User login
NAMDRC legislative initiatives take shape
Two priorities of NAMDRC have moved into the formal congressional arena. The issues focus on access to pulmonary rehabilitation and CMS’s move to include home mechanical ventilation in competitive bidding.
Pulmonary Rehabilitation – The Problem: One of the major concerns to CMS and Congress is the fact that different payment methodologies for the same service result in different payment amounts dependent upon the actual site of service. To address the phenomenon of hospitals purchasing certain physician practices to game the payment system, Congress included in the 2015 Budget Act a provision that would remove incentives for such hospital purchases by stating that new hospital outpatient services must be within 250 yards of the main hospital campus in order to receive payment based on the hospital outpatient prospective payment system methodology. If a hospital opens such services beyond that 250-yard threshold, the hospital would be reimbursed at the physician fee schedule amount for the same service. Likewise, if an off campus program moved its grandfathered location because of expansion, loss of lease, etc, the physician fee schedule would again kick in.
For pulmonary rehabilitation services, this is extremely problematic and is tying the hands of hospitals providing this service. The physician fee schedule payment for pulmonary rehabilitation is less than $30 for 1 hour of service, and it is, therefore, not surprising that the service is simply not provided in physician offices. In fact, Medicare data show that all physician specialties bill less than $1M for code G0424, and we believe that most of that is likely billing error. Pulmonologists bill less than $500K for code G0424, and putting that number in context, the entire Medicare program is approaching $700B in outlays.
Pulmonary Rehabilitation – The Solution: As a solution to this problem, HR 4838 has been introduced in the House of Representatives. There is no specific reference to pulmonary rehabilitation in the bill as our approach is based not only on substance but political considerations, as well. Using CMS’ own acknowledgment of “unintended consequences,” this legislation would exempt all CPT codes from the restrictions imposed by Section 603 of the 2015 Budget Act when the physician billings for that code are under $2M for the most recent year for which data are available. CMS has signaled to us that such a limitation would apply only to pulmonary and cardiac rehab services, but others may be affected, as well. By putting a dollar limit rather than identifying a specific service for such a “carve out,” it is a more politically viable approach.
Bills such as this rarely see the light of day; however, such provisions are often attached to larger, more substantive bills. For nearly 2 decades, the common legislative vehicle for such provisions is a larger Medicare bill, often including “must pass Medicare extender” provisions that are slated to expire on a particular date. Our goal is to include HR 4838 in such a package of extenders some time between now and the end of this Congress in 2020.
Home Mechanical Ventilation – The Problem: CMS has proposed inclusion of home mechanical ventilation in competitive bidding for durable medical equipment. Such a regulatory proposal is fraught with downside risk, most notably that such a policy would follow the history of liquid oxygen. Liquid 02 has virtually disappeared from the marketplace since it was included in competitive bidding as suppliers simply refused to provide liquid oxygen systems as their own bidding dropped the price to prohibitively low levels. Also, because there is a statutory requirement that such payment be made on the basis of “frequent and substantial servicing,” and that stipulation could trigger wide variations in actual bidding because some states require involvement of respiratory therapists in such services, while others do not.
It is critical to understand that the driving force behind all of this is the reality that CMS’ own coverage policies for home mechanical ventilation are seriously flawed and outdated, creating perverse incentives for physicians to order easily accessible systems rather than clinically appropriate ones. NAMDRC and its sister societies have been pushing CMS to revise those policies with no success.
Home Mechanical Ventilation – The Solution: Our solution is twofold. HR 4945 bill was introduced on November 1, 2019. First, the proposed legislation would create a blanket exemption for home mechanical ventilation from competitive bidding. Second, it requires CMS to convene a technical expert panel to craft up-to-date policies for home mechanical ventilation.
The political strategy here is slightly different. While passage of the bill is certainly our first choice, we believe that introduction of the bill is a red flag signal to CMS for the need to revise its coverage policies as those policies are the root cause of the growth of home mechanical ventilation outlays.
Two priorities of NAMDRC have moved into the formal congressional arena. The issues focus on access to pulmonary rehabilitation and CMS’s move to include home mechanical ventilation in competitive bidding.
Pulmonary Rehabilitation – The Problem: One of the major concerns to CMS and Congress is the fact that different payment methodologies for the same service result in different payment amounts dependent upon the actual site of service. To address the phenomenon of hospitals purchasing certain physician practices to game the payment system, Congress included in the 2015 Budget Act a provision that would remove incentives for such hospital purchases by stating that new hospital outpatient services must be within 250 yards of the main hospital campus in order to receive payment based on the hospital outpatient prospective payment system methodology. If a hospital opens such services beyond that 250-yard threshold, the hospital would be reimbursed at the physician fee schedule amount for the same service. Likewise, if an off campus program moved its grandfathered location because of expansion, loss of lease, etc, the physician fee schedule would again kick in.
For pulmonary rehabilitation services, this is extremely problematic and is tying the hands of hospitals providing this service. The physician fee schedule payment for pulmonary rehabilitation is less than $30 for 1 hour of service, and it is, therefore, not surprising that the service is simply not provided in physician offices. In fact, Medicare data show that all physician specialties bill less than $1M for code G0424, and we believe that most of that is likely billing error. Pulmonologists bill less than $500K for code G0424, and putting that number in context, the entire Medicare program is approaching $700B in outlays.
Pulmonary Rehabilitation – The Solution: As a solution to this problem, HR 4838 has been introduced in the House of Representatives. There is no specific reference to pulmonary rehabilitation in the bill as our approach is based not only on substance but political considerations, as well. Using CMS’ own acknowledgment of “unintended consequences,” this legislation would exempt all CPT codes from the restrictions imposed by Section 603 of the 2015 Budget Act when the physician billings for that code are under $2M for the most recent year for which data are available. CMS has signaled to us that such a limitation would apply only to pulmonary and cardiac rehab services, but others may be affected, as well. By putting a dollar limit rather than identifying a specific service for such a “carve out,” it is a more politically viable approach.
Bills such as this rarely see the light of day; however, such provisions are often attached to larger, more substantive bills. For nearly 2 decades, the common legislative vehicle for such provisions is a larger Medicare bill, often including “must pass Medicare extender” provisions that are slated to expire on a particular date. Our goal is to include HR 4838 in such a package of extenders some time between now and the end of this Congress in 2020.
Home Mechanical Ventilation – The Problem: CMS has proposed inclusion of home mechanical ventilation in competitive bidding for durable medical equipment. Such a regulatory proposal is fraught with downside risk, most notably that such a policy would follow the history of liquid oxygen. Liquid 02 has virtually disappeared from the marketplace since it was included in competitive bidding as suppliers simply refused to provide liquid oxygen systems as their own bidding dropped the price to prohibitively low levels. Also, because there is a statutory requirement that such payment be made on the basis of “frequent and substantial servicing,” and that stipulation could trigger wide variations in actual bidding because some states require involvement of respiratory therapists in such services, while others do not.
It is critical to understand that the driving force behind all of this is the reality that CMS’ own coverage policies for home mechanical ventilation are seriously flawed and outdated, creating perverse incentives for physicians to order easily accessible systems rather than clinically appropriate ones. NAMDRC and its sister societies have been pushing CMS to revise those policies with no success.
Home Mechanical Ventilation – The Solution: Our solution is twofold. HR 4945 bill was introduced on November 1, 2019. First, the proposed legislation would create a blanket exemption for home mechanical ventilation from competitive bidding. Second, it requires CMS to convene a technical expert panel to craft up-to-date policies for home mechanical ventilation.
The political strategy here is slightly different. While passage of the bill is certainly our first choice, we believe that introduction of the bill is a red flag signal to CMS for the need to revise its coverage policies as those policies are the root cause of the growth of home mechanical ventilation outlays.
Two priorities of NAMDRC have moved into the formal congressional arena. The issues focus on access to pulmonary rehabilitation and CMS’s move to include home mechanical ventilation in competitive bidding.
Pulmonary Rehabilitation – The Problem: One of the major concerns to CMS and Congress is the fact that different payment methodologies for the same service result in different payment amounts dependent upon the actual site of service. To address the phenomenon of hospitals purchasing certain physician practices to game the payment system, Congress included in the 2015 Budget Act a provision that would remove incentives for such hospital purchases by stating that new hospital outpatient services must be within 250 yards of the main hospital campus in order to receive payment based on the hospital outpatient prospective payment system methodology. If a hospital opens such services beyond that 250-yard threshold, the hospital would be reimbursed at the physician fee schedule amount for the same service. Likewise, if an off campus program moved its grandfathered location because of expansion, loss of lease, etc, the physician fee schedule would again kick in.
For pulmonary rehabilitation services, this is extremely problematic and is tying the hands of hospitals providing this service. The physician fee schedule payment for pulmonary rehabilitation is less than $30 for 1 hour of service, and it is, therefore, not surprising that the service is simply not provided in physician offices. In fact, Medicare data show that all physician specialties bill less than $1M for code G0424, and we believe that most of that is likely billing error. Pulmonologists bill less than $500K for code G0424, and putting that number in context, the entire Medicare program is approaching $700B in outlays.
Pulmonary Rehabilitation – The Solution: As a solution to this problem, HR 4838 has been introduced in the House of Representatives. There is no specific reference to pulmonary rehabilitation in the bill as our approach is based not only on substance but political considerations, as well. Using CMS’ own acknowledgment of “unintended consequences,” this legislation would exempt all CPT codes from the restrictions imposed by Section 603 of the 2015 Budget Act when the physician billings for that code are under $2M for the most recent year for which data are available. CMS has signaled to us that such a limitation would apply only to pulmonary and cardiac rehab services, but others may be affected, as well. By putting a dollar limit rather than identifying a specific service for such a “carve out,” it is a more politically viable approach.
Bills such as this rarely see the light of day; however, such provisions are often attached to larger, more substantive bills. For nearly 2 decades, the common legislative vehicle for such provisions is a larger Medicare bill, often including “must pass Medicare extender” provisions that are slated to expire on a particular date. Our goal is to include HR 4838 in such a package of extenders some time between now and the end of this Congress in 2020.
Home Mechanical Ventilation – The Problem: CMS has proposed inclusion of home mechanical ventilation in competitive bidding for durable medical equipment. Such a regulatory proposal is fraught with downside risk, most notably that such a policy would follow the history of liquid oxygen. Liquid 02 has virtually disappeared from the marketplace since it was included in competitive bidding as suppliers simply refused to provide liquid oxygen systems as their own bidding dropped the price to prohibitively low levels. Also, because there is a statutory requirement that such payment be made on the basis of “frequent and substantial servicing,” and that stipulation could trigger wide variations in actual bidding because some states require involvement of respiratory therapists in such services, while others do not.
It is critical to understand that the driving force behind all of this is the reality that CMS’ own coverage policies for home mechanical ventilation are seriously flawed and outdated, creating perverse incentives for physicians to order easily accessible systems rather than clinically appropriate ones. NAMDRC and its sister societies have been pushing CMS to revise those policies with no success.
Home Mechanical Ventilation – The Solution: Our solution is twofold. HR 4945 bill was introduced on November 1, 2019. First, the proposed legislation would create a blanket exemption for home mechanical ventilation from competitive bidding. Second, it requires CMS to convene a technical expert panel to craft up-to-date policies for home mechanical ventilation.
The political strategy here is slightly different. While passage of the bill is certainly our first choice, we believe that introduction of the bill is a red flag signal to CMS for the need to revise its coverage policies as those policies are the root cause of the growth of home mechanical ventilation outlays.
CMS proposal threatens entire landscape for home mechanical ventilators
CMS announced in a [press release in mid-March that as it revamped the competitive bidding program for durable medical equipment, it would move to include no invasive ventilation (NIV) in the revamped program, slated to take effect January 1, 2021.
While the implementation date is still more than 18 months in the future, the regulatory timetable for a formal announcement, as well as time for CMS to introduce its revamped bidding process, actually creates a relatively short window for aggressive action to thwart the CMS proposal.
In late November 2018, when CMS was seeking public comment on the idea of such a move, CHEST, NAMDRC and numerous other societies submitted strongly worded comments opposed to the recommendation, citing a wide array of clinical risks associated with such a proposal. The comments also highlighted CMS’ total failure to revamp its own coverage policies, frequently cited by the pulmonary medicine community and the Office of the Inspector General as the primary root cause for significant problems.
Background: Under current law, Medicare is required to pay for certain ventilators under a “frequent and substantial servicing” payment methodology, with payment continuing as long as medical necessity is documented. Nearly 2 decades ago, CMS (then HCFA) sought to circumvent those statutory requirements by declaring that some ventilators are really not ventilators (as FDA classifications indicate) but are actually “respiratory assist devices.” The long-term impact of that unilateral policy decision has been ongoing chaos, as well as flawed coverage policies. For example, it is much more challenging for a physician to order a cheaper bi-level device than to order a ventilator for treatment of “respiratory failure.” As there are no limitations or qualifying criteria tied to “respiratory failure,” the community has responded with the path of least resistance while pleading with CMS to restructure their coverage policies to reflect the standards of care for home mechanical ventilation.
Since 2014, the community has repeatedly tried to convince CMS of the importance, and cost savings, associated with such a revamp, to no avail. Given 5 years of well documented efforts, it is likely that the only genuine solution will be a legislative one that forces CMS to behave in certain ways.
The challenges: There are complicating variables that the clinical community will need to address:
1. If the term “ventilator” is included in any legislative effort, CMS could expand its infamous concept “just because FDA calls a device a ventilator doesn’t make it one.” Using particular CPT or HCPCS codes would open the door for CMS to simply change coding to circumvent legislative intent.
2. If a legislative effort receives serious support, it ought to include specific guidance to CMS to force it to change its coverage policies for home mechanical ventilation to reflect standards of care and state-of-the-art devices.
For example, because devices are designed today to serve a wide range of respiratory issues, one device may be used to provide critical life support for an ALS patient, while that same device could also be used to provide nocturnal or intermittent support for other neuromuscular or COPD patients. Because the durable medical equipment benefit is focused on devices, CMS’ move to change to focus from a device to a patient is questionable.
