User login
Leadership & Professional Development: New Team? No Problem. Creating Teams From Strangers
“Well begun is half done.” — Aristotle
In the clinical environment, team composition changes frequently and time is limited. As a result, teams often jump directly into patient care, addressing issues related to interpersonal dynamics only after they arise. Team leaders can accelerate the process of forming highly effective teams by deliberately leveraging principles of teaming, or the process of “how to turn a group of strangers into a team.”1
Setting the Stage
On the first day with a new team, a common misconception is that teaming will take away time, when in fact it will save time. Investing a few minutes before rounds to clarify roles and expectations can streamline subsequent shared work. For example, an attending might request to accompany residents and medical students for new admissions in the last 2 hours of the workday, rather than following the usual pattern of discussing the case after the team completes a full evaluation on their own. Importantly, attendings should clarify their intent—to preserve learning opportunities while helping teams wrap up on time—and their role, which is to provide real-time feedback, facilitate decision-making, or assist with documentation. This 2-minute upfront investment results in improved team camaraderie, better task coordination, and fewer late days in the hospital.
Uncovering Connections and Skills
By integrating a few positively framed, thoughtful questions into introductions, teams may also discover surprising expertise or valuable perspectives that positively impact team performance.2 For example, in lieu of questions about level of training or hometown, you might ask, “What is an experience outside the hospital that helps you inside the hospital?” or “What skills allow you to contribute best on teams?” These questions might lead, for example, a medical student to leverage her background in computer science to help her team design new electronic health record shortcuts. Or, they might enable a resident with a personal history of leukemia to help the team communicate with a young patient facing a prolonged hospitalization for a newly diagnosed serious illness. With typical introductions, these opportunities and unexpected solutions can easily be missed.
Creating Mutual Understanding and Focus
As part of teaming, members should also explicitly share individual work-style preferences to avoid misunderstandings that may adversely affect subsequent work. On new teams, members—especially trainees—expend considerable energy scrutinizing subtle behaviors, such as a clarifying question or a blank stare, to assess whether their performance is perceived favorably. That energy can be reallocated to more important tasks by encouraging each person to state nuances of their work style that may be misinterpreted. For example, an attending might share, “I ask questions to identify what to teach, not to judge knowledge, so don’t worry about saying you don’t know,” whereas a resident might warn, “I have trouble concentrating when I’m hungry, so I often get impatient if we don’t take a break for lunch.” Without this information, a student might feel unnecessarily embarrassed by an attending on rounds, and an attending might incorrectly interpret a resident’s impatience around lunchtime as a reflection of low commitment. Individual work styles vary, and recognizing these differences upfront allows teams to maintain a sharper focus on more important issues, such as clinical care.
A Winning Team
In the hospital, we find ourselves in perpetual motion, with frequent transitions of care and new team members. Teaming offers a concrete method to proactively avoid predictable challenges and to enable teams to become more efficient, effective, and connected. Furthermore, teaming empowers us to substitute the uncertainty of ever-changing teams with the excitement of discovering what each new team can achieve through intentional leadership at the outset.
1. Edmondson AC. How to turn a group of strangers into a team. Accessed March 1, 2021. https://www.ted.com/talks/amy_edmondson_how_to_turn_a_group_of_strangers_into_a_team?language=en
2. Edmondson AC. Teamwork on the fly. Harvard Business Review. Published April 2012. Accessed July 26, 2021. https://hbr.org/2012/04/teamwork-on-the-fly-2
“Well begun is half done.” — Aristotle
In the clinical environment, team composition changes frequently and time is limited. As a result, teams often jump directly into patient care, addressing issues related to interpersonal dynamics only after they arise. Team leaders can accelerate the process of forming highly effective teams by deliberately leveraging principles of teaming, or the process of “how to turn a group of strangers into a team.”1
Setting the Stage
On the first day with a new team, a common misconception is that teaming will take away time, when in fact it will save time. Investing a few minutes before rounds to clarify roles and expectations can streamline subsequent shared work. For example, an attending might request to accompany residents and medical students for new admissions in the last 2 hours of the workday, rather than following the usual pattern of discussing the case after the team completes a full evaluation on their own. Importantly, attendings should clarify their intent—to preserve learning opportunities while helping teams wrap up on time—and their role, which is to provide real-time feedback, facilitate decision-making, or assist with documentation. This 2-minute upfront investment results in improved team camaraderie, better task coordination, and fewer late days in the hospital.
Uncovering Connections and Skills
By integrating a few positively framed, thoughtful questions into introductions, teams may also discover surprising expertise or valuable perspectives that positively impact team performance.2 For example, in lieu of questions about level of training or hometown, you might ask, “What is an experience outside the hospital that helps you inside the hospital?” or “What skills allow you to contribute best on teams?” These questions might lead, for example, a medical student to leverage her background in computer science to help her team design new electronic health record shortcuts. Or, they might enable a resident with a personal history of leukemia to help the team communicate with a young patient facing a prolonged hospitalization for a newly diagnosed serious illness. With typical introductions, these opportunities and unexpected solutions can easily be missed.
Creating Mutual Understanding and Focus
As part of teaming, members should also explicitly share individual work-style preferences to avoid misunderstandings that may adversely affect subsequent work. On new teams, members—especially trainees—expend considerable energy scrutinizing subtle behaviors, such as a clarifying question or a blank stare, to assess whether their performance is perceived favorably. That energy can be reallocated to more important tasks by encouraging each person to state nuances of their work style that may be misinterpreted. For example, an attending might share, “I ask questions to identify what to teach, not to judge knowledge, so don’t worry about saying you don’t know,” whereas a resident might warn, “I have trouble concentrating when I’m hungry, so I often get impatient if we don’t take a break for lunch.” Without this information, a student might feel unnecessarily embarrassed by an attending on rounds, and an attending might incorrectly interpret a resident’s impatience around lunchtime as a reflection of low commitment. Individual work styles vary, and recognizing these differences upfront allows teams to maintain a sharper focus on more important issues, such as clinical care.
A Winning Team
In the hospital, we find ourselves in perpetual motion, with frequent transitions of care and new team members. Teaming offers a concrete method to proactively avoid predictable challenges and to enable teams to become more efficient, effective, and connected. Furthermore, teaming empowers us to substitute the uncertainty of ever-changing teams with the excitement of discovering what each new team can achieve through intentional leadership at the outset.
“Well begun is half done.” — Aristotle
In the clinical environment, team composition changes frequently and time is limited. As a result, teams often jump directly into patient care, addressing issues related to interpersonal dynamics only after they arise. Team leaders can accelerate the process of forming highly effective teams by deliberately leveraging principles of teaming, or the process of “how to turn a group of strangers into a team.”1
Setting the Stage
On the first day with a new team, a common misconception is that teaming will take away time, when in fact it will save time. Investing a few minutes before rounds to clarify roles and expectations can streamline subsequent shared work. For example, an attending might request to accompany residents and medical students for new admissions in the last 2 hours of the workday, rather than following the usual pattern of discussing the case after the team completes a full evaluation on their own. Importantly, attendings should clarify their intent—to preserve learning opportunities while helping teams wrap up on time—and their role, which is to provide real-time feedback, facilitate decision-making, or assist with documentation. This 2-minute upfront investment results in improved team camaraderie, better task coordination, and fewer late days in the hospital.
Uncovering Connections and Skills
By integrating a few positively framed, thoughtful questions into introductions, teams may also discover surprising expertise or valuable perspectives that positively impact team performance.2 For example, in lieu of questions about level of training or hometown, you might ask, “What is an experience outside the hospital that helps you inside the hospital?” or “What skills allow you to contribute best on teams?” These questions might lead, for example, a medical student to leverage her background in computer science to help her team design new electronic health record shortcuts. Or, they might enable a resident with a personal history of leukemia to help the team communicate with a young patient facing a prolonged hospitalization for a newly diagnosed serious illness. With typical introductions, these opportunities and unexpected solutions can easily be missed.
Creating Mutual Understanding and Focus
As part of teaming, members should also explicitly share individual work-style preferences to avoid misunderstandings that may adversely affect subsequent work. On new teams, members—especially trainees—expend considerable energy scrutinizing subtle behaviors, such as a clarifying question or a blank stare, to assess whether their performance is perceived favorably. That energy can be reallocated to more important tasks by encouraging each person to state nuances of their work style that may be misinterpreted. For example, an attending might share, “I ask questions to identify what to teach, not to judge knowledge, so don’t worry about saying you don’t know,” whereas a resident might warn, “I have trouble concentrating when I’m hungry, so I often get impatient if we don’t take a break for lunch.” Without this information, a student might feel unnecessarily embarrassed by an attending on rounds, and an attending might incorrectly interpret a resident’s impatience around lunchtime as a reflection of low commitment. Individual work styles vary, and recognizing these differences upfront allows teams to maintain a sharper focus on more important issues, such as clinical care.
A Winning Team
In the hospital, we find ourselves in perpetual motion, with frequent transitions of care and new team members. Teaming offers a concrete method to proactively avoid predictable challenges and to enable teams to become more efficient, effective, and connected. Furthermore, teaming empowers us to substitute the uncertainty of ever-changing teams with the excitement of discovering what each new team can achieve through intentional leadership at the outset.
1. Edmondson AC. How to turn a group of strangers into a team. Accessed March 1, 2021. https://www.ted.com/talks/amy_edmondson_how_to_turn_a_group_of_strangers_into_a_team?language=en
2. Edmondson AC. Teamwork on the fly. Harvard Business Review. Published April 2012. Accessed July 26, 2021. https://hbr.org/2012/04/teamwork-on-the-fly-2
1. Edmondson AC. How to turn a group of strangers into a team. Accessed March 1, 2021. https://www.ted.com/talks/amy_edmondson_how_to_turn_a_group_of_strangers_into_a_team?language=en
2. Edmondson AC. Teamwork on the fly. Harvard Business Review. Published April 2012. Accessed July 26, 2021. https://hbr.org/2012/04/teamwork-on-the-fly-2
© 2021 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: The Use of Intravenous Fluids in the Hospitalized Adult
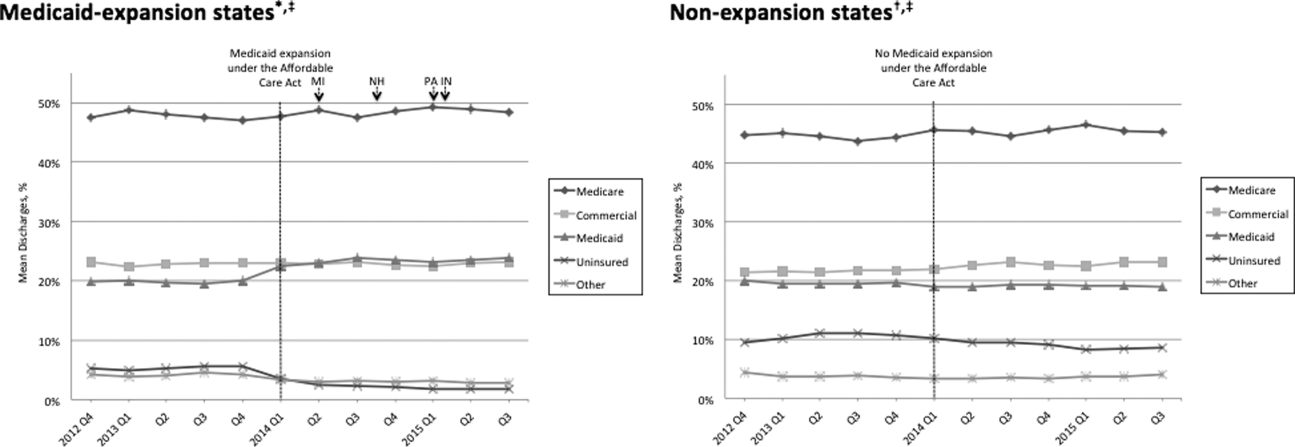
Hospitalized patients often receive intravenous fluids (IVF) when they cannot meet physiologic needs through oral intake in the setting of medical or surgical illness. Prescribing the optimal IVF solution to the appropriate patient is a complex decision and often occurs without the same degree of institutionalized restrictions or guidance developed for other inpatient pharmacologic agents. There is wide variation in clinical utilization of IVF due to the lack of data to guide decision making.1 When data do exist, they typically focus on a limited number of clinical situations.2 Thus, even though IVF are often considered low-risk, the frequency and lack of consistency with which they are used can result in errors, complications, and over-use of medical resources.3
KEY RECOMMENDATIONS FOR THE HOSPITALIST
(Evidence quality: not described in the guideline, recommendation strength: not described in the guideline)
Recommendation 1
To aid in fluid management and avoid complications, the guidelines recommend that patients on IVF require careful assessment of volume status, including a detailed history, physical exam, clinical monitoring, and daily labs.2
Clinical history should focus on understanding fluid losses and intake; physical exam should include vital signs, evidence of orthostatic hypotension, capillary refill, jugular venous pulsation, and assessment for pulmonary edema. Subsequent clinical monitoring should include fluid balance (Ins and Outs) and daily weights. All patients starting or continuing IVF should have a basic metabolic panel at least daily according to the guidelines, though the authors note this frequency may be too high for some patients and needs further study.2
Recommendation 2
The guidelines describe four types of IV fluids that can be administered: crystalloids, balanced crystalloids, glucose solutions, and non blood-product colloids.2
Crystalloids include isotonic saline with 154 millimoles (mmol) of sodium and chloride. Balanced crystalloids, such as lactated Ringer’s solution, are more physiologic, with less sodium and chloride, and the addition of magnesium, potassium, and calcium. Glucose solutions are quickly metabolized and, thus, are an effective way to deliver free water. Non blood-product colloids include particles that are retained within the circulation, including proteins such as human albumin.
