User login
Evaluation of Clonidine and Prazosin for the Treatment of Nighttime Posttraumatic Stress Disorder Symptoms
Posttraumatic stress disorder (PTSD) remains a significant health concern in veterans and military personnel. Whereas the lifetime incidence of PTSD in the U.S. general population is about 7% to 8%, the estimated prevalence of PTSD in deployed U.S. military personnel is higher than the national average, ranging from 11% to 17%.1,2 These numbers may be even higher, depending on the branch of service, responsibilities within the military, and specific conflict in which the veteran served. For example, one study found that 31% of Vietnam veterans have PTSD, and another recent study has reported PTSD in 28.7% of veterans returning from military service in Iraq and Afghanistan.3,4
Posttraumatic stress disorder treatment guidelines from both the American Psychiatric Association and the VA and DoD recommend the use of selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) as first-line pharmacotherapy for PTSD.5,6 However, SSRIs and SNRIs seem to be largely ineffective for the management of nighttime PTSD symptoms, such as insomnia and nightmares.7,8
Related: PTSD Increases Chance of Heart Failure
Researchers hypothesize that the sympathetic nervous system plays a significant role in the hyperarousal component of nighttime PTSD. The heightened responsiveness and disruption in restorative sleep seen in PTSD have been attributed to increased activity of norepinephrine in the central nervous system.9 Mechanistically, therapies that attenuate the increased noradrenergic signaling might be effective in the management of nighttime PTSD symptoms.
The body of evidence for the use of adrenergic agents for nighttime PTSD symptoms is growing. Prazosin, a peripherally acting α1-adrenergic receptor antagonist, has recently been demonstrated to be effective for nighttime PTSD symptoms in veterans in a series of small, randomized controlled trials.10-12 Data to support the use of clonidine, a centrally acting α2-adrenergic receptor agonist, are generally limited, with the most compelling data coming from a population of civilian Cambodian refugees.13,14 A 2007 article by Boehnlein and Kinzie includes a thorough review of the preclinical research, case reports, and early clinical studies that have led to the widespread use of these agents for PTSD despite the lack of FDA approval for this indication.13A previous retrospective review by Byers and colleagues compared the effectiveness and tolerability of prazosin and quetiapine for nighttime PTSD symptoms in veterans.15 The results of that review suggest that α1-adrenergic agents may be equally effective and better tolerated than alternative medication options (ie, atypical antipsychotics) for this purpose. The present study was adapted from this design to report concurrently on the real-world use of clonidine and prazosin for the treatment of nighttime PTSD symptoms.
Study Objectives
The primary objective of this retrospective chart review was to describe the experience of patients prescribed clonidine or prazosin for the treatment of nighttime PTSD symptoms, including initial effectiveness. The primary endpoint of initial drug effectiveness was documented improvement of nighttime PTSD symptoms in the patient’s chart within 6 months of the date of first prescription. Clonidine or prazosin was categorized as initially effective if a statement such as “frequency of nightmares decreased” or “patient’s nighttime PTSD symptoms have improved” was made within 6 months after initial prescription of the drug.
The secondary objectives of this study were to evaluate the long-term effectiveness and tolerability of prazosin. The endpoints used to assess these outcomes were the 2-year continuation rates of clonidine and prazosin (as a surrogate marker for long-term effectiveness) and the documented reasons for discontinuation of clonidine and prazosin for the treatment of nighttime PTSD symptoms (in order to assess tolerability).
Methods
An electronic database search was conducted to identify the VA Portland Health Care System (VAPHCS) patients with a diagnosis of PTSD who received a first prescription for clonidine or prazosin for nighttime PTSD symptoms from a VAPHCS mental health provider or primary care provider (PCP) from January 1, 2009, to December 31, 2011. Patients were excluded if they had any history of prior use of the drug being initiated, were co-initiated on both clonidine and prazosin (defined as starting the drugs within 30 days of each other), or had a concomitant diagnosis of schizophrenia, bipolar disorder, psychotic disorder, or cognitive disorder as defined in the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. Patients with traumatic brain injury (TBI) were excluded only if it could be determined that the event had resulted in lasting cognitive impairment.
Study Population
All patients with a diagnosis of PTSD who received a first prescription for clonidine during the period specified were screened for inclusion; patients with PTSD who were first prescribed prazosin during the same period were randomly sampled to equalize patient populations. This was done to maximize the data set while examining groups of roughly equal size for each drug, as prazosin is used much more commonly than clonidine for nighttime PTSD symptoms at VAPHCS. The patients in each resulting group were screened to determine whether they met inclusion and exclusion criteria. All subjects included were followed for 2 years from the date of the initial prescription.
Study Design
Initial effectiveness of each agent was determined by reviewing subjects’ progress notes after the initial prescription of clonidine or prazosin for documentation of improvement in symptoms within 6 months of the prescription start date. A decrease in frequency or intensity of nighttime PTSD symptoms, nightmares, or insomnia, as documented in the patient chart, was interpreted as improvement of symptoms.
Long-term continuation was assessed by reviewing subjects’ prescription records, to determine whether prescription(s) for clonidine or prazosin continued for 2 years after the date of the initial prescription.
Any gap between medication fills that resulted in an anticipated period without medication of ≥ 6 months (eg, 9 months after receiving a 90-day supply) was considered discontinuation of therapy. Prescription refill history was also reviewed, and medication possession ratio (MPR) was calculated to assess whether patients were adherent to the study drug as prescribed. Adherence was defined as an MPR of ≥ 80%. Patients who left the VAPHCS service area but continued to receive care at another VA were assessed for continuation of therapy, but refill data and/or MPR were not assessed.
Tolerability was assessed by reviewing subjects’ medical records to determine whether therapy with clonidine or prazosin was discontinued due to documented adverse effects (AEs). The occurrence of AEs was determined by reviewing progress notes and other chart documentation surrounding the date of discontinuation. If the drug was discontinued but the reason was not explicitly documented or if the prescription expired without a documented reason for nonrenewing, the reason for discontinuation was coded as “not specified.” Discontinuation due to treatment failure, change in symptoms, nonadherence, or other causes was also recorded. If multiple reasons for discontinuation were cited for a single patient, all were included in the data. This project was approved by the institutional review board at the VAPHCS.
Related:Depression and Substance Abuse Intensify Suicide Risk
Statistical Considerations
Based on clinical experience, it was presumed that many of the patients who were prescribed clonidine would be receiving it as a second-line therapy after failing prazosin. Therefore, statistical analysis of the relative effectiveness and tolerability of clonidine and prazosin could not be performed. Neither power nor sample size needed to demonstrate any difference in effectiveness or tolerability between the groups was calculated. All results are expressed using descriptive statistics.
Results
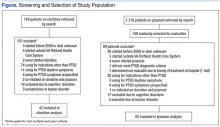
An initial database search for patients with PTSD who received a first prescription for clonidine between January 1, 2009, and December 31, 2011, from a VAPHCS provider yielded a list of 149 patients. The same search criteria applied for prazosin yielded 1,116 patients, 149 of whom were randomly selected for screening. After screening, 42 patients on clonidine and 60 patients on prazosin were included in this analysis (Figure).
