User login
FDA approves anti-CD38 with VMP in myeloma
The who are ineligible for autologous stem cell transplant (ASCT).
The drug is approved in combination with a standard VMP regimen – bortezomib (Velcade), melphalan, and prednisone. The FDA had granted priority review to the drug application in January 2018 based on the results of the phase 3 ALCYONE study (NCT02195479).
Daratumumab, an anti-CD38 monoclonal antibody, reduced the risk of disease progression or death by 50%, compared with VMP alone in the ALCYONE study. The median progression-free survival had not yet been reached in the daratumumab arm; the median progression-free survival was 18.1 months in the VMP-only arm (N Engl J Med. 2018;378:518-28).
Daratumumab is marketed by Janssen Biotech as Darzalex.
The who are ineligible for autologous stem cell transplant (ASCT).
The drug is approved in combination with a standard VMP regimen – bortezomib (Velcade), melphalan, and prednisone. The FDA had granted priority review to the drug application in January 2018 based on the results of the phase 3 ALCYONE study (NCT02195479).
Daratumumab, an anti-CD38 monoclonal antibody, reduced the risk of disease progression or death by 50%, compared with VMP alone in the ALCYONE study. The median progression-free survival had not yet been reached in the daratumumab arm; the median progression-free survival was 18.1 months in the VMP-only arm (N Engl J Med. 2018;378:518-28).
Daratumumab is marketed by Janssen Biotech as Darzalex.
The who are ineligible for autologous stem cell transplant (ASCT).
The drug is approved in combination with a standard VMP regimen – bortezomib (Velcade), melphalan, and prednisone. The FDA had granted priority review to the drug application in January 2018 based on the results of the phase 3 ALCYONE study (NCT02195479).
Daratumumab, an anti-CD38 monoclonal antibody, reduced the risk of disease progression or death by 50%, compared with VMP alone in the ALCYONE study. The median progression-free survival had not yet been reached in the daratumumab arm; the median progression-free survival was 18.1 months in the VMP-only arm (N Engl J Med. 2018;378:518-28).
Daratumumab is marketed by Janssen Biotech as Darzalex.
FDA places partial hold on trials after secondary lymphoma
The drugmaker after a pediatric patient developed a secondary T-cell lymphoma.
The Food and Drug Administration had issued a partial clinical hold in April on new enrollment of any patients with genetically defined solid tumors and hematologic malignancies. Patients already enrolled who have not had disease progression can continue to receive tazemetostat.
Tazemetostat is a first-in-class EZH2 inhibitor being studied as monotherapy in phase 1 and 2 trials for certain molecularly defined solid tumors, follicular lymphoma and diffuse large B-cell lymphoma, mesothelioma, and in combination studies of DLBCL and non–small cell lung cancer.
Epizyme is currently working to update informed consent, the investigator’s brochure, and study protocols, the company said in a statement.
The drugmaker after a pediatric patient developed a secondary T-cell lymphoma.
The Food and Drug Administration had issued a partial clinical hold in April on new enrollment of any patients with genetically defined solid tumors and hematologic malignancies. Patients already enrolled who have not had disease progression can continue to receive tazemetostat.
Tazemetostat is a first-in-class EZH2 inhibitor being studied as monotherapy in phase 1 and 2 trials for certain molecularly defined solid tumors, follicular lymphoma and diffuse large B-cell lymphoma, mesothelioma, and in combination studies of DLBCL and non–small cell lung cancer.
Epizyme is currently working to update informed consent, the investigator’s brochure, and study protocols, the company said in a statement.
The drugmaker after a pediatric patient developed a secondary T-cell lymphoma.
The Food and Drug Administration had issued a partial clinical hold in April on new enrollment of any patients with genetically defined solid tumors and hematologic malignancies. Patients already enrolled who have not had disease progression can continue to receive tazemetostat.
Tazemetostat is a first-in-class EZH2 inhibitor being studied as monotherapy in phase 1 and 2 trials for certain molecularly defined solid tumors, follicular lymphoma and diffuse large B-cell lymphoma, mesothelioma, and in combination studies of DLBCL and non–small cell lung cancer.
Epizyme is currently working to update informed consent, the investigator’s brochure, and study protocols, the company said in a statement.
