User login
Contribution of Predischarge ID Consult
With dramatically increasing costs of healthcare, it has become increasingly necessary for healthcare providers to demonstrate value in the delivery of care. Porter and Teisberg have strongly advocated that healthcare reform efforts should focus on improving value rather than limiting cost, with value being defined as quality per unit cost.1 However, it has been pointed out that value means different things to different people.2 The biggest challenge in defining value stems mainly from the difficulty in defining quality, because it, too, means vastly different things to different people. Modern medicine is increasingly characterized by multidisciplinary care. With limited or shrinking resources, it will become necessary for individual specialists to describe and articulate, in quantitative terms, their specific contributions to the overall outcome of individual patients.
Previous publications have provided broad descriptions of the value provided by infectious disease (ID) specialists in the domains of sepsis, infection control, outpatient antibiotic therapy, antimicrobial stewardship, and directive care and teaching.3, 4 Studies have also shown the value of ID physicians in specific disease conditions. ID consultation is associated with lower mortality5, 6 and lower relapse rates7 in hospitalized patients with Staphylococcus aureus bacteremia. In another study evaluating the impact of ID consultants, patients seen by ID consultants had longer lengths of hospital stay, longer intensive care unit lengths of stay, and higher antibiotic costs than matched controls not seen by ID consultants.8 It can be argued that a major limitation of the study was that controls were not matched for the ID diagnosis, nor for the causative microorganisms, but it is clear that ID physicians are challenged to demonstrate their contribution to the care of patients.
A unique activity of ID physicians is the management of community‐based parenteral anti‐infective therapy (CoPAT). At Baystate Medical Center, a policy of mandatory ID consultation was instituted for patients leaving hospital on parenteral antibiotics. A study was conducted on the impact of predischarge ID consultation for 44 patients who were not already being followed by the ID service. The study documented change from intravenous (IV) to oral formulation, change of antibiotic choice, and change of dose/duration of treatment in a substantial proportion of patients.9 These are significant changes, but ID consultation contributes more than the themes explored in the study.
The purpose of this study was to evaluate the contribution of ID consultation when consulted for CoPAT, an activity specific to ID practice, in a different institution, and using an expanded definition of medical contribution.
METHODS
The Cleveland Clinic's Department of Infectious Disease has 24 staff physicians and 11 inpatient ID consultative services. These include: 2 solid organ transplant services; a bone marrow transplant and oncologic service; 2 infective endocarditis/cardiac device infection services; an intensive care unit (ICU) service; a bone and joint infection service; a neuroinfection service; and 3 general ID consult services. Consultative services are provided 7 days a week. At the Cleveland Clinic, ID consultation is required prior to discharge on parenteral antibiotic therapy.10, 11 ID consultation for CoPAT usually occurs when the primary service deems the patient is close to being discharged from hospital. This circumstance allows for assessing the specific contribution of ID physicians beyond that of the primary service and other consulting services.
Case Ascertainment
The study was approved by the institutional review board. In February 2010, an electronic form for requesting ID consultations had been introduced into the computerized provider order entry (CPOE) system at the Cleveland Clinic. One of the required questions on the form was whether the consultation was regarding CoPAT, with options of Yes, No, or Not sure. These electronic ID consultation requests were screened to identify consultation requests for this study.
Inclusion and Exclusion Criteria
All adult ID consultations between February 11, 2010 and May 15, 2010 for which the CoPAT consult? field was marked Yes were included in the study. All other consultations, including not sure for CoPAT, were excluded.
Definitions
The first ID consultation during a hospitalization was considered an initial consultation. ID consultations for patients whom an ID service had previously seen during the same hospitalization were deemed reconsultations. Value provided was defined as contribution of the ID consultation team in the following domains: 1) optimization of antimicrobial therapy, 2) significant change in patient assessment, 3) additional medical care contribution. Specific contributions included in each domain are outlined in Table 1.
|
| Domain 1: Optimization of antibiotic therapy |
| Alteration of an antibiotic (change of antibiotic or route of administration) |
| Defining duration of therapy |
| Identification of psychosocial factors (eg, injection drug use) that influence treatment |
| Domain 2: Significant change in patient assessment |
| Diagnosis of an infectious process |
| Better appreciation of extent of disease |
| Refutation of a false infectious disease diagnosis |
| Recognition of a noninfectious process needing urgent attention |
| Identification of a positive culture as contaminant/colonization |
| Recognition of a need for additional testing (testing needed to arrive at a diagnosis or clarify a treatment plan before a patient could be safely discharged from hospital) |
| Recognition of need for surgery/emnvasive intervention |
| Refutation of antibiotic allergy by history or allergy testing |
| Domain 3: Additional medical care contribution |
| Administration of vaccines |
| Identification of an unrecognized medical problem that needed to be addressed after discharge from hospital |
| Provision of effective transition of care (ensuring that the same ID physician who saw the patient in hospital followed the patient after discharge from hospital) |
Data Collected
For each ID consultation episode, clinicians' notes were reviewed from the day of the ID consultation to the day the patient was discharged from hospital or the day the ID service signed off, whichever happened sooner. Results of recommended tests were followed up to determine if results led to a change in patient assessment. Data elements collected for each consultation episode included patient age, gender, race, date of hospitalization, date of discharge, date of ID consultation or reconsultation, primary service, and documentation of ID service contributions. Data were collected and entered in a Microsoft Access relational database. To minimize bias, the data collection was performed by physicians who had not participated in the care of the patient.
Analysis
The proportion of ID consultations in which the ID team contributed in the defined domains were enumerated, and described for the group overall and also separately for initial consultations and reconsultations.
RESULTS
In the time period studied, there were 1326 CPOE requests for ID consultation. The response to the question, CoPAT consult? was Yes for 304, No for 507, and Not sure for 515 requests. Of the 304 consultation requests marked Yes, 41 were excluded. Reasons for exclusion were: no ID consultation note (21), wrong service consulted (8), consultation request placed while the ID service was already following the patient (7), and duplicate consultation request (5). The remaining 263 consultation requests corresponded to 1 or more CoPAT consultation requests for 249 patients (across different hospitalizations). Of the 263 consultation requests, 172 were initial consultations, while the remaining 91 were reconsultations (patients not actively being followed by the ID service, but previously seen during the same hospitalization).
Consultation characteristics are outlined in Table 2. The most common group of infections for which CoPAT was sought was bone and joint infections, accounting for over 20% of the consultation requests. CoPAT consultations were requested a median of 4 days after hospitalization. Patients were discharged from hospital a median of 3 days after they were seen by the ID service. ID consultation did not delay discharge. The ID service usually saw the patient the same day, and followed the patient in hospital for a median of 1 day. There was no difference in hospital days after consult for patients who did not need antibiotics versus those who did.
