User login
Managing food allergy in children: An evidence-based update
Food allergy is a complex condition that has become a growing concern for parents and an increasing public health problem in the United States. Food allergy affects social interactions, school attendance, and quality of life, especially when associated with comorbid atopic conditions such as asthma, atopic dermatitis, and allergic rhinitis.1,2 It is the major cause of anaphylaxis in children, accounting for as many as 81% of cases.3 Societal costs of food allergy are great and are spread broadly across the health care system and the family. (See “What is the cost of food allergy?”2.)
SIDEBAR
What is the cost of food allergy?
Direct costs of food allergy to the health care system include medications, laboratory tests, office visits to primary care physicians and specialists, emergency department visits, and hospitalizations. Indirect costs include family medical and nonmedical expenses, lost work productivity, and job opportunity costs. Overall, the cost of food allergy in the United States is $24.8 billion annually—averaging $4184 for each affected child. Parents bear much of this expense.2
What a food allergy is—and isn’t
The National Institute of Allergy and Infectious Diseases (NIAID) defines food allergy as “an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food.”4 An adverse reaction to food or a food component that lacks an identified immunologic pathophysiology is not considered food allergy but is classified as food intolerance.4
Food allergy is caused by either immunoglobulin E (IgE)-mediated or non-IgE-mediated immunologic dysfunction. IgE antibodies can trigger an intense inflammatory response to certain allergens. Non-IgE-mediated food allergies are less common and not well understood.
This article focuses only on the diagnosis and management of IgE-mediated food allergy.
The culprits
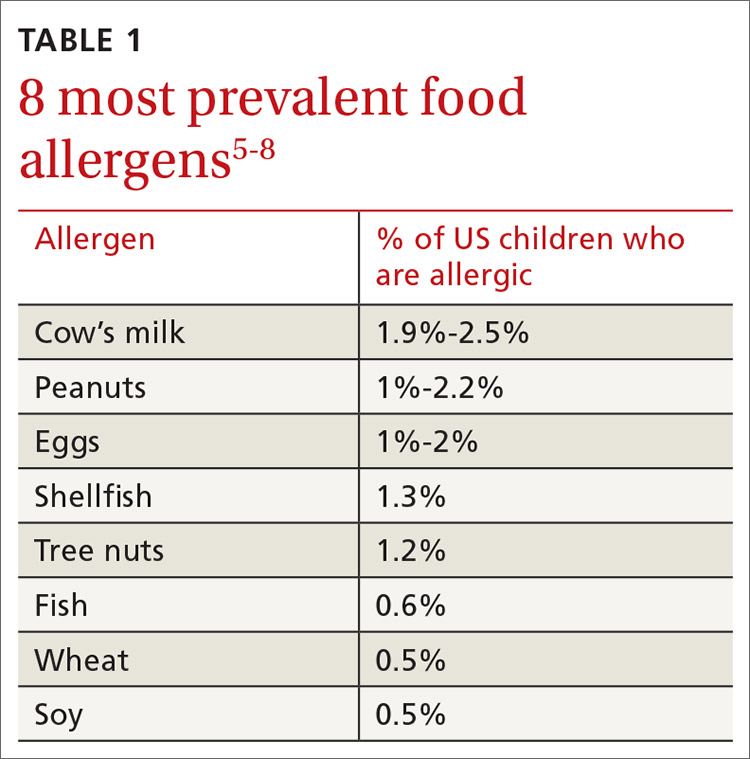
More than 170 foods have been reported to cause an IgE-mediated reaction. Table 15-8 lists the 8 foods that most commonly cause allergic reactions in the United States and that account for > 50% of allergies to food.9 Studies vary in their methodology for estimating the prevalence of allergy to individual foods, but cow’s milk and peanuts appear to be the most common, each affecting as many as 2% to 2.5% of children.7,8 In general, allergies to cow’s milk and to eggs are more prevalent in very young and preschool children, whereas allergies to peanuts, tree nuts, fish, and shellfish are more prevalent in older children.10 Labels on all packaged foods regulated by the US Food and Drug Administration must declare if the product contains even a trace of these 8 allergens.
How common is food allergy?
The Centers for Disease Control and Prevention (CDC) estimates that 4% to 6% of children in the United States have a food allergy.11,12 Almost 40% of food-allergic children have a history of severe food-induced reactions.13 Other developed countries cite similar estimates of overall prevalence.14,15
However, many estimates of the prevalence of food allergy are derived from self-reports, without objective data.9 Accurate evaluation of the prevalence of food allergy is challenging because of many factors, including differences in study methodology and the definition of allergy, geographic variation, racial and ethnic variations, and dietary exposure. Parents and children often confuse nonallergic food reactions, such as food intolerance, with food allergy. Precise determination of the prevalence and natural history of food allergy at the population level requires confirmatory oral food challenges of a representative sample of infants and young children with presumed food allergy.16
Continue to: The CDC concludes that the prevalence...
The CDC concludes that the prevalence of food allergy in children younger than 18 years increased by 18% from 1997 through 2007.17,18 The cause of this increase is unclear but likely multifactorial; hypotheses include an increase in associated atopic conditions, delayed introduction of allergenic foods, and living in an overly sterile environment with reduced exposure to microbes.19 A recent population-based study of food allergy among children in Olmsted County, Minnesota, found that the incidence of food allergy increased between 2002 and 2007, stabilized subsequently, and appears to be declining among children 1 to 4 years of age, following a peak in 2006-2007.19
What are the risk factors?
Proposed risk factors for food allergy include demographics, genetics, a history of atopic disease, and environmental factors. Food allergy might be more common in boys than in girls, and in African Americans and Asians than in Whites.12,16 A child is 7 times more likely to be allergic to peanuts if a parent or sibling has peanut allergy.20 Infants and children with eczema or asthma are more likely to develop food allergy; the severity of eczema correlates with risk.12,20 Improvements in hygiene in Western societies have decreased the spread of infection, but this has been accompanied by a rise in atopic disease. In countries where health standards are poor and exposure to pathogens is greater, the prevalence of allergy is low.21
Conversely, increased microbial exposure might help protect against atopy via a pathway in which T-helper cells prevent pro-allergic immune development and keep harmless environmental exposures from becoming allergens.22 Attendance at daycare and exposure to farm animals early in life reduces the likelihood of atopic disease.16,21 The presence of a dog in the home lessens the probability of egg allergy in infants.23 Food allergy is less common in younger siblings than in first-born children, possibly due to younger siblings’ increased exposure to infection and alterations in the gut microbiome.23,24
Diagnosis: Established by presentation, positive testing
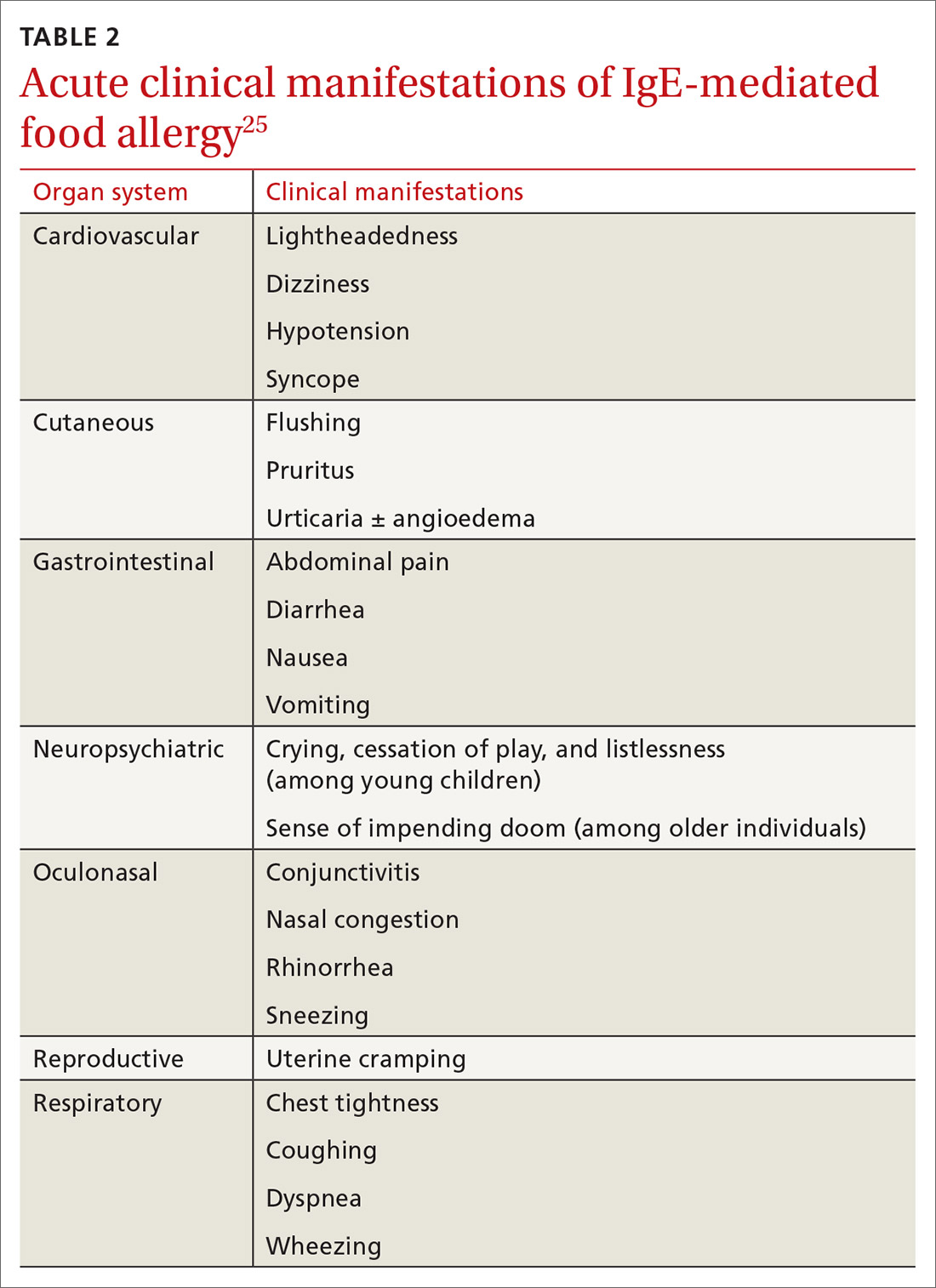
Onset of symptoms after exposure to a suspected food allergen almost always occurs within 2 hours and, typically, resolves within several hours. Symptoms should occur consistently after ingestion of the food allergen. Subsequent exposures can trigger more severe symptoms, depending on the amount, route, and duration of exposure to the allergen.25 Reactions typically follow ingestion or cutaneous exposures; inhalation rarely triggers a response.26 IgE-mediated release of histamine and other mediators from mast cells and basophils triggers reactions that typically involve one or more organ systems (Table 2).25
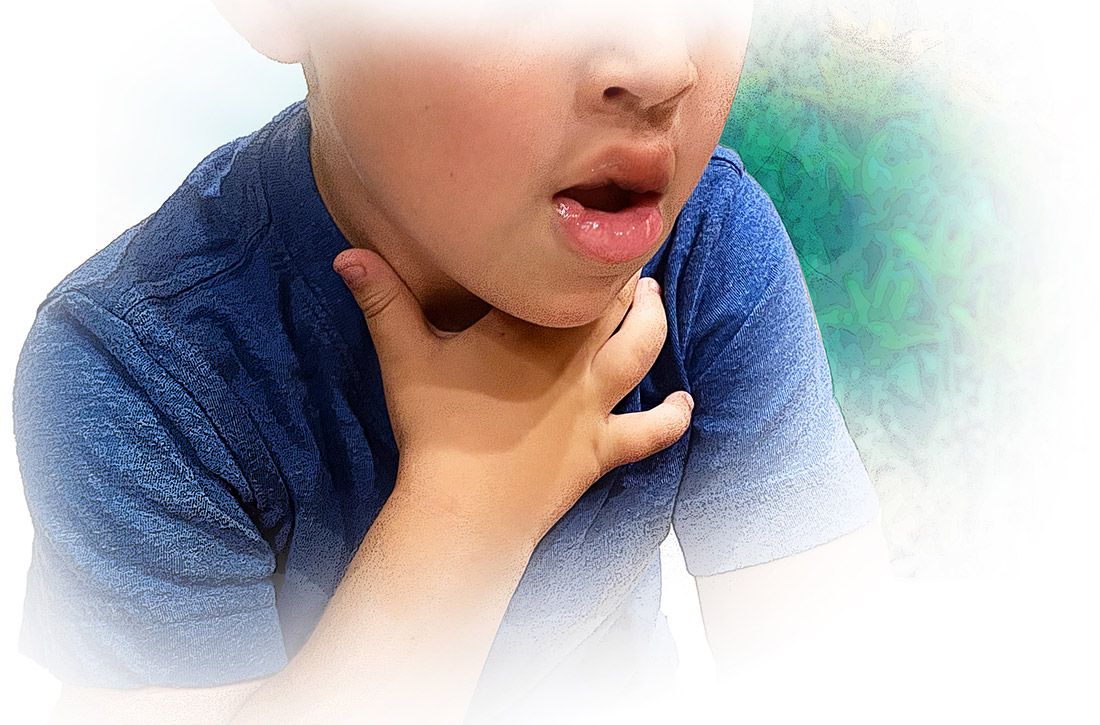
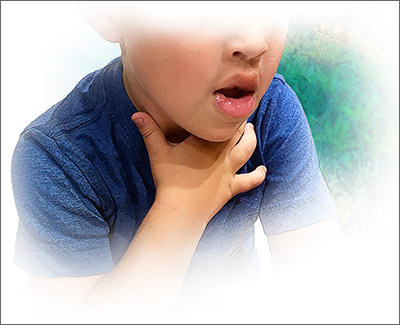
Cutaneous symptoms are the most common manifestations of food allergy, occurring in 70% to 80% of childhood reactions. Gastrointestinal and oral or respiratory symptoms occur in, respectively, 40% to 50% and 25% of allergic reactions to food. Cardiovascular symptoms develop in fewer than 10% of allergic reactions.26
Continue to: Anaphylaxis
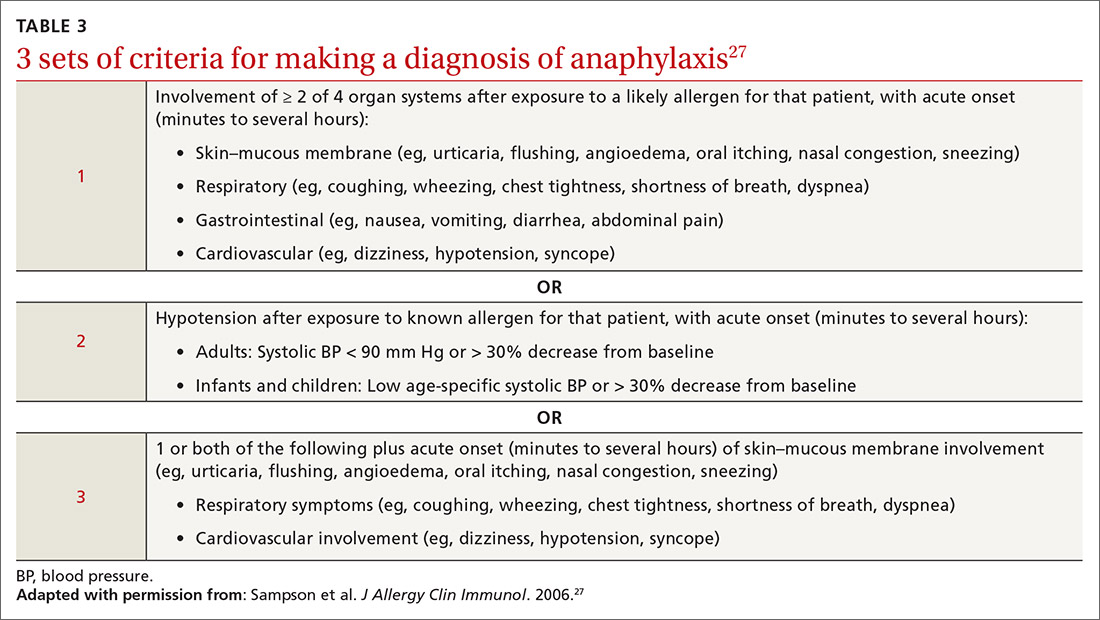
Anaphylaxis is a serious allergic reaction that develops rapidly and can cause death; diagnosis is based on specific criteria (Table 3).27 Data for rates of anaphylaxis due to food allergy are limited. The incidence of fatal reaction due to food allergy is estimated to be 1 in every 800,000 children annually.3
Clinical suspicion. Food allergy should be suspected in infants and children who present with anaphylaxis or other symptoms (Table 225) that occur within minutes to hours of ingesting food.4 Parental and self-reports alone are insufficient to diagnose food allergy. NIAID guidelines recommend that patient reports of food allergy be confirmed, because multiple studies demonstrate that 50% to 90% of presumed food allergies are not true allergy.4 Health care providers must obtain a detailed medical history and pertinent family history, plus perform a physical exam and allergy sensitivity testing. Methods to help diagnose food allergies include skin-prick tests, allergen-specific serum IgE tests, and oral food challenges.4
General principles and utility of testing
Before ordering tests, it’s important to distinguish between food sensitization and food allergy and to inform the families of children with suspected food allergy about the limitations of skin-prick tests and serum IgE tests. A child with IgE antibodies specific to a food or with a positive skin-prick test, but without symptoms upon ingestion of the food, is merely sensitized; food allergy indicates the appearance of symptoms following exposure to a specific food, in addition to the detection of specific IgE antibodies or a positive skin-prick test to that same food.28
Skin-prick testing. Skin-prick tests can be performed at any age. The procedure involves pricking or scratching the surface of the skin, usually the volar aspect of the forearm or the back, with a commercial extract. Testing should be performed by a physician or other provider who is properly trained in the technique and in interpreting results. The extract contains specific allergenic proteins that activate mast cells, resulting in a characteristic wheal-and-flare response that is typically measured 15 to 20 minutes after application. Some medications, such as H1- and H2-receptor blockers and tricyclic antidepressants, can interfere with results and need to be held for 3 to 5 days before testing.
A positive skin-prick test result is defined as a wheal ≥ 3 mm larger in diameter than the negative control. The larger the size of the wheal, the higher the likelihood of a reaction to the tested food.29 Patients who exhibit dermatographism might experience a wheal-and-flare response from the action of the skin-prick test, rather than from food-specific IgE antibodies. A negative skin-prick test has > 90% negative predictive value, so the test can rule out suspected food allergy.30 However, the skin-prick test alone cannot be used to diagnose food allergy because it has a high false-positive rate.
Continue to: Allergen-specific serum IgE testing
Allergen-specific serum IgE testing. Measurement of food-specific serum IgE levels is routinely available and requires only a blood specimen. The test can be used in patients with skin disease, and results are not affected by concurrent medications. The presence of food-specific IgE indicates that the patient is sensitized to that allergen and might react upon exposure; children with a higher level of antibody are more likely to react.29
Food-specific serum IgE tests are sensitive but nonspecific for food allergy.31 Broad food-allergy test panels often yield false-positive results that can lead to unnecessary dietary elimination, resulting in years of inconvenience, nutrition problems, and needless health care expense.32
It is appropriate to order tests of specific serum IgE to foods ingested within the 2 to 3–hour window before onset of symptoms to avoid broad food allergy test panels. Like skin-prick testing, positive allergen-specific serum IgE tests alone cannot diagnose food allergy.
Oral food challenge. The double-blind, placebo-controlled oral food challenge is the gold standard for the diagnosis of food allergy. Because this test is time-consuming and technically difficult, single-blind or open food challenges are more common. Oral food challenges should be performed only by a physician or other provider who can identify and treat anaphylaxis.
The oral challenge starts with a very low dose of suspected food allergen, which is gradually increased every 15 to 30 minutes as vital signs are monitored carefully. Patients are observed for an allergic reaction for 1 hour after the final dose.
Continue to: A retrospective study...
A retrospective study showed that, whereas 19% of patients reacted during an open food challenge, only 2% required epinephrine.33 Another study showed that 89% of children whose serum IgE testing was positive for specific foods were able to reintroduce those foods into the diet after a reassuring oral food challenge.34
Other diagnostic tests. The basophil activation assay, measurement of total serum IgE, atopy patch tests, and intradermal tests have been used, but are not recommended, for making the diagnosis of food allergy.4
How can food allergy be managed?
Medical options are few. No approved treatment exists for food allergy. However, it’s important to appropriately manage acute reactions and reduce the risk of subsequent reactions.1 Parents or other caregivers can give an H1 antihistamine, such as diphenhydramine, to infants and children with acute non-life-threatening symptoms. More severe symptoms require rapid administration of epinephrine.1 Auto-injectable epinephrine should be prescribed for parents and caregivers to use as needed for emergency treatment of anaphylaxis.
Team approach. A multidisciplinary approach to managing food allergy—involving physicians, school nurses, dietitians, and teachers, and using educational materials—is ideal. This strategy expands knowledge about food allergies, enhances correct administration of epinephrine, and reduces allergic reactions.1
Avoidance of food allergens can be challenging. Parents and caregivers should be taught to interpret the list of ingredients on food packages. Self-recognition of allergic reactions reduces the likelihood of a subsequent severe allergic reaction.35
Continue to: Importance of individualized care
Importance of individualized care. Health care providers should develop personalized management plans for their patients.1 (A good place to start is with the “Food Allergy & Anaphylaxis Emergency Care Plan”a developed by Food Allergy Research & Education [FARE]). Keep in mind that children with multiple food allergies consume less calcium and protein, and tend to be shorter4; therefore, it’s wise to closely monitor growth in these children and consider referral to a dietitian who is familiar with food allergy.
Potential of immunotherapy. Current research focuses on immunotherapy to induce tolerance to food allergens and protect against life-threatening allergic reactions. The goal of immunotherapy is to lessen adverse reactions to allergenic food proteins; the strategy is to have patients repeatedly ingest small but gradually increasing doses of the food allergen over many months.36 Although immunotherapy has successfully allowed some patients to consume larger quantities of a food without having an allergic reaction, it is unknown whether immunotherapy provides permanent resolution of food allergy. In addition, immunotherapy often causes serious systemic and local reactions.1,36,37
Is prevention possible?
Maternal diet during pregnancy and lactation does not affect development of food allergy in infants.38,39 Breastfeeding might prevent development of atopic disease, but evidence is insufficient to determine whether breastfeeding reduces the likelihood of food allergy.39 In nonbreastfed infants at high risk of food allergy, extensively or partially hydrolyzed formula might help protect against food allergy, compared to standard cow’s milk formula.9,39 Feeding with soy formula rather than cow’s milk formula does not help prevent food allergy.39,40 Pregnant and breastfeeding women should not restrict their diet as a means of preventing food allergy.39
Diet in infancy. Over the years, physicians have debated the proper timing of the introduction of solid foods into the diet of infants. Traditional teaching advocated delaying introduction of potentially allergenic foods to reduce the risk of food allergy; however, this guideline was based on inconsistent evidence,41 and the strategy did not reduce the incidence of food allergy. The prevalence of food allergy is lower in developing countries where caregivers introduce foods to infants at an earlier age.20
A recent large clinical trial indicates that early introduction of peanut-containing foods can help prevent peanut allergy. The study randomized 4- to 11-month-old infants with severe eczema, egg allergy, or both, to eat or avoid peanut products until 5 years of age. Infants assigned to eat peanuts were 81% less likely to develop peanut allergy than infants in the avoidance group. Absolute risk reduction was 14% (number need to treat = 7).42 Another study showed a nonsignificant (20%) lower relative risk of food allergy in breastfed infants who were fed potentially allergenic foods starting at 3 months of age, compared to being exclusively breastfed.43
Continue to: Based on these data...
Based on these data,42,43 NIAID instituted recommendations in 2017 aimed at preventing peanut allergy44:
- In healthy infants without known food allergy and those with mild or moderate eczema, caregivers can introduce peanut-containing foods at home with other solid foods.Parents who are anxious about a possible allergic reaction can introduce peanut products in a physician’s office.
- Infants at high risk of peanut allergy (those with severe eczema or egg allergy, or both) should undergo peanut-specific IgE or skin-prick testing:
- Negative test: indicates low risk of a reaction to peanuts; the infant should start consuming peanut-containing foods at 4 to 6 months of age, at home or in a physician’s office, depending on the parents’ preference
- Positive test: Referral to an allergist is recommended.
Do children outgrow food allergy?
Approximately 85% of children who have an allergy to milk, egg, soy, or wheat outgrow their allergy; however, only 15% to 20% who have an allergy to peanuts, tree nuts, fish, or shellfish eventually tolerate these foods. The time to resolution of food allergy varies with the food, and might not occur until adolescence.4 No test reliably predicts which children develop tolerance to any given food. A decrease in the food-specific serum IgE level or a decrease in the size of the wheal on skin-prick testing might portend the onset of tolerance to the food.4
CORRESPONDENCE
Catherine M. Bettcher, MD, FAAFP, Briarwood Family Medicine, 1801 Briarwood Circle, Building #10, Ann Arbor, MI 48108; [email protected].
1. Muraro A, Werfel T, Hoffmann-Sommergruber K, et al; . EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014;69:1008-1025.
2. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
3. Cianferoni A, Muraro A. Food-induced anaphylaxis. Immunol Allergy Clin North Am. 2012;32:165-195.
4., Boyce JA, Assa’ad A, Burks WA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
5. Vierk KA, Koehler KM, Fein SB, et al. Prevalence of self-reported food allergy in American adults and use of food labels. J Allergy Clin Immunol. 2007;119:1504-1510.
6. Allen KJ, Koplin JJ. The epidemiology of IgE-mediated food allergy and anaphylaxis. Immunol Allergy Clin North Am. 2012;32:35-50.
7. Iweala OI, Choudhary SK, Commins SP. Food allergy. Curr Gastroenterol Rep. 2018;20:17.
8. Gupta RS, Warren CM, Smith BM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. 2018;142:e20181235.
9. Chafen JJS, Newberry SJ, Riedl MA, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010;303:1848-1856.
10. Nwaru BI, Hickstein L, Panesar SS, et al. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69:992-1007.
11. Branum AM, Lukacs SL. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS Data Brief No. 10. National Center for Health Statistics. October 2008. www.cdc.gov/nchs/products/databriefs/db10.htm. Accessed August 19, 2020.
12. Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126:798-806.e13.
13. Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9-e17.
14. Soller L, Ben-Shoshan M, Harrington DW, et al. Overall prevalence of self-reported food allergy in Canada. J Allergy Clin Immunol. 2012;130:986-988.
15. Venter C, Pereira B, Voigt K, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008;63:354-359.
16. Savage J, Johns CB. Food allergy: epidemiology and natural history. Immunol Allergy Clin North Am. 2015;35:45-59.
17. Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124:1549-1555.
18. Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997-2011. NCHS Data Brief No. 121. National Center for Health Statistics. May 2013. www.cdc.gov/nchs/products/databriefs/db121.htm. Accessed August 19, 2020.
19. Willits EK, Park MA, Hartz MF, et al. Food allergy: a comprehensive population-based cohort study. Mayo Clin Proc. 2018;93:1423-1430.
20. Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol. 2008;121:1331-1336.
21. Okada H, Kuhn C, Feillet H, et al. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1-9.
22. Liu AH. Hygiene theory and allergy and asthma prevention. Paediatr Perinat Epidemiol. 2007;21 Suppl 3:2-7.
23. Prince BT, Mandel MJ, Nadeau K, et al. Gut microbiome and the development of food allergy and allergic disease. Pediatr Clin North Am. 2015;62:1479-1492.
24. Kusunoki T, Mukaida K, Morimoto T, et al. Birth order effect on childhood food allergy. Pediatr Allergy Immunol. 2012;23:250-254.
25. Abrams EM, Sicherer SH. Diagnosis and management of food allergy. CMAJ. 2016;188:1087-1093.
26. Perry TT, Matsui EC, Conover-Walker MK, et al. Risk of oral food challenges. J Allergy Clin Immunol. 2004;114:1164-1168.
27. Sampson HA, A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391-397.
28. Sampson HA. Food allergy. Part 2: diagnosis and management. J Allergy Clin Immunol. 1999;103:981-989.
29. Lieberman JA, Sicherer SH. Diagnosis of food allergy: epicutaneous skin tests, in vitro tests, and oral food challenge. Curr Allergy Asthma Rep. 2011;11:58-64.
30. Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S116-S125.
31. Soares-Weiser K, Takwoingi Y, Panesar SS, et al. The diagnosis of food allergy: a systematic review and meta-analysis. Allergy. 2014;69:76-86.
