extraocular muscle entrapment. She remained stable during the first 24 hours of monitoring and was given a diagnosis of

Repeat CT of the brain 5 days later revealed complete resolution of the subarachnoid hemorrhage. The patient's clinical condition improved 2 weeks later and included resolution of ptosis and recovery of ocular motility.
Key takeaways from the cases
Case 1: Herpes zoster ophthalmicus
Clinical diagnosis of HZO is straightforward, with painful vesicular lesions occurring along the trigeminal nerve (V1) dermatome, as was seen in this case. The oculomotor nerve is the CN most commonly involved; the trochlear nerve is the least-often affected.6 In a report from the Mayo Clinic, 3 of 86 patients with HZO had oculomotor nerve palsies (3.4%).7 A separate review from an eye hospital study stated that 9.8% (n = 133) of 1356 patients with HZO had extraocular muscle palsy, with TNP in 4 of the patients.8
Ocular complications such as blepharitis, keratoconjunctivits, or iritis occur in 20% to 70% of HZO cases.9 Ophthalmoplegia, which most often involves the oculomotor nerve, is seen in 7% to 31% of HZO cases (mostly in the elderly) and usually occurs within 1 to 3 weeks of the onset of rash.6 Our patient immediately underwent contrast CT of the brain to rule out meningitis and nerve compression.
Treatment with a systemic antiviral agent is crucial. Acyclovir, valaciclovir, and famciclovir are available treatment options, used for treating the skin lesions, reducing the viral load, and reducing the risk for ocular involvement or its progression. Our patient started a 2-week course of oral acyclovir 800 mg 5 times per day. Ophthalmoplegia is usually self-limiting and has a good prognosis. Time to resolution varies from 2 to 18 months. Diplopia, if present, resolves within 1 year.6 Our patient achieved full recovery of his extraocular movement after completing 4 weeks of antiviral treatment.
Continue to: Case 2
Case 2: Posterior communicating artery aneurysm
Given the patient’s high BP, ruling out a hypertensive emergency with CT was the first priority. TNP caused by microvascular ischemia is not uncommon in the elderly. However, her pupil involvement and persistent headache called for an MRI to better evaluate the soft tissues and to rule out possible vascular pathologies. Left posterior communicating artery aneurysm was discovered with MRI, and urgent cerebral angiography and coiling was performed successfully.
Incidence. One report of 1400 patients with TNP confirmed that aneurysm was the cause in 10% of cases, with posterior communicating artery aneurysm accounting for the greatest number, 119 (25.7%).10 Of these cases of posterior communicating artery aneurysm, pupillary involvement was detected in 108 (90.8%). The oculomotor nerve lies adjacent to the posterior communicating artery as it passes through the subarachnoid space of the basal cisterns, where it is susceptible to compression.3
A high index of suspicion for posterior communicating artery aneurysm is crucial for early detection and lifesaving treatment. The patient in this case did well after the coiling. Her ptosis resolved at 2 months, although she had residual left eye exotropia.
Case 3: Viral infection
We chose CTA of the brain instead of contrast CT to rule out the possibility of intracranial aneurysm. CTA has been shown to be an adequate first-line study to detect aneurysms, particularly those greater than 4 mm in diameter.2,11 One study demonstrated an 81.8% sensitivity for aneurysms smaller than 3 mm when performed on a 320-slice CT.12
Additional imaging selection. We also selected MRA to rule out berry aneurysm, which is often asymptomatic. We decided against MRI because of its higher cost and longer acquisition time. It is usually reserved for patients with a negative initial work-up with CT or cerebral angiography if suspicion of a possible aneurysm remains.11 The MRA finding in this case was negative, and we made a presumptive diagnosis of TNP secondary to viral infection.
Isolated TNP following viral infection is a clinical diagnosis of exclusion. In 1 reported case, a 39-year-old man developed a superior division palsy after a common cold without fever, underwent no serologic study, and recovered spontaneously 6 weeks later.13 A 5-year-old boy who experienced a superior division palsy immediately after a common cold with fever was found on serologic examination to have an increased titre of influenza A virus. His palsy resolved in 4 months.14
The exact mechanism of viral-induced palsy is unknown. The possibility of postinfectious cranial neuropathy has been postulated, as most reported cases following a flu-like illness resolved within a few months.15 Although the pathogenesis remains speculative, an autoimmune process might have been involved.16 Our patient recovered fully in 1 month following a short course of oral prednisolone 30 mg/d for 5 days.
Case 4: Trauma
Trauma accounts for approximately 12% of all TNP cases.17 Traumatic TNPs are usually sustained in severe, high-speed, closed-head injuries, and are often associated with other CN injuries and neurologic deficits. The damage may be caused indirectly by compression, hemorrhage, or ischemia, or directly at certain vulnerable points including the nerve’s exit from the brainstem and the point at which it crosses the petroclinoid ligament.17 In our case, despite the patient having complete TNP, there was no sign of localized orbital trauma on the CT other than the presence of subarachnoid hemorrhage at the right frontotemporal region.
