User login
Deep brain stimulation for movement disorders: Patient selection and technical options
Implantation of a deep brain stimulator is the most common surgical procedure performed in the United States and industrialized world for the management of advanced movement disorders. These procedures are US Food and Drug Administration (FDA)–approved for the management of the symptoms of Parkinson disease (PD) and essential tremor. Deep brain stimulation (DBS) is also approved for managing primary generalized dystonia and torticollis under a humanitarian device exemption.
Deep brain stimulation has largely replaced ablative procedures such as thalamotomy and pallidotomy. While ablative procedures can be effective for the symptoms of movement disorders, they cause a permanent lesion in the targeted nuclei and are therefore not reversible. DBS is considered safer because it can be adjusted over time and the location of the leads can be revised.1 On the other hand, regular maintenance of implanted hardware may be considered a disadvantage of DBS.
HARDWARE AND TARGETS
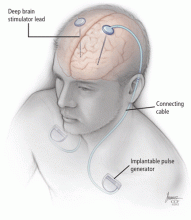
While ablative procedures do not require implantable hardware, DBS consists of permanently implanted neurostimulation systems. The battery-powered pulse generators typically last for several years but require multiple replacements during a lifetime. In addition, if other hardware components fail, surgical revision may be required to maintain treatment efficacy. Surgery involving implantation of hardware carries a higher risk of infection than does a nonimplantation procedure. If infections occur, removal of the hardware is often required, with reimplantation performed after the infection clears. In addition, the expense of DBS hardware may limit availability in some cases.
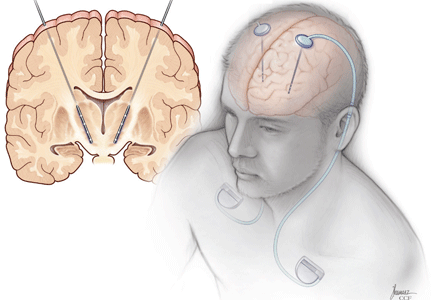
Three components
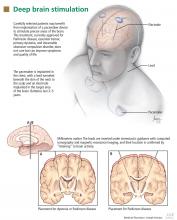
Target nuclei
Several nodes or nuclei can serve as targets for DBS. In patients with PD, the most common surgical target is the subthalamic nucleus (STN), either unilaterally or bilaterally.2 The globus pallidus pars interna (GPi) is also a viable target and is preferred for some patients with PD. The most common target for managing essential tremor is the ventral intermediate nucleus (VIM) of the thalamus, which can also be the target of choice for patients with tremor-predominant PD. However, the GPi and STN are usually preferred over the VIM in patients with PD because stimulation of these targets can relieve symptoms other than tremor, such as rigidity and bradykinesia. Bilateral stimulation of the GPi is the most frequent approach in patients with generalized torsion dystonia and torticollis, although the STN and thalamic nuclei (off-label) are also considered options.
PATIENT SELECTION
Patients are evaluated in our center at Cleveland Clinic by a multidisciplinary team that includes a movement disorder neurologist, a subspecialized neurosurgeon, a movement disorder neuropsychologist, and a psychiatrist with special interest in the behavioral comorbidities of movement disorders.3 Neuroimaging is included in this assessment. We have also included physical therapy as part of the initial evaluation in order to gain insight into the patient’s limitations and develop rehabilitation strategies that may enhance the outcomes of surgery or provide alternatives should surgery not be indicated. This evaluation provides extensive data that are then reviewed by the team in a conference dedicated to discussing candidacy for DBS or options for managing the symptoms of advanced movement disorders. Behavioral and cognitive issues are assessed in detail and, in our experience, are the most common reasons for not recommending DBS.
An important part of the evaluation of patients with PD is a formal test with rating of the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS) with the patient off medications for 8 to 12 hours and then after a test dose of levodopa. At our center, this off/on test is videotaped so that the responsiveness of individual symptoms to levodopa can be reviewed later in conference.
Risk of cognitive decline
While DBS is considered safe and effective, there is a risk of cognitive decline in some patients. In most patients, long-term stimulation-related cognitive decline may be detected with formal measures but is not clinically significant and is outweighed by the motor and quality-of-life benefits of surgery. In some patients, long-term cognitive decline can be significant and can limit function. Cognitive neuropsychologic testing provides valuable information in this regard. Patients with preserved cognitive function seldom experience significant decline with DBS while those with substantial baseline impairment are thought to be at greater risk. Patients who meet criteria for dementia are usually not considered candidates for DBS, but exceptions exist. Transient perioperative cognitive difficulties are more common than persistent deficits, and typically resolve within a few weeks (see “Complications of deep brain stimulation”).
Benefits in Parkinson disease
Deep brain stimulation can address several symptoms of PD but with varying effects. Tremor, rigidity, and bradykinesia usually improve substantially. Gait has a more variable response, and balance is typically refractory. A general rule is that symptoms that improve with a single dose of levodopa should also improve with DBS. (Tremor, however, will most often respond to DBS even if refractory to medication.) Good candidates for surgery typically have a greater than 30% improvement in UPDRS motor score with levodopa challenge, but sometimes, improvement in the total score is less informative than evaluation of the effects of levodopa on particular symptoms. Treatment effects can be compared with the patient’s expectations for surgery in order to infer whether the goals for symptom improvement are realistic.
Treatment outcomes depend on etiology
After programming, DBS can provide PD symptom control similar to that of medication “on time,” but with fewer on-off fluctuations and less on-time dyskinesia. Good surgical candidates are patients who once responded well to dopaminergic medications but who, after several years with the disease, present with increased duration of “off time,” unpredictable duration of on time, and medication side effects such as on-time dyskinesia. Patients who do not respond well to levodopa even in subscores of the UPDRS may not be good candidates for DBS, and in some cases the diagnosis itself needs to be reviewed.
Deep brain stimulation can improve quality of life and alleviate symptoms of essential tremor. Tremor control is best for the upper extremities and tends to be better for distal tremors than for proximal ones. Patients who are good candidates for surgery often have severe tremors. A substantial improvement in these symptoms often has a dramatic, positive effect on work and quality of life. In some patients, surgery is considered for mild tremor if it seriously disrupts the patient’s lifestyle or occupation and cannot be well controlled with medications. Often, in these cases, tremor that appears relatively mild to the examiner is significantly limiting for the patient.
Very severe and proximal tremor is more refractory, though it may also improve. The changes can be well documented with objective measures. In these cases, however, residual tremor can still be moderate to severe and can be functionally limiting. Head or vocal tremors are typically refractory. They may be improved with bilateral implantation, but this cannot be accurately predicted. Patients who present with head-only or head-predominant tremor are thought to be less likely to benefit than those with limb tremor. Nonetheless, tremors of the head can severely impair quality of life. Because there are few other treatment options, some patients choose DBS with the understanding that the outcome is uncertain and the benefit may be limited.
Tremor resulting from multiple sclerosis or other causes can be medically refractory and disabling. In our experience, DBS can be an off-label option for managing secondary tremors and good outcomes have been observed. However, outcomes are much less predictable and tremor control less effective than in patients with essential tremor.
Patients with primary generalized dystonia can be considered candidates for DBS and may experience improved symptom control and quality of life.4 Patients with the DYT1 mutation are more likely to respond well to DBS, as are those with other forms of primary generalized dystonia. In contrast to that seen in patients with PD and tremor, symptomatic improvement is frequently not observed during intraoperative testing. Several months of stimulation and programming may be required before significant improvements are detected.5 Surgery can also be considered for off-label use in the treatment of patients with secondary dystonia—such as that following injury or associated with cerebral palsy—but outcomes are less predictable and usually more limited. A possible exception may be seen in cases of tardive dystonia, for which there is increasing evidence6 for the effectiveness of DBS. This remains an off-label use of DBS.
Realistic expectations
An important aspect of the multidisciplinary evaluation includes a discussion of the expectations for surgery, the risks, and the requirements for postoperative care. As discussed above, DBS is reversible and adjustable, so outcomes depend not only on accurate implantation of the hardware but also on postoperative programming. Also, monitoring and maintenance of the implanted hardware are required in these patients. It is important that patients and families appreciate the fact that specialized, long-term postoperative follow-up is as much a part of the treatment as is the implantation itself.
UNILATERAL VERSUS BILATERAL DBS
Most patients with generalized dystonia undergo bilateral DBS. However, patients with PD or essential tremor may receive bilateral, staged, or unilateral implants. Some patients with PD present with either near-complete predominance of symptoms on one side or with symptoms that affect mostly the dominant extremity. In these patients, unilateral implantation is often recommended because it has less risk than the bilateral approach and may be sufficient to address the most limiting symptoms.
As the disease advances, an additional surgery may be required to accomplish bilateral symptom control. Nevertheless, we do not routinely recommend preventive implantation because it is not known whether second-side symptoms will become severe enough to require it. This strategy allows for deferring surgical risk, which is in itself advantageous. In our experience, bilateral implantation is often recommended to PD patients who present with symptoms such as freezing of gait.
Patients who have essential tremor often present with bilateral symptoms. Although many patients will indicate that they need symptom relief on both upper extremities in order to perform activities of daily living, our practice is to recommend surgery on one side at first and to suggest the patient consider contralateral implantation after weeks or months. Bilateral implantation may carry a risk for dysarthria and the risk is thought to be reduced if bilateral procedures are staged. Although high rates of dysarthria have been reported following bilateral surgery for tremor, its occurrence has been infrequent in our experience with bilateral staged DBS. Benefits of treating tremor in the dominant extremity usually exceed those of treating nondominant tremor, so most patients prefer that the dominant side be the first one treated.
TECHNICAL OPTIONS
There are several technical options for implantation of DBS systems. Stereotactic procedures rely on co-registration of preoperative imaging with external and internal fiducials, or points of reference. Targeting of the intended structures is performed by combining direct and indirect methods. Direct methods rely on identification of the target structures with imaging, such as visualization of the STN and GPi on preoperative magnetic resonance imaging (MRI). Indirect targeting relies on cadaveric anatomic atlases and coordinate systems that infer the location of the intended structures in relation to anatomical points of reference.
Frame-based systems
Frameless systems
The key advantage of the frameless system over the frame-based system is greater mobility of the head. Another important advantage is easier access to the airway, should an emergency situation occur. In our practice, patients with experience of both frameless and frame-based systems did not report significantly less discomfort with the frameless system.
The frameless system also has disadvantages, including less secure fixation of the head, which can add risk to the procedure. In addition, because of its lightweight, plastic construction, it provides less robust support to the instrumentation entering the brain than do metallic head frames and, in some cases, there is less flexibility for adjusting targets if needed during surgery. In addition, frameless systems are nonreusable and represent a substantial additional cost.
Microelectrode recording
Physiologic verification of anatomic targets identified by imaging can be accomplished with microelectrode recording (MER). This technique involves placing fine, high-impedance electrodes through the target area, so that anatomic structures can be recognized by characteristic electrical activity of individual neurons or groups of neurons. The locations of the structures are identified and the lengths of the electrode trajectories through the different structures—as well as the gaps between these structures—are recorded. The distances are then compared with the anatomy and a best-fit model is created to infer the location of the trajectory in the target area. Additional MER penetrations are made in order to further delineate the anatomy. Once a location for implantation has been selected, the DBS lead is inserted into the target area.
Electrode implantation
Lead implantation is often performed under fluoroscopic guidance in order to ensure accuracy and stability. When implanted, the electrode may cause a microlesional effect, manifested by transient improvement in symptoms.
The DBS leads are then connected to external pulse generators and assessed for clinical benefits and side effects. Amplitude, pulse width, and frequency are adjusted to test the therapeutic window of stimulation (clinical improvement thresholds versus side effect thresholds). Some PD patients develop dyskinesia during test stimulation, which may be a positive indicator for lead location. If good effects and a therapeutic window are observed, the location of the lead is considered to be satisfactory and the procedure is completed.
Pulse generator implantation
During the final step of surgery, performed under general anesthesia, the pulse generator is implanted. The extension cable that connects the DBS lead to the implantable pulse generator is tunneled subcutaneously, connecting the DBS lead to the pulse generator in the chest.
