User login
Neuromodulatory options for treatment-resistant depression
The emergence of treatment-resistant depression (TRD) poses a great clinical and public health challenge. There is no clear consensus on criteria to define TRD. The criteria range from failure to respond to 4 weeks of a single antidepressant to failure to respond to a single trial of electroconvulsive therapy (ECT).1
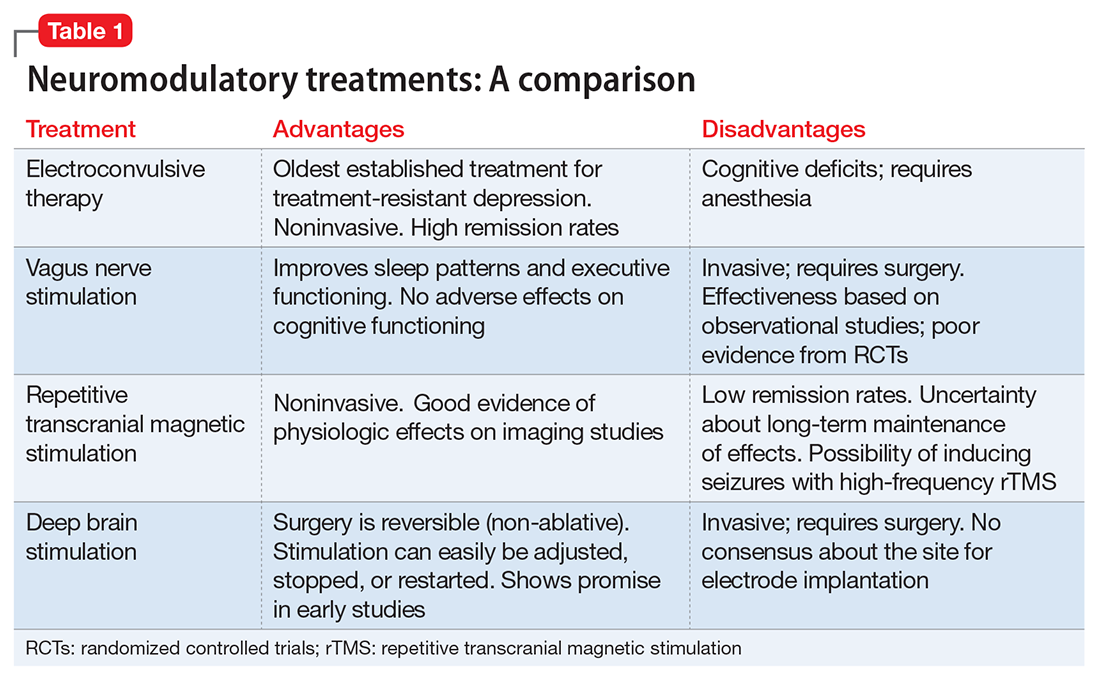
Neuromodulatory treatments for depression involve electrical stimulation of the brain through invasive or noninvasive methods. In this article, we discuss criteria for defining TRD, and compare the advantages and disadvantages of 4 neuromodulatory treatment options—ECT, vagus nerve stimulation (VNS), repetitive transcranial magnetic stimulation (rTMS), and deep brain stimulation (DBS)—for patients with depression who fail to respond to appropriate pharmacologic interventions (Table 1). Most of the studies we discuss selected patients who had severe depression and had not responded to numerous treatment trials.
Defining treatment resistance
Thase and Rush2 suggested progressive stages for categorizing TRD, ranging from Stage I (failure of at least 1 adequate trial of antidepressants) to Stage V (failure of adequate treatment with 2 selective serotonin reuptake inhibitors [SSRIs], a tricyclic antidepressant, a monoamine oxidase inhibitor, and a course of bilateral ECT). The Massachusetts General Hospital Staging Model suggested a quantitative scale to help characterize the degree of treatment resistance in which a higher score corresponds to a higher level of resistance.3 For every failed 6-week trial with adequate dose of an antidepressant, the patient is given a score of 1. The patient receives an extra .5 point for failure to respond to optimization of the dosage and augmentation with another medication. The patient also is given 3 points for failure to respond to ECT. Souery et al4,5 proposed a model in which they defined TRD as a failure to respond after ≥1 adequate antidepressant trials of ≥12 weeks.
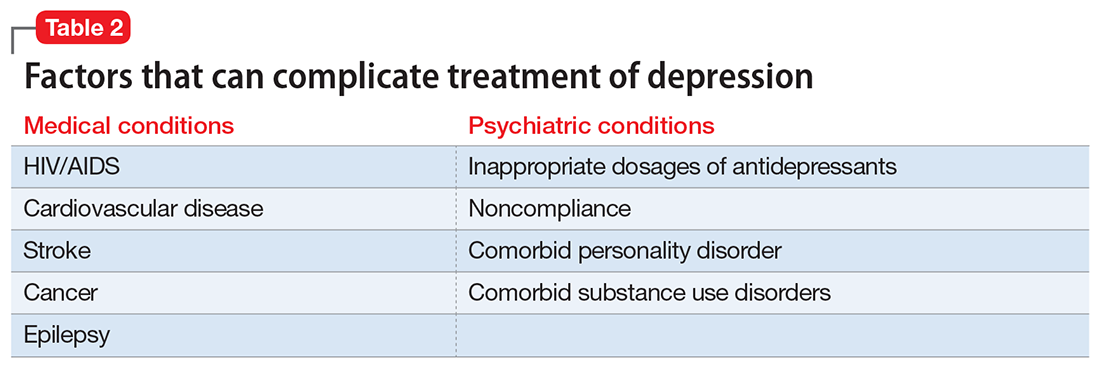
Treatment resistance often is the result of inadequate treatment of depressive symptoms. Inadequate treatment includes an inadequate dose of antidepressants and/or an inadequate duration of treatment. Treatment of depression also is often complicated by medical (cardiovascular, neurologic, endocrine disorders) and psychiatric (substance abuse disorders, personality disorders) comorbidities (Table 2). Patients with such comorbidities are at increased risk of mortality, and have lower response rates and increased morbidity.6
Electroconvulsive therapy
ECT involves the application of electric current to induce a self-limiting seizure. It affects multiple brain functions to produce its antidepressant effects. Patients with depression have a reduced concentration of γ-aminobutyric acid (GABA) in their plasma, CSF, and cortex. ECT increases GABAergic transmission in cortical circuits as demonstrated by increased levels of GABA in the occipital cortex, which may be responsible for ECT’s antidepressant effects.7 Sensitization of the 5-HT1A receptors and increased dopamine receptor binding in the striatum also have been associated with the antidepressant action of ECT.8 The antidepressant effects of ECT also can be attributed to increased neuroplasticity, as evidenced by increased neurotrophic factors and cell proliferation in animal models.9 Dysfunction of the HPA axis has long been associated with depressive disorders; ECT improves this dysfunction, as evidenced by normalization of the dexamethasone suppression test in patients who receive ECT.7
The results of neuroimaging studies exploring the effects of ECT vary widely based on the specific neuroimaging method, population, and statistical methods used to assess the changes. Some of the most consistent findings include reduced glucose metabolism in the frontal brain regions; reduced glucose metabolism in the hippocampus and medial temporal lobes; and reduction in functional connectivity in the anterior cingulate, parietal, medical frontal, and dorsolateral prefrontal cortex (DLPFC).10
Randomized control trials (RCTs) have established the superiority of ECT over pharmacotherapy and sham ECT. Compared with other neuromodulatory treatments, ECT has higher remission rates. On average, the remission rate among patients receiving ECT whose depression did not respond to pharmacotherapy is approximately 48%; this increases to 64.9% among patients who previously had responded to a medication.11
Some earlier trials found bilateral ECT to be more effective than unilateral ECT.12 Recent studies suggest that high-dose unilateral ECT (6 times the seizure threshold) is as effective as bilateral ECT.13 Studies have shown no significant differences in efficacy or treatment outcomes between twice- and thrice-weekly ECT regimens. Some studies suggest that twice-weekly ECT may be associated with a lower risk of short-term cognitive impairment compared with thrice-weekly ECT.14
In highly refractory cases, the effects of ECT can be augmented by using pre-treatment strategies such as hyperventilation, which may increase the duration of the seizure, and remifentanil, which helps reduce the anticonvulsant effect of agents used for anesthesia.15 Advanced age, psychotic features, resistance to pharmacotherapy, and comorbid personality disorders predict poor response to ECT.16
Adverse effects. Concerns about cognitive deficits secondary to ECT may curtail its use. Retrograde and anterograde amnesia are the most common deficits observed acutely after ECT.12 Other commonly affected cognitive functions include processing speed, attention/working memory, verbal and visual episodic memory, spatial problem solving, and executive functioning. The specific patterns of these deficits (in terms of duration and severity) vary between studies. In general, high-dose, thrice-weekly ECT and bilateral ECT are associated with greater cognitive deficits, whereas twice-weekly ECT and unilateral ECT are associated with a lower risk of cognitive adverse effects.12 A recent meta-analysis by Semkovska and McLoughlin17 found that most cognitive deficits seen after ECT are limited to the first 3 days after treatment. The authors of this meta-analysis concluded that these impairments improve over time and approach baseline 2 weeks after treatment. In fact, some of these impairments (processing speed, working memory, anterograde memory, and some aspects of executive function) improved beyond baseline after 15 days of treatment.17 The need for anesthesia and associated potential adverse effects also are a cause of concern with ECT.
Combining ECT with medication. Several patient-specific factors, including medication regimen and comorbid medical conditions, need to be considered before using ECT in combination with pharmacotherapy. Although most antipsychotics are safe to use with ECT, concomitant use of agents with higher antihistaminic properties may increase the risk of delirium. The risk of delirium also is increased with the use of anticonvulsants and mood stabilizers (eg, lithium) because these agents increase the seizure threshold. The potential for drug interactions may affect the choice of the anesthetic agents. Also, SSRIs and serotonin-norepinephrine reuptake inhibitors can increase the duration of induced seizures.18
Vagus nerve stimulation
VNS, in which an implanted device stimulates the vagus nerve with electrical impulses, initially was used to reduce the frequency of seizures in patients with epilepsy and treatment-resistant partial onset seizures.19 VNS was FDA-approved for TRD in July 2005.20 One VNS system, the NCP System, consists of an implantable, multi-programmable generator, known as a pulse generator, that is subcutaneously placed in the anterior chest wall during an outpatient surgical procedure. Separate bipolar nerve-stimulating electrodes are surgically wrapped around the left cervical vagus nerve, and then connected to the generator via a tunneling procedure. A telemetric wand is subsequently linked to a portable computer and used to adjust stimulation parameters.21,22
Support for using VNS for TRD came from a multitude of investigations and observations. Harden et al23 and Elger et al24 prospectively evaluated epileptic patients with standard depression symptom severity rating scales. They found that VNS was associated with statistically significant improvements in mood that were not related to reductions in seizures.23,24
The mechanism of action of VNS is not clear. Earlier researchers had found evidence that VNS affected brain regions associated with norepinephrine25 and serotonin systems26; both of these neurotransmitters have been implicated in the pathophysiology of depression. Positron emission tomography studies conducted during VNS treatment of epilepsy showed metabolic changes in cortical and subcortical areas of the brain, including the amygdala, hippocampus, and cingulate gyrus, all structures implicated in the pathophysiology of mood disorders.27
Most studies conducted to evaluate the efficacy of VNS have been observational, looking at depression ratings before and after treatment with VNS. The short-term studies measured the difference in depression rating scales at baseline and after 10 weeks of treatment. In most of these studies, treatment with VNS resulted in a statistically significant drop in depression rating scales scores, such as on the Hamilton Depression Rating Scale (HAM-D). Based on the study design and number of study participants, response rates have varied from 13%28 to 40%,29 whereas remission rates have varied from 15.3%30 to 28%.31 More than one-half of the reduction in symptoms occurred after 6 weeks of treatment.30 In longer-term follow-up studies, the antidepressant effect generally was sustained over time. Response rates remained essentially unchanged, but the remission rates increased to approximately 29%.29 Only 1 RCT has compared patients with controls; it found no significant differences in the response or remission rates between active VNS and sham VNS.32 In this study, all patients had VNS implanted, but in the control group, the VNS was never turned on.32 In a meta-analysis conducted by Martin and Martín-Sánchez,33 31.8% (95% confidence interval [CI], 23.2% to 41.8%; P < .001) of patients treated with VNS had a significant reduction in HAM-D scores. The response rate in patients with TRD ranged from 27% to 37% and the remission rate was approximately 13%. In studies that followed patients over longer periods, both the remission and response rates increased over time.34
Recent evidence suggests that the effectiveness of VNS may depend on the stimulation level. A multi-center double-blind study randomized patients to receive either a low (0.25 mA current, 130-millisecond pulse width), medium (0.5e1.0 mA, 250 millisecond), or high (1.25e1.5 mA, 250 millisecond) dose of VNS.35 Although all dose levels were associated with improvement in symptoms, a statistically significant durability in response was associated with the medium- and high-dose treatments.
