User login
Treatment of Diabetes in Emergency Dept.
Current consensus guidelines from the American Diabetes Association and the American Association of Clinical Endocrinologists recommend the use of insulin‐based treatment protocols for most hospitalized patients with hyperglycemia.1 For noncritically ill patients, it is recommended to target a fasting blood glucose (BG) < 140 mg/dL and a random BG < 180‐200 mg/dL, without excess hypoglycemia. Prior studies recommended using a basal‐bolus insulin protocol that specifies starting doses and parameters for dose adjustment, applied by well‐educated teams of physicians and nurses.27 We have shown that insulin detemir given as a once‐daily basal injection coupled with rapid‐acting insulin aspart with meals is an effective regimen for managing hyperglycemia in hospitalized patients with type 2 diabetes.7 We and others have shown that once‐daily basal insulin mealtime rapid‐acting insulin is significantly more effective than sliding‐scale regular insulin in the hospital setting.6, 8
The majority of patients admitted to general medical units are first evaluated in the emergency department (ED), and significant hyperglycemia is not uncommon in ED patients. However, protocols for the treatment of hyperglycemia in the ED have not been widely implemented. Ginde et al studied 160 ED patients with a history of diabetes and BG > 200 mg/dL and found that although 73% were admitted to the hospital, only 31% were treated with insulin, and only 18% had a diagnosis of diabetes charted.9 A recent survey of 152 residents and attendings in 3 academic EDs found that only 32% would give insulin for a BG > 200 mg/dL, 59% for a BG > 250 mg/dL, and 91% for a BG > 300 mg/dL to ED patients with known diabetes.10 We completed a preliminary study of a novel protocol for the administration of subcutaneous insulin aspart in the ED at Rush University in 2008.11 We found that the mean BG was significantly lowered during an ED stay, from 333 to 158 mg/dL, and that the protocol was easily adopted by ED staff, with a low rate of hypoglycemia. Historically, only 35% of hyperglycemic patients with diabetes received insulin in our ED.11 Reasons for limited ED management of hyperglycemia may include presence of more critical issues, time and resource restriction, unfamiliarity with glycemic targets, and concerns regarding hypoglycemia.9, 10
In the current study we focused on 3 questions:
Could we further reduce the risk of hypoglycemia by modifying our original Rush ED insulin protocol? We reduced insulin aspart from 0.1 to 0.05 units/kg for BG 200‐299 mg/dL, from 0.15 to 0.1 units/kg for BG 300‐399 mg/dL, and from 0.2 to 0.15 units/kg for BG 400 mg/dL.
Could we couple our ED insulin aspart protocol with prompt initiation of a detemir‐aspart protocol in those patients who were subsequently admitted to general medical units from the ED?
Would the hospital length of stay, mean BG, and incidence of hypoglycemia be improved by the use of 2 back‐to‐back subcutaneous insulin protocols in a randomized clinical trial compared with the usual care provided in the ED and general medical inpatient units?
Research Design and Methods
From May 2008 through June 2009, patients presenting to the Rush University Medical Center ED with a history of type 2 diabetes and an initial point‐of‐care BG 200 mg/dL were randomized to an intervention group (INT) or to a usual care group (UC) after giving informed consent. Inclusion criteria for the study were: ages 18‐80 years, history of type 2 diabetes for at least 3 months, and prior therapy with dietary management, oral agents, or insulin. Patients were excluded if subsequently found to have diabetic ketoacidosis, hyperosmolar nonketotic syndrome, or critical illness requiring intensive care unit admission/direct surgical intervention. Other exclusion criteria included a positive pregnancy test or an inability to give informed consent secondary to acute drug or alcohol intoxication or active mental illness. Patients with clinically significant liver disease, with ALT or AST > 3 times the upper limit of normal, or with a history of end‐stage renal disease requiring dialysis were also excluded because of their increased risk of hypoglycemia, as they have required a more conservative insulin regimen. Similarly, we excluded patients with a history of type 1 diabetes because our aspart algorithm for the ED had only been tested in a type 2 diabetes population, and we did not want to disrupt the insulin regimen of type 1 diabetes patients, usually glargine‐based or an insulin pump.
The study consisted of 2 phases. Patients randomized to INT or UC in phase 1 stayed in their respective groups through phase 2. After informed consent was obtained by the study staff, implementation of the protocol was carried out by the ED staff. ED nurses were trained in the study protocol. During phase 1, INT patients received subcutaneous aspart every 2 hours while in the ED if BG was > 200 mg/dL. Aspart dosing per protocol was limited to 2 doses. Uncommonly, when a third dose of aspart was needed, physician input was requested. Aspart dosing was weight‐ and BG level based (0.05 units/kg for BG 200‐299 mg/dL, 0.1 units/kg for BG 300‐399 mg/dL, or 0.15 units/kg for BG 400mg/dL; see Supporting Appendix Fig. 1). Regardless of BG level, the ED aspart protocol was discontinued on patient discharge home or admission to the hospital. UC patients received treatment for hyperglycemia at the discretion of their ED physicians. INT subjects who required hospital admission were transitioned to basal‐bolus insulin therapy with detemir and aspart, receiving their first dose of detemir in the ED. Detemir dosing was weight‐based (0.3 units/kg) if the patient was not on home insulin or based on the patient's home dose of insulin (same dose for detemir, unit‐for‐unit conversion from glargine to detemir, or 80% of total NPH dose). If a patient received basal insulin prior to arrival, the first dose of detemir was held until 12 hours after the last dose of NPH or until 20 hours after the last dose of glargine or detemir. We found from our preliminary study that inadvertent overlaps in long‐acting insulin were one cause of hypoglycemia. We compared differences between groups in the final ED BG level, frequency of hypoglycemia, and ED length of stay (LOS).
Patients subsequently admitted to the hospital entered phase 2. During phase 2, INT patients had detemir and premeal aspart titrated by study staff using a predefined protocol (see Supporting Appendix Fig. 2). Detemir was given once daily, 24 hours after the initial ED dose. UC patients had their diabetes managed by medical house staff teams. House staff members have been educated on the Rush inpatient insulin protocol on which the INT protocol was based. The Rush inpatient diabetes protocol is implemented via a single computerized order set in which all patients should receive mealtime insulin using aspart and a basal insulin (glargine or detemir or NPH). We compared differences between groups in the mean admission BG level, mean daily BG level, mean BG level before each meal, hospital LOS, and frequency of hypoglycemic events. Moderate hypoglycemia was defined as a BG between 50 and 69 mg/dL, and severe hypoglycemia was defined as a BG < 50 mg/dL. We also compared the frequency of BG < 60 mg/dL.
The Rush University Medical Center institutional review board approved the study. Statistical analysis was done using SPSS version 11.0. The Student t test was used to determine any significant difference in BG means between the INT and UC groups. The Fisher's exact test or the chi‐square test was used to determine any difference in proportions of hypoglycemic events between INT and UC patients.
