User login
Clinical Progress Note: Procalcitonin in the Identification of Invasive Bacterial Infections in Febrile Young Infants
Febrile infants 60 days of age or younger pose a significant diagnostic challenge for clinicians. Most of these infants are well appearing and do not have localizing signs or symptoms of infection, yet they may have serious bacterial infections (SBI) such as urinary tract infection (UTI), bacteremia, and meningitis. While urinalysis is highly sensitive for predicting UTI,1 older clinical decision rules and biomarkers such as white blood cell (WBC) count, absolute neutrophil count (ANC), and C-reactive protein (CRP) lack both appropriate sensitivity and specificity for identifying bacteremia and meningitis (ie, invasive bacterial infection [IBI]),2,3 which affect approximately 2.4% and 0.9% of febrile infants during the first 2 months of life, respectively.4 The lack of accurate diagnostic markers can drive overuse of laboratory testing, antibiotics, and hospitalization despite the low rates of these infections. As a result, procalcitonin (PCT) has generated interest because of its potential to serve as a more accurate biomarker for bacterial infections. This review summarizes recent literature on the diagnostic utility of PCT in the identification of IBI in febrile young infants 60 days or younger.
MECHANISM OF PROCALCITONIN
Procalcitonin is undetectable in noninflammatory states but can be detected in the blood within 4 to 6 hours after initial bacterial infection.5 Its production is stimulated throughout various tissues of the body by cytokines such as interleukin-6 and tumor necrosis factor, which are produced in response to bacterial infections. Interferon-γ, which is produced in response to viral infections, attenuates PCT production. While these characteristics suggest promise for PCT as a more specific screening test for underlying bacterial infection, there are caveats. PCT levels are physiologically elevated in the first 48 hours of life and vary with gestational age, factors that should be considered when interpreting results.6 Additionally, PCT levels can rise in other inflammatory states such as autoimmune conditions and certain malignancies,5 though these states are unlikely to confound the evaluation of febrile young infants.
DIAGNOSTIC ACCURACY OF PROCALCITONIN
Because of PCT’s potential to be more specific than other commonly used biomarkers, multiple studies have evaluated its performance characteristics in febrile young infants. Gomez et al retrospectively evaluated 1,112 well-appearing infants younger than 3 months with fever without a source in seven European emergency departments (EDs).7 Overall, 23 infants (2.1%) had IBI (1 with meningitis). A PCT level of 0.5 ng/mL or greater was the only independent risk factor for IBI (adjusted odds ratio, 21.69; 95% CI, 7.93-59.28). Four infants with IBI had a PCT level less than 0.5 ng/mL, and none of these four had meningitis. PCT was superior to CRP, ANC, and WBC in detecting IBI (area under the curve [AUC], 0.825; 95% CI, 0.698-0.952). PCT was the also the best marker for identifying IBI among 451 infants with a normal urine dipstick and fever detected ≤6 hours before presentation (AUC, 0.819; 95% CI, 0.551-1.087).
In the largest prospective study to date evaluating the diagnostic accuracy of PCT in febrile young infants, Milcent et al studied 2,047 previously healthy infants aged 7-91 days admitted for fever from 15 French EDs.8 In total, 21 (1%) had an IBI (8 with meningitis). PCT performed better than CRP, ANC, and WBC for the detection of IBI with an AUC of 0.91 (95% CI, 0.83-0.99). In a multivariable model, a PCT level of 0.3 ng/mL or greater was the only independent risk factor for IBI with an adjusted odds ratio of 40.3 (95% CI, 5.0-332). Only one infant with IBI had a PCT level less than 0.3 ng/mL. This infant was 83 days old, had 4 hours of fever, and became afebrile spontaneously prior to the blood culture revealing Streptococcus pneumoniae. PCT also performed better than CRP in the detection of IBI in infants 7-30 days of age and those with fever for less than 6 hours, though both subgroups had small numbers of infants with IBI. The authors determined that a PCT level of 0.3 ng/mL was the optimal cutoff for ruling out IBI; this cutoff had a sensitivity of 90% and negative likelihood ratio (LR) of 0.1 (Table). In contrast, the more commonly studied PCT cutoff of 0.5 ng/mL increased the negative LR to 0.2. The authors suggested that PCT, when used in the context of history, exam, and tests such as urinalysis, could identify infants at low risk of IBI.
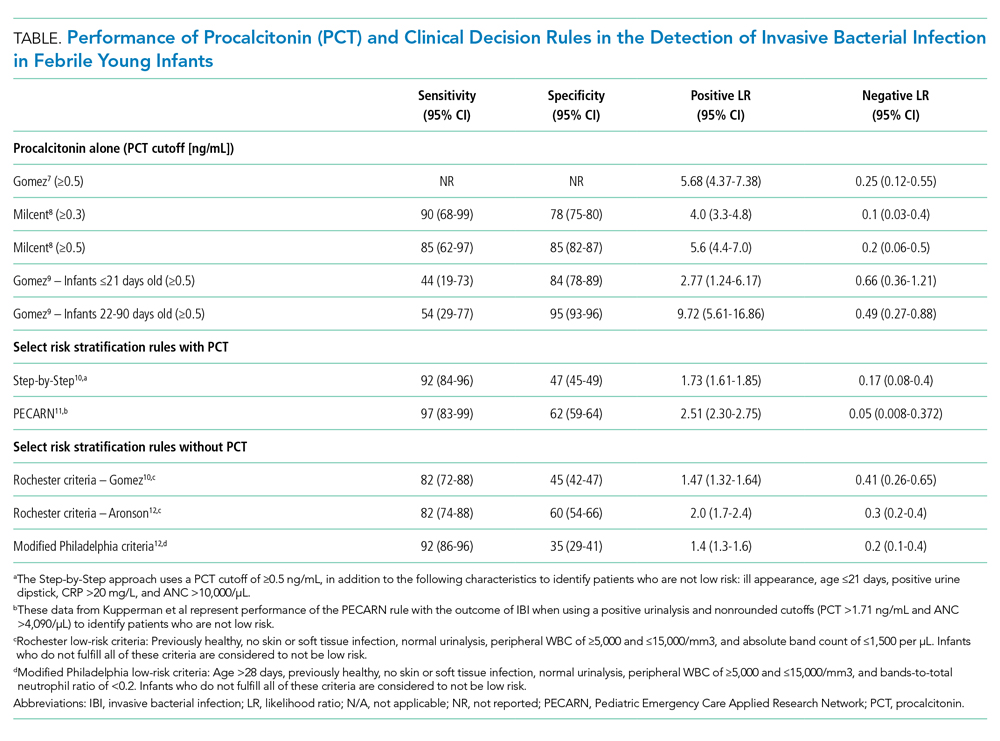
Gomez et al conducted a prospective, single-center study of well-appearing infants with fever without a source and negative urine dipsticks.9 They identified IBI in 9 of 196 infants (4.5%) 21 days or younger and 13 of 1,331 infants (1.0%) 22-90 days old. PCT was superior to CRP and ANC for IBI detection in both age groups. However, in infants 21 days or younger, both the positive and negative LRs for PCT levels of 0.5 ng/mL or greater were poor (Table). Differences in results from the prior two studies7,8 may be related to smaller sample size and differences in patient population because this study included infants younger than 7 days and a higher proportion of infants presenting within 6 hours of fever.
CLINICAL DECISION RULES
PCT has also been incorporated into clinical decision rules for febrile young infants, primarily to identify those at low risk of either IBI or SBI. The Step-by-Step approach10 classified well-appearing febrile infants 90 days or younger as having a high risk of IBI if they were ill appearing, younger than 21 days old, had a positive urine dipstick or a PCT level of 0.5 ng/mL or greater, and classified them as intermediate risk if they had a CRP level greater than 20 mg/L or ANC level greater than 10,000/µL. The remaining infants were classified as low risk and could be managed as outpatients without lumbar puncture or empiric antibiotics. Of note, derivation of this rule excluded patients with respiratory signs or symptoms. In a prospective validation study with 2,185 infants from 11 European EDs, 87 (4.0%) had an IBI (10 with bacterial meningitis). Sequentially identifying patients as high risk using general appearance, age, and urine dipstick alone identified 80% of infants with IBI and 90% of those with bacterial meningitis. The remaining case of meningitis would have been detected by an elevated PCT. A total of 7 of 991 infants (0.7%) classified as low risk had an IBI and none had meningitis. Six of these infants had a fever duration of less than 2 hours, which would not be enough time for PCT to rise. The Step-by-Step approach, with a sensitivity of 92% and negative LR of 0.17, performed well in the ability to rule out IBI.
A clinical prediction rule developed by the Pediatric Emergency Care Applied Research Network (PECARN) found that urinalysis, ANC, and PCT performed well in identifying infants 60 days or younger at low risk for SBI and IBI.11 This prospective observational study of 1,821 infants 60 days or younger in 26 US EDs found 170 (9.3%) with SBI and 30 (1.6%) with IBI; 10 had bacterial meningitis. Only one patient with IBI was classified as low risk, a 30-day-old whose blood culture grew Enterobacter cloacae and who had a negative repeat blood culture prior to antibiotic treatment. Together, a negative urinalysis, ANC of 4,090/µL or less, and PCT level of 1.71 ng/mL or less were excellent in predicting infants at low risk for both SBI and IBI, with a sensitivity of 97% and negative LR of 0.05 for the outcome of IBI. When applying these variables with “rounded cutoffs” of PCT levels less than 0.5 ng/mL (chosen by the authors because it is a more commonly used cutoff) and ANC of 4,000/µL or less to identify infants at low risk for SBI, their performance was similar to nonrounded cutoffs. Data for the rule with rounded cutoffs in identifying infants at low risk for IBI were not presented. The PECARN study was limited by the small numbers of infants with IBIs, and the authors recommended caution when applying the rule to infants 28 days or younger.
Older clinical decision rules without PCT, such as the Rochester and modified Philadelphia criteria, use clinical and laboratory features to assess risk of IBI.3 Recent studies have evaluated these criteria in cohorts with larger numbers of infants with IBI since the derivation studies included mostly infants with SBI and small numbers with IBI.3 Gomez et al demonstrated that the Rochester criteria had lower sensitivity and higher negative LR than the Step-by-Step approach in IBI detection.10 In a case-control study of 135 cases of IBI with 249 matched controls, Aronson et al reported that the modified Philadelphia criteria had higher sensitivity but lower specificity than the Rochester criteria for IBI detection.12 The ability of the Rochester and modified Philadelphia criteria to rule out IBI, as demonstrated by the negative LR (range 0.2-0.4), was inferior to the negative LRs documented by Milcent et al8 (PCT cutoff value of 0.3 ng/mL), the Step-by-Step approach,10 and the PECARN rule11 (range 0.05-0.17; Table). However, clinical decision rules with and without PCT suffer similar limitations in having poor specificity in identifying infants likely to have IBI.
GAPS IN THE LITERATURE
Several key knowledge gaps around PCT use for diagnosing neonatal infections exist. First, the optimal use of PCT in context with other biomarkers and clinical decision rules remains uncertain. A meta-analysis of 28 studies involving over 2,600 infants that compared PCT level (with and without CRP) with isolated CRP and presepsin levels found that PCT in combination with CRP had greater diagnostic accuracy than either PCT or CRP alone, which highlights a potential opportunity for prospective study.13 Second, more data are needed on the use of PCT in the ≤ 28-day age group given the increased risk of both IBI and neonatal herpes simplex virus infection (HSV), compared with that in the second month of life. Neonatal HSV poses diagnostic challenges because half of infants will initially present as afebrile,14 and delays in initiating antiviral treatment dramatically increase the risk of permanent disability or death.15 There have been no prospective studies evaluating PCT use as part of neonatal HSV evaluations.
CLINICAL APPLICATIONS AND CONCLUSIONS
In summary, PCT can play an important adjunctive diagnostic role in the evaluation of febrile young infants, especially during the second month of life when outpatient management is more likely to be considered. PCT is superior to other inflammatory markers in identifying IBI, though the optimal cutoffs to maximize sensitivity and specificity are uncertain. Its performance characteristics, both alone and within clinical decision rules, can help clinicians better identify children at low risk for IBI when compared with clinical decision rules without PCT. PCT measurement can help clinicians miss fewer infants with IBI and identify infants for whom safely doing less is an appropriate option, which can ultimately reduce costs and hospitalizations. PCT may be particularly helpful when the clinical history is difficult to assess or when other diagnostic test results are missing or give conflicting results. Centers that use PCT will need to ensure that results are available within a short turnaround time (a few hours) in order to meaningfully affect care. Future studies of PCT in febrile infant evaluations should focus on identifying optimal strategies for incorporating this biomarker into risk assessments that present information to parents in a way that enables them to understand their child’s risk of a serious infection.
1. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. https://doi.org/10.1542/peds.2017-3068
2. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
3. Hui C, Neto G, Tsertsvadze A, et al. Diagnosis and management of febrile infants (0-3 months). Evid Rep Technol Assess (Full Rep). 2012;(205):1-297.
4. Biondi EA, Lee B, Ralston SL, et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(3):e190874. https://doi.org/10.1001/jamanetworkopen.2019.0874
5. Fontela PS, Lacroix J. Procalcitonin: is this the promised biomarker for critically ill patients? J Pediatr Intensive Care. 2016;5(4):162-171. https://doi.org/10.1055/s-0036-1583279
6. Chiesa C, Natale F, Pascone R, et al. C reactive protein and procalcitonin: reference intervals for preterm and term newborns during the early neonatal period. Clin Chim Acta. 2011;412(11-12):1053-1059. https://doi.org/10.1016/j.cca.2011.02.020
7. Gomez B, Bressan S, Mintegi S, et al. Diagnostic value of procalcitonin in well-appearing young febrile infants. Pediatrics. 2012;130(5):815-822. https://doi.org/10.1542/peds.2011-3575
8. Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170(1):62-69. https://doi.org/10.1001/jamapediatrics.2015.3210
9. Gomez B, Diaz H, Carro A, Benito J, Mintegi S. Performance of blood biomarkers to rule out invasive bacterial infection in febrile infants under 21 days old. Arch Dis Child. 2019;104(6):547-551. https://doi.org/10.1136/archdischild-2018-315397
10. Gomez B, Mintegi S, Bressan S, et al. Validation of the “step-by-step” approach in the management of young febrile infants. Pediatrics. 2016;138(2):e20154381. https://doi.org/10.1542/peds.2015-4381
11. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
12. Aronson PL, Wang ME, Shapiro ED, et al. Risk stratification of febrile infants ≤60 days old without routine lumbar puncture. Pediatrics. 2018;142(6):e20181879. https://doi.org/10.1542/peds.2018-1879
13. Ruan L, Chen GY, Liu Z, et al. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: a meta-analysis and systematic review. Crit Care. 2018;22(1):316. https://doi.org/10.1186/s13054-018-2236-1
14. Brower L, Schondelmeyer A, Wilson P, Shah SS. Testing and empiric treatment for neonatal herpes simplex virus: challenges and opportunities for improving the value of care. Hosp Pediatr. 2016;6(2):108-111. https://doi.org/10.1542/hpeds.2015-0166
15. Long SS. Delayed acyclovir therapy in neonates with herpes simplex virus infection is associated with an increased odds of death compared with early therapy. Evid Based Med. 2013;18(2):e20. https://doi.org/10.1136/eb-2012-100674
Febrile infants 60 days of age or younger pose a significant diagnostic challenge for clinicians. Most of these infants are well appearing and do not have localizing signs or symptoms of infection, yet they may have serious bacterial infections (SBI) such as urinary tract infection (UTI), bacteremia, and meningitis. While urinalysis is highly sensitive for predicting UTI,1 older clinical decision rules and biomarkers such as white blood cell (WBC) count, absolute neutrophil count (ANC), and C-reactive protein (CRP) lack both appropriate sensitivity and specificity for identifying bacteremia and meningitis (ie, invasive bacterial infection [IBI]),2,3 which affect approximately 2.4% and 0.9% of febrile infants during the first 2 months of life, respectively.4 The lack of accurate diagnostic markers can drive overuse of laboratory testing, antibiotics, and hospitalization despite the low rates of these infections. As a result, procalcitonin (PCT) has generated interest because of its potential to serve as a more accurate biomarker for bacterial infections. This review summarizes recent literature on the diagnostic utility of PCT in the identification of IBI in febrile young infants 60 days or younger.
MECHANISM OF PROCALCITONIN
Procalcitonin is undetectable in noninflammatory states but can be detected in the blood within 4 to 6 hours after initial bacterial infection.5 Its production is stimulated throughout various tissues of the body by cytokines such as interleukin-6 and tumor necrosis factor, which are produced in response to bacterial infections. Interferon-γ, which is produced in response to viral infections, attenuates PCT production. While these characteristics suggest promise for PCT as a more specific screening test for underlying bacterial infection, there are caveats. PCT levels are physiologically elevated in the first 48 hours of life and vary with gestational age, factors that should be considered when interpreting results.6 Additionally, PCT levels can rise in other inflammatory states such as autoimmune conditions and certain malignancies,5 though these states are unlikely to confound the evaluation of febrile young infants.
DIAGNOSTIC ACCURACY OF PROCALCITONIN
Because of PCT’s potential to be more specific than other commonly used biomarkers, multiple studies have evaluated its performance characteristics in febrile young infants. Gomez et al retrospectively evaluated 1,112 well-appearing infants younger than 3 months with fever without a source in seven European emergency departments (EDs).7 Overall, 23 infants (2.1%) had IBI (1 with meningitis). A PCT level of 0.5 ng/mL or greater was the only independent risk factor for IBI (adjusted odds ratio, 21.69; 95% CI, 7.93-59.28). Four infants with IBI had a PCT level less than 0.5 ng/mL, and none of these four had meningitis. PCT was superior to CRP, ANC, and WBC in detecting IBI (area under the curve [AUC], 0.825; 95% CI, 0.698-0.952). PCT was the also the best marker for identifying IBI among 451 infants with a normal urine dipstick and fever detected ≤6 hours before presentation (AUC, 0.819; 95% CI, 0.551-1.087).
In the largest prospective study to date evaluating the diagnostic accuracy of PCT in febrile young infants, Milcent et al studied 2,047 previously healthy infants aged 7-91 days admitted for fever from 15 French EDs.8 In total, 21 (1%) had an IBI (8 with meningitis). PCT performed better than CRP, ANC, and WBC for the detection of IBI with an AUC of 0.91 (95% CI, 0.83-0.99). In a multivariable model, a PCT level of 0.3 ng/mL or greater was the only independent risk factor for IBI with an adjusted odds ratio of 40.3 (95% CI, 5.0-332). Only one infant with IBI had a PCT level less than 0.3 ng/mL. This infant was 83 days old, had 4 hours of fever, and became afebrile spontaneously prior to the blood culture revealing Streptococcus pneumoniae. PCT also performed better than CRP in the detection of IBI in infants 7-30 days of age and those with fever for less than 6 hours, though both subgroups had small numbers of infants with IBI. The authors determined that a PCT level of 0.3 ng/mL was the optimal cutoff for ruling out IBI; this cutoff had a sensitivity of 90% and negative likelihood ratio (LR) of 0.1 (Table). In contrast, the more commonly studied PCT cutoff of 0.5 ng/mL increased the negative LR to 0.2. The authors suggested that PCT, when used in the context of history, exam, and tests such as urinalysis, could identify infants at low risk of IBI.

Gomez et al conducted a prospective, single-center study of well-appearing infants with fever without a source and negative urine dipsticks.9 They identified IBI in 9 of 196 infants (4.5%) 21 days or younger and 13 of 1,331 infants (1.0%) 22-90 days old. PCT was superior to CRP and ANC for IBI detection in both age groups. However, in infants 21 days or younger, both the positive and negative LRs for PCT levels of 0.5 ng/mL or greater were poor (Table). Differences in results from the prior two studies7,8 may be related to smaller sample size and differences in patient population because this study included infants younger than 7 days and a higher proportion of infants presenting within 6 hours of fever.
CLINICAL DECISION RULES
PCT has also been incorporated into clinical decision rules for febrile young infants, primarily to identify those at low risk of either IBI or SBI. The Step-by-Step approach10 classified well-appearing febrile infants 90 days or younger as having a high risk of IBI if they were ill appearing, younger than 21 days old, had a positive urine dipstick or a PCT level of 0.5 ng/mL or greater, and classified them as intermediate risk if they had a CRP level greater than 20 mg/L or ANC level greater than 10,000/µL. The remaining infants were classified as low risk and could be managed as outpatients without lumbar puncture or empiric antibiotics. Of note, derivation of this rule excluded patients with respiratory signs or symptoms. In a prospective validation study with 2,185 infants from 11 European EDs, 87 (4.0%) had an IBI (10 with bacterial meningitis). Sequentially identifying patients as high risk using general appearance, age, and urine dipstick alone identified 80% of infants with IBI and 90% of those with bacterial meningitis. The remaining case of meningitis would have been detected by an elevated PCT. A total of 7 of 991 infants (0.7%) classified as low risk had an IBI and none had meningitis. Six of these infants had a fever duration of less than 2 hours, which would not be enough time for PCT to rise. The Step-by-Step approach, with a sensitivity of 92% and negative LR of 0.17, performed well in the ability to rule out IBI.
A clinical prediction rule developed by the Pediatric Emergency Care Applied Research Network (PECARN) found that urinalysis, ANC, and PCT performed well in identifying infants 60 days or younger at low risk for SBI and IBI.11 This prospective observational study of 1,821 infants 60 days or younger in 26 US EDs found 170 (9.3%) with SBI and 30 (1.6%) with IBI; 10 had bacterial meningitis. Only one patient with IBI was classified as low risk, a 30-day-old whose blood culture grew Enterobacter cloacae and who had a negative repeat blood culture prior to antibiotic treatment. Together, a negative urinalysis, ANC of 4,090/µL or less, and PCT level of 1.71 ng/mL or less were excellent in predicting infants at low risk for both SBI and IBI, with a sensitivity of 97% and negative LR of 0.05 for the outcome of IBI. When applying these variables with “rounded cutoffs” of PCT levels less than 0.5 ng/mL (chosen by the authors because it is a more commonly used cutoff) and ANC of 4,000/µL or less to identify infants at low risk for SBI, their performance was similar to nonrounded cutoffs. Data for the rule with rounded cutoffs in identifying infants at low risk for IBI were not presented. The PECARN study was limited by the small numbers of infants with IBIs, and the authors recommended caution when applying the rule to infants 28 days or younger.
Older clinical decision rules without PCT, such as the Rochester and modified Philadelphia criteria, use clinical and laboratory features to assess risk of IBI.3 Recent studies have evaluated these criteria in cohorts with larger numbers of infants with IBI since the derivation studies included mostly infants with SBI and small numbers with IBI.3 Gomez et al demonstrated that the Rochester criteria had lower sensitivity and higher negative LR than the Step-by-Step approach in IBI detection.10 In a case-control study of 135 cases of IBI with 249 matched controls, Aronson et al reported that the modified Philadelphia criteria had higher sensitivity but lower specificity than the Rochester criteria for IBI detection.12 The ability of the Rochester and modified Philadelphia criteria to rule out IBI, as demonstrated by the negative LR (range 0.2-0.4), was inferior to the negative LRs documented by Milcent et al8 (PCT cutoff value of 0.3 ng/mL), the Step-by-Step approach,10 and the PECARN rule11 (range 0.05-0.17; Table). However, clinical decision rules with and without PCT suffer similar limitations in having poor specificity in identifying infants likely to have IBI.
GAPS IN THE LITERATURE
Several key knowledge gaps around PCT use for diagnosing neonatal infections exist. First, the optimal use of PCT in context with other biomarkers and clinical decision rules remains uncertain. A meta-analysis of 28 studies involving over 2,600 infants that compared PCT level (with and without CRP) with isolated CRP and presepsin levels found that PCT in combination with CRP had greater diagnostic accuracy than either PCT or CRP alone, which highlights a potential opportunity for prospective study.13 Second, more data are needed on the use of PCT in the ≤ 28-day age group given the increased risk of both IBI and neonatal herpes simplex virus infection (HSV), compared with that in the second month of life. Neonatal HSV poses diagnostic challenges because half of infants will initially present as afebrile,14 and delays in initiating antiviral treatment dramatically increase the risk of permanent disability or death.15 There have been no prospective studies evaluating PCT use as part of neonatal HSV evaluations.
