User login
Dyspnea in Malignancy
Overview
Dyspnea, the subjective inability to breathe comfortably, is a common symptom, accounting for 3 to 4 million ED visits annually.1,2 Dyspnea can be acute, subacute, or chronic, with chronic dyspnea defined as the presence of symptoms for more than 1 to 2 months, and subacute dyspnea as symptoms lasting for hours to days.3,4
The reported prevalence of dyspnea based on etiology presenting to the ED is as follows: malignancy (16% to 77%), chronic heart failure (18% to 88%), chronic obstructive pulmonary disease (56% to 98%), or renal disease (11% to 82%).5 Irrespective of time course and etiology, dyspnea can be a debilitating symptom affecting up to 50% of patients admitted to acute, tertiary care hospitals, and a quarter of patients seeking care in the ambulatory setting.6-13
Although dyspnea is common in patients with cancer, it is often underreported.14-19 This article reviews the current information available regarding the epidemiology, pathophysiology, assessment, and treatment of dyspnea in the oncologic patient population. By providing insight into the unique nature of dyspnea for oncologic patients, this article illustrates why dyspnea is an important symptom to recognize, address, and treat.
Epidemiology, Background, and Recognition
Dyspnea has a significant impact on the quality of life for oncologic patients and often intensifies near the time of death. At one regional oncology center, dyspnea was present in 49% of general cancer patients.20 The percentage and severity of dyspnea increases as a patient’s cancer progresses. The National Hospice Study found that dyspnea occurred in 70% of 1,754 terminally ill cancer patients studied during their last 6 weeks of life; more than 28% of these patients graded the distress that they felt from dyspnea as moderate to severe. The incidence of dyspnea was exceeded only by the incidences of pain and anorexia.21
A study conducted by Roberts et al22 showed that dyspnea is a very common, undertreated, and underrecognized condition. In 62% of patients reporting dyspnea, the symptoms had been present for greater than 3 months before patients received medical or nursing intervention. In the inpatient hospital setting, 77% of patients claimed on interview to experience dyspnea, but only 39% of cases were actually reported by nursing staff. In addition, Reddy et al23 found that dyspnea can negatively influence activities of daily living in oncology patients. Moreover, as the patients’ dyspnea intensified, their ability to perform activities of daily living was increasingly affected.23,24
Differential Diagnosis
Studies demonstrate the presence of dyspnea in approximately 20% to 40% of patients at the time of diagnosis of advanced disease, with the percentage increasing to around 70% in the last 6 weeks of life.25-27 Symptom grading is at least moderate in more than 28% of terminally ill cancer patients.21 Although common in oncology patients, dyspnea is difficult to diagnose because it is seldom explained by a single, specific organic etiology.24,25,28 Extrinsic modulators that are difficult to quantify, such as psychological state, cultural background, and life experiences, can also attenuate the perception of dyspnea.18,25
The cause of dyspnea in cancer patients can be broken down into four categories to aid in the differential diagnosis as described by Dudgeon et al29 in 2001 (Table 1). The four categories consist of dyspnea as being directly due to the malignancy itself; indirectly due to the malignancy; a side effect of cancer treatment; or due to underlying lung and cardiac disease unrelated to the malignancy itself.21,29
Etiology
Dyspnea Directly Due to Cancer. Dudgeon et al29 reported that the top three malignancies that directly caused dyspnea are lung, head and neck cancer, and malignancies of genitourinary origin.29 Reuben21 showed that 75% of patients with tumors involving the lung reported dyspnea at some point. Metastasis to the mediastinum, especially to the ribs, was associated with higher levels of dyspnea when compared to metastasis to lung.29
Dyspnea Indirectly Due to Cancer. Dyspnea due to indirect causes such as anemia, pneumonia, and pulmonary emboli may be easier to rectify once diagnosed.
Dyspnea Due to Cancer Treatment. Treatment-related causes of dyspnea include the side effects of surgery and lung irradiation. Patients with a history of pneumonectomy, lobectomy, or pleurodesis report higher levels of dyspnea as do patients with a history of thoracic irradiation. Radiation pneumonitis can occur 6 to 12 weeks following treatment, and radiation fibrosis can occur 6 to 12 months following treatment.29
Dyspnea Unrelated to Cancer. Other medical conditions that must be considered when diagnosing dyspnea include chronic obstructive pulmonary disease, asthma, and congestive heart failure. Psychological distress—described as anxiety and depression in the cancer population—may also cause the sensation of dyspnea.25
Dyspnea can be constant or occur as breakthrough episodes. In the study by Reddy et al,23 39% of patients had constant dyspnea and 20% experienced breakthrough episodes. Breakthrough dyspnea is shorter in duration, lasting less than 5 minutes. Breakthrough dyspnea occurs approximately 5 to 6 times a day and is predominant with 80% of patients who are symptom-free between episodes. As opiates are the drug of choice for dyspnea treatment, differentiating the time course of dyspnea is important in terms of treatment strategy and response. For example, treating breakthrough dyspnea with standard opiate medications might not work given that symptom onset and resolution might occur even before the medication’s onset of action.
Pathophysiology and Psychology
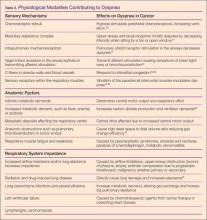
The cause of dyspnea in an oncology patient can be physiological and/or psychological in nature, with more numerous and unique presentations as compared to dyspnea associated with cardiac or lung disease. Chemoreceptor stimulation, mechanical stimuli originating in the lung and chest wall receptors, and neuroventilatory effects all contribute to dyspnea.12,16 Physiological modalities contributing to dyspnea are outlined in Table 2.
Psychological Contributions to the Etiology of Dyspnea
Dyspnea is subjective and biopsychosocial factors play a large role in an oncologic patient’s self-report of this condition. Multiple studies demonstrate that objective signs may not match the patient’s perception of dyspnea.18,21,25 Other studies report a correlation between psychological distress and worsened perception of dyspnea. Psychological distress is often measured by anxiety and depression and is also augmented by the presence of pain.
Assessment
Dyspnea is more challenging to assess in the ED than the laboratory setting36 as the condition is multidimensional in nature, often characterized by three factors consisting of breathing effort, chest tightness, and air hunger.37,38 Since these are subjective symptoms, assessment is difficult if a patient has delirium or other symptoms that alter the ability to provide a coherent response. Also, the subjective nature of dyspnea can lead to bias during symptom measurement and management.39
Research has documented factors such as lung involvement, anxiety, and maximum inspiratory pressure as influential to the perceived intensity of dyspnea for oncologic patients.40 Therefore, the sensation of dyspnea is multifactorial and includes physiological and psychological components for cancer patients.40 Subjective sensation does not always correlate with physiological measurements, adding to the difficulty of objective assessments.
Tanaka et al39 describe six criteria that must be met when attempting to create a scale to measure dyspnea in oncology patients. The Cancer Dyspnea Scale (CDS) is a self-rating scale that has been found to be acceptable and practicable in the clinical setting. The 12-item scale was originally conducted in Japanese and has been validated when translated into Swedish (CDS-S) and English (CDS-E) versions.39,41 Uronis et al41 further tested a reduced Cancer Dyspnea Scale that dropped three items from the original CDS scale (r-CDS-E). According to Uronis, the CDS-E better measures global dyspnea whereas the r-CDS-E can be used to measure effects of an intervention on dyspnea. One of the limitations of the CDS is that the scale was only validated in patients with lung cancer.
Since a universally useful scale has yet to be validated, the findings of a comprehensive history and thorough physical examination are the most important considerations when assessing oncologic patients for dyspnea.18,42 The history and physical examination must assess both physical factors and psychological factors. Physical factors include symptom quality and associated symptoms, provoking and relieving factors, previous treatments and response to treatments, and past medical history. Psychological factors include the patient’s emotional status at onset; as symptoms progress, the EP must assess whether perception of dyspnea is related to emotions such as anxiety and fear.
