User login
52-year-old man • syncopal episode • chest pain • mild lightheadedness • Dx?
THE CASE
A 52-year-old man with a history of hypertension and gastroesophageal reflux disease (GERD) presented to the emergency department (ED) after an episode of syncope. He reported that the syncope occurred soon after he stood up to go to the kitchen to make dinner but was without prodrome or associated symptoms. He recalled little of the event, and the episode was unwitnessed. He had a few bruises on his arms but no significant injuries.
On questioning, he reported occasional palpitations but no changes in his normal exercise tolerance. His only medication was lisinopril 10 mg/d.
In the ED, his vital signs, physical exam (including orthostatic vital signs), basic labs (including troponin I), and a 12-lead EKG were normal. After a cardiology consultation, he was discharged home with a 30-day ambulatory rhythm monitor.
A few days later, while walking up and down some hills, he experienced about 15 seconds of chest pain accompanied by mild lightheadedness. Thinking it might be related to his GERD, he took some over-the-counter antacids when he returned home, since these had been effective for him in the past.
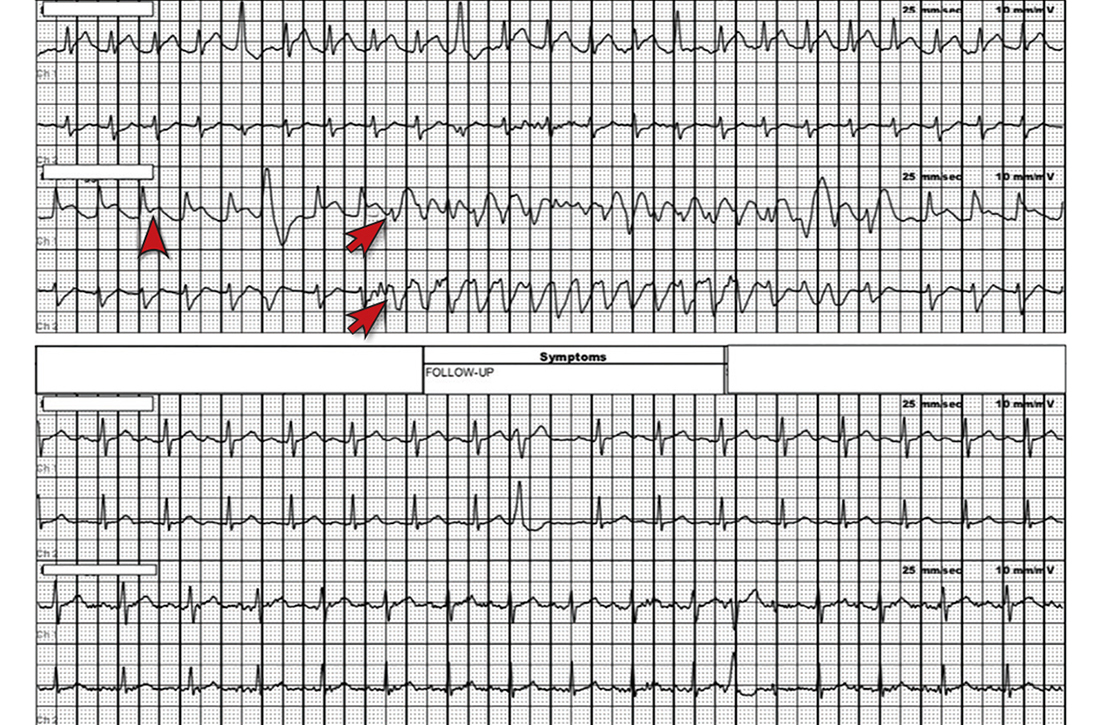
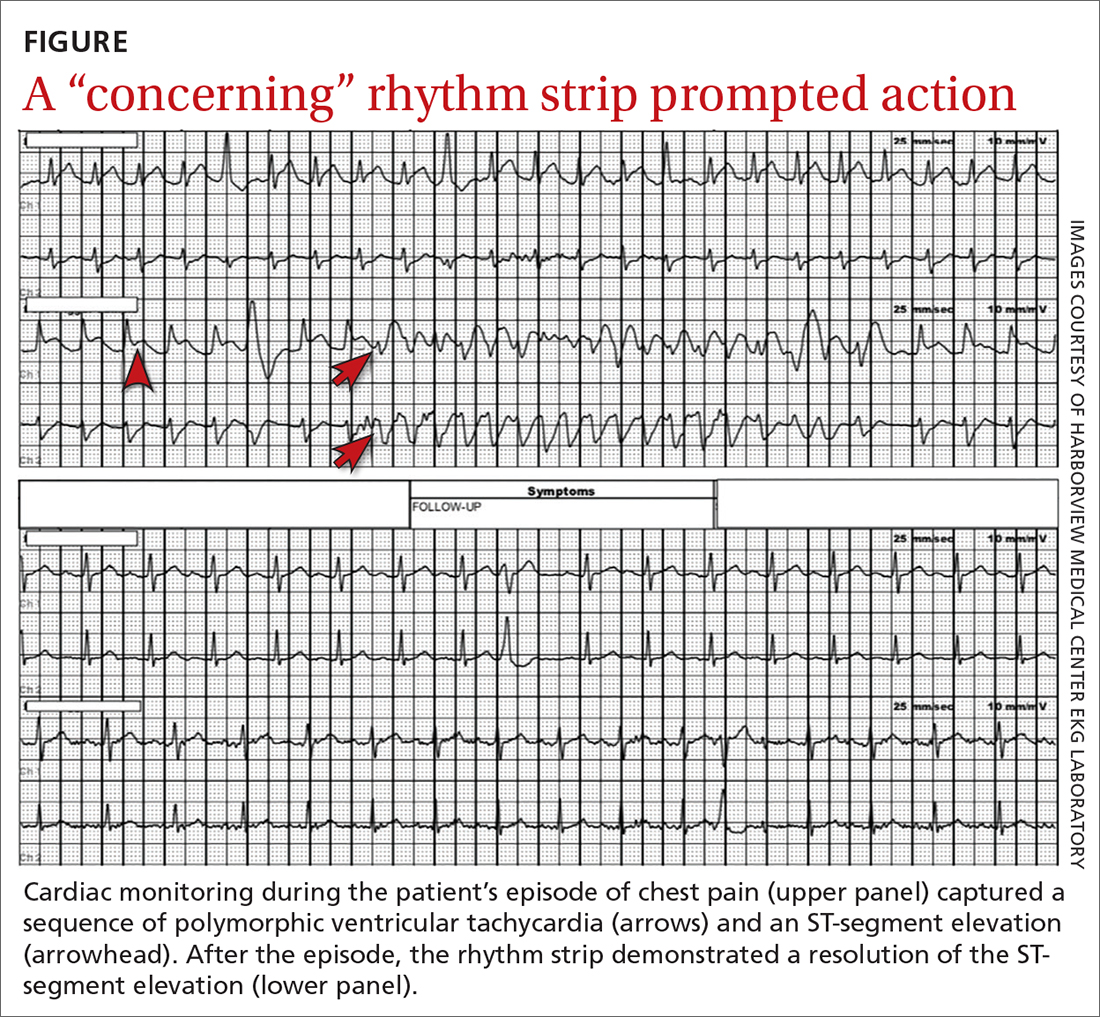
However, the rhythm monitoring company contacted the EKG lab to transmit a concerning strip (FIGURE). They also reported that the patient had been contacted and reported no further symptoms.
THE DIAGNOSIS
