User login
Suspected Orbital Compartment Syndrome Leading to Visual Loss After Pterional Craniotomy
Perioperative visual loss (POVL) is a well-documented yet uncommon complication of nonocular surgery. Patients undergoing cardiac and spinal surgery are at the greatest risk, though POVL may occur during other neurosurgical and vascular procedures as well. The most common causes of POVL are central retinal artery occlusion (CRAO) and ischemic optic neuropathy (ION),1-3 though cases of orbital compartment syndrome (OCS) have also been reported.4-7
We describe a case of POVL during a temporal meningioma excision using the pterional approach. Though the etiology is not fully understood, the patient’s clinical course was complicated by a third cranial nerve (CN III) palsy and CRAO, which, together with the patient’s presentation, were consistent with previously documented cases of OCS. The goals of this case report are to increase awareness of this surgical outcome, identify practices that may have contributed to its development, and delineate methods to minimize its occurrence.
Informed consent regarding this research was obtained from the patient and an institutional Health Insurance Portability and Accountability Act authorization form was completed. This manuscript adheres to the applicable Enhancing the Quality and Transparency of Health Research guideline.8
Case Presentation
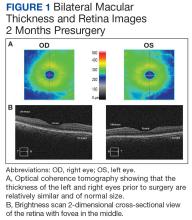
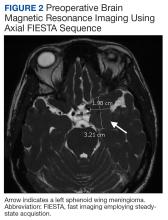
A 47-year-old woman underwent a left temporal craniotomy for resection of a sphenoid wing meningioma discovered during a workup for persistent headaches. She had no medical history of diabetes, hypertension, coronary artery disease, or ophthalmic disease. Two months before her scheduled surgery, the patient reported bilateral blurry vision and underwent ophthalmologic evaluation. Her intraocular pressure (IOP) was normal, and she had no pupillary or retinal disease. She showed evidence of decreased vision in her left eye, suggesting a possible mass effect from her meningioma. Subsequent imaging of the optic nerve and retina had unremarkable physiology (Figure 1). Preoperative magnetic resonance imaging (MRI) demonstrated a stable enhancing mass involving the left great sphenoid wing and left cavernous sinus(Figure 2). There was a superior mass effect on the left middle cerebral artery, but all vessels were patent without evidence of thrombosis.
The patient underwent general anesthesia with invasive hemodynamic monitoring used throughout the procedure. She was induced with fentanyl, propofol, and rocuronium; anesthesia was maintained with isoflurane and a remifentanil infusion. Hypotension was treated with phenylephrine and intravenous fluids. Intraoperative neuromonitoring with electroencephalogram (EEG) and somatosensory evoked potentials was performed. During the surgery, the patient was positioned supine in a Mayfield 3-point head fixation system. All pressure points were padded appropriately and continually checked. A standard left pterional craniotomy was performed, and the scalp was reflected anteriorly and secured using fish hooks with rubber bands. The operation did not violate the cavernous sinus or orbital compartment. There was no evidence of active bleeding upon inspection nor with the Valsalva maneuver. No changes were noted in EEG or somatosensory evoked potentials; blood pressure remained within 20 mm Hg of the patient’s baseline. She was extubated at the end of the 10-hour case and was hemodynamically stable upon transport to the surgical intensive care unit. Postoperative imaging confirmed the successful removal of the left sphenoid wing meningioma.
The patient’s postoperative examination demonstrated a 5 mm dilated, nonresponsive left pupil, though the patient did not report visual loss at that time. Defects were noted in the inferior oblique, superior, inferior, and medial rectus muscles, consistent with CN III palsy. The surgery included manipulation of CN III, which made this a possible outcome, but an alternate causative pathology like OCS was not immediately suspected. Postoperative computed tomography (CT) showed an expected pneumocephalus and left scalp swelling without evidence of mass effect or midline shift.
On the morning of postoperative Day 1, the patient reported vision loss in her left eye, while her clinical examination revealed erythema and conjunctival chemosis with left eyelid swelling. The ophthalmologic evaluation was notable for a continued leftCN III palsy with intact lateral rectus and superior oblique function, a nonreactive and dilated left eye with 3+ afferent pupillary defect by reverse (light perception), pallor throughout, a flat cherry red macula with blurred disc margins, left upper eyelid edema, and 18 mm Hg intraocular pressure bilaterally (reference range, 8 to 21 mm Hg). Fundoscopic examination showed a clear vitreous without plaques or occlusions, no perivascular sheathing, and no retinal hemorrhages. CT angiography revealed small outpouchings at the superolateral aspect of the left and right cavernous carotid, consistent with atherosclerotic calcifications. An echocardiogram revealed a Valsalva-dependent patent foramen ovale, but a venous Doppler ultrasound yielded negative results.
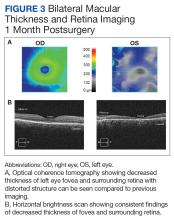
Repeat MRI showed denervation of the left medial rectus and minimal left-sided proptosis. A 3-month ophthalmologic follow-up revealed a persistent CN III palsy, including an afferent pupillary defect, absence of light perception in her left eye, and continued ophthalmoplegia. Repeat examination showed a left-sided 4+ afferent pupillary defect unreactive to light, 4+ pallor surrounding the optic nerve, macular atrophy, sclerotic vessels, and 17 mm Hg intraocular pressure bilaterally. The eye had diffuse atrophy of the inner retina and significant patchy atrophy of the outer retinal components without neovascularization of the iris. Postoperative retinal imaging can be seen in Figure 3. Her vision loss persisted at this encounter and has continued through subsequent follow-up examinations.
