User login
CAPO Aspiration Pneumonia
Pneumonia is a common clinical syndrome with well‐described epidemiology and microbiology. Aspiration pneumonia comprises 5% to 15% of patients with pneumonia acquired outside of the hospital,[1] but is less well characterized despite being a major syndrome of pneumonia in the elderly.[2, 3] Difficulties in studying aspiration pneumonia include the lack of a sensitive and specific marker for aspiration as well as the potential overlap between aspiration pneumonia and other forms of pneumonia.[4, 5, 6] Additionally, clinicians have difficulty distinguishing between aspiration pneumonia, which develops after the aspiration of oropharyngeal contents, and aspiration pneumonitis, wherein inhalation of gastric contents causes inflammation without the subsequent development of bacterial infection.[7, 8] Central to the study of aspiration pneumonia is whether it should exist as its own entity, or if aspiration is really a designation used for pneumonia in an older patient with greater comorbidities. The ability to clearly understand how a clinician diagnoses aspiration pneumonia, and whether that method has face validity with expert definitions may allow for improved future research, improved generalizability of current or past research, and possibly better clinical care.
Several validated mortality prediction models exist for community‐acquired pneumonia (CAP) using a variety of clinical predictors, but their performance in patients with aspiration pneumonia is less well characterized. Most studies validating pneumonia severity scoring systems excluded aspiration pneumonia from their study population.[9, 10, 11] Severity scoring systems for CAP may not accurately predict disease severity in patients with aspiration pneumonia. The CURB‐65[9] (confusion, uremia, respiratory rate, blood pressure, age 65 years) and the eCURB[12] scoring systems are poor predictors of mortality in patients with aspiration pneumonia, perhaps because they do not account for patient comorbidities.[13] The pneumonia severity index (PSI)[10] might predict mortality better than CURB‐65 in the aspiration population due to the inclusion of comorbidities.
Previous studies have demonstrated that patients with aspiration pneumonia are older and have greater disease severity and more comorbidities.[13, 14, 15] These single‐center studies also demonstrated greater mortality, more frequent admission to an intensive care unit (ICU), and longer hospital lengths of stay in patients with aspiration pneumonia. These studies identified aspiration pneumonia by the presence of a risk factor for aspiration[15] or by physician billing codes.[13] In practice, however, the bedside clinician diagnoses a patient as having aspiration pneumonia, but the logic is likely vague and inconsistent. Despite the potential for variability with individual judgment, an aggregate estimation from independent judgments may perform better than individual judgments.[16] Because there is no gold standard for defining aspiration pneumonia, all previous research has been limited to definitions created by investigators. This multicenter study seeks to determine what clinical characteristics lead physicians to diagnose a patient as having aspiration pneumonia, and whether or not the clinician‐derived diagnosis is distinct and clinically useful.
Our objectives were to: (1) identify covariates associated with bedside clinicians diagnosing a pneumonia patient as having aspiration pneumonia; (2) compare aspiration pneumonia and nonaspiration pneumonia in regard to disease severity, patient demographics, comorbidities, and clinical outcomes; and (3) measure the performance of the PSI in aspiration pneumonia versus nonaspiration pneumonia.
PATIENTS AND METHODS
Study Design and Setting
We performed a secondary analysis of the Community‐Acquired Pneumonia Organization (CAPO) database, which contains retrospectively collected data from 71 hospitals in 16 countries between June 2001 and December 2012. In each participating center, primary investigators selected nonconsecutive, adult hospitalized patients diagnosed with CAP. To decrease systematic selection biases, the selection of patients with CAP for enrollment in the trial was based on the date of hospital admission. Each investigator completed a case report form that was transferred via the internet to the CAPO study center at the University of Louisville (Louisville, KY). A sample of the data collection form is available at the study website (
Inclusion and Exclusion Criteria
Patients 18 years of age and satisfying criteria for CAP were included in this study. A diagnosis of CAP required a new pulmonary infiltrate at time of hospitalization, and at least 1 of the following: new or increased cough; leukocytosis; leukopenia, or left shift pattern on white blood cell count; and temperature >37.8C or <35.6 C. We excluded patients with pneumonia attributed to mycobacterial or fungal infection, and patients infected with human immunodeficiency virus, as we believed these types of pneumonia differ fundamentally from typical CAP.
Patient Variables
Patient variables included presence of aspiration pneumonia, laboratory data, comorbidities, and measures of disease severity, including the PSI. The clinician made a clinical diagnosis of the presence or absence of aspiration for each patient by marking a box on the case report form. Outcomes included in‐hospital mortality, hospital length of stay up to 14 days, and time to clinical stability up to 8 days. All variables were obtained directly from the case report form. In accordance with previously published definitions, we defined clinical stability as the day the following criteria were all met: improved clinical signs (improved cough and shortness of breath), lack of fever for >8 hours, improving leukocytosis (decreased at least 10% from the previous day), and tolerating oral intake.[17, 18]
Statistical Analysis
Baseline characteristics of patients with aspiration and nonaspiration CAP were compared using 2 or Fisher exact tests for categorical variables and the Mann‐Whitney U test for continuous variables.
To determine which patient variables were important in the physician diagnosis of aspiration pneumonia, we performed logistic regression with initial covariates comprising the demographic, comorbidity, and disease severity measurements listed in Table 1. We included interactions between cerebrovascular disease and age, nursing home status, and confusion to improve model fit. We centered all variables (including binary indicators) according to the method outlined by Kraemer and Blasey to improve interpretation of the main effects.[19]
| Aspiration Pneumonia, N=451 | Nonaspiration Pneumonia, N=4,734 | P Value | |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 79 (6587) | 69 (5380) | <0.001 |
| % Male | 59% | 60% | 0.58 |
| Nursing home residence | 25% | 5% | <0.001 |
| Recent (30 days) antibiotic use | 21% | 16% | 0.017 |
| Comorbidities | |||
| Cerebrovascular disease | 35% | 14% | <0.001 |
| Chronic obstructive pulmonary disease | 25% | 27% | 0.62 |
| Congestive heart failure | 23% | 19% | 0.027 |
| Diabetes | 18% | 18% | 0.85 |
| Cancer | 12% | 10% | 0.12 |
| Renal disease | 10% | 11% | 0.53 |
| Liver disease | 6% | 5% | 0.29 |
| Disease severity | |||
| Pneumonia severity index | 123 (99153) | 92 (68117) | <0.001 |
| Confusion | 49% | 12% | <0.001 |
| PaO2 <60 mm Hg | 43% | 33% | <0.001 |
| BUN >30 g/dL | 42% | 23% | <0.001 |
| Multilobar pneumonia | 34% | 28% | 0.003 |
| Pleural effusion | 25% | 21% | 0.07 |
| Respiratory rate >30 breaths/minute | 21% | 20% | 0.95 |
| pH <7.35 | 13% | 5% | <0.001 |
| Hematocrit <30% | 11% | 6% | 0.001 |
| Temperature >37.8C or <35.6C | 9% | 7% | 0.30 |
| Systolic blood pressure <90 mm Hg | 8% | 9% | 0.003 |
| Sodium <130 mEq/L | 8% | 6% | 0.08 |
| Heart rate >125 beats/minute | 8% | 5% | 0.71 |
| Glucose >250 mg/dL | 6% | 7% | 0.06 |
| Cavitary lesion | 0% | 0% | 0.67 |
| Clinical outcomes | |||
| In‐hospital mortality | 23% | 9% | <0.001 |
| Intensive care unit admission | 19% | 13% | 0.002 |
| Hospital length of stay, d | 9 (515) | 7 (412) | <0.001 |
| Time to clinical stability, d | 8 (48) | 4 (38) | <0.001 |
To determine if aspiration pneumonia had worse clinical outcomes compared to nonaspiration pneumonia, multiple methods were used. To compare the differences between the 2 groups with respect to time to clinical stability and length of hospital stay, we constructed Kaplan‐Meier survival curves and Cox proportional hazards regression models. The log‐rank test was used to determine statistical differences between the Kaplan‐Meier survival curves. To compare the impact of aspiration on mortality in patients with CAP, we conducted a propensity scorematched analysis. We chose propensity score matching over traditional logistic regression to balance variables among groups and to avoid the potential for overfit and multicollinearity. We considered a variable balanced after matching if its standardized difference was <10. All variables in the propensity scorematched analysis were balanced.
Although our dataset contained minimal missing data, we imputed any missing values to maintain the full study population in the creation of the propensity score. Missing data were imputed using the aregImpute function of the hmisc package of R (The R Foundation for Statistical Computing, Vienna, Austria).[20, 21] We built the propensity score model using a variable selection algorithm described by Bursac et al.[22] Our model included variables for region (United States/Canada, Europe, Asia/Africa or Latin America) and the variables listed in Table 1, with the exception of the PSI and the 4 clinical outcomes. Given that previous analyses accounting for clustering by physician did not substantially affect our results,[23] our model did not include physician‐level variables and did not account for the clustering effects of physicians. Using the propensity scores generated from this model, we matched a case of aspiration CAP with a case of nonaspiration CAP.[24] We then constructed a general linear model using the matched dataset to obtain the magnitude of effect of aspiration on mortality.
We used receiver operating characteristic curves to define the diagnostic accuracy of the pneumonia severity index for the prediction of mortality among patients with aspiration pneumonia and those with nonaspiration pneumonia. SAS version 9.3 (SAS Institute, Cary, NC) and R version 2.15.3 (The R Foundation for Statistical Computing) were used for all analyses. P values of 0.05 were considered statistically significant in all analyses.
RESULTS
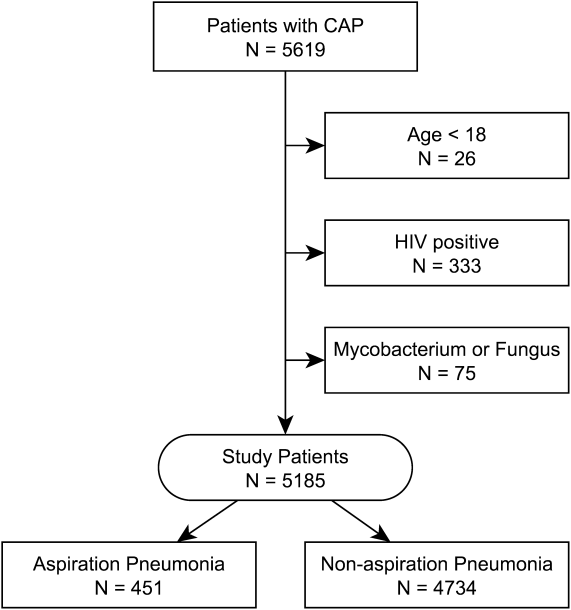
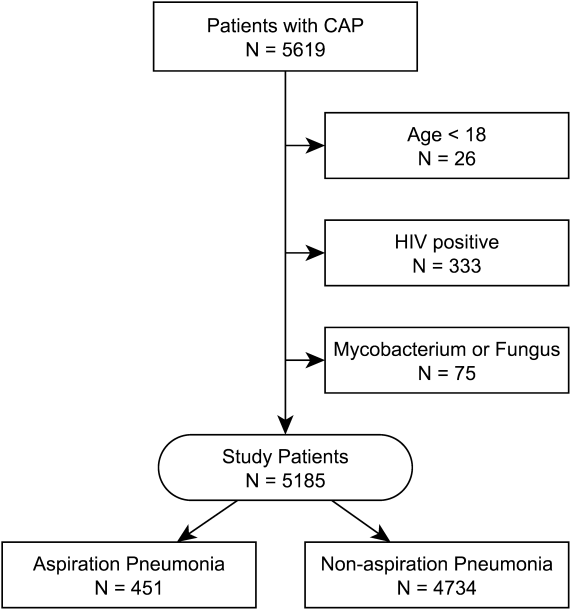
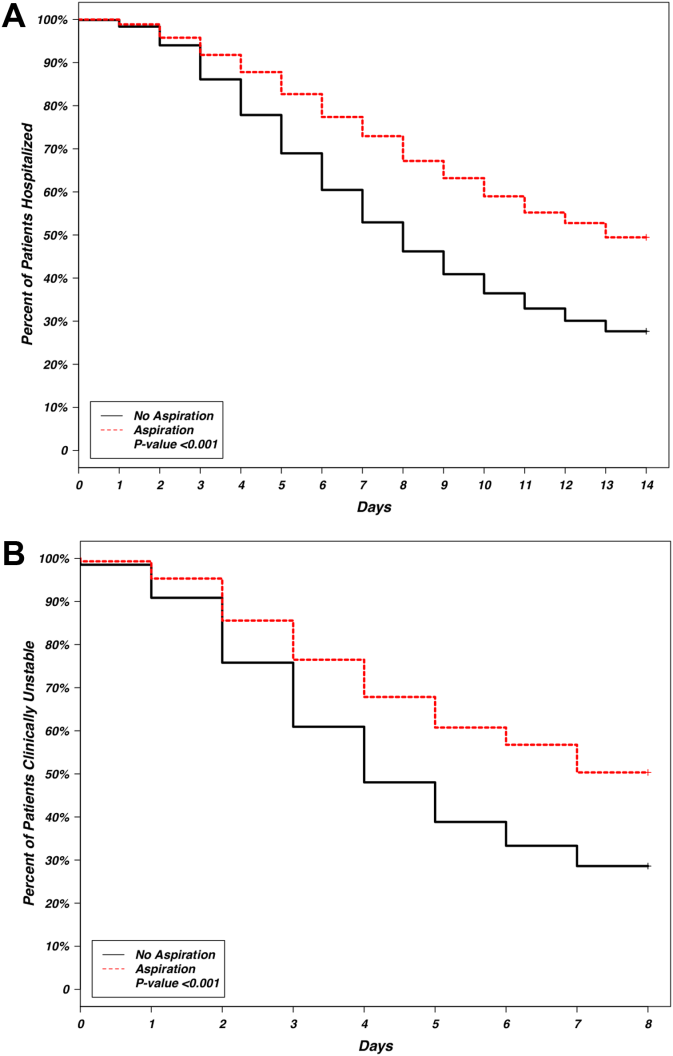
Our initial query, after exclusion criteria, yielded a study population of 5185 patients (Figure 1). We compared 451 patients diagnosed with aspiration pneumonia to 4734 with CAP (Figure 1). Patient characteristics are summarized in Table 1. Patients with aspiration pneumonia were older, more likely to live in a nursing home, had greater disease severity, and were more likely to be admitted to an ICU. Patients with aspiration pneumonia had longer adjusted hospital lengths of stay and took more days to achieve clinical stability than patients with nonaspiration pneumonia (Figure 2). After adjusting for all variables in Table 1, the Cox proportional hazards models demonstrated that aspiration pneumonia was associated with ongoing hospitalization (hazard ratio [HR] for discharge: 0.77, 95% confidence interval [CI]: 0.65‐0.91, P=0.002) and clinical instability (HR for attaining clinical stability: 0.72, 95% CI: 0.61‐0.84, P<0.001). Patients with aspiration pneumonia presented with greater disease severity than those with nonaspiration pneumonia. Although there was no difference between groups in regard to temperature, respiratory rate, hyponatremia, or presence of pleural effusions or cavitary lesions, all other measured indices of disease severity were worse in patients with aspiration pneumonia. Patients with aspiration pneumonia were more likely to have cerebrovascular disease than those with nonaspiration pneumonia. Aspiration pneumonia patients also had increased prevalence of congestive heart failure. There was no appreciable difference between groups among other measured comorbidities.
The patient characteristics most associated with a physician diagnosis of aspiration pneumonia, identified using logistic regression, were confusion, residence in nursing home, and presence of cerebrovascular disease (odds ratio [OR]: of 4.4, 2.9, and 2.3, respectively), whereas renal disease was associated with decreased physician diagnosis of aspiration pneumonia over nonaspiration pneumonia (OR: 0.58) (Table 2).
| Covariate | Odds Ratio | 95% Confidence Intervals | P Value |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 1.00 | 0.991.01 | 0.948 |
| Male | 1.20 | 0.941.54 | 0.148 |
| Nursing home residence | 2.93 | 2.134.00 | <0.001 |
| Comorbidities | |||
| Cerebrovascular disease | 2.26 | 1.533.32 | <0.001 |
| Renal disease | 0.58 | 0.390.85 | 0.006 |
| Disease severity | |||
| Confusion | 4.41 | 3.405.72 | <0.001 |
| Hematocrit <30% | 1.59 | 1.062.33 | 0.020 |
| pH <7.35 | 1.67 | 1.102.47 | 0.013 |
| Temperature >37.8C or <35.6C | 1.60 | 1.072.35 | 0.019 |
| Multilobar pneumonia | 1.29 | 1.001.65 | 0.047 |
| Interaction terms | |||
| Age * cerebrovascular disease | 0.98 | 0.960.99 | 0.011 |
| Nursing home * cerebrovascular disease | 0.51 | 0.270.96 | 0.037 |
| Confusion * cerebrovascular disease | 0.70 | 0.421.17 | 0.175 |
Observed in‐patient mortality of aspiration pneumonia was 23%. This mortality was considerably higher than a mean PSI score of 123 would predict (class IV risk group, with expected 30‐day mortality of 8%9%[25]). The PSI score's ability to predict inpatient mortality in patients with aspiration pneumonia was moderate, with an area under the curve (AUC) of 0.71. This was similar to its performance in patients with nonaspiration pneumonia (AUC of 0.75) (Figure 3). These values are lower than the AUC of 0.81 for the PSI in predicting mortality derived from a meta‐analysis of 31 other studies.[26]
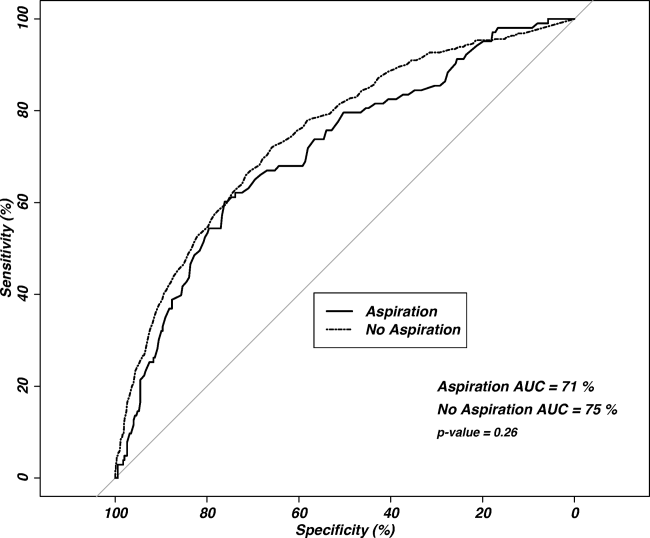
Our regression model after propensity score matching demonstrated that aspiration pneumonia independently confers a 2.3‐fold increased odds for inpatient mortality (95% CI: 1.56‐3.45, P<0.001).
DISCUSSION
Pneumonia patients with confusion, nursing home residence, or cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Although this is unsurprising, it is notable that these patients are more than twice as likely to die in the inpatient setting, even after accounting for age, comorbidities, and disease severity. These findings are similar to three previously published studies comparing aspiration and nonaspiration pneumonia at single institutions, albeit using different aspiration pneumonia definitions.[13, 14, 15] This study is the first large, multicenter, multinational study to demonstrate these findings.
Central to the interpretation of our results is the method of diagnosing aspiration versus nonaspiration. A bottom‐up method that relies on a clinician to check a box for aspiration may appear poorly reproducible. Because there is no diagnostic gold standard, clinicians may use different criteria to diagnose aspiration, creating potential for idiosyncratic noise. The strength of the wisdom of the crowd method used in this study is that an aggregate estimation from independent judgments may reduce the noise from individual judgments.[16] Although clinicians may vary in why they diagnose a particular patient as having aspiration pneumonia, it appears that the overwhelming reason for diagnosing a patient as having aspiration pneumonia is the presence of confusion, followed by previous nursing home residence or cerebrovascular disease. This finding has some face validity when compared with studies using an investigator definition, as altered mental status, chronic debility, and cerebrovascular disease are either prominent features of the definition of aspiration pneumonia[8] or frequently observed in patients with aspiration pneumonia.[13, 15] The distribution of cerebrovascular disease among our study's aspiration and nonaspiration pneumonia patients was similar to studies that used formal criteria in their definitions.[13, 15] Although nursing home residence was more likely in aspiration pneumonia patients, the majority of aspiration pneumonia patients were residing in the community, suggesting that aspiration is not simply a surrogate for healthcare‐associated pneumonia. Although patients with aspiration pneumonia are typically older than their nonaspiration counterparts, it appears that age is not a key determinant in the diagnosis of aspiration. With aspiration pneumonia, confusion, nursing home residence, and the presence of cerebrovascular disease are the greatest contributors in the clinical diagnosis, more than age.
Our data demonstrate that aspiration pneumonia confers increased odds for mortality, even after adjustment for age, disease severity, and comorbidities. These data suggest that aspiration pneumonia is a distinct entity from nonaspiration pneumonia, and that this disease is worse than nonaspiration CAP. If aspiration pneumonia is distinct from nonaspiration pneumonia, some unrecognized host factor other than age, disease severity, or the captured comorbidities decreases survival in aspiration pneumonia patients. However, it is also possible that aspiration pneumonia is merely a clinical designation for one end of the pneumonia spectrum, and we and others have failed to completely account for all measures of disease severity or all measures of comorbidities. Examples of unmeasured comorbidities would include presence of oropharyngeal dysphagia, which is not assessed in the database but could have a significant effect on clinical diagnosis. Unmeasured covariates can include measures beyond that of disease severity or comorbidity, such as the presence of a do not resuscitate (DNR) order, which could have a significant confounding effect on the observed association. A previous, single‐center study demonstrated that increased 30‐day mortality in aspiration pneumonia was mostly attributable to greater disease severity and comorbidities, although aspiration pneumonia independently conferred greater risk for adverse long‐term outcomes.[15] We propose that aspiration pneumonia represents a clinically distinct entity from nonaspiration pneumonia. Patients with chronic aspiration are often chronically malnourished and may have different oral flora than patients without chronic aspiration.[27, 28] Chronic aspiration has been associated with granulomatous reaction, organizing pneumonia, diffuse alveolar damage, and chronic bronchiolitis.[29] Chronic aspiration may elicit changes in the host physiology, and may render the host more susceptible to the development of secondary bacterial infection with morbid consequences.
The ability of the PSI to predict inpatient mortality was moderate (AUC only 0.7), with no significant additional discrimination between the aspiration and nonaspiration pneumonia groups. Although the PSI had moderate ability to predict inpatient mortality, the observed mortality was considerably higher than predicted. It is possible that the PSI incompletely captures clinically relevant comorbidities (eg, malnutrition). Further study to improve mortality prediction of aspiration pneumonia patients could employ sensitivity analysis to determine optimal thresholds and weighting of the PSI components.
