User login
Cracking the Case
A 43‐year‐old woman presented to an outside hospital with painful plaques and patches on her bilateral lower extremities. Two weeks prior to presentation, she had noticed a single red lesion on her left ankle. Over the next two weeks, the lesion enlarged to involve the lower half of her posterior calf and subsequently turned purple and became exquisitely tender. Similar but smaller purple, tender lesions simultaneously appeared, first over her right shin and then on her bilateral thighs and hips. She also reported fatigue as well as diffuse joint pains in her hands and wrists bilaterally for the past month. She denied any swelling of these joints or functional impairment. She denied fevers, weight loss, headache, sinus symptoms, difficulty breathing, or abdominal pain.
Although we do not yet have a physical exam, the tempo, pattern of spread, and accompanying features allow some early hypotheses to be considered. Distal lower extremity lesions which darkened and spread could be erythema nodosum or erythema induratum. Malignancies rarely have such prominent skin manifestations, although leukemia cutis or an aggressive cutaneous T cell lymphoma might present with disseminated and darkened plaques, and Kaposi's sarcoma is characteristically purple and multifocal. Autoimmune disorders such as sarcoidosis, cutaneous lupus, and psoriasis may similarly present with widespread plaques. Most disseminated infections that start with patches evolve to pustules, ulcers, bullae, or other forms that reflect the invasive nature of the infection; syphilis warrants consideration for any widespread eruption of unknown etiology. Antecedent arthralgias with fatigue suggest an autoimmune condition, although infections such as hepatitis or parvovirus can do the same. Systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA) would be favored initially on account of her demographics and the hand and wrist involvement, and each can be associated with vasculitis.
The significant pain as described is not compatible with most of the aforementioned diagnoses. Its presence, coupled with potential autoimmune symptoms, suggests a vasculitis such as polyarteritis nodosa (which can have prominent diffuse skin involvement), Henoch Schonlein purpura (with its predilection for the lower extremities, including extension to the hips and buttocks), cryoglobulinemia, or SLE‐ or RA‐associated vasculitis. Calciphylaxis is another ischemic vascular disorder that can cause diffuse dark painful lesions, but this only warrants consideration if advanced renal disease is present.
A skin biopsy ofher right hip was taken at the outside hospital. She was discharged on a two‐week course of prednisone for suspected vasculitis while biopsy results were pending. Over the next two weeks, none of the skin lesions improved, despite compliance with this treatment, and the skin over her left posterior calf and right shin lesions began to erode and bleed. In addition, small purple, tender lesions appeared over the pinnae of both ears. Three weeks after her initial evaluation, she presented to another emergency department for ulcerating skin lesions and worsening pain. At that point, the initial skin biopsy result was available and revealed vasculopathy of the small vessels with thrombi but no vasculitis.
The patient had no children,and denied a history of miscarriages. Her past medical history was unremarkable. She did not report any history of thrombotic events. She started a new job as a software engineer one month ago and was feeling stressed about her new responsibilities. She denied any high‐risk sexual behavior and any history of intravenous drug use. She had not traveled recently and did not own any pets. There was no family history of rheumatologic disorders, hypercoagulable states, or thrombotic events.
This picture of occluded but noninflamed vessels shifts the diagnosis away from vasculitis and focuses attention on hypercoagulable states with prominent dermal manifestations, including antiphospholipid antibody syndrome (APLS) and livedoid vasculopathy. In this young woman with arthralgias, consideration of SLE and APLS is warranted. Her recent increase in stress and widespread purpuric and ulcerative lesions could bring to mind a factitious disorder, but the histology results eliminate this possibility.
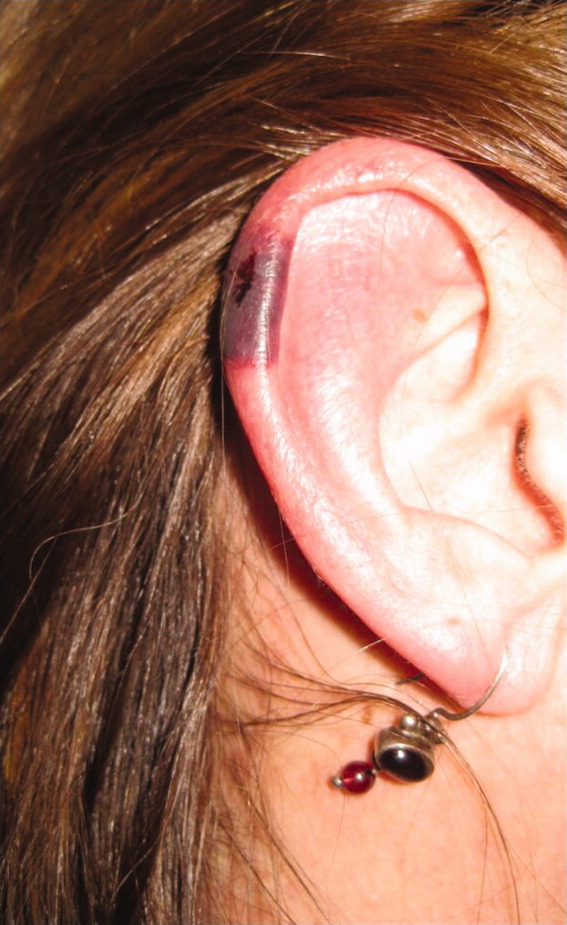
The patient's temperaturewas 36.5C, her blood pressure was 110/70 mmHg, respiratory rate was 16 breaths per minute, and her heart rate was 65 beats per minute. She was well‐appearing but in moderate pain. She did not have any oral lesions. Her cardiac, respiratory, and abdominal exams were normal. Skin exam revealed a 10‐cm by 4‐cm area of bloody granulation tissue draining serosanguinous fluid, surrounded by stellate palpable netlike purpura on her left posterior calf. There was a similar 4‐cm by 2‐cm ulcerated lesion on her right shin. Both lesions were exquisitely tender to palpation. On her bilateral thighs and hips, there were multiple stellate purpuric patches, all 4 cm in diameter or less, and only minimally tender to palpation. She also had 1‐cm purpuric bullae on the helices of both ears (Figure 1) which were slightly tender to palpation. Splinter hemorrhages were also noted on multiple nail beds bilaterally. Musculoskeletal exam did not reveal any synovitis.
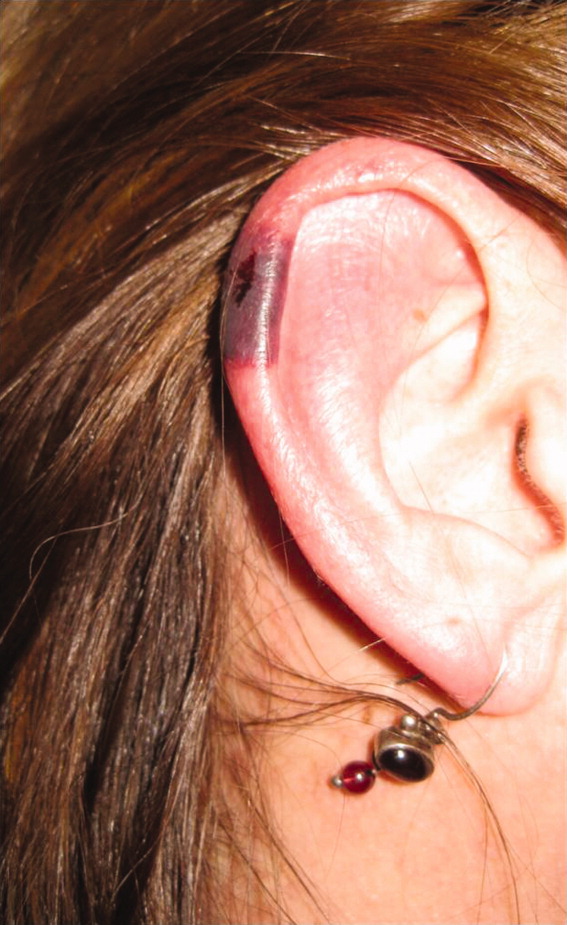
The original purpura on her calf and ear demonstrate a clear demarcation corresponding to cutaneous vascular insufficiency. The development of bullae (ear) and ulceration (calf) are compatible with ischemia. Despite the presence of multiple splinter hemorrhages, the distribution of lesions is very unusual for an embolic phenomenon (eg, endocarditis, cholesterol emboli, or atrial myxoma). The multifocal nature of the skin lesions with progression to well‐demarcated cutaneous necrosis is reminiscent of calciphylaxis or warfarin‐induced skin necrosis, although she lacks the relevant risk factors. A toxin such as cocaine or methamphetamine mediating multifocal vasoconstriction or hypercoagulability should be excluded.
The bilateral ear involvement remains decidedly unusual and makes me wonder if there is something about the ear, such as the nature of its circulation or its potentially lower temperature (as an acral organ) that might render it particularly susceptible, for instance, to cryoglobulinemia or cryofibrinogenemia‐mediated ischemia.
Laboratory studiesdemonstrated: white blood cell count of 1500/mm3 (37.3% neutrophils, 5.1% lymphocytes, 6.7% monocytes, and 1.3% eosinophils); hemoglobin 9.3 g/dl (mean corpuscular volume 91 fL); platelet count 212/mm3; erythrocyte sedimentation rate 62 mm/hr; C‐reactive protein 14.6 mg/L. Serum electrolytes, liver tests, coagulation studies, and urinalysis were normal. Fecal occult blood test was negative.
Her neutropenia and anemia suggest decreased production in the marrow by infection, malignancy, or toxin, or increased destruction, perhaps from an autoimmune process. The associated infections are usually viral, such as human immunodeficiency virus (HIV) and Epstein‐Barr virus (EBV), although their linkage with her cutaneous disease is tenuous. It is possible that malignancy could be present in the marrow with resultant dermal hypercoagulability and ischemia, but this seems unlikely. We do not know about any toxins that she has been exposed to, but these hematologic findings would mandate directed inquiry along those lines. In this young woman with cutaneous ulcers secondary to thrombotic vasculopathy, bicytopenia, antecedent arthralgias without synovitis, and elevated inflammatory markers, I favor an autoimmune process such as SLE, which I would evaluate with an antinuclear antibody (ANA) and antiphospholipid antibody studies.
She was admittedto the hospital and received hydromorphone for pain control. Corticosteroids were not administered. Peripheral blood morphology was normal. Antibodies against HIV1 and 2 were negative, as were antibodies against cytomegalovirus, EBV, parvovirus B19, mycoplasma pneumoniae, and hepatitis C virus. Bilateral lower extremity ultrasound was negative for deep vein thrombosis. Transthoracic echocardiogram was normal. Repeat skin biopsy confirmed small vessel vasculopathy without vasculitis (Figure 2). The results of the following investigations were also negative: ANA, rheumatoid factor, double‐stranded DNA (dsDNA), cyclic citrullinated peptide, ribonucleoprotein (RNP), and anti‐Smith antibodies. C3 and C4 complement levels were normal.
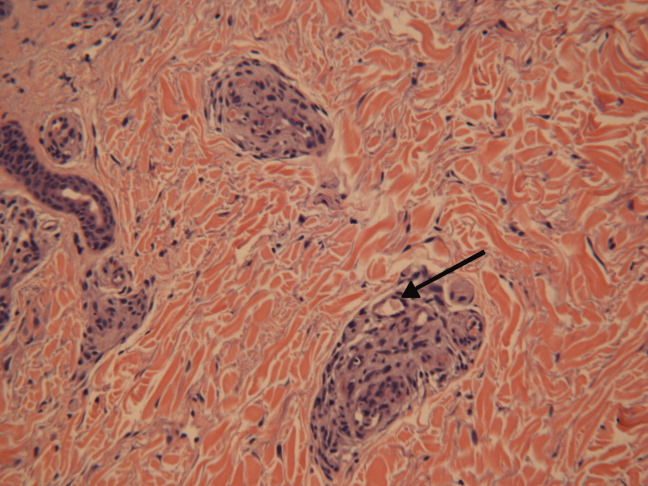
Given how much the histology is driving the clinical reasoning and focusing the differential diagnosis in this case, I agree with the decision to repeat the biopsy. In complex or undiagnosed cases, repeat histology samples allow for confirmation of the original interpretation (often with the perspective of new clinicians and pathologists) and sometimes reveal pathognomonic or additional findings that only appear after the disease has evolved over time. HIV seronegativity helps constrain the differential diagnosis, and parvovirus is another excellent consideration for arthralgias and cytopenias (with the predilection to involve cells lines other than RBCs particularly seen in HIV), although ulcers are not seen with this condition. Herpes simplex virus (HSV) is another viral infection that can cause painful skin ulcerations and cytopenias, although the duration and distribution are highly atypical. The negative ANA and dsDNA and normal complement levels make SLE unlikely. The negative lower extremity ultrasound helps frame the thromboses as a local cutaneous process rather than a systemic hypercoagulable state. Although the peripheral blood smear is normal, a bone marrow biopsy will be necessary to exclude a marrow invasive process, such as leukemia or lymphoma. A bone marrow biopsy would also provide another opportunity to examine tissue for mycobacteria or fungi which can cause ulcerations and cytopenias, although there is little reason to suspect she is susceptible to those pathogens. As this clinical picture fails to fit clearly with an infectious, autoimmune, or neoplastic disorder, I would revisit the possibility of toxinsprescription, complementary, over‐the‐counter, or illegal (eg, cocaine) at this time.
In further discussionwith the patient, she reported using cocaine intranasally for the past three months. Her urine toxicology was positive for cocaine. She was found to have positive perinuclear antineutrophil cytoplasmic antibodies (p‐ANCA), antimyeloperoxidase (MPO) antibodies, anticardiolipin (ACL) antibodies, and lupus anticoagulant (LAC). By hospital day 3, her lesions had significantly improved without any intervention, and her absolute neutrophil count increased to 1080/mm3.
The presence of widespread cutaneous ischemia (with bland thrombosis) and detectable ACL and LAC antibodies is compatible with APLS; the APLS could be deemed primary, because there is no clear associated rheumatologic or other systemic disease. However, neutropenia is not a characteristic of APLS, which has thrombocytopenia as its more frequently associated hematologic abnormality. Livedoid vasculopathy, a related disorder, is also supported by the ACL and LAC results, but also does not feature neutropenia. While the presence of diffuse thrombosis could be attributed to a widespread secondary effect of cocaine vasoconstriction, the appearance of ANCA (which can be drug‐induced, eg, propylthiouracil [PTU]) and the slowly resolving neutropenia during hospitalization without specific treatment is very suggestive of a toxin. The demographic, diffuse skin ulcers, and hematologic and serologic profile is compatible with the recently described toxidrome related to levamisole adulteration of cocaine.
A send‐out studyof a urine sample returned positive for levamisole. Based on purpuric skin lesions with a predilection for the ears, agranulocytosis, and skin biopsy revealing thrombotic vasculopathy, she was diagnosed with levamisole‐adulterated cocaine exposure. One week after discharge, her lower extremity pain and ulcerations were significantly improved. Her absolute neutrophil count increased to 2820/mm.3 Her urine toxicology screen was negative for cocaine.
DISCUSSION
Levamisole was initially developed in 1964 as an antihelminthic agent. Its incidentally discovered immunomodulatory effects led to trials for the treatment of chronic infections, inflammatory bowel disease, rheumatic diseases,1 and nephrotic syndrome in children.2 By 1990, 3 major studies supported levamisole as an adjunctive therapy in melanoma3 and colon cancer.4
Although levamisole appeared to be nontoxic at single or low doses, long‐term use in clinical trials demonstrated that 2.5%‐13% of patients developed life‐threatening agranulocytosis, and up to 10% of those instances resulted in death.5 A distinctive cutaneous pseudovasculitis was noted in children on therapeutic levamisole. They presented with purpura that had a predilection for the ears, cheeks, and thighs,6 and positive serologic markers for ANCA and antiphospholipid antibodies. Skin biopsies of the purpuric lesions revealed leukocytoclastic vasculitis, thrombotic vasculitis, and/or vascular occlusions.
Levamisole was withdrawn from the market in 2000 in the United States due to its side effects,7 but quickly found its way onto the black market. It was first detected in cocaine in 2002, and the percentage of cocaine containing levamisole has steadily been increasing since then. In July 2009, over 70% of cocaine seized by the Drug Enforcement Administration was found to contain levamisole.8 It is unclear exactly why this drug is used as an adulterant in cocaine. Theories include potentiation of the euphoric effects of cocaine, serving as a bulking agent, or functioning as a chemical signature to track distribution.9
The resurgence of levamisole has brought a new face to a problem seen over a decade ago. Current reports of levamisole toxicity describe adults presenting with purpura preferentially involving the ears, neutropenia, positive ANCA, and positive antiphospholipid antibodies.1012 Since 2002, there have been at least 20 confirmed cases of agranulocytosis and two deaths associated with levamisole‐adulterated cocaine.8, 13, 14 In September 2009, the Department of Health and Human Services issued a public health alert warning of an impending increase in levamisole‐related illness.
Levamisole is not detected on routine toxicology screens, but can be tested for using gas chromatography and mass spectrometry. Most laboratories do not offer testing for levamisole and send‐out testing is required. Given its half‐life of 5.6 hours, levamisole can only be detected in the blood within 24 hours, and in the urine within 48‐72 hours of exposure.15, 16 Urine samples are preferred over blood samples, since blood levels decline more rapidly and have lower sensitivity. Cocaine can also be sent out to local or state forensics laboratories to be tested for levamisole. The only definitive treatment for levamisole‐induced cutaneous pseudovasculitis and neutropenia is cessation of toxin exposure.
Although the discussant had familiarity with this toxidrome from local and published cases, he was only able to settle on levamisole toxicity after a series of competing hypotheses were ruled out on the basis of irreconcilable features (vasculitis and histology results; APLS and neutropenia; SLE and negative ANA with no visceral involvement) and by using analogical reasoning (eg, to infer the presence of a toxin on the basis of neutropenia [as seen with chemotherapy and other drugs] and ANCA induction [as seen with PTU]). It was a laborious process of hypothesis testing, but one that ultimately allowed him to crack the case.
Key Teaching Points
-
In patients presenting with neutropenia and purpuric skin lesionsparticularly with a predilection for the earsconsider levamisole‐adulterated cocaine exposure.
-
Tests supporting this diagnosis include positive serologies for ANCA and antiphospholipid antibodies, and skin biopsies that show leukocytoclastic vasculitis, thrombotic vasculitis, or vascular occlusion. Urine studies for levamisole are definitive if sent within 48 to 72 hours of exposure.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
- ,.Levamisole, the story and the lessons.Int J Immunopharmocol.1992;14(3):481–486.
- British Association for Paediatric Nephrology.Levamisole for corticosteroid‐dependent nephrotic syndrome in childhood.Lancet.1991;337:1555–1557.
- ,,,,.Improved survival in patients with poor‐prognosis malignant melanoma treated with adjuvant levamisole: a phase III study by the National Cancer Institute of Canada Clinical Trials Group.J Clin Oncol.1991;9:729–735.
- ,,.Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma.N Engl J Med.1990;322:352–358.
- ,,.Studies on levamisole‐induced agranulocytosis.Blood.1980;56(3):388–396.
- ,,.Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long‐term treatment with levamisole in children.Br J Dermatol.1999;140:948–951.
- . Janssen Discontinues Ergamisol. Available at: http://findarticles.com/p/articles/mi_m3374/is_18_22/ai_68536218/. Accessed July 25,2010.
- SAMHSA. Nationwide Public Health Alert Issued Concerning Life‐Threatening Risk Posed by Cocaine Laced with Veterinary Anti‐Parasite Drug. Available at: http://www.samhsa.gov/newsroom/advisories/090921vet5101.aspx. Accessed July 20,2010.
- .Unusual adulterants in cocaine seized on Italian clandestine market.Forensic Sci Int.2007;172(2–3):e1.
- ,,.Levamisole‐induced occlusive necrotizing vasculitis of the ears after use of cocaine contaminated with levamisole.J Med Toxicol.2010;Jun 12.
- ,,,.Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole.Ann Intern Med.2010;1;152(11):758–759.
- ,,,,.Cocaine‐associated retiform purpura and neutropenia: is levamisole the culprit?J Am Acad Dermatol.2010;Mar 19.
- ,,,.A confirmed case of agranulocytosis after use of cocaine contaminated with levamisole.J Med Toxicol.2010;Apr 1.