Discussion
Perioperative visual loss is a rare surgical complication, with an estimated incidence of once in every 60,000 to 125,000 cases.9 The mechanism of injury is variable and dependent upon the type of surgical intervention, with cardiac and spine surgeries carrying the greatest risk.10,11 The injury often results in either CRAO or ION, which may result in visual loss.1-3 POVL can also occur in the aftermath of rapid changes in intracranial pressure during decompressive craniotomies, though the pathophysiology in such cases is not well understood.5
Among the myriad ways in which POVL can occur, neurosurgical cases carry the unique risk of direct cranial nerve injury. Such an insult can lead to vision loss via optic nerve damage or ophthalmoplegia if damage occurs to CN III, IV, or VI. This can occur during manipulation or resection, especially if the surgical approach involves the orbital cavity or the cavernous sinus. Though neither space was entered in this patient, direct injury cannot be ruled out as the etiology for either her vision loss or persistent ophthalmoplegia. An alternate causative scenario for both symptoms involve an impaired blood supply, with the vision loss potentially occurring secondary to CRAO and the ophthalmoplegia to an alternate cause of decreased blood flow. It is unclear which of these 2 conditions occurred first or if they occurred due to the same insult, but OCS could lead to both. Though it is a less common etiology for POVL, this patient’s presentation was similar to those in previously reported cases, and OCS was identified as the likely diagnosis.
OCS is precipitated by an elevated orbital pressure, which leads to ischemia of the retina and damage to orbital contents. Though associated with retrobulbar hemorrhage and orbital trauma, another proposed mechanism for OCS is extrinsic orbital compression, resulting in increased IOP and subsequent CRAO.10 A cherry red spot is visible on fundoscopy, as only the macula with its thin retinal layer will permit the choroidal vessels to be visualized. In a separate process, the relative increase in orbital pressure may lead to impaired perfusion or damage of CN III. However, a causative relationship between the 2 may be difficult to establish. Such an injury to the oculomotor nerve is demonstrated by impaired function of the inferior oblique, superior rectus, inferior rectus, and medial rectus muscles, which may persist even after the compressive symptoms of OCS have resolved.12 Other reported symptoms of OCS include erythema, ophthalmoplegia, conjunctival chemosis, ptosis, corneal abrasion, and eyelid edema.12-15
Alternate Diagnoses
OCS is a diagnosis of exclusion, and several alternate mechanisms were considered before identifying it as the likely etiology. The patient’s preoperative imaging demonstrated a stable enhancing mass involving the left great sphenoid wing and left cavernous sinus, with displacement of the left middle cerebral artery, left cavernous internal carotid artery, and left optic canal. Dissection and removal of this tumor could have compromised the arterial or venous blood supply to the orbit, thus causing ischemia to the retina and other ocular structures. CN III was manipulated during surgery, and it may have been inadvertently damaged during exposure or resection of the tumor.
The patient’s Valsalva-dependent patent foramen ovale put her at risk of a paroxysmal embolus as an alternate explanation, particularly as a Valsalva maneuver was utilized to confirm hemostasis. The patient did not, however, demonstrate any evidence of venous thromboembolism (VTE) on ultrasound, nor did she have the common risk factors of hypertension, diabetes, or smoking history that would increase VTE risk.16 Her cancer diagnosis and surgical status may have put her at risk of VTE, but she did not have any clinical or laboratory values suggestive of hypercoagulability. Had an embolism occurred, it may have compromised the orbital blood supply and led to the CRAO. A similar scenario may have occurred from an atherosclerotic plaque in either of her carotid arteries, as she did have evidence of atherosclerosis on postoperative CT angiography. However, atherosclerosis as a risk factor for POVL appears to be related more to its impact upon impaired blood supply rather than as an embolic source. The patient did not have any significant intraoperative hypotensive episodes, making ION in the setting of atherosclerosis and hypotension a less likely etiology.17
This patient differed from other reported OCS cases. She was never placed in a prone or jackknife position, nor was she agitated or straining for a sustained period. These factors, along with the fact that the orbital compartment was not entered, decreased the likelihood of intraorbital hemorrhage and other intrinsic causes of elevated IOP.12 Additionally, the presentation of our patient’s vision loss was delayed compared with other cases, despite clinicians observing a dilated left pupil and CN III palsy on examination immediately after surgery.14 It is significant to note that OCS may not demonstrate a significant increase in IOP once the source of compression is removed, which may explain the absence of proptosis on her postoperative examination.
The diagnosis of OCS was primarily implicated by the positioning of the myocutaneous flap during the pterional approach to craniotomy. It was retracted anteriorly and superiorly, ultimately resting over her left orbit for most of the 10-hour surgery. Kim and colleagues found that myocutaneous flaps may increase IOP as much as 17.5 mm Hg if improperly positioned, providing an unrecognized source of compression and increasing the risk of damage to orbital contents. According to their review, elevated IOP > 40 mm Hg, particularly over several hours, can compromise blood flow to the optic nerve and increase the risk for POVL.18 The flap was secured using fish hooks and rubber bands. However, it is suspected that the orbital rim did not fully support its pressure, thereby resting to some degree directly on the globe for an extended period and compromising the orbital blood supply. There are no current methods for measuring intraoperative IOP, though surrogate markers are under investigation and may yield clinical utility.18 The myocutaneous flap was created and positioned by the surgeons, but it may be that increased vigilance and communication from the anesthesia and nursing teams could have prevented it from remaining in an improper position.
