User login
Salivary ductal adenocarcinoma with complete response to androgen blockade
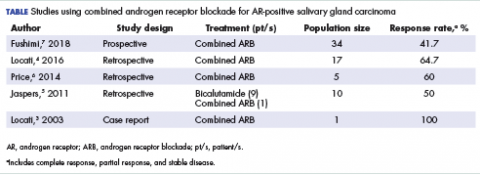
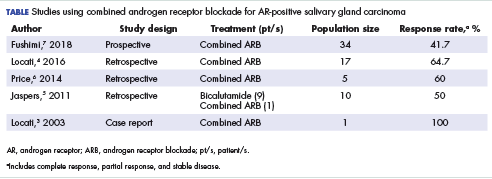
Salivary ductal adenocarcinomas make up about 9% of malignant salivary gland tumors and occur mostly in men older than 50 years, with a peak incidence in the sixth and seventh decades. It is the most aggressive of salivary gland tumors and is histologically similar to high-grade, invasive ductal carcinoma of the breast. In all, 65% of patients will die of the disease, and most will experience skin ulceration and nerve palsy.1 With such an aggressive clinical picture, the temptation for many oncologists and patients is to use aggressive cytotoxic chemotherapies. Considering the lack of large trials exploring treatment options in this less-common subtype of salivary gland carcinoma, practice guidelines also recommend the use of aggressive chemotherapies. Unlike other types of malignant cancers of the salivary glands, 70% to 90% of ductal adenocarcinomas express the androgen receptor (AR) by immunohistochemistry.2 There are reported cases of androgen deprivation therapy (ADT) as a successful treatment for salivary ductal adenocarcinomas that express the AR (Table).In 2003, Locati and colleagues reported the case of a man with salivary ductal adenocarcinomas who had a complete response with ADT.3 In 2016, the same group of authors published a retrospective analysis of 17 patients with recurrent or metastatic AR-positive salivary gland cancers who were treated with ADT and reported a 64.7% overall response rate among the patients.4 A 10-patient case series in the Netherlands demonstrated a 50% response rate to ADT plus bicalutamide, including a palliative effect in the form of pain relief.5 A retrospective analysis by Price and colleagues of 5 patients with AR-positive metastatic salivary duct adenocarcinoma showed a 60% response rate to a combination of leuprolide and bicalutamide.6
Case presentation and summary
A 91-year-old man was diagnosed with salivary ductal adenocarcinoma of the left parotid gland in September 2013 and underwent left parotidectomy and lymph node dissection, which revealed AJCC stage IVA (pT2 pN3 M0) disease. The following year, in December 2014, he had an enlarging left neck mass that was pathologically confirmed to be recurrent disease, and he underwent left level V neck dissection in February 2015. Five months after surgery, in July 2015, he presented with left neck fullness and new skin nodules, and the results of a biopsy confirmed recurrent disease. Given his relatively asymptomatic state and advanced age, the oncology care team decided to follow the patient without any pharmacologic therapy.
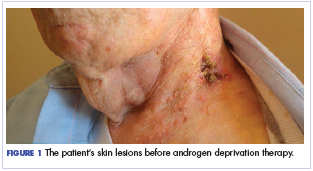
The patient felt relatively well for 11 months but slowly developed increasing pain in the left neck in June 2016. The skin nodules also began to spread inferiorly from his left neck to his upper chest with the development of open sores that wept serous fluid with scab formation (Figure 1). He and his wife lived independently and managed all their own instrumental activities of daily living (IADL). Eventually, the pain in his neck became so severe that it began to interfere with his ability to drive. He declined radiation therapy because of side effects and transportation issues, but he desired something to alleviate the burden of the disease. During a multidisciplinary cancer conference, the staff pathologist and oncologist discussed AR immunohistochemistry to assist with management. In June 2016, the patient’s tumor was found to have AR immunostaining (nuclear pattern) in 100% of cells, and he was treated with combined androgen blockade, consisting of monthly 3.6 mg goserelin injections and daily bicalutamide 50 mg orally.