Intraoperative, real-time MRI stereotaxis
Real-time intraoperative MRI has become available for DBS implantation with devices recently cleared for use by the FDA. The procedure, typically performed in a diagnostic MRI suite, uses MR images acquired during surgery to guide DBS lead implantation in the target area and to verify implantation accuracy.8
- Rezai AR, Machado AG, Azmi H, Kubu C, Boulis NM. Surgery for movement disorders. Neurosurg 2008; 62(SHC suppl 2):SHC809–SHC839.
- Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 2003; 349:1925–1934.
- Machado A, Fernandez HH, Deogaonkar M. Deep brain stimulation: what can patients expect from it? Cleve Clin J Med 2012; 79:113–120.
- Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 2005; 352:459–467.
- Kupsch A, Benecke R, Müller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006; 355:1978–1990.
- Gruber D, Trottenberg T, Kivi A, et al. Long-term effects of pallidal deep brain stimulation in tardive dystonia. Neurology 2009; 73:53–58.
- Gross RE, Sharan AD, Benabid AL. Deep brain stimulation for Parkinson’s disease: surgical technique and perioperative management. Mov Disord 2006; 21( suppl 14):S247–S258.
- Starr PA, Martin AJ, Ostrem JL, et al. Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: technique and application accuracy. J Neurosurg 2010; 112:479–490.
Implantation of a deep brain stimulator is the most common surgical procedure performed in the United States and industrialized world for the management of advanced movement disorders. These procedures are US Food and Drug Administration (FDA)–approved for the management of the symptoms of Parkinson disease (PD) and essential tremor. Deep brain stimulation (DBS) is also approved for managing primary generalized dystonia and torticollis under a humanitarian device exemption.
Deep brain stimulation has largely replaced ablative procedures such as thalamotomy and pallidotomy. While ablative procedures can be effective for the symptoms of movement disorders, they cause a permanent lesion in the targeted nuclei and are therefore not reversible. DBS is considered safer because it can be adjusted over time and the location of the leads can be revised.1 On the other hand, regular maintenance of implanted hardware may be considered a disadvantage of DBS.
HARDWARE AND TARGETS
While ablative procedures do not require implantable hardware, DBS consists of permanently implanted neurostimulation systems. The battery-powered pulse generators typically last for several years but require multiple replacements during a lifetime. In addition, if other hardware components fail, surgical revision may be required to maintain treatment efficacy. Surgery involving implantation of hardware carries a higher risk of infection than does a nonimplantation procedure. If infections occur, removal of the hardware is often required, with reimplantation performed after the infection clears. In addition, the expense of DBS hardware may limit availability in some cases.
Three components
Target nuclei
Several nodes or nuclei can serve as targets for DBS. In patients with PD, the most common surgical target is the subthalamic nucleus (STN), either unilaterally or bilaterally.2 The globus pallidus pars interna (GPi) is also a viable target and is preferred for some patients with PD. The most common target for managing essential tremor is the ventral intermediate nucleus (VIM) of the thalamus, which can also be the target of choice for patients with tremor-predominant PD. However, the GPi and STN are usually preferred over the VIM in patients with PD because stimulation of these targets can relieve symptoms other than tremor, such as rigidity and bradykinesia. Bilateral stimulation of the GPi is the most frequent approach in patients with generalized torsion dystonia and torticollis, although the STN and thalamic nuclei (off-label) are also considered options.
PATIENT SELECTION
Patients are evaluated in our center at Cleveland Clinic by a multidisciplinary team that includes a movement disorder neurologist, a subspecialized neurosurgeon, a movement disorder neuropsychologist, and a psychiatrist with special interest in the behavioral comorbidities of movement disorders.3 Neuroimaging is included in this assessment. We have also included physical therapy as part of the initial evaluation in order to gain insight into the patient’s limitations and develop rehabilitation strategies that may enhance the outcomes of surgery or provide alternatives should surgery not be indicated. This evaluation provides extensive data that are then reviewed by the team in a conference dedicated to discussing candidacy for DBS or options for managing the symptoms of advanced movement disorders. Behavioral and cognitive issues are assessed in detail and, in our experience, are the most common reasons for not recommending DBS.
An important part of the evaluation of patients with PD is a formal test with rating of the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS) with the patient off medications for 8 to 12 hours and then after a test dose of levodopa. At our center, this off/on test is videotaped so that the responsiveness of individual symptoms to levodopa can be reviewed later in conference.
Risk of cognitive decline
While DBS is considered safe and effective, there is a risk of cognitive decline in some patients. In most patients, long-term stimulation-related cognitive decline may be detected with formal measures but is not clinically significant and is outweighed by the motor and quality-of-life benefits of surgery. In some patients, long-term cognitive decline can be significant and can limit function. Cognitive neuropsychologic testing provides valuable information in this regard. Patients with preserved cognitive function seldom experience significant decline with DBS while those with substantial baseline impairment are thought to be at greater risk. Patients who meet criteria for dementia are usually not considered candidates for DBS, but exceptions exist. Transient perioperative cognitive difficulties are more common than persistent deficits, and typically resolve within a few weeks (see “Complications of deep brain stimulation”).
Benefits in Parkinson disease
Deep brain stimulation can address several symptoms of PD but with varying effects. Tremor, rigidity, and bradykinesia usually improve substantially. Gait has a more variable response, and balance is typically refractory. A general rule is that symptoms that improve with a single dose of levodopa should also improve with DBS. (Tremor, however, will most often respond to DBS even if refractory to medication.) Good candidates for surgery typically have a greater than 30% improvement in UPDRS motor score with levodopa challenge, but sometimes, improvement in the total score is less informative than evaluation of the effects of levodopa on particular symptoms. Treatment effects can be compared with the patient’s expectations for surgery in order to infer whether the goals for symptom improvement are realistic.
Treatment outcomes depend on etiology
After programming, DBS can provide PD symptom control similar to that of medication “on time,” but with fewer on-off fluctuations and less on-time dyskinesia. Good surgical candidates are patients who once responded well to dopaminergic medications but who, after several years with the disease, present with increased duration of “off time,” unpredictable duration of on time, and medication side effects such as on-time dyskinesia. Patients who do not respond well to levodopa even in subscores of the UPDRS may not be good candidates for DBS, and in some cases the diagnosis itself needs to be reviewed.
Deep brain stimulation can improve quality of life and alleviate symptoms of essential tremor. Tremor control is best for the upper extremities and tends to be better for distal tremors than for proximal ones. Patients who are good candidates for surgery often have severe tremors. A substantial improvement in these symptoms often has a dramatic, positive effect on work and quality of life. In some patients, surgery is considered for mild tremor if it seriously disrupts the patient’s lifestyle or occupation and cannot be well controlled with medications. Often, in these cases, tremor that appears relatively mild to the examiner is significantly limiting for the patient.
Very severe and proximal tremor is more refractory, though it may also improve. The changes can be well documented with objective measures. In these cases, however, residual tremor can still be moderate to severe and can be functionally limiting. Head or vocal tremors are typically refractory. They may be improved with bilateral implantation, but this cannot be accurately predicted. Patients who present with head-only or head-predominant tremor are thought to be less likely to benefit than those with limb tremor. Nonetheless, tremors of the head can severely impair quality of life. Because there are few other treatment options, some patients choose DBS with the understanding that the outcome is uncertain and the benefit may be limited.
Tremor resulting from multiple sclerosis or other causes can be medically refractory and disabling. In our experience, DBS can be an off-label option for managing secondary tremors and good outcomes have been observed. However, outcomes are much less predictable and tremor control less effective than in patients with essential tremor.
Patients with primary generalized dystonia can be considered candidates for DBS and may experience improved symptom control and quality of life.4 Patients with the DYT1 mutation are more likely to respond well to DBS, as are those with other forms of primary generalized dystonia. In contrast to that seen in patients with PD and tremor, symptomatic improvement is frequently not observed during intraoperative testing. Several months of stimulation and programming may be required before significant improvements are detected.5 Surgery can also be considered for off-label use in the treatment of patients with secondary dystonia—such as that following injury or associated with cerebral palsy—but outcomes are less predictable and usually more limited. A possible exception may be seen in cases of tardive dystonia, for which there is increasing evidence6 for the effectiveness of DBS. This remains an off-label use of DBS.
Realistic expectations
An important aspect of the multidisciplinary evaluation includes a discussion of the expectations for surgery, the risks, and the requirements for postoperative care. As discussed above, DBS is reversible and adjustable, so outcomes depend not only on accurate implantation of the hardware but also on postoperative programming. Also, monitoring and maintenance of the implanted hardware are required in these patients. It is important that patients and families appreciate the fact that specialized, long-term postoperative follow-up is as much a part of the treatment as is the implantation itself.

UNILATERAL VERSUS BILATERAL DBS
Most patients with generalized dystonia undergo bilateral DBS. However, patients with PD or essential tremor may receive bilateral, staged, or unilateral implants. Some patients with PD present with either near-complete predominance of symptoms on one side or with symptoms that affect mostly the dominant extremity. In these patients, unilateral implantation is often recommended because it has less risk than the bilateral approach and may be sufficient to address the most limiting symptoms.
As the disease advances, an additional surgery may be required to accomplish bilateral symptom control. Nevertheless, we do not routinely recommend preventive implantation because it is not known whether second-side symptoms will become severe enough to require it. This strategy allows for deferring surgical risk, which is in itself advantageous. In our experience, bilateral implantation is often recommended to PD patients who present with symptoms such as freezing of gait.
Patients who have essential tremor often present with bilateral symptoms. Although many patients will indicate that they need symptom relief on both upper extremities in order to perform activities of daily living, our practice is to recommend surgery on one side at first and to suggest the patient consider contralateral implantation after weeks or months. Bilateral implantation may carry a risk for dysarthria and the risk is thought to be reduced if bilateral procedures are staged. Although high rates of dysarthria have been reported following bilateral surgery for tremor, its occurrence has been infrequent in our experience with bilateral staged DBS. Benefits of treating tremor in the dominant extremity usually exceed those of treating nondominant tremor, so most patients prefer that the dominant side be the first one treated.
TECHNICAL OPTIONS
There are several technical options for implantation of DBS systems. Stereotactic procedures rely on co-registration of preoperative imaging with external and internal fiducials, or points of reference. Targeting of the intended structures is performed by combining direct and indirect methods. Direct methods rely on identification of the target structures with imaging, such as visualization of the STN and GPi on preoperative magnetic resonance imaging (MRI). Indirect targeting relies on cadaveric anatomic atlases and coordinate systems that infer the location of the intended structures in relation to anatomical points of reference.
Frame-based systems
Frameless systems
The key advantage of the frameless system over the frame-based system is greater mobility of the head. Another important advantage is easier access to the airway, should an emergency situation occur. In our practice, patients with experience of both frameless and frame-based systems did not report significantly less discomfort with the frameless system.
The frameless system also has disadvantages, including less secure fixation of the head, which can add risk to the procedure. In addition, because of its lightweight, plastic construction, it provides less robust support to the instrumentation entering the brain than do metallic head frames and, in some cases, there is less flexibility for adjusting targets if needed during surgery. In addition, frameless systems are nonreusable and represent a substantial additional cost.
Microelectrode recording
Physiologic verification of anatomic targets identified by imaging can be accomplished with microelectrode recording (MER). This technique involves placing fine, high-impedance electrodes through the target area, so that anatomic structures can be recognized by characteristic electrical activity of individual neurons or groups of neurons. The locations of the structures are identified and the lengths of the electrode trajectories through the different structures—as well as the gaps between these structures—are recorded. The distances are then compared with the anatomy and a best-fit model is created to infer the location of the trajectory in the target area. Additional MER penetrations are made in order to further delineate the anatomy. Once a location for implantation has been selected, the DBS lead is inserted into the target area.
Electrode implantation
Lead implantation is often performed under fluoroscopic guidance in order to ensure accuracy and stability. When implanted, the electrode may cause a microlesional effect, manifested by transient improvement in symptoms.
The DBS leads are then connected to external pulse generators and assessed for clinical benefits and side effects. Amplitude, pulse width, and frequency are adjusted to test the therapeutic window of stimulation (clinical improvement thresholds versus side effect thresholds). Some PD patients develop dyskinesia during test stimulation, which may be a positive indicator for lead location. If good effects and a therapeutic window are observed, the location of the lead is considered to be satisfactory and the procedure is completed.
