User login
The role of patient-reported outcomes in women’s health
In its landmark publication, “Crossing the quality chasm: A new health system for the 21st century,” the Institute of Medicine (now the National Academy of Medicine) called for an emphasis on patient-centered care that it defined as “Providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.”1 Studies suggest that the patient’s view of health care delivery determines outcome and satisfaction.2 Therefore, we need to expend more effort to understand what patients need or want from their treatment or interaction with the health care system.
Measuring patient-reported outcomes (PROs) is an attempt to recognize and address patient concerns. Although currently PROs are focused primarily in the arena of clinical research, their use has the potential to transform daily clinical patient encounters and improve the cost and quality of health care.3
In this article, we provide a brief overview of PROs and describe how they can be used to improve individual patient care, clinical research, and health care quality. We also offer examples of how PROs can be used in specific women’s health conditions.

What exactly are PROs?
PROs are reports of the status of a patient’s health condition, health behavior, or experience with health care; they come directly from the patient, without anyone else (such as a clinician or caregiver) interpreting the patient’s response.4 PROs usually pertain to general health, quality of life, functional status, or preferences associated with health care or treatment.5 Usually PROs are elicited via a self-administered survey and provide the patient’s perspective on treatment benefits, side effects, change in symptoms, general perceptions of feelings or well-being, or satisfaction with care. Often they represent the outcomes that are most important to patients.6 The survey usually consists of several questions or items. It can be general or condition specific, and it may represent one or more health care dimensions.
The term patient-reported outcome measure (PROM) refers to the survey instrument used to collect PROs. Patient-reported experience measures (PREMs), such as satisfaction surveys, are considered a subset of PROMs.7
Standardized PROs developed out of clinical trials
The use of PROs evolved from clinical trials. The proliferation of PROs resulted in an inability to compare outcomes across trials or different conditions. This led to a need to standardize and possibly harmonize measures and to reach consensus about properties required for a “good” measure and requirements needed for “adequate” reporting. Many investigators and several national and international organizations have provided iterative guidance, including the US Food and Drug Administration (FDA), European Medicines Agency, National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS), International Consortium for Health Outcomes Measurement (ICHOM), University of Oxford Patient Reported Outcomes Measurement Group, Cochrane Systematic Reviews, Consolidated Standards of Reporting Trials–Patient Reported Outcomes (CONSORT-PRO) extension (how to report PROs with the CONSORT checklist), and the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).4,5,8–18
In the United States, the RAND Medical Outcomes Study led to the development of the 12- and 36-item short form surveys, which are widely recognized and commonly used PROMs for health-related quality of life.19 The study generated multiple additional survey instruments that evaluate other domains and dimensions of health. These surveys have been translated into numerous languages, and the RAND website lists over 100 publications.19
In 2002, the NIH sponsored PROMIS, a cooperative program designed to develop, validate, and standardize item banks to measure PROs that were relevant across multiple, common medical conditions. Based on literature review, feedback from both healthy and sick patients, and clinical expert opinion, the PROMIS investigators developed a consensus-based framework for self-reported health that included the following domains: pain, fatigue, emotional distress, physical functioning, and social role participation; these domains were evaluated on paper or with computer-assisted technology.11–14 PROMIS is now a web-based resource with approximately 70 domains pertinent to children and adults in the general population and in those with chronic disease. Measures have been translated into more than 40 languages, and PROMIS-related work has resulted in more than 400 publications.14
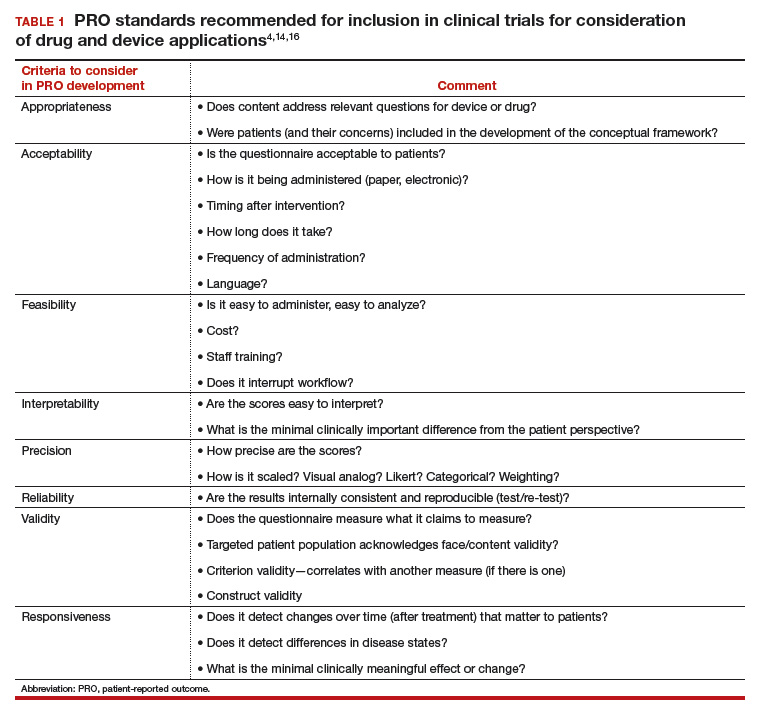
In 2006, the FDA issued a draft document regarding the PRO standards that should be included in clinical trials for consideration of drug and device applications (TABLE 1). These recommendations, updated in 2009, were largely drawn from work published by PROMIS and University of Oxford investigators.4,14,16
Because PROs are infrequently measured in routine clinical practice and PROMs that are used vary between countries, global comparison is difficult. Hence, ICHOM convened in 2012 to develop consensus-based, globally agreed on sets of outcomes that are intended to reflect what matters most to patients.
ICHOM specified 2 goals: 1) the core sets should be used in routine clinical practice, and 2) the core sets should be used as end points in clinical studies.15
As of May 2015, 12 standard sets of outcomes have been developed, representing 35% of the global burden of disease. ICHOM currently is creating networks of hospitals around the world to begin measuring, benchmarking, and performing outcome comparisons that can ultimately be used to inform global health system learning and clinical care improvement.15
Read about the evolving use of PROs
Use of PROs is evolving
Historically, PROMs have been used primarily in clinical trials to document the relative benefits of an intervention. With today’s focus on patient-centered care, however, there is a growing mandate to integrate PROMs into clinical care, quality improvement, and ultimately reimbursement. Recently, Basch and colleagues eloquently described the benefit of routine collection of PROs for cancer patients and the opportunity for improved care across the health system.20
PROs can be applied on various levels. For example, if a patient reports a symptom (X), or a change in symptom X, the following options are possible:
- Clinician level: Symptom management with altered dose or change in medication. This is associated with improved self-efficacy for the patient, a shift toward goal-oriented care, improved communication with the provider, and improved patient satisfaction.
- Researcher level: PROs should be used as a primary end point, in addition to traditional outcomes (mortality, survival, physiologic markers), to allow for comparative effectiveness studies or patient-centered outcomes research studies that evaluate what matters most to patients relative to the specific health condition, intervention, and symptom management.