Conclusions
Despite having few reported cases, OCS must be considered in neurosurgical patients with ophthalmoplegia and CRAO on postoperative examinations. Myocutaneous flaps that are retracted across the orbit can lead to significant elevations in IOP, leading to vision loss, which likely occurred with the patient in this case. Though protecting neurovascular structures is within the purview of the surgeon, all members of the intraoperative team should assist with ensuring proper flap positioning. These measures can help ensure adequate blood flow to the ophthalmic artery, decrease the likelihood of elevated IOP due to extrinsic compression, and help prevent the development of POVL and OCS in these patients.
1. Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia: follow the guidelines! Ophthalmology. 2018;125(10):1597-1607. doi:10.1016/j.ophtha.2018.03.054
2. Biousse V, Newman NJ. Ischemic optic neuropathies. N Engl J Med. 2015;372(25):2428-2436. doi:10.1056/NEJMra1413352
3. Shah SH, Chen YF, Moss HE, Rubin DS, Joslin CE, Roth S. Predicting risk of perioperative ischemic optic neuropathy in spine fusion surgery: a cohort study using the national inpatient sample. Anesth Analg. 2020;130(4):967-974. doi:10.1213/ANE.0000000000004383
4. Habets JGV, Haeren RHL, Lie SAN, Bauer NJC, Dings JTA. Acute monocular blindness due to orbital compartment syndrome following pterional craniotomy. World Neurosurg. 2018;114:72-75. doi:10.1016/j.wneu.2018.03.013
5. Vahedi P, Meshkini A, Mohajernezhadfard Z, Tubbs RS. Post-craniotomy blindness in the supine position: Unlikely or ignored? Asian J Neurosurg. 2013;8(1):36-41. doi:10.4103/1793-5482.110278
6. Kang S, Yang Y, Kim T, Kim J. Sudden unilateral blindness after intracranial aneurysm surgery. Acta Neurochir (Wien). 1997;139(3):221-226. doi:10.1007/BF01844755
7. Zimmerman CF, Van Patten PD, Golnik KC, Kopitnik TA Jr, Anand R. Orbital infarction syndrome after surgery for intracranial aneurysms. Ophthalmology. 1995;102(4):594-598. doi:10.1016/s0161-6420(95)30979-7
8. Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 23;2013:bcr2013201554. doi:10.1136/bcr-2013-201554
9. Raphael J, Moss HE, Roth S. Perioperative visual loss in cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33(5):1420-429. doi:10.1053/j.jvca.2018.11.035
10. Kansakar P, Sundar G. Vision loss associated with orbital surgery - a major review. Orbit. 2020;39(3):197-208. doi:10.1080/01676830.2019.1658790
11. Dohlman JC, Yoon MK. Principles of protection of the eye and vision in orbital surgery. J Neurol Surg B Skull Base. 2020;81(4):381-384. doi:10.1055/s-0040-1714077
12. Pahl FH, de Oliveira MF, Dal Col Lúcio JE, Souza E Castro EF. Orbital compartment syndrome after frontotemporal craniotomy: case report and review of literature. World Neurosurg. 2018;109:218-221. doi:10.1016/j.wneu.2017.09.167
13. Grossman W, Ward WT. Central retinal artery occlusion after scoliosis surgery with a horseshoe headrest. Case report and literature review. Spine (Phila Pa 1976). 1993;18(9):1226-1228. doi:10.1097/00007632-199307000-00017
14. Newman NJ. Perioperative visual loss after nonocular surgeries. Am J Ophthalmol. 2008;145(4):604-610. doi:10.1016/j.ajo.2007.09.016
15. Roth S, Tung A, Ksiazek S. Visual loss in a prone-positioned spine surgery patient with the head on a foam headrest and goggles covering the eyes: an old complication with a new mechanism. Anesth Analg. 2007;104(5):1185-1187. doi:10.1213/01.ane.0000264319.57758.55
16. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85. doi:10.1097/01.brs.0000152169.48117.c7
17. Nickels TJ, Manlapaz MR, Farag E. Perioperative visual loss after spine surgery. World J Orthop. 2014;5(2):100-106. Published 2014 April 18. doi:10.5312/wjo.v5.i2.100
18. Kim TS, Hur JW, Park DH, et al. Extraocular ressure measurements to avoid orbital compartment syndrome in aneurysm surgery. World Neurosurg. 2018;118:e601-e609. doi:10.1016/j.wneu.2018.06.248
Perioperative visual loss (POVL) is a well-documented yet uncommon complication of nonocular surgery. Patients undergoing cardiac and spinal surgery are at the greatest risk, though POVL may occur during other neurosurgical and vascular procedures as well. The most common causes of POVL are central retinal artery occlusion (CRAO) and ischemic optic neuropathy (ION),1-3 though cases of orbital compartment syndrome (OCS) have also been reported.4-7
We describe a case of POVL during a temporal meningioma excision using the pterional approach. Though the etiology is not fully understood, the patient’s clinical course was complicated by a third cranial nerve (CN III) palsy and CRAO, which, together with the patient’s presentation, were consistent with previously documented cases of OCS. The goals of this case report are to increase awareness of this surgical outcome, identify practices that may have contributed to its development, and delineate methods to minimize its occurrence.
Informed consent regarding this research was obtained from the patient and an institutional Health Insurance Portability and Accountability Act authorization form was completed. This manuscript adheres to the applicable Enhancing the Quality and Transparency of Health Research guideline.8
Case Presentation
A 47-year-old woman underwent a left temporal craniotomy for resection of a sphenoid wing meningioma discovered during a workup for persistent headaches. She had no medical history of diabetes, hypertension, coronary artery disease, or ophthalmic disease. Two months before her scheduled surgery, the patient reported bilateral blurry vision and underwent ophthalmologic evaluation. Her intraocular pressure (IOP) was normal, and she had no pupillary or retinal disease. She showed evidence of decreased vision in her left eye, suggesting a possible mass effect from her meningioma. Subsequent imaging of the optic nerve and retina had unremarkable physiology (Figure 1). Preoperative magnetic resonance imaging (MRI) demonstrated a stable enhancing mass involving the left great sphenoid wing and left cavernous sinus(Figure 2). There was a superior mass effect on the left middle cerebral artery, but all vessels were patent without evidence of thrombosis.