Pulse generator implantation
During the final step of surgery, performed under general anesthesia, the pulse generator is implanted. The extension cable that connects the DBS lead to the implantable pulse generator is tunneled subcutaneously, connecting the DBS lead to the pulse generator in the chest.
Intraoperative, real-time MRI stereotaxis
Real-time intraoperative MRI has become available for DBS implantation with devices recently cleared for use by the FDA. The procedure, typically performed in a diagnostic MRI suite, uses MR images acquired during surgery to guide DBS lead implantation in the target area and to verify implantation accuracy.8
Implantation of a deep brain stimulator is the most common surgical procedure performed in the United States and industrialized world for the management of advanced movement disorders. These procedures are US Food and Drug Administration (FDA)–approved for the management of the symptoms of Parkinson disease (PD) and essential tremor. Deep brain stimulation (DBS) is also approved for managing primary generalized dystonia and torticollis under a humanitarian device exemption.
Deep brain stimulation has largely replaced ablative procedures such as thalamotomy and pallidotomy. While ablative procedures can be effective for the symptoms of movement disorders, they cause a permanent lesion in the targeted nuclei and are therefore not reversible. DBS is considered safer because it can be adjusted over time and the location of the leads can be revised.1 On the other hand, regular maintenance of implanted hardware may be considered a disadvantage of DBS.
HARDWARE AND TARGETS
While ablative procedures do not require implantable hardware, DBS consists of permanently implanted neurostimulation systems. The battery-powered pulse generators typically last for several years but require multiple replacements during a lifetime. In addition, if other hardware components fail, surgical revision may be required to maintain treatment efficacy. Surgery involving implantation of hardware carries a higher risk of infection than does a nonimplantation procedure. If infections occur, removal of the hardware is often required, with reimplantation performed after the infection clears. In addition, the expense of DBS hardware may limit availability in some cases.
Three components
Target nuclei
Several nodes or nuclei can serve as targets for DBS. In patients with PD, the most common surgical target is the subthalamic nucleus (STN), either unilaterally or bilaterally.2 The globus pallidus pars interna (GPi) is also a viable target and is preferred for some patients with PD. The most common target for managing essential tremor is the ventral intermediate nucleus (VIM) of the thalamus, which can also be the target of choice for patients with tremor-predominant PD. However, the GPi and STN are usually preferred over the VIM in patients with PD because stimulation of these targets can relieve symptoms other than tremor, such as rigidity and bradykinesia. Bilateral stimulation of the GPi is the most frequent approach in patients with generalized torsion dystonia and torticollis, although the STN and thalamic nuclei (off-label) are also considered options.
PATIENT SELECTION
Patients are evaluated in our center at Cleveland Clinic by a multidisciplinary team that includes a movement disorder neurologist, a subspecialized neurosurgeon, a movement disorder neuropsychologist, and a psychiatrist with special interest in the behavioral comorbidities of movement disorders.3 Neuroimaging is included in this assessment. We have also included physical therapy as part of the initial evaluation in order to gain insight into the patient’s limitations and develop rehabilitation strategies that may enhance the outcomes of surgery or provide alternatives should surgery not be indicated. This evaluation provides extensive data that are then reviewed by the team in a conference dedicated to discussing candidacy for DBS or options for managing the symptoms of advanced movement disorders. Behavioral and cognitive issues are assessed in detail and, in our experience, are the most common reasons for not recommending DBS.
An important part of the evaluation of patients with PD is a formal test with rating of the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS) with the patient off medications for 8 to 12 hours and then after a test dose of levodopa. At our center, this off/on test is videotaped so that the responsiveness of individual symptoms to levodopa can be reviewed later in conference.
Risk of cognitive decline
While DBS is considered safe and effective, there is a risk of cognitive decline in some patients. In most patients, long-term stimulation-related cognitive decline may be detected with formal measures but is not clinically significant and is outweighed by the motor and quality-of-life benefits of surgery. In some patients, long-term cognitive decline can be significant and can limit function. Cognitive neuropsychologic testing provides valuable information in this regard. Patients with preserved cognitive function seldom experience significant decline with DBS while those with substantial baseline impairment are thought to be at greater risk. Patients who meet criteria for dementia are usually not considered candidates for DBS, but exceptions exist. Transient perioperative cognitive difficulties are more common than persistent deficits, and typically resolve within a few weeks (see “Complications of deep brain stimulation”).
Benefits in Parkinson disease
Deep brain stimulation can address several symptoms of PD but with varying effects. Tremor, rigidity, and bradykinesia usually improve substantially. Gait has a more variable response, and balance is typically refractory. A general rule is that symptoms that improve with a single dose of levodopa should also improve with DBS. (Tremor, however, will most often respond to DBS even if refractory to medication.) Good candidates for surgery typically have a greater than 30% improvement in UPDRS motor score with levodopa challenge, but sometimes, improvement in the total score is less informative than evaluation of the effects of levodopa on particular symptoms. Treatment effects can be compared with the patient’s expectations for surgery in order to infer whether the goals for symptom improvement are realistic.
Treatment outcomes depend on etiology
After programming, DBS can provide PD symptom control similar to that of medication “on time,” but with fewer on-off fluctuations and less on-time dyskinesia. Good surgical candidates are patients who once responded well to dopaminergic medications but who, after several years with the disease, present with increased duration of “off time,” unpredictable duration of on time, and medication side effects such as on-time dyskinesia. Patients who do not respond well to levodopa even in subscores of the UPDRS may not be good candidates for DBS, and in some cases the diagnosis itself needs to be reviewed.
Deep brain stimulation can improve quality of life and alleviate symptoms of essential tremor. Tremor control is best for the upper extremities and tends to be better for distal tremors than for proximal ones. Patients who are good candidates for surgery often have severe tremors. A substantial improvement in these symptoms often has a dramatic, positive effect on work and quality of life. In some patients, surgery is considered for mild tremor if it seriously disrupts the patient’s lifestyle or occupation and cannot be well controlled with medications. Often, in these cases, tremor that appears relatively mild to the examiner is significantly limiting for the patient.
Very severe and proximal tremor is more refractory, though it may also improve. The changes can be well documented with objective measures. In these cases, however, residual tremor can still be moderate to severe and can be functionally limiting. Head or vocal tremors are typically refractory. They may be improved with bilateral implantation, but this cannot be accurately predicted. Patients who present with head-only or head-predominant tremor are thought to be less likely to benefit than those with limb tremor. Nonetheless, tremors of the head can severely impair quality of life. Because there are few other treatment options, some patients choose DBS with the understanding that the outcome is uncertain and the benefit may be limited.
Tremor resulting from multiple sclerosis or other causes can be medically refractory and disabling. In our experience, DBS can be an off-label option for managing secondary tremors and good outcomes have been observed. However, outcomes are much less predictable and tremor control less effective than in patients with essential tremor.
Patients with primary generalized dystonia can be considered candidates for DBS and may experience improved symptom control and quality of life.4 Patients with the DYT1 mutation are more likely to respond well to DBS, as are those with other forms of primary generalized dystonia. In contrast to that seen in patients with PD and tremor, symptomatic improvement is frequently not observed during intraoperative testing. Several months of stimulation and programming may be required before significant improvements are detected.5 Surgery can also be considered for off-label use in the treatment of patients with secondary dystonia—such as that following injury or associated with cerebral palsy—but outcomes are less predictable and usually more limited. A possible exception may be seen in cases of tardive dystonia, for which there is increasing evidence6 for the effectiveness of DBS. This remains an off-label use of DBS.
Realistic expectations
An important aspect of the multidisciplinary evaluation includes a discussion of the expectations for surgery, the risks, and the requirements for postoperative care. As discussed above, DBS is reversible and adjustable, so outcomes depend not only on accurate implantation of the hardware but also on postoperative programming. Also, monitoring and maintenance of the implanted hardware are required in these patients. It is important that patients and families appreciate the fact that specialized, long-term postoperative follow-up is as much a part of the treatment as is the implantation itself.

UNILATERAL VERSUS BILATERAL DBS
Most patients with generalized dystonia undergo bilateral DBS. However, patients with PD or essential tremor may receive bilateral, staged, or unilateral implants. Some patients with PD present with either near-complete predominance of symptoms on one side or with symptoms that affect mostly the dominant extremity. In these patients, unilateral implantation is often recommended because it has less risk than the bilateral approach and may be sufficient to address the most limiting symptoms.
As the disease advances, an additional surgery may be required to accomplish bilateral symptom control. Nevertheless, we do not routinely recommend preventive implantation because it is not known whether second-side symptoms will become severe enough to require it. This strategy allows for deferring surgical risk, which is in itself advantageous. In our experience, bilateral implantation is often recommended to PD patients who present with symptoms such as freezing of gait.
Patients who have essential tremor often present with bilateral symptoms. Although many patients will indicate that they need symptom relief on both upper extremities in order to perform activities of daily living, our practice is to recommend surgery on one side at first and to suggest the patient consider contralateral implantation after weeks or months. Bilateral implantation may carry a risk for dysarthria and the risk is thought to be reduced if bilateral procedures are staged. Although high rates of dysarthria have been reported following bilateral surgery for tremor, its occurrence has been infrequent in our experience with bilateral staged DBS. Benefits of treating tremor in the dominant extremity usually exceed those of treating nondominant tremor, so most patients prefer that the dominant side be the first one treated.
TECHNICAL OPTIONS
There are several technical options for implantation of DBS systems. Stereotactic procedures rely on co-registration of preoperative imaging with external and internal fiducials, or points of reference. Targeting of the intended structures is performed by combining direct and indirect methods. Direct methods rely on identification of the target structures with imaging, such as visualization of the STN and GPi on preoperative magnetic resonance imaging (MRI). Indirect targeting relies on cadaveric anatomic atlases and coordinate systems that infer the location of the intended structures in relation to anatomical points of reference.
Frame-based systems
Frameless systems
The key advantage of the frameless system over the frame-based system is greater mobility of the head. Another important advantage is easier access to the airway, should an emergency situation occur. In our practice, patients with experience of both frameless and frame-based systems did not report significantly less discomfort with the frameless system.
The frameless system also has disadvantages, including less secure fixation of the head, which can add risk to the procedure. In addition, because of its lightweight, plastic construction, it provides less robust support to the instrumentation entering the brain than do metallic head frames and, in some cases, there is less flexibility for adjusting targets if needed during surgery. In addition, frameless systems are nonreusable and represent a substantial additional cost.
Microelectrode recording
Physiologic verification of anatomic targets identified by imaging can be accomplished with microelectrode recording (MER). This technique involves placing fine, high-impedance electrodes through the target area, so that anatomic structures can be recognized by characteristic electrical activity of individual neurons or groups of neurons. The locations of the structures are identified and the lengths of the electrode trajectories through the different structures—as well as the gaps between these structures—are recorded. The distances are then compared with the anatomy and a best-fit model is created to infer the location of the trajectory in the target area. Additional MER penetrations are made in order to further delineate the anatomy. Once a location for implantation has been selected, the DBS lead is inserted into the target area.
Electrode implantation
Lead implantation is often performed under fluoroscopic guidance in order to ensure accuracy and stability. When implanted, the electrode may cause a microlesional effect, manifested by transient improvement in symptoms.
The DBS leads are then connected to external pulse generators and assessed for clinical benefits and side effects. Amplitude, pulse width, and frequency are adjusted to test the therapeutic window of stimulation (clinical improvement thresholds versus side effect thresholds). Some PD patients develop dyskinesia during test stimulation, which may be a positive indicator for lead location. If good effects and a therapeutic window are observed, the location of the lead is considered to be satisfactory and the procedure is completed.
Pulse generator implantation
During the final step of surgery, performed under general anesthesia, the pulse generator is implanted. The extension cable that connects the DBS lead to the implantable pulse generator is tunneled subcutaneously, connecting the DBS lead to the pulse generator in the chest.
