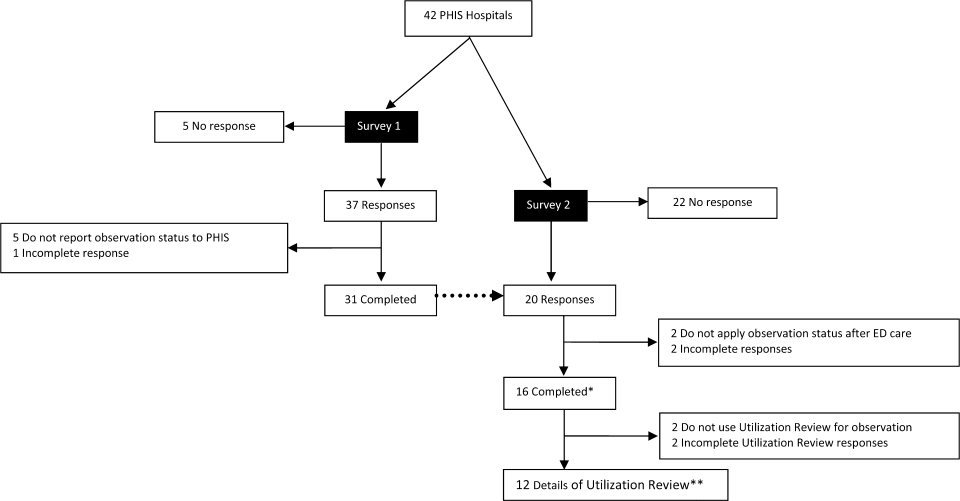
User login
Macrolides for Mycoplasmal Pneumonia
Mycoplasma pneumoniae is a common cause of community‐acquired pneumonia (CAP), among school‐age children and adolescents.14 Though pneumonia caused by M. pneumoniae is typically self‐limited, severe illness may occur.5 M. pneumoniae has also been implicated in airway inflammation, which may lead to the onset and development of chronic pulmonary disease.610 Few studies have directly addressed appropriate treatment strategies for M. pneumoniae pneumonia,11 and, despite its high prevalence and potential for causing severe complications, treatment recommendations remain inconsistent.
The efficacy of macrolide therapy in particular for M. pneumoniae remains unclear. In vitro susceptibility studies have shown bacteriostatic activity of erythromycin, clarithromycin, and azithromycin against M. pneumoniae.1218 Additionally, several small retrospective studies have shown that among children with atypical CAP (including M. pneumoniae pneumonia), those treated with macrolides were less likely to have persistence or progression of signs and symptoms after 3 days of therapy.19, 20 Lu et al21 found a shorter duration of fever among macrolide recipients compared with non‐recipients. In adults, Shames et al22 found a shorter duration of fever and hospitalization among erythromycin recipients compared with controls. Other randomized controlled trials have also addressed the use of macrolides in treatment of M. pneumoniae, but the ability to draw meaningful conclusions is limited by small samples sizes and by lack of details about the number of patients with M. pneumoniae.11
In addition to their antimicrobial effect, macrolides also have anti‐inflammatory properties.2327 The importance of these anti‐inflammatory properties is supported by studies showing clinical cure in patients treated with macrolides despite persistence of M. pneumoniae organisms,2831 clinical improvement despite the administration of doses that provide tissue levels below the minimum inhibitory concentration of the organism,3234 and clinical cure in patients with macrolide‐resistant M. pneumoniae.18, 35
The objectives of the current study were to examine the impact of macrolide therapy on the length of stay (LOS) and short‐ and longer‐term readmissions, including longer‐term asthma‐related readmissions, in children hospitalized with M. pneumoniae pneumonia.
METHODS
Data Source
Data for this retrospective cohort study were obtained from the Pediatric Health Information System (PHIS), which contains administrative data from 38 freestanding children's hospitals. Data quality and reliability are assured through a joint effort by the Child Health Corporation of America (Shawnee Mission, KS) and PHIS‐participating hospitals as described previously.36, 37 Encrypted medical record numbers allow for tracking of individual patients across hospitalizations. This study was reviewed and approved by the Committees for the Protection of Human Subjects at The Children's Hospital of Philadelphia (Philadelphia, PA).
Patients
Children 6‐18 years of age with CAP were eligible if they were discharged from a participating hospital between January 1, 2006 and December 31, 2008. Subjects were included if they received antibiotic therapy on the first day of hospitalization and if they satisfied one of the following International Classification of Diseases, 9th revision (ICD‐9) discharge diagnosis code criteria: 1) Principal diagnosis of M. pneumoniae pneumonia (483.0); 2) Principal diagnosis of a pneumonia‐related symptom (eg, fever, cough) (780.6 or 786.00‐786.52 [except 786.1]) and a secondary diagnosis of M. pneumoniae pneumonia; or 3) Principal diagnosis of pneumonia (481‐483.8 [except 483.0], 485‐486) and a secondary diagnosis of Mycoplasma (041.81).
Children younger than 6 years of age were excluded due to the low prevalence of M. pneumoniae infection.2, 38 Patients with comorbid conditions predisposing to severe or recurrent pneumonia (eg, cystic fibrosis, malignancy) were excluded using a previously reported classification scheme.39 In addition, we excluded patient data from 2 hospitals due to incomplete reporting of discharge information; thus data from 36 hospitals were included in this study.
Validation of Discharge Diagnosis Codes for Mycoplasma pneumoniae
To assess for misclassification of the diagnosis of M. pneumoniae, we reviewed records of a randomly selected subset of subjects from The Children's Hospital of Philadelphia; 14 of 15 patients had signs of lower respiratory tract infection in conjunction with a positive M. pneumoniae polymerase chain reaction test from nasopharyngeal washings to confirm the diagnosis of M. pneumoniae pneumonia. Hence, the positive predictive value of our algorithm for diagnosing M. pneumoniae pneumonia was 93.3%.
Study Definitions
We identified children with asthma in 2 ways. Asthma‐related hospitalizations were identified by an ICD‐9 code for asthma (493.0‐493.92) in any discharge diagnosis field during any hospitalization in the 24 months prior to the current hospitalization. Baseline controller medications were identified by receipt of inhaled corticosteroids (eg, fluticasone) or leukotriene receptor antagonists on the first day of hospitalization.
Systemic corticosteroids (either oral or intravenous) included dexamethasone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Measures of disease severity included admission to the intensive care unit within 48 hours of hospitalization, and administration of vancomycin or clindamycin, vasoactive infusions (epinephrine, norepinephrine, dopamine, and dobutamine), and invasive (endotracheal intubation) and noninvasive (continuous positive airway pressure) mechanical ventilation within 24 hours of hospitalization, as previously described.40, 41 Viral respiratory season was defined as October through March.
Measured Outcomes
The primary outcomes of interest were hospital LOS and all‐cause readmission within 28 days and 15 months after index discharge. We examined readmissions for asthma 15 months after index discharge as a secondary outcome measure because of the potential role for M. pneumoniae infection in long‐term lung dysfunction, including asthma.42 The 15‐month time frame was selected based on longitudinal data available in PHIS for the entire study cohort.
Measured Exposures
The main exposure was early initiation of macrolide therapy, defined as receipt of erythromycin, clarithromycin, or azithromycin on the first day of hospitalization.
Data Analysis
Continuous variables were described using median and interquartile range (IQR) or range values, and compared using the Wilcoxon rank‐sum test. Categorical variables were described using counts and frequencies, and compared using the chi‐square test. Multivariable linear (for LOS) and logistic (for readmission) regression analyses were performed to assess the independent association of macrolide therapy with the primary outcomes. Because the LOS data had a skewed distribution, our analyses were performed using logarithmically transformed LOS values as the dependent variable. The resulting beta‐coefficients were transformed to reflect the percent difference in LOS between subjects receiving and not receiving macrolide therapy.
Building of the multivariable models began with the inclusion of macrolide therapy. Variables associated with primary outcomes on univariate analysis (P < 0.20) were also considered for inclusion as potential confounders.43 These variables were included in the final multivariable model if they remained significant after adjusting for other factors, or if their inclusion in the model resulted in a 15% or greater change in the effect size of the primary association of interest (ie, macrolide therapy).44 Because corticosteroids also have anti‐inflammatory properties, we assessed for interactions with macrolide therapy. There was no interaction between macrolide and systemic corticosteroid therapy (P = 0.26, Likelihood ratio test), therefore our primary model adjusted for systemic corticosteroids.
Despite adjusting for systemic corticosteroid therapy in our primary analysis, residual confounding by indication for corticosteroid therapy might exist. We therefore repeated the analysis after stratifying by receipt or non‐receipt of systemic corticosteroid therapy. Because the benefit of macrolides in preventing long‐term dysfunction may be limited to those without a prior diagnosis of asthma, we repeated the analysis of readmissions within 15 months of index discharge (any readmission and asthma‐related readmissions) while limiting the cohort to those without evidence of asthma (ie, no prior asthma‐related hospitalizations and no chronic asthma medications). Because children with underlying conditions or circumstances that would predispose to prolonged hospitalizations may have been included, despite our restriction of the cohort to those without an identified chronic complex condition, we also repeated the analysis while limiting the cohort to those with a LOS 7 days. Finally, all analyses were clustered on hospital using the robust standard errors of Huber and White to account for the correlation of exposures and outcomes among children within centers.
Data were analyzed using Stata version 11 (Stata Corporation, College Station, TX). Statistical significance was determined a priori as a two‐tailed P value <0.05.
RESULTS
Patient Characteristics
During the study, 690 children ages 6 to 18 years met inclusion criteria. Characteristics of these patients are shown in Table 1. The median age was 10 years (IQR, 7‐13 years). Ten patients (1.4%) also had a concomitant discharge diagnosis of pneumococcal pneumonia, while 19 patients (2.7%) had a concomitant discharge diagnosis of viral pneumonia; 1 of these patients had discharge diagnoses of both viral and pneumococcal pneumonia.
| Empiric Macrolide Therapy | ||||
|---|---|---|---|---|
| Variable | All Subjects | Yes | No | P |
| ||||
| Demographics | ||||
| Male sex | 356 (51.6) | 200 (49.4) | 156 (54.7) | 0.166 |
| Race | ||||
| Black | 135 (19.6) | 81 (20.0) | 54 (19.0) | 0.506 |
| White | 484 (70.1) | 287 (70.9) | 197 (69.1) | |
| Other | 62 (9.0) | 31 (7.7) | 31 (10.9) | |
| Missing | 9 (1.3) | 6 (1.5) | 3 (1.1) | |
| Presentation during viral respiratory season | 420 (60.9) | 242 (59.8) | 178 (62.5) | |
| Prior asthma hospitalization | 41 (5.9) | 31 (7.7) | 10 (3.5) | 0.023 |
| Intensive care unit admission | 127 (18.4) | 74 (18.3) | 53 (18.6) | 0.914 |
| Laboratory tests and procedures | ||||
| Additional radiologic imaging* | 24 (3.5) | 13 (3.2) | 11 (3.9) | 0.646 |
| Arterial blood gas | 116 (17.3) | 72 (18.5) | 44 (15.6) | 0.316 |
| Complete blood count | 433 (64.4) | 249 (64.0) | 184 (65.0) | 0.788 |
| Blood culture | 280 (41.7) | 167 (42.9) | 113 (39.9) | 0.436 |
| Mechanical ventilation | 16 (2.3) | 5 (1.2) | 11 (3.86) | 0.024 |
| Medications | ||||
| Chronic asthma medication | 116 (16.8) | 72 (17.8) | 44 (15.4) | 0.419 |
| Beta‐agonist therapy | 328 (47.5) | 215 (53.1) | 113 (39.7) | 0.001 |
| Vasoactive infusions | 22 (3.2) | 13 (3.2) | 9 (3.2) | 0.969 |
| Systemic corticosteroids | 252 (36.5) | 191 (47.2) | 61 (21.4) | <0.001 |
| Clindamycin or vancomycin | 86 (12.5) | 24 (5.9) | 62 (21.8) | <0.001 |
Macrolide therapy was administered to 405 (58.7%) patients. Systemic corticosteroid therapy was administered to 252 (36.5%) patients. Overall, 191 (27.7%) of the 690 patients received both macrolides and systemic corticosteroids empirically, while 224 (32.5%) received neither; 61 (8.8%) received corticosteroids but not macrolides, while 214 (31.0%) received macrolides but not corticosteroids. Asthma hospitalization within the 24 months prior to admission was more common among those receiving macrolides (N = 60/405, 14.8%) than among those not receiving macrolides (N = 30/285, 10.5%) (P = 0.023). Macrolide recipients also more commonly received concomitant systemic corticosteroids (N = 191/405, 47.2%) than macrolide non‐recipients (N = 61/285, 21.4%) (P < 0.001) and more commonly received beta‐agonist therapy (N = 215/405, 53.1%) than macrolide non‐recipients (N = 113/285, 39.7%) (P = 0.001).
Length of Stay
The overall median LOS was 3 days (IQR, 2‐6 days); the median LOS was 3 days (IQR, 2‐5 days) for empiric macrolide recipients and 4 days (IQR, 2‐9 days) for non‐recipients (P < 0.001). Overall, 22.9% (N = 158) of children had an LOS 7 days and 8.8% (N = 61) of children had an LOS 14 days. The LOS was 7 days for 15.3% (N = 62) of macrolide recipients and 33.7% (N = 96) of non‐recipients. LOS was 7 days for 17.5% (N = 44) of systemic steroid recipients and 26% (N = 114) of non‐recipients. In unadjusted analysis, macrolide therapy (beta‐coefficient, 0.49; 95% confidence interval [CI]: 0.72 to 0.25; P < 0.001) and systemic corticosteroid administration (beta‐coefficient, 0.26; CI: 0.37 to 0.14; P < 0.001) were associated with shorter hospital LOS (Appendix 1).
In multivariable analysis, macrolide therapy remained associated with a shorter LOS (Table 2; Appendix 2). Systemic corticosteroid administration was associated with a 23% shorter LOS (adjusted beta‐coefficient, 0.26; 95% CI: 0.39 to 0.14; P < 0.001). In contrast, previous hospitalization for asthma was associated with a 31% longer LOS (adjusted beta‐coefficient, 0.27; 95% CI: 0.09‐0.045; P = 0.004). Receipt of beta‐agonist therapy or chronic asthma medications were not associated with significant differences in LOS. In analysis stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy, empiric macrolide therapy remained associated with a significantly shorter LOS in both systemic corticosteroid recipients and non‐recipient (Table 4). When the cohort was restricted to subjects with a LOS 7 days, macrolide therapy remained significantly associated with a shorter LOS (adjusted percent change, 20%; 95% CI: 32% to 5%; P = 0.015).
| Association of Empiric Macrolide Therapy With Outcomes* | |
|---|---|
| |
| Length of stay (days) | |
| Adjusted beta‐coefficient (95 % CI) | 0.38 (0.59 to 0.17) |
| Adjusted percent change (95% CI) | 32% (45% to 15%) |
| P value | 0.001 |
| Any readmission within 28 days | |
| Adjusted odds ratio (95% CI) | 1.12 (0.22 to 5.78) |
| P value | 0.890 |
| Any readmission within 15 mo | |
| Adjusted odds ratio (95% CI) | 1.00 (0.59 to 1.70) |
| P value | 0.991 |
| Asthma hospitalization within 15 mo | |
| Adjusted odds ratio (95% CI) | 1.09 (0.54 to 2.17) |
| P value | 0.820 |
Readmission
Overall, 8 children (1.2%) were readmitted for pneumonia‐associated conditions within 28 days of index discharge. Readmission occurred in 1.2% of macrolide recipients and 1.1% of non‐recipients (P = 0.83) (Table 4). In unadjusted analysis, neither macrolide therapy (odds ratio [OR], 1.18; 95% CI: 0.25‐5.45; P = 0.84) nor systemic corticosteroid administration (OR, 1.04; 95% CI: 0.27‐4.10; P = 0.95) was associated with 28‐day readmission (Appendix 3). In multivariable analysis, empiric macrolide therapy was not associated with 28‐day readmission in the overall cohort (Table 2; Appendix 4)), or when the analysis was stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy (Table 3).
| Concomitant Systemic Corticosteroid Therapy* | ||
|---|---|---|
| Yes | No | |
| ||
| Length of stay | ||
| Adjusted beta‐coefficient (95% CI) | 0.40 (0.74 to 0.07) | 0.37 (0.58 to 0.16) |
| Adjusted percent change (95% CI) | 33% (52% to 7%) | 31% (44% to 15%) |
| P value | 0.020 | 0.001 |
| Readmission within 28 days | ||
| Adjusted odds ratio (95% CI) | 1.09 (0.05 to 26.7) | 1.50 (0.21 to 10.8) |
| P value | 0.960 | 0.687 |
| Readmission within 15 mo | ||
| Adjusted odds ratio (95% CI) | 1.57 (0.65 to 3.82) | 0.81 (0.45 to 1.46) |
| P value | 0.32 | 0.49 |
| Asthma hospitalization within 15 mo | ||
| Adjusted odds ratio (95% CI) | 1.51 (0.58 to 3.93) | 0.85 (0.36 to 1.97) |
| P value | 0.395 | 0.700 |
| Empiric Macrolide Therapy | ||
|---|---|---|
| N/Total (%) | ||
| Readmission | Yes | No |
| Any readmission within 28 days | ||
| Overall | 5/405 (1.2) | 3/285 (1.1) |
| Systemic corticosteroid therapy | 2/186 (1.1) | 1/66 (1.5) |
| No systemic corticosteroid therapy | 3/177 (1.7) | 2/261 (0.8) |
| Any readmission within 15 mo | ||
| Overall | 96/405 (23.7) | 64/285 (22.5) |
| Systemic corticosteroid therapy | 52/186 (28.0) | 17/66 (25.8) |
| No systemic corticosteroid therapy | 32/177 (18.1) | 59/261 (22.6) |
| Asthma hospitalization within 15 mo | ||
| Overall | 61/405 (15.1) | 34/285 (11.9) |
| Systemic corticosteroid therapy | 39/186 (21.0) | 13/66 (19.7) |
| No systemic corticosteroid therapy | 14/177 (7.9) | 29/261 (11.1) |
Overall, 160 children (23.2%) were readmitted within 15 months of index discharge; 95 were readmitted for asthma during this time (Table 3). Overall readmission occurred in 23.7% of macrolide recipients and 22.5% of macrolide non‐recipients (P = 0.702). Asthma readmission occurred in 15.1% of macrolide recipients and 11.9% of macrolide non‐recipients (P = 0.240). In unadjusted analysis, empiric macrolide therapy was not significantly associated with any readmission within 15 months (OR, 1.07; 95% CI: 0.69‐1.68; P = 0.759) or with asthma‐related readmission within 15 months (OR, 1.31; 95% CI: 0.73‐ 2.36; P = 0.369). In multivariable analysis, neither any readmission nor asthma readmission within 15 months was associated with empiric macrolide therapy overall (Table 2) or when stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy (Table 3).
The analyses for readmissions within 15 months of index discharge were repeated while limiting the cohort to those without prior asthma hospitalizations or chronic asthma medications. In this subset of patients, readmissions for any reason occurred in 55 (18.6%) of 295 macrolide recipients and 50 (22.0%) of 227 non‐recipients. The difference was not statistically significant in multivariable analysis (adjusted odds ratio, 0.79; 95% CI: 0.41‐1.51; P = 0.47). Readmissions for asthma occurred in 30 (10.2%) of 295 macrolide recipients and 26 (11.5%) of 227 non‐recipients; this difference was also not significant in multivariable analysis (adjusted odds ratio, 0.83; 95% CI: 0.36‐1.93; P = 0.83). The magnitude of the estimate of effect for 28‐day and 15‐month readmissions, and 15‐month asthma hospitalizations, was similar to the primary analysis when the cohort was restricted to subjects with a LOS 7 days.
DISCUSSION
This multicenter study examined the role of macrolide therapy in children hospitalized with M. pneumoniae pneumonia. Empiric macrolide therapy was associated with an approximately 30% shorter hospital LOS and, in stratified analysis, remained associated with a significantly shorter hospital LOS in both systemic corticosteroid recipients and non‐recipients. Empiric macrolide therapy was not associated with short‐ or longer‐term hospital readmission.
Previous small randomized trials have been inconclusive regarding the potential benefit of macrolide therapy in M. pneumoniae pneumonia.11 Our study, which demonstrated a shorter LOS among macrolides recipients compared with non‐recipients, has several advantages over prior studies including a substantively larger sample size and multicenter design. Animal models support our observations regarding the potential beneficial antimicrobial role of macrolides. M. pneumoniae concentrations in bronchoalveolar lavage specimens were significantly lower among experimentally infected mice treated with clarithromycin, a macolide‐class antibiotic, compared with either placebo or dexamethasone.45 Combination therapy with clarithromycin and dexamethasone reduced histopathologic inflammation to a greater degree than dexamethasone alone.45
While the relative importance of the antimicrobial and anti‐inflammatory properties of macrolides is not known, observational studies of children infected with macrolide‐resistant M. pneumoniae suggest that the antimicrobial properties of macrolides may provide disproportionate clinical benefit. The duration of fever in macrolide recipients with macrolide‐resistant M. pneumoniae (median duration, 9 days) reported by Suzuki et al46 was significantly longer than those with macrolide‐susceptible infections (median duration, 5 days), and similar to the duration of fever in patients with M. pneumoniae infection treated with placebo (median duration, 8 days) reported by Kingston et al.47 Additionally, macrolide therapy was associated with significant improvements in lung function in patients with asthma and concomitant M. pneumoniae infection, but not in patients with asthma without documented M. pneumoniae infection.9 As corticosteroids also have anti‐inflammatory properties, we expect that any anti‐inflammatory benefit of macrolide therapy would be mitigated by the concomitant administration of corticosteroids. The shorter LOS associated with empiric macrolide therapy in our study was comparable among corticosteroid recipients and non‐recipients.
Atypical bacterial pathogens, including M. pneumoniae, are associated with diffuse lower airway inflammation6, 48 and airway hyperresponsiveness,6 and have been implicated as a cause of acute asthma exacerbations.7, 4954 Among patients with previously diagnosed asthma, acute M. pneumoniae infection was identified in up to 20% of those having acute exacerbations.7, 54 Macrolide therapy has a beneficial effect on lung function and airway hyperresponsiveness in adults with asthma.9, 55 Among mice infected with M. pneumoniae, 3 days of macrolide therapy resulted in a significant reduction in airway hyperresponsiveness compared with placebo or dexamethasone; however, after 6 days of therapy, there was no significant difference in airway hyperresponsiveness between those receiving macrolides, dexamethasone, or placebo, suggesting that the benefit of macrolides on airway hyperresponsiveness may be brief. Our findings of a shorter LOS but no difference in readmissions at 28 days or longer, for macrolide recipients compared with non‐recipients, support the limited benefit of macrolide therapy beyond the initial reduction in bacterial load seen in the first few days of therapy.
M. pneumoniae infection has also been implicated as a cause of chronic pulmonary disease, including asthma.610 In the mouse model, peribronchial and perivascular mononuclear infiltrates, increased airway methacholine reactivity, and increased airway obstruction were observed 530 days after M. pneumoniae inoculation.6 M. pneumoniae has been identified in 26 (50%) of 51 children experiencing their first asthma attack,7 and 23 (42%) of 55 adults with chronic, stable asthma.9 Nevertheless, results of other studies addressing the issue are inconsistent, and the role of M. pneumoniae in the development of asthma remains unclear.56 In order to investigate the impact of macrolide therapy on the development of chronic pulmonary disease requiring hospitalization, we examined the readmission rates in the 15 months following index discharge. The proportion of children hospitalized with asthma following the hospitalization for M. pneumoniae pneumonia was higher for both macrolide recipients and non‐recipients compared with the 24‐months prior to infection. These results support a possible role for M. pneumoniae in chronic pulmonary disease. However, macrolide therapy was not associated with long‐term overall hospital readmission or long‐term asthma readmission, either in the entire cohort or in the subset of patients without prior asthma hospitalizations or medications.
This study had several limitations. First, because the identification of children with M. pneumoniae pneumonia relied on ICD‐9 discharge diagnosis codes, it is possible that there was misclassification of disease. We minimized the inclusion of children without M. pneumoniae by including only children who received antibiotic therapy on the first day of hospitalization and by excluding patients younger than 6 years of age, a group at relatively low‐risk for M. pneumoniae infection. Further, our algorithm for identification of M. pneumoniae pneumonia was validated through review of the medical records at 1 institution and was found to have a high positive predictive value. However, the positive predictive value of these ICD‐9 codes may vary across institutions. Additionally, the sensitivity of ICD‐9 codes for identifying children with M. pneumoniae pneumonia is not known. Also, not all children with pneumonia undergo testing for M. pneumoniae, and different tests have varying sensitivity and specificity.57, 58 Thus, some children with M. pneumoniae pneumonia were not diagnosed and so were not included in our study. It is not known how inclusion of these children would affect our results.
Second, the antibiotic information used in this study was limited to empiric antibiotic therapy. It is possible that some patients received macrolide therapy before admission. It is also likely that identification of M. pneumoniae during the hospitalization prompted the addition or substitution of macrolide therapy for some patients. If this therapy was initiated beyond the first day of hospitalization, these children would be classified as macrolide non‐recipients. Since macrolide administration was associated with a shorter hospital LOS, such misclassification would bias our results towards finding no difference in LOS between macrolide recipients and non‐recipients. It is therefore possible that the benefit of macrolide therapy is even greater than found in our study.
Third, there may be unmeasured confounding or residual confounding by indication for adjunct corticosteroid therapy related to clinical presentation. We expect that corticosteroid recipients would be sicker than non‐recipients. We included variables associated with a greater severity of illness (such as intensive care unit admission) in the multivariable analysis. Additionally, the shorter LOS among macrolide recipients remained when the analysis was stratified by receipt or non‐receipt of systemic corticosteroid therapy.
Fourth, we were only able to record readmissions occurring at the same hospital as the index admission; any readmission presenting to a different hospital following their index admission did not appear in our records, and was therefore not counted. It is thus possible that the true number of readmissions is higher than that represented here. Finally, despite the large number of patients included in this study, the number of short‐term readmissions was relatively small. Thus, we may have been underpowered to detect small but significant differences in short‐term readmission rates.
In conclusion, macrolide therapy was associated with shorter hospital LOS, but not with short‐term or longer‐term readmission in children presenting with M. pneumoniae pneumonia.
Appendix
| Variable | Beta Coefficient | Confidence Interval | P Value |
|---|---|---|---|
| Demographics | |||
| Sex | 0.12 | (0.22, 0.02) | 0.022 |
| Race | |||
| Blackreference category | |||
| White | 0.01 | (0.21, 0.23) | 0.933 |
| Other | 0.13 | (0.39, 0.13) | 0.323 |
| Missing | 0.46 | (0.81, 0.11) | 0.012 |
| Presentation during viral respiratory season | 0.05 | (0.19, 0.09) | 0.462 |
| Prior asthma hospitalization | 0.36 | (0.64, 0.08) | 0.015 |
| Intensive care unit admission | 1.05 | (0.87, 1.23) | <0.001 |
| Labs and procedures performed | |||
| Additional radiologic imaging | 0.23 | (0.20, 0.67) | 0.287 |
| Arterial blood gas | 0.69 | (0.50, 0.87) | <0.001 |
| Complete blood count | 0.34 | (0.24, 0.45) | <0.001 |
| Blood culture | 0.17 | (0.98, 0.44) | 0.204 |
| Mechanical ventilation | 1.15 | (0.68, 1.63) | <0.001 |
| Therapies received | |||
| Empiric macrolide therapy | 0.49 | (0.72, 0.25) | <0.001 |
| Systemic steroids | 0.26 | (0.38, 0.14) | <0.001 |
| Chronic asthma medications | 0.20 | (0.38, 0.013) | 0.037 |
| Beta‐agonist therapy | 0.07 | (0.21, 0.08) | 0.357 |
| Vasoactive infusion | 1.08 | (0.727, 1.45) | <0.001 |
| Clindamycin or vancomycin | 0.55 | (0.34, 0.75) | <0.001 |
| Variable | Odds Ratio* | Confidence Interval | P Value |
|---|---|---|---|
| |||
| Demographics | |||
| Sex | 0.56 | (0.23, 1.33) | 0.190 |
| Race | |||
| Blackreference category | |||
| White | 0.46 | (0.19, 1.14) | 0.093 |
| Other | |||
| Missing | |||
| Presentation during viral respiratory season | 0.64 | (0.09, 4.75) | 0.662 |
| Prior asthma hospitalization | |||
| Intensive care unit admission | 4.54 | (1.21, 17.03) | 0.025 |
| Laboratory tests and procedures | |||
| Additional radiologic imaging | 10.00 | (2.25, 44.47) | 0.002 |
| Arterial blood gas | |||
| Complete blood count | 0.92 | (0.24, 3.48) | 0.901 |
| Blood culture | 0.85 | (0.30, 2.36) | 0.738 |
| Mechanical ventilation | |||
| Medications | |||
| Macrolide therapy | 1.18 | (0.25, 5.45) | 0.837 |
| Systemic corticosteroids | 1.04 | (0.276, 4.09) | 0.951 |
| Chronic asthma medication | 1.66 | (0.71, 3.88) | 0.242 |
| Beta‐agonist therapy | 0.66 | (0.16, 2.65) | 0.557 |
| Vasoactive infusions | |||
| Clindamycin or vancomycin | 1.00 | (0.10, 9.90) | 0.998 |
| Variable | Coefficient | Confidence Interval | P Value | % Change | Confidence Interval for % Change |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | 0.287 | (0.012, 0.045) | 0.001 | 2.9 | (1.2, 4.6) |
| Prior asthma hospitalization | 0.272 | (0.094, 0.45) | 0.004 | 31.3 | (9.9, 56.8) |
| Intensive care unit admission | 1.015 | (0.802, 1.23) | <0.001 | 175.9 | (123.0, 241.3) |
| Therapies received | |||||
| Macrolide therapy | 0.379 | (0.59, 0.166) | 0.001 | 31.6 | (44.6, 15.3) |
| Systemic corticosteroids | 0.264 | (0.391, 0.138) | <0.001 | 23.2 | (32.3, 12.9) |
| Chronic asthma medications | 0.056 | (0.255, 0.142) | 0.568 | 5.5 | (22.5, 15.2) |
| Albuterol | 0.07 | (0.059, 0.199) | 0.281 | 7.2 | (5.8, 22.0) |
| Clindamycin or vancomycin | 0.311 | (0.063, 0.559) | 0.015 | 36.5 | (6.5, 74.9) |
| Variable | Adjusted Odds Ratio | Confidence Interval | P Value |
|---|---|---|---|
| Demographics | |||
| Age | 0.91 | 0.72, 1.15 | 0.423 |
| Prior asthma hospitalization | 1.94 | 0.42, 8.90 | 0.394 |
| Intensive care unit admission | 5.73 | 2.03, 16.20 | 0.001 |
| Therapies received | |||
| Macrolide therapy | 1.12 | 0.22, 5.78 | 0.890 |
| Systemic corticosteroids | 0.696 | 0.10, 4.70 | 0.710 |
| Chronic asthma medications | 1.98 | 0.32, 12.20 | 0.460 |
| Albuterol | 0.519 | 0.081, 3.31 | 0.488 |
| Clindamycin or vancomycin | 0.904 | 0.07, 11.13 | 0.937 |
- ,,, et al.Prospective surveillance for atypical pathogens in children with community‐acquired pneumonia in Japan.J Infect Chemother.2006;12:36–41.
- ,,.Incidence of community‐acquired pneumonia in children caused by Mycoplasma pneumoniae: serological results of a prospective, population‐based study in primary health care.Respirology.2004;9:109–114.
- ,,.Mycoplasma pneumoniae infections in University of Wisconsin students.Am Rev Respir Dis.1967;96:237–244.
- .Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients.Clin Infect Dis.1993;17(suppl 1):S37–S46.
- .Mycoplasma pneumoniae. In: Long SS, Pickering LK, Prober CG, eds.Principles and Practice of Pediatric Infectious Diseases.3rd ed.Philadelphia, PA:Churchill Livingstone;2008:979–985.
- ,,, et al.Mycoplasma pneumoniae induces chronic respiratory infection, airway hyperreactivity, and pulmonary inflammation: a murine model of infection‐associated chronic reactive airway disease.Infect Immun.2002;70:649–654.
- ,,, et al.Mycoplasma pneumoniae and asthma in children.Clin Infect Dis.2004;38:1341–1346.
- ,,,.Isolation of Mycoplasma pneumoniae from asthmatic patients.Ann Allergy.1993;70:23–25.
- ,,,.Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin.Chest.2002;121:1782–1788.
- ,,,,.A link between chronic asthma and chronic infection.J Allergy Clin Immunol.2001;107:595–601.
- ,,.Antibiotics for community‐acquired lower respiratory tract infections secondary to Mycoplasma pneumoniae in children.Cochrane Database Syst Rev.2010;7:CD004875.
- ,,,.In vitro susceptibilities of mycoplasmas and ureaplasmas to new macrolides and aryl‐fluoroquinolones.Antimicrob Agents Chemother.1988;32:1500–1502.
- ,,.Inhibitory and bactericidal activities of gemifloxacin and other antimicrobials against Mycoplasma pneumoniae.Int J Antimicrob Agents.2003;21:574–577.
- ,,,,.The in vitro activity of some 14‐, 15‐ and 16‐ membered macrolides against Staphylococcus spp., Legionella spp., Mycoplasma spp. and Ureaplasma urealyticum.Drugs Exp Clin Res.1991;17:91–99.
- ,,, et al.In vitro and in vivo activities of macrolides against Mycoplasma pneumoniae.Antimicrob Agents Chemother.1994;38:790–798.
- ,.Comparative in vitro activity of azithromycin, clarithromycin, erythromycin and lomefloxacin against Mycoplasma pneumoniae, Mycoplasma hominis and Ureaplasma urealyticum.Eur J Clin Microbiol Infect Dis.1990;9:838–841.
- ,,, et al.Characteristics of macrolide‐resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro.Microbiol Immunol.2001;45:617–620.
- ,,, et al.Characterization and molecular analysis of macrolide‐resistant Mycoplasma pneumoniae clinical isolates obtained in Japan.Antimicrob Agents Chemother.2004;48:4624–4630.
- ,,,.Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community‐acquired lower respiratory tract infections.Clin Infect Dis.2001;32:1281–1289.
- ,,, et al.Characteristics of Streptococcus pneumoniae and atypical bacterial infections in children 2–5 years of age with community‐acquired pneumonia.Clin Infect Dis.2002;35:1345–1352.
- ,,,,.Macrolide use shortens fever duration in Mycoplasma pneumoniae infection in children: a 2‐year experience.J Microbiol Immunol Infect.2008;41:307–310.
- ,,,,.Comparison of antibiotics in the treatment of mycoplasmal pneumonia.Arch Intern Med.1970;125:680–684.
- ,,, et al.Antimicrobial and immunologic activities of clarithromycin in a murine model of Mycoplasma pneumoniae‐induced pneumonia.Antimicrob Agents Chemother.2003;47:1614–1620.
- ,.Antibiotics in asthma.Curr Allergy Asthma Rep.2004;4:132–138.
- ,.Immunomodulatory activity and effectiveness of macrolides in chronic airway disease.Chest.2004;125:70S–78S.
- ,,, et al.Interleukin‐8 gene repression by clarithromycin is mediated by the activator protein‐1 binding site in human bronchial epithelial cells.Am J Respir Cell Mol Biol.2000;22:51–60.
- ,,, et al.Clarithromycin inhibits NF‐kappaB activation in human peripheral blood mononuclear cells and pulmonary epithelial cells.Antimicrob Agents Chemother.2001;45:44–47.
- ,,,,.Epidemiology of Mycoplasma pneumoniae infection in families.JAMA.1966;197:859–866.
- ,,.Shedding of Mycoplasma pneumoniae after tetracycline and erythromycin therapy.N Engl J Med.1967;276:1172–1175.
- ,,.Mycoplasma pneumoniae disease: clinical spectrum, pathophysiology, epidemiology, and control.J Infect Dis.1971;123:74–92.
- .Is there a role for antibiotics in the treatment of asthma? Involvement of atypical organisms.BioDrugs.2000;14:349–354.
- ,.Diffuse panbronchiolitis: role of macrolides in therapy.Am J Respir Med.2002;1:119–131.
- ,,,,,.Long‐term low‐dose administration of erythromycin to patients with diffuse panbronchiolitis.Respiration.1991;58:145–149.
- ,,,,,.[Long‐term therapeutic effects of erythromycin and newquinolone antibacterial agents on diffuse panbronchiolitis].Nihon Kyobu Shikkan Gakkai Zasshi.1990;28:1305–1313.
- ,,, et al.A comparative clinical study of macrolide‐sensitive and macrolide‐resistant Mycoplasma pneumoniae infections in pediatric patients.J Infect Chemother.2009;15:380–383.
- ,,,.Corticosteroids and mortality in children with bacterial meningitis.JAMA.2008;299:2048–2055.
- ,,,,.Intravenous immunoglobulin in children with streptococcal toxic shock syndrome.Clin Infect Dis.2009;49:1369–1376.
- ,,, et al.Etiology of childhood pneumonia: serologic results of a prospective, population‐based study.Pediatr Infect Dis J.1998;17:986–991.
- ,,,,,.Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services.Pediatrics.2001;107:E99.
- ,,,,,.Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia.Pediatrics.2011;127:e255–e263.
- ,,, et al.Comparative effectiveness of pleural drainage procedures for the treatment of complicated pneumonia in childhood.J Hosp Med.2011;6:256–263.
- ,,, et al.Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma.Am J Respir Crit Care Med.1998;158:998–1001.
- ,.The impact of confounder selection criteria on effect estimation.Am J Epidemiol.1989;129:125–137.
- ,,.The risk of determining risk with multivariable models.Ann Intern Med.1993;118:201–210.
- ,,, et al.The impact of steroids given with macrolide therapy on experimental Mycoplasma pneumoniae respiratory infection.J Infect Dis.2008;198:1180–1188.
- ,,, et al.Clinical evaluation of macrolide‐resistant Mycoplasma pneumoniae.Antimicrob Agents Chemother.2006;50:709–712.
- ,,, et al.Eaton agent pneumonia.JAMA.1961;176:118–123.
- .The role of viral and atypical bacterial pathogens in asthma pathogenesis.Pediatr Pulmonol Suppl.1999;18:141–143.
- ,,.The association of viral and mycoplasma infections with recurrence of wheezing in the asthmatic child.Ann Allergy.1970;28:43–49.
- ,,,.Association of viral and mycoplasma infections with exacerbations of asthma.Ann Allergy.1974;33:145–149.
- ,,, et al.Acute Chlamydia pneumoniae and Mycoplasma pneumoniae infections in community‐acquired pneumonia and exacerbations of COPD or asthma: therapeutic considerations.J Chemother.2004;16:70–76.
- ,,, et al.Mycoplasma pneumoniae is a frequent cause of exacerbation of bronchial asthma in adults.Ann Allergy.1986;57:263–265.
- ,,, et al.Acute exacerbations of asthma in adults: role of Chlamydia pneumoniae infection.Eur Respir J.1994;7:2165–2168.
- ,,, et al.Atypical pathogen infection in adults with acute exacerbation of bronchial asthma.Am J Respir Crit Care Med.2003;167:406–410.
- ,,,,,.Erythromycin reduces the severity of bronchial hyperresponsiveness in asthma.Chest.1991;99:670–673.
- ,,,,.Atypical bacteria and macrolides in asthma.Allergy Asthma Clin Immunol.2008;4:111–116.
- ,,.Acute respiratory infection due to Mycoplasma pneumoniae: current status of diagnostic methods.Eur J Clin Microbiol Infect Dis.2010;29:1055–1069.
- ,,,,,.A multicenter pilot external quality assessment programme to assess the quality of molecular detection of Chlamydophila pneumoniae and Mycoplasma pneumoniae.J Microbiol Methods.2010;82:131–135.
Mycoplasma pneumoniae is a common cause of community‐acquired pneumonia (CAP), among school‐age children and adolescents.14 Though pneumonia caused by M. pneumoniae is typically self‐limited, severe illness may occur.5 M. pneumoniae has also been implicated in airway inflammation, which may lead to the onset and development of chronic pulmonary disease.610 Few studies have directly addressed appropriate treatment strategies for M. pneumoniae pneumonia,11 and, despite its high prevalence and potential for causing severe complications, treatment recommendations remain inconsistent.
The efficacy of macrolide therapy in particular for M. pneumoniae remains unclear. In vitro susceptibility studies have shown bacteriostatic activity of erythromycin, clarithromycin, and azithromycin against M. pneumoniae.1218 Additionally, several small retrospective studies have shown that among children with atypical CAP (including M. pneumoniae pneumonia), those treated with macrolides were less likely to have persistence or progression of signs and symptoms after 3 days of therapy.19, 20 Lu et al21 found a shorter duration of fever among macrolide recipients compared with non‐recipients. In adults, Shames et al22 found a shorter duration of fever and hospitalization among erythromycin recipients compared with controls. Other randomized controlled trials have also addressed the use of macrolides in treatment of M. pneumoniae, but the ability to draw meaningful conclusions is limited by small samples sizes and by lack of details about the number of patients with M. pneumoniae.11
In addition to their antimicrobial effect, macrolides also have anti‐inflammatory properties.2327 The importance of these anti‐inflammatory properties is supported by studies showing clinical cure in patients treated with macrolides despite persistence of M. pneumoniae organisms,2831 clinical improvement despite the administration of doses that provide tissue levels below the minimum inhibitory concentration of the organism,3234 and clinical cure in patients with macrolide‐resistant M. pneumoniae.18, 35
The objectives of the current study were to examine the impact of macrolide therapy on the length of stay (LOS) and short‐ and longer‐term readmissions, including longer‐term asthma‐related readmissions, in children hospitalized with M. pneumoniae pneumonia.
METHODS
Data Source
Data for this retrospective cohort study were obtained from the Pediatric Health Information System (PHIS), which contains administrative data from 38 freestanding children's hospitals. Data quality and reliability are assured through a joint effort by the Child Health Corporation of America (Shawnee Mission, KS) and PHIS‐participating hospitals as described previously.36, 37 Encrypted medical record numbers allow for tracking of individual patients across hospitalizations. This study was reviewed and approved by the Committees for the Protection of Human Subjects at The Children's Hospital of Philadelphia (Philadelphia, PA).
Patients
Children 6‐18 years of age with CAP were eligible if they were discharged from a participating hospital between January 1, 2006 and December 31, 2008. Subjects were included if they received antibiotic therapy on the first day of hospitalization and if they satisfied one of the following International Classification of Diseases, 9th revision (ICD‐9) discharge diagnosis code criteria: 1) Principal diagnosis of M. pneumoniae pneumonia (483.0); 2) Principal diagnosis of a pneumonia‐related symptom (eg, fever, cough) (780.6 or 786.00‐786.52 [except 786.1]) and a secondary diagnosis of M. pneumoniae pneumonia; or 3) Principal diagnosis of pneumonia (481‐483.8 [except 483.0], 485‐486) and a secondary diagnosis of Mycoplasma (041.81).
Children younger than 6 years of age were excluded due to the low prevalence of M. pneumoniae infection.2, 38 Patients with comorbid conditions predisposing to severe or recurrent pneumonia (eg, cystic fibrosis, malignancy) were excluded using a previously reported classification scheme.39 In addition, we excluded patient data from 2 hospitals due to incomplete reporting of discharge information; thus data from 36 hospitals were included in this study.
Validation of Discharge Diagnosis Codes for Mycoplasma pneumoniae
To assess for misclassification of the diagnosis of M. pneumoniae, we reviewed records of a randomly selected subset of subjects from The Children's Hospital of Philadelphia; 14 of 15 patients had signs of lower respiratory tract infection in conjunction with a positive M. pneumoniae polymerase chain reaction test from nasopharyngeal washings to confirm the diagnosis of M. pneumoniae pneumonia. Hence, the positive predictive value of our algorithm for diagnosing M. pneumoniae pneumonia was 93.3%.
Study Definitions
We identified children with asthma in 2 ways. Asthma‐related hospitalizations were identified by an ICD‐9 code for asthma (493.0‐493.92) in any discharge diagnosis field during any hospitalization in the 24 months prior to the current hospitalization. Baseline controller medications were identified by receipt of inhaled corticosteroids (eg, fluticasone) or leukotriene receptor antagonists on the first day of hospitalization.
Systemic corticosteroids (either oral or intravenous) included dexamethasone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Measures of disease severity included admission to the intensive care unit within 48 hours of hospitalization, and administration of vancomycin or clindamycin, vasoactive infusions (epinephrine, norepinephrine, dopamine, and dobutamine), and invasive (endotracheal intubation) and noninvasive (continuous positive airway pressure) mechanical ventilation within 24 hours of hospitalization, as previously described.40, 41 Viral respiratory season was defined as October through March.
Measured Outcomes
The primary outcomes of interest were hospital LOS and all‐cause readmission within 28 days and 15 months after index discharge. We examined readmissions for asthma 15 months after index discharge as a secondary outcome measure because of the potential role for M. pneumoniae infection in long‐term lung dysfunction, including asthma.42 The 15‐month time frame was selected based on longitudinal data available in PHIS for the entire study cohort.
Measured Exposures
The main exposure was early initiation of macrolide therapy, defined as receipt of erythromycin, clarithromycin, or azithromycin on the first day of hospitalization.
