User login
Falling Through the Cracks
A 61-year-old man presented to the emergency department (ED) for persistent headache that began after he fell in his bathroom 4 days earlier. He described the headache as generalized and constant, rating the severity as a 5 on a scale of 0 to 10. The patient denied any associated neck pain or changes in headache quality with position change. He reported a 3-day history of nausea and four episodes of vomiting.
Headache after a fall raises concern for intracranial hemorrhage, particularly if this patient is on anticoagulant or antiplatelet medications. Subdural hematoma (SDH) would be more likely than epidural or subarachnoid hematoma (SAH) given the duration of days without progression. While nausea and vomiting are nonspecific, persistent vomiting may indicate increased intracranial pressure (eg, from an intracranial mass or SDH), particularly if provoked by positional changes. Without a history of fever or neck stiffness, meningitis is less likely unless the patient has a history of immunosuppression. Secondary causes of headache include vascular etiologies (eg, hemorrhagic cerebrovascular accident [CVA], arterial dissection, aneurysm, vasculitis), systemic causes (eg, chronic hypoxia/hypercapnia, hypertension), or medication overuse or withdrawal. In this patient, traumatic head injury with resultant postconcussive symptoms, though a diagnosis of exclusion, should also be considered. If the patient has a history of migraines, it is essential to obtain a history of typical migraine symptoms. More information regarding the mechanism of the fall is also essential to help elucidate a potential cause.
The patient had a 1-year history of recurrent loss of consciousness resulting in falls. After each fall, he quickly regained consciousness and exhibited no residual deficits or confusion. These episodes occurred suddenly when the patient was performing normal daily activities such as walking, driving, doing light chores, and standing up from a seated position. Immediately before this most recent fall, the patient stood up from a chair, walked toward the bathroom and, without any warning signs, lost consciousness. He denied dizziness, lightheadedness, nausea, or diaphoresis immediately before or after the fall. He also reported experiencing intermittent palpitations, but these did not appear to be related to the syncopal episodes. He denied experiencing chest pain, shortness of breath, or seizures.
The differential diagnosis for syncope is broad; therefore, it is important to identify features that suggest an etiology requiring urgent management. In this patient, cardiac etiologies such as arrhythmia (eg, atrial fibrillation [AF], ventricular tachycardia, heart block), ischemia, heart failure, and structural heart disease (eg, valvular abnormalities, cardiomyopathies) must be considered. His complaints of intermittent palpitations could suggest arrhythmia; however, the absence of a correlation to the syncopal episodes and other associated cardiac symptoms makes arrhythmias such as AF less likely. Medication side effects provoking cardiac conduction disturbances, heart block, or hypotension should be considered. Ischemic heart disease and heart failure are possible causes despite the absence of chest pain and dyspnea. While the exertional nature of the patient’s symptoms could support cardiac etiologies, it could also be indicative of recurrent pulmonary embolism or right ventricular dysfunction/strain, such as chronic thromboembolic pulmonary hypertension (CTEPH).
Neurologic causes of syncope should also be included in the differential diagnosis. Seizure is less likely the underlying cause in this case since the patient regained consciousness quickly after each episode and reported no residual deficits, confusion, incontinence, or oral trauma. While less likely, other neurovascular causes can be considered, including transient ischemic attack (TIA), CVA, SAH, or vertebrobasilar insufficiency.
Neurocardiogenic syncope is less likely due to lack of a clear trigger or classical prodromal symptoms. Without a history of volume loss, orthostatic syncope is also unlikely. Other possibilities include adrenal insufficiency or an autonomic dysfunction resulting from diabetic neuropathy, chronic kidney disease, amyloidosis, spinal cord injury, or neurologic diseases (eg, Parkinson disease, Lewy body dementia). Thus far, the provided history is not suggestive of these etiologies. Other causes for loss of consciousness include hypoglycemia, sleep disorders (eg, narcolepsy), or psychiatric causes.
About 10 months prior to this presentation, the patient had presented to the hospital for evaluation of headache and was found to have bilateral SDH requiring burr hole evacuation. At that time, he was on anticoagulation therapy for a history of left superficial femoral vein thrombosis with negative workup for hypercoagulability. Warfarin was discontinued after the SDH was diagnosed. Regarding the patient’s social history, although he reported drinking two glasses of wine with dinner each night and smoking marijuana afterward, all syncopal events occurred during the daytime.
The history of prior SDH should raise suspicion for recurrent SDH, particularly considering the patient’s ongoing alcohol use. History of deep vein thrombosis (DVT) and possible exertional syncope might suggest recurrent pulmonary embolism or CTEPH as an etiology. DVT and TIA/CVA secondary to paradoxical embolism are also possible. Depending on extent of alcohol use, intoxication and cardiomyopathy with secondary arrhythmias are possibilities.
The basic workup should focus on identifying any acute intracranial processes that may explain the patient’s presentation and evaluating for syncope. This includes a complete blood count with differential, electrolytes, hepatic panel (based on patient’s history of alcohol use), and coagulation studies. Troponins and B-type natriuretic peptide would help assess for cardiac disease, and a urine/serum drug test would be beneficial to screen for substance use. Considering the patient’s prior history of SDH, head imaging should be obtained. If the patient were to exhibit focal neurologic deficits or persistent alterations in consciousness (thereby raising the index of suspicion for TIA or CVA), perfusion/diffusion-weighted magnetic resonance imaging (MRI) studies should be obtained. If obtaining a brain MRI is not practical, then a computed tomography angiogram (CTA) of the head and neck should be obtained. A noncontrast head CT would be sufficient to reveal the presence of SDH. An electroencephalogram (EEG) to assess for seizure should be performed if the patient is noted to have any focal neurologic findings or complaints consistent with seizure. With possible exertional syncope, an electrocardiogram (ECG) and transthoracic echocardiogram (with bubble study to assess for patent foramen ovale) should be obtained urgently.
The patient had a history of hypertension and irritable bowel syndrome, for which he took metoprolol and duloxetine, respectively. Eight months prior to the current ED presentation, he was admitted to the hospital for a syncope workup after falling and sustaining a fractured jaw and torn rotator cuff. ECG and continuous telemetry monitoring showed normal sinus rhythm, normal intervals, and rare episodes of sinus tachycardia, but no evidence of arrhythmia. An echocardiogram demonstrated normal ejection fraction and chamber sizes; CT and MRI of the brain showed no residual SDH; and EEG monitoring showed no seizure activity. It was determined that the patient’s syncopal episodes were multifactorial; possible etiologies included episodic hypotension from irritable bowel syndrome—related diarrhea, paroxysmal arrhythmias, and ongoing substance use.
The patient was discharged home with a 14-day Holter monitor. Rare episodes of AF (total burden 0.4%) were detected, and dronedarone was prescribed for rhythm control; he remained off anticoagulation therapy due to the history of SDH. Over the next few months, cardiology, electrophysiology, and neurology consultants concluded that paroxysmal AF was the likely etiology of the patient’s syncopal episodes. The patient was considered high risk for CVA, but the risk of bleeding from syncope-related falls was too high to resume anticoagulation therapy.
One month prior to the current ED presentation, the patient underwent a left atrial appendage closure with a WATCHMAN implant to avoid long-term anticoagulation. After the procedure, he was started on warfarin with plans to permanently discontinue anticoagulation after 6 to 8 weeks of completed therapy. He had been on warfarin for 3 weeks prior the most recent fall and current ED visit.At the time of this presentation, the patient was on dronedarone, duloxetine, metoprolol, and warfarin. On exam, he was alert and in no distress. His temperature was 36.8 °C, heart rate 98 beats per minute , blood pressure (BP) 110/75 mm Hg (with no orthostatic changes), respiratory rate 18 breaths per minute, and oxygen saturation 95% on room air. He had a regular heart rate and rhythm, clear lung fields, and a benign abdominal exam. He was oriented to time, place, and person. His pupils were equal in size and reactive to light, and sensation and strength were equal bilaterally with no focal neurologic deficits. His neck was supple, and head movements did not cause any symptoms. His musculoskeletal exam was notable for right supraspinatus weakness upon abduction of arm to 90° and a positive impingement sign. ECG showed normal sinus rhythm with normal intervals. Laboratory findings were notable only for an international normalized ratio of 4.9. CT of the head did not show any pathology. The patient was admitted to the medicine floor for further evaluation.
At this point in his clinical course, the patient has had a thorough workup—one that has largely been unrevealing aside from paroxysmal AF. With his current presentation, acute intracranial causes remain on the differential, but the normal CT scan essentially excludes hemorrhage or mass. Although previous MRI studies have been negative and no focal neurologic findings have been described throughout his course, given the patient’s repeated presentations for syncope, intracranial vessel imaging should be obtained to exclude anatomical abnormalities or focal stenosis that could cause recurrent TIAs.
Seizure is also a consideration, but prior EEG and normal neurologic exam makes this less likely. While cardiac workup for syncope has been reassuring, the patient’s history of AF should continue to remain a consideration even though this is less likely the underlying cause since he is now taking dronedarone. He should be placed on telemetry upon admission. While negative orthostatic vital signs make orthostatic syncope less likely, this could be confounded by use of beta-blockers. Overall, the patient’s case remains a challenging one, with the etiology of his syncope remaining unclear at this time.
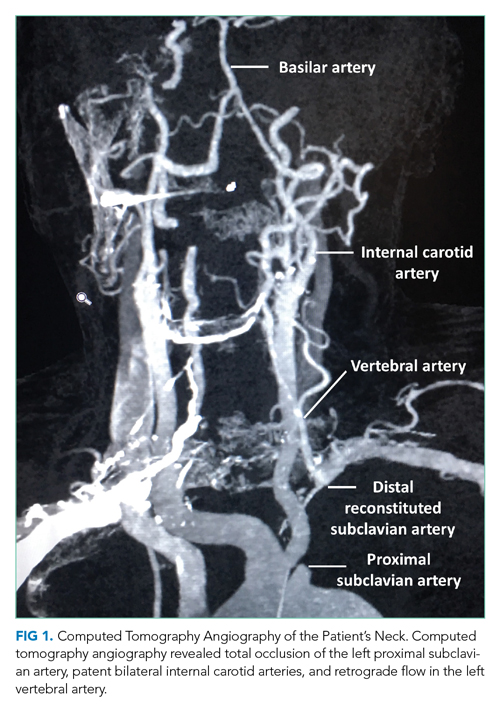
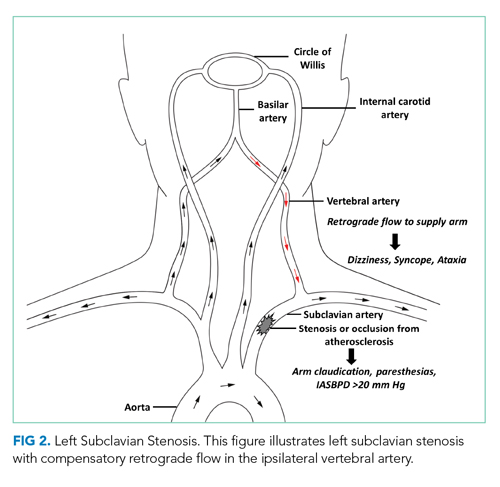
During this hospitalization, possible etiologies for recurrent syncope and falls were reviewed. The burden of verifiable AF was too low to explain the patient’s recurrent syncopal episodes. Further review of his medical record revealed that a carotid ultrasound was obtained a year earlier in the course of a previous hospitalization. The ultrasound report described patent carotid arteries and retrograde flow in the left vertebral artery consistent with ipsilateral subclavian stenosis. At the time, the ultrasound was interpreted as reassuring based on the lack of significant carotid stenosis; the findings were thought to be unrelated to the patient’s syncopal episodes. On further questioning, the patient noted that minimal exertion such as unloading a few items from the dishwasher caused left arm pain and paresthesia, accompanied by headache and lightheadedness. He also reported using his left arm more frequently following a right shoulder injury. Repeat physical exam found an inter-arm systolic BP difference (IASBPD) >40 mm Hg and left-arm claudication. CT-angiogram of the neck was obtained and showed total occlusion of the left proximal subclavian artery, patent bilateral internal carotid arteries, and retrograde flow in the left vertebral artery (Figure 1).
Subclavian steal syndrome (SSS) results from compromised flow to the distal arm or brainstem circulation due to a proximal subclavian artery occlusion or stenosis (prior to the origin of the vertebral artery).1,2 Subclavian stenosis may cause lowered pressure in the distal subclavian artery, creating a gradient for blood flow from the contralateral vertebral artery through the basilar artery to the ipsilateral vertebral artery, ultimately supplying blood flow to the affected subclavian artery distal to the occlusion (subclavian steal phenomenon). Flow reversal in the vertebrobasilar system can result in hypoperfusion of the brainstem (ie, vertebrobasilar insufficiency), which can cause a variety of neurologic symptoms, including SSS. While atherosclerosis is the most common cause of subclavian steal, it may be due to other conditions (eg, Takayasu arteritis, thoracic outlet syndrome, congenital heart disease).
Clinically, although many patients with proximal subclavian stenosis are asymptomatic (even in cases wherein angiographic flow reversal is detected), it is critical that clinicians be familiar with common symptoms associated with the diagnosis. Symptoms may include arm claudication related to hypoperfusion of the extremity, particularly when performing activities, as was observed in this patient. Neurologic symptoms are less common but include symptoms consistent with compromised posterior circulation such as dizziness, vertigo, ataxia, diplopia, nystagmus, impaired vision (blurring of vision, hemianopia), otologic symptoms (tinnitus, hearing loss), and/or syncope (ie, “drop attacks”). The patient’s initial complaints of sudden syncope are consistent with this presentation, as are his history of headache and lightheadedness upon use of his left arm.
Diagnostically, a gradient in upper extremity BP >15 mm Hg (as seen in this patient) or findings of arterial insufficiency would suggest subclavian stenosis. Duplex ultrasound is a reliable imaging modality and can demonstrate proximal subclavian stenosis (sensitivity of 90.9% and specificity of 82.5% for predicting >70% of stenosis cases) and ipsilateral vertebral artery flow reversal.1 Transcranial Doppler studies can be obtained to assess for basilar artery flow reversal as well. CTA/MRA can help delineate location, severity, and cause of stenosis. However, detection of vertebral or basilar artery flow reversal does not always correlate with the development of neurologic symptoms.
For patients with asymptomatic subclavian stenosis, medical management with aspirin, beta-blockers, angiotensin-converting enzyme inhibitors, and statins should be considered given the high likelihood for other atherosclerotic disease. Management of SSS may include percutaneous/surgical intervention in combination with medical therapy, particularly for patients with severe symptomatic disease (arm claudication, posterior circulation deficits, or coronary ischemia in patients with history of coronary bypass utilizing the left internal mammary artery).
The patient was diagnosed with SSS. Cardiovascular medicine and vascular surgery services were asked to evaluate the patient for a revascularization procedure. Because the patient’s anterior circulation was intact, several specialists remained skeptical of SSS as the cause of his syncope. As such, further evaluation for arrhythmia was recommended. The patient’s arm claudication was thought to be due to SSS; however, the well-established retrograde flow via the vertebral artery made a revascularization procedure nonurgent. Moreover, continuation of warfarin was necessary in the setting of his recent left atrial appendage closure and prior history of DVT. It was determined that the risks of discontinuing anticoagulation in order to surgically treat his subclavian stenosis outweighed the benefits. In the meantime, brachial-radial index measurement and a 30-day event monitor were ordered to further assess for arrhythmias. The patient reported being overwhelmed by diagnostic testing without resolution of his syncopal episodes and missed some of his scheduled appointments. One month later, he fell again and sustained vertebral fractures at C1, C4, and L1, and a subsequent SDH requiring craniotomy with a bone flap followed by clot evacuation. The 30-day event monitor report did not reveal any arrhythmias before, during, or after multiple syncopal events that occurred in the period leading up to this fall. The patient later died in a neurology intensive care unit.
DISCUSSION
SSS often stems from atherosclerotic arterial disease that leads to stenosis or occlusion of the proximal subclavian artery, causing decreased pressure distal to the lesion. The left subclavian artery is affected more often than the right because of its acute angle of origin, which presumably causes turbulence and predisposes to atherosclerosis.3 Compromised blood flow to the arm causes exertional arm claudication and paresthesia. The compensatory retrograde flow in the ipsilateral vertebral artery causes symptoms of vertebrobasilar insufficiency such as dizziness, vertigo, and syncope (Figure 2). This conglomerate of symptoms from subclavian steal, by definition, comprises SSS. The most remarkable signs of SSS are IASBPD >20 mm Hg and, less commonly, reproducible arm claudication.
Diagnosis of SSS requires a careful correlation of clinical history, physical examination, and radiologic findings. Over 80% of patients with subclavian disease have concomitant lesions (eg, in carotid arteries) that can affect collateral circulation.4 While symptoms of SSS may vary depending on the adequacy of collaterals, patent anterior circulation does not, by default, prevent SSS in patients with subclavian stenosis.3 In one study, neurologic symptoms were found in 36% of individuals with subclavian stenosis and concomitant carotid atherosclerotic lesions, and in only 5% in patients without carotid lesions.5
A key step in diagnosis is measurement of bilateral arm BP as elevated IASBPDs are highly sensitive for subclavian steal. More than 80% of patients with IASBPD >20 mm Hg have evidence of this condition on Doppler ultrasound.3 Higher differentials in BP correlate with occurrence of symptoms (~30% of patients with IASBPD 40-50 mm Hg, and ~40% of those with IASBPD >50 mm Hg).6
The severity of subclavian stenosis is traditionally classified by imaging into three separate grades or stages based on the direction of blood flow in vertebral arteries. Grade I involves no retrograde flow; grade II involves cardiac cycle dependent alternating antegrade and retrograde flow; and grade III involves permanent retrograde flow (complete steal).7 Our patient’s care was impacted by an unsupported conventional belief that grade II SSS may involve more hemodynamic instability and produce more severe symptoms compared to permanent retrograde flow (grade III), which would result in more stability with a reset of hemodynamics in posterior circulation.7 This hypothesis has been disproven in the past, and our patient’s tragic outcome also demonstrates that complete steal is not harmless.8 Our patient had permanent retrograde flow in the left vertebral artery, and he suffered classic symptoms of SSS, with devastating consequences. Moreover, increased demand or exertion can enhance the retrograde flow even in grade III stenosis and can precipitate neurologic symptoms of SSS, including syncope. This case provides an important lesson: Management of patients with SSS should depend on the severity of symptoms, not solely on radiologic grading.
Management of SSS is often medical for atherosclerosis and hypertension, especially if symptoms are mild and infrequent. Less than 10% patients with radiologic evidence of subclavian stenosis are symptomatic, and <20% patients with symptomatic SSS require revascularization.3 Percutaneous transluminal angioplasty (PTA) and stenting have become the most favored surgical approach rather than extra-anatomic revascularization techniques.7 Both endovascular interventions and open revascularization carry an excellent success rate with low morbidity. Patients undergoing PTA have a combined rate of 3.6% for CVA and death9 and a 5-year primary patency rate10 of 82%. Bypass surgery appears similarly well tolerated, with low perioperative CVA/mortality, and a 10-year primary patency rate of 92%.11 For patients with SSS and coexisting disease in the anterior circulation, carotid endarterectomy is prioritized over subclavian revascularization as repair of the anterior circulation often resolves symptoms of SSS.12
In our patient, SSS presented with classic vertebrobasilar and brachial symptoms, but several features of his presentation made the diagnosis a challenge. First, his history suggested several potential causes of syncope, including arrhythmia, orthostatic hypotension, and substance use. Second, he reported arm paresthesia and claudication only when specifically prompted and after a targeted history was obtained. Third, there were no consistent triggers for his syncopal episodes. The patient noted that he lost consciousness when walking, driving, doing light chores, and arising from a seated position. These atypical triggers of syncope were not consistent with any of the illnesses considered during the initial workup, and therefore resulted in a broad differential, delaying the targeted workup for SSS. The wisdom of parsimony may also have played an unintended role: In clinical practice, common things are common, and explanation of most or all symptoms with a known diagnosis is often correct rather than addition of uncommon disorders.
Unfortunately, this patient kept falling through the cracks. Several providers believed that AF and alcohol use were the likely causes of his syncope. This assumption enabled a less than rigorous appraisal of the critical ultrasound report. If SSS had been on the differential, assessing the patient for the associated signs and symptoms might have led to an earlier diagnosis.
KEY TEACHING POINTS
- SSS should be included in the differential diagnosis of patients with syncope, especially when common diagnoses have been ruled out.
- Incidentally detected retrograde vertebral flow on ultrasound should never be dismissed, and the patients should be assessed for signs and symptoms of subclavian steal.
- A difference in inter-arm systolic blood pressure >20 mm Hg is highly suggestive of subclavian stenosis.
- SSS has excellent prognosis with appropriate medical treatment or revascularization.
1. Mousa AY, Morkous R, Broce M, et al. Validation of subclavian duplex velocity criteria to grade severity of subclavian artery stenosis. J Vasc Surg. 2017;65(6):1779-1785. https://doi.org/10.1016/j.jvs.2016.12.098
2. Potter BJ, Pinto DS. Subclavian steal syndrome. Circulation. 2014;129(22):2320-2323. https://doi.org/10.1161/circulationaha.113.006653
3. Labropoulos N, Nandivada P, Bekelis K. Prevalence and impact of the subclavian steal syndrome. Ann Surg. 2010;252(1):166-170. https://doi.org/10.1097/sla.0b013e3181e3375a
4. Fields WS, Lemak NA. Joint study of extracranial arterial occlusion. VII. Subclavian steal--a review of 168 cases. JAMA. 1972;222(9):1139-1143. https://doi.org/10.1001/jama.1972.03210090019004
5. Hennerici M, Klemm C, Rautenberg W. The subclavian steal phenomenon: a common vascular disorder with rare neurologic deficits. Neurology. 1988;38(5): 669-673. https://doi.org/10.1212/wnl.38.5.669
6. Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet. 2012;379(9819):905-914. https://doi.org/10.1016/s0140-6736(11)61710-8
7. Osiro S, Zurada A, Gielecki J, Shoja MM, Tubbs RS, Loukas M. A review of subclavian steal syndrome with clinical correlation. Med Sci Monit. 2012;18(5):RA57-RA63. https://doi.org/10.12659/msm.882721
8. Thomassen L, Aarli JA. Subclavian steal phenomenon. Clinical and hemodynamic aspects. Acta Neurol Scand. 1994;90(4):241-244. https://doi.org/10.1111/j.1600-0404.1994.tb02714.x
9. De Vries JP, Jager LC, Van den Berg JC, et al. Durability of percutaneous transluminal angioplasty for obstructive lesions of proximal subclavian artery: long-term results. J Vasc Surg. 2005;41(1):19-23. https://doi.org/10.1016/j.jvs.2004.09.030
10. Wang KQ, Wang ZG, Yang BZ, et al. Long-term results of endovascular therapy for proximal subclavian arterial obstructive lesions. Chin Med J (Engl). 2010;123(1):45-50. https://doi.org/10.3760/cma.j.issn.0366-6999.2010.01.008
11. AbuRahma AF, Robinson PA, Jennings TG. Carotid-subclavian bypass grafting with polytetrafluoroethylene grafts for symptomatic subclavian artery stenosis or occlusion: a 20-year experience. J Vasc Surg. 2000;32(3):411-418; discussion 418-419. https://doi.org/10.1067/mva.2000.108644
12. Smith JM, Koury HI, Hafner CD, Welling RE. Subclavian steal syndrome. A review of 59 consecutive cases. J Cardiovasc Surg (Torino). 1994;35(1):11-14.
A 61-year-old man presented to the emergency department (ED) for persistent headache that began after he fell in his bathroom 4 days earlier. He described the headache as generalized and constant, rating the severity as a 5 on a scale of 0 to 10. The patient denied any associated neck pain or changes in headache quality with position change. He reported a 3-day history of nausea and four episodes of vomiting.
Headache after a fall raises concern for intracranial hemorrhage, particularly if this patient is on anticoagulant or antiplatelet medications. Subdural hematoma (SDH) would be more likely than epidural or subarachnoid hematoma (SAH) given the duration of days without progression. While nausea and vomiting are nonspecific, persistent vomiting may indicate increased intracranial pressure (eg, from an intracranial mass or SDH), particularly if provoked by positional changes. Without a history of fever or neck stiffness, meningitis is less likely unless the patient has a history of immunosuppression. Secondary causes of headache include vascular etiologies (eg, hemorrhagic cerebrovascular accident [CVA], arterial dissection, aneurysm, vasculitis), systemic causes (eg, chronic hypoxia/hypercapnia, hypertension), or medication overuse or withdrawal. In this patient, traumatic head injury with resultant postconcussive symptoms, though a diagnosis of exclusion, should also be considered. If the patient has a history of migraines, it is essential to obtain a history of typical migraine symptoms. More information regarding the mechanism of the fall is also essential to help elucidate a potential cause.
The patient had a 1-year history of recurrent loss of consciousness resulting in falls. After each fall, he quickly regained consciousness and exhibited no residual deficits or confusion. These episodes occurred suddenly when the patient was performing normal daily activities such as walking, driving, doing light chores, and standing up from a seated position. Immediately before this most recent fall, the patient stood up from a chair, walked toward the bathroom and, without any warning signs, lost consciousness. He denied dizziness, lightheadedness, nausea, or diaphoresis immediately before or after the fall. He also reported experiencing intermittent palpitations, but these did not appear to be related to the syncopal episodes. He denied experiencing chest pain, shortness of breath, or seizures.
The differential diagnosis for syncope is broad; therefore, it is important to identify features that suggest an etiology requiring urgent management. In this patient, cardiac etiologies such as arrhythmia (eg, atrial fibrillation [AF], ventricular tachycardia, heart block), ischemia, heart failure, and structural heart disease (eg, valvular abnormalities, cardiomyopathies) must be considered. His complaints of intermittent palpitations could suggest arrhythmia; however, the absence of a correlation to the syncopal episodes and other associated cardiac symptoms makes arrhythmias such as AF less likely. Medication side effects provoking cardiac conduction disturbances, heart block, or hypotension should be considered. Ischemic heart disease and heart failure are possible causes despite the absence of chest pain and dyspnea. While the exertional nature of the patient’s symptoms could support cardiac etiologies, it could also be indicative of recurrent pulmonary embolism or right ventricular dysfunction/strain, such as chronic thromboembolic pulmonary hypertension (CTEPH).
Neurologic causes of syncope should also be included in the differential diagnosis. Seizure is less likely the underlying cause in this case since the patient regained consciousness quickly after each episode and reported no residual deficits, confusion, incontinence, or oral trauma. While less likely, other neurovascular causes can be considered, including transient ischemic attack (TIA), CVA, SAH, or vertebrobasilar insufficiency.
Neurocardiogenic syncope is less likely due to lack of a clear trigger or classical prodromal symptoms. Without a history of volume loss, orthostatic syncope is also unlikely. Other possibilities include adrenal insufficiency or an autonomic dysfunction resulting from diabetic neuropathy, chronic kidney disease, amyloidosis, spinal cord injury, or neurologic diseases (eg, Parkinson disease, Lewy body dementia). Thus far, the provided history is not suggestive of these etiologies. Other causes for loss of consciousness include hypoglycemia, sleep disorders (eg, narcolepsy), or psychiatric causes.
About 10 months prior to this presentation, the patient had presented to the hospital for evaluation of headache and was found to have bilateral SDH requiring burr hole evacuation. At that time, he was on anticoagulation therapy for a history of left superficial femoral vein thrombosis with negative workup for hypercoagulability. Warfarin was discontinued after the SDH was diagnosed. Regarding the patient’s social history, although he reported drinking two glasses of wine with dinner each night and smoking marijuana afterward, all syncopal events occurred during the daytime.
The history of prior SDH should raise suspicion for recurrent SDH, particularly considering the patient’s ongoing alcohol use. History of deep vein thrombosis (DVT) and possible exertional syncope might suggest recurrent pulmonary embolism or CTEPH as an etiology. DVT and TIA/CVA secondary to paradoxical embolism are also possible. Depending on extent of alcohol use, intoxication and cardiomyopathy with secondary arrhythmias are possibilities.
The basic workup should focus on identifying any acute intracranial processes that may explain the patient’s presentation and evaluating for syncope. This includes a complete blood count with differential, electrolytes, hepatic panel (based on patient’s history of alcohol use), and coagulation studies. Troponins and B-type natriuretic peptide would help assess for cardiac disease, and a urine/serum drug test would be beneficial to screen for substance use. Considering the patient’s prior history of SDH, head imaging should be obtained. If the patient were to exhibit focal neurologic deficits or persistent alterations in consciousness (thereby raising the index of suspicion for TIA or CVA), perfusion/diffusion-weighted magnetic resonance imaging (MRI) studies should be obtained. If obtaining a brain MRI is not practical, then a computed tomography angiogram (CTA) of the head and neck should be obtained. A noncontrast head CT would be sufficient to reveal the presence of SDH. An electroencephalogram (EEG) to assess for seizure should be performed if the patient is noted to have any focal neurologic findings or complaints consistent with seizure. With possible exertional syncope, an electrocardiogram (ECG) and transthoracic echocardiogram (with bubble study to assess for patent foramen ovale) should be obtained urgently.
The patient had a history of hypertension and irritable bowel syndrome, for which he took metoprolol and duloxetine, respectively. Eight months prior to the current ED presentation, he was admitted to the hospital for a syncope workup after falling and sustaining a fractured jaw and torn rotator cuff. ECG and continuous telemetry monitoring showed normal sinus rhythm, normal intervals, and rare episodes of sinus tachycardia, but no evidence of arrhythmia. An echocardiogram demonstrated normal ejection fraction and chamber sizes; CT and MRI of the brain showed no residual SDH; and EEG monitoring showed no seizure activity. It was determined that the patient’s syncopal episodes were multifactorial; possible etiologies included episodic hypotension from irritable bowel syndrome—related diarrhea, paroxysmal arrhythmias, and ongoing substance use.
The patient was discharged home with a 14-day Holter monitor. Rare episodes of AF (total burden 0.4%) were detected, and dronedarone was prescribed for rhythm control; he remained off anticoagulation therapy due to the history of SDH. Over the next few months, cardiology, electrophysiology, and neurology consultants concluded that paroxysmal AF was the likely etiology of the patient’s syncopal episodes. The patient was considered high risk for CVA, but the risk of bleeding from syncope-related falls was too high to resume anticoagulation therapy.
One month prior to the current ED presentation, the patient underwent a left atrial appendage closure with a WATCHMAN implant to avoid long-term anticoagulation. After the procedure, he was started on warfarin with plans to permanently discontinue anticoagulation after 6 to 8 weeks of completed therapy. He had been on warfarin for 3 weeks prior the most recent fall and current ED visit.At the time of this presentation, the patient was on dronedarone, duloxetine, metoprolol, and warfarin. On exam, he was alert and in no distress. His temperature was 36.8 °C, heart rate 98 beats per minute , blood pressure (BP) 110/75 mm Hg (with no orthostatic changes), respiratory rate 18 breaths per minute, and oxygen saturation 95% on room air. He had a regular heart rate and rhythm, clear lung fields, and a benign abdominal exam. He was oriented to time, place, and person. His pupils were equal in size and reactive to light, and sensation and strength were equal bilaterally with no focal neurologic deficits. His neck was supple, and head movements did not cause any symptoms. His musculoskeletal exam was notable for right supraspinatus weakness upon abduction of arm to 90° and a positive impingement sign. ECG showed normal sinus rhythm with normal intervals. Laboratory findings were notable only for an international normalized ratio of 4.9. CT of the head did not show any pathology. The patient was admitted to the medicine floor for further evaluation.
At this point in his clinical course, the patient has had a thorough workup—one that has largely been unrevealing aside from paroxysmal AF. With his current presentation, acute intracranial causes remain on the differential, but the normal CT scan essentially excludes hemorrhage or mass. Although previous MRI studies have been negative and no focal neurologic findings have been described throughout his course, given the patient’s repeated presentations for syncope, intracranial vessel imaging should be obtained to exclude anatomical abnormalities or focal stenosis that could cause recurrent TIAs.
Seizure is also a consideration, but prior EEG and normal neurologic exam makes this less likely. While cardiac workup for syncope has been reassuring, the patient’s history of AF should continue to remain a consideration even though this is less likely the underlying cause since he is now taking dronedarone. He should be placed on telemetry upon admission. While negative orthostatic vital signs make orthostatic syncope less likely, this could be confounded by use of beta-blockers. Overall, the patient’s case remains a challenging one, with the etiology of his syncope remaining unclear at this time.
During this hospitalization, possible etiologies for recurrent syncope and falls were reviewed. The burden of verifiable AF was too low to explain the patient’s recurrent syncopal episodes. Further review of his medical record revealed that a carotid ultrasound was obtained a year earlier in the course of a previous hospitalization. The ultrasound report described patent carotid arteries and retrograde flow in the left vertebral artery consistent with ipsilateral subclavian stenosis. At the time, the ultrasound was interpreted as reassuring based on the lack of significant carotid stenosis; the findings were thought to be unrelated to the patient’s syncopal episodes. On further questioning, the patient noted that minimal exertion such as unloading a few items from the dishwasher caused left arm pain and paresthesia, accompanied by headache and lightheadedness. He also reported using his left arm more frequently following a right shoulder injury. Repeat physical exam found an inter-arm systolic BP difference (IASBPD) >40 mm Hg and left-arm claudication. CT-angiogram of the neck was obtained and showed total occlusion of the left proximal subclavian artery, patent bilateral internal carotid arteries, and retrograde flow in the left vertebral artery (Figure 1).

Subclavian steal syndrome (SSS) results from compromised flow to the distal arm or brainstem circulation due to a proximal subclavian artery occlusion or stenosis (prior to the origin of the vertebral artery).1,2 Subclavian stenosis may cause lowered pressure in the distal subclavian artery, creating a gradient for blood flow from the contralateral vertebral artery through the basilar artery to the ipsilateral vertebral artery, ultimately supplying blood flow to the affected subclavian artery distal to the occlusion (subclavian steal phenomenon). Flow reversal in the vertebrobasilar system can result in hypoperfusion of the brainstem (ie, vertebrobasilar insufficiency), which can cause a variety of neurologic symptoms, including SSS. While atherosclerosis is the most common cause of subclavian steal, it may be due to other conditions (eg, Takayasu arteritis, thoracic outlet syndrome, congenital heart disease).
Clinically, although many patients with proximal subclavian stenosis are asymptomatic (even in cases wherein angiographic flow reversal is detected), it is critical that clinicians be familiar with common symptoms associated with the diagnosis. Symptoms may include arm claudication related to hypoperfusion of the extremity, particularly when performing activities, as was observed in this patient. Neurologic symptoms are less common but include symptoms consistent with compromised posterior circulation such as dizziness, vertigo, ataxia, diplopia, nystagmus, impaired vision (blurring of vision, hemianopia), otologic symptoms (tinnitus, hearing loss), and/or syncope (ie, “drop attacks”). The patient’s initial complaints of sudden syncope are consistent with this presentation, as are his history of headache and lightheadedness upon use of his left arm.
Diagnostically, a gradient in upper extremity BP >15 mm Hg (as seen in this patient) or findings of arterial insufficiency would suggest subclavian stenosis. Duplex ultrasound is a reliable imaging modality and can demonstrate proximal subclavian stenosis (sensitivity of 90.9% and specificity of 82.5% for predicting >70% of stenosis cases) and ipsilateral vertebral artery flow reversal.1 Transcranial Doppler studies can be obtained to assess for basilar artery flow reversal as well. CTA/MRA can help delineate location, severity, and cause of stenosis. However, detection of vertebral or basilar artery flow reversal does not always correlate with the development of neurologic symptoms.
For patients with asymptomatic subclavian stenosis, medical management with aspirin, beta-blockers, angiotensin-converting enzyme inhibitors, and statins should be considered given the high likelihood for other atherosclerotic disease. Management of SSS may include percutaneous/surgical intervention in combination with medical therapy, particularly for patients with severe symptomatic disease (arm claudication, posterior circulation deficits, or coronary ischemia in patients with history of coronary bypass utilizing the left internal mammary artery).
The patient was diagnosed with SSS. Cardiovascular medicine and vascular surgery services were asked to evaluate the patient for a revascularization procedure. Because the patient’s anterior circulation was intact, several specialists remained skeptical of SSS as the cause of his syncope. As such, further evaluation for arrhythmia was recommended. The patient’s arm claudication was thought to be due to SSS; however, the well-established retrograde flow via the vertebral artery made a revascularization procedure nonurgent. Moreover, continuation of warfarin was necessary in the setting of his recent left atrial appendage closure and prior history of DVT. It was determined that the risks of discontinuing anticoagulation in order to surgically treat his subclavian stenosis outweighed the benefits. In the meantime, brachial-radial index measurement and a 30-day event monitor were ordered to further assess for arrhythmias. The patient reported being overwhelmed by diagnostic testing without resolution of his syncopal episodes and missed some of his scheduled appointments. One month later, he fell again and sustained vertebral fractures at C1, C4, and L1, and a subsequent SDH requiring craniotomy with a bone flap followed by clot evacuation. The 30-day event monitor report did not reveal any arrhythmias before, during, or after multiple syncopal events that occurred in the period leading up to this fall. The patient later died in a neurology intensive care unit.
DISCUSSION
SSS often stems from atherosclerotic arterial disease that leads to stenosis or occlusion of the proximal subclavian artery, causing decreased pressure distal to the lesion. The left subclavian artery is affected more often than the right because of its acute angle of origin, which presumably causes turbulence and predisposes to atherosclerosis.3 Compromised blood flow to the arm causes exertional arm claudication and paresthesia. The compensatory retrograde flow in the ipsilateral vertebral artery causes symptoms of vertebrobasilar insufficiency such as dizziness, vertigo, and syncope (Figure 2). This conglomerate of symptoms from subclavian steal, by definition, comprises SSS. The most remarkable signs of SSS are IASBPD >20 mm Hg and, less commonly, reproducible arm claudication.

