User login
A 74-year-old man with abdominal pain
A 74-year-old man presented to the emergency department in December 2011 with a 1-week history of worsening abdominal pain, nausea with emesis, and decreased appetite. The pain was dull, diffuse, and not related to oral intake or bowel movements. He denied any bloody stools, melena, or hematemesis, but he had not had a bowel movement in the past week.
He was already known to have stage IV colon cancer with metastases to the lungs and liver. He had undergone a partial colectomy in 2009 and was receiving chemotherapy at the time of admission.
He also had an infrarenal abdominal aortic aneurysm that had been repaired in 2003 with endovascular placement of a Gore Excluder stent graft. This was complicated by a type II endoleak, treated with coil embolization. The same endoleak later recurred and was treated with injection of Onyx liquid embolic agent.
His medical history also included hypertension, type 2 diabetes mellitus, and hyperlipidemia. He had undergone a laparoscopic cholecystectomy in 2007.
He denied any fevers, chills, headache, lightheadedness, or change in vision. He had no respiratory, cardiac, or urinary symptoms. He had been constipated for the past few weeks and had recently been started on a bowel regimen, with mild relief. There had been no other changes to his medications.
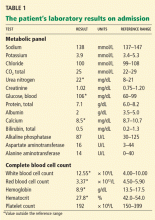
His temperature on presentation was 97.5°F (36.4°C), blood pressure 120/64 mm Hg, pulse 96, respiratory rate 22, and oxygen saturation 95% on room air. He was awake, alert, oriented, and in no acute distress. His mucous membranes were dry. His lungs were clear to auscultation, and his heart sounds were normal. His bowel sounds were hyperactive and his abdomen was slightly tender diffusely, but there was no abdominal distention, rebound tenderness, guarding, or palpable masses. His joints, muscle strength, and muscle tone were normal. Table 1 shows his initial laboratory values.
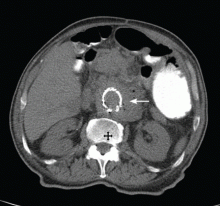
Given the patient’s history of colon cancer, the emergency department physician ordered computed tomography (CT) of the abdomen to assess the state of his disease and to evaluate for bowel obstruction. The scan revealed a large abdominal aortic aneurysm with foci of gas within the aneurysmal sac. Metastases in the liver, lung, and retroperitoneum appeared stable; abundant colonic stool suggested constipation (Figure 1).
CAUSES OF PERIAORTIC GAS AFTER ANEURYSM REPAIR
1. What is the most common cause of periaortic ectopic gas in a patient with a repaired abdominal aortic aneurysm?
- Endoleak
- Stent graft infection
- Retroperitoneal fibrosis
- Aortoenteric fistula
Endoleak
Endoleak, a complication of endovascular abdominal aortic aneurysm repair, is defined as blood flow within the aneurysm sac but outside the endoluminal graft.1 It occurs in up to 15% of patients after endograft placement in the first month alone, and in up to 47% of patients eventually.2 It can lead to aneurysm enlargement and rupture. Endoleaks are classified into five types, each with different causes and management options.3,4 Contrast-enhanced CT is the most commonly used diagnostic tool.5
Endoleak cannot be ruled out in our patient, since CT was done without contrast. However, gas within the aneurysm is not consistent with this diagnosis.
Stent graft infection
Infection has been reported in 1% to 6% of patients receiving a stent graft for aortic aneurysm.6 They occur most commonly in the first year after placement; one study showed that 42% of patients diagnosed with graft infection presented within 3 months of endovascular repair.7
The leading cause of graft infection is contamination during the original procedure, but secondary infection from hematologic seeding and contamination from adjacent bowel are also possible.8 In our patient, who underwent graft placement followed by endovascular repairs of endoleaks, bacterial seeding of his aortic aneurysm from the procedures should be considered.9
The most common organisms are staphylococcal species, with Staphylococcus aureus more common in early infection and coagulase-negative staphylococci more common in late infection.10 Methicillin-resistant S aureus has been reported in as many as 25% of cases of graft infection. Diphtheroids and gram-negative enteric organisms should also be considered.11
CT is the most effective imaging test for graft infection. Perigraft soft tissue, fluid, and gas are the major CT findings.12
Given that our patient presented with abdominal pain, leukocytosis, and the CT finding of perigraft gas, graft infection should be high on our list differential diagnoses.
Retroperitoneal fibrosis
Retroperitoneal fibrosis is most often idiopathic, although many believe it is due to an exaggerated local inflammatory reaction to aortic atherosclerosis or is a manifestation of a systemic autoimmune disease.13 Secondary retroperitoneal fibrosis may be due to drugs, infection, or malignancy.
Pathologic findings include sclerotic plaques, typically around the abdominal vessels and ureters. Clinical presentations are often nonspecific, with early symptoms that include back or abdominal pain, malaise, anorexia, edema, and hematuria.14,15 Progressive ureteral obstruction can occur in later stages. CT with contrast is the imaging test of choice to visualize the extent of disease, with the fibrosis exhibiting attenuation similar to that of muscle.16
Initial treatment of idiopathic retroperitoneal fibrosis is with a glucocorticoid or other immunosuppressive agent, whereas treatment of secondary retroperitoneal fibrosis is aimed at the underlying cause.17 Late stages complicated by ureteral obstruction typically require surgery.18
Our patient did have some nonspecific complaints that could be due to retroperitoneal fibrosis. He also had an intra-abdominal malignancy, which could lead to secondary retroperitoneal fibrosis. However, his CT findings of periaortic gas are not consistent with this diagnosis.19
Aortoenteric fistula
Aortoenteric fistulas can be either primary or secondary.
Primary aortoenteric fistulas occur de novo in patients who have never undergone any surgery or procedure in the aorta. This type of fistula usually results from pressure erosion of an atherosclerotic abdominal aortic aneurysm into the gastrointestinal tract. They are rare, with an annual incidence of 0.04% to 0.07% in the general population.20,21
Secondary aortoenteric fistulas are complications of aortic reconstructive therapy. After open repair, a perianastomotic or pseudoaneurysmal fistula can develop into the gastrointestinal tract.4 Endovascular repair leaves the aortic wall intact with no exposed suture lines, but an aortoenteric fistula can still develop22 and in fact occur in 0.4% to 3.1% of recipients of stent grafts for abdominal aortic aneurysm repair.23 In such cases, it is commonly thought that graft infection can lead to formation of an aortoenteric fistula, but a penetrating gastrointestinal ulcer, tumor invasion, radiation therapy, and trauma have also been implicated.19,24–26 An aortoenteric fistula can present several months to several years after either open or endovascular abdominal aortic aneurysm repair.4,23
One of the main CT signs of an aortoenteric fistula is periaortic ectopic gas at least 3 to 4 weeks after surgery or endovascular repair.19 Gas around the stent graft is most commonly caused by infection, but an aortoenteric fistula must also be considered in our patient, as roughly one-third of graft infections present as aortoenteric fistula.27 Our patient denied having any gastrointestinal bleeding, but his hemoglobin concentration at presentation was 8.9 g/dL.