Adverse effects. VNS has no major adverse effects on cognitive functioning, and some studies have found improvement in executive functioning that corresponded to improvement in depressive symptoms.30 VNS also may result in improved sleep patterns as evidenced by EEG changes.31 The most commonly reported adverse effects include pain in the incision site, hoarseness of voice, throat pain, and neck pain.36
Repetitive transcranial magnetic stimulation
rTMS is a noninvasive technique that uses high-intensity magnetic impulses to stimulate cortical neurons. A magnetic field is produced when current passes through a coil, which in turn causes electrical stimulation in the cortical neurons that results in transient changes in the excitability of the cortical neurons.37 Although many stimulation parameters exist for TMS, high-frequency stimulation to the left prefrontal cortex (HFL-rTMS) and low-frequency stimulation to the right prefrontal cortex (LFR-rTMS) have been shown most efficacious for treating depression.38 High-frequency (5 Hz to 20 Hz) stimulation using rTMS increases cortical neuron excitability, whereas low-frequency (approximately 1 Hz) is associated with reduced cortical neuron excitability.39 The choice of targeting the DLPFC stems from a large body of functional neuroimaging studies that have shown reduction in activity/blood flow in the left DLPFC and abnormal activity/blood flow in the right DLPFC.40
There is no dearth of RCTs evaluating the efficacy of rTMS vs sham rTMS (where no magnetic stimulation was provided). In a meta-analysis of 8 RCTs, low-frequency rTMS applied to the right DLPFC was associated with a remission rate of approximately 34.6%, compared with a 9.7% remission rate with sham rTMS.41 A response rate of approximately 38.2% was observed with HFL-rTMS, compared with a response rate of 15.1% for sham rTMS.41
Gaynes et al42 conducted a meta-analysis to determine the efficacy of rTMS in TRD. They found that for patients with TRD, rTMs produced a response rate of 29% and a remission rate of 30%. In long-term, naturalistic, observational studies, the response rates and remission rates were much higher (58% and 37.1%, respectively).43 Over a 1-year follow-up, almost two-thirds of patients continued to meet criteria for response to treatment.44 Trials comparing HFL-rTMS and LFR-rTMS have found no significant differences in efficacy.45
Advanced age, psychotic symptoms, and a longer duration of the current depressive episode predict poor response to rTMS. Also, imaging studies have shown that a lower metabolism in cerebellar, temporal, anterior cingulate, and occipital parts of the brain correlate with better response to HFL-rTMS.46,47
Adverse effects. The major adverse effect associated with rTMS is the risk of inducing seizures, which is more commonly associated with high-frequency rTMS. Other common adverse effects include headache, facial muscle twitching, and tinnitus.37
Deep brain stimulation
DBS is an invasive stereotactic surgical procedure. It involves unilateral or bilateral placement of electrodes at neuroanatomical locations to deliver continuous stimulation from a subcutaneously implanted pulse generator.48 In the past, destructive surgical procedures were used to treat intractable depression. Surgeries such as anterior cingulotomy, anterior capsulotomy, subcaudate tractotomy, and limbic leucotomy have been shown to effectively reduce depressive symptoms.49 The advantages of DBS over destructive procedures include the fact that DBS is reversible and that the stimulation levels can easily be adjusted, and the treatment can easily be stopped or restarted.
There is no consensus on the optimal anatomic locations for the electrode implantation in DBS. Electrodes have been implanted in the subcallosal cingulate gyrus, inferior thalamic peduncle, ventral capsule/ventral striatum, superolateral branch of the medial forebrain bundle (MFB), and nucleus accumbens.
The choice of anatomic locations stems from the large body of neuroimaging literature characterizing functional changes associated with acute depression and response to treatment. The electrode placement targets “nodes” that form an integral part of the affected neural circuits that are responsible for regulating depressive symptoms.50 Increased metabolic activity and blood flow to the subgenual cingulate gyrus and reduction in the blood flow to the DLPFC and the striatum have been associated with active depressed states. Response to antidepressant treatment has been associated with reversal of these findings.51 Functional magnetic resonance imaging studies have consistently shown increased activity in the amygdala in response to negative stimuli among patients with depression.
Regardless of the site of electrode placement, studies have reported symptomatic improvement among patients with depression who are treated with DBS. In 2 case reports, the electrode was implanted in the inferior thalamic peduncle.52,53 Each study had 1 participant, and each patient remitted.52,53
Placement of the electrodes in the nucleus accumbens resulted in a response rate of 45% in 1 study,54 whereas in a different study, all patients reported improvement in anhedonia.55 A response rate of 71% and a remission rate of 35% were observed in a study in which the electrode was implanted in the ventral capsule/ventral striatum area.56
Berlim et al57 published a systematic review and exploratory meta-analysis of studies in which the electrode had been implanted in the subgenual cingulate cortex. At 12 months, the response rate was 39.9% (95% CI, 28.4% to 52.8%), and 26.3% (95% CI, 13% to 45.9%) of patients achieved remission. The most significant drop in depression scores was observed 3 to 6 months after the surgery. No significant change in scores was observed between 6 to 12 months after surgery.57
The MFB, specifically the superolateral branch, is emerging as an exciting new target for electrode placement in DBS. Schlaepfer et al58 studied the effects of electrodes implanted bilaterally in the superolateral branch of the MFB. They observed an almost 50% reduction in symptoms by Day 7, and at the last follow-up visit (12 to 33 weeks) 4 of the 6 patients had achieved remission.58 In a recent systematic review, Gálvez et al59 found most studies had high response/remission rates without any significant adverse effects. In a recent study of DBS targeting the MFB, 3 of 4 patients had a >50% reduction in Montgomery-Åsberg Depression Rating Scale scores at the end of first week. Although 1 patient withdrew, 2 of the other 3 patients continued to report a >80% reduction in depressive symptoms, even at Week 26.60
Accurate localization of target areas (white matter tracts) and subsequent electrode placement might be an important factor governing treatment response. Riva-Posse et al61 found that clinical response was seen when the electrodes stimulated 3 specific white matter bundles. Interestingly, nonresponders were converted to responders simply by changing the position of the electrodes to include these white matter tracts.61
Adverse effects. The most common adverse effects noted during studies of DBS include pain at the site of implantation and wound infection. Other adverse effects include lead fracture, transient dysphagia, and other hardware-related problems.49
Sorting out the evidence
In the absence of head-to-head trials, it is difficult to establish a hierarchal algorithm for use of the 4 neuromodulatory treatments discussed in the article. If we were to base our decision solely on the current literature, ECT by far has the most evidence and highest remission rates.11 We can reduce the risk of cognitive deficits by using twice-weekly instead of thrice-weekly ECT, or by using unilateral instead of bilateral ECT.12 Another strategy for reducing adverse effects associated with long-term maintenance ECT is by using it in combination with VNS. ECT and VNS can be used safely concomitantly; ECT can be used to treat acutely worsening depression, and VNS for maintaining the antidepressant effect.62
Aside from ECT, rTMS is the only other treatment that has evidence from RCTs. Although the remission rates are not as high as ECT, its preferable adverse effects profile, noninvasive nature, and comparative low cost (compared with surgical procedures) make it a favorable choice. The Canadian Network for Mood and Anxiety Treatment guidelines suggest rTMS as the first-line treatment for patients who do not respond to pharmacologic treatments.63 ECT can be considered second-line treatment unless the patient has acute suicidal ideation, catatonia, psychotic features, greater treatment resistance, or physical deterioration, in which case ECT should be tried before TMS.63
Among the invasive options, VNS has more evidence and is FDA-approved for TRD. However, DBS has shown great promise in early studies, with remission rates as high as 35%.56 DBS has the advantage of being reversible, and the amount of stimulation can be adjusted easily. Despite early promise, more research is needed before DBS can be widely used in clinical settings.
1. Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol. 2007;17(11):696-707.
2. Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(suppl 13):23-29.
3. Petersen T, Papakostas GI, Posternak MA, et al. Empirical testing of two models for staging antidepressant treatment resistance. J Clin Psychopharmacol. 2005;25(4):336-341.
4. Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22.
5. Souery D, Amsterdam J, de Montigny C, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9(1-2):83-91.
6. Evans DL, Charney DS. Mood disorders and medical illness: a major public health problem. Biol. Psychiatry. 2003;54(3):177-180.
7. Sanacora G, Mason GF, Rothman DL, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160(3):577-579.
8. Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action, recent advances and limitations. Exp Neurol. 2009;219(1):20-26.
9. Perera TD, Coplan JD, Lisanby SH, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci. 2007;27(18):4894-4901.
10. Abbott CC, Gallegos P, Rediske N et al. A review of longitudinal electroconvulsive therapy: neuroimaging investigations. J Geriatr Psychiatry Neurol. 2014;27(1):33-46.
11. Heijnen WT, Birkenhäger TK, Wierdsma AI, et al. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol. 2010;30(5):616-619.
12. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
13. Semkovska M, Landau S, Dunne R et al. Bitemporal versus high-dose unilateral twice-weekly electroconvulsive therapy for depression (EFFECT-Dep): a pragmatic, randomized, non-inferiority trial. Am J Psychiatry. 2016;173(4):408-417.
14. Charlson F, Siskind D, Doi SA, et al. ECT efficacy and treatment course: a systematic review and meta-analysis of twice vs thrice weekly schedules. J Affect Disord. 2012;138(1-2):1-8.
15. Loo CK, Kaill A, Paton P, et al. The difficult-to-treat electroconvulsive therapy patient—strategies for augmenting outcomes. J Affect Disord. 2010;124(3):219-227.
16. de Vreede IM, Burger H, van Vliet IM. Prediction of response to ECT with routinely collected data in major depression. J Affect Disord. 2005;86(2-3):323-327.
17. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
18. Baghai TC, Marcuse A, Brosch M, et al. The influence of concomitant antidepressant medication on safety, tolerability and clinical effectiveness of electroconvulsive therapy. World J Biol Psychiatry. 2006;7(2):82-90.
19. Ben-Menachem E, Mañon-Espaillat R, Ristanovic R, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35(3):616-626.
20. Nemeroff CB, Mayberg HS, Krahl SE, et al. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31(7):1345-1355.
21. Matthews K, Eljamel MS. Vagus nerve stimulation and refractory depression: please can you switch me on doctor? Br J Psychiatry. 2003;183:181-183.
22. George MS, Rush AJ, Sackeim HA, et al. Vagus nerve stimulation (VNS): utility in neuropsychiatric disorders. Int J Neuropsychopharmacol. 2003;6(1):73-83.
23. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99.
24. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42(2-3):203-210.
25. Krahl SE, Clark KB, Smith DC, et al. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39(7):709-714.
26. Ben-Menachem E, Hamberger A, Hedner T, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20(3):221-227.
27. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia. 1998;39(9):983-990.
28. O’Keane V, Dinan TG, Scott L, et al. Changes in hypothalamic-pituitary-adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol Psychiatry. 2005;58(12):963-968.
29. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47(4):276-286.
30. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5):713-728.
31. Armitage R, Husain M, Hoffmann R, et al. The effects of vagus nerve stimulation on sleep EEG in depression: a preliminary report. J Psychosom Res. 2003;54(5):475-482.
32. Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58(5):347-354.
33. Martin JL, Martín-Sánchez E. Systematic review and meta-analysis of vagus nerve stimulation in the treatment of depression: variable results based on study designs. Eur Psychiatry. 2012;27(3):147-155.
34. Shah A, Carreno FR, Frazer A. Therapeutic modalities for treatment resistant depression: focus on vagal nerve stimulation and ketamine. Clin Psychopharmacol Neurosci. 2014;12(2):83-93.
35. Aaronson ST, Carpenter LL, Conway CR, et al. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul. 2013;6(4):631-640.
36. Daban C, Martinez-Aran A, Cruz N, et al. Safety and efficacy of vagus nerve stimulation in treatment-resistant depression. A systematic review. J Affect Disord. 2008;110(1-2):1-15.
37. Eitan R, Lerer B. Nonpharmacological, somatic treatments of depression: electroconvulsive therapy and novel brain stimulation modalities. Dialogues Clin Neurosci. 2006;8(2):241-258.
38. Lam RW, Chan P, Wilkins-Ho M, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Can J Psychiatry. 2008;53(9):621-631.
39. Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584-2596.
40. Fitzgerald PB, Oxley TJ, Laird AR, et al. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148(1):33-45.
41. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013;38(4):543-551.
42. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression. J Clin Psychiatry. 2014;75(5):477-489; quiz 489.
43. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
44. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder. J Clin Psychiatry. 2014;75(12):1394-1401.
45. Fitzgerald PB, Hoy K, Daskalakis ZJ, et al. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety. 2009;26(3):229-234.
46. Dumas R, Padovani R, Richieri R, et al. Repetitive transcranial magnetic stimulation in major depression: response factor [in French]. Encephale. 2012;38(4):360-368.
47. Fregni F, Marcolin MA, Myczkowski M, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int. J. Neuropsychopharmacol. 2006;9(6):641-654.
48. Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168(5):502-510.
49. Taghva AS, Malone DA, Rezai AR. Deep brain stimulation for treatment-resistant depression. World Neurosurg. 2013;80(3-4):S27.e17-S27.e24.
50. Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193-207.
51. Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682.
52. Jiménez F, Velasco F, Salín-Pascual R, et al. Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir Suppl. 2007;97(pt 2):393-398.
53. Jiménez F, Velasco F, Salin-Pascual R, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57(3):585-593; discussion 585-593.
54. Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110-116.
55. Schlaepfer TE, Bewernick BH, Kayser S, et al. Deep brain stimulation of the human reward system for major depression—rationale, outcomes and outlook. Neuropsychopharmacology. 2014;39(6):1303-1314.
56. Malone DA Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267-275.
57. Berlim MT, McGirr A, Van den Eynde F, et al. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31-38.
58. Schlaepfer TE, Bewernick BH, Kayser S, et al. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73(12):1204-1212.
59. Gálvez JF, Keser Z, Mwangi B, et al. The medial forebrain bundle as a deep brain stimulation target for treatment resistant depression: a review of published data. Prog Neuropsychopharmacol Biol Psychiatry. 2015;58:59-70.
60. Fenoy AJ, Schulz P, Selvaraj. Deep brain stimulation of the medial forebrain bundle: distinctive responses in resistant depression. J Affect Disord. 2016;203:143-151.
61. Riva-Posse P, Choi KS, Holtzheimer PE, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76(12):963-969.
62. Burke MJ, Husain MM. Concomitant use of vagus nerve stimulation and electroconvulsive therapy for treatment-resistant depression. J ECT. 2006;22(3):218-222.
63. Milev R V, Giacobbe P, Kennedy SH, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: section 4. Neurostimulation treatments. Can J Psychiatry. 2016;61:561-575.
The emergence of treatment-resistant depression (TRD) poses a great clinical and public health challenge. There is no clear consensus on criteria to define TRD. The criteria range from failure to respond to 4 weeks of a single antidepressant to failure to respond to a single trial of electroconvulsive therapy (ECT).1
Neuromodulatory treatments for depression involve electrical stimulation of the brain through invasive or noninvasive methods. In this article, we discuss criteria for defining TRD, and compare the advantages and disadvantages of 4 neuromodulatory treatment options—ECT, vagus nerve stimulation (VNS), repetitive transcranial magnetic stimulation (rTMS), and deep brain stimulation (DBS)—for patients with depression who fail to respond to appropriate pharmacologic interventions (Table 1). Most of the studies we discuss selected patients who had severe depression and had not responded to numerous treatment trials.

