User login
Critical Illness Outside the ICU
This issue of the Journal of Hospital Medicine describes 2 research and quality improvement demonstration projects funded by the Gordon and Betty Moore Foundation. Early detection is central to both projects. This introductory article does not provide a global review of the now voluminous literature on rapid response teams (RRTs), sepsis detection systems, or treatment protocols. Rather, it takes a step back and reassesses just what early detection and quantification of critical illness are. It then examines the implications of early detection and its quantification.
CONCEPTUAL FRAMEWORK
We define severe illness as the presence of acute disease such that a person can no longer expect to improve without dedicated hospital treatment but which is not inevitably associated with mortality, postdischarge morbidity, or major loss of autonomy. In contrast, we define critical illness as acute disease with high a priori risk of mortality, postdischarge morbidity, and major (possibly total) loss of autonomy. We accept that the boundaries between ordinary illness, severe illness, and critical illness are blurred. The basic assumption behind all efforts at early detection is that these edges can be made sharp, and that the knowledge base required to do so can also lead to improvements in treatment protocols and patient outcomes. Further, it is assumed that at least some forms of critical illness can be prevented or mitigated by earlier detection, identification, and treatment.
Research over the last 2 decades has provided important support for this intuitive view as well as making it more nuanced. With respect to epidemiology, the big news is that sepsis is the biggest culprit, and that it accounts for a substantial proportion of all hospital deaths, including many previously considered unexpected hospital deaths due to in‐hospital deterioration.[1] With respect to treatment, a number of studies have demonstrated that crucial therapies previously considered to be intensive care unit (ICU) therapies can be initiated in the emergency department or general medicalsurgical ward.[2]
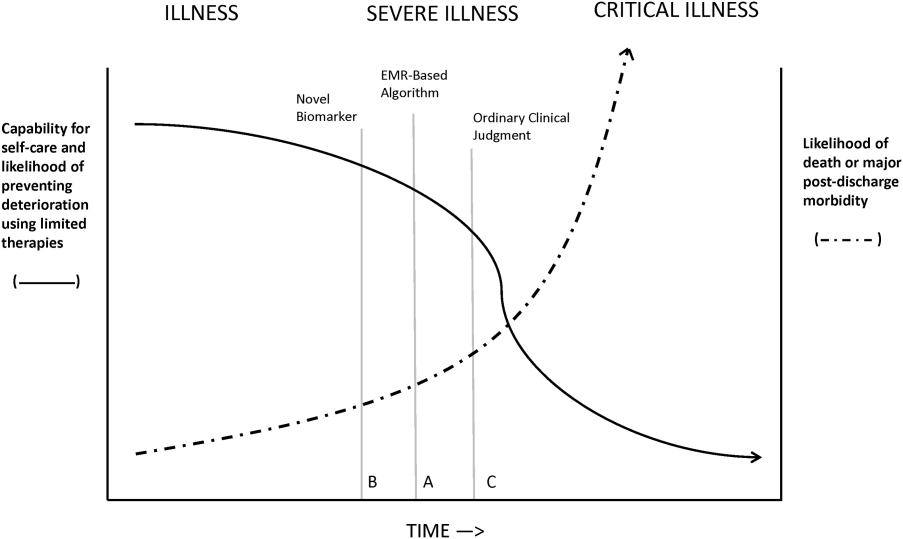
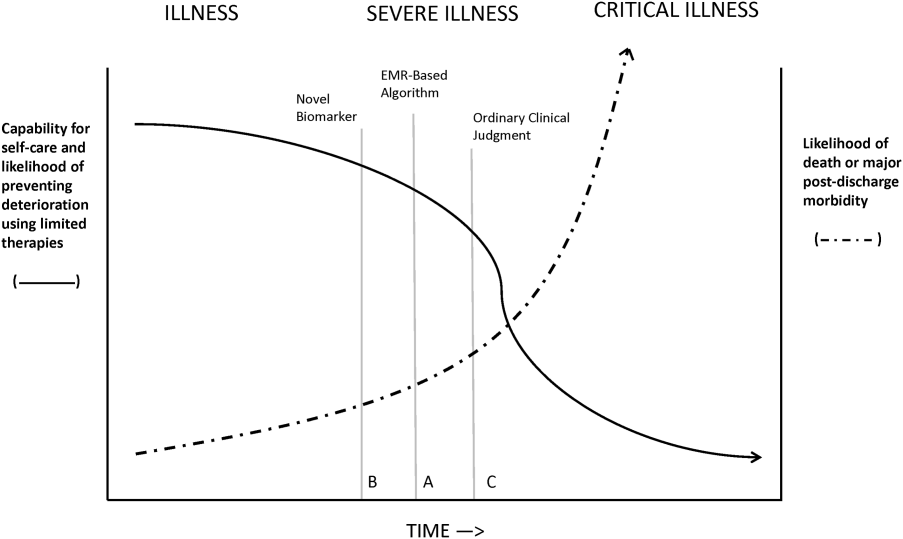
Figure 1 shows an idealized framework for illness presenting in the emergency department or general medicalsurgical wards. It illustrates the notion that a transition period exists when patients may be rescued with less intense therapy than will be required when condition progression occurs. Once a certain threshold is crossed, the risk of death or major postdischarge morbidity rises exponentially. Unaided human cognition's ability to determine where a given patient is in this continuum is dangerously variable and is highly dependent on the individuals training and experience. Consequently, as described in several of the articles in this issue as well as multiple other publications, health systems are employing comprehensive electronic medical records (EMRs) and are migrating to algorithmic approaches that combine multiple types of patient data.[3, 4] Although we are still some distance from being able to define exact boundaries between illness, severe illness, and critical illness, current EMRs permit much better definition of patient states, care processes, and short‐term outcomes.
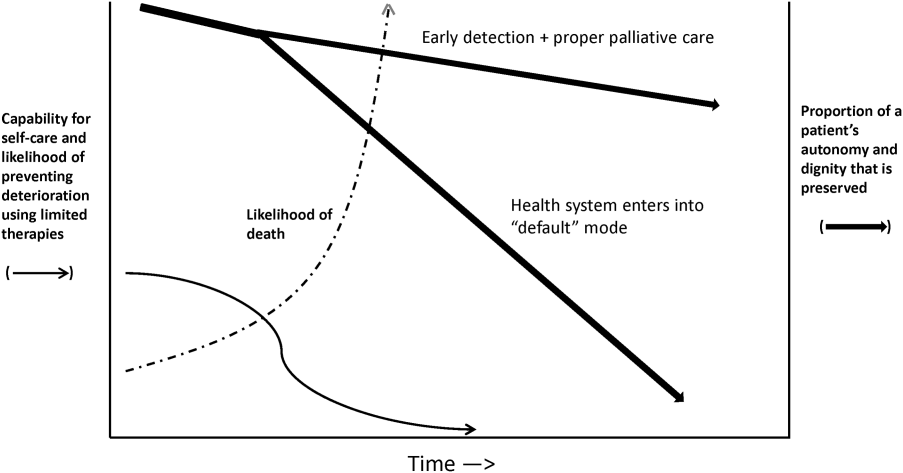
Whereas our ability to quantify many processes and short‐term outcomes is expanding rapidly, quantification of the possible benefit of early detection is complicated by the fact that, even in the best of circumstances, not all patients can be rescued. For some patients, rescue may be temporary, raising the prospect of repeated episodes of critical illness and prolonged intensive care without any hope of leaving the hospital. Figure 2 shows that, for these patients, the problem is no longer simply one of preventing death and preserving function but, rather, preserving autonomy and dignity. In this context, early detection means earlier specification of patient preferences.[5, 6]
JUST WHAT CONSTITUTES EARLY DETECTION (AND HOW DO WE QUANTIFY IT)?
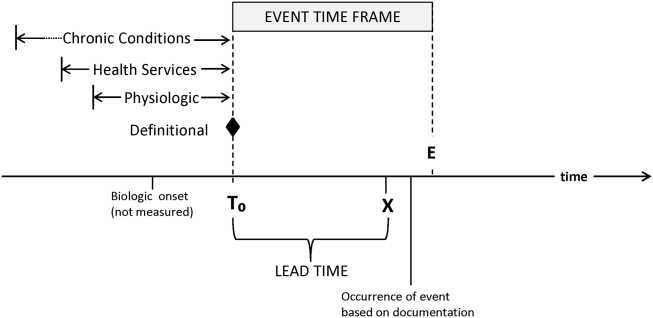
RRTs arose as the result of a number of studies showing thatin retrospectin‐hospital deteriorations should not have been unexpected. Given comprehensive inpatient EMRs, it is now possible to develop more rigorous definitions. A minimum set of parameters that one would need to specify for proper quantification of early detection is shown on Figure 3. The first is specifying a T0, that is, the moment when a prediction regarding event X (which needs to be defined) is issued. This is different from the (currently unmeasurable) biologic onset of illness as well as the first documented indication that critical illness was present. Further, it is important to be explicit about the event time frame (the time period during which a predicted event is expected to occur): we are predicting that X will occur within E hours of the T0. The time frame between the T0 and X, which we are referring to as lead time, is clinically very important, as it represents the time period during which the response arm (eg, RRT intervention) is to be instituted. Statistical approaches can be used to estimate it, but once an early detection system is in place, it can be quantified. Figure 3 is not restricted to electronic systems; all components shown can be and are used by unaided human cognition.
It is essential to specify what data are used to generate probability estimates as well as the time frames used, which we refer to as the look‐back time frames. Several types of data could be employed, with some data elements (eg, age or gender) being discrete data with a 1:1 fixed correspondence between the patient and the data. Other data have a many‐to‐1 relationship, and an exact look‐back time frame must be specified for each data type. For example, it seems reasonable to specify a short (1224 hours) look‐back period for some types of data (eg, vital signs, lactate, admission diagnosis or chief complaint), an intermediate time period (13 days) for information on the current encounter, and a longer (months to years) time period for preexisting illness or comorbidity burden.
Because many events are rare, traditional measures used to assess model performance, such as the area under the receiver operator characteristic curve (C statistic), are not as helpful.[7] Consequently, much more emphasis needs to be given to 2 key metrics: number needed to evaluate (or workup to detection ratio) and threshold‐specific sensitivity (ability of the alert to detect X at a given threshold). With these, one can answer 3 questions that will be asked by the physicians and nurses who are not likely to be researchers, and who will have little interest in the statistics: How many patients do I need to work up each day? How many patients will I need to work up for each possible outcome identified? For this amount of work, how many of the possible outcomes will we catch?
Data availability for the study of severe and critical illness continues to expand. Practically, this means that future research will require more nuanced ontologies for the classification of physiologic derangement. Current approaches to severity scoring (collapsing data into composite scores) need to be replaced by dynamic approaches that consider differential effects on organ systems as well as what can be measured. Severity scoring will also need to incorporate the rate of change of a score (or probability derived from a score) in predicting the occurrence of an event of interest as well as judging response to treatment. Thus, instead of at time of ICU admission, the patient had a severity score of 76, we may have although this patient's severity score at the time of admission was decreasing by 4 points per hour per 10 mL/kg fluid given, the probability for respiratory instability was increasing by 2.3% per hour given 3 L/min supplemental oxygen. This approach is concordant with work done in other clinical settings (eg, in addition to an absolute value of maximal negative inspiratory pressure or vital capacity, the rate of deterioration of neuromuscular weakness in Guillain‐Barr syndrome is also important in predicting respiratory failure[8]).
Electronic data also could permit better definition of patient preferences regarding escalation of care. At present, available electronic data are limited (primarily, orders such as do not resuscitate).[9] However, this EMR domain is gradually expanding.[10, 11] Entities such as the National Institutes of Health could develop sophisticated and rapid questionnaires around patient preferences that are similar to those developed for the Patient Reported Outcomes Measurement Information System.[12] Such tools could have a significant effect on our ability to quantify the benefits of early detection as it relates to a patient's preferences (including better delineation of what treatments they would and would not want).
ACTIVATING A RESPONSE ARM
Early identification, antibiotic administration, fluid resuscitation, and source control are now widely felt to constitute low‐hanging fruit for decreasing morbidity and mortality in severe sepsis. All these measures are included in quality improvement programs and sepsis bundles.[13, 14, 15] However, before early interventions can be instituted, sepsis must at least be suspected, hence the need for early detection. The situation with respect to patient deterioration (for reasons other than sepsis) in general medical surgical wards is less clear‐cut. Reasons for deterioration are much more heterogenous and, consequently, early detection is likely necessary but not sufficient for outcomes improvement.
The 2 projects described in this issue describe nonspecific (indicating elevated risk but not specifying what led to the elevation of risk) and sepsis‐specific alerting systems. In the case of the nonspecific system, detection may not lead to an immediate deployment of a response arm. Instead, a secondary evaluation process must be triggered first. Following this evaluation component, a response arm may or may not be required. In contrast, the sepsis‐specific project essentially transforms the general medicalsurgical ward into a screening system. This screening system then also triggers specific bundle components.
Neither of these systems relies on unaided human cognition. In the case of the nonspecific system, a complex equation generates a probability that is displayed in the EMR, with protocols specifying what actions are to be taken when that probability exceeds a prespecified threshold. With respect to the sepsis screening system, clinicians are supported by EMR alerts as well as protocols that increase nursing autonomy when sepsis is suspected.
The distinction between nonspecific (eg, acute respiratory failure or hemodynamic deterioration) and specific (eg, severe sepsis) alerting systems is likely to disappear as advances in the field occur. For example, incorporation of natural language processing would permit inclusion of semantic data, which could be processed so as to prebucket an alert into one that not just gave a probability, but also a likely cause for the elevated probability.
