User login
Risk Evaluation and Mitigation Strategy programs: How they can be improved
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program the FDA can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks (Box1). The FDA may require medication guides, patient package inserts, communication plans for health care professionals, and/or certain packaging and safe disposal technologies for medications that pose a serious risk of abuse or overdose. The FDA may also require elements to assure safe use and/or an implementation system be included in the REMS. Pharmaceutical manufacturers then develop a proposed REMS for FDA review.2 If the FDA approves the proposed REMS, the manufacturer is responsible for implementing the REMS requirements.
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
The FDA provides this description of a Risk Evaluation and Mitigation Strategy (REMS):
“A [REMS] is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication. While all medications have labeling that informs health care stakeholders about medication risks, only a few medications require a REMS. REMS are not designed to mitigate all the adverse events of a medication, these are communicated to health care providers in the medication’s prescribing information. Rather, REMS focus on preventing, monitoring and/or managing a specific serious risk by informing, educating and/or reinforcing actions to reduce the frequency and/or severity of the event.”1
The REMS program for clozapine3 has been the subject of much discussion in the psychiatric community. The adverse impact of the 2015 update to the clozapine REMS program was emphasized at meetings of both the American Psychiatric Association and the College of Psychiatric and Neurologic Pharmacists. A white paper published by the National Association of State Mental Health Program Directors shortly after the 2015 update concluded, “clozapine is underused due to a variety of barriers related to the drug and its properties, the health care system, regulatory requirements, and reimbursement issues.”4 After an update to the clozapine REMS program in 2021, the FDA temporarily suspended enforcement of certain requirements due to concerns from health care professionals about patient access to the medication because of problems with implementing the clozapine REMS program.5,6 In November 2022, the FDA issued a second announcement of enforcement discretion related to additional requirements of the REMS program.5 The FDA had previously announced a decision to not take action regarding adherence to REMS requirements for certain laboratory tests in March 2020, during the COVID-19 pandemic.7
REMS programs for other psychiatric medications may also present challenges. The REMS programs for esketamine8 and olanzapine for extended-release (ER) injectable suspension9 include certain risks that require postadministration monitoring. Some facilities have had to dedicate additional space and clinician time to ensure REMS requirements are met.
To further understand health care professionals’ perspectives regarding the value and burden of these REMS programs, a collaborative effort of the University of Maryland (College Park and Baltimore campuses) Center of Excellence in Regulatory Science and Innovation with the FDA was undertaken. The REMS for clozapine, olanzapine for ER injectable suspension, and esketamine were examined to develop recommendations for improving patient access while ensuring safe medication use and limiting the impact on health care professionals.
Assessing the REMS programs
Focus groups were held with health care professionals nominated by professional organizations to gather their perspectives on the REMS requirements. There was 1 focus group for each of the 3 medications. A facilitator’s guide was developed that contained the details of how to conduct the focus group along with the medication-specific questions. The questions were based on the REMS requirements as of May 2021 and assessed the impact of the REMS on patient safety, patient access, and health care professional workload; effects from the COVID-19 pandemic; and suggestions to improve the REMS programs. The University of Maryland Institutional Review Board reviewed the materials and processes and made the determination of exempt.
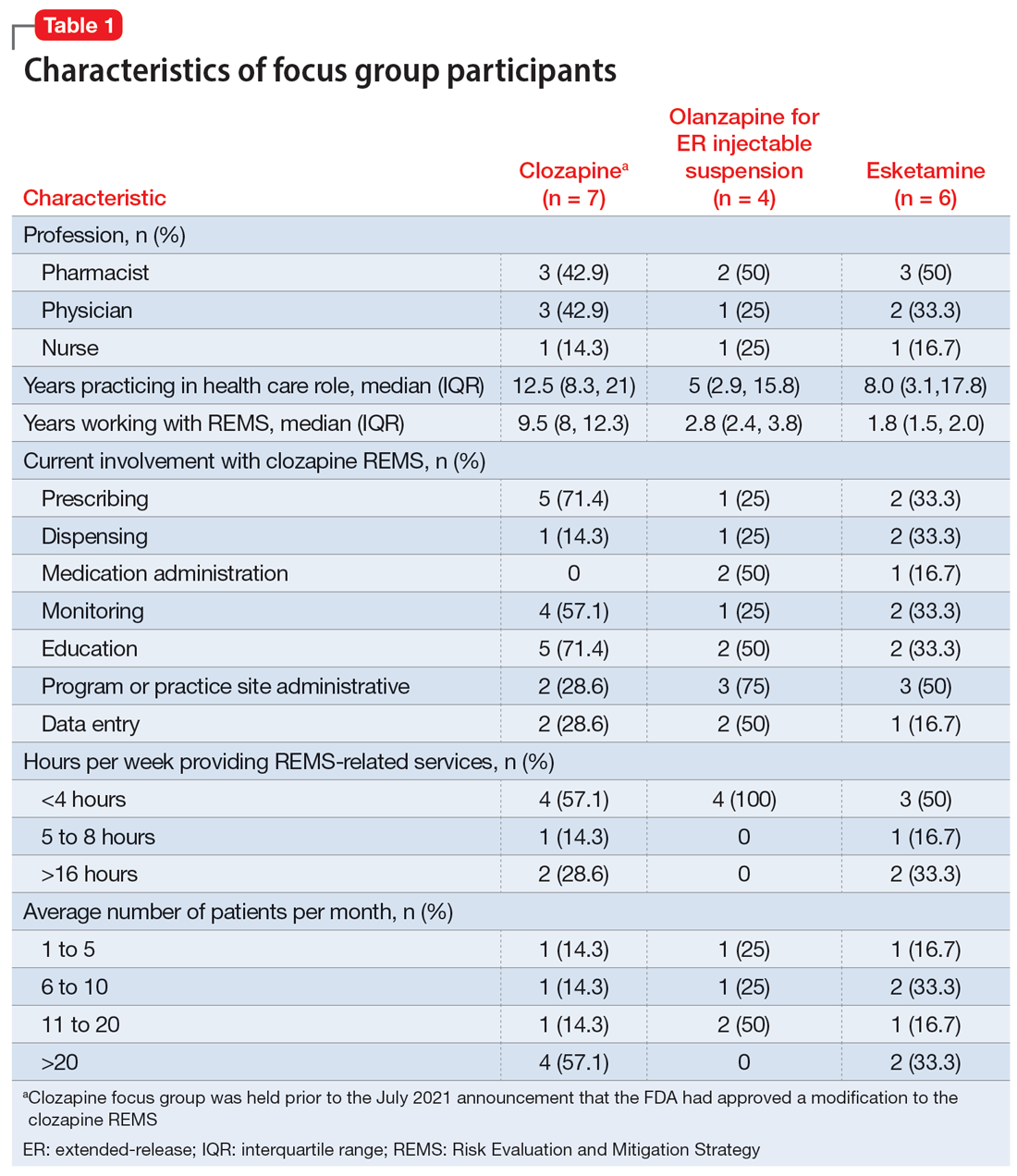
Health care professionals were eligible to participate in a focus group if they had ≥1 year of experience working with patients who use the specific medication and ≥6 months of experience within the past year working with the REMS program for that medication. Participants were excluded if they were employed by a pharmaceutical manufacturer or the FDA. The focus groups were conducted virtually using an online conferencing service during summer 2021 and were scheduled for 90 minutes. Prior to the focus group, participants received information from the “Goals” and “Summary” tabs of the FDA REMS website10 for the specific medication along with patient/caregiver guides, which were available for clozapine and olanzapine for ER injectable suspension. For each focus group, there was a target sample size of 6 to 9 participants. However, there were only 4 participants in the olanzapine for ER injectable suspension focus group, which we believed was due to lower national utilization of this medication. Individuals were only able to participate in 1 focus group, so the unique participant count for all 3 focus groups totaled 17 (Table 1).
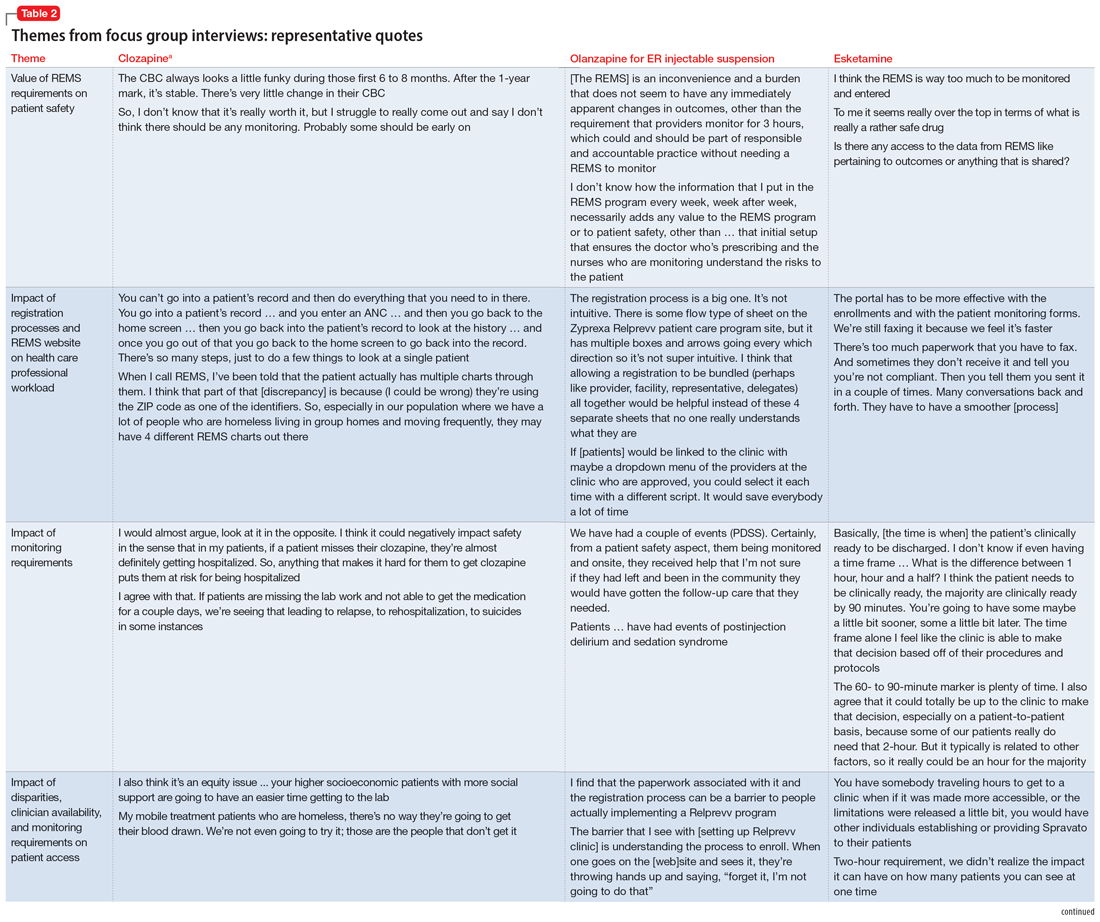
Themes extracted from qualitative analysis of the focus group responses were the value of the REMS programs; registration/enrollment processes and REMS websites; monitoring requirements; care transitions; and COVID considerations (Table 2). While the REMS programs were perceived to increase practitioner and patient awareness of potential harms, discussions centered on the relative cost-to-benefit of the required reporting and other REMS requirements. There were challenges with the registration/enrollment processes and REMS websites that also affected patient care during transitions to different health care settings or clinicians. Patient access was affected by disparities in care related to monitoring requirements and clinician availability.

Continue to: COVID impacted all REMS...
COVID impacted all REMS programs. Physical distancing was an issue for medications that required extensive postadministration monitoring (ie, esketamine and olanzapine for ER injectable suspension). Access to laboratory services was an issue for clozapine.