Defining treatment resistance
Thase and Rush2 suggested progressive stages for categorizing TRD, ranging from Stage I (failure of at least 1 adequate trial of antidepressants) to Stage V (failure of adequate treatment with 2 selective serotonin reuptake inhibitors [SSRIs], a tricyclic antidepressant, a monoamine oxidase inhibitor, and a course of bilateral ECT). The Massachusetts General Hospital Staging Model suggested a quantitative scale to help characterize the degree of treatment resistance in which a higher score corresponds to a higher level of resistance.3 For every failed 6-week trial with adequate dose of an antidepressant, the patient is given a score of 1. The patient receives an extra .5 point for failure to respond to optimization of the dosage and augmentation with another medication. The patient also is given 3 points for failure to respond to ECT. Souery et al4,5 proposed a model in which they defined TRD as a failure to respond after ≥1 adequate antidepressant trials of ≥12 weeks.
Treatment resistance often is the result of inadequate treatment of depressive symptoms. Inadequate treatment includes an inadequate dose of antidepressants and/or an inadequate duration of treatment. Treatment of depression also is often complicated by medical (cardiovascular, neurologic, endocrine disorders) and psychiatric (substance abuse disorders, personality disorders) comorbidities (Table 2). Patients with such comorbidities are at increased risk of mortality, and have lower response rates and increased morbidity.6

Electroconvulsive therapy
ECT involves the application of electric current to induce a self-limiting seizure. It affects multiple brain functions to produce its antidepressant effects. Patients with depression have a reduced concentration of γ-aminobutyric acid (GABA) in their plasma, CSF, and cortex. ECT increases GABAergic transmission in cortical circuits as demonstrated by increased levels of GABA in the occipital cortex, which may be responsible for ECT’s antidepressant effects.7 Sensitization of the 5-HT1A receptors and increased dopamine receptor binding in the striatum also have been associated with the antidepressant action of ECT.8 The antidepressant effects of ECT also can be attributed to increased neuroplasticity, as evidenced by increased neurotrophic factors and cell proliferation in animal models.9 Dysfunction of the HPA axis has long been associated with depressive disorders; ECT improves this dysfunction, as evidenced by normalization of the dexamethasone suppression test in patients who receive ECT.7
The results of neuroimaging studies exploring the effects of ECT vary widely based on the specific neuroimaging method, population, and statistical methods used to assess the changes. Some of the most consistent findings include reduced glucose metabolism in the frontal brain regions; reduced glucose metabolism in the hippocampus and medial temporal lobes; and reduction in functional connectivity in the anterior cingulate, parietal, medical frontal, and dorsolateral prefrontal cortex (DLPFC).10
Randomized control trials (RCTs) have established the superiority of ECT over pharmacotherapy and sham ECT. Compared with other neuromodulatory treatments, ECT has higher remission rates. On average, the remission rate among patients receiving ECT whose depression did not respond to pharmacotherapy is approximately 48%; this increases to 64.9% among patients who previously had responded to a medication.11
Some earlier trials found bilateral ECT to be more effective than unilateral ECT.12 Recent studies suggest that high-dose unilateral ECT (6 times the seizure threshold) is as effective as bilateral ECT.13 Studies have shown no significant differences in efficacy or treatment outcomes between twice- and thrice-weekly ECT regimens. Some studies suggest that twice-weekly ECT may be associated with a lower risk of short-term cognitive impairment compared with thrice-weekly ECT.14
In highly refractory cases, the effects of ECT can be augmented by using pre-treatment strategies such as hyperventilation, which may increase the duration of the seizure, and remifentanil, which helps reduce the anticonvulsant effect of agents used for anesthesia.15 Advanced age, psychotic features, resistance to pharmacotherapy, and comorbid personality disorders predict poor response to ECT.16
Adverse effects. Concerns about cognitive deficits secondary to ECT may curtail its use. Retrograde and anterograde amnesia are the most common deficits observed acutely after ECT.12 Other commonly affected cognitive functions include processing speed, attention/working memory, verbal and visual episodic memory, spatial problem solving, and executive functioning. The specific patterns of these deficits (in terms of duration and severity) vary between studies. In general, high-dose, thrice-weekly ECT and bilateral ECT are associated with greater cognitive deficits, whereas twice-weekly ECT and unilateral ECT are associated with a lower risk of cognitive adverse effects.12 A recent meta-analysis by Semkovska and McLoughlin17 found that most cognitive deficits seen after ECT are limited to the first 3 days after treatment. The authors of this meta-analysis concluded that these impairments improve over time and approach baseline 2 weeks after treatment. In fact, some of these impairments (processing speed, working memory, anterograde memory, and some aspects of executive function) improved beyond baseline after 15 days of treatment.17 The need for anesthesia and associated potential adverse effects also are a cause of concern with ECT.
Combining ECT with medication. Several patient-specific factors, including medication regimen and comorbid medical conditions, need to be considered before using ECT in combination with pharmacotherapy. Although most antipsychotics are safe to use with ECT, concomitant use of agents with higher antihistaminic properties may increase the risk of delirium. The risk of delirium also is increased with the use of anticonvulsants and mood stabilizers (eg, lithium) because these agents increase the seizure threshold. The potential for drug interactions may affect the choice of the anesthetic agents. Also, SSRIs and serotonin-norepinephrine reuptake inhibitors can increase the duration of induced seizures.18
Vagus nerve stimulation
VNS, in which an implanted device stimulates the vagus nerve with electrical impulses, initially was used to reduce the frequency of seizures in patients with epilepsy and treatment-resistant partial onset seizures.19 VNS was FDA-approved for TRD in July 2005.20 One VNS system, the NCP System, consists of an implantable, multi-programmable generator, known as a pulse generator, that is subcutaneously placed in the anterior chest wall during an outpatient surgical procedure. Separate bipolar nerve-stimulating electrodes are surgically wrapped around the left cervical vagus nerve, and then connected to the generator via a tunneling procedure. A telemetric wand is subsequently linked to a portable computer and used to adjust stimulation parameters.21,22
Support for using VNS for TRD came from a multitude of investigations and observations. Harden et al23 and Elger et al24 prospectively evaluated epileptic patients with standard depression symptom severity rating scales. They found that VNS was associated with statistically significant improvements in mood that were not related to reductions in seizures.23,24
The mechanism of action of VNS is not clear. Earlier researchers had found evidence that VNS affected brain regions associated with norepinephrine25 and serotonin systems26; both of these neurotransmitters have been implicated in the pathophysiology of depression. Positron emission tomography studies conducted during VNS treatment of epilepsy showed metabolic changes in cortical and subcortical areas of the brain, including the amygdala, hippocampus, and cingulate gyrus, all structures implicated in the pathophysiology of mood disorders.27
Most studies conducted to evaluate the efficacy of VNS have been observational, looking at depression ratings before and after treatment with VNS. The short-term studies measured the difference in depression rating scales at baseline and after 10 weeks of treatment. In most of these studies, treatment with VNS resulted in a statistically significant drop in depression rating scales scores, such as on the Hamilton Depression Rating Scale (HAM-D). Based on the study design and number of study participants, response rates have varied from 13%28 to 40%,29 whereas remission rates have varied from 15.3%30 to 28%.31 More than one-half of the reduction in symptoms occurred after 6 weeks of treatment.30 In longer-term follow-up studies, the antidepressant effect generally was sustained over time. Response rates remained essentially unchanged, but the remission rates increased to approximately 29%.29 Only 1 RCT has compared patients with controls; it found no significant differences in the response or remission rates between active VNS and sham VNS.32 In this study, all patients had VNS implanted, but in the control group, the VNS was never turned on.32 In a meta-analysis conducted by Martin and Martín-Sánchez,33 31.8% (95% confidence interval [CI], 23.2% to 41.8%; P < .001) of patients treated with VNS had a significant reduction in HAM-D scores. The response rate in patients with TRD ranged from 27% to 37% and the remission rate was approximately 13%. In studies that followed patients over longer periods, both the remission and response rates increased over time.34
Recent evidence suggests that the effectiveness of VNS may depend on the stimulation level. A multi-center double-blind study randomized patients to receive either a low (0.25 mA current, 130-millisecond pulse width), medium (0.5e1.0 mA, 250 millisecond), or high (1.25e1.5 mA, 250 millisecond) dose of VNS.35 Although all dose levels were associated with improvement in symptoms, a statistically significant durability in response was associated with the medium- and high-dose treatments.
Adverse effects. VNS has no major adverse effects on cognitive functioning, and some studies have found improvement in executive functioning that corresponded to improvement in depressive symptoms.30 VNS also may result in improved sleep patterns as evidenced by EEG changes.31 The most commonly reported adverse effects include pain in the incision site, hoarseness of voice, throat pain, and neck pain.36
Repetitive transcranial magnetic stimulation
rTMS is a noninvasive technique that uses high-intensity magnetic impulses to stimulate cortical neurons. A magnetic field is produced when current passes through a coil, which in turn causes electrical stimulation in the cortical neurons that results in transient changes in the excitability of the cortical neurons.37 Although many stimulation parameters exist for TMS, high-frequency stimulation to the left prefrontal cortex (HFL-rTMS) and low-frequency stimulation to the right prefrontal cortex (LFR-rTMS) have been shown most efficacious for treating depression.38 High-frequency (5 Hz to 20 Hz) stimulation using rTMS increases cortical neuron excitability, whereas low-frequency (approximately 1 Hz) is associated with reduced cortical neuron excitability.39 The choice of targeting the DLPFC stems from a large body of functional neuroimaging studies that have shown reduction in activity/blood flow in the left DLPFC and abnormal activity/blood flow in the right DLPFC.40
There is no dearth of RCTs evaluating the efficacy of rTMS vs sham rTMS (where no magnetic stimulation was provided). In a meta-analysis of 8 RCTs, low-frequency rTMS applied to the right DLPFC was associated with a remission rate of approximately 34.6%, compared with a 9.7% remission rate with sham rTMS.41 A response rate of approximately 38.2% was observed with HFL-rTMS, compared with a response rate of 15.1% for sham rTMS.41
Gaynes et al42 conducted a meta-analysis to determine the efficacy of rTMS in TRD. They found that for patients with TRD, rTMs produced a response rate of 29% and a remission rate of 30%. In long-term, naturalistic, observational studies, the response rates and remission rates were much higher (58% and 37.1%, respectively).43 Over a 1-year follow-up, almost two-thirds of patients continued to meet criteria for response to treatment.44 Trials comparing HFL-rTMS and LFR-rTMS have found no significant differences in efficacy.45
Advanced age, psychotic symptoms, and a longer duration of the current depressive episode predict poor response to rTMS. Also, imaging studies have shown that a lower metabolism in cerebellar, temporal, anterior cingulate, and occipital parts of the brain correlate with better response to HFL-rTMS.46,47
Adverse effects. The major adverse effect associated with rTMS is the risk of inducing seizures, which is more commonly associated with high-frequency rTMS. Other common adverse effects include headache, facial muscle twitching, and tinnitus.37
Deep brain stimulation
DBS is an invasive stereotactic surgical procedure. It involves unilateral or bilateral placement of electrodes at neuroanatomical locations to deliver continuous stimulation from a subcutaneously implanted pulse generator.48 In the past, destructive surgical procedures were used to treat intractable depression. Surgeries such as anterior cingulotomy, anterior capsulotomy, subcaudate tractotomy, and limbic leucotomy have been shown to effectively reduce depressive symptoms.49 The advantages of DBS over destructive procedures include the fact that DBS is reversible and that the stimulation levels can easily be adjusted, and the treatment can easily be stopped or restarted.
There is no consensus on the optimal anatomic locations for the electrode implantation in DBS. Electrodes have been implanted in the subcallosal cingulate gyrus, inferior thalamic peduncle, ventral capsule/ventral striatum, superolateral branch of the medial forebrain bundle (MFB), and nucleus accumbens.
The choice of anatomic locations stems from the large body of neuroimaging literature characterizing functional changes associated with acute depression and response to treatment. The electrode placement targets “nodes” that form an integral part of the affected neural circuits that are responsible for regulating depressive symptoms.50 Increased metabolic activity and blood flow to the subgenual cingulate gyrus and reduction in the blood flow to the DLPFC and the striatum have been associated with active depressed states. Response to antidepressant treatment has been associated with reversal of these findings.51 Functional magnetic resonance imaging studies have consistently shown increased activity in the amygdala in response to negative stimuli among patients with depression.
