User login
A look at the long-term safety of an extended-regimen OC
Abstract
Background: Oral contraceptives (OCs) are the most widely used method of reversible contraception. Recent alterations of the standard 28-day regimen have included shortening the traditional hormone-free interval (HFI), supplementing the HFI with low-dose estrogen, or increasing the number of active pills administered, thus extending the time between withdrawal bleeding episodes by a variable number of months. In light of these changes in regimens, clinicians may be seeking evidence that the new regimens are safe and will not result in unexpected adverse events.
Methods: We initiated a long-term extension trial to evaluate the safety of a 91-day extended-regimen OC containing 150 mcg levonorgestrel/30 mcg ethinyl estradiol (EE) for 84 days, followed by 7 days of 10 mcg EE. After participation in a 1-year, open-label, phase 3 contraceptive program, 320 women qualified for enrollment in a multicenter, nonrandomized study of 91-day extended-regimen OCs for up to 3 additional consecutive years; 116 completed the study. We evaluated incidence of reported adverse events (AEs), rates of study discontinuation, and reported bleeding patterns.
Results: Total exposure was equivalent to 8292 28-day cycles. Participants reported no thromboembolic events. Thirty-one (9.7%) women discontinued treatment due to AEs. Unscheduled bleeding and spotting diminished during the course of the trial. Overall rates of study discontinuation and incidence of AEs were consistent with those observed in the phase 3 clinical program.
Conclusion: This study demonstrated that the AE profile of the 91-day extended-regimen OC over 4 years was similar to that seen in the 1-year clinical trials, with no unexpected adverse events.
Two Phase 3 studies assessed a 91-day oral contraceptive (OC) regimen for 1 year—a multicenter, open-label trial that studied safety and efficacy,1 and a multicenter trial that evaluated endometrial safety.2 Results of both studies showed the regimen to be safe, effective, and well tolerated. The regimen: 84 days of combination tablets containing 150 mcg levonorgestrel (LNG) and 30 mcg ethinyl estradiol (EE), followed by 7 days of 10 mcg EE alone instead of placebo to maintain ovarian suppression,3,4 potentially reducing the incidence of intermenstrual bleeding or spotting. To gain longer experience with this regimen, we enrolled selected subjects from both studies in a 3-year extension trial.
Methods
Study design and population
In this nonrandomized, multicenter, open-label extension study, we invited women who had successfully completed 1 year of treatment in either of the Phase 3 trials to participate as part of a convenience sample for an additional 3 years of follow-up. We conducted this study in accordance with ethical guidelines for human subjects and applicable guidelines for good clinical practice.5
Inclusion and exclusion criteria were similar to those used in the Phase 3 studies.1,2 Participants agreed to use the study medication as their primary method of birth control throughout the study. We excluded women who were using a medication that might interfere with the efficacy of OCs, or who had any medical or lifestyle contraindications to OC use (eg, clinically significant abnormal Pap smear; cigarette use if older than 35 years).
We enrolled 320 subjects whose demographic characteristics were similar to those in the earlier Phase 3 trials (TABLE 1).2
TABLE 1
Demographic characteristics of all treated participants (N=320)
| Age at screening, y | |
| Mean (SD) | 28.1 (6.0) |
| Median | 27.5 |
| Min, Max | 18.2, 40.2 |
| Weight, lb | |
| Mean (SD) | 152.3 (37.6) |
| Median | 143.5 |
| Min, Max | 94.0, 360.0 |
| Body mass index, kg/m2 | |
| Mean (SD) | 25.5 (5.8) |
| Median | 24.1 |
| Min, Max | 16.8, 56.5 |
| OC use history, n (%) | |
| Recent user | 225 (70.3%) |
| Prior user | 67 (20.9%) |
| New start | 28 (8.8%) |
| Race, n (%) | |
| African American | 40 (12.5%) |
| Asian | 7 (2.2%) |
| Caucasian | 262 (81.9%) |
| Hispanic | 4 (1.3%) |
| Other | 7 (2.2%) |
| Cigarette use status, n (%) | |
| Nonsmoker | 269 (84.1%) |
| Smoker | 51 (15.9%) |
| OC, oral contraceptive; SD, standard deviation. | |
Regular evaluation of adherence and AEs
Every 3 months at the study site, we assessed adherence with the drug regimen by reviewing participants’ daily diaries and by counting pills in returned used pill packs. We also evaluated subject-reported adverse events (AEs)—side effects, as well as serious adverse events (SAEs) requiring treatment or drug discontinuation—and use of concomitant medications or cigarettes.
Factors in our safety assessment
Our safety analysis included any subject who took at least 1 dose of the study drug. We calculated the incidence rates of subject-reported AEs, overall rates of discontinuation, and cycles of exposure. These included incidence rates of AEs the investigators deemed to be at least “remotely” related to treatment. Safety analyses also included annual changes in laboratory values (complete blood count, serum chemistry, lipid profile, and urinalysis), vital signs, occurrence of pregnancy, and rates of reported bleeding or spotting.
The evaluation included bleeding/spotting that was scheduled—occurring on cycle days 85 through 91 (EE-only tablets)—and unscheduled—intermenstrual or “breakthrough” blood loss occurring on cycle days 1 through 84. We defined bleeding as any vaginal blood loss requiring the use of sanitary protection (pads or tampons); spotting was defined as vaginal blood loss not necessitating sanitary protection.