Intraoperative, real-time MRI stereotaxis
Real-time intraoperative MRI has become available for DBS implantation with devices recently cleared for use by the FDA. The procedure, typically performed in a diagnostic MRI suite, uses MR images acquired during surgery to guide DBS lead implantation in the target area and to verify implantation accuracy.8
- Rezai AR, Machado AG, Azmi H, Kubu C, Boulis NM. Surgery for movement disorders. Neurosurg 2008; 62(SHC suppl 2):SHC809–SHC839.
- Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 2003; 349:1925–1934.
- Machado A, Fernandez HH, Deogaonkar M. Deep brain stimulation: what can patients expect from it? Cleve Clin J Med 2012; 79:113–120.
- Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 2005; 352:459–467.
- Kupsch A, Benecke R, Müller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006; 355:1978–1990.
- Gruber D, Trottenberg T, Kivi A, et al. Long-term effects of pallidal deep brain stimulation in tardive dystonia. Neurology 2009; 73:53–58.
- Gross RE, Sharan AD, Benabid AL. Deep brain stimulation for Parkinson’s disease: surgical technique and perioperative management. Mov Disord 2006; 21( suppl 14):S247–S258.
- Starr PA, Martin AJ, Ostrem JL, et al. Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: technique and application accuracy. J Neurosurg 2010; 112:479–490.
- Rezai AR, Machado AG, Azmi H, Kubu C, Boulis NM. Surgery for movement disorders. Neurosurg 2008; 62(SHC suppl 2):SHC809–SHC839.
- Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 2003; 349:1925–1934.
- Machado A, Fernandez HH, Deogaonkar M. Deep brain stimulation: what can patients expect from it? Cleve Clin J Med 2012; 79:113–120.
- Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 2005; 352:459–467.
- Kupsch A, Benecke R, Müller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006; 355:1978–1990.
- Gruber D, Trottenberg T, Kivi A, et al. Long-term effects of pallidal deep brain stimulation in tardive dystonia. Neurology 2009; 73:53–58.
- Gross RE, Sharan AD, Benabid AL. Deep brain stimulation for Parkinson’s disease: surgical technique and perioperative management. Mov Disord 2006; 21( suppl 14):S247–S258.
- Starr PA, Martin AJ, Ostrem JL, et al. Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: technique and application accuracy. J Neurosurg 2010; 112:479–490.
Deep brain stimulation: What can patients expect from it?
Deep brain stimulation is an important therapy for Parkinson disease and other movement disorders. It involves implantation of a pulse generator that can be adjusted by telemetry and can be activated and deactivated by clinicians and patients. It is therefore also a good investigational tool, allowing for double-blind, sham-controlled clinical trials by testing the effects of the stimulation with optimal settings compared with no stimulation.
This article will discuss the approved indications for deep brain stimulation (particularly for managing movement disorders), the benefits that can be expected, the risks, the complications, the maintenance required, how candidates for this treatment are evaluated, and the surgical procedure for implantation of the devices.
DEVICE SIMILAR TO HEART PACEMAKERS
The deep brain stimulation system must be programmed by a physician or midlevel practitioner by observing a symptom and then changing the applied settings to the pulse generator until the symptom improves. This can be a very time-consuming process.
In contrast to heart pacemakers, which run at low frequencies, the brain devices for movement disorders are almost always set to a high frequency, greater than 100 Hz. For this reason, they consume more energy and need larger batteries than those in modern heart pacemakers.
The batteries in these generators typically last 3 to 5 years and are replaced in an outpatient procedure. Newer, smaller, rechargeable devices are expected to last longer but require more maintenance and care by patients, who have to recharge them at home periodically.
INDICATIONS FOR DEEP BRAIN STIMULATION
Deep brain stimulation is approved by the US Food and Drug Administration (FDA) for specific indications:
- Parkinson disease
- Essential tremor
- Primary dystonia (under a humanitarian device exemption)
- Intractable obsessive-compulsive disorder (also under a humanitarian device exemption). We will not discuss this indication further in this paper.
For each of these conditions, deep brain stimulation is considered when nonsurgical management has failed, as is the case for most functional neurosurgical treatments.
Investigations under way in other disorders
Several studies of deep brain stimulation are currently in progress under FDA-approved investigational device exemptions. Some, with funding from industry, are exploring its use in neuropsychiatric conditions other than parkinsonism. Two large clinical trials are evaluating its use for treatment-refractory depression, a common problem and a leading cause of disability in the industrialized world. Multiple investigators are also exploring novel uses of this technology in disorders ranging from obsessive-compulsive disorder to epilepsy.
Investigation is also under way at Cleveland Clinic in a federally funded, prospective, randomized clinical trial of deep brain stimulation for patients with thalamic pain syndrome. The primary hypothesis is that stimulation of the ventral striatal and ventral capsular area will modulate the affective component of this otherwise intractable pain syndrome, reducing pain-related disability and improving quality of life.
DEEP BRAIN STIMULATION VS ABLATION
Before deep brain stimulation became available, the only surgical options for patients with advanced Parkinson disease, tremor, or dystonia were ablative procedures such as pallidotomy (ablation of part of the globus pallidus) and thalamotomy (ablation of part of the thalamus). These procedures had been well known for several decades but fell out of favor when levodopa became available in the 1960s and revolutionized the medical treatment of Parkinson disease.
Surgery for movement disorders, in particular Parkinson disease, had a rebirth in the late 1980s when the limitations and complications associated with the pharmacologic management of Parkinson disease became increasingly evident. Ablative procedures are still used to treat advanced Parkinson disease, but much less commonly in industrialized countries.
Although pallidotomy and thalamotomy can have excellent results, they are not as safe as deep brain stimulation, which has the advantage of being reversible, modulating the function of an area rather than destroying it. Any unwanted effect can be immediately altered or reversed, unlike ablative procedures, in which any change is permanent. In addition, deep brain stimulation is adjustable, and the settings can be optimized as the disease progresses over the years.
Ablative procedures can be risky when performed bilaterally, while deep brain stimulation is routinely done on both hemispheres for patients with bilateral symptoms.
Although deep brain stimulation is today’s surgical treatment of choice, it is not perfect. It has the disadvantage of requiring lifelong maintenance of the hardware, for which the patient remains dependent on a medical center. Patients are usually seen more often at the specialized center in the first few months after surgery for optimization of programming and titration of drugs. (During this time, most patients see a gradual, substantial reduction in medication intake.) They are then followed by their physician and visit the center less often for monitoring of disease status and for further adjustments to the stimulator.
Most patients, to date, receive nonrechargeable pulse generators. As mentioned above, the batteries in these devices typically last 3 to 5 years. Preferably, batteries are replaced before they are completely depleted, to avoid interruption of therapy. Periodic visits to the center allow clinicians to estimate battery expiration ahead of time and plan replacements accordingly.
Rechargeable pulse generators have been recently introduced and are expected to last up to 9 years. They are an option for patients who can comply with the requirements for periodic home recharging of the hardware.
Patients are given a remote control so that they can turn the device on or off and check its status. Most patients keep it turned on all the time, although some turn it off at night to save battery life.
WHAT CAN PARKINSON PATIENTS EXPECT FROM THIS THERAPY?
Typically, some parkinsonian symptoms predominate over others, although some patients with advanced disease present with a severe combination of multiple disabling symptoms. Deep brain stimulation is best suited to address some of the cardinal motor symptoms, particularly tremor, rigidity, and bradykinesia, and motor fluctuations such as “wearing off” and dyskinesia.
Improvement in some motor symptoms
As a general rule, appendicular symptoms such as limb tremor and rigidity are more responsive to this therapy than axial symptoms such as gait and balance problems, but some patients experience improvement in gait as well. Other symptoms, such as swallowing or urinary symptoms, are seldom helped.
Although deep brain stimulation can help manage key motor symptoms and improve quality of life, it does not cure Parkinson disease. Also, there is no evidence to date that it slows disease progression, although this is a topic of ongoing investigation.
Fewer motor fluctuations
A common complaint of patients with advanced Parkinson disease is frequent—and often unpredictable—fluctuations between the “on” state (ie, when the effects of the patient’s levodopa therapy are apparent) and the “off” state (ie, when the levodopa doesn’t seem to be working). Sometimes, in the on state, patients experience involuntary choreic or ballistic movements, called dyskinesias. They also complain that the on time progressively lasts shorter and the day is spent alternating between shorter on states (during which the patient may be dyskinetic) and longer off states, limiting the patient’s independence and quality of life.
Deep brain stimulation can help patients prolong the on time while reducing the amplitude of these fluctuations so that the symptoms are not as severe in the off time and dyskinesias are reduced in the on time.
Some patients undergo deep brain stimulation primarily for managing the adverse effects of levodopa rather than for controlling the symptoms of the disease itself. While these patients need levodopa to address the disabling symptoms of the disease, they also present a greater sensitivity for developing levodopa-induced dyskinesias, quickly fluctuating from a lack of movement (the off state) to a state of uncontrollable movements (during the on state).
Deep brain stimulation typically allows the dosage of levodopa to be significantly reduced and gives patients more on time with fewer side effects and less fluctuation between the on and off states.
Response to levodopa predicts deep brain stimulation’s effects
Whether a patient is likely to be helped by deep brain stimulation can be tested with reasonable predictability by giving a single therapeutic dose of levodopa after the patient has been free of the drug for 12 hours. If there is an obvious difference on objective quantitative testing between the off and on states with a single dose, the patient is likely to benefit from deep brain stimulation. Those who do not respond well or are known to have never been well controlled by levodopa are likely poor candidates.
The test is also used as an indicator of whether the patient’s gait can be improved. Patients whose gait is substantially improved by levodopa, even for only a brief period of time, have a better chance of experiencing improvement in this domain with deep brain stimulation than those who do not show any gait improvement.
An important and notable exception to this rule is tremor control. Even Parkinson patients who do not experience significant improvement in tremor with levodopa (ie, who have medication-resistant tremors) are still likely to benefit from deep brain stimulation. Overall, tremor is the symptom that is most consistently improved with deep brain stimulation.
Results of clinical trials
Several clinical trials have demonstrated that deep brain stimulation plus medication works better than medications alone for advanced Parkinson disease.
Deuschl et al1 conducted a randomized trial in 156 patients with advanced Parkinson disease. Patients receiving subthalamic deep brain stimulation plus medication had significantly greater improvement in motor symptoms as measured by the Unified Parkinson’s Disease Rating Scale as well as in quality-of-life measures than patients receiving medications only.
Krack et al2 reported on the outcomes of 49 patients with advanced Parkinson disease who underwent deep brain stimulation and then were prospectively followed. At 5 years, motor function had improved by approximately 55% from baseline, activities-of-daily-living scores had improved by 49%, and patients continued to need significantly less levodopa and to experience less drug-induced dyskinesia.
Complications related to deep brain stimulation occurred in both studies, including two large intracerebral hemorrhages, one of which was fatal.
Weight gain. During the first 3 months after the device was implanted, patients tended to gain weight (mean 3 kg, maximum 5 kg). Although weight gain is considered an adverse effect, many patients are quite thin by the time they are candidates for deep brain stimulation, and in such cases gaining lean weight can be a benefit.
Patients with poorly controlled Parkinson disease lose weight for several reasons: increased calorie expenditure from shaking and excessive movements; diet modification and protein restriction for some patients who realize that protein competes with levodopa absorption; lack of appetite due to depression or from poor taste sensation (due to anosmia); and decreased overall food consumption due to difficulty swallowing.
DEEP BRAIN STIMULATION FOR ESSENTIAL TREMOR
Essential tremor is more common than Parkinson disease, with a prevalence in the United States estimated at approximately 4,000 per 100,000 people older than 65 years.
The tremor is often bilateral and is characteristically an action tremor, but in many patients it also has a postural, and sometimes a resting, component. It is distinct from parkinsonian tremor, which is usually predominantly a resting tremor. The differential diagnosis includes tremors secondary to central nervous system degenerative disorders as well as psychogenic tremors.
Drinking alcohol tends to relieve essential tremors, a finding that can often be elicited in the patient’s history. Patients whose symptoms improve with an alcoholic beverage are more likely to have essential tremor than another diagnosis.
Response to deep brain stimulation
Most patients with essential tremor respond well to deep brain stimulation of the contralateral ventral intermedius thalamic nucleus.
