User login
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Synthetic hair extensions are made from various plastic polymers (eg, modacrylic, vinyl chloride, and acrylonitrile) shaped into thin strands that mimic human hair and are used to add fullness, length, and manageability in individuals with textured hair.1-3 The plastic polymers used to make synthetic hair, most notably acrylonitrile and vinyl chloride, are known to be toxic to humans.1-4 The US Environmental Protection Agency classifies acrylonitrile as a probable carcinogen, and vinyl chloride is associated with the development of lymphoma; leukemia; and rare malignancies of the brain, liver, and lungs.1,4 According to the Occupational Safety and Health Administration, the maximum exposure limits of vinyl chloride and acrylonitrile vapor or gas over an 8-hour period are 1 ppm (0.001 g/L) and 2 ppm (0.002 g/L), respectively.5 Exposure levels from wearing synthetic hair extensions easily exceed these maximums; for example, a full head of braids requires application of multiple packets of synthetic hair, resulting in continuous exposure to carcinogenic materials that can last for weeks to months at a time.1 Furthermore, individuals as young as 3 years old can begin to style their hair with synthetic extensions, which not only leads to potentially harmful carcinogenic exposure in young children but also yields notably high levels of lifetime exposure in individuals who regularly style their hair with these products.
There currently are no regulations barring the use of potentially harmful materials from the manufacturing process for synthetic hair extensions.1 As a result, rinsing with apple cider vinegar (ACV) is a popular remedy that many users claim can effectively remove harmful chemicals from synthetic hair.6,7 As this is the only known remedy that aims to address this issue,
Methods
We conducted a search of Google Scholar, JSTOR, Science Direct, the Public Library of Science, and PubMed articles indexed for MEDLINE using the terms ACV, apple cider vinegar rinse, ACV rinse, synthetic hair carcinogens, synthetic fiber carcinogens, synthetic hair extension carcinogens, modacrylic fibers, Kanekalon (a flame-retardant modacrylic fiber), acrylonitrile, and vinyl chloride fibers to identify primary research articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions for inclusion in our review. To broaden our search, we did not establish a time frame for publication of the articles included in the study. Articles investigating the ACV rinse that were unrelated to carcinogenicity and synthetic hair extensions were excluded from this study.
Results
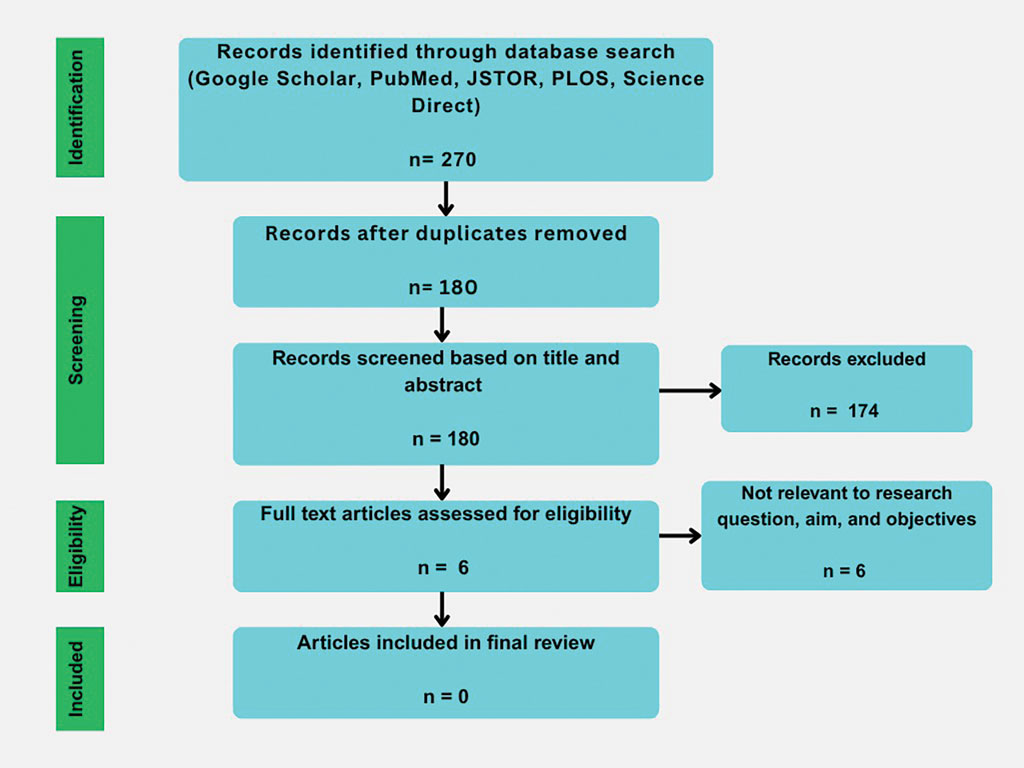
Our initial literature search identified 270 articles, which decreased to 180 after removal of duplicates. These 180 articles were screened for relevance based on title and abstract, which yielded 6 articles. None of the 6 articles identified through our literature search discussed synthetic hair and carcinogenicity in the context of the ACV rinse and were subsequently excluded from our review (eFigure 1).