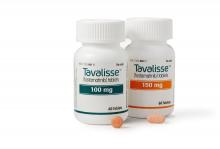
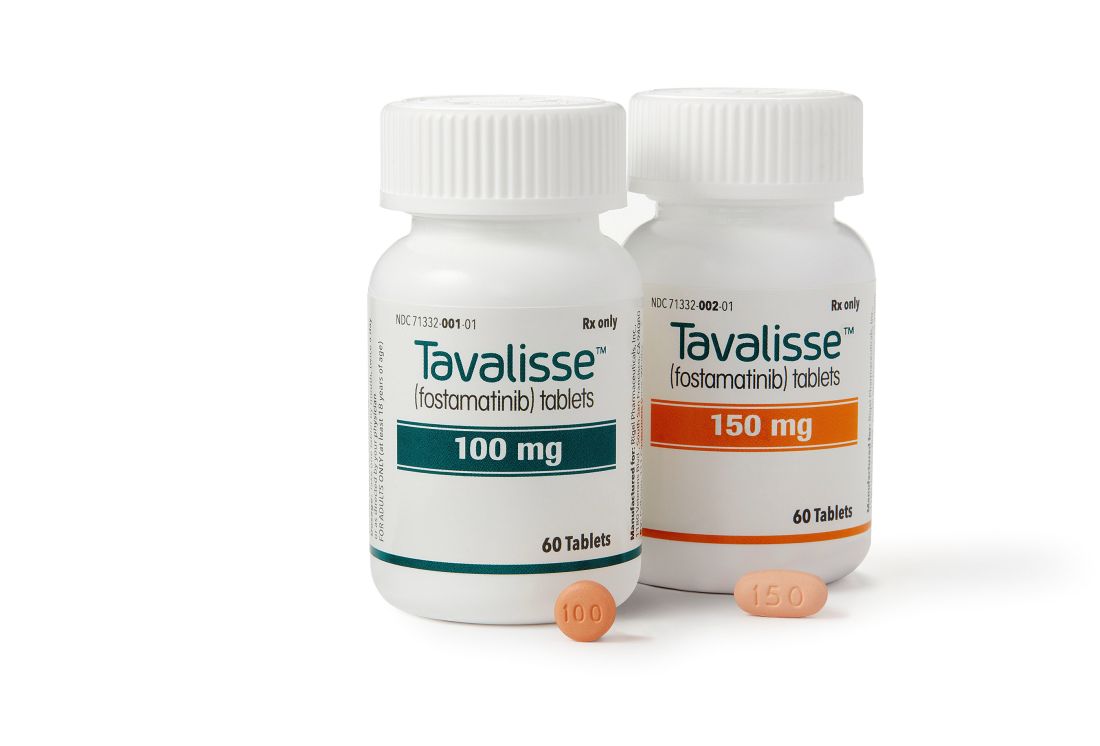
FDA approves new drug for thrombocytopenia
with chronic immune thrombocytopenia (ITP).
The Food and Drug Administration approved the oral spleen tyrosine kinase (SYK) inhibitor, which works by impeding platelet destruction, on April 17. Its approval was based on data from FIT clinical program, including two randomized placebo-controlled phase 3 trials.
Physicians are advised to monitor blood pressure and liver function with the drug. Fostamatinib may interact with CYP3A4 inhibitors and inducers. Concomitant use of fostamatinib with CYP3A4 inhibitors increases exposure to R406 – the drug’s major metabolite – and may increase the risk of adverse reactions. Use with strong CYP3A4 inducers is not recommended because it reduces exposure to R406.
Women of reproductive potential should be advised to use appropriate contraception while using fostamatinib and for a month after stopping the drug; pregnant women should be told of potential risk to the fetus. Advise women not to breastfeed during treatment and for at least 1 month after the last dose, according to a press statement.
Rigel Pharmaceuticals plans to release the drug in the United States in late May 2018.
with chronic immune thrombocytopenia (ITP).
The Food and Drug Administration approved the oral spleen tyrosine kinase (SYK) inhibitor, which works by impeding platelet destruction, on April 17. Its approval was based on data from FIT clinical program, including two randomized placebo-controlled phase 3 trials.
Physicians are advised to monitor blood pressure and liver function with the drug. Fostamatinib may interact with CYP3A4 inhibitors and inducers. Concomitant use of fostamatinib with CYP3A4 inhibitors increases exposure to R406 – the drug’s major metabolite – and may increase the risk of adverse reactions. Use with strong CYP3A4 inducers is not recommended because it reduces exposure to R406.