Patients with aspiration pneumonia had longer hospital lengths of stay and took longer to achieve clinical stability than their nonaspiration counterparts. Time to clinical stability has been associated with increased posthospitalization mortality and is associated with time to switch from intravenous to oral antibiotics.[17] Although some component of hospital length‐of‐stay is subject to local practice patterns, time to clinical stability has explicit criteria for clinical improvement and failure, and therefore is less likely to be affected by local practice patterns.
We noted a relatively high (16%21%) incidence of prior antibiotic use among patients in this database. Analysis of antibiotic prescription patterns was limited, given the several different countries from which the database draws its cases. Although we used accepted criteria to define CAP cases, it is possible that this population may have a higher rate of resistant or uncommon pathogens than other studies of CAP that have populations with lower incidence of prior antibiotic use. Although not assessed, we suspect a significant component of the prior antibiotic use represented outpatient pneumonia treatment during the few days prior to visiting the hospital.
This study has several limitations, of which the most important may be that we used clinical determination for defining presence of aspiration pneumonia. This method is susceptible to the subjective perceptions of the treating clinician. We did not account for the effect of individual physicians in our model, although we did adjust for regional differences. The retrospective identification of patients allows for the possibility of selection bias, and therefore we have not attempted to make inferences regarding the relative incidence of pneumonia, nor did we adjust for temporal trends in diagnosis. The ratio of aspiration pneumonia patients to nonaspiration pneumonia patients may not necessarily reflect that observed in reality. Microbiologic and antibiotic data were unavailable for analysis. This study cannot inform on nonhospitalized patients with aspiration pneumonia, as only hospitalized patients were enrolled. The database identified cases of pneumonia, so it is possible for a patient to enter into the database more than once. Detection of mortality was limited to the inpatient setting rather than a set interval of 30 days. Inpatient mortality depends on length‐of‐stay patterns that may bias the mortality endpoint.[30] Also not assessed was the presence of a DNR order. It is possible that an older patient with greater comorbidities and disease severity may have care intentionally limited or withdrawn early by the family or clinicians.
Strengths of the study include its size and its multicenter, multinational population. The CAPO database is a large and well‐described population of patients with CAP.[17, 31] These attributes, as well as the clinician‐determined diagnosis, increase the generalizability of the study compared to a single‐center, single‐country study that employs investigator‐defined criteria.
CONCLUSION
Pneumonia patients with confusion, who are nursing home residence, and have cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Our clinician‐diagnosed cohort appears similar to those derived using an investigator definition. Patients with aspiration pneumonia are older, and have greater disease severity and more comorbidities than patients with nonaspiration pneumonia. They have greater mortality than their PSI score class would predict. Even after accounting for age, disease severity, and comorbidities, the presence of aspiration pneumonia independently conferred a greater than 2‐fold increase in inpatient mortality. These findings together suggest that aspiration pneumonia should be considered a distinct entity from typical pneumonia, and that additional research should be done in this field.
ACKNOWLEDGMENTS
Disclosures: M.J.L. contributed to the study design, data analysis, statistical analysis, and writing of the manuscript. P.P. contributed to the study design and revision of the manuscript for important intellectual content. T.W. and E.W. contributed to the study design, statistical analysis, and revision of the manuscript for important intellectual content. J.A.R. and N.C.D. contributed to the study design and revision of the manuscript for important intellectual content. All authors read and approved the final manuscript. M.L. takes responsibility for the integrity of the work as a whole, from inception to published article. This investigation was partly supported with funding from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (grant 8UL1TR000105 [formerly UL1RR025764]). The authors report no conflicts of interest.
- , , , et al. Severe community‐acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis. 1991;144(2):312–318.
- , , . Risk factors for pneumonia in the elderly. Am J Med. 1994;96(4):313–320.
- , . Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328–336.
- , , . Pneumonia versus aspiration pneumonitis in nursing home residents: diagnosis and management. J Am Geriatr Soc. 2003;51(1):17–23.
- . Aspiration pneumonia: mixing apples with oranges and tangerines. Crit Care Med. 2004;32(5):1236; author reply 1236–1237.
- , , , , . Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999–2000. Crit Care Med. 2003;31(7):1930–1937.
- . Aspiration syndromes: aspiration pneumonia and pneumonitis. Hosp Pract (Minneap). 2010;38(1):35–42.
- . Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–671.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382.
- , , , et al. Comparison of a disease‐specific and a generic severity of illness measure for patients with community‐acquired pneumonia. J Gen Intern Med. 1995;10(7):359–368.
- , , , et al. Development and validation of a clinical prediction rule for severe community‐acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–1256.
- , , , et al. CURB‐65 pneumonia severity assessment adapted for electronic decision support. Chest. 2011;140(1):156–163.
- , , , . Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83–90.
- , , , , . Pneumonia Severity Index (PSI), CURB‐65, and mortality in hospitalized elderly patients with aspiration pneumonia [in German]. Z Gerontol Geriatr. 2011;44(4):229–234.
- , , , , . Risk factors for aspiration in community‐acquired pneumonia: analysis of a hospitalized UK cohort. Am J Med. 2013;126(11):995–1001.
- , , , . The wisdom of the crowd in combinatorial problems. Cogn Sci. 2012;36(3):452–470.
- , , , et al. Association between time to clinical stability and outcomes after discharge in hospitalized patients with community‐acquired pneumonia. Chest. 2011;140(2):482–488.
- . Clinical stability and switch therapy in hospitalised patients with community‐acquired pneumonia: are we there yet? Eur Respir J. 2013;41(1):5–6.
- , . Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13(3):141–151.
- . Hmisc: Harrell miscellaneous. Available at: http://CRAN.R‐project.org/package=Hmisc. Published Sept 12, 2014. Last accessed Oct 27, 2014.
- , . Multiple imputation for the fatal accident reporting system. J R Stat Soc Ser C Appl Stat. 1991;40(1):13–29.
- , , , . Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17.
- , , , , ; CAPO authors. Mortality differences among hospitalized patients with community‐acquired pneumonia in three world regions: results from the Community‐Acquired Pneumonia Organization (CAPO) International Cohort Study. Respir Med. 2013;107(7):1101–1111.
- . Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. In: Proceedings of the 26th Annual SAS Users Group International Conference. Cary, NC: SAS Institute Inc.; 2001:214–226. Available at: http://www2.sas.com/proceedings/sugi26/p214–26.pdf. Last accessed Oct 27, 2014.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336(4):243–250.
- , , , et al. Severity assessment tools for predicting mortality in hospitalised patients with community‐acquired pneumonia. Systematic review and meta‐analysis. Thorax. 2010;65(10):878–883.
- , , , , , . Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2010;39(1):39–45.
- , . The association between oral microorgansims and aspiration pneumonia in the institutionalized elderly: review and recommendations. Dysphagia. 2010;25(4):307–322.
- , . Histopathology of aspiration pneumonia not associated with food or other particulate matter: a clinicopathologic study of 10 cases diagnosed on biopsy. Am J Surg Pathol. 2011;35(3):426–431.
- , , , , , . Interpreting hospital mortality data. The role of clinical risk adjustment. JAMA. 1988;260(24):3611–3616.
- , , , . Hospitalization for community‐acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124(1):121–124.
Pneumonia is a common clinical syndrome with well‐described epidemiology and microbiology. Aspiration pneumonia comprises 5% to 15% of patients with pneumonia acquired outside of the hospital,[1] but is less well characterized despite being a major syndrome of pneumonia in the elderly.[2, 3] Difficulties in studying aspiration pneumonia include the lack of a sensitive and specific marker for aspiration as well as the potential overlap between aspiration pneumonia and other forms of pneumonia.[4, 5, 6] Additionally, clinicians have difficulty distinguishing between aspiration pneumonia, which develops after the aspiration of oropharyngeal contents, and aspiration pneumonitis, wherein inhalation of gastric contents causes inflammation without the subsequent development of bacterial infection.[7, 8] Central to the study of aspiration pneumonia is whether it should exist as its own entity, or if aspiration is really a designation used for pneumonia in an older patient with greater comorbidities. The ability to clearly understand how a clinician diagnoses aspiration pneumonia, and whether that method has face validity with expert definitions may allow for improved future research, improved generalizability of current or past research, and possibly better clinical care.
Several validated mortality prediction models exist for community‐acquired pneumonia (CAP) using a variety of clinical predictors, but their performance in patients with aspiration pneumonia is less well characterized. Most studies validating pneumonia severity scoring systems excluded aspiration pneumonia from their study population.[9, 10, 11] Severity scoring systems for CAP may not accurately predict disease severity in patients with aspiration pneumonia. The CURB‐65[9] (confusion, uremia, respiratory rate, blood pressure, age 65 years) and the eCURB[12] scoring systems are poor predictors of mortality in patients with aspiration pneumonia, perhaps because they do not account for patient comorbidities.[13] The pneumonia severity index (PSI)[10] might predict mortality better than CURB‐65 in the aspiration population due to the inclusion of comorbidities.
Previous studies have demonstrated that patients with aspiration pneumonia are older and have greater disease severity and more comorbidities.[13, 14, 15] These single‐center studies also demonstrated greater mortality, more frequent admission to an intensive care unit (ICU), and longer hospital lengths of stay in patients with aspiration pneumonia. These studies identified aspiration pneumonia by the presence of a risk factor for aspiration[15] or by physician billing codes.[13] In practice, however, the bedside clinician diagnoses a patient as having aspiration pneumonia, but the logic is likely vague and inconsistent. Despite the potential for variability with individual judgment, an aggregate estimation from independent judgments may perform better than individual judgments.[16] Because there is no gold standard for defining aspiration pneumonia, all previous research has been limited to definitions created by investigators. This multicenter study seeks to determine what clinical characteristics lead physicians to diagnose a patient as having aspiration pneumonia, and whether or not the clinician‐derived diagnosis is distinct and clinically useful.
Our objectives were to: (1) identify covariates associated with bedside clinicians diagnosing a pneumonia patient as having aspiration pneumonia; (2) compare aspiration pneumonia and nonaspiration pneumonia in regard to disease severity, patient demographics, comorbidities, and clinical outcomes; and (3) measure the performance of the PSI in aspiration pneumonia versus nonaspiration pneumonia.
PATIENTS AND METHODS
Study Design and Setting
We performed a secondary analysis of the Community‐Acquired Pneumonia Organization (CAPO) database, which contains retrospectively collected data from 71 hospitals in 16 countries between June 2001 and December 2012. In each participating center, primary investigators selected nonconsecutive, adult hospitalized patients diagnosed with CAP. To decrease systematic selection biases, the selection of patients with CAP for enrollment in the trial was based on the date of hospital admission. Each investigator completed a case report form that was transferred via the internet to the CAPO study center at the University of Louisville (Louisville, KY). A sample of the data collection form is available at the study website (
Inclusion and Exclusion Criteria
Patients 18 years of age and satisfying criteria for CAP were included in this study. A diagnosis of CAP required a new pulmonary infiltrate at time of hospitalization, and at least 1 of the following: new or increased cough; leukocytosis; leukopenia, or left shift pattern on white blood cell count; and temperature >37.8C or <35.6 C. We excluded patients with pneumonia attributed to mycobacterial or fungal infection, and patients infected with human immunodeficiency virus, as we believed these types of pneumonia differ fundamentally from typical CAP.
Patient Variables
Patient variables included presence of aspiration pneumonia, laboratory data, comorbidities, and measures of disease severity, including the PSI. The clinician made a clinical diagnosis of the presence or absence of aspiration for each patient by marking a box on the case report form. Outcomes included in‐hospital mortality, hospital length of stay up to 14 days, and time to clinical stability up to 8 days. All variables were obtained directly from the case report form. In accordance with previously published definitions, we defined clinical stability as the day the following criteria were all met: improved clinical signs (improved cough and shortness of breath), lack of fever for >8 hours, improving leukocytosis (decreased at least 10% from the previous day), and tolerating oral intake.[17, 18]
Statistical Analysis
Baseline characteristics of patients with aspiration and nonaspiration CAP were compared using 2 or Fisher exact tests for categorical variables and the Mann‐Whitney U test for continuous variables.
To determine which patient variables were important in the physician diagnosis of aspiration pneumonia, we performed logistic regression with initial covariates comprising the demographic, comorbidity, and disease severity measurements listed in Table 1. We included interactions between cerebrovascular disease and age, nursing home status, and confusion to improve model fit. We centered all variables (including binary indicators) according to the method outlined by Kraemer and Blasey to improve interpretation of the main effects.[19]
| Aspiration Pneumonia, N=451 | Nonaspiration Pneumonia, N=4,734 | P Value | |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 79 (6587) | 69 (5380) | <0.001 |
| % Male | 59% | 60% | 0.58 |
| Nursing home residence | 25% | 5% | <0.001 |
| Recent (30 days) antibiotic use | 21% | 16% | 0.017 |
| Comorbidities | |||
| Cerebrovascular disease | 35% | 14% | <0.001 |
| Chronic obstructive pulmonary disease | 25% | 27% | 0.62 |
| Congestive heart failure | 23% | 19% | 0.027 |
| Diabetes | 18% | 18% | 0.85 |
| Cancer | 12% | 10% | 0.12 |
| Renal disease | 10% | 11% | 0.53 |
| Liver disease | 6% | 5% | 0.29 |
| Disease severity | |||
| Pneumonia severity index | 123 (99153) | 92 (68117) | <0.001 |
| Confusion | 49% | 12% | <0.001 |
| PaO2 <60 mm Hg | 43% | 33% | <0.001 |
| BUN >30 g/dL | 42% | 23% | <0.001 |
| Multilobar pneumonia | 34% | 28% | 0.003 |
| Pleural effusion | 25% | 21% | 0.07 |
| Respiratory rate >30 breaths/minute | 21% | 20% | 0.95 |
| pH <7.35 | 13% | 5% | <0.001 |
| Hematocrit <30% | 11% | 6% | 0.001 |
| Temperature >37.8C or <35.6C | 9% | 7% | 0.30 |
| Systolic blood pressure <90 mm Hg | 8% | 9% | 0.003 |
| Sodium <130 mEq/L | 8% | 6% | 0.08 |
| Heart rate >125 beats/minute | 8% | 5% | 0.71 |
| Glucose >250 mg/dL | 6% | 7% | 0.06 |
| Cavitary lesion | 0% | 0% | 0.67 |
| Clinical outcomes | |||
| In‐hospital mortality | 23% | 9% | <0.001 |
| Intensive care unit admission | 19% | 13% | 0.002 |
| Hospital length of stay, d | 9 (515) | 7 (412) | <0.001 |
| Time to clinical stability, d | 8 (48) | 4 (38) | <0.001 |
To determine if aspiration pneumonia had worse clinical outcomes compared to nonaspiration pneumonia, multiple methods were used. To compare the differences between the 2 groups with respect to time to clinical stability and length of hospital stay, we constructed Kaplan‐Meier survival curves and Cox proportional hazards regression models. The log‐rank test was used to determine statistical differences between the Kaplan‐Meier survival curves. To compare the impact of aspiration on mortality in patients with CAP, we conducted a propensity scorematched analysis. We chose propensity score matching over traditional logistic regression to balance variables among groups and to avoid the potential for overfit and multicollinearity. We considered a variable balanced after matching if its standardized difference was <10. All variables in the propensity scorematched analysis were balanced.
Although our dataset contained minimal missing data, we imputed any missing values to maintain the full study population in the creation of the propensity score. Missing data were imputed using the aregImpute function of the hmisc package of R (The R Foundation for Statistical Computing, Vienna, Austria).[20, 21] We built the propensity score model using a variable selection algorithm described by Bursac et al.[22] Our model included variables for region (United States/Canada, Europe, Asia/Africa or Latin America) and the variables listed in Table 1, with the exception of the PSI and the 4 clinical outcomes. Given that previous analyses accounting for clustering by physician did not substantially affect our results,[23] our model did not include physician‐level variables and did not account for the clustering effects of physicians. Using the propensity scores generated from this model, we matched a case of aspiration CAP with a case of nonaspiration CAP.[24] We then constructed a general linear model using the matched dataset to obtain the magnitude of effect of aspiration on mortality.
We used receiver operating characteristic curves to define the diagnostic accuracy of the pneumonia severity index for the prediction of mortality among patients with aspiration pneumonia and those with nonaspiration pneumonia. SAS version 9.3 (SAS Institute, Cary, NC) and R version 2.15.3 (The R Foundation for Statistical Computing) were used for all analyses. P values of 0.05 were considered statistically significant in all analyses.
RESULTS
Our initial query, after exclusion criteria, yielded a study population of 5185 patients (Figure 1). We compared 451 patients diagnosed with aspiration pneumonia to 4734 with CAP (Figure 1). Patient characteristics are summarized in Table 1. Patients with aspiration pneumonia were older, more likely to live in a nursing home, had greater disease severity, and were more likely to be admitted to an ICU. Patients with aspiration pneumonia had longer adjusted hospital lengths of stay and took more days to achieve clinical stability than patients with nonaspiration pneumonia (Figure 2). After adjusting for all variables in Table 1, the Cox proportional hazards models demonstrated that aspiration pneumonia was associated with ongoing hospitalization (hazard ratio [HR] for discharge: 0.77, 95% confidence interval [CI]: 0.65‐0.91, P=0.002) and clinical instability (HR for attaining clinical stability: 0.72, 95% CI: 0.61‐0.84, P<0.001). Patients with aspiration pneumonia presented with greater disease severity than those with nonaspiration pneumonia. Although there was no difference between groups in regard to temperature, respiratory rate, hyponatremia, or presence of pleural effusions or cavitary lesions, all other measured indices of disease severity were worse in patients with aspiration pneumonia. Patients with aspiration pneumonia were more likely to have cerebrovascular disease than those with nonaspiration pneumonia. Aspiration pneumonia patients also had increased prevalence of congestive heart failure. There was no appreciable difference between groups among other measured comorbidities.


The patient characteristics most associated with a physician diagnosis of aspiration pneumonia, identified using logistic regression, were confusion, residence in nursing home, and presence of cerebrovascular disease (odds ratio [OR]: of 4.4, 2.9, and 2.3, respectively), whereas renal disease was associated with decreased physician diagnosis of aspiration pneumonia over nonaspiration pneumonia (OR: 0.58) (Table 2).
| Covariate | Odds Ratio | 95% Confidence Intervals | P Value |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 1.00 | 0.991.01 | 0.948 |
| Male | 1.20 | 0.941.54 | 0.148 |
| Nursing home residence | 2.93 | 2.134.00 | <0.001 |
| Comorbidities | |||
| Cerebrovascular disease | 2.26 | 1.533.32 | <0.001 |
| Renal disease | 0.58 | 0.390.85 | 0.006 |
| Disease severity | |||
| Confusion | 4.41 | 3.405.72 | <0.001 |
| Hematocrit <30% | 1.59 | 1.062.33 | 0.020 |
| pH <7.35 | 1.67 | 1.102.47 | 0.013 |
| Temperature >37.8C or <35.6C | 1.60 | 1.072.35 | 0.019 |
| Multilobar pneumonia | 1.29 | 1.001.65 | 0.047 |
| Interaction terms | |||
| Age * cerebrovascular disease | 0.98 | 0.960.99 | 0.011 |
| Nursing home * cerebrovascular disease | 0.51 | 0.270.96 | 0.037 |
| Confusion * cerebrovascular disease | 0.70 | 0.421.17 | 0.175 |
Observed in‐patient mortality of aspiration pneumonia was 23%. This mortality was considerably higher than a mean PSI score of 123 would predict (class IV risk group, with expected 30‐day mortality of 8%9%[25]). The PSI score's ability to predict inpatient mortality in patients with aspiration pneumonia was moderate, with an area under the curve (AUC) of 0.71. This was similar to its performance in patients with nonaspiration pneumonia (AUC of 0.75) (Figure 3). These values are lower than the AUC of 0.81 for the PSI in predicting mortality derived from a meta‐analysis of 31 other studies.[26]

Our regression model after propensity score matching demonstrated that aspiration pneumonia independently confers a 2.3‐fold increased odds for inpatient mortality (95% CI: 1.56‐3.45, P<0.001).
DISCUSSION
Pneumonia patients with confusion, nursing home residence, or cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Although this is unsurprising, it is notable that these patients are more than twice as likely to die in the inpatient setting, even after accounting for age, comorbidities, and disease severity. These findings are similar to three previously published studies comparing aspiration and nonaspiration pneumonia at single institutions, albeit using different aspiration pneumonia definitions.[13, 14, 15] This study is the first large, multicenter, multinational study to demonstrate these findings.
Central to the interpretation of our results is the method of diagnosing aspiration versus nonaspiration. A bottom‐up method that relies on a clinician to check a box for aspiration may appear poorly reproducible. Because there is no diagnostic gold standard, clinicians may use different criteria to diagnose aspiration, creating potential for idiosyncratic noise. The strength of the wisdom of the crowd method used in this study is that an aggregate estimation from independent judgments may reduce the noise from individual judgments.[16] Although clinicians may vary in why they diagnose a particular patient as having aspiration pneumonia, it appears that the overwhelming reason for diagnosing a patient as having aspiration pneumonia is the presence of confusion, followed by previous nursing home residence or cerebrovascular disease. This finding has some face validity when compared with studies using an investigator definition, as altered mental status, chronic debility, and cerebrovascular disease are either prominent features of the definition of aspiration pneumonia[8] or frequently observed in patients with aspiration pneumonia.[13, 15] The distribution of cerebrovascular disease among our study's aspiration and nonaspiration pneumonia patients was similar to studies that used formal criteria in their definitions.[13, 15] Although nursing home residence was more likely in aspiration pneumonia patients, the majority of aspiration pneumonia patients were residing in the community, suggesting that aspiration is not simply a surrogate for healthcare‐associated pneumonia. Although patients with aspiration pneumonia are typically older than their nonaspiration counterparts, it appears that age is not a key determinant in the diagnosis of aspiration. With aspiration pneumonia, confusion, nursing home residence, and the presence of cerebrovascular disease are the greatest contributors in the clinical diagnosis, more than age.