Comment
Potentially harmful chemicals and ingredients in hair care products marketed for textured hair are now established topics in public discourse among those familiar with textured hair care and maintenance1,8; however, the discourse remains limited. Our search for scientific articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions revealed a notable deficit in the literature regarding scientific studies assessing this practice. While the likelihood that the ACV rinse effectively alters the carcinogenicity of plastic polymers found in synthetic hair extensions and improves their safety seems improbable, the deficit of empirical data evaluating this practice is concerning given both the prevalence of this remedy and the sizable demographic of patients who practice styling with synthetic hair.1 Of the potential adverse outcomes (eg, contact dermatitis, traction alopecia) that are possible from styling with synthetic hair that have been reported in the literature, carcinogenic exposure from synthetic hair extensions is relatively absent, with the exception of a few publications,2,3,9 despite its potential to cause serious long-term consequences for hair stylists and those who regularly use these products.
Interestingly, individuals who style their hair with synthetic hair extensions frequently tout the efficacy of the ACV rinse for removal of mostly unidentified irritants, although the effects are unverified.6,7 While the ACV rinse may be an effective means of removing toxic chemicals from synthetic hair extensions, without verifiable data this method remains an unproven remedy whose perceived benefits could result from factors unrelated to the rinse itself. Theoretically, simply rinsing synthetic hair extensions with plain water prior to use may demonstrate similar efficacy to that of the ACV rinse.
An additional factor worth mentioning is the lack of government regulations concerning the manufacturing practices of synthetic hair extensions. Flame-retardant materials such as trichloroethylene, polyvinyl chloride, and hexabromocyclododecane frequently are used in synthetic hair extensions despite their known adverse effects, which include reproductive organ toxicity and links to various cancers, leading to them being banned in 5 states.1,10-12 With no federal ban on these materials, individuals using synthetic hair remain at risk.
It is unclear what chemicals, irritants, or toxic substances the ACV rinse method could potentially remove from synthetic hair. In general, manufacturers of synthetic hair extensions are not forthcoming with information regarding materials used in the processing of their products despite public inquiries into their manufacturing practices.6 Although Whitehurst’s3 curriculum details the process of making synthetic polymer fibers, the overall processes by which these plastics are made to resemble human hair have not been reviewed in academic publications. Should this information be made available to the public, consumers could potentially avoid specific irritants when purchasing synthetic hair extensions.
The most common management strategy observed in the literature for adverse outcomes attributable to synthetic hair is discontinuation of use2; however, the prevalence and cultural significance of styling with synthetic hair extensions, along with the convenience these styles offer, make this option suboptimal. The scarcity of publications concerning the management of adverse outcomes related to the use of synthetic hair extensions may explain the absence of alternative management recommendations in the literature. Notably, new synthetic hair extensions from manufacturers that exclude plastic polymers and other harmful additives are now available to the public13; however, these hair extensions are cost prohibitive and are less accessible compared to synthetic extensions made from modacrylic fibers (eFigures 2 and 3).1,13-16


Final Thoughts
- Thomas CG. Carcinogenic materials in synthetic braids: an unrecognized risk of hair products for Black women. Lancet Reg Health Am. 2023;22:100517.
- Dlova NC, Ferguson NN, Rorex JN, et al. Synthetic hair extensions causing irritant contact dermatitis in patients with a history of atopy: a report of 10 cases. Contact Dermatitis. 2021;85:141-145.