Highlight point. Perigraft gas after abdominal aortic aneurysm repair can be seen in graft infection and aortoenteric fistula.
SIGNS AND SYMPTOMS OF AORTOENTERIC FISTULA
2. What is the most common clinical sign or symptom of an aortoenteric fistula?
- Gastrointestinal bleeding
- Sepsis
- Abdominal pain
- Back pain
Gastrointestinal bleeding occurs in 80% of patients who have an aortoenteric fistula, sepsis in 40%, abdominal pain in 30%, and back pain in 15%.19 The classic triad of symptoms is gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass. However, symptoms can vary widely, and the classic triad is present in fewer than 25% of cases.28 Sepsis may be the predominant clinical manifestation, particularly in the early stages of fistula formation. Unexplained fever is an underrecognized early manifestation.24
Highlight point. The classic triad of symptoms of an aortoenteric fistula (gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass) is seen in fewer than 25% of cases.
Case continued: The patient develops frank bleeding
The vascular surgery service was consulted because of concern for an aortic graft infection, since surgical removal of the infected material is recommended.10 The patient was deemed to be a poor surgical candidate, given his stage IV colon cancer, so he was treated conservatively with broad-spectrum antibiotics.
Over the next 2 days, he had two episodes of dark, bloody bowel movements, but he remained hemodynamically stable. He subsequently developed frank bleeding per rectum with symptoms of lightheadedness, and his hemoglobin concentration fell to 6.9 g/dL. He was given a total of 3 units of packed red blood cells, which raised his hemoglobin level, but only to 8.3 g/dL. The gastroenterology service was consulted to evaluate for the source of the bleeding.
Comment. In a situation like this, an aortoenteric fistula is high on our list of differential diagnoses as the cause of bleeding, but other causes of frank bleeding per rectum such as diverticulosis, arteriovenous malformation, hemorrhoids, or a rapid upper-gastrointestinal bleed cannot be ruled out.
Upper-gastrointestinal endoscopy is the most commonly used diagnostic test for aortoenteric fistulas. It can also find other possible sources of gastrointestinal bleeding. CT with contrast is another option. It can depict the fistula itself or reveal signs of infection, such as gas or liquid surrounding the graft. In an emergency, when there is not enough time for diagnostic testing and an aortoenteric fistula is strongly suspected on clinical grounds, emergency surgical exploration is warranted.4,24
In our patient, the gastrointestinal service elected to first perform endoscopy to look for an aortoenteric fistula.
WHERE DO AORTOENTERIC FISTULAS OCCUR?
3. In which part of the gastrointestinal tract is an aortoenteric fistula most commonly located?
- Esophagus
- Stomach
- Duodenum
- Jejunum
Aortoenteric fistulas can occur at any of these locations, but 80% of cases of secondary aortoenteric fistula are in the duodenum, most often in the third or fourth (horizontal or ascending) part.19 Endoscopic visualization of a pulsatile bleeding mass in this area is diagnostic. However, even if no fistula is seen, upper endoscopy cannot rule out an aortoenteric fistula because the lesion can be located more distal than the scope can reach, which is typically no farther than the first or second parts.4,24
Case continued: What endoscopy showed
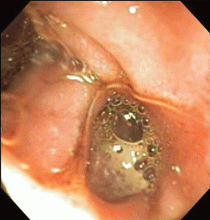
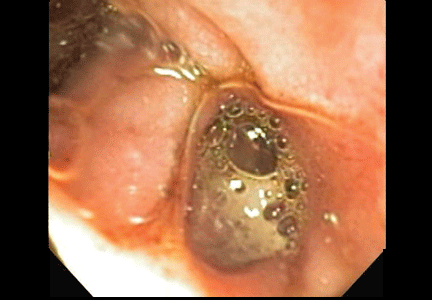
The esophagus was normal. There was old clotted blood in the stomach, but no lesions or ulcers. The duodenal bulb and second portion of the duodenum were normal. Three ulcers were noted in the third and fourth portions of the duodenum. The largest and deepest ulcer had an adherent blood clot, and the bowel wall was pulsatile in this region (Figure 2). These findings revealed the source of the gastrointestinal bleeding and were consistent with an aortoenteric fistula.
The patient’s initial bloody bowel movements were herald bleeds, ie, transient and self-limited episodes resulting from necrosis and mucosal ulceration. Herald bleeds can precede a massive gastrointestinal hemorrhage resulting from a true aortoenteric communication.19
Highlight point. Herald bleeds are self-limited and precede hemorrhage that results from a true aortoenteric communication.
TREATMENT OF AORTOENTERIC FISTULA
4. How are aortoenteric fistulas treated?
- Surgery
- Antibiotics
- Endoscopic intervention
Surgery is the definitive treatment. The traditional procedure is open surgical resection of the affected portion of the aorta followed by extra-anatomic (axillobifemoral) bypass or in situ aortic reconstruction using an antibiotic-impregnated prosthetic graft, autogenous femoral vein graft, or cryopreserved allograft.9,29 There have been cases of successful endovascular repair of aortoenteric fistulas, but this approach is generally used as a palliative bridge to definitive surgery.30
Antibiotics should be used if graft infection is suspected, ie, in most cases. However, surgery is still needed to repair the fistula and remove the source of infection. Cultures taken during surgical repair can help guide the choice of antibiotic after surgery.
Endoscopy can aid in diagnosing an aortoenteric fistula, as in the case of our patient. However, vascular surgery is necessary to close the communication between the aorta and the gastrointestinal tract.
Case continued: The patient declines treatment
In view of the patient’s enteroscopic findings, the vascular surgery service was again consulted for surgical correction of the aortoenteric fistula. Treatment was discussed with the patient and his family, but they declined any intervention in view of the high risk of morbidity and death that surgery would entail. Nearing the end of life with advanced cancer and a newly diagnosed aortoenteric fistula, the patient preferred comfort measures with hospice care.
Take-home points
Abdominal pain is the reason for 5% to 10% of emergency department visits, and between 35% to 41% of patients admitted to the hospital because of abdominal pain do not have a definitive diagnosis.31 It is crucial to think about an aortoenteric fistula in such patients who have a history of abdominal aortic aneurysm repair and gastrointestinal bleeding. Timely diagnosis and intervention are necessary to manage this otherwise-fatal condition.
- Hong C, Heiken JP, Sicard GA, Pilgram TK, Bae KT. Clinical significance of endoleak detected on follow-up CT after endovascular repair of abdominal aortic aneurysm. AJR Am J Roentgenol 2008; 191:808–813.
- Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg 2002; 35:1029–1035.
- Corriere MA, Feurer ID, Becker SY, et al. Endoleak following endovascular abdominal aortic aneurysm repair: implications for duration of screening. Ann Surg 2004; 239:800–805.
