User login
Spider Bite Wound Care and Review of Traditional and Advanced Treatment Options
The costs for wound care play a significant role in total health care costs and are expected to rise dramatically. A 2018 Medicare analysis estimated chronic wound care cost $28.1 to $96.8 billion in supplies, hospitalization, and nursing care: Most costs were accrued in outpatient wound care.1 The global market for advanced wound care supplies is projected to reach $13.7 billion by 2027, and negative wound pressure therapy alone is projected to grow at a compound annual growth rate of 5% over the analysis period 2020 to 2027.2 Chronic wound care also impacts the patient physiologically, socially, and psychologically. One study compared the 5-year mortality of a patient with a diabetic foot ulcer (30.5%) as similar to those patients with cancer (31%).3 Yet the investment in cancer research far outstrips wound care research.
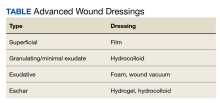
There is no perfect wound dressing for all chronic wounds, but there is expert consensus on interventions that facilitate wound healing. In 2021, Nuutila and Eriksson stated that wound dressings should fulfill the following criteria: protection against trauma, esthetically acceptable, painless to remove, easy to apply, protection for the wound from contamination and further trauma, a moist environment, and an optimal water vapor transmission rate.4 Balanced moisture control is considered essential for healing chronic wounds. Indeed, moisture control within the wound bed may be the most important factor in chronic wound management and healing. The body communicates through a liquid medium, and if that medium is compromised, communication and marshaling of the immune and healing responses may become inefficient.4 Too much moisture, exudate, or fluid in the wound, and the healing is slowed; too little moisture in the wound results in a compromised responses from the body’s immune system, thus delaying healing. In 1988, Dyson and colleagues demonstrated that moist wound care was superior for the inflammatory and proliferative phases of dermal repair compared with dry wound care. The results showed that 5 days after injury, 66% of the cells in the moist wound were fibroblasts and endothelial cells vs 48% of those in the dry wounds.5
The question of dry vs moist wound care has resulted in various wound dressings that produce favorable moisture balance. Moisture balance in a wound creates the ideal environment for wound healing. Sound wound care practices promote the following physiologic responses: increased probability of autolytic debridement; increased collagen synthesis; keratinocyte migration and reepithelization; decreased pain, inflammation, scarring, and necrosis;enhancement of cell-to-cell signaling; and increase in growth factors.5,6 All these processes are mediated through proper wound moisture control. In addition to proper moisture control, antibiotics added to the wound care milieu (either directly to the wound or systemically) may have a place in chronic wound care. In 2013, Junker and colleagues reported that low-dose antibiotics combined with appropriate moisture balance in wounds demonstrated less scar tissue compared with dry wound care.6
Approaches to chronic wound care are worlds apart: In developing nations the care of chronic wounds often involves traditional management with local products (eg, honey, boiled potato peels, aloe vera gel, banana leaves), whereas in developed nations, more expensive and technologically advanced products are available (eg, wound vacuum, saline wound chamber, hyperbaric oxygen therapy, antibacterial foam). Developing countries often do not have access to technologically advanced wound care products. Local products are often used by local healers, priests, and shamans. The use of these wound interventions in developing countries has produced satisfactory results. In contrast, developed countries have multiple chronic wound care products available (Table).
CASE Presentation
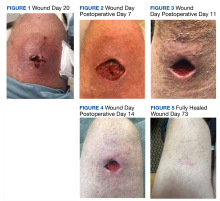
An athletic, healthy 60-year-old Utah National Guard member presented to the George E. Wahlen Department of Veterans Affairs Medical Center in Salt Lake City, Utah, 6 days after experiencing a spider bite. For the first 6 days, the patient applied bacitracin at home. On day 7, the patient noticed that the wound was enlarging and appeared to be fluctuant. The patient was prescribed clindamycin 300 mg 4 times daily on an outpatient basis, which was taken on days 7 to 14.
The wound’s total surface area continued to expand, and the patient returned to the Salt Lake City Veterans Hospital wound care clinic on day 17 stating that the wound was very painful and more fluctuant. The wound care nursing staff were consulted, the wound was debrided, and attempts to drain the wound resulted in minimal exudate expressed from the wound. Clindamycin was increased to 450 mg 4 times daily. However, the wound continued to enlarge and become more painful.
Discussion
Traditional Wound Care
Honey. Honey has been used as a treatment for wounds for almost 3000 years. It has antiseptic and antibacterial properties and contributes to a moist wound care environment. In 2011, Gupta and colleagues reported on the use of honey in 108 patients with burns of < 50% of the total body surface area.7 This report stated that delay in seeking medical care increased wound infection rates, contamination, time to sterilization, and healing. Compared with silver sulfadiazine cream, honey dressings improved the time to wound healing (33 days vs 18 days, respectively), decreased the time to wound sterilization (1 day vs no sterilization), and had better outcomes (37% vs 81%, respectively) with fewer hypertrophic scars and postburn contractures.7
Separate studies in 2011 and 2010 from Fukuda and colleagues and Majtan and colleagues, respectively, reported that honey eliminates pathogens from wounds, augments correct moisture balance, and elevates cytokine activity.8,9 Additional studies in 2006, 2008, and 2014 by Henriques and colleagues, Van den Berg and colleagues, and Majtan suggested that honey reduces reactive oxygen species, is responsible for direct antimicrobial effects in a healing wound, inhibits free radical production, and promotes antitumor activity, respectively.10-12 Van den Berg and colleagues suggested that buckwheat honey is the most effective honey in reducing reactive oxygen species.11
Sterile banana leaves. In medically underserved and rural areas, boiled banana leaves are used to treat burns and nonhealing wounds. In a 2015 study, Waffa and Hayah compared gauze dressings with sterile banana leaves wound dressing in patients with partial thickness burns. Topical antibiotics were added to each type of dressing. The results suggested that the banana leaf dressings were easier to remove, patients reported less pain overall, less pain with dressing changes, and demonstrated a decreased time to healing when contrasted with gauze.13 In 2003, Gore and Akolekar compared autoclaved banana leaves with boiled potato peels in the treatment of patients with partial thickness burns. The time to epithelialization, eschar formation, and skin graft healing were equal in both groups. However, banana leaves were 11 times cheaper and rated easier to prepare than boiled potato peels.14 In a study comparing petroleum gauze with sterile banana leaves, Chendake and colleagues reported that in measures of overall pain and trauma during dressing changes, patients with contused and sutured wounds on the face and neck achieved better outcomes with boiled banana leaves compared with petroleum gauze.15
Boiled potato peels. This treatment is used in rural areas of the world as an adjunct for wound care. In 2015, Manjunath and colleagues theorized that the use of boiled potato peels in patients with necrotizing fasciitis decreased the acidic environment created by the bacteria. Additionally, the study asserted that the toxic wound environment created by the bacteria was neutralized by the potassium content in the peel, and the flavonoids in the peel acted as a free radical scavenger.16 In 2011, Panda and colleagues, using povidone-iodine as a baseline control, reported that peel extract and a peel bandage of sweet potato showed an increased wound closure percentage measured by enhanced epithelialization.17 This increased epithelialization was attributed to the antioxidant effect of the peels enhancing collagen synthesis.17
In contrast, in 1996, a study by Subrahmanyam compared autoclaved potato peel bandages with honey dressings as adjuncts in burn patients with < 40% of the total body surface area affected. The author reported that 90% of the wounds treated with honey were sterile in 7 days, while infection persisted in the potato peel group after 7 days. In the same study, 100% of the wounds treated with honey were healed in 15 days vs 50% in the potato peel group.18 In 1990, Keswani and colleagues compared boiled potato peels with plain gauze as adjuncts in the treatment of burn patients and concluded that although the potato peels had no antibacterial effect, the wounds in both groups had identical bacterial species. But the wounds treated with the potato peels showed reduced desiccation, permitting the survival of skin cells, and enhanced epithelial regeneration.19
Aloe vera. First recorded by the Egyptians and Greeks, aloe vera gel has been used for centuries in many cultures for a variety of ailments, particularly burns and chronic wounds. In a 2016 wound healing study performed on rats, Oryan and colleagues demonstrated that aloe vera gel was superior to saline used as the baseline control. Aloe vera gel used in a dose-dependent fashion demonstrated increased tissue levels of collagen and glycosaminoglycans compared with controls. Aloe vera gel modulated wound inflammation, increased wound contraction, wound epithelialization, decreased scar tissue size, and increased alignment and organization of the scar tissue.20
Gauze. Iodoform gauze is a highly absorbent wound product. Sterile gauze promotes granulation and wound healing. It is well suited for wounds with minimal drainage. However, although gauze is inexpensive, it is easily overwhelmed by the moisture content in the wound, requiring frequent dressing changes (up to 3 times a day), ideally by nursing staff. The resulting increase in nursing care may actually increase the cost of wound care compared with other care modalities.