32. Bird JA, Crain M, Varshney P. Food allergen panel testing often results in misdiagnosis of food allergy. J Pediatr. 2015;166:97-100.
33. Lieberman JA, Cox AL, Vitale M, et al. Outcomes of office-based, open food challenges in the management of food allergy. J Allergy Clin Immunol. 2011;128:1120-1122.
34. Fleischer DM, Bock SA, Spears GC, et al. Oral food challenges in children with a diagnosis of food allergy. J Pediatr. 2011;158:578-583.e1.
35. Ewan PW, Clark AT. Long-term prospective observational study of patients with peanut and nut allergy after participation in a management plan. Lancet. 2001;357:111-115.
36. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
37. Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update—2014. J Allergy Clin Immunol. 2014;134:1016-1025.e43.
38. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;2012(9):CD000133.
39. de Silva D, Geromi M, Halken S, et al; . Primary prevention of food allergy in children and adults: systematic review. Allergy. 2014;69:581-589.
40. Osborn DA, Sinn J. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev. 2004;(3):CD003741.
41. Filipiak B, Zutavern A, Koletzko S, et al; GINI-Group. Solid food introduction in relation to eczema: results from a four-year prospective birth cohort study. J Pediatr. 2007;151:352-358.
42. Du Toit G, Roberts G, Sayre PH, et al; LEAP Study Team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
43. Perkin MR, Logan K, Tseng A, et al; EAT Study Team. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
44. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
Food allergy is a complex condition that has become a growing concern for parents and an increasing public health problem in the United States. Food allergy affects social interactions, school attendance, and quality of life, especially when associated with comorbid atopic conditions such as asthma, atopic dermatitis, and allergic rhinitis.1,2 It is the major cause of anaphylaxis in children, accounting for as many as 81% of cases.3 Societal costs of food allergy are great and are spread broadly across the health care system and the family. (See “What is the cost of food allergy?”2.)
SIDEBAR
What is the cost of food allergy?
Direct costs of food allergy to the health care system include medications, laboratory tests, office visits to primary care physicians and specialists, emergency department visits, and hospitalizations. Indirect costs include family medical and nonmedical expenses, lost work productivity, and job opportunity costs. Overall, the cost of food allergy in the United States is $24.8 billion annually—averaging $4184 for each affected child. Parents bear much of this expense.2
What a food allergy is—and isn’t
The National Institute of Allergy and Infectious Diseases (NIAID) defines food allergy as “an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food.”4 An adverse reaction to food or a food component that lacks an identified immunologic pathophysiology is not considered food allergy but is classified as food intolerance.4
Food allergy is caused by either immunoglobulin E (IgE)-mediated or non-IgE-mediated immunologic dysfunction. IgE antibodies can trigger an intense inflammatory response to certain allergens. Non-IgE-mediated food allergies are less common and not well understood.

This article focuses only on the diagnosis and management of IgE-mediated food allergy.
The culprits
More than 170 foods have been reported to cause an IgE-mediated reaction. Table 15-8 lists the 8 foods that most commonly cause allergic reactions in the United States and that account for > 50% of allergies to food.9 Studies vary in their methodology for estimating the prevalence of allergy to individual foods, but cow’s milk and peanuts appear to be the most common, each affecting as many as 2% to 2.5% of children.7,8 In general, allergies to cow’s milk and to eggs are more prevalent in very young and preschool children, whereas allergies to peanuts, tree nuts, fish, and shellfish are more prevalent in older children.10 Labels on all packaged foods regulated by the US Food and Drug Administration must declare if the product contains even a trace of these 8 allergens.

How common is food allergy?
The Centers for Disease Control and Prevention (CDC) estimates that 4% to 6% of children in the United States have a food allergy.11,12 Almost 40% of food-allergic children have a history of severe food-induced reactions.13 Other developed countries cite similar estimates of overall prevalence.14,15
However, many estimates of the prevalence of food allergy are derived from self-reports, without objective data.9 Accurate evaluation of the prevalence of food allergy is challenging because of many factors, including differences in study methodology and the definition of allergy, geographic variation, racial and ethnic variations, and dietary exposure. Parents and children often confuse nonallergic food reactions, such as food intolerance, with food allergy. Precise determination of the prevalence and natural history of food allergy at the population level requires confirmatory oral food challenges of a representative sample of infants and young children with presumed food allergy.16
Continue to: The CDC concludes that the prevalence...
The CDC concludes that the prevalence of food allergy in children younger than 18 years increased by 18% from 1997 through 2007.17,18 The cause of this increase is unclear but likely multifactorial; hypotheses include an increase in associated atopic conditions, delayed introduction of allergenic foods, and living in an overly sterile environment with reduced exposure to microbes.19 A recent population-based study of food allergy among children in Olmsted County, Minnesota, found that the incidence of food allergy increased between 2002 and 2007, stabilized subsequently, and appears to be declining among children 1 to 4 years of age, following a peak in 2006-2007.19
What are the risk factors?
Proposed risk factors for food allergy include demographics, genetics, a history of atopic disease, and environmental factors. Food allergy might be more common in boys than in girls, and in African Americans and Asians than in Whites.12,16 A child is 7 times more likely to be allergic to peanuts if a parent or sibling has peanut allergy.20 Infants and children with eczema or asthma are more likely to develop food allergy; the severity of eczema correlates with risk.12,20 Improvements in hygiene in Western societies have decreased the spread of infection, but this has been accompanied by a rise in atopic disease. In countries where health standards are poor and exposure to pathogens is greater, the prevalence of allergy is low.21
Conversely, increased microbial exposure might help protect against atopy via a pathway in which T-helper cells prevent pro-allergic immune development and keep harmless environmental exposures from becoming allergens.22 Attendance at daycare and exposure to farm animals early in life reduces the likelihood of atopic disease.16,21 The presence of a dog in the home lessens the probability of egg allergy in infants.23 Food allergy is less common in younger siblings than in first-born children, possibly due to younger siblings’ increased exposure to infection and alterations in the gut microbiome.23,24
Diagnosis: Established by presentation, positive testing
Onset of symptoms after exposure to a suspected food allergen almost always occurs within 2 hours and, typically, resolves within several hours. Symptoms should occur consistently after ingestion of the food allergen. Subsequent exposures can trigger more severe symptoms, depending on the amount, route, and duration of exposure to the allergen.25 Reactions typically follow ingestion or cutaneous exposures; inhalation rarely triggers a response.26 IgE-mediated release of histamine and other mediators from mast cells and basophils triggers reactions that typically involve one or more organ systems (Table 2).25

Cutaneous symptoms are the most common manifestations of food allergy, occurring in 70% to 80% of childhood reactions. Gastrointestinal and oral or respiratory symptoms occur in, respectively, 40% to 50% and 25% of allergic reactions to food. Cardiovascular symptoms develop in fewer than 10% of allergic reactions.26
Continue to: Anaphylaxis
Anaphylaxis is a serious allergic reaction that develops rapidly and can cause death; diagnosis is based on specific criteria (Table 3).27 Data for rates of anaphylaxis due to food allergy are limited. The incidence of fatal reaction due to food allergy is estimated to be 1 in every 800,000 children annually.3

Clinical suspicion. Food allergy should be suspected in infants and children who present with anaphylaxis or other symptoms (Table 225) that occur within minutes to hours of ingesting food.4 Parental and self-reports alone are insufficient to diagnose food allergy. NIAID guidelines recommend that patient reports of food allergy be confirmed, because multiple studies demonstrate that 50% to 90% of presumed food allergies are not true allergy.4 Health care providers must obtain a detailed medical history and pertinent family history, plus perform a physical exam and allergy sensitivity testing. Methods to help diagnose food allergies include skin-prick tests, allergen-specific serum IgE tests, and oral food challenges.4
General principles and utility of testing
Before ordering tests, it’s important to distinguish between food sensitization and food allergy and to inform the families of children with suspected food allergy about the limitations of skin-prick tests and serum IgE tests. A child with IgE antibodies specific to a food or with a positive skin-prick test, but without symptoms upon ingestion of the food, is merely sensitized; food allergy indicates the appearance of symptoms following exposure to a specific food, in addition to the detection of specific IgE antibodies or a positive skin-prick test to that same food.28
Skin-prick testing. Skin-prick tests can be performed at any age. The procedure involves pricking or scratching the surface of the skin, usually the volar aspect of the forearm or the back, with a commercial extract. Testing should be performed by a physician or other provider who is properly trained in the technique and in interpreting results. The extract contains specific allergenic proteins that activate mast cells, resulting in a characteristic wheal-and-flare response that is typically measured 15 to 20 minutes after application. Some medications, such as H1- and H2-receptor blockers and tricyclic antidepressants, can interfere with results and need to be held for 3 to 5 days before testing.
A positive skin-prick test result is defined as a wheal ≥ 3 mm larger in diameter than the negative control. The larger the size of the wheal, the higher the likelihood of a reaction to the tested food.29 Patients who exhibit dermatographism might experience a wheal-and-flare response from the action of the skin-prick test, rather than from food-specific IgE antibodies. A negative skin-prick test has > 90% negative predictive value, so the test can rule out suspected food allergy.30 However, the skin-prick test alone cannot be used to diagnose food allergy because it has a high false-positive rate.
Continue to: Allergen-specific serum IgE testing
Allergen-specific serum IgE testing. Measurement of food-specific serum IgE levels is routinely available and requires only a blood specimen. The test can be used in patients with skin disease, and results are not affected by concurrent medications. The presence of food-specific IgE indicates that the patient is sensitized to that allergen and might react upon exposure; children with a higher level of antibody are more likely to react.29
Food-specific serum IgE tests are sensitive but nonspecific for food allergy.31 Broad food-allergy test panels often yield false-positive results that can lead to unnecessary dietary elimination, resulting in years of inconvenience, nutrition problems, and needless health care expense.32
It is appropriate to order tests of specific serum IgE to foods ingested within the 2 to 3–hour window before onset of symptoms to avoid broad food allergy test panels. Like skin-prick testing, positive allergen-specific serum IgE tests alone cannot diagnose food allergy.
Oral food challenge. The double-blind, placebo-controlled oral food challenge is the gold standard for the diagnosis of food allergy. Because this test is time-consuming and technically difficult, single-blind or open food challenges are more common. Oral food challenges should be performed only by a physician or other provider who can identify and treat anaphylaxis.
The oral challenge starts with a very low dose of suspected food allergen, which is gradually increased every 15 to 30 minutes as vital signs are monitored carefully. Patients are observed for an allergic reaction for 1 hour after the final dose.
Continue to: A retrospective study...
A retrospective study showed that, whereas 19% of patients reacted during an open food challenge, only 2% required epinephrine.33 Another study showed that 89% of children whose serum IgE testing was positive for specific foods were able to reintroduce those foods into the diet after a reassuring oral food challenge.34
Other diagnostic tests. The basophil activation assay, measurement of total serum IgE, atopy patch tests, and intradermal tests have been used, but are not recommended, for making the diagnosis of food allergy.4
How can food allergy be managed?
Medical options are few. No approved treatment exists for food allergy. However, it’s important to appropriately manage acute reactions and reduce the risk of subsequent reactions.1 Parents or other caregivers can give an H1 antihistamine, such as diphenhydramine, to infants and children with acute non-life-threatening symptoms. More severe symptoms require rapid administration of epinephrine.1 Auto-injectable epinephrine should be prescribed for parents and caregivers to use as needed for emergency treatment of anaphylaxis.
Team approach. A multidisciplinary approach to managing food allergy—involving physicians, school nurses, dietitians, and teachers, and using educational materials—is ideal. This strategy expands knowledge about food allergies, enhances correct administration of epinephrine, and reduces allergic reactions.1
Avoidance of food allergens can be challenging. Parents and caregivers should be taught to interpret the list of ingredients on food packages. Self-recognition of allergic reactions reduces the likelihood of a subsequent severe allergic reaction.35
Continue to: Importance of individualized care
Importance of individualized care. Health care providers should develop personalized management plans for their patients.1 (A good place to start is with the “Food Allergy & Anaphylaxis Emergency Care Plan”a developed by Food Allergy Research & Education [FARE]). Keep in mind that children with multiple food allergies consume less calcium and protein, and tend to be shorter4; therefore, it’s wise to closely monitor growth in these children and consider referral to a dietitian who is familiar with food allergy.
Potential of immunotherapy. Current research focuses on immunotherapy to induce tolerance to food allergens and protect against life-threatening allergic reactions. The goal of immunotherapy is to lessen adverse reactions to allergenic food proteins; the strategy is to have patients repeatedly ingest small but gradually increasing doses of the food allergen over many months.36 Although immunotherapy has successfully allowed some patients to consume larger quantities of a food without having an allergic reaction, it is unknown whether immunotherapy provides permanent resolution of food allergy. In addition, immunotherapy often causes serious systemic and local reactions.1,36,37
Is prevention possible?
Maternal diet during pregnancy and lactation does not affect development of food allergy in infants.38,39 Breastfeeding might prevent development of atopic disease, but evidence is insufficient to determine whether breastfeeding reduces the likelihood of food allergy.39 In nonbreastfed infants at high risk of food allergy, extensively or partially hydrolyzed formula might help protect against food allergy, compared to standard cow’s milk formula.9,39 Feeding with soy formula rather than cow’s milk formula does not help prevent food allergy.39,40 Pregnant and breastfeeding women should not restrict their diet as a means of preventing food allergy.39
Diet in infancy. Over the years, physicians have debated the proper timing of the introduction of solid foods into the diet of infants. Traditional teaching advocated delaying introduction of potentially allergenic foods to reduce the risk of food allergy; however, this guideline was based on inconsistent evidence,41 and the strategy did not reduce the incidence of food allergy. The prevalence of food allergy is lower in developing countries where caregivers introduce foods to infants at an earlier age.20
A recent large clinical trial indicates that early introduction of peanut-containing foods can help prevent peanut allergy. The study randomized 4- to 11-month-old infants with severe eczema, egg allergy, or both, to eat or avoid peanut products until 5 years of age. Infants assigned to eat peanuts were 81% less likely to develop peanut allergy than infants in the avoidance group. Absolute risk reduction was 14% (number need to treat = 7).42 Another study showed a nonsignificant (20%) lower relative risk of food allergy in breastfed infants who were fed potentially allergenic foods starting at 3 months of age, compared to being exclusively breastfed.43
Continue to: Based on these data...
Based on these data,42,43 NIAID instituted recommendations in 2017 aimed at preventing peanut allergy44:
- In healthy infants without known food allergy and those with mild or moderate eczema, caregivers can introduce peanut-containing foods at home with other solid foods.Parents who are anxious about a possible allergic reaction can introduce peanut products in a physician’s office.
- Infants at high risk of peanut allergy (those with severe eczema or egg allergy, or both) should undergo peanut-specific IgE or skin-prick testing:
- Negative test: indicates low risk of a reaction to peanuts; the infant should start consuming peanut-containing foods at 4 to 6 months of age, at home or in a physician’s office, depending on the parents’ preference
- Positive test: Referral to an allergist is recommended.
Do children outgrow food allergy?
Approximately 85% of children who have an allergy to milk, egg, soy, or wheat outgrow their allergy; however, only 15% to 20% who have an allergy to peanuts, tree nuts, fish, or shellfish eventually tolerate these foods. The time to resolution of food allergy varies with the food, and might not occur until adolescence.4 No test reliably predicts which children develop tolerance to any given food. A decrease in the food-specific serum IgE level or a decrease in the size of the wheal on skin-prick testing might portend the onset of tolerance to the food.4
CORRESPONDENCE
Catherine M. Bettcher, MD, FAAFP, Briarwood Family Medicine, 1801 Briarwood Circle, Building #10, Ann Arbor, MI 48108; [email protected].
Food allergy is a complex condition that has become a growing concern for parents and an increasing public health problem in the United States. Food allergy affects social interactions, school attendance, and quality of life, especially when associated with comorbid atopic conditions such as asthma, atopic dermatitis, and allergic rhinitis.1,2 It is the major cause of anaphylaxis in children, accounting for as many as 81% of cases.3 Societal costs of food allergy are great and are spread broadly across the health care system and the family. (See “What is the cost of food allergy?”2.)
SIDEBAR
What is the cost of food allergy?
Direct costs of food allergy to the health care system include medications, laboratory tests, office visits to primary care physicians and specialists, emergency department visits, and hospitalizations. Indirect costs include family medical and nonmedical expenses, lost work productivity, and job opportunity costs. Overall, the cost of food allergy in the United States is $24.8 billion annually—averaging $4184 for each affected child. Parents bear much of this expense.2
What a food allergy is—and isn’t
The National Institute of Allergy and Infectious Diseases (NIAID) defines food allergy as “an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food.”4 An adverse reaction to food or a food component that lacks an identified immunologic pathophysiology is not considered food allergy but is classified as food intolerance.4
Food allergy is caused by either immunoglobulin E (IgE)-mediated or non-IgE-mediated immunologic dysfunction. IgE antibodies can trigger an intense inflammatory response to certain allergens. Non-IgE-mediated food allergies are less common and not well understood.

This article focuses only on the diagnosis and management of IgE-mediated food allergy.
The culprits
More than 170 foods have been reported to cause an IgE-mediated reaction. Table 15-8 lists the 8 foods that most commonly cause allergic reactions in the United States and that account for > 50% of allergies to food.9 Studies vary in their methodology for estimating the prevalence of allergy to individual foods, but cow’s milk and peanuts appear to be the most common, each affecting as many as 2% to 2.5% of children.7,8 In general, allergies to cow’s milk and to eggs are more prevalent in very young and preschool children, whereas allergies to peanuts, tree nuts, fish, and shellfish are more prevalent in older children.10 Labels on all packaged foods regulated by the US Food and Drug Administration must declare if the product contains even a trace of these 8 allergens.

How common is food allergy?
The Centers for Disease Control and Prevention (CDC) estimates that 4% to 6% of children in the United States have a food allergy.11,12 Almost 40% of food-allergic children have a history of severe food-induced reactions.13 Other developed countries cite similar estimates of overall prevalence.14,15
However, many estimates of the prevalence of food allergy are derived from self-reports, without objective data.9 Accurate evaluation of the prevalence of food allergy is challenging because of many factors, including differences in study methodology and the definition of allergy, geographic variation, racial and ethnic variations, and dietary exposure. Parents and children often confuse nonallergic food reactions, such as food intolerance, with food allergy. Precise determination of the prevalence and natural history of food allergy at the population level requires confirmatory oral food challenges of a representative sample of infants and young children with presumed food allergy.16
Continue to: The CDC concludes that the prevalence...
The CDC concludes that the prevalence of food allergy in children younger than 18 years increased by 18% from 1997 through 2007.17,18 The cause of this increase is unclear but likely multifactorial; hypotheses include an increase in associated atopic conditions, delayed introduction of allergenic foods, and living in an overly sterile environment with reduced exposure to microbes.19 A recent population-based study of food allergy among children in Olmsted County, Minnesota, found that the incidence of food allergy increased between 2002 and 2007, stabilized subsequently, and appears to be declining among children 1 to 4 years of age, following a peak in 2006-2007.19
What are the risk factors?
Proposed risk factors for food allergy include demographics, genetics, a history of atopic disease, and environmental factors. Food allergy might be more common in boys than in girls, and in African Americans and Asians than in Whites.12,16 A child is 7 times more likely to be allergic to peanuts if a parent or sibling has peanut allergy.20 Infants and children with eczema or asthma are more likely to develop food allergy; the severity of eczema correlates with risk.12,20 Improvements in hygiene in Western societies have decreased the spread of infection, but this has been accompanied by a rise in atopic disease. In countries where health standards are poor and exposure to pathogens is greater, the prevalence of allergy is low.21
Conversely, increased microbial exposure might help protect against atopy via a pathway in which T-helper cells prevent pro-allergic immune development and keep harmless environmental exposures from becoming allergens.22 Attendance at daycare and exposure to farm animals early in life reduces the likelihood of atopic disease.16,21 The presence of a dog in the home lessens the probability of egg allergy in infants.23 Food allergy is less common in younger siblings than in first-born children, possibly due to younger siblings’ increased exposure to infection and alterations in the gut microbiome.23,24
Diagnosis: Established by presentation, positive testing
Onset of symptoms after exposure to a suspected food allergen almost always occurs within 2 hours and, typically, resolves within several hours. Symptoms should occur consistently after ingestion of the food allergen. Subsequent exposures can trigger more severe symptoms, depending on the amount, route, and duration of exposure to the allergen.25 Reactions typically follow ingestion or cutaneous exposures; inhalation rarely triggers a response.26 IgE-mediated release of histamine and other mediators from mast cells and basophils triggers reactions that typically involve one or more organ systems (Table 2).25

Cutaneous symptoms are the most common manifestations of food allergy, occurring in 70% to 80% of childhood reactions. Gastrointestinal and oral or respiratory symptoms occur in, respectively, 40% to 50% and 25% of allergic reactions to food. Cardiovascular symptoms develop in fewer than 10% of allergic reactions.26
Continue to: Anaphylaxis
Anaphylaxis is a serious allergic reaction that develops rapidly and can cause death; diagnosis is based on specific criteria (Table 3).27 Data for rates of anaphylaxis due to food allergy are limited. The incidence of fatal reaction due to food allergy is estimated to be 1 in every 800,000 children annually.3

Clinical suspicion. Food allergy should be suspected in infants and children who present with anaphylaxis or other symptoms (Table 225) that occur within minutes to hours of ingesting food.4 Parental and self-reports alone are insufficient to diagnose food allergy. NIAID guidelines recommend that patient reports of food allergy be confirmed, because multiple studies demonstrate that 50% to 90% of presumed food allergies are not true allergy.4 Health care providers must obtain a detailed medical history and pertinent family history, plus perform a physical exam and allergy sensitivity testing. Methods to help diagnose food allergies include skin-prick tests, allergen-specific serum IgE tests, and oral food challenges.4
General principles and utility of testing
Before ordering tests, it’s important to distinguish between food sensitization and food allergy and to inform the families of children with suspected food allergy about the limitations of skin-prick tests and serum IgE tests. A child with IgE antibodies specific to a food or with a positive skin-prick test, but without symptoms upon ingestion of the food, is merely sensitized; food allergy indicates the appearance of symptoms following exposure to a specific food, in addition to the detection of specific IgE antibodies or a positive skin-prick test to that same food.28
Skin-prick testing. Skin-prick tests can be performed at any age. The procedure involves pricking or scratching the surface of the skin, usually the volar aspect of the forearm or the back, with a commercial extract. Testing should be performed by a physician or other provider who is properly trained in the technique and in interpreting results. The extract contains specific allergenic proteins that activate mast cells, resulting in a characteristic wheal-and-flare response that is typically measured 15 to 20 minutes after application. Some medications, such as H1- and H2-receptor blockers and tricyclic antidepressants, can interfere with results and need to be held for 3 to 5 days before testing.
A positive skin-prick test result is defined as a wheal ≥ 3 mm larger in diameter than the negative control. The larger the size of the wheal, the higher the likelihood of a reaction to the tested food.29 Patients who exhibit dermatographism might experience a wheal-and-flare response from the action of the skin-prick test, rather than from food-specific IgE antibodies. A negative skin-prick test has > 90% negative predictive value, so the test can rule out suspected food allergy.30 However, the skin-prick test alone cannot be used to diagnose food allergy because it has a high false-positive rate.
Continue to: Allergen-specific serum IgE testing
Allergen-specific serum IgE testing. Measurement of food-specific serum IgE levels is routinely available and requires only a blood specimen. The test can be used in patients with skin disease, and results are not affected by concurrent medications. The presence of food-specific IgE indicates that the patient is sensitized to that allergen and might react upon exposure; children with a higher level of antibody are more likely to react.29
Food-specific serum IgE tests are sensitive but nonspecific for food allergy.31 Broad food-allergy test panels often yield false-positive results that can lead to unnecessary dietary elimination, resulting in years of inconvenience, nutrition problems, and needless health care expense.32
It is appropriate to order tests of specific serum IgE to foods ingested within the 2 to 3–hour window before onset of symptoms to avoid broad food allergy test panels. Like skin-prick testing, positive allergen-specific serum IgE tests alone cannot diagnose food allergy.
Oral food challenge. The double-blind, placebo-controlled oral food challenge is the gold standard for the diagnosis of food allergy. Because this test is time-consuming and technically difficult, single-blind or open food challenges are more common. Oral food challenges should be performed only by a physician or other provider who can identify and treat anaphylaxis.
The oral challenge starts with a very low dose of suspected food allergen, which is gradually increased every 15 to 30 minutes as vital signs are monitored carefully. Patients are observed for an allergic reaction for 1 hour after the final dose.
Continue to: A retrospective study...
A retrospective study showed that, whereas 19% of patients reacted during an open food challenge, only 2% required epinephrine.33 Another study showed that 89% of children whose serum IgE testing was positive for specific foods were able to reintroduce those foods into the diet after a reassuring oral food challenge.34
Other diagnostic tests. The basophil activation assay, measurement of total serum IgE, atopy patch tests, and intradermal tests have been used, but are not recommended, for making the diagnosis of food allergy.4
How can food allergy be managed?
Medical options are few. No approved treatment exists for food allergy. However, it’s important to appropriately manage acute reactions and reduce the risk of subsequent reactions.1 Parents or other caregivers can give an H1 antihistamine, such as diphenhydramine, to infants and children with acute non-life-threatening symptoms. More severe symptoms require rapid administration of epinephrine.1 Auto-injectable epinephrine should be prescribed for parents and caregivers to use as needed for emergency treatment of anaphylaxis.
Team approach. A multidisciplinary approach to managing food allergy—involving physicians, school nurses, dietitians, and teachers, and using educational materials—is ideal. This strategy expands knowledge about food allergies, enhances correct administration of epinephrine, and reduces allergic reactions.1
Avoidance of food allergens can be challenging. Parents and caregivers should be taught to interpret the list of ingredients on food packages. Self-recognition of allergic reactions reduces the likelihood of a subsequent severe allergic reaction.35
Continue to: Importance of individualized care
Importance of individualized care. Health care providers should develop personalized management plans for their patients.1 (A good place to start is with the “Food Allergy & Anaphylaxis Emergency Care Plan”a developed by Food Allergy Research & Education [FARE]). Keep in mind that children with multiple food allergies consume less calcium and protein, and tend to be shorter4; therefore, it’s wise to closely monitor growth in these children and consider referral to a dietitian who is familiar with food allergy.
Potential of immunotherapy. Current research focuses on immunotherapy to induce tolerance to food allergens and protect against life-threatening allergic reactions. The goal of immunotherapy is to lessen adverse reactions to allergenic food proteins; the strategy is to have patients repeatedly ingest small but gradually increasing doses of the food allergen over many months.36 Although immunotherapy has successfully allowed some patients to consume larger quantities of a food without having an allergic reaction, it is unknown whether immunotherapy provides permanent resolution of food allergy. In addition, immunotherapy often causes serious systemic and local reactions.1,36,37
Is prevention possible?
Maternal diet during pregnancy and lactation does not affect development of food allergy in infants.38,39 Breastfeeding might prevent development of atopic disease, but evidence is insufficient to determine whether breastfeeding reduces the likelihood of food allergy.39 In nonbreastfed infants at high risk of food allergy, extensively or partially hydrolyzed formula might help protect against food allergy, compared to standard cow’s milk formula.9,39 Feeding with soy formula rather than cow’s milk formula does not help prevent food allergy.39,40 Pregnant and breastfeeding women should not restrict their diet as a means of preventing food allergy.39
Diet in infancy. Over the years, physicians have debated the proper timing of the introduction of solid foods into the diet of infants. Traditional teaching advocated delaying introduction of potentially allergenic foods to reduce the risk of food allergy; however, this guideline was based on inconsistent evidence,41 and the strategy did not reduce the incidence of food allergy. The prevalence of food allergy is lower in developing countries where caregivers introduce foods to infants at an earlier age.20
A recent large clinical trial indicates that early introduction of peanut-containing foods can help prevent peanut allergy. The study randomized 4- to 11-month-old infants with severe eczema, egg allergy, or both, to eat or avoid peanut products until 5 years of age. Infants assigned to eat peanuts were 81% less likely to develop peanut allergy than infants in the avoidance group. Absolute risk reduction was 14% (number need to treat = 7).42 Another study showed a nonsignificant (20%) lower relative risk of food allergy in breastfed infants who were fed potentially allergenic foods starting at 3 months of age, compared to being exclusively breastfed.43
Continue to: Based on these data...
Based on these data,42,43 NIAID instituted recommendations in 2017 aimed at preventing peanut allergy44:
- In healthy infants without known food allergy and those with mild or moderate eczema, caregivers can introduce peanut-containing foods at home with other solid foods.Parents who are anxious about a possible allergic reaction can introduce peanut products in a physician’s office.