Diagnosis of SSS requires a careful correlation of clinical history, physical examination, and radiologic findings. Over 80% of patients with subclavian disease have concomitant lesions (eg, in carotid arteries) that can affect collateral circulation.4 While symptoms of SSS may vary depending on the adequacy of collaterals, patent anterior circulation does not, by default, prevent SSS in patients with subclavian stenosis.3 In one study, neurologic symptoms were found in 36% of individuals with subclavian stenosis and concomitant carotid atherosclerotic lesions, and in only 5% in patients without carotid lesions.5
A key step in diagnosis is measurement of bilateral arm BP as elevated IASBPDs are highly sensitive for subclavian steal. More than 80% of patients with IASBPD >20 mm Hg have evidence of this condition on Doppler ultrasound.3 Higher differentials in BP correlate with occurrence of symptoms (~30% of patients with IASBPD 40-50 mm Hg, and ~40% of those with IASBPD >50 mm Hg).6
The severity of subclavian stenosis is traditionally classified by imaging into three separate grades or stages based on the direction of blood flow in vertebral arteries. Grade I involves no retrograde flow; grade II involves cardiac cycle dependent alternating antegrade and retrograde flow; and grade III involves permanent retrograde flow (complete steal).7 Our patient’s care was impacted by an unsupported conventional belief that grade II SSS may involve more hemodynamic instability and produce more severe symptoms compared to permanent retrograde flow (grade III), which would result in more stability with a reset of hemodynamics in posterior circulation.7 This hypothesis has been disproven in the past, and our patient’s tragic outcome also demonstrates that complete steal is not harmless.8 Our patient had permanent retrograde flow in the left vertebral artery, and he suffered classic symptoms of SSS, with devastating consequences. Moreover, increased demand or exertion can enhance the retrograde flow even in grade III stenosis and can precipitate neurologic symptoms of SSS, including syncope. This case provides an important lesson: Management of patients with SSS should depend on the severity of symptoms, not solely on radiologic grading.
Management of SSS is often medical for atherosclerosis and hypertension, especially if symptoms are mild and infrequent. Less than 10% patients with radiologic evidence of subclavian stenosis are symptomatic, and <20% patients with symptomatic SSS require revascularization.3 Percutaneous transluminal angioplasty (PTA) and stenting have become the most favored surgical approach rather than extra-anatomic revascularization techniques.7 Both endovascular interventions and open revascularization carry an excellent success rate with low morbidity. Patients undergoing PTA have a combined rate of 3.6% for CVA and death9 and a 5-year primary patency rate10 of 82%. Bypass surgery appears similarly well tolerated, with low perioperative CVA/mortality, and a 10-year primary patency rate of 92%.11 For patients with SSS and coexisting disease in the anterior circulation, carotid endarterectomy is prioritized over subclavian revascularization as repair of the anterior circulation often resolves symptoms of SSS.12
In our patient, SSS presented with classic vertebrobasilar and brachial symptoms, but several features of his presentation made the diagnosis a challenge. First, his history suggested several potential causes of syncope, including arrhythmia, orthostatic hypotension, and substance use. Second, he reported arm paresthesia and claudication only when specifically prompted and after a targeted history was obtained. Third, there were no consistent triggers for his syncopal episodes. The patient noted that he lost consciousness when walking, driving, doing light chores, and arising from a seated position. These atypical triggers of syncope were not consistent with any of the illnesses considered during the initial workup, and therefore resulted in a broad differential, delaying the targeted workup for SSS. The wisdom of parsimony may also have played an unintended role: In clinical practice, common things are common, and explanation of most or all symptoms with a known diagnosis is often correct rather than addition of uncommon disorders.
Unfortunately, this patient kept falling through the cracks. Several providers believed that AF and alcohol use were the likely causes of his syncope. This assumption enabled a less than rigorous appraisal of the critical ultrasound report. If SSS had been on the differential, assessing the patient for the associated signs and symptoms might have led to an earlier diagnosis.
KEY TEACHING POINTS
- SSS should be included in the differential diagnosis of patients with syncope, especially when common diagnoses have been ruled out.
- Incidentally detected retrograde vertebral flow on ultrasound should never be dismissed, and the patients should be assessed for signs and symptoms of subclavian steal.
- A difference in inter-arm systolic blood pressure >20 mm Hg is highly suggestive of subclavian stenosis.
- SSS has excellent prognosis with appropriate medical treatment or revascularization.
A 61-year-old man presented to the emergency department (ED) for persistent headache that began after he fell in his bathroom 4 days earlier. He described the headache as generalized and constant, rating the severity as a 5 on a scale of 0 to 10. The patient denied any associated neck pain or changes in headache quality with position change. He reported a 3-day history of nausea and four episodes of vomiting.
Headache after a fall raises concern for intracranial hemorrhage, particularly if this patient is on anticoagulant or antiplatelet medications. Subdural hematoma (SDH) would be more likely than epidural or subarachnoid hematoma (SAH) given the duration of days without progression. While nausea and vomiting are nonspecific, persistent vomiting may indicate increased intracranial pressure (eg, from an intracranial mass or SDH), particularly if provoked by positional changes. Without a history of fever or neck stiffness, meningitis is less likely unless the patient has a history of immunosuppression. Secondary causes of headache include vascular etiologies (eg, hemorrhagic cerebrovascular accident [CVA], arterial dissection, aneurysm, vasculitis), systemic causes (eg, chronic hypoxia/hypercapnia, hypertension), or medication overuse or withdrawal. In this patient, traumatic head injury with resultant postconcussive symptoms, though a diagnosis of exclusion, should also be considered. If the patient has a history of migraines, it is essential to obtain a history of typical migraine symptoms. More information regarding the mechanism of the fall is also essential to help elucidate a potential cause.
The patient had a 1-year history of recurrent loss of consciousness resulting in falls. After each fall, he quickly regained consciousness and exhibited no residual deficits or confusion. These episodes occurred suddenly when the patient was performing normal daily activities such as walking, driving, doing light chores, and standing up from a seated position. Immediately before this most recent fall, the patient stood up from a chair, walked toward the bathroom and, without any warning signs, lost consciousness. He denied dizziness, lightheadedness, nausea, or diaphoresis immediately before or after the fall. He also reported experiencing intermittent palpitations, but these did not appear to be related to the syncopal episodes. He denied experiencing chest pain, shortness of breath, or seizures.
The differential diagnosis for syncope is broad; therefore, it is important to identify features that suggest an etiology requiring urgent management. In this patient, cardiac etiologies such as arrhythmia (eg, atrial fibrillation [AF], ventricular tachycardia, heart block), ischemia, heart failure, and structural heart disease (eg, valvular abnormalities, cardiomyopathies) must be considered. His complaints of intermittent palpitations could suggest arrhythmia; however, the absence of a correlation to the syncopal episodes and other associated cardiac symptoms makes arrhythmias such as AF less likely. Medication side effects provoking cardiac conduction disturbances, heart block, or hypotension should be considered. Ischemic heart disease and heart failure are possible causes despite the absence of chest pain and dyspnea. While the exertional nature of the patient’s symptoms could support cardiac etiologies, it could also be indicative of recurrent pulmonary embolism or right ventricular dysfunction/strain, such as chronic thromboembolic pulmonary hypertension (CTEPH).
Neurologic causes of syncope should also be included in the differential diagnosis. Seizure is less likely the underlying cause in this case since the patient regained consciousness quickly after each episode and reported no residual deficits, confusion, incontinence, or oral trauma. While less likely, other neurovascular causes can be considered, including transient ischemic attack (TIA), CVA, SAH, or vertebrobasilar insufficiency.
Neurocardiogenic syncope is less likely due to lack of a clear trigger or classical prodromal symptoms. Without a history of volume loss, orthostatic syncope is also unlikely. Other possibilities include adrenal insufficiency or an autonomic dysfunction resulting from diabetic neuropathy, chronic kidney disease, amyloidosis, spinal cord injury, or neurologic diseases (eg, Parkinson disease, Lewy body dementia). Thus far, the provided history is not suggestive of these etiologies. Other causes for loss of consciousness include hypoglycemia, sleep disorders (eg, narcolepsy), or psychiatric causes.
About 10 months prior to this presentation, the patient had presented to the hospital for evaluation of headache and was found to have bilateral SDH requiring burr hole evacuation. At that time, he was on anticoagulation therapy for a history of left superficial femoral vein thrombosis with negative workup for hypercoagulability. Warfarin was discontinued after the SDH was diagnosed. Regarding the patient’s social history, although he reported drinking two glasses of wine with dinner each night and smoking marijuana afterward, all syncopal events occurred during the daytime.
The history of prior SDH should raise suspicion for recurrent SDH, particularly considering the patient’s ongoing alcohol use. History of deep vein thrombosis (DVT) and possible exertional syncope might suggest recurrent pulmonary embolism or CTEPH as an etiology. DVT and TIA/CVA secondary to paradoxical embolism are also possible. Depending on extent of alcohol use, intoxication and cardiomyopathy with secondary arrhythmias are possibilities.
The basic workup should focus on identifying any acute intracranial processes that may explain the patient’s presentation and evaluating for syncope. This includes a complete blood count with differential, electrolytes, hepatic panel (based on patient’s history of alcohol use), and coagulation studies. Troponins and B-type natriuretic peptide would help assess for cardiac disease, and a urine/serum drug test would be beneficial to screen for substance use. Considering the patient’s prior history of SDH, head imaging should be obtained. If the patient were to exhibit focal neurologic deficits or persistent alterations in consciousness (thereby raising the index of suspicion for TIA or CVA), perfusion/diffusion-weighted magnetic resonance imaging (MRI) studies should be obtained. If obtaining a brain MRI is not practical, then a computed tomography angiogram (CTA) of the head and neck should be obtained. A noncontrast head CT would be sufficient to reveal the presence of SDH. An electroencephalogram (EEG) to assess for seizure should be performed if the patient is noted to have any focal neurologic findings or complaints consistent with seizure. With possible exertional syncope, an electrocardiogram (ECG) and transthoracic echocardiogram (with bubble study to assess for patent foramen ovale) should be obtained urgently.
The patient had a history of hypertension and irritable bowel syndrome, for which he took metoprolol and duloxetine, respectively. Eight months prior to the current ED presentation, he was admitted to the hospital for a syncope workup after falling and sustaining a fractured jaw and torn rotator cuff. ECG and continuous telemetry monitoring showed normal sinus rhythm, normal intervals, and rare episodes of sinus tachycardia, but no evidence of arrhythmia. An echocardiogram demonstrated normal ejection fraction and chamber sizes; CT and MRI of the brain showed no residual SDH; and EEG monitoring showed no seizure activity. It was determined that the patient’s syncopal episodes were multifactorial; possible etiologies included episodic hypotension from irritable bowel syndrome—related diarrhea, paroxysmal arrhythmias, and ongoing substance use.
The patient was discharged home with a 14-day Holter monitor. Rare episodes of AF (total burden 0.4%) were detected, and dronedarone was prescribed for rhythm control; he remained off anticoagulation therapy due to the history of SDH. Over the next few months, cardiology, electrophysiology, and neurology consultants concluded that paroxysmal AF was the likely etiology of the patient’s syncopal episodes. The patient was considered high risk for CVA, but the risk of bleeding from syncope-related falls was too high to resume anticoagulation therapy.
One month prior to the current ED presentation, the patient underwent a left atrial appendage closure with a WATCHMAN implant to avoid long-term anticoagulation. After the procedure, he was started on warfarin with plans to permanently discontinue anticoagulation after 6 to 8 weeks of completed therapy. He had been on warfarin for 3 weeks prior the most recent fall and current ED visit.At the time of this presentation, the patient was on dronedarone, duloxetine, metoprolol, and warfarin. On exam, he was alert and in no distress. His temperature was 36.8 °C, heart rate 98 beats per minute , blood pressure (BP) 110/75 mm Hg (with no orthostatic changes), respiratory rate 18 breaths per minute, and oxygen saturation 95% on room air. He had a regular heart rate and rhythm, clear lung fields, and a benign abdominal exam. He was oriented to time, place, and person. His pupils were equal in size and reactive to light, and sensation and strength were equal bilaterally with no focal neurologic deficits. His neck was supple, and head movements did not cause any symptoms. His musculoskeletal exam was notable for right supraspinatus weakness upon abduction of arm to 90° and a positive impingement sign. ECG showed normal sinus rhythm with normal intervals. Laboratory findings were notable only for an international normalized ratio of 4.9. CT of the head did not show any pathology. The patient was admitted to the medicine floor for further evaluation.
At this point in his clinical course, the patient has had a thorough workup—one that has largely been unrevealing aside from paroxysmal AF. With his current presentation, acute intracranial causes remain on the differential, but the normal CT scan essentially excludes hemorrhage or mass. Although previous MRI studies have been negative and no focal neurologic findings have been described throughout his course, given the patient’s repeated presentations for syncope, intracranial vessel imaging should be obtained to exclude anatomical abnormalities or focal stenosis that could cause recurrent TIAs.
Seizure is also a consideration, but prior EEG and normal neurologic exam makes this less likely. While cardiac workup for syncope has been reassuring, the patient’s history of AF should continue to remain a consideration even though this is less likely the underlying cause since he is now taking dronedarone. He should be placed on telemetry upon admission. While negative orthostatic vital signs make orthostatic syncope less likely, this could be confounded by use of beta-blockers. Overall, the patient’s case remains a challenging one, with the etiology of his syncope remaining unclear at this time.
During this hospitalization, possible etiologies for recurrent syncope and falls were reviewed. The burden of verifiable AF was too low to explain the patient’s recurrent syncopal episodes. Further review of his medical record revealed that a carotid ultrasound was obtained a year earlier in the course of a previous hospitalization. The ultrasound report described patent carotid arteries and retrograde flow in the left vertebral artery consistent with ipsilateral subclavian stenosis. At the time, the ultrasound was interpreted as reassuring based on the lack of significant carotid stenosis; the findings were thought to be unrelated to the patient’s syncopal episodes. On further questioning, the patient noted that minimal exertion such as unloading a few items from the dishwasher caused left arm pain and paresthesia, accompanied by headache and lightheadedness. He also reported using his left arm more frequently following a right shoulder injury. Repeat physical exam found an inter-arm systolic BP difference (IASBPD) >40 mm Hg and left-arm claudication. CT-angiogram of the neck was obtained and showed total occlusion of the left proximal subclavian artery, patent bilateral internal carotid arteries, and retrograde flow in the left vertebral artery (Figure 1).

Subclavian steal syndrome (SSS) results from compromised flow to the distal arm or brainstem circulation due to a proximal subclavian artery occlusion or stenosis (prior to the origin of the vertebral artery).1,2 Subclavian stenosis may cause lowered pressure in the distal subclavian artery, creating a gradient for blood flow from the contralateral vertebral artery through the basilar artery to the ipsilateral vertebral artery, ultimately supplying blood flow to the affected subclavian artery distal to the occlusion (subclavian steal phenomenon). Flow reversal in the vertebrobasilar system can result in hypoperfusion of the brainstem (ie, vertebrobasilar insufficiency), which can cause a variety of neurologic symptoms, including SSS. While atherosclerosis is the most common cause of subclavian steal, it may be due to other conditions (eg, Takayasu arteritis, thoracic outlet syndrome, congenital heart disease).
Clinically, although many patients with proximal subclavian stenosis are asymptomatic (even in cases wherein angiographic flow reversal is detected), it is critical that clinicians be familiar with common symptoms associated with the diagnosis. Symptoms may include arm claudication related to hypoperfusion of the extremity, particularly when performing activities, as was observed in this patient. Neurologic symptoms are less common but include symptoms consistent with compromised posterior circulation such as dizziness, vertigo, ataxia, diplopia, nystagmus, impaired vision (blurring of vision, hemianopia), otologic symptoms (tinnitus, hearing loss), and/or syncope (ie, “drop attacks”). The patient’s initial complaints of sudden syncope are consistent with this presentation, as are his history of headache and lightheadedness upon use of his left arm.
Diagnostically, a gradient in upper extremity BP >15 mm Hg (as seen in this patient) or findings of arterial insufficiency would suggest subclavian stenosis. Duplex ultrasound is a reliable imaging modality and can demonstrate proximal subclavian stenosis (sensitivity of 90.9% and specificity of 82.5% for predicting >70% of stenosis cases) and ipsilateral vertebral artery flow reversal.1 Transcranial Doppler studies can be obtained to assess for basilar artery flow reversal as well. CTA/MRA can help delineate location, severity, and cause of stenosis. However, detection of vertebral or basilar artery flow reversal does not always correlate with the development of neurologic symptoms.
For patients with asymptomatic subclavian stenosis, medical management with aspirin, beta-blockers, angiotensin-converting enzyme inhibitors, and statins should be considered given the high likelihood for other atherosclerotic disease. Management of SSS may include percutaneous/surgical intervention in combination with medical therapy, particularly for patients with severe symptomatic disease (arm claudication, posterior circulation deficits, or coronary ischemia in patients with history of coronary bypass utilizing the left internal mammary artery).
The patient was diagnosed with SSS. Cardiovascular medicine and vascular surgery services were asked to evaluate the patient for a revascularization procedure. Because the patient’s anterior circulation was intact, several specialists remained skeptical of SSS as the cause of his syncope. As such, further evaluation for arrhythmia was recommended. The patient’s arm claudication was thought to be due to SSS; however, the well-established retrograde flow via the vertebral artery made a revascularization procedure nonurgent. Moreover, continuation of warfarin was necessary in the setting of his recent left atrial appendage closure and prior history of DVT. It was determined that the risks of discontinuing anticoagulation in order to surgically treat his subclavian stenosis outweighed the benefits. In the meantime, brachial-radial index measurement and a 30-day event monitor were ordered to further assess for arrhythmias. The patient reported being overwhelmed by diagnostic testing without resolution of his syncopal episodes and missed some of his scheduled appointments. One month later, he fell again and sustained vertebral fractures at C1, C4, and L1, and a subsequent SDH requiring craniotomy with a bone flap followed by clot evacuation. The 30-day event monitor report did not reveal any arrhythmias before, during, or after multiple syncopal events that occurred in the period leading up to this fall. The patient later died in a neurology intensive care unit.
DISCUSSION
SSS often stems from atherosclerotic arterial disease that leads to stenosis or occlusion of the proximal subclavian artery, causing decreased pressure distal to the lesion. The left subclavian artery is affected more often than the right because of its acute angle of origin, which presumably causes turbulence and predisposes to atherosclerosis.3 Compromised blood flow to the arm causes exertional arm claudication and paresthesia. The compensatory retrograde flow in the ipsilateral vertebral artery causes symptoms of vertebrobasilar insufficiency such as dizziness, vertigo, and syncope (Figure 2). This conglomerate of symptoms from subclavian steal, by definition, comprises SSS. The most remarkable signs of SSS are IASBPD >20 mm Hg and, less commonly, reproducible arm claudication.

Diagnosis of SSS requires a careful correlation of clinical history, physical examination, and radiologic findings. Over 80% of patients with subclavian disease have concomitant lesions (eg, in carotid arteries) that can affect collateral circulation.4 While symptoms of SSS may vary depending on the adequacy of collaterals, patent anterior circulation does not, by default, prevent SSS in patients with subclavian stenosis.3 In one study, neurologic symptoms were found in 36% of individuals with subclavian stenosis and concomitant carotid atherosclerotic lesions, and in only 5% in patients without carotid lesions.5
A key step in diagnosis is measurement of bilateral arm BP as elevated IASBPDs are highly sensitive for subclavian steal. More than 80% of patients with IASBPD >20 mm Hg have evidence of this condition on Doppler ultrasound.3 Higher differentials in BP correlate with occurrence of symptoms (~30% of patients with IASBPD 40-50 mm Hg, and ~40% of those with IASBPD >50 mm Hg).6
The severity of subclavian stenosis is traditionally classified by imaging into three separate grades or stages based on the direction of blood flow in vertebral arteries. Grade I involves no retrograde flow; grade II involves cardiac cycle dependent alternating antegrade and retrograde flow; and grade III involves permanent retrograde flow (complete steal).7 Our patient’s care was impacted by an unsupported conventional belief that grade II SSS may involve more hemodynamic instability and produce more severe symptoms compared to permanent retrograde flow (grade III), which would result in more stability with a reset of hemodynamics in posterior circulation.7 This hypothesis has been disproven in the past, and our patient’s tragic outcome also demonstrates that complete steal is not harmless.8 Our patient had permanent retrograde flow in the left vertebral artery, and he suffered classic symptoms of SSS, with devastating consequences. Moreover, increased demand or exertion can enhance the retrograde flow even in grade III stenosis and can precipitate neurologic symptoms of SSS, including syncope. This case provides an important lesson: Management of patients with SSS should depend on the severity of symptoms, not solely on radiologic grading.
Management of SSS is often medical for atherosclerosis and hypertension, especially if symptoms are mild and infrequent. Less than 10% patients with radiologic evidence of subclavian stenosis are symptomatic, and <20% patients with symptomatic SSS require revascularization.3 Percutaneous transluminal angioplasty (PTA) and stenting have become the most favored surgical approach rather than extra-anatomic revascularization techniques.7 Both endovascular interventions and open revascularization carry an excellent success rate with low morbidity. Patients undergoing PTA have a combined rate of 3.6% for CVA and death9 and a 5-year primary patency rate10 of 82%. Bypass surgery appears similarly well tolerated, with low perioperative CVA/mortality, and a 10-year primary patency rate of 92%.11 For patients with SSS and coexisting disease in the anterior circulation, carotid endarterectomy is prioritized over subclavian revascularization as repair of the anterior circulation often resolves symptoms of SSS.12
In our patient, SSS presented with classic vertebrobasilar and brachial symptoms, but several features of his presentation made the diagnosis a challenge. First, his history suggested several potential causes of syncope, including arrhythmia, orthostatic hypotension, and substance use. Second, he reported arm paresthesia and claudication only when specifically prompted and after a targeted history was obtained. Third, there were no consistent triggers for his syncopal episodes. The patient noted that he lost consciousness when walking, driving, doing light chores, and arising from a seated position. These atypical triggers of syncope were not consistent with any of the illnesses considered during the initial workup, and therefore resulted in a broad differential, delaying the targeted workup for SSS. The wisdom of parsimony may also have played an unintended role: In clinical practice, common things are common, and explanation of most or all symptoms with a known diagnosis is often correct rather than addition of uncommon disorders.
Unfortunately, this patient kept falling through the cracks. Several providers believed that AF and alcohol use were the likely causes of his syncope. This assumption enabled a less than rigorous appraisal of the critical ultrasound report. If SSS had been on the differential, assessing the patient for the associated signs and symptoms might have led to an earlier diagnosis.
KEY TEACHING POINTS
- SSS should be included in the differential diagnosis of patients with syncope, especially when common diagnoses have been ruled out.
- Incidentally detected retrograde vertebral flow on ultrasound should never be dismissed, and the patients should be assessed for signs and symptoms of subclavian steal.
- A difference in inter-arm systolic blood pressure >20 mm Hg is highly suggestive of subclavian stenosis.
- SSS has excellent prognosis with appropriate medical treatment or revascularization.
1. Mousa AY, Morkous R, Broce M, et al. Validation of subclavian duplex velocity criteria to grade severity of subclavian artery stenosis. J Vasc Surg. 2017;65(6):1779-1785. https://doi.org/10.1016/j.jvs.2016.12.098
2. Potter BJ, Pinto DS. Subclavian steal syndrome. Circulation. 2014;129(22):2320-2323. https://doi.org/10.1161/circulationaha.113.006653
3. Labropoulos N, Nandivada P, Bekelis K. Prevalence and impact of the subclavian steal syndrome. Ann Surg. 2010;252(1):166-170. https://doi.org/10.1097/sla.0b013e3181e3375a
4. Fields WS, Lemak NA. Joint study of extracranial arterial occlusion. VII. Subclavian steal--a review of 168 cases. JAMA. 1972;222(9):1139-1143. https://doi.org/10.1001/jama.1972.03210090019004
5. Hennerici M, Klemm C, Rautenberg W. The subclavian steal phenomenon: a common vascular disorder with rare neurologic deficits. Neurology. 1988;38(5): 669-673. https://doi.org/10.1212/wnl.38.5.669
6. Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet. 2012;379(9819):905-914. https://doi.org/10.1016/s0140-6736(11)61710-8
7. Osiro S, Zurada A, Gielecki J, Shoja MM, Tubbs RS, Loukas M. A review of subclavian steal syndrome with clinical correlation. Med Sci Monit. 2012;18(5):RA57-RA63. https://doi.org/10.12659/msm.882721
8. Thomassen L, Aarli JA. Subclavian steal phenomenon. Clinical and hemodynamic aspects. Acta Neurol Scand. 1994;90(4):241-244. https://doi.org/10.1111/j.1600-0404.1994.tb02714.x
9. De Vries JP, Jager LC, Van den Berg JC, et al. Durability of percutaneous transluminal angioplasty for obstructive lesions of proximal subclavian artery: long-term results. J Vasc Surg. 2005;41(1):19-23. https://doi.org/10.1016/j.jvs.2004.09.030
10. Wang KQ, Wang ZG, Yang BZ, et al. Long-term results of endovascular therapy for proximal subclavian arterial obstructive lesions. Chin Med J (Engl). 2010;123(1):45-50. https://doi.org/10.3760/cma.j.issn.0366-6999.2010.01.008
11. AbuRahma AF, Robinson PA, Jennings TG. Carotid-subclavian bypass grafting with polytetrafluoroethylene grafts for symptomatic subclavian artery stenosis or occlusion: a 20-year experience. J Vasc Surg. 2000;32(3):411-418; discussion 418-419. https://doi.org/10.1067/mva.2000.108644
12. Smith JM, Koury HI, Hafner CD, Welling RE. Subclavian steal syndrome. A review of 59 consecutive cases. J Cardiovasc Surg (Torino). 1994;35(1):11-14.
1. Mousa AY, Morkous R, Broce M, et al. Validation of subclavian duplex velocity criteria to grade severity of subclavian artery stenosis. J Vasc Surg. 2017;65(6):1779-1785. https://doi.org/10.1016/j.jvs.2016.12.098
2. Potter BJ, Pinto DS. Subclavian steal syndrome. Circulation. 2014;129(22):2320-2323. https://doi.org/10.1161/circulationaha.113.006653
3. Labropoulos N, Nandivada P, Bekelis K. Prevalence and impact of the subclavian steal syndrome. Ann Surg. 2010;252(1):166-170. https://doi.org/10.1097/sla.0b013e3181e3375a
4. Fields WS, Lemak NA. Joint study of extracranial arterial occlusion. VII. Subclavian steal--a review of 168 cases. JAMA. 1972;222(9):1139-1143. https://doi.org/10.1001/jama.1972.03210090019004
5. Hennerici M, Klemm C, Rautenberg W. The subclavian steal phenomenon: a common vascular disorder with rare neurologic deficits. Neurology. 1988;38(5): 669-673. https://doi.org/10.1212/wnl.38.5.669
6. Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet. 2012;379(9819):905-914. https://doi.org/10.1016/s0140-6736(11)61710-8
7. Osiro S, Zurada A, Gielecki J, Shoja MM, Tubbs RS, Loukas M. A review of subclavian steal syndrome with clinical correlation. Med Sci Monit. 2012;18(5):RA57-RA63. https://doi.org/10.12659/msm.882721
8. Thomassen L, Aarli JA. Subclavian steal phenomenon. Clinical and hemodynamic aspects. Acta Neurol Scand. 1994;90(4):241-244. https://doi.org/10.1111/j.1600-0404.1994.tb02714.x
9. De Vries JP, Jager LC, Van den Berg JC, et al. Durability of percutaneous transluminal angioplasty for obstructive lesions of proximal subclavian artery: long-term results. J Vasc Surg. 2005;41(1):19-23. https://doi.org/10.1016/j.jvs.2004.09.030
10. Wang KQ, Wang ZG, Yang BZ, et al. Long-term results of endovascular therapy for proximal subclavian arterial obstructive lesions. Chin Med J (Engl). 2010;123(1):45-50. https://doi.org/10.3760/cma.j.issn.0366-6999.2010.01.008
11. AbuRahma AF, Robinson PA, Jennings TG. Carotid-subclavian bypass grafting with polytetrafluoroethylene grafts for symptomatic subclavian artery stenosis or occlusion: a 20-year experience. J Vasc Surg. 2000;32(3):411-418; discussion 418-419. https://doi.org/10.1067/mva.2000.108644
12. Smith JM, Koury HI, Hafner CD, Welling RE. Subclavian steal syndrome. A review of 59 consecutive cases. J Cardiovasc Surg (Torino). 1994;35(1):11-14.
© 2021 Society of Hospital Medicine
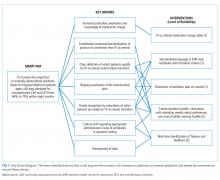
Decreasing Hospital Observation Time for Febrile Infants
Febrile infants aged 0 to 60 days often undergo diagnostic testing to evaluate for invasive bacterial infections (IBI; ie, bacteremia and meningitis) and are subsequently hospitalized pending culture results. Only 1% to 2% of infants 0 to 60 days old have an IBI,1-3 and most hospitalized infants are discharged once physicians feel confident that pathogens are unlikely to be isolated from blood and cerebrospinal fluid (CSF) cultures. Practice regarding duration of hospitalization while awaiting blood and CSF culture results is not standardized in this population. Longer hospitalizations can lead to increased costs and familial stress, including difficulty with breastfeeding and anxiety in newly postpartum mothers.4,5
In 2010, an institutional evidence-based guideline for the management of febrile infants aged 0 to 60 days recommended discharge after 36 hours of observation if all cultures were negative.6 However, recent studies demonstrate that 85% to 93% of pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 Assuming a 2% prevalence of IBI, if 15% of pathogens were identified after 24 hours of incubation, only one out of 333 infants would have an IBI identified after 24 hours of hospital observation.7
Furthermore, a review of our institution’s electronic health records (EHR) over the past 5 years revealed that an observation period of 24 hours would have resulted in the discharge of three infants with an IBI. Two infants had bacteremia; both were discharged from the emergency department (ED) without antibiotics, returned to care after cultures were reported positive at 27 hours, and had no adverse outcomes. The third infant had meningitis, but also had an abnormal CSF Gram stain, which led to a longer hospitalization.
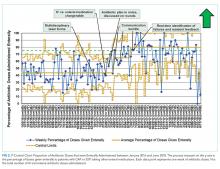
In 2019, our institution appraised the emerging literature and institutional data supporting the low absolute risk of missed IBI, and also leveraged local consensus among key stakeholders to update its evidence-based guideline for the evaluation and management of febrile infants aged 60 days and younger. The updated guideline recommends that clinicians consider discharging well-appearing neonates and infants if blood and CSF cultures remain negative at 24 hours.10 The objective of this study was to decrease the average hospital culture observation time (COT; culture incubation to hospital discharge) from 38 to 30 hours over a 12-month period in febrile infants aged 0 to 60 days.
METHODS
Context
Improvement efforts were conducted at Cincinnati Children’s Hospital Medical Center (CCHMC), a large, urban, academic hospital that admitted more than 8,000 noncritically ill patients to the hospital medicine (HM) service from July 1, 2018, through June 30, 2019. Hospital medicine teams, located at both the main and satellite campuses, are staffed by attending physicians, fellows, residents, medical students, and nurse practitioners. The two campuses, which are about 20 miles apart, share clinician providers but have distinct nursing pools.
Microbiology services for all CCHMC patients are provided at the main campus. Blood and CSF cultures at the satellite campus are transported to the main campus for incubation and monitoring via an urgent courier service. The microbiology laboratory at CCHMC uses a continuous monitoring system for blood cultures (BACT/ALERT Virtuo, BioMérieux). The system automatically alerts laboratory technicians of positive cultures; these results are reported to clinical providers within 30 minutes of detection. Laboratory technicians manually evaluate CSF cultures once daily for 5 days.
Improvement Team
Our improvement team included three HM attending physicians; two HM fellows; a pediatric chief resident; two nurses, who represented nursing pools at the main and satellite campuses; and a clinical pharmacist, who is a co-leader of the antimicrobial stewardship program at CCHMC. Supporting members for the improvement team included the CCHMC laboratory director; the microbiology laboratory director; an infectious disease physician, who is a co-leader of the antimicrobial stewardship program; and nursing directors of the HM units at both campuses.
Evidence-Based Guideline
Our improvement initiative was based on recommendations from the updated CCHMC Evidence-Based Care Guideline for Management of Infants 0 to 60 days with Fever of Unknown Source.10 This guideline, published in May 2019, was developed by a multidisciplinary working group composed of key stakeholders from HM, community pediatrics, emergency medicine, the pediatric residency program, infectious disease, and laboratory medicine. Several improvement team members were participants on the committee that published the evidence-based guideline. The committee first performed a systematic literature review and critical appraisal of the literature. Care recommendations were formulated via a consensus process directed by best evidence, patient and family preferences, and clinical expertise; the recommendations were subsequently reviewed and approved by clinical experts who were not involved in the development process.
Based on evidence review and multistakeholder consensus, the updated guideline recommends clinicians consider discharging neonates and infants aged 60 days and younger if there is no culture growth after an observation period of 24 hours (as documented in the EHR) and patients are otherwise medically ready for discharge (ie, well appearing with adequate oral intake).10,11 In addition, prior to discharge, there must be a documented working phone number on file for the patient’s parents/guardians, an established outpatient follow-up plan within 24 hours, and communication with the primary pediatrician who is in agreement with discharge at 24 hours.
Study Population
Infants 0 to 60 days old who had a documented or reported fever without an apparent source based on history and physical exam upon presentation to the ED, and who were subsequently admitted to the HM service at CCHMC between October 30, 2018, and July 10, 2020, were eligible for inclusion. We excluded infants who were admitted to other clinical services (eg, intensive care unit); had organisms identified on blood, urine, or CSF culture within 24 hours of incubation; had positive herpes simplex virus testing; had skin/soft tissue infections or another clearly documented source of bacterial infection; or had an alternative indication for hospitalization (eg, need for intravenous fluid or deep suctioning) after cultures had incubated for 24 hours. Infants who had a positive blood, urine, or CSF culture result after 24 hours of incubation were included in the study population. Organisms were classified as pathogen or contaminant based on treatment decisions made by the care team.
Improvement Activities
Key drivers critical to success of the improvement efforts were: (1) clearly defined standard of care for duration of observation in febrile infants 0 to 60 days old; (2) improved understanding of microbiology lab procedures; (3) effective communication of discharge criteria between providers and nurses; and (4) transparency of data with feedback (Figure 1).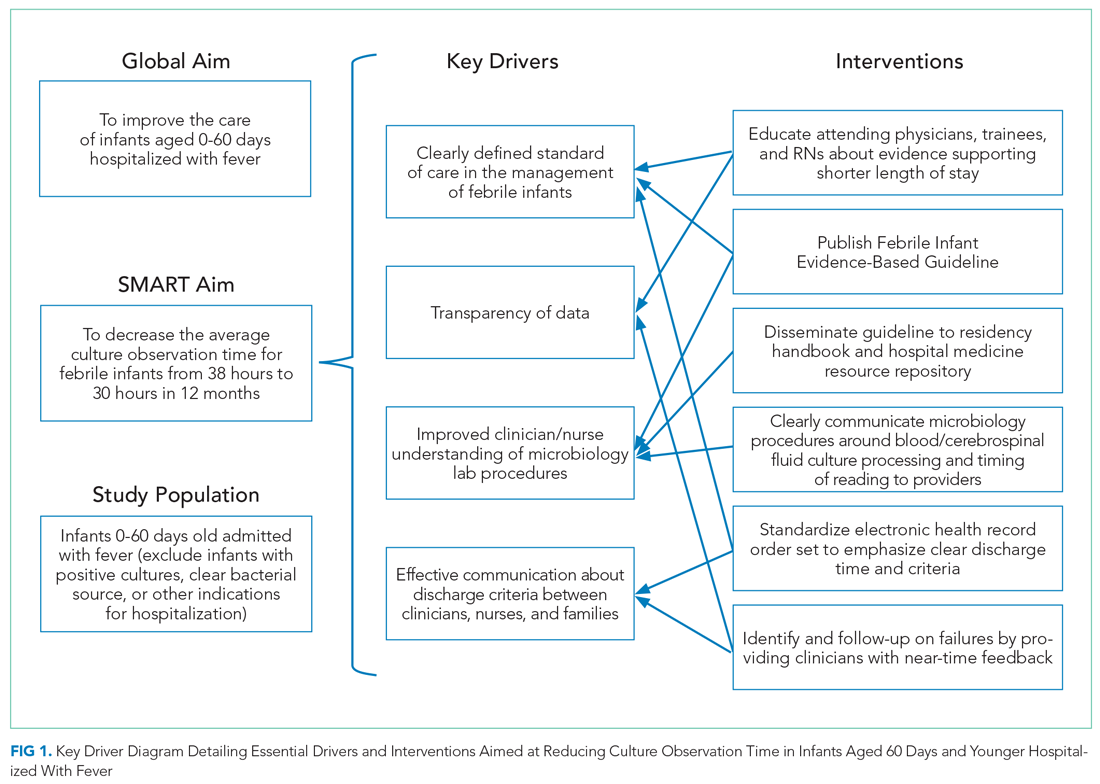
Education and Structured Dissemination of Evidence-Based Guideline
The CCHMC febrile infant guideline10 was disseminated to HM physicians, residents, and nurses via the following means: (1) in-person announcements at staff meetings and educational conferences, (2) published highlights from the guideline in weekly newsletters, and (3) email announcements. Additionally, members of the study team educated HM attending physicians, nursing staff from the medical units at both campuses, and resident physicians about recent studies demonstrating safety of shorter length of stay (LOS) in febrile infants aged 0 to 60 days. The study team also provided residents, physicians, and nurses with data on the number of positive blood and CSF cultures and outcomes of patients at CCHMC within the past 5 years. In addition, team members led a journal club for residents discussing an article7 describing time-to-positivity of blood and CSF cultures in febrile infants. For ongoing engagement, the evidence-based guideline and a detailed explanation of microbiology procedures were published in the resident handbook, an internal resource that includes vital clinical pearls and practice guidelines across specialties. (Each resident receives an updated hard copy each year, and there is also an online link to the resource in the EHR.) Information about the guideline and COT was also included in the monthly chief resident’s orientation script, which is relayed to all residents on the first day of their HM rotation.