Patient Demographics
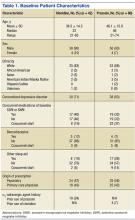
The average age of the clonidine patients was 38.5 years (range 21-65 years) (Table 1). The clonidine group was primarily male (90%) and white (83%). Eighteen of the 42 patients in the clonidine group had a baseline PTSD Checklist-Civilian version (PCL-C) score available within the 90 days before the first prescription of clonidine; the average baseline PCL-C score in this subgroup was 62 ± 12.0 (median 65.5, range 31-82). Most of the clonidine patients (71%) had a concomitant diagnosis of a depressive disorder. About one-quarter of the group (24%) had previously tried prazosin per prescription records. In 24 patients (57%), the first prescription for clonidine was written by a psychiatrist or psychiatric nurse practitioner; 18 patients (43%) were started on clonidine by PCPs.
The average age of the prazosin patients was 46.1 years (range 21-74 years). The prazosin group was also primarily male (93%) and white (88%). Twenty of the 60 patients in the prazosin group had a baseline PCL-C score available within the 90 days before the first prescription of prazosin; the average baseline PCL-C score in this subgroup was 55 ± 16.1 (median 64, range 30-72). Most of the prazosin patients (63%) had a concomitant diagnosis of a depressive disorder. Four patients (7%) had previously tried clonidine per prescription records. In 35 patients (58%), the first prescription for prazosin was written by a psychiatrist or psychiatric nurse practitioner; 25 patients (42%) were started on prazosin by PCPs.
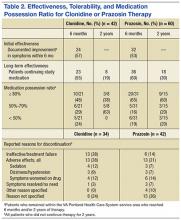
Data pertaining to initial and long-term effectiveness, tolerability, and MPR for both clonidine and prazosin are presented in Table 2.
Clonidine
Of the 42 clonidine patients assessed, 24 (57%) had a positive response to the medication for nighttime PTSD symptoms documented in the Computerized Patient Record System (CPRS) within 6 months of starting therapy. Six months after starting clonidine, 23 patients (55%) continued to take clonidine. Two years after starting therapy, 8 of the original 42 patients continued on clonidine for an overall 2-year continuation rate of 19%.
Tolerability
Of the 34 patients who discontinued clonidine within 2 years, 13 patients (38%) cited ineffectiveness of therapy as a reason for discontinuation. Another 13 patients (38%) reported discontinuing therapy due to AEs. Sedation (4 patients, 12%), dizziness/hypotension (3 patients, 9%), and paradoxical worsening of PTSD symptoms (4 patients, 12%) were the most common AEs leading to discontinuation. Other AEs cited as reasons for discontinuation were syncope (2 patients), erectile dysfunction (1 patient), rash (1 patient), myoclonus (1 patient), increased depression (1 patient), and fatigue (1 patient). One patient reported that he had discontinued clonidine due to symptom resolution/lack of need for treatment. In 8 of the 34 patients, no reason for discontinuation was found in chart documentation.
Medication Possession Ratio
Among the 21 evaluable patients who continued to receive clonidine 6 months after initiation, 10 (48%) were determined to be highly adherent to therapy, with an MPR of ≥ 80%. Six of the 21 patients (29%) had an MPR between 50% and 79%, and 5 patients (24%) had an MPR < 50%.
Of the 8 patients who continued on clonidine at the 2-year mark, 3 (38%) were adherent to therapy, with an MPR of ≥ 80%. Three more patients (38%) had a 2-year MPR between 50% and 80%, and 2 patients (25%) had an MPR < 50%.
Prazosin
Of the 60 prazosin patients assessed, 32 (53%) had a positive response to the medication for nighttime PTSD symptoms documented in the CPRS within 6 months of starting therapy. Six months after starting prazosin, 36 patients (60%) continued to take prazosin. Two years after starting therapy, 18 of the original 60 patients continued on prazosin for an overall 2-year continuation rate of 30%.
Tolerability
Of the 42 patients who discontinued prazosin within 2 years, six patients (14%) cited ineffectiveness of therapy as a reason for discontinuation. Thirteen patients (31%) reported discontinuing therapy due to AEs. Sedation (3 patients, 7%), dizziness/hypotension (3 patients, 7%), and paradoxical worsening of PTSD symptoms (6 patients, 14%) were the most common AEs leading to discontinuation. Other AEs cited as reasons for discontinuation were headache (2 patients), altered mental status (1 patient), and fatigue (1 patient). Three patients reported that they had discontinued clonidine due to symptom resolution/lack of need for treatment. Other reasons for discontinuation not related to AEs included flight rules (1 patient), changes to antihypertensive regimen (1 patient), refill issues (1 patient), and cost (1 patient). In 15 of the 42 patients, no reason for discontinuation was found in chart documentation.
Medication Possession Ratio
Among the 31 evaluable patients who continued to receive prazosin 6 months after initiation, 20 (65%) were determined to be highly adherent to therapy, with an MPR of ≥ 80%. Five of the 31 patients (16%) had an MPR between 50% and 80%, and 6 patients (19%) had an MPR < 50%.
Of the 15 evaluable patients who continued on prazosin at the 2-year mark, 9 (60%) were adherent to therapy, with an MPR of ≥ 80%. Three patients (20%) had a 2-year MPR between 50% and 80%, and 3 patients (20%) had an MPR < 50%.
Discussion
Although prazosin has been shown to be effective for nighttime PTSD symptoms in both prospective and retrospective evaluations in veterans, this study provides the first evidence to support the use of clonidine in a veteran population.10-12,15
Interestingly, 42% of the patients assessed received their first prescription of an α2-adrenergic agent for nighttime PTSD symptoms from a PCP. Even with the recent increased focus on integrating mental health into primary care within the VA, this was a surprising finding. Primary care providers at VAPHCS may have a greater role in the outpatient management of PTSD than previously suspected. The information presented here may prove useful and applicable in both psychiatric and primary care treatment settings.
The study results indicated that a majority of subjects initially reported effectiveness with either clonidine or prazosin (53% and 57%, respectively). The initial effectiveness rate for prazosin is similar to those described in previous studies.10-13,15 The data also support a viable role for clonidine in the treatment of nighttime PTSD symptoms.
Regardless of initial improvement, the study results also suggest that the therapeutic benefit may not persist in the long term, as evidenced by a significant percentage of discontinuations attributed to ineffectiveness (38% for clonidine and 14% for prazosin) and a very low rate of long-term continuation (19% for clonidine and 30% for prazosin at 2 years). This latter observation contrasts with findings from previous studies; Byers and colleagues reported a 2-year prazosin continuation rate of 48.4% in a similar analysis, and Boehnlein and colleagues reported a sustained benefit of clonidine in responders over a 10-year period.14,15 The wide variety of reasons for discontinuation reported here may help providers who are considering clonidine or prazosin for their patients to anticipate barriers to long-term success.
Part of the discrepancy between these results and previously reported successes with clonidine and prazosin may be attributable to the classic issue of efficacy vs effectiveness. Many of the studies that have informed us on the efficacy and tolerability of prazosin for nighttime PTSD symptoms described outcomes of prospective clinical research. Furthermore, these prospective trials were limited to < 6 months in duration. To date, neither clonidine nor prazosin has been evaluated for long-term efficacy and effectiveness in well-designed, prospective trials. This retrospective analysis may help provide a realistic estimate of the long-term effectiveness of these therapies, especially within the veteran population.
Limitations
This was a single-center, retrospective study conducted primarily in white male patients. Although likely applicable to the U.S. veteran population at large, these data may be poorly generalizable to patient populations outside the VA health care system.