Statistical analysis
Descriptive statistics included the number of subjects, and the mean, median (where appropriate), standard deviation or standard error of the mean (SE), or minimum and maximum values of patient characteristics. We summarized discrete events using frequencies or percentages. As this study was designed primarily to be observational and to gain further long-term experience with the regimen, we did not conduct formal power analyses and sample size calculations. For contraceptive trials, the US Food and Drug Administration typically requires a minimum exposure of 200 women using the method for 1 year. We also omitted a formal efficacy analysis, as efficacy was established in the Phase 3 clinical program.1
Results
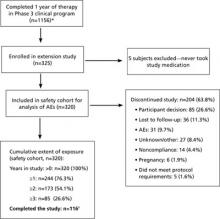
Of the 320 subjects enrolled and treated, 244 (76.3%) completed at least 1 year of treatment; 173 (54.1%) completed at least 2 years of treatment; and 85 (26.6%) completed 3 years of treatment in this extension study, beyond the 1 year completed in the Phase 3 clinical trials (FIGURE). A total of 204 women (63.8%) discontinued treatment; primarily due to personal decisions (26.6%), becoming lost to follow-up (11.3%), and adverse events (9.7%). These discontinuation rates are consistent with those in other long-term studies.6-8
FIGURE
Of the 320 participants enrolled, 116 completed the study
* In the pregnancy prevention study, 979 patients completed 1 year; in the endometrial safety study, 177 completed 1 year. Only 11 of the original 36 sites participated in the extension study, so not all 1156 subjects had the option of enrolling in the extension.
† Not all subjects enrolled at the same time. Thirty-one patients were participating in the study with various durations of exposure when the study was ended. Although they did not complete 3 full years of use, they did participate in the full course of the study that was available to them and were therefore classified as “completers.”
Serious adverse events were few
SAEs were reported by 12 subjects; 3 were possibly related to treatment—spontaneous abortion in a 33-year-old subject, nonthrombotic coronary artery spasm in a 40-year-old subject, and acute cholecystitis in a 37-year old subject. No venous thromboembolic events (VTEs) occurred; however, such events are rare (approximately 7-18 VTEs/100,000 OC users annually9) and would be unlikely in a study of 320 subjects.
Nonurgent adverse events comparable to earlier studies
The most commonly reported treatment-related AEs were headache (9.4%), metrorrhagia (9.1%), increased weight (6.9%), and dysmenorrhea (4.4%), as noted in TABLE 2. The most frequently reported treatment-emergent AEs (ie, regardless of relationship to study medication) were headache (21.9%), upper respiratory tract infection (18.4%), nasopharyngitis (15.0%), sinusitis (12.2%), and back pain (11.6%). A total of 31 subjects (9.7%) discontinued the study due to AEs. The incidence rates of treatment-emergent and treatment-related AEs in this study were not substantially higher than those in the Phase 3 trials.1
TABLE 2
Adverse events attributable to treatment occurred in ≥2% of participants (N=320)
| MedDRA System organ class and preferred term | n (%) |
|---|---|
| Reproductive system and breast disorders Metrorrhagia Dysmenorrhea | 29 (9.1) 14 (4.4) |
| Nervous system disorders Headache | 30 (9.4) |
| Investigations Weight increased | 22 (6.9) |
| Infections and infestations Vulvovaginal mycotic infection Vaginitis, bacterial Fungal infection | 13 (4.1) 9 (2.8) 7 (2.2) |
| Skin and subcutaneous tissue disorders Acne | 7 (2.2) |
| MedDRA, Medical Dictionary for Regulatory Activities. | |
Pregnancies due mostly to nonadherence
We conducted no formal efficacy analyses. Pregnancy was determined by a positive result on a pregnancy test conducted at the study site. Six subjects (1.9%) became pregnant during the study; 4 were noncompliant with the study medication, and 2 became pregnant at least 14 days after completing the study medication. One spontaneous abortion was reported. Among those participants who continued their pregnancies, none reported abnormal outcomes.
Laboratory values changed minimally, if at all
No notable changes occurred in serum chemistry, hematology, or urinalysis values. Specific mean changes from baseline included increases of 5.0 mg/dL for total cholesterol, 2.4 mg/dL for high-density lipoproteins, and 4.0 mg/dL for low-density lipoproteins; and decreases of 5.9 mg/dL for triglycerides and 0.1 g/dL for hemoglobin.
Vital signs remained stable
No notable changes occurred in systolic or diastolic blood pressure, heart rate, or temperature. The increase in mean weight that we observed (10.4 lb) is not unexpected, as the time period of evaluation was as long as 4 years after documentation of the baseline value.
Reported bleeding or spotting diminished over time
Median rates of unscheduled bleeding or spotting declined over the course of the study, from 4 days in 91 during cycle 1 to 1 day in 91 during cycle 11. In most of the 91-day cycles, participants consistently reported a median of 3 days of scheduled (withdrawal) bleeding or spotting.
Discussion
This 3-year study increased our experience with a novel extended-regimen OC to 4 years of continuous use. The results should reassure clinicians who are prescribing extended-regimen OCs that their patients are unlikely to experience side effects that differ significantly from traditional 28-day OC regimens. In other long-term studies of 28-day regimens, the most common AEs were headache, back pain, nausea, pharyngitis, and upper respiratory infection.7,8
Overall rates of study discontinuation and the incidence of AEs (including SAEs and AEs leading to discontinuation) were consistent with those observed in 1-year1,2,10,11 and 2-year6 studies of extended-regimen OCs.
