User login
Gastrointestinal Bleeding Caused by Large Intestine Amyloidosis
Gastrointestinal (GI) bleeding is a common cause of hospital admissions. The yearly incidence of upper GI bleeding is 80 to 150/100,000 people and lower GI bleeding is 87/100,000 people.1,2 The differential tends to initially be broad but narrows with good history followed by endoscopic findings. Getting an appropriate history can be difficult at times, which leads health care practitioners to rely more on interventional results.
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are 2 main types of amyloidosis, systemic and transthyretin, and 4 subtypes. Systemic amyloidosis includes amyloid light-chain (AL) deposition, caused by plasma cell dyscrasia, and amyloid A (AA) protein deposition, caused by systemic autoimmune illness or infections. Transthyretin amyloidosis is caused by changes and deposition of the transthyretin protein consisting of either unstable, mutant protein or wild type protein. Biopsy-proven amyloidosis of the GI tract is rare.4 About 60% of patients with AA amyloidosis and 8% with AL amyloidosis have GI involvement.5
We present a case of nonspecific symptoms that ultimately lined up perfectly with the official histologic confirmation of intestinal amyloidosis.
Case Presentation
A 79-year-old man with a history of type 2 diabetes mellitus, congestive heart failure, hyperlipidemia, obstructive sleep apnea, hypothyroidism, hypertension, coronary artery disease status postcoronary artery bypass grafting, and stent placements presented for 3 episodes of large, bright red bowel movements. He reported past bleeding and straining with stools, but bleeding of this amount had not been noted prior. He also reported dry heaves, lower abdominal pain, constipation with straining, early satiety with dysphagia, weakness, and decreased appetite. Lastly, he mentioned intentionally losing about 35 to 40 pounds in the past 3 to 4 months and over the past several months increased abdominal distention. However, he stated he had no history of alcohol misuse, liver or intestinal disease, cirrhosis, or other autoimmune diseases. His most recent colonoscopy was more than a decade prior and showed no acute process. The patient never had an esophagogastroduodenoscopy (EGD).
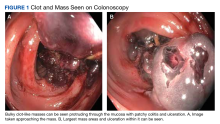
On initial presentation, the patient’s vital signs showed no acute findings. His physical examination noted a chronically ill–appearing male with decreased breath sounds to the bases bilaterally and noted abdominal distention with mild generalized tenderness. Laboratory findings were significant for a hemoglobin level, 9.4 g/dL (reference range, 11.6-15.3); iron, 23 ug/dL (reference range, 45-160); transferrin saturation, 8% (reference range, 15-50); ferritin level, 80 ng/mL (reference range, 30-300); and carcinoembryonic antigen level, 1.5 ng/mL (reference range, 0-2.9). Aspartate aminotransferase level was 54 IU/L (reference range, 0-40); alanine transaminase, 24 IU/L (reference range, 7-52); albumin, 2.7 g/dL (reference range, 3.4-5.7); international normalized ratio, 1.3 (reference range, 0-1.1); creatinine, 1.74 mg/dL (reference range, 0.44-1.27); alkaline phosphatase, 369 IU/L (reference range, 39-117). White blood cell count was 15.5 × 109/L (reference range, 3.5-10.3), and lactic acid was 2.5 mmol/L (reference range, 0.5-2.2). He was started on piperacillin/tazobactam in the emergency department and transitioned to ciprofloxacin and metronidazole for presumed intra-abdominal infection. Paracentesis showed a serum ascites albumin gradient of > 1.1 g/dL with no signs of spontaneous bacterial peritonitis. Computed tomography of the abdomen and pelvis with contrast was suspicious for colitis involving the proximal colon, and colonic mass could not be excluded. Also noted was hepatosplenomegaly with abdominopelvic ascites.
Based on these findings, an EGD and colonoscopy were done. The EGD showed mild portal hypertensive gastropathy.
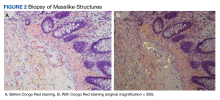
After the biopsy results, the patient was officially diagnosed with intestinal amyloidosis (Figure 2).
He returned to the gastroenterology clinic 2 months later. At that point, he had worsening symptoms, liver function test results, and international normalized ratio. He was admitted for further investigation. A bone biopsy was done to confirm the histology and define the underlying disorder. The biopsy returned showing Waldenstrom macroglobulinemia, and he was started on bortezomib. Unfortunately, his clinical status rapidly worsened, leading to acute renal and hepatic failure and the development of encephalopathy. He eventually died under palliative care services.