In addition, both types of systems suffer from the limitation of working off a limited database because, in general, current textbooks and training programs primary focus remains that of treatment of full‐blown clinical syndromes. For example, little is known about how one should manage patients with intermediate lactate values, despite evidence showing that a significant percentage of patients who die from sepsis will initially have such values, with 1 study showing 63% as many deaths with initial lactate of 2.5 to 4.0 mmol/L as occurred with an initial lactate of >4.0 mmol/L.[16] Lastly, as is discussed below, both systems will encounter similar problems when it comes to quantifying benefit.
QUANTIFYING BENEFIT
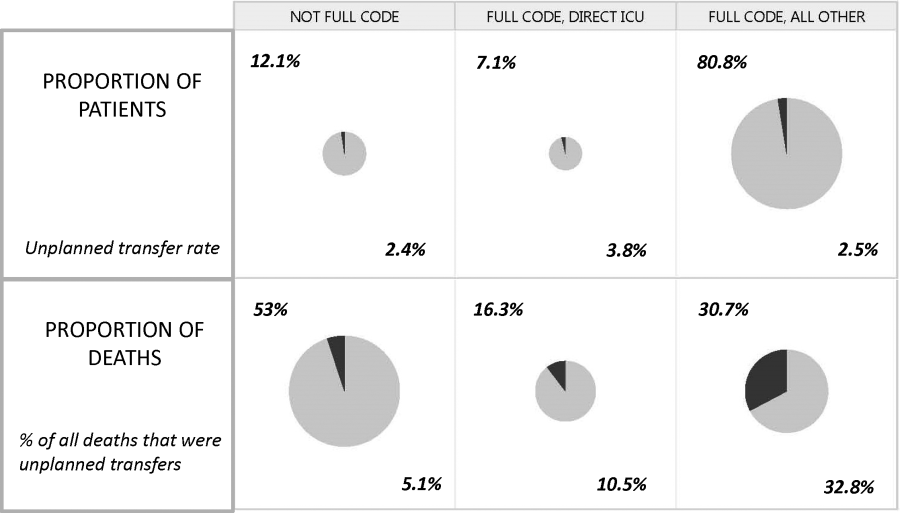
Whereas the notion of deploying RRTs has clearly been successful, success in demonstrating unequivocal benefit remains elusive.[17, 18, 19] Outcome measures vary dramatically across studies and have included the number of RRT calls, decreases in code blue events on the ward, and decreases in inpatient mortality.[20] We suspect that other reasons are behind this problem. First is the lack of adequate risk adjustment and ignoring the impact of patients near the end of life on the denominator. Figure 4 shows recent data from 21 Kaiser Permanente Northern California (KPNC) hospitals, which can now capture care directive orders electronically,[21] illustrates this problem. The majority (53%) of hospital deaths occur among a highly variable proportion (range across hospitals, 6.5%18.0%) of patients who arrive at the hospital with a restricted resuscitation preference (do not resuscitate, partial code, and comfort care only). These patients do not want to die or crash and burn but, were they to trigger an alert, they would not necessarily want to be rescued by being transferred to the ICU either; moreover, internal KPNC analyses show that large numbers of these patients have sepsis and refuse aggressive treatment. The second major confounder is that ICUs save lives. Consequently, although early detection could lead to fewer transfers to the ICU, using the end point of ICU admission is very problematic, because in many cases the goal of alerting systems should be to get patients to the ICU sooner, which would not affect the outcome of transfer to the ICU in a downward direction; in fact, such systems might increase transfer to the ICU.
The complexities summarized in Figure 4 mean that it is likely that formal quantification of benefit will require examination of multiple measures, including balancing measures as described below. It is also evident that, in this respectlack of agreement as to what constitutes a good outcomethe issues being faced here are a reflection of a broader area of disagreement within our profession and society at large that extends to medical conditions other than critical illness.
POTENTIAL HARMS OF EARLY DETECTION
Implementation of early detection and rapid response systems are not inherently free of harm. If these systems are not shown to have benefit, then the cost of operating them is moving resources away from other, possibly evidence‐based, interventions.[22] At the individual level, alerts could frighten patients and their families (for example, some people are very uncomfortable with the idea that one can predict events). Physicians and nurses who work in the hospital are already quite busy, so every time an alert is issued, it adds to the demand on their already limited time, hence, the critical importance of strategies to minimize false alarms and alert fatigue. Moreover, altering existing workflows can be disruptive and unpopular.
A potentially more quantifiable problem is the impact of early detection systems on ICU operations. For example, if an RRT decides to transfer a patient from the ward to the ICU as a preventive measure (soft landing) and this in turn ties up an ICU bed, that bed is then unavailable for a new patient in the emergency department. Similarly, early detection systems coupled with structured protocols for promoting soft landings could result in a change in ICU case mix, with greater patient flow due to increased numbers of patients with lower severity and lower ICU length of stay. These considerations suggest the need to couple early detection with other supportive data systems and workflows (eg, systems that monitor bed capacity proactively).
Lastly, if documentation protocols are not established and followed, early detection systems could expose both individual clinicians as well as healthcare institutions to medicallegal risk. This consideration could be particularly important in those instances where an alert is issued and, for whatever reasons, clinicians do not take action and do not document that decision. At present, early detection systems are relatively uncommon, but they may gradually become standard of care. This means that in‐house out of ICU deteriorations, which are generally considered to be bad luck or due to a specific error or oversight, may then be considered to be preventable. Another possible scenario that could arise is that of plaintiffs invoking enterprise liability, where a hospital's not having an early detection system becomes considered negligent.
ARTICLES IN THIS ISSUE
In this issue of the Journal of Hospital Medicine, we examine early detection from various perspectives but around a common theme that usually gets less attention in the academic literature: implementation. The article by Schorr et al.[23] describes a disease‐specific approach that can be instantiated using either electronic or paper tools. Escobar et al.[24] describe the quantitative as well as the electronic architecture of an early warning system (EWS) pilot at 2 hospitals that are part of an integrated healthcare delivery system. Dummett et al.[25] then show how a clinical rescue component was developed to take advantage of the EWS, whereas Granich et al.[26] describe the complementary component (integration of supportive care and ensuring that patient preferences are respected). The paper by Liu et al.[27] concludes by placing all of this work in a much broader context, that of the learning healthcare system.
FUTURE DIRECTIONS: KEY GAPS IN THE FIELD
Important gaps remain with respect to early detection and response systems. Future research will need to focus on a number of areas. First and foremost, better approaches to quantifying the costbenefit relationships of these systems are needed; somehow, we need to move beyond a purely intuitive sense that they are good things. Related to this is the need to establish metrics that would permit rigorous comparisons between different approaches; this work needs to go beyond simple comparisons of the statistical characteristics of different predictive models. Ideally, it should include comparisons of different approaches for the response arms as well. We also need to characterize clinician understanding about detection systems, what constitutes impending or incipient critical illness, and the optimum way to provide early detection. Finally, better approaches to integrating health services research with basic science work must be developed; for example, how should one test new biomarkers in settings with early detection and response systems?
The most important frontier, however, is how one can make early detection and response systems more patient centered and how one can enhance their ability to respect patient preferences. Developing systems to improve clinical management is laudable, but somehow we need to also find ways to have these systems make a better connection to what patients want most and what matters most to them, something that may need to include new ways that sometimes suspend use of these systems. At the end of the day, after early detection, patients must have a care experience that they see as an unequivocal improvement.
Acknowledgements
The authors thank our 2 foundation program officers, Dr. Marybeth Sharpe and Ms. Kate Weiland, for their administrative support and encouragement. The authors also thank Dr. Tracy Lieu, Dr. Michelle Caughey, Dr. Philip Madvig, and Ms. Barbara Crawford for their administrative assistance, Dr. Vincent Liu for comments on the manuscript, and Ms. Rachel Lesser for her help with formatting the manuscript and figures.
Disclosures
This work was supported by the Gordon and Betty Moore Foundation, The Permanente Medical Group, Inc., and Kaiser Foundation Hospitals, Inc. As part of our agreement with the Moore Foundation, we made a commitment to disseminate our findings in articles such as this one. However, the Gordon and Betty Moore Foundation and its staff played no role in how we actually structured our articles, nor did they review or preapprove any of the manuscripts submitted as part of the dissemination component. None of the authors has any conflicts of interest to declare of relevance to this work.
- , , , Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief. 2011(62):1–8.
- , , , et al. Surviving sepsis campaign: association between performance metrics and outcomes in a 7.5‐year study. Crit Care Med. 2015;43(1):3–12.
- , , , , , Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388–395.
- , , , et al. A randomized trial of real‐time automated clinical deterioration alerts sent to a rapid response team. J Hosp Med. 2014;9(7):424–429.
- , , , , , Enhanced end‐of‐life care associated with deploying a rapid response team: a pilot study. J Hosp Med. 2009;4(7):449–452.
- , , , , The medical emergency team call: a sentinel event that triggers goals of care discussion. Crit Care Med. 2014;42(2):322–327.
- , , , Why the C‐statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19:285.
- , , , , Anticipating mechanical ventilation in Guillain‐Barre syndrome. Arch Neurol. 2001;58(6):893–898.
- , , , , , The natural history of changes in preferences for life‐sustaining treatments and implications for inpatient mortality in younger and older hospitalized adults. J Am Geriatr Soc. 2016;64(5):981–989.
- , Remote collection of questionnaires. Clin Exp Rheumatol. 2014;32(5 suppl 85):S168–S172.
- Be prepared to make your health care wishes known. Health care directives. Allina Health website. Available at: http://www.allinahealth.org/Customer-Service/Be-prepared/Be-prepared-to-make-your-health-care-wishes-known. Accessed January 1, 2015.
- Patient Reported Outcomes Measurement Information System. Dynamic tools to measure health outcomes from the patient perspective. Available at: http://www.nihpromis.org. Accessed January 15, 2015.
- , , , , , Methodology of the surviving sepsis campaign global initiative for improving care of the patient with severe sepsis. Minerva Anestesiol. 2009;75(suppl 1):23–27.
- , , The Surviving Sepsis Campaign: a history and a perspective. Surg Infect (Larchmt). 2010;11(3):275–281.
- , The Surviving Sepsis Campaign: past, present and future. Trends Mol Med. 2014;20(4):192–194.
- , , , et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45(5):524–528.
- , , , et al. Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a Children's Hospital. JAMA. 2007;298(19):2267–2274.
- , , , , , Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387–390.
- , Rapid response teams: qualitative analysis of their effectiveness. Am J Crit Care. 2013;22(3):198–210.
- , , , , , Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300(21):2506–2513.
- , , , , Risk‐adjusting hospital mortality using a comprehensive electronic record in an integrated healthcare delivery system. Med Care. 2013;51(5):446–453.
- , , Rapid response teams—walk, don't run. JAMA. 2006;296(13):1645–1647.
- Schorr et al. J Hosp Med. 2016;11:000–000.
- , , , et al. Piloting electronic medical record–based early detection of inpatient deterioration in community hospitals. J Hosp Med. 2016;11:000–000.
- Dummett et al. J Hosp Med. 2016;11:000–000.
- Granich et al. J Hosp Med. 2016;11:000–000.
- Liu et al. , , , , , Data that drive: closing the loop in the learning hospital system. J Hosp Med. 2016;11:000–000.
This issue of the Journal of Hospital Medicine describes 2 research and quality improvement demonstration projects funded by the Gordon and Betty Moore Foundation. Early detection is central to both projects. This introductory article does not provide a global review of the now voluminous literature on rapid response teams (RRTs), sepsis detection systems, or treatment protocols. Rather, it takes a step back and reassesses just what early detection and quantification of critical illness are. It then examines the implications of early detection and its quantification.
CONCEPTUAL FRAMEWORK
We define severe illness as the presence of acute disease such that a person can no longer expect to improve without dedicated hospital treatment but which is not inevitably associated with mortality, postdischarge morbidity, or major loss of autonomy. In contrast, we define critical illness as acute disease with high a priori risk of mortality, postdischarge morbidity, and major (possibly total) loss of autonomy. We accept that the boundaries between ordinary illness, severe illness, and critical illness are blurred. The basic assumption behind all efforts at early detection is that these edges can be made sharp, and that the knowledge base required to do so can also lead to improvements in treatment protocols and patient outcomes. Further, it is assumed that at least some forms of critical illness can be prevented or mitigated by earlier detection, identification, and treatment.
Research over the last 2 decades has provided important support for this intuitive view as well as making it more nuanced. With respect to epidemiology, the big news is that sepsis is the biggest culprit, and that it accounts for a substantial proportion of all hospital deaths, including many previously considered unexpected hospital deaths due to in‐hospital deterioration.[1] With respect to treatment, a number of studies have demonstrated that crucial therapies previously considered to be intensive care unit (ICU) therapies can be initiated in the emergency department or general medicalsurgical ward.[2]
Figure 1 shows an idealized framework for illness presenting in the emergency department or general medicalsurgical wards. It illustrates the notion that a transition period exists when patients may be rescued with less intense therapy than will be required when condition progression occurs. Once a certain threshold is crossed, the risk of death or major postdischarge morbidity rises exponentially. Unaided human cognition's ability to determine where a given patient is in this continuum is dangerously variable and is highly dependent on the individuals training and experience. Consequently, as described in several of the articles in this issue as well as multiple other publications, health systems are employing comprehensive electronic medical records (EMRs) and are migrating to algorithmic approaches that combine multiple types of patient data.[3, 4] Although we are still some distance from being able to define exact boundaries between illness, severe illness, and critical illness, current EMRs permit much better definition of patient states, care processes, and short‐term outcomes.