Results
Phase 1: Emergency Department
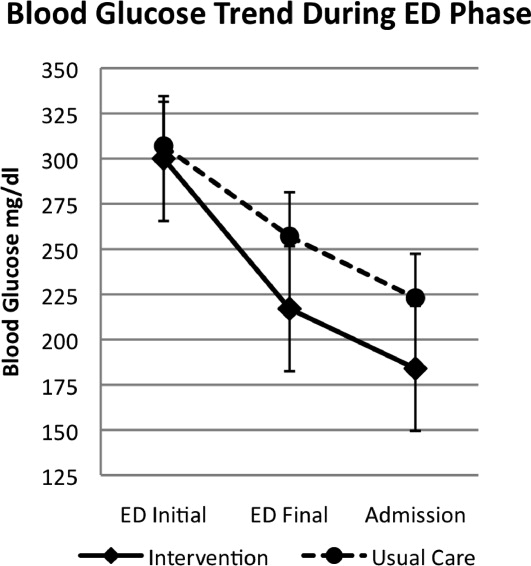
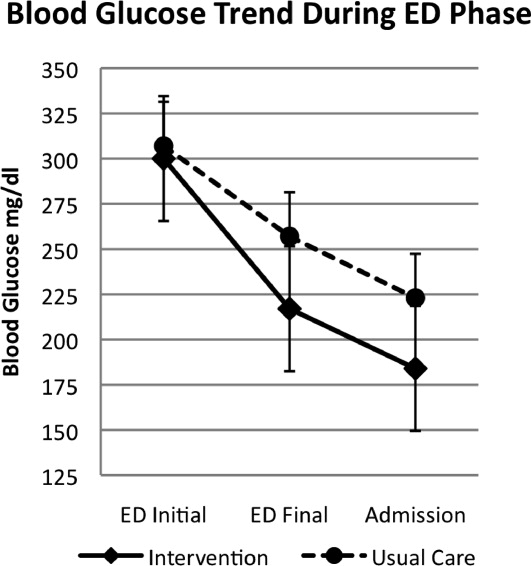
A total of 176 patients were randomized: 87 to the INT group and 89 to the UC group. Baseline characteristics were similar between groups (Table 1). Mean initial ED BG was similar: 300 70 mg/dL for INT patients and 307 82 mg/dL for UC patients. During phase 1, all INT patients were treated with aspart every 2 hours if BG > 200 mg/dL until discharge home or admission to the hospital. They received an initial mean insulin dose of 0.08 0.04 units/kg of subscutaneous aspart. Twenty‐five percent of INT patients received a second dose of aspart, and 3% received a third dose. For UC patients, only 55% received insulin therapy. Aspart was used for all UC patients who received insulin. Of those in the UC group who received insulin, 11% received a second dose for persistent hyperglycemia; none received a third dose. The mean initial ED BG for UC patients who received insulin was 358 73 mg/dL, and they received an initial mean dose of 0.11 0.05 units/kg. UC patients who did not receive insulin had a lower mean initial ED BG, 241 35 mg/dL. The mean final ED BG was 217 71 mg/dL for the INT group and 257 89 mg/dL for the UC group (P < .01; Fig. 1). The mean ED LOS was 30 minutes longer in the INT group (P = .06; Table 1). Sixty INT patients (69%) and 61 UC patients (69%) were admitted to the hospital. Fifty‐six percent of INT patients received the first dose of detemir based on their home insulin dose, and 44% received a weight‐based dose per protocol.
| Intervention (n = 87) | Usual Care (n = 89) | Significance | |
|---|---|---|---|
| |||
| Age (y) | 55 13 | 55 13 | |
| Sex (% male) | 48 | 39 | |
| BMI (kg/m2) | 34 9 | 33 9 | |
| Ethnicity (%) | |||
| African American | 58 | 66 | |
| Hispanic | 24 | 19 | |
| White | 15 | 11 | |
| Other | 3 | 4 | |
| Duration of diabetes (y) | 13 9 | 12 10 | |
| HA1C | 10.4 2.2 | 9.8 2.6 | |
| Insulin treatment at home (%) | 56 | 62 | |
| Presenting complaint/diagnosis (%) | |||
| Cardiac | 20 | 23 | |
| Gastrointestinal | 30 | 23 | |
| Hyperglycemia | 13 | 18 | |
| Infection | 9 | 11 | |
| Initial ED blood glucose (mg/dL) | 300 70 | 307 82 | |
| Final ED blood glucose (mg/dL) | 217 71 | 256 89 | P < .01 |
| ED length of stay (h) | 5.4 1.7 | 4.9 1.9 | P = .06 |
| Patients treated with insulin in ED (%) | 100 | 54 | |
| Initial dose of SQ aspart (units) | 7.9 4.2 | 9.5 4 | |
| ED patients admitted (%) | 69 | 69 | |
| Admission blood glucose (mg/dL) | 184 70 | 224 93 | P < .01 |
| Treatment of hyperglycemia in hospital (%) | |||
| Detemir aspart insulin | 100 | 7 | |
| Glargine aspart insulin | 0 | 36 | |
| NPH aspart insulin | 0 | 34 | |
| Oral agents | 0 | 8 | |
| None | 0 | 15 | |
| Hospital length of stay (days) | 2.7 2.0 | 3.1 1.9 | P = .58 |
Phase 2: Inpatient Setting
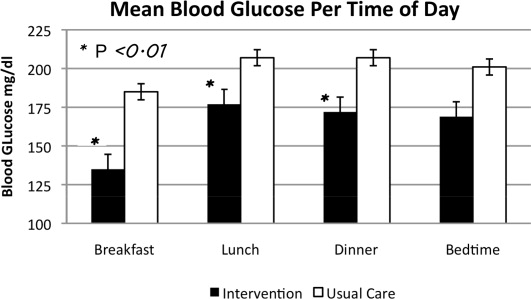
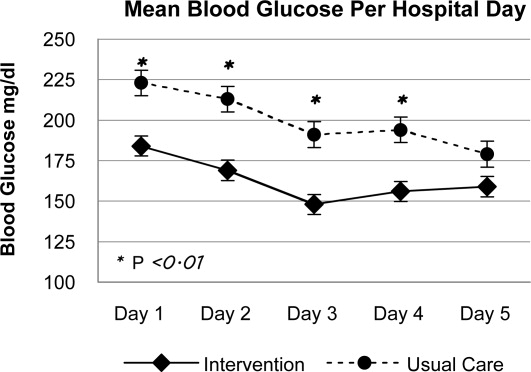
In phase 2, mean admission BG was significantly lower in the INT group (184 70 mg/dL) than in the UC group (223 93 mg/dL), P < .01, as a result of aspart given in the ED. The day 1 mean fasting BG for INT patients was 148 54 mg/dL, significantly lower than the day 1 mean fasting BG for UC patients: 212 81 mg/dL (P < .01). The mean fasting BG for the entire hospitalization was significantly lower for INT patients, 135 48 mg/dL, than for UC patients, 185 72 mg/dL (P < .01). During phase 2, all INT patients had detemir and aspart titrated daily per protocol. Treatment of UC patients was as follows: 78.5% with insulin, 8.2% with oral agents, and 11.5% did not receive medication for hyperglycemia. Of those in the UC group who received insulin, 36.0% were treated with lantus/aspart or detemir/aspart, 34.4% with NPH/aspart, 6.5% with lantus or detemir alone, and 1.6% with aspart alone. Overall, 76.9% of UC received basal insulin, and 70.4% received nutritional insulin. Only 47% of UC patients had insulin adjusted on a daily basis despite persistent hyperglycemia. Significant differences were also seen between INT and UC patients in mean prelunch and predinner BG levels, but not in mean bedtime BG level (Fig. 2). Mean daily BG levels for the initial 5 days of inpatient stay were significantly lower in the INT group (P < .01), except for day 5, when only 21 patients remained in the study (Fig. 3). Patient‐day weighted mean glucose was 163 39 mg/dL for INT patients versus 202 39 mg/dL for UC patients (P < .01). On admission, day 1 mean insulin total daily dose (TDD) was 0.65 0.26 units/kg for INT patients and 0.52 0.29 units/kg for UC patients. The final mean TDD was 0.75 0.35 units/kg for INT patients and 0.61 0.38 units/kg for UC patients. Mean hospital LOS was 9.6 hours shorter for INT patients (2.7 2 days) than for UC patients (3.1 1.9 days), P = .58.
Patient Safety: Frequency of Hypoglycemia
The frequency of hypoglycemia is shown in Table 2. During the ED phase, 3 UC patients (3.4%) had a BG < 50 mg/dL, and 2 INT patients (2.3%) had a BG of 67 mg/dL.
| Blood Glucose | Emergency Department (Number of Episodes) | Significance | Inpatient Phase (Patient Stays) | Significance | ||
|---|---|---|---|---|---|---|
| Usual Care | Intervention | Usual Care | Intervention | |||
| < 50 mg/dL | 3 | 0 | P = .50 | 6 | 1 | P = .11 |
| < 60 mg/dL | 3 | 0 | P = .50 | 7 | 8 | P = .98 |
| 5069 mg/dL | 0 | 2 | P = .23 | 6 | 12 | P = .20 |
During the hospital phase, INT patients had 4.3% of patient‐days and UC had 4.5% of patient‐days with any BG < 70 mg/dL. During 12 patient‐stays (20%) in the INT group there was an episode of moderate hypoglycemia, and during 1 patient‐stay (1.7%) there was an episode of severe hypoglycemia. During 6 patient‐stays (9.8%) in the UC group there was an episode of moderate hypoglycemia, and during 6 patient‐stays (9.8%) there was an episode of severe hypoglycemia (Table 2). The odds ratio (OR) for moderate hypoglycemia in the INT group compared with the UC group was 1.93 (95% CI, 0.7‐5.29), but for severe hypoglycemia the OR was 0.15 (95% CI, 0.018‐1.33). Moderate and severe hypoglycemic events in the UC group were split evenly between patients treated with glargine/detemir‐aspart and those treated with NPH‐aspart.
