User login
Age-related macular degeneration: Options for earlier detection and improved treatment
- Advanced age, positive family history, smoking, hypertension, and Caucasian ancestry are risk factors consistently associated with age-related macular degeneration (ARMD).
- With older patients, suspect ARMD if vision has decreased or if Amsler grid testing reveals scotoma or metamorphopsia (C).
- Refer any patient with suspected ARMD for an ophthalmology consultation (C).
- Lifestyle modifications, vitamin supplementation, and low-vision aids can slow or mitigate the effects of ARMD (A).
Age-related macular degeneration (ARMD) is no longer the ocular death sentence it once was. Therapies are improving and becoming more widely available.
Though everyone over 40 should have an ophthalmologic exam every year or 2, many patients that may routinely see their primary care physician will not consult an ophthalmologist until a problem manifests (see Demographics of ARMD). With simple, in-office testing described in this article, you can help alert patients to the need for ophthalmologic follow-up.
Age-related macular degeneration (ARMD) is a progressive eye disease that can result in the loss of the center of our field of vision due to damage to the macula, the most important portion of the retina. It is the leading cause of central vision loss in people over the age of 50 years in the United States1 and the resulting functional loss can be devastating. Over a 5-year time span, it is estimated that 1 in 3 people over the age of 70 years will develop signs of ARMD2 and that 315,000 Americans over the age of 74 years will develop signs of advanced ARMD.3 Since the risk of ARMD increases with age,1 the number of patients with this disease will increase as our population grows older.
5 Consistent risk factors
Several large, population-based studies have identified age, family history, smoking, hypertension, and Caucasian ancestry as risk factors consistently associated with ARMD.4 Other risk factors such as atherosclerosis, ultraviolet (UV) exposure, farsightedness, increased body-mass index, and cataracts4-6 have been suggested, but not consistently proven to increase risk.
The risk of developing ARMD increases with age.1 According to the Framingham Eye study, a person between the ages of 65 and 74 has a 6% chance of exhibiting ophthalmoscopic evidence of ARMD. For those 75 or older, the chances are almost 20%.1,7
The Beaver Dam Eye Study and Rotterdam Study looked at families with ARMD and found an odds ratio as high as 10:1 for siblings7,8 and 6:1 for the offspring of ARMD patients.7 Combining these risk factors, a person who lives to be 85 and has a first-degree relative with ARMD has a 48% chance of developing clinically detectable ARMD.7
Smoking and hypertension also correlate strongly with ARMD.4,6 Patients who have ARMD and continue to smoke are 3.6 times more likely to progress to the end stages of the disease with severe functional compromise. Even when patients quit smoking, the risk remains elevated for the next 20 years.9 Studies of ARMD and systemic hypertension show an odds ratio for clinically detectable dry ARMD at 2:1 for patients with a systolic reading of 160 mm Hg or greater, and an odds ratio of 4:1 for wet ARMD if the diastolic reading is greater than 95 mm Hg.10,11
Diagnosing ARMD
Though a dilated fundus exam remains the gold standard for diagnosing ARMD, other in-office diagnostic tools are also available.
Specific clues in the history
In addition to inquiring about a family history of ARMD, ask patients if they have difficulty recognizing faces or if words seem to disappear while reading. ARMD patients may also notice that telephone poles appear crooked.
Testing visual acuity
With the patient wearing a current pair of reading glasses, test one eye at a time in good lighting with a near vision card at 12 to 14 inches from the patient’s face. Though many disorders can cause decreased vision, a reading worse than 20/30 in an elderly patient could indicate undiagnosed ARMD. If the patient has noticed a recent decrease in vision, an immediate ophthalmology referral is indicated. If near vision has gradually worsened, referral is needed but perhaps not as urgently.
Amsler grid test for earlier diagnosis
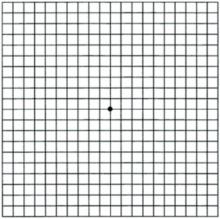
Amsler grid testing can detect ARMD (as well as other macular diseases) at a much earlier stage than can visual acuity testing alone. The grid (FIGURE 1; for a printable version, go to www.jfponline.com) is read with one eye at a time at a distance of 12 to 14 inches with reading glasses under good lighting conditions. Instruct the patient to focus only on the center dot in the grid and to view the remainder of the grid with peripheral vision and without looking around. If the patient reports that lines are missing (known as a scotoma) or appear wavy (known as metamorphopsia), the test is positive and prompt referral is indicated. The location of the distorted or missing lines corresponds closely with the location of the macular lesion.
FIGURE 1
Amsler grid
Patients with known ARMD can monitor their disease by performing this test once a day. This grid is shown actual size; a printable version is found at www.jfponline.com.
Changes seen on dilated fundus exam
To view the macula, ask the patient to look directly at the light during direct fundoscopy.
Dry ARMD displays drusen and changes in the retinal pigment epithelium. Drusen are discrete, round yellow lesions. Changes in the retinal pigment epithelium may be subtle tiny darkly colored patches in the macula near areas of lighter patches (see How ARMD develops).
Wet ARMD may appear as a dark red or green discoloration in the macula and requires immediate referral.
End-stage ARMD may demonstrate a well-delineated geographic pattern corresponding to retinal atrophy or scarring. Large blood vessels are usually visible in the base of these areas. Any macular lesions found in a patient without a diagnosis of ARMD or other maculopathy must have an ophthalmology referral (TABLE 1).