- Centers for Disease Control and Prevention.Agranulocytosis associated with cocaine use—four States, March 2008–November 2009.MMWR.2009;58(49):1381–1385.
- ,,.Levamisole as a contaminant of illicit cocaine.Journal of the Clandestine Laboratory Investigating Chemists Association.2006;16:6–11. Available at: http://www.tiaft2006.org/proceedings/pdf/PT‐p‐06.pdf. Accessed July 20, 2010.
- . Cocaine Cutting Agents—A Discussion. Laboratory Medicine and Pathology, University of Alberta. Available at: http://www.vandu.org/documents/Levamisole_Cocaine.pdf. Accessed July 20,2010.
A 43‐year‐old woman presented to an outside hospital with painful plaques and patches on her bilateral lower extremities. Two weeks prior to presentation, she had noticed a single red lesion on her left ankle. Over the next two weeks, the lesion enlarged to involve the lower half of her posterior calf and subsequently turned purple and became exquisitely tender. Similar but smaller purple, tender lesions simultaneously appeared, first over her right shin and then on her bilateral thighs and hips. She also reported fatigue as well as diffuse joint pains in her hands and wrists bilaterally for the past month. She denied any swelling of these joints or functional impairment. She denied fevers, weight loss, headache, sinus symptoms, difficulty breathing, or abdominal pain.
Although we do not yet have a physical exam, the tempo, pattern of spread, and accompanying features allow some early hypotheses to be considered. Distal lower extremity lesions which darkened and spread could be erythema nodosum or erythema induratum. Malignancies rarely have such prominent skin manifestations, although leukemia cutis or an aggressive cutaneous T cell lymphoma might present with disseminated and darkened plaques, and Kaposi's sarcoma is characteristically purple and multifocal. Autoimmune disorders such as sarcoidosis, cutaneous lupus, and psoriasis may similarly present with widespread plaques. Most disseminated infections that start with patches evolve to pustules, ulcers, bullae, or other forms that reflect the invasive nature of the infection; syphilis warrants consideration for any widespread eruption of unknown etiology. Antecedent arthralgias with fatigue suggest an autoimmune condition, although infections such as hepatitis or parvovirus can do the same. Systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA) would be favored initially on account of her demographics and the hand and wrist involvement, and each can be associated with vasculitis.
The significant pain as described is not compatible with most of the aforementioned diagnoses. Its presence, coupled with potential autoimmune symptoms, suggests a vasculitis such as polyarteritis nodosa (which can have prominent diffuse skin involvement), Henoch Schonlein purpura (with its predilection for the lower extremities, including extension to the hips and buttocks), cryoglobulinemia, or SLE‐ or RA‐associated vasculitis. Calciphylaxis is another ischemic vascular disorder that can cause diffuse dark painful lesions, but this only warrants consideration if advanced renal disease is present.
A skin biopsy ofher right hip was taken at the outside hospital. She was discharged on a two‐week course of prednisone for suspected vasculitis while biopsy results were pending. Over the next two weeks, none of the skin lesions improved, despite compliance with this treatment, and the skin over her left posterior calf and right shin lesions began to erode and bleed. In addition, small purple, tender lesions appeared over the pinnae of both ears. Three weeks after her initial evaluation, she presented to another emergency department for ulcerating skin lesions and worsening pain. At that point, the initial skin biopsy result was available and revealed vasculopathy of the small vessels with thrombi but no vasculitis.
The patient had no children,and denied a history of miscarriages. Her past medical history was unremarkable. She did not report any history of thrombotic events. She started a new job as a software engineer one month ago and was feeling stressed about her new responsibilities. She denied any high‐risk sexual behavior and any history of intravenous drug use. She had not traveled recently and did not own any pets. There was no family history of rheumatologic disorders, hypercoagulable states, or thrombotic events.
This picture of occluded but noninflamed vessels shifts the diagnosis away from vasculitis and focuses attention on hypercoagulable states with prominent dermal manifestations, including antiphospholipid antibody syndrome (APLS) and livedoid vasculopathy. In this young woman with arthralgias, consideration of SLE and APLS is warranted. Her recent increase in stress and widespread purpuric and ulcerative lesions could bring to mind a factitious disorder, but the histology results eliminate this possibility.
The patient's temperaturewas 36.5C, her blood pressure was 110/70 mmHg, respiratory rate was 16 breaths per minute, and her heart rate was 65 beats per minute. She was well‐appearing but in moderate pain. She did not have any oral lesions. Her cardiac, respiratory, and abdominal exams were normal. Skin exam revealed a 10‐cm by 4‐cm area of bloody granulation tissue draining serosanguinous fluid, surrounded by stellate palpable netlike purpura on her left posterior calf. There was a similar 4‐cm by 2‐cm ulcerated lesion on her right shin. Both lesions were exquisitely tender to palpation. On her bilateral thighs and hips, there were multiple stellate purpuric patches, all 4 cm in diameter or less, and only minimally tender to palpation. She also had 1‐cm purpuric bullae on the helices of both ears (Figure 1) which were slightly tender to palpation. Splinter hemorrhages were also noted on multiple nail beds bilaterally. Musculoskeletal exam did not reveal any synovitis.

The original purpura on her calf and ear demonstrate a clear demarcation corresponding to cutaneous vascular insufficiency. The development of bullae (ear) and ulceration (calf) are compatible with ischemia. Despite the presence of multiple splinter hemorrhages, the distribution of lesions is very unusual for an embolic phenomenon (eg, endocarditis, cholesterol emboli, or atrial myxoma). The multifocal nature of the skin lesions with progression to well‐demarcated cutaneous necrosis is reminiscent of calciphylaxis or warfarin‐induced skin necrosis, although she lacks the relevant risk factors. A toxin such as cocaine or methamphetamine mediating multifocal vasoconstriction or hypercoagulability should be excluded.
The bilateral ear involvement remains decidedly unusual and makes me wonder if there is something about the ear, such as the nature of its circulation or its potentially lower temperature (as an acral organ) that might render it particularly susceptible, for instance, to cryoglobulinemia or cryofibrinogenemia‐mediated ischemia.
Laboratory studiesdemonstrated: white blood cell count of 1500/mm3 (37.3% neutrophils, 5.1% lymphocytes, 6.7% monocytes, and 1.3% eosinophils); hemoglobin 9.3 g/dl (mean corpuscular volume 91 fL); platelet count 212/mm3; erythrocyte sedimentation rate 62 mm/hr; C‐reactive protein 14.6 mg/L. Serum electrolytes, liver tests, coagulation studies, and urinalysis were normal. Fecal occult blood test was negative.
Her neutropenia and anemia suggest decreased production in the marrow by infection, malignancy, or toxin, or increased destruction, perhaps from an autoimmune process. The associated infections are usually viral, such as human immunodeficiency virus (HIV) and Epstein‐Barr virus (EBV), although their linkage with her cutaneous disease is tenuous. It is possible that malignancy could be present in the marrow with resultant dermal hypercoagulability and ischemia, but this seems unlikely. We do not know about any toxins that she has been exposed to, but these hematologic findings would mandate directed inquiry along those lines. In this young woman with cutaneous ulcers secondary to thrombotic vasculopathy, bicytopenia, antecedent arthralgias without synovitis, and elevated inflammatory markers, I favor an autoimmune process such as SLE, which I would evaluate with an antinuclear antibody (ANA) and antiphospholipid antibody studies.
She was admittedto the hospital and received hydromorphone for pain control. Corticosteroids were not administered. Peripheral blood morphology was normal. Antibodies against HIV1 and 2 were negative, as were antibodies against cytomegalovirus, EBV, parvovirus B19, mycoplasma pneumoniae, and hepatitis C virus. Bilateral lower extremity ultrasound was negative for deep vein thrombosis. Transthoracic echocardiogram was normal. Repeat skin biopsy confirmed small vessel vasculopathy without vasculitis (Figure 2). The results of the following investigations were also negative: ANA, rheumatoid factor, double‐stranded DNA (dsDNA), cyclic citrullinated peptide, ribonucleoprotein (RNP), and anti‐Smith antibodies. C3 and C4 complement levels were normal.

Given how much the histology is driving the clinical reasoning and focusing the differential diagnosis in this case, I agree with the decision to repeat the biopsy. In complex or undiagnosed cases, repeat histology samples allow for confirmation of the original interpretation (often with the perspective of new clinicians and pathologists) and sometimes reveal pathognomonic or additional findings that only appear after the disease has evolved over time. HIV seronegativity helps constrain the differential diagnosis, and parvovirus is another excellent consideration for arthralgias and cytopenias (with the predilection to involve cells lines other than RBCs particularly seen in HIV), although ulcers are not seen with this condition. Herpes simplex virus (HSV) is another viral infection that can cause painful skin ulcerations and cytopenias, although the duration and distribution are highly atypical. The negative ANA and dsDNA and normal complement levels make SLE unlikely. The negative lower extremity ultrasound helps frame the thromboses as a local cutaneous process rather than a systemic hypercoagulable state. Although the peripheral blood smear is normal, a bone marrow biopsy will be necessary to exclude a marrow invasive process, such as leukemia or lymphoma. A bone marrow biopsy would also provide another opportunity to examine tissue for mycobacteria or fungi which can cause ulcerations and cytopenias, although there is little reason to suspect she is susceptible to those pathogens. As this clinical picture fails to fit clearly with an infectious, autoimmune, or neoplastic disorder, I would revisit the possibility of toxinsprescription, complementary, over‐the‐counter, or illegal (eg, cocaine) at this time.
In further discussionwith the patient, she reported using cocaine intranasally for the past three months. Her urine toxicology was positive for cocaine. She was found to have positive perinuclear antineutrophil cytoplasmic antibodies (p‐ANCA), antimyeloperoxidase (MPO) antibodies, anticardiolipin (ACL) antibodies, and lupus anticoagulant (LAC). By hospital day 3, her lesions had significantly improved without any intervention, and her absolute neutrophil count increased to 1080/mm3.
The presence of widespread cutaneous ischemia (with bland thrombosis) and detectable ACL and LAC antibodies is compatible with APLS; the APLS could be deemed primary, because there is no clear associated rheumatologic or other systemic disease. However, neutropenia is not a characteristic of APLS, which has thrombocytopenia as its more frequently associated hematologic abnormality. Livedoid vasculopathy, a related disorder, is also supported by the ACL and LAC results, but also does not feature neutropenia. While the presence of diffuse thrombosis could be attributed to a widespread secondary effect of cocaine vasoconstriction, the appearance of ANCA (which can be drug‐induced, eg, propylthiouracil [PTU]) and the slowly resolving neutropenia during hospitalization without specific treatment is very suggestive of a toxin. The demographic, diffuse skin ulcers, and hematologic and serologic profile is compatible with the recently described toxidrome related to levamisole adulteration of cocaine.
A send‐out studyof a urine sample returned positive for levamisole. Based on purpuric skin lesions with a predilection for the ears, agranulocytosis, and skin biopsy revealing thrombotic vasculopathy, she was diagnosed with levamisole‐adulterated cocaine exposure. One week after discharge, her lower extremity pain and ulcerations were significantly improved. Her absolute neutrophil count increased to 2820/mm.3 Her urine toxicology screen was negative for cocaine.
DISCUSSION
Levamisole was initially developed in 1964 as an antihelminthic agent. Its incidentally discovered immunomodulatory effects led to trials for the treatment of chronic infections, inflammatory bowel disease, rheumatic diseases,1 and nephrotic syndrome in children.2 By 1990, 3 major studies supported levamisole as an adjunctive therapy in melanoma3 and colon cancer.4
Although levamisole appeared to be nontoxic at single or low doses, long‐term use in clinical trials demonstrated that 2.5%‐13% of patients developed life‐threatening agranulocytosis, and up to 10% of those instances resulted in death.5 A distinctive cutaneous pseudovasculitis was noted in children on therapeutic levamisole. They presented with purpura that had a predilection for the ears, cheeks, and thighs,6 and positive serologic markers for ANCA and antiphospholipid antibodies. Skin biopsies of the purpuric lesions revealed leukocytoclastic vasculitis, thrombotic vasculitis, and/or vascular occlusions.
Levamisole was withdrawn from the market in 2000 in the United States due to its side effects,7 but quickly found its way onto the black market. It was first detected in cocaine in 2002, and the percentage of cocaine containing levamisole has steadily been increasing since then. In July 2009, over 70% of cocaine seized by the Drug Enforcement Administration was found to contain levamisole.8 It is unclear exactly why this drug is used as an adulterant in cocaine. Theories include potentiation of the euphoric effects of cocaine, serving as a bulking agent, or functioning as a chemical signature to track distribution.9
The resurgence of levamisole has brought a new face to a problem seen over a decade ago. Current reports of levamisole toxicity describe adults presenting with purpura preferentially involving the ears, neutropenia, positive ANCA, and positive antiphospholipid antibodies.1012 Since 2002, there have been at least 20 confirmed cases of agranulocytosis and two deaths associated with levamisole‐adulterated cocaine.8, 13, 14 In September 2009, the Department of Health and Human Services issued a public health alert warning of an impending increase in levamisole‐related illness.
Levamisole is not detected on routine toxicology screens, but can be tested for using gas chromatography and mass spectrometry. Most laboratories do not offer testing for levamisole and send‐out testing is required. Given its half‐life of 5.6 hours, levamisole can only be detected in the blood within 24 hours, and in the urine within 48‐72 hours of exposure.15, 16 Urine samples are preferred over blood samples, since blood levels decline more rapidly and have lower sensitivity. Cocaine can also be sent out to local or state forensics laboratories to be tested for levamisole. The only definitive treatment for levamisole‐induced cutaneous pseudovasculitis and neutropenia is cessation of toxin exposure.
Although the discussant had familiarity with this toxidrome from local and published cases, he was only able to settle on levamisole toxicity after a series of competing hypotheses were ruled out on the basis of irreconcilable features (vasculitis and histology results; APLS and neutropenia; SLE and negative ANA with no visceral involvement) and by using analogical reasoning (eg, to infer the presence of a toxin on the basis of neutropenia [as seen with chemotherapy and other drugs] and ANCA induction [as seen with PTU]). It was a laborious process of hypothesis testing, but one that ultimately allowed him to crack the case.
Key Teaching Points
-
In patients presenting with neutropenia and purpuric skin lesionsparticularly with a predilection for the earsconsider levamisole‐adulterated cocaine exposure.
-
Tests supporting this diagnosis include positive serologies for ANCA and antiphospholipid antibodies, and skin biopsies that show leukocytoclastic vasculitis, thrombotic vasculitis, or vascular occlusion. Urine studies for levamisole are definitive if sent within 48 to 72 hours of exposure.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 43‐year‐old woman presented to an outside hospital with painful plaques and patches on her bilateral lower extremities. Two weeks prior to presentation, she had noticed a single red lesion on her left ankle. Over the next two weeks, the lesion enlarged to involve the lower half of her posterior calf and subsequently turned purple and became exquisitely tender. Similar but smaller purple, tender lesions simultaneously appeared, first over her right shin and then on her bilateral thighs and hips. She also reported fatigue as well as diffuse joint pains in her hands and wrists bilaterally for the past month. She denied any swelling of these joints or functional impairment. She denied fevers, weight loss, headache, sinus symptoms, difficulty breathing, or abdominal pain.
Although we do not yet have a physical exam, the tempo, pattern of spread, and accompanying features allow some early hypotheses to be considered. Distal lower extremity lesions which darkened and spread could be erythema nodosum or erythema induratum. Malignancies rarely have such prominent skin manifestations, although leukemia cutis or an aggressive cutaneous T cell lymphoma might present with disseminated and darkened plaques, and Kaposi's sarcoma is characteristically purple and multifocal. Autoimmune disorders such as sarcoidosis, cutaneous lupus, and psoriasis may similarly present with widespread plaques. Most disseminated infections that start with patches evolve to pustules, ulcers, bullae, or other forms that reflect the invasive nature of the infection; syphilis warrants consideration for any widespread eruption of unknown etiology. Antecedent arthralgias with fatigue suggest an autoimmune condition, although infections such as hepatitis or parvovirus can do the same. Systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA) would be favored initially on account of her demographics and the hand and wrist involvement, and each can be associated with vasculitis.
The significant pain as described is not compatible with most of the aforementioned diagnoses. Its presence, coupled with potential autoimmune symptoms, suggests a vasculitis such as polyarteritis nodosa (which can have prominent diffuse skin involvement), Henoch Schonlein purpura (with its predilection for the lower extremities, including extension to the hips and buttocks), cryoglobulinemia, or SLE‐ or RA‐associated vasculitis. Calciphylaxis is another ischemic vascular disorder that can cause diffuse dark painful lesions, but this only warrants consideration if advanced renal disease is present.
A skin biopsy ofher right hip was taken at the outside hospital. She was discharged on a two‐week course of prednisone for suspected vasculitis while biopsy results were pending. Over the next two weeks, none of the skin lesions improved, despite compliance with this treatment, and the skin over her left posterior calf and right shin lesions began to erode and bleed. In addition, small purple, tender lesions appeared over the pinnae of both ears. Three weeks after her initial evaluation, she presented to another emergency department for ulcerating skin lesions and worsening pain. At that point, the initial skin biopsy result was available and revealed vasculopathy of the small vessels with thrombi but no vasculitis.
The patient had no children,and denied a history of miscarriages. Her past medical history was unremarkable. She did not report any history of thrombotic events. She started a new job as a software engineer one month ago and was feeling stressed about her new responsibilities. She denied any high‐risk sexual behavior and any history of intravenous drug use. She had not traveled recently and did not own any pets. There was no family history of rheumatologic disorders, hypercoagulable states, or thrombotic events.
This picture of occluded but noninflamed vessels shifts the diagnosis away from vasculitis and focuses attention on hypercoagulable states with prominent dermal manifestations, including antiphospholipid antibody syndrome (APLS) and livedoid vasculopathy. In this young woman with arthralgias, consideration of SLE and APLS is warranted. Her recent increase in stress and widespread purpuric and ulcerative lesions could bring to mind a factitious disorder, but the histology results eliminate this possibility.
The patient's temperaturewas 36.5C, her blood pressure was 110/70 mmHg, respiratory rate was 16 breaths per minute, and her heart rate was 65 beats per minute. She was well‐appearing but in moderate pain. She did not have any oral lesions. Her cardiac, respiratory, and abdominal exams were normal. Skin exam revealed a 10‐cm by 4‐cm area of bloody granulation tissue draining serosanguinous fluid, surrounded by stellate palpable netlike purpura on her left posterior calf. There was a similar 4‐cm by 2‐cm ulcerated lesion on her right shin. Both lesions were exquisitely tender to palpation. On her bilateral thighs and hips, there were multiple stellate purpuric patches, all 4 cm in diameter or less, and only minimally tender to palpation. She also had 1‐cm purpuric bullae on the helices of both ears (Figure 1) which were slightly tender to palpation. Splinter hemorrhages were also noted on multiple nail beds bilaterally. Musculoskeletal exam did not reveal any synovitis.

The original purpura on her calf and ear demonstrate a clear demarcation corresponding to cutaneous vascular insufficiency. The development of bullae (ear) and ulceration (calf) are compatible with ischemia. Despite the presence of multiple splinter hemorrhages, the distribution of lesions is very unusual for an embolic phenomenon (eg, endocarditis, cholesterol emboli, or atrial myxoma). The multifocal nature of the skin lesions with progression to well‐demarcated cutaneous necrosis is reminiscent of calciphylaxis or warfarin‐induced skin necrosis, although she lacks the relevant risk factors. A toxin such as cocaine or methamphetamine mediating multifocal vasoconstriction or hypercoagulability should be excluded.
The bilateral ear involvement remains decidedly unusual and makes me wonder if there is something about the ear, such as the nature of its circulation or its potentially lower temperature (as an acral organ) that might render it particularly susceptible, for instance, to cryoglobulinemia or cryofibrinogenemia‐mediated ischemia.
Laboratory studiesdemonstrated: white blood cell count of 1500/mm3 (37.3% neutrophils, 5.1% lymphocytes, 6.7% monocytes, and 1.3% eosinophils); hemoglobin 9.3 g/dl (mean corpuscular volume 91 fL); platelet count 212/mm3; erythrocyte sedimentation rate 62 mm/hr; C‐reactive protein 14.6 mg/L. Serum electrolytes, liver tests, coagulation studies, and urinalysis were normal. Fecal occult blood test was negative.