Clear Communication of Microbiology Procedures
Team members created a detailed process map describing the processing protocols for blood and CSF cultures collected at both CCHMC campuses. This information was shared with HM attending physicians and nurses via in-person announcements at staff meetings, flyers in team workrooms, and email communications. Residents received information on microbiology protocols via in-person announcements at educational conferences and dissemination in the weekly residency newsletter.Important information communicated included:
1. Definition of culture start time. We conveyed that there may be a delay of up to 4 hours between culture collection at the satellite campus and culture incubation at the main campus laboratory. As a result, the time of blood or CSF sample arrival to the main campus laboratory was a more accurate reflection of the culture incubation start time than the culture collection time.
2. Explanation of CSF culture processing. We discussed the process by which these cultures are plated upon arrival at the microbiology laboratory and read once per day in the morning. Therefore, a culture incubated at midnight would be evaluated once at 9 hours and not again until 33 hours.
Modification of Febrile Infant Order Set
Enhancements to the febrile infant order set improved communication and cultivated a shared mental model regarding discharge goals among all members of the care team. The EHR order set for febrile infants was updated as follows: (1) mandatory free-text fields that established the culture start time for blood and CSF cultures were added, (2) culture start time was clearly defined (ie, the time culture arrives at the main campus laboratory), and (3) a change was made in the default discharge criteria11 to “culture observation for 24 hours,” with the ability to modify COT (Appendix Figure 1). We embedded hyperlinks to the guideline and microbiology process map within the updated order set, which allowed providers to easily access this information and refresh their knowledge of the recommendations (Appendix Figure 1).
Identification of Failures and Follow-up With Near-Time Feedback
All cases of febrile infants were tracked weekly. For infants hospitalized longer than 24 hours, the study team contacted the discharging clinicians to discuss reasons for prolonged hospitalization, with an emphasis on identifying system-level barriers to earlier discharge.
Study of the Interventions
The institutional microbiology database was queried weekly to identify all infants 0 to 60 days old who had a blood culture obtained and were hospitalized on the HM service. Study team members conducted targeted EHR review to determine whether patients met exclusion criteria and to identify reasons for prolonged COT. Baseline data were collected retrospectively for a 3-month period prior to initiation of improvement activities. During the study period, queries were conducted weekly and reviewed by study team members to evaluate the impact of improvement activities and to inform new interventions.
Measures
Our primary outcome measure was COT, defined as the hours between final culture incubation and hospital discharge. The operational definition for “final culture incubation” was the documented time of arrival of the last collected culture to the microbiology laboratory. Our goal COT was 30 hours to account for a subset of patients whose blood and/or CSF culture were obtained overnight (ie, after 9
Analysis
Measures were evaluated using statistical process control charts and run charts, and Western Electric rules were employed to determine special cause variation.12 Annotated X-bar S control charts tracked the impact of improvement activities on average COT and LOS for all infants. Given that a relatively small number of patients (ie, two to four) met inclusion criteria each week, average COT was calculated per five patients.
This study was considered exempt from review by the CCHMC Institutional Review Board.
RESULTS
Of the 184 infants in this study, 46 were included as part of baseline data collection, and 138 were included during the intervention period. The median age was 26.6 days (range, 3-59 days); 52% of patients were female; two-thirds were non-Hispanic White; 22% were Black, and 5% were Hispanic (Appendix Table).
Average COT decreased from 38 hours to 32 hours with improvement activities (Figure 2) and was sustained for a total of 17 months. There were small decreases in COT after initial education was provided to attendings, nurses, and residents. 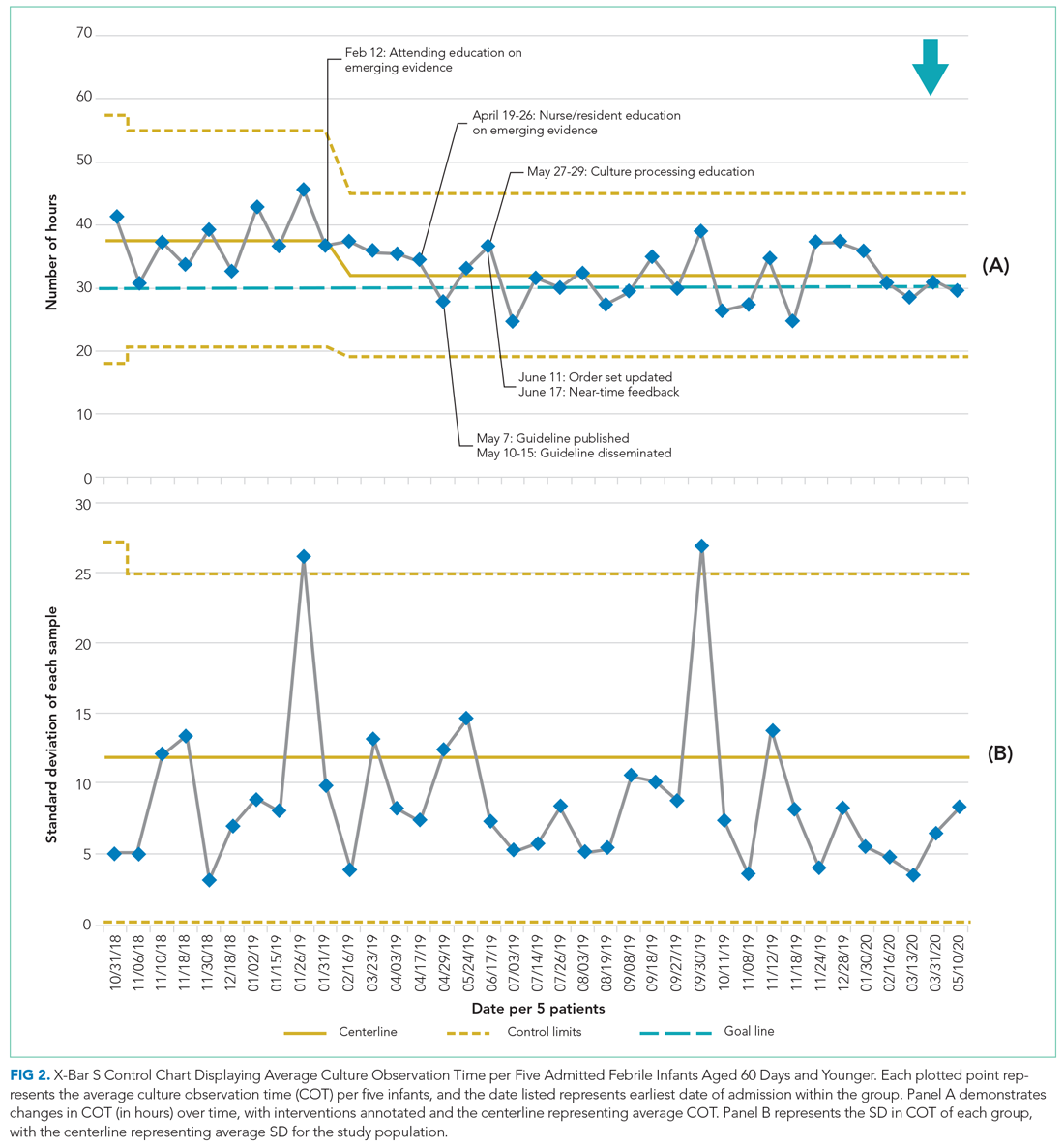

After the launch of the updated order set, median usage of the EHR order set increased from 50% to 80%. Medical discharge criteria were entered for 80 (96%) of the 83 patients for whom the updated order set was applied; culture incubation start times were entered for 78 (94%) of these patients.
No infants in our cohort were found to have IBI after hospital discharge. There were no ED revisits within 48 hours of discharge, and there were no hospital readmissions within 7 days of index discharge. Furthermore, none of the patients included in the study had growth of a pathogenic organism after 24 hours.
Of the 138 infants hospitalized during the intervention period, 77 (56%) had a COT greater than 30 hours. Among these 77 patients, 49 (64%) had their final culture incubated between 9
DISCUSSION
Our study aimed to decrease the average COT from 38 hours to 30 hours among hospitalized infants aged 60 days and younger over a period of 12 months. An intervention featuring implementation of an evidence-based guideline through education, laboratory procedure transparency, creation of a standardized EHR order set, and near-time feedback was associated with a shorter average COT of 32 hours, sustained over a 17-month period. No infants with bacteremia or meningitis were inappropriately discharged during this study.
Interpretation
Prior to our improvement efforts, most febrile infants at CCHMC were observed for at least 36 hours based on a prior institutional guideline,6 despite recent evidence suggesting that most pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 The goal of this improvement initiative was to bridge the gap between emerging evidence and clinical practice by developing and disseminating an updated evidence-based guideline to safely decrease the hospital observation time in febrile infants aged 60 days and younger.
Similar to previous studies aimed at improving diagnosis and management among febrile infants,13-16 generation and structured dissemination of an institutional evidence-based guideline was crucial to safely shortening COT in our population. These prior studies established a goal COT of 36 to 42 hours for hospitalized febrile infants.13,15,16 Our study incorporated emerging evidence and local experience into an updated evidence-based practice guideline to further reduce COT to 32 hours for hospitalized infants. Key factors contributing to our success included multidisciplinary engagement, specifically partnering with nurses and resident physicians in designing and implementing our initiatives. Furthermore, improved transparency of culture monitoring practices allowed clinicians to better understand the recommended observation periods. Finally, we employed a standardized EHR order set as a no-cost, one-time, high-reliability intervention to establish 24 hours of culture monitoring as the default and to enhance transparency around start time for culture incubation.
Average COT remained stable at 32 hours for 17 months after initiation of the intervention. During the intervention period, 64% patients with hospital stays longer than 30 hours had cultures obtained between 9
Limitations
The study has several limitations. First, this single-center study was conducted at a quaternary care medical center with a robust quality improvement infrastructure. Our interventions took advantage of the existing processes in place that ensure timely discharge of medically ready patients.11 Furthermore, microbiology laboratory practices are unique to our institution. These factors limit the generalizability of this work. Second, due to small numbers of eligible infants, analyses were conducted per five patients. Infrequent hospitalizations limited our ability to learn quickly from PDSA cycles. Finally, we did not measure cost savings attributable to shorter hospital stays. However, in addition to financial savings from charges and decreased nonmedical costs such as lost earnings and childcare,17 shorter hospitalizations have many additional benefits, such as promoting bonding and breastfeeding and decreasing exposure to nosocomial infections. Shorter hospitalizations, with clearly communicated discharge times, also serve to optimize patient throughput.
CONCLUSION
Implementation of a clinical practice guideline resulted in reduction of average COT from 38 to 32 hours in febrile infants aged 60 days and younger, with no cases of missed IBI. Engagement of multidisciplinary stakeholders in the generation and structured dissemination of the evidence-based guideline, improved transparency of the microbiological blood and CSF culture process, and standardization of EHR order sets were crucial to the success of this work. Cultures incubated overnight and daily CSF culture-monitoring practices primarily contributed to an average LOS of more than 30 hours.
Future work will include collaboration with emergency physicians to improve evaluation efficiency and decrease LOS in the ED for febrile infants. Additionally, creation of an automated data dashboard of COT and LOS will provide clinicians with real-time feedback on hospitalization practices.
Acknowledgments
The authors thank Dr Jeffrey Simmons, MD, MSc, as well as the members of the 2019 Fever of Uncertain Source Evidence-Based Guideline Committee. We also thank the James M Anderson Center for Health System Excellence and the Rapid Cycle Improvement Collaborative for their support with guideline development as well as design and execution of our improvement efforts.
1. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
2. Kuppermann N, Dayan PS, Levine DA, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
3. Nigrovic LE, Mahajan PV, Blumberg SM, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695
4. De S, Tong A, Isaacs D, Craig JC. Parental perspectives on evaluation and management of fever in young infants: an interview study. Arch Dis Child. 2014;99(8):717-723. https://doi.org/10.1136/archdischild-2013-305736
5. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202
6. FUS Team. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for fever of uncertain source in infants 60 days of age or less. Guideline 2. 2010:1-4.
7. Aronson PL, Wang ME, Nigrovic LE, et al; Febrile Young Infant Research Collaborative. Time to pathogen detection for non-ill versus ill-appearing infants ≤60 days old with bacteremia and meningitis. Hosp Pediatr. 2018;8(7):379-384. https://doi.org/10.1542/hpeds.2018-0002
8. Biondi EA, Mischler M, Jerardi KE, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844-849. https://doi.org/10.1001/jamapediatrics.2014.895
9. Lefebvre CE, Renaud C, Chartrand C. Time to positivity of blood cultures in infants 0 to 90 days old presenting to the emergency department: is 36 hours enough? J Pediatric Infect Dis Soc. 2017;6(1):28-32. https://doi.org/10.1093/jpids/piv078
10. Unaka N, Statile A, Bensman, R, et al. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for evidence-based care guideline for management of infants 0 to 60 days seen in emergency department for fever of unknown source. Guideline 10. 2019;1-42. http://www.cincinnatichildrens.org/service/j/anderson-center/evidence-based-care/recommendations/default/
11. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
12. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458-464. https://doi.org/10.1136/qhc.12.6.458
13. Biondi EA, McCulloh R, Staggs VS, et al; American Academy of Pediatrics’ Revise Collaborative. Reducing variability in the infant sepsis evaluation (REVISE): a national quality initiative. Pediatrics. 2019;144(3): e20182201. https://doi.org/10.1542/peds.2018-2201
14. McCulloh RJ, Commers T, Williams DD, Michael J, Mann K, Newland JG. Effect of combined clinical practice guideline and electronic order set implementation on febrile infant evaluation and management. Pediatr Emerg Care. 2021;37(1):e25-e31. https://doi.org/10.1097/pec.0000000000002012
15. Foster LZ, Beiner J, Duh-Leong C, et al. Implementation of febrile infant management guidelines reduces hospitalization. Pediatr Qual Saf. 2020;5(1):e252. https://doi.org/10.1097/pq9.0000000000000252
16. Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics. 2012;130(1):e16-e24. https://doi.org/10.1542/peds.2012-0127
17. Chang LV, Shah AN, Hoefgen ER, et al; H2O Study Group. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
Febrile infants aged 0 to 60 days often undergo diagnostic testing to evaluate for invasive bacterial infections (IBI; ie, bacteremia and meningitis) and are subsequently hospitalized pending culture results. Only 1% to 2% of infants 0 to 60 days old have an IBI,1-3 and most hospitalized infants are discharged once physicians feel confident that pathogens are unlikely to be isolated from blood and cerebrospinal fluid (CSF) cultures. Practice regarding duration of hospitalization while awaiting blood and CSF culture results is not standardized in this population. Longer hospitalizations can lead to increased costs and familial stress, including difficulty with breastfeeding and anxiety in newly postpartum mothers.4,5
In 2010, an institutional evidence-based guideline for the management of febrile infants aged 0 to 60 days recommended discharge after 36 hours of observation if all cultures were negative.6 However, recent studies demonstrate that 85% to 93% of pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 Assuming a 2% prevalence of IBI, if 15% of pathogens were identified after 24 hours of incubation, only one out of 333 infants would have an IBI identified after 24 hours of hospital observation.7
Furthermore, a review of our institution’s electronic health records (EHR) over the past 5 years revealed that an observation period of 24 hours would have resulted in the discharge of three infants with an IBI. Two infants had bacteremia; both were discharged from the emergency department (ED) without antibiotics, returned to care after cultures were reported positive at 27 hours, and had no adverse outcomes. The third infant had meningitis, but also had an abnormal CSF Gram stain, which led to a longer hospitalization.
In 2019, our institution appraised the emerging literature and institutional data supporting the low absolute risk of missed IBI, and also leveraged local consensus among key stakeholders to update its evidence-based guideline for the evaluation and management of febrile infants aged 60 days and younger. The updated guideline recommends that clinicians consider discharging well-appearing neonates and infants if blood and CSF cultures remain negative at 24 hours.10 The objective of this study was to decrease the average hospital culture observation time (COT; culture incubation to hospital discharge) from 38 to 30 hours over a 12-month period in febrile infants aged 0 to 60 days.
METHODS
Context
Improvement efforts were conducted at Cincinnati Children’s Hospital Medical Center (CCHMC), a large, urban, academic hospital that admitted more than 8,000 noncritically ill patients to the hospital medicine (HM) service from July 1, 2018, through June 30, 2019. Hospital medicine teams, located at both the main and satellite campuses, are staffed by attending physicians, fellows, residents, medical students, and nurse practitioners. The two campuses, which are about 20 miles apart, share clinician providers but have distinct nursing pools.
Microbiology services for all CCHMC patients are provided at the main campus. Blood and CSF cultures at the satellite campus are transported to the main campus for incubation and monitoring via an urgent courier service. The microbiology laboratory at CCHMC uses a continuous monitoring system for blood cultures (BACT/ALERT Virtuo, BioMérieux). The system automatically alerts laboratory technicians of positive cultures; these results are reported to clinical providers within 30 minutes of detection. Laboratory technicians manually evaluate CSF cultures once daily for 5 days.
Improvement Team
Our improvement team included three HM attending physicians; two HM fellows; a pediatric chief resident; two nurses, who represented nursing pools at the main and satellite campuses; and a clinical pharmacist, who is a co-leader of the antimicrobial stewardship program at CCHMC. Supporting members for the improvement team included the CCHMC laboratory director; the microbiology laboratory director; an infectious disease physician, who is a co-leader of the antimicrobial stewardship program; and nursing directors of the HM units at both campuses.
Evidence-Based Guideline
Our improvement initiative was based on recommendations from the updated CCHMC Evidence-Based Care Guideline for Management of Infants 0 to 60 days with Fever of Unknown Source.10 This guideline, published in May 2019, was developed by a multidisciplinary working group composed of key stakeholders from HM, community pediatrics, emergency medicine, the pediatric residency program, infectious disease, and laboratory medicine. Several improvement team members were participants on the committee that published the evidence-based guideline. The committee first performed a systematic literature review and critical appraisal of the literature. Care recommendations were formulated via a consensus process directed by best evidence, patient and family preferences, and clinical expertise; the recommendations were subsequently reviewed and approved by clinical experts who were not involved in the development process.
Based on evidence review and multistakeholder consensus, the updated guideline recommends clinicians consider discharging neonates and infants aged 60 days and younger if there is no culture growth after an observation period of 24 hours (as documented in the EHR) and patients are otherwise medically ready for discharge (ie, well appearing with adequate oral intake).10,11 In addition, prior to discharge, there must be a documented working phone number on file for the patient’s parents/guardians, an established outpatient follow-up plan within 24 hours, and communication with the primary pediatrician who is in agreement with discharge at 24 hours.
Study Population
Infants 0 to 60 days old who had a documented or reported fever without an apparent source based on history and physical exam upon presentation to the ED, and who were subsequently admitted to the HM service at CCHMC between October 30, 2018, and July 10, 2020, were eligible for inclusion. We excluded infants who were admitted to other clinical services (eg, intensive care unit); had organisms identified on blood, urine, or CSF culture within 24 hours of incubation; had positive herpes simplex virus testing; had skin/soft tissue infections or another clearly documented source of bacterial infection; or had an alternative indication for hospitalization (eg, need for intravenous fluid or deep suctioning) after cultures had incubated for 24 hours. Infants who had a positive blood, urine, or CSF culture result after 24 hours of incubation were included in the study population. Organisms were classified as pathogen or contaminant based on treatment decisions made by the care team.
Improvement Activities
Key drivers critical to success of the improvement efforts were: (1) clearly defined standard of care for duration of observation in febrile infants 0 to 60 days old; (2) improved understanding of microbiology lab procedures; (3) effective communication of discharge criteria between providers and nurses; and (4) transparency of data with feedback (Figure 1).
Education and Structured Dissemination of Evidence-Based Guideline
The CCHMC febrile infant guideline10 was disseminated to HM physicians, residents, and nurses via the following means: (1) in-person announcements at staff meetings and educational conferences, (2) published highlights from the guideline in weekly newsletters, and (3) email announcements. Additionally, members of the study team educated HM attending physicians, nursing staff from the medical units at both campuses, and resident physicians about recent studies demonstrating safety of shorter length of stay (LOS) in febrile infants aged 0 to 60 days. The study team also provided residents, physicians, and nurses with data on the number of positive blood and CSF cultures and outcomes of patients at CCHMC within the past 5 years. In addition, team members led a journal club for residents discussing an article7 describing time-to-positivity of blood and CSF cultures in febrile infants. For ongoing engagement, the evidence-based guideline and a detailed explanation of microbiology procedures were published in the resident handbook, an internal resource that includes vital clinical pearls and practice guidelines across specialties. (Each resident receives an updated hard copy each year, and there is also an online link to the resource in the EHR.) Information about the guideline and COT was also included in the monthly chief resident’s orientation script, which is relayed to all residents on the first day of their HM rotation.
Clear Communication of Microbiology Procedures
Team members created a detailed process map describing the processing protocols for blood and CSF cultures collected at both CCHMC campuses. This information was shared with HM attending physicians and nurses via in-person announcements at staff meetings, flyers in team workrooms, and email communications. Residents received information on microbiology protocols via in-person announcements at educational conferences and dissemination in the weekly residency newsletter.Important information communicated included:
1. Definition of culture start time. We conveyed that there may be a delay of up to 4 hours between culture collection at the satellite campus and culture incubation at the main campus laboratory. As a result, the time of blood or CSF sample arrival to the main campus laboratory was a more accurate reflection of the culture incubation start time than the culture collection time.
2. Explanation of CSF culture processing. We discussed the process by which these cultures are plated upon arrival at the microbiology laboratory and read once per day in the morning. Therefore, a culture incubated at midnight would be evaluated once at 9 hours and not again until 33 hours.
Modification of Febrile Infant Order Set
Enhancements to the febrile infant order set improved communication and cultivated a shared mental model regarding discharge goals among all members of the care team. The EHR order set for febrile infants was updated as follows: (1) mandatory free-text fields that established the culture start time for blood and CSF cultures were added, (2) culture start time was clearly defined (ie, the time culture arrives at the main campus laboratory), and (3) a change was made in the default discharge criteria11 to “culture observation for 24 hours,” with the ability to modify COT (Appendix Figure 1). We embedded hyperlinks to the guideline and microbiology process map within the updated order set, which allowed providers to easily access this information and refresh their knowledge of the recommendations (Appendix Figure 1).
Identification of Failures and Follow-up With Near-Time Feedback
All cases of febrile infants were tracked weekly. For infants hospitalized longer than 24 hours, the study team contacted the discharging clinicians to discuss reasons for prolonged hospitalization, with an emphasis on identifying system-level barriers to earlier discharge.
Study of the Interventions
The institutional microbiology database was queried weekly to identify all infants 0 to 60 days old who had a blood culture obtained and were hospitalized on the HM service. Study team members conducted targeted EHR review to determine whether patients met exclusion criteria and to identify reasons for prolonged COT. Baseline data were collected retrospectively for a 3-month period prior to initiation of improvement activities. During the study period, queries were conducted weekly and reviewed by study team members to evaluate the impact of improvement activities and to inform new interventions.
Measures
Our primary outcome measure was COT, defined as the hours between final culture incubation and hospital discharge. The operational definition for “final culture incubation” was the documented time of arrival of the last collected culture to the microbiology laboratory. Our goal COT was 30 hours to account for a subset of patients whose blood and/or CSF culture were obtained overnight (ie, after 9
Analysis
Measures were evaluated using statistical process control charts and run charts, and Western Electric rules were employed to determine special cause variation.12 Annotated X-bar S control charts tracked the impact of improvement activities on average COT and LOS for all infants. Given that a relatively small number of patients (ie, two to four) met inclusion criteria each week, average COT was calculated per five patients.
This study was considered exempt from review by the CCHMC Institutional Review Board.
RESULTS
Of the 184 infants in this study, 46 were included as part of baseline data collection, and 138 were included during the intervention period. The median age was 26.6 days (range, 3-59 days); 52% of patients were female; two-thirds were non-Hispanic White; 22% were Black, and 5% were Hispanic (Appendix Table).
Average COT decreased from 38 hours to 32 hours with improvement activities (Figure 2) and was sustained for a total of 17 months. There were small decreases in COT after initial education was provided to attendings, nurses, and residents. 

After the launch of the updated order set, median usage of the EHR order set increased from 50% to 80%. Medical discharge criteria were entered for 80 (96%) of the 83 patients for whom the updated order set was applied; culture incubation start times were entered for 78 (94%) of these patients.
No infants in our cohort were found to have IBI after hospital discharge. There were no ED revisits within 48 hours of discharge, and there were no hospital readmissions within 7 days of index discharge. Furthermore, none of the patients included in the study had growth of a pathogenic organism after 24 hours.
Of the 138 infants hospitalized during the intervention period, 77 (56%) had a COT greater than 30 hours. Among these 77 patients, 49 (64%) had their final culture incubated between 9
DISCUSSION
Our study aimed to decrease the average COT from 38 hours to 30 hours among hospitalized infants aged 60 days and younger over a period of 12 months. An intervention featuring implementation of an evidence-based guideline through education, laboratory procedure transparency, creation of a standardized EHR order set, and near-time feedback was associated with a shorter average COT of 32 hours, sustained over a 17-month period. No infants with bacteremia or meningitis were inappropriately discharged during this study.
Interpretation
Prior to our improvement efforts, most febrile infants at CCHMC were observed for at least 36 hours based on a prior institutional guideline,6 despite recent evidence suggesting that most pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 The goal of this improvement initiative was to bridge the gap between emerging evidence and clinical practice by developing and disseminating an updated evidence-based guideline to safely decrease the hospital observation time in febrile infants aged 60 days and younger.
Similar to previous studies aimed at improving diagnosis and management among febrile infants,13-16 generation and structured dissemination of an institutional evidence-based guideline was crucial to safely shortening COT in our population. These prior studies established a goal COT of 36 to 42 hours for hospitalized febrile infants.13,15,16 Our study incorporated emerging evidence and local experience into an updated evidence-based practice guideline to further reduce COT to 32 hours for hospitalized infants. Key factors contributing to our success included multidisciplinary engagement, specifically partnering with nurses and resident physicians in designing and implementing our initiatives. Furthermore, improved transparency of culture monitoring practices allowed clinicians to better understand the recommended observation periods. Finally, we employed a standardized EHR order set as a no-cost, one-time, high-reliability intervention to establish 24 hours of culture monitoring as the default and to enhance transparency around start time for culture incubation.
Average COT remained stable at 32 hours for 17 months after initiation of the intervention. During the intervention period, 64% patients with hospital stays longer than 30 hours had cultures obtained between 9
Limitations
The study has several limitations. First, this single-center study was conducted at a quaternary care medical center with a robust quality improvement infrastructure. Our interventions took advantage of the existing processes in place that ensure timely discharge of medically ready patients.11 Furthermore, microbiology laboratory practices are unique to our institution. These factors limit the generalizability of this work. Second, due to small numbers of eligible infants, analyses were conducted per five patients. Infrequent hospitalizations limited our ability to learn quickly from PDSA cycles. Finally, we did not measure cost savings attributable to shorter hospital stays. However, in addition to financial savings from charges and decreased nonmedical costs such as lost earnings and childcare,17 shorter hospitalizations have many additional benefits, such as promoting bonding and breastfeeding and decreasing exposure to nosocomial infections. Shorter hospitalizations, with clearly communicated discharge times, also serve to optimize patient throughput.
CONCLUSION
Implementation of a clinical practice guideline resulted in reduction of average COT from 38 to 32 hours in febrile infants aged 60 days and younger, with no cases of missed IBI. Engagement of multidisciplinary stakeholders in the generation and structured dissemination of the evidence-based guideline, improved transparency of the microbiological blood and CSF culture process, and standardization of EHR order sets were crucial to the success of this work. Cultures incubated overnight and daily CSF culture-monitoring practices primarily contributed to an average LOS of more than 30 hours.
Future work will include collaboration with emergency physicians to improve evaluation efficiency and decrease LOS in the ED for febrile infants. Additionally, creation of an automated data dashboard of COT and LOS will provide clinicians with real-time feedback on hospitalization practices.
Acknowledgments
The authors thank Dr Jeffrey Simmons, MD, MSc, as well as the members of the 2019 Fever of Uncertain Source Evidence-Based Guideline Committee. We also thank the James M Anderson Center for Health System Excellence and the Rapid Cycle Improvement Collaborative for their support with guideline development as well as design and execution of our improvement efforts.
Febrile infants aged 0 to 60 days often undergo diagnostic testing to evaluate for invasive bacterial infections (IBI; ie, bacteremia and meningitis) and are subsequently hospitalized pending culture results. Only 1% to 2% of infants 0 to 60 days old have an IBI,1-3 and most hospitalized infants are discharged once physicians feel confident that pathogens are unlikely to be isolated from blood and cerebrospinal fluid (CSF) cultures. Practice regarding duration of hospitalization while awaiting blood and CSF culture results is not standardized in this population. Longer hospitalizations can lead to increased costs and familial stress, including difficulty with breastfeeding and anxiety in newly postpartum mothers.4,5
In 2010, an institutional evidence-based guideline for the management of febrile infants aged 0 to 60 days recommended discharge after 36 hours of observation if all cultures were negative.6 However, recent studies demonstrate that 85% to 93% of pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 Assuming a 2% prevalence of IBI, if 15% of pathogens were identified after 24 hours of incubation, only one out of 333 infants would have an IBI identified after 24 hours of hospital observation.7
Furthermore, a review of our institution’s electronic health records (EHR) over the past 5 years revealed that an observation period of 24 hours would have resulted in the discharge of three infants with an IBI. Two infants had bacteremia; both were discharged from the emergency department (ED) without antibiotics, returned to care after cultures were reported positive at 27 hours, and had no adverse outcomes. The third infant had meningitis, but also had an abnormal CSF Gram stain, which led to a longer hospitalization.
In 2019, our institution appraised the emerging literature and institutional data supporting the low absolute risk of missed IBI, and also leveraged local consensus among key stakeholders to update its evidence-based guideline for the evaluation and management of febrile infants aged 60 days and younger. The updated guideline recommends that clinicians consider discharging well-appearing neonates and infants if blood and CSF cultures remain negative at 24 hours.10 The objective of this study was to decrease the average hospital culture observation time (COT; culture incubation to hospital discharge) from 38 to 30 hours over a 12-month period in febrile infants aged 0 to 60 days.
METHODS
Context
Improvement efforts were conducted at Cincinnati Children’s Hospital Medical Center (CCHMC), a large, urban, academic hospital that admitted more than 8,000 noncritically ill patients to the hospital medicine (HM) service from July 1, 2018, through June 30, 2019. Hospital medicine teams, located at both the main and satellite campuses, are staffed by attending physicians, fellows, residents, medical students, and nurse practitioners. The two campuses, which are about 20 miles apart, share clinician providers but have distinct nursing pools.
Microbiology services for all CCHMC patients are provided at the main campus. Blood and CSF cultures at the satellite campus are transported to the main campus for incubation and monitoring via an urgent courier service. The microbiology laboratory at CCHMC uses a continuous monitoring system for blood cultures (BACT/ALERT Virtuo, BioMérieux). The system automatically alerts laboratory technicians of positive cultures; these results are reported to clinical providers within 30 minutes of detection. Laboratory technicians manually evaluate CSF cultures once daily for 5 days.
Improvement Team
Our improvement team included three HM attending physicians; two HM fellows; a pediatric chief resident; two nurses, who represented nursing pools at the main and satellite campuses; and a clinical pharmacist, who is a co-leader of the antimicrobial stewardship program at CCHMC. Supporting members for the improvement team included the CCHMC laboratory director; the microbiology laboratory director; an infectious disease physician, who is a co-leader of the antimicrobial stewardship program; and nursing directors of the HM units at both campuses.
Evidence-Based Guideline
Our improvement initiative was based on recommendations from the updated CCHMC Evidence-Based Care Guideline for Management of Infants 0 to 60 days with Fever of Unknown Source.10 This guideline, published in May 2019, was developed by a multidisciplinary working group composed of key stakeholders from HM, community pediatrics, emergency medicine, the pediatric residency program, infectious disease, and laboratory medicine. Several improvement team members were participants on the committee that published the evidence-based guideline. The committee first performed a systematic literature review and critical appraisal of the literature. Care recommendations were formulated via a consensus process directed by best evidence, patient and family preferences, and clinical expertise; the recommendations were subsequently reviewed and approved by clinical experts who were not involved in the development process.
Based on evidence review and multistakeholder consensus, the updated guideline recommends clinicians consider discharging neonates and infants aged 60 days and younger if there is no culture growth after an observation period of 24 hours (as documented in the EHR) and patients are otherwise medically ready for discharge (ie, well appearing with adequate oral intake).10,11 In addition, prior to discharge, there must be a documented working phone number on file for the patient’s parents/guardians, an established outpatient follow-up plan within 24 hours, and communication with the primary pediatrician who is in agreement with discharge at 24 hours.
Study Population
Infants 0 to 60 days old who had a documented or reported fever without an apparent source based on history and physical exam upon presentation to the ED, and who were subsequently admitted to the HM service at CCHMC between October 30, 2018, and July 10, 2020, were eligible for inclusion. We excluded infants who were admitted to other clinical services (eg, intensive care unit); had organisms identified on blood, urine, or CSF culture within 24 hours of incubation; had positive herpes simplex virus testing; had skin/soft tissue infections or another clearly documented source of bacterial infection; or had an alternative indication for hospitalization (eg, need for intravenous fluid or deep suctioning) after cultures had incubated for 24 hours. Infants who had a positive blood, urine, or CSF culture result after 24 hours of incubation were included in the study population. Organisms were classified as pathogen or contaminant based on treatment decisions made by the care team.
Improvement Activities
Key drivers critical to success of the improvement efforts were: (1) clearly defined standard of care for duration of observation in febrile infants 0 to 60 days old; (2) improved understanding of microbiology lab procedures; (3) effective communication of discharge criteria between providers and nurses; and (4) transparency of data with feedback (Figure 1).
Education and Structured Dissemination of Evidence-Based Guideline
The CCHMC febrile infant guideline10 was disseminated to HM physicians, residents, and nurses via the following means: (1) in-person announcements at staff meetings and educational conferences, (2) published highlights from the guideline in weekly newsletters, and (3) email announcements. Additionally, members of the study team educated HM attending physicians, nursing staff from the medical units at both campuses, and resident physicians about recent studies demonstrating safety of shorter length of stay (LOS) in febrile infants aged 0 to 60 days. The study team also provided residents, physicians, and nurses with data on the number of positive blood and CSF cultures and outcomes of patients at CCHMC within the past 5 years. In addition, team members led a journal club for residents discussing an article7 describing time-to-positivity of blood and CSF cultures in febrile infants. For ongoing engagement, the evidence-based guideline and a detailed explanation of microbiology procedures were published in the resident handbook, an internal resource that includes vital clinical pearls and practice guidelines across specialties. (Each resident receives an updated hard copy each year, and there is also an online link to the resource in the EHR.) Information about the guideline and COT was also included in the monthly chief resident’s orientation script, which is relayed to all residents on the first day of their HM rotation.
Clear Communication of Microbiology Procedures
Team members created a detailed process map describing the processing protocols for blood and CSF cultures collected at both CCHMC campuses. This information was shared with HM attending physicians and nurses via in-person announcements at staff meetings, flyers in team workrooms, and email communications. Residents received information on microbiology protocols via in-person announcements at educational conferences and dissemination in the weekly residency newsletter.Important information communicated included:
1. Definition of culture start time. We conveyed that there may be a delay of up to 4 hours between culture collection at the satellite campus and culture incubation at the main campus laboratory. As a result, the time of blood or CSF sample arrival to the main campus laboratory was a more accurate reflection of the culture incubation start time than the culture collection time.
2. Explanation of CSF culture processing. We discussed the process by which these cultures are plated upon arrival at the microbiology laboratory and read once per day in the morning. Therefore, a culture incubated at midnight would be evaluated once at 9 hours and not again until 33 hours.
Modification of Febrile Infant Order Set
Enhancements to the febrile infant order set improved communication and cultivated a shared mental model regarding discharge goals among all members of the care team. The EHR order set for febrile infants was updated as follows: (1) mandatory free-text fields that established the culture start time for blood and CSF cultures were added, (2) culture start time was clearly defined (ie, the time culture arrives at the main campus laboratory), and (3) a change was made in the default discharge criteria11 to “culture observation for 24 hours,” with the ability to modify COT (Appendix Figure 1). We embedded hyperlinks to the guideline and microbiology process map within the updated order set, which allowed providers to easily access this information and refresh their knowledge of the recommendations (Appendix Figure 1).
Identification of Failures and Follow-up With Near-Time Feedback
All cases of febrile infants were tracked weekly. For infants hospitalized longer than 24 hours, the study team contacted the discharging clinicians to discuss reasons for prolonged hospitalization, with an emphasis on identifying system-level barriers to earlier discharge.
Study of the Interventions
The institutional microbiology database was queried weekly to identify all infants 0 to 60 days old who had a blood culture obtained and were hospitalized on the HM service. Study team members conducted targeted EHR review to determine whether patients met exclusion criteria and to identify reasons for prolonged COT. Baseline data were collected retrospectively for a 3-month period prior to initiation of improvement activities. During the study period, queries were conducted weekly and reviewed by study team members to evaluate the impact of improvement activities and to inform new interventions.
