User login
Keloids: Which treatment is best for your patient?
• Consider using corticosteroid injections to inhibit collagen synthesis and stimulate enzymatic degradation of existing keloid collagen. B
• Turn to treatment combinations for refractory keloids; regimens may include corticosteroid injections, surgical excision, pressure, occlusive dressings, or radiation. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Keloids are an ongoing clinical challenge. Despite the availability of multiple treatments, choosing an effective therapeutic regimen for these fibroproliferative scars can be vexing, as keloids usually recur.
This review provides a guide to determining which regimen is best for your patient. But first, a word about the causes of these claw-like growths and which patients are at highest risk of developing them.
Even superficial injuries can cause keloids
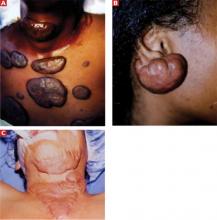
Keloids arise after a disruption of skin integrity following superficial or deep injuries (FIGURES 1A-C). Causes include physical trauma such as cuts, scratches, and insect bites; iatrogenic trauma as in vaccinations or surgical procedures; thermal or chemical burns; and skin eruptions such as chicken pox.
FIGURE 1
Keloids induced by scratches (A), ear piercing (B), and thermal burns (C)
Common sites of keloid development include the ears, jaw, neck, clavicle and sternum, and shoulders. Less commonly, keloids occur on the back, abdomen, and extremities. Rarely, keloids develop on the palms and soles, face, or mucous membranes.1
A minor keloid is smaller than 0.5 cm, while a major keloid is larger. No upper growth limit has been identified, as keloids seem to enlarge indefinitely.2 Keloid scars have a firm and inflexible texture, a shiny appearance, and are elevated above skin level. They are usually flesh-colored, but may be erythematous or hyperpigmented.1 (A similar aberrant wound healing condition often confused with keloids is hypertrophic scarring. See “Differentiating keloids from hypertrophic scars”).
Distinguishing between keloids and hypertrophic scars can be challenging, because both types of scars arise histologically from excessive collagen buildup during the wound healing process. However, there are 3 distinctive characteristics of keloids that hypertrophic scars lack:
- Keloids enlarge beyond wound margins, often with irregular shapes, while hypertrophic scars remain within the confines of the original wound and tend to be linear along scars.
- Keloids do not regress on their own; hypertrophic scars tend to regress spontaneously within a few years.
- Keloids may take months to years to develop, while hypertrophic scars usually appear within 4 to 6 weeks of a traumatic incident.
Source: Gauglitz G, Korting H, Pavicic T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113-125.
Although keloids have an aggressive growth pattern, they are considered benign tumors, because the development of malignant cells in a keloid is rare.1 Most patients seek medical attention for cosmetic concerns. However, tissue bulk can cause functional problems, and some keloids are symptomatic with pruritus (27%) or pain (19%).1,3
Who is affected?
The most consistent risk factor for keloid development is a previous keloid. Individuals with deeply pigmented skin appear to be at higher risk, with an estimated prevalence of 4% to 16% among individuals of African, Asian, and Hispanic ancestry.3 Although the rate of keloid occurrence among Caucasians has not been established, keloids have been reported in individuals of nearly every skin color, except albinos.3 Keloids occur in both sexes with similar frequency, and are most likely to develop during puberty and early adulthood—between the ages of 10 and 30 years—but can occur at any age.1,3
Although no clear familial inheritance pattern has been identified, keloids are associated with blood type A and human leukocyte antigens (HLA)-B14, -B21, -BW35, -DR5, -DRB1, -DQA1, -DQB1, and -DQW3.3,4 Emerging research indicates that mutations of the CDC2L1 gene—which encodes a protein kinase essential to cell cycle control—correlate with keloid formation.4
Wound healing gone awry
Normal wound healing involves an overlapping series of processes, including hemostasis, inflammation, and granulation and remodeling. Various mechanisms have been identified for keloid pathology, although no single definitive trigger is known.
Hemostasis. In normal hemostasis, platelets aggregate around a fibrin clot in a wound, stimulating the release of growth factors,3 which precipitate the migration of fibroblasts to the granulation-tissue scaffolding for wound healing. Keloids have increased fibroblast proliferation, with up to 20-fold increased production of Type 1 collagen.1
Inflammation. During the inflammation phase, cytokine cascades stimulate cell proliferation from inflammatory, endothelial, epithelial, and fibroblast cell lines. Keloids have altered levels of many cytokines, resulting in increased inflammatory activity, such as histamine release by mast cells.3
Granulation and remodeling. During normal granulation and remodeling, fibroblasts aggregate and produce extracellular matrix components. The keloid fibroblasts produce higher levels of collagen, elastin, fibronectin, and proteoglycans, but lower levels of hyaluronic acid.3,5 Keloid fibroblasts also make lower levels of tissue plasminogen activator inhibitor (TPA-I), with inferior breakdown of scaffold structures and collagens. While collagen bundles run parallel to the epithelial surface in normal skin, keloid collagen fibers are larger, thicker, and randomly oriented in dense sheets, swirls, or nodules.1
In normal tissue, wound healing stops after the wound has been fully epithelialized by keratinocytes, after which the scar tissue contracts to minimal size. However, keloid growth can continue for years.1
Numerous studies have inspired various hypotheses for keloid formation. Studies demonstrating that keloids contain an increased level of immunoglobulins suggest that they may be produced by an abnormal immune reaction. Microscopic examinations of keloids have shown that overabundant endothelial cells occlude microvessels, suggesting that keloids may occur in the context of wound hypoxia. In vitro studies showing that fibroblasts proliferate when cultured with keloidal keratinocytes suggest that keloids may be a result of abnormal epithelial–mesenchymal interactions.6,7 A hypothesis based on the body sites where keloids are observed is that mechanical stretch and tension across a wound may overstimulate collagen production, possibly as a result of mechanoreceptor damage or disorders.8
Treatment: Weighing the options
Due to the complex mechanisms of pathogenesis, many modalities are available for managing keloids, yet no definitive treatment protocols exist. Intradermal, extradermal, or systemic therapies may be used singly, although combination regimens are the most effective in treating keloids and preventing recurrences.5 The TABLE1,3,5,9-14 provides an overview of monotherapeutic treatment options, including efficacy and risks.
TABLE
Treatment options for keloids
| Treatment | Anticipated effects | Dosing and frequency | Efficacy in reducing keloid size | Adverse effects | SOR |
|---|---|---|---|---|---|
| Hygienic relief: washing & drying | Relief of pruritus, pain, general discomfort | As needed | N/A | Minimal | C |
| Antihistamines | Relief of pruritus, pain, burning sensation9 | As needed | N/A | Minimal | C |
| Corticosteroid injections | Reduction in keloid size, pruritus10 | Injections given every 4-8 wk for several months10 | >80% report moderate to marked regression10 | Local or systemic infection and allergy or anaphylaxis10 | B |
| Surgical excision | Temporary reduction in keloid size11 | Not successful as monotherapy; combine with alternative therapies as needed11 | Temporary; keloids always recur after excision11 | Chance of infection, excessive bleeding, or injury to adjacent tissues11 | C |
| Pressure therapy | Reduction in keloid size3,12 | Pressure of 25-40 mm Hg for 23-24 h/d over several months3,12 | 60%-80% of patients report at least partial improvement3 | Minimal; rarely, skin thinning and redness3,12 | B |
| Radiation therapy | Reduction in keloid size3 | 10-20 Gy fractionated over several weeks5 | Up to 94% of patients report improvement, but recurrence is common3 | Minimal; theoretical risk of malignancy13 | B |
| Silicone occlusive dressings | Reduction in pain, pruritus, and keloid size1,12,14 | Applied topically for 12-24 h/d for 18 mo1,12,14 | 68%-86% of patients report improvement in keloid texture, color, and size14; may be useful as a preventive measure14 | Skin breakdown, rash, pruritus12 | B |
| N/A, not applicable; SOR, strength of recommendation. | |||||
Offer symptomatic therapy routinely
Some patients find symptomatic relief through hygienic measures, such as regular washing and drying. Antihistamines can relieve symptoms of burning or pruritus,9 but are not expected to reduce keloid size.