The longitudinal progression of symptoms must be considered. Currow et al43 found that at days 10 and 3 before death, dyspnea increased in oncologic patients but remained unchanged in patients with a noncancer diagnosis. Thus, the longitudinal progression of dyspnea differs depending on the underlying condition. Nearing death, patients without cancer experience a sustained period of symptoms whereas patients with cancer frequently experience both increased symptom prevalence and intensity.44
Diagnostic testing may also help to identify treatable causes. Chest imaging can be performed by radiography and computed tomography. Complete blood counts and chemistry panels can assess for anemia and electrolyte abnormalities. Maximal inspiratory pressure (MIP), a measure that tests diaphragm and inspiratory muscle strength, is also helpful if no apparent cause is found using other diagnostic modalities.1
Treatment
Treatment of dyspnea in malignancy is uniquely challenging. Ideally, treatment should focus on correcting an underlying cause, but for malignancy, the cause is often not reversible. Therefore, dyspnea treatment often consists of palliative management to control the sensation of symptom burden.45 Terminal cancer patients oftentimes require hospitalization and sedation to adequately manage their symptoms.45
The American Thoracic Society has grouped dyspnea therapy into the following four categories: reducing ventilator demand, decreasing ventilator impedance, improving respiratory muscle strength, or altering central perception.1
Reducing Ventilator Demand
Reducing ventilator demand can be accomplished by decreasing metabolic load or altering central respiratory drive. Decreasing metabolic load by strengthening respiratory muscles helps to get rid of lactic acid accumulation.46 Exercise also helps to prevent overall sensations of fatigue.47,48
Central respiratory drive can be altered in two ways, either with pharmacotherapy or supplemental oxygen. Pharmacotherapy aids focus on either oxygenation or the removal of carbon dioxide. Studies show that opioids, although only with small statistical significance, can be beneficial in treating dyspnea. Morphine is currently the mainstay of pharmacotherapy, but studies have yet to identify the best standard for dosing or optimal route for administration. In 1990, Bruera et al49 found that morphine improved dyspnea without negative impact on respiratory rate, oxygen saturation, or expiratory carbon dioxide.
Benzodiazepines help manage dyspnea by mitigating associated anxiety. Benzodiazepines have yet to be proven beneficial in a study with high statistical significance; however, reassurance and patient education that dyspnea is a common symptom can help allay anxiety. Patients living at home should have strong family support and reassurance that someone will be available to assist if an episode of dyspnea occurs.50
Supplemental oxygen can decrease the sensation of dyspnea by altering central respiratory drive. Oxygen should be given at the lowest effective dose and administered with humidified air to prevent desiccation of the respiratory tract. In 1993, Bruera et al51 showed that oxygen decreases dyspnea in hypoxic cancer patients.
In 1996, a double-blind crossover trial of 14 oncologic patients by Dudgeon et al42 indicated that oxygen helps decrease the sensation of dyspnea, respiratory rate, and breathing effort when compared to air. Improvement in carbon dioxide elimination with the use of breathing techniques such as diaphragmatic breathing and pursed lip breathing are also methods to decrease dyspnea sensation through alteration of the central respiratory drive. These breath-retraining techniques promote relaxed and gentle breathing, help minimize the work of breathing, and promote a sensation of well-being.44,52
Decreasing Ventilator Impedance
Ventilator impedance can result from bronchospasm, airway obstruction, effusions, or increased secretions. Treat airway obstruction from extrinsic compression or an endobronchial tumor with procedures such as balloon bronchoplasty, tracheobronchial stent placement, and brachytherapy. Pleural effusions can be treated using repeat thoracentesis or a thoracentesis with the placement of a chronic indwelling catheter; long-term control can be achieved using procedures such as pleurodesis and a thoracotomy with decortication. Manage secretions by using anticholinergic agents (eg, scopolamine) along with physical suctioning. Corticosteroids also help to reduce ventilator impedance by reducing tumor edema, lymphangitis, and bronchospasm.44 Unfortunately, these treatments are not without side effects and additional risks such as bleeding, infection, and pneumothorax.53
Improving Respiratory Muscle Strength
Maximum inspiratory pressure measures respiratory muscle strength. Normal values of negative pressure are usually greater than –50 cm H2O, and the average MIP found in cancer patients is only –16 cm H2O. Any level below –25 cm H2O is associated with severe respiratory muscle impairment. Maintaining adequate nutrition is important to help improve respiratory muscle strength.
Altering Central Perception
Limited trials suggest altering perception with the use of acupuncture and guided imagery may be of benefit. Morphine and other opiates also help to decrease oxygen requirements by altering central perception.49 Relaxation methods are most effective after a patient has gained the ability to alter and control sensation with breathing techniques.
Dyspnea is a chief complaint reported by the oncologic patients presenting to the ED for evaluation. Despite its prevalence in this patient population, diagnosis can be difficult since it is rarely the result of a single etiology. Consequently, dyspnea is often underdiagnosed and undertreated in the cancer population. Moreover, this condition is a composite of manifestations that are unique to each patient and his or her corresponding disease. These composite manifestations differ in timing and severity and require targeted interventions. Dyspnea that is primarily physiological in nature can be managed by reversing the causative mechanisms through treatments including oxygen therapy and antibiotics. Psychological components of dyspnea can be modified with interventions for anxiety and depression. Dyspnea is a complex composite of problems that deserves more attention in the literature and in practice.
Dr Wattana is an assistant professor in the department of emergency medicine, division of internal medicine at The University of Texas MD Anderson Cancer Center, Houston. Dr Miller is an associate professor in the department of emergency medicine, division of internal medicine at The University of Texas MD Anderson Cancer Center, Houston.
- Parshall MB, Schwartzstein RM, Adams L, et al; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452.
- Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data. 2007;(386):1-32.
- Ekström MP, Abernethy AP, Currow DC. The management of chronic breathlessness in patients with advanced and terminal illness. BMJ. 2015;349:g7617.
- Karnani NG, Reisfield GM, Wilson GR. Evaluation of chronic dyspnea. Am Fam Physician. 2005;71(8):1529-1537.
- Moens K, Higginson IJ, Harding R; EURO IMPACT. Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage. 2014;48(4):660-677.
- Peters SP. When the chief complaint is (or should be) dyspnea in adults. J Allergy Clin Immunol Pract. 2013;1(2):129-136.
- Campbell ML. Dyspnea prevalence, trajectories, and measurement in critical care and at life’s end. Curr Opin Support Palliat Care. 2012;6(2):168-171.
- Argulian E, Agarwal V, Bangalore S, et al. Meta-analysis of prognostic implications of dyspnea versus chest pain in patients referred for stress testing. Am J Cardiol. 2014;113(3):559-564.
- van Mourik Y, Rutten FH, Moons KG, Bertens LC, Hoes AW, Reitsma JB. Prevalence and underlying causes of dyspnoea in older people: a systematic review. Age Ageing. 2014;43(3):319-326.
- Scano G, Gigliotti F, Stendardi L, Gagliardi E. Dyspnea and emotional states in health and disease. Respir Med. 2013;107(5):649-655.
- Murakami J, Ueda K, Sano F, Hayashi M, Tanaka N, Hamano K. Prediction of postoperative dyspnea and chronic respiratory failure. J Surg Res. 2015;195(1):303-310.
- Manning HL, Mahler DA. Pathophysiology of dyspnea. Monaldi Arch Chest Dis. 2001;56(4):325-330.
- Robinson LM. A Healthy 22-year-old woman with new onset dyspnea. J Emerg Nurs. 2010;36(2):146-147.
- Ben-Aharon I, Gafter-Gvili A, Leibovici L, Stemmer SM. Interventions for alleviating cancer-related dyspnea: a systematic review and meta-analysis. Acta Oncol. 2012;51(8):996-1008.
- Guirimand F, Sahut d’izarn M, Laporte L, Francillard M, Richard JF, Aegerter P. Sequential occurrence of dyspnea at the end of life in palliative care, according to the underlying cancer. Cancer Med. 2015;4(4):532-539.
- Fishbein D, Kearon C, Killian KJ. An approach to dyspnea in cancer patients. J Pain Symptom Manage. 1989;4(2):76-81.
- Ost DE, Ernst A, Grosu HB, et al; AQuIRE Brochoscopy Registry. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest. 2015;147(5):1282-1298.
- Ripamonti C, Bruera E. Dyspnea: pathophysiology and assessment. J Pain Symptom Manage. 1997;13(4):220-232.
- Petersen S, Ritz T. The role of fearful beliefs in the relationship between situational self-awareness and report of breathing-related sensations. Br J Health Psychol. 2011;16(Pt 2):359-372.