- Infants at high risk of peanut allergy (those with severe eczema or egg allergy, or both) should undergo peanut-specific IgE or skin-prick testing:
- Negative test: indicates low risk of a reaction to peanuts; the infant should start consuming peanut-containing foods at 4 to 6 months of age, at home or in a physician’s office, depending on the parents’ preference
- Positive test: Referral to an allergist is recommended.
Do children outgrow food allergy?
Approximately 85% of children who have an allergy to milk, egg, soy, or wheat outgrow their allergy; however, only 15% to 20% who have an allergy to peanuts, tree nuts, fish, or shellfish eventually tolerate these foods. The time to resolution of food allergy varies with the food, and might not occur until adolescence.4 No test reliably predicts which children develop tolerance to any given food. A decrease in the food-specific serum IgE level or a decrease in the size of the wheal on skin-prick testing might portend the onset of tolerance to the food.4
CORRESPONDENCE
Catherine M. Bettcher, MD, FAAFP, Briarwood Family Medicine, 1801 Briarwood Circle, Building #10, Ann Arbor, MI 48108; [email protected].
1. Muraro A, Werfel T, Hoffmann-Sommergruber K, et al; . EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014;69:1008-1025.
2. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
3. Cianferoni A, Muraro A. Food-induced anaphylaxis. Immunol Allergy Clin North Am. 2012;32:165-195.
4., Boyce JA, Assa’ad A, Burks WA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
5. Vierk KA, Koehler KM, Fein SB, et al. Prevalence of self-reported food allergy in American adults and use of food labels. J Allergy Clin Immunol. 2007;119:1504-1510.
6. Allen KJ, Koplin JJ. The epidemiology of IgE-mediated food allergy and anaphylaxis. Immunol Allergy Clin North Am. 2012;32:35-50.
7. Iweala OI, Choudhary SK, Commins SP. Food allergy. Curr Gastroenterol Rep. 2018;20:17.
8. Gupta RS, Warren CM, Smith BM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. 2018;142:e20181235.
9. Chafen JJS, Newberry SJ, Riedl MA, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010;303:1848-1856.
10. Nwaru BI, Hickstein L, Panesar SS, et al. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69:992-1007.
11. Branum AM, Lukacs SL. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS Data Brief No. 10. National Center for Health Statistics. October 2008. www.cdc.gov/nchs/products/databriefs/db10.htm. Accessed August 19, 2020.
12. Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126:798-806.e13.
13. Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9-e17.
14. Soller L, Ben-Shoshan M, Harrington DW, et al. Overall prevalence of self-reported food allergy in Canada. J Allergy Clin Immunol. 2012;130:986-988.
15. Venter C, Pereira B, Voigt K, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008;63:354-359.
16. Savage J, Johns CB. Food allergy: epidemiology and natural history. Immunol Allergy Clin North Am. 2015;35:45-59.
17. Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124:1549-1555.
18. Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997-2011. NCHS Data Brief No. 121. National Center for Health Statistics. May 2013. www.cdc.gov/nchs/products/databriefs/db121.htm. Accessed August 19, 2020.
19. Willits EK, Park MA, Hartz MF, et al. Food allergy: a comprehensive population-based cohort study. Mayo Clin Proc. 2018;93:1423-1430.
20. Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol. 2008;121:1331-1336.
21. Okada H, Kuhn C, Feillet H, et al. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1-9.
22. Liu AH. Hygiene theory and allergy and asthma prevention. Paediatr Perinat Epidemiol. 2007;21 Suppl 3:2-7.
23. Prince BT, Mandel MJ, Nadeau K, et al. Gut microbiome and the development of food allergy and allergic disease. Pediatr Clin North Am. 2015;62:1479-1492.
24. Kusunoki T, Mukaida K, Morimoto T, et al. Birth order effect on childhood food allergy. Pediatr Allergy Immunol. 2012;23:250-254.
25. Abrams EM, Sicherer SH. Diagnosis and management of food allergy. CMAJ. 2016;188:1087-1093.
26. Perry TT, Matsui EC, Conover-Walker MK, et al. Risk of oral food challenges. J Allergy Clin Immunol. 2004;114:1164-1168.
27. Sampson HA, A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391-397.
28. Sampson HA. Food allergy. Part 2: diagnosis and management. J Allergy Clin Immunol. 1999;103:981-989.
29. Lieberman JA, Sicherer SH. Diagnosis of food allergy: epicutaneous skin tests, in vitro tests, and oral food challenge. Curr Allergy Asthma Rep. 2011;11:58-64.
30. Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S116-S125.
31. Soares-Weiser K, Takwoingi Y, Panesar SS, et al. The diagnosis of food allergy: a systematic review and meta-analysis. Allergy. 2014;69:76-86.
32. Bird JA, Crain M, Varshney P. Food allergen panel testing often results in misdiagnosis of food allergy. J Pediatr. 2015;166:97-100.
33. Lieberman JA, Cox AL, Vitale M, et al. Outcomes of office-based, open food challenges in the management of food allergy. J Allergy Clin Immunol. 2011;128:1120-1122.
34. Fleischer DM, Bock SA, Spears GC, et al. Oral food challenges in children with a diagnosis of food allergy. J Pediatr. 2011;158:578-583.e1.
35. Ewan PW, Clark AT. Long-term prospective observational study of patients with peanut and nut allergy after participation in a management plan. Lancet. 2001;357:111-115.
36. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
37. Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update—2014. J Allergy Clin Immunol. 2014;134:1016-1025.e43.
38. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;2012(9):CD000133.
39. de Silva D, Geromi M, Halken S, et al; . Primary prevention of food allergy in children and adults: systematic review. Allergy. 2014;69:581-589.
40. Osborn DA, Sinn J. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev. 2004;(3):CD003741.
41. Filipiak B, Zutavern A, Koletzko S, et al; GINI-Group. Solid food introduction in relation to eczema: results from a four-year prospective birth cohort study. J Pediatr. 2007;151:352-358.
42. Du Toit G, Roberts G, Sayre PH, et al; LEAP Study Team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
43. Perkin MR, Logan K, Tseng A, et al; EAT Study Team. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
44. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
1. Muraro A, Werfel T, Hoffmann-Sommergruber K, et al; . EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014;69:1008-1025.
2. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
3. Cianferoni A, Muraro A. Food-induced anaphylaxis. Immunol Allergy Clin North Am. 2012;32:165-195.
4., Boyce JA, Assa’ad A, Burks WA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
5. Vierk KA, Koehler KM, Fein SB, et al. Prevalence of self-reported food allergy in American adults and use of food labels. J Allergy Clin Immunol. 2007;119:1504-1510.
6. Allen KJ, Koplin JJ. The epidemiology of IgE-mediated food allergy and anaphylaxis. Immunol Allergy Clin North Am. 2012;32:35-50.
7. Iweala OI, Choudhary SK, Commins SP. Food allergy. Curr Gastroenterol Rep. 2018;20:17.
8. Gupta RS, Warren CM, Smith BM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. 2018;142:e20181235.
9. Chafen JJS, Newberry SJ, Riedl MA, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010;303:1848-1856.
10. Nwaru BI, Hickstein L, Panesar SS, et al. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69:992-1007.
11. Branum AM, Lukacs SL. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS Data Brief No. 10. National Center for Health Statistics. October 2008. www.cdc.gov/nchs/products/databriefs/db10.htm. Accessed August 19, 2020.
12. Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126:798-806.e13.
13. Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9-e17.
14. Soller L, Ben-Shoshan M, Harrington DW, et al. Overall prevalence of self-reported food allergy in Canada. J Allergy Clin Immunol. 2012;130:986-988.
15. Venter C, Pereira B, Voigt K, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008;63:354-359.
16. Savage J, Johns CB. Food allergy: epidemiology and natural history. Immunol Allergy Clin North Am. 2015;35:45-59.
17. Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124:1549-1555.
18. Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997-2011. NCHS Data Brief No. 121. National Center for Health Statistics. May 2013. www.cdc.gov/nchs/products/databriefs/db121.htm. Accessed August 19, 2020.
19. Willits EK, Park MA, Hartz MF, et al. Food allergy: a comprehensive population-based cohort study. Mayo Clin Proc. 2018;93:1423-1430.
20. Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol. 2008;121:1331-1336.
21. Okada H, Kuhn C, Feillet H, et al. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1-9.
22. Liu AH. Hygiene theory and allergy and asthma prevention. Paediatr Perinat Epidemiol. 2007;21 Suppl 3:2-7.
23. Prince BT, Mandel MJ, Nadeau K, et al. Gut microbiome and the development of food allergy and allergic disease. Pediatr Clin North Am. 2015;62:1479-1492.
24. Kusunoki T, Mukaida K, Morimoto T, et al. Birth order effect on childhood food allergy. Pediatr Allergy Immunol. 2012;23:250-254.
25. Abrams EM, Sicherer SH. Diagnosis and management of food allergy. CMAJ. 2016;188:1087-1093.
26. Perry TT, Matsui EC, Conover-Walker MK, et al. Risk of oral food challenges. J Allergy Clin Immunol. 2004;114:1164-1168.
27. Sampson HA, A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391-397.
28. Sampson HA. Food allergy. Part 2: diagnosis and management. J Allergy Clin Immunol. 1999;103:981-989.
29. Lieberman JA, Sicherer SH. Diagnosis of food allergy: epicutaneous skin tests, in vitro tests, and oral food challenge. Curr Allergy Asthma Rep. 2011;11:58-64.
30. Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S116-S125.
31. Soares-Weiser K, Takwoingi Y, Panesar SS, et al. The diagnosis of food allergy: a systematic review and meta-analysis. Allergy. 2014;69:76-86.
32. Bird JA, Crain M, Varshney P. Food allergen panel testing often results in misdiagnosis of food allergy. J Pediatr. 2015;166:97-100.
33. Lieberman JA, Cox AL, Vitale M, et al. Outcomes of office-based, open food challenges in the management of food allergy. J Allergy Clin Immunol. 2011;128:1120-1122.
34. Fleischer DM, Bock SA, Spears GC, et al. Oral food challenges in children with a diagnosis of food allergy. J Pediatr. 2011;158:578-583.e1.
35. Ewan PW, Clark AT. Long-term prospective observational study of patients with peanut and nut allergy after participation in a management plan. Lancet. 2001;357:111-115.
36. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
37. Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update—2014. J Allergy Clin Immunol. 2014;134:1016-1025.e43.
38. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;2012(9):CD000133.
39. de Silva D, Geromi M, Halken S, et al; . Primary prevention of food allergy in children and adults: systematic review. Allergy. 2014;69:581-589.
40. Osborn DA, Sinn J. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev. 2004;(3):CD003741.
41. Filipiak B, Zutavern A, Koletzko S, et al; GINI-Group. Solid food introduction in relation to eczema: results from a four-year prospective birth cohort study. J Pediatr. 2007;151:352-358.
42. Du Toit G, Roberts G, Sayre PH, et al; LEAP Study Team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
43. Perkin MR, Logan K, Tseng A, et al; EAT Study Team. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
44. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
PRACTICE RECOMMENDATIONS
› Diagnose food allergy based on a convincing clinical history paired with positive diagnostic testing. A
› Use a multidisciplinary approach to improve caregiver and patient understanding of food allergy and to reduce allergic reactions. B
› Recommend early introduction of peanut products to infants to reduce the likelihood of peanut allergy. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What You Can Do To Improve Adult Immunization Rates
› Recommend immunization to patients routinely. Most adults believe vaccines are important and are likely to get them if recommended by their health care professionals. C
› Consider implementing standing orders that authorize nurses, pharmacists, or other trained health care personnel to assess a patient’s immunization status and administer vaccinations according to a protocol. C
› Explore the use of Web-based patient portals or other new-media communication formats to engage patients. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Vaccines have been proven effective in preventing disease and are one of the most cost-effective and successful public health initiatives of the 20th century. Nevertheless, adult vaccination rates in the United States for vaccine-preventable diseases are low for most routinely recommended vaccines.1 In 2013 alone, there were an estimated 3700 deaths in the United States (95% of which were adults) from pneumococcal infections—a vaccine-preventable disorder.2
Consider the threat posed by the flu. Annually, most people who die of influenza and its complications are adults, with estimates ranging from a low of 3000 to a high of 49,000 based on Centers for Disease Control and Prevention (CDC) data from the 1976-1977 flu season to the 2006-2007 season.3 Vaccination during the 2013-2014 season resulted in an estimated 7.2 million fewer cases of influenza, 90,000 fewer hospitalizations, and 3.1 million fewer medically attended cases than would have been expected without vaccination.4 If vaccination levels had reached the Healthy People 2020 target of 70%, an additional 5.9 million illnesses, 2.3 million medically attended illnesses, and 42,000 hospitalizations might have been averted.4
How are we doing with other vaccines? Based on the 2013 National Health Interview Survey, the CDC assessed vaccination coverage among adults ages ≥19 years for selected vaccines: pneumococcal vaccine, tetanus toxoid-containing vaccines (tetanus and diphtheria vaccine [Td] or tetanus and diphtheria with acellular pertussis vaccine [Tdap]), and vaccines for hepatitis A, hepatitis B, herpes zoster, and human papillomavirus (HPV). (With the exception of influenza vaccination, which is recommended annually for all adults, other vaccinations are directed at specific populations based on age, health conditions, behavioral risk factors, occupation, or travel conditions.)
Overall, coverage rates for hepatitis A and B, pneumococcal, Td, and human papillomavirus (HPV) for all adults did not improve from 2012 to 2013; rates increased only modestly for Tdap among adults ≥19 years, for herpes zoster among adults ≥60 years, and for HPV among men ages 19 to 26. Furthermore, racial and ethnic gaps in coverage are seen in all vaccines, and these gaps widened since 2012 for Tdap, herpes zoster, and HPV vaccination.1
Commonly cited barriers to improved vaccine uptake in adults include lack of regular assessment of vaccine status; lack of physician and other health care provider knowledge on current vaccine recommendations; cost; insufficient stocking of some vaccines; financial disincentives for vaccination in the primary care setting; limited use of electronic records, tools, and immunization registries; missed opportunities; and patient hesitancy and vaccine refusal.5
Removing barriers to immunization. Several recommendations on ways to improve adult vaccination rates are made by many federal organizations as well as by The Community Preventive Services Task Force (Task Force), an independent, nonfederal, unpaid panel of public health and prevention experts. The Task Force—which makes recommendations based on systematic reviews of the evidence of effectiveness, the applicability of the evidence, economic evaluations, and barriers to implementation of interventions6—advocates a 3-pronged approach to improve adult vaccination rates: 1) enhance access to vaccination services; 2) increase community demand for vaccinations; and 3) incorporate physician- or system-based interventions into practice.7
The CDC and other groups such as the National Vaccine Advisory Committee (NVAC) recommend that every routine adult office visit include a vaccination needs assessment, recommendation, and offer of vaccination.8 Additionally, the Task Force recommends 3 means of enhancing adult access to vaccination services: make home visits, reduce patient costs, and offer vaccination programs in the community.7
This article describes a number of simple steps physicians can take to increase the likelihood that adults will get their vaccines and reviews the literature on using new media such as smartphones and other Internet-based tools to improve immunization coverage.9
Increasing community demands for vaccinations
Physicians and other healthcare providers can increase community demand for vaccinations by improving their own knowledge on the subject, recommending vaccination to patients, and increasing their community and political involvement to strengthen or change laws to better support immunization uptake.
To increase awareness and education, keep abreast of the Advisory Committee on Immunization Practices (ACIP) recommendations and guidelines, which are updated annually and reported on in this journal’s Practice Alert column. Consider taking advantage of free immunization apps that are available from the CDC (“CDC Vaccine Schedules” http://www.cdc.gov/vaccines/schedules/hcp/schedule-app.html), the Society of Teachers of Family Medicine (STFM; “Shots Immunizations” http://www.immunizationed.org/Shots-Mobile-App), and the American College of Physicians (“ACP Immunization Advisor” http://immunization.acponline.org/app/).
Take steps to put guidelines into practice. Despite wide promulgation, clinical practice guidelines alone have had limited effect on changing physician behavior and improving patient outcomes. Interactive techniques are more effective than guidelines and didactic presentations alone at changing physician care and patient outcomes. Such techniques include audit/feedback (the reporting of an individual clinician’s vaccination rates compared with desired or target rates, for example), academic detailing/outreach, and reminders by way of electronic or other alerts.10,11
Promote immunization to patients. Physicians are highly influential in determining a patient’s decision to vaccinate, and it is well documented that a strong recommendation about the importance of immunizations makes a difference to patients.12,13
What you say and how you say it matters. A halfhearted recommendation for vaccination may result in the patient remaining unvaccinated.14 For example, “If you want, you can get your pneumonia shot today” is much less persuasive than, “I recommend you get your pneumonia vaccine today to prevent a potentially serious disease that affects thousands of adults each year.” Most adults believe that vaccines are important and are likely to get them if recommended by their health care professionals.15
The CDC recommends that physicians encourage patients to make an informed decision about vaccination by sharing critical information highlighting the importance of vaccinations and reminding patients what vaccines protect against while addressing their concerns (www.cdc.gov/vaccines/adultstandards). Free educational materials for patients can be found at www.cdc.gov/vaccines/AdultPatientEd.
Draw on community resources. Laws and policies that require vaccinations as a prerequisite for attending childcare, school, or college increase coverage. Community and faith-based organizations are likely to play an important role in reducing racial and ethnic disparities in adult immunizations because they can deliver education that is culturally sensitive and tailored to specific subpopulations.16,17 Physicians and other health care providers can get involved with community and faith-based groups and local and federal legislative efforts to improve immunization rates.
Consider implementing these system-based interventions
The following 6 system-based interventions can help improve adult immunization rates:
1. Develop a practice team. The practice team, based on the Patient-Centered Medical Home (PCMH), includes physicians, midlevel providers, nurses, medical assistants, pharmacists, social workers, and other staff. The PCMH team model can facilitate a shift of responsibilities among individuals to better orient the practice toward patients’ health and preventive services.18,19 While physicians have traditionally held all of the responsibility for patient care, including screening for disease and prevention, shifting the responsibility of vaccine screening to nurses or medical assistants can free up time for longer physician/patient interactions.18
The creation of a practice champion within the PCMH team—a physician, midlevel provider, or nurse—to oversee quality improvement for vaccine rates and work to generate support and cooperation from coworkers has also been shown to improve vaccination rates.20 The vaccine champion should keep abreast of new vaccine recommendations and relay that information to the practice through regular staff meetings, announcements, and office postings. The champion can also supervise pre-visit planning for immunizations.19
2. Use electronic immunization information systems (IIS). All states except New Hampshire have an IIS.21 Accurate tracking of adult immunizations in a registry provides a complete record and is essential to improving adult immunization rates,22 as does the use of chart notes, computerized alerts, checklists, and other tools that remind health care providers when patients are due for vaccinations.18 NVAC recommends that all physicians use their state IIS and create a process in their practice to include its use.
3. Incorporate physician feedback. Many health care systems and payers are using benchmarking and incentives to provide physician feedback on vaccination performance.23 Using achievable benchmarks enhances the effectiveness of physician performance feedback.24 The Task Force conducted a systematic review of the evidence on the effectiveness of health care provider assessment and feedback for increasing coverage rates and found that this strategy remains an effective means to increase vaccination rates.25
4. Use reminders/alerts. Even though you may intend to routinely recommend immunizations, remembering to do so at the time of each visit can be difficult when there are so many other issues to address. Reminders at the time of the visit can help. Some electronic records have reminder prompts, or “best practice alerts” (BPAs), programmed into their systems.26 These BPAs will prompt for needed immunizations whether the patient is being seen for a well, acute, or routine follow-up visit. These reminder/recall activities can be greatly simplified by participation in a population-based IIS.
Practices that don’t have an electronic health record can still improve vaccination rates by conveying the reminder with a brightly colored paper form attached to the front of a patient’s chart during the check-in process. One recent study showed that this approach increased rates of influenza vaccination in an urban practice by 12 percentage points.27
Furthermore, simply reminding patients to vaccinate increases the vaccination rate.28 Patient reminder/recall systems using telephone calls or mailings (phone calls are more effective than mailings) improve both childhood and adult vaccinations in all medical settings. More intensive systems using multiple reminders appear to be more effective than single reminders, and while costly, the benefits of increasing preventive visits/services and vaccine uptake help offset this cost.28
5. Implement standing orders. Standing orders—which allow nurses and other appropriately trained health care personnel to assess immunization status and administer vaccinations according to protocol—help improve immunization rates.29 ACIP advises that standing order programs be used in long-term care facilities under the supervision of a medical director to ensure the administration of recommended vaccinations for adults, and in inpatient and outpatient facilities. Because of the societal burden of influenza and pneumococcal disease, implementation of standing orders programs to improve adult vaccination coverage for these diseases is considered a national public health priority.30
6. Develop an encouraging communication style. Studies show that how one communicates with patients is just as important as what one communicates. Certain communication styles and techniques may be more or less effective when discussing vaccination needs with some patients, especially those with vaccine hesitancy or low confidence in vaccine safety or effectiveness. For example, styles that are “directing” are usually unhelpful in addressing concerns about vaccination. These styles typically use information and persuasion to achieve change and may be perceived as confrontational. This approach can lead to cues being missed, jargon being used, and vaccine safety being overstated.
Styles shown to be helpful are those that elicit patient concerns, ask permission to discuss, acknowledge/listen/empathize, determine readiness to change, inform about benefits and risks, and give appropriate resources. These helpful forms of communication are more of a “May I help you?” style vs a “This is what you should do” style of communication.31
Assure patients that recommendations are based on the best interest of their health and on the best available science. Listen to a patient’s concerns and acknowledge them in a nonconfrontational manner, allowing patients to express their concerns and thereby increase their willingness to listen.32 Saying that there is “absolutely no need to worry—vaccines are safe and you are silly not to get yours” is not as effective as saying, “What are your concerns regarding vaccines? Let’s talk about them.”
For the vaccine-hesitant group, building trust is essential through a respectful, nonjudgmental approach that aims to elicit and address specific concerns. For those who refuse vaccines, keep the consultation brief, keep the door open for further discussion, and provide appropriate resources if the patient wants them.33
Increase use of new media
Mass communication through smartphones and other Internet-based tools such as Facebook and Twitter brings a new dimension to health care, allowing patients and health professionals to communicate about health issues and possibly improve health outcomes.34 The number of people using social media increased by almost 570% worldwide between 2000 and 2012 and surpassed 2.75 billion in 2013.35
Sixty-one percent of adults in the United States look online for health information.36 In a survey conducted in September 2014, the Pew Research Center found that Facebook is the most popular social media site in the United States. Seventy-one percent of online-knowledgeable adults use Facebook, and multiplatform use is on the rise: 52% of adult Internet users now use 2 or more social media sites, a significant increase from 2013, when it stood at 42%. (Other platforms such as Twitter, Instagram, Pinterest, and LinkedIn saw significant increases over the past year in the proportion of online adults who use them).37
Health information provided by social media can answer medical questions and concerns and enhance health promotion and education.35 A recent review of 98 research studies provided evidence that social media can create a space to share, comment, and discuss health information.34 Compared with traditional communication methods, the widespread availability of social media makes health information more accessible, broadening access to various population groups, regardless of age, education, race, ethnicity, and locale.
New media platforms are proving effective. The first systematic assessment of available evidence on the use of new media to increase vaccine uptake and immunization coverage (a review of 7 randomized controlled trials [RCTs], 5 non-RCTs, 3 cross-sectional studies, one case-control study and 3 operational research studies published between 2000-2013) found that text messaging, accessing immunization campaign Web sites, using patient-held Web-based portals, computerized reminders, and standing orders increased immunization coverage rates.35 However, evidence was insufficient in this regard on the value of social networks, email communication, and smartphone applications.
One RCT showed that having access to a personalized Web-based portal where patients could manage health records as well as interact with both health care providers and other members of the community through social forums and messaging tools increased influenza vaccination rates.35
CORRESPONDENCE
Pamela G. Rockwell, DO, Department of Family Medicine, University of Michigan, 24 Frank Lloyd Wright Drive, P.O. Box 431, Ann Arbor, MI 48106-0795; [email protected].
1. Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:95-102.
2. Centers for Disease Control and Prevention. Active bacterial core surveillance (ABCs) report, emerging infections program network, Streptococcus pneumoniae, 2013. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Accessed August 20, 2015.
3. Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057-1062.
4. Reed C, Kim IK, Singleton JA, et al; Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by vaccination--United States, 2013-14 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:1151-1154.
5. Kimmel SR, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
6. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based Guide to Community Preventive Services—methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
7. The Guide to Community Preventive Services. Increasing appropriate vaccination. The Community Guide Web site. Available at: http://www.thecommunityguide.org/vaccines/index.html. Accessed August 20, 2015.
8. National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory committee: standards for adult immunization practice. Public Health Rep. 2014;129:115-123.
9. Househ M. The use of social media in healthcare: organizational, clinical, and patient perspectives. Stud Health Technol Inform. 2013;183:244-248.
10. Bloom BS. Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews. Int J Technol Assess Health Care. 2005;21:380-385.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458-1465.
12. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19-26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
13. Zimmerman RK, Santibanez TA, Janosky JE, et al. What affects influenza vaccination rates among older patients? An analysis from inner-city, suburban, rural, and Veterans Affairs practices. Am J Med. 2003;114:31-38.
14. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed August 20, 2015.
15. National Foundation for Infectious Diseases. Survey: adults do not recognize infectious disease risks. National Foundation for Infectious Diseases Web site. Available at: http://www.adultvaccination.org/newsroom/events/2009-vaccination-news-conference/NFID-Survey-Fact-Sheet.pdf. Accessed July 7, 2015.
16. Wang E, Clymer J, Davis-Hayes C, et al. Nonmedical exemptions from school immunization requirements: a systematic review. Am J Public Health. 2014;104:e62-e84.
17. National Vaccine Advisory Committee. A pathway to leadership for adult immunization: recommendations of the National Vaccine Advisory Committee: approved by the National Vaccine Advisory Committee on June 14, 2011. Public Health Rep. 2012;127:1-42.
18. Gannon M, Qaseem A, Snooks Q, et al. Improving adult immunization practices using a team approach in the primary care setting. Am J Public Health. 2012;102:e46-e52.
19. Bottino CJ, Cox JE, Kahlon PS, et al. Improving immunization rates in a hospital-based primary care practice. Pediatrics. 2014;133:e1047-e1054.
20. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
21. Centers for Disease Control and Prevention (CDC). Progress in immunization information systems - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:1005-1008.
22. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
23. Kerr EA, McGlynn EA, Adams J, et al. Profiling the quality of care in twelve communities: results from the CQI study. Health Aff (Millwood). 2004;23:247-256.
24. Kiefe CI, Allison JJ, Williams OD, et al. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA. 2001;285:2871-2879.
25. National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1-64.
26. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
27. Pierson RC, Malone AM, Haas DM. Increasing influenza vaccination rates in a busy urban clinic. J Nat Sci. 2015;1.
28. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;CD003941.
29. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Task Force on Community Preventive Services. Am J Prev Med. 2000;18:92-96.
30. McKibben LJ, Stange PV, Sneller VP, et al; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49:15-16.
31. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154.
32. Kimmel SR, Wolfe RM. Communicating the benefits and risks of vaccines. J Fam Pract. 2005;54:S51-S57.
33. Danchin M, Nolan T. A positive approach to parents with concerns about vaccination for the family physician. Aust Fam Physician. 2014;43:690-694.
34. Moorhead SA, Hazlett DE, Harrison L, et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
35. Odone A, Ferrari A, Spagnoli F, et al. Effectiveness of interventions that apply new media to improve vaccine uptake and vaccine coverage. Hum Vaccin Immunother. 2015;11:72-82.
36. Pew Research Center. Fox S. The Social Life of Health Information, 2011. Pew Research Center Web site. Available at: http://www.pewinternet.org/2011/05/12/the-social-life-of-health-information-2011/. Accessed August 20, 2015.
37. Pew Research Center. Duggan M, Ellison NB, Lampe C, et al. Social Media Update 2014. Pew Research Center Web site. Available at: http://www.pewinternet.org/2015/01/09/social-media-update-2014/. Accessed August 20, 2015.