- Saratzis N, Saratzis A, Melas N, Ktenidis K, Kiskinis D. Aortoduodenal fistulas after endovascular stent-graft repair of abdominal aortic aneurysms: single-center experience and review of the literature. J Endovasc Ther 2008; 15:441–448.
- Demko TM, Diamond JR, Groff J. Obstructive nephropathy as a result of retroperitoneal fibrosis: a review of its pathogenesis and associations. J Am Soc Nephrol 1997; 8:684–688.
- Zetrenne E, McIntosh BC, McRae MH, Gusberg R, Evans GR, Narayan D. Prosthetic vascular graft infection: a multi-center review of surgical management. Yale J Biol Med 2007; 80:113–121.
- Vogel TR, Symons R, Flum DR. The incidence and factors associated with graft infection after aortic aneurysm repair. J Vasc Surg 2008; 47:264–269.
- Swain TW, Calligaro KD, Dougherty MD. Management of infected aortic prosthetic grafts. Vasc Endovascular Surg 2004; 38:75–82.
- Cernohorsky P, Reijnen MM, Tielliu IF, van Sterkenburg SM, van den Dungen JJ, Zeebregts CJ. The relevance of aortic endograft prosthetic infection. J Vasc Surg 2011; 54:327–333.
- FitzGerald SF, Kelly C, Humphreys H. Diagnosis and treatment of prosthetic aortic graft infections: confusion and inconsistency in the absence of evidence or consensus. J Antimicrob Chemother 2005; 56:996–999.
- Orton DF, LeVeen RF, Saigh JA, et al. Aortic prosthetic graft infections: radiologic manifestations and implications for management. Radiographics 2000; 20:977–993.
- Pacanowski JP, Dieter RS, Stevens SL, Freeman MB, Goldman MH. Endoleak: the achilles heel of endovascular abdominal aortic aneurysm exclusion—a case report. WMJ 2002; 101:57–58,63.
- van Bommel EF. Retroperitoneal fibrosis. Neth J Med 2002; 60:231–242.
- Utz DC, Henry JD. Retroperitoneal fibrosis. Med Clin North Am 1966; 50:1091–1099.
- Dalla-Palma L, Rocca-Rossetti S, Pozzi-Mucelli RS, Rizzatto G. Computed tomography in the diagnosis of retroperitoneal fibrosis. Urol Radiol 1981; 3:77–83.
- Harreby M, Bilde T, Helin P, Meyhoff HH, Vinterberg H, Nielsen VA. Retroperitoneal fibrosis treated with methylprednisolon pulse and disease-modifying antirheumatic drugs. Scand J Urol Nephrol 1994; 28:237–242.
- Jois RN, Gaffney K, Marshall T, Scott DG. Chronic periaortitis. Rheumatology (Oxford) 2004; 43:1441–1446.
- Saers SJ, Scheltinga MR. Primary aortoenteric fistula. Br J Surg 2005; 92:143–152.
- Baril DT, Carroccio A, Ellozy SH, et al. Evolving strategies for the treatment of aortoenteric fistulas. J Vasc Surg 2006; 44:250–257.
- Vu QD, Menias CO, Bhalla S, Peterson C, Wang LL, Balfe DM. Aortoenteric fistulas: CT features and potential mimics. Radiographics 2009; 29:197–209.
- Jayarajan S, Napolitano LM, Rectenwald JE, Upchurch GR. Primary aortoenteric fistula and endovascular repair. Vasc Endovascular Surg 2009; 43:592–596.
- Ruby BJ, Cogbill TH. Aortoduodenal fistula 5 years after endovascular abdominal aortic aneurysm repair with the Ancure stent graft. J Vasc Surg 2007; 45:834–836.
- Senadhi V, Brown JC, Arora D, Shaffer R, Shetty D, Mackrell P. A mysterious cause of gastrointestinal bleeding disguising itself as diverticulosis and peptic ulcer disease: a review of diagnostic modalities for aortoenteric fistula. Case Rep Gastroenterol 2010; 4:510–517.
- Simon T, Feller E. Diverse presentation of secondary aortoenteric fistulae. Case Report Med 2011; 2011:406730.
- Schwab CW, McMahon DJ, Phillips G, Pentecost MJ. Aortic balloon control of a traumatic aortoenteric fistula after damage control laparotomy: a case report. J Trauma 1996; 40:1021–1023.
- Napoli PJ, Meade PC, Adams CW. Primary aortoenteric fistula from a posttraumatic pseudoaneurysm. J Trauma 1996; 41:149–152.
- Laser A, Baker N, Rectenwald J, Eliason JL, Criado-Pallares E, Upchurch GR. Graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2011; 54:58–63.
- Luo CY, Lai CH, Wen JS, Lin BW. Secondary aortocolic fistula: case report and review of the literature. Ann Vasc Surg 2010; 24:256.e5–256.e12.
- Kim JY, Kim YW, Kim CJ, Lim HI, Kim DI, Huh S. Successful surgical treatment of aortoenteric fistula. J Korean Med Sci 2007; 22:846–850.
- Verhey P, Best A, Lakin P, Nachiondo J, Petersen B. Successful endovascular treatment of aortoenteric fistula secondary to eroding duodenal stent. J Vasc Interv Radiol 2006; 17:1345–1348.
- Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. In:Hockberger RS, editor: UpToDate. Waltham, MA: UpToDate, 2012.
A 74-year-old man presented to the emergency department in December 2011 with a 1-week history of worsening abdominal pain, nausea with emesis, and decreased appetite. The pain was dull, diffuse, and not related to oral intake or bowel movements. He denied any bloody stools, melena, or hematemesis, but he had not had a bowel movement in the past week.
He was already known to have stage IV colon cancer with metastases to the lungs and liver. He had undergone a partial colectomy in 2009 and was receiving chemotherapy at the time of admission.
He also had an infrarenal abdominal aortic aneurysm that had been repaired in 2003 with endovascular placement of a Gore Excluder stent graft. This was complicated by a type II endoleak, treated with coil embolization. The same endoleak later recurred and was treated with injection of Onyx liquid embolic agent.
His medical history also included hypertension, type 2 diabetes mellitus, and hyperlipidemia. He had undergone a laparoscopic cholecystectomy in 2007.
He denied any fevers, chills, headache, lightheadedness, or change in vision. He had no respiratory, cardiac, or urinary symptoms. He had been constipated for the past few weeks and had recently been started on a bowel regimen, with mild relief. There had been no other changes to his medications.
His temperature on presentation was 97.5°F (36.4°C), blood pressure 120/64 mm Hg, pulse 96, respiratory rate 22, and oxygen saturation 95% on room air. He was awake, alert, oriented, and in no acute distress. His mucous membranes were dry. His lungs were clear to auscultation, and his heart sounds were normal. His bowel sounds were hyperactive and his abdomen was slightly tender diffusely, but there was no abdominal distention, rebound tenderness, guarding, or palpable masses. His joints, muscle strength, and muscle tone were normal. Table 1 shows his initial laboratory values.