Most notable on the patient’s rhythm strip was a continuously varying QRS complex, which was indicative of polymorphic ventricular tachycardia and consistent with the patient’s syncope and other symptoms. Less obvious at first glance was an ST-segment elevation in the preceding beats. Comparison to a post-episode tracing (FIGURE) highlights the abnormality. Polymorphic ventricular tachycardia resolves in 1 of 2 ways: It will either stop on its own (causing syncope if it lasts more than a few seconds) or it will devolve into ventricular fibrillation, causing cardiac arrest.1
The combination of these findings and the clinical scenario prompted a recommendation that the patient report to the ED for admission (his wife drove him). He was admitted to the intensive care unit (ICU) for continuous telemetry monitoring, and a cardiac catheterization was ordered. The procedure revealed a 99% thrombotic mid-right coronary artery lesion, for which aspiration thrombectomy and uncomplicated stenting were performed.
Continue to: DISCUSSION
DISCUSSION
Guidelines from the American College of Cardiology/American Heart Association/Heart Rhythm Society recommend a detailed history and physical exam, as well as an EKG, for the initial evaluation of syncope.2 If this does not point to a diagnosis (and depending on the presentation and other factors), an ambulatory rhythm monitor can be considered. Other possible testing modalities include stress testing, resting transthoracic echocardiography, electrophysiologic testing, and cardiac magnetic resonance imaging or computed tomography.
