User login
Mapping Pathology Work Associated With Precision Oncology Testing
Mapping Pathology Work Associated With Precision Oncology Testing
Comprehensive genomic profiling (CGP) is becoming progressively common and appropriate as the array of molecular targets expands. However, most hospital laboratories in the United States do not perform CGP assays in-house; instead, these tests are sent to reference laboratories. As evidenced by Inal et al, only a minority of guideline-indicated molecular testing is performed.1
The workload associated with referral testing is a barrier to increased use of such tests; streamlined processes in pathology might increase molecular test use. At 6 high-complexity US Department of Veterans Affairs (VA) medical centers (VAMCs) (Manhattan, Los Angeles, San Diego, Denver, Kansas City, and Salisbury, Maryland) ranging from 150 to 750 beds, a consult process for anatomic pathology molecular testing has increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care. This report comprehensively describes and maps the anatomic pathology molecular testing consult process at a VAMC. We present areas of inefficiency and a target state process map that incorporates best practices.
MOLECULAR TESTING CONSULT PROCESS
At the Kansas City VAMC (KCVAMC), a consult process for anatomic pathology molecular testing was introduced in 2021. Prior to this, requesting anatomic pathology molecular testing was not standardized. A variety of opportunities and methods were used for requests (eg, phone, page, Teams message, email, Computerized Patient Record System alert; or in-person during tumor board, an office meeting, or in passing). Requests were not documented in a standardized way, resulting in duplicate requests. Testing status and updates were documented outside the medical record, so requests for status updates (via various opportunities and methods) were common and redundant. Data from the year preceding consult implementation and the year following consult implementation have demonstrated increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care.
Consult Request
The precision oncology testing process starts with a health care practitioner (HCP) request on behalf of any physician or advanced practice registered nurse. It can be placed by any health care employee and directed to a designated employee in the pathology department. The request is ultimately reviewed by a pathologist (Figure 1). At KCVAMC, this request comes in the form of a consult in the electronic health record (EHR) from the ordering HCP to a pathologist. The KCVAMC pathology consult form was previously published with a discussion of the rationale for this process as opposed to a laboratory order process.2 This consult form ensures ordering HCPs supply all necessary information for the pathologist to approve the request and order the test without needing to, in most cases, contact the ordering HCP for clarification or additional information. The form asks the ordering HCP to specify which test is being requested and why. Within the Veterans Health Administration (VHA) there are local and national contracts with many laboratories with hundreds of precision oncology tests to choose from. Consulting with a pathologist is necessary to determine which test is most appropriate.
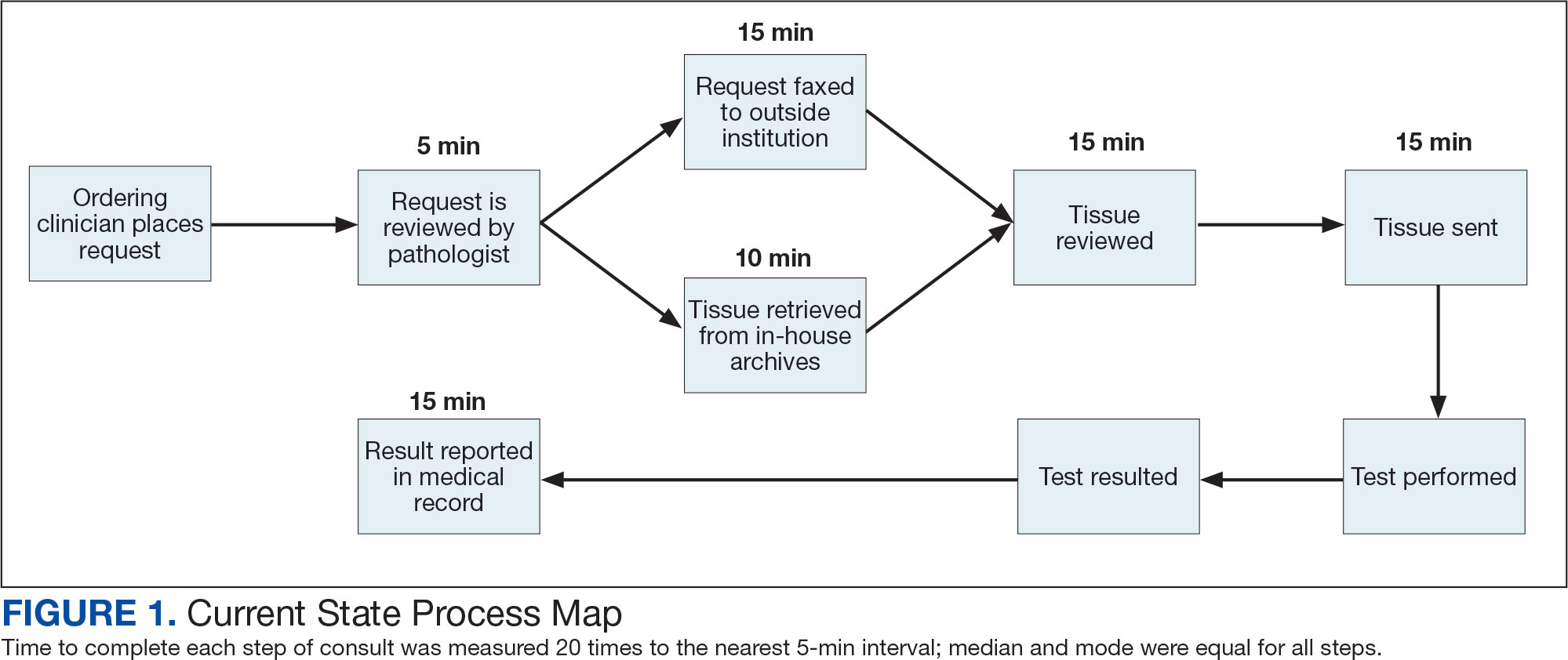
The precision oncology consult form cannot be submitted without completing all required fields. It also contains indications for the test the ordering HCP selects to minimize unintentionally inappropriate orders. The form asks which tissue the requestor expects the test to be performed on. The requestor must provide contact information for the originating institution when the tissue was collected outside the VHA. The consult form also asks whether another anatomic site is accessible and could be biopsied without unacceptable risk or impracticality, should all previously collected tissue be insufficient. For CGP requests, this allows the pathologist to determine the appropriateness of liquid biopsy without having to reach out to the ordering HCP or wait for the question to be addressed at a tumor board. When a companion diagnostic is available for a test, the ordering HCP is asked which drug will be used so that the most appropriate assay is chosen.
Consult Review
Pathology service involvement begins with pathologist review of the consult form to ensure that the correct test is indicated. Depending on the resources and preferences at a site, consults can be directed to and reviewed by the pathologist associated with the corresponding pathology specimen or to a single pathologist or group of pathologists charged with attending to consults.
The patient’s EHR is reviewed to verify that the test has not already been performed and to determine which tissue to review. Previous surgical pathology reports are examined to assess whether sufficient tissue is available for testing, which may be determined without the need for direct slide examination. Pathologists often use wording such as “rare cells” or in some cases specify that there are not enough lesional cells for ancillary testing. In biopsy reports, the percentage of tissue occupied by lesional cells or the greatest linear length of tumor cells is often documented. As for quality, pathologists may note that a specimen is largely necrotic, and gross descriptions will indicate if a specimen was compromised for molecular analysis by exposure to fixatives such as Bouin’s solution, B-5, or decalcifying agents that contain strong acids.
Tissue Retrieval
If, after such evaluation, the test is indicated and there is tissue that could be sufficient for testing, retrieval of the tissue is pursued. For in-house cases, the pathologist reviews the corresponding surgical pathology report to determine which blocks and slides to pull from the archives. In the cancer checklist, some pathologists specify the best block for subsequent ancillary studies. From the final diagnosis and gross description, the pathologist can determine which blocks are most likely to contain lesional tissue. These slides are retrieved from the archives.
For cases collected at an outside institution (other VHA facility or non-VHA facility/institution), the outside institution must be contacted to retrieve the needed slides and blocks. The phone numbers, fax numbers, email addresses, and mailing addresses for outside institutions are housed in an electronic file and are specific to the point of contact for such requests. Maintaining a record of contacts increases efficiency of the overall process; gathering contact information and successfully requesting tissue often involves multiple automated answering systems, misdirected calls, and failed attempts.
Tissue Review
After retrieving in-house tissue, the pathologist can proceed directly to slide review. For outside cases, the case must first be accessioned so that after review of the slides the pathologist can issue a report to confirm the outside diagnosis. In reviewing the slides, the pathologist looks to see that the diagnosis is correct, that there is a sufficient number of lesional cells in a section, that the lesional cells are of a sufficient concentration in a section, or subsection of the section that could be dissected, and that the cells are viable. Depending on the requested assay and the familiarity of the pathologist with that assay, the pathologist may need to look up the technical requirements of the assay and capabilities of the testing company. Assays vary in sensitivity and require differing amounts and concentrations of tumor. Some companies will dissect tissue, others will not.
If there is sufficient tissue in the material reviewed, the corresponding blocks are retrieved from in-house archives or requests are placed for outside blocks or unstained slides. If there was not enough tissue for testing, the same process is repeated to retrieve and evaluate any other specimens the patient may have. If there are no other specimens to review, this is simply communicated to the ordering HCP via the consult. If the patient is a candidate for liquid biopsy—ie, current specimens are of insufficient quality and/or quantity and a new tissue sample cannot be obtained due to unacceptable risk or impracticality—the order is placed at this time.
Tissue Transport and Testing
Unstained slides need to be cut unless blocks are sent. Slides, blocks, reports, and requisition forms are packaged for transport. An accession number is created for the precision oncology molecular laboratory test in the clinical laboratory section of the EHR system. The clinical laboratory accession number provides a way of tracking sendout testing status. The case is accessioned just prior to placement in the mail so that when an accession number appears in the EHR, the ordering HCP knows the case has been sent out. When results are received, the clinical laboratory accession is completed and a comment is added to indicate where in the EHR to find the report or, when applicable, notes that testing failed.
RESULT REPORTING
When a result becomes available, the report file is downloaded from the vendor portal. This full report is securely transmitted to the ordering HCP. The file is then scanned into the EHR. Additionally, salient findings from the report are abstracted by the pathologist for inclusion as a supplement to the anatomic pathology case. This step ensures that this information travels with the anatomic pathology report if the patient’s care is transferred elsewhere. Templates are used to ensure essential data is captured based on the type of test. The template reminds the pathologist to comment on things such as variants that may represent clonal hematopoiesis, variants that may be germline, and variants that qualify a patient for germline testing. Even with the template, the pathologist must spend significant time reviewing the chart for things such as personal cancer history, other medical history, other masses on imaging, family history, previous surgical pathology reports, and previous molecular testing.
If results are suboptimal, recommendations for repeat testing are made based on the consult response to the question of repeat biopsy feasibility and review of previous pathology reports. The final consult report is added as a consult note, the consult is completed, and the original vendor report file is associated with the consult note in the EHR.
Ancillary Testing Technician
Due to chronic KCVAMC understaffing in the clerical office, gross room, and histology, most of the consult tasks are performed by a pathologist. In an ideal scenario, the pathology staff would divide its time between a pathologist and another dedicated laboratory position, such as an ancillary testing technician (ATT). The ATT can assume responsibilities that do not require the expertise of a pathologist (Figure 2). In such a process, the only steps that would require a pathologist would be review of requests and slides and completion of the interpretive report. All other steps could be accomplished by someone who lacks certifications, laboratory experience, or postsecondary education.
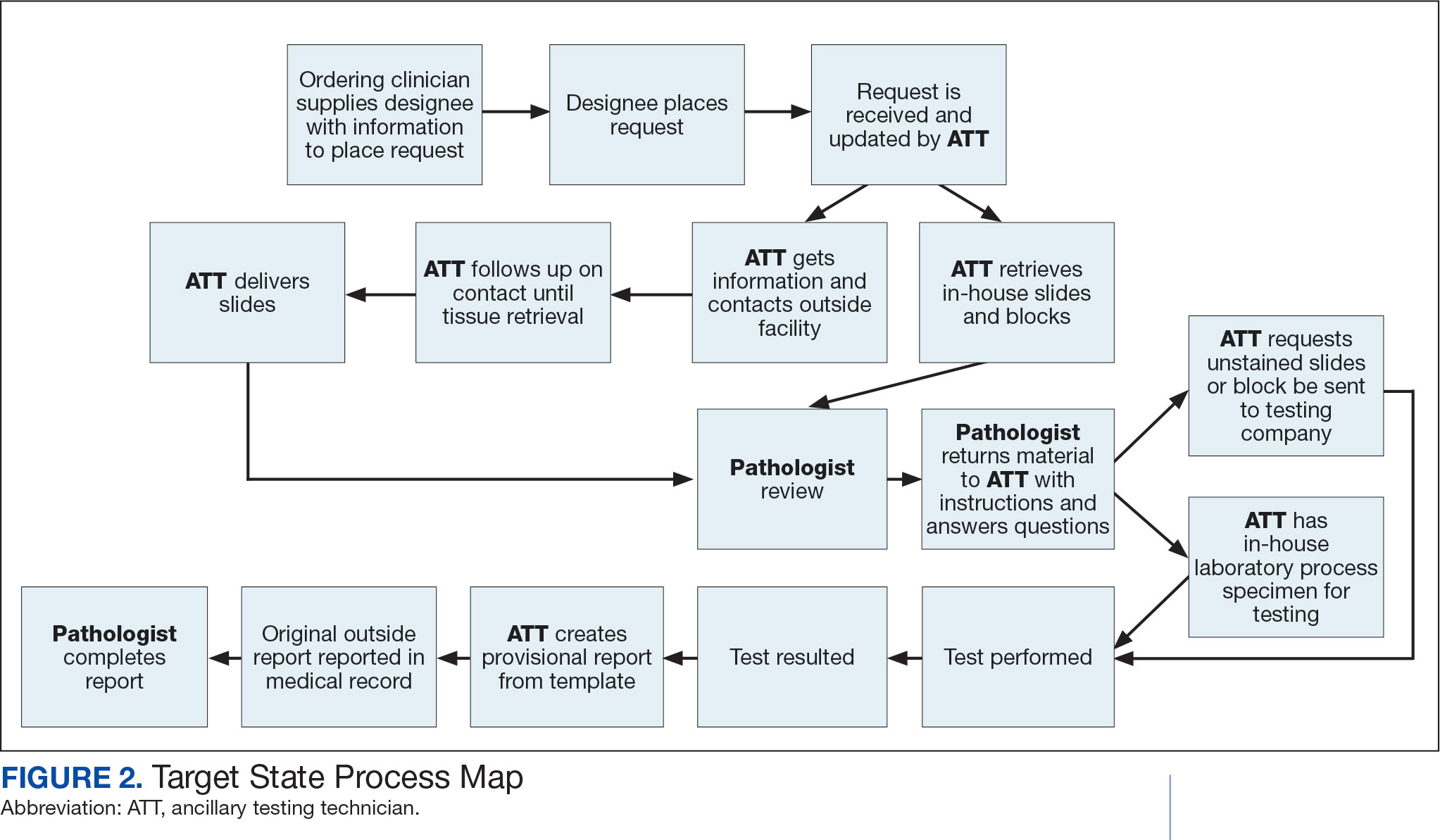
The ATT can receive the requests and retrieve slides and blocks. After slides have been reviewed by a pathologist, the pathologist can inform the ATT which slides or blocks testing will be performed on, provide any additional necessary information for completing the order, and answer any questions. For send-out tests, this allows the ATT to independently complete online portal forms and all other physical requirements prior to delivery of the slides and blocks to specimen processors in the laboratory.