The patient underwent general anesthesia with invasive hemodynamic monitoring used throughout the procedure. She was induced with fentanyl, propofol, and rocuronium; anesthesia was maintained with isoflurane and a remifentanil infusion. Hypotension was treated with phenylephrine and intravenous fluids. Intraoperative neuromonitoring with electroencephalogram (EEG) and somatosensory evoked potentials was performed. During the surgery, the patient was positioned supine in a Mayfield 3-point head fixation system. All pressure points were padded appropriately and continually checked. A standard left pterional craniotomy was performed, and the scalp was reflected anteriorly and secured using fish hooks with rubber bands. The operation did not violate the cavernous sinus or orbital compartment. There was no evidence of active bleeding upon inspection nor with the Valsalva maneuver. No changes were noted in EEG or somatosensory evoked potentials; blood pressure remained within 20 mm Hg of the patient’s baseline. She was extubated at the end of the 10-hour case and was hemodynamically stable upon transport to the surgical intensive care unit. Postoperative imaging confirmed the successful removal of the left sphenoid wing meningioma.
The patient’s postoperative examination demonstrated a 5 mm dilated, nonresponsive left pupil, though the patient did not report visual loss at that time. Defects were noted in the inferior oblique, superior, inferior, and medial rectus muscles, consistent with CN III palsy. The surgery included manipulation of CN III, which made this a possible outcome, but an alternate causative pathology like OCS was not immediately suspected. Postoperative computed tomography (CT) showed an expected pneumocephalus and left scalp swelling without evidence of mass effect or midline shift.
On the morning of postoperative Day 1, the patient reported vision loss in her left eye, while her clinical examination revealed erythema and conjunctival chemosis with left eyelid swelling. The ophthalmologic evaluation was notable for a continued leftCN III palsy with intact lateral rectus and superior oblique function, a nonreactive and dilated left eye with 3+ afferent pupillary defect by reverse (light perception), pallor throughout, a flat cherry red macula with blurred disc margins, left upper eyelid edema, and 18 mm Hg intraocular pressure bilaterally (reference range, 8 to 21 mm Hg). Fundoscopic examination showed a clear vitreous without plaques or occlusions, no perivascular sheathing, and no retinal hemorrhages. CT angiography revealed small outpouchings at the superolateral aspect of the left and right cavernous carotid, consistent with atherosclerotic calcifications. An echocardiogram revealed a Valsalva-dependent patent foramen ovale, but a venous Doppler ultrasound yielded negative results.
Repeat MRI showed denervation of the left medial rectus and minimal left-sided proptosis. A 3-month ophthalmologic follow-up revealed a persistent CN III palsy, including an afferent pupillary defect, absence of light perception in her left eye, and continued ophthalmoplegia. Repeat examination showed a left-sided 4+ afferent pupillary defect unreactive to light, 4+ pallor surrounding the optic nerve, macular atrophy, sclerotic vessels, and 17 mm Hg intraocular pressure bilaterally. The eye had diffuse atrophy of the inner retina and significant patchy atrophy of the outer retinal components without neovascularization of the iris. Postoperative retinal imaging can be seen in Figure 3. Her vision loss persisted at this encounter and has continued through subsequent follow-up examinations.
Discussion
Perioperative visual loss is a rare surgical complication, with an estimated incidence of once in every 60,000 to 125,000 cases.9 The mechanism of injury is variable and dependent upon the type of surgical intervention, with cardiac and spine surgeries carrying the greatest risk.10,11 The injury often results in either CRAO or ION, which may result in visual loss.1-3 POVL can also occur in the aftermath of rapid changes in intracranial pressure during decompressive craniotomies, though the pathophysiology in such cases is not well understood.5
Among the myriad ways in which POVL can occur, neurosurgical cases carry the unique risk of direct cranial nerve injury. Such an insult can lead to vision loss via optic nerve damage or ophthalmoplegia if damage occurs to CN III, IV, or VI. This can occur during manipulation or resection, especially if the surgical approach involves the orbital cavity or the cavernous sinus. Though neither space was entered in this patient, direct injury cannot be ruled out as the etiology for either her vision loss or persistent ophthalmoplegia. An alternate causative scenario for both symptoms involve an impaired blood supply, with the vision loss potentially occurring secondary to CRAO and the ophthalmoplegia to an alternate cause of decreased blood flow. It is unclear which of these 2 conditions occurred first or if they occurred due to the same insult, but OCS could lead to both. Though it is a less common etiology for POVL, this patient’s presentation was similar to those in previously reported cases, and OCS was identified as the likely diagnosis.
OCS is precipitated by an elevated orbital pressure, which leads to ischemia of the retina and damage to orbital contents. Though associated with retrobulbar hemorrhage and orbital trauma, another proposed mechanism for OCS is extrinsic orbital compression, resulting in increased IOP and subsequent CRAO.10 A cherry red spot is visible on fundoscopy, as only the macula with its thin retinal layer will permit the choroidal vessels to be visualized. In a separate process, the relative increase in orbital pressure may lead to impaired perfusion or damage of CN III. However, a causative relationship between the 2 may be difficult to establish. Such an injury to the oculomotor nerve is demonstrated by impaired function of the inferior oblique, superior rectus, inferior rectus, and medial rectus muscles, which may persist even after the compressive symptoms of OCS have resolved.12 Other reported symptoms of OCS include erythema, ophthalmoplegia, conjunctival chemosis, ptosis, corneal abrasion, and eyelid edema.12-15
Alternate Diagnoses
OCS is a diagnosis of exclusion, and several alternate mechanisms were considered before identifying it as the likely etiology. The patient’s preoperative imaging demonstrated a stable enhancing mass involving the left great sphenoid wing and left cavernous sinus, with displacement of the left middle cerebral artery, left cavernous internal carotid artery, and left optic canal. Dissection and removal of this tumor could have compromised the arterial or venous blood supply to the orbit, thus causing ischemia to the retina and other ocular structures. CN III was manipulated during surgery, and it may have been inadvertently damaged during exposure or resection of the tumor.