| Characteristic | Initial Consultation [172] n (%)* | Reconsultation [91] n (%)* | Overall [263] n (%)* |
|---|---|---|---|
| |||
| Patient age in years, mean (SD) | 58 (14) | 62 (13) | 59 (14) |
| Male gender | 98 (60) | 91 (56) | 149 (57) |
| Caucasian race | 126 (73) | 74 (81) | 200 (76) |
| Services requesting consults (5 most common overall) | |||
| Medicine | 41 (17) | 14 (15) | 55 (21) |
| Orthopedics | 34 (14) | 0 (0) | 34 (13) |
| Hematology/Oncology | 16 (7) | 10 (11) | 26 (10) |
| Cardiology | 9 (4) | 15 (16) | 24 (9) |
| Gastroenterology | 14 (6) | 5 (5) | 19 (7) |
| Consult diagnosis (5 most common overall) | |||
| Bone and joint infection | 45 (26) | 9 (10) | 54 (21) |
| Skin or soft tissue infection or rash | 21 (12) | 8 (9) | 29 (11) |
| Endocarditis or cardiac device infection | 7 (4) | 15 (16) | 22 (8) |
| IV catheter or other endovascular infection | 9 (5) | 8 (9) | 17 (6) |
| Urinary tract infection | 12 (7) | 5 (5) | 17 (6) |
| Days from admission to ID consult, median (IQR) | 4 (1‐11) | 7 (2‐19) | 4 (1‐14) |
| Days to respond to consult request, median (IQR) | 0 (0‐1) | 0 (0‐0) | 0 (0‐0) |
| Days from ID consult to discharge, median (IQR) | 3 (2‐7) | 2 (1‐4.5) | 3 (1‐6) |
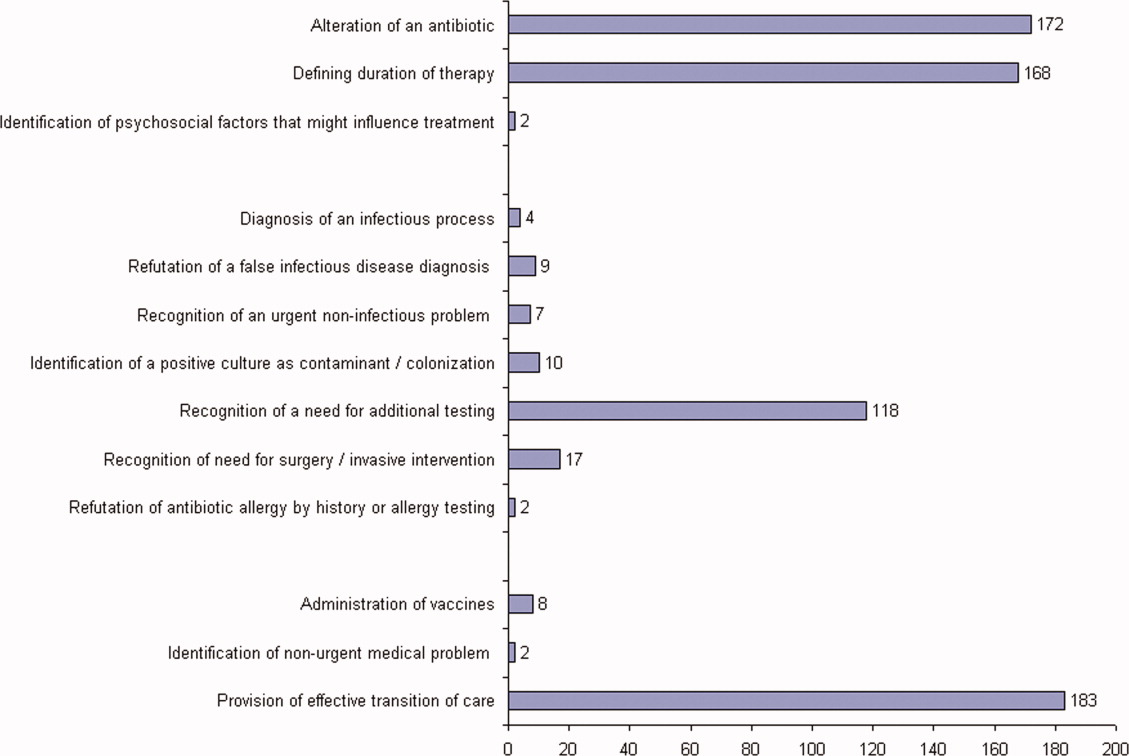
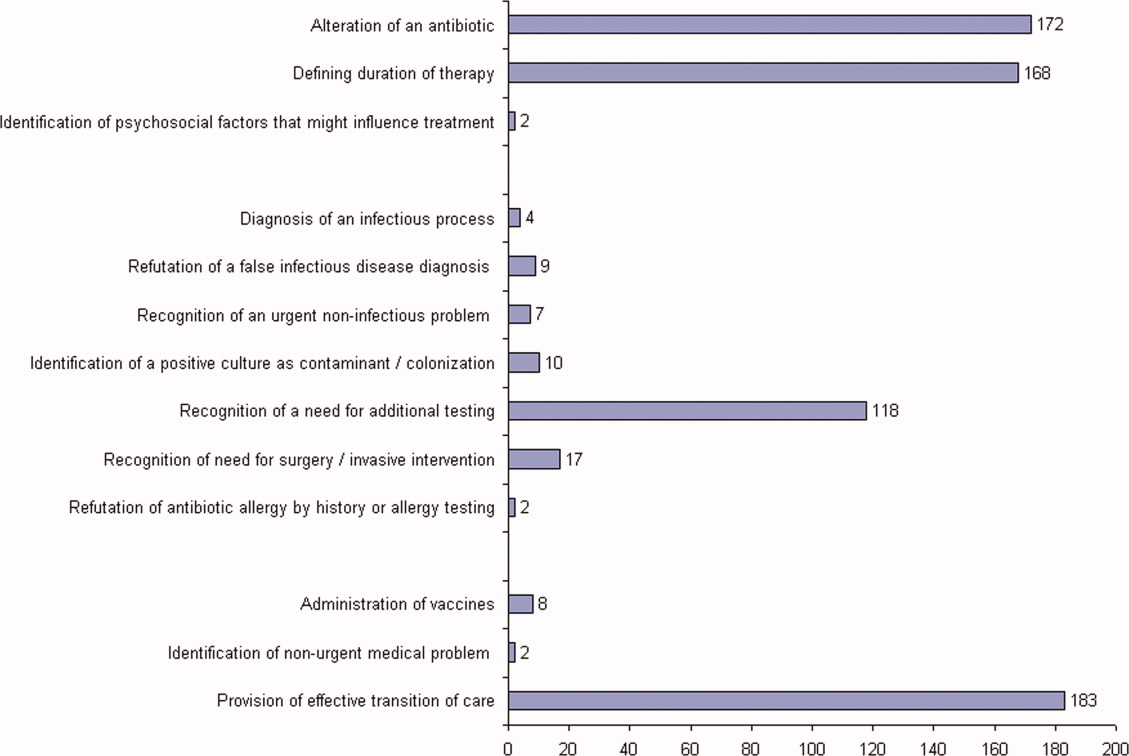
ID consultation provided value in at least 1 domain in 260 of the 263 consultations. This included optimization of antimicrobial treatment in 84%, significant alteration of patient assessment in 52%, and additional medical care contribution in 71% of consultations. Substantial contributions were made in all domains in both initial consultations and in reconsultations. Specific ID contributions within each of the domains are shown in Figure 1. There was wide overlap of contributions across the 3 domains for individual consultations (Figure 2), with contributions in all domains occurring in 34% of consultations. CoPAT was deemed not to be necessary in 27% of consultations. Among patients who did not require CoPAT, 60% received oral antibiotics and 40% were deemed not to need any antibiotics at hospital discharge. Among the patients discharged on CoPAT, a follow‐up appointment with a Cleveland Clinic ID physician familiar with the patient was set up 86% of the time; the rest either followed up with another physician or it was deemed that a scheduled follow‐up ID visit was not necessary.0

DISCUSSION
Physicians practicing in the specialty of infectious diseases face challenges and opportunities, as they adapt to changing demands within hospital practice in regard to reimbursement in an Accountable Care environment. Other challenges include emerging infections, antimicrobial resistance, need for antimicrobial stewardship, and increasing numbers of immunocompromised patients.12 From a health systems perspective, the overall value of care provided by the entire organization, and overall outcomes, are ultimately what matter. However, healthcare administrators need an appreciation of contributions of individual providers and specialties to fairly allocate resources and compensation for care provided. Articulating unique contributions is particularly challenging for individuals or services that provide purely cognitive input. Shrinking healthcare resources makes it critically important for cognitive specialists to be able to define their unique role in the care of patients with complex problems.
Our study found that a major contribution of ID consultation for CoPAT is that the process identifies a large number of patients who do not need CoPAT, thus effecting a powerful antimicrobial stewardship function. In our study, CoPAT was deemed unnecessary 27% of the time. The Infectious Diseases Society of America practice guidelines on outpatient parenteral antimicrobial therapy emphasize the importance of careful evaluation of patients considered for parenteral antibiotics outside the hospital setting.13 The focus on careful selection of appropriate patients for CoPAT has been a cornerstone of the Cleveland Clinic model of care. Nearly 30 years ago, we found that outpatient parenteral antibiotic therapy was unnecessary or not feasible in 40% of the patients referred for evaluation.10 If we adjust the numbers with the assumption that reimbursement issues present at that time are now less of an issue, the proportion of patients who were referred for CoPAT but not discharged on it was 29%, a figure remarkably similar to that found in the current study.
Another major contribution of ID consultation is the provision of effective transition of care from the inpatient to the outpatient setting. Frequent occurrence of postdischarge adverse events has been recognized as a problem in clinical practice.14 Primary care physicians are rarely involved in discussions about hospital discharge.15 A consensus conference including the American College of Physicians, Society of Hospital Medicine, and Society of General Internal Medicine, convened in July 2007 to address quality gaps in transitions of care between inpatient and outpatient settings. It identified 5 principles for effective care transitions: accountability, communication, timeliness, patient and family involvement, and respect for the hub of coordination of care.16 Recognizing gaps in care transition, hospitalists in a hospital‐based infusion program developed a model of care that successfully bridged the hospital‐to‐home care transition for patients who could return to hospital for daily antimicrobial infusions.17 In our system, ID physicians take ownership for directing parenteral antibiotic therapy for the episode of illness, specifying the physician, date, and time of follow‐up before the patient is discharged from hospital, thereby essentially satisfying the principles of effective care transitions identified. The purpose of the ID follow‐up is not to replace other follow‐up care for patients but to ensure safe transition of care while treating an episode of infection.
Attribution of identified contributions to the ID consultation could be done because our study was limited to CoPAT consultations. Such consultations typically occur when patients are deemed close to hospital discharge by the primary service. There should be little controversy about attribution of cognitive input in such consultations, because from the primary service's perspective, the patient is ready or almost ready to be discharged from hospital. It would be fair to state that most of the identified contributions in the study would not have occurred had it not been for the ID consultation.
We acknowledge that the study suffers from many limitations. The biggest limitation is that the contribution elements are defined by ID physicians and sought in the medical record by physicians from the same specialty. This arrangement certainly has potential for significant bias. To limit this bias, data collection was performed by physicians who had not participated in the care of the patient. In addition, we only could assess what was documented in the electronic health record. Our study found that alteration of antibiotic therapy was a substantial contribution, however, documentation of recommendation to change antibiotics in the medical record rarely specified exactly why the change was recommended. Reasons for antibiotic change recommendations included bug‐drug mismatch, minimum inhibitory concentration (MIC) considerations, pharmacokinetic considerations, adverse effects, convenience of dosing, drug interactions, and insurance coverage. However, it is not possible to quantify the specific contribution of each of these reasons, in a retrospective study, without making assumptions about why specific ID physicians made specific antibiotic change recommendations. There may have been more contributions that might not have been apparent on a retrospective chart review. The lack of a control group also lessens the impact of our findings. We could not have a control group, because no patient is discharged from the Cleveland Clinic on CoPAT without having been seen by an ID physician. Mandatory ID consultation for CoPAT has previously been shown to reduce costs,9 however, our study was not designed to evaluate cost.