› Recommend immunization to patients routinely. Most adults believe vaccines are important and are likely to get them if recommended by their health care professionals. C
› Consider implementing standing orders that authorize nurses, pharmacists, or other trained health care personnel to assess a patient’s immunization status and administer vaccinations according to a protocol. C
› Explore the use of Web-based patient portals or other new-media communication formats to engage patients. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Vaccines have been proven effective in preventing disease and are one of the most cost-effective and successful public health initiatives of the 20th century. Nevertheless, adult vaccination rates in the United States for vaccine-preventable diseases are low for most routinely recommended vaccines.1 In 2013 alone, there were an estimated 3700 deaths in the United States (95% of which were adults) from pneumococcal infections—a vaccine-preventable disorder.2
Consider the threat posed by the flu. Annually, most people who die of influenza and its complications are adults, with estimates ranging from a low of 3000 to a high of 49,000 based on Centers for Disease Control and Prevention (CDC) data from the 1976-1977 flu season to the 2006-2007 season.3 Vaccination during the 2013-2014 season resulted in an estimated 7.2 million fewer cases of influenza, 90,000 fewer hospitalizations, and 3.1 million fewer medically attended cases than would have been expected without vaccination.4 If vaccination levels had reached the Healthy People 2020 target of 70%, an additional 5.9 million illnesses, 2.3 million medically attended illnesses, and 42,000 hospitalizations might have been averted.4
How are we doing with other vaccines? Based on the 2013 National Health Interview Survey, the CDC assessed vaccination coverage among adults ages ≥19 years for selected vaccines: pneumococcal vaccine, tetanus toxoid-containing vaccines (tetanus and diphtheria vaccine [Td] or tetanus and diphtheria with acellular pertussis vaccine [Tdap]), and vaccines for hepatitis A, hepatitis B, herpes zoster, and human papillomavirus (HPV). (With the exception of influenza vaccination, which is recommended annually for all adults, other vaccinations are directed at specific populations based on age, health conditions, behavioral risk factors, occupation, or travel conditions.)
Overall, coverage rates for hepatitis A and B, pneumococcal, Td, and human papillomavirus (HPV) for all adults did not improve from 2012 to 2013; rates increased only modestly for Tdap among adults ≥19 years, for herpes zoster among adults ≥60 years, and for HPV among men ages 19 to 26. Furthermore, racial and ethnic gaps in coverage are seen in all vaccines, and these gaps widened since 2012 for Tdap, herpes zoster, and HPV vaccination.1
Commonly cited barriers to improved vaccine uptake in adults include lack of regular assessment of vaccine status; lack of physician and other health care provider knowledge on current vaccine recommendations; cost; insufficient stocking of some vaccines; financial disincentives for vaccination in the primary care setting; limited use of electronic records, tools, and immunization registries; missed opportunities; and patient hesitancy and vaccine refusal.5
Removing barriers to immunization. Several recommendations on ways to improve adult vaccination rates are made by many federal organizations as well as by The Community Preventive Services Task Force (Task Force), an independent, nonfederal, unpaid panel of public health and prevention experts. The Task Force—which makes recommendations based on systematic reviews of the evidence of effectiveness, the applicability of the evidence, economic evaluations, and barriers to implementation of interventions6—advocates a 3-pronged approach to improve adult vaccination rates: 1) enhance access to vaccination services; 2) increase community demand for vaccinations; and 3) incorporate physician- or system-based interventions into practice.7
The CDC and other groups such as the National Vaccine Advisory Committee (NVAC) recommend that every routine adult office visit include a vaccination needs assessment, recommendation, and offer of vaccination.8 Additionally, the Task Force recommends 3 means of enhancing adult access to vaccination services: make home visits, reduce patient costs, and offer vaccination programs in the community.7
This article describes a number of simple steps physicians can take to increase the likelihood that adults will get their vaccines and reviews the literature on using new media such as smartphones and other Internet-based tools to improve immunization coverage.9
Increasing community demands for vaccinations
Physicians and other healthcare providers can increase community demand for vaccinations by improving their own knowledge on the subject, recommending vaccination to patients, and increasing their community and political involvement to strengthen or change laws to better support immunization uptake.
To increase awareness and education, keep abreast of the Advisory Committee on Immunization Practices (ACIP) recommendations and guidelines, which are updated annually and reported on in this journal’s Practice Alert column. Consider taking advantage of free immunization apps that are available from the CDC (“CDC Vaccine Schedules” http://www.cdc.gov/vaccines/schedules/hcp/schedule-app.html), the Society of Teachers of Family Medicine (STFM; “Shots Immunizations” http://www.immunizationed.org/Shots-Mobile-App), and the American College of Physicians (“ACP Immunization Advisor” http://immunization.acponline.org/app/).
Take steps to put guidelines into practice. Despite wide promulgation, clinical practice guidelines alone have had limited effect on changing physician behavior and improving patient outcomes. Interactive techniques are more effective than guidelines and didactic presentations alone at changing physician care and patient outcomes. Such techniques include audit/feedback (the reporting of an individual clinician’s vaccination rates compared with desired or target rates, for example), academic detailing/outreach, and reminders by way of electronic or other alerts.10,11
Promote immunization to patients. Physicians are highly influential in determining a patient’s decision to vaccinate, and it is well documented that a strong recommendation about the importance of immunizations makes a difference to patients.12,13
What you say and how you say it matters. A halfhearted recommendation for vaccination may result in the patient remaining unvaccinated.14 For example, “If you want, you can get your pneumonia shot today” is much less persuasive than, “I recommend you get your pneumonia vaccine today to prevent a potentially serious disease that affects thousands of adults each year.” Most adults believe that vaccines are important and are likely to get them if recommended by their health care professionals.15
The CDC recommends that physicians encourage patients to make an informed decision about vaccination by sharing critical information highlighting the importance of vaccinations and reminding patients what vaccines protect against while addressing their concerns (www.cdc.gov/vaccines/adultstandards). Free educational materials for patients can be found at www.cdc.gov/vaccines/AdultPatientEd.
Draw on community resources. Laws and policies that require vaccinations as a prerequisite for attending childcare, school, or college increase coverage. Community and faith-based organizations are likely to play an important role in reducing racial and ethnic disparities in adult immunizations because they can deliver education that is culturally sensitive and tailored to specific subpopulations.16,17 Physicians and other health care providers can get involved with community and faith-based groups and local and federal legislative efforts to improve immunization rates.
Consider implementing these system-based interventions
The following 6 system-based interventions can help improve adult immunization rates:
1. Develop a practice team. The practice team, based on the Patient-Centered Medical Home (PCMH), includes physicians, midlevel providers, nurses, medical assistants, pharmacists, social workers, and other staff. The PCMH team model can facilitate a shift of responsibilities among individuals to better orient the practice toward patients’ health and preventive services.18,19 While physicians have traditionally held all of the responsibility for patient care, including screening for disease and prevention, shifting the responsibility of vaccine screening to nurses or medical assistants can free up time for longer physician/patient interactions.18
The creation of a practice champion within the PCMH team—a physician, midlevel provider, or nurse—to oversee quality improvement for vaccine rates and work to generate support and cooperation from coworkers has also been shown to improve vaccination rates.20 The vaccine champion should keep abreast of new vaccine recommendations and relay that information to the practice through regular staff meetings, announcements, and office postings. The champion can also supervise pre-visit planning for immunizations.19
2. Use electronic immunization information systems (IIS). All states except New Hampshire have an IIS.21 Accurate tracking of adult immunizations in a registry provides a complete record and is essential to improving adult immunization rates,22 as does the use of chart notes, computerized alerts, checklists, and other tools that remind health care providers when patients are due for vaccinations.18 NVAC recommends that all physicians use their state IIS and create a process in their practice to include its use.
3. Incorporate physician feedback. Many health care systems and payers are using benchmarking and incentives to provide physician feedback on vaccination performance.23 Using achievable benchmarks enhances the effectiveness of physician performance feedback.24 The Task Force conducted a systematic review of the evidence on the effectiveness of health care provider assessment and feedback for increasing coverage rates and found that this strategy remains an effective means to increase vaccination rates.25
4. Use reminders/alerts. Even though you may intend to routinely recommend immunizations, remembering to do so at the time of each visit can be difficult when there are so many other issues to address. Reminders at the time of the visit can help. Some electronic records have reminder prompts, or “best practice alerts” (BPAs), programmed into their systems.26 These BPAs will prompt for needed immunizations whether the patient is being seen for a well, acute, or routine follow-up visit. These reminder/recall activities can be greatly simplified by participation in a population-based IIS.
Practices that don’t have an electronic health record can still improve vaccination rates by conveying the reminder with a brightly colored paper form attached to the front of a patient’s chart during the check-in process. One recent study showed that this approach increased rates of influenza vaccination in an urban practice by 12 percentage points.27
Furthermore, simply reminding patients to vaccinate increases the vaccination rate.28 Patient reminder/recall systems using telephone calls or mailings (phone calls are more effective than mailings) improve both childhood and adult vaccinations in all medical settings. More intensive systems using multiple reminders appear to be more effective than single reminders, and while costly, the benefits of increasing preventive visits/services and vaccine uptake help offset this cost.28
5. Implement standing orders. Standing orders—which allow nurses and other appropriately trained health care personnel to assess immunization status and administer vaccinations according to protocol—help improve immunization rates.29 ACIP advises that standing order programs be used in long-term care facilities under the supervision of a medical director to ensure the administration of recommended vaccinations for adults, and in inpatient and outpatient facilities. Because of the societal burden of influenza and pneumococcal disease, implementation of standing orders programs to improve adult vaccination coverage for these diseases is considered a national public health priority.30
6. Develop an encouraging communication style. Studies show that how one communicates with patients is just as important as what one communicates. Certain communication styles and techniques may be more or less effective when discussing vaccination needs with some patients, especially those with vaccine hesitancy or low confidence in vaccine safety or effectiveness. For example, styles that are “directing” are usually unhelpful in addressing concerns about vaccination. These styles typically use information and persuasion to achieve change and may be perceived as confrontational. This approach can lead to cues being missed, jargon being used, and vaccine safety being overstated.
Styles shown to be helpful are those that elicit patient concerns, ask permission to discuss, acknowledge/listen/empathize, determine readiness to change, inform about benefits and risks, and give appropriate resources. These helpful forms of communication are more of a “May I help you?” style vs a “This is what you should do” style of communication.31
Assure patients that recommendations are based on the best interest of their health and on the best available science. Listen to a patient’s concerns and acknowledge them in a nonconfrontational manner, allowing patients to express their concerns and thereby increase their willingness to listen.32 Saying that there is “absolutely no need to worry—vaccines are safe and you are silly not to get yours” is not as effective as saying, “What are your concerns regarding vaccines? Let’s talk about them.”
For the vaccine-hesitant group, building trust is essential through a respectful, nonjudgmental approach that aims to elicit and address specific concerns. For those who refuse vaccines, keep the consultation brief, keep the door open for further discussion, and provide appropriate resources if the patient wants them.33
Increase use of new media
Mass communication through smartphones and other Internet-based tools such as Facebook and Twitter brings a new dimension to health care, allowing patients and health professionals to communicate about health issues and possibly improve health outcomes.34 The number of people using social media increased by almost 570% worldwide between 2000 and 2012 and surpassed 2.75 billion in 2013.35
Sixty-one percent of adults in the United States look online for health information.36 In a survey conducted in September 2014, the Pew Research Center found that Facebook is the most popular social media site in the United States. Seventy-one percent of online-knowledgeable adults use Facebook, and multiplatform use is on the rise: 52% of adult Internet users now use 2 or more social media sites, a significant increase from 2013, when it stood at 42%. (Other platforms such as Twitter, Instagram, Pinterest, and LinkedIn saw significant increases over the past year in the proportion of online adults who use them).37
Health information provided by social media can answer medical questions and concerns and enhance health promotion and education.35 A recent review of 98 research studies provided evidence that social media can create a space to share, comment, and discuss health information.34 Compared with traditional communication methods, the widespread availability of social media makes health information more accessible, broadening access to various population groups, regardless of age, education, race, ethnicity, and locale.
New media platforms are proving effective. The first systematic assessment of available evidence on the use of new media to increase vaccine uptake and immunization coverage (a review of 7 randomized controlled trials [RCTs], 5 non-RCTs, 3 cross-sectional studies, one case-control study and 3 operational research studies published between 2000-2013) found that text messaging, accessing immunization campaign Web sites, using patient-held Web-based portals, computerized reminders, and standing orders increased immunization coverage rates.35 However, evidence was insufficient in this regard on the value of social networks, email communication, and smartphone applications.
One RCT showed that having access to a personalized Web-based portal where patients could manage health records as well as interact with both health care providers and other members of the community through social forums and messaging tools increased influenza vaccination rates.35
CORRESPONDENCE
Pamela G. Rockwell, DO, Department of Family Medicine, University of Michigan, 24 Frank Lloyd Wright Drive, P.O. Box 431, Ann Arbor, MI 48106-0795; [email protected].
› Recommend immunization to patients routinely. Most adults believe vaccines are important and are likely to get them if recommended by their health care professionals. C
› Consider implementing standing orders that authorize nurses, pharmacists, or other trained health care personnel to assess a patient’s immunization status and administer vaccinations according to a protocol. C
› Explore the use of Web-based patient portals or other new-media communication formats to engage patients. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Vaccines have been proven effective in preventing disease and are one of the most cost-effective and successful public health initiatives of the 20th century. Nevertheless, adult vaccination rates in the United States for vaccine-preventable diseases are low for most routinely recommended vaccines.1 In 2013 alone, there were an estimated 3700 deaths in the United States (95% of which were adults) from pneumococcal infections—a vaccine-preventable disorder.2
Consider the threat posed by the flu. Annually, most people who die of influenza and its complications are adults, with estimates ranging from a low of 3000 to a high of 49,000 based on Centers for Disease Control and Prevention (CDC) data from the 1976-1977 flu season to the 2006-2007 season.3 Vaccination during the 2013-2014 season resulted in an estimated 7.2 million fewer cases of influenza, 90,000 fewer hospitalizations, and 3.1 million fewer medically attended cases than would have been expected without vaccination.4 If vaccination levels had reached the Healthy People 2020 target of 70%, an additional 5.9 million illnesses, 2.3 million medically attended illnesses, and 42,000 hospitalizations might have been averted.4
How are we doing with other vaccines? Based on the 2013 National Health Interview Survey, the CDC assessed vaccination coverage among adults ages ≥19 years for selected vaccines: pneumococcal vaccine, tetanus toxoid-containing vaccines (tetanus and diphtheria vaccine [Td] or tetanus and diphtheria with acellular pertussis vaccine [Tdap]), and vaccines for hepatitis A, hepatitis B, herpes zoster, and human papillomavirus (HPV). (With the exception of influenza vaccination, which is recommended annually for all adults, other vaccinations are directed at specific populations based on age, health conditions, behavioral risk factors, occupation, or travel conditions.)
Overall, coverage rates for hepatitis A and B, pneumococcal, Td, and human papillomavirus (HPV) for all adults did not improve from 2012 to 2013; rates increased only modestly for Tdap among adults ≥19 years, for herpes zoster among adults ≥60 years, and for HPV among men ages 19 to 26. Furthermore, racial and ethnic gaps in coverage are seen in all vaccines, and these gaps widened since 2012 for Tdap, herpes zoster, and HPV vaccination.1
Commonly cited barriers to improved vaccine uptake in adults include lack of regular assessment of vaccine status; lack of physician and other health care provider knowledge on current vaccine recommendations; cost; insufficient stocking of some vaccines; financial disincentives for vaccination in the primary care setting; limited use of electronic records, tools, and immunization registries; missed opportunities; and patient hesitancy and vaccine refusal.5
Removing barriers to immunization. Several recommendations on ways to improve adult vaccination rates are made by many federal organizations as well as by The Community Preventive Services Task Force (Task Force), an independent, nonfederal, unpaid panel of public health and prevention experts. The Task Force—which makes recommendations based on systematic reviews of the evidence of effectiveness, the applicability of the evidence, economic evaluations, and barriers to implementation of interventions6—advocates a 3-pronged approach to improve adult vaccination rates: 1) enhance access to vaccination services; 2) increase community demand for vaccinations; and 3) incorporate physician- or system-based interventions into practice.7
The CDC and other groups such as the National Vaccine Advisory Committee (NVAC) recommend that every routine adult office visit include a vaccination needs assessment, recommendation, and offer of vaccination.8 Additionally, the Task Force recommends 3 means of enhancing adult access to vaccination services: make home visits, reduce patient costs, and offer vaccination programs in the community.7
This article describes a number of simple steps physicians can take to increase the likelihood that adults will get their vaccines and reviews the literature on using new media such as smartphones and other Internet-based tools to improve immunization coverage.9
Increasing community demands for vaccinations
Physicians and other healthcare providers can increase community demand for vaccinations by improving their own knowledge on the subject, recommending vaccination to patients, and increasing their community and political involvement to strengthen or change laws to better support immunization uptake.
To increase awareness and education, keep abreast of the Advisory Committee on Immunization Practices (ACIP) recommendations and guidelines, which are updated annually and reported on in this journal’s Practice Alert column. Consider taking advantage of free immunization apps that are available from the CDC (“CDC Vaccine Schedules” http://www.cdc.gov/vaccines/schedules/hcp/schedule-app.html), the Society of Teachers of Family Medicine (STFM; “Shots Immunizations” http://www.immunizationed.org/Shots-Mobile-App), and the American College of Physicians (“ACP Immunization Advisor” http://immunization.acponline.org/app/).
Take steps to put guidelines into practice. Despite wide promulgation, clinical practice guidelines alone have had limited effect on changing physician behavior and improving patient outcomes. Interactive techniques are more effective than guidelines and didactic presentations alone at changing physician care and patient outcomes. Such techniques include audit/feedback (the reporting of an individual clinician’s vaccination rates compared with desired or target rates, for example), academic detailing/outreach, and reminders by way of electronic or other alerts.10,11
Promote immunization to patients. Physicians are highly influential in determining a patient’s decision to vaccinate, and it is well documented that a strong recommendation about the importance of immunizations makes a difference to patients.12,13
What you say and how you say it matters. A halfhearted recommendation for vaccination may result in the patient remaining unvaccinated.14 For example, “If you want, you can get your pneumonia shot today” is much less persuasive than, “I recommend you get your pneumonia vaccine today to prevent a potentially serious disease that affects thousands of adults each year.” Most adults believe that vaccines are important and are likely to get them if recommended by their health care professionals.15
The CDC recommends that physicians encourage patients to make an informed decision about vaccination by sharing critical information highlighting the importance of vaccinations and reminding patients what vaccines protect against while addressing their concerns (www.cdc.gov/vaccines/adultstandards). Free educational materials for patients can be found at www.cdc.gov/vaccines/AdultPatientEd.
Draw on community resources. Laws and policies that require vaccinations as a prerequisite for attending childcare, school, or college increase coverage. Community and faith-based organizations are likely to play an important role in reducing racial and ethnic disparities in adult immunizations because they can deliver education that is culturally sensitive and tailored to specific subpopulations.16,17 Physicians and other health care providers can get involved with community and faith-based groups and local and federal legislative efforts to improve immunization rates.
Consider implementing these system-based interventions
The following 6 system-based interventions can help improve adult immunization rates:
1. Develop a practice team. The practice team, based on the Patient-Centered Medical Home (PCMH), includes physicians, midlevel providers, nurses, medical assistants, pharmacists, social workers, and other staff. The PCMH team model can facilitate a shift of responsibilities among individuals to better orient the practice toward patients’ health and preventive services.18,19 While physicians have traditionally held all of the responsibility for patient care, including screening for disease and prevention, shifting the responsibility of vaccine screening to nurses or medical assistants can free up time for longer physician/patient interactions.18
The creation of a practice champion within the PCMH team—a physician, midlevel provider, or nurse—to oversee quality improvement for vaccine rates and work to generate support and cooperation from coworkers has also been shown to improve vaccination rates.20 The vaccine champion should keep abreast of new vaccine recommendations and relay that information to the practice through regular staff meetings, announcements, and office postings. The champion can also supervise pre-visit planning for immunizations.19
2. Use electronic immunization information systems (IIS). All states except New Hampshire have an IIS.21 Accurate tracking of adult immunizations in a registry provides a complete record and is essential to improving adult immunization rates,22 as does the use of chart notes, computerized alerts, checklists, and other tools that remind health care providers when patients are due for vaccinations.18 NVAC recommends that all physicians use their state IIS and create a process in their practice to include its use.
3. Incorporate physician feedback. Many health care systems and payers are using benchmarking and incentives to provide physician feedback on vaccination performance.23 Using achievable benchmarks enhances the effectiveness of physician performance feedback.24 The Task Force conducted a systematic review of the evidence on the effectiveness of health care provider assessment and feedback for increasing coverage rates and found that this strategy remains an effective means to increase vaccination rates.25
4. Use reminders/alerts. Even though you may intend to routinely recommend immunizations, remembering to do so at the time of each visit can be difficult when there are so many other issues to address. Reminders at the time of the visit can help. Some electronic records have reminder prompts, or “best practice alerts” (BPAs), programmed into their systems.26 These BPAs will prompt for needed immunizations whether the patient is being seen for a well, acute, or routine follow-up visit. These reminder/recall activities can be greatly simplified by participation in a population-based IIS.
Practices that don’t have an electronic health record can still improve vaccination rates by conveying the reminder with a brightly colored paper form attached to the front of a patient’s chart during the check-in process. One recent study showed that this approach increased rates of influenza vaccination in an urban practice by 12 percentage points.27
Furthermore, simply reminding patients to vaccinate increases the vaccination rate.28 Patient reminder/recall systems using telephone calls or mailings (phone calls are more effective than mailings) improve both childhood and adult vaccinations in all medical settings. More intensive systems using multiple reminders appear to be more effective than single reminders, and while costly, the benefits of increasing preventive visits/services and vaccine uptake help offset this cost.28
5. Implement standing orders. Standing orders—which allow nurses and other appropriately trained health care personnel to assess immunization status and administer vaccinations according to protocol—help improve immunization rates.29 ACIP advises that standing order programs be used in long-term care facilities under the supervision of a medical director to ensure the administration of recommended vaccinations for adults, and in inpatient and outpatient facilities. Because of the societal burden of influenza and pneumococcal disease, implementation of standing orders programs to improve adult vaccination coverage for these diseases is considered a national public health priority.30
6. Develop an encouraging communication style. Studies show that how one communicates with patients is just as important as what one communicates. Certain communication styles and techniques may be more or less effective when discussing vaccination needs with some patients, especially those with vaccine hesitancy or low confidence in vaccine safety or effectiveness. For example, styles that are “directing” are usually unhelpful in addressing concerns about vaccination. These styles typically use information and persuasion to achieve change and may be perceived as confrontational. This approach can lead to cues being missed, jargon being used, and vaccine safety being overstated.
Styles shown to be helpful are those that elicit patient concerns, ask permission to discuss, acknowledge/listen/empathize, determine readiness to change, inform about benefits and risks, and give appropriate resources. These helpful forms of communication are more of a “May I help you?” style vs a “This is what you should do” style of communication.31
Assure patients that recommendations are based on the best interest of their health and on the best available science. Listen to a patient’s concerns and acknowledge them in a nonconfrontational manner, allowing patients to express their concerns and thereby increase their willingness to listen.32 Saying that there is “absolutely no need to worry—vaccines are safe and you are silly not to get yours” is not as effective as saying, “What are your concerns regarding vaccines? Let’s talk about them.”
For the vaccine-hesitant group, building trust is essential through a respectful, nonjudgmental approach that aims to elicit and address specific concerns. For those who refuse vaccines, keep the consultation brief, keep the door open for further discussion, and provide appropriate resources if the patient wants them.33
Increase use of new media
Mass communication through smartphones and other Internet-based tools such as Facebook and Twitter brings a new dimension to health care, allowing patients and health professionals to communicate about health issues and possibly improve health outcomes.34 The number of people using social media increased by almost 570% worldwide between 2000 and 2012 and surpassed 2.75 billion in 2013.35
Sixty-one percent of adults in the United States look online for health information.36 In a survey conducted in September 2014, the Pew Research Center found that Facebook is the most popular social media site in the United States. Seventy-one percent of online-knowledgeable adults use Facebook, and multiplatform use is on the rise: 52% of adult Internet users now use 2 or more social media sites, a significant increase from 2013, when it stood at 42%. (Other platforms such as Twitter, Instagram, Pinterest, and LinkedIn saw significant increases over the past year in the proportion of online adults who use them).37
Health information provided by social media can answer medical questions and concerns and enhance health promotion and education.35 A recent review of 98 research studies provided evidence that social media can create a space to share, comment, and discuss health information.34 Compared with traditional communication methods, the widespread availability of social media makes health information more accessible, broadening access to various population groups, regardless of age, education, race, ethnicity, and locale.
New media platforms are proving effective. The first systematic assessment of available evidence on the use of new media to increase vaccine uptake and immunization coverage (a review of 7 randomized controlled trials [RCTs], 5 non-RCTs, 3 cross-sectional studies, one case-control study and 3 operational research studies published between 2000-2013) found that text messaging, accessing immunization campaign Web sites, using patient-held Web-based portals, computerized reminders, and standing orders increased immunization coverage rates.35 However, evidence was insufficient in this regard on the value of social networks, email communication, and smartphone applications.
One RCT showed that having access to a personalized Web-based portal where patients could manage health records as well as interact with both health care providers and other members of the community through social forums and messaging tools increased influenza vaccination rates.35
CORRESPONDENCE
Pamela G. Rockwell, DO, Department of Family Medicine, University of Michigan, 24 Frank Lloyd Wright Drive, P.O. Box 431, Ann Arbor, MI 48106-0795; [email protected].
1. Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:95-102.
2. Centers for Disease Control and Prevention. Active bacterial core surveillance (ABCs) report, emerging infections program network, Streptococcus pneumoniae, 2013. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Accessed August 20, 2015.
3. Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057-1062.
4. Reed C, Kim IK, Singleton JA, et al; Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by vaccination--United States, 2013-14 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:1151-1154.
5. Kimmel SR, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
6. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based Guide to Community Preventive Services—methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
7. The Guide to Community Preventive Services. Increasing appropriate vaccination. The Community Guide Web site. Available at: http://www.thecommunityguide.org/vaccines/index.html. Accessed August 20, 2015.
8. National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory committee: standards for adult immunization practice. Public Health Rep. 2014;129:115-123.
9. Househ M. The use of social media in healthcare: organizational, clinical, and patient perspectives. Stud Health Technol Inform. 2013;183:244-248.
10. Bloom BS. Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews. Int J Technol Assess Health Care. 2005;21:380-385.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458-1465.
12. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19-26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
13. Zimmerman RK, Santibanez TA, Janosky JE, et al. What affects influenza vaccination rates among older patients? An analysis from inner-city, suburban, rural, and Veterans Affairs practices. Am J Med. 2003;114:31-38.
14. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed August 20, 2015.
15. National Foundation for Infectious Diseases. Survey: adults do not recognize infectious disease risks. National Foundation for Infectious Diseases Web site. Available at: http://www.adultvaccination.org/newsroom/events/2009-vaccination-news-conference/NFID-Survey-Fact-Sheet.pdf. Accessed July 7, 2015.
16. Wang E, Clymer J, Davis-Hayes C, et al. Nonmedical exemptions from school immunization requirements: a systematic review. Am J Public Health. 2014;104:e62-e84.
17. National Vaccine Advisory Committee. A pathway to leadership for adult immunization: recommendations of the National Vaccine Advisory Committee: approved by the National Vaccine Advisory Committee on June 14, 2011. Public Health Rep. 2012;127:1-42.
18. Gannon M, Qaseem A, Snooks Q, et al. Improving adult immunization practices using a team approach in the primary care setting. Am J Public Health. 2012;102:e46-e52.
19. Bottino CJ, Cox JE, Kahlon PS, et al. Improving immunization rates in a hospital-based primary care practice. Pediatrics. 2014;133:e1047-e1054.
20. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
21. Centers for Disease Control and Prevention (CDC). Progress in immunization information systems - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:1005-1008.
22. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
23. Kerr EA, McGlynn EA, Adams J, et al. Profiling the quality of care in twelve communities: results from the CQI study. Health Aff (Millwood). 2004;23:247-256.
24. Kiefe CI, Allison JJ, Williams OD, et al. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA. 2001;285:2871-2879.
25. National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1-64.
26. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
27. Pierson RC, Malone AM, Haas DM. Increasing influenza vaccination rates in a busy urban clinic. J Nat Sci. 2015;1.
28. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;CD003941.
29. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Task Force on Community Preventive Services. Am J Prev Med. 2000;18:92-96.
30. McKibben LJ, Stange PV, Sneller VP, et al; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49:15-16.
31. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154.
32. Kimmel SR, Wolfe RM. Communicating the benefits and risks of vaccines. J Fam Pract. 2005;54:S51-S57.
33. Danchin M, Nolan T. A positive approach to parents with concerns about vaccination for the family physician. Aust Fam Physician. 2014;43:690-694.