TABLE 1
Referral guide for age-related macular degeneration
| GROUP 1 | IMMEDIATE REFERRAL (THAT DAY) |
| New onset of decreased vision of unknown cause | |
| New onset of metamorphopsia (crooked telephone poles or new findings on the Amsler grid test) | |
| New onset of a scotoma (losing words while reading) | |
| Retinal hemorrhages | |
| GROUP 2 | PROMPT REFERRAL (WITHIN 1 MONTH) |
| Drusen or retinal pigment epithelium changes seen without a previous diagnosis of ARMD | |
| History of long-standing metamorphopsia or scotoma without a previous diagnosis of ARMD | |
| GROUP 3 | ROUTINE REFERRAL (WITHIN 6 MONTHS) |
| Diagnosis of ARMD, but has not seen an ophthalmologist in more than 1 year | |
| Age over 60, but has not seen an ophthalmologist in more than 2 years | |
Pathologically, ARMD damages the macula in 1 of 2 ways, giving rise to the 2 forms of ARMD, wet and dry. Dry ARMD accounts for 80% of all cases of ARMD.12 In this form, the retinal pigment epithelium (RPE) degenerates as a result of the intracellular deposition of yellow metabolic waste products known as drusen. Drusen are typically accompanied by subtle, progressive changes in the pigmentation of the RPE (FIGURE 2). Without the nutritional support of the RPE, the overlying retina cells slowly atrophy causing a loss of central visual acuity. Wet ARMD is less common, but accounts for 80% of the severe vision loss from ARMD.12 In this form, neovascularization from the arterial system underlying the RPE breaks through the RPE barrier and quickly spreads underneath the retina, causing bleeding and fibrosis which can rapidly destroy the macula (FIGURE 3).
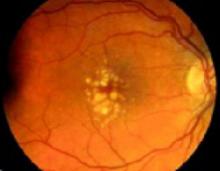
FIGURE 2
Dry ARMD
Dry ARMD demonstrating yellow-white drusen with areas of patchy hyperpigmentation of the retinal pigment epithelium. This patient has 20/30 vision and metamorphopsia on Amsler grid testing.
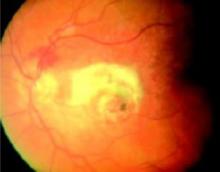
FIGURE 3
Wet ARMD
The greenish hue indicates bleeding under the retina. The yellow-white lesions represent sub-retinal scarring. This patient has 20/400 vision and a large scotoma on Amsler grid testing.
Treatment for dry macular degeneration
Vitamins confer modest benefit
Some patients with dry ARMD may benefit modestly from certain over-the-counter vitamin supplements. The Age-Related Eye Disease Study found that patients with extensive drusen but no advanced macular degeneration in either eye had an 11% chance of going from legal driving vision to legal blindness within 5 years. However, those who took the once-a-day formulation13 shown in TABLE 2 exhibited a modest drop in risk to 10% over 5 years. More encouraging, patients with advanced macular degeneration in the control eye reduced their chances of legal blindness from 28% to 17% with the supplement (strength of recommendation [SOR]: A14; level of evidence [LOE]: 1, randomized controlled trial [RCT]).
Ten mg of Lutein alone or combined with antioxidants has shown some promise in short-term studies15 (SOR: B; LOE: 2, RCT), but further research will be required.
TABLE 2
Recommended daily vitamin doses for dry ARMD patients
| VITAMIN | DOSE |
|---|---|
| Zinc | 80 mg |
| Vitamin C | 500 mg |
| Vitamin E | 400 IU |
| Beta-carotene | 15 mg |
| Copper | 2 mg |
| The recommended once a day dose from the Age-Related Eye Disease Study. Copper was included in the formulation to prevent a zinc-induced copper deficient anemia.13 It should also be noted that smokers may be at an increased risk of lung cancer from excessive beta-carotene use13 and special formulations for smokers are available. | |
Treatment for wet macular degeneration
Laser treatment most common choice
Though many types of therapy are available, patients with wet ARMD will usually undergo photodynamic therapy or conventional laser therapy.
Photodynamic therapy uses a photosensitizing agent with an affinity for neovascular tissue. After injection into the bloodstream, the agent is light-activated to induce thrombosis of the neovascular vessels.16 The Treatment of Age-Related Macular Degeneration with Photodynamic Therapy study showed that treated patients had a greater chance of retaining their vision compared with untreated patients, and that about 1 in 6 treated patients experienced some improvement in vision (SOR: A; LOE: 1, RCT).17 Unfortunately, recurrence of neovascular tissue is common and retreatment is often necessary.
Conventional thermal laser therapy directly coagulates the neovascular area. The Macular Photocoagulation Study showed that thermal laser therapy reduced the likelihood of losing 3 to 6 lines of vision (for example, going from 20/20 to 20/70) after 2 years of follow-up (SOR: A; LOE: 1, RCT).18 Conventional laser therapy requires retreatment less often than photodynamic therapy. However, it is applicable only to certain subtypes of wet ARMD and may immediately cause vision loss if used too close to the center of the macula.
Surgery usually not recommended
Surgical removal of neovascular tissue in wet ARMD has succeeded anatomically but has not yielded consistent results in protecting vision. Using microsurgical instruments, the surgeon can separate the macula from the retinal pigment epithelium and remove the neovascular tissue through a small incision in the retina. Though the Submacular Surgery Trials did find a subset of patients who benefited from this procedure, overall results did not support the use of this surgery (SOR: A; LOE: 1, RCT).19
Pegaptanib injections show promise
The newest treatment for wet ARMD is pegaptanib (Macugen). Pegaptanib is a macromolecule designed specifically to bind to VEGF165 isoform, thus blocking its angiogenic and permeability enhancing activity. It is injected directly into the vitreous cavity of patients with wet ARMD. Study results have encouragingly shown a significant reduction in moderate and severe visual loss at 12 months of follow-up (SOR: A; LOE: 1, RCT).20
Measures for wet and dry macular degeneration
Smoking cessation most important lifestyle change
Of all the treatment options for patients with wet or dry ARMD, the most effective means of preserving vision is smoking cessation. As mentioned, controlling blood pressure is also an important modifiable risk factor. Lowering cholesterol or decreasing body mass index may help prevent vision loss, but the literature does not consistently support these claims.