- Health system level: Quality assurance, quality improvement activities. How effective is the health system in the management of symptom X? Are all clinicians using the same medication or the same dose? Is there a best practice for managing symptom X?
- Population level: Provides evidence for other clinicians and patients to make decisions about what to expect with treatment for symptom X.
From a reimbursement level, clinicians and providers are paid based on performance—the more satisfied patients are about X, the higher the reimbursement. This has been pertinent particularly in high-volume orthopedic conditions in which anatomic correction of hip or knee joints has not consistently demonstrated improvement in quality of life as measured by the following PROs: perception of pain, mobility, physical functioning, social functioning, and emotional distress. Because of concerns about high volume, high cost, and inconsistent outcomes, the US Department of Health and Human Services has specified that 50% of Medicare and 90% of Medicaid reimbursements will be based on outcomes or value-based purchasing options.21
Studies have shown that it is possible to collect PRO data for cancer patients—despite age or severity of illness—and integrate it into clinical care delivery. These data can provide useful, actionable information, resulting in decreased emergency department visits, longer toleration of chemotherapy, and improved survival.22 Similar results have been demonstrated in other medical conditions, although challenges exist when transitioning from research settings to routine care. Challenges include privacy concerns, patient recruitment and tracking, encouraging patients to complete the PRO surveys (nonresponse leads to biased data), real and perceived administrative burden to staff, obtaining clinician buy in, and costs related to surveys and data analysis.23
Read about the benefits of PROs to patients and clients
Using PROs in women’s health care: Benefits for patients and clinicians
According to a study by Frosch, patients want to know if a prescribed therapy actually improves outcomes, not whether it changes an isolated biomarker that does not translate into subjective improvement.24 They want to know if the trade-off (adverse effects or higher cost) associated with a new drug or therapy is worth the improved mobility or time spent pain free.
Intuitively, all clinicians have similar opportunities for discussions with regard to the risks, benefits, and alternatives of medical treatment, surgical treatment, or expectant management. We routinely document this discussion daily. However, in this era of patient-centered care, when a patient asks, “What should I do, doctor?” we no longer can respond with a default recommendation. We must engage the patient and ask, “What do you want to do? What is most important to you?”
ObGyns are well suited to benefit from standardized efforts to collect PROs, as we frequently discuss with our patients trade-offs regarding treatment risks and benefits and their personal values and preferences. Examples include contraception options, hormone treatment for menopause, medication use during pregnancy, decisions at the limits of viability, preterm delivery for severe preeclampsia, induction/augmentation versus spontaneous labor, epidural versus physiologic labor, repeat cesarean versus vaginal birth after cesarean, and even elective primary cesarean versus vaginal birth.
Validated PROMs exist for benign gynecology, such as abnormal uterine bleeding, fibroids, polycystic ovary syndrome (PCOS), infertility, pelvic organ prolapse and/or urinary incontinence, and surgery for benign gynecology symptoms, as well as for cancer (breast, ovarian, cervical).25–39
From the PCOS literature we can glean a poignant example of the importance of PROs. Martin and colleagues compared patient and clinician interviews regarding important PROs from the patient perspective.29 Patients identified pain, cramping, heavy bleeding, and bloating as important, whereas clinicians did not consider these symptoms important to patients with PCOS. Clinicians thought “issues with menstruation,” characterized as irregular or no periods, were important, whereas patients were more concerned with heavy bleeding or bleeding of long duration. The authors concluded that concepts frequently expressed by patients and considered important from their perspective did not register with clinicians as being relevant and are not captured on current PRO instruments, emphasizing our knowledge gap and the need to pay attention to what patients want.29
Surprisingly, although pregnancy and childbirth is the number one cause for hospital admissions, a highly preference-driven condition, and a leading cause of morbidity, mortality, and costs, there are few published PROs in the field. In a systematic review of more than 1,700 articles describing PROs published in English through 2014, Martin found that fewer than 1% included PROs specific to pregnancy and childbirth.40
ICHOM has created a standard set of outcomes for pregnancy and childbirth based on consensus recommendations from physicians, measurement experts, and patients.41 The consortium describes 4 domains and 14 subdomains (TABLE 2) and provides suggestions for a validated PROM if known or where appropriate.
Similar domains and subdomains have been corroborated by our research team (the Maternal Quality Indicator [MQI] Work Group), the Childbirth Connection, and Gartner and colleagues.42–44 The MQI Work Group recently conducted a national survey of what women want and what they think is important for their childbirth experience. We identified 19 domains, consistent with those of other investigators.42 Gartner and colleagues advocate for a composite outcome measure that combines the core domains into one preference-based utility measure that is weighted.44 The rationale for this recommendation is that the levels of the domains might contribute differently to the overall birth experience. For example, communication might contribute more to an overall measure than pain management.44 The development of a childbirth-specific survey to evaluate patient-reported outcomes and patient-reported experiences with care is needed if we are to provide value-based care in this arena.45
Looking forward
PROs, PROMs, and PREMs are here to stay. They no longer are limited to clinical research, but increasingly will be incorporated into clinical care, providing us with opportunities to improve the quality of health care delivery, efficiency of patient/clinician interactions, and patients’ ratings of their health care experience.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001:6.
- Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- Rickert J. Patient-centered care: what it means and how to get there. Health Affairs website. http://healthaffairs.org/blog/2012/01/24/patient-centered-care-what-it-means-and-how-to-get-there/. Published January 24, 2012. Accessed October 15, 2017.
- US Food and Drug Administration. Guidance for industry: Patient reported outcome measures: use in medical product development to support labeling claims. https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf. Published December 2009. Accessed February 6, 2018.
- Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008. http://handbook.cochrane.org. Accessed October 15, 2017.
- Patrick DL, Guyatt PD, Acquadro C. Patient-reported outcomes. In: Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008:chap 17. http://handbook-5-1.cochrane.org/. Accessed October 15, 2017.
- Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights. 2013;6:61–68.
- McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):163–169.
- European Medicines Agency, Committee for Medicinal Products for Human Use. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. https://www.ispor.org/workpaper/emea-hrql-guidance.pdf. Published July 27, 2005. Accessed February 7, 2018.
- Venkatesan P. New European guidance on patient-reported outcomes. Lancet Oncol. 2016;17(6):e226.
- Cella D, Yount S, Rothrock N, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3–S11.
- Cella D, Riley W, Stone A, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194.
- Craig BM, Reeve BB, Brown PM, et al. US valuation of health outcomes measured using the PROMIS-29. Value Health. 2014;17(8):846–853.
- National Institutes of Health. Patient-Reported Outcomes Measurement Information System (PROMIS). https://commonfund.nih.gov/promis/index. Reviewed May 8, 2017. Accessed October 15, 2017.
- International Consortium for Health Outcomes Measurement (ICHOM). http://www.ichom.org/. Accessed October 15, 2017.
- University of Oxford, Patient Reported Outcomes Measurement Group http://phi.uhce.ox.ac.uk/. Accessed October 15, 2017.
- CONSORT. Patient-Reported Outcomes (CONSORT PRO). http://www.consort-statement.org/extensions/overview/consort-pro. Accessed October 15, 2017.