34. Moorhead SA, Hazlett DE, Harrison L, et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
35. Odone A, Ferrari A, Spagnoli F, et al. Effectiveness of interventions that apply new media to improve vaccine uptake and vaccine coverage. Hum Vaccin Immunother. 2015;11:72-82.
36. Pew Research Center. Fox S. The Social Life of Health Information, 2011. Pew Research Center Web site. Available at: http://www.pewinternet.org/2011/05/12/the-social-life-of-health-information-2011/. Accessed August 20, 2015.
37. Pew Research Center. Duggan M, Ellison NB, Lampe C, et al. Social Media Update 2014. Pew Research Center Web site. Available at: http://www.pewinternet.org/2015/01/09/social-media-update-2014/. Accessed August 20, 2015.
1. Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:95-102.
2. Centers for Disease Control and Prevention. Active bacterial core surveillance (ABCs) report, emerging infections program network, Streptococcus pneumoniae, 2013. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Accessed August 20, 2015.
3. Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057-1062.
4. Reed C, Kim IK, Singleton JA, et al; Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by vaccination--United States, 2013-14 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:1151-1154.
5. Kimmel SR, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
6. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based Guide to Community Preventive Services—methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
7. The Guide to Community Preventive Services. Increasing appropriate vaccination. The Community Guide Web site. Available at: http://www.thecommunityguide.org/vaccines/index.html. Accessed August 20, 2015.
8. National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory committee: standards for adult immunization practice. Public Health Rep. 2014;129:115-123.
9. Househ M. The use of social media in healthcare: organizational, clinical, and patient perspectives. Stud Health Technol Inform. 2013;183:244-248.
10. Bloom BS. Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews. Int J Technol Assess Health Care. 2005;21:380-385.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458-1465.
12. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19-26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
13. Zimmerman RK, Santibanez TA, Janosky JE, et al. What affects influenza vaccination rates among older patients? An analysis from inner-city, suburban, rural, and Veterans Affairs practices. Am J Med. 2003;114:31-38.
14. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed August 20, 2015.
15. National Foundation for Infectious Diseases. Survey: adults do not recognize infectious disease risks. National Foundation for Infectious Diseases Web site. Available at: http://www.adultvaccination.org/newsroom/events/2009-vaccination-news-conference/NFID-Survey-Fact-Sheet.pdf. Accessed July 7, 2015.
16. Wang E, Clymer J, Davis-Hayes C, et al. Nonmedical exemptions from school immunization requirements: a systematic review. Am J Public Health. 2014;104:e62-e84.
17. National Vaccine Advisory Committee. A pathway to leadership for adult immunization: recommendations of the National Vaccine Advisory Committee: approved by the National Vaccine Advisory Committee on June 14, 2011. Public Health Rep. 2012;127:1-42.
18. Gannon M, Qaseem A, Snooks Q, et al. Improving adult immunization practices using a team approach in the primary care setting. Am J Public Health. 2012;102:e46-e52.
19. Bottino CJ, Cox JE, Kahlon PS, et al. Improving immunization rates in a hospital-based primary care practice. Pediatrics. 2014;133:e1047-e1054.
20. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
21. Centers for Disease Control and Prevention (CDC). Progress in immunization information systems - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:1005-1008.
22. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
23. Kerr EA, McGlynn EA, Adams J, et al. Profiling the quality of care in twelve communities: results from the CQI study. Health Aff (Millwood). 2004;23:247-256.
24. Kiefe CI, Allison JJ, Williams OD, et al. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA. 2001;285:2871-2879.
25. National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1-64.
26. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
27. Pierson RC, Malone AM, Haas DM. Increasing influenza vaccination rates in a busy urban clinic. J Nat Sci. 2015;1.
28. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;CD003941.
29. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Task Force on Community Preventive Services. Am J Prev Med. 2000;18:92-96.
30. McKibben LJ, Stange PV, Sneller VP, et al; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49:15-16.
31. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154.
32. Kimmel SR, Wolfe RM. Communicating the benefits and risks of vaccines. J Fam Pract. 2005;54:S51-S57.
33. Danchin M, Nolan T. A positive approach to parents with concerns about vaccination for the family physician. Aust Fam Physician. 2014;43:690-694.
34. Moorhead SA, Hazlett DE, Harrison L, et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
35. Odone A, Ferrari A, Spagnoli F, et al. Effectiveness of interventions that apply new media to improve vaccine uptake and vaccine coverage. Hum Vaccin Immunother. 2015;11:72-82.
36. Pew Research Center. Fox S. The Social Life of Health Information, 2011. Pew Research Center Web site. Available at: http://www.pewinternet.org/2011/05/12/the-social-life-of-health-information-2011/. Accessed August 20, 2015.
37. Pew Research Center. Duggan M, Ellison NB, Lampe C, et al. Social Media Update 2014. Pew Research Center Web site. Available at: http://www.pewinternet.org/2015/01/09/social-media-update-2014/. Accessed August 20, 2015.
What you can do to improve adult immunization rates
› Recommend immunization to patients routinely. Most adults believe vaccines are important and are likely to get them if recommended by their health care professionals. C
› Consider implementing standing orders that authorize nurses, pharmacists, or other trained health care personnel to assess a patient’s immunization status and administer vaccinations according to a protocol. C
› Explore the use of Web-based patient portals or other new-media communication formats to engage patients. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Vaccines have been proven effective in preventing disease and are one of the most cost-effective and successful public health initiatives of the 20th century. Nevertheless, adult vaccination rates in the United States for vaccine-preventable diseases are low for most routinely recommended vaccines.1 In 2013 alone, there were an estimated 3700 deaths in the United States (95% of which were adults) from pneumococcal infections—a vaccine-preventable disorder.2
Consider the threat posed by the flu. Annually, most people who die of influenza and its complications are adults, with estimates ranging from a low of 3000 to a high of 49,000 based on Centers for Disease Control and Prevention (CDC) data from the 1976-1977 flu season to the 2006-2007 season.3 Vaccination during the 2013-2014 season resulted in an estimated 7.2 million fewer cases of influenza, 90,000 fewer hospitalizations, and 3.1 million fewer medically attended cases than would have been expected without vaccination.4 If vaccination levels had reached the Healthy People 2020 target of 70%, an additional 5.9 million illnesses, 2.3 million medically attended illnesses, and 42,000 hospitalizations might have been averted.4
How are we doing with other vaccines? Based on the 2013 National Health Interview Survey, the CDC assessed vaccination coverage among adults ages ≥19 years for selected vaccines: pneumococcal vaccine, tetanus toxoid-containing vaccines (tetanus and diphtheria vaccine [Td] or tetanus and diphtheria with acellular pertussis vaccine [Tdap]), and vaccines for hepatitis A, hepatitis B, herpes zoster, and human papillomavirus (HPV). (With the exception of influenza vaccination, which is recommended annually for all adults, other vaccinations are directed at specific populations based on age, health conditions, behavioral risk factors, occupation, or travel conditions.)
Overall, coverage rates for hepatitis A and B, pneumococcal, Td, and human papillomavirus (HPV) for all adults did not improve from 2012 to 2013; rates increased only modestly for Tdap among adults ≥19 years, for herpes zoster among adults ≥60 years, and for HPV among men ages 19 to 26. Furthermore, racial and ethnic gaps in coverage are seen in all vaccines, and these gaps widened since 2012 for Tdap, herpes zoster, and HPV vaccination.1
Commonly cited barriers to improved vaccine uptake in adults include lack of regular assessment of vaccine status; lack of physician and other health care provider knowledge on current vaccine recommendations; cost; insufficient stocking of some vaccines; financial disincentives for vaccination in the primary care setting; limited use of electronic records, tools, and immunization registries; missed opportunities; and patient hesitancy and vaccine refusal.5
Removing barriers to immunization. Several recommendations on ways to improve adult vaccination rates are made by many federal organizations as well as by The Community Preventive Services Task Force (Task Force), an independent, nonfederal, unpaid panel of public health and prevention experts. The Task Force—which makes recommendations based on systematic reviews of the evidence of effectiveness, the applicability of the evidence, economic evaluations, and barriers to implementation of interventions6—advocates a 3-pronged approach to improve adult vaccination rates: 1) enhance access to vaccination services; 2) increase community demand for vaccinations; and 3) incorporate physician- or system-based interventions into practice.7
The CDC and other groups such as the National Vaccine Advisory Committee (NVAC) recommend that every routine adult office visit include a vaccination needs assessment, recommendation, and offer of vaccination.8 Additionally, the Task Force recommends 3 means of enhancing adult access to vaccination services: make home visits, reduce patient costs, and offer vaccination programs in the community.7
This article describes a number of simple steps physicians can take to increase the likelihood that adults will get their vaccines and reviews the literature on using new media such as smartphones and other Internet-based tools to improve immunization coverage.9
Increasing community demands for vaccinations
Physicians and other healthcare providers can increase community demand for vaccinations by improving their own knowledge on the subject, recommending vaccination to patients, and increasing their community and political involvement to strengthen or change laws to better support immunization uptake.
To increase awareness and education, keep abreast of the Advisory Committee on Immunization Practices (ACIP) recommendations and guidelines, which are updated annually and reported on in this journal’s Practice Alert column. Consider taking advantage of free immunization apps that are available from the CDC (“CDC Vaccine Schedules” http://www.cdc.gov/vaccines/schedules/hcp/schedule-app.html), the Society of Teachers of Family Medicine (STFM; “Shots Immunizations” http://www.immunizationed.org/Shots-Mobile-App), and the American College of Physicians (“ACP Immunization Advisor” http://immunization.acponline.org/app/).
Take steps to put guidelines into practice. Despite wide promulgation, clinical practice guidelines alone have had limited effect on changing physician behavior and improving patient outcomes. Interactive techniques are more effective than guidelines and didactic presentations alone at changing physician care and patient outcomes. Such techniques include audit/feedback (the reporting of an individual clinician’s vaccination rates compared with desired or target rates, for example), academic detailing/outreach, and reminders by way of electronic or other alerts.10,11
Promote immunization to patients. Physicians are highly influential in determining a patient’s decision to vaccinate, and it is well documented that a strong recommendation about the importance of immunizations makes a difference to patients.12,13
What you say and how you say it matters. A halfhearted recommendation for vaccination may result in the patient remaining unvaccinated.14 For example, “If you want, you can get your pneumonia shot today” is much less persuasive than, “I recommend you get your pneumonia vaccine today to prevent a potentially serious disease that affects thousands of adults each year.” Most adults believe that vaccines are important and are likely to get them if recommended by their health care professionals.15
The CDC recommends that physicians encourage patients to make an informed decision about vaccination by sharing critical information highlighting the importance of vaccinations and reminding patients what vaccines protect against while addressing their concerns (www.cdc.gov/vaccines/adultstandards). Free educational materials for patients can be found at www.cdc.gov/vaccines/AdultPatientEd.
Draw on community resources. Laws and policies that require vaccinations as a prerequisite for attending childcare, school, or college increase coverage. Community and faith-based organizations are likely to play an important role in reducing racial and ethnic disparities in adult immunizations because they can deliver education that is culturally sensitive and tailored to specific subpopulations.16,17 Physicians and other health care providers can get involved with community and faith-based groups and local and federal legislative efforts to improve immunization rates.
Consider implementing these system-based interventions
The following 6 system-based interventions can help improve adult immunization rates:
1. Develop a practice team. The practice team, based on the Patient-Centered Medical Home (PCMH), includes physicians, midlevel providers, nurses, medical assistants, pharmacists, social workers, and other staff. The PCMH team model can facilitate a shift of responsibilities among individuals to better orient the practice toward patients’ health and preventive services.18,19 While physicians have traditionally held all of the responsibility for patient care, including screening for disease and prevention, shifting the responsibility of vaccine screening to nurses or medical assistants can free up time for longer physician/patient interactions.18
The creation of a practice champion within the PCMH team—a physician, midlevel provider, or nurse—to oversee quality improvement for vaccine rates and work to generate support and cooperation from coworkers has also been shown to improve vaccination rates.20 The vaccine champion should keep abreast of new vaccine recommendations and relay that information to the practice through regular staff meetings, announcements, and office postings. The champion can also supervise pre-visit planning for immunizations.19
2. Use electronic immunization information systems (IIS). All states except New Hampshire have an IIS.21 Accurate tracking of adult immunizations in a registry provides a complete record and is essential to improving adult immunization rates,22 as does the use of chart notes, computerized alerts, checklists, and other tools that remind health care providers when patients are due for vaccinations.18 NVAC recommends that all physicians use their state IIS and create a process in their practice to include its use.
3. Incorporate physician feedback. Many health care systems and payers are using benchmarking and incentives to provide physician feedback on vaccination performance.23 Using achievable benchmarks enhances the effectiveness of physician performance feedback.24 The Task Force conducted a systematic review of the evidence on the effectiveness of health care provider assessment and feedback for increasing coverage rates and found that this strategy remains an effective means to increase vaccination rates.25
4. Use reminders/alerts. Even though you may intend to routinely recommend immunizations, remembering to do so at the time of each visit can be difficult when there are so many other issues to address. Reminders at the time of the visit can help. Some electronic records have reminder prompts, or “best practice alerts” (BPAs), programmed into their systems.26 These BPAs will prompt for needed immunizations whether the patient is being seen for a well, acute, or routine follow-up visit. These reminder/recall activities can be greatly simplified by participation in a population-based IIS.
Practices that don’t have an electronic health record can still improve vaccination rates by conveying the reminder with a brightly colored paper form attached to the front of a patient’s chart during the check-in process. One recent study showed that this approach increased rates of influenza vaccination in an urban practice by 12 percentage points.27
Furthermore, simply reminding patients to vaccinate increases the vaccination rate.28 Patient reminder/recall systems using telephone calls or mailings (phone calls are more effective than mailings) improve both childhood and adult vaccinations in all medical settings. More intensive systems using multiple reminders appear to be more effective than single reminders, and while costly, the benefits of increasing preventive visits/services and vaccine uptake help offset this cost.28
5. Implement standing orders. Standing orders—which allow nurses and other appropriately trained health care personnel to assess immunization status and administer vaccinations according to protocol—help improve immunization rates.29 ACIP advises that standing order programs be used in long-term care facilities under the supervision of a medical director to ensure the administration of recommended vaccinations for adults, and in inpatient and outpatient facilities. Because of the societal burden of influenza and pneumococcal disease, implementation of standing orders programs to improve adult vaccination coverage for these diseases is considered a national public health priority.30
6. Develop an encouraging communication style. Studies show that how one communicates with patients is just as important as what one communicates. Certain communication styles and techniques may be more or less effective when discussing vaccination needs with some patients, especially those with vaccine hesitancy or low confidence in vaccine safety or effectiveness. For example, styles that are “directing” are usually unhelpful in addressing concerns about vaccination. These styles typically use information and persuasion to achieve change and may be perceived as confrontational. This approach can lead to cues being missed, jargon being used, and vaccine safety being overstated.
Styles shown to be helpful are those that elicit patient concerns, ask permission to discuss, acknowledge/listen/empathize, determine readiness to change, inform about benefits and risks, and give appropriate resources. These helpful forms of communication are more of a “May I help you?” style vs a “This is what you should do” style of communication.31
Assure patients that recommendations are based on the best interest of their health and on the best available science. Listen to a patient’s concerns and acknowledge them in a nonconfrontational manner, allowing patients to express their concerns and thereby increase their willingness to listen.32 Saying that there is “absolutely no need to worry—vaccines are safe and you are silly not to get yours” is not as effective as saying, “What are your concerns regarding vaccines? Let’s talk about them.”
For the vaccine-hesitant group, building trust is essential through a respectful, nonjudgmental approach that aims to elicit and address specific concerns. For those who refuse vaccines, keep the consultation brief, keep the door open for further discussion, and provide appropriate resources if the patient wants them.33
Increase use of new media
Mass communication through smartphones and other Internet-based tools such as Facebook and Twitter brings a new dimension to health care, allowing patients and health professionals to communicate about health issues and possibly improve health outcomes.34 The number of people using social media increased by almost 570% worldwide between 2000 and 2012 and surpassed 2.75 billion in 2013.35
Sixty-one percent of adults in the United States look online for health information.36 In a survey conducted in September 2014, the Pew Research Center found that Facebook is the most popular social media site in the United States. Seventy-one percent of online-knowledgeable adults use Facebook, and multiplatform use is on the rise: 52% of adult Internet users now use 2 or more social media sites, a significant increase from 2013, when it stood at 42%. (Other platforms such as Twitter, Instagram, Pinterest, and LinkedIn saw significant increases over the past year in the proportion of online adults who use them).37
Health information provided by social media can answer medical questions and concerns and enhance health promotion and education.35 A recent review of 98 research studies provided evidence that social media can create a space to share, comment, and discuss health information.34 Compared with traditional communication methods, the widespread availability of social media makes health information more accessible, broadening access to various population groups, regardless of age, education, race, ethnicity, and locale.
New media platforms are proving effective. The first systematic assessment of available evidence on the use of new media to increase vaccine uptake and immunization coverage (a review of 7 randomized controlled trials [RCTs], 5 non-RCTs, 3 cross-sectional studies, one case-control study and 3 operational research studies published between 2000-2013) found that text messaging, accessing immunization campaign Web sites, using patient-held Web-based portals, computerized reminders, and standing orders increased immunization coverage rates.35 However, evidence was insufficient in this regard on the value of social networks, email communication, and smartphone applications.
One RCT showed that having access to a personalized Web-based portal where patients could manage health records as well as interact with both health care providers and other members of the community through social forums and messaging tools increased influenza vaccination rates.35
CORRESPONDENCE
Pamela G. Rockwell, DO, Department of Family Medicine, University of Michigan, 24 Frank Lloyd Wright Drive, P.O. Box 431, Ann Arbor, MI 48106-0795; [email protected].
1. Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:95-102.
2. Centers for Disease Control and Prevention. Active bacterial core surveillance (ABCs) report, emerging infections program network, Streptococcus pneumoniae, 2013. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Accessed August 20, 2015.
3. Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057-1062.
4. Reed C, Kim IK, Singleton JA, et al; Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by vaccination--United States, 2013-14 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:1151-1154.
5. Kimmel SR, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
6. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based Guide to Community Preventive Services—methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
7. The Guide to Community Preventive Services. Increasing appropriate vaccination. The Community Guide Web site. Available at: http://www.thecommunityguide.org/vaccines/index.html. Accessed August 20, 2015.
8. National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory committee: standards for adult immunization practice. Public Health Rep. 2014;129:115-123.
9. Househ M. The use of social media in healthcare: organizational, clinical, and patient perspectives. Stud Health Technol Inform. 2013;183:244-248.
10. Bloom BS. Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews. Int J Technol Assess Health Care. 2005;21:380-385.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458-1465.
12. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19-26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
13. Zimmerman RK, Santibanez TA, Janosky JE, et al. What affects influenza vaccination rates among older patients? An analysis from inner-city, suburban, rural, and Veterans Affairs practices. Am J Med. 2003;114:31-38.
14. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed August 20, 2015.
15. National Foundation for Infectious Diseases. Survey: adults do not recognize infectious disease risks. National Foundation for Infectious Diseases Web site. Available at: http://www.adultvaccination.org/newsroom/events/2009-vaccination-news-conference/NFID-Survey-Fact-Sheet.pdf. Accessed July 7, 2015.
16. Wang E, Clymer J, Davis-Hayes C, et al. Nonmedical exemptions from school immunization requirements: a systematic review. Am J Public Health. 2014;104:e62-e84.
17. National Vaccine Advisory Committee. A pathway to leadership for adult immunization: recommendations of the National Vaccine Advisory Committee: approved by the National Vaccine Advisory Committee on June 14, 2011. Public Health Rep. 2012;127:1-42.
18. Gannon M, Qaseem A, Snooks Q, et al. Improving adult immunization practices using a team approach in the primary care setting. Am J Public Health. 2012;102:e46-e52.
19. Bottino CJ, Cox JE, Kahlon PS, et al. Improving immunization rates in a hospital-based primary care practice. Pediatrics. 2014;133:e1047-e1054.
20. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
21. Centers for Disease Control and Prevention (CDC). Progress in immunization information systems - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:1005-1008.
22. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
23. Kerr EA, McGlynn EA, Adams J, et al. Profiling the quality of care in twelve communities: results from the CQI study. Health Aff (Millwood). 2004;23:247-256.
24. Kiefe CI, Allison JJ, Williams OD, et al. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA. 2001;285:2871-2879.
25. National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1-64.
26. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
27. Pierson RC, Malone AM, Haas DM. Increasing influenza vaccination rates in a busy urban clinic. J Nat Sci. 2015;1.
28. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;CD003941.
29. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Task Force on Community Preventive Services. Am J Prev Med. 2000;18:92-96.
30. McKibben LJ, Stange PV, Sneller VP, et al; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49:15-16.
31. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154.
32. Kimmel SR, Wolfe RM. Communicating the benefits and risks of vaccines. J Fam Pract. 2005;54:S51-S57.
33. Danchin M, Nolan T. A positive approach to parents with concerns about vaccination for the family physician. Aust Fam Physician. 2014;43:690-694.
34. Moorhead SA, Hazlett DE, Harrison L, et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
35. Odone A, Ferrari A, Spagnoli F, et al. Effectiveness of interventions that apply new media to improve vaccine uptake and vaccine coverage. Hum Vaccin Immunother. 2015;11:72-82.
36. Pew Research Center. Fox S. The Social Life of Health Information, 2011. Pew Research Center Web site. Available at: http://www.pewinternet.org/2011/05/12/the-social-life-of-health-information-2011/. Accessed August 20, 2015.
37. Pew Research Center. Duggan M, Ellison NB, Lampe C, et al. Social Media Update 2014. Pew Research Center Web site. Available at: http://www.pewinternet.org/2015/01/09/social-media-update-2014/. Accessed August 20, 2015.
› Recommend immunization to patients routinely. Most adults believe vaccines are important and are likely to get them if recommended by their health care professionals. C
› Consider implementing standing orders that authorize nurses, pharmacists, or other trained health care personnel to assess a patient’s immunization status and administer vaccinations according to a protocol. C
› Explore the use of Web-based patient portals or other new-media communication formats to engage patients. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Vaccines have been proven effective in preventing disease and are one of the most cost-effective and successful public health initiatives of the 20th century. Nevertheless, adult vaccination rates in the United States for vaccine-preventable diseases are low for most routinely recommended vaccines.1 In 2013 alone, there were an estimated 3700 deaths in the United States (95% of which were adults) from pneumococcal infections—a vaccine-preventable disorder.2
Consider the threat posed by the flu. Annually, most people who die of influenza and its complications are adults, with estimates ranging from a low of 3000 to a high of 49,000 based on Centers for Disease Control and Prevention (CDC) data from the 1976-1977 flu season to the 2006-2007 season.3 Vaccination during the 2013-2014 season resulted in an estimated 7.2 million fewer cases of influenza, 90,000 fewer hospitalizations, and 3.1 million fewer medically attended cases than would have been expected without vaccination.4 If vaccination levels had reached the Healthy People 2020 target of 70%, an additional 5.9 million illnesses, 2.3 million medically attended illnesses, and 42,000 hospitalizations might have been averted.4
How are we doing with other vaccines? Based on the 2013 National Health Interview Survey, the CDC assessed vaccination coverage among adults ages ≥19 years for selected vaccines: pneumococcal vaccine, tetanus toxoid-containing vaccines (tetanus and diphtheria vaccine [Td] or tetanus and diphtheria with acellular pertussis vaccine [Tdap]), and vaccines for hepatitis A, hepatitis B, herpes zoster, and human papillomavirus (HPV). (With the exception of influenza vaccination, which is recommended annually for all adults, other vaccinations are directed at specific populations based on age, health conditions, behavioral risk factors, occupation, or travel conditions.)
Overall, coverage rates for hepatitis A and B, pneumococcal, Td, and human papillomavirus (HPV) for all adults did not improve from 2012 to 2013; rates increased only modestly for Tdap among adults ≥19 years, for herpes zoster among adults ≥60 years, and for HPV among men ages 19 to 26. Furthermore, racial and ethnic gaps in coverage are seen in all vaccines, and these gaps widened since 2012 for Tdap, herpes zoster, and HPV vaccination.1
Commonly cited barriers to improved vaccine uptake in adults include lack of regular assessment of vaccine status; lack of physician and other health care provider knowledge on current vaccine recommendations; cost; insufficient stocking of some vaccines; financial disincentives for vaccination in the primary care setting; limited use of electronic records, tools, and immunization registries; missed opportunities; and patient hesitancy and vaccine refusal.5
Removing barriers to immunization. Several recommendations on ways to improve adult vaccination rates are made by many federal organizations as well as by The Community Preventive Services Task Force (Task Force), an independent, nonfederal, unpaid panel of public health and prevention experts. The Task Force—which makes recommendations based on systematic reviews of the evidence of effectiveness, the applicability of the evidence, economic evaluations, and barriers to implementation of interventions6—advocates a 3-pronged approach to improve adult vaccination rates: 1) enhance access to vaccination services; 2) increase community demand for vaccinations; and 3) incorporate physician- or system-based interventions into practice.7
The CDC and other groups such as the National Vaccine Advisory Committee (NVAC) recommend that every routine adult office visit include a vaccination needs assessment, recommendation, and offer of vaccination.8 Additionally, the Task Force recommends 3 means of enhancing adult access to vaccination services: make home visits, reduce patient costs, and offer vaccination programs in the community.7
This article describes a number of simple steps physicians can take to increase the likelihood that adults will get their vaccines and reviews the literature on using new media such as smartphones and other Internet-based tools to improve immunization coverage.9
Increasing community demands for vaccinations
Physicians and other healthcare providers can increase community demand for vaccinations by improving their own knowledge on the subject, recommending vaccination to patients, and increasing their community and political involvement to strengthen or change laws to better support immunization uptake.
To increase awareness and education, keep abreast of the Advisory Committee on Immunization Practices (ACIP) recommendations and guidelines, which are updated annually and reported on in this journal’s Practice Alert column. Consider taking advantage of free immunization apps that are available from the CDC (“CDC Vaccine Schedules” http://www.cdc.gov/vaccines/schedules/hcp/schedule-app.html), the Society of Teachers of Family Medicine (STFM; “Shots Immunizations” http://www.immunizationed.org/Shots-Mobile-App), and the American College of Physicians (“ACP Immunization Advisor” http://immunization.acponline.org/app/).
Take steps to put guidelines into practice. Despite wide promulgation, clinical practice guidelines alone have had limited effect on changing physician behavior and improving patient outcomes. Interactive techniques are more effective than guidelines and didactic presentations alone at changing physician care and patient outcomes. Such techniques include audit/feedback (the reporting of an individual clinician’s vaccination rates compared with desired or target rates, for example), academic detailing/outreach, and reminders by way of electronic or other alerts.10,11
Promote immunization to patients. Physicians are highly influential in determining a patient’s decision to vaccinate, and it is well documented that a strong recommendation about the importance of immunizations makes a difference to patients.12,13
What you say and how you say it matters. A halfhearted recommendation for vaccination may result in the patient remaining unvaccinated.14 For example, “If you want, you can get your pneumonia shot today” is much less persuasive than, “I recommend you get your pneumonia vaccine today to prevent a potentially serious disease that affects thousands of adults each year.” Most adults believe that vaccines are important and are likely to get them if recommended by their health care professionals.15
The CDC recommends that physicians encourage patients to make an informed decision about vaccination by sharing critical information highlighting the importance of vaccinations and reminding patients what vaccines protect against while addressing their concerns (www.cdc.gov/vaccines/adultstandards). Free educational materials for patients can be found at www.cdc.gov/vaccines/AdultPatientEd.
Draw on community resources. Laws and policies that require vaccinations as a prerequisite for attending childcare, school, or college increase coverage. Community and faith-based organizations are likely to play an important role in reducing racial and ethnic disparities in adult immunizations because they can deliver education that is culturally sensitive and tailored to specific subpopulations.16,17 Physicians and other health care providers can get involved with community and faith-based groups and local and federal legislative efforts to improve immunization rates.