ATTs can keep the ordering HCPs informed of status and be identified as the point of contact for all status inquiries. ATTs can receive results and get outside reports scanned into the EHR. Finally, ATTs can use pathologistdesigned templates to transpose information from outside reports such that a provisional report is prepared and a pathologist does not spend time duplicating information from the outside report. The pathologist can then complete the report with information requiring medical judgment that enhances care.
Optimal Pathologist Involvement
Only 3 steps in the process (request review, tissue review, and completion of an interpretive report) require a pathologist, which are necessary for optimal care and to address barriers to precision oncology.3 While the laboratory may consume only 5% of a health system budget, optimal laboratory use could prevent as much as 30% of avoidable costs.4 These estimates are widely recognized and addressed by campaigns such as Choosing Wisely, as well as programming of alerts and hard stops in EHR systems to reduce duplicate or otherwise inappropriate orders. The tests associated with precision oncology, such as CGP assays, require more nuanced consideration that is best achieved through pathology consultation. In vetting requests for such tests, the pathologist needs information that ordering HCPs do not routinely provide when ordering other tests. A consult asking for such information allows an ordering HCP to efficiently convey this information without having to call the laboratory to circumvent a hard stop.
Regardless of whether a formal electronic consult is used, pathologists must be involved in the review of requests. Creation of an original in-house report also provides an opportunity for pathologists to offer their expertise and maximize the contribution of pathology to patient care. If outside (other VHA facility or non-VHA facility/institution) reports are simply scanned into the EHR without review and issuance of an interpretive report by an in-house pathologist, then an interpretation by a pathologist with access to the patient’s complete chart is never provided. Testing companies are not provided with every patient diagnosis, so in patients with multiple neoplastic conditions, a report may seem to indicate that a detected mutation is from 1 tumor when it is actually from another. Even when all known diagnoses are considered, a variant may be detected that the medical record could reveal to indicate a new diagnosis.
Variation in reporting between companies necessitates pathologist review to standardize care. Some companies indicate which variants may represent clonal hematopoiesis, while others will simply list the pathogenic variants. An oncologist who sees a high volume of hematolymphoid neoplasia may recognize which variants may represent clonal hematopoiesis, but others may not. Reports from the same company may vary, and their interpretation often requires a pathologist's expertise. For example, even if a sample meets the technical requirements for analysis, the report may indicate that the quality or quantity of DNA has reduced the sensitivity for genomic alteration detection. A pathologist would know how to use this information in deciding how to proceed. In a situation where quantity was the issue, the pathologist may know there is additional tissue that could be sent for testing. If quality is the issue, the pathologist may know that additional blocks from the same case likely have the same quality of DNA and would also be unsuitable for testing.
Pathologist input is necessary for precision oncology testing. Some tasks that would ideally be completed by a molecular pathologist (eg, creation of reports to indicate which variants may represent clonal hematopoiesis of indeterminate potential) may be sufficiently completed by a pathologist without fellowship training in molecular pathology.
There are about 15,000 full-time pathologists in the US.4 In the 20 years since molecular genetic pathology was formally recognized as a specialty, there have been < 500 pathologists who have pursued fellowship training in this specialty.5 With the inundation of molecular variants uncovered by routine next-generation sequencing (NGS), there are too few fellowship-trained molecular pathologists to provide all such aforementioned input; it is incumbent on surgical pathologists in general to take on such responsibilities.
Consult Implementation Data
These results support the feasibility and effectiveness of the consult process. Prior to consult implementation, many requests were not compliant with VHA National Precision Oncology Program (NPOP) testing guidelines. Since enactment of the consult, > 90% of requests have been in compliance. In the year preceding the consult (January 2020 to December 2021), 55 of 211 (26.1%) metastatic lung and prostate cancers samples eligible for NGS were tested and 126 (59.7%) NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 151 days. In the year following enactment of the consult (January 2021 to December 2022), 168 of 224 (75.0%) of metastatic lung and prostate cancers eligible for NGS were tested and all 224 NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 83 days. These data indicate that the practices recommended increase test use, appropriateness of orders, standardization of reporting, and efficiency of care.
CONCLUSIONS
Processing precision oncology testing requires substantial work for pathology departments. Laboratory workforce shortages and ever-expanding indications necessitate additional study of pathology processes to manage increasing workload and maintain the highest quality of cancer care through maximal efficiency and the development of appropriate staffing models. The use of a consult for anatomic pathology molecular testing is one process that can increase test use, appropriateness of orders, standardization of reporting, and efficiency of care. This report provides a comprehensive description and mapping of the process, highlights best practices, identifies inefficiencies, and provides a description and mapping of a target state.
- Inal C, Yilmaz E, Cheng H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: experience from a community-based academic center. J Clin Oncol. 2014;32(15 suppl):8098. doi:10.1200/jco.2014.32.15_suppl.8098
- Mettman D, Goodman M, Modzelewski J, et al. Streamlining institutional pathway processes: the development and implementation of a pathology molecular consult to facilitate convenient and efficient ordering, fulfillment, and reporting for tissue molecular tests. J Clin Pathw.Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633 2022;8(1):28-33.
- Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633
- Robboy SJ, Gupta S, Crawford JM, et al. The pathologist workforce in the United States: II. An interactive modeling tool for analyzing future qualitative and quantitative staffing demands for services. Arch Pathol Lab Med. 2015;139(11):1413-1430. doi:10.5858/arpa.2014-0559-OA doi:10.25270/jcp.2022.02.1
- Robboy SJ, Gross D, Park JY, et al. Reevaluation of the US pathologist workforce size. JAMA Netw Open. 2020;3(7): e2010648. doi:10.1001/jamanetworkopen.2020.10648
Comprehensive genomic profiling (CGP) is becoming progressively common and appropriate as the array of molecular targets expands. However, most hospital laboratories in the United States do not perform CGP assays in-house; instead, these tests are sent to reference laboratories. As evidenced by Inal et al, only a minority of guideline-indicated molecular testing is performed.1
The workload associated with referral testing is a barrier to increased use of such tests; streamlined processes in pathology might increase molecular test use. At 6 high-complexity US Department of Veterans Affairs (VA) medical centers (VAMCs) (Manhattan, Los Angeles, San Diego, Denver, Kansas City, and Salisbury, Maryland) ranging from 150 to 750 beds, a consult process for anatomic pathology molecular testing has increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care. This report comprehensively describes and maps the anatomic pathology molecular testing consult process at a VAMC. We present areas of inefficiency and a target state process map that incorporates best practices.
MOLECULAR TESTING CONSULT PROCESS
At the Kansas City VAMC (KCVAMC), a consult process for anatomic pathology molecular testing was introduced in 2021. Prior to this, requesting anatomic pathology molecular testing was not standardized. A variety of opportunities and methods were used for requests (eg, phone, page, Teams message, email, Computerized Patient Record System alert; or in-person during tumor board, an office meeting, or in passing). Requests were not documented in a standardized way, resulting in duplicate requests. Testing status and updates were documented outside the medical record, so requests for status updates (via various opportunities and methods) were common and redundant. Data from the year preceding consult implementation and the year following consult implementation have demonstrated increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care.
Consult Request
The precision oncology testing process starts with a health care practitioner (HCP) request on behalf of any physician or advanced practice registered nurse. It can be placed by any health care employee and directed to a designated employee in the pathology department. The request is ultimately reviewed by a pathologist (Figure 1). At KCVAMC, this request comes in the form of a consult in the electronic health record (EHR) from the ordering HCP to a pathologist. The KCVAMC pathology consult form was previously published with a discussion of the rationale for this process as opposed to a laboratory order process.2 This consult form ensures ordering HCPs supply all necessary information for the pathologist to approve the request and order the test without needing to, in most cases, contact the ordering HCP for clarification or additional information. The form asks the ordering HCP to specify which test is being requested and why. Within the Veterans Health Administration (VHA) there are local and national contracts with many laboratories with hundreds of precision oncology tests to choose from. Consulting with a pathologist is necessary to determine which test is most appropriate.

The precision oncology consult form cannot be submitted without completing all required fields. It also contains indications for the test the ordering HCP selects to minimize unintentionally inappropriate orders. The form asks which tissue the requestor expects the test to be performed on. The requestor must provide contact information for the originating institution when the tissue was collected outside the VHA. The consult form also asks whether another anatomic site is accessible and could be biopsied without unacceptable risk or impracticality, should all previously collected tissue be insufficient. For CGP requests, this allows the pathologist to determine the appropriateness of liquid biopsy without having to reach out to the ordering HCP or wait for the question to be addressed at a tumor board. When a companion diagnostic is available for a test, the ordering HCP is asked which drug will be used so that the most appropriate assay is chosen.
Consult Review
Pathology service involvement begins with pathologist review of the consult form to ensure that the correct test is indicated. Depending on the resources and preferences at a site, consults can be directed to and reviewed by the pathologist associated with the corresponding pathology specimen or to a single pathologist or group of pathologists charged with attending to consults.
The patient’s EHR is reviewed to verify that the test has not already been performed and to determine which tissue to review. Previous surgical pathology reports are examined to assess whether sufficient tissue is available for testing, which may be determined without the need for direct slide examination. Pathologists often use wording such as “rare cells” or in some cases specify that there are not enough lesional cells for ancillary testing. In biopsy reports, the percentage of tissue occupied by lesional cells or the greatest linear length of tumor cells is often documented. As for quality, pathologists may note that a specimen is largely necrotic, and gross descriptions will indicate if a specimen was compromised for molecular analysis by exposure to fixatives such as Bouin’s solution, B-5, or decalcifying agents that contain strong acids.
Tissue Retrieval
If, after such evaluation, the test is indicated and there is tissue that could be sufficient for testing, retrieval of the tissue is pursued. For in-house cases, the pathologist reviews the corresponding surgical pathology report to determine which blocks and slides to pull from the archives. In the cancer checklist, some pathologists specify the best block for subsequent ancillary studies. From the final diagnosis and gross description, the pathologist can determine which blocks are most likely to contain lesional tissue. These slides are retrieved from the archives.
For cases collected at an outside institution (other VHA facility or non-VHA facility/institution), the outside institution must be contacted to retrieve the needed slides and blocks. The phone numbers, fax numbers, email addresses, and mailing addresses for outside institutions are housed in an electronic file and are specific to the point of contact for such requests. Maintaining a record of contacts increases efficiency of the overall process; gathering contact information and successfully requesting tissue often involves multiple automated answering systems, misdirected calls, and failed attempts.
Tissue Review
After retrieving in-house tissue, the pathologist can proceed directly to slide review. For outside cases, the case must first be accessioned so that after review of the slides the pathologist can issue a report to confirm the outside diagnosis. In reviewing the slides, the pathologist looks to see that the diagnosis is correct, that there is a sufficient number of lesional cells in a section, that the lesional cells are of a sufficient concentration in a section, or subsection of the section that could be dissected, and that the cells are viable. Depending on the requested assay and the familiarity of the pathologist with that assay, the pathologist may need to look up the technical requirements of the assay and capabilities of the testing company. Assays vary in sensitivity and require differing amounts and concentrations of tumor. Some companies will dissect tissue, others will not.
If there is sufficient tissue in the material reviewed, the corresponding blocks are retrieved from in-house archives or requests are placed for outside blocks or unstained slides. If there was not enough tissue for testing, the same process is repeated to retrieve and evaluate any other specimens the patient may have. If there are no other specimens to review, this is simply communicated to the ordering HCP via the consult. If the patient is a candidate for liquid biopsy—ie, current specimens are of insufficient quality and/or quantity and a new tissue sample cannot be obtained due to unacceptable risk or impracticality—the order is placed at this time.
Tissue Transport and Testing
Unstained slides need to be cut unless blocks are sent. Slides, blocks, reports, and requisition forms are packaged for transport. An accession number is created for the precision oncology molecular laboratory test in the clinical laboratory section of the EHR system. The clinical laboratory accession number provides a way of tracking sendout testing status. The case is accessioned just prior to placement in the mail so that when an accession number appears in the EHR, the ordering HCP knows the case has been sent out. When results are received, the clinical laboratory accession is completed and a comment is added to indicate where in the EHR to find the report or, when applicable, notes that testing failed.
RESULT REPORTING
When a result becomes available, the report file is downloaded from the vendor portal. This full report is securely transmitted to the ordering HCP. The file is then scanned into the EHR. Additionally, salient findings from the report are abstracted by the pathologist for inclusion as a supplement to the anatomic pathology case. This step ensures that this information travels with the anatomic pathology report if the patient’s care is transferred elsewhere. Templates are used to ensure essential data is captured based on the type of test. The template reminds the pathologist to comment on things such as variants that may represent clonal hematopoiesis, variants that may be germline, and variants that qualify a patient for germline testing. Even with the template, the pathologist must spend significant time reviewing the chart for things such as personal cancer history, other medical history, other masses on imaging, family history, previous surgical pathology reports, and previous molecular testing.
If results are suboptimal, recommendations for repeat testing are made based on the consult response to the question of repeat biopsy feasibility and review of previous pathology reports. The final consult report is added as a consult note, the consult is completed, and the original vendor report file is associated with the consult note in the EHR.
Ancillary Testing Technician
Due to chronic KCVAMC understaffing in the clerical office, gross room, and histology, most of the consult tasks are performed by a pathologist. In an ideal scenario, the pathology staff would divide its time between a pathologist and another dedicated laboratory position, such as an ancillary testing technician (ATT). The ATT can assume responsibilities that do not require the expertise of a pathologist (Figure 2). In such a process, the only steps that would require a pathologist would be review of requests and slides and completion of the interpretive report. All other steps could be accomplished by someone who lacks certifications, laboratory experience, or postsecondary education.

The ATT can receive the requests and retrieve slides and blocks. After slides have been reviewed by a pathologist, the pathologist can inform the ATT which slides or blocks testing will be performed on, provide any additional necessary information for completing the order, and answer any questions. For send-out tests, this allows the ATT to independently complete online portal forms and all other physical requirements prior to delivery of the slides and blocks to specimen processors in the laboratory.