Discussion
This is the first randomized trial comparing the Rush Emergency Department Hyperglycemia Intervention (REDHI) protocol with usual care for the treatment of hyperglycemia in the ED. We believe this may be the first trial to initiate subcutaneous basal insulin therapy in the ED at the time of hospital admission. Initiation of our protocol for type 2 diabetic patients with BG > 200 mg/dL resulted in lower final ED and admission BGs compared with those in the UC group. Although a higher mean initial ED BG of 358 73 mg/dL was required to prompt initiation of insulin therapy for UC patients, 3 experienced severe hypoglycemia. By following the REDHI protocol, ED nurses avoided BG < 50 mg/dL in INT patients. Our first version of the REDHI protocol dosed more insulin than our current version, and we saw excess hypoglycemia.11 With a reduced dosing formula, there was less lowering of BG, but we eliminated all BG < 60 mg/dL. There was a trend toward an increased ED LOS in INT patients compared with UC patients. This may be because of delays in the administration of insulin or the requirement for a final BG check prior to discharge from the ED for INT patients. However, we did not receive feedback from ED nursing that either factor was a significant issue.
During phase 2, we observed improved glycemic control in INT, likely due to two factors: early initiation of basal insulin and protocol driven daily titration of both basal and mealtime insulin. We achieved a mean fasting BG of 148 54 mg/dL in INT the morning after the ED dose of detemir. BG levels in both groups continued to improve each day, but since the admission BG for INT was lower, this group maintained significantly lower BG levels throughout most of the hospitalization. There were also significant differences between groups at different times of day. Basal doses for INT patients were adjusted daily per fasting BG. Scheduled mealtime doses were based on the basal dose; each mealtime aspart dose was a third of the basal detemir dose. Therefore, patients who required larger doses for certain meals may have received less aspart than needed. This may explain why fasting BG control was better than BG control later in the day.
Current Rush guidelines recommend the same insulin doses as those that our intervention used, but patients in the UC group were less likely to have insulin titrated daily. Cook et al found that clinical inertia, or failure of health care providers to initiate or intensify therapy when indicated, is a common problem among medical residents treating inpatients with insulin.15 Reasons for clinical inertia may include unawareness of inpatient glycemic targets, lack of training or confidence in titrating insulin, and concerns regarding hypoglycemia. Our study shows that this is still an operative issue, even after residents have participated in multiple small‐group educational sessions. Details of the Rush inpatient insulin protocol are also on pocket cards distributed to residents. However, fewer than half of UC patients had insulin adjusted appropriately for persistent hyperglycemia. This may be one explanation for the improved control seen in the INT group and underscores the importance of daily dose titrations based on a uniform protocol.
During phase 2, despite improved glycemic control in INT, there was no significant difference in rates of hypoglycemia between the groups. The number of patient‐stays with moderate hypoglycemia was more in the INT group than in the UC group, 12 versus 6, respectively, but not statistically different (P = .20). There was a trend toward fewer patient‐stays with severe hypoglycemia in the INT group than in the UC group, 1 versus 6, respectively (P = .11).
Other studies have described improved inpatient glycemic control without excess hypoglycemia. In the RABBIT 2 trial, institution of a glargine‐glulisine insulin protocol, TTD of 0.4‐5 units/kg, among insulin‐naive inpatients resulted in a mean fasting BG of 147 36 mg/dL and a mean hospital BG of 166 32 mg/dL, with 3% of patient‐stays having a BG < 60 mg/dL.6 In a second trial, detemir‐aspart was compared with NPH‐aspart TTD of 0.4‐5 units/kg.7 Both groups achieved a similar mean fasting BG of 146 mg/dL and a mean hospital BG of 157 mg/dL. However, the rate of hypoglycemia was higher: 29% of patient‐stays overall. In our study, we achieved a mean fasting BG of 135 48 mg/dL and a mean hospital BG of 163 40 mg/dL in the INT group, using a mean initial TTD of 0.65 0.23 units/kg. The frequency of hypoglycemia in this trial, 22% of INT patient‐stays and 20% of UC patient‐stays, was less than that in Umpierrez et al,7 despite a lower mean fasting BG in our current trial. Maynard et al found 16% of patient‐stays and 3% of patient‐days had an episode of BG < 60 mg/dL in a trial of glarginerapid‐acting insulin (0.4‐5 units/kg TTD).12 Schnipper et al found that 6.1% of patient‐days had an episode of BG < 60 mg/dL using either glargine or NPH and a rapid‐acting insulin, TTD 0.6 units/kg.13 Our hypoglycemia rates were higher; however, we defined hypoglycemia as BG < 70 mg/dL, as suggested by the ADA workgroup.14 If we use a cutoff of < 60 mg/dL for hypoglycemia, it occurred in 13.3% of patient‐stays and 4.3% of patient‐days in the INT group, comparable to that in previous studies.
Our study has several limitations. First, this was a single‐center study, and our ED protocol should be tested in other ED settings, both academic and community. Second, although there were trends toward lower rates of severe hypoglycemia in the INT group, the study was underpowered to detect possible significant differences. Third, although ED nurses implemented the study protocol, study staff closely monitored nurses to ensure adherence. Therefore, it is difficult to speculate on protocol adherence under normal circumstances. Successful implementation requires ongoing nursing and medical staff education. A fourth limitation is the absence of patients with type 1 diabetes.
In conclusion we demonstrated that weight‐based subcutaneous aspart insulin therapy begun in the ED, coupled with prompt initiation of a detemir‐aspart insulin protocol, results in rapid correction of hyperglycemia and improved inpatient glycemic control without increasing hypoglycemia. Diabetes is a common comorbidity in patients presenting to the ED that is not uniformly addressed. These patients may present with uncontrolled hyperglycemia or diabetes‐related infections, and prompt, efficacious glucose control is important. The nurse‐driven Rush ED hyperglycemia protocol ensures that hyperglycemia is safely addressed, allowing the ED physician to address more critical issues. By initiating basal insulin in the ED, our protocol allows for a prompt and smooth transition to a basal‐bolus insulin regimen for the inpatient setting.
- ,,, et al.American Association of Clinical Endocrinologists and American Diabetes Association Consensus Statement on Inpatient Glycemic Control.Diabetes Care.2009;32:1119–1131.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,Eliminating inpatient sliding scale insulin: a re‐education project with medical house staff.Diabetes Care.2008;28:1008–1011.
- ,,, et al.Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy.Diabetes Care.2007;30:823–828.
- ,,.Comparison of once daily glargine insulin with twice‐daily NPH/Regular insulin for control of hyperglycemia in inpatients after cardiovascular surgery.Diabetes Technol Ther.2006;8:609–616.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,, et al.Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine Hagedorn plus regular in medical patients with type 2 diabetes.J Clin Endocrinol Metab.2009;94:564–569.
- ,,,.Once daily insulin glargine vs. six hourly sliding scale regular insulin for control of hyperglycemia after bariatric surgery: a randomized clinical trial.Endocr Pract.2007;13:225–231.
- ,,.Limited communication and management of emergency department hyperglycemia in hospitalized patients.J Hosp Med.2009;4:44–49.
- ,,,.Multicenter survey of emergency physician management and referral for hyperglycemia.J Emerg Med.2010;38:264–272.
- ,,,,Impact of a nurse‐driven subcutaneous insulin protocol: Rush Emergency Department Hyperglycemia Intervention (REDHI).J Emerg Med.2008 [Epub ahead of print].
- ,,,,.Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm.J Hosp Med.2009;4:3–15.
- ,,,.Effects of a subcutaneous insulin protocol, clinical education, and computerized order set on the quality of inpatient management of hyperglycemia: results of a clinical trial.J Hosp Med.2009;4:16–27.
- ADA Workgroup on Hypoglycemia.Defining and reporting hypoglycemia in diabetes.Diabetes Care.2005;28:1245–1249.