There was no suggestion of increased risk of serious estrogen-related AEs. There were no reports of endometrial abnormalities or hyperplasia, which is consistent with the results of endometrial biopsies in a previous study that compared before- and after-treatment biopsy samples from 63 subjects in the 1-year Phase 3 trial.2
A pharmacokinetic analysis of a similar extended-regimen OC demonstrated that estrogen levels, measured on days 1, 21, 84, and 91 of a 91-day extended-regimen cycle, did not build up over the course of the regimen.12
The risk of thromboembolic disease associated with OCs is not related to the length of use, and a 5-year case-control study found significantly decreasing odds ratios for reports of VTE in OC users over time.13 In this extension study, there were no reported thromboembolic AEs and there was no suggestion of an increased risk of thrombosis with the long-term use of this regimen, although such findings are not unexpected for a small-scale study.
Acknowledgements
The principal investigators and their locations are as follows: Angeli Adamczyk, Paige Brainard (Tucson, Ariz), Ted Anderson, Robert Rosenfeld, Shali Scott (Nashville, Tenn), Matthew Davis (Rochester, NY), William Gibbons, Laurel Stadtmauer (Norfolk, Va), James Lackey (Oklahoma City, Okla), Sooji Lee-Rugh (Arlington, Va), Thomas Littlejohn (Winston-Salem, NC), James Maly (Lincoln, Neb), David Portman (Columbus, Ohio), George Raad (Charlotte, NC), and Mark Shepard (Washington, DC).
CORRESPONDENCE Kathleen Reape, MD, Teva Branded Pharmaceutical Products R&D, Inc., 425 Privet Road, Horsham, PA 19044; Kathleen. [email protected]
1. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73:229-234.
2. Anderson FD, Feldman R, Reape KZ. Endometrial effects of a 91-day extended regimen oral contraceptive with low-dose estrogen in place of placebo. Contraception. 2008;77:91-96.
3. Vandever MA, Kuehl TJ, Sulak P, et al. Evaluation of pituitary-ovarian axis suppression with three oral contraceptive regimens. Contraception. 2008;77:162-170.
4. Reape KZ, DiLiberti CE, Hendy CH, et al. Effects on serum hormone levels of low-dose estrogen in place of placebo during the hormone-free interval of an oral contraceptive. Contraception. 2008;77:34-39.
5. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Available at: http://www.wma.net/en/30publications/10policies/b3/index.html. Accessed April 6, 2010.
6. Anderson FD, Gibbons W, Portman D. Long-term safety of an extended-cycle oral contraceptive (Seasonale): A 2-year multicenter open-label extension trial. Am J Obstet Gynecol. 2006;195:92-96.
7. Zahradnik HP, Hanjalic-Beck A. Efficacy, safety, and sustainability of treatment continuation and results of an oral contraceptive containing 30 mcg ethinyl estradiol and 2 mg chlormadinone acetate, in long-term usage (up to 45 cycles)—an open-label, prospective, noncontrolled, office-based Phase III study. Contraception. 2008;77:337-343.
8. Archer DF, Jensen JT, Johnson JV, et al. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74:439-445.
9. Burkman RT. Venous thromboembolism and oral contraceptives: Current status and clinical implications. Treat Endocrinol. 2002;1:143-147.
10. Anderson FD, Hait H. The Seasonale-301 Study Group. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
11. Anderson FD, Hait H, Hsiu J, et al. Endometrial microstructure after long-term use of a 91-day extended-cycle oral contraceptive regimen. Contraception. 2005;71:55-59.
12. Reape KZ, DiLiberti C. Steady-state pharmacokinetics of an extended-regimen oral contraceptive with continuous estrogen [abstract]. Obstet Gynecol. 2007;109(suppl 4):13S.-
13. Lidegaard O, Edstrom E, Kreiner S. Oral contraceptives and venous thromboembolism: a five-year national case-control study. Contraception. 2002;65:187-196.
Abstract
Background: Oral contraceptives (OCs) are the most widely used method of reversible contraception. Recent alterations of the standard 28-day regimen have included shortening the traditional hormone-free interval (HFI), supplementing the HFI with low-dose estrogen, or increasing the number of active pills administered, thus extending the time between withdrawal bleeding episodes by a variable number of months. In light of these changes in regimens, clinicians may be seeking evidence that the new regimens are safe and will not result in unexpected adverse events.
Methods: We initiated a long-term extension trial to evaluate the safety of a 91-day extended-regimen OC containing 150 mcg levonorgestrel/30 mcg ethinyl estradiol (EE) for 84 days, followed by 7 days of 10 mcg EE. After participation in a 1-year, open-label, phase 3 contraceptive program, 320 women qualified for enrollment in a multicenter, nonrandomized study of 91-day extended-regimen OCs for up to 3 additional consecutive years; 116 completed the study. We evaluated incidence of reported adverse events (AEs), rates of study discontinuation, and reported bleeding patterns.
Results: Total exposure was equivalent to 8292 28-day cycles. Participants reported no thromboembolic events. Thirty-one (9.7%) women discontinued treatment due to AEs. Unscheduled bleeding and spotting diminished during the course of the trial. Overall rates of study discontinuation and incidence of AEs were consistent with those observed in the phase 3 clinical program.
Conclusion: This study demonstrated that the AE profile of the 91-day extended-regimen OC over 4 years was similar to that seen in the 1-year clinical trials, with no unexpected adverse events.