ATTs can keep the ordering HCPs informed of status and be identified as the point of contact for all status inquiries. ATTs can receive results and get outside reports scanned into the EHR. Finally, ATTs can use pathologistdesigned templates to transpose information from outside reports such that a provisional report is prepared and a pathologist does not spend time duplicating information from the outside report. The pathologist can then complete the report with information requiring medical judgment that enhances care.
Optimal Pathologist Involvement
Only 3 steps in the process (request review, tissue review, and completion of an interpretive report) require a pathologist, which are necessary for optimal care and to address barriers to precision oncology.3 While the laboratory may consume only 5% of a health system budget, optimal laboratory use could prevent as much as 30% of avoidable costs.4 These estimates are widely recognized and addressed by campaigns such as Choosing Wisely, as well as programming of alerts and hard stops in EHR systems to reduce duplicate or otherwise inappropriate orders. The tests associated with precision oncology, such as CGP assays, require more nuanced consideration that is best achieved through pathology consultation. In vetting requests for such tests, the pathologist needs information that ordering HCPs do not routinely provide when ordering other tests. A consult asking for such information allows an ordering HCP to efficiently convey this information without having to call the laboratory to circumvent a hard stop.
Regardless of whether a formal electronic consult is used, pathologists must be involved in the review of requests. Creation of an original in-house report also provides an opportunity for pathologists to offer their expertise and maximize the contribution of pathology to patient care. If outside (other VHA facility or non-VHA facility/institution) reports are simply scanned into the EHR without review and issuance of an interpretive report by an in-house pathologist, then an interpretation by a pathologist with access to the patient’s complete chart is never provided. Testing companies are not provided with every patient diagnosis, so in patients with multiple neoplastic conditions, a report may seem to indicate that a detected mutation is from 1 tumor when it is actually from another. Even when all known diagnoses are considered, a variant may be detected that the medical record could reveal to indicate a new diagnosis.
Variation in reporting between companies necessitates pathologist review to standardize care. Some companies indicate which variants may represent clonal hematopoiesis, while others will simply list the pathogenic variants. An oncologist who sees a high volume of hematolymphoid neoplasia may recognize which variants may represent clonal hematopoiesis, but others may not. Reports from the same company may vary, and their interpretation often requires a pathologist's expertise. For example, even if a sample meets the technical requirements for analysis, the report may indicate that the quality or quantity of DNA has reduced the sensitivity for genomic alteration detection. A pathologist would know how to use this information in deciding how to proceed. In a situation where quantity was the issue, the pathologist may know there is additional tissue that could be sent for testing. If quality is the issue, the pathologist may know that additional blocks from the same case likely have the same quality of DNA and would also be unsuitable for testing.
Pathologist input is necessary for precision oncology testing. Some tasks that would ideally be completed by a molecular pathologist (eg, creation of reports to indicate which variants may represent clonal hematopoiesis of indeterminate potential) may be sufficiently completed by a pathologist without fellowship training in molecular pathology.
There are about 15,000 full-time pathologists in the US.4 In the 20 years since molecular genetic pathology was formally recognized as a specialty, there have been < 500 pathologists who have pursued fellowship training in this specialty.5 With the inundation of molecular variants uncovered by routine next-generation sequencing (NGS), there are too few fellowship-trained molecular pathologists to provide all such aforementioned input; it is incumbent on surgical pathologists in general to take on such responsibilities.
Consult Implementation Data
These results support the feasibility and effectiveness of the consult process. Prior to consult implementation, many requests were not compliant with VHA National Precision Oncology Program (NPOP) testing guidelines. Since enactment of the consult, > 90% of requests have been in compliance. In the year preceding the consult (January 2020 to December 2021), 55 of 211 (26.1%) metastatic lung and prostate cancers samples eligible for NGS were tested and 126 (59.7%) NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 151 days. In the year following enactment of the consult (January 2021 to December 2022), 168 of 224 (75.0%) of metastatic lung and prostate cancers eligible for NGS were tested and all 224 NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 83 days. These data indicate that the practices recommended increase test use, appropriateness of orders, standardization of reporting, and efficiency of care.
CONCLUSIONS
Processing precision oncology testing requires substantial work for pathology departments. Laboratory workforce shortages and ever-expanding indications necessitate additional study of pathology processes to manage increasing workload and maintain the highest quality of cancer care through maximal efficiency and the development of appropriate staffing models. The use of a consult for anatomic pathology molecular testing is one process that can increase test use, appropriateness of orders, standardization of reporting, and efficiency of care. This report provides a comprehensive description and mapping of the process, highlights best practices, identifies inefficiencies, and provides a description and mapping of a target state.
Comprehensive genomic profiling (CGP) is becoming progressively common and appropriate as the array of molecular targets expands. However, most hospital laboratories in the United States do not perform CGP assays in-house; instead, these tests are sent to reference laboratories. As evidenced by Inal et al, only a minority of guideline-indicated molecular testing is performed.1
The workload associated with referral testing is a barrier to increased use of such tests; streamlined processes in pathology might increase molecular test use. At 6 high-complexity US Department of Veterans Affairs (VA) medical centers (VAMCs) (Manhattan, Los Angeles, San Diego, Denver, Kansas City, and Salisbury, Maryland) ranging from 150 to 750 beds, a consult process for anatomic pathology molecular testing has increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care. This report comprehensively describes and maps the anatomic pathology molecular testing consult process at a VAMC. We present areas of inefficiency and a target state process map that incorporates best practices.
MOLECULAR TESTING CONSULT PROCESS
At the Kansas City VAMC (KCVAMC), a consult process for anatomic pathology molecular testing was introduced in 2021. Prior to this, requesting anatomic pathology molecular testing was not standardized. A variety of opportunities and methods were used for requests (eg, phone, page, Teams message, email, Computerized Patient Record System alert; or in-person during tumor board, an office meeting, or in passing). Requests were not documented in a standardized way, resulting in duplicate requests. Testing status and updates were documented outside the medical record, so requests for status updates (via various opportunities and methods) were common and redundant. Data from the year preceding consult implementation and the year following consult implementation have demonstrated increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care.
Consult Request
The precision oncology testing process starts with a health care practitioner (HCP) request on behalf of any physician or advanced practice registered nurse. It can be placed by any health care employee and directed to a designated employee in the pathology department. The request is ultimately reviewed by a pathologist (Figure 1). At KCVAMC, this request comes in the form of a consult in the electronic health record (EHR) from the ordering HCP to a pathologist. The KCVAMC pathology consult form was previously published with a discussion of the rationale for this process as opposed to a laboratory order process.2 This consult form ensures ordering HCPs supply all necessary information for the pathologist to approve the request and order the test without needing to, in most cases, contact the ordering HCP for clarification or additional information. The form asks the ordering HCP to specify which test is being requested and why. Within the Veterans Health Administration (VHA) there are local and national contracts with many laboratories with hundreds of precision oncology tests to choose from. Consulting with a pathologist is necessary to determine which test is most appropriate.

The precision oncology consult form cannot be submitted without completing all required fields. It also contains indications for the test the ordering HCP selects to minimize unintentionally inappropriate orders. The form asks which tissue the requestor expects the test to be performed on. The requestor must provide contact information for the originating institution when the tissue was collected outside the VHA. The consult form also asks whether another anatomic site is accessible and could be biopsied without unacceptable risk or impracticality, should all previously collected tissue be insufficient. For CGP requests, this allows the pathologist to determine the appropriateness of liquid biopsy without having to reach out to the ordering HCP or wait for the question to be addressed at a tumor board. When a companion diagnostic is available for a test, the ordering HCP is asked which drug will be used so that the most appropriate assay is chosen.
Consult Review
Pathology service involvement begins with pathologist review of the consult form to ensure that the correct test is indicated. Depending on the resources and preferences at a site, consults can be directed to and reviewed by the pathologist associated with the corresponding pathology specimen or to a single pathologist or group of pathologists charged with attending to consults.
The patient’s EHR is reviewed to verify that the test has not already been performed and to determine which tissue to review. Previous surgical pathology reports are examined to assess whether sufficient tissue is available for testing, which may be determined without the need for direct slide examination. Pathologists often use wording such as “rare cells” or in some cases specify that there are not enough lesional cells for ancillary testing. In biopsy reports, the percentage of tissue occupied by lesional cells or the greatest linear length of tumor cells is often documented. As for quality, pathologists may note that a specimen is largely necrotic, and gross descriptions will indicate if a specimen was compromised for molecular analysis by exposure to fixatives such as Bouin’s solution, B-5, or decalcifying agents that contain strong acids.
Tissue Retrieval
If, after such evaluation, the test is indicated and there is tissue that could be sufficient for testing, retrieval of the tissue is pursued. For in-house cases, the pathologist reviews the corresponding surgical pathology report to determine which blocks and slides to pull from the archives. In the cancer checklist, some pathologists specify the best block for subsequent ancillary studies. From the final diagnosis and gross description, the pathologist can determine which blocks are most likely to contain lesional tissue. These slides are retrieved from the archives.
For cases collected at an outside institution (other VHA facility or non-VHA facility/institution), the outside institution must be contacted to retrieve the needed slides and blocks. The phone numbers, fax numbers, email addresses, and mailing addresses for outside institutions are housed in an electronic file and are specific to the point of contact for such requests. Maintaining a record of contacts increases efficiency of the overall process; gathering contact information and successfully requesting tissue often involves multiple automated answering systems, misdirected calls, and failed attempts.
Tissue Review
After retrieving in-house tissue, the pathologist can proceed directly to slide review. For outside cases, the case must first be accessioned so that after review of the slides the pathologist can issue a report to confirm the outside diagnosis. In reviewing the slides, the pathologist looks to see that the diagnosis is correct, that there is a sufficient number of lesional cells in a section, that the lesional cells are of a sufficient concentration in a section, or subsection of the section that could be dissected, and that the cells are viable. Depending on the requested assay and the familiarity of the pathologist with that assay, the pathologist may need to look up the technical requirements of the assay and capabilities of the testing company. Assays vary in sensitivity and require differing amounts and concentrations of tumor. Some companies will dissect tissue, others will not.
If there is sufficient tissue in the material reviewed, the corresponding blocks are retrieved from in-house archives or requests are placed for outside blocks or unstained slides. If there was not enough tissue for testing, the same process is repeated to retrieve and evaluate any other specimens the patient may have. If there are no other specimens to review, this is simply communicated to the ordering HCP via the consult. If the patient is a candidate for liquid biopsy—ie, current specimens are of insufficient quality and/or quantity and a new tissue sample cannot be obtained due to unacceptable risk or impracticality—the order is placed at this time.
Tissue Transport and Testing
Unstained slides need to be cut unless blocks are sent. Slides, blocks, reports, and requisition forms are packaged for transport. An accession number is created for the precision oncology molecular laboratory test in the clinical laboratory section of the EHR system. The clinical laboratory accession number provides a way of tracking sendout testing status. The case is accessioned just prior to placement in the mail so that when an accession number appears in the EHR, the ordering HCP knows the case has been sent out. When results are received, the clinical laboratory accession is completed and a comment is added to indicate where in the EHR to find the report or, when applicable, notes that testing failed.
RESULT REPORTING
When a result becomes available, the report file is downloaded from the vendor portal. This full report is securely transmitted to the ordering HCP. The file is then scanned into the EHR. Additionally, salient findings from the report are abstracted by the pathologist for inclusion as a supplement to the anatomic pathology case. This step ensures that this information travels with the anatomic pathology report if the patient’s care is transferred elsewhere. Templates are used to ensure essential data is captured based on the type of test. The template reminds the pathologist to comment on things such as variants that may represent clonal hematopoiesis, variants that may be germline, and variants that qualify a patient for germline testing. Even with the template, the pathologist must spend significant time reviewing the chart for things such as personal cancer history, other medical history, other masses on imaging, family history, previous surgical pathology reports, and previous molecular testing.
If results are suboptimal, recommendations for repeat testing are made based on the consult response to the question of repeat biopsy feasibility and review of previous pathology reports. The final consult report is added as a consult note, the consult is completed, and the original vendor report file is associated with the consult note in the EHR.
Ancillary Testing Technician
Due to chronic KCVAMC understaffing in the clerical office, gross room, and histology, most of the consult tasks are performed by a pathologist. In an ideal scenario, the pathology staff would divide its time between a pathologist and another dedicated laboratory position, such as an ancillary testing technician (ATT). The ATT can assume responsibilities that do not require the expertise of a pathologist (Figure 2). In such a process, the only steps that would require a pathologist would be review of requests and slides and completion of the interpretive report. All other steps could be accomplished by someone who lacks certifications, laboratory experience, or postsecondary education.

The ATT can receive the requests and retrieve slides and blocks. After slides have been reviewed by a pathologist, the pathologist can inform the ATT which slides or blocks testing will be performed on, provide any additional necessary information for completing the order, and answer any questions. For send-out tests, this allows the ATT to independently complete online portal forms and all other physical requirements prior to delivery of the slides and blocks to specimen processors in the laboratory.
ATTs can keep the ordering HCPs informed of status and be identified as the point of contact for all status inquiries. ATTs can receive results and get outside reports scanned into the EHR. Finally, ATTs can use pathologistdesigned templates to transpose information from outside reports such that a provisional report is prepared and a pathologist does not spend time duplicating information from the outside report. The pathologist can then complete the report with information requiring medical judgment that enhances care.
Optimal Pathologist Involvement
Only 3 steps in the process (request review, tissue review, and completion of an interpretive report) require a pathologist, which are necessary for optimal care and to address barriers to precision oncology.3 While the laboratory may consume only 5% of a health system budget, optimal laboratory use could prevent as much as 30% of avoidable costs.4 These estimates are widely recognized and addressed by campaigns such as Choosing Wisely, as well as programming of alerts and hard stops in EHR systems to reduce duplicate or otherwise inappropriate orders. The tests associated with precision oncology, such as CGP assays, require more nuanced consideration that is best achieved through pathology consultation. In vetting requests for such tests, the pathologist needs information that ordering HCPs do not routinely provide when ordering other tests. A consult asking for such information allows an ordering HCP to efficiently convey this information without having to call the laboratory to circumvent a hard stop.
Regardless of whether a formal electronic consult is used, pathologists must be involved in the review of requests. Creation of an original in-house report also provides an opportunity for pathologists to offer their expertise and maximize the contribution of pathology to patient care. If outside (other VHA facility or non-VHA facility/institution) reports are simply scanned into the EHR without review and issuance of an interpretive report by an in-house pathologist, then an interpretation by a pathologist with access to the patient’s complete chart is never provided. Testing companies are not provided with every patient diagnosis, so in patients with multiple neoplastic conditions, a report may seem to indicate that a detected mutation is from 1 tumor when it is actually from another. Even when all known diagnoses are considered, a variant may be detected that the medical record could reveal to indicate a new diagnosis.