Women of reproductive potential should be advised to use appropriate contraception while using fostamatinib and for a month after stopping the drug; pregnant women should be told of potential risk to the fetus. Advise women not to breastfeed during treatment and for at least 1 month after the last dose, according to a press statement.
Rigel Pharmaceuticals plans to release the drug in the United States in late May 2018.
with chronic immune thrombocytopenia (ITP).
The Food and Drug Administration approved the oral spleen tyrosine kinase (SYK) inhibitor, which works by impeding platelet destruction, on April 17. Its approval was based on data from FIT clinical program, including two randomized placebo-controlled phase 3 trials.
Physicians are advised to monitor blood pressure and liver function with the drug. Fostamatinib may interact with CYP3A4 inhibitors and inducers. Concomitant use of fostamatinib with CYP3A4 inhibitors increases exposure to R406 – the drug’s major metabolite – and may increase the risk of adverse reactions. Use with strong CYP3A4 inducers is not recommended because it reduces exposure to R406.
Women of reproductive potential should be advised to use appropriate contraception while using fostamatinib and for a month after stopping the drug; pregnant women should be told of potential risk to the fetus. Advise women not to breastfeed during treatment and for at least 1 month after the last dose, according to a press statement.
Rigel Pharmaceuticals plans to release the drug in the United States in late May 2018.
PDPK1 could be novel target in MCL
Researchers may have found a new therapeutic approach for treating mantle cell lymphoma (MCL) by targeting 3-phosphoinositide-dependent protein kinase 1 (PDPK1).
Saori Maegawa and colleagues at Kyoto Prefectural University of Medicine in Japan, evaluated PDPK1 activity in patient-derived primary B-cell lymphoma cells by immunohistochemical staining of p-PDPK1Ser241 (p-PDPK1) in tissue specimens from seven patients with MCL, six patients with diffuse large B-cell lymphoma, and five patients with follicular lymphoma. All specimens were biopsied at initial diagnosis, before starting treatment.
“Our study showed that PDPK1 inhibition caused inactivation of RSK2-NTKD, as well as the decrease of total RSK2 protein, but not of AKT, in MCL-derived cells,” the researchers wrote in Experimental Hematology. “This implies that RSK2 activity is mainly regulated by PDPK1 at both the transcriptional expression and post-translational levels, but AKT activity is regulated by a signaling pathway that does not interact with a PDPK1-mediated pathway in MCL.”
If a PDPK1 inhibitor is pursued as clinical target, the researchers said careful monitoring for hyperglycemia may be required since impaired glucose metabolism is commonly seen with AKT inhibitors. Future research in MCL could also be directed toward the targeting of RSK2-NTKD, the researchers wrote.
SOURCE: Maegawa S et al. Exp Hematol. 2018 Mar;59:72-81.e2.
Researchers may have found a new therapeutic approach for treating mantle cell lymphoma (MCL) by targeting 3-phosphoinositide-dependent protein kinase 1 (PDPK1).
Saori Maegawa and colleagues at Kyoto Prefectural University of Medicine in Japan, evaluated PDPK1 activity in patient-derived primary B-cell lymphoma cells by immunohistochemical staining of p-PDPK1Ser241 (p-PDPK1) in tissue specimens from seven patients with MCL, six patients with diffuse large B-cell lymphoma, and five patients with follicular lymphoma. All specimens were biopsied at initial diagnosis, before starting treatment.
“Our study showed that PDPK1 inhibition caused inactivation of RSK2-NTKD, as well as the decrease of total RSK2 protein, but not of AKT, in MCL-derived cells,” the researchers wrote in Experimental Hematology. “This implies that RSK2 activity is mainly regulated by PDPK1 at both the transcriptional expression and post-translational levels, but AKT activity is regulated by a signaling pathway that does not interact with a PDPK1-mediated pathway in MCL.”
If a PDPK1 inhibitor is pursued as clinical target, the researchers said careful monitoring for hyperglycemia may be required since impaired glucose metabolism is commonly seen with AKT inhibitors. Future research in MCL could also be directed toward the targeting of RSK2-NTKD, the researchers wrote.
SOURCE: Maegawa S et al. Exp Hematol. 2018 Mar;59:72-81.e2.
Researchers may have found a new therapeutic approach for treating mantle cell lymphoma (MCL) by targeting 3-phosphoinositide-dependent protein kinase 1 (PDPK1).
