User login
Asthma: Resource use and costs for inhaled corticosteroid vs leukotriene modifier treatment—a meta-analysis
Objective To compare the effects of inhaled corticosteroid treatment with leukotriene modifier treatment on medical resource use and costs for asthma patients.
Study design Meta-analysis combining results from published and unpublished studies.
Data sources Studies were identified from the MEDLINE and EMBASE databases and the GlaxoSmithKline internal database study registers. Two independent reviewers evaluated the identified studies; studies meeting specified inclusion criteria were abstracted and summarized by meta-analysis with a random effects model.
Outcomes measured Hospitalization rate, emergency department visit rate, emergency department costs, drug costs, total asthma-related costs, and total medical care costs.
Results Patients taking inhaled corticosteroids had:
a significantly lower annual rate of hospitalization than those taking leukotriene modifiers (2.2% vs 4.3%, respectively;P<.05)
a greater decline in hospitalization rate (before vs after therapy initiation) than those taking leukotriene modifiers (decline of 2.4% vs 0.55%; P<.01)
a lower annual rate of emergency department visits than those taking leukotriene modifiers (6.2% vs 7.7%;P<.005).
lower total asthma-related medical costs than those taking leukotriene modifiers (P<.05) and a 17% reduction in overall total medical care costs (P not significant).
Conclusions Patients with asthma treated with inhaled corticosteroids had significantly fewer asthma-related hospitalizations and emergency department visits and lower total asthma-related health care costs than patients treated with leukotriene modifiers. These meta-analysis findings are consistent with results from randomized controlled trials showing improvements in lung function for patients taking inhaled corticosteroids as opposed to leukotriene modifiers.
Although many medications are available for patients with asthma, inhaled corticosteroids are generally the preferred treatment.1-4 Multiple studies have demonstrated that inhaled corticosteroid therapy improves patient outcomes.1 Inhaled corticosteroids have been shown to decrease costs5 and use of medical care resources.6-8
More recently, leukotriene modifiers have been introduced for asthma treatment. This class of medication has bronchodilator and anti-inflammatory effects.9 Although multiple studies have indicated improved outcomes and decreased costs associated with leukotriene modifier therapy incertain patient populations,10,11 its role in asthmamanagement is uncertain.9
Several studies have compared the clinical outcomes of these therapies12-15 and their impact on medical care resource use and costs.16,17 However, these studies were not powered specifically to detect significant differences in resource use or costs.
We performed a meta-analysis to (1) compare the rate of hospitalization among patients with asthma treated with inhaled corticosteroids vs those treated with leukotriene modifiers and (2) evaluate other resource use rates and costs for these patients.
Methods
The meta-analysis consisted of a literature search, the development of inclusion criteria, form development, and literature review.
Literature search
We searched the MEDLINE, EMBASE, Cochrane Collaboration Study Registry, and GlaxoSmithKline databases and consulted experts in this field. The GlaxoSmithKline database consists of studies sponsored by GlaxoSmithKline that met companywide minimum quality thresholds and were published in full or abstract form.
We also contacted the manufacturers of leukotriene modifiers available in the United States, AstraZeneca and Merck, to request published and unpublished information on studies comparing leukotriene modifiers with inhaled corticosteroids. To provide results corresponding to current treatment patterns, only studies from 1991 to 2001 were included. Published and unpublished materials were included.18,19
Inclusion criteria
Studies were included in the meta-analysis if they met the following criteria.
Population. Patients with diagnosed asthma. Only studies that did not restrict analyses to severe asthma patients or children were included.
Study design. Prospective and retrospective comparative studies of patients receiving inhaled corticosteroid or leukotriene modifier monotherapy (no other controller therapy) in the same study. Studies were required to have defined inclusion and exclusion criteria, defined number of patients in each study arm, defined treatment protocol (ie, medications and doses used), and separate results for each medication.
Only studies presenting primary research (hence excluding review articles and metaanalyses) were included. Only studies presenting data for at least 6 months on all participants were included.
Outcomes. Hospitalization visit rates and costs, emergency department visit rates and costs, pharmacy costs, total asthma-related costs, and total medical care costs. Because resource use patterns and medical care cost information differs substantially between countries, we only included US studies.
Study process
Each identified article was evaluated by 2 independent reviewers (KMS and MG); any differences were discussed with a project leader to reach a consensus. Documents selected for inclusion were then reviewed by the 2 reviewers, and differences in data abstraction were resolved before inclusion.
Analysis
We used the Q statistic20 to assess heterogeneity and, when appropriate, combined data from included studies with the use of a random effects model. Random effects methodology was used to assess the impact of inhaled corticosteroid vs leukotriene modifier therapy on the overall asthma population, not just the subpopulation of patients participating in included studies.21
Two sets of meta-analyses were performed with the abstracted data. First, the specified outcome measures were compared for patients taking inhaled corticosteroid vs leukotriene modifier therapy. Second, the impact (before vs after) of each treatment initiation was compared for each outcome.
Assessment of statistically significant differences between meta-analysis results was performed with the Student t test, with an Α of .05. Charges were used in the included studies as proxies for costs. These costs were inflated to 2000 values by using the medical care component of the consumer price index before inclusion.
Results
We identified 49 documents and reviewed them for inclusion in the meta-analysis; 6 (12.2%) met the inclusion criteria (Table 1). Five were retrospective cohort studies; only 1 study was identified as a prospective trial comparing inhaled corticosteroid and leukotriene modifier therapies and including results on resource use or medical care costs.16,17,22-25 All 6 studies were performed with support from GlaxoSmithKline.
Forty-three documents were excluded due to 1 or more of the following criteria: lack of primary results (9 documents, 21%); did not contain resource use rate or cost outcomes (22 documents, 51%); did not provide at least 6 months’ worth of data on resource use or cost outcomes (5 documents, 12%); did not meet the defined inclusion and exclusion criteria (primarily studies not including both inhaled corticosteroid and leukotriene modifiers or those restricted to patient clinical subgroups; 10 documents, 34%); or did not define the number of patients included in the study (1 document, 3%).
Because few studies presented data on the specified outcomes, we were unable to assess asthma-specific costs for subcategories of resource use. Too few studies included data on hospitalization costs (either asthma-specific or overall) to include in the analysis. Therefore, meta-analysis was performed on overall (ie, all causes) emergency department, pharmacy, and total medical care costs.
TABLE 1
Characteristics of studies included in the meta-analysis
| Duration (mo) | |||||
|---|---|---|---|---|---|
| Study | LOE* | Before therapy | After therapy | Treatment (N) | Comparison (N) |
| Oates and Gothard22 | 2b | 9 | 9 | Inhaled corticosteroids (546)† | Leukotriene modifiers (152)‡ |
| Pathak et al23 | 2b | 9 | 9 | Fluticasone propionate (284) | Leukotriene modifiers (497)‡ |
| Stanford et al24 | 1b | - | 6 | Fluticasone propionate (271) | Montelukast (262) |
| Stempel et al16 | 2b | 9 | 12 | Fluticasone propionate (602) | Zafirlukast (309) |
| Stempel et al17 | 2b | 9 | 9 | Fluticasone propionate (559) | Montelukast (382) |
| White et al25 | 2b | 9 | 9 | Inhaled corticosteroids (1305)† | Leukotriene modifiers (109)‡ |
| *LOE, level of evidence. For an explanation of levels of evidence. | |||||
| †Results were presented for all inhaled corticosteroids combined. | |||||
| ‡Results were presented for all leukotriene modifiers combined. | |||||
Primary analysis
The primary objective of this study was to evaluate the impact of inhaled corticosteroid and leukotriene modifier treatment on the mean annual hospitalization rate. Four of the 6 included studies contained information on hospitalization rate for each treatment. Results from the primary analysis are presented in Table 2.
Patients taking inhaled corticosteroids had a significantly lower annual rate of hospitalization than did patients taking leukotriene modifiers (2.23% vs 4.30%, respectively; P<.005). The absolute risk reduction was 2.07% (number needed to treat=48 for 1 year).
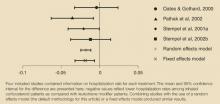
The difference in annual hospitalization visit rates for each study in the primary analysis is presented in the Figure, where negative values reflect lower hospitalization rates among patients taking inhaled corticosteroids than among those taking leukotriene modifiers. Two studies16,23 had statistically significant differences in hospitalization rates, whereas the differences in the other 2 studies were not statistically significant (P<.05). Combining studies with the use of a random effects model (the default methodology for this analysis) or a fixed effects model produced similar results. The Q statistic indicated no significant heterogeneity (P=.43).