Two Phase 3 studies assessed a 91-day oral contraceptive (OC) regimen for 1 year—a multicenter, open-label trial that studied safety and efficacy,1 and a multicenter trial that evaluated endometrial safety.2 Results of both studies showed the regimen to be safe, effective, and well tolerated. The regimen: 84 days of combination tablets containing 150 mcg levonorgestrel (LNG) and 30 mcg ethinyl estradiol (EE), followed by 7 days of 10 mcg EE alone instead of placebo to maintain ovarian suppression,3,4 potentially reducing the incidence of intermenstrual bleeding or spotting. To gain longer experience with this regimen, we enrolled selected subjects from both studies in a 3-year extension trial.
Methods
Study design and population
In this nonrandomized, multicenter, open-label extension study, we invited women who had successfully completed 1 year of treatment in either of the Phase 3 trials to participate as part of a convenience sample for an additional 3 years of follow-up. We conducted this study in accordance with ethical guidelines for human subjects and applicable guidelines for good clinical practice.5
Inclusion and exclusion criteria were similar to those used in the Phase 3 studies.1,2 Participants agreed to use the study medication as their primary method of birth control throughout the study. We excluded women who were using a medication that might interfere with the efficacy of OCs, or who had any medical or lifestyle contraindications to OC use (eg, clinically significant abnormal Pap smear; cigarette use if older than 35 years).
We enrolled 320 subjects whose demographic characteristics were similar to those in the earlier Phase 3 trials (TABLE 1).2
TABLE 1
Demographic characteristics of all treated participants (N=320)
| Age at screening, y | |
| Mean (SD) | 28.1 (6.0) |
| Median | 27.5 |
| Min, Max | 18.2, 40.2 |
| Weight, lb | |
| Mean (SD) | 152.3 (37.6) |
| Median | 143.5 |
| Min, Max | 94.0, 360.0 |
| Body mass index, kg/m2 | |
| Mean (SD) | 25.5 (5.8) |
| Median | 24.1 |
| Min, Max | 16.8, 56.5 |
| OC use history, n (%) | |
| Recent user | 225 (70.3%) |
| Prior user | 67 (20.9%) |
| New start | 28 (8.8%) |
| Race, n (%) | |
| African American | 40 (12.5%) |
| Asian | 7 (2.2%) |
| Caucasian | 262 (81.9%) |
| Hispanic | 4 (1.3%) |
| Other | 7 (2.2%) |
| Cigarette use status, n (%) | |
| Nonsmoker | 269 (84.1%) |
| Smoker | 51 (15.9%) |
| OC, oral contraceptive; SD, standard deviation. | |
Regular evaluation of adherence and AEs
Every 3 months at the study site, we assessed adherence with the drug regimen by reviewing participants’ daily diaries and by counting pills in returned used pill packs. We also evaluated subject-reported adverse events (AEs)—side effects, as well as serious adverse events (SAEs) requiring treatment or drug discontinuation—and use of concomitant medications or cigarettes.
Factors in our safety assessment
Our safety analysis included any subject who took at least 1 dose of the study drug. We calculated the incidence rates of subject-reported AEs, overall rates of discontinuation, and cycles of exposure. These included incidence rates of AEs the investigators deemed to be at least “remotely” related to treatment. Safety analyses also included annual changes in laboratory values (complete blood count, serum chemistry, lipid profile, and urinalysis), vital signs, occurrence of pregnancy, and rates of reported bleeding or spotting.
The evaluation included bleeding/spotting that was scheduled—occurring on cycle days 85 through 91 (EE-only tablets)—and unscheduled—intermenstrual or “breakthrough” blood loss occurring on cycle days 1 through 84. We defined bleeding as any vaginal blood loss requiring the use of sanitary protection (pads or tampons); spotting was defined as vaginal blood loss not necessitating sanitary protection.
Statistical analysis
Descriptive statistics included the number of subjects, and the mean, median (where appropriate), standard deviation or standard error of the mean (SE), or minimum and maximum values of patient characteristics. We summarized discrete events using frequencies or percentages. As this study was designed primarily to be observational and to gain further long-term experience with the regimen, we did not conduct formal power analyses and sample size calculations. For contraceptive trials, the US Food and Drug Administration typically requires a minimum exposure of 200 women using the method for 1 year. We also omitted a formal efficacy analysis, as efficacy was established in the Phase 3 clinical program.1
Results
Of the 320 subjects enrolled and treated, 244 (76.3%) completed at least 1 year of treatment; 173 (54.1%) completed at least 2 years of treatment; and 85 (26.6%) completed 3 years of treatment in this extension study, beyond the 1 year completed in the Phase 3 clinical trials (FIGURE). A total of 204 women (63.8%) discontinued treatment; primarily due to personal decisions (26.6%), becoming lost to follow-up (11.3%), and adverse events (9.7%). These discontinuation rates are consistent with those in other long-term studies.6-8
FIGURE
Of the 320 participants enrolled, 116 completed the study
* In the pregnancy prevention study, 979 patients completed 1 year; in the endometrial safety study, 177 completed 1 year. Only 11 of the original 36 sites participated in the extension study, so not all 1156 subjects had the option of enrolling in the extension.
† Not all subjects enrolled at the same time. Thirty-one patients were participating in the study with various durations of exposure when the study was ended. Although they did not complete 3 full years of use, they did participate in the full course of the study that was available to them and were therefore classified as “completers.”
Serious adverse events were few
SAEs were reported by 12 subjects; 3 were possibly related to treatment—spontaneous abortion in a 33-year-old subject, nonthrombotic coronary artery spasm in a 40-year-old subject, and acute cholecystitis in a 37-year old subject. No venous thromboembolic events (VTEs) occurred; however, such events are rare (approximately 7-18 VTEs/100,000 OC users annually9) and would be unlikely in a study of 320 subjects.