Measures
Our primary outcome measure was COT, defined as the hours between final culture incubation and hospital discharge. The operational definition for “final culture incubation” was the documented time of arrival of the last collected culture to the microbiology laboratory. Our goal COT was 30 hours to account for a subset of patients whose blood and/or CSF culture were obtained overnight (ie, after 9
Analysis
Measures were evaluated using statistical process control charts and run charts, and Western Electric rules were employed to determine special cause variation.12 Annotated X-bar S control charts tracked the impact of improvement activities on average COT and LOS for all infants. Given that a relatively small number of patients (ie, two to four) met inclusion criteria each week, average COT was calculated per five patients.
This study was considered exempt from review by the CCHMC Institutional Review Board.
RESULTS
Of the 184 infants in this study, 46 were included as part of baseline data collection, and 138 were included during the intervention period. The median age was 26.6 days (range, 3-59 days); 52% of patients were female; two-thirds were non-Hispanic White; 22% were Black, and 5% were Hispanic (Appendix Table).
Average COT decreased from 38 hours to 32 hours with improvement activities (Figure 2) and was sustained for a total of 17 months. There were small decreases in COT after initial education was provided to attendings, nurses, and residents. 

After the launch of the updated order set, median usage of the EHR order set increased from 50% to 80%. Medical discharge criteria were entered for 80 (96%) of the 83 patients for whom the updated order set was applied; culture incubation start times were entered for 78 (94%) of these patients.
No infants in our cohort were found to have IBI after hospital discharge. There were no ED revisits within 48 hours of discharge, and there were no hospital readmissions within 7 days of index discharge. Furthermore, none of the patients included in the study had growth of a pathogenic organism after 24 hours.
Of the 138 infants hospitalized during the intervention period, 77 (56%) had a COT greater than 30 hours. Among these 77 patients, 49 (64%) had their final culture incubated between 9
DISCUSSION
Our study aimed to decrease the average COT from 38 hours to 30 hours among hospitalized infants aged 60 days and younger over a period of 12 months. An intervention featuring implementation of an evidence-based guideline through education, laboratory procedure transparency, creation of a standardized EHR order set, and near-time feedback was associated with a shorter average COT of 32 hours, sustained over a 17-month period. No infants with bacteremia or meningitis were inappropriately discharged during this study.
Interpretation
Prior to our improvement efforts, most febrile infants at CCHMC were observed for at least 36 hours based on a prior institutional guideline,6 despite recent evidence suggesting that most pathogens in blood and CSF cultures grow within 24 hours of incubation.7-9 The goal of this improvement initiative was to bridge the gap between emerging evidence and clinical practice by developing and disseminating an updated evidence-based guideline to safely decrease the hospital observation time in febrile infants aged 60 days and younger.
Similar to previous studies aimed at improving diagnosis and management among febrile infants,13-16 generation and structured dissemination of an institutional evidence-based guideline was crucial to safely shortening COT in our population. These prior studies established a goal COT of 36 to 42 hours for hospitalized febrile infants.13,15,16 Our study incorporated emerging evidence and local experience into an updated evidence-based practice guideline to further reduce COT to 32 hours for hospitalized infants. Key factors contributing to our success included multidisciplinary engagement, specifically partnering with nurses and resident physicians in designing and implementing our initiatives. Furthermore, improved transparency of culture monitoring practices allowed clinicians to better understand the recommended observation periods. Finally, we employed a standardized EHR order set as a no-cost, one-time, high-reliability intervention to establish 24 hours of culture monitoring as the default and to enhance transparency around start time for culture incubation.
Average COT remained stable at 32 hours for 17 months after initiation of the intervention. During the intervention period, 64% patients with hospital stays longer than 30 hours had cultures obtained between 9
Limitations
The study has several limitations. First, this single-center study was conducted at a quaternary care medical center with a robust quality improvement infrastructure. Our interventions took advantage of the existing processes in place that ensure timely discharge of medically ready patients.11 Furthermore, microbiology laboratory practices are unique to our institution. These factors limit the generalizability of this work. Second, due to small numbers of eligible infants, analyses were conducted per five patients. Infrequent hospitalizations limited our ability to learn quickly from PDSA cycles. Finally, we did not measure cost savings attributable to shorter hospital stays. However, in addition to financial savings from charges and decreased nonmedical costs such as lost earnings and childcare,17 shorter hospitalizations have many additional benefits, such as promoting bonding and breastfeeding and decreasing exposure to nosocomial infections. Shorter hospitalizations, with clearly communicated discharge times, also serve to optimize patient throughput.
CONCLUSION
Implementation of a clinical practice guideline resulted in reduction of average COT from 38 to 32 hours in febrile infants aged 60 days and younger, with no cases of missed IBI. Engagement of multidisciplinary stakeholders in the generation and structured dissemination of the evidence-based guideline, improved transparency of the microbiological blood and CSF culture process, and standardization of EHR order sets were crucial to the success of this work. Cultures incubated overnight and daily CSF culture-monitoring practices primarily contributed to an average LOS of more than 30 hours.
Future work will include collaboration with emergency physicians to improve evaluation efficiency and decrease LOS in the ED for febrile infants. Additionally, creation of an automated data dashboard of COT and LOS will provide clinicians with real-time feedback on hospitalization practices.
Acknowledgments
The authors thank Dr Jeffrey Simmons, MD, MSc, as well as the members of the 2019 Fever of Uncertain Source Evidence-Based Guideline Committee. We also thank the James M Anderson Center for Health System Excellence and the Rapid Cycle Improvement Collaborative for their support with guideline development as well as design and execution of our improvement efforts.
1. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
2. Kuppermann N, Dayan PS, Levine DA, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
3. Nigrovic LE, Mahajan PV, Blumberg SM, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695
4. De S, Tong A, Isaacs D, Craig JC. Parental perspectives on evaluation and management of fever in young infants: an interview study. Arch Dis Child. 2014;99(8):717-723. https://doi.org/10.1136/archdischild-2013-305736
5. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202
6. FUS Team. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for fever of uncertain source in infants 60 days of age or less. Guideline 2. 2010:1-4.
7. Aronson PL, Wang ME, Nigrovic LE, et al; Febrile Young Infant Research Collaborative. Time to pathogen detection for non-ill versus ill-appearing infants ≤60 days old with bacteremia and meningitis. Hosp Pediatr. 2018;8(7):379-384. https://doi.org/10.1542/hpeds.2018-0002
8. Biondi EA, Mischler M, Jerardi KE, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844-849. https://doi.org/10.1001/jamapediatrics.2014.895
9. Lefebvre CE, Renaud C, Chartrand C. Time to positivity of blood cultures in infants 0 to 90 days old presenting to the emergency department: is 36 hours enough? J Pediatric Infect Dis Soc. 2017;6(1):28-32. https://doi.org/10.1093/jpids/piv078
10. Unaka N, Statile A, Bensman, R, et al. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for evidence-based care guideline for management of infants 0 to 60 days seen in emergency department for fever of unknown source. Guideline 10. 2019;1-42. http://www.cincinnatichildrens.org/service/j/anderson-center/evidence-based-care/recommendations/default/
11. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
12. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458-464. https://doi.org/10.1136/qhc.12.6.458
13. Biondi EA, McCulloh R, Staggs VS, et al; American Academy of Pediatrics’ Revise Collaborative. Reducing variability in the infant sepsis evaluation (REVISE): a national quality initiative. Pediatrics. 2019;144(3): e20182201. https://doi.org/10.1542/peds.2018-2201
14. McCulloh RJ, Commers T, Williams DD, Michael J, Mann K, Newland JG. Effect of combined clinical practice guideline and electronic order set implementation on febrile infant evaluation and management. Pediatr Emerg Care. 2021;37(1):e25-e31. https://doi.org/10.1097/pec.0000000000002012
15. Foster LZ, Beiner J, Duh-Leong C, et al. Implementation of febrile infant management guidelines reduces hospitalization. Pediatr Qual Saf. 2020;5(1):e252. https://doi.org/10.1097/pq9.0000000000000252
16. Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics. 2012;130(1):e16-e24. https://doi.org/10.1542/peds.2012-0127
17. Chang LV, Shah AN, Hoefgen ER, et al; H2O Study Group. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
1. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
2. Kuppermann N, Dayan PS, Levine DA, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
3. Nigrovic LE, Mahajan PV, Blumberg SM, et al; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695
4. De S, Tong A, Isaacs D, Craig JC. Parental perspectives on evaluation and management of fever in young infants: an interview study. Arch Dis Child. 2014;99(8):717-723. https://doi.org/10.1136/archdischild-2013-305736
5. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202
6. FUS Team. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for fever of uncertain source in infants 60 days of age or less. Guideline 2. 2010:1-4.
7. Aronson PL, Wang ME, Nigrovic LE, et al; Febrile Young Infant Research Collaborative. Time to pathogen detection for non-ill versus ill-appearing infants ≤60 days old with bacteremia and meningitis. Hosp Pediatr. 2018;8(7):379-384. https://doi.org/10.1542/hpeds.2018-0002
8. Biondi EA, Mischler M, Jerardi KE, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168(9):844-849. https://doi.org/10.1001/jamapediatrics.2014.895
9. Lefebvre CE, Renaud C, Chartrand C. Time to positivity of blood cultures in infants 0 to 90 days old presenting to the emergency department: is 36 hours enough? J Pediatric Infect Dis Soc. 2017;6(1):28-32. https://doi.org/10.1093/jpids/piv078
10. Unaka N, Statile A, Bensman, R, et al. Cincinnati Children’s Hospital Medical Center. Evidence-based clinical care guideline for evidence-based care guideline for management of infants 0 to 60 days seen in emergency department for fever of unknown source. Guideline 10. 2019;1-42. http://www.cincinnatichildrens.org/service/j/anderson-center/evidence-based-care/recommendations/default/
11. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
12. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458-464. https://doi.org/10.1136/qhc.12.6.458
13. Biondi EA, McCulloh R, Staggs VS, et al; American Academy of Pediatrics’ Revise Collaborative. Reducing variability in the infant sepsis evaluation (REVISE): a national quality initiative. Pediatrics. 2019;144(3): e20182201. https://doi.org/10.1542/peds.2018-2201
14. McCulloh RJ, Commers T, Williams DD, Michael J, Mann K, Newland JG. Effect of combined clinical practice guideline and electronic order set implementation on febrile infant evaluation and management. Pediatr Emerg Care. 2021;37(1):e25-e31. https://doi.org/10.1097/pec.0000000000002012
15. Foster LZ, Beiner J, Duh-Leong C, et al. Implementation of febrile infant management guidelines reduces hospitalization. Pediatr Qual Saf. 2020;5(1):e252. https://doi.org/10.1097/pq9.0000000000000252
16. Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics. 2012;130(1):e16-e24. https://doi.org/10.1542/peds.2012-0127
17. Chang LV, Shah AN, Hoefgen ER, et al; H2O Study Group. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
© 2021 Society of Hospital Medicine
Counterpoint: Prioritizing Healthcare Workers for Scarce Critical Care Resources Is Impractical and Unjust
The impact of the coronavirus disease 2019 (COVID-19) pandemic has been far reaching and devastating. As the pandemic reaches its 1-year mark, there have been more cases and deaths than most of us can comprehend: nearly 28 million cases and 497,000 deaths in the United States1 and more than 111 million cases and 2.4 million deaths globally.2 Frontline healthcare workers (HCWs) have struggled to provide compassionate care in the face of heavy workloads and risks to themselves and their loved ones. Sadly, more than 1,700 US HCWs have died from COVID-19.3 The pandemic has also taken a heavy emotional and psychological toll: HCWs have died by suicide, and others are leaving the profession in which they invested so much and formerly loved. Caring for ill colleagues and dying patients whose family members cannot visit has been particularly difficult. It is, therefore, understandable that some HCWs have called for their prioritization if it becomes necessary to implement crisis standards of care. Although Daffner’s4 reciprocity argument—HCWs should receive priority because of the risks that they have voluntarily accepted—has some appeal, it disregards several important considerations. First, it fails to consider the changing dynamics of viral transmission during the pandemic or alternative ways in which the duty of reciprocity may be fulfilled that do not involve prioritizing HCWs over others. Second, this position is both over- and underinclusive in ways that make it difficult to implement. Third, and most important, the inordinate attention to the prioritization of HCWs ignores the issues the pandemic raises regarding racism and inequity.
LIMITS OF RECIPROCITY AND ALTERNATIVES TO PRIORITIZATION
Although the reciprocity argument has some conceptual merit, there are several different ways that the duty of reciprocity can be fulfilled. One fundamental obligation of government agencies and healthcare systems is providing a safe work environment, including adequate personal protective equipment (PPE) and physical distancing. Before we understood the extent of the pandemic, modes of transmission, and effective preventative measures, hospital transmission was significant. For example, a single-center case series at Zhongan Hospital of Wuhan University, China, from January 1, 2020, to January 28, 2020, found that 29% (40 of 138) of hospitalized patients with COVID-19 were health professionals who were presumed to have been infected by patients.5 There were also significant shortages of PPE, and a number of frontline HCWs reported being dismissed for calling attention to unsafe conditions. Although professionals have an obligation to expose themselves to risk, they are not obligated to expose themselves to inordinate risk. Prioritizing HCWs in ventilator triage may have been justified during the initial surge.
The use of surgical masks by all employees and patients has substantially reduced hospital transmission. A study at Duke Health, Raleigh, North Carolina, of HCWs who tested positive for SARS-CoV-2 between March 15, 2020, and June 6, 2020, found 22% of cases were healthcare acquired, 38% were community acquired, and 40% were of unknown acquisition route. Of the healthcare-acquired cases, 30% were thought to be secondary to direct patient care and 70% to exposure to another worker. The cumulative incidence rate of healthcare-acquired infections among workers decreased significantly 1 week after universal masking was implemented on March 31, 2020. The cumulative incidence rates of community-acquired cases and those with unknown acquisition routes continued to mirror incidence rates in the community.6 There is substantially less justification for prioritizing HCWs during the current phase of the pandemic; reciprocity does not justify granting HCWs infected via community spread greater priority than non-HCWs similarly infected.
There are other means of reciprocating that do not involve prioritization. COVID-19 has exacted an immense toll on the mental well-being of frontline HCWs. They should be provided robust, comprehensive, and accessible mental health services. Additionally, reciprocity can be expressed by providing alternative housing options for HCWs who are concerned about infecting their family members, especially family members at higher risk of morbidity or mortality from COVID-19. Many HCWs have also died from COVID-193; providing life insurance would recognize the sacrifice of HCWs and support their survivors. None of these interventions would require prioritizing HCWs over others.
OVER- AND UNDERINCLUSIVENESS
As Daffner4 acknowledges, the category of “healthcare provider” is both over- and underinclusive. Healthcare providers are exposed to variable risks. Some physicians, for example, are no longer involved in direct patient care. It is unclear how triage teams will identify frontline HCWs or validate claims to being a frontline HCW, especially for individuals not employed by the hospital at which they are seeking care. Hence, triage protocols prioritizing healthcare providers are likely to be substantially overinclusive, which raises significant issues of fairness.
Moreover, the category “healthcare provider” is also underinclusive. Many essential, nonclinical hospital employees expose themselves to risk, including custodial and food service staff. As Daffner4 recognizes, there are also many other occupations outside of healthcare in which individuals voluntarily expose themselves to risks for the benefit of others, including police officers, firefighters, and clerks in grocery stores. We would add that workers in the food-supply system, transportation, and education face similar risks.7 Identifying the types of jobs that should confer priority and validating an individual’s employment also makes implementation difficult and risks injustice.
EQUITY AND JUSTICE
The COVID-19 pandemic and the murder of Black people by police have brought substantial attention to racism and racial inequities in the United States. We must, however, move from merely acknowledging existing inequities to dismantling structures that perpetuate them. The prioritization of HCWs may further privilege those who already have substantial advantages. This is especially true for physicians. For example, although state and federal laws pose limitations, physicians have historically extended one another professional courtesy by providing free or discounted services. Furthermore, HCWs and their family members are more likely to receive VIP treatment. For instance, when taken to the emergency department, children of physicians are less likely to have medical students and residents involved in their care and more likely to see attending physicians and consultants.8
In contrast, other categories of essential workers do not have such advantages. These workers are more likely to be members of marginalized racial and ethnic minority groups, have substantially lower wages, have less access to PPE, and work in more crowded conditions, and are less likely to have paid sick leave compared with HCWs.7 These workers are also more likely to lack access to quality healthcare. In fact, many safety net hospitals that provide care to marginalized communities have faced significant financial hardships as a result of the pandemic, and without additional support, some may close. Prioritizing HCWs will likely widen the gaps in health, economic, and social status among these groups.
With respect to allocation criteria, Black, Latinx, and Native American communities have more severe morbidity and mortality from COVID-19 as a result of racism and its interaction with other social determinants of health. Members of marginalized communities of color have a higher likelihood of becoming infected with COVID-19, a higher prevalence of comorbidities, and less access to treatment.7 Before her untimely death, Dr Susan Moore, a Black family physician, painfully described the racism to which she was subjected while being treated for COVID-19.9 The economic devastation caused by the pandemic, including unemployment, evictions, and food insecurity, compounds the impact of social determinants of health and disproportionately affects minority communities. Purely race- and ethnicity-based approaches to allocation to redress these inequities have potential limitations and obstacles, such as omission of other social determinants of health and legal challenges.7 While currently proposed for allocation of medications or vaccines, alternatives include using the Centers for Disease Control and Prevention’s Social Vulnerability Index8 or the Area Deprivation Index10 as a priority criterion. Most importantly, healthcare systems should more broadly demonstrate themselves trustworthy and assure that marginalized communities of color have access to quality healthcare services.
CONCLUSION
The United States has failed to adequately control the COVID-19 pandemic, and increasing numbers of admissions and staffing shortages have renewed concerns that hospitals will need to implement crisis standards of care. Daffner4 argues that healthcare providers should be prioritized in the allocation of critical care based on reciprocity. In the current phase of the pandemic, HCWs are more likely to be infected by one another or in the community than by patients. There are also other ways that hospitals can discharge this duty that do not require prioritizing HCWs over patients. The category of HCW is both over- and underinclusive, and Daffner4 has not shown that prioritization can be implemented fairly. Finally, inordinate attention has been paid to this topic. Much more attention should be focused on how to redress the ways in which the pandemic has exacerbated existing racial and ethnic inequities.
1. COVID data tracker: United States COVID-19 cases and deaths by state. Centers for Disease Control and Prevention . Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
2. WHO coronavirus disease (COVID-19) dashboard: overview. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
3. Sins of omission: how government failures to track Covid-19 data have led to more than 1,700 health care worker deaths and jeopardize public health. National Nurses United. September 2020. Accessed November 23, 2020. https://act.nationalnursesunited.org/page/-/files/graphics/0920_Covid19_SinsOfOmission_Data_Report.pdf
4. Daffner KR. Point: healthcare providers should receive treatment priority during a pandemic. J Hosp Med. Published online February 17, 2021. https://doi.org/10.12788/jhm.3596
5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585
6. Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
7. Gayle H, Foege W, Brown L, Kahn B, eds. Framework for Equitable Allocation of COVID-19 Vaccine. The National Academies Press; 2020. https://doi.org/10.17226/25917
8. Diekema DS, Cummings P, Quan L. Physicians’ children are treated differently in the emergency department. Am J Emerg Med. 1996;14(1):6-9. https://doi.org/10.1016/S0735-6757(96)90002-9
9. Maybank A, Jones CP, Blackstock U, Perry JC. Say her name: Dr. Susan Moore. The Washington Post. December 26, 2020. Accessed January 6, 2021. https://www.washingtonpost.com/opinions/2020/12/26/say-her-name-dr-susan-moore/
10. White DB, Schmidhofer M, McCreary E, et al. Model hospital policy for fair allocation of scarce medications to treat COVID-19. University of Pittsburgh. May 28, 2020. Accessed November 23, 2020. https://ccm.pitt.edu/sites/default/files/2020-05-28b%20Model%20hospital%20policy%20for%20allocating%20scarce%20COVID%20meds.pdf
The impact of the coronavirus disease 2019 (COVID-19) pandemic has been far reaching and devastating. As the pandemic reaches its 1-year mark, there have been more cases and deaths than most of us can comprehend: nearly 28 million cases and 497,000 deaths in the United States1 and more than 111 million cases and 2.4 million deaths globally.2 Frontline healthcare workers (HCWs) have struggled to provide compassionate care in the face of heavy workloads and risks to themselves and their loved ones. Sadly, more than 1,700 US HCWs have died from COVID-19.3 The pandemic has also taken a heavy emotional and psychological toll: HCWs have died by suicide, and others are leaving the profession in which they invested so much and formerly loved. Caring for ill colleagues and dying patients whose family members cannot visit has been particularly difficult. It is, therefore, understandable that some HCWs have called for their prioritization if it becomes necessary to implement crisis standards of care. Although Daffner’s4 reciprocity argument—HCWs should receive priority because of the risks that they have voluntarily accepted—has some appeal, it disregards several important considerations. First, it fails to consider the changing dynamics of viral transmission during the pandemic or alternative ways in which the duty of reciprocity may be fulfilled that do not involve prioritizing HCWs over others. Second, this position is both over- and underinclusive in ways that make it difficult to implement. Third, and most important, the inordinate attention to the prioritization of HCWs ignores the issues the pandemic raises regarding racism and inequity.
LIMITS OF RECIPROCITY AND ALTERNATIVES TO PRIORITIZATION
Although the reciprocity argument has some conceptual merit, there are several different ways that the duty of reciprocity can be fulfilled. One fundamental obligation of government agencies and healthcare systems is providing a safe work environment, including adequate personal protective equipment (PPE) and physical distancing. Before we understood the extent of the pandemic, modes of transmission, and effective preventative measures, hospital transmission was significant. For example, a single-center case series at Zhongan Hospital of Wuhan University, China, from January 1, 2020, to January 28, 2020, found that 29% (40 of 138) of hospitalized patients with COVID-19 were health professionals who were presumed to have been infected by patients.5 There were also significant shortages of PPE, and a number of frontline HCWs reported being dismissed for calling attention to unsafe conditions. Although professionals have an obligation to expose themselves to risk, they are not obligated to expose themselves to inordinate risk. Prioritizing HCWs in ventilator triage may have been justified during the initial surge.
The use of surgical masks by all employees and patients has substantially reduced hospital transmission. A study at Duke Health, Raleigh, North Carolina, of HCWs who tested positive for SARS-CoV-2 between March 15, 2020, and June 6, 2020, found 22% of cases were healthcare acquired, 38% were community acquired, and 40% were of unknown acquisition route. Of the healthcare-acquired cases, 30% were thought to be secondary to direct patient care and 70% to exposure to another worker. The cumulative incidence rate of healthcare-acquired infections among workers decreased significantly 1 week after universal masking was implemented on March 31, 2020. The cumulative incidence rates of community-acquired cases and those with unknown acquisition routes continued to mirror incidence rates in the community.6 There is substantially less justification for prioritizing HCWs during the current phase of the pandemic; reciprocity does not justify granting HCWs infected via community spread greater priority than non-HCWs similarly infected.
There are other means of reciprocating that do not involve prioritization. COVID-19 has exacted an immense toll on the mental well-being of frontline HCWs. They should be provided robust, comprehensive, and accessible mental health services. Additionally, reciprocity can be expressed by providing alternative housing options for HCWs who are concerned about infecting their family members, especially family members at higher risk of morbidity or mortality from COVID-19. Many HCWs have also died from COVID-193; providing life insurance would recognize the sacrifice of HCWs and support their survivors. None of these interventions would require prioritizing HCWs over others.
OVER- AND UNDERINCLUSIVENESS
As Daffner4 acknowledges, the category of “healthcare provider” is both over- and underinclusive. Healthcare providers are exposed to variable risks. Some physicians, for example, are no longer involved in direct patient care. It is unclear how triage teams will identify frontline HCWs or validate claims to being a frontline HCW, especially for individuals not employed by the hospital at which they are seeking care. Hence, triage protocols prioritizing healthcare providers are likely to be substantially overinclusive, which raises significant issues of fairness.
Moreover, the category “healthcare provider” is also underinclusive. Many essential, nonclinical hospital employees expose themselves to risk, including custodial and food service staff. As Daffner4 recognizes, there are also many other occupations outside of healthcare in which individuals voluntarily expose themselves to risks for the benefit of others, including police officers, firefighters, and clerks in grocery stores. We would add that workers in the food-supply system, transportation, and education face similar risks.7 Identifying the types of jobs that should confer priority and validating an individual’s employment also makes implementation difficult and risks injustice.
EQUITY AND JUSTICE
The COVID-19 pandemic and the murder of Black people by police have brought substantial attention to racism and racial inequities in the United States. We must, however, move from merely acknowledging existing inequities to dismantling structures that perpetuate them. The prioritization of HCWs may further privilege those who already have substantial advantages. This is especially true for physicians. For example, although state and federal laws pose limitations, physicians have historically extended one another professional courtesy by providing free or discounted services. Furthermore, HCWs and their family members are more likely to receive VIP treatment. For instance, when taken to the emergency department, children of physicians are less likely to have medical students and residents involved in their care and more likely to see attending physicians and consultants.8
In contrast, other categories of essential workers do not have such advantages. These workers are more likely to be members of marginalized racial and ethnic minority groups, have substantially lower wages, have less access to PPE, and work in more crowded conditions, and are less likely to have paid sick leave compared with HCWs.7 These workers are also more likely to lack access to quality healthcare. In fact, many safety net hospitals that provide care to marginalized communities have faced significant financial hardships as a result of the pandemic, and without additional support, some may close. Prioritizing HCWs will likely widen the gaps in health, economic, and social status among these groups.
With respect to allocation criteria, Black, Latinx, and Native American communities have more severe morbidity and mortality from COVID-19 as a result of racism and its interaction with other social determinants of health. Members of marginalized communities of color have a higher likelihood of becoming infected with COVID-19, a higher prevalence of comorbidities, and less access to treatment.7 Before her untimely death, Dr Susan Moore, a Black family physician, painfully described the racism to which she was subjected while being treated for COVID-19.9 The economic devastation caused by the pandemic, including unemployment, evictions, and food insecurity, compounds the impact of social determinants of health and disproportionately affects minority communities. Purely race- and ethnicity-based approaches to allocation to redress these inequities have potential limitations and obstacles, such as omission of other social determinants of health and legal challenges.7 While currently proposed for allocation of medications or vaccines, alternatives include using the Centers for Disease Control and Prevention’s Social Vulnerability Index8 or the Area Deprivation Index10 as a priority criterion. Most importantly, healthcare systems should more broadly demonstrate themselves trustworthy and assure that marginalized communities of color have access to quality healthcare services.
CONCLUSION
The United States has failed to adequately control the COVID-19 pandemic, and increasing numbers of admissions and staffing shortages have renewed concerns that hospitals will need to implement crisis standards of care. Daffner4 argues that healthcare providers should be prioritized in the allocation of critical care based on reciprocity. In the current phase of the pandemic, HCWs are more likely to be infected by one another or in the community than by patients. There are also other ways that hospitals can discharge this duty that do not require prioritizing HCWs over patients. The category of HCW is both over- and underinclusive, and Daffner4 has not shown that prioritization can be implemented fairly. Finally, inordinate attention has been paid to this topic. Much more attention should be focused on how to redress the ways in which the pandemic has exacerbated existing racial and ethnic inequities.
The impact of the coronavirus disease 2019 (COVID-19) pandemic has been far reaching and devastating. As the pandemic reaches its 1-year mark, there have been more cases and deaths than most of us can comprehend: nearly 28 million cases and 497,000 deaths in the United States1 and more than 111 million cases and 2.4 million deaths globally.2 Frontline healthcare workers (HCWs) have struggled to provide compassionate care in the face of heavy workloads and risks to themselves and their loved ones. Sadly, more than 1,700 US HCWs have died from COVID-19.3 The pandemic has also taken a heavy emotional and psychological toll: HCWs have died by suicide, and others are leaving the profession in which they invested so much and formerly loved. Caring for ill colleagues and dying patients whose family members cannot visit has been particularly difficult. It is, therefore, understandable that some HCWs have called for their prioritization if it becomes necessary to implement crisis standards of care. Although Daffner’s4 reciprocity argument—HCWs should receive priority because of the risks that they have voluntarily accepted—has some appeal, it disregards several important considerations. First, it fails to consider the changing dynamics of viral transmission during the pandemic or alternative ways in which the duty of reciprocity may be fulfilled that do not involve prioritizing HCWs over others. Second, this position is both over- and underinclusive in ways that make it difficult to implement. Third, and most important, the inordinate attention to the prioritization of HCWs ignores the issues the pandemic raises regarding racism and inequity.
LIMITS OF RECIPROCITY AND ALTERNATIVES TO PRIORITIZATION
Although the reciprocity argument has some conceptual merit, there are several different ways that the duty of reciprocity can be fulfilled. One fundamental obligation of government agencies and healthcare systems is providing a safe work environment, including adequate personal protective equipment (PPE) and physical distancing. Before we understood the extent of the pandemic, modes of transmission, and effective preventative measures, hospital transmission was significant. For example, a single-center case series at Zhongan Hospital of Wuhan University, China, from January 1, 2020, to January 28, 2020, found that 29% (40 of 138) of hospitalized patients with COVID-19 were health professionals who were presumed to have been infected by patients.5 There were also significant shortages of PPE, and a number of frontline HCWs reported being dismissed for calling attention to unsafe conditions. Although professionals have an obligation to expose themselves to risk, they are not obligated to expose themselves to inordinate risk. Prioritizing HCWs in ventilator triage may have been justified during the initial surge.
The use of surgical masks by all employees and patients has substantially reduced hospital transmission. A study at Duke Health, Raleigh, North Carolina, of HCWs who tested positive for SARS-CoV-2 between March 15, 2020, and June 6, 2020, found 22% of cases were healthcare acquired, 38% were community acquired, and 40% were of unknown acquisition route. Of the healthcare-acquired cases, 30% were thought to be secondary to direct patient care and 70% to exposure to another worker. The cumulative incidence rate of healthcare-acquired infections among workers decreased significantly 1 week after universal masking was implemented on March 31, 2020. The cumulative incidence rates of community-acquired cases and those with unknown acquisition routes continued to mirror incidence rates in the community.6 There is substantially less justification for prioritizing HCWs during the current phase of the pandemic; reciprocity does not justify granting HCWs infected via community spread greater priority than non-HCWs similarly infected.
There are other means of reciprocating that do not involve prioritization. COVID-19 has exacted an immense toll on the mental well-being of frontline HCWs. They should be provided robust, comprehensive, and accessible mental health services. Additionally, reciprocity can be expressed by providing alternative housing options for HCWs who are concerned about infecting their family members, especially family members at higher risk of morbidity or mortality from COVID-19. Many HCWs have also died from COVID-193; providing life insurance would recognize the sacrifice of HCWs and support their survivors. None of these interventions would require prioritizing HCWs over others.
OVER- AND UNDERINCLUSIVENESS
As Daffner4 acknowledges, the category of “healthcare provider” is both over- and underinclusive. Healthcare providers are exposed to variable risks. Some physicians, for example, are no longer involved in direct patient care. It is unclear how triage teams will identify frontline HCWs or validate claims to being a frontline HCW, especially for individuals not employed by the hospital at which they are seeking care. Hence, triage protocols prioritizing healthcare providers are likely to be substantially overinclusive, which raises significant issues of fairness.
Moreover, the category “healthcare provider” is also underinclusive. Many essential, nonclinical hospital employees expose themselves to risk, including custodial and food service staff. As Daffner4 recognizes, there are also many other occupations outside of healthcare in which individuals voluntarily expose themselves to risks for the benefit of others, including police officers, firefighters, and clerks in grocery stores. We would add that workers in the food-supply system, transportation, and education face similar risks.7 Identifying the types of jobs that should confer priority and validating an individual’s employment also makes implementation difficult and risks injustice.
EQUITY AND JUSTICE
The COVID-19 pandemic and the murder of Black people by police have brought substantial attention to racism and racial inequities in the United States. We must, however, move from merely acknowledging existing inequities to dismantling structures that perpetuate them. The prioritization of HCWs may further privilege those who already have substantial advantages. This is especially true for physicians. For example, although state and federal laws pose limitations, physicians have historically extended one another professional courtesy by providing free or discounted services. Furthermore, HCWs and their family members are more likely to receive VIP treatment. For instance, when taken to the emergency department, children of physicians are less likely to have medical students and residents involved in their care and more likely to see attending physicians and consultants.8
In contrast, other categories of essential workers do not have such advantages. These workers are more likely to be members of marginalized racial and ethnic minority groups, have substantially lower wages, have less access to PPE, and work in more crowded conditions, and are less likely to have paid sick leave compared with HCWs.7 These workers are also more likely to lack access to quality healthcare. In fact, many safety net hospitals that provide care to marginalized communities have faced significant financial hardships as a result of the pandemic, and without additional support, some may close. Prioritizing HCWs will likely widen the gaps in health, economic, and social status among these groups.
With respect to allocation criteria, Black, Latinx, and Native American communities have more severe morbidity and mortality from COVID-19 as a result of racism and its interaction with other social determinants of health. Members of marginalized communities of color have a higher likelihood of becoming infected with COVID-19, a higher prevalence of comorbidities, and less access to treatment.7 Before her untimely death, Dr Susan Moore, a Black family physician, painfully described the racism to which she was subjected while being treated for COVID-19.9 The economic devastation caused by the pandemic, including unemployment, evictions, and food insecurity, compounds the impact of social determinants of health and disproportionately affects minority communities. Purely race- and ethnicity-based approaches to allocation to redress these inequities have potential limitations and obstacles, such as omission of other social determinants of health and legal challenges.7 While currently proposed for allocation of medications or vaccines, alternatives include using the Centers for Disease Control and Prevention’s Social Vulnerability Index8 or the Area Deprivation Index10 as a priority criterion. Most importantly, healthcare systems should more broadly demonstrate themselves trustworthy and assure that marginalized communities of color have access to quality healthcare services.
CONCLUSION
The United States has failed to adequately control the COVID-19 pandemic, and increasing numbers of admissions and staffing shortages have renewed concerns that hospitals will need to implement crisis standards of care. Daffner4 argues that healthcare providers should be prioritized in the allocation of critical care based on reciprocity. In the current phase of the pandemic, HCWs are more likely to be infected by one another or in the community than by patients. There are also other ways that hospitals can discharge this duty that do not require prioritizing HCWs over patients. The category of HCW is both over- and underinclusive, and Daffner4 has not shown that prioritization can be implemented fairly. Finally, inordinate attention has been paid to this topic. Much more attention should be focused on how to redress the ways in which the pandemic has exacerbated existing racial and ethnic inequities.
1. COVID data tracker: United States COVID-19 cases and deaths by state. Centers for Disease Control and Prevention . Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
2. WHO coronavirus disease (COVID-19) dashboard: overview. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
3. Sins of omission: how government failures to track Covid-19 data have led to more than 1,700 health care worker deaths and jeopardize public health. National Nurses United. September 2020. Accessed November 23, 2020. https://act.nationalnursesunited.org/page/-/files/graphics/0920_Covid19_SinsOfOmission_Data_Report.pdf
4. Daffner KR. Point: healthcare providers should receive treatment priority during a pandemic. J Hosp Med. Published online February 17, 2021. https://doi.org/10.12788/jhm.3596
5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585
6. Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
7. Gayle H, Foege W, Brown L, Kahn B, eds. Framework for Equitable Allocation of COVID-19 Vaccine. The National Academies Press; 2020. https://doi.org/10.17226/25917
8. Diekema DS, Cummings P, Quan L. Physicians’ children are treated differently in the emergency department. Am J Emerg Med. 1996;14(1):6-9. https://doi.org/10.1016/S0735-6757(96)90002-9
9. Maybank A, Jones CP, Blackstock U, Perry JC. Say her name: Dr. Susan Moore. The Washington Post. December 26, 2020. Accessed January 6, 2021. https://www.washingtonpost.com/opinions/2020/12/26/say-her-name-dr-susan-moore/
10. White DB, Schmidhofer M, McCreary E, et al. Model hospital policy for fair allocation of scarce medications to treat COVID-19. University of Pittsburgh. May 28, 2020. Accessed November 23, 2020. https://ccm.pitt.edu/sites/default/files/2020-05-28b%20Model%20hospital%20policy%20for%20allocating%20scarce%20COVID%20meds.pdf
1. COVID data tracker: United States COVID-19 cases and deaths by state. Centers for Disease Control and Prevention . Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
2. WHO coronavirus disease (COVID-19) dashboard: overview. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
3. Sins of omission: how government failures to track Covid-19 data have led to more than 1,700 health care worker deaths and jeopardize public health. National Nurses United. September 2020. Accessed November 23, 2020. https://act.nationalnursesunited.org/page/-/files/graphics/0920_Covid19_SinsOfOmission_Data_Report.pdf
4. Daffner KR. Point: healthcare providers should receive treatment priority during a pandemic. J Hosp Med. Published online February 17, 2021. https://doi.org/10.12788/jhm.3596
5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585
6. Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
7. Gayle H, Foege W, Brown L, Kahn B, eds. Framework for Equitable Allocation of COVID-19 Vaccine. The National Academies Press; 2020. https://doi.org/10.17226/25917
8. Diekema DS, Cummings P, Quan L. Physicians’ children are treated differently in the emergency department. Am J Emerg Med. 1996;14(1):6-9. https://doi.org/10.1016/S0735-6757(96)90002-9
9. Maybank A, Jones CP, Blackstock U, Perry JC. Say her name: Dr. Susan Moore. The Washington Post. December 26, 2020. Accessed January 6, 2021. https://www.washingtonpost.com/opinions/2020/12/26/say-her-name-dr-susan-moore/
10. White DB, Schmidhofer M, McCreary E, et al. Model hospital policy for fair allocation of scarce medications to treat COVID-19. University of Pittsburgh. May 28, 2020. Accessed November 23, 2020. https://ccm.pitt.edu/sites/default/files/2020-05-28b%20Model%20hospital%20policy%20for%20allocating%20scarce%20COVID%20meds.pdf
© 2021 Society of Hospital Medicine
Truth in Tension: Reflections on Racism in Medicine
Core values should reflect our fundamental beliefs and serve as the building blocks of our behaviors and actions. Health systems across the United States define themselves by a myriad of guiding principles, which include patient-centeredness, dignity, respect, safety, and teamwork. On the surface, medicine’s ties to such altruistic values make intuitive sense. However, as Black physicians, we are in a state of cognitive dissonance as we wrestle with healthcare’s real identity and the principles it espouses. We know that within this psychological tension lies the truth: the US healthcare system was not designed to live up to these ideals. This truth is most evident in health inequities that exist among Black people and other marginalized communities of color. It is also the undeniable reality of Black physicians whose professional role is juxtaposed with recurring experiences that signal to us that we do not belong.