Regardless of the site of electrode placement, studies have reported symptomatic improvement among patients with depression who are treated with DBS. In 2 case reports, the electrode was implanted in the inferior thalamic peduncle.52,53 Each study had 1 participant, and each patient remitted.52,53
Placement of the electrodes in the nucleus accumbens resulted in a response rate of 45% in 1 study,54 whereas in a different study, all patients reported improvement in anhedonia.55 A response rate of 71% and a remission rate of 35% were observed in a study in which the electrode was implanted in the ventral capsule/ventral striatum area.56
Berlim et al57 published a systematic review and exploratory meta-analysis of studies in which the electrode had been implanted in the subgenual cingulate cortex. At 12 months, the response rate was 39.9% (95% CI, 28.4% to 52.8%), and 26.3% (95% CI, 13% to 45.9%) of patients achieved remission. The most significant drop in depression scores was observed 3 to 6 months after the surgery. No significant change in scores was observed between 6 to 12 months after surgery.57
The MFB, specifically the superolateral branch, is emerging as an exciting new target for electrode placement in DBS. Schlaepfer et al58 studied the effects of electrodes implanted bilaterally in the superolateral branch of the MFB. They observed an almost 50% reduction in symptoms by Day 7, and at the last follow-up visit (12 to 33 weeks) 4 of the 6 patients had achieved remission.58 In a recent systematic review, Gálvez et al59 found most studies had high response/remission rates without any significant adverse effects. In a recent study of DBS targeting the MFB, 3 of 4 patients had a >50% reduction in Montgomery-Åsberg Depression Rating Scale scores at the end of first week. Although 1 patient withdrew, 2 of the other 3 patients continued to report a >80% reduction in depressive symptoms, even at Week 26.60
Accurate localization of target areas (white matter tracts) and subsequent electrode placement might be an important factor governing treatment response. Riva-Posse et al61 found that clinical response was seen when the electrodes stimulated 3 specific white matter bundles. Interestingly, nonresponders were converted to responders simply by changing the position of the electrodes to include these white matter tracts.61
Adverse effects. The most common adverse effects noted during studies of DBS include pain at the site of implantation and wound infection. Other adverse effects include lead fracture, transient dysphagia, and other hardware-related problems.49
Sorting out the evidence
In the absence of head-to-head trials, it is difficult to establish a hierarchal algorithm for use of the 4 neuromodulatory treatments discussed in the article. If we were to base our decision solely on the current literature, ECT by far has the most evidence and highest remission rates.11 We can reduce the risk of cognitive deficits by using twice-weekly instead of thrice-weekly ECT, or by using unilateral instead of bilateral ECT.12 Another strategy for reducing adverse effects associated with long-term maintenance ECT is by using it in combination with VNS. ECT and VNS can be used safely concomitantly; ECT can be used to treat acutely worsening depression, and VNS for maintaining the antidepressant effect.62
Aside from ECT, rTMS is the only other treatment that has evidence from RCTs. Although the remission rates are not as high as ECT, its preferable adverse effects profile, noninvasive nature, and comparative low cost (compared with surgical procedures) make it a favorable choice. The Canadian Network for Mood and Anxiety Treatment guidelines suggest rTMS as the first-line treatment for patients who do not respond to pharmacologic treatments.63 ECT can be considered second-line treatment unless the patient has acute suicidal ideation, catatonia, psychotic features, greater treatment resistance, or physical deterioration, in which case ECT should be tried before TMS.63
Among the invasive options, VNS has more evidence and is FDA-approved for TRD. However, DBS has shown great promise in early studies, with remission rates as high as 35%.56 DBS has the advantage of being reversible, and the amount of stimulation can be adjusted easily. Despite early promise, more research is needed before DBS can be widely used in clinical settings.
The emergence of treatment-resistant depression (TRD) poses a great clinical and public health challenge. There is no clear consensus on criteria to define TRD. The criteria range from failure to respond to 4 weeks of a single antidepressant to failure to respond to a single trial of electroconvulsive therapy (ECT).1
Neuromodulatory treatments for depression involve electrical stimulation of the brain through invasive or noninvasive methods. In this article, we discuss criteria for defining TRD, and compare the advantages and disadvantages of 4 neuromodulatory treatment options—ECT, vagus nerve stimulation (VNS), repetitive transcranial magnetic stimulation (rTMS), and deep brain stimulation (DBS)—for patients with depression who fail to respond to appropriate pharmacologic interventions (Table 1). Most of the studies we discuss selected patients who had severe depression and had not responded to numerous treatment trials.

Defining treatment resistance
Thase and Rush2 suggested progressive stages for categorizing TRD, ranging from Stage I (failure of at least 1 adequate trial of antidepressants) to Stage V (failure of adequate treatment with 2 selective serotonin reuptake inhibitors [SSRIs], a tricyclic antidepressant, a monoamine oxidase inhibitor, and a course of bilateral ECT). The Massachusetts General Hospital Staging Model suggested a quantitative scale to help characterize the degree of treatment resistance in which a higher score corresponds to a higher level of resistance.3 For every failed 6-week trial with adequate dose of an antidepressant, the patient is given a score of 1. The patient receives an extra .5 point for failure to respond to optimization of the dosage and augmentation with another medication. The patient also is given 3 points for failure to respond to ECT. Souery et al4,5 proposed a model in which they defined TRD as a failure to respond after ≥1 adequate antidepressant trials of ≥12 weeks.
Treatment resistance often is the result of inadequate treatment of depressive symptoms. Inadequate treatment includes an inadequate dose of antidepressants and/or an inadequate duration of treatment. Treatment of depression also is often complicated by medical (cardiovascular, neurologic, endocrine disorders) and psychiatric (substance abuse disorders, personality disorders) comorbidities (Table 2). Patients with such comorbidities are at increased risk of mortality, and have lower response rates and increased morbidity.6

Electroconvulsive therapy
ECT involves the application of electric current to induce a self-limiting seizure. It affects multiple brain functions to produce its antidepressant effects. Patients with depression have a reduced concentration of γ-aminobutyric acid (GABA) in their plasma, CSF, and cortex. ECT increases GABAergic transmission in cortical circuits as demonstrated by increased levels of GABA in the occipital cortex, which may be responsible for ECT’s antidepressant effects.7 Sensitization of the 5-HT1A receptors and increased dopamine receptor binding in the striatum also have been associated with the antidepressant action of ECT.8 The antidepressant effects of ECT also can be attributed to increased neuroplasticity, as evidenced by increased neurotrophic factors and cell proliferation in animal models.9 Dysfunction of the HPA axis has long been associated with depressive disorders; ECT improves this dysfunction, as evidenced by normalization of the dexamethasone suppression test in patients who receive ECT.7
The results of neuroimaging studies exploring the effects of ECT vary widely based on the specific neuroimaging method, population, and statistical methods used to assess the changes. Some of the most consistent findings include reduced glucose metabolism in the frontal brain regions; reduced glucose metabolism in the hippocampus and medial temporal lobes; and reduction in functional connectivity in the anterior cingulate, parietal, medical frontal, and dorsolateral prefrontal cortex (DLPFC).10
Randomized control trials (RCTs) have established the superiority of ECT over pharmacotherapy and sham ECT. Compared with other neuromodulatory treatments, ECT has higher remission rates. On average, the remission rate among patients receiving ECT whose depression did not respond to pharmacotherapy is approximately 48%; this increases to 64.9% among patients who previously had responded to a medication.11
Some earlier trials found bilateral ECT to be more effective than unilateral ECT.12 Recent studies suggest that high-dose unilateral ECT (6 times the seizure threshold) is as effective as bilateral ECT.13 Studies have shown no significant differences in efficacy or treatment outcomes between twice- and thrice-weekly ECT regimens. Some studies suggest that twice-weekly ECT may be associated with a lower risk of short-term cognitive impairment compared with thrice-weekly ECT.14
In highly refractory cases, the effects of ECT can be augmented by using pre-treatment strategies such as hyperventilation, which may increase the duration of the seizure, and remifentanil, which helps reduce the anticonvulsant effect of agents used for anesthesia.15 Advanced age, psychotic features, resistance to pharmacotherapy, and comorbid personality disorders predict poor response to ECT.16
Adverse effects. Concerns about cognitive deficits secondary to ECT may curtail its use. Retrograde and anterograde amnesia are the most common deficits observed acutely after ECT.12 Other commonly affected cognitive functions include processing speed, attention/working memory, verbal and visual episodic memory, spatial problem solving, and executive functioning. The specific patterns of these deficits (in terms of duration and severity) vary between studies. In general, high-dose, thrice-weekly ECT and bilateral ECT are associated with greater cognitive deficits, whereas twice-weekly ECT and unilateral ECT are associated with a lower risk of cognitive adverse effects.12 A recent meta-analysis by Semkovska and McLoughlin17 found that most cognitive deficits seen after ECT are limited to the first 3 days after treatment. The authors of this meta-analysis concluded that these impairments improve over time and approach baseline 2 weeks after treatment. In fact, some of these impairments (processing speed, working memory, anterograde memory, and some aspects of executive function) improved beyond baseline after 15 days of treatment.17 The need for anesthesia and associated potential adverse effects also are a cause of concern with ECT.
Combining ECT with medication. Several patient-specific factors, including medication regimen and comorbid medical conditions, need to be considered before using ECT in combination with pharmacotherapy. Although most antipsychotics are safe to use with ECT, concomitant use of agents with higher antihistaminic properties may increase the risk of delirium. The risk of delirium also is increased with the use of anticonvulsants and mood stabilizers (eg, lithium) because these agents increase the seizure threshold. The potential for drug interactions may affect the choice of the anesthetic agents. Also, SSRIs and serotonin-norepinephrine reuptake inhibitors can increase the duration of induced seizures.18
Vagus nerve stimulation
VNS, in which an implanted device stimulates the vagus nerve with electrical impulses, initially was used to reduce the frequency of seizures in patients with epilepsy and treatment-resistant partial onset seizures.19 VNS was FDA-approved for TRD in July 2005.20 One VNS system, the NCP System, consists of an implantable, multi-programmable generator, known as a pulse generator, that is subcutaneously placed in the anterior chest wall during an outpatient surgical procedure. Separate bipolar nerve-stimulating electrodes are surgically wrapped around the left cervical vagus nerve, and then connected to the generator via a tunneling procedure. A telemetric wand is subsequently linked to a portable computer and used to adjust stimulation parameters.21,22
Support for using VNS for TRD came from a multitude of investigations and observations. Harden et al23 and Elger et al24 prospectively evaluated epileptic patients with standard depression symptom severity rating scales. They found that VNS was associated with statistically significant improvements in mood that were not related to reductions in seizures.23,24
The mechanism of action of VNS is not clear. Earlier researchers had found evidence that VNS affected brain regions associated with norepinephrine25 and serotonin systems26; both of these neurotransmitters have been implicated in the pathophysiology of depression. Positron emission tomography studies conducted during VNS treatment of epilepsy showed metabolic changes in cortical and subcortical areas of the brain, including the amygdala, hippocampus, and cingulate gyrus, all structures implicated in the pathophysiology of mood disorders.27
Most studies conducted to evaluate the efficacy of VNS have been observational, looking at depression ratings before and after treatment with VNS. The short-term studies measured the difference in depression rating scales at baseline and after 10 weeks of treatment. In most of these studies, treatment with VNS resulted in a statistically significant drop in depression rating scales scores, such as on the Hamilton Depression Rating Scale (HAM-D). Based on the study design and number of study participants, response rates have varied from 13%28 to 40%,29 whereas remission rates have varied from 15.3%30 to 28%.31 More than one-half of the reduction in symptoms occurred after 6 weeks of treatment.30 In longer-term follow-up studies, the antidepressant effect generally was sustained over time. Response rates remained essentially unchanged, but the remission rates increased to approximately 29%.29 Only 1 RCT has compared patients with controls; it found no significant differences in the response or remission rates between active VNS and sham VNS.32 In this study, all patients had VNS implanted, but in the control group, the VNS was never turned on.32 In a meta-analysis conducted by Martin and Martín-Sánchez,33 31.8% (95% confidence interval [CI], 23.2% to 41.8%; P < .001) of patients treated with VNS had a significant reduction in HAM-D scores. The response rate in patients with TRD ranged from 27% to 37% and the remission rate was approximately 13%. In studies that followed patients over longer periods, both the remission and response rates increased over time.34
Recent evidence suggests that the effectiveness of VNS may depend on the stimulation level. A multi-center double-blind study randomized patients to receive either a low (0.25 mA current, 130-millisecond pulse width), medium (0.5e1.0 mA, 250 millisecond), or high (1.25e1.5 mA, 250 millisecond) dose of VNS.35 Although all dose levels were associated with improvement in symptoms, a statistically significant durability in response was associated with the medium- and high-dose treatments.
Adverse effects. VNS has no major adverse effects on cognitive functioning, and some studies have found improvement in executive functioning that corresponded to improvement in depressive symptoms.30 VNS also may result in improved sleep patterns as evidenced by EEG changes.31 The most commonly reported adverse effects include pain in the incision site, hoarseness of voice, throat pain, and neck pain.36
Repetitive transcranial magnetic stimulation
rTMS is a noninvasive technique that uses high-intensity magnetic impulses to stimulate cortical neurons. A magnetic field is produced when current passes through a coil, which in turn causes electrical stimulation in the cortical neurons that results in transient changes in the excitability of the cortical neurons.37 Although many stimulation parameters exist for TMS, high-frequency stimulation to the left prefrontal cortex (HFL-rTMS) and low-frequency stimulation to the right prefrontal cortex (LFR-rTMS) have been shown most efficacious for treating depression.38 High-frequency (5 Hz to 20 Hz) stimulation using rTMS increases cortical neuron excitability, whereas low-frequency (approximately 1 Hz) is associated with reduced cortical neuron excitability.39 The choice of targeting the DLPFC stems from a large body of functional neuroimaging studies that have shown reduction in activity/blood flow in the left DLPFC and abnormal activity/blood flow in the right DLPFC.40
There is no dearth of RCTs evaluating the efficacy of rTMS vs sham rTMS (where no magnetic stimulation was provided). In a meta-analysis of 8 RCTs, low-frequency rTMS applied to the right DLPFC was associated with a remission rate of approximately 34.6%, compared with a 9.7% remission rate with sham rTMS.41 A response rate of approximately 38.2% was observed with HFL-rTMS, compared with a response rate of 15.1% for sham rTMS.41
Gaynes et al42 conducted a meta-analysis to determine the efficacy of rTMS in TRD. They found that for patients with TRD, rTMs produced a response rate of 29% and a remission rate of 30%. In long-term, naturalistic, observational studies, the response rates and remission rates were much higher (58% and 37.1%, respectively).43 Over a 1-year follow-up, almost two-thirds of patients continued to meet criteria for response to treatment.44 Trials comparing HFL-rTMS and LFR-rTMS have found no significant differences in efficacy.45
Advanced age, psychotic symptoms, and a longer duration of the current depressive episode predict poor response to rTMS. Also, imaging studies have shown that a lower metabolism in cerebellar, temporal, anterior cingulate, and occipital parts of the brain correlate with better response to HFL-rTMS.46,47
Adverse effects. The major adverse effect associated with rTMS is the risk of inducing seizures, which is more commonly associated with high-frequency rTMS. Other common adverse effects include headache, facial muscle twitching, and tinnitus.37
Deep brain stimulation
DBS is an invasive stereotactic surgical procedure. It involves unilateral or bilateral placement of electrodes at neuroanatomical locations to deliver continuous stimulation from a subcutaneously implanted pulse generator.48 In the past, destructive surgical procedures were used to treat intractable depression. Surgeries such as anterior cingulotomy, anterior capsulotomy, subcaudate tractotomy, and limbic leucotomy have been shown to effectively reduce depressive symptoms.49 The advantages of DBS over destructive procedures include the fact that DBS is reversible and that the stimulation levels can easily be adjusted, and the treatment can easily be stopped or restarted.