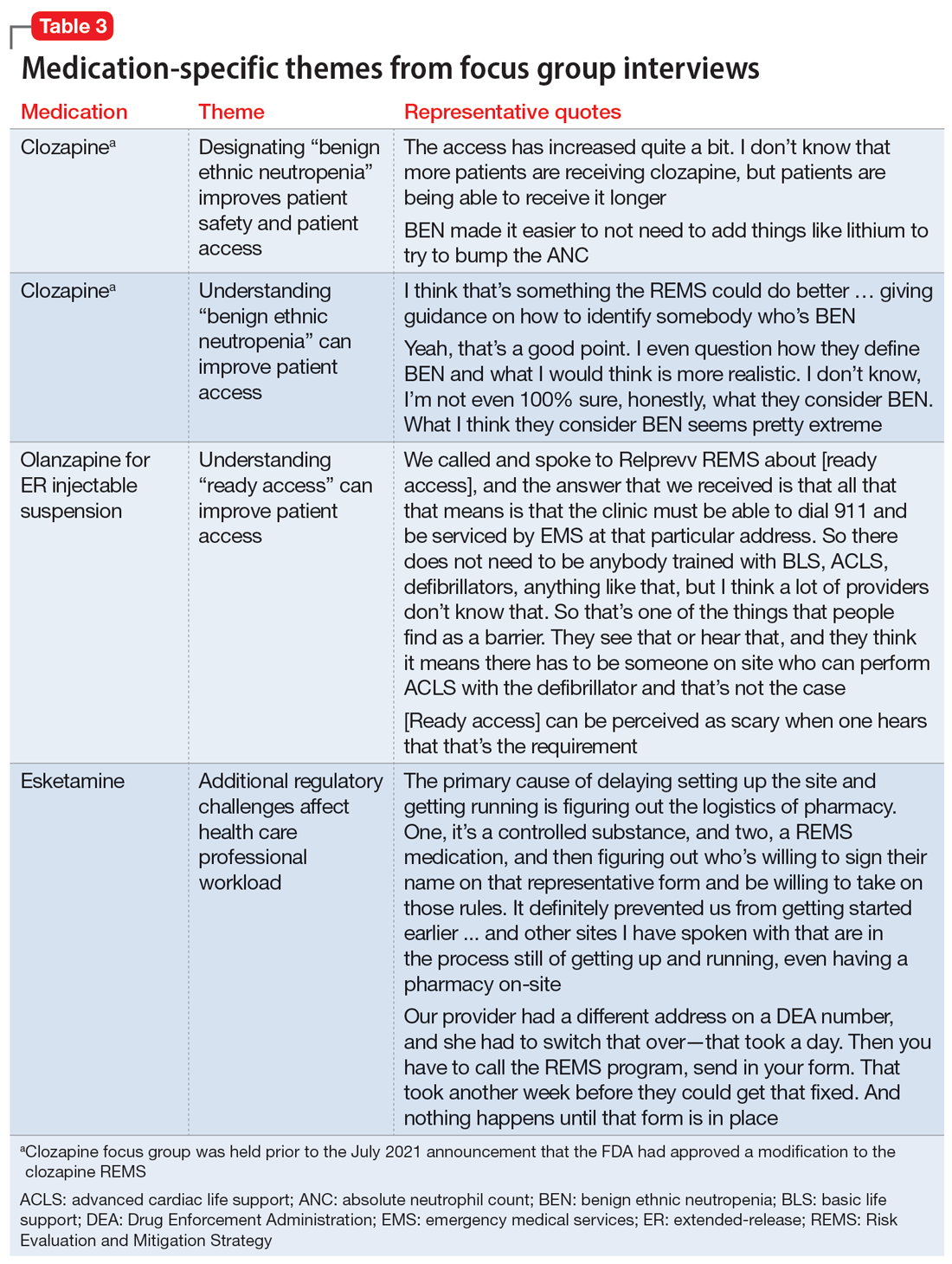
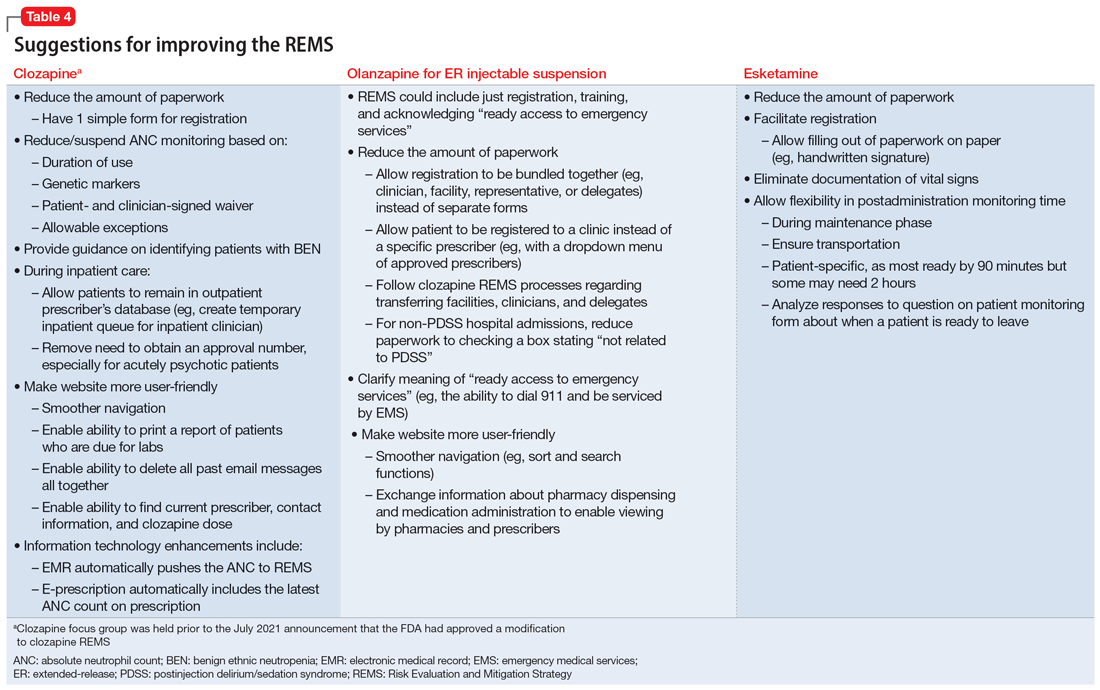
Medication-specific themes are listed in Table 3 and relate to terms and descriptions in the REMS or additional regulatory requirements from the Drug Enforcement Agency (DEA). Suggestions for improvement to the REMS are presented in Table 4.
Recommendations for improving REMS
A group consisting of health care professionals, policy experts, and mental health advocates reviewed the information provided by the focus groups and developed the following recommendations.
Overarching recommendations
Each REMS should include a section providing justification for its existence, including a risk analysis of the data regarding the risk the REMS is designed to mitigate. This analysis should be repeated on a regular basis as scientific evidence regarding the risk and its epidemiology evolves. This additional section should also explain how the program requirements of the REMS as implemented (or planned) will achieve the aims of the REMS and weigh the potential benefits of the REMS requirements as implemented (or planned) by the manufacturer vs the potential risks of the REMS requirements as implemented (or planned) by the manufacturer.
Each REMS should have specific quantifiable outcomes. For example, it should specify a reduction in occurrence of the rate of the concerned risk by a specified amount.
Continue to: Ensure adequate...
Ensure adequate stakeholder input during the REMS development and real-world testing in multiple environments before implementing the REMS to identify unanticipated consequences that might impact patient access, patient safety, and health care professional burden. Implementation testing should explore issues such as purchasing and procurement, billing and reimbursement, and relevant factors such as other federal regulations or requirements (eg, the DEA or Medicare).
Ensure harmonization of the REMS forms and processes (eg, initiation and monitoring) for different medications where possible. A prescriber, pharmacist, or system should not face additional barriers to participate in a REMS based on REMS-specific intricacies (ie, prescription systems, data submission systems, or ordering systems). This streamlining will likely decrease clinical inertia to initiate care with the REMS medication, decrease health care professional burden, and improve compliance with REMS requirements.
REMS should anticipate the need for care transitions and employ provisions to ensure seamless care. Considerations should be given to transitions that occur due to:
- Different care settings (eg, inpatient, outpatient, or long-term care)
- Different geographies (eg, patient moves)
- Changes in clinicians, including leaves or absences
- Changes in facilities (eg, pharmacies).
REMS should mirror normal health care professional workflow, including how monitoring data are collected and how and with which frequency pharmacies fill prescriptions.Enhanced information technology to support REMS programs is needed. For example, REMS should be integrated with major electronic patient health record and pharmacy systems to reduce the effort required for clinicians to supply data and automate REMS processes.
For medications that are subject to other agencies and their regulations (eg, the CDC, Centers for Medicare & Medicaid Services, or the DEA), REMS should be required to meet all standards of all agencies with a single system that accommodates normal health care professional workflow.
Continue to: REMS should have a...
REMS should have a standard disclaimer that allows the health care professional to waive certain provisions of the REMS in cases when the specific provisions of the REMS pose a greater risk to the patient than the risk posed by waiving the requirement.
Assure the actions implemented by the industry to meet the requirements for each REMS program are based on peer-reviewed evidence and provide a reasonable expectation to achieve the anticipated benefit.
Ensure that manufacturers make all accumulated REMS data available in a deidentified manner for use by qualified scientific researchers. Additionally, each REMS should have a plan for data access upon initiation and termination of the REMS.
Each REMS should collect data on the performance of the centers and/or personnel who operate the REMS and submit this data for review by qualified outside reviewers. Parameters to assess could include:
- timeliness of response
- timeliness of problem resolution
- data availability and its helpfulness to patient care
- adequacy of resources.
Recommendations for clozapine REMS
These comments relate to the clozapine REMS program prior to the July 2021 announcement that FDA had approved a modification.
Provide a clear definition for “benign ethnic neutropenia.”
Ensure the REMS includes patient-specific adjustments to allow flexibility for monitoring. During COVID, the FDA allowed clinicians to “use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of laboratory testing.”7 This guidance, which allowed flexibility to absolute neutrophil count (ANC) monitoring, was perceived as positive and safe. Before the changes in the REMS requirements, patients with benign ethnic neutropenia were restricted from accessing their medication or encountered harm from additional pharmacotherapy to mitigate ANC levels.
Continue to: Recommendations for olanzapine for ER injectable suspension REMS
Recommendations for olanzapine for ER injectable suspension REMS
Provide clear explicit instructions on what is required to have “ready access to emergency services.”
Ensure the REMS include patient-specific adjustments to allow flexibility for postadministration monitoring (eg, sedation or blood pressure). Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of olanzapine for ER injectable suspension by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness are included in the REMS. How was the 3-hour cut-point determined? Has it been reevaluated?
Ensure the REMS requirements allow for seamless care during transitions, particularly when clinicians are on vacation.
Continue to: Recommendations for esketamine REMS
Recommendations for esketamine REMS
Ensure the REMS includes patient-specific adjustments to allow flexibility for postadministration monitoring. Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of esketamine by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness of requirements are included in the REMS. How was the 2-hour cut-point determined? Has it been reevaluated?
Ensure that the REMS meet all standards of the DEA, with a single system that accommodates normal health care professional workflow.
A summary of the findings
Overall, the REMS programs for these 3 medications were positively perceived for raising awareness of safe medication use for clinicians and patients. Monitoring patients for safety concerns is important and REMS requirements provide accountability.
Continue to: The use of a single shared...
The use of a single shared REMS system for documenting requirements for clozapine (compared to separate systems for each manufacturer) was a positive move forward in implementation. The focus group welcomed the increased awareness of benign ethnic neutropenia as a result of this condition being incorporated in the revised monitoring requirements of the clozapine REMS.
Focus group participants raised the issue of the real-world efficiency of the REMS programs (reduced access and increased clinician workload) vs the benefits (patient safety). They noted that excessive workload could lead to clinicians becoming unwilling to use a medication that requires a REMS. Clinician workload may be further compromised when REMS logistics disrupt the normal workflow and transitions of care between clinicians or settings. This latter aspect is of particular concern for clozapine.
The complexities of the registration and reporting system for olanzapine for ER injectable suspension and the lack of clarity about monitoring were noted to have discouraged the opening of treatment sites. This scarcity of sites may make clinicians hesitant to use this medication, and instead opt for alternative treatments in patients who may be appropriate candidates.