SYSTEMIC RACISM, MISTRUST, AND HEALTH INEQUITIES
Racism in healthcare, laid bare by the well-documented exploitation of Black people by the medical community, adds to the not-so-subtle ways we are told our lives don’t matter.1 This mistreatment has resulted in a deep mistrust of healthcare providers that is legitimate and real. The 40-year Tuskegee Syphilis Study is infamous for breaking trust via the deception of hundreds of Black men. The study participants with syphilis were denied treatment despite a known and available cure; an act both unconscionable and inhumane. As recently as the 1990s, a study sought to identify a genetic origin for aggressive behavior; however, enrollment was restricted to Black and Latino boys, and families were incentivized with money. Furthermore, the children were taken off all medications, kept overnight without their parents, deprived of water, subjected to hourly blood draws, and given fenfluramine, a drug known to be associated with precipitating aggressive behavior.1 The study design perpetuated the stereotype of Black males as perpetrators of violence—a distorted and biased perception that continues to cost Black people their lives. This sobering example illustrates that even in the era of institutional review boards, the welfare and protection of Black people who participate in research is by no means guaranteed.
The very notion of social determinants of health exposes the underbelly of institutional racism and its pervasiveness in our healthcare system. As Black physicians, we see the flawed healthcare system’s disproportionate and devastating effects on patients who look like us: we have first-hand accounts as patients ourselves, and we have traversed the experiences endured by our loved ones. Broken trust and fractured care contribute to disparate rates of morbidity and mortality in Black men and women with cardiovascular disease, stroke, and diabetes.2 Black mothers have the highest rates of premature births and are three times more likely than White women to die from pregnancy-related complications.3 Black infants are two times more likely to die before their first birthday than are White infants.4 Children and adolescents from poor, predominantly Black and Latinx neighborhoods spend significantly more days in the hospital for various acute and chronic diagnoses than their counterparts from affluent, predominantly White neighborhoods.5 Not surprisingly, the COVID-19 pandemic’s effects on the Black community read like lines memorized from the same old, tired, script6: staggering mortality rates, extreme poverty, food insecurity, alarming education inequities, and a widening digital divide. And, as Black pediatricians, we hold our breath as we wait until the coast is clear to fully assess the overwhelming damage to our children caused by the pandemic’s tsunami.
ACADEMIC MEDICINE AND OUR INVISIBLE WOUNDS
In our roles as doctors, we experience first-hand the ills of academic medicine, an environment that poses significant challenges for those of us who are underrepresented in medicine (UIM). Despite an acute awareness of the need for Black physicians, little has changed over the past few decades. As of 2018, the percentages of Black or African American students who applied and were accepted to US medical schools were 8.4% and 7%, respectively.7 Diversity gains in the acceptance and matriculation rates of medical students were noted across multiple demographic groups over the past 40 years; however, Black applicants were the exception. In fact, the number of Black men enrolled in medical schools is currently less than it was in 1978, a dismal statistic that underscores this issue.8 Only 5% of US physicians identify as Black or African American.7 Furthermore, in academia, while 64% of faculty are White, only 3.6% are Black or African American.7 But there is more to it than just the numbers. Diversity means nothing without an inclusive environment. As Black physicians, we understand the power of visibility, and our strong desire to cultivate a safe and inclusive environment for students, trainees, and other faculty is a large part of why we remain in academia. Nevertheless, the experience in academic medicine for Black physicians and other UIMs is commonly one of isolation.
Lack of inclusivity and feelings of isolation are common themes among Black physicians in academia.9 They are intensified by microaggressions,10 shards of glass that slowly cut at our self-concept, confidence, and resolve. We nurse the wounds from the ones hurled at our Black patients as well as the ones directed our way. They are the microassaults from the mother who requests that a different physician care for her child; the father who proudly displays a swastika tattoo as you examine his newborn infant in the nursery; or the directive to empty out the garbage when you walk into a patient’s room. They are the microinsults from colleagues that convey our inferiority and associate our advancement with handouts because of our race; questions like, “How did you get that role?” and backhanded compliments such as, “You are so articulate,” as we exceed their mediocre expectations. They are the microinvalidations, for example being constantly confused with the few other Black physicians in the hospital, which sends the message that we are invisible. Likewise, our minority tax9—an underappreciated list of service-oriented expectations and responsibilities related to our UIM status—is paid in full via the call to put our “otherness” on display for the sake of diversity and when we speak out against racism and bias because no one else will. There are limited opportunities to establish strong relationships with Black physician mentors, who are more likely to understand the needs and identify with the differential experiences of Black physician mentees. Examples of authentic and effective cross-race mentorship relationships built on trust and psychological safety are scarce, and their rarity exacerbates feelings of isolation and disillusionment among Black physicians. And rare sponsorship—in the form of high visibility recognition or career advancing opportunities—is conflated with veiled tokenism. This atmosphere breeds hypervigilance for Black physicians in academia. The weight of our actions and performance being judged not on an individual level, but rather as a reflection of our entire race, is a heavy load to bear.
A CRITICAL JUNCTURE
Our country is at a crossroads, with resounding calls to dismantle systemic racism in all its forms. The call is greatest for those of us who fight to heal our patients yet work in a healthcare system that perpetuates inequity. Radical steps are needed to rebuild the system and include:
- Working relentlessly towards health equity in all phases and facets of patient care. This must involve mandating data transparency, defining clear measures, and implementing processes that make equitable practices the default.
- Moving beyond one-dimensional diversity initiatives that focus on recruitment, and investing in strategies that promote the inclusion, retention, and advancement of UIM faculty along leadership and academic ranks.
- Establishing specific experiences, opportunities, and support structures for UIMs that include Black students, trainees, and faculty to combat isolation and foster inclusivity.
- Developing and implementing comprehensive trainee and faculty education focused on implicit bias in general, and structural racism, medical mistrust, and racial bias in healthcare in particular.
- Cultivating an antiracist environment in which true and authentic allyship is widespread and macro- and microaggressions are not silently endured by UIMs but are immediately and effectively addressed by all.
We must reconcile the dissonance that currently exists in our healthcare system between lofty ideals of racial equity and opportunity with actual practice—and as a result, honor the dignity and worth of the people who experience and work in it.
1. Washington HA. Medical Apartheid: The Dark History Of Medical Experimentation On Black Americans From Colonial Times to the Present. Doubleday Books; 2006.
2. Calvin R, Winters K, Wyatt SB, Williams DR, Henderson FC, Walker ER. Racism and cardiovascular disease in African Americans. Am J Med Sci. 2003;325(6):315-331. https://doi.org/10.1097/00000441-200306000-00003
3. Petersen EE, Davis NL, Goodman D, et al. Vital signs: pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68(18):423. https://doi.org/10.15585/mmwr.mm6818e1
4. Centers for Disease Control and Prevention. Reproductive Health. Maternal and Infant Health. Infant Mortality Rates by Race and Ethnicity, 2016. Accessed June 6, 2020. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortality.htm
5. Beck AF, Anderson KL, Rich K, et al. Cooling the hot spots where child hospitalization rates are high: a neighborhood approach to population health. Health Aff. 2019;38(9):1433-1441. https://doi.org/10.1377/hlthaff.2018.05496
6. Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891-1892. https://doi.org/10.1001/jama.2020.6548
7. Diversity in Medicine: Facts and Figures 2019. Association of American Medical Colleges. Accessed June 6, 2020. https://www.aamc.org/data-reports/workforce/report/diversity-medicine-facts-and-figures-2019
8. Altering the Course: Black Males in Medicine. Association of American Medical Colleges; 2015.
9. Campbell KM, Rodríguez JE. Addressing the minority tax: perspectives from two diversity leaders on building minority faculty success in academic medicine. Acad Med. 2019;94(12):1854-1857. https://doi.org/10.1097/ACM.0000000000002839
10. Freeman L, Stewart H. Microaggressions in clinical medicine. Kennedy Inst Ethics J. 2018;28(4):411-449. https://doi.org/10.1353/ken.2018.0024
Core values should reflect our fundamental beliefs and serve as the building blocks of our behaviors and actions. Health systems across the United States define themselves by a myriad of guiding principles, which include patient-centeredness, dignity, respect, safety, and teamwork. On the surface, medicine’s ties to such altruistic values make intuitive sense. However, as Black physicians, we are in a state of cognitive dissonance as we wrestle with healthcare’s real identity and the principles it espouses. We know that within this psychological tension lies the truth: the US healthcare system was not designed to live up to these ideals. This truth is most evident in health inequities that exist among Black people and other marginalized communities of color. It is also the undeniable reality of Black physicians whose professional role is juxtaposed with recurring experiences that signal to us that we do not belong.
SYSTEMIC RACISM, MISTRUST, AND HEALTH INEQUITIES
Racism in healthcare, laid bare by the well-documented exploitation of Black people by the medical community, adds to the not-so-subtle ways we are told our lives don’t matter.1 This mistreatment has resulted in a deep mistrust of healthcare providers that is legitimate and real. The 40-year Tuskegee Syphilis Study is infamous for breaking trust via the deception of hundreds of Black men. The study participants with syphilis were denied treatment despite a known and available cure; an act both unconscionable and inhumane. As recently as the 1990s, a study sought to identify a genetic origin for aggressive behavior; however, enrollment was restricted to Black and Latino boys, and families were incentivized with money. Furthermore, the children were taken off all medications, kept overnight without their parents, deprived of water, subjected to hourly blood draws, and given fenfluramine, a drug known to be associated with precipitating aggressive behavior.1 The study design perpetuated the stereotype of Black males as perpetrators of violence—a distorted and biased perception that continues to cost Black people their lives. This sobering example illustrates that even in the era of institutional review boards, the welfare and protection of Black people who participate in research is by no means guaranteed.
The very notion of social determinants of health exposes the underbelly of institutional racism and its pervasiveness in our healthcare system. As Black physicians, we see the flawed healthcare system’s disproportionate and devastating effects on patients who look like us: we have first-hand accounts as patients ourselves, and we have traversed the experiences endured by our loved ones. Broken trust and fractured care contribute to disparate rates of morbidity and mortality in Black men and women with cardiovascular disease, stroke, and diabetes.2 Black mothers have the highest rates of premature births and are three times more likely than White women to die from pregnancy-related complications.3 Black infants are two times more likely to die before their first birthday than are White infants.4 Children and adolescents from poor, predominantly Black and Latinx neighborhoods spend significantly more days in the hospital for various acute and chronic diagnoses than their counterparts from affluent, predominantly White neighborhoods.5 Not surprisingly, the COVID-19 pandemic’s effects on the Black community read like lines memorized from the same old, tired, script6: staggering mortality rates, extreme poverty, food insecurity, alarming education inequities, and a widening digital divide. And, as Black pediatricians, we hold our breath as we wait until the coast is clear to fully assess the overwhelming damage to our children caused by the pandemic’s tsunami.
ACADEMIC MEDICINE AND OUR INVISIBLE WOUNDS
In our roles as doctors, we experience first-hand the ills of academic medicine, an environment that poses significant challenges for those of us who are underrepresented in medicine (UIM). Despite an acute awareness of the need for Black physicians, little has changed over the past few decades. As of 2018, the percentages of Black or African American students who applied and were accepted to US medical schools were 8.4% and 7%, respectively.7 Diversity gains in the acceptance and matriculation rates of medical students were noted across multiple demographic groups over the past 40 years; however, Black applicants were the exception. In fact, the number of Black men enrolled in medical schools is currently less than it was in 1978, a dismal statistic that underscores this issue.8 Only 5% of US physicians identify as Black or African American.7 Furthermore, in academia, while 64% of faculty are White, only 3.6% are Black or African American.7 But there is more to it than just the numbers. Diversity means nothing without an inclusive environment. As Black physicians, we understand the power of visibility, and our strong desire to cultivate a safe and inclusive environment for students, trainees, and other faculty is a large part of why we remain in academia. Nevertheless, the experience in academic medicine for Black physicians and other UIMs is commonly one of isolation.
Lack of inclusivity and feelings of isolation are common themes among Black physicians in academia.9 They are intensified by microaggressions,10 shards of glass that slowly cut at our self-concept, confidence, and resolve. We nurse the wounds from the ones hurled at our Black patients as well as the ones directed our way. They are the microassaults from the mother who requests that a different physician care for her child; the father who proudly displays a swastika tattoo as you examine his newborn infant in the nursery; or the directive to empty out the garbage when you walk into a patient’s room. They are the microinsults from colleagues that convey our inferiority and associate our advancement with handouts because of our race; questions like, “How did you get that role?” and backhanded compliments such as, “You are so articulate,” as we exceed their mediocre expectations. They are the microinvalidations, for example being constantly confused with the few other Black physicians in the hospital, which sends the message that we are invisible. Likewise, our minority tax9—an underappreciated list of service-oriented expectations and responsibilities related to our UIM status—is paid in full via the call to put our “otherness” on display for the sake of diversity and when we speak out against racism and bias because no one else will. There are limited opportunities to establish strong relationships with Black physician mentors, who are more likely to understand the needs and identify with the differential experiences of Black physician mentees. Examples of authentic and effective cross-race mentorship relationships built on trust and psychological safety are scarce, and their rarity exacerbates feelings of isolation and disillusionment among Black physicians. And rare sponsorship—in the form of high visibility recognition or career advancing opportunities—is conflated with veiled tokenism. This atmosphere breeds hypervigilance for Black physicians in academia. The weight of our actions and performance being judged not on an individual level, but rather as a reflection of our entire race, is a heavy load to bear.
A CRITICAL JUNCTURE
Our country is at a crossroads, with resounding calls to dismantle systemic racism in all its forms. The call is greatest for those of us who fight to heal our patients yet work in a healthcare system that perpetuates inequity. Radical steps are needed to rebuild the system and include:
- Working relentlessly towards health equity in all phases and facets of patient care. This must involve mandating data transparency, defining clear measures, and implementing processes that make equitable practices the default.
- Moving beyond one-dimensional diversity initiatives that focus on recruitment, and investing in strategies that promote the inclusion, retention, and advancement of UIM faculty along leadership and academic ranks.
- Establishing specific experiences, opportunities, and support structures for UIMs that include Black students, trainees, and faculty to combat isolation and foster inclusivity.
- Developing and implementing comprehensive trainee and faculty education focused on implicit bias in general, and structural racism, medical mistrust, and racial bias in healthcare in particular.
- Cultivating an antiracist environment in which true and authentic allyship is widespread and macro- and microaggressions are not silently endured by UIMs but are immediately and effectively addressed by all.
We must reconcile the dissonance that currently exists in our healthcare system between lofty ideals of racial equity and opportunity with actual practice—and as a result, honor the dignity and worth of the people who experience and work in it.
Core values should reflect our fundamental beliefs and serve as the building blocks of our behaviors and actions. Health systems across the United States define themselves by a myriad of guiding principles, which include patient-centeredness, dignity, respect, safety, and teamwork. On the surface, medicine’s ties to such altruistic values make intuitive sense. However, as Black physicians, we are in a state of cognitive dissonance as we wrestle with healthcare’s real identity and the principles it espouses. We know that within this psychological tension lies the truth: the US healthcare system was not designed to live up to these ideals. This truth is most evident in health inequities that exist among Black people and other marginalized communities of color. It is also the undeniable reality of Black physicians whose professional role is juxtaposed with recurring experiences that signal to us that we do not belong.
SYSTEMIC RACISM, MISTRUST, AND HEALTH INEQUITIES
Racism in healthcare, laid bare by the well-documented exploitation of Black people by the medical community, adds to the not-so-subtle ways we are told our lives don’t matter.1 This mistreatment has resulted in a deep mistrust of healthcare providers that is legitimate and real. The 40-year Tuskegee Syphilis Study is infamous for breaking trust via the deception of hundreds of Black men. The study participants with syphilis were denied treatment despite a known and available cure; an act both unconscionable and inhumane. As recently as the 1990s, a study sought to identify a genetic origin for aggressive behavior; however, enrollment was restricted to Black and Latino boys, and families were incentivized with money. Furthermore, the children were taken off all medications, kept overnight without their parents, deprived of water, subjected to hourly blood draws, and given fenfluramine, a drug known to be associated with precipitating aggressive behavior.1 The study design perpetuated the stereotype of Black males as perpetrators of violence—a distorted and biased perception that continues to cost Black people their lives. This sobering example illustrates that even in the era of institutional review boards, the welfare and protection of Black people who participate in research is by no means guaranteed.
The very notion of social determinants of health exposes the underbelly of institutional racism and its pervasiveness in our healthcare system. As Black physicians, we see the flawed healthcare system’s disproportionate and devastating effects on patients who look like us: we have first-hand accounts as patients ourselves, and we have traversed the experiences endured by our loved ones. Broken trust and fractured care contribute to disparate rates of morbidity and mortality in Black men and women with cardiovascular disease, stroke, and diabetes.2 Black mothers have the highest rates of premature births and are three times more likely than White women to die from pregnancy-related complications.3 Black infants are two times more likely to die before their first birthday than are White infants.4 Children and adolescents from poor, predominantly Black and Latinx neighborhoods spend significantly more days in the hospital for various acute and chronic diagnoses than their counterparts from affluent, predominantly White neighborhoods.5 Not surprisingly, the COVID-19 pandemic’s effects on the Black community read like lines memorized from the same old, tired, script6: staggering mortality rates, extreme poverty, food insecurity, alarming education inequities, and a widening digital divide. And, as Black pediatricians, we hold our breath as we wait until the coast is clear to fully assess the overwhelming damage to our children caused by the pandemic’s tsunami.
ACADEMIC MEDICINE AND OUR INVISIBLE WOUNDS
In our roles as doctors, we experience first-hand the ills of academic medicine, an environment that poses significant challenges for those of us who are underrepresented in medicine (UIM). Despite an acute awareness of the need for Black physicians, little has changed over the past few decades. As of 2018, the percentages of Black or African American students who applied and were accepted to US medical schools were 8.4% and 7%, respectively.7 Diversity gains in the acceptance and matriculation rates of medical students were noted across multiple demographic groups over the past 40 years; however, Black applicants were the exception. In fact, the number of Black men enrolled in medical schools is currently less than it was in 1978, a dismal statistic that underscores this issue.8 Only 5% of US physicians identify as Black or African American.7 Furthermore, in academia, while 64% of faculty are White, only 3.6% are Black or African American.7 But there is more to it than just the numbers. Diversity means nothing without an inclusive environment. As Black physicians, we understand the power of visibility, and our strong desire to cultivate a safe and inclusive environment for students, trainees, and other faculty is a large part of why we remain in academia. Nevertheless, the experience in academic medicine for Black physicians and other UIMs is commonly one of isolation.
Lack of inclusivity and feelings of isolation are common themes among Black physicians in academia.9 They are intensified by microaggressions,10 shards of glass that slowly cut at our self-concept, confidence, and resolve. We nurse the wounds from the ones hurled at our Black patients as well as the ones directed our way. They are the microassaults from the mother who requests that a different physician care for her child; the father who proudly displays a swastika tattoo as you examine his newborn infant in the nursery; or the directive to empty out the garbage when you walk into a patient’s room. They are the microinsults from colleagues that convey our inferiority and associate our advancement with handouts because of our race; questions like, “How did you get that role?” and backhanded compliments such as, “You are so articulate,” as we exceed their mediocre expectations. They are the microinvalidations, for example being constantly confused with the few other Black physicians in the hospital, which sends the message that we are invisible. Likewise, our minority tax9—an underappreciated list of service-oriented expectations and responsibilities related to our UIM status—is paid in full via the call to put our “otherness” on display for the sake of diversity and when we speak out against racism and bias because no one else will. There are limited opportunities to establish strong relationships with Black physician mentors, who are more likely to understand the needs and identify with the differential experiences of Black physician mentees. Examples of authentic and effective cross-race mentorship relationships built on trust and psychological safety are scarce, and their rarity exacerbates feelings of isolation and disillusionment among Black physicians. And rare sponsorship—in the form of high visibility recognition or career advancing opportunities—is conflated with veiled tokenism. This atmosphere breeds hypervigilance for Black physicians in academia. The weight of our actions and performance being judged not on an individual level, but rather as a reflection of our entire race, is a heavy load to bear.
A CRITICAL JUNCTURE
Our country is at a crossroads, with resounding calls to dismantle systemic racism in all its forms. The call is greatest for those of us who fight to heal our patients yet work in a healthcare system that perpetuates inequity. Radical steps are needed to rebuild the system and include:
- Working relentlessly towards health equity in all phases and facets of patient care. This must involve mandating data transparency, defining clear measures, and implementing processes that make equitable practices the default.
- Moving beyond one-dimensional diversity initiatives that focus on recruitment, and investing in strategies that promote the inclusion, retention, and advancement of UIM faculty along leadership and academic ranks.
- Establishing specific experiences, opportunities, and support structures for UIMs that include Black students, trainees, and faculty to combat isolation and foster inclusivity.
- Developing and implementing comprehensive trainee and faculty education focused on implicit bias in general, and structural racism, medical mistrust, and racial bias in healthcare in particular.
- Cultivating an antiracist environment in which true and authentic allyship is widespread and macro- and microaggressions are not silently endured by UIMs but are immediately and effectively addressed by all.
We must reconcile the dissonance that currently exists in our healthcare system between lofty ideals of racial equity and opportunity with actual practice—and as a result, honor the dignity and worth of the people who experience and work in it.
1. Washington HA. Medical Apartheid: The Dark History Of Medical Experimentation On Black Americans From Colonial Times to the Present. Doubleday Books; 2006.
2. Calvin R, Winters K, Wyatt SB, Williams DR, Henderson FC, Walker ER. Racism and cardiovascular disease in African Americans. Am J Med Sci. 2003;325(6):315-331. https://doi.org/10.1097/00000441-200306000-00003
3. Petersen EE, Davis NL, Goodman D, et al. Vital signs: pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68(18):423. https://doi.org/10.15585/mmwr.mm6818e1
4. Centers for Disease Control and Prevention. Reproductive Health. Maternal and Infant Health. Infant Mortality Rates by Race and Ethnicity, 2016. Accessed June 6, 2020. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortality.htm
5. Beck AF, Anderson KL, Rich K, et al. Cooling the hot spots where child hospitalization rates are high: a neighborhood approach to population health. Health Aff. 2019;38(9):1433-1441. https://doi.org/10.1377/hlthaff.2018.05496
6. Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891-1892. https://doi.org/10.1001/jama.2020.6548
7. Diversity in Medicine: Facts and Figures 2019. Association of American Medical Colleges. Accessed June 6, 2020. https://www.aamc.org/data-reports/workforce/report/diversity-medicine-facts-and-figures-2019
8. Altering the Course: Black Males in Medicine. Association of American Medical Colleges; 2015.
9. Campbell KM, Rodríguez JE. Addressing the minority tax: perspectives from two diversity leaders on building minority faculty success in academic medicine. Acad Med. 2019;94(12):1854-1857. https://doi.org/10.1097/ACM.0000000000002839
10. Freeman L, Stewart H. Microaggressions in clinical medicine. Kennedy Inst Ethics J. 2018;28(4):411-449. https://doi.org/10.1353/ken.2018.0024
1. Washington HA. Medical Apartheid: The Dark History Of Medical Experimentation On Black Americans From Colonial Times to the Present. Doubleday Books; 2006.
2. Calvin R, Winters K, Wyatt SB, Williams DR, Henderson FC, Walker ER. Racism and cardiovascular disease in African Americans. Am J Med Sci. 2003;325(6):315-331. https://doi.org/10.1097/00000441-200306000-00003
3. Petersen EE, Davis NL, Goodman D, et al. Vital signs: pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68(18):423. https://doi.org/10.15585/mmwr.mm6818e1
4. Centers for Disease Control and Prevention. Reproductive Health. Maternal and Infant Health. Infant Mortality Rates by Race and Ethnicity, 2016. Accessed June 6, 2020. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortality.htm
5. Beck AF, Anderson KL, Rich K, et al. Cooling the hot spots where child hospitalization rates are high: a neighborhood approach to population health. Health Aff. 2019;38(9):1433-1441. https://doi.org/10.1377/hlthaff.2018.05496
6. Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891-1892. https://doi.org/10.1001/jama.2020.6548
7. Diversity in Medicine: Facts and Figures 2019. Association of American Medical Colleges. Accessed June 6, 2020. https://www.aamc.org/data-reports/workforce/report/diversity-medicine-facts-and-figures-2019
8. Altering the Course: Black Males in Medicine. Association of American Medical Colleges; 2015.
9. Campbell KM, Rodríguez JE. Addressing the minority tax: perspectives from two diversity leaders on building minority faculty success in academic medicine. Acad Med. 2019;94(12):1854-1857. https://doi.org/10.1097/ACM.0000000000002839
10. Freeman L, Stewart H. Microaggressions in clinical medicine. Kennedy Inst Ethics J. 2018;28(4):411-449. https://doi.org/10.1353/ken.2018.0024
© 2020 Society of Hospital Medicine
Improving the Transition of Intravenous to Enteral Antibiotics in Pediatric Patients with Pneumonia or Skin and Soft Tissue Infections
Intravenous (IV) antibiotics are commonly used in hospitalized pediatric patients to treat bacterial infections. Antimicrobial stewardship guidelines published by the Infectious Diseases Society of America (IDSA) recommend institutions develop a systematic plan to convert from IV to enteral antibiotics, as early transition may reduce healthcare costs, decrease length of stay (LOS), and avoid prolonged IV access complications1 such as extravasation, thrombosis, and catheter-associated infections.2-5
Pediatric patients with community-acquired pneumonia (CAP) and mild skin and soft tissue infections (SSTI) may not require IV antibiotics, even if the patient is hospitalized.6 Although national guidelines for pediatric CAP and SSTI recommend IV antibiotics for hospitalized patients, these guidelines state that mild infections may be treated with enteral antibiotics and emphasize discontinuation of IV antibiotics when the patient meets discharge criteria.7,8 Furthermore, several enteral antibiotics used for the treatment of CAP and SSTI, such as cephalexin and clindamycin,9 have excellent bioavailability (>90%) or can achieve sufficient concentrations to attain the pharmacodynamic target (ie, amoxicillin and trimethoprim–sulfamethoxazole).10,11 Nonetheless, the guidelines do not explicitly outline criteria regarding the transition from IV to enteral antibiotics.7,8
At our institution, patients admitted to Hospital Medicine (HM) often remained on IV antibiotics until discharge. Data review revealed that antibiotic treatment of CAP and SSTI posed the greatest opportunity for early conversion to enteral therapy based on the high frequency of admissions and the ability of commonly used enteral antibiotics to attain pharmacodynamic targets. We sought to change practice culture by decoupling transition to enteral antibiotics from discharge and use administration of other enteral medications as an objective indicator for transition. Our aim was to increase the proportion of enterally administered antibiotic doses for HM patients aged >60 days admitted with uncomplicated CAP or SSTI from 44% to 75% in eight months.
METHODS
Context
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large, urban, academic hospital. The HM division has 45 attendings and admits >8,000 general pediatric patients annually. The five HM teams at the main campus consist of attendings, fellows, residents, and medical students. One HM team serves as the resident quality improvement (QI) team where residents collaborate in a longitudinal study under the guidance of QI-trained coaches. The focus of this QI initiative was determined by resident consensus and aligned with a high-value care curriculum.12
To identify the target patient population, we investigated IV antimicrobials frequently used in HM patients. Ampicillin and clindamycin are commonly used IV antibiotics, most frequently corresponding with the diagnoses of CAP and SSTI, respectively, accounting for half of all antibiotic use on the HM service. Amoxicillin, the enteral equivalent of ampicillin, can achieve sufficient concentrations to attain the pharmacodynamic target at infection sites, and clindamycin has high bioavailability, making them ideal options for early transition. Our institution’s robust antimicrobial stewardship program has published local guidelines on using amoxicillin as the enteral antibiotic of choice for uncomplicated CAP, but it does not provide guidance on the timing of transition for either CAP or SSTI; the clinical team makes this decision.
HM attendings were surveyed to determine the criteria used to transition from IV to enteral antibiotics for patients with CAP or SSTI. The survey illustrated practice variability with providers using differing clinical criteria to signal the timing of transition. Additionally, only 49% of respondents (n = 37) rated themselves as “very comfortable” with residents making autonomous decisions to transition to enteral antibiotics. We chose to use the administration of other enteral medications, instead of discharge readiness, as an objective indicator of a patient’s readiness to transition to enteral antibiotics, given the low-risk patient population and the ability of the enteral antibiotics commonly used for CAP and SSTI to achieve pharmacodynamic targets.
The study population included patients aged >60 days admitted to HM with CAP or SSTI treated with any antibiotic. We excluded patients with potential complications or significant progression of their disease process, including patients with parapneumonic effusions or chest tubes, patients who underwent bronchoscopy, and patients with osteomyelitis, septic arthritis, or preseptal or orbital cellulitis. Past medical history and clinical status on admission were not used to exclude patients.
Interventions
Our multidisciplinary team, formed in January 2017, included HM attendings, HM fellows, pediatric residents, a critical care attending, a pharmacy resident, and an antimicrobial stewardship pharmacist. Under the guidance of QI coaches, the residents on the HM QI team developed and tested all interventions on their team and then determined which interventions would spread to the other four teams. The nursing director of our primary HM unit disseminated project updates to bedside nurses. A simplified failure mode and effects analysis identified areas for improvement and potential interventions. Interventions focused on the following key drivers (Figure 1): increased prescriber awareness of medication charge, standardization of conversion from IV to enteral antibiotics, clear definition of the patients ready for transition, ongoing evaluation of the antimicrobial plan, timely recognition by prescribers of patients ready for transition, culture shift regarding the appropriate administration route in the inpatient setting, and transparency of data. The team implemented sequential Plan-Do-Study-Act (PDSA) cycles13 to test the interventions.
Charge Table
To improve knowledge about the increased charge for commonly used IV medications compared with enteral formulations, a table comparing relative charges was shared during monthly resident morning conferences and at an HM faculty meeting. The table included charge comparisons between ampicillin and amoxicillin and IV and enteral clindamycin.
Standardized Language in Electronic Health Record (EHR) Antibiotic Plan on Rounds
Standardized language to document antibiotic transition plans was added to admission and progress note templates in the EHR. The standard template prompted residents to (1) define clinical transition criteria, (2) discuss attending comfort with transition overnight (based on survey results), and (3) document patient preference of solid or liquid dosage forms. Plans were reviewed and updated daily. We hypothesized that since residents use the information in the daily progress notes, including assessments and plans, to present on rounds, inclusion of the transition criteria in the note would prompt transition plan discussions.
Communication Bundle
To promote early transition to enteral antibiotics, we standardized the discussion about antibiotic transition between residents and attendings. During a weekly preexisting meeting, the resident QI team reviewed preferences for transitions with the new service attending. By identifying attending preferences early, residents were able to proactively transition patients who met the criteria (eg, antibiotic transition in the evening instead of waiting until morning rounds). This discussion also provided an opportunity to engage service attendings in the QI efforts, which were also shared at HM faculty meetings quarterly.
Recognizing that in times of high census, discussion of patient plans may be abbreviated during rounds, residents were asked to identify all patients on IV antibiotics while reviewing patient medication orders prior to rounds. As part of an existing daily prerounds huddle to discuss rounding logistics, residents listed all patients on IV antibiotics and discussed which patients were ready for transition. If patients could not be transitioned immediately, the team identified the transition criteria.
At preexisting evening huddles between overnight shift HM residents and the evening HM attending, residents identified patients who were prescribed IV antibiotics and discussed readiness for enteral transition. If a patient could be transitioned overnight, enteral antibiotic orders were placed. Overnight residents were also encouraged to review the transition criteria with families upon admission.
Real-time Identification of Failures and Feedback
For two weeks, the EHR was queried daily to identify patients admitted for uncomplicated CAP and SSTI who were on antibiotics as well as other enteral medications. A failure was defined as an IV antibiotic dose given to a patient who was administered any enteral medication. Residents on the QI team approached residents on other HM teams whenever patients were identified as a failed transition to learn about failure reasons.
Study of the Interventions
Data for HM patients who met the inclusion criteria were collected weekly from January 2016 through June 2018 via EHR query. We initially searched for diagnoses that fit under the disease categories of pneumonia and SSTI in the EHR, which generated a list of International Classification of Disease-9 and -10 Diagnosis codes (Appendix Figure 1). The query identified patients based on these codes and reported whether the identified patients took a dose of any enteral medication, excluding nystatin, sildenafil, tacrolimus, and mouthwashes, which are commonly continued during NPO status due to no need for absorption or limited parenteral options. It also reported the ordered route of administration for the queried antibiotics (Appendix Figure 1).
The 2016 calendar year established our baseline to account for seasonal variability. Data were reported weekly and reviewed to evaluate the impact of PDSA cycles and inform new interventions.
Measures
Our process measure was the total number of enteral antibiotic doses divided by all antibiotic doses in patients receiving any enteral medication. We reasoned that if patients were well enough to take medications enterally, they could be given an enteral antibiotic that is highly bioavailable or readily achieves concentrations that attain pharmacodynamic targets. This practice change was a culture shift, decoupling the switch to enteral antibiotics from discharge readiness. Our EHR query reported only the antibiotic doses given to patients who took an enteral medication on the day of antibiotic administration and excluded patients who received only IV medications.
Outcome measures included antimicrobial costs per patient encounter using average wholesale prices, which were reported in our EHR query, and LOS. To ensure that transitions of IV to enteral antibiotics were not negatively impacting patient outcomes, patient readmissions within seven days served as a balancing measure.
Analysis
An annotated statistical process control p-chart tracked the impact of interventions on the proportion of antibiotic doses that were enterally administered during hospitalization. An x-bar and an s-chart tracked the impact of interventions on antimicrobial costs per patient encounter and on LOS. A p-chart and an encounters-between g-chart were used to evaluate the impact of our interventions on readmissions. Control chart rules for identifying special cause were used for center line shifts.14
Ethical Considerations
This study was part of a larger study of the residency high-value care curriculum,12 which was deemed exempt by the CCHMC IRB.
RESULTS
The baseline data collected included 372 patients and the postintervention period in 2017 included 326 patients (Table). Approximately two-thirds of patients had a diagnosis of CAP.
The percentage of antibiotic doses given enterally increased from 44% to 80% within eight months (Figure 2). When studying the impact of interventions, residents on the HM QI team found that the standard EHR template added to daily notes did not consistently prompt residents to discuss antibiotic plans and thus was abandoned. Initial improvement coincided with standardizing discussions between residents and attendings regarding transitions. Furthermore, discussion of all patients on IV antibiotics during the prerounds huddle allowed for reliable, daily communication about antibiotic plans and was subsequently spread to and adopted by all HM teams. The percentage of enterally administered antibiotic doses increased to >75% after the evening huddle, which involved all HM teams, and real-time identification of failures on all HM teams with provider feedback. Despite variability when the total number of antibiotic doses prescribed per week was low (<10), we demonstrated sustainability for 11 months (Figure 2), during which the prerounds and evening huddle discussions were continued and an updated control chart was shown monthly to residents during their educational conferences.
Residents on the QI team spoke directly with other HM residents when there were missed opportunities for transition. Based on these discussions and intermittent chart reviews, common reasons for failure to transition in patients with CAP included admission for failed outpatient enteral treatment, recent evaluation by critical care physicians for possible transfer to the intensive care unit, and difficulty weaning oxygen. For patients with SSTI, hand abscesses requiring drainage by surgery and treatment failure with other antibiotics constituted many of the IV antibiotic doses given to patients on enteral medications.
Antimicrobial costs per patient encounter decreased by 70% over one year; the shift in costs coincided with the second shift in our process measure (Appendix Figure 2A). Based on an estimate of 350 patients admitted per year for uncomplicated CAP or SSTI, this translates to an annual cost savings of approximately $29,000. The standard deviation of costs per patient encounter decreased by 84% (Appendix Figure 2B), suggesting a decrease in the variability of prescribing practices.
The average LOS in our patient population prior to intervention was 2.1 days and did not change (Appendix Figure 2C), but the standard deviation decreased by >50% (Appendix Figure 2D). There was no shift in the mean seven-day readmission rate or the number of encounters between readmissions (2.6% and 26, respectively; Appendix Figure 3). In addition, the hospital billing department did not identify an increase in insurance denials related to the route of antibiotic administration.
DISCUSSION
Summary
Using improvement science, we promoted earlier transition to enteral antibiotics for children hospitalized with uncomplicated CAP and SSTI by linking the decision for transition to the ability to take other enteral medications, rather than to discharge readiness. We increased the percentage of enterally administered antibiotic doses in this patient population from 44% to 80% in eight months. Although we did not observe a decrease in LOS as previously noted in a cost analysis study comparing pediatric patients with CAP treated with oral antibiotics versus those treated with IV antibiotics,15 we did find a decrease in LOS variability and in antimicrobial costs to our patients. These cost savings did not include potential savings from nursing or pharmacy labor. In addition, we noted a decrease in the variability in antibiotic prescribing practice, which demonstrates provider ability and willingness to couple antibiotic route transition to an objective characteristic (administration of other enteral medications).