There is no consensus on the optimal anatomic locations for the electrode implantation in DBS. Electrodes have been implanted in the subcallosal cingulate gyrus, inferior thalamic peduncle, ventral capsule/ventral striatum, superolateral branch of the medial forebrain bundle (MFB), and nucleus accumbens.
The choice of anatomic locations stems from the large body of neuroimaging literature characterizing functional changes associated with acute depression and response to treatment. The electrode placement targets “nodes” that form an integral part of the affected neural circuits that are responsible for regulating depressive symptoms.50 Increased metabolic activity and blood flow to the subgenual cingulate gyrus and reduction in the blood flow to the DLPFC and the striatum have been associated with active depressed states. Response to antidepressant treatment has been associated with reversal of these findings.51 Functional magnetic resonance imaging studies have consistently shown increased activity in the amygdala in response to negative stimuli among patients with depression.
Regardless of the site of electrode placement, studies have reported symptomatic improvement among patients with depression who are treated with DBS. In 2 case reports, the electrode was implanted in the inferior thalamic peduncle.52,53 Each study had 1 participant, and each patient remitted.52,53
Placement of the electrodes in the nucleus accumbens resulted in a response rate of 45% in 1 study,54 whereas in a different study, all patients reported improvement in anhedonia.55 A response rate of 71% and a remission rate of 35% were observed in a study in which the electrode was implanted in the ventral capsule/ventral striatum area.56
Berlim et al57 published a systematic review and exploratory meta-analysis of studies in which the electrode had been implanted in the subgenual cingulate cortex. At 12 months, the response rate was 39.9% (95% CI, 28.4% to 52.8%), and 26.3% (95% CI, 13% to 45.9%) of patients achieved remission. The most significant drop in depression scores was observed 3 to 6 months after the surgery. No significant change in scores was observed between 6 to 12 months after surgery.57
The MFB, specifically the superolateral branch, is emerging as an exciting new target for electrode placement in DBS. Schlaepfer et al58 studied the effects of electrodes implanted bilaterally in the superolateral branch of the MFB. They observed an almost 50% reduction in symptoms by Day 7, and at the last follow-up visit (12 to 33 weeks) 4 of the 6 patients had achieved remission.58 In a recent systematic review, Gálvez et al59 found most studies had high response/remission rates without any significant adverse effects. In a recent study of DBS targeting the MFB, 3 of 4 patients had a >50% reduction in Montgomery-Åsberg Depression Rating Scale scores at the end of first week. Although 1 patient withdrew, 2 of the other 3 patients continued to report a >80% reduction in depressive symptoms, even at Week 26.60
Accurate localization of target areas (white matter tracts) and subsequent electrode placement might be an important factor governing treatment response. Riva-Posse et al61 found that clinical response was seen when the electrodes stimulated 3 specific white matter bundles. Interestingly, nonresponders were converted to responders simply by changing the position of the electrodes to include these white matter tracts.61
Adverse effects. The most common adverse effects noted during studies of DBS include pain at the site of implantation and wound infection. Other adverse effects include lead fracture, transient dysphagia, and other hardware-related problems.49
Sorting out the evidence
In the absence of head-to-head trials, it is difficult to establish a hierarchal algorithm for use of the 4 neuromodulatory treatments discussed in the article. If we were to base our decision solely on the current literature, ECT by far has the most evidence and highest remission rates.11 We can reduce the risk of cognitive deficits by using twice-weekly instead of thrice-weekly ECT, or by using unilateral instead of bilateral ECT.12 Another strategy for reducing adverse effects associated with long-term maintenance ECT is by using it in combination with VNS. ECT and VNS can be used safely concomitantly; ECT can be used to treat acutely worsening depression, and VNS for maintaining the antidepressant effect.62
Aside from ECT, rTMS is the only other treatment that has evidence from RCTs. Although the remission rates are not as high as ECT, its preferable adverse effects profile, noninvasive nature, and comparative low cost (compared with surgical procedures) make it a favorable choice. The Canadian Network for Mood and Anxiety Treatment guidelines suggest rTMS as the first-line treatment for patients who do not respond to pharmacologic treatments.63 ECT can be considered second-line treatment unless the patient has acute suicidal ideation, catatonia, psychotic features, greater treatment resistance, or physical deterioration, in which case ECT should be tried before TMS.63
Among the invasive options, VNS has more evidence and is FDA-approved for TRD. However, DBS has shown great promise in early studies, with remission rates as high as 35%.56 DBS has the advantage of being reversible, and the amount of stimulation can be adjusted easily. Despite early promise, more research is needed before DBS can be widely used in clinical settings.
1. Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol. 2007;17(11):696-707.
2. Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(suppl 13):23-29.
3. Petersen T, Papakostas GI, Posternak MA, et al. Empirical testing of two models for staging antidepressant treatment resistance. J Clin Psychopharmacol. 2005;25(4):336-341.
4. Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22.
5. Souery D, Amsterdam J, de Montigny C, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9(1-2):83-91.
6. Evans DL, Charney DS. Mood disorders and medical illness: a major public health problem. Biol. Psychiatry. 2003;54(3):177-180.
7. Sanacora G, Mason GF, Rothman DL, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160(3):577-579.
8. Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action, recent advances and limitations. Exp Neurol. 2009;219(1):20-26.
9. Perera TD, Coplan JD, Lisanby SH, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci. 2007;27(18):4894-4901.
10. Abbott CC, Gallegos P, Rediske N et al. A review of longitudinal electroconvulsive therapy: neuroimaging investigations. J Geriatr Psychiatry Neurol. 2014;27(1):33-46.
11. Heijnen WT, Birkenhäger TK, Wierdsma AI, et al. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol. 2010;30(5):616-619.
12. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
13. Semkovska M, Landau S, Dunne R et al. Bitemporal versus high-dose unilateral twice-weekly electroconvulsive therapy for depression (EFFECT-Dep): a pragmatic, randomized, non-inferiority trial. Am J Psychiatry. 2016;173(4):408-417.
14. Charlson F, Siskind D, Doi SA, et al. ECT efficacy and treatment course: a systematic review and meta-analysis of twice vs thrice weekly schedules. J Affect Disord. 2012;138(1-2):1-8.
15. Loo CK, Kaill A, Paton P, et al. The difficult-to-treat electroconvulsive therapy patient—strategies for augmenting outcomes. J Affect Disord. 2010;124(3):219-227.
16. de Vreede IM, Burger H, van Vliet IM. Prediction of response to ECT with routinely collected data in major depression. J Affect Disord. 2005;86(2-3):323-327.
17. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
18. Baghai TC, Marcuse A, Brosch M, et al. The influence of concomitant antidepressant medication on safety, tolerability and clinical effectiveness of electroconvulsive therapy. World J Biol Psychiatry. 2006;7(2):82-90.
19. Ben-Menachem E, Mañon-Espaillat R, Ristanovic R, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35(3):616-626.
20. Nemeroff CB, Mayberg HS, Krahl SE, et al. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31(7):1345-1355.
21. Matthews K, Eljamel MS. Vagus nerve stimulation and refractory depression: please can you switch me on doctor? Br J Psychiatry. 2003;183:181-183.
22. George MS, Rush AJ, Sackeim HA, et al. Vagus nerve stimulation (VNS): utility in neuropsychiatric disorders. Int J Neuropsychopharmacol. 2003;6(1):73-83.
23. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99.
24. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42(2-3):203-210.
25. Krahl SE, Clark KB, Smith DC, et al. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39(7):709-714.
26. Ben-Menachem E, Hamberger A, Hedner T, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20(3):221-227.
27. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia. 1998;39(9):983-990.
28. O’Keane V, Dinan TG, Scott L, et al. Changes in hypothalamic-pituitary-adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol Psychiatry. 2005;58(12):963-968.
29. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47(4):276-286.
30. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5):713-728.
31. Armitage R, Husain M, Hoffmann R, et al. The effects of vagus nerve stimulation on sleep EEG in depression: a preliminary report. J Psychosom Res. 2003;54(5):475-482.
32. Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58(5):347-354.
33. Martin JL, Martín-Sánchez E. Systematic review and meta-analysis of vagus nerve stimulation in the treatment of depression: variable results based on study designs. Eur Psychiatry. 2012;27(3):147-155.
34. Shah A, Carreno FR, Frazer A. Therapeutic modalities for treatment resistant depression: focus on vagal nerve stimulation and ketamine. Clin Psychopharmacol Neurosci. 2014;12(2):83-93.
35. Aaronson ST, Carpenter LL, Conway CR, et al. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul. 2013;6(4):631-640.
36. Daban C, Martinez-Aran A, Cruz N, et al. Safety and efficacy of vagus nerve stimulation in treatment-resistant depression. A systematic review. J Affect Disord. 2008;110(1-2):1-15.
37. Eitan R, Lerer B. Nonpharmacological, somatic treatments of depression: electroconvulsive therapy and novel brain stimulation modalities. Dialogues Clin Neurosci. 2006;8(2):241-258.
38. Lam RW, Chan P, Wilkins-Ho M, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Can J Psychiatry. 2008;53(9):621-631.
39. Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584-2596.
40. Fitzgerald PB, Oxley TJ, Laird AR, et al. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148(1):33-45.
41. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013;38(4):543-551.
42. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression. J Clin Psychiatry. 2014;75(5):477-489; quiz 489.
43. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
44. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder. J Clin Psychiatry. 2014;75(12):1394-1401.
45. Fitzgerald PB, Hoy K, Daskalakis ZJ, et al. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety. 2009;26(3):229-234.
46. Dumas R, Padovani R, Richieri R, et al. Repetitive transcranial magnetic stimulation in major depression: response factor [in French]. Encephale. 2012;38(4):360-368.
47. Fregni F, Marcolin MA, Myczkowski M, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int. J. Neuropsychopharmacol. 2006;9(6):641-654.
48. Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168(5):502-510.
49. Taghva AS, Malone DA, Rezai AR. Deep brain stimulation for treatment-resistant depression. World Neurosurg. 2013;80(3-4):S27.e17-S27.e24.
50. Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193-207.
51. Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682.
52. Jiménez F, Velasco F, Salín-Pascual R, et al. Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir Suppl. 2007;97(pt 2):393-398.
53. Jiménez F, Velasco F, Salin-Pascual R, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57(3):585-593; discussion 585-593.
54. Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110-116.
55. Schlaepfer TE, Bewernick BH, Kayser S, et al. Deep brain stimulation of the human reward system for major depression—rationale, outcomes and outlook. Neuropsychopharmacology. 2014;39(6):1303-1314.
56. Malone DA Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267-275.
57. Berlim MT, McGirr A, Van den Eynde F, et al. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31-38.
58. Schlaepfer TE, Bewernick BH, Kayser S, et al. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73(12):1204-1212.
59. Gálvez JF, Keser Z, Mwangi B, et al. The medial forebrain bundle as a deep brain stimulation target for treatment resistant depression: a review of published data. Prog Neuropsychopharmacol Biol Psychiatry. 2015;58:59-70.
60. Fenoy AJ, Schulz P, Selvaraj. Deep brain stimulation of the medial forebrain bundle: distinctive responses in resistant depression. J Affect Disord. 2016;203:143-151.
61. Riva-Posse P, Choi KS, Holtzheimer PE, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76(12):963-969.
62. Burke MJ, Husain MM. Concomitant use of vagus nerve stimulation and electroconvulsive therapy for treatment-resistant depression. J ECT. 2006;22(3):218-222.
63. Milev R V, Giacobbe P, Kennedy SH, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: section 4. Neurostimulation treatments. Can J Psychiatry. 2016;61:561-575.
1. Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol. 2007;17(11):696-707.
2. Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(suppl 13):23-29.
3. Petersen T, Papakostas GI, Posternak MA, et al. Empirical testing of two models for staging antidepressant treatment resistance. J Clin Psychopharmacol. 2005;25(4):336-341.
4. Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22.
5. Souery D, Amsterdam J, de Montigny C, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9(1-2):83-91.
6. Evans DL, Charney DS. Mood disorders and medical illness: a major public health problem. Biol. Psychiatry. 2003;54(3):177-180.
7. Sanacora G, Mason GF, Rothman DL, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160(3):577-579.
8. Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action, recent advances and limitations. Exp Neurol. 2009;219(1):20-26.
9. Perera TD, Coplan JD, Lisanby SH, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci. 2007;27(18):4894-4901.
10. Abbott CC, Gallegos P, Rediske N et al. A review of longitudinal electroconvulsive therapy: neuroimaging investigations. J Geriatr Psychiatry Neurol. 2014;27(1):33-46.
11. Heijnen WT, Birkenhäger TK, Wierdsma AI, et al. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol. 2010;30(5):616-619.
12. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
13. Semkovska M, Landau S, Dunne R et al. Bitemporal versus high-dose unilateral twice-weekly electroconvulsive therapy for depression (EFFECT-Dep): a pragmatic, randomized, non-inferiority trial. Am J Psychiatry. 2016;173(4):408-417.
14. Charlson F, Siskind D, Doi SA, et al. ECT efficacy and treatment course: a systematic review and meta-analysis of twice vs thrice weekly schedules. J Affect Disord. 2012;138(1-2):1-8.
15. Loo CK, Kaill A, Paton P, et al. The difficult-to-treat electroconvulsive therapy patient—strategies for augmenting outcomes. J Affect Disord. 2010;124(3):219-227.
16. de Vreede IM, Burger H, van Vliet IM. Prediction of response to ECT with routinely collected data in major depression. J Affect Disord. 2005;86(2-3):323-327.
17. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
18. Baghai TC, Marcuse A, Brosch M, et al. The influence of concomitant antidepressant medication on safety, tolerability and clinical effectiveness of electroconvulsive therapy. World J Biol Psychiatry. 2006;7(2):82-90.
19. Ben-Menachem E, Mañon-Espaillat R, Ristanovic R, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35(3):616-626.
20. Nemeroff CB, Mayberg HS, Krahl SE, et al. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31(7):1345-1355.
21. Matthews K, Eljamel MS. Vagus nerve stimulation and refractory depression: please can you switch me on doctor? Br J Psychiatry. 2003;183:181-183.
22. George MS, Rush AJ, Sackeim HA, et al. Vagus nerve stimulation (VNS): utility in neuropsychiatric disorders. Int J Neuropsychopharmacol. 2003;6(1):73-83.
23. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99.
24. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42(2-3):203-210.
