User login
Current management of Barrett esophagus and esophageal adenocarcinoma
All cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus.1 But most cases of Barrett esophagus go undiagnosed. And Barrett esophagus is seen in 5% to 15% of patients with gastroesophageal reflux disease.2 These facts clearly emphasize the need for screening. Here, we review the rationale and recommendations for screening and surveillance, as well as the range of treatment options.
SCOPE OF THE PROBLEM
The American Cancer Society estimated there were 17,290 new cases of esophageal cancer and 15,850 deaths from it in the United States in 2018.3 Of the 2 main histologic types of esophageal cancer, adenocarcinoma and squamous cell cancer, adenocarcinoma is more common in the United States.
The precursor lesion is Barrett esophagus, defined as an extension of salmon-colored mucosa at least 1 cm into the tubular esophagus proximal to the gastroesophageal junction, with biopsy confirmation of intestinal metaplasia.4
The natural course of progression to dysplasia and cancer in Barrett esophagus is unknown but is thought to be stepwise, from no dysplasia to low-grade dysplasia to high-grade dysplasia and cancer, and the cancer risk depends on the degree of dysplasia: the annual risk is 0.33% if there is no dysplasia, 0.54% with low-grade dysplasia, and 7% with high-grade dysplasia.4
Although all cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus,1 more than 90% of patients with newly diagnosed esophageal adenocarcinoma do not have a prior diagnosis of Barrett esophagus.5 Therefore, there is a substantial unmet need to expand screening for Barrett esophagus in people at risk.
GASTROESOPHAGEAL REFLUX DISEASE IS A RISK FACTOR FOR CANCER
The rationale behind screening is that detecting Barrett esophagus early and intervening in a timely manner in patients at higher risk of developing adenocarcinoma will decrease mortality.
Chronic gastroesophageal reflux disease is a strong risk factor for esophageal adenocarcinoma (odds ratio [OR] 7.7, 95% confidence interval [CI] 5.3–11.4), and the risk increases when symptoms are long-standing (> 20 years) or severe (OR 43.5, 95% CI 18.3–103.5) or occur daily (OR 5.5, 95% CI 3.2–9.3).6
Reflux symptoms are scored as follows:
- Heartburn only, 1 point
- Regurgitation only, 1 point
- Heartburn with regurgitation, 1.5 points
- Nightly symptoms (2 points if yes, 0 if no)
- Symptoms once a week, 0 points; 2 to 6 times a week, 1 point; 7 to 15 times a week, 2 points; more than 15 times a week, 3 points.6
A score of 4.5 or higher indicates severe reflux disease. However, it is worth noting that the annual incidence of esophageal adenocarcinoma in patients with long-term gastroesophageal reflux disease is less than 0.001%.7
RISK FACTORS FOR BARRETT ESOPHAGUS
Risk factors for Barrett esophagus include:
Male sex. Barrett esophagus is more prevalent in men than in women, at a ratio of 2 to 1; but in individuals under age 50, the ratio is 4 to 1.8
Age 50 or older. Barrett esophagus is usually diagnosed in the sixth to seventh decade of life, and the prevalence increases from 2.1% in the third decade to 9.3% in the sixth decade.9
White race. It is more prevalent in whites than in blacks (5.0% vs 1.5%, P < .0001).10
Central obesity. Waist circumference is an independent risk factor: every 5-cm increase carries an OR of 1.14 (95% CI 1.03–1.27, P = .02).11
Cigarette smoking increases the risk of Barrett esophagus (OR 1.42; 95% CI 1.15–1.76).12
A family history of Barrett esophagus or esophageal adenocarcinoma is a strong risk factor (OR 12, 95% CI 3.3–44.8). In 1 study, the risk in first- and second-degree relatives of patients with Barrett esophagus was 24%, compared with 5% in a control population (P < .005).13
SCREENING GUIDELINES AND DRAWBACKS
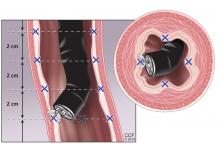
The standard screening method is esophagogastroduodenoscopy with sedation, with careful visual inspection and 4-quadrant biopsies every 2 cm using the Seattle protocol, ie, including biopsy of any mucosal irregularities in salmon-colored mucosa above the gastroesophageal junction (Figure 1).4
Endoscopic screening is cost-effective, costing $10,440 per quality-adjusted life-year saved, which is well below the accepted threshold of less than $100,000.14 However, it is still expensive, invasive, and not ideal for screening large populations.
Less-invasive methods under study
Less-invasive, less-expensive methods being tested for mass screening include:
Unsedated transnasal endoscopy. Done with only topical anesthesia, it has high diagnostic accuracy and is quicker and more cost-effective than standard esophagogastroduodenoscopy, with fewer adverse effects. However, the procedure has not yet gained widespread acceptance for regular use by gastroenterologists.15
A swallowable sponge. Another promising test is cell collection using the Cytosponge Cell Collection Device (Medtronic, Minneapolis, MN). An encapsulated compressed sponge with a string attached is swallowed; in the stomach, the capsule dissolves, and the sponge expands and is then withdrawn using the attached string. The obtained cytology sample from the lower esophagus is then tested for trefoil factor 3, a protein biomarker for Barrett esophagus.16
A retractable balloon. The EsoCheck Cell Collection Device is a retractable balloon attached to a string. When swallowed, it gathers distal esophageal cells for detecting methylated DNA markers for Barrett esophagus.17
Esophageal capsule endoscopy uses a camera to visualize the esophagus, but lacks the ability to obtain biopsy samples.
Other screening methods are being tested, although data are limited. Liquid biopsy uses a blood sample to detect microRNAs that are dysregulated in cancer. The “electronic nose” is a device that detects exhaled volatile organic compounds altered in Barrett esophagus. Another test involves taking an oral wash sample to study the oral microbiome for a pattern associated with adenocarcinoma.18–21
SURVEILLANCE: WHAT’S INVOLVED, WHAT’S AVAILABLE
Surveillance in Barrett esophagus aims to detect premalignant changes or early-stage adenocarcinoma to provide longer survival and lower cancer-related mortality. Recent evidence suggests that patients with esophageal adenocarcinoma that is diagnosed in a Barrett esophagus surveillance program have an earlier stage of disease and therefore a survival benefit.22
Patient education is essential
Before enrolling a patient in a surveillance program, the clinician should explain the risks, benefits, and limitations, the importance of periodic endoscopy, and the possible eventual need for endoscopic therapy or surgery.
The endoscopic procedure
Surveillance involves examination by high-definition white-light endoscopy, with random 4-quadrant biopsies every 2 cm (or every 1 cm in patients with a history of dysplasia) and biopsy of any mucosal irregularity (nodule, ulcer, or other visible lesion). The degree of dysplasia determines the frequency of follow-up surveillance intervals and the need for endoscopic eradication therapy, as presented in professional society guidelines (Table 1).4,23,24
Advanced methods for detecting dysplasia
Newer endoscopic surveillance techniques include dye-based chromoendoscopy, narrow-band imaging, confocal laser endomicroscopy, volumetric laser endomicroscopy, and wide-area transepithelial sampling with computer-assisted 3-dimensional analysis. All these techniques are used to increase the detection of dysplasia. Of these, dye-based chromoendoscopy, narrow-band imaging, and confocal laser endomicroscopy meet current criteria of the American Society for Gastrointestinal Endoscopy for preservation and incorporation of valuable endoscopic innovations.23
MANAGEMENT OF NONDYSPLASTIC BARRETT ESOPHAGUS
A proton pump inhibitor (PPI) is recommended to control reflux symptoms in patients with nondysplastic Barrett esophagus. But it is important to counsel patients on additional ways to protect against esophageal adenocarcinoma, such as:
- Low to moderate alcohol consumption
- Regular physical activity
- Increased dietary intake of fruits, vegetables, folate, fiber, beta-carotene, and vitamin C
- Weight control
- Smoking cessation.25
Surveillance endoscopy with 4-quadrant biopsies at 2-cm intervals is recommended every 3 to 5 years (Table 1).
DOES CHEMOPREVENTION HAVE A ROLE?
Chemoprevention is an exciting area of research in preventing progression to adenocarcinoma in patients with Barrett esophagus. Various drugs such as aspirin, other nonsteroidal anti-inflammatory drugs (NSAIDs), PPIs, metformin, and statins have been studied.
Aspirin
Aspirin has been shown to prevent development of Barrett esophagus in patients with reflux disease,26 but more studies are needed to validate those findings.
PPIs
Gastroesophageal reflux disease is a primary risk factor for esophageal adenocarcinoma, and gastric acid suppression with PPIs reduces cancer risk. PPI therapy is associated with a 71% decrease in the risk of high-grade dysplasia and adenocarcinoma in patients with Barrett esophagus (OR 0.29, 95% CI 0.12–0.79).27 Long-term therapy (> 2 to 3 years) has a higher protective effect (adjusted OR 0.45, 95% CI 0.19–1.06) than short-term therapy (< 2 to 3 years) (adjusted OR 1.09, 95% CI 0.47–2.56).27
NSAIDs
NSAIDs, including aspirin, have been associated with decreased risk of colon, stomach, lung, breast, and esophageal cancer due to their potential to inhibit cyclooxygenase 2 (COX-2) enzymes.
A meta-analysis demonstrated that aspirin and NSAIDs led to a 32% reduction in the risk of adenocarcinoma (OR 0.68, 95% CI 0.56–0.83). The benefit was even greater if the drug was taken daily or more frequently (OR 0.56, 95% CI 0.43–0.73, P < .001) or was taken for 10 or more years (OR 0.63, 95% CI 0.45–0.90, P = .04).28
PPI plus aspirin
The best evidence for the role of PPIs and aspirin in reducing the risk of dysplasia comes from the Aspirin and Esomeprazole Chemoprevention in Barrett’s Metaplasia Trial.29 This randomized, controlled trial compared 4 regimens consisting of esomeprazole (a PPI) in either a high dose (40 mg twice daily) or a low dose (20 mg once daily) plus either aspirin (300 or 320 mg per day) or no aspirin in 2,557 patients with Barrett esophagus. The composite end point was the time to all-cause mortality, adenocarcinoma, or high-grade dysplasia.
At a median follow-up of 8.9 years, the combination of high-dose esomeprazole plus aspirin had the strongest effect compared with low-dose esomeprazole without aspirin (time ratio 1.59, 95% CI 1.14–2.23, P = .0068). The number needed to treat was 34 for esomeprazole and 43 for aspirin.29
Based on these data, we can conclude that aspirin and PPIs can prevent dysplasia and all-cause mortality in Barrett esophagus.
Metformin: No evidence of benefit
Metformin was studied as a protective agent against obesity-associated cancers including esophageal adenocarcinoma, as it reduces insulin levels.
In a randomized controlled trial30 in 74 patients with Barrett esophagus, metformin (starting at 500 mg daily, increasing to 2,000 mg/day by week 4) was compared with placebo. At 12 weeks, the percent change in esophageal levels of the biomarker pS6K1—an intracellular mediator of insulin and insulin-like growth factor activation in Barrett epithelium—did not differ significantly between the 2 groups (1.4% with metformin vs −14.7% with placebo; 1-sided P = .80). This suggested that metformin did not significantly alter proliferation or apoptosis in Barrett epithelium, despite reducing serum insulin levels and insulin resistance. Thus, metformin did not demonstrate a chemoprotective effect in preventing the progression of Barrett esophagus to adenocarcinoma.
Vitamin D: No evidence of benefit
Vitamin D affects genes regulating proliferation, apoptosis, and differentiation, and has therefore been studied as a potential antineoplastic agent. Its deficiency has also been associated with increased risk of esophageal adenocarcinoma. However, its efficacy in chemoprevention is unclear.31
One study found no association between serum 25-hydroxyvitamin D levels and prevalence of dysplasia in Barrett esophagus (P = .90). An increase in vitamin D levels had no effect on progression to dysplasia or cancer (for every 5-nmol/L increase from baseline, hazard ratio 0.98, P = .62).32
In another study, supplementation with vitamin D3 (cholecalciferol 50,000 IU weekly) plus a PPI for 12 weeks significantly improved the serum 25-hydroxyvitamin D levels without significant changes in gene expression from Barrett epithelium.33 These findings were confirmed in a meta-analysis that showed no consistent association between vitamin D exposure and risk of esophageal neoplasm.34
Thus, there is currently no evidence to support vitamin D for chemoprevention in Barrett esophagus or esophageal adenocarcinoma.
Statins
In addition to lowering cholesterol, statins have antiproliferative, pro-apoptotic, anti-angiogenic, and immunomodulatory effects that prevent cancer, leading to a 41% reduction in the risk of adenocarcinoma in patients with Barrett esophagus in one study (adjusted OR 0.59, 95% CI 0.45–0.78); the number needed to treat with statins to prevent 1 case of adenocarcinoma was 389.35
A meta-analysis also showed that statin use was associated with a lower risk of progression of Barrett esophagus (OR 0.48, 95% CI 0.31–0.73).36
In general, statins appear promising for chemoprevention, but more study is needed.
When is chemoprevention appropriate?
Chemoprevention is not recommended for all patients with Barrett esophagus, given that the condition affects 1% to 2% of the US adult population, and very few patients have progression to esophageal adenocarcinoma. Rather, chemoprevention may be considered in patients with Barrett esophagus and multiple risk factors for adenocarcinoma.
INDEFINITE DYSPLASIA
In Barrett esophagus with indefinite dysplasia, either the epithelial abnormalities are insufficient for a diagnosis of dysplasia, or the nature of the epithelial abnormalities is uncertain due to inflammation or technical difficulties with specimen processing. The risk of high-grade dysplasia or cancer within 1 year of the diagnosis of indefinite dysplasia varies between 1.9% and 15%.37 The recommendation for management is to optimize acid-suppressive therapy for 3 to 6 months and then to repeat esophagogastroduodenoscopy. If indefinite dysplasia is noted again, repeat endoscopy in 12 months is recommended.2
ENDOSCOPIC ERADICATION: AN OVERVIEW
Because dysplasia in Barrett esophagus carries a high risk of progression to cancer, the standard of care is endoscopic mucosal resection of visible lesions, followed by ablation of the flat mucosa, with the aim of achieving complete eradication of intestinal metaplasia.4,38 The initial endoscopic treatment is followed by outpatient sessions every 8 to 10 weeks until the dysplasia is eradicated. A key part of treatment during this time is maximal acid suppression with a PPI twice daily and a histamine-2 blocker at night. In rare cases, fundoplication is required to control reflux refractory to medical therapy.