Whereas our ability to quantify many processes and short‐term outcomes is expanding rapidly, quantification of the possible benefit of early detection is complicated by the fact that, even in the best of circumstances, not all patients can be rescued. For some patients, rescue may be temporary, raising the prospect of repeated episodes of critical illness and prolonged intensive care without any hope of leaving the hospital. Figure 2 shows that, for these patients, the problem is no longer simply one of preventing death and preserving function but, rather, preserving autonomy and dignity. In this context, early detection means earlier specification of patient preferences.[5, 6]

JUST WHAT CONSTITUTES EARLY DETECTION (AND HOW DO WE QUANTIFY IT)?
RRTs arose as the result of a number of studies showing thatin retrospectin‐hospital deteriorations should not have been unexpected. Given comprehensive inpatient EMRs, it is now possible to develop more rigorous definitions. A minimum set of parameters that one would need to specify for proper quantification of early detection is shown on Figure 3. The first is specifying a T0, that is, the moment when a prediction regarding event X (which needs to be defined) is issued. This is different from the (currently unmeasurable) biologic onset of illness as well as the first documented indication that critical illness was present. Further, it is important to be explicit about the event time frame (the time period during which a predicted event is expected to occur): we are predicting that X will occur within E hours of the T0. The time frame between the T0 and X, which we are referring to as lead time, is clinically very important, as it represents the time period during which the response arm (eg, RRT intervention) is to be instituted. Statistical approaches can be used to estimate it, but once an early detection system is in place, it can be quantified. Figure 3 is not restricted to electronic systems; all components shown can be and are used by unaided human cognition.


It is essential to specify what data are used to generate probability estimates as well as the time frames used, which we refer to as the look‐back time frames. Several types of data could be employed, with some data elements (eg, age or gender) being discrete data with a 1:1 fixed correspondence between the patient and the data. Other data have a many‐to‐1 relationship, and an exact look‐back time frame must be specified for each data type. For example, it seems reasonable to specify a short (1224 hours) look‐back period for some types of data (eg, vital signs, lactate, admission diagnosis or chief complaint), an intermediate time period (13 days) for information on the current encounter, and a longer (months to years) time period for preexisting illness or comorbidity burden.
Because many events are rare, traditional measures used to assess model performance, such as the area under the receiver operator characteristic curve (C statistic), are not as helpful.[7] Consequently, much more emphasis needs to be given to 2 key metrics: number needed to evaluate (or workup to detection ratio) and threshold‐specific sensitivity (ability of the alert to detect X at a given threshold). With these, one can answer 3 questions that will be asked by the physicians and nurses who are not likely to be researchers, and who will have little interest in the statistics: How many patients do I need to work up each day? How many patients will I need to work up for each possible outcome identified? For this amount of work, how many of the possible outcomes will we catch?
Data availability for the study of severe and critical illness continues to expand. Practically, this means that future research will require more nuanced ontologies for the classification of physiologic derangement. Current approaches to severity scoring (collapsing data into composite scores) need to be replaced by dynamic approaches that consider differential effects on organ systems as well as what can be measured. Severity scoring will also need to incorporate the rate of change of a score (or probability derived from a score) in predicting the occurrence of an event of interest as well as judging response to treatment. Thus, instead of at time of ICU admission, the patient had a severity score of 76, we may have although this patient's severity score at the time of admission was decreasing by 4 points per hour per 10 mL/kg fluid given, the probability for respiratory instability was increasing by 2.3% per hour given 3 L/min supplemental oxygen. This approach is concordant with work done in other clinical settings (eg, in addition to an absolute value of maximal negative inspiratory pressure or vital capacity, the rate of deterioration of neuromuscular weakness in Guillain‐Barr syndrome is also important in predicting respiratory failure[8]).
Electronic data also could permit better definition of patient preferences regarding escalation of care. At present, available electronic data are limited (primarily, orders such as do not resuscitate).[9] However, this EMR domain is gradually expanding.[10, 11] Entities such as the National Institutes of Health could develop sophisticated and rapid questionnaires around patient preferences that are similar to those developed for the Patient Reported Outcomes Measurement Information System.[12] Such tools could have a significant effect on our ability to quantify the benefits of early detection as it relates to a patient's preferences (including better delineation of what treatments they would and would not want).
ACTIVATING A RESPONSE ARM
Early identification, antibiotic administration, fluid resuscitation, and source control are now widely felt to constitute low‐hanging fruit for decreasing morbidity and mortality in severe sepsis. All these measures are included in quality improvement programs and sepsis bundles.[13, 14, 15] However, before early interventions can be instituted, sepsis must at least be suspected, hence the need for early detection. The situation with respect to patient deterioration (for reasons other than sepsis) in general medical surgical wards is less clear‐cut. Reasons for deterioration are much more heterogenous and, consequently, early detection is likely necessary but not sufficient for outcomes improvement.
The 2 projects described in this issue describe nonspecific (indicating elevated risk but not specifying what led to the elevation of risk) and sepsis‐specific alerting systems. In the case of the nonspecific system, detection may not lead to an immediate deployment of a response arm. Instead, a secondary evaluation process must be triggered first. Following this evaluation component, a response arm may or may not be required. In contrast, the sepsis‐specific project essentially transforms the general medicalsurgical ward into a screening system. This screening system then also triggers specific bundle components.
Neither of these systems relies on unaided human cognition. In the case of the nonspecific system, a complex equation generates a probability that is displayed in the EMR, with protocols specifying what actions are to be taken when that probability exceeds a prespecified threshold. With respect to the sepsis screening system, clinicians are supported by EMR alerts as well as protocols that increase nursing autonomy when sepsis is suspected.
The distinction between nonspecific (eg, acute respiratory failure or hemodynamic deterioration) and specific (eg, severe sepsis) alerting systems is likely to disappear as advances in the field occur. For example, incorporation of natural language processing would permit inclusion of semantic data, which could be processed so as to prebucket an alert into one that not just gave a probability, but also a likely cause for the elevated probability.
In addition, both types of systems suffer from the limitation of working off a limited database because, in general, current textbooks and training programs primary focus remains that of treatment of full‐blown clinical syndromes. For example, little is known about how one should manage patients with intermediate lactate values, despite evidence showing that a significant percentage of patients who die from sepsis will initially have such values, with 1 study showing 63% as many deaths with initial lactate of 2.5 to 4.0 mmol/L as occurred with an initial lactate of >4.0 mmol/L.[16] Lastly, as is discussed below, both systems will encounter similar problems when it comes to quantifying benefit.
QUANTIFYING BENEFIT
Whereas the notion of deploying RRTs has clearly been successful, success in demonstrating unequivocal benefit remains elusive.[17, 18, 19] Outcome measures vary dramatically across studies and have included the number of RRT calls, decreases in code blue events on the ward, and decreases in inpatient mortality.[20] We suspect that other reasons are behind this problem. First is the lack of adequate risk adjustment and ignoring the impact of patients near the end of life on the denominator. Figure 4 shows recent data from 21 Kaiser Permanente Northern California (KPNC) hospitals, which can now capture care directive orders electronically,[21] illustrates this problem. The majority (53%) of hospital deaths occur among a highly variable proportion (range across hospitals, 6.5%18.0%) of patients who arrive at the hospital with a restricted resuscitation preference (do not resuscitate, partial code, and comfort care only). These patients do not want to die or crash and burn but, were they to trigger an alert, they would not necessarily want to be rescued by being transferred to the ICU either; moreover, internal KPNC analyses show that large numbers of these patients have sepsis and refuse aggressive treatment. The second major confounder is that ICUs save lives. Consequently, although early detection could lead to fewer transfers to the ICU, using the end point of ICU admission is very problematic, because in many cases the goal of alerting systems should be to get patients to the ICU sooner, which would not affect the outcome of transfer to the ICU in a downward direction; in fact, such systems might increase transfer to the ICU.
The complexities summarized in Figure 4 mean that it is likely that formal quantification of benefit will require examination of multiple measures, including balancing measures as described below. It is also evident that, in this respectlack of agreement as to what constitutes a good outcomethe issues being faced here are a reflection of a broader area of disagreement within our profession and society at large that extends to medical conditions other than critical illness.
POTENTIAL HARMS OF EARLY DETECTION
Implementation of early detection and rapid response systems are not inherently free of harm. If these systems are not shown to have benefit, then the cost of operating them is moving resources away from other, possibly evidence‐based, interventions.[22] At the individual level, alerts could frighten patients and their families (for example, some people are very uncomfortable with the idea that one can predict events). Physicians and nurses who work in the hospital are already quite busy, so every time an alert is issued, it adds to the demand on their already limited time, hence, the critical importance of strategies to minimize false alarms and alert fatigue. Moreover, altering existing workflows can be disruptive and unpopular.
A potentially more quantifiable problem is the impact of early detection systems on ICU operations. For example, if an RRT decides to transfer a patient from the ward to the ICU as a preventive measure (soft landing) and this in turn ties up an ICU bed, that bed is then unavailable for a new patient in the emergency department. Similarly, early detection systems coupled with structured protocols for promoting soft landings could result in a change in ICU case mix, with greater patient flow due to increased numbers of patients with lower severity and lower ICU length of stay. These considerations suggest the need to couple early detection with other supportive data systems and workflows (eg, systems that monitor bed capacity proactively).
Lastly, if documentation protocols are not established and followed, early detection systems could expose both individual clinicians as well as healthcare institutions to medicallegal risk. This consideration could be particularly important in those instances where an alert is issued and, for whatever reasons, clinicians do not take action and do not document that decision. At present, early detection systems are relatively uncommon, but they may gradually become standard of care. This means that in‐house out of ICU deteriorations, which are generally considered to be bad luck or due to a specific error or oversight, may then be considered to be preventable. Another possible scenario that could arise is that of plaintiffs invoking enterprise liability, where a hospital's not having an early detection system becomes considered negligent.
ARTICLES IN THIS ISSUE
In this issue of the Journal of Hospital Medicine, we examine early detection from various perspectives but around a common theme that usually gets less attention in the academic literature: implementation. The article by Schorr et al.[23] describes a disease‐specific approach that can be instantiated using either electronic or paper tools. Escobar et al.[24] describe the quantitative as well as the electronic architecture of an early warning system (EWS) pilot at 2 hospitals that are part of an integrated healthcare delivery system. Dummett et al.[25] then show how a clinical rescue component was developed to take advantage of the EWS, whereas Granich et al.[26] describe the complementary component (integration of supportive care and ensuring that patient preferences are respected). The paper by Liu et al.[27] concludes by placing all of this work in a much broader context, that of the learning healthcare system.
FUTURE DIRECTIONS: KEY GAPS IN THE FIELD
Important gaps remain with respect to early detection and response systems. Future research will need to focus on a number of areas. First and foremost, better approaches to quantifying the costbenefit relationships of these systems are needed; somehow, we need to move beyond a purely intuitive sense that they are good things. Related to this is the need to establish metrics that would permit rigorous comparisons between different approaches; this work needs to go beyond simple comparisons of the statistical characteristics of different predictive models. Ideally, it should include comparisons of different approaches for the response arms as well. We also need to characterize clinician understanding about detection systems, what constitutes impending or incipient critical illness, and the optimum way to provide early detection. Finally, better approaches to integrating health services research with basic science work must be developed; for example, how should one test new biomarkers in settings with early detection and response systems?
The most important frontier, however, is how one can make early detection and response systems more patient centered and how one can enhance their ability to respect patient preferences. Developing systems to improve clinical management is laudable, but somehow we need to also find ways to have these systems make a better connection to what patients want most and what matters most to them, something that may need to include new ways that sometimes suspend use of these systems. At the end of the day, after early detection, patients must have a care experience that they see as an unequivocal improvement.
Acknowledgements
The authors thank our 2 foundation program officers, Dr. Marybeth Sharpe and Ms. Kate Weiland, for their administrative support and encouragement. The authors also thank Dr. Tracy Lieu, Dr. Michelle Caughey, Dr. Philip Madvig, and Ms. Barbara Crawford for their administrative assistance, Dr. Vincent Liu for comments on the manuscript, and Ms. Rachel Lesser for her help with formatting the manuscript and figures.
Disclosures
This work was supported by the Gordon and Betty Moore Foundation, The Permanente Medical Group, Inc., and Kaiser Foundation Hospitals, Inc. As part of our agreement with the Moore Foundation, we made a commitment to disseminate our findings in articles such as this one. However, the Gordon and Betty Moore Foundation and its staff played no role in how we actually structured our articles, nor did they review or preapprove any of the manuscripts submitted as part of the dissemination component. None of the authors has any conflicts of interest to declare of relevance to this work.
This issue of the Journal of Hospital Medicine describes 2 research and quality improvement demonstration projects funded by the Gordon and Betty Moore Foundation. Early detection is central to both projects. This introductory article does not provide a global review of the now voluminous literature on rapid response teams (RRTs), sepsis detection systems, or treatment protocols. Rather, it takes a step back and reassesses just what early detection and quantification of critical illness are. It then examines the implications of early detection and its quantification.
CONCEPTUAL FRAMEWORK
We define severe illness as the presence of acute disease such that a person can no longer expect to improve without dedicated hospital treatment but which is not inevitably associated with mortality, postdischarge morbidity, or major loss of autonomy. In contrast, we define critical illness as acute disease with high a priori risk of mortality, postdischarge morbidity, and major (possibly total) loss of autonomy. We accept that the boundaries between ordinary illness, severe illness, and critical illness are blurred. The basic assumption behind all efforts at early detection is that these edges can be made sharp, and that the knowledge base required to do so can also lead to improvements in treatment protocols and patient outcomes. Further, it is assumed that at least some forms of critical illness can be prevented or mitigated by earlier detection, identification, and treatment.