- Dudgeon DJ, Kristjanson L, Sloan JA, Lertzman M, Clement K. Dyspnea in cancer patients: prevalence and associated factors. J Pain Symptom Manage. 2001;21(2):95-102.
- Reuben DB, Mor V. Dyspnea in terminally ill cancer patients. Chest. 1986;89(2):234-236.
- Roberts DK, Thorne SE, Pearson C. The experience of dyspnea in late-stage cancer. Patients’ and nurses’ perspectives. Cancer Nurs. 1993;16(4):310-320.
- Reddy SK, Parsons HA, Elsayem A, Palmer JL, Bruera E. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med. 2009;12(1):29-36.
- Hayen A, Herigstad M, Pattinson KT. Understanding dyspnea as a complex individual experience. Maturitas. 2013;76(1):45-50.
- Bruera E, Schmitz B, Pither J, Neumann CM, Hanson J. The frequency and correlates of dyspnea in patients with advanced cancer. J Pain Symptom Manage. 2000;19(5):357-362.
- Ripamonti C. Management of dyspnea in advanced cancer patients. Support Care Cancer. 1999;7(4):
- 233-243.
- Hui D, Morgado M, Vidal M, et al. Dyspnea in hospitalized advanced cancer patients: subjective and physiologic correlates. J Palliat Med. 2013;16(3):
- 274-280.
- Ripamonti C, Fulfaro F, Bruera E. Dyspnoea in patients with advanced cancer: incidence, causes and treatments. Cancer Treat Rev. 1998;24(1):69-80.
- Dudgeon DJ, Lertzman M, Askew GR. Physiological changes and clinical correlations of dyspnea in cancer outpatients. J Pain Symptom Manage. 2001;21(5):373-379.
- Swinburn CR, Wakefield JM, Jones PW. Relationship between ventilation and breathlessness during exercise in chronic obstructive airways disease is not altered by prevention of hypoxaemia. Clin Sci (Lond). 1984;67(5):515-519.
- Rock LK, Schwartzstein RM. Mechanisms of dyspnea in chronic lung disease. Curr Opin Support Palliat Care. 2007;1(2):102-108.
- Moy ML, Woodrow Weiss J, Sparrow D, Israel E, Schwartzstein RM. Quality of dyspnea in bronchoconstriction differs from external resistive loads. Am J Respir Crit Care Med. 2000;162(2 Pt 1):451-455.
- Nakayama H, Shibuya M, Yamada M, Suzuki H, Arakawa M, Homma I. In-phase chest wall vibration decreases dyspnea during arm elevation in chronic obstructive pulmonary disease patients. Intern Med. 1998;37(10):831-835.
- Killian KJ, Jones NL. Mechanisms of exertional dyspnea. Clin Chest Med. 1994;15(2):247-257
- Gross NJ. Chronic obstructive pulmonary disease. Current concepts and therapeutic approaches. Chest. 1990;97(2 Suppl):19S-23S.
- Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: review and hypotheses. Respir Physiol Neurobiol. 2009;167(1):53-60.
- Petersen S, Orth B, Ritz T. Awareness of breathing: the structure of language descriptors of respiratory sensations. Health Psychol. 2008;27(1):122-127.
- Parshall MB, Meek PM, Sklar D, Alcock J, Bittner P. Test-retest reliability of multidimensional dyspnea profile recall ratings in the emergency department: a prospective, longitudinal study. BMC Emerg Med. 2012;12:6.
- Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Development and validation of the Cancer Dyspnoea Scale: a multidimensional, brief, self-rating scale. Br J Cancer. 2000;82(4):800-805.
- Booth S. The management of dyspnoea in advanced cancer. Hosp Med. 1998;59(5):348-349.
- Uronis HE, Shelby RA, Currow DC, et al. Assessment of the psychometric properties of an English version of the cancer dyspnea scale in people with advanced lung cancer. J Pain Symptom Manage. 2012;44(5):741-749.
- Dudgeon DJ, Rosenthal S. Management of dyspnea and cough in patients with cancer. Hematol Oncol Clin North Am. 1996;10(1):157-171.
- Currow DC, Smith J, Davidson PM, Newton PJ, Agar MR, Abernethy AP. Do the trajectories of dyspnea differ in prevalence and intensity by diagnosis at the end of life? A consecutive cohort study. J Pain Symptom Manage. 2010;39(4):680.
- Acheson A, MacCormack D. Dyspnea and the cancer patient--an overview. Can Oncol Nurs J. 1997;7(4):209-213.
- Dudgeon DJ, Lertzman M. Dyspnea in the advanced cancer patient. J Pain Symptom Manage. 1998;16(4):212-219.
- Corner J, Plant H, A’Hern R, Bailey C. Non-pharmacological intervention for breathlessness in lung cancer. Palliat Med. 1996;10(4):299-305.
- LeGrand SB, Walsh D. Palliative management of dyspnea in advanced cancer. Curr Opin Oncol. 1999;11(4):250-254.
- LeGrand SB. Dyspnea: the continuing challenge of palliative management. Curr Opin Oncol. 2002;14(4):394-398.
- Bruera E, Macmillan K, Pither J, MacDonald RN. Effects of morphine on the dyspnea of terminal cancer patients. J Pain Symptom Manage. 1990;5(6):341-344.
- Held JL. Cancer care. Managing shortness of breath. Nursing. 1994;24(7):31.
- Bruera E, de Stoutz N, Velasco-Leiva A, Schoeller T, Hanson J. Effects of oxygen on dyspnoea in hypoxaemic terminal-cancer patients. Lancet. 1993;342(8862):13-14.
- Bailey C. Nursing as therapy in the management of breathlessness in lung cancer. Eur. J. Cancer Care (Engl.). 1995;4(4):184-190.
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage. 2012;(2):301-306.
Overview
Dyspnea, the subjective inability to breathe comfortably, is a common symptom, accounting for 3 to 4 million ED visits annually.1,2 Dyspnea can be acute, subacute, or chronic, with chronic dyspnea defined as the presence of symptoms for more than 1 to 2 months, and subacute dyspnea as symptoms lasting for hours to days.3,4
The reported prevalence of dyspnea based on etiology presenting to the ED is as follows: malignancy (16% to 77%), chronic heart failure (18% to 88%), chronic obstructive pulmonary disease (56% to 98%), or renal disease (11% to 82%).5 Irrespective of time course and etiology, dyspnea can be a debilitating symptom affecting up to 50% of patients admitted to acute, tertiary care hospitals, and a quarter of patients seeking care in the ambulatory setting.6-13
Although dyspnea is common in patients with cancer, it is often underreported.14-19 This article reviews the current information available regarding the epidemiology, pathophysiology, assessment, and treatment of dyspnea in the oncologic patient population. By providing insight into the unique nature of dyspnea for oncologic patients, this article illustrates why dyspnea is an important symptom to recognize, address, and treat.
Epidemiology, Background, and Recognition
Dyspnea has a significant impact on the quality of life for oncologic patients and often intensifies near the time of death. At one regional oncology center, dyspnea was present in 49% of general cancer patients.20 The percentage and severity of dyspnea increases as a patient’s cancer progresses. The National Hospice Study found that dyspnea occurred in 70% of 1,754 terminally ill cancer patients studied during their last 6 weeks of life; more than 28% of these patients graded the distress that they felt from dyspnea as moderate to severe. The incidence of dyspnea was exceeded only by the incidences of pain and anorexia.21
A study conducted by Roberts et al22 showed that dyspnea is a very common, undertreated, and underrecognized condition. In 62% of patients reporting dyspnea, the symptoms had been present for greater than 3 months before patients received medical or nursing intervention. In the inpatient hospital setting, 77% of patients claimed on interview to experience dyspnea, but only 39% of cases were actually reported by nursing staff. In addition, Reddy et al23 found that dyspnea can negatively influence activities of daily living in oncology patients. Moreover, as the patients’ dyspnea intensified, their ability to perform activities of daily living was increasingly affected.23,24
Differential Diagnosis
Studies demonstrate the presence of dyspnea in approximately 20% to 40% of patients at the time of diagnosis of advanced disease, with the percentage increasing to around 70% in the last 6 weeks of life.25-27 Symptom grading is at least moderate in more than 28% of terminally ill cancer patients.21 Although common in oncology patients, dyspnea is difficult to diagnose because it is seldom explained by a single, specific organic etiology.24,25,28 Extrinsic modulators that are difficult to quantify, such as psychological state, cultural background, and life experiences, can also attenuate the perception of dyspnea.18,25
The cause of dyspnea in cancer patients can be broken down into four categories to aid in the differential diagnosis as described by Dudgeon et al29 in 2001 (Table 1). The four categories consist of dyspnea as being directly due to the malignancy itself; indirectly due to the malignancy; a side effect of cancer treatment; or due to underlying lung and cardiac disease unrelated to the malignancy itself.21,29
Etiology
Dyspnea Directly Due to Cancer. Dudgeon et al29 reported that the top three malignancies that directly caused dyspnea are lung, head and neck cancer, and malignancies of genitourinary origin.29 Reuben21 showed that 75% of patients with tumors involving the lung reported dyspnea at some point. Metastasis to the mediastinum, especially to the ribs, was associated with higher levels of dyspnea when compared to metastasis to lung.29
Dyspnea Indirectly Due to Cancer. Dyspnea due to indirect causes such as anemia, pneumonia, and pulmonary emboli may be easier to rectify once diagnosed.