- Whitehurst L. Polytails and urban tumble weaves: the chemistry of synthetic hair fibers. Yale National Initiative. September 2011. Accessed September 29, 2025. teachers.yale.edu/curriculum/viewer/initiative_11.05.10_u
- Acrylonitrile. U.S. Environmental Protection Agency. April 1992. Updated January 2000. Accessed September 29, 2025. www.epa.gov/sites/default/files/2016-09/documents/acrylonitrile.pdf
- Permissible exposure limits – annotated tables. OSHA annotated table Z-1. Occupational Safety and Health Administration. Accessed September 29, 2025. www.osha.gov/annotated-pels/table-z-1
- Adesina P. Braids are causing unbearable itching & there’s a sinister reason behind it. Refinery29. August 19, 2019. Accessed September 29, 2025. www.refinery29.com/en-gb/itchy-braids-hair
- Boakye O. Here’s why you should always wash plastic synthetic braiding extensions. InStyle. February 27, 2023. Accessed September 29, 2025. https://www.instyle.com/synthetic-braiding-extensions-upkeep-7151722
- James-Todd T, Connolly L, Preston EV, et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J Expo Sci Environ Epidemiol. 2021;31:476-486.
- Ijere ND, Okereke JN, Ezeji EU. Potential hazards associated with wearing of synthetic hairs (wigs, weavons, hair extensions/attachments) in Nigeria. J Environ Sci Public Health. 2022;6:299-313.
- Kaminsky T. An act to amend the environmental conservation law, in relation to the regulation of chemicals in upholstered furniture, mattresses and electronic enclosures. S4630B (2021). Accessed October 2, 2025. www.nysenate.gov/legislation/bills/2021/S4630
- Shen Y. Hair extension standards and regulations in the US: an overview. Compliance Gate. December 20, 2022. Accessed September 29, 2025. www.compliancegate.com/hair-extension-regulations-united-states/
- Lienke J, Rothschild R. Regulating Risk From Toxic Substances: Best Practices for Economic Analysis of Risk Management Options Under the Toxic Substances Control Act. Institute of Policy Integrity; 2021.
- Rebundle. Accessed October 2, 2025. https://rebundle.co/
- About us. Kanekalon. Accessed October 2, 2025. https://www.kanekalon-hair.com/en/about
- Julianna wholesale smooth Kanekalon futura natural fiber heat resistant bone straight synthetic bundle weft hair extensions. Accessed October 2, 2025. https://www.alibaba.com/product-detail/Julianna-wholesale-Smooth-Kanekalon-Futura-Natural_1601335996748.html
- AIDUSA solid colors braiding hair 5pcs synthetic Afro braid hair extensions 24 inch 1 tone for women braids twist crochet braids 100g(#1B Natural Black). Accessed October 2, 2025. www.amazon.com/AIDUSA-Braiding-Synthetic-Extensions-Crochet/dp/B09TNB9LC8
Synthetic hair extensions are made from various plastic polymers (eg, modacrylic, vinyl chloride, and acrylonitrile) shaped into thin strands that mimic human hair and are used to add fullness, length, and manageability in individuals with textured hair.1-3 The plastic polymers used to make synthetic hair, most notably acrylonitrile and vinyl chloride, are known to be toxic to humans.1-4 The US Environmental Protection Agency classifies acrylonitrile as a probable carcinogen, and vinyl chloride is associated with the development of lymphoma; leukemia; and rare malignancies of the brain, liver, and lungs.1,4 According to the Occupational Safety and Health Administration, the maximum exposure limits of vinyl chloride and acrylonitrile vapor or gas over an 8-hour period are 1 ppm (0.001 g/L) and 2 ppm (0.002 g/L), respectively.5 Exposure levels from wearing synthetic hair extensions easily exceed these maximums; for example, a full head of braids requires application of multiple packets of synthetic hair, resulting in continuous exposure to carcinogenic materials that can last for weeks to months at a time.1 Furthermore, individuals as young as 3 years old can begin to style their hair with synthetic extensions, which not only leads to potentially harmful carcinogenic exposure in young children but also yields notably high levels of lifetime exposure in individuals who regularly style their hair with these products.