Research over the last 2 decades has provided important support for this intuitive view as well as making it more nuanced. With respect to epidemiology, the big news is that sepsis is the biggest culprit, and that it accounts for a substantial proportion of all hospital deaths, including many previously considered unexpected hospital deaths due to in‐hospital deterioration.[1] With respect to treatment, a number of studies have demonstrated that crucial therapies previously considered to be intensive care unit (ICU) therapies can be initiated in the emergency department or general medicalsurgical ward.[2]
Figure 1 shows an idealized framework for illness presenting in the emergency department or general medicalsurgical wards. It illustrates the notion that a transition period exists when patients may be rescued with less intense therapy than will be required when condition progression occurs. Once a certain threshold is crossed, the risk of death or major postdischarge morbidity rises exponentially. Unaided human cognition's ability to determine where a given patient is in this continuum is dangerously variable and is highly dependent on the individuals training and experience. Consequently, as described in several of the articles in this issue as well as multiple other publications, health systems are employing comprehensive electronic medical records (EMRs) and are migrating to algorithmic approaches that combine multiple types of patient data.[3, 4] Although we are still some distance from being able to define exact boundaries between illness, severe illness, and critical illness, current EMRs permit much better definition of patient states, care processes, and short‐term outcomes.

Whereas our ability to quantify many processes and short‐term outcomes is expanding rapidly, quantification of the possible benefit of early detection is complicated by the fact that, even in the best of circumstances, not all patients can be rescued. For some patients, rescue may be temporary, raising the prospect of repeated episodes of critical illness and prolonged intensive care without any hope of leaving the hospital. Figure 2 shows that, for these patients, the problem is no longer simply one of preventing death and preserving function but, rather, preserving autonomy and dignity. In this context, early detection means earlier specification of patient preferences.[5, 6]

JUST WHAT CONSTITUTES EARLY DETECTION (AND HOW DO WE QUANTIFY IT)?
RRTs arose as the result of a number of studies showing thatin retrospectin‐hospital deteriorations should not have been unexpected. Given comprehensive inpatient EMRs, it is now possible to develop more rigorous definitions. A minimum set of parameters that one would need to specify for proper quantification of early detection is shown on Figure 3. The first is specifying a T0, that is, the moment when a prediction regarding event X (which needs to be defined) is issued. This is different from the (currently unmeasurable) biologic onset of illness as well as the first documented indication that critical illness was present. Further, it is important to be explicit about the event time frame (the time period during which a predicted event is expected to occur): we are predicting that X will occur within E hours of the T0. The time frame between the T0 and X, which we are referring to as lead time, is clinically very important, as it represents the time period during which the response arm (eg, RRT intervention) is to be instituted. Statistical approaches can be used to estimate it, but once an early detection system is in place, it can be quantified. Figure 3 is not restricted to electronic systems; all components shown can be and are used by unaided human cognition.


It is essential to specify what data are used to generate probability estimates as well as the time frames used, which we refer to as the look‐back time frames. Several types of data could be employed, with some data elements (eg, age or gender) being discrete data with a 1:1 fixed correspondence between the patient and the data. Other data have a many‐to‐1 relationship, and an exact look‐back time frame must be specified for each data type. For example, it seems reasonable to specify a short (1224 hours) look‐back period for some types of data (eg, vital signs, lactate, admission diagnosis or chief complaint), an intermediate time period (13 days) for information on the current encounter, and a longer (months to years) time period for preexisting illness or comorbidity burden.
Because many events are rare, traditional measures used to assess model performance, such as the area under the receiver operator characteristic curve (C statistic), are not as helpful.[7] Consequently, much more emphasis needs to be given to 2 key metrics: number needed to evaluate (or workup to detection ratio) and threshold‐specific sensitivity (ability of the alert to detect X at a given threshold). With these, one can answer 3 questions that will be asked by the physicians and nurses who are not likely to be researchers, and who will have little interest in the statistics: How many patients do I need to work up each day? How many patients will I need to work up for each possible outcome identified? For this amount of work, how many of the possible outcomes will we catch?
Data availability for the study of severe and critical illness continues to expand. Practically, this means that future research will require more nuanced ontologies for the classification of physiologic derangement. Current approaches to severity scoring (collapsing data into composite scores) need to be replaced by dynamic approaches that consider differential effects on organ systems as well as what can be measured. Severity scoring will also need to incorporate the rate of change of a score (or probability derived from a score) in predicting the occurrence of an event of interest as well as judging response to treatment. Thus, instead of at time of ICU admission, the patient had a severity score of 76, we may have although this patient's severity score at the time of admission was decreasing by 4 points per hour per 10 mL/kg fluid given, the probability for respiratory instability was increasing by 2.3% per hour given 3 L/min supplemental oxygen. This approach is concordant with work done in other clinical settings (eg, in addition to an absolute value of maximal negative inspiratory pressure or vital capacity, the rate of deterioration of neuromuscular weakness in Guillain‐Barr syndrome is also important in predicting respiratory failure[8]).
Electronic data also could permit better definition of patient preferences regarding escalation of care. At present, available electronic data are limited (primarily, orders such as do not resuscitate).[9] However, this EMR domain is gradually expanding.[10, 11] Entities such as the National Institutes of Health could develop sophisticated and rapid questionnaires around patient preferences that are similar to those developed for the Patient Reported Outcomes Measurement Information System.[12] Such tools could have a significant effect on our ability to quantify the benefits of early detection as it relates to a patient's preferences (including better delineation of what treatments they would and would not want).
ACTIVATING A RESPONSE ARM
Early identification, antibiotic administration, fluid resuscitation, and source control are now widely felt to constitute low‐hanging fruit for decreasing morbidity and mortality in severe sepsis. All these measures are included in quality improvement programs and sepsis bundles.[13, 14, 15] However, before early interventions can be instituted, sepsis must at least be suspected, hence the need for early detection. The situation with respect to patient deterioration (for reasons other than sepsis) in general medical surgical wards is less clear‐cut. Reasons for deterioration are much more heterogenous and, consequently, early detection is likely necessary but not sufficient for outcomes improvement.
The 2 projects described in this issue describe nonspecific (indicating elevated risk but not specifying what led to the elevation of risk) and sepsis‐specific alerting systems. In the case of the nonspecific system, detection may not lead to an immediate deployment of a response arm. Instead, a secondary evaluation process must be triggered first. Following this evaluation component, a response arm may or may not be required. In contrast, the sepsis‐specific project essentially transforms the general medicalsurgical ward into a screening system. This screening system then also triggers specific bundle components.
Neither of these systems relies on unaided human cognition. In the case of the nonspecific system, a complex equation generates a probability that is displayed in the EMR, with protocols specifying what actions are to be taken when that probability exceeds a prespecified threshold. With respect to the sepsis screening system, clinicians are supported by EMR alerts as well as protocols that increase nursing autonomy when sepsis is suspected.
The distinction between nonspecific (eg, acute respiratory failure or hemodynamic deterioration) and specific (eg, severe sepsis) alerting systems is likely to disappear as advances in the field occur. For example, incorporation of natural language processing would permit inclusion of semantic data, which could be processed so as to prebucket an alert into one that not just gave a probability, but also a likely cause for the elevated probability.
In addition, both types of systems suffer from the limitation of working off a limited database because, in general, current textbooks and training programs primary focus remains that of treatment of full‐blown clinical syndromes. For example, little is known about how one should manage patients with intermediate lactate values, despite evidence showing that a significant percentage of patients who die from sepsis will initially have such values, with 1 study showing 63% as many deaths with initial lactate of 2.5 to 4.0 mmol/L as occurred with an initial lactate of >4.0 mmol/L.[16] Lastly, as is discussed below, both systems will encounter similar problems when it comes to quantifying benefit.
QUANTIFYING BENEFIT
Whereas the notion of deploying RRTs has clearly been successful, success in demonstrating unequivocal benefit remains elusive.[17, 18, 19] Outcome measures vary dramatically across studies and have included the number of RRT calls, decreases in code blue events on the ward, and decreases in inpatient mortality.[20] We suspect that other reasons are behind this problem. First is the lack of adequate risk adjustment and ignoring the impact of patients near the end of life on the denominator. Figure 4 shows recent data from 21 Kaiser Permanente Northern California (KPNC) hospitals, which can now capture care directive orders electronically,[21] illustrates this problem. The majority (53%) of hospital deaths occur among a highly variable proportion (range across hospitals, 6.5%18.0%) of patients who arrive at the hospital with a restricted resuscitation preference (do not resuscitate, partial code, and comfort care only). These patients do not want to die or crash and burn but, were they to trigger an alert, they would not necessarily want to be rescued by being transferred to the ICU either; moreover, internal KPNC analyses show that large numbers of these patients have sepsis and refuse aggressive treatment. The second major confounder is that ICUs save lives. Consequently, although early detection could lead to fewer transfers to the ICU, using the end point of ICU admission is very problematic, because in many cases the goal of alerting systems should be to get patients to the ICU sooner, which would not affect the outcome of transfer to the ICU in a downward direction; in fact, such systems might increase transfer to the ICU.
The complexities summarized in Figure 4 mean that it is likely that formal quantification of benefit will require examination of multiple measures, including balancing measures as described below. It is also evident that, in this respectlack of agreement as to what constitutes a good outcomethe issues being faced here are a reflection of a broader area of disagreement within our profession and society at large that extends to medical conditions other than critical illness.
POTENTIAL HARMS OF EARLY DETECTION
Implementation of early detection and rapid response systems are not inherently free of harm. If these systems are not shown to have benefit, then the cost of operating them is moving resources away from other, possibly evidence‐based, interventions.[22] At the individual level, alerts could frighten patients and their families (for example, some people are very uncomfortable with the idea that one can predict events). Physicians and nurses who work in the hospital are already quite busy, so every time an alert is issued, it adds to the demand on their already limited time, hence, the critical importance of strategies to minimize false alarms and alert fatigue. Moreover, altering existing workflows can be disruptive and unpopular.
A potentially more quantifiable problem is the impact of early detection systems on ICU operations. For example, if an RRT decides to transfer a patient from the ward to the ICU as a preventive measure (soft landing) and this in turn ties up an ICU bed, that bed is then unavailable for a new patient in the emergency department. Similarly, early detection systems coupled with structured protocols for promoting soft landings could result in a change in ICU case mix, with greater patient flow due to increased numbers of patients with lower severity and lower ICU length of stay. These considerations suggest the need to couple early detection with other supportive data systems and workflows (eg, systems that monitor bed capacity proactively).
Lastly, if documentation protocols are not established and followed, early detection systems could expose both individual clinicians as well as healthcare institutions to medicallegal risk. This consideration could be particularly important in those instances where an alert is issued and, for whatever reasons, clinicians do not take action and do not document that decision. At present, early detection systems are relatively uncommon, but they may gradually become standard of care. This means that in‐house out of ICU deteriorations, which are generally considered to be bad luck or due to a specific error or oversight, may then be considered to be preventable. Another possible scenario that could arise is that of plaintiffs invoking enterprise liability, where a hospital's not having an early detection system becomes considered negligent.
ARTICLES IN THIS ISSUE
In this issue of the Journal of Hospital Medicine, we examine early detection from various perspectives but around a common theme that usually gets less attention in the academic literature: implementation. The article by Schorr et al.[23] describes a disease‐specific approach that can be instantiated using either electronic or paper tools. Escobar et al.[24] describe the quantitative as well as the electronic architecture of an early warning system (EWS) pilot at 2 hospitals that are part of an integrated healthcare delivery system. Dummett et al.[25] then show how a clinical rescue component was developed to take advantage of the EWS, whereas Granich et al.[26] describe the complementary component (integration of supportive care and ensuring that patient preferences are respected). The paper by Liu et al.[27] concludes by placing all of this work in a much broader context, that of the learning healthcare system.
FUTURE DIRECTIONS: KEY GAPS IN THE FIELD
Important gaps remain with respect to early detection and response systems. Future research will need to focus on a number of areas. First and foremost, better approaches to quantifying the costbenefit relationships of these systems are needed; somehow, we need to move beyond a purely intuitive sense that they are good things. Related to this is the need to establish metrics that would permit rigorous comparisons between different approaches; this work needs to go beyond simple comparisons of the statistical characteristics of different predictive models. Ideally, it should include comparisons of different approaches for the response arms as well. We also need to characterize clinician understanding about detection systems, what constitutes impending or incipient critical illness, and the optimum way to provide early detection. Finally, better approaches to integrating health services research with basic science work must be developed; for example, how should one test new biomarkers in settings with early detection and response systems?
The most important frontier, however, is how one can make early detection and response systems more patient centered and how one can enhance their ability to respect patient preferences. Developing systems to improve clinical management is laudable, but somehow we need to also find ways to have these systems make a better connection to what patients want most and what matters most to them, something that may need to include new ways that sometimes suspend use of these systems. At the end of the day, after early detection, patients must have a care experience that they see as an unequivocal improvement.
Acknowledgements
The authors thank our 2 foundation program officers, Dr. Marybeth Sharpe and Ms. Kate Weiland, for their administrative support and encouragement. The authors also thank Dr. Tracy Lieu, Dr. Michelle Caughey, Dr. Philip Madvig, and Ms. Barbara Crawford for their administrative assistance, Dr. Vincent Liu for comments on the manuscript, and Ms. Rachel Lesser for her help with formatting the manuscript and figures.