Saori Maegawa and colleagues at Kyoto Prefectural University of Medicine in Japan, evaluated PDPK1 activity in patient-derived primary B-cell lymphoma cells by immunohistochemical staining of p-PDPK1Ser241 (p-PDPK1) in tissue specimens from seven patients with MCL, six patients with diffuse large B-cell lymphoma, and five patients with follicular lymphoma. All specimens were biopsied at initial diagnosis, before starting treatment.
“Our study showed that PDPK1 inhibition caused inactivation of RSK2-NTKD, as well as the decrease of total RSK2 protein, but not of AKT, in MCL-derived cells,” the researchers wrote in Experimental Hematology. “This implies that RSK2 activity is mainly regulated by PDPK1 at both the transcriptional expression and post-translational levels, but AKT activity is regulated by a signaling pathway that does not interact with a PDPK1-mediated pathway in MCL.”
If a PDPK1 inhibitor is pursued as clinical target, the researchers said careful monitoring for hyperglycemia may be required since impaired glucose metabolism is commonly seen with AKT inhibitors. Future research in MCL could also be directed toward the targeting of RSK2-NTKD, the researchers wrote.
SOURCE: Maegawa S et al. Exp Hematol. 2018 Mar;59:72-81.e2.
FROM EXPERIMENTAL HEMATOLOGY
FDA grants priority review of follicular lymphoma drug
by the Food and Drug Administration.
The biopharmaceutical company Verastem is seeking full approval for duvelisib for the treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and accelerated approval for the treatment of relapsed/refractory follicular lymphoma. The FDA has set Oct. 5, 2018, as the target action date, according to Verastem.
by the Food and Drug Administration.
The biopharmaceutical company Verastem is seeking full approval for duvelisib for the treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and accelerated approval for the treatment of relapsed/refractory follicular lymphoma. The FDA has set Oct. 5, 2018, as the target action date, according to Verastem.
by the Food and Drug Administration.
The biopharmaceutical company Verastem is seeking full approval for duvelisib for the treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and accelerated approval for the treatment of relapsed/refractory follicular lymphoma. The FDA has set Oct. 5, 2018, as the target action date, according to Verastem.
European Commission expands denosumab indication
The , making it is available for the prevention of skeletal-related events in adults with multiple myeloma and other advanced malignancies involving bone.
The European approval is based on the monoclonal antibody’s strong performance in a phase 3, international trial looking specifically at prevention of skeletal-related events in multiple myeloma patients.
During the trial, the drug demonstrated noninferiority to zoledronic acid in delaying the time to first skeletal-related event (hazard ratio, 0.98, 95% confidence interval: 0.85-1.14), according to Amgen, which markets denosumab. The median time to first skeletal-related event was 22.8 months for denosumab versus 24.0 months for zoledronic acid.
The denosumab indication was expanded to include prevention of skeletal-related events by the Food and Drug Administration in the United States in January 2018.
The , making it is available for the prevention of skeletal-related events in adults with multiple myeloma and other advanced malignancies involving bone.
The European approval is based on the monoclonal antibody’s strong performance in a phase 3, international trial looking specifically at prevention of skeletal-related events in multiple myeloma patients.
During the trial, the drug demonstrated noninferiority to zoledronic acid in delaying the time to first skeletal-related event (hazard ratio, 0.98, 95% confidence interval: 0.85-1.14), according to Amgen, which markets denosumab. The median time to first skeletal-related event was 22.8 months for denosumab versus 24.0 months for zoledronic acid.
The denosumab indication was expanded to include prevention of skeletal-related events by the Food and Drug Administration in the United States in January 2018.
The , making it is available for the prevention of skeletal-related events in adults with multiple myeloma and other advanced malignancies involving bone.
The European approval is based on the monoclonal antibody’s strong performance in a phase 3, international trial looking specifically at prevention of skeletal-related events in multiple myeloma patients.
During the trial, the drug demonstrated noninferiority to zoledronic acid in delaying the time to first skeletal-related event (hazard ratio, 0.98, 95% confidence interval: 0.85-1.14), according to Amgen, which markets denosumab. The median time to first skeletal-related event was 22.8 months for denosumab versus 24.0 months for zoledronic acid.
The denosumab indication was expanded to include prevention of skeletal-related events by the Food and Drug Administration in the United States in January 2018.