- International Society for Pharmacoeconomics and Outcomes Research. https://www.ispor.org/. Accessed October 15, 2017.
- RAND Health. RAND medical outcomes study: measures of quality of life core survey from RAND Health. https://www.rand.org/health/surveys_tools/mos.html. Accessed October 15, 2017.
- Basch EM, Deal AM, Dueck A, et al. Overall survival results of a randomized trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment [abstract LBA2]. J Clin Oncol. 2017;35(18)(suppl).
- Centers for Medicare and Medicaid Services. Better care. Smarter spending. Healthier people: paying providers for value, not volume. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-01-26-3.html. Accessed October 15, 2017.
- Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565.
- Chenok K, Teleki S, SooHoo NF, Huddleston J, Bozic KJ. Collecting patient-reported outcomes: lessons from the California Joint Replacement Registry. EGEMS (Wash DC). 2015;3(1):1196.
- Frosch DL. Patient-reported outcomes as a measure of healthcare quality. J Gen Intern Med. 2015;30(10):1383–1384.
- Gibbons E, Mackintosh A, Fitzpatrick R; Patient-Reported Outcome Measurement Group, Oxford. A structured review of patient-reported outcome measures for people undergoing elective procedures for benign gynaecological conditions of the uterus, 2010. http://phi.uhce.ox.ac.uk/pdf/ElectiveProcedures/PROMs_Oxford_Gynaecological%20procedures_012011.pdf. Accessed October 23, 2017.
- Matteson KA, Boardman LA, Munro MG, Clark MA. Abnormal uterine bleeding: a review of patient-based outcome measures. Fertil Steril. 2009;92(1):205–216.
- Matteson KA, Scott DM, Raker CA, Clark MA. The menstrual bleeding questionnaire: development and validation of a comprehensive patient-reported outcome instrument for heavy menstrual bleeding. BJOG. 2015;122(5):681–689.
- Coyne KS, Margolis MK, Bradley LD, Guido R, Maxwell GL, Spies JB. Further validation of the uterine fibroid symptom and quality-of-life questionnaire. Value Health. 2012;15(1):135–142.
- Martin ML, Halling K, Eek D, Krohe M, Paty J. Understanding polycystic ovary syndrome from the patient perspective: a concept elicitation patient interview study. Health Quality Life Outcomes. 2017;15(1):162.
- Malik-Aslam A, Reaney MD, Speight J. The suitability of polycystic ovary syndrome-specific questionnaires for measuring the impact of PCOS on quality of life in clinical trials. Value Health. 2010;13(4):440–446.
- Kitchen H, Aldhouse N, Trigg A, Palencia R, Mitchell S. A review of patient-reported outcome measures to assess female infertility-related quality of life. Health Qual Life Outcomes. 2017;15(1):86.
- Sung VW, Joo K, Marques F, Myers DL. Patient-reported outcomes after combined surgery for pelvic floor disorders in older compared to younger women. Am J Obstet Gynecol. 2009;201(5):534.e1–e5.
- Sung VW, Rogers RG, Barber MD, Clark MA. Conceptual framework for patient-important treatment outcomes for pelvic organ prolapse. Neurourol Urodynam. 2014;33(4):414–419.
- Sung VW, Wohlrab KJ, Madsen A, Raker C. Patient-reported goal attainment and comprehensive functioning outcomes after surgery compared with pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2016;215(5):659.e1–e7.
- Croke J. Cervical ca PROs in clinical practice. https://clinicaltrials.gov/ct2/show/NCT03048435. Accessed October 16, 2017.
- Catt S, Starkings R, Shilling V, Fallowfield L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: a systematic review. J Cancer Surviv. 2017;11(2):211–232.
- Jensen RE, Potosky AL, Moinpour CM, et al. United States population estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. J Clin Oncol. 2017;35(17):1913–1920.
- Friedlander M, Mercieca-Bebber RL, King MT. Patient-reported outcomes (PRO) in ovarian cancer clinical trials—lost opportunities and lessons learned. Ann Oncol. 2016;27(suppl 1):i66–i71.
- Joly F, Hilpert F, Okamoto A, Stuart G, Ochaia K, Friedlander M; 5th Ovarian Cancer Consensus Conference. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recommendations on incorporating patient-reported outcomes in clinical trials in epithelial ovarian cancer. Eur J Cancer. 2017;78:133–138.
- Martin A. Patient-reported outcomes in studies published in 2014: which disease areas have been the main focus of clinical research? Value Health. 2015;18(7):A742.
- International Consortium for Health Outcomes Management (ICHOM). Pregnancy and childbirth. http://www.ichom.org/medical-conditions/pregnancy-and-childbirth/. Accessed October 10, 2017.
- El Haj Ibrahim S, McCulloch J, Korst LM, Fridman M, Fink A, Gregory KD. Communication with staff during hospitalization for childbirth: the patient’s perspective [1R]. Obstet Gynecol. 2016;127.
- National Partnership for Women and Families. Childbirth Connection. Listening to mothers III: report of the third national US survey of women’s childbearing experience. http://transform.childbirthconnection.org/reports/listeningtomothers/. Accessed October 23, 2017.
- Gartner FR, Freeman LM, Rijnders ME, et al. A comprehensive representation of the birth-experience: identification and prioritization of birth-specific domains based on a mixed-method design. BMC Pregnancy Childbirth. 2014;14:147.
- National Partnership for Women and Families. The priority of developing and implementing CAHPS maternity care facility, clinician and health plan surveys. 2015. http://www.nationalpartnership.org/research-library/maternal-health/cahps-maternity-care-fact-sheet.pdf. Accessed October 23, 2017.
In its landmark publication, “Crossing the quality chasm: A new health system for the 21st century,” the Institute of Medicine (now the National Academy of Medicine) called for an emphasis on patient-centered care that it defined as “Providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.”1 Studies suggest that the patient’s view of health care delivery determines outcome and satisfaction.2 Therefore, we need to expend more effort to understand what patients need or want from their treatment or interaction with the health care system.
Measuring patient-reported outcomes (PROs) is an attempt to recognize and address patient concerns. Although currently PROs are focused primarily in the arena of clinical research, their use has the potential to transform daily clinical patient encounters and improve the cost and quality of health care.3
In this article, we provide a brief overview of PROs and describe how they can be used to improve individual patient care, clinical research, and health care quality. We also offer examples of how PROs can be used in specific women’s health conditions.

What exactly are PROs?
PROs are reports of the status of a patient’s health condition, health behavior, or experience with health care; they come directly from the patient, without anyone else (such as a clinician or caregiver) interpreting the patient’s response.4 PROs usually pertain to general health, quality of life, functional status, or preferences associated with health care or treatment.5 Usually PROs are elicited via a self-administered survey and provide the patient’s perspective on treatment benefits, side effects, change in symptoms, general perceptions of feelings or well-being, or satisfaction with care. Often they represent the outcomes that are most important to patients.6 The survey usually consists of several questions or items. It can be general or condition specific, and it may represent one or more health care dimensions.
