User login
Is marijuana a viable replacement for opioids in managing chronic non-cancer pain?
According to surveillance data from the Centers for Disease Control and Prevention (CDC), mortality from drug overdose has steadily increased since the dawn of the new millennium. More than 600,000 overdose deaths were reported from 2000 to 2016. According to the CDC, the first wave began with increased prescribing of opioids in the 1990s and then though the pill mills until approximately 2010. The second wave began shortly thereafter with increased overdose deaths involving heroin as the cost of prescription opioids increased when the pill mills were shut down. The third wave began in 2013 with increased overdose deaths involving synthetic opioids – particularly when illicitly manufactured fentanyl was first used as an additive to Mexican heroin. The illicitly manufactured opioid market continues to change, and is still found in combination with heroin, counterfeit “look-alike opioid prescription medication, spiked marijuana, and now cocaine.
In spite of this changing data, legally prescribed opioid use remains the focus of the most recent opioid “epidemic”. To wit, approximately 63% of the 52,404 overdose deaths in 2015 involved an opioid—but which one? By 2016, the trend was clear– heroin and fentanyl killed the majority of the 42,000 people who overdosed in 2016, more than any year on record. Further, 40% of opioid overdose deaths involved a prescription opioid. This does tell us whether the prescription opioid was attained legally or illegally. Pop star Prince died from an overdose of counterfeit oxycodone tablets, spiked with fentanyl.
Although various strategies have been introduced to address the crisis, including implementation of the CDC Guideline for Prescribing Opioids for Chronic Pain, as well as efforts to improve prescription drug monitoring programs and better access to treatment for opioid use disorder.
Figure 1 CDC Clinical Guidelines for Physicians Prescribing Opioids (CDC 2017):
| The CDC Guideline addresses patient-centered clinical practices including conducting thorough assessments, considering all possible treatments, closely monitoring risks, and safely discontinuing opioids. The three main focus areas in the Guideline include:
1. Determining when to initiate or continue opioids for chronic pain
2. Opioid selection, dosage, duration, follow-up, and discontinuation
3. Assessing risk and addressing harms of opioid use
|
These recommendations from the CDC are excellent but without adequate training for physicians who deal with chronic pain or addiction on a regular basis, they will likely not produce the change we hope for—and for good reason. The fear of suspicion and retribution from governing bodies who monitor prescription opioids and all scheduled medications have kept physicians from prescribing these drugs for their patients who need them. Many will recall the latter days of the pill mills, with surveillance videos of sketchy looking characters carrying trash bags full of hydrocodone and oxycodone out of a doctor’s office as part of a covert sting operation–followed by the physician in handcuffs being “perp walked” on the nightly news. These images became iconic in the American psyche and have changed medical practice. Yet for the millions of Americans who suffer from legitimate chronic, non-cancer pain, this is a frightening prospect. Why? Because for most pain patients, opioids have provided a viable means to a quality of life.
The facts are clear; patients with legitimate chronic pain who are cared for by boarded pain or addiction specialists do not abuse their opioid pain medication. In fact, recent data reveal that only between 4 and 10 percent of these patients will ever misuse or abuse their medication. Most of these individuals could not work, achieve self-efficacy, or have any quality of life without the pain relief opioids provide them. We all wish that alternatives were available—but they are not. As the baby boomers are living longer and have more wear and tear and injuries that require expert pain management, there is a legitimate need for these medications—until some alternative to opioids are found. Understandably, many pain patients and their doctors are afraid that government overreach, designed to stop drug dealers and drug addicts, will rob them of the little quality of life they have because opioids provide a temporary respite from severe pain.
In truth, the DEA has done a good job of shutting down the pill mills and most states now allow physicians to access each patient’s pharmacy records online. This process has also shut down “doctor shopping” for controlled substances and takes less than a minute to review all prescriptions for controlled substances for a single patient. Perhaps the epidemic of chronic pain should be the focus of our research efforts.
So why are people still dying from opioid overdose? The CDC report reveals part of the answer. As the pill mills shut down, the price of illicit prescription opioids increased substantially. In response, the Mexican cartel, which was losing billions due to legalized marijuana, flooded the US with cheap, powerful heroin. It was cheap and powerful because the cartel was spiking it with homemade fentanyl, which is approximately 100 times more potent that morphine. It is heroin and fentanyl that are killing thousands of Americans each month.
Cannabis and Pain
Is marijuana really a reasonable alternative to opioids for opioid addicts or for chronic pain? Maybe, but the science is woefully silent on the topic. What little science exists remains inconclusive. The recent JAMA article shows epidemiological trends among Medicare Part D recipients and state Medicaid recipients. The analysis attempts to statistically correlate states with liberal marijuana laws and a decrease in the number of opioid prescriptions written between 2010 and 2015.
Why Does This Matter?
Correlation is not causation. The conclusions reached by this investigation suggest that merely the decline of filling prescriptions for opioids by elderly infirmed adults is due to liberalized, legal access to cannabis, which is quite a reach in logic. This conclusion assumes these same adults voluntarily switched from their opioids to medical marijuana for pain control. In truth, there are hundreds of variants, including hordes of untrained and anxious physicians who live in fear that prescribing opioids sends a red flag to licensing boards and invites increased scrutiny and potential retribution. Plus, any physician who believes in evidence-based medicine is unlikely to prescribe medical marijuana for pain. At present, the research does not support this practice.
Double blind, placebo-controlled comparisons between medical marijuana and legally prescribed opioids for debilitating non-cancer pain syndromes will provide the science necessary to determine the efficacy and safety of marijuana as a medication for chronic pain.
Yes, it is feasible that the endocannabinoid system may provide new therapeutics for pain and other disease. However, the well-established risks associated with THC must be accounted for. It hard to believe that elderly patients would choose to be stoned for 3-5 hours and experience the cognitive “dulling” and short-term memory deficits over an opioid that if anything, gives them some energy, plus the best pain relief known.
The recent approval of the Cannabidiol (CBD) based medication Epiolidex by an FDA subcommittee, for treating two debilitating seizures disorders, has provided a model for assessing efficacy and risk for cannabis-based medicines. CBD is non-addictive and non-psychoactive. Until similar scientific scrutiny proves safety and efficacy of THC products, they should be considered harmful.
Reference
Bradford AC, Bradford WD, Abraham A, Bagwell Adams G . Association Between US State Medical Cannabis Laws and Opioid Prescribing in the Medicare Part D Population. JAMA Intern Med. 2018 Apr 2. doi: 10.1001/jamainternmed.2018.0266. [Epub ahead of print]
Reference
Bradford AC, Bradford WD, Abraham A, Bagwell Adams G . Association Between US State Medical Cannabis Laws and Opioid Prescribing in the Medicare Part D Population. JAMA Intern Med. 2018 Apr 2. doi: 10.1001/jamainternmed.2018.0266. [Epub ahead of print]
According to surveillance data from the Centers for Disease Control and Prevention (CDC), mortality from drug overdose has steadily increased since the dawn of the new millennium. More than 600,000 overdose deaths were reported from 2000 to 2016. According to the CDC, the first wave began with increased prescribing of opioids in the 1990s and then though the pill mills until approximately 2010. The second wave began shortly thereafter with increased overdose deaths involving heroin as the cost of prescription opioids increased when the pill mills were shut down. The third wave began in 2013 with increased overdose deaths involving synthetic opioids – particularly when illicitly manufactured fentanyl was first used as an additive to Mexican heroin. The illicitly manufactured opioid market continues to change, and is still found in combination with heroin, counterfeit “look-alike opioid prescription medication, spiked marijuana, and now cocaine.
In spite of this changing data, legally prescribed opioid use remains the focus of the most recent opioid “epidemic”. To wit, approximately 63% of the 52,404 overdose deaths in 2015 involved an opioid—but which one? By 2016, the trend was clear– heroin and fentanyl killed the majority of the 42,000 people who overdosed in 2016, more than any year on record. Further, 40% of opioid overdose deaths involved a prescription opioid. This does tell us whether the prescription opioid was attained legally or illegally. Pop star Prince died from an overdose of counterfeit oxycodone tablets, spiked with fentanyl.
Although various strategies have been introduced to address the crisis, including implementation of the CDC Guideline for Prescribing Opioids for Chronic Pain, as well as efforts to improve prescription drug monitoring programs and better access to treatment for opioid use disorder.
Figure 1 CDC Clinical Guidelines for Physicians Prescribing Opioids (CDC 2017):
| The CDC Guideline addresses patient-centered clinical practices including conducting thorough assessments, considering all possible treatments, closely monitoring risks, and safely discontinuing opioids. The three main focus areas in the Guideline include:
1. Determining when to initiate or continue opioids for chronic pain
2. Opioid selection, dosage, duration, follow-up, and discontinuation
3. Assessing risk and addressing harms of opioid use
|
These recommendations from the CDC are excellent but without adequate training for physicians who deal with chronic pain or addiction on a regular basis, they will likely not produce the change we hope for—and for good reason. The fear of suspicion and retribution from governing bodies who monitor prescription opioids and all scheduled medications have kept physicians from prescribing these drugs for their patients who need them. Many will recall the latter days of the pill mills, with surveillance videos of sketchy looking characters carrying trash bags full of hydrocodone and oxycodone out of a doctor’s office as part of a covert sting operation–followed by the physician in handcuffs being “perp walked” on the nightly news. These images became iconic in the American psyche and have changed medical practice. Yet for the millions of Americans who suffer from legitimate chronic, non-cancer pain, this is a frightening prospect. Why? Because for most pain patients, opioids have provided a viable means to a quality of life.