In a similar reported case, the patient had a right traumatic isolated TNP and was found to have left frontal subarachnoid hemorrhage with no sign of orbital trauma.18 However, the mechanisms of isolated TNP caused by traumatic brain injury are not clear. Possible causes include rootlet avulsion, distal fascicular damage, stretching of the nerve (including the parasellar segment), and decreased blood supply.18
It has been suggested that TNP is more frequently observed in cases of frontal region injury. As orbitofrontal regions are predominantly affected by cortical contusions, the risk for ocular involvement increases.19
Keep these fundamentals in mind
The diagnosis and management of isolated TNP are guided by the patient’s age, by the degree to which each of the oculomotor nerve’s 2 major functions—pupillomotor and oculomotor—are affected, and by the circumstances preceding the onset of TNP.2 Cases 1 and 3 in our series presented with partial TNP, while Cases 2 and 4 exhibited complete TNP. Pupillary involvement was detected only in Case 2. Nevertheless, radiologic imaging was ordered for all 4 cases after the diagnosis of TNP was made, to exclude the most worrying neurologic emergencies. The choice of imaging modality depends on not only the availability of the services but also the clinical signs and symptoms and presumptive clinical diagnosis. A tailored and thoughtful approach with consideration of the anatomy and varied pathologies help clinicians to skillfully discern emergencies from nonurgent cases.
CORRESPONDENCE
Lott Pooi Wah, MSOphth, FRCOphth, Department of Ophthalmology, Faculty of Medicine, Universiti Malaya, 50603 Kuala Lumpur, Malaysia; [email protected] Orcid no: 0000-0001-8746-1528
1. Radia M, Stahl M, Arunakirinathan M, et al. Examination of a third nerve palsy. Brit J Hosp Med. 2017;78:188-192. doi: 10.12968/hmed.2017.78.12.C188
2. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257-268. doi: 10.1055/s-2007-979681
3. Motoyama Y, Nonaka J, Hironaka Y, et al. Pupil-sparing oculomotor nerve palsy caused by upward compression of a large posterior communicating artery aneurysm. Case report. Neurol Med Chir (Tokyo). 2012;52:202-205. doi: 10.2176/nmc.52.202
4. Fang C, Leavitt JA, Hodge DO, et al. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23-28. doi: 10.1001/jamaophthal mol.2016.4456
5. Wyatt K. Three common ophthalmic emergencies. JAAPA. 2014;27:32-37. doi: 10.1097/01.JAA.0000447004.96714.34
6. Daswani M, Bhosale N, Shah VM. Rare case of herpes zoster ophthalmicus with orbital myositis, oculomotor nerve palsy and anterior uveitis. Indian J Dermatol Venereol Leprol. 2017;83:365-367. doi: 10.4103/0378-6323.199582
7. Womack LW, Liesegang TJ. Complications of herpes zoster ophthalmicus. Arch Ophthalmol. 1983;101:42-45. doi: 10.1001/archopht.1983.01040010044004
8. Marsh RJ, Dulley B, Kelly V. External ocular motor palsies in ophthalmic zoster: a review. Br J Ophthalmol. 1977;61:667-682. doi: 10.1136/bjo.61.11.677
9. Lim JJ, Ong YM, Zalina MCW, et al. Herpes zoster ophthalmicus with orbital apex syndrome – difference in outcomes and literature review. Ocul Immunol Inflamm. 2017;26:187-193. doi: 10.1080/09273948.2017.1327604
10. Keane JR. Third nerve palsy: analysis of 1400 personally-examined patients. Can J Neurol Sci. 2010;37:662-670. doi: 10.1017/s0317167100010866
11. Yoon NK, McNally S, Taussky P, et al. Imaging of cerebral aneurysms: a clinical perspective. Neurovasc Imaging. 2016;2:6. doi: 10.1186/s40809-016-0016-3
12. Wang H, Li W, He H, et al. 320-detector row CT angiography for detection and evaluation of intracranial aneurysms: comparison with conventional digital subtraction angiography. Clin Radiol. 2013;68:e15-20. doi: 10.1016/j.crad.2012.09.001
13. Derakhshan I. Superior branch palsy with spontaneous recovery. Ann Neurol. 1978;4:478-479. doi: 10.1002/ana.410040519
14. Engelhardt A, Credzich C, Kompf D. Isolated superior branch palsy of the oculomotor nerve in influenza A. Neuroophthalmol. 1989;9:233-235. doi: 10.3109/01658108908997359