Variation in reporting between companies necessitates pathologist review to standardize care. Some companies indicate which variants may represent clonal hematopoiesis, while others will simply list the pathogenic variants. An oncologist who sees a high volume of hematolymphoid neoplasia may recognize which variants may represent clonal hematopoiesis, but others may not. Reports from the same company may vary, and their interpretation often requires a pathologist's expertise. For example, even if a sample meets the technical requirements for analysis, the report may indicate that the quality or quantity of DNA has reduced the sensitivity for genomic alteration detection. A pathologist would know how to use this information in deciding how to proceed. In a situation where quantity was the issue, the pathologist may know there is additional tissue that could be sent for testing. If quality is the issue, the pathologist may know that additional blocks from the same case likely have the same quality of DNA and would also be unsuitable for testing.
Pathologist input is necessary for precision oncology testing. Some tasks that would ideally be completed by a molecular pathologist (eg, creation of reports to indicate which variants may represent clonal hematopoiesis of indeterminate potential) may be sufficiently completed by a pathologist without fellowship training in molecular pathology.
There are about 15,000 full-time pathologists in the US.4 In the 20 years since molecular genetic pathology was formally recognized as a specialty, there have been < 500 pathologists who have pursued fellowship training in this specialty.5 With the inundation of molecular variants uncovered by routine next-generation sequencing (NGS), there are too few fellowship-trained molecular pathologists to provide all such aforementioned input; it is incumbent on surgical pathologists in general to take on such responsibilities.
Consult Implementation Data
These results support the feasibility and effectiveness of the consult process. Prior to consult implementation, many requests were not compliant with VHA National Precision Oncology Program (NPOP) testing guidelines. Since enactment of the consult, > 90% of requests have been in compliance. In the year preceding the consult (January 2020 to December 2021), 55 of 211 (26.1%) metastatic lung and prostate cancers samples eligible for NGS were tested and 126 (59.7%) NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 151 days. In the year following enactment of the consult (January 2021 to December 2022), 168 of 224 (75.0%) of metastatic lung and prostate cancers eligible for NGS were tested and all 224 NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 83 days. These data indicate that the practices recommended increase test use, appropriateness of orders, standardization of reporting, and efficiency of care.
CONCLUSIONS
Processing precision oncology testing requires substantial work for pathology departments. Laboratory workforce shortages and ever-expanding indications necessitate additional study of pathology processes to manage increasing workload and maintain the highest quality of cancer care through maximal efficiency and the development of appropriate staffing models. The use of a consult for anatomic pathology molecular testing is one process that can increase test use, appropriateness of orders, standardization of reporting, and efficiency of care. This report provides a comprehensive description and mapping of the process, highlights best practices, identifies inefficiencies, and provides a description and mapping of a target state.
- Inal C, Yilmaz E, Cheng H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: experience from a community-based academic center. J Clin Oncol. 2014;32(15 suppl):8098. doi:10.1200/jco.2014.32.15_suppl.8098
- Mettman D, Goodman M, Modzelewski J, et al. Streamlining institutional pathway processes: the development and implementation of a pathology molecular consult to facilitate convenient and efficient ordering, fulfillment, and reporting for tissue molecular tests. J Clin Pathw.Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633 2022;8(1):28-33.
- Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633
- Robboy SJ, Gupta S, Crawford JM, et al. The pathologist workforce in the United States: II. An interactive modeling tool for analyzing future qualitative and quantitative staffing demands for services. Arch Pathol Lab Med. 2015;139(11):1413-1430. doi:10.5858/arpa.2014-0559-OA doi:10.25270/jcp.2022.02.1
- Robboy SJ, Gross D, Park JY, et al. Reevaluation of the US pathologist workforce size. JAMA Netw Open. 2020;3(7): e2010648. doi:10.1001/jamanetworkopen.2020.10648
- Inal C, Yilmaz E, Cheng H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: experience from a community-based academic center. J Clin Oncol. 2014;32(15 suppl):8098. doi:10.1200/jco.2014.32.15_suppl.8098
- Mettman D, Goodman M, Modzelewski J, et al. Streamlining institutional pathway processes: the development and implementation of a pathology molecular consult to facilitate convenient and efficient ordering, fulfillment, and reporting for tissue molecular tests. J Clin Pathw.Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633 2022;8(1):28-33.
- Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633
- Robboy SJ, Gupta S, Crawford JM, et al. The pathologist workforce in the United States: II. An interactive modeling tool for analyzing future qualitative and quantitative staffing demands for services. Arch Pathol Lab Med. 2015;139(11):1413-1430. doi:10.5858/arpa.2014-0559-OA doi:10.25270/jcp.2022.02.1
- Robboy SJ, Gross D, Park JY, et al. Reevaluation of the US pathologist workforce size. JAMA Netw Open. 2020;3(7): e2010648. doi:10.1001/jamanetworkopen.2020.10648
Mapping Pathology Work Associated With Precision Oncology Testing
Mapping Pathology Work Associated With Precision Oncology Testing
Integrating Germline Genetics Into Precision Oncology Practice in the Veterans Health Administration: Challenges and Opportunities (FULL)
The US Department of Veterans Affairs (VA) oversees the largest integrated health care system in the nation, administering care to 9 million veterans annually throughout its distributed network of 1,255 medical centers and outpatient facilities. Every year, about 50,000 veterans are diagnosed with and treated for cancer in the VA, representing about 3% of all cancer cases in the US.1 After skin cancer, prostate, colon, and lung cancers are the most common among veterans.1 One way that VA has sought to improve the care of its large cancer patient population is through the adoption of precision oncology, an ever-evolving practice of analyzing an individual patient’s cancer to inform clinical decision making. Most often, the analysis includes conducting genetic testing of the tumor itself. Here, we describe the opportunities and challenges of integrating germline genetics into precision oncology practice.
The Intersection of Precision Oncology and Germline Genetics
Precision oncology typically refers to genetic testing of tumor DNA to identify genetic variants with potential diagnostic, prognostic, or predictive therapeutic implications. It is enabled by a growing body of knowledge that identifies key drivers of cancer development, coupled with advances in tumor analysis by next-generation sequencing and other technologies and by the availability of new and repurposed therapeutic agents.2 Precision oncology has transformed cancer care by targeting both common and rare malignancies with specific therapies that improve clinical outcomes in patients.3
Testing of tumor DNA can reveal both somatic (acquired) and germline (inherited) gene variants. Precision oncology testing strategies can include tumor-only testing with or without subtraction of suspected germline variants, or paired tumor-normal testing with explicit analysis and reporting of genes associated with germline predisposition.2 With tumor-only testing, the germline status of variants may be inferred and follow-up germline testing in normal tissue such as blood or saliva can be considered. Paired tumor-normal testing provides distinct advantages over tumor-only testing, including improvement of the mutation detection rate in tumors and streamlining interpretation of results for both the tumor and germline tests.
Regardless of the strategy used, tumor testing has the potential to uncover clinically relevant germline variation associated with heritable cancer susceptibility and other conditions, as well as carrier status for autosomal recessive disorders (eAppendix
Germline genetic information, independent of somatic variation, can influence the choice of targeted cancer therapies. For example, Mandelker and colleagues identified germline variants that would impact the treatment of 38 (3.7%) of 1,040 patients with cancer.4 Individuals with a germline pathogenic variant in a DNA repair gene (eg, BRCA1, BRCA2, ATM, CHEK2) are candidates for platinum chemotherapy and poly-(adenosine diphosphate-ribose) polymerase (PARP) inhibitors that target the inability of a tumor to repair double-stranded DNA breaks.5,6 Individuals with a germline pathogenic variant in the MSH2, MLH1, MSH6, PMS2 or EPCAM genes (ie, Lynch syndrome) have tumors that are deficient in mismatch repair, and these tumors are responsive to inhibitors of the programmed death 1 (PD1) pathway.7,8
In addition to changing treatment decisions, identifying pathogenic germline variants can have health, reproductive, and psychosocial implications for the patient and the patient’s family members.9,10 A pathogenic germline variant can imply disease risk for both the patient and his or her relatives. In these cases, it is important to ascertain family history, understand the mode of inheritance, identify at-risk relatives, review the associated phenotype, and discuss management and prevention options for the patient and for family members. For example, a germline pathogenic variant in the BRCA2 gene is associated with increased risk for breast, ovarian, pancreatic, gastric, bile duct, and laryngeal cancer, and melanoma.11 Knowledge of these increased cancer risks could inform cancer prevention and early detection options, such as more frequent and intensive surveillance starting at younger ages compared with that of average-risk individuals, use of chemoprevention treatments, and for those at highest risk, risk-reducing surgical procedures. Therefore, reporting germline test results requires the clinician to take on additional responsibilities beyond those required when reporting only somatic variants.
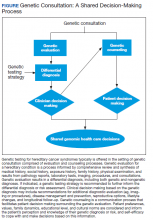
Because of the complexities inherent in germline genetic testing, it traditionally is offered in the context of a genetic consultation, comprised of genetic evaluation and genetic counseling (Figure). Clinical geneticists are physicians certified by the American Board of Medical Genetics and Genomics (a member board of the American Board of Medical Specialties) who received special training in the diagnosis and management of medical genetic conditions; they are trained to perform all aspects of a genetic consultation across the clinical spectrum and lifespan of a patient.12 In contrast, genetic counselors have a master’s degree in genetic counseling, a communication process that facilitates patient decision making surrounding the genetic evaluation.13 Most work as members of a team to ensure provision of comprehensive clinical genetic services. Genetic counselors are licensed in most states, and licensure in some states sanctions the ordering of genetic tests by genetic counselors. Genetics nurses are licensed professional nurses with special education and training in genetics who function in diverse roles in industry, education, research, and clinical care.14 Genetics nurses in clinical care perform risk assessment based on personal and family history, recognize and identify genetic conditions and predispositions, and discuss the implications of this with patients and their families. Advanced practice nurses (APRNs) have additional training that allows for diagnosis, interpretation of results, and surveillance and management recommendations.15
Germline Genetic Testing Challenges
Integrating germline genetic testing in precision oncology practice presents challenges at the patient, family, health care provider, and health system levels. Due to these challenges, implementation planning is obligatory, as germline testing has become a standard-of-care for certain tumor types and patients.2
On learning of a germline pathogenic variant or variant of uncertain significance, patients may experience distress and anxiety, especially in the short term.16-18 In addition, it can be difficult for patients to share germline genetic test results with their family; parents may feel guilty about the possibility of passing on a predisposition to children, and unaffected siblings may experience survivor guilt. For some veterans, there can be concerns about losing service-connected benefits if a genetic factor is found to contribute to their cancer history. In addition, patients may have concerns about discrimination by employers or insurers, including commercial health insurance or long-term care, disability, and life insurance. Yet there are many state and federal laws that ensure some protection from employment and health insurance discrimination based on genetic information.
For cancer care clinicians, incorporating germline testing requires additional responsibilities that can complicate care. Prior to germline genetic testing, genetic counseling with patients is recommended to review the potential benefits, harms, and limitations of genetic testing. Further, posttest genetic counseling is recommended to help the patient understand how the results may influence future cancer risks, provide recommendations for cancer management and prevention, and discuss implications for family members.9,10 While patients trust their health care providers to help them access and understand their genetic information, most health care providers are unprepared to integrate genetics into their practice; they lack adequate knowledge, skills, and confidence about genetics to effectively deliver genetic services.19-26 This leads to failure to recognize patients with indications for genetic testing, which often is due to insufficient family history collection. Other errors can include offering germline genetic testing to patients without appropriate indications and with inadequate informed consent procedures. When genetic testing is pursued, lack of knowledge about genetic principles and testing methods can lead to misinterpretation and miscommunication of results, contributing to inappropriate management recommendations. These errors can contribute to under-use, overuse, or misuse of genetic testing that can compromise the quality of patient care.27,28 With this in mind, thought must be given at the health care system level to develop effective strategies to deliver genetic services to patients. These strategies must address workforce capacity, organizational structure, and education.
Workforce Capacity
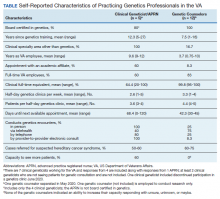
The VA clinical genetics workforce needs to expand to keep pace with increasing demand, which will be accelerated by the precision oncology programs for prostate and lung cancers and the VA Teleoncology initiative. In the US there are 10 to 15 genetics professionals per 1,000,000 residents.29-31 Most genetics professionals work in academic and metropolitan settings, leaving suburban and rural areas underserved. For example, in California, some patients travel up to 386 miles for genetics care (mean, 76.6 miles).32 In the VA, there are only 1 to 2 genetics professionals per 1 million enrollees, about 10-fold fewer than in community care. Meeting clinical needs of patients at the VA is particularly challenging because more than one-third of veterans live in rural areas.33
We recently surveyed genetics professionals in the VA about their practices and capacity to increase patient throughput (Table). Currently in the VA, there are 8 clinical geneticists, not all of whom practice clinical genetics, and 13 genetic counselors. Five VA programs provide clinical genetic services to local and nearby VA facilities near Boston, Massachusetts; Houston, Texas; Los Angeles and San Francisco, California; and Salt Lake City, Utah. These programs, first developed in 2008, typically are staffed by 1 or 2 genetics professionals. Most patients who are referred to the VA genetics programs are evaluated for hereditary cancer syndromes. Multiple modes of delivery may be used, including in-person, telehealth, telephone, and provider-to-provider e-consults in the EHR.
In 2010, in response to increased demand for clinical genetics services, the VA launched the Genomic Medicine Service (GMS), a national program with a centralized team of 9 genetic counselors based in Salt Lake City. GMS provides telehealth genetic counseling services exclusively to veterans onsite and at about 90 VA facilities across the country. More recently, the addition of a clinical geneticist and APRN with genetics expertise has allowed GMS to provide more comprehensive genetic consultative services.