25. Krahl SE, Clark KB, Smith DC, et al. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39(7):709-714.
26. Ben-Menachem E, Hamberger A, Hedner T, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20(3):221-227.
27. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia. 1998;39(9):983-990.
28. O’Keane V, Dinan TG, Scott L, et al. Changes in hypothalamic-pituitary-adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol Psychiatry. 2005;58(12):963-968.
29. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47(4):276-286.
30. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5):713-728.
31. Armitage R, Husain M, Hoffmann R, et al. The effects of vagus nerve stimulation on sleep EEG in depression: a preliminary report. J Psychosom Res. 2003;54(5):475-482.
32. Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58(5):347-354.
33. Martin JL, Martín-Sánchez E. Systematic review and meta-analysis of vagus nerve stimulation in the treatment of depression: variable results based on study designs. Eur Psychiatry. 2012;27(3):147-155.
34. Shah A, Carreno FR, Frazer A. Therapeutic modalities for treatment resistant depression: focus on vagal nerve stimulation and ketamine. Clin Psychopharmacol Neurosci. 2014;12(2):83-93.
35. Aaronson ST, Carpenter LL, Conway CR, et al. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul. 2013;6(4):631-640.
36. Daban C, Martinez-Aran A, Cruz N, et al. Safety and efficacy of vagus nerve stimulation in treatment-resistant depression. A systematic review. J Affect Disord. 2008;110(1-2):1-15.
37. Eitan R, Lerer B. Nonpharmacological, somatic treatments of depression: electroconvulsive therapy and novel brain stimulation modalities. Dialogues Clin Neurosci. 2006;8(2):241-258.
38. Lam RW, Chan P, Wilkins-Ho M, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Can J Psychiatry. 2008;53(9):621-631.
39. Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584-2596.
40. Fitzgerald PB, Oxley TJ, Laird AR, et al. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148(1):33-45.
41. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013;38(4):543-551.
42. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression. J Clin Psychiatry. 2014;75(5):477-489; quiz 489.
43. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
44. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder. J Clin Psychiatry. 2014;75(12):1394-1401.
45. Fitzgerald PB, Hoy K, Daskalakis ZJ, et al. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety. 2009;26(3):229-234.
46. Dumas R, Padovani R, Richieri R, et al. Repetitive transcranial magnetic stimulation in major depression: response factor [in French]. Encephale. 2012;38(4):360-368.
47. Fregni F, Marcolin MA, Myczkowski M, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int. J. Neuropsychopharmacol. 2006;9(6):641-654.
48. Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168(5):502-510.
49. Taghva AS, Malone DA, Rezai AR. Deep brain stimulation for treatment-resistant depression. World Neurosurg. 2013;80(3-4):S27.e17-S27.e24.
50. Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193-207.
51. Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682.
52. Jiménez F, Velasco F, Salín-Pascual R, et al. Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir Suppl. 2007;97(pt 2):393-398.
53. Jiménez F, Velasco F, Salin-Pascual R, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57(3):585-593; discussion 585-593.
54. Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110-116.
55. Schlaepfer TE, Bewernick BH, Kayser S, et al. Deep brain stimulation of the human reward system for major depression—rationale, outcomes and outlook. Neuropsychopharmacology. 2014;39(6):1303-1314.
56. Malone DA Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267-275.
57. Berlim MT, McGirr A, Van den Eynde F, et al. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31-38.
58. Schlaepfer TE, Bewernick BH, Kayser S, et al. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73(12):1204-1212.
59. Gálvez JF, Keser Z, Mwangi B, et al. The medial forebrain bundle as a deep brain stimulation target for treatment resistant depression: a review of published data. Prog Neuropsychopharmacol Biol Psychiatry. 2015;58:59-70.
60. Fenoy AJ, Schulz P, Selvaraj. Deep brain stimulation of the medial forebrain bundle: distinctive responses in resistant depression. J Affect Disord. 2016;203:143-151.
61. Riva-Posse P, Choi KS, Holtzheimer PE, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76(12):963-969.
62. Burke MJ, Husain MM. Concomitant use of vagus nerve stimulation and electroconvulsive therapy for treatment-resistant depression. J ECT. 2006;22(3):218-222.
63. Milev R V, Giacobbe P, Kennedy SH, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: section 4. Neurostimulation treatments. Can J Psychiatry. 2016;61:561-575.
Can what we learned about reducing no-shows in our clinic work for you?
The no-show rate is high in ambulatory psychiatric clinics, especially those associated with academic medical institutions, which usually accept all public insurance providers and do not maintain a strict rule by which patients are charged a penalty when they fail to keep a scheduled appointment—a policy that, to the contrary, is customary in private practice. The University of Texas (UT) Health Sciences Center at Houston is primarily an academic medical center with resident-managed, faculty-supervised clinics that provide care to a large volume of patients.
At the UT clinics, we have struggled with a high no-show rate, and were challenged to reduce that rate. Our study of the problem, formulation and application of strategies to reduce that rate, and a discussion of our results are provided here for the benefit of psychiatric clinicians who struggle with this problem, to the detriment of their patients’ health and the financial well-being of the practice.
For patients who have a severe psychiatric illness, such as schizophrenia or bipolar disorder, 60% to 70% of the direct cost of their care is attributable to inpatient services.1,2 Poor medication adherence is a critical factor: It results in exacerbation of symptoms, relapse, and hospitalization. The matter is compounded by patients’ failure to show up for scheduled follow-up appointments.
Studies show that failure to attend routinely scheduled outpatient appointments increases the risk of hospitalization. Recent research has shown that, among all causes of hospitalization, length of stay and relapse hospitalization are increased in patients with low adherence to their treatment regimen.3 Patients who miss an appointment also are more unwell and more functionally impaired—also contributing to a higher risk and rate of rehospitalization.4,5
To begin to address the problem at UT, we acknowledged that an elevated no-show rate is linked to medication nonadherence, increased risk of re-hospitalization, and increased costs associated with poor care.
Impact of nonadherence
Significant evidence supports the efficacy of antipsychotic medications for treating schizophrenia, of course,6 but that success story is undermined by the mean rate of medication nonadherence among schizophrenia patients, which can be as high as 49% in studies.7 (The actual rate might be higher because those studies do not account for persons who refuse treatment or drop out.)
Nonadherence increases the risk of relapse 3.7-fold, compared with what is seen in patients who adhere to treatment.8 Nonadherence to a medication regimen also can increase patients’ risk of engaging in assault and other dangerous behaviors, especially during periods of psychosis.8 Variables consistently associated with nonadherence include poor insight, negative attitude or subjective response toward medication, previous nonadherence, substance abuse, shorter duration of illness, inadequate discharge planning or after-care environment, and poorer therapeutic alliance.7,8
Investigation of medication adherence in bipolar disorder suggests that 1 in 3 patients fail to take at least 30% of their medication.9 In such patients, medication nonadherence can lead to mania, depression, hospital readmission, suicide, increased substance abuse, and nonresponse to treatment.10,11
Depression also is associated with an increased rate of health care utilization and severe limitation in daily functioning.12 Compared with non-depressed patients, depressed patients are 3 times more likely to be nonadherent with medical treatment recommendations.13 Estimates of medication nonadherence for unipolar and bipolar disorders range from 10% to 60% (median, 40%). This prevalence has not changed significantly with the introduction of new medications.14
Our literature review of research devoted to reducing no-shows found that few studies have explored this critical treatment concern. The no-show rate was higher among younger patients and slightly higher among women, but varied by diagnosis.15 The most common reason psychiatric patients gave for missing an appointment was “forgetting”—a response heard twice as often among no-show patients in psychiatry than in other specialties.4
Little has been tried to solve the problem. Often, community mental health centers and private practices double-book appointments. Double-booking is intended to reduce the financial burden on the practice when a patient misses an appointment. This approach fails to address nonadherence or the poor care that usually results when a patient misses regular outpatient appointments.
Several methods have been employed to improve adherence, such as electronic pill dispensing.16 Increasing medication adherence appears to be a key factor in improving quality-of-life measures in patients with schizophrenia.6
The UT project
Methods. This project was completed at the ambulatory psychiatry clinic at the UT Medical School at Houston. The clinic staff comprises residents and faculty members who provide outpatient care. During the study period, the clinic was scheduling as many as 800 office visits a month, including a mix of new and follow-up appointments. Two weeks’ retrospective data revealed a no-show rate of 31%.
For the project, we defined no-show rate as the total number of patients who missed an appointment or canceled fewer than 24 hours before the scheduled time, divided by the total number of patients scheduled that day.
Table 1 demonstrates the no-show rate calculations for 1 of the weeks preceding the start of the project. Given approximately 800 patient appointments a month, a 31% no-show rate meant that, first, 248 patients failed to receive recommended care and, second, 248 appointment slots were wasted.
Besides undermining such components of quality care as patient safety and medication compliance, the high no-show rate also harms employee morale and productivity; impairs medical education; and, possibly, increases the use of emergency and after-hour services.
We agreed that our current no-show rate of 31% was too high.
We then formed a team of residents, faculty members, therapists, front office staff, an office manager, and an office nurse. We explored and hypothesized what could be contributing to the high no-show rate (Table 2).
Several interventions were then devised and implemented:
• Patients. We increased patient education about 1) the need for regular follow-up and 2) risks associated with medication nonadherence.
• Environment. We explored environmental limitations to access and agreed that certain static factors could not be modified—eg, location of the clinic and lack of access to public transportation. We were able to make some changes to the environment (explained later) to reduce wait time.
• Staff. Some patients had complained of long wait times, which could hinder active participation in treatment. We agreed that the clinic nurse would make rounds through the waiting room every hour and talk to patients. The nurse would identify patients who had been waiting for longer than 30 minutes after their scheduled appointment time and notify the doctor accordingly. We also agreed to revise patient appointment reminder practices: instead of using an automated answering service, one of the staff members called patients personally to remind them about their appointments. (This also allowed us to update telephone numbers for many patients; numbers on record often were outdated.) We initially recruited summer interns and provided a written script to follow during calls to patients, which allowed patients to confirm, cancel, or reschedule their appointment. Once we demonstrated positive results from the change to personal calls, the department agreed to absorb the cost, and front desk personnel began making reminder calls.
• Policies and procedures. Although some practices are able to charge a small fine for missed appointments, this was not allowed at our institution. Instead, we had several departmental policies on the books, such as discharging patients from our clinics if they missed 3 consecutive appointments and limiting prescription refills to a maximum of 6 months. These policies were neither communicated to patients and staff, nor were they implemented. We decided to educate patients and staff and implement the policies.
• Transparency. We posted the no-show rate in common areas so that the team could review and follow the progression of that rate as we implemented the changes. This allowed team members to take ownership of the project and facilitated active participation.
By implementing these changes, we aimed to reduce the no-show rate to 20%.
Results. We were able to reduce the no-show rate from a documented average of 31% to an average of 12% during the study period after implementing all the proposed changes in the outpatient clinics.
We calculated the no-show rate (as shown in Table 1 for May 2013), then collected the daily no-show rate from June to September 2013 (Figure). With these calculations, we demonstrated a reduction in the no-show rate to 12%. Because of the time and effort required, we reduced data collection from daily to weekly, beginning in September.
Applying the changes required consistent effort and substantial input from various stakeholders—front desk staff, residents, the nurse, therapists, and faculty. Gradually, we were able to implement all the changes.
Keeping the no-show rate low required consistent effort and monitoring of the newly implemented procedures because even a slight change, such as failure to make reminder calls, resulted in a sudden increase in the no-show rate (that was the case in October of the study period, when we were short-staffed and could not call every patient). Patients told us that it was difficult to ignore a personal call; if they were not planning to keep the appointment, the call allowed them to reschedule on the spot.
We also made sure that current no-show rates were posted in common areas, visible to team members every day.
Discussion
We attempted a literature review of research exploring approaches to reducing the no-show rate but found few studies that explored this critical concern in patient treatment.15 Some data suggested that, in the setting studied, the no-show rate:
• was higher among younger patients (age 20 to 39) than older ones (age 60 to 79)
• was slightly higher in women than in men
• varied by diagnosis.
We found a paucity of data regarding interventions that can reduce the no-show rate.
Among the changes we made, the one that had the greatest impact was personalized appointment reminder calls, as evidenced by our patients’ reports and the increase in the no-show rate when personal calls were not made.
We also realized that, although we had several departmental policies in place regarding appointments, they were not being followed. Raising awareness among team members and their patients also was an effective deterrent to a no-show for an appointment. For example, patients were informed that 3 consecutive no-shows could lead to termination of care. Often, they reacted with surprise to this caution but also voiced a desire to improve their attendance to avoid such an outcome.
We found that establishing common operational definitions is important. It also was important to have a cohesive team, with every member agreeing on goals and changes to operational policies that needed to be implemented. Support from the department chair and the administration, we learned, is vital to the success of such an intervention.
A note about limitations. The goal of the project was limited to reducing the no-show rate. We demonstrated that this is possible among patients who have a severe mental illness, and that reducing the associated waste of time and resources can improve finances in an academic department of psychiatry. We would need additional measures, however, to quantify medication adherence and hospitalization; a larger, more inclusive project is needed to demonstrate that reducing the no-show rate reduces the symptomatic burden of psychiatric illness.
Comments in conclusion
This project was designed and conducted as a required part of a Clinical Safety and Effectiveness Program at Memorial Hermann Texas Medical Center and the UT Medical School at Houston.17 Although there was initial hesitancy about attempting to reduce the no-show rate in a chronically mentally ill population, the success of this project—indeed, it surpassed its proposed goals—demonstrates that operational changes in any clinic can reduce the no-show rate. It also is important to maintain operational changes, however; without consistent effort, desired results cannot be sustained.
Last, it is possible to replicate the methodology of this project and thereby attempt to reduce the no-show rate in other divisions of medicine that offer care to chronically ill patients, such as pediatrics and family medicine.
Bottom Line
Failure to attend routinely scheduled outpatient appointments increases a patient’s functional impairment and risk of hospitalization. Patient education, appointment reminder phone calls, revised policies and procedures, and transparency regarding the no-show rate can reduce the number of missed appointments and improve patient outcomes.
Related Resources
• Mitchell AJ, Selmes T. Why don’t patients attend their appointments? Maintaining engagement with psychiatric services. Advances in Psychiatric Treatment. 2007;13:423-434.