Counseling
Instruct patients with ARMD of either type on how to use the Amsler grid at home. They should understand that any changes on the grid should prompt a call to their ophthalmologist.
Patients with dry ARMD should understand that while the disease is progressive, it does so very slowly.
Patients with wet ARMD and those with end-stage dry ARMD should be counseled that, unless another ocular disease is present, they will not go blind. They can maintain normal peripheral vision. If their central vision is 20/200 or worse in the better eye with glasses, they are considered “legally blind” (in most states) and may qualify for certain disability help.
Low-vision aids
Even in the most advanced cases of ARMD, patients must never be told that nothing more can be done. Low-vision devices are available to aid and restore the functional needs of patients. These range from simple handheld magnifiers to closed-circuit television scanners. Inquire of your local ophthalmologist if he or she incorporates low-vision aid training into his practice. If not, it may be necessary to refer to a low-vision clinic at a larger institution.
Acknowledgments
This work was supported in part by unrestricted grants from Research to Prevent Blindness and the Pat & Willard Walker Eye Research Center.
CORRESPONDENCE
Michael N. Wiggins, MD, Jones Eye Institute, University of Arkansas for Medical Sciences, 4301 West Markham St. Slot 523, Little Rock, AR 72205-7199. E-mail: [email protected]
1. American Academy of Ophthalmology. Basic and Clinical Science Course, Section 12, Retina, ed. 2004-2005. San Francisco, Calif: LEO; 2004.
2. Mukesh BN, Dimitrov PN, Leikin S, et al. Five-year incidence of age-related maculopathy: the Visual Impairment Project. Ophthalmology 2004;111:1176-1182.
3. Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 1997;104:7-21.
4. Klein R, Peto T, Bird A, Vannewkirk M. The epidemiology of age related macular degeneration. Am J Ophthalmol 2004;137:486-495.
5. Tomany S, Cruickshanks K, Klein R, et al. Sunlight and the 10-year incidence of age-related maculopathy: The Beaver Dam Eye Study. Arch Ophthalmol 2004;122:750-757.
6. Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 2000;107:2224-2232.
7. Klaver C, Wolfs R, Assink J, et al. Genetic risk of age-related maculopathy: Population-based familial aggregation study. Arch Ophthalmol 1998;116:1646-1651.
8. Klein B, Klein R, Lee K, et al. Risk of incident age-related eye diseases in people with an affected sibling: The Beaver Dam Eye Study. Am J Epidemiol 2001;154:207-211.
9. Delcourt C, Diaz J, Ponton-Sanchez A, Papoz L. Smoking and age-related macular degeneration: The POLA Study. Arch Ophthalmol 1998;116:1031-1035.
10. van Leeuwen R, Ikram MK, Vingerling JR, Witteman JC, Hofman A, de Jong PT. Blood pressure, atherosclerosis, and the incidence of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci 2003;44:3771-3777.
11. Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol 2000;118:351-358.
12. Ferris FL, 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol 1984;102:1640-1642.
13. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001;119:1417-1436.
14. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53(2):111-120.
15. Richer S, Stiles W, Statkute L, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004;75:216-230.
16. Yanoff M, Duker J. Ophthalmology 2nd ed. St Louis, Mo: Mosby; 2004.
17. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol 1999;117:1329-1345.
18. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol 1993;111:1200-1209.
19. Hawkins BS, Bressler NM, Miskala PH, et al. Submacular Surgery Trials (SST) Research Group. Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: ophthalmic findings: SST report no. 11. Ophthalmology 2004;111:1967-1980.
20. Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805-2516.
- Advanced age, positive family history, smoking, hypertension, and Caucasian ancestry are risk factors consistently associated with age-related macular degeneration (ARMD).
- With older patients, suspect ARMD if vision has decreased or if Amsler grid testing reveals scotoma or metamorphopsia (C).
- Refer any patient with suspected ARMD for an ophthalmology consultation (C).
- Lifestyle modifications, vitamin supplementation, and low-vision aids can slow or mitigate the effects of ARMD (A).
Age-related macular degeneration (ARMD) is no longer the ocular death sentence it once was. Therapies are improving and becoming more widely available.
Though everyone over 40 should have an ophthalmologic exam every year or 2, many patients that may routinely see their primary care physician will not consult an ophthalmologist until a problem manifests (see Demographics of ARMD). With simple, in-office testing described in this article, you can help alert patients to the need for ophthalmologic follow-up.
Age-related macular degeneration (ARMD) is a progressive eye disease that can result in the loss of the center of our field of vision due to damage to the macula, the most important portion of the retina. It is the leading cause of central vision loss in people over the age of 50 years in the United States1 and the resulting functional loss can be devastating. Over a 5-year time span, it is estimated that 1 in 3 people over the age of 70 years will develop signs of ARMD2 and that 315,000 Americans over the age of 74 years will develop signs of advanced ARMD.3 Since the risk of ARMD increases with age,1 the number of patients with this disease will increase as our population grows older.