TABLE 2
Meta-analysis results for inhaled corticosteroid vs leukotriene modifier therapy*
| Inhaled corticosteroid vs leukotriene modifier patients † | ||||
|---|---|---|---|---|
| Inhaled corticosteroids | Leukotriene modifiers | Absolute difference | Relative difference | |
| Annual asthma hospitalizations‡ | 2.23% (1.69-2.78) | 4.30% (3.53-5.07) | -1.79% (-2.45 to -1.14) | -42.88% (-55.95 to -29.80) |
| Annual rate of visits to the emergency department due to asthma§ | 6.19% (4.84-7.53) | 7.74% (6.30-9.19) | -1.53% (-1.78 to -1.28) | -21.35% (-25.31 to -17.38) |
| Total annual costs of visits to the emergency department | $93 (38-148) | $73 (52-94) | $21(-17 to 59) | 1.00% (-38 to 40) |
| Total annual drug costs§ | $807 (548-1065) | $1062 (812-1312) | -$258 (-308 to -208) | -27.20% (-33.2 to -21.3) |
| Annual asthma-related cost‡ | $882 (613-1150) | $1393 (1143-1643) | $513 (-392 to -634) | -38.01% (-47.4 to -28.8) |
| Total annual cost | $5254 (4474-6033) | $7140 (4970-9311) | -$1918 (-3509 to -327) | -17.20% (-30.9 to -3.5) |
| *Data are presented as mean (95% confidence interval). | ||||
| †Absolute and relative differences were determined from meta-analyses of the absolute and relative differences for each included study. | ||||
| ‡Inhaled corticosteroid vs leukotriene modifier significant at P<.05. | ||||
| §Inhaled corticosteroid vs leukotriene modifier significant at P<.005. | ||||
FIGURE
Difference in hospitalization rates (mean, confidence interval)
Secondary outcomes
Results of secondary analyses for 5 study outcomes (annual visits to the emergency department due to asthma, total emergency department costs, total drug costs, total asthma-related costs, and overall total cost) are presented in Table 2.
Mean annual rates of visits to the emergency department and total annual drug costs were significantly higher for patients taking leukotriene modifiers than for those taking inhaled corticosteroids (P<.005 for each). Patients taking leukotriene modifiers had lower annual costs for visits to the emergency department than did those taking inhaled corticosteroids, although this difference was not statistically significant. The higher rate and lower cost of emergency department visits for patients taking leukotriene modifiers suggest that medical resources were used less at each visit as compared with those for patients taking inhaled corticosteroids.
Total asthma-related costs for patients taking inhaled corticosteroids were significantly lower than those for patients taking leukotriene modifiers (P<.05). Patients taking inhaled corticosteroids also incurred decreased annual total (allcause) medical care costs. Although this difference was qualitatively large (approximately $1900, or a decrease of over 17%), it did not reach statistical significance.
Pre- vs post-initiation of therapy
In addition to comparing the impact of the 2 therapies on resource use and costs, we were interested in the impact of initiating each therapy on the study outcomes. We therefore assessed resource use rates and costs before and after treatment initiation. These patients may have been receiving asthma therapies (or no asthma therapy) other than inhaled corticosteroids or leukotriene modifiers. The number of studies presenting data on costs was too small to allow comparison.
Results of this within-group analysis are presented in Table 3. For patients taking inhaled corticosteroids, hospitalization rates and emergency department visit rates decreased significantly after treatment initiation compared with the preinitiation values (P<.005 and P<.05, respectively). The decreases for patients taking leukotriene modifiers upon treatment initiation were smaller and not statistically significant.
Both groups showed similar small decreases in annual emergency department costs, neither of which was significant. The increases in annual total drug costs and annual total medical care costs after treatment initiation were significant for both groups of patients (all at P<.005). However, both increases were greater for patients taking leukotriene modifiers; the increase in drug costs was statistically significant (P<.001).
TABLE 3
Resource utilization and costs before and after therapy initiation for inhaled corticosteroids vs leukotriene modifiers*
| Before vs after treatment, mean (95% confidence interval) | |||
|---|---|---|---|
| Inhaled corticosteroid patients | Leukotriene modifier patients | Absolute difference | |
| Annual hospitalization rate | -2.37%† (-2.89 to -1.86) | -0.55% (-1.18 to 0.08) | -1.91%‡(-2.45 to -1.36) |
| Annual rate of visits to the emergency department due to asthma | -4.44%§ (-5.98 to -2.90) | -2.06% (-3.61 to -0.51) | -2.47%||(-3.09 to -1.86) |
| Total annual costs of the emergency despartment | -$5 (-21 to 10) | -$15 (-44 to 15) | $9 (-33 to 51) |
| Total annual drug costs | $415†(312-517) | $579† (472-686) | -$167||(-192 to 142) |
| Total annual medical care costs | $641† (113-1169) | $1712† (927-3529) | -$1080 (-2802 to 643) |
| *Post-therapy initiation values for inhaled corticosteroids and leukotriene modifiers are presented in Table 1. | |||
| †Before vs after treatment initiation significant at P<.005. | |||
| ‡Before vs after treatment initiation outcomes for inhaled corticosteroids vs leukotriene modifiers significant at P<.01. | |||
| §Before vs after treatment initiation significant at P<.05. | |||
| ||Before vs after treatment initiation outcomes for inhaled corticosteroids vs leukotriene modifiers significant at P<.001. | |||
Discussion
In this study, we used meta-analysis to combine data across studies and determine more robust estimates of the impact of inhaled corticosteroid vs leukotriene modifier therapy on medical resource use rates and costs. The primary analysis indicated that annual hospitalization rates among patients taking inhaled corticosteroids are significantly lower that those taking leukotriene modifiers. Other resource use rates and costs evaluated in this study also generally showed decreased values for patients taking inhaled corticosteroids.
Although meta-analysis generally has been used for clinical outcome measures, it is a highly appropriate method for resource use and cost outcomes. In general, studies of the impact of a particular treatment are powered to assess safety and efficacy or effectiveness; there is often insufficient power to detect differences in resource use or costs in any one study. Due to substantial variation in treatment patterns, the variance associated with resource use rates (and associated costs) may be substantially higher than that for clinical measures; such a wide variance adds to the difficulties in assessing differences for nonclinical outcomes.
Limitations
This study has a number of limitations. Only a few studies met inclusion criteria for the meta-analysis; the analysis should be replicated as additional studies become available. Data were abstracted from the included studies without modification (except for inflating costs to 2000 values when necessary). As in all meta-analyses, any problems present in the original data are present in the combined data; limitations of the original data are not addressed by this method.
Among the studies evaluated for the metaanalysis were a number published only as abstracts. Inclusion of unpublished literature in meta-analyses is controversial; however, several sources18,19 now recommend inclusion of published and unpublished studies. Egger and Smith26 found that studies with significant results are more likely to be published than are studies with nonsignificant results, leading to publication bias. Studies with significant results also may be more likely to be published in indexed journals, leading to “database bias.” As such, inclusion of unpublished studies is important to produce unbiased results.
Five of the 6 studies that met the inclusion criteria were observational, retrospective cohort analyses. Whereas many meta-analyses focus solely on prospective, randomized clinical trials, several have included retrospective data.27,28 Retrospective analyses and observational data have a number of limitations, in particular the lack of randomization that can lead to differences in characteristics of specified treatment groups. Further, as discussed by Egger et al,29 metaanalyses based on observational studies may involve bias and confounding.
However, observational data and retrospective analyses also have the advantage of reflecting “real world” treatment patterns and broader patient groups that increase the generalizability of the data, whereas clinical trials may include protocol-driven utilization and selected patient groups. Clinical trials also may occur in specialized health care settings, whereas observational (cohort) data may be more applicable to clinical practice. Due to these factors, meta-analysis of observational data has become common.29
The pre-initiation vs post-initiation analysis indicated that values for each treatment group provide information on the similarities between treatment groups before initiation of controller therapy. Even though the treatment groups were not randomized to each therapy and we have no means to ensure compatibility between groups, having similar rates of resource use between groups provides some evidence regarding similarity. Nonetheless, given the limitations of the retrospective data and meta-analysis in general, it will be important to validate the results of this meta-analysis in the future with naturalistic prospective studies.
Despite these limitations, this study provides important information on the impact of asthma therapies on resource use and costs. Specifically, the resource use and cost outcomes assessed in this study were lower for inhaled corticosteroid patients than for leukotriene modifier patients. This study also illustrates the usefulness of metaanalysis in evaluating resource use and costs. By selecting and combining outcomes across studies in a standardized, rigorous, and transparent manner, the effects of different therapies can be evaluated with greater precision.
