User login
At-Home Stroke Rehab Program as Effective as Outpatient Training
LOS ANGELES – An intensive poststroke home exercise program was as effective in improving walking speed as was a standard-care outpatient locomotor program in a randomized trial.
The learning platform did not matter nearly as much as the intervention’s timing, Pamela Duncan, Ph.D., reported at the International Stroke Conference. Patients who began to relearn walking 2 months after their stroke with the locomotor or home-based program progressed faster than did those randomized to the standard care of locomotor therapy beginning 6 months after their stroke.
"Patients recover faster and sustain recovery when the intervention is given early," she said.
Patients in the at-home exercise program also had significantly fewer falls than did those who underwent early outpatient locomotor training.
Dr. Duncan’s LEAPS (Locomotor Experience Applied Post Stroke) study randomized 408 patients to three groups: an early in-home program, an early outpatient locomotor program, and a wait-listed group that began outpatient locomotor training 6 months post stroke. Each training program lasted 12 weeks, and involved three 90-minute sessions per week.
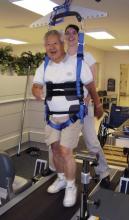
The locomotor program consisted of treadmill walking with the patient’s body weight fully or partially supported by a harness. As patients progressed, the harness supported less and less of their weight, until they were able to walk on the treadmill independently. This required two or three therapists for every patient.
The home-based program focused on strength and balance, with encouragement to walk every day. Exercises were intensive and challenging, including many repetitions and resistance training for lower-extremity muscles. One therapist was able to conduct the session, said Dr. Duncan, professor of physical therapy at Duke University, Durham, N.C.
At baseline, the patients’ mean age was 62 years, and 83% had suffered an ischemic stroke. At randomization, the mean time from stroke was 64 days.
Mobility was severely impaired in 53% of patients, who had a mean gait speed of less than 0.4 meters per second (m/s). The rest of the patients were moderately impaired with respect to walking, with a mean gait speed of 0.4-0.8 m/s. They took a median of 1,700 steps daily. "Overall, these patients were taking [fewer than] 20% of the 10,000 steps required to maintain physical health," Dr. Duncan said.
The final analysis included 139 patients who were randomized to early outpatient training, 126 to the early at-home training, and 143 who were wait-listed.
"At 6 months, we found a statistically – and clinically – significant difference between the two early-intervention groups and the wait-listed group," Dr. Duncan said. Patients in the locomotor-training program and the home exercise program improved similarly: The mean gait speed increases were 0.25 m/s and 0.23 m/s, respectively. However, by 6 months the wait-listed group had improved by a mean of only 0.13 m/s. "Both early groups also improved in their activities of daily living and quality of life scores, with the wait-listed group improving about half as much," she added.
Falls were another important end point of the LEAPS trial. "Individuals who are increasing mobility after stroke are at risk of falling," Dr. Duncan said. "Overall, we saw a 58% incidence of falls from 2 months to 1 year after the stroke." Most of these were single falls, but 34% of patients experienced multiple falls; 6% resulted in injury.
There were significantly more falls in the early locomotor-training group than in either the home exercise group or the late-intervention group. "This was largely attributed to multiple falls in the group of most severely disabled patients," Dr. Duncan said at the meeting, which was sponsored by the American Heart Association.
There were 10 patients who experienced serious adverse events, all of which were cardiovascular symptoms or blurred vision; all required hospital admission. They were similarly distributed, with three in the early-locomotor program, five in the late-locomotor program, and two in the home exercise program. Nine of the 10 patients returned to their walking programs after discharge. Minor adverse events occurred in 56% of patients.
Although both early-intervention groups were significantly improved at 6 months, compared with the wait-listed group, the late starters caught up quickly: At 12 months, the wait-listed patients had improved their gait speed by 0.25 m/s – the same rate as those in early therapy. These late improvements counter "the widely held assumption and reports that most functional improvements after stroke are complete by 6 months," Dr. Duncan said.
Dr. Ralph Sacco, president of the American Heart Association and the chair of neurology at the University of Miami, noted in a press conference that smaller studies of stroke patients have showed that intensive use of weak upper extremities vastly improves arm function, and "now we see for the first time that the same thing can occur in the lower extremities." He pointed out, however, that many of the services that patients receive for gait improvement are now fiscally restrained by Medicare and insurance companies. "Therapy is stopped at a fairly early stage," and thus, so is recovery, he said. "We need to have the payers reexamine how long they will fund these therapies, because the at-home option appears to be a very cost effective way to pursue this."
The main message is that a lot of therapy is better than a little, regardless of whether it’s delivered by a therapist in the home or by a locomotor-training apparatus in the outpatient setting, said Dr. Sacco.
The trial was funded by the National Institute of Neurological Disorders and Stroke. Neither Dr. Duncan nor Dr. Sacco had financial disclosures.
We have seen smaller studies of stroke patients showing that intensive use of weak upper extremities vastly improves arm function, and now we see for the first time that the same thing can occur in the lower extremities. However, many of the services that patients receive for gait improvement are now fiscally restrained by Medicare and insurance companies. Therapy is stopped at a fairly early stage, and thus, so is recovery. We now know for the very first time that a more prolonged and intensive course of therapy will yield important gains. We need to have the payers reexamine how long they will fund these therapies, because the at-home option appears to be a very cost effective way to pursue this.
The main message is that a lot of therapy is better than a little, regardless of whether it's delivered by a therapist in the home or by a locomotor-training apparatus in the outpatient setting.
I suspect we will find that most patients will benefit from home therapy. There might be subgroups with particular gait disorders for whom the locomotor programs might be more beneficial. But this trial will have a major impact because it shows us that, initially, more - and more intensive - therapy is better for everyone.
Ralph Sacco, M.D., is the president of the American Heart Association and the chair of neurology at the University of Miami. He had no financial disclosures.
Pamela Duncan, Ph.D., International Stroke Conference, physical therapy, neurology, stroke recovery, falls, LEAPS Locomotor Experience Applied Post Stroke study, treadmill,
strength, balance,
We have seen smaller studies of stroke patients showing that intensive use of weak upper extremities vastly improves arm function, and now we see for the first time that the same thing can occur in the lower extremities. However, many of the services that patients receive for gait improvement are now fiscally restrained by Medicare and insurance companies. Therapy is stopped at a fairly early stage, and thus, so is recovery. We now know for the very first time that a more prolonged and intensive course of therapy will yield important gains. We need to have the payers reexamine how long they will fund these therapies, because the at-home option appears to be a very cost effective way to pursue this.
The main message is that a lot of therapy is better than a little, regardless of whether it's delivered by a therapist in the home or by a locomotor-training apparatus in the outpatient setting.
I suspect we will find that most patients will benefit from home therapy. There might be subgroups with particular gait disorders for whom the locomotor programs might be more beneficial. But this trial will have a major impact because it shows us that, initially, more - and more intensive - therapy is better for everyone.
Ralph Sacco, M.D., is the president of the American Heart Association and the chair of neurology at the University of Miami. He had no financial disclosures.
We have seen smaller studies of stroke patients showing that intensive use of weak upper extremities vastly improves arm function, and now we see for the first time that the same thing can occur in the lower extremities. However, many of the services that patients receive for gait improvement are now fiscally restrained by Medicare and insurance companies. Therapy is stopped at a fairly early stage, and thus, so is recovery. We now know for the very first time that a more prolonged and intensive course of therapy will yield important gains. We need to have the payers reexamine how long they will fund these therapies, because the at-home option appears to be a very cost effective way to pursue this.
The main message is that a lot of therapy is better than a little, regardless of whether it's delivered by a therapist in the home or by a locomotor-training apparatus in the outpatient setting.
I suspect we will find that most patients will benefit from home therapy. There might be subgroups with particular gait disorders for whom the locomotor programs might be more beneficial. But this trial will have a major impact because it shows us that, initially, more - and more intensive - therapy is better for everyone.
Ralph Sacco, M.D., is the president of the American Heart Association and the chair of neurology at the University of Miami. He had no financial disclosures.
LOS ANGELES – An intensive poststroke home exercise program was as effective in improving walking speed as was a standard-care outpatient locomotor program in a randomized trial.
The learning platform did not matter nearly as much as the intervention’s timing, Pamela Duncan, Ph.D., reported at the International Stroke Conference. Patients who began to relearn walking 2 months after their stroke with the locomotor or home-based program progressed faster than did those randomized to the standard care of locomotor therapy beginning 6 months after their stroke.
"Patients recover faster and sustain recovery when the intervention is given early," she said.
Patients in the at-home exercise program also had significantly fewer falls than did those who underwent early outpatient locomotor training.
Dr. Duncan’s LEAPS (Locomotor Experience Applied Post Stroke) study randomized 408 patients to three groups: an early in-home program, an early outpatient locomotor program, and a wait-listed group that began outpatient locomotor training 6 months post stroke. Each training program lasted 12 weeks, and involved three 90-minute sessions per week.
The locomotor program consisted of treadmill walking with the patient’s body weight fully or partially supported by a harness. As patients progressed, the harness supported less and less of their weight, until they were able to walk on the treadmill independently. This required two or three therapists for every patient.
The home-based program focused on strength and balance, with encouragement to walk every day. Exercises were intensive and challenging, including many repetitions and resistance training for lower-extremity muscles. One therapist was able to conduct the session, said Dr. Duncan, professor of physical therapy at Duke University, Durham, N.C.
At baseline, the patients’ mean age was 62 years, and 83% had suffered an ischemic stroke. At randomization, the mean time from stroke was 64 days.
Mobility was severely impaired in 53% of patients, who had a mean gait speed of less than 0.4 meters per second (m/s). The rest of the patients were moderately impaired with respect to walking, with a mean gait speed of 0.4-0.8 m/s. They took a median of 1,700 steps daily. "Overall, these patients were taking [fewer than] 20% of the 10,000 steps required to maintain physical health," Dr. Duncan said.
The final analysis included 139 patients who were randomized to early outpatient training, 126 to the early at-home training, and 143 who were wait-listed.
"At 6 months, we found a statistically – and clinically – significant difference between the two early-intervention groups and the wait-listed group," Dr. Duncan said. Patients in the locomotor-training program and the home exercise program improved similarly: The mean gait speed increases were 0.25 m/s and 0.23 m/s, respectively. However, by 6 months the wait-listed group had improved by a mean of only 0.13 m/s. "Both early groups also improved in their activities of daily living and quality of life scores, with the wait-listed group improving about half as much," she added.
Falls were another important end point of the LEAPS trial. "Individuals who are increasing mobility after stroke are at risk of falling," Dr. Duncan said. "Overall, we saw a 58% incidence of falls from 2 months to 1 year after the stroke." Most of these were single falls, but 34% of patients experienced multiple falls; 6% resulted in injury.
There were significantly more falls in the early locomotor-training group than in either the home exercise group or the late-intervention group. "This was largely attributed to multiple falls in the group of most severely disabled patients," Dr. Duncan said at the meeting, which was sponsored by the American Heart Association.
There were 10 patients who experienced serious adverse events, all of which were cardiovascular symptoms or blurred vision; all required hospital admission. They were similarly distributed, with three in the early-locomotor program, five in the late-locomotor program, and two in the home exercise program. Nine of the 10 patients returned to their walking programs after discharge. Minor adverse events occurred in 56% of patients.
Although both early-intervention groups were significantly improved at 6 months, compared with the wait-listed group, the late starters caught up quickly: At 12 months, the wait-listed patients had improved their gait speed by 0.25 m/s – the same rate as those in early therapy. These late improvements counter "the widely held assumption and reports that most functional improvements after stroke are complete by 6 months," Dr. Duncan said.
Dr. Ralph Sacco, president of the American Heart Association and the chair of neurology at the University of Miami, noted in a press conference that smaller studies of stroke patients have showed that intensive use of weak upper extremities vastly improves arm function, and "now we see for the first time that the same thing can occur in the lower extremities." He pointed out, however, that many of the services that patients receive for gait improvement are now fiscally restrained by Medicare and insurance companies. "Therapy is stopped at a fairly early stage," and thus, so is recovery, he said. "We need to have the payers reexamine how long they will fund these therapies, because the at-home option appears to be a very cost effective way to pursue this."
The main message is that a lot of therapy is better than a little, regardless of whether it’s delivered by a therapist in the home or by a locomotor-training apparatus in the outpatient setting, said Dr. Sacco.
The trial was funded by the National Institute of Neurological Disorders and Stroke. Neither Dr. Duncan nor Dr. Sacco had financial disclosures.
LOS ANGELES – An intensive poststroke home exercise program was as effective in improving walking speed as was a standard-care outpatient locomotor program in a randomized trial.
The learning platform did not matter nearly as much as the intervention’s timing, Pamela Duncan, Ph.D., reported at the International Stroke Conference. Patients who began to relearn walking 2 months after their stroke with the locomotor or home-based program progressed faster than did those randomized to the standard care of locomotor therapy beginning 6 months after their stroke.
"Patients recover faster and sustain recovery when the intervention is given early," she said.
Patients in the at-home exercise program also had significantly fewer falls than did those who underwent early outpatient locomotor training.
Dr. Duncan’s LEAPS (Locomotor Experience Applied Post Stroke) study randomized 408 patients to three groups: an early in-home program, an early outpatient locomotor program, and a wait-listed group that began outpatient locomotor training 6 months post stroke. Each training program lasted 12 weeks, and involved three 90-minute sessions per week.
The locomotor program consisted of treadmill walking with the patient’s body weight fully or partially supported by a harness. As patients progressed, the harness supported less and less of their weight, until they were able to walk on the treadmill independently. This required two or three therapists for every patient.
The home-based program focused on strength and balance, with encouragement to walk every day. Exercises were intensive and challenging, including many repetitions and resistance training for lower-extremity muscles. One therapist was able to conduct the session, said Dr. Duncan, professor of physical therapy at Duke University, Durham, N.C.
At baseline, the patients’ mean age was 62 years, and 83% had suffered an ischemic stroke. At randomization, the mean time from stroke was 64 days.
Mobility was severely impaired in 53% of patients, who had a mean gait speed of less than 0.4 meters per second (m/s). The rest of the patients were moderately impaired with respect to walking, with a mean gait speed of 0.4-0.8 m/s. They took a median of 1,700 steps daily. "Overall, these patients were taking [fewer than] 20% of the 10,000 steps required to maintain physical health," Dr. Duncan said.
The final analysis included 139 patients who were randomized to early outpatient training, 126 to the early at-home training, and 143 who were wait-listed.
"At 6 months, we found a statistically – and clinically – significant difference between the two early-intervention groups and the wait-listed group," Dr. Duncan said. Patients in the locomotor-training program and the home exercise program improved similarly: The mean gait speed increases were 0.25 m/s and 0.23 m/s, respectively. However, by 6 months the wait-listed group had improved by a mean of only 0.13 m/s. "Both early groups also improved in their activities of daily living and quality of life scores, with the wait-listed group improving about half as much," she added.
Falls were another important end point of the LEAPS trial. "Individuals who are increasing mobility after stroke are at risk of falling," Dr. Duncan said. "Overall, we saw a 58% incidence of falls from 2 months to 1 year after the stroke." Most of these were single falls, but 34% of patients experienced multiple falls; 6% resulted in injury.
There were significantly more falls in the early locomotor-training group than in either the home exercise group or the late-intervention group. "This was largely attributed to multiple falls in the group of most severely disabled patients," Dr. Duncan said at the meeting, which was sponsored by the American Heart Association.
There were 10 patients who experienced serious adverse events, all of which were cardiovascular symptoms or blurred vision; all required hospital admission. They were similarly distributed, with three in the early-locomotor program, five in the late-locomotor program, and two in the home exercise program. Nine of the 10 patients returned to their walking programs after discharge. Minor adverse events occurred in 56% of patients.
Although both early-intervention groups were significantly improved at 6 months, compared with the wait-listed group, the late starters caught up quickly: At 12 months, the wait-listed patients had improved their gait speed by 0.25 m/s – the same rate as those in early therapy. These late improvements counter "the widely held assumption and reports that most functional improvements after stroke are complete by 6 months," Dr. Duncan said.
Dr. Ralph Sacco, president of the American Heart Association and the chair of neurology at the University of Miami, noted in a press conference that smaller studies of stroke patients have showed that intensive use of weak upper extremities vastly improves arm function, and "now we see for the first time that the same thing can occur in the lower extremities." He pointed out, however, that many of the services that patients receive for gait improvement are now fiscally restrained by Medicare and insurance companies. "Therapy is stopped at a fairly early stage," and thus, so is recovery, he said. "We need to have the payers reexamine how long they will fund these therapies, because the at-home option appears to be a very cost effective way to pursue this."
The main message is that a lot of therapy is better than a little, regardless of whether it’s delivered by a therapist in the home or by a locomotor-training apparatus in the outpatient setting, said Dr. Sacco.
The trial was funded by the National Institute of Neurological Disorders and Stroke. Neither Dr. Duncan nor Dr. Sacco had financial disclosures.
Pamela Duncan, Ph.D., International Stroke Conference, physical therapy, neurology, stroke recovery, falls, LEAPS Locomotor Experience Applied Post Stroke study, treadmill,
strength, balance,
Pamela Duncan, Ph.D., International Stroke Conference, physical therapy, neurology, stroke recovery, falls, LEAPS Locomotor Experience Applied Post Stroke study, treadmill,
strength, balance,
Major Finding: Patients who began an in-home exercise program 2 months after stroke improved their gait speed by 0.23 m/s at 6 months, which was similar to patients in an outpatient locomotor program.
Data Source: The randomized LEAPS trial of 408 patients.
Disclosures: The trial was funded by the National Institute of Neurological Disorders and Stroke. Dr. Duncan had no financial disclosures.
Rule Can Predict Walking Outcomes After Spinal Cord Injury
A new algorithm based on age and sensory and motor scores in four body areas may allow clinicians to predict with great accuracy which patients will be able to walk independently 1 year after spinal cord injury.
"We have developed a simple clinical prediction rule ... that can be used by physicians to counsel patients with traumatic spinal cord injury and their families during the initial phase after injury," Dr. Joost van Middendorp and his coauthors wrote in the March 4 online issue of the Lancet.
The rule, which "can predict a patient’s long-term probability of walking independently more accurately than ... the widely used AIS [American Spinal Injury Association/International Spinal Cord Society] grading system," was developed and validated in 492 trauma patients who were treated at 19 centers across Europe, according to Dr. van Middendorp of St. Radboud University Nijmegen (the Netherlands) and his coauthors (Lancet 2011 [doi:10.1016/S0140-6736(10)62276-3]).
The authors extracted data for their prediction algorithm from the European Multicenter Study on Human Spinal Cord Injury. The study included neurologic and functional status of 1,282 patients aged 18 years or older who had sustained a traumatic spinal cord injury and were treated and assessed according to each facility’s protocol at 15 days, and 1, 3, 6, and 12 months post injury.
Of the entire cohort, 118 had 6-month outcomes and 374 had 1-year outcomes available. Most of the patients were men (77%), with an average age at injury of 44 years. The AIS grade was A in 49%; B in 13%; C in 16%; and D in 22%. Of these 492 patients, 200 (41%) were able to walk independently after the injury.
The investigators’ prediction rule assumed an age cutoff point of 65 years, because studies have shown that patients aged 65 years and older have a lower functional recovery potential. They performed motor score testing; light-touch and pinprick-sensory testing; and determination of sacral function (voluntary anal contraction and sensation). In the final analysis, the authors included only the best scores at each level of the lower extremity that was being tested, and the binary (yes/no) score for sacral function.
The patients’ ability to walk independently at 1 year was measured by the Spinal Cord Independence Measure’s indoor mobility subscale. The scale ranges from 0 (requiring total assistance) to 8 (walking without any walking aids). Scores of 0-3 signified an inability to walk or a dependency on assistance, and 4-8 signified an ability to walk independently.
The investigators’ final logistic regression model, which was deemed highly accurate, included age and four neurologic predictors at 15 days after injury:
• Age of 65 or older (score of 0-1, multiplied by –10).
• Muscle grade for the quadriceps femoral muscle (score of 0-5, multiplied by 2).
• Muscle grade for the gastrocsoleus (score of 0-5, multiplied by 2).
• Light-touch sensation at spinal level L3 (score of 0-2, multiplied by 5).
• Light-touch sensation at spinal level S1 (score of 0-2, multiplied by 5).
The rule had a minimum score of –10 and a maximum score of 40. For patients with neurologic exams that were performed within the first 15 days after injury, the probability of independent walking rose as the total score increased, such that at –10 points there was no chance of independent walking at 1 year; at 10 points, there was 35% probability; at 15 points, about 65% probability; at 20 points, almost 90% probability; and at 30 points and greater, nearly 100% probability. The area under the receiver operating characteristic (ROC) curve for the prediction rule was 0.96, indicating excellent ability to discriminate between patients who would walk at 1 year and those would not, the authors said.
Additional analyses found that adding the level of spinal cord injury, the timing of the neurologic examination, or pinprick-sensation testing did not significantly change its predictive ability.
The investigators validated the accuracy of the prediction in a separate group of 214 patients who underwent neurologic evaluation within 15 days of their traumatic spinal cord injury.
"The prediction rule had a clear additional clinical value for the prediction of an individual’s ability to walk independently in each of the AIS grades," particularly in grade B and C patients, Dr. van Middendorp said in an interview. "These are the grades of severity [for which] physicians can experience difficulties in prognosticating."
"Although our prediction rule is more accurate and less time consuming than the AIS grading system, to do accurate and reliable assessments of the four [neurologic tests], a physician must have experience in the physical examination of patients with traumatic spinal cord injury," the authors noted.
In an accompanying commentary, Dr. Wagih Shafik El Masri and Dr. Naveen Kumar applauded the rule’s development, but cautioned that other factors, which the rule cannot address, play an important part in spinal cord injury recovery (Lancet 2011 [doi:10.1016/S0140-6736(11)60248-1]).
"Early prediction of ambulation outcome is important to patients," wrote the authors, both of whom are at the Robert Jones and Agnes Hunt Orthopaedic Hospital in Staffordshire, England. "However, several variables exist that affect the achievability and quality of such predictors," such as the patient’s preexisting health, associated comorbidities, quality of rehabilitation, and neurologic progress. "[Neurologic] and functional recovery should be thought of as two essential, distinct entities in the assessment of traumatic spinal cord injury outcomes."