CLINICAL APPLICATIONS AND CONCLUSIONS
In summary, PCT can play an important adjunctive diagnostic role in the evaluation of febrile young infants, especially during the second month of life when outpatient management is more likely to be considered. PCT is superior to other inflammatory markers in identifying IBI, though the optimal cutoffs to maximize sensitivity and specificity are uncertain. Its performance characteristics, both alone and within clinical decision rules, can help clinicians better identify children at low risk for IBI when compared with clinical decision rules without PCT. PCT measurement can help clinicians miss fewer infants with IBI and identify infants for whom safely doing less is an appropriate option, which can ultimately reduce costs and hospitalizations. PCT may be particularly helpful when the clinical history is difficult to assess or when other diagnostic test results are missing or give conflicting results. Centers that use PCT will need to ensure that results are available within a short turnaround time (a few hours) in order to meaningfully affect care. Future studies of PCT in febrile infant evaluations should focus on identifying optimal strategies for incorporating this biomarker into risk assessments that present information to parents in a way that enables them to understand their child’s risk of a serious infection.
Febrile infants 60 days of age or younger pose a significant diagnostic challenge for clinicians. Most of these infants are well appearing and do not have localizing signs or symptoms of infection, yet they may have serious bacterial infections (SBI) such as urinary tract infection (UTI), bacteremia, and meningitis. While urinalysis is highly sensitive for predicting UTI,1 older clinical decision rules and biomarkers such as white blood cell (WBC) count, absolute neutrophil count (ANC), and C-reactive protein (CRP) lack both appropriate sensitivity and specificity for identifying bacteremia and meningitis (ie, invasive bacterial infection [IBI]),2,3 which affect approximately 2.4% and 0.9% of febrile infants during the first 2 months of life, respectively.4 The lack of accurate diagnostic markers can drive overuse of laboratory testing, antibiotics, and hospitalization despite the low rates of these infections. As a result, procalcitonin (PCT) has generated interest because of its potential to serve as a more accurate biomarker for bacterial infections. This review summarizes recent literature on the diagnostic utility of PCT in the identification of IBI in febrile young infants 60 days or younger.
MECHANISM OF PROCALCITONIN
Procalcitonin is undetectable in noninflammatory states but can be detected in the blood within 4 to 6 hours after initial bacterial infection.5 Its production is stimulated throughout various tissues of the body by cytokines such as interleukin-6 and tumor necrosis factor, which are produced in response to bacterial infections. Interferon-γ, which is produced in response to viral infections, attenuates PCT production. While these characteristics suggest promise for PCT as a more specific screening test for underlying bacterial infection, there are caveats. PCT levels are physiologically elevated in the first 48 hours of life and vary with gestational age, factors that should be considered when interpreting results.6 Additionally, PCT levels can rise in other inflammatory states such as autoimmune conditions and certain malignancies,5 though these states are unlikely to confound the evaluation of febrile young infants.
DIAGNOSTIC ACCURACY OF PROCALCITONIN
Because of PCT’s potential to be more specific than other commonly used biomarkers, multiple studies have evaluated its performance characteristics in febrile young infants. Gomez et al retrospectively evaluated 1,112 well-appearing infants younger than 3 months with fever without a source in seven European emergency departments (EDs).7 Overall, 23 infants (2.1%) had IBI (1 with meningitis). A PCT level of 0.5 ng/mL or greater was the only independent risk factor for IBI (adjusted odds ratio, 21.69; 95% CI, 7.93-59.28). Four infants with IBI had a PCT level less than 0.5 ng/mL, and none of these four had meningitis. PCT was superior to CRP, ANC, and WBC in detecting IBI (area under the curve [AUC], 0.825; 95% CI, 0.698-0.952). PCT was the also the best marker for identifying IBI among 451 infants with a normal urine dipstick and fever detected ≤6 hours before presentation (AUC, 0.819; 95% CI, 0.551-1.087).
In the largest prospective study to date evaluating the diagnostic accuracy of PCT in febrile young infants, Milcent et al studied 2,047 previously healthy infants aged 7-91 days admitted for fever from 15 French EDs.8 In total, 21 (1%) had an IBI (8 with meningitis). PCT performed better than CRP, ANC, and WBC for the detection of IBI with an AUC of 0.91 (95% CI, 0.83-0.99). In a multivariable model, a PCT level of 0.3 ng/mL or greater was the only independent risk factor for IBI with an adjusted odds ratio of 40.3 (95% CI, 5.0-332). Only one infant with IBI had a PCT level less than 0.3 ng/mL. This infant was 83 days old, had 4 hours of fever, and became afebrile spontaneously prior to the blood culture revealing Streptococcus pneumoniae. PCT also performed better than CRP in the detection of IBI in infants 7-30 days of age and those with fever for less than 6 hours, though both subgroups had small numbers of infants with IBI. The authors determined that a PCT level of 0.3 ng/mL was the optimal cutoff for ruling out IBI; this cutoff had a sensitivity of 90% and negative likelihood ratio (LR) of 0.1 (Table). In contrast, the more commonly studied PCT cutoff of 0.5 ng/mL increased the negative LR to 0.2. The authors suggested that PCT, when used in the context of history, exam, and tests such as urinalysis, could identify infants at low risk of IBI.

Gomez et al conducted a prospective, single-center study of well-appearing infants with fever without a source and negative urine dipsticks.9 They identified IBI in 9 of 196 infants (4.5%) 21 days or younger and 13 of 1,331 infants (1.0%) 22-90 days old. PCT was superior to CRP and ANC for IBI detection in both age groups. However, in infants 21 days or younger, both the positive and negative LRs for PCT levels of 0.5 ng/mL or greater were poor (Table). Differences in results from the prior two studies7,8 may be related to smaller sample size and differences in patient population because this study included infants younger than 7 days and a higher proportion of infants presenting within 6 hours of fever.
CLINICAL DECISION RULES
PCT has also been incorporated into clinical decision rules for febrile young infants, primarily to identify those at low risk of either IBI or SBI. The Step-by-Step approach10 classified well-appearing febrile infants 90 days or younger as having a high risk of IBI if they were ill appearing, younger than 21 days old, had a positive urine dipstick or a PCT level of 0.5 ng/mL or greater, and classified them as intermediate risk if they had a CRP level greater than 20 mg/L or ANC level greater than 10,000/µL. The remaining infants were classified as low risk and could be managed as outpatients without lumbar puncture or empiric antibiotics. Of note, derivation of this rule excluded patients with respiratory signs or symptoms. In a prospective validation study with 2,185 infants from 11 European EDs, 87 (4.0%) had an IBI (10 with bacterial meningitis). Sequentially identifying patients as high risk using general appearance, age, and urine dipstick alone identified 80% of infants with IBI and 90% of those with bacterial meningitis. The remaining case of meningitis would have been detected by an elevated PCT. A total of 7 of 991 infants (0.7%) classified as low risk had an IBI and none had meningitis. Six of these infants had a fever duration of less than 2 hours, which would not be enough time for PCT to rise. The Step-by-Step approach, with a sensitivity of 92% and negative LR of 0.17, performed well in the ability to rule out IBI.
A clinical prediction rule developed by the Pediatric Emergency Care Applied Research Network (PECARN) found that urinalysis, ANC, and PCT performed well in identifying infants 60 days or younger at low risk for SBI and IBI.11 This prospective observational study of 1,821 infants 60 days or younger in 26 US EDs found 170 (9.3%) with SBI and 30 (1.6%) with IBI; 10 had bacterial meningitis. Only one patient with IBI was classified as low risk, a 30-day-old whose blood culture grew Enterobacter cloacae and who had a negative repeat blood culture prior to antibiotic treatment. Together, a negative urinalysis, ANC of 4,090/µL or less, and PCT level of 1.71 ng/mL or less were excellent in predicting infants at low risk for both SBI and IBI, with a sensitivity of 97% and negative LR of 0.05 for the outcome of IBI. When applying these variables with “rounded cutoffs” of PCT levels less than 0.5 ng/mL (chosen by the authors because it is a more commonly used cutoff) and ANC of 4,000/µL or less to identify infants at low risk for SBI, their performance was similar to nonrounded cutoffs. Data for the rule with rounded cutoffs in identifying infants at low risk for IBI were not presented. The PECARN study was limited by the small numbers of infants with IBIs, and the authors recommended caution when applying the rule to infants 28 days or younger.
Older clinical decision rules without PCT, such as the Rochester and modified Philadelphia criteria, use clinical and laboratory features to assess risk of IBI.3 Recent studies have evaluated these criteria in cohorts with larger numbers of infants with IBI since the derivation studies included mostly infants with SBI and small numbers with IBI.3 Gomez et al demonstrated that the Rochester criteria had lower sensitivity and higher negative LR than the Step-by-Step approach in IBI detection.10 In a case-control study of 135 cases of IBI with 249 matched controls, Aronson et al reported that the modified Philadelphia criteria had higher sensitivity but lower specificity than the Rochester criteria for IBI detection.12 The ability of the Rochester and modified Philadelphia criteria to rule out IBI, as demonstrated by the negative LR (range 0.2-0.4), was inferior to the negative LRs documented by Milcent et al8 (PCT cutoff value of 0.3 ng/mL), the Step-by-Step approach,10 and the PECARN rule11 (range 0.05-0.17; Table). However, clinical decision rules with and without PCT suffer similar limitations in having poor specificity in identifying infants likely to have IBI.
GAPS IN THE LITERATURE
Several key knowledge gaps around PCT use for diagnosing neonatal infections exist. First, the optimal use of PCT in context with other biomarkers and clinical decision rules remains uncertain. A meta-analysis of 28 studies involving over 2,600 infants that compared PCT level (with and without CRP) with isolated CRP and presepsin levels found that PCT in combination with CRP had greater diagnostic accuracy than either PCT or CRP alone, which highlights a potential opportunity for prospective study.13 Second, more data are needed on the use of PCT in the ≤ 28-day age group given the increased risk of both IBI and neonatal herpes simplex virus infection (HSV), compared with that in the second month of life. Neonatal HSV poses diagnostic challenges because half of infants will initially present as afebrile,14 and delays in initiating antiviral treatment dramatically increase the risk of permanent disability or death.15 There have been no prospective studies evaluating PCT use as part of neonatal HSV evaluations.
CLINICAL APPLICATIONS AND CONCLUSIONS
In summary, PCT can play an important adjunctive diagnostic role in the evaluation of febrile young infants, especially during the second month of life when outpatient management is more likely to be considered. PCT is superior to other inflammatory markers in identifying IBI, though the optimal cutoffs to maximize sensitivity and specificity are uncertain. Its performance characteristics, both alone and within clinical decision rules, can help clinicians better identify children at low risk for IBI when compared with clinical decision rules without PCT. PCT measurement can help clinicians miss fewer infants with IBI and identify infants for whom safely doing less is an appropriate option, which can ultimately reduce costs and hospitalizations. PCT may be particularly helpful when the clinical history is difficult to assess or when other diagnostic test results are missing or give conflicting results. Centers that use PCT will need to ensure that results are available within a short turnaround time (a few hours) in order to meaningfully affect care. Future studies of PCT in febrile infant evaluations should focus on identifying optimal strategies for incorporating this biomarker into risk assessments that present information to parents in a way that enables them to understand their child’s risk of a serious infection.
1. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. https://doi.org/10.1542/peds.2017-3068
2. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
3. Hui C, Neto G, Tsertsvadze A, et al. Diagnosis and management of febrile infants (0-3 months). Evid Rep Technol Assess (Full Rep). 2012;(205):1-297.
4. Biondi EA, Lee B, Ralston SL, et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(3):e190874. https://doi.org/10.1001/jamanetworkopen.2019.0874
5. Fontela PS, Lacroix J. Procalcitonin: is this the promised biomarker for critically ill patients? J Pediatr Intensive Care. 2016;5(4):162-171. https://doi.org/10.1055/s-0036-1583279
6. Chiesa C, Natale F, Pascone R, et al. C reactive protein and procalcitonin: reference intervals for preterm and term newborns during the early neonatal period. Clin Chim Acta. 2011;412(11-12):1053-1059. https://doi.org/10.1016/j.cca.2011.02.020
7. Gomez B, Bressan S, Mintegi S, et al. Diagnostic value of procalcitonin in well-appearing young febrile infants. Pediatrics. 2012;130(5):815-822. https://doi.org/10.1542/peds.2011-3575
8. Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170(1):62-69. https://doi.org/10.1001/jamapediatrics.2015.3210
9. Gomez B, Diaz H, Carro A, Benito J, Mintegi S. Performance of blood biomarkers to rule out invasive bacterial infection in febrile infants under 21 days old. Arch Dis Child. 2019;104(6):547-551. https://doi.org/10.1136/archdischild-2018-315397
10. Gomez B, Mintegi S, Bressan S, et al. Validation of the “step-by-step” approach in the management of young febrile infants. Pediatrics. 2016;138(2):e20154381. https://doi.org/10.1542/peds.2015-4381
11. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
12. Aronson PL, Wang ME, Shapiro ED, et al. Risk stratification of febrile infants ≤60 days old without routine lumbar puncture. Pediatrics. 2018;142(6):e20181879. https://doi.org/10.1542/peds.2018-1879
13. Ruan L, Chen GY, Liu Z, et al. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: a meta-analysis and systematic review. Crit Care. 2018;22(1):316. https://doi.org/10.1186/s13054-018-2236-1
14. Brower L, Schondelmeyer A, Wilson P, Shah SS. Testing and empiric treatment for neonatal herpes simplex virus: challenges and opportunities for improving the value of care. Hosp Pediatr. 2016;6(2):108-111. https://doi.org/10.1542/hpeds.2015-0166
15. Long SS. Delayed acyclovir therapy in neonates with herpes simplex virus infection is associated with an increased odds of death compared with early therapy. Evid Based Med. 2013;18(2):e20. https://doi.org/10.1136/eb-2012-100674
1. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. https://doi.org/10.1542/peds.2017-3068
2. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
3. Hui C, Neto G, Tsertsvadze A, et al. Diagnosis and management of febrile infants (0-3 months). Evid Rep Technol Assess (Full Rep). 2012;(205):1-297.
4. Biondi EA, Lee B, Ralston SL, et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(3):e190874. https://doi.org/10.1001/jamanetworkopen.2019.0874
5. Fontela PS, Lacroix J. Procalcitonin: is this the promised biomarker for critically ill patients? J Pediatr Intensive Care. 2016;5(4):162-171. https://doi.org/10.1055/s-0036-1583279
6. Chiesa C, Natale F, Pascone R, et al. C reactive protein and procalcitonin: reference intervals for preterm and term newborns during the early neonatal period. Clin Chim Acta. 2011;412(11-12):1053-1059. https://doi.org/10.1016/j.cca.2011.02.020
7. Gomez B, Bressan S, Mintegi S, et al. Diagnostic value of procalcitonin in well-appearing young febrile infants. Pediatrics. 2012;130(5):815-822. https://doi.org/10.1542/peds.2011-3575
8. Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170(1):62-69. https://doi.org/10.1001/jamapediatrics.2015.3210
9. Gomez B, Diaz H, Carro A, Benito J, Mintegi S. Performance of blood biomarkers to rule out invasive bacterial infection in febrile infants under 21 days old. Arch Dis Child. 2019;104(6):547-551. https://doi.org/10.1136/archdischild-2018-315397
10. Gomez B, Mintegi S, Bressan S, et al. Validation of the “step-by-step” approach in the management of young febrile infants. Pediatrics. 2016;138(2):e20154381. https://doi.org/10.1542/peds.2015-4381
11. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
12. Aronson PL, Wang ME, Shapiro ED, et al. Risk stratification of febrile infants ≤60 days old without routine lumbar puncture. Pediatrics. 2018;142(6):e20181879. https://doi.org/10.1542/peds.2018-1879
13. Ruan L, Chen GY, Liu Z, et al. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: a meta-analysis and systematic review. Crit Care. 2018;22(1):316. https://doi.org/10.1186/s13054-018-2236-1
14. Brower L, Schondelmeyer A, Wilson P, Shah SS. Testing and empiric treatment for neonatal herpes simplex virus: challenges and opportunities for improving the value of care. Hosp Pediatr. 2016;6(2):108-111. https://doi.org/10.1542/hpeds.2015-0166
15. Long SS. Delayed acyclovir therapy in neonates with herpes simplex virus infection is associated with an increased odds of death compared with early therapy. Evid Based Med. 2013;18(2):e20. https://doi.org/10.1136/eb-2012-100674
© 2020 Society of Hospital Medicine
Reconsidering Discharge Criteria in Children With Neurologic Impairment and Acute Respiratory Infections
Children with medical complexity account for 30% of pediatric hospitalizations and half of all pediatric hospital costs.1 They frequently experience long lengths of stay (LOS), which are associated with hospital-acquired infections, high costs, and family stress.
In this issue of the Journal of Hospital Medicine, Steuart and colleagues investigate one opportunity to decrease LOS in a subset of children with medical complexity by studying the impact of discharge before patients’ return to their respiratory baseline status.2 They examined 632 hospitalizations in children with neurologic impairment who required increased respiratory support for acute respiratory infections. After adjustment for demographic characteristics, clinical complexity, and acute illness severity, there was no difference in the risk of 30-day hospital reutilization (ie, emergency department revisits and readmissions) when comparing the 30% of children discharged before returning to their respiratory baseline with the 70% discharged at baseline (reutilization rates of 32.8% and 31.8%, respectively).
Twenty-six percent required readmission. This rate is four times that reported for children overall, and higher than the rate for children with the top 10 chronic conditions (range, 6%-21%).3 It also exceeds the median 30-day risk-standardized readmission rates for adult conditions targeted by the Centers for Medicare & Medicaid Services (range, 12%-22%).4 The high readmission rate demonstrates the vulnerability of this population and their need for support in hospital-to-home transitions.
These results suggest important areas for future research. First, the findings need to be replicated by multicenter studies to better understand their generalizability. Second, we need more information about the respiratory support required at discharge, which was not captured in this study. For example, clinicians and families may be more comfortable with discharge for a patient who needs slightly higher levels of their baseline support rather than a new modality of respiratory support. Third, we need to better understand the home context of patients discharged before return to respiratory baseline. Lack of home nursing, in particular, has been associated with discharge delays and prolonged LOS in this population.
This study prompts reconsideration of discharge criteria for acute respiratory infections, which often include return to respiratory baseline. Discharge before respiratory baseline for healthy children with bronchiolitis who were discharged on home supplemental oxygen has been associated with shorter hospitalizations and lower costs without differences in reutilization.5 Steuart and colleagues demonstrate the potential of this approach in children with neurologic impairment. One key question remains: Which children are most appropriate for discharge before return to respiratory baseline? Family engagement in discussions of goals of hospitalization, self-efficacy, and discharge readiness are important.6 These conversations provide context that informs discharge decisions. If the patient is stable and both the medical team and family are comfortable with discharge before respiratory baseline, there may be opportunities to engage in shared decision-making around discharge criteria.
The vulnerability of this population, evidenced by their high rates of readmission, reinforces the importance of family engagement, understanding these children’s diverse needs, and further research to identify effective interventions to support safe transitions from hospital to home.
1. Gold JM, Hall M, Shah SS, et al. Long length of hospital stay in children with medical complexity. J Hosp Med. 2016;11(11):750-756. https://doi.org/10.1002/jhm.2633
2. Steuart R, Tan R, Melink K, et al. Discharge before return to respiratory baseline in children with neurologic impairment. J Hosp Med. 2020; 15:531-537. https://doi.org/10.12788/jhm.3394
3. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
4. 2017 Medicare Hospital Quality Chartbook. Centers for Medicare & Medicaid Services. Last updated February 11, 2020. Accessed June 18, 2020. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures
5. Sandweiss DR, Mundorff MB, Hill T, et al. Decreasing hospital length of stay for bronchiolitis by using an observation unit and home oxygen therapy. JAMA Pediatr. 2013;167(5):422-428. https://doi.org/10.1001/jamapediatrics.2013.1435
6. Leyenaar JK, O’Brien ER, Leslie LK, Lindenauer PK, Mangione-Smith RM. Families’ priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics. 2017;139(1):e20161581. https://doi.org/10.1542/peds.2016-1581
Children with medical complexity account for 30% of pediatric hospitalizations and half of all pediatric hospital costs.1 They frequently experience long lengths of stay (LOS), which are associated with hospital-acquired infections, high costs, and family stress.
In this issue of the Journal of Hospital Medicine, Steuart and colleagues investigate one opportunity to decrease LOS in a subset of children with medical complexity by studying the impact of discharge before patients’ return to their respiratory baseline status.2 They examined 632 hospitalizations in children with neurologic impairment who required increased respiratory support for acute respiratory infections. After adjustment for demographic characteristics, clinical complexity, and acute illness severity, there was no difference in the risk of 30-day hospital reutilization (ie, emergency department revisits and readmissions) when comparing the 30% of children discharged before returning to their respiratory baseline with the 70% discharged at baseline (reutilization rates of 32.8% and 31.8%, respectively).
Twenty-six percent required readmission. This rate is four times that reported for children overall, and higher than the rate for children with the top 10 chronic conditions (range, 6%-21%).3 It also exceeds the median 30-day risk-standardized readmission rates for adult conditions targeted by the Centers for Medicare & Medicaid Services (range, 12%-22%).4 The high readmission rate demonstrates the vulnerability of this population and their need for support in hospital-to-home transitions.
These results suggest important areas for future research. First, the findings need to be replicated by multicenter studies to better understand their generalizability. Second, we need more information about the respiratory support required at discharge, which was not captured in this study. For example, clinicians and families may be more comfortable with discharge for a patient who needs slightly higher levels of their baseline support rather than a new modality of respiratory support. Third, we need to better understand the home context of patients discharged before return to respiratory baseline. Lack of home nursing, in particular, has been associated with discharge delays and prolonged LOS in this population.
This study prompts reconsideration of discharge criteria for acute respiratory infections, which often include return to respiratory baseline. Discharge before respiratory baseline for healthy children with bronchiolitis who were discharged on home supplemental oxygen has been associated with shorter hospitalizations and lower costs without differences in reutilization.5 Steuart and colleagues demonstrate the potential of this approach in children with neurologic impairment. One key question remains: Which children are most appropriate for discharge before return to respiratory baseline? Family engagement in discussions of goals of hospitalization, self-efficacy, and discharge readiness are important.6 These conversations provide context that informs discharge decisions. If the patient is stable and both the medical team and family are comfortable with discharge before respiratory baseline, there may be opportunities to engage in shared decision-making around discharge criteria.
The vulnerability of this population, evidenced by their high rates of readmission, reinforces the importance of family engagement, understanding these children’s diverse needs, and further research to identify effective interventions to support safe transitions from hospital to home.
Children with medical complexity account for 30% of pediatric hospitalizations and half of all pediatric hospital costs.1 They frequently experience long lengths of stay (LOS), which are associated with hospital-acquired infections, high costs, and family stress.
In this issue of the Journal of Hospital Medicine, Steuart and colleagues investigate one opportunity to decrease LOS in a subset of children with medical complexity by studying the impact of discharge before patients’ return to their respiratory baseline status.2 They examined 632 hospitalizations in children with neurologic impairment who required increased respiratory support for acute respiratory infections. After adjustment for demographic characteristics, clinical complexity, and acute illness severity, there was no difference in the risk of 30-day hospital reutilization (ie, emergency department revisits and readmissions) when comparing the 30% of children discharged before returning to their respiratory baseline with the 70% discharged at baseline (reutilization rates of 32.8% and 31.8%, respectively).