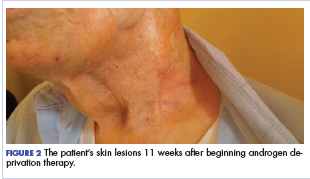
Within a week, the patient noticed that the skin lesions stopped weeping fluid. Within 2 weeks, the pain had begun to resolve. At his formal follow-up visit 11 weeks after starting treatment, he was not taking any pain medications and reported no pain. In addition, his visually apparent disease had almost completely resolved (Figure 2). He was fully able to manage his own IADL and reported a marked increase in satisfaction with the quality of his life.
Discussion
The oncology care team clearly defined the goal of care for this patient as palliative and conveyed as such to the patient. The team considered the risks and side effects of cytotoxic chemotherapy agents to be contrary to the patient’s stated primary goal of independence. We selected the combined androgen blockade because it has a low toxicity rate and thus met the primary goals of therapy.
The European Organization for Research and Treatment of Cancer is presently conducting a trial in which cytotoxic chemotherapy is being compared with ADT in AR-positive salivary duct tumors. Findings from a recent prospective, phase-2 trial conducted in Japan suggested that combined AR blockade has similar efficacy and less toxicity than conventional cytotoxic chemotherapy for recurrent and/or metastatic and unresectable locally advanced AR-positive salivary gland carcinoma.7 As more data become available from other studies, it is possible that practice guidelines will be revised to recommend this treatment approach for these cancers.
1. Eveson JW, Thompson LDR. Malignant neoplasms of the salivary glands. In: Thompson LDR, ed. Head and neck pathology. 2nd ed. Philadelphia, PA: Elsevier Inc; 2013:304-305.
2. Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(suppl 1):E1838-E1847.
3. Locati LD, Quattrone P, Bossi P, Marchianò AV, Cantù G, Licitra L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol. 2003;14(8):1327-1328.
4. Locati LD, Perrone F, Cortelazzi B, et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck. 2016;38(5):724-731.
5. Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473-e476.
6. Price KAR, Okuno SH, Molina JR, Garcia JJ. Treatment of metastatic salivary duct carcinoma with combined androgen blockade (CAB) with leuprolide acetate and bicalutamide. Int J Radiat Oncol Biol Phys. 2014;88(2):521-522.
7. Fushimi C, Tada Y, Takahashi H, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29(4):979-984.
Salivary ductal adenocarcinomas make up about 9% of malignant salivary gland tumors and occur mostly in men older than 50 years, with a peak incidence in the sixth and seventh decades. It is the most aggressive of salivary gland tumors and is histologically similar to high-grade, invasive ductal carcinoma of the breast. In all, 65% of patients will die of the disease, and most will experience skin ulceration and nerve palsy.1 With such an aggressive clinical picture, the temptation for many oncologists and patients is to use aggressive cytotoxic chemotherapies. Considering the lack of large trials exploring treatment options in this less-common subtype of salivary gland carcinoma, practice guidelines also recommend the use of aggressive chemotherapies. Unlike other types of malignant cancers of the salivary glands, 70% to 90% of ductal adenocarcinomas express the androgen receptor (AR) by immunohistochemistry.2 There are reported cases of androgen deprivation therapy (ADT) as a successful treatment for salivary ductal adenocarcinomas that express the AR (Table).In 2003, Locati and colleagues reported the case of a man with salivary ductal adenocarcinomas who had a complete response with ADT.3 In 2016, the same group of authors published a retrospective analysis of 17 patients with recurrent or metastatic AR-positive salivary gland cancers who were treated with ADT and reported a 64.7% overall response rate among the patients.4 A 10-patient case series in the Netherlands demonstrated a 50% response rate to ADT plus bicalutamide, including a palliative effect in the form of pain relief.5 A retrospective analysis by Price and colleagues of 5 patients with AR-positive metastatic salivary duct adenocarcinoma showed a 60% response rate to a combination of leuprolide and bicalutamide.6
Case presentation and summary
A 91-year-old man was diagnosed with salivary ductal adenocarcinoma of the left parotid gland in September 2013 and underwent left parotidectomy and lymph node dissection, which revealed AJCC stage IVA (pT2 pN3 M0) disease. The following year, in December 2014, he had an enlarging left neck mass that was pathologically confirmed to be recurrent disease, and he underwent left level V neck dissection in February 2015. Five months after surgery, in July 2015, he presented with left neck fullness and new skin nodules, and the results of a biopsy confirmed recurrent disease. Given his relatively asymptomatic state and advanced age, the oncology care team decided to follow the patient without any pharmacologic therapy.