There has also been limited growth of esketamine treatment sites, especially in comparison to ketamine treatment sites.11-14 Esketamine is FDA-approved for treatment-resistant depression in adults and for depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Ketamine is not FDA-approved for treating depression but is being used off-label to treat this disorder.15 The FDA determined that ketamine does not require a REMS to ensure the benefits outweigh the risks for its approved indications as an anesthetic agent, anesthesia-inducing agent, or supplement to anesthesia. Since ketamine has no REMS requirements, there may be a lower burden for its use. Thus, clinicians are treating patients for depression with this medication without needing to comply with a REMS.16
Technology plays a role in workload burden, and integrating health care processes within current workflow systems, such as using electronic patient health records and pharmacy systems, is recommended. The FDA has been exploring technologies to facilitate the completion of REMS requirements, including mandatory education within the prescribers’ and pharmacists’ workflow.17 This is a complex task that requires multiple stakeholders with differing perspectives and incentives to align.
Continue to: The data collected for the REMS...
The data collected for the REMS program belongs to the medication’s manufacturer. Current regulations do not require manufacturers to make this data available to qualified scientific researchers. A regulatory mandate to establish data sharing methods would improve transparency and enhance efforts to better understand the outcomes of the REMS programs.
A few caveats
Both the overarching and medication-specific recommendations were based on a small number of participants’ discussions related to clozapine, olanzapine for ER injectable suspension, and esketamine. These recommendations do not include other medications with REMS that are used to treat psychiatric disorders, such as loxapine, buprenorphine ER, and buprenorphine transmucosal products. Larger-scale qualitative and quantitative research is needed to better understand health care professionals’ perspectives. Lastly, some of the recommendations outlined in this article are beyond the current purview or authority of the FDA and may require legislative or regulatory action to implement.
Bottom Line
Risk Evaluation and Mitigation Strategy (REMS) programs are designed to help reduce the occurrence and/or severity of serious risks or to inform decision-making. However, REMS requirements may adversely impact patient access to certain REMS medications and clinician burden. Health care professionals can provide informed recommendations for improving the REMS programs for clozapine, olanzapine for extended-release injectable suspension, and esketamine.
Related Resources
- FDA. Frequently asked questions (FAQs) about REMS. www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/frequently-asked-questions-faqs-about-rems
Drug Brand Names
Buprenorphine extended-release • Sublocade
Buprenorphine transmucosal • Subutex, Suboxone
Clozapine • Clozaril
Esketamine • Spravato
Ketamine • Ketalar
Lithium • Eskalith, Lithobid
Loxapine • Adasuve
Olanzapine extended-release injectable suspension • Zyprexa Relprevv
1. U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategies. Accessed January 18, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
2. U.S. Department of Health and Human Services, Food and Drug Administration. Format and Content of a REMS Document. Guidance for Industry. Accessed January 18, 2023. https://www.fda.gov/media/77846/download
3. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Clozapine. Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=351
4. The National Association of State Mental Health Program Directors. Clozapine underutilization: addressing the barriers. Accessed September 30, 2019. https://nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
5. U.S. Food and Drug Administration. FDA is temporarily exercising enforcement discretion with respect to certain clozapine REMS program requirements to ensure continuity of care for patients taking clozapine. Updated November 22, 2022. Accessed June 1, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-temporarily-exercising-enforcement-discretion-respect-certain-clozapine-rems-program
6. Tanzi M. REMS issues affect clozapine, isotretinoin. Pharmacy Today. 2022;28(3):49.
7. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Accessed June 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
8. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Spravato (esketamine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386
9. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Zyprexa Relprevv (olanzapine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74
10. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm
11. Parikh SV, Lopez D, Vande Voort JL, et al. Developing an IV ketamine clinic for treatment-resistant depression: a primer. Psychopharmacol Bull. 2021;51(3):109-124.
12. Dodge D. The ketamine cure. The New York Times. November 4, 2021. Updated November 5, 2021. Accessed June 1, 2023. https://www.nytimes.com/2021/11/04/well/ketamine-therapy-depression.html
13. Burton KW. Time for a national ketamine registry, experts say. Medscape. February 15, 2023. Accessed June 1, 2023. https://www.medscape.com/viewarticle/988310
14. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039-1040. doi:10.1001/jama.2019.10728
15. Wilkinson ST, Toprak M, Turner MS, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174(7):695-696. doi:10.1176/appi.ajp.2017.17020239
16. Pai SM, Gries JM; ACCP Public Policy Committee. Off-label use of ketamine: a challenging drug treatment delivery model with an inherently unfavorable risk-benefit profile. J Clin Pharmacol. 2022;62(1):10-13. doi:10.1002/jcph.1983
17. Risk Evaluation and Mitigation Strategies (REMS) Integration. Accessed June 1, 2023. https://confluence.hl7.org/display/COD/Risk+Evaluation+and+Mitigation+Strategies+%28REMS%29+Integration
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program the FDA can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks (Box1). The FDA may require medication guides, patient package inserts, communication plans for health care professionals, and/or certain packaging and safe disposal technologies for medications that pose a serious risk of abuse or overdose. The FDA may also require elements to assure safe use and/or an implementation system be included in the REMS. Pharmaceutical manufacturers then develop a proposed REMS for FDA review.2 If the FDA approves the proposed REMS, the manufacturer is responsible for implementing the REMS requirements.
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
The FDA provides this description of a Risk Evaluation and Mitigation Strategy (REMS):
“A [REMS] is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication. While all medications have labeling that informs health care stakeholders about medication risks, only a few medications require a REMS. REMS are not designed to mitigate all the adverse events of a medication, these are communicated to health care providers in the medication’s prescribing information. Rather, REMS focus on preventing, monitoring and/or managing a specific serious risk by informing, educating and/or reinforcing actions to reduce the frequency and/or severity of the event.”1
The REMS program for clozapine3 has been the subject of much discussion in the psychiatric community. The adverse impact of the 2015 update to the clozapine REMS program was emphasized at meetings of both the American Psychiatric Association and the College of Psychiatric and Neurologic Pharmacists. A white paper published by the National Association of State Mental Health Program Directors shortly after the 2015 update concluded, “clozapine is underused due to a variety of barriers related to the drug and its properties, the health care system, regulatory requirements, and reimbursement issues.”4 After an update to the clozapine REMS program in 2021, the FDA temporarily suspended enforcement of certain requirements due to concerns from health care professionals about patient access to the medication because of problems with implementing the clozapine REMS program.5,6 In November 2022, the FDA issued a second announcement of enforcement discretion related to additional requirements of the REMS program.5 The FDA had previously announced a decision to not take action regarding adherence to REMS requirements for certain laboratory tests in March 2020, during the COVID-19 pandemic.7
REMS programs for other psychiatric medications may also present challenges. The REMS programs for esketamine8 and olanzapine for extended-release (ER) injectable suspension9 include certain risks that require postadministration monitoring. Some facilities have had to dedicate additional space and clinician time to ensure REMS requirements are met.
To further understand health care professionals’ perspectives regarding the value and burden of these REMS programs, a collaborative effort of the University of Maryland (College Park and Baltimore campuses) Center of Excellence in Regulatory Science and Innovation with the FDA was undertaken. The REMS for clozapine, olanzapine for ER injectable suspension, and esketamine were examined to develop recommendations for improving patient access while ensuring safe medication use and limiting the impact on health care professionals.
Assessing the REMS programs
Focus groups were held with health care professionals nominated by professional organizations to gather their perspectives on the REMS requirements. There was 1 focus group for each of the 3 medications. A facilitator’s guide was developed that contained the details of how to conduct the focus group along with the medication-specific questions. The questions were based on the REMS requirements as of May 2021 and assessed the impact of the REMS on patient safety, patient access, and health care professional workload; effects from the COVID-19 pandemic; and suggestions to improve the REMS programs. The University of Maryland Institutional Review Board reviewed the materials and processes and made the determination of exempt.
Health care professionals were eligible to participate in a focus group if they had ≥1 year of experience working with patients who use the specific medication and ≥6 months of experience within the past year working with the REMS program for that medication. Participants were excluded if they were employed by a pharmaceutical manufacturer or the FDA. The focus groups were conducted virtually using an online conferencing service during summer 2021 and were scheduled for 90 minutes. Prior to the focus group, participants received information from the “Goals” and “Summary” tabs of the FDA REMS website10 for the specific medication along with patient/caregiver guides, which were available for clozapine and olanzapine for ER injectable suspension. For each focus group, there was a target sample size of 6 to 9 participants. However, there were only 4 participants in the olanzapine for ER injectable suspension focus group, which we believed was due to lower national utilization of this medication. Individuals were only able to participate in 1 focus group, so the unique participant count for all 3 focus groups totaled 17 (Table 1).

Themes extracted from qualitative analysis of the focus group responses were the value of the REMS programs; registration/enrollment processes and REMS websites; monitoring requirements; care transitions; and COVID considerations (Table 2). While the REMS programs were perceived to increase practitioner and patient awareness of potential harms, discussions centered on the relative cost-to-benefit of the required reporting and other REMS requirements. There were challenges with the registration/enrollment processes and REMS websites that also affected patient care during transitions to different health care settings or clinicians. Patient access was affected by disparities in care related to monitoring requirements and clinician availability.


Continue to: COVID impacted all REMS...
COVID impacted all REMS programs. Physical distancing was an issue for medications that required extensive postadministration monitoring (ie, esketamine and olanzapine for ER injectable suspension). Access to laboratory services was an issue for clozapine.

Medication-specific themes are listed in Table 3 and relate to terms and descriptions in the REMS or additional regulatory requirements from the Drug Enforcement Agency (DEA). Suggestions for improvement to the REMS are presented in Table 4.

Recommendations for improving REMS
A group consisting of health care professionals, policy experts, and mental health advocates reviewed the information provided by the focus groups and developed the following recommendations.
Overarching recommendations
Each REMS should include a section providing justification for its existence, including a risk analysis of the data regarding the risk the REMS is designed to mitigate. This analysis should be repeated on a regular basis as scientific evidence regarding the risk and its epidemiology evolves. This additional section should also explain how the program requirements of the REMS as implemented (or planned) will achieve the aims of the REMS and weigh the potential benefits of the REMS requirements as implemented (or planned) by the manufacturer vs the potential risks of the REMS requirements as implemented (or planned) by the manufacturer.
Each REMS should have specific quantifiable outcomes. For example, it should specify a reduction in occurrence of the rate of the concerned risk by a specified amount.
Continue to: Ensure adequate...
Ensure adequate stakeholder input during the REMS development and real-world testing in multiple environments before implementing the REMS to identify unanticipated consequences that might impact patient access, patient safety, and health care professional burden. Implementation testing should explore issues such as purchasing and procurement, billing and reimbursement, and relevant factors such as other federal regulations or requirements (eg, the DEA or Medicare).
Ensure harmonization of the REMS forms and processes (eg, initiation and monitoring) for different medications where possible. A prescriber, pharmacist, or system should not face additional barriers to participate in a REMS based on REMS-specific intricacies (ie, prescription systems, data submission systems, or ordering systems). This streamlining will likely decrease clinical inertia to initiate care with the REMS medication, decrease health care professional burden, and improve compliance with REMS requirements.
REMS should anticipate the need for care transitions and employ provisions to ensure seamless care. Considerations should be given to transitions that occur due to:
- Different care settings (eg, inpatient, outpatient, or long-term care)
- Different geographies (eg, patient moves)
- Changes in clinicians, including leaves or absences
- Changes in facilities (eg, pharmacies).
REMS should mirror normal health care professional workflow, including how monitoring data are collected and how and with which frequency pharmacies fill prescriptions.Enhanced information technology to support REMS programs is needed. For example, REMS should be integrated with major electronic patient health record and pharmacy systems to reduce the effort required for clinicians to supply data and automate REMS processes.
For medications that are subject to other agencies and their regulations (eg, the CDC, Centers for Medicare & Medicaid Services, or the DEA), REMS should be required to meet all standards of all agencies with a single system that accommodates normal health care professional workflow.
Continue to: REMS should have a...
REMS should have a standard disclaimer that allows the health care professional to waive certain provisions of the REMS in cases when the specific provisions of the REMS pose a greater risk to the patient than the risk posed by waiving the requirement.
Assure the actions implemented by the industry to meet the requirements for each REMS program are based on peer-reviewed evidence and provide a reasonable expectation to achieve the anticipated benefit.