Further studies are necessary to assess the individual prognostic components of the rule and assess their value at different times during recovery, as are more studies of interventions that can help not only with ambulation, neurologic recovery, and functional recovery, but also with autonomic functions that can impact quality of life, including bladder, bowel, cardiorespiratory, vasomotor, erectile, and reproductive problems, Dr. El Masri and Dr. Kumar wrote.
Neither the authors of the study nor those of the commentary declared any conflicts of interest. The study was funded by the Internationale Stiftung für Forschung in Paraplegie.
A new algorithm based on age and sensory and motor scores in four body areas may allow clinicians to predict with great accuracy which patients will be able to walk independently 1 year after spinal cord injury.
"We have developed a simple clinical prediction rule ... that can be used by physicians to counsel patients with traumatic spinal cord injury and their families during the initial phase after injury," Dr. Joost van Middendorp and his coauthors wrote in the March 4 online issue of the Lancet.
The rule, which "can predict a patient’s long-term probability of walking independently more accurately than ... the widely used AIS [American Spinal Injury Association/International Spinal Cord Society] grading system," was developed and validated in 492 trauma patients who were treated at 19 centers across Europe, according to Dr. van Middendorp of St. Radboud University Nijmegen (the Netherlands) and his coauthors (Lancet 2011 [doi:10.1016/S0140-6736(10)62276-3]).
The authors extracted data for their prediction algorithm from the European Multicenter Study on Human Spinal Cord Injury. The study included neurologic and functional status of 1,282 patients aged 18 years or older who had sustained a traumatic spinal cord injury and were treated and assessed according to each facility’s protocol at 15 days, and 1, 3, 6, and 12 months post injury.
Of the entire cohort, 118 had 6-month outcomes and 374 had 1-year outcomes available. Most of the patients were men (77%), with an average age at injury of 44 years. The AIS grade was A in 49%; B in 13%; C in 16%; and D in 22%. Of these 492 patients, 200 (41%) were able to walk independently after the injury.
The investigators’ prediction rule assumed an age cutoff point of 65 years, because studies have shown that patients aged 65 years and older have a lower functional recovery potential. They performed motor score testing; light-touch and pinprick-sensory testing; and determination of sacral function (voluntary anal contraction and sensation). In the final analysis, the authors included only the best scores at each level of the lower extremity that was being tested, and the binary (yes/no) score for sacral function.
The patients’ ability to walk independently at 1 year was measured by the Spinal Cord Independence Measure’s indoor mobility subscale. The scale ranges from 0 (requiring total assistance) to 8 (walking without any walking aids). Scores of 0-3 signified an inability to walk or a dependency on assistance, and 4-8 signified an ability to walk independently.
The investigators’ final logistic regression model, which was deemed highly accurate, included age and four neurologic predictors at 15 days after injury:
• Age of 65 or older (score of 0-1, multiplied by –10).
• Muscle grade for the quadriceps femoral muscle (score of 0-5, multiplied by 2).
• Muscle grade for the gastrocsoleus (score of 0-5, multiplied by 2).
• Light-touch sensation at spinal level L3 (score of 0-2, multiplied by 5).
• Light-touch sensation at spinal level S1 (score of 0-2, multiplied by 5).
The rule had a minimum score of –10 and a maximum score of 40. For patients with neurologic exams that were performed within the first 15 days after injury, the probability of independent walking rose as the total score increased, such that at –10 points there was no chance of independent walking at 1 year; at 10 points, there was 35% probability; at 15 points, about 65% probability; at 20 points, almost 90% probability; and at 30 points and greater, nearly 100% probability. The area under the receiver operating characteristic (ROC) curve for the prediction rule was 0.96, indicating excellent ability to discriminate between patients who would walk at 1 year and those would not, the authors said.
Additional analyses found that adding the level of spinal cord injury, the timing of the neurologic examination, or pinprick-sensation testing did not significantly change its predictive ability.
The investigators validated the accuracy of the prediction in a separate group of 214 patients who underwent neurologic evaluation within 15 days of their traumatic spinal cord injury.
"The prediction rule had a clear additional clinical value for the prediction of an individual’s ability to walk independently in each of the AIS grades," particularly in grade B and C patients, Dr. van Middendorp said in an interview. "These are the grades of severity [for which] physicians can experience difficulties in prognosticating."
"Although our prediction rule is more accurate and less time consuming than the AIS grading system, to do accurate and reliable assessments of the four [neurologic tests], a physician must have experience in the physical examination of patients with traumatic spinal cord injury," the authors noted.
In an accompanying commentary, Dr. Wagih Shafik El Masri and Dr. Naveen Kumar applauded the rule’s development, but cautioned that other factors, which the rule cannot address, play an important part in spinal cord injury recovery (Lancet 2011 [doi:10.1016/S0140-6736(11)60248-1]).
"Early prediction of ambulation outcome is important to patients," wrote the authors, both of whom are at the Robert Jones and Agnes Hunt Orthopaedic Hospital in Staffordshire, England. "However, several variables exist that affect the achievability and quality of such predictors," such as the patient’s preexisting health, associated comorbidities, quality of rehabilitation, and neurologic progress. "[Neurologic] and functional recovery should be thought of as two essential, distinct entities in the assessment of traumatic spinal cord injury outcomes."
Further studies are necessary to assess the individual prognostic components of the rule and assess their value at different times during recovery, as are more studies of interventions that can help not only with ambulation, neurologic recovery, and functional recovery, but also with autonomic functions that can impact quality of life, including bladder, bowel, cardiorespiratory, vasomotor, erectile, and reproductive problems, Dr. El Masri and Dr. Kumar wrote.
Neither the authors of the study nor those of the commentary declared any conflicts of interest. The study was funded by the Internationale Stiftung für Forschung in Paraplegie.
A new algorithm based on age and sensory and motor scores in four body areas may allow clinicians to predict with great accuracy which patients will be able to walk independently 1 year after spinal cord injury.
"We have developed a simple clinical prediction rule ... that can be used by physicians to counsel patients with traumatic spinal cord injury and their families during the initial phase after injury," Dr. Joost van Middendorp and his coauthors wrote in the March 4 online issue of the Lancet.
The rule, which "can predict a patient’s long-term probability of walking independently more accurately than ... the widely used AIS [American Spinal Injury Association/International Spinal Cord Society] grading system," was developed and validated in 492 trauma patients who were treated at 19 centers across Europe, according to Dr. van Middendorp of St. Radboud University Nijmegen (the Netherlands) and his coauthors (Lancet 2011 [doi:10.1016/S0140-6736(10)62276-3]).
The authors extracted data for their prediction algorithm from the European Multicenter Study on Human Spinal Cord Injury. The study included neurologic and functional status of 1,282 patients aged 18 years or older who had sustained a traumatic spinal cord injury and were treated and assessed according to each facility’s protocol at 15 days, and 1, 3, 6, and 12 months post injury.
Of the entire cohort, 118 had 6-month outcomes and 374 had 1-year outcomes available. Most of the patients were men (77%), with an average age at injury of 44 years. The AIS grade was A in 49%; B in 13%; C in 16%; and D in 22%. Of these 492 patients, 200 (41%) were able to walk independently after the injury.
The investigators’ prediction rule assumed an age cutoff point of 65 years, because studies have shown that patients aged 65 years and older have a lower functional recovery potential. They performed motor score testing; light-touch and pinprick-sensory testing; and determination of sacral function (voluntary anal contraction and sensation). In the final analysis, the authors included only the best scores at each level of the lower extremity that was being tested, and the binary (yes/no) score for sacral function.
The patients’ ability to walk independently at 1 year was measured by the Spinal Cord Independence Measure’s indoor mobility subscale. The scale ranges from 0 (requiring total assistance) to 8 (walking without any walking aids). Scores of 0-3 signified an inability to walk or a dependency on assistance, and 4-8 signified an ability to walk independently.
The investigators’ final logistic regression model, which was deemed highly accurate, included age and four neurologic predictors at 15 days after injury:
• Age of 65 or older (score of 0-1, multiplied by –10).
• Muscle grade for the quadriceps femoral muscle (score of 0-5, multiplied by 2).
• Muscle grade for the gastrocsoleus (score of 0-5, multiplied by 2).
• Light-touch sensation at spinal level L3 (score of 0-2, multiplied by 5).
• Light-touch sensation at spinal level S1 (score of 0-2, multiplied by 5).
The rule had a minimum score of –10 and a maximum score of 40. For patients with neurologic exams that were performed within the first 15 days after injury, the probability of independent walking rose as the total score increased, such that at –10 points there was no chance of independent walking at 1 year; at 10 points, there was 35% probability; at 15 points, about 65% probability; at 20 points, almost 90% probability; and at 30 points and greater, nearly 100% probability. The area under the receiver operating characteristic (ROC) curve for the prediction rule was 0.96, indicating excellent ability to discriminate between patients who would walk at 1 year and those would not, the authors said.
Additional analyses found that adding the level of spinal cord injury, the timing of the neurologic examination, or pinprick-sensation testing did not significantly change its predictive ability.
The investigators validated the accuracy of the prediction in a separate group of 214 patients who underwent neurologic evaluation within 15 days of their traumatic spinal cord injury.
"The prediction rule had a clear additional clinical value for the prediction of an individual’s ability to walk independently in each of the AIS grades," particularly in grade B and C patients, Dr. van Middendorp said in an interview. "These are the grades of severity [for which] physicians can experience difficulties in prognosticating."
"Although our prediction rule is more accurate and less time consuming than the AIS grading system, to do accurate and reliable assessments of the four [neurologic tests], a physician must have experience in the physical examination of patients with traumatic spinal cord injury," the authors noted.
In an accompanying commentary, Dr. Wagih Shafik El Masri and Dr. Naveen Kumar applauded the rule’s development, but cautioned that other factors, which the rule cannot address, play an important part in spinal cord injury recovery (Lancet 2011 [doi:10.1016/S0140-6736(11)60248-1]).
"Early prediction of ambulation outcome is important to patients," wrote the authors, both of whom are at the Robert Jones and Agnes Hunt Orthopaedic Hospital in Staffordshire, England. "However, several variables exist that affect the achievability and quality of such predictors," such as the patient’s preexisting health, associated comorbidities, quality of rehabilitation, and neurologic progress. "[Neurologic] and functional recovery should be thought of as two essential, distinct entities in the assessment of traumatic spinal cord injury outcomes."
Further studies are necessary to assess the individual prognostic components of the rule and assess their value at different times during recovery, as are more studies of interventions that can help not only with ambulation, neurologic recovery, and functional recovery, but also with autonomic functions that can impact quality of life, including bladder, bowel, cardiorespiratory, vasomotor, erectile, and reproductive problems, Dr. El Masri and Dr. Kumar wrote.
Neither the authors of the study nor those of the commentary declared any conflicts of interest. The study was funded by the Internationale Stiftung für Forschung in Paraplegie.
Major Finding: A prediction rule using age and neurologic testing at four sites was highly accurate (with an area under the ROC curve of 0.96) in predicting walking outcomes at 1 year in 492 patients with spinal cord injury.
Data Source: A derivation cohort of 492 patients with traumatic spinal cord injury and a validation cohort of 214 similar patients.
Disclosures: Neither the authors of the study nor those of the commentary declared any conflicts of interest. The study was funded by the Internationale Stiftung für Forschung in Paraplegie.
Rule Can Predict Walking Outcomes After Spinal Cord Injury
A new algorithm based on age and sensory and motor scores in four body areas may allow clinicians to predict with great accuracy which patients will be able to walk independently 1 year after spinal cord injury.
"We have developed a simple clinical prediction rule ... that can be used by physicians to counsel patients with traumatic spinal cord injury and their families during the initial phase after injury," Dr. Joost van Middendorp and his coauthors wrote in the March 4 online issue of the Lancet.
The rule, which "can predict a patient’s long-term probability of walking independently more accurately than ... the widely used AIS [American Spinal Injury Association/International Spinal Cord Society] grading system," was developed and validated in 492 trauma patients who were treated at 19 centers across Europe, according to Dr. van Middendorp of St. Radboud University Nijmegen (the Netherlands) and his coauthors (Lancet 2011 [doi:10.1016/S0140-6736(10)62276-3]).
The authors extracted data for their prediction algorithm from the European Multicenter Study on Human Spinal Cord Injury. The study included neurologic and functional status of 1,282 patients aged 18 years or older who had sustained a traumatic spinal cord injury and were treated and assessed according to each facility’s protocol at 15 days, and 1, 3, 6, and 12 months post injury.
Of the entire cohort, 118 had 6-month outcomes and 374 had 1-year outcomes available. Most of the patients were men (77%), with an average age at injury of 44 years. The AIS grade was A in 49%; B in 13%; C in 16%; and D in 22%. Of these 492 patients, 200 (41%) were able to walk independently after the injury.
The investigators’ prediction rule assumed an age cutoff point of 65 years, because studies have shown that patients aged 65 years and older have a lower functional recovery potential. They performed motor score testing; light-touch and pinprick-sensory testing; and determination of sacral function (voluntary anal contraction and sensation). In the final analysis, the authors included only the best scores at each level of the lower extremity that was being tested, and the binary (yes/no) score for sacral function.
The patients’ ability to walk independently at 1 year was measured by the Spinal Cord Independence Measure’s indoor mobility subscale. The scale ranges from 0 (requiring total assistance) to 8 (walking without any walking aids). Scores of 0-3 signified an inability to walk or a dependency on assistance, and 4-8 signified an ability to walk independently.
The investigators’ final logistic regression model, which was deemed highly accurate, included age and four neurologic predictors at 15 days after injury:
• Age of 65 or older (score of 0-1, multiplied by –10).
• Muscle grade for the quadriceps femoral muscle (score of 0-5, multiplied by 2).
• Muscle grade for the gastrocsoleus (score of 0-5, multiplied by 2).
• Light-touch sensation at spinal level L3 (score of 0-2, multiplied by 5).
• Light-touch sensation at spinal level S1 (score of 0-2, multiplied by 5).
The rule had a minimum score of –10 and a maximum score of 40. For patients with neurologic exams that were performed within the first 15 days after injury, the probability of independent walking rose as the total score increased, such that at –10 points there was no chance of independent walking at 1 year; at 10 points, there was 35% probability; at 15 points, about 65% probability; at 20 points, almost 90% probability; and at 30 points and greater, nearly 100% probability. The area under the receiver operating characteristic (ROC) curve for the prediction rule was 0.96, indicating excellent ability to discriminate between patients who would walk at 1 year and those would not, the authors said.
Additional analyses found that adding the level of spinal cord injury, the timing of the neurologic examination, or pinprick-sensation testing did not significantly change its predictive ability.
The investigators validated the accuracy of the prediction in a separate group of 214 patients who underwent neurologic evaluation within 15 days of their traumatic spinal cord injury.
"The prediction rule had a clear additional clinical value for the prediction of an individual’s ability to walk independently in each of the AIS grades," particularly in grade B and C patients, Dr. van Middendorp said in an interview. "These are the grades of severity [for which] physicians can experience difficulties in prognosticating."
"Although our prediction rule is more accurate and less time consuming than the AIS grading system, to do accurate and reliable assessments of the four [neurologic tests], a physician must have experience in the physical examination of patients with traumatic spinal cord injury," the authors noted.
In an accompanying commentary, Dr. Wagih Shafik El Masri and Dr. Naveen Kumar applauded the rule’s development, but cautioned that other factors, which the rule cannot address, play an important part in spinal cord injury recovery (Lancet 2011 [doi:10.1016/S0140-6736(11)60248-1]).
"Early prediction of ambulation outcome is important to patients," wrote the authors, both of whom are at the Robert Jones and Agnes Hunt Orthopaedic Hospital in Staffordshire, England. "However, several variables exist that affect the achievability and quality of such predictors," such as the patient’s preexisting health, associated comorbidities, quality of rehabilitation, and neurologic progress. "[Neurologic] and functional recovery should be thought of as two essential, distinct entities in the assessment of traumatic spinal cord injury outcomes."
Further studies are necessary to assess the individual prognostic components of the rule and assess their value at different times during recovery, as are more studies of interventions that can help not only with ambulation, neurologic recovery, and functional recovery, but also with autonomic functions that can impact quality of life, including bladder, bowel, cardiorespiratory, vasomotor, erectile, and reproductive problems, Dr. El Masri and Dr. Kumar wrote.
Neither the authors of the study nor those of the commentary declared any conflicts of interest. The study was funded by the Internationale Stiftung für Forschung in Paraplegie.
A new algorithm based on age and sensory and motor scores in four body areas may allow clinicians to predict with great accuracy which patients will be able to walk independently 1 year after spinal cord injury.
"We have developed a simple clinical prediction rule ... that can be used by physicians to counsel patients with traumatic spinal cord injury and their families during the initial phase after injury," Dr. Joost van Middendorp and his coauthors wrote in the March 4 online issue of the Lancet.
The rule, which "can predict a patient’s long-term probability of walking independently more accurately than ... the widely used AIS [American Spinal Injury Association/International Spinal Cord Society] grading system," was developed and validated in 492 trauma patients who were treated at 19 centers across Europe, according to Dr. van Middendorp of St. Radboud University Nijmegen (the Netherlands) and his coauthors (Lancet 2011 [doi:10.1016/S0140-6736(10)62276-3]).
The authors extracted data for their prediction algorithm from the European Multicenter Study on Human Spinal Cord Injury. The study included neurologic and functional status of 1,282 patients aged 18 years or older who had sustained a traumatic spinal cord injury and were treated and assessed according to each facility’s protocol at 15 days, and 1, 3, 6, and 12 months post injury.
Of the entire cohort, 118 had 6-month outcomes and 374 had 1-year outcomes available. Most of the patients were men (77%), with an average age at injury of 44 years. The AIS grade was A in 49%; B in 13%; C in 16%; and D in 22%. Of these 492 patients, 200 (41%) were able to walk independently after the injury.
The investigators’ prediction rule assumed an age cutoff point of 65 years, because studies have shown that patients aged 65 years and older have a lower functional recovery potential. They performed motor score testing; light-touch and pinprick-sensory testing; and determination of sacral function (voluntary anal contraction and sensation). In the final analysis, the authors included only the best scores at each level of the lower extremity that was being tested, and the binary (yes/no) score for sacral function.
The patients’ ability to walk independently at 1 year was measured by the Spinal Cord Independence Measure’s indoor mobility subscale. The scale ranges from 0 (requiring total assistance) to 8 (walking without any walking aids). Scores of 0-3 signified an inability to walk or a dependency on assistance, and 4-8 signified an ability to walk independently.
The investigators’ final logistic regression model, which was deemed highly accurate, included age and four neurologic predictors at 15 days after injury:
• Age of 65 or older (score of 0-1, multiplied by –10).
• Muscle grade for the quadriceps femoral muscle (score of 0-5, multiplied by 2).
• Muscle grade for the gastrocsoleus (score of 0-5, multiplied by 2).
• Light-touch sensation at spinal level L3 (score of 0-2, multiplied by 5).
• Light-touch sensation at spinal level S1 (score of 0-2, multiplied by 5).
The rule had a minimum score of –10 and a maximum score of 40. For patients with neurologic exams that were performed within the first 15 days after injury, the probability of independent walking rose as the total score increased, such that at –10 points there was no chance of independent walking at 1 year; at 10 points, there was 35% probability; at 15 points, about 65% probability; at 20 points, almost 90% probability; and at 30 points and greater, nearly 100% probability. The area under the receiver operating characteristic (ROC) curve for the prediction rule was 0.96, indicating excellent ability to discriminate between patients who would walk at 1 year and those would not, the authors said.
Additional analyses found that adding the level of spinal cord injury, the timing of the neurologic examination, or pinprick-sensation testing did not significantly change its predictive ability.
The investigators validated the accuracy of the prediction in a separate group of 214 patients who underwent neurologic evaluation within 15 days of their traumatic spinal cord injury.
"The prediction rule had a clear additional clinical value for the prediction of an individual’s ability to walk independently in each of the AIS grades," particularly in grade B and C patients, Dr. van Middendorp said in an interview. "These are the grades of severity [for which] physicians can experience difficulties in prognosticating."
"Although our prediction rule is more accurate and less time consuming than the AIS grading system, to do accurate and reliable assessments of the four [neurologic tests], a physician must have experience in the physical examination of patients with traumatic spinal cord injury," the authors noted.
In an accompanying commentary, Dr. Wagih Shafik El Masri and Dr. Naveen Kumar applauded the rule’s development, but cautioned that other factors, which the rule cannot address, play an important part in spinal cord injury recovery (Lancet 2011 [doi:10.1016/S0140-6736(11)60248-1]).
"Early prediction of ambulation outcome is important to patients," wrote the authors, both of whom are at the Robert Jones and Agnes Hunt Orthopaedic Hospital in Staffordshire, England. "However, several variables exist that affect the achievability and quality of such predictors," such as the patient’s preexisting health, associated comorbidities, quality of rehabilitation, and neurologic progress. "[Neurologic] and functional recovery should be thought of as two essential, distinct entities in the assessment of traumatic spinal cord injury outcomes."
Further studies are necessary to assess the individual prognostic components of the rule and assess their value at different times during recovery, as are more studies of interventions that can help not only with ambulation, neurologic recovery, and functional recovery, but also with autonomic functions that can impact quality of life, including bladder, bowel, cardiorespiratory, vasomotor, erectile, and reproductive problems, Dr. El Masri and Dr. Kumar wrote.
Neither the authors of the study nor those of the commentary declared any conflicts of interest. The study was funded by the Internationale Stiftung für Forschung in Paraplegie.
A new algorithm based on age and sensory and motor scores in four body areas may allow clinicians to predict with great accuracy which patients will be able to walk independently 1 year after spinal cord injury.
"We have developed a simple clinical prediction rule ... that can be used by physicians to counsel patients with traumatic spinal cord injury and their families during the initial phase after injury," Dr. Joost van Middendorp and his coauthors wrote in the March 4 online issue of the Lancet.
The rule, which "can predict a patient’s long-term probability of walking independently more accurately than ... the widely used AIS [American Spinal Injury Association/International Spinal Cord Society] grading system," was developed and validated in 492 trauma patients who were treated at 19 centers across Europe, according to Dr. van Middendorp of St. Radboud University Nijmegen (the Netherlands) and his coauthors (Lancet 2011 [doi:10.1016/S0140-6736(10)62276-3]).