Her neutropenia and anemia suggest decreased production in the marrow by infection, malignancy, or toxin, or increased destruction, perhaps from an autoimmune process. The associated infections are usually viral, such as human immunodeficiency virus (HIV) and Epstein‐Barr virus (EBV), although their linkage with her cutaneous disease is tenuous. It is possible that malignancy could be present in the marrow with resultant dermal hypercoagulability and ischemia, but this seems unlikely. We do not know about any toxins that she has been exposed to, but these hematologic findings would mandate directed inquiry along those lines. In this young woman with cutaneous ulcers secondary to thrombotic vasculopathy, bicytopenia, antecedent arthralgias without synovitis, and elevated inflammatory markers, I favor an autoimmune process such as SLE, which I would evaluate with an antinuclear antibody (ANA) and antiphospholipid antibody studies.
She was admittedto the hospital and received hydromorphone for pain control. Corticosteroids were not administered. Peripheral blood morphology was normal. Antibodies against HIV1 and 2 were negative, as were antibodies against cytomegalovirus, EBV, parvovirus B19, mycoplasma pneumoniae, and hepatitis C virus. Bilateral lower extremity ultrasound was negative for deep vein thrombosis. Transthoracic echocardiogram was normal. Repeat skin biopsy confirmed small vessel vasculopathy without vasculitis (Figure 2). The results of the following investigations were also negative: ANA, rheumatoid factor, double‐stranded DNA (dsDNA), cyclic citrullinated peptide, ribonucleoprotein (RNP), and anti‐Smith antibodies. C3 and C4 complement levels were normal.

Given how much the histology is driving the clinical reasoning and focusing the differential diagnosis in this case, I agree with the decision to repeat the biopsy. In complex or undiagnosed cases, repeat histology samples allow for confirmation of the original interpretation (often with the perspective of new clinicians and pathologists) and sometimes reveal pathognomonic or additional findings that only appear after the disease has evolved over time. HIV seronegativity helps constrain the differential diagnosis, and parvovirus is another excellent consideration for arthralgias and cytopenias (with the predilection to involve cells lines other than RBCs particularly seen in HIV), although ulcers are not seen with this condition. Herpes simplex virus (HSV) is another viral infection that can cause painful skin ulcerations and cytopenias, although the duration and distribution are highly atypical. The negative ANA and dsDNA and normal complement levels make SLE unlikely. The negative lower extremity ultrasound helps frame the thromboses as a local cutaneous process rather than a systemic hypercoagulable state. Although the peripheral blood smear is normal, a bone marrow biopsy will be necessary to exclude a marrow invasive process, such as leukemia or lymphoma. A bone marrow biopsy would also provide another opportunity to examine tissue for mycobacteria or fungi which can cause ulcerations and cytopenias, although there is little reason to suspect she is susceptible to those pathogens. As this clinical picture fails to fit clearly with an infectious, autoimmune, or neoplastic disorder, I would revisit the possibility of toxinsprescription, complementary, over‐the‐counter, or illegal (eg, cocaine) at this time.
In further discussionwith the patient, she reported using cocaine intranasally for the past three months. Her urine toxicology was positive for cocaine. She was found to have positive perinuclear antineutrophil cytoplasmic antibodies (p‐ANCA), antimyeloperoxidase (MPO) antibodies, anticardiolipin (ACL) antibodies, and lupus anticoagulant (LAC). By hospital day 3, her lesions had significantly improved without any intervention, and her absolute neutrophil count increased to 1080/mm3.
The presence of widespread cutaneous ischemia (with bland thrombosis) and detectable ACL and LAC antibodies is compatible with APLS; the APLS could be deemed primary, because there is no clear associated rheumatologic or other systemic disease. However, neutropenia is not a characteristic of APLS, which has thrombocytopenia as its more frequently associated hematologic abnormality. Livedoid vasculopathy, a related disorder, is also supported by the ACL and LAC results, but also does not feature neutropenia. While the presence of diffuse thrombosis could be attributed to a widespread secondary effect of cocaine vasoconstriction, the appearance of ANCA (which can be drug‐induced, eg, propylthiouracil [PTU]) and the slowly resolving neutropenia during hospitalization without specific treatment is very suggestive of a toxin. The demographic, diffuse skin ulcers, and hematologic and serologic profile is compatible with the recently described toxidrome related to levamisole adulteration of cocaine.
A send‐out studyof a urine sample returned positive for levamisole. Based on purpuric skin lesions with a predilection for the ears, agranulocytosis, and skin biopsy revealing thrombotic vasculopathy, she was diagnosed with levamisole‐adulterated cocaine exposure. One week after discharge, her lower extremity pain and ulcerations were significantly improved. Her absolute neutrophil count increased to 2820/mm.3 Her urine toxicology screen was negative for cocaine.
DISCUSSION
Levamisole was initially developed in 1964 as an antihelminthic agent. Its incidentally discovered immunomodulatory effects led to trials for the treatment of chronic infections, inflammatory bowel disease, rheumatic diseases,1 and nephrotic syndrome in children.2 By 1990, 3 major studies supported levamisole as an adjunctive therapy in melanoma3 and colon cancer.4
Although levamisole appeared to be nontoxic at single or low doses, long‐term use in clinical trials demonstrated that 2.5%‐13% of patients developed life‐threatening agranulocytosis, and up to 10% of those instances resulted in death.5 A distinctive cutaneous pseudovasculitis was noted in children on therapeutic levamisole. They presented with purpura that had a predilection for the ears, cheeks, and thighs,6 and positive serologic markers for ANCA and antiphospholipid antibodies. Skin biopsies of the purpuric lesions revealed leukocytoclastic vasculitis, thrombotic vasculitis, and/or vascular occlusions.
Levamisole was withdrawn from the market in 2000 in the United States due to its side effects,7 but quickly found its way onto the black market. It was first detected in cocaine in 2002, and the percentage of cocaine containing levamisole has steadily been increasing since then. In July 2009, over 70% of cocaine seized by the Drug Enforcement Administration was found to contain levamisole.8 It is unclear exactly why this drug is used as an adulterant in cocaine. Theories include potentiation of the euphoric effects of cocaine, serving as a bulking agent, or functioning as a chemical signature to track distribution.9
The resurgence of levamisole has brought a new face to a problem seen over a decade ago. Current reports of levamisole toxicity describe adults presenting with purpura preferentially involving the ears, neutropenia, positive ANCA, and positive antiphospholipid antibodies.1012 Since 2002, there have been at least 20 confirmed cases of agranulocytosis and two deaths associated with levamisole‐adulterated cocaine.8, 13, 14 In September 2009, the Department of Health and Human Services issued a public health alert warning of an impending increase in levamisole‐related illness.
Levamisole is not detected on routine toxicology screens, but can be tested for using gas chromatography and mass spectrometry. Most laboratories do not offer testing for levamisole and send‐out testing is required. Given its half‐life of 5.6 hours, levamisole can only be detected in the blood within 24 hours, and in the urine within 48‐72 hours of exposure.15, 16 Urine samples are preferred over blood samples, since blood levels decline more rapidly and have lower sensitivity. Cocaine can also be sent out to local or state forensics laboratories to be tested for levamisole. The only definitive treatment for levamisole‐induced cutaneous pseudovasculitis and neutropenia is cessation of toxin exposure.
Although the discussant had familiarity with this toxidrome from local and published cases, he was only able to settle on levamisole toxicity after a series of competing hypotheses were ruled out on the basis of irreconcilable features (vasculitis and histology results; APLS and neutropenia; SLE and negative ANA with no visceral involvement) and by using analogical reasoning (eg, to infer the presence of a toxin on the basis of neutropenia [as seen with chemotherapy and other drugs] and ANCA induction [as seen with PTU]). It was a laborious process of hypothesis testing, but one that ultimately allowed him to crack the case.
Key Teaching Points
-
In patients presenting with neutropenia and purpuric skin lesionsparticularly with a predilection for the earsconsider levamisole‐adulterated cocaine exposure.
-
Tests supporting this diagnosis include positive serologies for ANCA and antiphospholipid antibodies, and skin biopsies that show leukocytoclastic vasculitis, thrombotic vasculitis, or vascular occlusion. Urine studies for levamisole are definitive if sent within 48 to 72 hours of exposure.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
- ,.Levamisole, the story and the lessons.Int J Immunopharmocol.1992;14(3):481–486.
- British Association for Paediatric Nephrology.Levamisole for corticosteroid‐dependent nephrotic syndrome in childhood.Lancet.1991;337:1555–1557.
- ,,,,.Improved survival in patients with poor‐prognosis malignant melanoma treated with adjuvant levamisole: a phase III study by the National Cancer Institute of Canada Clinical Trials Group.J Clin Oncol.1991;9:729–735.
- ,,.Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma.N Engl J Med.1990;322:352–358.
- ,,.Studies on levamisole‐induced agranulocytosis.Blood.1980;56(3):388–396.
- ,,.Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long‐term treatment with levamisole in children.Br J Dermatol.1999;140:948–951.
- . Janssen Discontinues Ergamisol. Available at: http://findarticles.com/p/articles/mi_m3374/is_18_22/ai_68536218/. Accessed July 25,2010.
- SAMHSA. Nationwide Public Health Alert Issued Concerning Life‐Threatening Risk Posed by Cocaine Laced with Veterinary Anti‐Parasite Drug. Available at: http://www.samhsa.gov/newsroom/advisories/090921vet5101.aspx. Accessed July 20,2010.
- .Unusual adulterants in cocaine seized on Italian clandestine market.Forensic Sci Int.2007;172(2–3):e1.
- ,,.Levamisole‐induced occlusive necrotizing vasculitis of the ears after use of cocaine contaminated with levamisole.J Med Toxicol.2010;Jun 12.
- ,,,.Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole.Ann Intern Med.2010;1;152(11):758–759.
- ,,,,.Cocaine‐associated retiform purpura and neutropenia: is levamisole the culprit?J Am Acad Dermatol.2010;Mar 19.
- ,,,.A confirmed case of agranulocytosis after use of cocaine contaminated with levamisole.J Med Toxicol.2010;Apr 1.
- Centers for Disease Control and Prevention.Agranulocytosis associated with cocaine use—four States, March 2008–November 2009.MMWR.2009;58(49):1381–1385.
- ,,.Levamisole as a contaminant of illicit cocaine.Journal of the Clandestine Laboratory Investigating Chemists Association.2006;16:6–11. Available at: http://www.tiaft2006.org/proceedings/pdf/PT‐p‐06.pdf. Accessed July 20, 2010.
- . Cocaine Cutting Agents—A Discussion. Laboratory Medicine and Pathology, University of Alberta. Available at: http://www.vandu.org/documents/Levamisole_Cocaine.pdf. Accessed July 20,2010.
- ,.Levamisole, the story and the lessons.Int J Immunopharmocol.1992;14(3):481–486.
- British Association for Paediatric Nephrology.Levamisole for corticosteroid‐dependent nephrotic syndrome in childhood.Lancet.1991;337:1555–1557.
- ,,,,.Improved survival in patients with poor‐prognosis malignant melanoma treated with adjuvant levamisole: a phase III study by the National Cancer Institute of Canada Clinical Trials Group.J Clin Oncol.1991;9:729–735.
- ,,.Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma.N Engl J Med.1990;322:352–358.
- ,,.Studies on levamisole‐induced agranulocytosis.Blood.1980;56(3):388–396.
- ,,.Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long‐term treatment with levamisole in children.Br J Dermatol.1999;140:948–951.
- . Janssen Discontinues Ergamisol. Available at: http://findarticles.com/p/articles/mi_m3374/is_18_22/ai_68536218/. Accessed July 25,2010.
- SAMHSA. Nationwide Public Health Alert Issued Concerning Life‐Threatening Risk Posed by Cocaine Laced with Veterinary Anti‐Parasite Drug. Available at: http://www.samhsa.gov/newsroom/advisories/090921vet5101.aspx. Accessed July 20,2010.
- .Unusual adulterants in cocaine seized on Italian clandestine market.Forensic Sci Int.2007;172(2–3):e1.
- ,,.Levamisole‐induced occlusive necrotizing vasculitis of the ears after use of cocaine contaminated with levamisole.J Med Toxicol.2010;Jun 12.
- ,,,.Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole.Ann Intern Med.2010;1;152(11):758–759.
- ,,,,.Cocaine‐associated retiform purpura and neutropenia: is levamisole the culprit?J Am Acad Dermatol.2010;Mar 19.
- ,,,.A confirmed case of agranulocytosis after use of cocaine contaminated with levamisole.J Med Toxicol.2010;Apr 1.
- Centers for Disease Control and Prevention.Agranulocytosis associated with cocaine use—four States, March 2008–November 2009.MMWR.2009;58(49):1381–1385.
- ,,.Levamisole as a contaminant of illicit cocaine.Journal of the Clandestine Laboratory Investigating Chemists Association.2006;16:6–11. Available at: http://www.tiaft2006.org/proceedings/pdf/PT‐p‐06.pdf. Accessed July 20, 2010.
- . Cocaine Cutting Agents—A Discussion. Laboratory Medicine and Pathology, University of Alberta. Available at: http://www.vandu.org/documents/Levamisole_Cocaine.pdf. Accessed July 20,2010.
A Novel Approach to Physician Shortages
The demand for physician talent is intensifying as the US healthcare system confronts an unprecedented confluence of demographic pressures. Not only will 78 million retiring baby‐boomers require significant healthcare resources, but tens of thousands of practicing physicians will themselves reach retirement age within the next decade.1 At the same time, factors like the large increase in the percentage of female physicians (who are more likely to work part time), the growth of nonpractice opportunities for MDs, and generational demands for greater worklife balance are creating a major supply‐demand mismatch within the physician workforce.2 In this demographic atmosphere, the ability to recruit and retain physician leaders confers tremendous value to healthcare enterprisesboth public and private. Recruiting and retaining strategies already weigh heavily in the most palpable shortage areas, like primary care, but the system faces widespread unmet demand for a variety of specialist and generalist practitioners.36
This article does not address the public policy implications of the upcoming physician shortage, recognition of which will lead to the largest increase in new medical school slots in decades.7 Rather, we set out to illustrate how successful nonmedical businesses are embracing a thoughtful, systematic approach to retaining talent, based on the philosophy that keeping and engaging valued employees is more efficient than recruiting and orienting replacements. We posit that the innovations used by progressive companies could apply to recruitment and retention challenges confronting medicine.
How Industry Approaches the Talent Vacuum: Talent Facilitation
Leaders outside of medicine have long acknowledged that changing demographics and a global economy are driving unprecedented employee turnover.8 In confronting a talent vacuum, forward‐thinking managers have prioritized retaining key talent (rather than hiring anew) in planning for the future.9, 10 Doing so begins with attempts to understand the relationship between workers and the workplace, with a particular emphasis on appreciating workers' priorities. Indeed, new executive positions with titles like Chief Learning Officer and Chief Experience Officer are appearing as companies realize a need for focused expertise beyond traditional human resource departments. These companies understand that offering higher salaries is not the only retention strategyand often not even the most effective one.
The Four Actions of Talent Facilitation
The talent facilitation process centers on four actions: attract, engage, develop, and retain. None of these actions can stand alone, and all should be present, to some degree, at all stages of a worker's tenure. To attract or engage an employee or practice partner is not a 1‐time hook, but a constant and dynamic process.
Importantly, the concepts addressed here are not specific to 1 type of corporate system, size, or management level. Although the early business focus had been on upper‐level and executive talent within large corporate settings, there is an increasing recognition that a dedicated talent strategy is useful wherever recruiting and retaining talented people is important (and where is it not?).
The ideas presented here may seem most applicable to leaders of large physician corporations, hospital‐owned physician groups, or large integrated healthcare systems (such as Kaiser) that employ physicians. However, we also believe that the ideas apply across‐the‐board in medicine, including entities such as small, private, physician‐owned groups. We argue that regardless of the exact practice structure, a limited pool of resources must be dedicated to the attraction and retention of talented partners or employees, or to the cost of replacing those people if they pursue other opportunities (or the cost of inefficient and disengaged physicians). While an integrated health system may have the resources and scale to hire a Chief Experience Officer, we do not anticipate that a 5‐partner private practice would. Rather, we point to examples to illustrate the talent facilitation paradigm as a tool to systematically frame the allocation of those resources. Undoubtedly, the specific shape of a thoughtful talent facilitation effort will vary when applied in a large urban academic medical center vs. an integrated healthcare system vs. a small physician private practice, but the basic principles remain the same.
Attract
Increasingly, companies approach talented prospects with dedicated marketing campaigns to convey the value of a work environment.11 Silicon Valley employee lounges with free massages and foosball tables are the iconic example of attraction, but the concept runs deeper. Today's workers seek access to state‐of‐the‐art ideas and technology and often want to be part of a larger vision. Many seek opportunities to integrate their own professional and personal aspirations into a particular job description.
Hospital executives have long recognized the importance of attracting physicians to their facility (after all, the physicians draw patients and thus generate revenue). The traditional approach has surrounded perks, from comfortable doctor's lounges to the latest in surgical technology. But, the stakes seem greater now than before, and successful talent facilitation strategies are going beyond the tried and true.
Clearly, physicians seek financially stable practice settings with historical success. But they may also seek evidence of a defined strategic plan focused on more than mere profitability. Physicians may gravitate to practice environments that endorse progressive movements like the No One Dies Alone campaign.12 Similarly, recognition of movements beyond healthcarea commitment to Leadership in Energy and Environmental Design (LEED) (Green Building Rating System; US Green Building Council [USGBC];
The current recruitment campaign of California's prison healthcare system offers an unlikely source of inspiration. The prison system was placed in receivership to address a shortage of competent physician staff and other inadequacies. A central feature of the campaign is an attractive starting salary and good benefits. But, the campaign does not rely on money alone. For example, the campaign's website (

Engage
The corporate tool being employed at this stage is a strategy called on‐boarding, which emphasizes a streamlined integration of newcomers with existing workers and culture, and prioritizes aligning organizational roles with a worker's specific skills and interests. On‐boarding also emphasizes the value of early and frequent provision of constructive feedback from same‐level peers or managers with advanced coaching skills.
Many companies use formal survey tools to measure employee engagement and regularly evaluate the proficiency of system leaders in the ability to engage their employees. An engineering firm executive recently told us (P.K., C.K.) that he performs detailed and frequent on‐the‐job interviews, even with company veterans. The primary goal of these interviews is to ensure that engineers spend at least 85% of their time on work that: (1) they find interesting and (2) allows for the application of their best skills. Wherever possible, traditional job descriptions are altered to achieve this. Inevitably, there is still work (15% in this particular corporate vision) that no one prefers but needs to get done, but this process of active recalibration minimizes this fraction to the degree possible.
Even within a small physician practice group, one can imagine how a strategic approach of inviting and acknowledging individual physician's professional goals and particular talents may challenge the long‐held belief that everyone within a group enjoys and must do the exact same job. Once these goals and talents are articulated, groups may find that allowing for more customized roles within the practice enhances professional satisfaction.
Social networking, collaboration, and sharing of best practices are staples of engaging companies. The Cisco and Qualcomm companies, for example, utilize elaborate e‐networks (rough corporate equivalents to Facebook) to foster collegial interaction within and across traditional hierarchical boundaries so that managers and executives directly engage the ideas of employees at every level.14 The premise is logical: engaged employees will be more likely to contribute innovative ideas which, when listened to, are more likely to engage employees.
Most physicians will recognize the traditional resident report as a model for engagement. Beyond its educational value, interaction with program leadership, social bonding, collaborative effort, and exploration of best practices add tremendous value. Many companies would jump at the chance to engrain a similar cultural staple. Enhancing this type of interaction in a postresidency setting may promote engagement in a given system, especially if it facilitates interactions between physicians and senior hospital leaders. Absent these types of interactions, ensuring regular provision of peer review and/or constructive feedback can help systematically enhance 2‐way communication and enhance engagement.
Develop
Talent development relies on mentorship reflecting a genuine interest in an individual's future. Development strategies include pairing formal annual talent reviews or (in the case of practice partners) formal peer review with strategic development plans. Effective development strategy may include transparent succession planning so that individuals are aware they are being groomed for future roles.