- ,, et al.Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
Current consensus guidelines from the American Diabetes Association and the American Association of Clinical Endocrinologists recommend the use of insulin‐based treatment protocols for most hospitalized patients with hyperglycemia.1 For noncritically ill patients, it is recommended to target a fasting blood glucose (BG) < 140 mg/dL and a random BG < 180‐200 mg/dL, without excess hypoglycemia. Prior studies recommended using a basal‐bolus insulin protocol that specifies starting doses and parameters for dose adjustment, applied by well‐educated teams of physicians and nurses.27 We have shown that insulin detemir given as a once‐daily basal injection coupled with rapid‐acting insulin aspart with meals is an effective regimen for managing hyperglycemia in hospitalized patients with type 2 diabetes.7 We and others have shown that once‐daily basal insulin mealtime rapid‐acting insulin is significantly more effective than sliding‐scale regular insulin in the hospital setting.6, 8
The majority of patients admitted to general medical units are first evaluated in the emergency department (ED), and significant hyperglycemia is not uncommon in ED patients. However, protocols for the treatment of hyperglycemia in the ED have not been widely implemented. Ginde et al studied 160 ED patients with a history of diabetes and BG > 200 mg/dL and found that although 73% were admitted to the hospital, only 31% were treated with insulin, and only 18% had a diagnosis of diabetes charted.9 A recent survey of 152 residents and attendings in 3 academic EDs found that only 32% would give insulin for a BG > 200 mg/dL, 59% for a BG > 250 mg/dL, and 91% for a BG > 300 mg/dL to ED patients with known diabetes.10 We completed a preliminary study of a novel protocol for the administration of subcutaneous insulin aspart in the ED at Rush University in 2008.11 We found that the mean BG was significantly lowered during an ED stay, from 333 to 158 mg/dL, and that the protocol was easily adopted by ED staff, with a low rate of hypoglycemia. Historically, only 35% of hyperglycemic patients with diabetes received insulin in our ED.11 Reasons for limited ED management of hyperglycemia may include presence of more critical issues, time and resource restriction, unfamiliarity with glycemic targets, and concerns regarding hypoglycemia.9, 10
In the current study we focused on 3 questions:
Could we further reduce the risk of hypoglycemia by modifying our original Rush ED insulin protocol? We reduced insulin aspart from 0.1 to 0.05 units/kg for BG 200‐299 mg/dL, from 0.15 to 0.1 units/kg for BG 300‐399 mg/dL, and from 0.2 to 0.15 units/kg for BG 400 mg/dL.
Could we couple our ED insulin aspart protocol with prompt initiation of a detemir‐aspart protocol in those patients who were subsequently admitted to general medical units from the ED?
Would the hospital length of stay, mean BG, and incidence of hypoglycemia be improved by the use of 2 back‐to‐back subcutaneous insulin protocols in a randomized clinical trial compared with the usual care provided in the ED and general medical inpatient units?
Research Design and Methods
From May 2008 through June 2009, patients presenting to the Rush University Medical Center ED with a history of type 2 diabetes and an initial point‐of‐care BG 200 mg/dL were randomized to an intervention group (INT) or to a usual care group (UC) after giving informed consent. Inclusion criteria for the study were: ages 18‐80 years, history of type 2 diabetes for at least 3 months, and prior therapy with dietary management, oral agents, or insulin. Patients were excluded if subsequently found to have diabetic ketoacidosis, hyperosmolar nonketotic syndrome, or critical illness requiring intensive care unit admission/direct surgical intervention. Other exclusion criteria included a positive pregnancy test or an inability to give informed consent secondary to acute drug or alcohol intoxication or active mental illness. Patients with clinically significant liver disease, with ALT or AST > 3 times the upper limit of normal, or with a history of end‐stage renal disease requiring dialysis were also excluded because of their increased risk of hypoglycemia, as they have required a more conservative insulin regimen. Similarly, we excluded patients with a history of type 1 diabetes because our aspart algorithm for the ED had only been tested in a type 2 diabetes population, and we did not want to disrupt the insulin regimen of type 1 diabetes patients, usually glargine‐based or an insulin pump.
The study consisted of 2 phases. Patients randomized to INT or UC in phase 1 stayed in their respective groups through phase 2. After informed consent was obtained by the study staff, implementation of the protocol was carried out by the ED staff. ED nurses were trained in the study protocol. During phase 1, INT patients received subcutaneous aspart every 2 hours while in the ED if BG was > 200 mg/dL. Aspart dosing per protocol was limited to 2 doses. Uncommonly, when a third dose of aspart was needed, physician input was requested. Aspart dosing was weight‐ and BG level based (0.05 units/kg for BG 200‐299 mg/dL, 0.1 units/kg for BG 300‐399 mg/dL, or 0.15 units/kg for BG 400mg/dL; see Supporting Appendix Fig. 1). Regardless of BG level, the ED aspart protocol was discontinued on patient discharge home or admission to the hospital. UC patients received treatment for hyperglycemia at the discretion of their ED physicians. INT subjects who required hospital admission were transitioned to basal‐bolus insulin therapy with detemir and aspart, receiving their first dose of detemir in the ED. Detemir dosing was weight‐based (0.3 units/kg) if the patient was not on home insulin or based on the patient's home dose of insulin (same dose for detemir, unit‐for‐unit conversion from glargine to detemir, or 80% of total NPH dose). If a patient received basal insulin prior to arrival, the first dose of detemir was held until 12 hours after the last dose of NPH or until 20 hours after the last dose of glargine or detemir. We found from our preliminary study that inadvertent overlaps in long‐acting insulin were one cause of hypoglycemia. We compared differences between groups in the final ED BG level, frequency of hypoglycemia, and ED length of stay (LOS).
Patients subsequently admitted to the hospital entered phase 2. During phase 2, INT patients had detemir and premeal aspart titrated by study staff using a predefined protocol (see Supporting Appendix Fig. 2). Detemir was given once daily, 24 hours after the initial ED dose. UC patients had their diabetes managed by medical house staff teams. House staff members have been educated on the Rush inpatient insulin protocol on which the INT protocol was based. The Rush inpatient diabetes protocol is implemented via a single computerized order set in which all patients should receive mealtime insulin using aspart and a basal insulin (glargine or detemir or NPH). We compared differences between groups in the mean admission BG level, mean daily BG level, mean BG level before each meal, hospital LOS, and frequency of hypoglycemic events. Moderate hypoglycemia was defined as a BG between 50 and 69 mg/dL, and severe hypoglycemia was defined as a BG < 50 mg/dL. We also compared the frequency of BG < 60 mg/dL.
The Rush University Medical Center institutional review board approved the study. Statistical analysis was done using SPSS version 11.0. The Student t test was used to determine any significant difference in BG means between the INT and UC groups. The Fisher's exact test or the chi‐square test was used to determine any difference in proportions of hypoglycemic events between INT and UC patients.
Results
Phase 1: Emergency Department
A total of 176 patients were randomized: 87 to the INT group and 89 to the UC group. Baseline characteristics were similar between groups (Table 1). Mean initial ED BG was similar: 300 70 mg/dL for INT patients and 307 82 mg/dL for UC patients. During phase 1, all INT patients were treated with aspart every 2 hours if BG > 200 mg/dL until discharge home or admission to the hospital. They received an initial mean insulin dose of 0.08 0.04 units/kg of subscutaneous aspart. Twenty‐five percent of INT patients received a second dose of aspart, and 3% received a third dose. For UC patients, only 55% received insulin therapy. Aspart was used for all UC patients who received insulin. Of those in the UC group who received insulin, 11% received a second dose for persistent hyperglycemia; none received a third dose. The mean initial ED BG for UC patients who received insulin was 358 73 mg/dL, and they received an initial mean dose of 0.11 0.05 units/kg. UC patients who did not receive insulin had a lower mean initial ED BG, 241 35 mg/dL. The mean final ED BG was 217 71 mg/dL for the INT group and 257 89 mg/dL for the UC group (P < .01; Fig. 1). The mean ED LOS was 30 minutes longer in the INT group (P = .06; Table 1). Sixty INT patients (69%) and 61 UC patients (69%) were admitted to the hospital. Fifty‐six percent of INT patients received the first dose of detemir based on their home insulin dose, and 44% received a weight‐based dose per protocol.