The authors extracted data for their prediction algorithm from the European Multicenter Study on Human Spinal Cord Injury. The study included neurologic and functional status of 1,282 patients aged 18 years or older who had sustained a traumatic spinal cord injury and were treated and assessed according to each facility’s protocol at 15 days, and 1, 3, 6, and 12 months post injury.
Of the entire cohort, 118 had 6-month outcomes and 374 had 1-year outcomes available. Most of the patients were men (77%), with an average age at injury of 44 years. The AIS grade was A in 49%; B in 13%; C in 16%; and D in 22%. Of these 492 patients, 200 (41%) were able to walk independently after the injury.
The investigators’ prediction rule assumed an age cutoff point of 65 years, because studies have shown that patients aged 65 years and older have a lower functional recovery potential. They performed motor score testing; light-touch and pinprick-sensory testing; and determination of sacral function (voluntary anal contraction and sensation). In the final analysis, the authors included only the best scores at each level of the lower extremity that was being tested, and the binary (yes/no) score for sacral function.
The patients’ ability to walk independently at 1 year was measured by the Spinal Cord Independence Measure’s indoor mobility subscale. The scale ranges from 0 (requiring total assistance) to 8 (walking without any walking aids). Scores of 0-3 signified an inability to walk or a dependency on assistance, and 4-8 signified an ability to walk independently.
The investigators’ final logistic regression model, which was deemed highly accurate, included age and four neurologic predictors at 15 days after injury:
• Age of 65 or older (score of 0-1, multiplied by –10).
• Muscle grade for the quadriceps femoral muscle (score of 0-5, multiplied by 2).
• Muscle grade for the gastrocsoleus (score of 0-5, multiplied by 2).
• Light-touch sensation at spinal level L3 (score of 0-2, multiplied by 5).
• Light-touch sensation at spinal level S1 (score of 0-2, multiplied by 5).
The rule had a minimum score of –10 and a maximum score of 40. For patients with neurologic exams that were performed within the first 15 days after injury, the probability of independent walking rose as the total score increased, such that at –10 points there was no chance of independent walking at 1 year; at 10 points, there was 35% probability; at 15 points, about 65% probability; at 20 points, almost 90% probability; and at 30 points and greater, nearly 100% probability. The area under the receiver operating characteristic (ROC) curve for the prediction rule was 0.96, indicating excellent ability to discriminate between patients who would walk at 1 year and those would not, the authors said.
Additional analyses found that adding the level of spinal cord injury, the timing of the neurologic examination, or pinprick-sensation testing did not significantly change its predictive ability.
The investigators validated the accuracy of the prediction in a separate group of 214 patients who underwent neurologic evaluation within 15 days of their traumatic spinal cord injury.
"The prediction rule had a clear additional clinical value for the prediction of an individual’s ability to walk independently in each of the AIS grades," particularly in grade B and C patients, Dr. van Middendorp said in an interview. "These are the grades of severity [for which] physicians can experience difficulties in prognosticating."
"Although our prediction rule is more accurate and less time consuming than the AIS grading system, to do accurate and reliable assessments of the four [neurologic tests], a physician must have experience in the physical examination of patients with traumatic spinal cord injury," the authors noted.
In an accompanying commentary, Dr. Wagih Shafik El Masri and Dr. Naveen Kumar applauded the rule’s development, but cautioned that other factors, which the rule cannot address, play an important part in spinal cord injury recovery (Lancet 2011 [doi:10.1016/S0140-6736(11)60248-1]).
"Early prediction of ambulation outcome is important to patients," wrote the authors, both of whom are at the Robert Jones and Agnes Hunt Orthopaedic Hospital in Staffordshire, England. "However, several variables exist that affect the achievability and quality of such predictors," such as the patient’s preexisting health, associated comorbidities, quality of rehabilitation, and neurologic progress. "[Neurologic] and functional recovery should be thought of as two essential, distinct entities in the assessment of traumatic spinal cord injury outcomes."
Further studies are necessary to assess the individual prognostic components of the rule and assess their value at different times during recovery, as are more studies of interventions that can help not only with ambulation, neurologic recovery, and functional recovery, but also with autonomic functions that can impact quality of life, including bladder, bowel, cardiorespiratory, vasomotor, erectile, and reproductive problems, Dr. El Masri and Dr. Kumar wrote.
Neither the authors of the study nor those of the commentary declared any conflicts of interest. The study was funded by the Internationale Stiftung für Forschung in Paraplegie.
Major Finding: A prediction rule using age and neurologic testing at four sites was highly accurate (with an area under the ROC curve of 0.96) in predicting walking outcomes at 1 year in 492 patients with spinal cord injury.
Data Source: A derivation cohort of 492 patients with traumatic spinal cord injury and a validation cohort of 214 similar patients.
Disclosures: Neither the authors of the study nor those of the commentary declared any conflicts of interest. The study was funded by the Internationale Stiftung für Forschung in Paraplegie.
Rule Can Predict Walking Outcomes After Spinal Cord Injury
A new algorithm based on age and sensory and motor scores in four body areas may allow clinicians to predict with great accuracy which patients will be able to walk independently 1 year after spinal cord injury.
"We have developed a simple clinical prediction rule ... that can be used by physicians to counsel patients with traumatic spinal cord injury and their families during the initial phase after injury," Dr. Joost van Middendorp and his coauthors wrote in the March 4 online issue of the Lancet.
The rule, which "can predict a patient’s long-term probability of walking independently more accurately than ... the widely used AIS [American Spinal Injury Association/International Spinal Cord Society] grading system," was developed and validated in 492 trauma patients who were treated at 19 centers across Europe, according to Dr. van Middendorp of St. Radboud University Nijmegen (the Netherlands) and his coauthors (Lancet 2011 [doi:10.1016/S0140-6736(10)62276-3]).
The authors extracted data for their prediction algorithm from the European Multicenter Study on Human Spinal Cord Injury. The study included neurologic and functional status of 1,282 patients aged 18 years or older who had sustained a traumatic spinal cord injury and were treated and assessed according to each facility’s protocol at 15 days, and 1, 3, 6, and 12 months post injury.
Of the entire cohort, 118 had 6-month outcomes and 374 had 1-year outcomes available. Most of the patients were men (77%), with an average age at injury of 44 years. The AIS grade was A in 49%; B in 13%; C in 16%; and D in 22%. Of these 492 patients, 200 (41%) were able to walk independently after the injury.
The investigators’ prediction rule assumed an age cutoff point of 65 years, because studies have shown that patients aged 65 years and older have a lower functional recovery potential. They performed motor score testing; light-touch and pinprick-sensory testing; and determination of sacral function (voluntary anal contraction and sensation). In the final analysis, the authors included only the best scores at each level of the lower extremity that was being tested, and the binary (yes/no) score for sacral function.
The patients’ ability to walk independently at 1 year was measured by the Spinal Cord Independence Measure’s indoor mobility subscale. The scale ranges from 0 (requiring total assistance) to 8 (walking without any walking aids). Scores of 0-3 signified an inability to walk or a dependency on assistance, and 4-8 signified an ability to walk independently.
The investigators’ final logistic regression model, which was deemed highly accurate, included age and four neurologic predictors at 15 days after injury:
• Age of 65 or older (score of 0-1, multiplied by –10).
• Muscle grade for the quadriceps femoral muscle (score of 0-5, multiplied by 2).
• Muscle grade for the gastrocsoleus (score of 0-5, multiplied by 2).
• Light-touch sensation at spinal level L3 (score of 0-2, multiplied by 5).
• Light-touch sensation at spinal level S1 (score of 0-2, multiplied by 5).
The rule had a minimum score of –10 and a maximum score of 40. For patients with neurologic exams that were performed within the first 15 days after injury, the probability of independent walking rose as the total score increased, such that at –10 points there was no chance of independent walking at 1 year; at 10 points, there was 35% probability; at 15 points, about 65% probability; at 20 points, almost 90% probability; and at 30 points and greater, nearly 100% probability. The area under the receiver operating characteristic (ROC) curve for the prediction rule was 0.96, indicating excellent ability to discriminate between patients who would walk at 1 year and those would not, the authors said.
Additional analyses found that adding the level of spinal cord injury, the timing of the neurologic examination, or pinprick-sensation testing did not significantly change its predictive ability.
The investigators validated the accuracy of the prediction in a separate group of 214 patients who underwent neurologic evaluation within 15 days of their traumatic spinal cord injury.
"The prediction rule had a clear additional clinical value for the prediction of an individual’s ability to walk independently in each of the AIS grades," particularly in grade B and C patients, Dr. van Middendorp said in an interview. "These are the grades of severity [for which] physicians can experience difficulties in prognosticating."
"Although our prediction rule is more accurate and less time consuming than the AIS grading system, to do accurate and reliable assessments of the four [neurologic tests], a physician must have experience in the physical examination of patients with traumatic spinal cord injury," the authors noted.
In an accompanying commentary, Dr. Wagih Shafik El Masri and Dr. Naveen Kumar applauded the rule’s development, but cautioned that other factors, which the rule cannot address, play an important part in spinal cord injury recovery (Lancet 2011 [doi:10.1016/S0140-6736(11)60248-1]).
"Early prediction of ambulation outcome is important to patients," wrote the authors, both of whom are at the Robert Jones and Agnes Hunt Orthopaedic Hospital in Staffordshire, England. "However, several variables exist that affect the achievability and quality of such predictors," such as the patient’s preexisting health, associated comorbidities, quality of rehabilitation, and neurologic progress. "[Neurologic] and functional recovery should be thought of as two essential, distinct entities in the assessment of traumatic spinal cord injury outcomes."
Further studies are necessary to assess the individual prognostic components of the rule and assess their value at different times during recovery, as are more studies of interventions that can help not only with ambulation, neurologic recovery, and functional recovery, but also with autonomic functions that can impact quality of life, including bladder, bowel, cardiorespiratory, vasomotor, erectile, and reproductive problems, Dr. El Masri and Dr. Kumar wrote.
Neither the authors of the study nor those of the commentary declared any conflicts of interest. The study was funded by the Internationale Stiftung für Forschung in Paraplegie.
A new algorithm based on age and sensory and motor scores in four body areas may allow clinicians to predict with great accuracy which patients will be able to walk independently 1 year after spinal cord injury.
"We have developed a simple clinical prediction rule ... that can be used by physicians to counsel patients with traumatic spinal cord injury and their families during the initial phase after injury," Dr. Joost van Middendorp and his coauthors wrote in the March 4 online issue of the Lancet.
The rule, which "can predict a patient’s long-term probability of walking independently more accurately than ... the widely used AIS [American Spinal Injury Association/International Spinal Cord Society] grading system," was developed and validated in 492 trauma patients who were treated at 19 centers across Europe, according to Dr. van Middendorp of St. Radboud University Nijmegen (the Netherlands) and his coauthors (Lancet 2011 [doi:10.1016/S0140-6736(10)62276-3]).
The authors extracted data for their prediction algorithm from the European Multicenter Study on Human Spinal Cord Injury. The study included neurologic and functional status of 1,282 patients aged 18 years or older who had sustained a traumatic spinal cord injury and were treated and assessed according to each facility’s protocol at 15 days, and 1, 3, 6, and 12 months post injury.
Of the entire cohort, 118 had 6-month outcomes and 374 had 1-year outcomes available. Most of the patients were men (77%), with an average age at injury of 44 years. The AIS grade was A in 49%; B in 13%; C in 16%; and D in 22%. Of these 492 patients, 200 (41%) were able to walk independently after the injury.
The investigators’ prediction rule assumed an age cutoff point of 65 years, because studies have shown that patients aged 65 years and older have a lower functional recovery potential. They performed motor score testing; light-touch and pinprick-sensory testing; and determination of sacral function (voluntary anal contraction and sensation). In the final analysis, the authors included only the best scores at each level of the lower extremity that was being tested, and the binary (yes/no) score for sacral function.
The patients’ ability to walk independently at 1 year was measured by the Spinal Cord Independence Measure’s indoor mobility subscale. The scale ranges from 0 (requiring total assistance) to 8 (walking without any walking aids). Scores of 0-3 signified an inability to walk or a dependency on assistance, and 4-8 signified an ability to walk independently.
The investigators’ final logistic regression model, which was deemed highly accurate, included age and four neurologic predictors at 15 days after injury:
• Age of 65 or older (score of 0-1, multiplied by –10).
• Muscle grade for the quadriceps femoral muscle (score of 0-5, multiplied by 2).
• Muscle grade for the gastrocsoleus (score of 0-5, multiplied by 2).
• Light-touch sensation at spinal level L3 (score of 0-2, multiplied by 5).
• Light-touch sensation at spinal level S1 (score of 0-2, multiplied by 5).
The rule had a minimum score of –10 and a maximum score of 40. For patients with neurologic exams that were performed within the first 15 days after injury, the probability of independent walking rose as the total score increased, such that at –10 points there was no chance of independent walking at 1 year; at 10 points, there was 35% probability; at 15 points, about 65% probability; at 20 points, almost 90% probability; and at 30 points and greater, nearly 100% probability. The area under the receiver operating characteristic (ROC) curve for the prediction rule was 0.96, indicating excellent ability to discriminate between patients who would walk at 1 year and those would not, the authors said.
Additional analyses found that adding the level of spinal cord injury, the timing of the neurologic examination, or pinprick-sensation testing did not significantly change its predictive ability.
The investigators validated the accuracy of the prediction in a separate group of 214 patients who underwent neurologic evaluation within 15 days of their traumatic spinal cord injury.
"The prediction rule had a clear additional clinical value for the prediction of an individual’s ability to walk independently in each of the AIS grades," particularly in grade B and C patients, Dr. van Middendorp said in an interview. "These are the grades of severity [for which] physicians can experience difficulties in prognosticating."
"Although our prediction rule is more accurate and less time consuming than the AIS grading system, to do accurate and reliable assessments of the four [neurologic tests], a physician must have experience in the physical examination of patients with traumatic spinal cord injury," the authors noted.
In an accompanying commentary, Dr. Wagih Shafik El Masri and Dr. Naveen Kumar applauded the rule’s development, but cautioned that other factors, which the rule cannot address, play an important part in spinal cord injury recovery (Lancet 2011 [doi:10.1016/S0140-6736(11)60248-1]).
"Early prediction of ambulation outcome is important to patients," wrote the authors, both of whom are at the Robert Jones and Agnes Hunt Orthopaedic Hospital in Staffordshire, England. "However, several variables exist that affect the achievability and quality of such predictors," such as the patient’s preexisting health, associated comorbidities, quality of rehabilitation, and neurologic progress. "[Neurologic] and functional recovery should be thought of as two essential, distinct entities in the assessment of traumatic spinal cord injury outcomes."
Further studies are necessary to assess the individual prognostic components of the rule and assess their value at different times during recovery, as are more studies of interventions that can help not only with ambulation, neurologic recovery, and functional recovery, but also with autonomic functions that can impact quality of life, including bladder, bowel, cardiorespiratory, vasomotor, erectile, and reproductive problems, Dr. El Masri and Dr. Kumar wrote.
Neither the authors of the study nor those of the commentary declared any conflicts of interest. The study was funded by the Internationale Stiftung für Forschung in Paraplegie.
A new algorithm based on age and sensory and motor scores in four body areas may allow clinicians to predict with great accuracy which patients will be able to walk independently 1 year after spinal cord injury.
"We have developed a simple clinical prediction rule ... that can be used by physicians to counsel patients with traumatic spinal cord injury and their families during the initial phase after injury," Dr. Joost van Middendorp and his coauthors wrote in the March 4 online issue of the Lancet.
The rule, which "can predict a patient’s long-term probability of walking independently more accurately than ... the widely used AIS [American Spinal Injury Association/International Spinal Cord Society] grading system," was developed and validated in 492 trauma patients who were treated at 19 centers across Europe, according to Dr. van Middendorp of St. Radboud University Nijmegen (the Netherlands) and his coauthors (Lancet 2011 [doi:10.1016/S0140-6736(10)62276-3]).
The authors extracted data for their prediction algorithm from the European Multicenter Study on Human Spinal Cord Injury. The study included neurologic and functional status of 1,282 patients aged 18 years or older who had sustained a traumatic spinal cord injury and were treated and assessed according to each facility’s protocol at 15 days, and 1, 3, 6, and 12 months post injury.
Of the entire cohort, 118 had 6-month outcomes and 374 had 1-year outcomes available. Most of the patients were men (77%), with an average age at injury of 44 years. The AIS grade was A in 49%; B in 13%; C in 16%; and D in 22%. Of these 492 patients, 200 (41%) were able to walk independently after the injury.
The investigators’ prediction rule assumed an age cutoff point of 65 years, because studies have shown that patients aged 65 years and older have a lower functional recovery potential. They performed motor score testing; light-touch and pinprick-sensory testing; and determination of sacral function (voluntary anal contraction and sensation). In the final analysis, the authors included only the best scores at each level of the lower extremity that was being tested, and the binary (yes/no) score for sacral function.
The patients’ ability to walk independently at 1 year was measured by the Spinal Cord Independence Measure’s indoor mobility subscale. The scale ranges from 0 (requiring total assistance) to 8 (walking without any walking aids). Scores of 0-3 signified an inability to walk or a dependency on assistance, and 4-8 signified an ability to walk independently.
The investigators’ final logistic regression model, which was deemed highly accurate, included age and four neurologic predictors at 15 days after injury:
• Age of 65 or older (score of 0-1, multiplied by –10).
• Muscle grade for the quadriceps femoral muscle (score of 0-5, multiplied by 2).
• Muscle grade for the gastrocsoleus (score of 0-5, multiplied by 2).
• Light-touch sensation at spinal level L3 (score of 0-2, multiplied by 5).
• Light-touch sensation at spinal level S1 (score of 0-2, multiplied by 5).
The rule had a minimum score of –10 and a maximum score of 40. For patients with neurologic exams that were performed within the first 15 days after injury, the probability of independent walking rose as the total score increased, such that at –10 points there was no chance of independent walking at 1 year; at 10 points, there was 35% probability; at 15 points, about 65% probability; at 20 points, almost 90% probability; and at 30 points and greater, nearly 100% probability. The area under the receiver operating characteristic (ROC) curve for the prediction rule was 0.96, indicating excellent ability to discriminate between patients who would walk at 1 year and those would not, the authors said.
Additional analyses found that adding the level of spinal cord injury, the timing of the neurologic examination, or pinprick-sensation testing did not significantly change its predictive ability.
The investigators validated the accuracy of the prediction in a separate group of 214 patients who underwent neurologic evaluation within 15 days of their traumatic spinal cord injury.
"The prediction rule had a clear additional clinical value for the prediction of an individual’s ability to walk independently in each of the AIS grades," particularly in grade B and C patients, Dr. van Middendorp said in an interview. "These are the grades of severity [for which] physicians can experience difficulties in prognosticating."
"Although our prediction rule is more accurate and less time consuming than the AIS grading system, to do accurate and reliable assessments of the four [neurologic tests], a physician must have experience in the physical examination of patients with traumatic spinal cord injury," the authors noted.
In an accompanying commentary, Dr. Wagih Shafik El Masri and Dr. Naveen Kumar applauded the rule’s development, but cautioned that other factors, which the rule cannot address, play an important part in spinal cord injury recovery (Lancet 2011 [doi:10.1016/S0140-6736(11)60248-1]).
"Early prediction of ambulation outcome is important to patients," wrote the authors, both of whom are at the Robert Jones and Agnes Hunt Orthopaedic Hospital in Staffordshire, England. "However, several variables exist that affect the achievability and quality of such predictors," such as the patient’s preexisting health, associated comorbidities, quality of rehabilitation, and neurologic progress. "[Neurologic] and functional recovery should be thought of as two essential, distinct entities in the assessment of traumatic spinal cord injury outcomes."
Further studies are necessary to assess the individual prognostic components of the rule and assess their value at different times during recovery, as are more studies of interventions that can help not only with ambulation, neurologic recovery, and functional recovery, but also with autonomic functions that can impact quality of life, including bladder, bowel, cardiorespiratory, vasomotor, erectile, and reproductive problems, Dr. El Masri and Dr. Kumar wrote.
Neither the authors of the study nor those of the commentary declared any conflicts of interest. The study was funded by the Internationale Stiftung für Forschung in Paraplegie.
Rule Can Predict Walking Outcomes After Spinal Cord Injury
A new algorithm based on age and sensory and motor scores in four body areas may allow clinicians to predict with great accuracy which patients will be able to walk independently 1 year after spinal cord injury.
"We have developed a simple clinical prediction rule ... that can be used by physicians to counsel patients with traumatic spinal cord injury and their families during the initial phase after injury," Dr. Joost van Middendorp and his coauthors wrote in the March 4 online issue of the Lancet.
The rule, which "can predict a patient’s long-term probability of walking independently more accurately than ... the widely used AIS [American Spinal Injury Association/International Spinal Cord Society] grading system," was developed and validated in 492 trauma patients who were treated at 19 centers across Europe, according to Dr. van Middendorp of St. Radboud University Nijmegen (the Netherlands) and his coauthors (Lancet 2011 [doi:10.1016/S0140-6736(10)62276-3]).
The authors extracted data for their prediction algorithm from the European Multicenter Study on Human Spinal Cord Injury. The study included neurologic and functional status of 1,282 patients aged 18 years or older who had sustained a traumatic spinal cord injury and were treated and assessed according to each facility’s protocol at 15 days, and 1, 3, 6, and 12 months post injury.
Of the entire cohort, 118 had 6-month outcomes and 374 had 1-year outcomes available. Most of the patients were men (77%), with an average age at injury of 44 years. The AIS grade was A in 49%; B in 13%; C in 16%; and D in 22%. Of these 492 patients, 200 (41%) were able to walk independently after the injury.
The investigators’ prediction rule assumed an age cutoff point of 65 years, because studies have shown that patients aged 65 years and older have a lower functional recovery potential. They performed motor score testing; light-touch and pinprick-sensory testing; and determination of sacral function (voluntary anal contraction and sensation). In the final analysis, the authors included only the best scores at each level of the lower extremity that was being tested, and the binary (yes/no) score for sacral function.
The patients’ ability to walk independently at 1 year was measured by the Spinal Cord Independence Measure’s indoor mobility subscale. The scale ranges from 0 (requiring total assistance) to 8 (walking without any walking aids). Scores of 0-3 signified an inability to walk or a dependency on assistance, and 4-8 signified an ability to walk independently.