Acknowledgments
We thank John O’Donnell and Layne Gothard for their assistance with this manuscript.
Corresponding author
Michael T. Halpern, MD, PhD, Principal Scientist, Exponent, Inc., 1800 Diagonal Road, Alexandria, VA 22314. E-mail: [email protected]
1. Janson S. National asthma education and prevention program, expert panel report. II: overview and application to primary care. Prim Care Pract 1998;2:578-588.
2. Lalloo UG, Bateman ED, Feldman C, et al. Guideline for the management of chronic asthma in adults—2000 update. South African Pulmonology Society Adult Asthma Working Group. S Afr Med J 2000;90:540-541-544-552.
3. Podell RN. National guidelines for the management of asthma in adults. Am Fam Physician 1992;46:1189-1196.
4. Veninga CC, Lagerlov P, Wahlstrom R, et al. Evaluating an educational intervention to improve the treatment of asthma in four European countries. Drug Education Project Group. Am J Respir Crit Care Med 1999;160:1254-1262.
5. Ozminkowski RJ, Wang S, Marder WD, Azzolini J, Schutt D. Cost implications for the use of inhaled anti-inflammatory medications in the treatment of asthma. Pharmacoeconomics 2000;18:253-264.
6. Paltiel AD, Fuhlbrigge AL, Kitch BT, et al. Cost-effectiveness of inhaled corticosteroids in adults with mild-to-moderate asthma: results from the asthma policy model. J Allergy Clin Immunol 2001;108:39-49.
7. Adams RJ, Fuhlbrigge A, Finkelstein JA, et al. Impact of inhaled antiinflammatory therapy on hospitalization and emergency department visits for children with asthma. Pediatrics 2001;107:706-711.
8. Donahue JG, Weiss ST, Livingston JM, Goetsch MA, Greineder DK, Platt R. Inhaled steroids and the risk of hospitalization for asthma. JAMA 1997;277:887-891.
9. Dempsey OJ. Leukotriene receptor antagonist therapy. Postgrad Med J 2000;76:767-773.
10. Klingman D, Bielory L, Wang Y, et al. Asthma outcome changes associated with use of the leukotriene-receptor antagonist zafirlukast. Manag Care Interface 2001;14:62-66.
11. Price DB, Ben-Joseph RH, Zhang Q. Changes in asthma drug therapy costs for patients receiving chronic montelukast therapy in the U.K. Resp Med 2001;95:83-89.
12. Bleecker ER, Welch MJ, Weinstein SF, et al. Low-dose inhaled fluticasone propionate versus oral zafirlukast in the treatment of persistent asthma. J Allergy Clin Immunol 2000;105:1123-1129.
13. Ind PW. Inhaled corticosteroids versus anti-leukotrienes: a literature review on the clinical effects. Allergy 1999;54(suppl 50):43-46.
14. Malmstrom K, Rodriguez-Gomez G, Guerra J, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med 1999;130:487-495.
15. Busse W, Nelson H, Wolfe J, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in patients with asthma. J Allergy Clin Immunol 1999;103:1075-1080.
16. Stempel DA, Meyer JW, Stanford RH, Yancey SW. One-year claims analysis comparing inhaled fluticasone propionate with zafirlukast for the treatment of asthma. J Allergy Clin Immunol 2001;107:94-98.
17. Stempel DA, Mauskopf J, McLaughlin T, Yazdani C, Stanford RH. Comparison of asthma costs in patients starting fluticasone propionate compared to patients starting montelukast. Respir Med 2001;95:227-234.
18. Cook DJ, Guyatt GH, Ryan G, et al. Should unpublished data be included in meta-analyses? Current convictions and controversies. JAMA 1993;269:2749-2753.
19. McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet 2000;356:1228-1231.
20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-188.
21. Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care 1990;6:5-30.
22. Oates V, Gothard L. PEER Study: New Starts on Inhaled Corticosteroids or Leukotriene Modifiers in an Asthmatic Population. Advanced Paradigm and GlaxoWellcome, Inc. July 19, 2000. Information available from GlaxoSmithKline at 1-800-825-5249.
23. Pathak DS, Davis EA, Stanford RH. Economic impact of asthma therapy with fluticasone propionate, montelukast, or zafirlukast in a managed care population. Pharmacotherapy 2002;22:166-174.
24. Stanford R, Davis A, Edwards L, Kalberg C, Rickard K. The costs and efficacy of fluticasone propionate 88 mcg twice daily and montelukast 10 mg once daily in patients needing single controller therapy. Chest 2001;120:225S.-
25. White TJ, Gothard L, Fontes CL, Juzba M, Berenbeim DM, Gilderman AM. A Retrospective Administrative Healthcare Claims Analysis to Describe and Assess Pharmaceutical and Medical Resource Utilization within the PacifiCare CA Healthplan PROJECT 2: Longitudinal Analysis of Newly Diagnosed Asthmatics. Prescription Solutions/PacifiCare Health Systems and GlaxoWellcome Inc. November 27, 2000. Information available from GlaxoSmithKline at 1-800-825-5249.
26. Egger M, Smith GD. Meta-analysis bias in location and selection of studies. BMJ 1998;316:61-66.
27. Ebell MH. Prearrest predictors of survival following inhospital cardiopulmonary resuscitation: a meta-analysis. J Fam Pract 1992;34:551-558.
28. Poynard T, Moussalli J, Ratziu V, et al. Is antiviral treatment (IFN alpha and/or ribavirin) justified in cirrhosis related to hepatitis C virus? Societe Royale Belge de Gastroenterologie. Acta Gastroenterol Belg 1998;61:431-437.
29. Egger M, Schneider M, Smith GD. Meta-analysis spurious precision? Meta-analysis of observational studies. BMJ 1998;316:140-144.
Objective To compare the effects of inhaled corticosteroid treatment with leukotriene modifier treatment on medical resource use and costs for asthma patients.
Study design Meta-analysis combining results from published and unpublished studies.
Data sources Studies were identified from the MEDLINE and EMBASE databases and the GlaxoSmithKline internal database study registers. Two independent reviewers evaluated the identified studies; studies meeting specified inclusion criteria were abstracted and summarized by meta-analysis with a random effects model.
Outcomes measured Hospitalization rate, emergency department visit rate, emergency department costs, drug costs, total asthma-related costs, and total medical care costs.
Results Patients taking inhaled corticosteroids had:
a significantly lower annual rate of hospitalization than those taking leukotriene modifiers (2.2% vs 4.3%, respectively;P<.05)
a greater decline in hospitalization rate (before vs after therapy initiation) than those taking leukotriene modifiers (decline of 2.4% vs 0.55%; P<.01)
a lower annual rate of emergency department visits than those taking leukotriene modifiers (6.2% vs 7.7%;P<.005).
lower total asthma-related medical costs than those taking leukotriene modifiers (P<.05) and a 17% reduction in overall total medical care costs (P not significant).
Conclusions Patients with asthma treated with inhaled corticosteroids had significantly fewer asthma-related hospitalizations and emergency department visits and lower total asthma-related health care costs than patients treated with leukotriene modifiers. These meta-analysis findings are consistent with results from randomized controlled trials showing improvements in lung function for patients taking inhaled corticosteroids as opposed to leukotriene modifiers.
Although many medications are available for patients with asthma, inhaled corticosteroids are generally the preferred treatment.1-4 Multiple studies have demonstrated that inhaled corticosteroid therapy improves patient outcomes.1 Inhaled corticosteroids have been shown to decrease costs5 and use of medical care resources.6-8
More recently, leukotriene modifiers have been introduced for asthma treatment. This class of medication has bronchodilator and anti-inflammatory effects.9 Although multiple studies have indicated improved outcomes and decreased costs associated with leukotriene modifier therapy incertain patient populations,10,11 its role in asthmamanagement is uncertain.9
Several studies have compared the clinical outcomes of these therapies12-15 and their impact on medical care resource use and costs.16,17 However, these studies were not powered specifically to detect significant differences in resource use or costs.
We performed a meta-analysis to (1) compare the rate of hospitalization among patients with asthma treated with inhaled corticosteroids vs those treated with leukotriene modifiers and (2) evaluate other resource use rates and costs for these patients.
Methods
The meta-analysis consisted of a literature search, the development of inclusion criteria, form development, and literature review.