After eradication is confirmed, continued surveillance is necessary, as recurrences have been reported at a rate of 4.8% per year for intestinal metaplasia, and 2% per year for dysplasia.39
Current endoscopic resection techniques
Endoscopic resection techniques include mucosal resection, submucosal dissection, radiofrequency ablation, cryotherapy, argon plasma coagulation, and photodynamic therapy (Figure 2).
In mucosal resection, the lesion is either suctioned into a band ligator, after which a band is placed around the lesion, or suctioned into a cap fitted at the end of the endoscope, after which the lesion is removed using a snare.
In submucosal dissection, a liquid is injected into the submucosa to lift the lesion, making it easier to remove. The procedure is technically complex and requires additional training.
In radiofrequency ablation, a special catheter is passed through the endoscope to ablate the affected epithelium by thermal injury. Argon plasma coagulation works in a similar way, but uses ionized argon gas to induce thermal coagulation of metaplastic epithelium.
Cryotherapy produces cellular injury by rapid freezing and thawing of tissue using a cryogen such as liquid nitrogen or nitrous oxide.
In photodynamic therapy, a photosensitizer (porfimer sodium) is administered and taken up preferentially by metaplastic epithelium; it is then activated by transmission of red light using the endoscope, leading to destruction of the metaplastic epithelium.
Of the different techniques, radiofrequency ablation has the most evidence for efficacy and hence is the most commonly used.
All of these procedures are generally well tolerated and have favorable side-effect profiles. After radiofrequency ablation with or without mucosal resection, esophageal strictures are noted in 5.6% of patients, and bleeding and perforation occur rarely (1% and 0.6% of patients, respectively).40 Submucosal dissection is associated with a higher rate of complications such as stricture formation (11% of patients) and bleeding or perforation (1.5% of patients).41
LOW-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
Most patients with low-grade dysplasia (73%) are down-staged to nondysplastic Barrett esophagus or to indefinite for dysplasia after review by expert pathologists.42 Patients with confirmed and persistent low-grade dysplasia are at higher risk of progression.43
Once low-grade dysplasia is confirmed by a second gastrointestinal pathologist, the patient should undergo endoscopic ablation. A landmark study by Shaheen et al44 demonstrated the benefit of radiofrequency ablation in achieving complete eradication of dysplasia (90.5% vs 22.7% for a sham procedure) and complete eradication of intestinal metaplasia (77.4% vs 2.3% for a sham procedure). In another trial of 136 patients with low-grade dysplasia followed for 3 years, Phoa et al45 demonstrated that radiofrequency ablation reduced the rate of progression to high-grade dysplasia by 25% and to adenocarcinoma by 7.4% compared with endoscopic surveillance.
Patients with confirmed low-grade dysplasia who do not undergo eradication therapy should have surveillance endoscopy every 6 to 12 months (Table 1).
HIGH-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
As with low-grade dysplasia, the diagnosis of high-grade dysplasia needs to be confirmed by a second pathologist with gastrointestinal expertise. In the past, the treatment was esophagectomy, but due to lower morbidity and equivalent efficacy of radiofrequency ablation,46 the current treatment of choice is endoscopic mucosal resection of raised lesions, followed by radiofrequency ablation of the entire affected segment.
In the study by Shaheen et al,44 42 patients with high-grade dysplasia were randomized to radiofrequency ablation and 21 to a sham procedure, and 81% of ablation patients achieved complete eradication of dysplasia vs 19% with the sham procedure. Eradication of intestinal metaplasia was achieved in 77% of ablation patients vs 2% of patients with the sham therapy. Results of 3-year follow-up from the same cohort showed complete eradication of dysplasia in 98% and of intestinal metaplasia in 91%.47
Endoscopic eradication therapy is recommended for all patients with Barrett esophagus and high-grade dysplasia without a life-limiting comorbidity. Alternatively, surveillance every 3 months is an option if the patient does not wish to undergo eradication therapy. Radiofrequency ablation is more cost-effective than esophagectomy or endoscopic surveillance followed by treatment once patients develop adenocarcinoma.48,49
EARLY ESOPHAGEAL ADENOCARCINOMA: RECOMMENDED MANAGEMENT
Adenocarcinoma limited to the mucosa and without evidence of nodal involvement can be resected endoscopically. In patients with localized cancer, mucosal resection is done not only for therapeutic purposes but also for staging. Ideal management is multidisciplinary, including a gastroenterologist, thoracic surgeon, oncologist, pathologist, and radiation oncologist.
If lesions have features suggesting submucosal invasion or are greater than 1.5 cm in size, or if it is difficult to separate (ie, lift) the mucosa from the submucosal layer with injection of saline, then submucosal dissection is recommended.50 Because of the risk of metachronous lesions, ablation of the remaining Barrett esophagus mucosa is recommended after resection of cancer.
Endoscopic eradication is highly effective and durable for the treatment of intramucosal esophageal adenocarcinoma. In a study of 1,000 patients, 963 patients (96.3%) had achieved a complete response; 12 patients (3.7%) underwent surgery after eradication failed during a follow-up of almost 5 years.51 Metachronous lesions or recurrence of cancer developed during the follow-up period in 140 patients (14.5%) but were successfully treated endoscopically in 115, resulting in a long-term complete remission rate of 93.8%.
POSTABLATION MANAGEMENT
Because of the risk of recurrence of dysplasia after ablation, long-term PPI therapy and surveillance are recommended.
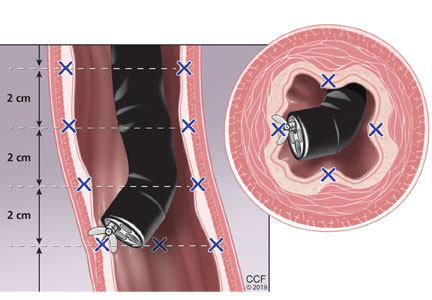
Surveillance endoscopy involves 4-quadrant biopsies taken every 1 cm from the entire length of segment where Barrett esophagus had been seen before ablation.
The timing of surveillance intervals depends on the preablation grade of dysplasia. For low-grade dysplasia, the recommendation is every 6 months for the first year after ablation and, if there is no recurrence of dysplasia, annually after that.2 After treatment of high-grade dysplasia or intramucosal adenocarcinoma, the recommendation is every 3 months for the first year, every 6 months in the second year, and then annually.2
- Mendes de Almeida JC, Chaves P, Pereira AD, Altorki NK. Is Barrett’s esophagus the precursor of most adenocarcinomas of the esophagus and cardia? A biochemical study. Ann Surg 1997; 226(6):725–733. pmid:9409571
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005; 61(2):226–231. pmid:15729230
- National Cancer Institute. Cancer stat facts: esophageal cancer. https://seer.cancer.gov/statfacts/html/esoph.html. Accessed August 6, 2019.
- Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016; 111(1):30–50. doi:10.1038/ajg.2015.322
- Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology 2002; 122(1):26–33. pmid:11781277
- Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999; 340(11):825–831. doi:10.1056/NEJM199903183401101
- Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: scientific review. JAMA 2002; 287(15):1972–1981. pmid:11960540
- van Blankenstein M, Looman CW, Johnston BJ, Caygill CP. Age and sex distribution of the prevalence of Barrett’s esophagus found in a primary referral endoscopy center. Am J Gastroenterol 2005; 100(3):568–576.
- Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett’s esophagus by endoscopy indication. Gastrointest Endosc 2010; 71(1):21–27. doi:10.1016/j.gie.2009.06.035
- Wang A, Mattek NC, Holub JL, Lieberman DA, Eisen GM. Prevalence of complicated gastroesophageal reflux disease and Barrett’s esophagus among racial groups in a multi-center consortium. Dig Dis Sci 2009; 54(5):964–971. doi:10.1007/s10620-009-0742-3
- Kubo A, Cook MB, Shaheen NJ, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett’s esophagus: a pooled analysis from the international BEACON consortium. Gut 2013; 62(12):1684–1691. doi:10.1136/gutjnl-2012-303753
- Andrici J, Cox MR, Eslick GD. Cigarette smoking and the risk of Barrett’s esophagus: a systematic review and meta-analysis. J Gastroenterol Hepatol 2013; 28(8):1258–1273. doi:10.1111/jgh.12230
- Chak A, Lee T, Kinnard MF, et al. Familial aggregation of Barrett’s esophagus, esophageal adenocarcinoma, and esophagogastric junctional adenocarcinoma in Caucasian adults. Gut 2002; 51(3):323–328. pmid:12171951
- Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med 2003; 138(3):176–186. pmid:12558356
- Jobe BA, Hunter JG, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. Am J Gastroenterol 2006; 101(12):2693–2703.
- Ross-Innes CS, Chettouh H, Achilleos A, et al; BEST2 study group. Risk stratification of Barrett’s esophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. Lancet Gastroenterol Hepatol 2017; 2(1):23–31. doi:10.1016/S2468-1253(16)30118-2
- Moinova HR, LaFramboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci Transl Med 2018; 10(424). pii:eaao5848. doi:10.1126/scitranslmed.aao5848
- Chan DK, Zakko L, Visrodia KH, et al. Breath testing for Barrett’s esophagus using exhaled volatile organic compound profiling with an electronic nose device. Gastroenterology 2017; 152(1):24–26. doi:10.1053/j.gastro.2016.11.001
- Kumar S, Huang J, Abbassi-Ghadi N, et al. Mass spectrometric analysis of exhaled breath for the identification of volatile organic compound biomarkers in esophageal and gastric adenocarcinoma. Ann Surg 2015; 262(6):981–990. doi:10.1097/SLA.0000000000001101
- Peters BA, Wu J, Pei Z, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res 2017; 77(23):6777–6787. doi:10.1158/0008-5472.CAN-17-1296
- Mallick R, Patnaik SK, Wani S, Bansal A. A systematic review of esophageal microrna markers for diagnosis and monitoring of Barrett’s esophagus. Dig Dis Sci 2016; 61(4):1039–1050. doi:10.1007/s10620-015-3959-3
- Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology 2018; 154(8):2068–2086.e5. doi:10.1053/j.gastro.2018.02.022
- ASGE Technology Committee; Thosani N, Abu Dayyeh BK, Sharma P, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE preservation and incorporation of valuable endoscopic innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett’s esophagus. Gastrointest Endosc 2016; 83(4):684–698.e7. doi:10.1016/j.gie.2016.01.007
- Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ; American Gastroenterological Association. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology 2011; 140(3):e18–e52. doi:10.1053/j.gastro.2011.01.031
- Castro C, Peleteiro B, Lunet N. Modifiable factors and esophageal cancer: a systematic review of published meta-analyses. J Gastroenterol 2018; 53(1):37–51. doi:10.1007/s00535-017-1375-5
- Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett’s esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol 2012; 10(7):722–727. doi:10.1016/j.cgh.2012.02.031
- Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gut 2014; 63(8):1229–1237. doi:10.1136/gutjnl-2013-305997
- Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 2012; 142(3):442–452.e5. doi:10.1053/j.gastro.2011.11.019
- Jankowski JAZ, de Caestecker J, Love SB, et al; AspECT Trial Team. Esomeprazole and aspirin in Barrett’s esophagus (AspECT): a randomised factorial trial. Lancet 2018; 392(10145):400–408. doi:10.1016/S0140-6736(18)31388-6
- Chak A, Buttar NS, Foster NR, et al; Cancer Prevention Network. Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2015; 13(4):665–672.e1–e4. doi:10.1016/j.cgh.2014.08.040
- Rouphael C, Kamal A, Sanaka MR, Thota PN. Vitamin D in esophageal cancer: is there a role for chemoprevention? World J Gastrointest Oncol 2018; 10(1):23–30. doi:10.4251/wjgo.v10.i1.23
- Thota PN, Kistangari G, Singh P, et al. Serum 25-hydroxyvitamin D levels and the risk of dysplasia and esophageal adenocarcinoma in patients with Barrett’s esophagus. Dig Dis Sci 2016; 61(1):247–254. doi:10.1007/s10620-015-3823-5
- Cummings LC, Thota PN, Willis JE, et al. A nonrandomized trial of vitamin D supplementation for Barrett’s esophagus. PLoS One 2017;1 2(9):e0184928. doi:10.1371/journal.pone.0184928
- Zgaga L, O’Sullivan F, Cantwell MM, Murray LJ, Thota PN, Coleman HG. Markers of vitamin D exposure and esophageal cancer risk: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2016; 25(6):877–886. doi:10.1158/1055-9965.EPI-15-1162
- Singh S, Singh AG, Singh PP, Murad MH, Iyer PG. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013; 11(6):620–629. doi:10.1016/j.cgh.2012.12.036
- Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors associated with progression of Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018; 6(7):1046–1055.e8. doi:10.1016/j.cgh.2017.11.044
- Thota PN, Kistangari G, Esnakula AK, Gonzalo DH, Liu XL. Clinical significance and management of Barrett’s esophagus with epithelial changes indefinite for dysplasia. World J Gastrointest Pharmacol Ther 2016; 7(3):406–411. doi:10.4292/wjgpt.v7.i3.406
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012; 143(2):336–346. doi:10.1053/j.gastro.2012.04.032
- Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett’s esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017; 85(3):482–495.e4. doi:10.1016/j.gie.2016.09.022
- Qumseya BJ, Wani S, Desai M, et al. Adverse events after radiofrequency ablation in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016; 14(8):1086–1095.e6. doi:10.1016/j.cgh.2016.04.001
- Yang D, Zou F, Xiong S, Forde JJ, Wang Y, Draganov PV. Endoscopic submucosal dissection for early Barrett’s neoplasia: a meta-analysis. Gastrointest Endosc 2018; 87(6):1383–1393. doi:10.1016/j.gie.2017.09.038
- Duits LC, Phoa KN, Curvers WL, et al. Barrett’s esophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015; 64(5):700–706. doi:10.1136/gutjnl-2014-307278
- Duits LC, van der Wel MJ, Cotton CC, et al. Patients with Barrett’s esophagus and confirmed persistent low-grade dysplasia are at increased risk for progression to neoplasia. Gastroenterology 2017; 152(5):993–1001.e1. doi:10.1053/j.gastro.2016.12.008
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009; 360(22):2277–2288. doi:10.1056/NEJMoa0808145
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014; 311(12):1209–1217. doi:10.1001/jama.2014.2511
- Hu Y, Puri V, Shami VM, Stukenborg GJ, Kozower BD. Comparative effectiveness of esophagectomy versus endoscopic treatment for esophageal high-grade dysplasia. Ann Surg 2016; 263(4):719–726. doi:10.1097/SLA.0000000000001387
- Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology 2011; 141(2):460–468. doi:10.1053/j.gastro.2011.04.061
- Hur C, Choi SE, Rubenstein JH, et al. The cost effectiveness of radiofrequency ablation for Barrett’s esophagus. Gastroenterology 2012; 143(3):567–575. doi:10.1053/j.gastro.2012.05.010
- Boger PC, Turner D, Roderick P, Patel P. A UK-based cost-utility analysis of radiofrequency ablation or oesophagectomy for the management of high-grade dysplasia in Barrett’s esophagus. Aliment Pharmacol Ther 2010; 32(11-12):1332–1342. doi:10.1111/j.1365-2036.2010.04450.x
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47(9):829–854. doi:10.1055/s-0034-1392882
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014; 146(3):652–660.e1. doi:10.1053/j.gastro.2013.11.006
All cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus.1 But most cases of Barrett esophagus go undiagnosed. And Barrett esophagus is seen in 5% to 15% of patients with gastroesophageal reflux disease.2 These facts clearly emphasize the need for screening. Here, we review the rationale and recommendations for screening and surveillance, as well as the range of treatment options.