The facts are clear; patients with legitimate chronic pain who are cared for by boarded pain or addiction specialists do not abuse their opioid pain medication. In fact, recent data reveal that only between 4 and 10 percent of these patients will ever misuse or abuse their medication. Most of these individuals could not work, achieve self-efficacy, or have any quality of life without the pain relief opioids provide them. We all wish that alternatives were available—but they are not. As the baby boomers are living longer and have more wear and tear and injuries that require expert pain management, there is a legitimate need for these medications—until some alternative to opioids are found. Understandably, many pain patients and their doctors are afraid that government overreach, designed to stop drug dealers and drug addicts, will rob them of the little quality of life they have because opioids provide a temporary respite from severe pain.
In truth, the DEA has done a good job of shutting down the pill mills and most states now allow physicians to access each patient’s pharmacy records online. This process has also shut down “doctor shopping” for controlled substances and takes less than a minute to review all prescriptions for controlled substances for a single patient. Perhaps the epidemic of chronic pain should be the focus of our research efforts.
So why are people still dying from opioid overdose? The CDC report reveals part of the answer. As the pill mills shut down, the price of illicit prescription opioids increased substantially. In response, the Mexican cartel, which was losing billions due to legalized marijuana, flooded the US with cheap, powerful heroin. It was cheap and powerful because the cartel was spiking it with homemade fentanyl, which is approximately 100 times more potent that morphine. It is heroin and fentanyl that are killing thousands of Americans each month.
Cannabis and Pain
Is marijuana really a reasonable alternative to opioids for opioid addicts or for chronic pain? Maybe, but the science is woefully silent on the topic. What little science exists remains inconclusive. The recent JAMA article shows epidemiological trends among Medicare Part D recipients and state Medicaid recipients. The analysis attempts to statistically correlate states with liberal marijuana laws and a decrease in the number of opioid prescriptions written between 2010 and 2015.
Why Does This Matter?
Correlation is not causation. The conclusions reached by this investigation suggest that merely the decline of filling prescriptions for opioids by elderly infirmed adults is due to liberalized, legal access to cannabis, which is quite a reach in logic. This conclusion assumes these same adults voluntarily switched from their opioids to medical marijuana for pain control. In truth, there are hundreds of variants, including hordes of untrained and anxious physicians who live in fear that prescribing opioids sends a red flag to licensing boards and invites increased scrutiny and potential retribution. Plus, any physician who believes in evidence-based medicine is unlikely to prescribe medical marijuana for pain. At present, the research does not support this practice.
Double blind, placebo-controlled comparisons between medical marijuana and legally prescribed opioids for debilitating non-cancer pain syndromes will provide the science necessary to determine the efficacy and safety of marijuana as a medication for chronic pain.
Yes, it is feasible that the endocannabinoid system may provide new therapeutics for pain and other disease. However, the well-established risks associated with THC must be accounted for. It hard to believe that elderly patients would choose to be stoned for 3-5 hours and experience the cognitive “dulling” and short-term memory deficits over an opioid that if anything, gives them some energy, plus the best pain relief known.
The recent approval of the Cannabidiol (CBD) based medication Epiolidex by an FDA subcommittee, for treating two debilitating seizures disorders, has provided a model for assessing efficacy and risk for cannabis-based medicines. CBD is non-addictive and non-psychoactive. Until similar scientific scrutiny proves safety and efficacy of THC products, they should be considered harmful.
Reference
Bradford AC, Bradford WD, Abraham A, Bagwell Adams G . Association Between US State Medical Cannabis Laws and Opioid Prescribing in the Medicare Part D Population. JAMA Intern Med. 2018 Apr 2. doi: 10.1001/jamainternmed.2018.0266. [Epub ahead of print]
According to surveillance data from the Centers for Disease Control and Prevention (CDC), mortality from drug overdose has steadily increased since the dawn of the new millennium. More than 600,000 overdose deaths were reported from 2000 to 2016. According to the CDC, the first wave began with increased prescribing of opioids in the 1990s and then though the pill mills until approximately 2010. The second wave began shortly thereafter with increased overdose deaths involving heroin as the cost of prescription opioids increased when the pill mills were shut down. The third wave began in 2013 with increased overdose deaths involving synthetic opioids – particularly when illicitly manufactured fentanyl was first used as an additive to Mexican heroin. The illicitly manufactured opioid market continues to change, and is still found in combination with heroin, counterfeit “look-alike opioid prescription medication, spiked marijuana, and now cocaine.
In spite of this changing data, legally prescribed opioid use remains the focus of the most recent opioid “epidemic”. To wit, approximately 63% of the 52,404 overdose deaths in 2015 involved an opioid—but which one? By 2016, the trend was clear– heroin and fentanyl killed the majority of the 42,000 people who overdosed in 2016, more than any year on record. Further, 40% of opioid overdose deaths involved a prescription opioid. This does tell us whether the prescription opioid was attained legally or illegally. Pop star Prince died from an overdose of counterfeit oxycodone tablets, spiked with fentanyl.
Although various strategies have been introduced to address the crisis, including implementation of the CDC Guideline for Prescribing Opioids for Chronic Pain, as well as efforts to improve prescription drug monitoring programs and better access to treatment for opioid use disorder.
Figure 1 CDC Clinical Guidelines for Physicians Prescribing Opioids (CDC 2017):
| The CDC Guideline addresses patient-centered clinical practices including conducting thorough assessments, considering all possible treatments, closely monitoring risks, and safely discontinuing opioids. The three main focus areas in the Guideline include:
1. Determining when to initiate or continue opioids for chronic pain
2. Opioid selection, dosage, duration, follow-up, and discontinuation
3. Assessing risk and addressing harms of opioid use
|
These recommendations from the CDC are excellent but without adequate training for physicians who deal with chronic pain or addiction on a regular basis, they will likely not produce the change we hope for—and for good reason. The fear of suspicion and retribution from governing bodies who monitor prescription opioids and all scheduled medications have kept physicians from prescribing these drugs for their patients who need them. Many will recall the latter days of the pill mills, with surveillance videos of sketchy looking characters carrying trash bags full of hydrocodone and oxycodone out of a doctor’s office as part of a covert sting operation–followed by the physician in handcuffs being “perp walked” on the nightly news. These images became iconic in the American psyche and have changed medical practice. Yet for the millions of Americans who suffer from legitimate chronic, non-cancer pain, this is a frightening prospect. Why? Because for most pain patients, opioids have provided a viable means to a quality of life.
The facts are clear; patients with legitimate chronic pain who are cared for by boarded pain or addiction specialists do not abuse their opioid pain medication. In fact, recent data reveal that only between 4 and 10 percent of these patients will ever misuse or abuse their medication. Most of these individuals could not work, achieve self-efficacy, or have any quality of life without the pain relief opioids provide them. We all wish that alternatives were available—but they are not. As the baby boomers are living longer and have more wear and tear and injuries that require expert pain management, there is a legitimate need for these medications—until some alternative to opioids are found. Understandably, many pain patients and their doctors are afraid that government overreach, designed to stop drug dealers and drug addicts, will rob them of the little quality of life they have because opioids provide a temporary respite from severe pain.
In truth, the DEA has done a good job of shutting down the pill mills and most states now allow physicians to access each patient’s pharmacy records online. This process has also shut down “doctor shopping” for controlled substances and takes less than a minute to review all prescriptions for controlled substances for a single patient. Perhaps the epidemic of chronic pain should be the focus of our research efforts.
So why are people still dying from opioid overdose? The CDC report reveals part of the answer. As the pill mills shut down, the price of illicit prescription opioids increased substantially. In response, the Mexican cartel, which was losing billions due to legalized marijuana, flooded the US with cheap, powerful heroin. It was cheap and powerful because the cartel was spiking it with homemade fentanyl, which is approximately 100 times more potent that morphine. It is heroin and fentanyl that are killing thousands of Americans each month.
Cannabis and Pain
Is marijuana really a reasonable alternative to opioids for opioid addicts or for chronic pain? Maybe, but the science is woefully silent on the topic. What little science exists remains inconclusive. The recent JAMA article shows epidemiological trends among Medicare Part D recipients and state Medicaid recipients. The analysis attempts to statistically correlate states with liberal marijuana laws and a decrease in the number of opioid prescriptions written between 2010 and 2015.
Why Does This Matter?
Correlation is not causation. The conclusions reached by this investigation suggest that merely the decline of filling prescriptions for opioids by elderly infirmed adults is due to liberalized, legal access to cannabis, which is quite a reach in logic. This conclusion assumes these same adults voluntarily switched from their opioids to medical marijuana for pain control. In truth, there are hundreds of variants, including hordes of untrained and anxious physicians who live in fear that prescribing opioids sends a red flag to licensing boards and invites increased scrutiny and potential retribution. Plus, any physician who believes in evidence-based medicine is unlikely to prescribe medical marijuana for pain. At present, the research does not support this practice.
Double blind, placebo-controlled comparisons between medical marijuana and legally prescribed opioids for debilitating non-cancer pain syndromes will provide the science necessary to determine the efficacy and safety of marijuana as a medication for chronic pain.
Yes, it is feasible that the endocannabinoid system may provide new therapeutics for pain and other disease. However, the well-established risks associated with THC must be accounted for. It hard to believe that elderly patients would choose to be stoned for 3-5 hours and experience the cognitive “dulling” and short-term memory deficits over an opioid that if anything, gives them some energy, plus the best pain relief known.
The recent approval of the Cannabidiol (CBD) based medication Epiolidex by an FDA subcommittee, for treating two debilitating seizures disorders, has provided a model for assessing efficacy and risk for cannabis-based medicines. CBD is non-addictive and non-psychoactive. Until similar scientific scrutiny proves safety and efficacy of THC products, they should be considered harmful.
