User login
Ablation of atrial fibrillation: What can we tell our patients?
More patients with atrial fibrillation are asking their physicians about catheter-based radiofrequency ablation as a treatment option.
Indeed, in the mere 10 years or so since this procedure was introduced, it has shown promising clinical results. Still, it is not yet available at many medical centers, and it is not yet considered the first-line treatment for atrial fibrillation.1 Moreover, some patients may have unrealistic expectations about it, such as being able to stop taking anticoagulant drugs afterward. It is therefore important for health care professionals not only to recognize which patients may benefit from catheter-based treatment, but also to educate them about it so they have reasonable expectations.
See related editorial and patient information at http://my.clevelandclinic.org/heart/services/tests/procedures/ablation.aspx
In this article, we briefly review the mechanisms of catheter ablation of atrial fibrillation and discuss its current indications, with an emphasis on how to determine which patients with atrial fibrillation are candidates for this new procedure.
NUMBERS ARE RISING, AND DRUG THERAPY HAS LIMITATIONS
Atrial fibrillation is a disease of the elderly: about 70% of patients are between the ages of 65 and 85.2 As the elderly segment of the US population increases, the number of people with atrial fibrillation is expected to more than double by the year 2050.3
This growing prevalence and the increasing socioeconomic burden are two reasons people are looking to new treatments such as catheter and surgical ablation.
Several randomized clinical trials,4–7 most importantly the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial,7 found that attempting to restore and maintain sinus rhythm with antiarrhythmic drugs imparts no significant benefit in terms of survival compared with a strategy of controlling the heart rate only. However, recent studies, including an analysis by the AFFIRM investigators,8 suggest that if sinus rhythm could be achieved without the adverse effects of antiarrythmic drugs, then rhythm control may have a survival benefit over rate control. These studies, combined with improving techniques and tools for catheter ablation of atrial fibrillation, have made ablative treatment an attractive option and an emerging trend.
HOW ATRIAL FIBRILLATION STARTS AND HOW IT IS MAINTAINED
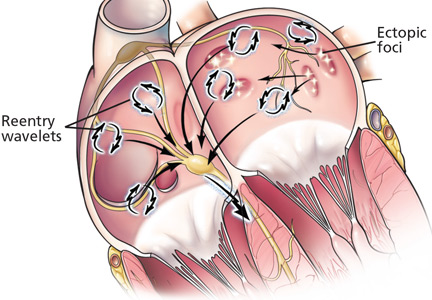
Several studies9–11 showed that left atrial myocardial cells extend into the pulmonary veins. These “myocardial sleeves,” which vary in extent between individuals, have short refractory periods and can cause conduction delays, which may create the conditions needed for arrhythmias.12,13
In landmark studies in the 1990s, Haïssaguerre et al14 and Jais et al15 showed that most focal triggers are in the myocardial sleeves at the junction of the pulmonary vein and the left atrium. These investigators went on to show that catheter-based ablation of these ectopic foci could eliminate atrial fibrillation in some patients.
Ectopic foci in the pulmonary veins fire rapidly and chaotically, generating impulses that enter the left atrium and begin to generate wavelets of reentry. These wavelets may be perpetuated if the conduction velocity is slow, the refractory period is short, and atrial mass is high.1,16,17
Some experts thought that by surgically interrupting the path of these wavelets and reducing the atrial mass, one could terminate atrial fibrillation. This model is the basis of the surgical maze procedure developed by Cox et al in the 1990s,18 which planted the seed for catheter ablation of atrial fibrillation.
DEFINITIONS OF ATRIAL FIBRILLATION
A 2007 consensus document1 prepared jointly by several heart societies emphasizes the need to classify the types of atrial fibrillation consistently, as recommendations for different treatments are based primarily on the type of atrial fibrillation. Although some patients may have atrial fibrillation that falls into more than one of these categories, it should be categorized by its most frequent pattern. These definitions apply only to episodes that last at least 30 seconds and have no identifiable reversible cause, such as acute pulmonary disease or hyperthyroidism.
Paroxysmal atrial fibrillation is defined as at least two episodes that terminate spontaneously within 7 days.
Persistent atrial fibrillation is defined as lasting more than 7 days, or lasting less than 7 days but necessitating pharmacologic or electrical cardioversion.
Permanent atrial fibrillation is defined as lasting more than 1 year.
THE TECHNIQUE
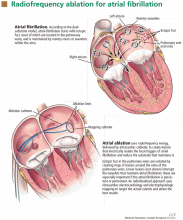
Catheter ablation is usually done as an outpatient procedure. As the procedure can take 3 to 5 hours, most patients receive conscious sedation or general anesthesia. A catheter is inserted into a femoral vein and advanced into the right atrium. Then, the atrial septum is punctured to gain access to the left atrium, and a radiofrequency ablation catheter and a mapping catheter are inserted (Figure 1).
What to ablate?
Various approaches are being used in catheter-based ablation of atrial fibrillation.1,12,19–29
Since most of the triggers of atrial fibrillation are located within the pulmonary veins, one can use an empiric anatomic approach, creating a ring of ablation lesions around the outside of the ostium of each of the four pulmonary veins (but not within the vein itself), or a single ring around the ostia of the two left pulmonary veins and another around the two right pulmonary veins. The aim is to electrically isolate these veins.
Refinements to this procedure involve making additional lines of lesions in the atrium, similar to those in the Cox maze procedure; a line across the roof of the left atrium connecting the ring of lesions around the left and right superior pulmonary veins; a line across the mitral isthmus (between the mitral valve and the left inferior pulmonary vein); and a line connecting either the roof line or the left or right circumferential lesion to the mitral annulus anteriorly. The aim of these additional lesions is to interrupt the re-entrant circuits that keep atrial fibrillation going, and they may make the procedure more effective in cases of persistent or permanent atrial fibrillation than it would be without these lesions.
An electrophysiologic approach involves using intracardiac electrocardiography to locate specific drivers of fibrillation and areas of complex fractionated atrial electrograms, which can be ablated. This is a more tailored approach, and it may be more effective. In addition, one can ablate, then attempt to induce fibrillation electrically or with drugs, and then, if fibrillation ensues, do more ablation.
Fluoroscopy vs intracardiac echocardiography
Fluoroscopy, intracardiac echocardiography, three-dimensional mapping, and pulmonary venography have all been used to guide left atrial pulmonary vein ablation.
Until recently, electrophysiologists used fluoroscopy, but now most use intracardiac echocardiography and other imaging techniques. Intracardiac echocardiography provides information that fluoroscopy cannot. It can show, from moment to moment, the anatomic structures, the position of the catheter, and if there are thrombi in the left atrium. It can also help optimize the use of radiofrequency energy by monitoring for microbubbles, which represent tissue overheating.1
Three-dimensional mapping and navigation techniques help define the anatomy and help guide the catheter, especially with previously acquired computed tomography (CT) or magnetic resonance imaging (MRI).30–32 Likewise, pulmonary venography can also show the shape and size of the pulmonary ostia. This modality can guide catheter manipulation and assessment for pulmonary venous stenosis resulting from prior ablation.1
INDICATIONS FOR CATHETER ABLATION
An absolute contraindication to catheter ablation is left atrial thrombus. Because of the risk of dislodging an existing thrombus during the procedure and causing a stroke, the committee recommends that patients with persistent atrial fibrillation who are in atrial fibrillation at the time of the procedure undergo transesophageal echocardiography to screen for thrombus.
An individualized decision
The decision to proceed with catheter ablation must be individualized on the basis of the risk of complications, the likely benefits, and the likelihood of success.1
Factors that increase the risk of iatrogenic complications such as myocardial perforation and thromboembolism include older age and comorbid conditions.
Factors that increase the chance of significant benefit include more severe symptoms and heart failure. Hsu et al33 found that the ejection fraction increased by 21 plus or minus 13 percentage points in heart failure patients who underwent the procedure.
Success rates for catheter-based ablation are lower in patients with persistent atrial fibrillation than in those with paroxysmal atrial fibrillation. Oral and colleagues34 reported that the recurrence rate in patients with persistent atrial fibrillation was 75%, compared with 29% in patients with paroxysmal atrial fibrillation. In addition, the chances of a successful outcome are lower in those with marked dilation of the left atrium.