A strength of our study was that residents, the most frequent prescribers of antibiotics on our HM service, were highly involved in the QI initiative, including defining the SMART aim, identifying key drivers, developing interventions, and completing sequential PDSA cycles. Under the guidance of QI-trained coaches, residents developed feasible interventions and assessed their success in real time. Consistent with other studies,16,17 resident buy-in and involvement led to the success of our improvement study.
Interpretation
Despite emerging evidence regarding the timing of transition to enteral antibiotics, several factors impeded early transition at our institution, including physician culture, variable practice habits, and hospital workflow. Evidence supports the use of enteral antibiotics in immunocompetent children hospitalized for uncomplicated CAP who do not have chronic lung disease, are not in shock, and have oxygen saturations >85%.6 Although existing literature suggests that in pediatric patients admitted for SSTIs not involving the eye or bone, IV antibiotics may be transitioned when clinical improvement, evidenced by a reduction in fever or erythema, is noted,6 enteral antibiotics that achieve appropriate concentrations to attain pharmacodynamic targets should have the same efficacy as that of IV antibiotics.9 Using the criterion of administration of any medication enterally to identify a patient’s readiness to transition, we were able to overcome practice variation among providers who may have differing opinions of what constitutes clinical improvement. Of note, new evidence is emerging on predictors of enteral antibiotic treatment failure in patients with CAP and SSTI to guide transition timing, but these studies have largely focused on the adult population or were performed in the outpatient and emergency department (ED) settings.18,19 Regardless, the stable number of encounters between readmissions in our patient population likely indicates that treatment failure in these patients was rare.
Rising healthcare costs have led to concerns around sustainability of the healthcare system;20,21 tackling overuse in clinical practice, as in our study, is one mitigation strategy. Several studies have used QI methods to facilitate the provision of high-value care through the decrease of continuous monitor overuse and extraneous ordering of electrolytes.22,23 Our QI study adds to the high-value care literature by safely decreasing the use of IV antibiotics. One retrospective study demonstrated that a one-day decrease in the use of IV antibiotics in pneumonia resulted in decreased costs without an increase in readmissions, similar to our findings.24 In adults, QI initiatives aimed at improving early transition of antibiotics utilized electronic trigger tools.25,26 Fischer et al. used active orders for scheduled enteral medications or an enteral diet as indication that a patient’s IV medications could be converted to enteral form.26
Our work is not without limitations. The list of ICD-9 and -10 codes used to query the EHR did not capture all diagnoses that would be considered as uncomplicated CAP or SSTI. However, we included an extensive list of diagnoses to ensure that the majority of patients meeting our inclusion criteria were captured. Our process measure did not account for patients on IV antibiotics who were not administered other enteral medications but tolerating an enteral diet. These patients were not identified in our EHR query and were not included in our process measure as a failure. However, in latter interventions, residents identified all patients on IV antibiotics, so that patients not identified by our EHR query benefited from our work. Furthermore, this QI study was conducted at a single institution and several interventions took advantage of preexisting structured huddles and a resident QI curriculum, which may not exist at other institutions. Our study does highlight that engaging frontline providers, such as residents, to review antibiotic orders consistently and question the appropriateness of the administration route is key to making incremental changes in prescribing practices.
CONCLUSIONS
Through a partnership between HM and Pharmacy and with substantial resident involvement, we improved the transition of IV antibiotics in patients with CAP or SSTI by increasing the percentage of enterally administered antibiotic doses and reducing antimicrobial costs and variability in antibiotic prescribing practices. This work illustrates how reducing overuse of IV antibiotics promotes high-value care and aligns with initiatives to prevent avoidable harm.27 Our work highlights that standardized discussions about medication orders to create consensus around enteral antibiotic transitions, real-time feedback, and challenging the status quo can influence practice habits and effect change.
Next steps include testing automated methods to notify providers of opportunities for transition from IV to enteral antibiotics through embedded clinical decision support, a method similar to the electronic trigger tools used in adult QI studies.25,26 Since our prerounds huddle includes identifying all patients on IV antibiotics, studying the transition to enteral antibiotics and its effect on prescribing practices in other diagnoses (ie, urinary tract infection and osteomyelitis) may contribute to spreading these efforts. Partnering with our ED colleagues may be an important next step, as several patients admitted to HM on IV antibiotics are given their first dose in the ED.
Acknowledgments
The authors would like to thank the faculty of the James M. Anderson Center for Health Systems Excellence Intermediate Improvement Science Series for their guidance in the planning of this project. The authors would also like to thank Ms. Ursula Bradshaw and Mr. Michael Ponti-Zins for obtaining the hospital data on length of stay and readmissions. The authors acknowledge Dr. Philip Hagedorn for his assistance with the software that queries the electronic health record and Dr. Laura Brower and Dr. Joanna Thomson for their assistance with statistical analysis. The authors are grateful to all the residents and coaches on the QI Hospital Medicine team who contributed ideas on study design and interventions.
1. Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393.
2. Shah SS, Srivastava R, Wu S, et al. Intravenous Versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6). https://doi.org/10.1542/peds.2016-1692.
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822.
4. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435.https://doi.org/10.1001/jamapediatrics.2013.775.
5. Zaoutis T, Localio AR, Leckerman K, et al. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596.
6. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X.
7. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
8. Stevens DL, Bisno AL, Chambers HF, et al. Executive summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):147-159. https://doi.org/10.1093/cid/ciu444.
9. MacGregor RR, Graziani AL. Oral administration of antibiotics: a rational alternative to the parenteral route. Clin Infect Dis. 1997;24(3):457-467. https://doi.org/10.1093/clinids/24.3.457.
10. Downes KJ, Hahn A, Wiles J, Courter JD, Vinks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in paediatrics. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006.
11. Autmizguine J, Melloni C, Hornik CP, et al. Population pharmacokinetics of trimethoprim-sulfamethoxazole in infants and children. Antimicrob Agents Chemother. 2018;62(1):e01813-e01817. https://doi.org/10.1128/AAC.01813-17.
12. Dewan M, Herrmann LE, Tchou MJ, et al. Development and evaluation of high-value pediatrics: a high-value care pediatric resident curriculum. Hosp Pediatr. 2018;8(12):785-792. https://doi.org/10.1542/hpeds.2018-0115
13. Langley GJ, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. New Jersey, US: John Wiley & Sons; 2009.
14. Benneyan JC. Use and interpretation of statistical quality control charts. Int J Qual Health Care. 1998;10(1):69-73. https://doi.org/10.1093/intqhc/10.1.69.
15. Lorgelly PK, Atkinson M, Lakhanpaul M, et al. Oral versus i.v. antibiotics for community-acquired pneumonia in children: a cost-minimisation analysis. Eur Respir J. 2010;35(4):858-864. https://doi.org/10.1183/09031936.00087209.
16. Vidyarthi AR, Green AL, Rosenbluth G, Baron RB. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89(3):460-468. https://doi.org/10.1097/ACM.0000000000000159.
17. Stinnett-Donnelly JM, Stevens PG, Hood VL. Developing a high value care programme from the bottom up: a programme of faculty-resident improvement projects targeting harmful or unnecessary care. BMJ Qual Saf. 2016;25(11):901-908. https://doi.org/10.1136/bmjqs-2015-004546.
18. Peterson D, McLeod S, Woolfrey K, McRae A. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21(5):526-531. https://doi.org/10.1111/acem.12371.
19. Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for non-purulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2018;20(S1):S24-S25. https://doi.org/10.1017/cem.2018.114.
20. Organisation for Economic Co-operation and Development. Healthcare costs unsustainable in advanced economies without reform. http://www.oecd.org/health/healthcarecostsunsustainableinadvancedeconomieswithoutreform.htm. Accessed June 28, 2018; 2015.
21. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516. https://doi.org/10.1001/jama.2012.362.
22. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135(4):e1044-e1051. https://doi.org/10.1542/peds.2014-2295.
23. Tchou MJ, Tang Girdwood S, Wormser B, et al. Reducing electrolyte testing in hospitalized children by using quality improvement methods. Pediatrics. 2018;141(5). https://doi.org/10.1542/peds.2017-3187.
24. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatr Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003.
25. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3.
26. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585.
27. Schroeder AR, Harris SJ, Newman TB. Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128(6):e1596-e1597. https://doi.org/10.1542/peds.2011-2726.
Intravenous (IV) antibiotics are commonly used in hospitalized pediatric patients to treat bacterial infections. Antimicrobial stewardship guidelines published by the Infectious Diseases Society of America (IDSA) recommend institutions develop a systematic plan to convert from IV to enteral antibiotics, as early transition may reduce healthcare costs, decrease length of stay (LOS), and avoid prolonged IV access complications1 such as extravasation, thrombosis, and catheter-associated infections.2-5
Pediatric patients with community-acquired pneumonia (CAP) and mild skin and soft tissue infections (SSTI) may not require IV antibiotics, even if the patient is hospitalized.6 Although national guidelines for pediatric CAP and SSTI recommend IV antibiotics for hospitalized patients, these guidelines state that mild infections may be treated with enteral antibiotics and emphasize discontinuation of IV antibiotics when the patient meets discharge criteria.7,8 Furthermore, several enteral antibiotics used for the treatment of CAP and SSTI, such as cephalexin and clindamycin,9 have excellent bioavailability (>90%) or can achieve sufficient concentrations to attain the pharmacodynamic target (ie, amoxicillin and trimethoprim–sulfamethoxazole).10,11 Nonetheless, the guidelines do not explicitly outline criteria regarding the transition from IV to enteral antibiotics.7,8
At our institution, patients admitted to Hospital Medicine (HM) often remained on IV antibiotics until discharge. Data review revealed that antibiotic treatment of CAP and SSTI posed the greatest opportunity for early conversion to enteral therapy based on the high frequency of admissions and the ability of commonly used enteral antibiotics to attain pharmacodynamic targets. We sought to change practice culture by decoupling transition to enteral antibiotics from discharge and use administration of other enteral medications as an objective indicator for transition. Our aim was to increase the proportion of enterally administered antibiotic doses for HM patients aged >60 days admitted with uncomplicated CAP or SSTI from 44% to 75% in eight months.
METHODS
Context
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large, urban, academic hospital. The HM division has 45 attendings and admits >8,000 general pediatric patients annually. The five HM teams at the main campus consist of attendings, fellows, residents, and medical students. One HM team serves as the resident quality improvement (QI) team where residents collaborate in a longitudinal study under the guidance of QI-trained coaches. The focus of this QI initiative was determined by resident consensus and aligned with a high-value care curriculum.12
To identify the target patient population, we investigated IV antimicrobials frequently used in HM patients. Ampicillin and clindamycin are commonly used IV antibiotics, most frequently corresponding with the diagnoses of CAP and SSTI, respectively, accounting for half of all antibiotic use on the HM service. Amoxicillin, the enteral equivalent of ampicillin, can achieve sufficient concentrations to attain the pharmacodynamic target at infection sites, and clindamycin has high bioavailability, making them ideal options for early transition. Our institution’s robust antimicrobial stewardship program has published local guidelines on using amoxicillin as the enteral antibiotic of choice for uncomplicated CAP, but it does not provide guidance on the timing of transition for either CAP or SSTI; the clinical team makes this decision.
HM attendings were surveyed to determine the criteria used to transition from IV to enteral antibiotics for patients with CAP or SSTI. The survey illustrated practice variability with providers using differing clinical criteria to signal the timing of transition. Additionally, only 49% of respondents (n = 37) rated themselves as “very comfortable” with residents making autonomous decisions to transition to enteral antibiotics. We chose to use the administration of other enteral medications, instead of discharge readiness, as an objective indicator of a patient’s readiness to transition to enteral antibiotics, given the low-risk patient population and the ability of the enteral antibiotics commonly used for CAP and SSTI to achieve pharmacodynamic targets.
The study population included patients aged >60 days admitted to HM with CAP or SSTI treated with any antibiotic. We excluded patients with potential complications or significant progression of their disease process, including patients with parapneumonic effusions or chest tubes, patients who underwent bronchoscopy, and patients with osteomyelitis, septic arthritis, or preseptal or orbital cellulitis. Past medical history and clinical status on admission were not used to exclude patients.
Interventions
Our multidisciplinary team, formed in January 2017, included HM attendings, HM fellows, pediatric residents, a critical care attending, a pharmacy resident, and an antimicrobial stewardship pharmacist. Under the guidance of QI coaches, the residents on the HM QI team developed and tested all interventions on their team and then determined which interventions would spread to the other four teams. The nursing director of our primary HM unit disseminated project updates to bedside nurses. A simplified failure mode and effects analysis identified areas for improvement and potential interventions. Interventions focused on the following key drivers (Figure 1): increased prescriber awareness of medication charge, standardization of conversion from IV to enteral antibiotics, clear definition of the patients ready for transition, ongoing evaluation of the antimicrobial plan, timely recognition by prescribers of patients ready for transition, culture shift regarding the appropriate administration route in the inpatient setting, and transparency of data. The team implemented sequential Plan-Do-Study-Act (PDSA) cycles13 to test the interventions.
Charge Table
To improve knowledge about the increased charge for commonly used IV medications compared with enteral formulations, a table comparing relative charges was shared during monthly resident morning conferences and at an HM faculty meeting. The table included charge comparisons between ampicillin and amoxicillin and IV and enteral clindamycin.
Standardized Language in Electronic Health Record (EHR) Antibiotic Plan on Rounds
Standardized language to document antibiotic transition plans was added to admission and progress note templates in the EHR. The standard template prompted residents to (1) define clinical transition criteria, (2) discuss attending comfort with transition overnight (based on survey results), and (3) document patient preference of solid or liquid dosage forms. Plans were reviewed and updated daily. We hypothesized that since residents use the information in the daily progress notes, including assessments and plans, to present on rounds, inclusion of the transition criteria in the note would prompt transition plan discussions.
Communication Bundle
To promote early transition to enteral antibiotics, we standardized the discussion about antibiotic transition between residents and attendings. During a weekly preexisting meeting, the resident QI team reviewed preferences for transitions with the new service attending. By identifying attending preferences early, residents were able to proactively transition patients who met the criteria (eg, antibiotic transition in the evening instead of waiting until morning rounds). This discussion also provided an opportunity to engage service attendings in the QI efforts, which were also shared at HM faculty meetings quarterly.
Recognizing that in times of high census, discussion of patient plans may be abbreviated during rounds, residents were asked to identify all patients on IV antibiotics while reviewing patient medication orders prior to rounds. As part of an existing daily prerounds huddle to discuss rounding logistics, residents listed all patients on IV antibiotics and discussed which patients were ready for transition. If patients could not be transitioned immediately, the team identified the transition criteria.
At preexisting evening huddles between overnight shift HM residents and the evening HM attending, residents identified patients who were prescribed IV antibiotics and discussed readiness for enteral transition. If a patient could be transitioned overnight, enteral antibiotic orders were placed. Overnight residents were also encouraged to review the transition criteria with families upon admission.
Real-time Identification of Failures and Feedback
For two weeks, the EHR was queried daily to identify patients admitted for uncomplicated CAP and SSTI who were on antibiotics as well as other enteral medications. A failure was defined as an IV antibiotic dose given to a patient who was administered any enteral medication. Residents on the QI team approached residents on other HM teams whenever patients were identified as a failed transition to learn about failure reasons.
Study of the Interventions
Data for HM patients who met the inclusion criteria were collected weekly from January 2016 through June 2018 via EHR query. We initially searched for diagnoses that fit under the disease categories of pneumonia and SSTI in the EHR, which generated a list of International Classification of Disease-9 and -10 Diagnosis codes (Appendix Figure 1). The query identified patients based on these codes and reported whether the identified patients took a dose of any enteral medication, excluding nystatin, sildenafil, tacrolimus, and mouthwashes, which are commonly continued during NPO status due to no need for absorption or limited parenteral options. It also reported the ordered route of administration for the queried antibiotics (Appendix Figure 1).
The 2016 calendar year established our baseline to account for seasonal variability. Data were reported weekly and reviewed to evaluate the impact of PDSA cycles and inform new interventions.
Measures
Our process measure was the total number of enteral antibiotic doses divided by all antibiotic doses in patients receiving any enteral medication. We reasoned that if patients were well enough to take medications enterally, they could be given an enteral antibiotic that is highly bioavailable or readily achieves concentrations that attain pharmacodynamic targets. This practice change was a culture shift, decoupling the switch to enteral antibiotics from discharge readiness. Our EHR query reported only the antibiotic doses given to patients who took an enteral medication on the day of antibiotic administration and excluded patients who received only IV medications.
Outcome measures included antimicrobial costs per patient encounter using average wholesale prices, which were reported in our EHR query, and LOS. To ensure that transitions of IV to enteral antibiotics were not negatively impacting patient outcomes, patient readmissions within seven days served as a balancing measure.
Analysis
An annotated statistical process control p-chart tracked the impact of interventions on the proportion of antibiotic doses that were enterally administered during hospitalization. An x-bar and an s-chart tracked the impact of interventions on antimicrobial costs per patient encounter and on LOS. A p-chart and an encounters-between g-chart were used to evaluate the impact of our interventions on readmissions. Control chart rules for identifying special cause were used for center line shifts.14
Ethical Considerations
This study was part of a larger study of the residency high-value care curriculum,12 which was deemed exempt by the CCHMC IRB.
RESULTS
The baseline data collected included 372 patients and the postintervention period in 2017 included 326 patients (Table). Approximately two-thirds of patients had a diagnosis of CAP.
The percentage of antibiotic doses given enterally increased from 44% to 80% within eight months (Figure 2). When studying the impact of interventions, residents on the HM QI team found that the standard EHR template added to daily notes did not consistently prompt residents to discuss antibiotic plans and thus was abandoned. Initial improvement coincided with standardizing discussions between residents and attendings regarding transitions. Furthermore, discussion of all patients on IV antibiotics during the prerounds huddle allowed for reliable, daily communication about antibiotic plans and was subsequently spread to and adopted by all HM teams. The percentage of enterally administered antibiotic doses increased to >75% after the evening huddle, which involved all HM teams, and real-time identification of failures on all HM teams with provider feedback. Despite variability when the total number of antibiotic doses prescribed per week was low (<10), we demonstrated sustainability for 11 months (Figure 2), during which the prerounds and evening huddle discussions were continued and an updated control chart was shown monthly to residents during their educational conferences.
Residents on the QI team spoke directly with other HM residents when there were missed opportunities for transition. Based on these discussions and intermittent chart reviews, common reasons for failure to transition in patients with CAP included admission for failed outpatient enteral treatment, recent evaluation by critical care physicians for possible transfer to the intensive care unit, and difficulty weaning oxygen. For patients with SSTI, hand abscesses requiring drainage by surgery and treatment failure with other antibiotics constituted many of the IV antibiotic doses given to patients on enteral medications.
Antimicrobial costs per patient encounter decreased by 70% over one year; the shift in costs coincided with the second shift in our process measure (Appendix Figure 2A). Based on an estimate of 350 patients admitted per year for uncomplicated CAP or SSTI, this translates to an annual cost savings of approximately $29,000. The standard deviation of costs per patient encounter decreased by 84% (Appendix Figure 2B), suggesting a decrease in the variability of prescribing practices.
The average LOS in our patient population prior to intervention was 2.1 days and did not change (Appendix Figure 2C), but the standard deviation decreased by >50% (Appendix Figure 2D). There was no shift in the mean seven-day readmission rate or the number of encounters between readmissions (2.6% and 26, respectively; Appendix Figure 3). In addition, the hospital billing department did not identify an increase in insurance denials related to the route of antibiotic administration.
DISCUSSION
Summary
Using improvement science, we promoted earlier transition to enteral antibiotics for children hospitalized with uncomplicated CAP and SSTI by linking the decision for transition to the ability to take other enteral medications, rather than to discharge readiness. We increased the percentage of enterally administered antibiotic doses in this patient population from 44% to 80% in eight months. Although we did not observe a decrease in LOS as previously noted in a cost analysis study comparing pediatric patients with CAP treated with oral antibiotics versus those treated with IV antibiotics,15 we did find a decrease in LOS variability and in antimicrobial costs to our patients. These cost savings did not include potential savings from nursing or pharmacy labor. In addition, we noted a decrease in the variability in antibiotic prescribing practice, which demonstrates provider ability and willingness to couple antibiotic route transition to an objective characteristic (administration of other enteral medications).
A strength of our study was that residents, the most frequent prescribers of antibiotics on our HM service, were highly involved in the QI initiative, including defining the SMART aim, identifying key drivers, developing interventions, and completing sequential PDSA cycles. Under the guidance of QI-trained coaches, residents developed feasible interventions and assessed their success in real time. Consistent with other studies,16,17 resident buy-in and involvement led to the success of our improvement study.
Interpretation
Despite emerging evidence regarding the timing of transition to enteral antibiotics, several factors impeded early transition at our institution, including physician culture, variable practice habits, and hospital workflow. Evidence supports the use of enteral antibiotics in immunocompetent children hospitalized for uncomplicated CAP who do not have chronic lung disease, are not in shock, and have oxygen saturations >85%.6 Although existing literature suggests that in pediatric patients admitted for SSTIs not involving the eye or bone, IV antibiotics may be transitioned when clinical improvement, evidenced by a reduction in fever or erythema, is noted,6 enteral antibiotics that achieve appropriate concentrations to attain pharmacodynamic targets should have the same efficacy as that of IV antibiotics.9 Using the criterion of administration of any medication enterally to identify a patient’s readiness to transition, we were able to overcome practice variation among providers who may have differing opinions of what constitutes clinical improvement. Of note, new evidence is emerging on predictors of enteral antibiotic treatment failure in patients with CAP and SSTI to guide transition timing, but these studies have largely focused on the adult population or were performed in the outpatient and emergency department (ED) settings.18,19 Regardless, the stable number of encounters between readmissions in our patient population likely indicates that treatment failure in these patients was rare.
Rising healthcare costs have led to concerns around sustainability of the healthcare system;20,21 tackling overuse in clinical practice, as in our study, is one mitigation strategy. Several studies have used QI methods to facilitate the provision of high-value care through the decrease of continuous monitor overuse and extraneous ordering of electrolytes.22,23 Our QI study adds to the high-value care literature by safely decreasing the use of IV antibiotics. One retrospective study demonstrated that a one-day decrease in the use of IV antibiotics in pneumonia resulted in decreased costs without an increase in readmissions, similar to our findings.24 In adults, QI initiatives aimed at improving early transition of antibiotics utilized electronic trigger tools.25,26 Fischer et al. used active orders for scheduled enteral medications or an enteral diet as indication that a patient’s IV medications could be converted to enteral form.26
Our work is not without limitations. The list of ICD-9 and -10 codes used to query the EHR did not capture all diagnoses that would be considered as uncomplicated CAP or SSTI. However, we included an extensive list of diagnoses to ensure that the majority of patients meeting our inclusion criteria were captured. Our process measure did not account for patients on IV antibiotics who were not administered other enteral medications but tolerating an enteral diet. These patients were not identified in our EHR query and were not included in our process measure as a failure. However, in latter interventions, residents identified all patients on IV antibiotics, so that patients not identified by our EHR query benefited from our work. Furthermore, this QI study was conducted at a single institution and several interventions took advantage of preexisting structured huddles and a resident QI curriculum, which may not exist at other institutions. Our study does highlight that engaging frontline providers, such as residents, to review antibiotic orders consistently and question the appropriateness of the administration route is key to making incremental changes in prescribing practices.
CONCLUSIONS
Through a partnership between HM and Pharmacy and with substantial resident involvement, we improved the transition of IV antibiotics in patients with CAP or SSTI by increasing the percentage of enterally administered antibiotic doses and reducing antimicrobial costs and variability in antibiotic prescribing practices. This work illustrates how reducing overuse of IV antibiotics promotes high-value care and aligns with initiatives to prevent avoidable harm.27 Our work highlights that standardized discussions about medication orders to create consensus around enteral antibiotic transitions, real-time feedback, and challenging the status quo can influence practice habits and effect change.
Next steps include testing automated methods to notify providers of opportunities for transition from IV to enteral antibiotics through embedded clinical decision support, a method similar to the electronic trigger tools used in adult QI studies.25,26 Since our prerounds huddle includes identifying all patients on IV antibiotics, studying the transition to enteral antibiotics and its effect on prescribing practices in other diagnoses (ie, urinary tract infection and osteomyelitis) may contribute to spreading these efforts. Partnering with our ED colleagues may be an important next step, as several patients admitted to HM on IV antibiotics are given their first dose in the ED.
Acknowledgments
The authors would like to thank the faculty of the James M. Anderson Center for Health Systems Excellence Intermediate Improvement Science Series for their guidance in the planning of this project. The authors would also like to thank Ms. Ursula Bradshaw and Mr. Michael Ponti-Zins for obtaining the hospital data on length of stay and readmissions. The authors acknowledge Dr. Philip Hagedorn for his assistance with the software that queries the electronic health record and Dr. Laura Brower and Dr. Joanna Thomson for their assistance with statistical analysis. The authors are grateful to all the residents and coaches on the QI Hospital Medicine team who contributed ideas on study design and interventions.
Intravenous (IV) antibiotics are commonly used in hospitalized pediatric patients to treat bacterial infections. Antimicrobial stewardship guidelines published by the Infectious Diseases Society of America (IDSA) recommend institutions develop a systematic plan to convert from IV to enteral antibiotics, as early transition may reduce healthcare costs, decrease length of stay (LOS), and avoid prolonged IV access complications1 such as extravasation, thrombosis, and catheter-associated infections.2-5
Pediatric patients with community-acquired pneumonia (CAP) and mild skin and soft tissue infections (SSTI) may not require IV antibiotics, even if the patient is hospitalized.6 Although national guidelines for pediatric CAP and SSTI recommend IV antibiotics for hospitalized patients, these guidelines state that mild infections may be treated with enteral antibiotics and emphasize discontinuation of IV antibiotics when the patient meets discharge criteria.7,8 Furthermore, several enteral antibiotics used for the treatment of CAP and SSTI, such as cephalexin and clindamycin,9 have excellent bioavailability (>90%) or can achieve sufficient concentrations to attain the pharmacodynamic target (ie, amoxicillin and trimethoprim–sulfamethoxazole).10,11 Nonetheless, the guidelines do not explicitly outline criteria regarding the transition from IV to enteral antibiotics.7,8
At our institution, patients admitted to Hospital Medicine (HM) often remained on IV antibiotics until discharge. Data review revealed that antibiotic treatment of CAP and SSTI posed the greatest opportunity for early conversion to enteral therapy based on the high frequency of admissions and the ability of commonly used enteral antibiotics to attain pharmacodynamic targets. We sought to change practice culture by decoupling transition to enteral antibiotics from discharge and use administration of other enteral medications as an objective indicator for transition. Our aim was to increase the proportion of enterally administered antibiotic doses for HM patients aged >60 days admitted with uncomplicated CAP or SSTI from 44% to 75% in eight months.
METHODS
Context
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large, urban, academic hospital. The HM division has 45 attendings and admits >8,000 general pediatric patients annually. The five HM teams at the main campus consist of attendings, fellows, residents, and medical students. One HM team serves as the resident quality improvement (QI) team where residents collaborate in a longitudinal study under the guidance of QI-trained coaches. The focus of this QI initiative was determined by resident consensus and aligned with a high-value care curriculum.12
To identify the target patient population, we investigated IV antimicrobials frequently used in HM patients. Ampicillin and clindamycin are commonly used IV antibiotics, most frequently corresponding with the diagnoses of CAP and SSTI, respectively, accounting for half of all antibiotic use on the HM service. Amoxicillin, the enteral equivalent of ampicillin, can achieve sufficient concentrations to attain the pharmacodynamic target at infection sites, and clindamycin has high bioavailability, making them ideal options for early transition. Our institution’s robust antimicrobial stewardship program has published local guidelines on using amoxicillin as the enteral antibiotic of choice for uncomplicated CAP, but it does not provide guidance on the timing of transition for either CAP or SSTI; the clinical team makes this decision.
HM attendings were surveyed to determine the criteria used to transition from IV to enteral antibiotics for patients with CAP or SSTI. The survey illustrated practice variability with providers using differing clinical criteria to signal the timing of transition. Additionally, only 49% of respondents (n = 37) rated themselves as “very comfortable” with residents making autonomous decisions to transition to enteral antibiotics. We chose to use the administration of other enteral medications, instead of discharge readiness, as an objective indicator of a patient’s readiness to transition to enteral antibiotics, given the low-risk patient population and the ability of the enteral antibiotics commonly used for CAP and SSTI to achieve pharmacodynamic targets.
The study population included patients aged >60 days admitted to HM with CAP or SSTI treated with any antibiotic. We excluded patients with potential complications or significant progression of their disease process, including patients with parapneumonic effusions or chest tubes, patients who underwent bronchoscopy, and patients with osteomyelitis, septic arthritis, or preseptal or orbital cellulitis. Past medical history and clinical status on admission were not used to exclude patients.
Interventions
Our multidisciplinary team, formed in January 2017, included HM attendings, HM fellows, pediatric residents, a critical care attending, a pharmacy resident, and an antimicrobial stewardship pharmacist. Under the guidance of QI coaches, the residents on the HM QI team developed and tested all interventions on their team and then determined which interventions would spread to the other four teams. The nursing director of our primary HM unit disseminated project updates to bedside nurses. A simplified failure mode and effects analysis identified areas for improvement and potential interventions. Interventions focused on the following key drivers (Figure 1): increased prescriber awareness of medication charge, standardization of conversion from IV to enteral antibiotics, clear definition of the patients ready for transition, ongoing evaluation of the antimicrobial plan, timely recognition by prescribers of patients ready for transition, culture shift regarding the appropriate administration route in the inpatient setting, and transparency of data. The team implemented sequential Plan-Do-Study-Act (PDSA) cycles13 to test the interventions.
Charge Table
To improve knowledge about the increased charge for commonly used IV medications compared with enteral formulations, a table comparing relative charges was shared during monthly resident morning conferences and at an HM faculty meeting. The table included charge comparisons between ampicillin and amoxicillin and IV and enteral clindamycin.
Standardized Language in Electronic Health Record (EHR) Antibiotic Plan on Rounds
Standardized language to document antibiotic transition plans was added to admission and progress note templates in the EHR. The standard template prompted residents to (1) define clinical transition criteria, (2) discuss attending comfort with transition overnight (based on survey results), and (3) document patient preference of solid or liquid dosage forms. Plans were reviewed and updated daily. We hypothesized that since residents use the information in the daily progress notes, including assessments and plans, to present on rounds, inclusion of the transition criteria in the note would prompt transition plan discussions.
Communication Bundle
To promote early transition to enteral antibiotics, we standardized the discussion about antibiotic transition between residents and attendings. During a weekly preexisting meeting, the resident QI team reviewed preferences for transitions with the new service attending. By identifying attending preferences early, residents were able to proactively transition patients who met the criteria (eg, antibiotic transition in the evening instead of waiting until morning rounds). This discussion also provided an opportunity to engage service attendings in the QI efforts, which were also shared at HM faculty meetings quarterly.
Recognizing that in times of high census, discussion of patient plans may be abbreviated during rounds, residents were asked to identify all patients on IV antibiotics while reviewing patient medication orders prior to rounds. As part of an existing daily prerounds huddle to discuss rounding logistics, residents listed all patients on IV antibiotics and discussed which patients were ready for transition. If patients could not be transitioned immediately, the team identified the transition criteria.
At preexisting evening huddles between overnight shift HM residents and the evening HM attending, residents identified patients who were prescribed IV antibiotics and discussed readiness for enteral transition. If a patient could be transitioned overnight, enteral antibiotic orders were placed. Overnight residents were also encouraged to review the transition criteria with families upon admission.
Real-time Identification of Failures and Feedback
For two weeks, the EHR was queried daily to identify patients admitted for uncomplicated CAP and SSTI who were on antibiotics as well as other enteral medications. A failure was defined as an IV antibiotic dose given to a patient who was administered any enteral medication. Residents on the QI team approached residents on other HM teams whenever patients were identified as a failed transition to learn about failure reasons.
Study of the Interventions
Data for HM patients who met the inclusion criteria were collected weekly from January 2016 through June 2018 via EHR query. We initially searched for diagnoses that fit under the disease categories of pneumonia and SSTI in the EHR, which generated a list of International Classification of Disease-9 and -10 Diagnosis codes (Appendix Figure 1). The query identified patients based on these codes and reported whether the identified patients took a dose of any enteral medication, excluding nystatin, sildenafil, tacrolimus, and mouthwashes, which are commonly continued during NPO status due to no need for absorption or limited parenteral options. It also reported the ordered route of administration for the queried antibiotics (Appendix Figure 1).
The 2016 calendar year established our baseline to account for seasonal variability. Data were reported weekly and reviewed to evaluate the impact of PDSA cycles and inform new interventions.
Measures
Our process measure was the total number of enteral antibiotic doses divided by all antibiotic doses in patients receiving any enteral medication. We reasoned that if patients were well enough to take medications enterally, they could be given an enteral antibiotic that is highly bioavailable or readily achieves concentrations that attain pharmacodynamic targets. This practice change was a culture shift, decoupling the switch to enteral antibiotics from discharge readiness. Our EHR query reported only the antibiotic doses given to patients who took an enteral medication on the day of antibiotic administration and excluded patients who received only IV medications.
Outcome measures included antimicrobial costs per patient encounter using average wholesale prices, which were reported in our EHR query, and LOS. To ensure that transitions of IV to enteral antibiotics were not negatively impacting patient outcomes, patient readmissions within seven days served as a balancing measure.
Analysis
An annotated statistical process control p-chart tracked the impact of interventions on the proportion of antibiotic doses that were enterally administered during hospitalization. An x-bar and an s-chart tracked the impact of interventions on antimicrobial costs per patient encounter and on LOS. A p-chart and an encounters-between g-chart were used to evaluate the impact of our interventions on readmissions. Control chart rules for identifying special cause were used for center line shifts.14
Ethical Considerations
This study was part of a larger study of the residency high-value care curriculum,12 which was deemed exempt by the CCHMC IRB.
RESULTS
The baseline data collected included 372 patients and the postintervention period in 2017 included 326 patients (Table). Approximately two-thirds of patients had a diagnosis of CAP.
The percentage of antibiotic doses given enterally increased from 44% to 80% within eight months (Figure 2). When studying the impact of interventions, residents on the HM QI team found that the standard EHR template added to daily notes did not consistently prompt residents to discuss antibiotic plans and thus was abandoned. Initial improvement coincided with standardizing discussions between residents and attendings regarding transitions. Furthermore, discussion of all patients on IV antibiotics during the prerounds huddle allowed for reliable, daily communication about antibiotic plans and was subsequently spread to and adopted by all HM teams. The percentage of enterally administered antibiotic doses increased to >75% after the evening huddle, which involved all HM teams, and real-time identification of failures on all HM teams with provider feedback. Despite variability when the total number of antibiotic doses prescribed per week was low (<10), we demonstrated sustainability for 11 months (Figure 2), during which the prerounds and evening huddle discussions were continued and an updated control chart was shown monthly to residents during their educational conferences.
Residents on the QI team spoke directly with other HM residents when there were missed opportunities for transition. Based on these discussions and intermittent chart reviews, common reasons for failure to transition in patients with CAP included admission for failed outpatient enteral treatment, recent evaluation by critical care physicians for possible transfer to the intensive care unit, and difficulty weaning oxygen. For patients with SSTI, hand abscesses requiring drainage by surgery and treatment failure with other antibiotics constituted many of the IV antibiotic doses given to patients on enteral medications.
Antimicrobial costs per patient encounter decreased by 70% over one year; the shift in costs coincided with the second shift in our process measure (Appendix Figure 2A). Based on an estimate of 350 patients admitted per year for uncomplicated CAP or SSTI, this translates to an annual cost savings of approximately $29,000. The standard deviation of costs per patient encounter decreased by 84% (Appendix Figure 2B), suggesting a decrease in the variability of prescribing practices.
The average LOS in our patient population prior to intervention was 2.1 days and did not change (Appendix Figure 2C), but the standard deviation decreased by >50% (Appendix Figure 2D). There was no shift in the mean seven-day readmission rate or the number of encounters between readmissions (2.6% and 26, respectively; Appendix Figure 3). In addition, the hospital billing department did not identify an increase in insurance denials related to the route of antibiotic administration.
DISCUSSION
Summary
Using improvement science, we promoted earlier transition to enteral antibiotics for children hospitalized with uncomplicated CAP and SSTI by linking the decision for transition to the ability to take other enteral medications, rather than to discharge readiness. We increased the percentage of enterally administered antibiotic doses in this patient population from 44% to 80% in eight months. Although we did not observe a decrease in LOS as previously noted in a cost analysis study comparing pediatric patients with CAP treated with oral antibiotics versus those treated with IV antibiotics,15 we did find a decrease in LOS variability and in antimicrobial costs to our patients. These cost savings did not include potential savings from nursing or pharmacy labor. In addition, we noted a decrease in the variability in antibiotic prescribing practice, which demonstrates provider ability and willingness to couple antibiotic route transition to an objective characteristic (administration of other enteral medications).
A strength of our study was that residents, the most frequent prescribers of antibiotics on our HM service, were highly involved in the QI initiative, including defining the SMART aim, identifying key drivers, developing interventions, and completing sequential PDSA cycles. Under the guidance of QI-trained coaches, residents developed feasible interventions and assessed their success in real time. Consistent with other studies,16,17 resident buy-in and involvement led to the success of our improvement study.