Data Analysis
Continuous variables were described using median and interquartile range (IQR) or range values, and compared using the Wilcoxon rank‐sum test. Categorical variables were described using counts and frequencies, and compared using the chi‐square test. Multivariable linear (for LOS) and logistic (for readmission) regression analyses were performed to assess the independent association of macrolide therapy with the primary outcomes. Because the LOS data had a skewed distribution, our analyses were performed using logarithmically transformed LOS values as the dependent variable. The resulting beta‐coefficients were transformed to reflect the percent difference in LOS between subjects receiving and not receiving macrolide therapy.
Building of the multivariable models began with the inclusion of macrolide therapy. Variables associated with primary outcomes on univariate analysis (P < 0.20) were also considered for inclusion as potential confounders.43 These variables were included in the final multivariable model if they remained significant after adjusting for other factors, or if their inclusion in the model resulted in a 15% or greater change in the effect size of the primary association of interest (ie, macrolide therapy).44 Because corticosteroids also have anti‐inflammatory properties, we assessed for interactions with macrolide therapy. There was no interaction between macrolide and systemic corticosteroid therapy (P = 0.26, Likelihood ratio test), therefore our primary model adjusted for systemic corticosteroids.
Despite adjusting for systemic corticosteroid therapy in our primary analysis, residual confounding by indication for corticosteroid therapy might exist. We therefore repeated the analysis after stratifying by receipt or non‐receipt of systemic corticosteroid therapy. Because the benefit of macrolides in preventing long‐term dysfunction may be limited to those without a prior diagnosis of asthma, we repeated the analysis of readmissions within 15 months of index discharge (any readmission and asthma‐related readmissions) while limiting the cohort to those without evidence of asthma (ie, no prior asthma‐related hospitalizations and no chronic asthma medications). Because children with underlying conditions or circumstances that would predispose to prolonged hospitalizations may have been included, despite our restriction of the cohort to those without an identified chronic complex condition, we also repeated the analysis while limiting the cohort to those with a LOS 7 days. Finally, all analyses were clustered on hospital using the robust standard errors of Huber and White to account for the correlation of exposures and outcomes among children within centers.
Data were analyzed using Stata version 11 (Stata Corporation, College Station, TX). Statistical significance was determined a priori as a two‐tailed P value <0.05.
RESULTS
Patient Characteristics
During the study, 690 children ages 6 to 18 years met inclusion criteria. Characteristics of these patients are shown in Table 1. The median age was 10 years (IQR, 7‐13 years). Ten patients (1.4%) also had a concomitant discharge diagnosis of pneumococcal pneumonia, while 19 patients (2.7%) had a concomitant discharge diagnosis of viral pneumonia; 1 of these patients had discharge diagnoses of both viral and pneumococcal pneumonia.
| Empiric Macrolide Therapy | ||||
|---|---|---|---|---|
| Variable | All Subjects | Yes | No | P |
| ||||
| Demographics | ||||
| Male sex | 356 (51.6) | 200 (49.4) | 156 (54.7) | 0.166 |
| Race | ||||
| Black | 135 (19.6) | 81 (20.0) | 54 (19.0) | 0.506 |
| White | 484 (70.1) | 287 (70.9) | 197 (69.1) | |
| Other | 62 (9.0) | 31 (7.7) | 31 (10.9) | |
| Missing | 9 (1.3) | 6 (1.5) | 3 (1.1) | |
| Presentation during viral respiratory season | 420 (60.9) | 242 (59.8) | 178 (62.5) | |
| Prior asthma hospitalization | 41 (5.9) | 31 (7.7) | 10 (3.5) | 0.023 |
| Intensive care unit admission | 127 (18.4) | 74 (18.3) | 53 (18.6) | 0.914 |
| Laboratory tests and procedures | ||||
| Additional radiologic imaging* | 24 (3.5) | 13 (3.2) | 11 (3.9) | 0.646 |
| Arterial blood gas | 116 (17.3) | 72 (18.5) | 44 (15.6) | 0.316 |
| Complete blood count | 433 (64.4) | 249 (64.0) | 184 (65.0) | 0.788 |
| Blood culture | 280 (41.7) | 167 (42.9) | 113 (39.9) | 0.436 |
| Mechanical ventilation | 16 (2.3) | 5 (1.2) | 11 (3.86) | 0.024 |
| Medications | ||||
| Chronic asthma medication | 116 (16.8) | 72 (17.8) | 44 (15.4) | 0.419 |
| Beta‐agonist therapy | 328 (47.5) | 215 (53.1) | 113 (39.7) | 0.001 |
| Vasoactive infusions | 22 (3.2) | 13 (3.2) | 9 (3.2) | 0.969 |
| Systemic corticosteroids | 252 (36.5) | 191 (47.2) | 61 (21.4) | <0.001 |
| Clindamycin or vancomycin | 86 (12.5) | 24 (5.9) | 62 (21.8) | <0.001 |
Macrolide therapy was administered to 405 (58.7%) patients. Systemic corticosteroid therapy was administered to 252 (36.5%) patients. Overall, 191 (27.7%) of the 690 patients received both macrolides and systemic corticosteroids empirically, while 224 (32.5%) received neither; 61 (8.8%) received corticosteroids but not macrolides, while 214 (31.0%) received macrolides but not corticosteroids. Asthma hospitalization within the 24 months prior to admission was more common among those receiving macrolides (N = 60/405, 14.8%) than among those not receiving macrolides (N = 30/285, 10.5%) (P = 0.023). Macrolide recipients also more commonly received concomitant systemic corticosteroids (N = 191/405, 47.2%) than macrolide non‐recipients (N = 61/285, 21.4%) (P < 0.001) and more commonly received beta‐agonist therapy (N = 215/405, 53.1%) than macrolide non‐recipients (N = 113/285, 39.7%) (P = 0.001).
Length of Stay
The overall median LOS was 3 days (IQR, 2‐6 days); the median LOS was 3 days (IQR, 2‐5 days) for empiric macrolide recipients and 4 days (IQR, 2‐9 days) for non‐recipients (P < 0.001). Overall, 22.9% (N = 158) of children had an LOS 7 days and 8.8% (N = 61) of children had an LOS 14 days. The LOS was 7 days for 15.3% (N = 62) of macrolide recipients and 33.7% (N = 96) of non‐recipients. LOS was 7 days for 17.5% (N = 44) of systemic steroid recipients and 26% (N = 114) of non‐recipients. In unadjusted analysis, macrolide therapy (beta‐coefficient, 0.49; 95% confidence interval [CI]: 0.72 to 0.25; P < 0.001) and systemic corticosteroid administration (beta‐coefficient, 0.26; CI: 0.37 to 0.14; P < 0.001) were associated with shorter hospital LOS (Appendix 1).
In multivariable analysis, macrolide therapy remained associated with a shorter LOS (Table 2; Appendix 2). Systemic corticosteroid administration was associated with a 23% shorter LOS (adjusted beta‐coefficient, 0.26; 95% CI: 0.39 to 0.14; P < 0.001). In contrast, previous hospitalization for asthma was associated with a 31% longer LOS (adjusted beta‐coefficient, 0.27; 95% CI: 0.09‐0.045; P = 0.004). Receipt of beta‐agonist therapy or chronic asthma medications were not associated with significant differences in LOS. In analysis stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy, empiric macrolide therapy remained associated with a significantly shorter LOS in both systemic corticosteroid recipients and non‐recipient (Table 4). When the cohort was restricted to subjects with a LOS 7 days, macrolide therapy remained significantly associated with a shorter LOS (adjusted percent change, 20%; 95% CI: 32% to 5%; P = 0.015).
| Association of Empiric Macrolide Therapy With Outcomes* | |
|---|---|
| |
| Length of stay (days) | |
| Adjusted beta‐coefficient (95 % CI) | 0.38 (0.59 to 0.17) |
| Adjusted percent change (95% CI) | 32% (45% to 15%) |
| P value | 0.001 |
| Any readmission within 28 days | |
| Adjusted odds ratio (95% CI) | 1.12 (0.22 to 5.78) |
| P value | 0.890 |
| Any readmission within 15 mo | |
| Adjusted odds ratio (95% CI) | 1.00 (0.59 to 1.70) |
| P value | 0.991 |
| Asthma hospitalization within 15 mo | |
| Adjusted odds ratio (95% CI) | 1.09 (0.54 to 2.17) |
| P value | 0.820 |
Readmission
Overall, 8 children (1.2%) were readmitted for pneumonia‐associated conditions within 28 days of index discharge. Readmission occurred in 1.2% of macrolide recipients and 1.1% of non‐recipients (P = 0.83) (Table 4). In unadjusted analysis, neither macrolide therapy (odds ratio [OR], 1.18; 95% CI: 0.25‐5.45; P = 0.84) nor systemic corticosteroid administration (OR, 1.04; 95% CI: 0.27‐4.10; P = 0.95) was associated with 28‐day readmission (Appendix 3). In multivariable analysis, empiric macrolide therapy was not associated with 28‐day readmission in the overall cohort (Table 2; Appendix 4)), or when the analysis was stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy (Table 3).
| Concomitant Systemic Corticosteroid Therapy* | ||
|---|---|---|
| Yes | No | |
| ||
| Length of stay | ||
| Adjusted beta‐coefficient (95% CI) | 0.40 (0.74 to 0.07) | 0.37 (0.58 to 0.16) |
| Adjusted percent change (95% CI) | 33% (52% to 7%) | 31% (44% to 15%) |
| P value | 0.020 | 0.001 |
| Readmission within 28 days | ||
| Adjusted odds ratio (95% CI) | 1.09 (0.05 to 26.7) | 1.50 (0.21 to 10.8) |
| P value | 0.960 | 0.687 |
| Readmission within 15 mo | ||
| Adjusted odds ratio (95% CI) | 1.57 (0.65 to 3.82) | 0.81 (0.45 to 1.46) |
| P value | 0.32 | 0.49 |
| Asthma hospitalization within 15 mo | ||
| Adjusted odds ratio (95% CI) | 1.51 (0.58 to 3.93) | 0.85 (0.36 to 1.97) |
| P value | 0.395 | 0.700 |
| Empiric Macrolide Therapy | ||
|---|---|---|
| N/Total (%) | ||
| Readmission | Yes | No |
| Any readmission within 28 days | ||
| Overall | 5/405 (1.2) | 3/285 (1.1) |
| Systemic corticosteroid therapy | 2/186 (1.1) | 1/66 (1.5) |
| No systemic corticosteroid therapy | 3/177 (1.7) | 2/261 (0.8) |
| Any readmission within 15 mo | ||
| Overall | 96/405 (23.7) | 64/285 (22.5) |
| Systemic corticosteroid therapy | 52/186 (28.0) | 17/66 (25.8) |
| No systemic corticosteroid therapy | 32/177 (18.1) | 59/261 (22.6) |
| Asthma hospitalization within 15 mo | ||
| Overall | 61/405 (15.1) | 34/285 (11.9) |
| Systemic corticosteroid therapy | 39/186 (21.0) | 13/66 (19.7) |
| No systemic corticosteroid therapy | 14/177 (7.9) | 29/261 (11.1) |
Overall, 160 children (23.2%) were readmitted within 15 months of index discharge; 95 were readmitted for asthma during this time (Table 3). Overall readmission occurred in 23.7% of macrolide recipients and 22.5% of macrolide non‐recipients (P = 0.702). Asthma readmission occurred in 15.1% of macrolide recipients and 11.9% of macrolide non‐recipients (P = 0.240). In unadjusted analysis, empiric macrolide therapy was not significantly associated with any readmission within 15 months (OR, 1.07; 95% CI: 0.69‐1.68; P = 0.759) or with asthma‐related readmission within 15 months (OR, 1.31; 95% CI: 0.73‐ 2.36; P = 0.369). In multivariable analysis, neither any readmission nor asthma readmission within 15 months was associated with empiric macrolide therapy overall (Table 2) or when stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy (Table 3).
The analyses for readmissions within 15 months of index discharge were repeated while limiting the cohort to those without prior asthma hospitalizations or chronic asthma medications. In this subset of patients, readmissions for any reason occurred in 55 (18.6%) of 295 macrolide recipients and 50 (22.0%) of 227 non‐recipients. The difference was not statistically significant in multivariable analysis (adjusted odds ratio, 0.79; 95% CI: 0.41‐1.51; P = 0.47). Readmissions for asthma occurred in 30 (10.2%) of 295 macrolide recipients and 26 (11.5%) of 227 non‐recipients; this difference was also not significant in multivariable analysis (adjusted odds ratio, 0.83; 95% CI: 0.36‐1.93; P = 0.83). The magnitude of the estimate of effect for 28‐day and 15‐month readmissions, and 15‐month asthma hospitalizations, was similar to the primary analysis when the cohort was restricted to subjects with a LOS 7 days.
DISCUSSION
This multicenter study examined the role of macrolide therapy in children hospitalized with M. pneumoniae pneumonia. Empiric macrolide therapy was associated with an approximately 30% shorter hospital LOS and, in stratified analysis, remained associated with a significantly shorter hospital LOS in both systemic corticosteroid recipients and non‐recipients. Empiric macrolide therapy was not associated with short‐ or longer‐term hospital readmission.
Previous small randomized trials have been inconclusive regarding the potential benefit of macrolide therapy in M. pneumoniae pneumonia.11 Our study, which demonstrated a shorter LOS among macrolides recipients compared with non‐recipients, has several advantages over prior studies including a substantively larger sample size and multicenter design. Animal models support our observations regarding the potential beneficial antimicrobial role of macrolides. M. pneumoniae concentrations in bronchoalveolar lavage specimens were significantly lower among experimentally infected mice treated with clarithromycin, a macolide‐class antibiotic, compared with either placebo or dexamethasone.45 Combination therapy with clarithromycin and dexamethasone reduced histopathologic inflammation to a greater degree than dexamethasone alone.45
While the relative importance of the antimicrobial and anti‐inflammatory properties of macrolides is not known, observational studies of children infected with macrolide‐resistant M. pneumoniae suggest that the antimicrobial properties of macrolides may provide disproportionate clinical benefit. The duration of fever in macrolide recipients with macrolide‐resistant M. pneumoniae (median duration, 9 days) reported by Suzuki et al46 was significantly longer than those with macrolide‐susceptible infections (median duration, 5 days), and similar to the duration of fever in patients with M. pneumoniae infection treated with placebo (median duration, 8 days) reported by Kingston et al.47 Additionally, macrolide therapy was associated with significant improvements in lung function in patients with asthma and concomitant M. pneumoniae infection, but not in patients with asthma without documented M. pneumoniae infection.9 As corticosteroids also have anti‐inflammatory properties, we expect that any anti‐inflammatory benefit of macrolide therapy would be mitigated by the concomitant administration of corticosteroids. The shorter LOS associated with empiric macrolide therapy in our study was comparable among corticosteroid recipients and non‐recipients.
Atypical bacterial pathogens, including M. pneumoniae, are associated with diffuse lower airway inflammation6, 48 and airway hyperresponsiveness,6 and have been implicated as a cause of acute asthma exacerbations.7, 4954 Among patients with previously diagnosed asthma, acute M. pneumoniae infection was identified in up to 20% of those having acute exacerbations.7, 54 Macrolide therapy has a beneficial effect on lung function and airway hyperresponsiveness in adults with asthma.9, 55 Among mice infected with M. pneumoniae, 3 days of macrolide therapy resulted in a significant reduction in airway hyperresponsiveness compared with placebo or dexamethasone; however, after 6 days of therapy, there was no significant difference in airway hyperresponsiveness between those receiving macrolides, dexamethasone, or placebo, suggesting that the benefit of macrolides on airway hyperresponsiveness may be brief. Our findings of a shorter LOS but no difference in readmissions at 28 days or longer, for macrolide recipients compared with non‐recipients, support the limited benefit of macrolide therapy beyond the initial reduction in bacterial load seen in the first few days of therapy.
M. pneumoniae infection has also been implicated as a cause of chronic pulmonary disease, including asthma.610 In the mouse model, peribronchial and perivascular mononuclear infiltrates, increased airway methacholine reactivity, and increased airway obstruction were observed 530 days after M. pneumoniae inoculation.6 M. pneumoniae has been identified in 26 (50%) of 51 children experiencing their first asthma attack,7 and 23 (42%) of 55 adults with chronic, stable asthma.9 Nevertheless, results of other studies addressing the issue are inconsistent, and the role of M. pneumoniae in the development of asthma remains unclear.56 In order to investigate the impact of macrolide therapy on the development of chronic pulmonary disease requiring hospitalization, we examined the readmission rates in the 15 months following index discharge. The proportion of children hospitalized with asthma following the hospitalization for M. pneumoniae pneumonia was higher for both macrolide recipients and non‐recipients compared with the 24‐months prior to infection. These results support a possible role for M. pneumoniae in chronic pulmonary disease. However, macrolide therapy was not associated with long‐term overall hospital readmission or long‐term asthma readmission, either in the entire cohort or in the subset of patients without prior asthma hospitalizations or medications.
This study had several limitations. First, because the identification of children with M. pneumoniae pneumonia relied on ICD‐9 discharge diagnosis codes, it is possible that there was misclassification of disease. We minimized the inclusion of children without M. pneumoniae by including only children who received antibiotic therapy on the first day of hospitalization and by excluding patients younger than 6 years of age, a group at relatively low‐risk for M. pneumoniae infection. Further, our algorithm for identification of M. pneumoniae pneumonia was validated through review of the medical records at 1 institution and was found to have a high positive predictive value. However, the positive predictive value of these ICD‐9 codes may vary across institutions. Additionally, the sensitivity of ICD‐9 codes for identifying children with M. pneumoniae pneumonia is not known. Also, not all children with pneumonia undergo testing for M. pneumoniae, and different tests have varying sensitivity and specificity.57, 58 Thus, some children with M. pneumoniae pneumonia were not diagnosed and so were not included in our study. It is not known how inclusion of these children would affect our results.
Second, the antibiotic information used in this study was limited to empiric antibiotic therapy. It is possible that some patients received macrolide therapy before admission. It is also likely that identification of M. pneumoniae during the hospitalization prompted the addition or substitution of macrolide therapy for some patients. If this therapy was initiated beyond the first day of hospitalization, these children would be classified as macrolide non‐recipients. Since macrolide administration was associated with a shorter hospital LOS, such misclassification would bias our results towards finding no difference in LOS between macrolide recipients and non‐recipients. It is therefore possible that the benefit of macrolide therapy is even greater than found in our study.
Third, there may be unmeasured confounding or residual confounding by indication for adjunct corticosteroid therapy related to clinical presentation. We expect that corticosteroid recipients would be sicker than non‐recipients. We included variables associated with a greater severity of illness (such as intensive care unit admission) in the multivariable analysis. Additionally, the shorter LOS among macrolide recipients remained when the analysis was stratified by receipt or non‐receipt of systemic corticosteroid therapy.
Fourth, we were only able to record readmissions occurring at the same hospital as the index admission; any readmission presenting to a different hospital following their index admission did not appear in our records, and was therefore not counted. It is thus possible that the true number of readmissions is higher than that represented here. Finally, despite the large number of patients included in this study, the number of short‐term readmissions was relatively small. Thus, we may have been underpowered to detect small but significant differences in short‐term readmission rates.
In conclusion, macrolide therapy was associated with shorter hospital LOS, but not with short‐term or longer‐term readmission in children presenting with M. pneumoniae pneumonia.
Appendix
| Variable | Beta Coefficient | Confidence Interval | P Value |
|---|---|---|---|
| Demographics | |||
| Sex | 0.12 | (0.22, 0.02) | 0.022 |
| Race | |||
| Blackreference category | |||
| White | 0.01 | (0.21, 0.23) | 0.933 |
| Other | 0.13 | (0.39, 0.13) | 0.323 |
| Missing | 0.46 | (0.81, 0.11) | 0.012 |
| Presentation during viral respiratory season | 0.05 | (0.19, 0.09) | 0.462 |
| Prior asthma hospitalization | 0.36 | (0.64, 0.08) | 0.015 |
| Intensive care unit admission | 1.05 | (0.87, 1.23) | <0.001 |
| Labs and procedures performed | |||
| Additional radiologic imaging | 0.23 | (0.20, 0.67) | 0.287 |
| Arterial blood gas | 0.69 | (0.50, 0.87) | <0.001 |
| Complete blood count | 0.34 | (0.24, 0.45) | <0.001 |
| Blood culture | 0.17 | (0.98, 0.44) | 0.204 |
| Mechanical ventilation | 1.15 | (0.68, 1.63) | <0.001 |
| Therapies received | |||
| Empiric macrolide therapy | 0.49 | (0.72, 0.25) | <0.001 |
| Systemic steroids | 0.26 | (0.38, 0.14) | <0.001 |
| Chronic asthma medications | 0.20 | (0.38, 0.013) | 0.037 |
| Beta‐agonist therapy | 0.07 | (0.21, 0.08) | 0.357 |
| Vasoactive infusion | 1.08 | (0.727, 1.45) | <0.001 |
| Clindamycin or vancomycin | 0.55 | (0.34, 0.75) | <0.001 |
| Variable | Odds Ratio* | Confidence Interval | P Value |
|---|---|---|---|
| |||
| Demographics | |||
| Sex | 0.56 | (0.23, 1.33) | 0.190 |
| Race | |||
| Blackreference category | |||
| White | 0.46 | (0.19, 1.14) | 0.093 |
| Other | |||
| Missing | |||
| Presentation during viral respiratory season | 0.64 | (0.09, 4.75) | 0.662 |
| Prior asthma hospitalization | |||
| Intensive care unit admission | 4.54 | (1.21, 17.03) | 0.025 |
| Laboratory tests and procedures | |||
| Additional radiologic imaging | 10.00 | (2.25, 44.47) | 0.002 |
| Arterial blood gas | |||
| Complete blood count | 0.92 | (0.24, 3.48) | 0.901 |
| Blood culture | 0.85 | (0.30, 2.36) | 0.738 |
| Mechanical ventilation | |||
| Medications | |||
| Macrolide therapy | 1.18 | (0.25, 5.45) | 0.837 |
| Systemic corticosteroids | 1.04 | (0.276, 4.09) | 0.951 |
| Chronic asthma medication | 1.66 | (0.71, 3.88) | 0.242 |
| Beta‐agonist therapy | 0.66 | (0.16, 2.65) | 0.557 |
| Vasoactive infusions | |||
| Clindamycin or vancomycin | 1.00 | (0.10, 9.90) | 0.998 |
| Variable | Coefficient | Confidence Interval | P Value | % Change | Confidence Interval for % Change |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | 0.287 | (0.012, 0.045) | 0.001 | 2.9 | (1.2, 4.6) |
| Prior asthma hospitalization | 0.272 | (0.094, 0.45) | 0.004 | 31.3 | (9.9, 56.8) |
| Intensive care unit admission | 1.015 | (0.802, 1.23) | <0.001 | 175.9 | (123.0, 241.3) |
| Therapies received | |||||
| Macrolide therapy | 0.379 | (0.59, 0.166) | 0.001 | 31.6 | (44.6, 15.3) |
| Systemic corticosteroids | 0.264 | (0.391, 0.138) | <0.001 | 23.2 | (32.3, 12.9) |
| Chronic asthma medications | 0.056 | (0.255, 0.142) | 0.568 | 5.5 | (22.5, 15.2) |
| Albuterol | 0.07 | (0.059, 0.199) | 0.281 | 7.2 | (5.8, 22.0) |
| Clindamycin or vancomycin | 0.311 | (0.063, 0.559) | 0.015 | 36.5 | (6.5, 74.9) |
| Variable | Adjusted Odds Ratio | Confidence Interval | P Value |
|---|---|---|---|
| Demographics | |||
| Age | 0.91 | 0.72, 1.15 | 0.423 |
| Prior asthma hospitalization | 1.94 | 0.42, 8.90 | 0.394 |
| Intensive care unit admission | 5.73 | 2.03, 16.20 | 0.001 |
| Therapies received | |||
| Macrolide therapy | 1.12 | 0.22, 5.78 | 0.890 |
| Systemic corticosteroids | 0.696 | 0.10, 4.70 | 0.710 |
| Chronic asthma medications | 1.98 | 0.32, 12.20 | 0.460 |
| Albuterol | 0.519 | 0.081, 3.31 | 0.488 |
| Clindamycin or vancomycin | 0.904 | 0.07, 11.13 | 0.937 |
Mycoplasma pneumoniae is a common cause of community‐acquired pneumonia (CAP), among school‐age children and adolescents.14 Though pneumonia caused by M. pneumoniae is typically self‐limited, severe illness may occur.5 M. pneumoniae has also been implicated in airway inflammation, which may lead to the onset and development of chronic pulmonary disease.610 Few studies have directly addressed appropriate treatment strategies for M. pneumoniae pneumonia,11 and, despite its high prevalence and potential for causing severe complications, treatment recommendations remain inconsistent.
The efficacy of macrolide therapy in particular for M. pneumoniae remains unclear. In vitro susceptibility studies have shown bacteriostatic activity of erythromycin, clarithromycin, and azithromycin against M. pneumoniae.1218 Additionally, several small retrospective studies have shown that among children with atypical CAP (including M. pneumoniae pneumonia), those treated with macrolides were less likely to have persistence or progression of signs and symptoms after 3 days of therapy.19, 20 Lu et al21 found a shorter duration of fever among macrolide recipients compared with non‐recipients. In adults, Shames et al22 found a shorter duration of fever and hospitalization among erythromycin recipients compared with controls. Other randomized controlled trials have also addressed the use of macrolides in treatment of M. pneumoniae, but the ability to draw meaningful conclusions is limited by small samples sizes and by lack of details about the number of patients with M. pneumoniae.11
In addition to their antimicrobial effect, macrolides also have anti‐inflammatory properties.2327 The importance of these anti‐inflammatory properties is supported by studies showing clinical cure in patients treated with macrolides despite persistence of M. pneumoniae organisms,2831 clinical improvement despite the administration of doses that provide tissue levels below the minimum inhibitory concentration of the organism,3234 and clinical cure in patients with macrolide‐resistant M. pneumoniae.18, 35
The objectives of the current study were to examine the impact of macrolide therapy on the length of stay (LOS) and short‐ and longer‐term readmissions, including longer‐term asthma‐related readmissions, in children hospitalized with M. pneumoniae pneumonia.
METHODS
Data Source
Data for this retrospective cohort study were obtained from the Pediatric Health Information System (PHIS), which contains administrative data from 38 freestanding children's hospitals. Data quality and reliability are assured through a joint effort by the Child Health Corporation of America (Shawnee Mission, KS) and PHIS‐participating hospitals as described previously.36, 37 Encrypted medical record numbers allow for tracking of individual patients across hospitalizations. This study was reviewed and approved by the Committees for the Protection of Human Subjects at The Children's Hospital of Philadelphia (Philadelphia, PA).
Patients
Children 6‐18 years of age with CAP were eligible if they were discharged from a participating hospital between January 1, 2006 and December 31, 2008. Subjects were included if they received antibiotic therapy on the first day of hospitalization and if they satisfied one of the following International Classification of Diseases, 9th revision (ICD‐9) discharge diagnosis code criteria: 1) Principal diagnosis of M. pneumoniae pneumonia (483.0); 2) Principal diagnosis of a pneumonia‐related symptom (eg, fever, cough) (780.6 or 786.00‐786.52 [except 786.1]) and a secondary diagnosis of M. pneumoniae pneumonia; or 3) Principal diagnosis of pneumonia (481‐483.8 [except 483.0], 485‐486) and a secondary diagnosis of Mycoplasma (041.81).
Children younger than 6 years of age were excluded due to the low prevalence of M. pneumoniae infection.2, 38 Patients with comorbid conditions predisposing to severe or recurrent pneumonia (eg, cystic fibrosis, malignancy) were excluded using a previously reported classification scheme.39 In addition, we excluded patient data from 2 hospitals due to incomplete reporting of discharge information; thus data from 36 hospitals were included in this study.
Validation of Discharge Diagnosis Codes for Mycoplasma pneumoniae
To assess for misclassification of the diagnosis of M. pneumoniae, we reviewed records of a randomly selected subset of subjects from The Children's Hospital of Philadelphia; 14 of 15 patients had signs of lower respiratory tract infection in conjunction with a positive M. pneumoniae polymerase chain reaction test from nasopharyngeal washings to confirm the diagnosis of M. pneumoniae pneumonia. Hence, the positive predictive value of our algorithm for diagnosing M. pneumoniae pneumonia was 93.3%.
Study Definitions
We identified children with asthma in 2 ways. Asthma‐related hospitalizations were identified by an ICD‐9 code for asthma (493.0‐493.92) in any discharge diagnosis field during any hospitalization in the 24 months prior to the current hospitalization. Baseline controller medications were identified by receipt of inhaled corticosteroids (eg, fluticasone) or leukotriene receptor antagonists on the first day of hospitalization.
Systemic corticosteroids (either oral or intravenous) included dexamethasone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Measures of disease severity included admission to the intensive care unit within 48 hours of hospitalization, and administration of vancomycin or clindamycin, vasoactive infusions (epinephrine, norepinephrine, dopamine, and dobutamine), and invasive (endotracheal intubation) and noninvasive (continuous positive airway pressure) mechanical ventilation within 24 hours of hospitalization, as previously described.40, 41 Viral respiratory season was defined as October through March.
Measured Outcomes
The primary outcomes of interest were hospital LOS and all‐cause readmission within 28 days and 15 months after index discharge. We examined readmissions for asthma 15 months after index discharge as a secondary outcome measure because of the potential role for M. pneumoniae infection in long‐term lung dysfunction, including asthma.42 The 15‐month time frame was selected based on longitudinal data available in PHIS for the entire study cohort.
Measured Exposures
The main exposure was early initiation of macrolide therapy, defined as receipt of erythromycin, clarithromycin, or azithromycin on the first day of hospitalization.
Data Analysis
Continuous variables were described using median and interquartile range (IQR) or range values, and compared using the Wilcoxon rank‐sum test. Categorical variables were described using counts and frequencies, and compared using the chi‐square test. Multivariable linear (for LOS) and logistic (for readmission) regression analyses were performed to assess the independent association of macrolide therapy with the primary outcomes. Because the LOS data had a skewed distribution, our analyses were performed using logarithmically transformed LOS values as the dependent variable. The resulting beta‐coefficients were transformed to reflect the percent difference in LOS between subjects receiving and not receiving macrolide therapy.
Building of the multivariable models began with the inclusion of macrolide therapy. Variables associated with primary outcomes on univariate analysis (P < 0.20) were also considered for inclusion as potential confounders.43 These variables were included in the final multivariable model if they remained significant after adjusting for other factors, or if their inclusion in the model resulted in a 15% or greater change in the effect size of the primary association of interest (ie, macrolide therapy).44 Because corticosteroids also have anti‐inflammatory properties, we assessed for interactions with macrolide therapy. There was no interaction between macrolide and systemic corticosteroid therapy (P = 0.26, Likelihood ratio test), therefore our primary model adjusted for systemic corticosteroids.
Despite adjusting for systemic corticosteroid therapy in our primary analysis, residual confounding by indication for corticosteroid therapy might exist. We therefore repeated the analysis after stratifying by receipt or non‐receipt of systemic corticosteroid therapy. Because the benefit of macrolides in preventing long‐term dysfunction may be limited to those without a prior diagnosis of asthma, we repeated the analysis of readmissions within 15 months of index discharge (any readmission and asthma‐related readmissions) while limiting the cohort to those without evidence of asthma (ie, no prior asthma‐related hospitalizations and no chronic asthma medications). Because children with underlying conditions or circumstances that would predispose to prolonged hospitalizations may have been included, despite our restriction of the cohort to those without an identified chronic complex condition, we also repeated the analysis while limiting the cohort to those with a LOS 7 days. Finally, all analyses were clustered on hospital using the robust standard errors of Huber and White to account for the correlation of exposures and outcomes among children within centers.
Data were analyzed using Stata version 11 (Stata Corporation, College Station, TX). Statistical significance was determined a priori as a two‐tailed P value <0.05.
RESULTS
Patient Characteristics
During the study, 690 children ages 6 to 18 years met inclusion criteria. Characteristics of these patients are shown in Table 1. The median age was 10 years (IQR, 7‐13 years). Ten patients (1.4%) also had a concomitant discharge diagnosis of pneumococcal pneumonia, while 19 patients (2.7%) had a concomitant discharge diagnosis of viral pneumonia; 1 of these patients had discharge diagnoses of both viral and pneumococcal pneumonia.
| Empiric Macrolide Therapy | ||||
|---|---|---|---|---|
| Variable | All Subjects | Yes | No | P |
| ||||
| Demographics | ||||
| Male sex | 356 (51.6) | 200 (49.4) | 156 (54.7) | 0.166 |
| Race | ||||
| Black | 135 (19.6) | 81 (20.0) | 54 (19.0) | 0.506 |
| White | 484 (70.1) | 287 (70.9) | 197 (69.1) | |
| Other | 62 (9.0) | 31 (7.7) | 31 (10.9) | |
| Missing | 9 (1.3) | 6 (1.5) | 3 (1.1) | |
| Presentation during viral respiratory season | 420 (60.9) | 242 (59.8) | 178 (62.5) | |
| Prior asthma hospitalization | 41 (5.9) | 31 (7.7) | 10 (3.5) | 0.023 |
| Intensive care unit admission | 127 (18.4) | 74 (18.3) | 53 (18.6) | 0.914 |
| Laboratory tests and procedures | ||||
| Additional radiologic imaging* | 24 (3.5) | 13 (3.2) | 11 (3.9) | 0.646 |
| Arterial blood gas | 116 (17.3) | 72 (18.5) | 44 (15.6) | 0.316 |
| Complete blood count | 433 (64.4) | 249 (64.0) | 184 (65.0) | 0.788 |
| Blood culture | 280 (41.7) | 167 (42.9) | 113 (39.9) | 0.436 |
| Mechanical ventilation | 16 (2.3) | 5 (1.2) | 11 (3.86) | 0.024 |
| Medications | ||||
| Chronic asthma medication | 116 (16.8) | 72 (17.8) | 44 (15.4) | 0.419 |
| Beta‐agonist therapy | 328 (47.5) | 215 (53.1) | 113 (39.7) | 0.001 |
| Vasoactive infusions | 22 (3.2) | 13 (3.2) | 9 (3.2) | 0.969 |
| Systemic corticosteroids | 252 (36.5) | 191 (47.2) | 61 (21.4) | <0.001 |
| Clindamycin or vancomycin | 86 (12.5) | 24 (5.9) | 62 (21.8) | <0.001 |
Macrolide therapy was administered to 405 (58.7%) patients. Systemic corticosteroid therapy was administered to 252 (36.5%) patients. Overall, 191 (27.7%) of the 690 patients received both macrolides and systemic corticosteroids empirically, while 224 (32.5%) received neither; 61 (8.8%) received corticosteroids but not macrolides, while 214 (31.0%) received macrolides but not corticosteroids. Asthma hospitalization within the 24 months prior to admission was more common among those receiving macrolides (N = 60/405, 14.8%) than among those not receiving macrolides (N = 30/285, 10.5%) (P = 0.023). Macrolide recipients also more commonly received concomitant systemic corticosteroids (N = 191/405, 47.2%) than macrolide non‐recipients (N = 61/285, 21.4%) (P < 0.001) and more commonly received beta‐agonist therapy (N = 215/405, 53.1%) than macrolide non‐recipients (N = 113/285, 39.7%) (P = 0.001).
Length of Stay
The overall median LOS was 3 days (IQR, 2‐6 days); the median LOS was 3 days (IQR, 2‐5 days) for empiric macrolide recipients and 4 days (IQR, 2‐9 days) for non‐recipients (P < 0.001). Overall, 22.9% (N = 158) of children had an LOS 7 days and 8.8% (N = 61) of children had an LOS 14 days. The LOS was 7 days for 15.3% (N = 62) of macrolide recipients and 33.7% (N = 96) of non‐recipients. LOS was 7 days for 17.5% (N = 44) of systemic steroid recipients and 26% (N = 114) of non‐recipients. In unadjusted analysis, macrolide therapy (beta‐coefficient, 0.49; 95% confidence interval [CI]: 0.72 to 0.25; P < 0.001) and systemic corticosteroid administration (beta‐coefficient, 0.26; CI: 0.37 to 0.14; P < 0.001) were associated with shorter hospital LOS (Appendix 1).
In multivariable analysis, macrolide therapy remained associated with a shorter LOS (Table 2; Appendix 2). Systemic corticosteroid administration was associated with a 23% shorter LOS (adjusted beta‐coefficient, 0.26; 95% CI: 0.39 to 0.14; P < 0.001). In contrast, previous hospitalization for asthma was associated with a 31% longer LOS (adjusted beta‐coefficient, 0.27; 95% CI: 0.09‐0.045; P = 0.004). Receipt of beta‐agonist therapy or chronic asthma medications were not associated with significant differences in LOS. In analysis stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy, empiric macrolide therapy remained associated with a significantly shorter LOS in both systemic corticosteroid recipients and non‐recipient (Table 4). When the cohort was restricted to subjects with a LOS 7 days, macrolide therapy remained significantly associated with a shorter LOS (adjusted percent change, 20%; 95% CI: 32% to 5%; P = 0.015).
| Association of Empiric Macrolide Therapy With Outcomes* | |
|---|---|
| |
| Length of stay (days) | |
| Adjusted beta‐coefficient (95 % CI) | 0.38 (0.59 to 0.17) |
| Adjusted percent change (95% CI) | 32% (45% to 15%) |
| P value | 0.001 |
| Any readmission within 28 days | |
| Adjusted odds ratio (95% CI) | 1.12 (0.22 to 5.78) |
| P value | 0.890 |
| Any readmission within 15 mo | |
| Adjusted odds ratio (95% CI) | 1.00 (0.59 to 1.70) |
| P value | 0.991 |
| Asthma hospitalization within 15 mo | |
| Adjusted odds ratio (95% CI) | 1.09 (0.54 to 2.17) |
| P value | 0.820 |
Readmission
Overall, 8 children (1.2%) were readmitted for pneumonia‐associated conditions within 28 days of index discharge. Readmission occurred in 1.2% of macrolide recipients and 1.1% of non‐recipients (P = 0.83) (Table 4). In unadjusted analysis, neither macrolide therapy (odds ratio [OR], 1.18; 95% CI: 0.25‐5.45; P = 0.84) nor systemic corticosteroid administration (OR, 1.04; 95% CI: 0.27‐4.10; P = 0.95) was associated with 28‐day readmission (Appendix 3). In multivariable analysis, empiric macrolide therapy was not associated with 28‐day readmission in the overall cohort (Table 2; Appendix 4)), or when the analysis was stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy (Table 3).
| Concomitant Systemic Corticosteroid Therapy* | ||
|---|---|---|
| Yes | No | |
| ||
| Length of stay | ||
| Adjusted beta‐coefficient (95% CI) | 0.40 (0.74 to 0.07) | 0.37 (0.58 to 0.16) |
| Adjusted percent change (95% CI) | 33% (52% to 7%) | 31% (44% to 15%) |
| P value | 0.020 | 0.001 |
| Readmission within 28 days | ||
| Adjusted odds ratio (95% CI) | 1.09 (0.05 to 26.7) | 1.50 (0.21 to 10.8) |
| P value | 0.960 | 0.687 |
| Readmission within 15 mo | ||
| Adjusted odds ratio (95% CI) | 1.57 (0.65 to 3.82) | 0.81 (0.45 to 1.46) |
| P value | 0.32 | 0.49 |
| Asthma hospitalization within 15 mo | ||
| Adjusted odds ratio (95% CI) | 1.51 (0.58 to 3.93) | 0.85 (0.36 to 1.97) |
| P value | 0.395 | 0.700 |
| Empiric Macrolide Therapy | ||
|---|---|---|
| N/Total (%) | ||
| Readmission | Yes | No |
| Any readmission within 28 days | ||
| Overall | 5/405 (1.2) | 3/285 (1.1) |
| Systemic corticosteroid therapy | 2/186 (1.1) | 1/66 (1.5) |
| No systemic corticosteroid therapy | 3/177 (1.7) | 2/261 (0.8) |
| Any readmission within 15 mo | ||
| Overall | 96/405 (23.7) | 64/285 (22.5) |
| Systemic corticosteroid therapy | 52/186 (28.0) | 17/66 (25.8) |
| No systemic corticosteroid therapy | 32/177 (18.1) | 59/261 (22.6) |
| Asthma hospitalization within 15 mo | ||
| Overall | 61/405 (15.1) | 34/285 (11.9) |
| Systemic corticosteroid therapy | 39/186 (21.0) | 13/66 (19.7) |
| No systemic corticosteroid therapy | 14/177 (7.9) | 29/261 (11.1) |
Overall, 160 children (23.2%) were readmitted within 15 months of index discharge; 95 were readmitted for asthma during this time (Table 3). Overall readmission occurred in 23.7% of macrolide recipients and 22.5% of macrolide non‐recipients (P = 0.702). Asthma readmission occurred in 15.1% of macrolide recipients and 11.9% of macrolide non‐recipients (P = 0.240). In unadjusted analysis, empiric macrolide therapy was not significantly associated with any readmission within 15 months (OR, 1.07; 95% CI: 0.69‐1.68; P = 0.759) or with asthma‐related readmission within 15 months (OR, 1.31; 95% CI: 0.73‐ 2.36; P = 0.369). In multivariable analysis, neither any readmission nor asthma readmission within 15 months was associated with empiric macrolide therapy overall (Table 2) or when stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy (Table 3).
The analyses for readmissions within 15 months of index discharge were repeated while limiting the cohort to those without prior asthma hospitalizations or chronic asthma medications. In this subset of patients, readmissions for any reason occurred in 55 (18.6%) of 295 macrolide recipients and 50 (22.0%) of 227 non‐recipients. The difference was not statistically significant in multivariable analysis (adjusted odds ratio, 0.79; 95% CI: 0.41‐1.51; P = 0.47). Readmissions for asthma occurred in 30 (10.2%) of 295 macrolide recipients and 26 (11.5%) of 227 non‐recipients; this difference was also not significant in multivariable analysis (adjusted odds ratio, 0.83; 95% CI: 0.36‐1.93; P = 0.83). The magnitude of the estimate of effect for 28‐day and 15‐month readmissions, and 15‐month asthma hospitalizations, was similar to the primary analysis when the cohort was restricted to subjects with a LOS 7 days.
DISCUSSION
This multicenter study examined the role of macrolide therapy in children hospitalized with M. pneumoniae pneumonia. Empiric macrolide therapy was associated with an approximately 30% shorter hospital LOS and, in stratified analysis, remained associated with a significantly shorter hospital LOS in both systemic corticosteroid recipients and non‐recipients. Empiric macrolide therapy was not associated with short‐ or longer‐term hospital readmission.
Previous small randomized trials have been inconclusive regarding the potential benefit of macrolide therapy in M. pneumoniae pneumonia.11 Our study, which demonstrated a shorter LOS among macrolides recipients compared with non‐recipients, has several advantages over prior studies including a substantively larger sample size and multicenter design. Animal models support our observations regarding the potential beneficial antimicrobial role of macrolides. M. pneumoniae concentrations in bronchoalveolar lavage specimens were significantly lower among experimentally infected mice treated with clarithromycin, a macolide‐class antibiotic, compared with either placebo or dexamethasone.45 Combination therapy with clarithromycin and dexamethasone reduced histopathologic inflammation to a greater degree than dexamethasone alone.45
While the relative importance of the antimicrobial and anti‐inflammatory properties of macrolides is not known, observational studies of children infected with macrolide‐resistant M. pneumoniae suggest that the antimicrobial properties of macrolides may provide disproportionate clinical benefit. The duration of fever in macrolide recipients with macrolide‐resistant M. pneumoniae (median duration, 9 days) reported by Suzuki et al46 was significantly longer than those with macrolide‐susceptible infections (median duration, 5 days), and similar to the duration of fever in patients with M. pneumoniae infection treated with placebo (median duration, 8 days) reported by Kingston et al.47 Additionally, macrolide therapy was associated with significant improvements in lung function in patients with asthma and concomitant M. pneumoniae infection, but not in patients with asthma without documented M. pneumoniae infection.9 As corticosteroids also have anti‐inflammatory properties, we expect that any anti‐inflammatory benefit of macrolide therapy would be mitigated by the concomitant administration of corticosteroids. The shorter LOS associated with empiric macrolide therapy in our study was comparable among corticosteroid recipients and non‐recipients.
Atypical bacterial pathogens, including M. pneumoniae, are associated with diffuse lower airway inflammation6, 48 and airway hyperresponsiveness,6 and have been implicated as a cause of acute asthma exacerbations.7, 4954 Among patients with previously diagnosed asthma, acute M. pneumoniae infection was identified in up to 20% of those having acute exacerbations.7, 54 Macrolide therapy has a beneficial effect on lung function and airway hyperresponsiveness in adults with asthma.9, 55 Among mice infected with M. pneumoniae, 3 days of macrolide therapy resulted in a significant reduction in airway hyperresponsiveness compared with placebo or dexamethasone; however, after 6 days of therapy, there was no significant difference in airway hyperresponsiveness between those receiving macrolides, dexamethasone, or placebo, suggesting that the benefit of macrolides on airway hyperresponsiveness may be brief. Our findings of a shorter LOS but no difference in readmissions at 28 days or longer, for macrolide recipients compared with non‐recipients, support the limited benefit of macrolide therapy beyond the initial reduction in bacterial load seen in the first few days of therapy.