The term patient-reported outcome measure (PROM) refers to the survey instrument used to collect PROs. Patient-reported experience measures (PREMs), such as satisfaction surveys, are considered a subset of PROMs.7
Standardized PROs developed out of clinical trials
The use of PROs evolved from clinical trials. The proliferation of PROs resulted in an inability to compare outcomes across trials or different conditions. This led to a need to standardize and possibly harmonize measures and to reach consensus about properties required for a “good” measure and requirements needed for “adequate” reporting. Many investigators and several national and international organizations have provided iterative guidance, including the US Food and Drug Administration (FDA), European Medicines Agency, National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS), International Consortium for Health Outcomes Measurement (ICHOM), University of Oxford Patient Reported Outcomes Measurement Group, Cochrane Systematic Reviews, Consolidated Standards of Reporting Trials–Patient Reported Outcomes (CONSORT-PRO) extension (how to report PROs with the CONSORT checklist), and the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).4,5,8–18
In the United States, the RAND Medical Outcomes Study led to the development of the 12- and 36-item short form surveys, which are widely recognized and commonly used PROMs for health-related quality of life.19 The study generated multiple additional survey instruments that evaluate other domains and dimensions of health. These surveys have been translated into numerous languages, and the RAND website lists over 100 publications.19
In 2002, the NIH sponsored PROMIS, a cooperative program designed to develop, validate, and standardize item banks to measure PROs that were relevant across multiple, common medical conditions. Based on literature review, feedback from both healthy and sick patients, and clinical expert opinion, the PROMIS investigators developed a consensus-based framework for self-reported health that included the following domains: pain, fatigue, emotional distress, physical functioning, and social role participation; these domains were evaluated on paper or with computer-assisted technology.11–14 PROMIS is now a web-based resource with approximately 70 domains pertinent to children and adults in the general population and in those with chronic disease. Measures have been translated into more than 40 languages, and PROMIS-related work has resulted in more than 400 publications.14
In 2006, the FDA issued a draft document regarding the PRO standards that should be included in clinical trials for consideration of drug and device applications (TABLE 1). These recommendations, updated in 2009, were largely drawn from work published by PROMIS and University of Oxford investigators.4,14,16

Because PROs are infrequently measured in routine clinical practice and PROMs that are used vary between countries, global comparison is difficult. Hence, ICHOM convened in 2012 to develop consensus-based, globally agreed on sets of outcomes that are intended to reflect what matters most to patients.
ICHOM specified 2 goals: 1) the core sets should be used in routine clinical practice, and 2) the core sets should be used as end points in clinical studies.15
As of May 2015, 12 standard sets of outcomes have been developed, representing 35% of the global burden of disease. ICHOM currently is creating networks of hospitals around the world to begin measuring, benchmarking, and performing outcome comparisons that can ultimately be used to inform global health system learning and clinical care improvement.15
Read about the evolving use of PROs
Use of PROs is evolving
Historically, PROMs have been used primarily in clinical trials to document the relative benefits of an intervention. With today’s focus on patient-centered care, however, there is a growing mandate to integrate PROMs into clinical care, quality improvement, and ultimately reimbursement. Recently, Basch and colleagues eloquently described the benefit of routine collection of PROs for cancer patients and the opportunity for improved care across the health system.20
PROs can be applied on various levels. For example, if a patient reports a symptom (X), or a change in symptom X, the following options are possible:
- Clinician level: Symptom management with altered dose or change in medication. This is associated with improved self-efficacy for the patient, a shift toward goal-oriented care, improved communication with the provider, and improved patient satisfaction.
- Researcher level: PROs should be used as a primary end point, in addition to traditional outcomes (mortality, survival, physiologic markers), to allow for comparative effectiveness studies or patient-centered outcomes research studies that evaluate what matters most to patients relative to the specific health condition, intervention, and symptom management.
- Health system level: Quality assurance, quality improvement activities. How effective is the health system in the management of symptom X? Are all clinicians using the same medication or the same dose? Is there a best practice for managing symptom X?
- Population level: Provides evidence for other clinicians and patients to make decisions about what to expect with treatment for symptom X.
From a reimbursement level, clinicians and providers are paid based on performance—the more satisfied patients are about X, the higher the reimbursement. This has been pertinent particularly in high-volume orthopedic conditions in which anatomic correction of hip or knee joints has not consistently demonstrated improvement in quality of life as measured by the following PROs: perception of pain, mobility, physical functioning, social functioning, and emotional distress. Because of concerns about high volume, high cost, and inconsistent outcomes, the US Department of Health and Human Services has specified that 50% of Medicare and 90% of Medicaid reimbursements will be based on outcomes or value-based purchasing options.21
Studies have shown that it is possible to collect PRO data for cancer patients—despite age or severity of illness—and integrate it into clinical care delivery. These data can provide useful, actionable information, resulting in decreased emergency department visits, longer toleration of chemotherapy, and improved survival.22 Similar results have been demonstrated in other medical conditions, although challenges exist when transitioning from research settings to routine care. Challenges include privacy concerns, patient recruitment and tracking, encouraging patients to complete the PRO surveys (nonresponse leads to biased data), real and perceived administrative burden to staff, obtaining clinician buy in, and costs related to surveys and data analysis.23
Read about the benefits of PROs to patients and clients
Using PROs in women’s health care: Benefits for patients and clinicians
According to a study by Frosch, patients want to know if a prescribed therapy actually improves outcomes, not whether it changes an isolated biomarker that does not translate into subjective improvement.24 They want to know if the trade-off (adverse effects or higher cost) associated with a new drug or therapy is worth the improved mobility or time spent pain free.
Intuitively, all clinicians have similar opportunities for discussions with regard to the risks, benefits, and alternatives of medical treatment, surgical treatment, or expectant management. We routinely document this discussion daily. However, in this era of patient-centered care, when a patient asks, “What should I do, doctor?” we no longer can respond with a default recommendation. We must engage the patient and ask, “What do you want to do? What is most important to you?”
ObGyns are well suited to benefit from standardized efforts to collect PROs, as we frequently discuss with our patients trade-offs regarding treatment risks and benefits and their personal values and preferences. Examples include contraception options, hormone treatment for menopause, medication use during pregnancy, decisions at the limits of viability, preterm delivery for severe preeclampsia, induction/augmentation versus spontaneous labor, epidural versus physiologic labor, repeat cesarean versus vaginal birth after cesarean, and even elective primary cesarean versus vaginal birth.