Recommendation 3
For each indication to administer IVF, the guidelines recommend the following formulations and considerations:2
For general resuscitation, use crystalloids with sodium content of 130-154 mmol, delivered in a bolus of at least 500 milliliters (mL) over 15 minutes or less. For sepsis, infuse at least 30 mL/kg.4 For routine maintenance, restrict the volume to 25-30 mL/kg/day of water, and include 1 mmol/kg/day of potassium, sodium, and chloride along with 50-100 g/day of glucose to prevent starvation ketosis, though glucose should be avoided in most diabetic patients. With obesity, adjust the IVF to ideal body weight, and for patients who are older, frail, or admitted with renal or cardiac impairment, consider prescribing a lower range of fluid (20-25 mL/kg/day). For redistribution or replacement, use sodium chloride or balanced crystalloids or consider colloids, which have a theoretical advantage in expanding intravascular volume while limiting interstitial edema. Note that colloids are more expensive, and definitive evidence supporting increased efficacy is lacking. Clinicians should monitor closely for hypovolemia, hypervolemia, and electrolyte abnormalities, particularly hypo- and hypernatremia that carry associated mental status implications and risk of central pontine myelinolysis. The inadvertent overuse of IVF is common in hospital settings, particularly when maintenance fluids are not discontinued upon patient improvement or when patients move between care areas. Thus, regular clinical reassessment of volume status is important.
Recommendation 4
In both noncritically ill and critically ill hospitalized patients, there is a benefit to using balanced crystalloids compared to isotonic saline in preventing major adverse kidney events and death.5,6
Two important studies in 2018 added new information to the existing NICE guidelines, addressing the previously unanswered question of the benefits of balanced crystalloids versus isotonic saline, one among non-critically ill patients and the other among critically ill patients.5,6 Prior data suggested that the use of isotonic saline is associated with multiple complications, including hyperchloremic metabolic acidosis, acute kidney injury, and death. In the non-critically ill population, the use of balanced crystalloids resulted in lower incidence of major adverse kidney events (absolute difference of 0.9%), but did not change the number of hospital days (the primary outcome).5 In the critically ill population the use of balanced crystalloids resulted in lower rates of death, new renal replacement therapy, or persistent renal dysfunction,6 and the authors found preferential use of balanced crystalloids could prevent one out of every 94 patients admitted to the ICU from experiencing these adverse outcomes. Given the similar cost associated with isotonic saline and balanced crystalloids, these new findings suggest hospitalists should select balanced crystalloids if there is no compelling clinical reason to use isotonic saline.
CRITIQUE
While conflicts of interest are often a concern in clinical guidelines due to influence by pharmaceutical, device, and specialty interests, the United Kingdom’s National Clinical Guideline Centre (NGC), which developed the NICE guidelines, is hosted by the Royal College of Physicians and has governance partnerships with the Royal College of Surgeons of England, Royal College of General Practitioners, and Royal College of Nursing. Each guideline produced by the NGC is overseen by an independent guideline committee comprised of healthcare professionals and patient representatives, and as a result, concern for conflicts of interest is low.
The NICE guidelines were created by a multidisciplinary team from multiple clinical specialties, and reviewed evidence addressing both clinical and health economic outcomes. Importantly, data from randomized controlled studies was relatively limited. The data excluded patients under 16 years of age, pregnant women, and those with severe liver or renal disease, diabetes or burns, as well as those in intensive care settings. Unfortunately, many medical patients cared for by hospitalists fall into one or more of these categories, limiting applicability of the guidelines.
Two important studies in 2018 added new information to the existing NICE guidelines, as outlined in Recommendation 4.5,6 Both of these studies occurred at a single institution, limiting their generalizability, though each study included a diverse patient population. In the ICU study, treating clinicians were aware of the composition of the assigned crystalloid so the decision to initiate renal-replacement therapy may have been susceptible to treatment bias. In addition, censoring of data collection at hospital discharge may have underestimated the true incidence of death at 30 days and overestimated persistent renal dysfunction at 30 days. Importantly, the trial design did not allow comparison of lactated Ringer’s solution versus Plasma-Lyte. The non-ICU study evaluated patients who began treatment in the emergency department and were subsequently admitted to non-ICU inpatient units—a population that mirrors much of hospitalist practice, however the un-blinded design makes bias a concern. Finally, lactated Ringer’s solution represented more than 95% of the balanced crystalloids used in the trial, so additional study is required to compare Plasma-Lyte with both saline and lactated Ringer’s solution.
AREAS IN NEED OF FUTURE STUDY
More evidence is needed to better understand the appropriate use of IVF in specific clinical scenarios, including to determine if balanced solutions, as compared with isotonic saline, are superior across a spectrum of clinical conditions. For patients with an indication for maintenance fluid administration, determining if a higher sodium content reduces the risk of hyponatremia without increasing the risk of volume overload will help guide practice. Finally, more comprehensive study of the incidence of overuse and complications as a consequence of IVF, as well as the optimal frequency of lab monitoring, is needed to guide understanding of how practicing hospitalists and health systems can help reduce harm and waste
Disclosures
The authors have nothing to disclose.
1. Minto G, Mythen MG. Perioperative fluid management: science, art or random chaos? Br J Anaesth. 2015;114(5):717–221. doi: 10.1093/bja/aev067. PubMed
2. National Clinical Guideline Centre. Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital, London: Royal College of Physicians (UK); 2013 Dec. Updated May 3, 2017. https://www.nice.org.uk/guidance/cg174. Accessed January 25, 2019.
3. Hall A, Ayus J, Moritz M. Things we do for no reason: the default use of hypotonic maintenance intravenous fluids in pediatrics. J Hosp Med. 2018;13(9):637-640. doi: 10.12788/jhm.3040. PubMed
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2016. Intensive Care Med. 2017;43(3):304-377. doi: 10.1007/s00134-017-4683-6. PubMed
5. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586. PubMed
6. Semler MW, Self WH, Rice TW. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi: 10.1056/NEJMoa1711584. PubMed
Hospitalized patients often receive intravenous fluids (IVF) when they cannot meet physiologic needs through oral intake in the setting of medical or surgical illness. Prescribing the optimal IVF solution to the appropriate patient is a complex decision and often occurs without the same degree of institutionalized restrictions or guidance developed for other inpatient pharmacologic agents. There is wide variation in clinical utilization of IVF due to the lack of data to guide decision making.1 When data do exist, they typically focus on a limited number of clinical situations.2 Thus, even though IVF are often considered low-risk, the frequency and lack of consistency with which they are used can result in errors, complications, and over-use of medical resources.3
KEY RECOMMENDATIONS FOR THE HOSPITALIST
(Evidence quality: not described in the guideline, recommendation strength: not described in the guideline)
Recommendation 1
To aid in fluid management and avoid complications, the guidelines recommend that patients on IVF require careful assessment of volume status, including a detailed history, physical exam, clinical monitoring, and daily labs.2
Clinical history should focus on understanding fluid losses and intake; physical exam should include vital signs, evidence of orthostatic hypotension, capillary refill, jugular venous pulsation, and assessment for pulmonary edema. Subsequent clinical monitoring should include fluid balance (Ins and Outs) and daily weights. All patients starting or continuing IVF should have a basic metabolic panel at least daily according to the guidelines, though the authors note this frequency may be too high for some patients and needs further study.2
Recommendation 2
The guidelines describe four types of IV fluids that can be administered: crystalloids, balanced crystalloids, glucose solutions, and non blood-product colloids.2
Crystalloids include isotonic saline with 154 millimoles (mmol) of sodium and chloride. Balanced crystalloids, such as lactated Ringer’s solution, are more physiologic, with less sodium and chloride, and the addition of magnesium, potassium, and calcium. Glucose solutions are quickly metabolized and, thus, are an effective way to deliver free water. Non blood-product colloids include particles that are retained within the circulation, including proteins such as human albumin.
Recommendation 3
For each indication to administer IVF, the guidelines recommend the following formulations and considerations:2
For general resuscitation, use crystalloids with sodium content of 130-154 mmol, delivered in a bolus of at least 500 milliliters (mL) over 15 minutes or less. For sepsis, infuse at least 30 mL/kg.4 For routine maintenance, restrict the volume to 25-30 mL/kg/day of water, and include 1 mmol/kg/day of potassium, sodium, and chloride along with 50-100 g/day of glucose to prevent starvation ketosis, though glucose should be avoided in most diabetic patients. With obesity, adjust the IVF to ideal body weight, and for patients who are older, frail, or admitted with renal or cardiac impairment, consider prescribing a lower range of fluid (20-25 mL/kg/day). For redistribution or replacement, use sodium chloride or balanced crystalloids or consider colloids, which have a theoretical advantage in expanding intravascular volume while limiting interstitial edema. Note that colloids are more expensive, and definitive evidence supporting increased efficacy is lacking. Clinicians should monitor closely for hypovolemia, hypervolemia, and electrolyte abnormalities, particularly hypo- and hypernatremia that carry associated mental status implications and risk of central pontine myelinolysis. The inadvertent overuse of IVF is common in hospital settings, particularly when maintenance fluids are not discontinued upon patient improvement or when patients move between care areas. Thus, regular clinical reassessment of volume status is important.
Recommendation 4
In both noncritically ill and critically ill hospitalized patients, there is a benefit to using balanced crystalloids compared to isotonic saline in preventing major adverse kidney events and death.5,6
Two important studies in 2018 added new information to the existing NICE guidelines, addressing the previously unanswered question of the benefits of balanced crystalloids versus isotonic saline, one among non-critically ill patients and the other among critically ill patients.5,6 Prior data suggested that the use of isotonic saline is associated with multiple complications, including hyperchloremic metabolic acidosis, acute kidney injury, and death. In the non-critically ill population, the use of balanced crystalloids resulted in lower incidence of major adverse kidney events (absolute difference of 0.9%), but did not change the number of hospital days (the primary outcome).5 In the critically ill population the use of balanced crystalloids resulted in lower rates of death, new renal replacement therapy, or persistent renal dysfunction,6 and the authors found preferential use of balanced crystalloids could prevent one out of every 94 patients admitted to the ICU from experiencing these adverse outcomes. Given the similar cost associated with isotonic saline and balanced crystalloids, these new findings suggest hospitalists should select balanced crystalloids if there is no compelling clinical reason to use isotonic saline.
CRITIQUE
While conflicts of interest are often a concern in clinical guidelines due to influence by pharmaceutical, device, and specialty interests, the United Kingdom’s National Clinical Guideline Centre (NGC), which developed the NICE guidelines, is hosted by the Royal College of Physicians and has governance partnerships with the Royal College of Surgeons of England, Royal College of General Practitioners, and Royal College of Nursing. Each guideline produced by the NGC is overseen by an independent guideline committee comprised of healthcare professionals and patient representatives, and as a result, concern for conflicts of interest is low.
The NICE guidelines were created by a multidisciplinary team from multiple clinical specialties, and reviewed evidence addressing both clinical and health economic outcomes. Importantly, data from randomized controlled studies was relatively limited. The data excluded patients under 16 years of age, pregnant women, and those with severe liver or renal disease, diabetes or burns, as well as those in intensive care settings. Unfortunately, many medical patients cared for by hospitalists fall into one or more of these categories, limiting applicability of the guidelines.
Two important studies in 2018 added new information to the existing NICE guidelines, as outlined in Recommendation 4.5,6 Both of these studies occurred at a single institution, limiting their generalizability, though each study included a diverse patient population. In the ICU study, treating clinicians were aware of the composition of the assigned crystalloid so the decision to initiate renal-replacement therapy may have been susceptible to treatment bias. In addition, censoring of data collection at hospital discharge may have underestimated the true incidence of death at 30 days and overestimated persistent renal dysfunction at 30 days. Importantly, the trial design did not allow comparison of lactated Ringer’s solution versus Plasma-Lyte. The non-ICU study evaluated patients who began treatment in the emergency department and were subsequently admitted to non-ICU inpatient units—a population that mirrors much of hospitalist practice, however the un-blinded design makes bias a concern. Finally, lactated Ringer’s solution represented more than 95% of the balanced crystalloids used in the trial, so additional study is required to compare Plasma-Lyte with both saline and lactated Ringer’s solution.
AREAS IN NEED OF FUTURE STUDY
More evidence is needed to better understand the appropriate use of IVF in specific clinical scenarios, including to determine if balanced solutions, as compared with isotonic saline, are superior across a spectrum of clinical conditions. For patients with an indication for maintenance fluid administration, determining if a higher sodium content reduces the risk of hyponatremia without increasing the risk of volume overload will help guide practice. Finally, more comprehensive study of the incidence of overuse and complications as a consequence of IVF, as well as the optimal frequency of lab monitoring, is needed to guide understanding of how practicing hospitalists and health systems can help reduce harm and waste
Disclosures
The authors have nothing to disclose.