| Intervention (n = 87) | Usual Care (n = 89) | Significance | |
|---|---|---|---|
| |||
| Age (y) | 55 13 | 55 13 | |
| Sex (% male) | 48 | 39 | |
| BMI (kg/m2) | 34 9 | 33 9 | |
| Ethnicity (%) | |||
| African American | 58 | 66 | |
| Hispanic | 24 | 19 | |
| White | 15 | 11 | |
| Other | 3 | 4 | |
| Duration of diabetes (y) | 13 9 | 12 10 | |
| HA1C | 10.4 2.2 | 9.8 2.6 | |
| Insulin treatment at home (%) | 56 | 62 | |
| Presenting complaint/diagnosis (%) | |||
| Cardiac | 20 | 23 | |
| Gastrointestinal | 30 | 23 | |
| Hyperglycemia | 13 | 18 | |
| Infection | 9 | 11 | |
| Initial ED blood glucose (mg/dL) | 300 70 | 307 82 | |
| Final ED blood glucose (mg/dL) | 217 71 | 256 89 | P < .01 |
| ED length of stay (h) | 5.4 1.7 | 4.9 1.9 | P = .06 |
| Patients treated with insulin in ED (%) | 100 | 54 | |
| Initial dose of SQ aspart (units) | 7.9 4.2 | 9.5 4 | |
| ED patients admitted (%) | 69 | 69 | |
| Admission blood glucose (mg/dL) | 184 70 | 224 93 | P < .01 |
| Treatment of hyperglycemia in hospital (%) | |||
| Detemir aspart insulin | 100 | 7 | |
| Glargine aspart insulin | 0 | 36 | |
| NPH aspart insulin | 0 | 34 | |
| Oral agents | 0 | 8 | |
| None | 0 | 15 | |
| Hospital length of stay (days) | 2.7 2.0 | 3.1 1.9 | P = .58 |
Phase 2: Inpatient Setting
In phase 2, mean admission BG was significantly lower in the INT group (184 70 mg/dL) than in the UC group (223 93 mg/dL), P < .01, as a result of aspart given in the ED. The day 1 mean fasting BG for INT patients was 148 54 mg/dL, significantly lower than the day 1 mean fasting BG for UC patients: 212 81 mg/dL (P < .01). The mean fasting BG for the entire hospitalization was significantly lower for INT patients, 135 48 mg/dL, than for UC patients, 185 72 mg/dL (P < .01). During phase 2, all INT patients had detemir and aspart titrated daily per protocol. Treatment of UC patients was as follows: 78.5% with insulin, 8.2% with oral agents, and 11.5% did not receive medication for hyperglycemia. Of those in the UC group who received insulin, 36.0% were treated with lantus/aspart or detemir/aspart, 34.4% with NPH/aspart, 6.5% with lantus or detemir alone, and 1.6% with aspart alone. Overall, 76.9% of UC received basal insulin, and 70.4% received nutritional insulin. Only 47% of UC patients had insulin adjusted on a daily basis despite persistent hyperglycemia. Significant differences were also seen between INT and UC patients in mean prelunch and predinner BG levels, but not in mean bedtime BG level (Fig. 2). Mean daily BG levels for the initial 5 days of inpatient stay were significantly lower in the INT group (P < .01), except for day 5, when only 21 patients remained in the study (Fig. 3). Patient‐day weighted mean glucose was 163 39 mg/dL for INT patients versus 202 39 mg/dL for UC patients (P < .01). On admission, day 1 mean insulin total daily dose (TDD) was 0.65 0.26 units/kg for INT patients and 0.52 0.29 units/kg for UC patients. The final mean TDD was 0.75 0.35 units/kg for INT patients and 0.61 0.38 units/kg for UC patients. Mean hospital LOS was 9.6 hours shorter for INT patients (2.7 2 days) than for UC patients (3.1 1.9 days), P = .58.


Patient Safety: Frequency of Hypoglycemia
The frequency of hypoglycemia is shown in Table 2. During the ED phase, 3 UC patients (3.4%) had a BG < 50 mg/dL, and 2 INT patients (2.3%) had a BG of 67 mg/dL.
| Blood Glucose | Emergency Department (Number of Episodes) | Significance | Inpatient Phase (Patient Stays) | Significance | ||
|---|---|---|---|---|---|---|
| Usual Care | Intervention | Usual Care | Intervention | |||
| < 50 mg/dL | 3 | 0 | P = .50 | 6 | 1 | P = .11 |
| < 60 mg/dL | 3 | 0 | P = .50 | 7 | 8 | P = .98 |
| 5069 mg/dL | 0 | 2 | P = .23 | 6 | 12 | P = .20 |
During the hospital phase, INT patients had 4.3% of patient‐days and UC had 4.5% of patient‐days with any BG < 70 mg/dL. During 12 patient‐stays (20%) in the INT group there was an episode of moderate hypoglycemia, and during 1 patient‐stay (1.7%) there was an episode of severe hypoglycemia. During 6 patient‐stays (9.8%) in the UC group there was an episode of moderate hypoglycemia, and during 6 patient‐stays (9.8%) there was an episode of severe hypoglycemia (Table 2). The odds ratio (OR) for moderate hypoglycemia in the INT group compared with the UC group was 1.93 (95% CI, 0.7‐5.29), but for severe hypoglycemia the OR was 0.15 (95% CI, 0.018‐1.33). Moderate and severe hypoglycemic events in the UC group were split evenly between patients treated with glargine/detemir‐aspart and those treated with NPH‐aspart.
Discussion
This is the first randomized trial comparing the Rush Emergency Department Hyperglycemia Intervention (REDHI) protocol with usual care for the treatment of hyperglycemia in the ED. We believe this may be the first trial to initiate subcutaneous basal insulin therapy in the ED at the time of hospital admission. Initiation of our protocol for type 2 diabetic patients with BG > 200 mg/dL resulted in lower final ED and admission BGs compared with those in the UC group. Although a higher mean initial ED BG of 358 73 mg/dL was required to prompt initiation of insulin therapy for UC patients, 3 experienced severe hypoglycemia. By following the REDHI protocol, ED nurses avoided BG < 50 mg/dL in INT patients. Our first version of the REDHI protocol dosed more insulin than our current version, and we saw excess hypoglycemia.11 With a reduced dosing formula, there was less lowering of BG, but we eliminated all BG < 60 mg/dL. There was a trend toward an increased ED LOS in INT patients compared with UC patients. This may be because of delays in the administration of insulin or the requirement for a final BG check prior to discharge from the ED for INT patients. However, we did not receive feedback from ED nursing that either factor was a significant issue.
During phase 2, we observed improved glycemic control in INT, likely due to two factors: early initiation of basal insulin and protocol driven daily titration of both basal and mealtime insulin. We achieved a mean fasting BG of 148 54 mg/dL in INT the morning after the ED dose of detemir. BG levels in both groups continued to improve each day, but since the admission BG for INT was lower, this group maintained significantly lower BG levels throughout most of the hospitalization. There were also significant differences between groups at different times of day. Basal doses for INT patients were adjusted daily per fasting BG. Scheduled mealtime doses were based on the basal dose; each mealtime aspart dose was a third of the basal detemir dose. Therefore, patients who required larger doses for certain meals may have received less aspart than needed. This may explain why fasting BG control was better than BG control later in the day.
Current Rush guidelines recommend the same insulin doses as those that our intervention used, but patients in the UC group were less likely to have insulin titrated daily. Cook et al found that clinical inertia, or failure of health care providers to initiate or intensify therapy when indicated, is a common problem among medical residents treating inpatients with insulin.15 Reasons for clinical inertia may include unawareness of inpatient glycemic targets, lack of training or confidence in titrating insulin, and concerns regarding hypoglycemia. Our study shows that this is still an operative issue, even after residents have participated in multiple small‐group educational sessions. Details of the Rush inpatient insulin protocol are also on pocket cards distributed to residents. However, fewer than half of UC patients had insulin adjusted appropriately for persistent hyperglycemia. This may be one explanation for the improved control seen in the INT group and underscores the importance of daily dose titrations based on a uniform protocol.
During phase 2, despite improved glycemic control in INT, there was no significant difference in rates of hypoglycemia between the groups. The number of patient‐stays with moderate hypoglycemia was more in the INT group than in the UC group, 12 versus 6, respectively, but not statistically different (P = .20). There was a trend toward fewer patient‐stays with severe hypoglycemia in the INT group than in the UC group, 1 versus 6, respectively (P = .11).
Other studies have described improved inpatient glycemic control without excess hypoglycemia. In the RABBIT 2 trial, institution of a glargine‐glulisine insulin protocol, TTD of 0.4‐5 units/kg, among insulin‐naive inpatients resulted in a mean fasting BG of 147 36 mg/dL and a mean hospital BG of 166 32 mg/dL, with 3% of patient‐stays having a BG < 60 mg/dL.6 In a second trial, detemir‐aspart was compared with NPH‐aspart TTD of 0.4‐5 units/kg.7 Both groups achieved a similar mean fasting BG of 146 mg/dL and a mean hospital BG of 157 mg/dL. However, the rate of hypoglycemia was higher: 29% of patient‐stays overall. In our study, we achieved a mean fasting BG of 135 48 mg/dL and a mean hospital BG of 163 40 mg/dL in the INT group, using a mean initial TTD of 0.65 0.23 units/kg. The frequency of hypoglycemia in this trial, 22% of INT patient‐stays and 20% of UC patient‐stays, was less than that in Umpierrez et al,7 despite a lower mean fasting BG in our current trial. Maynard et al found 16% of patient‐stays and 3% of patient‐days had an episode of BG < 60 mg/dL in a trial of glarginerapid‐acting insulin (0.4‐5 units/kg TTD).12 Schnipper et al found that 6.1% of patient‐days had an episode of BG < 60 mg/dL using either glargine or NPH and a rapid‐acting insulin, TTD 0.6 units/kg.13 Our hypoglycemia rates were higher; however, we defined hypoglycemia as BG < 70 mg/dL, as suggested by the ADA workgroup.14 If we use a cutoff of < 60 mg/dL for hypoglycemia, it occurred in 13.3% of patient‐stays and 4.3% of patient‐days in the INT group, comparable to that in previous studies.