Use corticosteroids for first-line treatment
Corticosteroids are the most widely used therapy for keloids.3 They decrease keloid bulking by inhibiting collagen synthesis1 and stimulate tissue collagenases and collagen degradation.5 Commonly used in the office setting are intralesional injections of triamcinolone acetonide, a potent anti-inflammatory and highly atrophogenic agent.
Administer injections every 4 to 8 weeks until clinical improvement (usually requiring several months) or prohibitive adverse effects occur. A general dosing guideline is about 0.1 to 0.2 mL of corticosteroid for every square centimeter of keloid tissue. Injecting higher doses can lead to accretions or deposits beneath the skin that might need to be unroofed. Additionally, excess corticosteroid can cause an atrophic contour deformity in the adjacent subcutaneous tissue. FIGURE 2 details steroid injection procedures and follow-up based on the author’s (SPD) experience.
FIGURE 2
A 5-step procedure for injecting keloids with corticosteroids*
*Based on the author’s (SPD) experience.
Contraindications to injections include local or systemic infection and known allergy or anaphylactic reaction to corticosteroids. Although injecting keloids can be difficult initially, the tissue tends to soften and become easier to inject with each successive treatment. Some clinicians advocate cryotherapy to soften the keloid before giving injections. Large trials, in which more than 80% of patients had moderate to marked keloid regression,10 have demonstrated the efficacy of this technique in reducing keloid size and itching.
Surgery alone is ineffective
The oldest remedy, simple scalpel excision of keloidal tissue, invariably results in regrowth, because the aberrant wound healing process is not remedied.11 Although surgical excision is a poor monotherapy, it may be useful in combination with other modalities. Repairs using meticulous atraumatic surgical techniques with minimal wound tension can lessen the risk of keloid recurrence. Absorbable monofilament sutures with a subcuticular stitch help to minimize further epidermal damage.6
Pressure is useful in certain cases
Applying pressure is a noninvasive method that produces initial tissue thinning and pliability, with 60% to 80% of patients reporting at least partial improvement.3 Pressure reduces keloids by decreasing tissue metabolism, so long-term therapy is required, with application of pressure 23 to 24 hours daily over several months.12 Pressure of 25 to 40 mm Hg is needed to exceed capillary pressure without damaging peripheral circulation.3
Custom pressure garments for burn patients have been used in specialized cases involving limbs and torso, face and neck, and hands and feet. For earlobe keloids, clip-on earring devices are the only practical solution. Compliance tends to wane after months of therapy, and cessation may be followed by rebound hypertrophy. The therapeutic effect of regular pressure massage has been poorly studied. Because pressure has minimal adverse effects, it is likely most useful as an adjunct therapy where practical.
Radiation has variable effectiveness
Radiation damages fibroblasts, inhibits proliferation, and may improve local oxygenation.13 Low megavolt electron beam radiation is used to limit depth of penetration, usually with a total dose of 10 to 20 Gy fractionated over several weeks.5 A dose-response effect is likely.3 Response rates vary from 10% to 94%,3 and keloid recurrence is common, making radiation a poor choice for primary treatment except in cases of a large-volume unresectable keloid (eg, post-burn keloids).13
Adverse effects can include nodule formation, hyperpigmentation, ulceration, pruritus, paresthesia, and wound dehiscence. Theoretical risks of malignancy with radiation treatment of keloids have not been borne out in large studies, which have shown a zero percent malignancy rate despite rare anecdotal reports.15 Nevertheless, radiation therapy for benign disease is not recommended for children or pregnant women, or in breast, thyroid, or other cancer-prone body sites.5
Occlusive dressings require patient compliance
An innovation in keloid treatment is the use of occlusive dressings, which started in 1981 and gained popularity in the 1990s.16 Gel, fluid, or rubbery sheeting, usually made from silicone, is applied topically for 12 to 24 hours daily for up to 18 months.1,12,14 Gel may be practical along creases or in areas of motion where silicone sheets are obtrusive. Because long-term therapy is recommended, occlusive dressings require active patient participation. Occlusive dressings can decrease pruritic symptoms by decreasing mast cell activity,12 and may do so by warmth, hydration, or occlusion effects.3
Although study methodology has been suboptimal and further research is required, some practitioners have reported efficacy of 86% for texture reduction, 84% improvement in color, and 68% in diminishing height of scars.14 However, silicone gel and sheeting may be most successful when applied to a wound before a keloid has formed, as a preventive measure for keloid-prone patients.14 Occlusive dressings are a relatively benign treatment because silicone rubber is inert, but adverse effects may include skin breakdown, rash, or pruritus that may require discontinuation of therapy for a few days or more.
Combination regimens may be most effective
If a keloid remains unresponsive to first-line therapies, combined therapies may reduce keloid size and prevent recurrence. Select regimens according to keloid characteristics and patient preferences.17 Corticosteroids used after surgical excision can produce cure rates exceeding 80%, making this a consistently successful management regimen for keloids and the standard of care in many primary care practices.3
Surgery and pressure are the preferred combination for earlobe keloids, as adverse effects are minimal and compliant patients may achieve response rates exceeding 80%.3,5
Surgery followed by immediate radiation reportedly has a response rate of 65% or higher.18 This regimen may be most successful in low-tension body areas, like the neck or lower limbs, where keloids are less likely to recur.18
A combination of surgery and occlusive dressings is also promising. Early studies have had recurrence-free rates of more than 80% when patients applied the dressings for up to 24 hours daily over 4 to 6 months.6
Emerging therapies that require further testing
Many promising treatments lack sufficiently rigorous evidence of efficacy.
- Intralesional calcium channel blockers, which depolymerize actin19 and inhibit protein synthesis,5 have shown promising results in nonrandomized early clinical trials.19
- Ultraviolet light is a potentially successful noninvasive method.
- Topical retinoids (vitamin A derivatives) may be effective but must be applied twice daily for several months to reduce collagen metabolism by fibroblasts5; they may also cause photosensitivity and skin irritation.7
- Intralesional fluorouracil (5-FU) (as an antimetabolite) successfully reduced keloid size after one year of treatment in a few small trials.5,20
- Oral lathyrogen, such as penicillamine with colchicine, can interfere with collagen cross-linking; in a small case series there was no keloid recurrence after treatment.5
- Topical imiquimod (Aldara) cream, an immune response modifier, showed promise in a few case reports.5,21
- Laser procedures are less likely than scalpel procedures to produce keloid scars and have been reported to successfully improve scar color, size, and texture, although the risk of hyperpigmentation ranges from 1% to 24%.2,3,22 (Lasers induce ischemia through blood vessel destruction and cause decreased fibroblast production and histamine release.23,24) Red flat scars (which triamcinolone injections may cause) can be lightened with yellow light laser, which has been used in the treatment of capillary malformations.25
Other methods may produce suboptimal results. Cryotherapy causes ischemic damage that reduces tumor bulk at least temporarily, but is inappropriate for darker skin due to a high risk of persistent hypopigmentation.1,26,27 Intralesional antifibrotic cytokines, such as interferon, reduce collagen synthesis in vitro,1 but lead to considerable systemic adverse effects including headache, myalgias, and influenza-like symptoms.3,5 Skin grafting is inappropriate as it may lead to keloid wave formation at the graft edges.