Nonurgent adverse events comparable to earlier studies
The most commonly reported treatment-related AEs were headache (9.4%), metrorrhagia (9.1%), increased weight (6.9%), and dysmenorrhea (4.4%), as noted in TABLE 2. The most frequently reported treatment-emergent AEs (ie, regardless of relationship to study medication) were headache (21.9%), upper respiratory tract infection (18.4%), nasopharyngitis (15.0%), sinusitis (12.2%), and back pain (11.6%). A total of 31 subjects (9.7%) discontinued the study due to AEs. The incidence rates of treatment-emergent and treatment-related AEs in this study were not substantially higher than those in the Phase 3 trials.1
TABLE 2
Adverse events attributable to treatment occurred in ≥2% of participants (N=320)
| MedDRA System organ class and preferred term | n (%) |
|---|---|
| Reproductive system and breast disorders Metrorrhagia Dysmenorrhea | 29 (9.1) 14 (4.4) |
| Nervous system disorders Headache | 30 (9.4) |
| Investigations Weight increased | 22 (6.9) |
| Infections and infestations Vulvovaginal mycotic infection Vaginitis, bacterial Fungal infection | 13 (4.1) 9 (2.8) 7 (2.2) |
| Skin and subcutaneous tissue disorders Acne | 7 (2.2) |
| MedDRA, Medical Dictionary for Regulatory Activities. | |
Pregnancies due mostly to nonadherence
We conducted no formal efficacy analyses. Pregnancy was determined by a positive result on a pregnancy test conducted at the study site. Six subjects (1.9%) became pregnant during the study; 4 were noncompliant with the study medication, and 2 became pregnant at least 14 days after completing the study medication. One spontaneous abortion was reported. Among those participants who continued their pregnancies, none reported abnormal outcomes.
Laboratory values changed minimally, if at all
No notable changes occurred in serum chemistry, hematology, or urinalysis values. Specific mean changes from baseline included increases of 5.0 mg/dL for total cholesterol, 2.4 mg/dL for high-density lipoproteins, and 4.0 mg/dL for low-density lipoproteins; and decreases of 5.9 mg/dL for triglycerides and 0.1 g/dL for hemoglobin.
Vital signs remained stable
No notable changes occurred in systolic or diastolic blood pressure, heart rate, or temperature. The increase in mean weight that we observed (10.4 lb) is not unexpected, as the time period of evaluation was as long as 4 years after documentation of the baseline value.
Reported bleeding or spotting diminished over time
Median rates of unscheduled bleeding or spotting declined over the course of the study, from 4 days in 91 during cycle 1 to 1 day in 91 during cycle 11. In most of the 91-day cycles, participants consistently reported a median of 3 days of scheduled (withdrawal) bleeding or spotting.
Discussion
This 3-year study increased our experience with a novel extended-regimen OC to 4 years of continuous use. The results should reassure clinicians who are prescribing extended-regimen OCs that their patients are unlikely to experience side effects that differ significantly from traditional 28-day OC regimens. In other long-term studies of 28-day regimens, the most common AEs were headache, back pain, nausea, pharyngitis, and upper respiratory infection.7,8
Overall rates of study discontinuation and the incidence of AEs (including SAEs and AEs leading to discontinuation) were consistent with those observed in 1-year1,2,10,11 and 2-year6 studies of extended-regimen OCs.
There was no suggestion of increased risk of serious estrogen-related AEs. There were no reports of endometrial abnormalities or hyperplasia, which is consistent with the results of endometrial biopsies in a previous study that compared before- and after-treatment biopsy samples from 63 subjects in the 1-year Phase 3 trial.2
A pharmacokinetic analysis of a similar extended-regimen OC demonstrated that estrogen levels, measured on days 1, 21, 84, and 91 of a 91-day extended-regimen cycle, did not build up over the course of the regimen.12
The risk of thromboembolic disease associated with OCs is not related to the length of use, and a 5-year case-control study found significantly decreasing odds ratios for reports of VTE in OC users over time.13 In this extension study, there were no reported thromboembolic AEs and there was no suggestion of an increased risk of thrombosis with the long-term use of this regimen, although such findings are not unexpected for a small-scale study.
Acknowledgements
The principal investigators and their locations are as follows: Angeli Adamczyk, Paige Brainard (Tucson, Ariz), Ted Anderson, Robert Rosenfeld, Shali Scott (Nashville, Tenn), Matthew Davis (Rochester, NY), William Gibbons, Laurel Stadtmauer (Norfolk, Va), James Lackey (Oklahoma City, Okla), Sooji Lee-Rugh (Arlington, Va), Thomas Littlejohn (Winston-Salem, NC), James Maly (Lincoln, Neb), David Portman (Columbus, Ohio), George Raad (Charlotte, NC), and Mark Shepard (Washington, DC).
CORRESPONDENCE Kathleen Reape, MD, Teva Branded Pharmaceutical Products R&D, Inc., 425 Privet Road, Horsham, PA 19044; Kathleen. [email protected]
Abstract
Background: Oral contraceptives (OCs) are the most widely used method of reversible contraception. Recent alterations of the standard 28-day regimen have included shortening the traditional hormone-free interval (HFI), supplementing the HFI with low-dose estrogen, or increasing the number of active pills administered, thus extending the time between withdrawal bleeding episodes by a variable number of months. In light of these changes in regimens, clinicians may be seeking evidence that the new regimens are safe and will not result in unexpected adverse events.