Aside from external validity, this study has several significant limitations. The primary limitation of this project is that it was not designed to allow for statistical comparison of clonidine and prazosin. Such an analysis would have better defined the role of clonidine in PTSD treatment, either by establishing similar effectiveness of clonidine and prazosin for nighttime symptoms or by providing evidence of the superiority of one over the other. In designing the project, investigators suspected based on experience that the majority of patients prescribed clonidine would receive the drug after having already failed first-line therapy with prazosin. Had this been the case, a direct comparison may have been biased in favor of prazosin. In retrospect, however, only 24% of the clonidine group had previously been prescribed prazosin, and only 7% of the prazosin group had been prescribed clonidine. This suggests that clonidine may be used first line more often than the investigators anticipated and that a future direct comparison would be worthwhile.
Second, the subjective data collected for this project required investigators to read and interpret chart notes, although the review of all records by a single investigator helped limit variability in interpretation. At times, information in the CPRS was incomplete in terms of determining continuation of therapy or cause for discontinuation.
Third, although it is implied that a significant number of veterans have combat-related PTSD, the nature of the traumatic event(s) leading to PTSD was not recorded in this study, and no subgroup analysis was done to compare the effect of α2-adrenergic agents between combat- and noncombat-related PTSD. Owing to their exclusion by design, it is also difficult to apply these results to veterans who have lasting cognitive impairment as a result of TBI, who are presumably among those most likely to have experienced traumas that could provoke PTSD.
The design of this project also did not include a subgroup analysis based on antidepressant type, and it is unclear whether the potential pharmacodynamic interaction between noradrenergic antidepressants (ie, SNRIs) and anti– α2-adrenergic agents had any impact on clinical outcomes. The use of complementary nonpharmacologic treatment modalities (ie, psychotherapy, eye movement desensitization and reprocessing) was also not evaluated.
Related: Female Service Members in the Long War
Finally, the primary outcome of patient-reported improvement in symptoms does not provide information on the magnitude or specific nature of benefits derived. Given the retrospective nature, data used in prospectively designed studies (eg, rating scales pertinent to PTSD), which might have helped to quantify the benefit of treatment, was not consistently available. Even a baseline PCL-C score, collected in order to describe the patient population, was available only in 37% of the patients assessed. Furthermore, nighttime PTSD symptoms vary among individuals, but the primary outcome of this study pools any benefits seen in areas such as nightmares, awakenings, night sweats, or sleep quality into a single outcome of symptom improvement.
Conclusions
This study indicates that both clonidine and prazosin may be effective for the treatment of nighttime PTSD symptoms in the veteran population but that their long-term utility may be limited by waning effectiveness, tolerability, and adherence issues. At this time, it is unclear whether either agent has an advantage over the other in terms of effectiveness or tolerability; further studies are needed to address that question.
Despite its limitations, the authors anticipate that this study will provide information regarding the effectiveness and tolerability of clonidine and prazosin to treat nighttime PTSD symptoms. Findings from this study may help clinicians to anticipate the needs and challenges of patients using β2-adrenergic agents for nighttime symptoms of PTSD.
Acknowledgements
The authors wish to acknowledge Brian Wilcox, PharmD, for his assistance in generating patient data reports, and Ronald Brown, RPh, MS, for his guidance regarding data analysis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental problems, and barriers to care. N Engl J Med. 2004;351(1):13-22.
2. Gates MA, Holowka DW, Vasterling JJ, Keane TM, Marx BP, Rosen RC. Posttraumatic stress disorder in veterans and military personnel: epidemiology, screening, and case recognition. Psychol Serv. 2012;9(4):361-382.
3. Kulka R, Schlenger WE, Fairbanks J, et al. Trauma and the Vietnam War Generation: Report of Findings From the National Vietnam Veterans Readjustment Study. New York, NY: Brunnel/Mazel; 1990.
4. Barrera TL, Graham DP, Dunn NJ, Teng EJ. Influence of trauma history on panic and posttraumatic stress disorder in returning veterans. Psychol Serv. 2013;10(2):168-176.
5. American Psychiatric Association. Practice Guideline for the Treatment of Patients With Acute Stress Disorder and Posttraumatic Stress Disorder. Arlington, VA: American Psychiatric Association; 2004.
6. U.S. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for management of post-traumatic stress. Version 2.0. U.S. Department of Veterans Affairs Website. http://www.healthquality.va.gov/guidelines/MH/ptsd/cpgPTSDFULL201011612c.pdf. Published October 2010. Accessed October 5, 2015.
7. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):169-180.
8. Ravindran LN, Stein MB. Pharmacotherapy of post-traumatic stress disorder. In: Stein MB, Steckler T, eds. Behavioral Neurobiology of Anxiety and Its Treatment. Vol 2. Heidelberg, Germany: Springer; 2010:505-525.
9. Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12(3):169-184.
10. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo controlled study. Am J Psychiatry. 2003;160(2):371-373.
11. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
12. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170:1003-1010.
13. Boehnlein JK, Kinzie JD. Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. J Psychiatr Pract. 2007;13(2):72-78.
14. Boehnlein JK, Kinzie JD, Sekiya U, Riley C, Pou K, Rosborough B. A ten-year treatment outcome study of traumatized Cambodian refugees. J Nerve Ment Dis. 2004;192(10):658-663.
15. Byers MG, Allison KM, Wendel CS, Lee JK. Prazosin versus quetiapine for nighttime posttraumatic stress disorder symptoms in veterans: an assessment of long-term comparative effectiveness and safety. J Clin Psychopharmacol. 2010;30(3):225-229.
Posttraumatic stress disorder (PTSD) remains a significant health concern in veterans and military personnel. Whereas the lifetime incidence of PTSD in the U.S. general population is about 7% to 8%, the estimated prevalence of PTSD in deployed U.S. military personnel is higher than the national average, ranging from 11% to 17%.1,2 These numbers may be even higher, depending on the branch of service, responsibilities within the military, and specific conflict in which the veteran served. For example, one study found that 31% of Vietnam veterans have PTSD, and another recent study has reported PTSD in 28.7% of veterans returning from military service in Iraq and Afghanistan.3,4
Posttraumatic stress disorder treatment guidelines from both the American Psychiatric Association and the VA and DoD recommend the use of selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) as first-line pharmacotherapy for PTSD.5,6 However, SSRIs and SNRIs seem to be largely ineffective for the management of nighttime PTSD symptoms, such as insomnia and nightmares.7,8
Related: PTSD Increases Chance of Heart Failure
Researchers hypothesize that the sympathetic nervous system plays a significant role in the hyperarousal component of nighttime PTSD. The heightened responsiveness and disruption in restorative sleep seen in PTSD have been attributed to increased activity of norepinephrine in the central nervous system.9 Mechanistically, therapies that attenuate the increased noradrenergic signaling might be effective in the management of nighttime PTSD symptoms.
The body of evidence for the use of adrenergic agents for nighttime PTSD symptoms is growing. Prazosin, a peripherally acting α1-adrenergic receptor antagonist, has recently been demonstrated to be effective for nighttime PTSD symptoms in veterans in a series of small, randomized controlled trials.10-12 Data to support the use of clonidine, a centrally acting α2-adrenergic receptor agonist, are generally limited, with the most compelling data coming from a population of civilian Cambodian refugees.13,14 A 2007 article by Boehnlein and Kinzie includes a thorough review of the preclinical research, case reports, and early clinical studies that have led to the widespread use of these agents for PTSD despite the lack of FDA approval for this indication.13A previous retrospective review by Byers and colleagues compared the effectiveness and tolerability of prazosin and quetiapine for nighttime PTSD symptoms in veterans.15 The results of that review suggest that α1-adrenergic agents may be equally effective and better tolerated than alternative medication options (ie, atypical antipsychotics) for this purpose. The present study was adapted from this design to report concurrently on the real-world use of clonidine and prazosin for the treatment of nighttime PTSD symptoms.