Ensure that manufacturers make all accumulated REMS data available in a deidentified manner for use by qualified scientific researchers. Additionally, each REMS should have a plan for data access upon initiation and termination of the REMS.
Each REMS should collect data on the performance of the centers and/or personnel who operate the REMS and submit this data for review by qualified outside reviewers. Parameters to assess could include:
- timeliness of response
- timeliness of problem resolution
- data availability and its helpfulness to patient care
- adequacy of resources.
Recommendations for clozapine REMS
These comments relate to the clozapine REMS program prior to the July 2021 announcement that FDA had approved a modification.
Provide a clear definition for “benign ethnic neutropenia.”
Ensure the REMS includes patient-specific adjustments to allow flexibility for monitoring. During COVID, the FDA allowed clinicians to “use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of laboratory testing.”7 This guidance, which allowed flexibility to absolute neutrophil count (ANC) monitoring, was perceived as positive and safe. Before the changes in the REMS requirements, patients with benign ethnic neutropenia were restricted from accessing their medication or encountered harm from additional pharmacotherapy to mitigate ANC levels.
Continue to: Recommendations for olanzapine for ER injectable suspension REMS
Recommendations for olanzapine for ER injectable suspension REMS
Provide clear explicit instructions on what is required to have “ready access to emergency services.”
Ensure the REMS include patient-specific adjustments to allow flexibility for postadministration monitoring (eg, sedation or blood pressure). Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of olanzapine for ER injectable suspension by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness are included in the REMS. How was the 3-hour cut-point determined? Has it been reevaluated?
Ensure the REMS requirements allow for seamless care during transitions, particularly when clinicians are on vacation.
Continue to: Recommendations for esketamine REMS
Recommendations for esketamine REMS
Ensure the REMS includes patient-specific adjustments to allow flexibility for postadministration monitoring. Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of esketamine by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness of requirements are included in the REMS. How was the 2-hour cut-point determined? Has it been reevaluated?
Ensure that the REMS meet all standards of the DEA, with a single system that accommodates normal health care professional workflow.
A summary of the findings
Overall, the REMS programs for these 3 medications were positively perceived for raising awareness of safe medication use for clinicians and patients. Monitoring patients for safety concerns is important and REMS requirements provide accountability.
Continue to: The use of a single shared...
The use of a single shared REMS system for documenting requirements for clozapine (compared to separate systems for each manufacturer) was a positive move forward in implementation. The focus group welcomed the increased awareness of benign ethnic neutropenia as a result of this condition being incorporated in the revised monitoring requirements of the clozapine REMS.
Focus group participants raised the issue of the real-world efficiency of the REMS programs (reduced access and increased clinician workload) vs the benefits (patient safety). They noted that excessive workload could lead to clinicians becoming unwilling to use a medication that requires a REMS. Clinician workload may be further compromised when REMS logistics disrupt the normal workflow and transitions of care between clinicians or settings. This latter aspect is of particular concern for clozapine.
The complexities of the registration and reporting system for olanzapine for ER injectable suspension and the lack of clarity about monitoring were noted to have discouraged the opening of treatment sites. This scarcity of sites may make clinicians hesitant to use this medication, and instead opt for alternative treatments in patients who may be appropriate candidates.
There has also been limited growth of esketamine treatment sites, especially in comparison to ketamine treatment sites.11-14 Esketamine is FDA-approved for treatment-resistant depression in adults and for depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Ketamine is not FDA-approved for treating depression but is being used off-label to treat this disorder.15 The FDA determined that ketamine does not require a REMS to ensure the benefits outweigh the risks for its approved indications as an anesthetic agent, anesthesia-inducing agent, or supplement to anesthesia. Since ketamine has no REMS requirements, there may be a lower burden for its use. Thus, clinicians are treating patients for depression with this medication without needing to comply with a REMS.16
Technology plays a role in workload burden, and integrating health care processes within current workflow systems, such as using electronic patient health records and pharmacy systems, is recommended. The FDA has been exploring technologies to facilitate the completion of REMS requirements, including mandatory education within the prescribers’ and pharmacists’ workflow.17 This is a complex task that requires multiple stakeholders with differing perspectives and incentives to align.
Continue to: The data collected for the REMS...
The data collected for the REMS program belongs to the medication’s manufacturer. Current regulations do not require manufacturers to make this data available to qualified scientific researchers. A regulatory mandate to establish data sharing methods would improve transparency and enhance efforts to better understand the outcomes of the REMS programs.
A few caveats
Both the overarching and medication-specific recommendations were based on a small number of participants’ discussions related to clozapine, olanzapine for ER injectable suspension, and esketamine. These recommendations do not include other medications with REMS that are used to treat psychiatric disorders, such as loxapine, buprenorphine ER, and buprenorphine transmucosal products. Larger-scale qualitative and quantitative research is needed to better understand health care professionals’ perspectives. Lastly, some of the recommendations outlined in this article are beyond the current purview or authority of the FDA and may require legislative or regulatory action to implement.
Bottom Line
Risk Evaluation and Mitigation Strategy (REMS) programs are designed to help reduce the occurrence and/or severity of serious risks or to inform decision-making. However, REMS requirements may adversely impact patient access to certain REMS medications and clinician burden. Health care professionals can provide informed recommendations for improving the REMS programs for clozapine, olanzapine for extended-release injectable suspension, and esketamine.
Related Resources
- FDA. Frequently asked questions (FAQs) about REMS. www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/frequently-asked-questions-faqs-about-rems
Drug Brand Names
Buprenorphine extended-release • Sublocade
Buprenorphine transmucosal • Subutex, Suboxone
Clozapine • Clozaril
Esketamine • Spravato
Ketamine • Ketalar
Lithium • Eskalith, Lithobid
Loxapine • Adasuve
Olanzapine extended-release injectable suspension • Zyprexa Relprevv
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program the FDA can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks (Box1). The FDA may require medication guides, patient package inserts, communication plans for health care professionals, and/or certain packaging and safe disposal technologies for medications that pose a serious risk of abuse or overdose. The FDA may also require elements to assure safe use and/or an implementation system be included in the REMS. Pharmaceutical manufacturers then develop a proposed REMS for FDA review.2 If the FDA approves the proposed REMS, the manufacturer is responsible for implementing the REMS requirements.
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
The FDA provides this description of a Risk Evaluation and Mitigation Strategy (REMS):
“A [REMS] is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication. While all medications have labeling that informs health care stakeholders about medication risks, only a few medications require a REMS. REMS are not designed to mitigate all the adverse events of a medication, these are communicated to health care providers in the medication’s prescribing information. Rather, REMS focus on preventing, monitoring and/or managing a specific serious risk by informing, educating and/or reinforcing actions to reduce the frequency and/or severity of the event.”1
The REMS program for clozapine3 has been the subject of much discussion in the psychiatric community. The adverse impact of the 2015 update to the clozapine REMS program was emphasized at meetings of both the American Psychiatric Association and the College of Psychiatric and Neurologic Pharmacists. A white paper published by the National Association of State Mental Health Program Directors shortly after the 2015 update concluded, “clozapine is underused due to a variety of barriers related to the drug and its properties, the health care system, regulatory requirements, and reimbursement issues.”4 After an update to the clozapine REMS program in 2021, the FDA temporarily suspended enforcement of certain requirements due to concerns from health care professionals about patient access to the medication because of problems with implementing the clozapine REMS program.5,6 In November 2022, the FDA issued a second announcement of enforcement discretion related to additional requirements of the REMS program.5 The FDA had previously announced a decision to not take action regarding adherence to REMS requirements for certain laboratory tests in March 2020, during the COVID-19 pandemic.7
REMS programs for other psychiatric medications may also present challenges. The REMS programs for esketamine8 and olanzapine for extended-release (ER) injectable suspension9 include certain risks that require postadministration monitoring. Some facilities have had to dedicate additional space and clinician time to ensure REMS requirements are met.
To further understand health care professionals’ perspectives regarding the value and burden of these REMS programs, a collaborative effort of the University of Maryland (College Park and Baltimore campuses) Center of Excellence in Regulatory Science and Innovation with the FDA was undertaken. The REMS for clozapine, olanzapine for ER injectable suspension, and esketamine were examined to develop recommendations for improving patient access while ensuring safe medication use and limiting the impact on health care professionals.
Assessing the REMS programs
Focus groups were held with health care professionals nominated by professional organizations to gather their perspectives on the REMS requirements. There was 1 focus group for each of the 3 medications. A facilitator’s guide was developed that contained the details of how to conduct the focus group along with the medication-specific questions. The questions were based on the REMS requirements as of May 2021 and assessed the impact of the REMS on patient safety, patient access, and health care professional workload; effects from the COVID-19 pandemic; and suggestions to improve the REMS programs. The University of Maryland Institutional Review Board reviewed the materials and processes and made the determination of exempt.
Health care professionals were eligible to participate in a focus group if they had ≥1 year of experience working with patients who use the specific medication and ≥6 months of experience within the past year working with the REMS program for that medication. Participants were excluded if they were employed by a pharmaceutical manufacturer or the FDA. The focus groups were conducted virtually using an online conferencing service during summer 2021 and were scheduled for 90 minutes. Prior to the focus group, participants received information from the “Goals” and “Summary” tabs of the FDA REMS website10 for the specific medication along with patient/caregiver guides, which were available for clozapine and olanzapine for ER injectable suspension. For each focus group, there was a target sample size of 6 to 9 participants. However, there were only 4 participants in the olanzapine for ER injectable suspension focus group, which we believed was due to lower national utilization of this medication. Individuals were only able to participate in 1 focus group, so the unique participant count for all 3 focus groups totaled 17 (Table 1).

Themes extracted from qualitative analysis of the focus group responses were the value of the REMS programs; registration/enrollment processes and REMS websites; monitoring requirements; care transitions; and COVID considerations (Table 2). While the REMS programs were perceived to increase practitioner and patient awareness of potential harms, discussions centered on the relative cost-to-benefit of the required reporting and other REMS requirements. There were challenges with the registration/enrollment processes and REMS websites that also affected patient care during transitions to different health care settings or clinicians. Patient access was affected by disparities in care related to monitoring requirements and clinician availability.


Continue to: COVID impacted all REMS...
COVID impacted all REMS programs. Physical distancing was an issue for medications that required extensive postadministration monitoring (ie, esketamine and olanzapine for ER injectable suspension). Access to laboratory services was an issue for clozapine.

Medication-specific themes are listed in Table 3 and relate to terms and descriptions in the REMS or additional regulatory requirements from the Drug Enforcement Agency (DEA). Suggestions for improvement to the REMS are presented in Table 4.

Recommendations for improving REMS
A group consisting of health care professionals, policy experts, and mental health advocates reviewed the information provided by the focus groups and developed the following recommendations.
Overarching recommendations
Each REMS should include a section providing justification for its existence, including a risk analysis of the data regarding the risk the REMS is designed to mitigate. This analysis should be repeated on a regular basis as scientific evidence regarding the risk and its epidemiology evolves. This additional section should also explain how the program requirements of the REMS as implemented (or planned) will achieve the aims of the REMS and weigh the potential benefits of the REMS requirements as implemented (or planned) by the manufacturer vs the potential risks of the REMS requirements as implemented (or planned) by the manufacturer.
Each REMS should have specific quantifiable outcomes. For example, it should specify a reduction in occurrence of the rate of the concerned risk by a specified amount.
Continue to: Ensure adequate...
Ensure adequate stakeholder input during the REMS development and real-world testing in multiple environments before implementing the REMS to identify unanticipated consequences that might impact patient access, patient safety, and health care professional burden. Implementation testing should explore issues such as purchasing and procurement, billing and reimbursement, and relevant factors such as other federal regulations or requirements (eg, the DEA or Medicare).
Ensure harmonization of the REMS forms and processes (eg, initiation and monitoring) for different medications where possible. A prescriber, pharmacist, or system should not face additional barriers to participate in a REMS based on REMS-specific intricacies (ie, prescription systems, data submission systems, or ordering systems). This streamlining will likely decrease clinical inertia to initiate care with the REMS medication, decrease health care professional burden, and improve compliance with REMS requirements.