Petroleum gauze is often used in the care of acute and chronic wounds. However, petroleum-impregnated gauze has a water vapor transmission rate that needs to be remoistened every 4 hours. If the affected area is not remoistened during the exudative phase of wound healing, it may precipitate a delay in healing and increase pain and the prevalence of clinical infections compared with hydrocolloid, film, or foam dressings. Bolton suggested stopping the use of petroleum gauze as the control in studies because it does not provide a balanced and moist wound healing environment.21
Advanced Wound Treatments
Film products. Film products, including plastic food wrap, can be used as wound dressings and meet many of the necessary criteria for enhancing wound healing. These include moisture permeability, carbon dioxide, oxygen transfer, and wound protection. Transmission of moisture varies among products known as the moisture vapor transpiration rate. Film dressings have no absorptive qualities and are unsuited for highly exudative wounds.22,23 Adding polymers, antibacterial, and bioactive agents may increase the wound care properties of film dressings.22 Film dressings excel in protecting shallow nonexudative wounds, are waterproof, and help protect the wound. These products are transparent, allowing clinicians to monitor the progress of the wound without removing the covering, and allowing the dressing to remain in place longer, which decreases the repeated trauma that can occur with dressing changes. Film dressings for wounds differ from those used for IV dressings and should not be used interchangeably.23
Bioactive wound care. These solutions contribute to a moist wound-healing environment. Found naturally in brown seaweed, alginate-containing compounds were used by sailors for centuries to heal wounds. This was known in traditional medicine as the mariner’s cure. Alginate dressings are highly absorbent and can absorb up to 20 times their weight, which makes them desirable for use in highly exudative wounds. First synthesized more than 50 years ago, newer products contain bioactive compounds that prevent tissue damage, stimulate wound healing, improve cell proliferation and migration, and enhance metabolite formation.24-26
In 2018, Aderibigbe and Buyana reported that polymers in the form of hydrogels were able to absorb fluid, making them a suitable choice for minimally exudative wounds. However, in their distended state, the hydrogel subgroup of these products became unstable (perhaps making them a poor choice for extensively exudative wounds), tended to dehydrate, and often needed a secondary dressing, which could lead to wound maceration.22 Most commonly used for wounds with minimal exudate, these dressings shine when used in nominally exudative dry wounds to promote autolytic debridement and hydrate the wound that has formed an eschar.
Hydrocolloid dressings are another type of bioactive wound dressing. These dressings are composed of 2 layers: an inner hydrophilic layer and an outer vapor-permeable layer that promote a moist wound environment. Hydrocolloid dressings assist in hydrating dry eschar wounds and have slight absorbency for exudative wounds. These dressings are not designed to be changed daily and can remain in place for 3 to 6 days. In a 2008 extensive review article, Thomas compared the utility of these dressings in patients with superficial or partial thickness burns, donor sites, surgical wounds, and minor traumatic wounds with basic wound dressings. The results of the review suggested that hydrocolloid dressings conferred statistically significant advantages in measures of decreased pain, healing times (decreased in donor sites by 40%), mobility restriction, and number of dressing changes.27 Although more expensive than basic dressings, the longevity of the hydrocolloid dressing helps defray the original cost. Unfortunately, as these dressings remain in place and continue absorbing exudate, they can take on a very unpleasant odor.
A 2013 Cochrane database review comparing hydrocolloids with foams, alginate, basic wound dressing, and topical treatment found no statistical difference between hydrocolloids and basic wound dressings in patients with diabetes who have noncomplex foot ulcers.28 In 2014, Pott and colleagues suggested a slight superiority in the performance of polyurethane foam dressings over hydrocolloid dressings used in pressure ulcers in older adults.29 In a large pooled analysis in 2010, Davies compared foam to hydrocolloid dressings used in exudative wounds and reported that in 11 of 12 studies, foam dressings were superior to hydrocolloid in terms of exudate management, conformity to the wound, ease of use, decreased trauma and pain at dressing changes, and reduced odor of the wound.30
Foam dressings. These products are typically composed of silicone or polyurethane. Consisting of 2 to 3 layers with a hydrophilic surface, foams are cut to approximate the wound size and serve to wick the macerated wound products to a secondary dressing above the foam. The micropores in the foam matrix absorb exudate from the wound bed while maintaining moisture equilibrium in the wound by donating back moisture to the wound, creating an environment conducive to wound healing. Foam dressings can be combined with various antiseptics (silver, GV/MB, etc) and serve as a delivery vehicle of those products directly to the wound surface.
A 2011 review comparing 8 studies found no difference among foam products available at that time in the use for chronic wounds.31 However, newer products on the market today have produced intriguing results with chronic wounds.
In 2017, Woo and Heil observed that chronic wounds treated with foam products containing GV/MB produced significant improvement when measured at week 4 in the areas of mean wound surface area (42.5%), decrease in baseline Pressure Ulcer Scale for Healing scores (from 13.3 to 10.7), wound coverage by devitalized tissue reduced (from 52.6% to 11.4%), and mean upper and lower wound infection scores were reduced by 75%.32 Further, the researchers reported a moist wound bed was achieved at dressing changes with polyvinyl alcohol (PVA) foam dressing. This led to the presumption that adequate moisture balance and autolytic debridement were facilitated using GV/MB antibacterial PVA foam dressings.
Many foam products on the market today exert an antibacterial effect on the wound bed. Antibiotic properties of various foam dressings create a microenvironment hostile to bacterial growth.32 In addition, the antibacterial properties combined with foam products contribute to the following: autolytic debridement, absorptive qualities (which reduce the bioburden of the wound), and maintenance of moisture in the wound bed. These qualities contribute significantly to the effectiveness of foam products with antibacterial properties.32 The correct balance of moisture in the wound has been identified as a superior environment and perhaps the most important component in chronic wounds.4 Foam dressings are less painful to change, easier to change, and in this case report, contributed to faster wound healing than gauze alone. In 2016, a study by Lee and colleagues suggested that the makeup of the foam product, defined as smaller pore and uniform cell size (foam density), resulted in greater permeability and better moisture absorption and retention capacity, contributing to improved wound healing.33
In 2004, Sibbald and colleagues reported that in a 4-week study of nonhealing chronic wounds, foam wound dressing impregnated with sustained-release silver compared with foam dressing without silver resulted in a reduction in wound size (50% vs 30%, respectively), decreased fluid leakage (27% vs 44% respectively), and reduction in ulcer size measured from baseline (45% vs 25%, respectively).34
In a 2006 study, Varma and colleagues compared sterilized, saline-soaked, nonmedicated polyurethane industrial upholstery foam in nonhealing wounds used in patients with diabetes with conventional techniques using topical antibiotics, hydrocolloid or hydrogel dressings as necessary, and desloughing agents as controls. At the end of a 3-month follow-up period, 100% of the wounds of the foam group had healed compared with 29.2% of the control group. Additionally, the time to wound healing was less than half for the foam group (22.5 days) compared with the control group (52 days), and the time to granulation and epithelialization was faster in the foam group.35
In a 2012 meta-analysis, Aziz and colleagues reported that silver-impregnated dressings and topical silver were no better or worse than controls in preventing wound infection and promoting the healing of burn wounds.36 The authors also noted that the nonsilver dressing groups continuing povidone-iodine, ionic hydrogel, or silicone-coated dressing showed reduced healing time compared with the silver-containing group.36 This is intriguing because silver has long been used as a standard for the treatment of burn wounds.
Conclusions
Although there is no perfect wound dressing, some wound care products seem to perform better due to fewer adverse effects and a much lesser cost. Important aspects of wound care appear to be time from injury to wound care, cleanliness of the wound, moist wound environment, cost, ease of use, and pain of dressing changes.
Primitive wound care products perform admirably in many situations. Modern medicated foam dressings containing antibacterial properties may have beneficial properties compared with other wound care products; however, comparison studies are lacking and need broad-based, randomized, controlled trials to confirm utility. Finally, any choice of wound care product must be tailored to the particular wound and individual patient needs. More large, robust, randomized controlled trials are needed.
Acknowledgments
The authors thank Sarah Maria Paulsen and Rosemary Ellen Brown Smith for their editing, proofreading, and preparation of the manuscript.
1. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost and Medicare policy implications of chronic non healing wounds. Value Health. 2018;21(1):27-32. doi:10.1016/j.jval.2017.07.007
3. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five-year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1)16. doi:10.1186/s13047-020-00383-2
4. Nuutila K, Eriksson E. Moist wound healing with commonly available dressings. Adv Wound Care (New Rochelle). 2021;10(12):685-698. doi:10.1089/wound.2020.1232
5. Dyson M, Young S, Pendle CL, Webster DF, Lang SM. Comparison of the effects of moist and dry conditions on dermal repair. J Investig Dermatol. 1988;91:434-439. doi:10.1111/1523-1747.ep1247646
6. Junker JPE, Kamel RA, Caterson EJ, Eriksson E. Clinical impact upon wound healing and inflammation in moist, wet and dry environments. Adv Wound Care (New Rochelle). 2013;2(7):348-356. doi:10.1089/wound.2012.0412
7. Gupta SS, Singh O, Bhagel PS, Moses S, Shukla S, Mathur RK. Honey dressing versus silver sulfadiazine dressing for wound healing in burn patients: a retrospective study. J Cutan Aesthet Surg. 2011;4(3):183-187. doi:10.4103/0974-2077.91249
8. Fukuda M, Kobayashi K, Hirono Y, et al. Jungle honey enhances immune function and antitumor activity. Evid Based Complement Alternat Med. 2011;2011:1-8. doi:10.1093/ecam/nen086
9. Majtan J, Kumar P, Majtan T, Walls AF, Klaudiny J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp Dermatol. 2010;19(8):e73-e79. doi:10.1111/j.1600-0625.2009.00994.x
10. Henriques A, Jackson S, Cooper R, Burton N. Free radical production and quenching in honeys with wound healing potential. J Antimicrob Chemother. 2006;58(4):773-777. doi:10.1093/jac/dkl336
11. Van den Berg AJJ, Van den Worm E, Quarles van Ufford HC, Halkes SBA, M J Hoekstra MJ, Beukelman C J. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J Wound Care. 2008;17(4):172-178. doi:10.12968/jowc.2008.17.4.28839
12. Majtan J. Honey: an immunomodulator in wound healing. Wound Repair Regen. 2014;22(2) 187-192. doi:10.1111/wrr.12117
13. Waffa GA, Hayah AEB. The effectiveness of using banana leaf dressing in management of partial thickness burns’ wound. IJND. 2015;5(4):22-27. doi:10.15520/ijnd.2015.vol5.iss04.70.
14. Gore MA, Akolekar D. Evaluation of banana leaf dressing for partial thickness burn wounds. Burns. 2003;29(5):487-492. doi:10.1016/s0305-4179(03)00050-0
15. Chendake S, Kale T, Manavadaria Y, Motimath AS. Evaluation of banana leaves (Musa paradisiaca) as an alternative wound dressing material compared to conventional petroleum jelly gauze dressing in contused, lacerated and sutured wounds over the head, neck and face region. Cureus. 2021;13(10):1-9. doi:10.7759/cureus.18552
16. Manjunath KS, Bhandage S, Kamat S. ‘Potato peel’ dressing: a novel adjunctive in the management of necrotizing fasciitis. J Maxillofacial Oral Surg. 2015;14(suppl 1):s352-s354. doi:10.1007/s12663-013-0590-8
17. Panda V, Sonkamble M, Patil S. Wound healing activity of Ipomoea batatas tubers (sweet potato). FFHDJ. 2011;1(10):403-415.
18. Subrahmanyam M. Honey dressing versus boiled potato peel in the treatment of burns: a prospective randomized study. Burns. 1996;22(6):491-493. doi:10.1016/0305-4179(96)00007-1.
19. Keswani M H, Vartak AM, Patil A, Davies JW. Histological and bacteriological studies of burn wounds treated with boiled potato peel dressings. Burns. 1990;16(2):137-143. doi:10.1016/0305-4179(90)90175-v
20. Oryan A, Mohammadalipour A, Moshiri A, MR Tabandeh. Topical application of aloe vera accelerated wound healing, modeling, and remodeling, an experimental study. Ann Plast Surg. 2016;77(1)37-46. doi:10.1097/SAP.0000000000000239
21. Bolton L. Evidence corner: April 2007. Wounds. 2007;19(4):A16-A22.
22. Aderibigbe BA, Buyana B. Alginate in wound dressings. Pharmaceutics. 2018;10(2):42. doi.10.3390/pharmaceutics10020042
23. Fletcher J. Using film dressings. Nurs Times. 2003;99(25):57.
24. Ranahewa TH, Premarathna AD, Wijesundara RMKK, Wijewardana V, Jayasooriya AP, Rajapakse RPVJ. Biochemical composition and anticancer effect of different seaweed species (in-vitro and in-vivo studies). Sustainable Marine Structures. 2019;1(2):5-11. doi:10.36956/sms.v1i2.94
25. El Gamal AA. Biological importance of marine algae. Saudi Pharmaceutical J. 2010;18(1):1-25. doi:10.1016/j.jsps.2009.12.001
26. Premarathna AD, Ranahewa TH, Wijesekera RRMKK, et al. Wound healing properties of aqueous extracts of Sargassum Illicifolium: an in vitro assay. Wound Medicine. 2019;24(1):1-7. doi:10.1016/j.wndm.2018.11.001
27. Thomas S. Hydrocolloid dressings in the management of acute wounds: a review of the literature. Int Wound J. 2008;5(5):602-613. doi:10.1111/j.1742-481X.2008.00541.x
28. Dumville JC, Deshpande S, O’Mera K, et al. Hydrocolloid dressing for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2013(8): CD009099. doi:10.1002/14651858.CD009099.pub3
29. Pott FS, Meier MJ, Stocco JGD, Crozeta K, Dayane Ribas J. The effectiveness of hydrocolloid dressings versus other dressings in the healing of pressure ulcers in adults and older adults: a systematic review and meta-analysis. Rev Lat-Am Enfermagem. 2014;22(3):511-520. doi:10.1590/0104-1169.3480.2445
30. Davies P, Rippon M. Comparison of foam and hydrocolloid dressings in the management of wounds: a review of the published literature. Accessed May 17, 2023. http://www.worldwidewounds.com/2010/July/DaviesRippon/DaviesRippon.html
31. Bianchi J, Gray D, Timmons J. Meaume S. Do all foam dressings have the same efficacy in the treatment of chronic wounds? Wounds UK. 2011;7(1):62-67.

33. Lee SM, Park IK, Kim HJ, et al. Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomaterials Res. 2016;20(15):1-11. doi:10.1186/s40824-016-0063-5
34. Sibbald RG, Meaume S, Kirsner RS, et al. Review of the clinical RCT evidence and cost-effectiveness data of a sustained-release silver foam dressing in the healing of critically colonized wounds. December 2005. Updated January 2006. Accessed May 18, 2023. http://www.worldwidewounds.com/2005/december/Sibbald/Silver-Foam-Dressings-Colonised-Wounds.html
35. Varma AK, Kumar H, Kesav Raiesh. Efficacy of polyurethane foam dressing in debrided diabetic lower limb wounds. Wounds. 2006;18(10):300-306.
36. Aziz Z, Abu SF, Chong NJ. A systematic review of silver-containing dressings and topical silver agents (used with dressings) for burn wounds. Burns. 2012;38(3):307-318. doi:10.1016/j.burns.2011.09.020
37. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
The costs for wound care play a significant role in total health care costs and are expected to rise dramatically. A 2018 Medicare analysis estimated chronic wound care cost $28.1 to $96.8 billion in supplies, hospitalization, and nursing care: Most costs were accrued in outpatient wound care.1 The global market for advanced wound care supplies is projected to reach $13.7 billion by 2027, and negative wound pressure therapy alone is projected to grow at a compound annual growth rate of 5% over the analysis period 2020 to 2027.2 Chronic wound care also impacts the patient physiologically, socially, and psychologically. One study compared the 5-year mortality of a patient with a diabetic foot ulcer (30.5%) as similar to those patients with cancer (31%).3 Yet the investment in cancer research far outstrips wound care research.
There is no perfect wound dressing for all chronic wounds, but there is expert consensus on interventions that facilitate wound healing. In 2021, Nuutila and Eriksson stated that wound dressings should fulfill the following criteria: protection against trauma, esthetically acceptable, painless to remove, easy to apply, protection for the wound from contamination and further trauma, a moist environment, and an optimal water vapor transmission rate.4 Balanced moisture control is considered essential for healing chronic wounds. Indeed, moisture control within the wound bed may be the most important factor in chronic wound management and healing. The body communicates through a liquid medium, and if that medium is compromised, communication and marshaling of the immune and healing responses may become inefficient.4 Too much moisture, exudate, or fluid in the wound, and the healing is slowed; too little moisture in the wound results in a compromised responses from the body’s immune system, thus delaying healing. In 1988, Dyson and colleagues demonstrated that moist wound care was superior for the inflammatory and proliferative phases of dermal repair compared with dry wound care. The results showed that 5 days after injury, 66% of the cells in the moist wound were fibroblasts and endothelial cells vs 48% of those in the dry wounds.5
The question of dry vs moist wound care has resulted in various wound dressings that produce favorable moisture balance. Moisture balance in a wound creates the ideal environment for wound healing. Sound wound care practices promote the following physiologic responses: increased probability of autolytic debridement; increased collagen synthesis; keratinocyte migration and reepithelization; decreased pain, inflammation, scarring, and necrosis;enhancement of cell-to-cell signaling; and increase in growth factors.5,6 All these processes are mediated through proper wound moisture control. In addition to proper moisture control, antibiotics added to the wound care milieu (either directly to the wound or systemically) may have a place in chronic wound care. In 2013, Junker and colleagues reported that low-dose antibiotics combined with appropriate moisture balance in wounds demonstrated less scar tissue compared with dry wound care.6
Approaches to chronic wound care are worlds apart: In developing nations the care of chronic wounds often involves traditional management with local products (eg, honey, boiled potato peels, aloe vera gel, banana leaves), whereas in developed nations, more expensive and technologically advanced products are available (eg, wound vacuum, saline wound chamber, hyperbaric oxygen therapy, antibacterial foam). Developing countries often do not have access to technologically advanced wound care products. Local products are often used by local healers, priests, and shamans. The use of these wound interventions in developing countries has produced satisfactory results. In contrast, developed countries have multiple chronic wound care products available (Table).