Discussion
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are several variations of amyloid, but the most common type is AL amyloidosis, which affects several organs, including the heart, kidney, liver, nervous system, and GI tract. When AL amyloidosis involves the liver, the median survival time is about 8.5 months.6 There are different ways to diagnose the disease, but a tissue biopsy and Congo Red staining can confirm specific organ involvement as seen in our case.
This case adds another layer to our constantly expanding differential as health care practitioners and proves that atypical patient presentations may not be atypical after all. GI amyloidosis tends to present similarly to our patient with bleeding, malabsorption, dysmotility, and protein-losing gastroenteropathy as ascites, edema, pericardial effusions, and laboratory evidence of hypoalbuminemia.7 Because amyloidosis is a systemic illness, early recognition is important as intestinal complications tend to present as symptoms, but mortality is more often caused by renal failure, cardiomyopathy, or ischemic heart disease, making early multispecialty involvement very important.8
Conclusions
Health care practitioners in all specialties should be aware of and include intestinal amyloidosis in their differential diagnosis when working up GI bleeds with the hope of identifying the disease early. With early recognition, rapid biopsy identification, and early specialist involvement, patients will get the opportunity for expedited multidisciplinary treatment and potentially delay rapid decompensation as shown by the evidence in this case.
1. Antunes C, Copelin II EL. Upper gastrointestinal bleeding. StatPearls [internet]. Updated July 18, 2022. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470300
2. Almaghrabi M, Gandhi M, Guizzetti L, et al. Comparison of risk scores for lower gastrointestinal bleeding: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(5):e2214253. doi:10.1001/jamanetworkopen.2022.14253
3. Pepys MB. Pathogenesis, diagnosis and treatment of systemic amyloidosis. Philos Trans R Soc Lond B Biol Sci. 2001;356(1406):203-211. doi:10.1098/rstb.2000.0766
4. Cowan AJ, Skinner M, Seldin DC, et al. Amyloidosis of the gastrointestinal tract: a 13-year, single-center, referral experience. Haematologica. 2013;98(1):141-146. doi:10.3324/haematol.2012.068155
5. Lee BS, Chudasama Y, Chen AI, Lim BS, Taira MT. Colonoscopy leading to the diagnosis of AL amyloidosis in the gastrointestinal tract mimicking an acute ulcerative colitis flare. ACG Case Rep J. 2019;6(11):e00289. doi:10.14309/crj.0000000000000289
6. Zhao L, Ren G, Guo J, Chen W, Xu W, Huang X. The clinical features and outcomes of systemic light chain amyloidosis with hepatic involvement. Ann Med. 2022;54(1):1226-1232. doi:10.1080/07853890.2022.2069281
7. Rowe K, Pankow J, Nehme F, Salyers W. Gastrointestinal amyloidosis: review of the literature. Cureus. 2017;9(5):e1228. doi:10.7759/cureus.1228
8. Kyle RA, Greipp PR, O’Fallon WM. Primary systemic amyloidosis: multivariate analysis for prognostic factors in 168 cases. Blood. 1986;68(1):220-224.
Gastrointestinal (GI) bleeding is a common cause of hospital admissions. The yearly incidence of upper GI bleeding is 80 to 150/100,000 people and lower GI bleeding is 87/100,000 people.1,2 The differential tends to initially be broad but narrows with good history followed by endoscopic findings. Getting an appropriate history can be difficult at times, which leads health care practitioners to rely more on interventional results.
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are 2 main types of amyloidosis, systemic and transthyretin, and 4 subtypes. Systemic amyloidosis includes amyloid light-chain (AL) deposition, caused by plasma cell dyscrasia, and amyloid A (AA) protein deposition, caused by systemic autoimmune illness or infections. Transthyretin amyloidosis is caused by changes and deposition of the transthyretin protein consisting of either unstable, mutant protein or wild type protein. Biopsy-proven amyloidosis of the GI tract is rare.4 About 60% of patients with AA amyloidosis and 8% with AL amyloidosis have GI involvement.5
We present a case of nonspecific symptoms that ultimately lined up perfectly with the official histologic confirmation of intestinal amyloidosis.