Consider implementing these system-based interventions
The following 6 system-based interventions can help improve adult immunization rates:
1. Develop a practice team. The practice team, based on the Patient-Centered Medical Home (PCMH), includes physicians, midlevel providers, nurses, medical assistants, pharmacists, social workers, and other staff. The PCMH team model can facilitate a shift of responsibilities among individuals to better orient the practice toward patients’ health and preventive services.18,19 While physicians have traditionally held all of the responsibility for patient care, including screening for disease and prevention, shifting the responsibility of vaccine screening to nurses or medical assistants can free up time for longer physician/patient interactions.18
The creation of a practice champion within the PCMH team—a physician, midlevel provider, or nurse—to oversee quality improvement for vaccine rates and work to generate support and cooperation from coworkers has also been shown to improve vaccination rates.20 The vaccine champion should keep abreast of new vaccine recommendations and relay that information to the practice through regular staff meetings, announcements, and office postings. The champion can also supervise pre-visit planning for immunizations.19
2. Use electronic immunization information systems (IIS). All states except New Hampshire have an IIS.21 Accurate tracking of adult immunizations in a registry provides a complete record and is essential to improving adult immunization rates,22 as does the use of chart notes, computerized alerts, checklists, and other tools that remind health care providers when patients are due for vaccinations.18 NVAC recommends that all physicians use their state IIS and create a process in their practice to include its use.
3. Incorporate physician feedback. Many health care systems and payers are using benchmarking and incentives to provide physician feedback on vaccination performance.23 Using achievable benchmarks enhances the effectiveness of physician performance feedback.24 The Task Force conducted a systematic review of the evidence on the effectiveness of health care provider assessment and feedback for increasing coverage rates and found that this strategy remains an effective means to increase vaccination rates.25
4. Use reminders/alerts. Even though you may intend to routinely recommend immunizations, remembering to do so at the time of each visit can be difficult when there are so many other issues to address. Reminders at the time of the visit can help. Some electronic records have reminder prompts, or “best practice alerts” (BPAs), programmed into their systems.26 These BPAs will prompt for needed immunizations whether the patient is being seen for a well, acute, or routine follow-up visit. These reminder/recall activities can be greatly simplified by participation in a population-based IIS.
Practices that don’t have an electronic health record can still improve vaccination rates by conveying the reminder with a brightly colored paper form attached to the front of a patient’s chart during the check-in process. One recent study showed that this approach increased rates of influenza vaccination in an urban practice by 12 percentage points.27
Furthermore, simply reminding patients to vaccinate increases the vaccination rate.28 Patient reminder/recall systems using telephone calls or mailings (phone calls are more effective than mailings) improve both childhood and adult vaccinations in all medical settings. More intensive systems using multiple reminders appear to be more effective than single reminders, and while costly, the benefits of increasing preventive visits/services and vaccine uptake help offset this cost.28
5. Implement standing orders. Standing orders—which allow nurses and other appropriately trained health care personnel to assess immunization status and administer vaccinations according to protocol—help improve immunization rates.29 ACIP advises that standing order programs be used in long-term care facilities under the supervision of a medical director to ensure the administration of recommended vaccinations for adults, and in inpatient and outpatient facilities. Because of the societal burden of influenza and pneumococcal disease, implementation of standing orders programs to improve adult vaccination coverage for these diseases is considered a national public health priority.30
6. Develop an encouraging communication style. Studies show that how one communicates with patients is just as important as what one communicates. Certain communication styles and techniques may be more or less effective when discussing vaccination needs with some patients, especially those with vaccine hesitancy or low confidence in vaccine safety or effectiveness. For example, styles that are “directing” are usually unhelpful in addressing concerns about vaccination. These styles typically use information and persuasion to achieve change and may be perceived as confrontational. This approach can lead to cues being missed, jargon being used, and vaccine safety being overstated.
Styles shown to be helpful are those that elicit patient concerns, ask permission to discuss, acknowledge/listen/empathize, determine readiness to change, inform about benefits and risks, and give appropriate resources. These helpful forms of communication are more of a “May I help you?” style vs a “This is what you should do” style of communication.31
Assure patients that recommendations are based on the best interest of their health and on the best available science. Listen to a patient’s concerns and acknowledge them in a nonconfrontational manner, allowing patients to express their concerns and thereby increase their willingness to listen.32 Saying that there is “absolutely no need to worry—vaccines are safe and you are silly not to get yours” is not as effective as saying, “What are your concerns regarding vaccines? Let’s talk about them.”
For the vaccine-hesitant group, building trust is essential through a respectful, nonjudgmental approach that aims to elicit and address specific concerns. For those who refuse vaccines, keep the consultation brief, keep the door open for further discussion, and provide appropriate resources if the patient wants them.33
Increase use of new media
Mass communication through smartphones and other Internet-based tools such as Facebook and Twitter brings a new dimension to health care, allowing patients and health professionals to communicate about health issues and possibly improve health outcomes.34 The number of people using social media increased by almost 570% worldwide between 2000 and 2012 and surpassed 2.75 billion in 2013.35
Sixty-one percent of adults in the United States look online for health information.36 In a survey conducted in September 2014, the Pew Research Center found that Facebook is the most popular social media site in the United States. Seventy-one percent of online-knowledgeable adults use Facebook, and multiplatform use is on the rise: 52% of adult Internet users now use 2 or more social media sites, a significant increase from 2013, when it stood at 42%. (Other platforms such as Twitter, Instagram, Pinterest, and LinkedIn saw significant increases over the past year in the proportion of online adults who use them).37
Health information provided by social media can answer medical questions and concerns and enhance health promotion and education.35 A recent review of 98 research studies provided evidence that social media can create a space to share, comment, and discuss health information.34 Compared with traditional communication methods, the widespread availability of social media makes health information more accessible, broadening access to various population groups, regardless of age, education, race, ethnicity, and locale.
New media platforms are proving effective. The first systematic assessment of available evidence on the use of new media to increase vaccine uptake and immunization coverage (a review of 7 randomized controlled trials [RCTs], 5 non-RCTs, 3 cross-sectional studies, one case-control study and 3 operational research studies published between 2000-2013) found that text messaging, accessing immunization campaign Web sites, using patient-held Web-based portals, computerized reminders, and standing orders increased immunization coverage rates.35 However, evidence was insufficient in this regard on the value of social networks, email communication, and smartphone applications.
One RCT showed that having access to a personalized Web-based portal where patients could manage health records as well as interact with both health care providers and other members of the community through social forums and messaging tools increased influenza vaccination rates.35
CORRESPONDENCE
Pamela G. Rockwell, DO, Department of Family Medicine, University of Michigan, 24 Frank Lloyd Wright Drive, P.O. Box 431, Ann Arbor, MI 48106-0795; [email protected].
› Recommend immunization to patients routinely. Most adults believe vaccines are important and are likely to get them if recommended by their health care professionals. C
› Consider implementing standing orders that authorize nurses, pharmacists, or other trained health care personnel to assess a patient’s immunization status and administer vaccinations according to a protocol. C
› Explore the use of Web-based patient portals or other new-media communication formats to engage patients. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Vaccines have been proven effective in preventing disease and are one of the most cost-effective and successful public health initiatives of the 20th century. Nevertheless, adult vaccination rates in the United States for vaccine-preventable diseases are low for most routinely recommended vaccines.1 In 2013 alone, there were an estimated 3700 deaths in the United States (95% of which were adults) from pneumococcal infections—a vaccine-preventable disorder.2
Consider the threat posed by the flu. Annually, most people who die of influenza and its complications are adults, with estimates ranging from a low of 3000 to a high of 49,000 based on Centers for Disease Control and Prevention (CDC) data from the 1976-1977 flu season to the 2006-2007 season.3 Vaccination during the 2013-2014 season resulted in an estimated 7.2 million fewer cases of influenza, 90,000 fewer hospitalizations, and 3.1 million fewer medically attended cases than would have been expected without vaccination.4 If vaccination levels had reached the Healthy People 2020 target of 70%, an additional 5.9 million illnesses, 2.3 million medically attended illnesses, and 42,000 hospitalizations might have been averted.4
How are we doing with other vaccines? Based on the 2013 National Health Interview Survey, the CDC assessed vaccination coverage among adults ages ≥19 years for selected vaccines: pneumococcal vaccine, tetanus toxoid-containing vaccines (tetanus and diphtheria vaccine [Td] or tetanus and diphtheria with acellular pertussis vaccine [Tdap]), and vaccines for hepatitis A, hepatitis B, herpes zoster, and human papillomavirus (HPV). (With the exception of influenza vaccination, which is recommended annually for all adults, other vaccinations are directed at specific populations based on age, health conditions, behavioral risk factors, occupation, or travel conditions.)
Overall, coverage rates for hepatitis A and B, pneumococcal, Td, and human papillomavirus (HPV) for all adults did not improve from 2012 to 2013; rates increased only modestly for Tdap among adults ≥19 years, for herpes zoster among adults ≥60 years, and for HPV among men ages 19 to 26. Furthermore, racial and ethnic gaps in coverage are seen in all vaccines, and these gaps widened since 2012 for Tdap, herpes zoster, and HPV vaccination.1
Commonly cited barriers to improved vaccine uptake in adults include lack of regular assessment of vaccine status; lack of physician and other health care provider knowledge on current vaccine recommendations; cost; insufficient stocking of some vaccines; financial disincentives for vaccination in the primary care setting; limited use of electronic records, tools, and immunization registries; missed opportunities; and patient hesitancy and vaccine refusal.5
Removing barriers to immunization. Several recommendations on ways to improve adult vaccination rates are made by many federal organizations as well as by The Community Preventive Services Task Force (Task Force), an independent, nonfederal, unpaid panel of public health and prevention experts. The Task Force—which makes recommendations based on systematic reviews of the evidence of effectiveness, the applicability of the evidence, economic evaluations, and barriers to implementation of interventions6—advocates a 3-pronged approach to improve adult vaccination rates: 1) enhance access to vaccination services; 2) increase community demand for vaccinations; and 3) incorporate physician- or system-based interventions into practice.7
The CDC and other groups such as the National Vaccine Advisory Committee (NVAC) recommend that every routine adult office visit include a vaccination needs assessment, recommendation, and offer of vaccination.8 Additionally, the Task Force recommends 3 means of enhancing adult access to vaccination services: make home visits, reduce patient costs, and offer vaccination programs in the community.7
This article describes a number of simple steps physicians can take to increase the likelihood that adults will get their vaccines and reviews the literature on using new media such as smartphones and other Internet-based tools to improve immunization coverage.9
Increasing community demands for vaccinations
Physicians and other healthcare providers can increase community demand for vaccinations by improving their own knowledge on the subject, recommending vaccination to patients, and increasing their community and political involvement to strengthen or change laws to better support immunization uptake.
To increase awareness and education, keep abreast of the Advisory Committee on Immunization Practices (ACIP) recommendations and guidelines, which are updated annually and reported on in this journal’s Practice Alert column. Consider taking advantage of free immunization apps that are available from the CDC (“CDC Vaccine Schedules” http://www.cdc.gov/vaccines/schedules/hcp/schedule-app.html), the Society of Teachers of Family Medicine (STFM; “Shots Immunizations” http://www.immunizationed.org/Shots-Mobile-App), and the American College of Physicians (“ACP Immunization Advisor” http://immunization.acponline.org/app/).
Take steps to put guidelines into practice. Despite wide promulgation, clinical practice guidelines alone have had limited effect on changing physician behavior and improving patient outcomes. Interactive techniques are more effective than guidelines and didactic presentations alone at changing physician care and patient outcomes. Such techniques include audit/feedback (the reporting of an individual clinician’s vaccination rates compared with desired or target rates, for example), academic detailing/outreach, and reminders by way of electronic or other alerts.10,11
Promote immunization to patients. Physicians are highly influential in determining a patient’s decision to vaccinate, and it is well documented that a strong recommendation about the importance of immunizations makes a difference to patients.12,13
What you say and how you say it matters. A halfhearted recommendation for vaccination may result in the patient remaining unvaccinated.14 For example, “If you want, you can get your pneumonia shot today” is much less persuasive than, “I recommend you get your pneumonia vaccine today to prevent a potentially serious disease that affects thousands of adults each year.” Most adults believe that vaccines are important and are likely to get them if recommended by their health care professionals.15
The CDC recommends that physicians encourage patients to make an informed decision about vaccination by sharing critical information highlighting the importance of vaccinations and reminding patients what vaccines protect against while addressing their concerns (www.cdc.gov/vaccines/adultstandards). Free educational materials for patients can be found at www.cdc.gov/vaccines/AdultPatientEd.
Draw on community resources. Laws and policies that require vaccinations as a prerequisite for attending childcare, school, or college increase coverage. Community and faith-based organizations are likely to play an important role in reducing racial and ethnic disparities in adult immunizations because they can deliver education that is culturally sensitive and tailored to specific subpopulations.16,17 Physicians and other health care providers can get involved with community and faith-based groups and local and federal legislative efforts to improve immunization rates.
Consider implementing these system-based interventions
The following 6 system-based interventions can help improve adult immunization rates:
1. Develop a practice team. The practice team, based on the Patient-Centered Medical Home (PCMH), includes physicians, midlevel providers, nurses, medical assistants, pharmacists, social workers, and other staff. The PCMH team model can facilitate a shift of responsibilities among individuals to better orient the practice toward patients’ health and preventive services.18,19 While physicians have traditionally held all of the responsibility for patient care, including screening for disease and prevention, shifting the responsibility of vaccine screening to nurses or medical assistants can free up time for longer physician/patient interactions.18
The creation of a practice champion within the PCMH team—a physician, midlevel provider, or nurse—to oversee quality improvement for vaccine rates and work to generate support and cooperation from coworkers has also been shown to improve vaccination rates.20 The vaccine champion should keep abreast of new vaccine recommendations and relay that information to the practice through regular staff meetings, announcements, and office postings. The champion can also supervise pre-visit planning for immunizations.19
2. Use electronic immunization information systems (IIS). All states except New Hampshire have an IIS.21 Accurate tracking of adult immunizations in a registry provides a complete record and is essential to improving adult immunization rates,22 as does the use of chart notes, computerized alerts, checklists, and other tools that remind health care providers when patients are due for vaccinations.18 NVAC recommends that all physicians use their state IIS and create a process in their practice to include its use.
3. Incorporate physician feedback. Many health care systems and payers are using benchmarking and incentives to provide physician feedback on vaccination performance.23 Using achievable benchmarks enhances the effectiveness of physician performance feedback.24 The Task Force conducted a systematic review of the evidence on the effectiveness of health care provider assessment and feedback for increasing coverage rates and found that this strategy remains an effective means to increase vaccination rates.25
4. Use reminders/alerts. Even though you may intend to routinely recommend immunizations, remembering to do so at the time of each visit can be difficult when there are so many other issues to address. Reminders at the time of the visit can help. Some electronic records have reminder prompts, or “best practice alerts” (BPAs), programmed into their systems.26 These BPAs will prompt for needed immunizations whether the patient is being seen for a well, acute, or routine follow-up visit. These reminder/recall activities can be greatly simplified by participation in a population-based IIS.
Practices that don’t have an electronic health record can still improve vaccination rates by conveying the reminder with a brightly colored paper form attached to the front of a patient’s chart during the check-in process. One recent study showed that this approach increased rates of influenza vaccination in an urban practice by 12 percentage points.27
Furthermore, simply reminding patients to vaccinate increases the vaccination rate.28 Patient reminder/recall systems using telephone calls or mailings (phone calls are more effective than mailings) improve both childhood and adult vaccinations in all medical settings. More intensive systems using multiple reminders appear to be more effective than single reminders, and while costly, the benefits of increasing preventive visits/services and vaccine uptake help offset this cost.28
5. Implement standing orders. Standing orders—which allow nurses and other appropriately trained health care personnel to assess immunization status and administer vaccinations according to protocol—help improve immunization rates.29 ACIP advises that standing order programs be used in long-term care facilities under the supervision of a medical director to ensure the administration of recommended vaccinations for adults, and in inpatient and outpatient facilities. Because of the societal burden of influenza and pneumococcal disease, implementation of standing orders programs to improve adult vaccination coverage for these diseases is considered a national public health priority.30
6. Develop an encouraging communication style. Studies show that how one communicates with patients is just as important as what one communicates. Certain communication styles and techniques may be more or less effective when discussing vaccination needs with some patients, especially those with vaccine hesitancy or low confidence in vaccine safety or effectiveness. For example, styles that are “directing” are usually unhelpful in addressing concerns about vaccination. These styles typically use information and persuasion to achieve change and may be perceived as confrontational. This approach can lead to cues being missed, jargon being used, and vaccine safety being overstated.
Styles shown to be helpful are those that elicit patient concerns, ask permission to discuss, acknowledge/listen/empathize, determine readiness to change, inform about benefits and risks, and give appropriate resources. These helpful forms of communication are more of a “May I help you?” style vs a “This is what you should do” style of communication.31
Assure patients that recommendations are based on the best interest of their health and on the best available science. Listen to a patient’s concerns and acknowledge them in a nonconfrontational manner, allowing patients to express their concerns and thereby increase their willingness to listen.32 Saying that there is “absolutely no need to worry—vaccines are safe and you are silly not to get yours” is not as effective as saying, “What are your concerns regarding vaccines? Let’s talk about them.”
For the vaccine-hesitant group, building trust is essential through a respectful, nonjudgmental approach that aims to elicit and address specific concerns. For those who refuse vaccines, keep the consultation brief, keep the door open for further discussion, and provide appropriate resources if the patient wants them.33
Increase use of new media
Mass communication through smartphones and other Internet-based tools such as Facebook and Twitter brings a new dimension to health care, allowing patients and health professionals to communicate about health issues and possibly improve health outcomes.34 The number of people using social media increased by almost 570% worldwide between 2000 and 2012 and surpassed 2.75 billion in 2013.35
Sixty-one percent of adults in the United States look online for health information.36 In a survey conducted in September 2014, the Pew Research Center found that Facebook is the most popular social media site in the United States. Seventy-one percent of online-knowledgeable adults use Facebook, and multiplatform use is on the rise: 52% of adult Internet users now use 2 or more social media sites, a significant increase from 2013, when it stood at 42%. (Other platforms such as Twitter, Instagram, Pinterest, and LinkedIn saw significant increases over the past year in the proportion of online adults who use them).37
Health information provided by social media can answer medical questions and concerns and enhance health promotion and education.35 A recent review of 98 research studies provided evidence that social media can create a space to share, comment, and discuss health information.34 Compared with traditional communication methods, the widespread availability of social media makes health information more accessible, broadening access to various population groups, regardless of age, education, race, ethnicity, and locale.
New media platforms are proving effective. The first systematic assessment of available evidence on the use of new media to increase vaccine uptake and immunization coverage (a review of 7 randomized controlled trials [RCTs], 5 non-RCTs, 3 cross-sectional studies, one case-control study and 3 operational research studies published between 2000-2013) found that text messaging, accessing immunization campaign Web sites, using patient-held Web-based portals, computerized reminders, and standing orders increased immunization coverage rates.35 However, evidence was insufficient in this regard on the value of social networks, email communication, and smartphone applications.
One RCT showed that having access to a personalized Web-based portal where patients could manage health records as well as interact with both health care providers and other members of the community through social forums and messaging tools increased influenza vaccination rates.35
CORRESPONDENCE
Pamela G. Rockwell, DO, Department of Family Medicine, University of Michigan, 24 Frank Lloyd Wright Drive, P.O. Box 431, Ann Arbor, MI 48106-0795; [email protected].
1. Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:95-102.
2. Centers for Disease Control and Prevention. Active bacterial core surveillance (ABCs) report, emerging infections program network, Streptococcus pneumoniae, 2013. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Accessed August 20, 2015.
3. Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057-1062.
4. Reed C, Kim IK, Singleton JA, et al; Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by vaccination--United States, 2013-14 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:1151-1154.
5. Kimmel SR, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
6. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based Guide to Community Preventive Services—methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
7. The Guide to Community Preventive Services. Increasing appropriate vaccination. The Community Guide Web site. Available at: http://www.thecommunityguide.org/vaccines/index.html. Accessed August 20, 2015.
8. National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory committee: standards for adult immunization practice. Public Health Rep. 2014;129:115-123.
9. Househ M. The use of social media in healthcare: organizational, clinical, and patient perspectives. Stud Health Technol Inform. 2013;183:244-248.
10. Bloom BS. Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews. Int J Technol Assess Health Care. 2005;21:380-385.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458-1465.
12. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19-26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
13. Zimmerman RK, Santibanez TA, Janosky JE, et al. What affects influenza vaccination rates among older patients? An analysis from inner-city, suburban, rural, and Veterans Affairs practices. Am J Med. 2003;114:31-38.
14. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed August 20, 2015.
15. National Foundation for Infectious Diseases. Survey: adults do not recognize infectious disease risks. National Foundation for Infectious Diseases Web site. Available at: http://www.adultvaccination.org/newsroom/events/2009-vaccination-news-conference/NFID-Survey-Fact-Sheet.pdf. Accessed July 7, 2015.
16. Wang E, Clymer J, Davis-Hayes C, et al. Nonmedical exemptions from school immunization requirements: a systematic review. Am J Public Health. 2014;104:e62-e84.
17. National Vaccine Advisory Committee. A pathway to leadership for adult immunization: recommendations of the National Vaccine Advisory Committee: approved by the National Vaccine Advisory Committee on June 14, 2011. Public Health Rep. 2012;127:1-42.
18. Gannon M, Qaseem A, Snooks Q, et al. Improving adult immunization practices using a team approach in the primary care setting. Am J Public Health. 2012;102:e46-e52.
19. Bottino CJ, Cox JE, Kahlon PS, et al. Improving immunization rates in a hospital-based primary care practice. Pediatrics. 2014;133:e1047-e1054.
20. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
21. Centers for Disease Control and Prevention (CDC). Progress in immunization information systems - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:1005-1008.
22. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
23. Kerr EA, McGlynn EA, Adams J, et al. Profiling the quality of care in twelve communities: results from the CQI study. Health Aff (Millwood). 2004;23:247-256.
24. Kiefe CI, Allison JJ, Williams OD, et al. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA. 2001;285:2871-2879.
25. National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1-64.
26. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
27. Pierson RC, Malone AM, Haas DM. Increasing influenza vaccination rates in a busy urban clinic. J Nat Sci. 2015;1.
28. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;CD003941.
29. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Task Force on Community Preventive Services. Am J Prev Med. 2000;18:92-96.
30. McKibben LJ, Stange PV, Sneller VP, et al; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49:15-16.
31. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154.
32. Kimmel SR, Wolfe RM. Communicating the benefits and risks of vaccines. J Fam Pract. 2005;54:S51-S57.
33. Danchin M, Nolan T. A positive approach to parents with concerns about vaccination for the family physician. Aust Fam Physician. 2014;43:690-694.
34. Moorhead SA, Hazlett DE, Harrison L, et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
35. Odone A, Ferrari A, Spagnoli F, et al. Effectiveness of interventions that apply new media to improve vaccine uptake and vaccine coverage. Hum Vaccin Immunother. 2015;11:72-82.
36. Pew Research Center. Fox S. The Social Life of Health Information, 2011. Pew Research Center Web site. Available at: http://www.pewinternet.org/2011/05/12/the-social-life-of-health-information-2011/. Accessed August 20, 2015.
37. Pew Research Center. Duggan M, Ellison NB, Lampe C, et al. Social Media Update 2014. Pew Research Center Web site. Available at: http://www.pewinternet.org/2015/01/09/social-media-update-2014/. Accessed August 20, 2015.
1. Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:95-102.
2. Centers for Disease Control and Prevention. Active bacterial core surveillance (ABCs) report, emerging infections program network, Streptococcus pneumoniae, 2013. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Accessed August 20, 2015.
3. Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057-1062.
4. Reed C, Kim IK, Singleton JA, et al; Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by vaccination--United States, 2013-14 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:1151-1154.
5. Kimmel SR, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
6. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based Guide to Community Preventive Services—methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
7. The Guide to Community Preventive Services. Increasing appropriate vaccination. The Community Guide Web site. Available at: http://www.thecommunityguide.org/vaccines/index.html. Accessed August 20, 2015.
8. National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory committee: standards for adult immunization practice. Public Health Rep. 2014;129:115-123.
9. Househ M. The use of social media in healthcare: organizational, clinical, and patient perspectives. Stud Health Technol Inform. 2013;183:244-248.
10. Bloom BS. Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews. Int J Technol Assess Health Care. 2005;21:380-385.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458-1465.
12. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19-26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
13. Zimmerman RK, Santibanez TA, Janosky JE, et al. What affects influenza vaccination rates among older patients? An analysis from inner-city, suburban, rural, and Veterans Affairs practices. Am J Med. 2003;114:31-38.
14. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed August 20, 2015.
15. National Foundation for Infectious Diseases. Survey: adults do not recognize infectious disease risks. National Foundation for Infectious Diseases Web site. Available at: http://www.adultvaccination.org/newsroom/events/2009-vaccination-news-conference/NFID-Survey-Fact-Sheet.pdf. Accessed July 7, 2015.
16. Wang E, Clymer J, Davis-Hayes C, et al. Nonmedical exemptions from school immunization requirements: a systematic review. Am J Public Health. 2014;104:e62-e84.
17. National Vaccine Advisory Committee. A pathway to leadership for adult immunization: recommendations of the National Vaccine Advisory Committee: approved by the National Vaccine Advisory Committee on June 14, 2011. Public Health Rep. 2012;127:1-42.
18. Gannon M, Qaseem A, Snooks Q, et al. Improving adult immunization practices using a team approach in the primary care setting. Am J Public Health. 2012;102:e46-e52.
19. Bottino CJ, Cox JE, Kahlon PS, et al. Improving immunization rates in a hospital-based primary care practice. Pediatrics. 2014;133:e1047-e1054.
20. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
21. Centers for Disease Control and Prevention (CDC). Progress in immunization information systems - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:1005-1008.
22. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
23. Kerr EA, McGlynn EA, Adams J, et al. Profiling the quality of care in twelve communities: results from the CQI study. Health Aff (Millwood). 2004;23:247-256.
24. Kiefe CI, Allison JJ, Williams OD, et al. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA. 2001;285:2871-2879.
25. National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1-64.
26. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
27. Pierson RC, Malone AM, Haas DM. Increasing influenza vaccination rates in a busy urban clinic. J Nat Sci. 2015;1.
28. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;CD003941.
29. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Task Force on Community Preventive Services. Am J Prev Med. 2000;18:92-96.
30. McKibben LJ, Stange PV, Sneller VP, et al; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49:15-16.
31. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154.
32. Kimmel SR, Wolfe RM. Communicating the benefits and risks of vaccines. J Fam Pract. 2005;54:S51-S57.
33. Danchin M, Nolan T. A positive approach to parents with concerns about vaccination for the family physician. Aust Fam Physician. 2014;43:690-694.
34. Moorhead SA, Hazlett DE, Harrison L, et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
35. Odone A, Ferrari A, Spagnoli F, et al. Effectiveness of interventions that apply new media to improve vaccine uptake and vaccine coverage. Hum Vaccin Immunother. 2015;11:72-82.
36. Pew Research Center. Fox S. The Social Life of Health Information, 2011. Pew Research Center Web site. Available at: http://www.pewinternet.org/2011/05/12/the-social-life-of-health-information-2011/. Accessed August 20, 2015.
37. Pew Research Center. Duggan M, Ellison NB, Lampe C, et al. Social Media Update 2014. Pew Research Center Web site. Available at: http://www.pewinternet.org/2015/01/09/social-media-update-2014/. Accessed August 20, 2015.
Ectopic pregnancy: Zero in on these lab and imaging clues
• Administer a urine pregnancy test for women of childbearing age who present with abdominal pain or vaginal bleeding. C
• Initiate quantitative beta-human chorionic gonadotropin testing and order transvaginal ultrasound for women with abdominal pain or vaginal bleeding and a positive urine pregnancy test, but no confirmation of intrauterine pregnancy by abdominal ultrasound. B
• Refer hemodynamically stable patients with ectopic pregnancy for laparoscopic salpingostomy. For selected patients, an alternative is medical treatment with methotrexate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Helen, who is 31 years old and G1P0, comes in to the office with a 10-day history of intermittent vaginal spotting without pelvic pain. A home pregnancy test 2 weeks earlier was positive, and this is a desired pregnancy. She has had no gynecologic disorders. It has been 6 weeks since her last menstrual period. Her vital signs are normal and her abdominal and pelvic exams are unremarkable. The cervical os is closed and there is a small amount of blood in the vaginal vault. Her family physician (FP) draws blood to measure the level of beta-human chorionic gonadotropin (β-hCG) and orders a transvaginal ultrasound (TVUS).
CASE 2 Mary is 28 years old and G2P1. She has experienced intermittent vaginal spotting and moderate pelvic discomfort for 3 days. She fears a return of pelvic inflammatory disease (PID). Her period is one week late and the office pregnancy test is positive. Her vital signs are normal. She has no cervical motion tenderness, but there is mild right adnexal tenderness to palpation. Her FP draws blood for a serum β-hCG level and orders a TVUS.
