User login
An easy approach to evaluating peripheral neuropathy
- Pain described by patients as electric shocks, burning, freezing, tightness, or throbbing suggests toxic, metabolic, or ischemic causes of neuropathy (C).
- When motor and sensory symptoms appear together, rank them in order of symptom predominance. Motor symptom supremacy may indicate an immune-related disorder (C).
- When measuring sensory thresholds, keep in mind that they normally increase with the patient’s age and height. Vibration sensation in the toes of elderly persons is often said to be decreased, when in fact it is only an age-related change (C).
Polyneuropathy has an estimated prevalence of about 2% in the general population.1,2 Despite being common, polyneuropathy remains a diagnostic challenge for most clinicians for many reasons, including the large number of potential causes and the fact that a specific cause often cannot be identified even after appropriate testing. These factors can contribute to uncertainty about the direction and level of aggressiveness of the evaluation. The result is often a “one size fits all” strategy—from an unnecessarily expensive “shotgun” approach to a “defeatist” attitude that too quickly deems a neuropathy as idiopathic.
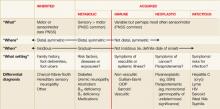
A number of experts have developed algorithms for the evaluation of neuropathy.1,3-8 We have incorporated and modified many of these suggestions to develop a simple, user-friendly approach; one that characterizes the neuropathy using “what?”, “where?”, “when?” and “what setting?” parameters (FIGURE).
The focus of this article is on the polyneuropathy that presents with widespread sensory or sensorimotor symptoms and signs. We will not discuss the evaluation of other peripheral neuropathic processes—eg, mononeuropathies, plexopathies, or motor neuronopathies such as amyotrophic lateral sclerosis. We emphasize the fundamental step of determining whether the polyneuropathy is a distal, symmetrical, sensory or sensorimotor polyneuropathy, in which case metabolic/toxic, inherited and idiopathic causes are more probable. In contrast, alternative presentations suggest immune-mediated and infectious causes.
What: Sensory, motor, or autonomic?
The first step is to assign the patient’s complaints and your examination findings to specific nerve fiber types of the peripheral nervous system: sensory, motor, or autonomic.5
FIGURE
Characterizing your patient’s neuropathy
Sensory findings help narrow your search
Once you have ruled out a nonneuropathic process (eg, arthritis) as the cause of sensory symptoms and signs, turn your attention to distinguishing between peripheral or central nervous system dysfunction. When the pathology resides in the central nervous system (eg, multiple sclerosis) accompanying symptoms and signs usually assist in localization. If a patient acknowledges clinical features suggesting past or present involvement of cerebellar, urinary, or visual (eg, optic neuritis) systems, for example, magnetic resonance imaging rather than electrodiagnostic testing may be warranted.
In a patient with peripheral nervous system dysfunction, sensory abnormalities help exclude neuromuscular diseases not associated with sensory dysfunction, such as myopathies, neuromuscular transmission disorders, or disease of the motor neuron.
Positive neuropathic sensory symptoms suggest acquired polyneuropathies. Patients may describe “prickling,” “tingling,” “swelling,” “asleep-like numbness,” or a sensation of “bunched-up socks.” Patients with acquired polyneuropathies usually complain of positive neuropathic sensory symptoms (PNSS), whereas patients with inherited polyneuropathies only rarely do (FIGURE).
Pain suggests toxic, metabolic, or ischemic causes. Patients may describe “electric shocks,” “burning,” “freezing,” “tightness,” or “throbbing.” They may complain of discomfort or pain to sensory stimuli that under normal circumstances would not be painful (allodynia)—eg, discomfort evoked by a bed sheet resting on the feet. They may also describe exaggerated pain to stimuli that would normally evoke low levels of discomfort or pain (hyperalgesia).
A painful neuropathy narrows the differential diagnosis to diseases that affect smaller nerve fibers, which generally convey pain and temperature input. Causes may be toxic, metabolic, ischemic, or idiopathic. For example, a pure “small-fiber” polyneuropathy commonly occurs in patients aged >60 years and typically causes painful feet. It is often idiopathic, but diabetes or impaired glucose tolerance and alcohol toxicity should be explored.9 Patients with painful neuropathy usually also exhibit reduced or absent sensation of pinprick and temperature in the distribution of their sensory complaints.
Negative neuropathic sensory symptoms. In addition to positive neuropathic symptoms, patients may complain of “negative” symptoms such as loss of sensation and imbalance. Examination usually reveals abnormalities of proprioception and sensation to vibration with reduced or absent deep tendon reflexes and ataxia. These features can occur in acquired or inherited causes of neuropathy.
Important sensory tests. Test sensation on the toes and fingertips, and more proximally (eg, ankle and shin) if any abnormality is found at these distal sites. Test vibration with a 128 Hz tuning fork, pinprick with disposable safety pin, and light touch with a cotton swab.
You may test temperature sensation by warming or cooling the handle or prong of the tuning fork and applying it to the patient’s skin.
Joint position testing is performed by asking the patient to avert his eyes, then moving the distal phalanx of a finger or toe up or down by small increments and asking the patient to tell you the direction of movement. Assess a patient’s casual and tandem gait for unsteadiness or ataxia.
Motor symptoms: Weigh them against sensory findings
Most patients with neuropathy have some degree of weakness, but it is usually overshadowed by sensory complaints. Distal lower extremity weakness may manifest as “foot drop,” which, if it affects ankle dorsiflexion, may cause a “slapping” or noisy step due to the forefoot hitting the ground with abnormal force.
Distal upper extremity weakness may cause trouble with fine motor skills of the hands.
Proximal weakness may present as difficulty in rising from a chair or lifting objects above the shoulders.
There may be muscle atrophy or fasciculations.
Motor symptoms are seldom the sole complaint. When motor and sensory symptoms are combined, it is helpful to rank them in order of symptom predominance—ie, motor greater than sensory, or vice versa. For instance, many immune-mediated disorders, such as Guillain-Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), produce chiefly motor abnormalities and fewer sensory symptoms. Sensory complaints predominate in many other polyneuropathies, especially the “length-dependent” polyneuropathies (ie, those affecting the longer nerves initially) caused by metabolic or toxic disorders.
Autonomic symptom evaluation
The number of processes that affect both autonomic and somatic nerves are relatively few (TABLE 1).5,7,10 It is particularly important to assess symptoms suggesting involvement of the autonomic nervous system.
Autonomic symptoms include lightheadedness, syncope, diarrhea, constipation, postprandial bloating, early satiety, urinary complaints, erectile dysfunction, abnormal or absent sweating, and dry mouth and eyes. Many of these complaints are common in the general population, so their relevance should be based on severity and temporal evolution, as well as comorbidities and medication use.
When complaints do not clearly implicate pathology in the autonomic nervous system, autonomic testing may be helpful, targeted for the domain that may be impaired. For example, bedside orthostatics or tilt-table testing are used for pre-syncopal symptoms, but gastric emptying testing can assist the evaluation of complaints of early satiety or postprandial bloating.
TABLE 1
Relatively common acquired polyneuropathies with autonomic nervous system involvement
| Diabetes mellitus |
| Amyloidosis |
| Guillain-Barré syndrome |
| Paraneoplastic neuropathy (usually small cell lung cancer) |
| Sjögren’s syndrome–associated neuropathy |
Where: The distribution of nerve involvement
“Where” refers to distribution of nerve involvement 1) globally throughout the body and 2) locally along the nerve(s). During the history taking and examination, determine the nature of general distribution (eg, symmetric or asymmetric) and where along the length of the nerve(s) (proximal and/or distal) the dysfunction exists.
Polyneuropathy most commonly presents in a “length-dependent” distribution, with clinical features appearing initially most distally and symmetrically (ie, in the feet). Asymmetry and involvement of the proximal parts of a nerve are “red flags” for an uncommon cause that may require referral to a neurologist (FIGURE).
Comparative vs absolute measurements. At bedside, 2 approaches are used to assess the distribution of nerve involvement: comparative and absolute. The comparative approach searches for a relative difference in sensory thresholds or weakness between sites. It can assess side-to-side or one nerve (or root or region) territory to another. It is useful for establishing sensory or motor impairment in a radicular, plexus, or single nerve distribution.
Testing for an absolute reduction in sensation (eg, decreased vibration in the toes) can be more challenging because it requires experience in judging what is normal and abnormal according to expectations for a particular site and modality. Take into account that sensory thresholds are normally increased with the patient’s age and height.11 For example, we commonly encounter elderly patients whose vibration sensation in the toes is said to be decreased, when in fact the reduced sensory threshold is only an age-related change.
Most assessments of sensory thresholds use the absolute approach because most generalized polyneuropathies are “length-dependent.”
Perform motor testing for appendicular (upper and lower extremities) and axial (neck and trunk) muscles, assessing particularly for weakness, atrophy, and fasciculations.
The typical polyneuropathy caused by metabolic, toxic, inherited, or unknown causes is distal and symmetric.12 Neuropathies caused by other mechanisms, such as immune-mediated or infectious, are rarely length-dependent. Examples include motor neuronopathies (eg, amyotrophic lateral sclerosis), sensory neuronopathies (eg, paraneoplastic), polyradiculoneuropathies (Guillain-Barré syndrome, CIDP), and mononeuritis multiplex (caused by vasculitis).
When: The time course of signs and symptoms
Knowing whether the onset of neuropathy was definite and abrupt or was gradual is the most helpful temporal clue to possible underlying causes. The time course (ie, tempo) following onset is also important.
An acute/subacute onset with a definite date often suggests an immune-mediated or an infectious process (FIGURE). With respect to immune-mediated neuropathies, consider primarily autoimmune conditions (eg, Guillain-Barré syndrome, vasculitic neuropathy) and also paraneoplastic autoimmune syndromes (eg, subacute sensory neuropathy). In the latter case, a cancer presents to the immune system an epitope that is similarly found in the nervous system, prompting an autoimmune attack of the nervous system. Both autoimmune and infectious processes almost always have a rapid start on a definite date.
With an insidious onset, the patient won’t recollect a definite date on which the neuropathy began. The underlying mechanism usually is an inherited, metabolic, or toxic process—or idiopathic, if a cause cannot be identified.
The disease course sheds additional light on the causative mechanism. Onset and subsequent progression often correlate in a predictable manner, owing in part to the underlying mechanism. For example, an acute onset neuropathy often is followed by rapid disease progression, especially when caused by an autoimmune process (Guillain-Barré and vasculitic neuropathy). On the other hand, a neuropathy of insidious onset usually follows a slow or even static course. But there are exceptions that may make the diagnosis challenging and illustrate the need for clinical follow-up.
What setting
The patient’s medical history, medications, social and family history, and a review of systems can uncover known risk factors for neuropathic processes.