MRD may indicate relapse risk in AML
Detecting molecular minimal residual disease among patients in complete remission was a significant independent predictor of relapse and survival in acute myeloid leukemia (AML), findings from a new study suggest.
Mojca Jongen-Lavrencic, MD, PhD, of the Erasmus University in the Netherlands, and colleagues conducted targeted next-generation sequencing on bone marrow or peripheral blood samples to detect minimal residual disease in 482 newly diagnosed AML patients aged 18-65 years. The sampling was conducted at diagnosis and again after induction therapy. The study endpoints included 4-year rates of relapse, relapse-free survival, and overall survival. The findings are reported in the New England Journal of Medicine.
Overall, at least one mutation was found in 430 patients (89.2%). Persistent DTA mutations, which are associated with age-related hematopoiesis, were not significantly associated with higher 4-year relapse rates, compared with no detection of DTA mutations (P = .29). However, having a persistent DTA mutation and a coexisting persistent non-DTA mutation was a significant predictor of relapse, with a 4-year relapse rate of 66.7% versus 39.4% for no detection (P = .002).
Similarly, having a persistent non-DTA mutation at any allele frequency was linked to an increase risk of relapse at 4 years (55.7% with detection versus 34.6% without detection, P = .001). Non-DTA mutation was also significantly associated with reduced relapsed-free survival and reduced overall survival.
The study was funded by the Queen Wilhelmina Fund Foundation of the Dutch Cancer, among others. There was no commercial funding for the study.
SOURCE: Jongen-Lavrencic M et al. N Engl J Med. 2018 Mar 29;378:1189-99.
Detecting molecular minimal residual disease among patients in complete remission was a significant independent predictor of relapse and survival in acute myeloid leukemia (AML), findings from a new study suggest.
Mojca Jongen-Lavrencic, MD, PhD, of the Erasmus University in the Netherlands, and colleagues conducted targeted next-generation sequencing on bone marrow or peripheral blood samples to detect minimal residual disease in 482 newly diagnosed AML patients aged 18-65 years. The sampling was conducted at diagnosis and again after induction therapy. The study endpoints included 4-year rates of relapse, relapse-free survival, and overall survival. The findings are reported in the New England Journal of Medicine.
Overall, at least one mutation was found in 430 patients (89.2%). Persistent DTA mutations, which are associated with age-related hematopoiesis, were not significantly associated with higher 4-year relapse rates, compared with no detection of DTA mutations (P = .29). However, having a persistent DTA mutation and a coexisting persistent non-DTA mutation was a significant predictor of relapse, with a 4-year relapse rate of 66.7% versus 39.4% for no detection (P = .002).
Similarly, having a persistent non-DTA mutation at any allele frequency was linked to an increase risk of relapse at 4 years (55.7% with detection versus 34.6% without detection, P = .001). Non-DTA mutation was also significantly associated with reduced relapsed-free survival and reduced overall survival.
The study was funded by the Queen Wilhelmina Fund Foundation of the Dutch Cancer, among others. There was no commercial funding for the study.
SOURCE: Jongen-Lavrencic M et al. N Engl J Med. 2018 Mar 29;378:1189-99.
Detecting molecular minimal residual disease among patients in complete remission was a significant independent predictor of relapse and survival in acute myeloid leukemia (AML), findings from a new study suggest.
Mojca Jongen-Lavrencic, MD, PhD, of the Erasmus University in the Netherlands, and colleagues conducted targeted next-generation sequencing on bone marrow or peripheral blood samples to detect minimal residual disease in 482 newly diagnosed AML patients aged 18-65 years. The sampling was conducted at diagnosis and again after induction therapy. The study endpoints included 4-year rates of relapse, relapse-free survival, and overall survival. The findings are reported in the New England Journal of Medicine.
Overall, at least one mutation was found in 430 patients (89.2%). Persistent DTA mutations, which are associated with age-related hematopoiesis, were not significantly associated with higher 4-year relapse rates, compared with no detection of DTA mutations (P = .29). However, having a persistent DTA mutation and a coexisting persistent non-DTA mutation was a significant predictor of relapse, with a 4-year relapse rate of 66.7% versus 39.4% for no detection (P = .002).
Similarly, having a persistent non-DTA mutation at any allele frequency was linked to an increase risk of relapse at 4 years (55.7% with detection versus 34.6% without detection, P = .001). Non-DTA mutation was also significantly associated with reduced relapsed-free survival and reduced overall survival.