Validated PROMs exist for benign gynecology, such as abnormal uterine bleeding, fibroids, polycystic ovary syndrome (PCOS), infertility, pelvic organ prolapse and/or urinary incontinence, and surgery for benign gynecology symptoms, as well as for cancer (breast, ovarian, cervical).25–39
From the PCOS literature we can glean a poignant example of the importance of PROs. Martin and colleagues compared patient and clinician interviews regarding important PROs from the patient perspective.29 Patients identified pain, cramping, heavy bleeding, and bloating as important, whereas clinicians did not consider these symptoms important to patients with PCOS. Clinicians thought “issues with menstruation,” characterized as irregular or no periods, were important, whereas patients were more concerned with heavy bleeding or bleeding of long duration. The authors concluded that concepts frequently expressed by patients and considered important from their perspective did not register with clinicians as being relevant and are not captured on current PRO instruments, emphasizing our knowledge gap and the need to pay attention to what patients want.29
Surprisingly, although pregnancy and childbirth is the number one cause for hospital admissions, a highly preference-driven condition, and a leading cause of morbidity, mortality, and costs, there are few published PROs in the field. In a systematic review of more than 1,700 articles describing PROs published in English through 2014, Martin found that fewer than 1% included PROs specific to pregnancy and childbirth.40
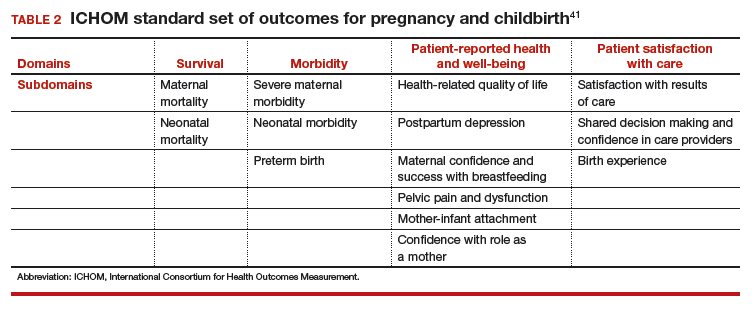
ICHOM has created a standard set of outcomes for pregnancy and childbirth based on consensus recommendations from physicians, measurement experts, and patients.41 The consortium describes 4 domains and 14 subdomains (TABLE 2) and provides suggestions for a validated PROM if known or where appropriate.

Similar domains and subdomains have been corroborated by our research team (the Maternal Quality Indicator [MQI] Work Group), the Childbirth Connection, and Gartner and colleagues.42–44 The MQI Work Group recently conducted a national survey of what women want and what they think is important for their childbirth experience. We identified 19 domains, consistent with those of other investigators.42 Gartner and colleagues advocate for a composite outcome measure that combines the core domains into one preference-based utility measure that is weighted.44 The rationale for this recommendation is that the levels of the domains might contribute differently to the overall birth experience. For example, communication might contribute more to an overall measure than pain management.44 The development of a childbirth-specific survey to evaluate patient-reported outcomes and patient-reported experiences with care is needed if we are to provide value-based care in this arena.45
Looking forward
PROs, PROMs, and PREMs are here to stay. They no longer are limited to clinical research, but increasingly will be incorporated into clinical care, providing us with opportunities to improve the quality of health care delivery, efficiency of patient/clinician interactions, and patients’ ratings of their health care experience.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In its landmark publication, “Crossing the quality chasm: A new health system for the 21st century,” the Institute of Medicine (now the National Academy of Medicine) called for an emphasis on patient-centered care that it defined as “Providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.”1 Studies suggest that the patient’s view of health care delivery determines outcome and satisfaction.2 Therefore, we need to expend more effort to understand what patients need or want from their treatment or interaction with the health care system.
Measuring patient-reported outcomes (PROs) is an attempt to recognize and address patient concerns. Although currently PROs are focused primarily in the arena of clinical research, their use has the potential to transform daily clinical patient encounters and improve the cost and quality of health care.3
In this article, we provide a brief overview of PROs and describe how they can be used to improve individual patient care, clinical research, and health care quality. We also offer examples of how PROs can be used in specific women’s health conditions.

What exactly are PROs?
PROs are reports of the status of a patient’s health condition, health behavior, or experience with health care; they come directly from the patient, without anyone else (such as a clinician or caregiver) interpreting the patient’s response.4 PROs usually pertain to general health, quality of life, functional status, or preferences associated with health care or treatment.5 Usually PROs are elicited via a self-administered survey and provide the patient’s perspective on treatment benefits, side effects, change in symptoms, general perceptions of feelings or well-being, or satisfaction with care. Often they represent the outcomes that are most important to patients.6 The survey usually consists of several questions or items. It can be general or condition specific, and it may represent one or more health care dimensions.
The term patient-reported outcome measure (PROM) refers to the survey instrument used to collect PROs. Patient-reported experience measures (PREMs), such as satisfaction surveys, are considered a subset of PROMs.7
Standardized PROs developed out of clinical trials
The use of PROs evolved from clinical trials. The proliferation of PROs resulted in an inability to compare outcomes across trials or different conditions. This led to a need to standardize and possibly harmonize measures and to reach consensus about properties required for a “good” measure and requirements needed for “adequate” reporting. Many investigators and several national and international organizations have provided iterative guidance, including the US Food and Drug Administration (FDA), European Medicines Agency, National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS), International Consortium for Health Outcomes Measurement (ICHOM), University of Oxford Patient Reported Outcomes Measurement Group, Cochrane Systematic Reviews, Consolidated Standards of Reporting Trials–Patient Reported Outcomes (CONSORT-PRO) extension (how to report PROs with the CONSORT checklist), and the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).4,5,8–18
In the United States, the RAND Medical Outcomes Study led to the development of the 12- and 36-item short form surveys, which are widely recognized and commonly used PROMs for health-related quality of life.19 The study generated multiple additional survey instruments that evaluate other domains and dimensions of health. These surveys have been translated into numerous languages, and the RAND website lists over 100 publications.19
In 2002, the NIH sponsored PROMIS, a cooperative program designed to develop, validate, and standardize item banks to measure PROs that were relevant across multiple, common medical conditions. Based on literature review, feedback from both healthy and sick patients, and clinical expert opinion, the PROMIS investigators developed a consensus-based framework for self-reported health that included the following domains: pain, fatigue, emotional distress, physical functioning, and social role participation; these domains were evaluated on paper or with computer-assisted technology.11–14 PROMIS is now a web-based resource with approximately 70 domains pertinent to children and adults in the general population and in those with chronic disease. Measures have been translated into more than 40 languages, and PROMIS-related work has resulted in more than 400 publications.14
In 2006, the FDA issued a draft document regarding the PRO standards that should be included in clinical trials for consideration of drug and device applications (TABLE 1). These recommendations, updated in 2009, were largely drawn from work published by PROMIS and University of Oxford investigators.4,14,16

Because PROs are infrequently measured in routine clinical practice and PROMs that are used vary between countries, global comparison is difficult. Hence, ICHOM convened in 2012 to develop consensus-based, globally agreed on sets of outcomes that are intended to reflect what matters most to patients.
ICHOM specified 2 goals: 1) the core sets should be used in routine clinical practice, and 2) the core sets should be used as end points in clinical studies.15
As of May 2015, 12 standard sets of outcomes have been developed, representing 35% of the global burden of disease. ICHOM currently is creating networks of hospitals around the world to begin measuring, benchmarking, and performing outcome comparisons that can ultimately be used to inform global health system learning and clinical care improvement.15
Read about the evolving use of PROs
Use of PROs is evolving
Historically, PROMs have been used primarily in clinical trials to document the relative benefits of an intervention. With today’s focus on patient-centered care, however, there is a growing mandate to integrate PROMs into clinical care, quality improvement, and ultimately reimbursement. Recently, Basch and colleagues eloquently described the benefit of routine collection of PROs for cancer patients and the opportunity for improved care across the health system.20
PROs can be applied on various levels. For example, if a patient reports a symptom (X), or a change in symptom X, the following options are possible:
- Clinician level: Symptom management with altered dose or change in medication. This is associated with improved self-efficacy for the patient, a shift toward goal-oriented care, improved communication with the provider, and improved patient satisfaction.