Our study has several limitations. First, this was a single‐center study, and our ED protocol should be tested in other ED settings, both academic and community. Second, although there were trends toward lower rates of severe hypoglycemia in the INT group, the study was underpowered to detect possible significant differences. Third, although ED nurses implemented the study protocol, study staff closely monitored nurses to ensure adherence. Therefore, it is difficult to speculate on protocol adherence under normal circumstances. Successful implementation requires ongoing nursing and medical staff education. A fourth limitation is the absence of patients with type 1 diabetes.
In conclusion we demonstrated that weight‐based subcutaneous aspart insulin therapy begun in the ED, coupled with prompt initiation of a detemir‐aspart insulin protocol, results in rapid correction of hyperglycemia and improved inpatient glycemic control without increasing hypoglycemia. Diabetes is a common comorbidity in patients presenting to the ED that is not uniformly addressed. These patients may present with uncontrolled hyperglycemia or diabetes‐related infections, and prompt, efficacious glucose control is important. The nurse‐driven Rush ED hyperglycemia protocol ensures that hyperglycemia is safely addressed, allowing the ED physician to address more critical issues. By initiating basal insulin in the ED, our protocol allows for a prompt and smooth transition to a basal‐bolus insulin regimen for the inpatient setting.
Current consensus guidelines from the American Diabetes Association and the American Association of Clinical Endocrinologists recommend the use of insulin‐based treatment protocols for most hospitalized patients with hyperglycemia.1 For noncritically ill patients, it is recommended to target a fasting blood glucose (BG) < 140 mg/dL and a random BG < 180‐200 mg/dL, without excess hypoglycemia. Prior studies recommended using a basal‐bolus insulin protocol that specifies starting doses and parameters for dose adjustment, applied by well‐educated teams of physicians and nurses.27 We have shown that insulin detemir given as a once‐daily basal injection coupled with rapid‐acting insulin aspart with meals is an effective regimen for managing hyperglycemia in hospitalized patients with type 2 diabetes.7 We and others have shown that once‐daily basal insulin mealtime rapid‐acting insulin is significantly more effective than sliding‐scale regular insulin in the hospital setting.6, 8
The majority of patients admitted to general medical units are first evaluated in the emergency department (ED), and significant hyperglycemia is not uncommon in ED patients. However, protocols for the treatment of hyperglycemia in the ED have not been widely implemented. Ginde et al studied 160 ED patients with a history of diabetes and BG > 200 mg/dL and found that although 73% were admitted to the hospital, only 31% were treated with insulin, and only 18% had a diagnosis of diabetes charted.9 A recent survey of 152 residents and attendings in 3 academic EDs found that only 32% would give insulin for a BG > 200 mg/dL, 59% for a BG > 250 mg/dL, and 91% for a BG > 300 mg/dL to ED patients with known diabetes.10 We completed a preliminary study of a novel protocol for the administration of subcutaneous insulin aspart in the ED at Rush University in 2008.11 We found that the mean BG was significantly lowered during an ED stay, from 333 to 158 mg/dL, and that the protocol was easily adopted by ED staff, with a low rate of hypoglycemia. Historically, only 35% of hyperglycemic patients with diabetes received insulin in our ED.11 Reasons for limited ED management of hyperglycemia may include presence of more critical issues, time and resource restriction, unfamiliarity with glycemic targets, and concerns regarding hypoglycemia.9, 10
In the current study we focused on 3 questions:
Could we further reduce the risk of hypoglycemia by modifying our original Rush ED insulin protocol? We reduced insulin aspart from 0.1 to 0.05 units/kg for BG 200‐299 mg/dL, from 0.15 to 0.1 units/kg for BG 300‐399 mg/dL, and from 0.2 to 0.15 units/kg for BG 400 mg/dL.
Could we couple our ED insulin aspart protocol with prompt initiation of a detemir‐aspart protocol in those patients who were subsequently admitted to general medical units from the ED?
Would the hospital length of stay, mean BG, and incidence of hypoglycemia be improved by the use of 2 back‐to‐back subcutaneous insulin protocols in a randomized clinical trial compared with the usual care provided in the ED and general medical inpatient units?
Research Design and Methods
From May 2008 through June 2009, patients presenting to the Rush University Medical Center ED with a history of type 2 diabetes and an initial point‐of‐care BG 200 mg/dL were randomized to an intervention group (INT) or to a usual care group (UC) after giving informed consent. Inclusion criteria for the study were: ages 18‐80 years, history of type 2 diabetes for at least 3 months, and prior therapy with dietary management, oral agents, or insulin. Patients were excluded if subsequently found to have diabetic ketoacidosis, hyperosmolar nonketotic syndrome, or critical illness requiring intensive care unit admission/direct surgical intervention. Other exclusion criteria included a positive pregnancy test or an inability to give informed consent secondary to acute drug or alcohol intoxication or active mental illness. Patients with clinically significant liver disease, with ALT or AST > 3 times the upper limit of normal, or with a history of end‐stage renal disease requiring dialysis were also excluded because of their increased risk of hypoglycemia, as they have required a more conservative insulin regimen. Similarly, we excluded patients with a history of type 1 diabetes because our aspart algorithm for the ED had only been tested in a type 2 diabetes population, and we did not want to disrupt the insulin regimen of type 1 diabetes patients, usually glargine‐based or an insulin pump.
The study consisted of 2 phases. Patients randomized to INT or UC in phase 1 stayed in their respective groups through phase 2. After informed consent was obtained by the study staff, implementation of the protocol was carried out by the ED staff. ED nurses were trained in the study protocol. During phase 1, INT patients received subcutaneous aspart every 2 hours while in the ED if BG was > 200 mg/dL. Aspart dosing per protocol was limited to 2 doses. Uncommonly, when a third dose of aspart was needed, physician input was requested. Aspart dosing was weight‐ and BG level based (0.05 units/kg for BG 200‐299 mg/dL, 0.1 units/kg for BG 300‐399 mg/dL, or 0.15 units/kg for BG 400mg/dL; see Supporting Appendix Fig. 1). Regardless of BG level, the ED aspart protocol was discontinued on patient discharge home or admission to the hospital. UC patients received treatment for hyperglycemia at the discretion of their ED physicians. INT subjects who required hospital admission were transitioned to basal‐bolus insulin therapy with detemir and aspart, receiving their first dose of detemir in the ED. Detemir dosing was weight‐based (0.3 units/kg) if the patient was not on home insulin or based on the patient's home dose of insulin (same dose for detemir, unit‐for‐unit conversion from glargine to detemir, or 80% of total NPH dose). If a patient received basal insulin prior to arrival, the first dose of detemir was held until 12 hours after the last dose of NPH or until 20 hours after the last dose of glargine or detemir. We found from our preliminary study that inadvertent overlaps in long‐acting insulin were one cause of hypoglycemia. We compared differences between groups in the final ED BG level, frequency of hypoglycemia, and ED length of stay (LOS).
Patients subsequently admitted to the hospital entered phase 2. During phase 2, INT patients had detemir and premeal aspart titrated by study staff using a predefined protocol (see Supporting Appendix Fig. 2). Detemir was given once daily, 24 hours after the initial ED dose. UC patients had their diabetes managed by medical house staff teams. House staff members have been educated on the Rush inpatient insulin protocol on which the INT protocol was based. The Rush inpatient diabetes protocol is implemented via a single computerized order set in which all patients should receive mealtime insulin using aspart and a basal insulin (glargine or detemir or NPH). We compared differences between groups in the mean admission BG level, mean daily BG level, mean BG level before each meal, hospital LOS, and frequency of hypoglycemic events. Moderate hypoglycemia was defined as a BG between 50 and 69 mg/dL, and severe hypoglycemia was defined as a BG < 50 mg/dL. We also compared the frequency of BG < 60 mg/dL.