Hospitalized patients often receive intravenous fluids (IVF) when they cannot meet physiologic needs through oral intake in the setting of medical or surgical illness. Prescribing the optimal IVF solution to the appropriate patient is a complex decision and often occurs without the same degree of institutionalized restrictions or guidance developed for other inpatient pharmacologic agents. There is wide variation in clinical utilization of IVF due to the lack of data to guide decision making.1 When data do exist, they typically focus on a limited number of clinical situations.2 Thus, even though IVF are often considered low-risk, the frequency and lack of consistency with which they are used can result in errors, complications, and over-use of medical resources.3
KEY RECOMMENDATIONS FOR THE HOSPITALIST
(Evidence quality: not described in the guideline, recommendation strength: not described in the guideline)
Recommendation 1
To aid in fluid management and avoid complications, the guidelines recommend that patients on IVF require careful assessment of volume status, including a detailed history, physical exam, clinical monitoring, and daily labs.2
Clinical history should focus on understanding fluid losses and intake; physical exam should include vital signs, evidence of orthostatic hypotension, capillary refill, jugular venous pulsation, and assessment for pulmonary edema. Subsequent clinical monitoring should include fluid balance (Ins and Outs) and daily weights. All patients starting or continuing IVF should have a basic metabolic panel at least daily according to the guidelines, though the authors note this frequency may be too high for some patients and needs further study.2
Recommendation 2
The guidelines describe four types of IV fluids that can be administered: crystalloids, balanced crystalloids, glucose solutions, and non blood-product colloids.2
Crystalloids include isotonic saline with 154 millimoles (mmol) of sodium and chloride. Balanced crystalloids, such as lactated Ringer’s solution, are more physiologic, with less sodium and chloride, and the addition of magnesium, potassium, and calcium. Glucose solutions are quickly metabolized and, thus, are an effective way to deliver free water. Non blood-product colloids include particles that are retained within the circulation, including proteins such as human albumin.
Recommendation 3
For each indication to administer IVF, the guidelines recommend the following formulations and considerations:2
For general resuscitation, use crystalloids with sodium content of 130-154 mmol, delivered in a bolus of at least 500 milliliters (mL) over 15 minutes or less. For sepsis, infuse at least 30 mL/kg.4 For routine maintenance, restrict the volume to 25-30 mL/kg/day of water, and include 1 mmol/kg/day of potassium, sodium, and chloride along with 50-100 g/day of glucose to prevent starvation ketosis, though glucose should be avoided in most diabetic patients. With obesity, adjust the IVF to ideal body weight, and for patients who are older, frail, or admitted with renal or cardiac impairment, consider prescribing a lower range of fluid (20-25 mL/kg/day). For redistribution or replacement, use sodium chloride or balanced crystalloids or consider colloids, which have a theoretical advantage in expanding intravascular volume while limiting interstitial edema. Note that colloids are more expensive, and definitive evidence supporting increased efficacy is lacking. Clinicians should monitor closely for hypovolemia, hypervolemia, and electrolyte abnormalities, particularly hypo- and hypernatremia that carry associated mental status implications and risk of central pontine myelinolysis. The inadvertent overuse of IVF is common in hospital settings, particularly when maintenance fluids are not discontinued upon patient improvement or when patients move between care areas. Thus, regular clinical reassessment of volume status is important.
Recommendation 4
In both noncritically ill and critically ill hospitalized patients, there is a benefit to using balanced crystalloids compared to isotonic saline in preventing major adverse kidney events and death.5,6
Two important studies in 2018 added new information to the existing NICE guidelines, addressing the previously unanswered question of the benefits of balanced crystalloids versus isotonic saline, one among non-critically ill patients and the other among critically ill patients.5,6 Prior data suggested that the use of isotonic saline is associated with multiple complications, including hyperchloremic metabolic acidosis, acute kidney injury, and death. In the non-critically ill population, the use of balanced crystalloids resulted in lower incidence of major adverse kidney events (absolute difference of 0.9%), but did not change the number of hospital days (the primary outcome).5 In the critically ill population the use of balanced crystalloids resulted in lower rates of death, new renal replacement therapy, or persistent renal dysfunction,6 and the authors found preferential use of balanced crystalloids could prevent one out of every 94 patients admitted to the ICU from experiencing these adverse outcomes. Given the similar cost associated with isotonic saline and balanced crystalloids, these new findings suggest hospitalists should select balanced crystalloids if there is no compelling clinical reason to use isotonic saline.
CRITIQUE
While conflicts of interest are often a concern in clinical guidelines due to influence by pharmaceutical, device, and specialty interests, the United Kingdom’s National Clinical Guideline Centre (NGC), which developed the NICE guidelines, is hosted by the Royal College of Physicians and has governance partnerships with the Royal College of Surgeons of England, Royal College of General Practitioners, and Royal College of Nursing. Each guideline produced by the NGC is overseen by an independent guideline committee comprised of healthcare professionals and patient representatives, and as a result, concern for conflicts of interest is low.
The NICE guidelines were created by a multidisciplinary team from multiple clinical specialties, and reviewed evidence addressing both clinical and health economic outcomes. Importantly, data from randomized controlled studies was relatively limited. The data excluded patients under 16 years of age, pregnant women, and those with severe liver or renal disease, diabetes or burns, as well as those in intensive care settings. Unfortunately, many medical patients cared for by hospitalists fall into one or more of these categories, limiting applicability of the guidelines.
Two important studies in 2018 added new information to the existing NICE guidelines, as outlined in Recommendation 4.5,6 Both of these studies occurred at a single institution, limiting their generalizability, though each study included a diverse patient population. In the ICU study, treating clinicians were aware of the composition of the assigned crystalloid so the decision to initiate renal-replacement therapy may have been susceptible to treatment bias. In addition, censoring of data collection at hospital discharge may have underestimated the true incidence of death at 30 days and overestimated persistent renal dysfunction at 30 days. Importantly, the trial design did not allow comparison of lactated Ringer’s solution versus Plasma-Lyte. The non-ICU study evaluated patients who began treatment in the emergency department and were subsequently admitted to non-ICU inpatient units—a population that mirrors much of hospitalist practice, however the un-blinded design makes bias a concern. Finally, lactated Ringer’s solution represented more than 95% of the balanced crystalloids used in the trial, so additional study is required to compare Plasma-Lyte with both saline and lactated Ringer’s solution.
AREAS IN NEED OF FUTURE STUDY
More evidence is needed to better understand the appropriate use of IVF in specific clinical scenarios, including to determine if balanced solutions, as compared with isotonic saline, are superior across a spectrum of clinical conditions. For patients with an indication for maintenance fluid administration, determining if a higher sodium content reduces the risk of hyponatremia without increasing the risk of volume overload will help guide practice. Finally, more comprehensive study of the incidence of overuse and complications as a consequence of IVF, as well as the optimal frequency of lab monitoring, is needed to guide understanding of how practicing hospitalists and health systems can help reduce harm and waste
Disclosures
The authors have nothing to disclose.
1. Minto G, Mythen MG. Perioperative fluid management: science, art or random chaos? Br J Anaesth. 2015;114(5):717–221. doi: 10.1093/bja/aev067. PubMed
2. National Clinical Guideline Centre. Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital, London: Royal College of Physicians (UK); 2013 Dec. Updated May 3, 2017. https://www.nice.org.uk/guidance/cg174. Accessed January 25, 2019.
3. Hall A, Ayus J, Moritz M. Things we do for no reason: the default use of hypotonic maintenance intravenous fluids in pediatrics. J Hosp Med. 2018;13(9):637-640. doi: 10.12788/jhm.3040. PubMed
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2016. Intensive Care Med. 2017;43(3):304-377. doi: 10.1007/s00134-017-4683-6. PubMed
5. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586. PubMed
6. Semler MW, Self WH, Rice TW. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi: 10.1056/NEJMoa1711584. PubMed
1. Minto G, Mythen MG. Perioperative fluid management: science, art or random chaos? Br J Anaesth. 2015;114(5):717–221. doi: 10.1093/bja/aev067. PubMed
2. National Clinical Guideline Centre. Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital, London: Royal College of Physicians (UK); 2013 Dec. Updated May 3, 2017. https://www.nice.org.uk/guidance/cg174. Accessed January 25, 2019.
3. Hall A, Ayus J, Moritz M. Things we do for no reason: the default use of hypotonic maintenance intravenous fluids in pediatrics. J Hosp Med. 2018;13(9):637-640. doi: 10.12788/jhm.3040. PubMed
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2016. Intensive Care Med. 2017;43(3):304-377. doi: 10.1007/s00134-017-4683-6. PubMed
5. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586. PubMed
6. Semler MW, Self WH, Rice TW. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi: 10.1056/NEJMoa1711584. PubMed
© 2019 Society of Hospital Medicine
What Is Career Success for Academic Hospitalists? A Qualitative Analysis of Early-Career Faculty Perspectives
Academic hospital medicine is a young specialty, with most faculty at the rank of instructor or assistant professor.1 Traditional markers of academic success for clinical and translational investigators emphasize progressive, externally funded grants, achievements in basic science research, and prolific publication in the peer-reviewed literature.2 Promotion is often used as a proxy measure for academic success.
Conceptual models of career success derived from nonhealthcare industries and for physician-scientists include both extrinsic and intrinsic domains.3,4 Extrinsic domains of career success include financial rewards (compensation) and progression in hierarchical status (advancement).3,4 Intrinsic domains of career success include pleasure derived from daily work (job satisfaction) and satisfaction derived from aspects of the career over time (career satisfaction).3,4
Research is limited regarding hospitalist faculty beliefs about career success. A better understanding of hospitalist perspectives can inform program development to support junior faculty in academic hospital medicine. In this phenomenological, qualitative study, we explore the global concept of career success as perceived by early-career clinician-educator hospitalists.
METHODS
Study Design, Setting, and Participants
We conducted interviews with hospitalists from 3 academic medical centers between May 2016 and October 2016. Purposeful sampling was used.5 Leaders within each hospital medicine group identified early-career faculty with approximately 2 to 5 years in academic medicine with a rank of instructor or assistant professor at each institution likely to self-identify as clinician-educators for targeted solicitation to enroll. Additional subjects were recruited until thematic saturation had been achieved on the personal definition of career success. Participants received disclosure and consent documents prior to enrollment. No compensation was provided to participants. This study was approved by the Colorado Multiple Institutional Review Board.
Interview Guide Development and Content
The semistructured interview format was developed and validated through an iterative process. Proposed questions were developed by study investigators on the basis of review of the literature on career success in nonhealthcare industries and academic hospitalist promotion. The questions were assessed for content validity through a review of interview domains by an academic hospitalist program director (R. P.). Cognitive interviewing with 3 representative academic hospitalists who were not part of the study cohort was done as an additional face-validation step of the question probe structure. As a result of the cognitive interviews, 1 question was eliminated, and a framework for clarifications and answer probes was derived prior to the enrollment of the first study subject. No changes were made to the interview format during the study period.
Data Collection
The principal investigator (E.C.) performed all interviews by using the interview tool consisting of 7 demographic questions and 11 open-ended questions and exploring aspects of the concept of career success. The initial open-ended question, “How would you personally define career success as an academic hospitalist at this stage in your career?” represented the primary question of interest. Follow-up questions were used to better understand responses to the primary question. All interviews were audio recorded, deidentified, and transcribed by the principal investigator. Transcripts were randomly audited by a second investigator (E.Y.) for accuracy and completeness.
Sample Size Determination
Interviews were continued to thematic saturation. After the first 3 interviews were transcribed, 2 members of the research team (E.C. and P.K.) reviewed the transcripts and developed a preliminary thematic codebook for the primary question. Subsequent interviews were reviewed and analyzed against these themes. Interviews were continued to thematic saturation, which was defined as more than 3 sequential interviews with no new identified themes.6
Data Analysis
By using qualitative data analysis software (ATLAS.ti version 7; ATLAS.ti Scientific Software Development GmbH, Berlin, Germany), transcriptions were analyzed with a team-based, mixed inductive-deductive approach. An inductive approach was utilized to allow basic theme codes to emerge from the raw text, and thus remaining open to unanticipated themes. Investigators assessed each distinct quote for new themes, confirmatory themes, and challenges to previously developed concepts. Basic themes were then discussed among research team members to determine prominent themes, with basic theme codes added, removed, or combined at this stage of the analysis. Responses to each follow-up question were subsequently assessed for new themes, confirmatory themes, or challenges to previously developed concepts related to the personal definition of career success. A deductive approach was then used to map our inductively generated themes back to the organizing themes of the existing conceptual framework.
RESULTS
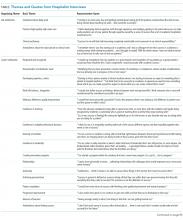
Thematic Mapping to Organizing Themes of the Conceptual Model (Table)
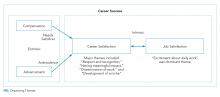
The single most dominant theme, “excitement about daily work” was connected to an intrinsic sense of job satisfaction. Career satisfaction emerged from interviews more frequently than extrinsic organizing themes, such as advancement or compensation. Advancement through promotion was infrequently referenced as part of success, and tenure was never raised despite being available for clinician-educators at 2 of the 3 institutions. Compensation was not referenced in any interviewee’s initial definition of career success, although in 1 interview, it came up in response to a follow-up question. The Figure visually represents the relative weighting (shown by the sizes of the boxes) of organizing themes to the early-career hospitalists’ self-concepts of career success. Relationships among organizing themes as they emerged from interviews are represented by arrows.
Intrinsic—Job Satisfaction
With regard to job satisfaction, early-career faculty often invoked words such as “excitement,” “enjoyment,” and “passionate” to describe an overall theme of “excitement about daily work.” A positive affective state created by the nature of daily work was described as integral to the personal sense of career success. It was also strongly associated with perception of sustainability in a hospitalist career.