Interpretation
Despite emerging evidence regarding the timing of transition to enteral antibiotics, several factors impeded early transition at our institution, including physician culture, variable practice habits, and hospital workflow. Evidence supports the use of enteral antibiotics in immunocompetent children hospitalized for uncomplicated CAP who do not have chronic lung disease, are not in shock, and have oxygen saturations >85%.6 Although existing literature suggests that in pediatric patients admitted for SSTIs not involving the eye or bone, IV antibiotics may be transitioned when clinical improvement, evidenced by a reduction in fever or erythema, is noted,6 enteral antibiotics that achieve appropriate concentrations to attain pharmacodynamic targets should have the same efficacy as that of IV antibiotics.9 Using the criterion of administration of any medication enterally to identify a patient’s readiness to transition, we were able to overcome practice variation among providers who may have differing opinions of what constitutes clinical improvement. Of note, new evidence is emerging on predictors of enteral antibiotic treatment failure in patients with CAP and SSTI to guide transition timing, but these studies have largely focused on the adult population or were performed in the outpatient and emergency department (ED) settings.18,19 Regardless, the stable number of encounters between readmissions in our patient population likely indicates that treatment failure in these patients was rare.
Rising healthcare costs have led to concerns around sustainability of the healthcare system;20,21 tackling overuse in clinical practice, as in our study, is one mitigation strategy. Several studies have used QI methods to facilitate the provision of high-value care through the decrease of continuous monitor overuse and extraneous ordering of electrolytes.22,23 Our QI study adds to the high-value care literature by safely decreasing the use of IV antibiotics. One retrospective study demonstrated that a one-day decrease in the use of IV antibiotics in pneumonia resulted in decreased costs without an increase in readmissions, similar to our findings.24 In adults, QI initiatives aimed at improving early transition of antibiotics utilized electronic trigger tools.25,26 Fischer et al. used active orders for scheduled enteral medications or an enteral diet as indication that a patient’s IV medications could be converted to enteral form.26
Our work is not without limitations. The list of ICD-9 and -10 codes used to query the EHR did not capture all diagnoses that would be considered as uncomplicated CAP or SSTI. However, we included an extensive list of diagnoses to ensure that the majority of patients meeting our inclusion criteria were captured. Our process measure did not account for patients on IV antibiotics who were not administered other enteral medications but tolerating an enteral diet. These patients were not identified in our EHR query and were not included in our process measure as a failure. However, in latter interventions, residents identified all patients on IV antibiotics, so that patients not identified by our EHR query benefited from our work. Furthermore, this QI study was conducted at a single institution and several interventions took advantage of preexisting structured huddles and a resident QI curriculum, which may not exist at other institutions. Our study does highlight that engaging frontline providers, such as residents, to review antibiotic orders consistently and question the appropriateness of the administration route is key to making incremental changes in prescribing practices.
CONCLUSIONS
Through a partnership between HM and Pharmacy and with substantial resident involvement, we improved the transition of IV antibiotics in patients with CAP or SSTI by increasing the percentage of enterally administered antibiotic doses and reducing antimicrobial costs and variability in antibiotic prescribing practices. This work illustrates how reducing overuse of IV antibiotics promotes high-value care and aligns with initiatives to prevent avoidable harm.27 Our work highlights that standardized discussions about medication orders to create consensus around enteral antibiotic transitions, real-time feedback, and challenging the status quo can influence practice habits and effect change.
Next steps include testing automated methods to notify providers of opportunities for transition from IV to enteral antibiotics through embedded clinical decision support, a method similar to the electronic trigger tools used in adult QI studies.25,26 Since our prerounds huddle includes identifying all patients on IV antibiotics, studying the transition to enteral antibiotics and its effect on prescribing practices in other diagnoses (ie, urinary tract infection and osteomyelitis) may contribute to spreading these efforts. Partnering with our ED colleagues may be an important next step, as several patients admitted to HM on IV antibiotics are given their first dose in the ED.
Acknowledgments
The authors would like to thank the faculty of the James M. Anderson Center for Health Systems Excellence Intermediate Improvement Science Series for their guidance in the planning of this project. The authors would also like to thank Ms. Ursula Bradshaw and Mr. Michael Ponti-Zins for obtaining the hospital data on length of stay and readmissions. The authors acknowledge Dr. Philip Hagedorn for his assistance with the software that queries the electronic health record and Dr. Laura Brower and Dr. Joanna Thomson for their assistance with statistical analysis. The authors are grateful to all the residents and coaches on the QI Hospital Medicine team who contributed ideas on study design and interventions.
1. Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393.
2. Shah SS, Srivastava R, Wu S, et al. Intravenous Versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6). https://doi.org/10.1542/peds.2016-1692.
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822.
4. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435.https://doi.org/10.1001/jamapediatrics.2013.775.
5. Zaoutis T, Localio AR, Leckerman K, et al. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596.
6. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X.
7. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
8. Stevens DL, Bisno AL, Chambers HF, et al. Executive summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):147-159. https://doi.org/10.1093/cid/ciu444.
9. MacGregor RR, Graziani AL. Oral administration of antibiotics: a rational alternative to the parenteral route. Clin Infect Dis. 1997;24(3):457-467. https://doi.org/10.1093/clinids/24.3.457.
10. Downes KJ, Hahn A, Wiles J, Courter JD, Vinks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in paediatrics. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006.
11. Autmizguine J, Melloni C, Hornik CP, et al. Population pharmacokinetics of trimethoprim-sulfamethoxazole in infants and children. Antimicrob Agents Chemother. 2018;62(1):e01813-e01817. https://doi.org/10.1128/AAC.01813-17.
12. Dewan M, Herrmann LE, Tchou MJ, et al. Development and evaluation of high-value pediatrics: a high-value care pediatric resident curriculum. Hosp Pediatr. 2018;8(12):785-792. https://doi.org/10.1542/hpeds.2018-0115
13. Langley GJ, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. New Jersey, US: John Wiley & Sons; 2009.
14. Benneyan JC. Use and interpretation of statistical quality control charts. Int J Qual Health Care. 1998;10(1):69-73. https://doi.org/10.1093/intqhc/10.1.69.
15. Lorgelly PK, Atkinson M, Lakhanpaul M, et al. Oral versus i.v. antibiotics for community-acquired pneumonia in children: a cost-minimisation analysis. Eur Respir J. 2010;35(4):858-864. https://doi.org/10.1183/09031936.00087209.
16. Vidyarthi AR, Green AL, Rosenbluth G, Baron RB. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89(3):460-468. https://doi.org/10.1097/ACM.0000000000000159.
17. Stinnett-Donnelly JM, Stevens PG, Hood VL. Developing a high value care programme from the bottom up: a programme of faculty-resident improvement projects targeting harmful or unnecessary care. BMJ Qual Saf. 2016;25(11):901-908. https://doi.org/10.1136/bmjqs-2015-004546.
18. Peterson D, McLeod S, Woolfrey K, McRae A. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21(5):526-531. https://doi.org/10.1111/acem.12371.
19. Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for non-purulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2018;20(S1):S24-S25. https://doi.org/10.1017/cem.2018.114.
20. Organisation for Economic Co-operation and Development. Healthcare costs unsustainable in advanced economies without reform. http://www.oecd.org/health/healthcarecostsunsustainableinadvancedeconomieswithoutreform.htm. Accessed June 28, 2018; 2015.
21. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516. https://doi.org/10.1001/jama.2012.362.
22. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135(4):e1044-e1051. https://doi.org/10.1542/peds.2014-2295.
23. Tchou MJ, Tang Girdwood S, Wormser B, et al. Reducing electrolyte testing in hospitalized children by using quality improvement methods. Pediatrics. 2018;141(5). https://doi.org/10.1542/peds.2017-3187.
24. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatr Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003.
25. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3.
26. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585.
27. Schroeder AR, Harris SJ, Newman TB. Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128(6):e1596-e1597. https://doi.org/10.1542/peds.2011-2726.
1. Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393.
2. Shah SS, Srivastava R, Wu S, et al. Intravenous Versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6). https://doi.org/10.1542/peds.2016-1692.
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822.
4. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435.https://doi.org/10.1001/jamapediatrics.2013.775.
5. Zaoutis T, Localio AR, Leckerman K, et al. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596.
6. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X.
7. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
8. Stevens DL, Bisno AL, Chambers HF, et al. Executive summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):147-159. https://doi.org/10.1093/cid/ciu444.
9. MacGregor RR, Graziani AL. Oral administration of antibiotics: a rational alternative to the parenteral route. Clin Infect Dis. 1997;24(3):457-467. https://doi.org/10.1093/clinids/24.3.457.
10. Downes KJ, Hahn A, Wiles J, Courter JD, Vinks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in paediatrics. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006.
11. Autmizguine J, Melloni C, Hornik CP, et al. Population pharmacokinetics of trimethoprim-sulfamethoxazole in infants and children. Antimicrob Agents Chemother. 2018;62(1):e01813-e01817. https://doi.org/10.1128/AAC.01813-17.
12. Dewan M, Herrmann LE, Tchou MJ, et al. Development and evaluation of high-value pediatrics: a high-value care pediatric resident curriculum. Hosp Pediatr. 2018;8(12):785-792. https://doi.org/10.1542/hpeds.2018-0115
13. Langley GJ, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. New Jersey, US: John Wiley & Sons; 2009.
14. Benneyan JC. Use and interpretation of statistical quality control charts. Int J Qual Health Care. 1998;10(1):69-73. https://doi.org/10.1093/intqhc/10.1.69.
15. Lorgelly PK, Atkinson M, Lakhanpaul M, et al. Oral versus i.v. antibiotics for community-acquired pneumonia in children: a cost-minimisation analysis. Eur Respir J. 2010;35(4):858-864. https://doi.org/10.1183/09031936.00087209.
16. Vidyarthi AR, Green AL, Rosenbluth G, Baron RB. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89(3):460-468. https://doi.org/10.1097/ACM.0000000000000159.
17. Stinnett-Donnelly JM, Stevens PG, Hood VL. Developing a high value care programme from the bottom up: a programme of faculty-resident improvement projects targeting harmful or unnecessary care. BMJ Qual Saf. 2016;25(11):901-908. https://doi.org/10.1136/bmjqs-2015-004546.
18. Peterson D, McLeod S, Woolfrey K, McRae A. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21(5):526-531. https://doi.org/10.1111/acem.12371.
19. Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for non-purulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2018;20(S1):S24-S25. https://doi.org/10.1017/cem.2018.114.
20. Organisation for Economic Co-operation and Development. Healthcare costs unsustainable in advanced economies without reform. http://www.oecd.org/health/healthcarecostsunsustainableinadvancedeconomieswithoutreform.htm. Accessed June 28, 2018; 2015.
21. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516. https://doi.org/10.1001/jama.2012.362.
22. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135(4):e1044-e1051. https://doi.org/10.1542/peds.2014-2295.
23. Tchou MJ, Tang Girdwood S, Wormser B, et al. Reducing electrolyte testing in hospitalized children by using quality improvement methods. Pediatrics. 2018;141(5). https://doi.org/10.1542/peds.2017-3187.
24. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatr Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003.
25. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3.
26. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585.
27. Schroeder AR, Harris SJ, Newman TB. Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128(6):e1596-e1597. https://doi.org/10.1542/peds.2011-2726.
© 2019 Society of Hospital Medicine
Inpatient Communication Barriers and Drivers When Caring for Limited English Proficiency Children
Immigrant children make up the fastest growing segment of the population in the United States.1 While most immigrant children are fluent in English, approximately 40% live with a parent who has limited English proficiency (LEP; ie, speaks English less than “very well”).2,3 In pediatrics, LEP status has been associated with longer hospitalizations,4 higher hospitalization costs,5 increased risk for serious adverse medical events,4,6 and more frequent emergency department reutilization.7 In the inpatient setting, multiple aspects of care present a variety of communication challenges,8 which are amplified by shift work and workflow complexity that result in patients and families interacting with numerous providers over the course of an inpatient stay.
Increasing access to trained professional interpreters when caring for LEP patients improves communication, patient satisfaction, adherence, and mortality.9-12 However, even when access to interpreter services is established, effective use is not guaranteed.13 Up to 57% of pediatricians report relying on family members to communicate with LEP patients and their caregivers;9 23% of pediatric residents categorized LEP encounters as frustrating while 78% perceived care of LEP patients to be “misdirected” (eg, delay in diagnosis or discharge) because of associated language barriers.14
Understanding experiences of frontline inpatient medical providers and interpreters is crucial in identifying challenges and ways to optimize communication for hospitalized LEP patients and families. However, there is a paucity of literature exploring the perspectives of medical providers and interpreters as it relates to communication with hospitalized LEP children and families. In this study, we sought to identify barriers and drivers of effective communication with pediatric patients and families with LEP in the inpatient setting from the perspective of frontline medical providers and interpreters.
METHODS
Study Design
This qualitative study used Group Level Assessment (GLA), a structured participatory methodology that allows diverse groups of stakeholders to generate and evaluate data in interactive sessions.15-18 GLA structure promotes active participation, group problem-solving, and development of actionable plans, distinguishing it from focus groups and in-depth semistructured interviews.15,19 This study received a human subject research exemption by the institutional review board.
Study Setting
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large quaternary care center with ~200 patient encounters each day who require the use of interpreter services. Interpreters (in-person, video, and phone) are utilized during admission, formal family-centered rounds, hospital discharge, and other encounters with physicians, nurses, and other healthcare professionals. In-person interpreters are available in-house for Spanish and Arabic, with 18 additional languages available through regional vendors. Despite available resources, there is no standard way in which medical providers and interpreters work with one another.
Study Participants and Recruitment
Medical providers who care for hospitalized general pediatric patients were eligible to participate, including attending physicians, resident physicians, bedside nurses, and inpatient ancillary staff (eg, respiratory therapists, physical therapists). Interpreters employed by CCHMC with experience in the inpatient setting were also eligible. Individuals were recruited based on published recommendations to optimize discussion and group-thinking.15 Each participant was asked to take part in one GLA session. Participants were assigned to specific sessions based on roles (ie, physicians, nurses, and interpreters) to maximize engagement and minimize the impact of hierarchy.
Study Procedure
GLA involves a seven-step structured process (Appendix 1): climate setting, generating, appreciating, reflecting, understanding, selecting, and action.15,18 Qualitative data were generated individually and anonymously by participants on flip charts in response to prompts such as: “I worry that LEP families___,” “The biggest challenge when using interpreter services is___,” and “I find___ works well in providing care for LEP families.” Prompts were developed by study investigators, modified based on input from nursing and interpreter services leadership, and finalized by GLA facilitators. Fifty-one unique prompts were utilized (Appendix 2); the number of prompts used (ranging from 15 to 32 prompts) per session was based on published recommendations.15 During sessions, study investigators took detailed notes, including verbatim transcription of participant quotes. Upon conclusion of the session, each participant completed a demographic survey, including years of experience, languages spoken and perceived fluency,20 and ethnicity.
Data Analysis
Within each session, under the guidance of trained and experienced GLA facilitators (WB, HV), participants distilled and summarized qualitative data into themes, discussed and prioritized themes, and generated action items. Following completion of all sessions, analyzed data was compiled by the research team to determine similarities and differences across groups based on participant roles, consolidate themes into barriers and drivers of communication with LEP families, and determine any overlap of priorities for action. Findings were shared back with each group to ensure accuracy and relevance.
RESULTS
Participants
A total of 64 individuals participated (Table 1): hospital medicine physicians and residents (56%), inpatient nurses and ancillary staff (16%), and interpreters (28%). While 81% of physicians spoke multiple languages, only 25% reported speaking them well; two physicians were certified to communicate medical information without an interpreter present.
Themes Resulting from GLA Sessions
A total of four barriers (Table 2) and four drivers (Table 3) of effective communication with pediatric LEP patients and their families in the inpatient setting were identified by participants. Participants across all groups, despite enthusiasm around improving communication, were concerned about quality of care LEP families received, noting that the system is “designed to deliver less-good care” and that “we really haven’t figured out how to care for [LEP patients and families] in a [high-]quality and reliable way.” Variation in theme discussion was noted between groups based on participant role: physicians voiced concern about rapport with LEP families, nurses emphasized actionable tasks, and interpreters focused on heightened challenges in times of stress.
Barrier 1: Difficulties Accessing Interpreter Services
Medical providers (physicians and nurses) identified the “opaque process to access [interpreter] services” as one of their biggest challenges when communicating with LEP families. In particular, the process of scheduling interpreters was described as a “black box,” with physicians and nurses expressing difficulty determining if and when in-person interpreters were scheduled and uncertainty about when to use modalities other than in-person interpretation. Participants across groups highlighted the lack of systems knowledge from medical providers and limitations within the system that make predictable, timely, and reliable access to interpreters challenging, especially for uncommon languages. Medical providers desired more in-person interpreters who can “stay as long as clinically indicated,” citing frustration associated with using phone- and video-interpretation (eg, challenges locating technology, unfamiliarity with use, unreliable functionality of equipment). Interpreters voiced wanting to take time to finish each encounter fully without “being in a hurry because the next appointment is coming soon” or “rushing… in [to the next] session sweating.”
Barrier 2: Uncertainty in Communication with LEP Families
Participants across all groups described three areas of uncertainty as detailed in Table 2: (1) what to share and how to prioritize information during encounters with LEP patients and families, (2) what is communicated during interpretation, and (3) what LEP patients and families understand.
Barrier 3: Unclear and Inconsistent Expectations and Roles of Team Members
Given the complexity involved in communication between medical providers, interpreters, and families, participants across all groups reported feeling ill-prepared when navigating hospital encounters with LEP patients and families. Interpreters reported having little to no clinical context, medical providers reported having no knowledge of the assigned interpreter’s style, and both interpreters and medical providers reported that families have little idea of what to expect or how to engage. All groups voiced frustration about the lack of clarity regarding specific roles and scope of practice for each team member during an encounter, where multiple people end up “talking [or] using the interpreter at once.” Interpreters shared their expectations of medical providers to set the pace and lead conversations with LEP families. On the other hand, medical providers expressed a desire for interpreters to provide cultural context to the team without prompting and to interrupt during encounters when necessary to voice concerns or redirect conversations.
Barrier 4: Unmet Family Engagement Expectations
Participants across all groups articulated challenges with establishing rapport with LEP patients and families, sharing concerns that “inadequate communication” due to “cultural or language barriers” ultimately impacts quality of care. Participants reported decreased bidirectional engagement with and from LEP families. Medical providers not only noted difficulty in connecting with LEP families “on a more personal level” and providing frequent medical updates, but also felt that LEP families do not ask questions even when uncertain. Interpreters expressed concerns about medical providers “not [having] enough patience to answer families’ questions” while LEP families “shy away from asking questions.”
Driver 1: Utilizing a Team-Based Approach between Medical Providers and Interpreters
Participants from all groups emphasized that a mutual understanding of roles and shared expectations regarding communication and interpretation style, clinical context, and time constraints would establish a foundation for respect between medical providers and interpreters. They reported that a team-based approach to LEP patient and family encounters were crucial to achieving effective communication.
Driver 2: Understanding the Role of Cultural Context in Providing Culturally Effective Care.
Participants across all groups highlighted three different aspects of cultural context that drive effective communication: (1) medical providers’ perception of the family’s culture; (2) LEP families’ knowledge about the culture and healthcare system in the US, and (3) medical providers insight into their own preconceived ideas about LEP families.
Driver 3: Practicing Empathy for Patients and Families
All participants reported that respect for diversity and consideration of the backgrounds and perspectives of LEP patients and families are necessary. Furthermore, both medical providers and interpreters articulated a need to remain patient and mindful when interacting with LEP families despite challenges, especially since, as noted by interpreters, encounters may “take longer, but it’s for a reason.”
Driver 4: Using Effective Family-Centered Communication Strategies
Participants identified the use of effective family-centered communication principles as a driver to optimal communication. Many of the principles identified by medical providers and interpreters are generally applicable to all hospitalized patients and families regardless of English proficiency: optimizing verbal communication (eg, using shorter sentences, pausing to allow for interpretation), optimizing nonverbal communication (eg, setting, position, and body language), and assessment of family understanding and engagement (eg, use of teach back).
DISCUSSION
Frontline medical providers and interpreters identified barriers and drivers that impact communication with LEP patients and families during hospitalization. To our knowledge, this is the first study that uses a participatory method to explore the perspectives of medical providers and interpreters who care for LEP children and families in the inpatient setting. Despite existing difficulties and concerns regarding language barriers and its impact on quality of care for hospitalized LEP patients and families, participants were enthusiastic about how identified barriers and drivers may inform future improvement efforts. Notable action steps for future improvement discussed by our participants included: increased use and functionality of technology for timely and predictable access to interpreters, deliberate training for providers focused on delivery of culturally-effective care, consistent use of family-centered communication strategies including teach-back, and implementing interdisciplinary expectation setting through “presessions” before encounters with LEP families.
Participants elaborated on several barriers previously described in the literature including time constraints and technical problems.14,21,22 Such barriers may serve as deterrents to consistent and appropriate use of interpreters in healthcare settings.9 A heavy reliance on off-site interpreters (including phone- or video-interpreters) and lack of knowledge regarding resource availability likely amplified frustration for medical providers. Communication with LEP families can be daunting, especially when medical providers do not care for LEP families or work with interpreters on a regular basis.14 Standardizing the education of medical providers regarding available resources, as well as the logistics, process, and parameters for scheduling interpreters and using technology, was an action step identified by our GLA participants. Targeted education about the logistics of accessing interpreter services and having standardized ways to make technology use easier (ie, one-touch dialing in hospital rooms) has been associated with increased interpreter use and decreased interpreter-related delays in care.23
Our frontline medical providers expressed added concern about not spending as much time with LEP families. In fact, LEP families in the literature have perceived medical providers to spend less time with their children compared to their English-proficient counterparts.24 Language and cultural barriers, both perceived and real, may limit medical provider rapport with LEP patients and families14 and likely contribute to medical providers relying on their preconceived assumptions instead.25 Cultural competency education for medical providers, as highlighted by our GLA participants as an action item, can be used to provide more comprehensive and effective care.26,27
In addition to enhancing cultural humility through education, our participants emphasized the use of family-centered communication strategies as a driver of optimal family engagement and understanding. Actively inviting questions from families and utilizing teach-back, an established evidence-based strategy28-30 discussed by our participants, can be particularly powerful in assessing family understanding and engagement. While information should be presented in plain language for families in all encounters,31 these evidence-based practices are of particular importance when communicating with LEP families. They promote effective communication, empower families to share concerns in a structured manner, and allow medical providers to address matters in real-time with interpreters present.
Finally, our participants highlighted the need for partnerships between providers and interpreter services, noting unclear roles and expectations among interpreters and medical providers as a major barrier. Specifically, physicians noted confusion regarding the scope of an interpreter’s practice. Participants from GLA sessions discussed the importance of a team-based approach and suggested implementing a “presession” prior to encounters with LEP patients and families. Presessions—a concept well accepted among interpreters and recommended by consensus-based practice guidelines—enable medical providers and interpreters to establish shared expectations about scope of practice, communication, interpretation style, time constraints, and medical context prior to patient encounters.32,33
There are several limitations to our study. First, individuals who chose to participate were likely highly motivated by their clinical experiences with LEP patients and invested in improving communication with LEP families. Second, the study is limited in generalizability, as it was conducted at a single academic institution in a Midwestern city. Despite regional variations in available resources as well as patient and workforce demographics, our findings regarding major themes are in agreement with previously published literature and further add to our understanding of ways to improve communication with this vulnerable population across the care spectrum. Lastly, we were logistically limited in our ability to elicit the perspectives of LEP families due to the participatory nature of GLA; the need for multiple interpreters to simultaneously interact with LEP individuals would have not only hindered active LEP family participation but may have also biased the data generated by patients and families, as the services interpreters provide during their inpatient stay was the focus of our study. Engaging LEP families in their preferred language using participatory methods should be considered for future studies.
In conclusion, frontline providers of medical and language services identified barriers and drivers impacting the effective use of interpreter services when communicating with LEP families during hospitalization. Our enhanced understanding of barriers and drivers, as well as identified actionable interventions, will inform future improvement of communication and interactions with LEP families that contributes to effective and efficient family centered care. A framework for the development and implementation of organizational strategies aimed at improving communication with LEP families must include a thorough assessment of impact, feasibility, stakeholder involvement, and sustainability of specific interventions. While there is no simple formula to improve language services, health systems should establish and adopt language access policies, standardize communication practices, and develop processes to optimize the use of language services in the hospital. Furthermore, engagement with LEP families to better understand their perceptions and experiences with the healthcare system is crucial to improve communication between medical providers and LEP families in the inpatient setting and should be the subject of future studies.
Disclosures
The authors have no conflicts of interest to disclose.
Funding
No external funding was secured for this study. Dr. Joanna Thomson is supported by the Agency for Healthcare Research and Quality (Grant #K08 HS025138). Dr. Raglin Bignall was supported through a Ruth L. Kirschstein National Research Service Award (T32HP10027) when the study was conducted. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations. The funding organizations had no role in the design, preparation, review, or approval of this paper.
1. The American Academy of Pediatrics Council on Community Pediatrics. Providing care for immigrant, migrant, and border children. Pediatrics. 2013;131(6):e2028-e2034. PubMed
2. Meneses C, Chilton L, Duffee J, et al. Council on Community Pediatrics Immigrant Health Tool Kit. The American Academy of Pediatrics. https://www.aap.org/en-us/Documents/cocp_toolkit_full.pdf. Accessed May 13, 2019.
3. Office for Civil Rights. Guidance to Federal Financial Assistance Recipients Regarding Title VI and the Prohibition Against National Origin Discrimination Affecting Limited English Proficient Persons. https://www.hhs.gov/civil-rights/for-individuals/special-topics/limited-english-proficiency/guidance-federal-financial-assistance-recipients-title-vi/index.html. Accessed May 13, 2019.
4. Lion KC, Rafton SA, Shafii J, et al. Association between language, serious adverse events, and length of stay Among hospitalized children. Hosp Pediatr. 2013;3(3):219-225. https://doi.org/10.1542/hpeds.2012-0091.
5. Lion KC, Wright DR, Desai AD, Mangione-Smith R. Costs of care for hospitalized children associated With preferred language and insurance type. Hosp Pediatr. 2017;7(2):70-78. https://doi.org/10.1542/hpeds.2016-0051.
6. Cohen AL, Rivara F, Marcuse EK, McPhillips H, Davis R. Are language barriers associated with serious medical events in hospitalized pediatric patients? Pediatrics. 2005;116(3):575-579. https://doi.org/10.1542/peds.2005-0521.
7. Samuels-Kalow ME, Stack AM, Amico K, Porter SC. Parental language and return visits to the Emergency Department After discharge. Pediatr Emerg Care. 2017;33(6):402-404. https://doi.org/10.1097/PEC.0000000000000592.
8. Unaka NI, Statile AM, Choe A, Shonna Yin H. Addressing health literacy in the inpatient setting. Curr Treat Options Pediatr. 2018;4(2):283-299. https://doi.org/10.1007/s40746-018-0122-3.
9. DeCamp LR, Kuo DZ, Flores G, O’Connor K, Minkovitz CS. Changes in language services use by US pediatricians. Pediatrics. 2013;132(2):e396-e406. https://doi.org/10.1542/peds.2012-2909.
10. Flores G. The impact of medical interpreter services on the quality of health care: A systematic review. Med Care Res Rev. 2005;62(3):255-299. https://doi.org/10.1177/1077558705275416.
11. Flores G, Abreu M, Barone CP, Bachur R, Lin H. Errors of medical interpretation and their potential clinical consequences: A comparison of professional versus hoc versus no interpreters. Ann Emerg Med. 2012;60(5):545-553. https://doi.org/10.1016/j.annemergmed.2012.01.025.
12. Anand KJ, Sepanski RJ, Giles K, Shah SH, Juarez PD. Pediatric intensive care unit mortality among Latino children before and after a multilevel health care delivery intervention. JAMA Pediatr. 2015;169(4):383-390. https://doi.org/10.1001/jamapediatrics.2014.3789.
13. The Joint Commission. Advancing Effective Communication, Cultural Competence, and Patient- and Family-Centered Care: A Roadmap for Hospitals. Oakbrook Terrace, IL: The Joint Commission; 2010.
14. Hernandez RG, Cowden JD, Moon M et al. Predictors of resident satisfaction in caring for limited English proficient families: a multisite study. Acad Pediatr. 2014;14(2):173-180. https://doi.org/10.1016/j.acap.2013.12.002.
15. Vaughn LM, Lohmueller M. Calling all stakeholders: group-level assessment (GLA)-a qualitative and participatory method for large groups. Eval Rev. 2014;38(4):336-355. https://doi.org/10.1177/0193841X14544903.
16. Vaughn LM, Jacquez F, Zhao J, Lang M. Partnering with students to explore the health needs of an ethnically diverse, low-resource school: an innovative large group assessment approach. Fam Commun Health. 2011;34(1):72-84. https://doi.org/10.1097/FCH.0b013e3181fded12.
17. Gosdin CH, Vaughn L. Perceptions of physician bedside handoff with nurse and family involvement. Hosp Pediatr. 2012;2(1):34-38. https://doi.org/10.1542/hpeds.2011-0008-2.
18. Graham KE, Schellinger AR, Vaughn LM. Developing strategies for positive change: transitioning foster youth to adulthood. Child Youth Serv Rev. 2015;54:71-79. https://doi.org/10.1016/j.childyouth.2015.04.014.
19. Vaughn LM. Group level assessment: A Large Group Method for Identifying Primary Issues and Needs within a community. London2014. http://methods.sagepub.com/case/group-level-assessment-large-group-primary-issues-needs-community. Accessed 2017/07/26.
20. Association of American Medical Colleges Electronic Residency Application Service. ERAS 2018 MyERAS Application Worksheet: Language Fluency. Washington, DC:: Association of American Medical Colleges; 2018:5.
21. Brisset C, Leanza Y, Laforest K. Working with interpreters in health care: A systematic review and meta-ethnography of qualitative studies. Patient Educ Couns. 2013;91(2):131-140. https://doi.org/10.1016/j.pec.2012.11.008.
22. Wiking E, Saleh-Stattin N, Johansson SE, Sundquist J. A description of some aspects of the triangular meeting between immigrant patients, their interpreters and GPs in primary health care in Stockholm, Sweden. Fam Pract. 2009;26(5):377-383. https://doi.org/10.1093/fampra/cmp052.
23. Lion KC, Ebel BE, Rafton S et al. Evaluation of a quality improvement intervention to increase use of telephonic interpretation. Pediatrics. 2015;135(3):e709-e716. https://doi.org/10.1542/peds.2014-2024.
24. Zurca AD, Fisher KR, Flor RJ, et al. Communication with limited English-proficient families in the PICU. Hosp Pediatr. 2017;7(1):9-15. https://doi.org/10.1542/hpeds.2016-0071.
25. Kodjo C. Cultural competence in clinician communication. Pediatr Rev. 2009;30(2):57-64. https://doi.org/10.1542/pir.30-2-57.
26. Britton CV, American Academy of Pediatrics Committee on Pediatric Workforce. Ensuring culturally effective pediatric care: implications for education and health policy. Pediatrics. 2004;114(6):1677-1685. https://doi.org/10.1542/peds.2004-2091.
27. The American Academy of Pediatrics. Culturally Effective Care Toolkit: Providing Cuturally Effective Pediatric Care; 2018. https://www.aap.org/en-us/professional-resources/practice-transformation/managing-patients/Pages/effective-care.aspx. Accessed May 13, 2019.
28. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
29. Jager AJ, Wynia MK. Who gets a teach-back? Patient-reported incidence of experiencing a teach-back. J Health Commun. 2012;17 Supplement 3:294-302. https://doi.org/10.1080/10810730.2012.712624.
30. Kornburger C, Gibson C, Sadowski S, Maletta K, Klingbeil C. Using “teach-back” to promote a safe transition from hospital to home: an evidence-based approach to improving the discharge process. J Pediatr Nurs. 2013;28(3):282-291. https://doi.org/10.1016/j.pedn.2012.10.007.
31. Abrams MA, Klass P, Dreyer BP. Health literacy and children: recommendations for action. Pediatrics. 2009;124 Supplement 3:S327-S331. https://doi.org/10.1542/peds.2009-1162I.
32. Betancourt JR, Renfrew MR, Green AR, Lopez L, Wasserman M. Improving Patient Safety Systems for Patients with Limited English Proficiency: a Guide for Hospitals. Agency for Healthcare Research and Quality; 2012. 33. The National Council on Interpreting in Health Care. Best Practices for Communicating Through an Interpreter . https://refugeehealthta.org/access-to-care/language-access/best-practices-communicating-through-an-interpreter/. Accessed May 19, 2019.
33. The National Council on Interpreting in Health Care. Best Practices for Communicating Through an Interpreter . https://refugeehealthta.org/access-to-care/language-access/best-practices-communicating-through-an-interpreter/. Accessed May 19, 2019.
Immigrant children make up the fastest growing segment of the population in the United States.1 While most immigrant children are fluent in English, approximately 40% live with a parent who has limited English proficiency (LEP; ie, speaks English less than “very well”).2,3 In pediatrics, LEP status has been associated with longer hospitalizations,4 higher hospitalization costs,5 increased risk for serious adverse medical events,4,6 and more frequent emergency department reutilization.7 In the inpatient setting, multiple aspects of care present a variety of communication challenges,8 which are amplified by shift work and workflow complexity that result in patients and families interacting with numerous providers over the course of an inpatient stay.
Increasing access to trained professional interpreters when caring for LEP patients improves communication, patient satisfaction, adherence, and mortality.9-12 However, even when access to interpreter services is established, effective use is not guaranteed.13 Up to 57% of pediatricians report relying on family members to communicate with LEP patients and their caregivers;9 23% of pediatric residents categorized LEP encounters as frustrating while 78% perceived care of LEP patients to be “misdirected” (eg, delay in diagnosis or discharge) because of associated language barriers.14
Understanding experiences of frontline inpatient medical providers and interpreters is crucial in identifying challenges and ways to optimize communication for hospitalized LEP patients and families. However, there is a paucity of literature exploring the perspectives of medical providers and interpreters as it relates to communication with hospitalized LEP children and families. In this study, we sought to identify barriers and drivers of effective communication with pediatric patients and families with LEP in the inpatient setting from the perspective of frontline medical providers and interpreters.
METHODS
Study Design
This qualitative study used Group Level Assessment (GLA), a structured participatory methodology that allows diverse groups of stakeholders to generate and evaluate data in interactive sessions.15-18 GLA structure promotes active participation, group problem-solving, and development of actionable plans, distinguishing it from focus groups and in-depth semistructured interviews.15,19 This study received a human subject research exemption by the institutional review board.
Study Setting
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large quaternary care center with ~200 patient encounters each day who require the use of interpreter services. Interpreters (in-person, video, and phone) are utilized during admission, formal family-centered rounds, hospital discharge, and other encounters with physicians, nurses, and other healthcare professionals. In-person interpreters are available in-house for Spanish and Arabic, with 18 additional languages available through regional vendors. Despite available resources, there is no standard way in which medical providers and interpreters work with one another.
Study Participants and Recruitment
Medical providers who care for hospitalized general pediatric patients were eligible to participate, including attending physicians, resident physicians, bedside nurses, and inpatient ancillary staff (eg, respiratory therapists, physical therapists). Interpreters employed by CCHMC with experience in the inpatient setting were also eligible. Individuals were recruited based on published recommendations to optimize discussion and group-thinking.15 Each participant was asked to take part in one GLA session. Participants were assigned to specific sessions based on roles (ie, physicians, nurses, and interpreters) to maximize engagement and minimize the impact of hierarchy.
Study Procedure
GLA involves a seven-step structured process (Appendix 1): climate setting, generating, appreciating, reflecting, understanding, selecting, and action.15,18 Qualitative data were generated individually and anonymously by participants on flip charts in response to prompts such as: “I worry that LEP families___,” “The biggest challenge when using interpreter services is___,” and “I find___ works well in providing care for LEP families.” Prompts were developed by study investigators, modified based on input from nursing and interpreter services leadership, and finalized by GLA facilitators. Fifty-one unique prompts were utilized (Appendix 2); the number of prompts used (ranging from 15 to 32 prompts) per session was based on published recommendations.15 During sessions, study investigators took detailed notes, including verbatim transcription of participant quotes. Upon conclusion of the session, each participant completed a demographic survey, including years of experience, languages spoken and perceived fluency,20 and ethnicity.
Data Analysis
Within each session, under the guidance of trained and experienced GLA facilitators (WB, HV), participants distilled and summarized qualitative data into themes, discussed and prioritized themes, and generated action items. Following completion of all sessions, analyzed data was compiled by the research team to determine similarities and differences across groups based on participant roles, consolidate themes into barriers and drivers of communication with LEP families, and determine any overlap of priorities for action. Findings were shared back with each group to ensure accuracy and relevance.
RESULTS
Participants
A total of 64 individuals participated (Table 1): hospital medicine physicians and residents (56%), inpatient nurses and ancillary staff (16%), and interpreters (28%). While 81% of physicians spoke multiple languages, only 25% reported speaking them well; two physicians were certified to communicate medical information without an interpreter present.
Themes Resulting from GLA Sessions
A total of four barriers (Table 2) and four drivers (Table 3) of effective communication with pediatric LEP patients and their families in the inpatient setting were identified by participants. Participants across all groups, despite enthusiasm around improving communication, were concerned about quality of care LEP families received, noting that the system is “designed to deliver less-good care” and that “we really haven’t figured out how to care for [LEP patients and families] in a [high-]quality and reliable way.” Variation in theme discussion was noted between groups based on participant role: physicians voiced concern about rapport with LEP families, nurses emphasized actionable tasks, and interpreters focused on heightened challenges in times of stress.
Barrier 1: Difficulties Accessing Interpreter Services
Medical providers (physicians and nurses) identified the “opaque process to access [interpreter] services” as one of their biggest challenges when communicating with LEP families. In particular, the process of scheduling interpreters was described as a “black box,” with physicians and nurses expressing difficulty determining if and when in-person interpreters were scheduled and uncertainty about when to use modalities other than in-person interpretation. Participants across groups highlighted the lack of systems knowledge from medical providers and limitations within the system that make predictable, timely, and reliable access to interpreters challenging, especially for uncommon languages. Medical providers desired more in-person interpreters who can “stay as long as clinically indicated,” citing frustration associated with using phone- and video-interpretation (eg, challenges locating technology, unfamiliarity with use, unreliable functionality of equipment). Interpreters voiced wanting to take time to finish each encounter fully without “being in a hurry because the next appointment is coming soon” or “rushing… in [to the next] session sweating.”