A well‐known adage suggests, People quit the manager or administrator, not the job. Development in this sense relies on presenting new opportunities and knowing that people flourish when allowed to explore multiple paths forward. In many companies, the role of Chief Experience or Chief Learning Officers is to enhance development planning. Consider how career coaching of young hospitalists could transform an infinitely portable and volatile commodity job, prone to burnout, into an engaged specialist of sorts with immense value to a hospital. Hospitalists have already demonstrated their potential as quality improvement leaders.15 Imagine if hospital leadership enlisted a young hospitalist in a relevant quality improvement task force, such as one working on preventing falls. With appropriate support, the physician could obtain skills for quality improvement evaluation that would not only enhance his or her engagement with the hospital system but also provide a valuable analysis for the hospital.
As an example of development strategy within a small practice setting, consider the following real‐life anecdote: a group of 4 physicians recently completed a long and expensive recruitment of a new partner. The new partner, intrigued by the local hospital's surgical robot technology, sought the support of her partners (who are not currently using the technology) to partake in an expensive robotics training program. The partners decided not to provide the financial support. The new partner subsequently left the group for a nearby practice opportunity that would provide for the training, and the group was faced with the loss of a partner (one‐fourth of the practice!) and the cost of repeating the recruiting process. A preemptive evaluation of the value of investing in the development of the new partner and enhancing that partner's professional development may have proven wise despite the significant up‐front costs. In this case the manager the new partner quit was the inflexibility of the practice trajectory.
Retain
The economic incentive to retain talented workers is not subtle. If it was, companies would not be funneling resources into Chief Experience Officers. Likewise, the estimated cost for a medical practice to replace an individual physician is as at least $250,000.16, 17
In retention, as in attraction, salary is only part of the equation.18 People want fair and competitive compensation, and may leave if they are not getting it, but they will not stay (and will not stay engaged) only for a salary bump. Retention is enhanced when workers can advance according to skills and talent, rather than mere tenure. An effective retention policy responds to people's desire to incorporate individual professional goals into their work and allows for people to customize their career rather than simply occupy a job class. Effective retention policy respects worklife balance and recognizes that this balance might look different for 2 people with the same job. It may take the form of positive reinforcement (rather than subtle disdain) for using vacation time or allowing for participation in international service projects. Many literally feel that they need to quit their job in order to take time off or explore other interests.
Worklife balance has been a longstanding issue in medicine, and innovative augmentation strategies may well help retain top talent. Today's successful medical school applicants not only show aptitude in the classroom, they often have many well‐developed nonmedical skills. No one can expect that medical training will somehow convince them to leave everything else behind. Moreover, today's residency graduates, already with Generation Y sensibilities, have completed their entire training under the auspices of the Accreditation Council for Graduate Medical Education (ACGME) duty hours regulations, which has made residents far more comfortable with shift work and defined hours.
At the other end of the generation spectrum, as large numbers of physicians ready for retirement, effective talent facilitation strategies may evaluate how to reoffer medicine as a valid option for senior physicians who still wish to work. Retaining these physicians will require an appreciation of their lifestyle goals, as they will likely find continuing a traditional practice role untenable. A recent survey of orthopedic surgeons 50 years of age or older found that having a part‐time option was a common reason they continued practicing, and that the option to work part‐time would have the most impact on keeping these surgeons working past age 65 years.19 Those working part‐time were doing so in a wide range of practice arrangements including private practice. However, one‐third of those surveyed said a part‐time option was not available to them. Clearly, in an environment of workforce shortages, physician‐leaders must begin to think about worklife balance not only for new doctors but for those considering retirement.
Critics will point out the financial drawbacks in the provision of worklife balance. But the cost may pale in comparison to the cost of replacing physician leaders. Moreover, engaged physicians are more likely to add value in the form of intangible capital such as patient satisfaction and practice innovation. As such, we argue that effective retention strategy in medicine is likely to be cost‐effective, even if it requires significant new up‐front resources.
Lessons From Industry
Doctors frequently assume that the challenges and obstacles confronted in healthcare are unique to medicine. But, for every phrase like When I started practice, I decided how long office visits were, not the insurance company, or Young doctors just don't want to work as hard, there is a parallel utterance in the greater business world. Luckily, there are now examples of the healthcare world learning lessons from business. For example, innovators in medical quality improvement found value in the experience of other industries.20 Airlines and automakers have long honed systems for error prevention and possess expertise that may curb errors in the hospital.2123
We suspect that the ideas and practice of talent facilitation have already made their way into some medical settings. A Google search reveals multiple opportunities for hospital‐based talent managers, and websites advertise the availability of talent consultants ready to lend their expertise to the medical world. In the arena of academic medicine, the University of California, San Francisco (UCSF) Division of Hospital Medicine put some of the ideas of talent facilitation into practice over the past year, in part in response to an increasingly competitive market for academic hospitalists.24 Leaders introduced a formal faculty development program that links junior faculty with mentors and facilitates early and frequent feedback across hierarchical boundaries.25 These more intentional mentoring efforts were accompanied by a seminar series aimed at the needs of new faculty members, a research incubator program, divisional grand rounds, and other web‐based and in‐person forums for sharing best practices and innovations. Less formal social events have also been promoted. Importantly, these sweeping strategies seek to encompass the needs of both teaching and nonteaching hospitalists within UCSF.26
Clearly, an academic hospitalist group with 45 faculty physicians has unique characteristics that inform the specifics of its talent facilitation strategy. The interventions discussed above are meant to represent examples of the types of strategies that may be utilized by physician groups once a decision is made to focus on talent management. Undoubtedly, the shape of such efforts will vary in diverse practice settings, but physician leaders have much to gain through further exploration of where these core principles already exist within medicine and where they may be more effectively deployed. By examining how multinational businesses are systematically applying the concepts of talent facilitation to address a global talent shortage, the doctoring profession might again take an outside hint to help inform its future.
- Long Term Care: Aging Baby Boom Generation Will Increase Demand and Burden on Federal and State Budgets. United States General Accounting Office Testimony before the Special Committee on Aging, US Senate. Hearing Before the Special Committee on Aging of the US Senate,2002. Available at:http://www.gao.gov/new.items/d02544t.pdf. Accessed July 2009.
- ,.Physician workforce shortages: implications and issues for academic health centers and policymakers.Acad Med.2006;81(9):782–787.
- .New York moves to tackle shortage of primary‐care doctors.Lancet.2008;371(9615):801–802.
- ,.The US dermatology workforce: a specialty remains in shortage.J Am Acad Dermatol.2008;59(5):741–745.
- ,.Challenges and opportunities for recruiting a new generation of neurosurgeons.Neurosurgery.2007;61(6):1314–1319.
- ,.The developing crisis in the national general surgery workforce.J Am Coll Surg.2008;206(5):790–795.
- Medical School Enrollment Plans: Analysis of the 2007 AAMC Survey. Publication of the Association of American Medical Colleges, Center for Workforce Studies, April2008. Available at:http://www.aamc.org/workforce. Accessed July 2009.
- It's 2008: Do You Know Where Your Talent Is? Why acquisition and retention strategies don't work. Part 1 of a Deloitte Research Series on Talent Management.2008. Available at: http://www.deloitte.com/dtt/cda/content/UKConsulting_TalentMgtResearchReport.pdf. Accessed August 2009.
- ,,.The race for talent: retaining and engaging workers in the 21st century.Hum Resour Plann.2004;27(3):12–25.
- Expecting sales growth, CEOs cite worker retention as critical to success. March 1,2004. Available at:http://www.barometersurveys.com/production/barsurv.nsf/89343582e94adb6185256b84006c8ffe/9672ab2f54cf99f885256e5500768232?OpenDocument. Accessed July 2009.
- Jet Blue announces aviation university gateway program for pilot candidates: airline partners with Embry‐Riddle Aeronautical University, University of North Dakota, and Cape Air to fill pilot pipeline. January 30, 2008. Available at:http://investor.jetblue.com/phoenix.zhtml?c=131045287(4):487–494.
- ,,.A review of physician turnover: rates, causes, and consequences.Am J Med Qual.2004;19(2):56–66.
- ,,.The impact on revenue of physician turnover: an assessment model and experience in a large healthcare center.J Med Pract Manage.2006;21(6):351–355.
- ,,.Employee motivation: a powerful new model.Harv Bus Rev.2008;86(7–8):78,84,160.
- ,,.Work satisfaction and retirement plans of orthopaedic surgeons 50 years of age or older.Clin Orthop Relat Res.2008;466(1):231–238.
- ,,,.The long road to patient safety: a status report on patient safety systems.JAMA.2005(22);294:2858–2865.
- .Error reduction through team leadership: what surgeons can learn from the airline industry.Clin Neurosurg.2007;54:195–199.
- ,.Applying the Toyota production system: using a patient safety alert system to reduce error.Jt Comm J Qual Patient Saf.2007;33(7):376–386.
- ,,,.Improving Papanikolaou test quality and reducing medical errors by using Toyota production system methods.Am J Obstet Gynecol.2006;194(1):57–64.
- Society of Hospital Medicine Career Satisfaction Task Force. White Paper on Hospitalist Career Satisfaction.2006; 1–45. Available at: http://www.hospitalmedicine.org. Accessed July 2009.
- UCSF Department of Medicine, Division of Hospital Medicine, Faculty Development. Available at: http://hospsrvr.ucsf.edu/cme/fds.html. Accessed July 2009.
- ,,, et al. Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3(3):247–245.
The demand for physician talent is intensifying as the US healthcare system confronts an unprecedented confluence of demographic pressures. Not only will 78 million retiring baby‐boomers require significant healthcare resources, but tens of thousands of practicing physicians will themselves reach retirement age within the next decade.1 At the same time, factors like the large increase in the percentage of female physicians (who are more likely to work part time), the growth of nonpractice opportunities for MDs, and generational demands for greater worklife balance are creating a major supply‐demand mismatch within the physician workforce.2 In this demographic atmosphere, the ability to recruit and retain physician leaders confers tremendous value to healthcare enterprisesboth public and private. Recruiting and retaining strategies already weigh heavily in the most palpable shortage areas, like primary care, but the system faces widespread unmet demand for a variety of specialist and generalist practitioners.36
This article does not address the public policy implications of the upcoming physician shortage, recognition of which will lead to the largest increase in new medical school slots in decades.7 Rather, we set out to illustrate how successful nonmedical businesses are embracing a thoughtful, systematic approach to retaining talent, based on the philosophy that keeping and engaging valued employees is more efficient than recruiting and orienting replacements. We posit that the innovations used by progressive companies could apply to recruitment and retention challenges confronting medicine.
How Industry Approaches the Talent Vacuum: Talent Facilitation
Leaders outside of medicine have long acknowledged that changing demographics and a global economy are driving unprecedented employee turnover.8 In confronting a talent vacuum, forward‐thinking managers have prioritized retaining key talent (rather than hiring anew) in planning for the future.9, 10 Doing so begins with attempts to understand the relationship between workers and the workplace, with a particular emphasis on appreciating workers' priorities. Indeed, new executive positions with titles like Chief Learning Officer and Chief Experience Officer are appearing as companies realize a need for focused expertise beyond traditional human resource departments. These companies understand that offering higher salaries is not the only retention strategyand often not even the most effective one.
The Four Actions of Talent Facilitation
The talent facilitation process centers on four actions: attract, engage, develop, and retain. None of these actions can stand alone, and all should be present, to some degree, at all stages of a worker's tenure. To attract or engage an employee or practice partner is not a 1‐time hook, but a constant and dynamic process.
Importantly, the concepts addressed here are not specific to 1 type of corporate system, size, or management level. Although the early business focus had been on upper‐level and executive talent within large corporate settings, there is an increasing recognition that a dedicated talent strategy is useful wherever recruiting and retaining talented people is important (and where is it not?).
The ideas presented here may seem most applicable to leaders of large physician corporations, hospital‐owned physician groups, or large integrated healthcare systems (such as Kaiser) that employ physicians. However, we also believe that the ideas apply across‐the‐board in medicine, including entities such as small, private, physician‐owned groups. We argue that regardless of the exact practice structure, a limited pool of resources must be dedicated to the attraction and retention of talented partners or employees, or to the cost of replacing those people if they pursue other opportunities (or the cost of inefficient and disengaged physicians). While an integrated health system may have the resources and scale to hire a Chief Experience Officer, we do not anticipate that a 5‐partner private practice would. Rather, we point to examples to illustrate the talent facilitation paradigm as a tool to systematically frame the allocation of those resources. Undoubtedly, the specific shape of a thoughtful talent facilitation effort will vary when applied in a large urban academic medical center vs. an integrated healthcare system vs. a small physician private practice, but the basic principles remain the same.
Attract
Increasingly, companies approach talented prospects with dedicated marketing campaigns to convey the value of a work environment.11 Silicon Valley employee lounges with free massages and foosball tables are the iconic example of attraction, but the concept runs deeper. Today's workers seek access to state‐of‐the‐art ideas and technology and often want to be part of a larger vision. Many seek opportunities to integrate their own professional and personal aspirations into a particular job description.
Hospital executives have long recognized the importance of attracting physicians to their facility (after all, the physicians draw patients and thus generate revenue). The traditional approach has surrounded perks, from comfortable doctor's lounges to the latest in surgical technology. But, the stakes seem greater now than before, and successful talent facilitation strategies are going beyond the tried and true.
Clearly, physicians seek financially stable practice settings with historical success. But they may also seek evidence of a defined strategic plan focused on more than mere profitability. Physicians may gravitate to practice environments that endorse progressive movements like the No One Dies Alone campaign.12 Similarly, recognition of movements beyond healthcarea commitment to Leadership in Energy and Environmental Design (LEED) (Green Building Rating System; US Green Building Council [USGBC];
The current recruitment campaign of California's prison healthcare system offers an unlikely source of inspiration. The prison system was placed in receivership to address a shortage of competent physician staff and other inadequacies. A central feature of the campaign is an attractive starting salary and good benefits. But, the campaign does not rely on money alone. For example, the campaign's website (

Engage
The corporate tool being employed at this stage is a strategy called on‐boarding, which emphasizes a streamlined integration of newcomers with existing workers and culture, and prioritizes aligning organizational roles with a worker's specific skills and interests. On‐boarding also emphasizes the value of early and frequent provision of constructive feedback from same‐level peers or managers with advanced coaching skills.
Many companies use formal survey tools to measure employee engagement and regularly evaluate the proficiency of system leaders in the ability to engage their employees. An engineering firm executive recently told us (P.K., C.K.) that he performs detailed and frequent on‐the‐job interviews, even with company veterans. The primary goal of these interviews is to ensure that engineers spend at least 85% of their time on work that: (1) they find interesting and (2) allows for the application of their best skills. Wherever possible, traditional job descriptions are altered to achieve this. Inevitably, there is still work (15% in this particular corporate vision) that no one prefers but needs to get done, but this process of active recalibration minimizes this fraction to the degree possible.
Even within a small physician practice group, one can imagine how a strategic approach of inviting and acknowledging individual physician's professional goals and particular talents may challenge the long‐held belief that everyone within a group enjoys and must do the exact same job. Once these goals and talents are articulated, groups may find that allowing for more customized roles within the practice enhances professional satisfaction.
Social networking, collaboration, and sharing of best practices are staples of engaging companies. The Cisco and Qualcomm companies, for example, utilize elaborate e‐networks (rough corporate equivalents to Facebook) to foster collegial interaction within and across traditional hierarchical boundaries so that managers and executives directly engage the ideas of employees at every level.14 The premise is logical: engaged employees will be more likely to contribute innovative ideas which, when listened to, are more likely to engage employees.
Most physicians will recognize the traditional resident report as a model for engagement. Beyond its educational value, interaction with program leadership, social bonding, collaborative effort, and exploration of best practices add tremendous value. Many companies would jump at the chance to engrain a similar cultural staple. Enhancing this type of interaction in a postresidency setting may promote engagement in a given system, especially if it facilitates interactions between physicians and senior hospital leaders. Absent these types of interactions, ensuring regular provision of peer review and/or constructive feedback can help systematically enhance 2‐way communication and enhance engagement.
Develop
Talent development relies on mentorship reflecting a genuine interest in an individual's future. Development strategies include pairing formal annual talent reviews or (in the case of practice partners) formal peer review with strategic development plans. Effective development strategy may include transparent succession planning so that individuals are aware they are being groomed for future roles.
A well‐known adage suggests, People quit the manager or administrator, not the job. Development in this sense relies on presenting new opportunities and knowing that people flourish when allowed to explore multiple paths forward. In many companies, the role of Chief Experience or Chief Learning Officers is to enhance development planning. Consider how career coaching of young hospitalists could transform an infinitely portable and volatile commodity job, prone to burnout, into an engaged specialist of sorts with immense value to a hospital. Hospitalists have already demonstrated their potential as quality improvement leaders.15 Imagine if hospital leadership enlisted a young hospitalist in a relevant quality improvement task force, such as one working on preventing falls. With appropriate support, the physician could obtain skills for quality improvement evaluation that would not only enhance his or her engagement with the hospital system but also provide a valuable analysis for the hospital.
As an example of development strategy within a small practice setting, consider the following real‐life anecdote: a group of 4 physicians recently completed a long and expensive recruitment of a new partner. The new partner, intrigued by the local hospital's surgical robot technology, sought the support of her partners (who are not currently using the technology) to partake in an expensive robotics training program. The partners decided not to provide the financial support. The new partner subsequently left the group for a nearby practice opportunity that would provide for the training, and the group was faced with the loss of a partner (one‐fourth of the practice!) and the cost of repeating the recruiting process. A preemptive evaluation of the value of investing in the development of the new partner and enhancing that partner's professional development may have proven wise despite the significant up‐front costs. In this case the manager the new partner quit was the inflexibility of the practice trajectory.
Retain
The economic incentive to retain talented workers is not subtle. If it was, companies would not be funneling resources into Chief Experience Officers. Likewise, the estimated cost for a medical practice to replace an individual physician is as at least $250,000.16, 17
In retention, as in attraction, salary is only part of the equation.18 People want fair and competitive compensation, and may leave if they are not getting it, but they will not stay (and will not stay engaged) only for a salary bump. Retention is enhanced when workers can advance according to skills and talent, rather than mere tenure. An effective retention policy responds to people's desire to incorporate individual professional goals into their work and allows for people to customize their career rather than simply occupy a job class. Effective retention policy respects worklife balance and recognizes that this balance might look different for 2 people with the same job. It may take the form of positive reinforcement (rather than subtle disdain) for using vacation time or allowing for participation in international service projects. Many literally feel that they need to quit their job in order to take time off or explore other interests.
Worklife balance has been a longstanding issue in medicine, and innovative augmentation strategies may well help retain top talent. Today's successful medical school applicants not only show aptitude in the classroom, they often have many well‐developed nonmedical skills. No one can expect that medical training will somehow convince them to leave everything else behind. Moreover, today's residency graduates, already with Generation Y sensibilities, have completed their entire training under the auspices of the Accreditation Council for Graduate Medical Education (ACGME) duty hours regulations, which has made residents far more comfortable with shift work and defined hours.
At the other end of the generation spectrum, as large numbers of physicians ready for retirement, effective talent facilitation strategies may evaluate how to reoffer medicine as a valid option for senior physicians who still wish to work. Retaining these physicians will require an appreciation of their lifestyle goals, as they will likely find continuing a traditional practice role untenable. A recent survey of orthopedic surgeons 50 years of age or older found that having a part‐time option was a common reason they continued practicing, and that the option to work part‐time would have the most impact on keeping these surgeons working past age 65 years.19 Those working part‐time were doing so in a wide range of practice arrangements including private practice. However, one‐third of those surveyed said a part‐time option was not available to them. Clearly, in an environment of workforce shortages, physician‐leaders must begin to think about worklife balance not only for new doctors but for those considering retirement.
Critics will point out the financial drawbacks in the provision of worklife balance. But the cost may pale in comparison to the cost of replacing physician leaders. Moreover, engaged physicians are more likely to add value in the form of intangible capital such as patient satisfaction and practice innovation. As such, we argue that effective retention strategy in medicine is likely to be cost‐effective, even if it requires significant new up‐front resources.