Lifelong anticoagulation is still needed
When weighing the pros and cons of catheter-based procedures, health care providers need to also emphasize to patients that the procedure does not allow them to forgo anticoagulation. Even after ablation, patients with atrial fibrillation still face a formidable risk of thromboembolic events, and most electrophysiologists suggest lifelong anticoagulation, especially in patients with other risk factors for stroke. We discuss the guidelines for anticoagulation later in this review.
EFFECTIVENESS
Crandall et al,35 in an excellent review of the literature, estimated that ablation treatment is successful in approximately 60% to 70% of patients, that 10% to 40% of patients require a second ablation procedure, and that 10% to 15% still need antiarrhythmic drugs.
The specifics of each procedure are beyond the scope of this review, but outcomes have been shown to be better with individualized ablation therapy than with an anatomic approach. Oral et al28 showed that this tailored approach could provide success rates up to 77%; the repeat-procedure rate was 18%, and the risk of complications was low. The tailored approach allows operators to target triggers in locations other than in the pulmonary veins, including those in the thoracic veins and superior vena cava, during mapping. In addition, this approach is often needed in persistent atrial fibrillation, where left atrial substrate is likely to play a larger role than in paroxysmal atrial fibrillation and can be specifically targeted using this method.
When using pulmonary vein isolation by itself, studies have shown better outcomes in treating paroxysmal atrial fibrillation than persistent atrial fibrillation.34 These outcomes have been shown to improve with an individualized approach to ablation therapy.
Haïssaguerre et al36 used various tailored ablation techniques to terminate persistent atrial fibrillation and were able to terminate the rhythm in 87% of their patients. Eleven months after the procedure, 95% of the patients in whom it succeeded remained free of arrhythmia.
Randomized controlled trials now confirm that left atrial ablation is superior to antiarrhythmic drug therapy in maintaining sinus rhythm over time.19,37,38
COMPLICATIONS
It is important that patients understand the risks associated with the procedure. Advancing technology in imaging and catheters and our growing understanding of atrial fibrillation are not only able to optimize ablation outcomes, but also to minimize complications.
Complications of catheter-based treatment of atrial fibrillation described by the expert consensus committee1 include:
- Cardiac tamponade
- Pulmonary vein stenosis
- Phrenic nerve injury
- Esophageal injury, atrioesophageal fistula
- Periesophageal vagal injury
- Thromboembolic events
- Vascular complications
- Acute coronary artery occlusion (rare)
- Air emboli from catheters and sheath
- Catheter entrapment in the mitral valve
- Tachyarrhythmias
- Radiation exposure
- Mitral valve trauma.
Cardiac tamponade
Cardiac tamponade due to accidental puncture or excessive heat accumulation, steam expansion, and perforation of the atrial wall occurs in about 6% of patients,20 but this number varies. Limiting the power delivered to tissue to less than 25 or 35 W may reduce the incidence of this complication.1 The expertise of the physician and the type of imaging used (eg, transesophageal or intracardiac echocardiography) are also factors.
Pulmonary vein stenosis
Pulmonary vein stenosis was seen after the first pulmonary vein isolation techniques were tried, when ablation within the pulmonary vein caused high rates of this complication.1 Improved knowledge of anatomy and better visualization using intracardiac echocardiography have led to a significantly lower rate of pulmonary vein stenosis.20 The current rate is 0.5% to 2%.12,20
Nevertheless, it is important for referring physicians to recognize the symptoms of pulmonary vein stenosis, as they will likely be the first providers to see a patient with these symptoms, which can be mistaken for pneumonia or congestive heart failure. The symptoms include cough, dyspnea, pneumonia, and hemoptysis that may occur early or late (weeks) after ablation.
CT, MRI, and ventilation-perfusion scanning can be used to diagnose pulmonary vein stenosis. Its treatment includes stenting the narrowed vein.
Esophageal injury
Esophageal injury, specifically formation of an atrioesophageal fistula, is a life-threatening complication of this treatment.1 The esophagus passes very close to the left atrial posterior wall and is therefore at risk of thermal injury during ablation.
Health care professionals should be alert to the symptoms of this complication, which include dysphagia, odynophagia, hematemesis, signs of intermittent cardiac or neurologic ischemia, persistent fever, bacteremia, fungemia, leukocytosis, and melena.12 These symptoms may arise weeks after the procedure.
Any patient who has recently undergone catheter ablation and who presents with some of these symptoms needs a prompt workup with MRI or CT. Endoscopy is contraindicated because it can introduce air into the esophagus, which may result in air embolism to the brain. Atrioesophageal fistula is generally fatal, but emergency surgery may be an option.
Thromboembolism
Thromboembolic events are another worrisome complication. The reported incidence rate ranges between 0% and 7%.1
Appropriate anticoagulation protocols can minimize the risk. Patients should take warfarin (Coumadin) for at least 3 weeks before undergoing ablation if they have paroxysmal atrial fibrillation and a CHADS2 score of 1 or higher (1 point each for having congestive heart failure, hypertension, age > 75 years, or diabetes; 2 points for having a prior stroke or transient ischemic attack), or if they have persistent atrial fibrillation regardless of the CHADS2 score. The target international normalized ratio (INR) is in the therapeutic range, ie, 2 to 3. Patients who have paroxysmal atrial fibrillation and a CHADS2 score of 0 may be treated with aspirin or warfarin before the procedure. Patients who have been taking warfarin should be “bridged” with subcutaneous low-molecular-weight heparin or intravenous unfractionated heparin before ablation, eg, by stopping the warfarin several days before the procedure and substituting enoxaparin (Lovenox) 0.5 to 1 mg/kg twice daily until the evening before the procedure.
To screen for thrombi in the left atrium, transesophageal echocardiography should be performed before the procedure in patients who have not been receiving warfarin, or whose INRs have not consistently been in the therapeutic range of 2 to 3, or who have persistent atrial fibrillation and are in atrial fibrillation at the time of the procedure.
During the procedure, anticoagulation is maintained with a heparin infusion. After the procedure, warfarin is restarted along with a low-molecular-weight heparin or unfractionated heparin. The heparin is stopped when the INR is in the therapeutic range, but warfarin should be continued for at least 3 months. Selected patients with a CHADS2 score of 1 may be switched to aspirin therapy after several months, and those with a score of 0 may be switched to aspirin or no therapy.12
We still lack data from large-scale trials about long-term thromboembolic complications of ablation therapy. Most electrophysiologists prefer to continue anticoagulation indefinitely and would consider terminating it only with great caution.
Arrhythmias
After ablation, new atrial arrhythmias such as atrial flutter are common, with a wide range of reported incidence rates.39 Most cases respond poorly to antiarrythmic drugs, but temporizing measures are recommended, since about half will resolve spontaneously. For this reason, experts generally recommend waiting 2 to 3 months after an ablation procedure before performing a repeat ablative procedure.1,12 Close monitoring is recommended during the months following catheter ablation.
COST AND QUALITY OF LIFE
The cost of catheter ablation needs to be taken into account when considering the procedure for an individual patient.
Catheter ablation is expensive, but so is ongoing medical treatment. In the United States, catheter ablation costs between $17,000 and $21,000 initially, with an ongoing cost of $1,500 to $2,000 per year.3 In comparison, medical therapy costs $4,000 to $5,000 per year. Therefore, catheter ablation would take 4 to 8 years to pay for itself.2
Quality of life also remains a key factor in determining whether to pursue this treatment option. Initial studies showed a trend toward better quality of life with catheter ablation than with medical therapy. In a nonrandomized study published in 2003, Pappone et al40 assessed quality of life in 109 patients who underwent ablation and in 102 medically treated patients, using the 36-item Short-Form General Health Survey. At baseline, both groups similarly rated their quality of life significantly lower than people of the same age and sex in the general population (P < .001). By 6 months, quality-of-life scores in the ablation group had risen to the same level as in the general population, while they stayed the same in the medically treated group. However, data are still limited, and, like the cost of the procedure, estimated quality of life needs to be weighed for the individual patient.
FUTURE DEVELOPMENTS
There are exciting developments in imaging and catheter systems for ablation of atrial fibrillation. It is hoped that these new technologies will improve success rates and reduce complication rates.
In imaging, digital fusion of CT and MRI with electroanatomic mapping shows the anatomy of the junction of the left atrium and pulmonary vein in real time. (Currently, CT and MRI have to be done prior to ablative techniques.)