Twenty-six percent required readmission. This rate is four times that reported for children overall, and higher than the rate for children with the top 10 chronic conditions (range, 6%-21%).3 It also exceeds the median 30-day risk-standardized readmission rates for adult conditions targeted by the Centers for Medicare & Medicaid Services (range, 12%-22%).4 The high readmission rate demonstrates the vulnerability of this population and their need for support in hospital-to-home transitions.
These results suggest important areas for future research. First, the findings need to be replicated by multicenter studies to better understand their generalizability. Second, we need more information about the respiratory support required at discharge, which was not captured in this study. For example, clinicians and families may be more comfortable with discharge for a patient who needs slightly higher levels of their baseline support rather than a new modality of respiratory support. Third, we need to better understand the home context of patients discharged before return to respiratory baseline. Lack of home nursing, in particular, has been associated with discharge delays and prolonged LOS in this population.
This study prompts reconsideration of discharge criteria for acute respiratory infections, which often include return to respiratory baseline. Discharge before respiratory baseline for healthy children with bronchiolitis who were discharged on home supplemental oxygen has been associated with shorter hospitalizations and lower costs without differences in reutilization.5 Steuart and colleagues demonstrate the potential of this approach in children with neurologic impairment. One key question remains: Which children are most appropriate for discharge before return to respiratory baseline? Family engagement in discussions of goals of hospitalization, self-efficacy, and discharge readiness are important.6 These conversations provide context that informs discharge decisions. If the patient is stable and both the medical team and family are comfortable with discharge before respiratory baseline, there may be opportunities to engage in shared decision-making around discharge criteria.
The vulnerability of this population, evidenced by their high rates of readmission, reinforces the importance of family engagement, understanding these children’s diverse needs, and further research to identify effective interventions to support safe transitions from hospital to home.
1. Gold JM, Hall M, Shah SS, et al. Long length of hospital stay in children with medical complexity. J Hosp Med. 2016;11(11):750-756. https://doi.org/10.1002/jhm.2633
2. Steuart R, Tan R, Melink K, et al. Discharge before return to respiratory baseline in children with neurologic impairment. J Hosp Med. 2020; 15:531-537. https://doi.org/10.12788/jhm.3394
3. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
4. 2017 Medicare Hospital Quality Chartbook. Centers for Medicare & Medicaid Services. Last updated February 11, 2020. Accessed June 18, 2020. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures
5. Sandweiss DR, Mundorff MB, Hill T, et al. Decreasing hospital length of stay for bronchiolitis by using an observation unit and home oxygen therapy. JAMA Pediatr. 2013;167(5):422-428. https://doi.org/10.1001/jamapediatrics.2013.1435
6. Leyenaar JK, O’Brien ER, Leslie LK, Lindenauer PK, Mangione-Smith RM. Families’ priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics. 2017;139(1):e20161581. https://doi.org/10.1542/peds.2016-1581
1. Gold JM, Hall M, Shah SS, et al. Long length of hospital stay in children with medical complexity. J Hosp Med. 2016;11(11):750-756. https://doi.org/10.1002/jhm.2633
2. Steuart R, Tan R, Melink K, et al. Discharge before return to respiratory baseline in children with neurologic impairment. J Hosp Med. 2020; 15:531-537. https://doi.org/10.12788/jhm.3394
3. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
4. 2017 Medicare Hospital Quality Chartbook. Centers for Medicare & Medicaid Services. Last updated February 11, 2020. Accessed June 18, 2020. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures
5. Sandweiss DR, Mundorff MB, Hill T, et al. Decreasing hospital length of stay for bronchiolitis by using an observation unit and home oxygen therapy. JAMA Pediatr. 2013;167(5):422-428. https://doi.org/10.1001/jamapediatrics.2013.1435
6. Leyenaar JK, O’Brien ER, Leslie LK, Lindenauer PK, Mangione-Smith RM. Families’ priorities regarding hospital-to-home transitions for children with medical complexity. Pediatrics. 2017;139(1):e20161581. https://doi.org/10.1542/peds.2016-1581
© 2020 Society of Hospital Medicine
Diagnosis and Management of UTI in Febrile Infants Age 0–2 Months: Applicability of the AAP Guideline
Urinary tract infections (UTIs) are the most common bacterial infection and one of the most common reasons for hospitalization in young infants.1,2 The American Academy of Pediatrics (AAP) has published several clinical practice guidelines for the evaluation and management of febrile children ages 2-24 months with first-time UTIs, most recently in 2011 and affirmed in 2016.3 These guidelines do not provide recommendations for infants aged <2 months, which leads to uncertainty regarding the diagnosis and management of UTIs for infants in this age group. We assess the applicability of the AAP UTI Guideline’s action statements for infants aged <2 months presenting with first-time UTIs, with an emphasis on recent evidence. Because the considerations for bacterial infections differ for febrile infants aged <2 months compared with older infants, we do not discuss action statements one and two (determination of the likelihood of UTIs and decision to test urine) and statement seven (medical evaluation for fever after first UTI).3 Additionally, because concomitant bacteremia and meningitis are more common in this age group than in older infants, we review some of the controversies surrounding the diagnosis and treatment of these disease entities.
DIAGNOSIS
“Action Statement 3: To establish the diagnosis of UTI, clinicians should require both urinalysis results that suggest infection (pyuria and/or bacteriuria) and the presence of at least 50,000 colony-forming units (CFUs) per mL of a uropathogen cultured from a urine specimen obtained through catheterization or SPA.”3
To distinguish asymptomatic bacteriuria or contamination from a true UTI, the AAP Guideline requires both a positive urinalysis (UA) and culture for a diagnosis of a UTI.3 Historically, the UA was considered to be poorly sensitive for infections in young infants, with older studies reporting sensitivities ranging from 40% to 82% using urine culture as the gold standard.4-7 Thus, infants aged <2 months with positive urine cultures and negative UAs are often treated as having true UTIs, though this practice varies by institution.8 Possible explanations for the low UA sensitivity in this population include rapid bladder emptying, immature immune systems, and inability to concentrate urine. However, a negative UA plus a positive urine culture could also represent a “true negative” UA and a “false positive” culture, a finding that may be more common in young infants in whom sterile urine obtainment is often challenging.
Two recent studies have addressed this issue by evaluating the UA sensitivity in patients with bacteremic UTIs, as growth of the same pathogenic organism from the blood and urine almost certainly represents true infection.9,10 In a retrospective study of 203 infants aged <3 months with bacteremic UTIs, the presence of any leukocyte esterase (LE) or pyuria (>3 white blood cells per high-powered field [WBC/HPF]) had a sensitivity of 99.5% (95% CI: 98.5%-100%) and specificity of 93.9% (95% CI: 87.8%-93.2%).9 In a prospective, multicenter study of 4,147 febrile infants aged ≤60 days, of whom 27 infants had bacteremic UTIs, a positive UA (any LE, >5 WBC/HPF, or nitrite) had a sensitivity and specificity of 1.00 (95% CI: 0.87-1.00) and 0.91 (95% CI: 0.90-0.91), respectively.10 Although screening tests may appear to have higher sensitivity in more severely diseased populations (“spectrum bias”),11 it is not clear that infants with bacteremic UTIs are definitively sicker than infants with nonbacteremic UTIs (see “bacteremic UTI” section below). Additionally, this study found similarly excellent sensitivity (0.94 [95% CI: 0.90-0.96]) and specificity (0.91 [95% CI: 0.90-0.91]) of the UA among infants with nonbacteremic UTIs, including infants <28 days old.10
UA sensitivity (using urine culture as the gold standard) may be lower for non-Escherichia coli UTIs.9,10,12 In a retrospective study that included 90 infants <2 months old with UTIs, urine cultures yielding Pseudomonas aeruginosa, Enterococcus, or Klebsiella species were significantly less likely (odds ratio [95% CI]: 0.19 [0.06-0.60]; 0.14 [0.07-0.28]; 0.34 [0.17-0.68], respectively) to have pyuria (≥5 WBC/HPF) or LE (1+ or greater) than urine cultures yielding E. coli.,12 though an alternative explanation for this finding is that these organisms may be more likely to cause asymptomatic bacteriuria or contamination.13
The appropriate CFU/mL threshold to define a UTI and the extent that this threshold should vary by urine collection methods are still unclear. In the aforementioned bacteremic UTI study,9 12 patients with E. coli bacteremia had urine cultures with <50,000 CFU/mL plus pyuria (WBC or LE) in the UA, indicating that true UTIs may occur with <50,000 CFU/mL.
Based on these recent studies, we believe that the recommendation to incorporate UA results into the diagnoses of UTIs can be applied to infants <2 months old, as well as consideration for a UTI for colony counts of ≥10,000 CFU/mL if the UA is positive. For infants with positive urine cultures and negative UAs who have not received antibiotics, we suggest repeating both studies if treatment is being considered. For those who have started antibiotics, the pretest probability of a UTI, initial illness severity, and risks and benefits of continuing treatment should be considered.
TREATMENT
“Action Statement 4a: When initiating treatment, the clinician should base the choice of route of administration on practical considerations. Initiating treatment orally or parenterally is equally efficacious. The clinician should base the choice of agent on local antimicrobial sensitivity patterns (if available) and should adjust the choice according to sensitivity testing of the isolated uropathogen.”3
Most infants <2 months old with UTIs are hospitalized initially because of fever. Therefore, the decision point for most clinicians is not whether to hospitalize but for how long to hospitalize and treat with intravenous (IV) antibiotics prior to discharging home on oral antibiotics. Although all-oral antibiotic regimens are used to treat UTIs in older infants and children,14-18 to our knowledge, there are no randomized controlled trials (RCTs) comparing all-IV vs all-oral antibiotics or a longer vs shorter initial IV course that include infants <1 month old. In the trials that do include infants aged 1-2 months,14,18 the number of subjects in this age group is too small to draw conclusions, a finding supported by a 2014 Cochrane review.19 An adequately powered RCT of different IV antibiotic durations in this age group would be challenging. For example, nearly 1,000 subjects would be needed to demonstrate a statistically significant difference between a 5% and 10% relapse risk between groups, a difference that some may find clinically important.
The paucity of evidence in this age group may explain the considerable variability in the approach to IV antibiotic duration in young infants. Concerns about enteral absorption and underdeveloped immune systems may prompt some physicians to treat the youngest patients more aggressively. One study demonstrated that the proportion of patients <2 months old receiving prolonged courses (≥4 days) of IV antibiotics for UTIs in 46 U.S. children’s hospitals ranged from 0% to 67%.20 Similar variability across hospitals has been described in other observational studies21,22 and across subspecialties in one survey of pediatricians.23
Several observational studies provide additional evidence supporting shorter IV courses. In two studies that examined administrative databases, there was no difference in treatment failure rates between infants aged <2 months20 and <6 months21 receiving longer (≥4 days) vs shorter IV courses. In a study of 172 infants <1 month old with UTIs, the median IV duration was 4 days (range 2-12 days), and no subjects experienced treatment failure or relapse.24 In a multicenter study of 251 infants <3 months old with bacteremic UTIs, mean IV antibiotic durations ranged from 5.5–12 days, and no patient had a relapsed bacteremic UTI. Six infants (2.4%) had a relapsed UTI without bacteremia, with no association between IV antibiotic duration and relapse.22
Based on the available data and known risks of hospitalization and prolonged IV therapy, a reasonable approach for infants <1 month old would be to hospitalize for two to three days while awaiting blood and cerebral spinal fluid (CSF) culture results. Given the possibility of Enterococcus or Enterobacteriaceae that are resistant to third-generation cephalosporins, standard therapy of ampicillin and gentamicin for febrile neonates is reasonable, assuming there is no concern for meningitis. Antibiotics should be narrowed when susceptibilities are known. Once culture results return and signs and symptoms have resolved, discharge home on oral antibiotics is justifiable based on the available literature. For well-appearing infants aged 1-2 months with a presumptive UTI (based on UA results), if hospitalization is not warranted for other reasons, then we recommend outpatient treatment with oral or intramuscular therapy based on local susceptibilities (typically a cephalosporin) and close follow-up for one to two days while awaiting culture results. Although empiric cephalosporin therapy may not provide 100% coverage for all potential organisms, clinical deterioration is uncommon in infants and children receiving discordant therapy.25
“Action Statement 4b: The clinician should choose 7 to 14 days as the duration of antimicrobial therapy.”3
The AAP’s recommendation to provide antibiotics (by oral or parenteral route) for a minimum of seven days total stems from a 2002 meta-analysis comparing long (7-14 days) vs short (≤3 days) courses, where the pooled relative risk of treatment failure with short-course therapy was 1.94 (95% CI: 1.19-3.15).26 However, in this analysis, the trials that demonstrated inferiority with short courses were all trials that used single doses of antibiotics, and a similar Cochrane review comparing 2-4 days with 7-14 days demonstrated no differences in outcomes.27 Therefore, shorter total courses, but not a single dose, are probably appropriate for most UTIs in children. Although there are no obvious biologic reasons why longer total courses would be needed in young infants, there are unfortunately limited data comparing different total antibiotic durations in this age group. We believe that 7-14 days of total therapy is a reasonable recommendation for infants <2 months old, and that future studies should investigate shorter total courses.
IMAGING
“Action Statement 5: Febrile infants with UTIs should undergo renal and bladder ultrasonography (RBUS).”3
The AAP Guideline acknowledges that the RBUS is a poor screening test for the detection of genitourinary abnormalities in infants.3 The RBUS can be normal in infants with vesicoureteral reflux (VUR) or show nonspecific findings of unclear clinical significance.28 In a prospective study of 220 infants <3 months old by Tsai et al, 9/39 infants (23%) with grade III-V VUR had normal RBUS.29 Studies that included older infants have found a similar false-negative rate of 0%-40% for detecting grade IV-V VUR by RBUS.28 Nonetheless, since a RBUS is safe and noninvasive, we feel that the benefits of screening for abnormalities such as hydronephrosis (that could indicate posterior urethral valves or ureteropelvic junction obstruction) outweigh the risks (eg, false positives, overdiagnosis, and cost) of performing a RBUS after a first-time UTI.
“Action Statement 6a: Voiding cystourethrography (VCUG) should not be performed routinely after the first febrile UTI; VCUG is indicated if RBUS reveals hydronephrosis, scarring, or other findings that would suggest either high-grade VUR or obstructive uropathy, as well as in other atypical or complex clinical circumstances.”3
“Action Statement 6b: Further evaluation should be conducted if there is a recurrence of febrile UTI.”3
The RBUS may be normal in infants with VUR. Therefore, the AAP’s recommendation to perform a VCUG only if the RBUS is abnormal or after a recurrent UTI concedes that there will be infants with VUR who are missed after the first UTI.3
The United Kingdom’s National Institute for Health and Care Excellence guideline recommends a VCUG for infants <6 months old with a bacteremic or non-E. coli UTI.30 Whether high-grade VUR is more common in young infants with bacteremic UTIs than nonbacteremic UTIs remains inconclusive. In the Honkinen et al. study that included 87 infants <3 months old with bacteremic UTIs, the prevalence of grade IV-V VUR (10%) and obstruction (7%) was higher than that of the 88 nonbacteremic infants (2% grade IV-V VUR and 2% with obstruction). In the multicenter study of 251 infants <3 months old with bacteremic UTIs, the prevalence of grade IV-V VUR was 12.1%.31 This is higher than that of the nonbacteremic infants in Honkinen et al.’s study32 but more similar to the prevalence of grade IV-V VUR found in Tsai et al. (8.2%) and Ismaili et al.’s (7.0%) studies of UTIs in general.29,33
There does appear to be a higher prevalence of urinary tract abnormalities in young infants with non-E. coli vs E. coli UTIs.31,32,34,35 The odds of an abnormal VCUG was 8.0 (95% CI: 2.3-28) times higher for non-E. coli than E. coli UTIs in the study of 251 bacteremic infants.31 In a study of 122 infants <3 months old, the odds of grade III-V VUR was 10 (95% CI 2.6-41) times higher for non-E. coli than E. coli UTIs.35
However, the need for early detection of VUR is controversial, and VCUGs are invasive, involve ionizing radiation, and may require sedation. Two recent trials (one which included only children with VUR and another in which 42% of subjects had VUR) demonstrated a modest effect of prophylactic antibiotics in preventing recurrent UTIs (>5,000 doses of antibiotics needed to prevent one UTI recurrence), but the effect size did not differ by the presence or degree of VUR, and neither demonstrated any benefit in reducing future renal scarring.36, 37 The benefit of surgical interventions for VUR also remains unclear, though studies are limited.38 Overall, there is no evidence suggesting that infants <2 months old require more vigilance for VUR detection than the 2-24 month age group.
SPECIAL CONSIDERATIONS
Bacteremic UTI
The prevalence of bacteremia in infants ≤60 days old with UTIs was 9% in a study conducted from 2008 to 2013 in 26 EDs and has ranged from 3% to 17% in older studies.10, 22 Many studies have described similar clinical and laboratory findings in young infants with bacteremic and nonbacteremic UTIs.39-41 Despite this, bacteremic UTIs have been associated with prolonged parenteral antibiotic courses, resulting in longer hospitalizations and increased costs.40 Two recent multicenter studies of infants with bacteremic UTIs (251 infants <3 months old22 and 115 infants ≤60 days old42) demonstrated variable IV courses and no association between IV duration and relapsed UTI. The latter study showed no risk difference in the adjusted 30-day UTI recurrence (risk difference 3%, 95% CI: −5.8 to 12.7) or all-cause reutilization (risk difference 3%, 95% CI: −14.5 to 20.6) between long and short IV groups.42 Neither study had patients with relapsed bacteremic UTIs or reported that patients suffered clinical deterioration while on oral antibiotics.22,42
Based on these data demonstrating that adverse outcomes are rare in infants with bacteremic UTIs and not associated with parenteral antibiotic duration, we recommend short parenteral courses (2-3 days) with conversion to oral therapy once infants have clinically improved.
Positive Urinalysis and Testing for Meningitis
Multiple risk stratification algorithms for febrile infants aged ≤60 days categorize infants with a positive UA (and therefore likely UTI) as high-risk for having concomitant bacteremia or meningitis, for which lumbar puncture (LP) is typically recommended.43-45 The risk of not testing CSF is the potential to insufficiently treat meningitis because treatment for UTIs and meningitis differ in dosing, route, and duration. Recent studies have challenged the practice of routine LPs for infants aged 1-2 months with a suspected UTI due to the low prevalence (0%-0.3%) of concomitant meningitis.39,46-48 A meta-analysis of 20 studies reporting rates of concomitant meningitis with UTI in infants aged 29-90 days found a pooled prevalence of 0.25% (95% CI: 0.09%-0.70%).49 Furthermore, a study of febrile infants ages 29-60 days found that the prevalence of meningitis did not differ between those with a positive vs negative UA (3/337 [0.9%] vs 5/498 [1.0%], respectively), suggesting that a positive UA alone should not modify the pretest probability of meningitis in this age group.50
Two studies have also examined the risk of delayed meningitis among infants ≤60 days old treated for UTIs without CSF testing. A northern California study that examined 345 episodes among 341 UA-positive infants aged 29-60 days found zero cases (95% CI: 0%-1.1%) of delayed meningitis within 30 days of evaluation.50 A multicenter study of well-appearing febrile infants aged 7-60 days found 0/505 cases (95% CI: 0%-0.6%) of delayed meningitis within 7 days of discharge; 407 (81%) were aged 31-60 days.51 In summary, studies have shown a low rate of concomitant meningitis and a low risk of delayed meningitis in infants aged 1-2 months treated for UTI without CSF testing. Given this, clinically targeted (eg, based on ill appearance and/or lethargy), rather than routine, CSF testing in this age group can be considered.
CONCLUSION
While the AAP UTI Guideline is directed toward 2-24-month-old infants, recent evidence suggests that action statements 3-6 apply to infants <2 months old. Incorporation of pyuria as a diagnostic criterion for UTIs, early transition to oral therapy, and selective VCUG testing are all warranted based on the available evidence and consideration of known risks and benefits. Future studies with larger sample sizes that include infants <2 months old would be beneficial to ensure that the available studies, which have relatively small cohorts, do not suffer from type II error. We propose that future studies examine shorter (<7 days) vs longer total antibiotic duration, shorter vs longer initial IV antibiotics (especially in infants <1 month old or with bacteremic UTIs), and whether RBUS can be performed in a targeted manner. RCTs comparing universal vs targeted imaging strategies would help ascertain whether the increased diagnostic yield that accompanies more aggressive imaging strategies translates into improved outcomes. Application of these AAP guidelines to the <2-month age group and enhancement of the evidence base can promote the high-value care of young infants with UTIs.
1. Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. https://doi.org/10.1097/INF.0000000000000225.
2. Spencer JD, Schwaderer A, McHugh K, Hains DS. Pediatric urinary tract infections: an analysis of hospitalizations, charges, and costs in the USA. Pediatr Nephrol. 2010;25(12):2469-2475. https://doi.org/10.1007/s00467-010-1625-8.
3. Subcommittee On Urinary Tract Infection. Reaffirmation of AAP Clinical Practice Guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138(6):1-5. https://doi.org/10.1542/peds.2016-3026.
4. Crain EF, Gershel JC. Urinary tract infections in febrile infants younger than 8 weeks of age. Pediatrics. 1990;86(3):363-367. https://doi.org/10.1542/peds.105.2.e20
5. Dayan PS, Bennett J, Best R, et al. Test characteristics of the urine Gram stain in infants <or= 60 days of age with fever. Pediatr Emerg Care. 2002;18(1):12-14. https://doi.org/10.1097/00006565-200202000-00004.
6. Bachur R, Harper MB. Reliability of the urinalysis for predicting urinary tract infections in young febrile children. Arch Pediatr Adolesc Med. 2001;155(1):60-65. https://doi.org/10.1001/archpedi.155.1.60.
7. Reardon JM, Carstairs KL, Rudinsky SL, Simon LV, Riffenburgh RH, Tanen DA. Urinalysis is not reliable to detect a urinary tract infection in febrile infants presenting to the ED. Am J Emerg Med. 2009;27(8):930-932. https://doi.org/10.1016/j.ajem.2008.07.015.
8. Schroeder AR, Lucas BP, Garber MD, McCulloh RJ, Joshi-Patel AA, Biondi EA. Negative urinalyses in febrile infants age 7 to 60 days treated for urinary tract infection. J Hosp Med. 2019;14(2):101-104. https://doi.org/10.12788/jhm.3120.
9. Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135(6):965-971. https://doi.org/10.1542/peds.2015-0012.
10. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. https://doi.org/10.1542/peds.2017-3068.
11. Newman TB, Kohn MA. Evidence-based diagnosis. Practical Guides to Biostatistics and Epidemiology. Cambridge; New York: Cambridge University Press, 2009.
12. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association Between Uropathogen and Pyuria. Pediatrics. 2016;138(1):e20160087. https://doi.org/10.1542/peds.2016-0087.
13. Eliacik K, Kanik A, Yavascan O, et al. A comparison of bladder catheterization and suprapubic aspiration methods for urine sample collection from infants with a suspected urinary tract infection. Clin Pediatr. 2016;55(9):819-824. https://doi.org/10.1177/0009922815608278.
14. Bocquet N, Sergent Alaoui A, Jais JP, et al. Randomized trial of oral versus sequential IV/oral antibiotic for acute pyelonephritis in children. Pediatrics. 2012;129(2):e269-e275. https://doi.org/10.1542/peds.2011-0814.
15. Bouissou F, Munzer C, Decramer S, et al. Prospective, randomized trial comparing short and long intravenous antibiotic treatment of acute pyelonephritis in children: dimercaptosuccinic acid scintigraphic evaluation at 9 months. Pediatrics. 2008;121(3):e553-e560. https://doi.org/10.1542/peds.2006-3632.
16. Hodson EM, Willis NS, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev. 2007(4):CD003772. https://doi.org/10.1002/14651858.CD003772.pub3.