Lessons From Industry
Doctors frequently assume that the challenges and obstacles confronted in healthcare are unique to medicine. But, for every phrase like When I started practice, I decided how long office visits were, not the insurance company, or Young doctors just don't want to work as hard, there is a parallel utterance in the greater business world. Luckily, there are now examples of the healthcare world learning lessons from business. For example, innovators in medical quality improvement found value in the experience of other industries.20 Airlines and automakers have long honed systems for error prevention and possess expertise that may curb errors in the hospital.2123
We suspect that the ideas and practice of talent facilitation have already made their way into some medical settings. A Google search reveals multiple opportunities for hospital‐based talent managers, and websites advertise the availability of talent consultants ready to lend their expertise to the medical world. In the arena of academic medicine, the University of California, San Francisco (UCSF) Division of Hospital Medicine put some of the ideas of talent facilitation into practice over the past year, in part in response to an increasingly competitive market for academic hospitalists.24 Leaders introduced a formal faculty development program that links junior faculty with mentors and facilitates early and frequent feedback across hierarchical boundaries.25 These more intentional mentoring efforts were accompanied by a seminar series aimed at the needs of new faculty members, a research incubator program, divisional grand rounds, and other web‐based and in‐person forums for sharing best practices and innovations. Less formal social events have also been promoted. Importantly, these sweeping strategies seek to encompass the needs of both teaching and nonteaching hospitalists within UCSF.26
Clearly, an academic hospitalist group with 45 faculty physicians has unique characteristics that inform the specifics of its talent facilitation strategy. The interventions discussed above are meant to represent examples of the types of strategies that may be utilized by physician groups once a decision is made to focus on talent management. Undoubtedly, the shape of such efforts will vary in diverse practice settings, but physician leaders have much to gain through further exploration of where these core principles already exist within medicine and where they may be more effectively deployed. By examining how multinational businesses are systematically applying the concepts of talent facilitation to address a global talent shortage, the doctoring profession might again take an outside hint to help inform its future.
The demand for physician talent is intensifying as the US healthcare system confronts an unprecedented confluence of demographic pressures. Not only will 78 million retiring baby‐boomers require significant healthcare resources, but tens of thousands of practicing physicians will themselves reach retirement age within the next decade.1 At the same time, factors like the large increase in the percentage of female physicians (who are more likely to work part time), the growth of nonpractice opportunities for MDs, and generational demands for greater worklife balance are creating a major supply‐demand mismatch within the physician workforce.2 In this demographic atmosphere, the ability to recruit and retain physician leaders confers tremendous value to healthcare enterprisesboth public and private. Recruiting and retaining strategies already weigh heavily in the most palpable shortage areas, like primary care, but the system faces widespread unmet demand for a variety of specialist and generalist practitioners.36
This article does not address the public policy implications of the upcoming physician shortage, recognition of which will lead to the largest increase in new medical school slots in decades.7 Rather, we set out to illustrate how successful nonmedical businesses are embracing a thoughtful, systematic approach to retaining talent, based on the philosophy that keeping and engaging valued employees is more efficient than recruiting and orienting replacements. We posit that the innovations used by progressive companies could apply to recruitment and retention challenges confronting medicine.
How Industry Approaches the Talent Vacuum: Talent Facilitation
Leaders outside of medicine have long acknowledged that changing demographics and a global economy are driving unprecedented employee turnover.8 In confronting a talent vacuum, forward‐thinking managers have prioritized retaining key talent (rather than hiring anew) in planning for the future.9, 10 Doing so begins with attempts to understand the relationship between workers and the workplace, with a particular emphasis on appreciating workers' priorities. Indeed, new executive positions with titles like Chief Learning Officer and Chief Experience Officer are appearing as companies realize a need for focused expertise beyond traditional human resource departments. These companies understand that offering higher salaries is not the only retention strategyand often not even the most effective one.
The Four Actions of Talent Facilitation
The talent facilitation process centers on four actions: attract, engage, develop, and retain. None of these actions can stand alone, and all should be present, to some degree, at all stages of a worker's tenure. To attract or engage an employee or practice partner is not a 1‐time hook, but a constant and dynamic process.
Importantly, the concepts addressed here are not specific to 1 type of corporate system, size, or management level. Although the early business focus had been on upper‐level and executive talent within large corporate settings, there is an increasing recognition that a dedicated talent strategy is useful wherever recruiting and retaining talented people is important (and where is it not?).
The ideas presented here may seem most applicable to leaders of large physician corporations, hospital‐owned physician groups, or large integrated healthcare systems (such as Kaiser) that employ physicians. However, we also believe that the ideas apply across‐the‐board in medicine, including entities such as small, private, physician‐owned groups. We argue that regardless of the exact practice structure, a limited pool of resources must be dedicated to the attraction and retention of talented partners or employees, or to the cost of replacing those people if they pursue other opportunities (or the cost of inefficient and disengaged physicians). While an integrated health system may have the resources and scale to hire a Chief Experience Officer, we do not anticipate that a 5‐partner private practice would. Rather, we point to examples to illustrate the talent facilitation paradigm as a tool to systematically frame the allocation of those resources. Undoubtedly, the specific shape of a thoughtful talent facilitation effort will vary when applied in a large urban academic medical center vs. an integrated healthcare system vs. a small physician private practice, but the basic principles remain the same.
Attract
Increasingly, companies approach talented prospects with dedicated marketing campaigns to convey the value of a work environment.11 Silicon Valley employee lounges with free massages and foosball tables are the iconic example of attraction, but the concept runs deeper. Today's workers seek access to state‐of‐the‐art ideas and technology and often want to be part of a larger vision. Many seek opportunities to integrate their own professional and personal aspirations into a particular job description.
Hospital executives have long recognized the importance of attracting physicians to their facility (after all, the physicians draw patients and thus generate revenue). The traditional approach has surrounded perks, from comfortable doctor's lounges to the latest in surgical technology. But, the stakes seem greater now than before, and successful talent facilitation strategies are going beyond the tried and true.
Clearly, physicians seek financially stable practice settings with historical success. But they may also seek evidence of a defined strategic plan focused on more than mere profitability. Physicians may gravitate to practice environments that endorse progressive movements like the No One Dies Alone campaign.12 Similarly, recognition of movements beyond healthcarea commitment to Leadership in Energy and Environmental Design (LEED) (Green Building Rating System; US Green Building Council [USGBC];
The current recruitment campaign of California's prison healthcare system offers an unlikely source of inspiration. The prison system was placed in receivership to address a shortage of competent physician staff and other inadequacies. A central feature of the campaign is an attractive starting salary and good benefits. But, the campaign does not rely on money alone. For example, the campaign's website (

Engage
The corporate tool being employed at this stage is a strategy called on‐boarding, which emphasizes a streamlined integration of newcomers with existing workers and culture, and prioritizes aligning organizational roles with a worker's specific skills and interests. On‐boarding also emphasizes the value of early and frequent provision of constructive feedback from same‐level peers or managers with advanced coaching skills.
Many companies use formal survey tools to measure employee engagement and regularly evaluate the proficiency of system leaders in the ability to engage their employees. An engineering firm executive recently told us (P.K., C.K.) that he performs detailed and frequent on‐the‐job interviews, even with company veterans. The primary goal of these interviews is to ensure that engineers spend at least 85% of their time on work that: (1) they find interesting and (2) allows for the application of their best skills. Wherever possible, traditional job descriptions are altered to achieve this. Inevitably, there is still work (15% in this particular corporate vision) that no one prefers but needs to get done, but this process of active recalibration minimizes this fraction to the degree possible.
Even within a small physician practice group, one can imagine how a strategic approach of inviting and acknowledging individual physician's professional goals and particular talents may challenge the long‐held belief that everyone within a group enjoys and must do the exact same job. Once these goals and talents are articulated, groups may find that allowing for more customized roles within the practice enhances professional satisfaction.
Social networking, collaboration, and sharing of best practices are staples of engaging companies. The Cisco and Qualcomm companies, for example, utilize elaborate e‐networks (rough corporate equivalents to Facebook) to foster collegial interaction within and across traditional hierarchical boundaries so that managers and executives directly engage the ideas of employees at every level.14 The premise is logical: engaged employees will be more likely to contribute innovative ideas which, when listened to, are more likely to engage employees.
Most physicians will recognize the traditional resident report as a model for engagement. Beyond its educational value, interaction with program leadership, social bonding, collaborative effort, and exploration of best practices add tremendous value. Many companies would jump at the chance to engrain a similar cultural staple. Enhancing this type of interaction in a postresidency setting may promote engagement in a given system, especially if it facilitates interactions between physicians and senior hospital leaders. Absent these types of interactions, ensuring regular provision of peer review and/or constructive feedback can help systematically enhance 2‐way communication and enhance engagement.
Develop
Talent development relies on mentorship reflecting a genuine interest in an individual's future. Development strategies include pairing formal annual talent reviews or (in the case of practice partners) formal peer review with strategic development plans. Effective development strategy may include transparent succession planning so that individuals are aware they are being groomed for future roles.
A well‐known adage suggests, People quit the manager or administrator, not the job. Development in this sense relies on presenting new opportunities and knowing that people flourish when allowed to explore multiple paths forward. In many companies, the role of Chief Experience or Chief Learning Officers is to enhance development planning. Consider how career coaching of young hospitalists could transform an infinitely portable and volatile commodity job, prone to burnout, into an engaged specialist of sorts with immense value to a hospital. Hospitalists have already demonstrated their potential as quality improvement leaders.15 Imagine if hospital leadership enlisted a young hospitalist in a relevant quality improvement task force, such as one working on preventing falls. With appropriate support, the physician could obtain skills for quality improvement evaluation that would not only enhance his or her engagement with the hospital system but also provide a valuable analysis for the hospital.
As an example of development strategy within a small practice setting, consider the following real‐life anecdote: a group of 4 physicians recently completed a long and expensive recruitment of a new partner. The new partner, intrigued by the local hospital's surgical robot technology, sought the support of her partners (who are not currently using the technology) to partake in an expensive robotics training program. The partners decided not to provide the financial support. The new partner subsequently left the group for a nearby practice opportunity that would provide for the training, and the group was faced with the loss of a partner (one‐fourth of the practice!) and the cost of repeating the recruiting process. A preemptive evaluation of the value of investing in the development of the new partner and enhancing that partner's professional development may have proven wise despite the significant up‐front costs. In this case the manager the new partner quit was the inflexibility of the practice trajectory.
Retain
The economic incentive to retain talented workers is not subtle. If it was, companies would not be funneling resources into Chief Experience Officers. Likewise, the estimated cost for a medical practice to replace an individual physician is as at least $250,000.16, 17
In retention, as in attraction, salary is only part of the equation.18 People want fair and competitive compensation, and may leave if they are not getting it, but they will not stay (and will not stay engaged) only for a salary bump. Retention is enhanced when workers can advance according to skills and talent, rather than mere tenure. An effective retention policy responds to people's desire to incorporate individual professional goals into their work and allows for people to customize their career rather than simply occupy a job class. Effective retention policy respects worklife balance and recognizes that this balance might look different for 2 people with the same job. It may take the form of positive reinforcement (rather than subtle disdain) for using vacation time or allowing for participation in international service projects. Many literally feel that they need to quit their job in order to take time off or explore other interests.
Worklife balance has been a longstanding issue in medicine, and innovative augmentation strategies may well help retain top talent. Today's successful medical school applicants not only show aptitude in the classroom, they often have many well‐developed nonmedical skills. No one can expect that medical training will somehow convince them to leave everything else behind. Moreover, today's residency graduates, already with Generation Y sensibilities, have completed their entire training under the auspices of the Accreditation Council for Graduate Medical Education (ACGME) duty hours regulations, which has made residents far more comfortable with shift work and defined hours.
At the other end of the generation spectrum, as large numbers of physicians ready for retirement, effective talent facilitation strategies may evaluate how to reoffer medicine as a valid option for senior physicians who still wish to work. Retaining these physicians will require an appreciation of their lifestyle goals, as they will likely find continuing a traditional practice role untenable. A recent survey of orthopedic surgeons 50 years of age or older found that having a part‐time option was a common reason they continued practicing, and that the option to work part‐time would have the most impact on keeping these surgeons working past age 65 years.19 Those working part‐time were doing so in a wide range of practice arrangements including private practice. However, one‐third of those surveyed said a part‐time option was not available to them. Clearly, in an environment of workforce shortages, physician‐leaders must begin to think about worklife balance not only for new doctors but for those considering retirement.
Critics will point out the financial drawbacks in the provision of worklife balance. But the cost may pale in comparison to the cost of replacing physician leaders. Moreover, engaged physicians are more likely to add value in the form of intangible capital such as patient satisfaction and practice innovation. As such, we argue that effective retention strategy in medicine is likely to be cost‐effective, even if it requires significant new up‐front resources.
Lessons From Industry
Doctors frequently assume that the challenges and obstacles confronted in healthcare are unique to medicine. But, for every phrase like When I started practice, I decided how long office visits were, not the insurance company, or Young doctors just don't want to work as hard, there is a parallel utterance in the greater business world. Luckily, there are now examples of the healthcare world learning lessons from business. For example, innovators in medical quality improvement found value in the experience of other industries.20 Airlines and automakers have long honed systems for error prevention and possess expertise that may curb errors in the hospital.2123
We suspect that the ideas and practice of talent facilitation have already made their way into some medical settings. A Google search reveals multiple opportunities for hospital‐based talent managers, and websites advertise the availability of talent consultants ready to lend their expertise to the medical world. In the arena of academic medicine, the University of California, San Francisco (UCSF) Division of Hospital Medicine put some of the ideas of talent facilitation into practice over the past year, in part in response to an increasingly competitive market for academic hospitalists.24 Leaders introduced a formal faculty development program that links junior faculty with mentors and facilitates early and frequent feedback across hierarchical boundaries.25 These more intentional mentoring efforts were accompanied by a seminar series aimed at the needs of new faculty members, a research incubator program, divisional grand rounds, and other web‐based and in‐person forums for sharing best practices and innovations. Less formal social events have also been promoted. Importantly, these sweeping strategies seek to encompass the needs of both teaching and nonteaching hospitalists within UCSF.26
Clearly, an academic hospitalist group with 45 faculty physicians has unique characteristics that inform the specifics of its talent facilitation strategy. The interventions discussed above are meant to represent examples of the types of strategies that may be utilized by physician groups once a decision is made to focus on talent management. Undoubtedly, the shape of such efforts will vary in diverse practice settings, but physician leaders have much to gain through further exploration of where these core principles already exist within medicine and where they may be more effectively deployed. By examining how multinational businesses are systematically applying the concepts of talent facilitation to address a global talent shortage, the doctoring profession might again take an outside hint to help inform its future.
- Long Term Care: Aging Baby Boom Generation Will Increase Demand and Burden on Federal and State Budgets. United States General Accounting Office Testimony before the Special Committee on Aging, US Senate. Hearing Before the Special Committee on Aging of the US Senate,2002. Available at:http://www.gao.gov/new.items/d02544t.pdf. Accessed July 2009.
- ,.Physician workforce shortages: implications and issues for academic health centers and policymakers.Acad Med.2006;81(9):782–787.
- .New York moves to tackle shortage of primary‐care doctors.Lancet.2008;371(9615):801–802.
- ,.The US dermatology workforce: a specialty remains in shortage.J Am Acad Dermatol.2008;59(5):741–745.
- ,.Challenges and opportunities for recruiting a new generation of neurosurgeons.Neurosurgery.2007;61(6):1314–1319.
- ,.The developing crisis in the national general surgery workforce.J Am Coll Surg.2008;206(5):790–795.
- Medical School Enrollment Plans: Analysis of the 2007 AAMC Survey. Publication of the Association of American Medical Colleges, Center for Workforce Studies, April2008. Available at:http://www.aamc.org/workforce. Accessed July 2009.
- It's 2008: Do You Know Where Your Talent Is? Why acquisition and retention strategies don't work. Part 1 of a Deloitte Research Series on Talent Management.2008. Available at: http://www.deloitte.com/dtt/cda/content/UKConsulting_TalentMgtResearchReport.pdf. Accessed August 2009.
- ,,.The race for talent: retaining and engaging workers in the 21st century.Hum Resour Plann.2004;27(3):12–25.
- Expecting sales growth, CEOs cite worker retention as critical to success. March 1,2004. Available at:http://www.barometersurveys.com/production/barsurv.nsf/89343582e94adb6185256b84006c8ffe/9672ab2f54cf99f885256e5500768232?OpenDocument. Accessed July 2009.
- Jet Blue announces aviation university gateway program for pilot candidates: airline partners with Embry‐Riddle Aeronautical University, University of North Dakota, and Cape Air to fill pilot pipeline. January 30, 2008. Available at:http://investor.jetblue.com/phoenix.zhtml?c=131045287(4):487–494.
- ,,.A review of physician turnover: rates, causes, and consequences.Am J Med Qual.2004;19(2):56–66.
- ,,.The impact on revenue of physician turnover: an assessment model and experience in a large healthcare center.J Med Pract Manage.2006;21(6):351–355.
- ,,.Employee motivation: a powerful new model.Harv Bus Rev.2008;86(7–8):78,84,160.
- ,,.Work satisfaction and retirement plans of orthopaedic surgeons 50 years of age or older.Clin Orthop Relat Res.2008;466(1):231–238.
- ,,,.The long road to patient safety: a status report on patient safety systems.JAMA.2005(22);294:2858–2865.
- .Error reduction through team leadership: what surgeons can learn from the airline industry.Clin Neurosurg.2007;54:195–199.
- ,.Applying the Toyota production system: using a patient safety alert system to reduce error.Jt Comm J Qual Patient Saf.2007;33(7):376–386.
- ,,,.Improving Papanikolaou test quality and reducing medical errors by using Toyota production system methods.Am J Obstet Gynecol.2006;194(1):57–64.
- Society of Hospital Medicine Career Satisfaction Task Force. White Paper on Hospitalist Career Satisfaction.2006; 1–45. Available at: http://www.hospitalmedicine.org. Accessed July 2009.
- UCSF Department of Medicine, Division of Hospital Medicine, Faculty Development. Available at: http://hospsrvr.ucsf.edu/cme/fds.html. Accessed July 2009.
- ,,, et al. Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3(3):247–245.
- Long Term Care: Aging Baby Boom Generation Will Increase Demand and Burden on Federal and State Budgets. United States General Accounting Office Testimony before the Special Committee on Aging, US Senate. Hearing Before the Special Committee on Aging of the US Senate,2002. Available at:http://www.gao.gov/new.items/d02544t.pdf. Accessed July 2009.
- ,.Physician workforce shortages: implications and issues for academic health centers and policymakers.Acad Med.2006;81(9):782–787.
- .New York moves to tackle shortage of primary‐care doctors.Lancet.2008;371(9615):801–802.
- ,.The US dermatology workforce: a specialty remains in shortage.J Am Acad Dermatol.2008;59(5):741–745.
- ,.Challenges and opportunities for recruiting a new generation of neurosurgeons.Neurosurgery.2007;61(6):1314–1319.
- ,.The developing crisis in the national general surgery workforce.J Am Coll Surg.2008;206(5):790–795.
- Medical School Enrollment Plans: Analysis of the 2007 AAMC Survey. Publication of the Association of American Medical Colleges, Center for Workforce Studies, April2008. Available at:http://www.aamc.org/workforce. Accessed July 2009.
- It's 2008: Do You Know Where Your Talent Is? Why acquisition and retention strategies don't work. Part 1 of a Deloitte Research Series on Talent Management.2008. Available at: http://www.deloitte.com/dtt/cda/content/UKConsulting_TalentMgtResearchReport.pdf. Accessed August 2009.
- ,,.The race for talent: retaining and engaging workers in the 21st century.Hum Resour Plann.2004;27(3):12–25.
- Expecting sales growth, CEOs cite worker retention as critical to success. March 1,2004. Available at:http://www.barometersurveys.com/production/barsurv.nsf/89343582e94adb6185256b84006c8ffe/9672ab2f54cf99f885256e5500768232?OpenDocument. Accessed July 2009.
- Jet Blue announces aviation university gateway program for pilot candidates: airline partners with Embry‐Riddle Aeronautical University, University of North Dakota, and Cape Air to fill pilot pipeline. January 30, 2008. Available at:http://investor.jetblue.com/phoenix.zhtml?c=131045287(4):487–494.
- ,,.A review of physician turnover: rates, causes, and consequences.Am J Med Qual.2004;19(2):56–66.
- ,,.The impact on revenue of physician turnover: an assessment model and experience in a large healthcare center.J Med Pract Manage.2006;21(6):351–355.