5 Consistent risk factors
Several large, population-based studies have identified age, family history, smoking, hypertension, and Caucasian ancestry as risk factors consistently associated with ARMD.4 Other risk factors such as atherosclerosis, ultraviolet (UV) exposure, farsightedness, increased body-mass index, and cataracts4-6 have been suggested, but not consistently proven to increase risk.
The risk of developing ARMD increases with age.1 According to the Framingham Eye study, a person between the ages of 65 and 74 has a 6% chance of exhibiting ophthalmoscopic evidence of ARMD. For those 75 or older, the chances are almost 20%.1,7
The Beaver Dam Eye Study and Rotterdam Study looked at families with ARMD and found an odds ratio as high as 10:1 for siblings7,8 and 6:1 for the offspring of ARMD patients.7 Combining these risk factors, a person who lives to be 85 and has a first-degree relative with ARMD has a 48% chance of developing clinically detectable ARMD.7
Smoking and hypertension also correlate strongly with ARMD.4,6 Patients who have ARMD and continue to smoke are 3.6 times more likely to progress to the end stages of the disease with severe functional compromise. Even when patients quit smoking, the risk remains elevated for the next 20 years.9 Studies of ARMD and systemic hypertension show an odds ratio for clinically detectable dry ARMD at 2:1 for patients with a systolic reading of 160 mm Hg or greater, and an odds ratio of 4:1 for wet ARMD if the diastolic reading is greater than 95 mm Hg.10,11
Diagnosing ARMD
Though a dilated fundus exam remains the gold standard for diagnosing ARMD, other in-office diagnostic tools are also available.
Specific clues in the history
In addition to inquiring about a family history of ARMD, ask patients if they have difficulty recognizing faces or if words seem to disappear while reading. ARMD patients may also notice that telephone poles appear crooked.
Testing visual acuity
With the patient wearing a current pair of reading glasses, test one eye at a time in good lighting with a near vision card at 12 to 14 inches from the patient’s face. Though many disorders can cause decreased vision, a reading worse than 20/30 in an elderly patient could indicate undiagnosed ARMD. If the patient has noticed a recent decrease in vision, an immediate ophthalmology referral is indicated. If near vision has gradually worsened, referral is needed but perhaps not as urgently.
Amsler grid test for earlier diagnosis
Amsler grid testing can detect ARMD (as well as other macular diseases) at a much earlier stage than can visual acuity testing alone. The grid (FIGURE 1; for a printable version, go to www.jfponline.com) is read with one eye at a time at a distance of 12 to 14 inches with reading glasses under good lighting conditions. Instruct the patient to focus only on the center dot in the grid and to view the remainder of the grid with peripheral vision and without looking around. If the patient reports that lines are missing (known as a scotoma) or appear wavy (known as metamorphopsia), the test is positive and prompt referral is indicated. The location of the distorted or missing lines corresponds closely with the location of the macular lesion.
FIGURE 1
Amsler grid
Patients with known ARMD can monitor their disease by performing this test once a day. This grid is shown actual size; a printable version is found at www.jfponline.com.
Changes seen on dilated fundus exam
To view the macula, ask the patient to look directly at the light during direct fundoscopy.
Dry ARMD displays drusen and changes in the retinal pigment epithelium. Drusen are discrete, round yellow lesions. Changes in the retinal pigment epithelium may be subtle tiny darkly colored patches in the macula near areas of lighter patches (see How ARMD develops).
Wet ARMD may appear as a dark red or green discoloration in the macula and requires immediate referral.
End-stage ARMD may demonstrate a well-delineated geographic pattern corresponding to retinal atrophy or scarring. Large blood vessels are usually visible in the base of these areas. Any macular lesions found in a patient without a diagnosis of ARMD or other maculopathy must have an ophthalmology referral (TABLE 1).
TABLE 1
Referral guide for age-related macular degeneration
| GROUP 1 | IMMEDIATE REFERRAL (THAT DAY) |
| New onset of decreased vision of unknown cause | |
| New onset of metamorphopsia (crooked telephone poles or new findings on the Amsler grid test) | |
| New onset of a scotoma (losing words while reading) | |
| Retinal hemorrhages | |
| GROUP 2 | PROMPT REFERRAL (WITHIN 1 MONTH) |
| Drusen or retinal pigment epithelium changes seen without a previous diagnosis of ARMD | |
| History of long-standing metamorphopsia or scotoma without a previous diagnosis of ARMD | |
| GROUP 3 | ROUTINE REFERRAL (WITHIN 6 MONTHS) |
| Diagnosis of ARMD, but has not seen an ophthalmologist in more than 1 year | |
| Age over 60, but has not seen an ophthalmologist in more than 2 years | |
Pathologically, ARMD damages the macula in 1 of 2 ways, giving rise to the 2 forms of ARMD, wet and dry. Dry ARMD accounts for 80% of all cases of ARMD.12 In this form, the retinal pigment epithelium (RPE) degenerates as a result of the intracellular deposition of yellow metabolic waste products known as drusen. Drusen are typically accompanied by subtle, progressive changes in the pigmentation of the RPE (FIGURE 2). Without the nutritional support of the RPE, the overlying retina cells slowly atrophy causing a loss of central visual acuity. Wet ARMD is less common, but accounts for 80% of the severe vision loss from ARMD.12 In this form, neovascularization from the arterial system underlying the RPE breaks through the RPE barrier and quickly spreads underneath the retina, causing bleeding and fibrosis which can rapidly destroy the macula (FIGURE 3).
FIGURE 2
Dry ARMD
Dry ARMD demonstrating yellow-white drusen with areas of patchy hyperpigmentation of the retinal pigment epithelium. This patient has 20/30 vision and metamorphopsia on Amsler grid testing.