Common causes of acquired polyneuropathies are diabetes mellitus, chronic renal disease, and alcohol dependence. If a patient with distal, symmetric sensory complaints also has any of these conditions, a causal relationship should be considered. Another example is the patient with a history of bariatric surgery, which could lead to neuropathy as a result of malnutrition, particularly vitamin B1 and B12 deficiencies.13
The clinical setting may indicate a need for further evaluation by a neurologist. For example, a family history of inherited neuropathy in a patient with high arches and curled “hammer” toes would strongly suggest Charcot-Marie-Tooth (CMT) disease, also known as hereditary motor and sensory neuropathy. A patient with a monoclonal gammopathy may have a paraproteinemic neuropathy that warrants further evaluation by a neuromuscular specialist. Although more rare, a paraneoplastic cause would warrant consideration in a smoker, especially if the neuropathy was subacute. In many cases, neuropathic symptoms are the first clue of a new medical condition (eg, impaired glucose tolerance).
Electrodiagnostic testing: What it can and can’t tell you
Most polyneuropathies warrant additional electrodiagnostic evaluation in an electromyography (EMG) laboratory. Electro-diagnostic testing comprises 2 procedures: nerve conduction studies and needle electrode examination.
Preparing your patient. When ordering this study, be sure to discuss it thoroughly with the patient. The entire study typically takes an hour or longer. And it can be painful, though in our experience nearly every patient tolerates the procedure. Most important for the patient to understand is that information gleaned from electrodiagnostic testing may be essential to the diagnosis, as explained below.
Many benefits of the study. First, electrodiagnostic testing can confirm a peripheral neuropathic basis for a patient’s complaints.
Second, electrodiagnostic testing helps characterize a neuropathy as primarily demyelinating, primarily axonal, or mixed demyelinating and axonal. An example of this benefit is that a primary demyelinating characterization greatly narrows the list of possible causes (eg, Guillain-Barré syndrome, chronic inflammatory demyelinating polyradiculoneuropathy, Charcot-Marie-Tooth disease type 1).
Third, electrodiagnostic testing assists in characterizing the neuropathic process as sensory, motor or sensorimotor.
Fourth, electrodiagnostic testing helps localize the neuropathic process (the “where”).
Fifth, electrodiagnostic testing can gauge the severity of the neuropathic process.
Limitations of the study. When the study returns normal results, keep in mind it has limited sensitivity. For example, nerve conduction studies are only able to assess the function of larger myelinated nerve fibers; a neuropathic process solely in small fibers will not be evident with this test. Likewise, the needle electrode examination is unable to assess small nerve fiber status. We include this caveat in our electrodiagnostic testing reports of patients who have symptoms suggestive of pure small fiber polyneuropathy.
When might blood studies be useful?
Laboratory testing of blood is often of great value, but only after a particular polyneuropathy has been characterized and placed into one or more potential etiologic subgroups.
For example, laboratory testing for a distal, symmetric sensory polyneuropathy (TABLE 2) should be much different than testing for another presentation (eg, mononeuritis multiplex). TABLE 3 details some of the laboratory tests we recommend for the more common polyneuropathies that don’t typically present in a distal, symmetric sensory fashion or that are accompanied by other distinctive features. In our experience, clinicians too frequently order unnecessary and expensive tests for disorders only rarely associated with neuropathy; the rare causes of neuropathy are intentionally not the subject of this review.
TABLE 2
Common blood tests for the evaluation of a distal, symmetrical neuropathy1,3,5,6,8
| TEST | POTENTIAL CONFIRMATORY VALUE OF TESTS |
|---|---|
| Fasting glucose or 2-hour oral glucose tolerance test | Diabetes mellitus and possibly impaired glucose tolerance (“pre-diabetes”) cause neuropathy |
| Serum protein electrophoresis | Paraproteinemias, including monoclonal gammopathy of undetermined significance, Waldenstrom’s macroglobulinemia, and osteosclerotic myeloma, are often associated with a demyelinating neuropathy. Amyloidosis and mixed cryoglobulinemiacan also cause an axonal neuropathy |
| Chemistry profile | Uremic neuropathy |
| Hepatitis C titer | Hepatitis C is associated with neuropathy, particularly when associated with mixed cryoglobulinemia. This neuropathy usually presents asymmetrically and often as mononeuritis multiplex but sometimes as a distal, symmetrical neuropathy |
| Serum B12 level | Vitamin B12 deficiency may cause neuropathy, often in association with symptoms and signs of myelopathy |
TABLE 3
Other diagnostic tests for acquired neuropathies in specific clinical situations2,4,5,7
| SUSPECTED PATHOLOGIC PROCESSES AND PERTINENT TESTS | POTENTIAL CONFIRMATORY VALUE OF TESTS |
|---|---|
| Metabolic/toxic | |
| Complete blood count | Elevated MCV may suggest alcoholism, vitamin B12 deficiency |
| Thiamine level | Thiamine deficiency (eg, alcoholics or following bariatric surgery) |
| Urine heavy metals | Heavy metal intoxication (rare; usually systemic symptoms too) |
| Thyroid function tests | Hypothyroid neuropathy (rare) |
| Inflammatory | |
| Complete blood count | If systemic vasculitic neuropathy suspected (eg, patient presents with painful “mononeuritis multiplex”) systemic vasculitic neuropathy |
| Markers of vasculitis or systemic inflammation (ESR, ANCA, RF, ANA, ENA, cryoglobulins, etc) | Cryoglobulinemic neuropathy is associated with hepatitis C |
| Cerebrospinal fluid (CSF) | CSF protein elevation in Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy |
| Neoplastic/paraneoplastic | |
| Paraneoplastic serology (technique and scope of testing varies between different labs) | Neuropathy associated with cancer, especially subacute and severe neuropathic processes in smokers (eg, subacute sensory neuronopathy associated with small cell lung cancer) |
| Chest X-ray and other imaging for cancer | Small cell lung cancer or other malignancy |
| Cerebrospinal cytology | Carcinomatous or lymphomatous polyradiculopathy. |
| Infectious | |
| Cerebrospinal fluid | CSF pleocytosis common in infectious polyradiculoneuropathies (Lyme, sarcoid, HIV) |
| Lyme titers (serum, cerebrospinal fluid) | Lyme neuroborreliosis |
| HIV testing | HIV-associated neuropathy |
| Hepatitis C (serum cryoglobulin testing) | Hepatitis C—mixed cryoglobulinemia |
Common causes of distal, symmetric polyneuropathies
Distal, symmetric polyneuropathies are usually due to metabolic/toxic, inherited, or idiopathic causes (FIGURE, TABLE 2). As such, it is often unnecessary to obtain diagnostic tests searching for active infectious, autoimmune or paraneoplastic etiologies.3 In electrodiagnostic terms, these neuropathies are almost always primarily axonal rather than demyelinating, usually involving both large and small nerve fibers.
Diabetes is the most common cause of neuropathy in developed Western countries, occurring in more than 50% of patients who require insulin.14 Diabetic neuropathy typically presents as a distal, symmetric neuropathy syndrome, though diabetic lumbosacral radiculoplexus neuropathy (diabetic amyotrophy) and other presentations may be seen less commonly.
Recent evidence suggests that neuropathy—particularly a sensory and often painful, distal, symmetric “small-fiber” neuropathy—sometimes occurs in patients with impaired fasting glucose (IFG) or impaired glucose tolerance (IGT).15-19 The 2-hour oral glucose tolerance test has been deemed more sensitive for the early diabetic state of IGT/IFG associated with neuropathy.19 It remains to be seen whether this early diabetic state neuropathy will turn out to represent a large percentage of cases of what were previously thought of as idiopathic small-fiber neuropathy.
Alcoholism is another common cause of a distal, symmetric neuropathy that is predominantly sensory with a painful, burning sensation.20-22 Alcohol abuse and dependence occurs in 10% to 20% of the primary care population.23,24 The prevalence of neuropathy in alcoholics is uncertain, though 1 study of hospitalized patients admitting to daily alcohol intake of over 100 g for men or 80 g for women (10 oz of beer, 1 oz liquor and 3–4 oz wine each have 10 g of alcohol) for 2 years or more (mean of 238±120 g for a period of 22.7±10.2 years) demonstrated that one third of patients had electrophysiologic evidence of peripheral neuropathy and one fourth of autonomic neuropathy.20 Most subjects in this cohort did not show any clinical or laboratory evidence of malnutrition.
Frequently alcoholic neuropathy coexists with thiamine-deficient neuropathy,22 warranting assessment of thiamine status in all alcoholics with neuropathy. Pain is a prominent complaint in alcoholic neuropathy but a less common complaint in thiamine-deficient neuropathy.22 Cobalamin (vitamin B12)-deficient neuropathy is more likely to occur suddenly and to involve the hands or hands and feet simultaneously, and is less likely to be painful.25 Myelopathy is frequent in cobalamin deficiency, sometimes serving as a clue to diagnosis. It may be difficult to determine whether sensory symptoms are caused by myelopathy or neuropathy. Electro-diagnostic testing, somatosensory evoked potentials, and radiological investigation may be helpful.
Uremic neuropathy may occur in patients with chronic renal failure on dialysis.26,27 Today it is less common by virtue of the widespread implementation of dialysis and renal transplantation. Generally reserve this diagnosis for patients with end-stage renal failure with a creatinine clearance of less than 10 mL/min. Other systemic disorders associated with renal failure must be considered; these include diabetes mellitus, amyloidosis, and vasculitis. Also consider drug-induced neuropathy in this population.
Pharmaceutical agents, industrial and environmental agents, and substances of abuse may cause neuropathy, most commonly in the form of a length-dependent axonal neuropathy. Offending chemotherapeutic agents include colchicine, pyridoxine, and amiodarone. However, toxic polyneuropathies probably represent a rather small proportion of cases.
The list of potentially offending substances is long and includes many medications and agents that the general population is commonly exposed to in low doses. Consider the inherent risk of neuropathy of the particular agent in question. To establish a causal link, verify exposure, determine that symptoms are temporally related to the toxin, and rule out other causes of neuropathy. Furthermore, some clinical improvement or at least stabilization should occur following removal of the offending agent, though this may take months to years.28
Medication-induced neuropathy is more common than industrial and environmental neuropathies. The neuropathy of heavy metal intoxication is rare, and is usually accompanied by a combination of gastrointestinal, hematologic, and central nervous system problems.
HIV neuropathy is predominantly sensory and length-dependent. It occurs in about one third of infected patients. This is an exception to the tenet that infectious neuropathies typically present in a non– length-dependent pattern. However, in almost all circumstances, the diagnosis of HIV is well established and so it usually doesn’t present a diagnostic challenge. It is not our routine practice to check for HIV in those presenting with a new-onset distal, symmetric neuropathy, though we will in the proper clinical setting. Whether HIV neuropathy should be included in the differential diagnosis depends on the incidence of HIV in your patient population and the patient’s specifics.6 Rarely, Guillain-Barré syndrome and CIDP can be the presentation of a recent HIV infection.