The study was funded by the Queen Wilhelmina Fund Foundation of the Dutch Cancer, among others. There was no commercial funding for the study.
SOURCE: Jongen-Lavrencic M et al. N Engl J Med. 2018 Mar 29;378:1189-99.
FROM NEW ENGLAND JOURNAL OF MEDICINE
VIDEO: How to prepare PTCL patients for transplant
LA JOLLA, CALIF. – according to Steven M. Horwitz, MD, of Memorial Sloan Kettering Cancer Center, New York.
“If you’re really trying to go to transplant, you want a complete remission or close to it. So that’s often been combination chemotherapy. But I think what we’re learning is, when some of the newer agents are combined, we’re seeing higher complete response rates. And we’re doing a better job at picking subtype specific approaches,” Dr. Horwitz said in a video interview at the annual T-cell Lymphoma Forum.
Dr. Horwitz also explored the role for reduced-intensity regimens in older patients, the use of radiation conditioning, and which new agents look most promising in peripheral T-cell lymphoma.
Dr. Horwitz had previously disclosed financial relationships with Celgene, Forty Seven, Huya Bioscience International, Infinity, Kyowa Hakko Kirin, Millennium, Seattle Genetics, and Takeda. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
SOURCE: Horwitz SM. TCLF 2018.
LA JOLLA, CALIF. – according to Steven M. Horwitz, MD, of Memorial Sloan Kettering Cancer Center, New York.
“If you’re really trying to go to transplant, you want a complete remission or close to it. So that’s often been combination chemotherapy. But I think what we’re learning is, when some of the newer agents are combined, we’re seeing higher complete response rates. And we’re doing a better job at picking subtype specific approaches,” Dr. Horwitz said in a video interview at the annual T-cell Lymphoma Forum.
Dr. Horwitz also explored the role for reduced-intensity regimens in older patients, the use of radiation conditioning, and which new agents look most promising in peripheral T-cell lymphoma.
Dr. Horwitz had previously disclosed financial relationships with Celgene, Forty Seven, Huya Bioscience International, Infinity, Kyowa Hakko Kirin, Millennium, Seattle Genetics, and Takeda. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
SOURCE: Horwitz SM. TCLF 2018.
LA JOLLA, CALIF. – according to Steven M. Horwitz, MD, of Memorial Sloan Kettering Cancer Center, New York.
“If you’re really trying to go to transplant, you want a complete remission or close to it. So that’s often been combination chemotherapy. But I think what we’re learning is, when some of the newer agents are combined, we’re seeing higher complete response rates. And we’re doing a better job at picking subtype specific approaches,” Dr. Horwitz said in a video interview at the annual T-cell Lymphoma Forum.
Dr. Horwitz also explored the role for reduced-intensity regimens in older patients, the use of radiation conditioning, and which new agents look most promising in peripheral T-cell lymphoma.
Dr. Horwitz had previously disclosed financial relationships with Celgene, Forty Seven, Huya Bioscience International, Infinity, Kyowa Hakko Kirin, Millennium, Seattle Genetics, and Takeda. The T-Cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
SOURCE: Horwitz SM. TCLF 2018.
REPORTING FROM TCLF 2018
FDA approves nilotinib for children with CML
Nilotinib is now approved for use by children aged 1 year and older with Philadelphia chromosome–positive chronic myeloid leukemia (Ph+ CML) in the chronic phase.
The Food and Drug Administration expanded the drug’s indication to include use as first- and second-line treatment in children.
Adverse events in the pediatric studies were similar to those observed in adults. However, children experienced hyperbilirubinemia (grade 3/4: 13%) and transaminase elevation (AST grade 3/4: 1%; ALT grade 3/4: 9%). Additionally, one previously treated pediatric patient progressed with advance phase/blast crisis after about 10 months of treatment.
Nilotinib (Tasigna) was already approved in adults with newly diagnosed Ph+ CML in the chronic phase and adults with chronic phase and accelerated phase Ph+ CML resistant or intolerant to prior therapy.
Nilotinib is now approved for use by children aged 1 year and older with Philadelphia chromosome–positive chronic myeloid leukemia (Ph+ CML) in the chronic phase.
The Food and Drug Administration expanded the drug’s indication to include use as first- and second-line treatment in children.