17. Neuhaus TJ, Berger C, Buechner K, et al. Randomised trial of oral versus sequential intravenous/oral cephalosporins in children with pyelonephritis. Eur J Pediatr. 2008;167(9):1037-1047. https://doi.org/10.1007/s00431-007-0638-1
18. Hoberman A, Wald ER, Hickey RW, et al. Oral versus initial intravenous therapy for urinary tract infections in young febrile children. Pediatrics. 1999;104(1 Pt 1):79-86. https://doi.org/10.1542/peds.104.1.79.
19. Strohmeier Y, Hodson EM, Willis NS, Webster AC, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev. 2014(7):CD003772. https://doi.org/10.1002/14651858.CD003772.pub4.
20. Lewis-de Los Angeles WW, Thurm C, Hersh AL, et al. Trends in intravenous antibiotic duration for urinary tract infections in young infants. Pediatrics. 2017;140(6):e20171021. https://doi.org/10.1542/peds.2017-1021.
21. Brady PW, Conway PH, Goudie A. Length of intravenous antibiotic therapy and treatment failure in infants with urinary tract infections. Pediatrics. 2010;126(2):196-203. https://doi.org/10.1542/peds.2009-2948.
22. Schroeder AR, Shen MW, Biondi EA, et al. Bacteraemic urinary tract infection: management and outcomes in young infants. Arch Dis Child. 2016;101(2):125-130. https://doi.org/10.1136/archdischild-2014-307997.
23. Joshi NS, Lucas BP, Schroeder AR. Physician preferences surrounding urinary tract infection management in neonates. Hosp Pediatr. 2018;8(1):21-27. https://doi.org/10.1542/hpeds.2017-0082.
24. Magin EC, Garcia-Garcia JJ, Sert SZ, Giralt AG, Cubells CL. Efficacy of short-term intravenous antibiotic in neonates with urinary tract infection. Pediatr Emerg Care. 2007;23(2):83-86. https://doi.org/10.1097/PEC.0b013e3180302c47.
25. Wang ME, Lee V, Greenhow TL, et al. Clinical response to discordant therapy in third-generation cephalosporin-resistant UTIs. Pediatrics. 2019; In press.
26. Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109(5):E70. https://doi.org/10.1542/peds.109.5.e70.
27. Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev. 2003(1):CD003966. https://doi.org/10.1002/14651858.CD003966.
28. Finnell SM, Carroll AE, Downs SM, Subcommittee on Urinary Tract I. Technical report-Diagnosis and management of an initial UTI in febrile infants and young children. Pediatrics. 2011;128(3):e749-e770. https://doi.org/10.1542/peds.2011-1332.
29. Tsai JD, Huang CT, Lin PY, et al. Screening high-grade vesicoureteral reflux in young infants with a febrile urinary tract infection. Pediatr Nephrol. 2012;27(6):955-963. https://doi.org/10.1007/s00467-012-2104-1.
30. National Institue for Health and Care Excellence. Urinary Tract Infection in Children. http://www.nice.org.uk/guidance/cg54/evidence/cg54-urinary-tract-infection-in-children-full-guideline2. Published August 2007. Accessed August 2019.
31. Chang PW, Abidari JM, Shen MW, et al. Urinary imaging findings in young infants with bacteremic urinary tract infection. Hosp Pediatr. 2016;6(11):647-652. https://doi.org/10.1542/hpeds.2015-0229.
32. Honkinen O, Jahnukainen T, Mertsola J, Eskola J, Ruuskanen O. Bacteremic urinary tract infection in children. Pediatr Infect Dis J. 2000;19(7):630-634. https://doi.org/10.1097/00006454-200007000-00009
33. Ismaili K, Lolin K, Damry N, Alexander M, Lepage P, Hall M. Febrile urinary tract infections in 0- to 3-month-old infants: a prospective follow-up study. J Pediatr. 2011;158(1):91-94. https://doi.org/10.1016/j.jpeds.2010.06.053.
34. Cleper R, Krause I, Eisenstein B, Davidovits M. Prevalence of vesicoureteral reflux in neonatal urinary tract infection. Clin Pediatr. 2004;43(7):619-625. https://doi.org/10.1177/000992280404300706.
35. Pauchard JY, Chehade H, Kies CZ, Girardin E, Cachat F, Gehri M. Avoidance of voiding cystourethrography in infants younger than 3 months with Escherichia coli urinary tract infection and normal renal ultrasound. Arch Dis Child. 2017;102(9):804-808. https://doi.org/10.1136/archdischild-2016-311587.
36. Craig JC, Simpson JM, Williams GJ, et al. Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med. 2009;361(18):1748-1759. https://doi.org/10.1056/NEJMoa0902295.
37. Hoberman A, Greenfield SP, Mattoo TK, et al. Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med. 2014;370(25):2367-2376. https://doi.org/10.1056/NEJMoa1401811.
38. Williams G, Hodson EM, Craig JC. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev. 2019;(2):CD001532. https://doi.org/10.1002/14651858.CD001532.pub4.
39. Schnadower D, Kuppermann N, Macias CG, et al. Febrile infants with urinary tract infections at very low risk for adverse events and bacteremia. Pediatrics. 2010;126(6):1074-1083. https://doi.org/10.1542/peds.2010-0479,
40. Roman HK, Chang PW, Schroeder AR. Diagnosis and management of bacteremic urinary tract infection in infants. Hosp Pediatr. 2015;5(1):1-8. https://doi.org/10.1542/hpeds.2014-0051.
41. Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in office settings: the Pediatric Research in Office Settings’ Febrile Infant Study. Arch Pediatr Adolesc Med. 2002;156(1):44-54. https://doi.org/10.1001/archpedi.156.1.44.
42. Desai S, Aronson PL, Shabanova V, et al. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844,
43. Gomez B, Mintegi S, Bressan S, et al. Validation of the “Step-by-Step” approach in the management of young febrile infants. Pediatrics. 2016;138(2):e20154381. https://doi.org/10.1542/peds.2015-4381.
44. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501.
45. DePorre AG, Aronson PL, McCulloh RJ. Facing the ongoing challenge of the febrile young infant. Crit Care. 2017;21(1):68. https://doi.org/10.1186/s13054-017-1646-9,
46. Tebruegge M, Pantazidou A, Clifford V, et al. The age-related risk of co-existing meningitis in children with urinary tract infection. PLoS One. 2011;6(11):e26576. https://doi.org/10.1371/journal.pone.0026576.
47. Thomson J, Cruz AT, Nigrovic LE, et al. Concomitant bacterial meningitis in infants with urinary tract infection. Pediatr Infect Dis J. 2017;36(9):908-910. https://doi.org/10.1097/INF.0000000000001626.
48. Wallace SS, Brown DN, Cruz AT. Prevalence of concomitant acute bacterial meningitis in neonates with febrile urinary tract infection: a retrospective cross-sectional study. J Pediatr. 2017;184:199-203. https://doi.org/10.1016/j.jpeds.2017.01.022.
49. Nugent J, Childers M, Singh-Miller N, Howard R, Allard R, Eberly M. Risk of meningitis in infants aged 29 to 90 days with urinary tract infection: a systematic review and meta-analysis. J Pediatr. 2019;212:102-110.e5. https://doi.org/10.1016/j.jpeds.2019.04.053.
50. Young BR, Nguyen THP, Alabaster A, Greenhow TL. The prevalence of bacterial meningitis in febrile infants 29-60 days with positive urinalysis. Hosp Pediatr. 2018;8(8):450-457. https
51. Wang ME, Biondi EA, McCulloh RJ, et al. Testing for meningitis in febrile well-appearing young infants with a positive urinalysis. Pediatrics. 2019;144(3):e20183979. https://doi.org/10.1542/peds.2018-3979.
Urinary tract infections (UTIs) are the most common bacterial infection and one of the most common reasons for hospitalization in young infants.1,2 The American Academy of Pediatrics (AAP) has published several clinical practice guidelines for the evaluation and management of febrile children ages 2-24 months with first-time UTIs, most recently in 2011 and affirmed in 2016.3 These guidelines do not provide recommendations for infants aged <2 months, which leads to uncertainty regarding the diagnosis and management of UTIs for infants in this age group. We assess the applicability of the AAP UTI Guideline’s action statements for infants aged <2 months presenting with first-time UTIs, with an emphasis on recent evidence. Because the considerations for bacterial infections differ for febrile infants aged <2 months compared with older infants, we do not discuss action statements one and two (determination of the likelihood of UTIs and decision to test urine) and statement seven (medical evaluation for fever after first UTI).3 Additionally, because concomitant bacteremia and meningitis are more common in this age group than in older infants, we review some of the controversies surrounding the diagnosis and treatment of these disease entities.
DIAGNOSIS
“Action Statement 3: To establish the diagnosis of UTI, clinicians should require both urinalysis results that suggest infection (pyuria and/or bacteriuria) and the presence of at least 50,000 colony-forming units (CFUs) per mL of a uropathogen cultured from a urine specimen obtained through catheterization or SPA.”3
To distinguish asymptomatic bacteriuria or contamination from a true UTI, the AAP Guideline requires both a positive urinalysis (UA) and culture for a diagnosis of a UTI.3 Historically, the UA was considered to be poorly sensitive for infections in young infants, with older studies reporting sensitivities ranging from 40% to 82% using urine culture as the gold standard.4-7 Thus, infants aged <2 months with positive urine cultures and negative UAs are often treated as having true UTIs, though this practice varies by institution.8 Possible explanations for the low UA sensitivity in this population include rapid bladder emptying, immature immune systems, and inability to concentrate urine. However, a negative UA plus a positive urine culture could also represent a “true negative” UA and a “false positive” culture, a finding that may be more common in young infants in whom sterile urine obtainment is often challenging.
Two recent studies have addressed this issue by evaluating the UA sensitivity in patients with bacteremic UTIs, as growth of the same pathogenic organism from the blood and urine almost certainly represents true infection.9,10 In a retrospective study of 203 infants aged <3 months with bacteremic UTIs, the presence of any leukocyte esterase (LE) or pyuria (>3 white blood cells per high-powered field [WBC/HPF]) had a sensitivity of 99.5% (95% CI: 98.5%-100%) and specificity of 93.9% (95% CI: 87.8%-93.2%).9 In a prospective, multicenter study of 4,147 febrile infants aged ≤60 days, of whom 27 infants had bacteremic UTIs, a positive UA (any LE, >5 WBC/HPF, or nitrite) had a sensitivity and specificity of 1.00 (95% CI: 0.87-1.00) and 0.91 (95% CI: 0.90-0.91), respectively.10 Although screening tests may appear to have higher sensitivity in more severely diseased populations (“spectrum bias”),11 it is not clear that infants with bacteremic UTIs are definitively sicker than infants with nonbacteremic UTIs (see “bacteremic UTI” section below). Additionally, this study found similarly excellent sensitivity (0.94 [95% CI: 0.90-0.96]) and specificity (0.91 [95% CI: 0.90-0.91]) of the UA among infants with nonbacteremic UTIs, including infants <28 days old.10
UA sensitivity (using urine culture as the gold standard) may be lower for non-Escherichia coli UTIs.9,10,12 In a retrospective study that included 90 infants <2 months old with UTIs, urine cultures yielding Pseudomonas aeruginosa, Enterococcus, or Klebsiella species were significantly less likely (odds ratio [95% CI]: 0.19 [0.06-0.60]; 0.14 [0.07-0.28]; 0.34 [0.17-0.68], respectively) to have pyuria (≥5 WBC/HPF) or LE (1+ or greater) than urine cultures yielding E. coli.,12 though an alternative explanation for this finding is that these organisms may be more likely to cause asymptomatic bacteriuria or contamination.13
The appropriate CFU/mL threshold to define a UTI and the extent that this threshold should vary by urine collection methods are still unclear. In the aforementioned bacteremic UTI study,9 12 patients with E. coli bacteremia had urine cultures with <50,000 CFU/mL plus pyuria (WBC or LE) in the UA, indicating that true UTIs may occur with <50,000 CFU/mL.
Based on these recent studies, we believe that the recommendation to incorporate UA results into the diagnoses of UTIs can be applied to infants <2 months old, as well as consideration for a UTI for colony counts of ≥10,000 CFU/mL if the UA is positive. For infants with positive urine cultures and negative UAs who have not received antibiotics, we suggest repeating both studies if treatment is being considered. For those who have started antibiotics, the pretest probability of a UTI, initial illness severity, and risks and benefits of continuing treatment should be considered.
TREATMENT
“Action Statement 4a: When initiating treatment, the clinician should base the choice of route of administration on practical considerations. Initiating treatment orally or parenterally is equally efficacious. The clinician should base the choice of agent on local antimicrobial sensitivity patterns (if available) and should adjust the choice according to sensitivity testing of the isolated uropathogen.”3
Most infants <2 months old with UTIs are hospitalized initially because of fever. Therefore, the decision point for most clinicians is not whether to hospitalize but for how long to hospitalize and treat with intravenous (IV) antibiotics prior to discharging home on oral antibiotics. Although all-oral antibiotic regimens are used to treat UTIs in older infants and children,14-18 to our knowledge, there are no randomized controlled trials (RCTs) comparing all-IV vs all-oral antibiotics or a longer vs shorter initial IV course that include infants <1 month old. In the trials that do include infants aged 1-2 months,14,18 the number of subjects in this age group is too small to draw conclusions, a finding supported by a 2014 Cochrane review.19 An adequately powered RCT of different IV antibiotic durations in this age group would be challenging. For example, nearly 1,000 subjects would be needed to demonstrate a statistically significant difference between a 5% and 10% relapse risk between groups, a difference that some may find clinically important.
The paucity of evidence in this age group may explain the considerable variability in the approach to IV antibiotic duration in young infants. Concerns about enteral absorption and underdeveloped immune systems may prompt some physicians to treat the youngest patients more aggressively. One study demonstrated that the proportion of patients <2 months old receiving prolonged courses (≥4 days) of IV antibiotics for UTIs in 46 U.S. children’s hospitals ranged from 0% to 67%.20 Similar variability across hospitals has been described in other observational studies21,22 and across subspecialties in one survey of pediatricians.23
Several observational studies provide additional evidence supporting shorter IV courses. In two studies that examined administrative databases, there was no difference in treatment failure rates between infants aged <2 months20 and <6 months21 receiving longer (≥4 days) vs shorter IV courses. In a study of 172 infants <1 month old with UTIs, the median IV duration was 4 days (range 2-12 days), and no subjects experienced treatment failure or relapse.24 In a multicenter study of 251 infants <3 months old with bacteremic UTIs, mean IV antibiotic durations ranged from 5.5–12 days, and no patient had a relapsed bacteremic UTI. Six infants (2.4%) had a relapsed UTI without bacteremia, with no association between IV antibiotic duration and relapse.22
Based on the available data and known risks of hospitalization and prolonged IV therapy, a reasonable approach for infants <1 month old would be to hospitalize for two to three days while awaiting blood and cerebral spinal fluid (CSF) culture results. Given the possibility of Enterococcus or Enterobacteriaceae that are resistant to third-generation cephalosporins, standard therapy of ampicillin and gentamicin for febrile neonates is reasonable, assuming there is no concern for meningitis. Antibiotics should be narrowed when susceptibilities are known. Once culture results return and signs and symptoms have resolved, discharge home on oral antibiotics is justifiable based on the available literature. For well-appearing infants aged 1-2 months with a presumptive UTI (based on UA results), if hospitalization is not warranted for other reasons, then we recommend outpatient treatment with oral or intramuscular therapy based on local susceptibilities (typically a cephalosporin) and close follow-up for one to two days while awaiting culture results. Although empiric cephalosporin therapy may not provide 100% coverage for all potential organisms, clinical deterioration is uncommon in infants and children receiving discordant therapy.25
“Action Statement 4b: The clinician should choose 7 to 14 days as the duration of antimicrobial therapy.”3
The AAP’s recommendation to provide antibiotics (by oral or parenteral route) for a minimum of seven days total stems from a 2002 meta-analysis comparing long (7-14 days) vs short (≤3 days) courses, where the pooled relative risk of treatment failure with short-course therapy was 1.94 (95% CI: 1.19-3.15).26 However, in this analysis, the trials that demonstrated inferiority with short courses were all trials that used single doses of antibiotics, and a similar Cochrane review comparing 2-4 days with 7-14 days demonstrated no differences in outcomes.27 Therefore, shorter total courses, but not a single dose, are probably appropriate for most UTIs in children. Although there are no obvious biologic reasons why longer total courses would be needed in young infants, there are unfortunately limited data comparing different total antibiotic durations in this age group. We believe that 7-14 days of total therapy is a reasonable recommendation for infants <2 months old, and that future studies should investigate shorter total courses.
IMAGING
“Action Statement 5: Febrile infants with UTIs should undergo renal and bladder ultrasonography (RBUS).”3
The AAP Guideline acknowledges that the RBUS is a poor screening test for the detection of genitourinary abnormalities in infants.3 The RBUS can be normal in infants with vesicoureteral reflux (VUR) or show nonspecific findings of unclear clinical significance.28 In a prospective study of 220 infants <3 months old by Tsai et al, 9/39 infants (23%) with grade III-V VUR had normal RBUS.29 Studies that included older infants have found a similar false-negative rate of 0%-40% for detecting grade IV-V VUR by RBUS.28 Nonetheless, since a RBUS is safe and noninvasive, we feel that the benefits of screening for abnormalities such as hydronephrosis (that could indicate posterior urethral valves or ureteropelvic junction obstruction) outweigh the risks (eg, false positives, overdiagnosis, and cost) of performing a RBUS after a first-time UTI.
“Action Statement 6a: Voiding cystourethrography (VCUG) should not be performed routinely after the first febrile UTI; VCUG is indicated if RBUS reveals hydronephrosis, scarring, or other findings that would suggest either high-grade VUR or obstructive uropathy, as well as in other atypical or complex clinical circumstances.”3
“Action Statement 6b: Further evaluation should be conducted if there is a recurrence of febrile UTI.”3
The RBUS may be normal in infants with VUR. Therefore, the AAP’s recommendation to perform a VCUG only if the RBUS is abnormal or after a recurrent UTI concedes that there will be infants with VUR who are missed after the first UTI.3
The United Kingdom’s National Institute for Health and Care Excellence guideline recommends a VCUG for infants <6 months old with a bacteremic or non-E. coli UTI.30 Whether high-grade VUR is more common in young infants with bacteremic UTIs than nonbacteremic UTIs remains inconclusive. In the Honkinen et al. study that included 87 infants <3 months old with bacteremic UTIs, the prevalence of grade IV-V VUR (10%) and obstruction (7%) was higher than that of the 88 nonbacteremic infants (2% grade IV-V VUR and 2% with obstruction). In the multicenter study of 251 infants <3 months old with bacteremic UTIs, the prevalence of grade IV-V VUR was 12.1%.31 This is higher than that of the nonbacteremic infants in Honkinen et al.’s study32 but more similar to the prevalence of grade IV-V VUR found in Tsai et al. (8.2%) and Ismaili et al.’s (7.0%) studies of UTIs in general.29,33
There does appear to be a higher prevalence of urinary tract abnormalities in young infants with non-E. coli vs E. coli UTIs.31,32,34,35 The odds of an abnormal VCUG was 8.0 (95% CI: 2.3-28) times higher for non-E. coli than E. coli UTIs in the study of 251 bacteremic infants.31 In a study of 122 infants <3 months old, the odds of grade III-V VUR was 10 (95% CI 2.6-41) times higher for non-E. coli than E. coli UTIs.35
However, the need for early detection of VUR is controversial, and VCUGs are invasive, involve ionizing radiation, and may require sedation. Two recent trials (one which included only children with VUR and another in which 42% of subjects had VUR) demonstrated a modest effect of prophylactic antibiotics in preventing recurrent UTIs (>5,000 doses of antibiotics needed to prevent one UTI recurrence), but the effect size did not differ by the presence or degree of VUR, and neither demonstrated any benefit in reducing future renal scarring.36, 37 The benefit of surgical interventions for VUR also remains unclear, though studies are limited.38 Overall, there is no evidence suggesting that infants <2 months old require more vigilance for VUR detection than the 2-24 month age group.
SPECIAL CONSIDERATIONS
Bacteremic UTI
The prevalence of bacteremia in infants ≤60 days old with UTIs was 9% in a study conducted from 2008 to 2013 in 26 EDs and has ranged from 3% to 17% in older studies.10, 22 Many studies have described similar clinical and laboratory findings in young infants with bacteremic and nonbacteremic UTIs.39-41 Despite this, bacteremic UTIs have been associated with prolonged parenteral antibiotic courses, resulting in longer hospitalizations and increased costs.40 Two recent multicenter studies of infants with bacteremic UTIs (251 infants <3 months old22 and 115 infants ≤60 days old42) demonstrated variable IV courses and no association between IV duration and relapsed UTI. The latter study showed no risk difference in the adjusted 30-day UTI recurrence (risk difference 3%, 95% CI: −5.8 to 12.7) or all-cause reutilization (risk difference 3%, 95% CI: −14.5 to 20.6) between long and short IV groups.42 Neither study had patients with relapsed bacteremic UTIs or reported that patients suffered clinical deterioration while on oral antibiotics.22,42
Based on these data demonstrating that adverse outcomes are rare in infants with bacteremic UTIs and not associated with parenteral antibiotic duration, we recommend short parenteral courses (2-3 days) with conversion to oral therapy once infants have clinically improved.
Positive Urinalysis and Testing for Meningitis
Multiple risk stratification algorithms for febrile infants aged ≤60 days categorize infants with a positive UA (and therefore likely UTI) as high-risk for having concomitant bacteremia or meningitis, for which lumbar puncture (LP) is typically recommended.43-45 The risk of not testing CSF is the potential to insufficiently treat meningitis because treatment for UTIs and meningitis differ in dosing, route, and duration. Recent studies have challenged the practice of routine LPs for infants aged 1-2 months with a suspected UTI due to the low prevalence (0%-0.3%) of concomitant meningitis.39,46-48 A meta-analysis of 20 studies reporting rates of concomitant meningitis with UTI in infants aged 29-90 days found a pooled prevalence of 0.25% (95% CI: 0.09%-0.70%).49 Furthermore, a study of febrile infants ages 29-60 days found that the prevalence of meningitis did not differ between those with a positive vs negative UA (3/337 [0.9%] vs 5/498 [1.0%], respectively), suggesting that a positive UA alone should not modify the pretest probability of meningitis in this age group.50
Two studies have also examined the risk of delayed meningitis among infants ≤60 days old treated for UTIs without CSF testing. A northern California study that examined 345 episodes among 341 UA-positive infants aged 29-60 days found zero cases (95% CI: 0%-1.1%) of delayed meningitis within 30 days of evaluation.50 A multicenter study of well-appearing febrile infants aged 7-60 days found 0/505 cases (95% CI: 0%-0.6%) of delayed meningitis within 7 days of discharge; 407 (81%) were aged 31-60 days.51 In summary, studies have shown a low rate of concomitant meningitis and a low risk of delayed meningitis in infants aged 1-2 months treated for UTI without CSF testing. Given this, clinically targeted (eg, based on ill appearance and/or lethargy), rather than routine, CSF testing in this age group can be considered.
CONCLUSION
While the AAP UTI Guideline is directed toward 2-24-month-old infants, recent evidence suggests that action statements 3-6 apply to infants <2 months old. Incorporation of pyuria as a diagnostic criterion for UTIs, early transition to oral therapy, and selective VCUG testing are all warranted based on the available evidence and consideration of known risks and benefits. Future studies with larger sample sizes that include infants <2 months old would be beneficial to ensure that the available studies, which have relatively small cohorts, do not suffer from type II error. We propose that future studies examine shorter (<7 days) vs longer total antibiotic duration, shorter vs longer initial IV antibiotics (especially in infants <1 month old or with bacteremic UTIs), and whether RBUS can be performed in a targeted manner. RCTs comparing universal vs targeted imaging strategies would help ascertain whether the increased diagnostic yield that accompanies more aggressive imaging strategies translates into improved outcomes. Application of these AAP guidelines to the <2-month age group and enhancement of the evidence base can promote the high-value care of young infants with UTIs.