- ,,.Employee motivation: a powerful new model.Harv Bus Rev.2008;86(7–8):78,84,160.
- ,,.Work satisfaction and retirement plans of orthopaedic surgeons 50 years of age or older.Clin Orthop Relat Res.2008;466(1):231–238.
- ,,,.The long road to patient safety: a status report on patient safety systems.JAMA.2005(22);294:2858–2865.
- .Error reduction through team leadership: what surgeons can learn from the airline industry.Clin Neurosurg.2007;54:195–199.
- ,.Applying the Toyota production system: using a patient safety alert system to reduce error.Jt Comm J Qual Patient Saf.2007;33(7):376–386.
- ,,,.Improving Papanikolaou test quality and reducing medical errors by using Toyota production system methods.Am J Obstet Gynecol.2006;194(1):57–64.
- Society of Hospital Medicine Career Satisfaction Task Force. White Paper on Hospitalist Career Satisfaction.2006; 1–45. Available at: http://www.hospitalmedicine.org. Accessed July 2009.
- UCSF Department of Medicine, Division of Hospital Medicine, Faculty Development. Available at: http://hospsrvr.ucsf.edu/cme/fds.html. Accessed July 2009.
- ,,, et al. Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3(3):247–245.
Trends in Catheter Ablation for AF
Atrial fibrillation (AF), the most common clinically significant cardiac arrhythmia, affects over 2.3 million people in the United States.1 AF is associated with an increased risk of stroke and heart failure and independently increases the risk of all cause mortality.26 As such, AF confers a staggering healthcare cost burden.7, 8 Pharmacologic treatments to restore sinus rhythm in patients with AF are associated with a considerable relapse rate911 and the development of nonpharmacologic treatments for AF, such as catheter ablation procedures,1214 may be significantly more successful in restoring and maintaining sinus rhythm.15, 16 Despite relatively poor results from early catheter ablation techniques, the practice has evolved and boasts short‐term success rates as high as 73% to 91% depending on the specific type of procedure.17
In light of the success of ablative therapy, this approach, which was once used primarily in younger patients with structurally intact hearts, has been expanded to include more medically complex patients, including elderly patients, those with cardiomyopathy, and those with implanted devices.16, 18 At the same time, catheter ablation is not without complications, with major complications observed in up to 6% of cases,19 and significant costs.20 Moreover, while the most optimistic randomized control data demonstrate the ability of catheter ablation to prevent the recurrence of AF at 1 year,12, 21, 22 long‐term outcome data are lacking, particularly in patients older than 65 years or those with heart failure.17, 23
The encouraging results supporting catheter ablation continue to stimulate the utilization of catheter ablation practices and spur innovations in ablation techniques.24 The American College of Cardiology/American Heart Association/European Society of Cardiology consensus guidelines recommend consideration of ablative therapy in many instances of AF.17 AF is primarily a disease of older adults25 and although most studies have focused on younger individuals,26 it is possible that increasing numbers of older patients are receiving ablation therapy.16 Although single center studies are available,16 there are few data about the characteristics of patients undergoing ablative therapy on a national level. In order to better understand the current use of catheter ablation treatment for AF, we analyzed data from the National Hospital Discharge Survey (NHDS) to explore trends in patient characteristics and rates of ablation procedures in hospitalized patients with AF from the years 1990 to 2005.
Methods
The NHDS is a nationally representative study of hospitalized patients conducted annually by the National Center for Health Statistics,27 which collects data from approximately 270,000 inpatient records using a representative sample of about 500 short‐stay nonfederal hospitals in the United States. Data for each patient are obtained for age, sex, hospital geographic region (Northeast, Midwest, South, West), and hospital bed size, as well as up to 7 diagnostic codes and 4 procedural codes using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM). Of note, data on race/ethnicity were not consistently coded in the NHDS and are therefore not included in this analysis.
We searched for all patients age 18 years or older who had an ICD‐9‐CM diagnosis of AF (427.31). Of these patients, we then identified those who had a procedure code for nonsurgical ablation of lesions or tissues of the heart via peripherally‐inserted catheter or an endovascular approach (37.34). We also searched for specific ICD‐9‐CM‐coded diagnoses corresponding to higher stroke risk according to the (CHADS2) risk index,28 where 1 point is assigned for congestive heart failure, hypertension, age >75 years, or diabetes mellitus, and 2 points for prior stroke or transient ischemic attack. We calculated a CHADS2 score for each patient.
Statistical Analysis
Ablation rates were calculated as the number of patients with a diagnosis of AF and a code for catheter ablation divided by all patients with AF. The change in ablation rate over time was determined using simple logistic regression. Differences in ablation rates by patient and hospital characteristics were tested using chi‐square tests for categorical variables and t‐tests for continuous variables. All variables that were tested in univariate analysis (age, sex, insurance status, year of procedure, hospital region, hospital bed‐size, and CHADS2 score) were forced into the final multivariable model examining predictors of ablation. The fit of the final model was tested using the Hosmer‐Lemeshow test for goodness‐of‐fit. Nationally representative estimates were calculated from the sample weights provided by the NHDS to account for the complex sampling design of the survey. All analyses were conducted using SAS Version 9.1 (SAS Institute, Inc., Cary, NC).
Results
From 1990 to 2005, we identified 269,471 hospitalizations in the NHDS with a diagnosis of AF, of which 1,144 (0.42%) had a procedure code for catheter ablation. When extrapolated to national estimates, this corresponds to 32 million hospitalizations of patients with AF in the United States during the time period, of which 133,003 underwent ablation. The proportion of patients with AF who had ablation increased significantly over time, from 0.06% in 1990 to 0.79% in 2005 (P < 0.001 for trend; Figure 1).
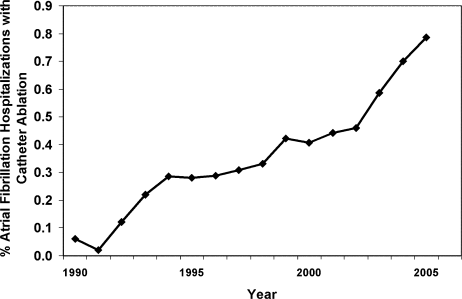
On univariate analysis, people with AF undergoing ablation were on average younger and more likely to be male than those who did not have ablation (Table 1). The rate of catheter ablation was higher in patients younger than 50 years (1.75%) compared to 0.55% in patients aged 50 to 79 years, and 0.16% in patients aged 80 years or older. However, ablation rates increased significantly in all age groups over time, with no one age group increasing at a significantly faster rate than the others (P value for interaction between age categories and hospitalization year = 0.7; Figure 2). People undergoing ablation tended to have lower CHADS2 stroke risk scores and fewer risk factors for stroke, including heart failure, coronary artery disease, and diabetes mellitus (Table 1).
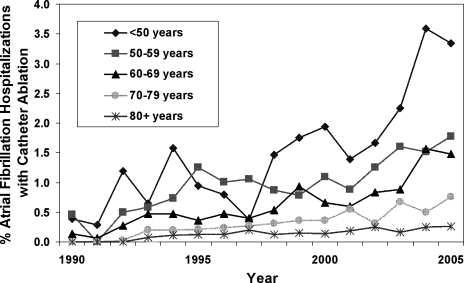
| Characteristic | Ablation (n = 1,144) | No Ablation (n = 268,327) | P Value |
|---|---|---|---|
| |||
| Age (years), mean (95% CI) | 66.0 (65.2‐66.8) | 75.9 (75.8‐75.9) | <0.001 |
| Male (%) | 56.6 | 43.4 | <0.001 |
| Insurance (%) | <0.001 | ||
| Private | 22.1 | 10.9 | |
| Medicare | 56.5 | 78.2 | |
| Medicaid | 2.2 | 2.5 | |
| Self‐pay | 0.7 | 1.2 | |
| Other/unknown | 18.5 | 7.2 | |
| Region (%) | <0.001 | ||
| West | 14.5 | 11.8 | |
| Midwest | 23.4 | 31.6 | |
| Northeast | 23.7 | 25.4 | |
| South | 39.3 | 31.2 | |
| Hospital bed size (%) | <0.001 | ||
| 6‐99 | 1.2 | 12.7 | |
| 100‐199 | 6.6 | 22.3 | |
| 200‐299 | 17.4 | 23.8 | |
| 300‐499 | 35.5 | 29.3 | |
| 500+ | 39.3 | 12.0 | |
| CHADS2 score, mean (95% CI) | 1.0 (0.9‐1.0) | 1.5 (1.5‐1.5) | <0.001 |
| CHADS2 = 0 (%) | 36.5 | 15.7 | <0.001 |
| Comorbid conditions | |||
| Heart failure (%) | 26.8 | 38.2 | <0.001 |
| Coronary artery disease (%) | 25.4 | 32.7 | <0.001 |
| Hypertension (%) | 30.8 | 29.2 | 0.24 |
| Diabetes mellitus (%) | 11.4 | 14.5 | 0.003 |
| Length of stay (days), mean (95% CI) | 5.1 (4.7‐5.5) | 7.4 (7.3‐7.4) | <0.001 |
| Discharge status (%) | <0.001 | ||
| Home | 88.8 | 58.7 | |
| Short‐term skilled facility | 0.8 | 4.06 | |
| Long‐term skilled facility | 4.0 | 18.3 | |
| Inpatient death | 1.0 | 6.7 | |
| Alive but status unknown | 5.0 | 10.9 | |
People who underwent ablation were more likely to have private insurance as their primary source of payment and less likely to have Medicare (Table 1). Ablation rates were higher among patients with AF hospitalized in the Western and Southern regions of the United States (0.52% and 0.53%, respectively), compared to rates in the Midwest (0.30%) and Northeast (0.40%). Hospital bed‐size was significantly related to the frequency of ablation, with the overall rate of ablation in patients with AF being 0.04% in hospitals with 6 to 99 beds compared to 1.37% in hospitals with at least 500 beds (P < 0.001). Length of stay was shorter in patients with ablations compared to patients without ablation therapy, and patients with ablation were more likely to be discharged home (Table 1). The inpatient mortality rate in patients undergoing ablation was quite low (0.96%).
In multivariate analysis, the likelihood of ablation therapy in a hospitalized patient with AF increased by 15% per year (95% confidence interval [CI], 13%‐16%) over the time period, adjusted for clinical and hospital characteristics. The likelihood of ablation decreased with older age (adjusted odds ratio [aOR], 0.7 [95% CI, 0.6‐0.7] for each decade of age over 50 years) and for each 1‐point increase in CHADS2 score (aOR, 0.7 [95% CI, 0.7‐0.8]). Ablation was significantly more likely to be performed in hospitals with larger bed‐sizes (aOR, 27.4 [95% CI, 16.1‐46.6] comparing bed‐size of 500+ to bed‐size of 6 to 99) and in patients with private insurance (aOR, 1.4 [95% CI, 1.2‐1.6]; Table 2). The goodness‐of‐fit of the model was appropriate, with a nonsignificant Hosmer‐Lemeshow test P value of 0.13.
| Characteristic | Adjusted Odds Ratio (95 % CI) | |
|---|---|---|
| All Patients (n = 269,471) | Subset* (n = 246,402) | |
| ||
| Age (per decade over 50 years) | 0.67 (0.64‐0.71) | 0.69 (0.64‐0.74) |
| Male | 1.0 (0.91‐1.2) | 0.88 (0.75‐1.0) |
| Insurance | ||
| Private | Ref | Ref |
| Not private | 0.73 (0.63‐0.85) | 0.70 (0.58‐0.86) |
| Other/unknown | 0.71 (0.38‐1.4) | 0.93 (0.45‐1.9) |
| Region | ||
| Northeast | Ref | Ref |
| West | 1.4 (1.2‐1.8) | 1.2 (0.95‐1.6) |
| Midwest | 0.84 (0.71‐1.0) | 0.81 (0.65‐1.0) |
| South | 1.3 (1.1‐1.5) | 1.1 (0.94‐1.4) |
| Hospital bed size | ||
| 6‐99 | Ref | Ref |
| 100‐199 | 2.8 (1.6‐4.9) | 5.0 (2.1‐11.5) |
| 200‐299 | 6.8 (4.0‐11.7) | 10.2 (4.5‐21.1) |
| 300‐499 | 11.1 (6.5‐19.0) | 16.6 (7.4‐37.3) |
| 500+ | 26.1 (15.3‐44.5) | 40.2 (17.9‐90.4) |
| CHADS2 score (per point increase) | 0.74 (0.69‐0.79) | 0.77 (0.71‐0.85) |
To account for the possibility that the ablation procedure was not specifically for AF, we performed a subgroup analysis that excluded all patients who also had diagnostic codes for supraventricular or ventricular tachycardias (427.0, 427.1, 427.2, and 427.4), or atrial flutter (427.32). Of the 269,471 hospitalizations with AF, 23,069 (8.6%) had a code for an arrhythmia in addition to AF. When we excluded patients with other arrhythmias, we identified 691 patients who underwent ablation and who only had a diagnosis of AF. An analysis of this subset yielded results similar to the full analysis (Table 2). The likelihood of ablation therapy in this subset of patients with only AF increased by 14% per year (95% CI, 11%‐16%), adjusting for patient age, sex, insurance status, CHADS2 score, hospital region, and hospital bed‐size.
Discussion
The proportion of hospitalized patients with AF who undergo ablation therapy in the United States has been increasing by approximately 15% per year over the last 15 years. Patients receiving ablation therapy are more likely to be younger, have private insurance, and have fewer stroke risk factors. These demographics likely reflect the fact that these ablations are elective procedures that are preferentially performed in healthier, lower‐risk patients. Despite these preferences, the rate of ablation therapy has been increasing significantly across all age groups, even in the oldest patients.
Though limited by relatively short follow‐up data, published studies of ablation therapies for AF show promising results,17, 26 and initial cost analyses suggest possible fiscal benefits of ablation for AF.20 Despite a paucity of randomized clinical trials comparing ablation to pharmacologic rhythm and rate control, studies suggest that quality of life may be significantly improved with ablation as compared to antiarrhythmic drugs.21 This may be because ablation may reduce AF‐related symptoms.12 As ablation becomes more widespread and recommended, physicians, including hospitalists, may be increasingly likely to refer their patients for ablation, even for patient subgroups who were not well‐represented in clinical trial settings.
The inpatient mortality rate in patients undergoing ablation therapy was quite low in our study, although ablation is not without some risk of procedure‐related stroke and other complications.19 An analysis of the compiled studies on ablation for AF estimates that major complication such as cardiac tamponade or thromboembolism occur in as many as 7% of patients.26 Patients are at highest risk for embolic events, such as transient ischemic attacks or ischemic strokes, in the immediate hours to weeks after ablation. An estimated 5% to 25% of patients will develop a new arrhythmia at some point in the postablation period and other complications, including esophageal injury, phrenic nerve injury, groin hematoma, and retroperitoneal bleed, have been observed.26 Increasing comanagement of postablation patients will necessitate that hospitalists understand the potential complications of ablation as well as current strategies for bridging anticoagulation therapy.
Few data are available about the safety and efficacy of catheter ablation for patients over the age of 65 years. In fact, the mean age of patients enrolled in most clinical trials of catheter ablation was younger than 60 years.26, 29 There are also limited data about the long‐term efficacy of ablation therapy in patients with structural heart disease30; despite this, our study shows that a quarter of patients with AF undergoing ablation therapy in the United States have diagnosed heart failure. As always, the optimistic introduction of new technologies to unstudied patient populations carries the risk of unintended harm. Hospitalists are well situated to collect and analyze outcome data for older patients with multiple comorbidities and to provide real‐time monitoring of potential complications.
Few studies have focused on the demographic and comorbid characteristics of patients undergoing ablation for AF on a national level. One study examined characteristics of patients referred to a single academic center for AF ablation from 1999 to 2005 and found that referred patients have, over time, been older (mean age 47 years in 1999 versus 56 years in 2005), have more persistent AF, larger atria, and were more likely to have had a history of cardiomyopathy (0% in 1999 versus 16% in 2006).16 This study also reported that men were consistently more likely to be referred for ablation than women. These results are generally consistent with our findings.
Our study has several limitations. The exact indication and specific type of ablation were not available in the NHDS, and it is possible that the ablation procedure was for an arrhythmia other than AF. However, our analysis of the subset of patients who only had AF as a diagnosis yielded results similar to the full analysis. We were unable to assess specific efficacy or complication data, but mortality was low and patients tended to have short hospital stays. Because the NHDS samples random hospitalizations, it is possible that some patients were overrepresented in the database if they were repeatedly hospitalized in a single year. This could potentially bias our results toward an overestimate of the number of patients who receive ablation.
It remains unclear what proportion of AF ablation procedures occur in the outpatient versus inpatient setting. Inpatient versus outpatient status is not specified in the few single‐center ablation experiences reported in the literature,16 and the few trials reported are not reliable for determining practice in a nonstudy setting. The most recent (2006) Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society Expert Consensus Statement on Catheter and Surgical Ablation of AF recommends aggressive anticoagulation in the periprocedure period with either heparin or low‐molecular‐weight heparins, followed by a bridge to warfarin.17 It makes intuitive sense that patients undergoing ablation for AF would be admitted at least overnight to bridge anticoagulation therapy and monitor for complications, but widespread use of low‐molecular‐weight heparin may make hospitalization less necessary. The observation that patients undergoing ablation had shorter hospital stays does not necessarily imply that ablation procedures shorten hospital stays. Rather, the data almost certainly reflect the fact that ablations are mostly elective procedures performed in the setting of planned short‐term admissions.
Our study provides important epidemiologic data about national trends in the use of ablation therapy in hospitalized patients with AF. We find that the rate of catheter ablation in patients with AF has been increasing significantly over time and across all age groups, including the oldest patients. As the proportion of patients with AF who receive ablation therapy continues to increase over time, comprehensive long‐term outcome data and cost‐effectiveness analyses will be important.
- ,,, et al.Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study.JAMA.2001;285(18):2370–2375.
- Atrial Fibrillation Investigators.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials.Arch Intern Med.1994;154(13):1449–1457.
- ,,,.A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study.Am J Med.2002;113(5):359–364.
- ,,,,.The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study.Am J Med.1995;98(5):476–484.
- ,,, et al.Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomized controlled trial.Lancet.2003;362(9377):7–13.
- ,,, et al.Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val‐HeFT).Am Heart J.2005;149(3):548–557.
- ,,,,.Impact of atrial fibrillation on mortality, stroke, and medical costs.Arch Intern Med.1998;158(3):229–234.
- ,,, et al.Cost of care distribution in atrial fibrillation patients: the COCAF study.Am Heart J.2004;147(1):121–126.
- ,,,,,.Serial antiarrhythmic drug treatment to maintain sinus rhythm after electrical cardioversion for chronic atrial fibrillation or atrial flutter.Am J Cardiol.1991;68(4):335–341.
- ,,, et al.Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators.N Engl J Med.2000;342(13):913–920.
- ,,, et al.Chronic atrial fibrillation. Success of serial cardioversion therapy and safety of oral anticoagulation.Arch Intern Med.1996;156(22):2585–2592.
- ,,, et al.Circumferential pulmonary‐vein ablation for chronic atrial fibrillation.N Engl J Med.2006;354(9):934–941.
- ,.Atrial fibrillation: catheter ablation.J Interv Card Electrophysiol.2006;16(1):15–26.
- ,,.Progress in nonpharmacologic therapy of atrial fibrillation.J Cardiovasc Electrophysiol.2003;14(12 Suppl):S296–S309.
- ,,,,,.Survey of physician experience, trends and outcomes with atrial fibrillation ablation.J Interv Card Electrophysiol.2005;12(3):213–220.
- ,,, et al.Characteristics of patients undergoing atrial fibrillation ablation: trends over a seven‐year period 1999–2005.J Cardiovasc Electrophysiol.2007;18(1):23–28.
- ,,, et al.ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society.Circulation.2006;114(7):e257–e354.
- ,,, et al.Safety and efficacy of radiofrequency energy catheter ablation of atrial fibrillation in patients with pacemakers and implantable cardiac defibrillators.Heart Rhythm.2005;2(12):1309–1316.
- ,,, et al.Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation.Circulation.2005;111(9):1100–1105.
- ,,,,,.Cost comparison of catheter ablation and medical therapy in atrial fibrillation.J Cardiovasc Electrophysiol.2007;18(9):907–913.
- ,,, et al.Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of symptomatic atrial fibrillation: a randomized trial.JAMA.2005;293(21):2634–2640.