Literature search
We searched the MEDLINE, EMBASE, Cochrane Collaboration Study Registry, and GlaxoSmithKline databases and consulted experts in this field. The GlaxoSmithKline database consists of studies sponsored by GlaxoSmithKline that met companywide minimum quality thresholds and were published in full or abstract form.
We also contacted the manufacturers of leukotriene modifiers available in the United States, AstraZeneca and Merck, to request published and unpublished information on studies comparing leukotriene modifiers with inhaled corticosteroids. To provide results corresponding to current treatment patterns, only studies from 1991 to 2001 were included. Published and unpublished materials were included.18,19
Inclusion criteria
Studies were included in the meta-analysis if they met the following criteria.
Population. Patients with diagnosed asthma. Only studies that did not restrict analyses to severe asthma patients or children were included.
Study design. Prospective and retrospective comparative studies of patients receiving inhaled corticosteroid or leukotriene modifier monotherapy (no other controller therapy) in the same study. Studies were required to have defined inclusion and exclusion criteria, defined number of patients in each study arm, defined treatment protocol (ie, medications and doses used), and separate results for each medication.
Only studies presenting primary research (hence excluding review articles and metaanalyses) were included. Only studies presenting data for at least 6 months on all participants were included.
Outcomes. Hospitalization visit rates and costs, emergency department visit rates and costs, pharmacy costs, total asthma-related costs, and total medical care costs. Because resource use patterns and medical care cost information differs substantially between countries, we only included US studies.
Study process
Each identified article was evaluated by 2 independent reviewers (KMS and MG); any differences were discussed with a project leader to reach a consensus. Documents selected for inclusion were then reviewed by the 2 reviewers, and differences in data abstraction were resolved before inclusion.
Analysis
We used the Q statistic20 to assess heterogeneity and, when appropriate, combined data from included studies with the use of a random effects model. Random effects methodology was used to assess the impact of inhaled corticosteroid vs leukotriene modifier therapy on the overall asthma population, not just the subpopulation of patients participating in included studies.21
Two sets of meta-analyses were performed with the abstracted data. First, the specified outcome measures were compared for patients taking inhaled corticosteroid vs leukotriene modifier therapy. Second, the impact (before vs after) of each treatment initiation was compared for each outcome.
Assessment of statistically significant differences between meta-analysis results was performed with the Student t test, with an Α of .05. Charges were used in the included studies as proxies for costs. These costs were inflated to 2000 values by using the medical care component of the consumer price index before inclusion.
Results
We identified 49 documents and reviewed them for inclusion in the meta-analysis; 6 (12.2%) met the inclusion criteria (Table 1). Five were retrospective cohort studies; only 1 study was identified as a prospective trial comparing inhaled corticosteroid and leukotriene modifier therapies and including results on resource use or medical care costs.16,17,22-25 All 6 studies were performed with support from GlaxoSmithKline.
Forty-three documents were excluded due to 1 or more of the following criteria: lack of primary results (9 documents, 21%); did not contain resource use rate or cost outcomes (22 documents, 51%); did not provide at least 6 months’ worth of data on resource use or cost outcomes (5 documents, 12%); did not meet the defined inclusion and exclusion criteria (primarily studies not including both inhaled corticosteroid and leukotriene modifiers or those restricted to patient clinical subgroups; 10 documents, 34%); or did not define the number of patients included in the study (1 document, 3%).
Because few studies presented data on the specified outcomes, we were unable to assess asthma-specific costs for subcategories of resource use. Too few studies included data on hospitalization costs (either asthma-specific or overall) to include in the analysis. Therefore, meta-analysis was performed on overall (ie, all causes) emergency department, pharmacy, and total medical care costs.
TABLE 1
Characteristics of studies included in the meta-analysis
| Duration (mo) | |||||
|---|---|---|---|---|---|
| Study | LOE* | Before therapy | After therapy | Treatment (N) | Comparison (N) |
| Oates and Gothard22 | 2b | 9 | 9 | Inhaled corticosteroids (546)† | Leukotriene modifiers (152)‡ |
| Pathak et al23 | 2b | 9 | 9 | Fluticasone propionate (284) | Leukotriene modifiers (497)‡ |
| Stanford et al24 | 1b | - | 6 | Fluticasone propionate (271) | Montelukast (262) |
| Stempel et al16 | 2b | 9 | 12 | Fluticasone propionate (602) | Zafirlukast (309) |
| Stempel et al17 | 2b | 9 | 9 | Fluticasone propionate (559) | Montelukast (382) |
| White et al25 | 2b | 9 | 9 | Inhaled corticosteroids (1305)† | Leukotriene modifiers (109)‡ |
| *LOE, level of evidence. For an explanation of levels of evidence. | |||||
| †Results were presented for all inhaled corticosteroids combined. | |||||
| ‡Results were presented for all leukotriene modifiers combined. | |||||
Primary analysis
The primary objective of this study was to evaluate the impact of inhaled corticosteroid and leukotriene modifier treatment on the mean annual hospitalization rate. Four of the 6 included studies contained information on hospitalization rate for each treatment. Results from the primary analysis are presented in Table 2.
Patients taking inhaled corticosteroids had a significantly lower annual rate of hospitalization than did patients taking leukotriene modifiers (2.23% vs 4.30%, respectively; P<.005). The absolute risk reduction was 2.07% (number needed to treat=48 for 1 year).
The difference in annual hospitalization visit rates for each study in the primary analysis is presented in the Figure, where negative values reflect lower hospitalization rates among patients taking inhaled corticosteroids than among those taking leukotriene modifiers. Two studies16,23 had statistically significant differences in hospitalization rates, whereas the differences in the other 2 studies were not statistically significant (P<.05). Combining studies with the use of a random effects model (the default methodology for this analysis) or a fixed effects model produced similar results. The Q statistic indicated no significant heterogeneity (P=.43).
TABLE 2
Meta-analysis results for inhaled corticosteroid vs leukotriene modifier therapy*
| Inhaled corticosteroid vs leukotriene modifier patients † | ||||
|---|---|---|---|---|
| Inhaled corticosteroids | Leukotriene modifiers | Absolute difference | Relative difference | |
| Annual asthma hospitalizations‡ | 2.23% (1.69-2.78) | 4.30% (3.53-5.07) | -1.79% (-2.45 to -1.14) | -42.88% (-55.95 to -29.80) |
| Annual rate of visits to the emergency department due to asthma§ | 6.19% (4.84-7.53) | 7.74% (6.30-9.19) | -1.53% (-1.78 to -1.28) | -21.35% (-25.31 to -17.38) |
| Total annual costs of visits to the emergency department | $93 (38-148) | $73 (52-94) | $21(-17 to 59) | 1.00% (-38 to 40) |
| Total annual drug costs§ | $807 (548-1065) | $1062 (812-1312) | -$258 (-308 to -208) | -27.20% (-33.2 to -21.3) |
| Annual asthma-related cost‡ | $882 (613-1150) | $1393 (1143-1643) | $513 (-392 to -634) | -38.01% (-47.4 to -28.8) |
| Total annual cost | $5254 (4474-6033) | $7140 (4970-9311) | -$1918 (-3509 to -327) | -17.20% (-30.9 to -3.5) |
| *Data are presented as mean (95% confidence interval). | ||||
| †Absolute and relative differences were determined from meta-analyses of the absolute and relative differences for each included study. | ||||
| ‡Inhaled corticosteroid vs leukotriene modifier significant at P<.05. | ||||
| §Inhaled corticosteroid vs leukotriene modifier significant at P<.005. | ||||
FIGURE
Difference in hospitalization rates (mean, confidence interval)
Secondary outcomes
Results of secondary analyses for 5 study outcomes (annual visits to the emergency department due to asthma, total emergency department costs, total drug costs, total asthma-related costs, and overall total cost) are presented in Table 2.
Mean annual rates of visits to the emergency department and total annual drug costs were significantly higher for patients taking leukotriene modifiers than for those taking inhaled corticosteroids (P<.005 for each). Patients taking leukotriene modifiers had lower annual costs for visits to the emergency department than did those taking inhaled corticosteroids, although this difference was not statistically significant. The higher rate and lower cost of emergency department visits for patients taking leukotriene modifiers suggest that medical resources were used less at each visit as compared with those for patients taking inhaled corticosteroids.
Total asthma-related costs for patients taking inhaled corticosteroids were significantly lower than those for patients taking leukotriene modifiers (P<.05). Patients taking inhaled corticosteroids also incurred decreased annual total (allcause) medical care costs. Although this difference was qualitatively large (approximately $1900, or a decrease of over 17%), it did not reach statistical significance.