CORRESPONDENCE
Stephen P. Daane, MD, 2186 Geary Boulevard #212, San Francisco, CA 94115; [email protected]
1. Shaffer JJ, Taylor SC, Cook-Bolden F. Keloid scars: a review with a critical look at therapeutic options. J Am Acad Dermatol. 2002;46(suppl):S63-S97.
2. Mutoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560-571.
3. Niessen FB, Pauwen PH, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104:1435-1458.
4. Zhang G, Jiang J, Luo S, et al. Analyses of CDC2L1 gene mutations in keloid tissue. Clin Exp Dermatol. 2012;37:277-283.
5. Al Attar A, Mess S, Thomassen JM, et al. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117:286-300.
6. Yang GP, Lim IJ, Phan TT, et al. From scarless fetal wounds to keloids: molecular studies in wound healing. Wound Repair Regen. 2003;11:411-418.
7. Lim IJ, Phan TT, Bay BH, et al. Fibroblast cocultured with keloid keratinocytes: normal fibroblasts secrete collagen in a keloid like manner. Am J Physiol Cell Physiol. 2002;283:C212-C222.
8. Ogawa R. Keloid and hypertrophic scarring may result from a mechanoreceptor or mechanosensitive nociceptor disorder. Med Hypotheses. 2008;71:493-500.
9. Davidson S, Aziz N, Rashid R, et al. A primary care perspective on keloids. Medscape J Med. 2009;11:18 [Epub].-Available at: http://www.medscape.com/viewarticle/582445. Accessed March 8, 2013.
10. Ketchum LD, Smith J, Robinson DW, et al. The treatment of hypertrophic scar, keloid and scar contracture by triamcinolone acetonide. Plast Reconstr Surg. 1996;38:209-218.
11. Cosman B, Crikelair GF, Ju MC, et al. The surgical treatment of keloidal scars. Plast Reconstr Surg. 1961;27:335-358.
12. Eishi K, Bae SJ, Ogawa F, et al. Silicone gel sheets relieve pain and pruritus with clinical improvement of keloid: possible target mast cells. J Dermatol Treat. 2003;14:248-252.
13. Malaker K, Vijayraghavan K, Hodson I, et al. Retrospective analysis of treatment of unresectable keloids with primary radiation over 25 years. Clin Oncol. 2004;26:290-298.
14. Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic and keloid scars. J Cutan Aesthet Surg. 2009;2:104-106.
15. Botwood N, Lewanski C, Lowdell C. The risks of treating keloids with radiotherapy. Br J Radiol. 1999;72:1222-1224.
16. O’Brien L, Pandit A. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2006;(1):CD003826.-
17. Juckett G, Hartman-Adams H. Management of keloids and hypertrophic scars. Am Fam Physician. 2009;80:253-260.
18. Ogawa R, Mitsuhasi K, Hyakusoku H, et al. Postoperative electron beam irradiation therapy for keloid scars. Plast Reconstr Surg. 2003;111:547-555.
19. D’Andrea F, Brongo S, Ferrano G, et al. Prevention and treatment of keloids with intralesional verapamil. Dermatology. 2002;204:60-62.
20. Kontochristopoulus G, Stefanaki C, Panagiotopoulos A, et al. Intralesional 5-fluorouracil in the treatment of keloids: an open clinical and histopathological study. J Am Acad Dermatol. 2005;52:474-479.
21. Berman B, Kaufman J. Pilot study of the effect of postoperative imiquimod 5% cream on the recurrence rate of excised keloids. J Am Acad Dermatol. 2002;47:2209-2211.
22. Fickerstrand EJ, Svaasand LO, Volden G. Pigmentary changes after pulsed dye laser treatment in 125 northern European patients with port wine stains. Br J Dermatol. 1998;138:477-479.
23. Alster TS. Laser treatment of hypertrophic scars, keloids and striae. Dermatol Clin. 1997;15:419-429.
24. Alster TS. Laser scar revision: comparison study of 585nm pulsed dye laser with and without intralesional corticosteroids. Dermatol Surg. 2003;29:25-29.
25. Astner S, Anderson RR. Treating vascular lesions. Dermatol Ther. 2005;18:267-281.
26. Har-Shai Y, Amar M, Sabo E. Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plast Reconstr Surg. 2003;111:1841-1852.
27. Williams C, De Groote S. What treatment is best for hypertrophic scars and keloids? J Fam Pract. 2011;60:757-758.
• Consider using corticosteroid injections to inhibit collagen synthesis and stimulate enzymatic degradation of existing keloid collagen. B
• Turn to treatment combinations for refractory keloids; regimens may include corticosteroid injections, surgical excision, pressure, occlusive dressings, or radiation. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Keloids are an ongoing clinical challenge. Despite the availability of multiple treatments, choosing an effective therapeutic regimen for these fibroproliferative scars can be vexing, as keloids usually recur.
This review provides a guide to determining which regimen is best for your patient. But first, a word about the causes of these claw-like growths and which patients are at highest risk of developing them.
Even superficial injuries can cause keloids
Keloids arise after a disruption of skin integrity following superficial or deep injuries (FIGURES 1A-C). Causes include physical trauma such as cuts, scratches, and insect bites; iatrogenic trauma as in vaccinations or surgical procedures; thermal or chemical burns; and skin eruptions such as chicken pox.
FIGURE 1
Keloids induced by scratches (A), ear piercing (B), and thermal burns (C)
Common sites of keloid development include the ears, jaw, neck, clavicle and sternum, and shoulders. Less commonly, keloids occur on the back, abdomen, and extremities. Rarely, keloids develop on the palms and soles, face, or mucous membranes.1
A minor keloid is smaller than 0.5 cm, while a major keloid is larger. No upper growth limit has been identified, as keloids seem to enlarge indefinitely.2 Keloid scars have a firm and inflexible texture, a shiny appearance, and are elevated above skin level. They are usually flesh-colored, but may be erythematous or hyperpigmented.1 (A similar aberrant wound healing condition often confused with keloids is hypertrophic scarring. See “Differentiating keloids from hypertrophic scars”).
Distinguishing between keloids and hypertrophic scars can be challenging, because both types of scars arise histologically from excessive collagen buildup during the wound healing process. However, there are 3 distinctive characteristics of keloids that hypertrophic scars lack:
- Keloids enlarge beyond wound margins, often with irregular shapes, while hypertrophic scars remain within the confines of the original wound and tend to be linear along scars.
- Keloids do not regress on their own; hypertrophic scars tend to regress spontaneously within a few years.
- Keloids may take months to years to develop, while hypertrophic scars usually appear within 4 to 6 weeks of a traumatic incident.
Source: Gauglitz G, Korting H, Pavicic T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113-125.