Treatment is usually started unilaterally, usually aimed at alleviating tremor in the patient’s dominant upper extremity. In selected cases, preference is given to treating the nondominant extremity when it is more severely affected than the dominant extremity.
Implantation of a device on the second side is offered to some patients who continue to be limited in activity and quality of life due to tremor of the untreated extremity. Surgery of the second side can be more complicated than the initial unilateral procedure. In particular, some patients may present with dysarthria, although that seems to be less common in our experience than initially estimated.
In practice, patients with moderate tremors tend to have an excellent response to deep brain stimulation. For this particular indication, if the response is not satisfactory, the treating team tends to consider surgically revising the placement of the lead rather than considering the patient a nonresponder. Patients with very severe tremors may have some residual tremor despite substantial improvement in severity. In our experience, patients with a greater proximal component of tremor tend to have less satisfactory results.
For challenging cases, implantation of additional electrodes in the thalamus or in new targets currently under investigation is sometimes considered, although this is an off-label use.
Treatment of secondary tremors, such as poststroke tremor or tremor due to multiple sclerosis, is sometimes attempted with deep brain stimulation. This is also an off-label option but is considered in selected cases for quality-of-life management.
Patients with axial tremors such as head or voice tremor are less likely to be helped by deep brain stimulation.
DEEP BRAIN STIMULATION FOR PRIMARY DYSTONIA
Generalized dystonia is a less common but severely impairing movement disorder.
Deep brain stimulation is approved for primary dystonia under a humanitarian device exemption, a regulatory mechanism for less common conditions. Deep brain stimulation is an option for patients who have significant impairment related to dystonia and who have not responded to conservative management such as anticholinergic agents, muscle relaxants, benzodiazepines, levodopa, or combinations of these drugs. Surgery has been shown to be effective for patients with primary generalized dystonia, whether or not they tested positive for a dystonia-related gene such as DYT1.
Kupsch et al3 evaluated 40 patients with primary dystonia in a randomized controlled trial of pallidal (globus pallidus pars interna) active deep brain stimulation vs sham stimulation (in which the device was implanted but not activated) for 3 months. Treated patients improved significantly more than controls (39% vs 5%) in the Burke-Fahn- Marsden Dystonia Rating Scale (BFMDRS).4 Similar improvement was noted when those receiving sham stimulation were switched to active stimulation.
During long-term follow-up, the results were generally sustained, with substantial improvement from deep brain stimulation in all movement symptoms evaluated except for speech and swallowing. Unlike improvement in tremor, which is quickly evident during testing in the operating room, the improvement in dystonia occurs gradually, and it may take months for patients to notice a change. Similarly, if stimulation stops because of device malfunction or dead batteries, symptoms sometimes do not recur for weeks or months.
Deep brain stimulation is sometimes offered to patients with dystonia secondary to conditions such as cerebral palsy or trauma (an off-label use). Although benefits are less consistent, deep brain stimulation remains an option for these individuals, aimed at alleviating some of the disabling symptoms. In patients with cerebral palsy or other secondary dystonias, it is sometimes difficult to distinguish how much of the disability is related to spasticity vs dystonia. Deep brain stimulation aims to alleviate the dystonic component; the spasticity may be managed with other options such as intrathecal baclofen (Lioresal).
Patients with tardive dystonia, which is usually secondary to treatment with antipsychotic agents, have been reported to respond well to bilateral deep brain stimulation. Gruber et al5 reported on a series of nine patients with a mean follow-up of 41 months. Patients improved by a mean of approximately 74% on the BFMDRS after 3 to 6 months of deep brain stimulation compared with baseline. None of the patients presented with long-term adverse effects, and quality of life and disability scores also improved significantly.
CANDIDATES ARE EVALUATED BY A MULTIDISCIPLINARY TEAM
Cleveland Clinic conducts a comprehensive 2-day evaluation for patients being considered for deep brain stimulation surgery, including consultations with specialists in neurology, neurosurgery, neuropsychology, and psychiatry.
Patients with significant cognitive deficits—near or meeting the diagnostic criteria for dementia—are usually not recommended to have surgery for Parkinson disease. Deep brain stimulation is not aimed at alleviating cognitive issues related to Parkinson disease or other concomitant dementia. In addition, there is a risk that neurostimulation could further worsen cognitive function in the already compromised brain. Moreover, patients with significant abnormalities detected by neuroimaging may have their diagnosis reconsidered in some cases, and some patients may not be deemed ideal candidates for surgery.
An important part of the process is a discussion with the patient and family about the risks and the potential short-term and long-term benefits. Informed consent requires a good understanding of this equation. Patients are counseled to have realistic expectations about what the procedure can offer. Deep brain stimulation can help some of the symptoms of Parkinson disease but will not cure it, and there is no evidence to date that it reduces its progress. At 5 or 10 years after surgery, patients are expected to be worse overall than they were in the first year after surgery, because of disease progression. However, patients who receive this treatment are expected, in general, to be doing better 5 or 10 years later (or longer) than those who do not receive it.
In addition to the discussion about risks, benefits, and expectations, a careful discussion is also devoted to hardware maintenance, including how to change the batteries. Particularly, younger patients should be informed about the risk of breakage of the leads and the extension wire, as they are likely to outlive their implant. Patients and caregivers should be able to come to the specialized center should hardware malfunction occur.
Patients are also informed that after the system is implanted they cannot undergo magnetic resonance imaging (MRI) except of the head, performed with a specific head coil and under specific parameters. MRI of any other body part and with a body coil is contraindicated.
HOW THE DEVICE IS IMPLANTED
There are several options for implanting a deep brain stimulation device.
Implantation with the patient awake, using a stereotactic headframe
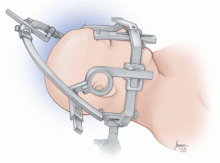
At Cleveland Clinic, we usually prefer implantation with a stereotactic headframe. The base or “halo” of the frame is applied to the head under local anesthesia, followed by imaging via computed tomography (Figure 1). Typically, the tomographic image is fused to a previously acquired MRI image, but the MRI is sometimes either initially performed or repeated on the day of surgery.
Patients are sedated for the beginning of the procedure, while the surgical team is opening the skin and drilling the opening in the skull for placement of the lead. The patient is awakened for placement of the electrodes, which is not painful.
Microelectrode recording is typically performed in order to refine the targeting based on the stereotactic coordinates derived from neuroimaging. Although cadaver atlases exist and provide a guide to the stereotactic localization of subcortical structures, they are not completely accurate in representing the brain anatomy of all patients.
By “listening” to cells and knowing their characteristic signals in specific areas, landmarks can be created, forming an individualized map of the patient’s brain target. Microelectrode recording is invasive and has risks, including the risk of a brain hemorrhage. It is routinely done in most specialized deep brain stimulation centers because it can provide better accuracy and precision in lead placement.
When the target has been located and refined by microelectrode recording, the permanent electrode is inserted. Fluoroscopy is usually used to verify the direction and stability of placement during the procedure.
An intraoperative test of the effects of deep brain stimulation is routinely performed to verify that some benefits can be achieved with the brain lead in its location, to determine the threshold for side effects, or both. For example, the patient may be asked to hold a cup as if trying to drink from it and to write or to draw a spiral on a clipboard to assess for improvements in tremor. Rigidity and bradykinesia can also be tested for improvements.
This intraoperative test is not aimed at assessing the best possible outcome of deep brain stimulation, and not even to see an improvement in all symptoms that burden the patient. Rather, it is to evaluate the likelihood that programming will be feasible with the implanted lead.
Subsequently, implantation of the pulse generator in the chest and connection to the brain lead is completed, usually with the patient under general anesthesia.
Implantation under general anesthesia, with intraoperative MRI
A new alternative to “awake stereotactic surgery” is implantation with the patient under general anesthesia, with intraoperative MRI. We have started to do this procedure in a new operating suite that is attached to an MRI suite. The magnet can be taken in and out of the operating room, allowing the surgeon to verify the location of the implanted leads right at the time of the procedure. In this fashion, intraoperative images are used to guide implantation instead of awake microelectrode recording. This is a new option for patients who cannot tolerate awake surgery and for those who have a contraindication to the regular stereotactic procedure with the patient awake.
Risks of bleeding and infection
The potential complications of implanting a device and leads in the brain can be significant.
Hemorrhage can occur, resulting in a superficial or deep hematoma.
Infection and erosion may require removal of the hardware for antibiotic treatment and possible reimplantation.
Other risks include those related to tunneling the wires from the head to the chest, to implanting the device in the chest, and to serious medical complications after surgery. Hardware failure can occur and requires additional surgery. Finally, environmental risks and risks related to medical devices such as MRI, electrocautery, and cardioversion should also be considered.
Deep brain stimulation is advantageous for its reversibility. If during postoperative programming the brain leads are considered not to be ideally placed, revisions can be done to reposition the leads.
- Deuschl G, Schade-Brittinger C, Krack P, et al; German Parkinson Study Group, Neurostimulation Section. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 2006; 355:896–908.
- Krack P, Batir A, Van Blercom N, et al. Five-year followup of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 2003; 349:1925–1934.
- Kupsch A, Benecke R, Müller J, et al; Deep-Brain Stimulation for Dystonia Study Group. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006; 355:1978–1990.
- Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scle for the primary torsion dystonias. Neurology 1985; 35:73–77.
- Gruber D, Trottenberg T, Kivi A, et al. Long-term effects of pallidal deep brain stimulation in tardive dystonia. Neurology 2009; 73:53–58.
Deep brain stimulation is an important therapy for Parkinson disease and other movement disorders. It involves implantation of a pulse generator that can be adjusted by telemetry and can be activated and deactivated by clinicians and patients. It is therefore also a good investigational tool, allowing for double-blind, sham-controlled clinical trials by testing the effects of the stimulation with optimal settings compared with no stimulation.
This article will discuss the approved indications for deep brain stimulation (particularly for managing movement disorders), the benefits that can be expected, the risks, the complications, the maintenance required, how candidates for this treatment are evaluated, and the surgical procedure for implantation of the devices.
DEVICE SIMILAR TO HEART PACEMAKERS
The deep brain stimulation system must be programmed by a physician or midlevel practitioner by observing a symptom and then changing the applied settings to the pulse generator until the symptom improves. This can be a very time-consuming process.
In contrast to heart pacemakers, which run at low frequencies, the brain devices for movement disorders are almost always set to a high frequency, greater than 100 Hz. For this reason, they consume more energy and need larger batteries than those in modern heart pacemakers.
The batteries in these generators typically last 3 to 5 years and are replaced in an outpatient procedure. Newer, smaller, rechargeable devices are expected to last longer but require more maintenance and care by patients, who have to recharge them at home periodically.
INDICATIONS FOR DEEP BRAIN STIMULATION
Deep brain stimulation is approved by the US Food and Drug Administration (FDA) for specific indications:
- Parkinson disease
- Essential tremor
- Primary dystonia (under a humanitarian device exemption)
- Intractable obsessive-compulsive disorder (also under a humanitarian device exemption). We will not discuss this indication further in this paper.
For each of these conditions, deep brain stimulation is considered when nonsurgical management has failed, as is the case for most functional neurosurgical treatments.
Investigations under way in other disorders
Several studies of deep brain stimulation are currently in progress under FDA-approved investigational device exemptions. Some, with funding from industry, are exploring its use in neuropsychiatric conditions other than parkinsonism. Two large clinical trials are evaluating its use for treatment-refractory depression, a common problem and a leading cause of disability in the industrialized world. Multiple investigators are also exploring novel uses of this technology in disorders ranging from obsessive-compulsive disorder to epilepsy.
Investigation is also under way at Cleveland Clinic in a federally funded, prospective, randomized clinical trial of deep brain stimulation for patients with thalamic pain syndrome. The primary hypothesis is that stimulation of the ventral striatal and ventral capsular area will modulate the affective component of this otherwise intractable pain syndrome, reducing pain-related disability and improving quality of life.
DEEP BRAIN STIMULATION VS ABLATION
Before deep brain stimulation became available, the only surgical options for patients with advanced Parkinson disease, tremor, or dystonia were ablative procedures such as pallidotomy (ablation of part of the globus pallidus) and thalamotomy (ablation of part of the thalamus). These procedures had been well known for several decades but fell out of favor when levodopa became available in the 1960s and revolutionized the medical treatment of Parkinson disease.