CASE Presentation
An athletic, healthy 60-year-old Utah National Guard member presented to the George E. Wahlen Department of Veterans Affairs Medical Center in Salt Lake City, Utah, 6 days after experiencing a spider bite. For the first 6 days, the patient applied bacitracin at home. On day 7, the patient noticed that the wound was enlarging and appeared to be fluctuant. The patient was prescribed clindamycin 300 mg 4 times daily on an outpatient basis, which was taken on days 7 to 14.
The wound’s total surface area continued to expand, and the patient returned to the Salt Lake City Veterans Hospital wound care clinic on day 17 stating that the wound was very painful and more fluctuant. The wound care nursing staff were consulted, the wound was debrided, and attempts to drain the wound resulted in minimal exudate expressed from the wound. Clindamycin was increased to 450 mg 4 times daily. However, the wound continued to enlarge and become more painful.
Discussion
Traditional Wound Care
Honey. Honey has been used as a treatment for wounds for almost 3000 years. It has antiseptic and antibacterial properties and contributes to a moist wound care environment. In 2011, Gupta and colleagues reported on the use of honey in 108 patients with burns of < 50% of the total body surface area.7 This report stated that delay in seeking medical care increased wound infection rates, contamination, time to sterilization, and healing. Compared with silver sulfadiazine cream, honey dressings improved the time to wound healing (33 days vs 18 days, respectively), decreased the time to wound sterilization (1 day vs no sterilization), and had better outcomes (37% vs 81%, respectively) with fewer hypertrophic scars and postburn contractures.7
Separate studies in 2011 and 2010 from Fukuda and colleagues and Majtan and colleagues, respectively, reported that honey eliminates pathogens from wounds, augments correct moisture balance, and elevates cytokine activity.8,9 Additional studies in 2006, 2008, and 2014 by Henriques and colleagues, Van den Berg and colleagues, and Majtan suggested that honey reduces reactive oxygen species, is responsible for direct antimicrobial effects in a healing wound, inhibits free radical production, and promotes antitumor activity, respectively.10-12 Van den Berg and colleagues suggested that buckwheat honey is the most effective honey in reducing reactive oxygen species.11
Sterile banana leaves. In medically underserved and rural areas, boiled banana leaves are used to treat burns and nonhealing wounds. In a 2015 study, Waffa and Hayah compared gauze dressings with sterile banana leaves wound dressing in patients with partial thickness burns. Topical antibiotics were added to each type of dressing. The results suggested that the banana leaf dressings were easier to remove, patients reported less pain overall, less pain with dressing changes, and demonstrated a decreased time to healing when contrasted with gauze.13 In 2003, Gore and Akolekar compared autoclaved banana leaves with boiled potato peels in the treatment of patients with partial thickness burns. The time to epithelialization, eschar formation, and skin graft healing were equal in both groups. However, banana leaves were 11 times cheaper and rated easier to prepare than boiled potato peels.14 In a study comparing petroleum gauze with sterile banana leaves, Chendake and colleagues reported that in measures of overall pain and trauma during dressing changes, patients with contused and sutured wounds on the face and neck achieved better outcomes with boiled banana leaves compared with petroleum gauze.15
Boiled potato peels. This treatment is used in rural areas of the world as an adjunct for wound care. In 2015, Manjunath and colleagues theorized that the use of boiled potato peels in patients with necrotizing fasciitis decreased the acidic environment created by the bacteria. Additionally, the study asserted that the toxic wound environment created by the bacteria was neutralized by the potassium content in the peel, and the flavonoids in the peel acted as a free radical scavenger.16 In 2011, Panda and colleagues, using povidone-iodine as a baseline control, reported that peel extract and a peel bandage of sweet potato showed an increased wound closure percentage measured by enhanced epithelialization.17 This increased epithelialization was attributed to the antioxidant effect of the peels enhancing collagen synthesis.17
In contrast, in 1996, a study by Subrahmanyam compared autoclaved potato peel bandages with honey dressings as adjuncts in burn patients with < 40% of the total body surface area affected. The author reported that 90% of the wounds treated with honey were sterile in 7 days, while infection persisted in the potato peel group after 7 days. In the same study, 100% of the wounds treated with honey were healed in 15 days vs 50% in the potato peel group.18 In 1990, Keswani and colleagues compared boiled potato peels with plain gauze as adjuncts in the treatment of burn patients and concluded that although the potato peels had no antibacterial effect, the wounds in both groups had identical bacterial species. But the wounds treated with the potato peels showed reduced desiccation, permitting the survival of skin cells, and enhanced epithelial regeneration.19
Aloe vera. First recorded by the Egyptians and Greeks, aloe vera gel has been used for centuries in many cultures for a variety of ailments, particularly burns and chronic wounds. In a 2016 wound healing study performed on rats, Oryan and colleagues demonstrated that aloe vera gel was superior to saline used as the baseline control. Aloe vera gel used in a dose-dependent fashion demonstrated increased tissue levels of collagen and glycosaminoglycans compared with controls. Aloe vera gel modulated wound inflammation, increased wound contraction, wound epithelialization, decreased scar tissue size, and increased alignment and organization of the scar tissue.20
Gauze. Iodoform gauze is a highly absorbent wound product. Sterile gauze promotes granulation and wound healing. It is well suited for wounds with minimal drainage. However, although gauze is inexpensive, it is easily overwhelmed by the moisture content in the wound, requiring frequent dressing changes (up to 3 times a day), ideally by nursing staff. The resulting increase in nursing care may actually increase the cost of wound care compared with other care modalities.
Petroleum gauze is often used in the care of acute and chronic wounds. However, petroleum-impregnated gauze has a water vapor transmission rate that needs to be remoistened every 4 hours. If the affected area is not remoistened during the exudative phase of wound healing, it may precipitate a delay in healing and increase pain and the prevalence of clinical infections compared with hydrocolloid, film, or foam dressings. Bolton suggested stopping the use of petroleum gauze as the control in studies because it does not provide a balanced and moist wound healing environment.21
Advanced Wound Treatments
Film products. Film products, including plastic food wrap, can be used as wound dressings and meet many of the necessary criteria for enhancing wound healing. These include moisture permeability, carbon dioxide, oxygen transfer, and wound protection. Transmission of moisture varies among products known as the moisture vapor transpiration rate. Film dressings have no absorptive qualities and are unsuited for highly exudative wounds.22,23 Adding polymers, antibacterial, and bioactive agents may increase the wound care properties of film dressings.22 Film dressings excel in protecting shallow nonexudative wounds, are waterproof, and help protect the wound. These products are transparent, allowing clinicians to monitor the progress of the wound without removing the covering, and allowing the dressing to remain in place longer, which decreases the repeated trauma that can occur with dressing changes. Film dressings for wounds differ from those used for IV dressings and should not be used interchangeably.23
Bioactive wound care. These solutions contribute to a moist wound-healing environment. Found naturally in brown seaweed, alginate-containing compounds were used by sailors for centuries to heal wounds. This was known in traditional medicine as the mariner’s cure. Alginate dressings are highly absorbent and can absorb up to 20 times their weight, which makes them desirable for use in highly exudative wounds. First synthesized more than 50 years ago, newer products contain bioactive compounds that prevent tissue damage, stimulate wound healing, improve cell proliferation and migration, and enhance metabolite formation.24-26
In 2018, Aderibigbe and Buyana reported that polymers in the form of hydrogels were able to absorb fluid, making them a suitable choice for minimally exudative wounds. However, in their distended state, the hydrogel subgroup of these products became unstable (perhaps making them a poor choice for extensively exudative wounds), tended to dehydrate, and often needed a secondary dressing, which could lead to wound maceration.22 Most commonly used for wounds with minimal exudate, these dressings shine when used in nominally exudative dry wounds to promote autolytic debridement and hydrate the wound that has formed an eschar.
Hydrocolloid dressings are another type of bioactive wound dressing. These dressings are composed of 2 layers: an inner hydrophilic layer and an outer vapor-permeable layer that promote a moist wound environment. Hydrocolloid dressings assist in hydrating dry eschar wounds and have slight absorbency for exudative wounds. These dressings are not designed to be changed daily and can remain in place for 3 to 6 days. In a 2008 extensive review article, Thomas compared the utility of these dressings in patients with superficial or partial thickness burns, donor sites, surgical wounds, and minor traumatic wounds with basic wound dressings. The results of the review suggested that hydrocolloid dressings conferred statistically significant advantages in measures of decreased pain, healing times (decreased in donor sites by 40%), mobility restriction, and number of dressing changes.27 Although more expensive than basic dressings, the longevity of the hydrocolloid dressing helps defray the original cost. Unfortunately, as these dressings remain in place and continue absorbing exudate, they can take on a very unpleasant odor.