Dyspnea Due to Cancer Treatment. Treatment-related causes of dyspnea include the side effects of surgery and lung irradiation. Patients with a history of pneumonectomy, lobectomy, or pleurodesis report higher levels of dyspnea as do patients with a history of thoracic irradiation. Radiation pneumonitis can occur 6 to 12 weeks following treatment, and radiation fibrosis can occur 6 to 12 months following treatment.29
Dyspnea Unrelated to Cancer. Other medical conditions that must be considered when diagnosing dyspnea include chronic obstructive pulmonary disease, asthma, and congestive heart failure. Psychological distress—described as anxiety and depression in the cancer population—may also cause the sensation of dyspnea.25
Dyspnea can be constant or occur as breakthrough episodes. In the study by Reddy et al,23 39% of patients had constant dyspnea and 20% experienced breakthrough episodes. Breakthrough dyspnea is shorter in duration, lasting less than 5 minutes. Breakthrough dyspnea occurs approximately 5 to 6 times a day and is predominant with 80% of patients who are symptom-free between episodes. As opiates are the drug of choice for dyspnea treatment, differentiating the time course of dyspnea is important in terms of treatment strategy and response. For example, treating breakthrough dyspnea with standard opiate medications might not work given that symptom onset and resolution might occur even before the medication’s onset of action.
Pathophysiology and Psychology
The cause of dyspnea in an oncology patient can be physiological and/or psychological in nature, with more numerous and unique presentations as compared to dyspnea associated with cardiac or lung disease. Chemoreceptor stimulation, mechanical stimuli originating in the lung and chest wall receptors, and neuroventilatory effects all contribute to dyspnea.12,16 Physiological modalities contributing to dyspnea are outlined in Table 2.
Psychological Contributions to the Etiology of Dyspnea
Dyspnea is subjective and biopsychosocial factors play a large role in an oncologic patient’s self-report of this condition. Multiple studies demonstrate that objective signs may not match the patient’s perception of dyspnea.18,21,25 Other studies report a correlation between psychological distress and worsened perception of dyspnea. Psychological distress is often measured by anxiety and depression and is also augmented by the presence of pain.
Assessment
Dyspnea is more challenging to assess in the ED than the laboratory setting36 as the condition is multidimensional in nature, often characterized by three factors consisting of breathing effort, chest tightness, and air hunger.37,38 Since these are subjective symptoms, assessment is difficult if a patient has delirium or other symptoms that alter the ability to provide a coherent response. Also, the subjective nature of dyspnea can lead to bias during symptom measurement and management.39
Research has documented factors such as lung involvement, anxiety, and maximum inspiratory pressure as influential to the perceived intensity of dyspnea for oncologic patients.40 Therefore, the sensation of dyspnea is multifactorial and includes physiological and psychological components for cancer patients.40 Subjective sensation does not always correlate with physiological measurements, adding to the difficulty of objective assessments.
Tanaka et al39 describe six criteria that must be met when attempting to create a scale to measure dyspnea in oncology patients. The Cancer Dyspnea Scale (CDS) is a self-rating scale that has been found to be acceptable and practicable in the clinical setting. The 12-item scale was originally conducted in Japanese and has been validated when translated into Swedish (CDS-S) and English (CDS-E) versions.39,41 Uronis et al41 further tested a reduced Cancer Dyspnea Scale that dropped three items from the original CDS scale (r-CDS-E). According to Uronis, the CDS-E better measures global dyspnea whereas the r-CDS-E can be used to measure effects of an intervention on dyspnea. One of the limitations of the CDS is that the scale was only validated in patients with lung cancer.
Since a universally useful scale has yet to be validated, the findings of a comprehensive history and thorough physical examination are the most important considerations when assessing oncologic patients for dyspnea.18,42 The history and physical examination must assess both physical factors and psychological factors. Physical factors include symptom quality and associated symptoms, provoking and relieving factors, previous treatments and response to treatments, and past medical history. Psychological factors include the patient’s emotional status at onset; as symptoms progress, the EP must assess whether perception of dyspnea is related to emotions such as anxiety and fear.
The longitudinal progression of symptoms must be considered. Currow et al43 found that at days 10 and 3 before death, dyspnea increased in oncologic patients but remained unchanged in patients with a noncancer diagnosis. Thus, the longitudinal progression of dyspnea differs depending on the underlying condition. Nearing death, patients without cancer experience a sustained period of symptoms whereas patients with cancer frequently experience both increased symptom prevalence and intensity.44
Diagnostic testing may also help to identify treatable causes. Chest imaging can be performed by radiography and computed tomography. Complete blood counts and chemistry panels can assess for anemia and electrolyte abnormalities. Maximal inspiratory pressure (MIP), a measure that tests diaphragm and inspiratory muscle strength, is also helpful if no apparent cause is found using other diagnostic modalities.1
Treatment
Treatment of dyspnea in malignancy is uniquely challenging. Ideally, treatment should focus on correcting an underlying cause, but for malignancy, the cause is often not reversible. Therefore, dyspnea treatment often consists of palliative management to control the sensation of symptom burden.45 Terminal cancer patients oftentimes require hospitalization and sedation to adequately manage their symptoms.45
The American Thoracic Society has grouped dyspnea therapy into the following four categories: reducing ventilator demand, decreasing ventilator impedance, improving respiratory muscle strength, or altering central perception.1
Reducing Ventilator Demand
Reducing ventilator demand can be accomplished by decreasing metabolic load or altering central respiratory drive. Decreasing metabolic load by strengthening respiratory muscles helps to get rid of lactic acid accumulation.46 Exercise also helps to prevent overall sensations of fatigue.47,48
Central respiratory drive can be altered in two ways, either with pharmacotherapy or supplemental oxygen. Pharmacotherapy aids focus on either oxygenation or the removal of carbon dioxide. Studies show that opioids, although only with small statistical significance, can be beneficial in treating dyspnea. Morphine is currently the mainstay of pharmacotherapy, but studies have yet to identify the best standard for dosing or optimal route for administration. In 1990, Bruera et al49 found that morphine improved dyspnea without negative impact on respiratory rate, oxygen saturation, or expiratory carbon dioxide.
Benzodiazepines help manage dyspnea by mitigating associated anxiety. Benzodiazepines have yet to be proven beneficial in a study with high statistical significance; however, reassurance and patient education that dyspnea is a common symptom can help allay anxiety. Patients living at home should have strong family support and reassurance that someone will be available to assist if an episode of dyspnea occurs.50
Supplemental oxygen can decrease the sensation of dyspnea by altering central respiratory drive. Oxygen should be given at the lowest effective dose and administered with humidified air to prevent desiccation of the respiratory tract. In 1993, Bruera et al51 showed that oxygen decreases dyspnea in hypoxic cancer patients.