SCOPE OF THE PROBLEM
The American Cancer Society estimated there were 17,290 new cases of esophageal cancer and 15,850 deaths from it in the United States in 2018.3 Of the 2 main histologic types of esophageal cancer, adenocarcinoma and squamous cell cancer, adenocarcinoma is more common in the United States.
The precursor lesion is Barrett esophagus, defined as an extension of salmon-colored mucosa at least 1 cm into the tubular esophagus proximal to the gastroesophageal junction, with biopsy confirmation of intestinal metaplasia.4
The natural course of progression to dysplasia and cancer in Barrett esophagus is unknown but is thought to be stepwise, from no dysplasia to low-grade dysplasia to high-grade dysplasia and cancer, and the cancer risk depends on the degree of dysplasia: the annual risk is 0.33% if there is no dysplasia, 0.54% with low-grade dysplasia, and 7% with high-grade dysplasia.4
Although all cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus,1 more than 90% of patients with newly diagnosed esophageal adenocarcinoma do not have a prior diagnosis of Barrett esophagus.5 Therefore, there is a substantial unmet need to expand screening for Barrett esophagus in people at risk.
GASTROESOPHAGEAL REFLUX DISEASE IS A RISK FACTOR FOR CANCER
The rationale behind screening is that detecting Barrett esophagus early and intervening in a timely manner in patients at higher risk of developing adenocarcinoma will decrease mortality.
Chronic gastroesophageal reflux disease is a strong risk factor for esophageal adenocarcinoma (odds ratio [OR] 7.7, 95% confidence interval [CI] 5.3–11.4), and the risk increases when symptoms are long-standing (> 20 years) or severe (OR 43.5, 95% CI 18.3–103.5) or occur daily (OR 5.5, 95% CI 3.2–9.3).6
Reflux symptoms are scored as follows:
- Heartburn only, 1 point
- Regurgitation only, 1 point
- Heartburn with regurgitation, 1.5 points
- Nightly symptoms (2 points if yes, 0 if no)
- Symptoms once a week, 0 points; 2 to 6 times a week, 1 point; 7 to 15 times a week, 2 points; more than 15 times a week, 3 points.6
A score of 4.5 or higher indicates severe reflux disease. However, it is worth noting that the annual incidence of esophageal adenocarcinoma in patients with long-term gastroesophageal reflux disease is less than 0.001%.7
RISK FACTORS FOR BARRETT ESOPHAGUS
Risk factors for Barrett esophagus include:
Male sex. Barrett esophagus is more prevalent in men than in women, at a ratio of 2 to 1; but in individuals under age 50, the ratio is 4 to 1.8
Age 50 or older. Barrett esophagus is usually diagnosed in the sixth to seventh decade of life, and the prevalence increases from 2.1% in the third decade to 9.3% in the sixth decade.9
White race. It is more prevalent in whites than in blacks (5.0% vs 1.5%, P < .0001).10
Central obesity. Waist circumference is an independent risk factor: every 5-cm increase carries an OR of 1.14 (95% CI 1.03–1.27, P = .02).11
Cigarette smoking increases the risk of Barrett esophagus (OR 1.42; 95% CI 1.15–1.76).12
A family history of Barrett esophagus or esophageal adenocarcinoma is a strong risk factor (OR 12, 95% CI 3.3–44.8). In 1 study, the risk in first- and second-degree relatives of patients with Barrett esophagus was 24%, compared with 5% in a control population (P < .005).13
SCREENING GUIDELINES AND DRAWBACKS
The standard screening method is esophagogastroduodenoscopy with sedation, with careful visual inspection and 4-quadrant biopsies every 2 cm using the Seattle protocol, ie, including biopsy of any mucosal irregularities in salmon-colored mucosa above the gastroesophageal junction (Figure 1).4
Endoscopic screening is cost-effective, costing $10,440 per quality-adjusted life-year saved, which is well below the accepted threshold of less than $100,000.14 However, it is still expensive, invasive, and not ideal for screening large populations.
Less-invasive methods under study
Less-invasive, less-expensive methods being tested for mass screening include:
Unsedated transnasal endoscopy. Done with only topical anesthesia, it has high diagnostic accuracy and is quicker and more cost-effective than standard esophagogastroduodenoscopy, with fewer adverse effects. However, the procedure has not yet gained widespread acceptance for regular use by gastroenterologists.15
A swallowable sponge. Another promising test is cell collection using the Cytosponge Cell Collection Device (Medtronic, Minneapolis, MN). An encapsulated compressed sponge with a string attached is swallowed; in the stomach, the capsule dissolves, and the sponge expands and is then withdrawn using the attached string. The obtained cytology sample from the lower esophagus is then tested for trefoil factor 3, a protein biomarker for Barrett esophagus.16
A retractable balloon. The EsoCheck Cell Collection Device is a retractable balloon attached to a string. When swallowed, it gathers distal esophageal cells for detecting methylated DNA markers for Barrett esophagus.17
Esophageal capsule endoscopy uses a camera to visualize the esophagus, but lacks the ability to obtain biopsy samples.
Other screening methods are being tested, although data are limited. Liquid biopsy uses a blood sample to detect microRNAs that are dysregulated in cancer. The “electronic nose” is a device that detects exhaled volatile organic compounds altered in Barrett esophagus. Another test involves taking an oral wash sample to study the oral microbiome for a pattern associated with adenocarcinoma.18–21
SURVEILLANCE: WHAT’S INVOLVED, WHAT’S AVAILABLE
Surveillance in Barrett esophagus aims to detect premalignant changes or early-stage adenocarcinoma to provide longer survival and lower cancer-related mortality. Recent evidence suggests that patients with esophageal adenocarcinoma that is diagnosed in a Barrett esophagus surveillance program have an earlier stage of disease and therefore a survival benefit.22
Patient education is essential
Before enrolling a patient in a surveillance program, the clinician should explain the risks, benefits, and limitations, the importance of periodic endoscopy, and the possible eventual need for endoscopic therapy or surgery.
The endoscopic procedure
Surveillance involves examination by high-definition white-light endoscopy, with random 4-quadrant biopsies every 2 cm (or every 1 cm in patients with a history of dysplasia) and biopsy of any mucosal irregularity (nodule, ulcer, or other visible lesion). The degree of dysplasia determines the frequency of follow-up surveillance intervals and the need for endoscopic eradication therapy, as presented in professional society guidelines (Table 1).4,23,24
Advanced methods for detecting dysplasia
Newer endoscopic surveillance techniques include dye-based chromoendoscopy, narrow-band imaging, confocal laser endomicroscopy, volumetric laser endomicroscopy, and wide-area transepithelial sampling with computer-assisted 3-dimensional analysis. All these techniques are used to increase the detection of dysplasia. Of these, dye-based chromoendoscopy, narrow-band imaging, and confocal laser endomicroscopy meet current criteria of the American Society for Gastrointestinal Endoscopy for preservation and incorporation of valuable endoscopic innovations.23
MANAGEMENT OF NONDYSPLASTIC BARRETT ESOPHAGUS
A proton pump inhibitor (PPI) is recommended to control reflux symptoms in patients with nondysplastic Barrett esophagus. But it is important to counsel patients on additional ways to protect against esophageal adenocarcinoma, such as:
- Low to moderate alcohol consumption
- Regular physical activity
- Increased dietary intake of fruits, vegetables, folate, fiber, beta-carotene, and vitamin C
- Weight control
- Smoking cessation.25
Surveillance endoscopy with 4-quadrant biopsies at 2-cm intervals is recommended every 3 to 5 years (Table 1).
DOES CHEMOPREVENTION HAVE A ROLE?
Chemoprevention is an exciting area of research in preventing progression to adenocarcinoma in patients with Barrett esophagus. Various drugs such as aspirin, other nonsteroidal anti-inflammatory drugs (NSAIDs), PPIs, metformin, and statins have been studied.
Aspirin
Aspirin has been shown to prevent development of Barrett esophagus in patients with reflux disease,26 but more studies are needed to validate those findings.
PPIs
Gastroesophageal reflux disease is a primary risk factor for esophageal adenocarcinoma, and gastric acid suppression with PPIs reduces cancer risk. PPI therapy is associated with a 71% decrease in the risk of high-grade dysplasia and adenocarcinoma in patients with Barrett esophagus (OR 0.29, 95% CI 0.12–0.79).27 Long-term therapy (> 2 to 3 years) has a higher protective effect (adjusted OR 0.45, 95% CI 0.19–1.06) than short-term therapy (< 2 to 3 years) (adjusted OR 1.09, 95% CI 0.47–2.56).27
NSAIDs
NSAIDs, including aspirin, have been associated with decreased risk of colon, stomach, lung, breast, and esophageal cancer due to their potential to inhibit cyclooxygenase 2 (COX-2) enzymes.
A meta-analysis demonstrated that aspirin and NSAIDs led to a 32% reduction in the risk of adenocarcinoma (OR 0.68, 95% CI 0.56–0.83). The benefit was even greater if the drug was taken daily or more frequently (OR 0.56, 95% CI 0.43–0.73, P < .001) or was taken for 10 or more years (OR 0.63, 95% CI 0.45–0.90, P = .04).28
PPI plus aspirin
The best evidence for the role of PPIs and aspirin in reducing the risk of dysplasia comes from the Aspirin and Esomeprazole Chemoprevention in Barrett’s Metaplasia Trial.29 This randomized, controlled trial compared 4 regimens consisting of esomeprazole (a PPI) in either a high dose (40 mg twice daily) or a low dose (20 mg once daily) plus either aspirin (300 or 320 mg per day) or no aspirin in 2,557 patients with Barrett esophagus. The composite end point was the time to all-cause mortality, adenocarcinoma, or high-grade dysplasia.
At a median follow-up of 8.9 years, the combination of high-dose esomeprazole plus aspirin had the strongest effect compared with low-dose esomeprazole without aspirin (time ratio 1.59, 95% CI 1.14–2.23, P = .0068). The number needed to treat was 34 for esomeprazole and 43 for aspirin.29
Based on these data, we can conclude that aspirin and PPIs can prevent dysplasia and all-cause mortality in Barrett esophagus.
Metformin: No evidence of benefit
Metformin was studied as a protective agent against obesity-associated cancers including esophageal adenocarcinoma, as it reduces insulin levels.
In a randomized controlled trial30 in 74 patients with Barrett esophagus, metformin (starting at 500 mg daily, increasing to 2,000 mg/day by week 4) was compared with placebo. At 12 weeks, the percent change in esophageal levels of the biomarker pS6K1—an intracellular mediator of insulin and insulin-like growth factor activation in Barrett epithelium—did not differ significantly between the 2 groups (1.4% with metformin vs −14.7% with placebo; 1-sided P = .80). This suggested that metformin did not significantly alter proliferation or apoptosis in Barrett epithelium, despite reducing serum insulin levels and insulin resistance. Thus, metformin did not demonstrate a chemoprotective effect in preventing the progression of Barrett esophagus to adenocarcinoma.
Vitamin D: No evidence of benefit
Vitamin D affects genes regulating proliferation, apoptosis, and differentiation, and has therefore been studied as a potential antineoplastic agent. Its deficiency has also been associated with increased risk of esophageal adenocarcinoma. However, its efficacy in chemoprevention is unclear.31
One study found no association between serum 25-hydroxyvitamin D levels and prevalence of dysplasia in Barrett esophagus (P = .90). An increase in vitamin D levels had no effect on progression to dysplasia or cancer (for every 5-nmol/L increase from baseline, hazard ratio 0.98, P = .62).32
In another study, supplementation with vitamin D3 (cholecalciferol 50,000 IU weekly) plus a PPI for 12 weeks significantly improved the serum 25-hydroxyvitamin D levels without significant changes in gene expression from Barrett epithelium.33 These findings were confirmed in a meta-analysis that showed no consistent association between vitamin D exposure and risk of esophageal neoplasm.34
Thus, there is currently no evidence to support vitamin D for chemoprevention in Barrett esophagus or esophageal adenocarcinoma.