Urinary tract infections (UTIs) are the most common bacterial infection and one of the most common reasons for hospitalization in young infants.1,2 The American Academy of Pediatrics (AAP) has published several clinical practice guidelines for the evaluation and management of febrile children ages 2-24 months with first-time UTIs, most recently in 2011 and affirmed in 2016.3 These guidelines do not provide recommendations for infants aged <2 months, which leads to uncertainty regarding the diagnosis and management of UTIs for infants in this age group. We assess the applicability of the AAP UTI Guideline’s action statements for infants aged <2 months presenting with first-time UTIs, with an emphasis on recent evidence. Because the considerations for bacterial infections differ for febrile infants aged <2 months compared with older infants, we do not discuss action statements one and two (determination of the likelihood of UTIs and decision to test urine) and statement seven (medical evaluation for fever after first UTI).3 Additionally, because concomitant bacteremia and meningitis are more common in this age group than in older infants, we review some of the controversies surrounding the diagnosis and treatment of these disease entities.
DIAGNOSIS
“Action Statement 3: To establish the diagnosis of UTI, clinicians should require both urinalysis results that suggest infection (pyuria and/or bacteriuria) and the presence of at least 50,000 colony-forming units (CFUs) per mL of a uropathogen cultured from a urine specimen obtained through catheterization or SPA.”3
To distinguish asymptomatic bacteriuria or contamination from a true UTI, the AAP Guideline requires both a positive urinalysis (UA) and culture for a diagnosis of a UTI.3 Historically, the UA was considered to be poorly sensitive for infections in young infants, with older studies reporting sensitivities ranging from 40% to 82% using urine culture as the gold standard.4-7 Thus, infants aged <2 months with positive urine cultures and negative UAs are often treated as having true UTIs, though this practice varies by institution.8 Possible explanations for the low UA sensitivity in this population include rapid bladder emptying, immature immune systems, and inability to concentrate urine. However, a negative UA plus a positive urine culture could also represent a “true negative” UA and a “false positive” culture, a finding that may be more common in young infants in whom sterile urine obtainment is often challenging.
Two recent studies have addressed this issue by evaluating the UA sensitivity in patients with bacteremic UTIs, as growth of the same pathogenic organism from the blood and urine almost certainly represents true infection.9,10 In a retrospective study of 203 infants aged <3 months with bacteremic UTIs, the presence of any leukocyte esterase (LE) or pyuria (>3 white blood cells per high-powered field [WBC/HPF]) had a sensitivity of 99.5% (95% CI: 98.5%-100%) and specificity of 93.9% (95% CI: 87.8%-93.2%).9 In a prospective, multicenter study of 4,147 febrile infants aged ≤60 days, of whom 27 infants had bacteremic UTIs, a positive UA (any LE, >5 WBC/HPF, or nitrite) had a sensitivity and specificity of 1.00 (95% CI: 0.87-1.00) and 0.91 (95% CI: 0.90-0.91), respectively.10 Although screening tests may appear to have higher sensitivity in more severely diseased populations (“spectrum bias”),11 it is not clear that infants with bacteremic UTIs are definitively sicker than infants with nonbacteremic UTIs (see “bacteremic UTI” section below). Additionally, this study found similarly excellent sensitivity (0.94 [95% CI: 0.90-0.96]) and specificity (0.91 [95% CI: 0.90-0.91]) of the UA among infants with nonbacteremic UTIs, including infants <28 days old.10
UA sensitivity (using urine culture as the gold standard) may be lower for non-Escherichia coli UTIs.9,10,12 In a retrospective study that included 90 infants <2 months old with UTIs, urine cultures yielding Pseudomonas aeruginosa, Enterococcus, or Klebsiella species were significantly less likely (odds ratio [95% CI]: 0.19 [0.06-0.60]; 0.14 [0.07-0.28]; 0.34 [0.17-0.68], respectively) to have pyuria (≥5 WBC/HPF) or LE (1+ or greater) than urine cultures yielding E. coli.,12 though an alternative explanation for this finding is that these organisms may be more likely to cause asymptomatic bacteriuria or contamination.13
The appropriate CFU/mL threshold to define a UTI and the extent that this threshold should vary by urine collection methods are still unclear. In the aforementioned bacteremic UTI study,9 12 patients with E. coli bacteremia had urine cultures with <50,000 CFU/mL plus pyuria (WBC or LE) in the UA, indicating that true UTIs may occur with <50,000 CFU/mL.
Based on these recent studies, we believe that the recommendation to incorporate UA results into the diagnoses of UTIs can be applied to infants <2 months old, as well as consideration for a UTI for colony counts of ≥10,000 CFU/mL if the UA is positive. For infants with positive urine cultures and negative UAs who have not received antibiotics, we suggest repeating both studies if treatment is being considered. For those who have started antibiotics, the pretest probability of a UTI, initial illness severity, and risks and benefits of continuing treatment should be considered.
TREATMENT
“Action Statement 4a: When initiating treatment, the clinician should base the choice of route of administration on practical considerations. Initiating treatment orally or parenterally is equally efficacious. The clinician should base the choice of agent on local antimicrobial sensitivity patterns (if available) and should adjust the choice according to sensitivity testing of the isolated uropathogen.”3
Most infants <2 months old with UTIs are hospitalized initially because of fever. Therefore, the decision point for most clinicians is not whether to hospitalize but for how long to hospitalize and treat with intravenous (IV) antibiotics prior to discharging home on oral antibiotics. Although all-oral antibiotic regimens are used to treat UTIs in older infants and children,14-18 to our knowledge, there are no randomized controlled trials (RCTs) comparing all-IV vs all-oral antibiotics or a longer vs shorter initial IV course that include infants <1 month old. In the trials that do include infants aged 1-2 months,14,18 the number of subjects in this age group is too small to draw conclusions, a finding supported by a 2014 Cochrane review.19 An adequately powered RCT of different IV antibiotic durations in this age group would be challenging. For example, nearly 1,000 subjects would be needed to demonstrate a statistically significant difference between a 5% and 10% relapse risk between groups, a difference that some may find clinically important.
The paucity of evidence in this age group may explain the considerable variability in the approach to IV antibiotic duration in young infants. Concerns about enteral absorption and underdeveloped immune systems may prompt some physicians to treat the youngest patients more aggressively. One study demonstrated that the proportion of patients <2 months old receiving prolonged courses (≥4 days) of IV antibiotics for UTIs in 46 U.S. children’s hospitals ranged from 0% to 67%.20 Similar variability across hospitals has been described in other observational studies21,22 and across subspecialties in one survey of pediatricians.23
Several observational studies provide additional evidence supporting shorter IV courses. In two studies that examined administrative databases, there was no difference in treatment failure rates between infants aged <2 months20 and <6 months21 receiving longer (≥4 days) vs shorter IV courses. In a study of 172 infants <1 month old with UTIs, the median IV duration was 4 days (range 2-12 days), and no subjects experienced treatment failure or relapse.24 In a multicenter study of 251 infants <3 months old with bacteremic UTIs, mean IV antibiotic durations ranged from 5.5–12 days, and no patient had a relapsed bacteremic UTI. Six infants (2.4%) had a relapsed UTI without bacteremia, with no association between IV antibiotic duration and relapse.22
Based on the available data and known risks of hospitalization and prolonged IV therapy, a reasonable approach for infants <1 month old would be to hospitalize for two to three days while awaiting blood and cerebral spinal fluid (CSF) culture results. Given the possibility of Enterococcus or Enterobacteriaceae that are resistant to third-generation cephalosporins, standard therapy of ampicillin and gentamicin for febrile neonates is reasonable, assuming there is no concern for meningitis. Antibiotics should be narrowed when susceptibilities are known. Once culture results return and signs and symptoms have resolved, discharge home on oral antibiotics is justifiable based on the available literature. For well-appearing infants aged 1-2 months with a presumptive UTI (based on UA results), if hospitalization is not warranted for other reasons, then we recommend outpatient treatment with oral or intramuscular therapy based on local susceptibilities (typically a cephalosporin) and close follow-up for one to two days while awaiting culture results. Although empiric cephalosporin therapy may not provide 100% coverage for all potential organisms, clinical deterioration is uncommon in infants and children receiving discordant therapy.25
“Action Statement 4b: The clinician should choose 7 to 14 days as the duration of antimicrobial therapy.”3
The AAP’s recommendation to provide antibiotics (by oral or parenteral route) for a minimum of seven days total stems from a 2002 meta-analysis comparing long (7-14 days) vs short (≤3 days) courses, where the pooled relative risk of treatment failure with short-course therapy was 1.94 (95% CI: 1.19-3.15).26 However, in this analysis, the trials that demonstrated inferiority with short courses were all trials that used single doses of antibiotics, and a similar Cochrane review comparing 2-4 days with 7-14 days demonstrated no differences in outcomes.27 Therefore, shorter total courses, but not a single dose, are probably appropriate for most UTIs in children. Although there are no obvious biologic reasons why longer total courses would be needed in young infants, there are unfortunately limited data comparing different total antibiotic durations in this age group. We believe that 7-14 days of total therapy is a reasonable recommendation for infants <2 months old, and that future studies should investigate shorter total courses.
IMAGING
“Action Statement 5: Febrile infants with UTIs should undergo renal and bladder ultrasonography (RBUS).”3
The AAP Guideline acknowledges that the RBUS is a poor screening test for the detection of genitourinary abnormalities in infants.3 The RBUS can be normal in infants with vesicoureteral reflux (VUR) or show nonspecific findings of unclear clinical significance.28 In a prospective study of 220 infants <3 months old by Tsai et al, 9/39 infants (23%) with grade III-V VUR had normal RBUS.29 Studies that included older infants have found a similar false-negative rate of 0%-40% for detecting grade IV-V VUR by RBUS.28 Nonetheless, since a RBUS is safe and noninvasive, we feel that the benefits of screening for abnormalities such as hydronephrosis (that could indicate posterior urethral valves or ureteropelvic junction obstruction) outweigh the risks (eg, false positives, overdiagnosis, and cost) of performing a RBUS after a first-time UTI.
“Action Statement 6a: Voiding cystourethrography (VCUG) should not be performed routinely after the first febrile UTI; VCUG is indicated if RBUS reveals hydronephrosis, scarring, or other findings that would suggest either high-grade VUR or obstructive uropathy, as well as in other atypical or complex clinical circumstances.”3
“Action Statement 6b: Further evaluation should be conducted if there is a recurrence of febrile UTI.”3
The RBUS may be normal in infants with VUR. Therefore, the AAP’s recommendation to perform a VCUG only if the RBUS is abnormal or after a recurrent UTI concedes that there will be infants with VUR who are missed after the first UTI.3
The United Kingdom’s National Institute for Health and Care Excellence guideline recommends a VCUG for infants <6 months old with a bacteremic or non-E. coli UTI.30 Whether high-grade VUR is more common in young infants with bacteremic UTIs than nonbacteremic UTIs remains inconclusive. In the Honkinen et al. study that included 87 infants <3 months old with bacteremic UTIs, the prevalence of grade IV-V VUR (10%) and obstruction (7%) was higher than that of the 88 nonbacteremic infants (2% grade IV-V VUR and 2% with obstruction). In the multicenter study of 251 infants <3 months old with bacteremic UTIs, the prevalence of grade IV-V VUR was 12.1%.31 This is higher than that of the nonbacteremic infants in Honkinen et al.’s study32 but more similar to the prevalence of grade IV-V VUR found in Tsai et al. (8.2%) and Ismaili et al.’s (7.0%) studies of UTIs in general.29,33
There does appear to be a higher prevalence of urinary tract abnormalities in young infants with non-E. coli vs E. coli UTIs.31,32,34,35 The odds of an abnormal VCUG was 8.0 (95% CI: 2.3-28) times higher for non-E. coli than E. coli UTIs in the study of 251 bacteremic infants.31 In a study of 122 infants <3 months old, the odds of grade III-V VUR was 10 (95% CI 2.6-41) times higher for non-E. coli than E. coli UTIs.35
However, the need for early detection of VUR is controversial, and VCUGs are invasive, involve ionizing radiation, and may require sedation. Two recent trials (one which included only children with VUR and another in which 42% of subjects had VUR) demonstrated a modest effect of prophylactic antibiotics in preventing recurrent UTIs (>5,000 doses of antibiotics needed to prevent one UTI recurrence), but the effect size did not differ by the presence or degree of VUR, and neither demonstrated any benefit in reducing future renal scarring.36, 37 The benefit of surgical interventions for VUR also remains unclear, though studies are limited.38 Overall, there is no evidence suggesting that infants <2 months old require more vigilance for VUR detection than the 2-24 month age group.
SPECIAL CONSIDERATIONS
Bacteremic UTI
The prevalence of bacteremia in infants ≤60 days old with UTIs was 9% in a study conducted from 2008 to 2013 in 26 EDs and has ranged from 3% to 17% in older studies.10, 22 Many studies have described similar clinical and laboratory findings in young infants with bacteremic and nonbacteremic UTIs.39-41 Despite this, bacteremic UTIs have been associated with prolonged parenteral antibiotic courses, resulting in longer hospitalizations and increased costs.40 Two recent multicenter studies of infants with bacteremic UTIs (251 infants <3 months old22 and 115 infants ≤60 days old42) demonstrated variable IV courses and no association between IV duration and relapsed UTI. The latter study showed no risk difference in the adjusted 30-day UTI recurrence (risk difference 3%, 95% CI: −5.8 to 12.7) or all-cause reutilization (risk difference 3%, 95% CI: −14.5 to 20.6) between long and short IV groups.42 Neither study had patients with relapsed bacteremic UTIs or reported that patients suffered clinical deterioration while on oral antibiotics.22,42
Based on these data demonstrating that adverse outcomes are rare in infants with bacteremic UTIs and not associated with parenteral antibiotic duration, we recommend short parenteral courses (2-3 days) with conversion to oral therapy once infants have clinically improved.
Positive Urinalysis and Testing for Meningitis
Multiple risk stratification algorithms for febrile infants aged ≤60 days categorize infants with a positive UA (and therefore likely UTI) as high-risk for having concomitant bacteremia or meningitis, for which lumbar puncture (LP) is typically recommended.43-45 The risk of not testing CSF is the potential to insufficiently treat meningitis because treatment for UTIs and meningitis differ in dosing, route, and duration. Recent studies have challenged the practice of routine LPs for infants aged 1-2 months with a suspected UTI due to the low prevalence (0%-0.3%) of concomitant meningitis.39,46-48 A meta-analysis of 20 studies reporting rates of concomitant meningitis with UTI in infants aged 29-90 days found a pooled prevalence of 0.25% (95% CI: 0.09%-0.70%).49 Furthermore, a study of febrile infants ages 29-60 days found that the prevalence of meningitis did not differ between those with a positive vs negative UA (3/337 [0.9%] vs 5/498 [1.0%], respectively), suggesting that a positive UA alone should not modify the pretest probability of meningitis in this age group.50
Two studies have also examined the risk of delayed meningitis among infants ≤60 days old treated for UTIs without CSF testing. A northern California study that examined 345 episodes among 341 UA-positive infants aged 29-60 days found zero cases (95% CI: 0%-1.1%) of delayed meningitis within 30 days of evaluation.50 A multicenter study of well-appearing febrile infants aged 7-60 days found 0/505 cases (95% CI: 0%-0.6%) of delayed meningitis within 7 days of discharge; 407 (81%) were aged 31-60 days.51 In summary, studies have shown a low rate of concomitant meningitis and a low risk of delayed meningitis in infants aged 1-2 months treated for UTI without CSF testing. Given this, clinically targeted (eg, based on ill appearance and/or lethargy), rather than routine, CSF testing in this age group can be considered.
CONCLUSION
While the AAP UTI Guideline is directed toward 2-24-month-old infants, recent evidence suggests that action statements 3-6 apply to infants <2 months old. Incorporation of pyuria as a diagnostic criterion for UTIs, early transition to oral therapy, and selective VCUG testing are all warranted based on the available evidence and consideration of known risks and benefits. Future studies with larger sample sizes that include infants <2 months old would be beneficial to ensure that the available studies, which have relatively small cohorts, do not suffer from type II error. We propose that future studies examine shorter (<7 days) vs longer total antibiotic duration, shorter vs longer initial IV antibiotics (especially in infants <1 month old or with bacteremic UTIs), and whether RBUS can be performed in a targeted manner. RCTs comparing universal vs targeted imaging strategies would help ascertain whether the increased diagnostic yield that accompanies more aggressive imaging strategies translates into improved outcomes. Application of these AAP guidelines to the <2-month age group and enhancement of the evidence base can promote the high-value care of young infants with UTIs.
1. Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. https://doi.org/10.1097/INF.0000000000000225.
2. Spencer JD, Schwaderer A, McHugh K, Hains DS. Pediatric urinary tract infections: an analysis of hospitalizations, charges, and costs in the USA. Pediatr Nephrol. 2010;25(12):2469-2475. https://doi.org/10.1007/s00467-010-1625-8.
3. Subcommittee On Urinary Tract Infection. Reaffirmation of AAP Clinical Practice Guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138(6):1-5. https://doi.org/10.1542/peds.2016-3026.
4. Crain EF, Gershel JC. Urinary tract infections in febrile infants younger than 8 weeks of age. Pediatrics. 1990;86(3):363-367. https://doi.org/10.1542/peds.105.2.e20
5. Dayan PS, Bennett J, Best R, et al. Test characteristics of the urine Gram stain in infants <or= 60 days of age with fever. Pediatr Emerg Care. 2002;18(1):12-14. https://doi.org/10.1097/00006565-200202000-00004.
6. Bachur R, Harper MB. Reliability of the urinalysis for predicting urinary tract infections in young febrile children. Arch Pediatr Adolesc Med. 2001;155(1):60-65. https://doi.org/10.1001/archpedi.155.1.60.
7. Reardon JM, Carstairs KL, Rudinsky SL, Simon LV, Riffenburgh RH, Tanen DA. Urinalysis is not reliable to detect a urinary tract infection in febrile infants presenting to the ED. Am J Emerg Med. 2009;27(8):930-932. https://doi.org/10.1016/j.ajem.2008.07.015.
8. Schroeder AR, Lucas BP, Garber MD, McCulloh RJ, Joshi-Patel AA, Biondi EA. Negative urinalyses in febrile infants age 7 to 60 days treated for urinary tract infection. J Hosp Med. 2019;14(2):101-104. https://doi.org/10.12788/jhm.3120.
9. Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135(6):965-971. https://doi.org/10.1542/peds.2015-0012.
10. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. https://doi.org/10.1542/peds.2017-3068.
11. Newman TB, Kohn MA. Evidence-based diagnosis. Practical Guides to Biostatistics and Epidemiology. Cambridge; New York: Cambridge University Press, 2009.
12. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association Between Uropathogen and Pyuria. Pediatrics. 2016;138(1):e20160087. https://doi.org/10.1542/peds.2016-0087.
13. Eliacik K, Kanik A, Yavascan O, et al. A comparison of bladder catheterization and suprapubic aspiration methods for urine sample collection from infants with a suspected urinary tract infection. Clin Pediatr. 2016;55(9):819-824. https://doi.org/10.1177/0009922815608278.
14. Bocquet N, Sergent Alaoui A, Jais JP, et al. Randomized trial of oral versus sequential IV/oral antibiotic for acute pyelonephritis in children. Pediatrics. 2012;129(2):e269-e275. https://doi.org/10.1542/peds.2011-0814.
15. Bouissou F, Munzer C, Decramer S, et al. Prospective, randomized trial comparing short and long intravenous antibiotic treatment of acute pyelonephritis in children: dimercaptosuccinic acid scintigraphic evaluation at 9 months. Pediatrics. 2008;121(3):e553-e560. https://doi.org/10.1542/peds.2006-3632.
16. Hodson EM, Willis NS, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev. 2007(4):CD003772. https://doi.org/10.1002/14651858.CD003772.pub3.
17. Neuhaus TJ, Berger C, Buechner K, et al. Randomised trial of oral versus sequential intravenous/oral cephalosporins in children with pyelonephritis. Eur J Pediatr. 2008;167(9):1037-1047. https://doi.org/10.1007/s00431-007-0638-1
18. Hoberman A, Wald ER, Hickey RW, et al. Oral versus initial intravenous therapy for urinary tract infections in young febrile children. Pediatrics. 1999;104(1 Pt 1):79-86. https://doi.org/10.1542/peds.104.1.79.
19. Strohmeier Y, Hodson EM, Willis NS, Webster AC, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev. 2014(7):CD003772. https://doi.org/10.1002/14651858.CD003772.pub4.
20. Lewis-de Los Angeles WW, Thurm C, Hersh AL, et al. Trends in intravenous antibiotic duration for urinary tract infections in young infants. Pediatrics. 2017;140(6):e20171021. https://doi.org/10.1542/peds.2017-1021.
21. Brady PW, Conway PH, Goudie A. Length of intravenous antibiotic therapy and treatment failure in infants with urinary tract infections. Pediatrics. 2010;126(2):196-203. https://doi.org/10.1542/peds.2009-2948.
22. Schroeder AR, Shen MW, Biondi EA, et al. Bacteraemic urinary tract infection: management and outcomes in young infants. Arch Dis Child. 2016;101(2):125-130. https://doi.org/10.1136/archdischild-2014-307997.
23. Joshi NS, Lucas BP, Schroeder AR. Physician preferences surrounding urinary tract infection management in neonates. Hosp Pediatr. 2018;8(1):21-27. https://doi.org/10.1542/hpeds.2017-0082.
24. Magin EC, Garcia-Garcia JJ, Sert SZ, Giralt AG, Cubells CL. Efficacy of short-term intravenous antibiotic in neonates with urinary tract infection. Pediatr Emerg Care. 2007;23(2):83-86. https://doi.org/10.1097/PEC.0b013e3180302c47.
25. Wang ME, Lee V, Greenhow TL, et al. Clinical response to discordant therapy in third-generation cephalosporin-resistant UTIs. Pediatrics. 2019; In press.
26. Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109(5):E70. https://doi.org/10.1542/peds.109.5.e70.
27. Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev. 2003(1):CD003966. https://doi.org/10.1002/14651858.CD003966.
28. Finnell SM, Carroll AE, Downs SM, Subcommittee on Urinary Tract I. Technical report-Diagnosis and management of an initial UTI in febrile infants and young children. Pediatrics. 2011;128(3):e749-e770. https://doi.org/10.1542/peds.2011-1332.
29. Tsai JD, Huang CT, Lin PY, et al. Screening high-grade vesicoureteral reflux in young infants with a febrile urinary tract infection. Pediatr Nephrol. 2012;27(6):955-963. https://doi.org/10.1007/s00467-012-2104-1.
30. National Institue for Health and Care Excellence. Urinary Tract Infection in Children. http://www.nice.org.uk/guidance/cg54/evidence/cg54-urinary-tract-infection-in-children-full-guideline2. Published August 2007. Accessed August 2019.
31. Chang PW, Abidari JM, Shen MW, et al. Urinary imaging findings in young infants with bacteremic urinary tract infection. Hosp Pediatr. 2016;6(11):647-652. https://doi.org/10.1542/hpeds.2015-0229.
32. Honkinen O, Jahnukainen T, Mertsola J, Eskola J, Ruuskanen O. Bacteremic urinary tract infection in children. Pediatr Infect Dis J. 2000;19(7):630-634. https://doi.org/10.1097/00006454-200007000-00009
33. Ismaili K, Lolin K, Damry N, Alexander M, Lepage P, Hall M. Febrile urinary tract infections in 0- to 3-month-old infants: a prospective follow-up study. J Pediatr. 2011;158(1):91-94. https://doi.org/10.1016/j.jpeds.2010.06.053.
34. Cleper R, Krause I, Eisenstein B, Davidovits M. Prevalence of vesicoureteral reflux in neonatal urinary tract infection. Clin Pediatr. 2004;43(7):619-625. https://doi.org/10.1177/000992280404300706.