3. Forcing CMS to move in a particular direction regarding coverage and device usage must be flexible enough to allow for technological and medical innovations; after all, no one wants to recommend legislative policies that would have to be revisited to address potential/likely advances in this field.
Broad strategies: While the durable medical equipment community is also challenging this proposal, they agreed that the medical and patient communities should take the lead. And, in principle, we agree. But implementation of that effort is a bit of a challenge as it requires a significant grassroots effort from concerned physicians, as well as patient groups to contact their legislators in Congress. After all, the worst case scenario is for a Senator to say, “How come I haven’t heard from any constituents about this problem if it is as bad as you say it is?” That is a fair and common refrain, and we must be prepared to engage the broad physician and patient communities to ensure success in this effort.
Once there is formal introduction of a proposal to move this matter forward, there will be outreach to physicians and respiratory therapists across the country to urge support of the legislation. Keep watching for such requests for action!
CMS announced in a [press release in mid-March that as it revamped the competitive bidding program for durable medical equipment, it would move to include no invasive ventilation (NIV) in the revamped program, slated to take effect January 1, 2021.
While the implementation date is still more than 18 months in the future, the regulatory timetable for a formal announcement, as well as time for CMS to introduce its revamped bidding process, actually creates a relatively short window for aggressive action to thwart the CMS proposal.
In late November 2018, when CMS was seeking public comment on the idea of such a move, CHEST, NAMDRC and numerous other societies submitted strongly worded comments opposed to the recommendation, citing a wide array of clinical risks associated with such a proposal. The comments also highlighted CMS’ total failure to revamp its own coverage policies, frequently cited by the pulmonary medicine community and the Office of the Inspector General as the primary root cause for significant problems.
Background: Under current law, Medicare is required to pay for certain ventilators under a “frequent and substantial servicing” payment methodology, with payment continuing as long as medical necessity is documented. Nearly 2 decades ago, CMS (then HCFA) sought to circumvent those statutory requirements by declaring that some ventilators are really not ventilators (as FDA classifications indicate) but are actually “respiratory assist devices.” The long-term impact of that unilateral policy decision has been ongoing chaos, as well as flawed coverage policies. For example, it is much more challenging for a physician to order a cheaper bi-level device than to order a ventilator for treatment of “respiratory failure.” As there are no limitations or qualifying criteria tied to “respiratory failure,” the community has responded with the path of least resistance while pleading with CMS to restructure their coverage policies to reflect the standards of care for home mechanical ventilation.
Since 2014, the community has repeatedly tried to convince CMS of the importance, and cost savings, associated with such a revamp, to no avail. Given 5 years of well documented efforts, it is likely that the only genuine solution will be a legislative one that forces CMS to behave in certain ways.
The challenges: There are complicating variables that the clinical community will need to address:
1. If the term “ventilator” is included in any legislative effort, CMS could expand its infamous concept “just because FDA calls a device a ventilator doesn’t make it one.” Using particular CPT or HCPCS codes would open the door for CMS to simply change coding to circumvent legislative intent.
2. If a legislative effort receives serious support, it ought to include specific guidance to CMS to force it to change its coverage policies for home mechanical ventilation to reflect standards of care and state-of-the-art devices.
For example, because devices are designed today to serve a wide range of respiratory issues, one device may be used to provide critical life support for an ALS patient, while that same device could also be used to provide nocturnal or intermittent support for other neuromuscular or COPD patients. Because the durable medical equipment benefit is focused on devices, CMS’ move to change to focus from a device to a patient is questionable.
3. Forcing CMS to move in a particular direction regarding coverage and device usage must be flexible enough to allow for technological and medical innovations; after all, no one wants to recommend legislative policies that would have to be revisited to address potential/likely advances in this field.
Broad strategies: While the durable medical equipment community is also challenging this proposal, they agreed that the medical and patient communities should take the lead. And, in principle, we agree. But implementation of that effort is a bit of a challenge as it requires a significant grassroots effort from concerned physicians, as well as patient groups to contact their legislators in Congress. After all, the worst case scenario is for a Senator to say, “How come I haven’t heard from any constituents about this problem if it is as bad as you say it is?” That is a fair and common refrain, and we must be prepared to engage the broad physician and patient communities to ensure success in this effort.
Once there is formal introduction of a proposal to move this matter forward, there will be outreach to physicians and respiratory therapists across the country to urge support of the legislation. Keep watching for such requests for action!
CMS announced in a [press release in mid-March that as it revamped the competitive bidding program for durable medical equipment, it would move to include no invasive ventilation (NIV) in the revamped program, slated to take effect January 1, 2021.
While the implementation date is still more than 18 months in the future, the regulatory timetable for a formal announcement, as well as time for CMS to introduce its revamped bidding process, actually creates a relatively short window for aggressive action to thwart the CMS proposal.
In late November 2018, when CMS was seeking public comment on the idea of such a move, CHEST, NAMDRC and numerous other societies submitted strongly worded comments opposed to the recommendation, citing a wide array of clinical risks associated with such a proposal. The comments also highlighted CMS’ total failure to revamp its own coverage policies, frequently cited by the pulmonary medicine community and the Office of the Inspector General as the primary root cause for significant problems.
Background: Under current law, Medicare is required to pay for certain ventilators under a “frequent and substantial servicing” payment methodology, with payment continuing as long as medical necessity is documented. Nearly 2 decades ago, CMS (then HCFA) sought to circumvent those statutory requirements by declaring that some ventilators are really not ventilators (as FDA classifications indicate) but are actually “respiratory assist devices.” The long-term impact of that unilateral policy decision has been ongoing chaos, as well as flawed coverage policies. For example, it is much more challenging for a physician to order a cheaper bi-level device than to order a ventilator for treatment of “respiratory failure.” As there are no limitations or qualifying criteria tied to “respiratory failure,” the community has responded with the path of least resistance while pleading with CMS to restructure their coverage policies to reflect the standards of care for home mechanical ventilation.
Since 2014, the community has repeatedly tried to convince CMS of the importance, and cost savings, associated with such a revamp, to no avail. Given 5 years of well documented efforts, it is likely that the only genuine solution will be a legislative one that forces CMS to behave in certain ways.
The challenges: There are complicating variables that the clinical community will need to address:
1. If the term “ventilator” is included in any legislative effort, CMS could expand its infamous concept “just because FDA calls a device a ventilator doesn’t make it one.” Using particular CPT or HCPCS codes would open the door for CMS to simply change coding to circumvent legislative intent.
2. If a legislative effort receives serious support, it ought to include specific guidance to CMS to force it to change its coverage policies for home mechanical ventilation to reflect standards of care and state-of-the-art devices.
For example, because devices are designed today to serve a wide range of respiratory issues, one device may be used to provide critical life support for an ALS patient, while that same device could also be used to provide nocturnal or intermittent support for other neuromuscular or COPD patients. Because the durable medical equipment benefit is focused on devices, CMS’ move to change to focus from a device to a patient is questionable.
3. Forcing CMS to move in a particular direction regarding coverage and device usage must be flexible enough to allow for technological and medical innovations; after all, no one wants to recommend legislative policies that would have to be revisited to address potential/likely advances in this field.
Broad strategies: While the durable medical equipment community is also challenging this proposal, they agreed that the medical and patient communities should take the lead. And, in principle, we agree. But implementation of that effort is a bit of a challenge as it requires a significant grassroots effort from concerned physicians, as well as patient groups to contact their legislators in Congress. After all, the worst case scenario is for a Senator to say, “How come I haven’t heard from any constituents about this problem if it is as bad as you say it is?” That is a fair and common refrain, and we must be prepared to engage the broad physician and patient communities to ensure success in this effort.
Once there is formal introduction of a proposal to move this matter forward, there will be outreach to physicians and respiratory therapists across the country to urge support of the legislation. Keep watching for such requests for action!
NAMDRC update
NAMDRC focuses on keeping its members informed on legislative and regulatory issues impacting their practices.
Today, those agreements have been replaced by employment contracts or simply disappeared entirely, replaced by various business models that have invariably shifted the focus of coverage and payment issues away from the group practice into significantly different financial incentives. The challenge for NAMDRC is to keep its members informed about structural changes in coverage and payment rules that could impact their decision making. In November 2018, CMS published three distinctly separate sets of rules slated to take effect in 2019, all of which affect physicians in the pulmonary, critical care, and sleep landscapes. Through the monthly membership publication, the Washington Watchline, members get timely information that impact their practices. Excerpts from a recent Watchline include:
Physician fee schedule: As most physicians know, CMS had proposed dramatic changes to payment for Level 4 and Level 5 E&M codes, but due to strong reaction from many within the medical community, CMS is withdrawing that specific proposal, at least in the short term. Related provisions include:
• For CY 2019 and 2020, CMS will continue the current coding and payment structure for E/M office/outpatient visits,
• Effective January 1, 2019, for new and established patients for E/M office/outpatient visits, practitioners need not re-enter in the medical record information on the patient’s chief complaint and history that has already been entered by ancillary staff or the beneficiary. The practitioner may simply indicate in the medical record that he or she reviewed and verified this information.
• For 2021, CMS is finalizing a significant reduction in the current payment variation in office/outpatient E/M visit levels by paying a single rate for E/M office/outpatient visit levels 2, 3, and 4 (one for established and another for new patients) beginning in 2021. However, CMS is not finalizing the inclusion of E/M office/outpatient level 5 visits in the single payment rate, to better account for the care and needs of particularly complex patients.
• CMS policy for 2021 will adopt add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of specialized medical care. As discussed further below, these codes will only be reportable with E/M office/outpatient level 2 through 4 visits, and their use generally will not impose new per-visit documentation requirements.
Hospital outpatient rules: There are two particularly relevant issues addressed in this final regulation. The payment rates for pulmonary rehab are:
Pulmonary Rehab via G0424 – APC 5733, $55.90 with co-pay of $11.18
Pulmonary Rehab via G0237, 38, 39 – APC 5732, $32.12 with co-pay of $6.43
This regulation is also the vehicle for CMS addressing issues related to Section 603/site of service payment issues. As physicians know, CMS enacted Section 603 of the 23015 Budget Act that puts notable restrictions on payment for certain hospital outpatient services provided off campus (more than 250 yards from main campus of the hospital). NAMDRC is most concerned about the impact on pulmonary rehab – under the rules, off-campus programs that are grandfathered (“excepted” is the CMS term) as long as they were billing for those services at that location November 2015. However, if a hospital chooses to open a new program, or relocate an existing program to a different location, the payment principles that apply are physician fee schedule rates rather than hospital outpatient rates. In the proposed rule posted this past July, CMS had proposed that even a new service provided in an excepted (grandfathered) setting would be subject to PFS payment rates rather than hospital outpatient rates. CMS has withdrawn that proposal for the coming year, so new services in excepted settings will be covered. “Excepted” is actually CMS’ terminology, which is used to refer to off-campus outpatient facilities that were offering services in November 2015. Services that do not meet that singular criterion are considered nonexcepted (not grandfathered), and those services are paid at the physician fee schedule rate.
DME: In its proposed rule this past summer, CMS actually acknowledged flaws in the structure of the competitive bidding system for DME (including oxygen, CPAP, and certain ventilators referred to by CMS as respiratory assist devices). Specifically, related to oxygen, there is also acknowledgement of reductions in liquid oxygen utilization, a story we have been pushing for years. The CMS proposed rule would have tied liquid portable payment rates to portable concentrator and transfill system payment rates, a genuine bump in actual $$. More than a dozen societies joined to respond to the proposed rule, including NAMDRC, CHEST, and ATS.
In the final rule, CMS is moving forward with its proposal, acknowledging that it will need to monitor shifts in the oxygen marketplace and adjust their payment policies accordingly.
NAMDRC focuses on keeping its members informed on legislative and regulatory issues impacting their practices.
Today, those agreements have been replaced by employment contracts or simply disappeared entirely, replaced by various business models that have invariably shifted the focus of coverage and payment issues away from the group practice into significantly different financial incentives. The challenge for NAMDRC is to keep its members informed about structural changes in coverage and payment rules that could impact their decision making. In November 2018, CMS published three distinctly separate sets of rules slated to take effect in 2019, all of which affect physicians in the pulmonary, critical care, and sleep landscapes. Through the monthly membership publication, the Washington Watchline, members get timely information that impact their practices. Excerpts from a recent Watchline include:
Physician fee schedule: As most physicians know, CMS had proposed dramatic changes to payment for Level 4 and Level 5 E&M codes, but due to strong reaction from many within the medical community, CMS is withdrawing that specific proposal, at least in the short term. Related provisions include:
• For CY 2019 and 2020, CMS will continue the current coding and payment structure for E/M office/outpatient visits,
• Effective January 1, 2019, for new and established patients for E/M office/outpatient visits, practitioners need not re-enter in the medical record information on the patient’s chief complaint and history that has already been entered by ancillary staff or the beneficiary. The practitioner may simply indicate in the medical record that he or she reviewed and verified this information.
• For 2021, CMS is finalizing a significant reduction in the current payment variation in office/outpatient E/M visit levels by paying a single rate for E/M office/outpatient visit levels 2, 3, and 4 (one for established and another for new patients) beginning in 2021. However, CMS is not finalizing the inclusion of E/M office/outpatient level 5 visits in the single payment rate, to better account for the care and needs of particularly complex patients.
• CMS policy for 2021 will adopt add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of specialized medical care. As discussed further below, these codes will only be reportable with E/M office/outpatient level 2 through 4 visits, and their use generally will not impose new per-visit documentation requirements.