Study Objectives
The primary objective of this retrospective chart review was to describe the experience of patients prescribed clonidine or prazosin for the treatment of nighttime PTSD symptoms, including initial effectiveness. The primary endpoint of initial drug effectiveness was documented improvement of nighttime PTSD symptoms in the patient’s chart within 6 months of the date of first prescription. Clonidine or prazosin was categorized as initially effective if a statement such as “frequency of nightmares decreased” or “patient’s nighttime PTSD symptoms have improved” was made within 6 months after initial prescription of the drug.
The secondary objectives of this study were to evaluate the long-term effectiveness and tolerability of prazosin. The endpoints used to assess these outcomes were the 2-year continuation rates of clonidine and prazosin (as a surrogate marker for long-term effectiveness) and the documented reasons for discontinuation of clonidine and prazosin for the treatment of nighttime PTSD symptoms (in order to assess tolerability).
Methods
An electronic database search was conducted to identify the VA Portland Health Care System (VAPHCS) patients with a diagnosis of PTSD who received a first prescription for clonidine or prazosin for nighttime PTSD symptoms from a VAPHCS mental health provider or primary care provider (PCP) from January 1, 2009, to December 31, 2011. Patients were excluded if they had any history of prior use of the drug being initiated, were co-initiated on both clonidine and prazosin (defined as starting the drugs within 30 days of each other), or had a concomitant diagnosis of schizophrenia, bipolar disorder, psychotic disorder, or cognitive disorder as defined in the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. Patients with traumatic brain injury (TBI) were excluded only if it could be determined that the event had resulted in lasting cognitive impairment.
Study Population
All patients with a diagnosis of PTSD who received a first prescription for clonidine during the period specified were screened for inclusion; patients with PTSD who were first prescribed prazosin during the same period were randomly sampled to equalize patient populations. This was done to maximize the data set while examining groups of roughly equal size for each drug, as prazosin is used much more commonly than clonidine for nighttime PTSD symptoms at VAPHCS. The patients in each resulting group were screened to determine whether they met inclusion and exclusion criteria. All subjects included were followed for 2 years from the date of the initial prescription.
Study Design
Initial effectiveness of each agent was determined by reviewing subjects’ progress notes after the initial prescription of clonidine or prazosin for documentation of improvement in symptoms within 6 months of the prescription start date. A decrease in frequency or intensity of nighttime PTSD symptoms, nightmares, or insomnia, as documented in the patient chart, was interpreted as improvement of symptoms.
Long-term continuation was assessed by reviewing subjects’ prescription records, to determine whether prescription(s) for clonidine or prazosin continued for 2 years after the date of the initial prescription.
Any gap between medication fills that resulted in an anticipated period without medication of ≥ 6 months (eg, 9 months after receiving a 90-day supply) was considered discontinuation of therapy. Prescription refill history was also reviewed, and medication possession ratio (MPR) was calculated to assess whether patients were adherent to the study drug as prescribed. Adherence was defined as an MPR of ≥ 80%. Patients who left the VAPHCS service area but continued to receive care at another VA were assessed for continuation of therapy, but refill data and/or MPR were not assessed.
Tolerability was assessed by reviewing subjects’ medical records to determine whether therapy with clonidine or prazosin was discontinued due to documented adverse effects (AEs). The occurrence of AEs was determined by reviewing progress notes and other chart documentation surrounding the date of discontinuation. If the drug was discontinued but the reason was not explicitly documented or if the prescription expired without a documented reason for nonrenewing, the reason for discontinuation was coded as “not specified.” Discontinuation due to treatment failure, change in symptoms, nonadherence, or other causes was also recorded. If multiple reasons for discontinuation were cited for a single patient, all were included in the data. This project was approved by the institutional review board at the VAPHCS.
Related:Depression and Substance Abuse Intensify Suicide Risk
Statistical Considerations
Based on clinical experience, it was presumed that many of the patients who were prescribed clonidine would be receiving it as a second-line therapy after failing prazosin. Therefore, statistical analysis of the relative effectiveness and tolerability of clonidine and prazosin could not be performed. Neither power nor sample size needed to demonstrate any difference in effectiveness or tolerability between the groups was calculated. All results are expressed using descriptive statistics.
Results
An initial database search for patients with PTSD who received a first prescription for clonidine between January 1, 2009, and December 31, 2011, from a VAPHCS provider yielded a list of 149 patients. The same search criteria applied for prazosin yielded 1,116 patients, 149 of whom were randomly selected for screening. After screening, 42 patients on clonidine and 60 patients on prazosin were included in this analysis (Figure).
Patient Demographics
The average age of the clonidine patients was 38.5 years (range 21-65 years) (Table 1). The clonidine group was primarily male (90%) and white (83%). Eighteen of the 42 patients in the clonidine group had a baseline PTSD Checklist-Civilian version (PCL-C) score available within the 90 days before the first prescription of clonidine; the average baseline PCL-C score in this subgroup was 62 ± 12.0 (median 65.5, range 31-82). Most of the clonidine patients (71%) had a concomitant diagnosis of a depressive disorder. About one-quarter of the group (24%) had previously tried prazosin per prescription records. In 24 patients (57%), the first prescription for clonidine was written by a psychiatrist or psychiatric nurse practitioner; 18 patients (43%) were started on clonidine by PCPs.
The average age of the prazosin patients was 46.1 years (range 21-74 years). The prazosin group was also primarily male (93%) and white (88%). Twenty of the 60 patients in the prazosin group had a baseline PCL-C score available within the 90 days before the first prescription of prazosin; the average baseline PCL-C score in this subgroup was 55 ± 16.1 (median 64, range 30-72). Most of the prazosin patients (63%) had a concomitant diagnosis of a depressive disorder. Four patients (7%) had previously tried clonidine per prescription records. In 35 patients (58%), the first prescription for prazosin was written by a psychiatrist or psychiatric nurse practitioner; 25 patients (42%) were started on prazosin by PCPs.
Data pertaining to initial and long-term effectiveness, tolerability, and MPR for both clonidine and prazosin are presented in Table 2.
Clonidine
Of the 42 clonidine patients assessed, 24 (57%) had a positive response to the medication for nighttime PTSD symptoms documented in the Computerized Patient Record System (CPRS) within 6 months of starting therapy. Six months after starting clonidine, 23 patients (55%) continued to take clonidine. Two years after starting therapy, 8 of the original 42 patients continued on clonidine for an overall 2-year continuation rate of 19%.
Tolerability
Of the 34 patients who discontinued clonidine within 2 years, 13 patients (38%) cited ineffectiveness of therapy as a reason for discontinuation. Another 13 patients (38%) reported discontinuing therapy due to AEs. Sedation (4 patients, 12%), dizziness/hypotension (3 patients, 9%), and paradoxical worsening of PTSD symptoms (4 patients, 12%) were the most common AEs leading to discontinuation. Other AEs cited as reasons for discontinuation were syncope (2 patients), erectile dysfunction (1 patient), rash (1 patient), myoclonus (1 patient), increased depression (1 patient), and fatigue (1 patient). One patient reported that he had discontinued clonidine due to symptom resolution/lack of need for treatment. In 8 of the 34 patients, no reason for discontinuation was found in chart documentation.