There currently are no regulations barring the use of potentially harmful materials from the manufacturing process for synthetic hair extensions.1 As a result, rinsing with apple cider vinegar (ACV) is a popular remedy that many users claim can effectively remove harmful chemicals from synthetic hair.6,7 As this is the only known remedy that aims to address this issue,
Methods
We conducted a search of Google Scholar, JSTOR, Science Direct, the Public Library of Science, and PubMed articles indexed for MEDLINE using the terms ACV, apple cider vinegar rinse, ACV rinse, synthetic hair carcinogens, synthetic fiber carcinogens, synthetic hair extension carcinogens, modacrylic fibers, Kanekalon (a flame-retardant modacrylic fiber), acrylonitrile, and vinyl chloride fibers to identify primary research articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions for inclusion in our review. To broaden our search, we did not establish a time frame for publication of the articles included in the study. Articles investigating the ACV rinse that were unrelated to carcinogenicity and synthetic hair extensions were excluded from this study.
Results
Our initial literature search identified 270 articles, which decreased to 180 after removal of duplicates. These 180 articles were screened for relevance based on title and abstract, which yielded 6 articles. None of the 6 articles identified through our literature search discussed synthetic hair and carcinogenicity in the context of the ACV rinse and were subsequently excluded from our review (eFigure 1).

Comment
Potentially harmful chemicals and ingredients in hair care products marketed for textured hair are now established topics in public discourse among those familiar with textured hair care and maintenance1,8; however, the discourse remains limited. Our search for scientific articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions revealed a notable deficit in the literature regarding scientific studies assessing this practice. While the likelihood that the ACV rinse effectively alters the carcinogenicity of plastic polymers found in synthetic hair extensions and improves their safety seems improbable, the deficit of empirical data evaluating this practice is concerning given both the prevalence of this remedy and the sizable demographic of patients who practice styling with synthetic hair.1 Of the potential adverse outcomes (eg, contact dermatitis, traction alopecia) that are possible from styling with synthetic hair that have been reported in the literature, carcinogenic exposure from synthetic hair extensions is relatively absent, with the exception of a few publications,2,3,9 despite its potential to cause serious long-term consequences for hair stylists and those who regularly use these products.
Interestingly, individuals who style their hair with synthetic hair extensions frequently tout the efficacy of the ACV rinse for removal of mostly unidentified irritants, although the effects are unverified.6,7 While the ACV rinse may be an effective means of removing toxic chemicals from synthetic hair extensions, without verifiable data this method remains an unproven remedy whose perceived benefits could result from factors unrelated to the rinse itself. Theoretically, simply rinsing synthetic hair extensions with plain water prior to use may demonstrate similar efficacy to that of the ACV rinse.
An additional factor worth mentioning is the lack of government regulations concerning the manufacturing practices of synthetic hair extensions. Flame-retardant materials such as trichloroethylene, polyvinyl chloride, and hexabromocyclododecane frequently are used in synthetic hair extensions despite their known adverse effects, which include reproductive organ toxicity and links to various cancers, leading to them being banned in 5 states.1,10-12 With no federal ban on these materials, individuals using synthetic hair remain at risk.
It is unclear what chemicals, irritants, or toxic substances the ACV rinse method could potentially remove from synthetic hair. In general, manufacturers of synthetic hair extensions are not forthcoming with information regarding materials used in the processing of their products despite public inquiries into their manufacturing practices.6 Although Whitehurst’s3 curriculum details the process of making synthetic polymer fibers, the overall processes by which these plastics are made to resemble human hair have not been reviewed in academic publications. Should this information be made available to the public, consumers could potentially avoid specific irritants when purchasing synthetic hair extensions.