REMS should anticipate the need for care transitions and employ provisions to ensure seamless care. Considerations should be given to transitions that occur due to:
- Different care settings (eg, inpatient, outpatient, or long-term care)
- Different geographies (eg, patient moves)
- Changes in clinicians, including leaves or absences
- Changes in facilities (eg, pharmacies).
REMS should mirror normal health care professional workflow, including how monitoring data are collected and how and with which frequency pharmacies fill prescriptions.Enhanced information technology to support REMS programs is needed. For example, REMS should be integrated with major electronic patient health record and pharmacy systems to reduce the effort required for clinicians to supply data and automate REMS processes.
For medications that are subject to other agencies and their regulations (eg, the CDC, Centers for Medicare & Medicaid Services, or the DEA), REMS should be required to meet all standards of all agencies with a single system that accommodates normal health care professional workflow.
Continue to: REMS should have a...
REMS should have a standard disclaimer that allows the health care professional to waive certain provisions of the REMS in cases when the specific provisions of the REMS pose a greater risk to the patient than the risk posed by waiving the requirement.
Assure the actions implemented by the industry to meet the requirements for each REMS program are based on peer-reviewed evidence and provide a reasonable expectation to achieve the anticipated benefit.
Ensure that manufacturers make all accumulated REMS data available in a deidentified manner for use by qualified scientific researchers. Additionally, each REMS should have a plan for data access upon initiation and termination of the REMS.
Each REMS should collect data on the performance of the centers and/or personnel who operate the REMS and submit this data for review by qualified outside reviewers. Parameters to assess could include:
- timeliness of response
- timeliness of problem resolution
- data availability and its helpfulness to patient care
- adequacy of resources.
Recommendations for clozapine REMS
These comments relate to the clozapine REMS program prior to the July 2021 announcement that FDA had approved a modification.
Provide a clear definition for “benign ethnic neutropenia.”
Ensure the REMS includes patient-specific adjustments to allow flexibility for monitoring. During COVID, the FDA allowed clinicians to “use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of laboratory testing.”7 This guidance, which allowed flexibility to absolute neutrophil count (ANC) monitoring, was perceived as positive and safe. Before the changes in the REMS requirements, patients with benign ethnic neutropenia were restricted from accessing their medication or encountered harm from additional pharmacotherapy to mitigate ANC levels.
Continue to: Recommendations for olanzapine for ER injectable suspension REMS
Recommendations for olanzapine for ER injectable suspension REMS
Provide clear explicit instructions on what is required to have “ready access to emergency services.”
Ensure the REMS include patient-specific adjustments to allow flexibility for postadministration monitoring (eg, sedation or blood pressure). Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of olanzapine for ER injectable suspension by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness are included in the REMS. How was the 3-hour cut-point determined? Has it been reevaluated?
Ensure the REMS requirements allow for seamless care during transitions, particularly when clinicians are on vacation.
Continue to: Recommendations for esketamine REMS
Recommendations for esketamine REMS
Ensure the REMS includes patient-specific adjustments to allow flexibility for postadministration monitoring. Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of esketamine by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness of requirements are included in the REMS. How was the 2-hour cut-point determined? Has it been reevaluated?
Ensure that the REMS meet all standards of the DEA, with a single system that accommodates normal health care professional workflow.
A summary of the findings
Overall, the REMS programs for these 3 medications were positively perceived for raising awareness of safe medication use for clinicians and patients. Monitoring patients for safety concerns is important and REMS requirements provide accountability.
Continue to: The use of a single shared...
The use of a single shared REMS system for documenting requirements for clozapine (compared to separate systems for each manufacturer) was a positive move forward in implementation. The focus group welcomed the increased awareness of benign ethnic neutropenia as a result of this condition being incorporated in the revised monitoring requirements of the clozapine REMS.
Focus group participants raised the issue of the real-world efficiency of the REMS programs (reduced access and increased clinician workload) vs the benefits (patient safety). They noted that excessive workload could lead to clinicians becoming unwilling to use a medication that requires a REMS. Clinician workload may be further compromised when REMS logistics disrupt the normal workflow and transitions of care between clinicians or settings. This latter aspect is of particular concern for clozapine.
The complexities of the registration and reporting system for olanzapine for ER injectable suspension and the lack of clarity about monitoring were noted to have discouraged the opening of treatment sites. This scarcity of sites may make clinicians hesitant to use this medication, and instead opt for alternative treatments in patients who may be appropriate candidates.
There has also been limited growth of esketamine treatment sites, especially in comparison to ketamine treatment sites.11-14 Esketamine is FDA-approved for treatment-resistant depression in adults and for depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Ketamine is not FDA-approved for treating depression but is being used off-label to treat this disorder.15 The FDA determined that ketamine does not require a REMS to ensure the benefits outweigh the risks for its approved indications as an anesthetic agent, anesthesia-inducing agent, or supplement to anesthesia. Since ketamine has no REMS requirements, there may be a lower burden for its use. Thus, clinicians are treating patients for depression with this medication without needing to comply with a REMS.16
Technology plays a role in workload burden, and integrating health care processes within current workflow systems, such as using electronic patient health records and pharmacy systems, is recommended. The FDA has been exploring technologies to facilitate the completion of REMS requirements, including mandatory education within the prescribers’ and pharmacists’ workflow.17 This is a complex task that requires multiple stakeholders with differing perspectives and incentives to align.
Continue to: The data collected for the REMS...
The data collected for the REMS program belongs to the medication’s manufacturer. Current regulations do not require manufacturers to make this data available to qualified scientific researchers. A regulatory mandate to establish data sharing methods would improve transparency and enhance efforts to better understand the outcomes of the REMS programs.
A few caveats
Both the overarching and medication-specific recommendations were based on a small number of participants’ discussions related to clozapine, olanzapine for ER injectable suspension, and esketamine. These recommendations do not include other medications with REMS that are used to treat psychiatric disorders, such as loxapine, buprenorphine ER, and buprenorphine transmucosal products. Larger-scale qualitative and quantitative research is needed to better understand health care professionals’ perspectives. Lastly, some of the recommendations outlined in this article are beyond the current purview or authority of the FDA and may require legislative or regulatory action to implement.
Bottom Line
Risk Evaluation and Mitigation Strategy (REMS) programs are designed to help reduce the occurrence and/or severity of serious risks or to inform decision-making. However, REMS requirements may adversely impact patient access to certain REMS medications and clinician burden. Health care professionals can provide informed recommendations for improving the REMS programs for clozapine, olanzapine for extended-release injectable suspension, and esketamine.
Related Resources
- FDA. Frequently asked questions (FAQs) about REMS. www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/frequently-asked-questions-faqs-about-rems
Drug Brand Names
Buprenorphine extended-release • Sublocade
Buprenorphine transmucosal • Subutex, Suboxone
Clozapine • Clozaril
Esketamine • Spravato
Ketamine • Ketalar
Lithium • Eskalith, Lithobid
Loxapine • Adasuve
Olanzapine extended-release injectable suspension • Zyprexa Relprevv
1. U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategies. Accessed January 18, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
2. U.S. Department of Health and Human Services, Food and Drug Administration. Format and Content of a REMS Document. Guidance for Industry. Accessed January 18, 2023. https://www.fda.gov/media/77846/download
3. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Clozapine. Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=351
4. The National Association of State Mental Health Program Directors. Clozapine underutilization: addressing the barriers. Accessed September 30, 2019. https://nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
5. U.S. Food and Drug Administration. FDA is temporarily exercising enforcement discretion with respect to certain clozapine REMS program requirements to ensure continuity of care for patients taking clozapine. Updated November 22, 2022. Accessed June 1, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-temporarily-exercising-enforcement-discretion-respect-certain-clozapine-rems-program
6. Tanzi M. REMS issues affect clozapine, isotretinoin. Pharmacy Today. 2022;28(3):49.
7. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Accessed June 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
8. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Spravato (esketamine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386
9. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Zyprexa Relprevv (olanzapine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74
10. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm
11. Parikh SV, Lopez D, Vande Voort JL, et al. Developing an IV ketamine clinic for treatment-resistant depression: a primer. Psychopharmacol Bull. 2021;51(3):109-124.
12. Dodge D. The ketamine cure. The New York Times. November 4, 2021. Updated November 5, 2021. Accessed June 1, 2023. https://www.nytimes.com/2021/11/04/well/ketamine-therapy-depression.html
13. Burton KW. Time for a national ketamine registry, experts say. Medscape. February 15, 2023. Accessed June 1, 2023. https://www.medscape.com/viewarticle/988310
14. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039-1040. doi:10.1001/jama.2019.10728
15. Wilkinson ST, Toprak M, Turner MS, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174(7):695-696. doi:10.1176/appi.ajp.2017.17020239
16. Pai SM, Gries JM; ACCP Public Policy Committee. Off-label use of ketamine: a challenging drug treatment delivery model with an inherently unfavorable risk-benefit profile. J Clin Pharmacol. 2022;62(1):10-13. doi:10.1002/jcph.1983
17. Risk Evaluation and Mitigation Strategies (REMS) Integration. Accessed June 1, 2023. https://confluence.hl7.org/display/COD/Risk+Evaluation+and+Mitigation+Strategies+%28REMS%29+Integration
1. U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategies. Accessed January 18, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
2. U.S. Department of Health and Human Services, Food and Drug Administration. Format and Content of a REMS Document. Guidance for Industry. Accessed January 18, 2023. https://www.fda.gov/media/77846/download
3. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Clozapine. Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=351
4. The National Association of State Mental Health Program Directors. Clozapine underutilization: addressing the barriers. Accessed September 30, 2019. https://nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
5. U.S. Food and Drug Administration. FDA is temporarily exercising enforcement discretion with respect to certain clozapine REMS program requirements to ensure continuity of care for patients taking clozapine. Updated November 22, 2022. Accessed June 1, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-temporarily-exercising-enforcement-discretion-respect-certain-clozapine-rems-program
6. Tanzi M. REMS issues affect clozapine, isotretinoin. Pharmacy Today. 2022;28(3):49.
7. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Accessed June 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
8. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Spravato (esketamine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386
9. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Zyprexa Relprevv (olanzapine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74
10. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm
11. Parikh SV, Lopez D, Vande Voort JL, et al. Developing an IV ketamine clinic for treatment-resistant depression: a primer. Psychopharmacol Bull. 2021;51(3):109-124.
12. Dodge D. The ketamine cure. The New York Times. November 4, 2021. Updated November 5, 2021. Accessed June 1, 2023. https://www.nytimes.com/2021/11/04/well/ketamine-therapy-depression.html
13. Burton KW. Time for a national ketamine registry, experts say. Medscape. February 15, 2023. Accessed June 1, 2023. https://www.medscape.com/viewarticle/988310
14. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039-1040. doi:10.1001/jama.2019.10728
15. Wilkinson ST, Toprak M, Turner MS, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174(7):695-696. doi:10.1176/appi.ajp.2017.17020239
16. Pai SM, Gries JM; ACCP Public Policy Committee. Off-label use of ketamine: a challenging drug treatment delivery model with an inherently unfavorable risk-benefit profile. J Clin Pharmacol. 2022;62(1):10-13. doi:10.1002/jcph.1983
17. Risk Evaluation and Mitigation Strategies (REMS) Integration. Accessed June 1, 2023. https://confluence.hl7.org/display/COD/Risk+Evaluation+and+Mitigation+Strategies+%28REMS%29+Integration
When a disaster disrupts access to psychiatric medications
In recent decades, disasters such as storms, earthquakes, and terrorism have occurred with increasing frequency. Disaster planners assess the needs and vulnerabilities of communities in order to save lives during these events. They focus on providing electricity and clean water and addressing other public health measures. What is not adequately planned for, in our opinion, is a disruption in the pharmaceutical supply chain, particularly supplies of psychiatric medications.
There is now a rich literature on disaster psychiatry.1-4 However, there’s been a lack of information about disrupted access to psychiatric medications. Disruptive behavior after Hurricanes Katrina, Maria, Rita, and others were a consequence of a lack of medications or difficulty obtaining medications following these disasters.5-7
This article discusses the pharmaceutical supply chain, the lack of stockpiles of psychiatric medications, and how clinicians can prepare themselves and their patients in the event a disaster strikes.