Case Presentation
A 79-year-old man with a history of type 2 diabetes mellitus, congestive heart failure, hyperlipidemia, obstructive sleep apnea, hypothyroidism, hypertension, coronary artery disease status postcoronary artery bypass grafting, and stent placements presented for 3 episodes of large, bright red bowel movements. He reported past bleeding and straining with stools, but bleeding of this amount had not been noted prior. He also reported dry heaves, lower abdominal pain, constipation with straining, early satiety with dysphagia, weakness, and decreased appetite. Lastly, he mentioned intentionally losing about 35 to 40 pounds in the past 3 to 4 months and over the past several months increased abdominal distention. However, he stated he had no history of alcohol misuse, liver or intestinal disease, cirrhosis, or other autoimmune diseases. His most recent colonoscopy was more than a decade prior and showed no acute process. The patient never had an esophagogastroduodenoscopy (EGD).
On initial presentation, the patient’s vital signs showed no acute findings. His physical examination noted a chronically ill–appearing male with decreased breath sounds to the bases bilaterally and noted abdominal distention with mild generalized tenderness. Laboratory findings were significant for a hemoglobin level, 9.4 g/dL (reference range, 11.6-15.3); iron, 23 ug/dL (reference range, 45-160); transferrin saturation, 8% (reference range, 15-50); ferritin level, 80 ng/mL (reference range, 30-300); and carcinoembryonic antigen level, 1.5 ng/mL (reference range, 0-2.9). Aspartate aminotransferase level was 54 IU/L (reference range, 0-40); alanine transaminase, 24 IU/L (reference range, 7-52); albumin, 2.7 g/dL (reference range, 3.4-5.7); international normalized ratio, 1.3 (reference range, 0-1.1); creatinine, 1.74 mg/dL (reference range, 0.44-1.27); alkaline phosphatase, 369 IU/L (reference range, 39-117). White blood cell count was 15.5 × 109/L (reference range, 3.5-10.3), and lactic acid was 2.5 mmol/L (reference range, 0.5-2.2). He was started on piperacillin/tazobactam in the emergency department and transitioned to ciprofloxacin and metronidazole for presumed intra-abdominal infection. Paracentesis showed a serum ascites albumin gradient of > 1.1 g/dL with no signs of spontaneous bacterial peritonitis. Computed tomography of the abdomen and pelvis with contrast was suspicious for colitis involving the proximal colon, and colonic mass could not be excluded. Also noted was hepatosplenomegaly with abdominopelvic ascites.
Based on these findings, an EGD and colonoscopy were done. The EGD showed mild portal hypertensive gastropathy.
After the biopsy results, the patient was officially diagnosed with intestinal amyloidosis (Figure 2).
He returned to the gastroenterology clinic 2 months later. At that point, he had worsening symptoms, liver function test results, and international normalized ratio. He was admitted for further investigation. A bone biopsy was done to confirm the histology and define the underlying disorder. The biopsy returned showing Waldenstrom macroglobulinemia, and he was started on bortezomib. Unfortunately, his clinical status rapidly worsened, leading to acute renal and hepatic failure and the development of encephalopathy. He eventually died under palliative care services.
Discussion
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are several variations of amyloid, but the most common type is AL amyloidosis, which affects several organs, including the heart, kidney, liver, nervous system, and GI tract. When AL amyloidosis involves the liver, the median survival time is about 8.5 months.6 There are different ways to diagnose the disease, but a tissue biopsy and Congo Red staining can confirm specific organ involvement as seen in our case.
This case adds another layer to our constantly expanding differential as health care practitioners and proves that atypical patient presentations may not be atypical after all. GI amyloidosis tends to present similarly to our patient with bleeding, malabsorption, dysmotility, and protein-losing gastroenteropathy as ascites, edema, pericardial effusions, and laboratory evidence of hypoalbuminemia.7 Because amyloidosis is a systemic illness, early recognition is important as intestinal complications tend to present as symptoms, but mortality is more often caused by renal failure, cardiomyopathy, or ischemic heart disease, making early multispecialty involvement very important.8
Conclusions
Health care practitioners in all specialties should be aware of and include intestinal amyloidosis in their differential diagnosis when working up GI bleeds with the hope of identifying the disease early. With early recognition, rapid biopsy identification, and early specialist involvement, patients will get the opportunity for expedited multidisciplinary treatment and potentially delay rapid decompensation as shown by the evidence in this case.
Gastrointestinal (GI) bleeding is a common cause of hospital admissions. The yearly incidence of upper GI bleeding is 80 to 150/100,000 people and lower GI bleeding is 87/100,000 people.1,2 The differential tends to initially be broad but narrows with good history followed by endoscopic findings. Getting an appropriate history can be difficult at times, which leads health care practitioners to rely more on interventional results.