Pre- vs post-initiation of therapy
In addition to comparing the impact of the 2 therapies on resource use and costs, we were interested in the impact of initiating each therapy on the study outcomes. We therefore assessed resource use rates and costs before and after treatment initiation. These patients may have been receiving asthma therapies (or no asthma therapy) other than inhaled corticosteroids or leukotriene modifiers. The number of studies presenting data on costs was too small to allow comparison.
Results of this within-group analysis are presented in Table 3. For patients taking inhaled corticosteroids, hospitalization rates and emergency department visit rates decreased significantly after treatment initiation compared with the preinitiation values (P<.005 and P<.05, respectively). The decreases for patients taking leukotriene modifiers upon treatment initiation were smaller and not statistically significant.
Both groups showed similar small decreases in annual emergency department costs, neither of which was significant. The increases in annual total drug costs and annual total medical care costs after treatment initiation were significant for both groups of patients (all at P<.005). However, both increases were greater for patients taking leukotriene modifiers; the increase in drug costs was statistically significant (P<.001).
TABLE 3
Resource utilization and costs before and after therapy initiation for inhaled corticosteroids vs leukotriene modifiers*
| Before vs after treatment, mean (95% confidence interval) | |||
|---|---|---|---|
| Inhaled corticosteroid patients | Leukotriene modifier patients | Absolute difference | |
| Annual hospitalization rate | -2.37%† (-2.89 to -1.86) | -0.55% (-1.18 to 0.08) | -1.91%‡(-2.45 to -1.36) |
| Annual rate of visits to the emergency department due to asthma | -4.44%§ (-5.98 to -2.90) | -2.06% (-3.61 to -0.51) | -2.47%||(-3.09 to -1.86) |
| Total annual costs of the emergency despartment | -$5 (-21 to 10) | -$15 (-44 to 15) | $9 (-33 to 51) |
| Total annual drug costs | $415†(312-517) | $579† (472-686) | -$167||(-192 to 142) |
| Total annual medical care costs | $641† (113-1169) | $1712† (927-3529) | -$1080 (-2802 to 643) |
| *Post-therapy initiation values for inhaled corticosteroids and leukotriene modifiers are presented in Table 1. | |||
| †Before vs after treatment initiation significant at P<.005. | |||
| ‡Before vs after treatment initiation outcomes for inhaled corticosteroids vs leukotriene modifiers significant at P<.01. | |||
| §Before vs after treatment initiation significant at P<.05. | |||
| ||Before vs after treatment initiation outcomes for inhaled corticosteroids vs leukotriene modifiers significant at P<.001. | |||
Discussion
In this study, we used meta-analysis to combine data across studies and determine more robust estimates of the impact of inhaled corticosteroid vs leukotriene modifier therapy on medical resource use rates and costs. The primary analysis indicated that annual hospitalization rates among patients taking inhaled corticosteroids are significantly lower that those taking leukotriene modifiers. Other resource use rates and costs evaluated in this study also generally showed decreased values for patients taking inhaled corticosteroids.
Although meta-analysis generally has been used for clinical outcome measures, it is a highly appropriate method for resource use and cost outcomes. In general, studies of the impact of a particular treatment are powered to assess safety and efficacy or effectiveness; there is often insufficient power to detect differences in resource use or costs in any one study. Due to substantial variation in treatment patterns, the variance associated with resource use rates (and associated costs) may be substantially higher than that for clinical measures; such a wide variance adds to the difficulties in assessing differences for nonclinical outcomes.
Limitations
This study has a number of limitations. Only a few studies met inclusion criteria for the meta-analysis; the analysis should be replicated as additional studies become available. Data were abstracted from the included studies without modification (except for inflating costs to 2000 values when necessary). As in all meta-analyses, any problems present in the original data are present in the combined data; limitations of the original data are not addressed by this method.
Among the studies evaluated for the metaanalysis were a number published only as abstracts. Inclusion of unpublished literature in meta-analyses is controversial; however, several sources18,19 now recommend inclusion of published and unpublished studies. Egger and Smith26 found that studies with significant results are more likely to be published than are studies with nonsignificant results, leading to publication bias. Studies with significant results also may be more likely to be published in indexed journals, leading to “database bias.” As such, inclusion of unpublished studies is important to produce unbiased results.
Five of the 6 studies that met the inclusion criteria were observational, retrospective cohort analyses. Whereas many meta-analyses focus solely on prospective, randomized clinical trials, several have included retrospective data.27,28 Retrospective analyses and observational data have a number of limitations, in particular the lack of randomization that can lead to differences in characteristics of specified treatment groups. Further, as discussed by Egger et al,29 metaanalyses based on observational studies may involve bias and confounding.
However, observational data and retrospective analyses also have the advantage of reflecting “real world” treatment patterns and broader patient groups that increase the generalizability of the data, whereas clinical trials may include protocol-driven utilization and selected patient groups. Clinical trials also may occur in specialized health care settings, whereas observational (cohort) data may be more applicable to clinical practice. Due to these factors, meta-analysis of observational data has become common.29
The pre-initiation vs post-initiation analysis indicated that values for each treatment group provide information on the similarities between treatment groups before initiation of controller therapy. Even though the treatment groups were not randomized to each therapy and we have no means to ensure compatibility between groups, having similar rates of resource use between groups provides some evidence regarding similarity. Nonetheless, given the limitations of the retrospective data and meta-analysis in general, it will be important to validate the results of this meta-analysis in the future with naturalistic prospective studies.
Despite these limitations, this study provides important information on the impact of asthma therapies on resource use and costs. Specifically, the resource use and cost outcomes assessed in this study were lower for inhaled corticosteroid patients than for leukotriene modifier patients. This study also illustrates the usefulness of metaanalysis in evaluating resource use and costs. By selecting and combining outcomes across studies in a standardized, rigorous, and transparent manner, the effects of different therapies can be evaluated with greater precision.
Acknowledgments
We thank John O’Donnell and Layne Gothard for their assistance with this manuscript.
Corresponding author
Michael T. Halpern, MD, PhD, Principal Scientist, Exponent, Inc., 1800 Diagonal Road, Alexandria, VA 22314. E-mail: [email protected]
Objective To compare the effects of inhaled corticosteroid treatment with leukotriene modifier treatment on medical resource use and costs for asthma patients.
Study design Meta-analysis combining results from published and unpublished studies.
Data sources Studies were identified from the MEDLINE and EMBASE databases and the GlaxoSmithKline internal database study registers. Two independent reviewers evaluated the identified studies; studies meeting specified inclusion criteria were abstracted and summarized by meta-analysis with a random effects model.
Outcomes measured Hospitalization rate, emergency department visit rate, emergency department costs, drug costs, total asthma-related costs, and total medical care costs.
Results Patients taking inhaled corticosteroids had:
a significantly lower annual rate of hospitalization than those taking leukotriene modifiers (2.2% vs 4.3%, respectively;P<.05)
a greater decline in hospitalization rate (before vs after therapy initiation) than those taking leukotriene modifiers (decline of 2.4% vs 0.55%; P<.01)
a lower annual rate of emergency department visits than those taking leukotriene modifiers (6.2% vs 7.7%;P<.005).
lower total asthma-related medical costs than those taking leukotriene modifiers (P<.05) and a 17% reduction in overall total medical care costs (P not significant).
Conclusions Patients with asthma treated with inhaled corticosteroids had significantly fewer asthma-related hospitalizations and emergency department visits and lower total asthma-related health care costs than patients treated with leukotriene modifiers. These meta-analysis findings are consistent with results from randomized controlled trials showing improvements in lung function for patients taking inhaled corticosteroids as opposed to leukotriene modifiers.
Although many medications are available for patients with asthma, inhaled corticosteroids are generally the preferred treatment.1-4 Multiple studies have demonstrated that inhaled corticosteroid therapy improves patient outcomes.1 Inhaled corticosteroids have been shown to decrease costs5 and use of medical care resources.6-8
More recently, leukotriene modifiers have been introduced for asthma treatment. This class of medication has bronchodilator and anti-inflammatory effects.9 Although multiple studies have indicated improved outcomes and decreased costs associated with leukotriene modifier therapy incertain patient populations,10,11 its role in asthmamanagement is uncertain.9
Several studies have compared the clinical outcomes of these therapies12-15 and their impact on medical care resource use and costs.16,17 However, these studies were not powered specifically to detect significant differences in resource use or costs.
We performed a meta-analysis to (1) compare the rate of hospitalization among patients with asthma treated with inhaled corticosteroids vs those treated with leukotriene modifiers and (2) evaluate other resource use rates and costs for these patients.