Reference
Bradford AC, Bradford WD, Abraham A, Bagwell Adams G . Association Between US State Medical Cannabis Laws and Opioid Prescribing in the Medicare Part D Population. JAMA Intern Med. 2018 Apr 2. doi: 10.1001/jamainternmed.2018.0266. [Epub ahead of print]
Reference
Bradford AC, Bradford WD, Abraham A, Bagwell Adams G . Association Between US State Medical Cannabis Laws and Opioid Prescribing in the Medicare Part D Population. JAMA Intern Med. 2018 Apr 2. doi: 10.1001/jamainternmed.2018.0266. [Epub ahead of print]
Reference
Bradford AC, Bradford WD, Abraham A, Bagwell Adams G . Association Between US State Medical Cannabis Laws and Opioid Prescribing in the Medicare Part D Population. JAMA Intern Med. 2018 Apr 2. doi: 10.1001/jamainternmed.2018.0266. [Epub ahead of print]
Pain, opioids and addiction
In the year 2017, a plethora of articles and commentaries on the “opioid crisis” have appeared in major medical journals, alongside the ongoing hyperbole seen daily in the lay media. But the pressing concern remains: How best to manage patients who are 1.) already taking opioids and 2.) those newly requesting relief of serious and chronic pain.
Opioid for Pain and Its Misuse
In this article by Volkow and Collins, both of whom are titans in neuroscience, we are reminded that despite all the warnings, opioids are being widely prescribed in the U.S. In a weighted national sample of over 50,000 adults, the investigators concluded that more than one-third of the adult population has taken an opioid at some point during 2015. Among these, 12.5% confirmed that they misused the drug, e.g., used them without a prescription or in any way contrary to the prescribed directions. Of these, 16.7% developed an opioid-use disorder, as defined in the DSM-IV.
In response, Volkow and Collins note that an increasing number of clinicians are attempting to control chronic or intractable pain with new anticonvulsants such as Pregabalin (Lyrica) and Gabapentin. Yet, these drugs have only been shown to be effective only for fibromyalgia and certain forms of neurogenic pain. In addition, the authors note that a multidisciplinary workgroup convened by the NIH Office of Disease Prevention (2014) found that there had been no randomized trials to evaluate the efficacy of long-term (>1 year) opioid treatment. Accordingly, the authors recommend short-term strategy to develop abuse-deterrent formulations that can minimize diversion and misuse.
What About Cannabis?
In a 2017 report from the National Academies of Sciences, Engineering, and Medicine, substantial evidence supports the effectiveness of cannabinoids in treating some types of pain. However, again there is scant research on phytocannabinoids as medicine. In addition, there are abundant research and legitimate concerns related to cognitive, motor and motivational impairment and the effects on brain development. However, the therapeutic potential of cannabinoids and mediators of the abundant endocannabinoid system warrants further exploration for alternatives to opioids.
Lastly, non-pharmacologic interventions, including behavioral, self-management interventions, may play an important role in pain management. The initiative described by the authors supports partnerships between the NIH and pharmaceutical and biotechnology companies to hasten medication and device development.
Why Does This Matter?
If these data are true, and one-third of the U.S. adult population suffers from chronic pain, we are duty bound to find therapeutic options with less risk, addictive potential and mortality. As I have argued for nearly 40 years, basic and translational research is desperately needed, as clinicians are in a conundrum between the worthy goals of alleviating pain and suffering and decreasing the risk for addiction and mortality. We can, and must do better.
Volkow ND, Collins FS. The Role of Science in Addressing the Opioid Crisis. N Engl J Med. 2017;377(4):391-394.
In the year 2017, a plethora of articles and commentaries on the “opioid crisis” have appeared in major medical journals, alongside the ongoing hyperbole seen daily in the lay media. But the pressing concern remains: How best to manage patients who are 1.) already taking opioids and 2.) those newly requesting relief of serious and chronic pain.
Opioid for Pain and Its Misuse
In this article by Volkow and Collins, both of whom are titans in neuroscience, we are reminded that despite all the warnings, opioids are being widely prescribed in the U.S. In a weighted national sample of over 50,000 adults, the investigators concluded that more than one-third of the adult population has taken an opioid at some point during 2015. Among these, 12.5% confirmed that they misused the drug, e.g., used them without a prescription or in any way contrary to the prescribed directions. Of these, 16.7% developed an opioid-use disorder, as defined in the DSM-IV.
In response, Volkow and Collins note that an increasing number of clinicians are attempting to control chronic or intractable pain with new anticonvulsants such as Pregabalin (Lyrica) and Gabapentin. Yet, these drugs have only been shown to be effective only for fibromyalgia and certain forms of neurogenic pain. In addition, the authors note that a multidisciplinary workgroup convened by the NIH Office of Disease Prevention (2014) found that there had been no randomized trials to evaluate the efficacy of long-term (>1 year) opioid treatment. Accordingly, the authors recommend short-term strategy to develop abuse-deterrent formulations that can minimize diversion and misuse.
What About Cannabis?
In a 2017 report from the National Academies of Sciences, Engineering, and Medicine, substantial evidence supports the effectiveness of cannabinoids in treating some types of pain. However, again there is scant research on phytocannabinoids as medicine. In addition, there are abundant research and legitimate concerns related to cognitive, motor and motivational impairment and the effects on brain development. However, the therapeutic potential of cannabinoids and mediators of the abundant endocannabinoid system warrants further exploration for alternatives to opioids.
Lastly, non-pharmacologic interventions, including behavioral, self-management interventions, may play an important role in pain management. The initiative described by the authors supports partnerships between the NIH and pharmaceutical and biotechnology companies to hasten medication and device development.
Why Does This Matter?
If these data are true, and one-third of the U.S. adult population suffers from chronic pain, we are duty bound to find therapeutic options with less risk, addictive potential and mortality. As I have argued for nearly 40 years, basic and translational research is desperately needed, as clinicians are in a conundrum between the worthy goals of alleviating pain and suffering and decreasing the risk for addiction and mortality. We can, and must do better.
In the year 2017, a plethora of articles and commentaries on the “opioid crisis” have appeared in major medical journals, alongside the ongoing hyperbole seen daily in the lay media. But the pressing concern remains: How best to manage patients who are 1.) already taking opioids and 2.) those newly requesting relief of serious and chronic pain.
Opioid for Pain and Its Misuse
In this article by Volkow and Collins, both of whom are titans in neuroscience, we are reminded that despite all the warnings, opioids are being widely prescribed in the U.S. In a weighted national sample of over 50,000 adults, the investigators concluded that more than one-third of the adult population has taken an opioid at some point during 2015. Among these, 12.5% confirmed that they misused the drug, e.g., used them without a prescription or in any way contrary to the prescribed directions. Of these, 16.7% developed an opioid-use disorder, as defined in the DSM-IV.
In response, Volkow and Collins note that an increasing number of clinicians are attempting to control chronic or intractable pain with new anticonvulsants such as Pregabalin (Lyrica) and Gabapentin. Yet, these drugs have only been shown to be effective only for fibromyalgia and certain forms of neurogenic pain. In addition, the authors note that a multidisciplinary workgroup convened by the NIH Office of Disease Prevention (2014) found that there had been no randomized trials to evaluate the efficacy of long-term (>1 year) opioid treatment. Accordingly, the authors recommend short-term strategy to develop abuse-deterrent formulations that can minimize diversion and misuse.
What About Cannabis?
In a 2017 report from the National Academies of Sciences, Engineering, and Medicine, substantial evidence supports the effectiveness of cannabinoids in treating some types of pain. However, again there is scant research on phytocannabinoids as medicine. In addition, there are abundant research and legitimate concerns related to cognitive, motor and motivational impairment and the effects on brain development. However, the therapeutic potential of cannabinoids and mediators of the abundant endocannabinoid system warrants further exploration for alternatives to opioids.
Lastly, non-pharmacologic interventions, including behavioral, self-management interventions, may play an important role in pain management. The initiative described by the authors supports partnerships between the NIH and pharmaceutical and biotechnology companies to hasten medication and device development.
Why Does This Matter?
If these data are true, and one-third of the U.S. adult population suffers from chronic pain, we are duty bound to find therapeutic options with less risk, addictive potential and mortality. As I have argued for nearly 40 years, basic and translational research is desperately needed, as clinicians are in a conundrum between the worthy goals of alleviating pain and suffering and decreasing the risk for addiction and mortality. We can, and must do better.
Volkow ND, Collins FS. The Role of Science in Addressing the Opioid Crisis. N Engl J Med. 2017;377(4):391-394.
Volkow ND, Collins FS. The Role of Science in Addressing the Opioid Crisis. N Engl J Med. 2017;377(4):391-394.
Methamphetamine-induced psychosis: Who says all drug use is reversible?
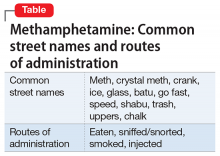
Use of methamphetamine, an N-methyl analog of amphetamine, is a serious public health problem; throughout the world an estimated 35.7 million people use the drug recreationally.1 Methamphetamine is easy to obtain because it is cheap to produce and can be synthesized anywhere. In the United States, methamphetamine is commonly manufactured in small-scale laboratories using relatively inexpensive, legally available ingredients. Large-scale manufacturing in clandestine laboratories also contributes to methamphetamine abuse. The drug, known as meth, crystal meth, ice, and other names, is available as a powder, tablet, or crystalline salt, and is used by various routes of administration (Table).