Our data demonstrate that aspiration pneumonia confers increased odds for mortality, even after adjustment for age, disease severity, and comorbidities. These data suggest that aspiration pneumonia is a distinct entity from nonaspiration pneumonia, and that this disease is worse than nonaspiration CAP. If aspiration pneumonia is distinct from nonaspiration pneumonia, some unrecognized host factor other than age, disease severity, or the captured comorbidities decreases survival in aspiration pneumonia patients. However, it is also possible that aspiration pneumonia is merely a clinical designation for one end of the pneumonia spectrum, and we and others have failed to completely account for all measures of disease severity or all measures of comorbidities. Examples of unmeasured comorbidities would include presence of oropharyngeal dysphagia, which is not assessed in the database but could have a significant effect on clinical diagnosis. Unmeasured covariates can include measures beyond that of disease severity or comorbidity, such as the presence of a do not resuscitate (DNR) order, which could have a significant confounding effect on the observed association. A previous, single‐center study demonstrated that increased 30‐day mortality in aspiration pneumonia was mostly attributable to greater disease severity and comorbidities, although aspiration pneumonia independently conferred greater risk for adverse long‐term outcomes.[15] We propose that aspiration pneumonia represents a clinically distinct entity from nonaspiration pneumonia. Patients with chronic aspiration are often chronically malnourished and may have different oral flora than patients without chronic aspiration.[27, 28] Chronic aspiration has been associated with granulomatous reaction, organizing pneumonia, diffuse alveolar damage, and chronic bronchiolitis.[29] Chronic aspiration may elicit changes in the host physiology, and may render the host more susceptible to the development of secondary bacterial infection with morbid consequences.
The ability of the PSI to predict inpatient mortality was moderate (AUC only 0.7), with no significant additional discrimination between the aspiration and nonaspiration pneumonia groups. Although the PSI had moderate ability to predict inpatient mortality, the observed mortality was considerably higher than predicted. It is possible that the PSI incompletely captures clinically relevant comorbidities (eg, malnutrition). Further study to improve mortality prediction of aspiration pneumonia patients could employ sensitivity analysis to determine optimal thresholds and weighting of the PSI components.
Patients with aspiration pneumonia had longer hospital lengths of stay and took longer to achieve clinical stability than their nonaspiration counterparts. Time to clinical stability has been associated with increased posthospitalization mortality and is associated with time to switch from intravenous to oral antibiotics.[17] Although some component of hospital length‐of‐stay is subject to local practice patterns, time to clinical stability has explicit criteria for clinical improvement and failure, and therefore is less likely to be affected by local practice patterns.
We noted a relatively high (16%21%) incidence of prior antibiotic use among patients in this database. Analysis of antibiotic prescription patterns was limited, given the several different countries from which the database draws its cases. Although we used accepted criteria to define CAP cases, it is possible that this population may have a higher rate of resistant or uncommon pathogens than other studies of CAP that have populations with lower incidence of prior antibiotic use. Although not assessed, we suspect a significant component of the prior antibiotic use represented outpatient pneumonia treatment during the few days prior to visiting the hospital.
This study has several limitations, of which the most important may be that we used clinical determination for defining presence of aspiration pneumonia. This method is susceptible to the subjective perceptions of the treating clinician. We did not account for the effect of individual physicians in our model, although we did adjust for regional differences. The retrospective identification of patients allows for the possibility of selection bias, and therefore we have not attempted to make inferences regarding the relative incidence of pneumonia, nor did we adjust for temporal trends in diagnosis. The ratio of aspiration pneumonia patients to nonaspiration pneumonia patients may not necessarily reflect that observed in reality. Microbiologic and antibiotic data were unavailable for analysis. This study cannot inform on nonhospitalized patients with aspiration pneumonia, as only hospitalized patients were enrolled. The database identified cases of pneumonia, so it is possible for a patient to enter into the database more than once. Detection of mortality was limited to the inpatient setting rather than a set interval of 30 days. Inpatient mortality depends on length‐of‐stay patterns that may bias the mortality endpoint.[30] Also not assessed was the presence of a DNR order. It is possible that an older patient with greater comorbidities and disease severity may have care intentionally limited or withdrawn early by the family or clinicians.
Strengths of the study include its size and its multicenter, multinational population. The CAPO database is a large and well‐described population of patients with CAP.[17, 31] These attributes, as well as the clinician‐determined diagnosis, increase the generalizability of the study compared to a single‐center, single‐country study that employs investigator‐defined criteria.
CONCLUSION
Pneumonia patients with confusion, who are nursing home residence, and have cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Our clinician‐diagnosed cohort appears similar to those derived using an investigator definition. Patients with aspiration pneumonia are older, and have greater disease severity and more comorbidities than patients with nonaspiration pneumonia. They have greater mortality than their PSI score class would predict. Even after accounting for age, disease severity, and comorbidities, the presence of aspiration pneumonia independently conferred a greater than 2‐fold increase in inpatient mortality. These findings together suggest that aspiration pneumonia should be considered a distinct entity from typical pneumonia, and that additional research should be done in this field.
ACKNOWLEDGMENTS
Disclosures: M.J.L. contributed to the study design, data analysis, statistical analysis, and writing of the manuscript. P.P. contributed to the study design and revision of the manuscript for important intellectual content. T.W. and E.W. contributed to the study design, statistical analysis, and revision of the manuscript for important intellectual content. J.A.R. and N.C.D. contributed to the study design and revision of the manuscript for important intellectual content. All authors read and approved the final manuscript. M.L. takes responsibility for the integrity of the work as a whole, from inception to published article. This investigation was partly supported with funding from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (grant 8UL1TR000105 [formerly UL1RR025764]). The authors report no conflicts of interest.
Pneumonia is a common clinical syndrome with well‐described epidemiology and microbiology. Aspiration pneumonia comprises 5% to 15% of patients with pneumonia acquired outside of the hospital,[1] but is less well characterized despite being a major syndrome of pneumonia in the elderly.[2, 3] Difficulties in studying aspiration pneumonia include the lack of a sensitive and specific marker for aspiration as well as the potential overlap between aspiration pneumonia and other forms of pneumonia.[4, 5, 6] Additionally, clinicians have difficulty distinguishing between aspiration pneumonia, which develops after the aspiration of oropharyngeal contents, and aspiration pneumonitis, wherein inhalation of gastric contents causes inflammation without the subsequent development of bacterial infection.[7, 8] Central to the study of aspiration pneumonia is whether it should exist as its own entity, or if aspiration is really a designation used for pneumonia in an older patient with greater comorbidities. The ability to clearly understand how a clinician diagnoses aspiration pneumonia, and whether that method has face validity with expert definitions may allow for improved future research, improved generalizability of current or past research, and possibly better clinical care.
Several validated mortality prediction models exist for community‐acquired pneumonia (CAP) using a variety of clinical predictors, but their performance in patients with aspiration pneumonia is less well characterized. Most studies validating pneumonia severity scoring systems excluded aspiration pneumonia from their study population.[9, 10, 11] Severity scoring systems for CAP may not accurately predict disease severity in patients with aspiration pneumonia. The CURB‐65[9] (confusion, uremia, respiratory rate, blood pressure, age 65 years) and the eCURB[12] scoring systems are poor predictors of mortality in patients with aspiration pneumonia, perhaps because they do not account for patient comorbidities.[13] The pneumonia severity index (PSI)[10] might predict mortality better than CURB‐65 in the aspiration population due to the inclusion of comorbidities.
Previous studies have demonstrated that patients with aspiration pneumonia are older and have greater disease severity and more comorbidities.[13, 14, 15] These single‐center studies also demonstrated greater mortality, more frequent admission to an intensive care unit (ICU), and longer hospital lengths of stay in patients with aspiration pneumonia. These studies identified aspiration pneumonia by the presence of a risk factor for aspiration[15] or by physician billing codes.[13] In practice, however, the bedside clinician diagnoses a patient as having aspiration pneumonia, but the logic is likely vague and inconsistent. Despite the potential for variability with individual judgment, an aggregate estimation from independent judgments may perform better than individual judgments.[16] Because there is no gold standard for defining aspiration pneumonia, all previous research has been limited to definitions created by investigators. This multicenter study seeks to determine what clinical characteristics lead physicians to diagnose a patient as having aspiration pneumonia, and whether or not the clinician‐derived diagnosis is distinct and clinically useful.
Our objectives were to: (1) identify covariates associated with bedside clinicians diagnosing a pneumonia patient as having aspiration pneumonia; (2) compare aspiration pneumonia and nonaspiration pneumonia in regard to disease severity, patient demographics, comorbidities, and clinical outcomes; and (3) measure the performance of the PSI in aspiration pneumonia versus nonaspiration pneumonia.
PATIENTS AND METHODS
Study Design and Setting
We performed a secondary analysis of the Community‐Acquired Pneumonia Organization (CAPO) database, which contains retrospectively collected data from 71 hospitals in 16 countries between June 2001 and December 2012. In each participating center, primary investigators selected nonconsecutive, adult hospitalized patients diagnosed with CAP. To decrease systematic selection biases, the selection of patients with CAP for enrollment in the trial was based on the date of hospital admission. Each investigator completed a case report form that was transferred via the internet to the CAPO study center at the University of Louisville (Louisville, KY). A sample of the data collection form is available at the study website (
Inclusion and Exclusion Criteria
Patients 18 years of age and satisfying criteria for CAP were included in this study. A diagnosis of CAP required a new pulmonary infiltrate at time of hospitalization, and at least 1 of the following: new or increased cough; leukocytosis; leukopenia, or left shift pattern on white blood cell count; and temperature >37.8C or <35.6 C. We excluded patients with pneumonia attributed to mycobacterial or fungal infection, and patients infected with human immunodeficiency virus, as we believed these types of pneumonia differ fundamentally from typical CAP.
Patient Variables
Patient variables included presence of aspiration pneumonia, laboratory data, comorbidities, and measures of disease severity, including the PSI. The clinician made a clinical diagnosis of the presence or absence of aspiration for each patient by marking a box on the case report form. Outcomes included in‐hospital mortality, hospital length of stay up to 14 days, and time to clinical stability up to 8 days. All variables were obtained directly from the case report form. In accordance with previously published definitions, we defined clinical stability as the day the following criteria were all met: improved clinical signs (improved cough and shortness of breath), lack of fever for >8 hours, improving leukocytosis (decreased at least 10% from the previous day), and tolerating oral intake.[17, 18]
Statistical Analysis
Baseline characteristics of patients with aspiration and nonaspiration CAP were compared using 2 or Fisher exact tests for categorical variables and the Mann‐Whitney U test for continuous variables.
To determine which patient variables were important in the physician diagnosis of aspiration pneumonia, we performed logistic regression with initial covariates comprising the demographic, comorbidity, and disease severity measurements listed in Table 1. We included interactions between cerebrovascular disease and age, nursing home status, and confusion to improve model fit. We centered all variables (including binary indicators) according to the method outlined by Kraemer and Blasey to improve interpretation of the main effects.[19]
| Aspiration Pneumonia, N=451 | Nonaspiration Pneumonia, N=4,734 | P Value | |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 79 (6587) | 69 (5380) | <0.001 |
| % Male | 59% | 60% | 0.58 |
| Nursing home residence | 25% | 5% | <0.001 |
| Recent (30 days) antibiotic use | 21% | 16% | 0.017 |
| Comorbidities | |||
| Cerebrovascular disease | 35% | 14% | <0.001 |
| Chronic obstructive pulmonary disease | 25% | 27% | 0.62 |
| Congestive heart failure | 23% | 19% | 0.027 |
| Diabetes | 18% | 18% | 0.85 |
| Cancer | 12% | 10% | 0.12 |
| Renal disease | 10% | 11% | 0.53 |
| Liver disease | 6% | 5% | 0.29 |
| Disease severity | |||
| Pneumonia severity index | 123 (99153) | 92 (68117) | <0.001 |
| Confusion | 49% | 12% | <0.001 |
| PaO2 <60 mm Hg | 43% | 33% | <0.001 |
| BUN >30 g/dL | 42% | 23% | <0.001 |
| Multilobar pneumonia | 34% | 28% | 0.003 |
| Pleural effusion | 25% | 21% | 0.07 |
| Respiratory rate >30 breaths/minute | 21% | 20% | 0.95 |
| pH <7.35 | 13% | 5% | <0.001 |
| Hematocrit <30% | 11% | 6% | 0.001 |
| Temperature >37.8C or <35.6C | 9% | 7% | 0.30 |
| Systolic blood pressure <90 mm Hg | 8% | 9% | 0.003 |
| Sodium <130 mEq/L | 8% | 6% | 0.08 |
| Heart rate >125 beats/minute | 8% | 5% | 0.71 |
| Glucose >250 mg/dL | 6% | 7% | 0.06 |
| Cavitary lesion | 0% | 0% | 0.67 |
| Clinical outcomes | |||
| In‐hospital mortality | 23% | 9% | <0.001 |
| Intensive care unit admission | 19% | 13% | 0.002 |
| Hospital length of stay, d | 9 (515) | 7 (412) | <0.001 |
| Time to clinical stability, d | 8 (48) | 4 (38) | <0.001 |
To determine if aspiration pneumonia had worse clinical outcomes compared to nonaspiration pneumonia, multiple methods were used. To compare the differences between the 2 groups with respect to time to clinical stability and length of hospital stay, we constructed Kaplan‐Meier survival curves and Cox proportional hazards regression models. The log‐rank test was used to determine statistical differences between the Kaplan‐Meier survival curves. To compare the impact of aspiration on mortality in patients with CAP, we conducted a propensity scorematched analysis. We chose propensity score matching over traditional logistic regression to balance variables among groups and to avoid the potential for overfit and multicollinearity. We considered a variable balanced after matching if its standardized difference was <10. All variables in the propensity scorematched analysis were balanced.
Although our dataset contained minimal missing data, we imputed any missing values to maintain the full study population in the creation of the propensity score. Missing data were imputed using the aregImpute function of the hmisc package of R (The R Foundation for Statistical Computing, Vienna, Austria).[20, 21] We built the propensity score model using a variable selection algorithm described by Bursac et al.[22] Our model included variables for region (United States/Canada, Europe, Asia/Africa or Latin America) and the variables listed in Table 1, with the exception of the PSI and the 4 clinical outcomes. Given that previous analyses accounting for clustering by physician did not substantially affect our results,[23] our model did not include physician‐level variables and did not account for the clustering effects of physicians. Using the propensity scores generated from this model, we matched a case of aspiration CAP with a case of nonaspiration CAP.[24] We then constructed a general linear model using the matched dataset to obtain the magnitude of effect of aspiration on mortality.
We used receiver operating characteristic curves to define the diagnostic accuracy of the pneumonia severity index for the prediction of mortality among patients with aspiration pneumonia and those with nonaspiration pneumonia. SAS version 9.3 (SAS Institute, Cary, NC) and R version 2.15.3 (The R Foundation for Statistical Computing) were used for all analyses. P values of 0.05 were considered statistically significant in all analyses.
RESULTS
Our initial query, after exclusion criteria, yielded a study population of 5185 patients (Figure 1). We compared 451 patients diagnosed with aspiration pneumonia to 4734 with CAP (Figure 1). Patient characteristics are summarized in Table 1. Patients with aspiration pneumonia were older, more likely to live in a nursing home, had greater disease severity, and were more likely to be admitted to an ICU. Patients with aspiration pneumonia had longer adjusted hospital lengths of stay and took more days to achieve clinical stability than patients with nonaspiration pneumonia (Figure 2). After adjusting for all variables in Table 1, the Cox proportional hazards models demonstrated that aspiration pneumonia was associated with ongoing hospitalization (hazard ratio [HR] for discharge: 0.77, 95% confidence interval [CI]: 0.65‐0.91, P=0.002) and clinical instability (HR for attaining clinical stability: 0.72, 95% CI: 0.61‐0.84, P<0.001). Patients with aspiration pneumonia presented with greater disease severity than those with nonaspiration pneumonia. Although there was no difference between groups in regard to temperature, respiratory rate, hyponatremia, or presence of pleural effusions or cavitary lesions, all other measured indices of disease severity were worse in patients with aspiration pneumonia. Patients with aspiration pneumonia were more likely to have cerebrovascular disease than those with nonaspiration pneumonia. Aspiration pneumonia patients also had increased prevalence of congestive heart failure. There was no appreciable difference between groups among other measured comorbidities.


The patient characteristics most associated with a physician diagnosis of aspiration pneumonia, identified using logistic regression, were confusion, residence in nursing home, and presence of cerebrovascular disease (odds ratio [OR]: of 4.4, 2.9, and 2.3, respectively), whereas renal disease was associated with decreased physician diagnosis of aspiration pneumonia over nonaspiration pneumonia (OR: 0.58) (Table 2).
| Covariate | Odds Ratio | 95% Confidence Intervals | P Value |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 1.00 | 0.991.01 | 0.948 |
| Male | 1.20 | 0.941.54 | 0.148 |
| Nursing home residence | 2.93 | 2.134.00 | <0.001 |
| Comorbidities | |||
| Cerebrovascular disease | 2.26 | 1.533.32 | <0.001 |
| Renal disease | 0.58 | 0.390.85 | 0.006 |
| Disease severity | |||
| Confusion | 4.41 | 3.405.72 | <0.001 |
| Hematocrit <30% | 1.59 | 1.062.33 | 0.020 |
| pH <7.35 | 1.67 | 1.102.47 | 0.013 |
| Temperature >37.8C or <35.6C | 1.60 | 1.072.35 | 0.019 |
| Multilobar pneumonia | 1.29 | 1.001.65 | 0.047 |
| Interaction terms | |||
| Age * cerebrovascular disease | 0.98 | 0.960.99 | 0.011 |
| Nursing home * cerebrovascular disease | 0.51 | 0.270.96 | 0.037 |
| Confusion * cerebrovascular disease | 0.70 | 0.421.17 | 0.175 |
Observed in‐patient mortality of aspiration pneumonia was 23%. This mortality was considerably higher than a mean PSI score of 123 would predict (class IV risk group, with expected 30‐day mortality of 8%9%[25]). The PSI score's ability to predict inpatient mortality in patients with aspiration pneumonia was moderate, with an area under the curve (AUC) of 0.71. This was similar to its performance in patients with nonaspiration pneumonia (AUC of 0.75) (Figure 3). These values are lower than the AUC of 0.81 for the PSI in predicting mortality derived from a meta‐analysis of 31 other studies.[26]

Our regression model after propensity score matching demonstrated that aspiration pneumonia independently confers a 2.3‐fold increased odds for inpatient mortality (95% CI: 1.56‐3.45, P<0.001).
DISCUSSION
Pneumonia patients with confusion, nursing home residence, or cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Although this is unsurprising, it is notable that these patients are more than twice as likely to die in the inpatient setting, even after accounting for age, comorbidities, and disease severity. These findings are similar to three previously published studies comparing aspiration and nonaspiration pneumonia at single institutions, albeit using different aspiration pneumonia definitions.[13, 14, 15] This study is the first large, multicenter, multinational study to demonstrate these findings.
Central to the interpretation of our results is the method of diagnosing aspiration versus nonaspiration. A bottom‐up method that relies on a clinician to check a box for aspiration may appear poorly reproducible. Because there is no diagnostic gold standard, clinicians may use different criteria to diagnose aspiration, creating potential for idiosyncratic noise. The strength of the wisdom of the crowd method used in this study is that an aggregate estimation from independent judgments may reduce the noise from individual judgments.[16] Although clinicians may vary in why they diagnose a particular patient as having aspiration pneumonia, it appears that the overwhelming reason for diagnosing a patient as having aspiration pneumonia is the presence of confusion, followed by previous nursing home residence or cerebrovascular disease. This finding has some face validity when compared with studies using an investigator definition, as altered mental status, chronic debility, and cerebrovascular disease are either prominent features of the definition of aspiration pneumonia[8] or frequently observed in patients with aspiration pneumonia.[13, 15] The distribution of cerebrovascular disease among our study's aspiration and nonaspiration pneumonia patients was similar to studies that used formal criteria in their definitions.[13, 15] Although nursing home residence was more likely in aspiration pneumonia patients, the majority of aspiration pneumonia patients were residing in the community, suggesting that aspiration is not simply a surrogate for healthcare‐associated pneumonia. Although patients with aspiration pneumonia are typically older than their nonaspiration counterparts, it appears that age is not a key determinant in the diagnosis of aspiration. With aspiration pneumonia, confusion, nursing home residence, and the presence of cerebrovascular disease are the greatest contributors in the clinical diagnosis, more than age.
Our data demonstrate that aspiration pneumonia confers increased odds for mortality, even after adjustment for age, disease severity, and comorbidities. These data suggest that aspiration pneumonia is a distinct entity from nonaspiration pneumonia, and that this disease is worse than nonaspiration CAP. If aspiration pneumonia is distinct from nonaspiration pneumonia, some unrecognized host factor other than age, disease severity, or the captured comorbidities decreases survival in aspiration pneumonia patients. However, it is also possible that aspiration pneumonia is merely a clinical designation for one end of the pneumonia spectrum, and we and others have failed to completely account for all measures of disease severity or all measures of comorbidities. Examples of unmeasured comorbidities would include presence of oropharyngeal dysphagia, which is not assessed in the database but could have a significant effect on clinical diagnosis. Unmeasured covariates can include measures beyond that of disease severity or comorbidity, such as the presence of a do not resuscitate (DNR) order, which could have a significant confounding effect on the observed association. A previous, single‐center study demonstrated that increased 30‐day mortality in aspiration pneumonia was mostly attributable to greater disease severity and comorbidities, although aspiration pneumonia independently conferred greater risk for adverse long‐term outcomes.[15] We propose that aspiration pneumonia represents a clinically distinct entity from nonaspiration pneumonia. Patients with chronic aspiration are often chronically malnourished and may have different oral flora than patients without chronic aspiration.[27, 28] Chronic aspiration has been associated with granulomatous reaction, organizing pneumonia, diffuse alveolar damage, and chronic bronchiolitis.[29] Chronic aspiration may elicit changes in the host physiology, and may render the host more susceptible to the development of secondary bacterial infection with morbid consequences.
The ability of the PSI to predict inpatient mortality was moderate (AUC only 0.7), with no significant additional discrimination between the aspiration and nonaspiration pneumonia groups. Although the PSI had moderate ability to predict inpatient mortality, the observed mortality was considerably higher than predicted. It is possible that the PSI incompletely captures clinically relevant comorbidities (eg, malnutrition). Further study to improve mortality prediction of aspiration pneumonia patients could employ sensitivity analysis to determine optimal thresholds and weighting of the PSI components.
Patients with aspiration pneumonia had longer hospital lengths of stay and took longer to achieve clinical stability than their nonaspiration counterparts. Time to clinical stability has been associated with increased posthospitalization mortality and is associated with time to switch from intravenous to oral antibiotics.[17] Although some component of hospital length‐of‐stay is subject to local practice patterns, time to clinical stability has explicit criteria for clinical improvement and failure, and therefore is less likely to be affected by local practice patterns.
We noted a relatively high (16%21%) incidence of prior antibiotic use among patients in this database. Analysis of antibiotic prescription patterns was limited, given the several different countries from which the database draws its cases. Although we used accepted criteria to define CAP cases, it is possible that this population may have a higher rate of resistant or uncommon pathogens than other studies of CAP that have populations with lower incidence of prior antibiotic use. Although not assessed, we suspect a significant component of the prior antibiotic use represented outpatient pneumonia treatment during the few days prior to visiting the hospital.