• Molfenter T. Reducing appointment no-shows: going from theory to practice. Subst Use Misuse. 2013;48(9):743-749.
• Williston MA, Block-Lerner J, Wolanin A, et al. Brief acceptance-based intervention for increasing intake attendance at a community mental health center. Psychol Serv. 2014;11(3):324-332.
Disclosure
Dr. Gajwani receives grant or research support from the National Institute on Mental Health, the National Institute of Drug Abuse, The Stanley Foundation, and Forest Laboratories, Inc. He is a member of the speakers’ bureau of AstraZeneca, Merck, Otsuka America Pharmaceutical, and Sunovion Pharmaceuticals.
1. Wyatt RJ, Henter I. An economic evaluation of manic-depressive illness—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5):213-219.
2. Wyatt RJ, Henter I, Leary MC, et al. An economic evaluation of schizophrenia—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5);196-205.
3. Offord S, Lin J, Wong B, et al. Impact of oral antipsychotic medication adherence on healthcare resource utilization among schizophrenic patients with medicare coverage. Community Ment Health J. 2013;49(6):625-629.
4. Killaspy H, Banerjee S, King M, et al. Prospective controlled study of psychiatric out-patient non-attendance: characteristics and outcome. Br J Psychiatry. 2000;176:160- 165.
5. Nelson EA, Maruish ME, Axler JL. Effects of discharge planning and compliance with outpatient appointments on readmission rates. Psychiatr Serv. 2000;51(7):885-889.
6. Thornley B, Adams C. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ. 1998;317(7167):1181-1184.
7. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892-909.
8. Fenton WS, Blyler C, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637-651.
9. Scott J, Pope M. Self-reported adherence to treatment with mood stabilizers, plasma levels, and psychiatric hospitalization. Am J Psychiatry. 2002;159(11):1927-1929.
10. Adams J, Scott J. Predicting medication adherence in severe mental disorders. Acta Psychiatr Scand. 2000;101(2):119-124.
11. Müller-Oerlinghausen B, Müser-Causemann B, Volk J. Suicides and parasuicides in a high-risk patient group on and off lithium long-term treatment. J Affect Disord. 1992;25(4):261-269.
12. Manning WG Jr, Wells KB. The effects of psychological distress and psychological well-being on use of medical services. Med Care. 1992;30(6):541-553.
13. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
14. Lingam R, Scott J. Treatment non‐adherence in affective disorders. Acta Psychiatr Scand. 2002;105(3):164-172.
15. Allan AT. No-shows at a community mental health clinic: a pilot study. Int J Soc Psychiatry. 1988;34(1):40-46.
16. Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv. 1998;49(2):196-201.
17. Gajwani P. Improving quality of care: reducing no-show rate in ambulatory psychiatry clinic. Poster presented at: American Psychiatric Association 166th Annual Meeting; May 18-22, 2013; San Francisco, CA.
The no-show rate is high in ambulatory psychiatric clinics, especially those associated with academic medical institutions, which usually accept all public insurance providers and do not maintain a strict rule by which patients are charged a penalty when they fail to keep a scheduled appointment—a policy that, to the contrary, is customary in private practice. The University of Texas (UT) Health Sciences Center at Houston is primarily an academic medical center with resident-managed, faculty-supervised clinics that provide care to a large volume of patients.
At the UT clinics, we have struggled with a high no-show rate, and were challenged to reduce that rate. Our study of the problem, formulation and application of strategies to reduce that rate, and a discussion of our results are provided here for the benefit of psychiatric clinicians who struggle with this problem, to the detriment of their patients’ health and the financial well-being of the practice.
For patients who have a severe psychiatric illness, such as schizophrenia or bipolar disorder, 60% to 70% of the direct cost of their care is attributable to inpatient services.1,2 Poor medication adherence is a critical factor: It results in exacerbation of symptoms, relapse, and hospitalization. The matter is compounded by patients’ failure to show up for scheduled follow-up appointments.
Studies show that failure to attend routinely scheduled outpatient appointments increases the risk of hospitalization. Recent research has shown that, among all causes of hospitalization, length of stay and relapse hospitalization are increased in patients with low adherence to their treatment regimen.3 Patients who miss an appointment also are more unwell and more functionally impaired—also contributing to a higher risk and rate of rehospitalization.4,5
To begin to address the problem at UT, we acknowledged that an elevated no-show rate is linked to medication nonadherence, increased risk of re-hospitalization, and increased costs associated with poor care.
Impact of nonadherence
Significant evidence supports the efficacy of antipsychotic medications for treating schizophrenia, of course,6 but that success story is undermined by the mean rate of medication nonadherence among schizophrenia patients, which can be as high as 49% in studies.7 (The actual rate might be higher because those studies do not account for persons who refuse treatment or drop out.)
Nonadherence increases the risk of relapse 3.7-fold, compared with what is seen in patients who adhere to treatment.8 Nonadherence to a medication regimen also can increase patients’ risk of engaging in assault and other dangerous behaviors, especially during periods of psychosis.8 Variables consistently associated with nonadherence include poor insight, negative attitude or subjective response toward medication, previous nonadherence, substance abuse, shorter duration of illness, inadequate discharge planning or after-care environment, and poorer therapeutic alliance.7,8
Investigation of medication adherence in bipolar disorder suggests that 1 in 3 patients fail to take at least 30% of their medication.9 In such patients, medication nonadherence can lead to mania, depression, hospital readmission, suicide, increased substance abuse, and nonresponse to treatment.10,11
Depression also is associated with an increased rate of health care utilization and severe limitation in daily functioning.12 Compared with non-depressed patients, depressed patients are 3 times more likely to be nonadherent with medical treatment recommendations.13 Estimates of medication nonadherence for unipolar and bipolar disorders range from 10% to 60% (median, 40%). This prevalence has not changed significantly with the introduction of new medications.14
Our literature review of research devoted to reducing no-shows found that few studies have explored this critical treatment concern. The no-show rate was higher among younger patients and slightly higher among women, but varied by diagnosis.15 The most common reason psychiatric patients gave for missing an appointment was “forgetting”—a response heard twice as often among no-show patients in psychiatry than in other specialties.4
Little has been tried to solve the problem. Often, community mental health centers and private practices double-book appointments. Double-booking is intended to reduce the financial burden on the practice when a patient misses an appointment. This approach fails to address nonadherence or the poor care that usually results when a patient misses regular outpatient appointments.
Several methods have been employed to improve adherence, such as electronic pill dispensing.16 Increasing medication adherence appears to be a key factor in improving quality-of-life measures in patients with schizophrenia.6
The UT project
Methods. This project was completed at the ambulatory psychiatry clinic at the UT Medical School at Houston. The clinic staff comprises residents and faculty members who provide outpatient care. During the study period, the clinic was scheduling as many as 800 office visits a month, including a mix of new and follow-up appointments. Two weeks’ retrospective data revealed a no-show rate of 31%.
For the project, we defined no-show rate as the total number of patients who missed an appointment or canceled fewer than 24 hours before the scheduled time, divided by the total number of patients scheduled that day.
Table 1 demonstrates the no-show rate calculations for 1 of the weeks preceding the start of the project. Given approximately 800 patient appointments a month, a 31% no-show rate meant that, first, 248 patients failed to receive recommended care and, second, 248 appointment slots were wasted.
Besides undermining such components of quality care as patient safety and medication compliance, the high no-show rate also harms employee morale and productivity; impairs medical education; and, possibly, increases the use of emergency and after-hour services.
We agreed that our current no-show rate of 31% was too high.
We then formed a team of residents, faculty members, therapists, front office staff, an office manager, and an office nurse. We explored and hypothesized what could be contributing to the high no-show rate (Table 2).
Several interventions were then devised and implemented:
• Patients. We increased patient education about 1) the need for regular follow-up and 2) risks associated with medication nonadherence.
• Environment. We explored environmental limitations to access and agreed that certain static factors could not be modified—eg, location of the clinic and lack of access to public transportation. We were able to make some changes to the environment (explained later) to reduce wait time.
• Staff. Some patients had complained of long wait times, which could hinder active participation in treatment. We agreed that the clinic nurse would make rounds through the waiting room every hour and talk to patients. The nurse would identify patients who had been waiting for longer than 30 minutes after their scheduled appointment time and notify the doctor accordingly. We also agreed to revise patient appointment reminder practices: instead of using an automated answering service, one of the staff members called patients personally to remind them about their appointments. (This also allowed us to update telephone numbers for many patients; numbers on record often were outdated.) We initially recruited summer interns and provided a written script to follow during calls to patients, which allowed patients to confirm, cancel, or reschedule their appointment. Once we demonstrated positive results from the change to personal calls, the department agreed to absorb the cost, and front desk personnel began making reminder calls.
• Policies and procedures. Although some practices are able to charge a small fine for missed appointments, this was not allowed at our institution. Instead, we had several departmental policies on the books, such as discharging patients from our clinics if they missed 3 consecutive appointments and limiting prescription refills to a maximum of 6 months. These policies were neither communicated to patients and staff, nor were they implemented. We decided to educate patients and staff and implement the policies.
• Transparency. We posted the no-show rate in common areas so that the team could review and follow the progression of that rate as we implemented the changes. This allowed team members to take ownership of the project and facilitated active participation.
By implementing these changes, we aimed to reduce the no-show rate to 20%.
Results. We were able to reduce the no-show rate from a documented average of 31% to an average of 12% during the study period after implementing all the proposed changes in the outpatient clinics.
We calculated the no-show rate (as shown in Table 1 for May 2013), then collected the daily no-show rate from June to September 2013 (Figure). With these calculations, we demonstrated a reduction in the no-show rate to 12%. Because of the time and effort required, we reduced data collection from daily to weekly, beginning in September.
Applying the changes required consistent effort and substantial input from various stakeholders—front desk staff, residents, the nurse, therapists, and faculty. Gradually, we were able to implement all the changes.
Keeping the no-show rate low required consistent effort and monitoring of the newly implemented procedures because even a slight change, such as failure to make reminder calls, resulted in a sudden increase in the no-show rate (that was the case in October of the study period, when we were short-staffed and could not call every patient). Patients told us that it was difficult to ignore a personal call; if they were not planning to keep the appointment, the call allowed them to reschedule on the spot.
We also made sure that current no-show rates were posted in common areas, visible to team members every day.
Discussion
We attempted a literature review of research exploring approaches to reducing the no-show rate but found few studies that explored this critical concern in patient treatment.15 Some data suggested that, in the setting studied, the no-show rate:
• was higher among younger patients (age 20 to 39) than older ones (age 60 to 79)
• was slightly higher in women than in men
• varied by diagnosis.
We found a paucity of data regarding interventions that can reduce the no-show rate.
Among the changes we made, the one that had the greatest impact was personalized appointment reminder calls, as evidenced by our patients’ reports and the increase in the no-show rate when personal calls were not made.
We also realized that, although we had several departmental policies in place regarding appointments, they were not being followed. Raising awareness among team members and their patients also was an effective deterrent to a no-show for an appointment. For example, patients were informed that 3 consecutive no-shows could lead to termination of care. Often, they reacted with surprise to this caution but also voiced a desire to improve their attendance to avoid such an outcome.
We found that establishing common operational definitions is important. It also was important to have a cohesive team, with every member agreeing on goals and changes to operational policies that needed to be implemented. Support from the department chair and the administration, we learned, is vital to the success of such an intervention.
A note about limitations. The goal of the project was limited to reducing the no-show rate. We demonstrated that this is possible among patients who have a severe mental illness, and that reducing the associated waste of time and resources can improve finances in an academic department of psychiatry. We would need additional measures, however, to quantify medication adherence and hospitalization; a larger, more inclusive project is needed to demonstrate that reducing the no-show rate reduces the symptomatic burden of psychiatric illness.
Comments in conclusion
This project was designed and conducted as a required part of a Clinical Safety and Effectiveness Program at Memorial Hermann Texas Medical Center and the UT Medical School at Houston.17 Although there was initial hesitancy about attempting to reduce the no-show rate in a chronically mentally ill population, the success of this project—indeed, it surpassed its proposed goals—demonstrates that operational changes in any clinic can reduce the no-show rate. It also is important to maintain operational changes, however; without consistent effort, desired results cannot be sustained.
Last, it is possible to replicate the methodology of this project and thereby attempt to reduce the no-show rate in other divisions of medicine that offer care to chronically ill patients, such as pediatrics and family medicine.
Bottom Line
Failure to attend routinely scheduled outpatient appointments increases a patient’s functional impairment and risk of hospitalization. Patient education, appointment reminder phone calls, revised policies and procedures, and transparency regarding the no-show rate can reduce the number of missed appointments and improve patient outcomes.
Related Resources
• Mitchell AJ, Selmes T. Why don’t patients attend their appointments? Maintaining engagement with psychiatric services. Advances in Psychiatric Treatment. 2007;13:423-434.
• Molfenter T. Reducing appointment no-shows: going from theory to practice. Subst Use Misuse. 2013;48(9):743-749.
• Williston MA, Block-Lerner J, Wolanin A, et al. Brief acceptance-based intervention for increasing intake attendance at a community mental health center. Psychol Serv. 2014;11(3):324-332.
Disclosure
Dr. Gajwani receives grant or research support from the National Institute on Mental Health, the National Institute of Drug Abuse, The Stanley Foundation, and Forest Laboratories, Inc. He is a member of the speakers’ bureau of AstraZeneca, Merck, Otsuka America Pharmaceutical, and Sunovion Pharmaceuticals.
The no-show rate is high in ambulatory psychiatric clinics, especially those associated with academic medical institutions, which usually accept all public insurance providers and do not maintain a strict rule by which patients are charged a penalty when they fail to keep a scheduled appointment—a policy that, to the contrary, is customary in private practice. The University of Texas (UT) Health Sciences Center at Houston is primarily an academic medical center with resident-managed, faculty-supervised clinics that provide care to a large volume of patients.
At the UT clinics, we have struggled with a high no-show rate, and were challenged to reduce that rate. Our study of the problem, formulation and application of strategies to reduce that rate, and a discussion of our results are provided here for the benefit of psychiatric clinicians who struggle with this problem, to the detriment of their patients’ health and the financial well-being of the practice.