The perceived value of ID consultation in our institution can be appreciated when one considers the longstanding institutional policy of requiring ID consultation for CoPAT.10, 11 The perpetuation of this tradition in the hospital is testament to the presumption that mandatory ID consultation is seen to be of value by the institution.
In summary, ID consultation in our institution contributes to the care of inpatients being considered for CoPAT by substantially reducing unnecessary parenteral antibiotic use, optimizing antibiotic therapy, recognizing need for additional testing before discharge from hospital, and by providing effective transition of care from the inpatient to the outpatient setting.
- ,.How physicians can change the future of health care.JAMA.2007;297:1103–1111.
- .Value of the infectious diseases specialist.Clin Infect Dis.1997;24:456.
- ,,, et al.The value of an infectious diseases specialist.Clin Infect Dis.2003;36:1013–1017.
- ,,,,,.The value of infectious diseases specialists: non‐patient care activities.Clin Infect Dis.2008;47:1051–1063.
- ,,,,.The value of infectious diseases consultation in Staphylococcus aureus bacteremia.Am J Med.2010;123:631–637.
- ,,,,.Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia.Medicine (Baltimore).2009;88:263–267.
- ,,, et al.Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients.Clin Infect Dis.1998;27:478–486.
- ,,.Infectious diseases consultation: impact on outcomes for hospitalized patients and results of a preliminary study.Clin Infect Dis.1997;24:468–470.
- ,,.Impact of mandatory inpatient infectious disease consultation on outpatient parenteral antibiotic therapy.Am J Med Sci.2005;330:60–64.
- ,.Home intravenous antibiotic therapy: a team approach.Ann Intern Med.1983;99:388–392.
- ,,.Transitioning antimicrobial stewardship beyond the hospital: the Cleveland Clinic's community‐based parenteral anti‐infective therapy (CoPAT) program.J Hosp Med.2011;6(suppl 1):S24–S30.
- ,,.Professional challenges and opportunities in clinical microbiology and infectious diseases in Europe.Lancet Infect Dis.2011;11:408–415.
- ,,, et al.Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines.Clin Infect Dis.2004;38:1651–1672.
- ,.Addressing postdischarge adverse events: a neglected area.Jt Comm J Qual Patient Saf.2008;34:85–97.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,, et al.Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine.J Hosp Med.2009;4:364–370.
- .Hospitalist to home: outpatient parenteral antimicrobial therapy at an academic center.Clin Infect Dis.2010;51(suppl 2):S220–S223.
With dramatically increasing costs of healthcare, it has become increasingly necessary for healthcare providers to demonstrate value in the delivery of care. Porter and Teisberg have strongly advocated that healthcare reform efforts should focus on improving value rather than limiting cost, with value being defined as quality per unit cost.1 However, it has been pointed out that value means different things to different people.2 The biggest challenge in defining value stems mainly from the difficulty in defining quality, because it, too, means vastly different things to different people. Modern medicine is increasingly characterized by multidisciplinary care. With limited or shrinking resources, it will become necessary for individual specialists to describe and articulate, in quantitative terms, their specific contributions to the overall outcome of individual patients.
Previous publications have provided broad descriptions of the value provided by infectious disease (ID) specialists in the domains of sepsis, infection control, outpatient antibiotic therapy, antimicrobial stewardship, and directive care and teaching.3, 4 Studies have also shown the value of ID physicians in specific disease conditions. ID consultation is associated with lower mortality5, 6 and lower relapse rates7 in hospitalized patients with Staphylococcus aureus bacteremia. In another study evaluating the impact of ID consultants, patients seen by ID consultants had longer lengths of hospital stay, longer intensive care unit lengths of stay, and higher antibiotic costs than matched controls not seen by ID consultants.8 It can be argued that a major limitation of the study was that controls were not matched for the ID diagnosis, nor for the causative microorganisms, but it is clear that ID physicians are challenged to demonstrate their contribution to the care of patients.
A unique activity of ID physicians is the management of community‐based parenteral anti‐infective therapy (CoPAT). At Baystate Medical Center, a policy of mandatory ID consultation was instituted for patients leaving hospital on parenteral antibiotics. A study was conducted on the impact of predischarge ID consultation for 44 patients who were not already being followed by the ID service. The study documented change from intravenous (IV) to oral formulation, change of antibiotic choice, and change of dose/duration of treatment in a substantial proportion of patients.9 These are significant changes, but ID consultation contributes more than the themes explored in the study.
The purpose of this study was to evaluate the contribution of ID consultation when consulted for CoPAT, an activity specific to ID practice, in a different institution, and using an expanded definition of medical contribution.
METHODS
The Cleveland Clinic's Department of Infectious Disease has 24 staff physicians and 11 inpatient ID consultative services. These include: 2 solid organ transplant services; a bone marrow transplant and oncologic service; 2 infective endocarditis/cardiac device infection services; an intensive care unit (ICU) service; a bone and joint infection service; a neuroinfection service; and 3 general ID consult services. Consultative services are provided 7 days a week. At the Cleveland Clinic, ID consultation is required prior to discharge on parenteral antibiotic therapy.10, 11 ID consultation for CoPAT usually occurs when the primary service deems the patient is close to being discharged from hospital. This circumstance allows for assessing the specific contribution of ID physicians beyond that of the primary service and other consulting services.
Case Ascertainment
The study was approved by the institutional review board. In February 2010, an electronic form for requesting ID consultations had been introduced into the computerized provider order entry (CPOE) system at the Cleveland Clinic. One of the required questions on the form was whether the consultation was regarding CoPAT, with options of Yes, No, or Not sure. These electronic ID consultation requests were screened to identify consultation requests for this study.
Inclusion and Exclusion Criteria
All adult ID consultations between February 11, 2010 and May 15, 2010 for which the CoPAT consult? field was marked Yes were included in the study. All other consultations, including not sure for CoPAT, were excluded.
Definitions
The first ID consultation during a hospitalization was considered an initial consultation. ID consultations for patients whom an ID service had previously seen during the same hospitalization were deemed reconsultations. Value provided was defined as contribution of the ID consultation team in the following domains: 1) optimization of antimicrobial therapy, 2) significant change in patient assessment, 3) additional medical care contribution. Specific contributions included in each domain are outlined in Table 1.
|
| Domain 1: Optimization of antibiotic therapy |
| Alteration of an antibiotic (change of antibiotic or route of administration) |
| Defining duration of therapy |
| Identification of psychosocial factors (eg, injection drug use) that influence treatment |
| Domain 2: Significant change in patient assessment |
| Diagnosis of an infectious process |
| Better appreciation of extent of disease |
| Refutation of a false infectious disease diagnosis |
| Recognition of a noninfectious process needing urgent attention |
| Identification of a positive culture as contaminant/colonization |
| Recognition of a need for additional testing (testing needed to arrive at a diagnosis or clarify a treatment plan before a patient could be safely discharged from hospital) |
| Recognition of need for surgery/emnvasive intervention |
| Refutation of antibiotic allergy by history or allergy testing |
| Domain 3: Additional medical care contribution |
| Administration of vaccines |
| Identification of an unrecognized medical problem that needed to be addressed after discharge from hospital |
| Provision of effective transition of care (ensuring that the same ID physician who saw the patient in hospital followed the patient after discharge from hospital) |
Data Collected
For each ID consultation episode, clinicians' notes were reviewed from the day of the ID consultation to the day the patient was discharged from hospital or the day the ID service signed off, whichever happened sooner. Results of recommended tests were followed up to determine if results led to a change in patient assessment. Data elements collected for each consultation episode included patient age, gender, race, date of hospitalization, date of discharge, date of ID consultation or reconsultation, primary service, and documentation of ID service contributions. Data were collected and entered in a Microsoft Access relational database. To minimize bias, the data collection was performed by physicians who had not participated in the care of the patient.
Analysis
The proportion of ID consultations in which the ID team contributed in the defined domains were enumerated, and described for the group overall and also separately for initial consultations and reconsultations.
RESULTS
In the time period studied, there were 1326 CPOE requests for ID consultation. The response to the question, CoPAT consult? was Yes for 304, No for 507, and Not sure for 515 requests. Of the 304 consultation requests marked Yes, 41 were excluded. Reasons for exclusion were: no ID consultation note (21), wrong service consulted (8), consultation request placed while the ID service was already following the patient (7), and duplicate consultation request (5). The remaining 263 consultation requests corresponded to 1 or more CoPAT consultation requests for 249 patients (across different hospitalizations). Of the 263 consultation requests, 172 were initial consultations, while the remaining 91 were reconsultations (patients not actively being followed by the ID service, but previously seen during the same hospitalization).