Is the cause cardiac? The guidelines suggest that a cardiac cause of syncope is more likely if several of the following factors are present: age > 60 years; male sex; presence of known heart disease (acquired or congenital); brief prodrome (eg, palpitations) or no prodrome; exertional or supine syncope; 1 to 2 episodes; an abnormal cardiac exam; and a family history of premature sudden death.2 A noncardiac cause is suggested by other factors: younger age; no known cardiac disease; standing or a position change from supine to sitting/standing; prodrome; specific triggers (eg, dehydration, pain); and frequent and prolonged stereotypic episodes.2
While the guidelines do not specify the number of factors or endorse a specific scoring system, such tools have been developed. For example, the EGSYS (Evaluation of Guidelines in Syncope Study) Score assigns 1 point for each of 6 factors: palpitations; heart disease and/or abnormal EKG; effort syncope; supine syncope; precipitating or predisposing factors; and autonomic prodromes. A score ≥ 3 identified cardiac syncope with a sensitivity of 95%, but with a specificity of only 61%. In the derivation study, patients with a score ≥ 3 had higher mortality than those with a lower score (17 vs 3%; P < .001).3
Myocardial ischemia can trigger ventricular arrhythmias. In the GUSTO-1 trial of fibrinolytic therapy in patients with acute ST-segment elevation myocardial infarction (n = 40,895), the incidence of ventricular tachycardia or ventricular fibrillation was 10.2%.4 In a pooled analysis (4 trials; n = 26,416) of patients who were treated for non–ST-segment elevation or unstable angina-type acute coronary syndromes, the rate of these arrhythmias was markedly lower (2.1%).5 The risk of ventricular arrhythmia is one reason close monitoring (eg, continuous telemetry, ICU admission) is the standard of care for patients with acute coronary syndromes.
Our patient experienced syncope upon standing, which suggested a noncardiac cause (usually orthostatic hypotension). However, the history of palpitations increased the suspicion for a cardiac cause, and thus the rhythm monitor was ordered.
THE TAKEAWAY
This case was unusual in that ambulatory monitoring captured electrocardiographic evidence of myocardial ischemia leading directly to a ventricular arrhythmia. In the evaluation of syncope, a detailed history, physical exam, and a baseline 12-lead EKG can sometimes give clues to an arrhythmic cause of syncope (eg, Brugada syndrome, prior infarct pattern, prolonged QTc, bradycardia, heart block, arrhythmogenic right ventricular cardiomyopathy)—but prolonged rhythm monitoring is sometimes needed to identify a cause.
Michael A. Chen, MD, PhD, Harborview Medical Center, University of Washington School of Medicine, 325 9th Avenue, Box 359748 (Cardiology), Seattle, WA 98104; [email protected]
1. Viskin S, Chorin E, Viskin D, et al. Polymorphic ventricular tachycardia: terminology, mechanism, diagnosis, and emergency therapy. Circulation. 2021;144:823-839. doi: 10.1161/CIRCULATIONAHA.121.055783
2. Shen W-K, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70:620-663. doi: 10.1016/j.jacc.2017.03.002
3. Del Rosso A, Ungar A, Maggi R, et al. Clinical predictors of cardiac syncope at initial evaluation in patients referred urgently to a general hospital: the EGSYS score. Heart. 2008;94:1528-1529. doi: 10.1136/hrt.2008.143123
4. Newby KH, Thompson T, Stebbins A, et al. Sustained ventricular arrhythmias in patients receiving thrombolytic therapy: incidence and outcomes. The GUSTO Investigators. Circulation. 1998;98:2567-2573. doi: 10.1161/01.cir.98.23.2567
5. Al-Khatib SM, Granger CB, Huang Y, et al. Sustained ventricular arrhythmias among patients with acute coronary syndromes with no ST-segment elevation: incidence, predictors, and outcomes. Circulation. 2002;106:309-12. doi: 10.1161/01.cir.0000022692.49934.e3
THE CASE
A 52-year-old man with a history of hypertension and gastroesophageal reflux disease (GERD) presented to the emergency department (ED) after an episode of syncope. He reported that the syncope occurred soon after he stood up to go to the kitchen to make dinner but was without prodrome or associated symptoms. He recalled little of the event, and the episode was unwitnessed. He had a few bruises on his arms but no significant injuries.