The patient felt relatively well for 11 months but slowly developed increasing pain in the left neck in June 2016. The skin nodules also began to spread inferiorly from his left neck to his upper chest with the development of open sores that wept serous fluid with scab formation (Figure 1). He and his wife lived independently and managed all their own instrumental activities of daily living (IADL). Eventually, the pain in his neck became so severe that it began to interfere with his ability to drive. He declined radiation therapy because of side effects and transportation issues, but he desired something to alleviate the burden of the disease. During a multidisciplinary cancer conference, the staff pathologist and oncologist discussed AR immunohistochemistry to assist with management. In June 2016, the patient’s tumor was found to have AR immunostaining (nuclear pattern) in 100% of cells, and he was treated with combined androgen blockade, consisting of monthly 3.6 mg goserelin injections and daily bicalutamide 50 mg orally.
Within a week, the patient noticed that the skin lesions stopped weeping fluid. Within 2 weeks, the pain had begun to resolve. At his formal follow-up visit 11 weeks after starting treatment, he was not taking any pain medications and reported no pain. In addition, his visually apparent disease had almost completely resolved (Figure 2). He was fully able to manage his own IADL and reported a marked increase in satisfaction with the quality of his life.
Discussion
The oncology care team clearly defined the goal of care for this patient as palliative and conveyed as such to the patient. The team considered the risks and side effects of cytotoxic chemotherapy agents to be contrary to the patient’s stated primary goal of independence. We selected the combined androgen blockade because it has a low toxicity rate and thus met the primary goals of therapy.
The European Organization for Research and Treatment of Cancer is presently conducting a trial in which cytotoxic chemotherapy is being compared with ADT in AR-positive salivary duct tumors. Findings from a recent prospective, phase-2 trial conducted in Japan suggested that combined AR blockade has similar efficacy and less toxicity than conventional cytotoxic chemotherapy for recurrent and/or metastatic and unresectable locally advanced AR-positive salivary gland carcinoma.7 As more data become available from other studies, it is possible that practice guidelines will be revised to recommend this treatment approach for these cancers.
Salivary ductal adenocarcinomas make up about 9% of malignant salivary gland tumors and occur mostly in men older than 50 years, with a peak incidence in the sixth and seventh decades. It is the most aggressive of salivary gland tumors and is histologically similar to high-grade, invasive ductal carcinoma of the breast. In all, 65% of patients will die of the disease, and most will experience skin ulceration and nerve palsy.1 With such an aggressive clinical picture, the temptation for many oncologists and patients is to use aggressive cytotoxic chemotherapies. Considering the lack of large trials exploring treatment options in this less-common subtype of salivary gland carcinoma, practice guidelines also recommend the use of aggressive chemotherapies. Unlike other types of malignant cancers of the salivary glands, 70% to 90% of ductal adenocarcinomas express the androgen receptor (AR) by immunohistochemistry.2 There are reported cases of androgen deprivation therapy (ADT) as a successful treatment for salivary ductal adenocarcinomas that express the AR (Table).In 2003, Locati and colleagues reported the case of a man with salivary ductal adenocarcinomas who had a complete response with ADT.3 In 2016, the same group of authors published a retrospective analysis of 17 patients with recurrent or metastatic AR-positive salivary gland cancers who were treated with ADT and reported a 64.7% overall response rate among the patients.4 A 10-patient case series in the Netherlands demonstrated a 50% response rate to ADT plus bicalutamide, including a palliative effect in the form of pain relief.5 A retrospective analysis by Price and colleagues of 5 patients with AR-positive metastatic salivary duct adenocarcinoma showed a 60% response rate to a combination of leuprolide and bicalutamide.6
Case presentation and summary
A 91-year-old man was diagnosed with salivary ductal adenocarcinoma of the left parotid gland in September 2013 and underwent left parotidectomy and lymph node dissection, which revealed AJCC stage IVA (pT2 pN3 M0) disease. The following year, in December 2014, he had an enlarging left neck mass that was pathologically confirmed to be recurrent disease, and he underwent left level V neck dissection in February 2015. Five months after surgery, in July 2015, he presented with left neck fullness and new skin nodules, and the results of a biopsy confirmed recurrent disease. Given his relatively asymptomatic state and advanced age, the oncology care team decided to follow the patient without any pharmacologic therapy.