Hospital outpatient rules: There are two particularly relevant issues addressed in this final regulation. The payment rates for pulmonary rehab are:
Pulmonary Rehab via G0424 – APC 5733, $55.90 with co-pay of $11.18
Pulmonary Rehab via G0237, 38, 39 – APC 5732, $32.12 with co-pay of $6.43
This regulation is also the vehicle for CMS addressing issues related to Section 603/site of service payment issues. As physicians know, CMS enacted Section 603 of the 23015 Budget Act that puts notable restrictions on payment for certain hospital outpatient services provided off campus (more than 250 yards from main campus of the hospital). NAMDRC is most concerned about the impact on pulmonary rehab – under the rules, off-campus programs that are grandfathered (“excepted” is the CMS term) as long as they were billing for those services at that location November 2015. However, if a hospital chooses to open a new program, or relocate an existing program to a different location, the payment principles that apply are physician fee schedule rates rather than hospital outpatient rates. In the proposed rule posted this past July, CMS had proposed that even a new service provided in an excepted (grandfathered) setting would be subject to PFS payment rates rather than hospital outpatient rates. CMS has withdrawn that proposal for the coming year, so new services in excepted settings will be covered. “Excepted” is actually CMS’ terminology, which is used to refer to off-campus outpatient facilities that were offering services in November 2015. Services that do not meet that singular criterion are considered nonexcepted (not grandfathered), and those services are paid at the physician fee schedule rate.
DME: In its proposed rule this past summer, CMS actually acknowledged flaws in the structure of the competitive bidding system for DME (including oxygen, CPAP, and certain ventilators referred to by CMS as respiratory assist devices). Specifically, related to oxygen, there is also acknowledgement of reductions in liquid oxygen utilization, a story we have been pushing for years. The CMS proposed rule would have tied liquid portable payment rates to portable concentrator and transfill system payment rates, a genuine bump in actual $$. More than a dozen societies joined to respond to the proposed rule, including NAMDRC, CHEST, and ATS.
In the final rule, CMS is moving forward with its proposal, acknowledging that it will need to monitor shifts in the oxygen marketplace and adjust their payment policies accordingly.
NAMDRC focuses on keeping its members informed on legislative and regulatory issues impacting their practices.
Today, those agreements have been replaced by employment contracts or simply disappeared entirely, replaced by various business models that have invariably shifted the focus of coverage and payment issues away from the group practice into significantly different financial incentives. The challenge for NAMDRC is to keep its members informed about structural changes in coverage and payment rules that could impact their decision making. In November 2018, CMS published three distinctly separate sets of rules slated to take effect in 2019, all of which affect physicians in the pulmonary, critical care, and sleep landscapes. Through the monthly membership publication, the Washington Watchline, members get timely information that impact their practices. Excerpts from a recent Watchline include:
Physician fee schedule: As most physicians know, CMS had proposed dramatic changes to payment for Level 4 and Level 5 E&M codes, but due to strong reaction from many within the medical community, CMS is withdrawing that specific proposal, at least in the short term. Related provisions include:
• For CY 2019 and 2020, CMS will continue the current coding and payment structure for E/M office/outpatient visits,
• Effective January 1, 2019, for new and established patients for E/M office/outpatient visits, practitioners need not re-enter in the medical record information on the patient’s chief complaint and history that has already been entered by ancillary staff or the beneficiary. The practitioner may simply indicate in the medical record that he or she reviewed and verified this information.
• For 2021, CMS is finalizing a significant reduction in the current payment variation in office/outpatient E/M visit levels by paying a single rate for E/M office/outpatient visit levels 2, 3, and 4 (one for established and another for new patients) beginning in 2021. However, CMS is not finalizing the inclusion of E/M office/outpatient level 5 visits in the single payment rate, to better account for the care and needs of particularly complex patients.
• CMS policy for 2021 will adopt add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of specialized medical care. As discussed further below, these codes will only be reportable with E/M office/outpatient level 2 through 4 visits, and their use generally will not impose new per-visit documentation requirements.
Hospital outpatient rules: There are two particularly relevant issues addressed in this final regulation. The payment rates for pulmonary rehab are:
Pulmonary Rehab via G0424 – APC 5733, $55.90 with co-pay of $11.18
Pulmonary Rehab via G0237, 38, 39 – APC 5732, $32.12 with co-pay of $6.43
This regulation is also the vehicle for CMS addressing issues related to Section 603/site of service payment issues. As physicians know, CMS enacted Section 603 of the 23015 Budget Act that puts notable restrictions on payment for certain hospital outpatient services provided off campus (more than 250 yards from main campus of the hospital). NAMDRC is most concerned about the impact on pulmonary rehab – under the rules, off-campus programs that are grandfathered (“excepted” is the CMS term) as long as they were billing for those services at that location November 2015. However, if a hospital chooses to open a new program, or relocate an existing program to a different location, the payment principles that apply are physician fee schedule rates rather than hospital outpatient rates. In the proposed rule posted this past July, CMS had proposed that even a new service provided in an excepted (grandfathered) setting would be subject to PFS payment rates rather than hospital outpatient rates. CMS has withdrawn that proposal for the coming year, so new services in excepted settings will be covered. “Excepted” is actually CMS’ terminology, which is used to refer to off-campus outpatient facilities that were offering services in November 2015. Services that do not meet that singular criterion are considered nonexcepted (not grandfathered), and those services are paid at the physician fee schedule rate.
DME: In its proposed rule this past summer, CMS actually acknowledged flaws in the structure of the competitive bidding system for DME (including oxygen, CPAP, and certain ventilators referred to by CMS as respiratory assist devices). Specifically, related to oxygen, there is also acknowledgement of reductions in liquid oxygen utilization, a story we have been pushing for years. The CMS proposed rule would have tied liquid portable payment rates to portable concentrator and transfill system payment rates, a genuine bump in actual $$. More than a dozen societies joined to respond to the proposed rule, including NAMDRC, CHEST, and ATS.
In the final rule, CMS is moving forward with its proposal, acknowledging that it will need to monitor shifts in the oxygen marketplace and adjust their payment policies accordingly.
NAMDRC update
NAMDRC focuses on keeping its members informed on legislative and regulatory issues impacting their practices
NAMDRC’s mission statement clearly signals its commitment to improve access to quality care for patients with respiratory disease by removing regulatory and legislative barriers to appropriate treatment. Adhering to that commitment presents challenges in the rapidly changing structure of the delivery of health care. For example, 10 years ago, the majority of NAMDRC members were private practitioners/group practices, many with contracts to provide a range of services to institutions. While those agreements varied, the underlying principles were relatively constant – structure your agreements that were mutually beneficial to physician and hospital.
Today, those agreements have been replaced by employment contracts or simply disappeared entirely, replaced by various business models that have invariably shifted the focus of coverage and payment issues away from the group practice into significantly different financial incentives. The challenge for NAMDRC is to keep its members informed about structural changes in coverage and payment rules that could impact their decision making. In November 2018, CMS published three distinctly separate sets of rules slated to take effect in 2019, all of which affect physicians in the pulmonary, critical care, and sleep landscapes. Through the monthly membership publication, the Washington Watchline, members get timely information that impact their practices. Excerpts from a recent Watchline include:
Physician fee schedule: As most physicians know, CMS had proposed dramatic changes to payment for Level 4 and Level % E&M codes, but due to strong reaction from man within the medical community, CMS is withdrawing that specific proposal, at least in the short term. Related provisions include:
• For CY 2019 and 2020, CMS will continue the current coding and payment structure for E/M office/outpatient visits,
• Effective January 1, 2019, for new and established patients for E/M office/outpatient visits, practitioners need not re-enter in the medical record information on the patient’s chief complaint and history that has already been entered by ancillary staff or the beneficiary. The practitioner may simply indicate in the medical record that he or she reviewed and verified this information.
• For 2021, CMS is finalizing a significant reduction in the current payment variation in office/outpatient E/M visit levels by paying a single rate for E/M office/outpatient visit levels 2, 3, and 4 (one for established and another for new patients) beginning in 2021. However, CMS is not finalizing the inclusion of E/M office/outpatient level 5 visits in the single payment rate, to better account for the care and needs of particularly complex patients.
• CMS policy for 2021 will adopt add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of specialized medical care. As discussed further below, these codes will only be reportable with E/M office/outpatient level 2 through 4 visits, and their use generally will not impose new per-visit documentation requirements.
Hospital outpatient rules: There are two particularly relevant issues addressed in this final regulation. The payment rates for pulmonary rehab are:
• Pulmonary Rehab via G0424 – APC 5733, $55.90 with co-pay of $11.18
• Pulmonary Rehab via G0237, 38, 39 – APC 5732, $32.12 with co-pay of $6.43
This regulation is also the vehicle for CMS addressing issues related to Section 603/site of service payment issues. As physicians know, CMS enacted Section 603 of the 23015 Budget Act that puts notable restrictions on payment for certain hospital outpatient services provided off campus (more than 250 yards from main campus of the hospital). NAMDRC is most concerned about the impact on pulmonary rehab – under the rules, off-campus programs that are grandfathered (“excepted” is the CMS term) as long as they were billing for those services at that location November 2015. However, if a hospital chooses to open a new program, or relocate an existing program to a different location, the payment principles that apply are physician fee schedule rates rather than hospital outpatient rates. In the proposed rule posted this past July, CMS had proposed that even a new service provided in an excepted setting would be subject to PFS payment rates rather than hospital outpatient rates. CMS has withdrawn that proposal for the coming year, so new services in excepted settings will be covered.
DME: In its proposed rule this past summer, CMS actually acknowledged flaws in the structure of the competitive bidding system for DME (including oxygen, CPAP, and certain ventilators referred to by CMS as respiratory assist devices). Specifically, related to oxygen, there is also acknowledgement of reductions in liquid oxygen utilization, a story we have been pushing for years. The CMS proposed rule would have tied liquid portable payment rates to portable concentrator and transfill system payment rates, a genuine bump in actual $$. More than a dozen societies joined to respond to the proposed rule, including NAMDRC, CHEST, and ATS.
In the final rule, CMS is moving forward with its proposal, acknowledging that it will need to monitor shifts in the oxygen marketplace and adjust their payment policies accordingly.
NAMDRC focuses on keeping its members informed on legislative and regulatory issues impacting their practices
NAMDRC’s mission statement clearly signals its commitment to improve access to quality care for patients with respiratory disease by removing regulatory and legislative barriers to appropriate treatment. Adhering to that commitment presents challenges in the rapidly changing structure of the delivery of health care. For example, 10 years ago, the majority of NAMDRC members were private practitioners/group practices, many with contracts to provide a range of services to institutions. While those agreements varied, the underlying principles were relatively constant – structure your agreements that were mutually beneficial to physician and hospital.
Today, those agreements have been replaced by employment contracts or simply disappeared entirely, replaced by various business models that have invariably shifted the focus of coverage and payment issues away from the group practice into significantly different financial incentives. The challenge for NAMDRC is to keep its members informed about structural changes in coverage and payment rules that could impact their decision making. In November 2018, CMS published three distinctly separate sets of rules slated to take effect in 2019, all of which affect physicians in the pulmonary, critical care, and sleep landscapes. Through the monthly membership publication, the Washington Watchline, members get timely information that impact their practices. Excerpts from a recent Watchline include:
Physician fee schedule: As most physicians know, CMS had proposed dramatic changes to payment for Level 4 and Level % E&M codes, but due to strong reaction from man within the medical community, CMS is withdrawing that specific proposal, at least in the short term. Related provisions include:
• For CY 2019 and 2020, CMS will continue the current coding and payment structure for E/M office/outpatient visits,
• Effective January 1, 2019, for new and established patients for E/M office/outpatient visits, practitioners need not re-enter in the medical record information on the patient’s chief complaint and history that has already been entered by ancillary staff or the beneficiary. The practitioner may simply indicate in the medical record that he or she reviewed and verified this information.
• For 2021, CMS is finalizing a significant reduction in the current payment variation in office/outpatient E/M visit levels by paying a single rate for E/M office/outpatient visit levels 2, 3, and 4 (one for established and another for new patients) beginning in 2021. However, CMS is not finalizing the inclusion of E/M office/outpatient level 5 visits in the single payment rate, to better account for the care and needs of particularly complex patients.
• CMS policy for 2021 will adopt add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of specialized medical care. As discussed further below, these codes will only be reportable with E/M office/outpatient level 2 through 4 visits, and their use generally will not impose new per-visit documentation requirements.
Hospital outpatient rules: There are two particularly relevant issues addressed in this final regulation. The payment rates for pulmonary rehab are:
• Pulmonary Rehab via G0424 – APC 5733, $55.90 with co-pay of $11.18
• Pulmonary Rehab via G0237, 38, 39 – APC 5732, $32.12 with co-pay of $6.43
This regulation is also the vehicle for CMS addressing issues related to Section 603/site of service payment issues. As physicians know, CMS enacted Section 603 of the 23015 Budget Act that puts notable restrictions on payment for certain hospital outpatient services provided off campus (more than 250 yards from main campus of the hospital). NAMDRC is most concerned about the impact on pulmonary rehab – under the rules, off-campus programs that are grandfathered (“excepted” is the CMS term) as long as they were billing for those services at that location November 2015. However, if a hospital chooses to open a new program, or relocate an existing program to a different location, the payment principles that apply are physician fee schedule rates rather than hospital outpatient rates. In the proposed rule posted this past July, CMS had proposed that even a new service provided in an excepted setting would be subject to PFS payment rates rather than hospital outpatient rates. CMS has withdrawn that proposal for the coming year, so new services in excepted settings will be covered.
DME: In its proposed rule this past summer, CMS actually acknowledged flaws in the structure of the competitive bidding system for DME (including oxygen, CPAP, and certain ventilators referred to by CMS as respiratory assist devices). Specifically, related to oxygen, there is also acknowledgement of reductions in liquid oxygen utilization, a story we have been pushing for years. The CMS proposed rule would have tied liquid portable payment rates to portable concentrator and transfill system payment rates, a genuine bump in actual $$. More than a dozen societies joined to respond to the proposed rule, including NAMDRC, CHEST, and ATS.
In the final rule, CMS is moving forward with its proposal, acknowledging that it will need to monitor shifts in the oxygen marketplace and adjust their payment policies accordingly.
NAMDRC focuses on keeping its members informed on legislative and regulatory issues impacting their practices
NAMDRC’s mission statement clearly signals its commitment to improve access to quality care for patients with respiratory disease by removing regulatory and legislative barriers to appropriate treatment. Adhering to that commitment presents challenges in the rapidly changing structure of the delivery of health care. For example, 10 years ago, the majority of NAMDRC members were private practitioners/group practices, many with contracts to provide a range of services to institutions. While those agreements varied, the underlying principles were relatively constant – structure your agreements that were mutually beneficial to physician and hospital.