This study has several limitations, of which the most important may be that we used clinical determination for defining presence of aspiration pneumonia. This method is susceptible to the subjective perceptions of the treating clinician. We did not account for the effect of individual physicians in our model, although we did adjust for regional differences. The retrospective identification of patients allows for the possibility of selection bias, and therefore we have not attempted to make inferences regarding the relative incidence of pneumonia, nor did we adjust for temporal trends in diagnosis. The ratio of aspiration pneumonia patients to nonaspiration pneumonia patients may not necessarily reflect that observed in reality. Microbiologic and antibiotic data were unavailable for analysis. This study cannot inform on nonhospitalized patients with aspiration pneumonia, as only hospitalized patients were enrolled. The database identified cases of pneumonia, so it is possible for a patient to enter into the database more than once. Detection of mortality was limited to the inpatient setting rather than a set interval of 30 days. Inpatient mortality depends on length‐of‐stay patterns that may bias the mortality endpoint.[30] Also not assessed was the presence of a DNR order. It is possible that an older patient with greater comorbidities and disease severity may have care intentionally limited or withdrawn early by the family or clinicians.
Strengths of the study include its size and its multicenter, multinational population. The CAPO database is a large and well‐described population of patients with CAP.[17, 31] These attributes, as well as the clinician‐determined diagnosis, increase the generalizability of the study compared to a single‐center, single‐country study that employs investigator‐defined criteria.
CONCLUSION
Pneumonia patients with confusion, who are nursing home residence, and have cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Our clinician‐diagnosed cohort appears similar to those derived using an investigator definition. Patients with aspiration pneumonia are older, and have greater disease severity and more comorbidities than patients with nonaspiration pneumonia. They have greater mortality than their PSI score class would predict. Even after accounting for age, disease severity, and comorbidities, the presence of aspiration pneumonia independently conferred a greater than 2‐fold increase in inpatient mortality. These findings together suggest that aspiration pneumonia should be considered a distinct entity from typical pneumonia, and that additional research should be done in this field.
ACKNOWLEDGMENTS
Disclosures: M.J.L. contributed to the study design, data analysis, statistical analysis, and writing of the manuscript. P.P. contributed to the study design and revision of the manuscript for important intellectual content. T.W. and E.W. contributed to the study design, statistical analysis, and revision of the manuscript for important intellectual content. J.A.R. and N.C.D. contributed to the study design and revision of the manuscript for important intellectual content. All authors read and approved the final manuscript. M.L. takes responsibility for the integrity of the work as a whole, from inception to published article. This investigation was partly supported with funding from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (grant 8UL1TR000105 [formerly UL1RR025764]). The authors report no conflicts of interest.
- , , , et al. Severe community‐acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis. 1991;144(2):312–318.
- , , . Risk factors for pneumonia in the elderly. Am J Med. 1994;96(4):313–320.
- , . Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328–336.
- , , . Pneumonia versus aspiration pneumonitis in nursing home residents: diagnosis and management. J Am Geriatr Soc. 2003;51(1):17–23.
- . Aspiration pneumonia: mixing apples with oranges and tangerines. Crit Care Med. 2004;32(5):1236; author reply 1236–1237.
- , , , , . Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999–2000. Crit Care Med. 2003;31(7):1930–1937.
- . Aspiration syndromes: aspiration pneumonia and pneumonitis. Hosp Pract (Minneap). 2010;38(1):35–42.
- . Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–671.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382.
- , , , et al. Comparison of a disease‐specific and a generic severity of illness measure for patients with community‐acquired pneumonia. J Gen Intern Med. 1995;10(7):359–368.
- , , , et al. Development and validation of a clinical prediction rule for severe community‐acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–1256.
- , , , et al. CURB‐65 pneumonia severity assessment adapted for electronic decision support. Chest. 2011;140(1):156–163.
- , , , . Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83–90.
- , , , , . Pneumonia Severity Index (PSI), CURB‐65, and mortality in hospitalized elderly patients with aspiration pneumonia [in German]. Z Gerontol Geriatr. 2011;44(4):229–234.
- , , , , . Risk factors for aspiration in community‐acquired pneumonia: analysis of a hospitalized UK cohort. Am J Med. 2013;126(11):995–1001.
- , , , . The wisdom of the crowd in combinatorial problems. Cogn Sci. 2012;36(3):452–470.
- , , , et al. Association between time to clinical stability and outcomes after discharge in hospitalized patients with community‐acquired pneumonia. Chest. 2011;140(2):482–488.
- . Clinical stability and switch therapy in hospitalised patients with community‐acquired pneumonia: are we there yet? Eur Respir J. 2013;41(1):5–6.
- , . Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13(3):141–151.
- . Hmisc: Harrell miscellaneous. Available at: http://CRAN.R‐project.org/package=Hmisc. Published Sept 12, 2014. Last accessed Oct 27, 2014.
- , . Multiple imputation for the fatal accident reporting system. J R Stat Soc Ser C Appl Stat. 1991;40(1):13–29.
- , , , . Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17.
- , , , , ; CAPO authors. Mortality differences among hospitalized patients with community‐acquired pneumonia in three world regions: results from the Community‐Acquired Pneumonia Organization (CAPO) International Cohort Study. Respir Med. 2013;107(7):1101–1111.
- . Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. In: Proceedings of the 26th Annual SAS Users Group International Conference. Cary, NC: SAS Institute Inc.; 2001:214–226. Available at: http://www2.sas.com/proceedings/sugi26/p214–26.pdf. Last accessed Oct 27, 2014.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336(4):243–250.
- , , , et al. Severity assessment tools for predicting mortality in hospitalised patients with community‐acquired pneumonia. Systematic review and meta‐analysis. Thorax. 2010;65(10):878–883.
- , , , , , . Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2010;39(1):39–45.
- , . The association between oral microorgansims and aspiration pneumonia in the institutionalized elderly: review and recommendations. Dysphagia. 2010;25(4):307–322.
- , . Histopathology of aspiration pneumonia not associated with food or other particulate matter: a clinicopathologic study of 10 cases diagnosed on biopsy. Am J Surg Pathol. 2011;35(3):426–431.
- , , , , , . Interpreting hospital mortality data. The role of clinical risk adjustment. JAMA. 1988;260(24):3611–3616.
- , , , . Hospitalization for community‐acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124(1):121–124.
- , , , et al. Severe community‐acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis. 1991;144(2):312–318.
- , , . Risk factors for pneumonia in the elderly. Am J Med. 1994;96(4):313–320.
- , . Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328–336.
- , , . Pneumonia versus aspiration pneumonitis in nursing home residents: diagnosis and management. J Am Geriatr Soc. 2003;51(1):17–23.
- . Aspiration pneumonia: mixing apples with oranges and tangerines. Crit Care Med. 2004;32(5):1236; author reply 1236–1237.
- , , , , . Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999–2000. Crit Care Med. 2003;31(7):1930–1937.
- . Aspiration syndromes: aspiration pneumonia and pneumonitis. Hosp Pract (Minneap). 2010;38(1):35–42.
- . Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–671.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382.
- , , , et al. Comparison of a disease‐specific and a generic severity of illness measure for patients with community‐acquired pneumonia. J Gen Intern Med. 1995;10(7):359–368.
- , , , et al. Development and validation of a clinical prediction rule for severe community‐acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–1256.
- , , , et al. CURB‐65 pneumonia severity assessment adapted for electronic decision support. Chest. 2011;140(1):156–163.
- , , , . Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83–90.
- , , , , . Pneumonia Severity Index (PSI), CURB‐65, and mortality in hospitalized elderly patients with aspiration pneumonia [in German]. Z Gerontol Geriatr. 2011;44(4):229–234.
- , , , , . Risk factors for aspiration in community‐acquired pneumonia: analysis of a hospitalized UK cohort. Am J Med. 2013;126(11):995–1001.
- , , , . The wisdom of the crowd in combinatorial problems. Cogn Sci. 2012;36(3):452–470.
- , , , et al. Association between time to clinical stability and outcomes after discharge in hospitalized patients with community‐acquired pneumonia. Chest. 2011;140(2):482–488.
- . Clinical stability and switch therapy in hospitalised patients with community‐acquired pneumonia: are we there yet? Eur Respir J. 2013;41(1):5–6.
- , . Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13(3):141–151.
- . Hmisc: Harrell miscellaneous. Available at: http://CRAN.R‐project.org/package=Hmisc. Published Sept 12, 2014. Last accessed Oct 27, 2014.
- , . Multiple imputation for the fatal accident reporting system. J R Stat Soc Ser C Appl Stat. 1991;40(1):13–29.
- , , , . Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17.
- , , , , ; CAPO authors. Mortality differences among hospitalized patients with community‐acquired pneumonia in three world regions: results from the Community‐Acquired Pneumonia Organization (CAPO) International Cohort Study. Respir Med. 2013;107(7):1101–1111.
- . Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. In: Proceedings of the 26th Annual SAS Users Group International Conference. Cary, NC: SAS Institute Inc.; 2001:214–226. Available at: http://www2.sas.com/proceedings/sugi26/p214–26.pdf. Last accessed Oct 27, 2014.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336(4):243–250.
- , , , et al. Severity assessment tools for predicting mortality in hospitalised patients with community‐acquired pneumonia. Systematic review and meta‐analysis. Thorax. 2010;65(10):878–883.
- , , , , , . Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2010;39(1):39–45.
- , . The association between oral microorgansims and aspiration pneumonia in the institutionalized elderly: review and recommendations. Dysphagia. 2010;25(4):307–322.
- , . Histopathology of aspiration pneumonia not associated with food or other particulate matter: a clinicopathologic study of 10 cases diagnosed on biopsy. Am J Surg Pathol. 2011;35(3):426–431.
- , , , , , . Interpreting hospital mortality data. The role of clinical risk adjustment. JAMA. 1988;260(24):3611–3616.
- , , , . Hospitalization for community‐acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124(1):121–124.
© 2014 Society of Hospital Medicine
Patients with Aspiration Pneumonia
Pneumonia is a common clinical syndrome with well‐described epidemiology and microbiology. Aspiration pneumonia comprises 5% to 15% of patients with pneumonia,[1] but is less well‐characterized despite being a major syndrome of pneumonia in the elderly.[2, 3] Difficulties in studying aspiration pneumonia include the lack of a sensitive and specific marker for aspiration, the overlap between aspiration pneumonia and other forms of pneumonia, and the lack of differentiation between aspiration pneumonia and aspiration pneumonitis by many clinicians.[4, 5, 6] Aspiration pneumonia, which develops after the aspiration of oropharyngeal contents, differs from aspiration pneumonitis, wherein inhalation of gastric contents causes inflammation without the subsequent development of bacterial infection.[7, 8]
A number of validated mortality prediction models exist for community‐acquired pneumonia (CAP), using a variety of clinical predictors. One clinical prediction rule endorsed by the British Thoracic Society is the CURB‐65, which assigns a score for Confusion, Uremia >19 mg/dL, Respiratory rate >= 30 breaths/min, Blood Pressure < 90 mmHg systolic or < 60 mmHg diastolic, and age 65). We favor eCURB, a version of the CURB‐65 model that uses continuously weighted variables to more accurately predict mortality, validated in CAP populations.[9] Most studies validating pneumonia severity scoring systems excluded aspiration pneumonia from their study population.[10, 11, 12] Severity scoring systems for CAP may not accurately predict disease severity patients with aspiration pneumonia.
The aims of our study were to: (1) identify a population of patients with aspiration pneumonia; (2) compare characteristics and outcomes in patients with community‐acquired aspiration pneumonia to those with CAP; and (3) study the performance of eCURB and CURB‐65 in predicting mortality for patients with community‐acquired aspiration pneumonia.
PATIENTS AND METHODS
Study Design and Setting
The study was performed at LDS Hospital, a university‐affiliated community teaching hospital in Salt Lake City, Utah, with 520 beds. In retrospective analysis of data from the electronic medical records, we identified all patients older than 18 years who were evaluated in the emergency department at LDS Hospital or admitted patients from other sources (direct admission, transfer from another hospital) from 1996 to 2006 with International Statistical Classification of Disease and Health Related Problems, 9th Revision (ICD‐9) codes specific for aspiration pneumonia and pneumonitis (507.x). The treating physicians were mostly hospitalists and intensivists. Two physicians (M.L. and N.D.) manually reviewed the electronic medical records, including the emergency room physician's notes, the admission histories and physicals, the discharge summaries, and radiographic reports of the patients identified in the query. Consensus regarding the diagnosis of aspiration pneumonia was achieved in all patients reviewed using criteria listed in Table 1. This study was approved by the LDS Hospital institutional review board, and permission was granted to use the Utah Population Database for determining mortality (#1008505), with a waiver of informed consent. For the contemporaneous group of CAP patients, we used a previously described population identified using ICD‐9 codes 481.x to 487.x, captured from the same hospital during the same period.[13]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| |
| 1. Age 18 years | 1. Absence of radiographic evidence of pneumonia within 48 hours after evaluation |
| 2. Either admitted to hospital or evaluated in emergency department | 2. Previous episode of aspiration pneumonia within 12 months |
| 3. 507.x code as primary diagnosis | 3. Initial admission date >48 hours before transfer to LDS Hospital |
| 4. 507.x code as secondary diagnosis with a primary diagnosis of pneumonia, respiratory failure, or septicemia | 4. AIDS 5. Receipt of antiretroviral therapy 6. History of solid organ transplant |
| 5. Treating physician indicated a diagnosis of aspiration pneumonia in the history and physical and/or discharge summary | 7. Hematologic malignancy 8. Witnessed isolated aspiration event within 24 hours prior to evaluation 9. Drug overdose, cardiopulmonary arrest, or seizure prior to hospital admission 10. Laryngoscopic or bronchoscopic evidence of food material in airway |
Inclusion and Exclusion Criteria
Inclusion and exclusion criteria are listed in Table 1. To exclude patients with recurrent pneumonia, we included only the first episode of pneumonia in a given 12‐month period. LDS Hospital frequently receives patients transferred from surrounding emergency departments and intensive care units. We excluded patients who were transferred >48 hours from their initial emergency department admission and therefore were late in their disease course. Exclusion criteria 8 to 10 were used to exclude patients with clinical presentations more consistent with aspiration pneumonitis. We also excluded immunocompromised patients (criteria 4 to 7).
Healthcare‐associated aspiration pneumonia was defined as receipt of chronic hemodialysis, residence in a nursing facility, or hospitalization within any Intermountain Healthcare‐affiliated hospital within the past 90 days.[14] The remaining patients were defined as community‐acquired aspiration pneumonia.
Measurements
The first vital signs, orientation status, and first 12 hours of routine laboratory results were extracted from the electronic medical records and used to calculate predicted mortality by eCURB and CURB‐65. We determined 30‐day mortality from the merger of the electronic medical records with vital status information from the Utah Population Database.[15] The first measured SpO2 and FiO2 were used to estimate the PaO2/FiO2 ratio, using the Severinghaus calculation[16] if no arterial blood gas was available. Presence of American Thoracic Society/Infectious Diseases Society of America (IDSA/ATS) 2007 minor criteria for severe community‐acquired pneumonia (SCAP)[17] were obtained from baseline patient characteristics (Table 2). A Charlson comorbidity index was calculated from ICD‐9 codes using published methodology.[18, 19] Presence of an abnormal swallow was defined as dysphagia or aspiration on modified barium swallow study, fiberoptic endoscopic evaluation, or clinical determination by a speech language pathologist during the index hospitalization. We also looked for causative pathogens, defined by a positive pneumococcus or legionella urinary antigen, or a positive culture from blood, bronchoalveolar lavage, pleural fluid, or tracheal aspirate, collected within 24 hours of admission. Antibiotics administered within the first 24 hours of admission were classified into 4 broad groups based on local physician prescribing patterns. Clindamycin and metronidazole were considered anaerobic‐specific antibiotics. Vancomycin or linezolid were considered methicillin‐resistant Staphylococcus aureus (MRSA) antibiotics. Broad‐spectrum antibiotics included any of the following: carbapenems, aztreonam, piperacillin/tazobactam, ticarcillin/clavulanate, cefepime, and ceftazidime. Macrolides, respiratory fluoroquinolones, and third‐generation cephalosporins were considered standard‐care antibiotics.
| Respiratory rate 30 breaths/minute |
| PaO2/FiO2 250 |
| Multilobar infiltrates |
| Confusion/disorientation |
| Uremia (blood urea nitrogen 20 mg/dL) |
| Leukopenia (white blood cell count <4000 cells/mm3) |
| Thrombocytopenia (platelet count <100,000 cells/mm3) |
| Hypothermia (core temperature 36C) |
| Hypotension requiring aggressive fluid resuscitation |
Statistical Analysis
We compared baseline patient characteristics and clinical outcomes using the Fisher exact test to compare proportions of categorical variables, and Mann‐Whitney U test or Student t test to compare central tendencies of continuous variables, as dictated by the normality of the data. Receiver operating characteristic curves calculated the ability of eCURB and CURB‐65 to predict 30‐day mortality prediction in patients with community‐acquired aspiration pneumonia and CAP, as well as the ability of IDSA/ATS minor criteria for SCAP to predict admission to the intensive care unit (ICU). We performed multivariate logistic regression to predict 30‐day mortality in patients with community‐acquired aspiration pneumonia and CAP, using stepwise backward elimination. Confounders were included if they were significant at a 0.05 level or if they altered the coefficient of the main variable by more than 10%. For logistic models, we evaluated goodness of fit with the Hosmer‐Lemeshow technique; comparisons of area under the curve (AUC) among models were made using the technique of DeLong.[20] Two‐tailed P values of 0.05 were considered statistically significant. Stata version 12 (StataCorp, College Station, TX) was used for all analyses.
RESULTS
Our initial query identified 1165 patients. Physician review of the medical records resulted in 628 patients, 118 of whom were classified as healthcare‐associated aspiration pneumonia (Figure 1, Table 3). Of all aspiration pneumonia patients, 80% were seen in the emergency department, 12.5% were directly admitted from the community, and 7.5% were transferred from another healthcare facility. Almost all patients seen in the emergency department (99.0%) were admitted to the hospital, with median length of hospitalization 6.7 days among survivors.
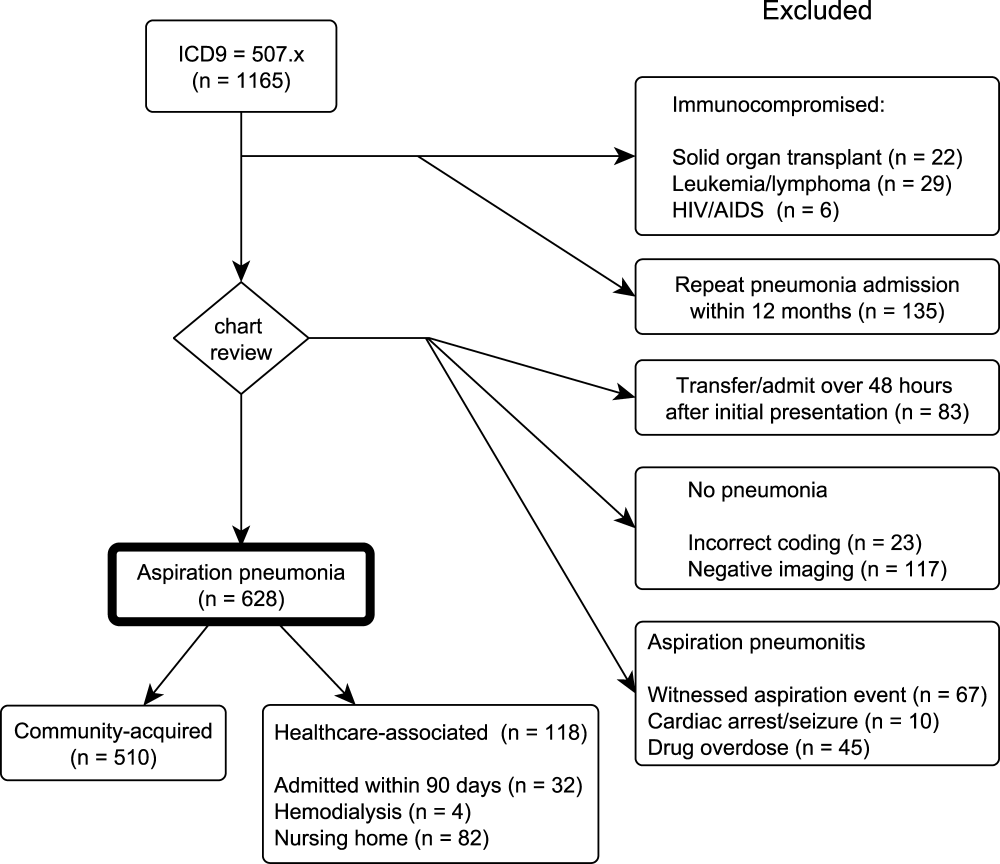
| Aspiration Pneumonia (N = 628) | Community‐Acquired Aspiration Pneumonia (N = 510) | Healthcare Associated Aspiration Pneumonia (N=118) | P Value | |
|---|---|---|---|---|
| ||||
| Age (range), y | 77 (6585) | 77 (6485) | 80 (6786) | 0.42 |
| Female, % | 49.8 | 50.2 | 48.3 | 0.76 |
| 30‐day mortality, % | 21.0% | 19.0% | 29.7% | 0.02 |
| CURB‐65 score | 2 (13) | 2 (13) | 2 (13) | 0.0012 |
| Confusion | 13.9% | 12.7% | 18.6% | 0.10 |
| Blood urea nitrogen (mg/dL) | 22 (1634) | 21 (1532) | 30 (2047) | <0.0001 |
| Respiratory rate (breaths/min) | 20 (1826) | 20 (1824) | 21 (1828) | 0.30 |
| Systolic blood pressure (mm Hg) | 128 (110149) | 129 (110150) | 127 (105146) | 0.28 |
| eCURB 30‐day mortality estimate (median, %) | 5.6 (2.414.2) | 5.2 (2.212.4) | 8.9 (4.222.5) | <0.0001 |
| eCURB 30‐day mortality estimate (mean, %) | 10.6 12.2 | 9.7 11.5 | 14.614.1 | <0.0001 |
| Hospital admission (of ED visits), % | 99.0 | 98.8 | 100 | 0.59 |
| Hospital LOS, d | 6.7 (4.111.1) | 6.5 (4.011.0) | 7.8 (5.412.3) | 0.05 |
| ICU admission, % | 37.9 | 37.1 | 41.5 | 0.21 |
| ICU LOS, d | 3.5 (1.98.8) | 3.1 (1.87.6) | 5.6 (3.810.8) | 0.02 |
| Mean ventilator‐free days (of ICU patients, out of 30 days) | 25.28.3 | 25.97.7 | 22.710.0 | 0.01 |
| Receipt of mechanical ventilation, % | 18.6 | 17.2 | 24.6 | 0.09 |
| Duration of ventilation, d | 2.8 (0.96.5) | 3.1 (1.06.6) | 1.9 (0.86.3) | 0.05 |
| Receipt of vasopressor, % | 1.8 | 1.4 | 3.4 | 0.13 |
| Charlson comorbidity index | 4 (26) | 3 (26) | 4 (36) | 0.0024 |
| Cerebrovascular disease, % | 33.9 | 32.4 | 40.7 | 0.11 |
| Chronic pulmonary disease, % | 51.0 | 51.8 | 47.5 | 0.42 |
| Congestive heart failure, % | 52.4 | 50.0 | 62.7 | 0.01 |
| Connective tissue disease, % | 8.4 | 8.8 | 6.8 | 0.58 |
| Dementia, % | 14.2 | 12.0 | 23.7 | 0.0019 |
| Hemiplegia/paraplegia | 9.4 | 8.0 | 15.2 | 0.02 |
| Myocardial infarction, % | 21.0 | 17.8 | 29.7 | 0.02 |
| Peripheral vascular disease, % | 17.7 | 16.3 | 23.7 | 0.06 |
| Peptic ulcer disease, % | 18.8 | 19.2 | 16.9 | 0.70 |
| Diabetes without complications, % | 10.7 | 9.2 | 16.9 | 0.02 |
| Diabetes with complications, % | 31.5 | 30.4 | 36.4 | 0.23 |
| Mild liver disease, % | 8.6 | 8.0 | 11.0 | 0.28 |
| Moderate or severe liver disease, % | 1.8 | 1.6 | 2.5 | 0.44 |
| Malignant solid tumor, % | 16.6 | 17.3 | 13.6 | 0.41 |
| Metastatic cancer, % | 5.4 | 5.7 | 4.2 | 0.66 |
| Renal disease, % | 14.7 | 4.2 | 18.6 | 0.19 |
| 3 or more minor SCAP criteria, %* | 24.7 | 23.1 | 31.4 | 0.08 |
| PaO2/FiO2 ratio | 221 (161280) | 226 (169280) | 181 (133245) | 0.0004 |
| Multilobar disease, % | 46.3 | 43.2 | 53.9 | 0.11 |
| Presence of an effusion, % | 23.1 | 19.7 | 31.9 | 0.03 |
| Swallow impairment (of tested survivors), % | 34.1 | 34.1 | 34.1 | 0.22 |
| Presence of a DNR/DNI order, % | 26.4 | 23.7 | 38.1 | 0.0024 |
| Mortality of patients with DNR/DNI order, % | 39.1 | 38.8 | 40.0 | 1.00 |
| Receipt of broad‐spectrum antibiotic, % | 35.4 | 32.5 | 47.5 | 0.0028 |
| Receipt of MRSA antibiotic, % | 7.5 | 5.7 | 15.3 | 0.0014 |
| Receipt of anaerobe antibiotic, % | 28.7 | 27.6 | 33.1 | 0.26 |
Observed mortality was 21.0%. eCURB significantly underestimated mortality in this group, predicting a mortality rate of 10.6%. When classifying patients by the 2007 IDSA/ATS guidelines, 24.7% of the patients had 3 or more minor criteria for SCAP.[17] The PaO2/FiO2 ratio was obtained in 99.7% of patients. The median PaO2/FiO2 ratio observed in this population was 221 mm Hg (equivalent to 260 mm Hg at sea level barometric pressure, adjusted for our altitude of 1400 meters), near the threshold sea level definition (250 mm Hg) for SCAP.[13, 17] Admission to the ICU was common, as were admission orders for do not resuscitate (DNR) or do not intubate (DNI). Patients with healthcare‐associated aspiration pneumonia had a higher comorbidity index and had a higher mortality rate than patients with community‐acquired aspiration pneumonia, although we found no significant difference in the rate of hospital or ICU admission or the receipt of critical care therapies. Inpatient assessment of dysphagia and aspiration was conducted in 177 patients. Abnormal swallow was noted in 96% of those tested.