M. pneumoniae infection has also been implicated as a cause of chronic pulmonary disease, including asthma.610 In the mouse model, peribronchial and perivascular mononuclear infiltrates, increased airway methacholine reactivity, and increased airway obstruction were observed 530 days after M. pneumoniae inoculation.6 M. pneumoniae has been identified in 26 (50%) of 51 children experiencing their first asthma attack,7 and 23 (42%) of 55 adults with chronic, stable asthma.9 Nevertheless, results of other studies addressing the issue are inconsistent, and the role of M. pneumoniae in the development of asthma remains unclear.56 In order to investigate the impact of macrolide therapy on the development of chronic pulmonary disease requiring hospitalization, we examined the readmission rates in the 15 months following index discharge. The proportion of children hospitalized with asthma following the hospitalization for M. pneumoniae pneumonia was higher for both macrolide recipients and non‐recipients compared with the 24‐months prior to infection. These results support a possible role for M. pneumoniae in chronic pulmonary disease. However, macrolide therapy was not associated with long‐term overall hospital readmission or long‐term asthma readmission, either in the entire cohort or in the subset of patients without prior asthma hospitalizations or medications.
This study had several limitations. First, because the identification of children with M. pneumoniae pneumonia relied on ICD‐9 discharge diagnosis codes, it is possible that there was misclassification of disease. We minimized the inclusion of children without M. pneumoniae by including only children who received antibiotic therapy on the first day of hospitalization and by excluding patients younger than 6 years of age, a group at relatively low‐risk for M. pneumoniae infection. Further, our algorithm for identification of M. pneumoniae pneumonia was validated through review of the medical records at 1 institution and was found to have a high positive predictive value. However, the positive predictive value of these ICD‐9 codes may vary across institutions. Additionally, the sensitivity of ICD‐9 codes for identifying children with M. pneumoniae pneumonia is not known. Also, not all children with pneumonia undergo testing for M. pneumoniae, and different tests have varying sensitivity and specificity.57, 58 Thus, some children with M. pneumoniae pneumonia were not diagnosed and so were not included in our study. It is not known how inclusion of these children would affect our results.
Second, the antibiotic information used in this study was limited to empiric antibiotic therapy. It is possible that some patients received macrolide therapy before admission. It is also likely that identification of M. pneumoniae during the hospitalization prompted the addition or substitution of macrolide therapy for some patients. If this therapy was initiated beyond the first day of hospitalization, these children would be classified as macrolide non‐recipients. Since macrolide administration was associated with a shorter hospital LOS, such misclassification would bias our results towards finding no difference in LOS between macrolide recipients and non‐recipients. It is therefore possible that the benefit of macrolide therapy is even greater than found in our study.
Third, there may be unmeasured confounding or residual confounding by indication for adjunct corticosteroid therapy related to clinical presentation. We expect that corticosteroid recipients would be sicker than non‐recipients. We included variables associated with a greater severity of illness (such as intensive care unit admission) in the multivariable analysis. Additionally, the shorter LOS among macrolide recipients remained when the analysis was stratified by receipt or non‐receipt of systemic corticosteroid therapy.
Fourth, we were only able to record readmissions occurring at the same hospital as the index admission; any readmission presenting to a different hospital following their index admission did not appear in our records, and was therefore not counted. It is thus possible that the true number of readmissions is higher than that represented here. Finally, despite the large number of patients included in this study, the number of short‐term readmissions was relatively small. Thus, we may have been underpowered to detect small but significant differences in short‐term readmission rates.
In conclusion, macrolide therapy was associated with shorter hospital LOS, but not with short‐term or longer‐term readmission in children presenting with M. pneumoniae pneumonia.
Appendix
| Variable | Beta Coefficient | Confidence Interval | P Value |
|---|---|---|---|
| Demographics | |||
| Sex | 0.12 | (0.22, 0.02) | 0.022 |
| Race | |||
| Blackreference category | |||
| White | 0.01 | (0.21, 0.23) | 0.933 |
| Other | 0.13 | (0.39, 0.13) | 0.323 |
| Missing | 0.46 | (0.81, 0.11) | 0.012 |
| Presentation during viral respiratory season | 0.05 | (0.19, 0.09) | 0.462 |
| Prior asthma hospitalization | 0.36 | (0.64, 0.08) | 0.015 |
| Intensive care unit admission | 1.05 | (0.87, 1.23) | <0.001 |
| Labs and procedures performed | |||
| Additional radiologic imaging | 0.23 | (0.20, 0.67) | 0.287 |
| Arterial blood gas | 0.69 | (0.50, 0.87) | <0.001 |
| Complete blood count | 0.34 | (0.24, 0.45) | <0.001 |
| Blood culture | 0.17 | (0.98, 0.44) | 0.204 |
| Mechanical ventilation | 1.15 | (0.68, 1.63) | <0.001 |
| Therapies received | |||
| Empiric macrolide therapy | 0.49 | (0.72, 0.25) | <0.001 |
| Systemic steroids | 0.26 | (0.38, 0.14) | <0.001 |
| Chronic asthma medications | 0.20 | (0.38, 0.013) | 0.037 |
| Beta‐agonist therapy | 0.07 | (0.21, 0.08) | 0.357 |
| Vasoactive infusion | 1.08 | (0.727, 1.45) | <0.001 |
| Clindamycin or vancomycin | 0.55 | (0.34, 0.75) | <0.001 |
| Variable | Odds Ratio* | Confidence Interval | P Value |
|---|---|---|---|
| |||
| Demographics | |||
| Sex | 0.56 | (0.23, 1.33) | 0.190 |
| Race | |||
| Blackreference category | |||
| White | 0.46 | (0.19, 1.14) | 0.093 |
| Other | |||
| Missing | |||
| Presentation during viral respiratory season | 0.64 | (0.09, 4.75) | 0.662 |
| Prior asthma hospitalization | |||
| Intensive care unit admission | 4.54 | (1.21, 17.03) | 0.025 |
| Laboratory tests and procedures | |||
| Additional radiologic imaging | 10.00 | (2.25, 44.47) | 0.002 |
| Arterial blood gas | |||
| Complete blood count | 0.92 | (0.24, 3.48) | 0.901 |
| Blood culture | 0.85 | (0.30, 2.36) | 0.738 |
| Mechanical ventilation | |||
| Medications | |||
| Macrolide therapy | 1.18 | (0.25, 5.45) | 0.837 |
| Systemic corticosteroids | 1.04 | (0.276, 4.09) | 0.951 |
| Chronic asthma medication | 1.66 | (0.71, 3.88) | 0.242 |
| Beta‐agonist therapy | 0.66 | (0.16, 2.65) | 0.557 |
| Vasoactive infusions | |||
| Clindamycin or vancomycin | 1.00 | (0.10, 9.90) | 0.998 |
| Variable | Coefficient | Confidence Interval | P Value | % Change | Confidence Interval for % Change |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | 0.287 | (0.012, 0.045) | 0.001 | 2.9 | (1.2, 4.6) |
| Prior asthma hospitalization | 0.272 | (0.094, 0.45) | 0.004 | 31.3 | (9.9, 56.8) |
| Intensive care unit admission | 1.015 | (0.802, 1.23) | <0.001 | 175.9 | (123.0, 241.3) |
| Therapies received | |||||
| Macrolide therapy | 0.379 | (0.59, 0.166) | 0.001 | 31.6 | (44.6, 15.3) |
| Systemic corticosteroids | 0.264 | (0.391, 0.138) | <0.001 | 23.2 | (32.3, 12.9) |
| Chronic asthma medications | 0.056 | (0.255, 0.142) | 0.568 | 5.5 | (22.5, 15.2) |
| Albuterol | 0.07 | (0.059, 0.199) | 0.281 | 7.2 | (5.8, 22.0) |
| Clindamycin or vancomycin | 0.311 | (0.063, 0.559) | 0.015 | 36.5 | (6.5, 74.9) |
| Variable | Adjusted Odds Ratio | Confidence Interval | P Value |
|---|---|---|---|
| Demographics | |||
| Age | 0.91 | 0.72, 1.15 | 0.423 |
| Prior asthma hospitalization | 1.94 | 0.42, 8.90 | 0.394 |
| Intensive care unit admission | 5.73 | 2.03, 16.20 | 0.001 |
| Therapies received | |||
| Macrolide therapy | 1.12 | 0.22, 5.78 | 0.890 |
| Systemic corticosteroids | 0.696 | 0.10, 4.70 | 0.710 |
| Chronic asthma medications | 1.98 | 0.32, 12.20 | 0.460 |
| Albuterol | 0.519 | 0.081, 3.31 | 0.488 |
| Clindamycin or vancomycin | 0.904 | 0.07, 11.13 | 0.937 |
- ,,, et al.Prospective surveillance for atypical pathogens in children with community‐acquired pneumonia in Japan.J Infect Chemother.2006;12:36–41.
- ,,.Incidence of community‐acquired pneumonia in children caused by Mycoplasma pneumoniae: serological results of a prospective, population‐based study in primary health care.Respirology.2004;9:109–114.
- ,,.Mycoplasma pneumoniae infections in University of Wisconsin students.Am Rev Respir Dis.1967;96:237–244.
- .Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients.Clin Infect Dis.1993;17(suppl 1):S37–S46.
- .Mycoplasma pneumoniae. In: Long SS, Pickering LK, Prober CG, eds.Principles and Practice of Pediatric Infectious Diseases.3rd ed.Philadelphia, PA:Churchill Livingstone;2008:979–985.
- ,,, et al.Mycoplasma pneumoniae induces chronic respiratory infection, airway hyperreactivity, and pulmonary inflammation: a murine model of infection‐associated chronic reactive airway disease.Infect Immun.2002;70:649–654.
- ,,, et al.Mycoplasma pneumoniae and asthma in children.Clin Infect Dis.2004;38:1341–1346.
- ,,,.Isolation of Mycoplasma pneumoniae from asthmatic patients.Ann Allergy.1993;70:23–25.
- ,,,.Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin.Chest.2002;121:1782–1788.
- ,,,,.A link between chronic asthma and chronic infection.J Allergy Clin Immunol.2001;107:595–601.
- ,,.Antibiotics for community‐acquired lower respiratory tract infections secondary to Mycoplasma pneumoniae in children.Cochrane Database Syst Rev.2010;7:CD004875.
- ,,,.In vitro susceptibilities of mycoplasmas and ureaplasmas to new macrolides and aryl‐fluoroquinolones.Antimicrob Agents Chemother.1988;32:1500–1502.
- ,,.Inhibitory and bactericidal activities of gemifloxacin and other antimicrobials against Mycoplasma pneumoniae.Int J Antimicrob Agents.2003;21:574–577.
- ,,,,.The in vitro activity of some 14‐, 15‐ and 16‐ membered macrolides against Staphylococcus spp., Legionella spp., Mycoplasma spp. and Ureaplasma urealyticum.Drugs Exp Clin Res.1991;17:91–99.
- ,,, et al.In vitro and in vivo activities of macrolides against Mycoplasma pneumoniae.Antimicrob Agents Chemother.1994;38:790–798.
- ,.Comparative in vitro activity of azithromycin, clarithromycin, erythromycin and lomefloxacin against Mycoplasma pneumoniae, Mycoplasma hominis and Ureaplasma urealyticum.Eur J Clin Microbiol Infect Dis.1990;9:838–841.
- ,,, et al.Characteristics of macrolide‐resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro.Microbiol Immunol.2001;45:617–620.
- ,,, et al.Characterization and molecular analysis of macrolide‐resistant Mycoplasma pneumoniae clinical isolates obtained in Japan.Antimicrob Agents Chemother.2004;48:4624–4630.
- ,,,.Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community‐acquired lower respiratory tract infections.Clin Infect Dis.2001;32:1281–1289.
- ,,, et al.Characteristics of Streptococcus pneumoniae and atypical bacterial infections in children 2–5 years of age with community‐acquired pneumonia.Clin Infect Dis.2002;35:1345–1352.
- ,,,,.Macrolide use shortens fever duration in Mycoplasma pneumoniae infection in children: a 2‐year experience.J Microbiol Immunol Infect.2008;41:307–310.
- ,,,,.Comparison of antibiotics in the treatment of mycoplasmal pneumonia.Arch Intern Med.1970;125:680–684.
- ,,, et al.Antimicrobial and immunologic activities of clarithromycin in a murine model of Mycoplasma pneumoniae‐induced pneumonia.Antimicrob Agents Chemother.2003;47:1614–1620.
- ,.Antibiotics in asthma.Curr Allergy Asthma Rep.2004;4:132–138.
- ,.Immunomodulatory activity and effectiveness of macrolides in chronic airway disease.Chest.2004;125:70S–78S.
- ,,, et al.Interleukin‐8 gene repression by clarithromycin is mediated by the activator protein‐1 binding site in human bronchial epithelial cells.Am J Respir Cell Mol Biol.2000;22:51–60.
- ,,, et al.Clarithromycin inhibits NF‐kappaB activation in human peripheral blood mononuclear cells and pulmonary epithelial cells.Antimicrob Agents Chemother.2001;45:44–47.
- ,,,,.Epidemiology of Mycoplasma pneumoniae infection in families.JAMA.1966;197:859–866.
- ,,.Shedding of Mycoplasma pneumoniae after tetracycline and erythromycin therapy.N Engl J Med.1967;276:1172–1175.
- ,,.Mycoplasma pneumoniae disease: clinical spectrum, pathophysiology, epidemiology, and control.J Infect Dis.1971;123:74–92.
- .Is there a role for antibiotics in the treatment of asthma? Involvement of atypical organisms.BioDrugs.2000;14:349–354.
- ,.Diffuse panbronchiolitis: role of macrolides in therapy.Am J Respir Med.2002;1:119–131.
- ,,,,,.Long‐term low‐dose administration of erythromycin to patients with diffuse panbronchiolitis.Respiration.1991;58:145–149.
- ,,,,,.[Long‐term therapeutic effects of erythromycin and newquinolone antibacterial agents on diffuse panbronchiolitis].Nihon Kyobu Shikkan Gakkai Zasshi.1990;28:1305–1313.
- ,,, et al.A comparative clinical study of macrolide‐sensitive and macrolide‐resistant Mycoplasma pneumoniae infections in pediatric patients.J Infect Chemother.2009;15:380–383.
- ,,,.Corticosteroids and mortality in children with bacterial meningitis.JAMA.2008;299:2048–2055.
- ,,,,.Intravenous immunoglobulin in children with streptococcal toxic shock syndrome.Clin Infect Dis.2009;49:1369–1376.
- ,,, et al.Etiology of childhood pneumonia: serologic results of a prospective, population‐based study.Pediatr Infect Dis J.1998;17:986–991.
- ,,,,,.Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services.Pediatrics.2001;107:E99.
- ,,,,,.Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia.Pediatrics.2011;127:e255–e263.
- ,,, et al.Comparative effectiveness of pleural drainage procedures for the treatment of complicated pneumonia in childhood.J Hosp Med.2011;6:256–263.
- ,,, et al.Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma.Am J Respir Crit Care Med.1998;158:998–1001.
- ,.The impact of confounder selection criteria on effect estimation.Am J Epidemiol.1989;129:125–137.
- ,,.The risk of determining risk with multivariable models.Ann Intern Med.1993;118:201–210.
- ,,, et al.The impact of steroids given with macrolide therapy on experimental Mycoplasma pneumoniae respiratory infection.J Infect Dis.2008;198:1180–1188.
- ,,, et al.Clinical evaluation of macrolide‐resistant Mycoplasma pneumoniae.Antimicrob Agents Chemother.2006;50:709–712.
- ,,, et al.Eaton agent pneumonia.JAMA.1961;176:118–123.
- .The role of viral and atypical bacterial pathogens in asthma pathogenesis.Pediatr Pulmonol Suppl.1999;18:141–143.
- ,,.The association of viral and mycoplasma infections with recurrence of wheezing in the asthmatic child.Ann Allergy.1970;28:43–49.
- ,,,.Association of viral and mycoplasma infections with exacerbations of asthma.Ann Allergy.1974;33:145–149.
- ,,, et al.Acute Chlamydia pneumoniae and Mycoplasma pneumoniae infections in community‐acquired pneumonia and exacerbations of COPD or asthma: therapeutic considerations.J Chemother.2004;16:70–76.
- ,,, et al.Mycoplasma pneumoniae is a frequent cause of exacerbation of bronchial asthma in adults.Ann Allergy.1986;57:263–265.
- ,,, et al.Acute exacerbations of asthma in adults: role of Chlamydia pneumoniae infection.Eur Respir J.1994;7:2165–2168.
- ,,, et al.Atypical pathogen infection in adults with acute exacerbation of bronchial asthma.Am J Respir Crit Care Med.2003;167:406–410.
- ,,,,,.Erythromycin reduces the severity of bronchial hyperresponsiveness in asthma.Chest.1991;99:670–673.
- ,,,,.Atypical bacteria and macrolides in asthma.Allergy Asthma Clin Immunol.2008;4:111–116.
- ,,.Acute respiratory infection due to Mycoplasma pneumoniae: current status of diagnostic methods.Eur J Clin Microbiol Infect Dis.2010;29:1055–1069.
- ,,,,,.A multicenter pilot external quality assessment programme to assess the quality of molecular detection of Chlamydophila pneumoniae and Mycoplasma pneumoniae.J Microbiol Methods.2010;82:131–135.
- ,,, et al.Prospective surveillance for atypical pathogens in children with community‐acquired pneumonia in Japan.J Infect Chemother.2006;12:36–41.
- ,,.Incidence of community‐acquired pneumonia in children caused by Mycoplasma pneumoniae: serological results of a prospective, population‐based study in primary health care.Respirology.2004;9:109–114.
- ,,.Mycoplasma pneumoniae infections in University of Wisconsin students.Am Rev Respir Dis.1967;96:237–244.
- .Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients.Clin Infect Dis.1993;17(suppl 1):S37–S46.
- .Mycoplasma pneumoniae. In: Long SS, Pickering LK, Prober CG, eds.Principles and Practice of Pediatric Infectious Diseases.3rd ed.Philadelphia, PA:Churchill Livingstone;2008:979–985.
- ,,, et al.Mycoplasma pneumoniae induces chronic respiratory infection, airway hyperreactivity, and pulmonary inflammation: a murine model of infection‐associated chronic reactive airway disease.Infect Immun.2002;70:649–654.
- ,,, et al.Mycoplasma pneumoniae and asthma in children.Clin Infect Dis.2004;38:1341–1346.
- ,,,.Isolation of Mycoplasma pneumoniae from asthmatic patients.Ann Allergy.1993;70:23–25.
- ,,,.Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin.Chest.2002;121:1782–1788.
- ,,,,.A link between chronic asthma and chronic infection.J Allergy Clin Immunol.2001;107:595–601.
- ,,.Antibiotics for community‐acquired lower respiratory tract infections secondary to Mycoplasma pneumoniae in children.Cochrane Database Syst Rev.2010;7:CD004875.
- ,,,.In vitro susceptibilities of mycoplasmas and ureaplasmas to new macrolides and aryl‐fluoroquinolones.Antimicrob Agents Chemother.1988;32:1500–1502.
- ,,.Inhibitory and bactericidal activities of gemifloxacin and other antimicrobials against Mycoplasma pneumoniae.Int J Antimicrob Agents.2003;21:574–577.
- ,,,,.The in vitro activity of some 14‐, 15‐ and 16‐ membered macrolides against Staphylococcus spp., Legionella spp., Mycoplasma spp. and Ureaplasma urealyticum.Drugs Exp Clin Res.1991;17:91–99.
- ,,, et al.In vitro and in vivo activities of macrolides against Mycoplasma pneumoniae.Antimicrob Agents Chemother.1994;38:790–798.
- ,.Comparative in vitro activity of azithromycin, clarithromycin, erythromycin and lomefloxacin against Mycoplasma pneumoniae, Mycoplasma hominis and Ureaplasma urealyticum.Eur J Clin Microbiol Infect Dis.1990;9:838–841.
- ,,, et al.Characteristics of macrolide‐resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro.Microbiol Immunol.2001;45:617–620.
- ,,, et al.Characterization and molecular analysis of macrolide‐resistant Mycoplasma pneumoniae clinical isolates obtained in Japan.Antimicrob Agents Chemother.2004;48:4624–4630.
- ,,,.Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community‐acquired lower respiratory tract infections.Clin Infect Dis.2001;32:1281–1289.
- ,,, et al.Characteristics of Streptococcus pneumoniae and atypical bacterial infections in children 2–5 years of age with community‐acquired pneumonia.Clin Infect Dis.2002;35:1345–1352.
- ,,,,.Macrolide use shortens fever duration in Mycoplasma pneumoniae infection in children: a 2‐year experience.J Microbiol Immunol Infect.2008;41:307–310.
- ,,,,.Comparison of antibiotics in the treatment of mycoplasmal pneumonia.Arch Intern Med.1970;125:680–684.
- ,,, et al.Antimicrobial and immunologic activities of clarithromycin in a murine model of Mycoplasma pneumoniae‐induced pneumonia.Antimicrob Agents Chemother.2003;47:1614–1620.
- ,.Antibiotics in asthma.Curr Allergy Asthma Rep.2004;4:132–138.
- ,.Immunomodulatory activity and effectiveness of macrolides in chronic airway disease.Chest.2004;125:70S–78S.
- ,,, et al.Interleukin‐8 gene repression by clarithromycin is mediated by the activator protein‐1 binding site in human bronchial epithelial cells.Am J Respir Cell Mol Biol.2000;22:51–60.
- ,,, et al.Clarithromycin inhibits NF‐kappaB activation in human peripheral blood mononuclear cells and pulmonary epithelial cells.Antimicrob Agents Chemother.2001;45:44–47.
- ,,,,.Epidemiology of Mycoplasma pneumoniae infection in families.JAMA.1966;197:859–866.
- ,,.Shedding of Mycoplasma pneumoniae after tetracycline and erythromycin therapy.N Engl J Med.1967;276:1172–1175.
- ,,.Mycoplasma pneumoniae disease: clinical spectrum, pathophysiology, epidemiology, and control.J Infect Dis.1971;123:74–92.
- .Is there a role for antibiotics in the treatment of asthma? Involvement of atypical organisms.BioDrugs.2000;14:349–354.
- ,.Diffuse panbronchiolitis: role of macrolides in therapy.Am J Respir Med.2002;1:119–131.
- ,,,,,.Long‐term low‐dose administration of erythromycin to patients with diffuse panbronchiolitis.Respiration.1991;58:145–149.
- ,,,,,.[Long‐term therapeutic effects of erythromycin and newquinolone antibacterial agents on diffuse panbronchiolitis].Nihon Kyobu Shikkan Gakkai Zasshi.1990;28:1305–1313.
- ,,, et al.A comparative clinical study of macrolide‐sensitive and macrolide‐resistant Mycoplasma pneumoniae infections in pediatric patients.J Infect Chemother.2009;15:380–383.
- ,,,.Corticosteroids and mortality in children with bacterial meningitis.JAMA.2008;299:2048–2055.
- ,,,,.Intravenous immunoglobulin in children with streptococcal toxic shock syndrome.Clin Infect Dis.2009;49:1369–1376.
- ,,, et al.Etiology of childhood pneumonia: serologic results of a prospective, population‐based study.Pediatr Infect Dis J.1998;17:986–991.
- ,,,,,.Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services.Pediatrics.2001;107:E99.
- ,,,,,.Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia.Pediatrics.2011;127:e255–e263.
- ,,, et al.Comparative effectiveness of pleural drainage procedures for the treatment of complicated pneumonia in childhood.J Hosp Med.2011;6:256–263.
- ,,, et al.Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma.Am J Respir Crit Care Med.1998;158:998–1001.
- ,.The impact of confounder selection criteria on effect estimation.Am J Epidemiol.1989;129:125–137.
- ,,.The risk of determining risk with multivariable models.Ann Intern Med.1993;118:201–210.
- ,,, et al.The impact of steroids given with macrolide therapy on experimental Mycoplasma pneumoniae respiratory infection.J Infect Dis.2008;198:1180–1188.
- ,,, et al.Clinical evaluation of macrolide‐resistant Mycoplasma pneumoniae.Antimicrob Agents Chemother.2006;50:709–712.
- ,,, et al.Eaton agent pneumonia.JAMA.1961;176:118–123.
- .The role of viral and atypical bacterial pathogens in asthma pathogenesis.Pediatr Pulmonol Suppl.1999;18:141–143.
- ,,.The association of viral and mycoplasma infections with recurrence of wheezing in the asthmatic child.Ann Allergy.1970;28:43–49.
- ,,,.Association of viral and mycoplasma infections with exacerbations of asthma.Ann Allergy.1974;33:145–149.
- ,,, et al.Acute Chlamydia pneumoniae and Mycoplasma pneumoniae infections in community‐acquired pneumonia and exacerbations of COPD or asthma: therapeutic considerations.J Chemother.2004;16:70–76.
- ,,, et al.Mycoplasma pneumoniae is a frequent cause of exacerbation of bronchial asthma in adults.Ann Allergy.1986;57:263–265.
- ,,, et al.Acute exacerbations of asthma in adults: role of Chlamydia pneumoniae infection.Eur Respir J.1994;7:2165–2168.
- ,,, et al.Atypical pathogen infection in adults with acute exacerbation of bronchial asthma.Am J Respir Crit Care Med.2003;167:406–410.
- ,,,,,.Erythromycin reduces the severity of bronchial hyperresponsiveness in asthma.Chest.1991;99:670–673.
- ,,,,.Atypical bacteria and macrolides in asthma.Allergy Asthma Clin Immunol.2008;4:111–116.
- ,,.Acute respiratory infection due to Mycoplasma pneumoniae: current status of diagnostic methods.Eur J Clin Microbiol Infect Dis.2010;29:1055–1069.
- ,,,,,.A multicenter pilot external quality assessment programme to assess the quality of molecular detection of Chlamydophila pneumoniae and Mycoplasma pneumoniae.J Microbiol Methods.2010;82:131–135.
Copyright © 2012 Society of Hospital Medicine
Observation Care in Children's Hospitals
Observation medicine has grown in recent decades out of changes in policies for hospital reimbursement, requirements for patients to meet admission criteria to qualify for inpatient admission, and efforts to avoid unnecessary or inappropriate admissions.1 Emergency physicians are frequently faced with patients who are too sick to be discharged home, but do not clearly meet criteria for an inpatient status admission. These patients often receive extended outpatient services (typically extending 24 to 48 hours) under the designation of observation status, in order to determine their response to treatment and need for hospitalization.
Observation care delivered to adult patients has increased substantially in recent years, and the confusion around the designation of observation versus inpatient care has received increasing attention in the lay press.27 According to the Centers for Medicare and Medicaid Services (CMS)8:
Observation care is a well‐defined set of specific, clinically appropriate services, which include ongoing short term treatment, assessment, and reassessment before a decision can be made regarding whether patients will require further treatment as hospital inpatients. Observation services are commonly ordered for patients who present to the emergency department and who then require a significant period of treatment or monitoring in order to make a decision concerning their admission or discharge.
Observation status is an administrative label that is applied to patients who do not meet inpatient level of care criteria, as defined by third parties such as InterQual. These criteria usually include a combination of the patient's clinical diagnoses, severity of illness, and expected needs for monitoring and interventions, in order to determine the admission status to which the patient may be assigned (eg, observation, inpatient, or intensive care). Observation services can be provided, in a variety of settings, to those patients who do not meet inpatient level of care but require a period of observation. Some hospitals provide observation care in discrete units in the emergency department (ED) or specific inpatient unit, and others have no designated unit but scatter observation patients throughout the institution, termed virtual observation units.9
For more than 30 years, observation unit (OU) admission has offered an alternative to traditional inpatient hospitalization for children with a variety of acute conditions.10, 11 Historically, the published literature on observation care for children in the United States has been largely based in dedicated emergency department OUs.12 Yet, in a 2001 survey of 21 pediatric EDs, just 6 reported the presence of a 23‐hour unit.13 There are single‐site examples of observation care delivered in other settings.14, 15 In 2 national surveys of US General Hospitals, 25% provided observation services in beds adjacent to the ED, and the remainder provided observation services in hospital inpatient units.16, 17 However, we are not aware of any previous multi‐institution studies exploring hospital‐wide practices related to observation care for children.
Recognizing that observation status can be designated using various standards, and that observation care can be delivered in locations outside of dedicated OUs,9 we developed 2 web‐based surveys to examine the current models of pediatric observation medicine in US children's hospitals. We hypothesized that observation care is most commonly applied as a billing designation and does not necessarily represent care delivered in a structurally or functionally distinct OU, nor does it represent a difference in care provided to those patients with inpatient designation.
METHODS
Study Design
Two web‐based surveys were distributed, in April 2010, to the 42 freestanding, tertiary care children's hospitals affiliated with the Child Health Corporation of America (CHCA; Shawnee Mission, KS) which contribute data to the Pediatric Health Information System (PHIS) database. The PHIS is a national administrative database that contains resource utilization data from participating hospitals located in noncompeting markets of 27 states plus the District of Columbia. These hospitals account for 20% of all tertiary care children's hospitals in the United States.
Survey Content
Survey 1
A survey of hospital observation status practices has been developed by CHCA as a part of the PHIS data quality initiative (see Supporting Appendix: Survey 1 in the online version of this article). Hospitals that did not provide observation patient data to PHIS were excluded after an initial screening question. This survey obtained information regarding the designation of observation status within each hospital. Hospitals provided free‐text responses to questions related to the criteria used to define observation, and to admit patients into observation status. Fixed‐choice response questions were used to determine specific observation status utilization criteria and clinical guidelines (eg, InterQual and Milliman) used by hospitals for the designation of observation status to patients.
Survey 2
We developed a detailed follow‐up survey in order to characterize the structures and processes of care associated with observation status (see Supporting Appendix: Survey 2 in the online version of this article). Within the follow‐up survey, an initial screening question was used to determine all types of patients to which observation status is assigned within the responding hospitals. All other questions in Survey 2 were focused specifically on those patients who required additional care following ED evaluation and treatment. Fixed‐choice response questions were used to explore differences in care for patients under observation and those admitted as inpatients. We also inquired of hospital practices related to boarding of patients in the ED while awaiting admission to an inpatient bed.
Survey Distribution
Two web‐based surveys were distributed to all 42 CHCA hospitals that contribute data to PHIS. During the month of April 2010, each hospital's designated PHIS operational contact received e‐mail correspondence requesting their participation in each survey. Within hospitals participating in PHIS, Operational Contacts have been assigned to serve as the day‐to‐day PHIS contact person based upon their experience working with the PHIS data. The Operational Contacts are CHCA's primary contact for issues related to the hospital's data quality and reporting to PHIS. Non‐responders were contacted by e‐mail for additional requests to complete the surveys. Each e‐mail provided an introduction to the topic of the survey and a link to complete the survey. The e‐mail requesting participation in Survey 1 was distributed the first week of April 2010, and the survey was open for responses during the first 3 weeks of the month. The e‐mail requesting participation in Survey 2 was sent the third week of April 2010, and the survey was open for responses during the subsequent 2 weeks.
DATA ANALYSIS
Survey responses were collected and are presented as a descriptive summary of results. Hospital characteristics were summarized with medians and interquartile ranges for continuous variables, and with percents for categorical variables. Characteristics were compared between hospitals that responded and those that did not respond to Survey 2 using Wilcoxon rank‐sum tests and chi‐square tests as appropriate. All analyses were performed using SAS v.9.2 (SAS Institute, Cary, NC), and a P value <0.05 was considered statistically significant. The study was reviewed by the University of Michigan Institutional Review Board and considered exempt.
RESULTS
Responses to Survey 1 were available from 37 of 42 (88%) of PHIS hospitals (Figure 1). For Survey 2, we received responses from 20 of 42 (48%) of PHIS hospitals. Based on information available from Survey 1, we know that 20 of the 31 (65%) PHIS hospitals that report observation status patient data to PHIS responded to Survey 2. Characteristics of the hospitals responding and not responding to Survey 2 are presented in Table 1. Respondents provided hospital identifying information which allowed for the linkage of data, from Survey 1, to 17 of the 20 hospitals responding to Survey 2. We did not have information available to link responses from 3 hospitals.
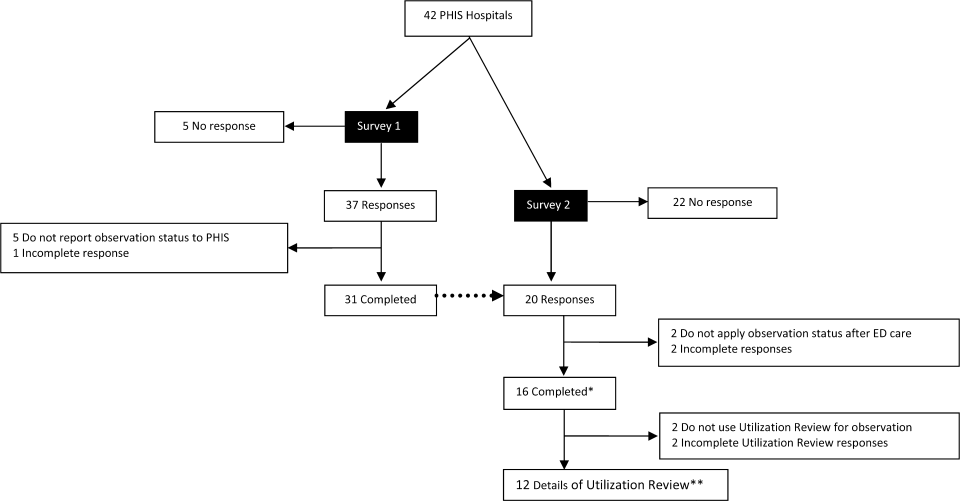
| Respondent N = 20 | Non‐Respondent N = 22 | P Value | |
|---|---|---|---|
| |||
| No. of inpatient beds Median [IQR] (excluding Obstetrics) | 245 [219283] | 282 [250381] | 0.076 |
| Annual admissions Median [IQR] (excluding births) | 11,658 [8,64213,213] | 13,522 [9,83018,705] | 0.106 |
| ED volume Median [IQR] | 60,528 [47,85082,955] | 64,486 [47,38684,450] | 0.640 |
| Percent government payer Median [IQR] | 53% [4662] | 49% [4158] | 0.528 |
| Region | |||
| Northeast | 37% | 0% | 0.021 |
| Midwest | 21% | 33% | |
| South | 21% | 50% | |
| West | 21% | 17% | |
| Reports observation status patients to PHIS | 85% | 90% | 0.555 |
Based on responses to the surveys and our knowledge of data reported to PHIS, our current understanding of patient flow from ED through observation to discharge home, and the application of observation status to the encounter, is presented in Figure 2. According to free‐text responses to Survey 1, various methods were applied to designate observation status (gray shaded boxes in Figure 2). Fixed‐choice responses to Survey 2 revealed that observation status patients were cared for in a variety of locations within hospitals, including ED beds, designated observation units, and inpatient beds (dashed boxes in Figure 2). Not every facility utilized all of the listed locations for observation care. Space constraints could dictate the location of care, regardless of patient status (eg, observation vs inpatient), in hospitals with more than one location of care available to observation patients. While patient status could change during a visit, only the final patient status at discharge enters the administrative record submitted to PHIS (black boxes in Figure 2). Facility charges for observation remained a part of the visit record and were reported to PHIS. Hospitals may or may not bill for all assigned charges depending on patient status, length of stay, or other specific criteria determined by contracts with individual payers.
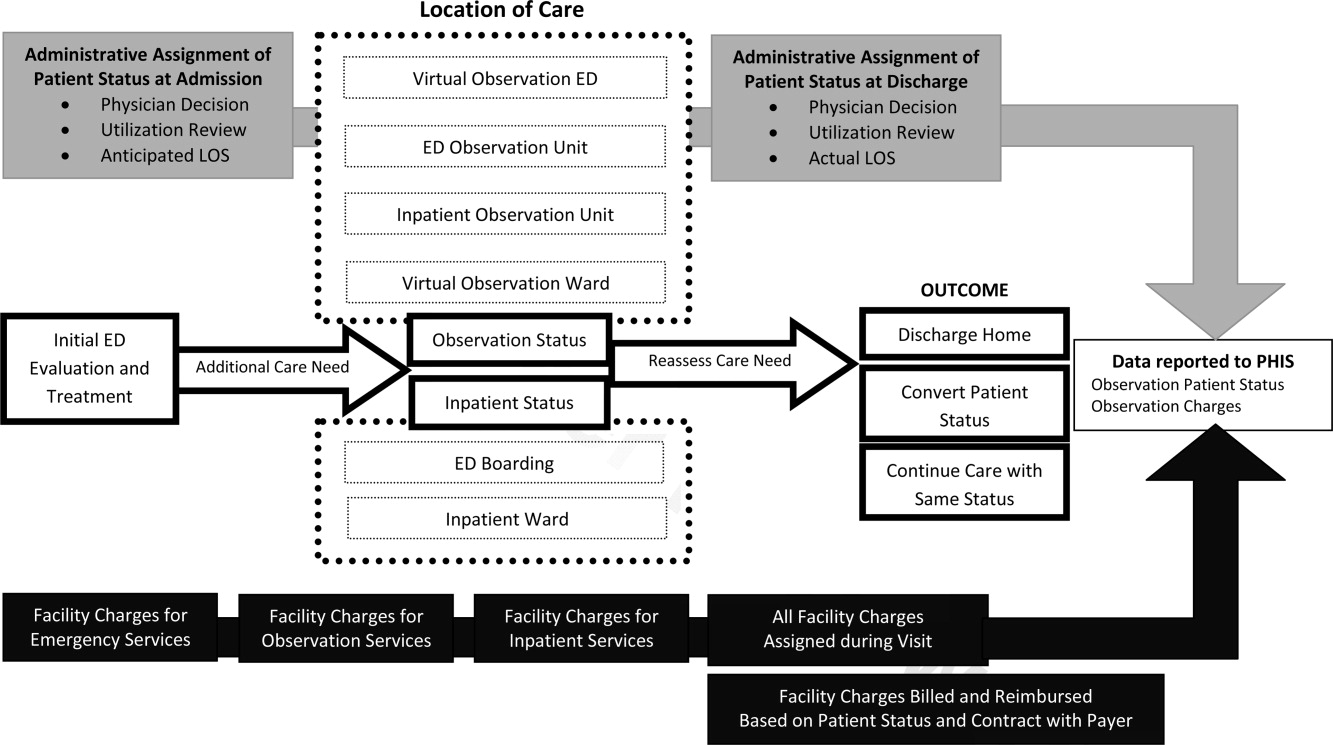
Survey 1: Classification of Observation Patients and Presence of Observation Units in PHIS Hospitals
According to responses to Survey 1, designated OUs were not widespread, present in only 12 of the 31 hospitals. No hospital reported treating all observation status patients exclusively in a designated OU. Observation status was defined by both duration of treatment and either level of care criteria or clinical care guidelines in 21 of the 31 hospitals responding to Survey 1. Of the remaining 10 hospitals, 1 reported that treatment duration alone defines observation status, and the others relied on prespecified observation criteria. When considering duration of treatment, hospitals variably indicated that anticipated or actual lengths of stay were used to determine observation status. Regarding the maximum hours a patient can be observed, 12 hospitals limited observation to 24 hours or fewer, 12 hospitals observed patients for no more than 36 to 48 hours, and the remaining 7 hospitals allowed observation periods of 72 hours or longer.
When admitting patients to observation status, 30 of 31 hospitals specified the criteria that were used to determine observation admissions. InterQual criteria, the most common response, were used by 23 of the 30 hospitals reporting specified criteria; the remaining 7 hospitals had developed hospital‐specific criteria or modified existing criteria, such as InterQual or Milliman, to determine observation status admissions. In addition to these criteria, 11 hospitals required a physician order for admission to observation status. Twenty‐four hospitals indicated that policies were in place to change patient status from observation to inpatient, or inpatient to observation, typically through processes of utilization review and application of criteria listed above.
Most hospitals indicated that they faced substantial variation in the standards used from one payer to another when considering reimbursement for care delivered under observation status. Hospitals noted that duration‐of‐carebased reimbursement practices included hourly rates, per diem, and reimbursement for only the first 24 or 48 hours of observation care. Hospitals identified that payers variably determined reimbursement for observation based on InterQual level of care criteria and Milliman care guidelines. One hospital reported that it was not their practice to bill for the observation bed.
Survey 2: Understanding Observation Patient Type Administrative Data Following ED Care Within PHIS Hospitals
Of the 20 hospitals responding to Survey 2, there were 2 hospitals that did not apply observation status to patients after ED care and 2 hospitals that did not provide complete responses. The remaining 16 hospitals provided information regarding observation status as applied to patients after receiving treatment in the ED. The settings available for observation care and patient groups treated within each area are presented in Table 2. In addition to the patient groups listed in Table 2, there were 4 hospitals where patients could be admitted to observation status directly from an outpatient clinic. All responding hospitals provided virtual observation care (ie, observation status is assigned but the patient is cared for in the existing ED or inpatient ward). Nine hospitals also provided observation care within a dedicated ED or ward‐based OU (ie, a separate clinical area in which observation patients are treated).
| Hospital No. | Available Observation Settings | Patient Groups Under Observation in Each Setting | UR to Assign Obs Status | When Obs Status Is Assigned | ||
|---|---|---|---|---|---|---|
| ED | Post‐Op | Test/Treat | ||||
| ||||||
| 1 | Virtual inpatient | X | X | X | Yes | Discharge |
| Ward‐based OU | X | X | No | |||
| 2 | Virtual inpatient | X | X | Yes | Admission | |
| Ward‐based OU | X | X | X | No | ||
| 3 | Virtual inpatient | X | X | X | Yes | Discharge |
| Ward‐based OU | X | X | X | Yes | ||
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 4 | Virtual inpatient | X | X | X | Yes | Discharge |
| ED OU | X | No | ||||
| Virtual ED | X | No | ||||
| 5 | Virtual inpatient | X | X | X | N/A | Discharge |
| 6 | Virtual inpatient | X | X | X | Yes | Discharge |
| 7 | Virtual inpatient | X | X | Yes | No response | |
| Ward‐based OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 8 | Virtual inpatient | X | X | X | Yes | Admission |
| 9 | Virtual inpatient | X | X | Yes | Discharge | |
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 10 | Virtual inpatient | X | X | X | Yes | Admission |
| ED OU | X | Yes | ||||
| 11 | Virtual inpatient | X | X | Yes | Discharge | |
| Ward‐based OU | X | X | Yes | |||
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 12 | Virtual inpatient | X | X | X | Yes | Admission |
| 13 | Virtual inpatient | X | X | N/A | Discharge | |
| Virtual ED | X | N/A | ||||
| 14 | Virtual inpatient | X | X | X | Yes | Both |
| 15 | Virtual inpatient | X | X | Yes | Admission | |
| Ward‐based OU | X | X | Yes | |||
| 16 | Virtual inpatient | X | Yes | Admission | ||
When asked to identify differences between clinical care delivered to patients admitted under virtual observation and those admitted under inpatient status, 14 of 16 hospitals selected the option There are no differences in the care delivery of these patients. The differences identified by 2 hospitals included patient care orders, treatment protocols, and physician documentation. Within the hospitals that reported utilization of virtual ED observation, 2 reported differences in care compared with other ED patients, including patient care orders, physician rounds, documentation, and discharge process. When admitted patients were boarded in the ED while awaiting an inpatient bed, 11 of 16 hospitals allowed for observation or inpatient level of care to be provided in the ED. Fourteen hospitals allow an admitted patient to be discharged home from boarding in the ED without ever receiving care in an inpatient bed. The discharge decision was made by ED providers in 7 hospitals, and inpatient providers in the other 7 hospitals.
Responses to questions providing detailed information on the process of utilization review were provided by 12 hospitals. Among this subset of hospitals, utilization review was consistently used to assign virtual inpatient observation status and was applied at admission (n = 6) or discharge (n = 8), depending on the hospital. One hospital applied observation status at both admission and discharge; 1 hospital did not provide a response. Responses to questions regarding utilization review are presented in Table 3.
| Survey Question | Yes N (%) | No N (%) |
|---|---|---|
| Preadmission utilization review is conducted at my hospital. | 3 (25) | 9 (75) |
| Utilization review occurs daily at my hospital. | 10 (83) | 2 (17) |
| A nonclinician can initiate an order for observation status. | 4 (33) | 8 (67) |
| Status can be changed after the patient has been discharged. | 10 (83) | 2 (17) |
| Inpatient status would always be assigned to a patient who receives less than 24 hours of care and meets inpatient criteria. | 9 (75) | 3 (25) |
| The same status would be assigned to different patients who received the same treatment of the same duration but have different payers. | 6 (50) | 6 (50) |
DISCUSSION
This is the largest descriptive study of pediatric observation status practices in US freestanding children's hospitals and, to our knowledge, the first to include information about both the ED and inpatient treatment environments. There are two important findings of this study. First, designated OUs were uncommon among the group of freestanding children's hospitals that reported observation patient data to PHIS in 2010. Second, despite the fact that hospitals reported observation care was delivered in a variety of settings, virtual inpatient observation status was nearly ubiquitous. Among the subset of hospitals that provided information about the clinical care delivered to patients admitted under virtual inpatient observation, hospitals frequently reported there were no differences in the care delivered to observation patients when compared with other inpatients.
The results of our survey indicate that designated OUs are not a commonly available model of observation care in the study hospitals. In fact, the vast majority of the hospitals used virtual inpatient observation care, which did not differ from the care delivered to a child admitted as an inpatient. ED‐based OUs, which often provide operationally and physically distinct care to observation patients, have been touted as cost‐effective alternatives to inpatient care,1820 resulting in fewer admissions and reductions in length of stay19, 20 without a resultant increase in return ED‐visits or readmissions.2123 Research is needed to determine the patient‐level outcomes for short‐stay patients in the variety of available treatment settings (eg, physically or operationally distinct OUs and virtual observation), and to evaluate these outcomes in comparison to results published from designated OUs. The operationally and physically distinct features of a designated OU may be required to realize the benefits of observation attributed to individual patients.