The patient’s Valsalva-dependent patent foramen ovale put her at risk of a paroxysmal embolus as an alternate explanation, particularly as a Valsalva maneuver was utilized to confirm hemostasis. The patient did not, however, demonstrate any evidence of venous thromboembolism (VTE) on ultrasound, nor did she have the common risk factors of hypertension, diabetes, or smoking history that would increase VTE risk.16 Her cancer diagnosis and surgical status may have put her at risk of VTE, but she did not have any clinical or laboratory values suggestive of hypercoagulability. Had an embolism occurred, it may have compromised the orbital blood supply and led to the CRAO. A similar scenario may have occurred from an atherosclerotic plaque in either of her carotid arteries, as she did have evidence of atherosclerosis on postoperative CT angiography. However, atherosclerosis as a risk factor for POVL appears to be related more to its impact upon impaired blood supply rather than as an embolic source. The patient did not have any significant intraoperative hypotensive episodes, making ION in the setting of atherosclerosis and hypotension a less likely etiology.17
This patient differed from other reported OCS cases. She was never placed in a prone or jackknife position, nor was she agitated or straining for a sustained period. These factors, along with the fact that the orbital compartment was not entered, decreased the likelihood of intraorbital hemorrhage and other intrinsic causes of elevated IOP.12 Additionally, the presentation of our patient’s vision loss was delayed compared with other cases, despite clinicians observing a dilated left pupil and CN III palsy on examination immediately after surgery.14 It is significant to note that OCS may not demonstrate a significant increase in IOP once the source of compression is removed, which may explain the absence of proptosis on her postoperative examination.
The diagnosis of OCS was primarily implicated by the positioning of the myocutaneous flap during the pterional approach to craniotomy. It was retracted anteriorly and superiorly, ultimately resting over her left orbit for most of the 10-hour surgery. Kim and colleagues found that myocutaneous flaps may increase IOP as much as 17.5 mm Hg if improperly positioned, providing an unrecognized source of compression and increasing the risk of damage to orbital contents. According to their review, elevated IOP > 40 mm Hg, particularly over several hours, can compromise blood flow to the optic nerve and increase the risk for POVL.18 The flap was secured using fish hooks and rubber bands. However, it is suspected that the orbital rim did not fully support its pressure, thereby resting to some degree directly on the globe for an extended period and compromising the orbital blood supply. There are no current methods for measuring intraoperative IOP, though surrogate markers are under investigation and may yield clinical utility.18 The myocutaneous flap was created and positioned by the surgeons, but it may be that increased vigilance and communication from the anesthesia and nursing teams could have prevented it from remaining in an improper position.
Conclusions
Despite having few reported cases, OCS must be considered in neurosurgical patients with ophthalmoplegia and CRAO on postoperative examinations. Myocutaneous flaps that are retracted across the orbit can lead to significant elevations in IOP, leading to vision loss, which likely occurred with the patient in this case. Though protecting neurovascular structures is within the purview of the surgeon, all members of the intraoperative team should assist with ensuring proper flap positioning. These measures can help ensure adequate blood flow to the ophthalmic artery, decrease the likelihood of elevated IOP due to extrinsic compression, and help prevent the development of POVL and OCS in these patients.
Perioperative visual loss (POVL) is a well-documented yet uncommon complication of nonocular surgery. Patients undergoing cardiac and spinal surgery are at the greatest risk, though POVL may occur during other neurosurgical and vascular procedures as well. The most common causes of POVL are central retinal artery occlusion (CRAO) and ischemic optic neuropathy (ION),1-3 though cases of orbital compartment syndrome (OCS) have also been reported.4-7
We describe a case of POVL during a temporal meningioma excision using the pterional approach. Though the etiology is not fully understood, the patient’s clinical course was complicated by a third cranial nerve (CN III) palsy and CRAO, which, together with the patient’s presentation, were consistent with previously documented cases of OCS. The goals of this case report are to increase awareness of this surgical outcome, identify practices that may have contributed to its development, and delineate methods to minimize its occurrence.
Informed consent regarding this research was obtained from the patient and an institutional Health Insurance Portability and Accountability Act authorization form was completed. This manuscript adheres to the applicable Enhancing the Quality and Transparency of Health Research guideline.8
Case Presentation
A 47-year-old woman underwent a left temporal craniotomy for resection of a sphenoid wing meningioma discovered during a workup for persistent headaches. She had no medical history of diabetes, hypertension, coronary artery disease, or ophthalmic disease. Two months before her scheduled surgery, the patient reported bilateral blurry vision and underwent ophthalmologic evaluation. Her intraocular pressure (IOP) was normal, and she had no pupillary or retinal disease. She showed evidence of decreased vision in her left eye, suggesting a possible mass effect from her meningioma. Subsequent imaging of the optic nerve and retina had unremarkable physiology (Figure 1). Preoperative magnetic resonance imaging (MRI) demonstrated a stable enhancing mass involving the left great sphenoid wing and left cavernous sinus(Figure 2). There was a superior mass effect on the left middle cerebral artery, but all vessels were patent without evidence of thrombosis.