In 1996, a double-blind crossover trial of 14 oncologic patients by Dudgeon et al42 indicated that oxygen helps decrease the sensation of dyspnea, respiratory rate, and breathing effort when compared to air. Improvement in carbon dioxide elimination with the use of breathing techniques such as diaphragmatic breathing and pursed lip breathing are also methods to decrease dyspnea sensation through alteration of the central respiratory drive. These breath-retraining techniques promote relaxed and gentle breathing, help minimize the work of breathing, and promote a sensation of well-being.44,52
Decreasing Ventilator Impedance
Ventilator impedance can result from bronchospasm, airway obstruction, effusions, or increased secretions. Treat airway obstruction from extrinsic compression or an endobronchial tumor with procedures such as balloon bronchoplasty, tracheobronchial stent placement, and brachytherapy. Pleural effusions can be treated using repeat thoracentesis or a thoracentesis with the placement of a chronic indwelling catheter; long-term control can be achieved using procedures such as pleurodesis and a thoracotomy with decortication. Manage secretions by using anticholinergic agents (eg, scopolamine) along with physical suctioning. Corticosteroids also help to reduce ventilator impedance by reducing tumor edema, lymphangitis, and bronchospasm.44 Unfortunately, these treatments are not without side effects and additional risks such as bleeding, infection, and pneumothorax.53
Improving Respiratory Muscle Strength
Maximum inspiratory pressure measures respiratory muscle strength. Normal values of negative pressure are usually greater than –50 cm H2O, and the average MIP found in cancer patients is only –16 cm H2O. Any level below –25 cm H2O is associated with severe respiratory muscle impairment. Maintaining adequate nutrition is important to help improve respiratory muscle strength.
Altering Central Perception
Limited trials suggest altering perception with the use of acupuncture and guided imagery may be of benefit. Morphine and other opiates also help to decrease oxygen requirements by altering central perception.49 Relaxation methods are most effective after a patient has gained the ability to alter and control sensation with breathing techniques.
Dyspnea is a chief complaint reported by the oncologic patients presenting to the ED for evaluation. Despite its prevalence in this patient population, diagnosis can be difficult since it is rarely the result of a single etiology. Consequently, dyspnea is often underdiagnosed and undertreated in the cancer population. Moreover, this condition is a composite of manifestations that are unique to each patient and his or her corresponding disease. These composite manifestations differ in timing and severity and require targeted interventions. Dyspnea that is primarily physiological in nature can be managed by reversing the causative mechanisms through treatments including oxygen therapy and antibiotics. Psychological components of dyspnea can be modified with interventions for anxiety and depression. Dyspnea is a complex composite of problems that deserves more attention in the literature and in practice.
Dr Wattana is an assistant professor in the department of emergency medicine, division of internal medicine at The University of Texas MD Anderson Cancer Center, Houston. Dr Miller is an associate professor in the department of emergency medicine, division of internal medicine at The University of Texas MD Anderson Cancer Center, Houston.
Overview
Dyspnea, the subjective inability to breathe comfortably, is a common symptom, accounting for 3 to 4 million ED visits annually.1,2 Dyspnea can be acute, subacute, or chronic, with chronic dyspnea defined as the presence of symptoms for more than 1 to 2 months, and subacute dyspnea as symptoms lasting for hours to days.3,4
The reported prevalence of dyspnea based on etiology presenting to the ED is as follows: malignancy (16% to 77%), chronic heart failure (18% to 88%), chronic obstructive pulmonary disease (56% to 98%), or renal disease (11% to 82%).5 Irrespective of time course and etiology, dyspnea can be a debilitating symptom affecting up to 50% of patients admitted to acute, tertiary care hospitals, and a quarter of patients seeking care in the ambulatory setting.6-13
Although dyspnea is common in patients with cancer, it is often underreported.14-19 This article reviews the current information available regarding the epidemiology, pathophysiology, assessment, and treatment of dyspnea in the oncologic patient population. By providing insight into the unique nature of dyspnea for oncologic patients, this article illustrates why dyspnea is an important symptom to recognize, address, and treat.
Epidemiology, Background, and Recognition
Dyspnea has a significant impact on the quality of life for oncologic patients and often intensifies near the time of death. At one regional oncology center, dyspnea was present in 49% of general cancer patients.20 The percentage and severity of dyspnea increases as a patient’s cancer progresses. The National Hospice Study found that dyspnea occurred in 70% of 1,754 terminally ill cancer patients studied during their last 6 weeks of life; more than 28% of these patients graded the distress that they felt from dyspnea as moderate to severe. The incidence of dyspnea was exceeded only by the incidences of pain and anorexia.21
A study conducted by Roberts et al22 showed that dyspnea is a very common, undertreated, and underrecognized condition. In 62% of patients reporting dyspnea, the symptoms had been present for greater than 3 months before patients received medical or nursing intervention. In the inpatient hospital setting, 77% of patients claimed on interview to experience dyspnea, but only 39% of cases were actually reported by nursing staff. In addition, Reddy et al23 found that dyspnea can negatively influence activities of daily living in oncology patients. Moreover, as the patients’ dyspnea intensified, their ability to perform activities of daily living was increasingly affected.23,24
Differential Diagnosis
Studies demonstrate the presence of dyspnea in approximately 20% to 40% of patients at the time of diagnosis of advanced disease, with the percentage increasing to around 70% in the last 6 weeks of life.25-27 Symptom grading is at least moderate in more than 28% of terminally ill cancer patients.21 Although common in oncology patients, dyspnea is difficult to diagnose because it is seldom explained by a single, specific organic etiology.24,25,28 Extrinsic modulators that are difficult to quantify, such as psychological state, cultural background, and life experiences, can also attenuate the perception of dyspnea.18,25
The cause of dyspnea in cancer patients can be broken down into four categories to aid in the differential diagnosis as described by Dudgeon et al29 in 2001 (Table 1). The four categories consist of dyspnea as being directly due to the malignancy itself; indirectly due to the malignancy; a side effect of cancer treatment; or due to underlying lung and cardiac disease unrelated to the malignancy itself.21,29
Etiology
Dyspnea Directly Due to Cancer. Dudgeon et al29 reported that the top three malignancies that directly caused dyspnea are lung, head and neck cancer, and malignancies of genitourinary origin.29 Reuben21 showed that 75% of patients with tumors involving the lung reported dyspnea at some point. Metastasis to the mediastinum, especially to the ribs, was associated with higher levels of dyspnea when compared to metastasis to lung.29
Dyspnea Indirectly Due to Cancer. Dyspnea due to indirect causes such as anemia, pneumonia, and pulmonary emboli may be easier to rectify once diagnosed.
Dyspnea Due to Cancer Treatment. Treatment-related causes of dyspnea include the side effects of surgery and lung irradiation. Patients with a history of pneumonectomy, lobectomy, or pleurodesis report higher levels of dyspnea as do patients with a history of thoracic irradiation. Radiation pneumonitis can occur 6 to 12 weeks following treatment, and radiation fibrosis can occur 6 to 12 months following treatment.29
Dyspnea Unrelated to Cancer. Other medical conditions that must be considered when diagnosing dyspnea include chronic obstructive pulmonary disease, asthma, and congestive heart failure. Psychological distress—described as anxiety and depression in the cancer population—may also cause the sensation of dyspnea.25
Dyspnea can be constant or occur as breakthrough episodes. In the study by Reddy et al,23 39% of patients had constant dyspnea and 20% experienced breakthrough episodes. Breakthrough dyspnea is shorter in duration, lasting less than 5 minutes. Breakthrough dyspnea occurs approximately 5 to 6 times a day and is predominant with 80% of patients who are symptom-free between episodes. As opiates are the drug of choice for dyspnea treatment, differentiating the time course of dyspnea is important in terms of treatment strategy and response. For example, treating breakthrough dyspnea with standard opiate medications might not work given that symptom onset and resolution might occur even before the medication’s onset of action.
Pathophysiology and Psychology
The cause of dyspnea in an oncology patient can be physiological and/or psychological in nature, with more numerous and unique presentations as compared to dyspnea associated with cardiac or lung disease. Chemoreceptor stimulation, mechanical stimuli originating in the lung and chest wall receptors, and neuroventilatory effects all contribute to dyspnea.12,16 Physiological modalities contributing to dyspnea are outlined in Table 2.
Psychological Contributions to the Etiology of Dyspnea
Dyspnea is subjective and biopsychosocial factors play a large role in an oncologic patient’s self-report of this condition. Multiple studies demonstrate that objective signs may not match the patient’s perception of dyspnea.18,21,25 Other studies report a correlation between psychological distress and worsened perception of dyspnea. Psychological distress is often measured by anxiety and depression and is also augmented by the presence of pain.