FIGURE 3
Wet ARMD
The greenish hue indicates bleeding under the retina. The yellow-white lesions represent sub-retinal scarring. This patient has 20/400 vision and a large scotoma on Amsler grid testing.
Treatment for dry macular degeneration
Vitamins confer modest benefit
Some patients with dry ARMD may benefit modestly from certain over-the-counter vitamin supplements. The Age-Related Eye Disease Study found that patients with extensive drusen but no advanced macular degeneration in either eye had an 11% chance of going from legal driving vision to legal blindness within 5 years. However, those who took the once-a-day formulation13 shown in TABLE 2 exhibited a modest drop in risk to 10% over 5 years. More encouraging, patients with advanced macular degeneration in the control eye reduced their chances of legal blindness from 28% to 17% with the supplement (strength of recommendation [SOR]: A14; level of evidence [LOE]: 1, randomized controlled trial [RCT]).
Ten mg of Lutein alone or combined with antioxidants has shown some promise in short-term studies15 (SOR: B; LOE: 2, RCT), but further research will be required.
TABLE 2
Recommended daily vitamin doses for dry ARMD patients
| VITAMIN | DOSE |
|---|---|
| Zinc | 80 mg |
| Vitamin C | 500 mg |
| Vitamin E | 400 IU |
| Beta-carotene | 15 mg |
| Copper | 2 mg |
| The recommended once a day dose from the Age-Related Eye Disease Study. Copper was included in the formulation to prevent a zinc-induced copper deficient anemia.13 It should also be noted that smokers may be at an increased risk of lung cancer from excessive beta-carotene use13 and special formulations for smokers are available. | |
Treatment for wet macular degeneration
Laser treatment most common choice
Though many types of therapy are available, patients with wet ARMD will usually undergo photodynamic therapy or conventional laser therapy.
Photodynamic therapy uses a photosensitizing agent with an affinity for neovascular tissue. After injection into the bloodstream, the agent is light-activated to induce thrombosis of the neovascular vessels.16 The Treatment of Age-Related Macular Degeneration with Photodynamic Therapy study showed that treated patients had a greater chance of retaining their vision compared with untreated patients, and that about 1 in 6 treated patients experienced some improvement in vision (SOR: A; LOE: 1, RCT).17 Unfortunately, recurrence of neovascular tissue is common and retreatment is often necessary.
Conventional thermal laser therapy directly coagulates the neovascular area. The Macular Photocoagulation Study showed that thermal laser therapy reduced the likelihood of losing 3 to 6 lines of vision (for example, going from 20/20 to 20/70) after 2 years of follow-up (SOR: A; LOE: 1, RCT).18 Conventional laser therapy requires retreatment less often than photodynamic therapy. However, it is applicable only to certain subtypes of wet ARMD and may immediately cause vision loss if used too close to the center of the macula.
Surgery usually not recommended
Surgical removal of neovascular tissue in wet ARMD has succeeded anatomically but has not yielded consistent results in protecting vision. Using microsurgical instruments, the surgeon can separate the macula from the retinal pigment epithelium and remove the neovascular tissue through a small incision in the retina. Though the Submacular Surgery Trials did find a subset of patients who benefited from this procedure, overall results did not support the use of this surgery (SOR: A; LOE: 1, RCT).19
Pegaptanib injections show promise
The newest treatment for wet ARMD is pegaptanib (Macugen). Pegaptanib is a macromolecule designed specifically to bind to VEGF165 isoform, thus blocking its angiogenic and permeability enhancing activity. It is injected directly into the vitreous cavity of patients with wet ARMD. Study results have encouragingly shown a significant reduction in moderate and severe visual loss at 12 months of follow-up (SOR: A; LOE: 1, RCT).20
Measures for wet and dry macular degeneration
Smoking cessation most important lifestyle change
Of all the treatment options for patients with wet or dry ARMD, the most effective means of preserving vision is smoking cessation. As mentioned, controlling blood pressure is also an important modifiable risk factor. Lowering cholesterol or decreasing body mass index may help prevent vision loss, but the literature does not consistently support these claims.
Counseling
Instruct patients with ARMD of either type on how to use the Amsler grid at home. They should understand that any changes on the grid should prompt a call to their ophthalmologist.
Patients with dry ARMD should understand that while the disease is progressive, it does so very slowly.
Patients with wet ARMD and those with end-stage dry ARMD should be counseled that, unless another ocular disease is present, they will not go blind. They can maintain normal peripheral vision. If their central vision is 20/200 or worse in the better eye with glasses, they are considered “legally blind” (in most states) and may qualify for certain disability help.
Low-vision aids
Even in the most advanced cases of ARMD, patients must never be told that nothing more can be done. Low-vision devices are available to aid and restore the functional needs of patients. These range from simple handheld magnifiers to closed-circuit television scanners. Inquire of your local ophthalmologist if he or she incorporates low-vision aid training into his practice. If not, it may be necessary to refer to a low-vision clinic at a larger institution.
Acknowledgments
This work was supported in part by unrestricted grants from Research to Prevent Blindness and the Pat & Willard Walker Eye Research Center.
CORRESPONDENCE
Michael N. Wiggins, MD, Jones Eye Institute, University of Arkansas for Medical Sciences, 4301 West Markham St. Slot 523, Little Rock, AR 72205-7199. E-mail: [email protected]
- Advanced age, positive family history, smoking, hypertension, and Caucasian ancestry are risk factors consistently associated with age-related macular degeneration (ARMD).
- With older patients, suspect ARMD if vision has decreased or if Amsler grid testing reveals scotoma or metamorphopsia (C).