We found several differences between patients with community‐acquired aspiration pneumonia and 2584 patients with CAP identified during the same time period[13] (Table 4). Patients with community‐acquired aspiration pneumonia were older, more likely to have multilobar disease or effusion on imaging, and had greater disease severity. They also had a higher frequency of ICU and hospital admission, IDSA/ATS minor criteria for SCAP, and higher Charlson comorbidity indices. Patients with community‐acquired aspiration pneumonia were more likely to receive mechanical ventilation than CAP patients, although there was no difference in 30‐day mortality among intubated patients or a difference in ventilator‐free days.
| Community‐Acquired Aspiration Pneumonia (N = 510) | Community‐ Acquired Pneumonia (N = 2584) | P Value | |
|---|---|---|---|
| |||
| Age (range), y | 77 (6485) | 59 (4176) | <0.0001 |
| Female, % | 50.2 | 49.5 | 0.81 |
| 30‐day mortality, % | 19.0 | 4.2 | <0.0001 |
| CURB‐65 score | 2 (13) | 1 (02) | <0.0001 |
| Confusion, % | 12.7 | 5.1 | <0.0001 |
| Blood urea nitrogen | 21 (1532) | 16 (1124) | <0.0001 |
| Respiratory rate | 20 (1824) | 20 (1824) | <0.0001 |
| Systolic blood pressure | 129 (110150) | 130 (112146) | 0.67 |
| eCURB 30‐day mortality estimate, median, % | 5.2 (2.212.4) | 1.7 (0.94.3) | <0.0001 |
| eCURB 30‐day mortality estimate, mean, % | 9.7 11.5 | 4.4 7.5 | <0.0001 |
| AUC of eCURB versus mortality | 0.71 (0.660.75) | 0.86 (0.830.90) | <0.0001 |
| Excluding DNR/DNI patients | 0.69 (0.650.74) | 0.87 (0.830.90) | 0.0001 |
| AUC of CURB‐65 versus mortality | 0.66 (0.620.69) | 0.81 (0.780.85) | <0.0001 |
| Excluding DNR/DNI patients | 0.65 (0.600.70) | 0.81 (0.760.85) | 0.0003 |
| Hospital admission (of ED visits), % | 98.8 | 57.8 | <0.0001 |
| Hospital LOS, d | 6.5 (4.011.0) | 3.3 (2.25.2) | <0.0001 |
| ICU admission, % | 37.1 | 14.2 | <0.0001 |
| ICU LOS, d | 3.1 (1.87.6) | 2.5 (1.17.7) | 0.01 |
| Mean ventilator‐free days (of ICU patients, out of 30 days) | 25.9 7.7 | 25 9 | 0.75 |
| Receipt of mechanical ventilation, % | 17.2 | 7.8 | <0.0001 |
| Duration of ventilation, d | 3.1 (1.06.6) | 3.5 (1.57.2) | 0.09 |
| Receipt of vasopressor, % | 1.4 | 3.3 | 0.02 |
| Charlson comorbidity index | 3 (26) | 1 (03) | <0.0001 |
| Cerebrovascular disease, % | 32.4 | 10.0 | <0.0001 |
| Chronic pulmonary disease, % | 51.8 | 42.5 | <0.0001 |
| Congestive heart failure, % | 50.0 | 22.1 | <0.0001 |
| Connective tissue disease, % | 8.8 | 5.6 | 0.0084 |
| Dementia, % | 12.0 | 2.8 | <0.0001 |
| Hemiplegia/paraplegia, % | 8.0 | 2.7 | <0.0001 |
| Myocardial infarction, % | 17.8 | 10.8 | <0.0001 |
| Peripheral vascular disease, % | 16.3 | 7.4 | <0.0001 |
| Peptic ulcer disease, % | 19.2 | 7.6 | <0.0001 |
| Diabetes without complications, % | 9.2 | 24.7 | <0.0001 |
| Diabetes with complications, % | 30.4 | 5.1 | <0.0001 |
| Mild liver disease, % | 8.0 | 6.2 | 0.14 |
| Moderate or severe liver disease, % | 1.6 | 0.8 | 0.13 |
| Malignant solid tumor, % | 17. | 8.9 | <0.0001 |
| Metastatic cancer, % | 5.7 | 1.3 | <0.0001 |
| Renal disease, % | 4.2 | 5.6 | <0.0001 |
| 3 or more minor SCAP criteria, %* | 24.7 | 19.1 | 0.01 |
| PaO2/FiO2 ratio | 226 (169280) | 260 (148338) | 0.0004 |
| Multilobar disease, % | 43.2 | 37.2 | 0.0012 |
| Presence of an effusion, % | 19.7 | 18.3 | <0.0001 |
| Presence of a DNR/DNI order, % | 23.7 | 9.7 | <0.0001 |
| Mortality of patients with DNR/DNI order, % | 38.8 | 12.4 | <0.0001 |
| Receipt of broad‐spectrum antibiotic, % | 32.5 | 8.4 | <0.0001 |
| Receipt of MRSA antibiotic, % | 5.7 | 2.2 | <0.0001 |
| Receipt of anaerobe antibiotic, % | 27.6 | 3.1 | <0.0001 |
Thirty‐day mortality for patients with community‐acquired aspiration pneumonia was significantly higher than in CAP patients. Patients with community‐acquired aspiration pneumonia also had higher eCURB and CURB‐65 scores. However, eCURB was a poor predictor of 30‐day mortality, with an AUC of 0.71, compared to 0.86 calculated for the CAP population (Figure 2). CURB‐65 performed similarly: AUC was 0.66 vs 0.81. The presence of a DNR/DNI order was twice as prevalent in the community‐acquired aspiration pneumonia population vs the CAP population; those patients with a DNR/DNI order were 3 times as likely to die. Excluding patients with a DNR/DNI order did not improve performance of eCURB or CURB‐65 (Table 4). The presence of IDSA/ATS minor criteria for SCAP was not predictive of triage to the ICU in the group of patients with community‐acquired aspiration pneumonia (AUC: 0.51), compared with CAP patients (AUC: 0.88, P < 0.01 for comparison, Figure 3). This finding persisted in the subset of patients without a DNR/DNI order (AUC: 0.52 in community‐acquired aspiration pneumonia vs 0.88 in CAP, P < 0.01).


Our regression model of mortality incorporated gender, presence of effusion or multilobar pneumonia, presence of a DNR/DNI order, and all the components of the CURB‐65, IDSA/ATS minor criteria for SCAP, and Charlson comorbidity index. The regression model demonstrated that even after adjustment for age, comorbidities, disease severity, and presence of a DNR/DNI order, the presence of aspiration pneumonia was associated with higher mortality than CAP (odds ratio [OR]: 3.46, P < 0.001, Table 5). In this model, systolic blood pressure did not predict mortality, and diabetes with complications was associated with decreased mortality.
| Odds Ratio | P Value | |
|---|---|---|
| ||
| Presence of aspiration pneumonia | 3.46 (2.115.67) | <0.001 |
| Age, y | 1.03 (1.011.04) | <0.001 |
| Confusion | 3.14 (1.955.05) | <0.001 |
| Blood urea nitrogen, mg/dL | 1.03 (1.021.04) | <0.001 |
| Respiratory rate, breaths/minute | 1.03 (1.001.06) | 0.04 |
| PaO2/FiO2 ratio, per 1 mm Hg | 0.99 (0.991.00) | <0.001 |
| Moderate or severe liver disease | 9.21 (3.1626.86) | <0.001 |
| Paraplegia/hemiplegia | 2.43 (1.135.27) | 0.02 |
| Diabetes with complications | 0.42 (0.200.87) | 0.02 |
| Leukocytosis | 4.47 (2.278.82) | <0.001 |
| DNR/DNI | 1.75 (1.112.75) | 0.02 |
Microbiological Findings
Blood cultures were performed at admission in 67.4% of aspiration‐pneumonia patients, and a tracheal aspirate in half (50.7%) of intubated patients with aspiration pneumonia. Organisms were recovered in 90 patients (14.3%), although 41 of those patients had tracheal aspirates of organisms commonly thought to be nonpathogenic (nonpneumococcal alpha‐hemolytic streptococcus, nonhemolytic streptococcus, diphtheroids, micrococci, coagulase negative staphylococccus). Tracheal aspirate was the most common method of recovering an organism (7.8% of patients), followed by blood culture (4.3%). Bronchoalveolar lavage, urinary antigen, and pleural fluid culture were less common (1.3%, 1.1%, 0.3%, respectively). The microbiologic results were grouped into: Staphylococcus aureus, Streptococcus pneumoniae, enteric bacilli, Haemophilus species, Neisseria species, Moraxella catarrhalis, and Pseudomonas aeruginosa (Figure 4). Comparing healthcare‐associated with community‐acquired aspiration pneumonia, healthcare‐associated patients were more likely to have a confirmed infection with MRSA (4.2% vs 1.4%, P = 0.06) and enteric bacteria (5.1% vs 1.6%, P = 0.03). There were no other statistically significant differences in microbiologic recovery between the 2 groups. Antibiotics targeting anaerobic pathogens were administered in 28.7% of patients with aspiration pneumonia, with no correlation to the presence of healthcare‐associated risks. Healthcare‐associated patients were more likely to receive broad‐spectrum antibiotics (47.5% vs 32.5%, P < 0.01) and MRSA coverage (15.3% vs 5.7%, P < 0.01) than patients with community‐acquired aspiration pneumonia.
DISCUSSION
Our study identifies a larger cohort of patients with aspiration pneumonia than previous studies.[21, 22, 23, 24, 25] Patients with community‐acquired aspiration pneumonia are older and more likely to die than CAP patients. They are more likely to be admitted to the hospital or ICU. Thirty‐day mortality in this patient population was significantly underestimated by CURB‐65 and eCURB, models developed and validated in CAP populations.[9, 26] This finding supports a prior study.[27] It appears that a traditional prognostic model assessing mortality risk in the CAP patient does not apply to the aspiration‐pneumonia patient. One reason for eCURB and CURB‐65s poor utility in community‐acquired aspiration pneumonia may be their reliance on objective clinical features rather than comorbidities, which may influence mortality to a greater degree in aspiration pneumonia.
This study has several limitations. There is no gold standard for the definition of aspiration pneumonia, and it is difficult to distinguish aspiration pneumonia from typical pneumonia. It is plausible that older patients with greater comorbidities are being designated as aspiration pneumonia. If this is the case, then aspiration pneumonia merely represents the end of the pneumonia spectrum with highest mortality risk, and it is no surprise that these patients fare poorly.
It appears that the hospitalist or emergency department physician implicitly appreciates that aspiration pneumonia has a higher mortality risk than predicted by traditional severity assessment. With such high mortality and morbidity, a patient presenting to the emergency room with aspiration pneumonia is almost always admitted to the hospital. Further work in this area should investigate other factors to improve prognostic modeling in patients with aspiration pneumonia, although the utility of such a model may be limited to determining ICU admission. Our data indicate that IDSA/ATS minor criteria for SCAP are not useful in predicting admission to the ICU in patients with aspiration pneumonia.
In this study, a DNR/DNI order was twice as common in the community‐acquired aspiration pneumonia population than the CAP population. However, patients with community‐acquired aspiration pneumonia and a DNR/DNI order were more than 3 times more likely to die than patients with CAP and a DNR/DNI order. Our regression model suggested that the presence of a DNR/DNI order was an independent predictor of mortality (OR: 1.75, P < 0.001). Although a DNR/DNI order may correlate with the withholding or withdrawal of medical therapy, it is also a surrogate for increased age or comorbidities.[28] In our study, however, the increased prevalence of DNR/DNI orders did not explain the poor mortality prediction of the eCURB or CURB‐65, as exclusion of those patients did not significantly alter the AUCs in either the aspiration group or the CAP group.
Controversy exists regarding treatment of aspiration pneumonia. Historically, some have advocated for treatment of aspiration pneumonia with a regimen designed to cover anaerobic bacteria.[29] This recommendation was based on early microbiologic studies that obtained the samples late in the course of illness, or other studies where the sample was obtained transtracheally, where oropharyngeal flora may contaminate the sample.[30, 31, 32] Our clinically obtained microbiologic recovery of organisms was similar to the flora recovered in more recent CAP studies, in respect to both the incidence of pathogen recovery and the relative frequencies of recovered organisms.[33, 34] Our data do not support inferences regarding the prevalence of anaerobic infections, as the recovery of anaerobic organisms was limited to blood and pleural fluid cultures in this study, rather than techniques used in research settings that might have greater yield. As expected, patients with healthcare‐associated risk factors trended toward increased incidence of MRSA. Given the similarity of the organisms recovered to those recovered in CAP,[35] this study supports IDSA/ATS recommendations that antibiotic therapy in aspiration pneumonia be similar to that of higher‐risk CAP, with the addition of vancomycin or linezolid for MRSA coverage in patients with risk factors for healthcare‐associated pneumonia.[17]
Our study is limited by its single‐center retrospective design. However, beginning in 1995, the LDS Hospital emergency department initiated a standardized pneumonia therapy protocol and deployed electronic medical records, which prospectively recorded a wide array of clinical, therapeutic, and biometric data. Most data elements used in this analysis were routinely charted for clinical purposes in real time. Although the eCURB, CURB‐65, and some comorbidities could be extracted electronically for each patient, it was not possible to calculate the pneumonia severity index score due to our inability to rigorously identify the necessary comorbid illness elements. Other comorbidities, not present in our model, may have been identified by the physician who makes a diagnosis of aspiration pneumonia. Our identification of swallow impairment is also methodologically limited. The decision to obtain a swallow study was clinical, usually occurring upon convalescence. Therefore, it is not possible to distinguish between antecedent oropharyngeal dysfunction and post‐critical illness dysfunction.
Our definition of aspiration pneumonia required the treating physician to diagnose and code the patient as having aspiration pneumonia, followed by excluding patients more likely to have aspiration pneumonitis. Although we relied on ICD‐9 codes to initially identify aspiration pneumonia, all patients in our database were confirmed by physician chart review. Our incidence of community‐acquired aspiration pneumonia is congruent with other studies using different methodologies.[1, 36, 37] Unfortunately, there is no standard and widely accepted definition for separating aspiration pneumonia from usual CAP. A younger and healthier patient who has developed pneumonia subsequent to aspiration may be more likely to be diagnosed with CAP, resulting in selection bias for older patients with greater comorbidities.
CONCLUSION
Patients diagnosed with aspiration pneumonia are older, have more comorbid conditions, and demonstrate greater disease severity and higher 30‐day mortality than CAP patients. Mortality prediction using CURB‐65 and eCURB in this population was poor, possibly due to a greater effect of comorbidities on mortality. The pneumonia severity index, which incorporates patient comorbidities, might perform better than the eCURB or CURB‐65, and should be studied in aspiration pneumonia populations where comorbid illness information is prospectively collected. Further areas of study include creating an improved mortality prediction model for aspiration pneumonia that incorporates comorbid conditions, DNR/DNI status, and disease severity.
Acknowledgments
The authors acknowledge Al Jephson for database support, Yao Li for statistical analysis, and Anita Austin for help reviewing the medical records. Dr. Lanspa had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure
Preliminary versions of this work were presented as posters at the American Thoracic Society Meeting, Denver, Colorado, May 17, 2011. This study was supported by grants from the Intermountain Research and Medical Foundation. Dr. Brown is supported by a career development award from National Institute of General Medical Sciences (K23GM094465). Dr. Dean served on an advisory board for Merck, has been a paid consultant for Cerexa, and has received an investigator‐initiated competitive grant from Pfizer for development of an electronic pneumonia decision support tool. All other authors report no relevant financial disclosures.
- , , , et al. Severe community‐acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis. 1991;144(2):312–318.
- , , . Risk factors for pneumonia in the elderly. Am J Med. 1994;96(4):313–320.
- , . Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328–336.
- , , . Pneumonia versus aspiration pneumonitis in nursing home residents: diagnosis and management. J Am Geriatr Soc. 2003;51(1):17–23.
- . Aspiration pneumonia: mixing apples with oranges and tangerines. Crit Care Med. 2004;32(5):1236; author reply 1236–1237.
- , , , , . Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999–2000. Crit Care Med. 2003;31(7):1930–1937.
- . Aspiration syndromes: aspiration pneumonia and pneumonitis. Hosp Pract (Minneap). 2010;38(1):35–42.
- . Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–671.
- , , , et al. CURB‐65 pneumonia severity assessment adapted for electronic decision support. Chest. 2011;140(1):156–163.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382.
- , , , et al. Comparison of a disease‐specific and a generic severity of illness measure for patients with community‐acquired pneumonia. J Gen Intern Med. 1995;10(7):359–368.
- , , , et al. Development and validation of a clinical prediction rule for severe community‐acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–1256.
- , , , . Validation of the Infectious Disease Society of America/American Thoracic Society 2007 guidelines for severe community‐acquired pneumonia. Crit Care Med. 2009;37(12):3010–3016.
- Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.
- .The Utah genealogical database: a resource for genetic epidemiology. In: Cairns JL, Skolnick M, eds. Banbury Report No 4; Cancer Incidence in Defined Populations. New York, NY:Cold Spring Harbor Laboratory;1980:285–297.
- . Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol. 1979;46(3):599–602.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , . Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845.
- , , . Prandial aspiration and pneumonia in an elderly population followed over 3 years. Dysphagia. 1996;11(2):104–109.
- , , , . Predictors of aspiration pneumonia in nursing home residents. Dysphagia. 2002;17(4):298–307.
- , , , , , . Aspiration pneumonia: dental and oral risk factors in an older veteran population. J Am Geriatr Soc. 2001;49(5):557–563.
- , , , et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167(12):1650–1654.
- , , . Aspiration pneumonia in stroke. Arch Phys Med Rehabil. 1993;74(9):973–976.
- , , , et al. Validation of a predictive rule for the management of community‐acquired pneumonia. Eur Respir J. 2006;27(1):151–157.
- , , , , . Pneumonia Severity Index (PSI), CURB‐65, and mortality in hospitalized elderly patients with aspiration pneumonia [in German]. Zeitschrift fur Gerontologie und Geriatrie. 2011;44(4):229–234.
- , , , . Do‐not‐resuscitate orders for critically ill patients in the hospital. How are they used and what is their impact?JAMA. 1986;256(2):233–237.
- , , , , . The Sanford Guide to Antimicrobial Therapy: 2010.40th ed.Sperryville, VA:Antimicrobial Therapy, Inc.;2009.
- , , . The bacteriology of aspiration pneumonia. Am J Med. 1974;56(2):202–207.
- , , . Bacteriologic flora of aspiration‐induced pulmonary infections. Arch Intern Med. 1975;135(5):711–714.