For inherited neuropathies, there are an overwhelming number of commercially available panels for DNA testing.3,29-31 A detailed discussion of inherited neuropathies is beyond the scope of this review, and many excellent reviews have been written on the subject. We will, however, make a few general comments.
First, Charcot-Marie-Tooth (CMT) disease is the most common inherited neuromuscular disorder, and it is encountered in primary care.
Second, foot deformities (eg, high arches, curled toes) commonly accompany an inherited polyneuropathy and are valuable clues.
Third, the inheritence pattern should be sought through a detailed family history and examination of other family members (including query and examination for foot deformities). Inherited neuropathies can be autosomal dominant, autosomal recessive, or X-linked, based largely on what gene harbors the mutation. Furthermore, spontaneous “de novo” mutations are not uncommon, being responsible for probably 25% of cases of CMT type 1.
Fourth, inherited neuropathies should be characterized just like acquired neuropathies, with particular attention paid to whether the neuropathy is demyelinating (eg, CMT type 1) or axonal (CMT type 2).
With respect to DNA testing of blood, focused genetic testing is almost always possible once you further characterize the inherited neuropathy and also take into account prevalence estimates of the various inherited neuropathies (eg, CMT type 1A caused by a duplication of the PMP-22 gene is responsible for the majority of cases on CMT), obviating the need for expensive, comprehensive genetic testing.
Other presentations
The evaluation of neuropathies that are not distal and symmetric can be complex and in many cases may warrant referral to a neurologist for further evaluation. With such presentations, diagnostic considerations include chest imaging and antineuronal (paraneoplastic) antibody serology for smokers with subacute neuropathies, cerebrospinal fluid analysis for an acquired demyelinating neuropathy, infectious neuropathy or a polyradiculopathy (eg, Lyme disease),3,7 and sensory nerve biopsy (eg, sural nerve) when vasculitis, amyloidosis, or sarcoidosis is suspected.
CORRESPONDENCE
Ted M. Burns, MD, University of Virginia Health System Department of Neurology, Box 800394, Charlottesville, VA 22908. E-mail: [email protected]
1. Hughes RAC. Peripheral neuropathy. BMJ 2002;324:466-469.
2. England JD, Asbury AK. Peripheral neuropathy. Lancet 2004;363:2151-2161.
3. Pourmand R. Evaluating patients with suspected peripheral neuropathy: Do the right thing, not everything. Muscle Nerve 2002;26:288-290.
4. Dyck PJ, Dyck PJB, Grant IA, Fealey RD. Ten steps in characterizing and diagnosing patients with peripheral neuropathy. Neurology 1996;47:10-17.
5. Barohn RJ. Approach to peripheral neuropathy and neuronopathy. Seminars Neurol 1998;18:7-18.
6. Rosenberg NR, Portegies P, de Visser M, Vermeulen M. Diagnostic investigation of patients with chronic polyneuropathy: evaluation of a clinical guideline. J Neurol Neurosurg Psych 2001;71:205-209.
7. Bromberg MB, Smith AG. Toward an efficient method to evaluate peripheral neuropathies. J Clin Neuromusc Dis 2002;3:172-182.
8. Hughes RAC, Umapathi T, Gray IA, et al. A controlled investigation of the cause of chronic idiopathic axonal polyneuropathy. Brain 2004;127:1723-1730.
9. Lacomis D. Small-fiber neuropathy. Muscle Nerve 2002;26:173-188.
10. Low PA, Vernino S, Suarez G. Autonomic dysfunction in peripheral nerve disease. Muscle Nerve 2003;27:646-661.
11. Burns TM, Taly A, O’Brien PC, Dyck PJ. Clinical versus quantitative vibration assessment: improving clinical performance. J Periph Nerv System 2002;7:112-117.
12. England JD, Gronseth GS, Franklin G, et al. Distal symmetric polyneuropathy: A definition for clinical research: Report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005;64:199-207.
13. Thaisetthawatkul P, Collazo-Clavell ML, Sarr MG, Norell JE, Dyck PJ. A controlled study of peripheral neuropathy after bariatric surgery. Neurology 2004;63:1462-1470.
14. Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973. Diabetes Care 1978;1:168-188.
15. Singleton JR, Smith AG, Russell JW, Feldman EL. Microvascular complications of impaired glucose tolerance. Diabetes 2003;52:2867-2873.
16. Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003;60:108-111.
17. Novella SP, Inzucchi SE, Goldstein JM. The frequency of undiagnosed diabetes and impaired glucose tolerance in patients with idiopathic sensory neuropathy. Muscle Nerve 2001;24:1229-1231.
18. Singleton JR, Smith AG, Bromberg MB. Painful sensory polyneuropathy associated with impaired glucose tolerance. Muscle Nerve 2001;24:1225-1228.
19. Polydefkis M, Griffin JW, McArthur J. New insights into diabetic polyneuropathy. JAMA 2003;290:1371-1376.
20. Monforte R, Estruch R, Valls-Sole J, Nicolas J, Villalta J, Urbano-Marquez A. Autonomic and peripheral neuropathies in patients with chronic alcoholism. A dose-related toxic effect of alcohol. Arch Neurol 1995;52:45-51.
21. Koike H, Mori K, Misu K, et al. Painful alcoholic polyneuropathy with predominant small-fiber loss and normal thiamine status. Neurol 2001;56:1727-1732.
22. Koike H, LiJima M, Sugiura M, et al. Alcoholic neuropathy is clinicopathologically distinct from thiamine-deficiency neuropathy. Ann Neurol 2003;54:19-29.
23. Coulehan JL, Zettler-Segal M, Block M, McClelland M, Schulberg HC. Recognition of alcoholism and substance abuse in primary care patients. Arch Intern Med 1987;147:349-352.
24. Brienza RS, Stein MD. Alcohol use disorders in primary care. Do gender-specific differences exist? J Gen Intern Med 2002;17:387-397.
25. Saperstein DS, Wolfe GI, Gronseth GS, et al. Challenges in the identification of cobalamin-deficiency polyneuropathy. Arch Neurol 2003;60:1296-1301.
26. Laaksonen S, Metsärinne K, Voipio-Pulkki LM, Falck B. Neurophysiologic parameters and symptoms in chronic renal failure. Muscle Nerve 2002;25:884-890.
27. Said G, Boudier L, Selva J, Zingraff J, Drueke T. Different patterns of uremic polyneuropathy: clinicopathologic study. Neurology 1983;33:567-574.
28. Hill AB. The environment and disease: association or causation? Proc Roy Soc Med 1965;58:295-300.
29. Donaghy M. Genes for peripheral neuropathy and their relevance to clinical practice. J Neurol Neurosurg Psychiatry 2004;75:1371-1372.
30. Lewis RA, Sumner AJ, Shy ME. Electrophysiological features of inherited demyelinating neuropathies: a reappraisal in the era of molecular diagnosis. Muscle Nerve 2000;23:1472-1487.
31. Pareyson D. Charcot-Marie-Tooth disease and related neuropathies: molecular basis and distinction and diagnosis. Muscle Nerve 1999;22:1498-1509.
32. Scherer SS. Finding the causes of inherited neuropathies. Arch Neurol 2006;63:812-816.
- Pain described by patients as electric shocks, burning, freezing, tightness, or throbbing suggests toxic, metabolic, or ischemic causes of neuropathy (C).
- When motor and sensory symptoms appear together, rank them in order of symptom predominance. Motor symptom supremacy may indicate an immune-related disorder (C).
- When measuring sensory thresholds, keep in mind that they normally increase with the patient’s age and height. Vibration sensation in the toes of elderly persons is often said to be decreased, when in fact it is only an age-related change (C).
Polyneuropathy has an estimated prevalence of about 2% in the general population.1,2 Despite being common, polyneuropathy remains a diagnostic challenge for most clinicians for many reasons, including the large number of potential causes and the fact that a specific cause often cannot be identified even after appropriate testing. These factors can contribute to uncertainty about the direction and level of aggressiveness of the evaluation. The result is often a “one size fits all” strategy—from an unnecessarily expensive “shotgun” approach to a “defeatist” attitude that too quickly deems a neuropathy as idiopathic.
A number of experts have developed algorithms for the evaluation of neuropathy.1,3-8 We have incorporated and modified many of these suggestions to develop a simple, user-friendly approach; one that characterizes the neuropathy using “what?”, “where?”, “when?” and “what setting?” parameters (FIGURE).
The focus of this article is on the polyneuropathy that presents with widespread sensory or sensorimotor symptoms and signs. We will not discuss the evaluation of other peripheral neuropathic processes—eg, mononeuropathies, plexopathies, or motor neuronopathies such as amyotrophic lateral sclerosis. We emphasize the fundamental step of determining whether the polyneuropathy is a distal, symmetrical, sensory or sensorimotor polyneuropathy, in which case metabolic/toxic, inherited and idiopathic causes are more probable. In contrast, alternative presentations suggest immune-mediated and infectious causes.
What: Sensory, motor, or autonomic?
The first step is to assign the patient’s complaints and your examination findings to specific nerve fiber types of the peripheral nervous system: sensory, motor, or autonomic.5
FIGURE
Characterizing your patient’s neuropathy
Sensory findings help narrow your search
Once you have ruled out a nonneuropathic process (eg, arthritis) as the cause of sensory symptoms and signs, turn your attention to distinguishing between peripheral or central nervous system dysfunction. When the pathology resides in the central nervous system (eg, multiple sclerosis) accompanying symptoms and signs usually assist in localization. If a patient acknowledges clinical features suggesting past or present involvement of cerebellar, urinary, or visual (eg, optic neuritis) systems, for example, magnetic resonance imaging rather than electrodiagnostic testing may be warranted.
In a patient with peripheral nervous system dysfunction, sensory abnormalities help exclude neuromuscular diseases not associated with sensory dysfunction, such as myopathies, neuromuscular transmission disorders, or disease of the motor neuron.
Positive neuropathic sensory symptoms suggest acquired polyneuropathies. Patients may describe “prickling,” “tingling,” “swelling,” “asleep-like numbness,” or a sensation of “bunched-up socks.” Patients with acquired polyneuropathies usually complain of positive neuropathic sensory symptoms (PNSS), whereas patients with inherited polyneuropathies only rarely do (FIGURE).
Pain suggests toxic, metabolic, or ischemic causes. Patients may describe “electric shocks,” “burning,” “freezing,” “tightness,” or “throbbing.” They may complain of discomfort or pain to sensory stimuli that under normal circumstances would not be painful (allodynia)—eg, discomfort evoked by a bed sheet resting on the feet. They may also describe exaggerated pain to stimuli that would normally evoke low levels of discomfort or pain (hyperalgesia).