On questioning, he reported occasional palpitations but no changes in his normal exercise tolerance. His only medication was lisinopril 10 mg/d.
In the ED, his vital signs, physical exam (including orthostatic vital signs), basic labs (including troponin I), and a 12-lead EKG were normal. After a cardiology consultation, he was discharged home with a 30-day ambulatory rhythm monitor.
A few days later, while walking up and down some hills, he experienced about 15 seconds of chest pain accompanied by mild lightheadedness. Thinking it might be related to his GERD, he took some over-the-counter antacids when he returned home, since these had been effective for him in the past.
However, the rhythm monitoring company contacted the EKG lab to transmit a concerning strip (FIGURE). They also reported that the patient had been contacted and reported no further symptoms.

THE DIAGNOSIS
Most notable on the patient’s rhythm strip was a continuously varying QRS complex, which was indicative of polymorphic ventricular tachycardia and consistent with the patient’s syncope and other symptoms. Less obvious at first glance was an ST-segment elevation in the preceding beats. Comparison to a post-episode tracing (FIGURE) highlights the abnormality. Polymorphic ventricular tachycardia resolves in 1 of 2 ways: It will either stop on its own (causing syncope if it lasts more than a few seconds) or it will devolve into ventricular fibrillation, causing cardiac arrest.1
The combination of these findings and the clinical scenario prompted a recommendation that the patient report to the ED for admission (his wife drove him). He was admitted to the intensive care unit (ICU) for continuous telemetry monitoring, and a cardiac catheterization was ordered. The procedure revealed a 99% thrombotic mid-right coronary artery lesion, for which aspiration thrombectomy and uncomplicated stenting were performed.
Continue to: DISCUSSION
DISCUSSION
Guidelines from the American College of Cardiology/American Heart Association/Heart Rhythm Society recommend a detailed history and physical exam, as well as an EKG, for the initial evaluation of syncope.2 If this does not point to a diagnosis (and depending on the presentation and other factors), an ambulatory rhythm monitor can be considered. Other possible testing modalities include stress testing, resting transthoracic echocardiography, electrophysiologic testing, and cardiac magnetic resonance imaging or computed tomography.
Is the cause cardiac? The guidelines suggest that a cardiac cause of syncope is more likely if several of the following factors are present: age > 60 years; male sex; presence of known heart disease (acquired or congenital); brief prodrome (eg, palpitations) or no prodrome; exertional or supine syncope; 1 to 2 episodes; an abnormal cardiac exam; and a family history of premature sudden death.2 A noncardiac cause is suggested by other factors: younger age; no known cardiac disease; standing or a position change from supine to sitting/standing; prodrome; specific triggers (eg, dehydration, pain); and frequent and prolonged stereotypic episodes.2
While the guidelines do not specify the number of factors or endorse a specific scoring system, such tools have been developed. For example, the EGSYS (Evaluation of Guidelines in Syncope Study) Score assigns 1 point for each of 6 factors: palpitations; heart disease and/or abnormal EKG; effort syncope; supine syncope; precipitating or predisposing factors; and autonomic prodromes. A score ≥ 3 identified cardiac syncope with a sensitivity of 95%, but with a specificity of only 61%. In the derivation study, patients with a score ≥ 3 had higher mortality than those with a lower score (17 vs 3%; P < .001).3
Myocardial ischemia can trigger ventricular arrhythmias. In the GUSTO-1 trial of fibrinolytic therapy in patients with acute ST-segment elevation myocardial infarction (n = 40,895), the incidence of ventricular tachycardia or ventricular fibrillation was 10.2%.4 In a pooled analysis (4 trials; n = 26,416) of patients who were treated for non–ST-segment elevation or unstable angina-type acute coronary syndromes, the rate of these arrhythmias was markedly lower (2.1%).5 The risk of ventricular arrhythmia is one reason close monitoring (eg, continuous telemetry, ICU admission) is the standard of care for patients with acute coronary syndromes.
Our patient experienced syncope upon standing, which suggested a noncardiac cause (usually orthostatic hypotension). However, the history of palpitations increased the suspicion for a cardiac cause, and thus the rhythm monitor was ordered.
THE TAKEAWAY
This case was unusual in that ambulatory monitoring captured electrocardiographic evidence of myocardial ischemia leading directly to a ventricular arrhythmia. In the evaluation of syncope, a detailed history, physical exam, and a baseline 12-lead EKG can sometimes give clues to an arrhythmic cause of syncope (eg, Brugada syndrome, prior infarct pattern, prolonged QTc, bradycardia, heart block, arrhythmogenic right ventricular cardiomyopathy)—but prolonged rhythm monitoring is sometimes needed to identify a cause.