Methods: We initiated a long-term extension trial to evaluate the safety of a 91-day extended-regimen OC containing 150 mcg levonorgestrel/30 mcg ethinyl estradiol (EE) for 84 days, followed by 7 days of 10 mcg EE. After participation in a 1-year, open-label, phase 3 contraceptive program, 320 women qualified for enrollment in a multicenter, nonrandomized study of 91-day extended-regimen OCs for up to 3 additional consecutive years; 116 completed the study. We evaluated incidence of reported adverse events (AEs), rates of study discontinuation, and reported bleeding patterns.
Results: Total exposure was equivalent to 8292 28-day cycles. Participants reported no thromboembolic events. Thirty-one (9.7%) women discontinued treatment due to AEs. Unscheduled bleeding and spotting diminished during the course of the trial. Overall rates of study discontinuation and incidence of AEs were consistent with those observed in the phase 3 clinical program.
Conclusion: This study demonstrated that the AE profile of the 91-day extended-regimen OC over 4 years was similar to that seen in the 1-year clinical trials, with no unexpected adverse events.
Two Phase 3 studies assessed a 91-day oral contraceptive (OC) regimen for 1 year—a multicenter, open-label trial that studied safety and efficacy,1 and a multicenter trial that evaluated endometrial safety.2 Results of both studies showed the regimen to be safe, effective, and well tolerated. The regimen: 84 days of combination tablets containing 150 mcg levonorgestrel (LNG) and 30 mcg ethinyl estradiol (EE), followed by 7 days of 10 mcg EE alone instead of placebo to maintain ovarian suppression,3,4 potentially reducing the incidence of intermenstrual bleeding or spotting. To gain longer experience with this regimen, we enrolled selected subjects from both studies in a 3-year extension trial.
Methods
Study design and population
In this nonrandomized, multicenter, open-label extension study, we invited women who had successfully completed 1 year of treatment in either of the Phase 3 trials to participate as part of a convenience sample for an additional 3 years of follow-up. We conducted this study in accordance with ethical guidelines for human subjects and applicable guidelines for good clinical practice.5
Inclusion and exclusion criteria were similar to those used in the Phase 3 studies.1,2 Participants agreed to use the study medication as their primary method of birth control throughout the study. We excluded women who were using a medication that might interfere with the efficacy of OCs, or who had any medical or lifestyle contraindications to OC use (eg, clinically significant abnormal Pap smear; cigarette use if older than 35 years).
We enrolled 320 subjects whose demographic characteristics were similar to those in the earlier Phase 3 trials (TABLE 1).2
TABLE 1
Demographic characteristics of all treated participants (N=320)
| Age at screening, y | |
| Mean (SD) | 28.1 (6.0) |
| Median | 27.5 |
| Min, Max | 18.2, 40.2 |
| Weight, lb | |
| Mean (SD) | 152.3 (37.6) |
| Median | 143.5 |
| Min, Max | 94.0, 360.0 |
| Body mass index, kg/m2 | |
| Mean (SD) | 25.5 (5.8) |
| Median | 24.1 |
| Min, Max | 16.8, 56.5 |
| OC use history, n (%) | |
| Recent user | 225 (70.3%) |
| Prior user | 67 (20.9%) |
| New start | 28 (8.8%) |
| Race, n (%) | |
| African American | 40 (12.5%) |
| Asian | 7 (2.2%) |
| Caucasian | 262 (81.9%) |
| Hispanic | 4 (1.3%) |
| Other | 7 (2.2%) |
| Cigarette use status, n (%) | |
| Nonsmoker | 269 (84.1%) |
| Smoker | 51 (15.9%) |
| OC, oral contraceptive; SD, standard deviation. | |
Regular evaluation of adherence and AEs
Every 3 months at the study site, we assessed adherence with the drug regimen by reviewing participants’ daily diaries and by counting pills in returned used pill packs. We also evaluated subject-reported adverse events (AEs)—side effects, as well as serious adverse events (SAEs) requiring treatment or drug discontinuation—and use of concomitant medications or cigarettes.
Factors in our safety assessment
Our safety analysis included any subject who took at least 1 dose of the study drug. We calculated the incidence rates of subject-reported AEs, overall rates of discontinuation, and cycles of exposure. These included incidence rates of AEs the investigators deemed to be at least “remotely” related to treatment. Safety analyses also included annual changes in laboratory values (complete blood count, serum chemistry, lipid profile, and urinalysis), vital signs, occurrence of pregnancy, and rates of reported bleeding or spotting.
The evaluation included bleeding/spotting that was scheduled—occurring on cycle days 85 through 91 (EE-only tablets)—and unscheduled—intermenstrual or “breakthrough” blood loss occurring on cycle days 1 through 84. We defined bleeding as any vaginal blood loss requiring the use of sanitary protection (pads or tampons); spotting was defined as vaginal blood loss not necessitating sanitary protection.