New ablation systems are being developed that use extreme cold, lasers, and ultrasound. An advantage of these new ablation systems is that they have balloon-tipped catheters, which are placed near the pulmonary vein ostium to deliver full circumferential ablation.41,42
- Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace 2007; 9:335–379.
- Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: full text: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace 2006; 8:651–745.
- Estes NA. Catheter ablation of atrial fibrillation: is the burn worth the buck? J Cardiovasc Electrophysiol 2007; 18:914–916.
- Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomized trial. Lancet 2000; 356:1789–1794.
- Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002; 347:1834–1840.
- Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003; 41:1690–1696.
- Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347:1825–1833.
- Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004; 109:1509–1513.
- Chugh A, Morady F. Atrial fibrillation: catheter ablation. J Interv Card Electrophysiol 2006; 16:15–26.
- Ho SY, Cabrera JA, Tran VH, Farré J, Anderson RH, Sànchez-Quintana D. Architecture of the pulmonary veins: relevance to radiofrequency ablation. Heart 2001; 86:265–270.
- Saito T, Waki K, Becker A. Left atrial myocardial extension onto pulmonary veins in humans: anatomic observations relevant for atrial arrhythmias. J Cardiovasc Electrophysiol 2000; 11:888–894.
- Natale A, Raviele A, Arentz T, et al. Venice Chart international consensus document on atrial fibrillation ablation. J Cardiovasc Electrophysiol 2007; 18:560–580.
- Hocini M, Ho SY, Kawara T, et al. Electrical conduction in canine pulmonary veins: electrophysiological and anatomic correlation. Circulation 2002; 105:2442–2448.
- Haïssaguerre M, Marcus FI, Fischer B, Clementy J. Radiofrequency catheter ablation in unusual mechanisms of atrial fibrillation: report of three cases. J Cardiovasc Electrophysiol 1994; 5:743–751.
- Jais P, Haïssaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997; 95:572–576.
- Moe GK, Rheinboldt WD, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964; 67:200–220.
- Allessie MA, Lammers WJEP, Bonke FIM, Hollen SJ. Experimental evaluation of Moe’s multiple wavelet hypothesis of atrial fibrillation. In:Zipes DP, Jalife J, editors. Cardiac Electrophysiology and Arrhythmias. New York: Grune & Stratton; 1985:265–275.
- Cox JL, Canavan TE, Schuessler RB, et al. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991; 101:406–426.
- Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol 2006; 48:2340–2347.
- Robbins IM, Colvin EV, Doyle TP, et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation. Circulation 1998; 98;1769–1775.
- Pappone C, Santinelli V, Manguso F, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 2004; 109:327–334.
- Pappone C, Manguso F, Vicedomini G, et al. Prevention of iatrogenic atrial tachycardia after ablation of atrial fibrillation: a prospective randomized study comparing circumferential pulmonary vein ablation with a modified approach. Circulation 2004; 110:3036–3042.
- Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm 2005; 2:624–631.
- Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol 1997; 273:H805–H816.
- Sanders P, Berenfeld O, Hocini M, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation 2005; 112:789–797.
- Lin YJ, Tai CT, Kao T, et al. Frequency analysis in different types of paroxysmal atrial fibrillation. J Am Coll Cardiol 2006; 47:1401–1407.
- Jais P, Hocini M, Hsu LF, et al. Technique and results of linear ablation at the mitral isthmus. Circulation 2004; 110:2996–3002.
- Oral H, Chugh A, Good E, et al. A tailored approach to catheter ablation of paroxysmal atrial fibrillation. Circulation 2006; 113:1824–1831.
- Macle L, Jais P, Weerasooriya R, et al. Irrigated-tip catheter ablation of pulmonary veins for treatment of atrial fibrillation. J Cardiovasc Electrophysiol 2002; 13:1067–1073.
- de Groot NM, Bootsma M, van der Velde ET, Schalij MJ. Three-dimensional catheter positioning during radiofrequency ablation in patients: first application of a real time position management system. J Cardiovasc Electrophysiol 2000; 11:1183–1192.
- Macle L, Jaïs P, Scavée C, et al. Pulmonary vein disconnection using the LocaLisa three-dimensional nonfluoroscopic catheter imaging system. J Cardiovasc Electrophysiol 2003; 14:693–697.
- Schreieck J, Ndrepepa G, Zrenner B, et al. Radiofrequency ablation of cardiac arrhythmias using a three-dimensional real time position management and mapping system. Pacing Clin Electrophysiol 2002; 25:1699–1707.
- Hsu LF, Jais P, Sanders P, et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med 2004; 351:2373–2383.
- Oral H, Knight BP, Tada H, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 2002; 105:1077–1081.
- Crandall MA, Bradley DJ, Packer DL, Asirvatham SJ. Contemporary management of atrial fibrillation: update on anticoagulation and invasive management strategies. Mayo Clin Proc 2009; 84:643–662.
- Haïssaguerre M, Hocini M, Sanders P, et al. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol 2005; 16:1138–1147.
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005; 293:2634–2640.
- Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008; 118:2498–2505.
- Chugh A, Oral H, Lemola K, et al. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm 2005; 2:464–471.
- Pappone C, Rosanio S, Augello G, et al. Mortality, morbidity, and qualityof lilfe after circumferential pulmonary vein ablation for atrial fibrillation. Outcomes from a controlled nonrandomized long-term study. J Am Coll Cardiol 2003; 42:185–197.
- Garan A, Al-Ahmad A, Mihalik T, et al. Cryoablation of the pulmonary veins using a novel balloon catheter. J Interv Card Electrophysiol 2006; 15:79–81.
- Meininger GR, Calkins H, Lickfett L, et al. Initial experience with a novel focused ultrasound ablation system for ring ablation outside the pulmonary vein. J Interv Card Electrophysiol 2003; 8:141–148.
More patients with atrial fibrillation are asking their physicians about catheter-based radiofrequency ablation as a treatment option.
Indeed, in the mere 10 years or so since this procedure was introduced, it has shown promising clinical results. Still, it is not yet available at many medical centers, and it is not yet considered the first-line treatment for atrial fibrillation.1 Moreover, some patients may have unrealistic expectations about it, such as being able to stop taking anticoagulant drugs afterward. It is therefore important for health care professionals not only to recognize which patients may benefit from catheter-based treatment, but also to educate them about it so they have reasonable expectations.
See related editorial and patient information at http://my.clevelandclinic.org/heart/services/tests/procedures/ablation.aspx
In this article, we briefly review the mechanisms of catheter ablation of atrial fibrillation and discuss its current indications, with an emphasis on how to determine which patients with atrial fibrillation are candidates for this new procedure.
NUMBERS ARE RISING, AND DRUG THERAPY HAS LIMITATIONS
Atrial fibrillation is a disease of the elderly: about 70% of patients are between the ages of 65 and 85.2 As the elderly segment of the US population increases, the number of people with atrial fibrillation is expected to more than double by the year 2050.3
This growing prevalence and the increasing socioeconomic burden are two reasons people are looking to new treatments such as catheter and surgical ablation.
Several randomized clinical trials,4–7 most importantly the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial,7 found that attempting to restore and maintain sinus rhythm with antiarrhythmic drugs imparts no significant benefit in terms of survival compared with a strategy of controlling the heart rate only. However, recent studies, including an analysis by the AFFIRM investigators,8 suggest that if sinus rhythm could be achieved without the adverse effects of antiarrythmic drugs, then rhythm control may have a survival benefit over rate control. These studies, combined with improving techniques and tools for catheter ablation of atrial fibrillation, have made ablative treatment an attractive option and an emerging trend.
HOW ATRIAL FIBRILLATION STARTS AND HOW IT IS MAINTAINED
Several studies9–11 showed that left atrial myocardial cells extend into the pulmonary veins. These “myocardial sleeves,” which vary in extent between individuals, have short refractory periods and can cause conduction delays, which may create the conditions needed for arrhythmias.12,13
In landmark studies in the 1990s, Haïssaguerre et al14 and Jais et al15 showed that most focal triggers are in the myocardial sleeves at the junction of the pulmonary vein and the left atrium. These investigators went on to show that catheter-based ablation of these ectopic foci could eliminate atrial fibrillation in some patients.