Surgery for movement disorders, in particular Parkinson disease, had a rebirth in the late 1980s when the limitations and complications associated with the pharmacologic management of Parkinson disease became increasingly evident. Ablative procedures are still used to treat advanced Parkinson disease, but much less commonly in industrialized countries.
Although pallidotomy and thalamotomy can have excellent results, they are not as safe as deep brain stimulation, which has the advantage of being reversible, modulating the function of an area rather than destroying it. Any unwanted effect can be immediately altered or reversed, unlike ablative procedures, in which any change is permanent. In addition, deep brain stimulation is adjustable, and the settings can be optimized as the disease progresses over the years.
Ablative procedures can be risky when performed bilaterally, while deep brain stimulation is routinely done on both hemispheres for patients with bilateral symptoms.
Although deep brain stimulation is today’s surgical treatment of choice, it is not perfect. It has the disadvantage of requiring lifelong maintenance of the hardware, for which the patient remains dependent on a medical center. Patients are usually seen more often at the specialized center in the first few months after surgery for optimization of programming and titration of drugs. (During this time, most patients see a gradual, substantial reduction in medication intake.) They are then followed by their physician and visit the center less often for monitoring of disease status and for further adjustments to the stimulator.
Most patients, to date, receive nonrechargeable pulse generators. As mentioned above, the batteries in these devices typically last 3 to 5 years. Preferably, batteries are replaced before they are completely depleted, to avoid interruption of therapy. Periodic visits to the center allow clinicians to estimate battery expiration ahead of time and plan replacements accordingly.
Rechargeable pulse generators have been recently introduced and are expected to last up to 9 years. They are an option for patients who can comply with the requirements for periodic home recharging of the hardware.
Patients are given a remote control so that they can turn the device on or off and check its status. Most patients keep it turned on all the time, although some turn it off at night to save battery life.
WHAT CAN PARKINSON PATIENTS EXPECT FROM THIS THERAPY?
Typically, some parkinsonian symptoms predominate over others, although some patients with advanced disease present with a severe combination of multiple disabling symptoms. Deep brain stimulation is best suited to address some of the cardinal motor symptoms, particularly tremor, rigidity, and bradykinesia, and motor fluctuations such as “wearing off” and dyskinesia.
Improvement in some motor symptoms
As a general rule, appendicular symptoms such as limb tremor and rigidity are more responsive to this therapy than axial symptoms such as gait and balance problems, but some patients experience improvement in gait as well. Other symptoms, such as swallowing or urinary symptoms, are seldom helped.
Although deep brain stimulation can help manage key motor symptoms and improve quality of life, it does not cure Parkinson disease. Also, there is no evidence to date that it slows disease progression, although this is a topic of ongoing investigation.
Fewer motor fluctuations
A common complaint of patients with advanced Parkinson disease is frequent—and often unpredictable—fluctuations between the “on” state (ie, when the effects of the patient’s levodopa therapy are apparent) and the “off” state (ie, when the levodopa doesn’t seem to be working). Sometimes, in the on state, patients experience involuntary choreic or ballistic movements, called dyskinesias. They also complain that the on time progressively lasts shorter and the day is spent alternating between shorter on states (during which the patient may be dyskinetic) and longer off states, limiting the patient’s independence and quality of life.
Deep brain stimulation can help patients prolong the on time while reducing the amplitude of these fluctuations so that the symptoms are not as severe in the off time and dyskinesias are reduced in the on time.
Some patients undergo deep brain stimulation primarily for managing the adverse effects of levodopa rather than for controlling the symptoms of the disease itself. While these patients need levodopa to address the disabling symptoms of the disease, they also present a greater sensitivity for developing levodopa-induced dyskinesias, quickly fluctuating from a lack of movement (the off state) to a state of uncontrollable movements (during the on state).
Deep brain stimulation typically allows the dosage of levodopa to be significantly reduced and gives patients more on time with fewer side effects and less fluctuation between the on and off states.
Response to levodopa predicts deep brain stimulation’s effects
Whether a patient is likely to be helped by deep brain stimulation can be tested with reasonable predictability by giving a single therapeutic dose of levodopa after the patient has been free of the drug for 12 hours. If there is an obvious difference on objective quantitative testing between the off and on states with a single dose, the patient is likely to benefit from deep brain stimulation. Those who do not respond well or are known to have never been well controlled by levodopa are likely poor candidates.
The test is also used as an indicator of whether the patient’s gait can be improved. Patients whose gait is substantially improved by levodopa, even for only a brief period of time, have a better chance of experiencing improvement in this domain with deep brain stimulation than those who do not show any gait improvement.
An important and notable exception to this rule is tremor control. Even Parkinson patients who do not experience significant improvement in tremor with levodopa (ie, who have medication-resistant tremors) are still likely to benefit from deep brain stimulation. Overall, tremor is the symptom that is most consistently improved with deep brain stimulation.
Results of clinical trials
Several clinical trials have demonstrated that deep brain stimulation plus medication works better than medications alone for advanced Parkinson disease.
Deuschl et al1 conducted a randomized trial in 156 patients with advanced Parkinson disease. Patients receiving subthalamic deep brain stimulation plus medication had significantly greater improvement in motor symptoms as measured by the Unified Parkinson’s Disease Rating Scale as well as in quality-of-life measures than patients receiving medications only.
Krack et al2 reported on the outcomes of 49 patients with advanced Parkinson disease who underwent deep brain stimulation and then were prospectively followed. At 5 years, motor function had improved by approximately 55% from baseline, activities-of-daily-living scores had improved by 49%, and patients continued to need significantly less levodopa and to experience less drug-induced dyskinesia.
Complications related to deep brain stimulation occurred in both studies, including two large intracerebral hemorrhages, one of which was fatal.
Weight gain. During the first 3 months after the device was implanted, patients tended to gain weight (mean 3 kg, maximum 5 kg). Although weight gain is considered an adverse effect, many patients are quite thin by the time they are candidates for deep brain stimulation, and in such cases gaining lean weight can be a benefit.
Patients with poorly controlled Parkinson disease lose weight for several reasons: increased calorie expenditure from shaking and excessive movements; diet modification and protein restriction for some patients who realize that protein competes with levodopa absorption; lack of appetite due to depression or from poor taste sensation (due to anosmia); and decreased overall food consumption due to difficulty swallowing.
DEEP BRAIN STIMULATION FOR ESSENTIAL TREMOR
Essential tremor is more common than Parkinson disease, with a prevalence in the United States estimated at approximately 4,000 per 100,000 people older than 65 years.
The tremor is often bilateral and is characteristically an action tremor, but in many patients it also has a postural, and sometimes a resting, component. It is distinct from parkinsonian tremor, which is usually predominantly a resting tremor. The differential diagnosis includes tremors secondary to central nervous system degenerative disorders as well as psychogenic tremors.
Drinking alcohol tends to relieve essential tremors, a finding that can often be elicited in the patient’s history. Patients whose symptoms improve with an alcoholic beverage are more likely to have essential tremor than another diagnosis.
Response to deep brain stimulation
Most patients with essential tremor respond well to deep brain stimulation of the contralateral ventral intermedius thalamic nucleus.
Treatment is usually started unilaterally, usually aimed at alleviating tremor in the patient’s dominant upper extremity. In selected cases, preference is given to treating the nondominant extremity when it is more severely affected than the dominant extremity.
Implantation of a device on the second side is offered to some patients who continue to be limited in activity and quality of life due to tremor of the untreated extremity. Surgery of the second side can be more complicated than the initial unilateral procedure. In particular, some patients may present with dysarthria, although that seems to be less common in our experience than initially estimated.
In practice, patients with moderate tremors tend to have an excellent response to deep brain stimulation. For this particular indication, if the response is not satisfactory, the treating team tends to consider surgically revising the placement of the lead rather than considering the patient a nonresponder. Patients with very severe tremors may have some residual tremor despite substantial improvement in severity. In our experience, patients with a greater proximal component of tremor tend to have less satisfactory results.
For challenging cases, implantation of additional electrodes in the thalamus or in new targets currently under investigation is sometimes considered, although this is an off-label use.
Treatment of secondary tremors, such as poststroke tremor or tremor due to multiple sclerosis, is sometimes attempted with deep brain stimulation. This is also an off-label option but is considered in selected cases for quality-of-life management.
Patients with axial tremors such as head or voice tremor are less likely to be helped by deep brain stimulation.
DEEP BRAIN STIMULATION FOR PRIMARY DYSTONIA
Generalized dystonia is a less common but severely impairing movement disorder.
Deep brain stimulation is approved for primary dystonia under a humanitarian device exemption, a regulatory mechanism for less common conditions. Deep brain stimulation is an option for patients who have significant impairment related to dystonia and who have not responded to conservative management such as anticholinergic agents, muscle relaxants, benzodiazepines, levodopa, or combinations of these drugs. Surgery has been shown to be effective for patients with primary generalized dystonia, whether or not they tested positive for a dystonia-related gene such as DYT1.
Kupsch et al3 evaluated 40 patients with primary dystonia in a randomized controlled trial of pallidal (globus pallidus pars interna) active deep brain stimulation vs sham stimulation (in which the device was implanted but not activated) for 3 months. Treated patients improved significantly more than controls (39% vs 5%) in the Burke-Fahn- Marsden Dystonia Rating Scale (BFMDRS).4 Similar improvement was noted when those receiving sham stimulation were switched to active stimulation.
During long-term follow-up, the results were generally sustained, with substantial improvement from deep brain stimulation in all movement symptoms evaluated except for speech and swallowing. Unlike improvement in tremor, which is quickly evident during testing in the operating room, the improvement in dystonia occurs gradually, and it may take months for patients to notice a change. Similarly, if stimulation stops because of device malfunction or dead batteries, symptoms sometimes do not recur for weeks or months.
Deep brain stimulation is sometimes offered to patients with dystonia secondary to conditions such as cerebral palsy or trauma (an off-label use). Although benefits are less consistent, deep brain stimulation remains an option for these individuals, aimed at alleviating some of the disabling symptoms. In patients with cerebral palsy or other secondary dystonias, it is sometimes difficult to distinguish how much of the disability is related to spasticity vs dystonia. Deep brain stimulation aims to alleviate the dystonic component; the spasticity may be managed with other options such as intrathecal baclofen (Lioresal).
Patients with tardive dystonia, which is usually secondary to treatment with antipsychotic agents, have been reported to respond well to bilateral deep brain stimulation. Gruber et al5 reported on a series of nine patients with a mean follow-up of 41 months. Patients improved by a mean of approximately 74% on the BFMDRS after 3 to 6 months of deep brain stimulation compared with baseline. None of the patients presented with long-term adverse effects, and quality of life and disability scores also improved significantly.
CANDIDATES ARE EVALUATED BY A MULTIDISCIPLINARY TEAM
Cleveland Clinic conducts a comprehensive 2-day evaluation for patients being considered for deep brain stimulation surgery, including consultations with specialists in neurology, neurosurgery, neuropsychology, and psychiatry.
Patients with significant cognitive deficits—near or meeting the diagnostic criteria for dementia—are usually not recommended to have surgery for Parkinson disease. Deep brain stimulation is not aimed at alleviating cognitive issues related to Parkinson disease or other concomitant dementia. In addition, there is a risk that neurostimulation could further worsen cognitive function in the already compromised brain. Moreover, patients with significant abnormalities detected by neuroimaging may have their diagnosis reconsidered in some cases, and some patients may not be deemed ideal candidates for surgery.
An important part of the process is a discussion with the patient and family about the risks and the potential short-term and long-term benefits. Informed consent requires a good understanding of this equation. Patients are counseled to have realistic expectations about what the procedure can offer. Deep brain stimulation can help some of the symptoms of Parkinson disease but will not cure it, and there is no evidence to date that it reduces its progress. At 5 or 10 years after surgery, patients are expected to be worse overall than they were in the first year after surgery, because of disease progression. However, patients who receive this treatment are expected, in general, to be doing better 5 or 10 years later (or longer) than those who do not receive it.