- , . Bacteriology of aspiration pneumonia. A prospective study of community‐ and hospital‐acquired cases. Ann Intern Med. 1974;81(3):329–331.
- , . The role of anaerobes in patients with ventilator‐associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115(1):178–183.
- , , , et al. Is penicillin G an adequate initial treatment for aspiration pneumonia? A prospective evaluation using a protected specimen brush and quantitative cultures. Intensive Care Med. 1993;19(5):279–284.
- , , , , . Community‐acquired pneumonia in the elderly. Semin Respir Infect. 1999;14(2):173–183.
- , , . Nursing home‐acquired pneumonia. A case‐control study. J Am Geriatr Soc. 1986;34(10):697–702.
- , , , , . Severe community‐acquired pneumonia. Etiology, epidemiology, and prognosis factors. French Study Group for Community‐Acquired Pneumonia in the Intensive Care Unit. Chest. 1994;105(5):1487–1495.
Pneumonia is a common clinical syndrome with well‐described epidemiology and microbiology. Aspiration pneumonia comprises 5% to 15% of patients with pneumonia,[1] but is less well‐characterized despite being a major syndrome of pneumonia in the elderly.[2, 3] Difficulties in studying aspiration pneumonia include the lack of a sensitive and specific marker for aspiration, the overlap between aspiration pneumonia and other forms of pneumonia, and the lack of differentiation between aspiration pneumonia and aspiration pneumonitis by many clinicians.[4, 5, 6] Aspiration pneumonia, which develops after the aspiration of oropharyngeal contents, differs from aspiration pneumonitis, wherein inhalation of gastric contents causes inflammation without the subsequent development of bacterial infection.[7, 8]
A number of validated mortality prediction models exist for community‐acquired pneumonia (CAP), using a variety of clinical predictors. One clinical prediction rule endorsed by the British Thoracic Society is the CURB‐65, which assigns a score for Confusion, Uremia >19 mg/dL, Respiratory rate >= 30 breaths/min, Blood Pressure < 90 mmHg systolic or < 60 mmHg diastolic, and age 65). We favor eCURB, a version of the CURB‐65 model that uses continuously weighted variables to more accurately predict mortality, validated in CAP populations.[9] Most studies validating pneumonia severity scoring systems excluded aspiration pneumonia from their study population.[10, 11, 12] Severity scoring systems for CAP may not accurately predict disease severity patients with aspiration pneumonia.
The aims of our study were to: (1) identify a population of patients with aspiration pneumonia; (2) compare characteristics and outcomes in patients with community‐acquired aspiration pneumonia to those with CAP; and (3) study the performance of eCURB and CURB‐65 in predicting mortality for patients with community‐acquired aspiration pneumonia.
PATIENTS AND METHODS
Study Design and Setting
The study was performed at LDS Hospital, a university‐affiliated community teaching hospital in Salt Lake City, Utah, with 520 beds. In retrospective analysis of data from the electronic medical records, we identified all patients older than 18 years who were evaluated in the emergency department at LDS Hospital or admitted patients from other sources (direct admission, transfer from another hospital) from 1996 to 2006 with International Statistical Classification of Disease and Health Related Problems, 9th Revision (ICD‐9) codes specific for aspiration pneumonia and pneumonitis (507.x). The treating physicians were mostly hospitalists and intensivists. Two physicians (M.L. and N.D.) manually reviewed the electronic medical records, including the emergency room physician's notes, the admission histories and physicals, the discharge summaries, and radiographic reports of the patients identified in the query. Consensus regarding the diagnosis of aspiration pneumonia was achieved in all patients reviewed using criteria listed in Table 1. This study was approved by the LDS Hospital institutional review board, and permission was granted to use the Utah Population Database for determining mortality (#1008505), with a waiver of informed consent. For the contemporaneous group of CAP patients, we used a previously described population identified using ICD‐9 codes 481.x to 487.x, captured from the same hospital during the same period.[13]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| |
| 1. Age 18 years | 1. Absence of radiographic evidence of pneumonia within 48 hours after evaluation |
| 2. Either admitted to hospital or evaluated in emergency department | 2. Previous episode of aspiration pneumonia within 12 months |
| 3. 507.x code as primary diagnosis | 3. Initial admission date >48 hours before transfer to LDS Hospital |
| 4. 507.x code as secondary diagnosis with a primary diagnosis of pneumonia, respiratory failure, or septicemia | 4. AIDS 5. Receipt of antiretroviral therapy 6. History of solid organ transplant |
| 5. Treating physician indicated a diagnosis of aspiration pneumonia in the history and physical and/or discharge summary | 7. Hematologic malignancy 8. Witnessed isolated aspiration event within 24 hours prior to evaluation 9. Drug overdose, cardiopulmonary arrest, or seizure prior to hospital admission 10. Laryngoscopic or bronchoscopic evidence of food material in airway |
Inclusion and Exclusion Criteria
Inclusion and exclusion criteria are listed in Table 1. To exclude patients with recurrent pneumonia, we included only the first episode of pneumonia in a given 12‐month period. LDS Hospital frequently receives patients transferred from surrounding emergency departments and intensive care units. We excluded patients who were transferred >48 hours from their initial emergency department admission and therefore were late in their disease course. Exclusion criteria 8 to 10 were used to exclude patients with clinical presentations more consistent with aspiration pneumonitis. We also excluded immunocompromised patients (criteria 4 to 7).
Healthcare‐associated aspiration pneumonia was defined as receipt of chronic hemodialysis, residence in a nursing facility, or hospitalization within any Intermountain Healthcare‐affiliated hospital within the past 90 days.[14] The remaining patients were defined as community‐acquired aspiration pneumonia.
Measurements
The first vital signs, orientation status, and first 12 hours of routine laboratory results were extracted from the electronic medical records and used to calculate predicted mortality by eCURB and CURB‐65. We determined 30‐day mortality from the merger of the electronic medical records with vital status information from the Utah Population Database.[15] The first measured SpO2 and FiO2 were used to estimate the PaO2/FiO2 ratio, using the Severinghaus calculation[16] if no arterial blood gas was available. Presence of American Thoracic Society/Infectious Diseases Society of America (IDSA/ATS) 2007 minor criteria for severe community‐acquired pneumonia (SCAP)[17] were obtained from baseline patient characteristics (Table 2). A Charlson comorbidity index was calculated from ICD‐9 codes using published methodology.[18, 19] Presence of an abnormal swallow was defined as dysphagia or aspiration on modified barium swallow study, fiberoptic endoscopic evaluation, or clinical determination by a speech language pathologist during the index hospitalization. We also looked for causative pathogens, defined by a positive pneumococcus or legionella urinary antigen, or a positive culture from blood, bronchoalveolar lavage, pleural fluid, or tracheal aspirate, collected within 24 hours of admission. Antibiotics administered within the first 24 hours of admission were classified into 4 broad groups based on local physician prescribing patterns. Clindamycin and metronidazole were considered anaerobic‐specific antibiotics. Vancomycin or linezolid were considered methicillin‐resistant Staphylococcus aureus (MRSA) antibiotics. Broad‐spectrum antibiotics included any of the following: carbapenems, aztreonam, piperacillin/tazobactam, ticarcillin/clavulanate, cefepime, and ceftazidime. Macrolides, respiratory fluoroquinolones, and third‐generation cephalosporins were considered standard‐care antibiotics.
| Respiratory rate 30 breaths/minute |
| PaO2/FiO2 250 |
| Multilobar infiltrates |
| Confusion/disorientation |
| Uremia (blood urea nitrogen 20 mg/dL) |
| Leukopenia (white blood cell count <4000 cells/mm3) |
| Thrombocytopenia (platelet count <100,000 cells/mm3) |
| Hypothermia (core temperature 36C) |
| Hypotension requiring aggressive fluid resuscitation |
Statistical Analysis
We compared baseline patient characteristics and clinical outcomes using the Fisher exact test to compare proportions of categorical variables, and Mann‐Whitney U test or Student t test to compare central tendencies of continuous variables, as dictated by the normality of the data. Receiver operating characteristic curves calculated the ability of eCURB and CURB‐65 to predict 30‐day mortality prediction in patients with community‐acquired aspiration pneumonia and CAP, as well as the ability of IDSA/ATS minor criteria for SCAP to predict admission to the intensive care unit (ICU). We performed multivariate logistic regression to predict 30‐day mortality in patients with community‐acquired aspiration pneumonia and CAP, using stepwise backward elimination. Confounders were included if they were significant at a 0.05 level or if they altered the coefficient of the main variable by more than 10%. For logistic models, we evaluated goodness of fit with the Hosmer‐Lemeshow technique; comparisons of area under the curve (AUC) among models were made using the technique of DeLong.[20] Two‐tailed P values of 0.05 were considered statistically significant. Stata version 12 (StataCorp, College Station, TX) was used for all analyses.
RESULTS
Our initial query identified 1165 patients. Physician review of the medical records resulted in 628 patients, 118 of whom were classified as healthcare‐associated aspiration pneumonia (Figure 1, Table 3). Of all aspiration pneumonia patients, 80% were seen in the emergency department, 12.5% were directly admitted from the community, and 7.5% were transferred from another healthcare facility. Almost all patients seen in the emergency department (99.0%) were admitted to the hospital, with median length of hospitalization 6.7 days among survivors.

| Aspiration Pneumonia (N = 628) | Community‐Acquired Aspiration Pneumonia (N = 510) | Healthcare Associated Aspiration Pneumonia (N=118) | P Value | |
|---|---|---|---|---|
| ||||
| Age (range), y | 77 (6585) | 77 (6485) | 80 (6786) | 0.42 |
| Female, % | 49.8 | 50.2 | 48.3 | 0.76 |
| 30‐day mortality, % | 21.0% | 19.0% | 29.7% | 0.02 |
| CURB‐65 score | 2 (13) | 2 (13) | 2 (13) | 0.0012 |
| Confusion | 13.9% | 12.7% | 18.6% | 0.10 |
| Blood urea nitrogen (mg/dL) | 22 (1634) | 21 (1532) | 30 (2047) | <0.0001 |
| Respiratory rate (breaths/min) | 20 (1826) | 20 (1824) | 21 (1828) | 0.30 |
| Systolic blood pressure (mm Hg) | 128 (110149) | 129 (110150) | 127 (105146) | 0.28 |
| eCURB 30‐day mortality estimate (median, %) | 5.6 (2.414.2) | 5.2 (2.212.4) | 8.9 (4.222.5) | <0.0001 |
| eCURB 30‐day mortality estimate (mean, %) | 10.6 12.2 | 9.7 11.5 | 14.614.1 | <0.0001 |
| Hospital admission (of ED visits), % | 99.0 | 98.8 | 100 | 0.59 |
| Hospital LOS, d | 6.7 (4.111.1) | 6.5 (4.011.0) | 7.8 (5.412.3) | 0.05 |
| ICU admission, % | 37.9 | 37.1 | 41.5 | 0.21 |
| ICU LOS, d | 3.5 (1.98.8) | 3.1 (1.87.6) | 5.6 (3.810.8) | 0.02 |
| Mean ventilator‐free days (of ICU patients, out of 30 days) | 25.28.3 | 25.97.7 | 22.710.0 | 0.01 |
| Receipt of mechanical ventilation, % | 18.6 | 17.2 | 24.6 | 0.09 |
| Duration of ventilation, d | 2.8 (0.96.5) | 3.1 (1.06.6) | 1.9 (0.86.3) | 0.05 |
| Receipt of vasopressor, % | 1.8 | 1.4 | 3.4 | 0.13 |
| Charlson comorbidity index | 4 (26) | 3 (26) | 4 (36) | 0.0024 |
| Cerebrovascular disease, % | 33.9 | 32.4 | 40.7 | 0.11 |
| Chronic pulmonary disease, % | 51.0 | 51.8 | 47.5 | 0.42 |
| Congestive heart failure, % | 52.4 | 50.0 | 62.7 | 0.01 |
| Connective tissue disease, % | 8.4 | 8.8 | 6.8 | 0.58 |
| Dementia, % | 14.2 | 12.0 | 23.7 | 0.0019 |
| Hemiplegia/paraplegia | 9.4 | 8.0 | 15.2 | 0.02 |
| Myocardial infarction, % | 21.0 | 17.8 | 29.7 | 0.02 |
| Peripheral vascular disease, % | 17.7 | 16.3 | 23.7 | 0.06 |
| Peptic ulcer disease, % | 18.8 | 19.2 | 16.9 | 0.70 |
| Diabetes without complications, % | 10.7 | 9.2 | 16.9 | 0.02 |
| Diabetes with complications, % | 31.5 | 30.4 | 36.4 | 0.23 |
| Mild liver disease, % | 8.6 | 8.0 | 11.0 | 0.28 |
| Moderate or severe liver disease, % | 1.8 | 1.6 | 2.5 | 0.44 |
| Malignant solid tumor, % | 16.6 | 17.3 | 13.6 | 0.41 |
| Metastatic cancer, % | 5.4 | 5.7 | 4.2 | 0.66 |
| Renal disease, % | 14.7 | 4.2 | 18.6 | 0.19 |
| 3 or more minor SCAP criteria, %* | 24.7 | 23.1 | 31.4 | 0.08 |
| PaO2/FiO2 ratio | 221 (161280) | 226 (169280) | 181 (133245) | 0.0004 |
| Multilobar disease, % | 46.3 | 43.2 | 53.9 | 0.11 |
| Presence of an effusion, % | 23.1 | 19.7 | 31.9 | 0.03 |
| Swallow impairment (of tested survivors), % | 34.1 | 34.1 | 34.1 | 0.22 |
| Presence of a DNR/DNI order, % | 26.4 | 23.7 | 38.1 | 0.0024 |
| Mortality of patients with DNR/DNI order, % | 39.1 | 38.8 | 40.0 | 1.00 |
| Receipt of broad‐spectrum antibiotic, % | 35.4 | 32.5 | 47.5 | 0.0028 |
| Receipt of MRSA antibiotic, % | 7.5 | 5.7 | 15.3 | 0.0014 |
| Receipt of anaerobe antibiotic, % | 28.7 | 27.6 | 33.1 | 0.26 |
Observed mortality was 21.0%. eCURB significantly underestimated mortality in this group, predicting a mortality rate of 10.6%. When classifying patients by the 2007 IDSA/ATS guidelines, 24.7% of the patients had 3 or more minor criteria for SCAP.[17] The PaO2/FiO2 ratio was obtained in 99.7% of patients. The median PaO2/FiO2 ratio observed in this population was 221 mm Hg (equivalent to 260 mm Hg at sea level barometric pressure, adjusted for our altitude of 1400 meters), near the threshold sea level definition (250 mm Hg) for SCAP.[13, 17] Admission to the ICU was common, as were admission orders for do not resuscitate (DNR) or do not intubate (DNI). Patients with healthcare‐associated aspiration pneumonia had a higher comorbidity index and had a higher mortality rate than patients with community‐acquired aspiration pneumonia, although we found no significant difference in the rate of hospital or ICU admission or the receipt of critical care therapies. Inpatient assessment of dysphagia and aspiration was conducted in 177 patients. Abnormal swallow was noted in 96% of those tested.
We found several differences between patients with community‐acquired aspiration pneumonia and 2584 patients with CAP identified during the same time period[13] (Table 4). Patients with community‐acquired aspiration pneumonia were older, more likely to have multilobar disease or effusion on imaging, and had greater disease severity. They also had a higher frequency of ICU and hospital admission, IDSA/ATS minor criteria for SCAP, and higher Charlson comorbidity indices. Patients with community‐acquired aspiration pneumonia were more likely to receive mechanical ventilation than CAP patients, although there was no difference in 30‐day mortality among intubated patients or a difference in ventilator‐free days.
| Community‐Acquired Aspiration Pneumonia (N = 510) | Community‐ Acquired Pneumonia (N = 2584) | P Value | |
|---|---|---|---|
| |||
| Age (range), y | 77 (6485) | 59 (4176) | <0.0001 |
| Female, % | 50.2 | 49.5 | 0.81 |
| 30‐day mortality, % | 19.0 | 4.2 | <0.0001 |
| CURB‐65 score | 2 (13) | 1 (02) | <0.0001 |
| Confusion, % | 12.7 | 5.1 | <0.0001 |
| Blood urea nitrogen | 21 (1532) | 16 (1124) | <0.0001 |
| Respiratory rate | 20 (1824) | 20 (1824) | <0.0001 |
| Systolic blood pressure | 129 (110150) | 130 (112146) | 0.67 |
| eCURB 30‐day mortality estimate, median, % | 5.2 (2.212.4) | 1.7 (0.94.3) | <0.0001 |
| eCURB 30‐day mortality estimate, mean, % | 9.7 11.5 | 4.4 7.5 | <0.0001 |
| AUC of eCURB versus mortality | 0.71 (0.660.75) | 0.86 (0.830.90) | <0.0001 |
| Excluding DNR/DNI patients | 0.69 (0.650.74) | 0.87 (0.830.90) | 0.0001 |
| AUC of CURB‐65 versus mortality | 0.66 (0.620.69) | 0.81 (0.780.85) | <0.0001 |
| Excluding DNR/DNI patients | 0.65 (0.600.70) | 0.81 (0.760.85) | 0.0003 |
| Hospital admission (of ED visits), % | 98.8 | 57.8 | <0.0001 |
| Hospital LOS, d | 6.5 (4.011.0) | 3.3 (2.25.2) | <0.0001 |
| ICU admission, % | 37.1 | 14.2 | <0.0001 |
| ICU LOS, d | 3.1 (1.87.6) | 2.5 (1.17.7) | 0.01 |
| Mean ventilator‐free days (of ICU patients, out of 30 days) | 25.9 7.7 | 25 9 | 0.75 |
| Receipt of mechanical ventilation, % | 17.2 | 7.8 | <0.0001 |
| Duration of ventilation, d | 3.1 (1.06.6) | 3.5 (1.57.2) | 0.09 |
| Receipt of vasopressor, % | 1.4 | 3.3 | 0.02 |
| Charlson comorbidity index | 3 (26) | 1 (03) | <0.0001 |
| Cerebrovascular disease, % | 32.4 | 10.0 | <0.0001 |
| Chronic pulmonary disease, % | 51.8 | 42.5 | <0.0001 |
| Congestive heart failure, % | 50.0 | 22.1 | <0.0001 |
| Connective tissue disease, % | 8.8 | 5.6 | 0.0084 |
| Dementia, % | 12.0 | 2.8 | <0.0001 |
| Hemiplegia/paraplegia, % | 8.0 | 2.7 | <0.0001 |
| Myocardial infarction, % | 17.8 | 10.8 | <0.0001 |
| Peripheral vascular disease, % | 16.3 | 7.4 | <0.0001 |
| Peptic ulcer disease, % | 19.2 | 7.6 | <0.0001 |
| Diabetes without complications, % | 9.2 | 24.7 | <0.0001 |
| Diabetes with complications, % | 30.4 | 5.1 | <0.0001 |
| Mild liver disease, % | 8.0 | 6.2 | 0.14 |
| Moderate or severe liver disease, % | 1.6 | 0.8 | 0.13 |
| Malignant solid tumor, % | 17. | 8.9 | <0.0001 |
| Metastatic cancer, % | 5.7 | 1.3 | <0.0001 |
| Renal disease, % | 4.2 | 5.6 | <0.0001 |
| 3 or more minor SCAP criteria, %* | 24.7 | 19.1 | 0.01 |
| PaO2/FiO2 ratio | 226 (169280) | 260 (148338) | 0.0004 |
| Multilobar disease, % | 43.2 | 37.2 | 0.0012 |
| Presence of an effusion, % | 19.7 | 18.3 | <0.0001 |
| Presence of a DNR/DNI order, % | 23.7 | 9.7 | <0.0001 |
| Mortality of patients with DNR/DNI order, % | 38.8 | 12.4 | <0.0001 |
| Receipt of broad‐spectrum antibiotic, % | 32.5 | 8.4 | <0.0001 |
| Receipt of MRSA antibiotic, % | 5.7 | 2.2 | <0.0001 |
| Receipt of anaerobe antibiotic, % | 27.6 | 3.1 | <0.0001 |
Thirty‐day mortality for patients with community‐acquired aspiration pneumonia was significantly higher than in CAP patients. Patients with community‐acquired aspiration pneumonia also had higher eCURB and CURB‐65 scores. However, eCURB was a poor predictor of 30‐day mortality, with an AUC of 0.71, compared to 0.86 calculated for the CAP population (Figure 2). CURB‐65 performed similarly: AUC was 0.66 vs 0.81. The presence of a DNR/DNI order was twice as prevalent in the community‐acquired aspiration pneumonia population vs the CAP population; those patients with a DNR/DNI order were 3 times as likely to die. Excluding patients with a DNR/DNI order did not improve performance of eCURB or CURB‐65 (Table 4). The presence of IDSA/ATS minor criteria for SCAP was not predictive of triage to the ICU in the group of patients with community‐acquired aspiration pneumonia (AUC: 0.51), compared with CAP patients (AUC: 0.88, P < 0.01 for comparison, Figure 3). This finding persisted in the subset of patients without a DNR/DNI order (AUC: 0.52 in community‐acquired aspiration pneumonia vs 0.88 in CAP, P < 0.01).


Our regression model of mortality incorporated gender, presence of effusion or multilobar pneumonia, presence of a DNR/DNI order, and all the components of the CURB‐65, IDSA/ATS minor criteria for SCAP, and Charlson comorbidity index. The regression model demonstrated that even after adjustment for age, comorbidities, disease severity, and presence of a DNR/DNI order, the presence of aspiration pneumonia was associated with higher mortality than CAP (odds ratio [OR]: 3.46, P < 0.001, Table 5). In this model, systolic blood pressure did not predict mortality, and diabetes with complications was associated with decreased mortality.
| Odds Ratio | P Value | |
|---|---|---|
| ||
| Presence of aspiration pneumonia | 3.46 (2.115.67) | <0.001 |
| Age, y | 1.03 (1.011.04) | <0.001 |
| Confusion | 3.14 (1.955.05) | <0.001 |
| Blood urea nitrogen, mg/dL | 1.03 (1.021.04) | <0.001 |
| Respiratory rate, breaths/minute | 1.03 (1.001.06) | 0.04 |
| PaO2/FiO2 ratio, per 1 mm Hg | 0.99 (0.991.00) | <0.001 |
| Moderate or severe liver disease | 9.21 (3.1626.86) | <0.001 |
| Paraplegia/hemiplegia | 2.43 (1.135.27) | 0.02 |
| Diabetes with complications | 0.42 (0.200.87) | 0.02 |
| Leukocytosis | 4.47 (2.278.82) | <0.001 |
| DNR/DNI | 1.75 (1.112.75) | 0.02 |
Microbiological Findings
Blood cultures were performed at admission in 67.4% of aspiration‐pneumonia patients, and a tracheal aspirate in half (50.7%) of intubated patients with aspiration pneumonia. Organisms were recovered in 90 patients (14.3%), although 41 of those patients had tracheal aspirates of organisms commonly thought to be nonpathogenic (nonpneumococcal alpha‐hemolytic streptococcus, nonhemolytic streptococcus, diphtheroids, micrococci, coagulase negative staphylococccus). Tracheal aspirate was the most common method of recovering an organism (7.8% of patients), followed by blood culture (4.3%). Bronchoalveolar lavage, urinary antigen, and pleural fluid culture were less common (1.3%, 1.1%, 0.3%, respectively). The microbiologic results were grouped into: Staphylococcus aureus, Streptococcus pneumoniae, enteric bacilli, Haemophilus species, Neisseria species, Moraxella catarrhalis, and Pseudomonas aeruginosa (Figure 4). Comparing healthcare‐associated with community‐acquired aspiration pneumonia, healthcare‐associated patients were more likely to have a confirmed infection with MRSA (4.2% vs 1.4%, P = 0.06) and enteric bacteria (5.1% vs 1.6%, P = 0.03). There were no other statistically significant differences in microbiologic recovery between the 2 groups. Antibiotics targeting anaerobic pathogens were administered in 28.7% of patients with aspiration pneumonia, with no correlation to the presence of healthcare‐associated risks. Healthcare‐associated patients were more likely to receive broad‐spectrum antibiotics (47.5% vs 32.5%, P < 0.01) and MRSA coverage (15.3% vs 5.7%, P < 0.01) than patients with community‐acquired aspiration pneumonia.