Today, those agreements have been replaced by employment contracts or simply disappeared entirely, replaced by various business models that have invariably shifted the focus of coverage and payment issues away from the group practice into significantly different financial incentives. The challenge for NAMDRC is to keep its members informed about structural changes in coverage and payment rules that could impact their decision making. In November 2018, CMS published three distinctly separate sets of rules slated to take effect in 2019, all of which affect physicians in the pulmonary, critical care, and sleep landscapes. Through the monthly membership publication, the Washington Watchline, members get timely information that impact their practices. Excerpts from a recent Watchline include:
Physician fee schedule: As most physicians know, CMS had proposed dramatic changes to payment for Level 4 and Level % E&M codes, but due to strong reaction from man within the medical community, CMS is withdrawing that specific proposal, at least in the short term. Related provisions include:
• For CY 2019 and 2020, CMS will continue the current coding and payment structure for E/M office/outpatient visits,
• Effective January 1, 2019, for new and established patients for E/M office/outpatient visits, practitioners need not re-enter in the medical record information on the patient’s chief complaint and history that has already been entered by ancillary staff or the beneficiary. The practitioner may simply indicate in the medical record that he or she reviewed and verified this information.
• For 2021, CMS is finalizing a significant reduction in the current payment variation in office/outpatient E/M visit levels by paying a single rate for E/M office/outpatient visit levels 2, 3, and 4 (one for established and another for new patients) beginning in 2021. However, CMS is not finalizing the inclusion of E/M office/outpatient level 5 visits in the single payment rate, to better account for the care and needs of particularly complex patients.
• CMS policy for 2021 will adopt add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of specialized medical care. As discussed further below, these codes will only be reportable with E/M office/outpatient level 2 through 4 visits, and their use generally will not impose new per-visit documentation requirements.
Hospital outpatient rules: There are two particularly relevant issues addressed in this final regulation. The payment rates for pulmonary rehab are:
• Pulmonary Rehab via G0424 – APC 5733, $55.90 with co-pay of $11.18
• Pulmonary Rehab via G0237, 38, 39 – APC 5732, $32.12 with co-pay of $6.43
This regulation is also the vehicle for CMS addressing issues related to Section 603/site of service payment issues. As physicians know, CMS enacted Section 603 of the 23015 Budget Act that puts notable restrictions on payment for certain hospital outpatient services provided off campus (more than 250 yards from main campus of the hospital). NAMDRC is most concerned about the impact on pulmonary rehab – under the rules, off-campus programs that are grandfathered (“excepted” is the CMS term) as long as they were billing for those services at that location November 2015. However, if a hospital chooses to open a new program, or relocate an existing program to a different location, the payment principles that apply are physician fee schedule rates rather than hospital outpatient rates. In the proposed rule posted this past July, CMS had proposed that even a new service provided in an excepted setting would be subject to PFS payment rates rather than hospital outpatient rates. CMS has withdrawn that proposal for the coming year, so new services in excepted settings will be covered.
DME: In its proposed rule this past summer, CMS actually acknowledged flaws in the structure of the competitive bidding system for DME (including oxygen, CPAP, and certain ventilators referred to by CMS as respiratory assist devices). Specifically, related to oxygen, there is also acknowledgement of reductions in liquid oxygen utilization, a story we have been pushing for years. The CMS proposed rule would have tied liquid portable payment rates to portable concentrator and transfill system payment rates, a genuine bump in actual $$. More than a dozen societies joined to respond to the proposed rule, including NAMDRC, CHEST, and ATS.
In the final rule, CMS is moving forward with its proposal, acknowledging that it will need to monitor shifts in the oxygen marketplace and adjust their payment policies accordingly.
NAMDRC News
NAMDRC will host its 42nd Annual Educational Conference March 14-16, 2019, in Sonoma, California, with a blue chip program featuring nationally recognized speakers. Keynote speakers include Bartolome Celli, MD, FCCP; E. Wesley Ely Jr., MD, FCCP; and a special “Conversation on Health Care Strategies” with Troyen Brennan, MD, Executive Vice President and Chief Medical Officer of CVS Health.
The NAMDRC conference format is unlike other pulmonary focused conferences. All sessions are plenary, and speakers are encouraged to take advantage of our wireless audience response system by simply texting their responses to questions. Sessions begin by 8:00 AM each day and conclude by 12:30 PM to provide ample time for all attendees to enjoy the Napa Sonoma region.
Details regarding registration, lodging, and more specifics regarding the program, social events, and related matters are available at the NAMDRC website at www.namdrc.org.
A few highlights:
• Thursday, March 14
Wesley Ely, MD – ICU Liberation and the ABCDEF Bundle – New Data; and ICU Delirium in Ventilated Patients – New Data
Neil MacIntyre, MD – Managing Severe Hypoxemic Respiratory Failure: The Ever Expanding Evidence Base
Samuel Hammerman, MD – Role of Long Term Acute Care
A Panel with Drs. Ely and MacIntyre – Challenges in Critical Care: Spontaneous Ventilation in Lung Injury, ECMO and Other.
Troyen Brennan, MD – A Conversation on Health Care Strategies
• Friday, March 15
Peter Gay, MD, FCCP – Heart Failure in Central Sleep Apnea
Susan Jacobs, RN, Christine Garvey, FNP, MSN, Phil Porte – Optimizing Oxygen Therapy
Bartolome Celli, MD, FCCP – Changing the Natural Course of COPD
Alan Plummer, MD, FCCP – Coding Update, 2019
Steve Peters, MD, FCCP – Practice Management Update
Phillip Porte – Legislative and Regulatory Updates
• Saturday, March 16
Bartolome Celli, MD, FCCP -- Pharmacological Therapy of COPD: Reasons for Optimism
Richard Channick, MD – Management of Acute Pulmonary Embolism: New Approaches
Colleen Channick, MD – The Role of Interventional Pulmonology in the Management of Cancer: From Diagnosis to Palliation
Stanley Yung-Chuan Lui, MD – Surgical Approach to OSA
Daniel Culver, DO, FCCP – Sarcoidosis
Regulatory proposals from CMS trigger NAMDRC responses
CMS has released several proposed rules to take effect January 1 that, if implemented as proposed, will impact patients, as well as physicians. The first regulation recommends important changes in the durable medical equipment competitive bidding program in general, with specific recommendations related to improving availability of liquid oxygen. CMS acknowledges that access to liquid oxygen has become problematic and is seeking comment on a proposal that would bump payment for liquid oxygen, including high flow, approximately 50%.
While the acknowledgement is important, the proposed solution falls far short of what virtually everyone in the industry believes is workable. For perspective, allowable charges for 2016 for liquid portable systems was just over $2 million, less than 2% of all outlays for portable equipment. Statutory language would require “budget neutrality,” thereby reducing payment for all other oxygen systems to bump liquid payment. Experts in the field agree that the proposed 50% bump is nowhere near the bump necessary to address the costs to suppliers to provide oxygen. Just as most oxygen modalities fit into the “nondelivery business model” that has reduced direct contact with patients, liquid fits into a “delivery business model” that necessitates constant refills by the supplier. That added cost needs to be reflected in any payment, and competitive bidding has eviscerated that payment.
NAMDRC and other societies recommend a “carve out” for liquid oxygen, removing it entirely from competitive bidding. While this approach would revert to a 1986 payment methodology, adjusted over time, it could be enough incentive for some suppliers to re-enter the liquid arena.
The second proposal espoused by CMS reduces payment for Level 4 and Level 5 office visits, with extra dollars going to lower intensity visits. Depending on a physician’s particular practice, the impact could be minimal or, at the other end of the spectrum, quite damaging. The proposal has its origins with the family practice community, long frustrated by the relatively low payment for Level 1 and level 2 visits. CMS ostensibly refers to reduced paperwork, but most physician groups see the real impact affecting their memberships.
CMS will publish final rules, reflecting public comment, around November 1, with an implementation date of January 1, 2019.
NAMDRC will host its 42nd Annual Educational Conference March 14-16, 2019, in Sonoma, California, with a blue chip program featuring nationally recognized speakers. Keynote speakers include Bartolome Celli, MD, FCCP; E. Wesley Ely Jr., MD, FCCP; and a special “Conversation on Health Care Strategies” with Troyen Brennan, MD, Executive Vice President and Chief Medical Officer of CVS Health.
The NAMDRC conference format is unlike other pulmonary focused conferences. All sessions are plenary, and speakers are encouraged to take advantage of our wireless audience response system by simply texting their responses to questions. Sessions begin by 8:00 AM each day and conclude by 12:30 PM to provide ample time for all attendees to enjoy the Napa Sonoma region.
Details regarding registration, lodging, and more specifics regarding the program, social events, and related matters are available at the NAMDRC website at www.namdrc.org.
A few highlights:
• Thursday, March 14
Wesley Ely, MD – ICU Liberation and the ABCDEF Bundle – New Data; and ICU Delirium in Ventilated Patients – New Data
Neil MacIntyre, MD – Managing Severe Hypoxemic Respiratory Failure: The Ever Expanding Evidence Base
Samuel Hammerman, MD – Role of Long Term Acute Care
A Panel with Drs. Ely and MacIntyre – Challenges in Critical Care: Spontaneous Ventilation in Lung Injury, ECMO and Other.
Troyen Brennan, MD – A Conversation on Health Care Strategies
• Friday, March 15
Peter Gay, MD, FCCP – Heart Failure in Central Sleep Apnea
Susan Jacobs, RN, Christine Garvey, FNP, MSN, Phil Porte – Optimizing Oxygen Therapy
Bartolome Celli, MD, FCCP – Changing the Natural Course of COPD
Alan Plummer, MD, FCCP – Coding Update, 2019
Steve Peters, MD, FCCP – Practice Management Update
Phillip Porte – Legislative and Regulatory Updates
• Saturday, March 16
Bartolome Celli, MD, FCCP -- Pharmacological Therapy of COPD: Reasons for Optimism
Richard Channick, MD – Management of Acute Pulmonary Embolism: New Approaches
Colleen Channick, MD – The Role of Interventional Pulmonology in the Management of Cancer: From Diagnosis to Palliation
Stanley Yung-Chuan Lui, MD – Surgical Approach to OSA
Daniel Culver, DO, FCCP – Sarcoidosis
Regulatory proposals from CMS trigger NAMDRC responses
CMS has released several proposed rules to take effect January 1 that, if implemented as proposed, will impact patients, as well as physicians. The first regulation recommends important changes in the durable medical equipment competitive bidding program in general, with specific recommendations related to improving availability of liquid oxygen. CMS acknowledges that access to liquid oxygen has become problematic and is seeking comment on a proposal that would bump payment for liquid oxygen, including high flow, approximately 50%.
While the acknowledgement is important, the proposed solution falls far short of what virtually everyone in the industry believes is workable. For perspective, allowable charges for 2016 for liquid portable systems was just over $2 million, less than 2% of all outlays for portable equipment. Statutory language would require “budget neutrality,” thereby reducing payment for all other oxygen systems to bump liquid payment. Experts in the field agree that the proposed 50% bump is nowhere near the bump necessary to address the costs to suppliers to provide oxygen. Just as most oxygen modalities fit into the “nondelivery business model” that has reduced direct contact with patients, liquid fits into a “delivery business model” that necessitates constant refills by the supplier. That added cost needs to be reflected in any payment, and competitive bidding has eviscerated that payment.
NAMDRC and other societies recommend a “carve out” for liquid oxygen, removing it entirely from competitive bidding. While this approach would revert to a 1986 payment methodology, adjusted over time, it could be enough incentive for some suppliers to re-enter the liquid arena.
The second proposal espoused by CMS reduces payment for Level 4 and Level 5 office visits, with extra dollars going to lower intensity visits. Depending on a physician’s particular practice, the impact could be minimal or, at the other end of the spectrum, quite damaging. The proposal has its origins with the family practice community, long frustrated by the relatively low payment for Level 1 and level 2 visits. CMS ostensibly refers to reduced paperwork, but most physician groups see the real impact affecting their memberships.
CMS will publish final rules, reflecting public comment, around November 1, with an implementation date of January 1, 2019.
NAMDRC will host its 42nd Annual Educational Conference March 14-16, 2019, in Sonoma, California, with a blue chip program featuring nationally recognized speakers. Keynote speakers include Bartolome Celli, MD, FCCP; E. Wesley Ely Jr., MD, FCCP; and a special “Conversation on Health Care Strategies” with Troyen Brennan, MD, Executive Vice President and Chief Medical Officer of CVS Health.
The NAMDRC conference format is unlike other pulmonary focused conferences. All sessions are plenary, and speakers are encouraged to take advantage of our wireless audience response system by simply texting their responses to questions. Sessions begin by 8:00 AM each day and conclude by 12:30 PM to provide ample time for all attendees to enjoy the Napa Sonoma region.
Details regarding registration, lodging, and more specifics regarding the program, social events, and related matters are available at the NAMDRC website at www.namdrc.org.
A few highlights:
• Thursday, March 14
Wesley Ely, MD – ICU Liberation and the ABCDEF Bundle – New Data; and ICU Delirium in Ventilated Patients – New Data
Neil MacIntyre, MD – Managing Severe Hypoxemic Respiratory Failure: The Ever Expanding Evidence Base
Samuel Hammerman, MD – Role of Long Term Acute Care
A Panel with Drs. Ely and MacIntyre – Challenges in Critical Care: Spontaneous Ventilation in Lung Injury, ECMO and Other.