Although FDA-approved for treating attention-deficit/hyperactivity disorder, methamphetamine is taken recreationally for its euphoric effects; however, it also produces anhedonia, paranoia, and a host of cognitive deficits and other adverse effects.
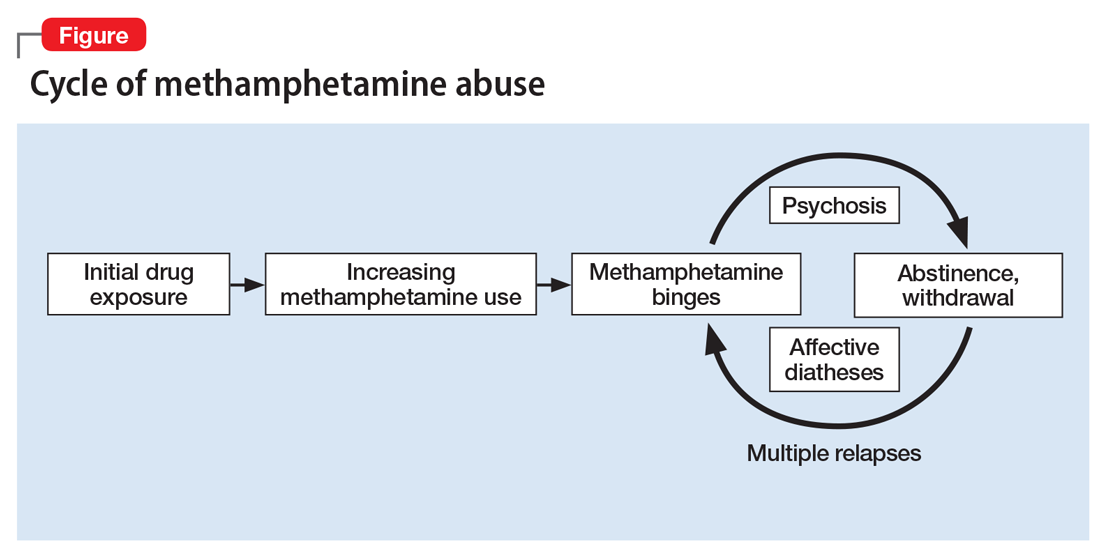
Methamphetamine causes psychiatric diseases that resemble naturally occurring illnesses but are more difficult to treat. Dependence occurs over a period of escalating use (Figure). Long-term exposure to the drug has been shown to cause severe neurotoxic and neuropathological effects with consequent disturbances in several cognitive domains.4
Despite advances in understanding the basic neurobiology of methamphetamine-induced effects on the brain, much remains to be done to translate this knowledge to treating patients and the complications that result from chronic abuse of this stimulant. In this review, we:
- provide a brief synopsis of the clinical presentation of patients who use methamphetamine
- describe some of the complications of methamphetamine abuse/dependence, focusing on methamphetamine-induced psychosis
- suggest ways to approach the treatment of these patients, including those with methamphetamine-induced psychosis.
Acute effects of methamphetamine use
Psychiatric symptoms. Patients under the influence of methamphetamine may present with clinical symptoms that mimic psychiatric disorders. For example, the drug can cause marked euphoria, hyperactivity, and disturbed speech patterns, thus mimicking a manic state. Patients also may present with anxiety, agitation, and irritability or aggressiveness. Although an individual may take methamphetamine for sexual enhancement, the drug can cause hypersexuality, which often is associated with unintended and unsafe sexual activities. These signs and symptoms are exacerbated during drug binges that can last for days, during which time large quantities of the drug are consumed.
Methamphetamine users may become preoccupied with their own thought patterns, and their actions can become compulsive and nonsensical. For example, a patient may become obsessed with an object of no specific value in his (her) environment, such as a doorknob or a cloud. Patients also may become suspicious of their friends and family members or think that police officers are after them. Less commonly, a patient also may suffer from poverty of speech, psychomotor retardation, and diminished social engagement similar to that reported in some patients with schizophrenia with deficit syndrome. Usually, acute symptoms will last 4 to 7 days after drug cessation, and then resolve completely with protracted abstinence from the drug.
Neurologic signs of methamphetamine use include hemorrhagic strokes in young people without any evidence of previous neurologic impairments. Studies have documented similarities between methamphetamine-induced neurotoxicity and traumatic brain injury.5 Postmortem studies have reported the presence of arteriovenous malformation in some patients with hemorrhagic strokes.
Hyperthermia is a dangerous acute effect of methamphetamine use. High body temperatures can cause both peripheral and central abnormalities, including muscular and cardiovascular dysfunction, renal failure secondary to rhabdomyolysis, heat stroke, and other heat-induced malignant syndromes. Some of the central dysfunctions may be related to heat-induced production of free radicals in various brain regions. There are no pharmacologic treatments for methamphetamine-induced thermal dysregulation.6 Therefore, clinicians need to focus on reducing body temperature by using cooling fans or cold water baths. Efforts should be made to avoid overhydrating patients because of the risk of developing the syndrome of inappropriate antidiuretic hormone secretion.
Chronic methamphetamine abuse
Psychosis is a long-term complication of chronic abuse of the drug.7 Although psychosis has been a reported complication of methamphetamine use since the 1950s,8 most of the subsequent literature is from Japan, where methamphetamine use was highly prevalent after World War II.9,10 The prevalence of methamphetamine-induced psychosis in methamphetamine-dependent patients varies from 13% (in the United States11) to 50% (in Asia12). This difference might be related to variability in the purity of methamphetamine used in different locations.
Methamphetamine users may experience a pre-psychotic state that consists of ideas of reference and delusional moods. This is followed by a psychotic state that includes hallucinations and delusions. The time it takes to develop these symptoms can vary from a few months up to >20 years after starting to use methamphetamine.10,13 Psychosis can occur in patients who do not have a history of psychiatric illness.10
The clinical presentation of methamphetamine-induced psychosis includes delusions of reference and persecutions.8-10 Paranoid delusions may be accompanied by violent behavior. Some patients may present with grandiose or jealousy delusions. Patients may experience auditory, tactile, or visual hallucinations. They may exhibit mania and logorrheic verbal outputs, symptoms consistent with a diagnosis of methamphetamine-induced mood disorder with manic features. Patients who use large daily doses of the drug also may report that there are ants or other parasites crawling under their skin (eg, formication, “meth mites”) and might present with infected excoriations of their skin as a result of attempting to remove insects. This is clinically important because penicillin-resistant bacteria are common in patients who use methamphetamine, and strains tend to be virulent.
Psychotic symptoms can last from a few days to several weeks after stopping methamphetamine use, although methamphetamine-induced psychosis can persist after long periods of abstinence.14 Psychotic symptoms may recur with re-exposure to the drug9 or repeated stressful life events.15 Patients with recurrent psychosis in the absence of a drug trigger appear to have high levels of peripheral norepinephrine.15 Patients with psychosis caused by long-term methamphetamine use will not necessarily show signs of sympathomimetic dysfunction because they may not have any methamphetamine in the body when they first present for clinical evaluation. Importantly, patients with methamphetamine-induced psychosis have been reported to have poor outcomes at follow-up.16 They have an increased risk of suicide, recurrent drug-induced psychosis, and comorbid alcohol abuse.16
Doses required to induce psychosis vary from patient to patient and may depend on the patient’s genetic background and/or environmental conditions. Methamphetamine can increase the severity of many psychiatric symptoms17 and may expedite the development of schizophrenia in first-degree relatives of patients with schizophrenia.18
The diagnosis of methamphetamine-induced psychosis should focus on differentiating it from schizophrenia. Wang et al19 found similar patterns of delusions in patients with schizophrenia and those with methamphetamine-induced psychosis. However, compared with patients with schizophrenia, patients with methamphetamine-induced psychosis have a higher prevalence of visual and tactile hallucinations, and less disorganization, blunted affect, and motor retardation. Some patients may present with depression and suicidal ideation; these features may be more prominent during withdrawal, but also may be obvious during periods of active use.16
Although these clinical features may be helpful initially, more comparative neurobiologic investigations are needed to identify potential biologic differences between schizophrenia and methamphetamine-induced psychosis because these differences will impact therapeutic approaches to these diverse population groups.
Neurologic complications. Chronic methamphetamine users may develop various neurologic disorders.20 They may present with stereotypies involving finger movements or repeated rubbing of mouth or face, orofacial dyskinesia, and choreoathetoid movements reminiscent of classical neurologic disorders. These movement disorders can persist after cessation of methamphetamine use. In some cases, these movement abnormalities may respond to dopamine receptor antagonists such as haloperidol.
Neuropsychological findings. Chronic methamphetamine users show mild signs of cognitive decline that affects a broad range of neuropsychological functions.21-23 There are deficits in several cognitive processes that are dependent on the function of frontostriatal and limbic circuits.24-26 Specifically, episodic memory, executive functions, complex information processing speed, and psychomotor functions all have been reported to be negatively impacted.
Methamphetamine use often results in psychiatric distress that impacts users’ interpersonal relationships.27 Additionally, impulsivity may exacerbate their psychosocial difficulties and promote maintenance of drug-seeking behaviors.28 Cognitive deficits lead to poor health outcomes, high-risk behaviors, employment difficulties, and repeated relapse.29,30
Partial recovery of neuropsychological functioning and improvement in affective distress can be achieved after sustained abstinence from methamphetamine, but recovery may not be complete. Because cognitive dysfunction can influence treatment outcomes, clinicians need to be fully aware of the cognitive status of those patients, and a thorough neuropsychological evaluation is necessary before initiating treatment.