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are 2 main types of amyloidosis, systemic and transthyretin, and 4 subtypes. Systemic amyloidosis includes amyloid light-chain (AL) deposition, caused by plasma cell dyscrasia, and amyloid A (AA) protein deposition, caused by systemic autoimmune illness or infections. Transthyretin amyloidosis is caused by changes and deposition of the transthyretin protein consisting of either unstable, mutant protein or wild type protein. Biopsy-proven amyloidosis of the GI tract is rare.4 About 60% of patients with AA amyloidosis and 8% with AL amyloidosis have GI involvement.5
We present a case of nonspecific symptoms that ultimately lined up perfectly with the official histologic confirmation of intestinal amyloidosis.
Case Presentation
A 79-year-old man with a history of type 2 diabetes mellitus, congestive heart failure, hyperlipidemia, obstructive sleep apnea, hypothyroidism, hypertension, coronary artery disease status postcoronary artery bypass grafting, and stent placements presented for 3 episodes of large, bright red bowel movements. He reported past bleeding and straining with stools, but bleeding of this amount had not been noted prior. He also reported dry heaves, lower abdominal pain, constipation with straining, early satiety with dysphagia, weakness, and decreased appetite. Lastly, he mentioned intentionally losing about 35 to 40 pounds in the past 3 to 4 months and over the past several months increased abdominal distention. However, he stated he had no history of alcohol misuse, liver or intestinal disease, cirrhosis, or other autoimmune diseases. His most recent colonoscopy was more than a decade prior and showed no acute process. The patient never had an esophagogastroduodenoscopy (EGD).
On initial presentation, the patient’s vital signs showed no acute findings. His physical examination noted a chronically ill–appearing male with decreased breath sounds to the bases bilaterally and noted abdominal distention with mild generalized tenderness. Laboratory findings were significant for a hemoglobin level, 9.4 g/dL (reference range, 11.6-15.3); iron, 23 ug/dL (reference range, 45-160); transferrin saturation, 8% (reference range, 15-50); ferritin level, 80 ng/mL (reference range, 30-300); and carcinoembryonic antigen level, 1.5 ng/mL (reference range, 0-2.9). Aspartate aminotransferase level was 54 IU/L (reference range, 0-40); alanine transaminase, 24 IU/L (reference range, 7-52); albumin, 2.7 g/dL (reference range, 3.4-5.7); international normalized ratio, 1.3 (reference range, 0-1.1); creatinine, 1.74 mg/dL (reference range, 0.44-1.27); alkaline phosphatase, 369 IU/L (reference range, 39-117). White blood cell count was 15.5 × 109/L (reference range, 3.5-10.3), and lactic acid was 2.5 mmol/L (reference range, 0.5-2.2). He was started on piperacillin/tazobactam in the emergency department and transitioned to ciprofloxacin and metronidazole for presumed intra-abdominal infection. Paracentesis showed a serum ascites albumin gradient of > 1.1 g/dL with no signs of spontaneous bacterial peritonitis. Computed tomography of the abdomen and pelvis with contrast was suspicious for colitis involving the proximal colon, and colonic mass could not be excluded. Also noted was hepatosplenomegaly with abdominopelvic ascites.
Based on these findings, an EGD and colonoscopy were done. The EGD showed mild portal hypertensive gastropathy.
After the biopsy results, the patient was officially diagnosed with intestinal amyloidosis (Figure 2).
He returned to the gastroenterology clinic 2 months later. At that point, he had worsening symptoms, liver function test results, and international normalized ratio. He was admitted for further investigation. A bone biopsy was done to confirm the histology and define the underlying disorder. The biopsy returned showing Waldenstrom macroglobulinemia, and he was started on bortezomib. Unfortunately, his clinical status rapidly worsened, leading to acute renal and hepatic failure and the development of encephalopathy. He eventually died under palliative care services.
Discussion
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are several variations of amyloid, but the most common type is AL amyloidosis, which affects several organs, including the heart, kidney, liver, nervous system, and GI tract. When AL amyloidosis involves the liver, the median survival time is about 8.5 months.6 There are different ways to diagnose the disease, but a tissue biopsy and Congo Red staining can confirm specific organ involvement as seen in our case.