A painful neuropathy narrows the differential diagnosis to diseases that affect smaller nerve fibers, which generally convey pain and temperature input. Causes may be toxic, metabolic, ischemic, or idiopathic. For example, a pure “small-fiber” polyneuropathy commonly occurs in patients aged >60 years and typically causes painful feet. It is often idiopathic, but diabetes or impaired glucose tolerance and alcohol toxicity should be explored.9 Patients with painful neuropathy usually also exhibit reduced or absent sensation of pinprick and temperature in the distribution of their sensory complaints.
Negative neuropathic sensory symptoms. In addition to positive neuropathic symptoms, patients may complain of “negative” symptoms such as loss of sensation and imbalance. Examination usually reveals abnormalities of proprioception and sensation to vibration with reduced or absent deep tendon reflexes and ataxia. These features can occur in acquired or inherited causes of neuropathy.
Important sensory tests. Test sensation on the toes and fingertips, and more proximally (eg, ankle and shin) if any abnormality is found at these distal sites. Test vibration with a 128 Hz tuning fork, pinprick with disposable safety pin, and light touch with a cotton swab.
You may test temperature sensation by warming or cooling the handle or prong of the tuning fork and applying it to the patient’s skin.
Joint position testing is performed by asking the patient to avert his eyes, then moving the distal phalanx of a finger or toe up or down by small increments and asking the patient to tell you the direction of movement. Assess a patient’s casual and tandem gait for unsteadiness or ataxia.
Motor symptoms: Weigh them against sensory findings
Most patients with neuropathy have some degree of weakness, but it is usually overshadowed by sensory complaints. Distal lower extremity weakness may manifest as “foot drop,” which, if it affects ankle dorsiflexion, may cause a “slapping” or noisy step due to the forefoot hitting the ground with abnormal force.
Distal upper extremity weakness may cause trouble with fine motor skills of the hands.
Proximal weakness may present as difficulty in rising from a chair or lifting objects above the shoulders.
There may be muscle atrophy or fasciculations.
Motor symptoms are seldom the sole complaint. When motor and sensory symptoms are combined, it is helpful to rank them in order of symptom predominance—ie, motor greater than sensory, or vice versa. For instance, many immune-mediated disorders, such as Guillain-Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), produce chiefly motor abnormalities and fewer sensory symptoms. Sensory complaints predominate in many other polyneuropathies, especially the “length-dependent” polyneuropathies (ie, those affecting the longer nerves initially) caused by metabolic or toxic disorders.
Autonomic symptom evaluation
The number of processes that affect both autonomic and somatic nerves are relatively few (TABLE 1).5,7,10 It is particularly important to assess symptoms suggesting involvement of the autonomic nervous system.
Autonomic symptoms include lightheadedness, syncope, diarrhea, constipation, postprandial bloating, early satiety, urinary complaints, erectile dysfunction, abnormal or absent sweating, and dry mouth and eyes. Many of these complaints are common in the general population, so their relevance should be based on severity and temporal evolution, as well as comorbidities and medication use.
When complaints do not clearly implicate pathology in the autonomic nervous system, autonomic testing may be helpful, targeted for the domain that may be impaired. For example, bedside orthostatics or tilt-table testing are used for pre-syncopal symptoms, but gastric emptying testing can assist the evaluation of complaints of early satiety or postprandial bloating.
TABLE 1
Relatively common acquired polyneuropathies with autonomic nervous system involvement
| Diabetes mellitus |
| Amyloidosis |
| Guillain-Barré syndrome |
| Paraneoplastic neuropathy (usually small cell lung cancer) |
| Sjögren’s syndrome–associated neuropathy |
Where: The distribution of nerve involvement
“Where” refers to distribution of nerve involvement 1) globally throughout the body and 2) locally along the nerve(s). During the history taking and examination, determine the nature of general distribution (eg, symmetric or asymmetric) and where along the length of the nerve(s) (proximal and/or distal) the dysfunction exists.
Polyneuropathy most commonly presents in a “length-dependent” distribution, with clinical features appearing initially most distally and symmetrically (ie, in the feet). Asymmetry and involvement of the proximal parts of a nerve are “red flags” for an uncommon cause that may require referral to a neurologist (FIGURE).
Comparative vs absolute measurements. At bedside, 2 approaches are used to assess the distribution of nerve involvement: comparative and absolute. The comparative approach searches for a relative difference in sensory thresholds or weakness between sites. It can assess side-to-side or one nerve (or root or region) territory to another. It is useful for establishing sensory or motor impairment in a radicular, plexus, or single nerve distribution.
Testing for an absolute reduction in sensation (eg, decreased vibration in the toes) can be more challenging because it requires experience in judging what is normal and abnormal according to expectations for a particular site and modality. Take into account that sensory thresholds are normally increased with the patient’s age and height.11 For example, we commonly encounter elderly patients whose vibration sensation in the toes is said to be decreased, when in fact the reduced sensory threshold is only an age-related change.
Most assessments of sensory thresholds use the absolute approach because most generalized polyneuropathies are “length-dependent.”
Perform motor testing for appendicular (upper and lower extremities) and axial (neck and trunk) muscles, assessing particularly for weakness, atrophy, and fasciculations.
The typical polyneuropathy caused by metabolic, toxic, inherited, or unknown causes is distal and symmetric.12 Neuropathies caused by other mechanisms, such as immune-mediated or infectious, are rarely length-dependent. Examples include motor neuronopathies (eg, amyotrophic lateral sclerosis), sensory neuronopathies (eg, paraneoplastic), polyradiculoneuropathies (Guillain-Barré syndrome, CIDP), and mononeuritis multiplex (caused by vasculitis).
When: The time course of signs and symptoms
Knowing whether the onset of neuropathy was definite and abrupt or was gradual is the most helpful temporal clue to possible underlying causes. The time course (ie, tempo) following onset is also important.
An acute/subacute onset with a definite date often suggests an immune-mediated or an infectious process (FIGURE). With respect to immune-mediated neuropathies, consider primarily autoimmune conditions (eg, Guillain-Barré syndrome, vasculitic neuropathy) and also paraneoplastic autoimmune syndromes (eg, subacute sensory neuropathy). In the latter case, a cancer presents to the immune system an epitope that is similarly found in the nervous system, prompting an autoimmune attack of the nervous system. Both autoimmune and infectious processes almost always have a rapid start on a definite date.
With an insidious onset, the patient won’t recollect a definite date on which the neuropathy began. The underlying mechanism usually is an inherited, metabolic, or toxic process—or idiopathic, if a cause cannot be identified.
The disease course sheds additional light on the causative mechanism. Onset and subsequent progression often correlate in a predictable manner, owing in part to the underlying mechanism. For example, an acute onset neuropathy often is followed by rapid disease progression, especially when caused by an autoimmune process (Guillain-Barré and vasculitic neuropathy). On the other hand, a neuropathy of insidious onset usually follows a slow or even static course. But there are exceptions that may make the diagnosis challenging and illustrate the need for clinical follow-up.
What setting
The patient’s medical history, medications, social and family history, and a review of systems can uncover known risk factors for neuropathic processes.
Common causes of acquired polyneuropathies are diabetes mellitus, chronic renal disease, and alcohol dependence. If a patient with distal, symmetric sensory complaints also has any of these conditions, a causal relationship should be considered. Another example is the patient with a history of bariatric surgery, which could lead to neuropathy as a result of malnutrition, particularly vitamin B1 and B12 deficiencies.13
The clinical setting may indicate a need for further evaluation by a neurologist. For example, a family history of inherited neuropathy in a patient with high arches and curled “hammer” toes would strongly suggest Charcot-Marie-Tooth (CMT) disease, also known as hereditary motor and sensory neuropathy. A patient with a monoclonal gammopathy may have a paraproteinemic neuropathy that warrants further evaluation by a neuromuscular specialist. Although more rare, a paraneoplastic cause would warrant consideration in a smoker, especially if the neuropathy was subacute. In many cases, neuropathic symptoms are the first clue of a new medical condition (eg, impaired glucose tolerance).
Electrodiagnostic testing: What it can and can’t tell you
Most polyneuropathies warrant additional electrodiagnostic evaluation in an electromyography (EMG) laboratory. Electro-diagnostic testing comprises 2 procedures: nerve conduction studies and needle electrode examination.
Preparing your patient. When ordering this study, be sure to discuss it thoroughly with the patient. The entire study typically takes an hour or longer. And it can be painful, though in our experience nearly every patient tolerates the procedure. Most important for the patient to understand is that information gleaned from electrodiagnostic testing may be essential to the diagnosis, as explained below.
Many benefits of the study. First, electrodiagnostic testing can confirm a peripheral neuropathic basis for a patient’s complaints.
Second, electrodiagnostic testing helps characterize a neuropathy as primarily demyelinating, primarily axonal, or mixed demyelinating and axonal. An example of this benefit is that a primary demyelinating characterization greatly narrows the list of possible causes (eg, Guillain-Barré syndrome, chronic inflammatory demyelinating polyradiculoneuropathy, Charcot-Marie-Tooth disease type 1).
Third, electrodiagnostic testing assists in characterizing the neuropathic process as sensory, motor or sensorimotor.
Fourth, electrodiagnostic testing helps localize the neuropathic process (the “where”).
Fifth, electrodiagnostic testing can gauge the severity of the neuropathic process.
Limitations of the study. When the study returns normal results, keep in mind it has limited sensitivity. For example, nerve conduction studies are only able to assess the function of larger myelinated nerve fibers; a neuropathic process solely in small fibers will not be evident with this test. Likewise, the needle electrode examination is unable to assess small nerve fiber status. We include this caveat in our electrodiagnostic testing reports of patients who have symptoms suggestive of pure small fiber polyneuropathy.
When might blood studies be useful?
Laboratory testing of blood is often of great value, but only after a particular polyneuropathy has been characterized and placed into one or more potential etiologic subgroups.
For example, laboratory testing for a distal, symmetric sensory polyneuropathy (TABLE 2) should be much different than testing for another presentation (eg, mononeuritis multiplex). TABLE 3 details some of the laboratory tests we recommend for the more common polyneuropathies that don’t typically present in a distal, symmetric sensory fashion or that are accompanied by other distinctive features. In our experience, clinicians too frequently order unnecessary and expensive tests for disorders only rarely associated with neuropathy; the rare causes of neuropathy are intentionally not the subject of this review.