Troyen Brennan, MD – A Conversation on Health Care Strategies
• Friday, March 15
Peter Gay, MD, FCCP – Heart Failure in Central Sleep Apnea
Susan Jacobs, RN, Christine Garvey, FNP, MSN, Phil Porte – Optimizing Oxygen Therapy
Bartolome Celli, MD, FCCP – Changing the Natural Course of COPD
Alan Plummer, MD, FCCP – Coding Update, 2019
Steve Peters, MD, FCCP – Practice Management Update
Phillip Porte – Legislative and Regulatory Updates
• Saturday, March 16
Bartolome Celli, MD, FCCP -- Pharmacological Therapy of COPD: Reasons for Optimism
Richard Channick, MD – Management of Acute Pulmonary Embolism: New Approaches
Colleen Channick, MD – The Role of Interventional Pulmonology in the Management of Cancer: From Diagnosis to Palliation
Stanley Yung-Chuan Lui, MD – Surgical Approach to OSA
Daniel Culver, DO, FCCP – Sarcoidosis
Regulatory proposals from CMS trigger NAMDRC responses
CMS has released several proposed rules to take effect January 1 that, if implemented as proposed, will impact patients, as well as physicians. The first regulation recommends important changes in the durable medical equipment competitive bidding program in general, with specific recommendations related to improving availability of liquid oxygen. CMS acknowledges that access to liquid oxygen has become problematic and is seeking comment on a proposal that would bump payment for liquid oxygen, including high flow, approximately 50%.
While the acknowledgement is important, the proposed solution falls far short of what virtually everyone in the industry believes is workable. For perspective, allowable charges for 2016 for liquid portable systems was just over $2 million, less than 2% of all outlays for portable equipment. Statutory language would require “budget neutrality,” thereby reducing payment for all other oxygen systems to bump liquid payment. Experts in the field agree that the proposed 50% bump is nowhere near the bump necessary to address the costs to suppliers to provide oxygen. Just as most oxygen modalities fit into the “nondelivery business model” that has reduced direct contact with patients, liquid fits into a “delivery business model” that necessitates constant refills by the supplier. That added cost needs to be reflected in any payment, and competitive bidding has eviscerated that payment.
NAMDRC and other societies recommend a “carve out” for liquid oxygen, removing it entirely from competitive bidding. While this approach would revert to a 1986 payment methodology, adjusted over time, it could be enough incentive for some suppliers to re-enter the liquid arena.
The second proposal espoused by CMS reduces payment for Level 4 and Level 5 office visits, with extra dollars going to lower intensity visits. Depending on a physician’s particular practice, the impact could be minimal or, at the other end of the spectrum, quite damaging. The proposal has its origins with the family practice community, long frustrated by the relatively low payment for Level 1 and level 2 visits. CMS ostensibly refers to reduced paperwork, but most physician groups see the real impact affecting their memberships.
CMS will publish final rules, reflecting public comment, around November 1, with an implementation date of January 1, 2019.
NAMDRC Legislative and Regulatory Agenda Once Again Focuses on Patient Access
NAMDRC’s Mission Statement declares, “NAMDRC’s primary mission is to improve access to quality care for patients with respiratory disease by removing regulatory and legislative barriers to appropriate treatment.” This mission is clear as we review our legislative and regulatory agenda on an ongoing and continuing basis.
Home Mechanical Ventilation: Close to 20 years ago, HCFA (now CMS) was faced with an important reality: advances in technology related to home mechanical ventilation are triggering an exponential growth in availability of these life supporting devices, but a price would be paid. At that time, Medicare law was quite explicit, indicating that certain ventilators would be paid under a “frequent and substantial servicing” payment methodology, authorizing payment on an ongoing basis as long as the prescribing physician documented medical necessity. To circumvent that statutory reality, the agency created a new category of medical device – a respiratory assist device/RAD – and declared that these devices are no longer ventilators and are now subject to capped rental rules and regulations.
NAMDRC was determined to work within the system, but roadblocks were consistently encountered, ie, contractor policies that did not reflect current medical standards of care, peer reviewed literature, etc. Even defining a “respiratory assist device” was (and still is) a challenge, as the term does not appear in the medical literature or in FDA vernacular.
Spin forward to 2018 and numerous realities come into play. Physicians still struggle with the concept of RADs without a definitive, consistent definition and no FDA language to guide usage. Today, it is easier to secure a ventilator if a physician documents the patient experiences some level of respiratory failure than it is to prescribe a simple ventilator with a back-up rate. Because of that dichotomy, the growth of life support ventilator usage is well documented.
If one takes the approach that a device should be paired with the actual clinical characteristics/medical need of the patient, changes in policy are necessary. While CMS clearly has the authority to act to improve policy and match clinical need to patient access, years and years of back and forth have signaled a definite unwillingness of the agency to move in that direction; therefore, the only genuine recourse is to seek legislative relief.
NAMDRC is working closely with the United States Senate, particularly the Finance Committee, Senator Cassidy (R-LA), and the Office of Senate Legislative Counsel to craft legislative language to address the myriad of issues associated with home mechanical ventilation.
Home Oxygen Therapy: In 1986, Congress revamped the statute governing coverage and payment of home oxygen. Pondering the reality of a segment of pulmonary medicine that has seen dramatic technological improvements and enhancements over the past 30-plus years, coupled with a payment system that is stuck with e-cylinders and competitive bidding, it is no wonder that both patients and physicians experience ongoing frustration trying to match a patient’s needs with an oxygen system that reflects the patient’s needs.
It’s a challenge to even consider where to start a reasonable discussion of home oxygen therapy. While the concept of supplemental oxygen is well accepted, the actual clinical evidence relies heavily on a very small number of studies. While virtually no one challenges the concept of the therapy, the actual science has progressed modestly in 30-plus years. But the technology surrounding oxygen therapy has become an industry all to itself. There are concentrators, portable oxygen concentrators, liquid systems, transfill systems, transtracheal oxygen therapy, and so on.
Add to the environment the growing demand for high flow systems that would deliver continuous flow oxygen at rates in excess of 4 L/min, and you begin to realize that the current payment system is a barrier to access. After all, the current payment system has problematic characteristics:
1. A flawed competitive bidding methodology;
2. Payment tied to liter flow pegged at a baseline of 2 L/min, regardless of actual patient need;
3. The major shift from a “delivery model” of care to a nondelivery model that reflects these newer technologies;
4. Virtual disappearance of liquid system availability as an option for physicians/patients;
5. The total failure of CMS to monitor, let alone act on, patient concerns.
Again, taking the NAMDRC Mission Statement into context, NAMDRC is working with all the key societies to craft a broad strategy to address these problems, acknowledging that it will likely take a mix of legislative and regulatory actions to bring home oxygen therapy into the 21st century, let alone to reflect realities of care in 2018.
NAMDRC’s Mission Statement declares, “NAMDRC’s primary mission is to improve access to quality care for patients with respiratory disease by removing regulatory and legislative barriers to appropriate treatment.” This mission is clear as we review our legislative and regulatory agenda on an ongoing and continuing basis.
Home Mechanical Ventilation: Close to 20 years ago, HCFA (now CMS) was faced with an important reality: advances in technology related to home mechanical ventilation are triggering an exponential growth in availability of these life supporting devices, but a price would be paid. At that time, Medicare law was quite explicit, indicating that certain ventilators would be paid under a “frequent and substantial servicing” payment methodology, authorizing payment on an ongoing basis as long as the prescribing physician documented medical necessity. To circumvent that statutory reality, the agency created a new category of medical device – a respiratory assist device/RAD – and declared that these devices are no longer ventilators and are now subject to capped rental rules and regulations.
NAMDRC was determined to work within the system, but roadblocks were consistently encountered, ie, contractor policies that did not reflect current medical standards of care, peer reviewed literature, etc. Even defining a “respiratory assist device” was (and still is) a challenge, as the term does not appear in the medical literature or in FDA vernacular.
Spin forward to 2018 and numerous realities come into play. Physicians still struggle with the concept of RADs without a definitive, consistent definition and no FDA language to guide usage. Today, it is easier to secure a ventilator if a physician documents the patient experiences some level of respiratory failure than it is to prescribe a simple ventilator with a back-up rate. Because of that dichotomy, the growth of life support ventilator usage is well documented.
If one takes the approach that a device should be paired with the actual clinical characteristics/medical need of the patient, changes in policy are necessary. While CMS clearly has the authority to act to improve policy and match clinical need to patient access, years and years of back and forth have signaled a definite unwillingness of the agency to move in that direction; therefore, the only genuine recourse is to seek legislative relief.
NAMDRC is working closely with the United States Senate, particularly the Finance Committee, Senator Cassidy (R-LA), and the Office of Senate Legislative Counsel to craft legislative language to address the myriad of issues associated with home mechanical ventilation.
Home Oxygen Therapy: In 1986, Congress revamped the statute governing coverage and payment of home oxygen. Pondering the reality of a segment of pulmonary medicine that has seen dramatic technological improvements and enhancements over the past 30-plus years, coupled with a payment system that is stuck with e-cylinders and competitive bidding, it is no wonder that both patients and physicians experience ongoing frustration trying to match a patient’s needs with an oxygen system that reflects the patient’s needs.
It’s a challenge to even consider where to start a reasonable discussion of home oxygen therapy. While the concept of supplemental oxygen is well accepted, the actual clinical evidence relies heavily on a very small number of studies. While virtually no one challenges the concept of the therapy, the actual science has progressed modestly in 30-plus years. But the technology surrounding oxygen therapy has become an industry all to itself. There are concentrators, portable oxygen concentrators, liquid systems, transfill systems, transtracheal oxygen therapy, and so on.
Add to the environment the growing demand for high flow systems that would deliver continuous flow oxygen at rates in excess of 4 L/min, and you begin to realize that the current payment system is a barrier to access. After all, the current payment system has problematic characteristics:
1. A flawed competitive bidding methodology;
2. Payment tied to liter flow pegged at a baseline of 2 L/min, regardless of actual patient need;
3. The major shift from a “delivery model” of care to a nondelivery model that reflects these newer technologies;
4. Virtual disappearance of liquid system availability as an option for physicians/patients;
5. The total failure of CMS to monitor, let alone act on, patient concerns.
Again, taking the NAMDRC Mission Statement into context, NAMDRC is working with all the key societies to craft a broad strategy to address these problems, acknowledging that it will likely take a mix of legislative and regulatory actions to bring home oxygen therapy into the 21st century, let alone to reflect realities of care in 2018.
NAMDRC’s Mission Statement declares, “NAMDRC’s primary mission is to improve access to quality care for patients with respiratory disease by removing regulatory and legislative barriers to appropriate treatment.” This mission is clear as we review our legislative and regulatory agenda on an ongoing and continuing basis.
Home Mechanical Ventilation: Close to 20 years ago, HCFA (now CMS) was faced with an important reality: advances in technology related to home mechanical ventilation are triggering an exponential growth in availability of these life supporting devices, but a price would be paid. At that time, Medicare law was quite explicit, indicating that certain ventilators would be paid under a “frequent and substantial servicing” payment methodology, authorizing payment on an ongoing basis as long as the prescribing physician documented medical necessity. To circumvent that statutory reality, the agency created a new category of medical device – a respiratory assist device/RAD – and declared that these devices are no longer ventilators and are now subject to capped rental rules and regulations.
NAMDRC was determined to work within the system, but roadblocks were consistently encountered, ie, contractor policies that did not reflect current medical standards of care, peer reviewed literature, etc. Even defining a “respiratory assist device” was (and still is) a challenge, as the term does not appear in the medical literature or in FDA vernacular.
Spin forward to 2018 and numerous realities come into play. Physicians still struggle with the concept of RADs without a definitive, consistent definition and no FDA language to guide usage. Today, it is easier to secure a ventilator if a physician documents the patient experiences some level of respiratory failure than it is to prescribe a simple ventilator with a back-up rate. Because of that dichotomy, the growth of life support ventilator usage is well documented.
If one takes the approach that a device should be paired with the actual clinical characteristics/medical need of the patient, changes in policy are necessary. While CMS clearly has the authority to act to improve policy and match clinical need to patient access, years and years of back and forth have signaled a definite unwillingness of the agency to move in that direction; therefore, the only genuine recourse is to seek legislative relief.
NAMDRC is working closely with the United States Senate, particularly the Finance Committee, Senator Cassidy (R-LA), and the Office of Senate Legislative Counsel to craft legislative language to address the myriad of issues associated with home mechanical ventilation.
Home Oxygen Therapy: In 1986, Congress revamped the statute governing coverage and payment of home oxygen. Pondering the reality of a segment of pulmonary medicine that has seen dramatic technological improvements and enhancements over the past 30-plus years, coupled with a payment system that is stuck with e-cylinders and competitive bidding, it is no wonder that both patients and physicians experience ongoing frustration trying to match a patient’s needs with an oxygen system that reflects the patient’s needs.
It’s a challenge to even consider where to start a reasonable discussion of home oxygen therapy. While the concept of supplemental oxygen is well accepted, the actual clinical evidence relies heavily on a very small number of studies. While virtually no one challenges the concept of the therapy, the actual science has progressed modestly in 30-plus years. But the technology surrounding oxygen therapy has become an industry all to itself. There are concentrators, portable oxygen concentrators, liquid systems, transfill systems, transtracheal oxygen therapy, and so on.
Add to the environment the growing demand for high flow systems that would deliver continuous flow oxygen at rates in excess of 4 L/min, and you begin to realize that the current payment system is a barrier to access. After all, the current payment system has problematic characteristics:
1. A flawed competitive bidding methodology;
2. Payment tied to liter flow pegged at a baseline of 2 L/min, regardless of actual patient need;
3. The major shift from a “delivery model” of care to a nondelivery model that reflects these newer technologies;
4. Virtual disappearance of liquid system availability as an option for physicians/patients;
5. The total failure of CMS to monitor, let alone act on, patient concerns.
Again, taking the NAMDRC Mission Statement into context, NAMDRC is working with all the key societies to craft a broad strategy to address these problems, acknowledging that it will likely take a mix of legislative and regulatory actions to bring home oxygen therapy into the 21st century, let alone to reflect realities of care in 2018.
Low payment for pulmonary rehab explained
A new review of 2015 Medicare data clearly points fingers at hospitals for the historically low payment rates for pulmonary rehabilitation.
To fully understand these data, everyone involved in the delivery of pulmonary rehabilitation services needs to know some of the specifics regarding Medicare’s rate setting process for hospital outpatient services. Those services are paid on the basis of a prospective payment methodology, similar to the DRG system for inpatient services. Under the outpatient system, APCs (ambulatory payment classifications) are computed with two key data sources, both provided by hospitals.