Disclosures
This work was supported by the Gordon and Betty Moore Foundation, The Permanente Medical Group, Inc., and Kaiser Foundation Hospitals, Inc. As part of our agreement with the Moore Foundation, we made a commitment to disseminate our findings in articles such as this one. However, the Gordon and Betty Moore Foundation and its staff played no role in how we actually structured our articles, nor did they review or preapprove any of the manuscripts submitted as part of the dissemination component. None of the authors has any conflicts of interest to declare of relevance to this work.
- , , , Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief. 2011(62):1–8.
- , , , et al. Surviving sepsis campaign: association between performance metrics and outcomes in a 7.5‐year study. Crit Care Med. 2015;43(1):3–12.
- , , , , , Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388–395.
- , , , et al. A randomized trial of real‐time automated clinical deterioration alerts sent to a rapid response team. J Hosp Med. 2014;9(7):424–429.
- , , , , , Enhanced end‐of‐life care associated with deploying a rapid response team: a pilot study. J Hosp Med. 2009;4(7):449–452.
- , , , , The medical emergency team call: a sentinel event that triggers goals of care discussion. Crit Care Med. 2014;42(2):322–327.
- , , , Why the C‐statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19:285.
- , , , , Anticipating mechanical ventilation in Guillain‐Barre syndrome. Arch Neurol. 2001;58(6):893–898.
- , , , , , The natural history of changes in preferences for life‐sustaining treatments and implications for inpatient mortality in younger and older hospitalized adults. J Am Geriatr Soc. 2016;64(5):981–989.
- , Remote collection of questionnaires. Clin Exp Rheumatol. 2014;32(5 suppl 85):S168–S172.
- Be prepared to make your health care wishes known. Health care directives. Allina Health website. Available at: http://www.allinahealth.org/Customer-Service/Be-prepared/Be-prepared-to-make-your-health-care-wishes-known. Accessed January 1, 2015.
- Patient Reported Outcomes Measurement Information System. Dynamic tools to measure health outcomes from the patient perspective. Available at: http://www.nihpromis.org. Accessed January 15, 2015.
- , , , , , Methodology of the surviving sepsis campaign global initiative for improving care of the patient with severe sepsis. Minerva Anestesiol. 2009;75(suppl 1):23–27.
- , , The Surviving Sepsis Campaign: a history and a perspective. Surg Infect (Larchmt). 2010;11(3):275–281.
- , The Surviving Sepsis Campaign: past, present and future. Trends Mol Med. 2014;20(4):192–194.
- , , , et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45(5):524–528.
- , , , et al. Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a Children's Hospital. JAMA. 2007;298(19):2267–2274.
- , , , , , Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387–390.
- , Rapid response teams: qualitative analysis of their effectiveness. Am J Crit Care. 2013;22(3):198–210.
- , , , , , Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300(21):2506–2513.
- , , , , Risk‐adjusting hospital mortality using a comprehensive electronic record in an integrated healthcare delivery system. Med Care. 2013;51(5):446–453.
- , , Rapid response teams—walk, don't run. JAMA. 2006;296(13):1645–1647.
- Schorr et al. J Hosp Med. 2016;11:000–000.
- , , , et al. Piloting electronic medical record–based early detection of inpatient deterioration in community hospitals. J Hosp Med. 2016;11:000–000.
- Dummett et al. J Hosp Med. 2016;11:000–000.
- Granich et al. J Hosp Med. 2016;11:000–000.
- Liu et al. , , , , , Data that drive: closing the loop in the learning hospital system. J Hosp Med. 2016;11:000–000.
- , , , Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief. 2011(62):1–8.
- , , , et al. Surviving sepsis campaign: association between performance metrics and outcomes in a 7.5‐year study. Crit Care Med. 2015;43(1):3–12.
- , , , , , Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388–395.
- , , , et al. A randomized trial of real‐time automated clinical deterioration alerts sent to a rapid response team. J Hosp Med. 2014;9(7):424–429.
- , , , , , Enhanced end‐of‐life care associated with deploying a rapid response team: a pilot study. J Hosp Med. 2009;4(7):449–452.
- , , , , The medical emergency team call: a sentinel event that triggers goals of care discussion. Crit Care Med. 2014;42(2):322–327.
- , , , Why the C‐statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19:285.
- , , , , Anticipating mechanical ventilation in Guillain‐Barre syndrome. Arch Neurol. 2001;58(6):893–898.
- , , , , , The natural history of changes in preferences for life‐sustaining treatments and implications for inpatient mortality in younger and older hospitalized adults. J Am Geriatr Soc. 2016;64(5):981–989.
- , Remote collection of questionnaires. Clin Exp Rheumatol. 2014;32(5 suppl 85):S168–S172.
- Be prepared to make your health care wishes known. Health care directives. Allina Health website. Available at: http://www.allinahealth.org/Customer-Service/Be-prepared/Be-prepared-to-make-your-health-care-wishes-known. Accessed January 1, 2015.
- Patient Reported Outcomes Measurement Information System. Dynamic tools to measure health outcomes from the patient perspective. Available at: http://www.nihpromis.org. Accessed January 15, 2015.
- , , , , , Methodology of the surviving sepsis campaign global initiative for improving care of the patient with severe sepsis. Minerva Anestesiol. 2009;75(suppl 1):23–27.
- , , The Surviving Sepsis Campaign: a history and a perspective. Surg Infect (Larchmt). 2010;11(3):275–281.
- , The Surviving Sepsis Campaign: past, present and future. Trends Mol Med. 2014;20(4):192–194.
- , , , et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45(5):524–528.
- , , , et al. Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a Children's Hospital. JAMA. 2007;298(19):2267–2274.
- , , , , , Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387–390.
- , Rapid response teams: qualitative analysis of their effectiveness. Am J Crit Care. 2013;22(3):198–210.
- , , , , , Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300(21):2506–2513.
- , , , , Risk‐adjusting hospital mortality using a comprehensive electronic record in an integrated healthcare delivery system. Med Care. 2013;51(5):446–453.
- , , Rapid response teams—walk, don't run. JAMA. 2006;296(13):1645–1647.
- Schorr et al. J Hosp Med. 2016;11:000–000.
- , , , et al. Piloting electronic medical record–based early detection of inpatient deterioration in community hospitals. J Hosp Med. 2016;11:000–000.
- Dummett et al. J Hosp Med. 2016;11:000–000.
- Granich et al. J Hosp Med. 2016;11:000–000.
- Liu et al. , , , , , Data that drive: closing the loop in the learning hospital system. J Hosp Med. 2016;11:000–000.
The Surviving Sepsis Campaign
The incidence of severe sepsis (sepsis with organ dysfunction) is increasing.1 The initial diagnosis and management of severe sepsis may occur in the ED, the ICU, or the hospital ward.
Several recently published studies have demonstrated decreased mortality and morbidity as a result of interventions and therapeutics applied to patients with sepsis.2-5 These new data, resulting from rigorously performed, randomized controlled trials, combined with previous data for beneficial interventions not specific to sepsis management (such as DVT and stress ulcer prophylaxis) and consensus opinion where no evidence exists lend significant weight to the belief that critical care clinicians can now significantly reduce mortality in patients with severe sepsis and septic shock.6-9
Protocolized care now exists for heart attack and stroke, which is based on recent advances as demonstrated by the medical literature. Until now there has been no attempt to reproduce such an approach in severe sepsis. The Surviving Sepsis Campaign hopes to change that.
The Surviving Sepsis Campaign is administered by the Society of Critical Care Medicine (SCCM), the European Society of Intensive Care Medicine (ESICM), and the International Sepsis Forum (ISF) and is open to all industry for funding through unrestricted educational grants. Contributors to date include Baxter, Edwards, and Eli Lilly.
The first phase was the introduction of the campaign at several major international critical care medicine conferences, the ESICM meeting in Barcelona in 2002, and the SCCM meeting in 2003. The stated goal of the campaign is to decrease the mortality from severe sepsis by 25% in five years.
Phase 2 of the campaign was aimed at producing guidelines for the management of sepsis. In 2003, critical care and infectious disease experts representing 11 international organizations developed evidence-based management guidelines for severe sepsis and septic shock for practical use for the bedside clinician, under the auspices of the Surviving Sepsis Campaign.
Pediatric considerations were provided to contrast adult and pediatric management. The resulting recommendations represent an attempt to facilitate a rapid change in the standard of care for management of sepsis, based on the quality of available published data and expert opinion where no literature guidance is available. The guidelines manuscript was published in both Critical Care Medicine and Intensive Care Medicine.10,11 The publication of this manuscript represents an historic step for critical care worldwide. These guidelines represent an international consensus on the best available standard for management of sepsis.
Key Recommendations
Key recommendations (listed by category and not by hierarchy) include:
- Early goal-directed resuscitation of the septic patient during the first six hours after recognition;
- Appropriate diagnostic studies to ascertain causative organisms before starting antibiotics;
- Early administration of broad-spectrum antibiotic therapy;
- Reassessment of antibiotic therapy with microbiology and clinical data to narrow coverage, when appropriate;
- A usual seven to 10 days of antibiotic therapy guided by clinical response;
- Source control with attention to the method that balances risks and benefits;
- Equivalence of crystalloid and colloid resuscitation;
- Aggressive fluid challenge to restore mean circulating filling pressure;
- Vasopressor preference for norepinephrine and dopamine;
- Cautious use of vasopressin pending further studies;
- Avoidance of low-dose dopamine administration for renal protection;
- Consideration of dobutamine inotropic therapy in some clinical situations;
- Avoidance of supranormal oxygen delivery as a goal of therapy;
- Stress-dose steroid therapy for septic shock;
- Use of recombinant activated protein C in patients with severe sepsis and high risk for death;
- Resolution of tissue hypoperfusion and targeting a hemoglobin of 7-9 g/dL in the absence of coronary artery disease or acute hemorrhage;
- Appropriate use of fresh frozen plasma and platelets;
- A low tidal volume and limitation of inspiratory plateau pressure strategy for acute lung injury and acute respiratory distress syndrome;
- Application of a minimal amount of positive end expiratory pressure in acute lung injury/acute respiratory distress syndrome;
- A semi-recumbent bed position unless contraindicated;
- Protocols for weaning and sedation/analgesia, using either intermittent bolus sedation or continuous infusion sedation with daily interruptions/lightening;
- Avoidance of neuromuscular blockers, if at all possible;
- Maintenance of blood glucose <150 mg/dL after initial stabilization;
- Equivalence of continuous veno-veno hemofiltration (CVVH) and intermittent hemodialysis;
- Lack of utility of bicarbonate use for pH 7.15 or greater;
- Use of DVT/stress ulcer prophylaxis; and
- Consideration of limitation of support where appropriate.
Pediatric considerations include a more likely need for intubation due to low functional residual capacity; more difficult intravenous access; fluid resuscitation based on weight with 40-60 mL/kg or higher needed; decreased cardiac output and increased systemic vascular resistance as the most common hemodynamic profile; greater use of physical examination therapeutic endpoints; unsettled issue of high-dose steroids for therapy of septic shock; and greater risk of hypoglycemia with aggressive glucose control.
Operationalizing the Guidelines
Unfortunately, clinicians change slowly. Historically, transfer of research from the bench to the bedside is a long, tortuous process—one that is not driven by anything clear and that seems to be based more on fad and coincidence than on a keen, evidence-based evaluation of the literature. Phase 3 of the campaign hopes to change that.
Phase 3 of the campaign (www.survivingsepsis.org) aims to operationalize the guidelines to create a global standard of care for sepsis management.12 The guidelines will be transformed into user-friendly tools that allow clinicians to easily incorporate these new recommendations into bedside care. The first step in this next phase has been a joint effort with the Institute of Healthcare Improvement (IHI) to deploy a “change bundle” based on a core set of the previous recommendations into the IHI’s collaborative system. Chart review or concurrent data gathering will identify and track changes in practice and clinical outcomes. Engendering evidence-based change through motivational strategies while monitoring and sharing the results with healthcare practitioners is the key to improving outcomes in severe sepsis.
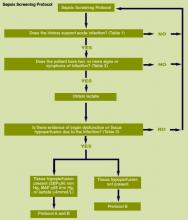
The severe sepsis bundles form the core of the Surviving Sepsis Campaign. A “bundle” is a group of interventions related to a disease process. When executed together, the interventions produce better outcomes than when implemented individually. The individual bundle elements are built on evidence-based practices. The science behind the elements of a bundle is so well established that their implementation should be considered a generally accepted practice. Develop a bundle process in the following way:
- Identify a set of four to six evidence-based interventions that apply to a cohort of patients with a common disease or a common location. An example might be patients with sepsis admitted to the ICU;
- Develop the will in the providers to deliver the interventions every time they are indicated;
- Redesign the delivery system to ensure the interventions in the bundle are delivered; and
- Measure related outcomes to ascertain the effects of the changes in the delivery system.
The sepsis bundles were developed in just such a manner, based on the experience of the ventilator bundle. The goal now is to motivate providers to deliver the sepsis interventions every time they are indicated and measure them in an all-or-nothing way. We believe that if the bundle elements are reliably performed we can achieve the desired outcome of reducing sepsis-related deaths by 25%.
These elements distill the Surviving Sepsis Campaign practice guidelines into a manageable format for use at most institutions. The bundles represent the specific changes the campaign has identified as essential to the care of severely septic patients. Following the severe sepsis bundles will eliminate the piecemeal or inappropriate application of standards for sepsis care that characterize most clinical environments today.