Michael A. Chen, MD, PhD, Harborview Medical Center, University of Washington School of Medicine, 325 9th Avenue, Box 359748 (Cardiology), Seattle, WA 98104; [email protected]
THE CASE
A 52-year-old man with a history of hypertension and gastroesophageal reflux disease (GERD) presented to the emergency department (ED) after an episode of syncope. He reported that the syncope occurred soon after he stood up to go to the kitchen to make dinner but was without prodrome or associated symptoms. He recalled little of the event, and the episode was unwitnessed. He had a few bruises on his arms but no significant injuries.
On questioning, he reported occasional palpitations but no changes in his normal exercise tolerance. His only medication was lisinopril 10 mg/d.
In the ED, his vital signs, physical exam (including orthostatic vital signs), basic labs (including troponin I), and a 12-lead EKG were normal. After a cardiology consultation, he was discharged home with a 30-day ambulatory rhythm monitor.
A few days later, while walking up and down some hills, he experienced about 15 seconds of chest pain accompanied by mild lightheadedness. Thinking it might be related to his GERD, he took some over-the-counter antacids when he returned home, since these had been effective for him in the past.
However, the rhythm monitoring company contacted the EKG lab to transmit a concerning strip (FIGURE). They also reported that the patient had been contacted and reported no further symptoms.

THE DIAGNOSIS
Most notable on the patient’s rhythm strip was a continuously varying QRS complex, which was indicative of polymorphic ventricular tachycardia and consistent with the patient’s syncope and other symptoms. Less obvious at first glance was an ST-segment elevation in the preceding beats. Comparison to a post-episode tracing (FIGURE) highlights the abnormality. Polymorphic ventricular tachycardia resolves in 1 of 2 ways: It will either stop on its own (causing syncope if it lasts more than a few seconds) or it will devolve into ventricular fibrillation, causing cardiac arrest.1
The combination of these findings and the clinical scenario prompted a recommendation that the patient report to the ED for admission (his wife drove him). He was admitted to the intensive care unit (ICU) for continuous telemetry monitoring, and a cardiac catheterization was ordered. The procedure revealed a 99% thrombotic mid-right coronary artery lesion, for which aspiration thrombectomy and uncomplicated stenting were performed.
Continue to: DISCUSSION
DISCUSSION
Guidelines from the American College of Cardiology/American Heart Association/Heart Rhythm Society recommend a detailed history and physical exam, as well as an EKG, for the initial evaluation of syncope.2 If this does not point to a diagnosis (and depending on the presentation and other factors), an ambulatory rhythm monitor can be considered. Other possible testing modalities include stress testing, resting transthoracic echocardiography, electrophysiologic testing, and cardiac magnetic resonance imaging or computed tomography.
Is the cause cardiac? The guidelines suggest that a cardiac cause of syncope is more likely if several of the following factors are present: age > 60 years; male sex; presence of known heart disease (acquired or congenital); brief prodrome (eg, palpitations) or no prodrome; exertional or supine syncope; 1 to 2 episodes; an abnormal cardiac exam; and a family history of premature sudden death.2 A noncardiac cause is suggested by other factors: younger age; no known cardiac disease; standing or a position change from supine to sitting/standing; prodrome; specific triggers (eg, dehydration, pain); and frequent and prolonged stereotypic episodes.2
While the guidelines do not specify the number of factors or endorse a specific scoring system, such tools have been developed. For example, the EGSYS (Evaluation of Guidelines in Syncope Study) Score assigns 1 point for each of 6 factors: palpitations; heart disease and/or abnormal EKG; effort syncope; supine syncope; precipitating or predisposing factors; and autonomic prodromes. A score ≥ 3 identified cardiac syncope with a sensitivity of 95%, but with a specificity of only 61%. In the derivation study, patients with a score ≥ 3 had higher mortality than those with a lower score (17 vs 3%; P < .001).3
Myocardial ischemia can trigger ventricular arrhythmias. In the GUSTO-1 trial of fibrinolytic therapy in patients with acute ST-segment elevation myocardial infarction (n = 40,895), the incidence of ventricular tachycardia or ventricular fibrillation was 10.2%.4 In a pooled analysis (4 trials; n = 26,416) of patients who were treated for non–ST-segment elevation or unstable angina-type acute coronary syndromes, the rate of these arrhythmias was markedly lower (2.1%).5 The risk of ventricular arrhythmia is one reason close monitoring (eg, continuous telemetry, ICU admission) is the standard of care for patients with acute coronary syndromes.