Adverse events in the pediatric studies were similar to those observed in adults. However, children experienced hyperbilirubinemia (grade 3/4: 13%) and transaminase elevation (AST grade 3/4: 1%; ALT grade 3/4: 9%). Additionally, one previously treated pediatric patient progressed with advance phase/blast crisis after about 10 months of treatment.
Nilotinib (Tasigna) was already approved in adults with newly diagnosed Ph+ CML in the chronic phase and adults with chronic phase and accelerated phase Ph+ CML resistant or intolerant to prior therapy.
Nilotinib is now approved for use by children aged 1 year and older with Philadelphia chromosome–positive chronic myeloid leukemia (Ph+ CML) in the chronic phase.
The Food and Drug Administration expanded the drug’s indication to include use as first- and second-line treatment in children.
Adverse events in the pediatric studies were similar to those observed in adults. However, children experienced hyperbilirubinemia (grade 3/4: 13%) and transaminase elevation (AST grade 3/4: 1%; ALT grade 3/4: 9%). Additionally, one previously treated pediatric patient progressed with advance phase/blast crisis after about 10 months of treatment.
Nilotinib (Tasigna) was already approved in adults with newly diagnosed Ph+ CML in the chronic phase and adults with chronic phase and accelerated phase Ph+ CML resistant or intolerant to prior therapy.
FDA approves new option in Hodgkin lymphoma treatment
The Food and Drug Administration has approved brentuximab vedotin, in combination with chemotherapy, for previously untreated adults with stage III or IV classical Hodgkin lymphoma.
The drug, which is marketed by Seattle Genetics as Adcetris, is already approved in classical Hodgkin lymphoma after relapse and after stem cell transplant when the patient is at risk of relapse or progression. The drug is also approved to treat both systemic anaplastic large cell lymphoma (ALCL) and primary cutaneous ALCL after failure on other treatments.
The modified 2-year progression-free survival in the trial was 82.1% for patients receiving brentuximab plus AVD versus 77.2% for ABVD (P = .03), a 23% relative risk reduction (N Engl J Med. 2018;378:331-44).
Common side effects of brentuximab vedotin include neutropenia, anemia, peripheral neuropathy, nausea, fatigue, constipation, diarrhea, vomiting, and pyrexia. The drug carries a boxed warning highlighting the risk of John Cunningham virus infection resulting in progressive multifocal leukoencephalopathy.
The Food and Drug Administration has approved brentuximab vedotin, in combination with chemotherapy, for previously untreated adults with stage III or IV classical Hodgkin lymphoma.
The drug, which is marketed by Seattle Genetics as Adcetris, is already approved in classical Hodgkin lymphoma after relapse and after stem cell transplant when the patient is at risk of relapse or progression. The drug is also approved to treat both systemic anaplastic large cell lymphoma (ALCL) and primary cutaneous ALCL after failure on other treatments.
The modified 2-year progression-free survival in the trial was 82.1% for patients receiving brentuximab plus AVD versus 77.2% for ABVD (P = .03), a 23% relative risk reduction (N Engl J Med. 2018;378:331-44).
Common side effects of brentuximab vedotin include neutropenia, anemia, peripheral neuropathy, nausea, fatigue, constipation, diarrhea, vomiting, and pyrexia. The drug carries a boxed warning highlighting the risk of John Cunningham virus infection resulting in progressive multifocal leukoencephalopathy.
The Food and Drug Administration has approved brentuximab vedotin, in combination with chemotherapy, for previously untreated adults with stage III or IV classical Hodgkin lymphoma.
The drug, which is marketed by Seattle Genetics as Adcetris, is already approved in classical Hodgkin lymphoma after relapse and after stem cell transplant when the patient is at risk of relapse or progression. The drug is also approved to treat both systemic anaplastic large cell lymphoma (ALCL) and primary cutaneous ALCL after failure on other treatments.
The modified 2-year progression-free survival in the trial was 82.1% for patients receiving brentuximab plus AVD versus 77.2% for ABVD (P = .03), a 23% relative risk reduction (N Engl J Med. 2018;378:331-44).
Common side effects of brentuximab vedotin include neutropenia, anemia, peripheral neuropathy, nausea, fatigue, constipation, diarrhea, vomiting, and pyrexia. The drug carries a boxed warning highlighting the risk of John Cunningham virus infection resulting in progressive multifocal leukoencephalopathy.