Supply chains
Each day, nearly 12 million prescriptions are filled in the United States, with gratifying swiftness, efficiency, and accuracy. Our confidence in the nation’s pharmaceutical dependability, however, rests squarely upon the strength and resilience of vast, interconnected supply chains that involve the myriad aspects of private industry—from manufacturing to shipping and transport to last-mile delivery from pharmacy to patient. The failure of any one of the links in any of these supply chains can result in the instant unavailability of critical medications.
Supply chains are fundamental to modern life and must fluctuate to address disruptions; however, common supplemental and gap-filling functions that address minor changes may be insufficient to mitigate supply chain disruptions during a disaster. While supply chains can be extremely complex and can vary significantly from product to product, all supply chains can generally be presented through the components found in Table 1.
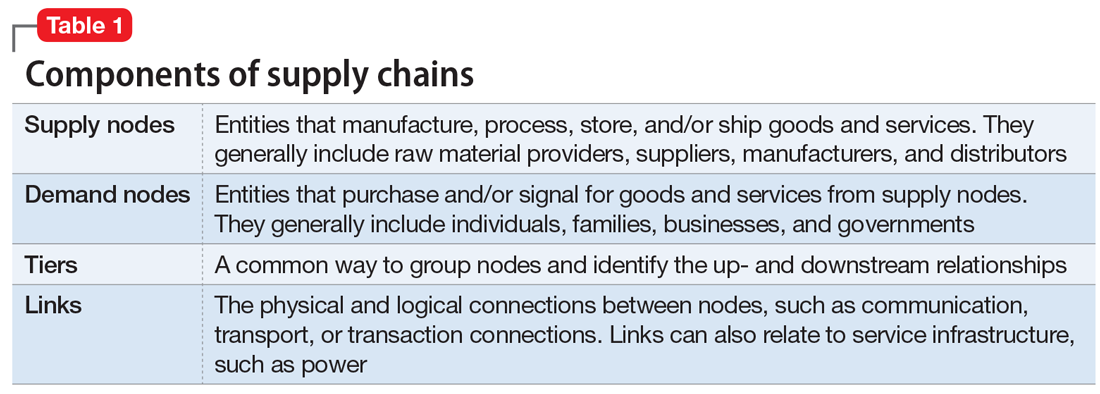
All components within a supply chain, such as the transportation mechanisms between nodes, facilities, people, and communication networks, can affect a supply chain’s resilience. For a supply chain to be resilient, key players—in this case, psychiatrists and associated medical professionals—must be acutely aware of the supply chain elements within their vision and reasonable anticipation: known nodes and links, their potential vulnerabilities, and ways and means to mitigate expected disruption.
Recent natural disasters, especially Hurricanes Katrina, Sandy, Harvey, and Maria, have given both government emergency management (at all levels) and clinicians the opportunity to understand the full effects of broken pharmaceutical supply chains under varying and extreme circumstances.
Continue to: As stated in a...
As stated in a recent Department of Homeland Security health care supply chain report, “Pharmaceuticals are one of the top concerns for healthcare providers in terms of supply chain disruptions. They are prone to various supply chain problems, including limited sources, lack of alternatives, time sensitivity, frequent shortages, and minimal on-site inventories. Each stakeholder along the pharmaceutical supply chain faces challenges with understanding and planning for possible disruptions emerging further up the chain. The rapidly expanding use of just-in-time inventory practices by distributors and healthcare customers is creating an increasingly fragile supply-demand balance that could be highly disrupted by a major event either further up the supply chain or within the last mile of delivery.”8,9
No national stockpiles of psychiatric medications
The CDC maintains stockpiles of emergency medications, but these supplies focus on medications to combat infection. In these caches, there are no psychiatric medications other than diazepam, which is stocked for its ability to combat the effects of nerve agents.
In major storm-related events, such as Hurricane Katrina in New Orleans in 2005, the disruptions in all supply chains included psychiatric medications. In the aftermath, many people with addictions and/or severe mental illnesses did not receive either their drugs of choice and/or antimanic and antipsychotic medications. As a result, disruptive behavior became common, especially in the shelters.5-7
During a widespread public emergency, police and emergency services are often stretched very thin. In calmer times, police or emergency services may take a person with disruptive and aggressive behavior to a local emergency department. However, in times of chaos, such as during Hurricane Katrina, patients with aggressive or disruptive behaviors were forcefully incapacitated (ie, “tased”) or shot.
Withdrawal from antidepressants, opiates, alcohol, and benzodiazepines has its own risks. Withdrawal from alcohol or benzodiazepines can be life-threatening. Therefore, it is critically important that clinicians think about how to ensure their patients have a supply of their medications. This may imply stockpiling on a personal or community basis.
Continue to: What to consider before disruption
What to consider before disruption
Many psychiatrists, especially those who have not practiced through a local disaster, may have never contemplated how they would support their patients during a disruptive event. Psychiatrists should carefully consider the questions outlined in Table 2 before a disaster strikes.

Medication-specific issues
During major disasters, patients may not have access to their medications, or the medications may not be able to be fed into the health care system for dispersion. Other issues include closed pharmacies, expired medications as a result of limited refrigeration service, inability to deliver medications to an affected area, and the inability of manufacturing plants to produce medications. For example, after Hurricane Maria, sterile water was in short supply.
After a major disaster, clinicians often leave their communities because they cannot support themselves or their practices. Thus, clinicians may not be available to prescribe needed medications. Available clinicians—often primary care physicians—may not be aware of a patient’s medication history, or they may be uncomfortable prescribing psychiatric medications, especially antipsychotics.
Abrupt discontinuation of psychiatric medications can have severe consequences. Patients may experience withdrawal symptoms, worsening psychiatric symptoms, new-onset psychiatric symptoms, thoughts of harm to self or others, psychosis, or cravings. These issues may be particularly problematic for patients receiving antidepressants, antipsychotics, benzodiazepines, or medication-assisted treatment for opioid use disorder.
Antidepressants. Patients experiencing antidepressant withdrawal, particularly withdrawal from selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, may exhibit severe symptoms. In addition to the potential recurrence of depressive or anxiety symptoms and suicidal thoughts, patients may experience irritability, insomnia, headache, nausea, and electric shock–like sensations. Prescribing an antidepressant with a longer half-life could potentially prevent an abrupt withdrawal in the event a disaster occurs.
Continue to: Antipsychotics
Antipsychotics. Rapid or abrupt withdrawal of antipsychotics could lead to an increase in psychosis, paranoia, hallucinations, or delusions. Withdrawal of antipsychotics could also lead to agitation, restlessness, insomnia, paresthesia, and anxiety. If a known disaster is likely to occur, such as in the case of a hurricane forecast, clinicians may consider switching a patient a long-acting injectable antipsychotic to minimize the risk of withdrawal and symptom exacerbation.
Benzodiazepines. The abrupt withdrawal of benzodiazepines could result in symptoms that include rebound anxiety, insomnia, restlessness, muscle tension, irritability, nausea, malaise, blurred vision, diaphoresis, nightmares, and seizures. Additionally, many people use benzodiazepines recreationally, and their illicit supply may run out during disasters, which could lead to untreated withdrawal and violence in the community.
Clinicians need to develop action plans for any patients who are receiving scheduled benzodiazepine dosing in order to prevent abrupt withdrawal if a disaster occurs.
Opioids. Opioid cravings and withdrawal are also a major concern during times of disrupted supply. Patients receiving chronic opioid therapy may not be able to receive their maintenance medications, which could lead to withdrawal. Additionally, patients taking illicit opioids may also be at risk of withdrawal.
Early symptoms of opioid withdrawal include watery eyes, runny nose, sweating, anxiety and irritability, poor sleep, and muscle pain. Later symptoms could include cramping, diarrhea, vomiting, increased heart rate and blood pressure, restlessness, shakiness, chills, sweating, and dilated pupils.
Continue to: Contingency planning...
Contingency planning should be a part of the treatment plan for every patient receiving chronic opioid therapy who lives in an area where major disasters are likely to occur.
Medication-assisted treatment for opioid use disorder. Patients receiving treatment for opioid use disorder may be prescribed the partial opioid agonist buprenorphine, either by itself or in combination with the opioid antagonist naloxone. This could be particularly problematic to continue in a major disaster due to the lack of credentialed clinicians, limited supplies, and patients only receiving small amounts of the medication at a time due to the risk of diversion.
Symptoms of buprenorphine withdrawal are similar to those associated with opioid withdrawal. Developing a thoughtful plan in case of a disaster should be part of all buprenorphine prescribing. Patients should be aware of withdrawal symptoms and what to do if they run out of medication.
Additionally, emergency clinicians should have access to buprenorphine and buprenorphine/naloxone and the ability to prescribe them in disaster situations. As with all aspects of disaster response, it is wise to work out issues in advance.
Help your patients get ready
Advise your patients to prepare emergency kits that contain their psychiatric medications that they could quickly grab and go if needed. Because there may be times when it is not possible to gather all necessary medications, having even a small supply ready to go at a moment’s notice would be beneficial. If permitted, patients should also consider keeping medications in multiple locations, including at their place of work, home, or a family member’s home.
Continue to: Additionally, instruct patients...
Additionally, instruct patients to always carry a list of all medications they currently take. Ideally, this list should also include past medications and responses, allergies, and provider contact information. During a disaster, this information could prove vital to an emergency clinician. At a minimum, verify that your patient maintains a list of current medications.
Clinicians should develop emergency plans for all psychiatric medications they prescribe. Document and discuss with your patients any necessary considerations for patients who take medications that require more intensive monitoring, such as lithium or clozapine.
Clinicians, patients, emergency responders, and health care workers need to work together to prepare for major disasters to avoid withdrawal and other consequences of disrupted access to psychiatric medications.
Bottom Line
Consult with local public health officials to determine and develop contingency plans to provide psychiatric medications to your patients in the event of a disaster. Discuss treatment plans and contingency planning with patients, particularly those in regions most likely to be affected by a disaster. Instruct patients to refill medications prior to a foreseeable disaster and to maintain a personal stockpile of medications when appropriate.
Related Resources
- Ochi S, Hodgson S, Landeg O, et al. Disaster-driven evacuation and medication loss: A systematic literature review. PLoS Curr. 2014;6.b. doi: 10.1371/currents.dis.fa417630b566a0c7dfdbf945910edd96.
- Pate JE, Fisher JW. Disaster ethics: What are the ground rules? Current Psychiatry. 2007;6(6):69-78.
Drug Brand Names
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone
Clozapine • Clozaril
Diazepam • Valium
Lithium • Eskalith, Lithobid
1. National Institute of Mental Health. Mental health and mass violence: evidence based early psychological intervention for victims/survivors of mass violence. A workshop to reach consensus on best practices. https://cpa.ca/docs/File/Emergencies/massviolence.pdf. Published 2002. Accessed March 11, 2019.
2. Ritchie EC, Friedman M, Watson P. Interventions following mass violence and disasters: strategies for mental health practice. New York, NY: Guilford Press; 2006.
3. Ritchie EC, O’Brien K, Grant M, et al. Disaster psychiatry. In: Stern TA, Rosenbaum JF, Fava M, et al. The Massachusetts General Hospital textbook of comprehensive clinical psychiatry, 2nd edition. Philadelphia, PA: Mosby/Elsevier; 2016:968-974.
4. Ritchie EC, Hamilton S. Early interventions and risk assessment following disaster. Psychiatric Annals. 2004;34(8):605-610.
5. Kessler RC, Galea S, Gruber MJ, et al. Trends in mental illness and suicidality after Hurricane Katrina. Mol Psychiatry. 2008;13(4):374-384.
6. Weisler RH, Barbee JG IV, Townsend MH. Mental health and recovery in the Gulf Coast after Hurricanes Katrina and Rita. JAMA. 2006;296(5):585-588.
7. Galea S, Brewin CR, Gruber M, et al. Exposure to hurricane-related stressors and mental illness after Hurricane Katrina. Arch Gen Psychiatry. 2007;64(12).1427-1434.
8. Federal Emergency Management Agency. Supply Chain Resilience Guide Department of Homeland Security. https://www.fema.gov/media-library-data/1544795397837-767851ba177c7097bf8672aadf8a93c9/NE_DRAFT_Supply_Chain_Resilience.pdf. Published December 17, 2018. Accessed January 2, 2019.