The investigators’ final logistic regression model, which was deemed highly accurate, included age and four neurologic predictors at 15 days after injury:
• Age of 65 or older (score of 0-1, multiplied by –10).
• Muscle grade for the quadriceps femoral muscle (score of 0-5, multiplied by 2).
• Muscle grade for the gastrocsoleus (score of 0-5, multiplied by 2).
• Light-touch sensation at spinal level L3 (score of 0-2, multiplied by 5).
• Light-touch sensation at spinal level S1 (score of 0-2, multiplied by 5).
The rule had a minimum score of –10 and a maximum score of 40. For patients with neurologic exams that were performed within the first 15 days after injury, the probability of independent walking rose as the total score increased, such that at –10 points there was no chance of independent walking at 1 year; at 10 points, there was 35% probability; at 15 points, about 65% probability; at 20 points, almost 90% probability; and at 30 points and greater, nearly 100% probability. The area under the receiver operating characteristic (ROC) curve for the prediction rule was 0.96, indicating excellent ability to discriminate between patients who would walk at 1 year and those would not, the authors said.
Additional analyses found that adding the level of spinal cord injury, the timing of the neurologic examination, or pinprick-sensation testing did not significantly change its predictive ability.
The investigators validated the accuracy of the prediction in a separate group of 214 patients who underwent neurologic evaluation within 15 days of their traumatic spinal cord injury.
"The prediction rule had a clear additional clinical value for the prediction of an individual’s ability to walk independently in each of the AIS grades," particularly in grade B and C patients, Dr. van Middendorp said in an interview. "These are the grades of severity [for which] physicians can experience difficulties in prognosticating."
"Although our prediction rule is more accurate and less time consuming than the AIS grading system, to do accurate and reliable assessments of the four [neurologic tests], a physician must have experience in the physical examination of patients with traumatic spinal cord injury," the authors noted.
In an accompanying commentary, Dr. Wagih Shafik El Masri and Dr. Naveen Kumar applauded the rule’s development, but cautioned that other factors, which the rule cannot address, play an important part in spinal cord injury recovery (Lancet 2011 [doi:10.1016/S0140-6736(11)60248-1]).
"Early prediction of ambulation outcome is important to patients," wrote the authors, both of whom are at the Robert Jones and Agnes Hunt Orthopaedic Hospital in Staffordshire, England. "However, several variables exist that affect the achievability and quality of such predictors," such as the patient’s preexisting health, associated comorbidities, quality of rehabilitation, and neurologic progress. "[Neurologic] and functional recovery should be thought of as two essential, distinct entities in the assessment of traumatic spinal cord injury outcomes."
Further studies are necessary to assess the individual prognostic components of the rule and assess their value at different times during recovery, as are more studies of interventions that can help not only with ambulation, neurologic recovery, and functional recovery, but also with autonomic functions that can impact quality of life, including bladder, bowel, cardiorespiratory, vasomotor, erectile, and reproductive problems, Dr. El Masri and Dr. Kumar wrote.
Neither the authors of the study nor those of the commentary declared any conflicts of interest. The study was funded by the Internationale Stiftung für Forschung in Paraplegie.
A new algorithm based on age and sensory and motor scores in four body areas may allow clinicians to predict with great accuracy which patients will be able to walk independently 1 year after spinal cord injury.
"We have developed a simple clinical prediction rule ... that can be used by physicians to counsel patients with traumatic spinal cord injury and their families during the initial phase after injury," Dr. Joost van Middendorp and his coauthors wrote in the March 4 online issue of the Lancet.
The rule, which "can predict a patient’s long-term probability of walking independently more accurately than ... the widely used AIS [American Spinal Injury Association/International Spinal Cord Society] grading system," was developed and validated in 492 trauma patients who were treated at 19 centers across Europe, according to Dr. van Middendorp of St. Radboud University Nijmegen (the Netherlands) and his coauthors (Lancet 2011 [doi:10.1016/S0140-6736(10)62276-3]).
The authors extracted data for their prediction algorithm from the European Multicenter Study on Human Spinal Cord Injury. The study included neurologic and functional status of 1,282 patients aged 18 years or older who had sustained a traumatic spinal cord injury and were treated and assessed according to each facility’s protocol at 15 days, and 1, 3, 6, and 12 months post injury.
Of the entire cohort, 118 had 6-month outcomes and 374 had 1-year outcomes available. Most of the patients were men (77%), with an average age at injury of 44 years. The AIS grade was A in 49%; B in 13%; C in 16%; and D in 22%. Of these 492 patients, 200 (41%) were able to walk independently after the injury.
The investigators’ prediction rule assumed an age cutoff point of 65 years, because studies have shown that patients aged 65 years and older have a lower functional recovery potential. They performed motor score testing; light-touch and pinprick-sensory testing; and determination of sacral function (voluntary anal contraction and sensation). In the final analysis, the authors included only the best scores at each level of the lower extremity that was being tested, and the binary (yes/no) score for sacral function.
The patients’ ability to walk independently at 1 year was measured by the Spinal Cord Independence Measure’s indoor mobility subscale. The scale ranges from 0 (requiring total assistance) to 8 (walking without any walking aids). Scores of 0-3 signified an inability to walk or a dependency on assistance, and 4-8 signified an ability to walk independently.
The investigators’ final logistic regression model, which was deemed highly accurate, included age and four neurologic predictors at 15 days after injury:
• Age of 65 or older (score of 0-1, multiplied by –10).
• Muscle grade for the quadriceps femoral muscle (score of 0-5, multiplied by 2).
• Muscle grade for the gastrocsoleus (score of 0-5, multiplied by 2).
• Light-touch sensation at spinal level L3 (score of 0-2, multiplied by 5).
• Light-touch sensation at spinal level S1 (score of 0-2, multiplied by 5).
The rule had a minimum score of –10 and a maximum score of 40. For patients with neurologic exams that were performed within the first 15 days after injury, the probability of independent walking rose as the total score increased, such that at –10 points there was no chance of independent walking at 1 year; at 10 points, there was 35% probability; at 15 points, about 65% probability; at 20 points, almost 90% probability; and at 30 points and greater, nearly 100% probability. The area under the receiver operating characteristic (ROC) curve for the prediction rule was 0.96, indicating excellent ability to discriminate between patients who would walk at 1 year and those would not, the authors said.
Additional analyses found that adding the level of spinal cord injury, the timing of the neurologic examination, or pinprick-sensation testing did not significantly change its predictive ability.
The investigators validated the accuracy of the prediction in a separate group of 214 patients who underwent neurologic evaluation within 15 days of their traumatic spinal cord injury.
"The prediction rule had a clear additional clinical value for the prediction of an individual’s ability to walk independently in each of the AIS grades," particularly in grade B and C patients, Dr. van Middendorp said in an interview. "These are the grades of severity [for which] physicians can experience difficulties in prognosticating."
"Although our prediction rule is more accurate and less time consuming than the AIS grading system, to do accurate and reliable assessments of the four [neurologic tests], a physician must have experience in the physical examination of patients with traumatic spinal cord injury," the authors noted.
In an accompanying commentary, Dr. Wagih Shafik El Masri and Dr. Naveen Kumar applauded the rule’s development, but cautioned that other factors, which the rule cannot address, play an important part in spinal cord injury recovery (Lancet 2011 [doi:10.1016/S0140-6736(11)60248-1]).
"Early prediction of ambulation outcome is important to patients," wrote the authors, both of whom are at the Robert Jones and Agnes Hunt Orthopaedic Hospital in Staffordshire, England. "However, several variables exist that affect the achievability and quality of such predictors," such as the patient’s preexisting health, associated comorbidities, quality of rehabilitation, and neurologic progress. "[Neurologic] and functional recovery should be thought of as two essential, distinct entities in the assessment of traumatic spinal cord injury outcomes."
Further studies are necessary to assess the individual prognostic components of the rule and assess their value at different times during recovery, as are more studies of interventions that can help not only with ambulation, neurologic recovery, and functional recovery, but also with autonomic functions that can impact quality of life, including bladder, bowel, cardiorespiratory, vasomotor, erectile, and reproductive problems, Dr. El Masri and Dr. Kumar wrote.
Neither the authors of the study nor those of the commentary declared any conflicts of interest. The study was funded by the Internationale Stiftung für Forschung in Paraplegie.
A new algorithm based on age and sensory and motor scores in four body areas may allow clinicians to predict with great accuracy which patients will be able to walk independently 1 year after spinal cord injury.
"We have developed a simple clinical prediction rule ... that can be used by physicians to counsel patients with traumatic spinal cord injury and their families during the initial phase after injury," Dr. Joost van Middendorp and his coauthors wrote in the March 4 online issue of the Lancet.
The rule, which "can predict a patient’s long-term probability of walking independently more accurately than ... the widely used AIS [American Spinal Injury Association/International Spinal Cord Society] grading system," was developed and validated in 492 trauma patients who were treated at 19 centers across Europe, according to Dr. van Middendorp of St. Radboud University Nijmegen (the Netherlands) and his coauthors (Lancet 2011 [doi:10.1016/S0140-6736(10)62276-3]).
The authors extracted data for their prediction algorithm from the European Multicenter Study on Human Spinal Cord Injury. The study included neurologic and functional status of 1,282 patients aged 18 years or older who had sustained a traumatic spinal cord injury and were treated and assessed according to each facility’s protocol at 15 days, and 1, 3, 6, and 12 months post injury.
Of the entire cohort, 118 had 6-month outcomes and 374 had 1-year outcomes available. Most of the patients were men (77%), with an average age at injury of 44 years. The AIS grade was A in 49%; B in 13%; C in 16%; and D in 22%. Of these 492 patients, 200 (41%) were able to walk independently after the injury.
The investigators’ prediction rule assumed an age cutoff point of 65 years, because studies have shown that patients aged 65 years and older have a lower functional recovery potential. They performed motor score testing; light-touch and pinprick-sensory testing; and determination of sacral function (voluntary anal contraction and sensation). In the final analysis, the authors included only the best scores at each level of the lower extremity that was being tested, and the binary (yes/no) score for sacral function.
The patients’ ability to walk independently at 1 year was measured by the Spinal Cord Independence Measure’s indoor mobility subscale. The scale ranges from 0 (requiring total assistance) to 8 (walking without any walking aids). Scores of 0-3 signified an inability to walk or a dependency on assistance, and 4-8 signified an ability to walk independently.
The investigators’ final logistic regression model, which was deemed highly accurate, included age and four neurologic predictors at 15 days after injury:
• Age of 65 or older (score of 0-1, multiplied by –10).
• Muscle grade for the quadriceps femoral muscle (score of 0-5, multiplied by 2).
• Muscle grade for the gastrocsoleus (score of 0-5, multiplied by 2).
• Light-touch sensation at spinal level L3 (score of 0-2, multiplied by 5).
• Light-touch sensation at spinal level S1 (score of 0-2, multiplied by 5).
The rule had a minimum score of –10 and a maximum score of 40. For patients with neurologic exams that were performed within the first 15 days after injury, the probability of independent walking rose as the total score increased, such that at –10 points there was no chance of independent walking at 1 year; at 10 points, there was 35% probability; at 15 points, about 65% probability; at 20 points, almost 90% probability; and at 30 points and greater, nearly 100% probability. The area under the receiver operating characteristic (ROC) curve for the prediction rule was 0.96, indicating excellent ability to discriminate between patients who would walk at 1 year and those would not, the authors said.
Additional analyses found that adding the level of spinal cord injury, the timing of the neurologic examination, or pinprick-sensation testing did not significantly change its predictive ability.
The investigators validated the accuracy of the prediction in a separate group of 214 patients who underwent neurologic evaluation within 15 days of their traumatic spinal cord injury.
"The prediction rule had a clear additional clinical value for the prediction of an individual’s ability to walk independently in each of the AIS grades," particularly in grade B and C patients, Dr. van Middendorp said in an interview. "These are the grades of severity [for which] physicians can experience difficulties in prognosticating."
"Although our prediction rule is more accurate and less time consuming than the AIS grading system, to do accurate and reliable assessments of the four [neurologic tests], a physician must have experience in the physical examination of patients with traumatic spinal cord injury," the authors noted.
In an accompanying commentary, Dr. Wagih Shafik El Masri and Dr. Naveen Kumar applauded the rule’s development, but cautioned that other factors, which the rule cannot address, play an important part in spinal cord injury recovery (Lancet 2011 [doi:10.1016/S0140-6736(11)60248-1]).
"Early prediction of ambulation outcome is important to patients," wrote the authors, both of whom are at the Robert Jones and Agnes Hunt Orthopaedic Hospital in Staffordshire, England. "However, several variables exist that affect the achievability and quality of such predictors," such as the patient’s preexisting health, associated comorbidities, quality of rehabilitation, and neurologic progress. "[Neurologic] and functional recovery should be thought of as two essential, distinct entities in the assessment of traumatic spinal cord injury outcomes."
Further studies are necessary to assess the individual prognostic components of the rule and assess their value at different times during recovery, as are more studies of interventions that can help not only with ambulation, neurologic recovery, and functional recovery, but also with autonomic functions that can impact quality of life, including bladder, bowel, cardiorespiratory, vasomotor, erectile, and reproductive problems, Dr. El Masri and Dr. Kumar wrote.
Neither the authors of the study nor those of the commentary declared any conflicts of interest. The study was funded by the Internationale Stiftung für Forschung in Paraplegie.
Major Finding: A prediction rule using age and neurologic testing at four sites was highly accurate (with an area under the ROC curve of 0.96) in predicting walking outcomes at 1 year in 492 patients with spinal cord injury.
Data Source: A derivation cohort of 492 patients with traumatic spinal cord injury and a validation cohort of 214 similar patients.
Disclosures: Neither the authors of the study nor those of the commentary declared any conflicts of interest. The study was funded by the Internationale Stiftung für Forschung in Paraplegie.
Registry Supports MERCI Trials Data, but Methods Criticized
LOS ANGELES - Results of the MERCI patient registry appear to uphold findings from the device’s two pivotal trials of patients with acute ischemic stroke, which demonstrated that successful recanalization is significantly associated with good outcome.
But audience members who spoke at the International Stroke Conference following the presentation of the registry results emphasized – to the applause of others in the audience – that the MERCI device lacks randomized data proving its safety and efficacy, and that reported outcomes for patients have not been stratified according to intubation status.
The registry is a large, nonrandomized case series documenting the postapproval use of the devices, whereas the two pivotal trials (MERCI and Multi MERCI) had strict inclusion and exclusion criteria and protocols but did not compare the device vs. medical therapy. Device-treated patients in the trials were instead compared with a placebo group from a randomized medical trial, called PROACT II (Prolyse in Acute Cerebral Thromboembolism).
At the conference, Dr. Marilyn Rymer presented the 90-day outcomes of 1,000 patients with acute ischemic stroke in the registry who were treated with the MERCI clot retriever embolectomy device. The MERCI (Mechanical Embolus Removal in Cerebral Ischemia) and Multi MERCI studies examined embolectomy with the device in similar patient groups, with a total of 305 patients.
"The registry is designed to answer the question, ‘What does the real-world, unrestrained treatment of ischemic stroke with this device look like?’" said Dr. Rymer, a medical director of the Brain and Stroke Institute at St. Luke’s Hospital in Kansas City, Mo. However, she noted that because the registry consists of nonrandomized cases, the efficacy implied in it "can’t be compared to a medical therapy."
The participating sites included every consecutive patient who was treated with the device. Treatment remained a clinical decision guided by each site’s general practice. The inclusion criteria were a diagnosis of acute ischemic stroke and at least one pass with the tool.
The primary end point was revascularization with a TICI (Thrombolysis in Cerebral Infarction) scale grade of 2a or higher; there was also a secondary functional outcome, which was the modified Rankin Scale (mRS) score at 90 days. The final analysis included 872 patients as a result of excluding 4 with insufficient procedural data and 90 who were disabled before their stroke with an mRS of 2 or more, as well as losing 34 to follow-up.
The patients’ median age was 68 years, compared with the median age of 72 in the trials. There was wide intersite variability with regard to age: At one site, the median age of patients treated was 58, and at another the median age of patients treated was 72.
"We saw the same kind of variation in terms of baseline National Institutes of Health Stroke Scale [NIHSS] score," Dr. Rymer said at the conference, which was sponsored by the American Heart Association. "The median in the registry was 17, while it was 21 in the trials." Median NIHSS scores also varied across the registry sites (range, 14-21), she said.
Overall, 305 patients received intravenous thrombolytic therapy, "But there was an incredible variation among sites" in the use of thrombolytics in conjunction with embolectomy (range, 0%-71%). Intra-arterial thrombolytic therapy also varied widely: Some 47% of patients overall received it, but the intersite rate varied from 7% to 100%.
Overall, 63% of patients were intubated, with the rate varying from 12% to 100%. "Several sites used intubation routinely in 100% of their patients, and some intubated only for airway protection. This becomes important as we begin to understand that intubation is associated with a worse outcome," Dr. Rymer said.
Most sites treated fewer than 19% of patients with angioplasty or stenting in addition to clot retrieval, but one site employed these additional treatments in 64% of patients.
The time from symptom onset to groin insertion was 6.3 hours, compared with 4.5 hours in the MERCI trials. Most patients (71%) were treated 3-8 hours after symptom onset.
Overall in the registry, 80% of patients were successfully recanalized, which was significantly more than in the two MERCI trials combined (65%). Similar numbers of patients had good 90-day outcomes (32% in both the registry and combined trials). Mortality at 90 days also was not significantly different between the registry and the trials (33% and 38%).
Symptomatic brain hemorrhage in the registry was 7% overall, not significantly different from the 8.8% seen in the MERCI trials, Dr. Rymer said. "It is notable that in the patients who were well recanalized (those with a TICI grade of 2b to 3), the symptomatic hemorrhage rate was lower (3.7% in TICI 2b and 5.4% in TICI 3)."
When the investigators examined the rate of good 90-day outcome (defined as an mRS of 0-2), they found the best outcomes in patients with the lowest baseline NIHSS scores. "As the stroke became more severe, the likelihood of good outcome went down," she said. In cases with NIHSS scores lower than 16, "the outcomes were excellent," she said, with up to 70% of those with a TICI grade of 2b or 3 experiencing a good outcome. TICI 2a provided only modest benefit, but it was consistent across the whole range of NIHSS scores, she added.
Age and recanalization status also affected mortality. "Age was a predictor of worse outcome, but recanalization did provide benefit across all ages except for the very young, who had low mortality rates in any case," she said.
A multivariate analysis identified several factors that significantly affected mortality both negatively and positively, including advancing age (odds ratio, 1.05), worse baseline NIHSS score (OR, 1.08), revascularization to a TICI grade of 2a, 2b, or 3 (OR, 0.33), heart failure (OR, 2.85), blood glucose above 140 mg/dL (OR 2.0), and intubation during the revascularization procedure (OR, 2.20)
The same multivariate model also identified factors that negatively impacted good 90-day outcomes, including worse baseline NIHSS score (OR, 0.88), advancing age (OR, 0.96), intubation during the procedure (OR, 0.43), longer duration of procedure (OR, 0.66), and a blood glucose level of 140 mg/dL or greater (OR, 0.59).
During the discussion period, several audience members questioned the relationship between intubation and poor outcomes. Dr. Joseph Broderick, chair of the department of neurology at the University of Cincinnati, said that the intubation data were interesting but could throw a statistical kink into the risk analysis. "How do you take into account those centers with a 100% intubation rate?" he asked. "Why don’t you analyze the data separately and see if the risk of intubation still holds up? There is always a risk of selection bias unless you compare the [sites] that always intubated against those that did not. Otherwise, you might be including people who were intubated because they looked dead, had heart failure, or weren’t breathing."
Three other speakers echoed the same concern, with one suggesting that intubation could lead to aspiration pneumonia, which in turn could contribute to a bad outcome.
"We don’t know what all the facts are" in relation to intubation, Dr. Rymer said. "We can only speculate."
However, the discussants also emphasized that there are still no randomized data confirming the device’s efficacy when compared with medical therapies. "There is, however, a clear association of improved outcomes with better revascularization," Dr. Rymer said in an interview.
Both MERCI and Multi MERCI were criticized for comparing their device-treated populations with only a placebo arm of PROACT II, according to an editorial by Dr. Kyra J. Becker of the University of Washington and Dr. Thomas G. Brott of the Mayo Clinic in Jacksonville, Fla. (Stroke 2005;36:400-3).
They said that the data presented to the Food and Drug Administration for the device’s approval did not meet the standards that could be achieved in a randomized trial.
"These data certainly provide no reassurance about the safety of mechanical embolectomy, yet alone the efficacy," the authors said. "The risks of treatment with the Concentric clot retriever might not be limited to arterial perforation, dissection, or distal embolization; there could be unidentified risks associated with the required immediate poststroke angiography as well as with endovascular instrumentation and injury to the arterial endothelium. Unfortunately, these questions cannot be addressed with the data presented ... by the MERCI investigators. Randomized trials have a distinct advantage in this setting; they can control for variables we know are likely to relate to procedure success and outcome, and they can control for important variables we have yet to identify."
Concentric Medical, the manufacturer of the MERCI device, sponsored the MERCI registry. Dr. Rymer reported being on the speakers bureau of the company. The five other coauthors reported varying relationships with Concentric, including being consultants or medical advisors, and having an ownership interest in Concentric.
LOS ANGELES - Results of the MERCI patient registry appear to uphold findings from the device’s two pivotal trials of patients with acute ischemic stroke, which demonstrated that successful recanalization is significantly associated with good outcome.
But audience members who spoke at the International Stroke Conference following the presentation of the registry results emphasized – to the applause of others in the audience – that the MERCI device lacks randomized data proving its safety and efficacy, and that reported outcomes for patients have not been stratified according to intubation status.
The registry is a large, nonrandomized case series documenting the postapproval use of the devices, whereas the two pivotal trials (MERCI and Multi MERCI) had strict inclusion and exclusion criteria and protocols but did not compare the device vs. medical therapy. Device-treated patients in the trials were instead compared with a placebo group from a randomized medical trial, called PROACT II (Prolyse in Acute Cerebral Thromboembolism).
At the conference, Dr. Marilyn Rymer presented the 90-day outcomes of 1,000 patients with acute ischemic stroke in the registry who were treated with the MERCI clot retriever embolectomy device. The MERCI (Mechanical Embolus Removal in Cerebral Ischemia) and Multi MERCI studies examined embolectomy with the device in similar patient groups, with a total of 305 patients.