Consultation characteristics are outlined in Table 2. The most common group of infections for which CoPAT was sought was bone and joint infections, accounting for over 20% of the consultation requests. CoPAT consultations were requested a median of 4 days after hospitalization. Patients were discharged from hospital a median of 3 days after they were seen by the ID service. ID consultation did not delay discharge. The ID service usually saw the patient the same day, and followed the patient in hospital for a median of 1 day. There was no difference in hospital days after consult for patients who did not need antibiotics versus those who did.
| Characteristic | Initial Consultation [172] n (%)* | Reconsultation [91] n (%)* | Overall [263] n (%)* |
|---|---|---|---|
| |||
| Patient age in years, mean (SD) | 58 (14) | 62 (13) | 59 (14) |
| Male gender | 98 (60) | 91 (56) | 149 (57) |
| Caucasian race | 126 (73) | 74 (81) | 200 (76) |
| Services requesting consults (5 most common overall) | |||
| Medicine | 41 (17) | 14 (15) | 55 (21) |
| Orthopedics | 34 (14) | 0 (0) | 34 (13) |
| Hematology/Oncology | 16 (7) | 10 (11) | 26 (10) |
| Cardiology | 9 (4) | 15 (16) | 24 (9) |
| Gastroenterology | 14 (6) | 5 (5) | 19 (7) |
| Consult diagnosis (5 most common overall) | |||
| Bone and joint infection | 45 (26) | 9 (10) | 54 (21) |
| Skin or soft tissue infection or rash | 21 (12) | 8 (9) | 29 (11) |
| Endocarditis or cardiac device infection | 7 (4) | 15 (16) | 22 (8) |
| IV catheter or other endovascular infection | 9 (5) | 8 (9) | 17 (6) |
| Urinary tract infection | 12 (7) | 5 (5) | 17 (6) |
| Days from admission to ID consult, median (IQR) | 4 (1‐11) | 7 (2‐19) | 4 (1‐14) |
| Days to respond to consult request, median (IQR) | 0 (0‐1) | 0 (0‐0) | 0 (0‐0) |
| Days from ID consult to discharge, median (IQR) | 3 (2‐7) | 2 (1‐4.5) | 3 (1‐6) |
ID consultation provided value in at least 1 domain in 260 of the 263 consultations. This included optimization of antimicrobial treatment in 84%, significant alteration of patient assessment in 52%, and additional medical care contribution in 71% of consultations. Substantial contributions were made in all domains in both initial consultations and in reconsultations. Specific ID contributions within each of the domains are shown in Figure 1. There was wide overlap of contributions across the 3 domains for individual consultations (Figure 2), with contributions in all domains occurring in 34% of consultations. CoPAT was deemed not to be necessary in 27% of consultations. Among patients who did not require CoPAT, 60% received oral antibiotics and 40% were deemed not to need any antibiotics at hospital discharge. Among the patients discharged on CoPAT, a follow‐up appointment with a Cleveland Clinic ID physician familiar with the patient was set up 86% of the time; the rest either followed up with another physician or it was deemed that a scheduled follow‐up ID visit was not necessary.0


DISCUSSION
Physicians practicing in the specialty of infectious diseases face challenges and opportunities, as they adapt to changing demands within hospital practice in regard to reimbursement in an Accountable Care environment. Other challenges include emerging infections, antimicrobial resistance, need for antimicrobial stewardship, and increasing numbers of immunocompromised patients.12 From a health systems perspective, the overall value of care provided by the entire organization, and overall outcomes, are ultimately what matter. However, healthcare administrators need an appreciation of contributions of individual providers and specialties to fairly allocate resources and compensation for care provided. Articulating unique contributions is particularly challenging for individuals or services that provide purely cognitive input. Shrinking healthcare resources makes it critically important for cognitive specialists to be able to define their unique role in the care of patients with complex problems.
Our study found that a major contribution of ID consultation for CoPAT is that the process identifies a large number of patients who do not need CoPAT, thus effecting a powerful antimicrobial stewardship function. In our study, CoPAT was deemed unnecessary 27% of the time. The Infectious Diseases Society of America practice guidelines on outpatient parenteral antimicrobial therapy emphasize the importance of careful evaluation of patients considered for parenteral antibiotics outside the hospital setting.13 The focus on careful selection of appropriate patients for CoPAT has been a cornerstone of the Cleveland Clinic model of care. Nearly 30 years ago, we found that outpatient parenteral antibiotic therapy was unnecessary or not feasible in 40% of the patients referred for evaluation.10 If we adjust the numbers with the assumption that reimbursement issues present at that time are now less of an issue, the proportion of patients who were referred for CoPAT but not discharged on it was 29%, a figure remarkably similar to that found in the current study.
Another major contribution of ID consultation is the provision of effective transition of care from the inpatient to the outpatient setting. Frequent occurrence of postdischarge adverse events has been recognized as a problem in clinical practice.14 Primary care physicians are rarely involved in discussions about hospital discharge.15 A consensus conference including the American College of Physicians, Society of Hospital Medicine, and Society of General Internal Medicine, convened in July 2007 to address quality gaps in transitions of care between inpatient and outpatient settings. It identified 5 principles for effective care transitions: accountability, communication, timeliness, patient and family involvement, and respect for the hub of coordination of care.16 Recognizing gaps in care transition, hospitalists in a hospital‐based infusion program developed a model of care that successfully bridged the hospital‐to‐home care transition for patients who could return to hospital for daily antimicrobial infusions.17 In our system, ID physicians take ownership for directing parenteral antibiotic therapy for the episode of illness, specifying the physician, date, and time of follow‐up before the patient is discharged from hospital, thereby essentially satisfying the principles of effective care transitions identified. The purpose of the ID follow‐up is not to replace other follow‐up care for patients but to ensure safe transition of care while treating an episode of infection.
Attribution of identified contributions to the ID consultation could be done because our study was limited to CoPAT consultations. Such consultations typically occur when patients are deemed close to hospital discharge by the primary service. There should be little controversy about attribution of cognitive input in such consultations, because from the primary service's perspective, the patient is ready or almost ready to be discharged from hospital. It would be fair to state that most of the identified contributions in the study would not have occurred had it not been for the ID consultation.
We acknowledge that the study suffers from many limitations. The biggest limitation is that the contribution elements are defined by ID physicians and sought in the medical record by physicians from the same specialty. This arrangement certainly has potential for significant bias. To limit this bias, data collection was performed by physicians who had not participated in the care of the patient. In addition, we only could assess what was documented in the electronic health record. Our study found that alteration of antibiotic therapy was a substantial contribution, however, documentation of recommendation to change antibiotics in the medical record rarely specified exactly why the change was recommended. Reasons for antibiotic change recommendations included bug‐drug mismatch, minimum inhibitory concentration (MIC) considerations, pharmacokinetic considerations, adverse effects, convenience of dosing, drug interactions, and insurance coverage. However, it is not possible to quantify the specific contribution of each of these reasons, in a retrospective study, without making assumptions about why specific ID physicians made specific antibiotic change recommendations. There may have been more contributions that might not have been apparent on a retrospective chart review. The lack of a control group also lessens the impact of our findings. We could not have a control group, because no patient is discharged from the Cleveland Clinic on CoPAT without having been seen by an ID physician. Mandatory ID consultation for CoPAT has previously been shown to reduce costs,9 however, our study was not designed to evaluate cost.
The perceived value of ID consultation in our institution can be appreciated when one considers the longstanding institutional policy of requiring ID consultation for CoPAT.10, 11 The perpetuation of this tradition in the hospital is testament to the presumption that mandatory ID consultation is seen to be of value by the institution.
In summary, ID consultation in our institution contributes to the care of inpatients being considered for CoPAT by substantially reducing unnecessary parenteral antibiotic use, optimizing antibiotic therapy, recognizing need for additional testing before discharge from hospital, and by providing effective transition of care from the inpatient to the outpatient setting.
With dramatically increasing costs of healthcare, it has become increasingly necessary for healthcare providers to demonstrate value in the delivery of care. Porter and Teisberg have strongly advocated that healthcare reform efforts should focus on improving value rather than limiting cost, with value being defined as quality per unit cost.1 However, it has been pointed out that value means different things to different people.2 The biggest challenge in defining value stems mainly from the difficulty in defining quality, because it, too, means vastly different things to different people. Modern medicine is increasingly characterized by multidisciplinary care. With limited or shrinking resources, it will become necessary for individual specialists to describe and articulate, in quantitative terms, their specific contributions to the overall outcome of individual patients.
Previous publications have provided broad descriptions of the value provided by infectious disease (ID) specialists in the domains of sepsis, infection control, outpatient antibiotic therapy, antimicrobial stewardship, and directive care and teaching.3, 4 Studies have also shown the value of ID physicians in specific disease conditions. ID consultation is associated with lower mortality5, 6 and lower relapse rates7 in hospitalized patients with Staphylococcus aureus bacteremia. In another study evaluating the impact of ID consultants, patients seen by ID consultants had longer lengths of hospital stay, longer intensive care unit lengths of stay, and higher antibiotic costs than matched controls not seen by ID consultants.8 It can be argued that a major limitation of the study was that controls were not matched for the ID diagnosis, nor for the causative microorganisms, but it is clear that ID physicians are challenged to demonstrate their contribution to the care of patients.
A unique activity of ID physicians is the management of community‐based parenteral anti‐infective therapy (CoPAT). At Baystate Medical Center, a policy of mandatory ID consultation was instituted for patients leaving hospital on parenteral antibiotics. A study was conducted on the impact of predischarge ID consultation for 44 patients who were not already being followed by the ID service. The study documented change from intravenous (IV) to oral formulation, change of antibiotic choice, and change of dose/duration of treatment in a substantial proportion of patients.9 These are significant changes, but ID consultation contributes more than the themes explored in the study.