A 2013 Cochrane database review comparing hydrocolloids with foams, alginate, basic wound dressing, and topical treatment found no statistical difference between hydrocolloids and basic wound dressings in patients with diabetes who have noncomplex foot ulcers.28 In 2014, Pott and colleagues suggested a slight superiority in the performance of polyurethane foam dressings over hydrocolloid dressings used in pressure ulcers in older adults.29 In a large pooled analysis in 2010, Davies compared foam to hydrocolloid dressings used in exudative wounds and reported that in 11 of 12 studies, foam dressings were superior to hydrocolloid in terms of exudate management, conformity to the wound, ease of use, decreased trauma and pain at dressing changes, and reduced odor of the wound.30
Foam dressings. These products are typically composed of silicone or polyurethane. Consisting of 2 to 3 layers with a hydrophilic surface, foams are cut to approximate the wound size and serve to wick the macerated wound products to a secondary dressing above the foam. The micropores in the foam matrix absorb exudate from the wound bed while maintaining moisture equilibrium in the wound by donating back moisture to the wound, creating an environment conducive to wound healing. Foam dressings can be combined with various antiseptics (silver, GV/MB, etc) and serve as a delivery vehicle of those products directly to the wound surface.
A 2011 review comparing 8 studies found no difference among foam products available at that time in the use for chronic wounds.31 However, newer products on the market today have produced intriguing results with chronic wounds.
In 2017, Woo and Heil observed that chronic wounds treated with foam products containing GV/MB produced significant improvement when measured at week 4 in the areas of mean wound surface area (42.5%), decrease in baseline Pressure Ulcer Scale for Healing scores (from 13.3 to 10.7), wound coverage by devitalized tissue reduced (from 52.6% to 11.4%), and mean upper and lower wound infection scores were reduced by 75%.32 Further, the researchers reported a moist wound bed was achieved at dressing changes with polyvinyl alcohol (PVA) foam dressing. This led to the presumption that adequate moisture balance and autolytic debridement were facilitated using GV/MB antibacterial PVA foam dressings.
Many foam products on the market today exert an antibacterial effect on the wound bed. Antibiotic properties of various foam dressings create a microenvironment hostile to bacterial growth.32 In addition, the antibacterial properties combined with foam products contribute to the following: autolytic debridement, absorptive qualities (which reduce the bioburden of the wound), and maintenance of moisture in the wound bed. These qualities contribute significantly to the effectiveness of foam products with antibacterial properties.32 The correct balance of moisture in the wound has been identified as a superior environment and perhaps the most important component in chronic wounds.4 Foam dressings are less painful to change, easier to change, and in this case report, contributed to faster wound healing than gauze alone. In 2016, a study by Lee and colleagues suggested that the makeup of the foam product, defined as smaller pore and uniform cell size (foam density), resulted in greater permeability and better moisture absorption and retention capacity, contributing to improved wound healing.33
In 2004, Sibbald and colleagues reported that in a 4-week study of nonhealing chronic wounds, foam wound dressing impregnated with sustained-release silver compared with foam dressing without silver resulted in a reduction in wound size (50% vs 30%, respectively), decreased fluid leakage (27% vs 44% respectively), and reduction in ulcer size measured from baseline (45% vs 25%, respectively).34
In a 2006 study, Varma and colleagues compared sterilized, saline-soaked, nonmedicated polyurethane industrial upholstery foam in nonhealing wounds used in patients with diabetes with conventional techniques using topical antibiotics, hydrocolloid or hydrogel dressings as necessary, and desloughing agents as controls. At the end of a 3-month follow-up period, 100% of the wounds of the foam group had healed compared with 29.2% of the control group. Additionally, the time to wound healing was less than half for the foam group (22.5 days) compared with the control group (52 days), and the time to granulation and epithelialization was faster in the foam group.35
In a 2012 meta-analysis, Aziz and colleagues reported that silver-impregnated dressings and topical silver were no better or worse than controls in preventing wound infection and promoting the healing of burn wounds.36 The authors also noted that the nonsilver dressing groups continuing povidone-iodine, ionic hydrogel, or silicone-coated dressing showed reduced healing time compared with the silver-containing group.36 This is intriguing because silver has long been used as a standard for the treatment of burn wounds.
Conclusions
Although there is no perfect wound dressing, some wound care products seem to perform better due to fewer adverse effects and a much lesser cost. Important aspects of wound care appear to be time from injury to wound care, cleanliness of the wound, moist wound environment, cost, ease of use, and pain of dressing changes.
Primitive wound care products perform admirably in many situations. Modern medicated foam dressings containing antibacterial properties may have beneficial properties compared with other wound care products; however, comparison studies are lacking and need broad-based, randomized, controlled trials to confirm utility. Finally, any choice of wound care product must be tailored to the particular wound and individual patient needs. More large, robust, randomized controlled trials are needed.
Acknowledgments
The authors thank Sarah Maria Paulsen and Rosemary Ellen Brown Smith for their editing, proofreading, and preparation of the manuscript.
The costs for wound care play a significant role in total health care costs and are expected to rise dramatically. A 2018 Medicare analysis estimated chronic wound care cost $28.1 to $96.8 billion in supplies, hospitalization, and nursing care: Most costs were accrued in outpatient wound care.1 The global market for advanced wound care supplies is projected to reach $13.7 billion by 2027, and negative wound pressure therapy alone is projected to grow at a compound annual growth rate of 5% over the analysis period 2020 to 2027.2 Chronic wound care also impacts the patient physiologically, socially, and psychologically. One study compared the 5-year mortality of a patient with a diabetic foot ulcer (30.5%) as similar to those patients with cancer (31%).3 Yet the investment in cancer research far outstrips wound care research.
There is no perfect wound dressing for all chronic wounds, but there is expert consensus on interventions that facilitate wound healing. In 2021, Nuutila and Eriksson stated that wound dressings should fulfill the following criteria: protection against trauma, esthetically acceptable, painless to remove, easy to apply, protection for the wound from contamination and further trauma, a moist environment, and an optimal water vapor transmission rate.4 Balanced moisture control is considered essential for healing chronic wounds. Indeed, moisture control within the wound bed may be the most important factor in chronic wound management and healing. The body communicates through a liquid medium, and if that medium is compromised, communication and marshaling of the immune and healing responses may become inefficient.4 Too much moisture, exudate, or fluid in the wound, and the healing is slowed; too little moisture in the wound results in a compromised responses from the body’s immune system, thus delaying healing. In 1988, Dyson and colleagues demonstrated that moist wound care was superior for the inflammatory and proliferative phases of dermal repair compared with dry wound care. The results showed that 5 days after injury, 66% of the cells in the moist wound were fibroblasts and endothelial cells vs 48% of those in the dry wounds.5
The question of dry vs moist wound care has resulted in various wound dressings that produce favorable moisture balance. Moisture balance in a wound creates the ideal environment for wound healing. Sound wound care practices promote the following physiologic responses: increased probability of autolytic debridement; increased collagen synthesis; keratinocyte migration and reepithelization; decreased pain, inflammation, scarring, and necrosis;enhancement of cell-to-cell signaling; and increase in growth factors.5,6 All these processes are mediated through proper wound moisture control. In addition to proper moisture control, antibiotics added to the wound care milieu (either directly to the wound or systemically) may have a place in chronic wound care. In 2013, Junker and colleagues reported that low-dose antibiotics combined with appropriate moisture balance in wounds demonstrated less scar tissue compared with dry wound care.6
Approaches to chronic wound care are worlds apart: In developing nations the care of chronic wounds often involves traditional management with local products (eg, honey, boiled potato peels, aloe vera gel, banana leaves), whereas in developed nations, more expensive and technologically advanced products are available (eg, wound vacuum, saline wound chamber, hyperbaric oxygen therapy, antibacterial foam). Developing countries often do not have access to technologically advanced wound care products. Local products are often used by local healers, priests, and shamans. The use of these wound interventions in developing countries has produced satisfactory results. In contrast, developed countries have multiple chronic wound care products available (Table).
CASE Presentation
An athletic, healthy 60-year-old Utah National Guard member presented to the George E. Wahlen Department of Veterans Affairs Medical Center in Salt Lake City, Utah, 6 days after experiencing a spider bite. For the first 6 days, the patient applied bacitracin at home. On day 7, the patient noticed that the wound was enlarging and appeared to be fluctuant. The patient was prescribed clindamycin 300 mg 4 times daily on an outpatient basis, which was taken on days 7 to 14.
The wound’s total surface area continued to expand, and the patient returned to the Salt Lake City Veterans Hospital wound care clinic on day 17 stating that the wound was very painful and more fluctuant. The wound care nursing staff were consulted, the wound was debrided, and attempts to drain the wound resulted in minimal exudate expressed from the wound. Clindamycin was increased to 450 mg 4 times daily. However, the wound continued to enlarge and become more painful.