Statins
In addition to lowering cholesterol, statins have antiproliferative, pro-apoptotic, anti-angiogenic, and immunomodulatory effects that prevent cancer, leading to a 41% reduction in the risk of adenocarcinoma in patients with Barrett esophagus in one study (adjusted OR 0.59, 95% CI 0.45–0.78); the number needed to treat with statins to prevent 1 case of adenocarcinoma was 389.35
A meta-analysis also showed that statin use was associated with a lower risk of progression of Barrett esophagus (OR 0.48, 95% CI 0.31–0.73).36
In general, statins appear promising for chemoprevention, but more study is needed.
When is chemoprevention appropriate?
Chemoprevention is not recommended for all patients with Barrett esophagus, given that the condition affects 1% to 2% of the US adult population, and very few patients have progression to esophageal adenocarcinoma. Rather, chemoprevention may be considered in patients with Barrett esophagus and multiple risk factors for adenocarcinoma.
INDEFINITE DYSPLASIA
In Barrett esophagus with indefinite dysplasia, either the epithelial abnormalities are insufficient for a diagnosis of dysplasia, or the nature of the epithelial abnormalities is uncertain due to inflammation or technical difficulties with specimen processing. The risk of high-grade dysplasia or cancer within 1 year of the diagnosis of indefinite dysplasia varies between 1.9% and 15%.37 The recommendation for management is to optimize acid-suppressive therapy for 3 to 6 months and then to repeat esophagogastroduodenoscopy. If indefinite dysplasia is noted again, repeat endoscopy in 12 months is recommended.2
ENDOSCOPIC ERADICATION: AN OVERVIEW
Because dysplasia in Barrett esophagus carries a high risk of progression to cancer, the standard of care is endoscopic mucosal resection of visible lesions, followed by ablation of the flat mucosa, with the aim of achieving complete eradication of intestinal metaplasia.4,38 The initial endoscopic treatment is followed by outpatient sessions every 8 to 10 weeks until the dysplasia is eradicated. A key part of treatment during this time is maximal acid suppression with a PPI twice daily and a histamine-2 blocker at night. In rare cases, fundoplication is required to control reflux refractory to medical therapy.
After eradication is confirmed, continued surveillance is necessary, as recurrences have been reported at a rate of 4.8% per year for intestinal metaplasia, and 2% per year for dysplasia.39
Current endoscopic resection techniques
Endoscopic resection techniques include mucosal resection, submucosal dissection, radiofrequency ablation, cryotherapy, argon plasma coagulation, and photodynamic therapy (Figure 2).
In mucosal resection, the lesion is either suctioned into a band ligator, after which a band is placed around the lesion, or suctioned into a cap fitted at the end of the endoscope, after which the lesion is removed using a snare.
In submucosal dissection, a liquid is injected into the submucosa to lift the lesion, making it easier to remove. The procedure is technically complex and requires additional training.
In radiofrequency ablation, a special catheter is passed through the endoscope to ablate the affected epithelium by thermal injury. Argon plasma coagulation works in a similar way, but uses ionized argon gas to induce thermal coagulation of metaplastic epithelium.
Cryotherapy produces cellular injury by rapid freezing and thawing of tissue using a cryogen such as liquid nitrogen or nitrous oxide.
In photodynamic therapy, a photosensitizer (porfimer sodium) is administered and taken up preferentially by metaplastic epithelium; it is then activated by transmission of red light using the endoscope, leading to destruction of the metaplastic epithelium.
Of the different techniques, radiofrequency ablation has the most evidence for efficacy and hence is the most commonly used.
All of these procedures are generally well tolerated and have favorable side-effect profiles. After radiofrequency ablation with or without mucosal resection, esophageal strictures are noted in 5.6% of patients, and bleeding and perforation occur rarely (1% and 0.6% of patients, respectively).40 Submucosal dissection is associated with a higher rate of complications such as stricture formation (11% of patients) and bleeding or perforation (1.5% of patients).41
LOW-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
Most patients with low-grade dysplasia (73%) are down-staged to nondysplastic Barrett esophagus or to indefinite for dysplasia after review by expert pathologists.42 Patients with confirmed and persistent low-grade dysplasia are at higher risk of progression.43
Once low-grade dysplasia is confirmed by a second gastrointestinal pathologist, the patient should undergo endoscopic ablation. A landmark study by Shaheen et al44 demonstrated the benefit of radiofrequency ablation in achieving complete eradication of dysplasia (90.5% vs 22.7% for a sham procedure) and complete eradication of intestinal metaplasia (77.4% vs 2.3% for a sham procedure). In another trial of 136 patients with low-grade dysplasia followed for 3 years, Phoa et al45 demonstrated that radiofrequency ablation reduced the rate of progression to high-grade dysplasia by 25% and to adenocarcinoma by 7.4% compared with endoscopic surveillance.
Patients with confirmed low-grade dysplasia who do not undergo eradication therapy should have surveillance endoscopy every 6 to 12 months (Table 1).
HIGH-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
As with low-grade dysplasia, the diagnosis of high-grade dysplasia needs to be confirmed by a second pathologist with gastrointestinal expertise. In the past, the treatment was esophagectomy, but due to lower morbidity and equivalent efficacy of radiofrequency ablation,46 the current treatment of choice is endoscopic mucosal resection of raised lesions, followed by radiofrequency ablation of the entire affected segment.
In the study by Shaheen et al,44 42 patients with high-grade dysplasia were randomized to radiofrequency ablation and 21 to a sham procedure, and 81% of ablation patients achieved complete eradication of dysplasia vs 19% with the sham procedure. Eradication of intestinal metaplasia was achieved in 77% of ablation patients vs 2% of patients with the sham therapy. Results of 3-year follow-up from the same cohort showed complete eradication of dysplasia in 98% and of intestinal metaplasia in 91%.47
Endoscopic eradication therapy is recommended for all patients with Barrett esophagus and high-grade dysplasia without a life-limiting comorbidity. Alternatively, surveillance every 3 months is an option if the patient does not wish to undergo eradication therapy. Radiofrequency ablation is more cost-effective than esophagectomy or endoscopic surveillance followed by treatment once patients develop adenocarcinoma.48,49
EARLY ESOPHAGEAL ADENOCARCINOMA: RECOMMENDED MANAGEMENT
Adenocarcinoma limited to the mucosa and without evidence of nodal involvement can be resected endoscopically. In patients with localized cancer, mucosal resection is done not only for therapeutic purposes but also for staging. Ideal management is multidisciplinary, including a gastroenterologist, thoracic surgeon, oncologist, pathologist, and radiation oncologist.
If lesions have features suggesting submucosal invasion or are greater than 1.5 cm in size, or if it is difficult to separate (ie, lift) the mucosa from the submucosal layer with injection of saline, then submucosal dissection is recommended.50 Because of the risk of metachronous lesions, ablation of the remaining Barrett esophagus mucosa is recommended after resection of cancer.
Endoscopic eradication is highly effective and durable for the treatment of intramucosal esophageal adenocarcinoma. In a study of 1,000 patients, 963 patients (96.3%) had achieved a complete response; 12 patients (3.7%) underwent surgery after eradication failed during a follow-up of almost 5 years.51 Metachronous lesions or recurrence of cancer developed during the follow-up period in 140 patients (14.5%) but were successfully treated endoscopically in 115, resulting in a long-term complete remission rate of 93.8%.
POSTABLATION MANAGEMENT
Because of the risk of recurrence of dysplasia after ablation, long-term PPI therapy and surveillance are recommended.
Surveillance endoscopy involves 4-quadrant biopsies taken every 1 cm from the entire length of segment where Barrett esophagus had been seen before ablation.
The timing of surveillance intervals depends on the preablation grade of dysplasia. For low-grade dysplasia, the recommendation is every 6 months for the first year after ablation and, if there is no recurrence of dysplasia, annually after that.2 After treatment of high-grade dysplasia or intramucosal adenocarcinoma, the recommendation is every 3 months for the first year, every 6 months in the second year, and then annually.2
All cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus.1 But most cases of Barrett esophagus go undiagnosed. And Barrett esophagus is seen in 5% to 15% of patients with gastroesophageal reflux disease.2 These facts clearly emphasize the need for screening. Here, we review the rationale and recommendations for screening and surveillance, as well as the range of treatment options.
SCOPE OF THE PROBLEM
The American Cancer Society estimated there were 17,290 new cases of esophageal cancer and 15,850 deaths from it in the United States in 2018.3 Of the 2 main histologic types of esophageal cancer, adenocarcinoma and squamous cell cancer, adenocarcinoma is more common in the United States.
The precursor lesion is Barrett esophagus, defined as an extension of salmon-colored mucosa at least 1 cm into the tubular esophagus proximal to the gastroesophageal junction, with biopsy confirmation of intestinal metaplasia.4
The natural course of progression to dysplasia and cancer in Barrett esophagus is unknown but is thought to be stepwise, from no dysplasia to low-grade dysplasia to high-grade dysplasia and cancer, and the cancer risk depends on the degree of dysplasia: the annual risk is 0.33% if there is no dysplasia, 0.54% with low-grade dysplasia, and 7% with high-grade dysplasia.4
Although all cases of esophageal adenocarcinoma are thought to arise from Barrett esophagus,1 more than 90% of patients with newly diagnosed esophageal adenocarcinoma do not have a prior diagnosis of Barrett esophagus.5 Therefore, there is a substantial unmet need to expand screening for Barrett esophagus in people at risk.
GASTROESOPHAGEAL REFLUX DISEASE IS A RISK FACTOR FOR CANCER
The rationale behind screening is that detecting Barrett esophagus early and intervening in a timely manner in patients at higher risk of developing adenocarcinoma will decrease mortality.
Chronic gastroesophageal reflux disease is a strong risk factor for esophageal adenocarcinoma (odds ratio [OR] 7.7, 95% confidence interval [CI] 5.3–11.4), and the risk increases when symptoms are long-standing (> 20 years) or severe (OR 43.5, 95% CI 18.3–103.5) or occur daily (OR 5.5, 95% CI 3.2–9.3).6
Reflux symptoms are scored as follows:
- Heartburn only, 1 point
- Regurgitation only, 1 point
- Heartburn with regurgitation, 1.5 points
- Nightly symptoms (2 points if yes, 0 if no)
- Symptoms once a week, 0 points; 2 to 6 times a week, 1 point; 7 to 15 times a week, 2 points; more than 15 times a week, 3 points.6
A score of 4.5 or higher indicates severe reflux disease. However, it is worth noting that the annual incidence of esophageal adenocarcinoma in patients with long-term gastroesophageal reflux disease is less than 0.001%.7
RISK FACTORS FOR BARRETT ESOPHAGUS
Risk factors for Barrett esophagus include:
Male sex. Barrett esophagus is more prevalent in men than in women, at a ratio of 2 to 1; but in individuals under age 50, the ratio is 4 to 1.8
Age 50 or older. Barrett esophagus is usually diagnosed in the sixth to seventh decade of life, and the prevalence increases from 2.1% in the third decade to 9.3% in the sixth decade.9
White race. It is more prevalent in whites than in blacks (5.0% vs 1.5%, P < .0001).10
Central obesity. Waist circumference is an independent risk factor: every 5-cm increase carries an OR of 1.14 (95% CI 1.03–1.27, P = .02).11
Cigarette smoking increases the risk of Barrett esophagus (OR 1.42; 95% CI 1.15–1.76).12
A family history of Barrett esophagus or esophageal adenocarcinoma is a strong risk factor (OR 12, 95% CI 3.3–44.8). In 1 study, the risk in first- and second-degree relatives of patients with Barrett esophagus was 24%, compared with 5% in a control population (P < .005).13
SCREENING GUIDELINES AND DRAWBACKS
The standard screening method is esophagogastroduodenoscopy with sedation, with careful visual inspection and 4-quadrant biopsies every 2 cm using the Seattle protocol, ie, including biopsy of any mucosal irregularities in salmon-colored mucosa above the gastroesophageal junction (Figure 1).4
Endoscopic screening is cost-effective, costing $10,440 per quality-adjusted life-year saved, which is well below the accepted threshold of less than $100,000.14 However, it is still expensive, invasive, and not ideal for screening large populations.
Less-invasive methods under study
Less-invasive, less-expensive methods being tested for mass screening include:
Unsedated transnasal endoscopy. Done with only topical anesthesia, it has high diagnostic accuracy and is quicker and more cost-effective than standard esophagogastroduodenoscopy, with fewer adverse effects. However, the procedure has not yet gained widespread acceptance for regular use by gastroenterologists.15
A swallowable sponge. Another promising test is cell collection using the Cytosponge Cell Collection Device (Medtronic, Minneapolis, MN). An encapsulated compressed sponge with a string attached is swallowed; in the stomach, the capsule dissolves, and the sponge expands and is then withdrawn using the attached string. The obtained cytology sample from the lower esophagus is then tested for trefoil factor 3, a protein biomarker for Barrett esophagus.16
A retractable balloon. The EsoCheck Cell Collection Device is a retractable balloon attached to a string. When swallowed, it gathers distal esophageal cells for detecting methylated DNA markers for Barrett esophagus.17
Esophageal capsule endoscopy uses a camera to visualize the esophagus, but lacks the ability to obtain biopsy samples.
Other screening methods are being tested, although data are limited. Liquid biopsy uses a blood sample to detect microRNAs that are dysregulated in cancer. The “electronic nose” is a device that detects exhaled volatile organic compounds altered in Barrett esophagus. Another test involves taking an oral wash sample to study the oral microbiome for a pattern associated with adenocarcinoma.18–21
SURVEILLANCE: WHAT’S INVOLVED, WHAT’S AVAILABLE
Surveillance in Barrett esophagus aims to detect premalignant changes or early-stage adenocarcinoma to provide longer survival and lower cancer-related mortality. Recent evidence suggests that patients with esophageal adenocarcinoma that is diagnosed in a Barrett esophagus surveillance program have an earlier stage of disease and therefore a survival benefit.22
Patient education is essential
Before enrolling a patient in a surveillance program, the clinician should explain the risks, benefits, and limitations, the importance of periodic endoscopy, and the possible eventual need for endoscopic therapy or surgery.