All VA genetics programs are currently at full capacity with long waits for an appointment. To expand clinical genetic services, the VA genetics professionals responding to our survey reported a need for additional support (eg, administrative, care coordination, clinical), resources (eg, clinical space, salary support), and organizational change (eg, division of Medical Genetics at facility level, services provided at the level of the Veterans Integrated Service Network). Given the dearth of genetic care providers in the community, referral to non-VA care is not a viable option in many markets. In addition, avoiding referral outside of the VA could help to ensure continuity of care, more efficient care, and reduce the risk of duplication of testing, and polypharmacy.34-37
As part of its precision oncology initiative, VA is focusing on building clinical genetics services capacity. To increase access to clinical genetic services and appropriate genetic testing, the VA needs more genetics professionals, including clinical geneticists, genetic counselors, and genetic nurses–ideally a workforce study could be performed to inform the right staffing mix needed. To grow the genetics workforce in the long term, the VA could leverage its academic affiliations to train the next generation of genetics professionals. The VA has an important role in training medical professionals. By forming affiliations with medical schools and universities, the VA has become the largest provider of health care training in the US.38
Genetic Health Care Organization in the VA
Understanding a patient’s genetic background increasingly has become more and more important in the clinic, which necessitates a major shift in health care. Unfortunately, on a national scale, the number of clinical genetics professionals has not kept pace with the need-limiting the ability to grow the traditional genetics workforce in the VA in the near term.29-31 Thus, we must look to alternative genetic health care models in which other members of the health care team assume some of the genetic evaluation and counseling activities while caring for their cancer patients with referral to a clinical genetics team, as needed.39
Two genetic health care models have been described.40 Traditionally, clinical genetic services are coordinated between genetics professionals and other clinicians, organized as a regional genetics center and usually affiliated with an academic medical center. By contrast, the nontraditional genetic health care model integrates genetic services within primary and specialty care. Under the new approach, nongeneticists can be assisted by decision support tools in the EHR that help with assessing family history risk, identifying indications for genetic testing, and suggesting management options based on genetic test results.41-43
The VA National Precision Oncology Program (NPOP) is shaped by a commitment to be a high reliability organization (HRO). As such, the goal is to create a system of excellence that integrates precision medicine, implementation science, and the learning health care system to improve the health and health care of veterans with cancer. This initiative is establishing the foundations for best-in-class cancer care to enable veterans access to life-saving therapies through a concerted effort that began with the Cancer Moonshot, development of the NPOP, and collaborations with the VA Office of Research and Development. One of the fundamental objectives of this initiative is to implement strategies that ensure clinical genetic services are available to veterans receiving cancer care at all VA facilities and to extend these services to veterans in remote geographic locations nationwide. The initiative aims to synergize VA Teleoncology services that seek to deliver best-in-class oncology care across the VA enterprise using cutting-edge technologies.
Conclusions
To accomplish the goal of delivering world-class clinical genetic services to veterans and meet the increasing needs of precision oncology and support quality genetic health care, the VA must develop an integrated system of genetic health care that will have a network of clinical genetics that interfaces with other clinical and operational programs, genomics researchers, and educational programs to support quality genetic health care. The VA has highly qualified and dedicated genetics professionals at many sites across the country. Connecting them could create powerful synergies that would benefit patients and strengthen the genetics workforce. The clinical genetics network will enable development and dissemination of evidence-based policies, protocols, and clinical pathways for genomic medicine. This will help to identify, benchmark, and promote best practices for clinical genetic services, and increase access, increase efficiencies, and reduce variability in the care delivered.
The VA is well positioned to achieve successful implementation of genetic services given its investment in genomic medicine and the commitment of the VA NPOP. However, there is a need for structured and targeted implementation strategies for genetic services in the VA, as uptake of this innovation will not occur by passive diffusion.44,45 To keep pace with the demand for germline testing in veterans, VA may want to consider an outsized focus on training genetics professionals, given the high demand for this expertise. Perhaps most importantly, the VA will need to better prepare its frontline clinical workforce to integrate genetics into their practice. This could be facilitated by identifying implementation strategies and educational programs for genomic medicine that help clinicians to think genetically while caring for their patients, performing aspects of family history risk assessment and pre- and posttest genetic counseling as they are able, and referring complex cases to the clinical genetics network when needed.
Much is already known on how best to accomplish this through studies conducted by many talented VA health services researchers.46 Crucially, clinical tools embedded within the VA EHR will be fundamental to these efforts by facilitating identification of patients who can benefit from genetic services and genetic testing at the point of care. Through integration of VA research with clinical genetic services, the VA will become more prepared to realize the promise of genomic medicine for veterans.
Acknowledgments
We thank the members of the Genomic Medicine Program Advisory Committee, Clinical Genetics Subcommittee for providing input and guidance on the topics included in this article.
1. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891. doi:10.7205/MILMED-D-16-00371
2. Li MM, Chao E, Esplin ED, et al. Points to consider for reporting of germline variation in patients undergoing tumor testing: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2020;22(7):1142-1148. doi:10.1038/s41436-020-0783-8
3. Malone ER, Oliva M, Sabatini PJB, Stockley TL, Siu LL. Molecular profiling for precision cancer therapies. Genome Med. 2020;12(1):8. Published 2020 Jan 14. doi:10.1186/s13073-019-0703-1
4. Mandelker D, Zhang L, Kemel Y, et al. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing [published correction appears in JAMA. 2018 Dec 11;320(22):2381]. JAMA. 2017;318(9):825-835. doi:10.1001/jama.2017.11137
5. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697-1708. doi:10.1056/NEJMoa1506859
6. Ratta R, Guida A, Scotté F, et al. PARP inhibitors as a new therapeutic option in metastatic prostate cancer: a systematic review [published online ahead of print, 2020 May 4]. Prostate Cancer Prostatic Dis. 2020;10.1038/s41391-020-0233-3. doi:10.1038/s41391-020-0233-3
7. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. doi:10.1056/NEJMoa1500596
8. Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS One. 2020;15(5):e0233260. doi:10.1371/journal.pone.0233260
9. Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K; American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28(5):893-901. doi:10.1200/JCO.2009.27.0660
10. Riley BD, Culver JO, Skrzynia C, et al. Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2012;21(2):151-161. doi:10.1007/s10897-011-9462-x
11. Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993.
12. ACMG Board of Directors. Scope of practice: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2015;17(9):e3. doi:10.1038/gim.2015.94
13. National Society of Genetic Counselors’ Definition Task Force, Resta R, Biesecker BB, et al. A new definition of Genetic Counseling: National Society of Genetic Counselors’ Task Force report. J Genet Couns. 2006;15(2):77-83. doi:10.1007/s10897-005-9014-3
14. Calzone KA, Cashion A, Feetham S, et al. Nurses transforming health care using genetics and genomics [published correction appears in Nurs Outlook. 2010;58(3):163]. Nurs Outlook. 2010;58(1):26-35. doi:10.1016/j.outlook.2009.05.001
15. US Department of Veterans Affairs, Veterans Health Administration, Office of Nursing Services. 2018 Office of Nursing Services (ONS) Annual Brief. https://www.va.gov/nursing/docs/about/2018_ONS_Annual_Report_Brief.pdf. Accessed July 21, 2020.
16. Lerman C, Croyle RT. Emotional and behavioral responses to genetic testing for susceptibility to cancer. Oncology (Williston Park). 1996;10(2):191-202.
17. Bonadona V, Saltel P, Desseigne F, et al. Cancer patients who experienced diagnostic genetic testing for cancer susceptibility: reactions and behavior after the disclosure of a positive test result. Cancer Epidemiol Biomarkers Prev. 2002;11(1):97-104.
18. Murakami Y, Okamura H, Sugano K, et al. Psychologic distress after disclosure of genetic test results regarding hereditary nonpolyposis colorectal carcinoma. Cancer. 2004;101(2):395-403. doi:10.1002/cncr.20363
19. Brierley KL, Campfield D, Ducaine W, et al. Errors in delivery of cancer genetics services: implications for practice. Conn Med. 2010;74(7):413-423.
20. Dhar SU, Cooper HP, Wang T, et al. Significant differences among physician specialties in management recommendations of BRCA1 mutation carriers. Breast Cancer Res Treat. 2011;129(1):221-227. doi:10.1007/s10549-011-1449-7
21. Plon SE, Cooper HP, Parks B, et al. Genetic testing and cancer risk management recommendations by physicians for at-risk relatives. Genet Med. 2011;13(2):148-154. doi:10.1097/GIM.0b013e318207f564
22. Bellcross CA, Kolor K, Goddard KA, Coates RJ, Reyes M, Khoury MJ. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40(1):61-66. doi:10.1016/j.amepre.2010.09.027
23. Pal T, Cragun D, Lewis C, et al. A statewide survey of practitioners to assess knowledge and clinical practices regarding hereditary breast and ovarian cancer. Genet Test Mol Biomarkers. 2013;17(5):367-375. doi:10.1089/gtmb.2012.0381
24. Bensend TA, Veach PM, Niendorf KB. What’s the harm? Genetic counselor perceptions of adverse effects of genetics service provision by non-genetics professionals. J Genet Couns. 2014;23(1):48-63. doi:10.1007/s10897-013-9605-3
25. Teng I, Spigelman A. Attitudes and knowledge of medical practitioners to hereditary cancer clinics and cancer genetic testing. Fam Cancer. 2014;13(2):311-324. doi:10.1007/s10689-013-9695-y
26. Mikat-Stevens NA, Larson IA, Tarini BA. Primary-care providers’ perceived barriers to integration of genetics services: a systematic review of the literature. Genet Med. 2015;17(3):169-176. doi:10.1038/gim.2014.101
27. Scheuner MT, Hilborne L, Brown J, Lubin IM; members of the RAND Molecular Genetic Test Report Advisory Board. A report template for molecular genetic tests designed to improve communication between the clinician and laboratory. Genet Test Mol Biomarkers. 2012;16(7):761-769. doi:10.1089/gtmb.2011.0328
28. Scheuner MT, Peredo J, Tangney K, et al. Electronic health record interventions at the point of care improve documentation of care processes and decrease orders for genetic tests commonly ordered by nongeneticists. Genet Med. 2017;19(1):112-120. doi:10.1038/gim.2016.73
29. Cooksey JA, Forte G, Benkendorf J, Blitzer MG. The state of the medical geneticist workforce: findings of the 2003 survey of American Board of Medical Genetics certified geneticists. Genet Med. 2005;7(6):439-443. doi:10.1097/01.gim.0000172416.35285.9f
30. Institute of Medicine. Roundtable on Translating Genomic-Based Research for Health. Washington, DC: National Academies Press; 2009. https://www.ncbi.nlm.nih.gov/books/NBK26394. Accessed July 22, 2020.
31. Hoskovec JM, Bennett RL, Carey ME, et al. Projecting the supply and demand for certified genetic counselors: a workforce study. J Genet Couns. 2018;27(1):16-20. doi:10.1007/s10897-017-0158-8
32. Penon-Portmann M, Chang J, Cheng M, Shieh JT. Genetics workforce: distribution of genetics services and challenges to health care in California. Genet Med. 2020;22(1):227-231. doi:10.1038/s41436-019-0628-5

33. Spoont M, Greer N, Su J, Fitzgerald P, Rutks I, Wilt TJ. Rural vs. Urban Ambulatory Health Care: A Systematic Review. Washington, DC: US Department of Veterans Affairs; 2011. https://www.hsrd.research.va.gov/publications/esp/ambulatory.cfm. Accessed July 21, 2020.
34. Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89(1):39-68. doi:10.1111/j.1468-0009.2011.00619.x
35. Walsh J, Harrison JD, Young JM, Butow PN, Solomon MJ, Masya L. What are the current barriers to effective cancer care coordination? A qualitative study. BMC Health Serv Res. 2010;10:132. Published 2010 May 20. doi:10.1186/1472-6963-10-132
36. McDonald KM, Schultz E, Albin L, et al. Care Coordination Measures Atlas. Version 4. Agency for Healthcare Research and Quality Publication No. 14-0037. https://www.ahrq.gov/sites/default/files/publications/files/ccm_atlas.pdf. Updated June 2014. Accessed July 22, 2020.
37. Greenwood-Lee J, Jewett L, Woodhouse L, Marshall DA. A categorisation of problems and solutions to improve patient referrals from primary to specialty care. BMC Health Serv Res. 2018;18(1):986. Published 2018 Dec 20. doi:10.1186/s12913-018-3745-y
38. US Department of Veterans Affairs, Office of Academic Affiliations. Our medical and dental training program. https://www.va.gov/oaa/gme_default.asp. Updated January 7, 2020. Accessed July 21, 2020.
39. Scheuner MT, Marshall N, Lanto A, et al. Delivery of clinical genetic consultative services in the Veterans Health Administration. Genet Med. 2014;16(8):609-619. doi:10.1038/gim.2013.202.
40. Battista RN, Blancquaert I, Laberge AM, van Schendel N, Leduc N. Genetics in health care: an overview of current and emerging models. Public Health Genomics. 2012;15(1):34-45. doi:10.1159/000328846
41. Emery J. The GRAIDS Trial: the development and evaluation of computer decision support for cancer genetic risk assessment in primary care. Ann Hum Biol. 2005;32(2):218-227. doi:10.1080/03014460500074921
42. Scheuner MT, Hamilton AB, Peredo J, et al. A cancer genetics toolkit improves access to genetic services through documentation and use of the family history by primary-care clinicians. Genet Med. 2014;16(1):60-69. doi:10.1038/gim.2013.75
43. Scheuner MT, Peredo J, Tangney K, et al. Electronic health record interventions at the point of care improve documentation of care processes and decrease orders for genetic tests commonly ordered by nongeneticists. Genet Med. 2017;19(1):112-120. doi:10.1038/gim.2016.73
44. Hamilton AB, Oishi S, Yano EM, Gammage CE, Marshall NJ, Scheuner MT. Factors influencing organizational adoption and implementation of clinical genetic services. Genet Med. 2014;16(3):238-245. doi:10.1038/gim.2013.101
45. Sperber NR, Andrews SM, Voils CI, Green GL, Provenzale D, Knight S. Barriers and facilitators to adoption of genomic services for colorectal care within the Veterans Health Administration. J Pers Med. 2016;6(2):16. Published 2016 Apr 28. doi:10.3390/jpm6020016
46. US Department of Veterans Affairs, Health Services Research and Development. Genomics. https://www.hsrd.research.va.gov/research/portfolio_description.cfm?Sulu=17. Updated July 21, 2020. Accessed June 22, 2020.
The US Department of Veterans Affairs (VA) oversees the largest integrated health care system in the nation, administering care to 9 million veterans annually throughout its distributed network of 1,255 medical centers and outpatient facilities. Every year, about 50,000 veterans are diagnosed with and treated for cancer in the VA, representing about 3% of all cancer cases in the US.1 After skin cancer, prostate, colon, and lung cancers are the most common among veterans.1 One way that VA has sought to improve the care of its large cancer patient population is through the adoption of precision oncology, an ever-evolving practice of analyzing an individual patient’s cancer to inform clinical decision making. Most often, the analysis includes conducting genetic testing of the tumor itself. Here, we describe the opportunities and challenges of integrating germline genetics into precision oncology practice.
The Intersection of Precision Oncology and Germline Genetics
Precision oncology typically refers to genetic testing of tumor DNA to identify genetic variants with potential diagnostic, prognostic, or predictive therapeutic implications. It is enabled by a growing body of knowledge that identifies key drivers of cancer development, coupled with advances in tumor analysis by next-generation sequencing and other technologies and by the availability of new and repurposed therapeutic agents.2 Precision oncology has transformed cancer care by targeting both common and rare malignancies with specific therapies that improve clinical outcomes in patients.3
Testing of tumor DNA can reveal both somatic (acquired) and germline (inherited) gene variants. Precision oncology testing strategies can include tumor-only testing with or without subtraction of suspected germline variants, or paired tumor-normal testing with explicit analysis and reporting of genes associated with germline predisposition.2 With tumor-only testing, the germline status of variants may be inferred and follow-up germline testing in normal tissue such as blood or saliva can be considered. Paired tumor-normal testing provides distinct advantages over tumor-only testing, including improvement of the mutation detection rate in tumors and streamlining interpretation of results for both the tumor and germline tests.