Given the patient’s history of colon cancer, the emergency department physician ordered computed tomography (CT) of the abdomen to assess the state of his disease and to evaluate for bowel obstruction. The scan revealed a large abdominal aortic aneurysm with foci of gas within the aneurysmal sac. Metastases in the liver, lung, and retroperitoneum appeared stable; abundant colonic stool suggested constipation (Figure 1).
CAUSES OF PERIAORTIC GAS AFTER ANEURYSM REPAIR
1. What is the most common cause of periaortic ectopic gas in a patient with a repaired abdominal aortic aneurysm?
- Endoleak
- Stent graft infection
- Retroperitoneal fibrosis
- Aortoenteric fistula
Endoleak
Endoleak, a complication of endovascular abdominal aortic aneurysm repair, is defined as blood flow within the aneurysm sac but outside the endoluminal graft.1 It occurs in up to 15% of patients after endograft placement in the first month alone, and in up to 47% of patients eventually.2 It can lead to aneurysm enlargement and rupture. Endoleaks are classified into five types, each with different causes and management options.3,4 Contrast-enhanced CT is the most commonly used diagnostic tool.5
Endoleak cannot be ruled out in our patient, since CT was done without contrast. However, gas within the aneurysm is not consistent with this diagnosis.
Stent graft infection
Infection has been reported in 1% to 6% of patients receiving a stent graft for aortic aneurysm.6 They occur most commonly in the first year after placement; one study showed that 42% of patients diagnosed with graft infection presented within 3 months of endovascular repair.7
The leading cause of graft infection is contamination during the original procedure, but secondary infection from hematologic seeding and contamination from adjacent bowel are also possible.8 In our patient, who underwent graft placement followed by endovascular repairs of endoleaks, bacterial seeding of his aortic aneurysm from the procedures should be considered.9
The most common organisms are staphylococcal species, with Staphylococcus aureus more common in early infection and coagulase-negative staphylococci more common in late infection.10 Methicillin-resistant S aureus has been reported in as many as 25% of cases of graft infection. Diphtheroids and gram-negative enteric organisms should also be considered.11
CT is the most effective imaging test for graft infection. Perigraft soft tissue, fluid, and gas are the major CT findings.12
Given that our patient presented with abdominal pain, leukocytosis, and the CT finding of perigraft gas, graft infection should be high on our list differential diagnoses.
Retroperitoneal fibrosis
Retroperitoneal fibrosis is most often idiopathic, although many believe it is due to an exaggerated local inflammatory reaction to aortic atherosclerosis or is a manifestation of a systemic autoimmune disease.13 Secondary retroperitoneal fibrosis may be due to drugs, infection, or malignancy.
Pathologic findings include sclerotic plaques, typically around the abdominal vessels and ureters. Clinical presentations are often nonspecific, with early symptoms that include back or abdominal pain, malaise, anorexia, edema, and hematuria.14,15 Progressive ureteral obstruction can occur in later stages. CT with contrast is the imaging test of choice to visualize the extent of disease, with the fibrosis exhibiting attenuation similar to that of muscle.16
Initial treatment of idiopathic retroperitoneal fibrosis is with a glucocorticoid or other immunosuppressive agent, whereas treatment of secondary retroperitoneal fibrosis is aimed at the underlying cause.17 Late stages complicated by ureteral obstruction typically require surgery.18
Our patient did have some nonspecific complaints that could be due to retroperitoneal fibrosis. He also had an intra-abdominal malignancy, which could lead to secondary retroperitoneal fibrosis. However, his CT findings of periaortic gas are not consistent with this diagnosis.19
Aortoenteric fistula
Aortoenteric fistulas can be either primary or secondary.
Primary aortoenteric fistulas occur de novo in patients who have never undergone any surgery or procedure in the aorta. This type of fistula usually results from pressure erosion of an atherosclerotic abdominal aortic aneurysm into the gastrointestinal tract. They are rare, with an annual incidence of 0.04% to 0.07% in the general population.20,21
Secondary aortoenteric fistulas are complications of aortic reconstructive therapy. After open repair, a perianastomotic or pseudoaneurysmal fistula can develop into the gastrointestinal tract.4 Endovascular repair leaves the aortic wall intact with no exposed suture lines, but an aortoenteric fistula can still develop22 and in fact occur in 0.4% to 3.1% of recipients of stent grafts for abdominal aortic aneurysm repair.23 In such cases, it is commonly thought that graft infection can lead to formation of an aortoenteric fistula, but a penetrating gastrointestinal ulcer, tumor invasion, radiation therapy, and trauma have also been implicated.19,24–26 An aortoenteric fistula can present several months to several years after either open or endovascular abdominal aortic aneurysm repair.4,23
One of the main CT signs of an aortoenteric fistula is periaortic ectopic gas at least 3 to 4 weeks after surgery or endovascular repair.19 Gas around the stent graft is most commonly caused by infection, but an aortoenteric fistula must also be considered in our patient, as roughly one-third of graft infections present as aortoenteric fistula.27 Our patient denied having any gastrointestinal bleeding, but his hemoglobin concentration at presentation was 8.9 g/dL.
Highlight point. Perigraft gas after abdominal aortic aneurysm repair can be seen in graft infection and aortoenteric fistula.
SIGNS AND SYMPTOMS OF AORTOENTERIC FISTULA
2. What is the most common clinical sign or symptom of an aortoenteric fistula?
- Gastrointestinal bleeding
- Sepsis
- Abdominal pain
- Back pain
Gastrointestinal bleeding occurs in 80% of patients who have an aortoenteric fistula, sepsis in 40%, abdominal pain in 30%, and back pain in 15%.19 The classic triad of symptoms is gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass. However, symptoms can vary widely, and the classic triad is present in fewer than 25% of cases.28 Sepsis may be the predominant clinical manifestation, particularly in the early stages of fistula formation. Unexplained fever is an underrecognized early manifestation.24
Highlight point. The classic triad of symptoms of an aortoenteric fistula (gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass) is seen in fewer than 25% of cases.
Case continued: The patient develops frank bleeding
The vascular surgery service was consulted because of concern for an aortic graft infection, since surgical removal of the infected material is recommended.10 The patient was deemed to be a poor surgical candidate, given his stage IV colon cancer, so he was treated conservatively with broad-spectrum antibiotics.
Over the next 2 days, he had two episodes of dark, bloody bowel movements, but he remained hemodynamically stable. He subsequently developed frank bleeding per rectum with symptoms of lightheadedness, and his hemoglobin concentration fell to 6.9 g/dL. He was given a total of 3 units of packed red blood cells, which raised his hemoglobin level, but only to 8.3 g/dL. The gastroenterology service was consulted to evaluate for the source of the bleeding.
Comment. In a situation like this, an aortoenteric fistula is high on our list of differential diagnoses as the cause of bleeding, but other causes of frank bleeding per rectum such as diverticulosis, arteriovenous malformation, hemorrhoids, or a rapid upper-gastrointestinal bleed cannot be ruled out.