The most common management strategy observed in the literature for adverse outcomes attributable to synthetic hair is discontinuation of use2; however, the prevalence and cultural significance of styling with synthetic hair extensions, along with the convenience these styles offer, make this option suboptimal. The scarcity of publications concerning the management of adverse outcomes related to the use of synthetic hair extensions may explain the absence of alternative management recommendations in the literature. Notably, new synthetic hair extensions from manufacturers that exclude plastic polymers and other harmful additives are now available to the public13; however, these hair extensions are cost prohibitive and are less accessible compared to synthetic extensions made from modacrylic fibers (eFigures 2 and 3).1,13-16


Final Thoughts
Synthetic hair extensions are made from various plastic polymers (eg, modacrylic, vinyl chloride, and acrylonitrile) shaped into thin strands that mimic human hair and are used to add fullness, length, and manageability in individuals with textured hair.1-3 The plastic polymers used to make synthetic hair, most notably acrylonitrile and vinyl chloride, are known to be toxic to humans.1-4 The US Environmental Protection Agency classifies acrylonitrile as a probable carcinogen, and vinyl chloride is associated with the development of lymphoma; leukemia; and rare malignancies of the brain, liver, and lungs.1,4 According to the Occupational Safety and Health Administration, the maximum exposure limits of vinyl chloride and acrylonitrile vapor or gas over an 8-hour period are 1 ppm (0.001 g/L) and 2 ppm (0.002 g/L), respectively.5 Exposure levels from wearing synthetic hair extensions easily exceed these maximums; for example, a full head of braids requires application of multiple packets of synthetic hair, resulting in continuous exposure to carcinogenic materials that can last for weeks to months at a time.1 Furthermore, individuals as young as 3 years old can begin to style their hair with synthetic extensions, which not only leads to potentially harmful carcinogenic exposure in young children but also yields notably high levels of lifetime exposure in individuals who regularly style their hair with these products.
There currently are no regulations barring the use of potentially harmful materials from the manufacturing process for synthetic hair extensions.1 As a result, rinsing with apple cider vinegar (ACV) is a popular remedy that many users claim can effectively remove harmful chemicals from synthetic hair.6,7 As this is the only known remedy that aims to address this issue,
Methods
We conducted a search of Google Scholar, JSTOR, Science Direct, the Public Library of Science, and PubMed articles indexed for MEDLINE using the terms ACV, apple cider vinegar rinse, ACV rinse, synthetic hair carcinogens, synthetic fiber carcinogens, synthetic hair extension carcinogens, modacrylic fibers, Kanekalon (a flame-retardant modacrylic fiber), acrylonitrile, and vinyl chloride fibers to identify primary research articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions for inclusion in our review. To broaden our search, we did not establish a time frame for publication of the articles included in the study. Articles investigating the ACV rinse that were unrelated to carcinogenicity and synthetic hair extensions were excluded from this study.
Results
Our initial literature search identified 270 articles, which decreased to 180 after removal of duplicates. These 180 articles were screened for relevance based on title and abstract, which yielded 6 articles. None of the 6 articles identified through our literature search discussed synthetic hair and carcinogenicity in the context of the ACV rinse and were subsequently excluded from our review (eFigure 1).

Comment
Potentially harmful chemicals and ingredients in hair care products marketed for textured hair are now established topics in public discourse among those familiar with textured hair care and maintenance1,8; however, the discourse remains limited. Our search for scientific articles investigating the effects of the ACV rinse on the carcinogenicity of synthetic hair extensions revealed a notable deficit in the literature regarding scientific studies assessing this practice. While the likelihood that the ACV rinse effectively alters the carcinogenicity of plastic polymers found in synthetic hair extensions and improves their safety seems improbable, the deficit of empirical data evaluating this practice is concerning given both the prevalence of this remedy and the sizable demographic of patients who practice styling with synthetic hair.1 Of the potential adverse outcomes (eg, contact dermatitis, traction alopecia) that are possible from styling with synthetic hair that have been reported in the literature, carcinogenic exposure from synthetic hair extensions is relatively absent, with the exception of a few publications,2,3,9 despite its potential to cause serious long-term consequences for hair stylists and those who regularly use these products.
Interestingly, individuals who style their hair with synthetic hair extensions frequently tout the efficacy of the ACV rinse for removal of mostly unidentified irritants, although the effects are unverified.6,7 While the ACV rinse may be an effective means of removing toxic chemicals from synthetic hair extensions, without verifiable data this method remains an unproven remedy whose perceived benefits could result from factors unrelated to the rinse itself. Theoretically, simply rinsing synthetic hair extensions with plain water prior to use may demonstrate similar efficacy to that of the ACV rinse.