The endoscopic procedure
Surveillance involves examination by high-definition white-light endoscopy, with random 4-quadrant biopsies every 2 cm (or every 1 cm in patients with a history of dysplasia) and biopsy of any mucosal irregularity (nodule, ulcer, or other visible lesion). The degree of dysplasia determines the frequency of follow-up surveillance intervals and the need for endoscopic eradication therapy, as presented in professional society guidelines (Table 1).4,23,24
Advanced methods for detecting dysplasia
Newer endoscopic surveillance techniques include dye-based chromoendoscopy, narrow-band imaging, confocal laser endomicroscopy, volumetric laser endomicroscopy, and wide-area transepithelial sampling with computer-assisted 3-dimensional analysis. All these techniques are used to increase the detection of dysplasia. Of these, dye-based chromoendoscopy, narrow-band imaging, and confocal laser endomicroscopy meet current criteria of the American Society for Gastrointestinal Endoscopy for preservation and incorporation of valuable endoscopic innovations.23
MANAGEMENT OF NONDYSPLASTIC BARRETT ESOPHAGUS
A proton pump inhibitor (PPI) is recommended to control reflux symptoms in patients with nondysplastic Barrett esophagus. But it is important to counsel patients on additional ways to protect against esophageal adenocarcinoma, such as:
- Low to moderate alcohol consumption
- Regular physical activity
- Increased dietary intake of fruits, vegetables, folate, fiber, beta-carotene, and vitamin C
- Weight control
- Smoking cessation.25
Surveillance endoscopy with 4-quadrant biopsies at 2-cm intervals is recommended every 3 to 5 years (Table 1).
DOES CHEMOPREVENTION HAVE A ROLE?
Chemoprevention is an exciting area of research in preventing progression to adenocarcinoma in patients with Barrett esophagus. Various drugs such as aspirin, other nonsteroidal anti-inflammatory drugs (NSAIDs), PPIs, metformin, and statins have been studied.
Aspirin
Aspirin has been shown to prevent development of Barrett esophagus in patients with reflux disease,26 but more studies are needed to validate those findings.
PPIs
Gastroesophageal reflux disease is a primary risk factor for esophageal adenocarcinoma, and gastric acid suppression with PPIs reduces cancer risk. PPI therapy is associated with a 71% decrease in the risk of high-grade dysplasia and adenocarcinoma in patients with Barrett esophagus (OR 0.29, 95% CI 0.12–0.79).27 Long-term therapy (> 2 to 3 years) has a higher protective effect (adjusted OR 0.45, 95% CI 0.19–1.06) than short-term therapy (< 2 to 3 years) (adjusted OR 1.09, 95% CI 0.47–2.56).27
NSAIDs
NSAIDs, including aspirin, have been associated with decreased risk of colon, stomach, lung, breast, and esophageal cancer due to their potential to inhibit cyclooxygenase 2 (COX-2) enzymes.
A meta-analysis demonstrated that aspirin and NSAIDs led to a 32% reduction in the risk of adenocarcinoma (OR 0.68, 95% CI 0.56–0.83). The benefit was even greater if the drug was taken daily or more frequently (OR 0.56, 95% CI 0.43–0.73, P < .001) or was taken for 10 or more years (OR 0.63, 95% CI 0.45–0.90, P = .04).28
PPI plus aspirin
The best evidence for the role of PPIs and aspirin in reducing the risk of dysplasia comes from the Aspirin and Esomeprazole Chemoprevention in Barrett’s Metaplasia Trial.29 This randomized, controlled trial compared 4 regimens consisting of esomeprazole (a PPI) in either a high dose (40 mg twice daily) or a low dose (20 mg once daily) plus either aspirin (300 or 320 mg per day) or no aspirin in 2,557 patients with Barrett esophagus. The composite end point was the time to all-cause mortality, adenocarcinoma, or high-grade dysplasia.
At a median follow-up of 8.9 years, the combination of high-dose esomeprazole plus aspirin had the strongest effect compared with low-dose esomeprazole without aspirin (time ratio 1.59, 95% CI 1.14–2.23, P = .0068). The number needed to treat was 34 for esomeprazole and 43 for aspirin.29
Based on these data, we can conclude that aspirin and PPIs can prevent dysplasia and all-cause mortality in Barrett esophagus.
Metformin: No evidence of benefit
Metformin was studied as a protective agent against obesity-associated cancers including esophageal adenocarcinoma, as it reduces insulin levels.
In a randomized controlled trial30 in 74 patients with Barrett esophagus, metformin (starting at 500 mg daily, increasing to 2,000 mg/day by week 4) was compared with placebo. At 12 weeks, the percent change in esophageal levels of the biomarker pS6K1—an intracellular mediator of insulin and insulin-like growth factor activation in Barrett epithelium—did not differ significantly between the 2 groups (1.4% with metformin vs −14.7% with placebo; 1-sided P = .80). This suggested that metformin did not significantly alter proliferation or apoptosis in Barrett epithelium, despite reducing serum insulin levels and insulin resistance. Thus, metformin did not demonstrate a chemoprotective effect in preventing the progression of Barrett esophagus to adenocarcinoma.
Vitamin D: No evidence of benefit
Vitamin D affects genes regulating proliferation, apoptosis, and differentiation, and has therefore been studied as a potential antineoplastic agent. Its deficiency has also been associated with increased risk of esophageal adenocarcinoma. However, its efficacy in chemoprevention is unclear.31
One study found no association between serum 25-hydroxyvitamin D levels and prevalence of dysplasia in Barrett esophagus (P = .90). An increase in vitamin D levels had no effect on progression to dysplasia or cancer (for every 5-nmol/L increase from baseline, hazard ratio 0.98, P = .62).32
In another study, supplementation with vitamin D3 (cholecalciferol 50,000 IU weekly) plus a PPI for 12 weeks significantly improved the serum 25-hydroxyvitamin D levels without significant changes in gene expression from Barrett epithelium.33 These findings were confirmed in a meta-analysis that showed no consistent association between vitamin D exposure and risk of esophageal neoplasm.34
Thus, there is currently no evidence to support vitamin D for chemoprevention in Barrett esophagus or esophageal adenocarcinoma.
Statins
In addition to lowering cholesterol, statins have antiproliferative, pro-apoptotic, anti-angiogenic, and immunomodulatory effects that prevent cancer, leading to a 41% reduction in the risk of adenocarcinoma in patients with Barrett esophagus in one study (adjusted OR 0.59, 95% CI 0.45–0.78); the number needed to treat with statins to prevent 1 case of adenocarcinoma was 389.35
A meta-analysis also showed that statin use was associated with a lower risk of progression of Barrett esophagus (OR 0.48, 95% CI 0.31–0.73).36
In general, statins appear promising for chemoprevention, but more study is needed.
When is chemoprevention appropriate?
Chemoprevention is not recommended for all patients with Barrett esophagus, given that the condition affects 1% to 2% of the US adult population, and very few patients have progression to esophageal adenocarcinoma. Rather, chemoprevention may be considered in patients with Barrett esophagus and multiple risk factors for adenocarcinoma.
INDEFINITE DYSPLASIA
In Barrett esophagus with indefinite dysplasia, either the epithelial abnormalities are insufficient for a diagnosis of dysplasia, or the nature of the epithelial abnormalities is uncertain due to inflammation or technical difficulties with specimen processing. The risk of high-grade dysplasia or cancer within 1 year of the diagnosis of indefinite dysplasia varies between 1.9% and 15%.37 The recommendation for management is to optimize acid-suppressive therapy for 3 to 6 months and then to repeat esophagogastroduodenoscopy. If indefinite dysplasia is noted again, repeat endoscopy in 12 months is recommended.2
ENDOSCOPIC ERADICATION: AN OVERVIEW
Because dysplasia in Barrett esophagus carries a high risk of progression to cancer, the standard of care is endoscopic mucosal resection of visible lesions, followed by ablation of the flat mucosa, with the aim of achieving complete eradication of intestinal metaplasia.4,38 The initial endoscopic treatment is followed by outpatient sessions every 8 to 10 weeks until the dysplasia is eradicated. A key part of treatment during this time is maximal acid suppression with a PPI twice daily and a histamine-2 blocker at night. In rare cases, fundoplication is required to control reflux refractory to medical therapy.
After eradication is confirmed, continued surveillance is necessary, as recurrences have been reported at a rate of 4.8% per year for intestinal metaplasia, and 2% per year for dysplasia.39
Current endoscopic resection techniques
Endoscopic resection techniques include mucosal resection, submucosal dissection, radiofrequency ablation, cryotherapy, argon plasma coagulation, and photodynamic therapy (Figure 2).
In mucosal resection, the lesion is either suctioned into a band ligator, after which a band is placed around the lesion, or suctioned into a cap fitted at the end of the endoscope, after which the lesion is removed using a snare.
In submucosal dissection, a liquid is injected into the submucosa to lift the lesion, making it easier to remove. The procedure is technically complex and requires additional training.
In radiofrequency ablation, a special catheter is passed through the endoscope to ablate the affected epithelium by thermal injury. Argon plasma coagulation works in a similar way, but uses ionized argon gas to induce thermal coagulation of metaplastic epithelium.
Cryotherapy produces cellular injury by rapid freezing and thawing of tissue using a cryogen such as liquid nitrogen or nitrous oxide.
In photodynamic therapy, a photosensitizer (porfimer sodium) is administered and taken up preferentially by metaplastic epithelium; it is then activated by transmission of red light using the endoscope, leading to destruction of the metaplastic epithelium.
Of the different techniques, radiofrequency ablation has the most evidence for efficacy and hence is the most commonly used.
All of these procedures are generally well tolerated and have favorable side-effect profiles. After radiofrequency ablation with or without mucosal resection, esophageal strictures are noted in 5.6% of patients, and bleeding and perforation occur rarely (1% and 0.6% of patients, respectively).40 Submucosal dissection is associated with a higher rate of complications such as stricture formation (11% of patients) and bleeding or perforation (1.5% of patients).41
LOW-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
Most patients with low-grade dysplasia (73%) are down-staged to nondysplastic Barrett esophagus or to indefinite for dysplasia after review by expert pathologists.42 Patients with confirmed and persistent low-grade dysplasia are at higher risk of progression.43
Once low-grade dysplasia is confirmed by a second gastrointestinal pathologist, the patient should undergo endoscopic ablation. A landmark study by Shaheen et al44 demonstrated the benefit of radiofrequency ablation in achieving complete eradication of dysplasia (90.5% vs 22.7% for a sham procedure) and complete eradication of intestinal metaplasia (77.4% vs 2.3% for a sham procedure). In another trial of 136 patients with low-grade dysplasia followed for 3 years, Phoa et al45 demonstrated that radiofrequency ablation reduced the rate of progression to high-grade dysplasia by 25% and to adenocarcinoma by 7.4% compared with endoscopic surveillance.
Patients with confirmed low-grade dysplasia who do not undergo eradication therapy should have surveillance endoscopy every 6 to 12 months (Table 1).
HIGH-GRADE DYSPLASIA: RECOMMENDED MANAGEMENT
As with low-grade dysplasia, the diagnosis of high-grade dysplasia needs to be confirmed by a second pathologist with gastrointestinal expertise. In the past, the treatment was esophagectomy, but due to lower morbidity and equivalent efficacy of radiofrequency ablation,46 the current treatment of choice is endoscopic mucosal resection of raised lesions, followed by radiofrequency ablation of the entire affected segment.
In the study by Shaheen et al,44 42 patients with high-grade dysplasia were randomized to radiofrequency ablation and 21 to a sham procedure, and 81% of ablation patients achieved complete eradication of dysplasia vs 19% with the sham procedure. Eradication of intestinal metaplasia was achieved in 77% of ablation patients vs 2% of patients with the sham therapy. Results of 3-year follow-up from the same cohort showed complete eradication of dysplasia in 98% and of intestinal metaplasia in 91%.47
Endoscopic eradication therapy is recommended for all patients with Barrett esophagus and high-grade dysplasia without a life-limiting comorbidity. Alternatively, surveillance every 3 months is an option if the patient does not wish to undergo eradication therapy. Radiofrequency ablation is more cost-effective than esophagectomy or endoscopic surveillance followed by treatment once patients develop adenocarcinoma.48,49
EARLY ESOPHAGEAL ADENOCARCINOMA: RECOMMENDED MANAGEMENT
Adenocarcinoma limited to the mucosa and without evidence of nodal involvement can be resected endoscopically. In patients with localized cancer, mucosal resection is done not only for therapeutic purposes but also for staging. Ideal management is multidisciplinary, including a gastroenterologist, thoracic surgeon, oncologist, pathologist, and radiation oncologist.
If lesions have features suggesting submucosal invasion or are greater than 1.5 cm in size, or if it is difficult to separate (ie, lift) the mucosa from the submucosal layer with injection of saline, then submucosal dissection is recommended.50 Because of the risk of metachronous lesions, ablation of the remaining Barrett esophagus mucosa is recommended after resection of cancer.
Endoscopic eradication is highly effective and durable for the treatment of intramucosal esophageal adenocarcinoma. In a study of 1,000 patients, 963 patients (96.3%) had achieved a complete response; 12 patients (3.7%) underwent surgery after eradication failed during a follow-up of almost 5 years.51 Metachronous lesions or recurrence of cancer developed during the follow-up period in 140 patients (14.5%) but were successfully treated endoscopically in 115, resulting in a long-term complete remission rate of 93.8%.
POSTABLATION MANAGEMENT
Because of the risk of recurrence of dysplasia after ablation, long-term PPI therapy and surveillance are recommended.
Surveillance endoscopy involves 4-quadrant biopsies taken every 1 cm from the entire length of segment where Barrett esophagus had been seen before ablation.
The timing of surveillance intervals depends on the preablation grade of dysplasia. For low-grade dysplasia, the recommendation is every 6 months for the first year after ablation and, if there is no recurrence of dysplasia, annually after that.2 After treatment of high-grade dysplasia or intramucosal adenocarcinoma, the recommendation is every 3 months for the first year, every 6 months in the second year, and then annually.2
- Mendes de Almeida JC, Chaves P, Pereira AD, Altorki NK. Is Barrett’s esophagus the precursor of most adenocarcinomas of the esophagus and cardia? A biochemical study. Ann Surg 1997; 226(6):725–733. pmid:9409571
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005; 61(2):226–231. pmid:15729230
- National Cancer Institute. Cancer stat facts: esophageal cancer. https://seer.cancer.gov/statfacts/html/esoph.html. Accessed August 6, 2019.
- Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016; 111(1):30–50. doi:10.1038/ajg.2015.322
- Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology 2002; 122(1):26–33. pmid:11781277
- Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999; 340(11):825–831. doi:10.1056/NEJM199903183401101
- Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: scientific review. JAMA 2002; 287(15):1972–1981. pmid:11960540
- van Blankenstein M, Looman CW, Johnston BJ, Caygill CP. Age and sex distribution of the prevalence of Barrett’s esophagus found in a primary referral endoscopy center. Am J Gastroenterol 2005; 100(3):568–576.
- Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett’s esophagus by endoscopy indication. Gastrointest Endosc 2010; 71(1):21–27. doi:10.1016/j.gie.2009.06.035
- Wang A, Mattek NC, Holub JL, Lieberman DA, Eisen GM. Prevalence of complicated gastroesophageal reflux disease and Barrett’s esophagus among racial groups in a multi-center consortium. Dig Dis Sci 2009; 54(5):964–971. doi:10.1007/s10620-009-0742-3
- Kubo A, Cook MB, Shaheen NJ, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett’s esophagus: a pooled analysis from the international BEACON consortium. Gut 2013; 62(12):1684–1691. doi:10.1136/gutjnl-2012-303753
- Andrici J, Cox MR, Eslick GD. Cigarette smoking and the risk of Barrett’s esophagus: a systematic review and meta-analysis. J Gastroenterol Hepatol 2013; 28(8):1258–1273. doi:10.1111/jgh.12230
- Chak A, Lee T, Kinnard MF, et al. Familial aggregation of Barrett’s esophagus, esophageal adenocarcinoma, and esophagogastric junctional adenocarcinoma in Caucasian adults. Gut 2002; 51(3):323–328. pmid:12171951
- Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med 2003; 138(3):176–186. pmid:12558356
- Jobe BA, Hunter JG, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. Am J Gastroenterol 2006; 101(12):2693–2703.
- Ross-Innes CS, Chettouh H, Achilleos A, et al; BEST2 study group. Risk stratification of Barrett’s esophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. Lancet Gastroenterol Hepatol 2017; 2(1):23–31. doi:10.1016/S2468-1253(16)30118-2
- Moinova HR, LaFramboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci Transl Med 2018; 10(424). pii:eaao5848. doi:10.1126/scitranslmed.aao5848
- Chan DK, Zakko L, Visrodia KH, et al. Breath testing for Barrett’s esophagus using exhaled volatile organic compound profiling with an electronic nose device. Gastroenterology 2017; 152(1):24–26. doi:10.1053/j.gastro.2016.11.001
- Kumar S, Huang J, Abbassi-Ghadi N, et al. Mass spectrometric analysis of exhaled breath for the identification of volatile organic compound biomarkers in esophageal and gastric adenocarcinoma. Ann Surg 2015; 262(6):981–990. doi:10.1097/SLA.0000000000001101
- Peters BA, Wu J, Pei Z, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res 2017; 77(23):6777–6787. doi:10.1158/0008-5472.CAN-17-1296
- Mallick R, Patnaik SK, Wani S, Bansal A. A systematic review of esophageal microrna markers for diagnosis and monitoring of Barrett’s esophagus. Dig Dis Sci 2016; 61(4):1039–1050. doi:10.1007/s10620-015-3959-3
- Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology 2018; 154(8):2068–2086.e5. doi:10.1053/j.gastro.2018.02.022
- ASGE Technology Committee; Thosani N, Abu Dayyeh BK, Sharma P, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE preservation and incorporation of valuable endoscopic innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett’s esophagus. Gastrointest Endosc 2016; 83(4):684–698.e7. doi:10.1016/j.gie.2016.01.007
- Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ; American Gastroenterological Association. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology 2011; 140(3):e18–e52. doi:10.1053/j.gastro.2011.01.031
- Castro C, Peleteiro B, Lunet N. Modifiable factors and esophageal cancer: a systematic review of published meta-analyses. J Gastroenterol 2018; 53(1):37–51. doi:10.1007/s00535-017-1375-5
- Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett’s esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol 2012; 10(7):722–727. doi:10.1016/j.cgh.2012.02.031
- Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gut 2014; 63(8):1229–1237. doi:10.1136/gutjnl-2013-305997
- Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 2012; 142(3):442–452.e5. doi:10.1053/j.gastro.2011.11.019
- Jankowski JAZ, de Caestecker J, Love SB, et al; AspECT Trial Team. Esomeprazole and aspirin in Barrett’s esophagus (AspECT): a randomised factorial trial. Lancet 2018; 392(10145):400–408. doi:10.1016/S0140-6736(18)31388-6
- Chak A, Buttar NS, Foster NR, et al; Cancer Prevention Network. Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2015; 13(4):665–672.e1–e4. doi:10.1016/j.cgh.2014.08.040
- Rouphael C, Kamal A, Sanaka MR, Thota PN. Vitamin D in esophageal cancer: is there a role for chemoprevention? World J Gastrointest Oncol 2018; 10(1):23–30. doi:10.4251/wjgo.v10.i1.23
- Thota PN, Kistangari G, Singh P, et al. Serum 25-hydroxyvitamin D levels and the risk of dysplasia and esophageal adenocarcinoma in patients with Barrett’s esophagus. Dig Dis Sci 2016; 61(1):247–254. doi:10.1007/s10620-015-3823-5
- Cummings LC, Thota PN, Willis JE, et al. A nonrandomized trial of vitamin D supplementation for Barrett’s esophagus. PLoS One 2017;1 2(9):e0184928. doi:10.1371/journal.pone.0184928
- Zgaga L, O’Sullivan F, Cantwell MM, Murray LJ, Thota PN, Coleman HG. Markers of vitamin D exposure and esophageal cancer risk: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2016; 25(6):877–886. doi:10.1158/1055-9965.EPI-15-1162
- Singh S, Singh AG, Singh PP, Murad MH, Iyer PG. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013; 11(6):620–629. doi:10.1016/j.cgh.2012.12.036
- Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors associated with progression of Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018; 6(7):1046–1055.e8. doi:10.1016/j.cgh.2017.11.044
- Thota PN, Kistangari G, Esnakula AK, Gonzalo DH, Liu XL. Clinical significance and management of Barrett’s esophagus with epithelial changes indefinite for dysplasia. World J Gastrointest Pharmacol Ther 2016; 7(3):406–411. doi:10.4292/wjgpt.v7.i3.406
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012; 143(2):336–346. doi:10.1053/j.gastro.2012.04.032
- Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett’s esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017; 85(3):482–495.e4. doi:10.1016/j.gie.2016.09.022
- Qumseya BJ, Wani S, Desai M, et al. Adverse events after radiofrequency ablation in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016; 14(8):1086–1095.e6. doi:10.1016/j.cgh.2016.04.001
- Yang D, Zou F, Xiong S, Forde JJ, Wang Y, Draganov PV. Endoscopic submucosal dissection for early Barrett’s neoplasia: a meta-analysis. Gastrointest Endosc 2018; 87(6):1383–1393. doi:10.1016/j.gie.2017.09.038
- Duits LC, Phoa KN, Curvers WL, et al. Barrett’s esophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015; 64(5):700–706. doi:10.1136/gutjnl-2014-307278
- Duits LC, van der Wel MJ, Cotton CC, et al. Patients with Barrett’s esophagus and confirmed persistent low-grade dysplasia are at increased risk for progression to neoplasia. Gastroenterology 2017; 152(5):993–1001.e1. doi:10.1053/j.gastro.2016.12.008
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009; 360(22):2277–2288. doi:10.1056/NEJMoa0808145
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014; 311(12):1209–1217. doi:10.1001/jama.2014.2511
- Hu Y, Puri V, Shami VM, Stukenborg GJ, Kozower BD. Comparative effectiveness of esophagectomy versus endoscopic treatment for esophageal high-grade dysplasia. Ann Surg 2016; 263(4):719–726. doi:10.1097/SLA.0000000000001387
- Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology 2011; 141(2):460–468. doi:10.1053/j.gastro.2011.04.061
- Hur C, Choi SE, Rubenstein JH, et al. The cost effectiveness of radiofrequency ablation for Barrett’s esophagus. Gastroenterology 2012; 143(3):567–575. doi:10.1053/j.gastro.2012.05.010
- Boger PC, Turner D, Roderick P, Patel P. A UK-based cost-utility analysis of radiofrequency ablation or oesophagectomy for the management of high-grade dysplasia in Barrett’s esophagus. Aliment Pharmacol Ther 2010; 32(11-12):1332–1342. doi:10.1111/j.1365-2036.2010.04450.x
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47(9):829–854. doi:10.1055/s-0034-1392882
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014; 146(3):652–660.e1. doi:10.1053/j.gastro.2013.11.006
- Mendes de Almeida JC, Chaves P, Pereira AD, Altorki NK. Is Barrett’s esophagus the precursor of most adenocarcinomas of the esophagus and cardia? A biochemical study. Ann Surg 1997; 226(6):725–733. pmid:9409571
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005; 61(2):226–231. pmid:15729230
- National Cancer Institute. Cancer stat facts: esophageal cancer. https://seer.cancer.gov/statfacts/html/esoph.html. Accessed August 6, 2019.
- Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016; 111(1):30–50. doi:10.1038/ajg.2015.322
- Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology 2002; 122(1):26–33. pmid:11781277
- Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999; 340(11):825–831. doi:10.1056/NEJM199903183401101
- Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: scientific review. JAMA 2002; 287(15):1972–1981. pmid:11960540
- van Blankenstein M, Looman CW, Johnston BJ, Caygill CP. Age and sex distribution of the prevalence of Barrett’s esophagus found in a primary referral endoscopy center. Am J Gastroenterol 2005; 100(3):568–576.
- Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett’s esophagus by endoscopy indication. Gastrointest Endosc 2010; 71(1):21–27. doi:10.1016/j.gie.2009.06.035
- Wang A, Mattek NC, Holub JL, Lieberman DA, Eisen GM. Prevalence of complicated gastroesophageal reflux disease and Barrett’s esophagus among racial groups in a multi-center consortium. Dig Dis Sci 2009; 54(5):964–971. doi:10.1007/s10620-009-0742-3
- Kubo A, Cook MB, Shaheen NJ, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett’s esophagus: a pooled analysis from the international BEACON consortium. Gut 2013; 62(12):1684–1691. doi:10.1136/gutjnl-2012-303753
- Andrici J, Cox MR, Eslick GD. Cigarette smoking and the risk of Barrett’s esophagus: a systematic review and meta-analysis. J Gastroenterol Hepatol 2013; 28(8):1258–1273. doi:10.1111/jgh.12230
- Chak A, Lee T, Kinnard MF, et al. Familial aggregation of Barrett’s esophagus, esophageal adenocarcinoma, and esophagogastric junctional adenocarcinoma in Caucasian adults. Gut 2002; 51(3):323–328. pmid:12171951
- Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med 2003; 138(3):176–186. pmid:12558356
- Jobe BA, Hunter JG, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. Am J Gastroenterol 2006; 101(12):2693–2703.