Medication Possession Ratio
Among the 21 evaluable patients who continued to receive clonidine 6 months after initiation, 10 (48%) were determined to be highly adherent to therapy, with an MPR of ≥ 80%. Six of the 21 patients (29%) had an MPR between 50% and 79%, and 5 patients (24%) had an MPR < 50%.
Of the 8 patients who continued on clonidine at the 2-year mark, 3 (38%) were adherent to therapy, with an MPR of ≥ 80%. Three more patients (38%) had a 2-year MPR between 50% and 80%, and 2 patients (25%) had an MPR < 50%.
Prazosin
Of the 60 prazosin patients assessed, 32 (53%) had a positive response to the medication for nighttime PTSD symptoms documented in the CPRS within 6 months of starting therapy. Six months after starting prazosin, 36 patients (60%) continued to take prazosin. Two years after starting therapy, 18 of the original 60 patients continued on prazosin for an overall 2-year continuation rate of 30%.
Tolerability
Of the 42 patients who discontinued prazosin within 2 years, six patients (14%) cited ineffectiveness of therapy as a reason for discontinuation. Thirteen patients (31%) reported discontinuing therapy due to AEs. Sedation (3 patients, 7%), dizziness/hypotension (3 patients, 7%), and paradoxical worsening of PTSD symptoms (6 patients, 14%) were the most common AEs leading to discontinuation. Other AEs cited as reasons for discontinuation were headache (2 patients), altered mental status (1 patient), and fatigue (1 patient). Three patients reported that they had discontinued clonidine due to symptom resolution/lack of need for treatment. Other reasons for discontinuation not related to AEs included flight rules (1 patient), changes to antihypertensive regimen (1 patient), refill issues (1 patient), and cost (1 patient). In 15 of the 42 patients, no reason for discontinuation was found in chart documentation.
Medication Possession Ratio
Among the 31 evaluable patients who continued to receive prazosin 6 months after initiation, 20 (65%) were determined to be highly adherent to therapy, with an MPR of ≥ 80%. Five of the 31 patients (16%) had an MPR between 50% and 80%, and 6 patients (19%) had an MPR < 50%.
Of the 15 evaluable patients who continued on prazosin at the 2-year mark, 9 (60%) were adherent to therapy, with an MPR of ≥ 80%. Three patients (20%) had a 2-year MPR between 50% and 80%, and 3 patients (20%) had an MPR < 50%.
Discussion
Although prazosin has been shown to be effective for nighttime PTSD symptoms in both prospective and retrospective evaluations in veterans, this study provides the first evidence to support the use of clonidine in a veteran population.10-12,15
Interestingly, 42% of the patients assessed received their first prescription of an α2-adrenergic agent for nighttime PTSD symptoms from a PCP. Even with the recent increased focus on integrating mental health into primary care within the VA, this was a surprising finding. Primary care providers at VAPHCS may have a greater role in the outpatient management of PTSD than previously suspected. The information presented here may prove useful and applicable in both psychiatric and primary care treatment settings.
The study results indicated that a majority of subjects initially reported effectiveness with either clonidine or prazosin (53% and 57%, respectively). The initial effectiveness rate for prazosin is similar to those described in previous studies.10-13,15 The data also support a viable role for clonidine in the treatment of nighttime PTSD symptoms.
Regardless of initial improvement, the study results also suggest that the therapeutic benefit may not persist in the long term, as evidenced by a significant percentage of discontinuations attributed to ineffectiveness (38% for clonidine and 14% for prazosin) and a very low rate of long-term continuation (19% for clonidine and 30% for prazosin at 2 years). This latter observation contrasts with findings from previous studies; Byers and colleagues reported a 2-year prazosin continuation rate of 48.4% in a similar analysis, and Boehnlein and colleagues reported a sustained benefit of clonidine in responders over a 10-year period.14,15 The wide variety of reasons for discontinuation reported here may help providers who are considering clonidine or prazosin for their patients to anticipate barriers to long-term success.
Part of the discrepancy between these results and previously reported successes with clonidine and prazosin may be attributable to the classic issue of efficacy vs effectiveness. Many of the studies that have informed us on the efficacy and tolerability of prazosin for nighttime PTSD symptoms described outcomes of prospective clinical research. Furthermore, these prospective trials were limited to < 6 months in duration. To date, neither clonidine nor prazosin has been evaluated for long-term efficacy and effectiveness in well-designed, prospective trials. This retrospective analysis may help provide a realistic estimate of the long-term effectiveness of these therapies, especially within the veteran population.
Limitations
This was a single-center, retrospective study conducted primarily in white male patients. Although likely applicable to the U.S. veteran population at large, these data may be poorly generalizable to patient populations outside the VA health care system.
Aside from external validity, this study has several significant limitations. The primary limitation of this project is that it was not designed to allow for statistical comparison of clonidine and prazosin. Such an analysis would have better defined the role of clonidine in PTSD treatment, either by establishing similar effectiveness of clonidine and prazosin for nighttime symptoms or by providing evidence of the superiority of one over the other. In designing the project, investigators suspected based on experience that the majority of patients prescribed clonidine would receive the drug after having already failed first-line therapy with prazosin. Had this been the case, a direct comparison may have been biased in favor of prazosin. In retrospect, however, only 24% of the clonidine group had previously been prescribed prazosin, and only 7% of the prazosin group had been prescribed clonidine. This suggests that clonidine may be used first line more often than the investigators anticipated and that a future direct comparison would be worthwhile.
Second, the subjective data collected for this project required investigators to read and interpret chart notes, although the review of all records by a single investigator helped limit variability in interpretation. At times, information in the CPRS was incomplete in terms of determining continuation of therapy or cause for discontinuation.
Third, although it is implied that a significant number of veterans have combat-related PTSD, the nature of the traumatic event(s) leading to PTSD was not recorded in this study, and no subgroup analysis was done to compare the effect of α2-adrenergic agents between combat- and noncombat-related PTSD. Owing to their exclusion by design, it is also difficult to apply these results to veterans who have lasting cognitive impairment as a result of TBI, who are presumably among those most likely to have experienced traumas that could provoke PTSD.
The design of this project also did not include a subgroup analysis based on antidepressant type, and it is unclear whether the potential pharmacodynamic interaction between noradrenergic antidepressants (ie, SNRIs) and anti– α2-adrenergic agents had any impact on clinical outcomes. The use of complementary nonpharmacologic treatment modalities (ie, psychotherapy, eye movement desensitization and reprocessing) was also not evaluated.
Related: Female Service Members in the Long War
Finally, the primary outcome of patient-reported improvement in symptoms does not provide information on the magnitude or specific nature of benefits derived. Given the retrospective nature, data used in prospectively designed studies (eg, rating scales pertinent to PTSD), which might have helped to quantify the benefit of treatment, was not consistently available. Even a baseline PCL-C score, collected in order to describe the patient population, was available only in 37% of the patients assessed. Furthermore, nighttime PTSD symptoms vary among individuals, but the primary outcome of this study pools any benefits seen in areas such as nightmares, awakenings, night sweats, or sleep quality into a single outcome of symptom improvement.
Conclusions
This study indicates that both clonidine and prazosin may be effective for the treatment of nighttime PTSD symptoms in the veteran population but that their long-term utility may be limited by waning effectiveness, tolerability, and adherence issues. At this time, it is unclear whether either agent has an advantage over the other in terms of effectiveness or tolerability; further studies are needed to address that question.