“I think [career success] would be job satisfaction. …So, for me, that would be happiness with my job. I like coming to work. I like doing what I do and at the end of the day going home and saying that was a good day. I like to think that would be success at work…is how I would define it.”
This theme was also related to a negative aspect often referred to as burnout, which many identified as antithetical to career success. More often, they described success as a heightened state of enthusiasm for the daily work experience.
“I am staying engaged and excited. So, I am not just taking care of patients; I am not just teaching. Having enough excitement from my work to come home and talk about it at dinner. To enjoy my days off but at the same time being excited to get back to work.”
This description of passion toward the work of being a hospitalist was often linked to a sense of deeper purpose found through the delivery of clinical care and education of learners.
“I really feel that we have the opportunity to very meaningfully and powerfully impact people’s lives, and that to me is meaningful. …That’s value. ...That’s coming home at the end of the day and thinking that you have had a positive impact.”
The interviews reflected that core to meaningful work was a sense of personal efficacy as a clinician, which was reflected in the themes of clinical proficiency and practicing high-quality care.
“I think developing clinical expertise, both through experience and studying. Getting to the point to where you can take really excellent care of your patient through expertise would be a sense of success that a lot of academic hospitalists would strive for.”
Intrinsic—Career Satisfaction
Within career satisfaction, participants described that “being respected and recognized” and “dissemination of work” were important contributors to career success. Reputation was frequently referenced as a measure of career success. Reputation was defined by some in a local context of having the respect of learners, peers, and others as a national renown. As a prerequisite for developing a reputation beyond the local academic environment, dissemination of work was often referenced as an important component of satisfaction in the career. This dissemination extended beyond peer-reviewed publications and included other forms of scholarship, presentations at conferences, and sharing clinical innovations between hospitals.
“For me personally, I have less of an emphasis on research and some of the more, I don’t want to say ‘academic’ because I think education is academic, but maybe some of the more scholarly practice of medicine, doing research and the writing of papers and things like that, although I certainly view some of that as a part of career success.”
Within career satisfaction, participants also described a diverse set of themes, including progressive improvement in skills, developing a self-perception of excellence in 1 or more arenas of academic medicine, leadership, work–life integration, innovation, and relationships. The concept of developing a niche, or becoming an expert in a particular domain of hospital medicine, was frequently referenced.
“I think part of [success] is ‘Have they identified a niche?’ Because I think if you want to be in an academic center, as much as I value teaching and taking care of patients, I think 1 of the advantages is the opportunity to potentially identify an area of expertise.”
Participants frequently alluded to the idea that the most important aspects of career satisfaction are not static phenomena but rather values that could evolve over the course of a career. For instance, in the early-career, making a difference with individual learners or patients could have greater valence, but as the career progressed, finding a niche, disseminating work, and building a national reputation would gain importance to a personal sense of career satisfaction.
Extrinsic—Advancement
Promotion was typically referenced when discussing career success, but it was not uniformly valued by early career hospitalists. Some expressed significant ambivalence about its effect on their personal sense of career success. Academic hospitalists identified a number of organizations with definitions of success that influence them. Definitions of success for the university were more relevant to interviewees compared to those of the hospital or professional societies. Interviewees were able to describe a variety of criteria by which their universities define or recognize career success. These commonly included promotion, publications and/or scholarship, and research. The list of factors perceived as success by the hospital were often distinct from those of the university and included cost-effective care, patient safety, and clinical leadership roles.
Participants described a sense of internal conflict when external-stakeholder definitions of success diverged from internal motivators. This was particularly true when this divergence led academic hospitalists to engage in activities for advancement that they did not find personally fulfilling. Academic hospitalists recognized that advancement was central to the concept of career success for organizations even if this was not identified as being core to their personal definitions of success.
“I think that for me, the idea of being promoted and being a leader in the field is less important to me than...for the organization.”
Hospitalists expressed that objective markers, such as promotion and publications, were perceived as more important at higher levels of the academic organization, whereas more subjective aspects of success, aligned with intrinsic personal definitions, were more valued within the hospital medicine group.
Extrinsic—Compensation
Compensation was notable for its absence in participants’ discussion of career success. When asked about their definitions of career success, academic hospitalists did not spontaneously raise the topic of compensation. The only mention of compensation was in response to a question about how personal and external definitions of career success differ.
Unexpected Findings
While it was almost universally recognized by participants as important, ambivalence toward the “academic value of clinical work,” “scholarship,” and especially “promotion” represented an unexpected thematic family.
“I can’t quite get excited about a title attached to my name or the number of times my name pops up when I enter it into PubMed. My personal definition is more…where do I have something that I am interested [in] that someone else values. And that value is not shown as an associate professorship or an assistant professorship next to my name. …When you push me on it, you could call me clinical instructor forever, and I don’t think I would care too much.”
The interaction between work and personal activities as representing complementary aspects of a global sense of success was also unexpected and ran contrary to a simplistic conception of work and life in conflict. Academic hospitalists referenced that the ability to participate in aspects of life external to the workplace was important to their sense of career success. Participants frequently used phrases such as “work–life balance” to encompass a larger sense that work and nonwork life needed to merge to form a holistic sense of having a positive impact.
“Personal success is becoming what I have termed a ‘man of worth.’ I think [that is] someone who feels as though they make a positive impact in the world. Through both my career, but I guess the things that I do that are external to my career. Those would be defined by being a good husband, a good son, a philanthropist out in the community…sometimes, these are not things that can necessarily go on a [curriculum vitae].”
Conflict Among Organizing Themes
At times, academic hospitalists described a tension between day-to-day job satisfaction and what would be necessary to accomplish longer-term career success in the other organizing themes. This was reflected by a sense of trade-off. For instance, activities that lead to some aspects of career satisfaction or advancement would take time away from the direct exposure to learners and clinical care that currently drive job satisfaction.
“If the institution wanted me to be more productive from a research standpoint or…advocate that I receive funding so I could buy down clinical time and interactions I have with my students and my patients, then I can see my satisfaction going down.”
Many described a sense of engaging in activities they did not find personally fulfilling because of a sense of expectation that those activities were considered successful by others. Some described a state in which the drive toward advancement as an extrinsic incentive could come at the expense of the intrinsic rewards of being an academic hospitalist.
DISCUSSION
Career success has been defined as “the positive psychological or work-related outcomes or achievements one accumulates as a result of work experiences.”4,7,8 Academic career success for hospitalist faculty isn’t as well defined and has not been examined from the perspectives of early-career clinician-educator hospitalist faculty themselves.
The themes that emerged in this study describe a definition of success anchored in the daily work of striving to become an exceptional clinician and teacher. The major themes included (1) having excitement about daily work, (2) having meaningful impact, (3) development of a niche (4) a sense of respect within the sphere of academic medicine, and (5) disseminating work.
Success was very much internally defined as having a positive, meaningful impact on patients, learners, and the systems in which they practice. The faculty had a conception of what promotion committees value and often internalized aspects of this, such as developing a national reputation and giving talks at national meetings. Participants typically self-identified as clinician-educators, and yet dissemination of work remained an important component of personal success. While promotion was clearly identified as a marker of success, academic hospitalists often rejected the supposition of promotion itself as a professional goal. They expressed hope, and some skepticism, that external recognition of career success would follow the pursuit of internally meaningful goals.
While promotion and peer-reviewed publications represent easily measured markers often used as proxies for individual career and programmatic success, our research demonstrates that there is a deep well of externally imperceptible influences on an individual’s sense of success as an academic hospitalist. In our analysis, intrinsic elements of career success received far greater weight with early-career academic hospitalists. Our findings are supported by a prior survey of academic physicians that similarly found that faculty with >50% of their time devoted to clinical care placed greater career value in patient care, relationships with patients, and recognition by patients and residents compared to national reputation.9 Similar to our own findings, highly clinical faculty in that study were also less likely to value promotion and tenure as indicators of career success.9
The main focus of our questions was how early-career faculty define success at this point in their careers. When asked to extrapolate to a future state of career success, the concept of progression was repeatedly raised. This included successive promotions to higher academic ranks, increasing responsibility, titles, leadership, and achieving competitive roles or awards. It also included a progressively increasing impact of scholarship, growing national reputation, and becoming part of a network of accomplished academic hospitalists across the country. Looking forward, our early-career hospitalists felt that long-term career success would represent accomplishing these things and still being able to be focused on being excellent clinicians to patients, having a work–life balance, and keeping joy and excitement in daily activities.
Our work has limitations, including a focus on early-career clinician-educator hospitalists. The perception of career success may evolve over time, and future work to examine perceptions in more advanced academic hospitalists would be of interest. Our work used purposeful sampling to capture individuals who were likely to self-identify as academic clinician-educators, and results may not generalize to hospitalist physician-scientists or hospitalists in community practices.
Our analysis suggests that external organizations influence internal perceptions of career success. However, success is ultimately defined by the individual and not the institution. Efforts to measure and improve academic hospitalists’ attainment of career success should attend to intrinsic aspects of satisfaction in addition to objective measures, such as publications and promotion. This may provide a mechanism to address burnout and improve retention. As important as commonality in themes is the variation in self-definitions of career success among individuals. This suggests the value of inquiry by academic leadership in exploring and understanding what success is from the individual faculty perspective. This may enhance the alignment among personal definitions, organizational values, and, ultimately, sustainable, successful careers.
Disclosure: The authors have nothing to disclose.
1. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US Academic Hospitalist Leaders About Mentorship and Academic Activities in Hospitalist Groups. J Hosp Med. 2011;6(1):5-9. PubMed
2. Buddeberg-Fischer B, Stamm M, Buddeberg C, Klaghofer R. Career-Success Scale. A New Instrument to Assess Young Physicians Academic Career Steps. BMC Health Serv Res. 2008;8:120. PubMed
3. Rubio DM, Primack BA, Switzer GE, Bryce CL, Selzer DL, Kapoor WN. A Comprehensive Career-Success Model for Physician-Scientists. Acad Med. 2011;86(12):1571-1576. PubMed
4. Judge TA, Cable DM, Boudreau JW, Bretz RD. An empirical investigation of the predictors of executive career success (CAHRS Working Paper #94-08). Ithaca, NY: Cornell University, School of Industrial and Labor Relations, Center for Advanced Human Resource Studies. 1994. http://digitalcommons.ilr.cornell.edu/cahrswp/233. Accessed November 27, 2017.
5. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42(5):533-544. PubMed
6. Francis JJ, Johnston M, Robertson C, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health. 2010;25(10):1229-1245. PubMed
7. Abele AE, Spurk, D. The longitudinal impact of self-efficacy and career goals on objective and subjective career success. J Vocat Behav. 2009;74(1):53-62.
8. Seibert SE, Kraimer ML. The five-factor model of personality and career success. J Vocat Behav. 2011;58(1):1-21.
9. Buckley, LM, Sanders K, Shih M, Hampton CL. Attitudes of Clinical Faculty About Career Progress, Career Success, and Commitment to Academic Medicine: Results of a Survey. Arch Intern Med. 2000;160(17):2625-2629. PubMed
Academic hospital medicine is a young specialty, with most faculty at the rank of instructor or assistant professor.1 Traditional markers of academic success for clinical and translational investigators emphasize progressive, externally funded grants, achievements in basic science research, and prolific publication in the peer-reviewed literature.2 Promotion is often used as a proxy measure for academic success.
Conceptual models of career success derived from nonhealthcare industries and for physician-scientists include both extrinsic and intrinsic domains.3,4 Extrinsic domains of career success include financial rewards (compensation) and progression in hierarchical status (advancement).3,4 Intrinsic domains of career success include pleasure derived from daily work (job satisfaction) and satisfaction derived from aspects of the career over time (career satisfaction).3,4
Research is limited regarding hospitalist faculty beliefs about career success. A better understanding of hospitalist perspectives can inform program development to support junior faculty in academic hospital medicine. In this phenomenological, qualitative study, we explore the global concept of career success as perceived by early-career clinician-educator hospitalists.
METHODS
Study Design, Setting, and Participants
We conducted interviews with hospitalists from 3 academic medical centers between May 2016 and October 2016. Purposeful sampling was used.5 Leaders within each hospital medicine group identified early-career faculty with approximately 2 to 5 years in academic medicine with a rank of instructor or assistant professor at each institution likely to self-identify as clinician-educators for targeted solicitation to enroll. Additional subjects were recruited until thematic saturation had been achieved on the personal definition of career success. Participants received disclosure and consent documents prior to enrollment. No compensation was provided to participants. This study was approved by the Colorado Multiple Institutional Review Board.
Interview Guide Development and Content
The semistructured interview format was developed and validated through an iterative process. Proposed questions were developed by study investigators on the basis of review of the literature on career success in nonhealthcare industries and academic hospitalist promotion. The questions were assessed for content validity through a review of interview domains by an academic hospitalist program director (R. P.). Cognitive interviewing with 3 representative academic hospitalists who were not part of the study cohort was done as an additional face-validation step of the question probe structure. As a result of the cognitive interviews, 1 question was eliminated, and a framework for clarifications and answer probes was derived prior to the enrollment of the first study subject. No changes were made to the interview format during the study period.
Data Collection
The principal investigator (E.C.) performed all interviews by using the interview tool consisting of 7 demographic questions and 11 open-ended questions and exploring aspects of the concept of career success. The initial open-ended question, “How would you personally define career success as an academic hospitalist at this stage in your career?” represented the primary question of interest. Follow-up questions were used to better understand responses to the primary question. All interviews were audio recorded, deidentified, and transcribed by the principal investigator. Transcripts were randomly audited by a second investigator (E.Y.) for accuracy and completeness.