While observation care has been historically provided by emergency physicians, there is increasing interest in the role of inpatient providers in observation care.9 According to our survey, children were admitted to observation status directly from clinics, following surgical procedures, scheduled tests and treatment, or after evaluation and treatment in the ED. As many of these children undergo virtual observation in inpatient areas, the role of inpatient providers, such as pediatric hospitalists, in observation care may be an important area for future study, education, and professional development. Novel models of care, with hospitalists collaborating with emergency physicians, may be of benefit to the children who require observation following initial stabilization and treatment in the ED.24, 25
We identified variation between hospitals in the methods used to assign observation status to an episode of care, including a wide range of length of stay criteria and different approaches to utilization review. In addition, the criteria payers use to reimburse for observation varied between payers, even within individual hospitals. The results of our survey may be driven by issues of reimbursement and not based on a model of optimizing patient care outcomes using designated OUs. Variations in reimbursement may limit hospital efforts to refine models of observation care for children. Designated OUs have been suggested as a method for improving ED patient flow,26 increasing inpatient capacity,27 and reducing costs of care.28 Standardization of observation status criteria and consistent reimbursement for observation services may be necessary for hospitals to develop operationally and physically distinct OUs, which may be essential to achieving the proposed benefits of observation medicine on costs of care, patient flow, and hospital capacity.
LIMITATIONS
Our study results should be interpreted with the following limitations in mind. First, the surveys were distributed only to freestanding children's hospitals who participate in PHIS. As a result, our findings may not be generalizable to the experiences of other children's hospitals or general hospitals caring for children. Questions in Survey 2 were focused on understanding observation care, delivered to patients following ED care, which may differ from observation practices related to a direct admission or following scheduled procedures, tests, or treatments. It is important to note that, hospitals that do not report observation status patient data to PHIS are still providing care to children with acute conditions that respond to brief periods of hospital treatment, even though it is not labeled observation. However, it was beyond the scope of this study to characterize the care delivered to all patients who experience a short stay.
The second main limitation of our study is the lower response rate to Survey 2. In addition, several surveys contained incomplete responses which further limits our sample size for some questions, specifically those related to utilization review. The lower response to Survey 2 could be related to the timing of the distribution of the 2 surveys, or to the information contained in the introductory e‐mail describing Survey 2. Hospitals with designated observation units, or where observation status care has been receiving attention, may have been more likely to respond to our survey, which may bias our results to reflect the experiences of hospitals experiencing particular successes or challenges with observation status care. A comparison of known hospital characteristics revealed no differences between hospitals that did and did not provide responses to Survey 2, but other unmeasured differences may exist.
CONCLUSION
Observation status is assigned using duration of treatment, clinical care guidelines, and level of care criteria, and is defined differently by individual hospitals and payers. Currently, the most widely available setting for pediatric observation status is within a virtual inpatient unit. Our results suggest that the care delivered to observation patients in virtual inpatient units is consistent with care provided to other inpatients. As such, observation status is largely an administrative/billing designation, which does not appear to reflect differences in clinical care. A consistent approach to the assignment of patients to observation status, and treatment of patients under observation among hospitals and payers, may be necessary to compare quality outcomes. Studies of the clinical care delivery and processes of care for short‐stay patients are needed to optimize models of pediatric observation care.
- .Observation medicine: the healthcare system's tincture of time. In: Graff LG, ed.Principles of Observation Medicine.Dallas, TX:American College of Emergency Physicians;2010. Available at: http://www.acep.org/content.aspx?id=46142. Accessed February 18,year="2011"2011.
- .Hospital ‘observation’ status a matter of billing.The Columbus Dispatch. February 14,2011.
- .Hospital payments downgraded.Philadelphia Business Journal. February 18,2011.
- .Medicare rules give full hospital benefits only to those with ‘inpatient’ status.The Washington Post. September 7,2010.
- .Hospitals caught between a rock and a hard place over observation.Health Leaders Media. September 15,2010.
- .AHA: observation status fears on the rise.Health Leaders Media. October 29,2010.
- .Put your hospital bill under a microscope.The New York Times. September 13,2010.
- Medicare Hospital Manual Section 455.Washington, DC:Department of Health and Human Services, Centers for Medicare and Medicaid Services;2001.
- ,,,,.The Observation Unit: An Operational Overview for the Hospitalist. Society of Hospital Medicine White Paper. May 21, 2009. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Publications/White Papers/White_Papers.htm. Accessed May 21,2009.
- ,,,,.Utilization and unexpected hospitalization rates of a pediatric emergency department 23‐hour observation unit.Pediatr Emerg Care.2008;24(9):589–594.
- ,,.The pediatric hybrid observation unit: an analysis of 6477 consecutive patient encounters.Pediatrics.2005;115(5):e535–e542.
- ,,,,.Pediatric observation units in the United States: a systematic review.J Hosp Med.2010;5(3):172–182.
- ,,.Pediatric emergency department directors' benchmarking survey: fiscal year 2001.Pediatr Emerg Care.2003;19(3):143–147.
- ,,,.Pediatric observation status beds on an inpatient unit: an integrated care model.Pediatr Emerg Care.2004;20(1):17–21.
- ,,,.Impact of a short stay unit on asthma patients admitted to a tertiary pediatric hospital.Qual Manag Health Care.1997;6(1):14–22.
- ,,,.A national survey of observation units in the United States.Am J Emerg Med.2003;21(7):529–533.
- ,,,.A survey of observation units in the United States.Am J Emerg Med.1989;7(6):576–580.
- ,,.When the patient requires observation not hospitalization.J Nurs Admin.1988;18(10):20–23.
- ,,.A reduction in hospitalization, length of stay, and hospital charges for croup with the institution of a pediatric observation unit.Am J Emerg Med.2006;24(7):818–821.
- ,,.Outpatient oral rehydration in the United States.Am J Dis Child.1986;140(3):211–215.
- ,,,,.Pediatric closed head injuries treated in an observation unit.Pediatr Emerg Care.2005;21(10):639–644.
- ,,,.Use of pediatric observation unit for treatment of children with dehydration caused by gastroenteritis.Pediatr Emerg Care.2006;22(1):1–6.
- ,,,.Children with asthma admitted to a pediatric observation unit.Pediatr Emerg Care.2005;21(10):645–649.
- ,,,.Redefining the community pediatric hospitalist: the combined pediatric ED/inpatient unit.Pediatr Emerg Care.2007;23(1):33–37.
- ,,,.Program description: a hospitalist‐run, medical short‐stay unit in a teaching hospital.Can Med Assoc J.2000;163(11):1477–1480.
- ,.Impact of an observation unit and an emergency department‐admitted patient transfer mandate in decreasing overcrowding in a pediatric emergency department: a discrete event simulation exercise.Pediatr Emerg Care.2009;25(3):160–163.
- ,,, et al.Children's hospitals do not acutely respond to high occupancy.Pediatrics.125(5):974–981.
- ,,,,,.Trends in high‐turnover stays among children hospitalized in the United States, 1993‐2003.Pediatrics.2009;123(3):996–1002.
Observation medicine has grown in recent decades out of changes in policies for hospital reimbursement, requirements for patients to meet admission criteria to qualify for inpatient admission, and efforts to avoid unnecessary or inappropriate admissions.1 Emergency physicians are frequently faced with patients who are too sick to be discharged home, but do not clearly meet criteria for an inpatient status admission. These patients often receive extended outpatient services (typically extending 24 to 48 hours) under the designation of observation status, in order to determine their response to treatment and need for hospitalization.
Observation care delivered to adult patients has increased substantially in recent years, and the confusion around the designation of observation versus inpatient care has received increasing attention in the lay press.27 According to the Centers for Medicare and Medicaid Services (CMS)8:
Observation care is a well‐defined set of specific, clinically appropriate services, which include ongoing short term treatment, assessment, and reassessment before a decision can be made regarding whether patients will require further treatment as hospital inpatients. Observation services are commonly ordered for patients who present to the emergency department and who then require a significant period of treatment or monitoring in order to make a decision concerning their admission or discharge.
Observation status is an administrative label that is applied to patients who do not meet inpatient level of care criteria, as defined by third parties such as InterQual. These criteria usually include a combination of the patient's clinical diagnoses, severity of illness, and expected needs for monitoring and interventions, in order to determine the admission status to which the patient may be assigned (eg, observation, inpatient, or intensive care). Observation services can be provided, in a variety of settings, to those patients who do not meet inpatient level of care but require a period of observation. Some hospitals provide observation care in discrete units in the emergency department (ED) or specific inpatient unit, and others have no designated unit but scatter observation patients throughout the institution, termed virtual observation units.9
For more than 30 years, observation unit (OU) admission has offered an alternative to traditional inpatient hospitalization for children with a variety of acute conditions.10, 11 Historically, the published literature on observation care for children in the United States has been largely based in dedicated emergency department OUs.12 Yet, in a 2001 survey of 21 pediatric EDs, just 6 reported the presence of a 23‐hour unit.13 There are single‐site examples of observation care delivered in other settings.14, 15 In 2 national surveys of US General Hospitals, 25% provided observation services in beds adjacent to the ED, and the remainder provided observation services in hospital inpatient units.16, 17 However, we are not aware of any previous multi‐institution studies exploring hospital‐wide practices related to observation care for children.
Recognizing that observation status can be designated using various standards, and that observation care can be delivered in locations outside of dedicated OUs,9 we developed 2 web‐based surveys to examine the current models of pediatric observation medicine in US children's hospitals. We hypothesized that observation care is most commonly applied as a billing designation and does not necessarily represent care delivered in a structurally or functionally distinct OU, nor does it represent a difference in care provided to those patients with inpatient designation.
METHODS
Study Design
Two web‐based surveys were distributed, in April 2010, to the 42 freestanding, tertiary care children's hospitals affiliated with the Child Health Corporation of America (CHCA; Shawnee Mission, KS) which contribute data to the Pediatric Health Information System (PHIS) database. The PHIS is a national administrative database that contains resource utilization data from participating hospitals located in noncompeting markets of 27 states plus the District of Columbia. These hospitals account for 20% of all tertiary care children's hospitals in the United States.
Survey Content
Survey 1
A survey of hospital observation status practices has been developed by CHCA as a part of the PHIS data quality initiative (see Supporting Appendix: Survey 1 in the online version of this article). Hospitals that did not provide observation patient data to PHIS were excluded after an initial screening question. This survey obtained information regarding the designation of observation status within each hospital. Hospitals provided free‐text responses to questions related to the criteria used to define observation, and to admit patients into observation status. Fixed‐choice response questions were used to determine specific observation status utilization criteria and clinical guidelines (eg, InterQual and Milliman) used by hospitals for the designation of observation status to patients.
Survey 2
We developed a detailed follow‐up survey in order to characterize the structures and processes of care associated with observation status (see Supporting Appendix: Survey 2 in the online version of this article). Within the follow‐up survey, an initial screening question was used to determine all types of patients to which observation status is assigned within the responding hospitals. All other questions in Survey 2 were focused specifically on those patients who required additional care following ED evaluation and treatment. Fixed‐choice response questions were used to explore differences in care for patients under observation and those admitted as inpatients. We also inquired of hospital practices related to boarding of patients in the ED while awaiting admission to an inpatient bed.
Survey Distribution
Two web‐based surveys were distributed to all 42 CHCA hospitals that contribute data to PHIS. During the month of April 2010, each hospital's designated PHIS operational contact received e‐mail correspondence requesting their participation in each survey. Within hospitals participating in PHIS, Operational Contacts have been assigned to serve as the day‐to‐day PHIS contact person based upon their experience working with the PHIS data. The Operational Contacts are CHCA's primary contact for issues related to the hospital's data quality and reporting to PHIS. Non‐responders were contacted by e‐mail for additional requests to complete the surveys. Each e‐mail provided an introduction to the topic of the survey and a link to complete the survey. The e‐mail requesting participation in Survey 1 was distributed the first week of April 2010, and the survey was open for responses during the first 3 weeks of the month. The e‐mail requesting participation in Survey 2 was sent the third week of April 2010, and the survey was open for responses during the subsequent 2 weeks.
DATA ANALYSIS
Survey responses were collected and are presented as a descriptive summary of results. Hospital characteristics were summarized with medians and interquartile ranges for continuous variables, and with percents for categorical variables. Characteristics were compared between hospitals that responded and those that did not respond to Survey 2 using Wilcoxon rank‐sum tests and chi‐square tests as appropriate. All analyses were performed using SAS v.9.2 (SAS Institute, Cary, NC), and a P value <0.05 was considered statistically significant. The study was reviewed by the University of Michigan Institutional Review Board and considered exempt.
RESULTS
Responses to Survey 1 were available from 37 of 42 (88%) of PHIS hospitals (Figure 1). For Survey 2, we received responses from 20 of 42 (48%) of PHIS hospitals. Based on information available from Survey 1, we know that 20 of the 31 (65%) PHIS hospitals that report observation status patient data to PHIS responded to Survey 2. Characteristics of the hospitals responding and not responding to Survey 2 are presented in Table 1. Respondents provided hospital identifying information which allowed for the linkage of data, from Survey 1, to 17 of the 20 hospitals responding to Survey 2. We did not have information available to link responses from 3 hospitals.

| Respondent N = 20 | Non‐Respondent N = 22 | P Value | |
|---|---|---|---|
| |||
| No. of inpatient beds Median [IQR] (excluding Obstetrics) | 245 [219283] | 282 [250381] | 0.076 |
| Annual admissions Median [IQR] (excluding births) | 11,658 [8,64213,213] | 13,522 [9,83018,705] | 0.106 |
| ED volume Median [IQR] | 60,528 [47,85082,955] | 64,486 [47,38684,450] | 0.640 |
| Percent government payer Median [IQR] | 53% [4662] | 49% [4158] | 0.528 |
| Region | |||
| Northeast | 37% | 0% | 0.021 |
| Midwest | 21% | 33% | |
| South | 21% | 50% | |
| West | 21% | 17% | |
| Reports observation status patients to PHIS | 85% | 90% | 0.555 |
Based on responses to the surveys and our knowledge of data reported to PHIS, our current understanding of patient flow from ED through observation to discharge home, and the application of observation status to the encounter, is presented in Figure 2. According to free‐text responses to Survey 1, various methods were applied to designate observation status (gray shaded boxes in Figure 2). Fixed‐choice responses to Survey 2 revealed that observation status patients were cared for in a variety of locations within hospitals, including ED beds, designated observation units, and inpatient beds (dashed boxes in Figure 2). Not every facility utilized all of the listed locations for observation care. Space constraints could dictate the location of care, regardless of patient status (eg, observation vs inpatient), in hospitals with more than one location of care available to observation patients. While patient status could change during a visit, only the final patient status at discharge enters the administrative record submitted to PHIS (black boxes in Figure 2). Facility charges for observation remained a part of the visit record and were reported to PHIS. Hospitals may or may not bill for all assigned charges depending on patient status, length of stay, or other specific criteria determined by contracts with individual payers.

Survey 1: Classification of Observation Patients and Presence of Observation Units in PHIS Hospitals
According to responses to Survey 1, designated OUs were not widespread, present in only 12 of the 31 hospitals. No hospital reported treating all observation status patients exclusively in a designated OU. Observation status was defined by both duration of treatment and either level of care criteria or clinical care guidelines in 21 of the 31 hospitals responding to Survey 1. Of the remaining 10 hospitals, 1 reported that treatment duration alone defines observation status, and the others relied on prespecified observation criteria. When considering duration of treatment, hospitals variably indicated that anticipated or actual lengths of stay were used to determine observation status. Regarding the maximum hours a patient can be observed, 12 hospitals limited observation to 24 hours or fewer, 12 hospitals observed patients for no more than 36 to 48 hours, and the remaining 7 hospitals allowed observation periods of 72 hours or longer.
When admitting patients to observation status, 30 of 31 hospitals specified the criteria that were used to determine observation admissions. InterQual criteria, the most common response, were used by 23 of the 30 hospitals reporting specified criteria; the remaining 7 hospitals had developed hospital‐specific criteria or modified existing criteria, such as InterQual or Milliman, to determine observation status admissions. In addition to these criteria, 11 hospitals required a physician order for admission to observation status. Twenty‐four hospitals indicated that policies were in place to change patient status from observation to inpatient, or inpatient to observation, typically through processes of utilization review and application of criteria listed above.
Most hospitals indicated that they faced substantial variation in the standards used from one payer to another when considering reimbursement for care delivered under observation status. Hospitals noted that duration‐of‐carebased reimbursement practices included hourly rates, per diem, and reimbursement for only the first 24 or 48 hours of observation care. Hospitals identified that payers variably determined reimbursement for observation based on InterQual level of care criteria and Milliman care guidelines. One hospital reported that it was not their practice to bill for the observation bed.
Survey 2: Understanding Observation Patient Type Administrative Data Following ED Care Within PHIS Hospitals
Of the 20 hospitals responding to Survey 2, there were 2 hospitals that did not apply observation status to patients after ED care and 2 hospitals that did not provide complete responses. The remaining 16 hospitals provided information regarding observation status as applied to patients after receiving treatment in the ED. The settings available for observation care and patient groups treated within each area are presented in Table 2. In addition to the patient groups listed in Table 2, there were 4 hospitals where patients could be admitted to observation status directly from an outpatient clinic. All responding hospitals provided virtual observation care (ie, observation status is assigned but the patient is cared for in the existing ED or inpatient ward). Nine hospitals also provided observation care within a dedicated ED or ward‐based OU (ie, a separate clinical area in which observation patients are treated).
| Hospital No. | Available Observation Settings | Patient Groups Under Observation in Each Setting | UR to Assign Obs Status | When Obs Status Is Assigned | ||
|---|---|---|---|---|---|---|
| ED | Post‐Op | Test/Treat | ||||
| ||||||
| 1 | Virtual inpatient | X | X | X | Yes | Discharge |
| Ward‐based OU | X | X | No | |||
| 2 | Virtual inpatient | X | X | Yes | Admission | |
| Ward‐based OU | X | X | X | No | ||
| 3 | Virtual inpatient | X | X | X | Yes | Discharge |
| Ward‐based OU | X | X | X | Yes | ||
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 4 | Virtual inpatient | X | X | X | Yes | Discharge |
| ED OU | X | No | ||||
| Virtual ED | X | No | ||||
| 5 | Virtual inpatient | X | X | X | N/A | Discharge |
| 6 | Virtual inpatient | X | X | X | Yes | Discharge |
| 7 | Virtual inpatient | X | X | Yes | No response | |
| Ward‐based OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 8 | Virtual inpatient | X | X | X | Yes | Admission |
| 9 | Virtual inpatient | X | X | Yes | Discharge | |
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 10 | Virtual inpatient | X | X | X | Yes | Admission |
| ED OU | X | Yes | ||||
| 11 | Virtual inpatient | X | X | Yes | Discharge | |
| Ward‐based OU | X | X | Yes | |||
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 12 | Virtual inpatient | X | X | X | Yes | Admission |
| 13 | Virtual inpatient | X | X | N/A | Discharge | |
| Virtual ED | X | N/A | ||||
| 14 | Virtual inpatient | X | X | X | Yes | Both |
| 15 | Virtual inpatient | X | X | Yes | Admission | |
| Ward‐based OU | X | X | Yes | |||
| 16 | Virtual inpatient | X | Yes | Admission | ||
When asked to identify differences between clinical care delivered to patients admitted under virtual observation and those admitted under inpatient status, 14 of 16 hospitals selected the option There are no differences in the care delivery of these patients. The differences identified by 2 hospitals included patient care orders, treatment protocols, and physician documentation. Within the hospitals that reported utilization of virtual ED observation, 2 reported differences in care compared with other ED patients, including patient care orders, physician rounds, documentation, and discharge process. When admitted patients were boarded in the ED while awaiting an inpatient bed, 11 of 16 hospitals allowed for observation or inpatient level of care to be provided in the ED. Fourteen hospitals allow an admitted patient to be discharged home from boarding in the ED without ever receiving care in an inpatient bed. The discharge decision was made by ED providers in 7 hospitals, and inpatient providers in the other 7 hospitals.
Responses to questions providing detailed information on the process of utilization review were provided by 12 hospitals. Among this subset of hospitals, utilization review was consistently used to assign virtual inpatient observation status and was applied at admission (n = 6) or discharge (n = 8), depending on the hospital. One hospital applied observation status at both admission and discharge; 1 hospital did not provide a response. Responses to questions regarding utilization review are presented in Table 3.
| Survey Question | Yes N (%) | No N (%) |
|---|---|---|
| Preadmission utilization review is conducted at my hospital. | 3 (25) | 9 (75) |
| Utilization review occurs daily at my hospital. | 10 (83) | 2 (17) |
| A nonclinician can initiate an order for observation status. | 4 (33) | 8 (67) |
| Status can be changed after the patient has been discharged. | 10 (83) | 2 (17) |
| Inpatient status would always be assigned to a patient who receives less than 24 hours of care and meets inpatient criteria. | 9 (75) | 3 (25) |
| The same status would be assigned to different patients who received the same treatment of the same duration but have different payers. | 6 (50) | 6 (50) |
DISCUSSION
This is the largest descriptive study of pediatric observation status practices in US freestanding children's hospitals and, to our knowledge, the first to include information about both the ED and inpatient treatment environments. There are two important findings of this study. First, designated OUs were uncommon among the group of freestanding children's hospitals that reported observation patient data to PHIS in 2010. Second, despite the fact that hospitals reported observation care was delivered in a variety of settings, virtual inpatient observation status was nearly ubiquitous. Among the subset of hospitals that provided information about the clinical care delivered to patients admitted under virtual inpatient observation, hospitals frequently reported there were no differences in the care delivered to observation patients when compared with other inpatients.
The results of our survey indicate that designated OUs are not a commonly available model of observation care in the study hospitals. In fact, the vast majority of the hospitals used virtual inpatient observation care, which did not differ from the care delivered to a child admitted as an inpatient. ED‐based OUs, which often provide operationally and physically distinct care to observation patients, have been touted as cost‐effective alternatives to inpatient care,1820 resulting in fewer admissions and reductions in length of stay19, 20 without a resultant increase in return ED‐visits or readmissions.2123 Research is needed to determine the patient‐level outcomes for short‐stay patients in the variety of available treatment settings (eg, physically or operationally distinct OUs and virtual observation), and to evaluate these outcomes in comparison to results published from designated OUs. The operationally and physically distinct features of a designated OU may be required to realize the benefits of observation attributed to individual patients.
While observation care has been historically provided by emergency physicians, there is increasing interest in the role of inpatient providers in observation care.9 According to our survey, children were admitted to observation status directly from clinics, following surgical procedures, scheduled tests and treatment, or after evaluation and treatment in the ED. As many of these children undergo virtual observation in inpatient areas, the role of inpatient providers, such as pediatric hospitalists, in observation care may be an important area for future study, education, and professional development. Novel models of care, with hospitalists collaborating with emergency physicians, may be of benefit to the children who require observation following initial stabilization and treatment in the ED.24, 25
We identified variation between hospitals in the methods used to assign observation status to an episode of care, including a wide range of length of stay criteria and different approaches to utilization review. In addition, the criteria payers use to reimburse for observation varied between payers, even within individual hospitals. The results of our survey may be driven by issues of reimbursement and not based on a model of optimizing patient care outcomes using designated OUs. Variations in reimbursement may limit hospital efforts to refine models of observation care for children. Designated OUs have been suggested as a method for improving ED patient flow,26 increasing inpatient capacity,27 and reducing costs of care.28 Standardization of observation status criteria and consistent reimbursement for observation services may be necessary for hospitals to develop operationally and physically distinct OUs, which may be essential to achieving the proposed benefits of observation medicine on costs of care, patient flow, and hospital capacity.
LIMITATIONS
Our study results should be interpreted with the following limitations in mind. First, the surveys were distributed only to freestanding children's hospitals who participate in PHIS. As a result, our findings may not be generalizable to the experiences of other children's hospitals or general hospitals caring for children. Questions in Survey 2 were focused on understanding observation care, delivered to patients following ED care, which may differ from observation practices related to a direct admission or following scheduled procedures, tests, or treatments. It is important to note that, hospitals that do not report observation status patient data to PHIS are still providing care to children with acute conditions that respond to brief periods of hospital treatment, even though it is not labeled observation. However, it was beyond the scope of this study to characterize the care delivered to all patients who experience a short stay.
The second main limitation of our study is the lower response rate to Survey 2. In addition, several surveys contained incomplete responses which further limits our sample size for some questions, specifically those related to utilization review. The lower response to Survey 2 could be related to the timing of the distribution of the 2 surveys, or to the information contained in the introductory e‐mail describing Survey 2. Hospitals with designated observation units, or where observation status care has been receiving attention, may have been more likely to respond to our survey, which may bias our results to reflect the experiences of hospitals experiencing particular successes or challenges with observation status care. A comparison of known hospital characteristics revealed no differences between hospitals that did and did not provide responses to Survey 2, but other unmeasured differences may exist.
CONCLUSION
Observation status is assigned using duration of treatment, clinical care guidelines, and level of care criteria, and is defined differently by individual hospitals and payers. Currently, the most widely available setting for pediatric observation status is within a virtual inpatient unit. Our results suggest that the care delivered to observation patients in virtual inpatient units is consistent with care provided to other inpatients. As such, observation status is largely an administrative/billing designation, which does not appear to reflect differences in clinical care. A consistent approach to the assignment of patients to observation status, and treatment of patients under observation among hospitals and payers, may be necessary to compare quality outcomes. Studies of the clinical care delivery and processes of care for short‐stay patients are needed to optimize models of pediatric observation care.
Observation medicine has grown in recent decades out of changes in policies for hospital reimbursement, requirements for patients to meet admission criteria to qualify for inpatient admission, and efforts to avoid unnecessary or inappropriate admissions.1 Emergency physicians are frequently faced with patients who are too sick to be discharged home, but do not clearly meet criteria for an inpatient status admission. These patients often receive extended outpatient services (typically extending 24 to 48 hours) under the designation of observation status, in order to determine their response to treatment and need for hospitalization.
Observation care delivered to adult patients has increased substantially in recent years, and the confusion around the designation of observation versus inpatient care has received increasing attention in the lay press.27 According to the Centers for Medicare and Medicaid Services (CMS)8:
Observation care is a well‐defined set of specific, clinically appropriate services, which include ongoing short term treatment, assessment, and reassessment before a decision can be made regarding whether patients will require further treatment as hospital inpatients. Observation services are commonly ordered for patients who present to the emergency department and who then require a significant period of treatment or monitoring in order to make a decision concerning their admission or discharge.
Observation status is an administrative label that is applied to patients who do not meet inpatient level of care criteria, as defined by third parties such as InterQual. These criteria usually include a combination of the patient's clinical diagnoses, severity of illness, and expected needs for monitoring and interventions, in order to determine the admission status to which the patient may be assigned (eg, observation, inpatient, or intensive care). Observation services can be provided, in a variety of settings, to those patients who do not meet inpatient level of care but require a period of observation. Some hospitals provide observation care in discrete units in the emergency department (ED) or specific inpatient unit, and others have no designated unit but scatter observation patients throughout the institution, termed virtual observation units.9
For more than 30 years, observation unit (OU) admission has offered an alternative to traditional inpatient hospitalization for children with a variety of acute conditions.10, 11 Historically, the published literature on observation care for children in the United States has been largely based in dedicated emergency department OUs.12 Yet, in a 2001 survey of 21 pediatric EDs, just 6 reported the presence of a 23‐hour unit.13 There are single‐site examples of observation care delivered in other settings.14, 15 In 2 national surveys of US General Hospitals, 25% provided observation services in beds adjacent to the ED, and the remainder provided observation services in hospital inpatient units.16, 17 However, we are not aware of any previous multi‐institution studies exploring hospital‐wide practices related to observation care for children.
Recognizing that observation status can be designated using various standards, and that observation care can be delivered in locations outside of dedicated OUs,9 we developed 2 web‐based surveys to examine the current models of pediatric observation medicine in US children's hospitals. We hypothesized that observation care is most commonly applied as a billing designation and does not necessarily represent care delivered in a structurally or functionally distinct OU, nor does it represent a difference in care provided to those patients with inpatient designation.
METHODS
Study Design
Two web‐based surveys were distributed, in April 2010, to the 42 freestanding, tertiary care children's hospitals affiliated with the Child Health Corporation of America (CHCA; Shawnee Mission, KS) which contribute data to the Pediatric Health Information System (PHIS) database. The PHIS is a national administrative database that contains resource utilization data from participating hospitals located in noncompeting markets of 27 states plus the District of Columbia. These hospitals account for 20% of all tertiary care children's hospitals in the United States.
Survey Content
Survey 1
A survey of hospital observation status practices has been developed by CHCA as a part of the PHIS data quality initiative (see Supporting Appendix: Survey 1 in the online version of this article). Hospitals that did not provide observation patient data to PHIS were excluded after an initial screening question. This survey obtained information regarding the designation of observation status within each hospital. Hospitals provided free‐text responses to questions related to the criteria used to define observation, and to admit patients into observation status. Fixed‐choice response questions were used to determine specific observation status utilization criteria and clinical guidelines (eg, InterQual and Milliman) used by hospitals for the designation of observation status to patients.
Survey 2
We developed a detailed follow‐up survey in order to characterize the structures and processes of care associated with observation status (see Supporting Appendix: Survey 2 in the online version of this article). Within the follow‐up survey, an initial screening question was used to determine all types of patients to which observation status is assigned within the responding hospitals. All other questions in Survey 2 were focused specifically on those patients who required additional care following ED evaluation and treatment. Fixed‐choice response questions were used to explore differences in care for patients under observation and those admitted as inpatients. We also inquired of hospital practices related to boarding of patients in the ED while awaiting admission to an inpatient bed.
Survey Distribution
Two web‐based surveys were distributed to all 42 CHCA hospitals that contribute data to PHIS. During the month of April 2010, each hospital's designated PHIS operational contact received e‐mail correspondence requesting their participation in each survey. Within hospitals participating in PHIS, Operational Contacts have been assigned to serve as the day‐to‐day PHIS contact person based upon their experience working with the PHIS data. The Operational Contacts are CHCA's primary contact for issues related to the hospital's data quality and reporting to PHIS. Non‐responders were contacted by e‐mail for additional requests to complete the surveys. Each e‐mail provided an introduction to the topic of the survey and a link to complete the survey. The e‐mail requesting participation in Survey 1 was distributed the first week of April 2010, and the survey was open for responses during the first 3 weeks of the month. The e‐mail requesting participation in Survey 2 was sent the third week of April 2010, and the survey was open for responses during the subsequent 2 weeks.
DATA ANALYSIS
Survey responses were collected and are presented as a descriptive summary of results. Hospital characteristics were summarized with medians and interquartile ranges for continuous variables, and with percents for categorical variables. Characteristics were compared between hospitals that responded and those that did not respond to Survey 2 using Wilcoxon rank‐sum tests and chi‐square tests as appropriate. All analyses were performed using SAS v.9.2 (SAS Institute, Cary, NC), and a P value <0.05 was considered statistically significant. The study was reviewed by the University of Michigan Institutional Review Board and considered exempt.
RESULTS
Responses to Survey 1 were available from 37 of 42 (88%) of PHIS hospitals (Figure 1). For Survey 2, we received responses from 20 of 42 (48%) of PHIS hospitals. Based on information available from Survey 1, we know that 20 of the 31 (65%) PHIS hospitals that report observation status patient data to PHIS responded to Survey 2. Characteristics of the hospitals responding and not responding to Survey 2 are presented in Table 1. Respondents provided hospital identifying information which allowed for the linkage of data, from Survey 1, to 17 of the 20 hospitals responding to Survey 2. We did not have information available to link responses from 3 hospitals.

| Respondent N = 20 | Non‐Respondent N = 22 | P Value | |
|---|---|---|---|
| |||
| No. of inpatient beds Median [IQR] (excluding Obstetrics) | 245 [219283] | 282 [250381] | 0.076 |
| Annual admissions Median [IQR] (excluding births) | 11,658 [8,64213,213] | 13,522 [9,83018,705] | 0.106 |
| ED volume Median [IQR] | 60,528 [47,85082,955] | 64,486 [47,38684,450] | 0.640 |
| Percent government payer Median [IQR] | 53% [4662] | 49% [4158] | 0.528 |
| Region | |||
| Northeast | 37% | 0% | 0.021 |
| Midwest | 21% | 33% | |
| South | 21% | 50% | |
| West | 21% | 17% | |
| Reports observation status patients to PHIS | 85% | 90% | 0.555 |
Based on responses to the surveys and our knowledge of data reported to PHIS, our current understanding of patient flow from ED through observation to discharge home, and the application of observation status to the encounter, is presented in Figure 2. According to free‐text responses to Survey 1, various methods were applied to designate observation status (gray shaded boxes in Figure 2). Fixed‐choice responses to Survey 2 revealed that observation status patients were cared for in a variety of locations within hospitals, including ED beds, designated observation units, and inpatient beds (dashed boxes in Figure 2). Not every facility utilized all of the listed locations for observation care. Space constraints could dictate the location of care, regardless of patient status (eg, observation vs inpatient), in hospitals with more than one location of care available to observation patients. While patient status could change during a visit, only the final patient status at discharge enters the administrative record submitted to PHIS (black boxes in Figure 2). Facility charges for observation remained a part of the visit record and were reported to PHIS. Hospitals may or may not bill for all assigned charges depending on patient status, length of stay, or other specific criteria determined by contracts with individual payers.

Survey 1: Classification of Observation Patients and Presence of Observation Units in PHIS Hospitals
According to responses to Survey 1, designated OUs were not widespread, present in only 12 of the 31 hospitals. No hospital reported treating all observation status patients exclusively in a designated OU. Observation status was defined by both duration of treatment and either level of care criteria or clinical care guidelines in 21 of the 31 hospitals responding to Survey 1. Of the remaining 10 hospitals, 1 reported that treatment duration alone defines observation status, and the others relied on prespecified observation criteria. When considering duration of treatment, hospitals variably indicated that anticipated or actual lengths of stay were used to determine observation status. Regarding the maximum hours a patient can be observed, 12 hospitals limited observation to 24 hours or fewer, 12 hospitals observed patients for no more than 36 to 48 hours, and the remaining 7 hospitals allowed observation periods of 72 hours or longer.
When admitting patients to observation status, 30 of 31 hospitals specified the criteria that were used to determine observation admissions. InterQual criteria, the most common response, were used by 23 of the 30 hospitals reporting specified criteria; the remaining 7 hospitals had developed hospital‐specific criteria or modified existing criteria, such as InterQual or Milliman, to determine observation status admissions. In addition to these criteria, 11 hospitals required a physician order for admission to observation status. Twenty‐four hospitals indicated that policies were in place to change patient status from observation to inpatient, or inpatient to observation, typically through processes of utilization review and application of criteria listed above.
Most hospitals indicated that they faced substantial variation in the standards used from one payer to another when considering reimbursement for care delivered under observation status. Hospitals noted that duration‐of‐carebased reimbursement practices included hourly rates, per diem, and reimbursement for only the first 24 or 48 hours of observation care. Hospitals identified that payers variably determined reimbursement for observation based on InterQual level of care criteria and Milliman care guidelines. One hospital reported that it was not their practice to bill for the observation bed.
Survey 2: Understanding Observation Patient Type Administrative Data Following ED Care Within PHIS Hospitals
Of the 20 hospitals responding to Survey 2, there were 2 hospitals that did not apply observation status to patients after ED care and 2 hospitals that did not provide complete responses. The remaining 16 hospitals provided information regarding observation status as applied to patients after receiving treatment in the ED. The settings available for observation care and patient groups treated within each area are presented in Table 2. In addition to the patient groups listed in Table 2, there were 4 hospitals where patients could be admitted to observation status directly from an outpatient clinic. All responding hospitals provided virtual observation care (ie, observation status is assigned but the patient is cared for in the existing ED or inpatient ward). Nine hospitals also provided observation care within a dedicated ED or ward‐based OU (ie, a separate clinical area in which observation patients are treated).
| Hospital No. | Available Observation Settings | Patient Groups Under Observation in Each Setting | UR to Assign Obs Status | When Obs Status Is Assigned | ||
|---|---|---|---|---|---|---|
| ED | Post‐Op | Test/Treat | ||||
| ||||||
| 1 | Virtual inpatient | X | X | X | Yes | Discharge |
| Ward‐based OU | X | X | No | |||
| 2 | Virtual inpatient | X | X | Yes | Admission | |
| Ward‐based OU | X | X | X | No | ||
| 3 | Virtual inpatient | X | X | X | Yes | Discharge |
| Ward‐based OU | X | X | X | Yes | ||
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 4 | Virtual inpatient | X | X | X | Yes | Discharge |
| ED OU | X | No | ||||
| Virtual ED | X | No | ||||
| 5 | Virtual inpatient | X | X | X | N/A | Discharge |
| 6 | Virtual inpatient | X | X | X | Yes | Discharge |
| 7 | Virtual inpatient | X | X | Yes | No response | |
| Ward‐based OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 8 | Virtual inpatient | X | X | X | Yes | Admission |
| 9 | Virtual inpatient | X | X | Yes | Discharge | |
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 10 | Virtual inpatient | X | X | X | Yes | Admission |
| ED OU | X | Yes | ||||
| 11 | Virtual inpatient | X | X | Yes | Discharge | |
| Ward‐based OU | X | X | Yes | |||
| ED OU | X | Yes | ||||
| Virtual ED | X | Yes | ||||
| 12 | Virtual inpatient | X | X | X | Yes | Admission |
| 13 | Virtual inpatient | X | X | N/A | Discharge | |
| Virtual ED | X | N/A | ||||
| 14 | Virtual inpatient | X | X | X | Yes | Both |
| 15 | Virtual inpatient | X | X | Yes | Admission | |
| Ward‐based OU | X | X | Yes | |||
| 16 | Virtual inpatient | X | Yes | Admission | ||
When asked to identify differences between clinical care delivered to patients admitted under virtual observation and those admitted under inpatient status, 14 of 16 hospitals selected the option There are no differences in the care delivery of these patients. The differences identified by 2 hospitals included patient care orders, treatment protocols, and physician documentation. Within the hospitals that reported utilization of virtual ED observation, 2 reported differences in care compared with other ED patients, including patient care orders, physician rounds, documentation, and discharge process. When admitted patients were boarded in the ED while awaiting an inpatient bed, 11 of 16 hospitals allowed for observation or inpatient level of care to be provided in the ED. Fourteen hospitals allow an admitted patient to be discharged home from boarding in the ED without ever receiving care in an inpatient bed. The discharge decision was made by ED providers in 7 hospitals, and inpatient providers in the other 7 hospitals.
Responses to questions providing detailed information on the process of utilization review were provided by 12 hospitals. Among this subset of hospitals, utilization review was consistently used to assign virtual inpatient observation status and was applied at admission (n = 6) or discharge (n = 8), depending on the hospital. One hospital applied observation status at both admission and discharge; 1 hospital did not provide a response. Responses to questions regarding utilization review are presented in Table 3.
| Survey Question | Yes N (%) | No N (%) |
|---|---|---|
| Preadmission utilization review is conducted at my hospital. | 3 (25) | 9 (75) |
| Utilization review occurs daily at my hospital. | 10 (83) | 2 (17) |
| A nonclinician can initiate an order for observation status. | 4 (33) | 8 (67) |
| Status can be changed after the patient has been discharged. | 10 (83) | 2 (17) |
| Inpatient status would always be assigned to a patient who receives less than 24 hours of care and meets inpatient criteria. | 9 (75) | 3 (25) |
| The same status would be assigned to different patients who received the same treatment of the same duration but have different payers. | 6 (50) | 6 (50) |
DISCUSSION
This is the largest descriptive study of pediatric observation status practices in US freestanding children's hospitals and, to our knowledge, the first to include information about both the ED and inpatient treatment environments. There are two important findings of this study. First, designated OUs were uncommon among the group of freestanding children's hospitals that reported observation patient data to PHIS in 2010. Second, despite the fact that hospitals reported observation care was delivered in a variety of settings, virtual inpatient observation status was nearly ubiquitous. Among the subset of hospitals that provided information about the clinical care delivered to patients admitted under virtual inpatient observation, hospitals frequently reported there were no differences in the care delivered to observation patients when compared with other inpatients.
The results of our survey indicate that designated OUs are not a commonly available model of observation care in the study hospitals. In fact, the vast majority of the hospitals used virtual inpatient observation care, which did not differ from the care delivered to a child admitted as an inpatient. ED‐based OUs, which often provide operationally and physically distinct care to observation patients, have been touted as cost‐effective alternatives to inpatient care,1820 resulting in fewer admissions and reductions in length of stay19, 20 without a resultant increase in return ED‐visits or readmissions.2123 Research is needed to determine the patient‐level outcomes for short‐stay patients in the variety of available treatment settings (eg, physically or operationally distinct OUs and virtual observation), and to evaluate these outcomes in comparison to results published from designated OUs. The operationally and physically distinct features of a designated OU may be required to realize the benefits of observation attributed to individual patients.
While observation care has been historically provided by emergency physicians, there is increasing interest in the role of inpatient providers in observation care.9 According to our survey, children were admitted to observation status directly from clinics, following surgical procedures, scheduled tests and treatment, or after evaluation and treatment in the ED. As many of these children undergo virtual observation in inpatient areas, the role of inpatient providers, such as pediatric hospitalists, in observation care may be an important area for future study, education, and professional development. Novel models of care, with hospitalists collaborating with emergency physicians, may be of benefit to the children who require observation following initial stabilization and treatment in the ED.24, 25
We identified variation between hospitals in the methods used to assign observation status to an episode of care, including a wide range of length of stay criteria and different approaches to utilization review. In addition, the criteria payers use to reimburse for observation varied between payers, even within individual hospitals. The results of our survey may be driven by issues of reimbursement and not based on a model of optimizing patient care outcomes using designated OUs. Variations in reimbursement may limit hospital efforts to refine models of observation care for children. Designated OUs have been suggested as a method for improving ED patient flow,26 increasing inpatient capacity,27 and reducing costs of care.28 Standardization of observation status criteria and consistent reimbursement for observation services may be necessary for hospitals to develop operationally and physically distinct OUs, which may be essential to achieving the proposed benefits of observation medicine on costs of care, patient flow, and hospital capacity.
LIMITATIONS
Our study results should be interpreted with the following limitations in mind. First, the surveys were distributed only to freestanding children's hospitals who participate in PHIS. As a result, our findings may not be generalizable to the experiences of other children's hospitals or general hospitals caring for children. Questions in Survey 2 were focused on understanding observation care, delivered to patients following ED care, which may differ from observation practices related to a direct admission or following scheduled procedures, tests, or treatments. It is important to note that, hospitals that do not report observation status patient data to PHIS are still providing care to children with acute conditions that respond to brief periods of hospital treatment, even though it is not labeled observation. However, it was beyond the scope of this study to characterize the care delivered to all patients who experience a short stay.
The second main limitation of our study is the lower response rate to Survey 2. In addition, several surveys contained incomplete responses which further limits our sample size for some questions, specifically those related to utilization review. The lower response to Survey 2 could be related to the timing of the distribution of the 2 surveys, or to the information contained in the introductory e‐mail describing Survey 2. Hospitals with designated observation units, or where observation status care has been receiving attention, may have been more likely to respond to our survey, which may bias our results to reflect the experiences of hospitals experiencing particular successes or challenges with observation status care. A comparison of known hospital characteristics revealed no differences between hospitals that did and did not provide responses to Survey 2, but other unmeasured differences may exist.
CONCLUSION
Observation status is assigned using duration of treatment, clinical care guidelines, and level of care criteria, and is defined differently by individual hospitals and payers. Currently, the most widely available setting for pediatric observation status is within a virtual inpatient unit. Our results suggest that the care delivered to observation patients in virtual inpatient units is consistent with care provided to other inpatients. As such, observation status is largely an administrative/billing designation, which does not appear to reflect differences in clinical care. A consistent approach to the assignment of patients to observation status, and treatment of patients under observation among hospitals and payers, may be necessary to compare quality outcomes. Studies of the clinical care delivery and processes of care for short‐stay patients are needed to optimize models of pediatric observation care.
- .Observation medicine: the healthcare system's tincture of time. In: Graff LG, ed.Principles of Observation Medicine.Dallas, TX:American College of Emergency Physicians;2010. Available at: http://www.acep.org/content.aspx?id=46142. Accessed February 18,year="2011"2011.
- .Hospital ‘observation’ status a matter of billing.The Columbus Dispatch. February 14,2011.
- .Hospital payments downgraded.Philadelphia Business Journal. February 18,2011.
- .Medicare rules give full hospital benefits only to those with ‘inpatient’ status.The Washington Post. September 7,2010.
- .Hospitals caught between a rock and a hard place over observation.Health Leaders Media. September 15,2010.
- .AHA: observation status fears on the rise.Health Leaders Media. October 29,2010.
- .Put your hospital bill under a microscope.The New York Times. September 13,2010.
- Medicare Hospital Manual Section 455.Washington, DC:Department of Health and Human Services, Centers for Medicare and Medicaid Services;2001.
- ,,,,.The Observation Unit: An Operational Overview for the Hospitalist. Society of Hospital Medicine White Paper. May 21, 2009. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Publications/White Papers/White_Papers.htm. Accessed May 21,2009.
- ,,,,.Utilization and unexpected hospitalization rates of a pediatric emergency department 23‐hour observation unit.Pediatr Emerg Care.2008;24(9):589–594.
- ,,.The pediatric hybrid observation unit: an analysis of 6477 consecutive patient encounters.Pediatrics.2005;115(5):e535–e542.
- ,,,,.Pediatric observation units in the United States: a systematic review.J Hosp Med.2010;5(3):172–182.
- ,,.Pediatric emergency department directors' benchmarking survey: fiscal year 2001.Pediatr Emerg Care.2003;19(3):143–147.