An additional factor worth mentioning is the lack of government regulations concerning the manufacturing practices of synthetic hair extensions. Flame-retardant materials such as trichloroethylene, polyvinyl chloride, and hexabromocyclododecane frequently are used in synthetic hair extensions despite their known adverse effects, which include reproductive organ toxicity and links to various cancers, leading to them being banned in 5 states.1,10-12 With no federal ban on these materials, individuals using synthetic hair remain at risk.
It is unclear what chemicals, irritants, or toxic substances the ACV rinse method could potentially remove from synthetic hair. In general, manufacturers of synthetic hair extensions are not forthcoming with information regarding materials used in the processing of their products despite public inquiries into their manufacturing practices.6 Although Whitehurst’s3 curriculum details the process of making synthetic polymer fibers, the overall processes by which these plastics are made to resemble human hair have not been reviewed in academic publications. Should this information be made available to the public, consumers could potentially avoid specific irritants when purchasing synthetic hair extensions.
The most common management strategy observed in the literature for adverse outcomes attributable to synthetic hair is discontinuation of use2; however, the prevalence and cultural significance of styling with synthetic hair extensions, along with the convenience these styles offer, make this option suboptimal. The scarcity of publications concerning the management of adverse outcomes related to the use of synthetic hair extensions may explain the absence of alternative management recommendations in the literature. Notably, new synthetic hair extensions from manufacturers that exclude plastic polymers and other harmful additives are now available to the public13; however, these hair extensions are cost prohibitive and are less accessible compared to synthetic extensions made from modacrylic fibers (eFigures 2 and 3).1,13-16


Final Thoughts
- Thomas CG. Carcinogenic materials in synthetic braids: an unrecognized risk of hair products for Black women. Lancet Reg Health Am. 2023;22:100517.
- Dlova NC, Ferguson NN, Rorex JN, et al. Synthetic hair extensions causing irritant contact dermatitis in patients with a history of atopy: a report of 10 cases. Contact Dermatitis. 2021;85:141-145.
- Whitehurst L. Polytails and urban tumble weaves: the chemistry of synthetic hair fibers. Yale National Initiative. September 2011. Accessed September 29, 2025. teachers.yale.edu/curriculum/viewer/initiative_11.05.10_u
- Acrylonitrile. U.S. Environmental Protection Agency. April 1992. Updated January 2000. Accessed September 29, 2025. www.epa.gov/sites/default/files/2016-09/documents/acrylonitrile.pdf
- Permissible exposure limits – annotated tables. OSHA annotated table Z-1. Occupational Safety and Health Administration. Accessed September 29, 2025. www.osha.gov/annotated-pels/table-z-1
- Adesina P. Braids are causing unbearable itching & there’s a sinister reason behind it. Refinery29. August 19, 2019. Accessed September 29, 2025. www.refinery29.com/en-gb/itchy-braids-hair
- Boakye O. Here’s why you should always wash plastic synthetic braiding extensions. InStyle. February 27, 2023. Accessed September 29, 2025. https://www.instyle.com/synthetic-braiding-extensions-upkeep-7151722
- James-Todd T, Connolly L, Preston EV, et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J Expo Sci Environ Epidemiol. 2021;31:476-486.
- Ijere ND, Okereke JN, Ezeji EU. Potential hazards associated with wearing of synthetic hairs (wigs, weavons, hair extensions/attachments) in Nigeria. J Environ Sci Public Health. 2022;6:299-313.
- Kaminsky T. An act to amend the environmental conservation law, in relation to the regulation of chemicals in upholstered furniture, mattresses and electronic enclosures. S4630B (2021). Accessed October 2, 2025. www.nysenate.gov/legislation/bills/2021/S4630
- Shen Y. Hair extension standards and regulations in the US: an overview. Compliance Gate. December 20, 2022. Accessed September 29, 2025. www.compliancegate.com/hair-extension-regulations-united-states/
- Lienke J, Rothschild R. Regulating Risk From Toxic Substances: Best Practices for Economic Analysis of Risk Management Options Under the Toxic Substances Control Act. Institute of Policy Integrity; 2021.