Methods
The meta-analysis consisted of a literature search, the development of inclusion criteria, form development, and literature review.
Literature search
We searched the MEDLINE, EMBASE, Cochrane Collaboration Study Registry, and GlaxoSmithKline databases and consulted experts in this field. The GlaxoSmithKline database consists of studies sponsored by GlaxoSmithKline that met companywide minimum quality thresholds and were published in full or abstract form.
We also contacted the manufacturers of leukotriene modifiers available in the United States, AstraZeneca and Merck, to request published and unpublished information on studies comparing leukotriene modifiers with inhaled corticosteroids. To provide results corresponding to current treatment patterns, only studies from 1991 to 2001 were included. Published and unpublished materials were included.18,19
Inclusion criteria
Studies were included in the meta-analysis if they met the following criteria.
Population. Patients with diagnosed asthma. Only studies that did not restrict analyses to severe asthma patients or children were included.
Study design. Prospective and retrospective comparative studies of patients receiving inhaled corticosteroid or leukotriene modifier monotherapy (no other controller therapy) in the same study. Studies were required to have defined inclusion and exclusion criteria, defined number of patients in each study arm, defined treatment protocol (ie, medications and doses used), and separate results for each medication.
Only studies presenting primary research (hence excluding review articles and metaanalyses) were included. Only studies presenting data for at least 6 months on all participants were included.
Outcomes. Hospitalization visit rates and costs, emergency department visit rates and costs, pharmacy costs, total asthma-related costs, and total medical care costs. Because resource use patterns and medical care cost information differs substantially between countries, we only included US studies.
Study process
Each identified article was evaluated by 2 independent reviewers (KMS and MG); any differences were discussed with a project leader to reach a consensus. Documents selected for inclusion were then reviewed by the 2 reviewers, and differences in data abstraction were resolved before inclusion.
Analysis
We used the Q statistic20 to assess heterogeneity and, when appropriate, combined data from included studies with the use of a random effects model. Random effects methodology was used to assess the impact of inhaled corticosteroid vs leukotriene modifier therapy on the overall asthma population, not just the subpopulation of patients participating in included studies.21
Two sets of meta-analyses were performed with the abstracted data. First, the specified outcome measures were compared for patients taking inhaled corticosteroid vs leukotriene modifier therapy. Second, the impact (before vs after) of each treatment initiation was compared for each outcome.
Assessment of statistically significant differences between meta-analysis results was performed with the Student t test, with an Α of .05. Charges were used in the included studies as proxies for costs. These costs were inflated to 2000 values by using the medical care component of the consumer price index before inclusion.
Results
We identified 49 documents and reviewed them for inclusion in the meta-analysis; 6 (12.2%) met the inclusion criteria (Table 1). Five were retrospective cohort studies; only 1 study was identified as a prospective trial comparing inhaled corticosteroid and leukotriene modifier therapies and including results on resource use or medical care costs.16,17,22-25 All 6 studies were performed with support from GlaxoSmithKline.
Forty-three documents were excluded due to 1 or more of the following criteria: lack of primary results (9 documents, 21%); did not contain resource use rate or cost outcomes (22 documents, 51%); did not provide at least 6 months’ worth of data on resource use or cost outcomes (5 documents, 12%); did not meet the defined inclusion and exclusion criteria (primarily studies not including both inhaled corticosteroid and leukotriene modifiers or those restricted to patient clinical subgroups; 10 documents, 34%); or did not define the number of patients included in the study (1 document, 3%).
Because few studies presented data on the specified outcomes, we were unable to assess asthma-specific costs for subcategories of resource use. Too few studies included data on hospitalization costs (either asthma-specific or overall) to include in the analysis. Therefore, meta-analysis was performed on overall (ie, all causes) emergency department, pharmacy, and total medical care costs.
TABLE 1
Characteristics of studies included in the meta-analysis
| Duration (mo) | |||||
|---|---|---|---|---|---|
| Study | LOE* | Before therapy | After therapy | Treatment (N) | Comparison (N) |
| Oates and Gothard22 | 2b | 9 | 9 | Inhaled corticosteroids (546)† | Leukotriene modifiers (152)‡ |
| Pathak et al23 | 2b | 9 | 9 | Fluticasone propionate (284) | Leukotriene modifiers (497)‡ |
| Stanford et al24 | 1b | - | 6 | Fluticasone propionate (271) | Montelukast (262) |
| Stempel et al16 | 2b | 9 | 12 | Fluticasone propionate (602) | Zafirlukast (309) |
| Stempel et al17 | 2b | 9 | 9 | Fluticasone propionate (559) | Montelukast (382) |
| White et al25 | 2b | 9 | 9 | Inhaled corticosteroids (1305)† | Leukotriene modifiers (109)‡ |
| *LOE, level of evidence. For an explanation of levels of evidence. | |||||
| †Results were presented for all inhaled corticosteroids combined. | |||||
| ‡Results were presented for all leukotriene modifiers combined. | |||||
Primary analysis
The primary objective of this study was to evaluate the impact of inhaled corticosteroid and leukotriene modifier treatment on the mean annual hospitalization rate. Four of the 6 included studies contained information on hospitalization rate for each treatment. Results from the primary analysis are presented in Table 2.
Patients taking inhaled corticosteroids had a significantly lower annual rate of hospitalization than did patients taking leukotriene modifiers (2.23% vs 4.30%, respectively; P<.005). The absolute risk reduction was 2.07% (number needed to treat=48 for 1 year).
The difference in annual hospitalization visit rates for each study in the primary analysis is presented in the Figure, where negative values reflect lower hospitalization rates among patients taking inhaled corticosteroids than among those taking leukotriene modifiers. Two studies16,23 had statistically significant differences in hospitalization rates, whereas the differences in the other 2 studies were not statistically significant (P<.05). Combining studies with the use of a random effects model (the default methodology for this analysis) or a fixed effects model produced similar results. The Q statistic indicated no significant heterogeneity (P=.43).
TABLE 2
Meta-analysis results for inhaled corticosteroid vs leukotriene modifier therapy*
| Inhaled corticosteroid vs leukotriene modifier patients † | ||||
|---|---|---|---|---|
| Inhaled corticosteroids | Leukotriene modifiers | Absolute difference | Relative difference | |
| Annual asthma hospitalizations‡ | 2.23% (1.69-2.78) | 4.30% (3.53-5.07) | -1.79% (-2.45 to -1.14) | -42.88% (-55.95 to -29.80) |
| Annual rate of visits to the emergency department due to asthma§ | 6.19% (4.84-7.53) | 7.74% (6.30-9.19) | -1.53% (-1.78 to -1.28) | -21.35% (-25.31 to -17.38) |
| Total annual costs of visits to the emergency department | $93 (38-148) | $73 (52-94) | $21(-17 to 59) | 1.00% (-38 to 40) |
| Total annual drug costs§ | $807 (548-1065) | $1062 (812-1312) | -$258 (-308 to -208) | -27.20% (-33.2 to -21.3) |
| Annual asthma-related cost‡ | $882 (613-1150) | $1393 (1143-1643) | $513 (-392 to -634) | -38.01% (-47.4 to -28.8) |
| Total annual cost | $5254 (4474-6033) | $7140 (4970-9311) | -$1918 (-3509 to -327) | -17.20% (-30.9 to -3.5) |
| *Data are presented as mean (95% confidence interval). | ||||
| †Absolute and relative differences were determined from meta-analyses of the absolute and relative differences for each included study. | ||||
| ‡Inhaled corticosteroid vs leukotriene modifier significant at P<.05. | ||||
| §Inhaled corticosteroid vs leukotriene modifier significant at P<.005. | ||||
FIGURE
Difference in hospitalization rates (mean, confidence interval)
Secondary outcomes
Results of secondary analyses for 5 study outcomes (annual visits to the emergency department due to asthma, total emergency department costs, total drug costs, total asthma-related costs, and overall total cost) are presented in Table 2.
Mean annual rates of visits to the emergency department and total annual drug costs were significantly higher for patients taking leukotriene modifiers than for those taking inhaled corticosteroids (P<.005 for each). Patients taking leukotriene modifiers had lower annual costs for visits to the emergency department than did those taking inhaled corticosteroids, although this difference was not statistically significant. The higher rate and lower cost of emergency department visits for patients taking leukotriene modifiers suggest that medical resources were used less at each visit as compared with those for patients taking inhaled corticosteroids.
Total asthma-related costs for patients taking inhaled corticosteroids were significantly lower than those for patients taking leukotriene modifiers (P<.05). Patients taking inhaled corticosteroids also incurred decreased annual total (allcause) medical care costs. Although this difference was qualitatively large (approximately $1900, or a decrease of over 17%), it did not reach statistical significance.