"The registry is designed to answer the question, ‘What does the real-world, unrestrained treatment of ischemic stroke with this device look like?’" said Dr. Rymer, a medical director of the Brain and Stroke Institute at St. Luke’s Hospital in Kansas City, Mo. However, she noted that because the registry consists of nonrandomized cases, the efficacy implied in it "can’t be compared to a medical therapy."
The participating sites included every consecutive patient who was treated with the device. Treatment remained a clinical decision guided by each site’s general practice. The inclusion criteria were a diagnosis of acute ischemic stroke and at least one pass with the tool.
The primary end point was revascularization with a TICI (Thrombolysis in Cerebral Infarction) scale grade of 2a or higher; there was also a secondary functional outcome, which was the modified Rankin Scale (mRS) score at 90 days. The final analysis included 872 patients as a result of excluding 4 with insufficient procedural data and 90 who were disabled before their stroke with an mRS of 2 or more, as well as losing 34 to follow-up.
The patients’ median age was 68 years, compared with the median age of 72 in the trials. There was wide intersite variability with regard to age: At one site, the median age of patients treated was 58, and at another the median age of patients treated was 72.
"We saw the same kind of variation in terms of baseline National Institutes of Health Stroke Scale [NIHSS] score," Dr. Rymer said at the conference, which was sponsored by the American Heart Association. "The median in the registry was 17, while it was 21 in the trials." Median NIHSS scores also varied across the registry sites (range, 14-21), she said.
Overall, 305 patients received intravenous thrombolytic therapy, "But there was an incredible variation among sites" in the use of thrombolytics in conjunction with embolectomy (range, 0%-71%). Intra-arterial thrombolytic therapy also varied widely: Some 47% of patients overall received it, but the intersite rate varied from 7% to 100%.
Overall, 63% of patients were intubated, with the rate varying from 12% to 100%. "Several sites used intubation routinely in 100% of their patients, and some intubated only for airway protection. This becomes important as we begin to understand that intubation is associated with a worse outcome," Dr. Rymer said.
Most sites treated fewer than 19% of patients with angioplasty or stenting in addition to clot retrieval, but one site employed these additional treatments in 64% of patients.
The time from symptom onset to groin insertion was 6.3 hours, compared with 4.5 hours in the MERCI trials. Most patients (71%) were treated 3-8 hours after symptom onset.
Overall in the registry, 80% of patients were successfully recanalized, which was significantly more than in the two MERCI trials combined (65%). Similar numbers of patients had good 90-day outcomes (32% in both the registry and combined trials). Mortality at 90 days also was not significantly different between the registry and the trials (33% and 38%).
Symptomatic brain hemorrhage in the registry was 7% overall, not significantly different from the 8.8% seen in the MERCI trials, Dr. Rymer said. "It is notable that in the patients who were well recanalized (those with a TICI grade of 2b to 3), the symptomatic hemorrhage rate was lower (3.7% in TICI 2b and 5.4% in TICI 3)."
When the investigators examined the rate of good 90-day outcome (defined as an mRS of 0-2), they found the best outcomes in patients with the lowest baseline NIHSS scores. "As the stroke became more severe, the likelihood of good outcome went down," she said. In cases with NIHSS scores lower than 16, "the outcomes were excellent," she said, with up to 70% of those with a TICI grade of 2b or 3 experiencing a good outcome. TICI 2a provided only modest benefit, but it was consistent across the whole range of NIHSS scores, she added.
Age and recanalization status also affected mortality. "Age was a predictor of worse outcome, but recanalization did provide benefit across all ages except for the very young, who had low mortality rates in any case," she said.
A multivariate analysis identified several factors that significantly affected mortality both negatively and positively, including advancing age (odds ratio, 1.05), worse baseline NIHSS score (OR, 1.08), revascularization to a TICI grade of 2a, 2b, or 3 (OR, 0.33), heart failure (OR, 2.85), blood glucose above 140 mg/dL (OR 2.0), and intubation during the revascularization procedure (OR, 2.20)
The same multivariate model also identified factors that negatively impacted good 90-day outcomes, including worse baseline NIHSS score (OR, 0.88), advancing age (OR, 0.96), intubation during the procedure (OR, 0.43), longer duration of procedure (OR, 0.66), and a blood glucose level of 140 mg/dL or greater (OR, 0.59).
During the discussion period, several audience members questioned the relationship between intubation and poor outcomes. Dr. Joseph Broderick, chair of the department of neurology at the University of Cincinnati, said that the intubation data were interesting but could throw a statistical kink into the risk analysis. "How do you take into account those centers with a 100% intubation rate?" he asked. "Why don’t you analyze the data separately and see if the risk of intubation still holds up? There is always a risk of selection bias unless you compare the [sites] that always intubated against those that did not. Otherwise, you might be including people who were intubated because they looked dead, had heart failure, or weren’t breathing."
Three other speakers echoed the same concern, with one suggesting that intubation could lead to aspiration pneumonia, which in turn could contribute to a bad outcome.
"We don’t know what all the facts are" in relation to intubation, Dr. Rymer said. "We can only speculate."
However, the discussants also emphasized that there are still no randomized data confirming the device’s efficacy when compared with medical therapies. "There is, however, a clear association of improved outcomes with better revascularization," Dr. Rymer said in an interview.
Both MERCI and Multi MERCI were criticized for comparing their device-treated populations with only a placebo arm of PROACT II, according to an editorial by Dr. Kyra J. Becker of the University of Washington and Dr. Thomas G. Brott of the Mayo Clinic in Jacksonville, Fla. (Stroke 2005;36:400-3).
They said that the data presented to the Food and Drug Administration for the device’s approval did not meet the standards that could be achieved in a randomized trial.
"These data certainly provide no reassurance about the safety of mechanical embolectomy, yet alone the efficacy," the authors said. "The risks of treatment with the Concentric clot retriever might not be limited to arterial perforation, dissection, or distal embolization; there could be unidentified risks associated with the required immediate poststroke angiography as well as with endovascular instrumentation and injury to the arterial endothelium. Unfortunately, these questions cannot be addressed with the data presented ... by the MERCI investigators. Randomized trials have a distinct advantage in this setting; they can control for variables we know are likely to relate to procedure success and outcome, and they can control for important variables we have yet to identify."
Concentric Medical, the manufacturer of the MERCI device, sponsored the MERCI registry. Dr. Rymer reported being on the speakers bureau of the company. The five other coauthors reported varying relationships with Concentric, including being consultants or medical advisors, and having an ownership interest in Concentric.
LOS ANGELES - Results of the MERCI patient registry appear to uphold findings from the device’s two pivotal trials of patients with acute ischemic stroke, which demonstrated that successful recanalization is significantly associated with good outcome.
But audience members who spoke at the International Stroke Conference following the presentation of the registry results emphasized – to the applause of others in the audience – that the MERCI device lacks randomized data proving its safety and efficacy, and that reported outcomes for patients have not been stratified according to intubation status.
The registry is a large, nonrandomized case series documenting the postapproval use of the devices, whereas the two pivotal trials (MERCI and Multi MERCI) had strict inclusion and exclusion criteria and protocols but did not compare the device vs. medical therapy. Device-treated patients in the trials were instead compared with a placebo group from a randomized medical trial, called PROACT II (Prolyse in Acute Cerebral Thromboembolism).
At the conference, Dr. Marilyn Rymer presented the 90-day outcomes of 1,000 patients with acute ischemic stroke in the registry who were treated with the MERCI clot retriever embolectomy device. The MERCI (Mechanical Embolus Removal in Cerebral Ischemia) and Multi MERCI studies examined embolectomy with the device in similar patient groups, with a total of 305 patients.
"The registry is designed to answer the question, ‘What does the real-world, unrestrained treatment of ischemic stroke with this device look like?’" said Dr. Rymer, a medical director of the Brain and Stroke Institute at St. Luke’s Hospital in Kansas City, Mo. However, she noted that because the registry consists of nonrandomized cases, the efficacy implied in it "can’t be compared to a medical therapy."
The participating sites included every consecutive patient who was treated with the device. Treatment remained a clinical decision guided by each site’s general practice. The inclusion criteria were a diagnosis of acute ischemic stroke and at least one pass with the tool.
The primary end point was revascularization with a TICI (Thrombolysis in Cerebral Infarction) scale grade of 2a or higher; there was also a secondary functional outcome, which was the modified Rankin Scale (mRS) score at 90 days. The final analysis included 872 patients as a result of excluding 4 with insufficient procedural data and 90 who were disabled before their stroke with an mRS of 2 or more, as well as losing 34 to follow-up.
The patients’ median age was 68 years, compared with the median age of 72 in the trials. There was wide intersite variability with regard to age: At one site, the median age of patients treated was 58, and at another the median age of patients treated was 72.
"We saw the same kind of variation in terms of baseline National Institutes of Health Stroke Scale [NIHSS] score," Dr. Rymer said at the conference, which was sponsored by the American Heart Association. "The median in the registry was 17, while it was 21 in the trials." Median NIHSS scores also varied across the registry sites (range, 14-21), she said.
Overall, 305 patients received intravenous thrombolytic therapy, "But there was an incredible variation among sites" in the use of thrombolytics in conjunction with embolectomy (range, 0%-71%). Intra-arterial thrombolytic therapy also varied widely: Some 47% of patients overall received it, but the intersite rate varied from 7% to 100%.
Overall, 63% of patients were intubated, with the rate varying from 12% to 100%. "Several sites used intubation routinely in 100% of their patients, and some intubated only for airway protection. This becomes important as we begin to understand that intubation is associated with a worse outcome," Dr. Rymer said.
Most sites treated fewer than 19% of patients with angioplasty or stenting in addition to clot retrieval, but one site employed these additional treatments in 64% of patients.
The time from symptom onset to groin insertion was 6.3 hours, compared with 4.5 hours in the MERCI trials. Most patients (71%) were treated 3-8 hours after symptom onset.
Overall in the registry, 80% of patients were successfully recanalized, which was significantly more than in the two MERCI trials combined (65%). Similar numbers of patients had good 90-day outcomes (32% in both the registry and combined trials). Mortality at 90 days also was not significantly different between the registry and the trials (33% and 38%).
Symptomatic brain hemorrhage in the registry was 7% overall, not significantly different from the 8.8% seen in the MERCI trials, Dr. Rymer said. "It is notable that in the patients who were well recanalized (those with a TICI grade of 2b to 3), the symptomatic hemorrhage rate was lower (3.7% in TICI 2b and 5.4% in TICI 3)."
When the investigators examined the rate of good 90-day outcome (defined as an mRS of 0-2), they found the best outcomes in patients with the lowest baseline NIHSS scores. "As the stroke became more severe, the likelihood of good outcome went down," she said. In cases with NIHSS scores lower than 16, "the outcomes were excellent," she said, with up to 70% of those with a TICI grade of 2b or 3 experiencing a good outcome. TICI 2a provided only modest benefit, but it was consistent across the whole range of NIHSS scores, she added.
Age and recanalization status also affected mortality. "Age was a predictor of worse outcome, but recanalization did provide benefit across all ages except for the very young, who had low mortality rates in any case," she said.
A multivariate analysis identified several factors that significantly affected mortality both negatively and positively, including advancing age (odds ratio, 1.05), worse baseline NIHSS score (OR, 1.08), revascularization to a TICI grade of 2a, 2b, or 3 (OR, 0.33), heart failure (OR, 2.85), blood glucose above 140 mg/dL (OR 2.0), and intubation during the revascularization procedure (OR, 2.20)
The same multivariate model also identified factors that negatively impacted good 90-day outcomes, including worse baseline NIHSS score (OR, 0.88), advancing age (OR, 0.96), intubation during the procedure (OR, 0.43), longer duration of procedure (OR, 0.66), and a blood glucose level of 140 mg/dL or greater (OR, 0.59).
During the discussion period, several audience members questioned the relationship between intubation and poor outcomes. Dr. Joseph Broderick, chair of the department of neurology at the University of Cincinnati, said that the intubation data were interesting but could throw a statistical kink into the risk analysis. "How do you take into account those centers with a 100% intubation rate?" he asked. "Why don’t you analyze the data separately and see if the risk of intubation still holds up? There is always a risk of selection bias unless you compare the [sites] that always intubated against those that did not. Otherwise, you might be including people who were intubated because they looked dead, had heart failure, or weren’t breathing."
Three other speakers echoed the same concern, with one suggesting that intubation could lead to aspiration pneumonia, which in turn could contribute to a bad outcome.
"We don’t know what all the facts are" in relation to intubation, Dr. Rymer said. "We can only speculate."
However, the discussants also emphasized that there are still no randomized data confirming the device’s efficacy when compared with medical therapies. "There is, however, a clear association of improved outcomes with better revascularization," Dr. Rymer said in an interview.
Both MERCI and Multi MERCI were criticized for comparing their device-treated populations with only a placebo arm of PROACT II, according to an editorial by Dr. Kyra J. Becker of the University of Washington and Dr. Thomas G. Brott of the Mayo Clinic in Jacksonville, Fla. (Stroke 2005;36:400-3).
They said that the data presented to the Food and Drug Administration for the device’s approval did not meet the standards that could be achieved in a randomized trial.
"These data certainly provide no reassurance about the safety of mechanical embolectomy, yet alone the efficacy," the authors said. "The risks of treatment with the Concentric clot retriever might not be limited to arterial perforation, dissection, or distal embolization; there could be unidentified risks associated with the required immediate poststroke angiography as well as with endovascular instrumentation and injury to the arterial endothelium. Unfortunately, these questions cannot be addressed with the data presented ... by the MERCI investigators. Randomized trials have a distinct advantage in this setting; they can control for variables we know are likely to relate to procedure success and outcome, and they can control for important variables we have yet to identify."
Concentric Medical, the manufacturer of the MERCI device, sponsored the MERCI registry. Dr. Rymer reported being on the speakers bureau of the company. The five other coauthors reported varying relationships with Concentric, including being consultants or medical advisors, and having an ownership interest in Concentric.
Major Finding: Use of the MERCI clot retrieval device resulted in successful recanalization in 80% of patients, good 90-day functional outcomes in 32%, and 33% mortality.
Data Source: The MERCI patient registry of 1,000 patients with acute ischemic stroke who were treated consecutively at 37 U.S. centers.
Disclosures: Concentric Medical, the manufacturer of the MERCI device, sponsored the MERCI registry. Dr. Rymer reported being on the speakers bureau of the company. The five other coauthors reported varying relationships with Concentric, including being consultants or medical advisors, and having an ownership interest in Concentric.
Registry Supports MERCI Trials Data, but Methods Criticized
LOS ANGELES - Results of the MERCI patient registry appear to uphold findings from the device’s two pivotal trials of patients with acute ischemic stroke, which demonstrated that successful recanalization is significantly associated with good outcome.
But audience members who spoke at the International Stroke Conference following the presentation of the registry results emphasized – to the applause of others in the audience – that the MERCI device lacks randomized data proving its safety and efficacy, and that reported outcomes for patients have not been stratified according to intubation status.
The registry is a large, nonrandomized case series documenting the postapproval use of the devices, whereas the two pivotal trials (MERCI and Multi MERCI) had strict inclusion and exclusion criteria and protocols but did not compare the device vs. medical therapy. Device-treated patients in the trials were instead compared with a placebo group from a randomized medical trial, called PROACT II (Prolyse in Acute Cerebral Thromboembolism).
At the conference, Dr. Marilyn Rymer presented the 90-day outcomes of 1,000 patients with acute ischemic stroke in the registry who were treated with the MERCI clot retriever embolectomy device. The MERCI (Mechanical Embolus Removal in Cerebral Ischemia) and Multi MERCI studies examined embolectomy with the device in similar patient groups, with a total of 305 patients.
"The registry is designed to answer the question, ‘What does the real-world, unrestrained treatment of ischemic stroke with this device look like?’" said Dr. Rymer, a medical director of the Brain and Stroke Institute at St. Luke’s Hospital in Kansas City, Mo. However, she noted that because the registry consists of nonrandomized cases, the efficacy implied in it "can’t be compared to a medical therapy."
The participating sites included every consecutive patient who was treated with the device. Treatment remained a clinical decision guided by each site’s general practice. The inclusion criteria were a diagnosis of acute ischemic stroke and at least one pass with the tool.
The primary end point was revascularization with a TICI (Thrombolysis in Cerebral Infarction) scale grade of 2a or higher; there was also a secondary functional outcome, which was the modified Rankin Scale (mRS) score at 90 days. The final analysis included 872 patients as a result of excluding 4 with insufficient procedural data and 90 who were disabled before their stroke with an mRS of 2 or more, as well as losing 34 to follow-up.
The patients’ median age was 68 years, compared with the median age of 72 in the trials. There was wide intersite variability with regard to age: At one site, the median age of patients treated was 58, and at another the median age of patients treated was 72.
"We saw the same kind of variation in terms of baseline National Institutes of Health Stroke Scale [NIHSS] score," Dr. Rymer said at the conference, which was sponsored by the American Heart Association. "The median in the registry was 17, while it was 21 in the trials." Median NIHSS scores also varied across the registry sites (range, 14-21), she said.
Overall, 305 patients received intravenous thrombolytic therapy, "But there was an incredible variation among sites" in the use of thrombolytics in conjunction with embolectomy (range, 0%-71%). Intra-arterial thrombolytic therapy also varied widely: Some 47% of patients overall received it, but the intersite rate varied from 7% to 100%.
Overall, 63% of patients were intubated, with the rate varying from 12% to 100%. "Several sites used intubation routinely in 100% of their patients, and some intubated only for airway protection. This becomes important as we begin to understand that intubation is associated with a worse outcome," Dr. Rymer said.
Most sites treated fewer than 19% of patients with angioplasty or stenting in addition to clot retrieval, but one site employed these additional treatments in 64% of patients.
The time from symptom onset to groin insertion was 6.3 hours, compared with 4.5 hours in the MERCI trials. Most patients (71%) were treated 3-8 hours after symptom onset.
Overall in the registry, 80% of patients were successfully recanalized, which was significantly more than in the two MERCI trials combined (65%). Similar numbers of patients had good 90-day outcomes (32% in both the registry and combined trials). Mortality at 90 days also was not significantly different between the registry and the trials (33% and 38%).
Symptomatic brain hemorrhage in the registry was 7% overall, not significantly different from the 8.8% seen in the MERCI trials, Dr. Rymer said. "It is notable that in the patients who were well recanalized (those with a TICI grade of 2b to 3), the symptomatic hemorrhage rate was lower (3.7% in TICI 2b and 5.4% in TICI 3)."
When the investigators examined the rate of good 90-day outcome (defined as an mRS of 0-2), they found the best outcomes in patients with the lowest baseline NIHSS scores. "As the stroke became more severe, the likelihood of good outcome went down," she said. In cases with NIHSS scores lower than 16, "the outcomes were excellent," she said, with up to 70% of those with a TICI grade of 2b or 3 experiencing a good outcome. TICI 2a provided only modest benefit, but it was consistent across the whole range of NIHSS scores, she added.
Age and recanalization status also affected mortality. "Age was a predictor of worse outcome, but recanalization did provide benefit across all ages except for the very young, who had low mortality rates in any case," she said.
A multivariate analysis identified several factors that significantly affected mortality both negatively and positively, including advancing age (odds ratio, 1.05), worse baseline NIHSS score (OR, 1.08), revascularization to a TICI grade of 2a, 2b, or 3 (OR, 0.33), heart failure (OR, 2.85), blood glucose above 140 mg/dL (OR 2.0), and intubation during the revascularization procedure (OR, 2.20)
The same multivariate model also identified factors that negatively impacted good 90-day outcomes, including worse baseline NIHSS score (OR, 0.88), advancing age (OR, 0.96), intubation during the procedure (OR, 0.43), longer duration of procedure (OR, 0.66), and a blood glucose level of 140 mg/dL or greater (OR, 0.59).
During the discussion period, several audience members questioned the relationship between intubation and poor outcomes. Dr. Joseph Broderick, chair of the department of neurology at the University of Cincinnati, said that the intubation data were interesting but could throw a statistical kink into the risk analysis. "How do you take into account those centers with a 100% intubation rate?" he asked. "Why don’t you analyze the data separately and see if the risk of intubation still holds up? There is always a risk of selection bias unless you compare the [sites] that always intubated against those that did not. Otherwise, you might be including people who were intubated because they looked dead, had heart failure, or weren’t breathing."
Three other speakers echoed the same concern, with one suggesting that intubation could lead to aspiration pneumonia, which in turn could contribute to a bad outcome.
"We don’t know what all the facts are" in relation to intubation, Dr. Rymer said. "We can only speculate."
However, the discussants also emphasized that there are still no randomized data confirming the device’s efficacy when compared with medical therapies. "There is, however, a clear association of improved outcomes with better revascularization," Dr. Rymer said in an interview.
Both MERCI and Multi MERCI were criticized for comparing their device-treated populations with only a placebo arm of PROACT II, according to an editorial by Dr. Kyra J. Becker of the University of Washington and Dr. Thomas G. Brott of the Mayo Clinic in Jacksonville, Fla. (Stroke 2005;36:400-3).
They said that the data presented to the Food and Drug Administration for the device’s approval did not meet the standards that could be achieved in a randomized trial.
"These data certainly provide no reassurance about the safety of mechanical embolectomy, yet alone the efficacy," the authors said. "The risks of treatment with the Concentric clot retriever might not be limited to arterial perforation, dissection, or distal embolization; there could be unidentified risks associated with the required immediate poststroke angiography as well as with endovascular instrumentation and injury to the arterial endothelium. Unfortunately, these questions cannot be addressed with the data presented ... by the MERCI investigators. Randomized trials have a distinct advantage in this setting; they can control for variables we know are likely to relate to procedure success and outcome, and they can control for important variables we have yet to identify."
Concentric Medical, the manufacturer of the MERCI device, sponsored the MERCI registry. Dr. Rymer reported being on the speakers bureau of the company. The five other coauthors reported varying relationships with Concentric, including being consultants or medical advisors, and having an ownership interest in Concentric.
LOS ANGELES - Results of the MERCI patient registry appear to uphold findings from the device’s two pivotal trials of patients with acute ischemic stroke, which demonstrated that successful recanalization is significantly associated with good outcome.
But audience members who spoke at the International Stroke Conference following the presentation of the registry results emphasized – to the applause of others in the audience – that the MERCI device lacks randomized data proving its safety and efficacy, and that reported outcomes for patients have not been stratified according to intubation status.