The purpose of this study was to evaluate the contribution of ID consultation when consulted for CoPAT, an activity specific to ID practice, in a different institution, and using an expanded definition of medical contribution.
METHODS
The Cleveland Clinic's Department of Infectious Disease has 24 staff physicians and 11 inpatient ID consultative services. These include: 2 solid organ transplant services; a bone marrow transplant and oncologic service; 2 infective endocarditis/cardiac device infection services; an intensive care unit (ICU) service; a bone and joint infection service; a neuroinfection service; and 3 general ID consult services. Consultative services are provided 7 days a week. At the Cleveland Clinic, ID consultation is required prior to discharge on parenteral antibiotic therapy.10, 11 ID consultation for CoPAT usually occurs when the primary service deems the patient is close to being discharged from hospital. This circumstance allows for assessing the specific contribution of ID physicians beyond that of the primary service and other consulting services.
Case Ascertainment
The study was approved by the institutional review board. In February 2010, an electronic form for requesting ID consultations had been introduced into the computerized provider order entry (CPOE) system at the Cleveland Clinic. One of the required questions on the form was whether the consultation was regarding CoPAT, with options of Yes, No, or Not sure. These electronic ID consultation requests were screened to identify consultation requests for this study.
Inclusion and Exclusion Criteria
All adult ID consultations between February 11, 2010 and May 15, 2010 for which the CoPAT consult? field was marked Yes were included in the study. All other consultations, including not sure for CoPAT, were excluded.
Definitions
The first ID consultation during a hospitalization was considered an initial consultation. ID consultations for patients whom an ID service had previously seen during the same hospitalization were deemed reconsultations. Value provided was defined as contribution of the ID consultation team in the following domains: 1) optimization of antimicrobial therapy, 2) significant change in patient assessment, 3) additional medical care contribution. Specific contributions included in each domain are outlined in Table 1.
|
| Domain 1: Optimization of antibiotic therapy |
| Alteration of an antibiotic (change of antibiotic or route of administration) |
| Defining duration of therapy |
| Identification of psychosocial factors (eg, injection drug use) that influence treatment |
| Domain 2: Significant change in patient assessment |
| Diagnosis of an infectious process |
| Better appreciation of extent of disease |
| Refutation of a false infectious disease diagnosis |
| Recognition of a noninfectious process needing urgent attention |
| Identification of a positive culture as contaminant/colonization |
| Recognition of a need for additional testing (testing needed to arrive at a diagnosis or clarify a treatment plan before a patient could be safely discharged from hospital) |
| Recognition of need for surgery/emnvasive intervention |
| Refutation of antibiotic allergy by history or allergy testing |
| Domain 3: Additional medical care contribution |
| Administration of vaccines |
| Identification of an unrecognized medical problem that needed to be addressed after discharge from hospital |
| Provision of effective transition of care (ensuring that the same ID physician who saw the patient in hospital followed the patient after discharge from hospital) |
Data Collected
For each ID consultation episode, clinicians' notes were reviewed from the day of the ID consultation to the day the patient was discharged from hospital or the day the ID service signed off, whichever happened sooner. Results of recommended tests were followed up to determine if results led to a change in patient assessment. Data elements collected for each consultation episode included patient age, gender, race, date of hospitalization, date of discharge, date of ID consultation or reconsultation, primary service, and documentation of ID service contributions. Data were collected and entered in a Microsoft Access relational database. To minimize bias, the data collection was performed by physicians who had not participated in the care of the patient.
Analysis
The proportion of ID consultations in which the ID team contributed in the defined domains were enumerated, and described for the group overall and also separately for initial consultations and reconsultations.
RESULTS
In the time period studied, there were 1326 CPOE requests for ID consultation. The response to the question, CoPAT consult? was Yes for 304, No for 507, and Not sure for 515 requests. Of the 304 consultation requests marked Yes, 41 were excluded. Reasons for exclusion were: no ID consultation note (21), wrong service consulted (8), consultation request placed while the ID service was already following the patient (7), and duplicate consultation request (5). The remaining 263 consultation requests corresponded to 1 or more CoPAT consultation requests for 249 patients (across different hospitalizations). Of the 263 consultation requests, 172 were initial consultations, while the remaining 91 were reconsultations (patients not actively being followed by the ID service, but previously seen during the same hospitalization).
Consultation characteristics are outlined in Table 2. The most common group of infections for which CoPAT was sought was bone and joint infections, accounting for over 20% of the consultation requests. CoPAT consultations were requested a median of 4 days after hospitalization. Patients were discharged from hospital a median of 3 days after they were seen by the ID service. ID consultation did not delay discharge. The ID service usually saw the patient the same day, and followed the patient in hospital for a median of 1 day. There was no difference in hospital days after consult for patients who did not need antibiotics versus those who did.
| Characteristic | Initial Consultation [172] n (%)* | Reconsultation [91] n (%)* | Overall [263] n (%)* |
|---|---|---|---|
| |||
| Patient age in years, mean (SD) | 58 (14) | 62 (13) | 59 (14) |
| Male gender | 98 (60) | 91 (56) | 149 (57) |
| Caucasian race | 126 (73) | 74 (81) | 200 (76) |
| Services requesting consults (5 most common overall) | |||
| Medicine | 41 (17) | 14 (15) | 55 (21) |
| Orthopedics | 34 (14) | 0 (0) | 34 (13) |
| Hematology/Oncology | 16 (7) | 10 (11) | 26 (10) |
| Cardiology | 9 (4) | 15 (16) | 24 (9) |
| Gastroenterology | 14 (6) | 5 (5) | 19 (7) |
| Consult diagnosis (5 most common overall) | |||
| Bone and joint infection | 45 (26) | 9 (10) | 54 (21) |
| Skin or soft tissue infection or rash | 21 (12) | 8 (9) | 29 (11) |
| Endocarditis or cardiac device infection | 7 (4) | 15 (16) | 22 (8) |
| IV catheter or other endovascular infection | 9 (5) | 8 (9) | 17 (6) |
| Urinary tract infection | 12 (7) | 5 (5) | 17 (6) |
| Days from admission to ID consult, median (IQR) | 4 (1‐11) | 7 (2‐19) | 4 (1‐14) |
| Days to respond to consult request, median (IQR) | 0 (0‐1) | 0 (0‐0) | 0 (0‐0) |
| Days from ID consult to discharge, median (IQR) | 3 (2‐7) | 2 (1‐4.5) | 3 (1‐6) |
ID consultation provided value in at least 1 domain in 260 of the 263 consultations. This included optimization of antimicrobial treatment in 84%, significant alteration of patient assessment in 52%, and additional medical care contribution in 71% of consultations. Substantial contributions were made in all domains in both initial consultations and in reconsultations. Specific ID contributions within each of the domains are shown in Figure 1. There was wide overlap of contributions across the 3 domains for individual consultations (Figure 2), with contributions in all domains occurring in 34% of consultations. CoPAT was deemed not to be necessary in 27% of consultations. Among patients who did not require CoPAT, 60% received oral antibiotics and 40% were deemed not to need any antibiotics at hospital discharge. Among the patients discharged on CoPAT, a follow‐up appointment with a Cleveland Clinic ID physician familiar with the patient was set up 86% of the time; the rest either followed up with another physician or it was deemed that a scheduled follow‐up ID visit was not necessary.0


DISCUSSION
Physicians practicing in the specialty of infectious diseases face challenges and opportunities, as they adapt to changing demands within hospital practice in regard to reimbursement in an Accountable Care environment. Other challenges include emerging infections, antimicrobial resistance, need for antimicrobial stewardship, and increasing numbers of immunocompromised patients.12 From a health systems perspective, the overall value of care provided by the entire organization, and overall outcomes, are ultimately what matter. However, healthcare administrators need an appreciation of contributions of individual providers and specialties to fairly allocate resources and compensation for care provided. Articulating unique contributions is particularly challenging for individuals or services that provide purely cognitive input. Shrinking healthcare resources makes it critically important for cognitive specialists to be able to define their unique role in the care of patients with complex problems.
Our study found that a major contribution of ID consultation for CoPAT is that the process identifies a large number of patients who do not need CoPAT, thus effecting a powerful antimicrobial stewardship function. In our study, CoPAT was deemed unnecessary 27% of the time. The Infectious Diseases Society of America practice guidelines on outpatient parenteral antimicrobial therapy emphasize the importance of careful evaluation of patients considered for parenteral antibiotics outside the hospital setting.13 The focus on careful selection of appropriate patients for CoPAT has been a cornerstone of the Cleveland Clinic model of care. Nearly 30 years ago, we found that outpatient parenteral antibiotic therapy was unnecessary or not feasible in 40% of the patients referred for evaluation.10 If we adjust the numbers with the assumption that reimbursement issues present at that time are now less of an issue, the proportion of patients who were referred for CoPAT but not discharged on it was 29%, a figure remarkably similar to that found in the current study.
Another major contribution of ID consultation is the provision of effective transition of care from the inpatient to the outpatient setting. Frequent occurrence of postdischarge adverse events has been recognized as a problem in clinical practice.14 Primary care physicians are rarely involved in discussions about hospital discharge.15 A consensus conference including the American College of Physicians, Society of Hospital Medicine, and Society of General Internal Medicine, convened in July 2007 to address quality gaps in transitions of care between inpatient and outpatient settings. It identified 5 principles for effective care transitions: accountability, communication, timeliness, patient and family involvement, and respect for the hub of coordination of care.16 Recognizing gaps in care transition, hospitalists in a hospital‐based infusion program developed a model of care that successfully bridged the hospital‐to‐home care transition for patients who could return to hospital for daily antimicrobial infusions.17 In our system, ID physicians take ownership for directing parenteral antibiotic therapy for the episode of illness, specifying the physician, date, and time of follow‐up before the patient is discharged from hospital, thereby essentially satisfying the principles of effective care transitions identified. The purpose of the ID follow‐up is not to replace other follow‐up care for patients but to ensure safe transition of care while treating an episode of infection.