Treatment
Methamphetamine abuse. Because patients who abuse methamphetamine are at high risk of developing psychosis, neurologic complications, and neuropsychological disorders, initiating treatment early in the course of their addiction is of paramount importance. Treatment of methamphetamine addiction is complicated by the fact that these patients have a high prevalence of comorbid psychiatric disorders, which clinicians need to keep in mind when selecting therapeutic interventions.
There are no FDA-approved agents for treating methamphetamine abuse.31 Several drugs have been tried with varying degrees of success, including bupropion, modafinil, and naltrexone. A study of modafinil found no clinically significant effects for treating methamphetamine abuse; however, only approximately one-half of participants in this study took modafinil as instructed.32 Certain selective serotonin reuptake inhibitors, including fluoxetine and paroxetine, have not been shown to be effective in treating these patients. Naltrexone may be a reasonable medication to consider because of the high prevalence of comorbid alcohol abuse among methamphetamine users.
Other treatments for methamphetamine addiction consist of behavioral interventions such as cognitive-behavioral therapy. Clinical experience has shown that the risk of relapse depends on how long the patient has been abstinent prior to entering a treatment program, the presence of attention and memory deficits, and findings of poor decision-making on neuropsychological tests.
The presence of cognitive abnormalities has been reported to impact methamphetamine abusers’ response to treatment.33 These findings suggest the need to develop approaches that might improve cognition in patients who are undergoing treatment for methamphetamine abuse. The monoaminergic agent modafinil and similar drugs need to be evaluated in large populations to increase the possibility of identifying characteristics of patients who might respond to cognitive enhancement.34
Methamphetamine-induced psychosis. First-generation antipsychotics, such as haloperidol or fluphenazine, need to be used sparingly in patients with methamphetamine-induced psychosis because of the risk of developing extrapyramidal symptoms (EPS) and because these patients are prone to develop motor complications as a result of methamphetamine abuse. Second-generation antipsychotics, such as risperidone and olanzapine, may be more appropriate because of the lower risks of EPS.35 The presence of high norepinephrine levels in some patients with recurrent methamphetamine psychosis suggests that drugs that block norepinephrine receptors, such as prazosin or propranolol, might be of therapeutic benefit if they are shown to be effective in controlled clinical trials.
1. United Nations Office on Drugs and Crime. World Drug Report 2016. United Nations publication, Sales No. E.16.XI.7. http://www.unodc.org/wdr2016. Published 2016. Accessed September 28, 2017.
2. Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60(2):379-407.
3. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760-773.
4. Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol. 2014;127(1):91-107.
5. Gold MS, Kobeissy FH, Wang KK, et al. Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol Psychiatry. 2009;66(2):118-127.
6. Gold MS, Graham NA, Kobeissy FH, et al. Speed, cocaine, and other psychostimulants death rates. Am J Cardiol. 2007;100(7):1184.
7. Shelly J, Uhlmann A, Sinclair H, et al. First-rank symptoms in methamphetamine psychosis and schizophrenia. Psychopathology. 2016;49(6):429-435.
8. Connell PH. Amphetamine psychosis. In: Connell PH. Maudsley monographs. No. 5. London, United Kingdom: Oxford Press; 1958:5.
9. Sato M. A lasting vulnerability to psychosis in patients with previous methamphetamine psychosis. Ann N Y Acad Sci. 1992;654(1):160-170.
10. Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann N Y Acad Sci. 2004;1025(1):279-287.
11. Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, et al; Methamphetamine Treatment Project Corporate Authors. Psychopathology in methamphetamine-dependent adults 3 years after treatment. Drug Alcohol Rev. 2010;29(1):12-20.
12. Sulaiman AH, Said MA, Habil MH, et al. The risk and associated factors of methamphetamine psychosis in methamphetamine-dependent patients in Malaysia. Compr Psychiatry. 2014;55(suppl 1):S89-S94.
13. Fasihpour B, Molavi S, Shariat SV. Clinical features of inpatients with methamphetamine-induced psychosis. J Ment Health. 2013;22(4):341-349.
14. Akiyama K, Saito A, Shimoda K. Chronic methamphetamine psychosis after long-term abstinence in Japanese incarcerated patients. Am J Addict. 2011;20(3):240-249.
15. Yui K, Goto K, Ikemoto S, et al. Methamphetamine psychosis: spontaneous recurrence of paranoid-hallucinatory states and monoamine neurotransmitter function. J Clin Psychopharmacol. 1997;17(1):34-43.
16. Kittirattanapaiboon P, Mahatnirunkul S, Booncharoen H, et al. Long-term outcomes in methamphetamine psychosis patients after first hospitalisation. Drug Alcohol Rev. 2010;29(4):456-461.
17. McKetin R, Dawe S, Burns RA, et al. The profile of psychiatric symptoms exacerbated by methamphetamine use. Drug Alcohol Depend. 2016;161:104-109.
18. Li H, Lu Q, Xiao E, et al. Methamphetamine enhances the development of schizophrenia in first-degree relatives of patients with schizophrenia. Can J Psychiatry. 2014;59(2):107-113.
19. Wang LJ, Lin SK, Chen YC, et al. Differences in clinical features of methamphetamine users with persistent psychosis and patients with schizophrenia. Psychopathology. 2016;49(2):108-115.
20. Rusyniak DE. Neurologic manifestations of chronic methamphetamine abuse. Psychiatr Clin North Am. 2013;36(2):261-275.
21. Simon SL, Domier C, Carnell J, et al. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9(3):222-231.
22. Paulus MP, Hozack NE, Zauscher BE, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26(1):53-63.
23. Rendell PG, Mazur M, Henry JD. Prospective memory impairment in former users of methamphetamine. Psychopharmacology (Berl). 2009;203(3):609-616.
24. Monterosso JR, Ainslie G, Xu J, et al. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28(5):383-393.
25. Nestor LJ, Ghahremani DG, Monterosso J, et al. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194(3):287-295.
26. Scott JC, Woods SP, Matt GE, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275-297.
27. Cretzmeyer M, Sarrazin MV, Huber DL, et al. Treatment of methamphetamine abuse: research findings and clinical directions. J Subst Abuse Treat. 2003;24(3):267-277.
28. Semple SJ, Zians J, Grant I, et al. Impulsivity and methamphetamine use. J Subst Abuse Treat. 2005;29(2):85-93.
29. Hester R, Lee N, Pennay A, et al. The effects of modafinil treatment on neuropsychological and attentional bias performance during 7-day inpatient withdrawal from methamphetamine dependence. Exp Clin Psychopharmacol. 2010;18(6):489-497.
30. Weber E, Blackstone K, Iudicello JE, et al; Translational Methamphetamine AIDS Research Center (TMARC) Group. Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend. 2012;125(1-2):146-153.
31. Ballester J, Valentine G, Sofuoglu M. Pharmacological treatments for methamphetamine addiction: current status and future directions. Expert Rev Clin Pharmacol. 2017;10(3):305-314.
32. Anderson AL, Li SH, Biswas K, et al. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120(1-3):135-141.
33. Cadet JL, Bisagno V. Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front Psychiatry. 2016;6:189.
34. Loland CJ, Mereu M, Okunola OM, et al. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry. 2012;72(5):405-413.
35. Farnia V, Shakeri J, Tatari F, et al. Randomized controlled trial of aripiprazole versus risperidone for the treatment of amphetamine-induced psychosis. Am J Drug Alcohol Abuse. 2014;40(1):10-15.
Use of methamphetamine, an N-methyl analog of amphetamine, is a serious public health problem; throughout the world an estimated 35.7 million people use the drug recreationally.1 Methamphetamine is easy to obtain because it is cheap to produce and can be synthesized anywhere. In the United States, methamphetamine is commonly manufactured in small-scale laboratories using relatively inexpensive, legally available ingredients. Large-scale manufacturing in clandestine laboratories also contributes to methamphetamine abuse. The drug, known as meth, crystal meth, ice, and other names, is available as a powder, tablet, or crystalline salt, and is used by various routes of administration (Table).
Although FDA-approved for treating attention-deficit/hyperactivity disorder, methamphetamine is taken recreationally for its euphoric effects; however, it also produces anhedonia, paranoia, and a host of cognitive deficits and other adverse effects.
Methamphetamine causes psychiatric diseases that resemble naturally occurring illnesses but are more difficult to treat. Dependence occurs over a period of escalating use (Figure). Long-term exposure to the drug has been shown to cause severe neurotoxic and neuropathological effects with consequent disturbances in several cognitive domains.4

Despite advances in understanding the basic neurobiology of methamphetamine-induced effects on the brain, much remains to be done to translate this knowledge to treating patients and the complications that result from chronic abuse of this stimulant. In this review, we:
- provide a brief synopsis of the clinical presentation of patients who use methamphetamine
- describe some of the complications of methamphetamine abuse/dependence, focusing on methamphetamine-induced psychosis
- suggest ways to approach the treatment of these patients, including those with methamphetamine-induced psychosis.
Acute effects of methamphetamine use
Psychiatric symptoms. Patients under the influence of methamphetamine may present with clinical symptoms that mimic psychiatric disorders. For example, the drug can cause marked euphoria, hyperactivity, and disturbed speech patterns, thus mimicking a manic state. Patients also may present with anxiety, agitation, and irritability or aggressiveness. Although an individual may take methamphetamine for sexual enhancement, the drug can cause hypersexuality, which often is associated with unintended and unsafe sexual activities. These signs and symptoms are exacerbated during drug binges that can last for days, during which time large quantities of the drug are consumed.