Sample Size Determination
Interviews were continued to thematic saturation. After the first 3 interviews were transcribed, 2 members of the research team (E.C. and P.K.) reviewed the transcripts and developed a preliminary thematic codebook for the primary question. Subsequent interviews were reviewed and analyzed against these themes. Interviews were continued to thematic saturation, which was defined as more than 3 sequential interviews with no new identified themes.6
Data Analysis
By using qualitative data analysis software (ATLAS.ti version 7; ATLAS.ti Scientific Software Development GmbH, Berlin, Germany), transcriptions were analyzed with a team-based, mixed inductive-deductive approach. An inductive approach was utilized to allow basic theme codes to emerge from the raw text, and thus remaining open to unanticipated themes. Investigators assessed each distinct quote for new themes, confirmatory themes, and challenges to previously developed concepts. Basic themes were then discussed among research team members to determine prominent themes, with basic theme codes added, removed, or combined at this stage of the analysis. Responses to each follow-up question were subsequently assessed for new themes, confirmatory themes, or challenges to previously developed concepts related to the personal definition of career success. A deductive approach was then used to map our inductively generated themes back to the organizing themes of the existing conceptual framework.
RESULTS
Thematic Mapping to Organizing Themes of the Conceptual Model (Table)
The single most dominant theme, “excitement about daily work” was connected to an intrinsic sense of job satisfaction. Career satisfaction emerged from interviews more frequently than extrinsic organizing themes, such as advancement or compensation. Advancement through promotion was infrequently referenced as part of success, and tenure was never raised despite being available for clinician-educators at 2 of the 3 institutions. Compensation was not referenced in any interviewee’s initial definition of career success, although in 1 interview, it came up in response to a follow-up question. The Figure visually represents the relative weighting (shown by the sizes of the boxes) of organizing themes to the early-career hospitalists’ self-concepts of career success. Relationships among organizing themes as they emerged from interviews are represented by arrows.
Intrinsic—Job Satisfaction
With regard to job satisfaction, early-career faculty often invoked words such as “excitement,” “enjoyment,” and “passionate” to describe an overall theme of “excitement about daily work.” A positive affective state created by the nature of daily work was described as integral to the personal sense of career success. It was also strongly associated with perception of sustainability in a hospitalist career.
“I think [career success] would be job satisfaction. …So, for me, that would be happiness with my job. I like coming to work. I like doing what I do and at the end of the day going home and saying that was a good day. I like to think that would be success at work…is how I would define it.”
This theme was also related to a negative aspect often referred to as burnout, which many identified as antithetical to career success. More often, they described success as a heightened state of enthusiasm for the daily work experience.
“I am staying engaged and excited. So, I am not just taking care of patients; I am not just teaching. Having enough excitement from my work to come home and talk about it at dinner. To enjoy my days off but at the same time being excited to get back to work.”
This description of passion toward the work of being a hospitalist was often linked to a sense of deeper purpose found through the delivery of clinical care and education of learners.
“I really feel that we have the opportunity to very meaningfully and powerfully impact people’s lives, and that to me is meaningful. …That’s value. ...That’s coming home at the end of the day and thinking that you have had a positive impact.”
The interviews reflected that core to meaningful work was a sense of personal efficacy as a clinician, which was reflected in the themes of clinical proficiency and practicing high-quality care.
“I think developing clinical expertise, both through experience and studying. Getting to the point to where you can take really excellent care of your patient through expertise would be a sense of success that a lot of academic hospitalists would strive for.”
Intrinsic—Career Satisfaction
Within career satisfaction, participants described that “being respected and recognized” and “dissemination of work” were important contributors to career success. Reputation was frequently referenced as a measure of career success. Reputation was defined by some in a local context of having the respect of learners, peers, and others as a national renown. As a prerequisite for developing a reputation beyond the local academic environment, dissemination of work was often referenced as an important component of satisfaction in the career. This dissemination extended beyond peer-reviewed publications and included other forms of scholarship, presentations at conferences, and sharing clinical innovations between hospitals.
“For me personally, I have less of an emphasis on research and some of the more, I don’t want to say ‘academic’ because I think education is academic, but maybe some of the more scholarly practice of medicine, doing research and the writing of papers and things like that, although I certainly view some of that as a part of career success.”
Within career satisfaction, participants also described a diverse set of themes, including progressive improvement in skills, developing a self-perception of excellence in 1 or more arenas of academic medicine, leadership, work–life integration, innovation, and relationships. The concept of developing a niche, or becoming an expert in a particular domain of hospital medicine, was frequently referenced.
“I think part of [success] is ‘Have they identified a niche?’ Because I think if you want to be in an academic center, as much as I value teaching and taking care of patients, I think 1 of the advantages is the opportunity to potentially identify an area of expertise.”
Participants frequently alluded to the idea that the most important aspects of career satisfaction are not static phenomena but rather values that could evolve over the course of a career. For instance, in the early-career, making a difference with individual learners or patients could have greater valence, but as the career progressed, finding a niche, disseminating work, and building a national reputation would gain importance to a personal sense of career satisfaction.
Extrinsic—Advancement
Promotion was typically referenced when discussing career success, but it was not uniformly valued by early career hospitalists. Some expressed significant ambivalence about its effect on their personal sense of career success. Academic hospitalists identified a number of organizations with definitions of success that influence them. Definitions of success for the university were more relevant to interviewees compared to those of the hospital or professional societies. Interviewees were able to describe a variety of criteria by which their universities define or recognize career success. These commonly included promotion, publications and/or scholarship, and research. The list of factors perceived as success by the hospital were often distinct from those of the university and included cost-effective care, patient safety, and clinical leadership roles.
Participants described a sense of internal conflict when external-stakeholder definitions of success diverged from internal motivators. This was particularly true when this divergence led academic hospitalists to engage in activities for advancement that they did not find personally fulfilling. Academic hospitalists recognized that advancement was central to the concept of career success for organizations even if this was not identified as being core to their personal definitions of success.
“I think that for me, the idea of being promoted and being a leader in the field is less important to me than...for the organization.”
Hospitalists expressed that objective markers, such as promotion and publications, were perceived as more important at higher levels of the academic organization, whereas more subjective aspects of success, aligned with intrinsic personal definitions, were more valued within the hospital medicine group.
Extrinsic—Compensation
Compensation was notable for its absence in participants’ discussion of career success. When asked about their definitions of career success, academic hospitalists did not spontaneously raise the topic of compensation. The only mention of compensation was in response to a question about how personal and external definitions of career success differ.
Unexpected Findings
While it was almost universally recognized by participants as important, ambivalence toward the “academic value of clinical work,” “scholarship,” and especially “promotion” represented an unexpected thematic family.
“I can’t quite get excited about a title attached to my name or the number of times my name pops up when I enter it into PubMed. My personal definition is more…where do I have something that I am interested [in] that someone else values. And that value is not shown as an associate professorship or an assistant professorship next to my name. …When you push me on it, you could call me clinical instructor forever, and I don’t think I would care too much.”
The interaction between work and personal activities as representing complementary aspects of a global sense of success was also unexpected and ran contrary to a simplistic conception of work and life in conflict. Academic hospitalists referenced that the ability to participate in aspects of life external to the workplace was important to their sense of career success. Participants frequently used phrases such as “work–life balance” to encompass a larger sense that work and nonwork life needed to merge to form a holistic sense of having a positive impact.
“Personal success is becoming what I have termed a ‘man of worth.’ I think [that is] someone who feels as though they make a positive impact in the world. Through both my career, but I guess the things that I do that are external to my career. Those would be defined by being a good husband, a good son, a philanthropist out in the community…sometimes, these are not things that can necessarily go on a [curriculum vitae].”
Conflict Among Organizing Themes
At times, academic hospitalists described a tension between day-to-day job satisfaction and what would be necessary to accomplish longer-term career success in the other organizing themes. This was reflected by a sense of trade-off. For instance, activities that lead to some aspects of career satisfaction or advancement would take time away from the direct exposure to learners and clinical care that currently drive job satisfaction.
“If the institution wanted me to be more productive from a research standpoint or…advocate that I receive funding so I could buy down clinical time and interactions I have with my students and my patients, then I can see my satisfaction going down.”
Many described a sense of engaging in activities they did not find personally fulfilling because of a sense of expectation that those activities were considered successful by others. Some described a state in which the drive toward advancement as an extrinsic incentive could come at the expense of the intrinsic rewards of being an academic hospitalist.
DISCUSSION
Career success has been defined as “the positive psychological or work-related outcomes or achievements one accumulates as a result of work experiences.”4,7,8 Academic career success for hospitalist faculty isn’t as well defined and has not been examined from the perspectives of early-career clinician-educator hospitalist faculty themselves.
The themes that emerged in this study describe a definition of success anchored in the daily work of striving to become an exceptional clinician and teacher. The major themes included (1) having excitement about daily work, (2) having meaningful impact, (3) development of a niche (4) a sense of respect within the sphere of academic medicine, and (5) disseminating work.
Success was very much internally defined as having a positive, meaningful impact on patients, learners, and the systems in which they practice. The faculty had a conception of what promotion committees value and often internalized aspects of this, such as developing a national reputation and giving talks at national meetings. Participants typically self-identified as clinician-educators, and yet dissemination of work remained an important component of personal success. While promotion was clearly identified as a marker of success, academic hospitalists often rejected the supposition of promotion itself as a professional goal. They expressed hope, and some skepticism, that external recognition of career success would follow the pursuit of internally meaningful goals.
While promotion and peer-reviewed publications represent easily measured markers often used as proxies for individual career and programmatic success, our research demonstrates that there is a deep well of externally imperceptible influences on an individual’s sense of success as an academic hospitalist. In our analysis, intrinsic elements of career success received far greater weight with early-career academic hospitalists. Our findings are supported by a prior survey of academic physicians that similarly found that faculty with >50% of their time devoted to clinical care placed greater career value in patient care, relationships with patients, and recognition by patients and residents compared to national reputation.9 Similar to our own findings, highly clinical faculty in that study were also less likely to value promotion and tenure as indicators of career success.9
The main focus of our questions was how early-career faculty define success at this point in their careers. When asked to extrapolate to a future state of career success, the concept of progression was repeatedly raised. This included successive promotions to higher academic ranks, increasing responsibility, titles, leadership, and achieving competitive roles or awards. It also included a progressively increasing impact of scholarship, growing national reputation, and becoming part of a network of accomplished academic hospitalists across the country. Looking forward, our early-career hospitalists felt that long-term career success would represent accomplishing these things and still being able to be focused on being excellent clinicians to patients, having a work–life balance, and keeping joy and excitement in daily activities.
Our work has limitations, including a focus on early-career clinician-educator hospitalists. The perception of career success may evolve over time, and future work to examine perceptions in more advanced academic hospitalists would be of interest. Our work used purposeful sampling to capture individuals who were likely to self-identify as academic clinician-educators, and results may not generalize to hospitalist physician-scientists or hospitalists in community practices.
Our analysis suggests that external organizations influence internal perceptions of career success. However, success is ultimately defined by the individual and not the institution. Efforts to measure and improve academic hospitalists’ attainment of career success should attend to intrinsic aspects of satisfaction in addition to objective measures, such as publications and promotion. This may provide a mechanism to address burnout and improve retention. As important as commonality in themes is the variation in self-definitions of career success among individuals. This suggests the value of inquiry by academic leadership in exploring and understanding what success is from the individual faculty perspective. This may enhance the alignment among personal definitions, organizational values, and, ultimately, sustainable, successful careers.
Disclosure: The authors have nothing to disclose.
Academic hospital medicine is a young specialty, with most faculty at the rank of instructor or assistant professor.1 Traditional markers of academic success for clinical and translational investigators emphasize progressive, externally funded grants, achievements in basic science research, and prolific publication in the peer-reviewed literature.2 Promotion is often used as a proxy measure for academic success.
Conceptual models of career success derived from nonhealthcare industries and for physician-scientists include both extrinsic and intrinsic domains.3,4 Extrinsic domains of career success include financial rewards (compensation) and progression in hierarchical status (advancement).3,4 Intrinsic domains of career success include pleasure derived from daily work (job satisfaction) and satisfaction derived from aspects of the career over time (career satisfaction).3,4
Research is limited regarding hospitalist faculty beliefs about career success. A better understanding of hospitalist perspectives can inform program development to support junior faculty in academic hospital medicine. In this phenomenological, qualitative study, we explore the global concept of career success as perceived by early-career clinician-educator hospitalists.
METHODS
Study Design, Setting, and Participants
We conducted interviews with hospitalists from 3 academic medical centers between May 2016 and October 2016. Purposeful sampling was used.5 Leaders within each hospital medicine group identified early-career faculty with approximately 2 to 5 years in academic medicine with a rank of instructor or assistant professor at each institution likely to self-identify as clinician-educators for targeted solicitation to enroll. Additional subjects were recruited until thematic saturation had been achieved on the personal definition of career success. Participants received disclosure and consent documents prior to enrollment. No compensation was provided to participants. This study was approved by the Colorado Multiple Institutional Review Board.