Regardless of the strategy used, tumor testing has the potential to uncover clinically relevant germline variation associated with heritable cancer susceptibility and other conditions, as well as carrier status for autosomal recessive disorders (eAppendix
Germline genetic information, independent of somatic variation, can influence the choice of targeted cancer therapies. For example, Mandelker and colleagues identified germline variants that would impact the treatment of 38 (3.7%) of 1,040 patients with cancer.4 Individuals with a germline pathogenic variant in a DNA repair gene (eg, BRCA1, BRCA2, ATM, CHEK2) are candidates for platinum chemotherapy and poly-(adenosine diphosphate-ribose) polymerase (PARP) inhibitors that target the inability of a tumor to repair double-stranded DNA breaks.5,6 Individuals with a germline pathogenic variant in the MSH2, MLH1, MSH6, PMS2 or EPCAM genes (ie, Lynch syndrome) have tumors that are deficient in mismatch repair, and these tumors are responsive to inhibitors of the programmed death 1 (PD1) pathway.7,8
In addition to changing treatment decisions, identifying pathogenic germline variants can have health, reproductive, and psychosocial implications for the patient and the patient’s family members.9,10 A pathogenic germline variant can imply disease risk for both the patient and his or her relatives. In these cases, it is important to ascertain family history, understand the mode of inheritance, identify at-risk relatives, review the associated phenotype, and discuss management and prevention options for the patient and for family members. For example, a germline pathogenic variant in the BRCA2 gene is associated with increased risk for breast, ovarian, pancreatic, gastric, bile duct, and laryngeal cancer, and melanoma.11 Knowledge of these increased cancer risks could inform cancer prevention and early detection options, such as more frequent and intensive surveillance starting at younger ages compared with that of average-risk individuals, use of chemoprevention treatments, and for those at highest risk, risk-reducing surgical procedures. Therefore, reporting germline test results requires the clinician to take on additional responsibilities beyond those required when reporting only somatic variants.
Because of the complexities inherent in germline genetic testing, it traditionally is offered in the context of a genetic consultation, comprised of genetic evaluation and genetic counseling (Figure). Clinical geneticists are physicians certified by the American Board of Medical Genetics and Genomics (a member board of the American Board of Medical Specialties) who received special training in the diagnosis and management of medical genetic conditions; they are trained to perform all aspects of a genetic consultation across the clinical spectrum and lifespan of a patient.12 In contrast, genetic counselors have a master’s degree in genetic counseling, a communication process that facilitates patient decision making surrounding the genetic evaluation.13 Most work as members of a team to ensure provision of comprehensive clinical genetic services. Genetic counselors are licensed in most states, and licensure in some states sanctions the ordering of genetic tests by genetic counselors. Genetics nurses are licensed professional nurses with special education and training in genetics who function in diverse roles in industry, education, research, and clinical care.14 Genetics nurses in clinical care perform risk assessment based on personal and family history, recognize and identify genetic conditions and predispositions, and discuss the implications of this with patients and their families. Advanced practice nurses (APRNs) have additional training that allows for diagnosis, interpretation of results, and surveillance and management recommendations.15
Germline Genetic Testing Challenges
Integrating germline genetic testing in precision oncology practice presents challenges at the patient, family, health care provider, and health system levels. Due to these challenges, implementation planning is obligatory, as germline testing has become a standard-of-care for certain tumor types and patients.2
On learning of a germline pathogenic variant or variant of uncertain significance, patients may experience distress and anxiety, especially in the short term.16-18 In addition, it can be difficult for patients to share germline genetic test results with their family; parents may feel guilty about the possibility of passing on a predisposition to children, and unaffected siblings may experience survivor guilt. For some veterans, there can be concerns about losing service-connected benefits if a genetic factor is found to contribute to their cancer history. In addition, patients may have concerns about discrimination by employers or insurers, including commercial health insurance or long-term care, disability, and life insurance. Yet there are many state and federal laws that ensure some protection from employment and health insurance discrimination based on genetic information.
For cancer care clinicians, incorporating germline testing requires additional responsibilities that can complicate care. Prior to germline genetic testing, genetic counseling with patients is recommended to review the potential benefits, harms, and limitations of genetic testing. Further, posttest genetic counseling is recommended to help the patient understand how the results may influence future cancer risks, provide recommendations for cancer management and prevention, and discuss implications for family members.9,10 While patients trust their health care providers to help them access and understand their genetic information, most health care providers are unprepared to integrate genetics into their practice; they lack adequate knowledge, skills, and confidence about genetics to effectively deliver genetic services.19-26 This leads to failure to recognize patients with indications for genetic testing, which often is due to insufficient family history collection. Other errors can include offering germline genetic testing to patients without appropriate indications and with inadequate informed consent procedures. When genetic testing is pursued, lack of knowledge about genetic principles and testing methods can lead to misinterpretation and miscommunication of results, contributing to inappropriate management recommendations. These errors can contribute to under-use, overuse, or misuse of genetic testing that can compromise the quality of patient care.27,28 With this in mind, thought must be given at the health care system level to develop effective strategies to deliver genetic services to patients. These strategies must address workforce capacity, organizational structure, and education.
Workforce Capacity
The VA clinical genetics workforce needs to expand to keep pace with increasing demand, which will be accelerated by the precision oncology programs for prostate and lung cancers and the VA Teleoncology initiative. In the US there are 10 to 15 genetics professionals per 1,000,000 residents.29-31 Most genetics professionals work in academic and metropolitan settings, leaving suburban and rural areas underserved. For example, in California, some patients travel up to 386 miles for genetics care (mean, 76.6 miles).32 In the VA, there are only 1 to 2 genetics professionals per 1 million enrollees, about 10-fold fewer than in community care. Meeting clinical needs of patients at the VA is particularly challenging because more than one-third of veterans live in rural areas.33
We recently surveyed genetics professionals in the VA about their practices and capacity to increase patient throughput (Table). Currently in the VA, there are 8 clinical geneticists, not all of whom practice clinical genetics, and 13 genetic counselors. Five VA programs provide clinical genetic services to local and nearby VA facilities near Boston, Massachusetts; Houston, Texas; Los Angeles and San Francisco, California; and Salt Lake City, Utah. These programs, first developed in 2008, typically are staffed by 1 or 2 genetics professionals. Most patients who are referred to the VA genetics programs are evaluated for hereditary cancer syndromes. Multiple modes of delivery may be used, including in-person, telehealth, telephone, and provider-to-provider e-consults in the EHR.
In 2010, in response to increased demand for clinical genetics services, the VA launched the Genomic Medicine Service (GMS), a national program with a centralized team of 9 genetic counselors based in Salt Lake City. GMS provides telehealth genetic counseling services exclusively to veterans onsite and at about 90 VA facilities across the country. More recently, the addition of a clinical geneticist and APRN with genetics expertise has allowed GMS to provide more comprehensive genetic consultative services.
All VA genetics programs are currently at full capacity with long waits for an appointment. To expand clinical genetic services, the VA genetics professionals responding to our survey reported a need for additional support (eg, administrative, care coordination, clinical), resources (eg, clinical space, salary support), and organizational change (eg, division of Medical Genetics at facility level, services provided at the level of the Veterans Integrated Service Network). Given the dearth of genetic care providers in the community, referral to non-VA care is not a viable option in many markets. In addition, avoiding referral outside of the VA could help to ensure continuity of care, more efficient care, and reduce the risk of duplication of testing, and polypharmacy.34-37
As part of its precision oncology initiative, VA is focusing on building clinical genetics services capacity. To increase access to clinical genetic services and appropriate genetic testing, the VA needs more genetics professionals, including clinical geneticists, genetic counselors, and genetic nurses–ideally a workforce study could be performed to inform the right staffing mix needed. To grow the genetics workforce in the long term, the VA could leverage its academic affiliations to train the next generation of genetics professionals. The VA has an important role in training medical professionals. By forming affiliations with medical schools and universities, the VA has become the largest provider of health care training in the US.38
Genetic Health Care Organization in the VA
Understanding a patient’s genetic background increasingly has become more and more important in the clinic, which necessitates a major shift in health care. Unfortunately, on a national scale, the number of clinical genetics professionals has not kept pace with the need-limiting the ability to grow the traditional genetics workforce in the VA in the near term.29-31 Thus, we must look to alternative genetic health care models in which other members of the health care team assume some of the genetic evaluation and counseling activities while caring for their cancer patients with referral to a clinical genetics team, as needed.39
Two genetic health care models have been described.40 Traditionally, clinical genetic services are coordinated between genetics professionals and other clinicians, organized as a regional genetics center and usually affiliated with an academic medical center. By contrast, the nontraditional genetic health care model integrates genetic services within primary and specialty care. Under the new approach, nongeneticists can be assisted by decision support tools in the EHR that help with assessing family history risk, identifying indications for genetic testing, and suggesting management options based on genetic test results.41-43
The VA National Precision Oncology Program (NPOP) is shaped by a commitment to be a high reliability organization (HRO). As such, the goal is to create a system of excellence that integrates precision medicine, implementation science, and the learning health care system to improve the health and health care of veterans with cancer. This initiative is establishing the foundations for best-in-class cancer care to enable veterans access to life-saving therapies through a concerted effort that began with the Cancer Moonshot, development of the NPOP, and collaborations with the VA Office of Research and Development. One of the fundamental objectives of this initiative is to implement strategies that ensure clinical genetic services are available to veterans receiving cancer care at all VA facilities and to extend these services to veterans in remote geographic locations nationwide. The initiative aims to synergize VA Teleoncology services that seek to deliver best-in-class oncology care across the VA enterprise using cutting-edge technologies.
Conclusions
To accomplish the goal of delivering world-class clinical genetic services to veterans and meet the increasing needs of precision oncology and support quality genetic health care, the VA must develop an integrated system of genetic health care that will have a network of clinical genetics that interfaces with other clinical and operational programs, genomics researchers, and educational programs to support quality genetic health care. The VA has highly qualified and dedicated genetics professionals at many sites across the country. Connecting them could create powerful synergies that would benefit patients and strengthen the genetics workforce. The clinical genetics network will enable development and dissemination of evidence-based policies, protocols, and clinical pathways for genomic medicine. This will help to identify, benchmark, and promote best practices for clinical genetic services, and increase access, increase efficiencies, and reduce variability in the care delivered.
The VA is well positioned to achieve successful implementation of genetic services given its investment in genomic medicine and the commitment of the VA NPOP. However, there is a need for structured and targeted implementation strategies for genetic services in the VA, as uptake of this innovation will not occur by passive diffusion.44,45 To keep pace with the demand for germline testing in veterans, VA may want to consider an outsized focus on training genetics professionals, given the high demand for this expertise. Perhaps most importantly, the VA will need to better prepare its frontline clinical workforce to integrate genetics into their practice. This could be facilitated by identifying implementation strategies and educational programs for genomic medicine that help clinicians to think genetically while caring for their patients, performing aspects of family history risk assessment and pre- and posttest genetic counseling as they are able, and referring complex cases to the clinical genetics network when needed.
Much is already known on how best to accomplish this through studies conducted by many talented VA health services researchers.46 Crucially, clinical tools embedded within the VA EHR will be fundamental to these efforts by facilitating identification of patients who can benefit from genetic services and genetic testing at the point of care. Through integration of VA research with clinical genetic services, the VA will become more prepared to realize the promise of genomic medicine for veterans.
Acknowledgments
We thank the members of the Genomic Medicine Program Advisory Committee, Clinical Genetics Subcommittee for providing input and guidance on the topics included in this article.
The US Department of Veterans Affairs (VA) oversees the largest integrated health care system in the nation, administering care to 9 million veterans annually throughout its distributed network of 1,255 medical centers and outpatient facilities. Every year, about 50,000 veterans are diagnosed with and treated for cancer in the VA, representing about 3% of all cancer cases in the US.1 After skin cancer, prostate, colon, and lung cancers are the most common among veterans.1 One way that VA has sought to improve the care of its large cancer patient population is through the adoption of precision oncology, an ever-evolving practice of analyzing an individual patient’s cancer to inform clinical decision making. Most often, the analysis includes conducting genetic testing of the tumor itself. Here, we describe the opportunities and challenges of integrating germline genetics into precision oncology practice.
The Intersection of Precision Oncology and Germline Genetics
Precision oncology typically refers to genetic testing of tumor DNA to identify genetic variants with potential diagnostic, prognostic, or predictive therapeutic implications. It is enabled by a growing body of knowledge that identifies key drivers of cancer development, coupled with advances in tumor analysis by next-generation sequencing and other technologies and by the availability of new and repurposed therapeutic agents.2 Precision oncology has transformed cancer care by targeting both common and rare malignancies with specific therapies that improve clinical outcomes in patients.3
Testing of tumor DNA can reveal both somatic (acquired) and germline (inherited) gene variants. Precision oncology testing strategies can include tumor-only testing with or without subtraction of suspected germline variants, or paired tumor-normal testing with explicit analysis and reporting of genes associated with germline predisposition.2 With tumor-only testing, the germline status of variants may be inferred and follow-up germline testing in normal tissue such as blood or saliva can be considered. Paired tumor-normal testing provides distinct advantages over tumor-only testing, including improvement of the mutation detection rate in tumors and streamlining interpretation of results for both the tumor and germline tests.
Regardless of the strategy used, tumor testing has the potential to uncover clinically relevant germline variation associated with heritable cancer susceptibility and other conditions, as well as carrier status for autosomal recessive disorders (eAppendix
Germline genetic information, independent of somatic variation, can influence the choice of targeted cancer therapies. For example, Mandelker and colleagues identified germline variants that would impact the treatment of 38 (3.7%) of 1,040 patients with cancer.4 Individuals with a germline pathogenic variant in a DNA repair gene (eg, BRCA1, BRCA2, ATM, CHEK2) are candidates for platinum chemotherapy and poly-(adenosine diphosphate-ribose) polymerase (PARP) inhibitors that target the inability of a tumor to repair double-stranded DNA breaks.5,6 Individuals with a germline pathogenic variant in the MSH2, MLH1, MSH6, PMS2 or EPCAM genes (ie, Lynch syndrome) have tumors that are deficient in mismatch repair, and these tumors are responsive to inhibitors of the programmed death 1 (PD1) pathway.7,8
In addition to changing treatment decisions, identifying pathogenic germline variants can have health, reproductive, and psychosocial implications for the patient and the patient’s family members.9,10 A pathogenic germline variant can imply disease risk for both the patient and his or her relatives. In these cases, it is important to ascertain family history, understand the mode of inheritance, identify at-risk relatives, review the associated phenotype, and discuss management and prevention options for the patient and for family members. For example, a germline pathogenic variant in the BRCA2 gene is associated with increased risk for breast, ovarian, pancreatic, gastric, bile duct, and laryngeal cancer, and melanoma.11 Knowledge of these increased cancer risks could inform cancer prevention and early detection options, such as more frequent and intensive surveillance starting at younger ages compared with that of average-risk individuals, use of chemoprevention treatments, and for those at highest risk, risk-reducing surgical procedures. Therefore, reporting germline test results requires the clinician to take on additional responsibilities beyond those required when reporting only somatic variants.