The second key component used by CMS for rate setting is the hospital cost report, submitted annually to CMS tied to the individual hospital’s fiscal year. This flow of data to CMS is ongoing because of differing fiscal years and is somewhat attributable to changes in Medicare proposed rates for the following year, published in July, compared with final rates, published in early November.
The other key historical fact that needs emphasis is what happened in 2010 when CMS began reimbursing for pulmonary rehab under new HCPCS code G0424. Clearly, there were no charge data to examine, so the Agency had to do a bit of guesswork, estimating what would be a reasonable payment. CMS turned to payment information tied to codes G0237 and G0238, codes that had been used by many institutions for the previous decade for billing pulmonary rehab. But one critical difference existed. The new code, G0424, was a 1-hour code, while G0237-38 were 15-minute codes. Over the next 2 years, even CMS cited the failure of hospitals to adjust their charges to reflect all the component services included in this new, bundled 1-hour code, compared with the unbundled 15-minute code.
The new review of CMS data bears out this problem. With approximately 1,350 institutions billing for hospital outpatient pulmonary rehab via code G0424, there is incredibly wide variance in charge data. The range is from a high of $1,981 to a low of $44, with 1,350 institutions in-between. The average charge was $247, but the difference between the lowest charge and the highest charge is approximately 44-fold.
For cost report data, the spread is from $1,265 to $4 (yes, $4, based on data provided to CMS). Approximately 750 hospitals, more than half, submit data to CMS reflecting costs associated with the delivery of pulmonary rehab, per hour, at $50 or less.
There are probably several reasons why hospitals behave this way. First, there is the historical phenomenon cited by CMS that it often takes years for hospitals to adjust charges appropriately when any new HCPCS code is adopted by CMS. And, in fact, CMS cited pulmonary rehab as a glaring example of that failure by hospitals. Second, there is the cost report data, and we believe it, too, falls victim to hospital neglect. We can understand that a service such as pulmonary rehab falls so far below the radar by chargemasters, hospital administrators and others associated with information submitted to CMS that little attention is paid to accuracy of charges or administrative costs culled from the hospital cost report. And then, there is the matter of community relations. The hospitals at the very high end of the spectrum in terms of charges ($1,100 and up) are unlikely to build good community relations if they let people know of those charges. Ironically, it is fair to presume that hospitals do pay very close attention to their charges and cost report data for very high-end hospital outpatient services, micro-examining that information to ensure desirable payment rates.
So, the critical challenge to the pulmonary community is to focus on those two very specific bits of data submitted by hospitals to CMS: what a hospital identifies as the “charge” for code G0424 and is then entered on every claim submitted to G0424; and second, information correlated to the administrative aspects of pulmonary rehab that hospitals submit to CMS annually in their cost report to CMS. Until those adjustments are made, pulmonary rehab will live with unacceptable payment rates.
A new review of 2015 Medicare data clearly points fingers at hospitals for the historically low payment rates for pulmonary rehabilitation.
To fully understand these data, everyone involved in the delivery of pulmonary rehabilitation services needs to know some of the specifics regarding Medicare’s rate setting process for hospital outpatient services. Those services are paid on the basis of a prospective payment methodology, similar to the DRG system for inpatient services. Under the outpatient system, APCs (ambulatory payment classifications) are computed with two key data sources, both provided by hospitals.
The second key component used by CMS for rate setting is the hospital cost report, submitted annually to CMS tied to the individual hospital’s fiscal year. This flow of data to CMS is ongoing because of differing fiscal years and is somewhat attributable to changes in Medicare proposed rates for the following year, published in July, compared with final rates, published in early November.
The other key historical fact that needs emphasis is what happened in 2010 when CMS began reimbursing for pulmonary rehab under new HCPCS code G0424. Clearly, there were no charge data to examine, so the Agency had to do a bit of guesswork, estimating what would be a reasonable payment. CMS turned to payment information tied to codes G0237 and G0238, codes that had been used by many institutions for the previous decade for billing pulmonary rehab. But one critical difference existed. The new code, G0424, was a 1-hour code, while G0237-38 were 15-minute codes. Over the next 2 years, even CMS cited the failure of hospitals to adjust their charges to reflect all the component services included in this new, bundled 1-hour code, compared with the unbundled 15-minute code.
The new review of CMS data bears out this problem. With approximately 1,350 institutions billing for hospital outpatient pulmonary rehab via code G0424, there is incredibly wide variance in charge data. The range is from a high of $1,981 to a low of $44, with 1,350 institutions in-between. The average charge was $247, but the difference between the lowest charge and the highest charge is approximately 44-fold.
For cost report data, the spread is from $1,265 to $4 (yes, $4, based on data provided to CMS). Approximately 750 hospitals, more than half, submit data to CMS reflecting costs associated with the delivery of pulmonary rehab, per hour, at $50 or less.
There are probably several reasons why hospitals behave this way. First, there is the historical phenomenon cited by CMS that it often takes years for hospitals to adjust charges appropriately when any new HCPCS code is adopted by CMS. And, in fact, CMS cited pulmonary rehab as a glaring example of that failure by hospitals. Second, there is the cost report data, and we believe it, too, falls victim to hospital neglect. We can understand that a service such as pulmonary rehab falls so far below the radar by chargemasters, hospital administrators and others associated with information submitted to CMS that little attention is paid to accuracy of charges or administrative costs culled from the hospital cost report. And then, there is the matter of community relations. The hospitals at the very high end of the spectrum in terms of charges ($1,100 and up) are unlikely to build good community relations if they let people know of those charges. Ironically, it is fair to presume that hospitals do pay very close attention to their charges and cost report data for very high-end hospital outpatient services, micro-examining that information to ensure desirable payment rates.
So, the critical challenge to the pulmonary community is to focus on those two very specific bits of data submitted by hospitals to CMS: what a hospital identifies as the “charge” for code G0424 and is then entered on every claim submitted to G0424; and second, information correlated to the administrative aspects of pulmonary rehab that hospitals submit to CMS annually in their cost report to CMS. Until those adjustments are made, pulmonary rehab will live with unacceptable payment rates.
A new review of 2015 Medicare data clearly points fingers at hospitals for the historically low payment rates for pulmonary rehabilitation.
To fully understand these data, everyone involved in the delivery of pulmonary rehabilitation services needs to know some of the specifics regarding Medicare’s rate setting process for hospital outpatient services. Those services are paid on the basis of a prospective payment methodology, similar to the DRG system for inpatient services. Under the outpatient system, APCs (ambulatory payment classifications) are computed with two key data sources, both provided by hospitals.
The second key component used by CMS for rate setting is the hospital cost report, submitted annually to CMS tied to the individual hospital’s fiscal year. This flow of data to CMS is ongoing because of differing fiscal years and is somewhat attributable to changes in Medicare proposed rates for the following year, published in July, compared with final rates, published in early November.
The other key historical fact that needs emphasis is what happened in 2010 when CMS began reimbursing for pulmonary rehab under new HCPCS code G0424. Clearly, there were no charge data to examine, so the Agency had to do a bit of guesswork, estimating what would be a reasonable payment. CMS turned to payment information tied to codes G0237 and G0238, codes that had been used by many institutions for the previous decade for billing pulmonary rehab. But one critical difference existed. The new code, G0424, was a 1-hour code, while G0237-38 were 15-minute codes. Over the next 2 years, even CMS cited the failure of hospitals to adjust their charges to reflect all the component services included in this new, bundled 1-hour code, compared with the unbundled 15-minute code.
The new review of CMS data bears out this problem. With approximately 1,350 institutions billing for hospital outpatient pulmonary rehab via code G0424, there is incredibly wide variance in charge data. The range is from a high of $1,981 to a low of $44, with 1,350 institutions in-between. The average charge was $247, but the difference between the lowest charge and the highest charge is approximately 44-fold.
For cost report data, the spread is from $1,265 to $4 (yes, $4, based on data provided to CMS). Approximately 750 hospitals, more than half, submit data to CMS reflecting costs associated with the delivery of pulmonary rehab, per hour, at $50 or less.
There are probably several reasons why hospitals behave this way. First, there is the historical phenomenon cited by CMS that it often takes years for hospitals to adjust charges appropriately when any new HCPCS code is adopted by CMS. And, in fact, CMS cited pulmonary rehab as a glaring example of that failure by hospitals. Second, there is the cost report data, and we believe it, too, falls victim to hospital neglect. We can understand that a service such as pulmonary rehab falls so far below the radar by chargemasters, hospital administrators and others associated with information submitted to CMS that little attention is paid to accuracy of charges or administrative costs culled from the hospital cost report. And then, there is the matter of community relations. The hospitals at the very high end of the spectrum in terms of charges ($1,100 and up) are unlikely to build good community relations if they let people know of those charges. Ironically, it is fair to presume that hospitals do pay very close attention to their charges and cost report data for very high-end hospital outpatient services, micro-examining that information to ensure desirable payment rates.
So, the critical challenge to the pulmonary community is to focus on those two very specific bits of data submitted by hospitals to CMS: what a hospital identifies as the “charge” for code G0424 and is then entered on every claim submitted to G0424; and second, information correlated to the administrative aspects of pulmonary rehab that hospitals submit to CMS annually in their cost report to CMS. Until those adjustments are made, pulmonary rehab will live with unacceptable payment rates.
Bipartisan Budget Act (BBA) of 2015 threatens growth of pulmonary rehab
In late 2015, Congress passed the Bipartisan Budget Act (BBA) to address numerous wide-ranging budget concerns, including issues related to agriculture, pensions, the strategic petroleum reserve, along with some Medicare issues. Section 603 of BBA is now coming back to haunt pulmonary rehabilitation services.
The intent of Section 603 is reasonable – to address the phenomenon of hospitals purchasing physician practices to take advantage of payment differentials between identical or virtually identical services when comparing the hospital outpatient prospective payment system (HOPPS) and the physician fee schedule (PFS). For example, an orthopedic practice might own its own MRI and related support services. It will bill for those services under the PFS. However, if the practice sells that segment of the revenue stream (the MRI assets, etc) to a hospital, the hospital can bill Medicare for those same services under the hospital outpatient prospective payment system at an amount notably higher than the PFS payment.
To address this payment aberration, Congress instructed the Centers for Medicare & Medicaid Services to craft a system to preclude a hospital from such behavior. If a hospital offers new or expanded outpatient services, it could NOT bill Medicare under the hospital outpatient services methodology and would be required to bill under the PFS payment methodology. Importantly, a few exemptions exist. If the new or expanded service is within 250 yards of the main hospital campus, the outpatient billing methodology is permitted. Likewise, if expansion of a current off-campus service occurs at the same location of the current off-site service, the hospital may continue to bill under the outpatient rules. Several other technical exceptions are permitted, for example construction planned prior to passage of BBA.
The implications for pulmonary rehabilitation are critical to its growth. A hospital that wishes to expand its current program and bill under the hospital outpatient methodology MUST do so by expanding at its current location. An expansion at a new location that is not within 250 yards of the main hospital campus triggers Section 603 provisions, and the hospital will bill at the physician fee schedule rate. Because the PFS payment rate is just over half of the payment rate for HOPPS payment, it is unlikely that a hospital would expand an existing program or establish a new one if it would be forced to bill under the lower rate.
While congressional logic may be relatively understandable, for pulmonary medicine, it is based on the premise that a hospital would purchase a pulmonary practice because that practice had a lucrative pulmonary rehabilitation services cash flow. NAMDRC and other societies were able to document major flaws in the basic premise, resulting in very problematic unintended consequences. A detailed review of Medicare claims data provides strong evidence that pulmonary practices simply do not provide pulmonary rehab services.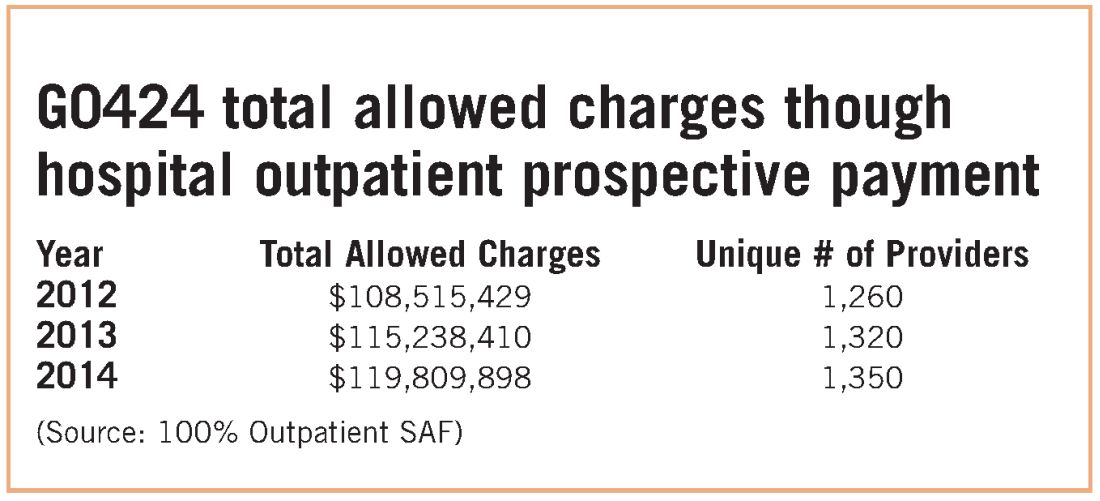
These data strongly indicate that G0424 pulmonary practice physician office billing for the most recent year data are available ($230K), compared with hospital outpatient allowed charges ($119M), is less than two-tenths of 1% of billing through the hospital setting. To argue that hospitals are purchasing pulmonary practices for financial gain tied to pulmonary rehab services defies Medicare data, as well as financial logic. If the CMS premise was valid, one would expect the aggregate physician office billing to be much greater than $535K. In discussions with CMS, the Agency did agree that there are likely to be unintended consequences related to Section 603 implementation. The Agency also emphasizes that it does not have the statutory authority for a “carve out” exemption. CMS stated that even if it agreed with us, it simply lacked the authority to exempt pulmonary rehab services. CMS also agreed that there is growing evidence that pulmonary rehab is a underutilized service that may very well save the program money through reduced hospitalizations and rehospitalizations, but it has little choice to implement the statute as Congress so mandated.