- Researcher level: PROs should be used as a primary end point, in addition to traditional outcomes (mortality, survival, physiologic markers), to allow for comparative effectiveness studies or patient-centered outcomes research studies that evaluate what matters most to patients relative to the specific health condition, intervention, and symptom management.
- Health system level: Quality assurance, quality improvement activities. How effective is the health system in the management of symptom X? Are all clinicians using the same medication or the same dose? Is there a best practice for managing symptom X?
- Population level: Provides evidence for other clinicians and patients to make decisions about what to expect with treatment for symptom X.
From a reimbursement level, clinicians and providers are paid based on performance—the more satisfied patients are about X, the higher the reimbursement. This has been pertinent particularly in high-volume orthopedic conditions in which anatomic correction of hip or knee joints has not consistently demonstrated improvement in quality of life as measured by the following PROs: perception of pain, mobility, physical functioning, social functioning, and emotional distress. Because of concerns about high volume, high cost, and inconsistent outcomes, the US Department of Health and Human Services has specified that 50% of Medicare and 90% of Medicaid reimbursements will be based on outcomes or value-based purchasing options.21
Studies have shown that it is possible to collect PRO data for cancer patients—despite age or severity of illness—and integrate it into clinical care delivery. These data can provide useful, actionable information, resulting in decreased emergency department visits, longer toleration of chemotherapy, and improved survival.22 Similar results have been demonstrated in other medical conditions, although challenges exist when transitioning from research settings to routine care. Challenges include privacy concerns, patient recruitment and tracking, encouraging patients to complete the PRO surveys (nonresponse leads to biased data), real and perceived administrative burden to staff, obtaining clinician buy in, and costs related to surveys and data analysis.23
Read about the benefits of PROs to patients and clients
Using PROs in women’s health care: Benefits for patients and clinicians
According to a study by Frosch, patients want to know if a prescribed therapy actually improves outcomes, not whether it changes an isolated biomarker that does not translate into subjective improvement.24 They want to know if the trade-off (adverse effects or higher cost) associated with a new drug or therapy is worth the improved mobility or time spent pain free.
Intuitively, all clinicians have similar opportunities for discussions with regard to the risks, benefits, and alternatives of medical treatment, surgical treatment, or expectant management. We routinely document this discussion daily. However, in this era of patient-centered care, when a patient asks, “What should I do, doctor?” we no longer can respond with a default recommendation. We must engage the patient and ask, “What do you want to do? What is most important to you?”
ObGyns are well suited to benefit from standardized efforts to collect PROs, as we frequently discuss with our patients trade-offs regarding treatment risks and benefits and their personal values and preferences. Examples include contraception options, hormone treatment for menopause, medication use during pregnancy, decisions at the limits of viability, preterm delivery for severe preeclampsia, induction/augmentation versus spontaneous labor, epidural versus physiologic labor, repeat cesarean versus vaginal birth after cesarean, and even elective primary cesarean versus vaginal birth.
Validated PROMs exist for benign gynecology, such as abnormal uterine bleeding, fibroids, polycystic ovary syndrome (PCOS), infertility, pelvic organ prolapse and/or urinary incontinence, and surgery for benign gynecology symptoms, as well as for cancer (breast, ovarian, cervical).25–39
From the PCOS literature we can glean a poignant example of the importance of PROs. Martin and colleagues compared patient and clinician interviews regarding important PROs from the patient perspective.29 Patients identified pain, cramping, heavy bleeding, and bloating as important, whereas clinicians did not consider these symptoms important to patients with PCOS. Clinicians thought “issues with menstruation,” characterized as irregular or no periods, were important, whereas patients were more concerned with heavy bleeding or bleeding of long duration. The authors concluded that concepts frequently expressed by patients and considered important from their perspective did not register with clinicians as being relevant and are not captured on current PRO instruments, emphasizing our knowledge gap and the need to pay attention to what patients want.29
Surprisingly, although pregnancy and childbirth is the number one cause for hospital admissions, a highly preference-driven condition, and a leading cause of morbidity, mortality, and costs, there are few published PROs in the field. In a systematic review of more than 1,700 articles describing PROs published in English through 2014, Martin found that fewer than 1% included PROs specific to pregnancy and childbirth.40
ICHOM has created a standard set of outcomes for pregnancy and childbirth based on consensus recommendations from physicians, measurement experts, and patients.41 The consortium describes 4 domains and 14 subdomains (TABLE 2) and provides suggestions for a validated PROM if known or where appropriate.

Similar domains and subdomains have been corroborated by our research team (the Maternal Quality Indicator [MQI] Work Group), the Childbirth Connection, and Gartner and colleagues.42–44 The MQI Work Group recently conducted a national survey of what women want and what they think is important for their childbirth experience. We identified 19 domains, consistent with those of other investigators.42 Gartner and colleagues advocate for a composite outcome measure that combines the core domains into one preference-based utility measure that is weighted.44 The rationale for this recommendation is that the levels of the domains might contribute differently to the overall birth experience. For example, communication might contribute more to an overall measure than pain management.44 The development of a childbirth-specific survey to evaluate patient-reported outcomes and patient-reported experiences with care is needed if we are to provide value-based care in this arena.45
Looking forward
PROs, PROMs, and PREMs are here to stay. They no longer are limited to clinical research, but increasingly will be incorporated into clinical care, providing us with opportunities to improve the quality of health care delivery, efficiency of patient/clinician interactions, and patients’ ratings of their health care experience.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001:6.
- Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- Rickert J. Patient-centered care: what it means and how to get there. Health Affairs website. http://healthaffairs.org/blog/2012/01/24/patient-centered-care-what-it-means-and-how-to-get-there/. Published January 24, 2012. Accessed October 15, 2017.
- US Food and Drug Administration. Guidance for industry: Patient reported outcome measures: use in medical product development to support labeling claims. https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf. Published December 2009. Accessed February 6, 2018.
- Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008. http://handbook.cochrane.org. Accessed October 15, 2017.
- Patrick DL, Guyatt PD, Acquadro C. Patient-reported outcomes. In: Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008:chap 17. http://handbook-5-1.cochrane.org/. Accessed October 15, 2017.
- Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights. 2013;6:61–68.
- McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):163–169.
- European Medicines Agency, Committee for Medicinal Products for Human Use. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. https://www.ispor.org/workpaper/emea-hrql-guidance.pdf. Published July 27, 2005. Accessed February 7, 2018.
- Venkatesan P. New European guidance on patient-reported outcomes. Lancet Oncol. 2016;17(6):e226.
- Cella D, Yount S, Rothrock N, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3–S11.
- Cella D, Riley W, Stone A, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194.