This case adds another layer to our constantly expanding differential as health care practitioners and proves that atypical patient presentations may not be atypical after all. GI amyloidosis tends to present similarly to our patient with bleeding, malabsorption, dysmotility, and protein-losing gastroenteropathy as ascites, edema, pericardial effusions, and laboratory evidence of hypoalbuminemia.7 Because amyloidosis is a systemic illness, early recognition is important as intestinal complications tend to present as symptoms, but mortality is more often caused by renal failure, cardiomyopathy, or ischemic heart disease, making early multispecialty involvement very important.8
Conclusions
Health care practitioners in all specialties should be aware of and include intestinal amyloidosis in their differential diagnosis when working up GI bleeds with the hope of identifying the disease early. With early recognition, rapid biopsy identification, and early specialist involvement, patients will get the opportunity for expedited multidisciplinary treatment and potentially delay rapid decompensation as shown by the evidence in this case.
1. Antunes C, Copelin II EL. Upper gastrointestinal bleeding. StatPearls [internet]. Updated July 18, 2022. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470300
2. Almaghrabi M, Gandhi M, Guizzetti L, et al. Comparison of risk scores for lower gastrointestinal bleeding: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(5):e2214253. doi:10.1001/jamanetworkopen.2022.14253
3. Pepys MB. Pathogenesis, diagnosis and treatment of systemic amyloidosis. Philos Trans R Soc Lond B Biol Sci. 2001;356(1406):203-211. doi:10.1098/rstb.2000.0766
4. Cowan AJ, Skinner M, Seldin DC, et al. Amyloidosis of the gastrointestinal tract: a 13-year, single-center, referral experience. Haematologica. 2013;98(1):141-146. doi:10.3324/haematol.2012.068155
5. Lee BS, Chudasama Y, Chen AI, Lim BS, Taira MT. Colonoscopy leading to the diagnosis of AL amyloidosis in the gastrointestinal tract mimicking an acute ulcerative colitis flare. ACG Case Rep J. 2019;6(11):e00289. doi:10.14309/crj.0000000000000289
6. Zhao L, Ren G, Guo J, Chen W, Xu W, Huang X. The clinical features and outcomes of systemic light chain amyloidosis with hepatic involvement. Ann Med. 2022;54(1):1226-1232. doi:10.1080/07853890.2022.2069281
7. Rowe K, Pankow J, Nehme F, Salyers W. Gastrointestinal amyloidosis: review of the literature. Cureus. 2017;9(5):e1228. doi:10.7759/cureus.1228
8. Kyle RA, Greipp PR, O’Fallon WM. Primary systemic amyloidosis: multivariate analysis for prognostic factors in 168 cases. Blood. 1986;68(1):220-224.
1. Antunes C, Copelin II EL. Upper gastrointestinal bleeding. StatPearls [internet]. Updated July 18, 2022. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470300
2. Almaghrabi M, Gandhi M, Guizzetti L, et al. Comparison of risk scores for lower gastrointestinal bleeding: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(5):e2214253. doi:10.1001/jamanetworkopen.2022.14253
3. Pepys MB. Pathogenesis, diagnosis and treatment of systemic amyloidosis. Philos Trans R Soc Lond B Biol Sci. 2001;356(1406):203-211. doi:10.1098/rstb.2000.0766
4. Cowan AJ, Skinner M, Seldin DC, et al. Amyloidosis of the gastrointestinal tract: a 13-year, single-center, referral experience. Haematologica. 2013;98(1):141-146. doi:10.3324/haematol.2012.068155
5. Lee BS, Chudasama Y, Chen AI, Lim BS, Taira MT. Colonoscopy leading to the diagnosis of AL amyloidosis in the gastrointestinal tract mimicking an acute ulcerative colitis flare. ACG Case Rep J. 2019;6(11):e00289. doi:10.14309/crj.0000000000000289
6. Zhao L, Ren G, Guo J, Chen W, Xu W, Huang X. The clinical features and outcomes of systemic light chain amyloidosis with hepatic involvement. Ann Med. 2022;54(1):1226-1232. doi:10.1080/07853890.2022.2069281
7. Rowe K, Pankow J, Nehme F, Salyers W. Gastrointestinal amyloidosis: review of the literature. Cureus. 2017;9(5):e1228. doi:10.7759/cureus.1228
8. Kyle RA, Greipp PR, O’Fallon WM. Primary systemic amyloidosis: multivariate analysis for prognostic factors in 168 cases. Blood. 1986;68(1):220-224.