TABLE 2
Common blood tests for the evaluation of a distal, symmetrical neuropathy1,3,5,6,8
| TEST | POTENTIAL CONFIRMATORY VALUE OF TESTS |
|---|---|
| Fasting glucose or 2-hour oral glucose tolerance test | Diabetes mellitus and possibly impaired glucose tolerance (“pre-diabetes”) cause neuropathy |
| Serum protein electrophoresis | Paraproteinemias, including monoclonal gammopathy of undetermined significance, Waldenstrom’s macroglobulinemia, and osteosclerotic myeloma, are often associated with a demyelinating neuropathy. Amyloidosis and mixed cryoglobulinemiacan also cause an axonal neuropathy |
| Chemistry profile | Uremic neuropathy |
| Hepatitis C titer | Hepatitis C is associated with neuropathy, particularly when associated with mixed cryoglobulinemia. This neuropathy usually presents asymmetrically and often as mononeuritis multiplex but sometimes as a distal, symmetrical neuropathy |
| Serum B12 level | Vitamin B12 deficiency may cause neuropathy, often in association with symptoms and signs of myelopathy |
TABLE 3
Other diagnostic tests for acquired neuropathies in specific clinical situations2,4,5,7
| SUSPECTED PATHOLOGIC PROCESSES AND PERTINENT TESTS | POTENTIAL CONFIRMATORY VALUE OF TESTS |
|---|---|
| Metabolic/toxic | |
| Complete blood count | Elevated MCV may suggest alcoholism, vitamin B12 deficiency |
| Thiamine level | Thiamine deficiency (eg, alcoholics or following bariatric surgery) |
| Urine heavy metals | Heavy metal intoxication (rare; usually systemic symptoms too) |
| Thyroid function tests | Hypothyroid neuropathy (rare) |
| Inflammatory | |
| Complete blood count | If systemic vasculitic neuropathy suspected (eg, patient presents with painful “mononeuritis multiplex”) systemic vasculitic neuropathy |
| Markers of vasculitis or systemic inflammation (ESR, ANCA, RF, ANA, ENA, cryoglobulins, etc) | Cryoglobulinemic neuropathy is associated with hepatitis C |
| Cerebrospinal fluid (CSF) | CSF protein elevation in Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy |
| Neoplastic/paraneoplastic | |
| Paraneoplastic serology (technique and scope of testing varies between different labs) | Neuropathy associated with cancer, especially subacute and severe neuropathic processes in smokers (eg, subacute sensory neuronopathy associated with small cell lung cancer) |
| Chest X-ray and other imaging for cancer | Small cell lung cancer or other malignancy |
| Cerebrospinal cytology | Carcinomatous or lymphomatous polyradiculopathy. |
| Infectious | |
| Cerebrospinal fluid | CSF pleocytosis common in infectious polyradiculoneuropathies (Lyme, sarcoid, HIV) |
| Lyme titers (serum, cerebrospinal fluid) | Lyme neuroborreliosis |
| HIV testing | HIV-associated neuropathy |
| Hepatitis C (serum cryoglobulin testing) | Hepatitis C—mixed cryoglobulinemia |
Common causes of distal, symmetric polyneuropathies
Distal, symmetric polyneuropathies are usually due to metabolic/toxic, inherited, or idiopathic causes (FIGURE, TABLE 2). As such, it is often unnecessary to obtain diagnostic tests searching for active infectious, autoimmune or paraneoplastic etiologies.3 In electrodiagnostic terms, these neuropathies are almost always primarily axonal rather than demyelinating, usually involving both large and small nerve fibers.
Diabetes is the most common cause of neuropathy in developed Western countries, occurring in more than 50% of patients who require insulin.14 Diabetic neuropathy typically presents as a distal, symmetric neuropathy syndrome, though diabetic lumbosacral radiculoplexus neuropathy (diabetic amyotrophy) and other presentations may be seen less commonly.
Recent evidence suggests that neuropathy—particularly a sensory and often painful, distal, symmetric “small-fiber” neuropathy—sometimes occurs in patients with impaired fasting glucose (IFG) or impaired glucose tolerance (IGT).15-19 The 2-hour oral glucose tolerance test has been deemed more sensitive for the early diabetic state of IGT/IFG associated with neuropathy.19 It remains to be seen whether this early diabetic state neuropathy will turn out to represent a large percentage of cases of what were previously thought of as idiopathic small-fiber neuropathy.
Alcoholism is another common cause of a distal, symmetric neuropathy that is predominantly sensory with a painful, burning sensation.20-22 Alcohol abuse and dependence occurs in 10% to 20% of the primary care population.23,24 The prevalence of neuropathy in alcoholics is uncertain, though 1 study of hospitalized patients admitting to daily alcohol intake of over 100 g for men or 80 g for women (10 oz of beer, 1 oz liquor and 3–4 oz wine each have 10 g of alcohol) for 2 years or more (mean of 238±120 g for a period of 22.7±10.2 years) demonstrated that one third of patients had electrophysiologic evidence of peripheral neuropathy and one fourth of autonomic neuropathy.20 Most subjects in this cohort did not show any clinical or laboratory evidence of malnutrition.
Frequently alcoholic neuropathy coexists with thiamine-deficient neuropathy,22 warranting assessment of thiamine status in all alcoholics with neuropathy. Pain is a prominent complaint in alcoholic neuropathy but a less common complaint in thiamine-deficient neuropathy.22 Cobalamin (vitamin B12)-deficient neuropathy is more likely to occur suddenly and to involve the hands or hands and feet simultaneously, and is less likely to be painful.25 Myelopathy is frequent in cobalamin deficiency, sometimes serving as a clue to diagnosis. It may be difficult to determine whether sensory symptoms are caused by myelopathy or neuropathy. Electro-diagnostic testing, somatosensory evoked potentials, and radiological investigation may be helpful.
Uremic neuropathy may occur in patients with chronic renal failure on dialysis.26,27 Today it is less common by virtue of the widespread implementation of dialysis and renal transplantation. Generally reserve this diagnosis for patients with end-stage renal failure with a creatinine clearance of less than 10 mL/min. Other systemic disorders associated with renal failure must be considered; these include diabetes mellitus, amyloidosis, and vasculitis. Also consider drug-induced neuropathy in this population.
Pharmaceutical agents, industrial and environmental agents, and substances of abuse may cause neuropathy, most commonly in the form of a length-dependent axonal neuropathy. Offending chemotherapeutic agents include colchicine, pyridoxine, and amiodarone. However, toxic polyneuropathies probably represent a rather small proportion of cases.
The list of potentially offending substances is long and includes many medications and agents that the general population is commonly exposed to in low doses. Consider the inherent risk of neuropathy of the particular agent in question. To establish a causal link, verify exposure, determine that symptoms are temporally related to the toxin, and rule out other causes of neuropathy. Furthermore, some clinical improvement or at least stabilization should occur following removal of the offending agent, though this may take months to years.28
Medication-induced neuropathy is more common than industrial and environmental neuropathies. The neuropathy of heavy metal intoxication is rare, and is usually accompanied by a combination of gastrointestinal, hematologic, and central nervous system problems.
HIV neuropathy is predominantly sensory and length-dependent. It occurs in about one third of infected patients. This is an exception to the tenet that infectious neuropathies typically present in a non– length-dependent pattern. However, in almost all circumstances, the diagnosis of HIV is well established and so it usually doesn’t present a diagnostic challenge. It is not our routine practice to check for HIV in those presenting with a new-onset distal, symmetric neuropathy, though we will in the proper clinical setting. Whether HIV neuropathy should be included in the differential diagnosis depends on the incidence of HIV in your patient population and the patient’s specifics.6 Rarely, Guillain-Barré syndrome and CIDP can be the presentation of a recent HIV infection.
For inherited neuropathies, there are an overwhelming number of commercially available panels for DNA testing.3,29-31 A detailed discussion of inherited neuropathies is beyond the scope of this review, and many excellent reviews have been written on the subject. We will, however, make a few general comments.
First, Charcot-Marie-Tooth (CMT) disease is the most common inherited neuromuscular disorder, and it is encountered in primary care.
Second, foot deformities (eg, high arches, curled toes) commonly accompany an inherited polyneuropathy and are valuable clues.
Third, the inheritence pattern should be sought through a detailed family history and examination of other family members (including query and examination for foot deformities). Inherited neuropathies can be autosomal dominant, autosomal recessive, or X-linked, based largely on what gene harbors the mutation. Furthermore, spontaneous “de novo” mutations are not uncommon, being responsible for probably 25% of cases of CMT type 1.
Fourth, inherited neuropathies should be characterized just like acquired neuropathies, with particular attention paid to whether the neuropathy is demyelinating (eg, CMT type 1) or axonal (CMT type 2).
With respect to DNA testing of blood, focused genetic testing is almost always possible once you further characterize the inherited neuropathy and also take into account prevalence estimates of the various inherited neuropathies (eg, CMT type 1A caused by a duplication of the PMP-22 gene is responsible for the majority of cases on CMT), obviating the need for expensive, comprehensive genetic testing.
Other presentations
The evaluation of neuropathies that are not distal and symmetric can be complex and in many cases may warrant referral to a neurologist for further evaluation. With such presentations, diagnostic considerations include chest imaging and antineuronal (paraneoplastic) antibody serology for smokers with subacute neuropathies, cerebrospinal fluid analysis for an acquired demyelinating neuropathy, infectious neuropathy or a polyradiculopathy (eg, Lyme disease),3,7 and sensory nerve biopsy (eg, sural nerve) when vasculitis, amyloidosis, or sarcoidosis is suspected.
CORRESPONDENCE
Ted M. Burns, MD, University of Virginia Health System Department of Neurology, Box 800394, Charlottesville, VA 22908. E-mail: [email protected]
- Pain described by patients as electric shocks, burning, freezing, tightness, or throbbing suggests toxic, metabolic, or ischemic causes of neuropathy (C).
- When motor and sensory symptoms appear together, rank them in order of symptom predominance. Motor symptom supremacy may indicate an immune-related disorder (C).
- When measuring sensory thresholds, keep in mind that they normally increase with the patient’s age and height. Vibration sensation in the toes of elderly persons is often said to be decreased, when in fact it is only an age-related change (C).
Polyneuropathy has an estimated prevalence of about 2% in the general population.1,2 Despite being common, polyneuropathy remains a diagnostic challenge for most clinicians for many reasons, including the large number of potential causes and the fact that a specific cause often cannot be identified even after appropriate testing. These factors can contribute to uncertainty about the direction and level of aggressiveness of the evaluation. The result is often a “one size fits all” strategy—from an unnecessarily expensive “shotgun” approach to a “defeatist” attitude that too quickly deems a neuropathy as idiopathic.
A number of experts have developed algorithms for the evaluation of neuropathy.1,3-8 We have incorporated and modified many of these suggestions to develop a simple, user-friendly approach; one that characterizes the neuropathy using “what?”, “where?”, “when?” and “what setting?” parameters (FIGURE).