Although keloids have an aggressive growth pattern, they are considered benign tumors, because the development of malignant cells in a keloid is rare.1 Most patients seek medical attention for cosmetic concerns. However, tissue bulk can cause functional problems, and some keloids are symptomatic with pruritus (27%) or pain (19%).1,3
Who is affected?
The most consistent risk factor for keloid development is a previous keloid. Individuals with deeply pigmented skin appear to be at higher risk, with an estimated prevalence of 4% to 16% among individuals of African, Asian, and Hispanic ancestry.3 Although the rate of keloid occurrence among Caucasians has not been established, keloids have been reported in individuals of nearly every skin color, except albinos.3 Keloids occur in both sexes with similar frequency, and are most likely to develop during puberty and early adulthood—between the ages of 10 and 30 years—but can occur at any age.1,3
Although no clear familial inheritance pattern has been identified, keloids are associated with blood type A and human leukocyte antigens (HLA)-B14, -B21, -BW35, -DR5, -DRB1, -DQA1, -DQB1, and -DQW3.3,4 Emerging research indicates that mutations of the CDC2L1 gene—which encodes a protein kinase essential to cell cycle control—correlate with keloid formation.4
Wound healing gone awry
Normal wound healing involves an overlapping series of processes, including hemostasis, inflammation, and granulation and remodeling. Various mechanisms have been identified for keloid pathology, although no single definitive trigger is known.
Hemostasis. In normal hemostasis, platelets aggregate around a fibrin clot in a wound, stimulating the release of growth factors,3 which precipitate the migration of fibroblasts to the granulation-tissue scaffolding for wound healing. Keloids have increased fibroblast proliferation, with up to 20-fold increased production of Type 1 collagen.1
Inflammation. During the inflammation phase, cytokine cascades stimulate cell proliferation from inflammatory, endothelial, epithelial, and fibroblast cell lines. Keloids have altered levels of many cytokines, resulting in increased inflammatory activity, such as histamine release by mast cells.3
Granulation and remodeling. During normal granulation and remodeling, fibroblasts aggregate and produce extracellular matrix components. The keloid fibroblasts produce higher levels of collagen, elastin, fibronectin, and proteoglycans, but lower levels of hyaluronic acid.3,5 Keloid fibroblasts also make lower levels of tissue plasminogen activator inhibitor (TPA-I), with inferior breakdown of scaffold structures and collagens. While collagen bundles run parallel to the epithelial surface in normal skin, keloid collagen fibers are larger, thicker, and randomly oriented in dense sheets, swirls, or nodules.1
In normal tissue, wound healing stops after the wound has been fully epithelialized by keratinocytes, after which the scar tissue contracts to minimal size. However, keloid growth can continue for years.1
Numerous studies have inspired various hypotheses for keloid formation. Studies demonstrating that keloids contain an increased level of immunoglobulins suggest that they may be produced by an abnormal immune reaction. Microscopic examinations of keloids have shown that overabundant endothelial cells occlude microvessels, suggesting that keloids may occur in the context of wound hypoxia. In vitro studies showing that fibroblasts proliferate when cultured with keloidal keratinocytes suggest that keloids may be a result of abnormal epithelial–mesenchymal interactions.6,7 A hypothesis based on the body sites where keloids are observed is that mechanical stretch and tension across a wound may overstimulate collagen production, possibly as a result of mechanoreceptor damage or disorders.8
Treatment: Weighing the options
Due to the complex mechanisms of pathogenesis, many modalities are available for managing keloids, yet no definitive treatment protocols exist. Intradermal, extradermal, or systemic therapies may be used singly, although combination regimens are the most effective in treating keloids and preventing recurrences.5 The TABLE1,3,5,9-14 provides an overview of monotherapeutic treatment options, including efficacy and risks.
TABLE
Treatment options for keloids
| Treatment | Anticipated effects | Dosing and frequency | Efficacy in reducing keloid size | Adverse effects | SOR |
|---|---|---|---|---|---|
| Hygienic relief: washing & drying | Relief of pruritus, pain, general discomfort | As needed | N/A | Minimal | C |
| Antihistamines | Relief of pruritus, pain, burning sensation9 | As needed | N/A | Minimal | C |
| Corticosteroid injections | Reduction in keloid size, pruritus10 | Injections given every 4-8 wk for several months10 | >80% report moderate to marked regression10 | Local or systemic infection and allergy or anaphylaxis10 | B |
| Surgical excision | Temporary reduction in keloid size11 | Not successful as monotherapy; combine with alternative therapies as needed11 | Temporary; keloids always recur after excision11 | Chance of infection, excessive bleeding, or injury to adjacent tissues11 | C |
| Pressure therapy | Reduction in keloid size3,12 | Pressure of 25-40 mm Hg for 23-24 h/d over several months3,12 | 60%-80% of patients report at least partial improvement3 | Minimal; rarely, skin thinning and redness3,12 | B |
| Radiation therapy | Reduction in keloid size3 | 10-20 Gy fractionated over several weeks5 | Up to 94% of patients report improvement, but recurrence is common3 | Minimal; theoretical risk of malignancy13 | B |
| Silicone occlusive dressings | Reduction in pain, pruritus, and keloid size1,12,14 | Applied topically for 12-24 h/d for 18 mo1,12,14 | 68%-86% of patients report improvement in keloid texture, color, and size14; may be useful as a preventive measure14 | Skin breakdown, rash, pruritus12 | B |
| N/A, not applicable; SOR, strength of recommendation. | |||||
Offer symptomatic therapy routinely
Some patients find symptomatic relief through hygienic measures, such as regular washing and drying. Antihistamines can relieve symptoms of burning or pruritus,9 but are not expected to reduce keloid size.
Use corticosteroids for first-line treatment
Corticosteroids are the most widely used therapy for keloids.3 They decrease keloid bulking by inhibiting collagen synthesis1 and stimulate tissue collagenases and collagen degradation.5 Commonly used in the office setting are intralesional injections of triamcinolone acetonide, a potent anti-inflammatory and highly atrophogenic agent.
Administer injections every 4 to 8 weeks until clinical improvement (usually requiring several months) or prohibitive adverse effects occur. A general dosing guideline is about 0.1 to 0.2 mL of corticosteroid for every square centimeter of keloid tissue. Injecting higher doses can lead to accretions or deposits beneath the skin that might need to be unroofed. Additionally, excess corticosteroid can cause an atrophic contour deformity in the adjacent subcutaneous tissue. FIGURE 2 details steroid injection procedures and follow-up based on the author’s (SPD) experience.
FIGURE 2
A 5-step procedure for injecting keloids with corticosteroids*
*Based on the author’s (SPD) experience.
Contraindications to injections include local or systemic infection and known allergy or anaphylactic reaction to corticosteroids. Although injecting keloids can be difficult initially, the tissue tends to soften and become easier to inject with each successive treatment. Some clinicians advocate cryotherapy to soften the keloid before giving injections. Large trials, in which more than 80% of patients had moderate to marked keloid regression,10 have demonstrated the efficacy of this technique in reducing keloid size and itching.