The registry is a large, nonrandomized case series documenting the postapproval use of the devices, whereas the two pivotal trials (MERCI and Multi MERCI) had strict inclusion and exclusion criteria and protocols but did not compare the device vs. medical therapy. Device-treated patients in the trials were instead compared with a placebo group from a randomized medical trial, called PROACT II (Prolyse in Acute Cerebral Thromboembolism).
At the conference, Dr. Marilyn Rymer presented the 90-day outcomes of 1,000 patients with acute ischemic stroke in the registry who were treated with the MERCI clot retriever embolectomy device. The MERCI (Mechanical Embolus Removal in Cerebral Ischemia) and Multi MERCI studies examined embolectomy with the device in similar patient groups, with a total of 305 patients.
"The registry is designed to answer the question, ‘What does the real-world, unrestrained treatment of ischemic stroke with this device look like?’" said Dr. Rymer, a medical director of the Brain and Stroke Institute at St. Luke’s Hospital in Kansas City, Mo. However, she noted that because the registry consists of nonrandomized cases, the efficacy implied in it "can’t be compared to a medical therapy."
The participating sites included every consecutive patient who was treated with the device. Treatment remained a clinical decision guided by each site’s general practice. The inclusion criteria were a diagnosis of acute ischemic stroke and at least one pass with the tool.
The primary end point was revascularization with a TICI (Thrombolysis in Cerebral Infarction) scale grade of 2a or higher; there was also a secondary functional outcome, which was the modified Rankin Scale (mRS) score at 90 days. The final analysis included 872 patients as a result of excluding 4 with insufficient procedural data and 90 who were disabled before their stroke with an mRS of 2 or more, as well as losing 34 to follow-up.
The patients’ median age was 68 years, compared with the median age of 72 in the trials. There was wide intersite variability with regard to age: At one site, the median age of patients treated was 58, and at another the median age of patients treated was 72.
"We saw the same kind of variation in terms of baseline National Institutes of Health Stroke Scale [NIHSS] score," Dr. Rymer said at the conference, which was sponsored by the American Heart Association. "The median in the registry was 17, while it was 21 in the trials." Median NIHSS scores also varied across the registry sites (range, 14-21), she said.
Overall, 305 patients received intravenous thrombolytic therapy, "But there was an incredible variation among sites" in the use of thrombolytics in conjunction with embolectomy (range, 0%-71%). Intra-arterial thrombolytic therapy also varied widely: Some 47% of patients overall received it, but the intersite rate varied from 7% to 100%.
Overall, 63% of patients were intubated, with the rate varying from 12% to 100%. "Several sites used intubation routinely in 100% of their patients, and some intubated only for airway protection. This becomes important as we begin to understand that intubation is associated with a worse outcome," Dr. Rymer said.
Most sites treated fewer than 19% of patients with angioplasty or stenting in addition to clot retrieval, but one site employed these additional treatments in 64% of patients.
The time from symptom onset to groin insertion was 6.3 hours, compared with 4.5 hours in the MERCI trials. Most patients (71%) were treated 3-8 hours after symptom onset.
Overall in the registry, 80% of patients were successfully recanalized, which was significantly more than in the two MERCI trials combined (65%). Similar numbers of patients had good 90-day outcomes (32% in both the registry and combined trials). Mortality at 90 days also was not significantly different between the registry and the trials (33% and 38%).
Symptomatic brain hemorrhage in the registry was 7% overall, not significantly different from the 8.8% seen in the MERCI trials, Dr. Rymer said. "It is notable that in the patients who were well recanalized (those with a TICI grade of 2b to 3), the symptomatic hemorrhage rate was lower (3.7% in TICI 2b and 5.4% in TICI 3)."
When the investigators examined the rate of good 90-day outcome (defined as an mRS of 0-2), they found the best outcomes in patients with the lowest baseline NIHSS scores. "As the stroke became more severe, the likelihood of good outcome went down," she said. In cases with NIHSS scores lower than 16, "the outcomes were excellent," she said, with up to 70% of those with a TICI grade of 2b or 3 experiencing a good outcome. TICI 2a provided only modest benefit, but it was consistent across the whole range of NIHSS scores, she added.
Age and recanalization status also affected mortality. "Age was a predictor of worse outcome, but recanalization did provide benefit across all ages except for the very young, who had low mortality rates in any case," she said.
A multivariate analysis identified several factors that significantly affected mortality both negatively and positively, including advancing age (odds ratio, 1.05), worse baseline NIHSS score (OR, 1.08), revascularization to a TICI grade of 2a, 2b, or 3 (OR, 0.33), heart failure (OR, 2.85), blood glucose above 140 mg/dL (OR 2.0), and intubation during the revascularization procedure (OR, 2.20)
The same multivariate model also identified factors that negatively impacted good 90-day outcomes, including worse baseline NIHSS score (OR, 0.88), advancing age (OR, 0.96), intubation during the procedure (OR, 0.43), longer duration of procedure (OR, 0.66), and a blood glucose level of 140 mg/dL or greater (OR, 0.59).
During the discussion period, several audience members questioned the relationship between intubation and poor outcomes. Dr. Joseph Broderick, chair of the department of neurology at the University of Cincinnati, said that the intubation data were interesting but could throw a statistical kink into the risk analysis. "How do you take into account those centers with a 100% intubation rate?" he asked. "Why don’t you analyze the data separately and see if the risk of intubation still holds up? There is always a risk of selection bias unless you compare the [sites] that always intubated against those that did not. Otherwise, you might be including people who were intubated because they looked dead, had heart failure, or weren’t breathing."
Three other speakers echoed the same concern, with one suggesting that intubation could lead to aspiration pneumonia, which in turn could contribute to a bad outcome.
"We don’t know what all the facts are" in relation to intubation, Dr. Rymer said. "We can only speculate."
However, the discussants also emphasized that there are still no randomized data confirming the device’s efficacy when compared with medical therapies. "There is, however, a clear association of improved outcomes with better revascularization," Dr. Rymer said in an interview.
Both MERCI and Multi MERCI were criticized for comparing their device-treated populations with only a placebo arm of PROACT II, according to an editorial by Dr. Kyra J. Becker of the University of Washington and Dr. Thomas G. Brott of the Mayo Clinic in Jacksonville, Fla. (Stroke 2005;36:400-3).
They said that the data presented to the Food and Drug Administration for the device’s approval did not meet the standards that could be achieved in a randomized trial.
"These data certainly provide no reassurance about the safety of mechanical embolectomy, yet alone the efficacy," the authors said. "The risks of treatment with the Concentric clot retriever might not be limited to arterial perforation, dissection, or distal embolization; there could be unidentified risks associated with the required immediate poststroke angiography as well as with endovascular instrumentation and injury to the arterial endothelium. Unfortunately, these questions cannot be addressed with the data presented ... by the MERCI investigators. Randomized trials have a distinct advantage in this setting; they can control for variables we know are likely to relate to procedure success and outcome, and they can control for important variables we have yet to identify."
Concentric Medical, the manufacturer of the MERCI device, sponsored the MERCI registry. Dr. Rymer reported being on the speakers bureau of the company. The five other coauthors reported varying relationships with Concentric, including being consultants or medical advisors, and having an ownership interest in Concentric.
LOS ANGELES - Results of the MERCI patient registry appear to uphold findings from the device’s two pivotal trials of patients with acute ischemic stroke, which demonstrated that successful recanalization is significantly associated with good outcome.
But audience members who spoke at the International Stroke Conference following the presentation of the registry results emphasized – to the applause of others in the audience – that the MERCI device lacks randomized data proving its safety and efficacy, and that reported outcomes for patients have not been stratified according to intubation status.
The registry is a large, nonrandomized case series documenting the postapproval use of the devices, whereas the two pivotal trials (MERCI and Multi MERCI) had strict inclusion and exclusion criteria and protocols but did not compare the device vs. medical therapy. Device-treated patients in the trials were instead compared with a placebo group from a randomized medical trial, called PROACT II (Prolyse in Acute Cerebral Thromboembolism).
At the conference, Dr. Marilyn Rymer presented the 90-day outcomes of 1,000 patients with acute ischemic stroke in the registry who were treated with the MERCI clot retriever embolectomy device. The MERCI (Mechanical Embolus Removal in Cerebral Ischemia) and Multi MERCI studies examined embolectomy with the device in similar patient groups, with a total of 305 patients.
"The registry is designed to answer the question, ‘What does the real-world, unrestrained treatment of ischemic stroke with this device look like?’" said Dr. Rymer, a medical director of the Brain and Stroke Institute at St. Luke’s Hospital in Kansas City, Mo. However, she noted that because the registry consists of nonrandomized cases, the efficacy implied in it "can’t be compared to a medical therapy."
The participating sites included every consecutive patient who was treated with the device. Treatment remained a clinical decision guided by each site’s general practice. The inclusion criteria were a diagnosis of acute ischemic stroke and at least one pass with the tool.
The primary end point was revascularization with a TICI (Thrombolysis in Cerebral Infarction) scale grade of 2a or higher; there was also a secondary functional outcome, which was the modified Rankin Scale (mRS) score at 90 days. The final analysis included 872 patients as a result of excluding 4 with insufficient procedural data and 90 who were disabled before their stroke with an mRS of 2 or more, as well as losing 34 to follow-up.
The patients’ median age was 68 years, compared with the median age of 72 in the trials. There was wide intersite variability with regard to age: At one site, the median age of patients treated was 58, and at another the median age of patients treated was 72.
"We saw the same kind of variation in terms of baseline National Institutes of Health Stroke Scale [NIHSS] score," Dr. Rymer said at the conference, which was sponsored by the American Heart Association. "The median in the registry was 17, while it was 21 in the trials." Median NIHSS scores also varied across the registry sites (range, 14-21), she said.
Overall, 305 patients received intravenous thrombolytic therapy, "But there was an incredible variation among sites" in the use of thrombolytics in conjunction with embolectomy (range, 0%-71%). Intra-arterial thrombolytic therapy also varied widely: Some 47% of patients overall received it, but the intersite rate varied from 7% to 100%.
Overall, 63% of patients were intubated, with the rate varying from 12% to 100%. "Several sites used intubation routinely in 100% of their patients, and some intubated only for airway protection. This becomes important as we begin to understand that intubation is associated with a worse outcome," Dr. Rymer said.
Most sites treated fewer than 19% of patients with angioplasty or stenting in addition to clot retrieval, but one site employed these additional treatments in 64% of patients.
The time from symptom onset to groin insertion was 6.3 hours, compared with 4.5 hours in the MERCI trials. Most patients (71%) were treated 3-8 hours after symptom onset.
Overall in the registry, 80% of patients were successfully recanalized, which was significantly more than in the two MERCI trials combined (65%). Similar numbers of patients had good 90-day outcomes (32% in both the registry and combined trials). Mortality at 90 days also was not significantly different between the registry and the trials (33% and 38%).
Symptomatic brain hemorrhage in the registry was 7% overall, not significantly different from the 8.8% seen in the MERCI trials, Dr. Rymer said. "It is notable that in the patients who were well recanalized (those with a TICI grade of 2b to 3), the symptomatic hemorrhage rate was lower (3.7% in TICI 2b and 5.4% in TICI 3)."
When the investigators examined the rate of good 90-day outcome (defined as an mRS of 0-2), they found the best outcomes in patients with the lowest baseline NIHSS scores. "As the stroke became more severe, the likelihood of good outcome went down," she said. In cases with NIHSS scores lower than 16, "the outcomes were excellent," she said, with up to 70% of those with a TICI grade of 2b or 3 experiencing a good outcome. TICI 2a provided only modest benefit, but it was consistent across the whole range of NIHSS scores, she added.
Age and recanalization status also affected mortality. "Age was a predictor of worse outcome, but recanalization did provide benefit across all ages except for the very young, who had low mortality rates in any case," she said.
A multivariate analysis identified several factors that significantly affected mortality both negatively and positively, including advancing age (odds ratio, 1.05), worse baseline NIHSS score (OR, 1.08), revascularization to a TICI grade of 2a, 2b, or 3 (OR, 0.33), heart failure (OR, 2.85), blood glucose above 140 mg/dL (OR 2.0), and intubation during the revascularization procedure (OR, 2.20)
The same multivariate model also identified factors that negatively impacted good 90-day outcomes, including worse baseline NIHSS score (OR, 0.88), advancing age (OR, 0.96), intubation during the procedure (OR, 0.43), longer duration of procedure (OR, 0.66), and a blood glucose level of 140 mg/dL or greater (OR, 0.59).
During the discussion period, several audience members questioned the relationship between intubation and poor outcomes. Dr. Joseph Broderick, chair of the department of neurology at the University of Cincinnati, said that the intubation data were interesting but could throw a statistical kink into the risk analysis. "How do you take into account those centers with a 100% intubation rate?" he asked. "Why don’t you analyze the data separately and see if the risk of intubation still holds up? There is always a risk of selection bias unless you compare the [sites] that always intubated against those that did not. Otherwise, you might be including people who were intubated because they looked dead, had heart failure, or weren’t breathing."
Three other speakers echoed the same concern, with one suggesting that intubation could lead to aspiration pneumonia, which in turn could contribute to a bad outcome.
"We don’t know what all the facts are" in relation to intubation, Dr. Rymer said. "We can only speculate."
However, the discussants also emphasized that there are still no randomized data confirming the device’s efficacy when compared with medical therapies. "There is, however, a clear association of improved outcomes with better revascularization," Dr. Rymer said in an interview.
Both MERCI and Multi MERCI were criticized for comparing their device-treated populations with only a placebo arm of PROACT II, according to an editorial by Dr. Kyra J. Becker of the University of Washington and Dr. Thomas G. Brott of the Mayo Clinic in Jacksonville, Fla. (Stroke 2005;36:400-3).
They said that the data presented to the Food and Drug Administration for the device’s approval did not meet the standards that could be achieved in a randomized trial.
"These data certainly provide no reassurance about the safety of mechanical embolectomy, yet alone the efficacy," the authors said. "The risks of treatment with the Concentric clot retriever might not be limited to arterial perforation, dissection, or distal embolization; there could be unidentified risks associated with the required immediate poststroke angiography as well as with endovascular instrumentation and injury to the arterial endothelium. Unfortunately, these questions cannot be addressed with the data presented ... by the MERCI investigators. Randomized trials have a distinct advantage in this setting; they can control for variables we know are likely to relate to procedure success and outcome, and they can control for important variables we have yet to identify."
Concentric Medical, the manufacturer of the MERCI device, sponsored the MERCI registry. Dr. Rymer reported being on the speakers bureau of the company. The five other coauthors reported varying relationships with Concentric, including being consultants or medical advisors, and having an ownership interest in Concentric.
Major Finding: Use of the MERCI clot retrieval device resulted in successful recanalization in 80% of patients, good 90-day functional outcomes in 32%, and 33% mortality.
Data Source: The MERCI patient registry of 1,000 patients with acute ischemic stroke who were treated consecutively at 37 U.S. centers.
Disclosures: Concentric Medical, the manufacturer of the MERCI device, sponsored the MERCI registry. Dr. Rymer reported being on the speakers bureau of the company. The five other coauthors reported varying relationships with Concentric, including being consultants or medical advisors, and having an ownership interest in Concentric.
Registry Supports MERCI Trials Data, but Methods Criticized
LOS ANGELES - Results of the MERCI patient registry appear to uphold findings from the device’s two pivotal trials of patients with acute ischemic stroke, which demonstrated that successful recanalization is significantly associated with good outcome.
But audience members who spoke at the International Stroke Conference following the presentation of the registry results emphasized – to the applause of others in the audience – that the MERCI device lacks randomized data proving its safety and efficacy, and that reported outcomes for patients have not been stratified according to intubation status.
The registry is a large, nonrandomized case series documenting the postapproval use of the devices, whereas the two pivotal trials (MERCI and Multi MERCI) had strict inclusion and exclusion criteria and protocols but did not compare the device vs. medical therapy. Device-treated patients in the trials were instead compared with a placebo group from a randomized medical trial, called PROACT II (Prolyse in Acute Cerebral Thromboembolism).
At the conference, Dr. Marilyn Rymer presented the 90-day outcomes of 1,000 patients with acute ischemic stroke in the registry who were treated with the MERCI clot retriever embolectomy device. The MERCI (Mechanical Embolus Removal in Cerebral Ischemia) and Multi MERCI studies examined embolectomy with the device in similar patient groups, with a total of 305 patients.
"The registry is designed to answer the question, ‘What does the real-world, unrestrained treatment of ischemic stroke with this device look like?’" said Dr. Rymer, a medical director of the Brain and Stroke Institute at St. Luke’s Hospital in Kansas City, Mo. However, she noted that because the registry consists of nonrandomized cases, the efficacy implied in it "can’t be compared to a medical therapy."
The participating sites included every consecutive patient who was treated with the device. Treatment remained a clinical decision guided by each site’s general practice. The inclusion criteria were a diagnosis of acute ischemic stroke and at least one pass with the tool.
The primary end point was revascularization with a TICI (Thrombolysis in Cerebral Infarction) scale grade of 2a or higher; there was also a secondary functional outcome, which was the modified Rankin Scale (mRS) score at 90 days. The final analysis included 872 patients as a result of excluding 4 with insufficient procedural data and 90 who were disabled before their stroke with an mRS of 2 or more, as well as losing 34 to follow-up.
The patients’ median age was 68 years, compared with the median age of 72 in the trials. There was wide intersite variability with regard to age: At one site, the median age of patients treated was 58, and at another the median age of patients treated was 72.
"We saw the same kind of variation in terms of baseline National Institutes of Health Stroke Scale [NIHSS] score," Dr. Rymer said at the conference, which was sponsored by the American Heart Association. "The median in the registry was 17, while it was 21 in the trials." Median NIHSS scores also varied across the registry sites (range, 14-21), she said.
Overall, 305 patients received intravenous thrombolytic therapy, "But there was an incredible variation among sites" in the use of thrombolytics in conjunction with embolectomy (range, 0%-71%). Intra-arterial thrombolytic therapy also varied widely: Some 47% of patients overall received it, but the intersite rate varied from 7% to 100%.
Overall, 63% of patients were intubated, with the rate varying from 12% to 100%. "Several sites used intubation routinely in 100% of their patients, and some intubated only for airway protection. This becomes important as we begin to understand that intubation is associated with a worse outcome," Dr. Rymer said.
Most sites treated fewer than 19% of patients with angioplasty or stenting in addition to clot retrieval, but one site employed these additional treatments in 64% of patients.
The time from symptom onset to groin insertion was 6.3 hours, compared with 4.5 hours in the MERCI trials. Most patients (71%) were treated 3-8 hours after symptom onset.
Overall in the registry, 80% of patients were successfully recanalized, which was significantly more than in the two MERCI trials combined (65%). Similar numbers of patients had good 90-day outcomes (32% in both the registry and combined trials). Mortality at 90 days also was not significantly different between the registry and the trials (33% and 38%).
Symptomatic brain hemorrhage in the registry was 7% overall, not significantly different from the 8.8% seen in the MERCI trials, Dr. Rymer said. "It is notable that in the patients who were well recanalized (those with a TICI grade of 2b to 3), the symptomatic hemorrhage rate was lower (3.7% in TICI 2b and 5.4% in TICI 3)."
When the investigators examined the rate of good 90-day outcome (defined as an mRS of 0-2), they found the best outcomes in patients with the lowest baseline NIHSS scores. "As the stroke became more severe, the likelihood of good outcome went down," she said. In cases with NIHSS scores lower than 16, "the outcomes were excellent," she said, with up to 70% of those with a TICI grade of 2b or 3 experiencing a good outcome. TICI 2a provided only modest benefit, but it was consistent across the whole range of NIHSS scores, she added.
Age and recanalization status also affected mortality. "Age was a predictor of worse outcome, but recanalization did provide benefit across all ages except for the very young, who had low mortality rates in any case," she said.
A multivariate analysis identified several factors that significantly affected mortality both negatively and positively, including advancing age (odds ratio, 1.05), worse baseline NIHSS score (OR, 1.08), revascularization to a TICI grade of 2a, 2b, or 3 (OR, 0.33), heart failure (OR, 2.85), blood glucose above 140 mg/dL (OR 2.0), and intubation during the revascularization procedure (OR, 2.20)
The same multivariate model also identified factors that negatively impacted good 90-day outcomes, including worse baseline NIHSS score (OR, 0.88), advancing age (OR, 0.96), intubation during the procedure (OR, 0.43), longer duration of procedure (OR, 0.66), and a blood glucose level of 140 mg/dL or greater (OR, 0.59).
During the discussion period, several audience members questioned the relationship between intubation and poor outcomes. Dr. Joseph Broderick, chair of the department of neurology at the University of Cincinnati, said that the intubation data were interesting but could throw a statistical kink into the risk analysis. "How do you take into account those centers with a 100% intubation rate?" he asked. "Why don’t you analyze the data separately and see if the risk of intubation still holds up? There is always a risk of selection bias unless you compare the [sites] that always intubated against those that did not. Otherwise, you might be including people who were intubated because they looked dead, had heart failure, or weren’t breathing."
Three other speakers echoed the same concern, with one suggesting that intubation could lead to aspiration pneumonia, which in turn could contribute to a bad outcome.
"We don’t know what all the facts are" in relation to intubation, Dr. Rymer said. "We can only speculate."
However, the discussants also emphasized that there are still no randomized data confirming the device’s efficacy when compared with medical therapies. "There is, however, a clear association of improved outcomes with better revascularization," Dr. Rymer said in an interview.
Both MERCI and Multi MERCI were criticized for comparing their device-treated populations with only a placebo arm of PROACT II, according to an editorial by Dr. Kyra J. Becker of the University of Washington and Dr. Thomas G. Brott of the Mayo Clinic in Jacksonville, Fla. (Stroke 2005;36:400-3).
They said that the data presented to the Food and Drug Administration for the device’s approval did not meet the standards that could be achieved in a randomized trial.
"These data certainly provide no reassurance about the safety of mechanical embolectomy, yet alone the efficacy," the authors said. "The risks of treatment with the Concentric clot retriever might not be limited to arterial perforation, dissection, or distal embolization; there could be unidentified risks associated with the required immediate poststroke angiography as well as with endovascular instrumentation and injury to the arterial endothelium. Unfortunately, these questions cannot be addressed with the data presented ... by the MERCI investigators. Randomized trials have a distinct advantage in this setting; they can control for variables we know are likely to relate to procedure success and outcome, and they can control for important variables we have yet to identify."