- Ross-Innes CS, Chettouh H, Achilleos A, et al; BEST2 study group. Risk stratification of Barrett’s esophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. Lancet Gastroenterol Hepatol 2017; 2(1):23–31. doi:10.1016/S2468-1253(16)30118-2
- Moinova HR, LaFramboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci Transl Med 2018; 10(424). pii:eaao5848. doi:10.1126/scitranslmed.aao5848
- Chan DK, Zakko L, Visrodia KH, et al. Breath testing for Barrett’s esophagus using exhaled volatile organic compound profiling with an electronic nose device. Gastroenterology 2017; 152(1):24–26. doi:10.1053/j.gastro.2016.11.001
- Kumar S, Huang J, Abbassi-Ghadi N, et al. Mass spectrometric analysis of exhaled breath for the identification of volatile organic compound biomarkers in esophageal and gastric adenocarcinoma. Ann Surg 2015; 262(6):981–990. doi:10.1097/SLA.0000000000001101
- Peters BA, Wu J, Pei Z, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res 2017; 77(23):6777–6787. doi:10.1158/0008-5472.CAN-17-1296
- Mallick R, Patnaik SK, Wani S, Bansal A. A systematic review of esophageal microrna markers for diagnosis and monitoring of Barrett’s esophagus. Dig Dis Sci 2016; 61(4):1039–1050. doi:10.1007/s10620-015-3959-3
- Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology 2018; 154(8):2068–2086.e5. doi:10.1053/j.gastro.2018.02.022
- ASGE Technology Committee; Thosani N, Abu Dayyeh BK, Sharma P, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE preservation and incorporation of valuable endoscopic innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett’s esophagus. Gastrointest Endosc 2016; 83(4):684–698.e7. doi:10.1016/j.gie.2016.01.007
- Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ; American Gastroenterological Association. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology 2011; 140(3):e18–e52. doi:10.1053/j.gastro.2011.01.031
- Castro C, Peleteiro B, Lunet N. Modifiable factors and esophageal cancer: a systematic review of published meta-analyses. J Gastroenterol 2018; 53(1):37–51. doi:10.1007/s00535-017-1375-5
- Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett’s esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol 2012; 10(7):722–727. doi:10.1016/j.cgh.2012.02.031
- Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gut 2014; 63(8):1229–1237. doi:10.1136/gutjnl-2013-305997
- Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 2012; 142(3):442–452.e5. doi:10.1053/j.gastro.2011.11.019
- Jankowski JAZ, de Caestecker J, Love SB, et al; AspECT Trial Team. Esomeprazole and aspirin in Barrett’s esophagus (AspECT): a randomised factorial trial. Lancet 2018; 392(10145):400–408. doi:10.1016/S0140-6736(18)31388-6
- Chak A, Buttar NS, Foster NR, et al; Cancer Prevention Network. Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2015; 13(4):665–672.e1–e4. doi:10.1016/j.cgh.2014.08.040
- Rouphael C, Kamal A, Sanaka MR, Thota PN. Vitamin D in esophageal cancer: is there a role for chemoprevention? World J Gastrointest Oncol 2018; 10(1):23–30. doi:10.4251/wjgo.v10.i1.23
- Thota PN, Kistangari G, Singh P, et al. Serum 25-hydroxyvitamin D levels and the risk of dysplasia and esophageal adenocarcinoma in patients with Barrett’s esophagus. Dig Dis Sci 2016; 61(1):247–254. doi:10.1007/s10620-015-3823-5
- Cummings LC, Thota PN, Willis JE, et al. A nonrandomized trial of vitamin D supplementation for Barrett’s esophagus. PLoS One 2017;1 2(9):e0184928. doi:10.1371/journal.pone.0184928
- Zgaga L, O’Sullivan F, Cantwell MM, Murray LJ, Thota PN, Coleman HG. Markers of vitamin D exposure and esophageal cancer risk: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2016; 25(6):877–886. doi:10.1158/1055-9965.EPI-15-1162
- Singh S, Singh AG, Singh PP, Murad MH, Iyer PG. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013; 11(6):620–629. doi:10.1016/j.cgh.2012.12.036
- Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors associated with progression of Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018; 6(7):1046–1055.e8. doi:10.1016/j.cgh.2017.11.044
- Thota PN, Kistangari G, Esnakula AK, Gonzalo DH, Liu XL. Clinical significance and management of Barrett’s esophagus with epithelial changes indefinite for dysplasia. World J Gastrointest Pharmacol Ther 2016; 7(3):406–411. doi:10.4292/wjgpt.v7.i3.406
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012; 143(2):336–346. doi:10.1053/j.gastro.2012.04.032
- Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett’s esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017; 85(3):482–495.e4. doi:10.1016/j.gie.2016.09.022
- Qumseya BJ, Wani S, Desai M, et al. Adverse events after radiofrequency ablation in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016; 14(8):1086–1095.e6. doi:10.1016/j.cgh.2016.04.001
- Yang D, Zou F, Xiong S, Forde JJ, Wang Y, Draganov PV. Endoscopic submucosal dissection for early Barrett’s neoplasia: a meta-analysis. Gastrointest Endosc 2018; 87(6):1383–1393. doi:10.1016/j.gie.2017.09.038
- Duits LC, Phoa KN, Curvers WL, et al. Barrett’s esophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015; 64(5):700–706. doi:10.1136/gutjnl-2014-307278
- Duits LC, van der Wel MJ, Cotton CC, et al. Patients with Barrett’s esophagus and confirmed persistent low-grade dysplasia are at increased risk for progression to neoplasia. Gastroenterology 2017; 152(5):993–1001.e1. doi:10.1053/j.gastro.2016.12.008
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009; 360(22):2277–2288. doi:10.1056/NEJMoa0808145
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014; 311(12):1209–1217. doi:10.1001/jama.2014.2511
- Hu Y, Puri V, Shami VM, Stukenborg GJ, Kozower BD. Comparative effectiveness of esophagectomy versus endoscopic treatment for esophageal high-grade dysplasia. Ann Surg 2016; 263(4):719–726. doi:10.1097/SLA.0000000000001387
- Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology 2011; 141(2):460–468. doi:10.1053/j.gastro.2011.04.061
- Hur C, Choi SE, Rubenstein JH, et al. The cost effectiveness of radiofrequency ablation for Barrett’s esophagus. Gastroenterology 2012; 143(3):567–575. doi:10.1053/j.gastro.2012.05.010
- Boger PC, Turner D, Roderick P, Patel P. A UK-based cost-utility analysis of radiofrequency ablation or oesophagectomy for the management of high-grade dysplasia in Barrett’s esophagus. Aliment Pharmacol Ther 2010; 32(11-12):1332–1342. doi:10.1111/j.1365-2036.2010.04450.x
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47(9):829–854. doi:10.1055/s-0034-1392882
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014; 146(3):652–660.e1. doi:10.1053/j.gastro.2013.11.006
KEY POINTS
- Screening is recommended for patients with long-standing reflux symptoms (> 5 years) and 1 or more key risk factors: male sex, age over 50, white race, central obesity, and history of smoking.
- In Barrett esophagus without dysplasia, surveillance endoscopy is recommended every 3 to 5 years to detect dysplasia and early esophageal adenocarcinoma.
- The recommended treatment of dysplasia is endoscopic eradication followed by surveillance endoscopy.
Are there alternatives to surgery for Zenker diverticulum?
Conventional treatment for a large symptomatic Zenker diverticulum is to surgically either remove it (diverticulectomy) or obliterate it by repositioning and securing it after cutting into the cricopharyngeus muscle (diverticulopexy with cricopharyngeal myotomy). But high rates of complications with these procedures in elderly patients have led to the development of endoscopic procedures that are safer and have similar or higher success rates.
ESOPHAGEAL POUCH IN SPACE BETWEEN MUSCLES
Zenker diverticulum is an outpouching of the esophageal mucosa into the potential space of the Killian triangle, the area between the inferior pharyngeal constrictor and the cricopharyngeus muscle. Increased cricopharyngeal tone coupled with insufficient relaxation results in a pressure gradient that eventually causes esophageal outpouchings. The problem is induced by age-related fibrosis and atrophy, esophageal spasms related to gastroesophageal reflux disease, and idiopathic cricopharyngeal spasms.1
USUALLY PRESENTS WITH DYSPHAGIA
Zenker diverticulum has an estimated incidence of about 2 per 100,000 per year, but this is likely low because many cases are asymptomatic. It occurs mostly in men and people of Northern European descent in their 60s and 70s and rarely before age 40.2
About 80% to 90% of patients present with dysphagia. Other signs and symptoms include regurgitation of undigested foods and medications, halitosis, hoarseness, chronic cough, and aspiration.
Rare complications of Zenker diverticulum include recurrent pulmonary infection, tracheal fistula, ulceration, hemorrhage, squamous cell carcinoma (incidence 0.4% to 1.5%), vocal cord paralysis, and fistula to the prevertebral ligament with cervical osteomyelitis.1
The diagnosis is suspected based on symptoms but should be confirmed with a barium esophagram that shows an outpouching of contrast from the main contrast column.
A watch-and-wait approach should be used for patients with mild symptoms (ie, mild or intermittent dysphagia) and minor functional limitations. Patients should be counseled to eat small amounts of food at a time, to chew thoroughly, and to sip liquids between bites.
TREATMENT IS SURGERY OR ENDOSCOPY
Patients with more than mild symptoms who are candidates for intervention should be offered treatment. The goal is to open the septum between the diverticulum and the main esophageal lumen so that food can be propelled from the hypopharynx to the main esophageal lumen without obstruction. Because increased cricopharyngeal tone plays a large role in the development and propagation of a diverticulum, most experts recommend cricopharyngomyotomy in addition to any treatment strategy.
Open surgery
In centers that do not offer flexible endoscopy, and for patients with a large diverticulum, open surgery is the sole option. It is done under general anesthesia with a left cervical approach. The length of the cricopharyngeal myotomy can vary from 2 to 6 cm.3,4 This is followed by one of three options:
- Diverticulectomy
- Diverticulopexy, in which the diverticulum is repositioned and sutured against the prevertebral fascia
- Diverticular inversion, in which the diverticulum is inverted into the esophageal lumen and then oversewn.4
Success rates for open surgery vary from study to study but are usually around 90%.4 Complications are reported in 10% to 30% of cases and include mediastinitis, severe recurrent laryngeal nerve injury, and a 1% to 2% chance of death. These rates seem high, but the older age of most of these patients puts them at high risk.5
Endoscopic procedures
All endoscopic procedures involve incision of the wall separating the diverticulum from the esophageal lumen to relieve the obstruction.
Rigid endoscopy is used in centers that do not offer flexible endoscopy for patients who are not candidates for surgery. It is done under general anesthesia. With the patient’s neck completely hyperextended, a rigid endoscope is passed into the oral cavity, and diverticulotomy and cricopharyngeal myotomy are performed.
This technique has been extensively evaluated and, although effective, carries up to an 8% risk of complications, including perforation. It is not recommended for patients with a small diverticulum, a high body mass index, or difficulty with neck extension.6
Flexible endoscopy is the first-line therapy for most patients in many centers. With the flexible endoscope, the diverticulotomy and the cricopharyngeal myotomy can be performed in an outpatient endoscopy suite, and general anesthesia is not required.3
Initial studies of outcomes using these approaches have been promising,3,7–9 with substantial reduction in dysphagia, regurgitation, and chronic cough. Unfortunately, this technique is associated with a recurrence rate as high as 25%. However, repeat endoscopic therapy in patients with recurrence results in success rates similar to those for first-time treatment.
Complication rates are low. In a series of 150 patients, four adverse events occurred—three cases of fever and one of pneumonia. In addition, one patient had subcutaneous emphysema that resolved spontaneously.7
Improved device on horizon
A potentially major advance in the endoscopic treatment of Zenker diverticulum is the development of a diverticuloscope, a soft tube with a V-shaped end. This tube is inserted through the mouth with one leg of the “V” protecting the anterior esophageal wall and the other leg protecting the posterior diverticular lumen. The endoscope is passed through this tube to perform the diverticulotomy. In one report,10 diverticuloscope-guided diverticulotomy led to fewer complications, shorter procedural time, and higher success rates compared with the standard technique.10 This device is not yet available in the United States.
No head-to-head comparison of outcomes with various flexible endoscopic techniques for treating Zenker diverticulum has been published, nor are there data yet on the success of surgery if endoscopic therapy fails.
We recommend flexible endoscopy for the initial treatment of Zenker diverticulum in most patients, but its availability is limited in the United States, as many practitioners do not have adequate experience with the technique.
TAKE-HOME POINTS
- Zenker diverticulum is an outpouching of the esophageal mucosa. It is often asymptomatic. Patients with minimal symptoms and with no functional impairment can be followed conservatively.
- A head-to-head comparison of surgical and endoscopic therapy has not yet been done, and optimal patient selection criteria are lacking. Therefore, treatment recommendations are based primarily on expert opinion.
- We recommend flexible endoscopic therapy as initial treatment in patients with a small diverticulum, but this may be limited by the unavailability of a surgeon experienced in this technique.
- For a large diverticulum, we recommend an open cervical procedure involving excision of the diverticulum or a diverticulopexy for patients who are good surgical candidates.
- Ferreira LE, Simmons DT, Baron TH. Zenker’s diverticula: pathophysiology, clinical presentation, and flexible endoscopic management. Dis Esophagus 2008; 21:1–8.
- Manno M, Manta R, Caruso A, et al. Alternative endoscopic treatment of Zenker’s diverticulum: a case series (with video). Gastrointest Endosc 2014; 79:168–170.
- Yuan Y, Zhao YF, Hu Y, Chen LQ. Surgical treatment of Zenker’s diverticulum. Dig Surg 2013; 30:207–218.
- Bizzotto A, Iacopini F, Landi R, Costamagna G. Zenker’s diverticulum: exploring treatment options. Acta Otorhinolaryngol Ital 2013; 33:219–229.
- Repici A, Pagano N, Fumagalli U, et. al. Transoral treatment of Zenker diverticulum: flexible endoscopy versus endoscopic stapling. A retrospective comparison of outcomes. Dis Esophagus 2011; 24:235–239.
- Law R, Katzka DA, Baron TH. Zenker’s diverticulum. Clin Gastroenterol Hepatol 2014; 12:1773–1782.
- Huberty V, El Bacha S, Blero D, Le Moine O, Hassid S, Devière J. Endoscopic treatment for Zenker’s diverticulum: long-term results (with video). Gastrointest Endosc 2013; 77:701–707.
- Case DJ, Baron TH. Flexible endoscopic management of Zenker diverticulum: the Mayo Clinic experience. Mayo Clin Proc 2010; 85:719–722.
- Al-Kadi AS, Maghrabi AA, Thomson D, Gillman LM, Dhalla S. Endoscopic treatment of Zenker diverticulum: results of a 7-year experience. J Am Coll Surg 2010; 211:239–243.
- Costamagna G, Iacopini F, Tringali A, et al. Flexible endoscopic Zenker’s diverticulotomy: cap-assisted technique vs diverticuloscope-assisted technique. Endoscopy 2007; 39:146–152.
Conventional treatment for a large symptomatic Zenker diverticulum is to surgically either remove it (diverticulectomy) or obliterate it by repositioning and securing it after cutting into the cricopharyngeus muscle (diverticulopexy with cricopharyngeal myotomy). But high rates of complications with these procedures in elderly patients have led to the development of endoscopic procedures that are safer and have similar or higher success rates.
ESOPHAGEAL POUCH IN SPACE BETWEEN MUSCLES
Zenker diverticulum is an outpouching of the esophageal mucosa into the potential space of the Killian triangle, the area between the inferior pharyngeal constrictor and the cricopharyngeus muscle. Increased cricopharyngeal tone coupled with insufficient relaxation results in a pressure gradient that eventually causes esophageal outpouchings. The problem is induced by age-related fibrosis and atrophy, esophageal spasms related to gastroesophageal reflux disease, and idiopathic cricopharyngeal spasms.1
USUALLY PRESENTS WITH DYSPHAGIA
Zenker diverticulum has an estimated incidence of about 2 per 100,000 per year, but this is likely low because many cases are asymptomatic. It occurs mostly in men and people of Northern European descent in their 60s and 70s and rarely before age 40.2
About 80% to 90% of patients present with dysphagia. Other signs and symptoms include regurgitation of undigested foods and medications, halitosis, hoarseness, chronic cough, and aspiration.
Rare complications of Zenker diverticulum include recurrent pulmonary infection, tracheal fistula, ulceration, hemorrhage, squamous cell carcinoma (incidence 0.4% to 1.5%), vocal cord paralysis, and fistula to the prevertebral ligament with cervical osteomyelitis.1
The diagnosis is suspected based on symptoms but should be confirmed with a barium esophagram that shows an outpouching of contrast from the main contrast column.