- Refer any patient with suspected ARMD for an ophthalmology consultation (C).
- Lifestyle modifications, vitamin supplementation, and low-vision aids can slow or mitigate the effects of ARMD (A).
Age-related macular degeneration (ARMD) is no longer the ocular death sentence it once was. Therapies are improving and becoming more widely available.
Though everyone over 40 should have an ophthalmologic exam every year or 2, many patients that may routinely see their primary care physician will not consult an ophthalmologist until a problem manifests (see Demographics of ARMD). With simple, in-office testing described in this article, you can help alert patients to the need for ophthalmologic follow-up.
Age-related macular degeneration (ARMD) is a progressive eye disease that can result in the loss of the center of our field of vision due to damage to the macula, the most important portion of the retina. It is the leading cause of central vision loss in people over the age of 50 years in the United States1 and the resulting functional loss can be devastating. Over a 5-year time span, it is estimated that 1 in 3 people over the age of 70 years will develop signs of ARMD2 and that 315,000 Americans over the age of 74 years will develop signs of advanced ARMD.3 Since the risk of ARMD increases with age,1 the number of patients with this disease will increase as our population grows older.
5 Consistent risk factors
Several large, population-based studies have identified age, family history, smoking, hypertension, and Caucasian ancestry as risk factors consistently associated with ARMD.4 Other risk factors such as atherosclerosis, ultraviolet (UV) exposure, farsightedness, increased body-mass index, and cataracts4-6 have been suggested, but not consistently proven to increase risk.
The risk of developing ARMD increases with age.1 According to the Framingham Eye study, a person between the ages of 65 and 74 has a 6% chance of exhibiting ophthalmoscopic evidence of ARMD. For those 75 or older, the chances are almost 20%.1,7
The Beaver Dam Eye Study and Rotterdam Study looked at families with ARMD and found an odds ratio as high as 10:1 for siblings7,8 and 6:1 for the offspring of ARMD patients.7 Combining these risk factors, a person who lives to be 85 and has a first-degree relative with ARMD has a 48% chance of developing clinically detectable ARMD.7
Smoking and hypertension also correlate strongly with ARMD.4,6 Patients who have ARMD and continue to smoke are 3.6 times more likely to progress to the end stages of the disease with severe functional compromise. Even when patients quit smoking, the risk remains elevated for the next 20 years.9 Studies of ARMD and systemic hypertension show an odds ratio for clinically detectable dry ARMD at 2:1 for patients with a systolic reading of 160 mm Hg or greater, and an odds ratio of 4:1 for wet ARMD if the diastolic reading is greater than 95 mm Hg.10,11
Diagnosing ARMD
Though a dilated fundus exam remains the gold standard for diagnosing ARMD, other in-office diagnostic tools are also available.
Specific clues in the history
In addition to inquiring about a family history of ARMD, ask patients if they have difficulty recognizing faces or if words seem to disappear while reading. ARMD patients may also notice that telephone poles appear crooked.
Testing visual acuity
With the patient wearing a current pair of reading glasses, test one eye at a time in good lighting with a near vision card at 12 to 14 inches from the patient’s face. Though many disorders can cause decreased vision, a reading worse than 20/30 in an elderly patient could indicate undiagnosed ARMD. If the patient has noticed a recent decrease in vision, an immediate ophthalmology referral is indicated. If near vision has gradually worsened, referral is needed but perhaps not as urgently.
Amsler grid test for earlier diagnosis
Amsler grid testing can detect ARMD (as well as other macular diseases) at a much earlier stage than can visual acuity testing alone. The grid (FIGURE 1; for a printable version, go to www.jfponline.com) is read with one eye at a time at a distance of 12 to 14 inches with reading glasses under good lighting conditions. Instruct the patient to focus only on the center dot in the grid and to view the remainder of the grid with peripheral vision and without looking around. If the patient reports that lines are missing (known as a scotoma) or appear wavy (known as metamorphopsia), the test is positive and prompt referral is indicated. The location of the distorted or missing lines corresponds closely with the location of the macular lesion.
FIGURE 1
Amsler grid
Patients with known ARMD can monitor their disease by performing this test once a day. This grid is shown actual size; a printable version is found at www.jfponline.com.
Changes seen on dilated fundus exam
To view the macula, ask the patient to look directly at the light during direct fundoscopy.
Dry ARMD displays drusen and changes in the retinal pigment epithelium. Drusen are discrete, round yellow lesions. Changes in the retinal pigment epithelium may be subtle tiny darkly colored patches in the macula near areas of lighter patches (see How ARMD develops).
Wet ARMD may appear as a dark red or green discoloration in the macula and requires immediate referral.
End-stage ARMD may demonstrate a well-delineated geographic pattern corresponding to retinal atrophy or scarring. Large blood vessels are usually visible in the base of these areas. Any macular lesions found in a patient without a diagnosis of ARMD or other maculopathy must have an ophthalmology referral (TABLE 1).