Statistical analysis
Descriptive statistics included the number of subjects, and the mean, median (where appropriate), standard deviation or standard error of the mean (SE), or minimum and maximum values of patient characteristics. We summarized discrete events using frequencies or percentages. As this study was designed primarily to be observational and to gain further long-term experience with the regimen, we did not conduct formal power analyses and sample size calculations. For contraceptive trials, the US Food and Drug Administration typically requires a minimum exposure of 200 women using the method for 1 year. We also omitted a formal efficacy analysis, as efficacy was established in the Phase 3 clinical program.1
Results
Of the 320 subjects enrolled and treated, 244 (76.3%) completed at least 1 year of treatment; 173 (54.1%) completed at least 2 years of treatment; and 85 (26.6%) completed 3 years of treatment in this extension study, beyond the 1 year completed in the Phase 3 clinical trials (FIGURE). A total of 204 women (63.8%) discontinued treatment; primarily due to personal decisions (26.6%), becoming lost to follow-up (11.3%), and adverse events (9.7%). These discontinuation rates are consistent with those in other long-term studies.6-8
FIGURE
Of the 320 participants enrolled, 116 completed the study
* In the pregnancy prevention study, 979 patients completed 1 year; in the endometrial safety study, 177 completed 1 year. Only 11 of the original 36 sites participated in the extension study, so not all 1156 subjects had the option of enrolling in the extension.
† Not all subjects enrolled at the same time. Thirty-one patients were participating in the study with various durations of exposure when the study was ended. Although they did not complete 3 full years of use, they did participate in the full course of the study that was available to them and were therefore classified as “completers.”
Serious adverse events were few
SAEs were reported by 12 subjects; 3 were possibly related to treatment—spontaneous abortion in a 33-year-old subject, nonthrombotic coronary artery spasm in a 40-year-old subject, and acute cholecystitis in a 37-year old subject. No venous thromboembolic events (VTEs) occurred; however, such events are rare (approximately 7-18 VTEs/100,000 OC users annually9) and would be unlikely in a study of 320 subjects.
Nonurgent adverse events comparable to earlier studies
The most commonly reported treatment-related AEs were headache (9.4%), metrorrhagia (9.1%), increased weight (6.9%), and dysmenorrhea (4.4%), as noted in TABLE 2. The most frequently reported treatment-emergent AEs (ie, regardless of relationship to study medication) were headache (21.9%), upper respiratory tract infection (18.4%), nasopharyngitis (15.0%), sinusitis (12.2%), and back pain (11.6%). A total of 31 subjects (9.7%) discontinued the study due to AEs. The incidence rates of treatment-emergent and treatment-related AEs in this study were not substantially higher than those in the Phase 3 trials.1
TABLE 2
Adverse events attributable to treatment occurred in ≥2% of participants (N=320)
| MedDRA System organ class and preferred term | n (%) |
|---|---|
| Reproductive system and breast disorders Metrorrhagia Dysmenorrhea | 29 (9.1) 14 (4.4) |
| Nervous system disorders Headache | 30 (9.4) |
| Investigations Weight increased | 22 (6.9) |
| Infections and infestations Vulvovaginal mycotic infection Vaginitis, bacterial Fungal infection | 13 (4.1) 9 (2.8) 7 (2.2) |
| Skin and subcutaneous tissue disorders Acne | 7 (2.2) |
| MedDRA, Medical Dictionary for Regulatory Activities. | |
Pregnancies due mostly to nonadherence
We conducted no formal efficacy analyses. Pregnancy was determined by a positive result on a pregnancy test conducted at the study site. Six subjects (1.9%) became pregnant during the study; 4 were noncompliant with the study medication, and 2 became pregnant at least 14 days after completing the study medication. One spontaneous abortion was reported. Among those participants who continued their pregnancies, none reported abnormal outcomes.
Laboratory values changed minimally, if at all
No notable changes occurred in serum chemistry, hematology, or urinalysis values. Specific mean changes from baseline included increases of 5.0 mg/dL for total cholesterol, 2.4 mg/dL for high-density lipoproteins, and 4.0 mg/dL for low-density lipoproteins; and decreases of 5.9 mg/dL for triglycerides and 0.1 g/dL for hemoglobin.
Vital signs remained stable
No notable changes occurred in systolic or diastolic blood pressure, heart rate, or temperature. The increase in mean weight that we observed (10.4 lb) is not unexpected, as the time period of evaluation was as long as 4 years after documentation of the baseline value.
Reported bleeding or spotting diminished over time
Median rates of unscheduled bleeding or spotting declined over the course of the study, from 4 days in 91 during cycle 1 to 1 day in 91 during cycle 11. In most of the 91-day cycles, participants consistently reported a median of 3 days of scheduled (withdrawal) bleeding or spotting.
Discussion
This 3-year study increased our experience with a novel extended-regimen OC to 4 years of continuous use. The results should reassure clinicians who are prescribing extended-regimen OCs that their patients are unlikely to experience side effects that differ significantly from traditional 28-day OC regimens. In other long-term studies of 28-day regimens, the most common AEs were headache, back pain, nausea, pharyngitis, and upper respiratory infection.7,8
Overall rates of study discontinuation and the incidence of AEs (including SAEs and AEs leading to discontinuation) were consistent with those observed in 1-year1,2,10,11 and 2-year6 studies of extended-regimen OCs.
There was no suggestion of increased risk of serious estrogen-related AEs. There were no reports of endometrial abnormalities or hyperplasia, which is consistent with the results of endometrial biopsies in a previous study that compared before- and after-treatment biopsy samples from 63 subjects in the 1-year Phase 3 trial.2
A pharmacokinetic analysis of a similar extended-regimen OC demonstrated that estrogen levels, measured on days 1, 21, 84, and 91 of a 91-day extended-regimen cycle, did not build up over the course of the regimen.12
The risk of thromboembolic disease associated with OCs is not related to the length of use, and a 5-year case-control study found significantly decreasing odds ratios for reports of VTE in OC users over time.13 In this extension study, there were no reported thromboembolic AEs and there was no suggestion of an increased risk of thrombosis with the long-term use of this regimen, although such findings are not unexpected for a small-scale study.