Because of the complexities inherent in germline genetic testing, it traditionally is offered in the context of a genetic consultation, comprised of genetic evaluation and genetic counseling (Figure). Clinical geneticists are physicians certified by the American Board of Medical Genetics and Genomics (a member board of the American Board of Medical Specialties) who received special training in the diagnosis and management of medical genetic conditions; they are trained to perform all aspects of a genetic consultation across the clinical spectrum and lifespan of a patient.12 In contrast, genetic counselors have a master’s degree in genetic counseling, a communication process that facilitates patient decision making surrounding the genetic evaluation.13 Most work as members of a team to ensure provision of comprehensive clinical genetic services. Genetic counselors are licensed in most states, and licensure in some states sanctions the ordering of genetic tests by genetic counselors. Genetics nurses are licensed professional nurses with special education and training in genetics who function in diverse roles in industry, education, research, and clinical care.14 Genetics nurses in clinical care perform risk assessment based on personal and family history, recognize and identify genetic conditions and predispositions, and discuss the implications of this with patients and their families. Advanced practice nurses (APRNs) have additional training that allows for diagnosis, interpretation of results, and surveillance and management recommendations.15
Germline Genetic Testing Challenges
Integrating germline genetic testing in precision oncology practice presents challenges at the patient, family, health care provider, and health system levels. Due to these challenges, implementation planning is obligatory, as germline testing has become a standard-of-care for certain tumor types and patients.2
On learning of a germline pathogenic variant or variant of uncertain significance, patients may experience distress and anxiety, especially in the short term.16-18 In addition, it can be difficult for patients to share germline genetic test results with their family; parents may feel guilty about the possibility of passing on a predisposition to children, and unaffected siblings may experience survivor guilt. For some veterans, there can be concerns about losing service-connected benefits if a genetic factor is found to contribute to their cancer history. In addition, patients may have concerns about discrimination by employers or insurers, including commercial health insurance or long-term care, disability, and life insurance. Yet there are many state and federal laws that ensure some protection from employment and health insurance discrimination based on genetic information.
For cancer care clinicians, incorporating germline testing requires additional responsibilities that can complicate care. Prior to germline genetic testing, genetic counseling with patients is recommended to review the potential benefits, harms, and limitations of genetic testing. Further, posttest genetic counseling is recommended to help the patient understand how the results may influence future cancer risks, provide recommendations for cancer management and prevention, and discuss implications for family members.9,10 While patients trust their health care providers to help them access and understand their genetic information, most health care providers are unprepared to integrate genetics into their practice; they lack adequate knowledge, skills, and confidence about genetics to effectively deliver genetic services.19-26 This leads to failure to recognize patients with indications for genetic testing, which often is due to insufficient family history collection. Other errors can include offering germline genetic testing to patients without appropriate indications and with inadequate informed consent procedures. When genetic testing is pursued, lack of knowledge about genetic principles and testing methods can lead to misinterpretation and miscommunication of results, contributing to inappropriate management recommendations. These errors can contribute to under-use, overuse, or misuse of genetic testing that can compromise the quality of patient care.27,28 With this in mind, thought must be given at the health care system level to develop effective strategies to deliver genetic services to patients. These strategies must address workforce capacity, organizational structure, and education.
Workforce Capacity
The VA clinical genetics workforce needs to expand to keep pace with increasing demand, which will be accelerated by the precision oncology programs for prostate and lung cancers and the VA Teleoncology initiative. In the US there are 10 to 15 genetics professionals per 1,000,000 residents.29-31 Most genetics professionals work in academic and metropolitan settings, leaving suburban and rural areas underserved. For example, in California, some patients travel up to 386 miles for genetics care (mean, 76.6 miles).32 In the VA, there are only 1 to 2 genetics professionals per 1 million enrollees, about 10-fold fewer than in community care. Meeting clinical needs of patients at the VA is particularly challenging because more than one-third of veterans live in rural areas.33
We recently surveyed genetics professionals in the VA about their practices and capacity to increase patient throughput (Table). Currently in the VA, there are 8 clinical geneticists, not all of whom practice clinical genetics, and 13 genetic counselors. Five VA programs provide clinical genetic services to local and nearby VA facilities near Boston, Massachusetts; Houston, Texas; Los Angeles and San Francisco, California; and Salt Lake City, Utah. These programs, first developed in 2008, typically are staffed by 1 or 2 genetics professionals. Most patients who are referred to the VA genetics programs are evaluated for hereditary cancer syndromes. Multiple modes of delivery may be used, including in-person, telehealth, telephone, and provider-to-provider e-consults in the EHR.
In 2010, in response to increased demand for clinical genetics services, the VA launched the Genomic Medicine Service (GMS), a national program with a centralized team of 9 genetic counselors based in Salt Lake City. GMS provides telehealth genetic counseling services exclusively to veterans onsite and at about 90 VA facilities across the country. More recently, the addition of a clinical geneticist and APRN with genetics expertise has allowed GMS to provide more comprehensive genetic consultative services.
All VA genetics programs are currently at full capacity with long waits for an appointment. To expand clinical genetic services, the VA genetics professionals responding to our survey reported a need for additional support (eg, administrative, care coordination, clinical), resources (eg, clinical space, salary support), and organizational change (eg, division of Medical Genetics at facility level, services provided at the level of the Veterans Integrated Service Network). Given the dearth of genetic care providers in the community, referral to non-VA care is not a viable option in many markets. In addition, avoiding referral outside of the VA could help to ensure continuity of care, more efficient care, and reduce the risk of duplication of testing, and polypharmacy.34-37
As part of its precision oncology initiative, VA is focusing on building clinical genetics services capacity. To increase access to clinical genetic services and appropriate genetic testing, the VA needs more genetics professionals, including clinical geneticists, genetic counselors, and genetic nurses–ideally a workforce study could be performed to inform the right staffing mix needed. To grow the genetics workforce in the long term, the VA could leverage its academic affiliations to train the next generation of genetics professionals. The VA has an important role in training medical professionals. By forming affiliations with medical schools and universities, the VA has become the largest provider of health care training in the US.38
Genetic Health Care Organization in the VA
Understanding a patient’s genetic background increasingly has become more and more important in the clinic, which necessitates a major shift in health care. Unfortunately, on a national scale, the number of clinical genetics professionals has not kept pace with the need-limiting the ability to grow the traditional genetics workforce in the VA in the near term.29-31 Thus, we must look to alternative genetic health care models in which other members of the health care team assume some of the genetic evaluation and counseling activities while caring for their cancer patients with referral to a clinical genetics team, as needed.39
Two genetic health care models have been described.40 Traditionally, clinical genetic services are coordinated between genetics professionals and other clinicians, organized as a regional genetics center and usually affiliated with an academic medical center. By contrast, the nontraditional genetic health care model integrates genetic services within primary and specialty care. Under the new approach, nongeneticists can be assisted by decision support tools in the EHR that help with assessing family history risk, identifying indications for genetic testing, and suggesting management options based on genetic test results.41-43
The VA National Precision Oncology Program (NPOP) is shaped by a commitment to be a high reliability organization (HRO). As such, the goal is to create a system of excellence that integrates precision medicine, implementation science, and the learning health care system to improve the health and health care of veterans with cancer. This initiative is establishing the foundations for best-in-class cancer care to enable veterans access to life-saving therapies through a concerted effort that began with the Cancer Moonshot, development of the NPOP, and collaborations with the VA Office of Research and Development. One of the fundamental objectives of this initiative is to implement strategies that ensure clinical genetic services are available to veterans receiving cancer care at all VA facilities and to extend these services to veterans in remote geographic locations nationwide. The initiative aims to synergize VA Teleoncology services that seek to deliver best-in-class oncology care across the VA enterprise using cutting-edge technologies.
Conclusions
To accomplish the goal of delivering world-class clinical genetic services to veterans and meet the increasing needs of precision oncology and support quality genetic health care, the VA must develop an integrated system of genetic health care that will have a network of clinical genetics that interfaces with other clinical and operational programs, genomics researchers, and educational programs to support quality genetic health care. The VA has highly qualified and dedicated genetics professionals at many sites across the country. Connecting them could create powerful synergies that would benefit patients and strengthen the genetics workforce. The clinical genetics network will enable development and dissemination of evidence-based policies, protocols, and clinical pathways for genomic medicine. This will help to identify, benchmark, and promote best practices for clinical genetic services, and increase access, increase efficiencies, and reduce variability in the care delivered.
The VA is well positioned to achieve successful implementation of genetic services given its investment in genomic medicine and the commitment of the VA NPOP. However, there is a need for structured and targeted implementation strategies for genetic services in the VA, as uptake of this innovation will not occur by passive diffusion.44,45 To keep pace with the demand for germline testing in veterans, VA may want to consider an outsized focus on training genetics professionals, given the high demand for this expertise. Perhaps most importantly, the VA will need to better prepare its frontline clinical workforce to integrate genetics into their practice. This could be facilitated by identifying implementation strategies and educational programs for genomic medicine that help clinicians to think genetically while caring for their patients, performing aspects of family history risk assessment and pre- and posttest genetic counseling as they are able, and referring complex cases to the clinical genetics network when needed.
Much is already known on how best to accomplish this through studies conducted by many talented VA health services researchers.46 Crucially, clinical tools embedded within the VA EHR will be fundamental to these efforts by facilitating identification of patients who can benefit from genetic services and genetic testing at the point of care. Through integration of VA research with clinical genetic services, the VA will become more prepared to realize the promise of genomic medicine for veterans.
Acknowledgments
We thank the members of the Genomic Medicine Program Advisory Committee, Clinical Genetics Subcommittee for providing input and guidance on the topics included in this article.
1. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891. doi:10.7205/MILMED-D-16-00371
2. Li MM, Chao E, Esplin ED, et al. Points to consider for reporting of germline variation in patients undergoing tumor testing: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2020;22(7):1142-1148. doi:10.1038/s41436-020-0783-8
3. Malone ER, Oliva M, Sabatini PJB, Stockley TL, Siu LL. Molecular profiling for precision cancer therapies. Genome Med. 2020;12(1):8. Published 2020 Jan 14. doi:10.1186/s13073-019-0703-1
4. Mandelker D, Zhang L, Kemel Y, et al. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing [published correction appears in JAMA. 2018 Dec 11;320(22):2381]. JAMA. 2017;318(9):825-835. doi:10.1001/jama.2017.11137
5. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697-1708. doi:10.1056/NEJMoa1506859
6. Ratta R, Guida A, Scotté F, et al. PARP inhibitors as a new therapeutic option in metastatic prostate cancer: a systematic review [published online ahead of print, 2020 May 4]. Prostate Cancer Prostatic Dis. 2020;10.1038/s41391-020-0233-3. doi:10.1038/s41391-020-0233-3
7. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. doi:10.1056/NEJMoa1500596
8. Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS One. 2020;15(5):e0233260. doi:10.1371/journal.pone.0233260
9. Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K; American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28(5):893-901. doi:10.1200/JCO.2009.27.0660
10. Riley BD, Culver JO, Skrzynia C, et al. Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2012;21(2):151-161. doi:10.1007/s10897-011-9462-x
11. Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993.
12. ACMG Board of Directors. Scope of practice: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2015;17(9):e3. doi:10.1038/gim.2015.94
13. National Society of Genetic Counselors’ Definition Task Force, Resta R, Biesecker BB, et al. A new definition of Genetic Counseling: National Society of Genetic Counselors’ Task Force report. J Genet Couns. 2006;15(2):77-83. doi:10.1007/s10897-005-9014-3
14. Calzone KA, Cashion A, Feetham S, et al. Nurses transforming health care using genetics and genomics [published correction appears in Nurs Outlook. 2010;58(3):163]. Nurs Outlook. 2010;58(1):26-35. doi:10.1016/j.outlook.2009.05.001
15. US Department of Veterans Affairs, Veterans Health Administration, Office of Nursing Services. 2018 Office of Nursing Services (ONS) Annual Brief. https://www.va.gov/nursing/docs/about/2018_ONS_Annual_Report_Brief.pdf. Accessed July 21, 2020.
16. Lerman C, Croyle RT. Emotional and behavioral responses to genetic testing for susceptibility to cancer. Oncology (Williston Park). 1996;10(2):191-202.
17. Bonadona V, Saltel P, Desseigne F, et al. Cancer patients who experienced diagnostic genetic testing for cancer susceptibility: reactions and behavior after the disclosure of a positive test result. Cancer Epidemiol Biomarkers Prev. 2002;11(1):97-104.
18. Murakami Y, Okamura H, Sugano K, et al. Psychologic distress after disclosure of genetic test results regarding hereditary nonpolyposis colorectal carcinoma. Cancer. 2004;101(2):395-403. doi:10.1002/cncr.20363
19. Brierley KL, Campfield D, Ducaine W, et al. Errors in delivery of cancer genetics services: implications for practice. Conn Med. 2010;74(7):413-423.
20. Dhar SU, Cooper HP, Wang T, et al. Significant differences among physician specialties in management recommendations of BRCA1 mutation carriers. Breast Cancer Res Treat. 2011;129(1):221-227. doi:10.1007/s10549-011-1449-7
21. Plon SE, Cooper HP, Parks B, et al. Genetic testing and cancer risk management recommendations by physicians for at-risk relatives. Genet Med. 2011;13(2):148-154. doi:10.1097/GIM.0b013e318207f564
22. Bellcross CA, Kolor K, Goddard KA, Coates RJ, Reyes M, Khoury MJ. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40(1):61-66. doi:10.1016/j.amepre.2010.09.027
23. Pal T, Cragun D, Lewis C, et al. A statewide survey of practitioners to assess knowledge and clinical practices regarding hereditary breast and ovarian cancer. Genet Test Mol Biomarkers. 2013;17(5):367-375. doi:10.1089/gtmb.2012.0381
24. Bensend TA, Veach PM, Niendorf KB. What’s the harm? Genetic counselor perceptions of adverse effects of genetics service provision by non-genetics professionals. J Genet Couns. 2014;23(1):48-63. doi:10.1007/s10897-013-9605-3
25. Teng I, Spigelman A. Attitudes and knowledge of medical practitioners to hereditary cancer clinics and cancer genetic testing. Fam Cancer. 2014;13(2):311-324. doi:10.1007/s10689-013-9695-y
26. Mikat-Stevens NA, Larson IA, Tarini BA. Primary-care providers’ perceived barriers to integration of genetics services: a systematic review of the literature. Genet Med. 2015;17(3):169-176. doi:10.1038/gim.2014.101
27. Scheuner MT, Hilborne L, Brown J, Lubin IM; members of the RAND Molecular Genetic Test Report Advisory Board. A report template for molecular genetic tests designed to improve communication between the clinician and laboratory. Genet Test Mol Biomarkers. 2012;16(7):761-769. doi:10.1089/gtmb.2011.0328
28. Scheuner MT, Peredo J, Tangney K, et al. Electronic health record interventions at the point of care improve documentation of care processes and decrease orders for genetic tests commonly ordered by nongeneticists. Genet Med. 2017;19(1):112-120. doi:10.1038/gim.2016.73
29. Cooksey JA, Forte G, Benkendorf J, Blitzer MG. The state of the medical geneticist workforce: findings of the 2003 survey of American Board of Medical Genetics certified geneticists. Genet Med. 2005;7(6):439-443. doi:10.1097/01.gim.0000172416.35285.9f
30. Institute of Medicine. Roundtable on Translating Genomic-Based Research for Health. Washington, DC: National Academies Press; 2009. https://www.ncbi.nlm.nih.gov/books/NBK26394. Accessed July 22, 2020.