Concentric Medical, the manufacturer of the MERCI device, sponsored the MERCI registry. Dr. Rymer reported being on the speakers bureau of the company. The five other coauthors reported varying relationships with Concentric, including being consultants or medical advisors, and having an ownership interest in Concentric.
LOS ANGELES - Results of the MERCI patient registry appear to uphold findings from the device’s two pivotal trials of patients with acute ischemic stroke, which demonstrated that successful recanalization is significantly associated with good outcome.
But audience members who spoke at the International Stroke Conference following the presentation of the registry results emphasized – to the applause of others in the audience – that the MERCI device lacks randomized data proving its safety and efficacy, and that reported outcomes for patients have not been stratified according to intubation status.
The registry is a large, nonrandomized case series documenting the postapproval use of the devices, whereas the two pivotal trials (MERCI and Multi MERCI) had strict inclusion and exclusion criteria and protocols but did not compare the device vs. medical therapy. Device-treated patients in the trials were instead compared with a placebo group from a randomized medical trial, called PROACT II (Prolyse in Acute Cerebral Thromboembolism).
At the conference, Dr. Marilyn Rymer presented the 90-day outcomes of 1,000 patients with acute ischemic stroke in the registry who were treated with the MERCI clot retriever embolectomy device. The MERCI (Mechanical Embolus Removal in Cerebral Ischemia) and Multi MERCI studies examined embolectomy with the device in similar patient groups, with a total of 305 patients.
"The registry is designed to answer the question, ‘What does the real-world, unrestrained treatment of ischemic stroke with this device look like?’" said Dr. Rymer, a medical director of the Brain and Stroke Institute at St. Luke’s Hospital in Kansas City, Mo. However, she noted that because the registry consists of nonrandomized cases, the efficacy implied in it "can’t be compared to a medical therapy."
The participating sites included every consecutive patient who was treated with the device. Treatment remained a clinical decision guided by each site’s general practice. The inclusion criteria were a diagnosis of acute ischemic stroke and at least one pass with the tool.
The primary end point was revascularization with a TICI (Thrombolysis in Cerebral Infarction) scale grade of 2a or higher; there was also a secondary functional outcome, which was the modified Rankin Scale (mRS) score at 90 days. The final analysis included 872 patients as a result of excluding 4 with insufficient procedural data and 90 who were disabled before their stroke with an mRS of 2 or more, as well as losing 34 to follow-up.
The patients’ median age was 68 years, compared with the median age of 72 in the trials. There was wide intersite variability with regard to age: At one site, the median age of patients treated was 58, and at another the median age of patients treated was 72.
"We saw the same kind of variation in terms of baseline National Institutes of Health Stroke Scale [NIHSS] score," Dr. Rymer said at the conference, which was sponsored by the American Heart Association. "The median in the registry was 17, while it was 21 in the trials." Median NIHSS scores also varied across the registry sites (range, 14-21), she said.
Overall, 305 patients received intravenous thrombolytic therapy, "But there was an incredible variation among sites" in the use of thrombolytics in conjunction with embolectomy (range, 0%-71%). Intra-arterial thrombolytic therapy also varied widely: Some 47% of patients overall received it, but the intersite rate varied from 7% to 100%.
Overall, 63% of patients were intubated, with the rate varying from 12% to 100%. "Several sites used intubation routinely in 100% of their patients, and some intubated only for airway protection. This becomes important as we begin to understand that intubation is associated with a worse outcome," Dr. Rymer said.
Most sites treated fewer than 19% of patients with angioplasty or stenting in addition to clot retrieval, but one site employed these additional treatments in 64% of patients.
The time from symptom onset to groin insertion was 6.3 hours, compared with 4.5 hours in the MERCI trials. Most patients (71%) were treated 3-8 hours after symptom onset.
Overall in the registry, 80% of patients were successfully recanalized, which was significantly more than in the two MERCI trials combined (65%). Similar numbers of patients had good 90-day outcomes (32% in both the registry and combined trials). Mortality at 90 days also was not significantly different between the registry and the trials (33% and 38%).
Symptomatic brain hemorrhage in the registry was 7% overall, not significantly different from the 8.8% seen in the MERCI trials, Dr. Rymer said. "It is notable that in the patients who were well recanalized (those with a TICI grade of 2b to 3), the symptomatic hemorrhage rate was lower (3.7% in TICI 2b and 5.4% in TICI 3)."
When the investigators examined the rate of good 90-day outcome (defined as an mRS of 0-2), they found the best outcomes in patients with the lowest baseline NIHSS scores. "As the stroke became more severe, the likelihood of good outcome went down," she said. In cases with NIHSS scores lower than 16, "the outcomes were excellent," she said, with up to 70% of those with a TICI grade of 2b or 3 experiencing a good outcome. TICI 2a provided only modest benefit, but it was consistent across the whole range of NIHSS scores, she added.
Age and recanalization status also affected mortality. "Age was a predictor of worse outcome, but recanalization did provide benefit across all ages except for the very young, who had low mortality rates in any case," she said.
A multivariate analysis identified several factors that significantly affected mortality both negatively and positively, including advancing age (odds ratio, 1.05), worse baseline NIHSS score (OR, 1.08), revascularization to a TICI grade of 2a, 2b, or 3 (OR, 0.33), heart failure (OR, 2.85), blood glucose above 140 mg/dL (OR 2.0), and intubation during the revascularization procedure (OR, 2.20)
The same multivariate model also identified factors that negatively impacted good 90-day outcomes, including worse baseline NIHSS score (OR, 0.88), advancing age (OR, 0.96), intubation during the procedure (OR, 0.43), longer duration of procedure (OR, 0.66), and a blood glucose level of 140 mg/dL or greater (OR, 0.59).
During the discussion period, several audience members questioned the relationship between intubation and poor outcomes. Dr. Joseph Broderick, chair of the department of neurology at the University of Cincinnati, said that the intubation data were interesting but could throw a statistical kink into the risk analysis. "How do you take into account those centers with a 100% intubation rate?" he asked. "Why don’t you analyze the data separately and see if the risk of intubation still holds up? There is always a risk of selection bias unless you compare the [sites] that always intubated against those that did not. Otherwise, you might be including people who were intubated because they looked dead, had heart failure, or weren’t breathing."
Three other speakers echoed the same concern, with one suggesting that intubation could lead to aspiration pneumonia, which in turn could contribute to a bad outcome.
"We don’t know what all the facts are" in relation to intubation, Dr. Rymer said. "We can only speculate."
However, the discussants also emphasized that there are still no randomized data confirming the device’s efficacy when compared with medical therapies. "There is, however, a clear association of improved outcomes with better revascularization," Dr. Rymer said in an interview.
Both MERCI and Multi MERCI were criticized for comparing their device-treated populations with only a placebo arm of PROACT II, according to an editorial by Dr. Kyra J. Becker of the University of Washington and Dr. Thomas G. Brott of the Mayo Clinic in Jacksonville, Fla. (Stroke 2005;36:400-3).
They said that the data presented to the Food and Drug Administration for the device’s approval did not meet the standards that could be achieved in a randomized trial.
"These data certainly provide no reassurance about the safety of mechanical embolectomy, yet alone the efficacy," the authors said. "The risks of treatment with the Concentric clot retriever might not be limited to arterial perforation, dissection, or distal embolization; there could be unidentified risks associated with the required immediate poststroke angiography as well as with endovascular instrumentation and injury to the arterial endothelium. Unfortunately, these questions cannot be addressed with the data presented ... by the MERCI investigators. Randomized trials have a distinct advantage in this setting; they can control for variables we know are likely to relate to procedure success and outcome, and they can control for important variables we have yet to identify."
Concentric Medical, the manufacturer of the MERCI device, sponsored the MERCI registry. Dr. Rymer reported being on the speakers bureau of the company. The five other coauthors reported varying relationships with Concentric, including being consultants or medical advisors, and having an ownership interest in Concentric.
LOS ANGELES - Results of the MERCI patient registry appear to uphold findings from the device’s two pivotal trials of patients with acute ischemic stroke, which demonstrated that successful recanalization is significantly associated with good outcome.
But audience members who spoke at the International Stroke Conference following the presentation of the registry results emphasized – to the applause of others in the audience – that the MERCI device lacks randomized data proving its safety and efficacy, and that reported outcomes for patients have not been stratified according to intubation status.
The registry is a large, nonrandomized case series documenting the postapproval use of the devices, whereas the two pivotal trials (MERCI and Multi MERCI) had strict inclusion and exclusion criteria and protocols but did not compare the device vs. medical therapy. Device-treated patients in the trials were instead compared with a placebo group from a randomized medical trial, called PROACT II (Prolyse in Acute Cerebral Thromboembolism).
At the conference, Dr. Marilyn Rymer presented the 90-day outcomes of 1,000 patients with acute ischemic stroke in the registry who were treated with the MERCI clot retriever embolectomy device. The MERCI (Mechanical Embolus Removal in Cerebral Ischemia) and Multi MERCI studies examined embolectomy with the device in similar patient groups, with a total of 305 patients.
"The registry is designed to answer the question, ‘What does the real-world, unrestrained treatment of ischemic stroke with this device look like?’" said Dr. Rymer, a medical director of the Brain and Stroke Institute at St. Luke’s Hospital in Kansas City, Mo. However, she noted that because the registry consists of nonrandomized cases, the efficacy implied in it "can’t be compared to a medical therapy."
The participating sites included every consecutive patient who was treated with the device. Treatment remained a clinical decision guided by each site’s general practice. The inclusion criteria were a diagnosis of acute ischemic stroke and at least one pass with the tool.
The primary end point was revascularization with a TICI (Thrombolysis in Cerebral Infarction) scale grade of 2a or higher; there was also a secondary functional outcome, which was the modified Rankin Scale (mRS) score at 90 days. The final analysis included 872 patients as a result of excluding 4 with insufficient procedural data and 90 who were disabled before their stroke with an mRS of 2 or more, as well as losing 34 to follow-up.
The patients’ median age was 68 years, compared with the median age of 72 in the trials. There was wide intersite variability with regard to age: At one site, the median age of patients treated was 58, and at another the median age of patients treated was 72.
"We saw the same kind of variation in terms of baseline National Institutes of Health Stroke Scale [NIHSS] score," Dr. Rymer said at the conference, which was sponsored by the American Heart Association. "The median in the registry was 17, while it was 21 in the trials." Median NIHSS scores also varied across the registry sites (range, 14-21), she said.
Overall, 305 patients received intravenous thrombolytic therapy, "But there was an incredible variation among sites" in the use of thrombolytics in conjunction with embolectomy (range, 0%-71%). Intra-arterial thrombolytic therapy also varied widely: Some 47% of patients overall received it, but the intersite rate varied from 7% to 100%.
Overall, 63% of patients were intubated, with the rate varying from 12% to 100%. "Several sites used intubation routinely in 100% of their patients, and some intubated only for airway protection. This becomes important as we begin to understand that intubation is associated with a worse outcome," Dr. Rymer said.
Most sites treated fewer than 19% of patients with angioplasty or stenting in addition to clot retrieval, but one site employed these additional treatments in 64% of patients.
The time from symptom onset to groin insertion was 6.3 hours, compared with 4.5 hours in the MERCI trials. Most patients (71%) were treated 3-8 hours after symptom onset.
Overall in the registry, 80% of patients were successfully recanalized, which was significantly more than in the two MERCI trials combined (65%). Similar numbers of patients had good 90-day outcomes (32% in both the registry and combined trials). Mortality at 90 days also was not significantly different between the registry and the trials (33% and 38%).
Symptomatic brain hemorrhage in the registry was 7% overall, not significantly different from the 8.8% seen in the MERCI trials, Dr. Rymer said. "It is notable that in the patients who were well recanalized (those with a TICI grade of 2b to 3), the symptomatic hemorrhage rate was lower (3.7% in TICI 2b and 5.4% in TICI 3)."
When the investigators examined the rate of good 90-day outcome (defined as an mRS of 0-2), they found the best outcomes in patients with the lowest baseline NIHSS scores. "As the stroke became more severe, the likelihood of good outcome went down," she said. In cases with NIHSS scores lower than 16, "the outcomes were excellent," she said, with up to 70% of those with a TICI grade of 2b or 3 experiencing a good outcome. TICI 2a provided only modest benefit, but it was consistent across the whole range of NIHSS scores, she added.
Age and recanalization status also affected mortality. "Age was a predictor of worse outcome, but recanalization did provide benefit across all ages except for the very young, who had low mortality rates in any case," she said.
A multivariate analysis identified several factors that significantly affected mortality both negatively and positively, including advancing age (odds ratio, 1.05), worse baseline NIHSS score (OR, 1.08), revascularization to a TICI grade of 2a, 2b, or 3 (OR, 0.33), heart failure (OR, 2.85), blood glucose above 140 mg/dL (OR 2.0), and intubation during the revascularization procedure (OR, 2.20)
The same multivariate model also identified factors that negatively impacted good 90-day outcomes, including worse baseline NIHSS score (OR, 0.88), advancing age (OR, 0.96), intubation during the procedure (OR, 0.43), longer duration of procedure (OR, 0.66), and a blood glucose level of 140 mg/dL or greater (OR, 0.59).
During the discussion period, several audience members questioned the relationship between intubation and poor outcomes. Dr. Joseph Broderick, chair of the department of neurology at the University of Cincinnati, said that the intubation data were interesting but could throw a statistical kink into the risk analysis. "How do you take into account those centers with a 100% intubation rate?" he asked. "Why don’t you analyze the data separately and see if the risk of intubation still holds up? There is always a risk of selection bias unless you compare the [sites] that always intubated against those that did not. Otherwise, you might be including people who were intubated because they looked dead, had heart failure, or weren’t breathing."
Three other speakers echoed the same concern, with one suggesting that intubation could lead to aspiration pneumonia, which in turn could contribute to a bad outcome.
"We don’t know what all the facts are" in relation to intubation, Dr. Rymer said. "We can only speculate."
However, the discussants also emphasized that there are still no randomized data confirming the device’s efficacy when compared with medical therapies. "There is, however, a clear association of improved outcomes with better revascularization," Dr. Rymer said in an interview.
Both MERCI and Multi MERCI were criticized for comparing their device-treated populations with only a placebo arm of PROACT II, according to an editorial by Dr. Kyra J. Becker of the University of Washington and Dr. Thomas G. Brott of the Mayo Clinic in Jacksonville, Fla. (Stroke 2005;36:400-3).
They said that the data presented to the Food and Drug Administration for the device’s approval did not meet the standards that could be achieved in a randomized trial.
"These data certainly provide no reassurance about the safety of mechanical embolectomy, yet alone the efficacy," the authors said. "The risks of treatment with the Concentric clot retriever might not be limited to arterial perforation, dissection, or distal embolization; there could be unidentified risks associated with the required immediate poststroke angiography as well as with endovascular instrumentation and injury to the arterial endothelium. Unfortunately, these questions cannot be addressed with the data presented ... by the MERCI investigators. Randomized trials have a distinct advantage in this setting; they can control for variables we know are likely to relate to procedure success and outcome, and they can control for important variables we have yet to identify."
Concentric Medical, the manufacturer of the MERCI device, sponsored the MERCI registry. Dr. Rymer reported being on the speakers bureau of the company. The five other coauthors reported varying relationships with Concentric, including being consultants or medical advisors, and having an ownership interest in Concentric.
Major Finding: Use of the MERCI clot retrieval device resulted in successful recanalization in 80% of patients, good 90-day functional outcomes in 32%, and 33% mortality.
Data Source: The MERCI patient registry of 1,000 patients with acute ischemic stroke who were treated consecutively at 37 U.S. centers.
Disclosures: Concentric Medical, the manufacturer of the MERCI device, sponsored the MERCI registry. Dr. Rymer reported being on the speakers bureau of the company. The five other coauthors reported varying relationships with Concentric, including being consultants or medical advisors, and having an ownership interest in Concentric.
Referenced EEGs May Guide Depression Treatment Better Than STAR*D Algorithm
Treatment-resistant depression seems to respond better when medication decisions are made by referenced electroencephalogram than by the STAR*D algorithm, according to a 12-week randomized trial.
Among 114 patients randomized to the two treatment methods, those whose medication was guided by referenced-EEG (rEEG) improved significantly more than did those treated by Sequenced Treatment Alternatives to Relieve Depression (STAR*D) guidelines in both primary measures of depression, as well as in most of the secondary measurements, Dr. Charles DeBattista and his colleagues wrote in the January issue of Journal of Psychiatric Research (doi:10.1016/j.jpsychires.2010.05.009).
"These results warrant additional studies to determine the role of rEEG-guided psychopharmacology in the treatment of depression, and perhaps in other psychiatric disorders," wrote Dr. DeBattista of Stanford (Calif.) University and his coauthors. "If these results were confirmed, rEEG-guided pharmacotherapy would represent an easy, relatively inexpensive, predictive, objective office procedure that builds upon clinical judgment to guide antidepressant medication choice."
The rEEG relies on a large database built over an 18-year period. It now contains information on more than 1,800 patients who were followed for an average of 405 days. The database includes the patients’ unmedicated EEGs, along with outcomes of more than 17,000 medical trials. The result, according to Dr. DeBattista and his colleagues, is the availability of 74 EEG biomarkers that "provide a very large collection of outcomes data allowing calculation of statistical correlation between the biomarkers found in a patient’s EEG and the treatment response predictions to many medications, based on patients who had similar EEG biomarkers."
The STAR*D algorithm study (pdf), published in 2006, is a four-step treatment algorithm; most patients start at level 1 with citalopram. If they do not enter remission within 14 weeks, they either switch drugs or add another medication; this pattern can continue for four levels, ending with tranylcypromine or mirtazapine plus extended-release venlafaxine.
The trial by Dr. DeBattista and his colleagues randomized 114 patients with treatment-resistant depression. After undergoing a drug washout period consisting of five consecutive half-life days, patients in the active group were randomized to a treatment regimen based on rEEG, constructed after review of their baseline EEG.
Control patients who had failed only selective serotonin reuptake inhibitors received extended-release venlafaxine; those who had failed two or more classes of antidepressants received a medication regimen from steps 2-4 of STAR*D.
The primary end points were changes in the Quick Inventory of Depressive Symptomatology (pdf) (QIDS-SR16) and the Quality of Life Enjoyment and Satisfaction Questionnaire Short Form (pdf) (Q-LES-Q SF).
Secondary end points were changes in the Clinical Global Impression severity scale (pdf) (CGI-severity), the Clinical Global Impression improvement scale (pdf) (CGI-improvement), the Montgomery-Asberg Depression Rating Scale (MADRS), and the Patient Health Questionnaire-9 (PHQ-9).
The patients’ mean age was 44 years; most (63%) were women and white (66%). Overall, they had failed a mean of four previous central nervous system medications.
After 12 weeks, those in the rEEG group had improved significantly more than the control patients on the QIDS-16 (–7 vs. –4.5) and the Q-LES-Q-SF (18 vs. 9).
The rEEG group also improved significantly more in most of the secondary end points, including the CGI-improvement scores, the CGI-severity scores, and the final number of responders as measured by the MADRS system. However, the authors pointed out, the mean MADRS change from baseline to the study’s end was not significantly greater in the rEEG group than in the control groups.
The PHQ-9 end point also showed significant benefit in favor of rEEG, but in its remission measurement, rEEG was not significantly different from the control group (47% vs. 37%).
In a treatment curve, the authors noted that the groups began to separate as early as 2 weeks after the initiation of therapy, with the rEEG group continuing to improve more than the control group each week.
Treatment-related adverse events occurred in 45% of the rEEG group and 48% of the control groups – not significantly different. The most common in both groups were nausea/vomiting, fatigue, headache, anxiety, and insomnia. None of the adverse events or their rates was different from what could have been expected given the drugs employed, the authors noted.
Even in the absence of final remission superiority, the benefit of rEEG-guided treatment over STAR*D-guided treatment was clear and clinically relevant, the investigators said. "For example, the magnitude of improvement on the QIDS-SR16 response rate was 65% for rEEG compared with only 39% for the controls. Likewise the secondary measures on the Q-LES-Q-SF were also quite consistent in supporting the efficacy of the rEEG-guided therapy."
In addition, the investigators said, the early and continuous separation of treatment curves suggested that "rEEG’s superiority in reducing patients’ depressive symptoms might have continued had the trial continued."
The study was sponsored by CNS Response Inc., the company that owns the rEEG platform. Dr. DeBattista said he has received research support from the company, as have 4 of the other 14 investigators. Four other investigators are company employees and own stock in CNS Response.
Treatment-resistant depression seems to respond better when medication decisions are made by referenced electroencephalogram than by the STAR*D algorithm, according to a 12-week randomized trial.
Among 114 patients randomized to the two treatment methods, those whose medication was guided by referenced-EEG (rEEG) improved significantly more than did those treated by Sequenced Treatment Alternatives to Relieve Depression (STAR*D) guidelines in both primary measures of depression, as well as in most of the secondary measurements, Dr. Charles DeBattista and his colleagues wrote in the January issue of Journal of Psychiatric Research (doi:10.1016/j.jpsychires.2010.05.009).
"These results warrant additional studies to determine the role of rEEG-guided psychopharmacology in the treatment of depression, and perhaps in other psychiatric disorders," wrote Dr. DeBattista of Stanford (Calif.) University and his coauthors. "If these results were confirmed, rEEG-guided pharmacotherapy would represent an easy, relatively inexpensive, predictive, objective office procedure that builds upon clinical judgment to guide antidepressant medication choice."
The rEEG relies on a large database built over an 18-year period. It now contains information on more than 1,800 patients who were followed for an average of 405 days. The database includes the patients’ unmedicated EEGs, along with outcomes of more than 17,000 medical trials. The result, according to Dr. DeBattista and his colleagues, is the availability of 74 EEG biomarkers that "provide a very large collection of outcomes data allowing calculation of statistical correlation between the biomarkers found in a patient’s EEG and the treatment response predictions to many medications, based on patients who had similar EEG biomarkers."
The STAR*D algorithm study (pdf), published in 2006, is a four-step treatment algorithm; most patients start at level 1 with citalopram. If they do not enter remission within 14 weeks, they either switch drugs or add another medication; this pattern can continue for four levels, ending with tranylcypromine or mirtazapine plus extended-release venlafaxine.