Upper-gastrointestinal endoscopy is the most commonly used diagnostic test for aortoenteric fistulas. It can also find other possible sources of gastrointestinal bleeding. CT with contrast is another option. It can depict the fistula itself or reveal signs of infection, such as gas or liquid surrounding the graft. In an emergency, when there is not enough time for diagnostic testing and an aortoenteric fistula is strongly suspected on clinical grounds, emergency surgical exploration is warranted.4,24
In our patient, the gastrointestinal service elected to first perform endoscopy to look for an aortoenteric fistula.
WHERE DO AORTOENTERIC FISTULAS OCCUR?
3. In which part of the gastrointestinal tract is an aortoenteric fistula most commonly located?
- Esophagus
- Stomach
- Duodenum
- Jejunum
Aortoenteric fistulas can occur at any of these locations, but 80% of cases of secondary aortoenteric fistula are in the duodenum, most often in the third or fourth (horizontal or ascending) part.19 Endoscopic visualization of a pulsatile bleeding mass in this area is diagnostic. However, even if no fistula is seen, upper endoscopy cannot rule out an aortoenteric fistula because the lesion can be located more distal than the scope can reach, which is typically no farther than the first or second parts.4,24
Case continued: What endoscopy showed
The esophagus was normal. There was old clotted blood in the stomach, but no lesions or ulcers. The duodenal bulb and second portion of the duodenum were normal. Three ulcers were noted in the third and fourth portions of the duodenum. The largest and deepest ulcer had an adherent blood clot, and the bowel wall was pulsatile in this region (Figure 2). These findings revealed the source of the gastrointestinal bleeding and were consistent with an aortoenteric fistula.
The patient’s initial bloody bowel movements were herald bleeds, ie, transient and self-limited episodes resulting from necrosis and mucosal ulceration. Herald bleeds can precede a massive gastrointestinal hemorrhage resulting from a true aortoenteric communication.19
Highlight point. Herald bleeds are self-limited and precede hemorrhage that results from a true aortoenteric communication.
TREATMENT OF AORTOENTERIC FISTULA
4. How are aortoenteric fistulas treated?
- Surgery
- Antibiotics
- Endoscopic intervention
Surgery is the definitive treatment. The traditional procedure is open surgical resection of the affected portion of the aorta followed by extra-anatomic (axillobifemoral) bypass or in situ aortic reconstruction using an antibiotic-impregnated prosthetic graft, autogenous femoral vein graft, or cryopreserved allograft.9,29 There have been cases of successful endovascular repair of aortoenteric fistulas, but this approach is generally used as a palliative bridge to definitive surgery.30
Antibiotics should be used if graft infection is suspected, ie, in most cases. However, surgery is still needed to repair the fistula and remove the source of infection. Cultures taken during surgical repair can help guide the choice of antibiotic after surgery.
Endoscopy can aid in diagnosing an aortoenteric fistula, as in the case of our patient. However, vascular surgery is necessary to close the communication between the aorta and the gastrointestinal tract.
Case continued: The patient declines treatment
In view of the patient’s enteroscopic findings, the vascular surgery service was again consulted for surgical correction of the aortoenteric fistula. Treatment was discussed with the patient and his family, but they declined any intervention in view of the high risk of morbidity and death that surgery would entail. Nearing the end of life with advanced cancer and a newly diagnosed aortoenteric fistula, the patient preferred comfort measures with hospice care.
Take-home points
Abdominal pain is the reason for 5% to 10% of emergency department visits, and between 35% to 41% of patients admitted to the hospital because of abdominal pain do not have a definitive diagnosis.31 It is crucial to think about an aortoenteric fistula in such patients who have a history of abdominal aortic aneurysm repair and gastrointestinal bleeding. Timely diagnosis and intervention are necessary to manage this otherwise-fatal condition.
A 74-year-old man presented to the emergency department in December 2011 with a 1-week history of worsening abdominal pain, nausea with emesis, and decreased appetite. The pain was dull, diffuse, and not related to oral intake or bowel movements. He denied any bloody stools, melena, or hematemesis, but he had not had a bowel movement in the past week.
He was already known to have stage IV colon cancer with metastases to the lungs and liver. He had undergone a partial colectomy in 2009 and was receiving chemotherapy at the time of admission.
He also had an infrarenal abdominal aortic aneurysm that had been repaired in 2003 with endovascular placement of a Gore Excluder stent graft. This was complicated by a type II endoleak, treated with coil embolization. The same endoleak later recurred and was treated with injection of Onyx liquid embolic agent.
His medical history also included hypertension, type 2 diabetes mellitus, and hyperlipidemia. He had undergone a laparoscopic cholecystectomy in 2007.
He denied any fevers, chills, headache, lightheadedness, or change in vision. He had no respiratory, cardiac, or urinary symptoms. He had been constipated for the past few weeks and had recently been started on a bowel regimen, with mild relief. There had been no other changes to his medications.
His temperature on presentation was 97.5°F (36.4°C), blood pressure 120/64 mm Hg, pulse 96, respiratory rate 22, and oxygen saturation 95% on room air. He was awake, alert, oriented, and in no acute distress. His mucous membranes were dry. His lungs were clear to auscultation, and his heart sounds were normal. His bowel sounds were hyperactive and his abdomen was slightly tender diffusely, but there was no abdominal distention, rebound tenderness, guarding, or palpable masses. His joints, muscle strength, and muscle tone were normal. Table 1 shows his initial laboratory values.
Given the patient’s history of colon cancer, the emergency department physician ordered computed tomography (CT) of the abdomen to assess the state of his disease and to evaluate for bowel obstruction. The scan revealed a large abdominal aortic aneurysm with foci of gas within the aneurysmal sac. Metastases in the liver, lung, and retroperitoneum appeared stable; abundant colonic stool suggested constipation (Figure 1).
CAUSES OF PERIAORTIC GAS AFTER ANEURYSM REPAIR
1. What is the most common cause of periaortic ectopic gas in a patient with a repaired abdominal aortic aneurysm?
- Endoleak
- Stent graft infection
- Retroperitoneal fibrosis
- Aortoenteric fistula
Endoleak
Endoleak, a complication of endovascular abdominal aortic aneurysm repair, is defined as blood flow within the aneurysm sac but outside the endoluminal graft.1 It occurs in up to 15% of patients after endograft placement in the first month alone, and in up to 47% of patients eventually.2 It can lead to aneurysm enlargement and rupture. Endoleaks are classified into five types, each with different causes and management options.3,4 Contrast-enhanced CT is the most commonly used diagnostic tool.5
Endoleak cannot be ruled out in our patient, since CT was done without contrast. However, gas within the aneurysm is not consistent with this diagnosis.