Assessment
Dyspnea is more challenging to assess in the ED than the laboratory setting36 as the condition is multidimensional in nature, often characterized by three factors consisting of breathing effort, chest tightness, and air hunger.37,38 Since these are subjective symptoms, assessment is difficult if a patient has delirium or other symptoms that alter the ability to provide a coherent response. Also, the subjective nature of dyspnea can lead to bias during symptom measurement and management.39
Research has documented factors such as lung involvement, anxiety, and maximum inspiratory pressure as influential to the perceived intensity of dyspnea for oncologic patients.40 Therefore, the sensation of dyspnea is multifactorial and includes physiological and psychological components for cancer patients.40 Subjective sensation does not always correlate with physiological measurements, adding to the difficulty of objective assessments.
Tanaka et al39 describe six criteria that must be met when attempting to create a scale to measure dyspnea in oncology patients. The Cancer Dyspnea Scale (CDS) is a self-rating scale that has been found to be acceptable and practicable in the clinical setting. The 12-item scale was originally conducted in Japanese and has been validated when translated into Swedish (CDS-S) and English (CDS-E) versions.39,41 Uronis et al41 further tested a reduced Cancer Dyspnea Scale that dropped three items from the original CDS scale (r-CDS-E). According to Uronis, the CDS-E better measures global dyspnea whereas the r-CDS-E can be used to measure effects of an intervention on dyspnea. One of the limitations of the CDS is that the scale was only validated in patients with lung cancer.
Since a universally useful scale has yet to be validated, the findings of a comprehensive history and thorough physical examination are the most important considerations when assessing oncologic patients for dyspnea.18,42 The history and physical examination must assess both physical factors and psychological factors. Physical factors include symptom quality and associated symptoms, provoking and relieving factors, previous treatments and response to treatments, and past medical history. Psychological factors include the patient’s emotional status at onset; as symptoms progress, the EP must assess whether perception of dyspnea is related to emotions such as anxiety and fear.
The longitudinal progression of symptoms must be considered. Currow et al43 found that at days 10 and 3 before death, dyspnea increased in oncologic patients but remained unchanged in patients with a noncancer diagnosis. Thus, the longitudinal progression of dyspnea differs depending on the underlying condition. Nearing death, patients without cancer experience a sustained period of symptoms whereas patients with cancer frequently experience both increased symptom prevalence and intensity.44
Diagnostic testing may also help to identify treatable causes. Chest imaging can be performed by radiography and computed tomography. Complete blood counts and chemistry panels can assess for anemia and electrolyte abnormalities. Maximal inspiratory pressure (MIP), a measure that tests diaphragm and inspiratory muscle strength, is also helpful if no apparent cause is found using other diagnostic modalities.1
Treatment
Treatment of dyspnea in malignancy is uniquely challenging. Ideally, treatment should focus on correcting an underlying cause, but for malignancy, the cause is often not reversible. Therefore, dyspnea treatment often consists of palliative management to control the sensation of symptom burden.45 Terminal cancer patients oftentimes require hospitalization and sedation to adequately manage their symptoms.45
The American Thoracic Society has grouped dyspnea therapy into the following four categories: reducing ventilator demand, decreasing ventilator impedance, improving respiratory muscle strength, or altering central perception.1
Reducing Ventilator Demand
Reducing ventilator demand can be accomplished by decreasing metabolic load or altering central respiratory drive. Decreasing metabolic load by strengthening respiratory muscles helps to get rid of lactic acid accumulation.46 Exercise also helps to prevent overall sensations of fatigue.47,48
Central respiratory drive can be altered in two ways, either with pharmacotherapy or supplemental oxygen. Pharmacotherapy aids focus on either oxygenation or the removal of carbon dioxide. Studies show that opioids, although only with small statistical significance, can be beneficial in treating dyspnea. Morphine is currently the mainstay of pharmacotherapy, but studies have yet to identify the best standard for dosing or optimal route for administration. In 1990, Bruera et al49 found that morphine improved dyspnea without negative impact on respiratory rate, oxygen saturation, or expiratory carbon dioxide.
Benzodiazepines help manage dyspnea by mitigating associated anxiety. Benzodiazepines have yet to be proven beneficial in a study with high statistical significance; however, reassurance and patient education that dyspnea is a common symptom can help allay anxiety. Patients living at home should have strong family support and reassurance that someone will be available to assist if an episode of dyspnea occurs.50
Supplemental oxygen can decrease the sensation of dyspnea by altering central respiratory drive. Oxygen should be given at the lowest effective dose and administered with humidified air to prevent desiccation of the respiratory tract. In 1993, Bruera et al51 showed that oxygen decreases dyspnea in hypoxic cancer patients.
In 1996, a double-blind crossover trial of 14 oncologic patients by Dudgeon et al42 indicated that oxygen helps decrease the sensation of dyspnea, respiratory rate, and breathing effort when compared to air. Improvement in carbon dioxide elimination with the use of breathing techniques such as diaphragmatic breathing and pursed lip breathing are also methods to decrease dyspnea sensation through alteration of the central respiratory drive. These breath-retraining techniques promote relaxed and gentle breathing, help minimize the work of breathing, and promote a sensation of well-being.44,52
Decreasing Ventilator Impedance
Ventilator impedance can result from bronchospasm, airway obstruction, effusions, or increased secretions. Treat airway obstruction from extrinsic compression or an endobronchial tumor with procedures such as balloon bronchoplasty, tracheobronchial stent placement, and brachytherapy. Pleural effusions can be treated using repeat thoracentesis or a thoracentesis with the placement of a chronic indwelling catheter; long-term control can be achieved using procedures such as pleurodesis and a thoracotomy with decortication. Manage secretions by using anticholinergic agents (eg, scopolamine) along with physical suctioning. Corticosteroids also help to reduce ventilator impedance by reducing tumor edema, lymphangitis, and bronchospasm.44 Unfortunately, these treatments are not without side effects and additional risks such as bleeding, infection, and pneumothorax.53
Improving Respiratory Muscle Strength
Maximum inspiratory pressure measures respiratory muscle strength. Normal values of negative pressure are usually greater than –50 cm H2O, and the average MIP found in cancer patients is only –16 cm H2O. Any level below –25 cm H2O is associated with severe respiratory muscle impairment. Maintaining adequate nutrition is important to help improve respiratory muscle strength.
Altering Central Perception
Limited trials suggest altering perception with the use of acupuncture and guided imagery may be of benefit. Morphine and other opiates also help to decrease oxygen requirements by altering central perception.49 Relaxation methods are most effective after a patient has gained the ability to alter and control sensation with breathing techniques.
Dyspnea is a chief complaint reported by the oncologic patients presenting to the ED for evaluation. Despite its prevalence in this patient population, diagnosis can be difficult since it is rarely the result of a single etiology. Consequently, dyspnea is often underdiagnosed and undertreated in the cancer population. Moreover, this condition is a composite of manifestations that are unique to each patient and his or her corresponding disease. These composite manifestations differ in timing and severity and require targeted interventions. Dyspnea that is primarily physiological in nature can be managed by reversing the causative mechanisms through treatments including oxygen therapy and antibiotics. Psychological components of dyspnea can be modified with interventions for anxiety and depression. Dyspnea is a complex composite of problems that deserves more attention in the literature and in practice.
Dr Wattana is an assistant professor in the department of emergency medicine, division of internal medicine at The University of Texas MD Anderson Cancer Center, Houston. Dr Miller is an associate professor in the department of emergency medicine, division of internal medicine at The University of Texas MD Anderson Cancer Center, Houston.
- Parshall MB, Schwartzstein RM, Adams L, et al; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452.
- Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data. 2007;(386):1-32.
- Ekström MP, Abernethy AP, Currow DC. The management of chronic breathlessness in patients with advanced and terminal illness. BMJ. 2015;349:g7617.
- Karnani NG, Reisfield GM, Wilson GR. Evaluation of chronic dyspnea. Am Fam Physician. 2005;71(8):1529-1537.
- Moens K, Higginson IJ, Harding R; EURO IMPACT. Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage. 2014;48(4):660-677.
- Peters SP. When the chief complaint is (or should be) dyspnea in adults. J Allergy Clin Immunol Pract. 2013;1(2):129-136.
- Campbell ML. Dyspnea prevalence, trajectories, and measurement in critical care and at life’s end. Curr Opin Support Palliat Care. 2012;6(2):168-171.