35. Pauchard JY, Chehade H, Kies CZ, Girardin E, Cachat F, Gehri M. Avoidance of voiding cystourethrography in infants younger than 3 months with Escherichia coli urinary tract infection and normal renal ultrasound. Arch Dis Child. 2017;102(9):804-808. https://doi.org/10.1136/archdischild-2016-311587.
36. Craig JC, Simpson JM, Williams GJ, et al. Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med. 2009;361(18):1748-1759. https://doi.org/10.1056/NEJMoa0902295.
37. Hoberman A, Greenfield SP, Mattoo TK, et al. Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med. 2014;370(25):2367-2376. https://doi.org/10.1056/NEJMoa1401811.
38. Williams G, Hodson EM, Craig JC. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev. 2019;(2):CD001532. https://doi.org/10.1002/14651858.CD001532.pub4.
39. Schnadower D, Kuppermann N, Macias CG, et al. Febrile infants with urinary tract infections at very low risk for adverse events and bacteremia. Pediatrics. 2010;126(6):1074-1083. https://doi.org/10.1542/peds.2010-0479,
40. Roman HK, Chang PW, Schroeder AR. Diagnosis and management of bacteremic urinary tract infection in infants. Hosp Pediatr. 2015;5(1):1-8. https://doi.org/10.1542/hpeds.2014-0051.
41. Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in office settings: the Pediatric Research in Office Settings’ Febrile Infant Study. Arch Pediatr Adolesc Med. 2002;156(1):44-54. https://doi.org/10.1001/archpedi.156.1.44.
42. Desai S, Aronson PL, Shabanova V, et al. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844,
43. Gomez B, Mintegi S, Bressan S, et al. Validation of the “Step-by-Step” approach in the management of young febrile infants. Pediatrics. 2016;138(2):e20154381. https://doi.org/10.1542/peds.2015-4381.
44. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501.
45. DePorre AG, Aronson PL, McCulloh RJ. Facing the ongoing challenge of the febrile young infant. Crit Care. 2017;21(1):68. https://doi.org/10.1186/s13054-017-1646-9,
46. Tebruegge M, Pantazidou A, Clifford V, et al. The age-related risk of co-existing meningitis in children with urinary tract infection. PLoS One. 2011;6(11):e26576. https://doi.org/10.1371/journal.pone.0026576.
47. Thomson J, Cruz AT, Nigrovic LE, et al. Concomitant bacterial meningitis in infants with urinary tract infection. Pediatr Infect Dis J. 2017;36(9):908-910. https://doi.org/10.1097/INF.0000000000001626.
48. Wallace SS, Brown DN, Cruz AT. Prevalence of concomitant acute bacterial meningitis in neonates with febrile urinary tract infection: a retrospective cross-sectional study. J Pediatr. 2017;184:199-203. https://doi.org/10.1016/j.jpeds.2017.01.022.
49. Nugent J, Childers M, Singh-Miller N, Howard R, Allard R, Eberly M. Risk of meningitis in infants aged 29 to 90 days with urinary tract infection: a systematic review and meta-analysis. J Pediatr. 2019;212:102-110.e5. https://doi.org/10.1016/j.jpeds.2019.04.053.
50. Young BR, Nguyen THP, Alabaster A, Greenhow TL. The prevalence of bacterial meningitis in febrile infants 29-60 days with positive urinalysis. Hosp Pediatr. 2018;8(8):450-457. https
51. Wang ME, Biondi EA, McCulloh RJ, et al. Testing for meningitis in febrile well-appearing young infants with a positive urinalysis. Pediatrics. 2019;144(3):e20183979. https://doi.org/10.1542/peds.2018-3979.
1. Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. https://doi.org/10.1097/INF.0000000000000225.
2. Spencer JD, Schwaderer A, McHugh K, Hains DS. Pediatric urinary tract infections: an analysis of hospitalizations, charges, and costs in the USA. Pediatr Nephrol. 2010;25(12):2469-2475. https://doi.org/10.1007/s00467-010-1625-8.
3. Subcommittee On Urinary Tract Infection. Reaffirmation of AAP Clinical Practice Guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138(6):1-5. https://doi.org/10.1542/peds.2016-3026.
4. Crain EF, Gershel JC. Urinary tract infections in febrile infants younger than 8 weeks of age. Pediatrics. 1990;86(3):363-367. https://doi.org/10.1542/peds.105.2.e20
5. Dayan PS, Bennett J, Best R, et al. Test characteristics of the urine Gram stain in infants <or= 60 days of age with fever. Pediatr Emerg Care. 2002;18(1):12-14. https://doi.org/10.1097/00006565-200202000-00004.
6. Bachur R, Harper MB. Reliability of the urinalysis for predicting urinary tract infections in young febrile children. Arch Pediatr Adolesc Med. 2001;155(1):60-65. https://doi.org/10.1001/archpedi.155.1.60.
7. Reardon JM, Carstairs KL, Rudinsky SL, Simon LV, Riffenburgh RH, Tanen DA. Urinalysis is not reliable to detect a urinary tract infection in febrile infants presenting to the ED. Am J Emerg Med. 2009;27(8):930-932. https://doi.org/10.1016/j.ajem.2008.07.015.
8. Schroeder AR, Lucas BP, Garber MD, McCulloh RJ, Joshi-Patel AA, Biondi EA. Negative urinalyses in febrile infants age 7 to 60 days treated for urinary tract infection. J Hosp Med. 2019;14(2):101-104. https://doi.org/10.12788/jhm.3120.
9. Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135(6):965-971. https://doi.org/10.1542/peds.2015-0012.
10. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. https://doi.org/10.1542/peds.2017-3068.
11. Newman TB, Kohn MA. Evidence-based diagnosis. Practical Guides to Biostatistics and Epidemiology. Cambridge; New York: Cambridge University Press, 2009.
12. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association Between Uropathogen and Pyuria. Pediatrics. 2016;138(1):e20160087. https://doi.org/10.1542/peds.2016-0087.
13. Eliacik K, Kanik A, Yavascan O, et al. A comparison of bladder catheterization and suprapubic aspiration methods for urine sample collection from infants with a suspected urinary tract infection. Clin Pediatr. 2016;55(9):819-824. https://doi.org/10.1177/0009922815608278.
14. Bocquet N, Sergent Alaoui A, Jais JP, et al. Randomized trial of oral versus sequential IV/oral antibiotic for acute pyelonephritis in children. Pediatrics. 2012;129(2):e269-e275. https://doi.org/10.1542/peds.2011-0814.
15. Bouissou F, Munzer C, Decramer S, et al. Prospective, randomized trial comparing short and long intravenous antibiotic treatment of acute pyelonephritis in children: dimercaptosuccinic acid scintigraphic evaluation at 9 months. Pediatrics. 2008;121(3):e553-e560. https://doi.org/10.1542/peds.2006-3632.
16. Hodson EM, Willis NS, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev. 2007(4):CD003772. https://doi.org/10.1002/14651858.CD003772.pub3.
17. Neuhaus TJ, Berger C, Buechner K, et al. Randomised trial of oral versus sequential intravenous/oral cephalosporins in children with pyelonephritis. Eur J Pediatr. 2008;167(9):1037-1047. https://doi.org/10.1007/s00431-007-0638-1
18. Hoberman A, Wald ER, Hickey RW, et al. Oral versus initial intravenous therapy for urinary tract infections in young febrile children. Pediatrics. 1999;104(1 Pt 1):79-86. https://doi.org/10.1542/peds.104.1.79.
19. Strohmeier Y, Hodson EM, Willis NS, Webster AC, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev. 2014(7):CD003772. https://doi.org/10.1002/14651858.CD003772.pub4.
20. Lewis-de Los Angeles WW, Thurm C, Hersh AL, et al. Trends in intravenous antibiotic duration for urinary tract infections in young infants. Pediatrics. 2017;140(6):e20171021. https://doi.org/10.1542/peds.2017-1021.
21. Brady PW, Conway PH, Goudie A. Length of intravenous antibiotic therapy and treatment failure in infants with urinary tract infections. Pediatrics. 2010;126(2):196-203. https://doi.org/10.1542/peds.2009-2948.
22. Schroeder AR, Shen MW, Biondi EA, et al. Bacteraemic urinary tract infection: management and outcomes in young infants. Arch Dis Child. 2016;101(2):125-130. https://doi.org/10.1136/archdischild-2014-307997.
23. Joshi NS, Lucas BP, Schroeder AR. Physician preferences surrounding urinary tract infection management in neonates. Hosp Pediatr. 2018;8(1):21-27. https://doi.org/10.1542/hpeds.2017-0082.
24. Magin EC, Garcia-Garcia JJ, Sert SZ, Giralt AG, Cubells CL. Efficacy of short-term intravenous antibiotic in neonates with urinary tract infection. Pediatr Emerg Care. 2007;23(2):83-86. https://doi.org/10.1097/PEC.0b013e3180302c47.
25. Wang ME, Lee V, Greenhow TL, et al. Clinical response to discordant therapy in third-generation cephalosporin-resistant UTIs. Pediatrics. 2019; In press.
26. Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109(5):E70. https://doi.org/10.1542/peds.109.5.e70.
27. Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev. 2003(1):CD003966. https://doi.org/10.1002/14651858.CD003966.
28. Finnell SM, Carroll AE, Downs SM, Subcommittee on Urinary Tract I. Technical report-Diagnosis and management of an initial UTI in febrile infants and young children. Pediatrics. 2011;128(3):e749-e770. https://doi.org/10.1542/peds.2011-1332.
29. Tsai JD, Huang CT, Lin PY, et al. Screening high-grade vesicoureteral reflux in young infants with a febrile urinary tract infection. Pediatr Nephrol. 2012;27(6):955-963. https://doi.org/10.1007/s00467-012-2104-1.
30. National Institue for Health and Care Excellence. Urinary Tract Infection in Children. http://www.nice.org.uk/guidance/cg54/evidence/cg54-urinary-tract-infection-in-children-full-guideline2. Published August 2007. Accessed August 2019.
31. Chang PW, Abidari JM, Shen MW, et al. Urinary imaging findings in young infants with bacteremic urinary tract infection. Hosp Pediatr. 2016;6(11):647-652. https://doi.org/10.1542/hpeds.2015-0229.
32. Honkinen O, Jahnukainen T, Mertsola J, Eskola J, Ruuskanen O. Bacteremic urinary tract infection in children. Pediatr Infect Dis J. 2000;19(7):630-634. https://doi.org/10.1097/00006454-200007000-00009
33. Ismaili K, Lolin K, Damry N, Alexander M, Lepage P, Hall M. Febrile urinary tract infections in 0- to 3-month-old infants: a prospective follow-up study. J Pediatr. 2011;158(1):91-94. https://doi.org/10.1016/j.jpeds.2010.06.053.
34. Cleper R, Krause I, Eisenstein B, Davidovits M. Prevalence of vesicoureteral reflux in neonatal urinary tract infection. Clin Pediatr. 2004;43(7):619-625. https://doi.org/10.1177/000992280404300706.
35. Pauchard JY, Chehade H, Kies CZ, Girardin E, Cachat F, Gehri M. Avoidance of voiding cystourethrography in infants younger than 3 months with Escherichia coli urinary tract infection and normal renal ultrasound. Arch Dis Child. 2017;102(9):804-808. https://doi.org/10.1136/archdischild-2016-311587.
36. Craig JC, Simpson JM, Williams GJ, et al. Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med. 2009;361(18):1748-1759. https://doi.org/10.1056/NEJMoa0902295.
37. Hoberman A, Greenfield SP, Mattoo TK, et al. Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med. 2014;370(25):2367-2376. https://doi.org/10.1056/NEJMoa1401811.
38. Williams G, Hodson EM, Craig JC. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev. 2019;(2):CD001532. https://doi.org/10.1002/14651858.CD001532.pub4.
39. Schnadower D, Kuppermann N, Macias CG, et al. Febrile infants with urinary tract infections at very low risk for adverse events and bacteremia. Pediatrics. 2010;126(6):1074-1083. https://doi.org/10.1542/peds.2010-0479,
40. Roman HK, Chang PW, Schroeder AR. Diagnosis and management of bacteremic urinary tract infection in infants. Hosp Pediatr. 2015;5(1):1-8. https://doi.org/10.1542/hpeds.2014-0051.
41. Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in office settings: the Pediatric Research in Office Settings’ Febrile Infant Study. Arch Pediatr Adolesc Med. 2002;156(1):44-54. https://doi.org/10.1001/archpedi.156.1.44.
42. Desai S, Aronson PL, Shabanova V, et al. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844,
43. Gomez B, Mintegi S, Bressan S, et al. Validation of the “Step-by-Step” approach in the management of young febrile infants. Pediatrics. 2016;138(2):e20154381. https://doi.org/10.1542/peds.2015-4381.
44. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501.
45. DePorre AG, Aronson PL, McCulloh RJ. Facing the ongoing challenge of the febrile young infant. Crit Care. 2017;21(1):68. https://doi.org/10.1186/s13054-017-1646-9,
46. Tebruegge M, Pantazidou A, Clifford V, et al. The age-related risk of co-existing meningitis in children with urinary tract infection. PLoS One. 2011;6(11):e26576. https://doi.org/10.1371/journal.pone.0026576.
47. Thomson J, Cruz AT, Nigrovic LE, et al. Concomitant bacterial meningitis in infants with urinary tract infection. Pediatr Infect Dis J. 2017;36(9):908-910. https://doi.org/10.1097/INF.0000000000001626.
48. Wallace SS, Brown DN, Cruz AT. Prevalence of concomitant acute bacterial meningitis in neonates with febrile urinary tract infection: a retrospective cross-sectional study. J Pediatr. 2017;184:199-203. https://doi.org/10.1016/j.jpeds.2017.01.022.
49. Nugent J, Childers M, Singh-Miller N, Howard R, Allard R, Eberly M. Risk of meningitis in infants aged 29 to 90 days with urinary tract infection: a systematic review and meta-analysis. J Pediatr. 2019;212:102-110.e5. https://doi.org/10.1016/j.jpeds.2019.04.053.
50. Young BR, Nguyen THP, Alabaster A, Greenhow TL. The prevalence of bacterial meningitis in febrile infants 29-60 days with positive urinalysis. Hosp Pediatr. 2018;8(8):450-457. https
51. Wang ME, Biondi EA, McCulloh RJ, et al. Testing for meningitis in febrile well-appearing young infants with a positive urinalysis. Pediatrics. 2019;144(3):e20183979. https://doi.org/10.1542/peds.2018-3979.
© 2020 Society of Hospital Medicine
The Future of Pediatric Hospital Medicine: Challenges and Opportunities
Pediatric hospital medicine (PHM) is in the midst of an exciting period of growth. In 2016, the American Board of Medical Specialties approved the petition for PHM to become the newest pediatric subspecialty, taking PHM on a divergent path from the Focused Practice in Hospital Medicine designation established for adult hospitalists. Establishment as a subspecialty has allowed PHM to define the unique skills and qualifications that hospitalists bring to patients and the healthcare system. These skills and qualifications are delineated in the PHM core competencies and national fellowship curriculum.1,2 In order to realize the vision of PHM to improve care for hospitalized children described by Roberts et al.,3 concerted efforts are needed to train and retain a workforce that is equipped with the skills to catalyze improvements in inpatient pediatric care. We discuss challenges and opportunities facing PHM in workforce development, sustainability of clinical work models, and interhospital collaboration.
FELLOWSHIP TRAINING AND THE PHM PIPELINE
The development of PHM as a subspecialty was driven by a number of factors.4 The acuity of hospitalized children has increased significantly, with a population comprised of more children with complex chronic conditions and/or technology dependence, serious complications of acute conditions, and acute mental health problems. At the same time, the medical and behavioral conditions seen by outpatient general pediatricians have become more complex and time intensive, with these practitioners less likely to work in inpatient settings. Hospitalist care has positive impacts on healthcare efficiency and value, and both parents and primary care pediatricians report high levels of satisfaction with the healthcare delivered by PHM services.4
A national count of the number of pediatric hospitalists is currently lacking. Conservative estimates suggest that at least 3,000 pediatric hospitalists currently practice in the United States.5 These hospitalists have highly varied scopes of practice and work across diverse settings—more diverse, perhaps, than any other pediatric subspecialty. Although difficult to quantify, we estimate that approximately one-third of pediatric hospitalists in the US work in community hospitals and the remainder practice at children’s hospitals.6 Many of the needs of hospitalized children differ across these settings, and the roles and challenges faced by hospitalists in these settings correspondingly differ. Community hospitalists frequently take active roles in newborn care and emergency department consultation, often without the support of other pediatric subspecialties.7 In contrast, hospitalists working at children’s hospitals more frequently care for highly complex patients, often collaborate across multiple specialties and assume nonclinical roles in quality improvement (QI), research, and medical education.
Residents graduating in July 2019 were the last cohort of residents eligible to pursue PHM subspecialty certification via the practice pathway. Accordingly, future residency graduates interested in PHM subspecialty certification will need to complete a PHM fellowship at an accredited program in the US or Canada. Since 2008, PHM fellowship directors have met yearly to collaborate and share best practices,8 developing the two-year fellowship curriculum that forms the basis for the American Board of Pediatrics training pathway.2 The curriculum allows significant flexibility to meet diverse needs, including tailored content for fellows planning to practice in community settings, fellows planning research careers, medicine-pediatrics hospitalist careers, and those desiring increased training in QI, medical education, or leadership/administration.2 In the spring of 2019, Pediatric Research in Inpatient Settings (PRIS) leadership, directors of existing PHM fellowship programs, and national academic society representatives met to develop a fellows’ research curriculum, training resources, and guidelines around scholarship expectations.9 This collaboration aims to accelerate the growth of high-quality clinical training and scholarship to benefit hospitalized children across many different settings.
Such collaboration is essential to address an emerging workforce challenge in PHM. Although the number of PHM fellowship positions is expected to grow in the coming years, there is currently a shortage relative to the anticipated demand. With approximately 2,800 US pediatric residents graduating annually and data indicating that 7% of graduating residents enter and remain in PHM for at least five years,10,11 almost 200 fellowship spots may be needed each year. As of November 2019, 77 fellowship positions were available for residents graduating in 2020,12 which is less than half of the potential demand. To address this mismatch, the PHM Fellowship Directors’ Council has led an annual training for new and potential fellowship directors, and 18 new programs are under development.13 However, this growth may be inadequate to meet the needs of the field. The extent to which limited PHM fellowship positions will adversely affect the pipeline of pediatricians pursuing PHM is unknown.
Efforts to support institutions in creating and expanding fellowship programs will be needed to address the potential shortage of fellowship positions. Continued guidance from the PHM Fellowship Directors’ Council in the many aspects of fellowship program development (eg, curriculum design, assessment) will be crucial in this endeavor. Furthermore, given that fellowships must support fellows to conduct scholarly work and demonstrate evidence of robust faculty scholarly activities to attain accreditation, an essential area of focus is faculty development. Considering barriers such as lack of time, mentorship, and resources, some divisions interested in starting a fellowship may find it challenging to achieve these standards.14 However, hospitalists are often engaged in areas such as QI and medical education, and there is potential to turn ongoing work into meaningful scholarship with appropriate guidance. Many of our supporting organizations (eg, Academic Pediatric Association, American Academy of Pediatrics, and Society of Hospital Medicine) provide training programs for faculty in areas such as educational scholarship, research, and QI; however, more may be needed. Leaders of PHM programs will need to be mindful and creative in accessing local, regional, and national resources to invest in faculty development.
CLINICAL WORK MODELS AND SUSTAINABILITY
As a group, pediatric hospitalists report high levels of satisfaction with their jobs.11 Despite this finding, there are a number of threats to the sustainability of current work models, some of which are unique to pediatrics given the overall lower patient volumes and greater seasonal variation compared with adult hospital medicine. Both university and community-based hospitalist programs report high weekend, overnight, and in-house clinical effort.7,15 Recent studies reported that a significant proportion of PHM program leaders (50% of division directors at university-affiliated programs and 37% of community program leaders) perceive their program to be unsustainable.7,15 Among university-affiliated programs, a higher burden of weekend work as well as university employment were associated with perceived unsustainability, while no specific program or employer characteristic was associated with this perception in community programs.
These findings indicate that efforts are needed to address PHM program sustainability and that different work models and interventions may be needed for university-based and community PHM programs. Wide variability exists in the ways that programs address overall clinical burden, with strategies including census caps, seasonal expansion of coverage, and formal back-up systems.7,15 Additional potential solutions may include differential weighting or financial incentives for nights and weekends, support for nonclinical work, loan repayment programs, and competitive salaries.11 In addition, structuring clinical and nonclinical roles to facilitate career development and advancement may enhance career longevity.15 Lessons learned from pediatric emergency medicine (PEM), which developed as a field a few decades ahead of PHM, may predict future challenges. A 2015 survey of PEM faculty found that despite a 15% decrease in weekly work hours over a 15-year period, a substantial number of PEM faculty report concerns about burnout, with 40% reporting a plan to decrease their clinical workload and 13% planning to leave the field within five years.16 Like PEM, the field of PHM may benefit from the development of best practice guidelines to improve well-being and career longevity.17
INTERHOSPITAL COLLABORATION
The culture of collaboration within PHM places the field in a solid position to address both workforce challenges and barriers to high-quality care for hospitalized children. There are several hospital-based learning networks actively working to strengthen our knowledge base and improve healthcare quality. The PRIS network (www.prisnetwork.org) aims to improve healthcare for children through multihospital studies, boasting 114 sites in the US and Canada. Numerous collaborative projects have linked hospitalists across programs to tackle problems ranging from handoff communication18 to eliminating monitor overuse.19 The Value in Inpatient Pediatrics network has similarly leveraged collaborations across multiple children’s and community hospitals to improve transitions of care20 and care for common conditions such as bronchiolitis, febrile infants, and asthma.21 These networks serve as models of effective collaboration between children’s hospitals and community hospitals, more of which is needed to increase research and QI initiatives in community hospitals, where the majority of US children receive their hospital-based care.6,22
With the rapid growth of scholarly networks in research, QI, and education, PHM has a solid infrastructure on which to base continued development as a subspeciality. Building on this infrastructure will be essential in order to address current challenges in workforce development, fellowship training, and program sustainability. Ultimately, achieving a strong, stable, and skilled workforce will enable PHM to fulfill its promise of improving the care of children across the diversity of settings where they receive their hospital-based care.
Disclosures
Dr. Leyenaar provides consultative services to the American Board of Pediatrics Foundation, which is not associated with this manuscript. Drs. Wang and Shaughnessy have no disclosures
1. Stucky ER, Maniscalco J, Ottolini MC, et al. The Pediatric Hospital Medicine Core Competencies Supplement: a framework for curriculum development by the Society of Hospital Medicine with acknowledgement to pediatric hospitalists from the American Academy of Pediatrics and the Academic Pediatric Association. J Hosp Med. 2010;5(S2):1-114. https://doi.org/10.1002/jhm.776.
2. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1). https://doi.org/10.1542/peds.2017-0698.
3. Roberts KB, Fisher ER, Rauch DA. A history of pediatric hospital medicine in the United States, 1996-2019. J Hosp Med. 2019.
4. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017;139(3). https://doi.org/10.1542/peds.2016-1823.
5. American Board of Medical Specialities. American Board of Medical Specialities application for a new subspecialty certificate: Pediatric hospital medicine. http://www.abms.org/media/114649/abpeds-application-for-pediatric-hospital-medicine.pdf. Accessed November 6, 2019.
6. Leyenaar JK, Ralston SL, Shieh MS, et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
7. Alvarez F, McDaniel CE, Birnie K, et al. Community pediatric hospitalist workload: results from a national survey. J Hosp Med. 2019;14(10):682-685. https://doi.org/10.12788/jhm.3263.
8. Shah NH, Rhim HJ, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-328. https://doi.org/10.1002/jhm.2571.
9. Pediatric Hospital Medicine Fellowship Research Training Development. https://projectreporter.nih.gov/project_info_description.cfm?aid=9593276&icde=47889643. Accessed December 10, 2019.
10. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001.
11. House S, Frintner MP, Leyenaar JK. Factors influencing career longevity in pediatric hospital medicine. Hosp Pediatr. 2019;9(12):983-988. https://doi.org/10.1542/hpeds.2019-0151.
12. PHM Fellowship Programs. http://phmfellows.org/phm-programs/. Accessed November 6, 2019.
13. Rassbach C [Personal communication]; 2019.
14. Bekmezian A, Teufel RJ, 2nd, Wilson KM. Research needs of pediatric hospitalists. Hosp Pediatr. 2011;1(1):38-44. https://doi.org/10.1542/hpeds.2011-0006.
15. Fromme HB, Chen CO, Fine BR, Gosdin C, Shaughnessy EE. Pediatric hospitalist workload and sustainability in university-based programs: Results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. https://doi.org/10.12788/jhm.2977.
16. Gorelick MH, Schremmer R, Ruch-Ross H, Radabaugh C, Selbst S. Current workforce characteristics and burnout in pediatric emergency medicine. Acad Emerg Med. 2016;23(1):48-54. https://doi.org/10.1111/acem.12845.
17. American College of Emergency Physicians. Policy Statement: Emergency Physician Shift Work; June 2017.
18. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
19. Rasooly IR, Beidas RS, Wolk CB, et al. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: Study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5:68. https://doi.org/10.1186/s40814-019-0453-2.
20. Coghlin DT, Leyenaar JK, Shen M, et al. Pediatric discharge content: a multisite assessment of physician preferences and experiences. Hosp Pediatr. 2014;4(1):9-15. https://doi.org/10.1542/hpeds.2013-0022.
21. Value in inpatient pediatrics (VIP) Network. 2019. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed October 13, 2019.
22. McDaniel CE, Jennings R, Schroeder AR, et al. Aligning inpatient pediatric research with settings of care: A call to action. Pediatrics. 2019;143(5). https://doi.org/10.1542/peds.2018-2648.
Pediatric hospital medicine (PHM) is in the midst of an exciting period of growth. In 2016, the American Board of Medical Specialties approved the petition for PHM to become the newest pediatric subspecialty, taking PHM on a divergent path from the Focused Practice in Hospital Medicine designation established for adult hospitalists. Establishment as a subspecialty has allowed PHM to define the unique skills and qualifications that hospitalists bring to patients and the healthcare system. These skills and qualifications are delineated in the PHM core competencies and national fellowship curriculum.1,2 In order to realize the vision of PHM to improve care for hospitalized children described by Roberts et al.,3 concerted efforts are needed to train and retain a workforce that is equipped with the skills to catalyze improvements in inpatient pediatric care. We discuss challenges and opportunities facing PHM in workforce development, sustainability of clinical work models, and interhospital collaboration.
FELLOWSHIP TRAINING AND THE PHM PIPELINE
The development of PHM as a subspecialty was driven by a number of factors.4 The acuity of hospitalized children has increased significantly, with a population comprised of more children with complex chronic conditions and/or technology dependence, serious complications of acute conditions, and acute mental health problems. At the same time, the medical and behavioral conditions seen by outpatient general pediatricians have become more complex and time intensive, with these practitioners less likely to work in inpatient settings. Hospitalist care has positive impacts on healthcare efficiency and value, and both parents and primary care pediatricians report high levels of satisfaction with the healthcare delivered by PHM services.4
A national count of the number of pediatric hospitalists is currently lacking. Conservative estimates suggest that at least 3,000 pediatric hospitalists currently practice in the United States.5 These hospitalists have highly varied scopes of practice and work across diverse settings—more diverse, perhaps, than any other pediatric subspecialty. Although difficult to quantify, we estimate that approximately one-third of pediatric hospitalists in the US work in community hospitals and the remainder practice at children’s hospitals.6 Many of the needs of hospitalized children differ across these settings, and the roles and challenges faced by hospitalists in these settings correspondingly differ. Community hospitalists frequently take active roles in newborn care and emergency department consultation, often without the support of other pediatric subspecialties.7 In contrast, hospitalists working at children’s hospitals more frequently care for highly complex patients, often collaborate across multiple specialties and assume nonclinical roles in quality improvement (QI), research, and medical education.
Residents graduating in July 2019 were the last cohort of residents eligible to pursue PHM subspecialty certification via the practice pathway. Accordingly, future residency graduates interested in PHM subspecialty certification will need to complete a PHM fellowship at an accredited program in the US or Canada. Since 2008, PHM fellowship directors have met yearly to collaborate and share best practices,8 developing the two-year fellowship curriculum that forms the basis for the American Board of Pediatrics training pathway.2 The curriculum allows significant flexibility to meet diverse needs, including tailored content for fellows planning to practice in community settings, fellows planning research careers, medicine-pediatrics hospitalist careers, and those desiring increased training in QI, medical education, or leadership/administration.2 In the spring of 2019, Pediatric Research in Inpatient Settings (PRIS) leadership, directors of existing PHM fellowship programs, and national academic society representatives met to develop a fellows’ research curriculum, training resources, and guidelines around scholarship expectations.9 This collaboration aims to accelerate the growth of high-quality clinical training and scholarship to benefit hospitalized children across many different settings.
Such collaboration is essential to address an emerging workforce challenge in PHM. Although the number of PHM fellowship positions is expected to grow in the coming years, there is currently a shortage relative to the anticipated demand. With approximately 2,800 US pediatric residents graduating annually and data indicating that 7% of graduating residents enter and remain in PHM for at least five years,10,11 almost 200 fellowship spots may be needed each year. As of November 2019, 77 fellowship positions were available for residents graduating in 2020,12 which is less than half of the potential demand. To address this mismatch, the PHM Fellowship Directors’ Council has led an annual training for new and potential fellowship directors, and 18 new programs are under development.13 However, this growth may be inadequate to meet the needs of the field. The extent to which limited PHM fellowship positions will adversely affect the pipeline of pediatricians pursuing PHM is unknown.
Efforts to support institutions in creating and expanding fellowship programs will be needed to address the potential shortage of fellowship positions. Continued guidance from the PHM Fellowship Directors’ Council in the many aspects of fellowship program development (eg, curriculum design, assessment) will be crucial in this endeavor. Furthermore, given that fellowships must support fellows to conduct scholarly work and demonstrate evidence of robust faculty scholarly activities to attain accreditation, an essential area of focus is faculty development. Considering barriers such as lack of time, mentorship, and resources, some divisions interested in starting a fellowship may find it challenging to achieve these standards.14 However, hospitalists are often engaged in areas such as QI and medical education, and there is potential to turn ongoing work into meaningful scholarship with appropriate guidance. Many of our supporting organizations (eg, Academic Pediatric Association, American Academy of Pediatrics, and Society of Hospital Medicine) provide training programs for faculty in areas such as educational scholarship, research, and QI; however, more may be needed. Leaders of PHM programs will need to be mindful and creative in accessing local, regional, and national resources to invest in faculty development.
CLINICAL WORK MODELS AND SUSTAINABILITY
As a group, pediatric hospitalists report high levels of satisfaction with their jobs.11 Despite this finding, there are a number of threats to the sustainability of current work models, some of which are unique to pediatrics given the overall lower patient volumes and greater seasonal variation compared with adult hospital medicine. Both university and community-based hospitalist programs report high weekend, overnight, and in-house clinical effort.7,15 Recent studies reported that a significant proportion of PHM program leaders (50% of division directors at university-affiliated programs and 37% of community program leaders) perceive their program to be unsustainable.7,15 Among university-affiliated programs, a higher burden of weekend work as well as university employment were associated with perceived unsustainability, while no specific program or employer characteristic was associated with this perception in community programs.
These findings indicate that efforts are needed to address PHM program sustainability and that different work models and interventions may be needed for university-based and community PHM programs. Wide variability exists in the ways that programs address overall clinical burden, with strategies including census caps, seasonal expansion of coverage, and formal back-up systems.7,15 Additional potential solutions may include differential weighting or financial incentives for nights and weekends, support for nonclinical work, loan repayment programs, and competitive salaries.11 In addition, structuring clinical and nonclinical roles to facilitate career development and advancement may enhance career longevity.15 Lessons learned from pediatric emergency medicine (PEM), which developed as a field a few decades ahead of PHM, may predict future challenges. A 2015 survey of PEM faculty found that despite a 15% decrease in weekly work hours over a 15-year period, a substantial number of PEM faculty report concerns about burnout, with 40% reporting a plan to decrease their clinical workload and 13% planning to leave the field within five years.16 Like PEM, the field of PHM may benefit from the development of best practice guidelines to improve well-being and career longevity.17
INTERHOSPITAL COLLABORATION
The culture of collaboration within PHM places the field in a solid position to address both workforce challenges and barriers to high-quality care for hospitalized children. There are several hospital-based learning networks actively working to strengthen our knowledge base and improve healthcare quality. The PRIS network (www.prisnetwork.org) aims to improve healthcare for children through multihospital studies, boasting 114 sites in the US and Canada. Numerous collaborative projects have linked hospitalists across programs to tackle problems ranging from handoff communication18 to eliminating monitor overuse.19 The Value in Inpatient Pediatrics network has similarly leveraged collaborations across multiple children’s and community hospitals to improve transitions of care20 and care for common conditions such as bronchiolitis, febrile infants, and asthma.21 These networks serve as models of effective collaboration between children’s hospitals and community hospitals, more of which is needed to increase research and QI initiatives in community hospitals, where the majority of US children receive their hospital-based care.6,22
With the rapid growth of scholarly networks in research, QI, and education, PHM has a solid infrastructure on which to base continued development as a subspeciality. Building on this infrastructure will be essential in order to address current challenges in workforce development, fellowship training, and program sustainability. Ultimately, achieving a strong, stable, and skilled workforce will enable PHM to fulfill its promise of improving the care of children across the diversity of settings where they receive their hospital-based care.
Disclosures
Dr. Leyenaar provides consultative services to the American Board of Pediatrics Foundation, which is not associated with this manuscript. Drs. Wang and Shaughnessy have no disclosures
Pediatric hospital medicine (PHM) is in the midst of an exciting period of growth. In 2016, the American Board of Medical Specialties approved the petition for PHM to become the newest pediatric subspecialty, taking PHM on a divergent path from the Focused Practice in Hospital Medicine designation established for adult hospitalists. Establishment as a subspecialty has allowed PHM to define the unique skills and qualifications that hospitalists bring to patients and the healthcare system. These skills and qualifications are delineated in the PHM core competencies and national fellowship curriculum.1,2 In order to realize the vision of PHM to improve care for hospitalized children described by Roberts et al.,3 concerted efforts are needed to train and retain a workforce that is equipped with the skills to catalyze improvements in inpatient pediatric care. We discuss challenges and opportunities facing PHM in workforce development, sustainability of clinical work models, and interhospital collaboration.
FELLOWSHIP TRAINING AND THE PHM PIPELINE
The development of PHM as a subspecialty was driven by a number of factors.4 The acuity of hospitalized children has increased significantly, with a population comprised of more children with complex chronic conditions and/or technology dependence, serious complications of acute conditions, and acute mental health problems. At the same time, the medical and behavioral conditions seen by outpatient general pediatricians have become more complex and time intensive, with these practitioners less likely to work in inpatient settings. Hospitalist care has positive impacts on healthcare efficiency and value, and both parents and primary care pediatricians report high levels of satisfaction with the healthcare delivered by PHM services.4
A national count of the number of pediatric hospitalists is currently lacking. Conservative estimates suggest that at least 3,000 pediatric hospitalists currently practice in the United States.5 These hospitalists have highly varied scopes of practice and work across diverse settings—more diverse, perhaps, than any other pediatric subspecialty. Although difficult to quantify, we estimate that approximately one-third of pediatric hospitalists in the US work in community hospitals and the remainder practice at children’s hospitals.6 Many of the needs of hospitalized children differ across these settings, and the roles and challenges faced by hospitalists in these settings correspondingly differ. Community hospitalists frequently take active roles in newborn care and emergency department consultation, often without the support of other pediatric subspecialties.7 In contrast, hospitalists working at children’s hospitals more frequently care for highly complex patients, often collaborate across multiple specialties and assume nonclinical roles in quality improvement (QI), research, and medical education.
Residents graduating in July 2019 were the last cohort of residents eligible to pursue PHM subspecialty certification via the practice pathway. Accordingly, future residency graduates interested in PHM subspecialty certification will need to complete a PHM fellowship at an accredited program in the US or Canada. Since 2008, PHM fellowship directors have met yearly to collaborate and share best practices,8 developing the two-year fellowship curriculum that forms the basis for the American Board of Pediatrics training pathway.2 The curriculum allows significant flexibility to meet diverse needs, including tailored content for fellows planning to practice in community settings, fellows planning research careers, medicine-pediatrics hospitalist careers, and those desiring increased training in QI, medical education, or leadership/administration.2 In the spring of 2019, Pediatric Research in Inpatient Settings (PRIS) leadership, directors of existing PHM fellowship programs, and national academic society representatives met to develop a fellows’ research curriculum, training resources, and guidelines around scholarship expectations.9 This collaboration aims to accelerate the growth of high-quality clinical training and scholarship to benefit hospitalized children across many different settings.
Such collaboration is essential to address an emerging workforce challenge in PHM. Although the number of PHM fellowship positions is expected to grow in the coming years, there is currently a shortage relative to the anticipated demand. With approximately 2,800 US pediatric residents graduating annually and data indicating that 7% of graduating residents enter and remain in PHM for at least five years,10,11 almost 200 fellowship spots may be needed each year. As of November 2019, 77 fellowship positions were available for residents graduating in 2020,12 which is less than half of the potential demand. To address this mismatch, the PHM Fellowship Directors’ Council has led an annual training for new and potential fellowship directors, and 18 new programs are under development.13 However, this growth may be inadequate to meet the needs of the field. The extent to which limited PHM fellowship positions will adversely affect the pipeline of pediatricians pursuing PHM is unknown.
Efforts to support institutions in creating and expanding fellowship programs will be needed to address the potential shortage of fellowship positions. Continued guidance from the PHM Fellowship Directors’ Council in the many aspects of fellowship program development (eg, curriculum design, assessment) will be crucial in this endeavor. Furthermore, given that fellowships must support fellows to conduct scholarly work and demonstrate evidence of robust faculty scholarly activities to attain accreditation, an essential area of focus is faculty development. Considering barriers such as lack of time, mentorship, and resources, some divisions interested in starting a fellowship may find it challenging to achieve these standards.14 However, hospitalists are often engaged in areas such as QI and medical education, and there is potential to turn ongoing work into meaningful scholarship with appropriate guidance. Many of our supporting organizations (eg, Academic Pediatric Association, American Academy of Pediatrics, and Society of Hospital Medicine) provide training programs for faculty in areas such as educational scholarship, research, and QI; however, more may be needed. Leaders of PHM programs will need to be mindful and creative in accessing local, regional, and national resources to invest in faculty development.
CLINICAL WORK MODELS AND SUSTAINABILITY
As a group, pediatric hospitalists report high levels of satisfaction with their jobs.11 Despite this finding, there are a number of threats to the sustainability of current work models, some of which are unique to pediatrics given the overall lower patient volumes and greater seasonal variation compared with adult hospital medicine. Both university and community-based hospitalist programs report high weekend, overnight, and in-house clinical effort.7,15 Recent studies reported that a significant proportion of PHM program leaders (50% of division directors at university-affiliated programs and 37% of community program leaders) perceive their program to be unsustainable.7,15 Among university-affiliated programs, a higher burden of weekend work as well as university employment were associated with perceived unsustainability, while no specific program or employer characteristic was associated with this perception in community programs.
These findings indicate that efforts are needed to address PHM program sustainability and that different work models and interventions may be needed for university-based and community PHM programs. Wide variability exists in the ways that programs address overall clinical burden, with strategies including census caps, seasonal expansion of coverage, and formal back-up systems.7,15 Additional potential solutions may include differential weighting or financial incentives for nights and weekends, support for nonclinical work, loan repayment programs, and competitive salaries.11 In addition, structuring clinical and nonclinical roles to facilitate career development and advancement may enhance career longevity.15 Lessons learned from pediatric emergency medicine (PEM), which developed as a field a few decades ahead of PHM, may predict future challenges. A 2015 survey of PEM faculty found that despite a 15% decrease in weekly work hours over a 15-year period, a substantial number of PEM faculty report concerns about burnout, with 40% reporting a plan to decrease their clinical workload and 13% planning to leave the field within five years.16 Like PEM, the field of PHM may benefit from the development of best practice guidelines to improve well-being and career longevity.17
INTERHOSPITAL COLLABORATION
The culture of collaboration within PHM places the field in a solid position to address both workforce challenges and barriers to high-quality care for hospitalized children. There are several hospital-based learning networks actively working to strengthen our knowledge base and improve healthcare quality. The PRIS network (www.prisnetwork.org) aims to improve healthcare for children through multihospital studies, boasting 114 sites in the US and Canada. Numerous collaborative projects have linked hospitalists across programs to tackle problems ranging from handoff communication18 to eliminating monitor overuse.19 The Value in Inpatient Pediatrics network has similarly leveraged collaborations across multiple children’s and community hospitals to improve transitions of care20 and care for common conditions such as bronchiolitis, febrile infants, and asthma.21 These networks serve as models of effective collaboration between children’s hospitals and community hospitals, more of which is needed to increase research and QI initiatives in community hospitals, where the majority of US children receive their hospital-based care.6,22
With the rapid growth of scholarly networks in research, QI, and education, PHM has a solid infrastructure on which to base continued development as a subspeciality. Building on this infrastructure will be essential in order to address current challenges in workforce development, fellowship training, and program sustainability. Ultimately, achieving a strong, stable, and skilled workforce will enable PHM to fulfill its promise of improving the care of children across the diversity of settings where they receive their hospital-based care.
Disclosures
Dr. Leyenaar provides consultative services to the American Board of Pediatrics Foundation, which is not associated with this manuscript. Drs. Wang and Shaughnessy have no disclosures
1. Stucky ER, Maniscalco J, Ottolini MC, et al. The Pediatric Hospital Medicine Core Competencies Supplement: a framework for curriculum development by the Society of Hospital Medicine with acknowledgement to pediatric hospitalists from the American Academy of Pediatrics and the Academic Pediatric Association. J Hosp Med. 2010;5(S2):1-114. https://doi.org/10.1002/jhm.776.
2. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1). https://doi.org/10.1542/peds.2017-0698.
3. Roberts KB, Fisher ER, Rauch DA. A history of pediatric hospital medicine in the United States, 1996-2019. J Hosp Med. 2019.
4. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017;139(3). https://doi.org/10.1542/peds.2016-1823.
5. American Board of Medical Specialities. American Board of Medical Specialities application for a new subspecialty certificate: Pediatric hospital medicine. http://www.abms.org/media/114649/abpeds-application-for-pediatric-hospital-medicine.pdf. Accessed November 6, 2019.
6. Leyenaar JK, Ralston SL, Shieh MS, et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
7. Alvarez F, McDaniel CE, Birnie K, et al. Community pediatric hospitalist workload: results from a national survey. J Hosp Med. 2019;14(10):682-685. https://doi.org/10.12788/jhm.3263.
8. Shah NH, Rhim HJ, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-328. https://doi.org/10.1002/jhm.2571.
9. Pediatric Hospital Medicine Fellowship Research Training Development. https://projectreporter.nih.gov/project_info_description.cfm?aid=9593276&icde=47889643. Accessed December 10, 2019.
10. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001.
11. House S, Frintner MP, Leyenaar JK. Factors influencing career longevity in pediatric hospital medicine. Hosp Pediatr. 2019;9(12):983-988. https://doi.org/10.1542/hpeds.2019-0151.
12. PHM Fellowship Programs. http://phmfellows.org/phm-programs/. Accessed November 6, 2019.
13. Rassbach C [Personal communication]; 2019.
14. Bekmezian A, Teufel RJ, 2nd, Wilson KM. Research needs of pediatric hospitalists. Hosp Pediatr. 2011;1(1):38-44. https://doi.org/10.1542/hpeds.2011-0006.
15. Fromme HB, Chen CO, Fine BR, Gosdin C, Shaughnessy EE. Pediatric hospitalist workload and sustainability in university-based programs: Results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. https://doi.org/10.12788/jhm.2977.
16. Gorelick MH, Schremmer R, Ruch-Ross H, Radabaugh C, Selbst S. Current workforce characteristics and burnout in pediatric emergency medicine. Acad Emerg Med. 2016;23(1):48-54. https://doi.org/10.1111/acem.12845.
17. American College of Emergency Physicians. Policy Statement: Emergency Physician Shift Work; June 2017.
18. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
19. Rasooly IR, Beidas RS, Wolk CB, et al. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: Study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5:68. https://doi.org/10.1186/s40814-019-0453-2.
20. Coghlin DT, Leyenaar JK, Shen M, et al. Pediatric discharge content: a multisite assessment of physician preferences and experiences. Hosp Pediatr. 2014;4(1):9-15. https://doi.org/10.1542/hpeds.2013-0022.
21. Value in inpatient pediatrics (VIP) Network. 2019. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed October 13, 2019.
22. McDaniel CE, Jennings R, Schroeder AR, et al. Aligning inpatient pediatric research with settings of care: A call to action. Pediatrics. 2019;143(5). https://doi.org/10.1542/peds.2018-2648.
1. Stucky ER, Maniscalco J, Ottolini MC, et al. The Pediatric Hospital Medicine Core Competencies Supplement: a framework for curriculum development by the Society of Hospital Medicine with acknowledgement to pediatric hospitalists from the American Academy of Pediatrics and the Academic Pediatric Association. J Hosp Med. 2010;5(S2):1-114. https://doi.org/10.1002/jhm.776.
2. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1). https://doi.org/10.1542/peds.2017-0698.
3. Roberts KB, Fisher ER, Rauch DA. A history of pediatric hospital medicine in the United States, 1996-2019. J Hosp Med. 2019.
4. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017;139(3). https://doi.org/10.1542/peds.2016-1823.
5. American Board of Medical Specialities. American Board of Medical Specialities application for a new subspecialty certificate: Pediatric hospital medicine. http://www.abms.org/media/114649/abpeds-application-for-pediatric-hospital-medicine.pdf. Accessed November 6, 2019.
6. Leyenaar JK, Ralston SL, Shieh MS, et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
7. Alvarez F, McDaniel CE, Birnie K, et al. Community pediatric hospitalist workload: results from a national survey. J Hosp Med. 2019;14(10):682-685. https://doi.org/10.12788/jhm.3263.
8. Shah NH, Rhim HJ, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-328. https://doi.org/10.1002/jhm.2571.
9. Pediatric Hospital Medicine Fellowship Research Training Development. https://projectreporter.nih.gov/project_info_description.cfm?aid=9593276&icde=47889643. Accessed December 10, 2019.
10. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001.
11. House S, Frintner MP, Leyenaar JK. Factors influencing career longevity in pediatric hospital medicine. Hosp Pediatr. 2019;9(12):983-988. https://doi.org/10.1542/hpeds.2019-0151.
12. PHM Fellowship Programs. http://phmfellows.org/phm-programs/. Accessed November 6, 2019.
13. Rassbach C [Personal communication]; 2019.
14. Bekmezian A, Teufel RJ, 2nd, Wilson KM. Research needs of pediatric hospitalists. Hosp Pediatr. 2011;1(1):38-44. https://doi.org/10.1542/hpeds.2011-0006.
15. Fromme HB, Chen CO, Fine BR, Gosdin C, Shaughnessy EE. Pediatric hospitalist workload and sustainability in university-based programs: Results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. https://doi.org/10.12788/jhm.2977.
16. Gorelick MH, Schremmer R, Ruch-Ross H, Radabaugh C, Selbst S. Current workforce characteristics and burnout in pediatric emergency medicine. Acad Emerg Med. 2016;23(1):48-54. https://doi.org/10.1111/acem.12845.