The patient underwent general anesthesia with invasive hemodynamic monitoring used throughout the procedure. She was induced with fentanyl, propofol, and rocuronium; anesthesia was maintained with isoflurane and a remifentanil infusion. Hypotension was treated with phenylephrine and intravenous fluids. Intraoperative neuromonitoring with electroencephalogram (EEG) and somatosensory evoked potentials was performed. During the surgery, the patient was positioned supine in a Mayfield 3-point head fixation system. All pressure points were padded appropriately and continually checked. A standard left pterional craniotomy was performed, and the scalp was reflected anteriorly and secured using fish hooks with rubber bands. The operation did not violate the cavernous sinus or orbital compartment. There was no evidence of active bleeding upon inspection nor with the Valsalva maneuver. No changes were noted in EEG or somatosensory evoked potentials; blood pressure remained within 20 mm Hg of the patient’s baseline. She was extubated at the end of the 10-hour case and was hemodynamically stable upon transport to the surgical intensive care unit. Postoperative imaging confirmed the successful removal of the left sphenoid wing meningioma.
The patient’s postoperative examination demonstrated a 5 mm dilated, nonresponsive left pupil, though the patient did not report visual loss at that time. Defects were noted in the inferior oblique, superior, inferior, and medial rectus muscles, consistent with CN III palsy. The surgery included manipulation of CN III, which made this a possible outcome, but an alternate causative pathology like OCS was not immediately suspected. Postoperative computed tomography (CT) showed an expected pneumocephalus and left scalp swelling without evidence of mass effect or midline shift.
On the morning of postoperative Day 1, the patient reported vision loss in her left eye, while her clinical examination revealed erythema and conjunctival chemosis with left eyelid swelling. The ophthalmologic evaluation was notable for a continued leftCN III palsy with intact lateral rectus and superior oblique function, a nonreactive and dilated left eye with 3+ afferent pupillary defect by reverse (light perception), pallor throughout, a flat cherry red macula with blurred disc margins, left upper eyelid edema, and 18 mm Hg intraocular pressure bilaterally (reference range, 8 to 21 mm Hg). Fundoscopic examination showed a clear vitreous without plaques or occlusions, no perivascular sheathing, and no retinal hemorrhages. CT angiography revealed small outpouchings at the superolateral aspect of the left and right cavernous carotid, consistent with atherosclerotic calcifications. An echocardiogram revealed a Valsalva-dependent patent foramen ovale, but a venous Doppler ultrasound yielded negative results.
Repeat MRI showed denervation of the left medial rectus and minimal left-sided proptosis. A 3-month ophthalmologic follow-up revealed a persistent CN III palsy, including an afferent pupillary defect, absence of light perception in her left eye, and continued ophthalmoplegia. Repeat examination showed a left-sided 4+ afferent pupillary defect unreactive to light, 4+ pallor surrounding the optic nerve, macular atrophy, sclerotic vessels, and 17 mm Hg intraocular pressure bilaterally. The eye had diffuse atrophy of the inner retina and significant patchy atrophy of the outer retinal components without neovascularization of the iris. Postoperative retinal imaging can be seen in Figure 3. Her vision loss persisted at this encounter and has continued through subsequent follow-up examinations.
Discussion
Perioperative visual loss is a rare surgical complication, with an estimated incidence of once in every 60,000 to 125,000 cases.9 The mechanism of injury is variable and dependent upon the type of surgical intervention, with cardiac and spine surgeries carrying the greatest risk.10,11 The injury often results in either CRAO or ION, which may result in visual loss.1-3 POVL can also occur in the aftermath of rapid changes in intracranial pressure during decompressive craniotomies, though the pathophysiology in such cases is not well understood.5
Among the myriad ways in which POVL can occur, neurosurgical cases carry the unique risk of direct cranial nerve injury. Such an insult can lead to vision loss via optic nerve damage or ophthalmoplegia if damage occurs to CN III, IV, or VI. This can occur during manipulation or resection, especially if the surgical approach involves the orbital cavity or the cavernous sinus. Though neither space was entered in this patient, direct injury cannot be ruled out as the etiology for either her vision loss or persistent ophthalmoplegia. An alternate causative scenario for both symptoms involve an impaired blood supply, with the vision loss potentially occurring secondary to CRAO and the ophthalmoplegia to an alternate cause of decreased blood flow. It is unclear which of these 2 conditions occurred first or if they occurred due to the same insult, but OCS could lead to both. Though it is a less common etiology for POVL, this patient’s presentation was similar to those in previously reported cases, and OCS was identified as the likely diagnosis.
OCS is precipitated by an elevated orbital pressure, which leads to ischemia of the retina and damage to orbital contents. Though associated with retrobulbar hemorrhage and orbital trauma, another proposed mechanism for OCS is extrinsic orbital compression, resulting in increased IOP and subsequent CRAO.10 A cherry red spot is visible on fundoscopy, as only the macula with its thin retinal layer will permit the choroidal vessels to be visualized. In a separate process, the relative increase in orbital pressure may lead to impaired perfusion or damage of CN III. However, a causative relationship between the 2 may be difficult to establish. Such an injury to the oculomotor nerve is demonstrated by impaired function of the inferior oblique, superior rectus, inferior rectus, and medial rectus muscles, which may persist even after the compressive symptoms of OCS have resolved.12 Other reported symptoms of OCS include erythema, ophthalmoplegia, conjunctival chemosis, ptosis, corneal abrasion, and eyelid edema.12-15
Alternate Diagnoses
OCS is a diagnosis of exclusion, and several alternate mechanisms were considered before identifying it as the likely etiology. The patient’s preoperative imaging demonstrated a stable enhancing mass involving the left great sphenoid wing and left cavernous sinus, with displacement of the left middle cerebral artery, left cavernous internal carotid artery, and left optic canal. Dissection and removal of this tumor could have compromised the arterial or venous blood supply to the orbit, thus causing ischemia to the retina and other ocular structures. CN III was manipulated during surgery, and it may have been inadvertently damaged during exposure or resection of the tumor.