Interview Guide Development and Content
The semistructured interview format was developed and validated through an iterative process. Proposed questions were developed by study investigators on the basis of review of the literature on career success in nonhealthcare industries and academic hospitalist promotion. The questions were assessed for content validity through a review of interview domains by an academic hospitalist program director (R. P.). Cognitive interviewing with 3 representative academic hospitalists who were not part of the study cohort was done as an additional face-validation step of the question probe structure. As a result of the cognitive interviews, 1 question was eliminated, and a framework for clarifications and answer probes was derived prior to the enrollment of the first study subject. No changes were made to the interview format during the study period.
Data Collection
The principal investigator (E.C.) performed all interviews by using the interview tool consisting of 7 demographic questions and 11 open-ended questions and exploring aspects of the concept of career success. The initial open-ended question, “How would you personally define career success as an academic hospitalist at this stage in your career?” represented the primary question of interest. Follow-up questions were used to better understand responses to the primary question. All interviews were audio recorded, deidentified, and transcribed by the principal investigator. Transcripts were randomly audited by a second investigator (E.Y.) for accuracy and completeness.
Sample Size Determination
Interviews were continued to thematic saturation. After the first 3 interviews were transcribed, 2 members of the research team (E.C. and P.K.) reviewed the transcripts and developed a preliminary thematic codebook for the primary question. Subsequent interviews were reviewed and analyzed against these themes. Interviews were continued to thematic saturation, which was defined as more than 3 sequential interviews with no new identified themes.6
Data Analysis
By using qualitative data analysis software (ATLAS.ti version 7; ATLAS.ti Scientific Software Development GmbH, Berlin, Germany), transcriptions were analyzed with a team-based, mixed inductive-deductive approach. An inductive approach was utilized to allow basic theme codes to emerge from the raw text, and thus remaining open to unanticipated themes. Investigators assessed each distinct quote for new themes, confirmatory themes, and challenges to previously developed concepts. Basic themes were then discussed among research team members to determine prominent themes, with basic theme codes added, removed, or combined at this stage of the analysis. Responses to each follow-up question were subsequently assessed for new themes, confirmatory themes, or challenges to previously developed concepts related to the personal definition of career success. A deductive approach was then used to map our inductively generated themes back to the organizing themes of the existing conceptual framework.
RESULTS
Thematic Mapping to Organizing Themes of the Conceptual Model (Table)
The single most dominant theme, “excitement about daily work” was connected to an intrinsic sense of job satisfaction. Career satisfaction emerged from interviews more frequently than extrinsic organizing themes, such as advancement or compensation. Advancement through promotion was infrequently referenced as part of success, and tenure was never raised despite being available for clinician-educators at 2 of the 3 institutions. Compensation was not referenced in any interviewee’s initial definition of career success, although in 1 interview, it came up in response to a follow-up question. The Figure visually represents the relative weighting (shown by the sizes of the boxes) of organizing themes to the early-career hospitalists’ self-concepts of career success. Relationships among organizing themes as they emerged from interviews are represented by arrows.
Intrinsic—Job Satisfaction
With regard to job satisfaction, early-career faculty often invoked words such as “excitement,” “enjoyment,” and “passionate” to describe an overall theme of “excitement about daily work.” A positive affective state created by the nature of daily work was described as integral to the personal sense of career success. It was also strongly associated with perception of sustainability in a hospitalist career.
“I think [career success] would be job satisfaction. …So, for me, that would be happiness with my job. I like coming to work. I like doing what I do and at the end of the day going home and saying that was a good day. I like to think that would be success at work…is how I would define it.”
This theme was also related to a negative aspect often referred to as burnout, which many identified as antithetical to career success. More often, they described success as a heightened state of enthusiasm for the daily work experience.
“I am staying engaged and excited. So, I am not just taking care of patients; I am not just teaching. Having enough excitement from my work to come home and talk about it at dinner. To enjoy my days off but at the same time being excited to get back to work.”
This description of passion toward the work of being a hospitalist was often linked to a sense of deeper purpose found through the delivery of clinical care and education of learners.
“I really feel that we have the opportunity to very meaningfully and powerfully impact people’s lives, and that to me is meaningful. …That’s value. ...That’s coming home at the end of the day and thinking that you have had a positive impact.”
The interviews reflected that core to meaningful work was a sense of personal efficacy as a clinician, which was reflected in the themes of clinical proficiency and practicing high-quality care.
“I think developing clinical expertise, both through experience and studying. Getting to the point to where you can take really excellent care of your patient through expertise would be a sense of success that a lot of academic hospitalists would strive for.”
Intrinsic—Career Satisfaction
Within career satisfaction, participants described that “being respected and recognized” and “dissemination of work” were important contributors to career success. Reputation was frequently referenced as a measure of career success. Reputation was defined by some in a local context of having the respect of learners, peers, and others as a national renown. As a prerequisite for developing a reputation beyond the local academic environment, dissemination of work was often referenced as an important component of satisfaction in the career. This dissemination extended beyond peer-reviewed publications and included other forms of scholarship, presentations at conferences, and sharing clinical innovations between hospitals.
“For me personally, I have less of an emphasis on research and some of the more, I don’t want to say ‘academic’ because I think education is academic, but maybe some of the more scholarly practice of medicine, doing research and the writing of papers and things like that, although I certainly view some of that as a part of career success.”
Within career satisfaction, participants also described a diverse set of themes, including progressive improvement in skills, developing a self-perception of excellence in 1 or more arenas of academic medicine, leadership, work–life integration, innovation, and relationships. The concept of developing a niche, or becoming an expert in a particular domain of hospital medicine, was frequently referenced.
“I think part of [success] is ‘Have they identified a niche?’ Because I think if you want to be in an academic center, as much as I value teaching and taking care of patients, I think 1 of the advantages is the opportunity to potentially identify an area of expertise.”
Participants frequently alluded to the idea that the most important aspects of career satisfaction are not static phenomena but rather values that could evolve over the course of a career. For instance, in the early-career, making a difference with individual learners or patients could have greater valence, but as the career progressed, finding a niche, disseminating work, and building a national reputation would gain importance to a personal sense of career satisfaction.
Extrinsic—Advancement
Promotion was typically referenced when discussing career success, but it was not uniformly valued by early career hospitalists. Some expressed significant ambivalence about its effect on their personal sense of career success. Academic hospitalists identified a number of organizations with definitions of success that influence them. Definitions of success for the university were more relevant to interviewees compared to those of the hospital or professional societies. Interviewees were able to describe a variety of criteria by which their universities define or recognize career success. These commonly included promotion, publications and/or scholarship, and research. The list of factors perceived as success by the hospital were often distinct from those of the university and included cost-effective care, patient safety, and clinical leadership roles.
Participants described a sense of internal conflict when external-stakeholder definitions of success diverged from internal motivators. This was particularly true when this divergence led academic hospitalists to engage in activities for advancement that they did not find personally fulfilling. Academic hospitalists recognized that advancement was central to the concept of career success for organizations even if this was not identified as being core to their personal definitions of success.
“I think that for me, the idea of being promoted and being a leader in the field is less important to me than...for the organization.”
Hospitalists expressed that objective markers, such as promotion and publications, were perceived as more important at higher levels of the academic organization, whereas more subjective aspects of success, aligned with intrinsic personal definitions, were more valued within the hospital medicine group.
Extrinsic—Compensation
Compensation was notable for its absence in participants’ discussion of career success. When asked about their definitions of career success, academic hospitalists did not spontaneously raise the topic of compensation. The only mention of compensation was in response to a question about how personal and external definitions of career success differ.
Unexpected Findings
While it was almost universally recognized by participants as important, ambivalence toward the “academic value of clinical work,” “scholarship,” and especially “promotion” represented an unexpected thematic family.
“I can’t quite get excited about a title attached to my name or the number of times my name pops up when I enter it into PubMed. My personal definition is more…where do I have something that I am interested [in] that someone else values. And that value is not shown as an associate professorship or an assistant professorship next to my name. …When you push me on it, you could call me clinical instructor forever, and I don’t think I would care too much.”
The interaction between work and personal activities as representing complementary aspects of a global sense of success was also unexpected and ran contrary to a simplistic conception of work and life in conflict. Academic hospitalists referenced that the ability to participate in aspects of life external to the workplace was important to their sense of career success. Participants frequently used phrases such as “work–life balance” to encompass a larger sense that work and nonwork life needed to merge to form a holistic sense of having a positive impact.
“Personal success is becoming what I have termed a ‘man of worth.’ I think [that is] someone who feels as though they make a positive impact in the world. Through both my career, but I guess the things that I do that are external to my career. Those would be defined by being a good husband, a good son, a philanthropist out in the community…sometimes, these are not things that can necessarily go on a [curriculum vitae].”
Conflict Among Organizing Themes
At times, academic hospitalists described a tension between day-to-day job satisfaction and what would be necessary to accomplish longer-term career success in the other organizing themes. This was reflected by a sense of trade-off. For instance, activities that lead to some aspects of career satisfaction or advancement would take time away from the direct exposure to learners and clinical care that currently drive job satisfaction.
“If the institution wanted me to be more productive from a research standpoint or…advocate that I receive funding so I could buy down clinical time and interactions I have with my students and my patients, then I can see my satisfaction going down.”
Many described a sense of engaging in activities they did not find personally fulfilling because of a sense of expectation that those activities were considered successful by others. Some described a state in which the drive toward advancement as an extrinsic incentive could come at the expense of the intrinsic rewards of being an academic hospitalist.
DISCUSSION
Career success has been defined as “the positive psychological or work-related outcomes or achievements one accumulates as a result of work experiences.”4,7,8 Academic career success for hospitalist faculty isn’t as well defined and has not been examined from the perspectives of early-career clinician-educator hospitalist faculty themselves.
The themes that emerged in this study describe a definition of success anchored in the daily work of striving to become an exceptional clinician and teacher. The major themes included (1) having excitement about daily work, (2) having meaningful impact, (3) development of a niche (4) a sense of respect within the sphere of academic medicine, and (5) disseminating work.
Success was very much internally defined as having a positive, meaningful impact on patients, learners, and the systems in which they practice. The faculty had a conception of what promotion committees value and often internalized aspects of this, such as developing a national reputation and giving talks at national meetings. Participants typically self-identified as clinician-educators, and yet dissemination of work remained an important component of personal success. While promotion was clearly identified as a marker of success, academic hospitalists often rejected the supposition of promotion itself as a professional goal. They expressed hope, and some skepticism, that external recognition of career success would follow the pursuit of internally meaningful goals.
While promotion and peer-reviewed publications represent easily measured markers often used as proxies for individual career and programmatic success, our research demonstrates that there is a deep well of externally imperceptible influences on an individual’s sense of success as an academic hospitalist. In our analysis, intrinsic elements of career success received far greater weight with early-career academic hospitalists. Our findings are supported by a prior survey of academic physicians that similarly found that faculty with >50% of their time devoted to clinical care placed greater career value in patient care, relationships with patients, and recognition by patients and residents compared to national reputation.9 Similar to our own findings, highly clinical faculty in that study were also less likely to value promotion and tenure as indicators of career success.9
The main focus of our questions was how early-career faculty define success at this point in their careers. When asked to extrapolate to a future state of career success, the concept of progression was repeatedly raised. This included successive promotions to higher academic ranks, increasing responsibility, titles, leadership, and achieving competitive roles or awards. It also included a progressively increasing impact of scholarship, growing national reputation, and becoming part of a network of accomplished academic hospitalists across the country. Looking forward, our early-career hospitalists felt that long-term career success would represent accomplishing these things and still being able to be focused on being excellent clinicians to patients, having a work–life balance, and keeping joy and excitement in daily activities.
Our work has limitations, including a focus on early-career clinician-educator hospitalists. The perception of career success may evolve over time, and future work to examine perceptions in more advanced academic hospitalists would be of interest. Our work used purposeful sampling to capture individuals who were likely to self-identify as academic clinician-educators, and results may not generalize to hospitalist physician-scientists or hospitalists in community practices.
Our analysis suggests that external organizations influence internal perceptions of career success. However, success is ultimately defined by the individual and not the institution. Efforts to measure and improve academic hospitalists’ attainment of career success should attend to intrinsic aspects of satisfaction in addition to objective measures, such as publications and promotion. This may provide a mechanism to address burnout and improve retention. As important as commonality in themes is the variation in self-definitions of career success among individuals. This suggests the value of inquiry by academic leadership in exploring and understanding what success is from the individual faculty perspective. This may enhance the alignment among personal definitions, organizational values, and, ultimately, sustainable, successful careers.
Disclosure: The authors have nothing to disclose.
1. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US Academic Hospitalist Leaders About Mentorship and Academic Activities in Hospitalist Groups. J Hosp Med. 2011;6(1):5-9. PubMed
2. Buddeberg-Fischer B, Stamm M, Buddeberg C, Klaghofer R. Career-Success Scale. A New Instrument to Assess Young Physicians Academic Career Steps. BMC Health Serv Res. 2008;8:120. PubMed
3. Rubio DM, Primack BA, Switzer GE, Bryce CL, Selzer DL, Kapoor WN. A Comprehensive Career-Success Model for Physician-Scientists. Acad Med. 2011;86(12):1571-1576. PubMed
4. Judge TA, Cable DM, Boudreau JW, Bretz RD. An empirical investigation of the predictors of executive career success (CAHRS Working Paper #94-08). Ithaca, NY: Cornell University, School of Industrial and Labor Relations, Center for Advanced Human Resource Studies. 1994. http://digitalcommons.ilr.cornell.edu/cahrswp/233. Accessed November 27, 2017.
5. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42(5):533-544. PubMed
6. Francis JJ, Johnston M, Robertson C, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health. 2010;25(10):1229-1245. PubMed
7. Abele AE, Spurk, D. The longitudinal impact of self-efficacy and career goals on objective and subjective career success. J Vocat Behav. 2009;74(1):53-62.
8. Seibert SE, Kraimer ML. The five-factor model of personality and career success. J Vocat Behav. 2011;58(1):1-21.
9. Buckley, LM, Sanders K, Shih M, Hampton CL. Attitudes of Clinical Faculty About Career Progress, Career Success, and Commitment to Academic Medicine: Results of a Survey. Arch Intern Med. 2000;160(17):2625-2629. PubMed
1. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US Academic Hospitalist Leaders About Mentorship and Academic Activities in Hospitalist Groups. J Hosp Med. 2011;6(1):5-9. PubMed
2. Buddeberg-Fischer B, Stamm M, Buddeberg C, Klaghofer R. Career-Success Scale. A New Instrument to Assess Young Physicians Academic Career Steps. BMC Health Serv Res. 2008;8:120. PubMed
3. Rubio DM, Primack BA, Switzer GE, Bryce CL, Selzer DL, Kapoor WN. A Comprehensive Career-Success Model for Physician-Scientists. Acad Med. 2011;86(12):1571-1576. PubMed
4. Judge TA, Cable DM, Boudreau JW, Bretz RD. An empirical investigation of the predictors of executive career success (CAHRS Working Paper #94-08). Ithaca, NY: Cornell University, School of Industrial and Labor Relations, Center for Advanced Human Resource Studies. 1994. http://digitalcommons.ilr.cornell.edu/cahrswp/233. Accessed November 27, 2017.
5. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42(5):533-544. PubMed
6. Francis JJ, Johnston M, Robertson C, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health. 2010;25(10):1229-1245. PubMed
7. Abele AE, Spurk, D. The longitudinal impact of self-efficacy and career goals on objective and subjective career success. J Vocat Behav. 2009;74(1):53-62.
8. Seibert SE, Kraimer ML. The five-factor model of personality and career success. J Vocat Behav. 2011;58(1):1-21.
9. Buckley, LM, Sanders K, Shih M, Hampton CL. Attitudes of Clinical Faculty About Career Progress, Career Success, and Commitment to Academic Medicine: Results of a Survey. Arch Intern Med. 2000;160(17):2625-2629. PubMed
©2018 Society of Hospital Medicine
State Medicaid Expansion Status
On January 1, 2014, several major provisions of the Affordable Care Act (ACA) took effect, including introduction of the individual mandate for health insurance coverage, opening of the Health Insurance Marketplace, and expansion of Medicaid eligibility to Americans earning up to 133% of the federal poverty level.[1] Nearly 9 million US adults have enrolled in Medicaid since that time, primarily in the 31 states and Washington, DC that have opted into Medicaid expansion.[2, 3] ACA implementation has also had a significant impact on hospital payer mix, primarily by reducing the volume of uncompensated care in Medicaid‐expansion states.[4, 5]
The differential shift in payer mix in Medicaid‐expansion versus nonexpansion states may be relevant to hospitals beyond reimbursement. Medicaid insurance has historically been associated with longer hospitalizations and higher in‐hospital mortality in diverse patient populations, more so than commercial insurance and often even uninsured payer status.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15] The disparity in outcomes between patients with Medicaid versus other insurance persists even after adjustment for disease severity and baseline comorbidities. Insurance type may influence the delivery of inpatient care through variation in access to invasive procedures and adherence to guideline‐concordant medical therapies.[9, 10, 11, 12] Medicaid patients may be more likely than uninsured patients to remain hospitalized pending postacute care placement rather than be discharged home with family support.[16] Medicaid patients are also less likely to leave against medical advice than uninsured patients.[17]
Currently, little is known about the impact of state Medicaid expansion status on length of stay (LOS) or mortality nationally. It is possible that hospitals in Medicaid‐expansion states have experienced relative worsening in LOS and mortality as their share of Medicaid patients has grown. Determining the impact of ACA implementation on payer mix and patient outcomes is particularly important for academic medical centers (AMCs), as they traditionally care for the greatest percentage of both Medicaid and uninsured patients.[18] We sought to characterize the impact of state Medicaid expansion status on payer mix, LOS, and in‐hospital mortality for general medicine patients at AMCs in the United States.
METHODS
The University HealthSystem Consortium (UHC) is an alliance of 117 AMCs and 310 affiliated hospitals, representing >90% of such institutions in the US. We queried the online UHC Clinical Data Base/Resource Manager (CDB/RM) to obtain hospital‐level insurance, LOS, and mortality data for inpatients discharged from a general medicine service between October 1, 2012 and September 30, 2015. We excluded hospitals that were missing data for any month within the study period. No patient‐level data were accessed.
Our outcomes of interest were the proportion of discharges by primary payer (Medicare, commercial, Medicaid, uninsured, or other [eg, Tri‐Care or Workers' Compensation]), as well as the LOS index and mortality index. Both indices were defined as the ratio of the observed to expected values. To determine the expected LOS and mortality, the UHC 2015 risk adjustment models were applied to all cases, adjusting for variables such as patient demographics, low socioeconomic status, admit source and status, severity of illness, and comorbid conditions, as described by International Classification of Diseases, Ninth Revision codes. These models have been validated and are used for research and quality benchmarking for member institutions.[19]
We next stratified hospitals according to state Medicaid expansion status. We defined Medicaid‐expansion states as those that had expanded Medicaid by the end of the study period: Arizona, Arkansas, California, Colorado, Connecticut, Illinois, Indiana, Iowa, Kentucky, Maryland, Massachusetts, Michigan, Minnesota, Nevada, New Hampshire, New Jersey, New Mexico, New York, Ohio, Oregon, Pennsylvania, Rhode Island, Washington, Washington DC, and West Virginia. Nonexpansion states included Alabama, Florida, Georgia, Kansas, Louisiana, Missouri, Nebraska, North Carolina, South Carolina, Tennessee, Texas, Utah, Virginia, and Wisconsin. We excluded 12 states due to incomplete data: Alaska, Delaware, Hawaii, Idaho, North Dakota, Maine, Mississippi, Montana, Oklahoma, South Dakota, Vermont, and Wyoming.
We then identified our pre‐ and post‐ACA implementation periods. Medicaid coverage expansion took effect in all expansion states on January 1, 2014, with the exception of Michigan (April 1, 2014), New Hampshire (August 15, 2014), Pennsylvania (January 1, 2015), and Indiana (February 1, 2015).[3] We therefore defined October 1, 2012 to December 31, 2013 as the pre‐ACA implementation period and January 1, 2014 to September 30, 2015 as the post‐ACA implementation period for all states except for Michigan, New Hampshire, Pennsylvania, and Indiana. For these 4 states, we customized the pre‐ and post‐ACA implementation periods to their respective dates of Medicaid expansion; for New Hampshire, we designated October 1, 2012 to July 31, 2014 as the pre‐ACA implementation period and September 1, 2014 to September 30, 2015 as the post‐ACA implementation period, as we were unable to distinguish before versus after data in August 2014 based on the midmonth expansion of Medicaid.
After stratifying hospitals into groups based on whether they were located in Medicaid‐expansion or nonexpansion states, the proportion of discharges by payer was compared between pre‐ and post‐ACA implementation periods both graphically by quarter and using linear regression models weighted for the number of cases from each hospital. Next, for both Medicaid‐expansion and nonexpansion hospitals, LOS index and mortality index were compared before and after ACA implementation using linear regression models weighted for the number of cases from each hospital, both overall and by payer. Difference‐in‐differences estimations were then completed to compare the proportion of discharges by payer, LOS index, and mortality index between Medicaid‐expansion and nonexpansion hospitals before and after ACA implementation. Post hoc linear regression analyses were completed to evaluate the effect of clustering by state level strata on payer mix and LOS and mortality indices. A 2‐sided P value of 0.05 was considered statistically significant. Data analyses were performed using Stata 12.0 (StataCorp, College Station, TX).
RESULTS
We identified 4,258,952 discharges among general medicine patients from 211 hospitals in 38 states and Washington, DC between October 1, 2012, and September 30, 2015. This included 3,144,488 discharges from 156 hospitals in 24 Medicaid‐expansion states and Washington, DC and 1,114,464 discharges from 55 hospitals in 14 nonexpansion states.
Figure 1 shows the trends in payer mix over time for hospitals in both Medicaid‐expansion and nonexpansion states. As summarized in Table 1, hospitals in Medicaid‐expansion states experienced a significant 3.7‐percentage point increase in Medicaid discharges (P = 0.013) and 2.9‐percentage point decrease in uninsured discharges (P 0.001) after ACA implementation. This represented an approximately 19% jump and 60% drop in Medicaid and uninsured discharges, respectively. Hospitals in nonexpansion states saw no significant change in the proportion of discharges by payer after ACA implementation. In the difference‐in‐differences analysis, there was a trend toward a greater change in the proportion of Medicaid discharges pre‐ to post‐ACA implementation among hospitals in Medicaid‐expansion states compared to hospitals in nonexpansion states (mean difference‐in‐differences 4.1%, 95% confidence interval [CI]: 0.3%, 8.6%, P = 0.070).
| Medicaid‐expansion n=156 hospitals; 3,144,488 cases | Non‐expansion n=55 hospitals; 1,114,464 cases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐ACA Implementation (1,453,090 Cases) | Post‐ACA Implementation (1,691,398 Cases) | Mean Difference | P Value | Pre‐ACA Implementation (455,440 Cases) | Post‐ACA Implementation (659,024 Cases) | Mean Difference | P Value | Mean Difference‐in‐Differences | P Value | |
| ||||||||||
| Payer mix, % (95% CI) | ||||||||||
| Medicare | 48.6 (46.2, 51.0)* | 48.3 (45.9, 50.7) | 0.3 (3.6, 3.1) | 0.865 | 44.3 (40.7, 47.7)* | 45.3 (41.9, 48.6) | 1.0 (3.8, 5.8) | 0.671 | 1.3 (7.1, 4.5) | 0.655 |
| Commercial | 23.1 (21.4, 24.7) | 23.2 (21.8, 24.6) | 0.2 (2.0, 2.3) | 0.882 | 21.5 (18.5, 24.6) | 22.7 (19.7, 25.8) | 1.2 (3.0, 5.4) | 0.574 | 1.0 (5.7, 3.6) | 0.662 |
| Medicaid | 19.6 (17.6, 21.6) | 23.3 (21.2, 25.5) | 3.7 (0.8, 6.6) | 0.013 | 19.4 (16.9, 21.9) | 19.0 (16.5, 21.4) | 0.4 (3.8, 3.0) | 0.812 | 4.1 (0.3, 8.6) | 0.070 |
| Uninsured | 5.0 (4.0, 5.9) | 2.0 (1.7, 2.3) | 2.9 (3.9, 2.0) | 0.001 | 10.9 (8.1, 13.7) | 9.4 (7.0, 11.7) | 1.5 (5.1, 2.1) | 0.407 | 1.4 (5.1, 2.2) | 0.442 |
| Other | 3.8 (2.6, 4.9) | 3.1 (2.0, 4.3) | 0.7 (2.3, 1.0) | 0.435 | 4.0 (2.9, 5.0) | 3.7 (2.6, 4.7) | 0.3 (1.7, 1.1) | 0.662 | 0.3 (2.5, 1.8) | 0.762 |
| LOS index, mean (95% CI) | ||||||||||
| Overall | 1.017 (0.996, 1.038) | 1.006 (0.981, 1.031) | 0.011 (0.044, 0.021) | 0.488 | 1.008 (0.974, 1.042) | 0.995 (0.961, 1.029) | 0.013 (0.061, 0.034) | 0.574 | 0.002 (0.055, 0.059) | 0.943 |
| Medicare | 1.012 (0.989, 1.035) | 0.999 (0.971, 1.027) | 0.013 (0.049, 0.023) | 0.488 | 0.982 (0.946, 1.017) | 0.979 (0.944, 1.013) | 0.003 (0.052, 0.046) | 0.899 | 0.010 (0.070, 0.051) | 0.754 |
| Commercial | 0.993 (0.974, 1.012) | 0.977 (0.955, 0.998) | 0.016 (0.045, 0.013) | 0.271 | 1.009 (0.978, 1.039) | 0.986 (0.956, 1.016) | 0.022 (0.065, 0.020) | 0.298 | 0.006 (0.044, 0.057) | 0.809 |
| Medicaid | 1.059 (1.036, 1.082) | 1.043 (1.018, 1.067) | 0.016 (0.049, 0.017) | 0.349 | 1.064 (1.020, 1.108) | 1.060 (1.015, 1.106) | 0.004 (0.066, 0.059) | 0.911 | 0.012 (0.082, 0.057) | 0.727 |
| Uninsured | 0.960 (0.933, 0.988) | 0.925 (0.890, 0.961) | 0.035 (0.080, 0.010) | 0.126 | 0.972 (0.935, 1.009) | 0.944 (0.909, 0.979) | 0.028 (0.078, 0.022) | 0.273 | 0.007 (0.074, 0.060) | 0.835 |
| Other | 0.988 (0.960, 1.017) | 0.984 (0.952, 1.015) | 0.005 (0.047, 0.037) | 0.822 | 1.022 (0.973, 1.071) | 0.984 (0.944, 1.024) | 0.038 (0.100, 0.024) | 0.232 | 0.033 (0.042, 0.107) | 0.386 |