Acknowledgements
The principal investigators and their locations are as follows: Angeli Adamczyk, Paige Brainard (Tucson, Ariz), Ted Anderson, Robert Rosenfeld, Shali Scott (Nashville, Tenn), Matthew Davis (Rochester, NY), William Gibbons, Laurel Stadtmauer (Norfolk, Va), James Lackey (Oklahoma City, Okla), Sooji Lee-Rugh (Arlington, Va), Thomas Littlejohn (Winston-Salem, NC), James Maly (Lincoln, Neb), David Portman (Columbus, Ohio), George Raad (Charlotte, NC), and Mark Shepard (Washington, DC).
CORRESPONDENCE Kathleen Reape, MD, Teva Branded Pharmaceutical Products R&D, Inc., 425 Privet Road, Horsham, PA 19044; Kathleen. [email protected]
1. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73:229-234.
2. Anderson FD, Feldman R, Reape KZ. Endometrial effects of a 91-day extended regimen oral contraceptive with low-dose estrogen in place of placebo. Contraception. 2008;77:91-96.
3. Vandever MA, Kuehl TJ, Sulak P, et al. Evaluation of pituitary-ovarian axis suppression with three oral contraceptive regimens. Contraception. 2008;77:162-170.
4. Reape KZ, DiLiberti CE, Hendy CH, et al. Effects on serum hormone levels of low-dose estrogen in place of placebo during the hormone-free interval of an oral contraceptive. Contraception. 2008;77:34-39.
5. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Available at: http://www.wma.net/en/30publications/10policies/b3/index.html. Accessed April 6, 2010.
6. Anderson FD, Gibbons W, Portman D. Long-term safety of an extended-cycle oral contraceptive (Seasonale): A 2-year multicenter open-label extension trial. Am J Obstet Gynecol. 2006;195:92-96.
7. Zahradnik HP, Hanjalic-Beck A. Efficacy, safety, and sustainability of treatment continuation and results of an oral contraceptive containing 30 mcg ethinyl estradiol and 2 mg chlormadinone acetate, in long-term usage (up to 45 cycles)—an open-label, prospective, noncontrolled, office-based Phase III study. Contraception. 2008;77:337-343.
8. Archer DF, Jensen JT, Johnson JV, et al. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74:439-445.
9. Burkman RT. Venous thromboembolism and oral contraceptives: Current status and clinical implications. Treat Endocrinol. 2002;1:143-147.
10. Anderson FD, Hait H. The Seasonale-301 Study Group. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
11. Anderson FD, Hait H, Hsiu J, et al. Endometrial microstructure after long-term use of a 91-day extended-cycle oral contraceptive regimen. Contraception. 2005;71:55-59.
12. Reape KZ, DiLiberti C. Steady-state pharmacokinetics of an extended-regimen oral contraceptive with continuous estrogen [abstract]. Obstet Gynecol. 2007;109(suppl 4):13S.-
13. Lidegaard O, Edstrom E, Kreiner S. Oral contraceptives and venous thromboembolism: a five-year national case-control study. Contraception. 2002;65:187-196.
1. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73:229-234.
2. Anderson FD, Feldman R, Reape KZ. Endometrial effects of a 91-day extended regimen oral contraceptive with low-dose estrogen in place of placebo. Contraception. 2008;77:91-96.
3. Vandever MA, Kuehl TJ, Sulak P, et al. Evaluation of pituitary-ovarian axis suppression with three oral contraceptive regimens. Contraception. 2008;77:162-170.
4. Reape KZ, DiLiberti CE, Hendy CH, et al. Effects on serum hormone levels of low-dose estrogen in place of placebo during the hormone-free interval of an oral contraceptive. Contraception. 2008;77:34-39.
5. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Available at: http://www.wma.net/en/30publications/10policies/b3/index.html. Accessed April 6, 2010.
6. Anderson FD, Gibbons W, Portman D. Long-term safety of an extended-cycle oral contraceptive (Seasonale): A 2-year multicenter open-label extension trial. Am J Obstet Gynecol. 2006;195:92-96.
7. Zahradnik HP, Hanjalic-Beck A. Efficacy, safety, and sustainability of treatment continuation and results of an oral contraceptive containing 30 mcg ethinyl estradiol and 2 mg chlormadinone acetate, in long-term usage (up to 45 cycles)—an open-label, prospective, noncontrolled, office-based Phase III study. Contraception. 2008;77:337-343.
8. Archer DF, Jensen JT, Johnson JV, et al. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74:439-445.
9. Burkman RT. Venous thromboembolism and oral contraceptives: Current status and clinical implications. Treat Endocrinol. 2002;1:143-147.
10. Anderson FD, Hait H. The Seasonale-301 Study Group. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
11. Anderson FD, Hait H, Hsiu J, et al. Endometrial microstructure after long-term use of a 91-day extended-cycle oral contraceptive regimen. Contraception. 2005;71:55-59.
12. Reape KZ, DiLiberti C. Steady-state pharmacokinetics of an extended-regimen oral contraceptive with continuous estrogen [abstract]. Obstet Gynecol. 2007;109(suppl 4):13S.-
13. Lidegaard O, Edstrom E, Kreiner S. Oral contraceptives and venous thromboembolism: a five-year national case-control study. Contraception. 2002;65:187-196.