The Rush University Medical Center institutional review board approved the study. Statistical analysis was done using SPSS version 11.0. The Student t test was used to determine any significant difference in BG means between the INT and UC groups. The Fisher's exact test or the chi‐square test was used to determine any difference in proportions of hypoglycemic events between INT and UC patients.
Results
Phase 1: Emergency Department
A total of 176 patients were randomized: 87 to the INT group and 89 to the UC group. Baseline characteristics were similar between groups (Table 1). Mean initial ED BG was similar: 300 70 mg/dL for INT patients and 307 82 mg/dL for UC patients. During phase 1, all INT patients were treated with aspart every 2 hours if BG > 200 mg/dL until discharge home or admission to the hospital. They received an initial mean insulin dose of 0.08 0.04 units/kg of subscutaneous aspart. Twenty‐five percent of INT patients received a second dose of aspart, and 3% received a third dose. For UC patients, only 55% received insulin therapy. Aspart was used for all UC patients who received insulin. Of those in the UC group who received insulin, 11% received a second dose for persistent hyperglycemia; none received a third dose. The mean initial ED BG for UC patients who received insulin was 358 73 mg/dL, and they received an initial mean dose of 0.11 0.05 units/kg. UC patients who did not receive insulin had a lower mean initial ED BG, 241 35 mg/dL. The mean final ED BG was 217 71 mg/dL for the INT group and 257 89 mg/dL for the UC group (P < .01; Fig. 1). The mean ED LOS was 30 minutes longer in the INT group (P = .06; Table 1). Sixty INT patients (69%) and 61 UC patients (69%) were admitted to the hospital. Fifty‐six percent of INT patients received the first dose of detemir based on their home insulin dose, and 44% received a weight‐based dose per protocol.

| Intervention (n = 87) | Usual Care (n = 89) | Significance | |
|---|---|---|---|
| |||
| Age (y) | 55 13 | 55 13 | |
| Sex (% male) | 48 | 39 | |
| BMI (kg/m2) | 34 9 | 33 9 | |
| Ethnicity (%) | |||
| African American | 58 | 66 | |
| Hispanic | 24 | 19 | |
| White | 15 | 11 | |
| Other | 3 | 4 | |
| Duration of diabetes (y) | 13 9 | 12 10 | |
| HA1C | 10.4 2.2 | 9.8 2.6 | |
| Insulin treatment at home (%) | 56 | 62 | |
| Presenting complaint/diagnosis (%) | |||
| Cardiac | 20 | 23 | |
| Gastrointestinal | 30 | 23 | |
| Hyperglycemia | 13 | 18 | |
| Infection | 9 | 11 | |
| Initial ED blood glucose (mg/dL) | 300 70 | 307 82 | |
| Final ED blood glucose (mg/dL) | 217 71 | 256 89 | P < .01 |
| ED length of stay (h) | 5.4 1.7 | 4.9 1.9 | P = .06 |
| Patients treated with insulin in ED (%) | 100 | 54 | |
| Initial dose of SQ aspart (units) | 7.9 4.2 | 9.5 4 | |
| ED patients admitted (%) | 69 | 69 | |
| Admission blood glucose (mg/dL) | 184 70 | 224 93 | P < .01 |
| Treatment of hyperglycemia in hospital (%) | |||
| Detemir aspart insulin | 100 | 7 | |
| Glargine aspart insulin | 0 | 36 | |
| NPH aspart insulin | 0 | 34 | |
| Oral agents | 0 | 8 | |
| None | 0 | 15 | |
| Hospital length of stay (days) | 2.7 2.0 | 3.1 1.9 | P = .58 |
Phase 2: Inpatient Setting
In phase 2, mean admission BG was significantly lower in the INT group (184 70 mg/dL) than in the UC group (223 93 mg/dL), P < .01, as a result of aspart given in the ED. The day 1 mean fasting BG for INT patients was 148 54 mg/dL, significantly lower than the day 1 mean fasting BG for UC patients: 212 81 mg/dL (P < .01). The mean fasting BG for the entire hospitalization was significantly lower for INT patients, 135 48 mg/dL, than for UC patients, 185 72 mg/dL (P < .01). During phase 2, all INT patients had detemir and aspart titrated daily per protocol. Treatment of UC patients was as follows: 78.5% with insulin, 8.2% with oral agents, and 11.5% did not receive medication for hyperglycemia. Of those in the UC group who received insulin, 36.0% were treated with lantus/aspart or detemir/aspart, 34.4% with NPH/aspart, 6.5% with lantus or detemir alone, and 1.6% with aspart alone. Overall, 76.9% of UC received basal insulin, and 70.4% received nutritional insulin. Only 47% of UC patients had insulin adjusted on a daily basis despite persistent hyperglycemia. Significant differences were also seen between INT and UC patients in mean prelunch and predinner BG levels, but not in mean bedtime BG level (Fig. 2). Mean daily BG levels for the initial 5 days of inpatient stay were significantly lower in the INT group (P < .01), except for day 5, when only 21 patients remained in the study (Fig. 3). Patient‐day weighted mean glucose was 163 39 mg/dL for INT patients versus 202 39 mg/dL for UC patients (P < .01). On admission, day 1 mean insulin total daily dose (TDD) was 0.65 0.26 units/kg for INT patients and 0.52 0.29 units/kg for UC patients. The final mean TDD was 0.75 0.35 units/kg for INT patients and 0.61 0.38 units/kg for UC patients. Mean hospital LOS was 9.6 hours shorter for INT patients (2.7 2 days) than for UC patients (3.1 1.9 days), P = .58.


Patient Safety: Frequency of Hypoglycemia
The frequency of hypoglycemia is shown in Table 2. During the ED phase, 3 UC patients (3.4%) had a BG < 50 mg/dL, and 2 INT patients (2.3%) had a BG of 67 mg/dL.
| Blood Glucose | Emergency Department (Number of Episodes) | Significance | Inpatient Phase (Patient Stays) | Significance | ||
|---|---|---|---|---|---|---|
| Usual Care | Intervention | Usual Care | Intervention | |||
| < 50 mg/dL | 3 | 0 | P = .50 | 6 | 1 | P = .11 |
| < 60 mg/dL | 3 | 0 | P = .50 | 7 | 8 | P = .98 |
| 5069 mg/dL | 0 | 2 | P = .23 | 6 | 12 | P = .20 |
During the hospital phase, INT patients had 4.3% of patient‐days and UC had 4.5% of patient‐days with any BG < 70 mg/dL. During 12 patient‐stays (20%) in the INT group there was an episode of moderate hypoglycemia, and during 1 patient‐stay (1.7%) there was an episode of severe hypoglycemia. During 6 patient‐stays (9.8%) in the UC group there was an episode of moderate hypoglycemia, and during 6 patient‐stays (9.8%) there was an episode of severe hypoglycemia (Table 2). The odds ratio (OR) for moderate hypoglycemia in the INT group compared with the UC group was 1.93 (95% CI, 0.7‐5.29), but for severe hypoglycemia the OR was 0.15 (95% CI, 0.018‐1.33). Moderate and severe hypoglycemic events in the UC group were split evenly between patients treated with glargine/detemir‐aspart and those treated with NPH‐aspart.
Discussion
This is the first randomized trial comparing the Rush Emergency Department Hyperglycemia Intervention (REDHI) protocol with usual care for the treatment of hyperglycemia in the ED. We believe this may be the first trial to initiate subcutaneous basal insulin therapy in the ED at the time of hospital admission. Initiation of our protocol for type 2 diabetic patients with BG > 200 mg/dL resulted in lower final ED and admission BGs compared with those in the UC group. Although a higher mean initial ED BG of 358 73 mg/dL was required to prompt initiation of insulin therapy for UC patients, 3 experienced severe hypoglycemia. By following the REDHI protocol, ED nurses avoided BG < 50 mg/dL in INT patients. Our first version of the REDHI protocol dosed more insulin than our current version, and we saw excess hypoglycemia.11 With a reduced dosing formula, there was less lowering of BG, but we eliminated all BG < 60 mg/dL. There was a trend toward an increased ED LOS in INT patients compared with UC patients. This may be because of delays in the administration of insulin or the requirement for a final BG check prior to discharge from the ED for INT patients. However, we did not receive feedback from ED nursing that either factor was a significant issue.