Therefore, the only solution is a legislative one. NAMDRC and other societies are seriously considering approaching Congress for such resolution.
In late 2015, Congress passed the Bipartisan Budget Act (BBA) to address numerous wide-ranging budget concerns, including issues related to agriculture, pensions, the strategic petroleum reserve, along with some Medicare issues. Section 603 of BBA is now coming back to haunt pulmonary rehabilitation services.
The intent of Section 603 is reasonable – to address the phenomenon of hospitals purchasing physician practices to take advantage of payment differentials between identical or virtually identical services when comparing the hospital outpatient prospective payment system (HOPPS) and the physician fee schedule (PFS). For example, an orthopedic practice might own its own MRI and related support services. It will bill for those services under the PFS. However, if the practice sells that segment of the revenue stream (the MRI assets, etc) to a hospital, the hospital can bill Medicare for those same services under the hospital outpatient prospective payment system at an amount notably higher than the PFS payment.
To address this payment aberration, Congress instructed the Centers for Medicare & Medicaid Services to craft a system to preclude a hospital from such behavior. If a hospital offers new or expanded outpatient services, it could NOT bill Medicare under the hospital outpatient services methodology and would be required to bill under the PFS payment methodology. Importantly, a few exemptions exist. If the new or expanded service is within 250 yards of the main hospital campus, the outpatient billing methodology is permitted. Likewise, if expansion of a current off-campus service occurs at the same location of the current off-site service, the hospital may continue to bill under the outpatient rules. Several other technical exceptions are permitted, for example construction planned prior to passage of BBA.
The implications for pulmonary rehabilitation are critical to its growth. A hospital that wishes to expand its current program and bill under the hospital outpatient methodology MUST do so by expanding at its current location. An expansion at a new location that is not within 250 yards of the main hospital campus triggers Section 603 provisions, and the hospital will bill at the physician fee schedule rate. Because the PFS payment rate is just over half of the payment rate for HOPPS payment, it is unlikely that a hospital would expand an existing program or establish a new one if it would be forced to bill under the lower rate.
While congressional logic may be relatively understandable, for pulmonary medicine, it is based on the premise that a hospital would purchase a pulmonary practice because that practice had a lucrative pulmonary rehabilitation services cash flow. NAMDRC and other societies were able to document major flaws in the basic premise, resulting in very problematic unintended consequences. A detailed review of Medicare claims data provides strong evidence that pulmonary practices simply do not provide pulmonary rehab services.
These data strongly indicate that G0424 pulmonary practice physician office billing for the most recent year data are available ($230K), compared with hospital outpatient allowed charges ($119M), is less than two-tenths of 1% of billing through the hospital setting. To argue that hospitals are purchasing pulmonary practices for financial gain tied to pulmonary rehab services defies Medicare data, as well as financial logic. If the CMS premise was valid, one would expect the aggregate physician office billing to be much greater than $535K. In discussions with CMS, the Agency did agree that there are likely to be unintended consequences related to Section 603 implementation. The Agency also emphasizes that it does not have the statutory authority for a “carve out” exemption. CMS stated that even if it agreed with us, it simply lacked the authority to exempt pulmonary rehab services. CMS also agreed that there is growing evidence that pulmonary rehab is a underutilized service that may very well save the program money through reduced hospitalizations and rehospitalizations, but it has little choice to implement the statute as Congress so mandated.
Therefore, the only solution is a legislative one. NAMDRC and other societies are seriously considering approaching Congress for such resolution.
In late 2015, Congress passed the Bipartisan Budget Act (BBA) to address numerous wide-ranging budget concerns, including issues related to agriculture, pensions, the strategic petroleum reserve, along with some Medicare issues. Section 603 of BBA is now coming back to haunt pulmonary rehabilitation services.
The intent of Section 603 is reasonable – to address the phenomenon of hospitals purchasing physician practices to take advantage of payment differentials between identical or virtually identical services when comparing the hospital outpatient prospective payment system (HOPPS) and the physician fee schedule (PFS). For example, an orthopedic practice might own its own MRI and related support services. It will bill for those services under the PFS. However, if the practice sells that segment of the revenue stream (the MRI assets, etc) to a hospital, the hospital can bill Medicare for those same services under the hospital outpatient prospective payment system at an amount notably higher than the PFS payment.
To address this payment aberration, Congress instructed the Centers for Medicare & Medicaid Services to craft a system to preclude a hospital from such behavior. If a hospital offers new or expanded outpatient services, it could NOT bill Medicare under the hospital outpatient services methodology and would be required to bill under the PFS payment methodology. Importantly, a few exemptions exist. If the new or expanded service is within 250 yards of the main hospital campus, the outpatient billing methodology is permitted. Likewise, if expansion of a current off-campus service occurs at the same location of the current off-site service, the hospital may continue to bill under the outpatient rules. Several other technical exceptions are permitted, for example construction planned prior to passage of BBA.
The implications for pulmonary rehabilitation are critical to its growth. A hospital that wishes to expand its current program and bill under the hospital outpatient methodology MUST do so by expanding at its current location. An expansion at a new location that is not within 250 yards of the main hospital campus triggers Section 603 provisions, and the hospital will bill at the physician fee schedule rate. Because the PFS payment rate is just over half of the payment rate for HOPPS payment, it is unlikely that a hospital would expand an existing program or establish a new one if it would be forced to bill under the lower rate.
While congressional logic may be relatively understandable, for pulmonary medicine, it is based on the premise that a hospital would purchase a pulmonary practice because that practice had a lucrative pulmonary rehabilitation services cash flow. NAMDRC and other societies were able to document major flaws in the basic premise, resulting in very problematic unintended consequences. A detailed review of Medicare claims data provides strong evidence that pulmonary practices simply do not provide pulmonary rehab services.
These data strongly indicate that G0424 pulmonary practice physician office billing for the most recent year data are available ($230K), compared with hospital outpatient allowed charges ($119M), is less than two-tenths of 1% of billing through the hospital setting. To argue that hospitals are purchasing pulmonary practices for financial gain tied to pulmonary rehab services defies Medicare data, as well as financial logic. If the CMS premise was valid, one would expect the aggregate physician office billing to be much greater than $535K. In discussions with CMS, the Agency did agree that there are likely to be unintended consequences related to Section 603 implementation. The Agency also emphasizes that it does not have the statutory authority for a “carve out” exemption. CMS stated that even if it agreed with us, it simply lacked the authority to exempt pulmonary rehab services. CMS also agreed that there is growing evidence that pulmonary rehab is a underutilized service that may very well save the program money through reduced hospitalizations and rehospitalizations, but it has little choice to implement the statute as Congress so mandated.
Therefore, the only solution is a legislative one. NAMDRC and other societies are seriously considering approaching Congress for such resolution.
NAMDRC and Partners Focus on CMS Threat to Pulmonary Rehabilitation
In a genuine very good news, very bad news proposal included in the 2017 hospital outpatient regulations, the Centers for Medicare & Medicaid Services (CMS) has proposed a major payment boost for pulmonary rehab services billed through hospital outpatient departments, but, simultaneously, the Agency proposes to preclude certain programs from utilizing that long- standing payment mechanism.
In November 2015, Congress authorized CMS to take action on the growing trend of hospitals purchasing certain physician practices so that the hospital can bill for certain services at a notably higher rate than the same service when provided in the physician office setting. CMS Section 603 pf P.L. 114-74 authorizes such action, and “These proposals are made in accordance with our belief that section 603… is intended to curb the practice of hospital acquisition of physician practices that result in receiving additional Medicare payment for similar services.” While we recognize that the congressional intent has some level of legitimacy, as is often the case, the CMS approach is too inclusive, especially as it applies to pulmonary rehabilitation services billed through HCPCS code G0424.
This problem has evolved because of two distinctly different formulas for determining payment. The physician fee schedule is based on the concept of RVUs, practice expense, and malpractice expense. Hospital outpatient services that may be virtually identical are based on a formula that includes charge data from Medicare claims forms and the annual hospital cost report identifying overhead.
If adopted as proposed, hospital outpatient programs in place on the date of enactment of P.L. 114-74 (early November 2015) are grandfathered into the hospital outpatient methodology. However, new programs that are not part of the main hospital campus (or within 250 yards of the campus) will only be able to bill at the physician office setting rate. Likewise, an existing program that moves to a new location that is beyond the 250-yard threshold will lose its “grandfather” status and be forced to bill at the physician office setting payment rate.
For practical purposes, the 2017 proposed rate for G0424 in the hospital setting is $160+, while the same service in the physician office setting is $30+.
While there is certainly understandable logic in the Congressional mandate, the CMS approach that includes pulmonary rehab is fraught with basic flaws in logic, strongly supported by CMS data. For example, in 2014 only 231 distinct providers billed for a total of 22,603 services. That translates into an outlay of approximately $535,000. Compare that outlay for 2014 with the outlay for hospital outpatient pulmonary rehab at just under $120 million, billed by 1350 distinct providers.
Those data alone strongly support the contention that a business model of pulmonary rehab in a physician office setting is rarely viable. Space, capital investment, and staffing, coupled with low payment, hardly create an incentive for a hospital to purchase a pulmonary practice because of lucrative pulmonary rehab services.
Other Medicare data also work in our favor. An examination of the physician specialties that actually bill G0424 through the physician fee schedule also punches a large hole in the CMS argument. The top five physician specialties that billed G0424 through the physician office setting include:
2012 2013 2014
TOTAL $688,489 $589,116 $535,512
Pulmonary $340,805 $310,065 $229,832
Family Practice $175,788 $116,681 $183,499
Internal Medicine $79,053 $78,211 $52,943
Crit Care (intensivists) $29,964 $29,139 $18,723
Cardiology $31,947 $17,729 $17,242
Source: Physician Supplier Procedure Summary File (PSPS), 2012-2014
These data speak volumes, or perhaps an absolute lack of volume. How does one support the concept that this proposed action is necessary to stem the tide of hospital acquisition of pulmonary practices when the total volume, notably declining over the past 3 years, of actual billings for pulmonary rehab is valued at under $230,000? The comparison with actual hospital billing in 2014 of just under $120 million is critical. There is no rhyme or reason to the CMS proposal as it applies to pulmonary rehabilitation services.
Unintended consequences are not difficult to imagine. With the new payment rates, hospitals may choose to expand their programs but cannot do so unless that physical location in on the main campus or within 250 yards of the campus. An off-site program that must move to accommodate larger space would be precluded from such a move. Likewise, hospitals that may want to open a new program must do so within the confines of the hospital campus/250-yard perimeter. Otherwise, these programs would be required to bill at the physician fee schedule rate.
In a genuine very good news, very bad news proposal included in the 2017 hospital outpatient regulations, the Centers for Medicare & Medicaid Services (CMS) has proposed a major payment boost for pulmonary rehab services billed through hospital outpatient departments, but, simultaneously, the Agency proposes to preclude certain programs from utilizing that long- standing payment mechanism.
In November 2015, Congress authorized CMS to take action on the growing trend of hospitals purchasing certain physician practices so that the hospital can bill for certain services at a notably higher rate than the same service when provided in the physician office setting. CMS Section 603 pf P.L. 114-74 authorizes such action, and “These proposals are made in accordance with our belief that section 603… is intended to curb the practice of hospital acquisition of physician practices that result in receiving additional Medicare payment for similar services.” While we recognize that the congressional intent has some level of legitimacy, as is often the case, the CMS approach is too inclusive, especially as it applies to pulmonary rehabilitation services billed through HCPCS code G0424.
This problem has evolved because of two distinctly different formulas for determining payment. The physician fee schedule is based on the concept of RVUs, practice expense, and malpractice expense. Hospital outpatient services that may be virtually identical are based on a formula that includes charge data from Medicare claims forms and the annual hospital cost report identifying overhead.
If adopted as proposed, hospital outpatient programs in place on the date of enactment of P.L. 114-74 (early November 2015) are grandfathered into the hospital outpatient methodology. However, new programs that are not part of the main hospital campus (or within 250 yards of the campus) will only be able to bill at the physician office setting rate. Likewise, an existing program that moves to a new location that is beyond the 250-yard threshold will lose its “grandfather” status and be forced to bill at the physician office setting payment rate.
For practical purposes, the 2017 proposed rate for G0424 in the hospital setting is $160+, while the same service in the physician office setting is $30+.
While there is certainly understandable logic in the Congressional mandate, the CMS approach that includes pulmonary rehab is fraught with basic flaws in logic, strongly supported by CMS data. For example, in 2014 only 231 distinct providers billed for a total of 22,603 services. That translates into an outlay of approximately $535,000. Compare that outlay for 2014 with the outlay for hospital outpatient pulmonary rehab at just under $120 million, billed by 1350 distinct providers.
Those data alone strongly support the contention that a business model of pulmonary rehab in a physician office setting is rarely viable. Space, capital investment, and staffing, coupled with low payment, hardly create an incentive for a hospital to purchase a pulmonary practice because of lucrative pulmonary rehab services.
Other Medicare data also work in our favor. An examination of the physician specialties that actually bill G0424 through the physician fee schedule also punches a large hole in the CMS argument. The top five physician specialties that billed G0424 through the physician office setting include:
2012 2013 2014
TOTAL $688,489 $589,116 $535,512
Pulmonary $340,805 $310,065 $229,832
Family Practice $175,788 $116,681 $183,499
Internal Medicine $79,053 $78,211 $52,943
Crit Care (intensivists) $29,964 $29,139 $18,723
Cardiology $31,947 $17,729 $17,242
Source: Physician Supplier Procedure Summary File (PSPS), 2012-2014
These data speak volumes, or perhaps an absolute lack of volume. How does one support the concept that this proposed action is necessary to stem the tide of hospital acquisition of pulmonary practices when the total volume, notably declining over the past 3 years, of actual billings for pulmonary rehab is valued at under $230,000? The comparison with actual hospital billing in 2014 of just under $120 million is critical. There is no rhyme or reason to the CMS proposal as it applies to pulmonary rehabilitation services.