Surgery alone is ineffective
The oldest remedy, simple scalpel excision of keloidal tissue, invariably results in regrowth, because the aberrant wound healing process is not remedied.11 Although surgical excision is a poor monotherapy, it may be useful in combination with other modalities. Repairs using meticulous atraumatic surgical techniques with minimal wound tension can lessen the risk of keloid recurrence. Absorbable monofilament sutures with a subcuticular stitch help to minimize further epidermal damage.6
Pressure is useful in certain cases
Applying pressure is a noninvasive method that produces initial tissue thinning and pliability, with 60% to 80% of patients reporting at least partial improvement.3 Pressure reduces keloids by decreasing tissue metabolism, so long-term therapy is required, with application of pressure 23 to 24 hours daily over several months.12 Pressure of 25 to 40 mm Hg is needed to exceed capillary pressure without damaging peripheral circulation.3
Custom pressure garments for burn patients have been used in specialized cases involving limbs and torso, face and neck, and hands and feet. For earlobe keloids, clip-on earring devices are the only practical solution. Compliance tends to wane after months of therapy, and cessation may be followed by rebound hypertrophy. The therapeutic effect of regular pressure massage has been poorly studied. Because pressure has minimal adverse effects, it is likely most useful as an adjunct therapy where practical.
Radiation has variable effectiveness
Radiation damages fibroblasts, inhibits proliferation, and may improve local oxygenation.13 Low megavolt electron beam radiation is used to limit depth of penetration, usually with a total dose of 10 to 20 Gy fractionated over several weeks.5 A dose-response effect is likely.3 Response rates vary from 10% to 94%,3 and keloid recurrence is common, making radiation a poor choice for primary treatment except in cases of a large-volume unresectable keloid (eg, post-burn keloids).13
Adverse effects can include nodule formation, hyperpigmentation, ulceration, pruritus, paresthesia, and wound dehiscence. Theoretical risks of malignancy with radiation treatment of keloids have not been borne out in large studies, which have shown a zero percent malignancy rate despite rare anecdotal reports.15 Nevertheless, radiation therapy for benign disease is not recommended for children or pregnant women, or in breast, thyroid, or other cancer-prone body sites.5
Occlusive dressings require patient compliance
An innovation in keloid treatment is the use of occlusive dressings, which started in 1981 and gained popularity in the 1990s.16 Gel, fluid, or rubbery sheeting, usually made from silicone, is applied topically for 12 to 24 hours daily for up to 18 months.1,12,14 Gel may be practical along creases or in areas of motion where silicone sheets are obtrusive. Because long-term therapy is recommended, occlusive dressings require active patient participation. Occlusive dressings can decrease pruritic symptoms by decreasing mast cell activity,12 and may do so by warmth, hydration, or occlusion effects.3
Although study methodology has been suboptimal and further research is required, some practitioners have reported efficacy of 86% for texture reduction, 84% improvement in color, and 68% in diminishing height of scars.14 However, silicone gel and sheeting may be most successful when applied to a wound before a keloid has formed, as a preventive measure for keloid-prone patients.14 Occlusive dressings are a relatively benign treatment because silicone rubber is inert, but adverse effects may include skin breakdown, rash, or pruritus that may require discontinuation of therapy for a few days or more.
Combination regimens may be most effective
If a keloid remains unresponsive to first-line therapies, combined therapies may reduce keloid size and prevent recurrence. Select regimens according to keloid characteristics and patient preferences.17 Corticosteroids used after surgical excision can produce cure rates exceeding 80%, making this a consistently successful management regimen for keloids and the standard of care in many primary care practices.3
Surgery and pressure are the preferred combination for earlobe keloids, as adverse effects are minimal and compliant patients may achieve response rates exceeding 80%.3,5
Surgery followed by immediate radiation reportedly has a response rate of 65% or higher.18 This regimen may be most successful in low-tension body areas, like the neck or lower limbs, where keloids are less likely to recur.18
A combination of surgery and occlusive dressings is also promising. Early studies have had recurrence-free rates of more than 80% when patients applied the dressings for up to 24 hours daily over 4 to 6 months.6
Emerging therapies that require further testing
Many promising treatments lack sufficiently rigorous evidence of efficacy.
- Intralesional calcium channel blockers, which depolymerize actin19 and inhibit protein synthesis,5 have shown promising results in nonrandomized early clinical trials.19
- Ultraviolet light is a potentially successful noninvasive method.
- Topical retinoids (vitamin A derivatives) may be effective but must be applied twice daily for several months to reduce collagen metabolism by fibroblasts5; they may also cause photosensitivity and skin irritation.7
- Intralesional fluorouracil (5-FU) (as an antimetabolite) successfully reduced keloid size after one year of treatment in a few small trials.5,20
- Oral lathyrogen, such as penicillamine with colchicine, can interfere with collagen cross-linking; in a small case series there was no keloid recurrence after treatment.5
- Topical imiquimod (Aldara) cream, an immune response modifier, showed promise in a few case reports.5,21
- Laser procedures are less likely than scalpel procedures to produce keloid scars and have been reported to successfully improve scar color, size, and texture, although the risk of hyperpigmentation ranges from 1% to 24%.2,3,22 (Lasers induce ischemia through blood vessel destruction and cause decreased fibroblast production and histamine release.23,24) Red flat scars (which triamcinolone injections may cause) can be lightened with yellow light laser, which has been used in the treatment of capillary malformations.25
Other methods may produce suboptimal results. Cryotherapy causes ischemic damage that reduces tumor bulk at least temporarily, but is inappropriate for darker skin due to a high risk of persistent hypopigmentation.1,26,27 Intralesional antifibrotic cytokines, such as interferon, reduce collagen synthesis in vitro,1 but lead to considerable systemic adverse effects including headache, myalgias, and influenza-like symptoms.3,5 Skin grafting is inappropriate as it may lead to keloid wave formation at the graft edges.
CORRESPONDENCE
Stephen P. Daane, MD, 2186 Geary Boulevard #212, San Francisco, CA 94115; [email protected]
• Consider using corticosteroid injections to inhibit collagen synthesis and stimulate enzymatic degradation of existing keloid collagen. B
• Turn to treatment combinations for refractory keloids; regimens may include corticosteroid injections, surgical excision, pressure, occlusive dressings, or radiation. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Keloids are an ongoing clinical challenge. Despite the availability of multiple treatments, choosing an effective therapeutic regimen for these fibroproliferative scars can be vexing, as keloids usually recur.
This review provides a guide to determining which regimen is best for your patient. But first, a word about the causes of these claw-like growths and which patients are at highest risk of developing them.
Even superficial injuries can cause keloids
Keloids arise after a disruption of skin integrity following superficial or deep injuries (FIGURES 1A-C). Causes include physical trauma such as cuts, scratches, and insect bites; iatrogenic trauma as in vaccinations or surgical procedures; thermal or chemical burns; and skin eruptions such as chicken pox.
FIGURE 1
Keloids induced by scratches (A), ear piercing (B), and thermal burns (C)
Common sites of keloid development include the ears, jaw, neck, clavicle and sternum, and shoulders. Less commonly, keloids occur on the back, abdomen, and extremities. Rarely, keloids develop on the palms and soles, face, or mucous membranes.1
A minor keloid is smaller than 0.5 cm, while a major keloid is larger. No upper growth limit has been identified, as keloids seem to enlarge indefinitely.2 Keloid scars have a firm and inflexible texture, a shiny appearance, and are elevated above skin level. They are usually flesh-colored, but may be erythematous or hyperpigmented.1 (A similar aberrant wound healing condition often confused with keloids is hypertrophic scarring. See “Differentiating keloids from hypertrophic scars”).