The patient’s Valsalva-dependent patent foramen ovale put her at risk of a paroxysmal embolus as an alternate explanation, particularly as a Valsalva maneuver was utilized to confirm hemostasis. The patient did not, however, demonstrate any evidence of venous thromboembolism (VTE) on ultrasound, nor did she have the common risk factors of hypertension, diabetes, or smoking history that would increase VTE risk.16 Her cancer diagnosis and surgical status may have put her at risk of VTE, but she did not have any clinical or laboratory values suggestive of hypercoagulability. Had an embolism occurred, it may have compromised the orbital blood supply and led to the CRAO. A similar scenario may have occurred from an atherosclerotic plaque in either of her carotid arteries, as she did have evidence of atherosclerosis on postoperative CT angiography. However, atherosclerosis as a risk factor for POVL appears to be related more to its impact upon impaired blood supply rather than as an embolic source. The patient did not have any significant intraoperative hypotensive episodes, making ION in the setting of atherosclerosis and hypotension a less likely etiology.17
This patient differed from other reported OCS cases. She was never placed in a prone or jackknife position, nor was she agitated or straining for a sustained period. These factors, along with the fact that the orbital compartment was not entered, decreased the likelihood of intraorbital hemorrhage and other intrinsic causes of elevated IOP.12 Additionally, the presentation of our patient’s vision loss was delayed compared with other cases, despite clinicians observing a dilated left pupil and CN III palsy on examination immediately after surgery.14 It is significant to note that OCS may not demonstrate a significant increase in IOP once the source of compression is removed, which may explain the absence of proptosis on her postoperative examination.
The diagnosis of OCS was primarily implicated by the positioning of the myocutaneous flap during the pterional approach to craniotomy. It was retracted anteriorly and superiorly, ultimately resting over her left orbit for most of the 10-hour surgery. Kim and colleagues found that myocutaneous flaps may increase IOP as much as 17.5 mm Hg if improperly positioned, providing an unrecognized source of compression and increasing the risk of damage to orbital contents. According to their review, elevated IOP > 40 mm Hg, particularly over several hours, can compromise blood flow to the optic nerve and increase the risk for POVL.18 The flap was secured using fish hooks and rubber bands. However, it is suspected that the orbital rim did not fully support its pressure, thereby resting to some degree directly on the globe for an extended period and compromising the orbital blood supply. There are no current methods for measuring intraoperative IOP, though surrogate markers are under investigation and may yield clinical utility.18 The myocutaneous flap was created and positioned by the surgeons, but it may be that increased vigilance and communication from the anesthesia and nursing teams could have prevented it from remaining in an improper position.
Conclusions
Despite having few reported cases, OCS must be considered in neurosurgical patients with ophthalmoplegia and CRAO on postoperative examinations. Myocutaneous flaps that are retracted across the orbit can lead to significant elevations in IOP, leading to vision loss, which likely occurred with the patient in this case. Though protecting neurovascular structures is within the purview of the surgeon, all members of the intraoperative team should assist with ensuring proper flap positioning. These measures can help ensure adequate blood flow to the ophthalmic artery, decrease the likelihood of elevated IOP due to extrinsic compression, and help prevent the development of POVL and OCS in these patients.
1. Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia: follow the guidelines! Ophthalmology. 2018;125(10):1597-1607. doi:10.1016/j.ophtha.2018.03.054
2. Biousse V, Newman NJ. Ischemic optic neuropathies. N Engl J Med. 2015;372(25):2428-2436. doi:10.1056/NEJMra1413352
3. Shah SH, Chen YF, Moss HE, Rubin DS, Joslin CE, Roth S. Predicting risk of perioperative ischemic optic neuropathy in spine fusion surgery: a cohort study using the national inpatient sample. Anesth Analg. 2020;130(4):967-974. doi:10.1213/ANE.0000000000004383
4. Habets JGV, Haeren RHL, Lie SAN, Bauer NJC, Dings JTA. Acute monocular blindness due to orbital compartment syndrome following pterional craniotomy. World Neurosurg. 2018;114:72-75. doi:10.1016/j.wneu.2018.03.013
5. Vahedi P, Meshkini A, Mohajernezhadfard Z, Tubbs RS. Post-craniotomy blindness in the supine position: Unlikely or ignored? Asian J Neurosurg. 2013;8(1):36-41. doi:10.4103/1793-5482.110278
6. Kang S, Yang Y, Kim T, Kim J. Sudden unilateral blindness after intracranial aneurysm surgery. Acta Neurochir (Wien). 1997;139(3):221-226. doi:10.1007/BF01844755
7. Zimmerman CF, Van Patten PD, Golnik KC, Kopitnik TA Jr, Anand R. Orbital infarction syndrome after surgery for intracranial aneurysms. Ophthalmology. 1995;102(4):594-598. doi:10.1016/s0161-6420(95)30979-7
8. Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 23;2013:bcr2013201554. doi:10.1136/bcr-2013-201554
9. Raphael J, Moss HE, Roth S. Perioperative visual loss in cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33(5):1420-429. doi:10.1053/j.jvca.2018.11.035
10. Kansakar P, Sundar G. Vision loss associated with orbital surgery - a major review. Orbit. 2020;39(3):197-208. doi:10.1080/01676830.2019.1658790
11. Dohlman JC, Yoon MK. Principles of protection of the eye and vision in orbital surgery. J Neurol Surg B Skull Base. 2020;81(4):381-384. doi:10.1055/s-0040-1714077
12. Pahl FH, de Oliveira MF, Dal Col Lúcio JE, Souza E Castro EF. Orbital compartment syndrome after frontotemporal craniotomy: case report and review of literature. World Neurosurg. 2018;109:218-221. doi:10.1016/j.wneu.2017.09.167
13. Grossman W, Ward WT. Central retinal artery occlusion after scoliosis surgery with a horseshoe headrest. Case report and literature review. Spine (Phila Pa 1976). 1993;18(9):1226-1228. doi:10.1097/00007632-199307000-00017
14. Newman NJ. Perioperative visual loss after nonocular surgeries. Am J Ophthalmol. 2008;145(4):604-610. doi:10.1016/j.ajo.2007.09.016
15. Roth S, Tung A, Ksiazek S. Visual loss in a prone-positioned spine surgery patient with the head on a foam headrest and goggles covering the eyes: an old complication with a new mechanism. Anesth Analg. 2007;104(5):1185-1187. doi:10.1213/01.ane.0000264319.57758.55
16. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85. doi:10.1097/01.brs.0000152169.48117.c7
17. Nickels TJ, Manlapaz MR, Farag E. Perioperative visual loss after spine surgery. World J Orthop. 2014;5(2):100-106. Published 2014 April 18. doi:10.5312/wjo.v5.i2.100
18. Kim TS, Hur JW, Park DH, et al. Extraocular ressure measurements to avoid orbital compartment syndrome in aneurysm surgery. World Neurosurg. 2018;118:e601-e609. doi:10.1016/j.wneu.2018.06.248
1. Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia: follow the guidelines! Ophthalmology. 2018;125(10):1597-1607. doi:10.1016/j.ophtha.2018.03.054
2. Biousse V, Newman NJ. Ischemic optic neuropathies. N Engl J Med. 2015;372(25):2428-2436. doi:10.1056/NEJMra1413352
3. Shah SH, Chen YF, Moss HE, Rubin DS, Joslin CE, Roth S. Predicting risk of perioperative ischemic optic neuropathy in spine fusion surgery: a cohort study using the national inpatient sample. Anesth Analg. 2020;130(4):967-974. doi:10.1213/ANE.0000000000004383
4. Habets JGV, Haeren RHL, Lie SAN, Bauer NJC, Dings JTA. Acute monocular blindness due to orbital compartment syndrome following pterional craniotomy. World Neurosurg. 2018;114:72-75. doi:10.1016/j.wneu.2018.03.013
5. Vahedi P, Meshkini A, Mohajernezhadfard Z, Tubbs RS. Post-craniotomy blindness in the supine position: Unlikely or ignored? Asian J Neurosurg. 2013;8(1):36-41. doi:10.4103/1793-5482.110278
6. Kang S, Yang Y, Kim T, Kim J. Sudden unilateral blindness after intracranial aneurysm surgery. Acta Neurochir (Wien). 1997;139(3):221-226. doi:10.1007/BF01844755
7. Zimmerman CF, Van Patten PD, Golnik KC, Kopitnik TA Jr, Anand R. Orbital infarction syndrome after surgery for intracranial aneurysms. Ophthalmology. 1995;102(4):594-598. doi:10.1016/s0161-6420(95)30979-7
8. Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 23;2013:bcr2013201554. doi:10.1136/bcr-2013-201554
9. Raphael J, Moss HE, Roth S. Perioperative visual loss in cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33(5):1420-429. doi:10.1053/j.jvca.2018.11.035
10. Kansakar P, Sundar G. Vision loss associated with orbital surgery - a major review. Orbit. 2020;39(3):197-208. doi:10.1080/01676830.2019.1658790
11. Dohlman JC, Yoon MK. Principles of protection of the eye and vision in orbital surgery. J Neurol Surg B Skull Base. 2020;81(4):381-384. doi:10.1055/s-0040-1714077
12. Pahl FH, de Oliveira MF, Dal Col Lúcio JE, Souza E Castro EF. Orbital compartment syndrome after frontotemporal craniotomy: case report and review of literature. World Neurosurg. 2018;109:218-221. doi:10.1016/j.wneu.2017.09.167
13. Grossman W, Ward WT. Central retinal artery occlusion after scoliosis surgery with a horseshoe headrest. Case report and literature review. Spine (Phila Pa 1976). 1993;18(9):1226-1228. doi:10.1097/00007632-199307000-00017
14. Newman NJ. Perioperative visual loss after nonocular surgeries. Am J Ophthalmol. 2008;145(4):604-610. doi:10.1016/j.ajo.2007.09.016
15. Roth S, Tung A, Ksiazek S. Visual loss in a prone-positioned spine surgery patient with the head on a foam headrest and goggles covering the eyes: an old complication with a new mechanism. Anesth Analg. 2007;104(5):1185-1187. doi:10.1213/01.ane.0000264319.57758.55
16. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85. doi:10.1097/01.brs.0000152169.48117.c7
17. Nickels TJ, Manlapaz MR, Farag E. Perioperative visual loss after spine surgery. World J Orthop. 2014;5(2):100-106. Published 2014 April 18. doi:10.5312/wjo.v5.i2.100
18. Kim TS, Hur JW, Park DH, et al. Extraocular ressure measurements to avoid orbital compartment syndrome in aneurysm surgery. World Neurosurg. 2018;118:e601-e609. doi:10.1016/j.wneu.2018.06.248