Despite its limitations, the authors anticipate that this study will provide information regarding the effectiveness and tolerability of clonidine and prazosin to treat nighttime PTSD symptoms. Findings from this study may help clinicians to anticipate the needs and challenges of patients using β2-adrenergic agents for nighttime symptoms of PTSD.
Acknowledgements
The authors wish to acknowledge Brian Wilcox, PharmD, for his assistance in generating patient data reports, and Ronald Brown, RPh, MS, for his guidance regarding data analysis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Posttraumatic stress disorder (PTSD) remains a significant health concern in veterans and military personnel. Whereas the lifetime incidence of PTSD in the U.S. general population is about 7% to 8%, the estimated prevalence of PTSD in deployed U.S. military personnel is higher than the national average, ranging from 11% to 17%.1,2 These numbers may be even higher, depending on the branch of service, responsibilities within the military, and specific conflict in which the veteran served. For example, one study found that 31% of Vietnam veterans have PTSD, and another recent study has reported PTSD in 28.7% of veterans returning from military service in Iraq and Afghanistan.3,4
Posttraumatic stress disorder treatment guidelines from both the American Psychiatric Association and the VA and DoD recommend the use of selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) as first-line pharmacotherapy for PTSD.5,6 However, SSRIs and SNRIs seem to be largely ineffective for the management of nighttime PTSD symptoms, such as insomnia and nightmares.7,8
Related: PTSD Increases Chance of Heart Failure
Researchers hypothesize that the sympathetic nervous system plays a significant role in the hyperarousal component of nighttime PTSD. The heightened responsiveness and disruption in restorative sleep seen in PTSD have been attributed to increased activity of norepinephrine in the central nervous system.9 Mechanistically, therapies that attenuate the increased noradrenergic signaling might be effective in the management of nighttime PTSD symptoms.
The body of evidence for the use of adrenergic agents for nighttime PTSD symptoms is growing. Prazosin, a peripherally acting α1-adrenergic receptor antagonist, has recently been demonstrated to be effective for nighttime PTSD symptoms in veterans in a series of small, randomized controlled trials.10-12 Data to support the use of clonidine, a centrally acting α2-adrenergic receptor agonist, are generally limited, with the most compelling data coming from a population of civilian Cambodian refugees.13,14 A 2007 article by Boehnlein and Kinzie includes a thorough review of the preclinical research, case reports, and early clinical studies that have led to the widespread use of these agents for PTSD despite the lack of FDA approval for this indication.13A previous retrospective review by Byers and colleagues compared the effectiveness and tolerability of prazosin and quetiapine for nighttime PTSD symptoms in veterans.15 The results of that review suggest that α1-adrenergic agents may be equally effective and better tolerated than alternative medication options (ie, atypical antipsychotics) for this purpose. The present study was adapted from this design to report concurrently on the real-world use of clonidine and prazosin for the treatment of nighttime PTSD symptoms.
Study Objectives
The primary objective of this retrospective chart review was to describe the experience of patients prescribed clonidine or prazosin for the treatment of nighttime PTSD symptoms, including initial effectiveness. The primary endpoint of initial drug effectiveness was documented improvement of nighttime PTSD symptoms in the patient’s chart within 6 months of the date of first prescription. Clonidine or prazosin was categorized as initially effective if a statement such as “frequency of nightmares decreased” or “patient’s nighttime PTSD symptoms have improved” was made within 6 months after initial prescription of the drug.
The secondary objectives of this study were to evaluate the long-term effectiveness and tolerability of prazosin. The endpoints used to assess these outcomes were the 2-year continuation rates of clonidine and prazosin (as a surrogate marker for long-term effectiveness) and the documented reasons for discontinuation of clonidine and prazosin for the treatment of nighttime PTSD symptoms (in order to assess tolerability).
Methods
An electronic database search was conducted to identify the VA Portland Health Care System (VAPHCS) patients with a diagnosis of PTSD who received a first prescription for clonidine or prazosin for nighttime PTSD symptoms from a VAPHCS mental health provider or primary care provider (PCP) from January 1, 2009, to December 31, 2011. Patients were excluded if they had any history of prior use of the drug being initiated, were co-initiated on both clonidine and prazosin (defined as starting the drugs within 30 days of each other), or had a concomitant diagnosis of schizophrenia, bipolar disorder, psychotic disorder, or cognitive disorder as defined in the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. Patients with traumatic brain injury (TBI) were excluded only if it could be determined that the event had resulted in lasting cognitive impairment.
Study Population
All patients with a diagnosis of PTSD who received a first prescription for clonidine during the period specified were screened for inclusion; patients with PTSD who were first prescribed prazosin during the same period were randomly sampled to equalize patient populations. This was done to maximize the data set while examining groups of roughly equal size for each drug, as prazosin is used much more commonly than clonidine for nighttime PTSD symptoms at VAPHCS. The patients in each resulting group were screened to determine whether they met inclusion and exclusion criteria. All subjects included were followed for 2 years from the date of the initial prescription.
Study Design
Initial effectiveness of each agent was determined by reviewing subjects’ progress notes after the initial prescription of clonidine or prazosin for documentation of improvement in symptoms within 6 months of the prescription start date. A decrease in frequency or intensity of nighttime PTSD symptoms, nightmares, or insomnia, as documented in the patient chart, was interpreted as improvement of symptoms.
Long-term continuation was assessed by reviewing subjects’ prescription records, to determine whether prescription(s) for clonidine or prazosin continued for 2 years after the date of the initial prescription.
Any gap between medication fills that resulted in an anticipated period without medication of ≥ 6 months (eg, 9 months after receiving a 90-day supply) was considered discontinuation of therapy. Prescription refill history was also reviewed, and medication possession ratio (MPR) was calculated to assess whether patients were adherent to the study drug as prescribed. Adherence was defined as an MPR of ≥ 80%. Patients who left the VAPHCS service area but continued to receive care at another VA were assessed for continuation of therapy, but refill data and/or MPR were not assessed.
Tolerability was assessed by reviewing subjects’ medical records to determine whether therapy with clonidine or prazosin was discontinued due to documented adverse effects (AEs). The occurrence of AEs was determined by reviewing progress notes and other chart documentation surrounding the date of discontinuation. If the drug was discontinued but the reason was not explicitly documented or if the prescription expired without a documented reason for nonrenewing, the reason for discontinuation was coded as “not specified.” Discontinuation due to treatment failure, change in symptoms, nonadherence, or other causes was also recorded. If multiple reasons for discontinuation were cited for a single patient, all were included in the data. This project was approved by the institutional review board at the VAPHCS.
Related:Depression and Substance Abuse Intensify Suicide Risk
Statistical Considerations
Based on clinical experience, it was presumed that many of the patients who were prescribed clonidine would be receiving it as a second-line therapy after failing prazosin. Therefore, statistical analysis of the relative effectiveness and tolerability of clonidine and prazosin could not be performed. Neither power nor sample size needed to demonstrate any difference in effectiveness or tolerability between the groups was calculated. All results are expressed using descriptive statistics.
Results
An initial database search for patients with PTSD who received a first prescription for clonidine between January 1, 2009, and December 31, 2011, from a VAPHCS provider yielded a list of 149 patients. The same search criteria applied for prazosin yielded 1,116 patients, 149 of whom were randomly selected for screening. After screening, 42 patients on clonidine and 60 patients on prazosin were included in this analysis (Figure).