- Craig BM, Reeve BB, Brown PM, et al. US valuation of health outcomes measured using the PROMIS-29. Value Health. 2014;17(8):846–853.
- National Institutes of Health. Patient-Reported Outcomes Measurement Information System (PROMIS). https://commonfund.nih.gov/promis/index. Reviewed May 8, 2017. Accessed October 15, 2017.
- International Consortium for Health Outcomes Measurement (ICHOM). http://www.ichom.org/. Accessed October 15, 2017.
- University of Oxford, Patient Reported Outcomes Measurement Group http://phi.uhce.ox.ac.uk/. Accessed October 15, 2017.
- CONSORT. Patient-Reported Outcomes (CONSORT PRO). http://www.consort-statement.org/extensions/overview/consort-pro. Accessed October 15, 2017.
- International Society for Pharmacoeconomics and Outcomes Research. https://www.ispor.org/. Accessed October 15, 2017.
- RAND Health. RAND medical outcomes study: measures of quality of life core survey from RAND Health. https://www.rand.org/health/surveys_tools/mos.html. Accessed October 15, 2017.
- Basch EM, Deal AM, Dueck A, et al. Overall survival results of a randomized trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment [abstract LBA2]. J Clin Oncol. 2017;35(18)(suppl).
- Centers for Medicare and Medicaid Services. Better care. Smarter spending. Healthier people: paying providers for value, not volume. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-01-26-3.html. Accessed October 15, 2017.
- Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565.
- Chenok K, Teleki S, SooHoo NF, Huddleston J, Bozic KJ. Collecting patient-reported outcomes: lessons from the California Joint Replacement Registry. EGEMS (Wash DC). 2015;3(1):1196.
- Frosch DL. Patient-reported outcomes as a measure of healthcare quality. J Gen Intern Med. 2015;30(10):1383–1384.
- Gibbons E, Mackintosh A, Fitzpatrick R; Patient-Reported Outcome Measurement Group, Oxford. A structured review of patient-reported outcome measures for people undergoing elective procedures for benign gynaecological conditions of the uterus, 2010. http://phi.uhce.ox.ac.uk/pdf/ElectiveProcedures/PROMs_Oxford_Gynaecological%20procedures_012011.pdf. Accessed October 23, 2017.
- Matteson KA, Boardman LA, Munro MG, Clark MA. Abnormal uterine bleeding: a review of patient-based outcome measures. Fertil Steril. 2009;92(1):205–216.
- Matteson KA, Scott DM, Raker CA, Clark MA. The menstrual bleeding questionnaire: development and validation of a comprehensive patient-reported outcome instrument for heavy menstrual bleeding. BJOG. 2015;122(5):681–689.
- Coyne KS, Margolis MK, Bradley LD, Guido R, Maxwell GL, Spies JB. Further validation of the uterine fibroid symptom and quality-of-life questionnaire. Value Health. 2012;15(1):135–142.
- Martin ML, Halling K, Eek D, Krohe M, Paty J. Understanding polycystic ovary syndrome from the patient perspective: a concept elicitation patient interview study. Health Quality Life Outcomes. 2017;15(1):162.
- Malik-Aslam A, Reaney MD, Speight J. The suitability of polycystic ovary syndrome-specific questionnaires for measuring the impact of PCOS on quality of life in clinical trials. Value Health. 2010;13(4):440–446.
- Kitchen H, Aldhouse N, Trigg A, Palencia R, Mitchell S. A review of patient-reported outcome measures to assess female infertility-related quality of life. Health Qual Life Outcomes. 2017;15(1):86.
- Sung VW, Joo K, Marques F, Myers DL. Patient-reported outcomes after combined surgery for pelvic floor disorders in older compared to younger women. Am J Obstet Gynecol. 2009;201(5):534.e1–e5.
- Sung VW, Rogers RG, Barber MD, Clark MA. Conceptual framework for patient-important treatment outcomes for pelvic organ prolapse. Neurourol Urodynam. 2014;33(4):414–419.
- Sung VW, Wohlrab KJ, Madsen A, Raker C. Patient-reported goal attainment and comprehensive functioning outcomes after surgery compared with pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2016;215(5):659.e1–e7.
- Croke J. Cervical ca PROs in clinical practice. https://clinicaltrials.gov/ct2/show/NCT03048435. Accessed October 16, 2017.
- Catt S, Starkings R, Shilling V, Fallowfield L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: a systematic review. J Cancer Surviv. 2017;11(2):211–232.
- Jensen RE, Potosky AL, Moinpour CM, et al. United States population estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. J Clin Oncol. 2017;35(17):1913–1920.
- Friedlander M, Mercieca-Bebber RL, King MT. Patient-reported outcomes (PRO) in ovarian cancer clinical trials—lost opportunities and lessons learned. Ann Oncol. 2016;27(suppl 1):i66–i71.
- Joly F, Hilpert F, Okamoto A, Stuart G, Ochaia K, Friedlander M; 5th Ovarian Cancer Consensus Conference. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recommendations on incorporating patient-reported outcomes in clinical trials in epithelial ovarian cancer. Eur J Cancer. 2017;78:133–138.
- Martin A. Patient-reported outcomes in studies published in 2014: which disease areas have been the main focus of clinical research? Value Health. 2015;18(7):A742.
- International Consortium for Health Outcomes Management (ICHOM). Pregnancy and childbirth. http://www.ichom.org/medical-conditions/pregnancy-and-childbirth/. Accessed October 10, 2017.
- El Haj Ibrahim S, McCulloch J, Korst LM, Fridman M, Fink A, Gregory KD. Communication with staff during hospitalization for childbirth: the patient’s perspective [1R]. Obstet Gynecol. 2016;127.
- National Partnership for Women and Families. Childbirth Connection. Listening to mothers III: report of the third national US survey of women’s childbearing experience. http://transform.childbirthconnection.org/reports/listeningtomothers/. Accessed October 23, 2017.
- Gartner FR, Freeman LM, Rijnders ME, et al. A comprehensive representation of the birth-experience: identification and prioritization of birth-specific domains based on a mixed-method design. BMC Pregnancy Childbirth. 2014;14:147.
- National Partnership for Women and Families. The priority of developing and implementing CAHPS maternity care facility, clinician and health plan surveys. 2015. http://www.nationalpartnership.org/research-library/maternal-health/cahps-maternity-care-fact-sheet.pdf. Accessed October 23, 2017.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001:6.
- Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- Rickert J. Patient-centered care: what it means and how to get there. Health Affairs website. http://healthaffairs.org/blog/2012/01/24/patient-centered-care-what-it-means-and-how-to-get-there/. Published January 24, 2012. Accessed October 15, 2017.
- US Food and Drug Administration. Guidance for industry: Patient reported outcome measures: use in medical product development to support labeling claims. https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf. Published December 2009. Accessed February 6, 2018.
- Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008. http://handbook.cochrane.org. Accessed October 15, 2017.
- Patrick DL, Guyatt PD, Acquadro C. Patient-reported outcomes. In: Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008:chap 17. http://handbook-5-1.cochrane.org/. Accessed October 15, 2017.
- Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights. 2013;6:61–68.
- McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):163–169.