Pre- vs post-initiation of therapy
In addition to comparing the impact of the 2 therapies on resource use and costs, we were interested in the impact of initiating each therapy on the study outcomes. We therefore assessed resource use rates and costs before and after treatment initiation. These patients may have been receiving asthma therapies (or no asthma therapy) other than inhaled corticosteroids or leukotriene modifiers. The number of studies presenting data on costs was too small to allow comparison.
Results of this within-group analysis are presented in Table 3. For patients taking inhaled corticosteroids, hospitalization rates and emergency department visit rates decreased significantly after treatment initiation compared with the preinitiation values (P<.005 and P<.05, respectively). The decreases for patients taking leukotriene modifiers upon treatment initiation were smaller and not statistically significant.
Both groups showed similar small decreases in annual emergency department costs, neither of which was significant. The increases in annual total drug costs and annual total medical care costs after treatment initiation were significant for both groups of patients (all at P<.005). However, both increases were greater for patients taking leukotriene modifiers; the increase in drug costs was statistically significant (P<.001).
TABLE 3
Resource utilization and costs before and after therapy initiation for inhaled corticosteroids vs leukotriene modifiers*
| Before vs after treatment, mean (95% confidence interval) | |||
|---|---|---|---|
| Inhaled corticosteroid patients | Leukotriene modifier patients | Absolute difference | |
| Annual hospitalization rate | -2.37%† (-2.89 to -1.86) | -0.55% (-1.18 to 0.08) | -1.91%‡(-2.45 to -1.36) |
| Annual rate of visits to the emergency department due to asthma | -4.44%§ (-5.98 to -2.90) | -2.06% (-3.61 to -0.51) | -2.47%||(-3.09 to -1.86) |
| Total annual costs of the emergency despartment | -$5 (-21 to 10) | -$15 (-44 to 15) | $9 (-33 to 51) |
| Total annual drug costs | $415†(312-517) | $579† (472-686) | -$167||(-192 to 142) |
| Total annual medical care costs | $641† (113-1169) | $1712† (927-3529) | -$1080 (-2802 to 643) |
| *Post-therapy initiation values for inhaled corticosteroids and leukotriene modifiers are presented in Table 1. | |||
| †Before vs after treatment initiation significant at P<.005. | |||
| ‡Before vs after treatment initiation outcomes for inhaled corticosteroids vs leukotriene modifiers significant at P<.01. | |||
| §Before vs after treatment initiation significant at P<.05. | |||
| ||Before vs after treatment initiation outcomes for inhaled corticosteroids vs leukotriene modifiers significant at P<.001. | |||
Discussion
In this study, we used meta-analysis to combine data across studies and determine more robust estimates of the impact of inhaled corticosteroid vs leukotriene modifier therapy on medical resource use rates and costs. The primary analysis indicated that annual hospitalization rates among patients taking inhaled corticosteroids are significantly lower that those taking leukotriene modifiers. Other resource use rates and costs evaluated in this study also generally showed decreased values for patients taking inhaled corticosteroids.
Although meta-analysis generally has been used for clinical outcome measures, it is a highly appropriate method for resource use and cost outcomes. In general, studies of the impact of a particular treatment are powered to assess safety and efficacy or effectiveness; there is often insufficient power to detect differences in resource use or costs in any one study. Due to substantial variation in treatment patterns, the variance associated with resource use rates (and associated costs) may be substantially higher than that for clinical measures; such a wide variance adds to the difficulties in assessing differences for nonclinical outcomes.
Limitations
This study has a number of limitations. Only a few studies met inclusion criteria for the meta-analysis; the analysis should be replicated as additional studies become available. Data were abstracted from the included studies without modification (except for inflating costs to 2000 values when necessary). As in all meta-analyses, any problems present in the original data are present in the combined data; limitations of the original data are not addressed by this method.
Among the studies evaluated for the metaanalysis were a number published only as abstracts. Inclusion of unpublished literature in meta-analyses is controversial; however, several sources18,19 now recommend inclusion of published and unpublished studies. Egger and Smith26 found that studies with significant results are more likely to be published than are studies with nonsignificant results, leading to publication bias. Studies with significant results also may be more likely to be published in indexed journals, leading to “database bias.” As such, inclusion of unpublished studies is important to produce unbiased results.
Five of the 6 studies that met the inclusion criteria were observational, retrospective cohort analyses. Whereas many meta-analyses focus solely on prospective, randomized clinical trials, several have included retrospective data.27,28 Retrospective analyses and observational data have a number of limitations, in particular the lack of randomization that can lead to differences in characteristics of specified treatment groups. Further, as discussed by Egger et al,29 metaanalyses based on observational studies may involve bias and confounding.
However, observational data and retrospective analyses also have the advantage of reflecting “real world” treatment patterns and broader patient groups that increase the generalizability of the data, whereas clinical trials may include protocol-driven utilization and selected patient groups. Clinical trials also may occur in specialized health care settings, whereas observational (cohort) data may be more applicable to clinical practice. Due to these factors, meta-analysis of observational data has become common.29
The pre-initiation vs post-initiation analysis indicated that values for each treatment group provide information on the similarities between treatment groups before initiation of controller therapy. Even though the treatment groups were not randomized to each therapy and we have no means to ensure compatibility between groups, having similar rates of resource use between groups provides some evidence regarding similarity. Nonetheless, given the limitations of the retrospective data and meta-analysis in general, it will be important to validate the results of this meta-analysis in the future with naturalistic prospective studies.
Despite these limitations, this study provides important information on the impact of asthma therapies on resource use and costs. Specifically, the resource use and cost outcomes assessed in this study were lower for inhaled corticosteroid patients than for leukotriene modifier patients. This study also illustrates the usefulness of metaanalysis in evaluating resource use and costs. By selecting and combining outcomes across studies in a standardized, rigorous, and transparent manner, the effects of different therapies can be evaluated with greater precision.
Acknowledgments
We thank John O’Donnell and Layne Gothard for their assistance with this manuscript.
Corresponding author
Michael T. Halpern, MD, PhD, Principal Scientist, Exponent, Inc., 1800 Diagonal Road, Alexandria, VA 22314. E-mail: [email protected]
1. Janson S. National asthma education and prevention program, expert panel report. II: overview and application to primary care. Prim Care Pract 1998;2:578-588.
2. Lalloo UG, Bateman ED, Feldman C, et al. Guideline for the management of chronic asthma in adults—2000 update. South African Pulmonology Society Adult Asthma Working Group. S Afr Med J 2000;90:540-541-544-552.
3. Podell RN. National guidelines for the management of asthma in adults. Am Fam Physician 1992;46:1189-1196.
4. Veninga CC, Lagerlov P, Wahlstrom R, et al. Evaluating an educational intervention to improve the treatment of asthma in four European countries. Drug Education Project Group. Am J Respir Crit Care Med 1999;160:1254-1262.
5. Ozminkowski RJ, Wang S, Marder WD, Azzolini J, Schutt D. Cost implications for the use of inhaled anti-inflammatory medications in the treatment of asthma. Pharmacoeconomics 2000;18:253-264.
6. Paltiel AD, Fuhlbrigge AL, Kitch BT, et al. Cost-effectiveness of inhaled corticosteroids in adults with mild-to-moderate asthma: results from the asthma policy model. J Allergy Clin Immunol 2001;108:39-49.
7. Adams RJ, Fuhlbrigge A, Finkelstein JA, et al. Impact of inhaled antiinflammatory therapy on hospitalization and emergency department visits for children with asthma. Pediatrics 2001;107:706-711.
8. Donahue JG, Weiss ST, Livingston JM, Goetsch MA, Greineder DK, Platt R. Inhaled steroids and the risk of hospitalization for asthma. JAMA 1997;277:887-891.
9. Dempsey OJ. Leukotriene receptor antagonist therapy. Postgrad Med J 2000;76:767-773.
10. Klingman D, Bielory L, Wang Y, et al. Asthma outcome changes associated with use of the leukotriene-receptor antagonist zafirlukast. Manag Care Interface 2001;14:62-66.
11. Price DB, Ben-Joseph RH, Zhang Q. Changes in asthma drug therapy costs for patients receiving chronic montelukast therapy in the U.K. Resp Med 2001;95:83-89.
12. Bleecker ER, Welch MJ, Weinstein SF, et al. Low-dose inhaled fluticasone propionate versus oral zafirlukast in the treatment of persistent asthma. J Allergy Clin Immunol 2000;105:1123-1129.