Ectopic foci in the pulmonary veins fire rapidly and chaotically, generating impulses that enter the left atrium and begin to generate wavelets of reentry. These wavelets may be perpetuated if the conduction velocity is slow, the refractory period is short, and atrial mass is high.1,16,17
Some experts thought that by surgically interrupting the path of these wavelets and reducing the atrial mass, one could terminate atrial fibrillation. This model is the basis of the surgical maze procedure developed by Cox et al in the 1990s,18 which planted the seed for catheter ablation of atrial fibrillation.
DEFINITIONS OF ATRIAL FIBRILLATION
A 2007 consensus document1 prepared jointly by several heart societies emphasizes the need to classify the types of atrial fibrillation consistently, as recommendations for different treatments are based primarily on the type of atrial fibrillation. Although some patients may have atrial fibrillation that falls into more than one of these categories, it should be categorized by its most frequent pattern. These definitions apply only to episodes that last at least 30 seconds and have no identifiable reversible cause, such as acute pulmonary disease or hyperthyroidism.
Paroxysmal atrial fibrillation is defined as at least two episodes that terminate spontaneously within 7 days.
Persistent atrial fibrillation is defined as lasting more than 7 days, or lasting less than 7 days but necessitating pharmacologic or electrical cardioversion.
Permanent atrial fibrillation is defined as lasting more than 1 year.
THE TECHNIQUE
Catheter ablation is usually done as an outpatient procedure. As the procedure can take 3 to 5 hours, most patients receive conscious sedation or general anesthesia. A catheter is inserted into a femoral vein and advanced into the right atrium. Then, the atrial septum is punctured to gain access to the left atrium, and a radiofrequency ablation catheter and a mapping catheter are inserted (Figure 1).
What to ablate?
Various approaches are being used in catheter-based ablation of atrial fibrillation.1,12,19–29
Since most of the triggers of atrial fibrillation are located within the pulmonary veins, one can use an empiric anatomic approach, creating a ring of ablation lesions around the outside of the ostium of each of the four pulmonary veins (but not within the vein itself), or a single ring around the ostia of the two left pulmonary veins and another around the two right pulmonary veins. The aim is to electrically isolate these veins.
Refinements to this procedure involve making additional lines of lesions in the atrium, similar to those in the Cox maze procedure; a line across the roof of the left atrium connecting the ring of lesions around the left and right superior pulmonary veins; a line across the mitral isthmus (between the mitral valve and the left inferior pulmonary vein); and a line connecting either the roof line or the left or right circumferential lesion to the mitral annulus anteriorly. The aim of these additional lesions is to interrupt the re-entrant circuits that keep atrial fibrillation going, and they may make the procedure more effective in cases of persistent or permanent atrial fibrillation than it would be without these lesions.
An electrophysiologic approach involves using intracardiac electrocardiography to locate specific drivers of fibrillation and areas of complex fractionated atrial electrograms, which can be ablated. This is a more tailored approach, and it may be more effective. In addition, one can ablate, then attempt to induce fibrillation electrically or with drugs, and then, if fibrillation ensues, do more ablation.
Fluoroscopy vs intracardiac echocardiography
Fluoroscopy, intracardiac echocardiography, three-dimensional mapping, and pulmonary venography have all been used to guide left atrial pulmonary vein ablation.
Until recently, electrophysiologists used fluoroscopy, but now most use intracardiac echocardiography and other imaging techniques. Intracardiac echocardiography provides information that fluoroscopy cannot. It can show, from moment to moment, the anatomic structures, the position of the catheter, and if there are thrombi in the left atrium. It can also help optimize the use of radiofrequency energy by monitoring for microbubbles, which represent tissue overheating.1
Three-dimensional mapping and navigation techniques help define the anatomy and help guide the catheter, especially with previously acquired computed tomography (CT) or magnetic resonance imaging (MRI).30–32 Likewise, pulmonary venography can also show the shape and size of the pulmonary ostia. This modality can guide catheter manipulation and assessment for pulmonary venous stenosis resulting from prior ablation.1
INDICATIONS FOR CATHETER ABLATION
An absolute contraindication to catheter ablation is left atrial thrombus. Because of the risk of dislodging an existing thrombus during the procedure and causing a stroke, the committee recommends that patients with persistent atrial fibrillation who are in atrial fibrillation at the time of the procedure undergo transesophageal echocardiography to screen for thrombus.
An individualized decision
The decision to proceed with catheter ablation must be individualized on the basis of the risk of complications, the likely benefits, and the likelihood of success.1
Factors that increase the risk of iatrogenic complications such as myocardial perforation and thromboembolism include older age and comorbid conditions.
Factors that increase the chance of significant benefit include more severe symptoms and heart failure. Hsu et al33 found that the ejection fraction increased by 21 plus or minus 13 percentage points in heart failure patients who underwent the procedure.
Success rates for catheter-based ablation are lower in patients with persistent atrial fibrillation than in those with paroxysmal atrial fibrillation. Oral and colleagues34 reported that the recurrence rate in patients with persistent atrial fibrillation was 75%, compared with 29% in patients with paroxysmal atrial fibrillation. In addition, the chances of a successful outcome are lower in those with marked dilation of the left atrium.
Lifelong anticoagulation is still needed
When weighing the pros and cons of catheter-based procedures, health care providers need to also emphasize to patients that the procedure does not allow them to forgo anticoagulation. Even after ablation, patients with atrial fibrillation still face a formidable risk of thromboembolic events, and most electrophysiologists suggest lifelong anticoagulation, especially in patients with other risk factors for stroke. We discuss the guidelines for anticoagulation later in this review.
EFFECTIVENESS
Crandall et al,35 in an excellent review of the literature, estimated that ablation treatment is successful in approximately 60% to 70% of patients, that 10% to 40% of patients require a second ablation procedure, and that 10% to 15% still need antiarrhythmic drugs.
The specifics of each procedure are beyond the scope of this review, but outcomes have been shown to be better with individualized ablation therapy than with an anatomic approach. Oral et al28 showed that this tailored approach could provide success rates up to 77%; the repeat-procedure rate was 18%, and the risk of complications was low. The tailored approach allows operators to target triggers in locations other than in the pulmonary veins, including those in the thoracic veins and superior vena cava, during mapping. In addition, this approach is often needed in persistent atrial fibrillation, where left atrial substrate is likely to play a larger role than in paroxysmal atrial fibrillation and can be specifically targeted using this method.
When using pulmonary vein isolation by itself, studies have shown better outcomes in treating paroxysmal atrial fibrillation than persistent atrial fibrillation.34 These outcomes have been shown to improve with an individualized approach to ablation therapy.
Haïssaguerre et al36 used various tailored ablation techniques to terminate persistent atrial fibrillation and were able to terminate the rhythm in 87% of their patients. Eleven months after the procedure, 95% of the patients in whom it succeeded remained free of arrhythmia.
Randomized controlled trials now confirm that left atrial ablation is superior to antiarrhythmic drug therapy in maintaining sinus rhythm over time.19,37,38
COMPLICATIONS
It is important that patients understand the risks associated with the procedure. Advancing technology in imaging and catheters and our growing understanding of atrial fibrillation are not only able to optimize ablation outcomes, but also to minimize complications.
Complications of catheter-based treatment of atrial fibrillation described by the expert consensus committee1 include:
- Cardiac tamponade
- Pulmonary vein stenosis
- Phrenic nerve injury
- Esophageal injury, atrioesophageal fistula
- Periesophageal vagal injury
- Thromboembolic events
- Vascular complications
- Acute coronary artery occlusion (rare)
- Air emboli from catheters and sheath
- Catheter entrapment in the mitral valve
- Tachyarrhythmias
- Radiation exposure
- Mitral valve trauma.
Cardiac tamponade
Cardiac tamponade due to accidental puncture or excessive heat accumulation, steam expansion, and perforation of the atrial wall occurs in about 6% of patients,20 but this number varies. Limiting the power delivered to tissue to less than 25 or 35 W may reduce the incidence of this complication.1 The expertise of the physician and the type of imaging used (eg, transesophageal or intracardiac echocardiography) are also factors.