- European Medicines Agency, Committee for Medicinal Products for Human Use. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. https://www.ispor.org/workpaper/emea-hrql-guidance.pdf. Published July 27, 2005. Accessed February 7, 2018.
- Venkatesan P. New European guidance on patient-reported outcomes. Lancet Oncol. 2016;17(6):e226.
- Cella D, Yount S, Rothrock N, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3–S11.
- Cella D, Riley W, Stone A, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194.
- Craig BM, Reeve BB, Brown PM, et al. US valuation of health outcomes measured using the PROMIS-29. Value Health. 2014;17(8):846–853.
- National Institutes of Health. Patient-Reported Outcomes Measurement Information System (PROMIS). https://commonfund.nih.gov/promis/index. Reviewed May 8, 2017. Accessed October 15, 2017.
- International Consortium for Health Outcomes Measurement (ICHOM). http://www.ichom.org/. Accessed October 15, 2017.
- University of Oxford, Patient Reported Outcomes Measurement Group http://phi.uhce.ox.ac.uk/. Accessed October 15, 2017.
- CONSORT. Patient-Reported Outcomes (CONSORT PRO). http://www.consort-statement.org/extensions/overview/consort-pro. Accessed October 15, 2017.
- International Society for Pharmacoeconomics and Outcomes Research. https://www.ispor.org/. Accessed October 15, 2017.
- RAND Health. RAND medical outcomes study: measures of quality of life core survey from RAND Health. https://www.rand.org/health/surveys_tools/mos.html. Accessed October 15, 2017.
- Basch EM, Deal AM, Dueck A, et al. Overall survival results of a randomized trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment [abstract LBA2]. J Clin Oncol. 2017;35(18)(suppl).
- Centers for Medicare and Medicaid Services. Better care. Smarter spending. Healthier people: paying providers for value, not volume. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-01-26-3.html. Accessed October 15, 2017.
- Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565.
- Chenok K, Teleki S, SooHoo NF, Huddleston J, Bozic KJ. Collecting patient-reported outcomes: lessons from the California Joint Replacement Registry. EGEMS (Wash DC). 2015;3(1):1196.
- Frosch DL. Patient-reported outcomes as a measure of healthcare quality. J Gen Intern Med. 2015;30(10):1383–1384.
- Gibbons E, Mackintosh A, Fitzpatrick R; Patient-Reported Outcome Measurement Group, Oxford. A structured review of patient-reported outcome measures for people undergoing elective procedures for benign gynaecological conditions of the uterus, 2010. http://phi.uhce.ox.ac.uk/pdf/ElectiveProcedures/PROMs_Oxford_Gynaecological%20procedures_012011.pdf. Accessed October 23, 2017.
- Matteson KA, Boardman LA, Munro MG, Clark MA. Abnormal uterine bleeding: a review of patient-based outcome measures. Fertil Steril. 2009;92(1):205–216.
- Matteson KA, Scott DM, Raker CA, Clark MA. The menstrual bleeding questionnaire: development and validation of a comprehensive patient-reported outcome instrument for heavy menstrual bleeding. BJOG. 2015;122(5):681–689.
- Coyne KS, Margolis MK, Bradley LD, Guido R, Maxwell GL, Spies JB. Further validation of the uterine fibroid symptom and quality-of-life questionnaire. Value Health. 2012;15(1):135–142.
- Martin ML, Halling K, Eek D, Krohe M, Paty J. Understanding polycystic ovary syndrome from the patient perspective: a concept elicitation patient interview study. Health Quality Life Outcomes. 2017;15(1):162.
- Malik-Aslam A, Reaney MD, Speight J. The suitability of polycystic ovary syndrome-specific questionnaires for measuring the impact of PCOS on quality of life in clinical trials. Value Health. 2010;13(4):440–446.
- Kitchen H, Aldhouse N, Trigg A, Palencia R, Mitchell S. A review of patient-reported outcome measures to assess female infertility-related quality of life. Health Qual Life Outcomes. 2017;15(1):86.
- Sung VW, Joo K, Marques F, Myers DL. Patient-reported outcomes after combined surgery for pelvic floor disorders in older compared to younger women. Am J Obstet Gynecol. 2009;201(5):534.e1–e5.
- Sung VW, Rogers RG, Barber MD, Clark MA. Conceptual framework for patient-important treatment outcomes for pelvic organ prolapse. Neurourol Urodynam. 2014;33(4):414–419.
- Sung VW, Wohlrab KJ, Madsen A, Raker C. Patient-reported goal attainment and comprehensive functioning outcomes after surgery compared with pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2016;215(5):659.e1–e7.
- Croke J. Cervical ca PROs in clinical practice. https://clinicaltrials.gov/ct2/show/NCT03048435. Accessed October 16, 2017.
- Catt S, Starkings R, Shilling V, Fallowfield L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: a systematic review. J Cancer Surviv. 2017;11(2):211–232.
- Jensen RE, Potosky AL, Moinpour CM, et al. United States population estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. J Clin Oncol. 2017;35(17):1913–1920.
- Friedlander M, Mercieca-Bebber RL, King MT. Patient-reported outcomes (PRO) in ovarian cancer clinical trials—lost opportunities and lessons learned. Ann Oncol. 2016;27(suppl 1):i66–i71.
- Joly F, Hilpert F, Okamoto A, Stuart G, Ochaia K, Friedlander M; 5th Ovarian Cancer Consensus Conference. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recommendations on incorporating patient-reported outcomes in clinical trials in epithelial ovarian cancer. Eur J Cancer. 2017;78:133–138.
- Martin A. Patient-reported outcomes in studies published in 2014: which disease areas have been the main focus of clinical research? Value Health. 2015;18(7):A742.
- International Consortium for Health Outcomes Management (ICHOM). Pregnancy and childbirth. http://www.ichom.org/medical-conditions/pregnancy-and-childbirth/. Accessed October 10, 2017.
- El Haj Ibrahim S, McCulloch J, Korst LM, Fridman M, Fink A, Gregory KD. Communication with staff during hospitalization for childbirth: the patient’s perspective [1R]. Obstet Gynecol. 2016;127.
- National Partnership for Women and Families. Childbirth Connection. Listening to mothers III: report of the third national US survey of women’s childbearing experience. http://transform.childbirthconnection.org/reports/listeningtomothers/. Accessed October 23, 2017.
- Gartner FR, Freeman LM, Rijnders ME, et al. A comprehensive representation of the birth-experience: identification and prioritization of birth-specific domains based on a mixed-method design. BMC Pregnancy Childbirth. 2014;14:147.
- National Partnership for Women and Families. The priority of developing and implementing CAHPS maternity care facility, clinician and health plan surveys. 2015. http://www.nationalpartnership.org/research-library/maternal-health/cahps-maternity-care-fact-sheet.pdf. Accessed October 23, 2017.
Read all parts of this series
PART 1 Value-based payment: What does it mean and how can ObGyns get out ahead
PART 2 What makes a “quality” quality measure?
PART 3 The role of patient-reported outcomes in women’s health
PART 4 It costs what?! How we can educate residents and students on how much things cost