The focus of this article is on the polyneuropathy that presents with widespread sensory or sensorimotor symptoms and signs. We will not discuss the evaluation of other peripheral neuropathic processes—eg, mononeuropathies, plexopathies, or motor neuronopathies such as amyotrophic lateral sclerosis. We emphasize the fundamental step of determining whether the polyneuropathy is a distal, symmetrical, sensory or sensorimotor polyneuropathy, in which case metabolic/toxic, inherited and idiopathic causes are more probable. In contrast, alternative presentations suggest immune-mediated and infectious causes.
What: Sensory, motor, or autonomic?
The first step is to assign the patient’s complaints and your examination findings to specific nerve fiber types of the peripheral nervous system: sensory, motor, or autonomic.5
FIGURE
Characterizing your patient’s neuropathy
Sensory findings help narrow your search
Once you have ruled out a nonneuropathic process (eg, arthritis) as the cause of sensory symptoms and signs, turn your attention to distinguishing between peripheral or central nervous system dysfunction. When the pathology resides in the central nervous system (eg, multiple sclerosis) accompanying symptoms and signs usually assist in localization. If a patient acknowledges clinical features suggesting past or present involvement of cerebellar, urinary, or visual (eg, optic neuritis) systems, for example, magnetic resonance imaging rather than electrodiagnostic testing may be warranted.
In a patient with peripheral nervous system dysfunction, sensory abnormalities help exclude neuromuscular diseases not associated with sensory dysfunction, such as myopathies, neuromuscular transmission disorders, or disease of the motor neuron.
Positive neuropathic sensory symptoms suggest acquired polyneuropathies. Patients may describe “prickling,” “tingling,” “swelling,” “asleep-like numbness,” or a sensation of “bunched-up socks.” Patients with acquired polyneuropathies usually complain of positive neuropathic sensory symptoms (PNSS), whereas patients with inherited polyneuropathies only rarely do (FIGURE).
Pain suggests toxic, metabolic, or ischemic causes. Patients may describe “electric shocks,” “burning,” “freezing,” “tightness,” or “throbbing.” They may complain of discomfort or pain to sensory stimuli that under normal circumstances would not be painful (allodynia)—eg, discomfort evoked by a bed sheet resting on the feet. They may also describe exaggerated pain to stimuli that would normally evoke low levels of discomfort or pain (hyperalgesia).
A painful neuropathy narrows the differential diagnosis to diseases that affect smaller nerve fibers, which generally convey pain and temperature input. Causes may be toxic, metabolic, ischemic, or idiopathic. For example, a pure “small-fiber” polyneuropathy commonly occurs in patients aged >60 years and typically causes painful feet. It is often idiopathic, but diabetes or impaired glucose tolerance and alcohol toxicity should be explored.9 Patients with painful neuropathy usually also exhibit reduced or absent sensation of pinprick and temperature in the distribution of their sensory complaints.
Negative neuropathic sensory symptoms. In addition to positive neuropathic symptoms, patients may complain of “negative” symptoms such as loss of sensation and imbalance. Examination usually reveals abnormalities of proprioception and sensation to vibration with reduced or absent deep tendon reflexes and ataxia. These features can occur in acquired or inherited causes of neuropathy.
Important sensory tests. Test sensation on the toes and fingertips, and more proximally (eg, ankle and shin) if any abnormality is found at these distal sites. Test vibration with a 128 Hz tuning fork, pinprick with disposable safety pin, and light touch with a cotton swab.
You may test temperature sensation by warming or cooling the handle or prong of the tuning fork and applying it to the patient’s skin.
Joint position testing is performed by asking the patient to avert his eyes, then moving the distal phalanx of a finger or toe up or down by small increments and asking the patient to tell you the direction of movement. Assess a patient’s casual and tandem gait for unsteadiness or ataxia.
Motor symptoms: Weigh them against sensory findings
Most patients with neuropathy have some degree of weakness, but it is usually overshadowed by sensory complaints. Distal lower extremity weakness may manifest as “foot drop,” which, if it affects ankle dorsiflexion, may cause a “slapping” or noisy step due to the forefoot hitting the ground with abnormal force.
Distal upper extremity weakness may cause trouble with fine motor skills of the hands.
Proximal weakness may present as difficulty in rising from a chair or lifting objects above the shoulders.
There may be muscle atrophy or fasciculations.
Motor symptoms are seldom the sole complaint. When motor and sensory symptoms are combined, it is helpful to rank them in order of symptom predominance—ie, motor greater than sensory, or vice versa. For instance, many immune-mediated disorders, such as Guillain-Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), produce chiefly motor abnormalities and fewer sensory symptoms. Sensory complaints predominate in many other polyneuropathies, especially the “length-dependent” polyneuropathies (ie, those affecting the longer nerves initially) caused by metabolic or toxic disorders.
Autonomic symptom evaluation
The number of processes that affect both autonomic and somatic nerves are relatively few (TABLE 1).5,7,10 It is particularly important to assess symptoms suggesting involvement of the autonomic nervous system.
Autonomic symptoms include lightheadedness, syncope, diarrhea, constipation, postprandial bloating, early satiety, urinary complaints, erectile dysfunction, abnormal or absent sweating, and dry mouth and eyes. Many of these complaints are common in the general population, so their relevance should be based on severity and temporal evolution, as well as comorbidities and medication use.
When complaints do not clearly implicate pathology in the autonomic nervous system, autonomic testing may be helpful, targeted for the domain that may be impaired. For example, bedside orthostatics or tilt-table testing are used for pre-syncopal symptoms, but gastric emptying testing can assist the evaluation of complaints of early satiety or postprandial bloating.
TABLE 1
Relatively common acquired polyneuropathies with autonomic nervous system involvement
| Diabetes mellitus |
| Amyloidosis |
| Guillain-Barré syndrome |
| Paraneoplastic neuropathy (usually small cell lung cancer) |
| Sjögren’s syndrome–associated neuropathy |
Where: The distribution of nerve involvement
“Where” refers to distribution of nerve involvement 1) globally throughout the body and 2) locally along the nerve(s). During the history taking and examination, determine the nature of general distribution (eg, symmetric or asymmetric) and where along the length of the nerve(s) (proximal and/or distal) the dysfunction exists.
Polyneuropathy most commonly presents in a “length-dependent” distribution, with clinical features appearing initially most distally and symmetrically (ie, in the feet). Asymmetry and involvement of the proximal parts of a nerve are “red flags” for an uncommon cause that may require referral to a neurologist (FIGURE).
Comparative vs absolute measurements. At bedside, 2 approaches are used to assess the distribution of nerve involvement: comparative and absolute. The comparative approach searches for a relative difference in sensory thresholds or weakness between sites. It can assess side-to-side or one nerve (or root or region) territory to another. It is useful for establishing sensory or motor impairment in a radicular, plexus, or single nerve distribution.
Testing for an absolute reduction in sensation (eg, decreased vibration in the toes) can be more challenging because it requires experience in judging what is normal and abnormal according to expectations for a particular site and modality. Take into account that sensory thresholds are normally increased with the patient’s age and height.11 For example, we commonly encounter elderly patients whose vibration sensation in the toes is said to be decreased, when in fact the reduced sensory threshold is only an age-related change.
Most assessments of sensory thresholds use the absolute approach because most generalized polyneuropathies are “length-dependent.”
Perform motor testing for appendicular (upper and lower extremities) and axial (neck and trunk) muscles, assessing particularly for weakness, atrophy, and fasciculations.
The typical polyneuropathy caused by metabolic, toxic, inherited, or unknown causes is distal and symmetric.12 Neuropathies caused by other mechanisms, such as immune-mediated or infectious, are rarely length-dependent. Examples include motor neuronopathies (eg, amyotrophic lateral sclerosis), sensory neuronopathies (eg, paraneoplastic), polyradiculoneuropathies (Guillain-Barré syndrome, CIDP), and mononeuritis multiplex (caused by vasculitis).
When: The time course of signs and symptoms
Knowing whether the onset of neuropathy was definite and abrupt or was gradual is the most helpful temporal clue to possible underlying causes. The time course (ie, tempo) following onset is also important.
An acute/subacute onset with a definite date often suggests an immune-mediated or an infectious process (FIGURE). With respect to immune-mediated neuropathies, consider primarily autoimmune conditions (eg, Guillain-Barré syndrome, vasculitic neuropathy) and also paraneoplastic autoimmune syndromes (eg, subacute sensory neuropathy). In the latter case, a cancer presents to the immune system an epitope that is similarly found in the nervous system, prompting an autoimmune attack of the nervous system. Both autoimmune and infectious processes almost always have a rapid start on a definite date.
With an insidious onset, the patient won’t recollect a definite date on which the neuropathy began. The underlying mechanism usually is an inherited, metabolic, or toxic process—or idiopathic, if a cause cannot be identified.
The disease course sheds additional light on the causative mechanism. Onset and subsequent progression often correlate in a predictable manner, owing in part to the underlying mechanism. For example, an acute onset neuropathy often is followed by rapid disease progression, especially when caused by an autoimmune process (Guillain-Barré and vasculitic neuropathy). On the other hand, a neuropathy of insidious onset usually follows a slow or even static course. But there are exceptions that may make the diagnosis challenging and illustrate the need for clinical follow-up.
What setting
The patient’s medical history, medications, social and family history, and a review of systems can uncover known risk factors for neuropathic processes.
Common causes of acquired polyneuropathies are diabetes mellitus, chronic renal disease, and alcohol dependence. If a patient with distal, symmetric sensory complaints also has any of these conditions, a causal relationship should be considered. Another example is the patient with a history of bariatric surgery, which could lead to neuropathy as a result of malnutrition, particularly vitamin B1 and B12 deficiencies.13
The clinical setting may indicate a need for further evaluation by a neurologist. For example, a family history of inherited neuropathy in a patient with high arches and curled “hammer” toes would strongly suggest Charcot-Marie-Tooth (CMT) disease, also known as hereditary motor and sensory neuropathy. A patient with a monoclonal gammopathy may have a paraproteinemic neuropathy that warrants further evaluation by a neuromuscular specialist. Although more rare, a paraneoplastic cause would warrant consideration in a smoker, especially if the neuropathy was subacute. In many cases, neuropathic symptoms are the first clue of a new medical condition (eg, impaired glucose tolerance).
Electrodiagnostic testing: What it can and can’t tell you
Most polyneuropathies warrant additional electrodiagnostic evaluation in an electromyography (EMG) laboratory. Electro-diagnostic testing comprises 2 procedures: nerve conduction studies and needle electrode examination.
Preparing your patient. When ordering this study, be sure to discuss it thoroughly with the patient. The entire study typically takes an hour or longer. And it can be painful, though in our experience nearly every patient tolerates the procedure. Most important for the patient to understand is that information gleaned from electrodiagnostic testing may be essential to the diagnosis, as explained below.
Many benefits of the study. First, electrodiagnostic testing can confirm a peripheral neuropathic basis for a patient’s complaints.