15. Knox DL, Clark DB, Schuster FF. Benign VI nerve palsies in children. Pediatrics. 1967;40:560-564.
16. Saeki N, Yotsukura J, Adachi E, et al. Isolated superior division oculomotor palsy in a child with spontaneous recovery. J Clin Neurosci. 2000;7:62-64. doi: 10.1054/jocn.1998.0152
17. Nagendran ST, Lee V, Perry M. Traumatic orbital third nerve palsy. Brit J Oral Maxillofac Surg. 2019;57:578-581. doi: 10.1016/j.bjoms.2019.01.029
18. Kim T, Nam K, Kwon BS. Isolated oculomotor nerve palsy in mild traumatic brain injury: a literature review. Am J Phys Med Rehabil. 2020;99:430-435. doi: 10.1097/PHM.0000000000001316
19. Sharma B, Gupta R, Anand R, et al. Ocular manifestations of head injury and incidence of post-traumatic ocular motor nerve involvement in cases of head injury: a clinical review. Int Ophthalmol. 2014;34:893-900. doi: 10.1007/s10792-014-9898-8
1. Radia M, Stahl M, Arunakirinathan M, et al. Examination of a third nerve palsy. Brit J Hosp Med. 2017;78:188-192. doi: 10.12968/hmed.2017.78.12.C188
2. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257-268. doi: 10.1055/s-2007-979681
3. Motoyama Y, Nonaka J, Hironaka Y, et al. Pupil-sparing oculomotor nerve palsy caused by upward compression of a large posterior communicating artery aneurysm. Case report. Neurol Med Chir (Tokyo). 2012;52:202-205. doi: 10.2176/nmc.52.202
4. Fang C, Leavitt JA, Hodge DO, et al. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23-28. doi: 10.1001/jamaophthal mol.2016.4456
5. Wyatt K. Three common ophthalmic emergencies. JAAPA. 2014;27:32-37. doi: 10.1097/01.JAA.0000447004.96714.34
6. Daswani M, Bhosale N, Shah VM. Rare case of herpes zoster ophthalmicus with orbital myositis, oculomotor nerve palsy and anterior uveitis. Indian J Dermatol Venereol Leprol. 2017;83:365-367. doi: 10.4103/0378-6323.199582
7. Womack LW, Liesegang TJ. Complications of herpes zoster ophthalmicus. Arch Ophthalmol. 1983;101:42-45. doi: 10.1001/archopht.1983.01040010044004
8. Marsh RJ, Dulley B, Kelly V. External ocular motor palsies in ophthalmic zoster: a review. Br J Ophthalmol. 1977;61:667-682. doi: 10.1136/bjo.61.11.677
9. Lim JJ, Ong YM, Zalina MCW, et al. Herpes zoster ophthalmicus with orbital apex syndrome – difference in outcomes and literature review. Ocul Immunol Inflamm. 2017;26:187-193. doi: 10.1080/09273948.2017.1327604
10. Keane JR. Third nerve palsy: analysis of 1400 personally-examined patients. Can J Neurol Sci. 2010;37:662-670. doi: 10.1017/s0317167100010866
11. Yoon NK, McNally S, Taussky P, et al. Imaging of cerebral aneurysms: a clinical perspective. Neurovasc Imaging. 2016;2:6. doi: 10.1186/s40809-016-0016-3
12. Wang H, Li W, He H, et al. 320-detector row CT angiography for detection and evaluation of intracranial aneurysms: comparison with conventional digital subtraction angiography. Clin Radiol. 2013;68:e15-20. doi: 10.1016/j.crad.2012.09.001
13. Derakhshan I. Superior branch palsy with spontaneous recovery. Ann Neurol. 1978;4:478-479. doi: 10.1002/ana.410040519
14. Engelhardt A, Credzich C, Kompf D. Isolated superior branch palsy of the oculomotor nerve in influenza A. Neuroophthalmol. 1989;9:233-235. doi: 10.3109/01658108908997359
15. Knox DL, Clark DB, Schuster FF. Benign VI nerve palsies in children. Pediatrics. 1967;40:560-564.
16. Saeki N, Yotsukura J, Adachi E, et al. Isolated superior division oculomotor palsy in a child with spontaneous recovery. J Clin Neurosci. 2000;7:62-64. doi: 10.1054/jocn.1998.0152
17. Nagendran ST, Lee V, Perry M. Traumatic orbital third nerve palsy. Brit J Oral Maxillofac Surg. 2019;57:578-581. doi: 10.1016/j.bjoms.2019.01.029
18. Kim T, Nam K, Kwon BS. Isolated oculomotor nerve palsy in mild traumatic brain injury: a literature review. Am J Phys Med Rehabil. 2020;99:430-435. doi: 10.1097/PHM.0000000000001316
19. Sharma B, Gupta R, Anand R, et al. Ocular manifestations of head injury and incidence of post-traumatic ocular motor nerve involvement in cases of head injury: a clinical review. Int Ophthalmol. 2014;34:893-900. doi: 10.1007/s10792-014-9898-8
PRACTICE RECOMMENDATIONS
› Consider microvascular ischemia if third nerve palsy is pupil sparing. C
› Consider computerized tomography (CT) angiography as an alternative to plain CT for first-line study of suspected aneurysm. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series