DISCUSSION
Our study identifies a larger cohort of patients with aspiration pneumonia than previous studies.[21, 22, 23, 24, 25] Patients with community‐acquired aspiration pneumonia are older and more likely to die than CAP patients. They are more likely to be admitted to the hospital or ICU. Thirty‐day mortality in this patient population was significantly underestimated by CURB‐65 and eCURB, models developed and validated in CAP populations.[9, 26] This finding supports a prior study.[27] It appears that a traditional prognostic model assessing mortality risk in the CAP patient does not apply to the aspiration‐pneumonia patient. One reason for eCURB and CURB‐65s poor utility in community‐acquired aspiration pneumonia may be their reliance on objective clinical features rather than comorbidities, which may influence mortality to a greater degree in aspiration pneumonia.
This study has several limitations. There is no gold standard for the definition of aspiration pneumonia, and it is difficult to distinguish aspiration pneumonia from typical pneumonia. It is plausible that older patients with greater comorbidities are being designated as aspiration pneumonia. If this is the case, then aspiration pneumonia merely represents the end of the pneumonia spectrum with highest mortality risk, and it is no surprise that these patients fare poorly.
It appears that the hospitalist or emergency department physician implicitly appreciates that aspiration pneumonia has a higher mortality risk than predicted by traditional severity assessment. With such high mortality and morbidity, a patient presenting to the emergency room with aspiration pneumonia is almost always admitted to the hospital. Further work in this area should investigate other factors to improve prognostic modeling in patients with aspiration pneumonia, although the utility of such a model may be limited to determining ICU admission. Our data indicate that IDSA/ATS minor criteria for SCAP are not useful in predicting admission to the ICU in patients with aspiration pneumonia.
In this study, a DNR/DNI order was twice as common in the community‐acquired aspiration pneumonia population than the CAP population. However, patients with community‐acquired aspiration pneumonia and a DNR/DNI order were more than 3 times more likely to die than patients with CAP and a DNR/DNI order. Our regression model suggested that the presence of a DNR/DNI order was an independent predictor of mortality (OR: 1.75, P < 0.001). Although a DNR/DNI order may correlate with the withholding or withdrawal of medical therapy, it is also a surrogate for increased age or comorbidities.[28] In our study, however, the increased prevalence of DNR/DNI orders did not explain the poor mortality prediction of the eCURB or CURB‐65, as exclusion of those patients did not significantly alter the AUCs in either the aspiration group or the CAP group.
Controversy exists regarding treatment of aspiration pneumonia. Historically, some have advocated for treatment of aspiration pneumonia with a regimen designed to cover anaerobic bacteria.[29] This recommendation was based on early microbiologic studies that obtained the samples late in the course of illness, or other studies where the sample was obtained transtracheally, where oropharyngeal flora may contaminate the sample.[30, 31, 32] Our clinically obtained microbiologic recovery of organisms was similar to the flora recovered in more recent CAP studies, in respect to both the incidence of pathogen recovery and the relative frequencies of recovered organisms.[33, 34] Our data do not support inferences regarding the prevalence of anaerobic infections, as the recovery of anaerobic organisms was limited to blood and pleural fluid cultures in this study, rather than techniques used in research settings that might have greater yield. As expected, patients with healthcare‐associated risk factors trended toward increased incidence of MRSA. Given the similarity of the organisms recovered to those recovered in CAP,[35] this study supports IDSA/ATS recommendations that antibiotic therapy in aspiration pneumonia be similar to that of higher‐risk CAP, with the addition of vancomycin or linezolid for MRSA coverage in patients with risk factors for healthcare‐associated pneumonia.[17]
Our study is limited by its single‐center retrospective design. However, beginning in 1995, the LDS Hospital emergency department initiated a standardized pneumonia therapy protocol and deployed electronic medical records, which prospectively recorded a wide array of clinical, therapeutic, and biometric data. Most data elements used in this analysis were routinely charted for clinical purposes in real time. Although the eCURB, CURB‐65, and some comorbidities could be extracted electronically for each patient, it was not possible to calculate the pneumonia severity index score due to our inability to rigorously identify the necessary comorbid illness elements. Other comorbidities, not present in our model, may have been identified by the physician who makes a diagnosis of aspiration pneumonia. Our identification of swallow impairment is also methodologically limited. The decision to obtain a swallow study was clinical, usually occurring upon convalescence. Therefore, it is not possible to distinguish between antecedent oropharyngeal dysfunction and post‐critical illness dysfunction.
Our definition of aspiration pneumonia required the treating physician to diagnose and code the patient as having aspiration pneumonia, followed by excluding patients more likely to have aspiration pneumonitis. Although we relied on ICD‐9 codes to initially identify aspiration pneumonia, all patients in our database were confirmed by physician chart review. Our incidence of community‐acquired aspiration pneumonia is congruent with other studies using different methodologies.[1, 36, 37] Unfortunately, there is no standard and widely accepted definition for separating aspiration pneumonia from usual CAP. A younger and healthier patient who has developed pneumonia subsequent to aspiration may be more likely to be diagnosed with CAP, resulting in selection bias for older patients with greater comorbidities.
CONCLUSION
Patients diagnosed with aspiration pneumonia are older, have more comorbid conditions, and demonstrate greater disease severity and higher 30‐day mortality than CAP patients. Mortality prediction using CURB‐65 and eCURB in this population was poor, possibly due to a greater effect of comorbidities on mortality. The pneumonia severity index, which incorporates patient comorbidities, might perform better than the eCURB or CURB‐65, and should be studied in aspiration pneumonia populations where comorbid illness information is prospectively collected. Further areas of study include creating an improved mortality prediction model for aspiration pneumonia that incorporates comorbid conditions, DNR/DNI status, and disease severity.
Acknowledgments
The authors acknowledge Al Jephson for database support, Yao Li for statistical analysis, and Anita Austin for help reviewing the medical records. Dr. Lanspa had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure
Preliminary versions of this work were presented as posters at the American Thoracic Society Meeting, Denver, Colorado, May 17, 2011. This study was supported by grants from the Intermountain Research and Medical Foundation. Dr. Brown is supported by a career development award from National Institute of General Medical Sciences (K23GM094465). Dr. Dean served on an advisory board for Merck, has been a paid consultant for Cerexa, and has received an investigator‐initiated competitive grant from Pfizer for development of an electronic pneumonia decision support tool. All other authors report no relevant financial disclosures.
Pneumonia is a common clinical syndrome with well‐described epidemiology and microbiology. Aspiration pneumonia comprises 5% to 15% of patients with pneumonia,[1] but is less well‐characterized despite being a major syndrome of pneumonia in the elderly.[2, 3] Difficulties in studying aspiration pneumonia include the lack of a sensitive and specific marker for aspiration, the overlap between aspiration pneumonia and other forms of pneumonia, and the lack of differentiation between aspiration pneumonia and aspiration pneumonitis by many clinicians.[4, 5, 6] Aspiration pneumonia, which develops after the aspiration of oropharyngeal contents, differs from aspiration pneumonitis, wherein inhalation of gastric contents causes inflammation without the subsequent development of bacterial infection.[7, 8]
A number of validated mortality prediction models exist for community‐acquired pneumonia (CAP), using a variety of clinical predictors. One clinical prediction rule endorsed by the British Thoracic Society is the CURB‐65, which assigns a score for Confusion, Uremia >19 mg/dL, Respiratory rate >= 30 breaths/min, Blood Pressure < 90 mmHg systolic or < 60 mmHg diastolic, and age 65). We favor eCURB, a version of the CURB‐65 model that uses continuously weighted variables to more accurately predict mortality, validated in CAP populations.[9] Most studies validating pneumonia severity scoring systems excluded aspiration pneumonia from their study population.[10, 11, 12] Severity scoring systems for CAP may not accurately predict disease severity patients with aspiration pneumonia.
The aims of our study were to: (1) identify a population of patients with aspiration pneumonia; (2) compare characteristics and outcomes in patients with community‐acquired aspiration pneumonia to those with CAP; and (3) study the performance of eCURB and CURB‐65 in predicting mortality for patients with community‐acquired aspiration pneumonia.
PATIENTS AND METHODS
Study Design and Setting
The study was performed at LDS Hospital, a university‐affiliated community teaching hospital in Salt Lake City, Utah, with 520 beds. In retrospective analysis of data from the electronic medical records, we identified all patients older than 18 years who were evaluated in the emergency department at LDS Hospital or admitted patients from other sources (direct admission, transfer from another hospital) from 1996 to 2006 with International Statistical Classification of Disease and Health Related Problems, 9th Revision (ICD‐9) codes specific for aspiration pneumonia and pneumonitis (507.x). The treating physicians were mostly hospitalists and intensivists. Two physicians (M.L. and N.D.) manually reviewed the electronic medical records, including the emergency room physician's notes, the admission histories and physicals, the discharge summaries, and radiographic reports of the patients identified in the query. Consensus regarding the diagnosis of aspiration pneumonia was achieved in all patients reviewed using criteria listed in Table 1. This study was approved by the LDS Hospital institutional review board, and permission was granted to use the Utah Population Database for determining mortality (#1008505), with a waiver of informed consent. For the contemporaneous group of CAP patients, we used a previously described population identified using ICD‐9 codes 481.x to 487.x, captured from the same hospital during the same period.[13]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| |
| 1. Age 18 years | 1. Absence of radiographic evidence of pneumonia within 48 hours after evaluation |
| 2. Either admitted to hospital or evaluated in emergency department | 2. Previous episode of aspiration pneumonia within 12 months |
| 3. 507.x code as primary diagnosis | 3. Initial admission date >48 hours before transfer to LDS Hospital |
| 4. 507.x code as secondary diagnosis with a primary diagnosis of pneumonia, respiratory failure, or septicemia | 4. AIDS 5. Receipt of antiretroviral therapy 6. History of solid organ transplant |
| 5. Treating physician indicated a diagnosis of aspiration pneumonia in the history and physical and/or discharge summary | 7. Hematologic malignancy 8. Witnessed isolated aspiration event within 24 hours prior to evaluation 9. Drug overdose, cardiopulmonary arrest, or seizure prior to hospital admission 10. Laryngoscopic or bronchoscopic evidence of food material in airway |
Inclusion and Exclusion Criteria
Inclusion and exclusion criteria are listed in Table 1. To exclude patients with recurrent pneumonia, we included only the first episode of pneumonia in a given 12‐month period. LDS Hospital frequently receives patients transferred from surrounding emergency departments and intensive care units. We excluded patients who were transferred >48 hours from their initial emergency department admission and therefore were late in their disease course. Exclusion criteria 8 to 10 were used to exclude patients with clinical presentations more consistent with aspiration pneumonitis. We also excluded immunocompromised patients (criteria 4 to 7).
Healthcare‐associated aspiration pneumonia was defined as receipt of chronic hemodialysis, residence in a nursing facility, or hospitalization within any Intermountain Healthcare‐affiliated hospital within the past 90 days.[14] The remaining patients were defined as community‐acquired aspiration pneumonia.
Measurements
The first vital signs, orientation status, and first 12 hours of routine laboratory results were extracted from the electronic medical records and used to calculate predicted mortality by eCURB and CURB‐65. We determined 30‐day mortality from the merger of the electronic medical records with vital status information from the Utah Population Database.[15] The first measured SpO2 and FiO2 were used to estimate the PaO2/FiO2 ratio, using the Severinghaus calculation[16] if no arterial blood gas was available. Presence of American Thoracic Society/Infectious Diseases Society of America (IDSA/ATS) 2007 minor criteria for severe community‐acquired pneumonia (SCAP)[17] were obtained from baseline patient characteristics (Table 2). A Charlson comorbidity index was calculated from ICD‐9 codes using published methodology.[18, 19] Presence of an abnormal swallow was defined as dysphagia or aspiration on modified barium swallow study, fiberoptic endoscopic evaluation, or clinical determination by a speech language pathologist during the index hospitalization. We also looked for causative pathogens, defined by a positive pneumococcus or legionella urinary antigen, or a positive culture from blood, bronchoalveolar lavage, pleural fluid, or tracheal aspirate, collected within 24 hours of admission. Antibiotics administered within the first 24 hours of admission were classified into 4 broad groups based on local physician prescribing patterns. Clindamycin and metronidazole were considered anaerobic‐specific antibiotics. Vancomycin or linezolid were considered methicillin‐resistant Staphylococcus aureus (MRSA) antibiotics. Broad‐spectrum antibiotics included any of the following: carbapenems, aztreonam, piperacillin/tazobactam, ticarcillin/clavulanate, cefepime, and ceftazidime. Macrolides, respiratory fluoroquinolones, and third‐generation cephalosporins were considered standard‐care antibiotics.
| Respiratory rate 30 breaths/minute |
| PaO2/FiO2 250 |
| Multilobar infiltrates |
| Confusion/disorientation |
| Uremia (blood urea nitrogen 20 mg/dL) |
| Leukopenia (white blood cell count <4000 cells/mm3) |
| Thrombocytopenia (platelet count <100,000 cells/mm3) |
| Hypothermia (core temperature 36C) |
| Hypotension requiring aggressive fluid resuscitation |
Statistical Analysis
We compared baseline patient characteristics and clinical outcomes using the Fisher exact test to compare proportions of categorical variables, and Mann‐Whitney U test or Student t test to compare central tendencies of continuous variables, as dictated by the normality of the data. Receiver operating characteristic curves calculated the ability of eCURB and CURB‐65 to predict 30‐day mortality prediction in patients with community‐acquired aspiration pneumonia and CAP, as well as the ability of IDSA/ATS minor criteria for SCAP to predict admission to the intensive care unit (ICU). We performed multivariate logistic regression to predict 30‐day mortality in patients with community‐acquired aspiration pneumonia and CAP, using stepwise backward elimination. Confounders were included if they were significant at a 0.05 level or if they altered the coefficient of the main variable by more than 10%. For logistic models, we evaluated goodness of fit with the Hosmer‐Lemeshow technique; comparisons of area under the curve (AUC) among models were made using the technique of DeLong.[20] Two‐tailed P values of 0.05 were considered statistically significant. Stata version 12 (StataCorp, College Station, TX) was used for all analyses.
RESULTS
Our initial query identified 1165 patients. Physician review of the medical records resulted in 628 patients, 118 of whom were classified as healthcare‐associated aspiration pneumonia (Figure 1, Table 3). Of all aspiration pneumonia patients, 80% were seen in the emergency department, 12.5% were directly admitted from the community, and 7.5% were transferred from another healthcare facility. Almost all patients seen in the emergency department (99.0%) were admitted to the hospital, with median length of hospitalization 6.7 days among survivors.

| Aspiration Pneumonia (N = 628) | Community‐Acquired Aspiration Pneumonia (N = 510) | Healthcare Associated Aspiration Pneumonia (N=118) | P Value | |
|---|---|---|---|---|
| ||||
| Age (range), y | 77 (6585) | 77 (6485) | 80 (6786) | 0.42 |
| Female, % | 49.8 | 50.2 | 48.3 | 0.76 |
| 30‐day mortality, % | 21.0% | 19.0% | 29.7% | 0.02 |
| CURB‐65 score | 2 (13) | 2 (13) | 2 (13) | 0.0012 |
| Confusion | 13.9% | 12.7% | 18.6% | 0.10 |
| Blood urea nitrogen (mg/dL) | 22 (1634) | 21 (1532) | 30 (2047) | <0.0001 |
| Respiratory rate (breaths/min) | 20 (1826) | 20 (1824) | 21 (1828) | 0.30 |
| Systolic blood pressure (mm Hg) | 128 (110149) | 129 (110150) | 127 (105146) | 0.28 |
| eCURB 30‐day mortality estimate (median, %) | 5.6 (2.414.2) | 5.2 (2.212.4) | 8.9 (4.222.5) | <0.0001 |
| eCURB 30‐day mortality estimate (mean, %) | 10.6 12.2 | 9.7 11.5 | 14.614.1 | <0.0001 |
| Hospital admission (of ED visits), % | 99.0 | 98.8 | 100 | 0.59 |
| Hospital LOS, d | 6.7 (4.111.1) | 6.5 (4.011.0) | 7.8 (5.412.3) | 0.05 |
| ICU admission, % | 37.9 | 37.1 | 41.5 | 0.21 |
| ICU LOS, d | 3.5 (1.98.8) | 3.1 (1.87.6) | 5.6 (3.810.8) | 0.02 |
| Mean ventilator‐free days (of ICU patients, out of 30 days) | 25.28.3 | 25.97.7 | 22.710.0 | 0.01 |
| Receipt of mechanical ventilation, % | 18.6 | 17.2 | 24.6 | 0.09 |
| Duration of ventilation, d | 2.8 (0.96.5) | 3.1 (1.06.6) | 1.9 (0.86.3) | 0.05 |
| Receipt of vasopressor, % | 1.8 | 1.4 | 3.4 | 0.13 |
| Charlson comorbidity index | 4 (26) | 3 (26) | 4 (36) | 0.0024 |
| Cerebrovascular disease, % | 33.9 | 32.4 | 40.7 | 0.11 |
| Chronic pulmonary disease, % | 51.0 | 51.8 | 47.5 | 0.42 |
| Congestive heart failure, % | 52.4 | 50.0 | 62.7 | 0.01 |
| Connective tissue disease, % | 8.4 | 8.8 | 6.8 | 0.58 |
| Dementia, % | 14.2 | 12.0 | 23.7 | 0.0019 |
| Hemiplegia/paraplegia | 9.4 | 8.0 | 15.2 | 0.02 |
| Myocardial infarction, % | 21.0 | 17.8 | 29.7 | 0.02 |
| Peripheral vascular disease, % | 17.7 | 16.3 | 23.7 | 0.06 |
| Peptic ulcer disease, % | 18.8 | 19.2 | 16.9 | 0.70 |
| Diabetes without complications, % | 10.7 | 9.2 | 16.9 | 0.02 |
| Diabetes with complications, % | 31.5 | 30.4 | 36.4 | 0.23 |
| Mild liver disease, % | 8.6 | 8.0 | 11.0 | 0.28 |
| Moderate or severe liver disease, % | 1.8 | 1.6 | 2.5 | 0.44 |
| Malignant solid tumor, % | 16.6 | 17.3 | 13.6 | 0.41 |
| Metastatic cancer, % | 5.4 | 5.7 | 4.2 | 0.66 |
| Renal disease, % | 14.7 | 4.2 | 18.6 | 0.19 |
| 3 or more minor SCAP criteria, %* | 24.7 | 23.1 | 31.4 | 0.08 |
| PaO2/FiO2 ratio | 221 (161280) | 226 (169280) | 181 (133245) | 0.0004 |
| Multilobar disease, % | 46.3 | 43.2 | 53.9 | 0.11 |
| Presence of an effusion, % | 23.1 | 19.7 | 31.9 | 0.03 |
| Swallow impairment (of tested survivors), % | 34.1 | 34.1 | 34.1 | 0.22 |
| Presence of a DNR/DNI order, % | 26.4 | 23.7 | 38.1 | 0.0024 |
| Mortality of patients with DNR/DNI order, % | 39.1 | 38.8 | 40.0 | 1.00 |
| Receipt of broad‐spectrum antibiotic, % | 35.4 | 32.5 | 47.5 | 0.0028 |
| Receipt of MRSA antibiotic, % | 7.5 | 5.7 | 15.3 | 0.0014 |
| Receipt of anaerobe antibiotic, % | 28.7 | 27.6 | 33.1 | 0.26 |
Observed mortality was 21.0%. eCURB significantly underestimated mortality in this group, predicting a mortality rate of 10.6%. When classifying patients by the 2007 IDSA/ATS guidelines, 24.7% of the patients had 3 or more minor criteria for SCAP.[17] The PaO2/FiO2 ratio was obtained in 99.7% of patients. The median PaO2/FiO2 ratio observed in this population was 221 mm Hg (equivalent to 260 mm Hg at sea level barometric pressure, adjusted for our altitude of 1400 meters), near the threshold sea level definition (250 mm Hg) for SCAP.[13, 17] Admission to the ICU was common, as were admission orders for do not resuscitate (DNR) or do not intubate (DNI). Patients with healthcare‐associated aspiration pneumonia had a higher comorbidity index and had a higher mortality rate than patients with community‐acquired aspiration pneumonia, although we found no significant difference in the rate of hospital or ICU admission or the receipt of critical care therapies. Inpatient assessment of dysphagia and aspiration was conducted in 177 patients. Abnormal swallow was noted in 96% of those tested.