In addition to the discussion about risks, benefits, and expectations, a careful discussion is also devoted to hardware maintenance, including how to change the batteries. Particularly, younger patients should be informed about the risk of breakage of the leads and the extension wire, as they are likely to outlive their implant. Patients and caregivers should be able to come to the specialized center should hardware malfunction occur.
Patients are also informed that after the system is implanted they cannot undergo magnetic resonance imaging (MRI) except of the head, performed with a specific head coil and under specific parameters. MRI of any other body part and with a body coil is contraindicated.
HOW THE DEVICE IS IMPLANTED
There are several options for implanting a deep brain stimulation device.
Implantation with the patient awake, using a stereotactic headframe
At Cleveland Clinic, we usually prefer implantation with a stereotactic headframe. The base or “halo” of the frame is applied to the head under local anesthesia, followed by imaging via computed tomography (Figure 1). Typically, the tomographic image is fused to a previously acquired MRI image, but the MRI is sometimes either initially performed or repeated on the day of surgery.
Patients are sedated for the beginning of the procedure, while the surgical team is opening the skin and drilling the opening in the skull for placement of the lead. The patient is awakened for placement of the electrodes, which is not painful.
Microelectrode recording is typically performed in order to refine the targeting based on the stereotactic coordinates derived from neuroimaging. Although cadaver atlases exist and provide a guide to the stereotactic localization of subcortical structures, they are not completely accurate in representing the brain anatomy of all patients.
By “listening” to cells and knowing their characteristic signals in specific areas, landmarks can be created, forming an individualized map of the patient’s brain target. Microelectrode recording is invasive and has risks, including the risk of a brain hemorrhage. It is routinely done in most specialized deep brain stimulation centers because it can provide better accuracy and precision in lead placement.
When the target has been located and refined by microelectrode recording, the permanent electrode is inserted. Fluoroscopy is usually used to verify the direction and stability of placement during the procedure.
An intraoperative test of the effects of deep brain stimulation is routinely performed to verify that some benefits can be achieved with the brain lead in its location, to determine the threshold for side effects, or both. For example, the patient may be asked to hold a cup as if trying to drink from it and to write or to draw a spiral on a clipboard to assess for improvements in tremor. Rigidity and bradykinesia can also be tested for improvements.
This intraoperative test is not aimed at assessing the best possible outcome of deep brain stimulation, and not even to see an improvement in all symptoms that burden the patient. Rather, it is to evaluate the likelihood that programming will be feasible with the implanted lead.
Subsequently, implantation of the pulse generator in the chest and connection to the brain lead is completed, usually with the patient under general anesthesia.
Implantation under general anesthesia, with intraoperative MRI
A new alternative to “awake stereotactic surgery” is implantation with the patient under general anesthesia, with intraoperative MRI. We have started to do this procedure in a new operating suite that is attached to an MRI suite. The magnet can be taken in and out of the operating room, allowing the surgeon to verify the location of the implanted leads right at the time of the procedure. In this fashion, intraoperative images are used to guide implantation instead of awake microelectrode recording. This is a new option for patients who cannot tolerate awake surgery and for those who have a contraindication to the regular stereotactic procedure with the patient awake.
Risks of bleeding and infection
The potential complications of implanting a device and leads in the brain can be significant.
Hemorrhage can occur, resulting in a superficial or deep hematoma.
Infection and erosion may require removal of the hardware for antibiotic treatment and possible reimplantation.
Other risks include those related to tunneling the wires from the head to the chest, to implanting the device in the chest, and to serious medical complications after surgery. Hardware failure can occur and requires additional surgery. Finally, environmental risks and risks related to medical devices such as MRI, electrocautery, and cardioversion should also be considered.
Deep brain stimulation is advantageous for its reversibility. If during postoperative programming the brain leads are considered not to be ideally placed, revisions can be done to reposition the leads.
Deep brain stimulation is an important therapy for Parkinson disease and other movement disorders. It involves implantation of a pulse generator that can be adjusted by telemetry and can be activated and deactivated by clinicians and patients. It is therefore also a good investigational tool, allowing for double-blind, sham-controlled clinical trials by testing the effects of the stimulation with optimal settings compared with no stimulation.
This article will discuss the approved indications for deep brain stimulation (particularly for managing movement disorders), the benefits that can be expected, the risks, the complications, the maintenance required, how candidates for this treatment are evaluated, and the surgical procedure for implantation of the devices.
DEVICE SIMILAR TO HEART PACEMAKERS
The deep brain stimulation system must be programmed by a physician or midlevel practitioner by observing a symptom and then changing the applied settings to the pulse generator until the symptom improves. This can be a very time-consuming process.
In contrast to heart pacemakers, which run at low frequencies, the brain devices for movement disorders are almost always set to a high frequency, greater than 100 Hz. For this reason, they consume more energy and need larger batteries than those in modern heart pacemakers.
The batteries in these generators typically last 3 to 5 years and are replaced in an outpatient procedure. Newer, smaller, rechargeable devices are expected to last longer but require more maintenance and care by patients, who have to recharge them at home periodically.
INDICATIONS FOR DEEP BRAIN STIMULATION
Deep brain stimulation is approved by the US Food and Drug Administration (FDA) for specific indications:
- Parkinson disease
- Essential tremor
- Primary dystonia (under a humanitarian device exemption)
- Intractable obsessive-compulsive disorder (also under a humanitarian device exemption). We will not discuss this indication further in this paper.
For each of these conditions, deep brain stimulation is considered when nonsurgical management has failed, as is the case for most functional neurosurgical treatments.
Investigations under way in other disorders
Several studies of deep brain stimulation are currently in progress under FDA-approved investigational device exemptions. Some, with funding from industry, are exploring its use in neuropsychiatric conditions other than parkinsonism. Two large clinical trials are evaluating its use for treatment-refractory depression, a common problem and a leading cause of disability in the industrialized world. Multiple investigators are also exploring novel uses of this technology in disorders ranging from obsessive-compulsive disorder to epilepsy.
Investigation is also under way at Cleveland Clinic in a federally funded, prospective, randomized clinical trial of deep brain stimulation for patients with thalamic pain syndrome. The primary hypothesis is that stimulation of the ventral striatal and ventral capsular area will modulate the affective component of this otherwise intractable pain syndrome, reducing pain-related disability and improving quality of life.
DEEP BRAIN STIMULATION VS ABLATION
Before deep brain stimulation became available, the only surgical options for patients with advanced Parkinson disease, tremor, or dystonia were ablative procedures such as pallidotomy (ablation of part of the globus pallidus) and thalamotomy (ablation of part of the thalamus). These procedures had been well known for several decades but fell out of favor when levodopa became available in the 1960s and revolutionized the medical treatment of Parkinson disease.
Surgery for movement disorders, in particular Parkinson disease, had a rebirth in the late 1980s when the limitations and complications associated with the pharmacologic management of Parkinson disease became increasingly evident. Ablative procedures are still used to treat advanced Parkinson disease, but much less commonly in industrialized countries.
Although pallidotomy and thalamotomy can have excellent results, they are not as safe as deep brain stimulation, which has the advantage of being reversible, modulating the function of an area rather than destroying it. Any unwanted effect can be immediately altered or reversed, unlike ablative procedures, in which any change is permanent. In addition, deep brain stimulation is adjustable, and the settings can be optimized as the disease progresses over the years.
Ablative procedures can be risky when performed bilaterally, while deep brain stimulation is routinely done on both hemispheres for patients with bilateral symptoms.
Although deep brain stimulation is today’s surgical treatment of choice, it is not perfect. It has the disadvantage of requiring lifelong maintenance of the hardware, for which the patient remains dependent on a medical center. Patients are usually seen more often at the specialized center in the first few months after surgery for optimization of programming and titration of drugs. (During this time, most patients see a gradual, substantial reduction in medication intake.) They are then followed by their physician and visit the center less often for monitoring of disease status and for further adjustments to the stimulator.
Most patients, to date, receive nonrechargeable pulse generators. As mentioned above, the batteries in these devices typically last 3 to 5 years. Preferably, batteries are replaced before they are completely depleted, to avoid interruption of therapy. Periodic visits to the center allow clinicians to estimate battery expiration ahead of time and plan replacements accordingly.
Rechargeable pulse generators have been recently introduced and are expected to last up to 9 years. They are an option for patients who can comply with the requirements for periodic home recharging of the hardware.
Patients are given a remote control so that they can turn the device on or off and check its status. Most patients keep it turned on all the time, although some turn it off at night to save battery life.
WHAT CAN PARKINSON PATIENTS EXPECT FROM THIS THERAPY?
Typically, some parkinsonian symptoms predominate over others, although some patients with advanced disease present with a severe combination of multiple disabling symptoms. Deep brain stimulation is best suited to address some of the cardinal motor symptoms, particularly tremor, rigidity, and bradykinesia, and motor fluctuations such as “wearing off” and dyskinesia.
Improvement in some motor symptoms
As a general rule, appendicular symptoms such as limb tremor and rigidity are more responsive to this therapy than axial symptoms such as gait and balance problems, but some patients experience improvement in gait as well. Other symptoms, such as swallowing or urinary symptoms, are seldom helped.
Although deep brain stimulation can help manage key motor symptoms and improve quality of life, it does not cure Parkinson disease. Also, there is no evidence to date that it slows disease progression, although this is a topic of ongoing investigation.
Fewer motor fluctuations
A common complaint of patients with advanced Parkinson disease is frequent—and often unpredictable—fluctuations between the “on” state (ie, when the effects of the patient’s levodopa therapy are apparent) and the “off” state (ie, when the levodopa doesn’t seem to be working). Sometimes, in the on state, patients experience involuntary choreic or ballistic movements, called dyskinesias. They also complain that the on time progressively lasts shorter and the day is spent alternating between shorter on states (during which the patient may be dyskinetic) and longer off states, limiting the patient’s independence and quality of life.
Deep brain stimulation can help patients prolong the on time while reducing the amplitude of these fluctuations so that the symptoms are not as severe in the off time and dyskinesias are reduced in the on time.
Some patients undergo deep brain stimulation primarily for managing the adverse effects of levodopa rather than for controlling the symptoms of the disease itself. While these patients need levodopa to address the disabling symptoms of the disease, they also present a greater sensitivity for developing levodopa-induced dyskinesias, quickly fluctuating from a lack of movement (the off state) to a state of uncontrollable movements (during the on state).
Deep brain stimulation typically allows the dosage of levodopa to be significantly reduced and gives patients more on time with fewer side effects and less fluctuation between the on and off states.
Response to levodopa predicts deep brain stimulation’s effects
Whether a patient is likely to be helped by deep brain stimulation can be tested with reasonable predictability by giving a single therapeutic dose of levodopa after the patient has been free of the drug for 12 hours. If there is an obvious difference on objective quantitative testing between the off and on states with a single dose, the patient is likely to benefit from deep brain stimulation. Those who do not respond well or are known to have never been well controlled by levodopa are likely poor candidates.
The test is also used as an indicator of whether the patient’s gait can be improved. Patients whose gait is substantially improved by levodopa, even for only a brief period of time, have a better chance of experiencing improvement in this domain with deep brain stimulation than those who do not show any gait improvement.
An important and notable exception to this rule is tremor control. Even Parkinson patients who do not experience significant improvement in tremor with levodopa (ie, who have medication-resistant tremors) are still likely to benefit from deep brain stimulation. Overall, tremor is the symptom that is most consistently improved with deep brain stimulation.
Results of clinical trials
Several clinical trials have demonstrated that deep brain stimulation plus medication works better than medications alone for advanced Parkinson disease.
Deuschl et al1 conducted a randomized trial in 156 patients with advanced Parkinson disease. Patients receiving subthalamic deep brain stimulation plus medication had significantly greater improvement in motor symptoms as measured by the Unified Parkinson’s Disease Rating Scale as well as in quality-of-life measures than patients receiving medications only.
Krack et al2 reported on the outcomes of 49 patients with advanced Parkinson disease who underwent deep brain stimulation and then were prospectively followed. At 5 years, motor function had improved by approximately 55% from baseline, activities-of-daily-living scores had improved by 49%, and patients continued to need significantly less levodopa and to experience less drug-induced dyskinesia.
Complications related to deep brain stimulation occurred in both studies, including two large intracerebral hemorrhages, one of which was fatal.