31. Hoskovec JM, Bennett RL, Carey ME, et al. Projecting the supply and demand for certified genetic counselors: a workforce study. J Genet Couns. 2018;27(1):16-20. doi:10.1007/s10897-017-0158-8
32. Penon-Portmann M, Chang J, Cheng M, Shieh JT. Genetics workforce: distribution of genetics services and challenges to health care in California. Genet Med. 2020;22(1):227-231. doi:10.1038/s41436-019-0628-5

33. Spoont M, Greer N, Su J, Fitzgerald P, Rutks I, Wilt TJ. Rural vs. Urban Ambulatory Health Care: A Systematic Review. Washington, DC: US Department of Veterans Affairs; 2011. https://www.hsrd.research.va.gov/publications/esp/ambulatory.cfm. Accessed July 21, 2020.
34. Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89(1):39-68. doi:10.1111/j.1468-0009.2011.00619.x
35. Walsh J, Harrison JD, Young JM, Butow PN, Solomon MJ, Masya L. What are the current barriers to effective cancer care coordination? A qualitative study. BMC Health Serv Res. 2010;10:132. Published 2010 May 20. doi:10.1186/1472-6963-10-132
36. McDonald KM, Schultz E, Albin L, et al. Care Coordination Measures Atlas. Version 4. Agency for Healthcare Research and Quality Publication No. 14-0037. https://www.ahrq.gov/sites/default/files/publications/files/ccm_atlas.pdf. Updated June 2014. Accessed July 22, 2020.
37. Greenwood-Lee J, Jewett L, Woodhouse L, Marshall DA. A categorisation of problems and solutions to improve patient referrals from primary to specialty care. BMC Health Serv Res. 2018;18(1):986. Published 2018 Dec 20. doi:10.1186/s12913-018-3745-y
38. US Department of Veterans Affairs, Office of Academic Affiliations. Our medical and dental training program. https://www.va.gov/oaa/gme_default.asp. Updated January 7, 2020. Accessed July 21, 2020.
39. Scheuner MT, Marshall N, Lanto A, et al. Delivery of clinical genetic consultative services in the Veterans Health Administration. Genet Med. 2014;16(8):609-619. doi:10.1038/gim.2013.202.
40. Battista RN, Blancquaert I, Laberge AM, van Schendel N, Leduc N. Genetics in health care: an overview of current and emerging models. Public Health Genomics. 2012;15(1):34-45. doi:10.1159/000328846
41. Emery J. The GRAIDS Trial: the development and evaluation of computer decision support for cancer genetic risk assessment in primary care. Ann Hum Biol. 2005;32(2):218-227. doi:10.1080/03014460500074921
42. Scheuner MT, Hamilton AB, Peredo J, et al. A cancer genetics toolkit improves access to genetic services through documentation and use of the family history by primary-care clinicians. Genet Med. 2014;16(1):60-69. doi:10.1038/gim.2013.75
43. Scheuner MT, Peredo J, Tangney K, et al. Electronic health record interventions at the point of care improve documentation of care processes and decrease orders for genetic tests commonly ordered by nongeneticists. Genet Med. 2017;19(1):112-120. doi:10.1038/gim.2016.73
44. Hamilton AB, Oishi S, Yano EM, Gammage CE, Marshall NJ, Scheuner MT. Factors influencing organizational adoption and implementation of clinical genetic services. Genet Med. 2014;16(3):238-245. doi:10.1038/gim.2013.101
45. Sperber NR, Andrews SM, Voils CI, Green GL, Provenzale D, Knight S. Barriers and facilitators to adoption of genomic services for colorectal care within the Veterans Health Administration. J Pers Med. 2016;6(2):16. Published 2016 Apr 28. doi:10.3390/jpm6020016
46. US Department of Veterans Affairs, Health Services Research and Development. Genomics. https://www.hsrd.research.va.gov/research/portfolio_description.cfm?Sulu=17. Updated July 21, 2020. Accessed June 22, 2020.
1. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891. doi:10.7205/MILMED-D-16-00371
2. Li MM, Chao E, Esplin ED, et al. Points to consider for reporting of germline variation in patients undergoing tumor testing: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2020;22(7):1142-1148. doi:10.1038/s41436-020-0783-8
3. Malone ER, Oliva M, Sabatini PJB, Stockley TL, Siu LL. Molecular profiling for precision cancer therapies. Genome Med. 2020;12(1):8. Published 2020 Jan 14. doi:10.1186/s13073-019-0703-1
4. Mandelker D, Zhang L, Kemel Y, et al. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing [published correction appears in JAMA. 2018 Dec 11;320(22):2381]. JAMA. 2017;318(9):825-835. doi:10.1001/jama.2017.11137
5. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697-1708. doi:10.1056/NEJMoa1506859
6. Ratta R, Guida A, Scotté F, et al. PARP inhibitors as a new therapeutic option in metastatic prostate cancer: a systematic review [published online ahead of print, 2020 May 4]. Prostate Cancer Prostatic Dis. 2020;10.1038/s41391-020-0233-3. doi:10.1038/s41391-020-0233-3
7. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. doi:10.1056/NEJMoa1500596
8. Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS One. 2020;15(5):e0233260. doi:10.1371/journal.pone.0233260
9. Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K; American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28(5):893-901. doi:10.1200/JCO.2009.27.0660
10. Riley BD, Culver JO, Skrzynia C, et al. Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2012;21(2):151-161. doi:10.1007/s10897-011-9462-x
11. Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993.
12. ACMG Board of Directors. Scope of practice: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2015;17(9):e3. doi:10.1038/gim.2015.94
13. National Society of Genetic Counselors’ Definition Task Force, Resta R, Biesecker BB, et al. A new definition of Genetic Counseling: National Society of Genetic Counselors’ Task Force report. J Genet Couns. 2006;15(2):77-83. doi:10.1007/s10897-005-9014-3
14. Calzone KA, Cashion A, Feetham S, et al. Nurses transforming health care using genetics and genomics [published correction appears in Nurs Outlook. 2010;58(3):163]. Nurs Outlook. 2010;58(1):26-35. doi:10.1016/j.outlook.2009.05.001
15. US Department of Veterans Affairs, Veterans Health Administration, Office of Nursing Services. 2018 Office of Nursing Services (ONS) Annual Brief. https://www.va.gov/nursing/docs/about/2018_ONS_Annual_Report_Brief.pdf. Accessed July 21, 2020.
16. Lerman C, Croyle RT. Emotional and behavioral responses to genetic testing for susceptibility to cancer. Oncology (Williston Park). 1996;10(2):191-202.
17. Bonadona V, Saltel P, Desseigne F, et al. Cancer patients who experienced diagnostic genetic testing for cancer susceptibility: reactions and behavior after the disclosure of a positive test result. Cancer Epidemiol Biomarkers Prev. 2002;11(1):97-104.
18. Murakami Y, Okamura H, Sugano K, et al. Psychologic distress after disclosure of genetic test results regarding hereditary nonpolyposis colorectal carcinoma. Cancer. 2004;101(2):395-403. doi:10.1002/cncr.20363
19. Brierley KL, Campfield D, Ducaine W, et al. Errors in delivery of cancer genetics services: implications for practice. Conn Med. 2010;74(7):413-423.
20. Dhar SU, Cooper HP, Wang T, et al. Significant differences among physician specialties in management recommendations of BRCA1 mutation carriers. Breast Cancer Res Treat. 2011;129(1):221-227. doi:10.1007/s10549-011-1449-7
21. Plon SE, Cooper HP, Parks B, et al. Genetic testing and cancer risk management recommendations by physicians for at-risk relatives. Genet Med. 2011;13(2):148-154. doi:10.1097/GIM.0b013e318207f564
22. Bellcross CA, Kolor K, Goddard KA, Coates RJ, Reyes M, Khoury MJ. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40(1):61-66. doi:10.1016/j.amepre.2010.09.027
23. Pal T, Cragun D, Lewis C, et al. A statewide survey of practitioners to assess knowledge and clinical practices regarding hereditary breast and ovarian cancer. Genet Test Mol Biomarkers. 2013;17(5):367-375. doi:10.1089/gtmb.2012.0381
24. Bensend TA, Veach PM, Niendorf KB. What’s the harm? Genetic counselor perceptions of adverse effects of genetics service provision by non-genetics professionals. J Genet Couns. 2014;23(1):48-63. doi:10.1007/s10897-013-9605-3
25. Teng I, Spigelman A. Attitudes and knowledge of medical practitioners to hereditary cancer clinics and cancer genetic testing. Fam Cancer. 2014;13(2):311-324. doi:10.1007/s10689-013-9695-y
26. Mikat-Stevens NA, Larson IA, Tarini BA. Primary-care providers’ perceived barriers to integration of genetics services: a systematic review of the literature. Genet Med. 2015;17(3):169-176. doi:10.1038/gim.2014.101
27. Scheuner MT, Hilborne L, Brown J, Lubin IM; members of the RAND Molecular Genetic Test Report Advisory Board. A report template for molecular genetic tests designed to improve communication between the clinician and laboratory. Genet Test Mol Biomarkers. 2012;16(7):761-769. doi:10.1089/gtmb.2011.0328
28. Scheuner MT, Peredo J, Tangney K, et al. Electronic health record interventions at the point of care improve documentation of care processes and decrease orders for genetic tests commonly ordered by nongeneticists. Genet Med. 2017;19(1):112-120. doi:10.1038/gim.2016.73
29. Cooksey JA, Forte G, Benkendorf J, Blitzer MG. The state of the medical geneticist workforce: findings of the 2003 survey of American Board of Medical Genetics certified geneticists. Genet Med. 2005;7(6):439-443. doi:10.1097/01.gim.0000172416.35285.9f
30. Institute of Medicine. Roundtable on Translating Genomic-Based Research for Health. Washington, DC: National Academies Press; 2009. https://www.ncbi.nlm.nih.gov/books/NBK26394. Accessed July 22, 2020.
31. Hoskovec JM, Bennett RL, Carey ME, et al. Projecting the supply and demand for certified genetic counselors: a workforce study. J Genet Couns. 2018;27(1):16-20. doi:10.1007/s10897-017-0158-8
32. Penon-Portmann M, Chang J, Cheng M, Shieh JT. Genetics workforce: distribution of genetics services and challenges to health care in California. Genet Med. 2020;22(1):227-231. doi:10.1038/s41436-019-0628-5

33. Spoont M, Greer N, Su J, Fitzgerald P, Rutks I, Wilt TJ. Rural vs. Urban Ambulatory Health Care: A Systematic Review. Washington, DC: US Department of Veterans Affairs; 2011. https://www.hsrd.research.va.gov/publications/esp/ambulatory.cfm. Accessed July 21, 2020.
34. Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89(1):39-68. doi:10.1111/j.1468-0009.2011.00619.x
35. Walsh J, Harrison JD, Young JM, Butow PN, Solomon MJ, Masya L. What are the current barriers to effective cancer care coordination? A qualitative study. BMC Health Serv Res. 2010;10:132. Published 2010 May 20. doi:10.1186/1472-6963-10-132
36. McDonald KM, Schultz E, Albin L, et al. Care Coordination Measures Atlas. Version 4. Agency for Healthcare Research and Quality Publication No. 14-0037. https://www.ahrq.gov/sites/default/files/publications/files/ccm_atlas.pdf. Updated June 2014. Accessed July 22, 2020.
37. Greenwood-Lee J, Jewett L, Woodhouse L, Marshall DA. A categorisation of problems and solutions to improve patient referrals from primary to specialty care. BMC Health Serv Res. 2018;18(1):986. Published 2018 Dec 20. doi:10.1186/s12913-018-3745-y
38. US Department of Veterans Affairs, Office of Academic Affiliations. Our medical and dental training program. https://www.va.gov/oaa/gme_default.asp. Updated January 7, 2020. Accessed July 21, 2020.
39. Scheuner MT, Marshall N, Lanto A, et al. Delivery of clinical genetic consultative services in the Veterans Health Administration. Genet Med. 2014;16(8):609-619. doi:10.1038/gim.2013.202.
40. Battista RN, Blancquaert I, Laberge AM, van Schendel N, Leduc N. Genetics in health care: an overview of current and emerging models. Public Health Genomics. 2012;15(1):34-45. doi:10.1159/000328846
41. Emery J. The GRAIDS Trial: the development and evaluation of computer decision support for cancer genetic risk assessment in primary care. Ann Hum Biol. 2005;32(2):218-227. doi:10.1080/03014460500074921
42. Scheuner MT, Hamilton AB, Peredo J, et al. A cancer genetics toolkit improves access to genetic services through documentation and use of the family history by primary-care clinicians. Genet Med. 2014;16(1):60-69. doi:10.1038/gim.2013.75
43. Scheuner MT, Peredo J, Tangney K, et al. Electronic health record interventions at the point of care improve documentation of care processes and decrease orders for genetic tests commonly ordered by nongeneticists. Genet Med. 2017;19(1):112-120. doi:10.1038/gim.2016.73
44. Hamilton AB, Oishi S, Yano EM, Gammage CE, Marshall NJ, Scheuner MT. Factors influencing organizational adoption and implementation of clinical genetic services. Genet Med. 2014;16(3):238-245. doi:10.1038/gim.2013.101
45. Sperber NR, Andrews SM, Voils CI, Green GL, Provenzale D, Knight S. Barriers and facilitators to adoption of genomic services for colorectal care within the Veterans Health Administration. J Pers Med. 2016;6(2):16. Published 2016 Apr 28. doi:10.3390/jpm6020016
46. US Department of Veterans Affairs, Health Services Research and Development. Genomics. https://www.hsrd.research.va.gov/research/portfolio_description.cfm?Sulu=17. Updated July 21, 2020. Accessed June 22, 2020.