- ,,,.Pediatric observation status beds on an inpatient unit: an integrated care model.Pediatr Emerg Care.2004;20(1):17–21.
- ,,,.Impact of a short stay unit on asthma patients admitted to a tertiary pediatric hospital.Qual Manag Health Care.1997;6(1):14–22.
- ,,,.A national survey of observation units in the United States.Am J Emerg Med.2003;21(7):529–533.
- ,,,.A survey of observation units in the United States.Am J Emerg Med.1989;7(6):576–580.
- ,,.When the patient requires observation not hospitalization.J Nurs Admin.1988;18(10):20–23.
- ,,.A reduction in hospitalization, length of stay, and hospital charges for croup with the institution of a pediatric observation unit.Am J Emerg Med.2006;24(7):818–821.
- ,,.Outpatient oral rehydration in the United States.Am J Dis Child.1986;140(3):211–215.
- ,,,,.Pediatric closed head injuries treated in an observation unit.Pediatr Emerg Care.2005;21(10):639–644.
- ,,,.Use of pediatric observation unit for treatment of children with dehydration caused by gastroenteritis.Pediatr Emerg Care.2006;22(1):1–6.
- ,,,.Children with asthma admitted to a pediatric observation unit.Pediatr Emerg Care.2005;21(10):645–649.
- ,,,.Redefining the community pediatric hospitalist: the combined pediatric ED/inpatient unit.Pediatr Emerg Care.2007;23(1):33–37.
- ,,,.Program description: a hospitalist‐run, medical short‐stay unit in a teaching hospital.Can Med Assoc J.2000;163(11):1477–1480.
- ,.Impact of an observation unit and an emergency department‐admitted patient transfer mandate in decreasing overcrowding in a pediatric emergency department: a discrete event simulation exercise.Pediatr Emerg Care.2009;25(3):160–163.
- ,,, et al.Children's hospitals do not acutely respond to high occupancy.Pediatrics.125(5):974–981.
- ,,,,,.Trends in high‐turnover stays among children hospitalized in the United States, 1993‐2003.Pediatrics.2009;123(3):996–1002.
- .Observation medicine: the healthcare system's tincture of time. In: Graff LG, ed.Principles of Observation Medicine.Dallas, TX:American College of Emergency Physicians;2010. Available at: http://www.acep.org/content.aspx?id=46142. Accessed February 18,year="2011"2011.
- .Hospital ‘observation’ status a matter of billing.The Columbus Dispatch. February 14,2011.
- .Hospital payments downgraded.Philadelphia Business Journal. February 18,2011.
- .Medicare rules give full hospital benefits only to those with ‘inpatient’ status.The Washington Post. September 7,2010.
- .Hospitals caught between a rock and a hard place over observation.Health Leaders Media. September 15,2010.
- .AHA: observation status fears on the rise.Health Leaders Media. October 29,2010.
- .Put your hospital bill under a microscope.The New York Times. September 13,2010.
- Medicare Hospital Manual Section 455.Washington, DC:Department of Health and Human Services, Centers for Medicare and Medicaid Services;2001.
- ,,,,.The Observation Unit: An Operational Overview for the Hospitalist. Society of Hospital Medicine White Paper. May 21, 2009. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Publications/White Papers/White_Papers.htm. Accessed May 21,2009.
- ,,,,.Utilization and unexpected hospitalization rates of a pediatric emergency department 23‐hour observation unit.Pediatr Emerg Care.2008;24(9):589–594.
- ,,.The pediatric hybrid observation unit: an analysis of 6477 consecutive patient encounters.Pediatrics.2005;115(5):e535–e542.
- ,,,,.Pediatric observation units in the United States: a systematic review.J Hosp Med.2010;5(3):172–182.
- ,,.Pediatric emergency department directors' benchmarking survey: fiscal year 2001.Pediatr Emerg Care.2003;19(3):143–147.
- ,,,.Pediatric observation status beds on an inpatient unit: an integrated care model.Pediatr Emerg Care.2004;20(1):17–21.
- ,,,.Impact of a short stay unit on asthma patients admitted to a tertiary pediatric hospital.Qual Manag Health Care.1997;6(1):14–22.
- ,,,.A national survey of observation units in the United States.Am J Emerg Med.2003;21(7):529–533.
- ,,,.A survey of observation units in the United States.Am J Emerg Med.1989;7(6):576–580.
- ,,.When the patient requires observation not hospitalization.J Nurs Admin.1988;18(10):20–23.
- ,,.A reduction in hospitalization, length of stay, and hospital charges for croup with the institution of a pediatric observation unit.Am J Emerg Med.2006;24(7):818–821.
- ,,.Outpatient oral rehydration in the United States.Am J Dis Child.1986;140(3):211–215.
- ,,,,.Pediatric closed head injuries treated in an observation unit.Pediatr Emerg Care.2005;21(10):639–644.
- ,,,.Use of pediatric observation unit for treatment of children with dehydration caused by gastroenteritis.Pediatr Emerg Care.2006;22(1):1–6.
- ,,,.Children with asthma admitted to a pediatric observation unit.Pediatr Emerg Care.2005;21(10):645–649.
- ,,,.Redefining the community pediatric hospitalist: the combined pediatric ED/inpatient unit.Pediatr Emerg Care.2007;23(1):33–37.
- ,,,.Program description: a hospitalist‐run, medical short‐stay unit in a teaching hospital.Can Med Assoc J.2000;163(11):1477–1480.
- ,.Impact of an observation unit and an emergency department‐admitted patient transfer mandate in decreasing overcrowding in a pediatric emergency department: a discrete event simulation exercise.Pediatr Emerg Care.2009;25(3):160–163.
- ,,, et al.Children's hospitals do not acutely respond to high occupancy.Pediatrics.125(5):974–981.
- ,,,,,.Trends in high‐turnover stays among children hospitalized in the United States, 1993‐2003.Pediatrics.2009;123(3):996–1002.
Copyright © 2011 Society of Hospital Medicine
Addressing Inpatient Crowding
High levels of hospital occupancy are associated with compromises to quality of care and access (often referred to as crowding), 18 while low occupancy may be inefficient and also impact quality. 9, 10 Despite this, hospitals typically have uneven occupancy. Although some demand for services is driven by factors beyond the control of a hospital (eg, seasonal variation in viral illness), approximately 15%30% of admissions to children's hospitals are scheduled from days to months in advance, with usual arrivals on weekdays. 1114 For example, of the 3.4 million elective admissions in the 2006 Healthcare Cost and Utilization Project Kids Inpatient Database (HCUP KID), only 13% were admitted on weekends. 14 Combined with short length of stay (LOS) for such patients, this leads to higher midweek and lower weekend occupancy. 12
Hospitals respond to crowding in a number of ways, but often focus on reducing LOS to make room for new patients. 11, 15, 16 For hospitals that are relatively efficient in terms of LOS, efforts to reduce it may not increase functional capacity adequately. In children's hospitals, median lengths of stay are 2 to 3 days, and one‐third of hospitalizations are 1 day or less. 17 Thus, even 10%20% reductions in LOS trims hours, not days, from typical stays. Practical barriers (eg, reluctance to discharge in the middle of the night, or family preferences and work schedules) and undesired outcomes (eg, increased hospital re‐visits) are additional pitfalls encountered by relying on throughput enhancement alone.
Managing scheduled admissions through smoothing is an alternative strategy to reduce variability and high occupancy. 6, 12, 1820 The concept is to proactively control the entry of patients, when possible, to achieve more even levels of occupancy, instead of the peaks and troughs commonly encountered. Nonetheless, it is not a widely used approach. 18, 20, 21 We hypothesized that children's hospitals had substantial unused capacity that could be used to smooth occupancy, which would reduce weekday crowding. While it is obvious that smoothing will reduce peaks to average levels (and also raise troughs), we sought to quantify just how large this difference wasand thereby quantify the potential of smoothing to reduce inpatient crowding (or, conversely, expose more patients to high levels of occupancy). Is there enough variation to justify smoothing, and, if a hospital does smooth, what is the expected result? If the number of patients removed from exposure to high occupancy is not substantial, other means to address inpatient crowding might be of more value. Our aims were to quantify the difference in weekday versus weekend occupancy, report on mathematical feasibility of such an approach, and determine the difference in number of patients exposed to various levels of high occupancy.
Methods
Data Source
This retrospective study was conducted with resource‐utilization data from 39 freestanding, tertiary‐care children's hospitals in the Pediatric Health Information System (PHIS). Participating hospitals are located in noncompeting markets of 23 states, plus the District of Columbia, and affiliated with the Child Health Corporation of America (CHCA, Shawnee Mission, KS). They account for 80% of freestanding, and 20% of all general, tertiary‐care children's hospitals. Data quality and reliability are assured through joint ongoing, systematic monitoring. The Children's Hospital of Philadelphia Committees for the Protection of Human Subjects approved the protocol with a waiver of informed consent.
Patients
Patients admitted January 1December 31, 2007 were eligible for inclusion. Due to variation in the presence of birthing, neonatal intensive care, and behavioral health units across hospitals, these beds and associated patients were excluded. Inpatients enter hospitals either as scheduled (often referred to as elective) or unscheduled (emergent or urgent) admissions. Because PHIS does not include these data, KID was used to standardize the PHIS data for proportion of scheduled admissions. 22 (KID is a healthcare database of 23 million pediatric inpatient discharges developed through federalstateindustry partnership, and sponsored by the Agency for Healthcare Research and Quality [AHRQ].) Each encounter in KID includes a principal International Classification of Diseases, 9th revision (ICD‐9) discharge diagnosis code, and is designated by the hospital as elective (ranging from chemotherapy to tonsillectomy) or not elective. Because admissions, rather than diagnoses, are scheduled, a proportion of patients with each primary diagnosis in KID are scheduled (eg, 28% of patients with a primary diagnosis of esophageal reflux). Proportions in KID were matched to principal diagnoses in PHIS.
Definitions
The census was the number of patients registered as inpatients (including those physically in the emergency department [ED] from time of ED arrival)whether observation or inpatient statusat midnight, the conclusion of the day. Hospital capacity was set using CHCA data (and confirmed by each hospital's administrative personnel) as the number of licensed in‐service beds available for patients in 2007; we assumed beds were staffed and capacity fixed for the year. Occupancy was calculated by dividing census by capacity. Maximum occupancy in a week referred to the highest occupancy level achieved in a seven‐day period (MondaySunday). We analyzed a set of thresholds for high‐occupancy (85%, 90%, 95%, and 100%), because there is no consistent definition for when hospitals are at high occupancy or when crowding occurs, though crowding has been described as starting at 85% occupancy. 2325
Analysis
The hospital was the unit of analysis. We report hospital characteristics, including capacity, number of discharges, and census region, and annual standardized length of stay ratio (SLOSR) as observed‐to‐expected LOS.
Smoothing Technique
A retrospective smoothing algorithm set each hospital's daily occupancy during a week to that hospital's mean occupancy for the week; effectively spreading the week's volume of patients evenly across the days of the week. While inter‐week and inter‐month smoothing were considered, intra‐week smoothing was deemed more practical for the largest number of patients, as it would not mean delaying care by more than one week. In the case of a planned treatment course (eg, chemotherapy), only intra‐week smoothing would maintain the necessary scheduled intervals of treatment.
Mathematical Feasibility
To approximate the number of patient admissions that would require different scheduling during a particular week to achieve smoothed weekly occupancy, we determined the total number of patient‐days in the week that required different scheduling and divided by the average LOS for the week. We then divided the number of admissions‐to‐move by total weekly admissions to compute the percentage at each hospital across 52 weeks of the year.
Measuring the Impact of Smoothing
We focused on the frequency and severity of high occupancy and the number of patients exposed to it. This framework led to 4 measures that assess the opportunity and effect of smoothing:
Difference in hospital weekdayweekend occupancy: Equal to 12‐month median of difference between mean weekday occupancy and mean weekend occupancy for each hospital‐week.
Difference in hospital maximummean occupancy: Equal to median of difference between maximum one‐day occupancy and weekly mean (smoothed) occupancy for each hospital‐week. A regression line was derived from the data for the 39 hospitals to report expected reduction in peak occupancy based on the magnitude of the difference between weekday and weekend occupancy.
Difference in number of hospitals exposed to above‐threshold occupancy: Equal to difference, pre‐ and post‐smoothing, in number of hospitals facing high‐occupancy conditions on an average of at least one weekday midnight per week during the year at different occupancy thresholds.
Difference in number of patients exposed to above‐threshold occupancy: Equal to difference, pre‐ and post‐smoothing, in number of patients exposed to hospital midnight occupancy at the thresholds. We utilized patient‐days for the calculation to avoid double‐counting, and divided this by average LOS, in order to determine the number of patients who would no longer be exposed to over‐threshold occupancy after smoothing, while also adjusting for patients newly exposed to over‐threshold occupancy levels.
All analyses were performed separately for each hospital for the entire year and then for winter (DecemberMarch), the period during which most crowding occurred. Analyses were performed using SAS (version 9.2, SAS Institute, Inc, Cary, NC); P values <0.05 were considered statistically significant.
Results
The characteristics of the 39 hospitals are provided in Table 1. Based on standardization with KID, 23.6% of PHIS admissions were scheduled (range: 18.1%35.8%) or a median of 81.5 scheduled admissions per week per hospital; 26.6% of weekday admissions were scheduled versus 16.1% for weekends. Overall, 12.4% of scheduled admissions entered on weekends. For all patients, median LOS was three days (interquartile range [IQR]: twofive days), but median LOS for scheduled admissions was two days (IQR: onefour days). The median LOS and IQR were the same by day of admission for all days of the week. Most hospitals had an overall SLOSR close to one (median: 0.9, IQR: 0.91.1). Overall, hospital mean midnight occupancy ranged from 70.9% to 108.1% on weekdays and 65.7% to 94.9% on weekends. Uniformly, weekday occupancy exceeded weekend occupancy, with a median difference of 8.2% points (IQR: 7.2%9.5% points). There was a wide range of median hospital weekdayweekend occupancy differences across hospitals (Figure 1). The overall difference was less in winter (median difference: 7.7% points; IQR: 6.3%8.8% points) than in summer (median difference: 8.6% points; IQR: 7.4%9.8% points (Wilcoxon Sign Rank test, P < 0.001). Thirty‐five hospitals (89.7%) exceeded the 85% occupancy threshold and 29 (74.4%) exceeded the 95% occupancy threshold on at least 20% of weekdays (Table 2). Across all the hospitals, the median difference in weekly maximum and weekly mean occupancy was 6.6% points (IQR: 6.2%7.4% points) (Figure 2).
| Characteristics | No. (%) |
|---|---|
| |
| Licensed in‐service beds | n = 39 hospitals |
| <200 beds | 6 (15.4) |
| 200249 beds | 10 (25.6) |
| 250300 beds | 14 (35.9) |
| >300 beds | 9 (23.1) |
| No. of discharges | |
| <10,000 | 5 (12.8) |
| 10,00013,999 | 14 (35.9) |
| 14,00017,999 | 11 (28.2) |
| >18,000 | 9 (23.1) |
| Census region | |
| West | 9 (23.1) |
| Midwest | 11 (28.2) |
| Northeast | 6 (15.4) |
| South | 13 (33.3) |
| Admissions | n = 590,352 admissions |
| Medical scheduled admissions* | 79,683 |
| Surgical scheduled admissions* | 59,640 |
| Total scheduled admissions* (% of all admissions) | 139,323 (23.6) |
| Weekend medical scheduled admissions* (% of all medical scheduled admissions) | 13,546 (17.0) |
| Weekend surgical scheduled admissions* (% of all surgical scheduled admissions) | 3,757 (6.3) |
| Weekend total scheduled admissions* (% of total scheduled admissions) | 17,276 (12.4) |
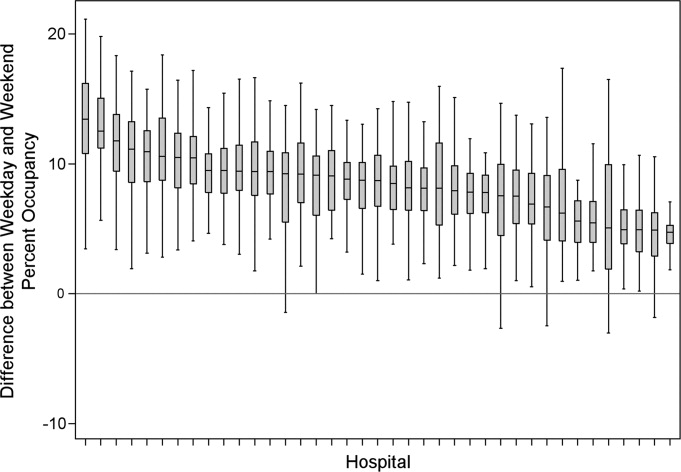
| Entire Year | >85% | Occupancy Threshold | >95% | >100% |
|---|---|---|---|---|
| >90% | ||||
| ||||
| No. of hospitals (n = 39) with mean weekday occupancy above threshold | ||||
| Before smoothing (current state) | 33 | 25 | 14 | 6 |
| After smoothing | 32 | 22 | 10 | 1 |
| No. of hospitals (n = 39) above threshold 20% of weekdays | ||||
| Before smoothing (current state) | 35 | 34 | 29 | 14 |
| After smoothing | 35 | 32 | 21 | 9 |
| Median (IQR) no. of patient‐days per hospital not exposed to occupancy above threshold by smoothing | 3,071 | 281 | 3236 | 3281 |
| (5,552, 919) | (5,288, 3,103) | (0, 7,083) | (962, 8,517) | |
| Median (IQR) no. of patients per hospital not exposed to occupancy above threshold by smoothing | 596 | 50 | 630 | 804 |
| (1,190, 226) | (916, 752) | (0, 1,492) | (231, 2,195) | |
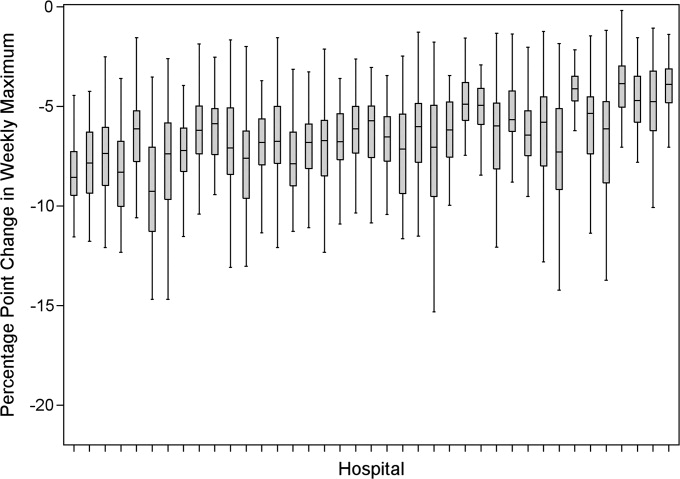
Smoothing reduced the number of hospitals at each occupancy threshold, except 85% (Table 2). As a linear relationship, the reduction in weekday peak occupancy (y) based on a hospital's median difference in weekly maximum and weekly mean occupancy (x) was y = 2.69 + 0.48x. Thus, a hospital with a 10% point difference between weekday and weekend occupancy could reduce weekday peak by 7.5% points.
Smoothing increased the number of patients exposed to the lower thresholds (85% and 90%), but decreased the number of patients exposed to >95% occupancy (Table 2). For example, smoothing at the 95% threshold resulted in 630 fewer patients per hospital exposed to that threshold. If all 39 hospitals had within‐week smoothing, a net of 39,607 patients would have been protected from exposure to >95% occupancy and a net of 50,079 patients from 100% occupancy.
To demonstrate the varied effects of smoothing, Table 3 and Figure 3 present representative categories of response to smoothing depending on pre‐smoothing patterns. While not all hospitals decreased occupancy to below thresholds after smoothing (Types B and D), the overall occupancy was reduced and fewer patients were exposed to extreme levels of high occupancy (eg, >100%).
| Category | Before Smoothing Hospital Description | After Smoothing Hospital Description | No. of Hospitals at 85% Threshold (n = 39) | No. of Hospitals at 95% Threshold (n = 39) |
|---|---|---|---|---|
| ||||
| Type A | Weekdays above threshold | All days below threshold, resulting in net decrease in patients exposed to occupancies above threshold | 3 | 1 |
| Weekends below threshold | ||||
| Type B | Weekdays above threshold | All days above threshold, resulting in net increase in patients exposed to occupancies above threshold | 12 | 18 |
| Weekends below threshold | ||||
| Type C | All days of week below threshold | All days of week below threshold | 6 | 19 |
| Type D | All days of week above threshold | All days of week above threshold, resulting in net decrease in patients exposed to extreme high occupancy | 18 | 1 |
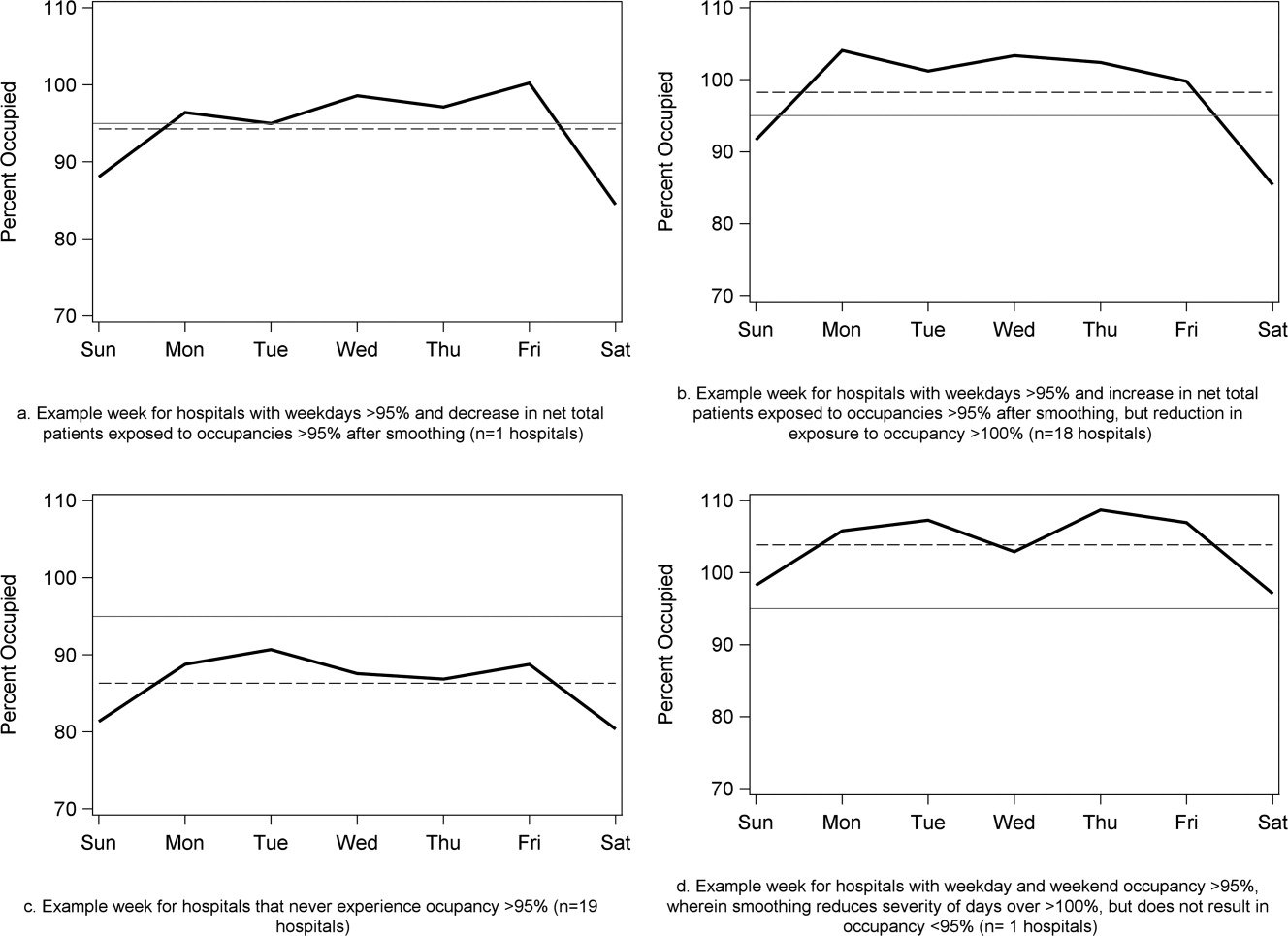
To achieve within‐week smoothing, a median of 7.4 patient‐admissions per week (range: 2.314.4) would have to be scheduled on a different day of the week. This equates to a median of 2.6% (IQR: 2.25%, 2.99%; range: 0.02%9.2%) of all admissionsor 9% of a typical hospital‐week's scheduled admissions.
Discussion
This analysis of 39 children's hospitals found high levels of occupancy and weekend occupancy lower than weekday occupancy (median difference: 8.2% points). Only 12.4% of scheduled admissions entered on weekends. Thus, weekend capacity is available to offset high weekday occupancy. Hospitals at the higher end of the occupancy thresholds (95%, 100%) would reduce the number of days operating at very high occupancy and the number of patients exposed to such levels by smoothing. This change is mathematically feasible, as a median of 7.4 patients would have to be proactively scheduled differently each week, just under one‐tenth of scheduled admissions. Since LOS by day of admission was the same (median: two days), the opportunity to affect occupancy by shifting patients should be relatively similar for all days of the week. In addition, these admissions were short, conferring greater flexibility. Implementing smoothing over the course of the week does not necessarily require admitting patients on weekends. For example, Monday admissions with an anticipated three‐day LOS could enter on Friday with anticipated discharge on Monday to alleviate midweek crowding and take advantage of unoccupied weekend beds. 26
At the highest levels of occupancy, smoothing reduces the frequency of reaching these maximum levels, but can have the effect of actually exposing more patient‐days to a higher occupancy. For example, for nine hospitals in our analysis with >20% of days over 100%, smoothing decreased days over 100%, but exposed weekend patients to higher levels of occupancy (Figure 3). Since most admissions are short and most scheduled admissions currently occur on weekdays, the number of individual patients (not patient‐days) newly exposed to such high occupancy may not increase much after smoothing at these facilities. Regardless, hospitals with such a pattern may not be able to rely solely on smoothing to avoid weekday crowding, and, if they are operating efficiently in terms of SLOSR, might be justified in building more capacity.
Consistent with our findings, the Institute for Healthcare Improvement, the Institute for Healthcare Optimization, and the American Hospital Association Quality Center stress that addressing artificial variability of scheduled admissions is a critical first step to improving patient flow and quality of care while reducing costs. 18, 21, 27 Our study suggests that small numbers of patients need to be proactively scheduled differently to decrease midweek peak occupancy, so only a small proportion of families would need to find this desirable to make it attractive for hospitals and patients. This type of proactive smoothing decreases peak occupancy on weekdays, reducing the safety risks associated with high occupancy, improving acute access for emergent patients, shortening wait‐times and loss of scheduled patients to another facility, and increasing procedure volume (3%74% in one study). 28 Smoothing may also increase quality and safety on weekends, as emergent patients admitted on weekends experience more delays in necessary treatment and have worse outcomes. 2932 In addition, increasing scheduled admissions to span weekends may appeal to some families wishing to avoid absence from work to be with their hospitalized child, to parents concerned about school performanceand may also appeal to staff members seeking flexible schedules. Increasing weekend hospital capacity is safe, feasible, and economical, even when considering the increased wages for weekend work. 33, 34 Finally, smoothing over the whole week allows fixed costs (eg, surgical suites, imaging equipment) to be allocated over 7 days rather than 5, and allows for better matching of revenue to the fixed expenses.
Rather than a prescriptive approach, our work suggests hospitals need to identify only a small number of patients to proactively shift, providing them opportunities to adapt the approach to local circumstances. The particular patients to move around may also depend on the costs and benefits of services (eg, radiologic, laboratory, operative) and the hospital's existing patterns of staffing. A number of hospitals that have engaged in similar work have achieved sustainable results, such as Seattle Children's Hospital, Boston Medical Center, St. John's Regional Health Center, and New York University Langone Medical Center. 19, 26, 3537 In these cases, proactive smoothing took advantage of unused capacity and decreased crowding on days that had been traditionally very full. Hospitals that rarely or never have high‐occupancy days, and that do not expect growth in volume, may not need to employ smoothing, whereas others that have crowding issues primarily in the winter may wish to implement smoothing techniques seasonally.
Aside from attempting to reduce high‐occupancy through modification of admission patterns, other proactive approaches include optimizing staffing and processes around care, improving efficiency of care, and building additional beds. 16, 25, 38, 39 However, the expense of construction and the scarcity of capital often preclude this last option. Among children's hospitals, with SLOSR close to one, implementing strategies to reduce the LOS during periods of high occupancy may not result in meaningful reductions in LOS, as such approaches would only decrease the typical child's hospitalization by hours, not days. In addition to proactive strategies, hospitals also rely on reactive approaches, such as ED boarding, placing patients in hallways on units, diverting ambulances or transfers, or canceling scheduled admissions at the last moment, to decrease crowding. 16, 39, 40
This study has several limitations. First, use of administrative data precluded modeling all responses. For example, some hospitals may be better able to accommodate fluctuations in census or high occupancy without compromising quality or access. Second, we only considered intra‐week smoothing, but hospitals may benefit from smoothing over longer periods of time, especially since children's hospitals are busier in winter months, but incoming scheduled volume is often not reduced. 11 Hospitals with large occupancy variations across months may want to consider broadening the time horizon for smoothing, and weigh the costs and benefits over that period of time, including parental and clinician concerns and preferences for not delaying treatment. At the individual hospital level, discrete‐event simulation would likely be useful to consider the trade‐offs of smoothing to different levels and over different periods of time. Third, we assumed a fixed number of beds for the year, an approach that may not accurately reflect actual available beds on specific days. This limitation was minimized by counting all beds for each hospital as available for all the days of the year, so that hospitals with a high census when all available beds are included would have an even higher percent occupancy if some of those beds were not actually open. In a related way, then, we also do not consider how staffing may need to be altered or augmented to care for additional patients on certain days. Fourth, midnight census, the only universally available measure, was used to determine occupancy rather than peak census. Midnight census provides a standard snapshot, but is lower than mid‐day peak census. 41 In order to account for these limitations, we considered several different thresholds of high occupancy. Fifth, we smoothed at the hospital level, but differential effects may exist at the unit level. Sixth, to determine proportion of scheduled admissions, we used HCUP KID proportions on PHIS admissions. Overall, this approach likely overestimated scheduled medical admissions on weekends, thus biasing our result towards the null hypothesis. Finally, only freestanding children's hospitals were included in this study. While this may limit generalizability, the general concept of smoothing occupancy should apply in any setting with substantial and consistent variation.
In summary, our study revealed that children's hospitals often face high midweek occupancy, but also have substantial unused weekend capacity. Hospitals facing challenges with high weekday occupancy could proactively use a smoothing approach to decrease the frequency and severity of high occupancy. Further qualitative evaluation is also warranted around child, family, and staff preferences concerning scheduled admissions, school, and work.
- , , , . A comparison of in‐hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Medical Care. 2010;48(3):224–232.
- , , , et al. Hospital workload and adverse events. Med Care. 2007;45(5):448–455.
- , , , , . Impact of admission‐day crowding on the length of stay of pediatric hospitalizations. Pediatrics. 2008;121(4):e718–e730.
- , , , . The effect of hospital bed occupancy on throughput in the pediatric emergency department. Ann Emerg Med. 2009;53(6):767–776.
- . The tipping point: the relationship between volume and patient harm. Am J Med Qual. 2008;23(5):336–341.
- , , , , , . Managing unnecessary variability in patient demand to reduce nursing stress and improve patient safety. Jt Comm J Qual Patient Saf. 2005;31(6):330–338.
- Hospital‐Based Emergency Care: At the Breaking Point. Washington, DC: Institute of Medicine Committee on the Future of Emergency Care in the United States Health System; 2006.
- , , , , . The effect of hospital occupancy on emergency department length of stay and patient disposition. Acad Emerg Med. 2003;10(2):127–133.
- . Interpreting the Volume‐Outcome Relationship in the Context of Health Care Quality: Workshop Summary. Washington, DC: National Academies Press; 2000.
- , , , , . Has recognition of the relationship between mortality rates and hospital volume for major cancer surgery in California made a difference? A follow‐up analysis of another decade. Ann Surg. 2009;250(3):472–483.
- , , , et al. Children's hospitals do not acutely respond to high occupancy. Pediatrics. 2010;125:974–981.
- , , , , , . Scheduled admissions and high occupancy at a children's hospital. J Hosp Med. 2011;6(2):81–87.
- , , . Characteristics of weekday and weekend hospital admissions. HCUP Statistical Brief. 2010;87.
- Agency for Healthcare Research and Quality. HCUP databases, Healthcare Cost and Utilization Project (HCUP); 2008. Available at: http://www.hcup‐us.ahrq.gov/kidoverview.jsp. Accessed July 15, 2009.
- , et al. Managing capacity to reduce emergency department overcrowding and ambulance diversions. J Qual Patient Saf. 2006;32(5):239–245.
- Institute for Healthcare Improvement. Flow initiatives; 2008. Available at: http://www.ihi.org/IHI/Topics/Flow. Accessed February 20, 2008.
- , , , , , . Trends in high‐turnover stays among children hospitalized in the United States, 1993–2003. Pediatrics. 2009;123(3):996–1002.
- Institute for Healthcare Improvement. Smoothing elective surgical admissions. Available at: http://www.ihi.org/IHI/Topics/Flow/PatientFlow/EmergingContent/SmoothingElectiveSurgicalAdmissions.htm. Accessed October 24, 2008.
- Boston hospital sees big impact from smoothing elective schedule. OR Manager. 2004;20:12.
- . Managing Variability in Patient Flow Is the Key to Improving Access to Care, Nursing Staffing, Quality of Care, and Reducing Its Cost. Paper presented at Institute of Medicine, Washington, DC; June 24, 2004.
- American Hospital Association Quality Center. Available at: http://www.ahaqualitycenter.org/ahaqualitycenter/. Accessed October 14, 2008.
- Healthcare Cost and Utilization Project (HCUP). Kids' Inpatient Database (KID); July 2008. Available at: http://www.hcup‐us.ahrq.gov/kidoverview.jsp. Accessed September 10, 2008.
- , , . Using a queuing model to help plan bed allocation in a department of geriatric medicine. Health Care Manag Sci. 2002;5(4):307–313.
- . How many hospital beds? Inquiry. 2002;39(4):400–412.
- . Institute for Healthcare Improvement. Patient flow comments. Available at: http://www.ihi.org/IHI/Topics/Flow. Accessed September 10, 2008.
- . Factory efficiency comes to the hospital. New York Times. July 9, 2010.
- Institute for Healthcare Improvement. Re‐engineering the operating room. Available at: http://www.ihi.org/IHI/Programs/ConferencesAndSeminars/ReengineeringtheOperatingRoomSept08.htm. Accessed November 8, 2008.
- , . Enhanced weekend service: an affordable means to increased hospital procedure volume. CMAJ. 2005;172(4):503–504.
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345:663–668.
- , , , , , . Weekend versus weekday admission and mortality from myocardial infarction. N Engl J Med. 2007;356:1099–1109.
- , . Waiting for urgent procedures on the weekend among emergently hospitalized patients. Am J Med. 2004;117:175–181.
- . Do hospitals provide lower quality care on weekends? Health Serv Res. 2007;42:1589–1612.
- . Hospital saves by working weekends. Mod Healthc. 1996;26:82–99.
- , , , , . Weekend and holiday exercise testing in patients with chest pain. J Gen Intern Med. 1999;14:10–14.
- . Boston Medical Center Case Study: Institute of Healthcare Optimization; 2006. Available at: http://www.ihoptimize.org/8f16e142‐eeaa‐4898–9e62–660218f19ffb/download.htm. Accessed October 3, 2010.
- , , , . The impact of IMPACT on St John's Regional Health Center. Mo Med. 2003;100:590–592.
- NYU Langone Medical Center Extends Access to Non‐Emergent Care as Part of Commitment to Patient‐Centered Care (June 23, 2010). Available at: http://communications.med.nyu.edu/news/2010/nyu‐langone‐medical‐center‐extends‐access‐non‐emergent‐care‐part‐commitment‐patient‐center. Accessed October 3, 2010.
- Carondelet St. Mary's Hospital. A pragmatic approach to improving patient efficiency throughput. Improvement Report 2005. Available at: http://www.ihi.org/IHI/Topics/Flow/PatientFlow/ImprovementStories/APragmaticApproachtoImprovingPatientEfficiencyThroughput.htm. Accessed October 3, 2010.
- AHA Solutions. Patient Flow Challenges Assessment 2009. Chicago, IL; 2009.
- , , , , , . A conceptual model of emergency department crowding. Ann Emerg Med. 2003;42(2):173–180.
- . Annual bed statistics give a misleading picture of hospital surge capacity. Ann Emerg Med. 2006;48(4):384–388.
High levels of hospital occupancy are associated with compromises to quality of care and access (often referred to as crowding), 18 while low occupancy may be inefficient and also impact quality. 9, 10 Despite this, hospitals typically have uneven occupancy. Although some demand for services is driven by factors beyond the control of a hospital (eg, seasonal variation in viral illness), approximately 15%30% of admissions to children's hospitals are scheduled from days to months in advance, with usual arrivals on weekdays. 1114 For example, of the 3.4 million elective admissions in the 2006 Healthcare Cost and Utilization Project Kids Inpatient Database (HCUP KID), only 13% were admitted on weekends. 14 Combined with short length of stay (LOS) for such patients, this leads to higher midweek and lower weekend occupancy. 12
Hospitals respond to crowding in a number of ways, but often focus on reducing LOS to make room for new patients. 11, 15, 16 For hospitals that are relatively efficient in terms of LOS, efforts to reduce it may not increase functional capacity adequately. In children's hospitals, median lengths of stay are 2 to 3 days, and one‐third of hospitalizations are 1 day or less. 17 Thus, even 10%20% reductions in LOS trims hours, not days, from typical stays. Practical barriers (eg, reluctance to discharge in the middle of the night, or family preferences and work schedules) and undesired outcomes (eg, increased hospital re‐visits) are additional pitfalls encountered by relying on throughput enhancement alone.
Managing scheduled admissions through smoothing is an alternative strategy to reduce variability and high occupancy. 6, 12, 1820 The concept is to proactively control the entry of patients, when possible, to achieve more even levels of occupancy, instead of the peaks and troughs commonly encountered. Nonetheless, it is not a widely used approach. 18, 20, 21 We hypothesized that children's hospitals had substantial unused capacity that could be used to smooth occupancy, which would reduce weekday crowding. While it is obvious that smoothing will reduce peaks to average levels (and also raise troughs), we sought to quantify just how large this difference wasand thereby quantify the potential of smoothing to reduce inpatient crowding (or, conversely, expose more patients to high levels of occupancy). Is there enough variation to justify smoothing, and, if a hospital does smooth, what is the expected result? If the number of patients removed from exposure to high occupancy is not substantial, other means to address inpatient crowding might be of more value. Our aims were to quantify the difference in weekday versus weekend occupancy, report on mathematical feasibility of such an approach, and determine the difference in number of patients exposed to various levels of high occupancy.
Methods
Data Source
This retrospective study was conducted with resource‐utilization data from 39 freestanding, tertiary‐care children's hospitals in the Pediatric Health Information System (PHIS). Participating hospitals are located in noncompeting markets of 23 states, plus the District of Columbia, and affiliated with the Child Health Corporation of America (CHCA, Shawnee Mission, KS). They account for 80% of freestanding, and 20% of all general, tertiary‐care children's hospitals. Data quality and reliability are assured through joint ongoing, systematic monitoring. The Children's Hospital of Philadelphia Committees for the Protection of Human Subjects approved the protocol with a waiver of informed consent.
Patients
Patients admitted January 1December 31, 2007 were eligible for inclusion. Due to variation in the presence of birthing, neonatal intensive care, and behavioral health units across hospitals, these beds and associated patients were excluded. Inpatients enter hospitals either as scheduled (often referred to as elective) or unscheduled (emergent or urgent) admissions. Because PHIS does not include these data, KID was used to standardize the PHIS data for proportion of scheduled admissions. 22 (KID is a healthcare database of 23 million pediatric inpatient discharges developed through federalstateindustry partnership, and sponsored by the Agency for Healthcare Research and Quality [AHRQ].) Each encounter in KID includes a principal International Classification of Diseases, 9th revision (ICD‐9) discharge diagnosis code, and is designated by the hospital as elective (ranging from chemotherapy to tonsillectomy) or not elective. Because admissions, rather than diagnoses, are scheduled, a proportion of patients with each primary diagnosis in KID are scheduled (eg, 28% of patients with a primary diagnosis of esophageal reflux). Proportions in KID were matched to principal diagnoses in PHIS.
Definitions
The census was the number of patients registered as inpatients (including those physically in the emergency department [ED] from time of ED arrival)whether observation or inpatient statusat midnight, the conclusion of the day. Hospital capacity was set using CHCA data (and confirmed by each hospital's administrative personnel) as the number of licensed in‐service beds available for patients in 2007; we assumed beds were staffed and capacity fixed for the year. Occupancy was calculated by dividing census by capacity. Maximum occupancy in a week referred to the highest occupancy level achieved in a seven‐day period (MondaySunday). We analyzed a set of thresholds for high‐occupancy (85%, 90%, 95%, and 100%), because there is no consistent definition for when hospitals are at high occupancy or when crowding occurs, though crowding has been described as starting at 85% occupancy. 2325
Analysis
The hospital was the unit of analysis. We report hospital characteristics, including capacity, number of discharges, and census region, and annual standardized length of stay ratio (SLOSR) as observed‐to‐expected LOS.
Smoothing Technique
A retrospective smoothing algorithm set each hospital's daily occupancy during a week to that hospital's mean occupancy for the week; effectively spreading the week's volume of patients evenly across the days of the week. While inter‐week and inter‐month smoothing were considered, intra‐week smoothing was deemed more practical for the largest number of patients, as it would not mean delaying care by more than one week. In the case of a planned treatment course (eg, chemotherapy), only intra‐week smoothing would maintain the necessary scheduled intervals of treatment.
Mathematical Feasibility
To approximate the number of patient admissions that would require different scheduling during a particular week to achieve smoothed weekly occupancy, we determined the total number of patient‐days in the week that required different scheduling and divided by the average LOS for the week. We then divided the number of admissions‐to‐move by total weekly admissions to compute the percentage at each hospital across 52 weeks of the year.
Measuring the Impact of Smoothing
We focused on the frequency and severity of high occupancy and the number of patients exposed to it. This framework led to 4 measures that assess the opportunity and effect of smoothing:
Difference in hospital weekdayweekend occupancy: Equal to 12‐month median of difference between mean weekday occupancy and mean weekend occupancy for each hospital‐week.
Difference in hospital maximummean occupancy: Equal to median of difference between maximum one‐day occupancy and weekly mean (smoothed) occupancy for each hospital‐week. A regression line was derived from the data for the 39 hospitals to report expected reduction in peak occupancy based on the magnitude of the difference between weekday and weekend occupancy.
Difference in number of hospitals exposed to above‐threshold occupancy: Equal to difference, pre‐ and post‐smoothing, in number of hospitals facing high‐occupancy conditions on an average of at least one weekday midnight per week during the year at different occupancy thresholds.
Difference in number of patients exposed to above‐threshold occupancy: Equal to difference, pre‐ and post‐smoothing, in number of patients exposed to hospital midnight occupancy at the thresholds. We utilized patient‐days for the calculation to avoid double‐counting, and divided this by average LOS, in order to determine the number of patients who would no longer be exposed to over‐threshold occupancy after smoothing, while also adjusting for patients newly exposed to over‐threshold occupancy levels.
All analyses were performed separately for each hospital for the entire year and then for winter (DecemberMarch), the period during which most crowding occurred. Analyses were performed using SAS (version 9.2, SAS Institute, Inc, Cary, NC); P values <0.05 were considered statistically significant.