Distinguishing between keloids and hypertrophic scars can be challenging, because both types of scars arise histologically from excessive collagen buildup during the wound healing process. However, there are 3 distinctive characteristics of keloids that hypertrophic scars lack:
- Keloids enlarge beyond wound margins, often with irregular shapes, while hypertrophic scars remain within the confines of the original wound and tend to be linear along scars.
- Keloids do not regress on their own; hypertrophic scars tend to regress spontaneously within a few years.
- Keloids may take months to years to develop, while hypertrophic scars usually appear within 4 to 6 weeks of a traumatic incident.
Source: Gauglitz G, Korting H, Pavicic T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113-125.
Although keloids have an aggressive growth pattern, they are considered benign tumors, because the development of malignant cells in a keloid is rare.1 Most patients seek medical attention for cosmetic concerns. However, tissue bulk can cause functional problems, and some keloids are symptomatic with pruritus (27%) or pain (19%).1,3
Who is affected?
The most consistent risk factor for keloid development is a previous keloid. Individuals with deeply pigmented skin appear to be at higher risk, with an estimated prevalence of 4% to 16% among individuals of African, Asian, and Hispanic ancestry.3 Although the rate of keloid occurrence among Caucasians has not been established, keloids have been reported in individuals of nearly every skin color, except albinos.3 Keloids occur in both sexes with similar frequency, and are most likely to develop during puberty and early adulthood—between the ages of 10 and 30 years—but can occur at any age.1,3
Although no clear familial inheritance pattern has been identified, keloids are associated with blood type A and human leukocyte antigens (HLA)-B14, -B21, -BW35, -DR5, -DRB1, -DQA1, -DQB1, and -DQW3.3,4 Emerging research indicates that mutations of the CDC2L1 gene—which encodes a protein kinase essential to cell cycle control—correlate with keloid formation.4
Wound healing gone awry
Normal wound healing involves an overlapping series of processes, including hemostasis, inflammation, and granulation and remodeling. Various mechanisms have been identified for keloid pathology, although no single definitive trigger is known.
Hemostasis. In normal hemostasis, platelets aggregate around a fibrin clot in a wound, stimulating the release of growth factors,3 which precipitate the migration of fibroblasts to the granulation-tissue scaffolding for wound healing. Keloids have increased fibroblast proliferation, with up to 20-fold increased production of Type 1 collagen.1
Inflammation. During the inflammation phase, cytokine cascades stimulate cell proliferation from inflammatory, endothelial, epithelial, and fibroblast cell lines. Keloids have altered levels of many cytokines, resulting in increased inflammatory activity, such as histamine release by mast cells.3
Granulation and remodeling. During normal granulation and remodeling, fibroblasts aggregate and produce extracellular matrix components. The keloid fibroblasts produce higher levels of collagen, elastin, fibronectin, and proteoglycans, but lower levels of hyaluronic acid.3,5 Keloid fibroblasts also make lower levels of tissue plasminogen activator inhibitor (TPA-I), with inferior breakdown of scaffold structures and collagens. While collagen bundles run parallel to the epithelial surface in normal skin, keloid collagen fibers are larger, thicker, and randomly oriented in dense sheets, swirls, or nodules.1
In normal tissue, wound healing stops after the wound has been fully epithelialized by keratinocytes, after which the scar tissue contracts to minimal size. However, keloid growth can continue for years.1
Numerous studies have inspired various hypotheses for keloid formation. Studies demonstrating that keloids contain an increased level of immunoglobulins suggest that they may be produced by an abnormal immune reaction. Microscopic examinations of keloids have shown that overabundant endothelial cells occlude microvessels, suggesting that keloids may occur in the context of wound hypoxia. In vitro studies showing that fibroblasts proliferate when cultured with keloidal keratinocytes suggest that keloids may be a result of abnormal epithelial–mesenchymal interactions.6,7 A hypothesis based on the body sites where keloids are observed is that mechanical stretch and tension across a wound may overstimulate collagen production, possibly as a result of mechanoreceptor damage or disorders.8
Treatment: Weighing the options
Due to the complex mechanisms of pathogenesis, many modalities are available for managing keloids, yet no definitive treatment protocols exist. Intradermal, extradermal, or systemic therapies may be used singly, although combination regimens are the most effective in treating keloids and preventing recurrences.5 The TABLE1,3,5,9-14 provides an overview of monotherapeutic treatment options, including efficacy and risks.
TABLE
Treatment options for keloids
| Treatment | Anticipated effects | Dosing and frequency | Efficacy in reducing keloid size | Adverse effects | SOR |
|---|---|---|---|---|---|
| Hygienic relief: washing & drying | Relief of pruritus, pain, general discomfort | As needed | N/A | Minimal | C |
| Antihistamines | Relief of pruritus, pain, burning sensation9 | As needed | N/A | Minimal | C |
| Corticosteroid injections | Reduction in keloid size, pruritus10 | Injections given every 4-8 wk for several months10 | >80% report moderate to marked regression10 | Local or systemic infection and allergy or anaphylaxis10 | B |
| Surgical excision | Temporary reduction in keloid size11 | Not successful as monotherapy; combine with alternative therapies as needed11 | Temporary; keloids always recur after excision11 | Chance of infection, excessive bleeding, or injury to adjacent tissues11 | C |
| Pressure therapy | Reduction in keloid size3,12 | Pressure of 25-40 mm Hg for 23-24 h/d over several months3,12 | 60%-80% of patients report at least partial improvement3 | Minimal; rarely, skin thinning and redness3,12 | B |
| Radiation therapy | Reduction in keloid size3 | 10-20 Gy fractionated over several weeks5 | Up to 94% of patients report improvement, but recurrence is common3 | Minimal; theoretical risk of malignancy13 | B |
| Silicone occlusive dressings | Reduction in pain, pruritus, and keloid size1,12,14 | Applied topically for 12-24 h/d for 18 mo1,12,14 | 68%-86% of patients report improvement in keloid texture, color, and size14; may be useful as a preventive measure14 | Skin breakdown, rash, pruritus12 | B |
| N/A, not applicable; SOR, strength of recommendation. | |||||
Offer symptomatic therapy routinely
Some patients find symptomatic relief through hygienic measures, such as regular washing and drying. Antihistamines can relieve symptoms of burning or pruritus,9 but are not expected to reduce keloid size.
Use corticosteroids for first-line treatment
Corticosteroids are the most widely used therapy for keloids.3 They decrease keloid bulking by inhibiting collagen synthesis1 and stimulate tissue collagenases and collagen degradation.5 Commonly used in the office setting are intralesional injections of triamcinolone acetonide, a potent anti-inflammatory and highly atrophogenic agent.
Administer injections every 4 to 8 weeks until clinical improvement (usually requiring several months) or prohibitive adverse effects occur. A general dosing guideline is about 0.1 to 0.2 mL of corticosteroid for every square centimeter of keloid tissue. Injecting higher doses can lead to accretions or deposits beneath the skin that might need to be unroofed. Additionally, excess corticosteroid can cause an atrophic contour deformity in the adjacent subcutaneous tissue. FIGURE 2 details steroid injection procedures and follow-up based on the author’s (SPD) experience.
FIGURE 2
A 5-step procedure for injecting keloids with corticosteroids*
*Based on the author’s (SPD) experience.