9. Durkin J, Telab M, Fitzmaurice P, et al. Only as strong as its weakest link: resilience of the healthcare supply chain in New York. https://www.hstoday.us/subject-matter-areas/emergency-preparedness/only-as-strong-as-its-weakest-link-the-resilience-of-the-healthcare-supply-chain-in-new-york/. Published October 26, 2018. Accessed February 14, 2019.
In recent decades, disasters such as storms, earthquakes, and terrorism have occurred with increasing frequency. Disaster planners assess the needs and vulnerabilities of communities in order to save lives during these events. They focus on providing electricity and clean water and addressing other public health measures. What is not adequately planned for, in our opinion, is a disruption in the pharmaceutical supply chain, particularly supplies of psychiatric medications.
There is now a rich literature on disaster psychiatry.1-4 However, there’s been a lack of information about disrupted access to psychiatric medications. Disruptive behavior after Hurricanes Katrina, Maria, Rita, and others were a consequence of a lack of medications or difficulty obtaining medications following these disasters.5-7
This article discusses the pharmaceutical supply chain, the lack of stockpiles of psychiatric medications, and how clinicians can prepare themselves and their patients in the event a disaster strikes.
Supply chains
Each day, nearly 12 million prescriptions are filled in the United States, with gratifying swiftness, efficiency, and accuracy. Our confidence in the nation’s pharmaceutical dependability, however, rests squarely upon the strength and resilience of vast, interconnected supply chains that involve the myriad aspects of private industry—from manufacturing to shipping and transport to last-mile delivery from pharmacy to patient. The failure of any one of the links in any of these supply chains can result in the instant unavailability of critical medications.
Supply chains are fundamental to modern life and must fluctuate to address disruptions; however, common supplemental and gap-filling functions that address minor changes may be insufficient to mitigate supply chain disruptions during a disaster. While supply chains can be extremely complex and can vary significantly from product to product, all supply chains can generally be presented through the components found in Table 1.

All components within a supply chain, such as the transportation mechanisms between nodes, facilities, people, and communication networks, can affect a supply chain’s resilience. For a supply chain to be resilient, key players—in this case, psychiatrists and associated medical professionals—must be acutely aware of the supply chain elements within their vision and reasonable anticipation: known nodes and links, their potential vulnerabilities, and ways and means to mitigate expected disruption.
Recent natural disasters, especially Hurricanes Katrina, Sandy, Harvey, and Maria, have given both government emergency management (at all levels) and clinicians the opportunity to understand the full effects of broken pharmaceutical supply chains under varying and extreme circumstances.
Continue to: As stated in a...
As stated in a recent Department of Homeland Security health care supply chain report, “Pharmaceuticals are one of the top concerns for healthcare providers in terms of supply chain disruptions. They are prone to various supply chain problems, including limited sources, lack of alternatives, time sensitivity, frequent shortages, and minimal on-site inventories. Each stakeholder along the pharmaceutical supply chain faces challenges with understanding and planning for possible disruptions emerging further up the chain. The rapidly expanding use of just-in-time inventory practices by distributors and healthcare customers is creating an increasingly fragile supply-demand balance that could be highly disrupted by a major event either further up the supply chain or within the last mile of delivery.”8,9
No national stockpiles of psychiatric medications
The CDC maintains stockpiles of emergency medications, but these supplies focus on medications to combat infection. In these caches, there are no psychiatric medications other than diazepam, which is stocked for its ability to combat the effects of nerve agents.
In major storm-related events, such as Hurricane Katrina in New Orleans in 2005, the disruptions in all supply chains included psychiatric medications. In the aftermath, many people with addictions and/or severe mental illnesses did not receive either their drugs of choice and/or antimanic and antipsychotic medications. As a result, disruptive behavior became common, especially in the shelters.5-7
During a widespread public emergency, police and emergency services are often stretched very thin. In calmer times, police or emergency services may take a person with disruptive and aggressive behavior to a local emergency department. However, in times of chaos, such as during Hurricane Katrina, patients with aggressive or disruptive behaviors were forcefully incapacitated (ie, “tased”) or shot.
Withdrawal from antidepressants, opiates, alcohol, and benzodiazepines has its own risks. Withdrawal from alcohol or benzodiazepines can be life-threatening. Therefore, it is critically important that clinicians think about how to ensure their patients have a supply of their medications. This may imply stockpiling on a personal or community basis.
Continue to: What to consider before disruption
What to consider before disruption
Many psychiatrists, especially those who have not practiced through a local disaster, may have never contemplated how they would support their patients during a disruptive event. Psychiatrists should carefully consider the questions outlined in Table 2 before a disaster strikes.

Medication-specific issues
During major disasters, patients may not have access to their medications, or the medications may not be able to be fed into the health care system for dispersion. Other issues include closed pharmacies, expired medications as a result of limited refrigeration service, inability to deliver medications to an affected area, and the inability of manufacturing plants to produce medications. For example, after Hurricane Maria, sterile water was in short supply.
After a major disaster, clinicians often leave their communities because they cannot support themselves or their practices. Thus, clinicians may not be available to prescribe needed medications. Available clinicians—often primary care physicians—may not be aware of a patient’s medication history, or they may be uncomfortable prescribing psychiatric medications, especially antipsychotics.
Abrupt discontinuation of psychiatric medications can have severe consequences. Patients may experience withdrawal symptoms, worsening psychiatric symptoms, new-onset psychiatric symptoms, thoughts of harm to self or others, psychosis, or cravings. These issues may be particularly problematic for patients receiving antidepressants, antipsychotics, benzodiazepines, or medication-assisted treatment for opioid use disorder.
Antidepressants. Patients experiencing antidepressant withdrawal, particularly withdrawal from selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, may exhibit severe symptoms. In addition to the potential recurrence of depressive or anxiety symptoms and suicidal thoughts, patients may experience irritability, insomnia, headache, nausea, and electric shock–like sensations. Prescribing an antidepressant with a longer half-life could potentially prevent an abrupt withdrawal in the event a disaster occurs.
Continue to: Antipsychotics
Antipsychotics. Rapid or abrupt withdrawal of antipsychotics could lead to an increase in psychosis, paranoia, hallucinations, or delusions. Withdrawal of antipsychotics could also lead to agitation, restlessness, insomnia, paresthesia, and anxiety. If a known disaster is likely to occur, such as in the case of a hurricane forecast, clinicians may consider switching a patient a long-acting injectable antipsychotic to minimize the risk of withdrawal and symptom exacerbation.
Benzodiazepines. The abrupt withdrawal of benzodiazepines could result in symptoms that include rebound anxiety, insomnia, restlessness, muscle tension, irritability, nausea, malaise, blurred vision, diaphoresis, nightmares, and seizures. Additionally, many people use benzodiazepines recreationally, and their illicit supply may run out during disasters, which could lead to untreated withdrawal and violence in the community.
Clinicians need to develop action plans for any patients who are receiving scheduled benzodiazepine dosing in order to prevent abrupt withdrawal if a disaster occurs.
Opioids. Opioid cravings and withdrawal are also a major concern during times of disrupted supply. Patients receiving chronic opioid therapy may not be able to receive their maintenance medications, which could lead to withdrawal. Additionally, patients taking illicit opioids may also be at risk of withdrawal.
Early symptoms of opioid withdrawal include watery eyes, runny nose, sweating, anxiety and irritability, poor sleep, and muscle pain. Later symptoms could include cramping, diarrhea, vomiting, increased heart rate and blood pressure, restlessness, shakiness, chills, sweating, and dilated pupils.
Continue to: Contingency planning...
Contingency planning should be a part of the treatment plan for every patient receiving chronic opioid therapy who lives in an area where major disasters are likely to occur.
Medication-assisted treatment for opioid use disorder. Patients receiving treatment for opioid use disorder may be prescribed the partial opioid agonist buprenorphine, either by itself or in combination with the opioid antagonist naloxone. This could be particularly problematic to continue in a major disaster due to the lack of credentialed clinicians, limited supplies, and patients only receiving small amounts of the medication at a time due to the risk of diversion.
Symptoms of buprenorphine withdrawal are similar to those associated with opioid withdrawal. Developing a thoughtful plan in case of a disaster should be part of all buprenorphine prescribing. Patients should be aware of withdrawal symptoms and what to do if they run out of medication.
Additionally, emergency clinicians should have access to buprenorphine and buprenorphine/naloxone and the ability to prescribe them in disaster situations. As with all aspects of disaster response, it is wise to work out issues in advance.
Help your patients get ready
Advise your patients to prepare emergency kits that contain their psychiatric medications that they could quickly grab and go if needed. Because there may be times when it is not possible to gather all necessary medications, having even a small supply ready to go at a moment’s notice would be beneficial. If permitted, patients should also consider keeping medications in multiple locations, including at their place of work, home, or a family member’s home.
Continue to: Additionally, instruct patients...
Additionally, instruct patients to always carry a list of all medications they currently take. Ideally, this list should also include past medications and responses, allergies, and provider contact information. During a disaster, this information could prove vital to an emergency clinician. At a minimum, verify that your patient maintains a list of current medications.
Clinicians should develop emergency plans for all psychiatric medications they prescribe. Document and discuss with your patients any necessary considerations for patients who take medications that require more intensive monitoring, such as lithium or clozapine.
Clinicians, patients, emergency responders, and health care workers need to work together to prepare for major disasters to avoid withdrawal and other consequences of disrupted access to psychiatric medications.
Bottom Line
Consult with local public health officials to determine and develop contingency plans to provide psychiatric medications to your patients in the event of a disaster. Discuss treatment plans and contingency planning with patients, particularly those in regions most likely to be affected by a disaster. Instruct patients to refill medications prior to a foreseeable disaster and to maintain a personal stockpile of medications when appropriate.
Related Resources
- Ochi S, Hodgson S, Landeg O, et al. Disaster-driven evacuation and medication loss: A systematic literature review. PLoS Curr. 2014;6.b. doi: 10.1371/currents.dis.fa417630b566a0c7dfdbf945910edd96.
- Pate JE, Fisher JW. Disaster ethics: What are the ground rules? Current Psychiatry. 2007;6(6):69-78.
Drug Brand Names
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone
Clozapine • Clozaril
Diazepam • Valium
Lithium • Eskalith, Lithobid
In recent decades, disasters such as storms, earthquakes, and terrorism have occurred with increasing frequency. Disaster planners assess the needs and vulnerabilities of communities in order to save lives during these events. They focus on providing electricity and clean water and addressing other public health measures. What is not adequately planned for, in our opinion, is a disruption in the pharmaceutical supply chain, particularly supplies of psychiatric medications.
There is now a rich literature on disaster psychiatry.1-4 However, there’s been a lack of information about disrupted access to psychiatric medications. Disruptive behavior after Hurricanes Katrina, Maria, Rita, and others were a consequence of a lack of medications or difficulty obtaining medications following these disasters.5-7
This article discusses the pharmaceutical supply chain, the lack of stockpiles of psychiatric medications, and how clinicians can prepare themselves and their patients in the event a disaster strikes.
Supply chains
Each day, nearly 12 million prescriptions are filled in the United States, with gratifying swiftness, efficiency, and accuracy. Our confidence in the nation’s pharmaceutical dependability, however, rests squarely upon the strength and resilience of vast, interconnected supply chains that involve the myriad aspects of private industry—from manufacturing to shipping and transport to last-mile delivery from pharmacy to patient. The failure of any one of the links in any of these supply chains can result in the instant unavailability of critical medications.
Supply chains are fundamental to modern life and must fluctuate to address disruptions; however, common supplemental and gap-filling functions that address minor changes may be insufficient to mitigate supply chain disruptions during a disaster. While supply chains can be extremely complex and can vary significantly from product to product, all supply chains can generally be presented through the components found in Table 1.

All components within a supply chain, such as the transportation mechanisms between nodes, facilities, people, and communication networks, can affect a supply chain’s resilience. For a supply chain to be resilient, key players—in this case, psychiatrists and associated medical professionals—must be acutely aware of the supply chain elements within their vision and reasonable anticipation: known nodes and links, their potential vulnerabilities, and ways and means to mitigate expected disruption.