Weight gain. During the first 3 months after the device was implanted, patients tended to gain weight (mean 3 kg, maximum 5 kg). Although weight gain is considered an adverse effect, many patients are quite thin by the time they are candidates for deep brain stimulation, and in such cases gaining lean weight can be a benefit.
Patients with poorly controlled Parkinson disease lose weight for several reasons: increased calorie expenditure from shaking and excessive movements; diet modification and protein restriction for some patients who realize that protein competes with levodopa absorption; lack of appetite due to depression or from poor taste sensation (due to anosmia); and decreased overall food consumption due to difficulty swallowing.
DEEP BRAIN STIMULATION FOR ESSENTIAL TREMOR
Essential tremor is more common than Parkinson disease, with a prevalence in the United States estimated at approximately 4,000 per 100,000 people older than 65 years.
The tremor is often bilateral and is characteristically an action tremor, but in many patients it also has a postural, and sometimes a resting, component. It is distinct from parkinsonian tremor, which is usually predominantly a resting tremor. The differential diagnosis includes tremors secondary to central nervous system degenerative disorders as well as psychogenic tremors.
Drinking alcohol tends to relieve essential tremors, a finding that can often be elicited in the patient’s history. Patients whose symptoms improve with an alcoholic beverage are more likely to have essential tremor than another diagnosis.
Response to deep brain stimulation
Most patients with essential tremor respond well to deep brain stimulation of the contralateral ventral intermedius thalamic nucleus.
Treatment is usually started unilaterally, usually aimed at alleviating tremor in the patient’s dominant upper extremity. In selected cases, preference is given to treating the nondominant extremity when it is more severely affected than the dominant extremity.
Implantation of a device on the second side is offered to some patients who continue to be limited in activity and quality of life due to tremor of the untreated extremity. Surgery of the second side can be more complicated than the initial unilateral procedure. In particular, some patients may present with dysarthria, although that seems to be less common in our experience than initially estimated.
In practice, patients with moderate tremors tend to have an excellent response to deep brain stimulation. For this particular indication, if the response is not satisfactory, the treating team tends to consider surgically revising the placement of the lead rather than considering the patient a nonresponder. Patients with very severe tremors may have some residual tremor despite substantial improvement in severity. In our experience, patients with a greater proximal component of tremor tend to have less satisfactory results.
For challenging cases, implantation of additional electrodes in the thalamus or in new targets currently under investigation is sometimes considered, although this is an off-label use.
Treatment of secondary tremors, such as poststroke tremor or tremor due to multiple sclerosis, is sometimes attempted with deep brain stimulation. This is also an off-label option but is considered in selected cases for quality-of-life management.
Patients with axial tremors such as head or voice tremor are less likely to be helped by deep brain stimulation.
DEEP BRAIN STIMULATION FOR PRIMARY DYSTONIA
Generalized dystonia is a less common but severely impairing movement disorder.
Deep brain stimulation is approved for primary dystonia under a humanitarian device exemption, a regulatory mechanism for less common conditions. Deep brain stimulation is an option for patients who have significant impairment related to dystonia and who have not responded to conservative management such as anticholinergic agents, muscle relaxants, benzodiazepines, levodopa, or combinations of these drugs. Surgery has been shown to be effective for patients with primary generalized dystonia, whether or not they tested positive for a dystonia-related gene such as DYT1.
Kupsch et al3 evaluated 40 patients with primary dystonia in a randomized controlled trial of pallidal (globus pallidus pars interna) active deep brain stimulation vs sham stimulation (in which the device was implanted but not activated) for 3 months. Treated patients improved significantly more than controls (39% vs 5%) in the Burke-Fahn- Marsden Dystonia Rating Scale (BFMDRS).4 Similar improvement was noted when those receiving sham stimulation were switched to active stimulation.
During long-term follow-up, the results were generally sustained, with substantial improvement from deep brain stimulation in all movement symptoms evaluated except for speech and swallowing. Unlike improvement in tremor, which is quickly evident during testing in the operating room, the improvement in dystonia occurs gradually, and it may take months for patients to notice a change. Similarly, if stimulation stops because of device malfunction or dead batteries, symptoms sometimes do not recur for weeks or months.
Deep brain stimulation is sometimes offered to patients with dystonia secondary to conditions such as cerebral palsy or trauma (an off-label use). Although benefits are less consistent, deep brain stimulation remains an option for these individuals, aimed at alleviating some of the disabling symptoms. In patients with cerebral palsy or other secondary dystonias, it is sometimes difficult to distinguish how much of the disability is related to spasticity vs dystonia. Deep brain stimulation aims to alleviate the dystonic component; the spasticity may be managed with other options such as intrathecal baclofen (Lioresal).
Patients with tardive dystonia, which is usually secondary to treatment with antipsychotic agents, have been reported to respond well to bilateral deep brain stimulation. Gruber et al5 reported on a series of nine patients with a mean follow-up of 41 months. Patients improved by a mean of approximately 74% on the BFMDRS after 3 to 6 months of deep brain stimulation compared with baseline. None of the patients presented with long-term adverse effects, and quality of life and disability scores also improved significantly.
CANDIDATES ARE EVALUATED BY A MULTIDISCIPLINARY TEAM
Cleveland Clinic conducts a comprehensive 2-day evaluation for patients being considered for deep brain stimulation surgery, including consultations with specialists in neurology, neurosurgery, neuropsychology, and psychiatry.
Patients with significant cognitive deficits—near or meeting the diagnostic criteria for dementia—are usually not recommended to have surgery for Parkinson disease. Deep brain stimulation is not aimed at alleviating cognitive issues related to Parkinson disease or other concomitant dementia. In addition, there is a risk that neurostimulation could further worsen cognitive function in the already compromised brain. Moreover, patients with significant abnormalities detected by neuroimaging may have their diagnosis reconsidered in some cases, and some patients may not be deemed ideal candidates for surgery.
An important part of the process is a discussion with the patient and family about the risks and the potential short-term and long-term benefits. Informed consent requires a good understanding of this equation. Patients are counseled to have realistic expectations about what the procedure can offer. Deep brain stimulation can help some of the symptoms of Parkinson disease but will not cure it, and there is no evidence to date that it reduces its progress. At 5 or 10 years after surgery, patients are expected to be worse overall than they were in the first year after surgery, because of disease progression. However, patients who receive this treatment are expected, in general, to be doing better 5 or 10 years later (or longer) than those who do not receive it.
In addition to the discussion about risks, benefits, and expectations, a careful discussion is also devoted to hardware maintenance, including how to change the batteries. Particularly, younger patients should be informed about the risk of breakage of the leads and the extension wire, as they are likely to outlive their implant. Patients and caregivers should be able to come to the specialized center should hardware malfunction occur.
Patients are also informed that after the system is implanted they cannot undergo magnetic resonance imaging (MRI) except of the head, performed with a specific head coil and under specific parameters. MRI of any other body part and with a body coil is contraindicated.
HOW THE DEVICE IS IMPLANTED
There are several options for implanting a deep brain stimulation device.
Implantation with the patient awake, using a stereotactic headframe
At Cleveland Clinic, we usually prefer implantation with a stereotactic headframe. The base or “halo” of the frame is applied to the head under local anesthesia, followed by imaging via computed tomography (Figure 1). Typically, the tomographic image is fused to a previously acquired MRI image, but the MRI is sometimes either initially performed or repeated on the day of surgery.
Patients are sedated for the beginning of the procedure, while the surgical team is opening the skin and drilling the opening in the skull for placement of the lead. The patient is awakened for placement of the electrodes, which is not painful.
Microelectrode recording is typically performed in order to refine the targeting based on the stereotactic coordinates derived from neuroimaging. Although cadaver atlases exist and provide a guide to the stereotactic localization of subcortical structures, they are not completely accurate in representing the brain anatomy of all patients.
By “listening” to cells and knowing their characteristic signals in specific areas, landmarks can be created, forming an individualized map of the patient’s brain target. Microelectrode recording is invasive and has risks, including the risk of a brain hemorrhage. It is routinely done in most specialized deep brain stimulation centers because it can provide better accuracy and precision in lead placement.
When the target has been located and refined by microelectrode recording, the permanent electrode is inserted. Fluoroscopy is usually used to verify the direction and stability of placement during the procedure.
An intraoperative test of the effects of deep brain stimulation is routinely performed to verify that some benefits can be achieved with the brain lead in its location, to determine the threshold for side effects, or both. For example, the patient may be asked to hold a cup as if trying to drink from it and to write or to draw a spiral on a clipboard to assess for improvements in tremor. Rigidity and bradykinesia can also be tested for improvements.
This intraoperative test is not aimed at assessing the best possible outcome of deep brain stimulation, and not even to see an improvement in all symptoms that burden the patient. Rather, it is to evaluate the likelihood that programming will be feasible with the implanted lead.
Subsequently, implantation of the pulse generator in the chest and connection to the brain lead is completed, usually with the patient under general anesthesia.
Implantation under general anesthesia, with intraoperative MRI
A new alternative to “awake stereotactic surgery” is implantation with the patient under general anesthesia, with intraoperative MRI. We have started to do this procedure in a new operating suite that is attached to an MRI suite. The magnet can be taken in and out of the operating room, allowing the surgeon to verify the location of the implanted leads right at the time of the procedure. In this fashion, intraoperative images are used to guide implantation instead of awake microelectrode recording. This is a new option for patients who cannot tolerate awake surgery and for those who have a contraindication to the regular stereotactic procedure with the patient awake.
Risks of bleeding and infection
The potential complications of implanting a device and leads in the brain can be significant.
Hemorrhage can occur, resulting in a superficial or deep hematoma.
Infection and erosion may require removal of the hardware for antibiotic treatment and possible reimplantation.
Other risks include those related to tunneling the wires from the head to the chest, to implanting the device in the chest, and to serious medical complications after surgery. Hardware failure can occur and requires additional surgery. Finally, environmental risks and risks related to medical devices such as MRI, electrocautery, and cardioversion should also be considered.
Deep brain stimulation is advantageous for its reversibility. If during postoperative programming the brain leads are considered not to be ideally placed, revisions can be done to reposition the leads.
- Deuschl G, Schade-Brittinger C, Krack P, et al; German Parkinson Study Group, Neurostimulation Section. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 2006; 355:896–908.
- Krack P, Batir A, Van Blercom N, et al. Five-year followup of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 2003; 349:1925–1934.
- Kupsch A, Benecke R, Müller J, et al; Deep-Brain Stimulation for Dystonia Study Group. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006; 355:1978–1990.
- Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scle for the primary torsion dystonias. Neurology 1985; 35:73–77.
- Gruber D, Trottenberg T, Kivi A, et al. Long-term effects of pallidal deep brain stimulation in tardive dystonia. Neurology 2009; 73:53–58.
- Deuschl G, Schade-Brittinger C, Krack P, et al; German Parkinson Study Group, Neurostimulation Section. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 2006; 355:896–908.
- Krack P, Batir A, Van Blercom N, et al. Five-year followup of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 2003; 349:1925–1934.
- Kupsch A, Benecke R, Müller J, et al; Deep-Brain Stimulation for Dystonia Study Group. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006; 355:1978–1990.
- Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scle for the primary torsion dystonias. Neurology 1985; 35:73–77.
- Gruber D, Trottenberg T, Kivi A, et al. Long-term effects of pallidal deep brain stimulation in tardive dystonia. Neurology 2009; 73:53–58.
KEY POINTS
- Compared with ablative procedures, deep brain stimulation has the advantage of being reversible and adjustable. It is considered safer than ablative surgery, in particular for bilateral procedures, which are often needed for patients with advanced Parkinson disease and other movement disorders.
- For Parkinson disease, deep brain stimulation improves the cardinal motor symptoms, extends medication “on” time, and reduces motor fluctuations during the day.
- In general, patients with Parkinson disease are likely to benefit from this therapy if they show a clear response to levodopa. Patients are therefore asked to stop their Parkinson medications overnight to permit a formal evaluation of their motor response before and after a dose of levodopa.
- Candidates require a thorough evaluation to assess whether they are likely to benefit from deep brain stimulation and if they can comply with the maintenance often required for a successful outcome.