Hospitals should implement two different severe sepsis bundles. Each bundle articulates objectives to be accomplished within specific time frames.
Sepsis Resuscitation Bundle
The severe sepsis resuscitation bundle describes seven tasks that should begin immediately but must be accomplished within the first six hours of presentation for patients with severe sepsis or septic shock. Some items may not be completed if the clinical conditions described in the bundle do not prevail in a particular case, but clinicians must assess for them. The goal is to perform all indicated tasks 100% of the time within the first six hours of identification of severe sepsis. The tasks are:
- Measure serum lactate;
- Obtain blood cultures prior to antibiotic administration;
- Administer broad-spectrum antibiotics within three hours from time of presentation for ED admissions and one hour for non-ED ICU admissions;
- In the event of hypotension and/or lactate >4 mmol/L (36 mg/dL):
- Deliver an initial minimum of 20 ml/kg of crystalloid (or colloid equivalent); and
- Apply vasopressors for hypotension not responding to initial fluid resuscitation to maintain mean arterial pressure (MAP) ≥65 mm Hg;
- In the event of persistent hypotension despite fluid resuscitation (septic shock) and/or lactate > 4 mmol/L (36 mg/dL):
- Achieve central venous pressure (CVP) of ≥8-12 mm Hg; and
- Achieve central venous oxygen saturation (ScvO2) of Surviving Sepsis Campaign70%. (Achieving a mixed venous oxygen saturation (SvO2) of 65% is an acceptable alternative.)
Sepsis Management Bundle
The severe sepsis management bundle lists four management goals. Efforts to accomplish these tasks should also begin immediately, but these items may be completed within 24 hours of presentation for patients with severe sepsis or septic shock.
- Administer low-dose steroids for septic shock in accordance with a standardized ICU policy;
- Administer drotrecogin alfa (activated) in accordance with a standardized ICU policy;
- Maintain glucose control ≥ lower limit of normal, but <150 mg/dL (8.3 mmol/L); and
- Maintain inspiratory plateau pressures <30 cm H2O for mechanically ventilated patients.
Team Effort
To achieve the goal of reducing mortality by 25% by 2008, everyone involved with the care of severe sepsis patients must be included, work processes must be carefully scripted and standardized, and commitment to this effort must be elevated. This must be a team effort that crosses disciplines and departments; it requires leadership, support from the entire organization, and buy-in from all stakeholders involved with the care of these patients.
Three levels of participation exist in creating successful change:
1) Active working teams are responsible for daily planning, documentation, communication, education, monitoring, and evaluation of activities. The working team must have representation from all departments involved in the change processes ICU, ED, pulmonary department, pharmacy, etc. The team should also be multidisciplinary, comprising physicians, nurses, pharmacists, respiratory therapists, and other staff with roles in the specific change process, such as clerks and technicians. Team members should be knowledgeable about the specific aims, the current local work processes, the associated literature, and any environmental issues that will be affected by these changes.
2) A leadership group or person within the team helps remove barriers, provides resources, monitors global progress, and gives suggestions from an institutional perspective. The working team needs someone with authority in the organization to overcome barriers and to allocate the time and resources the team needs to achieve its aim. Leadership needs to understand how the proposed changes will affect various parts of the system and the more remote consequences such changes might trigger.
3) Providers and stakeholders must be kept informed. Procedures are needed to keep them informed, to receive their feedback, and to ensure them that their responses are respected. This gives stakeholders a sense of ownership and facilitates implementation of the new processes.
Protocols
Teams should use the bundles to create customized protocols and pathways that will function well within their institutions. However, all of the elements in the bundles must be incorporated into the protocols. The protocols should mirror the bundles but allow flexibility to accommodate the specific needs of a local hospital. The severe sepsis bundles (and thereby the hospital’s protocol) form the basis for the measurements the team will conduct. If all of the elements of the bundles are not incorporated into your customized protocol, your performance on the measures will suffer.
A strong protocol will accomplish all of the items listed in the severe sepsis bundles. If the protocol designer pays careful attention to the details in the bundles, the protocol will score well on the severe sepsis quality indicators. Hospitals will want to publicize their efforts with regard to improving sepsis care and make the protocol an integral part of their rollout strategy. It is imperative to launch an educational initiative regarding the effort.
Examples of sepsis screening and management protocols are available on the Surviving Sepsis Campaign IHI Web site and are rendered on this page as “Protocol A: Create a protocol and educate users” and as “Prot0col B.” The easiest way to get to that page of the IHI Web site is through the home page link from the Surviving Sepsis Campaign Web site, www.survivingsepsis.org. These highly visual and easy-to-follow pathways exemplify ways to encourage adherence to a protocol. Notice that the “Sepsis Screening Protocol” (p. 25) complies with the terms of the severe sepsis bundles. Posting these types of algorithms prominently in the ED, hospital wards, and ICU, and making them readily available in laminated and PDA format, can have a significant impact on performance improvement programs.
These flow diagrams may be incorporated into lectures and training programs to support your efforts to change care at the bedside. You can adapt the algorithms to fit the needs of your individual institution, but keep in mind the need to comply with the overall structure of the severe sepsis bundles.
Data Collection
Data collection can seem like an onerous duty in any quality improvement project. Nevertheless, it is essential for improvement. Without attention to measurement, how will you know that your efforts are leading to improvement? At most hospitals, the magnitude of the data collection effort will not be huge as it will be relative to the number of severely septic patients cared for in the ICU.
Generally, hospitals report three to four severely septic patients are treated in one week’s time. This means that zero, one, or two severely septic patients’ charts will need to be abstracted each day in an average-size hospital. If abstraction takes between 20–30 minutes per chart, the daily time for this effort may range from 30–90 minutes daily. This relatively small burden is likely to represent an initial challenge to anyone unfamiliar with the organization of the chart and the measurement forms, or tools, used by the Surviving Sepsis Campaign for data collection. In time, however, data collection will become easier as the chart and the tools provided by the Surviving Sepsis Campaign will become more familiar. Bundle implementation and data collection have begun in hospitals throughout Europe, Latin America, the United Kingdom, and the United States.
The measurement tools were created to achieve a uniform system of data gathering, collation, and calculation across hospitals. Without the measurement tools, teams armed with only the concepts in the severe sepsis bundles would need to decide how to gather data from charts and put it in a format consistent with the calculations listed in the severe sepsis quality indicators. If any hospital were to undertake such a task on its own, it would quickly find that its results were not comparable across institutions because scores of other hospitals would have derived their results by entirely different means.
The Surviving Sepsis Campaign aims to make using the measurement tools as easy as possible for those involved in collecting data. Several basic tools organize data from the patient’s chart. Initially, a paper set of measurement tools was developed to help hospitals orchestrate data collection. Although a database now performs much of the work formerly done on paper, some use of paper tools may be helpful.
For example, the Surviving Sepsis Campaign’s screening tool for severe sepsis is integrated into the database. However, a paper version readily accessible to nurses and clinicians in the ED triage area, the medical and surgical nursing stations, and even the ICU itself will still be practical.
Likewise, some data collectors might find that first capturing on paper the data abstracted from the chart and subsequently entering it into the database is preferable. Most users are likely to find, however, that bringing the database to the ICU on a laptop and directly entering data is the easiest solution.
The most up-to-date paper versions of the tools and the Surviving Sepsis Campaign database can be found on the Institute of Healthcare Improvement Web site. The easiest way to get to that page of the Institute of Healthcare Improvement Web site is by home page linkage from the Surviving Sepsis Campaign Web site, www.survivingsepsis.org. An implementation manual is also available that will facilitate initiation of the Surviving Sepsis Campaign performance improvement program as well as installation and use of the associated electronic database.
Conclusion
The Surviving Sepsis Campaign represents an important step for international critical care societies. Recognizing the long history of delay in incorporating research into bedside care, these critical care societies have committed to working together to facilitate bench-to-bedside transfer of recent research. Thus, the campaign represents an ongoing commitment to excellence in patient care. The Surviving Sepsis Campaign has established a target of a 25% reduction in mortality worldwide from sepsis over the next five years. If the Surviving Sepsis Campaign is able to bring the guidelines into routine use, it is possible to achieve this goal. For the campaign to be successful, it will require more than good publicity. It will require a further commitment from bedside clinicians to appraise new research critically and adopt interventions proven to be effective rapidly.
Hospitalists interested in more information about instituting the Surviving Sepsis Campaign and performance improvement package in their hospital should e-mail the Surviving Sepsis Campaign user group program manager at [email protected]. TH
References
- Angus DC, Linde-Zwirble WT, Lidicer J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377.
- Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862-871.
- Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10)699-709.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301-1308.
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion in critical care. N Engl J Med. 1999;340:409-4178.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359-1367.
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341:793-800.
- Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med. 1998;338:791-797.
- Dellinger RP, Carlet JM, Masur H, et al: Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858-873.
- Dellinger RP, Carlet JM, Masur H, et al: Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536-55.
- Levy MM, Pronovost PJ, Dellinger RP, et al. Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome. Crit Care Med. 2004;32(suppl):S595-S597.
The incidence of severe sepsis (sepsis with organ dysfunction) is increasing.1 The initial diagnosis and management of severe sepsis may occur in the ED, the ICU, or the hospital ward.
Several recently published studies have demonstrated decreased mortality and morbidity as a result of interventions and therapeutics applied to patients with sepsis.2-5 These new data, resulting from rigorously performed, randomized controlled trials, combined with previous data for beneficial interventions not specific to sepsis management (such as DVT and stress ulcer prophylaxis) and consensus opinion where no evidence exists lend significant weight to the belief that critical care clinicians can now significantly reduce mortality in patients with severe sepsis and septic shock.6-9
Protocolized care now exists for heart attack and stroke, which is based on recent advances as demonstrated by the medical literature. Until now there has been no attempt to reproduce such an approach in severe sepsis. The Surviving Sepsis Campaign hopes to change that.
The Surviving Sepsis Campaign is administered by the Society of Critical Care Medicine (SCCM), the European Society of Intensive Care Medicine (ESICM), and the International Sepsis Forum (ISF) and is open to all industry for funding through unrestricted educational grants. Contributors to date include Baxter, Edwards, and Eli Lilly.
The first phase was the introduction of the campaign at several major international critical care medicine conferences, the ESICM meeting in Barcelona in 2002, and the SCCM meeting in 2003. The stated goal of the campaign is to decrease the mortality from severe sepsis by 25% in five years.
Phase 2 of the campaign was aimed at producing guidelines for the management of sepsis. In 2003, critical care and infectious disease experts representing 11 international organizations developed evidence-based management guidelines for severe sepsis and septic shock for practical use for the bedside clinician, under the auspices of the Surviving Sepsis Campaign.
Pediatric considerations were provided to contrast adult and pediatric management. The resulting recommendations represent an attempt to facilitate a rapid change in the standard of care for management of sepsis, based on the quality of available published data and expert opinion where no literature guidance is available. The guidelines manuscript was published in both Critical Care Medicine and Intensive Care Medicine.10,11 The publication of this manuscript represents an historic step for critical care worldwide. These guidelines represent an international consensus on the best available standard for management of sepsis.
Key Recommendations
Key recommendations (listed by category and not by hierarchy) include:
- Early goal-directed resuscitation of the septic patient during the first six hours after recognition;
- Appropriate diagnostic studies to ascertain causative organisms before starting antibiotics;
- Early administration of broad-spectrum antibiotic therapy;
- Reassessment of antibiotic therapy with microbiology and clinical data to narrow coverage, when appropriate;
- A usual seven to 10 days of antibiotic therapy guided by clinical response;
- Source control with attention to the method that balances risks and benefits;
- Equivalence of crystalloid and colloid resuscitation;
- Aggressive fluid challenge to restore mean circulating filling pressure;
- Vasopressor preference for norepinephrine and dopamine;
- Cautious use of vasopressin pending further studies;
- Avoidance of low-dose dopamine administration for renal protection;
- Consideration of dobutamine inotropic therapy in some clinical situations;
- Avoidance of supranormal oxygen delivery as a goal of therapy;
- Stress-dose steroid therapy for septic shock;
- Use of recombinant activated protein C in patients with severe sepsis and high risk for death;
- Resolution of tissue hypoperfusion and targeting a hemoglobin of 7-9 g/dL in the absence of coronary artery disease or acute hemorrhage;
- Appropriate use of fresh frozen plasma and platelets;
- A low tidal volume and limitation of inspiratory plateau pressure strategy for acute lung injury and acute respiratory distress syndrome;
- Application of a minimal amount of positive end expiratory pressure in acute lung injury/acute respiratory distress syndrome;
- A semi-recumbent bed position unless contraindicated;
- Protocols for weaning and sedation/analgesia, using either intermittent bolus sedation or continuous infusion sedation with daily interruptions/lightening;
- Avoidance of neuromuscular blockers, if at all possible;
- Maintenance of blood glucose <150 mg/dL after initial stabilization;
- Equivalence of continuous veno-veno hemofiltration (CVVH) and intermittent hemodialysis;
- Lack of utility of bicarbonate use for pH 7.15 or greater;
- Use of DVT/stress ulcer prophylaxis; and
- Consideration of limitation of support where appropriate.
Pediatric considerations include a more likely need for intubation due to low functional residual capacity; more difficult intravenous access; fluid resuscitation based on weight with 40-60 mL/kg or higher needed; decreased cardiac output and increased systemic vascular resistance as the most common hemodynamic profile; greater use of physical examination therapeutic endpoints; unsettled issue of high-dose steroids for therapy of septic shock; and greater risk of hypoglycemia with aggressive glucose control.