Assess physical and history findings for perspective
Abdominal or pelvic pain and vaginal bleeding in the first trimester are the most common presenting symptoms of ectopic pregnancies.1 Physical examination will often elicit lateral or bilateral abdominal or pelvic tenderness, peritoneal signs, and cervical motion tenderness. But such findings (or their absence) cannot confirm (or exclude) the diagnosis with a high level of reliability.2 A woman with a positive pregnancy test and pelvic pain or vaginal bleeding may instead have a normal pregnancy, spontaneous abortion (failing intrauterine pregnancy), or a disorder such as PID, acute appendicitis, tubo-ovarian abscess, or ovarian torsion.
In an early ectopic pregnancy, vital signs are usually normal. Even in cases of ruptured ectopic pregnancy, hypotension or tachycardia is present in <40% of cases.3
Factors conferring a relative risk ratio >2 for ectopic pregnancy are a previous ectopic pregnancy; documented tubal pathology or tubal instrumentation (eg, tubal sterilization or tubal corrective surgery); assisted reproductive technology such as in vitro fertilization; history of infertility; smoking; or a history of PID.4-11
Proceed with a laboratory and imaging strategy
When a woman who has tested positive for pregnancy presents with abdominal pain or vaginal bleeding and a normal intrauterine pregnancy (IUP) has not been confirmed by abdominal ultrasound, request a quantitative measurement of the β-hCG level and arrange for urgent TVUS.12,13 If pregnancy has been unsuspected in a patient with these symptoms, perform a urine test for pregnancy immediately and follow up with ultrasound.14
If TVUS reveals either IUP or ectopic pregnancy, management is relatively straightforward. However, an inconclusive TVUS result indicates a “pregnancy of unknown location” (PUL) and necessitates further testing and follow-up to achieve a final diagnosis.15
Monitor β-hCG levels
Valuable diagnostic measures include documenting the initial serum level of β-hCG, monitoring the subsequent rise-or-fall pattern in the level, and making use of the “discriminatory cutoff” value.
β-hCG, made by placental cells, can be detected in the mother’s blood approximately 11 days after conception, and in the urine 12 to 14 days after conception. The serum β-hCG level normally doubles every 48 to 72 hours until it reaches its peak in the first 8 to 11 weeks of pregnancy. The level then declines and plateaus.
”Discriminatory cutoff” is a widely accepted concept signifying the level of β-hCG at which a normal IUP can be visualized by ultrasonography with sensitivity approaching 100%.16 Generally an intrauterine sac can by visualized by abdominal ultrasound when the serum β-hCG level is >6500 mIU/mL.17 Visualization with TVUS (the preferred modality) has been demonstrated when the serum β-hCG level is as low as 1000 mIU/mL.17 However, the generally accepted cutoff range is 1500 to 2500 mIU/mL, based on several studies.13,18-20 The absence of an IUP in a pregnant woman with pain or bleeding and a β-hCG level above the cutoff implies an ectopic pregnancy18 or a failing IUP (spontaneous abortion).
Serial ß-hCG levels. When the β-hCG level is below the discriminatory cutoff, serial β-hCG measurements every 2 to 3 days are needed to assess viability of the pregnancy. A “normal rise” of β-hCG indicates early viable pregnancy and “normal fall” indicates spontaneous abortion. An analysis of 287 women with abdominal pain or vaginal bleeding who ultimately had normal uterine pregnancies found that the median slope for rise of β-hCG was 1.5 times (50% increase) in 1 day, and 2.24 times (124% rise) in 2 days.21 A rapid fall in β-hCG is consistent with a miscarriage that may resolve spontaneously. However, if the β-hCG level does not decline by 21% to 35% in 2 days, suspect ectopic pregnancy.21
Arrange for transvaginal ultrasound
TVUS is the imaging modality of choice for diagnosis of ectopic pregnancy, with a sensitivity of 87.0% to 99.0% and specificity of 94.0% to 99.9%.22 Arrange for TVUS when a women has abdominal pain or vaginal bleeding and a positive urine pregnancy test, even if the β-hCG level is lower than the discriminatory cutoff of 1500 to 2500 mIU/mL.13,18-20 Ordering TVUS and β-hCG level at the same time yields the best outcome for diagnosis,19 while varying the discriminatory zone alone has not improved diagnosis.18,23
Other novel markers
The use of serum progesterone and other novel markers such as inhibin A, activin A, creatinine kinase, vascular endothelial growth factor, and cancer antigen 125 in the diagnosis of ectopic pregnancy has been studied extensively. To date, no single marker has demonstrated high sensitivity and specificity in differentiating ectopic pregnancy.24 However, when the initial progesterone level is ≤10 nmol/L (equivalent to 31.4 ng/mL) in a woman with a PUL, the probability that she will require any intervention is reported to be low (4 cases out of 227 PUL cases).25 Multiplex tools to combine multiple biomarkers may become available in the future.
Evacuation of uterine contents
When the β-hCG level is above the discriminatory cutoff but no evidence of an extrauterine or intrauterine pregnancy can be found by TVUS, the patient likely has a failing IUP or impending abortion. Some experts suggest considering evaluation of the uterine contents by dilation and curettage (D&C) or manual vacuum extraction at this time, to differentiate an abnormal intrauterine gestation from an ectopic pregnancy. Barnhart found that more than one-third of such cases were due to a failed uterine pregnancy, not ectopic pregnancy.26
If, after a D&C or manual extraction, chorionic villi are not confirmed by pathologic examination of the uterine contents, then treat as an ectopic pregnancy. Some clinicians alternatively recommend checking the β-hCG level again in 12 to 24 hours, expecting ≥15% decline with a spontaneous abortion.27 Alternatively, some recommend using methotrexate (MTX) without D&C to avoid unnecessary medical and surgical treatment.26
CASE 1 Helen’s serum β-hCG level is 4500 mIU/mL, and the TVUS image the next day shows an echogenic mass next to the right ovary—highly suspicious for ectopic pregnancy.
CASE 2 Mary’s TVUS does not show any evidence of IUP or any abnormality in either adnexa. Her serum β-hCG level is 650 mIU/mL. She has a PUL. Her FP informs her that she may have an early normal pregnancy, a failed IUP, or an ectopic pregnancy. She agrees to have her serum β-hCG measured every 2 days. Her β-hCG level increases to 1100, 2000, and 3500 mIU/mL, in 2, 4, and 6 days, respectively. TVUS on the sixth day is still nondiagnostic.
Treatment of ectopic pregnancy: Surgical vs medical
For hemodynamically unstable patients, laparotomy is still the mainstay of therapy. However, with early diagnosis and a stable patient, options are minimally invasive surgical intervention via laparostomy or medical management with MTX in a single or multidose regimen. Surgical and medical treatments have comparable outcomes, as documented by a Cochrane review.28
The risk of recurrent ectopic pregnancy after MTX treatment and salpingostomy is similar—about 10%.29 Ipsilateral tubal patency as documented by hysterosalpingography after MTX treatment or salpingostomy was reported to be equal.28 Reproductive outcomes after either treatment were similar, as well.30
We recommend urgent referral for OB/GYN consultation if the diagnosis of ectopic pregnancy is made by TVUS, since the recommended treatment is laparoscopic salpingostomy. In the case of a PUL, we recommend referral to an OB/GYN when the serum β-hCG level is above the discriminatory cutoff of 1500 to 2500 mIU/mL without signs of IUP as seen by a gestational sac via TVUS. When an urgent referral is not possible, initiate medical treatment. Regardless of the treatment method, give anti-D immunoglobulin to any woman whose blood is Rh negative (no D-antigen) and who has not been sensitized to D-antigen.
Surgical management
Laparoscopic salpingostomy is the preferred surgical treatment for ectopic pregnancy. A Cochrane review meta-analysis of 35 randomized controlled trials (RCTs) on intervention of ectopic pregnancy concluded that, compared with laparotomy, laparoscopy results in shorter operative time, less blood loss, less analgesia, shorter hospital stays, and greater cost effectiveness.28 Another meta-analysis of 15 RCTs concluded that laparoscopic salpingostomy is the most cost-effective treatment for ectopic pregnancy. 31
Medical management with methotrexate
This folic acid antagonist is highly effective in treating ectopic pregnancy, and is usually given intramuscularly for this indication. Clinicians who use this chemotherapeutic agent must be familiar with its dosing regimen, contraindications, and possible adverse effects. Multidose MTX is more effective than surgery, but more expensive.32 Single-dose MTX has a higher failure rate than laparoscopic salpingostomy, especially in patients with higher β-hCG levels.32
The best candidate for medical therapy is the woman who is asymptomatic, motivated, and compliant. Absolute contraindications to single-dose MTX include the following:
- breastfeeding
- overt or lab evidence of immunodeficiency
- alcoholism, alcoholic liver disease, or other chronic liver disease
- preexisting blood dyscrasias, such as bone marrow hypoplasia, leucopenia, thrombocytopenia, or significant anemia
- known sensitivity for methotrexate
- acute pulmonary disease
- peptic ulcer disease
- hepatic, renal, or hematologic dysfunction, and several metabolic diseases.33
Dosing regimen. The 3 general dosing schemes of single dose, 2-dose, and multidose (up to 4 doses) are shown in the TABLE. These were recommended by the American College of Obstetrician and Gynecologists (ACOG).33
Single dose vs multidose. The single-dose treatment is easier to administer and monitor and is most cost effective, but it may have a higher failure rate than the multidose regimens.28 The best prognostic indicator of successful treatment with single-dose MTX is the initial β-hCG level. The lower the initial level, the higher the success rate. The reported failure rate is 1.5% if the initial β-hCG level is <1000 mIU/mL; 5.6% with 1000 to 2000 mIU/mL; 3.8% with 2000 to 5000 mIU/mL; and 14.3% with 5000 to 10,000 mIU/mL.34 ACOG has outlined relative contraindications to single-dose MTX: ectopic pregnancy larger than 3.5 cm and the presence of fetal cardiac activity. Both correlate with an increased failure rate. Patients with PUL and low β-hCG levels are good candidates for single-dose MTX treatment.
Monitoring efficacy of treatment
Serum β-hCG levels indicate response to medical and surgical therapy. After salpingostomy, the serum β-hCG level declines rapidly within the first 4 days, and then more gradually, with mean resolution occurring at about 20 days. In contrast, after single-dose MTX, the mean serum β-hCG level increases for the first 4 days and then gradually declines, with a mean resolution at 27 days.35 The guideline for surveillance is shown in the TABLE.
CASE 1 The FP counsels Helen on the risks and benefits of surgery and MTX treatment for her ectopic pregnancy, and she elects to have a laparoscopic salpingostomy. The FP refers Helen to an OB/GYN via the emergency department on the same day. Helen does well. After the surgery, her β-hCG is monitored every 2 days until it decreases to 1000 mIU/mL, then every week until it is negative.
CASE 2 The FP advises Mary that an OB/GYN would likely recommend a D&C for her PUL, as her β-hCG level is above the discriminatory cutoff and the TVUS does not show a viable IUP. After discussing MTX treatment and manual vacuum aspiration of the uterine contents, Mary elects to have the MTX treatment and receives the 2-dose protocol. Her β-hCG level is 4210 mIU/mL on Day 1—higher than her level prior to the methotrexate treatment, but expected. Levels drop to 3635, 3102, and 2214 mIU/mL on Days 4, 7, and 10, respectively. Mary receives weekly surveillance until her level decreases to 0, which it did in a month.
TABLE
Monitoring methotrexate therapy for ectopic pregnancy
| Regimen | Surveillance |
|---|---|
| Single dose* Methotrexate, 50 mg/m2 IM | Measure β-hCG level on Days 4 and 7: If difference ≥15%, repeat weekly until undetectable
|
| 2 dose Methotrexate, 50 mg/m2 IM, Days 0, 4 | Follow up as for single-dose regimen |
| Multidose (up to 4 doses) Methotrexate, 1 mg/kg IM, Days 1, 3, 5, 7 Leucovorin, 0.1 mg/kg IM, Days 2, 4, 6, 8 | Measure β-hCG level on Days 1, 3, 5, and 7
|
| β-hCG, beta-human chorionic gonadotropin; IM, intramuscularly. *Preferred treatment if low initial β-hCG level. Adapted from: Seeber BE, et al. Obstet Gynecol. 2006.27 | |
CORRESPONDENCE Sahoko H. Little, MD, Room 2300, Lobby H, 24 Frank Lloyd Wright Drive, Ann Arbor, MI 48105-9755; [email protected]
1. Aboud E, Chaliha C. Nine year survey of 138 ectopic pregnancies. Arch Gynecol Obstet. 1998;261:83-87.
2. Dart GD, Kaplan B, Varaklis K. Predictive value of history and physical examination in patients with suspected ectopic pregnancy. Ann Emerg Med. 1999;33:283-290.
3. Birkhahn RH, Gaeta TJ, Van Deusen SK, et al. The ability of traditional vital signs and shock index to identify ruptured ectopic pregnancy. Am J Obstet Gynecol. 2003;189:1293-1296.
4. Cunningham FG, Leveno KJ, Bloom SL, et al. Ectopic pregnancy. In: Cunningham FG, Leveno KJ, Bloom SL, et al, eds. Williams Obstetrics. 23rd ed. New York, NY: McGraw-Hill; 2010:238–256.
5. Bakken IJ, Skjeldestad FE, Lydersen S, et al. Births and ectopic pregnancies in a large cohort of women tested for Chlamydia trachomatis. Sex Transm Dis. 2007;34:739-743.
6. Bakken IJ, Skjeldestad FE, Nordbo SA. Chlamydia trachomatis infections increase the risk for ectopic pregnancy: a population-based, nested case–control study. Sex Transm Dis. 2007;34:166-169.
7. Barnhart KT, Sammel MD, Gracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril. 2006;86:36-43.
8. Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185-194.
9. Gala RB. Ectopic pregnancy. In: Schorge JO, Schaffer JI, Halvorson LM, et al, eds. Williams Gynecology. New York, NY: McGraw-Hill; 2008:160-175.
10. Karaer A, Avsar FA, Batioglu S. Risk factors for ectopic pregnancy: a case-control study. Aust N Z J Obstet Gynaecol. 2006;46:521-527.
11. Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653.
12. Dart RG, Kaplan B, Cox C. Transvaginal ultrasound in patients with low beta-human chorionic gonadotropin values: how often is the study diagnostic? Ann Emerg Med. 1997;30:135-140.
13. Kaplan BC, Dart RG, Moskos M, et al. Ectopic pregnancy: prospective study with improved diagnostic accuracy. Ann Emerg Med. 1996;28:10-17.
14. Clinical policy: critical issues for the initial evaluation and management of patients presenting with a chief complaint of nontraumatic acute abdominal pain Ann Emerg Med. 2000;36:406-415.
15. Barnhart K, van Mello NM, Bourne T, et al. Pregnancy of unknown location: a consensus statement of nomenclature, definitions, and outcome. Fertil Steril. 2011;95:857-866.
16. Barnhart K, Mennuti MT, Benjamin I, et al. Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol. 1994;84:1010-1015.
17. Aleem FA, DeFazio M, Gintautas J. Endovaginal sonography for the early diagnosis of intrauterine and ectopic pregnancies. Hum Reprod. 1990;5:755-758.
18. Coundous G, Kirk E, Lu C, et al. Diagnostic accuracy of varying discriminatory zones for the prediction of ectopic pregnancy in women with a pregnancy of unknown location. Ultrasound Obstet Gynecol. 2005;26:770-775.
19. Gracia CR, Kurt TB. Diagnosing ectopic pregnancy: decision analysis comparing six strategies. Obstet Gynecol. 2001;97:464-470.
20. Kohn MA, Kerr K, Malkevich D, et al. Beta-human chorionic gonadotropin levels and the likelihood of ectopic pregnancy in emergency department patients with abdominal pain or vaginal bleeding. Acad Emerg Med. 2003;10:119-126.
21. Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104:975-981.
22. Kirk E, Bourne T. Diagnosis of ectopic pregnancy with ultrasound. Best Pract Res Clin Obstet Gynaecol. 2009;23:501-508.
23. van Mello NM, Mol F, Opmeer BC, et al. Diagnostic value of serum hCG on the outcome of pregnancy of unknown location: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:603-617.
24. Segal S, Mercado R, Rivnay B. Ectopic pregnancy early diagnosis markers. Minerva Ginecol. 2010;62:49-62.
25. Cordina M, Schramm-Gajraj K, Ross JA, et al. Introduction of a single visit protocol in the management of selected patients with pregnancy of unknown location: a prospective study. BJOG. 2011;118:693-697.
26. Barnhart KT, Katz I, Hummel A. Presumed diagnosis of ectopic pregnancy. Obstet Gynecol. 2002;100:505-510.
27. Seeber BE, Barnhart KT. Suspected ectopic pregnancy. Obstet Gynecol. 2006;107:399-413.
28. Hajenius PJ, Mol F, Mol BW, et al. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2007;(1):CD000324.-
29. Stovall TG. Medical management should be routinely used as primary therapy for ectopic pregnancy. Clin Obstet Gynecol. 1995;38:346-352.
30. Fernandez H, Yves Vincent SC, Pauthier S, et al. Randomized trial of conservative laparoscopic treatment and methotrexate administration in ectopic pregnancy and subsequent fertility. Hum Reprod. 1998;13:3239-3243.
31. Mol F, Strandell A, Jurkovic D, et al. The ESEP study: salpingostomy versus salpingectomy for tubal ectopic pregnancy; the impact on future fertility: a randomised controlled trial. BMC Womens Health. 2008;8:11.-
32. Mol F, Mol BW, Ankum WM, et al. Current evidence on surgery, systemic methotrexate and expectant management in the treatment of tubal ectopic pregnancy: a systematic review and meta-analysis. Hum Reprod Update. 2008;14:309-319.
33. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 94. Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111:1479-1485.
34. Menon S, Colins J, Barnhart KT. Establishing a human chorionic gonadotropin cutoff to guide methotrexate treatment of ectopic pregnancy: a systematic review. Fertil Steril. 2007;87:481-484.
35. Saraj AJ, Wilcox JG, Najmabadi S, et al. Resolution of hormonal markers of ectopic gestation: a randomized trial comparing single-dose intramuscular methotrexate with salpingostomy. Obstet Gynecol. 1998;92:989-994.
• Administer a urine pregnancy test for women of childbearing age who present with abdominal pain or vaginal bleeding. C
• Initiate quantitative beta-human chorionic gonadotropin testing and order transvaginal ultrasound for women with abdominal pain or vaginal bleeding and a positive urine pregnancy test, but no confirmation of intrauterine pregnancy by abdominal ultrasound. B
• Refer hemodynamically stable patients with ectopic pregnancy for laparoscopic salpingostomy. For selected patients, an alternative is medical treatment with methotrexate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Helen, who is 31 years old and G1P0, comes in to the office with a 10-day history of intermittent vaginal spotting without pelvic pain. A home pregnancy test 2 weeks earlier was positive, and this is a desired pregnancy. She has had no gynecologic disorders. It has been 6 weeks since her last menstrual period. Her vital signs are normal and her abdominal and pelvic exams are unremarkable. The cervical os is closed and there is a small amount of blood in the vaginal vault. Her family physician (FP) draws blood to measure the level of beta-human chorionic gonadotropin (β-hCG) and orders a transvaginal ultrasound (TVUS).
CASE 2 Mary is 28 years old and G2P1. She has experienced intermittent vaginal spotting and moderate pelvic discomfort for 3 days. She fears a return of pelvic inflammatory disease (PID). Her period is one week late and the office pregnancy test is positive. Her vital signs are normal. She has no cervical motion tenderness, but there is mild right adnexal tenderness to palpation. Her FP draws blood for a serum β-hCG level and orders a TVUS.
Assess physical and history findings for perspective
Abdominal or pelvic pain and vaginal bleeding in the first trimester are the most common presenting symptoms of ectopic pregnancies.1 Physical examination will often elicit lateral or bilateral abdominal or pelvic tenderness, peritoneal signs, and cervical motion tenderness. But such findings (or their absence) cannot confirm (or exclude) the diagnosis with a high level of reliability.2 A woman with a positive pregnancy test and pelvic pain or vaginal bleeding may instead have a normal pregnancy, spontaneous abortion (failing intrauterine pregnancy), or a disorder such as PID, acute appendicitis, tubo-ovarian abscess, or ovarian torsion.
In an early ectopic pregnancy, vital signs are usually normal. Even in cases of ruptured ectopic pregnancy, hypotension or tachycardia is present in <40% of cases.3
Factors conferring a relative risk ratio >2 for ectopic pregnancy are a previous ectopic pregnancy; documented tubal pathology or tubal instrumentation (eg, tubal sterilization or tubal corrective surgery); assisted reproductive technology such as in vitro fertilization; history of infertility; smoking; or a history of PID.4-11
Proceed with a laboratory and imaging strategy
When a woman who has tested positive for pregnancy presents with abdominal pain or vaginal bleeding and a normal intrauterine pregnancy (IUP) has not been confirmed by abdominal ultrasound, request a quantitative measurement of the β-hCG level and arrange for urgent TVUS.12,13 If pregnancy has been unsuspected in a patient with these symptoms, perform a urine test for pregnancy immediately and follow up with ultrasound.14
If TVUS reveals either IUP or ectopic pregnancy, management is relatively straightforward. However, an inconclusive TVUS result indicates a “pregnancy of unknown location” (PUL) and necessitates further testing and follow-up to achieve a final diagnosis.15
Monitor β-hCG levels
Valuable diagnostic measures include documenting the initial serum level of β-hCG, monitoring the subsequent rise-or-fall pattern in the level, and making use of the “discriminatory cutoff” value.
β-hCG, made by placental cells, can be detected in the mother’s blood approximately 11 days after conception, and in the urine 12 to 14 days after conception. The serum β-hCG level normally doubles every 48 to 72 hours until it reaches its peak in the first 8 to 11 weeks of pregnancy. The level then declines and plateaus.
”Discriminatory cutoff” is a widely accepted concept signifying the level of β-hCG at which a normal IUP can be visualized by ultrasonography with sensitivity approaching 100%.16 Generally an intrauterine sac can by visualized by abdominal ultrasound when the serum β-hCG level is >6500 mIU/mL.17 Visualization with TVUS (the preferred modality) has been demonstrated when the serum β-hCG level is as low as 1000 mIU/mL.17 However, the generally accepted cutoff range is 1500 to 2500 mIU/mL, based on several studies.13,18-20 The absence of an IUP in a pregnant woman with pain or bleeding and a β-hCG level above the cutoff implies an ectopic pregnancy18 or a failing IUP (spontaneous abortion).
Serial ß-hCG levels. When the β-hCG level is below the discriminatory cutoff, serial β-hCG measurements every 2 to 3 days are needed to assess viability of the pregnancy. A “normal rise” of β-hCG indicates early viable pregnancy and “normal fall” indicates spontaneous abortion. An analysis of 287 women with abdominal pain or vaginal bleeding who ultimately had normal uterine pregnancies found that the median slope for rise of β-hCG was 1.5 times (50% increase) in 1 day, and 2.24 times (124% rise) in 2 days.21 A rapid fall in β-hCG is consistent with a miscarriage that may resolve spontaneously. However, if the β-hCG level does not decline by 21% to 35% in 2 days, suspect ectopic pregnancy.21
Arrange for transvaginal ultrasound
TVUS is the imaging modality of choice for diagnosis of ectopic pregnancy, with a sensitivity of 87.0% to 99.0% and specificity of 94.0% to 99.9%.22 Arrange for TVUS when a women has abdominal pain or vaginal bleeding and a positive urine pregnancy test, even if the β-hCG level is lower than the discriminatory cutoff of 1500 to 2500 mIU/mL.13,18-20 Ordering TVUS and β-hCG level at the same time yields the best outcome for diagnosis,19 while varying the discriminatory zone alone has not improved diagnosis.18,23
Other novel markers
The use of serum progesterone and other novel markers such as inhibin A, activin A, creatinine kinase, vascular endothelial growth factor, and cancer antigen 125 in the diagnosis of ectopic pregnancy has been studied extensively. To date, no single marker has demonstrated high sensitivity and specificity in differentiating ectopic pregnancy.24 However, when the initial progesterone level is ≤10 nmol/L (equivalent to 31.4 ng/mL) in a woman with a PUL, the probability that she will require any intervention is reported to be low (4 cases out of 227 PUL cases).25 Multiplex tools to combine multiple biomarkers may become available in the future.
Evacuation of uterine contents
When the β-hCG level is above the discriminatory cutoff but no evidence of an extrauterine or intrauterine pregnancy can be found by TVUS, the patient likely has a failing IUP or impending abortion. Some experts suggest considering evaluation of the uterine contents by dilation and curettage (D&C) or manual vacuum extraction at this time, to differentiate an abnormal intrauterine gestation from an ectopic pregnancy. Barnhart found that more than one-third of such cases were due to a failed uterine pregnancy, not ectopic pregnancy.26
If, after a D&C or manual extraction, chorionic villi are not confirmed by pathologic examination of the uterine contents, then treat as an ectopic pregnancy. Some clinicians alternatively recommend checking the β-hCG level again in 12 to 24 hours, expecting ≥15% decline with a spontaneous abortion.27 Alternatively, some recommend using methotrexate (MTX) without D&C to avoid unnecessary medical and surgical treatment.26
CASE 1 Helen’s serum β-hCG level is 4500 mIU/mL, and the TVUS image the next day shows an echogenic mass next to the right ovary—highly suspicious for ectopic pregnancy.
CASE 2 Mary’s TVUS does not show any evidence of IUP or any abnormality in either adnexa. Her serum β-hCG level is 650 mIU/mL. She has a PUL. Her FP informs her that she may have an early normal pregnancy, a failed IUP, or an ectopic pregnancy. She agrees to have her serum β-hCG measured every 2 days. Her β-hCG level increases to 1100, 2000, and 3500 mIU/mL, in 2, 4, and 6 days, respectively. TVUS on the sixth day is still nondiagnostic.
Treatment of ectopic pregnancy: Surgical vs medical
For hemodynamically unstable patients, laparotomy is still the mainstay of therapy. However, with early diagnosis and a stable patient, options are minimally invasive surgical intervention via laparostomy or medical management with MTX in a single or multidose regimen. Surgical and medical treatments have comparable outcomes, as documented by a Cochrane review.28
The risk of recurrent ectopic pregnancy after MTX treatment and salpingostomy is similar—about 10%.29 Ipsilateral tubal patency as documented by hysterosalpingography after MTX treatment or salpingostomy was reported to be equal.28 Reproductive outcomes after either treatment were similar, as well.30
We recommend urgent referral for OB/GYN consultation if the diagnosis of ectopic pregnancy is made by TVUS, since the recommended treatment is laparoscopic salpingostomy. In the case of a PUL, we recommend referral to an OB/GYN when the serum β-hCG level is above the discriminatory cutoff of 1500 to 2500 mIU/mL without signs of IUP as seen by a gestational sac via TVUS. When an urgent referral is not possible, initiate medical treatment. Regardless of the treatment method, give anti-D immunoglobulin to any woman whose blood is Rh negative (no D-antigen) and who has not been sensitized to D-antigen.
Surgical management
Laparoscopic salpingostomy is the preferred surgical treatment for ectopic pregnancy. A Cochrane review meta-analysis of 35 randomized controlled trials (RCTs) on intervention of ectopic pregnancy concluded that, compared with laparotomy, laparoscopy results in shorter operative time, less blood loss, less analgesia, shorter hospital stays, and greater cost effectiveness.28 Another meta-analysis of 15 RCTs concluded that laparoscopic salpingostomy is the most cost-effective treatment for ectopic pregnancy. 31
Medical management with methotrexate
This folic acid antagonist is highly effective in treating ectopic pregnancy, and is usually given intramuscularly for this indication. Clinicians who use this chemotherapeutic agent must be familiar with its dosing regimen, contraindications, and possible adverse effects. Multidose MTX is more effective than surgery, but more expensive.32 Single-dose MTX has a higher failure rate than laparoscopic salpingostomy, especially in patients with higher β-hCG levels.32
The best candidate for medical therapy is the woman who is asymptomatic, motivated, and compliant. Absolute contraindications to single-dose MTX include the following:
- breastfeeding
- overt or lab evidence of immunodeficiency
- alcoholism, alcoholic liver disease, or other chronic liver disease
- preexisting blood dyscrasias, such as bone marrow hypoplasia, leucopenia, thrombocytopenia, or significant anemia
- known sensitivity for methotrexate
- acute pulmonary disease
- peptic ulcer disease
- hepatic, renal, or hematologic dysfunction, and several metabolic diseases.33
Dosing regimen. The 3 general dosing schemes of single dose, 2-dose, and multidose (up to 4 doses) are shown in the TABLE. These were recommended by the American College of Obstetrician and Gynecologists (ACOG).33
Single dose vs multidose. The single-dose treatment is easier to administer and monitor and is most cost effective, but it may have a higher failure rate than the multidose regimens.28 The best prognostic indicator of successful treatment with single-dose MTX is the initial β-hCG level. The lower the initial level, the higher the success rate. The reported failure rate is 1.5% if the initial β-hCG level is <1000 mIU/mL; 5.6% with 1000 to 2000 mIU/mL; 3.8% with 2000 to 5000 mIU/mL; and 14.3% with 5000 to 10,000 mIU/mL.34 ACOG has outlined relative contraindications to single-dose MTX: ectopic pregnancy larger than 3.5 cm and the presence of fetal cardiac activity. Both correlate with an increased failure rate. Patients with PUL and low β-hCG levels are good candidates for single-dose MTX treatment.