Unintended consequences are not difficult to imagine. With the new payment rates, hospitals may choose to expand their programs but cannot do so unless that physical location in on the main campus or within 250 yards of the campus. An off-site program that must move to accommodate larger space would be precluded from such a move. Likewise, hospitals that may want to open a new program must do so within the confines of the hospital campus/250-yard perimeter. Otherwise, these programs would be required to bill at the physician fee schedule rate.
In a genuine very good news, very bad news proposal included in the 2017 hospital outpatient regulations, the Centers for Medicare & Medicaid Services (CMS) has proposed a major payment boost for pulmonary rehab services billed through hospital outpatient departments, but, simultaneously, the Agency proposes to preclude certain programs from utilizing that long- standing payment mechanism.
In November 2015, Congress authorized CMS to take action on the growing trend of hospitals purchasing certain physician practices so that the hospital can bill for certain services at a notably higher rate than the same service when provided in the physician office setting. CMS Section 603 pf P.L. 114-74 authorizes such action, and “These proposals are made in accordance with our belief that section 603… is intended to curb the practice of hospital acquisition of physician practices that result in receiving additional Medicare payment for similar services.” While we recognize that the congressional intent has some level of legitimacy, as is often the case, the CMS approach is too inclusive, especially as it applies to pulmonary rehabilitation services billed through HCPCS code G0424.
This problem has evolved because of two distinctly different formulas for determining payment. The physician fee schedule is based on the concept of RVUs, practice expense, and malpractice expense. Hospital outpatient services that may be virtually identical are based on a formula that includes charge data from Medicare claims forms and the annual hospital cost report identifying overhead.
If adopted as proposed, hospital outpatient programs in place on the date of enactment of P.L. 114-74 (early November 2015) are grandfathered into the hospital outpatient methodology. However, new programs that are not part of the main hospital campus (or within 250 yards of the campus) will only be able to bill at the physician office setting rate. Likewise, an existing program that moves to a new location that is beyond the 250-yard threshold will lose its “grandfather” status and be forced to bill at the physician office setting payment rate.
For practical purposes, the 2017 proposed rate for G0424 in the hospital setting is $160+, while the same service in the physician office setting is $30+.
While there is certainly understandable logic in the Congressional mandate, the CMS approach that includes pulmonary rehab is fraught with basic flaws in logic, strongly supported by CMS data. For example, in 2014 only 231 distinct providers billed for a total of 22,603 services. That translates into an outlay of approximately $535,000. Compare that outlay for 2014 with the outlay for hospital outpatient pulmonary rehab at just under $120 million, billed by 1350 distinct providers.
Those data alone strongly support the contention that a business model of pulmonary rehab in a physician office setting is rarely viable. Space, capital investment, and staffing, coupled with low payment, hardly create an incentive for a hospital to purchase a pulmonary practice because of lucrative pulmonary rehab services.
Other Medicare data also work in our favor. An examination of the physician specialties that actually bill G0424 through the physician fee schedule also punches a large hole in the CMS argument. The top five physician specialties that billed G0424 through the physician office setting include:
2012 2013 2014
TOTAL $688,489 $589,116 $535,512
Pulmonary $340,805 $310,065 $229,832
Family Practice $175,788 $116,681 $183,499
Internal Medicine $79,053 $78,211 $52,943
Crit Care (intensivists) $29,964 $29,139 $18,723
Cardiology $31,947 $17,729 $17,242
Source: Physician Supplier Procedure Summary File (PSPS), 2012-2014
These data speak volumes, or perhaps an absolute lack of volume. How does one support the concept that this proposed action is necessary to stem the tide of hospital acquisition of pulmonary practices when the total volume, notably declining over the past 3 years, of actual billings for pulmonary rehab is valued at under $230,000? The comparison with actual hospital billing in 2014 of just under $120 million is critical. There is no rhyme or reason to the CMS proposal as it applies to pulmonary rehabilitation services.
Unintended consequences are not difficult to imagine. With the new payment rates, hospitals may choose to expand their programs but cannot do so unless that physical location in on the main campus or within 250 yards of the campus. An off-site program that must move to accommodate larger space would be precluded from such a move. Likewise, hospitals that may want to open a new program must do so within the confines of the hospital campus/250-yard perimeter. Otherwise, these programs would be required to bill at the physician fee schedule rate.
NAMDRC Signals Concern Over CMS Proposed Changes in Payment Methodology
CMS has proposed a dramatic change in the methodology used to determine payment to physician offices that provide drugs covered under the Part B Medicare benefit. Under current policy, physician offices are reimbursed at a rate of the average sales price (ASP) +6%. While not exactly a “moneymaker” in the pulmonary space, this policy has been particularly attractive to oncologists who have the opportunity to select expensive medicines that, according to CMS, may overlook very similar, less expensive drugs.
The proposal, in effect a nationwide pilot, reduces the payment to ASP + 3%, plus a $16 administrative fee. The response from the oncology community has been vociferous opposition, and it has garnered significant support on Capitol Hill. But the impact on the pulmonary community has, for the most part, been muted, thanks to uncertainty about the impact such a policy change may trigger. NAMDRC submitted detailed comments to CMS, highlighting concerns that this initiative, which is mandatory and nationwide in scope, could adversely impact the medical care of seniors who suffer from COPD and other related pulmonary diseases. NAMDRC is also concerned about the precedent this policy sets by using limited demonstration authority to change statutory payment policy nationwide.
While NAMDRC supports the goals of developing new health-care delivery methods to increase quality and provide more efficient patient care, it is nevertheless troubled by the Part B drug reimbursement policy proposed by CMS, as it appears to have been created in a vacuum without any input from stakeholders involved, particularly beneficiaries and their physicians. Forcing vulnerable Medicare beneficiaries, many with potentially life-threatening conditions, including COPD, the third leading cause of death in the United States, and asthma, to be exposed to a new mandatory payment initiative that runs the notable risk of impeding access to life-saving therapies runs counter to the initiatives that Congress has put forth.
While NAMDRC understands the need to look seriously at cost issues within our core health programs, we must not subject beneficiaries and their physicians to the problematic choice between practice economics and prescribing the most medically appropriate treatment for each individual patient. As CMS knows, biologic medications for treatment of asthma are likely to take an important role in treatment protocols in the immediate future; one new biologic for asthma was approved recently, and two new biologics in the pipeline are likely to be approved by the end of the year. Beyond asthma, the development of new biologics in the pulmonary field is likely to expand in the foreseeable future.
As noted above, in the proposed rule, CMS expresses concern that the current 6% ASP add-on payment “may encourage the use of more expensive drugs because the 6% add-on generates more revenue for more expensive drugs.” In addition to lacking any data to support this premise, the reimbursement changes contemplated under this model may actually increase overall health-care spending by causing patients to receive care in more expensive settings.
Most importantly, there is no evidence indicating that the payment changes contemplated by the model will improve quality of care and may adversely impact those patients who lose access to their most appropriate treatments. Instead, NAMDRC believes that Medicare beneficiaries would be best served by a more patient-centric approach with appropriate safeguards, while also fostering physician-patient collaboration and ensuring that the unique needs of seniors are met. Therefore, NAMDRC strongly requested that CMS withdraw the proposed rule and obtain meaningful stakeholder input, including from patients and providers, before proceeding with Phase 2 of the proposed pilot.
2017 Educational Conference planning underway: NAMDRC’s 2017 Program Committee is targeting the middle of July for completion of the primary program for the 2017 conference to be held at the Meritage Resort, Napa, CA March 23-25, 2017. For information on the program, visit www.namdrc.org or call the Executive Office at 703/752-4359.
CMS has proposed a dramatic change in the methodology used to determine payment to physician offices that provide drugs covered under the Part B Medicare benefit. Under current policy, physician offices are reimbursed at a rate of the average sales price (ASP) +6%. While not exactly a “moneymaker” in the pulmonary space, this policy has been particularly attractive to oncologists who have the opportunity to select expensive medicines that, according to CMS, may overlook very similar, less expensive drugs.
The proposal, in effect a nationwide pilot, reduces the payment to ASP + 3%, plus a $16 administrative fee. The response from the oncology community has been vociferous opposition, and it has garnered significant support on Capitol Hill. But the impact on the pulmonary community has, for the most part, been muted, thanks to uncertainty about the impact such a policy change may trigger. NAMDRC submitted detailed comments to CMS, highlighting concerns that this initiative, which is mandatory and nationwide in scope, could adversely impact the medical care of seniors who suffer from COPD and other related pulmonary diseases. NAMDRC is also concerned about the precedent this policy sets by using limited demonstration authority to change statutory payment policy nationwide.
While NAMDRC supports the goals of developing new health-care delivery methods to increase quality and provide more efficient patient care, it is nevertheless troubled by the Part B drug reimbursement policy proposed by CMS, as it appears to have been created in a vacuum without any input from stakeholders involved, particularly beneficiaries and their physicians. Forcing vulnerable Medicare beneficiaries, many with potentially life-threatening conditions, including COPD, the third leading cause of death in the United States, and asthma, to be exposed to a new mandatory payment initiative that runs the notable risk of impeding access to life-saving therapies runs counter to the initiatives that Congress has put forth.
While NAMDRC understands the need to look seriously at cost issues within our core health programs, we must not subject beneficiaries and their physicians to the problematic choice between practice economics and prescribing the most medically appropriate treatment for each individual patient. As CMS knows, biologic medications for treatment of asthma are likely to take an important role in treatment protocols in the immediate future; one new biologic for asthma was approved recently, and two new biologics in the pipeline are likely to be approved by the end of the year. Beyond asthma, the development of new biologics in the pulmonary field is likely to expand in the foreseeable future.
As noted above, in the proposed rule, CMS expresses concern that the current 6% ASP add-on payment “may encourage the use of more expensive drugs because the 6% add-on generates more revenue for more expensive drugs.” In addition to lacking any data to support this premise, the reimbursement changes contemplated under this model may actually increase overall health-care spending by causing patients to receive care in more expensive settings.
Most importantly, there is no evidence indicating that the payment changes contemplated by the model will improve quality of care and may adversely impact those patients who lose access to their most appropriate treatments. Instead, NAMDRC believes that Medicare beneficiaries would be best served by a more patient-centric approach with appropriate safeguards, while also fostering physician-patient collaboration and ensuring that the unique needs of seniors are met. Therefore, NAMDRC strongly requested that CMS withdraw the proposed rule and obtain meaningful stakeholder input, including from patients and providers, before proceeding with Phase 2 of the proposed pilot.
2017 Educational Conference planning underway: NAMDRC’s 2017 Program Committee is targeting the middle of July for completion of the primary program for the 2017 conference to be held at the Meritage Resort, Napa, CA March 23-25, 2017. For information on the program, visit www.namdrc.org or call the Executive Office at 703/752-4359.
CMS has proposed a dramatic change in the methodology used to determine payment to physician offices that provide drugs covered under the Part B Medicare benefit. Under current policy, physician offices are reimbursed at a rate of the average sales price (ASP) +6%. While not exactly a “moneymaker” in the pulmonary space, this policy has been particularly attractive to oncologists who have the opportunity to select expensive medicines that, according to CMS, may overlook very similar, less expensive drugs.
The proposal, in effect a nationwide pilot, reduces the payment to ASP + 3%, plus a $16 administrative fee. The response from the oncology community has been vociferous opposition, and it has garnered significant support on Capitol Hill. But the impact on the pulmonary community has, for the most part, been muted, thanks to uncertainty about the impact such a policy change may trigger. NAMDRC submitted detailed comments to CMS, highlighting concerns that this initiative, which is mandatory and nationwide in scope, could adversely impact the medical care of seniors who suffer from COPD and other related pulmonary diseases. NAMDRC is also concerned about the precedent this policy sets by using limited demonstration authority to change statutory payment policy nationwide.
While NAMDRC supports the goals of developing new health-care delivery methods to increase quality and provide more efficient patient care, it is nevertheless troubled by the Part B drug reimbursement policy proposed by CMS, as it appears to have been created in a vacuum without any input from stakeholders involved, particularly beneficiaries and their physicians. Forcing vulnerable Medicare beneficiaries, many with potentially life-threatening conditions, including COPD, the third leading cause of death in the United States, and asthma, to be exposed to a new mandatory payment initiative that runs the notable risk of impeding access to life-saving therapies runs counter to the initiatives that Congress has put forth.
While NAMDRC understands the need to look seriously at cost issues within our core health programs, we must not subject beneficiaries and their physicians to the problematic choice between practice economics and prescribing the most medically appropriate treatment for each individual patient. As CMS knows, biologic medications for treatment of asthma are likely to take an important role in treatment protocols in the immediate future; one new biologic for asthma was approved recently, and two new biologics in the pipeline are likely to be approved by the end of the year. Beyond asthma, the development of new biologics in the pulmonary field is likely to expand in the foreseeable future.
As noted above, in the proposed rule, CMS expresses concern that the current 6% ASP add-on payment “may encourage the use of more expensive drugs because the 6% add-on generates more revenue for more expensive drugs.” In addition to lacking any data to support this premise, the reimbursement changes contemplated under this model may actually increase overall health-care spending by causing patients to receive care in more expensive settings.
Most importantly, there is no evidence indicating that the payment changes contemplated by the model will improve quality of care and may adversely impact those patients who lose access to their most appropriate treatments. Instead, NAMDRC believes that Medicare beneficiaries would be best served by a more patient-centric approach with appropriate safeguards, while also fostering physician-patient collaboration and ensuring that the unique needs of seniors are met. Therefore, NAMDRC strongly requested that CMS withdraw the proposed rule and obtain meaningful stakeholder input, including from patients and providers, before proceeding with Phase 2 of the proposed pilot.
2017 Educational Conference planning underway: NAMDRC’s 2017 Program Committee is targeting the middle of July for completion of the primary program for the 2017 conference to be held at the Meritage Resort, Napa, CA March 23-25, 2017. For information on the program, visit www.namdrc.org or call the Executive Office at 703/752-4359.