Pulmonary vein stenosis
Pulmonary vein stenosis was seen after the first pulmonary vein isolation techniques were tried, when ablation within the pulmonary vein caused high rates of this complication.1 Improved knowledge of anatomy and better visualization using intracardiac echocardiography have led to a significantly lower rate of pulmonary vein stenosis.20 The current rate is 0.5% to 2%.12,20
Nevertheless, it is important for referring physicians to recognize the symptoms of pulmonary vein stenosis, as they will likely be the first providers to see a patient with these symptoms, which can be mistaken for pneumonia or congestive heart failure. The symptoms include cough, dyspnea, pneumonia, and hemoptysis that may occur early or late (weeks) after ablation.
CT, MRI, and ventilation-perfusion scanning can be used to diagnose pulmonary vein stenosis. Its treatment includes stenting the narrowed vein.
Esophageal injury
Esophageal injury, specifically formation of an atrioesophageal fistula, is a life-threatening complication of this treatment.1 The esophagus passes very close to the left atrial posterior wall and is therefore at risk of thermal injury during ablation.
Health care professionals should be alert to the symptoms of this complication, which include dysphagia, odynophagia, hematemesis, signs of intermittent cardiac or neurologic ischemia, persistent fever, bacteremia, fungemia, leukocytosis, and melena.12 These symptoms may arise weeks after the procedure.
Any patient who has recently undergone catheter ablation and who presents with some of these symptoms needs a prompt workup with MRI or CT. Endoscopy is contraindicated because it can introduce air into the esophagus, which may result in air embolism to the brain. Atrioesophageal fistula is generally fatal, but emergency surgery may be an option.
Thromboembolism
Thromboembolic events are another worrisome complication. The reported incidence rate ranges between 0% and 7%.1
Appropriate anticoagulation protocols can minimize the risk. Patients should take warfarin (Coumadin) for at least 3 weeks before undergoing ablation if they have paroxysmal atrial fibrillation and a CHADS2 score of 1 or higher (1 point each for having congestive heart failure, hypertension, age > 75 years, or diabetes; 2 points for having a prior stroke or transient ischemic attack), or if they have persistent atrial fibrillation regardless of the CHADS2 score. The target international normalized ratio (INR) is in the therapeutic range, ie, 2 to 3. Patients who have paroxysmal atrial fibrillation and a CHADS2 score of 0 may be treated with aspirin or warfarin before the procedure. Patients who have been taking warfarin should be “bridged” with subcutaneous low-molecular-weight heparin or intravenous unfractionated heparin before ablation, eg, by stopping the warfarin several days before the procedure and substituting enoxaparin (Lovenox) 0.5 to 1 mg/kg twice daily until the evening before the procedure.
To screen for thrombi in the left atrium, transesophageal echocardiography should be performed before the procedure in patients who have not been receiving warfarin, or whose INRs have not consistently been in the therapeutic range of 2 to 3, or who have persistent atrial fibrillation and are in atrial fibrillation at the time of the procedure.
During the procedure, anticoagulation is maintained with a heparin infusion. After the procedure, warfarin is restarted along with a low-molecular-weight heparin or unfractionated heparin. The heparin is stopped when the INR is in the therapeutic range, but warfarin should be continued for at least 3 months. Selected patients with a CHADS2 score of 1 may be switched to aspirin therapy after several months, and those with a score of 0 may be switched to aspirin or no therapy.12
We still lack data from large-scale trials about long-term thromboembolic complications of ablation therapy. Most electrophysiologists prefer to continue anticoagulation indefinitely and would consider terminating it only with great caution.
Arrhythmias
After ablation, new atrial arrhythmias such as atrial flutter are common, with a wide range of reported incidence rates.39 Most cases respond poorly to antiarrythmic drugs, but temporizing measures are recommended, since about half will resolve spontaneously. For this reason, experts generally recommend waiting 2 to 3 months after an ablation procedure before performing a repeat ablative procedure.1,12 Close monitoring is recommended during the months following catheter ablation.
COST AND QUALITY OF LIFE
The cost of catheter ablation needs to be taken into account when considering the procedure for an individual patient.
Catheter ablation is expensive, but so is ongoing medical treatment. In the United States, catheter ablation costs between $17,000 and $21,000 initially, with an ongoing cost of $1,500 to $2,000 per year.3 In comparison, medical therapy costs $4,000 to $5,000 per year. Therefore, catheter ablation would take 4 to 8 years to pay for itself.2
Quality of life also remains a key factor in determining whether to pursue this treatment option. Initial studies showed a trend toward better quality of life with catheter ablation than with medical therapy. In a nonrandomized study published in 2003, Pappone et al40 assessed quality of life in 109 patients who underwent ablation and in 102 medically treated patients, using the 36-item Short-Form General Health Survey. At baseline, both groups similarly rated their quality of life significantly lower than people of the same age and sex in the general population (P < .001). By 6 months, quality-of-life scores in the ablation group had risen to the same level as in the general population, while they stayed the same in the medically treated group. However, data are still limited, and, like the cost of the procedure, estimated quality of life needs to be weighed for the individual patient.
FUTURE DEVELOPMENTS
There are exciting developments in imaging and catheter systems for ablation of atrial fibrillation. It is hoped that these new technologies will improve success rates and reduce complication rates.
In imaging, digital fusion of CT and MRI with electroanatomic mapping shows the anatomy of the junction of the left atrium and pulmonary vein in real time. (Currently, CT and MRI have to be done prior to ablative techniques.)
New ablation systems are being developed that use extreme cold, lasers, and ultrasound. An advantage of these new ablation systems is that they have balloon-tipped catheters, which are placed near the pulmonary vein ostium to deliver full circumferential ablation.41,42
More patients with atrial fibrillation are asking their physicians about catheter-based radiofrequency ablation as a treatment option.
Indeed, in the mere 10 years or so since this procedure was introduced, it has shown promising clinical results. Still, it is not yet available at many medical centers, and it is not yet considered the first-line treatment for atrial fibrillation.1 Moreover, some patients may have unrealistic expectations about it, such as being able to stop taking anticoagulant drugs afterward. It is therefore important for health care professionals not only to recognize which patients may benefit from catheter-based treatment, but also to educate them about it so they have reasonable expectations.
See related editorial and patient information at http://my.clevelandclinic.org/heart/services/tests/procedures/ablation.aspx
In this article, we briefly review the mechanisms of catheter ablation of atrial fibrillation and discuss its current indications, with an emphasis on how to determine which patients with atrial fibrillation are candidates for this new procedure.
NUMBERS ARE RISING, AND DRUG THERAPY HAS LIMITATIONS
Atrial fibrillation is a disease of the elderly: about 70% of patients are between the ages of 65 and 85.2 As the elderly segment of the US population increases, the number of people with atrial fibrillation is expected to more than double by the year 2050.3
This growing prevalence and the increasing socioeconomic burden are two reasons people are looking to new treatments such as catheter and surgical ablation.
Several randomized clinical trials,4–7 most importantly the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial,7 found that attempting to restore and maintain sinus rhythm with antiarrhythmic drugs imparts no significant benefit in terms of survival compared with a strategy of controlling the heart rate only. However, recent studies, including an analysis by the AFFIRM investigators,8 suggest that if sinus rhythm could be achieved without the adverse effects of antiarrythmic drugs, then rhythm control may have a survival benefit over rate control. These studies, combined with improving techniques and tools for catheter ablation of atrial fibrillation, have made ablative treatment an attractive option and an emerging trend.
HOW ATRIAL FIBRILLATION STARTS AND HOW IT IS MAINTAINED
Several studies9–11 showed that left atrial myocardial cells extend into the pulmonary veins. These “myocardial sleeves,” which vary in extent between individuals, have short refractory periods and can cause conduction delays, which may create the conditions needed for arrhythmias.12,13
In landmark studies in the 1990s, Haïssaguerre et al14 and Jais et al15 showed that most focal triggers are in the myocardial sleeves at the junction of the pulmonary vein and the left atrium. These investigators went on to show that catheter-based ablation of these ectopic foci could eliminate atrial fibrillation in some patients.
Ectopic foci in the pulmonary veins fire rapidly and chaotically, generating impulses that enter the left atrium and begin to generate wavelets of reentry. These wavelets may be perpetuated if the conduction velocity is slow, the refractory period is short, and atrial mass is high.1,16,17
Some experts thought that by surgically interrupting the path of these wavelets and reducing the atrial mass, one could terminate atrial fibrillation. This model is the basis of the surgical maze procedure developed by Cox et al in the 1990s,18 which planted the seed for catheter ablation of atrial fibrillation.