For patients who have a severe psychiatric illness, such as schizophrenia or bipolar disorder, 60% to 70% of the direct cost of their care is attributable to inpatient services.1,2 Poor medication adherence is a critical factor: It results in exacerbation of symptoms, relapse, and hospitalization. The matter is compounded by patients’ failure to show up for scheduled follow-up appointments.
Studies show that failure to attend routinely scheduled outpatient appointments increases the risk of hospitalization. Recent research has shown that, among all causes of hospitalization, length of stay and relapse hospitalization are increased in patients with low adherence to their treatment regimen.3 Patients who miss an appointment also are more unwell and more functionally impaired—also contributing to a higher risk and rate of rehospitalization.4,5
To begin to address the problem at UT, we acknowledged that an elevated no-show rate is linked to medication nonadherence, increased risk of re-hospitalization, and increased costs associated with poor care.
Impact of nonadherence
Significant evidence supports the efficacy of antipsychotic medications for treating schizophrenia, of course,6 but that success story is undermined by the mean rate of medication nonadherence among schizophrenia patients, which can be as high as 49% in studies.7 (The actual rate might be higher because those studies do not account for persons who refuse treatment or drop out.)
Nonadherence increases the risk of relapse 3.7-fold, compared with what is seen in patients who adhere to treatment.8 Nonadherence to a medication regimen also can increase patients’ risk of engaging in assault and other dangerous behaviors, especially during periods of psychosis.8 Variables consistently associated with nonadherence include poor insight, negative attitude or subjective response toward medication, previous nonadherence, substance abuse, shorter duration of illness, inadequate discharge planning or after-care environment, and poorer therapeutic alliance.7,8
Investigation of medication adherence in bipolar disorder suggests that 1 in 3 patients fail to take at least 30% of their medication.9 In such patients, medication nonadherence can lead to mania, depression, hospital readmission, suicide, increased substance abuse, and nonresponse to treatment.10,11
Depression also is associated with an increased rate of health care utilization and severe limitation in daily functioning.12 Compared with non-depressed patients, depressed patients are 3 times more likely to be nonadherent with medical treatment recommendations.13 Estimates of medication nonadherence for unipolar and bipolar disorders range from 10% to 60% (median, 40%). This prevalence has not changed significantly with the introduction of new medications.14
Our literature review of research devoted to reducing no-shows found that few studies have explored this critical treatment concern. The no-show rate was higher among younger patients and slightly higher among women, but varied by diagnosis.15 The most common reason psychiatric patients gave for missing an appointment was “forgetting”—a response heard twice as often among no-show patients in psychiatry than in other specialties.4
Little has been tried to solve the problem. Often, community mental health centers and private practices double-book appointments. Double-booking is intended to reduce the financial burden on the practice when a patient misses an appointment. This approach fails to address nonadherence or the poor care that usually results when a patient misses regular outpatient appointments.
Several methods have been employed to improve adherence, such as electronic pill dispensing.16 Increasing medication adherence appears to be a key factor in improving quality-of-life measures in patients with schizophrenia.6
The UT project
Methods. This project was completed at the ambulatory psychiatry clinic at the UT Medical School at Houston. The clinic staff comprises residents and faculty members who provide outpatient care. During the study period, the clinic was scheduling as many as 800 office visits a month, including a mix of new and follow-up appointments. Two weeks’ retrospective data revealed a no-show rate of 31%.
For the project, we defined no-show rate as the total number of patients who missed an appointment or canceled fewer than 24 hours before the scheduled time, divided by the total number of patients scheduled that day.
Table 1 demonstrates the no-show rate calculations for 1 of the weeks preceding the start of the project. Given approximately 800 patient appointments a month, a 31% no-show rate meant that, first, 248 patients failed to receive recommended care and, second, 248 appointment slots were wasted.
Besides undermining such components of quality care as patient safety and medication compliance, the high no-show rate also harms employee morale and productivity; impairs medical education; and, possibly, increases the use of emergency and after-hour services.
We agreed that our current no-show rate of 31% was too high.
We then formed a team of residents, faculty members, therapists, front office staff, an office manager, and an office nurse. We explored and hypothesized what could be contributing to the high no-show rate (Table 2).
Several interventions were then devised and implemented:
• Patients. We increased patient education about 1) the need for regular follow-up and 2) risks associated with medication nonadherence.
• Environment. We explored environmental limitations to access and agreed that certain static factors could not be modified—eg, location of the clinic and lack of access to public transportation. We were able to make some changes to the environment (explained later) to reduce wait time.
• Staff. Some patients had complained of long wait times, which could hinder active participation in treatment. We agreed that the clinic nurse would make rounds through the waiting room every hour and talk to patients. The nurse would identify patients who had been waiting for longer than 30 minutes after their scheduled appointment time and notify the doctor accordingly. We also agreed to revise patient appointment reminder practices: instead of using an automated answering service, one of the staff members called patients personally to remind them about their appointments. (This also allowed us to update telephone numbers for many patients; numbers on record often were outdated.) We initially recruited summer interns and provided a written script to follow during calls to patients, which allowed patients to confirm, cancel, or reschedule their appointment. Once we demonstrated positive results from the change to personal calls, the department agreed to absorb the cost, and front desk personnel began making reminder calls.
• Policies and procedures. Although some practices are able to charge a small fine for missed appointments, this was not allowed at our institution. Instead, we had several departmental policies on the books, such as discharging patients from our clinics if they missed 3 consecutive appointments and limiting prescription refills to a maximum of 6 months. These policies were neither communicated to patients and staff, nor were they implemented. We decided to educate patients and staff and implement the policies.
• Transparency. We posted the no-show rate in common areas so that the team could review and follow the progression of that rate as we implemented the changes. This allowed team members to take ownership of the project and facilitated active participation.
By implementing these changes, we aimed to reduce the no-show rate to 20%.
Results. We were able to reduce the no-show rate from a documented average of 31% to an average of 12% during the study period after implementing all the proposed changes in the outpatient clinics.
We calculated the no-show rate (as shown in Table 1 for May 2013), then collected the daily no-show rate from June to September 2013 (Figure). With these calculations, we demonstrated a reduction in the no-show rate to 12%. Because of the time and effort required, we reduced data collection from daily to weekly, beginning in September.
Applying the changes required consistent effort and substantial input from various stakeholders—front desk staff, residents, the nurse, therapists, and faculty. Gradually, we were able to implement all the changes.
Keeping the no-show rate low required consistent effort and monitoring of the newly implemented procedures because even a slight change, such as failure to make reminder calls, resulted in a sudden increase in the no-show rate (that was the case in October of the study period, when we were short-staffed and could not call every patient). Patients told us that it was difficult to ignore a personal call; if they were not planning to keep the appointment, the call allowed them to reschedule on the spot.
We also made sure that current no-show rates were posted in common areas, visible to team members every day.
Discussion
We attempted a literature review of research exploring approaches to reducing the no-show rate but found few studies that explored this critical concern in patient treatment.15 Some data suggested that, in the setting studied, the no-show rate:
• was higher among younger patients (age 20 to 39) than older ones (age 60 to 79)
• was slightly higher in women than in men
• varied by diagnosis.
We found a paucity of data regarding interventions that can reduce the no-show rate.
Among the changes we made, the one that had the greatest impact was personalized appointment reminder calls, as evidenced by our patients’ reports and the increase in the no-show rate when personal calls were not made.
We also realized that, although we had several departmental policies in place regarding appointments, they were not being followed. Raising awareness among team members and their patients also was an effective deterrent to a no-show for an appointment. For example, patients were informed that 3 consecutive no-shows could lead to termination of care. Often, they reacted with surprise to this caution but also voiced a desire to improve their attendance to avoid such an outcome.
We found that establishing common operational definitions is important. It also was important to have a cohesive team, with every member agreeing on goals and changes to operational policies that needed to be implemented. Support from the department chair and the administration, we learned, is vital to the success of such an intervention.
A note about limitations. The goal of the project was limited to reducing the no-show rate. We demonstrated that this is possible among patients who have a severe mental illness, and that reducing the associated waste of time and resources can improve finances in an academic department of psychiatry. We would need additional measures, however, to quantify medication adherence and hospitalization; a larger, more inclusive project is needed to demonstrate that reducing the no-show rate reduces the symptomatic burden of psychiatric illness.
Comments in conclusion
This project was designed and conducted as a required part of a Clinical Safety and Effectiveness Program at Memorial Hermann Texas Medical Center and the UT Medical School at Houston.17 Although there was initial hesitancy about attempting to reduce the no-show rate in a chronically mentally ill population, the success of this project—indeed, it surpassed its proposed goals—demonstrates that operational changes in any clinic can reduce the no-show rate. It also is important to maintain operational changes, however; without consistent effort, desired results cannot be sustained.
Last, it is possible to replicate the methodology of this project and thereby attempt to reduce the no-show rate in other divisions of medicine that offer care to chronically ill patients, such as pediatrics and family medicine.
Bottom Line
Failure to attend routinely scheduled outpatient appointments increases a patient’s functional impairment and risk of hospitalization. Patient education, appointment reminder phone calls, revised policies and procedures, and transparency regarding the no-show rate can reduce the number of missed appointments and improve patient outcomes.
Related Resources
• Mitchell AJ, Selmes T. Why don’t patients attend their appointments? Maintaining engagement with psychiatric services. Advances in Psychiatric Treatment. 2007;13:423-434.
• Molfenter T. Reducing appointment no-shows: going from theory to practice. Subst Use Misuse. 2013;48(9):743-749.
• Williston MA, Block-Lerner J, Wolanin A, et al. Brief acceptance-based intervention for increasing intake attendance at a community mental health center. Psychol Serv. 2014;11(3):324-332.
Disclosure
Dr. Gajwani receives grant or research support from the National Institute on Mental Health, the National Institute of Drug Abuse, The Stanley Foundation, and Forest Laboratories, Inc. He is a member of the speakers’ bureau of AstraZeneca, Merck, Otsuka America Pharmaceutical, and Sunovion Pharmaceuticals.
1. Wyatt RJ, Henter I. An economic evaluation of manic-depressive illness—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5):213-219.
2. Wyatt RJ, Henter I, Leary MC, et al. An economic evaluation of schizophrenia—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5);196-205.
3. Offord S, Lin J, Wong B, et al. Impact of oral antipsychotic medication adherence on healthcare resource utilization among schizophrenic patients with medicare coverage. Community Ment Health J. 2013;49(6):625-629.
4. Killaspy H, Banerjee S, King M, et al. Prospective controlled study of psychiatric out-patient non-attendance: characteristics and outcome. Br J Psychiatry. 2000;176:160- 165.
5. Nelson EA, Maruish ME, Axler JL. Effects of discharge planning and compliance with outpatient appointments on readmission rates. Psychiatr Serv. 2000;51(7):885-889.
6. Thornley B, Adams C. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ. 1998;317(7167):1181-1184.
7. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892-909.
8. Fenton WS, Blyler C, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637-651.
9. Scott J, Pope M. Self-reported adherence to treatment with mood stabilizers, plasma levels, and psychiatric hospitalization. Am J Psychiatry. 2002;159(11):1927-1929.
10. Adams J, Scott J. Predicting medication adherence in severe mental disorders. Acta Psychiatr Scand. 2000;101(2):119-124.
11. Müller-Oerlinghausen B, Müser-Causemann B, Volk J. Suicides and parasuicides in a high-risk patient group on and off lithium long-term treatment. J Affect Disord. 1992;25(4):261-269.
12. Manning WG Jr, Wells KB. The effects of psychological distress and psychological well-being on use of medical services. Med Care. 1992;30(6):541-553.
13. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
14. Lingam R, Scott J. Treatment non‐adherence in affective disorders. Acta Psychiatr Scand. 2002;105(3):164-172.
15. Allan AT. No-shows at a community mental health clinic: a pilot study. Int J Soc Psychiatry. 1988;34(1):40-46.
16. Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv. 1998;49(2):196-201.
17. Gajwani P. Improving quality of care: reducing no-show rate in ambulatory psychiatry clinic. Poster presented at: American Psychiatric Association 166th Annual Meeting; May 18-22, 2013; San Francisco, CA.
1. Wyatt RJ, Henter I. An economic evaluation of manic-depressive illness—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5):213-219.
2. Wyatt RJ, Henter I, Leary MC, et al. An economic evaluation of schizophrenia—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5);196-205.
3. Offord S, Lin J, Wong B, et al. Impact of oral antipsychotic medication adherence on healthcare resource utilization among schizophrenic patients with medicare coverage. Community Ment Health J. 2013;49(6):625-629.
4. Killaspy H, Banerjee S, King M, et al. Prospective controlled study of psychiatric out-patient non-attendance: characteristics and outcome. Br J Psychiatry. 2000;176:160- 165.
5. Nelson EA, Maruish ME, Axler JL. Effects of discharge planning and compliance with outpatient appointments on readmission rates. Psychiatr Serv. 2000;51(7):885-889.
6. Thornley B, Adams C. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ. 1998;317(7167):1181-1184.
7. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892-909.
8. Fenton WS, Blyler C, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637-651.
9. Scott J, Pope M. Self-reported adherence to treatment with mood stabilizers, plasma levels, and psychiatric hospitalization. Am J Psychiatry. 2002;159(11):1927-1929.
10. Adams J, Scott J. Predicting medication adherence in severe mental disorders. Acta Psychiatr Scand. 2000;101(2):119-124.
11. Müller-Oerlinghausen B, Müser-Causemann B, Volk J. Suicides and parasuicides in a high-risk patient group on and off lithium long-term treatment. J Affect Disord. 1992;25(4):261-269.
12. Manning WG Jr, Wells KB. The effects of psychological distress and psychological well-being on use of medical services. Med Care. 1992;30(6):541-553.
13. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
14. Lingam R, Scott J. Treatment non‐adherence in affective disorders. Acta Psychiatr Scand. 2002;105(3):164-172.
15. Allan AT. No-shows at a community mental health clinic: a pilot study. Int J Soc Psychiatry. 1988;34(1):40-46.
16. Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv. 1998;49(2):196-201.
17. Gajwani P. Improving quality of care: reducing no-show rate in ambulatory psychiatry clinic. Poster presented at: American Psychiatric Association 166th Annual Meeting; May 18-22, 2013; San Francisco, CA.