Second, electrodiagnostic testing helps characterize a neuropathy as primarily demyelinating, primarily axonal, or mixed demyelinating and axonal. An example of this benefit is that a primary demyelinating characterization greatly narrows the list of possible causes (eg, Guillain-Barré syndrome, chronic inflammatory demyelinating polyradiculoneuropathy, Charcot-Marie-Tooth disease type 1).
Third, electrodiagnostic testing assists in characterizing the neuropathic process as sensory, motor or sensorimotor.
Fourth, electrodiagnostic testing helps localize the neuropathic process (the “where”).
Fifth, electrodiagnostic testing can gauge the severity of the neuropathic process.
Limitations of the study. When the study returns normal results, keep in mind it has limited sensitivity. For example, nerve conduction studies are only able to assess the function of larger myelinated nerve fibers; a neuropathic process solely in small fibers will not be evident with this test. Likewise, the needle electrode examination is unable to assess small nerve fiber status. We include this caveat in our electrodiagnostic testing reports of patients who have symptoms suggestive of pure small fiber polyneuropathy.
When might blood studies be useful?
Laboratory testing of blood is often of great value, but only after a particular polyneuropathy has been characterized and placed into one or more potential etiologic subgroups.
For example, laboratory testing for a distal, symmetric sensory polyneuropathy (TABLE 2) should be much different than testing for another presentation (eg, mononeuritis multiplex). TABLE 3 details some of the laboratory tests we recommend for the more common polyneuropathies that don’t typically present in a distal, symmetric sensory fashion or that are accompanied by other distinctive features. In our experience, clinicians too frequently order unnecessary and expensive tests for disorders only rarely associated with neuropathy; the rare causes of neuropathy are intentionally not the subject of this review.
TABLE 2
Common blood tests for the evaluation of a distal, symmetrical neuropathy1,3,5,6,8
| TEST | POTENTIAL CONFIRMATORY VALUE OF TESTS |
|---|---|
| Fasting glucose or 2-hour oral glucose tolerance test | Diabetes mellitus and possibly impaired glucose tolerance (“pre-diabetes”) cause neuropathy |
| Serum protein electrophoresis | Paraproteinemias, including monoclonal gammopathy of undetermined significance, Waldenstrom’s macroglobulinemia, and osteosclerotic myeloma, are often associated with a demyelinating neuropathy. Amyloidosis and mixed cryoglobulinemiacan also cause an axonal neuropathy |
| Chemistry profile | Uremic neuropathy |
| Hepatitis C titer | Hepatitis C is associated with neuropathy, particularly when associated with mixed cryoglobulinemia. This neuropathy usually presents asymmetrically and often as mononeuritis multiplex but sometimes as a distal, symmetrical neuropathy |
| Serum B12 level | Vitamin B12 deficiency may cause neuropathy, often in association with symptoms and signs of myelopathy |
TABLE 3
Other diagnostic tests for acquired neuropathies in specific clinical situations2,4,5,7
| SUSPECTED PATHOLOGIC PROCESSES AND PERTINENT TESTS | POTENTIAL CONFIRMATORY VALUE OF TESTS |
|---|---|
| Metabolic/toxic | |
| Complete blood count | Elevated MCV may suggest alcoholism, vitamin B12 deficiency |
| Thiamine level | Thiamine deficiency (eg, alcoholics or following bariatric surgery) |
| Urine heavy metals | Heavy metal intoxication (rare; usually systemic symptoms too) |
| Thyroid function tests | Hypothyroid neuropathy (rare) |
| Inflammatory | |
| Complete blood count | If systemic vasculitic neuropathy suspected (eg, patient presents with painful “mononeuritis multiplex”) systemic vasculitic neuropathy |
| Markers of vasculitis or systemic inflammation (ESR, ANCA, RF, ANA, ENA, cryoglobulins, etc) | Cryoglobulinemic neuropathy is associated with hepatitis C |
| Cerebrospinal fluid (CSF) | CSF protein elevation in Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy |
| Neoplastic/paraneoplastic | |
| Paraneoplastic serology (technique and scope of testing varies between different labs) | Neuropathy associated with cancer, especially subacute and severe neuropathic processes in smokers (eg, subacute sensory neuronopathy associated with small cell lung cancer) |
| Chest X-ray and other imaging for cancer | Small cell lung cancer or other malignancy |
| Cerebrospinal cytology | Carcinomatous or lymphomatous polyradiculopathy. |
| Infectious | |
| Cerebrospinal fluid | CSF pleocytosis common in infectious polyradiculoneuropathies (Lyme, sarcoid, HIV) |
| Lyme titers (serum, cerebrospinal fluid) | Lyme neuroborreliosis |
| HIV testing | HIV-associated neuropathy |
| Hepatitis C (serum cryoglobulin testing) | Hepatitis C—mixed cryoglobulinemia |
Common causes of distal, symmetric polyneuropathies
Distal, symmetric polyneuropathies are usually due to metabolic/toxic, inherited, or idiopathic causes (FIGURE, TABLE 2). As such, it is often unnecessary to obtain diagnostic tests searching for active infectious, autoimmune or paraneoplastic etiologies.3 In electrodiagnostic terms, these neuropathies are almost always primarily axonal rather than demyelinating, usually involving both large and small nerve fibers.
Diabetes is the most common cause of neuropathy in developed Western countries, occurring in more than 50% of patients who require insulin.14 Diabetic neuropathy typically presents as a distal, symmetric neuropathy syndrome, though diabetic lumbosacral radiculoplexus neuropathy (diabetic amyotrophy) and other presentations may be seen less commonly.
Recent evidence suggests that neuropathy—particularly a sensory and often painful, distal, symmetric “small-fiber” neuropathy—sometimes occurs in patients with impaired fasting glucose (IFG) or impaired glucose tolerance (IGT).15-19 The 2-hour oral glucose tolerance test has been deemed more sensitive for the early diabetic state of IGT/IFG associated with neuropathy.19 It remains to be seen whether this early diabetic state neuropathy will turn out to represent a large percentage of cases of what were previously thought of as idiopathic small-fiber neuropathy.
Alcoholism is another common cause of a distal, symmetric neuropathy that is predominantly sensory with a painful, burning sensation.20-22 Alcohol abuse and dependence occurs in 10% to 20% of the primary care population.23,24 The prevalence of neuropathy in alcoholics is uncertain, though 1 study of hospitalized patients admitting to daily alcohol intake of over 100 g for men or 80 g for women (10 oz of beer, 1 oz liquor and 3–4 oz wine each have 10 g of alcohol) for 2 years or more (mean of 238±120 g for a period of 22.7±10.2 years) demonstrated that one third of patients had electrophysiologic evidence of peripheral neuropathy and one fourth of autonomic neuropathy.20 Most subjects in this cohort did not show any clinical or laboratory evidence of malnutrition.
Frequently alcoholic neuropathy coexists with thiamine-deficient neuropathy,22 warranting assessment of thiamine status in all alcoholics with neuropathy. Pain is a prominent complaint in alcoholic neuropathy but a less common complaint in thiamine-deficient neuropathy.22 Cobalamin (vitamin B12)-deficient neuropathy is more likely to occur suddenly and to involve the hands or hands and feet simultaneously, and is less likely to be painful.25 Myelopathy is frequent in cobalamin deficiency, sometimes serving as a clue to diagnosis. It may be difficult to determine whether sensory symptoms are caused by myelopathy or neuropathy. Electro-diagnostic testing, somatosensory evoked potentials, and radiological investigation may be helpful.
Uremic neuropathy may occur in patients with chronic renal failure on dialysis.26,27 Today it is less common by virtue of the widespread implementation of dialysis and renal transplantation. Generally reserve this diagnosis for patients with end-stage renal failure with a creatinine clearance of less than 10 mL/min. Other systemic disorders associated with renal failure must be considered; these include diabetes mellitus, amyloidosis, and vasculitis. Also consider drug-induced neuropathy in this population.
Pharmaceutical agents, industrial and environmental agents, and substances of abuse may cause neuropathy, most commonly in the form of a length-dependent axonal neuropathy. Offending chemotherapeutic agents include colchicine, pyridoxine, and amiodarone. However, toxic polyneuropathies probably represent a rather small proportion of cases.
The list of potentially offending substances is long and includes many medications and agents that the general population is commonly exposed to in low doses. Consider the inherent risk of neuropathy of the particular agent in question. To establish a causal link, verify exposure, determine that symptoms are temporally related to the toxin, and rule out other causes of neuropathy. Furthermore, some clinical improvement or at least stabilization should occur following removal of the offending agent, though this may take months to years.28
Medication-induced neuropathy is more common than industrial and environmental neuropathies. The neuropathy of heavy metal intoxication is rare, and is usually accompanied by a combination of gastrointestinal, hematologic, and central nervous system problems.
HIV neuropathy is predominantly sensory and length-dependent. It occurs in about one third of infected patients. This is an exception to the tenet that infectious neuropathies typically present in a non– length-dependent pattern. However, in almost all circumstances, the diagnosis of HIV is well established and so it usually doesn’t present a diagnostic challenge. It is not our routine practice to check for HIV in those presenting with a new-onset distal, symmetric neuropathy, though we will in the proper clinical setting. Whether HIV neuropathy should be included in the differential diagnosis depends on the incidence of HIV in your patient population and the patient’s specifics.6 Rarely, Guillain-Barré syndrome and CIDP can be the presentation of a recent HIV infection.
For inherited neuropathies, there are an overwhelming number of commercially available panels for DNA testing.3,29-31 A detailed discussion of inherited neuropathies is beyond the scope of this review, and many excellent reviews have been written on the subject. We will, however, make a few general comments.
First, Charcot-Marie-Tooth (CMT) disease is the most common inherited neuromuscular disorder, and it is encountered in primary care.
Second, foot deformities (eg, high arches, curled toes) commonly accompany an inherited polyneuropathy and are valuable clues.
Third, the inheritence pattern should be sought through a detailed family history and examination of other family members (including query and examination for foot deformities). Inherited neuropathies can be autosomal dominant, autosomal recessive, or X-linked, based largely on what gene harbors the mutation. Furthermore, spontaneous “de novo” mutations are not uncommon, being responsible for probably 25% of cases of CMT type 1.
Fourth, inherited neuropathies should be characterized just like acquired neuropathies, with particular attention paid to whether the neuropathy is demyelinating (eg, CMT type 1) or axonal (CMT type 2).
With respect to DNA testing of blood, focused genetic testing is almost always possible once you further characterize the inherited neuropathy and also take into account prevalence estimates of the various inherited neuropathies (eg, CMT type 1A caused by a duplication of the PMP-22 gene is responsible for the majority of cases on CMT), obviating the need for expensive, comprehensive genetic testing.