Contraindications to injections include local or systemic infection and known allergy or anaphylactic reaction to corticosteroids. Although injecting keloids can be difficult initially, the tissue tends to soften and become easier to inject with each successive treatment. Some clinicians advocate cryotherapy to soften the keloid before giving injections. Large trials, in which more than 80% of patients had moderate to marked keloid regression,10 have demonstrated the efficacy of this technique in reducing keloid size and itching.
Surgery alone is ineffective
The oldest remedy, simple scalpel excision of keloidal tissue, invariably results in regrowth, because the aberrant wound healing process is not remedied.11 Although surgical excision is a poor monotherapy, it may be useful in combination with other modalities. Repairs using meticulous atraumatic surgical techniques with minimal wound tension can lessen the risk of keloid recurrence. Absorbable monofilament sutures with a subcuticular stitch help to minimize further epidermal damage.6
Pressure is useful in certain cases
Applying pressure is a noninvasive method that produces initial tissue thinning and pliability, with 60% to 80% of patients reporting at least partial improvement.3 Pressure reduces keloids by decreasing tissue metabolism, so long-term therapy is required, with application of pressure 23 to 24 hours daily over several months.12 Pressure of 25 to 40 mm Hg is needed to exceed capillary pressure without damaging peripheral circulation.3
Custom pressure garments for burn patients have been used in specialized cases involving limbs and torso, face and neck, and hands and feet. For earlobe keloids, clip-on earring devices are the only practical solution. Compliance tends to wane after months of therapy, and cessation may be followed by rebound hypertrophy. The therapeutic effect of regular pressure massage has been poorly studied. Because pressure has minimal adverse effects, it is likely most useful as an adjunct therapy where practical.
Radiation has variable effectiveness
Radiation damages fibroblasts, inhibits proliferation, and may improve local oxygenation.13 Low megavolt electron beam radiation is used to limit depth of penetration, usually with a total dose of 10 to 20 Gy fractionated over several weeks.5 A dose-response effect is likely.3 Response rates vary from 10% to 94%,3 and keloid recurrence is common, making radiation a poor choice for primary treatment except in cases of a large-volume unresectable keloid (eg, post-burn keloids).13
Adverse effects can include nodule formation, hyperpigmentation, ulceration, pruritus, paresthesia, and wound dehiscence. Theoretical risks of malignancy with radiation treatment of keloids have not been borne out in large studies, which have shown a zero percent malignancy rate despite rare anecdotal reports.15 Nevertheless, radiation therapy for benign disease is not recommended for children or pregnant women, or in breast, thyroid, or other cancer-prone body sites.5
Occlusive dressings require patient compliance
An innovation in keloid treatment is the use of occlusive dressings, which started in 1981 and gained popularity in the 1990s.16 Gel, fluid, or rubbery sheeting, usually made from silicone, is applied topically for 12 to 24 hours daily for up to 18 months.1,12,14 Gel may be practical along creases or in areas of motion where silicone sheets are obtrusive. Because long-term therapy is recommended, occlusive dressings require active patient participation. Occlusive dressings can decrease pruritic symptoms by decreasing mast cell activity,12 and may do so by warmth, hydration, or occlusion effects.3
Although study methodology has been suboptimal and further research is required, some practitioners have reported efficacy of 86% for texture reduction, 84% improvement in color, and 68% in diminishing height of scars.14 However, silicone gel and sheeting may be most successful when applied to a wound before a keloid has formed, as a preventive measure for keloid-prone patients.14 Occlusive dressings are a relatively benign treatment because silicone rubber is inert, but adverse effects may include skin breakdown, rash, or pruritus that may require discontinuation of therapy for a few days or more.
Combination regimens may be most effective
If a keloid remains unresponsive to first-line therapies, combined therapies may reduce keloid size and prevent recurrence. Select regimens according to keloid characteristics and patient preferences.17 Corticosteroids used after surgical excision can produce cure rates exceeding 80%, making this a consistently successful management regimen for keloids and the standard of care in many primary care practices.3
Surgery and pressure are the preferred combination for earlobe keloids, as adverse effects are minimal and compliant patients may achieve response rates exceeding 80%.3,5
Surgery followed by immediate radiation reportedly has a response rate of 65% or higher.18 This regimen may be most successful in low-tension body areas, like the neck or lower limbs, where keloids are less likely to recur.18
A combination of surgery and occlusive dressings is also promising. Early studies have had recurrence-free rates of more than 80% when patients applied the dressings for up to 24 hours daily over 4 to 6 months.6
Emerging therapies that require further testing
Many promising treatments lack sufficiently rigorous evidence of efficacy.
- Intralesional calcium channel blockers, which depolymerize actin19 and inhibit protein synthesis,5 have shown promising results in nonrandomized early clinical trials.19
- Ultraviolet light is a potentially successful noninvasive method.
- Topical retinoids (vitamin A derivatives) may be effective but must be applied twice daily for several months to reduce collagen metabolism by fibroblasts5; they may also cause photosensitivity and skin irritation.7
- Intralesional fluorouracil (5-FU) (as an antimetabolite) successfully reduced keloid size after one year of treatment in a few small trials.5,20
- Oral lathyrogen, such as penicillamine with colchicine, can interfere with collagen cross-linking; in a small case series there was no keloid recurrence after treatment.5
- Topical imiquimod (Aldara) cream, an immune response modifier, showed promise in a few case reports.5,21
- Laser procedures are less likely than scalpel procedures to produce keloid scars and have been reported to successfully improve scar color, size, and texture, although the risk of hyperpigmentation ranges from 1% to 24%.2,3,22 (Lasers induce ischemia through blood vessel destruction and cause decreased fibroblast production and histamine release.23,24) Red flat scars (which triamcinolone injections may cause) can be lightened with yellow light laser, which has been used in the treatment of capillary malformations.25
Other methods may produce suboptimal results. Cryotherapy causes ischemic damage that reduces tumor bulk at least temporarily, but is inappropriate for darker skin due to a high risk of persistent hypopigmentation.1,26,27 Intralesional antifibrotic cytokines, such as interferon, reduce collagen synthesis in vitro,1 but lead to considerable systemic adverse effects including headache, myalgias, and influenza-like symptoms.3,5 Skin grafting is inappropriate as it may lead to keloid wave formation at the graft edges.
CORRESPONDENCE
Stephen P. Daane, MD, 2186 Geary Boulevard #212, San Francisco, CA 94115; [email protected]
1. Shaffer JJ, Taylor SC, Cook-Bolden F. Keloid scars: a review with a critical look at therapeutic options. J Am Acad Dermatol. 2002;46(suppl):S63-S97.
2. Mutoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560-571.
3. Niessen FB, Pauwen PH, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104:1435-1458.
4. Zhang G, Jiang J, Luo S, et al. Analyses of CDC2L1 gene mutations in keloid tissue. Clin Exp Dermatol. 2012;37:277-283.
5. Al Attar A, Mess S, Thomassen JM, et al. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117:286-300.
6. Yang GP, Lim IJ, Phan TT, et al. From scarless fetal wounds to keloids: molecular studies in wound healing. Wound Repair Regen. 2003;11:411-418.
7. Lim IJ, Phan TT, Bay BH, et al. Fibroblast cocultured with keloid keratinocytes: normal fibroblasts secrete collagen in a keloid like manner. Am J Physiol Cell Physiol. 2002;283:C212-C222.
8. Ogawa R. Keloid and hypertrophic scarring may result from a mechanoreceptor or mechanosensitive nociceptor disorder. Med Hypotheses. 2008;71:493-500.