Patient Demographics
The average age of the clonidine patients was 38.5 years (range 21-65 years) (Table 1). The clonidine group was primarily male (90%) and white (83%). Eighteen of the 42 patients in the clonidine group had a baseline PTSD Checklist-Civilian version (PCL-C) score available within the 90 days before the first prescription of clonidine; the average baseline PCL-C score in this subgroup was 62 ± 12.0 (median 65.5, range 31-82). Most of the clonidine patients (71%) had a concomitant diagnosis of a depressive disorder. About one-quarter of the group (24%) had previously tried prazosin per prescription records. In 24 patients (57%), the first prescription for clonidine was written by a psychiatrist or psychiatric nurse practitioner; 18 patients (43%) were started on clonidine by PCPs.
The average age of the prazosin patients was 46.1 years (range 21-74 years). The prazosin group was also primarily male (93%) and white (88%). Twenty of the 60 patients in the prazosin group had a baseline PCL-C score available within the 90 days before the first prescription of prazosin; the average baseline PCL-C score in this subgroup was 55 ± 16.1 (median 64, range 30-72). Most of the prazosin patients (63%) had a concomitant diagnosis of a depressive disorder. Four patients (7%) had previously tried clonidine per prescription records. In 35 patients (58%), the first prescription for prazosin was written by a psychiatrist or psychiatric nurse practitioner; 25 patients (42%) were started on prazosin by PCPs.
Data pertaining to initial and long-term effectiveness, tolerability, and MPR for both clonidine and prazosin are presented in Table 2.
Clonidine
Of the 42 clonidine patients assessed, 24 (57%) had a positive response to the medication for nighttime PTSD symptoms documented in the Computerized Patient Record System (CPRS) within 6 months of starting therapy. Six months after starting clonidine, 23 patients (55%) continued to take clonidine. Two years after starting therapy, 8 of the original 42 patients continued on clonidine for an overall 2-year continuation rate of 19%.
Tolerability
Of the 34 patients who discontinued clonidine within 2 years, 13 patients (38%) cited ineffectiveness of therapy as a reason for discontinuation. Another 13 patients (38%) reported discontinuing therapy due to AEs. Sedation (4 patients, 12%), dizziness/hypotension (3 patients, 9%), and paradoxical worsening of PTSD symptoms (4 patients, 12%) were the most common AEs leading to discontinuation. Other AEs cited as reasons for discontinuation were syncope (2 patients), erectile dysfunction (1 patient), rash (1 patient), myoclonus (1 patient), increased depression (1 patient), and fatigue (1 patient). One patient reported that he had discontinued clonidine due to symptom resolution/lack of need for treatment. In 8 of the 34 patients, no reason for discontinuation was found in chart documentation.
Medication Possession Ratio
Among the 21 evaluable patients who continued to receive clonidine 6 months after initiation, 10 (48%) were determined to be highly adherent to therapy, with an MPR of ≥ 80%. Six of the 21 patients (29%) had an MPR between 50% and 79%, and 5 patients (24%) had an MPR < 50%.
Of the 8 patients who continued on clonidine at the 2-year mark, 3 (38%) were adherent to therapy, with an MPR of ≥ 80%. Three more patients (38%) had a 2-year MPR between 50% and 80%, and 2 patients (25%) had an MPR < 50%.
Prazosin
Of the 60 prazosin patients assessed, 32 (53%) had a positive response to the medication for nighttime PTSD symptoms documented in the CPRS within 6 months of starting therapy. Six months after starting prazosin, 36 patients (60%) continued to take prazosin. Two years after starting therapy, 18 of the original 60 patients continued on prazosin for an overall 2-year continuation rate of 30%.
Tolerability
Of the 42 patients who discontinued prazosin within 2 years, six patients (14%) cited ineffectiveness of therapy as a reason for discontinuation. Thirteen patients (31%) reported discontinuing therapy due to AEs. Sedation (3 patients, 7%), dizziness/hypotension (3 patients, 7%), and paradoxical worsening of PTSD symptoms (6 patients, 14%) were the most common AEs leading to discontinuation. Other AEs cited as reasons for discontinuation were headache (2 patients), altered mental status (1 patient), and fatigue (1 patient). Three patients reported that they had discontinued clonidine due to symptom resolution/lack of need for treatment. Other reasons for discontinuation not related to AEs included flight rules (1 patient), changes to antihypertensive regimen (1 patient), refill issues (1 patient), and cost (1 patient). In 15 of the 42 patients, no reason for discontinuation was found in chart documentation.
Medication Possession Ratio
Among the 31 evaluable patients who continued to receive prazosin 6 months after initiation, 20 (65%) were determined to be highly adherent to therapy, with an MPR of ≥ 80%. Five of the 31 patients (16%) had an MPR between 50% and 80%, and 6 patients (19%) had an MPR < 50%.
Of the 15 evaluable patients who continued on prazosin at the 2-year mark, 9 (60%) were adherent to therapy, with an MPR of ≥ 80%. Three patients (20%) had a 2-year MPR between 50% and 80%, and 3 patients (20%) had an MPR < 50%.
Discussion
Although prazosin has been shown to be effective for nighttime PTSD symptoms in both prospective and retrospective evaluations in veterans, this study provides the first evidence to support the use of clonidine in a veteran population.10-12,15
Interestingly, 42% of the patients assessed received their first prescription of an α2-adrenergic agent for nighttime PTSD symptoms from a PCP. Even with the recent increased focus on integrating mental health into primary care within the VA, this was a surprising finding. Primary care providers at VAPHCS may have a greater role in the outpatient management of PTSD than previously suspected. The information presented here may prove useful and applicable in both psychiatric and primary care treatment settings.
The study results indicated that a majority of subjects initially reported effectiveness with either clonidine or prazosin (53% and 57%, respectively). The initial effectiveness rate for prazosin is similar to those described in previous studies.10-13,15 The data also support a viable role for clonidine in the treatment of nighttime PTSD symptoms.
Regardless of initial improvement, the study results also suggest that the therapeutic benefit may not persist in the long term, as evidenced by a significant percentage of discontinuations attributed to ineffectiveness (38% for clonidine and 14% for prazosin) and a very low rate of long-term continuation (19% for clonidine and 30% for prazosin at 2 years). This latter observation contrasts with findings from previous studies; Byers and colleagues reported a 2-year prazosin continuation rate of 48.4% in a similar analysis, and Boehnlein and colleagues reported a sustained benefit of clonidine in responders over a 10-year period.14,15 The wide variety of reasons for discontinuation reported here may help providers who are considering clonidine or prazosin for their patients to anticipate barriers to long-term success.
Part of the discrepancy between these results and previously reported successes with clonidine and prazosin may be attributable to the classic issue of efficacy vs effectiveness. Many of the studies that have informed us on the efficacy and tolerability of prazosin for nighttime PTSD symptoms described outcomes of prospective clinical research. Furthermore, these prospective trials were limited to < 6 months in duration. To date, neither clonidine nor prazosin has been evaluated for long-term efficacy and effectiveness in well-designed, prospective trials. This retrospective analysis may help provide a realistic estimate of the long-term effectiveness of these therapies, especially within the veteran population.
Limitations
This was a single-center, retrospective study conducted primarily in white male patients. Although likely applicable to the U.S. veteran population at large, these data may be poorly generalizable to patient populations outside the VA health care system.