Operationalizing the Guidelines
Unfortunately, clinicians change slowly. Historically, transfer of research from the bench to the bedside is a long, tortuous process—one that is not driven by anything clear and that seems to be based more on fad and coincidence than on a keen, evidence-based evaluation of the literature. Phase 3 of the campaign hopes to change that.
Phase 3 of the campaign (www.survivingsepsis.org) aims to operationalize the guidelines to create a global standard of care for sepsis management.12 The guidelines will be transformed into user-friendly tools that allow clinicians to easily incorporate these new recommendations into bedside care. The first step in this next phase has been a joint effort with the Institute of Healthcare Improvement (IHI) to deploy a “change bundle” based on a core set of the previous recommendations into the IHI’s collaborative system. Chart review or concurrent data gathering will identify and track changes in practice and clinical outcomes. Engendering evidence-based change through motivational strategies while monitoring and sharing the results with healthcare practitioners is the key to improving outcomes in severe sepsis.
The severe sepsis bundles form the core of the Surviving Sepsis Campaign. A “bundle” is a group of interventions related to a disease process. When executed together, the interventions produce better outcomes than when implemented individually. The individual bundle elements are built on evidence-based practices. The science behind the elements of a bundle is so well established that their implementation should be considered a generally accepted practice. Develop a bundle process in the following way:
- Identify a set of four to six evidence-based interventions that apply to a cohort of patients with a common disease or a common location. An example might be patients with sepsis admitted to the ICU;
- Develop the will in the providers to deliver the interventions every time they are indicated;
- Redesign the delivery system to ensure the interventions in the bundle are delivered; and
- Measure related outcomes to ascertain the effects of the changes in the delivery system.
The sepsis bundles were developed in just such a manner, based on the experience of the ventilator bundle. The goal now is to motivate providers to deliver the sepsis interventions every time they are indicated and measure them in an all-or-nothing way. We believe that if the bundle elements are reliably performed we can achieve the desired outcome of reducing sepsis-related deaths by 25%.
These elements distill the Surviving Sepsis Campaign practice guidelines into a manageable format for use at most institutions. The bundles represent the specific changes the campaign has identified as essential to the care of severely septic patients. Following the severe sepsis bundles will eliminate the piecemeal or inappropriate application of standards for sepsis care that characterize most clinical environments today.
Hospitals should implement two different severe sepsis bundles. Each bundle articulates objectives to be accomplished within specific time frames.
Sepsis Resuscitation Bundle
The severe sepsis resuscitation bundle describes seven tasks that should begin immediately but must be accomplished within the first six hours of presentation for patients with severe sepsis or septic shock. Some items may not be completed if the clinical conditions described in the bundle do not prevail in a particular case, but clinicians must assess for them. The goal is to perform all indicated tasks 100% of the time within the first six hours of identification of severe sepsis. The tasks are:
- Measure serum lactate;
- Obtain blood cultures prior to antibiotic administration;
- Administer broad-spectrum antibiotics within three hours from time of presentation for ED admissions and one hour for non-ED ICU admissions;
- In the event of hypotension and/or lactate >4 mmol/L (36 mg/dL):
- Deliver an initial minimum of 20 ml/kg of crystalloid (or colloid equivalent); and
- Apply vasopressors for hypotension not responding to initial fluid resuscitation to maintain mean arterial pressure (MAP) ≥65 mm Hg;
- In the event of persistent hypotension despite fluid resuscitation (septic shock) and/or lactate > 4 mmol/L (36 mg/dL):
- Achieve central venous pressure (CVP) of ≥8-12 mm Hg; and
- Achieve central venous oxygen saturation (ScvO2) of Surviving Sepsis Campaign70%. (Achieving a mixed venous oxygen saturation (SvO2) of 65% is an acceptable alternative.)
Sepsis Management Bundle
The severe sepsis management bundle lists four management goals. Efforts to accomplish these tasks should also begin immediately, but these items may be completed within 24 hours of presentation for patients with severe sepsis or septic shock.
- Administer low-dose steroids for septic shock in accordance with a standardized ICU policy;
- Administer drotrecogin alfa (activated) in accordance with a standardized ICU policy;
- Maintain glucose control ≥ lower limit of normal, but <150 mg/dL (8.3 mmol/L); and
- Maintain inspiratory plateau pressures <30 cm H2O for mechanically ventilated patients.
Team Effort
To achieve the goal of reducing mortality by 25% by 2008, everyone involved with the care of severe sepsis patients must be included, work processes must be carefully scripted and standardized, and commitment to this effort must be elevated. This must be a team effort that crosses disciplines and departments; it requires leadership, support from the entire organization, and buy-in from all stakeholders involved with the care of these patients.
Three levels of participation exist in creating successful change:
1) Active working teams are responsible for daily planning, documentation, communication, education, monitoring, and evaluation of activities. The working team must have representation from all departments involved in the change processes ICU, ED, pulmonary department, pharmacy, etc. The team should also be multidisciplinary, comprising physicians, nurses, pharmacists, respiratory therapists, and other staff with roles in the specific change process, such as clerks and technicians. Team members should be knowledgeable about the specific aims, the current local work processes, the associated literature, and any environmental issues that will be affected by these changes.
2) A leadership group or person within the team helps remove barriers, provides resources, monitors global progress, and gives suggestions from an institutional perspective. The working team needs someone with authority in the organization to overcome barriers and to allocate the time and resources the team needs to achieve its aim. Leadership needs to understand how the proposed changes will affect various parts of the system and the more remote consequences such changes might trigger.
3) Providers and stakeholders must be kept informed. Procedures are needed to keep them informed, to receive their feedback, and to ensure them that their responses are respected. This gives stakeholders a sense of ownership and facilitates implementation of the new processes.
Protocols
Teams should use the bundles to create customized protocols and pathways that will function well within their institutions. However, all of the elements in the bundles must be incorporated into the protocols. The protocols should mirror the bundles but allow flexibility to accommodate the specific needs of a local hospital. The severe sepsis bundles (and thereby the hospital’s protocol) form the basis for the measurements the team will conduct. If all of the elements of the bundles are not incorporated into your customized protocol, your performance on the measures will suffer.
A strong protocol will accomplish all of the items listed in the severe sepsis bundles. If the protocol designer pays careful attention to the details in the bundles, the protocol will score well on the severe sepsis quality indicators. Hospitals will want to publicize their efforts with regard to improving sepsis care and make the protocol an integral part of their rollout strategy. It is imperative to launch an educational initiative regarding the effort.
Examples of sepsis screening and management protocols are available on the Surviving Sepsis Campaign IHI Web site and are rendered on this page as “Protocol A: Create a protocol and educate users” and as “Prot0col B.” The easiest way to get to that page of the IHI Web site is through the home page link from the Surviving Sepsis Campaign Web site, www.survivingsepsis.org. These highly visual and easy-to-follow pathways exemplify ways to encourage adherence to a protocol. Notice that the “Sepsis Screening Protocol” (p. 25) complies with the terms of the severe sepsis bundles. Posting these types of algorithms prominently in the ED, hospital wards, and ICU, and making them readily available in laminated and PDA format, can have a significant impact on performance improvement programs.
These flow diagrams may be incorporated into lectures and training programs to support your efforts to change care at the bedside. You can adapt the algorithms to fit the needs of your individual institution, but keep in mind the need to comply with the overall structure of the severe sepsis bundles.
Data Collection
Data collection can seem like an onerous duty in any quality improvement project. Nevertheless, it is essential for improvement. Without attention to measurement, how will you know that your efforts are leading to improvement? At most hospitals, the magnitude of the data collection effort will not be huge as it will be relative to the number of severely septic patients cared for in the ICU.
Generally, hospitals report three to four severely septic patients are treated in one week’s time. This means that zero, one, or two severely septic patients’ charts will need to be abstracted each day in an average-size hospital. If abstraction takes between 20–30 minutes per chart, the daily time for this effort may range from 30–90 minutes daily. This relatively small burden is likely to represent an initial challenge to anyone unfamiliar with the organization of the chart and the measurement forms, or tools, used by the Surviving Sepsis Campaign for data collection. In time, however, data collection will become easier as the chart and the tools provided by the Surviving Sepsis Campaign will become more familiar. Bundle implementation and data collection have begun in hospitals throughout Europe, Latin America, the United Kingdom, and the United States.
The measurement tools were created to achieve a uniform system of data gathering, collation, and calculation across hospitals. Without the measurement tools, teams armed with only the concepts in the severe sepsis bundles would need to decide how to gather data from charts and put it in a format consistent with the calculations listed in the severe sepsis quality indicators. If any hospital were to undertake such a task on its own, it would quickly find that its results were not comparable across institutions because scores of other hospitals would have derived their results by entirely different means.
The Surviving Sepsis Campaign aims to make using the measurement tools as easy as possible for those involved in collecting data. Several basic tools organize data from the patient’s chart. Initially, a paper set of measurement tools was developed to help hospitals orchestrate data collection. Although a database now performs much of the work formerly done on paper, some use of paper tools may be helpful.
For example, the Surviving Sepsis Campaign’s screening tool for severe sepsis is integrated into the database. However, a paper version readily accessible to nurses and clinicians in the ED triage area, the medical and surgical nursing stations, and even the ICU itself will still be practical.
Likewise, some data collectors might find that first capturing on paper the data abstracted from the chart and subsequently entering it into the database is preferable. Most users are likely to find, however, that bringing the database to the ICU on a laptop and directly entering data is the easiest solution.
The most up-to-date paper versions of the tools and the Surviving Sepsis Campaign database can be found on the Institute of Healthcare Improvement Web site. The easiest way to get to that page of the Institute of Healthcare Improvement Web site is by home page linkage from the Surviving Sepsis Campaign Web site, www.survivingsepsis.org. An implementation manual is also available that will facilitate initiation of the Surviving Sepsis Campaign performance improvement program as well as installation and use of the associated electronic database.
Conclusion
The Surviving Sepsis Campaign represents an important step for international critical care societies. Recognizing the long history of delay in incorporating research into bedside care, these critical care societies have committed to working together to facilitate bench-to-bedside transfer of recent research. Thus, the campaign represents an ongoing commitment to excellence in patient care. The Surviving Sepsis Campaign has established a target of a 25% reduction in mortality worldwide from sepsis over the next five years. If the Surviving Sepsis Campaign is able to bring the guidelines into routine use, it is possible to achieve this goal. For the campaign to be successful, it will require more than good publicity. It will require a further commitment from bedside clinicians to appraise new research critically and adopt interventions proven to be effective rapidly.
Hospitalists interested in more information about instituting the Surviving Sepsis Campaign and performance improvement package in their hospital should e-mail the Surviving Sepsis Campaign user group program manager at [email protected]. TH
References
- Angus DC, Linde-Zwirble WT, Lidicer J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377.
- Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862-871.
- Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10)699-709.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301-1308.
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion in critical care. N Engl J Med. 1999;340:409-4178.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359-1367.
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341:793-800.
- Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med. 1998;338:791-797.
- Dellinger RP, Carlet JM, Masur H, et al: Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858-873.
- Dellinger RP, Carlet JM, Masur H, et al: Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536-55.
- Levy MM, Pronovost PJ, Dellinger RP, et al. Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome. Crit Care Med. 2004;32(suppl):S595-S597.
The incidence of severe sepsis (sepsis with organ dysfunction) is increasing.1 The initial diagnosis and management of severe sepsis may occur in the ED, the ICU, or the hospital ward.
Several recently published studies have demonstrated decreased mortality and morbidity as a result of interventions and therapeutics applied to patients with sepsis.2-5 These new data, resulting from rigorously performed, randomized controlled trials, combined with previous data for beneficial interventions not specific to sepsis management (such as DVT and stress ulcer prophylaxis) and consensus opinion where no evidence exists lend significant weight to the belief that critical care clinicians can now significantly reduce mortality in patients with severe sepsis and septic shock.6-9
Protocolized care now exists for heart attack and stroke, which is based on recent advances as demonstrated by the medical literature. Until now there has been no attempt to reproduce such an approach in severe sepsis. The Surviving Sepsis Campaign hopes to change that.
The Surviving Sepsis Campaign is administered by the Society of Critical Care Medicine (SCCM), the European Society of Intensive Care Medicine (ESICM), and the International Sepsis Forum (ISF) and is open to all industry for funding through unrestricted educational grants. Contributors to date include Baxter, Edwards, and Eli Lilly.
The first phase was the introduction of the campaign at several major international critical care medicine conferences, the ESICM meeting in Barcelona in 2002, and the SCCM meeting in 2003. The stated goal of the campaign is to decrease the mortality from severe sepsis by 25% in five years.
Phase 2 of the campaign was aimed at producing guidelines for the management of sepsis. In 2003, critical care and infectious disease experts representing 11 international organizations developed evidence-based management guidelines for severe sepsis and septic shock for practical use for the bedside clinician, under the auspices of the Surviving Sepsis Campaign.
Pediatric considerations were provided to contrast adult and pediatric management. The resulting recommendations represent an attempt to facilitate a rapid change in the standard of care for management of sepsis, based on the quality of available published data and expert opinion where no literature guidance is available. The guidelines manuscript was published in both Critical Care Medicine and Intensive Care Medicine.10,11 The publication of this manuscript represents an historic step for critical care worldwide. These guidelines represent an international consensus on the best available standard for management of sepsis.