Monitoring efficacy of treatment
Serum β-hCG levels indicate response to medical and surgical therapy. After salpingostomy, the serum β-hCG level declines rapidly within the first 4 days, and then more gradually, with mean resolution occurring at about 20 days. In contrast, after single-dose MTX, the mean serum β-hCG level increases for the first 4 days and then gradually declines, with a mean resolution at 27 days.35 The guideline for surveillance is shown in the TABLE.
CASE 1 The FP counsels Helen on the risks and benefits of surgery and MTX treatment for her ectopic pregnancy, and she elects to have a laparoscopic salpingostomy. The FP refers Helen to an OB/GYN via the emergency department on the same day. Helen does well. After the surgery, her β-hCG is monitored every 2 days until it decreases to 1000 mIU/mL, then every week until it is negative.
CASE 2 The FP advises Mary that an OB/GYN would likely recommend a D&C for her PUL, as her β-hCG level is above the discriminatory cutoff and the TVUS does not show a viable IUP. After discussing MTX treatment and manual vacuum aspiration of the uterine contents, Mary elects to have the MTX treatment and receives the 2-dose protocol. Her β-hCG level is 4210 mIU/mL on Day 1—higher than her level prior to the methotrexate treatment, but expected. Levels drop to 3635, 3102, and 2214 mIU/mL on Days 4, 7, and 10, respectively. Mary receives weekly surveillance until her level decreases to 0, which it did in a month.
TABLE
Monitoring methotrexate therapy for ectopic pregnancy
| Regimen | Surveillance |
|---|---|
| Single dose* Methotrexate, 50 mg/m2 IM | Measure β-hCG level on Days 4 and 7: If difference ≥15%, repeat weekly until undetectable
|
| 2 dose Methotrexate, 50 mg/m2 IM, Days 0, 4 | Follow up as for single-dose regimen |
| Multidose (up to 4 doses) Methotrexate, 1 mg/kg IM, Days 1, 3, 5, 7 Leucovorin, 0.1 mg/kg IM, Days 2, 4, 6, 8 | Measure β-hCG level on Days 1, 3, 5, and 7
|
| β-hCG, beta-human chorionic gonadotropin; IM, intramuscularly. *Preferred treatment if low initial β-hCG level. Adapted from: Seeber BE, et al. Obstet Gynecol. 2006.27 | |
CORRESPONDENCE Sahoko H. Little, MD, Room 2300, Lobby H, 24 Frank Lloyd Wright Drive, Ann Arbor, MI 48105-9755; [email protected]
• Administer a urine pregnancy test for women of childbearing age who present with abdominal pain or vaginal bleeding. C
• Initiate quantitative beta-human chorionic gonadotropin testing and order transvaginal ultrasound for women with abdominal pain or vaginal bleeding and a positive urine pregnancy test, but no confirmation of intrauterine pregnancy by abdominal ultrasound. B
• Refer hemodynamically stable patients with ectopic pregnancy for laparoscopic salpingostomy. For selected patients, an alternative is medical treatment with methotrexate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Helen, who is 31 years old and G1P0, comes in to the office with a 10-day history of intermittent vaginal spotting without pelvic pain. A home pregnancy test 2 weeks earlier was positive, and this is a desired pregnancy. She has had no gynecologic disorders. It has been 6 weeks since her last menstrual period. Her vital signs are normal and her abdominal and pelvic exams are unremarkable. The cervical os is closed and there is a small amount of blood in the vaginal vault. Her family physician (FP) draws blood to measure the level of beta-human chorionic gonadotropin (β-hCG) and orders a transvaginal ultrasound (TVUS).
CASE 2 Mary is 28 years old and G2P1. She has experienced intermittent vaginal spotting and moderate pelvic discomfort for 3 days. She fears a return of pelvic inflammatory disease (PID). Her period is one week late and the office pregnancy test is positive. Her vital signs are normal. She has no cervical motion tenderness, but there is mild right adnexal tenderness to palpation. Her FP draws blood for a serum β-hCG level and orders a TVUS.
Assess physical and history findings for perspective
Abdominal or pelvic pain and vaginal bleeding in the first trimester are the most common presenting symptoms of ectopic pregnancies.1 Physical examination will often elicit lateral or bilateral abdominal or pelvic tenderness, peritoneal signs, and cervical motion tenderness. But such findings (or their absence) cannot confirm (or exclude) the diagnosis with a high level of reliability.2 A woman with a positive pregnancy test and pelvic pain or vaginal bleeding may instead have a normal pregnancy, spontaneous abortion (failing intrauterine pregnancy), or a disorder such as PID, acute appendicitis, tubo-ovarian abscess, or ovarian torsion.
In an early ectopic pregnancy, vital signs are usually normal. Even in cases of ruptured ectopic pregnancy, hypotension or tachycardia is present in <40% of cases.3
Factors conferring a relative risk ratio >2 for ectopic pregnancy are a previous ectopic pregnancy; documented tubal pathology or tubal instrumentation (eg, tubal sterilization or tubal corrective surgery); assisted reproductive technology such as in vitro fertilization; history of infertility; smoking; or a history of PID.4-11
Proceed with a laboratory and imaging strategy
When a woman who has tested positive for pregnancy presents with abdominal pain or vaginal bleeding and a normal intrauterine pregnancy (IUP) has not been confirmed by abdominal ultrasound, request a quantitative measurement of the β-hCG level and arrange for urgent TVUS.12,13 If pregnancy has been unsuspected in a patient with these symptoms, perform a urine test for pregnancy immediately and follow up with ultrasound.14
If TVUS reveals either IUP or ectopic pregnancy, management is relatively straightforward. However, an inconclusive TVUS result indicates a “pregnancy of unknown location” (PUL) and necessitates further testing and follow-up to achieve a final diagnosis.15
Monitor β-hCG levels
Valuable diagnostic measures include documenting the initial serum level of β-hCG, monitoring the subsequent rise-or-fall pattern in the level, and making use of the “discriminatory cutoff” value.
β-hCG, made by placental cells, can be detected in the mother’s blood approximately 11 days after conception, and in the urine 12 to 14 days after conception. The serum β-hCG level normally doubles every 48 to 72 hours until it reaches its peak in the first 8 to 11 weeks of pregnancy. The level then declines and plateaus.
”Discriminatory cutoff” is a widely accepted concept signifying the level of β-hCG at which a normal IUP can be visualized by ultrasonography with sensitivity approaching 100%.16 Generally an intrauterine sac can by visualized by abdominal ultrasound when the serum β-hCG level is >6500 mIU/mL.17 Visualization with TVUS (the preferred modality) has been demonstrated when the serum β-hCG level is as low as 1000 mIU/mL.17 However, the generally accepted cutoff range is 1500 to 2500 mIU/mL, based on several studies.13,18-20 The absence of an IUP in a pregnant woman with pain or bleeding and a β-hCG level above the cutoff implies an ectopic pregnancy18 or a failing IUP (spontaneous abortion).
Serial ß-hCG levels. When the β-hCG level is below the discriminatory cutoff, serial β-hCG measurements every 2 to 3 days are needed to assess viability of the pregnancy. A “normal rise” of β-hCG indicates early viable pregnancy and “normal fall” indicates spontaneous abortion. An analysis of 287 women with abdominal pain or vaginal bleeding who ultimately had normal uterine pregnancies found that the median slope for rise of β-hCG was 1.5 times (50% increase) in 1 day, and 2.24 times (124% rise) in 2 days.21 A rapid fall in β-hCG is consistent with a miscarriage that may resolve spontaneously. However, if the β-hCG level does not decline by 21% to 35% in 2 days, suspect ectopic pregnancy.21
Arrange for transvaginal ultrasound
TVUS is the imaging modality of choice for diagnosis of ectopic pregnancy, with a sensitivity of 87.0% to 99.0% and specificity of 94.0% to 99.9%.22 Arrange for TVUS when a women has abdominal pain or vaginal bleeding and a positive urine pregnancy test, even if the β-hCG level is lower than the discriminatory cutoff of 1500 to 2500 mIU/mL.13,18-20 Ordering TVUS and β-hCG level at the same time yields the best outcome for diagnosis,19 while varying the discriminatory zone alone has not improved diagnosis.18,23
Other novel markers
The use of serum progesterone and other novel markers such as inhibin A, activin A, creatinine kinase, vascular endothelial growth factor, and cancer antigen 125 in the diagnosis of ectopic pregnancy has been studied extensively. To date, no single marker has demonstrated high sensitivity and specificity in differentiating ectopic pregnancy.24 However, when the initial progesterone level is ≤10 nmol/L (equivalent to 31.4 ng/mL) in a woman with a PUL, the probability that she will require any intervention is reported to be low (4 cases out of 227 PUL cases).25 Multiplex tools to combine multiple biomarkers may become available in the future.
Evacuation of uterine contents
When the β-hCG level is above the discriminatory cutoff but no evidence of an extrauterine or intrauterine pregnancy can be found by TVUS, the patient likely has a failing IUP or impending abortion. Some experts suggest considering evaluation of the uterine contents by dilation and curettage (D&C) or manual vacuum extraction at this time, to differentiate an abnormal intrauterine gestation from an ectopic pregnancy. Barnhart found that more than one-third of such cases were due to a failed uterine pregnancy, not ectopic pregnancy.26
If, after a D&C or manual extraction, chorionic villi are not confirmed by pathologic examination of the uterine contents, then treat as an ectopic pregnancy. Some clinicians alternatively recommend checking the β-hCG level again in 12 to 24 hours, expecting ≥15% decline with a spontaneous abortion.27 Alternatively, some recommend using methotrexate (MTX) without D&C to avoid unnecessary medical and surgical treatment.26
CASE 1 Helen’s serum β-hCG level is 4500 mIU/mL, and the TVUS image the next day shows an echogenic mass next to the right ovary—highly suspicious for ectopic pregnancy.
CASE 2 Mary’s TVUS does not show any evidence of IUP or any abnormality in either adnexa. Her serum β-hCG level is 650 mIU/mL. She has a PUL. Her FP informs her that she may have an early normal pregnancy, a failed IUP, or an ectopic pregnancy. She agrees to have her serum β-hCG measured every 2 days. Her β-hCG level increases to 1100, 2000, and 3500 mIU/mL, in 2, 4, and 6 days, respectively. TVUS on the sixth day is still nondiagnostic.
Treatment of ectopic pregnancy: Surgical vs medical
For hemodynamically unstable patients, laparotomy is still the mainstay of therapy. However, with early diagnosis and a stable patient, options are minimally invasive surgical intervention via laparostomy or medical management with MTX in a single or multidose regimen. Surgical and medical treatments have comparable outcomes, as documented by a Cochrane review.28
The risk of recurrent ectopic pregnancy after MTX treatment and salpingostomy is similar—about 10%.29 Ipsilateral tubal patency as documented by hysterosalpingography after MTX treatment or salpingostomy was reported to be equal.28 Reproductive outcomes after either treatment were similar, as well.30
We recommend urgent referral for OB/GYN consultation if the diagnosis of ectopic pregnancy is made by TVUS, since the recommended treatment is laparoscopic salpingostomy. In the case of a PUL, we recommend referral to an OB/GYN when the serum β-hCG level is above the discriminatory cutoff of 1500 to 2500 mIU/mL without signs of IUP as seen by a gestational sac via TVUS. When an urgent referral is not possible, initiate medical treatment. Regardless of the treatment method, give anti-D immunoglobulin to any woman whose blood is Rh negative (no D-antigen) and who has not been sensitized to D-antigen.
Surgical management
Laparoscopic salpingostomy is the preferred surgical treatment for ectopic pregnancy. A Cochrane review meta-analysis of 35 randomized controlled trials (RCTs) on intervention of ectopic pregnancy concluded that, compared with laparotomy, laparoscopy results in shorter operative time, less blood loss, less analgesia, shorter hospital stays, and greater cost effectiveness.28 Another meta-analysis of 15 RCTs concluded that laparoscopic salpingostomy is the most cost-effective treatment for ectopic pregnancy. 31
Medical management with methotrexate
This folic acid antagonist is highly effective in treating ectopic pregnancy, and is usually given intramuscularly for this indication. Clinicians who use this chemotherapeutic agent must be familiar with its dosing regimen, contraindications, and possible adverse effects. Multidose MTX is more effective than surgery, but more expensive.32 Single-dose MTX has a higher failure rate than laparoscopic salpingostomy, especially in patients with higher β-hCG levels.32
The best candidate for medical therapy is the woman who is asymptomatic, motivated, and compliant. Absolute contraindications to single-dose MTX include the following:
- breastfeeding
- overt or lab evidence of immunodeficiency
- alcoholism, alcoholic liver disease, or other chronic liver disease
- preexisting blood dyscrasias, such as bone marrow hypoplasia, leucopenia, thrombocytopenia, or significant anemia
- known sensitivity for methotrexate
- acute pulmonary disease
- peptic ulcer disease
- hepatic, renal, or hematologic dysfunction, and several metabolic diseases.33
Dosing regimen. The 3 general dosing schemes of single dose, 2-dose, and multidose (up to 4 doses) are shown in the TABLE. These were recommended by the American College of Obstetrician and Gynecologists (ACOG).33
Single dose vs multidose. The single-dose treatment is easier to administer and monitor and is most cost effective, but it may have a higher failure rate than the multidose regimens.28 The best prognostic indicator of successful treatment with single-dose MTX is the initial β-hCG level. The lower the initial level, the higher the success rate. The reported failure rate is 1.5% if the initial β-hCG level is <1000 mIU/mL; 5.6% with 1000 to 2000 mIU/mL; 3.8% with 2000 to 5000 mIU/mL; and 14.3% with 5000 to 10,000 mIU/mL.34 ACOG has outlined relative contraindications to single-dose MTX: ectopic pregnancy larger than 3.5 cm and the presence of fetal cardiac activity. Both correlate with an increased failure rate. Patients with PUL and low β-hCG levels are good candidates for single-dose MTX treatment.
Monitoring efficacy of treatment
Serum β-hCG levels indicate response to medical and surgical therapy. After salpingostomy, the serum β-hCG level declines rapidly within the first 4 days, and then more gradually, with mean resolution occurring at about 20 days. In contrast, after single-dose MTX, the mean serum β-hCG level increases for the first 4 days and then gradually declines, with a mean resolution at 27 days.35 The guideline for surveillance is shown in the TABLE.
CASE 1 The FP counsels Helen on the risks and benefits of surgery and MTX treatment for her ectopic pregnancy, and she elects to have a laparoscopic salpingostomy. The FP refers Helen to an OB/GYN via the emergency department on the same day. Helen does well. After the surgery, her β-hCG is monitored every 2 days until it decreases to 1000 mIU/mL, then every week until it is negative.
CASE 2 The FP advises Mary that an OB/GYN would likely recommend a D&C for her PUL, as her β-hCG level is above the discriminatory cutoff and the TVUS does not show a viable IUP. After discussing MTX treatment and manual vacuum aspiration of the uterine contents, Mary elects to have the MTX treatment and receives the 2-dose protocol. Her β-hCG level is 4210 mIU/mL on Day 1—higher than her level prior to the methotrexate treatment, but expected. Levels drop to 3635, 3102, and 2214 mIU/mL on Days 4, 7, and 10, respectively. Mary receives weekly surveillance until her level decreases to 0, which it did in a month.
TABLE
Monitoring methotrexate therapy for ectopic pregnancy
| Regimen | Surveillance |
|---|---|
| Single dose* Methotrexate, 50 mg/m2 IM | Measure β-hCG level on Days 4 and 7: If difference ≥15%, repeat weekly until undetectable
|
| 2 dose Methotrexate, 50 mg/m2 IM, Days 0, 4 | Follow up as for single-dose regimen |
| Multidose (up to 4 doses) Methotrexate, 1 mg/kg IM, Days 1, 3, 5, 7 Leucovorin, 0.1 mg/kg IM, Days 2, 4, 6, 8 | Measure β-hCG level on Days 1, 3, 5, and 7
|
| β-hCG, beta-human chorionic gonadotropin; IM, intramuscularly. *Preferred treatment if low initial β-hCG level. Adapted from: Seeber BE, et al. Obstet Gynecol. 2006.27 | |
CORRESPONDENCE Sahoko H. Little, MD, Room 2300, Lobby H, 24 Frank Lloyd Wright Drive, Ann Arbor, MI 48105-9755; [email protected]
1. Aboud E, Chaliha C. Nine year survey of 138 ectopic pregnancies. Arch Gynecol Obstet. 1998;261:83-87.
2. Dart GD, Kaplan B, Varaklis K. Predictive value of history and physical examination in patients with suspected ectopic pregnancy. Ann Emerg Med. 1999;33:283-290.
3. Birkhahn RH, Gaeta TJ, Van Deusen SK, et al. The ability of traditional vital signs and shock index to identify ruptured ectopic pregnancy. Am J Obstet Gynecol. 2003;189:1293-1296.
4. Cunningham FG, Leveno KJ, Bloom SL, et al. Ectopic pregnancy. In: Cunningham FG, Leveno KJ, Bloom SL, et al, eds. Williams Obstetrics. 23rd ed. New York, NY: McGraw-Hill; 2010:238–256.
5. Bakken IJ, Skjeldestad FE, Lydersen S, et al. Births and ectopic pregnancies in a large cohort of women tested for Chlamydia trachomatis. Sex Transm Dis. 2007;34:739-743.
6. Bakken IJ, Skjeldestad FE, Nordbo SA. Chlamydia trachomatis infections increase the risk for ectopic pregnancy: a population-based, nested case–control study. Sex Transm Dis. 2007;34:166-169.
7. Barnhart KT, Sammel MD, Gracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril. 2006;86:36-43.
8. Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185-194.
9. Gala RB. Ectopic pregnancy. In: Schorge JO, Schaffer JI, Halvorson LM, et al, eds. Williams Gynecology. New York, NY: McGraw-Hill; 2008:160-175.
10. Karaer A, Avsar FA, Batioglu S. Risk factors for ectopic pregnancy: a case-control study. Aust N Z J Obstet Gynaecol. 2006;46:521-527.
11. Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653.
12. Dart RG, Kaplan B, Cox C. Transvaginal ultrasound in patients with low beta-human chorionic gonadotropin values: how often is the study diagnostic? Ann Emerg Med. 1997;30:135-140.
13. Kaplan BC, Dart RG, Moskos M, et al. Ectopic pregnancy: prospective study with improved diagnostic accuracy. Ann Emerg Med. 1996;28:10-17.
14. Clinical policy: critical issues for the initial evaluation and management of patients presenting with a chief complaint of nontraumatic acute abdominal pain Ann Emerg Med. 2000;36:406-415.
15. Barnhart K, van Mello NM, Bourne T, et al. Pregnancy of unknown location: a consensus statement of nomenclature, definitions, and outcome. Fertil Steril. 2011;95:857-866.
16. Barnhart K, Mennuti MT, Benjamin I, et al. Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol. 1994;84:1010-1015.
17. Aleem FA, DeFazio M, Gintautas J. Endovaginal sonography for the early diagnosis of intrauterine and ectopic pregnancies. Hum Reprod. 1990;5:755-758.
18. Coundous G, Kirk E, Lu C, et al. Diagnostic accuracy of varying discriminatory zones for the prediction of ectopic pregnancy in women with a pregnancy of unknown location. Ultrasound Obstet Gynecol. 2005;26:770-775.
19. Gracia CR, Kurt TB. Diagnosing ectopic pregnancy: decision analysis comparing six strategies. Obstet Gynecol. 2001;97:464-470.
20. Kohn MA, Kerr K, Malkevich D, et al. Beta-human chorionic gonadotropin levels and the likelihood of ectopic pregnancy in emergency department patients with abdominal pain or vaginal bleeding. Acad Emerg Med. 2003;10:119-126.
21. Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104:975-981.
22. Kirk E, Bourne T. Diagnosis of ectopic pregnancy with ultrasound. Best Pract Res Clin Obstet Gynaecol. 2009;23:501-508.
23. van Mello NM, Mol F, Opmeer BC, et al. Diagnostic value of serum hCG on the outcome of pregnancy of unknown location: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:603-617.
24. Segal S, Mercado R, Rivnay B. Ectopic pregnancy early diagnosis markers. Minerva Ginecol. 2010;62:49-62.
25. Cordina M, Schramm-Gajraj K, Ross JA, et al. Introduction of a single visit protocol in the management of selected patients with pregnancy of unknown location: a prospective study. BJOG. 2011;118:693-697.
26. Barnhart KT, Katz I, Hummel A. Presumed diagnosis of ectopic pregnancy. Obstet Gynecol. 2002;100:505-510.
27. Seeber BE, Barnhart KT. Suspected ectopic pregnancy. Obstet Gynecol. 2006;107:399-413.
28. Hajenius PJ, Mol F, Mol BW, et al. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2007;(1):CD000324.-
29. Stovall TG. Medical management should be routinely used as primary therapy for ectopic pregnancy. Clin Obstet Gynecol. 1995;38:346-352.
30. Fernandez H, Yves Vincent SC, Pauthier S, et al. Randomized trial of conservative laparoscopic treatment and methotrexate administration in ectopic pregnancy and subsequent fertility. Hum Reprod. 1998;13:3239-3243.
31. Mol F, Strandell A, Jurkovic D, et al. The ESEP study: salpingostomy versus salpingectomy for tubal ectopic pregnancy; the impact on future fertility: a randomised controlled trial. BMC Womens Health. 2008;8:11.-
32. Mol F, Mol BW, Ankum WM, et al. Current evidence on surgery, systemic methotrexate and expectant management in the treatment of tubal ectopic pregnancy: a systematic review and meta-analysis. Hum Reprod Update. 2008;14:309-319.
33. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 94. Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111:1479-1485.
34. Menon S, Colins J, Barnhart KT. Establishing a human chorionic gonadotropin cutoff to guide methotrexate treatment of ectopic pregnancy: a systematic review. Fertil Steril. 2007;87:481-484.
35. Saraj AJ, Wilcox JG, Najmabadi S, et al. Resolution of hormonal markers of ectopic gestation: a randomized trial comparing single-dose intramuscular methotrexate with salpingostomy. Obstet Gynecol. 1998;92:989-994.
1. Aboud E, Chaliha C. Nine year survey of 138 ectopic pregnancies. Arch Gynecol Obstet. 1998;261:83-87.
2. Dart GD, Kaplan B, Varaklis K. Predictive value of history and physical examination in patients with suspected ectopic pregnancy. Ann Emerg Med. 1999;33:283-290.
3. Birkhahn RH, Gaeta TJ, Van Deusen SK, et al. The ability of traditional vital signs and shock index to identify ruptured ectopic pregnancy. Am J Obstet Gynecol. 2003;189:1293-1296.
4. Cunningham FG, Leveno KJ, Bloom SL, et al. Ectopic pregnancy. In: Cunningham FG, Leveno KJ, Bloom SL, et al, eds. Williams Obstetrics. 23rd ed. New York, NY: McGraw-Hill; 2010:238–256.
5. Bakken IJ, Skjeldestad FE, Lydersen S, et al. Births and ectopic pregnancies in a large cohort of women tested for Chlamydia trachomatis. Sex Transm Dis. 2007;34:739-743.
6. Bakken IJ, Skjeldestad FE, Nordbo SA. Chlamydia trachomatis infections increase the risk for ectopic pregnancy: a population-based, nested case–control study. Sex Transm Dis. 2007;34:166-169.
7. Barnhart KT, Sammel MD, Gracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril. 2006;86:36-43.
8. Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157:185-194.
9. Gala RB. Ectopic pregnancy. In: Schorge JO, Schaffer JI, Halvorson LM, et al, eds. Williams Gynecology. New York, NY: McGraw-Hill; 2008:160-175.
10. Karaer A, Avsar FA, Batioglu S. Risk factors for ectopic pregnancy: a case-control study. Aust N Z J Obstet Gynaecol. 2006;46:521-527.
11. Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653.
12. Dart RG, Kaplan B, Cox C. Transvaginal ultrasound in patients with low beta-human chorionic gonadotropin values: how often is the study diagnostic? Ann Emerg Med. 1997;30:135-140.
13. Kaplan BC, Dart RG, Moskos M, et al. Ectopic pregnancy: prospective study with improved diagnostic accuracy. Ann Emerg Med. 1996;28:10-17.
14. Clinical policy: critical issues for the initial evaluation and management of patients presenting with a chief complaint of nontraumatic acute abdominal pain Ann Emerg Med. 2000;36:406-415.
15. Barnhart K, van Mello NM, Bourne T, et al. Pregnancy of unknown location: a consensus statement of nomenclature, definitions, and outcome. Fertil Steril. 2011;95:857-866.
16. Barnhart K, Mennuti MT, Benjamin I, et al. Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol. 1994;84:1010-1015.
17. Aleem FA, DeFazio M, Gintautas J. Endovaginal sonography for the early diagnosis of intrauterine and ectopic pregnancies. Hum Reprod. 1990;5:755-758.
18. Coundous G, Kirk E, Lu C, et al. Diagnostic accuracy of varying discriminatory zones for the prediction of ectopic pregnancy in women with a pregnancy of unknown location. Ultrasound Obstet Gynecol. 2005;26:770-775.
19. Gracia CR, Kurt TB. Diagnosing ectopic pregnancy: decision analysis comparing six strategies. Obstet Gynecol. 2001;97:464-470.
20. Kohn MA, Kerr K, Malkevich D, et al. Beta-human chorionic gonadotropin levels and the likelihood of ectopic pregnancy in emergency department patients with abdominal pain or vaginal bleeding. Acad Emerg Med. 2003;10:119-126.
21. Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104:975-981.
22. Kirk E, Bourne T. Diagnosis of ectopic pregnancy with ultrasound. Best Pract Res Clin Obstet Gynaecol. 2009;23:501-508.
23. van Mello NM, Mol F, Opmeer BC, et al. Diagnostic value of serum hCG on the outcome of pregnancy of unknown location: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:603-617.
24. Segal S, Mercado R, Rivnay B. Ectopic pregnancy early diagnosis markers. Minerva Ginecol. 2010;62:49-62.
25. Cordina M, Schramm-Gajraj K, Ross JA, et al. Introduction of a single visit protocol in the management of selected patients with pregnancy of unknown location: a prospective study. BJOG. 2011;118:693-697.
26. Barnhart KT, Katz I, Hummel A. Presumed diagnosis of ectopic pregnancy. Obstet Gynecol. 2002;100:505-510.
27. Seeber BE, Barnhart KT. Suspected ectopic pregnancy. Obstet Gynecol. 2006;107:399-413.
28. Hajenius PJ, Mol F, Mol BW, et al. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2007;(1):CD000324.-
29. Stovall TG. Medical management should be routinely used as primary therapy for ectopic pregnancy. Clin Obstet Gynecol. 1995;38:346-352.
30. Fernandez H, Yves Vincent SC, Pauthier S, et al. Randomized trial of conservative laparoscopic treatment and methotrexate administration in ectopic pregnancy and subsequent fertility. Hum Reprod. 1998;13:3239-3243.
31. Mol F, Strandell A, Jurkovic D, et al. The ESEP study: salpingostomy versus salpingectomy for tubal ectopic pregnancy; the impact on future fertility: a randomised controlled trial. BMC Womens Health. 2008;8:11.-
32. Mol F, Mol BW, Ankum WM, et al. Current evidence on surgery, systemic methotrexate and expectant management in the treatment of tubal ectopic pregnancy: a systematic review and meta-analysis. Hum Reprod Update. 2008;14:309-319.
33. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 94. Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111:1479-1485.
34. Menon S, Colins J, Barnhart KT. Establishing a human chorionic gonadotropin cutoff to guide methotrexate treatment of ectopic pregnancy: a systematic review. Fertil Steril. 2007;87:481-484.
35. Saraj AJ, Wilcox JG, Najmabadi S, et al. Resolution of hormonal markers of ectopic gestation: a randomized trial comparing single-dose intramuscular methotrexate with salpingostomy. Obstet Gynecol. 1998;92:989-994.