Our patient experienced syncope upon standing, which suggested a noncardiac cause (usually orthostatic hypotension). However, the history of palpitations increased the suspicion for a cardiac cause, and thus the rhythm monitor was ordered.
THE TAKEAWAY
This case was unusual in that ambulatory monitoring captured electrocardiographic evidence of myocardial ischemia leading directly to a ventricular arrhythmia. In the evaluation of syncope, a detailed history, physical exam, and a baseline 12-lead EKG can sometimes give clues to an arrhythmic cause of syncope (eg, Brugada syndrome, prior infarct pattern, prolonged QTc, bradycardia, heart block, arrhythmogenic right ventricular cardiomyopathy)—but prolonged rhythm monitoring is sometimes needed to identify a cause.
Michael A. Chen, MD, PhD, Harborview Medical Center, University of Washington School of Medicine, 325 9th Avenue, Box 359748 (Cardiology), Seattle, WA 98104; [email protected]
1. Viskin S, Chorin E, Viskin D, et al. Polymorphic ventricular tachycardia: terminology, mechanism, diagnosis, and emergency therapy. Circulation. 2021;144:823-839. doi: 10.1161/CIRCULATIONAHA.121.055783
2. Shen W-K, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70:620-663. doi: 10.1016/j.jacc.2017.03.002
3. Del Rosso A, Ungar A, Maggi R, et al. Clinical predictors of cardiac syncope at initial evaluation in patients referred urgently to a general hospital: the EGSYS score. Heart. 2008;94:1528-1529. doi: 10.1136/hrt.2008.143123
4. Newby KH, Thompson T, Stebbins A, et al. Sustained ventricular arrhythmias in patients receiving thrombolytic therapy: incidence and outcomes. The GUSTO Investigators. Circulation. 1998;98:2567-2573. doi: 10.1161/01.cir.98.23.2567
5. Al-Khatib SM, Granger CB, Huang Y, et al. Sustained ventricular arrhythmias among patients with acute coronary syndromes with no ST-segment elevation: incidence, predictors, and outcomes. Circulation. 2002;106:309-12. doi: 10.1161/01.cir.0000022692.49934.e3
1. Viskin S, Chorin E, Viskin D, et al. Polymorphic ventricular tachycardia: terminology, mechanism, diagnosis, and emergency therapy. Circulation. 2021;144:823-839. doi: 10.1161/CIRCULATIONAHA.121.055783
2. Shen W-K, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70:620-663. doi: 10.1016/j.jacc.2017.03.002
3. Del Rosso A, Ungar A, Maggi R, et al. Clinical predictors of cardiac syncope at initial evaluation in patients referred urgently to a general hospital: the EGSYS score. Heart. 2008;94:1528-1529. doi: 10.1136/hrt.2008.143123
4. Newby KH, Thompson T, Stebbins A, et al. Sustained ventricular arrhythmias in patients receiving thrombolytic therapy: incidence and outcomes. The GUSTO Investigators. Circulation. 1998;98:2567-2573. doi: 10.1161/01.cir.98.23.2567
5. Al-Khatib SM, Granger CB, Huang Y, et al. Sustained ventricular arrhythmias among patients with acute coronary syndromes with no ST-segment elevation: incidence, predictors, and outcomes. Circulation. 2002;106:309-12. doi: 10.1161/01.cir.0000022692.49934.e3
A “Routine” Electrocardiogram (ECG)
Patient Presentation
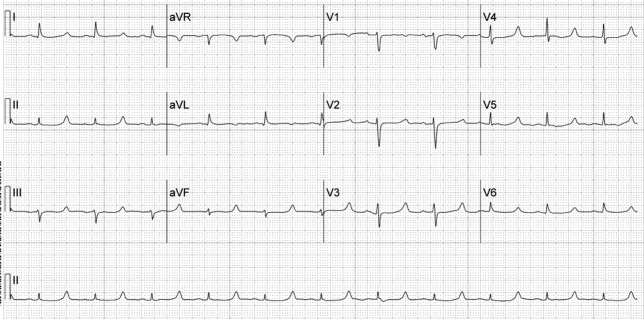
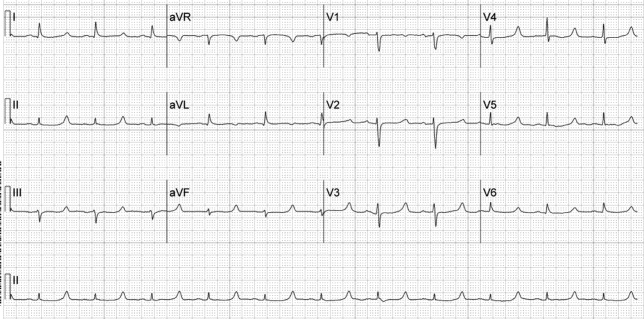
A 52‐year‐old woman with cirrhosis presented with a large‐volume upper gastrointestinal (GI) bleed. After receiving massive volume and blood product resuscitation (18 U packed red blood cells [PRBCs], 17 U fresh frozen plasma [FFP], 2 U cryoprecipitate, 1 U platelets, and 14 L normal saline), a routine admission electrocardiogram (ECG) was obtained (Figure 1).