- Argulian E, Agarwal V, Bangalore S, et al. Meta-analysis of prognostic implications of dyspnea versus chest pain in patients referred for stress testing. Am J Cardiol. 2014;113(3):559-564.
- van Mourik Y, Rutten FH, Moons KG, Bertens LC, Hoes AW, Reitsma JB. Prevalence and underlying causes of dyspnoea in older people: a systematic review. Age Ageing. 2014;43(3):319-326.
- Scano G, Gigliotti F, Stendardi L, Gagliardi E. Dyspnea and emotional states in health and disease. Respir Med. 2013;107(5):649-655.
- Murakami J, Ueda K, Sano F, Hayashi M, Tanaka N, Hamano K. Prediction of postoperative dyspnea and chronic respiratory failure. J Surg Res. 2015;195(1):303-310.
- Manning HL, Mahler DA. Pathophysiology of dyspnea. Monaldi Arch Chest Dis. 2001;56(4):325-330.
- Robinson LM. A Healthy 22-year-old woman with new onset dyspnea. J Emerg Nurs. 2010;36(2):146-147.
- Ben-Aharon I, Gafter-Gvili A, Leibovici L, Stemmer SM. Interventions for alleviating cancer-related dyspnea: a systematic review and meta-analysis. Acta Oncol. 2012;51(8):996-1008.
- Guirimand F, Sahut d’izarn M, Laporte L, Francillard M, Richard JF, Aegerter P. Sequential occurrence of dyspnea at the end of life in palliative care, according to the underlying cancer. Cancer Med. 2015;4(4):532-539.
- Fishbein D, Kearon C, Killian KJ. An approach to dyspnea in cancer patients. J Pain Symptom Manage. 1989;4(2):76-81.
- Ost DE, Ernst A, Grosu HB, et al; AQuIRE Brochoscopy Registry. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest. 2015;147(5):1282-1298.
- Ripamonti C, Bruera E. Dyspnea: pathophysiology and assessment. J Pain Symptom Manage. 1997;13(4):220-232.
- Petersen S, Ritz T. The role of fearful beliefs in the relationship between situational self-awareness and report of breathing-related sensations. Br J Health Psychol. 2011;16(Pt 2):359-372.
- Dudgeon DJ, Kristjanson L, Sloan JA, Lertzman M, Clement K. Dyspnea in cancer patients: prevalence and associated factors. J Pain Symptom Manage. 2001;21(2):95-102.
- Reuben DB, Mor V. Dyspnea in terminally ill cancer patients. Chest. 1986;89(2):234-236.
- Roberts DK, Thorne SE, Pearson C. The experience of dyspnea in late-stage cancer. Patients’ and nurses’ perspectives. Cancer Nurs. 1993;16(4):310-320.
- Reddy SK, Parsons HA, Elsayem A, Palmer JL, Bruera E. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med. 2009;12(1):29-36.
- Hayen A, Herigstad M, Pattinson KT. Understanding dyspnea as a complex individual experience. Maturitas. 2013;76(1):45-50.
- Bruera E, Schmitz B, Pither J, Neumann CM, Hanson J. The frequency and correlates of dyspnea in patients with advanced cancer. J Pain Symptom Manage. 2000;19(5):357-362.
- Ripamonti C. Management of dyspnea in advanced cancer patients. Support Care Cancer. 1999;7(4):
- 233-243.
- Hui D, Morgado M, Vidal M, et al. Dyspnea in hospitalized advanced cancer patients: subjective and physiologic correlates. J Palliat Med. 2013;16(3):
- 274-280.
- Ripamonti C, Fulfaro F, Bruera E. Dyspnoea in patients with advanced cancer: incidence, causes and treatments. Cancer Treat Rev. 1998;24(1):69-80.
- Dudgeon DJ, Lertzman M, Askew GR. Physiological changes and clinical correlations of dyspnea in cancer outpatients. J Pain Symptom Manage. 2001;21(5):373-379.
- Swinburn CR, Wakefield JM, Jones PW. Relationship between ventilation and breathlessness during exercise in chronic obstructive airways disease is not altered by prevention of hypoxaemia. Clin Sci (Lond). 1984;67(5):515-519.
- Rock LK, Schwartzstein RM. Mechanisms of dyspnea in chronic lung disease. Curr Opin Support Palliat Care. 2007;1(2):102-108.
- Moy ML, Woodrow Weiss J, Sparrow D, Israel E, Schwartzstein RM. Quality of dyspnea in bronchoconstriction differs from external resistive loads. Am J Respir Crit Care Med. 2000;162(2 Pt 1):451-455.
- Nakayama H, Shibuya M, Yamada M, Suzuki H, Arakawa M, Homma I. In-phase chest wall vibration decreases dyspnea during arm elevation in chronic obstructive pulmonary disease patients. Intern Med. 1998;37(10):831-835.
- Killian KJ, Jones NL. Mechanisms of exertional dyspnea. Clin Chest Med. 1994;15(2):247-257
- Gross NJ. Chronic obstructive pulmonary disease. Current concepts and therapeutic approaches. Chest. 1990;97(2 Suppl):19S-23S.
- Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: review and hypotheses. Respir Physiol Neurobiol. 2009;167(1):53-60.
- Petersen S, Orth B, Ritz T. Awareness of breathing: the structure of language descriptors of respiratory sensations. Health Psychol. 2008;27(1):122-127.
- Parshall MB, Meek PM, Sklar D, Alcock J, Bittner P. Test-retest reliability of multidimensional dyspnea profile recall ratings in the emergency department: a prospective, longitudinal study. BMC Emerg Med. 2012;12:6.
- Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Development and validation of the Cancer Dyspnoea Scale: a multidimensional, brief, self-rating scale. Br J Cancer. 2000;82(4):800-805.
- Booth S. The management of dyspnoea in advanced cancer. Hosp Med. 1998;59(5):348-349.
- Uronis HE, Shelby RA, Currow DC, et al. Assessment of the psychometric properties of an English version of the cancer dyspnea scale in people with advanced lung cancer. J Pain Symptom Manage. 2012;44(5):741-749.
- Dudgeon DJ, Rosenthal S. Management of dyspnea and cough in patients with cancer. Hematol Oncol Clin North Am. 1996;10(1):157-171.
- Currow DC, Smith J, Davidson PM, Newton PJ, Agar MR, Abernethy AP. Do the trajectories of dyspnea differ in prevalence and intensity by diagnosis at the end of life? A consecutive cohort study. J Pain Symptom Manage. 2010;39(4):680.
- Acheson A, MacCormack D. Dyspnea and the cancer patient--an overview. Can Oncol Nurs J. 1997;7(4):209-213.
- Dudgeon DJ, Lertzman M. Dyspnea in the advanced cancer patient. J Pain Symptom Manage. 1998;16(4):212-219.
- Corner J, Plant H, A’Hern R, Bailey C. Non-pharmacological intervention for breathlessness in lung cancer. Palliat Med. 1996;10(4):299-305.
- LeGrand SB, Walsh D. Palliative management of dyspnea in advanced cancer. Curr Opin Oncol. 1999;11(4):250-254.
- LeGrand SB. Dyspnea: the continuing challenge of palliative management. Curr Opin Oncol. 2002;14(4):394-398.
- Bruera E, Macmillan K, Pither J, MacDonald RN. Effects of morphine on the dyspnea of terminal cancer patients. J Pain Symptom Manage. 1990;5(6):341-344.
- Held JL. Cancer care. Managing shortness of breath. Nursing. 1994;24(7):31.
- Bruera E, de Stoutz N, Velasco-Leiva A, Schoeller T, Hanson J. Effects of oxygen on dyspnoea in hypoxaemic terminal-cancer patients. Lancet. 1993;342(8862):13-14.
- Bailey C. Nursing as therapy in the management of breathlessness in lung cancer. Eur. J. Cancer Care (Engl.). 1995;4(4):184-190.
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage. 2012;(2):301-306.
- Parshall MB, Schwartzstein RM, Adams L, et al; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452.
- Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data. 2007;(386):1-32.
- Ekström MP, Abernethy AP, Currow DC. The management of chronic breathlessness in patients with advanced and terminal illness. BMJ. 2015;349:g7617.
- Karnani NG, Reisfield GM, Wilson GR. Evaluation of chronic dyspnea. Am Fam Physician. 2005;71(8):1529-1537.