Methamphetamine users may become preoccupied with their own thought patterns, and their actions can become compulsive and nonsensical. For example, a patient may become obsessed with an object of no specific value in his (her) environment, such as a doorknob or a cloud. Patients also may become suspicious of their friends and family members or think that police officers are after them. Less commonly, a patient also may suffer from poverty of speech, psychomotor retardation, and diminished social engagement similar to that reported in some patients with schizophrenia with deficit syndrome. Usually, acute symptoms will last 4 to 7 days after drug cessation, and then resolve completely with protracted abstinence from the drug.
Neurologic signs of methamphetamine use include hemorrhagic strokes in young people without any evidence of previous neurologic impairments. Studies have documented similarities between methamphetamine-induced neurotoxicity and traumatic brain injury.5 Postmortem studies have reported the presence of arteriovenous malformation in some patients with hemorrhagic strokes.
Hyperthermia is a dangerous acute effect of methamphetamine use. High body temperatures can cause both peripheral and central abnormalities, including muscular and cardiovascular dysfunction, renal failure secondary to rhabdomyolysis, heat stroke, and other heat-induced malignant syndromes. Some of the central dysfunctions may be related to heat-induced production of free radicals in various brain regions. There are no pharmacologic treatments for methamphetamine-induced thermal dysregulation.6 Therefore, clinicians need to focus on reducing body temperature by using cooling fans or cold water baths. Efforts should be made to avoid overhydrating patients because of the risk of developing the syndrome of inappropriate antidiuretic hormone secretion.
Chronic methamphetamine abuse
Psychosis is a long-term complication of chronic abuse of the drug.7 Although psychosis has been a reported complication of methamphetamine use since the 1950s,8 most of the subsequent literature is from Japan, where methamphetamine use was highly prevalent after World War II.9,10 The prevalence of methamphetamine-induced psychosis in methamphetamine-dependent patients varies from 13% (in the United States11) to 50% (in Asia12). This difference might be related to variability in the purity of methamphetamine used in different locations.
Methamphetamine users may experience a pre-psychotic state that consists of ideas of reference and delusional moods. This is followed by a psychotic state that includes hallucinations and delusions. The time it takes to develop these symptoms can vary from a few months up to >20 years after starting to use methamphetamine.10,13 Psychosis can occur in patients who do not have a history of psychiatric illness.10
The clinical presentation of methamphetamine-induced psychosis includes delusions of reference and persecutions.8-10 Paranoid delusions may be accompanied by violent behavior. Some patients may present with grandiose or jealousy delusions. Patients may experience auditory, tactile, or visual hallucinations. They may exhibit mania and logorrheic verbal outputs, symptoms consistent with a diagnosis of methamphetamine-induced mood disorder with manic features. Patients who use large daily doses of the drug also may report that there are ants or other parasites crawling under their skin (eg, formication, “meth mites”) and might present with infected excoriations of their skin as a result of attempting to remove insects. This is clinically important because penicillin-resistant bacteria are common in patients who use methamphetamine, and strains tend to be virulent.
Psychotic symptoms can last from a few days to several weeks after stopping methamphetamine use, although methamphetamine-induced psychosis can persist after long periods of abstinence.14 Psychotic symptoms may recur with re-exposure to the drug9 or repeated stressful life events.15 Patients with recurrent psychosis in the absence of a drug trigger appear to have high levels of peripheral norepinephrine.15 Patients with psychosis caused by long-term methamphetamine use will not necessarily show signs of sympathomimetic dysfunction because they may not have any methamphetamine in the body when they first present for clinical evaluation. Importantly, patients with methamphetamine-induced psychosis have been reported to have poor outcomes at follow-up.16 They have an increased risk of suicide, recurrent drug-induced psychosis, and comorbid alcohol abuse.16
Doses required to induce psychosis vary from patient to patient and may depend on the patient’s genetic background and/or environmental conditions. Methamphetamine can increase the severity of many psychiatric symptoms17 and may expedite the development of schizophrenia in first-degree relatives of patients with schizophrenia.18
The diagnosis of methamphetamine-induced psychosis should focus on differentiating it from schizophrenia. Wang et al19 found similar patterns of delusions in patients with schizophrenia and those with methamphetamine-induced psychosis. However, compared with patients with schizophrenia, patients with methamphetamine-induced psychosis have a higher prevalence of visual and tactile hallucinations, and less disorganization, blunted affect, and motor retardation. Some patients may present with depression and suicidal ideation; these features may be more prominent during withdrawal, but also may be obvious during periods of active use.16
Although these clinical features may be helpful initially, more comparative neurobiologic investigations are needed to identify potential biologic differences between schizophrenia and methamphetamine-induced psychosis because these differences will impact therapeutic approaches to these diverse population groups.
Neurologic complications. Chronic methamphetamine users may develop various neurologic disorders.20 They may present with stereotypies involving finger movements or repeated rubbing of mouth or face, orofacial dyskinesia, and choreoathetoid movements reminiscent of classical neurologic disorders. These movement disorders can persist after cessation of methamphetamine use. In some cases, these movement abnormalities may respond to dopamine receptor antagonists such as haloperidol.
Neuropsychological findings. Chronic methamphetamine users show mild signs of cognitive decline that affects a broad range of neuropsychological functions.21-23 There are deficits in several cognitive processes that are dependent on the function of frontostriatal and limbic circuits.24-26 Specifically, episodic memory, executive functions, complex information processing speed, and psychomotor functions all have been reported to be negatively impacted.
Methamphetamine use often results in psychiatric distress that impacts users’ interpersonal relationships.27 Additionally, impulsivity may exacerbate their psychosocial difficulties and promote maintenance of drug-seeking behaviors.28 Cognitive deficits lead to poor health outcomes, high-risk behaviors, employment difficulties, and repeated relapse.29,30
Partial recovery of neuropsychological functioning and improvement in affective distress can be achieved after sustained abstinence from methamphetamine, but recovery may not be complete. Because cognitive dysfunction can influence treatment outcomes, clinicians need to be fully aware of the cognitive status of those patients, and a thorough neuropsychological evaluation is necessary before initiating treatment.
Treatment
Methamphetamine abuse. Because patients who abuse methamphetamine are at high risk of developing psychosis, neurologic complications, and neuropsychological disorders, initiating treatment early in the course of their addiction is of paramount importance. Treatment of methamphetamine addiction is complicated by the fact that these patients have a high prevalence of comorbid psychiatric disorders, which clinicians need to keep in mind when selecting therapeutic interventions.
There are no FDA-approved agents for treating methamphetamine abuse.31 Several drugs have been tried with varying degrees of success, including bupropion, modafinil, and naltrexone. A study of modafinil found no clinically significant effects for treating methamphetamine abuse; however, only approximately one-half of participants in this study took modafinil as instructed.32 Certain selective serotonin reuptake inhibitors, including fluoxetine and paroxetine, have not been shown to be effective in treating these patients. Naltrexone may be a reasonable medication to consider because of the high prevalence of comorbid alcohol abuse among methamphetamine users.
Other treatments for methamphetamine addiction consist of behavioral interventions such as cognitive-behavioral therapy. Clinical experience has shown that the risk of relapse depends on how long the patient has been abstinent prior to entering a treatment program, the presence of attention and memory deficits, and findings of poor decision-making on neuropsychological tests.
The presence of cognitive abnormalities has been reported to impact methamphetamine abusers’ response to treatment.33 These findings suggest the need to develop approaches that might improve cognition in patients who are undergoing treatment for methamphetamine abuse. The monoaminergic agent modafinil and similar drugs need to be evaluated in large populations to increase the possibility of identifying characteristics of patients who might respond to cognitive enhancement.34
Methamphetamine-induced psychosis. First-generation antipsychotics, such as haloperidol or fluphenazine, need to be used sparingly in patients with methamphetamine-induced psychosis because of the risk of developing extrapyramidal symptoms (EPS) and because these patients are prone to develop motor complications as a result of methamphetamine abuse. Second-generation antipsychotics, such as risperidone and olanzapine, may be more appropriate because of the lower risks of EPS.35 The presence of high norepinephrine levels in some patients with recurrent methamphetamine psychosis suggests that drugs that block norepinephrine receptors, such as prazosin or propranolol, might be of therapeutic benefit if they are shown to be effective in controlled clinical trials.
Use of methamphetamine, an N-methyl analog of amphetamine, is a serious public health problem; throughout the world an estimated 35.7 million people use the drug recreationally.1 Methamphetamine is easy to obtain because it is cheap to produce and can be synthesized anywhere. In the United States, methamphetamine is commonly manufactured in small-scale laboratories using relatively inexpensive, legally available ingredients. Large-scale manufacturing in clandestine laboratories also contributes to methamphetamine abuse. The drug, known as meth, crystal meth, ice, and other names, is available as a powder, tablet, or crystalline salt, and is used by various routes of administration (Table).
Although FDA-approved for treating attention-deficit/hyperactivity disorder, methamphetamine is taken recreationally for its euphoric effects; however, it also produces anhedonia, paranoia, and a host of cognitive deficits and other adverse effects.
Methamphetamine causes psychiatric diseases that resemble naturally occurring illnesses but are more difficult to treat. Dependence occurs over a period of escalating use (Figure). Long-term exposure to the drug has been shown to cause severe neurotoxic and neuropathological effects with consequent disturbances in several cognitive domains.4

Despite advances in understanding the basic neurobiology of methamphetamine-induced effects on the brain, much remains to be done to translate this knowledge to treating patients and the complications that result from chronic abuse of this stimulant. In this review, we:
- provide a brief synopsis of the clinical presentation of patients who use methamphetamine
- describe some of the complications of methamphetamine abuse/dependence, focusing on methamphetamine-induced psychosis
- suggest ways to approach the treatment of these patients, including those with methamphetamine-induced psychosis.