Other presentations
The evaluation of neuropathies that are not distal and symmetric can be complex and in many cases may warrant referral to a neurologist for further evaluation. With such presentations, diagnostic considerations include chest imaging and antineuronal (paraneoplastic) antibody serology for smokers with subacute neuropathies, cerebrospinal fluid analysis for an acquired demyelinating neuropathy, infectious neuropathy or a polyradiculopathy (eg, Lyme disease),3,7 and sensory nerve biopsy (eg, sural nerve) when vasculitis, amyloidosis, or sarcoidosis is suspected.
CORRESPONDENCE
Ted M. Burns, MD, University of Virginia Health System Department of Neurology, Box 800394, Charlottesville, VA 22908. E-mail: [email protected]
1. Hughes RAC. Peripheral neuropathy. BMJ 2002;324:466-469.
2. England JD, Asbury AK. Peripheral neuropathy. Lancet 2004;363:2151-2161.
3. Pourmand R. Evaluating patients with suspected peripheral neuropathy: Do the right thing, not everything. Muscle Nerve 2002;26:288-290.
4. Dyck PJ, Dyck PJB, Grant IA, Fealey RD. Ten steps in characterizing and diagnosing patients with peripheral neuropathy. Neurology 1996;47:10-17.
5. Barohn RJ. Approach to peripheral neuropathy and neuronopathy. Seminars Neurol 1998;18:7-18.
6. Rosenberg NR, Portegies P, de Visser M, Vermeulen M. Diagnostic investigation of patients with chronic polyneuropathy: evaluation of a clinical guideline. J Neurol Neurosurg Psych 2001;71:205-209.
7. Bromberg MB, Smith AG. Toward an efficient method to evaluate peripheral neuropathies. J Clin Neuromusc Dis 2002;3:172-182.
8. Hughes RAC, Umapathi T, Gray IA, et al. A controlled investigation of the cause of chronic idiopathic axonal polyneuropathy. Brain 2004;127:1723-1730.
9. Lacomis D. Small-fiber neuropathy. Muscle Nerve 2002;26:173-188.
10. Low PA, Vernino S, Suarez G. Autonomic dysfunction in peripheral nerve disease. Muscle Nerve 2003;27:646-661.
11. Burns TM, Taly A, O’Brien PC, Dyck PJ. Clinical versus quantitative vibration assessment: improving clinical performance. J Periph Nerv System 2002;7:112-117.
12. England JD, Gronseth GS, Franklin G, et al. Distal symmetric polyneuropathy: A definition for clinical research: Report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005;64:199-207.
13. Thaisetthawatkul P, Collazo-Clavell ML, Sarr MG, Norell JE, Dyck PJ. A controlled study of peripheral neuropathy after bariatric surgery. Neurology 2004;63:1462-1470.
14. Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973. Diabetes Care 1978;1:168-188.
15. Singleton JR, Smith AG, Russell JW, Feldman EL. Microvascular complications of impaired glucose tolerance. Diabetes 2003;52:2867-2873.
16. Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003;60:108-111.
17. Novella SP, Inzucchi SE, Goldstein JM. The frequency of undiagnosed diabetes and impaired glucose tolerance in patients with idiopathic sensory neuropathy. Muscle Nerve 2001;24:1229-1231.
18. Singleton JR, Smith AG, Bromberg MB. Painful sensory polyneuropathy associated with impaired glucose tolerance. Muscle Nerve 2001;24:1225-1228.
19. Polydefkis M, Griffin JW, McArthur J. New insights into diabetic polyneuropathy. JAMA 2003;290:1371-1376.
20. Monforte R, Estruch R, Valls-Sole J, Nicolas J, Villalta J, Urbano-Marquez A. Autonomic and peripheral neuropathies in patients with chronic alcoholism. A dose-related toxic effect of alcohol. Arch Neurol 1995;52:45-51.
21. Koike H, Mori K, Misu K, et al. Painful alcoholic polyneuropathy with predominant small-fiber loss and normal thiamine status. Neurol 2001;56:1727-1732.
22. Koike H, LiJima M, Sugiura M, et al. Alcoholic neuropathy is clinicopathologically distinct from thiamine-deficiency neuropathy. Ann Neurol 2003;54:19-29.
23. Coulehan JL, Zettler-Segal M, Block M, McClelland M, Schulberg HC. Recognition of alcoholism and substance abuse in primary care patients. Arch Intern Med 1987;147:349-352.
24. Brienza RS, Stein MD. Alcohol use disorders in primary care. Do gender-specific differences exist? J Gen Intern Med 2002;17:387-397.
25. Saperstein DS, Wolfe GI, Gronseth GS, et al. Challenges in the identification of cobalamin-deficiency polyneuropathy. Arch Neurol 2003;60:1296-1301.
26. Laaksonen S, Metsärinne K, Voipio-Pulkki LM, Falck B. Neurophysiologic parameters and symptoms in chronic renal failure. Muscle Nerve 2002;25:884-890.
27. Said G, Boudier L, Selva J, Zingraff J, Drueke T. Different patterns of uremic polyneuropathy: clinicopathologic study. Neurology 1983;33:567-574.
28. Hill AB. The environment and disease: association or causation? Proc Roy Soc Med 1965;58:295-300.
29. Donaghy M. Genes for peripheral neuropathy and their relevance to clinical practice. J Neurol Neurosurg Psychiatry 2004;75:1371-1372.
30. Lewis RA, Sumner AJ, Shy ME. Electrophysiological features of inherited demyelinating neuropathies: a reappraisal in the era of molecular diagnosis. Muscle Nerve 2000;23:1472-1487.
31. Pareyson D. Charcot-Marie-Tooth disease and related neuropathies: molecular basis and distinction and diagnosis. Muscle Nerve 1999;22:1498-1509.
32. Scherer SS. Finding the causes of inherited neuropathies. Arch Neurol 2006;63:812-816.
1. Hughes RAC. Peripheral neuropathy. BMJ 2002;324:466-469.
2. England JD, Asbury AK. Peripheral neuropathy. Lancet 2004;363:2151-2161.
3. Pourmand R. Evaluating patients with suspected peripheral neuropathy: Do the right thing, not everything. Muscle Nerve 2002;26:288-290.
4. Dyck PJ, Dyck PJB, Grant IA, Fealey RD. Ten steps in characterizing and diagnosing patients with peripheral neuropathy. Neurology 1996;47:10-17.
5. Barohn RJ. Approach to peripheral neuropathy and neuronopathy. Seminars Neurol 1998;18:7-18.
6. Rosenberg NR, Portegies P, de Visser M, Vermeulen M. Diagnostic investigation of patients with chronic polyneuropathy: evaluation of a clinical guideline. J Neurol Neurosurg Psych 2001;71:205-209.
7. Bromberg MB, Smith AG. Toward an efficient method to evaluate peripheral neuropathies. J Clin Neuromusc Dis 2002;3:172-182.
8. Hughes RAC, Umapathi T, Gray IA, et al. A controlled investigation of the cause of chronic idiopathic axonal polyneuropathy. Brain 2004;127:1723-1730.
9. Lacomis D. Small-fiber neuropathy. Muscle Nerve 2002;26:173-188.
10. Low PA, Vernino S, Suarez G. Autonomic dysfunction in peripheral nerve disease. Muscle Nerve 2003;27:646-661.
11. Burns TM, Taly A, O’Brien PC, Dyck PJ. Clinical versus quantitative vibration assessment: improving clinical performance. J Periph Nerv System 2002;7:112-117.
12. England JD, Gronseth GS, Franklin G, et al. Distal symmetric polyneuropathy: A definition for clinical research: Report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005;64:199-207.
13. Thaisetthawatkul P, Collazo-Clavell ML, Sarr MG, Norell JE, Dyck PJ. A controlled study of peripheral neuropathy after bariatric surgery. Neurology 2004;63:1462-1470.
14. Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973. Diabetes Care 1978;1:168-188.
15. Singleton JR, Smith AG, Russell JW, Feldman EL. Microvascular complications of impaired glucose tolerance. Diabetes 2003;52:2867-2873.
16. Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003;60:108-111.
17. Novella SP, Inzucchi SE, Goldstein JM. The frequency of undiagnosed diabetes and impaired glucose tolerance in patients with idiopathic sensory neuropathy. Muscle Nerve 2001;24:1229-1231.
18. Singleton JR, Smith AG, Bromberg MB. Painful sensory polyneuropathy associated with impaired glucose tolerance. Muscle Nerve 2001;24:1225-1228.
19. Polydefkis M, Griffin JW, McArthur J. New insights into diabetic polyneuropathy. JAMA 2003;290:1371-1376.
20. Monforte R, Estruch R, Valls-Sole J, Nicolas J, Villalta J, Urbano-Marquez A. Autonomic and peripheral neuropathies in patients with chronic alcoholism. A dose-related toxic effect of alcohol. Arch Neurol 1995;52:45-51.
21. Koike H, Mori K, Misu K, et al. Painful alcoholic polyneuropathy with predominant small-fiber loss and normal thiamine status. Neurol 2001;56:1727-1732.
22. Koike H, LiJima M, Sugiura M, et al. Alcoholic neuropathy is clinicopathologically distinct from thiamine-deficiency neuropathy. Ann Neurol 2003;54:19-29.
23. Coulehan JL, Zettler-Segal M, Block M, McClelland M, Schulberg HC. Recognition of alcoholism and substance abuse in primary care patients. Arch Intern Med 1987;147:349-352.
24. Brienza RS, Stein MD. Alcohol use disorders in primary care. Do gender-specific differences exist? J Gen Intern Med 2002;17:387-397.
25. Saperstein DS, Wolfe GI, Gronseth GS, et al. Challenges in the identification of cobalamin-deficiency polyneuropathy. Arch Neurol 2003;60:1296-1301.
26. Laaksonen S, Metsärinne K, Voipio-Pulkki LM, Falck B. Neurophysiologic parameters and symptoms in chronic renal failure. Muscle Nerve 2002;25:884-890.
27. Said G, Boudier L, Selva J, Zingraff J, Drueke T. Different patterns of uremic polyneuropathy: clinicopathologic study. Neurology 1983;33:567-574.
28. Hill AB. The environment and disease: association or causation? Proc Roy Soc Med 1965;58:295-300.
29. Donaghy M. Genes for peripheral neuropathy and their relevance to clinical practice. J Neurol Neurosurg Psychiatry 2004;75:1371-1372.
30. Lewis RA, Sumner AJ, Shy ME. Electrophysiological features of inherited demyelinating neuropathies: a reappraisal in the era of molecular diagnosis. Muscle Nerve 2000;23:1472-1487.
31. Pareyson D. Charcot-Marie-Tooth disease and related neuropathies: molecular basis and distinction and diagnosis. Muscle Nerve 1999;22:1498-1509.
32. Scherer SS. Finding the causes of inherited neuropathies. Arch Neurol 2006;63:812-816.