- Moens K, Higginson IJ, Harding R; EURO IMPACT. Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage. 2014;48(4):660-677.
- Peters SP. When the chief complaint is (or should be) dyspnea in adults. J Allergy Clin Immunol Pract. 2013;1(2):129-136.
- Campbell ML. Dyspnea prevalence, trajectories, and measurement in critical care and at life’s end. Curr Opin Support Palliat Care. 2012;6(2):168-171.
- Argulian E, Agarwal V, Bangalore S, et al. Meta-analysis of prognostic implications of dyspnea versus chest pain in patients referred for stress testing. Am J Cardiol. 2014;113(3):559-564.
- van Mourik Y, Rutten FH, Moons KG, Bertens LC, Hoes AW, Reitsma JB. Prevalence and underlying causes of dyspnoea in older people: a systematic review. Age Ageing. 2014;43(3):319-326.
- Scano G, Gigliotti F, Stendardi L, Gagliardi E. Dyspnea and emotional states in health and disease. Respir Med. 2013;107(5):649-655.
- Murakami J, Ueda K, Sano F, Hayashi M, Tanaka N, Hamano K. Prediction of postoperative dyspnea and chronic respiratory failure. J Surg Res. 2015;195(1):303-310.
- Manning HL, Mahler DA. Pathophysiology of dyspnea. Monaldi Arch Chest Dis. 2001;56(4):325-330.
- Robinson LM. A Healthy 22-year-old woman with new onset dyspnea. J Emerg Nurs. 2010;36(2):146-147.
- Ben-Aharon I, Gafter-Gvili A, Leibovici L, Stemmer SM. Interventions for alleviating cancer-related dyspnea: a systematic review and meta-analysis. Acta Oncol. 2012;51(8):996-1008.
- Guirimand F, Sahut d’izarn M, Laporte L, Francillard M, Richard JF, Aegerter P. Sequential occurrence of dyspnea at the end of life in palliative care, according to the underlying cancer. Cancer Med. 2015;4(4):532-539.
- Fishbein D, Kearon C, Killian KJ. An approach to dyspnea in cancer patients. J Pain Symptom Manage. 1989;4(2):76-81.
- Ost DE, Ernst A, Grosu HB, et al; AQuIRE Brochoscopy Registry. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest. 2015;147(5):1282-1298.
- Ripamonti C, Bruera E. Dyspnea: pathophysiology and assessment. J Pain Symptom Manage. 1997;13(4):220-232.
- Petersen S, Ritz T. The role of fearful beliefs in the relationship between situational self-awareness and report of breathing-related sensations. Br J Health Psychol. 2011;16(Pt 2):359-372.
- Dudgeon DJ, Kristjanson L, Sloan JA, Lertzman M, Clement K. Dyspnea in cancer patients: prevalence and associated factors. J Pain Symptom Manage. 2001;21(2):95-102.
- Reuben DB, Mor V. Dyspnea in terminally ill cancer patients. Chest. 1986;89(2):234-236.
- Roberts DK, Thorne SE, Pearson C. The experience of dyspnea in late-stage cancer. Patients’ and nurses’ perspectives. Cancer Nurs. 1993;16(4):310-320.
- Reddy SK, Parsons HA, Elsayem A, Palmer JL, Bruera E. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med. 2009;12(1):29-36.
- Hayen A, Herigstad M, Pattinson KT. Understanding dyspnea as a complex individual experience. Maturitas. 2013;76(1):45-50.
- Bruera E, Schmitz B, Pither J, Neumann CM, Hanson J. The frequency and correlates of dyspnea in patients with advanced cancer. J Pain Symptom Manage. 2000;19(5):357-362.
- Ripamonti C. Management of dyspnea in advanced cancer patients. Support Care Cancer. 1999;7(4):
- 233-243.
- Hui D, Morgado M, Vidal M, et al. Dyspnea in hospitalized advanced cancer patients: subjective and physiologic correlates. J Palliat Med. 2013;16(3):
- 274-280.
- Ripamonti C, Fulfaro F, Bruera E. Dyspnoea in patients with advanced cancer: incidence, causes and treatments. Cancer Treat Rev. 1998;24(1):69-80.
- Dudgeon DJ, Lertzman M, Askew GR. Physiological changes and clinical correlations of dyspnea in cancer outpatients. J Pain Symptom Manage. 2001;21(5):373-379.
- Swinburn CR, Wakefield JM, Jones PW. Relationship between ventilation and breathlessness during exercise in chronic obstructive airways disease is not altered by prevention of hypoxaemia. Clin Sci (Lond). 1984;67(5):515-519.
- Rock LK, Schwartzstein RM. Mechanisms of dyspnea in chronic lung disease. Curr Opin Support Palliat Care. 2007;1(2):102-108.
- Moy ML, Woodrow Weiss J, Sparrow D, Israel E, Schwartzstein RM. Quality of dyspnea in bronchoconstriction differs from external resistive loads. Am J Respir Crit Care Med. 2000;162(2 Pt 1):451-455.
- Nakayama H, Shibuya M, Yamada M, Suzuki H, Arakawa M, Homma I. In-phase chest wall vibration decreases dyspnea during arm elevation in chronic obstructive pulmonary disease patients. Intern Med. 1998;37(10):831-835.
- Killian KJ, Jones NL. Mechanisms of exertional dyspnea. Clin Chest Med. 1994;15(2):247-257
- Gross NJ. Chronic obstructive pulmonary disease. Current concepts and therapeutic approaches. Chest. 1990;97(2 Suppl):19S-23S.
- Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: review and hypotheses. Respir Physiol Neurobiol. 2009;167(1):53-60.
- Petersen S, Orth B, Ritz T. Awareness of breathing: the structure of language descriptors of respiratory sensations. Health Psychol. 2008;27(1):122-127.
- Parshall MB, Meek PM, Sklar D, Alcock J, Bittner P. Test-retest reliability of multidimensional dyspnea profile recall ratings in the emergency department: a prospective, longitudinal study. BMC Emerg Med. 2012;12:6.
- Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Development and validation of the Cancer Dyspnoea Scale: a multidimensional, brief, self-rating scale. Br J Cancer. 2000;82(4):800-805.
- Booth S. The management of dyspnoea in advanced cancer. Hosp Med. 1998;59(5):348-349.
- Uronis HE, Shelby RA, Currow DC, et al. Assessment of the psychometric properties of an English version of the cancer dyspnea scale in people with advanced lung cancer. J Pain Symptom Manage. 2012;44(5):741-749.
- Dudgeon DJ, Rosenthal S. Management of dyspnea and cough in patients with cancer. Hematol Oncol Clin North Am. 1996;10(1):157-171.
- Currow DC, Smith J, Davidson PM, Newton PJ, Agar MR, Abernethy AP. Do the trajectories of dyspnea differ in prevalence and intensity by diagnosis at the end of life? A consecutive cohort study. J Pain Symptom Manage. 2010;39(4):680.
- Acheson A, MacCormack D. Dyspnea and the cancer patient--an overview. Can Oncol Nurs J. 1997;7(4):209-213.
- Dudgeon DJ, Lertzman M. Dyspnea in the advanced cancer patient. J Pain Symptom Manage. 1998;16(4):212-219.
- Corner J, Plant H, A’Hern R, Bailey C. Non-pharmacological intervention for breathlessness in lung cancer. Palliat Med. 1996;10(4):299-305.
- LeGrand SB, Walsh D. Palliative management of dyspnea in advanced cancer. Curr Opin Oncol. 1999;11(4):250-254.
- LeGrand SB. Dyspnea: the continuing challenge of palliative management. Curr Opin Oncol. 2002;14(4):394-398.
- Bruera E, Macmillan K, Pither J, MacDonald RN. Effects of morphine on the dyspnea of terminal cancer patients. J Pain Symptom Manage. 1990;5(6):341-344.
- Held JL. Cancer care. Managing shortness of breath. Nursing. 1994;24(7):31.
- Bruera E, de Stoutz N, Velasco-Leiva A, Schoeller T, Hanson J. Effects of oxygen on dyspnoea in hypoxaemic terminal-cancer patients. Lancet. 1993;342(8862):13-14.
- Bailey C. Nursing as therapy in the management of breathlessness in lung cancer. Eur. J. Cancer Care (Engl.). 1995;4(4):184-190.
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage. 2012;(2):301-306.