Aside from external validity, this study has several significant limitations. The primary limitation of this project is that it was not designed to allow for statistical comparison of clonidine and prazosin. Such an analysis would have better defined the role of clonidine in PTSD treatment, either by establishing similar effectiveness of clonidine and prazosin for nighttime symptoms or by providing evidence of the superiority of one over the other. In designing the project, investigators suspected based on experience that the majority of patients prescribed clonidine would receive the drug after having already failed first-line therapy with prazosin. Had this been the case, a direct comparison may have been biased in favor of prazosin. In retrospect, however, only 24% of the clonidine group had previously been prescribed prazosin, and only 7% of the prazosin group had been prescribed clonidine. This suggests that clonidine may be used first line more often than the investigators anticipated and that a future direct comparison would be worthwhile.
Second, the subjective data collected for this project required investigators to read and interpret chart notes, although the review of all records by a single investigator helped limit variability in interpretation. At times, information in the CPRS was incomplete in terms of determining continuation of therapy or cause for discontinuation.
Third, although it is implied that a significant number of veterans have combat-related PTSD, the nature of the traumatic event(s) leading to PTSD was not recorded in this study, and no subgroup analysis was done to compare the effect of α2-adrenergic agents between combat- and noncombat-related PTSD. Owing to their exclusion by design, it is also difficult to apply these results to veterans who have lasting cognitive impairment as a result of TBI, who are presumably among those most likely to have experienced traumas that could provoke PTSD.
The design of this project also did not include a subgroup analysis based on antidepressant type, and it is unclear whether the potential pharmacodynamic interaction between noradrenergic antidepressants (ie, SNRIs) and anti– α2-adrenergic agents had any impact on clinical outcomes. The use of complementary nonpharmacologic treatment modalities (ie, psychotherapy, eye movement desensitization and reprocessing) was also not evaluated.
Related: Female Service Members in the Long War
Finally, the primary outcome of patient-reported improvement in symptoms does not provide information on the magnitude or specific nature of benefits derived. Given the retrospective nature, data used in prospectively designed studies (eg, rating scales pertinent to PTSD), which might have helped to quantify the benefit of treatment, was not consistently available. Even a baseline PCL-C score, collected in order to describe the patient population, was available only in 37% of the patients assessed. Furthermore, nighttime PTSD symptoms vary among individuals, but the primary outcome of this study pools any benefits seen in areas such as nightmares, awakenings, night sweats, or sleep quality into a single outcome of symptom improvement.
Conclusions
This study indicates that both clonidine and prazosin may be effective for the treatment of nighttime PTSD symptoms in the veteran population but that their long-term utility may be limited by waning effectiveness, tolerability, and adherence issues. At this time, it is unclear whether either agent has an advantage over the other in terms of effectiveness or tolerability; further studies are needed to address that question.
Despite its limitations, the authors anticipate that this study will provide information regarding the effectiveness and tolerability of clonidine and prazosin to treat nighttime PTSD symptoms. Findings from this study may help clinicians to anticipate the needs and challenges of patients using β2-adrenergic agents for nighttime symptoms of PTSD.
Acknowledgements
The authors wish to acknowledge Brian Wilcox, PharmD, for his assistance in generating patient data reports, and Ronald Brown, RPh, MS, for his guidance regarding data analysis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental problems, and barriers to care. N Engl J Med. 2004;351(1):13-22.
2. Gates MA, Holowka DW, Vasterling JJ, Keane TM, Marx BP, Rosen RC. Posttraumatic stress disorder in veterans and military personnel: epidemiology, screening, and case recognition. Psychol Serv. 2012;9(4):361-382.
3. Kulka R, Schlenger WE, Fairbanks J, et al. Trauma and the Vietnam War Generation: Report of Findings From the National Vietnam Veterans Readjustment Study. New York, NY: Brunnel/Mazel; 1990.
4. Barrera TL, Graham DP, Dunn NJ, Teng EJ. Influence of trauma history on panic and posttraumatic stress disorder in returning veterans. Psychol Serv. 2013;10(2):168-176.
5. American Psychiatric Association. Practice Guideline for the Treatment of Patients With Acute Stress Disorder and Posttraumatic Stress Disorder. Arlington, VA: American Psychiatric Association; 2004.
6. U.S. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for management of post-traumatic stress. Version 2.0. U.S. Department of Veterans Affairs Website. http://www.healthquality.va.gov/guidelines/MH/ptsd/cpgPTSDFULL201011612c.pdf. Published October 2010. Accessed October 5, 2015.
7. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):169-180.
8. Ravindran LN, Stein MB. Pharmacotherapy of post-traumatic stress disorder. In: Stein MB, Steckler T, eds. Behavioral Neurobiology of Anxiety and Its Treatment. Vol 2. Heidelberg, Germany: Springer; 2010:505-525.
9. Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12(3):169-184.
10. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo controlled study. Am J Psychiatry. 2003;160(2):371-373.
11. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
12. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170:1003-1010.
13. Boehnlein JK, Kinzie JD. Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. J Psychiatr Pract. 2007;13(2):72-78.
14. Boehnlein JK, Kinzie JD, Sekiya U, Riley C, Pou K, Rosborough B. A ten-year treatment outcome study of traumatized Cambodian refugees. J Nerve Ment Dis. 2004;192(10):658-663.
15. Byers MG, Allison KM, Wendel CS, Lee JK. Prazosin versus quetiapine for nighttime posttraumatic stress disorder symptoms in veterans: an assessment of long-term comparative effectiveness and safety. J Clin Psychopharmacol. 2010;30(3):225-229.
1. Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental problems, and barriers to care. N Engl J Med. 2004;351(1):13-22.
2. Gates MA, Holowka DW, Vasterling JJ, Keane TM, Marx BP, Rosen RC. Posttraumatic stress disorder in veterans and military personnel: epidemiology, screening, and case recognition. Psychol Serv. 2012;9(4):361-382.
3. Kulka R, Schlenger WE, Fairbanks J, et al. Trauma and the Vietnam War Generation: Report of Findings From the National Vietnam Veterans Readjustment Study. New York, NY: Brunnel/Mazel; 1990.
4. Barrera TL, Graham DP, Dunn NJ, Teng EJ. Influence of trauma history on panic and posttraumatic stress disorder in returning veterans. Psychol Serv. 2013;10(2):168-176.
5. American Psychiatric Association. Practice Guideline for the Treatment of Patients With Acute Stress Disorder and Posttraumatic Stress Disorder. Arlington, VA: American Psychiatric Association; 2004.
6. U.S. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for management of post-traumatic stress. Version 2.0. U.S. Department of Veterans Affairs Website. http://www.healthquality.va.gov/guidelines/MH/ptsd/cpgPTSDFULL201011612c.pdf. Published October 2010. Accessed October 5, 2015.
7. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):169-180.
8. Ravindran LN, Stein MB. Pharmacotherapy of post-traumatic stress disorder. In: Stein MB, Steckler T, eds. Behavioral Neurobiology of Anxiety and Its Treatment. Vol 2. Heidelberg, Germany: Springer; 2010:505-525.
9. Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12(3):169-184.
10. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo controlled study. Am J Psychiatry. 2003;160(2):371-373.
11. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
12. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170:1003-1010.
13. Boehnlein JK, Kinzie JD. Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. J Psychiatr Pract. 2007;13(2):72-78.
14. Boehnlein JK, Kinzie JD, Sekiya U, Riley C, Pou K, Rosborough B. A ten-year treatment outcome study of traumatized Cambodian refugees. J Nerve Ment Dis. 2004;192(10):658-663.
15. Byers MG, Allison KM, Wendel CS, Lee JK. Prazosin versus quetiapine for nighttime posttraumatic stress disorder symptoms in veterans: an assessment of long-term comparative effectiveness and safety. J Clin Psychopharmacol. 2010;30(3):225-229.