Attribution of identified contributions to the ID consultation could be done because our study was limited to CoPAT consultations. Such consultations typically occur when patients are deemed close to hospital discharge by the primary service. There should be little controversy about attribution of cognitive input in such consultations, because from the primary service's perspective, the patient is ready or almost ready to be discharged from hospital. It would be fair to state that most of the identified contributions in the study would not have occurred had it not been for the ID consultation.
We acknowledge that the study suffers from many limitations. The biggest limitation is that the contribution elements are defined by ID physicians and sought in the medical record by physicians from the same specialty. This arrangement certainly has potential for significant bias. To limit this bias, data collection was performed by physicians who had not participated in the care of the patient. In addition, we only could assess what was documented in the electronic health record. Our study found that alteration of antibiotic therapy was a substantial contribution, however, documentation of recommendation to change antibiotics in the medical record rarely specified exactly why the change was recommended. Reasons for antibiotic change recommendations included bug‐drug mismatch, minimum inhibitory concentration (MIC) considerations, pharmacokinetic considerations, adverse effects, convenience of dosing, drug interactions, and insurance coverage. However, it is not possible to quantify the specific contribution of each of these reasons, in a retrospective study, without making assumptions about why specific ID physicians made specific antibiotic change recommendations. There may have been more contributions that might not have been apparent on a retrospective chart review. The lack of a control group also lessens the impact of our findings. We could not have a control group, because no patient is discharged from the Cleveland Clinic on CoPAT without having been seen by an ID physician. Mandatory ID consultation for CoPAT has previously been shown to reduce costs,9 however, our study was not designed to evaluate cost.
The perceived value of ID consultation in our institution can be appreciated when one considers the longstanding institutional policy of requiring ID consultation for CoPAT.10, 11 The perpetuation of this tradition in the hospital is testament to the presumption that mandatory ID consultation is seen to be of value by the institution.
In summary, ID consultation in our institution contributes to the care of inpatients being considered for CoPAT by substantially reducing unnecessary parenteral antibiotic use, optimizing antibiotic therapy, recognizing need for additional testing before discharge from hospital, and by providing effective transition of care from the inpatient to the outpatient setting.
- ,.How physicians can change the future of health care.JAMA.2007;297:1103–1111.
- .Value of the infectious diseases specialist.Clin Infect Dis.1997;24:456.
- ,,, et al.The value of an infectious diseases specialist.Clin Infect Dis.2003;36:1013–1017.
- ,,,,,.The value of infectious diseases specialists: non‐patient care activities.Clin Infect Dis.2008;47:1051–1063.
- ,,,,.The value of infectious diseases consultation in Staphylococcus aureus bacteremia.Am J Med.2010;123:631–637.
- ,,,,.Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia.Medicine (Baltimore).2009;88:263–267.
- ,,, et al.Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients.Clin Infect Dis.1998;27:478–486.
- ,,.Infectious diseases consultation: impact on outcomes for hospitalized patients and results of a preliminary study.Clin Infect Dis.1997;24:468–470.
- ,,.Impact of mandatory inpatient infectious disease consultation on outpatient parenteral antibiotic therapy.Am J Med Sci.2005;330:60–64.
- ,.Home intravenous antibiotic therapy: a team approach.Ann Intern Med.1983;99:388–392.
- ,,.Transitioning antimicrobial stewardship beyond the hospital: the Cleveland Clinic's community‐based parenteral anti‐infective therapy (CoPAT) program.J Hosp Med.2011;6(suppl 1):S24–S30.
- ,,.Professional challenges and opportunities in clinical microbiology and infectious diseases in Europe.Lancet Infect Dis.2011;11:408–415.
- ,,, et al.Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines.Clin Infect Dis.2004;38:1651–1672.
- ,.Addressing postdischarge adverse events: a neglected area.Jt Comm J Qual Patient Saf.2008;34:85–97.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,, et al.Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine.J Hosp Med.2009;4:364–370.
- .Hospitalist to home: outpatient parenteral antimicrobial therapy at an academic center.Clin Infect Dis.2010;51(suppl 2):S220–S223.
- ,.How physicians can change the future of health care.JAMA.2007;297:1103–1111.
- .Value of the infectious diseases specialist.Clin Infect Dis.1997;24:456.
- ,,, et al.The value of an infectious diseases specialist.Clin Infect Dis.2003;36:1013–1017.
- ,,,,,.The value of infectious diseases specialists: non‐patient care activities.Clin Infect Dis.2008;47:1051–1063.
- ,,,,.The value of infectious diseases consultation in Staphylococcus aureus bacteremia.Am J Med.2010;123:631–637.
- ,,,,.Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia.Medicine (Baltimore).2009;88:263–267.
- ,,, et al.Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients.Clin Infect Dis.1998;27:478–486.
- ,,.Infectious diseases consultation: impact on outcomes for hospitalized patients and results of a preliminary study.Clin Infect Dis.1997;24:468–470.
- ,,.Impact of mandatory inpatient infectious disease consultation on outpatient parenteral antibiotic therapy.Am J Med Sci.2005;330:60–64.
- ,.Home intravenous antibiotic therapy: a team approach.Ann Intern Med.1983;99:388–392.
- ,,.Transitioning antimicrobial stewardship beyond the hospital: the Cleveland Clinic's community‐based parenteral anti‐infective therapy (CoPAT) program.J Hosp Med.2011;6(suppl 1):S24–S30.
- ,,.Professional challenges and opportunities in clinical microbiology and infectious diseases in Europe.Lancet Infect Dis.2011;11:408–415.
- ,,, et al.Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines.Clin Infect Dis.2004;38:1651–1672.
- ,.Addressing postdischarge adverse events: a neglected area.Jt Comm J Qual Patient Saf.2008;34:85–97.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,, et al.Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine.J Hosp Med.2009;4:364–370.
- .Hospitalist to home: outpatient parenteral antimicrobial therapy at an academic center.Clin Infect Dis.2010;51(suppl 2):S220–S223.
Cardiovascular implantable electronic device infection: A complication of medical progress
The term cardiovascular implantable electronic device (CIED) includes both permanent pacemakers and implantable cardioverter-defibrillators. These devices are being implanted in more people every year.1 They have also become increasingly sophisticated, with newer devices capable of both pacing and cardioversion-defibrillation functions.2 Patients receiving these devices are also increasingly older and have more comorbid conditions.3,4 As more CIEDs are placed in older and sicker patients, infections of these devices can be expected to be encountered with increasing frequency.
In this issue of the Cleveland Clinic Journal of Medicine, Dababneh and Sohail5 review CIED infections and provide a stepwise approach to their diagnosis and treatment.
HOW THE DEVICES BECOME INFECTED
CIEDs can become infected during implantation, in which case the infection presents early on, usually with pocket manifestations, or by secondary hematogenous seeding, in which case the infection generally presents with endovascular manifestations. Dababneh and Sohail have elegantly outlined the risk factors that predispose to infection of these devices.
If there are no early complications, patients generally do well with these devices. However, many patients do fine with their first device but develop a pocket infection when the pulse generator is changed because of battery depletion or other reasons. When patients with a CIED develop bacteremia as a complication of a vascular catheter infection or other infection, particularly with Staphylococcus aureus, they are at increased risk of having the intravascular portion of their device seeded.
PATIENTS MAY NOT APPEAR VERY ILL AT PRESENTATION
Dababneh and Sohail divide the clinical presentations of CIED infection into two broad categories: pocket infection and endovascular infection with an intact pocket. This is a useful categorization, as it provides a clue to pathogenesis.
As the authors point out, most patients with CIED infection present first to their primary care physician when they develop symptoms. An understanding of this infection by primary care physicians will allow for early recognition and more timely treatment, thus avoiding unnecessary complications.
Patients with pocket infection may not appear ill, but this should not lead a clinician away from the diagnosis. A pocket hematoma is an important differential diagnosis in the early postoperative period after device implantation or pulse generator change, and it may be difficult to decide if pocket changes are from an uninfected hematoma or from an infection.
Patients with endovascular infection are more likely to have systemic symptoms such as fever, fatigue, and malaise. However, absence of systemic features does not necessarily exclude endovascular infection.
BLOOD CULTURES AND TEE ARE KEY DIAGNOSTIC TESTS
All patients with suspected CIED infection should have at least two sets of blood cultures checked, even if they appear to be reasonably well. If there is any suspicion of endovascular infection, echocardiography should be performed.
Transesophageal echocardiography (TEE) is far superior to transthoracic echocardiography (TTE) for detecting lead vegetations.6 TEE should be carefully performed whenever endovascular infection is suspected, including all patients with positive blood cultures and all patients with systemic signs and symptoms.
Purulent drainage should be cultured, and when the device is removed, cultures of lead tips and pocket tissue should be done as well.