- Rebundle. Accessed October 2, 2025. https://rebundle.co/
- About us. Kanekalon. Accessed October 2, 2025. https://www.kanekalon-hair.com/en/about
- Julianna wholesale smooth Kanekalon futura natural fiber heat resistant bone straight synthetic bundle weft hair extensions. Accessed October 2, 2025. https://www.alibaba.com/product-detail/Julianna-wholesale-Smooth-Kanekalon-Futura-Natural_1601335996748.html
- AIDUSA solid colors braiding hair 5pcs synthetic Afro braid hair extensions 24 inch 1 tone for women braids twist crochet braids 100g(#1B Natural Black). Accessed October 2, 2025. www.amazon.com/AIDUSA-Braiding-Synthetic-Extensions-Crochet/dp/B09TNB9LC8
- Thomas CG. Carcinogenic materials in synthetic braids: an unrecognized risk of hair products for Black women. Lancet Reg Health Am. 2023;22:100517.
- Dlova NC, Ferguson NN, Rorex JN, et al. Synthetic hair extensions causing irritant contact dermatitis in patients with a history of atopy: a report of 10 cases. Contact Dermatitis. 2021;85:141-145.
- Whitehurst L. Polytails and urban tumble weaves: the chemistry of synthetic hair fibers. Yale National Initiative. September 2011. Accessed September 29, 2025. teachers.yale.edu/curriculum/viewer/initiative_11.05.10_u
- Acrylonitrile. U.S. Environmental Protection Agency. April 1992. Updated January 2000. Accessed September 29, 2025. www.epa.gov/sites/default/files/2016-09/documents/acrylonitrile.pdf
- Permissible exposure limits – annotated tables. OSHA annotated table Z-1. Occupational Safety and Health Administration. Accessed September 29, 2025. www.osha.gov/annotated-pels/table-z-1
- Adesina P. Braids are causing unbearable itching & there’s a sinister reason behind it. Refinery29. August 19, 2019. Accessed September 29, 2025. www.refinery29.com/en-gb/itchy-braids-hair
- Boakye O. Here’s why you should always wash plastic synthetic braiding extensions. InStyle. February 27, 2023. Accessed September 29, 2025. https://www.instyle.com/synthetic-braiding-extensions-upkeep-7151722
- James-Todd T, Connolly L, Preston EV, et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J Expo Sci Environ Epidemiol. 2021;31:476-486.
- Ijere ND, Okereke JN, Ezeji EU. Potential hazards associated with wearing of synthetic hairs (wigs, weavons, hair extensions/attachments) in Nigeria. J Environ Sci Public Health. 2022;6:299-313.
- Kaminsky T. An act to amend the environmental conservation law, in relation to the regulation of chemicals in upholstered furniture, mattresses and electronic enclosures. S4630B (2021). Accessed October 2, 2025. www.nysenate.gov/legislation/bills/2021/S4630
- Shen Y. Hair extension standards and regulations in the US: an overview. Compliance Gate. December 20, 2022. Accessed September 29, 2025. www.compliancegate.com/hair-extension-regulations-united-states/
- Lienke J, Rothschild R. Regulating Risk From Toxic Substances: Best Practices for Economic Analysis of Risk Management Options Under the Toxic Substances Control Act. Institute of Policy Integrity; 2021.
- Rebundle. Accessed October 2, 2025. https://rebundle.co/
- About us. Kanekalon. Accessed October 2, 2025. https://www.kanekalon-hair.com/en/about
- Julianna wholesale smooth Kanekalon futura natural fiber heat resistant bone straight synthetic bundle weft hair extensions. Accessed October 2, 2025. https://www.alibaba.com/product-detail/Julianna-wholesale-Smooth-Kanekalon-Futura-Natural_1601335996748.html
- AIDUSA solid colors braiding hair 5pcs synthetic Afro braid hair extensions 24 inch 1 tone for women braids twist crochet braids 100g(#1B Natural Black). Accessed October 2, 2025. www.amazon.com/AIDUSA-Braiding-Synthetic-Extensions-Crochet/dp/B09TNB9LC8
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Assessing the Merit of the Apple Cider Vinegar Rinse Method for Synthetic Hair Extensions
Practice Points
- Synthetic hair extensions are made from materials that can expose patients to high levels of carcinogens beginning in early childhood.
- The apple cider vinegar rinse method is an anecdotal remedy lacking data validating its ability to mitigate adverse reactions and complications associated with synthetic hair extensions, including carcinogenic exposure to materials they comprise.
- Dermatologists should inform patients of the potential exposure risks when using synthetic hair extensions to help patients make informed decisions regarding future styling habits and hair care choices.