Discussion
Traditional Wound Care
Honey. Honey has been used as a treatment for wounds for almost 3000 years. It has antiseptic and antibacterial properties and contributes to a moist wound care environment. In 2011, Gupta and colleagues reported on the use of honey in 108 patients with burns of < 50% of the total body surface area.7 This report stated that delay in seeking medical care increased wound infection rates, contamination, time to sterilization, and healing. Compared with silver sulfadiazine cream, honey dressings improved the time to wound healing (33 days vs 18 days, respectively), decreased the time to wound sterilization (1 day vs no sterilization), and had better outcomes (37% vs 81%, respectively) with fewer hypertrophic scars and postburn contractures.7
Separate studies in 2011 and 2010 from Fukuda and colleagues and Majtan and colleagues, respectively, reported that honey eliminates pathogens from wounds, augments correct moisture balance, and elevates cytokine activity.8,9 Additional studies in 2006, 2008, and 2014 by Henriques and colleagues, Van den Berg and colleagues, and Majtan suggested that honey reduces reactive oxygen species, is responsible for direct antimicrobial effects in a healing wound, inhibits free radical production, and promotes antitumor activity, respectively.10-12 Van den Berg and colleagues suggested that buckwheat honey is the most effective honey in reducing reactive oxygen species.11
Sterile banana leaves. In medically underserved and rural areas, boiled banana leaves are used to treat burns and nonhealing wounds. In a 2015 study, Waffa and Hayah compared gauze dressings with sterile banana leaves wound dressing in patients with partial thickness burns. Topical antibiotics were added to each type of dressing. The results suggested that the banana leaf dressings were easier to remove, patients reported less pain overall, less pain with dressing changes, and demonstrated a decreased time to healing when contrasted with gauze.13 In 2003, Gore and Akolekar compared autoclaved banana leaves with boiled potato peels in the treatment of patients with partial thickness burns. The time to epithelialization, eschar formation, and skin graft healing were equal in both groups. However, banana leaves were 11 times cheaper and rated easier to prepare than boiled potato peels.14 In a study comparing petroleum gauze with sterile banana leaves, Chendake and colleagues reported that in measures of overall pain and trauma during dressing changes, patients with contused and sutured wounds on the face and neck achieved better outcomes with boiled banana leaves compared with petroleum gauze.15
Boiled potato peels. This treatment is used in rural areas of the world as an adjunct for wound care. In 2015, Manjunath and colleagues theorized that the use of boiled potato peels in patients with necrotizing fasciitis decreased the acidic environment created by the bacteria. Additionally, the study asserted that the toxic wound environment created by the bacteria was neutralized by the potassium content in the peel, and the flavonoids in the peel acted as a free radical scavenger.16 In 2011, Panda and colleagues, using povidone-iodine as a baseline control, reported that peel extract and a peel bandage of sweet potato showed an increased wound closure percentage measured by enhanced epithelialization.17 This increased epithelialization was attributed to the antioxidant effect of the peels enhancing collagen synthesis.17
In contrast, in 1996, a study by Subrahmanyam compared autoclaved potato peel bandages with honey dressings as adjuncts in burn patients with < 40% of the total body surface area affected. The author reported that 90% of the wounds treated with honey were sterile in 7 days, while infection persisted in the potato peel group after 7 days. In the same study, 100% of the wounds treated with honey were healed in 15 days vs 50% in the potato peel group.18 In 1990, Keswani and colleagues compared boiled potato peels with plain gauze as adjuncts in the treatment of burn patients and concluded that although the potato peels had no antibacterial effect, the wounds in both groups had identical bacterial species. But the wounds treated with the potato peels showed reduced desiccation, permitting the survival of skin cells, and enhanced epithelial regeneration.19
Aloe vera. First recorded by the Egyptians and Greeks, aloe vera gel has been used for centuries in many cultures for a variety of ailments, particularly burns and chronic wounds. In a 2016 wound healing study performed on rats, Oryan and colleagues demonstrated that aloe vera gel was superior to saline used as the baseline control. Aloe vera gel used in a dose-dependent fashion demonstrated increased tissue levels of collagen and glycosaminoglycans compared with controls. Aloe vera gel modulated wound inflammation, increased wound contraction, wound epithelialization, decreased scar tissue size, and increased alignment and organization of the scar tissue.20
Gauze. Iodoform gauze is a highly absorbent wound product. Sterile gauze promotes granulation and wound healing. It is well suited for wounds with minimal drainage. However, although gauze is inexpensive, it is easily overwhelmed by the moisture content in the wound, requiring frequent dressing changes (up to 3 times a day), ideally by nursing staff. The resulting increase in nursing care may actually increase the cost of wound care compared with other care modalities.
Petroleum gauze is often used in the care of acute and chronic wounds. However, petroleum-impregnated gauze has a water vapor transmission rate that needs to be remoistened every 4 hours. If the affected area is not remoistened during the exudative phase of wound healing, it may precipitate a delay in healing and increase pain and the prevalence of clinical infections compared with hydrocolloid, film, or foam dressings. Bolton suggested stopping the use of petroleum gauze as the control in studies because it does not provide a balanced and moist wound healing environment.21
Advanced Wound Treatments
Film products. Film products, including plastic food wrap, can be used as wound dressings and meet many of the necessary criteria for enhancing wound healing. These include moisture permeability, carbon dioxide, oxygen transfer, and wound protection. Transmission of moisture varies among products known as the moisture vapor transpiration rate. Film dressings have no absorptive qualities and are unsuited for highly exudative wounds.22,23 Adding polymers, antibacterial, and bioactive agents may increase the wound care properties of film dressings.22 Film dressings excel in protecting shallow nonexudative wounds, are waterproof, and help protect the wound. These products are transparent, allowing clinicians to monitor the progress of the wound without removing the covering, and allowing the dressing to remain in place longer, which decreases the repeated trauma that can occur with dressing changes. Film dressings for wounds differ from those used for IV dressings and should not be used interchangeably.23
Bioactive wound care. These solutions contribute to a moist wound-healing environment. Found naturally in brown seaweed, alginate-containing compounds were used by sailors for centuries to heal wounds. This was known in traditional medicine as the mariner’s cure. Alginate dressings are highly absorbent and can absorb up to 20 times their weight, which makes them desirable for use in highly exudative wounds. First synthesized more than 50 years ago, newer products contain bioactive compounds that prevent tissue damage, stimulate wound healing, improve cell proliferation and migration, and enhance metabolite formation.24-26
In 2018, Aderibigbe and Buyana reported that polymers in the form of hydrogels were able to absorb fluid, making them a suitable choice for minimally exudative wounds. However, in their distended state, the hydrogel subgroup of these products became unstable (perhaps making them a poor choice for extensively exudative wounds), tended to dehydrate, and often needed a secondary dressing, which could lead to wound maceration.22 Most commonly used for wounds with minimal exudate, these dressings shine when used in nominally exudative dry wounds to promote autolytic debridement and hydrate the wound that has formed an eschar.
Hydrocolloid dressings are another type of bioactive wound dressing. These dressings are composed of 2 layers: an inner hydrophilic layer and an outer vapor-permeable layer that promote a moist wound environment. Hydrocolloid dressings assist in hydrating dry eschar wounds and have slight absorbency for exudative wounds. These dressings are not designed to be changed daily and can remain in place for 3 to 6 days. In a 2008 extensive review article, Thomas compared the utility of these dressings in patients with superficial or partial thickness burns, donor sites, surgical wounds, and minor traumatic wounds with basic wound dressings. The results of the review suggested that hydrocolloid dressings conferred statistically significant advantages in measures of decreased pain, healing times (decreased in donor sites by 40%), mobility restriction, and number of dressing changes.27 Although more expensive than basic dressings, the longevity of the hydrocolloid dressing helps defray the original cost. Unfortunately, as these dressings remain in place and continue absorbing exudate, they can take on a very unpleasant odor.
A 2013 Cochrane database review comparing hydrocolloids with foams, alginate, basic wound dressing, and topical treatment found no statistical difference between hydrocolloids and basic wound dressings in patients with diabetes who have noncomplex foot ulcers.28 In 2014, Pott and colleagues suggested a slight superiority in the performance of polyurethane foam dressings over hydrocolloid dressings used in pressure ulcers in older adults.29 In a large pooled analysis in 2010, Davies compared foam to hydrocolloid dressings used in exudative wounds and reported that in 11 of 12 studies, foam dressings were superior to hydrocolloid in terms of exudate management, conformity to the wound, ease of use, decreased trauma and pain at dressing changes, and reduced odor of the wound.30
Foam dressings. These products are typically composed of silicone or polyurethane. Consisting of 2 to 3 layers with a hydrophilic surface, foams are cut to approximate the wound size and serve to wick the macerated wound products to a secondary dressing above the foam. The micropores in the foam matrix absorb exudate from the wound bed while maintaining moisture equilibrium in the wound by donating back moisture to the wound, creating an environment conducive to wound healing. Foam dressings can be combined with various antiseptics (silver, GV/MB, etc) and serve as a delivery vehicle of those products directly to the wound surface.
A 2011 review comparing 8 studies found no difference among foam products available at that time in the use for chronic wounds.31 However, newer products on the market today have produced intriguing results with chronic wounds.