TREATMENT USUALLY REQUIRES COMPLETE DEVICE REMOVAL
A superficial infection in the early postoperative period may respond to antibiotic therapy alone. But in all other patients, the device must be removed to cure the infection. In referral centers, it is not unusual to see patients who have been referred after having been treated with antibiotics for weeks and sometimes months in the mistaken belief that the infection would be cured with antibiotics alone.
In some patients presenting with only pocket findings in the early postoperative period, it may be difficult indeed to tell if there is pocket infection. In such patients, it is not necessary to make a hasty decision to remove the device, but it is important to monitor them closely until the presence or absence of infection becomes clear. Also, erosion of the device through the skin represents pocket infection even if the patient appears otherwise healthy.
When removing the device, it is necessary to remove the generator and all leads to treat the infection effectively.
If patients are device-dependent, it is usually safe to place a new device with the new pulse generator pocket in a different location from the infected one a few days after the infected device is removed.
AREAS OF UNCERTAINTY AND CHALLENGE
Although there is no controversy about the need for complete removal of infected devices in order to effect a cure, the appropriate duration of antibiotic therapy after device removal is less clear. Dababneh and Sohail provide a useful algorithm to help with this decision. Patients usually need a new device to replace the infected one and there is a legitimate reason for concern about undertreating, since one would not want the new device to become infected because of inadequate antibiotic therapy. When endovascular infection is suspected or documented, patients are probably best treated as they would be for infective endocarditis.
Difficulties arise when patients with a CIED develop bacteremia with no echocardiographic evidence of device infection. Finding the source of bacteremia is very important because a diagnosis of CIED infection indicates that the device has to be removed. When there is a clear alternative explanation for the bacteremia, the CIED does not have to be removed. The type of bacterium helps clinicians to gauge the likelihood of CIED infection and to decide on the appropriate course of action. These cases should always be managed in conjunction with an infectious disease specialist and a cardiac electrophysiologist.
Another concern is secondary seeding of an uninfected CIED caused by bacteremia from another source. This concern is particularly acute with S aureus bacteremia. When patients with a CIED and S aureus bacteremia have been studied, endovascular CIED infection was documented in about half, although only a few had evidence of pocket inflammation.7,8 This suggests that the devices were seeded via the endovascular route.
Medical procedures such as dialysis and total parenteral nutrition require frequent intravascular access—often facilitated by leaving an indwelling vascular catheter in place. Frequent entry into the intravascular compartment puts patients at substantial risk of bloodstream infection, and in patients with a CIED this can be complicated by device infection. In patients with a CIED and an indwelling vascular catheter who develop bacteremia, determining the source of the bacteremia is particularly challenging, as is the treatment. Thus, preventing endovascular infection in such patients is extremely desirable, but there are no easy solutions.
PLACING CIED INFECTIONS IN PERSPECTIVE
The vast majority of patients with a CIED never develop a device infection. Those unfortunate enough to have a CIED infection have little choice other than to have the device removed, but those diagnosed early and treated appropriately generally do well. The development of CIEDs has been an important advance in the practice of cardiac electrophysiology. An appropriate understanding of CIED infection and its treatment will help optimize the diagnosis and management of this complication when it does occur.
- Zhan C, Baine WB, Sedrakyan A, Steiner C. Cardiac device implantation in the United States from 1997 through 2004: a population-based analysis. J Gen Intern Med 2008; 23(suppl 1):13–19.
- Hayes DL, Furman S. Cardiac pacing: how it started, where we are, where we are going. J Cardiovasc Electrophysiol 2004; 15:619–627.
- Lin G, Meverden RA, Hodge DO, Uslan DZ, Hayes DL, Brady PA. Age and gender trends in implantable cardioverter defibrillator utilization: a population based study. J Interv Card Electrophysiol 2008; 22:65–70.
- Uslan DZ, Tleyjeh IM, Baddour LM, et al. Temporal trends in permanent pacemaker implantation: a population-based study. Am Heart J 2008; 155:896–903.
- Dababneh SR, Sohail MR. Cardiovascular implantable electronic device infection: a stepwise approach to diagnosis and management. Cleve Clin J Med 2011; 78:529–537.
- Victor F, De Place C, Camus C, et al. Pacemaker lead infection: echocardiographic features, management, and outcome. Heart 1999; 81:82–97.
- Chamis AL, Peterson GE, Cabell CH, et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation 2001; 104:1029–1033.
- Uslan DZ, Sohail MR, St Sauver JL, et a.l Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med 2007; 167:669–675.
The term cardiovascular implantable electronic device (CIED) includes both permanent pacemakers and implantable cardioverter-defibrillators. These devices are being implanted in more people every year.1 They have also become increasingly sophisticated, with newer devices capable of both pacing and cardioversion-defibrillation functions.2 Patients receiving these devices are also increasingly older and have more comorbid conditions.3,4 As more CIEDs are placed in older and sicker patients, infections of these devices can be expected to be encountered with increasing frequency.
In this issue of the Cleveland Clinic Journal of Medicine, Dababneh and Sohail5 review CIED infections and provide a stepwise approach to their diagnosis and treatment.
HOW THE DEVICES BECOME INFECTED
CIEDs can become infected during implantation, in which case the infection presents early on, usually with pocket manifestations, or by secondary hematogenous seeding, in which case the infection generally presents with endovascular manifestations. Dababneh and Sohail have elegantly outlined the risk factors that predispose to infection of these devices.
If there are no early complications, patients generally do well with these devices. However, many patients do fine with their first device but develop a pocket infection when the pulse generator is changed because of battery depletion or other reasons. When patients with a CIED develop bacteremia as a complication of a vascular catheter infection or other infection, particularly with Staphylococcus aureus, they are at increased risk of having the intravascular portion of their device seeded.
PATIENTS MAY NOT APPEAR VERY ILL AT PRESENTATION
Dababneh and Sohail divide the clinical presentations of CIED infection into two broad categories: pocket infection and endovascular infection with an intact pocket. This is a useful categorization, as it provides a clue to pathogenesis.
As the authors point out, most patients with CIED infection present first to their primary care physician when they develop symptoms. An understanding of this infection by primary care physicians will allow for early recognition and more timely treatment, thus avoiding unnecessary complications.
Patients with pocket infection may not appear ill, but this should not lead a clinician away from the diagnosis. A pocket hematoma is an important differential diagnosis in the early postoperative period after device implantation or pulse generator change, and it may be difficult to decide if pocket changes are from an uninfected hematoma or from an infection.
Patients with endovascular infection are more likely to have systemic symptoms such as fever, fatigue, and malaise. However, absence of systemic features does not necessarily exclude endovascular infection.
BLOOD CULTURES AND TEE ARE KEY DIAGNOSTIC TESTS
All patients with suspected CIED infection should have at least two sets of blood cultures checked, even if they appear to be reasonably well. If there is any suspicion of endovascular infection, echocardiography should be performed.
Transesophageal echocardiography (TEE) is far superior to transthoracic echocardiography (TTE) for detecting lead vegetations.6 TEE should be carefully performed whenever endovascular infection is suspected, including all patients with positive blood cultures and all patients with systemic signs and symptoms.
Purulent drainage should be cultured, and when the device is removed, cultures of lead tips and pocket tissue should be done as well.
TREATMENT USUALLY REQUIRES COMPLETE DEVICE REMOVAL
A superficial infection in the early postoperative period may respond to antibiotic therapy alone. But in all other patients, the device must be removed to cure the infection. In referral centers, it is not unusual to see patients who have been referred after having been treated with antibiotics for weeks and sometimes months in the mistaken belief that the infection would be cured with antibiotics alone.
In some patients presenting with only pocket findings in the early postoperative period, it may be difficult indeed to tell if there is pocket infection. In such patients, it is not necessary to make a hasty decision to remove the device, but it is important to monitor them closely until the presence or absence of infection becomes clear. Also, erosion of the device through the skin represents pocket infection even if the patient appears otherwise healthy.
When removing the device, it is necessary to remove the generator and all leads to treat the infection effectively.
If patients are device-dependent, it is usually safe to place a new device with the new pulse generator pocket in a different location from the infected one a few days after the infected device is removed.
AREAS OF UNCERTAINTY AND CHALLENGE
Although there is no controversy about the need for complete removal of infected devices in order to effect a cure, the appropriate duration of antibiotic therapy after device removal is less clear. Dababneh and Sohail provide a useful algorithm to help with this decision. Patients usually need a new device to replace the infected one and there is a legitimate reason for concern about undertreating, since one would not want the new device to become infected because of inadequate antibiotic therapy. When endovascular infection is suspected or documented, patients are probably best treated as they would be for infective endocarditis.
Difficulties arise when patients with a CIED develop bacteremia with no echocardiographic evidence of device infection. Finding the source of bacteremia is very important because a diagnosis of CIED infection indicates that the device has to be removed. When there is a clear alternative explanation for the bacteremia, the CIED does not have to be removed. The type of bacterium helps clinicians to gauge the likelihood of CIED infection and to decide on the appropriate course of action. These cases should always be managed in conjunction with an infectious disease specialist and a cardiac electrophysiologist.
Another concern is secondary seeding of an uninfected CIED caused by bacteremia from another source. This concern is particularly acute with S aureus bacteremia. When patients with a CIED and S aureus bacteremia have been studied, endovascular CIED infection was documented in about half, although only a few had evidence of pocket inflammation.7,8 This suggests that the devices were seeded via the endovascular route.