Acute effects of methamphetamine use
Psychiatric symptoms. Patients under the influence of methamphetamine may present with clinical symptoms that mimic psychiatric disorders. For example, the drug can cause marked euphoria, hyperactivity, and disturbed speech patterns, thus mimicking a manic state. Patients also may present with anxiety, agitation, and irritability or aggressiveness. Although an individual may take methamphetamine for sexual enhancement, the drug can cause hypersexuality, which often is associated with unintended and unsafe sexual activities. These signs and symptoms are exacerbated during drug binges that can last for days, during which time large quantities of the drug are consumed.
Methamphetamine users may become preoccupied with their own thought patterns, and their actions can become compulsive and nonsensical. For example, a patient may become obsessed with an object of no specific value in his (her) environment, such as a doorknob or a cloud. Patients also may become suspicious of their friends and family members or think that police officers are after them. Less commonly, a patient also may suffer from poverty of speech, psychomotor retardation, and diminished social engagement similar to that reported in some patients with schizophrenia with deficit syndrome. Usually, acute symptoms will last 4 to 7 days after drug cessation, and then resolve completely with protracted abstinence from the drug.
Neurologic signs of methamphetamine use include hemorrhagic strokes in young people without any evidence of previous neurologic impairments. Studies have documented similarities between methamphetamine-induced neurotoxicity and traumatic brain injury.5 Postmortem studies have reported the presence of arteriovenous malformation in some patients with hemorrhagic strokes.
Hyperthermia is a dangerous acute effect of methamphetamine use. High body temperatures can cause both peripheral and central abnormalities, including muscular and cardiovascular dysfunction, renal failure secondary to rhabdomyolysis, heat stroke, and other heat-induced malignant syndromes. Some of the central dysfunctions may be related to heat-induced production of free radicals in various brain regions. There are no pharmacologic treatments for methamphetamine-induced thermal dysregulation.6 Therefore, clinicians need to focus on reducing body temperature by using cooling fans or cold water baths. Efforts should be made to avoid overhydrating patients because of the risk of developing the syndrome of inappropriate antidiuretic hormone secretion.
Chronic methamphetamine abuse
Psychosis is a long-term complication of chronic abuse of the drug.7 Although psychosis has been a reported complication of methamphetamine use since the 1950s,8 most of the subsequent literature is from Japan, where methamphetamine use was highly prevalent after World War II.9,10 The prevalence of methamphetamine-induced psychosis in methamphetamine-dependent patients varies from 13% (in the United States11) to 50% (in Asia12). This difference might be related to variability in the purity of methamphetamine used in different locations.
Methamphetamine users may experience a pre-psychotic state that consists of ideas of reference and delusional moods. This is followed by a psychotic state that includes hallucinations and delusions. The time it takes to develop these symptoms can vary from a few months up to >20 years after starting to use methamphetamine.10,13 Psychosis can occur in patients who do not have a history of psychiatric illness.10
The clinical presentation of methamphetamine-induced psychosis includes delusions of reference and persecutions.8-10 Paranoid delusions may be accompanied by violent behavior. Some patients may present with grandiose or jealousy delusions. Patients may experience auditory, tactile, or visual hallucinations. They may exhibit mania and logorrheic verbal outputs, symptoms consistent with a diagnosis of methamphetamine-induced mood disorder with manic features. Patients who use large daily doses of the drug also may report that there are ants or other parasites crawling under their skin (eg, formication, “meth mites”) and might present with infected excoriations of their skin as a result of attempting to remove insects. This is clinically important because penicillin-resistant bacteria are common in patients who use methamphetamine, and strains tend to be virulent.
Psychotic symptoms can last from a few days to several weeks after stopping methamphetamine use, although methamphetamine-induced psychosis can persist after long periods of abstinence.14 Psychotic symptoms may recur with re-exposure to the drug9 or repeated stressful life events.15 Patients with recurrent psychosis in the absence of a drug trigger appear to have high levels of peripheral norepinephrine.15 Patients with psychosis caused by long-term methamphetamine use will not necessarily show signs of sympathomimetic dysfunction because they may not have any methamphetamine in the body when they first present for clinical evaluation. Importantly, patients with methamphetamine-induced psychosis have been reported to have poor outcomes at follow-up.16 They have an increased risk of suicide, recurrent drug-induced psychosis, and comorbid alcohol abuse.16
Doses required to induce psychosis vary from patient to patient and may depend on the patient’s genetic background and/or environmental conditions. Methamphetamine can increase the severity of many psychiatric symptoms17 and may expedite the development of schizophrenia in first-degree relatives of patients with schizophrenia.18
The diagnosis of methamphetamine-induced psychosis should focus on differentiating it from schizophrenia. Wang et al19 found similar patterns of delusions in patients with schizophrenia and those with methamphetamine-induced psychosis. However, compared with patients with schizophrenia, patients with methamphetamine-induced psychosis have a higher prevalence of visual and tactile hallucinations, and less disorganization, blunted affect, and motor retardation. Some patients may present with depression and suicidal ideation; these features may be more prominent during withdrawal, but also may be obvious during periods of active use.16
Although these clinical features may be helpful initially, more comparative neurobiologic investigations are needed to identify potential biologic differences between schizophrenia and methamphetamine-induced psychosis because these differences will impact therapeutic approaches to these diverse population groups.
Neurologic complications. Chronic methamphetamine users may develop various neurologic disorders.20 They may present with stereotypies involving finger movements or repeated rubbing of mouth or face, orofacial dyskinesia, and choreoathetoid movements reminiscent of classical neurologic disorders. These movement disorders can persist after cessation of methamphetamine use. In some cases, these movement abnormalities may respond to dopamine receptor antagonists such as haloperidol.
Neuropsychological findings. Chronic methamphetamine users show mild signs of cognitive decline that affects a broad range of neuropsychological functions.21-23 There are deficits in several cognitive processes that are dependent on the function of frontostriatal and limbic circuits.24-26 Specifically, episodic memory, executive functions, complex information processing speed, and psychomotor functions all have been reported to be negatively impacted.
Methamphetamine use often results in psychiatric distress that impacts users’ interpersonal relationships.27 Additionally, impulsivity may exacerbate their psychosocial difficulties and promote maintenance of drug-seeking behaviors.28 Cognitive deficits lead to poor health outcomes, high-risk behaviors, employment difficulties, and repeated relapse.29,30
Partial recovery of neuropsychological functioning and improvement in affective distress can be achieved after sustained abstinence from methamphetamine, but recovery may not be complete. Because cognitive dysfunction can influence treatment outcomes, clinicians need to be fully aware of the cognitive status of those patients, and a thorough neuropsychological evaluation is necessary before initiating treatment.
Treatment
Methamphetamine abuse. Because patients who abuse methamphetamine are at high risk of developing psychosis, neurologic complications, and neuropsychological disorders, initiating treatment early in the course of their addiction is of paramount importance. Treatment of methamphetamine addiction is complicated by the fact that these patients have a high prevalence of comorbid psychiatric disorders, which clinicians need to keep in mind when selecting therapeutic interventions.
There are no FDA-approved agents for treating methamphetamine abuse.31 Several drugs have been tried with varying degrees of success, including bupropion, modafinil, and naltrexone. A study of modafinil found no clinically significant effects for treating methamphetamine abuse; however, only approximately one-half of participants in this study took modafinil as instructed.32 Certain selective serotonin reuptake inhibitors, including fluoxetine and paroxetine, have not been shown to be effective in treating these patients. Naltrexone may be a reasonable medication to consider because of the high prevalence of comorbid alcohol abuse among methamphetamine users.
Other treatments for methamphetamine addiction consist of behavioral interventions such as cognitive-behavioral therapy. Clinical experience has shown that the risk of relapse depends on how long the patient has been abstinent prior to entering a treatment program, the presence of attention and memory deficits, and findings of poor decision-making on neuropsychological tests.
The presence of cognitive abnormalities has been reported to impact methamphetamine abusers’ response to treatment.33 These findings suggest the need to develop approaches that might improve cognition in patients who are undergoing treatment for methamphetamine abuse. The monoaminergic agent modafinil and similar drugs need to be evaluated in large populations to increase the possibility of identifying characteristics of patients who might respond to cognitive enhancement.34
Methamphetamine-induced psychosis. First-generation antipsychotics, such as haloperidol or fluphenazine, need to be used sparingly in patients with methamphetamine-induced psychosis because of the risk of developing extrapyramidal symptoms (EPS) and because these patients are prone to develop motor complications as a result of methamphetamine abuse. Second-generation antipsychotics, such as risperidone and olanzapine, may be more appropriate because of the lower risks of EPS.35 The presence of high norepinephrine levels in some patients with recurrent methamphetamine psychosis suggests that drugs that block norepinephrine receptors, such as prazosin or propranolol, might be of therapeutic benefit if they are shown to be effective in controlled clinical trials.
1. United Nations Office on Drugs and Crime. World Drug Report 2016. United Nations publication, Sales No. E.16.XI.7. http://www.unodc.org/wdr2016. Published 2016. Accessed September 28, 2017.
2. Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60(2):379-407.
3. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760-773.
4. Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol. 2014;127(1):91-107.
5. Gold MS, Kobeissy FH, Wang KK, et al. Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol Psychiatry. 2009;66(2):118-127.
6. Gold MS, Graham NA, Kobeissy FH, et al. Speed, cocaine, and other psychostimulants death rates. Am J Cardiol. 2007;100(7):1184.
7. Shelly J, Uhlmann A, Sinclair H, et al. First-rank symptoms in methamphetamine psychosis and schizophrenia. Psychopathology. 2016;49(6):429-435.