In 2017, Woo and Heil observed that chronic wounds treated with foam products containing GV/MB produced significant improvement when measured at week 4 in the areas of mean wound surface area (42.5%), decrease in baseline Pressure Ulcer Scale for Healing scores (from 13.3 to 10.7), wound coverage by devitalized tissue reduced (from 52.6% to 11.4%), and mean upper and lower wound infection scores were reduced by 75%.32 Further, the researchers reported a moist wound bed was achieved at dressing changes with polyvinyl alcohol (PVA) foam dressing. This led to the presumption that adequate moisture balance and autolytic debridement were facilitated using GV/MB antibacterial PVA foam dressings.
Many foam products on the market today exert an antibacterial effect on the wound bed. Antibiotic properties of various foam dressings create a microenvironment hostile to bacterial growth.32 In addition, the antibacterial properties combined with foam products contribute to the following: autolytic debridement, absorptive qualities (which reduce the bioburden of the wound), and maintenance of moisture in the wound bed. These qualities contribute significantly to the effectiveness of foam products with antibacterial properties.32 The correct balance of moisture in the wound has been identified as a superior environment and perhaps the most important component in chronic wounds.4 Foam dressings are less painful to change, easier to change, and in this case report, contributed to faster wound healing than gauze alone. In 2016, a study by Lee and colleagues suggested that the makeup of the foam product, defined as smaller pore and uniform cell size (foam density), resulted in greater permeability and better moisture absorption and retention capacity, contributing to improved wound healing.33
In 2004, Sibbald and colleagues reported that in a 4-week study of nonhealing chronic wounds, foam wound dressing impregnated with sustained-release silver compared with foam dressing without silver resulted in a reduction in wound size (50% vs 30%, respectively), decreased fluid leakage (27% vs 44% respectively), and reduction in ulcer size measured from baseline (45% vs 25%, respectively).34
In a 2006 study, Varma and colleagues compared sterilized, saline-soaked, nonmedicated polyurethane industrial upholstery foam in nonhealing wounds used in patients with diabetes with conventional techniques using topical antibiotics, hydrocolloid or hydrogel dressings as necessary, and desloughing agents as controls. At the end of a 3-month follow-up period, 100% of the wounds of the foam group had healed compared with 29.2% of the control group. Additionally, the time to wound healing was less than half for the foam group (22.5 days) compared with the control group (52 days), and the time to granulation and epithelialization was faster in the foam group.35
In a 2012 meta-analysis, Aziz and colleagues reported that silver-impregnated dressings and topical silver were no better or worse than controls in preventing wound infection and promoting the healing of burn wounds.36 The authors also noted that the nonsilver dressing groups continuing povidone-iodine, ionic hydrogel, or silicone-coated dressing showed reduced healing time compared with the silver-containing group.36 This is intriguing because silver has long been used as a standard for the treatment of burn wounds.
Conclusions
Although there is no perfect wound dressing, some wound care products seem to perform better due to fewer adverse effects and a much lesser cost. Important aspects of wound care appear to be time from injury to wound care, cleanliness of the wound, moist wound environment, cost, ease of use, and pain of dressing changes.
Primitive wound care products perform admirably in many situations. Modern medicated foam dressings containing antibacterial properties may have beneficial properties compared with other wound care products; however, comparison studies are lacking and need broad-based, randomized, controlled trials to confirm utility. Finally, any choice of wound care product must be tailored to the particular wound and individual patient needs. More large, robust, randomized controlled trials are needed.
Acknowledgments
The authors thank Sarah Maria Paulsen and Rosemary Ellen Brown Smith for their editing, proofreading, and preparation of the manuscript.
1. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost and Medicare policy implications of chronic non healing wounds. Value Health. 2018;21(1):27-32. doi:10.1016/j.jval.2017.07.007
3. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five-year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1)16. doi:10.1186/s13047-020-00383-2
4. Nuutila K, Eriksson E. Moist wound healing with commonly available dressings. Adv Wound Care (New Rochelle). 2021;10(12):685-698. doi:10.1089/wound.2020.1232
5. Dyson M, Young S, Pendle CL, Webster DF, Lang SM. Comparison of the effects of moist and dry conditions on dermal repair. J Investig Dermatol. 1988;91:434-439. doi:10.1111/1523-1747.ep1247646
6. Junker JPE, Kamel RA, Caterson EJ, Eriksson E. Clinical impact upon wound healing and inflammation in moist, wet and dry environments. Adv Wound Care (New Rochelle). 2013;2(7):348-356. doi:10.1089/wound.2012.0412
7. Gupta SS, Singh O, Bhagel PS, Moses S, Shukla S, Mathur RK. Honey dressing versus silver sulfadiazine dressing for wound healing in burn patients: a retrospective study. J Cutan Aesthet Surg. 2011;4(3):183-187. doi:10.4103/0974-2077.91249
8. Fukuda M, Kobayashi K, Hirono Y, et al. Jungle honey enhances immune function and antitumor activity. Evid Based Complement Alternat Med. 2011;2011:1-8. doi:10.1093/ecam/nen086
9. Majtan J, Kumar P, Majtan T, Walls AF, Klaudiny J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp Dermatol. 2010;19(8):e73-e79. doi:10.1111/j.1600-0625.2009.00994.x
10. Henriques A, Jackson S, Cooper R, Burton N. Free radical production and quenching in honeys with wound healing potential. J Antimicrob Chemother. 2006;58(4):773-777. doi:10.1093/jac/dkl336
11. Van den Berg AJJ, Van den Worm E, Quarles van Ufford HC, Halkes SBA, M J Hoekstra MJ, Beukelman C J. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J Wound Care. 2008;17(4):172-178. doi:10.12968/jowc.2008.17.4.28839
12. Majtan J. Honey: an immunomodulator in wound healing. Wound Repair Regen. 2014;22(2) 187-192. doi:10.1111/wrr.12117
13. Waffa GA, Hayah AEB. The effectiveness of using banana leaf dressing in management of partial thickness burns’ wound. IJND. 2015;5(4):22-27. doi:10.15520/ijnd.2015.vol5.iss04.70.
14. Gore MA, Akolekar D. Evaluation of banana leaf dressing for partial thickness burn wounds. Burns. 2003;29(5):487-492. doi:10.1016/s0305-4179(03)00050-0
15. Chendake S, Kale T, Manavadaria Y, Motimath AS. Evaluation of banana leaves (Musa paradisiaca) as an alternative wound dressing material compared to conventional petroleum jelly gauze dressing in contused, lacerated and sutured wounds over the head, neck and face region. Cureus. 2021;13(10):1-9. doi:10.7759/cureus.18552
16. Manjunath KS, Bhandage S, Kamat S. ‘Potato peel’ dressing: a novel adjunctive in the management of necrotizing fasciitis. J Maxillofacial Oral Surg. 2015;14(suppl 1):s352-s354. doi:10.1007/s12663-013-0590-8
17. Panda V, Sonkamble M, Patil S. Wound healing activity of Ipomoea batatas tubers (sweet potato). FFHDJ. 2011;1(10):403-415.
18. Subrahmanyam M. Honey dressing versus boiled potato peel in the treatment of burns: a prospective randomized study. Burns. 1996;22(6):491-493. doi:10.1016/0305-4179(96)00007-1.
19. Keswani M H, Vartak AM, Patil A, Davies JW. Histological and bacteriological studies of burn wounds treated with boiled potato peel dressings. Burns. 1990;16(2):137-143. doi:10.1016/0305-4179(90)90175-v
20. Oryan A, Mohammadalipour A, Moshiri A, MR Tabandeh. Topical application of aloe vera accelerated wound healing, modeling, and remodeling, an experimental study. Ann Plast Surg. 2016;77(1)37-46. doi:10.1097/SAP.0000000000000239
21. Bolton L. Evidence corner: April 2007. Wounds. 2007;19(4):A16-A22.
22. Aderibigbe BA, Buyana B. Alginate in wound dressings. Pharmaceutics. 2018;10(2):42. doi.10.3390/pharmaceutics10020042
23. Fletcher J. Using film dressings. Nurs Times. 2003;99(25):57.
24. Ranahewa TH, Premarathna AD, Wijesundara RMKK, Wijewardana V, Jayasooriya AP, Rajapakse RPVJ. Biochemical composition and anticancer effect of different seaweed species (in-vitro and in-vivo studies). Sustainable Marine Structures. 2019;1(2):5-11. doi:10.36956/sms.v1i2.94
25. El Gamal AA. Biological importance of marine algae. Saudi Pharmaceutical J. 2010;18(1):1-25. doi:10.1016/j.jsps.2009.12.001
26. Premarathna AD, Ranahewa TH, Wijesekera RRMKK, et al. Wound healing properties of aqueous extracts of Sargassum Illicifolium: an in vitro assay. Wound Medicine. 2019;24(1):1-7. doi:10.1016/j.wndm.2018.11.001
27. Thomas S. Hydrocolloid dressings in the management of acute wounds: a review of the literature. Int Wound J. 2008;5(5):602-613. doi:10.1111/j.1742-481X.2008.00541.x
28. Dumville JC, Deshpande S, O’Mera K, et al. Hydrocolloid dressing for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2013(8): CD009099. doi:10.1002/14651858.CD009099.pub3
29. Pott FS, Meier MJ, Stocco JGD, Crozeta K, Dayane Ribas J. The effectiveness of hydrocolloid dressings versus other dressings in the healing of pressure ulcers in adults and older adults: a systematic review and meta-analysis. Rev Lat-Am Enfermagem. 2014;22(3):511-520. doi:10.1590/0104-1169.3480.2445
30. Davies P, Rippon M. Comparison of foam and hydrocolloid dressings in the management of wounds: a review of the published literature. Accessed May 17, 2023. http://www.worldwidewounds.com/2010/July/DaviesRippon/DaviesRippon.html
31. Bianchi J, Gray D, Timmons J. Meaume S. Do all foam dressings have the same efficacy in the treatment of chronic wounds? Wounds UK. 2011;7(1):62-67.