The trial by Dr. DeBattista and his colleagues randomized 114 patients with treatment-resistant depression. After undergoing a drug washout period consisting of five consecutive half-life days, patients in the active group were randomized to a treatment regimen based on rEEG, constructed after review of their baseline EEG.
Control patients who had failed only selective serotonin reuptake inhibitors received extended-release venlafaxine; those who had failed two or more classes of antidepressants received a medication regimen from steps 2-4 of STAR*D.
The primary end points were changes in the Quick Inventory of Depressive Symptomatology (pdf) (QIDS-SR16) and the Quality of Life Enjoyment and Satisfaction Questionnaire Short Form (pdf) (Q-LES-Q SF).
Secondary end points were changes in the Clinical Global Impression severity scale (pdf) (CGI-severity), the Clinical Global Impression improvement scale (pdf) (CGI-improvement), the Montgomery-Asberg Depression Rating Scale (MADRS), and the Patient Health Questionnaire-9 (PHQ-9).
The patients’ mean age was 44 years; most (63%) were women and white (66%). Overall, they had failed a mean of four previous central nervous system medications.
After 12 weeks, those in the rEEG group had improved significantly more than the control patients on the QIDS-16 (–7 vs. –4.5) and the Q-LES-Q-SF (18 vs. 9).
The rEEG group also improved significantly more in most of the secondary end points, including the CGI-improvement scores, the CGI-severity scores, and the final number of responders as measured by the MADRS system. However, the authors pointed out, the mean MADRS change from baseline to the study’s end was not significantly greater in the rEEG group than in the control groups.
The PHQ-9 end point also showed significant benefit in favor of rEEG, but in its remission measurement, rEEG was not significantly different from the control group (47% vs. 37%).
In a treatment curve, the authors noted that the groups began to separate as early as 2 weeks after the initiation of therapy, with the rEEG group continuing to improve more than the control group each week.
Treatment-related adverse events occurred in 45% of the rEEG group and 48% of the control groups – not significantly different. The most common in both groups were nausea/vomiting, fatigue, headache, anxiety, and insomnia. None of the adverse events or their rates was different from what could have been expected given the drugs employed, the authors noted.
Even in the absence of final remission superiority, the benefit of rEEG-guided treatment over STAR*D-guided treatment was clear and clinically relevant, the investigators said. "For example, the magnitude of improvement on the QIDS-SR16 response rate was 65% for rEEG compared with only 39% for the controls. Likewise the secondary measures on the Q-LES-Q-SF were also quite consistent in supporting the efficacy of the rEEG-guided therapy."
In addition, the investigators said, the early and continuous separation of treatment curves suggested that "rEEG’s superiority in reducing patients’ depressive symptoms might have continued had the trial continued."
The study was sponsored by CNS Response Inc., the company that owns the rEEG platform. Dr. DeBattista said he has received research support from the company, as have 4 of the other 14 investigators. Four other investigators are company employees and own stock in CNS Response.
Treatment-resistant depression seems to respond better when medication decisions are made by referenced electroencephalogram than by the STAR*D algorithm, according to a 12-week randomized trial.
Among 114 patients randomized to the two treatment methods, those whose medication was guided by referenced-EEG (rEEG) improved significantly more than did those treated by Sequenced Treatment Alternatives to Relieve Depression (STAR*D) guidelines in both primary measures of depression, as well as in most of the secondary measurements, Dr. Charles DeBattista and his colleagues wrote in the January issue of Journal of Psychiatric Research (doi:10.1016/j.jpsychires.2010.05.009).
"These results warrant additional studies to determine the role of rEEG-guided psychopharmacology in the treatment of depression, and perhaps in other psychiatric disorders," wrote Dr. DeBattista of Stanford (Calif.) University and his coauthors. "If these results were confirmed, rEEG-guided pharmacotherapy would represent an easy, relatively inexpensive, predictive, objective office procedure that builds upon clinical judgment to guide antidepressant medication choice."
The rEEG relies on a large database built over an 18-year period. It now contains information on more than 1,800 patients who were followed for an average of 405 days. The database includes the patients’ unmedicated EEGs, along with outcomes of more than 17,000 medical trials. The result, according to Dr. DeBattista and his colleagues, is the availability of 74 EEG biomarkers that "provide a very large collection of outcomes data allowing calculation of statistical correlation between the biomarkers found in a patient’s EEG and the treatment response predictions to many medications, based on patients who had similar EEG biomarkers."
The STAR*D algorithm study (pdf), published in 2006, is a four-step treatment algorithm; most patients start at level 1 with citalopram. If they do not enter remission within 14 weeks, they either switch drugs or add another medication; this pattern can continue for four levels, ending with tranylcypromine or mirtazapine plus extended-release venlafaxine.
The trial by Dr. DeBattista and his colleagues randomized 114 patients with treatment-resistant depression. After undergoing a drug washout period consisting of five consecutive half-life days, patients in the active group were randomized to a treatment regimen based on rEEG, constructed after review of their baseline EEG.
Control patients who had failed only selective serotonin reuptake inhibitors received extended-release venlafaxine; those who had failed two or more classes of antidepressants received a medication regimen from steps 2-4 of STAR*D.
The primary end points were changes in the Quick Inventory of Depressive Symptomatology (pdf) (QIDS-SR16) and the Quality of Life Enjoyment and Satisfaction Questionnaire Short Form (pdf) (Q-LES-Q SF).
Secondary end points were changes in the Clinical Global Impression severity scale (pdf) (CGI-severity), the Clinical Global Impression improvement scale (pdf) (CGI-improvement), the Montgomery-Asberg Depression Rating Scale (MADRS), and the Patient Health Questionnaire-9 (PHQ-9).
The patients’ mean age was 44 years; most (63%) were women and white (66%). Overall, they had failed a mean of four previous central nervous system medications.
After 12 weeks, those in the rEEG group had improved significantly more than the control patients on the QIDS-16 (–7 vs. –4.5) and the Q-LES-Q-SF (18 vs. 9).
The rEEG group also improved significantly more in most of the secondary end points, including the CGI-improvement scores, the CGI-severity scores, and the final number of responders as measured by the MADRS system. However, the authors pointed out, the mean MADRS change from baseline to the study’s end was not significantly greater in the rEEG group than in the control groups.
The PHQ-9 end point also showed significant benefit in favor of rEEG, but in its remission measurement, rEEG was not significantly different from the control group (47% vs. 37%).
In a treatment curve, the authors noted that the groups began to separate as early as 2 weeks after the initiation of therapy, with the rEEG group continuing to improve more than the control group each week.
Treatment-related adverse events occurred in 45% of the rEEG group and 48% of the control groups – not significantly different. The most common in both groups were nausea/vomiting, fatigue, headache, anxiety, and insomnia. None of the adverse events or their rates was different from what could have been expected given the drugs employed, the authors noted.
Even in the absence of final remission superiority, the benefit of rEEG-guided treatment over STAR*D-guided treatment was clear and clinically relevant, the investigators said. "For example, the magnitude of improvement on the QIDS-SR16 response rate was 65% for rEEG compared with only 39% for the controls. Likewise the secondary measures on the Q-LES-Q-SF were also quite consistent in supporting the efficacy of the rEEG-guided therapy."
In addition, the investigators said, the early and continuous separation of treatment curves suggested that "rEEG’s superiority in reducing patients’ depressive symptoms might have continued had the trial continued."
The study was sponsored by CNS Response Inc., the company that owns the rEEG platform. Dr. DeBattista said he has received research support from the company, as have 4 of the other 14 investigators. Four other investigators are company employees and own stock in CNS Response.
FROM JOURNAL OF PSYCHIATRIC RESEARCH
Major Finding: Treatment-resistant depression improved significantly more in 114 patients randomized to medications guided by referenced EEG than to STAR*D criteria.
Data Source: A randomized, parallel group study of patients with treatment-resistant depression.
Disclosures: The study was sponsored by CNS Response Inc., the company that owns the rEEG platform. Dr. DeBattista said he has received research support from the company, as have 4 of the other 14 investigators. Four other investigators are company employees and own stock in CNS Response.
Referenced EEGs May Guide Depression Treatment Better Than STAR*D Algorithm
Treatment-resistant depression seems to respond better when medication decisions are made by referenced electroencephalogram than by the STAR*D algorithm, according to a 12-week randomized trial.
Among 114 patients randomized to the two treatment methods, those whose medication was guided by referenced-EEG (rEEG) improved significantly more than did those treated by Sequenced Treatment Alternatives to Relieve Depression (STAR*D) guidelines in both primary measures of depression, as well as in most of the secondary measurements, Dr. Charles DeBattista and his colleagues wrote in the January issue of Journal of Psychiatric Research (doi:10.1016/j.jpsychires.2010.05.009).
"These results warrant additional studies to determine the role of rEEG-guided psychopharmacology in the treatment of depression, and perhaps in other psychiatric disorders," wrote Dr. DeBattista of Stanford (Calif.) University and his coauthors. "If these results were confirmed, rEEG-guided pharmacotherapy would represent an easy, relatively inexpensive, predictive, objective office procedure that builds upon clinical judgment to guide antidepressant medication choice."
The rEEG relies on a large database built over an 18-year period. It now contains information on more than 1,800 patients who were followed for an average of 405 days. The database includes the patients’ unmedicated EEGs, along with outcomes of more than 17,000 medical trials. The result, according to Dr. DeBattista and his colleagues, is the availability of 74 EEG biomarkers that "provide a very large collection of outcomes data allowing calculation of statistical correlation between the biomarkers found in a patient’s EEG and the treatment response predictions to many medications, based on patients who had similar EEG biomarkers."
The STAR*D algorithm study (pdf), published in 2006, is a four-step treatment algorithm; most patients start at level 1 with citalopram. If they do not enter remission within 14 weeks, they either switch drugs or add another medication; this pattern can continue for four levels, ending with tranylcypromine or mirtazapine plus extended-release venlafaxine.
The trial by Dr. DeBattista and his colleagues randomized 114 patients with treatment-resistant depression. After undergoing a drug washout period consisting of five consecutive half-life days, patients in the active group were randomized to a treatment regimen based on rEEG, constructed after review of their baseline EEG.
Control patients who had failed only selective serotonin reuptake inhibitors received extended-release venlafaxine; those who had failed two or more classes of antidepressants received a medication regimen from steps 2-4 of STAR*D.
The primary end points were changes in the Quick Inventory of Depressive Symptomatology (pdf) (QIDS-SR16) and the Quality of Life Enjoyment and Satisfaction Questionnaire Short Form (pdf) (Q-LES-Q SF).
Secondary end points were changes in the Clinical Global Impression severity scale (pdf) (CGI-severity), the Clinical Global Impression improvement scale (pdf) (CGI-improvement), the Montgomery-Asberg Depression Rating Scale (MADRS), and the Patient Health Questionnaire-9 (PHQ-9).
The patients’ mean age was 44 years; most (63%) were women and white (66%). Overall, they had failed a mean of four previous central nervous system medications.
After 12 weeks, those in the rEEG group had improved significantly more than the control patients on the QIDS-16 (–7 vs. –4.5) and the Q-LES-Q-SF (18 vs. 9).
The rEEG group also improved significantly more in most of the secondary end points, including the CGI-improvement scores, the CGI-severity scores, and the final number of responders as measured by the MADRS system. However, the authors pointed out, the mean MADRS change from baseline to the study’s end was not significantly greater in the rEEG group than in the control groups.
The PHQ-9 end point also showed significant benefit in favor of rEEG, but in its remission measurement, rEEG was not significantly different from the control group (47% vs. 37%).
In a treatment curve, the authors noted that the groups began to separate as early as 2 weeks after the initiation of therapy, with the rEEG group continuing to improve more than the control group each week.
Treatment-related adverse events occurred in 45% of the rEEG group and 48% of the control groups – not significantly different. The most common in both groups were nausea/vomiting, fatigue, headache, anxiety, and insomnia. None of the adverse events or their rates was different from what could have been expected given the drugs employed, the authors noted.
Even in the absence of final remission superiority, the benefit of rEEG-guided treatment over STAR*D-guided treatment was clear and clinically relevant, the investigators said. "For example, the magnitude of improvement on the QIDS-SR16 response rate was 65% for rEEG compared with only 39% for the controls. Likewise the secondary measures on the Q-LES-Q-SF were also quite consistent in supporting the efficacy of the rEEG-guided therapy."
In addition, the investigators said, the early and continuous separation of treatment curves suggested that "rEEG’s superiority in reducing patients’ depressive symptoms might have continued had the trial continued."
The study was sponsored by CNS Response Inc., the company that owns the rEEG platform. Dr. DeBattista said he has received research support from the company, as have 4 of the other 14 investigators. Four other investigators are company employees and own stock in CNS Response.
Treatment-resistant depression seems to respond better when medication decisions are made by referenced electroencephalogram than by the STAR*D algorithm, according to a 12-week randomized trial.
Among 114 patients randomized to the two treatment methods, those whose medication was guided by referenced-EEG (rEEG) improved significantly more than did those treated by Sequenced Treatment Alternatives to Relieve Depression (STAR*D) guidelines in both primary measures of depression, as well as in most of the secondary measurements, Dr. Charles DeBattista and his colleagues wrote in the January issue of Journal of Psychiatric Research (doi:10.1016/j.jpsychires.2010.05.009).
"These results warrant additional studies to determine the role of rEEG-guided psychopharmacology in the treatment of depression, and perhaps in other psychiatric disorders," wrote Dr. DeBattista of Stanford (Calif.) University and his coauthors. "If these results were confirmed, rEEG-guided pharmacotherapy would represent an easy, relatively inexpensive, predictive, objective office procedure that builds upon clinical judgment to guide antidepressant medication choice."
The rEEG relies on a large database built over an 18-year period. It now contains information on more than 1,800 patients who were followed for an average of 405 days. The database includes the patients’ unmedicated EEGs, along with outcomes of more than 17,000 medical trials. The result, according to Dr. DeBattista and his colleagues, is the availability of 74 EEG biomarkers that "provide a very large collection of outcomes data allowing calculation of statistical correlation between the biomarkers found in a patient’s EEG and the treatment response predictions to many medications, based on patients who had similar EEG biomarkers."
The STAR*D algorithm study (pdf), published in 2006, is a four-step treatment algorithm; most patients start at level 1 with citalopram. If they do not enter remission within 14 weeks, they either switch drugs or add another medication; this pattern can continue for four levels, ending with tranylcypromine or mirtazapine plus extended-release venlafaxine.
The trial by Dr. DeBattista and his colleagues randomized 114 patients with treatment-resistant depression. After undergoing a drug washout period consisting of five consecutive half-life days, patients in the active group were randomized to a treatment regimen based on rEEG, constructed after review of their baseline EEG.
Control patients who had failed only selective serotonin reuptake inhibitors received extended-release venlafaxine; those who had failed two or more classes of antidepressants received a medication regimen from steps 2-4 of STAR*D.
The primary end points were changes in the Quick Inventory of Depressive Symptomatology (pdf) (QIDS-SR16) and the Quality of Life Enjoyment and Satisfaction Questionnaire Short Form (pdf) (Q-LES-Q SF).
Secondary end points were changes in the Clinical Global Impression severity scale (pdf) (CGI-severity), the Clinical Global Impression improvement scale (pdf) (CGI-improvement), the Montgomery-Asberg Depression Rating Scale (MADRS), and the Patient Health Questionnaire-9 (PHQ-9).
The patients’ mean age was 44 years; most (63%) were women and white (66%). Overall, they had failed a mean of four previous central nervous system medications.
After 12 weeks, those in the rEEG group had improved significantly more than the control patients on the QIDS-16 (–7 vs. –4.5) and the Q-LES-Q-SF (18 vs. 9).
The rEEG group also improved significantly more in most of the secondary end points, including the CGI-improvement scores, the CGI-severity scores, and the final number of responders as measured by the MADRS system. However, the authors pointed out, the mean MADRS change from baseline to the study’s end was not significantly greater in the rEEG group than in the control groups.
The PHQ-9 end point also showed significant benefit in favor of rEEG, but in its remission measurement, rEEG was not significantly different from the control group (47% vs. 37%).
In a treatment curve, the authors noted that the groups began to separate as early as 2 weeks after the initiation of therapy, with the rEEG group continuing to improve more than the control group each week.
Treatment-related adverse events occurred in 45% of the rEEG group and 48% of the control groups – not significantly different. The most common in both groups were nausea/vomiting, fatigue, headache, anxiety, and insomnia. None of the adverse events or their rates was different from what could have been expected given the drugs employed, the authors noted.
Even in the absence of final remission superiority, the benefit of rEEG-guided treatment over STAR*D-guided treatment was clear and clinically relevant, the investigators said. "For example, the magnitude of improvement on the QIDS-SR16 response rate was 65% for rEEG compared with only 39% for the controls. Likewise the secondary measures on the Q-LES-Q-SF were also quite consistent in supporting the efficacy of the rEEG-guided therapy."
In addition, the investigators said, the early and continuous separation of treatment curves suggested that "rEEG’s superiority in reducing patients’ depressive symptoms might have continued had the trial continued."
The study was sponsored by CNS Response Inc., the company that owns the rEEG platform. Dr. DeBattista said he has received research support from the company, as have 4 of the other 14 investigators. Four other investigators are company employees and own stock in CNS Response.
Treatment-resistant depression seems to respond better when medication decisions are made by referenced electroencephalogram than by the STAR*D algorithm, according to a 12-week randomized trial.
Among 114 patients randomized to the two treatment methods, those whose medication was guided by referenced-EEG (rEEG) improved significantly more than did those treated by Sequenced Treatment Alternatives to Relieve Depression (STAR*D) guidelines in both primary measures of depression, as well as in most of the secondary measurements, Dr. Charles DeBattista and his colleagues wrote in the January issue of Journal of Psychiatric Research (doi:10.1016/j.jpsychires.2010.05.009).
"These results warrant additional studies to determine the role of rEEG-guided psychopharmacology in the treatment of depression, and perhaps in other psychiatric disorders," wrote Dr. DeBattista of Stanford (Calif.) University and his coauthors. "If these results were confirmed, rEEG-guided pharmacotherapy would represent an easy, relatively inexpensive, predictive, objective office procedure that builds upon clinical judgment to guide antidepressant medication choice."
The rEEG relies on a large database built over an 18-year period. It now contains information on more than 1,800 patients who were followed for an average of 405 days. The database includes the patients’ unmedicated EEGs, along with outcomes of more than 17,000 medical trials. The result, according to Dr. DeBattista and his colleagues, is the availability of 74 EEG biomarkers that "provide a very large collection of outcomes data allowing calculation of statistical correlation between the biomarkers found in a patient’s EEG and the treatment response predictions to many medications, based on patients who had similar EEG biomarkers."
The STAR*D algorithm study (pdf), published in 2006, is a four-step treatment algorithm; most patients start at level 1 with citalopram. If they do not enter remission within 14 weeks, they either switch drugs or add another medication; this pattern can continue for four levels, ending with tranylcypromine or mirtazapine plus extended-release venlafaxine.
The trial by Dr. DeBattista and his colleagues randomized 114 patients with treatment-resistant depression. After undergoing a drug washout period consisting of five consecutive half-life days, patients in the active group were randomized to a treatment regimen based on rEEG, constructed after review of their baseline EEG.
Control patients who had failed only selective serotonin reuptake inhibitors received extended-release venlafaxine; those who had failed two or more classes of antidepressants received a medication regimen from steps 2-4 of STAR*D.
The primary end points were changes in the Quick Inventory of Depressive Symptomatology (pdf) (QIDS-SR16) and the Quality of Life Enjoyment and Satisfaction Questionnaire Short Form (pdf) (Q-LES-Q SF).
Secondary end points were changes in the Clinical Global Impression severity scale (pdf) (CGI-severity), the Clinical Global Impression improvement scale (pdf) (CGI-improvement), the Montgomery-Asberg Depression Rating Scale (MADRS), and the Patient Health Questionnaire-9 (PHQ-9).
The patients’ mean age was 44 years; most (63%) were women and white (66%). Overall, they had failed a mean of four previous central nervous system medications.
After 12 weeks, those in the rEEG group had improved significantly more than the control patients on the QIDS-16 (–7 vs. –4.5) and the Q-LES-Q-SF (18 vs. 9).
The rEEG group also improved significantly more in most of the secondary end points, including the CGI-improvement scores, the CGI-severity scores, and the final number of responders as measured by the MADRS system. However, the authors pointed out, the mean MADRS change from baseline to the study’s end was not significantly greater in the rEEG group than in the control groups.
The PHQ-9 end point also showed significant benefit in favor of rEEG, but in its remission measurement, rEEG was not significantly different from the control group (47% vs. 37%).
In a treatment curve, the authors noted that the groups began to separate as early as 2 weeks after the initiation of therapy, with the rEEG group continuing to improve more than the control group each week.
Treatment-related adverse events occurred in 45% of the rEEG group and 48% of the control groups – not significantly different. The most common in both groups were nausea/vomiting, fatigue, headache, anxiety, and insomnia. None of the adverse events or their rates was different from what could have been expected given the drugs employed, the authors noted.
Even in the absence of final remission superiority, the benefit of rEEG-guided treatment over STAR*D-guided treatment was clear and clinically relevant, the investigators said. "For example, the magnitude of improvement on the QIDS-SR16 response rate was 65% for rEEG compared with only 39% for the controls. Likewise the secondary measures on the Q-LES-Q-SF were also quite consistent in supporting the efficacy of the rEEG-guided therapy."
In addition, the investigators said, the early and continuous separation of treatment curves suggested that "rEEG’s superiority in reducing patients’ depressive symptoms might have continued had the trial continued."
The study was sponsored by CNS Response Inc., the company that owns the rEEG platform. Dr. DeBattista said he has received research support from the company, as have 4 of the other 14 investigators. Four other investigators are company employees and own stock in CNS Response.
FROM JOURNAL OF PSYCHIATRIC RESEARCH
Major Finding: Treatment-resistant depression improved significantly more in 114 patients randomized to medications guided by referenced EEG than to STAR*D criteria.
Data Source: A randomized, parallel group study of patients with treatment-resistant depression.
Disclosures: The study was sponsored by CNS Response Inc., the company that owns the rEEG platform. Dr. DeBattista said he has received research support from the company, as have 4 of the other 14 investigators. Four other investigators are company employees and own stock in CNS Response.