DEFINITIONS OF ATRIAL FIBRILLATION
A 2007 consensus document1 prepared jointly by several heart societies emphasizes the need to classify the types of atrial fibrillation consistently, as recommendations for different treatments are based primarily on the type of atrial fibrillation. Although some patients may have atrial fibrillation that falls into more than one of these categories, it should be categorized by its most frequent pattern. These definitions apply only to episodes that last at least 30 seconds and have no identifiable reversible cause, such as acute pulmonary disease or hyperthyroidism.
Paroxysmal atrial fibrillation is defined as at least two episodes that terminate spontaneously within 7 days.
Persistent atrial fibrillation is defined as lasting more than 7 days, or lasting less than 7 days but necessitating pharmacologic or electrical cardioversion.
Permanent atrial fibrillation is defined as lasting more than 1 year.
THE TECHNIQUE
Catheter ablation is usually done as an outpatient procedure. As the procedure can take 3 to 5 hours, most patients receive conscious sedation or general anesthesia. A catheter is inserted into a femoral vein and advanced into the right atrium. Then, the atrial septum is punctured to gain access to the left atrium, and a radiofrequency ablation catheter and a mapping catheter are inserted (Figure 1).
What to ablate?
Various approaches are being used in catheter-based ablation of atrial fibrillation.1,12,19–29
Since most of the triggers of atrial fibrillation are located within the pulmonary veins, one can use an empiric anatomic approach, creating a ring of ablation lesions around the outside of the ostium of each of the four pulmonary veins (but not within the vein itself), or a single ring around the ostia of the two left pulmonary veins and another around the two right pulmonary veins. The aim is to electrically isolate these veins.
Refinements to this procedure involve making additional lines of lesions in the atrium, similar to those in the Cox maze procedure; a line across the roof of the left atrium connecting the ring of lesions around the left and right superior pulmonary veins; a line across the mitral isthmus (between the mitral valve and the left inferior pulmonary vein); and a line connecting either the roof line or the left or right circumferential lesion to the mitral annulus anteriorly. The aim of these additional lesions is to interrupt the re-entrant circuits that keep atrial fibrillation going, and they may make the procedure more effective in cases of persistent or permanent atrial fibrillation than it would be without these lesions.
An electrophysiologic approach involves using intracardiac electrocardiography to locate specific drivers of fibrillation and areas of complex fractionated atrial electrograms, which can be ablated. This is a more tailored approach, and it may be more effective. In addition, one can ablate, then attempt to induce fibrillation electrically or with drugs, and then, if fibrillation ensues, do more ablation.
Fluoroscopy vs intracardiac echocardiography
Fluoroscopy, intracardiac echocardiography, three-dimensional mapping, and pulmonary venography have all been used to guide left atrial pulmonary vein ablation.
Until recently, electrophysiologists used fluoroscopy, but now most use intracardiac echocardiography and other imaging techniques. Intracardiac echocardiography provides information that fluoroscopy cannot. It can show, from moment to moment, the anatomic structures, the position of the catheter, and if there are thrombi in the left atrium. It can also help optimize the use of radiofrequency energy by monitoring for microbubbles, which represent tissue overheating.1
Three-dimensional mapping and navigation techniques help define the anatomy and help guide the catheter, especially with previously acquired computed tomography (CT) or magnetic resonance imaging (MRI).30–32 Likewise, pulmonary venography can also show the shape and size of the pulmonary ostia. This modality can guide catheter manipulation and assessment for pulmonary venous stenosis resulting from prior ablation.1
INDICATIONS FOR CATHETER ABLATION
An absolute contraindication to catheter ablation is left atrial thrombus. Because of the risk of dislodging an existing thrombus during the procedure and causing a stroke, the committee recommends that patients with persistent atrial fibrillation who are in atrial fibrillation at the time of the procedure undergo transesophageal echocardiography to screen for thrombus.
An individualized decision
The decision to proceed with catheter ablation must be individualized on the basis of the risk of complications, the likely benefits, and the likelihood of success.1
Factors that increase the risk of iatrogenic complications such as myocardial perforation and thromboembolism include older age and comorbid conditions.
Factors that increase the chance of significant benefit include more severe symptoms and heart failure. Hsu et al33 found that the ejection fraction increased by 21 plus or minus 13 percentage points in heart failure patients who underwent the procedure.
Success rates for catheter-based ablation are lower in patients with persistent atrial fibrillation than in those with paroxysmal atrial fibrillation. Oral and colleagues34 reported that the recurrence rate in patients with persistent atrial fibrillation was 75%, compared with 29% in patients with paroxysmal atrial fibrillation. In addition, the chances of a successful outcome are lower in those with marked dilation of the left atrium.
Lifelong anticoagulation is still needed
When weighing the pros and cons of catheter-based procedures, health care providers need to also emphasize to patients that the procedure does not allow them to forgo anticoagulation. Even after ablation, patients with atrial fibrillation still face a formidable risk of thromboembolic events, and most electrophysiologists suggest lifelong anticoagulation, especially in patients with other risk factors for stroke. We discuss the guidelines for anticoagulation later in this review.
EFFECTIVENESS
Crandall et al,35 in an excellent review of the literature, estimated that ablation treatment is successful in approximately 60% to 70% of patients, that 10% to 40% of patients require a second ablation procedure, and that 10% to 15% still need antiarrhythmic drugs.
The specifics of each procedure are beyond the scope of this review, but outcomes have been shown to be better with individualized ablation therapy than with an anatomic approach. Oral et al28 showed that this tailored approach could provide success rates up to 77%; the repeat-procedure rate was 18%, and the risk of complications was low. The tailored approach allows operators to target triggers in locations other than in the pulmonary veins, including those in the thoracic veins and superior vena cava, during mapping. In addition, this approach is often needed in persistent atrial fibrillation, where left atrial substrate is likely to play a larger role than in paroxysmal atrial fibrillation and can be specifically targeted using this method.
When using pulmonary vein isolation by itself, studies have shown better outcomes in treating paroxysmal atrial fibrillation than persistent atrial fibrillation.34 These outcomes have been shown to improve with an individualized approach to ablation therapy.
Haïssaguerre et al36 used various tailored ablation techniques to terminate persistent atrial fibrillation and were able to terminate the rhythm in 87% of their patients. Eleven months after the procedure, 95% of the patients in whom it succeeded remained free of arrhythmia.
Randomized controlled trials now confirm that left atrial ablation is superior to antiarrhythmic drug therapy in maintaining sinus rhythm over time.19,37,38
COMPLICATIONS
It is important that patients understand the risks associated with the procedure. Advancing technology in imaging and catheters and our growing understanding of atrial fibrillation are not only able to optimize ablation outcomes, but also to minimize complications.
Complications of catheter-based treatment of atrial fibrillation described by the expert consensus committee1 include:
- Cardiac tamponade
- Pulmonary vein stenosis
- Phrenic nerve injury
- Esophageal injury, atrioesophageal fistula
- Periesophageal vagal injury
- Thromboembolic events
- Vascular complications
- Acute coronary artery occlusion (rare)
- Air emboli from catheters and sheath
- Catheter entrapment in the mitral valve
- Tachyarrhythmias
- Radiation exposure
- Mitral valve trauma.
Cardiac tamponade
Cardiac tamponade due to accidental puncture or excessive heat accumulation, steam expansion, and perforation of the atrial wall occurs in about 6% of patients,20 but this number varies. Limiting the power delivered to tissue to less than 25 or 35 W may reduce the incidence of this complication.1 The expertise of the physician and the type of imaging used (eg, transesophageal or intracardiac echocardiography) are also factors.
Pulmonary vein stenosis
Pulmonary vein stenosis was seen after the first pulmonary vein isolation techniques were tried, when ablation within the pulmonary vein caused high rates of this complication.1 Improved knowledge of anatomy and better visualization using intracardiac echocardiography have led to a significantly lower rate of pulmonary vein stenosis.20 The current rate is 0.5% to 2%.12,20
Nevertheless, it is important for referring physicians to recognize the symptoms of pulmonary vein stenosis, as they will likely be the first providers to see a patient with these symptoms, which can be mistaken for pneumonia or congestive heart failure. The symptoms include cough, dyspnea, pneumonia, and hemoptysis that may occur early or late (weeks) after ablation.