During phase 2, we observed improved glycemic control in INT, likely due to two factors: early initiation of basal insulin and protocol driven daily titration of both basal and mealtime insulin. We achieved a mean fasting BG of 148 54 mg/dL in INT the morning after the ED dose of detemir. BG levels in both groups continued to improve each day, but since the admission BG for INT was lower, this group maintained significantly lower BG levels throughout most of the hospitalization. There were also significant differences between groups at different times of day. Basal doses for INT patients were adjusted daily per fasting BG. Scheduled mealtime doses were based on the basal dose; each mealtime aspart dose was a third of the basal detemir dose. Therefore, patients who required larger doses for certain meals may have received less aspart than needed. This may explain why fasting BG control was better than BG control later in the day.
Current Rush guidelines recommend the same insulin doses as those that our intervention used, but patients in the UC group were less likely to have insulin titrated daily. Cook et al found that clinical inertia, or failure of health care providers to initiate or intensify therapy when indicated, is a common problem among medical residents treating inpatients with insulin.15 Reasons for clinical inertia may include unawareness of inpatient glycemic targets, lack of training or confidence in titrating insulin, and concerns regarding hypoglycemia. Our study shows that this is still an operative issue, even after residents have participated in multiple small‐group educational sessions. Details of the Rush inpatient insulin protocol are also on pocket cards distributed to residents. However, fewer than half of UC patients had insulin adjusted appropriately for persistent hyperglycemia. This may be one explanation for the improved control seen in the INT group and underscores the importance of daily dose titrations based on a uniform protocol.
During phase 2, despite improved glycemic control in INT, there was no significant difference in rates of hypoglycemia between the groups. The number of patient‐stays with moderate hypoglycemia was more in the INT group than in the UC group, 12 versus 6, respectively, but not statistically different (P = .20). There was a trend toward fewer patient‐stays with severe hypoglycemia in the INT group than in the UC group, 1 versus 6, respectively (P = .11).
Other studies have described improved inpatient glycemic control without excess hypoglycemia. In the RABBIT 2 trial, institution of a glargine‐glulisine insulin protocol, TTD of 0.4‐5 units/kg, among insulin‐naive inpatients resulted in a mean fasting BG of 147 36 mg/dL and a mean hospital BG of 166 32 mg/dL, with 3% of patient‐stays having a BG < 60 mg/dL.6 In a second trial, detemir‐aspart was compared with NPH‐aspart TTD of 0.4‐5 units/kg.7 Both groups achieved a similar mean fasting BG of 146 mg/dL and a mean hospital BG of 157 mg/dL. However, the rate of hypoglycemia was higher: 29% of patient‐stays overall. In our study, we achieved a mean fasting BG of 135 48 mg/dL and a mean hospital BG of 163 40 mg/dL in the INT group, using a mean initial TTD of 0.65 0.23 units/kg. The frequency of hypoglycemia in this trial, 22% of INT patient‐stays and 20% of UC patient‐stays, was less than that in Umpierrez et al,7 despite a lower mean fasting BG in our current trial. Maynard et al found 16% of patient‐stays and 3% of patient‐days had an episode of BG < 60 mg/dL in a trial of glarginerapid‐acting insulin (0.4‐5 units/kg TTD).12 Schnipper et al found that 6.1% of patient‐days had an episode of BG < 60 mg/dL using either glargine or NPH and a rapid‐acting insulin, TTD 0.6 units/kg.13 Our hypoglycemia rates were higher; however, we defined hypoglycemia as BG < 70 mg/dL, as suggested by the ADA workgroup.14 If we use a cutoff of < 60 mg/dL for hypoglycemia, it occurred in 13.3% of patient‐stays and 4.3% of patient‐days in the INT group, comparable to that in previous studies.
Our study has several limitations. First, this was a single‐center study, and our ED protocol should be tested in other ED settings, both academic and community. Second, although there were trends toward lower rates of severe hypoglycemia in the INT group, the study was underpowered to detect possible significant differences. Third, although ED nurses implemented the study protocol, study staff closely monitored nurses to ensure adherence. Therefore, it is difficult to speculate on protocol adherence under normal circumstances. Successful implementation requires ongoing nursing and medical staff education. A fourth limitation is the absence of patients with type 1 diabetes.
In conclusion we demonstrated that weight‐based subcutaneous aspart insulin therapy begun in the ED, coupled with prompt initiation of a detemir‐aspart insulin protocol, results in rapid correction of hyperglycemia and improved inpatient glycemic control without increasing hypoglycemia. Diabetes is a common comorbidity in patients presenting to the ED that is not uniformly addressed. These patients may present with uncontrolled hyperglycemia or diabetes‐related infections, and prompt, efficacious glucose control is important. The nurse‐driven Rush ED hyperglycemia protocol ensures that hyperglycemia is safely addressed, allowing the ED physician to address more critical issues. By initiating basal insulin in the ED, our protocol allows for a prompt and smooth transition to a basal‐bolus insulin regimen for the inpatient setting.
- ,,, et al.American Association of Clinical Endocrinologists and American Diabetes Association Consensus Statement on Inpatient Glycemic Control.Diabetes Care.2009;32:1119–1131.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,Eliminating inpatient sliding scale insulin: a re‐education project with medical house staff.Diabetes Care.2008;28:1008–1011.
- ,,, et al.Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy.Diabetes Care.2007;30:823–828.
- ,,.Comparison of once daily glargine insulin with twice‐daily NPH/Regular insulin for control of hyperglycemia in inpatients after cardiovascular surgery.Diabetes Technol Ther.2006;8:609–616.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,, et al.Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine Hagedorn plus regular in medical patients with type 2 diabetes.J Clin Endocrinol Metab.2009;94:564–569.
- ,,,.Once daily insulin glargine vs. six hourly sliding scale regular insulin for control of hyperglycemia after bariatric surgery: a randomized clinical trial.Endocr Pract.2007;13:225–231.
- ,,.Limited communication and management of emergency department hyperglycemia in hospitalized patients.J Hosp Med.2009;4:44–49.
- ,,,.Multicenter survey of emergency physician management and referral for hyperglycemia.J Emerg Med.2010;38:264–272.
- ,,,,Impact of a nurse‐driven subcutaneous insulin protocol: Rush Emergency Department Hyperglycemia Intervention (REDHI).J Emerg Med.2008 [Epub ahead of print].
- ,,,,.Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm.J Hosp Med.2009;4:3–15.
- ,,,.Effects of a subcutaneous insulin protocol, clinical education, and computerized order set on the quality of inpatient management of hyperglycemia: results of a clinical trial.J Hosp Med.2009;4:16–27.
- ADA Workgroup on Hypoglycemia.Defining and reporting hypoglycemia in diabetes.Diabetes Care.2005;28:1245–1249.
- ,, et al.Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- ,,, et al.American Association of Clinical Endocrinologists and American Diabetes Association Consensus Statement on Inpatient Glycemic Control.Diabetes Care.2009;32:1119–1131.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,Eliminating inpatient sliding scale insulin: a re‐education project with medical house staff.Diabetes Care.2008;28:1008–1011.
- ,,, et al.Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy.Diabetes Care.2007;30:823–828.
- ,,.Comparison of once daily glargine insulin with twice‐daily NPH/Regular insulin for control of hyperglycemia in inpatients after cardiovascular surgery.Diabetes Technol Ther.2006;8:609–616.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,, et al.Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine Hagedorn plus regular in medical patients with type 2 diabetes.J Clin Endocrinol Metab.2009;94:564–569.
- ,,,.Once daily insulin glargine vs. six hourly sliding scale regular insulin for control of hyperglycemia after bariatric surgery: a randomized clinical trial.Endocr Pract.2007;13:225–231.
- ,,.Limited communication and management of emergency department hyperglycemia in hospitalized patients.J Hosp Med.2009;4:44–49.
- ,,,.Multicenter survey of emergency physician management and referral for hyperglycemia.J Emerg Med.2010;38:264–272.
- ,,,,Impact of a nurse‐driven subcutaneous insulin protocol: Rush Emergency Department Hyperglycemia Intervention (REDHI).J Emerg Med.2008 [Epub ahead of print].
- ,,,,.Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm.J Hosp Med.2009;4:3–15.
- ,,,.Effects of a subcutaneous insulin protocol, clinical education, and computerized order set on the quality of inpatient management of hyperglycemia: results of a clinical trial.J Hosp Med.2009;4:16–27.
- ADA Workgroup on Hypoglycemia.Defining and reporting hypoglycemia in diabetes.Diabetes Care.2005;28:1245–1249.
- ,, et al.Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
Copyright © 2010 Society of Hospital Medicine