9. Davidson S, Aziz N, Rashid R, et al. A primary care perspective on keloids. Medscape J Med. 2009;11:18 [Epub].-Available at: http://www.medscape.com/viewarticle/582445. Accessed March 8, 2013.
10. Ketchum LD, Smith J, Robinson DW, et al. The treatment of hypertrophic scar, keloid and scar contracture by triamcinolone acetonide. Plast Reconstr Surg. 1996;38:209-218.
11. Cosman B, Crikelair GF, Ju MC, et al. The surgical treatment of keloidal scars. Plast Reconstr Surg. 1961;27:335-358.
12. Eishi K, Bae SJ, Ogawa F, et al. Silicone gel sheets relieve pain and pruritus with clinical improvement of keloid: possible target mast cells. J Dermatol Treat. 2003;14:248-252.
13. Malaker K, Vijayraghavan K, Hodson I, et al. Retrospective analysis of treatment of unresectable keloids with primary radiation over 25 years. Clin Oncol. 2004;26:290-298.
14. Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic and keloid scars. J Cutan Aesthet Surg. 2009;2:104-106.
15. Botwood N, Lewanski C, Lowdell C. The risks of treating keloids with radiotherapy. Br J Radiol. 1999;72:1222-1224.
16. O’Brien L, Pandit A. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2006;(1):CD003826.-
17. Juckett G, Hartman-Adams H. Management of keloids and hypertrophic scars. Am Fam Physician. 2009;80:253-260.
18. Ogawa R, Mitsuhasi K, Hyakusoku H, et al. Postoperative electron beam irradiation therapy for keloid scars. Plast Reconstr Surg. 2003;111:547-555.
19. D’Andrea F, Brongo S, Ferrano G, et al. Prevention and treatment of keloids with intralesional verapamil. Dermatology. 2002;204:60-62.
20. Kontochristopoulus G, Stefanaki C, Panagiotopoulos A, et al. Intralesional 5-fluorouracil in the treatment of keloids: an open clinical and histopathological study. J Am Acad Dermatol. 2005;52:474-479.
21. Berman B, Kaufman J. Pilot study of the effect of postoperative imiquimod 5% cream on the recurrence rate of excised keloids. J Am Acad Dermatol. 2002;47:2209-2211.
22. Fickerstrand EJ, Svaasand LO, Volden G. Pigmentary changes after pulsed dye laser treatment in 125 northern European patients with port wine stains. Br J Dermatol. 1998;138:477-479.
23. Alster TS. Laser treatment of hypertrophic scars, keloids and striae. Dermatol Clin. 1997;15:419-429.
24. Alster TS. Laser scar revision: comparison study of 585nm pulsed dye laser with and without intralesional corticosteroids. Dermatol Surg. 2003;29:25-29.
25. Astner S, Anderson RR. Treating vascular lesions. Dermatol Ther. 2005;18:267-281.
26. Har-Shai Y, Amar M, Sabo E. Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plast Reconstr Surg. 2003;111:1841-1852.
27. Williams C, De Groote S. What treatment is best for hypertrophic scars and keloids? J Fam Pract. 2011;60:757-758.
1. Shaffer JJ, Taylor SC, Cook-Bolden F. Keloid scars: a review with a critical look at therapeutic options. J Am Acad Dermatol. 2002;46(suppl):S63-S97.
2. Mutoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560-571.
3. Niessen FB, Pauwen PH, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104:1435-1458.
4. Zhang G, Jiang J, Luo S, et al. Analyses of CDC2L1 gene mutations in keloid tissue. Clin Exp Dermatol. 2012;37:277-283.
5. Al Attar A, Mess S, Thomassen JM, et al. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117:286-300.
6. Yang GP, Lim IJ, Phan TT, et al. From scarless fetal wounds to keloids: molecular studies in wound healing. Wound Repair Regen. 2003;11:411-418.
7. Lim IJ, Phan TT, Bay BH, et al. Fibroblast cocultured with keloid keratinocytes: normal fibroblasts secrete collagen in a keloid like manner. Am J Physiol Cell Physiol. 2002;283:C212-C222.
8. Ogawa R. Keloid and hypertrophic scarring may result from a mechanoreceptor or mechanosensitive nociceptor disorder. Med Hypotheses. 2008;71:493-500.
9. Davidson S, Aziz N, Rashid R, et al. A primary care perspective on keloids. Medscape J Med. 2009;11:18 [Epub].-Available at: http://www.medscape.com/viewarticle/582445. Accessed March 8, 2013.
10. Ketchum LD, Smith J, Robinson DW, et al. The treatment of hypertrophic scar, keloid and scar contracture by triamcinolone acetonide. Plast Reconstr Surg. 1996;38:209-218.
11. Cosman B, Crikelair GF, Ju MC, et al. The surgical treatment of keloidal scars. Plast Reconstr Surg. 1961;27:335-358.
12. Eishi K, Bae SJ, Ogawa F, et al. Silicone gel sheets relieve pain and pruritus with clinical improvement of keloid: possible target mast cells. J Dermatol Treat. 2003;14:248-252.
13. Malaker K, Vijayraghavan K, Hodson I, et al. Retrospective analysis of treatment of unresectable keloids with primary radiation over 25 years. Clin Oncol. 2004;26:290-298.
14. Puri N, Talwar A. The efficacy of silicone gel for the treatment of hypertrophic and keloid scars. J Cutan Aesthet Surg. 2009;2:104-106.
15. Botwood N, Lewanski C, Lowdell C. The risks of treating keloids with radiotherapy. Br J Radiol. 1999;72:1222-1224.
16. O’Brien L, Pandit A. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2006;(1):CD003826.-
17. Juckett G, Hartman-Adams H. Management of keloids and hypertrophic scars. Am Fam Physician. 2009;80:253-260.
18. Ogawa R, Mitsuhasi K, Hyakusoku H, et al. Postoperative electron beam irradiation therapy for keloid scars. Plast Reconstr Surg. 2003;111:547-555.
19. D’Andrea F, Brongo S, Ferrano G, et al. Prevention and treatment of keloids with intralesional verapamil. Dermatology. 2002;204:60-62.
20. Kontochristopoulus G, Stefanaki C, Panagiotopoulos A, et al. Intralesional 5-fluorouracil in the treatment of keloids: an open clinical and histopathological study. J Am Acad Dermatol. 2005;52:474-479.
21. Berman B, Kaufman J. Pilot study of the effect of postoperative imiquimod 5% cream on the recurrence rate of excised keloids. J Am Acad Dermatol. 2002;47:2209-2211.
22. Fickerstrand EJ, Svaasand LO, Volden G. Pigmentary changes after pulsed dye laser treatment in 125 northern European patients with port wine stains. Br J Dermatol. 1998;138:477-479.
23. Alster TS. Laser treatment of hypertrophic scars, keloids and striae. Dermatol Clin. 1997;15:419-429.
24. Alster TS. Laser scar revision: comparison study of 585nm pulsed dye laser with and without intralesional corticosteroids. Dermatol Surg. 2003;29:25-29.
25. Astner S, Anderson RR. Treating vascular lesions. Dermatol Ther. 2005;18:267-281.
26. Har-Shai Y, Amar M, Sabo E. Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plast Reconstr Surg. 2003;111:1841-1852.
27. Williams C, De Groote S. What treatment is best for hypertrophic scars and keloids? J Fam Pract. 2011;60:757-758.