13. Ind PW. Inhaled corticosteroids versus anti-leukotrienes: a literature review on the clinical effects. Allergy 1999;54(suppl 50):43-46.
14. Malmstrom K, Rodriguez-Gomez G, Guerra J, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med 1999;130:487-495.
15. Busse W, Nelson H, Wolfe J, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in patients with asthma. J Allergy Clin Immunol 1999;103:1075-1080.
16. Stempel DA, Meyer JW, Stanford RH, Yancey SW. One-year claims analysis comparing inhaled fluticasone propionate with zafirlukast for the treatment of asthma. J Allergy Clin Immunol 2001;107:94-98.
17. Stempel DA, Mauskopf J, McLaughlin T, Yazdani C, Stanford RH. Comparison of asthma costs in patients starting fluticasone propionate compared to patients starting montelukast. Respir Med 2001;95:227-234.
18. Cook DJ, Guyatt GH, Ryan G, et al. Should unpublished data be included in meta-analyses? Current convictions and controversies. JAMA 1993;269:2749-2753.
19. McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet 2000;356:1228-1231.
20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-188.
21. Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care 1990;6:5-30.
22. Oates V, Gothard L. PEER Study: New Starts on Inhaled Corticosteroids or Leukotriene Modifiers in an Asthmatic Population. Advanced Paradigm and GlaxoWellcome, Inc. July 19, 2000. Information available from GlaxoSmithKline at 1-800-825-5249.
23. Pathak DS, Davis EA, Stanford RH. Economic impact of asthma therapy with fluticasone propionate, montelukast, or zafirlukast in a managed care population. Pharmacotherapy 2002;22:166-174.
24. Stanford R, Davis A, Edwards L, Kalberg C, Rickard K. The costs and efficacy of fluticasone propionate 88 mcg twice daily and montelukast 10 mg once daily in patients needing single controller therapy. Chest 2001;120:225S.-
25. White TJ, Gothard L, Fontes CL, Juzba M, Berenbeim DM, Gilderman AM. A Retrospective Administrative Healthcare Claims Analysis to Describe and Assess Pharmaceutical and Medical Resource Utilization within the PacifiCare CA Healthplan PROJECT 2: Longitudinal Analysis of Newly Diagnosed Asthmatics. Prescription Solutions/PacifiCare Health Systems and GlaxoWellcome Inc. November 27, 2000. Information available from GlaxoSmithKline at 1-800-825-5249.
26. Egger M, Smith GD. Meta-analysis bias in location and selection of studies. BMJ 1998;316:61-66.
27. Ebell MH. Prearrest predictors of survival following inhospital cardiopulmonary resuscitation: a meta-analysis. J Fam Pract 1992;34:551-558.
28. Poynard T, Moussalli J, Ratziu V, et al. Is antiviral treatment (IFN alpha and/or ribavirin) justified in cirrhosis related to hepatitis C virus? Societe Royale Belge de Gastroenterologie. Acta Gastroenterol Belg 1998;61:431-437.
29. Egger M, Schneider M, Smith GD. Meta-analysis spurious precision? Meta-analysis of observational studies. BMJ 1998;316:140-144.
1. Janson S. National asthma education and prevention program, expert panel report. II: overview and application to primary care. Prim Care Pract 1998;2:578-588.
2. Lalloo UG, Bateman ED, Feldman C, et al. Guideline for the management of chronic asthma in adults—2000 update. South African Pulmonology Society Adult Asthma Working Group. S Afr Med J 2000;90:540-541-544-552.
3. Podell RN. National guidelines for the management of asthma in adults. Am Fam Physician 1992;46:1189-1196.
4. Veninga CC, Lagerlov P, Wahlstrom R, et al. Evaluating an educational intervention to improve the treatment of asthma in four European countries. Drug Education Project Group. Am J Respir Crit Care Med 1999;160:1254-1262.
5. Ozminkowski RJ, Wang S, Marder WD, Azzolini J, Schutt D. Cost implications for the use of inhaled anti-inflammatory medications in the treatment of asthma. Pharmacoeconomics 2000;18:253-264.
6. Paltiel AD, Fuhlbrigge AL, Kitch BT, et al. Cost-effectiveness of inhaled corticosteroids in adults with mild-to-moderate asthma: results from the asthma policy model. J Allergy Clin Immunol 2001;108:39-49.
7. Adams RJ, Fuhlbrigge A, Finkelstein JA, et al. Impact of inhaled antiinflammatory therapy on hospitalization and emergency department visits for children with asthma. Pediatrics 2001;107:706-711.
8. Donahue JG, Weiss ST, Livingston JM, Goetsch MA, Greineder DK, Platt R. Inhaled steroids and the risk of hospitalization for asthma. JAMA 1997;277:887-891.
9. Dempsey OJ. Leukotriene receptor antagonist therapy. Postgrad Med J 2000;76:767-773.
10. Klingman D, Bielory L, Wang Y, et al. Asthma outcome changes associated with use of the leukotriene-receptor antagonist zafirlukast. Manag Care Interface 2001;14:62-66.
11. Price DB, Ben-Joseph RH, Zhang Q. Changes in asthma drug therapy costs for patients receiving chronic montelukast therapy in the U.K. Resp Med 2001;95:83-89.
12. Bleecker ER, Welch MJ, Weinstein SF, et al. Low-dose inhaled fluticasone propionate versus oral zafirlukast in the treatment of persistent asthma. J Allergy Clin Immunol 2000;105:1123-1129.
13. Ind PW. Inhaled corticosteroids versus anti-leukotrienes: a literature review on the clinical effects. Allergy 1999;54(suppl 50):43-46.
14. Malmstrom K, Rodriguez-Gomez G, Guerra J, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med 1999;130:487-495.
15. Busse W, Nelson H, Wolfe J, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in patients with asthma. J Allergy Clin Immunol 1999;103:1075-1080.
16. Stempel DA, Meyer JW, Stanford RH, Yancey SW. One-year claims analysis comparing inhaled fluticasone propionate with zafirlukast for the treatment of asthma. J Allergy Clin Immunol 2001;107:94-98.
17. Stempel DA, Mauskopf J, McLaughlin T, Yazdani C, Stanford RH. Comparison of asthma costs in patients starting fluticasone propionate compared to patients starting montelukast. Respir Med 2001;95:227-234.
18. Cook DJ, Guyatt GH, Ryan G, et al. Should unpublished data be included in meta-analyses? Current convictions and controversies. JAMA 1993;269:2749-2753.
19. McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet 2000;356:1228-1231.
20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-188.
21. Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care 1990;6:5-30.
22. Oates V, Gothard L. PEER Study: New Starts on Inhaled Corticosteroids or Leukotriene Modifiers in an Asthmatic Population. Advanced Paradigm and GlaxoWellcome, Inc. July 19, 2000. Information available from GlaxoSmithKline at 1-800-825-5249.
23. Pathak DS, Davis EA, Stanford RH. Economic impact of asthma therapy with fluticasone propionate, montelukast, or zafirlukast in a managed care population. Pharmacotherapy 2002;22:166-174.
24. Stanford R, Davis A, Edwards L, Kalberg C, Rickard K. The costs and efficacy of fluticasone propionate 88 mcg twice daily and montelukast 10 mg once daily in patients needing single controller therapy. Chest 2001;120:225S.-
25. White TJ, Gothard L, Fontes CL, Juzba M, Berenbeim DM, Gilderman AM. A Retrospective Administrative Healthcare Claims Analysis to Describe and Assess Pharmaceutical and Medical Resource Utilization within the PacifiCare CA Healthplan PROJECT 2: Longitudinal Analysis of Newly Diagnosed Asthmatics. Prescription Solutions/PacifiCare Health Systems and GlaxoWellcome Inc. November 27, 2000. Information available from GlaxoSmithKline at 1-800-825-5249.
26. Egger M, Smith GD. Meta-analysis bias in location and selection of studies. BMJ 1998;316:61-66.
27. Ebell MH. Prearrest predictors of survival following inhospital cardiopulmonary resuscitation: a meta-analysis. J Fam Pract 1992;34:551-558.
28. Poynard T, Moussalli J, Ratziu V, et al. Is antiviral treatment (IFN alpha and/or ribavirin) justified in cirrhosis related to hepatitis C virus? Societe Royale Belge de Gastroenterologie. Acta Gastroenterol Belg 1998;61:431-437.
29. Egger M, Schneider M, Smith GD. Meta-analysis spurious precision? Meta-analysis of observational studies. BMJ 1998;316:140-144.