TABLE 1
Referral guide for age-related macular degeneration
| GROUP 1 | IMMEDIATE REFERRAL (THAT DAY) |
| New onset of decreased vision of unknown cause | |
| New onset of metamorphopsia (crooked telephone poles or new findings on the Amsler grid test) | |
| New onset of a scotoma (losing words while reading) | |
| Retinal hemorrhages | |
| GROUP 2 | PROMPT REFERRAL (WITHIN 1 MONTH) |
| Drusen or retinal pigment epithelium changes seen without a previous diagnosis of ARMD | |
| History of long-standing metamorphopsia or scotoma without a previous diagnosis of ARMD | |
| GROUP 3 | ROUTINE REFERRAL (WITHIN 6 MONTHS) |
| Diagnosis of ARMD, but has not seen an ophthalmologist in more than 1 year | |
| Age over 60, but has not seen an ophthalmologist in more than 2 years | |
Pathologically, ARMD damages the macula in 1 of 2 ways, giving rise to the 2 forms of ARMD, wet and dry. Dry ARMD accounts for 80% of all cases of ARMD.12 In this form, the retinal pigment epithelium (RPE) degenerates as a result of the intracellular deposition of yellow metabolic waste products known as drusen. Drusen are typically accompanied by subtle, progressive changes in the pigmentation of the RPE (FIGURE 2). Without the nutritional support of the RPE, the overlying retina cells slowly atrophy causing a loss of central visual acuity. Wet ARMD is less common, but accounts for 80% of the severe vision loss from ARMD.12 In this form, neovascularization from the arterial system underlying the RPE breaks through the RPE barrier and quickly spreads underneath the retina, causing bleeding and fibrosis which can rapidly destroy the macula (FIGURE 3).
FIGURE 2
Dry ARMD
Dry ARMD demonstrating yellow-white drusen with areas of patchy hyperpigmentation of the retinal pigment epithelium. This patient has 20/30 vision and metamorphopsia on Amsler grid testing.
FIGURE 3
Wet ARMD
The greenish hue indicates bleeding under the retina. The yellow-white lesions represent sub-retinal scarring. This patient has 20/400 vision and a large scotoma on Amsler grid testing.
Treatment for dry macular degeneration
Vitamins confer modest benefit
Some patients with dry ARMD may benefit modestly from certain over-the-counter vitamin supplements. The Age-Related Eye Disease Study found that patients with extensive drusen but no advanced macular degeneration in either eye had an 11% chance of going from legal driving vision to legal blindness within 5 years. However, those who took the once-a-day formulation13 shown in TABLE 2 exhibited a modest drop in risk to 10% over 5 years. More encouraging, patients with advanced macular degeneration in the control eye reduced their chances of legal blindness from 28% to 17% with the supplement (strength of recommendation [SOR]: A14; level of evidence [LOE]: 1, randomized controlled trial [RCT]).
Ten mg of Lutein alone or combined with antioxidants has shown some promise in short-term studies15 (SOR: B; LOE: 2, RCT), but further research will be required.
TABLE 2
Recommended daily vitamin doses for dry ARMD patients
| VITAMIN | DOSE |
|---|---|
| Zinc | 80 mg |
| Vitamin C | 500 mg |
| Vitamin E | 400 IU |
| Beta-carotene | 15 mg |
| Copper | 2 mg |
| The recommended once a day dose from the Age-Related Eye Disease Study. Copper was included in the formulation to prevent a zinc-induced copper deficient anemia.13 It should also be noted that smokers may be at an increased risk of lung cancer from excessive beta-carotene use13 and special formulations for smokers are available. | |
Treatment for wet macular degeneration
Laser treatment most common choice
Though many types of therapy are available, patients with wet ARMD will usually undergo photodynamic therapy or conventional laser therapy.
Photodynamic therapy uses a photosensitizing agent with an affinity for neovascular tissue. After injection into the bloodstream, the agent is light-activated to induce thrombosis of the neovascular vessels.16 The Treatment of Age-Related Macular Degeneration with Photodynamic Therapy study showed that treated patients had a greater chance of retaining their vision compared with untreated patients, and that about 1 in 6 treated patients experienced some improvement in vision (SOR: A; LOE: 1, RCT).17 Unfortunately, recurrence of neovascular tissue is common and retreatment is often necessary.
Conventional thermal laser therapy directly coagulates the neovascular area. The Macular Photocoagulation Study showed that thermal laser therapy reduced the likelihood of losing 3 to 6 lines of vision (for example, going from 20/20 to 20/70) after 2 years of follow-up (SOR: A; LOE: 1, RCT).18 Conventional laser therapy requires retreatment less often than photodynamic therapy. However, it is applicable only to certain subtypes of wet ARMD and may immediately cause vision loss if used too close to the center of the macula.
Surgery usually not recommended
Surgical removal of neovascular tissue in wet ARMD has succeeded anatomically but has not yielded consistent results in protecting vision. Using microsurgical instruments, the surgeon can separate the macula from the retinal pigment epithelium and remove the neovascular tissue through a small incision in the retina. Though the Submacular Surgery Trials did find a subset of patients who benefited from this procedure, overall results did not support the use of this surgery (SOR: A; LOE: 1, RCT).19
Pegaptanib injections show promise
The newest treatment for wet ARMD is pegaptanib (Macugen). Pegaptanib is a macromolecule designed specifically to bind to VEGF165 isoform, thus blocking its angiogenic and permeability enhancing activity. It is injected directly into the vitreous cavity of patients with wet ARMD. Study results have encouragingly shown a significant reduction in moderate and severe visual loss at 12 months of follow-up (SOR: A; LOE: 1, RCT).20
Measures for wet and dry macular degeneration
Smoking cessation most important lifestyle change
Of all the treatment options for patients with wet or dry ARMD, the most effective means of preserving vision is smoking cessation. As mentioned, controlling blood pressure is also an important modifiable risk factor. Lowering cholesterol or decreasing body mass index may help prevent vision loss, but the literature does not consistently support these claims.
Counseling
Instruct patients with ARMD of either type on how to use the Amsler grid at home. They should understand that any changes on the grid should prompt a call to their ophthalmologist.
Patients with dry ARMD should understand that while the disease is progressive, it does so very slowly.