Results
The characteristics of the 39 hospitals are provided in Table 1. Based on standardization with KID, 23.6% of PHIS admissions were scheduled (range: 18.1%35.8%) or a median of 81.5 scheduled admissions per week per hospital; 26.6% of weekday admissions were scheduled versus 16.1% for weekends. Overall, 12.4% of scheduled admissions entered on weekends. For all patients, median LOS was three days (interquartile range [IQR]: twofive days), but median LOS for scheduled admissions was two days (IQR: onefour days). The median LOS and IQR were the same by day of admission for all days of the week. Most hospitals had an overall SLOSR close to one (median: 0.9, IQR: 0.91.1). Overall, hospital mean midnight occupancy ranged from 70.9% to 108.1% on weekdays and 65.7% to 94.9% on weekends. Uniformly, weekday occupancy exceeded weekend occupancy, with a median difference of 8.2% points (IQR: 7.2%9.5% points). There was a wide range of median hospital weekdayweekend occupancy differences across hospitals (Figure 1). The overall difference was less in winter (median difference: 7.7% points; IQR: 6.3%8.8% points) than in summer (median difference: 8.6% points; IQR: 7.4%9.8% points (Wilcoxon Sign Rank test, P < 0.001). Thirty‐five hospitals (89.7%) exceeded the 85% occupancy threshold and 29 (74.4%) exceeded the 95% occupancy threshold on at least 20% of weekdays (Table 2). Across all the hospitals, the median difference in weekly maximum and weekly mean occupancy was 6.6% points (IQR: 6.2%7.4% points) (Figure 2).
| Characteristics | No. (%) |
|---|---|
| |
| Licensed in‐service beds | n = 39 hospitals |
| <200 beds | 6 (15.4) |
| 200249 beds | 10 (25.6) |
| 250300 beds | 14 (35.9) |
| >300 beds | 9 (23.1) |
| No. of discharges | |
| <10,000 | 5 (12.8) |
| 10,00013,999 | 14 (35.9) |
| 14,00017,999 | 11 (28.2) |
| >18,000 | 9 (23.1) |
| Census region | |
| West | 9 (23.1) |
| Midwest | 11 (28.2) |
| Northeast | 6 (15.4) |
| South | 13 (33.3) |
| Admissions | n = 590,352 admissions |
| Medical scheduled admissions* | 79,683 |
| Surgical scheduled admissions* | 59,640 |
| Total scheduled admissions* (% of all admissions) | 139,323 (23.6) |
| Weekend medical scheduled admissions* (% of all medical scheduled admissions) | 13,546 (17.0) |
| Weekend surgical scheduled admissions* (% of all surgical scheduled admissions) | 3,757 (6.3) |
| Weekend total scheduled admissions* (% of total scheduled admissions) | 17,276 (12.4) |

| Entire Year | >85% | Occupancy Threshold | >95% | >100% |
|---|---|---|---|---|
| >90% | ||||
| ||||
| No. of hospitals (n = 39) with mean weekday occupancy above threshold | ||||
| Before smoothing (current state) | 33 | 25 | 14 | 6 |
| After smoothing | 32 | 22 | 10 | 1 |
| No. of hospitals (n = 39) above threshold 20% of weekdays | ||||
| Before smoothing (current state) | 35 | 34 | 29 | 14 |
| After smoothing | 35 | 32 | 21 | 9 |
| Median (IQR) no. of patient‐days per hospital not exposed to occupancy above threshold by smoothing | 3,071 | 281 | 3236 | 3281 |
| (5,552, 919) | (5,288, 3,103) | (0, 7,083) | (962, 8,517) | |
| Median (IQR) no. of patients per hospital not exposed to occupancy above threshold by smoothing | 596 | 50 | 630 | 804 |
| (1,190, 226) | (916, 752) | (0, 1,492) | (231, 2,195) | |

Smoothing reduced the number of hospitals at each occupancy threshold, except 85% (Table 2). As a linear relationship, the reduction in weekday peak occupancy (y) based on a hospital's median difference in weekly maximum and weekly mean occupancy (x) was y = 2.69 + 0.48x. Thus, a hospital with a 10% point difference between weekday and weekend occupancy could reduce weekday peak by 7.5% points.
Smoothing increased the number of patients exposed to the lower thresholds (85% and 90%), but decreased the number of patients exposed to >95% occupancy (Table 2). For example, smoothing at the 95% threshold resulted in 630 fewer patients per hospital exposed to that threshold. If all 39 hospitals had within‐week smoothing, a net of 39,607 patients would have been protected from exposure to >95% occupancy and a net of 50,079 patients from 100% occupancy.
To demonstrate the varied effects of smoothing, Table 3 and Figure 3 present representative categories of response to smoothing depending on pre‐smoothing patterns. While not all hospitals decreased occupancy to below thresholds after smoothing (Types B and D), the overall occupancy was reduced and fewer patients were exposed to extreme levels of high occupancy (eg, >100%).
| Category | Before Smoothing Hospital Description | After Smoothing Hospital Description | No. of Hospitals at 85% Threshold (n = 39) | No. of Hospitals at 95% Threshold (n = 39) |
|---|---|---|---|---|
| ||||
| Type A | Weekdays above threshold | All days below threshold, resulting in net decrease in patients exposed to occupancies above threshold | 3 | 1 |
| Weekends below threshold | ||||
| Type B | Weekdays above threshold | All days above threshold, resulting in net increase in patients exposed to occupancies above threshold | 12 | 18 |
| Weekends below threshold | ||||
| Type C | All days of week below threshold | All days of week below threshold | 6 | 19 |
| Type D | All days of week above threshold | All days of week above threshold, resulting in net decrease in patients exposed to extreme high occupancy | 18 | 1 |

To achieve within‐week smoothing, a median of 7.4 patient‐admissions per week (range: 2.314.4) would have to be scheduled on a different day of the week. This equates to a median of 2.6% (IQR: 2.25%, 2.99%; range: 0.02%9.2%) of all admissionsor 9% of a typical hospital‐week's scheduled admissions.
Discussion
This analysis of 39 children's hospitals found high levels of occupancy and weekend occupancy lower than weekday occupancy (median difference: 8.2% points). Only 12.4% of scheduled admissions entered on weekends. Thus, weekend capacity is available to offset high weekday occupancy. Hospitals at the higher end of the occupancy thresholds (95%, 100%) would reduce the number of days operating at very high occupancy and the number of patients exposed to such levels by smoothing. This change is mathematically feasible, as a median of 7.4 patients would have to be proactively scheduled differently each week, just under one‐tenth of scheduled admissions. Since LOS by day of admission was the same (median: two days), the opportunity to affect occupancy by shifting patients should be relatively similar for all days of the week. In addition, these admissions were short, conferring greater flexibility. Implementing smoothing over the course of the week does not necessarily require admitting patients on weekends. For example, Monday admissions with an anticipated three‐day LOS could enter on Friday with anticipated discharge on Monday to alleviate midweek crowding and take advantage of unoccupied weekend beds. 26
At the highest levels of occupancy, smoothing reduces the frequency of reaching these maximum levels, but can have the effect of actually exposing more patient‐days to a higher occupancy. For example, for nine hospitals in our analysis with >20% of days over 100%, smoothing decreased days over 100%, but exposed weekend patients to higher levels of occupancy (Figure 3). Since most admissions are short and most scheduled admissions currently occur on weekdays, the number of individual patients (not patient‐days) newly exposed to such high occupancy may not increase much after smoothing at these facilities. Regardless, hospitals with such a pattern may not be able to rely solely on smoothing to avoid weekday crowding, and, if they are operating efficiently in terms of SLOSR, might be justified in building more capacity.
Consistent with our findings, the Institute for Healthcare Improvement, the Institute for Healthcare Optimization, and the American Hospital Association Quality Center stress that addressing artificial variability of scheduled admissions is a critical first step to improving patient flow and quality of care while reducing costs. 18, 21, 27 Our study suggests that small numbers of patients need to be proactively scheduled differently to decrease midweek peak occupancy, so only a small proportion of families would need to find this desirable to make it attractive for hospitals and patients. This type of proactive smoothing decreases peak occupancy on weekdays, reducing the safety risks associated with high occupancy, improving acute access for emergent patients, shortening wait‐times and loss of scheduled patients to another facility, and increasing procedure volume (3%74% in one study). 28 Smoothing may also increase quality and safety on weekends, as emergent patients admitted on weekends experience more delays in necessary treatment and have worse outcomes. 2932 In addition, increasing scheduled admissions to span weekends may appeal to some families wishing to avoid absence from work to be with their hospitalized child, to parents concerned about school performanceand may also appeal to staff members seeking flexible schedules. Increasing weekend hospital capacity is safe, feasible, and economical, even when considering the increased wages for weekend work. 33, 34 Finally, smoothing over the whole week allows fixed costs (eg, surgical suites, imaging equipment) to be allocated over 7 days rather than 5, and allows for better matching of revenue to the fixed expenses.
Rather than a prescriptive approach, our work suggests hospitals need to identify only a small number of patients to proactively shift, providing them opportunities to adapt the approach to local circumstances. The particular patients to move around may also depend on the costs and benefits of services (eg, radiologic, laboratory, operative) and the hospital's existing patterns of staffing. A number of hospitals that have engaged in similar work have achieved sustainable results, such as Seattle Children's Hospital, Boston Medical Center, St. John's Regional Health Center, and New York University Langone Medical Center. 19, 26, 3537 In these cases, proactive smoothing took advantage of unused capacity and decreased crowding on days that had been traditionally very full. Hospitals that rarely or never have high‐occupancy days, and that do not expect growth in volume, may not need to employ smoothing, whereas others that have crowding issues primarily in the winter may wish to implement smoothing techniques seasonally.
Aside from attempting to reduce high‐occupancy through modification of admission patterns, other proactive approaches include optimizing staffing and processes around care, improving efficiency of care, and building additional beds. 16, 25, 38, 39 However, the expense of construction and the scarcity of capital often preclude this last option. Among children's hospitals, with SLOSR close to one, implementing strategies to reduce the LOS during periods of high occupancy may not result in meaningful reductions in LOS, as such approaches would only decrease the typical child's hospitalization by hours, not days. In addition to proactive strategies, hospitals also rely on reactive approaches, such as ED boarding, placing patients in hallways on units, diverting ambulances or transfers, or canceling scheduled admissions at the last moment, to decrease crowding. 16, 39, 40
This study has several limitations. First, use of administrative data precluded modeling all responses. For example, some hospitals may be better able to accommodate fluctuations in census or high occupancy without compromising quality or access. Second, we only considered intra‐week smoothing, but hospitals may benefit from smoothing over longer periods of time, especially since children's hospitals are busier in winter months, but incoming scheduled volume is often not reduced. 11 Hospitals with large occupancy variations across months may want to consider broadening the time horizon for smoothing, and weigh the costs and benefits over that period of time, including parental and clinician concerns and preferences for not delaying treatment. At the individual hospital level, discrete‐event simulation would likely be useful to consider the trade‐offs of smoothing to different levels and over different periods of time. Third, we assumed a fixed number of beds for the year, an approach that may not accurately reflect actual available beds on specific days. This limitation was minimized by counting all beds for each hospital as available for all the days of the year, so that hospitals with a high census when all available beds are included would have an even higher percent occupancy if some of those beds were not actually open. In a related way, then, we also do not consider how staffing may need to be altered or augmented to care for additional patients on certain days. Fourth, midnight census, the only universally available measure, was used to determine occupancy rather than peak census. Midnight census provides a standard snapshot, but is lower than mid‐day peak census. 41 In order to account for these limitations, we considered several different thresholds of high occupancy. Fifth, we smoothed at the hospital level, but differential effects may exist at the unit level. Sixth, to determine proportion of scheduled admissions, we used HCUP KID proportions on PHIS admissions. Overall, this approach likely overestimated scheduled medical admissions on weekends, thus biasing our result towards the null hypothesis. Finally, only freestanding children's hospitals were included in this study. While this may limit generalizability, the general concept of smoothing occupancy should apply in any setting with substantial and consistent variation.
In summary, our study revealed that children's hospitals often face high midweek occupancy, but also have substantial unused weekend capacity. Hospitals facing challenges with high weekday occupancy could proactively use a smoothing approach to decrease the frequency and severity of high occupancy. Further qualitative evaluation is also warranted around child, family, and staff preferences concerning scheduled admissions, school, and work.
High levels of hospital occupancy are associated with compromises to quality of care and access (often referred to as crowding), 18 while low occupancy may be inefficient and also impact quality. 9, 10 Despite this, hospitals typically have uneven occupancy. Although some demand for services is driven by factors beyond the control of a hospital (eg, seasonal variation in viral illness), approximately 15%30% of admissions to children's hospitals are scheduled from days to months in advance, with usual arrivals on weekdays. 1114 For example, of the 3.4 million elective admissions in the 2006 Healthcare Cost and Utilization Project Kids Inpatient Database (HCUP KID), only 13% were admitted on weekends. 14 Combined with short length of stay (LOS) for such patients, this leads to higher midweek and lower weekend occupancy. 12
Hospitals respond to crowding in a number of ways, but often focus on reducing LOS to make room for new patients. 11, 15, 16 For hospitals that are relatively efficient in terms of LOS, efforts to reduce it may not increase functional capacity adequately. In children's hospitals, median lengths of stay are 2 to 3 days, and one‐third of hospitalizations are 1 day or less. 17 Thus, even 10%20% reductions in LOS trims hours, not days, from typical stays. Practical barriers (eg, reluctance to discharge in the middle of the night, or family preferences and work schedules) and undesired outcomes (eg, increased hospital re‐visits) are additional pitfalls encountered by relying on throughput enhancement alone.
Managing scheduled admissions through smoothing is an alternative strategy to reduce variability and high occupancy. 6, 12, 1820 The concept is to proactively control the entry of patients, when possible, to achieve more even levels of occupancy, instead of the peaks and troughs commonly encountered. Nonetheless, it is not a widely used approach. 18, 20, 21 We hypothesized that children's hospitals had substantial unused capacity that could be used to smooth occupancy, which would reduce weekday crowding. While it is obvious that smoothing will reduce peaks to average levels (and also raise troughs), we sought to quantify just how large this difference wasand thereby quantify the potential of smoothing to reduce inpatient crowding (or, conversely, expose more patients to high levels of occupancy). Is there enough variation to justify smoothing, and, if a hospital does smooth, what is the expected result? If the number of patients removed from exposure to high occupancy is not substantial, other means to address inpatient crowding might be of more value. Our aims were to quantify the difference in weekday versus weekend occupancy, report on mathematical feasibility of such an approach, and determine the difference in number of patients exposed to various levels of high occupancy.
Methods
Data Source
This retrospective study was conducted with resource‐utilization data from 39 freestanding, tertiary‐care children's hospitals in the Pediatric Health Information System (PHIS). Participating hospitals are located in noncompeting markets of 23 states, plus the District of Columbia, and affiliated with the Child Health Corporation of America (CHCA, Shawnee Mission, KS). They account for 80% of freestanding, and 20% of all general, tertiary‐care children's hospitals. Data quality and reliability are assured through joint ongoing, systematic monitoring. The Children's Hospital of Philadelphia Committees for the Protection of Human Subjects approved the protocol with a waiver of informed consent.
Patients
Patients admitted January 1December 31, 2007 were eligible for inclusion. Due to variation in the presence of birthing, neonatal intensive care, and behavioral health units across hospitals, these beds and associated patients were excluded. Inpatients enter hospitals either as scheduled (often referred to as elective) or unscheduled (emergent or urgent) admissions. Because PHIS does not include these data, KID was used to standardize the PHIS data for proportion of scheduled admissions. 22 (KID is a healthcare database of 23 million pediatric inpatient discharges developed through federalstateindustry partnership, and sponsored by the Agency for Healthcare Research and Quality [AHRQ].) Each encounter in KID includes a principal International Classification of Diseases, 9th revision (ICD‐9) discharge diagnosis code, and is designated by the hospital as elective (ranging from chemotherapy to tonsillectomy) or not elective. Because admissions, rather than diagnoses, are scheduled, a proportion of patients with each primary diagnosis in KID are scheduled (eg, 28% of patients with a primary diagnosis of esophageal reflux). Proportions in KID were matched to principal diagnoses in PHIS.
Definitions
The census was the number of patients registered as inpatients (including those physically in the emergency department [ED] from time of ED arrival)whether observation or inpatient statusat midnight, the conclusion of the day. Hospital capacity was set using CHCA data (and confirmed by each hospital's administrative personnel) as the number of licensed in‐service beds available for patients in 2007; we assumed beds were staffed and capacity fixed for the year. Occupancy was calculated by dividing census by capacity. Maximum occupancy in a week referred to the highest occupancy level achieved in a seven‐day period (MondaySunday). We analyzed a set of thresholds for high‐occupancy (85%, 90%, 95%, and 100%), because there is no consistent definition for when hospitals are at high occupancy or when crowding occurs, though crowding has been described as starting at 85% occupancy. 2325
Analysis
The hospital was the unit of analysis. We report hospital characteristics, including capacity, number of discharges, and census region, and annual standardized length of stay ratio (SLOSR) as observed‐to‐expected LOS.
Smoothing Technique
A retrospective smoothing algorithm set each hospital's daily occupancy during a week to that hospital's mean occupancy for the week; effectively spreading the week's volume of patients evenly across the days of the week. While inter‐week and inter‐month smoothing were considered, intra‐week smoothing was deemed more practical for the largest number of patients, as it would not mean delaying care by more than one week. In the case of a planned treatment course (eg, chemotherapy), only intra‐week smoothing would maintain the necessary scheduled intervals of treatment.
Mathematical Feasibility
To approximate the number of patient admissions that would require different scheduling during a particular week to achieve smoothed weekly occupancy, we determined the total number of patient‐days in the week that required different scheduling and divided by the average LOS for the week. We then divided the number of admissions‐to‐move by total weekly admissions to compute the percentage at each hospital across 52 weeks of the year.
Measuring the Impact of Smoothing
We focused on the frequency and severity of high occupancy and the number of patients exposed to it. This framework led to 4 measures that assess the opportunity and effect of smoothing:
Difference in hospital weekdayweekend occupancy: Equal to 12‐month median of difference between mean weekday occupancy and mean weekend occupancy for each hospital‐week.
Difference in hospital maximummean occupancy: Equal to median of difference between maximum one‐day occupancy and weekly mean (smoothed) occupancy for each hospital‐week. A regression line was derived from the data for the 39 hospitals to report expected reduction in peak occupancy based on the magnitude of the difference between weekday and weekend occupancy.
Difference in number of hospitals exposed to above‐threshold occupancy: Equal to difference, pre‐ and post‐smoothing, in number of hospitals facing high‐occupancy conditions on an average of at least one weekday midnight per week during the year at different occupancy thresholds.
Difference in number of patients exposed to above‐threshold occupancy: Equal to difference, pre‐ and post‐smoothing, in number of patients exposed to hospital midnight occupancy at the thresholds. We utilized patient‐days for the calculation to avoid double‐counting, and divided this by average LOS, in order to determine the number of patients who would no longer be exposed to over‐threshold occupancy after smoothing, while also adjusting for patients newly exposed to over‐threshold occupancy levels.
All analyses were performed separately for each hospital for the entire year and then for winter (DecemberMarch), the period during which most crowding occurred. Analyses were performed using SAS (version 9.2, SAS Institute, Inc, Cary, NC); P values <0.05 were considered statistically significant.
Results
The characteristics of the 39 hospitals are provided in Table 1. Based on standardization with KID, 23.6% of PHIS admissions were scheduled (range: 18.1%35.8%) or a median of 81.5 scheduled admissions per week per hospital; 26.6% of weekday admissions were scheduled versus 16.1% for weekends. Overall, 12.4% of scheduled admissions entered on weekends. For all patients, median LOS was three days (interquartile range [IQR]: twofive days), but median LOS for scheduled admissions was two days (IQR: onefour days). The median LOS and IQR were the same by day of admission for all days of the week. Most hospitals had an overall SLOSR close to one (median: 0.9, IQR: 0.91.1). Overall, hospital mean midnight occupancy ranged from 70.9% to 108.1% on weekdays and 65.7% to 94.9% on weekends. Uniformly, weekday occupancy exceeded weekend occupancy, with a median difference of 8.2% points (IQR: 7.2%9.5% points). There was a wide range of median hospital weekdayweekend occupancy differences across hospitals (Figure 1). The overall difference was less in winter (median difference: 7.7% points; IQR: 6.3%8.8% points) than in summer (median difference: 8.6% points; IQR: 7.4%9.8% points (Wilcoxon Sign Rank test, P < 0.001). Thirty‐five hospitals (89.7%) exceeded the 85% occupancy threshold and 29 (74.4%) exceeded the 95% occupancy threshold on at least 20% of weekdays (Table 2). Across all the hospitals, the median difference in weekly maximum and weekly mean occupancy was 6.6% points (IQR: 6.2%7.4% points) (Figure 2).
| Characteristics | No. (%) |
|---|---|
| |
| Licensed in‐service beds | n = 39 hospitals |
| <200 beds | 6 (15.4) |
| 200249 beds | 10 (25.6) |
| 250300 beds | 14 (35.9) |
| >300 beds | 9 (23.1) |
| No. of discharges | |
| <10,000 | 5 (12.8) |
| 10,00013,999 | 14 (35.9) |
| 14,00017,999 | 11 (28.2) |
| >18,000 | 9 (23.1) |
| Census region | |
| West | 9 (23.1) |
| Midwest | 11 (28.2) |
| Northeast | 6 (15.4) |
| South | 13 (33.3) |
| Admissions | n = 590,352 admissions |
| Medical scheduled admissions* | 79,683 |
| Surgical scheduled admissions* | 59,640 |
| Total scheduled admissions* (% of all admissions) | 139,323 (23.6) |
| Weekend medical scheduled admissions* (% of all medical scheduled admissions) | 13,546 (17.0) |
| Weekend surgical scheduled admissions* (% of all surgical scheduled admissions) | 3,757 (6.3) |
| Weekend total scheduled admissions* (% of total scheduled admissions) | 17,276 (12.4) |

| Entire Year | >85% | Occupancy Threshold | >95% | >100% |
|---|---|---|---|---|
| >90% | ||||
| ||||
| No. of hospitals (n = 39) with mean weekday occupancy above threshold | ||||
| Before smoothing (current state) | 33 | 25 | 14 | 6 |
| After smoothing | 32 | 22 | 10 | 1 |
| No. of hospitals (n = 39) above threshold 20% of weekdays | ||||
| Before smoothing (current state) | 35 | 34 | 29 | 14 |
| After smoothing | 35 | 32 | 21 | 9 |
| Median (IQR) no. of patient‐days per hospital not exposed to occupancy above threshold by smoothing | 3,071 | 281 | 3236 | 3281 |
| (5,552, 919) | (5,288, 3,103) | (0, 7,083) | (962, 8,517) | |
| Median (IQR) no. of patients per hospital not exposed to occupancy above threshold by smoothing | 596 | 50 | 630 | 804 |
| (1,190, 226) | (916, 752) | (0, 1,492) | (231, 2,195) | |

Smoothing reduced the number of hospitals at each occupancy threshold, except 85% (Table 2). As a linear relationship, the reduction in weekday peak occupancy (y) based on a hospital's median difference in weekly maximum and weekly mean occupancy (x) was y = 2.69 + 0.48x. Thus, a hospital with a 10% point difference between weekday and weekend occupancy could reduce weekday peak by 7.5% points.
Smoothing increased the number of patients exposed to the lower thresholds (85% and 90%), but decreased the number of patients exposed to >95% occupancy (Table 2). For example, smoothing at the 95% threshold resulted in 630 fewer patients per hospital exposed to that threshold. If all 39 hospitals had within‐week smoothing, a net of 39,607 patients would have been protected from exposure to >95% occupancy and a net of 50,079 patients from 100% occupancy.
To demonstrate the varied effects of smoothing, Table 3 and Figure 3 present representative categories of response to smoothing depending on pre‐smoothing patterns. While not all hospitals decreased occupancy to below thresholds after smoothing (Types B and D), the overall occupancy was reduced and fewer patients were exposed to extreme levels of high occupancy (eg, >100%).
| Category | Before Smoothing Hospital Description | After Smoothing Hospital Description | No. of Hospitals at 85% Threshold (n = 39) | No. of Hospitals at 95% Threshold (n = 39) |
|---|---|---|---|---|
| ||||
| Type A | Weekdays above threshold | All days below threshold, resulting in net decrease in patients exposed to occupancies above threshold | 3 | 1 |
| Weekends below threshold | ||||
| Type B | Weekdays above threshold | All days above threshold, resulting in net increase in patients exposed to occupancies above threshold | 12 | 18 |
| Weekends below threshold | ||||
| Type C | All days of week below threshold | All days of week below threshold | 6 | 19 |
| Type D | All days of week above threshold | All days of week above threshold, resulting in net decrease in patients exposed to extreme high occupancy | 18 | 1 |

To achieve within‐week smoothing, a median of 7.4 patient‐admissions per week (range: 2.314.4) would have to be scheduled on a different day of the week. This equates to a median of 2.6% (IQR: 2.25%, 2.99%; range: 0.02%9.2%) of all admissionsor 9% of a typical hospital‐week's scheduled admissions.
Discussion
This analysis of 39 children's hospitals found high levels of occupancy and weekend occupancy lower than weekday occupancy (median difference: 8.2% points). Only 12.4% of scheduled admissions entered on weekends. Thus, weekend capacity is available to offset high weekday occupancy. Hospitals at the higher end of the occupancy thresholds (95%, 100%) would reduce the number of days operating at very high occupancy and the number of patients exposed to such levels by smoothing. This change is mathematically feasible, as a median of 7.4 patients would have to be proactively scheduled differently each week, just under one‐tenth of scheduled admissions. Since LOS by day of admission was the same (median: two days), the opportunity to affect occupancy by shifting patients should be relatively similar for all days of the week. In addition, these admissions were short, conferring greater flexibility. Implementing smoothing over the course of the week does not necessarily require admitting patients on weekends. For example, Monday admissions with an anticipated three‐day LOS could enter on Friday with anticipated discharge on Monday to alleviate midweek crowding and take advantage of unoccupied weekend beds. 26
At the highest levels of occupancy, smoothing reduces the frequency of reaching these maximum levels, but can have the effect of actually exposing more patient‐days to a higher occupancy. For example, for nine hospitals in our analysis with >20% of days over 100%, smoothing decreased days over 100%, but exposed weekend patients to higher levels of occupancy (Figure 3). Since most admissions are short and most scheduled admissions currently occur on weekdays, the number of individual patients (not patient‐days) newly exposed to such high occupancy may not increase much after smoothing at these facilities. Regardless, hospitals with such a pattern may not be able to rely solely on smoothing to avoid weekday crowding, and, if they are operating efficiently in terms of SLOSR, might be justified in building more capacity.
Consistent with our findings, the Institute for Healthcare Improvement, the Institute for Healthcare Optimization, and the American Hospital Association Quality Center stress that addressing artificial variability of scheduled admissions is a critical first step to improving patient flow and quality of care while reducing costs. 18, 21, 27 Our study suggests that small numbers of patients need to be proactively scheduled differently to decrease midweek peak occupancy, so only a small proportion of families would need to find this desirable to make it attractive for hospitals and patients. This type of proactive smoothing decreases peak occupancy on weekdays, reducing the safety risks associated with high occupancy, improving acute access for emergent patients, shortening wait‐times and loss of scheduled patients to another facility, and increasing procedure volume (3%74% in one study). 28 Smoothing may also increase quality and safety on weekends, as emergent patients admitted on weekends experience more delays in necessary treatment and have worse outcomes. 2932 In addition, increasing scheduled admissions to span weekends may appeal to some families wishing to avoid absence from work to be with their hospitalized child, to parents concerned about school performanceand may also appeal to staff members seeking flexible schedules. Increasing weekend hospital capacity is safe, feasible, and economical, even when considering the increased wages for weekend work. 33, 34 Finally, smoothing over the whole week allows fixed costs (eg, surgical suites, imaging equipment) to be allocated over 7 days rather than 5, and allows for better matching of revenue to the fixed expenses.
Rather than a prescriptive approach, our work suggests hospitals need to identify only a small number of patients to proactively shift, providing them opportunities to adapt the approach to local circumstances. The particular patients to move around may also depend on the costs and benefits of services (eg, radiologic, laboratory, operative) and the hospital's existing patterns of staffing. A number of hospitals that have engaged in similar work have achieved sustainable results, such as Seattle Children's Hospital, Boston Medical Center, St. John's Regional Health Center, and New York University Langone Medical Center. 19, 26, 3537 In these cases, proactive smoothing took advantage of unused capacity and decreased crowding on days that had been traditionally very full. Hospitals that rarely or never have high‐occupancy days, and that do not expect growth in volume, may not need to employ smoothing, whereas others that have crowding issues primarily in the winter may wish to implement smoothing techniques seasonally.
Aside from attempting to reduce high‐occupancy through modification of admission patterns, other proactive approaches include optimizing staffing and processes around care, improving efficiency of care, and building additional beds. 16, 25, 38, 39 However, the expense of construction and the scarcity of capital often preclude this last option. Among children's hospitals, with SLOSR close to one, implementing strategies to reduce the LOS during periods of high occupancy may not result in meaningful reductions in LOS, as such approaches would only decrease the typical child's hospitalization by hours, not days. In addition to proactive strategies, hospitals also rely on reactive approaches, such as ED boarding, placing patients in hallways on units, diverting ambulances or transfers, or canceling scheduled admissions at the last moment, to decrease crowding. 16, 39, 40
This study has several limitations. First, use of administrative data precluded modeling all responses. For example, some hospitals may be better able to accommodate fluctuations in census or high occupancy without compromising quality or access. Second, we only considered intra‐week smoothing, but hospitals may benefit from smoothing over longer periods of time, especially since children's hospitals are busier in winter months, but incoming scheduled volume is often not reduced. 11 Hospitals with large occupancy variations across months may want to consider broadening the time horizon for smoothing, and weigh the costs and benefits over that period of time, including parental and clinician concerns and preferences for not delaying treatment. At the individual hospital level, discrete‐event simulation would likely be useful to consider the trade‐offs of smoothing to different levels and over different periods of time. Third, we assumed a fixed number of beds for the year, an approach that may not accurately reflect actual available beds on specific days. This limitation was minimized by counting all beds for each hospital as available for all the days of the year, so that hospitals with a high census when all available beds are included would have an even higher percent occupancy if some of those beds were not actually open. In a related way, then, we also do not consider how staffing may need to be altered or augmented to care for additional patients on certain days. Fourth, midnight census, the only universally available measure, was used to determine occupancy rather than peak census. Midnight census provides a standard snapshot, but is lower than mid‐day peak census. 41 In order to account for these limitations, we considered several different thresholds of high occupancy. Fifth, we smoothed at the hospital level, but differential effects may exist at the unit level. Sixth, to determine proportion of scheduled admissions, we used HCUP KID proportions on PHIS admissions. Overall, this approach likely overestimated scheduled medical admissions on weekends, thus biasing our result towards the null hypothesis. Finally, only freestanding children's hospitals were included in this study. While this may limit generalizability, the general concept of smoothing occupancy should apply in any setting with substantial and consistent variation.
In summary, our study revealed that children's hospitals often face high midweek occupancy, but also have substantial unused weekend capacity. Hospitals facing challenges with high weekday occupancy could proactively use a smoothing approach to decrease the frequency and severity of high occupancy. Further qualitative evaluation is also warranted around child, family, and staff preferences concerning scheduled admissions, school, and work.
- , , , . A comparison of in‐hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Medical Care. 2010;48(3):224–232.
- , , , et al. Hospital workload and adverse events. Med Care. 2007;45(5):448–455.
- , , , , . Impact of admission‐day crowding on the length of stay of pediatric hospitalizations. Pediatrics. 2008;121(4):e718–e730.
- , , , . The effect of hospital bed occupancy on throughput in the pediatric emergency department. Ann Emerg Med. 2009;53(6):767–776.
- . The tipping point: the relationship between volume and patient harm. Am J Med Qual. 2008;23(5):336–341.
- , , , , , . Managing unnecessary variability in patient demand to reduce nursing stress and improve patient safety. Jt Comm J Qual Patient Saf. 2005;31(6):330–338.
- Hospital‐Based Emergency Care: At the Breaking Point. Washington, DC: Institute of Medicine Committee on the Future of Emergency Care in the United States Health System; 2006.
- , , , , . The effect of hospital occupancy on emergency department length of stay and patient disposition. Acad Emerg Med. 2003;10(2):127–133.
- . Interpreting the Volume‐Outcome Relationship in the Context of Health Care Quality: Workshop Summary. Washington, DC: National Academies Press; 2000.
- , , , , . Has recognition of the relationship between mortality rates and hospital volume for major cancer surgery in California made a difference? A follow‐up analysis of another decade. Ann Surg. 2009;250(3):472–483.
- , , , et al. Children's hospitals do not acutely respond to high occupancy. Pediatrics. 2010;125:974–981.
- , , , , , . Scheduled admissions and high occupancy at a children's hospital. J Hosp Med. 2011;6(2):81–87.
- , , . Characteristics of weekday and weekend hospital admissions. HCUP Statistical Brief. 2010;87.
- Agency for Healthcare Research and Quality. HCUP databases, Healthcare Cost and Utilization Project (HCUP); 2008. Available at: http://www.hcup‐us.ahrq.gov/kidoverview.jsp. Accessed July 15, 2009.
- , et al. Managing capacity to reduce emergency department overcrowding and ambulance diversions. J Qual Patient Saf. 2006;32(5):239–245.
- Institute for Healthcare Improvement. Flow initiatives; 2008. Available at: http://www.ihi.org/IHI/Topics/Flow. Accessed February 20, 2008.
- , , , , , . Trends in high‐turnover stays among children hospitalized in the United States, 1993–2003. Pediatrics. 2009;123(3):996–1002.
- Institute for Healthcare Improvement. Smoothing elective surgical admissions. Available at: http://www.ihi.org/IHI/Topics/Flow/PatientFlow/EmergingContent/SmoothingElectiveSurgicalAdmissions.htm. Accessed October 24, 2008.
- Boston hospital sees big impact from smoothing elective schedule. OR Manager. 2004;20:12.
- . Managing Variability in Patient Flow Is the Key to Improving Access to Care, Nursing Staffing, Quality of Care, and Reducing Its Cost. Paper presented at Institute of Medicine, Washington, DC; June 24, 2004.
- American Hospital Association Quality Center. Available at: http://www.ahaqualitycenter.org/ahaqualitycenter/. Accessed October 14, 2008.
- Healthcare Cost and Utilization Project (HCUP). Kids' Inpatient Database (KID); July 2008. Available at: http://www.hcup‐us.ahrq.gov/kidoverview.jsp. Accessed September 10, 2008.
- , , . Using a queuing model to help plan bed allocation in a department of geriatric medicine. Health Care Manag Sci. 2002;5(4):307–313.
- . How many hospital beds? Inquiry. 2002;39(4):400–412.
- . Institute for Healthcare Improvement. Patient flow comments. Available at: http://www.ihi.org/IHI/Topics/Flow. Accessed September 10, 2008.
- . Factory efficiency comes to the hospital. New York Times. July 9, 2010.
- Institute for Healthcare Improvement. Re‐engineering the operating room. Available at: http://www.ihi.org/IHI/Programs/ConferencesAndSeminars/ReengineeringtheOperatingRoomSept08.htm. Accessed November 8, 2008.
- , . Enhanced weekend service: an affordable means to increased hospital procedure volume. CMAJ. 2005;172(4):503–504.
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345:663–668.
- , , , , , . Weekend versus weekday admission and mortality from myocardial infarction. N Engl J Med. 2007;356:1099–1109.
- , . Waiting for urgent procedures on the weekend among emergently hospitalized patients. Am J Med. 2004;117:175–181.
- . Do hospitals provide lower quality care on weekends? Health Serv Res. 2007;42:1589–1612.
- . Hospital saves by working weekends. Mod Healthc. 1996;26:82–99.
- , , , , . Weekend and holiday exercise testing in patients with chest pain. J Gen Intern Med. 1999;14:10–14.
- . Boston Medical Center Case Study: Institute of Healthcare Optimization; 2006. Available at: http://www.ihoptimize.org/8f16e142‐eeaa‐4898–9e62–660218f19ffb/download.htm. Accessed October 3, 2010.
- , , , . The impact of IMPACT on St John's Regional Health Center. Mo Med. 2003;100:590–592.
- NYU Langone Medical Center Extends Access to Non‐Emergent Care as Part of Commitment to Patient‐Centered Care (June 23, 2010). Available at: http://communications.med.nyu.edu/news/2010/nyu‐langone‐medical‐center‐extends‐access‐non‐emergent‐care‐part‐commitment‐patient‐center. Accessed October 3, 2010.
- Carondelet St. Mary's Hospital. A pragmatic approach to improving patient efficiency throughput. Improvement Report 2005. Available at: http://www.ihi.org/IHI/Topics/Flow/PatientFlow/ImprovementStories/APragmaticApproachtoImprovingPatientEfficiencyThroughput.htm. Accessed October 3, 2010.
- AHA Solutions. Patient Flow Challenges Assessment 2009. Chicago, IL; 2009.
- , , , , , . A conceptual model of emergency department crowding. Ann Emerg Med. 2003;42(2):173–180.
- . Annual bed statistics give a misleading picture of hospital surge capacity. Ann Emerg Med. 2006;48(4):384–388.
- , , , . A comparison of in‐hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Medical Care. 2010;48(3):224–232.
- , , , et al. Hospital workload and adverse events. Med Care. 2007;45(5):448–455.
- , , , , . Impact of admission‐day crowding on the length of stay of pediatric hospitalizations. Pediatrics. 2008;121(4):e718–e730.
- , , , . The effect of hospital bed occupancy on throughput in the pediatric emergency department. Ann Emerg Med. 2009;53(6):767–776.
- . The tipping point: the relationship between volume and patient harm. Am J Med Qual. 2008;23(5):336–341.
- , , , , , . Managing unnecessary variability in patient demand to reduce nursing stress and improve patient safety. Jt Comm J Qual Patient Saf. 2005;31(6):330–338.
- Hospital‐Based Emergency Care: At the Breaking Point. Washington, DC: Institute of Medicine Committee on the Future of Emergency Care in the United States Health System; 2006.
- , , , , . The effect of hospital occupancy on emergency department length of stay and patient disposition. Acad Emerg Med. 2003;10(2):127–133.
- . Interpreting the Volume‐Outcome Relationship in the Context of Health Care Quality: Workshop Summary. Washington, DC: National Academies Press; 2000.
- , , , , . Has recognition of the relationship between mortality rates and hospital volume for major cancer surgery in California made a difference? A follow‐up analysis of another decade. Ann Surg. 2009;250(3):472–483.
- , , , et al. Children's hospitals do not acutely respond to high occupancy. Pediatrics. 2010;125:974–981.
- , , , , , . Scheduled admissions and high occupancy at a children's hospital. J Hosp Med. 2011;6(2):81–87.
- , , . Characteristics of weekday and weekend hospital admissions. HCUP Statistical Brief. 2010;87.
- Agency for Healthcare Research and Quality. HCUP databases, Healthcare Cost and Utilization Project (HCUP); 2008. Available at: http://www.hcup‐us.ahrq.gov/kidoverview.jsp. Accessed July 15, 2009.
- , et al. Managing capacity to reduce emergency department overcrowding and ambulance diversions. J Qual Patient Saf. 2006;32(5):239–245.
- Institute for Healthcare Improvement. Flow initiatives; 2008. Available at: http://www.ihi.org/IHI/Topics/Flow. Accessed February 20, 2008.
- , , , , , . Trends in high‐turnover stays among children hospitalized in the United States, 1993–2003. Pediatrics. 2009;123(3):996–1002.
- Institute for Healthcare Improvement. Smoothing elective surgical admissions. Available at: http://www.ihi.org/IHI/Topics/Flow/PatientFlow/EmergingContent/SmoothingElectiveSurgicalAdmissions.htm. Accessed October 24, 2008.
- Boston hospital sees big impact from smoothing elective schedule. OR Manager. 2004;20:12.
- . Managing Variability in Patient Flow Is the Key to Improving Access to Care, Nursing Staffing, Quality of Care, and Reducing Its Cost. Paper presented at Institute of Medicine, Washington, DC; June 24, 2004.
- American Hospital Association Quality Center. Available at: http://www.ahaqualitycenter.org/ahaqualitycenter/. Accessed October 14, 2008.
- Healthcare Cost and Utilization Project (HCUP). Kids' Inpatient Database (KID); July 2008. Available at: http://www.hcup‐us.ahrq.gov/kidoverview.jsp. Accessed September 10, 2008.
- , , . Using a queuing model to help plan bed allocation in a department of geriatric medicine. Health Care Manag Sci. 2002;5(4):307–313.
- . How many hospital beds? Inquiry. 2002;39(4):400–412.
- . Institute for Healthcare Improvement. Patient flow comments. Available at: http://www.ihi.org/IHI/Topics/Flow. Accessed September 10, 2008.
- . Factory efficiency comes to the hospital. New York Times. July 9, 2010.
- Institute for Healthcare Improvement. Re‐engineering the operating room. Available at: http://www.ihi.org/IHI/Programs/ConferencesAndSeminars/ReengineeringtheOperatingRoomSept08.htm. Accessed November 8, 2008.
- , . Enhanced weekend service: an affordable means to increased hospital procedure volume. CMAJ. 2005;172(4):503–504.
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345:663–668.
- , , , , , . Weekend versus weekday admission and mortality from myocardial infarction. N Engl J Med. 2007;356:1099–1109.
- , . Waiting for urgent procedures on the weekend among emergently hospitalized patients. Am J Med. 2004;117:175–181.
- . Do hospitals provide lower quality care on weekends? Health Serv Res. 2007;42:1589–1612.
- . Hospital saves by working weekends. Mod Healthc. 1996;26:82–99.
- , , , , . Weekend and holiday exercise testing in patients with chest pain. J Gen Intern Med. 1999;14:10–14.
- . Boston Medical Center Case Study: Institute of Healthcare Optimization; 2006. Available at: http://www.ihoptimize.org/8f16e142‐eeaa‐4898–9e62–660218f19ffb/download.htm. Accessed October 3, 2010.
- , , , . The impact of IMPACT on St John's Regional Health Center. Mo Med. 2003;100:590–592.
- NYU Langone Medical Center Extends Access to Non‐Emergent Care as Part of Commitment to Patient‐Centered Care (June 23, 2010). Available at: http://communications.med.nyu.edu/news/2010/nyu‐langone‐medical‐center‐extends‐access‐non‐emergent‐care‐part‐commitment‐patient‐center. Accessed October 3, 2010.
- Carondelet St. Mary's Hospital. A pragmatic approach to improving patient efficiency throughput. Improvement Report 2005. Available at: http://www.ihi.org/IHI/Topics/Flow/PatientFlow/ImprovementStories/APragmaticApproachtoImprovingPatientEfficiencyThroughput.htm. Accessed October 3, 2010.
- AHA Solutions. Patient Flow Challenges Assessment 2009. Chicago, IL; 2009.
- , , , , , . A conceptual model of emergency department crowding. Ann Emerg Med. 2003;42(2):173–180.
- . Annual bed statistics give a misleading picture of hospital surge capacity. Ann Emerg Med. 2006;48(4):384–388.
Copyright © 2011 Society of Hospital Medicine
Treatment of Complicated Pneumonia
Community‐acquired pneumonia, the most common serious bacterial infection in childhood, may be complicated by parapneumonic effusion (ie, complicated pneumonia).1 Children with complicated pneumonia require prolonged hospitalization and frequently undergo multiple pleural fluid drainage procedures.2 Additionally, the incidence of complicated pneumonia has increased,37 making the need to define appropriate therapy even more pressing. Defining appropriate therapy is challenging for the individual physician as a result of inconsistent and insufficient evidence, and wide variation in treatment practices.2, 8
Historically, thoracotomy was performed only if initial chest tube placement did not lead to clinical improvement.9, 10 Several authors, noting the rapid resolution of symptoms in children undergoing earlier thoracotomy, advocated for the use of thoracotomy as initial therapy rather than as a procedure of last resort.114 The advent of less invasive techniques such as video‐assisted thoracoscopic surgery (VATS) has served as an additional impetus to consider surgical drainage as the initial treatment strategy.1518 Few well‐designed studies have examined the relative efficacy of these interventions.2, 1922 Published randomized trials were single center, enrolled few patients, and arrived at different conclusions.19, 21, 22 In addition, these trials did not examine other important outcomes such as requirement for additional pleural fluid drainage procedures and hospital readmission. Two large retrospective multicenter studies found modest reductions in length of stay (LOS) and substantial decreases in the requirement for additional pleural fluid drainage procedures in children undergoing initial VATS compared with initial chest tube placement.2, 20 However, Shah et al2 included relatively few patients undergoing VATS. Li et al20 combined patients undergoing initial thoracentesis, initial chest tube placement, late pleural fluid drainage (by any method), and no pleural fluid drainage into a single non‐operative management category, precluding conclusions about the relative benefits of chest tube placement compared with VATS. Neither study2, 20 examined the role of chemical fibrinolysis, a therapy which has been associated with outcomes comparable to VATS in two small randomized trials.21, 22
The objectives of this multicenter study were to describe the variation in the initial management strategy along with associated outcomes of complicated pneumonia in childhood and to determine the comparative effectiveness of different pleural fluid drainage procedures.
Methods
Data Source
The Pediatric Health Information System (PHIS), which contains resource utilization data from 40 freestanding children's hospitals, provided data for this multicenter retrospective cohort study. Participating hospitals are located in noncompeting markets of 27 states plus the District of Columbia. The PHIS database includes patient demographics, diagnoses, and procedures as well as data for all drugs, radiologic studies, laboratory tests, and supplies charged to each patient. Data are de‐identified, however encrypted medical record numbers allow for tracking individual patients across admissions. The Child Health Corporation of America (Shawnee Mission, KS) and participating hospitals jointly assure data quality and reliability as described previously.23, 24 The Children's Hospital of Philadelphia Institutional Review Board reviewed and approved this study.
Patients
Children 18 years of age receiving a pleural drainage procedure for complicated pneumonia were eligible if they were discharged from participating hospitals between January 1, 2004 and June 30, 2009. Study participants met the following criteria: 1) discharge diagnosis of pneumonia (International Classification of Diseases, 9th revision [ICD‐9] discharge diagnosis codes 480.x‐483.x, 485.x‐487.x), 2) discharge diagnosis of pleural effusion (ICD‐9 codes 510.0, 510.9, 511.0, 511.1, or 511.9), and 3) billing charge for antibiotics on the first day of hospitalization. Additionally, the primary discharge diagnosis had to be either pneumonia or pleural effusion. Patients were excluded if they did not undergo pleural fluid drainage or if their initial pleural fluid drainage procedure was thoracentesis.
Study Definitions
Pleural drainage procedures were identified using ICD‐9 procedure codes for thoracentesis (34.91), chest tube placement (34.04), VATS (34.21), and thoracotomy (34.02 or 34.09). Fibrinolysis was defined as receipt of urokinase, streptokinase, or alteplase within two days of initial chest tube placement.