Key Recommendations
Key recommendations (listed by category and not by hierarchy) include:
- Early goal-directed resuscitation of the septic patient during the first six hours after recognition;
- Appropriate diagnostic studies to ascertain causative organisms before starting antibiotics;
- Early administration of broad-spectrum antibiotic therapy;
- Reassessment of antibiotic therapy with microbiology and clinical data to narrow coverage, when appropriate;
- A usual seven to 10 days of antibiotic therapy guided by clinical response;
- Source control with attention to the method that balances risks and benefits;
- Equivalence of crystalloid and colloid resuscitation;
- Aggressive fluid challenge to restore mean circulating filling pressure;
- Vasopressor preference for norepinephrine and dopamine;
- Cautious use of vasopressin pending further studies;
- Avoidance of low-dose dopamine administration for renal protection;
- Consideration of dobutamine inotropic therapy in some clinical situations;
- Avoidance of supranormal oxygen delivery as a goal of therapy;
- Stress-dose steroid therapy for septic shock;
- Use of recombinant activated protein C in patients with severe sepsis and high risk for death;
- Resolution of tissue hypoperfusion and targeting a hemoglobin of 7-9 g/dL in the absence of coronary artery disease or acute hemorrhage;
- Appropriate use of fresh frozen plasma and platelets;
- A low tidal volume and limitation of inspiratory plateau pressure strategy for acute lung injury and acute respiratory distress syndrome;
- Application of a minimal amount of positive end expiratory pressure in acute lung injury/acute respiratory distress syndrome;
- A semi-recumbent bed position unless contraindicated;
- Protocols for weaning and sedation/analgesia, using either intermittent bolus sedation or continuous infusion sedation with daily interruptions/lightening;
- Avoidance of neuromuscular blockers, if at all possible;
- Maintenance of blood glucose <150 mg/dL after initial stabilization;
- Equivalence of continuous veno-veno hemofiltration (CVVH) and intermittent hemodialysis;
- Lack of utility of bicarbonate use for pH 7.15 or greater;
- Use of DVT/stress ulcer prophylaxis; and
- Consideration of limitation of support where appropriate.
Pediatric considerations include a more likely need for intubation due to low functional residual capacity; more difficult intravenous access; fluid resuscitation based on weight with 40-60 mL/kg or higher needed; decreased cardiac output and increased systemic vascular resistance as the most common hemodynamic profile; greater use of physical examination therapeutic endpoints; unsettled issue of high-dose steroids for therapy of septic shock; and greater risk of hypoglycemia with aggressive glucose control.
Operationalizing the Guidelines
Unfortunately, clinicians change slowly. Historically, transfer of research from the bench to the bedside is a long, tortuous process—one that is not driven by anything clear and that seems to be based more on fad and coincidence than on a keen, evidence-based evaluation of the literature. Phase 3 of the campaign hopes to change that.
Phase 3 of the campaign (www.survivingsepsis.org) aims to operationalize the guidelines to create a global standard of care for sepsis management.12 The guidelines will be transformed into user-friendly tools that allow clinicians to easily incorporate these new recommendations into bedside care. The first step in this next phase has been a joint effort with the Institute of Healthcare Improvement (IHI) to deploy a “change bundle” based on a core set of the previous recommendations into the IHI’s collaborative system. Chart review or concurrent data gathering will identify and track changes in practice and clinical outcomes. Engendering evidence-based change through motivational strategies while monitoring and sharing the results with healthcare practitioners is the key to improving outcomes in severe sepsis.
The severe sepsis bundles form the core of the Surviving Sepsis Campaign. A “bundle” is a group of interventions related to a disease process. When executed together, the interventions produce better outcomes than when implemented individually. The individual bundle elements are built on evidence-based practices. The science behind the elements of a bundle is so well established that their implementation should be considered a generally accepted practice. Develop a bundle process in the following way:
- Identify a set of four to six evidence-based interventions that apply to a cohort of patients with a common disease or a common location. An example might be patients with sepsis admitted to the ICU;
- Develop the will in the providers to deliver the interventions every time they are indicated;
- Redesign the delivery system to ensure the interventions in the bundle are delivered; and
- Measure related outcomes to ascertain the effects of the changes in the delivery system.
The sepsis bundles were developed in just such a manner, based on the experience of the ventilator bundle. The goal now is to motivate providers to deliver the sepsis interventions every time they are indicated and measure them in an all-or-nothing way. We believe that if the bundle elements are reliably performed we can achieve the desired outcome of reducing sepsis-related deaths by 25%.
These elements distill the Surviving Sepsis Campaign practice guidelines into a manageable format for use at most institutions. The bundles represent the specific changes the campaign has identified as essential to the care of severely septic patients. Following the severe sepsis bundles will eliminate the piecemeal or inappropriate application of standards for sepsis care that characterize most clinical environments today.
Hospitals should implement two different severe sepsis bundles. Each bundle articulates objectives to be accomplished within specific time frames.
Sepsis Resuscitation Bundle
The severe sepsis resuscitation bundle describes seven tasks that should begin immediately but must be accomplished within the first six hours of presentation for patients with severe sepsis or septic shock. Some items may not be completed if the clinical conditions described in the bundle do not prevail in a particular case, but clinicians must assess for them. The goal is to perform all indicated tasks 100% of the time within the first six hours of identification of severe sepsis. The tasks are:
- Measure serum lactate;
- Obtain blood cultures prior to antibiotic administration;
- Administer broad-spectrum antibiotics within three hours from time of presentation for ED admissions and one hour for non-ED ICU admissions;
- In the event of hypotension and/or lactate >4 mmol/L (36 mg/dL):
- Deliver an initial minimum of 20 ml/kg of crystalloid (or colloid equivalent); and
- Apply vasopressors for hypotension not responding to initial fluid resuscitation to maintain mean arterial pressure (MAP) ≥65 mm Hg;
- In the event of persistent hypotension despite fluid resuscitation (septic shock) and/or lactate > 4 mmol/L (36 mg/dL):
- Achieve central venous pressure (CVP) of ≥8-12 mm Hg; and
- Achieve central venous oxygen saturation (ScvO2) of Surviving Sepsis Campaign70%. (Achieving a mixed venous oxygen saturation (SvO2) of 65% is an acceptable alternative.)
Sepsis Management Bundle
The severe sepsis management bundle lists four management goals. Efforts to accomplish these tasks should also begin immediately, but these items may be completed within 24 hours of presentation for patients with severe sepsis or septic shock.
- Administer low-dose steroids for septic shock in accordance with a standardized ICU policy;
- Administer drotrecogin alfa (activated) in accordance with a standardized ICU policy;
- Maintain glucose control ≥ lower limit of normal, but <150 mg/dL (8.3 mmol/L); and
- Maintain inspiratory plateau pressures <30 cm H2O for mechanically ventilated patients.
Team Effort
To achieve the goal of reducing mortality by 25% by 2008, everyone involved with the care of severe sepsis patients must be included, work processes must be carefully scripted and standardized, and commitment to this effort must be elevated. This must be a team effort that crosses disciplines and departments; it requires leadership, support from the entire organization, and buy-in from all stakeholders involved with the care of these patients.
Three levels of participation exist in creating successful change:
1) Active working teams are responsible for daily planning, documentation, communication, education, monitoring, and evaluation of activities. The working team must have representation from all departments involved in the change processes ICU, ED, pulmonary department, pharmacy, etc. The team should also be multidisciplinary, comprising physicians, nurses, pharmacists, respiratory therapists, and other staff with roles in the specific change process, such as clerks and technicians. Team members should be knowledgeable about the specific aims, the current local work processes, the associated literature, and any environmental issues that will be affected by these changes.
2) A leadership group or person within the team helps remove barriers, provides resources, monitors global progress, and gives suggestions from an institutional perspective. The working team needs someone with authority in the organization to overcome barriers and to allocate the time and resources the team needs to achieve its aim. Leadership needs to understand how the proposed changes will affect various parts of the system and the more remote consequences such changes might trigger.
3) Providers and stakeholders must be kept informed. Procedures are needed to keep them informed, to receive their feedback, and to ensure them that their responses are respected. This gives stakeholders a sense of ownership and facilitates implementation of the new processes.
Protocols
Teams should use the bundles to create customized protocols and pathways that will function well within their institutions. However, all of the elements in the bundles must be incorporated into the protocols. The protocols should mirror the bundles but allow flexibility to accommodate the specific needs of a local hospital. The severe sepsis bundles (and thereby the hospital’s protocol) form the basis for the measurements the team will conduct. If all of the elements of the bundles are not incorporated into your customized protocol, your performance on the measures will suffer.
A strong protocol will accomplish all of the items listed in the severe sepsis bundles. If the protocol designer pays careful attention to the details in the bundles, the protocol will score well on the severe sepsis quality indicators. Hospitals will want to publicize their efforts with regard to improving sepsis care and make the protocol an integral part of their rollout strategy. It is imperative to launch an educational initiative regarding the effort.
Examples of sepsis screening and management protocols are available on the Surviving Sepsis Campaign IHI Web site and are rendered on this page as “Protocol A: Create a protocol and educate users” and as “Prot0col B.” The easiest way to get to that page of the IHI Web site is through the home page link from the Surviving Sepsis Campaign Web site, www.survivingsepsis.org. These highly visual and easy-to-follow pathways exemplify ways to encourage adherence to a protocol. Notice that the “Sepsis Screening Protocol” (p. 25) complies with the terms of the severe sepsis bundles. Posting these types of algorithms prominently in the ED, hospital wards, and ICU, and making them readily available in laminated and PDA format, can have a significant impact on performance improvement programs.
These flow diagrams may be incorporated into lectures and training programs to support your efforts to change care at the bedside. You can adapt the algorithms to fit the needs of your individual institution, but keep in mind the need to comply with the overall structure of the severe sepsis bundles.
Data Collection
Data collection can seem like an onerous duty in any quality improvement project. Nevertheless, it is essential for improvement. Without attention to measurement, how will you know that your efforts are leading to improvement? At most hospitals, the magnitude of the data collection effort will not be huge as it will be relative to the number of severely septic patients cared for in the ICU.
Generally, hospitals report three to four severely septic patients are treated in one week’s time. This means that zero, one, or two severely septic patients’ charts will need to be abstracted each day in an average-size hospital. If abstraction takes between 20–30 minutes per chart, the daily time for this effort may range from 30–90 minutes daily. This relatively small burden is likely to represent an initial challenge to anyone unfamiliar with the organization of the chart and the measurement forms, or tools, used by the Surviving Sepsis Campaign for data collection. In time, however, data collection will become easier as the chart and the tools provided by the Surviving Sepsis Campaign will become more familiar. Bundle implementation and data collection have begun in hospitals throughout Europe, Latin America, the United Kingdom, and the United States.
The measurement tools were created to achieve a uniform system of data gathering, collation, and calculation across hospitals. Without the measurement tools, teams armed with only the concepts in the severe sepsis bundles would need to decide how to gather data from charts and put it in a format consistent with the calculations listed in the severe sepsis quality indicators. If any hospital were to undertake such a task on its own, it would quickly find that its results were not comparable across institutions because scores of other hospitals would have derived their results by entirely different means.
The Surviving Sepsis Campaign aims to make using the measurement tools as easy as possible for those involved in collecting data. Several basic tools organize data from the patient’s chart. Initially, a paper set of measurement tools was developed to help hospitals orchestrate data collection. Although a database now performs much of the work formerly done on paper, some use of paper tools may be helpful.
For example, the Surviving Sepsis Campaign’s screening tool for severe sepsis is integrated into the database. However, a paper version readily accessible to nurses and clinicians in the ED triage area, the medical and surgical nursing stations, and even the ICU itself will still be practical.
Likewise, some data collectors might find that first capturing on paper the data abstracted from the chart and subsequently entering it into the database is preferable. Most users are likely to find, however, that bringing the database to the ICU on a laptop and directly entering data is the easiest solution.
The most up-to-date paper versions of the tools and the Surviving Sepsis Campaign database can be found on the Institute of Healthcare Improvement Web site. The easiest way to get to that page of the Institute of Healthcare Improvement Web site is by home page linkage from the Surviving Sepsis Campaign Web site, www.survivingsepsis.org. An implementation manual is also available that will facilitate initiation of the Surviving Sepsis Campaign performance improvement program as well as installation and use of the associated electronic database.
Conclusion
The Surviving Sepsis Campaign represents an important step for international critical care societies. Recognizing the long history of delay in incorporating research into bedside care, these critical care societies have committed to working together to facilitate bench-to-bedside transfer of recent research. Thus, the campaign represents an ongoing commitment to excellence in patient care. The Surviving Sepsis Campaign has established a target of a 25% reduction in mortality worldwide from sepsis over the next five years. If the Surviving Sepsis Campaign is able to bring the guidelines into routine use, it is possible to achieve this goal. For the campaign to be successful, it will require more than good publicity. It will require a further commitment from bedside clinicians to appraise new research critically and adopt interventions proven to be effective rapidly.
Hospitalists interested in more information about instituting the Surviving Sepsis Campaign and performance improvement package in their hospital should e-mail the Surviving Sepsis Campaign user group program manager at [email protected]. TH
References
- Angus DC, Linde-Zwirble WT, Lidicer J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377.
- Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862-871.
- Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10)699-709.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301-1308.
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion in critical care. N Engl J Med. 1999;340:409-4178.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359-1367.
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341:793-800.
- Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med. 1998;338:791-797.
- Dellinger RP, Carlet JM, Masur H, et al: Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858-873.
- Dellinger RP, Carlet JM, Masur H, et al: Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536-55.
- Levy MM, Pronovost PJ, Dellinger RP, et al. Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome. Crit Care Med. 2004;32(suppl):S595-S597.