Stent graft infection
Infection has been reported in 1% to 6% of patients receiving a stent graft for aortic aneurysm.6 They occur most commonly in the first year after placement; one study showed that 42% of patients diagnosed with graft infection presented within 3 months of endovascular repair.7
The leading cause of graft infection is contamination during the original procedure, but secondary infection from hematologic seeding and contamination from adjacent bowel are also possible.8 In our patient, who underwent graft placement followed by endovascular repairs of endoleaks, bacterial seeding of his aortic aneurysm from the procedures should be considered.9
The most common organisms are staphylococcal species, with Staphylococcus aureus more common in early infection and coagulase-negative staphylococci more common in late infection.10 Methicillin-resistant S aureus has been reported in as many as 25% of cases of graft infection. Diphtheroids and gram-negative enteric organisms should also be considered.11
CT is the most effective imaging test for graft infection. Perigraft soft tissue, fluid, and gas are the major CT findings.12
Given that our patient presented with abdominal pain, leukocytosis, and the CT finding of perigraft gas, graft infection should be high on our list differential diagnoses.
Retroperitoneal fibrosis
Retroperitoneal fibrosis is most often idiopathic, although many believe it is due to an exaggerated local inflammatory reaction to aortic atherosclerosis or is a manifestation of a systemic autoimmune disease.13 Secondary retroperitoneal fibrosis may be due to drugs, infection, or malignancy.
Pathologic findings include sclerotic plaques, typically around the abdominal vessels and ureters. Clinical presentations are often nonspecific, with early symptoms that include back or abdominal pain, malaise, anorexia, edema, and hematuria.14,15 Progressive ureteral obstruction can occur in later stages. CT with contrast is the imaging test of choice to visualize the extent of disease, with the fibrosis exhibiting attenuation similar to that of muscle.16
Initial treatment of idiopathic retroperitoneal fibrosis is with a glucocorticoid or other immunosuppressive agent, whereas treatment of secondary retroperitoneal fibrosis is aimed at the underlying cause.17 Late stages complicated by ureteral obstruction typically require surgery.18
Our patient did have some nonspecific complaints that could be due to retroperitoneal fibrosis. He also had an intra-abdominal malignancy, which could lead to secondary retroperitoneal fibrosis. However, his CT findings of periaortic gas are not consistent with this diagnosis.19
Aortoenteric fistula
Aortoenteric fistulas can be either primary or secondary.
Primary aortoenteric fistulas occur de novo in patients who have never undergone any surgery or procedure in the aorta. This type of fistula usually results from pressure erosion of an atherosclerotic abdominal aortic aneurysm into the gastrointestinal tract. They are rare, with an annual incidence of 0.04% to 0.07% in the general population.20,21
Secondary aortoenteric fistulas are complications of aortic reconstructive therapy. After open repair, a perianastomotic or pseudoaneurysmal fistula can develop into the gastrointestinal tract.4 Endovascular repair leaves the aortic wall intact with no exposed suture lines, but an aortoenteric fistula can still develop22 and in fact occur in 0.4% to 3.1% of recipients of stent grafts for abdominal aortic aneurysm repair.23 In such cases, it is commonly thought that graft infection can lead to formation of an aortoenteric fistula, but a penetrating gastrointestinal ulcer, tumor invasion, radiation therapy, and trauma have also been implicated.19,24–26 An aortoenteric fistula can present several months to several years after either open or endovascular abdominal aortic aneurysm repair.4,23
One of the main CT signs of an aortoenteric fistula is periaortic ectopic gas at least 3 to 4 weeks after surgery or endovascular repair.19 Gas around the stent graft is most commonly caused by infection, but an aortoenteric fistula must also be considered in our patient, as roughly one-third of graft infections present as aortoenteric fistula.27 Our patient denied having any gastrointestinal bleeding, but his hemoglobin concentration at presentation was 8.9 g/dL.
Highlight point. Perigraft gas after abdominal aortic aneurysm repair can be seen in graft infection and aortoenteric fistula.
SIGNS AND SYMPTOMS OF AORTOENTERIC FISTULA
2. What is the most common clinical sign or symptom of an aortoenteric fistula?
- Gastrointestinal bleeding
- Sepsis
- Abdominal pain
- Back pain
Gastrointestinal bleeding occurs in 80% of patients who have an aortoenteric fistula, sepsis in 40%, abdominal pain in 30%, and back pain in 15%.19 The classic triad of symptoms is gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass. However, symptoms can vary widely, and the classic triad is present in fewer than 25% of cases.28 Sepsis may be the predominant clinical manifestation, particularly in the early stages of fistula formation. Unexplained fever is an underrecognized early manifestation.24
Highlight point. The classic triad of symptoms of an aortoenteric fistula (gastrointestinal bleeding, abdominal pain, and a pulsatile abdominal mass) is seen in fewer than 25% of cases.
Case continued: The patient develops frank bleeding
The vascular surgery service was consulted because of concern for an aortic graft infection, since surgical removal of the infected material is recommended.10 The patient was deemed to be a poor surgical candidate, given his stage IV colon cancer, so he was treated conservatively with broad-spectrum antibiotics.
Over the next 2 days, he had two episodes of dark, bloody bowel movements, but he remained hemodynamically stable. He subsequently developed frank bleeding per rectum with symptoms of lightheadedness, and his hemoglobin concentration fell to 6.9 g/dL. He was given a total of 3 units of packed red blood cells, which raised his hemoglobin level, but only to 8.3 g/dL. The gastroenterology service was consulted to evaluate for the source of the bleeding.
Comment. In a situation like this, an aortoenteric fistula is high on our list of differential diagnoses as the cause of bleeding, but other causes of frank bleeding per rectum such as diverticulosis, arteriovenous malformation, hemorrhoids, or a rapid upper-gastrointestinal bleed cannot be ruled out.
Upper-gastrointestinal endoscopy is the most commonly used diagnostic test for aortoenteric fistulas. It can also find other possible sources of gastrointestinal bleeding. CT with contrast is another option. It can depict the fistula itself or reveal signs of infection, such as gas or liquid surrounding the graft. In an emergency, when there is not enough time for diagnostic testing and an aortoenteric fistula is strongly suspected on clinical grounds, emergency surgical exploration is warranted.4,24
In our patient, the gastrointestinal service elected to first perform endoscopy to look for an aortoenteric fistula.
WHERE DO AORTOENTERIC FISTULAS OCCUR?
3. In which part of the gastrointestinal tract is an aortoenteric fistula most commonly located?
- Esophagus
- Stomach
- Duodenum
- Jejunum
Aortoenteric fistulas can occur at any of these locations, but 80% of cases of secondary aortoenteric fistula are in the duodenum, most often in the third or fourth (horizontal or ascending) part.19 Endoscopic visualization of a pulsatile bleeding mass in this area is diagnostic. However, even if no fistula is seen, upper endoscopy cannot rule out an aortoenteric fistula because the lesion can be located more distal than the scope can reach, which is typically no farther than the first or second parts.4,24
Case continued: What endoscopy showed
The esophagus was normal. There was old clotted blood in the stomach, but no lesions or ulcers. The duodenal bulb and second portion of the duodenum were normal. Three ulcers were noted in the third and fourth portions of the duodenum. The largest and deepest ulcer had an adherent blood clot, and the bowel wall was pulsatile in this region (Figure 2). These findings revealed the source of the gastrointestinal bleeding and were consistent with an aortoenteric fistula.