33. Lee SM, Park IK, Kim HJ, et al. Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomaterials Res. 2016;20(15):1-11. doi:10.1186/s40824-016-0063-5
34. Sibbald RG, Meaume S, Kirsner RS, et al. Review of the clinical RCT evidence and cost-effectiveness data of a sustained-release silver foam dressing in the healing of critically colonized wounds. December 2005. Updated January 2006. Accessed May 18, 2023. http://www.worldwidewounds.com/2005/december/Sibbald/Silver-Foam-Dressings-Colonised-Wounds.html
35. Varma AK, Kumar H, Kesav Raiesh. Efficacy of polyurethane foam dressing in debrided diabetic lower limb wounds. Wounds. 2006;18(10):300-306.
36. Aziz Z, Abu SF, Chong NJ. A systematic review of silver-containing dressings and topical silver agents (used with dressings) for burn wounds. Burns. 2012;38(3):307-318. doi:10.1016/j.burns.2011.09.020
37. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
1. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost and Medicare policy implications of chronic non healing wounds. Value Health. 2018;21(1):27-32. doi:10.1016/j.jval.2017.07.007
3. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five-year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1)16. doi:10.1186/s13047-020-00383-2
4. Nuutila K, Eriksson E. Moist wound healing with commonly available dressings. Adv Wound Care (New Rochelle). 2021;10(12):685-698. doi:10.1089/wound.2020.1232
5. Dyson M, Young S, Pendle CL, Webster DF, Lang SM. Comparison of the effects of moist and dry conditions on dermal repair. J Investig Dermatol. 1988;91:434-439. doi:10.1111/1523-1747.ep1247646
6. Junker JPE, Kamel RA, Caterson EJ, Eriksson E. Clinical impact upon wound healing and inflammation in moist, wet and dry environments. Adv Wound Care (New Rochelle). 2013;2(7):348-356. doi:10.1089/wound.2012.0412
7. Gupta SS, Singh O, Bhagel PS, Moses S, Shukla S, Mathur RK. Honey dressing versus silver sulfadiazine dressing for wound healing in burn patients: a retrospective study. J Cutan Aesthet Surg. 2011;4(3):183-187. doi:10.4103/0974-2077.91249
8. Fukuda M, Kobayashi K, Hirono Y, et al. Jungle honey enhances immune function and antitumor activity. Evid Based Complement Alternat Med. 2011;2011:1-8. doi:10.1093/ecam/nen086
9. Majtan J, Kumar P, Majtan T, Walls AF, Klaudiny J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp Dermatol. 2010;19(8):e73-e79. doi:10.1111/j.1600-0625.2009.00994.x
10. Henriques A, Jackson S, Cooper R, Burton N. Free radical production and quenching in honeys with wound healing potential. J Antimicrob Chemother. 2006;58(4):773-777. doi:10.1093/jac/dkl336
11. Van den Berg AJJ, Van den Worm E, Quarles van Ufford HC, Halkes SBA, M J Hoekstra MJ, Beukelman C J. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J Wound Care. 2008;17(4):172-178. doi:10.12968/jowc.2008.17.4.28839
12. Majtan J. Honey: an immunomodulator in wound healing. Wound Repair Regen. 2014;22(2) 187-192. doi:10.1111/wrr.12117
13. Waffa GA, Hayah AEB. The effectiveness of using banana leaf dressing in management of partial thickness burns’ wound. IJND. 2015;5(4):22-27. doi:10.15520/ijnd.2015.vol5.iss04.70.
14. Gore MA, Akolekar D. Evaluation of banana leaf dressing for partial thickness burn wounds. Burns. 2003;29(5):487-492. doi:10.1016/s0305-4179(03)00050-0
15. Chendake S, Kale T, Manavadaria Y, Motimath AS. Evaluation of banana leaves (Musa paradisiaca) as an alternative wound dressing material compared to conventional petroleum jelly gauze dressing in contused, lacerated and sutured wounds over the head, neck and face region. Cureus. 2021;13(10):1-9. doi:10.7759/cureus.18552
16. Manjunath KS, Bhandage S, Kamat S. ‘Potato peel’ dressing: a novel adjunctive in the management of necrotizing fasciitis. J Maxillofacial Oral Surg. 2015;14(suppl 1):s352-s354. doi:10.1007/s12663-013-0590-8
17. Panda V, Sonkamble M, Patil S. Wound healing activity of Ipomoea batatas tubers (sweet potato). FFHDJ. 2011;1(10):403-415.
18. Subrahmanyam M. Honey dressing versus boiled potato peel in the treatment of burns: a prospective randomized study. Burns. 1996;22(6):491-493. doi:10.1016/0305-4179(96)00007-1.
19. Keswani M H, Vartak AM, Patil A, Davies JW. Histological and bacteriological studies of burn wounds treated with boiled potato peel dressings. Burns. 1990;16(2):137-143. doi:10.1016/0305-4179(90)90175-v
20. Oryan A, Mohammadalipour A, Moshiri A, MR Tabandeh. Topical application of aloe vera accelerated wound healing, modeling, and remodeling, an experimental study. Ann Plast Surg. 2016;77(1)37-46. doi:10.1097/SAP.0000000000000239
21. Bolton L. Evidence corner: April 2007. Wounds. 2007;19(4):A16-A22.
22. Aderibigbe BA, Buyana B. Alginate in wound dressings. Pharmaceutics. 2018;10(2):42. doi.10.3390/pharmaceutics10020042
23. Fletcher J. Using film dressings. Nurs Times. 2003;99(25):57.
24. Ranahewa TH, Premarathna AD, Wijesundara RMKK, Wijewardana V, Jayasooriya AP, Rajapakse RPVJ. Biochemical composition and anticancer effect of different seaweed species (in-vitro and in-vivo studies). Sustainable Marine Structures. 2019;1(2):5-11. doi:10.36956/sms.v1i2.94
25. El Gamal AA. Biological importance of marine algae. Saudi Pharmaceutical J. 2010;18(1):1-25. doi:10.1016/j.jsps.2009.12.001
26. Premarathna AD, Ranahewa TH, Wijesekera RRMKK, et al. Wound healing properties of aqueous extracts of Sargassum Illicifolium: an in vitro assay. Wound Medicine. 2019;24(1):1-7. doi:10.1016/j.wndm.2018.11.001
27. Thomas S. Hydrocolloid dressings in the management of acute wounds: a review of the literature. Int Wound J. 2008;5(5):602-613. doi:10.1111/j.1742-481X.2008.00541.x
28. Dumville JC, Deshpande S, O’Mera K, et al. Hydrocolloid dressing for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2013(8): CD009099. doi:10.1002/14651858.CD009099.pub3
29. Pott FS, Meier MJ, Stocco JGD, Crozeta K, Dayane Ribas J. The effectiveness of hydrocolloid dressings versus other dressings in the healing of pressure ulcers in adults and older adults: a systematic review and meta-analysis. Rev Lat-Am Enfermagem. 2014;22(3):511-520. doi:10.1590/0104-1169.3480.2445
30. Davies P, Rippon M. Comparison of foam and hydrocolloid dressings in the management of wounds: a review of the published literature. Accessed May 17, 2023. http://www.worldwidewounds.com/2010/July/DaviesRippon/DaviesRippon.html
31. Bianchi J, Gray D, Timmons J. Meaume S. Do all foam dressings have the same efficacy in the treatment of chronic wounds? Wounds UK. 2011;7(1):62-67.

33. Lee SM, Park IK, Kim HJ, et al. Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomaterials Res. 2016;20(15):1-11. doi:10.1186/s40824-016-0063-5
34. Sibbald RG, Meaume S, Kirsner RS, et al. Review of the clinical RCT evidence and cost-effectiveness data of a sustained-release silver foam dressing in the healing of critically colonized wounds. December 2005. Updated January 2006. Accessed May 18, 2023. http://www.worldwidewounds.com/2005/december/Sibbald/Silver-Foam-Dressings-Colonised-Wounds.html
35. Varma AK, Kumar H, Kesav Raiesh. Efficacy of polyurethane foam dressing in debrided diabetic lower limb wounds. Wounds. 2006;18(10):300-306.
36. Aziz Z, Abu SF, Chong NJ. A systematic review of silver-containing dressings and topical silver agents (used with dressings) for burn wounds. Burns. 2012;38(3):307-318. doi:10.1016/j.burns.2011.09.020
37. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015