Discussion
The ECG shows normal sinus rhythm with a prolonged QT interval (specifically due to a long ST segment). The differential diagnosis of this pattern of QT prolongation includes hypocalcemia, long QT syndrome (variant 3), and hypothermia. Massive transfusion can result in the chelation of calcium by citrate resulting in hypocalcemia. This patient's serum calcium was 7 mg/dL (8.9‐10.2) (ionized 0.21 mmol/L; 1.18‐1.38), her pH was 7.03. There were no overt signs or symptoms of hypocalcemia (paresthesias, twitching, tetany, or seizures.) and with aggressive replacement the calcium and the ECG normalized. Transfusions are an uncommon cause of hypocalcemia, with hypomagnesemia or hypermagnesemia, acute pancreatitis, rhabdomyolysis, tumor lysis syndrome, renal failure, vitamin D deficiency, pseudoparathyroidism and hypoparathyroidism being the more common disorders caused.1, 2 This patient's magnesium was normal on admission (2.1 mg/dL) and she did not have any of the other conditions mentioned. It is possible that hemodilution may also have played a role.
Genetic long QT syndrome (variant 3) can manifest with a similar ECG pattern, although the T‐wave is sometimes peaked or biphasic in that condition. Our patient had no personal or family history of syncope or sudden death, and her ECG normalized with calcium replacement.3
Hypothermia can also present with a long QT interval secondary to a long ST segment. Our patient was normothermic, and had no other ECG findings suggestive of hypothermia (Osborn waves: notching of terminal portion of QRS), shivering artifacts, sinus bradycardia, atrial fibrillation, QRS prolongation, or prolongation of the PR interval.4
The differential diagnosis of a long QT interval with a long ST segment is short, and the clinical scenario will provide the etiology in most cases.
- .Hypercalcemia and hypocalcemia. In:Fauci AS,Braunwald E,Kasper DL, et al., eds.Harrison's Principles of Internal Medicine. Available at: http://www.accessmedicine.com/content.aspx?aID=2864354.
- ,,.Massive transfusion.Crit Care Clin.1986;2:791–805.
- ,,,.Novel insights in the congenital long QT syndrome.Ann Intern Med.2002;137:981–992.
- ,,.The clinical value of the ECG in noncardiac conditions.Chest.2004;125:1561–1576.
Patient Presentation
A 52‐year‐old woman with cirrhosis presented with a large‐volume upper gastrointestinal (GI) bleed. After receiving massive volume and blood product resuscitation (18 U packed red blood cells [PRBCs], 17 U fresh frozen plasma [FFP], 2 U cryoprecipitate, 1 U platelets, and 14 L normal saline), a routine admission electrocardiogram (ECG) was obtained (Figure 1).

Discussion
The ECG shows normal sinus rhythm with a prolonged QT interval (specifically due to a long ST segment). The differential diagnosis of this pattern of QT prolongation includes hypocalcemia, long QT syndrome (variant 3), and hypothermia. Massive transfusion can result in the chelation of calcium by citrate resulting in hypocalcemia. This patient's serum calcium was 7 mg/dL (8.9‐10.2) (ionized 0.21 mmol/L; 1.18‐1.38), her pH was 7.03. There were no overt signs or symptoms of hypocalcemia (paresthesias, twitching, tetany, or seizures.) and with aggressive replacement the calcium and the ECG normalized. Transfusions are an uncommon cause of hypocalcemia, with hypomagnesemia or hypermagnesemia, acute pancreatitis, rhabdomyolysis, tumor lysis syndrome, renal failure, vitamin D deficiency, pseudoparathyroidism and hypoparathyroidism being the more common disorders caused.1, 2 This patient's magnesium was normal on admission (2.1 mg/dL) and she did not have any of the other conditions mentioned. It is possible that hemodilution may also have played a role.