A watch-and-wait approach should be used for patients with mild symptoms (ie, mild or intermittent dysphagia) and minor functional limitations. Patients should be counseled to eat small amounts of food at a time, to chew thoroughly, and to sip liquids between bites.
TREATMENT IS SURGERY OR ENDOSCOPY
Patients with more than mild symptoms who are candidates for intervention should be offered treatment. The goal is to open the septum between the diverticulum and the main esophageal lumen so that food can be propelled from the hypopharynx to the main esophageal lumen without obstruction. Because increased cricopharyngeal tone plays a large role in the development and propagation of a diverticulum, most experts recommend cricopharyngomyotomy in addition to any treatment strategy.
Open surgery
In centers that do not offer flexible endoscopy, and for patients with a large diverticulum, open surgery is the sole option. It is done under general anesthesia with a left cervical approach. The length of the cricopharyngeal myotomy can vary from 2 to 6 cm.3,4 This is followed by one of three options:
- Diverticulectomy
- Diverticulopexy, in which the diverticulum is repositioned and sutured against the prevertebral fascia
- Diverticular inversion, in which the diverticulum is inverted into the esophageal lumen and then oversewn.4
Success rates for open surgery vary from study to study but are usually around 90%.4 Complications are reported in 10% to 30% of cases and include mediastinitis, severe recurrent laryngeal nerve injury, and a 1% to 2% chance of death. These rates seem high, but the older age of most of these patients puts them at high risk.5
Endoscopic procedures
All endoscopic procedures involve incision of the wall separating the diverticulum from the esophageal lumen to relieve the obstruction.
Rigid endoscopy is used in centers that do not offer flexible endoscopy for patients who are not candidates for surgery. It is done under general anesthesia. With the patient’s neck completely hyperextended, a rigid endoscope is passed into the oral cavity, and diverticulotomy and cricopharyngeal myotomy are performed.
This technique has been extensively evaluated and, although effective, carries up to an 8% risk of complications, including perforation. It is not recommended for patients with a small diverticulum, a high body mass index, or difficulty with neck extension.6
Flexible endoscopy is the first-line therapy for most patients in many centers. With the flexible endoscope, the diverticulotomy and the cricopharyngeal myotomy can be performed in an outpatient endoscopy suite, and general anesthesia is not required.3
Initial studies of outcomes using these approaches have been promising,3,7–9 with substantial reduction in dysphagia, regurgitation, and chronic cough. Unfortunately, this technique is associated with a recurrence rate as high as 25%. However, repeat endoscopic therapy in patients with recurrence results in success rates similar to those for first-time treatment.
Complication rates are low. In a series of 150 patients, four adverse events occurred—three cases of fever and one of pneumonia. In addition, one patient had subcutaneous emphysema that resolved spontaneously.7
Improved device on horizon
A potentially major advance in the endoscopic treatment of Zenker diverticulum is the development of a diverticuloscope, a soft tube with a V-shaped end. This tube is inserted through the mouth with one leg of the “V” protecting the anterior esophageal wall and the other leg protecting the posterior diverticular lumen. The endoscope is passed through this tube to perform the diverticulotomy. In one report,10 diverticuloscope-guided diverticulotomy led to fewer complications, shorter procedural time, and higher success rates compared with the standard technique.10 This device is not yet available in the United States.
No head-to-head comparison of outcomes with various flexible endoscopic techniques for treating Zenker diverticulum has been published, nor are there data yet on the success of surgery if endoscopic therapy fails.
We recommend flexible endoscopy for the initial treatment of Zenker diverticulum in most patients, but its availability is limited in the United States, as many practitioners do not have adequate experience with the technique.
TAKE-HOME POINTS
- Zenker diverticulum is an outpouching of the esophageal mucosa. It is often asymptomatic. Patients with minimal symptoms and with no functional impairment can be followed conservatively.
- A head-to-head comparison of surgical and endoscopic therapy has not yet been done, and optimal patient selection criteria are lacking. Therefore, treatment recommendations are based primarily on expert opinion.
- We recommend flexible endoscopic therapy as initial treatment in patients with a small diverticulum, but this may be limited by the unavailability of a surgeon experienced in this technique.
- For a large diverticulum, we recommend an open cervical procedure involving excision of the diverticulum or a diverticulopexy for patients who are good surgical candidates.
Conventional treatment for a large symptomatic Zenker diverticulum is to surgically either remove it (diverticulectomy) or obliterate it by repositioning and securing it after cutting into the cricopharyngeus muscle (diverticulopexy with cricopharyngeal myotomy). But high rates of complications with these procedures in elderly patients have led to the development of endoscopic procedures that are safer and have similar or higher success rates.
ESOPHAGEAL POUCH IN SPACE BETWEEN MUSCLES
Zenker diverticulum is an outpouching of the esophageal mucosa into the potential space of the Killian triangle, the area between the inferior pharyngeal constrictor and the cricopharyngeus muscle. Increased cricopharyngeal tone coupled with insufficient relaxation results in a pressure gradient that eventually causes esophageal outpouchings. The problem is induced by age-related fibrosis and atrophy, esophageal spasms related to gastroesophageal reflux disease, and idiopathic cricopharyngeal spasms.1
USUALLY PRESENTS WITH DYSPHAGIA
Zenker diverticulum has an estimated incidence of about 2 per 100,000 per year, but this is likely low because many cases are asymptomatic. It occurs mostly in men and people of Northern European descent in their 60s and 70s and rarely before age 40.2
About 80% to 90% of patients present with dysphagia. Other signs and symptoms include regurgitation of undigested foods and medications, halitosis, hoarseness, chronic cough, and aspiration.
Rare complications of Zenker diverticulum include recurrent pulmonary infection, tracheal fistula, ulceration, hemorrhage, squamous cell carcinoma (incidence 0.4% to 1.5%), vocal cord paralysis, and fistula to the prevertebral ligament with cervical osteomyelitis.1
The diagnosis is suspected based on symptoms but should be confirmed with a barium esophagram that shows an outpouching of contrast from the main contrast column.
A watch-and-wait approach should be used for patients with mild symptoms (ie, mild or intermittent dysphagia) and minor functional limitations. Patients should be counseled to eat small amounts of food at a time, to chew thoroughly, and to sip liquids between bites.
TREATMENT IS SURGERY OR ENDOSCOPY
Patients with more than mild symptoms who are candidates for intervention should be offered treatment. The goal is to open the septum between the diverticulum and the main esophageal lumen so that food can be propelled from the hypopharynx to the main esophageal lumen without obstruction. Because increased cricopharyngeal tone plays a large role in the development and propagation of a diverticulum, most experts recommend cricopharyngomyotomy in addition to any treatment strategy.
Open surgery
In centers that do not offer flexible endoscopy, and for patients with a large diverticulum, open surgery is the sole option. It is done under general anesthesia with a left cervical approach. The length of the cricopharyngeal myotomy can vary from 2 to 6 cm.3,4 This is followed by one of three options:
- Diverticulectomy
- Diverticulopexy, in which the diverticulum is repositioned and sutured against the prevertebral fascia
- Diverticular inversion, in which the diverticulum is inverted into the esophageal lumen and then oversewn.4
Success rates for open surgery vary from study to study but are usually around 90%.4 Complications are reported in 10% to 30% of cases and include mediastinitis, severe recurrent laryngeal nerve injury, and a 1% to 2% chance of death. These rates seem high, but the older age of most of these patients puts them at high risk.5
Endoscopic procedures
All endoscopic procedures involve incision of the wall separating the diverticulum from the esophageal lumen to relieve the obstruction.
Rigid endoscopy is used in centers that do not offer flexible endoscopy for patients who are not candidates for surgery. It is done under general anesthesia. With the patient’s neck completely hyperextended, a rigid endoscope is passed into the oral cavity, and diverticulotomy and cricopharyngeal myotomy are performed.
This technique has been extensively evaluated and, although effective, carries up to an 8% risk of complications, including perforation. It is not recommended for patients with a small diverticulum, a high body mass index, or difficulty with neck extension.6
Flexible endoscopy is the first-line therapy for most patients in many centers. With the flexible endoscope, the diverticulotomy and the cricopharyngeal myotomy can be performed in an outpatient endoscopy suite, and general anesthesia is not required.3
Initial studies of outcomes using these approaches have been promising,3,7–9 with substantial reduction in dysphagia, regurgitation, and chronic cough. Unfortunately, this technique is associated with a recurrence rate as high as 25%. However, repeat endoscopic therapy in patients with recurrence results in success rates similar to those for first-time treatment.
Complication rates are low. In a series of 150 patients, four adverse events occurred—three cases of fever and one of pneumonia. In addition, one patient had subcutaneous emphysema that resolved spontaneously.7
Improved device on horizon
A potentially major advance in the endoscopic treatment of Zenker diverticulum is the development of a diverticuloscope, a soft tube with a V-shaped end. This tube is inserted through the mouth with one leg of the “V” protecting the anterior esophageal wall and the other leg protecting the posterior diverticular lumen. The endoscope is passed through this tube to perform the diverticulotomy. In one report,10 diverticuloscope-guided diverticulotomy led to fewer complications, shorter procedural time, and higher success rates compared with the standard technique.10 This device is not yet available in the United States.
No head-to-head comparison of outcomes with various flexible endoscopic techniques for treating Zenker diverticulum has been published, nor are there data yet on the success of surgery if endoscopic therapy fails.
We recommend flexible endoscopy for the initial treatment of Zenker diverticulum in most patients, but its availability is limited in the United States, as many practitioners do not have adequate experience with the technique.
TAKE-HOME POINTS
- Zenker diverticulum is an outpouching of the esophageal mucosa. It is often asymptomatic. Patients with minimal symptoms and with no functional impairment can be followed conservatively.
- A head-to-head comparison of surgical and endoscopic therapy has not yet been done, and optimal patient selection criteria are lacking. Therefore, treatment recommendations are based primarily on expert opinion.
- We recommend flexible endoscopic therapy as initial treatment in patients with a small diverticulum, but this may be limited by the unavailability of a surgeon experienced in this technique.
- For a large diverticulum, we recommend an open cervical procedure involving excision of the diverticulum or a diverticulopexy for patients who are good surgical candidates.
- Ferreira LE, Simmons DT, Baron TH. Zenker’s diverticula: pathophysiology, clinical presentation, and flexible endoscopic management. Dis Esophagus 2008; 21:1–8.
- Manno M, Manta R, Caruso A, et al. Alternative endoscopic treatment of Zenker’s diverticulum: a case series (with video). Gastrointest Endosc 2014; 79:168–170.
- Yuan Y, Zhao YF, Hu Y, Chen LQ. Surgical treatment of Zenker’s diverticulum. Dig Surg 2013; 30:207–218.
- Bizzotto A, Iacopini F, Landi R, Costamagna G. Zenker’s diverticulum: exploring treatment options. Acta Otorhinolaryngol Ital 2013; 33:219–229.
- Repici A, Pagano N, Fumagalli U, et. al. Transoral treatment of Zenker diverticulum: flexible endoscopy versus endoscopic stapling. A retrospective comparison of outcomes. Dis Esophagus 2011; 24:235–239.
- Law R, Katzka DA, Baron TH. Zenker’s diverticulum. Clin Gastroenterol Hepatol 2014; 12:1773–1782.
- Huberty V, El Bacha S, Blero D, Le Moine O, Hassid S, Devière J. Endoscopic treatment for Zenker’s diverticulum: long-term results (with video). Gastrointest Endosc 2013; 77:701–707.
- Case DJ, Baron TH. Flexible endoscopic management of Zenker diverticulum: the Mayo Clinic experience. Mayo Clin Proc 2010; 85:719–722.
- Al-Kadi AS, Maghrabi AA, Thomson D, Gillman LM, Dhalla S. Endoscopic treatment of Zenker diverticulum: results of a 7-year experience. J Am Coll Surg 2010; 211:239–243.
- Costamagna G, Iacopini F, Tringali A, et al. Flexible endoscopic Zenker’s diverticulotomy: cap-assisted technique vs diverticuloscope-assisted technique. Endoscopy 2007; 39:146–152.
- Ferreira LE, Simmons DT, Baron TH. Zenker’s diverticula: pathophysiology, clinical presentation, and flexible endoscopic management. Dis Esophagus 2008; 21:1–8.
- Manno M, Manta R, Caruso A, et al. Alternative endoscopic treatment of Zenker’s diverticulum: a case series (with video). Gastrointest Endosc 2014; 79:168–170.
- Yuan Y, Zhao YF, Hu Y, Chen LQ. Surgical treatment of Zenker’s diverticulum. Dig Surg 2013; 30:207–218.
- Bizzotto A, Iacopini F, Landi R, Costamagna G. Zenker’s diverticulum: exploring treatment options. Acta Otorhinolaryngol Ital 2013; 33:219–229.
- Repici A, Pagano N, Fumagalli U, et. al. Transoral treatment of Zenker diverticulum: flexible endoscopy versus endoscopic stapling. A retrospective comparison of outcomes. Dis Esophagus 2011; 24:235–239.
- Law R, Katzka DA, Baron TH. Zenker’s diverticulum. Clin Gastroenterol Hepatol 2014; 12:1773–1782.
- Huberty V, El Bacha S, Blero D, Le Moine O, Hassid S, Devière J. Endoscopic treatment for Zenker’s diverticulum: long-term results (with video). Gastrointest Endosc 2013; 77:701–707.
- Case DJ, Baron TH. Flexible endoscopic management of Zenker diverticulum: the Mayo Clinic experience. Mayo Clin Proc 2010; 85:719–722.
- Al-Kadi AS, Maghrabi AA, Thomson D, Gillman LM, Dhalla S. Endoscopic treatment of Zenker diverticulum: results of a 7-year experience. J Am Coll Surg 2010; 211:239–243.
- Costamagna G, Iacopini F, Tringali A, et al. Flexible endoscopic Zenker’s diverticulotomy: cap-assisted technique vs diverticuloscope-assisted technique. Endoscopy 2007; 39:146–152.