The patient’s initial bloody bowel movements were herald bleeds, ie, transient and self-limited episodes resulting from necrosis and mucosal ulceration. Herald bleeds can precede a massive gastrointestinal hemorrhage resulting from a true aortoenteric communication.19
Highlight point. Herald bleeds are self-limited and precede hemorrhage that results from a true aortoenteric communication.
TREATMENT OF AORTOENTERIC FISTULA
4. How are aortoenteric fistulas treated?
- Surgery
- Antibiotics
- Endoscopic intervention
Surgery is the definitive treatment. The traditional procedure is open surgical resection of the affected portion of the aorta followed by extra-anatomic (axillobifemoral) bypass or in situ aortic reconstruction using an antibiotic-impregnated prosthetic graft, autogenous femoral vein graft, or cryopreserved allograft.9,29 There have been cases of successful endovascular repair of aortoenteric fistulas, but this approach is generally used as a palliative bridge to definitive surgery.30
Antibiotics should be used if graft infection is suspected, ie, in most cases. However, surgery is still needed to repair the fistula and remove the source of infection. Cultures taken during surgical repair can help guide the choice of antibiotic after surgery.
Endoscopy can aid in diagnosing an aortoenteric fistula, as in the case of our patient. However, vascular surgery is necessary to close the communication between the aorta and the gastrointestinal tract.
Case continued: The patient declines treatment
In view of the patient’s enteroscopic findings, the vascular surgery service was again consulted for surgical correction of the aortoenteric fistula. Treatment was discussed with the patient and his family, but they declined any intervention in view of the high risk of morbidity and death that surgery would entail. Nearing the end of life with advanced cancer and a newly diagnosed aortoenteric fistula, the patient preferred comfort measures with hospice care.
Take-home points
Abdominal pain is the reason for 5% to 10% of emergency department visits, and between 35% to 41% of patients admitted to the hospital because of abdominal pain do not have a definitive diagnosis.31 It is crucial to think about an aortoenteric fistula in such patients who have a history of abdominal aortic aneurysm repair and gastrointestinal bleeding. Timely diagnosis and intervention are necessary to manage this otherwise-fatal condition.
- Hong C, Heiken JP, Sicard GA, Pilgram TK, Bae KT. Clinical significance of endoleak detected on follow-up CT after endovascular repair of abdominal aortic aneurysm. AJR Am J Roentgenol 2008; 191:808–813.
- Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg 2002; 35:1029–1035.
- Corriere MA, Feurer ID, Becker SY, et al. Endoleak following endovascular abdominal aortic aneurysm repair: implications for duration of screening. Ann Surg 2004; 239:800–805.
- Saratzis N, Saratzis A, Melas N, Ktenidis K, Kiskinis D. Aortoduodenal fistulas after endovascular stent-graft repair of abdominal aortic aneurysms: single-center experience and review of the literature. J Endovasc Ther 2008; 15:441–448.
- Demko TM, Diamond JR, Groff J. Obstructive nephropathy as a result of retroperitoneal fibrosis: a review of its pathogenesis and associations. J Am Soc Nephrol 1997; 8:684–688.
- Zetrenne E, McIntosh BC, McRae MH, Gusberg R, Evans GR, Narayan D. Prosthetic vascular graft infection: a multi-center review of surgical management. Yale J Biol Med 2007; 80:113–121.
- Vogel TR, Symons R, Flum DR. The incidence and factors associated with graft infection after aortic aneurysm repair. J Vasc Surg 2008; 47:264–269.
- Swain TW, Calligaro KD, Dougherty MD. Management of infected aortic prosthetic grafts. Vasc Endovascular Surg 2004; 38:75–82.
- Cernohorsky P, Reijnen MM, Tielliu IF, van Sterkenburg SM, van den Dungen JJ, Zeebregts CJ. The relevance of aortic endograft prosthetic infection. J Vasc Surg 2011; 54:327–333.
- FitzGerald SF, Kelly C, Humphreys H. Diagnosis and treatment of prosthetic aortic graft infections: confusion and inconsistency in the absence of evidence or consensus. J Antimicrob Chemother 2005; 56:996–999.
- Orton DF, LeVeen RF, Saigh JA, et al. Aortic prosthetic graft infections: radiologic manifestations and implications for management. Radiographics 2000; 20:977–993.
- Pacanowski JP, Dieter RS, Stevens SL, Freeman MB, Goldman MH. Endoleak: the achilles heel of endovascular abdominal aortic aneurysm exclusion—a case report. WMJ 2002; 101:57–58,63.
- van Bommel EF. Retroperitoneal fibrosis. Neth J Med 2002; 60:231–242.
- Utz DC, Henry JD. Retroperitoneal fibrosis. Med Clin North Am 1966; 50:1091–1099.
- Dalla-Palma L, Rocca-Rossetti S, Pozzi-Mucelli RS, Rizzatto G. Computed tomography in the diagnosis of retroperitoneal fibrosis. Urol Radiol 1981; 3:77–83.
- Harreby M, Bilde T, Helin P, Meyhoff HH, Vinterberg H, Nielsen VA. Retroperitoneal fibrosis treated with methylprednisolon pulse and disease-modifying antirheumatic drugs. Scand J Urol Nephrol 1994; 28:237–242.
- Jois RN, Gaffney K, Marshall T, Scott DG. Chronic periaortitis. Rheumatology (Oxford) 2004; 43:1441–1446.
- Saers SJ, Scheltinga MR. Primary aortoenteric fistula. Br J Surg 2005; 92:143–152.
- Baril DT, Carroccio A, Ellozy SH, et al. Evolving strategies for the treatment of aortoenteric fistulas. J Vasc Surg 2006; 44:250–257.
- Vu QD, Menias CO, Bhalla S, Peterson C, Wang LL, Balfe DM. Aortoenteric fistulas: CT features and potential mimics. Radiographics 2009; 29:197–209.
- Jayarajan S, Napolitano LM, Rectenwald JE, Upchurch GR. Primary aortoenteric fistula and endovascular repair. Vasc Endovascular Surg 2009; 43:592–596.
- Ruby BJ, Cogbill TH. Aortoduodenal fistula 5 years after endovascular abdominal aortic aneurysm repair with the Ancure stent graft. J Vasc Surg 2007; 45:834–836.
- Senadhi V, Brown JC, Arora D, Shaffer R, Shetty D, Mackrell P. A mysterious cause of gastrointestinal bleeding disguising itself as diverticulosis and peptic ulcer disease: a review of diagnostic modalities for aortoenteric fistula. Case Rep Gastroenterol 2010; 4:510–517.
- Simon T, Feller E. Diverse presentation of secondary aortoenteric fistulae. Case Report Med 2011; 2011:406730.