- ,,, et al.A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study.J Am Coll Cardiol.2006;48(11):2340–2347.
- ,,.Atrial fibrillation in the elderly.Am J Med.2007;120(6):481–487.
- ,,,,,,.Catheter ablation for atrial fibrillation.Circulation.2007;116(13):1515–1523.
- ,,,,,.Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study).Am J Cardiol.1994;74(3):236–241.
- ,,, et al.HRS/EHRA/ECAS Expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow‐up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. European Heart Rhythm Association (EHRA), European Cardiac Arrhythmia Scoiety (ECAS), American College of Cardiology (ACC), American Heart Association (AHA), Society of Thoracic Surgeons (STS).Heart Rhythm.2007;4(6):816–861.
- U.S. Department of Health and Human Services, Public Health Service, National Center for Health Statistics National Hospital Discharge Survey 1990–2005. Multi‐Year Public‐Use Data File Documentation. Available at: http://www.cdc.gov/nchs/about/major/hdasd/nhds.htm. Accessed December2008.
- ,,,,,.Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation.JAMA.2001;285(22):2864–2870.
- ,,,.Clinical outcomes after ablation and pacing therapy for atrial fibrillation: a meta‐analysis.Circulation.2000;101(10):1138–1144.
- ,,, et al.Catheter ablation for atrial fibrillation in congestive heart failure.N Engl J Med.2004;351(23):2373–2383.
Atrial fibrillation (AF), the most common clinically significant cardiac arrhythmia, affects over 2.3 million people in the United States.1 AF is associated with an increased risk of stroke and heart failure and independently increases the risk of all cause mortality.26 As such, AF confers a staggering healthcare cost burden.7, 8 Pharmacologic treatments to restore sinus rhythm in patients with AF are associated with a considerable relapse rate911 and the development of nonpharmacologic treatments for AF, such as catheter ablation procedures,1214 may be significantly more successful in restoring and maintaining sinus rhythm.15, 16 Despite relatively poor results from early catheter ablation techniques, the practice has evolved and boasts short‐term success rates as high as 73% to 91% depending on the specific type of procedure.17
In light of the success of ablative therapy, this approach, which was once used primarily in younger patients with structurally intact hearts, has been expanded to include more medically complex patients, including elderly patients, those with cardiomyopathy, and those with implanted devices.16, 18 At the same time, catheter ablation is not without complications, with major complications observed in up to 6% of cases,19 and significant costs.20 Moreover, while the most optimistic randomized control data demonstrate the ability of catheter ablation to prevent the recurrence of AF at 1 year,12, 21, 22 long‐term outcome data are lacking, particularly in patients older than 65 years or those with heart failure.17, 23
The encouraging results supporting catheter ablation continue to stimulate the utilization of catheter ablation practices and spur innovations in ablation techniques.24 The American College of Cardiology/American Heart Association/European Society of Cardiology consensus guidelines recommend consideration of ablative therapy in many instances of AF.17 AF is primarily a disease of older adults25 and although most studies have focused on younger individuals,26 it is possible that increasing numbers of older patients are receiving ablation therapy.16 Although single center studies are available,16 there are few data about the characteristics of patients undergoing ablative therapy on a national level. In order to better understand the current use of catheter ablation treatment for AF, we analyzed data from the National Hospital Discharge Survey (NHDS) to explore trends in patient characteristics and rates of ablation procedures in hospitalized patients with AF from the years 1990 to 2005.
Methods
The NHDS is a nationally representative study of hospitalized patients conducted annually by the National Center for Health Statistics,27 which collects data from approximately 270,000 inpatient records using a representative sample of about 500 short‐stay nonfederal hospitals in the United States. Data for each patient are obtained for age, sex, hospital geographic region (Northeast, Midwest, South, West), and hospital bed size, as well as up to 7 diagnostic codes and 4 procedural codes using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM). Of note, data on race/ethnicity were not consistently coded in the NHDS and are therefore not included in this analysis.
We searched for all patients age 18 years or older who had an ICD‐9‐CM diagnosis of AF (427.31). Of these patients, we then identified those who had a procedure code for nonsurgical ablation of lesions or tissues of the heart via peripherally‐inserted catheter or an endovascular approach (37.34). We also searched for specific ICD‐9‐CM‐coded diagnoses corresponding to higher stroke risk according to the (CHADS2) risk index,28 where 1 point is assigned for congestive heart failure, hypertension, age >75 years, or diabetes mellitus, and 2 points for prior stroke or transient ischemic attack. We calculated a CHADS2 score for each patient.
Statistical Analysis
Ablation rates were calculated as the number of patients with a diagnosis of AF and a code for catheter ablation divided by all patients with AF. The change in ablation rate over time was determined using simple logistic regression. Differences in ablation rates by patient and hospital characteristics were tested using chi‐square tests for categorical variables and t‐tests for continuous variables. All variables that were tested in univariate analysis (age, sex, insurance status, year of procedure, hospital region, hospital bed‐size, and CHADS2 score) were forced into the final multivariable model examining predictors of ablation. The fit of the final model was tested using the Hosmer‐Lemeshow test for goodness‐of‐fit. Nationally representative estimates were calculated from the sample weights provided by the NHDS to account for the complex sampling design of the survey. All analyses were conducted using SAS Version 9.1 (SAS Institute, Inc., Cary, NC).
Results
From 1990 to 2005, we identified 269,471 hospitalizations in the NHDS with a diagnosis of AF, of which 1,144 (0.42%) had a procedure code for catheter ablation. When extrapolated to national estimates, this corresponds to 32 million hospitalizations of patients with AF in the United States during the time period, of which 133,003 underwent ablation. The proportion of patients with AF who had ablation increased significantly over time, from 0.06% in 1990 to 0.79% in 2005 (P < 0.001 for trend; Figure 1).

On univariate analysis, people with AF undergoing ablation were on average younger and more likely to be male than those who did not have ablation (Table 1). The rate of catheter ablation was higher in patients younger than 50 years (1.75%) compared to 0.55% in patients aged 50 to 79 years, and 0.16% in patients aged 80 years or older. However, ablation rates increased significantly in all age groups over time, with no one age group increasing at a significantly faster rate than the others (P value for interaction between age categories and hospitalization year = 0.7; Figure 2). People undergoing ablation tended to have lower CHADS2 stroke risk scores and fewer risk factors for stroke, including heart failure, coronary artery disease, and diabetes mellitus (Table 1).

| Characteristic | Ablation (n = 1,144) | No Ablation (n = 268,327) | P Value |
|---|---|---|---|
| |||
| Age (years), mean (95% CI) | 66.0 (65.2‐66.8) | 75.9 (75.8‐75.9) | <0.001 |
| Male (%) | 56.6 | 43.4 | <0.001 |
| Insurance (%) | <0.001 | ||
| Private | 22.1 | 10.9 | |
| Medicare | 56.5 | 78.2 | |
| Medicaid | 2.2 | 2.5 | |
| Self‐pay | 0.7 | 1.2 | |
| Other/unknown | 18.5 | 7.2 | |
| Region (%) | <0.001 | ||
| West | 14.5 | 11.8 | |
| Midwest | 23.4 | 31.6 | |
| Northeast | 23.7 | 25.4 | |
| South | 39.3 | 31.2 | |
| Hospital bed size (%) | <0.001 | ||
| 6‐99 | 1.2 | 12.7 | |
| 100‐199 | 6.6 | 22.3 | |
| 200‐299 | 17.4 | 23.8 | |
| 300‐499 | 35.5 | 29.3 | |
| 500+ | 39.3 | 12.0 | |
| CHADS2 score, mean (95% CI) | 1.0 (0.9‐1.0) | 1.5 (1.5‐1.5) | <0.001 |
| CHADS2 = 0 (%) | 36.5 | 15.7 | <0.001 |
| Comorbid conditions | |||
| Heart failure (%) | 26.8 | 38.2 | <0.001 |
| Coronary artery disease (%) | 25.4 | 32.7 | <0.001 |
| Hypertension (%) | 30.8 | 29.2 | 0.24 |
| Diabetes mellitus (%) | 11.4 | 14.5 | 0.003 |
| Length of stay (days), mean (95% CI) | 5.1 (4.7‐5.5) | 7.4 (7.3‐7.4) | <0.001 |
| Discharge status (%) | <0.001 | ||
| Home | 88.8 | 58.7 | |
| Short‐term skilled facility | 0.8 | 4.06 | |
| Long‐term skilled facility | 4.0 | 18.3 | |
| Inpatient death | 1.0 | 6.7 | |
| Alive but status unknown | 5.0 | 10.9 | |
People who underwent ablation were more likely to have private insurance as their primary source of payment and less likely to have Medicare (Table 1). Ablation rates were higher among patients with AF hospitalized in the Western and Southern regions of the United States (0.52% and 0.53%, respectively), compared to rates in the Midwest (0.30%) and Northeast (0.40%). Hospital bed‐size was significantly related to the frequency of ablation, with the overall rate of ablation in patients with AF being 0.04% in hospitals with 6 to 99 beds compared to 1.37% in hospitals with at least 500 beds (P < 0.001). Length of stay was shorter in patients with ablations compared to patients without ablation therapy, and patients with ablation were more likely to be discharged home (Table 1). The inpatient mortality rate in patients undergoing ablation was quite low (0.96%).
In multivariate analysis, the likelihood of ablation therapy in a hospitalized patient with AF increased by 15% per year (95% confidence interval [CI], 13%‐16%) over the time period, adjusted for clinical and hospital characteristics. The likelihood of ablation decreased with older age (adjusted odds ratio [aOR], 0.7 [95% CI, 0.6‐0.7] for each decade of age over 50 years) and for each 1‐point increase in CHADS2 score (aOR, 0.7 [95% CI, 0.7‐0.8]). Ablation was significantly more likely to be performed in hospitals with larger bed‐sizes (aOR, 27.4 [95% CI, 16.1‐46.6] comparing bed‐size of 500+ to bed‐size of 6 to 99) and in patients with private insurance (aOR, 1.4 [95% CI, 1.2‐1.6]; Table 2). The goodness‐of‐fit of the model was appropriate, with a nonsignificant Hosmer‐Lemeshow test P value of 0.13.
| Characteristic | Adjusted Odds Ratio (95 % CI) | |
|---|---|---|
| All Patients (n = 269,471) | Subset* (n = 246,402) | |
| ||
| Age (per decade over 50 years) | 0.67 (0.64‐0.71) | 0.69 (0.64‐0.74) |
| Male | 1.0 (0.91‐1.2) | 0.88 (0.75‐1.0) |
| Insurance | ||
| Private | Ref | Ref |
| Not private | 0.73 (0.63‐0.85) | 0.70 (0.58‐0.86) |
| Other/unknown | 0.71 (0.38‐1.4) | 0.93 (0.45‐1.9) |
| Region | ||
| Northeast | Ref | Ref |
| West | 1.4 (1.2‐1.8) | 1.2 (0.95‐1.6) |
| Midwest | 0.84 (0.71‐1.0) | 0.81 (0.65‐1.0) |
| South | 1.3 (1.1‐1.5) | 1.1 (0.94‐1.4) |
| Hospital bed size | ||
| 6‐99 | Ref | Ref |
| 100‐199 | 2.8 (1.6‐4.9) | 5.0 (2.1‐11.5) |
| 200‐299 | 6.8 (4.0‐11.7) | 10.2 (4.5‐21.1) |
| 300‐499 | 11.1 (6.5‐19.0) | 16.6 (7.4‐37.3) |
| 500+ | 26.1 (15.3‐44.5) | 40.2 (17.9‐90.4) |
| CHADS2 score (per point increase) | 0.74 (0.69‐0.79) | 0.77 (0.71‐0.85) |
To account for the possibility that the ablation procedure was not specifically for AF, we performed a subgroup analysis that excluded all patients who also had diagnostic codes for supraventricular or ventricular tachycardias (427.0, 427.1, 427.2, and 427.4), or atrial flutter (427.32). Of the 269,471 hospitalizations with AF, 23,069 (8.6%) had a code for an arrhythmia in addition to AF. When we excluded patients with other arrhythmias, we identified 691 patients who underwent ablation and who only had a diagnosis of AF. An analysis of this subset yielded results similar to the full analysis (Table 2). The likelihood of ablation therapy in this subset of patients with only AF increased by 14% per year (95% CI, 11%‐16%), adjusting for patient age, sex, insurance status, CHADS2 score, hospital region, and hospital bed‐size.
Discussion
The proportion of hospitalized patients with AF who undergo ablation therapy in the United States has been increasing by approximately 15% per year over the last 15 years. Patients receiving ablation therapy are more likely to be younger, have private insurance, and have fewer stroke risk factors. These demographics likely reflect the fact that these ablations are elective procedures that are preferentially performed in healthier, lower‐risk patients. Despite these preferences, the rate of ablation therapy has been increasing significantly across all age groups, even in the oldest patients.
Though limited by relatively short follow‐up data, published studies of ablation therapies for AF show promising results,17, 26 and initial cost analyses suggest possible fiscal benefits of ablation for AF.20 Despite a paucity of randomized clinical trials comparing ablation to pharmacologic rhythm and rate control, studies suggest that quality of life may be significantly improved with ablation as compared to antiarrhythmic drugs.21 This may be because ablation may reduce AF‐related symptoms.12 As ablation becomes more widespread and recommended, physicians, including hospitalists, may be increasingly likely to refer their patients for ablation, even for patient subgroups who were not well‐represented in clinical trial settings.
The inpatient mortality rate in patients undergoing ablation therapy was quite low in our study, although ablation is not without some risk of procedure‐related stroke and other complications.19 An analysis of the compiled studies on ablation for AF estimates that major complication such as cardiac tamponade or thromboembolism occur in as many as 7% of patients.26 Patients are at highest risk for embolic events, such as transient ischemic attacks or ischemic strokes, in the immediate hours to weeks after ablation. An estimated 5% to 25% of patients will develop a new arrhythmia at some point in the postablation period and other complications, including esophageal injury, phrenic nerve injury, groin hematoma, and retroperitoneal bleed, have been observed.26 Increasing comanagement of postablation patients will necessitate that hospitalists understand the potential complications of ablation as well as current strategies for bridging anticoagulation therapy.
Few data are available about the safety and efficacy of catheter ablation for patients over the age of 65 years. In fact, the mean age of patients enrolled in most clinical trials of catheter ablation was younger than 60 years.26, 29 There are also limited data about the long‐term efficacy of ablation therapy in patients with structural heart disease30; despite this, our study shows that a quarter of patients with AF undergoing ablation therapy in the United States have diagnosed heart failure. As always, the optimistic introduction of new technologies to unstudied patient populations carries the risk of unintended harm. Hospitalists are well situated to collect and analyze outcome data for older patients with multiple comorbidities and to provide real‐time monitoring of potential complications.
Few studies have focused on the demographic and comorbid characteristics of patients undergoing ablation for AF on a national level. One study examined characteristics of patients referred to a single academic center for AF ablation from 1999 to 2005 and found that referred patients have, over time, been older (mean age 47 years in 1999 versus 56 years in 2005), have more persistent AF, larger atria, and were more likely to have had a history of cardiomyopathy (0% in 1999 versus 16% in 2006).16 This study also reported that men were consistently more likely to be referred for ablation than women. These results are generally consistent with our findings.
Our study has several limitations. The exact indication and specific type of ablation were not available in the NHDS, and it is possible that the ablation procedure was for an arrhythmia other than AF. However, our analysis of the subset of patients who only had AF as a diagnosis yielded results similar to the full analysis. We were unable to assess specific efficacy or complication data, but mortality was low and patients tended to have short hospital stays. Because the NHDS samples random hospitalizations, it is possible that some patients were overrepresented in the database if they were repeatedly hospitalized in a single year. This could potentially bias our results toward an overestimate of the number of patients who receive ablation.
It remains unclear what proportion of AF ablation procedures occur in the outpatient versus inpatient setting. Inpatient versus outpatient status is not specified in the few single‐center ablation experiences reported in the literature,16 and the few trials reported are not reliable for determining practice in a nonstudy setting. The most recent (2006) Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society Expert Consensus Statement on Catheter and Surgical Ablation of AF recommends aggressive anticoagulation in the periprocedure period with either heparin or low‐molecular‐weight heparins, followed by a bridge to warfarin.17 It makes intuitive sense that patients undergoing ablation for AF would be admitted at least overnight to bridge anticoagulation therapy and monitor for complications, but widespread use of low‐molecular‐weight heparin may make hospitalization less necessary. The observation that patients undergoing ablation had shorter hospital stays does not necessarily imply that ablation procedures shorten hospital stays. Rather, the data almost certainly reflect the fact that ablations are mostly elective procedures performed in the setting of planned short‐term admissions.
Our study provides important epidemiologic data about national trends in the use of ablation therapy in hospitalized patients with AF. We find that the rate of catheter ablation in patients with AF has been increasing significantly over time and across all age groups, including the oldest patients. As the proportion of patients with AF who receive ablation therapy continues to increase over time, comprehensive long‐term outcome data and cost‐effectiveness analyses will be important.
Atrial fibrillation (AF), the most common clinically significant cardiac arrhythmia, affects over 2.3 million people in the United States.1 AF is associated with an increased risk of stroke and heart failure and independently increases the risk of all cause mortality.26 As such, AF confers a staggering healthcare cost burden.7, 8 Pharmacologic treatments to restore sinus rhythm in patients with AF are associated with a considerable relapse rate911 and the development of nonpharmacologic treatments for AF, such as catheter ablation procedures,1214 may be significantly more successful in restoring and maintaining sinus rhythm.15, 16 Despite relatively poor results from early catheter ablation techniques, the practice has evolved and boasts short‐term success rates as high as 73% to 91% depending on the specific type of procedure.17
In light of the success of ablative therapy, this approach, which was once used primarily in younger patients with structurally intact hearts, has been expanded to include more medically complex patients, including elderly patients, those with cardiomyopathy, and those with implanted devices.16, 18 At the same time, catheter ablation is not without complications, with major complications observed in up to 6% of cases,19 and significant costs.20 Moreover, while the most optimistic randomized control data demonstrate the ability of catheter ablation to prevent the recurrence of AF at 1 year,12, 21, 22 long‐term outcome data are lacking, particularly in patients older than 65 years or those with heart failure.17, 23
The encouraging results supporting catheter ablation continue to stimulate the utilization of catheter ablation practices and spur innovations in ablation techniques.24 The American College of Cardiology/American Heart Association/European Society of Cardiology consensus guidelines recommend consideration of ablative therapy in many instances of AF.17 AF is primarily a disease of older adults25 and although most studies have focused on younger individuals,26 it is possible that increasing numbers of older patients are receiving ablation therapy.16 Although single center studies are available,16 there are few data about the characteristics of patients undergoing ablative therapy on a national level. In order to better understand the current use of catheter ablation treatment for AF, we analyzed data from the National Hospital Discharge Survey (NHDS) to explore trends in patient characteristics and rates of ablation procedures in hospitalized patients with AF from the years 1990 to 2005.
Methods
The NHDS is a nationally representative study of hospitalized patients conducted annually by the National Center for Health Statistics,27 which collects data from approximately 270,000 inpatient records using a representative sample of about 500 short‐stay nonfederal hospitals in the United States. Data for each patient are obtained for age, sex, hospital geographic region (Northeast, Midwest, South, West), and hospital bed size, as well as up to 7 diagnostic codes and 4 procedural codes using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM). Of note, data on race/ethnicity were not consistently coded in the NHDS and are therefore not included in this analysis.
We searched for all patients age 18 years or older who had an ICD‐9‐CM diagnosis of AF (427.31). Of these patients, we then identified those who had a procedure code for nonsurgical ablation of lesions or tissues of the heart via peripherally‐inserted catheter or an endovascular approach (37.34). We also searched for specific ICD‐9‐CM‐coded diagnoses corresponding to higher stroke risk according to the (CHADS2) risk index,28 where 1 point is assigned for congestive heart failure, hypertension, age >75 years, or diabetes mellitus, and 2 points for prior stroke or transient ischemic attack. We calculated a CHADS2 score for each patient.