Barrier 2: Uncertainty in Communication with LEP Families
Participants across all groups described three areas of uncertainty as detailed in Table 2: (1) what to share and how to prioritize information during encounters with LEP patients and families, (2) what is communicated during interpretation, and (3) what LEP patients and families understand.
Barrier 3: Unclear and Inconsistent Expectations and Roles of Team Members
Given the complexity involved in communication between medical providers, interpreters, and families, participants across all groups reported feeling ill-prepared when navigating hospital encounters with LEP patients and families. Interpreters reported having little to no clinical context, medical providers reported having no knowledge of the assigned interpreter’s style, and both interpreters and medical providers reported that families have little idea of what to expect or how to engage. All groups voiced frustration about the lack of clarity regarding specific roles and scope of practice for each team member during an encounter, where multiple people end up “talking [or] using the interpreter at once.” Interpreters shared their expectations of medical providers to set the pace and lead conversations with LEP families. On the other hand, medical providers expressed a desire for interpreters to provide cultural context to the team without prompting and to interrupt during encounters when necessary to voice concerns or redirect conversations.
Barrier 4: Unmet Family Engagement Expectations
Participants across all groups articulated challenges with establishing rapport with LEP patients and families, sharing concerns that “inadequate communication” due to “cultural or language barriers” ultimately impacts quality of care. Participants reported decreased bidirectional engagement with and from LEP families. Medical providers not only noted difficulty in connecting with LEP families “on a more personal level” and providing frequent medical updates, but also felt that LEP families do not ask questions even when uncertain. Interpreters expressed concerns about medical providers “not [having] enough patience to answer families’ questions” while LEP families “shy away from asking questions.”
Driver 1: Utilizing a Team-Based Approach between Medical Providers and Interpreters
Participants from all groups emphasized that a mutual understanding of roles and shared expectations regarding communication and interpretation style, clinical context, and time constraints would establish a foundation for respect between medical providers and interpreters. They reported that a team-based approach to LEP patient and family encounters were crucial to achieving effective communication.
Driver 2: Understanding the Role of Cultural Context in Providing Culturally Effective Care.
Participants across all groups highlighted three different aspects of cultural context that drive effective communication: (1) medical providers’ perception of the family’s culture; (2) LEP families’ knowledge about the culture and healthcare system in the US, and (3) medical providers insight into their own preconceived ideas about LEP families.
Driver 3: Practicing Empathy for Patients and Families
All participants reported that respect for diversity and consideration of the backgrounds and perspectives of LEP patients and families are necessary. Furthermore, both medical providers and interpreters articulated a need to remain patient and mindful when interacting with LEP families despite challenges, especially since, as noted by interpreters, encounters may “take longer, but it’s for a reason.”
Driver 4: Using Effective Family-Centered Communication Strategies
Participants identified the use of effective family-centered communication principles as a driver to optimal communication. Many of the principles identified by medical providers and interpreters are generally applicable to all hospitalized patients and families regardless of English proficiency: optimizing verbal communication (eg, using shorter sentences, pausing to allow for interpretation), optimizing nonverbal communication (eg, setting, position, and body language), and assessment of family understanding and engagement (eg, use of teach back).
DISCUSSION
Frontline medical providers and interpreters identified barriers and drivers that impact communication with LEP patients and families during hospitalization. To our knowledge, this is the first study that uses a participatory method to explore the perspectives of medical providers and interpreters who care for LEP children and families in the inpatient setting. Despite existing difficulties and concerns regarding language barriers and its impact on quality of care for hospitalized LEP patients and families, participants were enthusiastic about how identified barriers and drivers may inform future improvement efforts. Notable action steps for future improvement discussed by our participants included: increased use and functionality of technology for timely and predictable access to interpreters, deliberate training for providers focused on delivery of culturally-effective care, consistent use of family-centered communication strategies including teach-back, and implementing interdisciplinary expectation setting through “presessions” before encounters with LEP families.
Participants elaborated on several barriers previously described in the literature including time constraints and technical problems.14,21,22 Such barriers may serve as deterrents to consistent and appropriate use of interpreters in healthcare settings.9 A heavy reliance on off-site interpreters (including phone- or video-interpreters) and lack of knowledge regarding resource availability likely amplified frustration for medical providers. Communication with LEP families can be daunting, especially when medical providers do not care for LEP families or work with interpreters on a regular basis.14 Standardizing the education of medical providers regarding available resources, as well as the logistics, process, and parameters for scheduling interpreters and using technology, was an action step identified by our GLA participants. Targeted education about the logistics of accessing interpreter services and having standardized ways to make technology use easier (ie, one-touch dialing in hospital rooms) has been associated with increased interpreter use and decreased interpreter-related delays in care.23
Our frontline medical providers expressed added concern about not spending as much time with LEP families. In fact, LEP families in the literature have perceived medical providers to spend less time with their children compared to their English-proficient counterparts.24 Language and cultural barriers, both perceived and real, may limit medical provider rapport with LEP patients and families14 and likely contribute to medical providers relying on their preconceived assumptions instead.25 Cultural competency education for medical providers, as highlighted by our GLA participants as an action item, can be used to provide more comprehensive and effective care.26,27
In addition to enhancing cultural humility through education, our participants emphasized the use of family-centered communication strategies as a driver of optimal family engagement and understanding. Actively inviting questions from families and utilizing teach-back, an established evidence-based strategy28-30 discussed by our participants, can be particularly powerful in assessing family understanding and engagement. While information should be presented in plain language for families in all encounters,31 these evidence-based practices are of particular importance when communicating with LEP families. They promote effective communication, empower families to share concerns in a structured manner, and allow medical providers to address matters in real-time with interpreters present.
Finally, our participants highlighted the need for partnerships between providers and interpreter services, noting unclear roles and expectations among interpreters and medical providers as a major barrier. Specifically, physicians noted confusion regarding the scope of an interpreter’s practice. Participants from GLA sessions discussed the importance of a team-based approach and suggested implementing a “presession” prior to encounters with LEP patients and families. Presessions—a concept well accepted among interpreters and recommended by consensus-based practice guidelines—enable medical providers and interpreters to establish shared expectations about scope of practice, communication, interpretation style, time constraints, and medical context prior to patient encounters.32,33
There are several limitations to our study. First, individuals who chose to participate were likely highly motivated by their clinical experiences with LEP patients and invested in improving communication with LEP families. Second, the study is limited in generalizability, as it was conducted at a single academic institution in a Midwestern city. Despite regional variations in available resources as well as patient and workforce demographics, our findings regarding major themes are in agreement with previously published literature and further add to our understanding of ways to improve communication with this vulnerable population across the care spectrum. Lastly, we were logistically limited in our ability to elicit the perspectives of LEP families due to the participatory nature of GLA; the need for multiple interpreters to simultaneously interact with LEP individuals would have not only hindered active LEP family participation but may have also biased the data generated by patients and families, as the services interpreters provide during their inpatient stay was the focus of our study. Engaging LEP families in their preferred language using participatory methods should be considered for future studies.
In conclusion, frontline providers of medical and language services identified barriers and drivers impacting the effective use of interpreter services when communicating with LEP families during hospitalization. Our enhanced understanding of barriers and drivers, as well as identified actionable interventions, will inform future improvement of communication and interactions with LEP families that contributes to effective and efficient family centered care. A framework for the development and implementation of organizational strategies aimed at improving communication with LEP families must include a thorough assessment of impact, feasibility, stakeholder involvement, and sustainability of specific interventions. While there is no simple formula to improve language services, health systems should establish and adopt language access policies, standardize communication practices, and develop processes to optimize the use of language services in the hospital. Furthermore, engagement with LEP families to better understand their perceptions and experiences with the healthcare system is crucial to improve communication between medical providers and LEP families in the inpatient setting and should be the subject of future studies.
Disclosures
The authors have no conflicts of interest to disclose.
Funding
No external funding was secured for this study. Dr. Joanna Thomson is supported by the Agency for Healthcare Research and Quality (Grant #K08 HS025138). Dr. Raglin Bignall was supported through a Ruth L. Kirschstein National Research Service Award (T32HP10027) when the study was conducted. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations. The funding organizations had no role in the design, preparation, review, or approval of this paper.
Immigrant children make up the fastest growing segment of the population in the United States.1 While most immigrant children are fluent in English, approximately 40% live with a parent who has limited English proficiency (LEP; ie, speaks English less than “very well”).2,3 In pediatrics, LEP status has been associated with longer hospitalizations,4 higher hospitalization costs,5 increased risk for serious adverse medical events,4,6 and more frequent emergency department reutilization.7 In the inpatient setting, multiple aspects of care present a variety of communication challenges,8 which are amplified by shift work and workflow complexity that result in patients and families interacting with numerous providers over the course of an inpatient stay.
Increasing access to trained professional interpreters when caring for LEP patients improves communication, patient satisfaction, adherence, and mortality.9-12 However, even when access to interpreter services is established, effective use is not guaranteed.13 Up to 57% of pediatricians report relying on family members to communicate with LEP patients and their caregivers;9 23% of pediatric residents categorized LEP encounters as frustrating while 78% perceived care of LEP patients to be “misdirected” (eg, delay in diagnosis or discharge) because of associated language barriers.14
Understanding experiences of frontline inpatient medical providers and interpreters is crucial in identifying challenges and ways to optimize communication for hospitalized LEP patients and families. However, there is a paucity of literature exploring the perspectives of medical providers and interpreters as it relates to communication with hospitalized LEP children and families. In this study, we sought to identify barriers and drivers of effective communication with pediatric patients and families with LEP in the inpatient setting from the perspective of frontline medical providers and interpreters.
METHODS
Study Design
This qualitative study used Group Level Assessment (GLA), a structured participatory methodology that allows diverse groups of stakeholders to generate and evaluate data in interactive sessions.15-18 GLA structure promotes active participation, group problem-solving, and development of actionable plans, distinguishing it from focus groups and in-depth semistructured interviews.15,19 This study received a human subject research exemption by the institutional review board.
Study Setting
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large quaternary care center with ~200 patient encounters each day who require the use of interpreter services. Interpreters (in-person, video, and phone) are utilized during admission, formal family-centered rounds, hospital discharge, and other encounters with physicians, nurses, and other healthcare professionals. In-person interpreters are available in-house for Spanish and Arabic, with 18 additional languages available through regional vendors. Despite available resources, there is no standard way in which medical providers and interpreters work with one another.
Study Participants and Recruitment
Medical providers who care for hospitalized general pediatric patients were eligible to participate, including attending physicians, resident physicians, bedside nurses, and inpatient ancillary staff (eg, respiratory therapists, physical therapists). Interpreters employed by CCHMC with experience in the inpatient setting were also eligible. Individuals were recruited based on published recommendations to optimize discussion and group-thinking.15 Each participant was asked to take part in one GLA session. Participants were assigned to specific sessions based on roles (ie, physicians, nurses, and interpreters) to maximize engagement and minimize the impact of hierarchy.
Study Procedure
GLA involves a seven-step structured process (Appendix 1): climate setting, generating, appreciating, reflecting, understanding, selecting, and action.15,18 Qualitative data were generated individually and anonymously by participants on flip charts in response to prompts such as: “I worry that LEP families___,” “The biggest challenge when using interpreter services is___,” and “I find___ works well in providing care for LEP families.” Prompts were developed by study investigators, modified based on input from nursing and interpreter services leadership, and finalized by GLA facilitators. Fifty-one unique prompts were utilized (Appendix 2); the number of prompts used (ranging from 15 to 32 prompts) per session was based on published recommendations.15 During sessions, study investigators took detailed notes, including verbatim transcription of participant quotes. Upon conclusion of the session, each participant completed a demographic survey, including years of experience, languages spoken and perceived fluency,20 and ethnicity.
Data Analysis
Within each session, under the guidance of trained and experienced GLA facilitators (WB, HV), participants distilled and summarized qualitative data into themes, discussed and prioritized themes, and generated action items. Following completion of all sessions, analyzed data was compiled by the research team to determine similarities and differences across groups based on participant roles, consolidate themes into barriers and drivers of communication with LEP families, and determine any overlap of priorities for action. Findings were shared back with each group to ensure accuracy and relevance.
RESULTS
Participants
A total of 64 individuals participated (Table 1): hospital medicine physicians and residents (56%), inpatient nurses and ancillary staff (16%), and interpreters (28%). While 81% of physicians spoke multiple languages, only 25% reported speaking them well; two physicians were certified to communicate medical information without an interpreter present.
Themes Resulting from GLA Sessions
A total of four barriers (Table 2) and four drivers (Table 3) of effective communication with pediatric LEP patients and their families in the inpatient setting were identified by participants. Participants across all groups, despite enthusiasm around improving communication, were concerned about quality of care LEP families received, noting that the system is “designed to deliver less-good care” and that “we really haven’t figured out how to care for [LEP patients and families] in a [high-]quality and reliable way.” Variation in theme discussion was noted between groups based on participant role: physicians voiced concern about rapport with LEP families, nurses emphasized actionable tasks, and interpreters focused on heightened challenges in times of stress.
Barrier 1: Difficulties Accessing Interpreter Services
Medical providers (physicians and nurses) identified the “opaque process to access [interpreter] services” as one of their biggest challenges when communicating with LEP families. In particular, the process of scheduling interpreters was described as a “black box,” with physicians and nurses expressing difficulty determining if and when in-person interpreters were scheduled and uncertainty about when to use modalities other than in-person interpretation. Participants across groups highlighted the lack of systems knowledge from medical providers and limitations within the system that make predictable, timely, and reliable access to interpreters challenging, especially for uncommon languages. Medical providers desired more in-person interpreters who can “stay as long as clinically indicated,” citing frustration associated with using phone- and video-interpretation (eg, challenges locating technology, unfamiliarity with use, unreliable functionality of equipment). Interpreters voiced wanting to take time to finish each encounter fully without “being in a hurry because the next appointment is coming soon” or “rushing… in [to the next] session sweating.”
Barrier 2: Uncertainty in Communication with LEP Families
Participants across all groups described three areas of uncertainty as detailed in Table 2: (1) what to share and how to prioritize information during encounters with LEP patients and families, (2) what is communicated during interpretation, and (3) what LEP patients and families understand.
Barrier 3: Unclear and Inconsistent Expectations and Roles of Team Members
Given the complexity involved in communication between medical providers, interpreters, and families, participants across all groups reported feeling ill-prepared when navigating hospital encounters with LEP patients and families. Interpreters reported having little to no clinical context, medical providers reported having no knowledge of the assigned interpreter’s style, and both interpreters and medical providers reported that families have little idea of what to expect or how to engage. All groups voiced frustration about the lack of clarity regarding specific roles and scope of practice for each team member during an encounter, where multiple people end up “talking [or] using the interpreter at once.” Interpreters shared their expectations of medical providers to set the pace and lead conversations with LEP families. On the other hand, medical providers expressed a desire for interpreters to provide cultural context to the team without prompting and to interrupt during encounters when necessary to voice concerns or redirect conversations.
Barrier 4: Unmet Family Engagement Expectations
Participants across all groups articulated challenges with establishing rapport with LEP patients and families, sharing concerns that “inadequate communication” due to “cultural or language barriers” ultimately impacts quality of care. Participants reported decreased bidirectional engagement with and from LEP families. Medical providers not only noted difficulty in connecting with LEP families “on a more personal level” and providing frequent medical updates, but also felt that LEP families do not ask questions even when uncertain. Interpreters expressed concerns about medical providers “not [having] enough patience to answer families’ questions” while LEP families “shy away from asking questions.”
Driver 1: Utilizing a Team-Based Approach between Medical Providers and Interpreters
Participants from all groups emphasized that a mutual understanding of roles and shared expectations regarding communication and interpretation style, clinical context, and time constraints would establish a foundation for respect between medical providers and interpreters. They reported that a team-based approach to LEP patient and family encounters were crucial to achieving effective communication.
Driver 2: Understanding the Role of Cultural Context in Providing Culturally Effective Care.
Participants across all groups highlighted three different aspects of cultural context that drive effective communication: (1) medical providers’ perception of the family’s culture; (2) LEP families’ knowledge about the culture and healthcare system in the US, and (3) medical providers insight into their own preconceived ideas about LEP families.
Driver 3: Practicing Empathy for Patients and Families
All participants reported that respect for diversity and consideration of the backgrounds and perspectives of LEP patients and families are necessary. Furthermore, both medical providers and interpreters articulated a need to remain patient and mindful when interacting with LEP families despite challenges, especially since, as noted by interpreters, encounters may “take longer, but it’s for a reason.”
Driver 4: Using Effective Family-Centered Communication Strategies
Participants identified the use of effective family-centered communication principles as a driver to optimal communication. Many of the principles identified by medical providers and interpreters are generally applicable to all hospitalized patients and families regardless of English proficiency: optimizing verbal communication (eg, using shorter sentences, pausing to allow for interpretation), optimizing nonverbal communication (eg, setting, position, and body language), and assessment of family understanding and engagement (eg, use of teach back).
DISCUSSION
Frontline medical providers and interpreters identified barriers and drivers that impact communication with LEP patients and families during hospitalization. To our knowledge, this is the first study that uses a participatory method to explore the perspectives of medical providers and interpreters who care for LEP children and families in the inpatient setting. Despite existing difficulties and concerns regarding language barriers and its impact on quality of care for hospitalized LEP patients and families, participants were enthusiastic about how identified barriers and drivers may inform future improvement efforts. Notable action steps for future improvement discussed by our participants included: increased use and functionality of technology for timely and predictable access to interpreters, deliberate training for providers focused on delivery of culturally-effective care, consistent use of family-centered communication strategies including teach-back, and implementing interdisciplinary expectation setting through “presessions” before encounters with LEP families.
Participants elaborated on several barriers previously described in the literature including time constraints and technical problems.14,21,22 Such barriers may serve as deterrents to consistent and appropriate use of interpreters in healthcare settings.9 A heavy reliance on off-site interpreters (including phone- or video-interpreters) and lack of knowledge regarding resource availability likely amplified frustration for medical providers. Communication with LEP families can be daunting, especially when medical providers do not care for LEP families or work with interpreters on a regular basis.14 Standardizing the education of medical providers regarding available resources, as well as the logistics, process, and parameters for scheduling interpreters and using technology, was an action step identified by our GLA participants. Targeted education about the logistics of accessing interpreter services and having standardized ways to make technology use easier (ie, one-touch dialing in hospital rooms) has been associated with increased interpreter use and decreased interpreter-related delays in care.23
Our frontline medical providers expressed added concern about not spending as much time with LEP families. In fact, LEP families in the literature have perceived medical providers to spend less time with their children compared to their English-proficient counterparts.24 Language and cultural barriers, both perceived and real, may limit medical provider rapport with LEP patients and families14 and likely contribute to medical providers relying on their preconceived assumptions instead.25 Cultural competency education for medical providers, as highlighted by our GLA participants as an action item, can be used to provide more comprehensive and effective care.26,27
In addition to enhancing cultural humility through education, our participants emphasized the use of family-centered communication strategies as a driver of optimal family engagement and understanding. Actively inviting questions from families and utilizing teach-back, an established evidence-based strategy28-30 discussed by our participants, can be particularly powerful in assessing family understanding and engagement. While information should be presented in plain language for families in all encounters,31 these evidence-based practices are of particular importance when communicating with LEP families. They promote effective communication, empower families to share concerns in a structured manner, and allow medical providers to address matters in real-time with interpreters present.
Finally, our participants highlighted the need for partnerships between providers and interpreter services, noting unclear roles and expectations among interpreters and medical providers as a major barrier. Specifically, physicians noted confusion regarding the scope of an interpreter’s practice. Participants from GLA sessions discussed the importance of a team-based approach and suggested implementing a “presession” prior to encounters with LEP patients and families. Presessions—a concept well accepted among interpreters and recommended by consensus-based practice guidelines—enable medical providers and interpreters to establish shared expectations about scope of practice, communication, interpretation style, time constraints, and medical context prior to patient encounters.32,33
There are several limitations to our study. First, individuals who chose to participate were likely highly motivated by their clinical experiences with LEP patients and invested in improving communication with LEP families. Second, the study is limited in generalizability, as it was conducted at a single academic institution in a Midwestern city. Despite regional variations in available resources as well as patient and workforce demographics, our findings regarding major themes are in agreement with previously published literature and further add to our understanding of ways to improve communication with this vulnerable population across the care spectrum. Lastly, we were logistically limited in our ability to elicit the perspectives of LEP families due to the participatory nature of GLA; the need for multiple interpreters to simultaneously interact with LEP individuals would have not only hindered active LEP family participation but may have also biased the data generated by patients and families, as the services interpreters provide during their inpatient stay was the focus of our study. Engaging LEP families in their preferred language using participatory methods should be considered for future studies.
In conclusion, frontline providers of medical and language services identified barriers and drivers impacting the effective use of interpreter services when communicating with LEP families during hospitalization. Our enhanced understanding of barriers and drivers, as well as identified actionable interventions, will inform future improvement of communication and interactions with LEP families that contributes to effective and efficient family centered care. A framework for the development and implementation of organizational strategies aimed at improving communication with LEP families must include a thorough assessment of impact, feasibility, stakeholder involvement, and sustainability of specific interventions. While there is no simple formula to improve language services, health systems should establish and adopt language access policies, standardize communication practices, and develop processes to optimize the use of language services in the hospital. Furthermore, engagement with LEP families to better understand their perceptions and experiences with the healthcare system is crucial to improve communication between medical providers and LEP families in the inpatient setting and should be the subject of future studies.
Disclosures
The authors have no conflicts of interest to disclose.
Funding
No external funding was secured for this study. Dr. Joanna Thomson is supported by the Agency for Healthcare Research and Quality (Grant #K08 HS025138). Dr. Raglin Bignall was supported through a Ruth L. Kirschstein National Research Service Award (T32HP10027) when the study was conducted. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations. The funding organizations had no role in the design, preparation, review, or approval of this paper.
1. The American Academy of Pediatrics Council on Community Pediatrics. Providing care for immigrant, migrant, and border children. Pediatrics. 2013;131(6):e2028-e2034. PubMed
2. Meneses C, Chilton L, Duffee J, et al. Council on Community Pediatrics Immigrant Health Tool Kit. The American Academy of Pediatrics. https://www.aap.org/en-us/Documents/cocp_toolkit_full.pdf. Accessed May 13, 2019.
3. Office for Civil Rights. Guidance to Federal Financial Assistance Recipients Regarding Title VI and the Prohibition Against National Origin Discrimination Affecting Limited English Proficient Persons. https://www.hhs.gov/civil-rights/for-individuals/special-topics/limited-english-proficiency/guidance-federal-financial-assistance-recipients-title-vi/index.html. Accessed May 13, 2019.
4. Lion KC, Rafton SA, Shafii J, et al. Association between language, serious adverse events, and length of stay Among hospitalized children. Hosp Pediatr. 2013;3(3):219-225. https://doi.org/10.1542/hpeds.2012-0091.
5. Lion KC, Wright DR, Desai AD, Mangione-Smith R. Costs of care for hospitalized children associated With preferred language and insurance type. Hosp Pediatr. 2017;7(2):70-78. https://doi.org/10.1542/hpeds.2016-0051.
6. Cohen AL, Rivara F, Marcuse EK, McPhillips H, Davis R. Are language barriers associated with serious medical events in hospitalized pediatric patients? Pediatrics. 2005;116(3):575-579. https://doi.org/10.1542/peds.2005-0521.
7. Samuels-Kalow ME, Stack AM, Amico K, Porter SC. Parental language and return visits to the Emergency Department After discharge. Pediatr Emerg Care. 2017;33(6):402-404. https://doi.org/10.1097/PEC.0000000000000592.
8. Unaka NI, Statile AM, Choe A, Shonna Yin H. Addressing health literacy in the inpatient setting. Curr Treat Options Pediatr. 2018;4(2):283-299. https://doi.org/10.1007/s40746-018-0122-3.
9. DeCamp LR, Kuo DZ, Flores G, O’Connor K, Minkovitz CS. Changes in language services use by US pediatricians. Pediatrics. 2013;132(2):e396-e406. https://doi.org/10.1542/peds.2012-2909.
10. Flores G. The impact of medical interpreter services on the quality of health care: A systematic review. Med Care Res Rev. 2005;62(3):255-299. https://doi.org/10.1177/1077558705275416.
11. Flores G, Abreu M, Barone CP, Bachur R, Lin H. Errors of medical interpretation and their potential clinical consequences: A comparison of professional versus hoc versus no interpreters. Ann Emerg Med. 2012;60(5):545-553. https://doi.org/10.1016/j.annemergmed.2012.01.025.
12. Anand KJ, Sepanski RJ, Giles K, Shah SH, Juarez PD. Pediatric intensive care unit mortality among Latino children before and after a multilevel health care delivery intervention. JAMA Pediatr. 2015;169(4):383-390. https://doi.org/10.1001/jamapediatrics.2014.3789.
13. The Joint Commission. Advancing Effective Communication, Cultural Competence, and Patient- and Family-Centered Care: A Roadmap for Hospitals. Oakbrook Terrace, IL: The Joint Commission; 2010.
14. Hernandez RG, Cowden JD, Moon M et al. Predictors of resident satisfaction in caring for limited English proficient families: a multisite study. Acad Pediatr. 2014;14(2):173-180. https://doi.org/10.1016/j.acap.2013.12.002.
15. Vaughn LM, Lohmueller M. Calling all stakeholders: group-level assessment (GLA)-a qualitative and participatory method for large groups. Eval Rev. 2014;38(4):336-355. https://doi.org/10.1177/0193841X14544903.
16. Vaughn LM, Jacquez F, Zhao J, Lang M. Partnering with students to explore the health needs of an ethnically diverse, low-resource school: an innovative large group assessment approach. Fam Commun Health. 2011;34(1):72-84. https://doi.org/10.1097/FCH.0b013e3181fded12.
17. Gosdin CH, Vaughn L. Perceptions of physician bedside handoff with nurse and family involvement. Hosp Pediatr. 2012;2(1):34-38. https://doi.org/10.1542/hpeds.2011-0008-2.
18. Graham KE, Schellinger AR, Vaughn LM. Developing strategies for positive change: transitioning foster youth to adulthood. Child Youth Serv Rev. 2015;54:71-79. https://doi.org/10.1016/j.childyouth.2015.04.014.
19. Vaughn LM. Group level assessment: A Large Group Method for Identifying Primary Issues and Needs within a community. London2014. http://methods.sagepub.com/case/group-level-assessment-large-group-primary-issues-needs-community. Accessed 2017/07/26.
20. Association of American Medical Colleges Electronic Residency Application Service. ERAS 2018 MyERAS Application Worksheet: Language Fluency. Washington, DC:: Association of American Medical Colleges; 2018:5.
21. Brisset C, Leanza Y, Laforest K. Working with interpreters in health care: A systematic review and meta-ethnography of qualitative studies. Patient Educ Couns. 2013;91(2):131-140. https://doi.org/10.1016/j.pec.2012.11.008.
22. Wiking E, Saleh-Stattin N, Johansson SE, Sundquist J. A description of some aspects of the triangular meeting between immigrant patients, their interpreters and GPs in primary health care in Stockholm, Sweden. Fam Pract. 2009;26(5):377-383. https://doi.org/10.1093/fampra/cmp052.
23. Lion KC, Ebel BE, Rafton S et al. Evaluation of a quality improvement intervention to increase use of telephonic interpretation. Pediatrics. 2015;135(3):e709-e716. https://doi.org/10.1542/peds.2014-2024.
24. Zurca AD, Fisher KR, Flor RJ, et al. Communication with limited English-proficient families in the PICU. Hosp Pediatr. 2017;7(1):9-15. https://doi.org/10.1542/hpeds.2016-0071.
25. Kodjo C. Cultural competence in clinician communication. Pediatr Rev. 2009;30(2):57-64. https://doi.org/10.1542/pir.30-2-57.
26. Britton CV, American Academy of Pediatrics Committee on Pediatric Workforce. Ensuring culturally effective pediatric care: implications for education and health policy. Pediatrics. 2004;114(6):1677-1685. https://doi.org/10.1542/peds.2004-2091.
27. The American Academy of Pediatrics. Culturally Effective Care Toolkit: Providing Cuturally Effective Pediatric Care; 2018. https://www.aap.org/en-us/professional-resources/practice-transformation/managing-patients/Pages/effective-care.aspx. Accessed May 13, 2019.
28. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
29. Jager AJ, Wynia MK. Who gets a teach-back? Patient-reported incidence of experiencing a teach-back. J Health Commun. 2012;17 Supplement 3:294-302. https://doi.org/10.1080/10810730.2012.712624.
30. Kornburger C, Gibson C, Sadowski S, Maletta K, Klingbeil C. Using “teach-back” to promote a safe transition from hospital to home: an evidence-based approach to improving the discharge process. J Pediatr Nurs. 2013;28(3):282-291. https://doi.org/10.1016/j.pedn.2012.10.007.
31. Abrams MA, Klass P, Dreyer BP. Health literacy and children: recommendations for action. Pediatrics. 2009;124 Supplement 3:S327-S331. https://doi.org/10.1542/peds.2009-1162I.
32. Betancourt JR, Renfrew MR, Green AR, Lopez L, Wasserman M. Improving Patient Safety Systems for Patients with Limited English Proficiency: a Guide for Hospitals. Agency for Healthcare Research and Quality; 2012. 33. The National Council on Interpreting in Health Care. Best Practices for Communicating Through an Interpreter . https://refugeehealthta.org/access-to-care/language-access/best-practices-communicating-through-an-interpreter/. Accessed May 19, 2019.
33. The National Council on Interpreting in Health Care. Best Practices for Communicating Through an Interpreter . https://refugeehealthta.org/access-to-care/language-access/best-practices-communicating-through-an-interpreter/. Accessed May 19, 2019.
1. The American Academy of Pediatrics Council on Community Pediatrics. Providing care for immigrant, migrant, and border children. Pediatrics. 2013;131(6):e2028-e2034. PubMed
2. Meneses C, Chilton L, Duffee J, et al. Council on Community Pediatrics Immigrant Health Tool Kit. The American Academy of Pediatrics. https://www.aap.org/en-us/Documents/cocp_toolkit_full.pdf. Accessed May 13, 2019.
3. Office for Civil Rights. Guidance to Federal Financial Assistance Recipients Regarding Title VI and the Prohibition Against National Origin Discrimination Affecting Limited English Proficient Persons. https://www.hhs.gov/civil-rights/for-individuals/special-topics/limited-english-proficiency/guidance-federal-financial-assistance-recipients-title-vi/index.html. Accessed May 13, 2019.
4. Lion KC, Rafton SA, Shafii J, et al. Association between language, serious adverse events, and length of stay Among hospitalized children. Hosp Pediatr. 2013;3(3):219-225. https://doi.org/10.1542/hpeds.2012-0091.
5. Lion KC, Wright DR, Desai AD, Mangione-Smith R. Costs of care for hospitalized children associated With preferred language and insurance type. Hosp Pediatr. 2017;7(2):70-78. https://doi.org/10.1542/hpeds.2016-0051.
6. Cohen AL, Rivara F, Marcuse EK, McPhillips H, Davis R. Are language barriers associated with serious medical events in hospitalized pediatric patients? Pediatrics. 2005;116(3):575-579. https://doi.org/10.1542/peds.2005-0521.
7. Samuels-Kalow ME, Stack AM, Amico K, Porter SC. Parental language and return visits to the Emergency Department After discharge. Pediatr Emerg Care. 2017;33(6):402-404. https://doi.org/10.1097/PEC.0000000000000592.
8. Unaka NI, Statile AM, Choe A, Shonna Yin H. Addressing health literacy in the inpatient setting. Curr Treat Options Pediatr. 2018;4(2):283-299. https://doi.org/10.1007/s40746-018-0122-3.
9. DeCamp LR, Kuo DZ, Flores G, O’Connor K, Minkovitz CS. Changes in language services use by US pediatricians. Pediatrics. 2013;132(2):e396-e406. https://doi.org/10.1542/peds.2012-2909.
10. Flores G. The impact of medical interpreter services on the quality of health care: A systematic review. Med Care Res Rev. 2005;62(3):255-299. https://doi.org/10.1177/1077558705275416.
11. Flores G, Abreu M, Barone CP, Bachur R, Lin H. Errors of medical interpretation and their potential clinical consequences: A comparison of professional versus hoc versus no interpreters. Ann Emerg Med. 2012;60(5):545-553. https://doi.org/10.1016/j.annemergmed.2012.01.025.
12. Anand KJ, Sepanski RJ, Giles K, Shah SH, Juarez PD. Pediatric intensive care unit mortality among Latino children before and after a multilevel health care delivery intervention. JAMA Pediatr. 2015;169(4):383-390. https://doi.org/10.1001/jamapediatrics.2014.3789.
13. The Joint Commission. Advancing Effective Communication, Cultural Competence, and Patient- and Family-Centered Care: A Roadmap for Hospitals. Oakbrook Terrace, IL: The Joint Commission; 2010.
14. Hernandez RG, Cowden JD, Moon M et al. Predictors of resident satisfaction in caring for limited English proficient families: a multisite study. Acad Pediatr. 2014;14(2):173-180. https://doi.org/10.1016/j.acap.2013.12.002.
15. Vaughn LM, Lohmueller M. Calling all stakeholders: group-level assessment (GLA)-a qualitative and participatory method for large groups. Eval Rev. 2014;38(4):336-355. https://doi.org/10.1177/0193841X14544903.
16. Vaughn LM, Jacquez F, Zhao J, Lang M. Partnering with students to explore the health needs of an ethnically diverse, low-resource school: an innovative large group assessment approach. Fam Commun Health. 2011;34(1):72-84. https://doi.org/10.1097/FCH.0b013e3181fded12.
17. Gosdin CH, Vaughn L. Perceptions of physician bedside handoff with nurse and family involvement. Hosp Pediatr. 2012;2(1):34-38. https://doi.org/10.1542/hpeds.2011-0008-2.
18. Graham KE, Schellinger AR, Vaughn LM. Developing strategies for positive change: transitioning foster youth to adulthood. Child Youth Serv Rev. 2015;54:71-79. https://doi.org/10.1016/j.childyouth.2015.04.014.
19. Vaughn LM. Group level assessment: A Large Group Method for Identifying Primary Issues and Needs within a community. London2014. http://methods.sagepub.com/case/group-level-assessment-large-group-primary-issues-needs-community. Accessed 2017/07/26.
20. Association of American Medical Colleges Electronic Residency Application Service. ERAS 2018 MyERAS Application Worksheet: Language Fluency. Washington, DC:: Association of American Medical Colleges; 2018:5.
21. Brisset C, Leanza Y, Laforest K. Working with interpreters in health care: A systematic review and meta-ethnography of qualitative studies. Patient Educ Couns. 2013;91(2):131-140. https://doi.org/10.1016/j.pec.2012.11.008.
22. Wiking E, Saleh-Stattin N, Johansson SE, Sundquist J. A description of some aspects of the triangular meeting between immigrant patients, their interpreters and GPs in primary health care in Stockholm, Sweden. Fam Pract. 2009;26(5):377-383. https://doi.org/10.1093/fampra/cmp052.
23. Lion KC, Ebel BE, Rafton S et al. Evaluation of a quality improvement intervention to increase use of telephonic interpretation. Pediatrics. 2015;135(3):e709-e716. https://doi.org/10.1542/peds.2014-2024.
24. Zurca AD, Fisher KR, Flor RJ, et al. Communication with limited English-proficient families in the PICU. Hosp Pediatr. 2017;7(1):9-15. https://doi.org/10.1542/hpeds.2016-0071.
25. Kodjo C. Cultural competence in clinician communication. Pediatr Rev. 2009;30(2):57-64. https://doi.org/10.1542/pir.30-2-57.
26. Britton CV, American Academy of Pediatrics Committee on Pediatric Workforce. Ensuring culturally effective pediatric care: implications for education and health policy. Pediatrics. 2004;114(6):1677-1685. https://doi.org/10.1542/peds.2004-2091.
27. The American Academy of Pediatrics. Culturally Effective Care Toolkit: Providing Cuturally Effective Pediatric Care; 2018. https://www.aap.org/en-us/professional-resources/practice-transformation/managing-patients/Pages/effective-care.aspx. Accessed May 13, 2019.
28. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
29. Jager AJ, Wynia MK. Who gets a teach-back? Patient-reported incidence of experiencing a teach-back. J Health Commun. 2012;17 Supplement 3:294-302. https://doi.org/10.1080/10810730.2012.712624.
30. Kornburger C, Gibson C, Sadowski S, Maletta K, Klingbeil C. Using “teach-back” to promote a safe transition from hospital to home: an evidence-based approach to improving the discharge process. J Pediatr Nurs. 2013;28(3):282-291. https://doi.org/10.1016/j.pedn.2012.10.007.
31. Abrams MA, Klass P, Dreyer BP. Health literacy and children: recommendations for action. Pediatrics. 2009;124 Supplement 3:S327-S331. https://doi.org/10.1542/peds.2009-1162I.
32. Betancourt JR, Renfrew MR, Green AR, Lopez L, Wasserman M. Improving Patient Safety Systems for Patients with Limited English Proficiency: a Guide for Hospitals. Agency for Healthcare Research and Quality; 2012. 33. The National Council on Interpreting in Health Care. Best Practices for Communicating Through an Interpreter . https://refugeehealthta.org/access-to-care/language-access/best-practices-communicating-through-an-interpreter/. Accessed May 19, 2019.
33. The National Council on Interpreting in Health Care. Best Practices for Communicating Through an Interpreter . https://refugeehealthta.org/access-to-care/language-access/best-practices-communicating-through-an-interpreter/. Accessed May 19, 2019.
© 2019 Society of Hospital Medicine