CT, MRI, and ventilation-perfusion scanning can be used to diagnose pulmonary vein stenosis. Its treatment includes stenting the narrowed vein.
Esophageal injury
Esophageal injury, specifically formation of an atrioesophageal fistula, is a life-threatening complication of this treatment.1 The esophagus passes very close to the left atrial posterior wall and is therefore at risk of thermal injury during ablation.
Health care professionals should be alert to the symptoms of this complication, which include dysphagia, odynophagia, hematemesis, signs of intermittent cardiac or neurologic ischemia, persistent fever, bacteremia, fungemia, leukocytosis, and melena.12 These symptoms may arise weeks after the procedure.
Any patient who has recently undergone catheter ablation and who presents with some of these symptoms needs a prompt workup with MRI or CT. Endoscopy is contraindicated because it can introduce air into the esophagus, which may result in air embolism to the brain. Atrioesophageal fistula is generally fatal, but emergency surgery may be an option.
Thromboembolism
Thromboembolic events are another worrisome complication. The reported incidence rate ranges between 0% and 7%.1
Appropriate anticoagulation protocols can minimize the risk. Patients should take warfarin (Coumadin) for at least 3 weeks before undergoing ablation if they have paroxysmal atrial fibrillation and a CHADS2 score of 1 or higher (1 point each for having congestive heart failure, hypertension, age > 75 years, or diabetes; 2 points for having a prior stroke or transient ischemic attack), or if they have persistent atrial fibrillation regardless of the CHADS2 score. The target international normalized ratio (INR) is in the therapeutic range, ie, 2 to 3. Patients who have paroxysmal atrial fibrillation and a CHADS2 score of 0 may be treated with aspirin or warfarin before the procedure. Patients who have been taking warfarin should be “bridged” with subcutaneous low-molecular-weight heparin or intravenous unfractionated heparin before ablation, eg, by stopping the warfarin several days before the procedure and substituting enoxaparin (Lovenox) 0.5 to 1 mg/kg twice daily until the evening before the procedure.
To screen for thrombi in the left atrium, transesophageal echocardiography should be performed before the procedure in patients who have not been receiving warfarin, or whose INRs have not consistently been in the therapeutic range of 2 to 3, or who have persistent atrial fibrillation and are in atrial fibrillation at the time of the procedure.
During the procedure, anticoagulation is maintained with a heparin infusion. After the procedure, warfarin is restarted along with a low-molecular-weight heparin or unfractionated heparin. The heparin is stopped when the INR is in the therapeutic range, but warfarin should be continued for at least 3 months. Selected patients with a CHADS2 score of 1 may be switched to aspirin therapy after several months, and those with a score of 0 may be switched to aspirin or no therapy.12
We still lack data from large-scale trials about long-term thromboembolic complications of ablation therapy. Most electrophysiologists prefer to continue anticoagulation indefinitely and would consider terminating it only with great caution.
Arrhythmias
After ablation, new atrial arrhythmias such as atrial flutter are common, with a wide range of reported incidence rates.39 Most cases respond poorly to antiarrythmic drugs, but temporizing measures are recommended, since about half will resolve spontaneously. For this reason, experts generally recommend waiting 2 to 3 months after an ablation procedure before performing a repeat ablative procedure.1,12 Close monitoring is recommended during the months following catheter ablation.
COST AND QUALITY OF LIFE
The cost of catheter ablation needs to be taken into account when considering the procedure for an individual patient.
Catheter ablation is expensive, but so is ongoing medical treatment. In the United States, catheter ablation costs between $17,000 and $21,000 initially, with an ongoing cost of $1,500 to $2,000 per year.3 In comparison, medical therapy costs $4,000 to $5,000 per year. Therefore, catheter ablation would take 4 to 8 years to pay for itself.2
Quality of life also remains a key factor in determining whether to pursue this treatment option. Initial studies showed a trend toward better quality of life with catheter ablation than with medical therapy. In a nonrandomized study published in 2003, Pappone et al40 assessed quality of life in 109 patients who underwent ablation and in 102 medically treated patients, using the 36-item Short-Form General Health Survey. At baseline, both groups similarly rated their quality of life significantly lower than people of the same age and sex in the general population (P < .001). By 6 months, quality-of-life scores in the ablation group had risen to the same level as in the general population, while they stayed the same in the medically treated group. However, data are still limited, and, like the cost of the procedure, estimated quality of life needs to be weighed for the individual patient.
FUTURE DEVELOPMENTS
There are exciting developments in imaging and catheter systems for ablation of atrial fibrillation. It is hoped that these new technologies will improve success rates and reduce complication rates.
In imaging, digital fusion of CT and MRI with electroanatomic mapping shows the anatomy of the junction of the left atrium and pulmonary vein in real time. (Currently, CT and MRI have to be done prior to ablative techniques.)
New ablation systems are being developed that use extreme cold, lasers, and ultrasound. An advantage of these new ablation systems is that they have balloon-tipped catheters, which are placed near the pulmonary vein ostium to deliver full circumferential ablation.41,42
- Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace 2007; 9:335–379.
- Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: full text: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace 2006; 8:651–745.
- Estes NA. Catheter ablation of atrial fibrillation: is the burn worth the buck? J Cardiovasc Electrophysiol 2007; 18:914–916.
- Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomized trial. Lancet 2000; 356:1789–1794.
- Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002; 347:1834–1840.
- Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003; 41:1690–1696.
- Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347:1825–1833.
- Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004; 109:1509–1513.
- Chugh A, Morady F. Atrial fibrillation: catheter ablation. J Interv Card Electrophysiol 2006; 16:15–26.
- Ho SY, Cabrera JA, Tran VH, Farré J, Anderson RH, Sànchez-Quintana D. Architecture of the pulmonary veins: relevance to radiofrequency ablation. Heart 2001; 86:265–270.
- Saito T, Waki K, Becker A. Left atrial myocardial extension onto pulmonary veins in humans: anatomic observations relevant for atrial arrhythmias. J Cardiovasc Electrophysiol 2000; 11:888–894.
- Natale A, Raviele A, Arentz T, et al. Venice Chart international consensus document on atrial fibrillation ablation. J Cardiovasc Electrophysiol 2007; 18:560–580.
- Hocini M, Ho SY, Kawara T, et al. Electrical conduction in canine pulmonary veins: electrophysiological and anatomic correlation. Circulation 2002; 105:2442–2448.
- Haïssaguerre M, Marcus FI, Fischer B, Clementy J. Radiofrequency catheter ablation in unusual mechanisms of atrial fibrillation: report of three cases. J Cardiovasc Electrophysiol 1994; 5:743–751.
- Jais P, Haïssaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997; 95:572–576.
- Moe GK, Rheinboldt WD, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964; 67:200–220.
- Allessie MA, Lammers WJEP, Bonke FIM, Hollen SJ. Experimental evaluation of Moe’s multiple wavelet hypothesis of atrial fibrillation. In:Zipes DP, Jalife J, editors. Cardiac Electrophysiology and Arrhythmias. New York: Grune & Stratton; 1985:265–275.
- Cox JL, Canavan TE, Schuessler RB, et al. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991; 101:406–426.
- Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol 2006; 48:2340–2347.
- Robbins IM, Colvin EV, Doyle TP, et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation. Circulation 1998; 98;1769–1775.
- Pappone C, Santinelli V, Manguso F, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 2004; 109:327–334.
- Pappone C, Manguso F, Vicedomini G, et al. Prevention of iatrogenic atrial tachycardia after ablation of atrial fibrillation: a prospective randomized study comparing circumferential pulmonary vein ablation with a modified approach. Circulation 2004; 110:3036–3042.
- Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm 2005; 2:624–631.
- Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol 1997; 273:H805–H816.
- Sanders P, Berenfeld O, Hocini M, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation 2005; 112:789–797.
- Lin YJ, Tai CT, Kao T, et al. Frequency analysis in different types of paroxysmal atrial fibrillation. J Am Coll Cardiol 2006; 47:1401–1407.
- Jais P, Hocini M, Hsu LF, et al. Technique and results of linear ablation at the mitral isthmus. Circulation 2004; 110:2996–3002.
- Oral H, Chugh A, Good E, et al. A tailored approach to catheter ablation of paroxysmal atrial fibrillation. Circulation 2006; 113:1824–1831.