We found several differences between patients with community‐acquired aspiration pneumonia and 2584 patients with CAP identified during the same time period[13] (Table 4). Patients with community‐acquired aspiration pneumonia were older, more likely to have multilobar disease or effusion on imaging, and had greater disease severity. They also had a higher frequency of ICU and hospital admission, IDSA/ATS minor criteria for SCAP, and higher Charlson comorbidity indices. Patients with community‐acquired aspiration pneumonia were more likely to receive mechanical ventilation than CAP patients, although there was no difference in 30‐day mortality among intubated patients or a difference in ventilator‐free days.
| Community‐Acquired Aspiration Pneumonia (N = 510) | Community‐ Acquired Pneumonia (N = 2584) | P Value | |
|---|---|---|---|
| |||
| Age (range), y | 77 (6485) | 59 (4176) | <0.0001 |
| Female, % | 50.2 | 49.5 | 0.81 |
| 30‐day mortality, % | 19.0 | 4.2 | <0.0001 |
| CURB‐65 score | 2 (13) | 1 (02) | <0.0001 |
| Confusion, % | 12.7 | 5.1 | <0.0001 |
| Blood urea nitrogen | 21 (1532) | 16 (1124) | <0.0001 |
| Respiratory rate | 20 (1824) | 20 (1824) | <0.0001 |
| Systolic blood pressure | 129 (110150) | 130 (112146) | 0.67 |
| eCURB 30‐day mortality estimate, median, % | 5.2 (2.212.4) | 1.7 (0.94.3) | <0.0001 |
| eCURB 30‐day mortality estimate, mean, % | 9.7 11.5 | 4.4 7.5 | <0.0001 |
| AUC of eCURB versus mortality | 0.71 (0.660.75) | 0.86 (0.830.90) | <0.0001 |
| Excluding DNR/DNI patients | 0.69 (0.650.74) | 0.87 (0.830.90) | 0.0001 |
| AUC of CURB‐65 versus mortality | 0.66 (0.620.69) | 0.81 (0.780.85) | <0.0001 |
| Excluding DNR/DNI patients | 0.65 (0.600.70) | 0.81 (0.760.85) | 0.0003 |
| Hospital admission (of ED visits), % | 98.8 | 57.8 | <0.0001 |
| Hospital LOS, d | 6.5 (4.011.0) | 3.3 (2.25.2) | <0.0001 |
| ICU admission, % | 37.1 | 14.2 | <0.0001 |
| ICU LOS, d | 3.1 (1.87.6) | 2.5 (1.17.7) | 0.01 |
| Mean ventilator‐free days (of ICU patients, out of 30 days) | 25.9 7.7 | 25 9 | 0.75 |
| Receipt of mechanical ventilation, % | 17.2 | 7.8 | <0.0001 |
| Duration of ventilation, d | 3.1 (1.06.6) | 3.5 (1.57.2) | 0.09 |
| Receipt of vasopressor, % | 1.4 | 3.3 | 0.02 |
| Charlson comorbidity index | 3 (26) | 1 (03) | <0.0001 |
| Cerebrovascular disease, % | 32.4 | 10.0 | <0.0001 |
| Chronic pulmonary disease, % | 51.8 | 42.5 | <0.0001 |
| Congestive heart failure, % | 50.0 | 22.1 | <0.0001 |
| Connective tissue disease, % | 8.8 | 5.6 | 0.0084 |
| Dementia, % | 12.0 | 2.8 | <0.0001 |
| Hemiplegia/paraplegia, % | 8.0 | 2.7 | <0.0001 |
| Myocardial infarction, % | 17.8 | 10.8 | <0.0001 |
| Peripheral vascular disease, % | 16.3 | 7.4 | <0.0001 |
| Peptic ulcer disease, % | 19.2 | 7.6 | <0.0001 |
| Diabetes without complications, % | 9.2 | 24.7 | <0.0001 |
| Diabetes with complications, % | 30.4 | 5.1 | <0.0001 |
| Mild liver disease, % | 8.0 | 6.2 | 0.14 |
| Moderate or severe liver disease, % | 1.6 | 0.8 | 0.13 |
| Malignant solid tumor, % | 17. | 8.9 | <0.0001 |
| Metastatic cancer, % | 5.7 | 1.3 | <0.0001 |
| Renal disease, % | 4.2 | 5.6 | <0.0001 |
| 3 or more minor SCAP criteria, %* | 24.7 | 19.1 | 0.01 |
| PaO2/FiO2 ratio | 226 (169280) | 260 (148338) | 0.0004 |
| Multilobar disease, % | 43.2 | 37.2 | 0.0012 |
| Presence of an effusion, % | 19.7 | 18.3 | <0.0001 |
| Presence of a DNR/DNI order, % | 23.7 | 9.7 | <0.0001 |
| Mortality of patients with DNR/DNI order, % | 38.8 | 12.4 | <0.0001 |
| Receipt of broad‐spectrum antibiotic, % | 32.5 | 8.4 | <0.0001 |
| Receipt of MRSA antibiotic, % | 5.7 | 2.2 | <0.0001 |
| Receipt of anaerobe antibiotic, % | 27.6 | 3.1 | <0.0001 |
Thirty‐day mortality for patients with community‐acquired aspiration pneumonia was significantly higher than in CAP patients. Patients with community‐acquired aspiration pneumonia also had higher eCURB and CURB‐65 scores. However, eCURB was a poor predictor of 30‐day mortality, with an AUC of 0.71, compared to 0.86 calculated for the CAP population (Figure 2). CURB‐65 performed similarly: AUC was 0.66 vs 0.81. The presence of a DNR/DNI order was twice as prevalent in the community‐acquired aspiration pneumonia population vs the CAP population; those patients with a DNR/DNI order were 3 times as likely to die. Excluding patients with a DNR/DNI order did not improve performance of eCURB or CURB‐65 (Table 4). The presence of IDSA/ATS minor criteria for SCAP was not predictive of triage to the ICU in the group of patients with community‐acquired aspiration pneumonia (AUC: 0.51), compared with CAP patients (AUC: 0.88, P < 0.01 for comparison, Figure 3). This finding persisted in the subset of patients without a DNR/DNI order (AUC: 0.52 in community‐acquired aspiration pneumonia vs 0.88 in CAP, P < 0.01).


Our regression model of mortality incorporated gender, presence of effusion or multilobar pneumonia, presence of a DNR/DNI order, and all the components of the CURB‐65, IDSA/ATS minor criteria for SCAP, and Charlson comorbidity index. The regression model demonstrated that even after adjustment for age, comorbidities, disease severity, and presence of a DNR/DNI order, the presence of aspiration pneumonia was associated with higher mortality than CAP (odds ratio [OR]: 3.46, P < 0.001, Table 5). In this model, systolic blood pressure did not predict mortality, and diabetes with complications was associated with decreased mortality.
| Odds Ratio | P Value | |
|---|---|---|
| ||
| Presence of aspiration pneumonia | 3.46 (2.115.67) | <0.001 |
| Age, y | 1.03 (1.011.04) | <0.001 |
| Confusion | 3.14 (1.955.05) | <0.001 |
| Blood urea nitrogen, mg/dL | 1.03 (1.021.04) | <0.001 |
| Respiratory rate, breaths/minute | 1.03 (1.001.06) | 0.04 |
| PaO2/FiO2 ratio, per 1 mm Hg | 0.99 (0.991.00) | <0.001 |
| Moderate or severe liver disease | 9.21 (3.1626.86) | <0.001 |
| Paraplegia/hemiplegia | 2.43 (1.135.27) | 0.02 |
| Diabetes with complications | 0.42 (0.200.87) | 0.02 |
| Leukocytosis | 4.47 (2.278.82) | <0.001 |
| DNR/DNI | 1.75 (1.112.75) | 0.02 |
Microbiological Findings
Blood cultures were performed at admission in 67.4% of aspiration‐pneumonia patients, and a tracheal aspirate in half (50.7%) of intubated patients with aspiration pneumonia. Organisms were recovered in 90 patients (14.3%), although 41 of those patients had tracheal aspirates of organisms commonly thought to be nonpathogenic (nonpneumococcal alpha‐hemolytic streptococcus, nonhemolytic streptococcus, diphtheroids, micrococci, coagulase negative staphylococccus). Tracheal aspirate was the most common method of recovering an organism (7.8% of patients), followed by blood culture (4.3%). Bronchoalveolar lavage, urinary antigen, and pleural fluid culture were less common (1.3%, 1.1%, 0.3%, respectively). The microbiologic results were grouped into: Staphylococcus aureus, Streptococcus pneumoniae, enteric bacilli, Haemophilus species, Neisseria species, Moraxella catarrhalis, and Pseudomonas aeruginosa (Figure 4). Comparing healthcare‐associated with community‐acquired aspiration pneumonia, healthcare‐associated patients were more likely to have a confirmed infection with MRSA (4.2% vs 1.4%, P = 0.06) and enteric bacteria (5.1% vs 1.6%, P = 0.03). There were no other statistically significant differences in microbiologic recovery between the 2 groups. Antibiotics targeting anaerobic pathogens were administered in 28.7% of patients with aspiration pneumonia, with no correlation to the presence of healthcare‐associated risks. Healthcare‐associated patients were more likely to receive broad‐spectrum antibiotics (47.5% vs 32.5%, P < 0.01) and MRSA coverage (15.3% vs 5.7%, P < 0.01) than patients with community‐acquired aspiration pneumonia.

DISCUSSION
Our study identifies a larger cohort of patients with aspiration pneumonia than previous studies.[21, 22, 23, 24, 25] Patients with community‐acquired aspiration pneumonia are older and more likely to die than CAP patients. They are more likely to be admitted to the hospital or ICU. Thirty‐day mortality in this patient population was significantly underestimated by CURB‐65 and eCURB, models developed and validated in CAP populations.[9, 26] This finding supports a prior study.[27] It appears that a traditional prognostic model assessing mortality risk in the CAP patient does not apply to the aspiration‐pneumonia patient. One reason for eCURB and CURB‐65s poor utility in community‐acquired aspiration pneumonia may be their reliance on objective clinical features rather than comorbidities, which may influence mortality to a greater degree in aspiration pneumonia.
This study has several limitations. There is no gold standard for the definition of aspiration pneumonia, and it is difficult to distinguish aspiration pneumonia from typical pneumonia. It is plausible that older patients with greater comorbidities are being designated as aspiration pneumonia. If this is the case, then aspiration pneumonia merely represents the end of the pneumonia spectrum with highest mortality risk, and it is no surprise that these patients fare poorly.
It appears that the hospitalist or emergency department physician implicitly appreciates that aspiration pneumonia has a higher mortality risk than predicted by traditional severity assessment. With such high mortality and morbidity, a patient presenting to the emergency room with aspiration pneumonia is almost always admitted to the hospital. Further work in this area should investigate other factors to improve prognostic modeling in patients with aspiration pneumonia, although the utility of such a model may be limited to determining ICU admission. Our data indicate that IDSA/ATS minor criteria for SCAP are not useful in predicting admission to the ICU in patients with aspiration pneumonia.
In this study, a DNR/DNI order was twice as common in the community‐acquired aspiration pneumonia population than the CAP population. However, patients with community‐acquired aspiration pneumonia and a DNR/DNI order were more than 3 times more likely to die than patients with CAP and a DNR/DNI order. Our regression model suggested that the presence of a DNR/DNI order was an independent predictor of mortality (OR: 1.75, P < 0.001). Although a DNR/DNI order may correlate with the withholding or withdrawal of medical therapy, it is also a surrogate for increased age or comorbidities.[28] In our study, however, the increased prevalence of DNR/DNI orders did not explain the poor mortality prediction of the eCURB or CURB‐65, as exclusion of those patients did not significantly alter the AUCs in either the aspiration group or the CAP group.
Controversy exists regarding treatment of aspiration pneumonia. Historically, some have advocated for treatment of aspiration pneumonia with a regimen designed to cover anaerobic bacteria.[29] This recommendation was based on early microbiologic studies that obtained the samples late in the course of illness, or other studies where the sample was obtained transtracheally, where oropharyngeal flora may contaminate the sample.[30, 31, 32] Our clinically obtained microbiologic recovery of organisms was similar to the flora recovered in more recent CAP studies, in respect to both the incidence of pathogen recovery and the relative frequencies of recovered organisms.[33, 34] Our data do not support inferences regarding the prevalence of anaerobic infections, as the recovery of anaerobic organisms was limited to blood and pleural fluid cultures in this study, rather than techniques used in research settings that might have greater yield. As expected, patients with healthcare‐associated risk factors trended toward increased incidence of MRSA. Given the similarity of the organisms recovered to those recovered in CAP,[35] this study supports IDSA/ATS recommendations that antibiotic therapy in aspiration pneumonia be similar to that of higher‐risk CAP, with the addition of vancomycin or linezolid for MRSA coverage in patients with risk factors for healthcare‐associated pneumonia.[17]
Our study is limited by its single‐center retrospective design. However, beginning in 1995, the LDS Hospital emergency department initiated a standardized pneumonia therapy protocol and deployed electronic medical records, which prospectively recorded a wide array of clinical, therapeutic, and biometric data. Most data elements used in this analysis were routinely charted for clinical purposes in real time. Although the eCURB, CURB‐65, and some comorbidities could be extracted electronically for each patient, it was not possible to calculate the pneumonia severity index score due to our inability to rigorously identify the necessary comorbid illness elements. Other comorbidities, not present in our model, may have been identified by the physician who makes a diagnosis of aspiration pneumonia. Our identification of swallow impairment is also methodologically limited. The decision to obtain a swallow study was clinical, usually occurring upon convalescence. Therefore, it is not possible to distinguish between antecedent oropharyngeal dysfunction and post‐critical illness dysfunction.
Our definition of aspiration pneumonia required the treating physician to diagnose and code the patient as having aspiration pneumonia, followed by excluding patients more likely to have aspiration pneumonitis. Although we relied on ICD‐9 codes to initially identify aspiration pneumonia, all patients in our database were confirmed by physician chart review. Our incidence of community‐acquired aspiration pneumonia is congruent with other studies using different methodologies.[1, 36, 37] Unfortunately, there is no standard and widely accepted definition for separating aspiration pneumonia from usual CAP. A younger and healthier patient who has developed pneumonia subsequent to aspiration may be more likely to be diagnosed with CAP, resulting in selection bias for older patients with greater comorbidities.
CONCLUSION
Patients diagnosed with aspiration pneumonia are older, have more comorbid conditions, and demonstrate greater disease severity and higher 30‐day mortality than CAP patients. Mortality prediction using CURB‐65 and eCURB in this population was poor, possibly due to a greater effect of comorbidities on mortality. The pneumonia severity index, which incorporates patient comorbidities, might perform better than the eCURB or CURB‐65, and should be studied in aspiration pneumonia populations where comorbid illness information is prospectively collected. Further areas of study include creating an improved mortality prediction model for aspiration pneumonia that incorporates comorbid conditions, DNR/DNI status, and disease severity.
Acknowledgments
The authors acknowledge Al Jephson for database support, Yao Li for statistical analysis, and Anita Austin for help reviewing the medical records. Dr. Lanspa had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure
Preliminary versions of this work were presented as posters at the American Thoracic Society Meeting, Denver, Colorado, May 17, 2011. This study was supported by grants from the Intermountain Research and Medical Foundation. Dr. Brown is supported by a career development award from National Institute of General Medical Sciences (K23GM094465). Dr. Dean served on an advisory board for Merck, has been a paid consultant for Cerexa, and has received an investigator‐initiated competitive grant from Pfizer for development of an electronic pneumonia decision support tool. All other authors report no relevant financial disclosures.
- , , , et al. Severe community‐acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis. 1991;144(2):312–318.
- , , . Risk factors for pneumonia in the elderly. Am J Med. 1994;96(4):313–320.
- , . Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328–336.
- , , . Pneumonia versus aspiration pneumonitis in nursing home residents: diagnosis and management. J Am Geriatr Soc. 2003;51(1):17–23.
- . Aspiration pneumonia: mixing apples with oranges and tangerines. Crit Care Med. 2004;32(5):1236; author reply 1236–1237.
- , , , , . Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999–2000. Crit Care Med. 2003;31(7):1930–1937.
- . Aspiration syndromes: aspiration pneumonia and pneumonitis. Hosp Pract (Minneap). 2010;38(1):35–42.
- . Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–671.
- , , , et al. CURB‐65 pneumonia severity assessment adapted for electronic decision support. Chest. 2011;140(1):156–163.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382.
- , , , et al. Comparison of a disease‐specific and a generic severity of illness measure for patients with community‐acquired pneumonia. J Gen Intern Med. 1995;10(7):359–368.
- , , , et al. Development and validation of a clinical prediction rule for severe community‐acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–1256.
- , , , . Validation of the Infectious Disease Society of America/American Thoracic Society 2007 guidelines for severe community‐acquired pneumonia. Crit Care Med. 2009;37(12):3010–3016.
- Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.
- .The Utah genealogical database: a resource for genetic epidemiology. In: Cairns JL, Skolnick M, eds. Banbury Report No 4; Cancer Incidence in Defined Populations. New York, NY:Cold Spring Harbor Laboratory;1980:285–297.
- . Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol. 1979;46(3):599–602.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , . Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845.
- , , . Prandial aspiration and pneumonia in an elderly population followed over 3 years. Dysphagia. 1996;11(2):104–109.
- , , , . Predictors of aspiration pneumonia in nursing home residents. Dysphagia. 2002;17(4):298–307.
- , , , , , . Aspiration pneumonia: dental and oral risk factors in an older veteran population. J Am Geriatr Soc. 2001;49(5):557–563.
- , , , et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167(12):1650–1654.
- , , . Aspiration pneumonia in stroke. Arch Phys Med Rehabil. 1993;74(9):973–976.
- , , , et al. Validation of a predictive rule for the management of community‐acquired pneumonia. Eur Respir J. 2006;27(1):151–157.
- , , , , . Pneumonia Severity Index (PSI), CURB‐65, and mortality in hospitalized elderly patients with aspiration pneumonia [in German]. Zeitschrift fur Gerontologie und Geriatrie. 2011;44(4):229–234.
- , , , . Do‐not‐resuscitate orders for critically ill patients in the hospital. How are they used and what is their impact?JAMA. 1986;256(2):233–237.
- , , , , . The Sanford Guide to Antimicrobial Therapy: 2010.40th ed.Sperryville, VA:Antimicrobial Therapy, Inc.;2009.
- , , . The bacteriology of aspiration pneumonia. Am J Med. 1974;56(2):202–207.
- , , . Bacteriologic flora of aspiration‐induced pulmonary infections. Arch Intern Med. 1975;135(5):711–714.
- , . Bacteriology of aspiration pneumonia. A prospective study of community‐ and hospital‐acquired cases. Ann Intern Med. 1974;81(3):329–331.
- , . The role of anaerobes in patients with ventilator‐associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115(1):178–183.
- , , , et al. Is penicillin G an adequate initial treatment for aspiration pneumonia? A prospective evaluation using a protected specimen brush and quantitative cultures. Intensive Care Med. 1993;19(5):279–284.
- , , , , . Community‐acquired pneumonia in the elderly. Semin Respir Infect. 1999;14(2):173–183.
- , , . Nursing home‐acquired pneumonia. A case‐control study. J Am Geriatr Soc. 1986;34(10):697–702.
- , , , , . Severe community‐acquired pneumonia. Etiology, epidemiology, and prognosis factors. French Study Group for Community‐Acquired Pneumonia in the Intensive Care Unit. Chest. 1994;105(5):1487–1495.
- , , , et al. Severe community‐acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis. 1991;144(2):312–318.
- , , . Risk factors for pneumonia in the elderly. Am J Med. 1994;96(4):313–320.
- , . Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328–336.
- , , . Pneumonia versus aspiration pneumonitis in nursing home residents: diagnosis and management. J Am Geriatr Soc. 2003;51(1):17–23.
- . Aspiration pneumonia: mixing apples with oranges and tangerines. Crit Care Med. 2004;32(5):1236; author reply 1236–1237.
- , , , , . Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999–2000. Crit Care Med. 2003;31(7):1930–1937.
- . Aspiration syndromes: aspiration pneumonia and pneumonitis. Hosp Pract (Minneap). 2010;38(1):35–42.
- . Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–671.
- , , , et al. CURB‐65 pneumonia severity assessment adapted for electronic decision support. Chest. 2011;140(1):156–163.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382.
- , , , et al. Comparison of a disease‐specific and a generic severity of illness measure for patients with community‐acquired pneumonia. J Gen Intern Med. 1995;10(7):359–368.
- , , , et al. Development and validation of a clinical prediction rule for severe community‐acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–1256.
- , , , . Validation of the Infectious Disease Society of America/American Thoracic Society 2007 guidelines for severe community‐acquired pneumonia. Crit Care Med. 2009;37(12):3010–3016.
- Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.
- .The Utah genealogical database: a resource for genetic epidemiology. In: Cairns JL, Skolnick M, eds. Banbury Report No 4; Cancer Incidence in Defined Populations. New York, NY:Cold Spring Harbor Laboratory;1980:285–297.
- . Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol. 1979;46(3):599–602.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , . Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845.
- , , . Prandial aspiration and pneumonia in an elderly population followed over 3 years. Dysphagia. 1996;11(2):104–109.
- , , , . Predictors of aspiration pneumonia in nursing home residents. Dysphagia. 2002;17(4):298–307.
- , , , , , . Aspiration pneumonia: dental and oral risk factors in an older veteran population. J Am Geriatr Soc. 2001;49(5):557–563.
- , , , et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167(12):1650–1654.
- , , . Aspiration pneumonia in stroke. Arch Phys Med Rehabil. 1993;74(9):973–976.
- , , , et al. Validation of a predictive rule for the management of community‐acquired pneumonia. Eur Respir J. 2006;27(1):151–157.
- , , , , . Pneumonia Severity Index (PSI), CURB‐65, and mortality in hospitalized elderly patients with aspiration pneumonia [in German]. Zeitschrift fur Gerontologie und Geriatrie. 2011;44(4):229–234.
- , , , . Do‐not‐resuscitate orders for critically ill patients in the hospital. How are they used and what is their impact?JAMA. 1986;256(2):233–237.
- , , , , . The Sanford Guide to Antimicrobial Therapy: 2010.40th ed.Sperryville, VA:Antimicrobial Therapy, Inc.;2009.
- , , . The bacteriology of aspiration pneumonia. Am J Med. 1974;56(2):202–207.
- , , . Bacteriologic flora of aspiration‐induced pulmonary infections. Arch Intern Med. 1975;135(5):711–714.
- , . Bacteriology of aspiration pneumonia. A prospective study of community‐ and hospital‐acquired cases. Ann Intern Med. 1974;81(3):329–331.
- , . The role of anaerobes in patients with ventilator‐associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115(1):178–183.
- , , , et al. Is penicillin G an adequate initial treatment for aspiration pneumonia? A prospective evaluation using a protected specimen brush and quantitative cultures. Intensive Care Med. 1993;19(5):279–284.
- , , , , . Community‐acquired pneumonia in the elderly. Semin Respir Infect. 1999;14(2):173–183.
- , , . Nursing home‐acquired pneumonia. A case‐control study. J Am Geriatr Soc. 1986;34(10):697–702.
- , , , , . Severe community‐acquired pneumonia. Etiology, epidemiology, and prognosis factors. French Study Group for Community‐Acquired Pneumonia in the Intensive Care Unit. Chest. 1994;105(5):1487–1495.
Copyright © 2012 Society of Hospital Medicine