Patients with wet ARMD and those with end-stage dry ARMD should be counseled that, unless another ocular disease is present, they will not go blind. They can maintain normal peripheral vision. If their central vision is 20/200 or worse in the better eye with glasses, they are considered “legally blind” (in most states) and may qualify for certain disability help.
Low-vision aids
Even in the most advanced cases of ARMD, patients must never be told that nothing more can be done. Low-vision devices are available to aid and restore the functional needs of patients. These range from simple handheld magnifiers to closed-circuit television scanners. Inquire of your local ophthalmologist if he or she incorporates low-vision aid training into his practice. If not, it may be necessary to refer to a low-vision clinic at a larger institution.
Acknowledgments
This work was supported in part by unrestricted grants from Research to Prevent Blindness and the Pat & Willard Walker Eye Research Center.
CORRESPONDENCE
Michael N. Wiggins, MD, Jones Eye Institute, University of Arkansas for Medical Sciences, 4301 West Markham St. Slot 523, Little Rock, AR 72205-7199. E-mail: [email protected]
1. American Academy of Ophthalmology. Basic and Clinical Science Course, Section 12, Retina, ed. 2004-2005. San Francisco, Calif: LEO; 2004.
2. Mukesh BN, Dimitrov PN, Leikin S, et al. Five-year incidence of age-related maculopathy: the Visual Impairment Project. Ophthalmology 2004;111:1176-1182.
3. Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 1997;104:7-21.
4. Klein R, Peto T, Bird A, Vannewkirk M. The epidemiology of age related macular degeneration. Am J Ophthalmol 2004;137:486-495.
5. Tomany S, Cruickshanks K, Klein R, et al. Sunlight and the 10-year incidence of age-related maculopathy: The Beaver Dam Eye Study. Arch Ophthalmol 2004;122:750-757.
6. Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 2000;107:2224-2232.
7. Klaver C, Wolfs R, Assink J, et al. Genetic risk of age-related maculopathy: Population-based familial aggregation study. Arch Ophthalmol 1998;116:1646-1651.
8. Klein B, Klein R, Lee K, et al. Risk of incident age-related eye diseases in people with an affected sibling: The Beaver Dam Eye Study. Am J Epidemiol 2001;154:207-211.
9. Delcourt C, Diaz J, Ponton-Sanchez A, Papoz L. Smoking and age-related macular degeneration: The POLA Study. Arch Ophthalmol 1998;116:1031-1035.
10. van Leeuwen R, Ikram MK, Vingerling JR, Witteman JC, Hofman A, de Jong PT. Blood pressure, atherosclerosis, and the incidence of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci 2003;44:3771-3777.
11. Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol 2000;118:351-358.
12. Ferris FL, 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol 1984;102:1640-1642.
13. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001;119:1417-1436.
14. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53(2):111-120.
15. Richer S, Stiles W, Statkute L, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004;75:216-230.
16. Yanoff M, Duker J. Ophthalmology 2nd ed. St Louis, Mo: Mosby; 2004.
17. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol 1999;117:1329-1345.
18. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol 1993;111:1200-1209.
19. Hawkins BS, Bressler NM, Miskala PH, et al. Submacular Surgery Trials (SST) Research Group. Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: ophthalmic findings: SST report no. 11. Ophthalmology 2004;111:1967-1980.
20. Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805-2516.
1. American Academy of Ophthalmology. Basic and Clinical Science Course, Section 12, Retina, ed. 2004-2005. San Francisco, Calif: LEO; 2004.
2. Mukesh BN, Dimitrov PN, Leikin S, et al. Five-year incidence of age-related maculopathy: the Visual Impairment Project. Ophthalmology 2004;111:1176-1182.
3. Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 1997;104:7-21.
4. Klein R, Peto T, Bird A, Vannewkirk M. The epidemiology of age related macular degeneration. Am J Ophthalmol 2004;137:486-495.
5. Tomany S, Cruickshanks K, Klein R, et al. Sunlight and the 10-year incidence of age-related maculopathy: The Beaver Dam Eye Study. Arch Ophthalmol 2004;122:750-757.
6. Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 2000;107:2224-2232.
7. Klaver C, Wolfs R, Assink J, et al. Genetic risk of age-related maculopathy: Population-based familial aggregation study. Arch Ophthalmol 1998;116:1646-1651.
8. Klein B, Klein R, Lee K, et al. Risk of incident age-related eye diseases in people with an affected sibling: The Beaver Dam Eye Study. Am J Epidemiol 2001;154:207-211.
9. Delcourt C, Diaz J, Ponton-Sanchez A, Papoz L. Smoking and age-related macular degeneration: The POLA Study. Arch Ophthalmol 1998;116:1031-1035.
10. van Leeuwen R, Ikram MK, Vingerling JR, Witteman JC, Hofman A, de Jong PT. Blood pressure, atherosclerosis, and the incidence of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci 2003;44:3771-3777.
11. Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol 2000;118:351-358.
12. Ferris FL, 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol 1984;102:1640-1642.
13. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001;119:1417-1436.
14. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53(2):111-120.
15. Richer S, Stiles W, Statkute L, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004;75:216-230.
16. Yanoff M, Duker J. Ophthalmology 2nd ed. St Louis, Mo: Mosby; 2004.
17. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol 1999;117:1329-1345.
18. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol 1993;111:1200-1209.
19. Hawkins BS, Bressler NM, Miskala PH, et al. Submacular Surgery Trials (SST) Research Group. Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: ophthalmic findings: SST report no. 11. Ophthalmology 2004;111:1967-1980.
20. Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805-2516.