Acute conditions or complications included influenza (487, 487.0, 487.1, 487.8, 488, or V04.81) and hemolytic‐uremic syndrome (283.11). Chronic comorbid conditions (CCCs) (eg, malignancy) were identified using a previously reported classification scheme.25 Billing data were used to classify receipt of mechanical ventilation and medications on the first day of hospitalization.
Measured Outcomes
The primary outcomes were hospital LOS (both overall and post‐initial procedure), requirement for additional pleural drainage procedures, total cost for index hospitalization, all‐cause readmission within 14 days after index hospital discharge, and total cost of the episode (accounting for the cost of readmissions).
Measured Exposures
The primary exposure of interest was the initial pleural fluid drainage procedure, classified as chest tube placement without fibrinolysis, chest tube placement with fibrinolysis, VATS, or thoracotomy.
Statistical Analysis
Variables were summarized using frequencies and percentages for categorical variables, and median, interquartile range (IQR), and range for continuous variables. Outcomes by initial pleural drainage procedure were compared using chi‐squared tests for categorical variables and Kruskal‐Wallis tests for continuous variables.
Multivariable analysis was performed to account for potential confounding by observed baseline variables. For dichotomous outcome variables, modeling consisted of logistic regression using generalized estimating equations to account for hospital clustering. For continuous variables, a mixed model approach was used, treating hospital as a random effect. Log transformation was applied to the right‐skewed outcome variables (LOS and cost). Cost outcomes remained skewed following log transformation, thus gamma mixed models were applied.2629 Odds ratios and 95% confidence intervals (CIs) were reported for comparison of dichotomous outcomes and the adjusted means and 95% CIs were reported for continuous outcomes after appropriate back transformation.
Additional analyses addressed the potential impact of confounding by indication inherent in any observational study. First, patients with an underlying CCC were excluded to ensure that our results would be generalizable to otherwise healthy children with community‐acquired pneumonia. Second, patients undergoing pleural drainage >2 days after hospitalization were excluded to minimize the effect of residual confounding related to differences in timing of the initial drainage procedure. Third, the analysis was repeated using a generalized propensity score as an additional method to account for confounding by indication for the initial drainage procedure.30 Propensity scores, constructed using a multivariable generalized logit model, included all variables listed in Table 1. The inverse of the propensity score was included as a weight in each multivariable model described previously. Only the primary multivariable analyses are presented as the results of the propensity score analysis were nearly identical to the primary analyses.
| Overall | Chest Tube Without Fibrinolysis | Chest Tube With Fibrinolysis | Thoracotomy | VATS | P Value | |
|---|---|---|---|---|---|---|
| ||||||
| N | 3500 | 1672 (47.8) | 623 (17.8) | 797 (22.8) | 408 (11.7) | |
| Age | ||||||
| <1 year | 335 (9.6) | 176 (10.5) | 56 (9.0) | 78 (9.8) | 25 (6.1) | |
| 1 year | 475 (13.6) | 238 (14.2) | 98 (15.7) | 92 (11.5) | 47 (11.5) | 0.003 |
| 24 years | 1230 (35.1) | 548 (32.8) | 203 (32.6) | 310 (38.9) | 169 (41.4) | |
| 59 years | 897 (25.6) | 412 (24.6) | 170 (27.3) | 199 (25.0) | 116 (28.4) | |
| 1014 years | 324 (9.3) | 167 (10.0) | 61 (9.8) | 65 (8.2) | 31 (7.6) | |
| 1518 years | 193 (5.5) | 106 (6.3) | 29 (4.6) | 40 (5.0) | 18 (4.4) | |
| >18 years | 46 (1.3) | 25 (1.5) | 6 (0.96) | 13 (1.6) | 2 (0.5) | |
| Comorbid Conditions | ||||||
| Cardiac | 69 (2.0) | 43 (2.6) | 14 (2.3) | 12 (1.5) | 0 (0.0) | 0.006 |
| Malignancy | 81 (2.3) | 31 (1.9) | 18 (2.9) | 21 (2.6) | 11 (2.7) | 0.375 |
| Neurological | 138 (3.9) | 73 (4.4) | 20 (3.2) | 34 (4.3) | 11 (2.7) | 0.313 |
| Any Other Condition | 202 (5.8) | 96 (5.7) | 40 (6.4) | 47 (5.9) | 19 (4.7) | 0.696 |
| Payer | ||||||
| Government | 1240 (35.6) | 630 (37.8) | 224 (36.0) | 259 (32.7) | 127 (31.3) | <0.001 |
| Private | 1383 (39.7) | 607 (36.4) | 283 (45.4) | 310 (39.2) | 183 (45.07) | |
| Other | 864 (24.8) | 430 (25.8) | 116 (18.6) | 222 (28.1) | 96 (23.65) | |
| Race | ||||||
| Non‐Hispanic White | 1746 (51.9) | 838 (51.6) | 358 (59.7) | 361 (47.8) | 189 (48.7) | <0.001 |
| Non‐Hispanic Black | 601 (17.9) | 318 (19.6) | 90 (15.0) | 128 (17.0) | 65 (16.8) | |
| Hispanic | 588 (17.5) | 280 (17.3) | 73 (12.2) | 155 (20.5) | 80 (20.6) | |
| Asian | 117 (3.5) | 47 (2.9) | 20 (3.3) | 37 (4.9) | 13 (3.4) | |
| Other | 314 (9.3) | 140 (8.6) | 59 (9.8) | 74 (9.8) | 41 (10.6) | |
| Male Sex | 1912 (54.6) | 923 (55.2) | 336 (53.9) | 439 (55.1) | 214 (52.5) | 0.755 |
| Radiology | ||||||
| CT, no US | 1200 (34.3) | 600 (35.9) | 184 (29.5) | 280 (35.1) | 136 (33.3) | <0.001 |
| CT and US | 221 (6.3) | 84 (5.0) | 53 (8.5) | 61 (7.7) | 23 (5.6) | |
| US, no CT | 799 (22.8) | 324 (19.4) | 178 (28.6) | 200 (25.1) | 97 (23.8) | |
| No US, no CT | 1280 (36.6) | 664 (39.7) | 208 (33.4) | 256 (32.1) | 152 (37.3) | |
| Empiric Antibiotic Regimen | ||||||
| Cephalosporins alone | 448 (12.8) | 181 (10.83) | 126 (20.2) | 73 (9.2) | 68 (16.7) | <0.001 |
| Cephalosporin and clindamycin | 797 (22.8) | 359 (21.5) | 145 (23.3) | 184 (23.1) | 109 (26.7) | |
| Other antibiotic combination | 167 (4.8) | 82 (4.9) | 30 (4.8) | 38 (4.8) | 17 (4.2) | |
| Cephalosporin and vancomycin | 2088 (59.7) | 1050 (62.8) | 322 (51.7) | 502 (63.0) | 214 (52.5) | |
| Mechanical ventilation | 494 (14.1) | 251 (15.0) | 75 (12.0) | 114 (14.3) | 54 (13.2) | 0.307 |
| Corticosteroids | 520 (14.9) | 291 (17.4) | 72 (11.6) | 114 (14.3) | 43 (10.5) | <0.001 |
| Blood product transfusionsb | 761 (21.7) | 387 (23.2) | 145 (23.3) | 161 (20.2) | 68 (16.7) | 0.018 |
| Vasoactive infusionsc | 381 (10.9) | 223 (13.3) | 63 (10.1) | 72 (9.0) | 23 (5.6) | <0.001 |
| Admission to intensive care | 1397 (39.9) | 731 (43.7) | 234 (37.6) | 296 (37.1) | 136 (33.3) | <0.001 |
| Extracorporeal membranous oxygenation | 18 (0.5) | 13 (0.8) | 2 (0.3) | 3 (0.4) | 0 (0.0) | 0.163 |
| Hemolytic‐uremic syndrome | 31 (0.9) | 15 (0.9) | 6 (1.0) | 7 (0.9) | 3 (0.7) | 0.985 |
| Influenza | 108 (3.1) | 53 (3.2) | 27 (4.3) | 23 (2.9) | 5 (1.2) | 0.044 |
| Arterial blood gas measurements | 0 (0,1) | 0 (0, 2) | 0 (0,1) | 0 (0, 1) | 0 (0, 1) | <0.001 |
| Days to first procedure | 1 (0, 3) | 1 (0, 2) | 1 (1, 3) | 1 (1, 3) | 1 (1, 3) | <0.001 |
Medical records of a randomly selected subset of subjects from 6 hospitals were reviewed to determine the accuracy of our algorithm in identifying patients with complicated pneumonia; these subjects represented 1% of the study population. For the purposes of medical record review, complicated pneumonia was defined by the following: 1) radiologically‐confirmed lung infiltrate; 2) moderate or large pleural effusion; and 3) signs and symptoms of lower respiratory tract infection. Complicated pneumonia was identified in 118 of 120 reviewed subjects for a positive predictive value of 98.3%.
All analyses were clustered by hospital. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). A two‐tailed P < 0.05 was considered statistically significant.
Results
Patient Characteristics
During the study period, 9,680 subjects had complicated pneumonia. Subjects were excluded if they did not have a pleural drainage procedure (n = 5798), or if thoracentesis was the first pleural fluid drainage procedure performed (n = 382). The remaining 3500 patients were included. Demographic characteristics are summarized in Table 1. The median patient age was 4.1 years (IQR: 2.17.2 years). An underlying CCC was present in 424 (12.1%) patients. There was no association between type of drainage procedure and mechanical ventilation. However, factors associated with more severe systemic illness, such as blood product transfusion, were more common among those undergoing initial chest tube placement with or without fibrinolysis (Table 1).
Initial Pleural Fluid Drainage Procedures
The primary procedures included chest tube without fibrinolysis (47.8%); chest tube with fibrinolysis (17.8%); thoracotomy (22.8%); and VATS (11.7%) (Table 1). The proportion of patients undergoing primary chest tube placement with fibrinolysis increased over time from 14.2% in 2004 to 30.0% in 2009 (P < 0.001; chi‐squared test for trend). The initial procedure varied by hospital with the greatest proportion of patients undergoing primary chest tube placement without fibrinolysis at 28 (70.0%) hospitals, chest tube placement with fibrinolysis at 5 (12.5%) hospitals, thoracotomy at 5 (12.5%) hospitals, and VATS at 2 (5.0%) hospitals (Figure 1). The median proportion of patients undergoing primary VATS across all hospitals was 11.5% (IQR: 3.9%‐26.5%) (Figure 1). The median time to first procedure was 1 day (IQR: 03 days).
Outcome Measures
Variation in outcomes occurred across hospitals. Additional pleural drainage procedures were performed in a median of 20.9% of patients with a range of 6.8% to 44.8% (IQR: 14.5%‐25.3%) of patients across all hospitals. Median LOS was 10 days with a range of 714 days (IQR: 8.511 days) and the median LOS following the initial pleural fluid drainage procedure was 8 days with a range of 6 to 13 days (IQR: 78 days). Variation in timing of the initial pleural fluid drainage procedure explained 9.6% of the variability in LOS (Spearman rho, 0.31; P < 0.001).
Overall, 118 (3.4%) patients were readmitted within 14 days of index discharge; the median readmission rate was 3.8% with a range of 0.8% to 33.3% (IQR: 2.1%‐5.8%) across hospitals. The median total cost of the index hospitalization was $19,574 (IQR: $13,791‐$31,063). The total cost for the index hospitalization exceeded $54,215 for 10% of patients and the total cost of the episode exceeded $55,208 for 10% of patients. Unadjusted outcomes, stratified by primary pleural fluid drainage procedure, are summarized in Table 2.
| Overall | Chest Tube Without Fibrinolysis | Chest Tube With Fibrinolysis | Thoracotomy | VATS | P Valueb | |
|---|---|---|---|---|---|---|
| ||||||
| Additional Procedure | 716 (20.5) | 331 (19.8) | 144 (23.1) | 197 (24.7) | 44 (10.8) | <0.001 |
| Readmission within 14 days | 118 (3.4) | 54 (3.3) | 13 (2.1) | 32 (4.0) | 19 (4.7) | 0.096 |
| Total LOS (days) | 10 (7, 14) | 10 (7, 14) | 9 (7, 13) | 10 (7, 14) | 9 (7, 12) | <.001 |
| Post‐initial Procedure LOS (days) | 8 (5, 12) | 8 (6, 12) | 7 (5, 10) | 8 (5, 12) | 7 (5, 10) | <0.001 |
| Total Cost, Index Hospitalization ($)e | 19319 (13358, 30955) | 19951 (13576, 32018)c | 19565 (13209, 32778)d | 20352 (14351, 31343) | 17918 (13531, 25166) | 0.016 |
| Total Cost, Episode of Illness ($)e | 19831 (13927, 31749) | 20151 (13764, 32653) | 19593 (13210, 32861) | 20573 (14419, 31753) | 18344 (13835, 25462) | 0.029 |
In multivariable analysis, differences in total LOS and post‐procedure LOS were not significant (Table 3). The odds of additional drainage procedures were higher for all drainage procedures compared with initial VATS (Table 3). Patients undergoing initial chest tube placement with fibrinolysis were less likely to require readmission compared with patients undergoing initial VATS (Table 3). The total cost for the episode of illness (including the cost of readmission) was significantly less for those undergoing primary chest tube placement without fibrinolysis compared with primary VATS. The results of subanalyses excluding patients with an underlying CCC (Supporting Appendix online, Table 4) and restricting the cohort to patients undergoing pleural drainage within two days of admission (Supporting Appendix online, Table 5) were similar to the results of our primary analysis with one exception; in the latter subanalysis, children undergoing initial chest tube placement without fibrinolysis were also less likely to require readmission compared with patients undergoing initial VATS.
| Adjusted OR (95% CI)a | P Value | |
|---|---|---|
| ||
| Additional pleural drainage procedure | ||
| Chest tube without fibrinolysis | 1.82 (1.103.00) | .019 |
| Chest tube with fibrinolysis | 2.31 (1.443.72) | <0.001 |
| Thoracotomy | 2.59 (1.624.14) | <0.001 |
| VATS | Reference | |
| Readmission within 14 days | ||
| Chest tube without fibrinolysis | 0.61 (0.361.05) | .077 |
| Chest tube with fibrinolysis | 0.45 (0.230.86) | .015 |
| Thoracotomy | 0.85 (0.521.39) | .521 |
| VATS | Reference | |
| Adjusted Mean (95% CI)a | P Value | |
| Total LOS (days) | ||
| Chest tube without fibrinolysis | 8.0 (7.88.2) | .339 |
| Chest tube with fibrinolysis | 8.1 (7.98.3) | .812 |
| Thoracotomy | 8.1 (7.98.3) | .632 |
| VATS | 8.1 (7.98.3) | Ref |
| Post‐initial procedure LOS (days) | ||
| Chest tube without fibrinolysis | 7.3 (7.07.5) | .512 |
| Chest tube with fibrinolysis | 7.5 (7.27.8) | .239 |
| Thoracotomy | 7.3 (7.07.6) | .841 |
| VATS | 7.3 (7.17.6) | Reference |
| Total cost, index hospitalization ($) | ||
| Chest tube without fibrinolysis | 22928 (2200023895 | .012 |
| Chest tube with fibrinolysis | 23621 (2263124655) | .657 |
| Thoracotomy | 23386 (2241924395 | .262 |
| VATS | 23820 (2280824878) | Reference |
| Total cost, episode of illness ($) | ||
| Chest tube without fibrinolysis | 23218 (2227824199) | .004 |
| Chest tube with fibrinolysis | 23749 (2275224790) | .253 |
| Thoracotomy | 23673 (2269324696) | .131 |
| VATS | 24280 (2324425362) | Reference |
Discussion
This multicenter study is the largest to evaluate the management of children hospitalized with complicated pneumonia. We found considerable variation in initial management and outcomes across hospitals. Differences in timing of the initial drainage procedure explained only a small amount of the variability in outcomes. Children undergoing initial VATS less commonly required additional drainage procedures while children undergoing initial chest tube placement with fibrinolysis less commonly required readmission. Differences in total and post‐procedure LOS were not statistically significant. Differences in cost, while statistically significant, were of marginal relevance.
Previous studies have also shown significant variation in treatment and outcomes of children with complicated pneumonia across hospitals.2, 8 Our study provides data from additional hospitals, includes a substantially larger number of patients undergoing initial VATS, distinguishes between fibrinolysis recipients and nonrecipients, and is the first to compare outcomes between four different initial drainage strategies. The creation of national consensus guidelines might reduce variability in initial management strategies, although the variability in outcomes across hospitals in the current study could not be explained simply by differences in the type or timing of the initial drainage procedure. Thus, future studies examining hospital‐level factors may play an important role in improving quality of care for children with complicated pneumonia.
Patients with initial thoracotomy or chest tube placement with or without fibrinolysis more commonly received additional drainage procedures than patients with initial VATS. This difference remained when patients with CCCs were excluded from the analysis and when the analysis was limited to patients undergoing pleural fluid drainage within 2 days of hospitalization. Several small, randomized trials demonstrated conflicting results when comparing initial chest tube placement with fibrinolysis and VATS. St. Peter et al22 reported that 3 (17%) of 18 patients undergoing initial chest tube placement with fibrinolysis and none of the 18 patients undergoing initial VATS received additional pleural drainage procedures. Sonnappa et al21 found no differences between the two groups. Kurt et al19 did not state the proportion of patients receiving additional procedures. However, the mean number of drainage procedures was 2.25 among the 8 patients undergoing initial chest tube placement while none of the 10 patients with VATS received additional drainage.19
Thoracotomy is often perceived as a definitive procedure for treatment of complicated pneumonia. However, several possibilities exist to explain why additional procedures were performed less frequently in patients undergoing initial VATS compared with initial thoracotomy. The limited visual field in thoracotomy may lead to greater residual disease post‐operatively in those receiving thoracotomy compared with VATS.31 Additionally, thoracotomy substantially disrupts the integrity of the chest wall and is consequently associated with complications such as bleeding and air leak into the pleural cavity more often than VATS.31, 32 It is thus possible that some of the additional procedures in patients receiving initial thoracotomy were necessary for management of thoracotomy‐associated complications rather than for failure of the initial drainage procedure.
Similar to the randomized trials by Sonnappa et al21 and St. Peter et al,22 differences in the overall and post‐procedure LOS were not significant among patients undergoing initial VATS compared with initial chest tube placement with fibrinolysis. However, chest tube placement without fibrinolysis did not result in significant differences in LOS compared with initial VATS. In the only pediatric randomized trial, the 29 intrapleural urokinase recipients had a 2 day shorter LOS compared with the 29 intrapleural saline recipients.33 Several small, randomized controlled trials of adults with complicated pneumonia reported improved pleural fluid drainage among intrapleural fibrinolysis recipients compared with non‐recipients.3436 However, a large multicenter randomized trial in adults found no differences in mortality, requirement for surgical drainage, or LOS between intrapleural streptokinase and placebo recipients.37 Subsequent meta‐analyses of randomized trials in adults also demonstrated no benefit to fibrinolysis.38, 39 In the context of the increasing use of intrapleural fibrinolysis in children with complicated pneumonia, our results highlight the need for a large, multicenter randomized controlled trial to determine whether chest tube with fibrinolysis is superior to chest tube alone.
Two small randomized trials21, 22 and a decision analysis40 identified chest tube with fibrinolysis as the most economical approach to children with complicated pneumonia. However, the costs did not differ significantly between patients undergoing initial VATS or initial chest tube placement with fibrinolysis in our study. The least costly approach was initial chest tube placement without fibrinolysis. Unlike the randomized controlled trials, we considered costs associated with readmissions in determining the total costs. Shah et al41 found no difference in total charges for patients undergoing initial VATS compared with initial chest tube placement; however, patients undergoing initial VATS were concentrated in a few centers, making it difficult to determine the relative importance of procedural and hospital factors.
This multicenter observational study has several limitations. First, discharge diagnosis coding may be unreliable for specific diseases. However, our rigorous definition of complicated pneumonia, supported by the high positive predictive value as verified by medical record review, minimizes the likelihood of misclassification.
Second, unmeasured confounding or residual confounding by indication for the method of pleural drainage may occur, potentially influencing our results in two disparate ways. If patients with more severe systemic illness were too unstable for operative interventions, then our results would be biased towards worse outcomes for children undergoing initial chest tube placement. We adjusted for several variables associated with a greater systemic severity of illness, including intensive care unit admission, making this possibility less likely. We also could not account for some factors associated with more severe local disease such as the size and character of the effusion. We suspect that patients with more extensive local disease (ie, loculated effusions) would have worse outcomes than other patients, regardless of initial procedure, and that these patients would also be more likely to undergo primary surgical drainage. Thus, this study may have underestimated the benefit of initial surgical drainage (eg, VATS) compared with nonsurgical drainage (ie, chest tube placement).
Third, misclassification of the method of initial pleural drainage may have occurred. Patients transferred from another institution following chest tube placement could either be classified as not receiving pleural drainage and thus excluded from the study or classified as having initial VATS or thoracotomy if the reason for transfer was chest tube treatment failure. Additionally, we could not distinguish routine use of fibrinolysis from fibrinolysis to maintain chest tube patency. Whether such misclassification would falsely minimize or maximize differences in outcomes between the various groups remains uncertain. Fourth, because this study only included tertiary care children's hospitals, these data are not generalizable to community settings. VATS requires specialized surgical training that may be unavailable in some areas. Finally, this study demonstrates the relative efficacy of various pleural fluid drainage procedures on short‐term clinical outcomes and resource utilization. However, long‐term functional outcomes should be measured in future prospective studies.
Conclusions
In conclusion, emphasis on evidence driven treatment to optimize care has led to an increasing examination of unwarranted practice variation.42 The lack of evidence for best practice makes it difficult to define unwarranted variation in the treatment of complicated pneumonia. Our study demonstrates the large variability in practice and raises additional questions regarding the optimal drainage strategies. Published randomized trials have focused on comparisons between chest tube placement with fibrinolysis and VATS. However, our data suggest that future randomized trials should include chest tube placement without fibrinolysis as a treatment strategy. In determining the current best treatment for patients with complicated pneumonia, a clinician must weigh the impact of needing an additional procedure in approximately one‐quarter of patients undergoing initial chest tube placement (with or without fibrinolysis) with the risks of general anesthesia and readmission in patients undergoing initial VATS.
Acknowledgements
Dr. Hall had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the analysis.
- ,.Parapneumonic pleural effusion and empyema in children. Review of a 19‐year experience, 1962–1980.Clin Pediatr (Phila).1983;22:414–419.
- ,,,,.Primary early thoracoscopy and reduction in length of hospital stay and additional procedures among children with complicated pneumonia: Results of a multicenter retrospective cohort study.Arch Pediatr Adolesc Med.2008;162:675–681.
- ,.Empyema hospitalizations increased in US children despite pneumococcal conjugate vaccine.Pediatrics.2010;125:26–33.
- ,,, et al.Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema.Pediatr Infect Dis J.2006;25:250–254.
- ,,,,.Five‐fold increase in pediatric parapneumonic empyema since introduction of pneumococcal conjugate vaccine.Pediatric Infect Dis J.2008;27:1030–1032.
- ,,,.Increasing incidence of empyema complicating childhood community‐acquired pneumonia in the United States.Clin Infect Dis.2010;50:805–813.
- ,,,,.National hospitalization trends for pediatric pneumonia and associated complications.Pediatrics.2010;126:204–213.
- ,,, et al.Empyema associated with community‐acquired pneumonia: A Pediatric Investigator's Collaborative Network on Infections in Canada (PICNIC) study.BMC Infect Dis.2008;8:129.
- ,,,,.Pleural empyema in children.Ann Thorac Surg.1970;10:37–44.
- ,,.Management of streptococcal empyema.Ann Thorac Surg.1966;2:658–664.
- ,.Thoracoscopy in the management of empyema in children.J Pediatr Surg.1993;28:1128–1132.
- ,,,.Surgical treatment of parapneumonic empyema.Pediatr Pulmonol.1996;22:348–356.
- ,.The controversial role of decortication in the management of pediatric empyema.J Thorac Cardiovasc Surg.1988;96:166–170.
- ,,,,,.Postpneumonic empyema in children treated by early decortication.Eur J Pediatr Surg.1997;7:135–137.
- ,.Video‐assisted thoracoscopic surgery in the management of pediatric empyema.JSLS.1997;1:251–3.
- ,,,.Early video‐assisted thoracic surgery in the management of empyema.Pediatrics.1999;103:e63.
- ,,,,.Early definitive intervention by thoracoscopy in pediatric empyema.J Pediatr Surg.1999;34:178–180; discussion80–81.
- ,,,,.Thoracoscopy in the management of pediatric empyema.J Pediatr Surg.1995;30:1211–1215.
- ,,,,.Therapy of parapneumonic effusions in children: Video‐assisted thoracoscopic surgery versus conventional thoracostomy drainage.Pediatrics.2006;118:e547–e553.
- ,.Primary operative management for pediatric empyema: Decreases in hospital length of stay and charges in a national sample.Arch Pediatr Adolesc Med.2008;162:44–48.
- ,,, et al.Comparison of urokinase and video‐assisted thoracoscopic surgery for treatment of childhood empyema.Am J Respir Crit Care Med.2006;174:221–227.
- ,,, et al.Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: A prospective, randomized trial.J Pediatr Surg.2009;44:106–111; discussion11.
- ,,,.Corticosteroids and mortality in children with bacterial meningitis.JAMA.2008;299:2048–2055.
- ,,,,.Intravenous immunoglobulin in children with streptococcal toxic shock syndrome.Clin Infect Dis.2009;49:1369–1376.
- ,,, et al.Deaths attributed to pediatric complex chronic conditions: National trends and implications for supportive care services.Pediatrics.2001;107:e99.
- ,.Multiple regression of cost data: Use of generalised linear models.J Health Serv Res Policy.2004;9:197–204.
- ,,,.A robustified modeling approach to analyze pediatric length of stay.Ann Epidemiol.2005;15:673–677.
- ,,.Correlates of length of stay, cost of care, and mortality among patients hospitalized for necrotizing fasciitis.Epidemiol Infect.2007;135:868–876.
- ,,,,,.Health care costs of adults treated for attention‐deficit/hyperactivity disorder who received alternative drug therapies.J Manag Care Pharm.2007;13:561–569.
- .The role of the propensity score in estimating dose‐response functions.Biometrika.2000;87:706–710.
- ,,,,.Experience with video‐assisted thoracoscopic surgery in the management of complicated pneumonia in children.J Pediatr Surg.2001;36:316–319.
- ,,, et al.VATS debridement versus thoracotomy in the treatment of loculated postpneumonia empyema.Ann Thorac Surg.1996;61:1626–1630.
- ,,,,.Randomised trial of intrapleural urokinase in the treatment of childhood empyema.Thorax.2002;57:343–347.
- ,,,,,.Intrapleural urokinase versus normal saline in the treatment of complicated parapneumonic effusions and empyema. A randomized, double‐blind study.Am J Respir Crit Care Med.1999;159:37–42.
- ,,.Randomised controlled trial of intrapleural streptokinase in community acquired pleural infection.Thorax.1997;52:416–421.
- ,,,,.Intrapleural streptokinase for empyema and complicated parapneumonic effusions.Am J Respir Crit Care Med.2004;170:49–53.
- ,,, et al.U.K. Controlled trial of intrapleural streptokinase for pleural infection.N Engl J Med.2005;352:865–874.
- ,.Intra‐pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema.Cochrane Database Syst Rev.2008:CD002312.
- ,,,.Intrapleural fibrinolytic agents for empyema and complicated parapneumonic effusions: A meta‐analysis.Chest.2006;129:783–790.
- ,,.Cost‐effectiveness of competing strategies for the treatment of pediatric empyema.Pediatrics.2008;121:e1250–e1257.
- ,,.Costs of treating children with complicated pneumonia: A comparison of primary video‐assisted thoracoscopic surgery and chest tube placement.Pediatr Pulmonol.2010;45:71–77.
- .Unwarranted variation in pediatric medical care.Pediatr Clin North Am.2009;56:745–755.
Community‐acquired pneumonia, the most common serious bacterial infection in childhood, may be complicated by parapneumonic effusion (ie, complicated pneumonia).1 Children with complicated pneumonia require prolonged hospitalization and frequently undergo multiple pleural fluid drainage procedures.2 Additionally, the incidence of complicated pneumonia has increased,37 making the need to define appropriate therapy even more pressing. Defining appropriate therapy is challenging for the individual physician as a result of inconsistent and insufficient evidence, and wide variation in treatment practices.2, 8
Historically, thoracotomy was performed only if initial chest tube placement did not lead to clinical improvement.9, 10 Several authors, noting the rapid resolution of symptoms in children undergoing earlier thoracotomy, advocated for the use of thoracotomy as initial therapy rather than as a procedure of last resort.114 The advent of less invasive techniques such as video‐assisted thoracoscopic surgery (VATS) has served as an additional impetus to consider surgical drainage as the initial treatment strategy.1518 Few well‐designed studies have examined the relative efficacy of these interventions.2, 1922 Published randomized trials were single center, enrolled few patients, and arrived at different conclusions.19, 21, 22 In addition, these trials did not examine other important outcomes such as requirement for additional pleural fluid drainage procedures and hospital readmission. Two large retrospective multicenter studies found modest reductions in length of stay (LOS) and substantial decreases in the requirement for additional pleural fluid drainage procedures in children undergoing initial VATS compared with initial chest tube placement.2, 20 However, Shah et al2 included relatively few patients undergoing VATS. Li et al20 combined patients undergoing initial thoracentesis, initial chest tube placement, late pleural fluid drainage (by any method), and no pleural fluid drainage into a single non‐operative management category, precluding conclusions about the relative benefits of chest tube placement compared with VATS. Neither study2, 20 examined the role of chemical fibrinolysis, a therapy which has been associated with outcomes comparable to VATS in two small randomized trials.21, 22
The objectives of this multicenter study were to describe the variation in the initial management strategy along with associated outcomes of complicated pneumonia in childhood and to determine the comparative effectiveness of different pleural fluid drainage procedures.
Methods
Data Source
The Pediatric Health Information System (PHIS), which contains resource utilization data from 40 freestanding children's hospitals, provided data for this multicenter retrospective cohort study. Participating hospitals are located in noncompeting markets of 27 states plus the District of Columbia. The PHIS database includes patient demographics, diagnoses, and procedures as well as data for all drugs, radiologic studies, laboratory tests, and supplies charged to each patient. Data are de‐identified, however encrypted medical record numbers allow for tracking individual patients across admissions. The Child Health Corporation of America (Shawnee Mission, KS) and participating hospitals jointly assure data quality and reliability as described previously.23, 24 The Children's Hospital of Philadelphia Institutional Review Board reviewed and approved this study.
Patients
Children 18 years of age receiving a pleural drainage procedure for complicated pneumonia were eligible if they were discharged from participating hospitals between January 1, 2004 and June 30, 2009. Study participants met the following criteria: 1) discharge diagnosis of pneumonia (International Classification of Diseases, 9th revision [ICD‐9] discharge diagnosis codes 480.x‐483.x, 485.x‐487.x), 2) discharge diagnosis of pleural effusion (ICD‐9 codes 510.0, 510.9, 511.0, 511.1, or 511.9), and 3) billing charge for antibiotics on the first day of hospitalization. Additionally, the primary discharge diagnosis had to be either pneumonia or pleural effusion. Patients were excluded if they did not undergo pleural fluid drainage or if their initial pleural fluid drainage procedure was thoracentesis.
Study Definitions
Pleural drainage procedures were identified using ICD‐9 procedure codes for thoracentesis (34.91), chest tube placement (34.04), VATS (34.21), and thoracotomy (34.02 or 34.09). Fibrinolysis was defined as receipt of urokinase, streptokinase, or alteplase within two days of initial chest tube placement.
Acute conditions or complications included influenza (487, 487.0, 487.1, 487.8, 488, or V04.81) and hemolytic‐uremic syndrome (283.11). Chronic comorbid conditions (CCCs) (eg, malignancy) were identified using a previously reported classification scheme.25 Billing data were used to classify receipt of mechanical ventilation and medications on the first day of hospitalization.
Measured Outcomes
The primary outcomes were hospital LOS (both overall and post‐initial procedure), requirement for additional pleural drainage procedures, total cost for index hospitalization, all‐cause readmission within 14 days after index hospital discharge, and total cost of the episode (accounting for the cost of readmissions).
Measured Exposures
The primary exposure of interest was the initial pleural fluid drainage procedure, classified as chest tube placement without fibrinolysis, chest tube placement with fibrinolysis, VATS, or thoracotomy.
Statistical Analysis
Variables were summarized using frequencies and percentages for categorical variables, and median, interquartile range (IQR), and range for continuous variables. Outcomes by initial pleural drainage procedure were compared using chi‐squared tests for categorical variables and Kruskal‐Wallis tests for continuous variables.
Multivariable analysis was performed to account for potential confounding by observed baseline variables. For dichotomous outcome variables, modeling consisted of logistic regression using generalized estimating equations to account for hospital clustering. For continuous variables, a mixed model approach was used, treating hospital as a random effect. Log transformation was applied to the right‐skewed outcome variables (LOS and cost). Cost outcomes remained skewed following log transformation, thus gamma mixed models were applied.2629 Odds ratios and 95% confidence intervals (CIs) were reported for comparison of dichotomous outcomes and the adjusted means and 95% CIs were reported for continuous outcomes after appropriate back transformation.
Additional analyses addressed the potential impact of confounding by indication inherent in any observational study. First, patients with an underlying CCC were excluded to ensure that our results would be generalizable to otherwise healthy children with community‐acquired pneumonia. Second, patients undergoing pleural drainage >2 days after hospitalization were excluded to minimize the effect of residual confounding related to differences in timing of the initial drainage procedure. Third, the analysis was repeated using a generalized propensity score as an additional method to account for confounding by indication for the initial drainage procedure.30 Propensity scores, constructed using a multivariable generalized logit model, included all variables listed in Table 1. The inverse of the propensity score was included as a weight in each multivariable model described previously. Only the primary multivariable analyses are presented as the results of the propensity score analysis were nearly identical to the primary analyses.
| Overall | Chest Tube Without Fibrinolysis | Chest Tube With Fibrinolysis | Thoracotomy | VATS | P Value | |
|---|---|---|---|---|---|---|
| ||||||
| N | 3500 | 1672 (47.8) | 623 (17.8) | 797 (22.8) | 408 (11.7) | |
| Age | ||||||
| <1 year | 335 (9.6) | 176 (10.5) | 56 (9.0) | 78 (9.8) | 25 (6.1) | |
| 1 year | 475 (13.6) | 238 (14.2) | 98 (15.7) | 92 (11.5) | 47 (11.5) | 0.003 |
| 24 years | 1230 (35.1) | 548 (32.8) | 203 (32.6) | 310 (38.9) | 169 (41.4) | |
| 59 years | 897 (25.6) | 412 (24.6) | 170 (27.3) | 199 (25.0) | 116 (28.4) | |
| 1014 years | 324 (9.3) | 167 (10.0) | 61 (9.8) | 65 (8.2) | 31 (7.6) | |
| 1518 years | 193 (5.5) | 106 (6.3) | 29 (4.6) | 40 (5.0) | 18 (4.4) | |
| >18 years | 46 (1.3) | 25 (1.5) | 6 (0.96) | 13 (1.6) | 2 (0.5) | |
| Comorbid Conditions | ||||||
| Cardiac | 69 (2.0) | 43 (2.6) | 14 (2.3) | 12 (1.5) | 0 (0.0) | 0.006 |
| Malignancy | 81 (2.3) | 31 (1.9) | 18 (2.9) | 21 (2.6) | 11 (2.7) | 0.375 |
| Neurological | 138 (3.9) | 73 (4.4) | 20 (3.2) | 34 (4.3) | 11 (2.7) | 0.313 |
| Any Other Condition | 202 (5.8) | 96 (5.7) | 40 (6.4) | 47 (5.9) | 19 (4.7) | 0.696 |
| Payer | ||||||
| Government | 1240 (35.6) | 630 (37.8) | 224 (36.0) | 259 (32.7) | 127 (31.3) | <0.001 |
| Private | 1383 (39.7) | 607 (36.4) | 283 (45.4) | 310 (39.2) | 183 (45.07) | |
| Other | 864 (24.8) | 430 (25.8) | 116 (18.6) | 222 (28.1) | 96 (23.65) | |
| Race | ||||||
| Non‐Hispanic White | 1746 (51.9) | 838 (51.6) | 358 (59.7) | 361 (47.8) | 189 (48.7) | <0.001 |
| Non‐Hispanic Black | 601 (17.9) | 318 (19.6) | 90 (15.0) | 128 (17.0) | 65 (16.8) | |
| Hispanic | 588 (17.5) | 280 (17.3) | 73 (12.2) | 155 (20.5) | 80 (20.6) | |
| Asian | 117 (3.5) | 47 (2.9) | 20 (3.3) | 37 (4.9) | 13 (3.4) | |
| Other | 314 (9.3) | 140 (8.6) | 59 (9.8) | 74 (9.8) | 41 (10.6) | |
| Male Sex | 1912 (54.6) | 923 (55.2) | 336 (53.9) | 439 (55.1) | 214 (52.5) | 0.755 |
| Radiology | ||||||
| CT, no US | 1200 (34.3) | 600 (35.9) | 184 (29.5) | 280 (35.1) | 136 (33.3) | <0.001 |
| CT and US | 221 (6.3) | 84 (5.0) | 53 (8.5) | 61 (7.7) | 23 (5.6) | |
| US, no CT | 799 (22.8) | 324 (19.4) | 178 (28.6) | 200 (25.1) | 97 (23.8) | |
| No US, no CT | 1280 (36.6) | 664 (39.7) | 208 (33.4) | 256 (32.1) | 152 (37.3) | |
| Empiric Antibiotic Regimen | ||||||
| Cephalosporins alone | 448 (12.8) | 181 (10.83) | 126 (20.2) | 73 (9.2) | 68 (16.7) | <0.001 |
| Cephalosporin and clindamycin | 797 (22.8) | 359 (21.5) | 145 (23.3) | 184 (23.1) | 109 (26.7) | |
| Other antibiotic combination | 167 (4.8) | 82 (4.9) | 30 (4.8) | 38 (4.8) | 17 (4.2) | |
| Cephalosporin and vancomycin | 2088 (59.7) | 1050 (62.8) | 322 (51.7) | 502 (63.0) | 214 (52.5) | |
| Mechanical ventilation | 494 (14.1) | 251 (15.0) | 75 (12.0) | 114 (14.3) | 54 (13.2) | 0.307 |
| Corticosteroids | 520 (14.9) | 291 (17.4) | 72 (11.6) | 114 (14.3) | 43 (10.5) | <0.001 |
| Blood product transfusionsb | 761 (21.7) | 387 (23.2) | 145 (23.3) | 161 (20.2) | 68 (16.7) | 0.018 |
| Vasoactive infusionsc | 381 (10.9) | 223 (13.3) | 63 (10.1) | 72 (9.0) | 23 (5.6) | <0.001 |
| Admission to intensive care | 1397 (39.9) | 731 (43.7) | 234 (37.6) | 296 (37.1) | 136 (33.3) | <0.001 |
| Extracorporeal membranous oxygenation | 18 (0.5) | 13 (0.8) | 2 (0.3) | 3 (0.4) | 0 (0.0) | 0.163 |
| Hemolytic‐uremic syndrome | 31 (0.9) | 15 (0.9) | 6 (1.0) | 7 (0.9) | 3 (0.7) | 0.985 |
| Influenza | 108 (3.1) | 53 (3.2) | 27 (4.3) | 23 (2.9) | 5 (1.2) | 0.044 |
| Arterial blood gas measurements | 0 (0,1) | 0 (0, 2) | 0 (0,1) | 0 (0, 1) | 0 (0, 1) | <0.001 |
| Days to first procedure | 1 (0, 3) | 1 (0, 2) | 1 (1, 3) | 1 (1, 3) | 1 (1, 3) | <0.001 |
Medical records of a randomly selected subset of subjects from 6 hospitals were reviewed to determine the accuracy of our algorithm in identifying patients with complicated pneumonia; these subjects represented 1% of the study population. For the purposes of medical record review, complicated pneumonia was defined by the following: 1) radiologically‐confirmed lung infiltrate; 2) moderate or large pleural effusion; and 3) signs and symptoms of lower respiratory tract infection. Complicated pneumonia was identified in 118 of 120 reviewed subjects for a positive predictive value of 98.3%.
All analyses were clustered by hospital. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). A two‐tailed P < 0.05 was considered statistically significant.
Results
Patient Characteristics
During the study period, 9,680 subjects had complicated pneumonia. Subjects were excluded if they did not have a pleural drainage procedure (n = 5798), or if thoracentesis was the first pleural fluid drainage procedure performed (n = 382). The remaining 3500 patients were included. Demographic characteristics are summarized in Table 1. The median patient age was 4.1 years (IQR: 2.17.2 years). An underlying CCC was present in 424 (12.1%) patients. There was no association between type of drainage procedure and mechanical ventilation. However, factors associated with more severe systemic illness, such as blood product transfusion, were more common among those undergoing initial chest tube placement with or without fibrinolysis (Table 1).
Initial Pleural Fluid Drainage Procedures
The primary procedures included chest tube without fibrinolysis (47.8%); chest tube with fibrinolysis (17.8%); thoracotomy (22.8%); and VATS (11.7%) (Table 1). The proportion of patients undergoing primary chest tube placement with fibrinolysis increased over time from 14.2% in 2004 to 30.0% in 2009 (P < 0.001; chi‐squared test for trend). The initial procedure varied by hospital with the greatest proportion of patients undergoing primary chest tube placement without fibrinolysis at 28 (70.0%) hospitals, chest tube placement with fibrinolysis at 5 (12.5%) hospitals, thoracotomy at 5 (12.5%) hospitals, and VATS at 2 (5.0%) hospitals (Figure 1). The median proportion of patients undergoing primary VATS across all hospitals was 11.5% (IQR: 3.9%‐26.5%) (Figure 1). The median time to first procedure was 1 day (IQR: 03 days).

Outcome Measures
Variation in outcomes occurred across hospitals. Additional pleural drainage procedures were performed in a median of 20.9% of patients with a range of 6.8% to 44.8% (IQR: 14.5%‐25.3%) of patients across all hospitals. Median LOS was 10 days with a range of 714 days (IQR: 8.511 days) and the median LOS following the initial pleural fluid drainage procedure was 8 days with a range of 6 to 13 days (IQR: 78 days). Variation in timing of the initial pleural fluid drainage procedure explained 9.6% of the variability in LOS (Spearman rho, 0.31; P < 0.001).
Overall, 118 (3.4%) patients were readmitted within 14 days of index discharge; the median readmission rate was 3.8% with a range of 0.8% to 33.3% (IQR: 2.1%‐5.8%) across hospitals. The median total cost of the index hospitalization was $19,574 (IQR: $13,791‐$31,063). The total cost for the index hospitalization exceeded $54,215 for 10% of patients and the total cost of the episode exceeded $55,208 for 10% of patients. Unadjusted outcomes, stratified by primary pleural fluid drainage procedure, are summarized in Table 2.
| Overall | Chest Tube Without Fibrinolysis | Chest Tube With Fibrinolysis | Thoracotomy | VATS | P Valueb | |
|---|---|---|---|---|---|---|
| ||||||
| Additional Procedure | 716 (20.5) | 331 (19.8) | 144 (23.1) | 197 (24.7) | 44 (10.8) | <0.001 |
| Readmission within 14 days | 118 (3.4) | 54 (3.3) | 13 (2.1) | 32 (4.0) | 19 (4.7) | 0.096 |
| Total LOS (days) | 10 (7, 14) | 10 (7, 14) | 9 (7, 13) | 10 (7, 14) | 9 (7, 12) | <.001 |
| Post‐initial Procedure LOS (days) | 8 (5, 12) | 8 (6, 12) | 7 (5, 10) | 8 (5, 12) | 7 (5, 10) | <0.001 |
| Total Cost, Index Hospitalization ($)e | 19319 (13358, 30955) | 19951 (13576, 32018)c | 19565 (13209, 32778)d | 20352 (14351, 31343) | 17918 (13531, 25166) | 0.016 |
| Total Cost, Episode of Illness ($)e | 19831 (13927, 31749) | 20151 (13764, 32653) | 19593 (13210, 32861) | 20573 (14419, 31753) | 18344 (13835, 25462) | 0.029 |
In multivariable analysis, differences in total LOS and post‐procedure LOS were not significant (Table 3). The odds of additional drainage procedures were higher for all drainage procedures compared with initial VATS (Table 3). Patients undergoing initial chest tube placement with fibrinolysis were less likely to require readmission compared with patients undergoing initial VATS (Table 3). The total cost for the episode of illness (including the cost of readmission) was significantly less for those undergoing primary chest tube placement without fibrinolysis compared with primary VATS. The results of subanalyses excluding patients with an underlying CCC (Supporting Appendix online, Table 4) and restricting the cohort to patients undergoing pleural drainage within two days of admission (Supporting Appendix online, Table 5) were similar to the results of our primary analysis with one exception; in the latter subanalysis, children undergoing initial chest tube placement without fibrinolysis were also less likely to require readmission compared with patients undergoing initial VATS.
| Adjusted OR (95% CI)a | P Value | |
|---|---|---|
| ||
| Additional pleural drainage procedure | ||
| Chest tube without fibrinolysis | 1.82 (1.103.00) | .019 |
| Chest tube with fibrinolysis | 2.31 (1.443.72) | <0.001 |
| Thoracotomy | 2.59 (1.624.14) | <0.001 |
| VATS | Reference | |
| Readmission within 14 days | ||
| Chest tube without fibrinolysis | 0.61 (0.361.05) | .077 |
| Chest tube with fibrinolysis | 0.45 (0.230.86) | .015 |
| Thoracotomy | 0.85 (0.521.39) | .521 |
| VATS | Reference | |
| Adjusted Mean (95% CI)a | P Value | |
| Total LOS (days) | ||
| Chest tube without fibrinolysis | 8.0 (7.88.2) | .339 |
| Chest tube with fibrinolysis | 8.1 (7.98.3) | .812 |
| Thoracotomy | 8.1 (7.98.3) | .632 |
| VATS | 8.1 (7.98.3) | Ref |
| Post‐initial procedure LOS (days) | ||
| Chest tube without fibrinolysis | 7.3 (7.07.5) | .512 |
| Chest tube with fibrinolysis | 7.5 (7.27.8) | .239 |
| Thoracotomy | 7.3 (7.07.6) | .841 |
| VATS | 7.3 (7.17.6) | Reference |
| Total cost, index hospitalization ($) | ||
| Chest tube without fibrinolysis | 22928 (2200023895 | .012 |
| Chest tube with fibrinolysis | 23621 (2263124655) | .657 |
| Thoracotomy | 23386 (2241924395 | .262 |
| VATS | 23820 (2280824878) | Reference |
| Total cost, episode of illness ($) | ||
| Chest tube without fibrinolysis | 23218 (2227824199) | .004 |
| Chest tube with fibrinolysis | 23749 (2275224790) | .253 |
| Thoracotomy | 23673 (2269324696) | .131 |
| VATS | 24280 (2324425362) | Reference |
Discussion
This multicenter study is the largest to evaluate the management of children hospitalized with complicated pneumonia. We found considerable variation in initial management and outcomes across hospitals. Differences in timing of the initial drainage procedure explained only a small amount of the variability in outcomes. Children undergoing initial VATS less commonly required additional drainage procedures while children undergoing initial chest tube placement with fibrinolysis less commonly required readmission. Differences in total and post‐procedure LOS were not statistically significant. Differences in cost, while statistically significant, were of marginal relevance.