Genetic long QT syndrome (variant 3) can manifest with a similar ECG pattern, although the T‐wave is sometimes peaked or biphasic in that condition. Our patient had no personal or family history of syncope or sudden death, and her ECG normalized with calcium replacement.3
Hypothermia can also present with a long QT interval secondary to a long ST segment. Our patient was normothermic, and had no other ECG findings suggestive of hypothermia (Osborn waves: notching of terminal portion of QRS), shivering artifacts, sinus bradycardia, atrial fibrillation, QRS prolongation, or prolongation of the PR interval.4
The differential diagnosis of a long QT interval with a long ST segment is short, and the clinical scenario will provide the etiology in most cases.
Patient Presentation
A 52‐year‐old woman with cirrhosis presented with a large‐volume upper gastrointestinal (GI) bleed. After receiving massive volume and blood product resuscitation (18 U packed red blood cells [PRBCs], 17 U fresh frozen plasma [FFP], 2 U cryoprecipitate, 1 U platelets, and 14 L normal saline), a routine admission electrocardiogram (ECG) was obtained (Figure 1).

Discussion
The ECG shows normal sinus rhythm with a prolonged QT interval (specifically due to a long ST segment). The differential diagnosis of this pattern of QT prolongation includes hypocalcemia, long QT syndrome (variant 3), and hypothermia. Massive transfusion can result in the chelation of calcium by citrate resulting in hypocalcemia. This patient's serum calcium was 7 mg/dL (8.9‐10.2) (ionized 0.21 mmol/L; 1.18‐1.38), her pH was 7.03. There were no overt signs or symptoms of hypocalcemia (paresthesias, twitching, tetany, or seizures.) and with aggressive replacement the calcium and the ECG normalized. Transfusions are an uncommon cause of hypocalcemia, with hypomagnesemia or hypermagnesemia, acute pancreatitis, rhabdomyolysis, tumor lysis syndrome, renal failure, vitamin D deficiency, pseudoparathyroidism and hypoparathyroidism being the more common disorders caused.1, 2 This patient's magnesium was normal on admission (2.1 mg/dL) and she did not have any of the other conditions mentioned. It is possible that hemodilution may also have played a role.
Genetic long QT syndrome (variant 3) can manifest with a similar ECG pattern, although the T‐wave is sometimes peaked or biphasic in that condition. Our patient had no personal or family history of syncope or sudden death, and her ECG normalized with calcium replacement.3
Hypothermia can also present with a long QT interval secondary to a long ST segment. Our patient was normothermic, and had no other ECG findings suggestive of hypothermia (Osborn waves: notching of terminal portion of QRS), shivering artifacts, sinus bradycardia, atrial fibrillation, QRS prolongation, or prolongation of the PR interval.4
The differential diagnosis of a long QT interval with a long ST segment is short, and the clinical scenario will provide the etiology in most cases.
- .Hypercalcemia and hypocalcemia. In:Fauci AS,Braunwald E,Kasper DL, et al., eds.Harrison's Principles of Internal Medicine. Available at: http://www.accessmedicine.com/content.aspx?aID=2864354.
- ,,.Massive transfusion.Crit Care Clin.1986;2:791–805.
- ,,,.Novel insights in the congenital long QT syndrome.Ann Intern Med.2002;137:981–992.
- ,,.The clinical value of the ECG in noncardiac conditions.Chest.2004;125:1561–1576.
- .Hypercalcemia and hypocalcemia. In:Fauci AS,Braunwald E,Kasper DL, et al., eds.Harrison's Principles of Internal Medicine. Available at: http://www.accessmedicine.com/content.aspx?aID=2864354.
- ,,.Massive transfusion.Crit Care Clin.1986;2:791–805.
- ,,,.Novel insights in the congenital long QT syndrome.Ann Intern Med.2002;137:981–992.
- ,,.The clinical value of the ECG in noncardiac conditions.Chest.2004;125:1561–1576.