- Schwab CW, McMahon DJ, Phillips G, Pentecost MJ. Aortic balloon control of a traumatic aortoenteric fistula after damage control laparotomy: a case report. J Trauma 1996; 40:1021–1023.
- Napoli PJ, Meade PC, Adams CW. Primary aortoenteric fistula from a posttraumatic pseudoaneurysm. J Trauma 1996; 41:149–152.
- Laser A, Baker N, Rectenwald J, Eliason JL, Criado-Pallares E, Upchurch GR. Graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2011; 54:58–63.
- Luo CY, Lai CH, Wen JS, Lin BW. Secondary aortocolic fistula: case report and review of the literature. Ann Vasc Surg 2010; 24:256.e5–256.e12.
- Kim JY, Kim YW, Kim CJ, Lim HI, Kim DI, Huh S. Successful surgical treatment of aortoenteric fistula. J Korean Med Sci 2007; 22:846–850.
- Verhey P, Best A, Lakin P, Nachiondo J, Petersen B. Successful endovascular treatment of aortoenteric fistula secondary to eroding duodenal stent. J Vasc Interv Radiol 2006; 17:1345–1348.
- Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. In:Hockberger RS, editor: UpToDate. Waltham, MA: UpToDate, 2012.
- Hong C, Heiken JP, Sicard GA, Pilgram TK, Bae KT. Clinical significance of endoleak detected on follow-up CT after endovascular repair of abdominal aortic aneurysm. AJR Am J Roentgenol 2008; 191:808–813.
- Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg 2002; 35:1029–1035.
- Corriere MA, Feurer ID, Becker SY, et al. Endoleak following endovascular abdominal aortic aneurysm repair: implications for duration of screening. Ann Surg 2004; 239:800–805.
- Saratzis N, Saratzis A, Melas N, Ktenidis K, Kiskinis D. Aortoduodenal fistulas after endovascular stent-graft repair of abdominal aortic aneurysms: single-center experience and review of the literature. J Endovasc Ther 2008; 15:441–448.
- Demko TM, Diamond JR, Groff J. Obstructive nephropathy as a result of retroperitoneal fibrosis: a review of its pathogenesis and associations. J Am Soc Nephrol 1997; 8:684–688.
- Zetrenne E, McIntosh BC, McRae MH, Gusberg R, Evans GR, Narayan D. Prosthetic vascular graft infection: a multi-center review of surgical management. Yale J Biol Med 2007; 80:113–121.
- Vogel TR, Symons R, Flum DR. The incidence and factors associated with graft infection after aortic aneurysm repair. J Vasc Surg 2008; 47:264–269.
- Swain TW, Calligaro KD, Dougherty MD. Management of infected aortic prosthetic grafts. Vasc Endovascular Surg 2004; 38:75–82.
- Cernohorsky P, Reijnen MM, Tielliu IF, van Sterkenburg SM, van den Dungen JJ, Zeebregts CJ. The relevance of aortic endograft prosthetic infection. J Vasc Surg 2011; 54:327–333.
- FitzGerald SF, Kelly C, Humphreys H. Diagnosis and treatment of prosthetic aortic graft infections: confusion and inconsistency in the absence of evidence or consensus. J Antimicrob Chemother 2005; 56:996–999.
- Orton DF, LeVeen RF, Saigh JA, et al. Aortic prosthetic graft infections: radiologic manifestations and implications for management. Radiographics 2000; 20:977–993.
- Pacanowski JP, Dieter RS, Stevens SL, Freeman MB, Goldman MH. Endoleak: the achilles heel of endovascular abdominal aortic aneurysm exclusion—a case report. WMJ 2002; 101:57–58,63.
- van Bommel EF. Retroperitoneal fibrosis. Neth J Med 2002; 60:231–242.
- Utz DC, Henry JD. Retroperitoneal fibrosis. Med Clin North Am 1966; 50:1091–1099.
- Dalla-Palma L, Rocca-Rossetti S, Pozzi-Mucelli RS, Rizzatto G. Computed tomography in the diagnosis of retroperitoneal fibrosis. Urol Radiol 1981; 3:77–83.
- Harreby M, Bilde T, Helin P, Meyhoff HH, Vinterberg H, Nielsen VA. Retroperitoneal fibrosis treated with methylprednisolon pulse and disease-modifying antirheumatic drugs. Scand J Urol Nephrol 1994; 28:237–242.
- Jois RN, Gaffney K, Marshall T, Scott DG. Chronic periaortitis. Rheumatology (Oxford) 2004; 43:1441–1446.
- Saers SJ, Scheltinga MR. Primary aortoenteric fistula. Br J Surg 2005; 92:143–152.
- Baril DT, Carroccio A, Ellozy SH, et al. Evolving strategies for the treatment of aortoenteric fistulas. J Vasc Surg 2006; 44:250–257.
- Vu QD, Menias CO, Bhalla S, Peterson C, Wang LL, Balfe DM. Aortoenteric fistulas: CT features and potential mimics. Radiographics 2009; 29:197–209.
- Jayarajan S, Napolitano LM, Rectenwald JE, Upchurch GR. Primary aortoenteric fistula and endovascular repair. Vasc Endovascular Surg 2009; 43:592–596.
- Ruby BJ, Cogbill TH. Aortoduodenal fistula 5 years after endovascular abdominal aortic aneurysm repair with the Ancure stent graft. J Vasc Surg 2007; 45:834–836.
- Senadhi V, Brown JC, Arora D, Shaffer R, Shetty D, Mackrell P. A mysterious cause of gastrointestinal bleeding disguising itself as diverticulosis and peptic ulcer disease: a review of diagnostic modalities for aortoenteric fistula. Case Rep Gastroenterol 2010; 4:510–517.
- Simon T, Feller E. Diverse presentation of secondary aortoenteric fistulae. Case Report Med 2011; 2011:406730.
- Schwab CW, McMahon DJ, Phillips G, Pentecost MJ. Aortic balloon control of a traumatic aortoenteric fistula after damage control laparotomy: a case report. J Trauma 1996; 40:1021–1023.
- Napoli PJ, Meade PC, Adams CW. Primary aortoenteric fistula from a posttraumatic pseudoaneurysm. J Trauma 1996; 41:149–152.
- Laser A, Baker N, Rectenwald J, Eliason JL, Criado-Pallares E, Upchurch GR. Graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2011; 54:58–63.
- Luo CY, Lai CH, Wen JS, Lin BW. Secondary aortocolic fistula: case report and review of the literature. Ann Vasc Surg 2010; 24:256.e5–256.e12.
- Kim JY, Kim YW, Kim CJ, Lim HI, Kim DI, Huh S. Successful surgical treatment of aortoenteric fistula. J Korean Med Sci 2007; 22:846–850.
- Verhey P, Best A, Lakin P, Nachiondo J, Petersen B. Successful endovascular treatment of aortoenteric fistula secondary to eroding duodenal stent. J Vasc Interv Radiol 2006; 17:1345–1348.
- Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. In:Hockberger RS, editor: UpToDate. Waltham, MA: UpToDate, 2012.