The patient felt relatively well for 11 months but slowly developed increasing pain in the left neck in June 2016. The skin nodules also began to spread inferiorly from his left neck to his upper chest with the development of open sores that wept serous fluid with scab formation (Figure 1). He and his wife lived independently and managed all their own instrumental activities of daily living (IADL). Eventually, the pain in his neck became so severe that it began to interfere with his ability to drive. He declined radiation therapy because of side effects and transportation issues, but he desired something to alleviate the burden of the disease. During a multidisciplinary cancer conference, the staff pathologist and oncologist discussed AR immunohistochemistry to assist with management. In June 2016, the patient’s tumor was found to have AR immunostaining (nuclear pattern) in 100% of cells, and he was treated with combined androgen blockade, consisting of monthly 3.6 mg goserelin injections and daily bicalutamide 50 mg orally.
Within a week, the patient noticed that the skin lesions stopped weeping fluid. Within 2 weeks, the pain had begun to resolve. At his formal follow-up visit 11 weeks after starting treatment, he was not taking any pain medications and reported no pain. In addition, his visually apparent disease had almost completely resolved (Figure 2). He was fully able to manage his own IADL and reported a marked increase in satisfaction with the quality of his life.
Discussion
The oncology care team clearly defined the goal of care for this patient as palliative and conveyed as such to the patient. The team considered the risks and side effects of cytotoxic chemotherapy agents to be contrary to the patient’s stated primary goal of independence. We selected the combined androgen blockade because it has a low toxicity rate and thus met the primary goals of therapy.
The European Organization for Research and Treatment of Cancer is presently conducting a trial in which cytotoxic chemotherapy is being compared with ADT in AR-positive salivary duct tumors. Findings from a recent prospective, phase-2 trial conducted in Japan suggested that combined AR blockade has similar efficacy and less toxicity than conventional cytotoxic chemotherapy for recurrent and/or metastatic and unresectable locally advanced AR-positive salivary gland carcinoma.7 As more data become available from other studies, it is possible that practice guidelines will be revised to recommend this treatment approach for these cancers.
1. Eveson JW, Thompson LDR. Malignant neoplasms of the salivary glands. In: Thompson LDR, ed. Head and neck pathology. 2nd ed. Philadelphia, PA: Elsevier Inc; 2013:304-305.
2. Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(suppl 1):E1838-E1847.
3. Locati LD, Quattrone P, Bossi P, Marchianò AV, Cantù G, Licitra L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol. 2003;14(8):1327-1328.
4. Locati LD, Perrone F, Cortelazzi B, et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck. 2016;38(5):724-731.
5. Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473-e476.
6. Price KAR, Okuno SH, Molina JR, Garcia JJ. Treatment of metastatic salivary duct carcinoma with combined androgen blockade (CAB) with leuprolide acetate and bicalutamide. Int J Radiat Oncol Biol Phys. 2014;88(2):521-522.
7. Fushimi C, Tada Y, Takahashi H, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29(4):979-984.
1. Eveson JW, Thompson LDR. Malignant neoplasms of the salivary glands. In: Thompson LDR, ed. Head and neck pathology. 2nd ed. Philadelphia, PA: Elsevier Inc; 2013:304-305.
2. Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(suppl 1):E1838-E1847.
3. Locati LD, Quattrone P, Bossi P, Marchianò AV, Cantù G, Licitra L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol. 2003;14(8):1327-1328.
4. Locati LD, Perrone F, Cortelazzi B, et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck. 2016;38(5):724-731.
5. Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473-e476.
6. Price KAR, Okuno SH, Molina JR, Garcia JJ. Treatment of metastatic salivary duct carcinoma with combined androgen blockade (CAB) with leuprolide acetate and bicalutamide. Int J Radiat Oncol Biol Phys. 2014;88(2):521-522.
7. Fushimi C, Tada Y, Takahashi H, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29(4):979-984.