Statistical Analysis
Ablation rates were calculated as the number of patients with a diagnosis of AF and a code for catheter ablation divided by all patients with AF. The change in ablation rate over time was determined using simple logistic regression. Differences in ablation rates by patient and hospital characteristics were tested using chi‐square tests for categorical variables and t‐tests for continuous variables. All variables that were tested in univariate analysis (age, sex, insurance status, year of procedure, hospital region, hospital bed‐size, and CHADS2 score) were forced into the final multivariable model examining predictors of ablation. The fit of the final model was tested using the Hosmer‐Lemeshow test for goodness‐of‐fit. Nationally representative estimates were calculated from the sample weights provided by the NHDS to account for the complex sampling design of the survey. All analyses were conducted using SAS Version 9.1 (SAS Institute, Inc., Cary, NC).
Results
From 1990 to 2005, we identified 269,471 hospitalizations in the NHDS with a diagnosis of AF, of which 1,144 (0.42%) had a procedure code for catheter ablation. When extrapolated to national estimates, this corresponds to 32 million hospitalizations of patients with AF in the United States during the time period, of which 133,003 underwent ablation. The proportion of patients with AF who had ablation increased significantly over time, from 0.06% in 1990 to 0.79% in 2005 (P < 0.001 for trend; Figure 1).

On univariate analysis, people with AF undergoing ablation were on average younger and more likely to be male than those who did not have ablation (Table 1). The rate of catheter ablation was higher in patients younger than 50 years (1.75%) compared to 0.55% in patients aged 50 to 79 years, and 0.16% in patients aged 80 years or older. However, ablation rates increased significantly in all age groups over time, with no one age group increasing at a significantly faster rate than the others (P value for interaction between age categories and hospitalization year = 0.7; Figure 2). People undergoing ablation tended to have lower CHADS2 stroke risk scores and fewer risk factors for stroke, including heart failure, coronary artery disease, and diabetes mellitus (Table 1).

| Characteristic | Ablation (n = 1,144) | No Ablation (n = 268,327) | P Value |
|---|---|---|---|
| |||
| Age (years), mean (95% CI) | 66.0 (65.2‐66.8) | 75.9 (75.8‐75.9) | <0.001 |
| Male (%) | 56.6 | 43.4 | <0.001 |
| Insurance (%) | <0.001 | ||
| Private | 22.1 | 10.9 | |
| Medicare | 56.5 | 78.2 | |
| Medicaid | 2.2 | 2.5 | |
| Self‐pay | 0.7 | 1.2 | |
| Other/unknown | 18.5 | 7.2 | |
| Region (%) | <0.001 | ||
| West | 14.5 | 11.8 | |
| Midwest | 23.4 | 31.6 | |
| Northeast | 23.7 | 25.4 | |
| South | 39.3 | 31.2 | |
| Hospital bed size (%) | <0.001 | ||
| 6‐99 | 1.2 | 12.7 | |
| 100‐199 | 6.6 | 22.3 | |
| 200‐299 | 17.4 | 23.8 | |
| 300‐499 | 35.5 | 29.3 | |
| 500+ | 39.3 | 12.0 | |
| CHADS2 score, mean (95% CI) | 1.0 (0.9‐1.0) | 1.5 (1.5‐1.5) | <0.001 |
| CHADS2 = 0 (%) | 36.5 | 15.7 | <0.001 |
| Comorbid conditions | |||
| Heart failure (%) | 26.8 | 38.2 | <0.001 |
| Coronary artery disease (%) | 25.4 | 32.7 | <0.001 |
| Hypertension (%) | 30.8 | 29.2 | 0.24 |
| Diabetes mellitus (%) | 11.4 | 14.5 | 0.003 |
| Length of stay (days), mean (95% CI) | 5.1 (4.7‐5.5) | 7.4 (7.3‐7.4) | <0.001 |
| Discharge status (%) | <0.001 | ||
| Home | 88.8 | 58.7 | |
| Short‐term skilled facility | 0.8 | 4.06 | |
| Long‐term skilled facility | 4.0 | 18.3 | |
| Inpatient death | 1.0 | 6.7 | |
| Alive but status unknown | 5.0 | 10.9 | |
People who underwent ablation were more likely to have private insurance as their primary source of payment and less likely to have Medicare (Table 1). Ablation rates were higher among patients with AF hospitalized in the Western and Southern regions of the United States (0.52% and 0.53%, respectively), compared to rates in the Midwest (0.30%) and Northeast (0.40%). Hospital bed‐size was significantly related to the frequency of ablation, with the overall rate of ablation in patients with AF being 0.04% in hospitals with 6 to 99 beds compared to 1.37% in hospitals with at least 500 beds (P < 0.001). Length of stay was shorter in patients with ablations compared to patients without ablation therapy, and patients with ablation were more likely to be discharged home (Table 1). The inpatient mortality rate in patients undergoing ablation was quite low (0.96%).
In multivariate analysis, the likelihood of ablation therapy in a hospitalized patient with AF increased by 15% per year (95% confidence interval [CI], 13%‐16%) over the time period, adjusted for clinical and hospital characteristics. The likelihood of ablation decreased with older age (adjusted odds ratio [aOR], 0.7 [95% CI, 0.6‐0.7] for each decade of age over 50 years) and for each 1‐point increase in CHADS2 score (aOR, 0.7 [95% CI, 0.7‐0.8]). Ablation was significantly more likely to be performed in hospitals with larger bed‐sizes (aOR, 27.4 [95% CI, 16.1‐46.6] comparing bed‐size of 500+ to bed‐size of 6 to 99) and in patients with private insurance (aOR, 1.4 [95% CI, 1.2‐1.6]; Table 2). The goodness‐of‐fit of the model was appropriate, with a nonsignificant Hosmer‐Lemeshow test P value of 0.13.
| Characteristic | Adjusted Odds Ratio (95 % CI) | |
|---|---|---|
| All Patients (n = 269,471) | Subset* (n = 246,402) | |
| ||
| Age (per decade over 50 years) | 0.67 (0.64‐0.71) | 0.69 (0.64‐0.74) |
| Male | 1.0 (0.91‐1.2) | 0.88 (0.75‐1.0) |
| Insurance | ||
| Private | Ref | Ref |
| Not private | 0.73 (0.63‐0.85) | 0.70 (0.58‐0.86) |
| Other/unknown | 0.71 (0.38‐1.4) | 0.93 (0.45‐1.9) |
| Region | ||
| Northeast | Ref | Ref |
| West | 1.4 (1.2‐1.8) | 1.2 (0.95‐1.6) |
| Midwest | 0.84 (0.71‐1.0) | 0.81 (0.65‐1.0) |
| South | 1.3 (1.1‐1.5) | 1.1 (0.94‐1.4) |
| Hospital bed size | ||
| 6‐99 | Ref | Ref |
| 100‐199 | 2.8 (1.6‐4.9) | 5.0 (2.1‐11.5) |
| 200‐299 | 6.8 (4.0‐11.7) | 10.2 (4.5‐21.1) |
| 300‐499 | 11.1 (6.5‐19.0) | 16.6 (7.4‐37.3) |
| 500+ | 26.1 (15.3‐44.5) | 40.2 (17.9‐90.4) |
| CHADS2 score (per point increase) | 0.74 (0.69‐0.79) | 0.77 (0.71‐0.85) |
To account for the possibility that the ablation procedure was not specifically for AF, we performed a subgroup analysis that excluded all patients who also had diagnostic codes for supraventricular or ventricular tachycardias (427.0, 427.1, 427.2, and 427.4), or atrial flutter (427.32). Of the 269,471 hospitalizations with AF, 23,069 (8.6%) had a code for an arrhythmia in addition to AF. When we excluded patients with other arrhythmias, we identified 691 patients who underwent ablation and who only had a diagnosis of AF. An analysis of this subset yielded results similar to the full analysis (Table 2). The likelihood of ablation therapy in this subset of patients with only AF increased by 14% per year (95% CI, 11%‐16%), adjusting for patient age, sex, insurance status, CHADS2 score, hospital region, and hospital bed‐size.
Discussion
The proportion of hospitalized patients with AF who undergo ablation therapy in the United States has been increasing by approximately 15% per year over the last 15 years. Patients receiving ablation therapy are more likely to be younger, have private insurance, and have fewer stroke risk factors. These demographics likely reflect the fact that these ablations are elective procedures that are preferentially performed in healthier, lower‐risk patients. Despite these preferences, the rate of ablation therapy has been increasing significantly across all age groups, even in the oldest patients.
Though limited by relatively short follow‐up data, published studies of ablation therapies for AF show promising results,17, 26 and initial cost analyses suggest possible fiscal benefits of ablation for AF.20 Despite a paucity of randomized clinical trials comparing ablation to pharmacologic rhythm and rate control, studies suggest that quality of life may be significantly improved with ablation as compared to antiarrhythmic drugs.21 This may be because ablation may reduce AF‐related symptoms.12 As ablation becomes more widespread and recommended, physicians, including hospitalists, may be increasingly likely to refer their patients for ablation, even for patient subgroups who were not well‐represented in clinical trial settings.
The inpatient mortality rate in patients undergoing ablation therapy was quite low in our study, although ablation is not without some risk of procedure‐related stroke and other complications.19 An analysis of the compiled studies on ablation for AF estimates that major complication such as cardiac tamponade or thromboembolism occur in as many as 7% of patients.26 Patients are at highest risk for embolic events, such as transient ischemic attacks or ischemic strokes, in the immediate hours to weeks after ablation. An estimated 5% to 25% of patients will develop a new arrhythmia at some point in the postablation period and other complications, including esophageal injury, phrenic nerve injury, groin hematoma, and retroperitoneal bleed, have been observed.26 Increasing comanagement of postablation patients will necessitate that hospitalists understand the potential complications of ablation as well as current strategies for bridging anticoagulation therapy.
Few data are available about the safety and efficacy of catheter ablation for patients over the age of 65 years. In fact, the mean age of patients enrolled in most clinical trials of catheter ablation was younger than 60 years.26, 29 There are also limited data about the long‐term efficacy of ablation therapy in patients with structural heart disease30; despite this, our study shows that a quarter of patients with AF undergoing ablation therapy in the United States have diagnosed heart failure. As always, the optimistic introduction of new technologies to unstudied patient populations carries the risk of unintended harm. Hospitalists are well situated to collect and analyze outcome data for older patients with multiple comorbidities and to provide real‐time monitoring of potential complications.
Few studies have focused on the demographic and comorbid characteristics of patients undergoing ablation for AF on a national level. One study examined characteristics of patients referred to a single academic center for AF ablation from 1999 to 2005 and found that referred patients have, over time, been older (mean age 47 years in 1999 versus 56 years in 2005), have more persistent AF, larger atria, and were more likely to have had a history of cardiomyopathy (0% in 1999 versus 16% in 2006).16 This study also reported that men were consistently more likely to be referred for ablation than women. These results are generally consistent with our findings.
Our study has several limitations. The exact indication and specific type of ablation were not available in the NHDS, and it is possible that the ablation procedure was for an arrhythmia other than AF. However, our analysis of the subset of patients who only had AF as a diagnosis yielded results similar to the full analysis. We were unable to assess specific efficacy or complication data, but mortality was low and patients tended to have short hospital stays. Because the NHDS samples random hospitalizations, it is possible that some patients were overrepresented in the database if they were repeatedly hospitalized in a single year. This could potentially bias our results toward an overestimate of the number of patients who receive ablation.
It remains unclear what proportion of AF ablation procedures occur in the outpatient versus inpatient setting. Inpatient versus outpatient status is not specified in the few single‐center ablation experiences reported in the literature,16 and the few trials reported are not reliable for determining practice in a nonstudy setting. The most recent (2006) Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society Expert Consensus Statement on Catheter and Surgical Ablation of AF recommends aggressive anticoagulation in the periprocedure period with either heparin or low‐molecular‐weight heparins, followed by a bridge to warfarin.17 It makes intuitive sense that patients undergoing ablation for AF would be admitted at least overnight to bridge anticoagulation therapy and monitor for complications, but widespread use of low‐molecular‐weight heparin may make hospitalization less necessary. The observation that patients undergoing ablation had shorter hospital stays does not necessarily imply that ablation procedures shorten hospital stays. Rather, the data almost certainly reflect the fact that ablations are mostly elective procedures performed in the setting of planned short‐term admissions.
Our study provides important epidemiologic data about national trends in the use of ablation therapy in hospitalized patients with AF. We find that the rate of catheter ablation in patients with AF has been increasing significantly over time and across all age groups, including the oldest patients. As the proportion of patients with AF who receive ablation therapy continues to increase over time, comprehensive long‐term outcome data and cost‐effectiveness analyses will be important.
- ,,, et al.Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study.JAMA.2001;285(18):2370–2375.
- Atrial Fibrillation Investigators.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials.Arch Intern Med.1994;154(13):1449–1457.
- ,,,.A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study.Am J Med.2002;113(5):359–364.
- ,,,,.The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study.Am J Med.1995;98(5):476–484.
- ,,, et al.Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomized controlled trial.Lancet.2003;362(9377):7–13.
- ,,, et al.Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val‐HeFT).Am Heart J.2005;149(3):548–557.
- ,,,,.Impact of atrial fibrillation on mortality, stroke, and medical costs.Arch Intern Med.1998;158(3):229–234.
- ,,, et al.Cost of care distribution in atrial fibrillation patients: the COCAF study.Am Heart J.2004;147(1):121–126.
- ,,,,,.Serial antiarrhythmic drug treatment to maintain sinus rhythm after electrical cardioversion for chronic atrial fibrillation or atrial flutter.Am J Cardiol.1991;68(4):335–341.
- ,,, et al.Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators.N Engl J Med.2000;342(13):913–920.
- ,,, et al.Chronic atrial fibrillation. Success of serial cardioversion therapy and safety of oral anticoagulation.Arch Intern Med.1996;156(22):2585–2592.
- ,,, et al.Circumferential pulmonary‐vein ablation for chronic atrial fibrillation.N Engl J Med.2006;354(9):934–941.
- ,.Atrial fibrillation: catheter ablation.J Interv Card Electrophysiol.2006;16(1):15–26.
- ,,.Progress in nonpharmacologic therapy of atrial fibrillation.J Cardiovasc Electrophysiol.2003;14(12 Suppl):S296–S309.
- ,,,,,.Survey of physician experience, trends and outcomes with atrial fibrillation ablation.J Interv Card Electrophysiol.2005;12(3):213–220.
- ,,, et al.Characteristics of patients undergoing atrial fibrillation ablation: trends over a seven‐year period 1999–2005.J Cardiovasc Electrophysiol.2007;18(1):23–28.
- ,,, et al.ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society.Circulation.2006;114(7):e257–e354.
- ,,, et al.Safety and efficacy of radiofrequency energy catheter ablation of atrial fibrillation in patients with pacemakers and implantable cardiac defibrillators.Heart Rhythm.2005;2(12):1309–1316.
- ,,, et al.Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation.Circulation.2005;111(9):1100–1105.
- ,,,,,.Cost comparison of catheter ablation and medical therapy in atrial fibrillation.J Cardiovasc Electrophysiol.2007;18(9):907–913.
- ,,, et al.Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of symptomatic atrial fibrillation: a randomized trial.JAMA.2005;293(21):2634–2640.
- ,,, et al.A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study.J Am Coll Cardiol.2006;48(11):2340–2347.
- ,,.Atrial fibrillation in the elderly.Am J Med.2007;120(6):481–487.
- ,,,,,,.Catheter ablation for atrial fibrillation.Circulation.2007;116(13):1515–1523.
- ,,,,,.Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study).Am J Cardiol.1994;74(3):236–241.
- ,,, et al.HRS/EHRA/ECAS Expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow‐up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. European Heart Rhythm Association (EHRA), European Cardiac Arrhythmia Scoiety (ECAS), American College of Cardiology (ACC), American Heart Association (AHA), Society of Thoracic Surgeons (STS).Heart Rhythm.2007;4(6):816–861.
- U.S. Department of Health and Human Services, Public Health Service, National Center for Health Statistics National Hospital Discharge Survey 1990–2005. Multi‐Year Public‐Use Data File Documentation. Available at: http://www.cdc.gov/nchs/about/major/hdasd/nhds.htm. Accessed December2008.
- ,,,,,.Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation.JAMA.2001;285(22):2864–2870.
- ,,,.Clinical outcomes after ablation and pacing therapy for atrial fibrillation: a meta‐analysis.Circulation.2000;101(10):1138–1144.
- ,,, et al.Catheter ablation for atrial fibrillation in congestive heart failure.N Engl J Med.2004;351(23):2373–2383.
- ,,, et al.Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study.JAMA.2001;285(18):2370–2375.
- Atrial Fibrillation Investigators.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials.Arch Intern Med.1994;154(13):1449–1457.
- ,,,.A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study.Am J Med.2002;113(5):359–364.
- ,,,,.The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study.Am J Med.1995;98(5):476–484.
- ,,, et al.Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomized controlled trial.Lancet.2003;362(9377):7–13.
- ,,, et al.Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val‐HeFT).Am Heart J.2005;149(3):548–557.
- ,,,,.Impact of atrial fibrillation on mortality, stroke, and medical costs.Arch Intern Med.1998;158(3):229–234.
- ,,, et al.Cost of care distribution in atrial fibrillation patients: the COCAF study.Am Heart J.2004;147(1):121–126.
- ,,,,,.Serial antiarrhythmic drug treatment to maintain sinus rhythm after electrical cardioversion for chronic atrial fibrillation or atrial flutter.Am J Cardiol.1991;68(4):335–341.
- ,,, et al.Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators.N Engl J Med.2000;342(13):913–920.
- ,,, et al.Chronic atrial fibrillation. Success of serial cardioversion therapy and safety of oral anticoagulation.Arch Intern Med.1996;156(22):2585–2592.
- ,,, et al.Circumferential pulmonary‐vein ablation for chronic atrial fibrillation.N Engl J Med.2006;354(9):934–941.
- ,.Atrial fibrillation: catheter ablation.J Interv Card Electrophysiol.2006;16(1):15–26.
- ,,.Progress in nonpharmacologic therapy of atrial fibrillation.J Cardiovasc Electrophysiol.2003;14(12 Suppl):S296–S309.
- ,,,,,.Survey of physician experience, trends and outcomes with atrial fibrillation ablation.J Interv Card Electrophysiol.2005;12(3):213–220.
- ,,, et al.Characteristics of patients undergoing atrial fibrillation ablation: trends over a seven‐year period 1999–2005.J Cardiovasc Electrophysiol.2007;18(1):23–28.
- ,,, et al.ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society.Circulation.2006;114(7):e257–e354.
- ,,, et al.Safety and efficacy of radiofrequency energy catheter ablation of atrial fibrillation in patients with pacemakers and implantable cardiac defibrillators.Heart Rhythm.2005;2(12):1309–1316.
- ,,, et al.Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation.Circulation.2005;111(9):1100–1105.
- ,,,,,.Cost comparison of catheter ablation and medical therapy in atrial fibrillation.J Cardiovasc Electrophysiol.2007;18(9):907–913.
- ,,, et al.Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of symptomatic atrial fibrillation: a randomized trial.JAMA.2005;293(21):2634–2640.
- ,,, et al.A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study.J Am Coll Cardiol.2006;48(11):2340–2347.
- ,,.Atrial fibrillation in the elderly.Am J Med.2007;120(6):481–487.
- ,,,,,,.Catheter ablation for atrial fibrillation.Circulation.2007;116(13):1515–1523.
- ,,,,,.Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study).Am J Cardiol.1994;74(3):236–241.
- ,,, et al.HRS/EHRA/ECAS Expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow‐up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. European Heart Rhythm Association (EHRA), European Cardiac Arrhythmia Scoiety (ECAS), American College of Cardiology (ACC), American Heart Association (AHA), Society of Thoracic Surgeons (STS).Heart Rhythm.2007;4(6):816–861.
- U.S. Department of Health and Human Services, Public Health Service, National Center for Health Statistics National Hospital Discharge Survey 1990–2005. Multi‐Year Public‐Use Data File Documentation. Available at: http://www.cdc.gov/nchs/about/major/hdasd/nhds.htm. Accessed December2008.
- ,,,,,.Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation.JAMA.2001;285(22):2864–2870.
- ,,,.Clinical outcomes after ablation and pacing therapy for atrial fibrillation: a meta‐analysis.Circulation.2000;101(10):1138–1144.
- ,,, et al.Catheter ablation for atrial fibrillation in congestive heart failure.N Engl J Med.2004;351(23):2373–2383.
Copyright © 2009 Society of Hospital Medicine