8. Connell PH. Amphetamine psychosis. In: Connell PH. Maudsley monographs. No. 5. London, United Kingdom: Oxford Press; 1958:5.
9. Sato M. A lasting vulnerability to psychosis in patients with previous methamphetamine psychosis. Ann N Y Acad Sci. 1992;654(1):160-170.
10. Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann N Y Acad Sci. 2004;1025(1):279-287.
11. Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, et al; Methamphetamine Treatment Project Corporate Authors. Psychopathology in methamphetamine-dependent adults 3 years after treatment. Drug Alcohol Rev. 2010;29(1):12-20.
12. Sulaiman AH, Said MA, Habil MH, et al. The risk and associated factors of methamphetamine psychosis in methamphetamine-dependent patients in Malaysia. Compr Psychiatry. 2014;55(suppl 1):S89-S94.
13. Fasihpour B, Molavi S, Shariat SV. Clinical features of inpatients with methamphetamine-induced psychosis. J Ment Health. 2013;22(4):341-349.
14. Akiyama K, Saito A, Shimoda K. Chronic methamphetamine psychosis after long-term abstinence in Japanese incarcerated patients. Am J Addict. 2011;20(3):240-249.
15. Yui K, Goto K, Ikemoto S, et al. Methamphetamine psychosis: spontaneous recurrence of paranoid-hallucinatory states and monoamine neurotransmitter function. J Clin Psychopharmacol. 1997;17(1):34-43.
16. Kittirattanapaiboon P, Mahatnirunkul S, Booncharoen H, et al. Long-term outcomes in methamphetamine psychosis patients after first hospitalisation. Drug Alcohol Rev. 2010;29(4):456-461.
17. McKetin R, Dawe S, Burns RA, et al. The profile of psychiatric symptoms exacerbated by methamphetamine use. Drug Alcohol Depend. 2016;161:104-109.
18. Li H, Lu Q, Xiao E, et al. Methamphetamine enhances the development of schizophrenia in first-degree relatives of patients with schizophrenia. Can J Psychiatry. 2014;59(2):107-113.
19. Wang LJ, Lin SK, Chen YC, et al. Differences in clinical features of methamphetamine users with persistent psychosis and patients with schizophrenia. Psychopathology. 2016;49(2):108-115.
20. Rusyniak DE. Neurologic manifestations of chronic methamphetamine abuse. Psychiatr Clin North Am. 2013;36(2):261-275.
21. Simon SL, Domier C, Carnell J, et al. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9(3):222-231.
22. Paulus MP, Hozack NE, Zauscher BE, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26(1):53-63.
23. Rendell PG, Mazur M, Henry JD. Prospective memory impairment in former users of methamphetamine. Psychopharmacology (Berl). 2009;203(3):609-616.
24. Monterosso JR, Ainslie G, Xu J, et al. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28(5):383-393.
25. Nestor LJ, Ghahremani DG, Monterosso J, et al. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194(3):287-295.
26. Scott JC, Woods SP, Matt GE, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275-297.
27. Cretzmeyer M, Sarrazin MV, Huber DL, et al. Treatment of methamphetamine abuse: research findings and clinical directions. J Subst Abuse Treat. 2003;24(3):267-277.
28. Semple SJ, Zians J, Grant I, et al. Impulsivity and methamphetamine use. J Subst Abuse Treat. 2005;29(2):85-93.
29. Hester R, Lee N, Pennay A, et al. The effects of modafinil treatment on neuropsychological and attentional bias performance during 7-day inpatient withdrawal from methamphetamine dependence. Exp Clin Psychopharmacol. 2010;18(6):489-497.
30. Weber E, Blackstone K, Iudicello JE, et al; Translational Methamphetamine AIDS Research Center (TMARC) Group. Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend. 2012;125(1-2):146-153.
31. Ballester J, Valentine G, Sofuoglu M. Pharmacological treatments for methamphetamine addiction: current status and future directions. Expert Rev Clin Pharmacol. 2017;10(3):305-314.
32. Anderson AL, Li SH, Biswas K, et al. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120(1-3):135-141.
33. Cadet JL, Bisagno V. Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front Psychiatry. 2016;6:189.
34. Loland CJ, Mereu M, Okunola OM, et al. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry. 2012;72(5):405-413.
35. Farnia V, Shakeri J, Tatari F, et al. Randomized controlled trial of aripiprazole versus risperidone for the treatment of amphetamine-induced psychosis. Am J Drug Alcohol Abuse. 2014;40(1):10-15.
1. United Nations Office on Drugs and Crime. World Drug Report 2016. United Nations publication, Sales No. E.16.XI.7. http://www.unodc.org/wdr2016. Published 2016. Accessed September 28, 2017.
2. Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60(2):379-407.
3. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760-773.
4. Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol. 2014;127(1):91-107.
5. Gold MS, Kobeissy FH, Wang KK, et al. Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol Psychiatry. 2009;66(2):118-127.
6. Gold MS, Graham NA, Kobeissy FH, et al. Speed, cocaine, and other psychostimulants death rates. Am J Cardiol. 2007;100(7):1184.
7. Shelly J, Uhlmann A, Sinclair H, et al. First-rank symptoms in methamphetamine psychosis and schizophrenia. Psychopathology. 2016;49(6):429-435.
8. Connell PH. Amphetamine psychosis. In: Connell PH. Maudsley monographs. No. 5. London, United Kingdom: Oxford Press; 1958:5.
9. Sato M. A lasting vulnerability to psychosis in patients with previous methamphetamine psychosis. Ann N Y Acad Sci. 1992;654(1):160-170.
10. Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann N Y Acad Sci. 2004;1025(1):279-287.
11. Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, et al; Methamphetamine Treatment Project Corporate Authors. Psychopathology in methamphetamine-dependent adults 3 years after treatment. Drug Alcohol Rev. 2010;29(1):12-20.
12. Sulaiman AH, Said MA, Habil MH, et al. The risk and associated factors of methamphetamine psychosis in methamphetamine-dependent patients in Malaysia. Compr Psychiatry. 2014;55(suppl 1):S89-S94.
13. Fasihpour B, Molavi S, Shariat SV. Clinical features of inpatients with methamphetamine-induced psychosis. J Ment Health. 2013;22(4):341-349.
14. Akiyama K, Saito A, Shimoda K. Chronic methamphetamine psychosis after long-term abstinence in Japanese incarcerated patients. Am J Addict. 2011;20(3):240-249.
15. Yui K, Goto K, Ikemoto S, et al. Methamphetamine psychosis: spontaneous recurrence of paranoid-hallucinatory states and monoamine neurotransmitter function. J Clin Psychopharmacol. 1997;17(1):34-43.
16. Kittirattanapaiboon P, Mahatnirunkul S, Booncharoen H, et al. Long-term outcomes in methamphetamine psychosis patients after first hospitalisation. Drug Alcohol Rev. 2010;29(4):456-461.
17. McKetin R, Dawe S, Burns RA, et al. The profile of psychiatric symptoms exacerbated by methamphetamine use. Drug Alcohol Depend. 2016;161:104-109.
18. Li H, Lu Q, Xiao E, et al. Methamphetamine enhances the development of schizophrenia in first-degree relatives of patients with schizophrenia. Can J Psychiatry. 2014;59(2):107-113.
19. Wang LJ, Lin SK, Chen YC, et al. Differences in clinical features of methamphetamine users with persistent psychosis and patients with schizophrenia. Psychopathology. 2016;49(2):108-115.
20. Rusyniak DE. Neurologic manifestations of chronic methamphetamine abuse. Psychiatr Clin North Am. 2013;36(2):261-275.
21. Simon SL, Domier C, Carnell J, et al. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9(3):222-231.
22. Paulus MP, Hozack NE, Zauscher BE, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26(1):53-63.
23. Rendell PG, Mazur M, Henry JD. Prospective memory impairment in former users of methamphetamine. Psychopharmacology (Berl). 2009;203(3):609-616.
24. Monterosso JR, Ainslie G, Xu J, et al. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28(5):383-393.
25. Nestor LJ, Ghahremani DG, Monterosso J, et al. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194(3):287-295.
26. Scott JC, Woods SP, Matt GE, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275-297.
27. Cretzmeyer M, Sarrazin MV, Huber DL, et al. Treatment of methamphetamine abuse: research findings and clinical directions. J Subst Abuse Treat. 2003;24(3):267-277.
28. Semple SJ, Zians J, Grant I, et al. Impulsivity and methamphetamine use. J Subst Abuse Treat. 2005;29(2):85-93.
29. Hester R, Lee N, Pennay A, et al. The effects of modafinil treatment on neuropsychological and attentional bias performance during 7-day inpatient withdrawal from methamphetamine dependence. Exp Clin Psychopharmacol. 2010;18(6):489-497.
30. Weber E, Blackstone K, Iudicello JE, et al; Translational Methamphetamine AIDS Research Center (TMARC) Group. Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend. 2012;125(1-2):146-153.
31. Ballester J, Valentine G, Sofuoglu M. Pharmacological treatments for methamphetamine addiction: current status and future directions. Expert Rev Clin Pharmacol. 2017;10(3):305-314.
32. Anderson AL, Li SH, Biswas K, et al. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120(1-3):135-141.
33. Cadet JL, Bisagno V. Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front Psychiatry. 2016;6:189.
34. Loland CJ, Mereu M, Okunola OM, et al. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry. 2012;72(5):405-413.
35. Farnia V, Shakeri J, Tatari F, et al. Randomized controlled trial of aripiprazole versus risperidone for the treatment of amphetamine-induced psychosis. Am J Drug Alcohol Abuse. 2014;40(1):10-15.