Previous studies have also shown significant variation in treatment and outcomes of children with complicated pneumonia across hospitals.2, 8 Our study provides data from additional hospitals, includes a substantially larger number of patients undergoing initial VATS, distinguishes between fibrinolysis recipients and nonrecipients, and is the first to compare outcomes between four different initial drainage strategies. The creation of national consensus guidelines might reduce variability in initial management strategies, although the variability in outcomes across hospitals in the current study could not be explained simply by differences in the type or timing of the initial drainage procedure. Thus, future studies examining hospital‐level factors may play an important role in improving quality of care for children with complicated pneumonia.
Patients with initial thoracotomy or chest tube placement with or without fibrinolysis more commonly received additional drainage procedures than patients with initial VATS. This difference remained when patients with CCCs were excluded from the analysis and when the analysis was limited to patients undergoing pleural fluid drainage within 2 days of hospitalization. Several small, randomized trials demonstrated conflicting results when comparing initial chest tube placement with fibrinolysis and VATS. St. Peter et al22 reported that 3 (17%) of 18 patients undergoing initial chest tube placement with fibrinolysis and none of the 18 patients undergoing initial VATS received additional pleural drainage procedures. Sonnappa et al21 found no differences between the two groups. Kurt et al19 did not state the proportion of patients receiving additional procedures. However, the mean number of drainage procedures was 2.25 among the 8 patients undergoing initial chest tube placement while none of the 10 patients with VATS received additional drainage.19
Thoracotomy is often perceived as a definitive procedure for treatment of complicated pneumonia. However, several possibilities exist to explain why additional procedures were performed less frequently in patients undergoing initial VATS compared with initial thoracotomy. The limited visual field in thoracotomy may lead to greater residual disease post‐operatively in those receiving thoracotomy compared with VATS.31 Additionally, thoracotomy substantially disrupts the integrity of the chest wall and is consequently associated with complications such as bleeding and air leak into the pleural cavity more often than VATS.31, 32 It is thus possible that some of the additional procedures in patients receiving initial thoracotomy were necessary for management of thoracotomy‐associated complications rather than for failure of the initial drainage procedure.
Similar to the randomized trials by Sonnappa et al21 and St. Peter et al,22 differences in the overall and post‐procedure LOS were not significant among patients undergoing initial VATS compared with initial chest tube placement with fibrinolysis. However, chest tube placement without fibrinolysis did not result in significant differences in LOS compared with initial VATS. In the only pediatric randomized trial, the 29 intrapleural urokinase recipients had a 2 day shorter LOS compared with the 29 intrapleural saline recipients.33 Several small, randomized controlled trials of adults with complicated pneumonia reported improved pleural fluid drainage among intrapleural fibrinolysis recipients compared with non‐recipients.3436 However, a large multicenter randomized trial in adults found no differences in mortality, requirement for surgical drainage, or LOS between intrapleural streptokinase and placebo recipients.37 Subsequent meta‐analyses of randomized trials in adults also demonstrated no benefit to fibrinolysis.38, 39 In the context of the increasing use of intrapleural fibrinolysis in children with complicated pneumonia, our results highlight the need for a large, multicenter randomized controlled trial to determine whether chest tube with fibrinolysis is superior to chest tube alone.
Two small randomized trials21, 22 and a decision analysis40 identified chest tube with fibrinolysis as the most economical approach to children with complicated pneumonia. However, the costs did not differ significantly between patients undergoing initial VATS or initial chest tube placement with fibrinolysis in our study. The least costly approach was initial chest tube placement without fibrinolysis. Unlike the randomized controlled trials, we considered costs associated with readmissions in determining the total costs. Shah et al41 found no difference in total charges for patients undergoing initial VATS compared with initial chest tube placement; however, patients undergoing initial VATS were concentrated in a few centers, making it difficult to determine the relative importance of procedural and hospital factors.
This multicenter observational study has several limitations. First, discharge diagnosis coding may be unreliable for specific diseases. However, our rigorous definition of complicated pneumonia, supported by the high positive predictive value as verified by medical record review, minimizes the likelihood of misclassification.
Second, unmeasured confounding or residual confounding by indication for the method of pleural drainage may occur, potentially influencing our results in two disparate ways. If patients with more severe systemic illness were too unstable for operative interventions, then our results would be biased towards worse outcomes for children undergoing initial chest tube placement. We adjusted for several variables associated with a greater systemic severity of illness, including intensive care unit admission, making this possibility less likely. We also could not account for some factors associated with more severe local disease such as the size and character of the effusion. We suspect that patients with more extensive local disease (ie, loculated effusions) would have worse outcomes than other patients, regardless of initial procedure, and that these patients would also be more likely to undergo primary surgical drainage. Thus, this study may have underestimated the benefit of initial surgical drainage (eg, VATS) compared with nonsurgical drainage (ie, chest tube placement).
Third, misclassification of the method of initial pleural drainage may have occurred. Patients transferred from another institution following chest tube placement could either be classified as not receiving pleural drainage and thus excluded from the study or classified as having initial VATS or thoracotomy if the reason for transfer was chest tube treatment failure. Additionally, we could not distinguish routine use of fibrinolysis from fibrinolysis to maintain chest tube patency. Whether such misclassification would falsely minimize or maximize differences in outcomes between the various groups remains uncertain. Fourth, because this study only included tertiary care children's hospitals, these data are not generalizable to community settings. VATS requires specialized surgical training that may be unavailable in some areas. Finally, this study demonstrates the relative efficacy of various pleural fluid drainage procedures on short‐term clinical outcomes and resource utilization. However, long‐term functional outcomes should be measured in future prospective studies.
Conclusions
In conclusion, emphasis on evidence driven treatment to optimize care has led to an increasing examination of unwarranted practice variation.42 The lack of evidence for best practice makes it difficult to define unwarranted variation in the treatment of complicated pneumonia. Our study demonstrates the large variability in practice and raises additional questions regarding the optimal drainage strategies. Published randomized trials have focused on comparisons between chest tube placement with fibrinolysis and VATS. However, our data suggest that future randomized trials should include chest tube placement without fibrinolysis as a treatment strategy. In determining the current best treatment for patients with complicated pneumonia, a clinician must weigh the impact of needing an additional procedure in approximately one‐quarter of patients undergoing initial chest tube placement (with or without fibrinolysis) with the risks of general anesthesia and readmission in patients undergoing initial VATS.
Acknowledgements
Dr. Hall had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the analysis.
Community‐acquired pneumonia, the most common serious bacterial infection in childhood, may be complicated by parapneumonic effusion (ie, complicated pneumonia).1 Children with complicated pneumonia require prolonged hospitalization and frequently undergo multiple pleural fluid drainage procedures.2 Additionally, the incidence of complicated pneumonia has increased,37 making the need to define appropriate therapy even more pressing. Defining appropriate therapy is challenging for the individual physician as a result of inconsistent and insufficient evidence, and wide variation in treatment practices.2, 8
Historically, thoracotomy was performed only if initial chest tube placement did not lead to clinical improvement.9, 10 Several authors, noting the rapid resolution of symptoms in children undergoing earlier thoracotomy, advocated for the use of thoracotomy as initial therapy rather than as a procedure of last resort.114 The advent of less invasive techniques such as video‐assisted thoracoscopic surgery (VATS) has served as an additional impetus to consider surgical drainage as the initial treatment strategy.1518 Few well‐designed studies have examined the relative efficacy of these interventions.2, 1922 Published randomized trials were single center, enrolled few patients, and arrived at different conclusions.19, 21, 22 In addition, these trials did not examine other important outcomes such as requirement for additional pleural fluid drainage procedures and hospital readmission. Two large retrospective multicenter studies found modest reductions in length of stay (LOS) and substantial decreases in the requirement for additional pleural fluid drainage procedures in children undergoing initial VATS compared with initial chest tube placement.2, 20 However, Shah et al2 included relatively few patients undergoing VATS. Li et al20 combined patients undergoing initial thoracentesis, initial chest tube placement, late pleural fluid drainage (by any method), and no pleural fluid drainage into a single non‐operative management category, precluding conclusions about the relative benefits of chest tube placement compared with VATS. Neither study2, 20 examined the role of chemical fibrinolysis, a therapy which has been associated with outcomes comparable to VATS in two small randomized trials.21, 22
The objectives of this multicenter study were to describe the variation in the initial management strategy along with associated outcomes of complicated pneumonia in childhood and to determine the comparative effectiveness of different pleural fluid drainage procedures.
Methods
Data Source
The Pediatric Health Information System (PHIS), which contains resource utilization data from 40 freestanding children's hospitals, provided data for this multicenter retrospective cohort study. Participating hospitals are located in noncompeting markets of 27 states plus the District of Columbia. The PHIS database includes patient demographics, diagnoses, and procedures as well as data for all drugs, radiologic studies, laboratory tests, and supplies charged to each patient. Data are de‐identified, however encrypted medical record numbers allow for tracking individual patients across admissions. The Child Health Corporation of America (Shawnee Mission, KS) and participating hospitals jointly assure data quality and reliability as described previously.23, 24 The Children's Hospital of Philadelphia Institutional Review Board reviewed and approved this study.
Patients
Children 18 years of age receiving a pleural drainage procedure for complicated pneumonia were eligible if they were discharged from participating hospitals between January 1, 2004 and June 30, 2009. Study participants met the following criteria: 1) discharge diagnosis of pneumonia (International Classification of Diseases, 9th revision [ICD‐9] discharge diagnosis codes 480.x‐483.x, 485.x‐487.x), 2) discharge diagnosis of pleural effusion (ICD‐9 codes 510.0, 510.9, 511.0, 511.1, or 511.9), and 3) billing charge for antibiotics on the first day of hospitalization. Additionally, the primary discharge diagnosis had to be either pneumonia or pleural effusion. Patients were excluded if they did not undergo pleural fluid drainage or if their initial pleural fluid drainage procedure was thoracentesis.
Study Definitions
Pleural drainage procedures were identified using ICD‐9 procedure codes for thoracentesis (34.91), chest tube placement (34.04), VATS (34.21), and thoracotomy (34.02 or 34.09). Fibrinolysis was defined as receipt of urokinase, streptokinase, or alteplase within two days of initial chest tube placement.
Acute conditions or complications included influenza (487, 487.0, 487.1, 487.8, 488, or V04.81) and hemolytic‐uremic syndrome (283.11). Chronic comorbid conditions (CCCs) (eg, malignancy) were identified using a previously reported classification scheme.25 Billing data were used to classify receipt of mechanical ventilation and medications on the first day of hospitalization.
Measured Outcomes
The primary outcomes were hospital LOS (both overall and post‐initial procedure), requirement for additional pleural drainage procedures, total cost for index hospitalization, all‐cause readmission within 14 days after index hospital discharge, and total cost of the episode (accounting for the cost of readmissions).
Measured Exposures
The primary exposure of interest was the initial pleural fluid drainage procedure, classified as chest tube placement without fibrinolysis, chest tube placement with fibrinolysis, VATS, or thoracotomy.
Statistical Analysis
Variables were summarized using frequencies and percentages for categorical variables, and median, interquartile range (IQR), and range for continuous variables. Outcomes by initial pleural drainage procedure were compared using chi‐squared tests for categorical variables and Kruskal‐Wallis tests for continuous variables.
Multivariable analysis was performed to account for potential confounding by observed baseline variables. For dichotomous outcome variables, modeling consisted of logistic regression using generalized estimating equations to account for hospital clustering. For continuous variables, a mixed model approach was used, treating hospital as a random effect. Log transformation was applied to the right‐skewed outcome variables (LOS and cost). Cost outcomes remained skewed following log transformation, thus gamma mixed models were applied.2629 Odds ratios and 95% confidence intervals (CIs) were reported for comparison of dichotomous outcomes and the adjusted means and 95% CIs were reported for continuous outcomes after appropriate back transformation.
Additional analyses addressed the potential impact of confounding by indication inherent in any observational study. First, patients with an underlying CCC were excluded to ensure that our results would be generalizable to otherwise healthy children with community‐acquired pneumonia. Second, patients undergoing pleural drainage >2 days after hospitalization were excluded to minimize the effect of residual confounding related to differences in timing of the initial drainage procedure. Third, the analysis was repeated using a generalized propensity score as an additional method to account for confounding by indication for the initial drainage procedure.30 Propensity scores, constructed using a multivariable generalized logit model, included all variables listed in Table 1. The inverse of the propensity score was included as a weight in each multivariable model described previously. Only the primary multivariable analyses are presented as the results of the propensity score analysis were nearly identical to the primary analyses.
| Overall | Chest Tube Without Fibrinolysis | Chest Tube With Fibrinolysis | Thoracotomy | VATS | P Value | |
|---|---|---|---|---|---|---|
| ||||||
| N | 3500 | 1672 (47.8) | 623 (17.8) | 797 (22.8) | 408 (11.7) | |
| Age | ||||||
| <1 year | 335 (9.6) | 176 (10.5) | 56 (9.0) | 78 (9.8) | 25 (6.1) | |
| 1 year | 475 (13.6) | 238 (14.2) | 98 (15.7) | 92 (11.5) | 47 (11.5) | 0.003 |
| 24 years | 1230 (35.1) | 548 (32.8) | 203 (32.6) | 310 (38.9) | 169 (41.4) | |
| 59 years | 897 (25.6) | 412 (24.6) | 170 (27.3) | 199 (25.0) | 116 (28.4) | |
| 1014 years | 324 (9.3) | 167 (10.0) | 61 (9.8) | 65 (8.2) | 31 (7.6) | |
| 1518 years | 193 (5.5) | 106 (6.3) | 29 (4.6) | 40 (5.0) | 18 (4.4) | |
| >18 years | 46 (1.3) | 25 (1.5) | 6 (0.96) | 13 (1.6) | 2 (0.5) | |
| Comorbid Conditions | ||||||
| Cardiac | 69 (2.0) | 43 (2.6) | 14 (2.3) | 12 (1.5) | 0 (0.0) | 0.006 |
| Malignancy | 81 (2.3) | 31 (1.9) | 18 (2.9) | 21 (2.6) | 11 (2.7) | 0.375 |
| Neurological | 138 (3.9) | 73 (4.4) | 20 (3.2) | 34 (4.3) | 11 (2.7) | 0.313 |
| Any Other Condition | 202 (5.8) | 96 (5.7) | 40 (6.4) | 47 (5.9) | 19 (4.7) | 0.696 |
| Payer | ||||||
| Government | 1240 (35.6) | 630 (37.8) | 224 (36.0) | 259 (32.7) | 127 (31.3) | <0.001 |
| Private | 1383 (39.7) | 607 (36.4) | 283 (45.4) | 310 (39.2) | 183 (45.07) | |
| Other | 864 (24.8) | 430 (25.8) | 116 (18.6) | 222 (28.1) | 96 (23.65) | |
| Race | ||||||
| Non‐Hispanic White | 1746 (51.9) | 838 (51.6) | 358 (59.7) | 361 (47.8) | 189 (48.7) | <0.001 |
| Non‐Hispanic Black | 601 (17.9) | 318 (19.6) | 90 (15.0) | 128 (17.0) | 65 (16.8) | |
| Hispanic | 588 (17.5) | 280 (17.3) | 73 (12.2) | 155 (20.5) | 80 (20.6) | |
| Asian | 117 (3.5) | 47 (2.9) | 20 (3.3) | 37 (4.9) | 13 (3.4) | |
| Other | 314 (9.3) | 140 (8.6) | 59 (9.8) | 74 (9.8) | 41 (10.6) | |
| Male Sex | 1912 (54.6) | 923 (55.2) | 336 (53.9) | 439 (55.1) | 214 (52.5) | 0.755 |
| Radiology | ||||||
| CT, no US | 1200 (34.3) | 600 (35.9) | 184 (29.5) | 280 (35.1) | 136 (33.3) | <0.001 |
| CT and US | 221 (6.3) | 84 (5.0) | 53 (8.5) | 61 (7.7) | 23 (5.6) | |
| US, no CT | 799 (22.8) | 324 (19.4) | 178 (28.6) | 200 (25.1) | 97 (23.8) | |
| No US, no CT | 1280 (36.6) | 664 (39.7) | 208 (33.4) | 256 (32.1) | 152 (37.3) | |
| Empiric Antibiotic Regimen | ||||||
| Cephalosporins alone | 448 (12.8) | 181 (10.83) | 126 (20.2) | 73 (9.2) | 68 (16.7) | <0.001 |
| Cephalosporin and clindamycin | 797 (22.8) | 359 (21.5) | 145 (23.3) | 184 (23.1) | 109 (26.7) | |
| Other antibiotic combination | 167 (4.8) | 82 (4.9) | 30 (4.8) | 38 (4.8) | 17 (4.2) | |
| Cephalosporin and vancomycin | 2088 (59.7) | 1050 (62.8) | 322 (51.7) | 502 (63.0) | 214 (52.5) | |
| Mechanical ventilation | 494 (14.1) | 251 (15.0) | 75 (12.0) | 114 (14.3) | 54 (13.2) | 0.307 |
| Corticosteroids | 520 (14.9) | 291 (17.4) | 72 (11.6) | 114 (14.3) | 43 (10.5) | <0.001 |
| Blood product transfusionsb | 761 (21.7) | 387 (23.2) | 145 (23.3) | 161 (20.2) | 68 (16.7) | 0.018 |
| Vasoactive infusionsc | 381 (10.9) | 223 (13.3) | 63 (10.1) | 72 (9.0) | 23 (5.6) | <0.001 |
| Admission to intensive care | 1397 (39.9) | 731 (43.7) | 234 (37.6) | 296 (37.1) | 136 (33.3) | <0.001 |
| Extracorporeal membranous oxygenation | 18 (0.5) | 13 (0.8) | 2 (0.3) | 3 (0.4) | 0 (0.0) | 0.163 |
| Hemolytic‐uremic syndrome | 31 (0.9) | 15 (0.9) | 6 (1.0) | 7 (0.9) | 3 (0.7) | 0.985 |
| Influenza | 108 (3.1) | 53 (3.2) | 27 (4.3) | 23 (2.9) | 5 (1.2) | 0.044 |
| Arterial blood gas measurements | 0 (0,1) | 0 (0, 2) | 0 (0,1) | 0 (0, 1) | 0 (0, 1) | <0.001 |
| Days to first procedure | 1 (0, 3) | 1 (0, 2) | 1 (1, 3) | 1 (1, 3) | 1 (1, 3) | <0.001 |
Medical records of a randomly selected subset of subjects from 6 hospitals were reviewed to determine the accuracy of our algorithm in identifying patients with complicated pneumonia; these subjects represented 1% of the study population. For the purposes of medical record review, complicated pneumonia was defined by the following: 1) radiologically‐confirmed lung infiltrate; 2) moderate or large pleural effusion; and 3) signs and symptoms of lower respiratory tract infection. Complicated pneumonia was identified in 118 of 120 reviewed subjects for a positive predictive value of 98.3%.
All analyses were clustered by hospital. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). A two‐tailed P < 0.05 was considered statistically significant.
Results
Patient Characteristics
During the study period, 9,680 subjects had complicated pneumonia. Subjects were excluded if they did not have a pleural drainage procedure (n = 5798), or if thoracentesis was the first pleural fluid drainage procedure performed (n = 382). The remaining 3500 patients were included. Demographic characteristics are summarized in Table 1. The median patient age was 4.1 years (IQR: 2.17.2 years). An underlying CCC was present in 424 (12.1%) patients. There was no association between type of drainage procedure and mechanical ventilation. However, factors associated with more severe systemic illness, such as blood product transfusion, were more common among those undergoing initial chest tube placement with or without fibrinolysis (Table 1).
Initial Pleural Fluid Drainage Procedures
The primary procedures included chest tube without fibrinolysis (47.8%); chest tube with fibrinolysis (17.8%); thoracotomy (22.8%); and VATS (11.7%) (Table 1). The proportion of patients undergoing primary chest tube placement with fibrinolysis increased over time from 14.2% in 2004 to 30.0% in 2009 (P < 0.001; chi‐squared test for trend). The initial procedure varied by hospital with the greatest proportion of patients undergoing primary chest tube placement without fibrinolysis at 28 (70.0%) hospitals, chest tube placement with fibrinolysis at 5 (12.5%) hospitals, thoracotomy at 5 (12.5%) hospitals, and VATS at 2 (5.0%) hospitals (Figure 1). The median proportion of patients undergoing primary VATS across all hospitals was 11.5% (IQR: 3.9%‐26.5%) (Figure 1). The median time to first procedure was 1 day (IQR: 03 days).

Outcome Measures
Variation in outcomes occurred across hospitals. Additional pleural drainage procedures were performed in a median of 20.9% of patients with a range of 6.8% to 44.8% (IQR: 14.5%‐25.3%) of patients across all hospitals. Median LOS was 10 days with a range of 714 days (IQR: 8.511 days) and the median LOS following the initial pleural fluid drainage procedure was 8 days with a range of 6 to 13 days (IQR: 78 days). Variation in timing of the initial pleural fluid drainage procedure explained 9.6% of the variability in LOS (Spearman rho, 0.31; P < 0.001).
Overall, 118 (3.4%) patients were readmitted within 14 days of index discharge; the median readmission rate was 3.8% with a range of 0.8% to 33.3% (IQR: 2.1%‐5.8%) across hospitals. The median total cost of the index hospitalization was $19,574 (IQR: $13,791‐$31,063). The total cost for the index hospitalization exceeded $54,215 for 10% of patients and the total cost of the episode exceeded $55,208 for 10% of patients. Unadjusted outcomes, stratified by primary pleural fluid drainage procedure, are summarized in Table 2.
| Overall | Chest Tube Without Fibrinolysis | Chest Tube With Fibrinolysis | Thoracotomy | VATS | P Valueb | |
|---|---|---|---|---|---|---|
| ||||||
| Additional Procedure | 716 (20.5) | 331 (19.8) | 144 (23.1) | 197 (24.7) | 44 (10.8) | <0.001 |
| Readmission within 14 days | 118 (3.4) | 54 (3.3) | 13 (2.1) | 32 (4.0) | 19 (4.7) | 0.096 |
| Total LOS (days) | 10 (7, 14) | 10 (7, 14) | 9 (7, 13) | 10 (7, 14) | 9 (7, 12) | <.001 |
| Post‐initial Procedure LOS (days) | 8 (5, 12) | 8 (6, 12) | 7 (5, 10) | 8 (5, 12) | 7 (5, 10) | <0.001 |
| Total Cost, Index Hospitalization ($)e | 19319 (13358, 30955) | 19951 (13576, 32018)c | 19565 (13209, 32778)d | 20352 (14351, 31343) | 17918 (13531, 25166) | 0.016 |
| Total Cost, Episode of Illness ($)e | 19831 (13927, 31749) | 20151 (13764, 32653) | 19593 (13210, 32861) | 20573 (14419, 31753) | 18344 (13835, 25462) | 0.029 |
In multivariable analysis, differences in total LOS and post‐procedure LOS were not significant (Table 3). The odds of additional drainage procedures were higher for all drainage procedures compared with initial VATS (Table 3). Patients undergoing initial chest tube placement with fibrinolysis were less likely to require readmission compared with patients undergoing initial VATS (Table 3). The total cost for the episode of illness (including the cost of readmission) was significantly less for those undergoing primary chest tube placement without fibrinolysis compared with primary VATS. The results of subanalyses excluding patients with an underlying CCC (Supporting Appendix online, Table 4) and restricting the cohort to patients undergoing pleural drainage within two days of admission (Supporting Appendix online, Table 5) were similar to the results of our primary analysis with one exception; in the latter subanalysis, children undergoing initial chest tube placement without fibrinolysis were also less likely to require readmission compared with patients undergoing initial VATS.
| Adjusted OR (95% CI)a | P Value | |
|---|---|---|
| ||
| Additional pleural drainage procedure | ||
| Chest tube without fibrinolysis | 1.82 (1.103.00) | .019 |
| Chest tube with fibrinolysis | 2.31 (1.443.72) | <0.001 |
| Thoracotomy | 2.59 (1.624.14) | <0.001 |
| VATS | Reference | |
| Readmission within 14 days | ||
| Chest tube without fibrinolysis | 0.61 (0.361.05) | .077 |
| Chest tube with fibrinolysis | 0.45 (0.230.86) | .015 |
| Thoracotomy | 0.85 (0.521.39) | .521 |
| VATS | Reference | |
| Adjusted Mean (95% CI)a | P Value | |
| Total LOS (days) | ||
| Chest tube without fibrinolysis | 8.0 (7.88.2) | .339 |
| Chest tube with fibrinolysis | 8.1 (7.98.3) | .812 |
| Thoracotomy | 8.1 (7.98.3) | .632 |
| VATS | 8.1 (7.98.3) | Ref |
| Post‐initial procedure LOS (days) | ||
| Chest tube without fibrinolysis | 7.3 (7.07.5) | .512 |
| Chest tube with fibrinolysis | 7.5 (7.27.8) | .239 |
| Thoracotomy | 7.3 (7.07.6) | .841 |
| VATS | 7.3 (7.17.6) | Reference |
| Total cost, index hospitalization ($) | ||
| Chest tube without fibrinolysis | 22928 (2200023895 | .012 |
| Chest tube with fibrinolysis | 23621 (2263124655) | .657 |
| Thoracotomy | 23386 (2241924395 | .262 |
| VATS | 23820 (2280824878) | Reference |
| Total cost, episode of illness ($) | ||
| Chest tube without fibrinolysis | 23218 (2227824199) | .004 |
| Chest tube with fibrinolysis | 23749 (2275224790) | .253 |
| Thoracotomy | 23673 (2269324696) | .131 |
| VATS | 24280 (2324425362) | Reference |
Discussion
This multicenter study is the largest to evaluate the management of children hospitalized with complicated pneumonia. We found considerable variation in initial management and outcomes across hospitals. Differences in timing of the initial drainage procedure explained only a small amount of the variability in outcomes. Children undergoing initial VATS less commonly required additional drainage procedures while children undergoing initial chest tube placement with fibrinolysis less commonly required readmission. Differences in total and post‐procedure LOS were not statistically significant. Differences in cost, while statistically significant, were of marginal relevance.
Previous studies have also shown significant variation in treatment and outcomes of children with complicated pneumonia across hospitals.2, 8 Our study provides data from additional hospitals, includes a substantially larger number of patients undergoing initial VATS, distinguishes between fibrinolysis recipients and nonrecipients, and is the first to compare outcomes between four different initial drainage strategies. The creation of national consensus guidelines might reduce variability in initial management strategies, although the variability in outcomes across hospitals in the current study could not be explained simply by differences in the type or timing of the initial drainage procedure. Thus, future studies examining hospital‐level factors may play an important role in improving quality of care for children with complicated pneumonia.
Patients with initial thoracotomy or chest tube placement with or without fibrinolysis more commonly received additional drainage procedures than patients with initial VATS. This difference remained when patients with CCCs were excluded from the analysis and when the analysis was limited to patients undergoing pleural fluid drainage within 2 days of hospitalization. Several small, randomized trials demonstrated conflicting results when comparing initial chest tube placement with fibrinolysis and VATS. St. Peter et al22 reported that 3 (17%) of 18 patients undergoing initial chest tube placement with fibrinolysis and none of the 18 patients undergoing initial VATS received additional pleural drainage procedures. Sonnappa et al21 found no differences between the two groups. Kurt et al19 did not state the proportion of patients receiving additional procedures. However, the mean number of drainage procedures was 2.25 among the 8 patients undergoing initial chest tube placement while none of the 10 patients with VATS received additional drainage.19
Thoracotomy is often perceived as a definitive procedure for treatment of complicated pneumonia. However, several possibilities exist to explain why additional procedures were performed less frequently in patients undergoing initial VATS compared with initial thoracotomy. The limited visual field in thoracotomy may lead to greater residual disease post‐operatively in those receiving thoracotomy compared with VATS.31 Additionally, thoracotomy substantially disrupts the integrity of the chest wall and is consequently associated with complications such as bleeding and air leak into the pleural cavity more often than VATS.31, 32 It is thus possible that some of the additional procedures in patients receiving initial thoracotomy were necessary for management of thoracotomy‐associated complications rather than for failure of the initial drainage procedure.
Similar to the randomized trials by Sonnappa et al21 and St. Peter et al,22 differences in the overall and post‐procedure LOS were not significant among patients undergoing initial VATS compared with initial chest tube placement with fibrinolysis. However, chest tube placement without fibrinolysis did not result in significant differences in LOS compared with initial VATS. In the only pediatric randomized trial, the 29 intrapleural urokinase recipients had a 2 day shorter LOS compared with the 29 intrapleural saline recipients.33 Several small, randomized controlled trials of adults with complicated pneumonia reported improved pleural fluid drainage among intrapleural fibrinolysis recipients compared with non‐recipients.3436 However, a large multicenter randomized trial in adults found no differences in mortality, requirement for surgical drainage, or LOS between intrapleural streptokinase and placebo recipients.37 Subsequent meta‐analyses of randomized trials in adults also demonstrated no benefit to fibrinolysis.38, 39 In the context of the increasing use of intrapleural fibrinolysis in children with complicated pneumonia, our results highlight the need for a large, multicenter randomized controlled trial to determine whether chest tube with fibrinolysis is superior to chest tube alone.
Two small randomized trials21, 22 and a decision analysis40 identified chest tube with fibrinolysis as the most economical approach to children with complicated pneumonia. However, the costs did not differ significantly between patients undergoing initial VATS or initial chest tube placement with fibrinolysis in our study. The least costly approach was initial chest tube placement without fibrinolysis. Unlike the randomized controlled trials, we considered costs associated with readmissions in determining the total costs. Shah et al41 found no difference in total charges for patients undergoing initial VATS compared with initial chest tube placement; however, patients undergoing initial VATS were concentrated in a few centers, making it difficult to determine the relative importance of procedural and hospital factors.
This multicenter observational study has several limitations. First, discharge diagnosis coding may be unreliable for specific diseases. However, our rigorous definition of complicated pneumonia, supported by the high positive predictive value as verified by medical record review, minimizes the likelihood of misclassification.
Second, unmeasured confounding or residual confounding by indication for the method of pleural drainage may occur, potentially influencing our results in two disparate ways. If patients with more severe systemic illness were too unstable for operative interventions, then our results would be biased towards worse outcomes for children undergoing initial chest tube placement. We adjusted for several variables associated with a greater systemic severity of illness, including intensive care unit admission, making this possibility less likely. We also could not account for some factors associated with more severe local disease such as the size and character of the effusion. We suspect that patients with more extensive local disease (ie, loculated effusions) would have worse outcomes than other patients, regardless of initial procedure, and that these patients would also be more likely to undergo primary surgical drainage. Thus, this study may have underestimated the benefit of initial surgical drainage (eg, VATS) compared with nonsurgical drainage (ie, chest tube placement).
Third, misclassification of the method of initial pleural drainage may have occurred. Patients transferred from another institution following chest tube placement could either be classified as not receiving pleural drainage and thus excluded from the study or classified as having initial VATS or thoracotomy if the reason for transfer was chest tube treatment failure. Additionally, we could not distinguish routine use of fibrinolysis from fibrinolysis to maintain chest tube patency. Whether such misclassification would falsely minimize or maximize differences in outcomes between the various groups remains uncertain. Fourth, because this study only included tertiary care children's hospitals, these data are not generalizable to community settings. VATS requires specialized surgical training that may be unavailable in some areas. Finally, this study demonstrates the relative efficacy of various pleural fluid drainage procedures on short‐term clinical outcomes and resource utilization. However, long‐term functional outcomes should be measured in future prospective studies.
Conclusions
In conclusion, emphasis on evidence driven treatment to optimize care has led to an increasing examination of unwarranted practice variation.42 The lack of evidence for best practice makes it difficult to define unwarranted variation in the treatment of complicated pneumonia. Our study demonstrates the large variability in practice and raises additional questions regarding the optimal drainage strategies. Published randomized trials have focused on comparisons between chest tube placement with fibrinolysis and VATS. However, our data suggest that future randomized trials should include chest tube placement without fibrinolysis as a treatment strategy. In determining the current best treatment for patients with complicated pneumonia, a clinician must weigh the impact of needing an additional procedure in approximately one‐quarter of patients undergoing initial chest tube placement (with or without fibrinolysis) with the risks of general anesthesia and readmission in patients undergoing initial VATS.
Acknowledgements
Dr. Hall had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the analysis.
- ,.Parapneumonic pleural effusion and empyema in children. Review of a 19‐year experience, 1962–1980.Clin Pediatr (Phila).1983;22:414–419.
- ,,,,.Primary early thoracoscopy and reduction in length of hospital stay and additional procedures among children with complicated pneumonia: Results of a multicenter retrospective cohort study.Arch Pediatr Adolesc Med.2008;162:675–681.
- ,.Empyema hospitalizations increased in US children despite pneumococcal conjugate vaccine.Pediatrics.2010;125:26–33.
- ,,, et al.Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema.Pediatr Infect Dis J.2006;25:250–254.
- ,,,,.Five‐fold increase in pediatric parapneumonic empyema since introduction of pneumococcal conjugate vaccine.Pediatric Infect Dis J.2008;27:1030–1032.
- ,,,.Increasing incidence of empyema complicating childhood community‐acquired pneumonia in the United States.Clin Infect Dis.2010;50:805–813.
- ,,,,.National hospitalization trends for pediatric pneumonia and associated complications.Pediatrics.2010;126:204–213.
- ,,, et al.Empyema associated with community‐acquired pneumonia: A Pediatric Investigator's Collaborative Network on Infections in Canada (PICNIC) study.BMC Infect Dis.2008;8:129.
- ,,,,.Pleural empyema in children.Ann Thorac Surg.1970;10:37–44.
- ,,.Management of streptococcal empyema.Ann Thorac Surg.1966;2:658–664.
- ,.Thoracoscopy in the management of empyema in children.J Pediatr Surg.1993;28:1128–1132.
- ,,,.Surgical treatment of parapneumonic empyema.Pediatr Pulmonol.1996;22:348–356.
- ,.The controversial role of decortication in the management of pediatric empyema.J Thorac Cardiovasc Surg.1988;96:166–170.
- ,,,,,.Postpneumonic empyema in children treated by early decortication.Eur J Pediatr Surg.1997;7:135–137.
- ,.Video‐assisted thoracoscopic surgery in the management of pediatric empyema.JSLS.1997;1:251–3.
- ,,,.Early video‐assisted thoracic surgery in the management of empyema.Pediatrics.1999;103:e63.
- ,,,,.Early definitive intervention by thoracoscopy in pediatric empyema.J Pediatr Surg.1999;34:178–180; discussion80–81.
- ,,,,.Thoracoscopy in the management of pediatric empyema.J Pediatr Surg.1995;30:1211–1215.
- ,,,,.Therapy of parapneumonic effusions in children: Video‐assisted thoracoscopic surgery versus conventional thoracostomy drainage.Pediatrics.2006;118:e547–e553.
- ,.Primary operative management for pediatric empyema: Decreases in hospital length of stay and charges in a national sample.Arch Pediatr Adolesc Med.2008;162:44–48.
- ,,, et al.Comparison of urokinase and video‐assisted thoracoscopic surgery for treatment of childhood empyema.Am J Respir Crit Care Med.2006;174:221–227.
- ,,, et al.Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: A prospective, randomized trial.J Pediatr Surg.2009;44:106–111; discussion11.
- ,,,.Corticosteroids and mortality in children with bacterial meningitis.JAMA.2008;299:2048–2055.
- ,,,,.Intravenous immunoglobulin in children with streptococcal toxic shock syndrome.Clin Infect Dis.2009;49:1369–1376.
- ,,, et al.Deaths attributed to pediatric complex chronic conditions: National trends and implications for supportive care services.Pediatrics.2001;107:e99.
- ,.Multiple regression of cost data: Use of generalised linear models.J Health Serv Res Policy.2004;9:197–204.
- ,,,.A robustified modeling approach to analyze pediatric length of stay.Ann Epidemiol.2005;15:673–677.
- ,,.Correlates of length of stay, cost of care, and mortality among patients hospitalized for necrotizing fasciitis.Epidemiol Infect.2007;135:868–876.
- ,,,,,.Health care costs of adults treated for attention‐deficit/hyperactivity disorder who received alternative drug therapies.J Manag Care Pharm.2007;13:561–569.
- .The role of the propensity score in estimating dose‐response functions.Biometrika.2000;87:706–710.
- ,,,,.Experience with video‐assisted thoracoscopic surgery in the management of complicated pneumonia in children.J Pediatr Surg.2001;36:316–319.
- ,,, et al.VATS debridement versus thoracotomy in the treatment of loculated postpneumonia empyema.Ann Thorac Surg.1996;61:1626–1630.
- ,,,,.Randomised trial of intrapleural urokinase in the treatment of childhood empyema.Thorax.2002;57:343–347.
- ,,,,,.Intrapleural urokinase versus normal saline in the treatment of complicated parapneumonic effusions and empyema. A randomized, double‐blind study.Am J Respir Crit Care Med.1999;159:37–42.
- ,,.Randomised controlled trial of intrapleural streptokinase in community acquired pleural infection.Thorax.1997;52:416–421.
- ,,,,.Intrapleural streptokinase for empyema and complicated parapneumonic effusions.Am J Respir Crit Care Med.2004;170:49–53.
- ,,, et al.U.K. Controlled trial of intrapleural streptokinase for pleural infection.N Engl J Med.2005;352:865–874.
- ,.Intra‐pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema.Cochrane Database Syst Rev.2008:CD002312.
- ,,,.Intrapleural fibrinolytic agents for empyema and complicated parapneumonic effusions: A meta‐analysis.Chest.2006;129:783–790.
- ,,.Cost‐effectiveness of competing strategies for the treatment of pediatric empyema.Pediatrics.2008;121:e1250–e1257.
- ,,.Costs of treating children with complicated pneumonia: A comparison of primary video‐assisted thoracoscopic surgery and chest tube placement.Pediatr Pulmonol.2010;45:71–77.
- .Unwarranted variation in pediatric medical care.Pediatr Clin North Am.2009;56:745–755.
- ,.Parapneumonic pleural effusion and empyema in children. Review of a 19‐year experience, 1962–1980.Clin Pediatr (Phila).1983;22:414–419.
- ,,,,.Primary early thoracoscopy and reduction in length of hospital stay and additional procedures among children with complicated pneumonia: Results of a multicenter retrospective cohort study.Arch Pediatr Adolesc Med.2008;162:675–681.
- ,.Empyema hospitalizations increased in US children despite pneumococcal conjugate vaccine.Pediatrics.2010;125:26–33.
- ,,, et al.Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema.Pediatr Infect Dis J.2006;25:250–254.
- ,,,,.Five‐fold increase in pediatric parapneumonic empyema since introduction of pneumococcal conjugate vaccine.Pediatric Infect Dis J.2008;27:1030–1032.
- ,,,.Increasing incidence of empyema complicating childhood community‐acquired pneumonia in the United States.Clin Infect Dis.2010;50:805–813.
- ,,,,.National hospitalization trends for pediatric pneumonia and associated complications.Pediatrics.2010;126:204–213.
- ,,, et al.Empyema associated with community‐acquired pneumonia: A Pediatric Investigator's Collaborative Network on Infections in Canada (PICNIC) study.BMC Infect Dis.2008;8:129.
- ,,,,.Pleural empyema in children.Ann Thorac Surg.1970;10:37–44.
- ,,.Management of streptococcal empyema.Ann Thorac Surg.1966;2:658–664.
- ,.Thoracoscopy in the management of empyema in children.J Pediatr Surg.1993;28:1128–1132.
- ,,,.Surgical treatment of parapneumonic empyema.Pediatr Pulmonol.1996;22:348–356.
- ,.The controversial role of decortication in the management of pediatric empyema.J Thorac Cardiovasc Surg.1988;96:166–170.
- ,,,,,.Postpneumonic empyema in children treated by early decortication.Eur J Pediatr Surg.1997;7:135–137.
- ,.Video‐assisted thoracoscopic surgery in the management of pediatric empyema.JSLS.1997;1:251–3.
- ,,,.Early video‐assisted thoracic surgery in the management of empyema.Pediatrics.1999;103:e63.
- ,,,,.Early definitive intervention by thoracoscopy in pediatric empyema.J Pediatr Surg.1999;34:178–180; discussion80–81.
- ,,,,.Thoracoscopy in the management of pediatric empyema.J Pediatr Surg.1995;30:1211–1215.
- ,,,,.Therapy of parapneumonic effusions in children: Video‐assisted thoracoscopic surgery versus conventional thoracostomy drainage.Pediatrics.2006;118:e547–e553.
- ,.Primary operative management for pediatric empyema: Decreases in hospital length of stay and charges in a national sample.Arch Pediatr Adolesc Med.2008;162:44–48.
- ,,, et al.Comparison of urokinase and video‐assisted thoracoscopic surgery for treatment of childhood empyema.Am J Respir Crit Care Med.2006;174:221–227.
- ,,, et al.Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: A prospective, randomized trial.J Pediatr Surg.2009;44:106–111; discussion11.
- ,,,.Corticosteroids and mortality in children with bacterial meningitis.JAMA.2008;299:2048–2055.
- ,,,,.Intravenous immunoglobulin in children with streptococcal toxic shock syndrome.Clin Infect Dis.2009;49:1369–1376.
- ,,, et al.Deaths attributed to pediatric complex chronic conditions: National trends and implications for supportive care services.Pediatrics.2001;107:e99.
- ,.Multiple regression of cost data: Use of generalised linear models.J Health Serv Res Policy.2004;9:197–204.
- ,,,.A robustified modeling approach to analyze pediatric length of stay.Ann Epidemiol.2005;15:673–677.
- ,,.Correlates of length of stay, cost of care, and mortality among patients hospitalized for necrotizing fasciitis.Epidemiol Infect.2007;135:868–876.
- ,,,,,.Health care costs of adults treated for attention‐deficit/hyperactivity disorder who received alternative drug therapies.J Manag Care Pharm.2007;13:561–569.
- .The role of the propensity score in estimating dose‐response functions.Biometrika.2000;87:706–710.
- ,,,,.Experience with video‐assisted thoracoscopic surgery in the management of complicated pneumonia in children.J Pediatr Surg.2001;36:316–319.
- ,,, et al.VATS debridement versus thoracotomy in the treatment of loculated postpneumonia empyema.Ann Thorac Surg.1996;61:1626–1630.
- ,,,,.Randomised trial of intrapleural urokinase in the treatment of childhood empyema.Thorax.2002;57:343–347.
- ,,,,,.Intrapleural urokinase versus normal saline in the treatment of complicated parapneumonic effusions and empyema. A randomized, double‐blind study.Am J Respir Crit Care Med.1999;159:37–42.
- ,,.Randomised controlled trial of intrapleural streptokinase in community acquired pleural infection.Thorax.1997;52:416–421.
- ,,,,.Intrapleural streptokinase for empyema and complicated parapneumonic effusions.Am J Respir Crit Care Med.2004;170:49–53.
- ,,, et al.U.K. Controlled trial of intrapleural streptokinase for pleural infection.N Engl J Med.2005;352:865–874.
- ,.Intra‐pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema.Cochrane Database Syst Rev.2008:CD002312.
- ,,,.Intrapleural fibrinolytic agents for empyema and complicated parapneumonic effusions: A meta‐analysis.Chest.2006;129:783–790.
- ,,.Cost‐effectiveness of competing strategies for the treatment of pediatric empyema.Pediatrics.2008;121:e1250–e1257.
- ,,.Costs of treating children with complicated pneumonia: A comparison of primary video‐assisted thoracoscopic surgery and chest tube placement.Pediatr Pulmonol.2010;45:71–77.
- .Unwarranted variation in pediatric medical care.Pediatr Clin North Am.2009;56:745–755.
Copyright © 2011 Society of Hospital Medicine