17. American College of Emergency Physicians. Policy Statement: Emergency Physician Shift Work; June 2017.
18. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
19. Rasooly IR, Beidas RS, Wolk CB, et al. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: Study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5:68. https://doi.org/10.1186/s40814-019-0453-2.
20. Coghlin DT, Leyenaar JK, Shen M, et al. Pediatric discharge content: a multisite assessment of physician preferences and experiences. Hosp Pediatr. 2014;4(1):9-15. https://doi.org/10.1542/hpeds.2013-0022.
21. Value in inpatient pediatrics (VIP) Network. 2019. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed October 13, 2019.
22. McDaniel CE, Jennings R, Schroeder AR, et al. Aligning inpatient pediatric research with settings of care: A call to action. Pediatrics. 2019;143(5). https://doi.org/10.1542/peds.2018-2648.
© 2020 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Diagnosis and Management of Measles
Measles is a highly contagious acute respiratory illness that can cause complications in multiple organ systems. Measles was declared eliminated in the United States in 2000; however, outbreaks still occur, especially in unvaccinated populations. The Centers for Disease Control and Prevention (CDC) reported that as of October 3, 2019, 1,250 cases of measles had been confirmed in 31 states in 2019, which represents the greatest number of cases reported in the US since 1992.1 Although the disease is often self-limited, infected individuals can also develop complications requiring hospitalization, which occurred in 10% of confirmed cases this year.1 In February 2018, the CDC updated their recommendations about measles diagnosis and treatment on their website,2 adding an interim update in July 2019 to include new guidelines about infection control and prevention.3 This highlight reviews those recommendations most relevant to hospitalists, who can play a critical role in the diagnosis and management of patients with suspected and/or confirmed measles.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Healthcare providers should consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness. Healthcare providers should report suspected measles cases to their local health department within 24 hours.
Measles is an acute febrile illness that begins with a prodrome of fever, followed by one or more of the following three “C’s”: cough, coryza (rhinitis), and conjunctivitis. Koplik spots, a pathognomonic buccal enanthem consisting of white lesions on an erythematous base, can appear shortly thereafter. An erythematous, maculopapular rash develops three to four days after the onset of the fever. The rash starts on the face and then spreads over the next few days to the trunk and extremities. Clinical recovery generally occurs within one week of rash onset in uncomplicated measles. Complications can affect almost any organ system. The most common complications are pneumonia, often caused by secondary viral or bacterial pathogens, diarrhea, otitis media, and laryngotracheobronchitis. Rare but serious complications include acute encephalitis and subacute sclerosing panencephalitis. Groups at the highest risk for serious disease include children aged <5 years, adults aged >20 years, pregnant women, and immunocompromised individuals.
When encountering patients with a febrile rash and compatible symptoms, clinicians should also have a high index of suspicion for measles in patients who are unvaccinated or undervaccinated, since the majority of measles cases have occurred in the unvaccinated population. Providers should contact their local health department and infectious diseases/infection control team as soon as suspected measles cases are identified. Laboratory confirmation is necessary for all suspected cases and should typically consist of measles IgM antibody testing from serum and real-time polymerase chain reaction (RT-PCR) from respiratory and urine specimens.
Recommendation 2. Adhere to airborne precautions for anyone with known or suspected measles.
Measles is highly contagious, and infectious particles can remain in the air for up to two hours after a person with measles leaves a room. From 2001 to 2014, 6% (78/1,318) of nonimported measles cases in the US were transmitted in healthcare settings.4 Key steps in preventing the spread of measles within hospitals include ensuring that all healthcare personnel have evidence of immunity to measles and rapid identification and isolation of suspect cases. Patients with suspected measles should be given a facemask and moved immediately into a single-patient airborne infection isolation room. Personnel, even those with presumptive evidence of immunity, should use N95 respirators or the equivalent when caring for patients with suspected or confirmed measles. Patients with measles are contagious from four days before to four days after rash onset; therefore, airborne precautions should be continued for four days following the onset of rash in immunocompetent patients. For immunocompromised patients, airborne precautions should be continued for the duration of the illness based on data suggesting prolonged shedding, particularly in the setting of altered T-cell immunity.4
Recommendation 3. People exposed to measles who cannot readily show that they have evidence of immunity against measles should be offered postexposure prophylaxis (PEP) or be excluded from the setting (school, hospital, childcare). To potentially provide protection or modify the clinical course of disease among susceptible persons, either administer a measles, mumps, and rubella (MMR) vaccine within 72 hours of initial measles exposure or immunoglobulin (IG) within six days of exposure.
MMR vaccine is recommended for vaccine-eligible, exposed individuals aged ≥6 months within 72 hours of measles exposure. IG, which contains measles antibody due to widespread immunization in the US, is recommended for individuals at high risk for serious illness, including infants aged ≤12 months, pregnant women without evidence of measles immunity, and severely immunocompromised patients regardless of vaccination status. For infants aged 6-11 months, MMR vaccine can be given in place of IG if done within 72 hours of exposure. PEP for children during the 2013 New York City outbreak reduced the risk of measles by 83.4% (95% CI: 34.4%-95.8%) in recipients of MMR vaccine and by 100% (95% CI: 56.2%-99.8%) in recipients of IG compared with those without prophylaxis.5 A 2014 Cochrane Review found that IG reduced the risk of measles by 83% (95% CI: 64%-92%).6
Recommendation 4. Severe measles cases among children, such as those who are hospitalized, should be treated with vitamin A. Vitamin A should be administered immediately on diagnosis and repeated the next day.
In children, vitamin A deficiency, even if clinically inapparent, leads to increased measles severity, and randomized controlled trial data suggest that supplementation reduces measles-related morbidity and mortality.4 Even in high-income countries, children with measles have high rates of vitamin A deficiency, which is associated with increased morbidity.7 A Cochrane review found that two-dose regimens of vitamin A reduced the overall mortality (RR 0.21; 95% CI: 0.07-0.66) in children with measles aged <2 years.8 World Health Organization guidelines suggest vitamin A therapy for all children with acute measles infection, and the AAP Committee on Infectious Diseases recommends vitamin A for severe (ie, hospitalized) cases. Vitamin A is given orally once daily for two days at the following doses: 50,000 international units (IU) for infants aged <6 months, 100,000 IU for infants aged 6-11 months, and 200,000 IU for children aged ≥12 months. A third dose can be given two to four weeks later for children with signs and symptoms of vitamin A deficiency (eg, corneal clouding or conjunctival plaques).
CRITIQUE
In outbreak settings, hospitalists may find challenges with having a sufficient number of single negative-pressure rooms for patients with suspected or confirmed measles and providing IG prophylaxis given the recent national shortages of intravenous immunoglobulin. Collaboration with the infection control team, pharmacy, and the local public health department is essential to appropriately address these challenges. With regard to treatment recommendations, randomized studies on the impact of vitamin A treatment in children have been primarily conducted in resource-limited settings.8 However, these data, in combination with observational data from resource-rich settings,7 support its use given the favorable risk-benefit profile. The role of vitamin A therapy in adults with measles infection is considerably less clear, although there are reports of its use in severe cases.
AREAS OF FUTURE STUDY
Much of our knowledge regarding measles complications and treatment outcomes comes from resource-limited settings or from older data before widespread vaccination. Data suggest that prophylactic antibiotics may prevent complications; however, currently available data are insufficient to support routine use.9 Coordination and collaboration between public health, infectious diseases, and hospital medicine would enhance the ability to conduct detailed epidemiologic studies during outbreak situations. Further studies examining treatment and outcomes in hospitalized patients, including the role of prophylactic antibiotics in the prevention of complications, would provide valuable guidance for hospitalists caring for patients with severe measles.
1. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. 2019; https://www.cdc.gov/measles/cases-outbreaks.html. Accessed October 14, 2019.
2. Centers for Disease Control and Prevention. Measles (Rubeola): For Healthcare Professionals. 2019; https://www.cdc.gov/measles/hcp/index.html. Accessed October 14, 2019.
3. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings. 2019.
4. Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61(4):615-618. https://doi.org/10.1093/cid/civ387.
5. Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City, 2013. Clin Infect Dis. 2017;65(11):1843-1847. https://doi.org/10.1093/cid/cix639.
6. Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014;(4):Cd010056. https://doi.org/10.1002/14651858.CD010056.pub2.
7. Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. New York City. Am J Dis Child. 1992;146(2):182-186. https://doi.org/10.1001/archpedi.1992.02160140048019.
8. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005(4):Cd001479. https://doi.org/10.1002/14651858.CD001479.pub3.
9. Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. Cochrane Database Syst Rev. 2013(8):Cd001477. https://doi.org/10.1002/14651858.CD001477.pub3.
Measles is a highly contagious acute respiratory illness that can cause complications in multiple organ systems. Measles was declared eliminated in the United States in 2000; however, outbreaks still occur, especially in unvaccinated populations. The Centers for Disease Control and Prevention (CDC) reported that as of October 3, 2019, 1,250 cases of measles had been confirmed in 31 states in 2019, which represents the greatest number of cases reported in the US since 1992.1 Although the disease is often self-limited, infected individuals can also develop complications requiring hospitalization, which occurred in 10% of confirmed cases this year.1 In February 2018, the CDC updated their recommendations about measles diagnosis and treatment on their website,2 adding an interim update in July 2019 to include new guidelines about infection control and prevention.3 This highlight reviews those recommendations most relevant to hospitalists, who can play a critical role in the diagnosis and management of patients with suspected and/or confirmed measles.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Healthcare providers should consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness. Healthcare providers should report suspected measles cases to their local health department within 24 hours.
Measles is an acute febrile illness that begins with a prodrome of fever, followed by one or more of the following three “C’s”: cough, coryza (rhinitis), and conjunctivitis. Koplik spots, a pathognomonic buccal enanthem consisting of white lesions on an erythematous base, can appear shortly thereafter. An erythematous, maculopapular rash develops three to four days after the onset of the fever. The rash starts on the face and then spreads over the next few days to the trunk and extremities. Clinical recovery generally occurs within one week of rash onset in uncomplicated measles. Complications can affect almost any organ system. The most common complications are pneumonia, often caused by secondary viral or bacterial pathogens, diarrhea, otitis media, and laryngotracheobronchitis. Rare but serious complications include acute encephalitis and subacute sclerosing panencephalitis. Groups at the highest risk for serious disease include children aged <5 years, adults aged >20 years, pregnant women, and immunocompromised individuals.
When encountering patients with a febrile rash and compatible symptoms, clinicians should also have a high index of suspicion for measles in patients who are unvaccinated or undervaccinated, since the majority of measles cases have occurred in the unvaccinated population. Providers should contact their local health department and infectious diseases/infection control team as soon as suspected measles cases are identified. Laboratory confirmation is necessary for all suspected cases and should typically consist of measles IgM antibody testing from serum and real-time polymerase chain reaction (RT-PCR) from respiratory and urine specimens.
Recommendation 2. Adhere to airborne precautions for anyone with known or suspected measles.
Measles is highly contagious, and infectious particles can remain in the air for up to two hours after a person with measles leaves a room. From 2001 to 2014, 6% (78/1,318) of nonimported measles cases in the US were transmitted in healthcare settings.4 Key steps in preventing the spread of measles within hospitals include ensuring that all healthcare personnel have evidence of immunity to measles and rapid identification and isolation of suspect cases. Patients with suspected measles should be given a facemask and moved immediately into a single-patient airborne infection isolation room. Personnel, even those with presumptive evidence of immunity, should use N95 respirators or the equivalent when caring for patients with suspected or confirmed measles. Patients with measles are contagious from four days before to four days after rash onset; therefore, airborne precautions should be continued for four days following the onset of rash in immunocompetent patients. For immunocompromised patients, airborne precautions should be continued for the duration of the illness based on data suggesting prolonged shedding, particularly in the setting of altered T-cell immunity.4
Recommendation 3. People exposed to measles who cannot readily show that they have evidence of immunity against measles should be offered postexposure prophylaxis (PEP) or be excluded from the setting (school, hospital, childcare). To potentially provide protection or modify the clinical course of disease among susceptible persons, either administer a measles, mumps, and rubella (MMR) vaccine within 72 hours of initial measles exposure or immunoglobulin (IG) within six days of exposure.
MMR vaccine is recommended for vaccine-eligible, exposed individuals aged ≥6 months within 72 hours of measles exposure. IG, which contains measles antibody due to widespread immunization in the US, is recommended for individuals at high risk for serious illness, including infants aged ≤12 months, pregnant women without evidence of measles immunity, and severely immunocompromised patients regardless of vaccination status. For infants aged 6-11 months, MMR vaccine can be given in place of IG if done within 72 hours of exposure. PEP for children during the 2013 New York City outbreak reduced the risk of measles by 83.4% (95% CI: 34.4%-95.8%) in recipients of MMR vaccine and by 100% (95% CI: 56.2%-99.8%) in recipients of IG compared with those without prophylaxis.5 A 2014 Cochrane Review found that IG reduced the risk of measles by 83% (95% CI: 64%-92%).6
Recommendation 4. Severe measles cases among children, such as those who are hospitalized, should be treated with vitamin A. Vitamin A should be administered immediately on diagnosis and repeated the next day.
In children, vitamin A deficiency, even if clinically inapparent, leads to increased measles severity, and randomized controlled trial data suggest that supplementation reduces measles-related morbidity and mortality.4 Even in high-income countries, children with measles have high rates of vitamin A deficiency, which is associated with increased morbidity.7 A Cochrane review found that two-dose regimens of vitamin A reduced the overall mortality (RR 0.21; 95% CI: 0.07-0.66) in children with measles aged <2 years.8 World Health Organization guidelines suggest vitamin A therapy for all children with acute measles infection, and the AAP Committee on Infectious Diseases recommends vitamin A for severe (ie, hospitalized) cases. Vitamin A is given orally once daily for two days at the following doses: 50,000 international units (IU) for infants aged <6 months, 100,000 IU for infants aged 6-11 months, and 200,000 IU for children aged ≥12 months. A third dose can be given two to four weeks later for children with signs and symptoms of vitamin A deficiency (eg, corneal clouding or conjunctival plaques).
CRITIQUE
In outbreak settings, hospitalists may find challenges with having a sufficient number of single negative-pressure rooms for patients with suspected or confirmed measles and providing IG prophylaxis given the recent national shortages of intravenous immunoglobulin. Collaboration with the infection control team, pharmacy, and the local public health department is essential to appropriately address these challenges. With regard to treatment recommendations, randomized studies on the impact of vitamin A treatment in children have been primarily conducted in resource-limited settings.8 However, these data, in combination with observational data from resource-rich settings,7 support its use given the favorable risk-benefit profile. The role of vitamin A therapy in adults with measles infection is considerably less clear, although there are reports of its use in severe cases.
AREAS OF FUTURE STUDY
Much of our knowledge regarding measles complications and treatment outcomes comes from resource-limited settings or from older data before widespread vaccination. Data suggest that prophylactic antibiotics may prevent complications; however, currently available data are insufficient to support routine use.9 Coordination and collaboration between public health, infectious diseases, and hospital medicine would enhance the ability to conduct detailed epidemiologic studies during outbreak situations. Further studies examining treatment and outcomes in hospitalized patients, including the role of prophylactic antibiotics in the prevention of complications, would provide valuable guidance for hospitalists caring for patients with severe measles.
Measles is a highly contagious acute respiratory illness that can cause complications in multiple organ systems. Measles was declared eliminated in the United States in 2000; however, outbreaks still occur, especially in unvaccinated populations. The Centers for Disease Control and Prevention (CDC) reported that as of October 3, 2019, 1,250 cases of measles had been confirmed in 31 states in 2019, which represents the greatest number of cases reported in the US since 1992.1 Although the disease is often self-limited, infected individuals can also develop complications requiring hospitalization, which occurred in 10% of confirmed cases this year.1 In February 2018, the CDC updated their recommendations about measles diagnosis and treatment on their website,2 adding an interim update in July 2019 to include new guidelines about infection control and prevention.3 This highlight reviews those recommendations most relevant to hospitalists, who can play a critical role in the diagnosis and management of patients with suspected and/or confirmed measles.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Healthcare providers should consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness. Healthcare providers should report suspected measles cases to their local health department within 24 hours.
Measles is an acute febrile illness that begins with a prodrome of fever, followed by one or more of the following three “C’s”: cough, coryza (rhinitis), and conjunctivitis. Koplik spots, a pathognomonic buccal enanthem consisting of white lesions on an erythematous base, can appear shortly thereafter. An erythematous, maculopapular rash develops three to four days after the onset of the fever. The rash starts on the face and then spreads over the next few days to the trunk and extremities. Clinical recovery generally occurs within one week of rash onset in uncomplicated measles. Complications can affect almost any organ system. The most common complications are pneumonia, often caused by secondary viral or bacterial pathogens, diarrhea, otitis media, and laryngotracheobronchitis. Rare but serious complications include acute encephalitis and subacute sclerosing panencephalitis. Groups at the highest risk for serious disease include children aged <5 years, adults aged >20 years, pregnant women, and immunocompromised individuals.
When encountering patients with a febrile rash and compatible symptoms, clinicians should also have a high index of suspicion for measles in patients who are unvaccinated or undervaccinated, since the majority of measles cases have occurred in the unvaccinated population. Providers should contact their local health department and infectious diseases/infection control team as soon as suspected measles cases are identified. Laboratory confirmation is necessary for all suspected cases and should typically consist of measles IgM antibody testing from serum and real-time polymerase chain reaction (RT-PCR) from respiratory and urine specimens.
Recommendation 2. Adhere to airborne precautions for anyone with known or suspected measles.
Measles is highly contagious, and infectious particles can remain in the air for up to two hours after a person with measles leaves a room. From 2001 to 2014, 6% (78/1,318) of nonimported measles cases in the US were transmitted in healthcare settings.4 Key steps in preventing the spread of measles within hospitals include ensuring that all healthcare personnel have evidence of immunity to measles and rapid identification and isolation of suspect cases. Patients with suspected measles should be given a facemask and moved immediately into a single-patient airborne infection isolation room. Personnel, even those with presumptive evidence of immunity, should use N95 respirators or the equivalent when caring for patients with suspected or confirmed measles. Patients with measles are contagious from four days before to four days after rash onset; therefore, airborne precautions should be continued for four days following the onset of rash in immunocompetent patients. For immunocompromised patients, airborne precautions should be continued for the duration of the illness based on data suggesting prolonged shedding, particularly in the setting of altered T-cell immunity.4
Recommendation 3. People exposed to measles who cannot readily show that they have evidence of immunity against measles should be offered postexposure prophylaxis (PEP) or be excluded from the setting (school, hospital, childcare). To potentially provide protection or modify the clinical course of disease among susceptible persons, either administer a measles, mumps, and rubella (MMR) vaccine within 72 hours of initial measles exposure or immunoglobulin (IG) within six days of exposure.
MMR vaccine is recommended for vaccine-eligible, exposed individuals aged ≥6 months within 72 hours of measles exposure. IG, which contains measles antibody due to widespread immunization in the US, is recommended for individuals at high risk for serious illness, including infants aged ≤12 months, pregnant women without evidence of measles immunity, and severely immunocompromised patients regardless of vaccination status. For infants aged 6-11 months, MMR vaccine can be given in place of IG if done within 72 hours of exposure. PEP for children during the 2013 New York City outbreak reduced the risk of measles by 83.4% (95% CI: 34.4%-95.8%) in recipients of MMR vaccine and by 100% (95% CI: 56.2%-99.8%) in recipients of IG compared with those without prophylaxis.5 A 2014 Cochrane Review found that IG reduced the risk of measles by 83% (95% CI: 64%-92%).6
Recommendation 4. Severe measles cases among children, such as those who are hospitalized, should be treated with vitamin A. Vitamin A should be administered immediately on diagnosis and repeated the next day.
In children, vitamin A deficiency, even if clinically inapparent, leads to increased measles severity, and randomized controlled trial data suggest that supplementation reduces measles-related morbidity and mortality.4 Even in high-income countries, children with measles have high rates of vitamin A deficiency, which is associated with increased morbidity.7 A Cochrane review found that two-dose regimens of vitamin A reduced the overall mortality (RR 0.21; 95% CI: 0.07-0.66) in children with measles aged <2 years.8 World Health Organization guidelines suggest vitamin A therapy for all children with acute measles infection, and the AAP Committee on Infectious Diseases recommends vitamin A for severe (ie, hospitalized) cases. Vitamin A is given orally once daily for two days at the following doses: 50,000 international units (IU) for infants aged <6 months, 100,000 IU for infants aged 6-11 months, and 200,000 IU for children aged ≥12 months. A third dose can be given two to four weeks later for children with signs and symptoms of vitamin A deficiency (eg, corneal clouding or conjunctival plaques).
CRITIQUE
In outbreak settings, hospitalists may find challenges with having a sufficient number of single negative-pressure rooms for patients with suspected or confirmed measles and providing IG prophylaxis given the recent national shortages of intravenous immunoglobulin. Collaboration with the infection control team, pharmacy, and the local public health department is essential to appropriately address these challenges. With regard to treatment recommendations, randomized studies on the impact of vitamin A treatment in children have been primarily conducted in resource-limited settings.8 However, these data, in combination with observational data from resource-rich settings,7 support its use given the favorable risk-benefit profile. The role of vitamin A therapy in adults with measles infection is considerably less clear, although there are reports of its use in severe cases.
AREAS OF FUTURE STUDY
Much of our knowledge regarding measles complications and treatment outcomes comes from resource-limited settings or from older data before widespread vaccination. Data suggest that prophylactic antibiotics may prevent complications; however, currently available data are insufficient to support routine use.9 Coordination and collaboration between public health, infectious diseases, and hospital medicine would enhance the ability to conduct detailed epidemiologic studies during outbreak situations. Further studies examining treatment and outcomes in hospitalized patients, including the role of prophylactic antibiotics in the prevention of complications, would provide valuable guidance for hospitalists caring for patients with severe measles.
1. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. 2019; https://www.cdc.gov/measles/cases-outbreaks.html. Accessed October 14, 2019.
2. Centers for Disease Control and Prevention. Measles (Rubeola): For Healthcare Professionals. 2019; https://www.cdc.gov/measles/hcp/index.html. Accessed October 14, 2019.
3. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings. 2019.
4. Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61(4):615-618. https://doi.org/10.1093/cid/civ387.
5. Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City, 2013. Clin Infect Dis. 2017;65(11):1843-1847. https://doi.org/10.1093/cid/cix639.
6. Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014;(4):Cd010056. https://doi.org/10.1002/14651858.CD010056.pub2.
7. Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. New York City. Am J Dis Child. 1992;146(2):182-186. https://doi.org/10.1001/archpedi.1992.02160140048019.
8. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005(4):Cd001479. https://doi.org/10.1002/14651858.CD001479.pub3.
9. Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. Cochrane Database Syst Rev. 2013(8):Cd001477. https://doi.org/10.1002/14651858.CD001477.pub3.
1. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. 2019; https://www.cdc.gov/measles/cases-outbreaks.html. Accessed October 14, 2019.
2. Centers for Disease Control and Prevention. Measles (Rubeola): For Healthcare Professionals. 2019; https://www.cdc.gov/measles/hcp/index.html. Accessed October 14, 2019.
3. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings. 2019.
4. Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61(4):615-618. https://doi.org/10.1093/cid/civ387.
5. Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City, 2013. Clin Infect Dis. 2017;65(11):1843-1847. https://doi.org/10.1093/cid/cix639.
6. Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014;(4):Cd010056. https://doi.org/10.1002/14651858.CD010056.pub2.
7. Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. New York City. Am J Dis Child. 1992;146(2):182-186. https://doi.org/10.1001/archpedi.1992.02160140048019.
8. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005(4):Cd001479. https://doi.org/10.1002/14651858.CD001479.pub3.
9. Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. Cochrane Database Syst Rev. 2013(8):Cd001477. https://doi.org/10.1002/14651858.CD001477.pub3.
© 2020 Society of Hospital Medicine