Medical procedures such as dialysis and total parenteral nutrition require frequent intravascular access—often facilitated by leaving an indwelling vascular catheter in place. Frequent entry into the intravascular compartment puts patients at substantial risk of bloodstream infection, and in patients with a CIED this can be complicated by device infection. In patients with a CIED and an indwelling vascular catheter who develop bacteremia, determining the source of the bacteremia is particularly challenging, as is the treatment. Thus, preventing endovascular infection in such patients is extremely desirable, but there are no easy solutions.
PLACING CIED INFECTIONS IN PERSPECTIVE
The vast majority of patients with a CIED never develop a device infection. Those unfortunate enough to have a CIED infection have little choice other than to have the device removed, but those diagnosed early and treated appropriately generally do well. The development of CIEDs has been an important advance in the practice of cardiac electrophysiology. An appropriate understanding of CIED infection and its treatment will help optimize the diagnosis and management of this complication when it does occur.
The term cardiovascular implantable electronic device (CIED) includes both permanent pacemakers and implantable cardioverter-defibrillators. These devices are being implanted in more people every year.1 They have also become increasingly sophisticated, with newer devices capable of both pacing and cardioversion-defibrillation functions.2 Patients receiving these devices are also increasingly older and have more comorbid conditions.3,4 As more CIEDs are placed in older and sicker patients, infections of these devices can be expected to be encountered with increasing frequency.
In this issue of the Cleveland Clinic Journal of Medicine, Dababneh and Sohail5 review CIED infections and provide a stepwise approach to their diagnosis and treatment.
HOW THE DEVICES BECOME INFECTED
CIEDs can become infected during implantation, in which case the infection presents early on, usually with pocket manifestations, or by secondary hematogenous seeding, in which case the infection generally presents with endovascular manifestations. Dababneh and Sohail have elegantly outlined the risk factors that predispose to infection of these devices.
If there are no early complications, patients generally do well with these devices. However, many patients do fine with their first device but develop a pocket infection when the pulse generator is changed because of battery depletion or other reasons. When patients with a CIED develop bacteremia as a complication of a vascular catheter infection or other infection, particularly with Staphylococcus aureus, they are at increased risk of having the intravascular portion of their device seeded.
PATIENTS MAY NOT APPEAR VERY ILL AT PRESENTATION
Dababneh and Sohail divide the clinical presentations of CIED infection into two broad categories: pocket infection and endovascular infection with an intact pocket. This is a useful categorization, as it provides a clue to pathogenesis.
As the authors point out, most patients with CIED infection present first to their primary care physician when they develop symptoms. An understanding of this infection by primary care physicians will allow for early recognition and more timely treatment, thus avoiding unnecessary complications.
Patients with pocket infection may not appear ill, but this should not lead a clinician away from the diagnosis. A pocket hematoma is an important differential diagnosis in the early postoperative period after device implantation or pulse generator change, and it may be difficult to decide if pocket changes are from an uninfected hematoma or from an infection.
Patients with endovascular infection are more likely to have systemic symptoms such as fever, fatigue, and malaise. However, absence of systemic features does not necessarily exclude endovascular infection.
BLOOD CULTURES AND TEE ARE KEY DIAGNOSTIC TESTS
All patients with suspected CIED infection should have at least two sets of blood cultures checked, even if they appear to be reasonably well. If there is any suspicion of endovascular infection, echocardiography should be performed.
Transesophageal echocardiography (TEE) is far superior to transthoracic echocardiography (TTE) for detecting lead vegetations.6 TEE should be carefully performed whenever endovascular infection is suspected, including all patients with positive blood cultures and all patients with systemic signs and symptoms.
Purulent drainage should be cultured, and when the device is removed, cultures of lead tips and pocket tissue should be done as well.
TREATMENT USUALLY REQUIRES COMPLETE DEVICE REMOVAL
A superficial infection in the early postoperative period may respond to antibiotic therapy alone. But in all other patients, the device must be removed to cure the infection. In referral centers, it is not unusual to see patients who have been referred after having been treated with antibiotics for weeks and sometimes months in the mistaken belief that the infection would be cured with antibiotics alone.
In some patients presenting with only pocket findings in the early postoperative period, it may be difficult indeed to tell if there is pocket infection. In such patients, it is not necessary to make a hasty decision to remove the device, but it is important to monitor them closely until the presence or absence of infection becomes clear. Also, erosion of the device through the skin represents pocket infection even if the patient appears otherwise healthy.
When removing the device, it is necessary to remove the generator and all leads to treat the infection effectively.
If patients are device-dependent, it is usually safe to place a new device with the new pulse generator pocket in a different location from the infected one a few days after the infected device is removed.
AREAS OF UNCERTAINTY AND CHALLENGE
Although there is no controversy about the need for complete removal of infected devices in order to effect a cure, the appropriate duration of antibiotic therapy after device removal is less clear. Dababneh and Sohail provide a useful algorithm to help with this decision. Patients usually need a new device to replace the infected one and there is a legitimate reason for concern about undertreating, since one would not want the new device to become infected because of inadequate antibiotic therapy. When endovascular infection is suspected or documented, patients are probably best treated as they would be for infective endocarditis.
Difficulties arise when patients with a CIED develop bacteremia with no echocardiographic evidence of device infection. Finding the source of bacteremia is very important because a diagnosis of CIED infection indicates that the device has to be removed. When there is a clear alternative explanation for the bacteremia, the CIED does not have to be removed. The type of bacterium helps clinicians to gauge the likelihood of CIED infection and to decide on the appropriate course of action. These cases should always be managed in conjunction with an infectious disease specialist and a cardiac electrophysiologist.
Another concern is secondary seeding of an uninfected CIED caused by bacteremia from another source. This concern is particularly acute with S aureus bacteremia. When patients with a CIED and S aureus bacteremia have been studied, endovascular CIED infection was documented in about half, although only a few had evidence of pocket inflammation.7,8 This suggests that the devices were seeded via the endovascular route.
Medical procedures such as dialysis and total parenteral nutrition require frequent intravascular access—often facilitated by leaving an indwelling vascular catheter in place. Frequent entry into the intravascular compartment puts patients at substantial risk of bloodstream infection, and in patients with a CIED this can be complicated by device infection. In patients with a CIED and an indwelling vascular catheter who develop bacteremia, determining the source of the bacteremia is particularly challenging, as is the treatment. Thus, preventing endovascular infection in such patients is extremely desirable, but there are no easy solutions.
PLACING CIED INFECTIONS IN PERSPECTIVE
The vast majority of patients with a CIED never develop a device infection. Those unfortunate enough to have a CIED infection have little choice other than to have the device removed, but those diagnosed early and treated appropriately generally do well. The development of CIEDs has been an important advance in the practice of cardiac electrophysiology. An appropriate understanding of CIED infection and its treatment will help optimize the diagnosis and management of this complication when it does occur.
- Zhan C, Baine WB, Sedrakyan A, Steiner C. Cardiac device implantation in the United States from 1997 through 2004: a population-based analysis. J Gen Intern Med 2008; 23(suppl 1):13–19.
- Hayes DL, Furman S. Cardiac pacing: how it started, where we are, where we are going. J Cardiovasc Electrophysiol 2004; 15:619–627.
- Lin G, Meverden RA, Hodge DO, Uslan DZ, Hayes DL, Brady PA. Age and gender trends in implantable cardioverter defibrillator utilization: a population based study. J Interv Card Electrophysiol 2008; 22:65–70.
- Uslan DZ, Tleyjeh IM, Baddour LM, et al. Temporal trends in permanent pacemaker implantation: a population-based study. Am Heart J 2008; 155:896–903.
- Dababneh SR, Sohail MR. Cardiovascular implantable electronic device infection: a stepwise approach to diagnosis and management. Cleve Clin J Med 2011; 78:529–537.
- Victor F, De Place C, Camus C, et al. Pacemaker lead infection: echocardiographic features, management, and outcome. Heart 1999; 81:82–97.
- Chamis AL, Peterson GE, Cabell CH, et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation 2001; 104:1029–1033.
- Uslan DZ, Sohail MR, St Sauver JL, et a.l Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med 2007; 167:669–675.
- Zhan C, Baine WB, Sedrakyan A, Steiner C. Cardiac device implantation in the United States from 1997 through 2004: a population-based analysis. J Gen Intern Med 2008; 23(suppl 1):13–19.
- Hayes DL, Furman S. Cardiac pacing: how it started, where we are, where we are going. J Cardiovasc Electrophysiol 2004; 15:619–627.
- Lin G, Meverden RA, Hodge DO, Uslan DZ, Hayes DL, Brady PA. Age and gender trends in implantable cardioverter defibrillator utilization: a population based study. J Interv Card Electrophysiol 2008; 22:65–70.
- Uslan DZ, Tleyjeh IM, Baddour LM, et al. Temporal trends in permanent pacemaker implantation: a population-based study. Am Heart J 2008; 155:896–903.
- Dababneh SR, Sohail MR. Cardiovascular implantable electronic device infection: a stepwise approach to diagnosis and management. Cleve Clin J Med 2011; 78:529–537.
- Victor F, De Place C, Camus C, et al. Pacemaker lead infection: echocardiographic features, management, and outcome. Heart 1999; 81:82–97.
- Chamis AL, Peterson GE, Cabell CH, et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation 2001; 104:1029–1033.
- Uslan DZ, Sohail MR, St Sauver JL, et a.l Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med 2007; 167:669–675.