- Macle L, Jais P, Weerasooriya R, et al. Irrigated-tip catheter ablation of pulmonary veins for treatment of atrial fibrillation. J Cardiovasc Electrophysiol 2002; 13:1067–1073.
- de Groot NM, Bootsma M, van der Velde ET, Schalij MJ. Three-dimensional catheter positioning during radiofrequency ablation in patients: first application of a real time position management system. J Cardiovasc Electrophysiol 2000; 11:1183–1192.
- Macle L, Jaïs P, Scavée C, et al. Pulmonary vein disconnection using the LocaLisa three-dimensional nonfluoroscopic catheter imaging system. J Cardiovasc Electrophysiol 2003; 14:693–697.
- Schreieck J, Ndrepepa G, Zrenner B, et al. Radiofrequency ablation of cardiac arrhythmias using a three-dimensional real time position management and mapping system. Pacing Clin Electrophysiol 2002; 25:1699–1707.
- Hsu LF, Jais P, Sanders P, et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med 2004; 351:2373–2383.
- Oral H, Knight BP, Tada H, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 2002; 105:1077–1081.
- Crandall MA, Bradley DJ, Packer DL, Asirvatham SJ. Contemporary management of atrial fibrillation: update on anticoagulation and invasive management strategies. Mayo Clin Proc 2009; 84:643–662.
- Haïssaguerre M, Hocini M, Sanders P, et al. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol 2005; 16:1138–1147.
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005; 293:2634–2640.
- Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008; 118:2498–2505.
- Chugh A, Oral H, Lemola K, et al. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm 2005; 2:464–471.
- Pappone C, Rosanio S, Augello G, et al. Mortality, morbidity, and qualityof lilfe after circumferential pulmonary vein ablation for atrial fibrillation. Outcomes from a controlled nonrandomized long-term study. J Am Coll Cardiol 2003; 42:185–197.
- Garan A, Al-Ahmad A, Mihalik T, et al. Cryoablation of the pulmonary veins using a novel balloon catheter. J Interv Card Electrophysiol 2006; 15:79–81.
- Meininger GR, Calkins H, Lickfett L, et al. Initial experience with a novel focused ultrasound ablation system for ring ablation outside the pulmonary vein. J Interv Card Electrophysiol 2003; 8:141–148.
- Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace 2007; 9:335–379.
- Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: full text: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace 2006; 8:651–745.
- Estes NA. Catheter ablation of atrial fibrillation: is the burn worth the buck? J Cardiovasc Electrophysiol 2007; 18:914–916.
- Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomized trial. Lancet 2000; 356:1789–1794.
- Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002; 347:1834–1840.
- Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003; 41:1690–1696.
- Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347:1825–1833.
- Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004; 109:1509–1513.
- Chugh A, Morady F. Atrial fibrillation: catheter ablation. J Interv Card Electrophysiol 2006; 16:15–26.
- Ho SY, Cabrera JA, Tran VH, Farré J, Anderson RH, Sànchez-Quintana D. Architecture of the pulmonary veins: relevance to radiofrequency ablation. Heart 2001; 86:265–270.
- Saito T, Waki K, Becker A. Left atrial myocardial extension onto pulmonary veins in humans: anatomic observations relevant for atrial arrhythmias. J Cardiovasc Electrophysiol 2000; 11:888–894.
- Natale A, Raviele A, Arentz T, et al. Venice Chart international consensus document on atrial fibrillation ablation. J Cardiovasc Electrophysiol 2007; 18:560–580.
- Hocini M, Ho SY, Kawara T, et al. Electrical conduction in canine pulmonary veins: electrophysiological and anatomic correlation. Circulation 2002; 105:2442–2448.
- Haïssaguerre M, Marcus FI, Fischer B, Clementy J. Radiofrequency catheter ablation in unusual mechanisms of atrial fibrillation: report of three cases. J Cardiovasc Electrophysiol 1994; 5:743–751.
- Jais P, Haïssaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997; 95:572–576.
- Moe GK, Rheinboldt WD, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964; 67:200–220.
- Allessie MA, Lammers WJEP, Bonke FIM, Hollen SJ. Experimental evaluation of Moe’s multiple wavelet hypothesis of atrial fibrillation. In:Zipes DP, Jalife J, editors. Cardiac Electrophysiology and Arrhythmias. New York: Grune & Stratton; 1985:265–275.
- Cox JL, Canavan TE, Schuessler RB, et al. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991; 101:406–426.
- Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol 2006; 48:2340–2347.
- Robbins IM, Colvin EV, Doyle TP, et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation. Circulation 1998; 98;1769–1775.
- Pappone C, Santinelli V, Manguso F, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 2004; 109:327–334.
- Pappone C, Manguso F, Vicedomini G, et al. Prevention of iatrogenic atrial tachycardia after ablation of atrial fibrillation: a prospective randomized study comparing circumferential pulmonary vein ablation with a modified approach. Circulation 2004; 110:3036–3042.
- Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm 2005; 2:624–631.
- Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol 1997; 273:H805–H816.
- Sanders P, Berenfeld O, Hocini M, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation 2005; 112:789–797.
- Lin YJ, Tai CT, Kao T, et al. Frequency analysis in different types of paroxysmal atrial fibrillation. J Am Coll Cardiol 2006; 47:1401–1407.
- Jais P, Hocini M, Hsu LF, et al. Technique and results of linear ablation at the mitral isthmus. Circulation 2004; 110:2996–3002.
- Oral H, Chugh A, Good E, et al. A tailored approach to catheter ablation of paroxysmal atrial fibrillation. Circulation 2006; 113:1824–1831.
- Macle L, Jais P, Weerasooriya R, et al. Irrigated-tip catheter ablation of pulmonary veins for treatment of atrial fibrillation. J Cardiovasc Electrophysiol 2002; 13:1067–1073.
- de Groot NM, Bootsma M, van der Velde ET, Schalij MJ. Three-dimensional catheter positioning during radiofrequency ablation in patients: first application of a real time position management system. J Cardiovasc Electrophysiol 2000; 11:1183–1192.
- Macle L, Jaïs P, Scavée C, et al. Pulmonary vein disconnection using the LocaLisa three-dimensional nonfluoroscopic catheter imaging system. J Cardiovasc Electrophysiol 2003; 14:693–697.
- Schreieck J, Ndrepepa G, Zrenner B, et al. Radiofrequency ablation of cardiac arrhythmias using a three-dimensional real time position management and mapping system. Pacing Clin Electrophysiol 2002; 25:1699–1707.
- Hsu LF, Jais P, Sanders P, et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med 2004; 351:2373–2383.
- Oral H, Knight BP, Tada H, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 2002; 105:1077–1081.
- Crandall MA, Bradley DJ, Packer DL, Asirvatham SJ. Contemporary management of atrial fibrillation: update on anticoagulation and invasive management strategies. Mayo Clin Proc 2009; 84:643–662.
- Haïssaguerre M, Hocini M, Sanders P, et al. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol 2005; 16:1138–1147.
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005; 293:2634–2640.
- Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008; 118:2498–2505.
- Chugh A, Oral H, Lemola K, et al. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm 2005; 2:464–471.
- Pappone C, Rosanio S, Augello G, et al. Mortality, morbidity, and qualityof lilfe after circumferential pulmonary vein ablation for atrial fibrillation. Outcomes from a controlled nonrandomized long-term study. J Am Coll Cardiol 2003; 42:185–197.
- Garan A, Al-Ahmad A, Mihalik T, et al. Cryoablation of the pulmonary veins using a novel balloon catheter. J Interv Card Electrophysiol 2006; 15:79–81.
- Meininger GR, Calkins H, Lickfett L, et al. Initial experience with a novel focused ultrasound ablation system for ring ablation outside the pulmonary vein. J Interv Card Electrophysiol 2003; 8:141–148.
KEY POINTS
- During the procedure, scar tissue is created in rings around the ostia of the pulmonary veins and in other locations in the left atrium to electrically isolate triggers of fibrillation and areas that maintain it.
- Results of the procedure are superior to those of drug therapy. Success rates are higher for those with paroxysmal atrial fibrillation than for those with persistent atrial fibrillation.
- The main indication for this procedure is failure of drug therapy or inability to tolerate drug therapy.
- Patients must understand that ablation therapy will not eliminate the need to take anticoagulant drugs.