Recent natural disasters, especially Hurricanes Katrina, Sandy, Harvey, and Maria, have given both government emergency management (at all levels) and clinicians the opportunity to understand the full effects of broken pharmaceutical supply chains under varying and extreme circumstances.
Continue to: As stated in a...
As stated in a recent Department of Homeland Security health care supply chain report, “Pharmaceuticals are one of the top concerns for healthcare providers in terms of supply chain disruptions. They are prone to various supply chain problems, including limited sources, lack of alternatives, time sensitivity, frequent shortages, and minimal on-site inventories. Each stakeholder along the pharmaceutical supply chain faces challenges with understanding and planning for possible disruptions emerging further up the chain. The rapidly expanding use of just-in-time inventory practices by distributors and healthcare customers is creating an increasingly fragile supply-demand balance that could be highly disrupted by a major event either further up the supply chain or within the last mile of delivery.”8,9
No national stockpiles of psychiatric medications
The CDC maintains stockpiles of emergency medications, but these supplies focus on medications to combat infection. In these caches, there are no psychiatric medications other than diazepam, which is stocked for its ability to combat the effects of nerve agents.
In major storm-related events, such as Hurricane Katrina in New Orleans in 2005, the disruptions in all supply chains included psychiatric medications. In the aftermath, many people with addictions and/or severe mental illnesses did not receive either their drugs of choice and/or antimanic and antipsychotic medications. As a result, disruptive behavior became common, especially in the shelters.5-7
During a widespread public emergency, police and emergency services are often stretched very thin. In calmer times, police or emergency services may take a person with disruptive and aggressive behavior to a local emergency department. However, in times of chaos, such as during Hurricane Katrina, patients with aggressive or disruptive behaviors were forcefully incapacitated (ie, “tased”) or shot.
Withdrawal from antidepressants, opiates, alcohol, and benzodiazepines has its own risks. Withdrawal from alcohol or benzodiazepines can be life-threatening. Therefore, it is critically important that clinicians think about how to ensure their patients have a supply of their medications. This may imply stockpiling on a personal or community basis.
Continue to: What to consider before disruption
What to consider before disruption
Many psychiatrists, especially those who have not practiced through a local disaster, may have never contemplated how they would support their patients during a disruptive event. Psychiatrists should carefully consider the questions outlined in Table 2 before a disaster strikes.

Medication-specific issues
During major disasters, patients may not have access to their medications, or the medications may not be able to be fed into the health care system for dispersion. Other issues include closed pharmacies, expired medications as a result of limited refrigeration service, inability to deliver medications to an affected area, and the inability of manufacturing plants to produce medications. For example, after Hurricane Maria, sterile water was in short supply.
After a major disaster, clinicians often leave their communities because they cannot support themselves or their practices. Thus, clinicians may not be available to prescribe needed medications. Available clinicians—often primary care physicians—may not be aware of a patient’s medication history, or they may be uncomfortable prescribing psychiatric medications, especially antipsychotics.
Abrupt discontinuation of psychiatric medications can have severe consequences. Patients may experience withdrawal symptoms, worsening psychiatric symptoms, new-onset psychiatric symptoms, thoughts of harm to self or others, psychosis, or cravings. These issues may be particularly problematic for patients receiving antidepressants, antipsychotics, benzodiazepines, or medication-assisted treatment for opioid use disorder.
Antidepressants. Patients experiencing antidepressant withdrawal, particularly withdrawal from selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, may exhibit severe symptoms. In addition to the potential recurrence of depressive or anxiety symptoms and suicidal thoughts, patients may experience irritability, insomnia, headache, nausea, and electric shock–like sensations. Prescribing an antidepressant with a longer half-life could potentially prevent an abrupt withdrawal in the event a disaster occurs.
Continue to: Antipsychotics
Antipsychotics. Rapid or abrupt withdrawal of antipsychotics could lead to an increase in psychosis, paranoia, hallucinations, or delusions. Withdrawal of antipsychotics could also lead to agitation, restlessness, insomnia, paresthesia, and anxiety. If a known disaster is likely to occur, such as in the case of a hurricane forecast, clinicians may consider switching a patient a long-acting injectable antipsychotic to minimize the risk of withdrawal and symptom exacerbation.
Benzodiazepines. The abrupt withdrawal of benzodiazepines could result in symptoms that include rebound anxiety, insomnia, restlessness, muscle tension, irritability, nausea, malaise, blurred vision, diaphoresis, nightmares, and seizures. Additionally, many people use benzodiazepines recreationally, and their illicit supply may run out during disasters, which could lead to untreated withdrawal and violence in the community.
Clinicians need to develop action plans for any patients who are receiving scheduled benzodiazepine dosing in order to prevent abrupt withdrawal if a disaster occurs.
Opioids. Opioid cravings and withdrawal are also a major concern during times of disrupted supply. Patients receiving chronic opioid therapy may not be able to receive their maintenance medications, which could lead to withdrawal. Additionally, patients taking illicit opioids may also be at risk of withdrawal.
Early symptoms of opioid withdrawal include watery eyes, runny nose, sweating, anxiety and irritability, poor sleep, and muscle pain. Later symptoms could include cramping, diarrhea, vomiting, increased heart rate and blood pressure, restlessness, shakiness, chills, sweating, and dilated pupils.
Continue to: Contingency planning...
Contingency planning should be a part of the treatment plan for every patient receiving chronic opioid therapy who lives in an area where major disasters are likely to occur.
Medication-assisted treatment for opioid use disorder. Patients receiving treatment for opioid use disorder may be prescribed the partial opioid agonist buprenorphine, either by itself or in combination with the opioid antagonist naloxone. This could be particularly problematic to continue in a major disaster due to the lack of credentialed clinicians, limited supplies, and patients only receiving small amounts of the medication at a time due to the risk of diversion.
Symptoms of buprenorphine withdrawal are similar to those associated with opioid withdrawal. Developing a thoughtful plan in case of a disaster should be part of all buprenorphine prescribing. Patients should be aware of withdrawal symptoms and what to do if they run out of medication.
Additionally, emergency clinicians should have access to buprenorphine and buprenorphine/naloxone and the ability to prescribe them in disaster situations. As with all aspects of disaster response, it is wise to work out issues in advance.
Help your patients get ready
Advise your patients to prepare emergency kits that contain their psychiatric medications that they could quickly grab and go if needed. Because there may be times when it is not possible to gather all necessary medications, having even a small supply ready to go at a moment’s notice would be beneficial. If permitted, patients should also consider keeping medications in multiple locations, including at their place of work, home, or a family member’s home.
Continue to: Additionally, instruct patients...
Additionally, instruct patients to always carry a list of all medications they currently take. Ideally, this list should also include past medications and responses, allergies, and provider contact information. During a disaster, this information could prove vital to an emergency clinician. At a minimum, verify that your patient maintains a list of current medications.
Clinicians should develop emergency plans for all psychiatric medications they prescribe. Document and discuss with your patients any necessary considerations for patients who take medications that require more intensive monitoring, such as lithium or clozapine.
Clinicians, patients, emergency responders, and health care workers need to work together to prepare for major disasters to avoid withdrawal and other consequences of disrupted access to psychiatric medications.
Bottom Line
Consult with local public health officials to determine and develop contingency plans to provide psychiatric medications to your patients in the event of a disaster. Discuss treatment plans and contingency planning with patients, particularly those in regions most likely to be affected by a disaster. Instruct patients to refill medications prior to a foreseeable disaster and to maintain a personal stockpile of medications when appropriate.
Related Resources
- Ochi S, Hodgson S, Landeg O, et al. Disaster-driven evacuation and medication loss: A systematic literature review. PLoS Curr. 2014;6.b. doi: 10.1371/currents.dis.fa417630b566a0c7dfdbf945910edd96.
- Pate JE, Fisher JW. Disaster ethics: What are the ground rules? Current Psychiatry. 2007;6(6):69-78.
Drug Brand Names
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone
Clozapine • Clozaril
Diazepam • Valium
Lithium • Eskalith, Lithobid
1. National Institute of Mental Health. Mental health and mass violence: evidence based early psychological intervention for victims/survivors of mass violence. A workshop to reach consensus on best practices. https://cpa.ca/docs/File/Emergencies/massviolence.pdf. Published 2002. Accessed March 11, 2019.
2. Ritchie EC, Friedman M, Watson P. Interventions following mass violence and disasters: strategies for mental health practice. New York, NY: Guilford Press; 2006.
3. Ritchie EC, O’Brien K, Grant M, et al. Disaster psychiatry. In: Stern TA, Rosenbaum JF, Fava M, et al. The Massachusetts General Hospital textbook of comprehensive clinical psychiatry, 2nd edition. Philadelphia, PA: Mosby/Elsevier; 2016:968-974.
4. Ritchie EC, Hamilton S. Early interventions and risk assessment following disaster. Psychiatric Annals. 2004;34(8):605-610.
5. Kessler RC, Galea S, Gruber MJ, et al. Trends in mental illness and suicidality after Hurricane Katrina. Mol Psychiatry. 2008;13(4):374-384.
6. Weisler RH, Barbee JG IV, Townsend MH. Mental health and recovery in the Gulf Coast after Hurricanes Katrina and Rita. JAMA. 2006;296(5):585-588.
7. Galea S, Brewin CR, Gruber M, et al. Exposure to hurricane-related stressors and mental illness after Hurricane Katrina. Arch Gen Psychiatry. 2007;64(12).1427-1434.
8. Federal Emergency Management Agency. Supply Chain Resilience Guide Department of Homeland Security. https://www.fema.gov/media-library-data/1544795397837-767851ba177c7097bf8672aadf8a93c9/NE_DRAFT_Supply_Chain_Resilience.pdf. Published December 17, 2018. Accessed January 2, 2019.
9. Durkin J, Telab M, Fitzmaurice P, et al. Only as strong as its weakest link: resilience of the healthcare supply chain in New York. https://www.hstoday.us/subject-matter-areas/emergency-preparedness/only-as-strong-as-its-weakest-link-the-resilience-of-the-healthcare-supply-chain-in-new-york/. Published October 26, 2018. Accessed February 14, 2019.
1. National Institute of Mental Health. Mental health and mass violence: evidence based early psychological intervention for victims/survivors of mass violence. A workshop to reach consensus on best practices. https://cpa.ca/docs/File/Emergencies/massviolence.pdf. Published 2002. Accessed March 11, 2019.
2. Ritchie EC, Friedman M, Watson P. Interventions following mass violence and disasters: strategies for mental health practice. New York, NY: Guilford Press; 2006.
3. Ritchie EC, O’Brien K, Grant M, et al. Disaster psychiatry. In: Stern TA, Rosenbaum JF, Fava M, et al. The Massachusetts General Hospital textbook of comprehensive clinical psychiatry, 2nd edition. Philadelphia, PA: Mosby/Elsevier; 2016:968-974.
4. Ritchie EC, Hamilton S. Early interventions and risk assessment following disaster. Psychiatric Annals. 2004;34(8):605-610.
5. Kessler RC, Galea S, Gruber MJ, et al. Trends in mental illness and suicidality after Hurricane Katrina. Mol Psychiatry. 2008;13(4):374-384.
6. Weisler RH, Barbee JG IV, Townsend MH. Mental health and recovery in the Gulf Coast after Hurricanes Katrina and Rita. JAMA. 2006;296(5):585-588.
7. Galea S, Brewin CR, Gruber M, et al. Exposure to hurricane-related stressors and mental illness after Hurricane Katrina. Arch Gen Psychiatry. 2007;64(12).1427-1434.
8. Federal Emergency Management Agency. Supply Chain Resilience Guide Department of Homeland Security. https://www.fema.gov/media-library-data/1544795397837-767851ba177c7097bf8672aadf8a93c9/NE_DRAFT_Supply_Chain_Resilience.pdf. Published December 17, 2018. Accessed January 2, 2019.
9. Durkin J, Telab M, Fitzmaurice P, et al. Only as strong as its weakest link: resilience of the healthcare supply chain in New York. https://www.hstoday.us/subject-matter-areas/emergency-preparedness/only-as-strong-as-its-weakest-link-the-resilience-of-the-healthcare-supply-chain-in-new-york/. Published October 26, 2018. Accessed February 14, 2019.