| Mortality index, mean (95% CI) | ||||||||||
| Overall | 1.000 (0.955, 1.045) | 0.878 (0.836, 0.921) | 0.122 (0.183, 0.061) | 0.001 | 0.997 (0.931, 1.062) | 0.850 (0.800, 0.900) | 0.147 (0.227, 0.066) | 0.001 | 0.025 (0.076, 0.125) | 0.628 |
| Medicare | 0.990 (0.942, 1.038) | 0.871 (0.826, 0.917) | 0.119 (0.185, 0.053) | 0.001 | 1.000 (0.925, 1.076) | 0.844 (0.788, 0.900) | 0.156 (0.249, 0.064) | 0.001 | 0.038 (0.075, 0.150) | 0.513 |
| Commercial | 1.045 (0.934, 1.155) | 0.908 (0.842, 0.975) | 0.136 (0.264, 0.008) | 0.037 | 1.023 (0.935, 1.111) | 0.820 (0.758, 0.883) | 0.203 (0.309, 0.096) | 0.001 | 0.067 (0.099, 0.232) | 0.430 |
| Medicaid | 0.894 (0.845, 0.942) | 0.786 (0.748, 0.824) | 0.107 (0.168, 0.046) | 0.001 | 0.937 (0.861, 1.013) | 0.789 (0.733, 0.844) | 0.148 (0.242, 0.055) | 0.002 | 0.041 (0.069, 0.151) | 0.464 |
| Uninsured | 1.172 (1.007, 1.337)∥ | 1.136 (0.968, 1.303) | 0.037 (0.271, 0.197) | 0.758 | 0.868 (0.768, 0.968)∥ | 0.850 (0.761, 0.939) | 0.017 (0.149, 0.115) | 0.795 | 0.019 (0.287, 0.248) | 0.887 |
| Other | 1.376 (1.052, 1.700)# | 1.156 (0.910, 1.402) | 0.220 (0.624, 0.184) | 0.285 | 1.009 (0.868, 1.150) # | 0.874 (0.682, 1.066) | 0.135 (0.369, 0.099) | 0.254 | 0.085 (0.555, 0.380) | 0.720 |
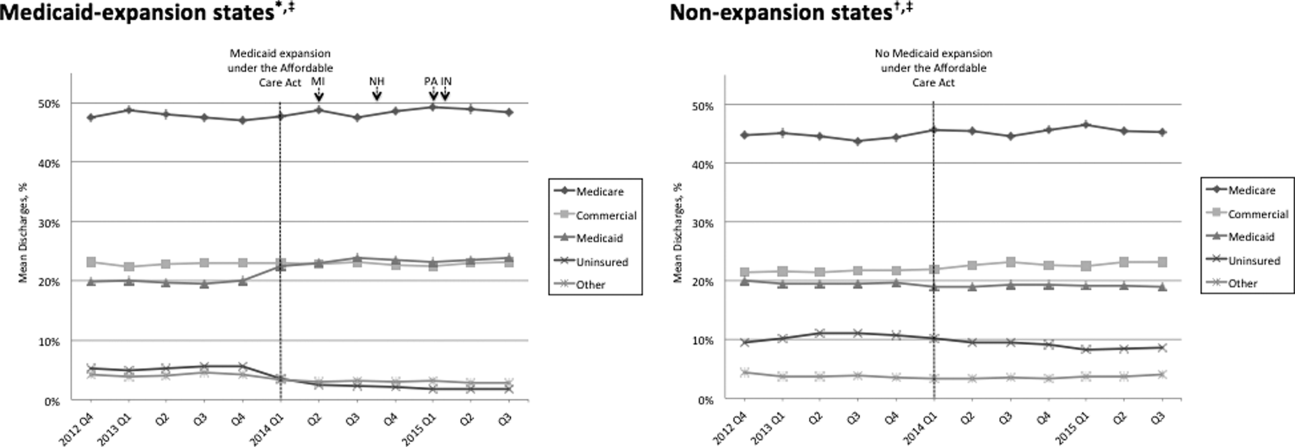
Table 1 shows that the overall LOS index remained unchanged pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.017 to 1.006, P = 0.488) and nonexpansion hospitals (1.008 to 0.995, P = 0.574). LOS indices for each payer type also remained unchanged. The overall mortality index significantly improved pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.000 to 0.878, P 0.001) and nonexpansion hospitals (0.997 to 0.850, P = 0.001). Among both Medicaid‐expansion and nonexpansion hospitals, the mortality index significantly improved for Medicare, commercial, and Medicaid discharges but not for uninsured or other discharges. In the difference‐in‐differences analysis, the changes in LOS indices and mortality indices pre‐ to post‐ACA implementation did not differ significantly between hospitals in Medicaid‐expansion versus nonexpansion states.
In post hoc linear regression analyses of payer mix and LOS and mortality indices clustered by state‐level strata, point estimates were minimally changed. Although 95% CIs were slightly wider, statistical significance was unchanged from our primary analyses (data not shown).
DISCUSSION
We found that ACA implementation had a significant impact on payer mix for general medicine patients at AMCs in the United States, primarily by increasing the number of Medicaid beneficiaries and by decreasing the number of uninsured patients in Medicaid‐expansion states. State Medicaid expansion status did not appear to influence either LOS or in‐hospital mortality.
Our study offers some of the longest‐term data currently available on the impact of ACA implementation on payer mix trends and encompasses more states than others have previously. Although we uniquely focused on general medicine patients at AMCs, our results are similar to those seen for US hospitals overall. Nikpay and colleagues evaluated payer mix trends for non‐Medicare adult inpatient stays in 16 states through the second quarter of 2014 using the Healthcare Cost and Utilization Project database through the Agency for Healthcare Research and Quality.[4] They found a relative 20% increase and 50% decrease in Medicaid and uninsured discharges in Medicaid‐expansion states, along with nonsignificant changes in nonexpansion states. Hempstead and Cantor assessed payer mix for non‐Medicare discharges using state hospital association data from 21 states through the fourth quarter of 2014 and found a significant increase in Medicaid patients as well as a nearly significant decrease in uninsured patients in expansion states relative to nonexpansion states.[5] The Department of Health and Human Services also reported that uninsured/self‐pay discharges fell substantially (65%73%) in Medicaid‐expansion states by the end of 2014, with slight decreases in nonexpansion states.[20]
In contrast to our hypothesis, the overall LOS and in‐hospital mortality indices were not influenced by state Medicaid expansion status. From a purely mathematical standpoint, the contribution of Medicaid patients to the overall LOS and mortality indices may have been eclipsed by Medicare and commercially insured patients, who represented a higher proportion of total discharges. The lack of impact of state Medicaid expansion status on overall LOS and mortality indices did not appear to occur as a result of indices for Medicaid patients trending toward the mean. As predicted based on observational studies, Medicaid patients in our study tended to have a higher LOS index than those with other insurance types. Medicaid patients actually tended to have a lower mortality index in our analysis; the reason for this latter finding is unclear and in contrast to other published studies.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 21]
To our knowledge, no other studies have evaluated the effect of payer mix changes under the ACA on inpatient outcomes. However, new evidence is emerging on outpatient outcomes. Low‐income adults in Medicaid‐expansion states have reported greater gains in access to primary care services and in the diagnosis of certain chronic health conditions than those in nonexpansion states as a result of ACA implementation.[22, 23] Such improvements in the outpatient setting might be expected to reduce patient acuity on admission. However, they would not necessarily translate to relative improvements in LOS or mortality indices for Medicaid‐expansion hospitals, as the UHC risk adjustment models controlled for disease severity on admission.
Similarly, few studies have assessed the impact of payer mix changes under previous state Medicaid expansions on inpatient outcomes. After Massachusetts expanded Medicaid and enacted near‐universal healthcare coverage in 2006, a minimal LOS reduction of just 0.05 days was observed.[24] New York expanded Medicaid eligibility to nondisabled childless adults with incomes below 100% of the federal poverty level in September 2001, whereas Arizona did so in November 2001 and Maine in October 2002. A study comparing outcomes in these states to 4 neighboring nonexpansion states found a relative reduction in annual all‐cause mortality of 6.1% population wide; however, it did not assess in‐hospital mortality.[25] The Oregon Health Insurance Experiment that randomized low‐income adults to expanded Medicaid coverage or not in 2008 has also reported on outpatient rather than inpatient outcomes.[26]
Our findings have potential implications for health policymakers. That Medicaid expansion status had a neutral effect on both LOS and mortality indices in our analysis should be reassuring for states contemplating Medicaid expansion in the future. Our results also highlight the need for further efforts to reduce disparities in inpatient care based on payer status. For example, although Medicare, commercially insured, and Medicaid patients witnessed significant improvements in mortality indices pre‐ to post‐ACA implementation in hospitals in both Medicaid‐expansion and nonexpansion states, uninsured patients did not.
This study has several limitations. First, our analysis of the impact of ACA implementation on payer mix did not account for concurrent socioeconomic trends that may have influenced insurance coverage across the United States. However, the main goal of this analysis was to demonstrate that changes in payer mix did in fact occur over time, to provide rationale for our subsequent LOS and mortality analyses. Second, we could not control for variation in the design and implementation of Medicaid expansions across states as permitted under the federal Section 1115 waiver process. Third, we only had access to hospital‐level data through the UHC CDB/RM, rather than individual patient data. We attempted to mitigate this limitation by weighting data according to the number of cases per hospital. Lastly, additional patient‐level factors that may influence LOS or mortality may not be included in the UHC risk adjustment models.
In summary, the differential shift in payer mix between Medicaid‐expansion and nonexpansion states did not influence overall LOS or in‐hospital mortality for general medicine patients at AMCs in the United States. Additional research could help to determine the impact of ACA implementation on other patient outcomes that may be dependent on insurance status, such as readmissions or hospital‐acquired complications.
Disclosures: M.E.A. conceived of the study concept and design, assisted with data acquisition, and drafted the manuscript. J.J.G. assisted with study design and made critical revisions to the manuscript. D.A. assisted with study design and made critical revisions to the manuscript. R.P. assisted with study design and made critical revisions to the manuscript. M.L. assisted with study design and data acquisition and made critical revisions to the manuscript. C.D.J. assisted with study design, performed data analyses, and made critical revisions to the manuscript. A modified abstract was presented in poster format at the University HealthSystem Consortium Annual Conference held September 30 to October 2, 2015 in Orlando, Florida, as well as at the Society of Hospital Medicine Research, Innovations, and Vignettes 2016 Annual Meeting held March 69, 2016, in San Diego, California. The authors report no conflicts of interest.
- Department of Health and Human Services. Key features of the Affordable Care Act by year. Available at: http://www.hhs.gov/healthcare/facts‐and‐features/key‐features‐of‐aca‐by‐year/index.html#2014. Accessed April 4, 2016.
- Centers for Medicare and Medicaid Services. Medicaid enrollment data collected through MBES. Available at: https://www.medicaid.gov/medicaid‐chip‐program‐information/program‐information/medicaid‐and‐chip‐enrollment‐data/medicaid‐enrollment‐data‐collected‐through‐mbes.html. Accessed April 4, 2016.
- The Henry J. Kaiser Family Foundation. Status of state action on the Medicaid expansion decision. Available at: http://kff.org/health‐reform/state‐indicator/state‐activity‐around‐expanding‐medicaid‐under‐the‐affordable‐care‐act. Accessed April 4, 2016.
- , , . Affordable Care Act Medicaid expansion reduced uninsured hospital stays in 2014. Health Aff (Millwood). 2016;35(1):106–110.
- , . State Medicaid expansion and changes in hospital volume according to payer. N Engl J Med. 2016;374(2):196–198.
- , , , , , . Understanding predictors of prolonged hospitalizations among general medicine patients: a guide and preliminary analysis. J Hosp Med. 2015;10(9):623–626.
- , , , . Impact of insurance and hospital ownership on hospital length of stay among patients with ambulatory care‐sensitive conditions. Ann Fam Med. 2011;9:489–495.
- , , . Insurance status and hospital care for myocardial infarction, stroke, and pneumonia. J Hosp Med. 2010;5:452–459.
- , , , , , . Payment source, quality of care, and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2011;58(14):1465–1471.
- , , , , . The inpatient experience and predictors of length of stay for patients hospitalized with systolic heart failure: comparison by commercial, Medicaid, and Medicare payer type. J Med Econ. 2013;16(1):43–54.
- , , et al. Insurance coverage and care of patients with non‐ST‐segment elevation acute coronary syndromes. Ann Intern Med. 2006;145:739–748.
- , , , et al. Association of insurance status with inpatient treatment for coronary artery disease: findings from the Get with the Guidelines Program. Am Heart J. 2010;159:1026–1036.
- , , , et al. Primary payer status affects mortality for major surgical operations. Ann Surg. 2010;252:544–551.
- , , . Medicaid payer status is associated with in‐hospital morbidity and resource utilization following primary total joint arthroplasty. J Bone Joint Surg Am. 2014;96(21):e180.
- , , . The quality of care delivered to patients within the same hospital varies by insurance type. Health Aff (Millwood). 2013;32(10):1731–1739.
- , , , , . Effect of insurance status on postacute care among working age stroke survivors. Neurology. 2012;78(20):1590–1595.
- , , , . Hospitalizations in which patients leave the hospital against medical advice (AMA), 2007. HCUP statistical brief #78. August 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2009. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb78.pdf. Accessed May 12, 2016.
- , , , . Characteristics of Medicaid and uninsured hospitalizations, 2012. HCUP statistical brief #182. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb182‐Medicaid‐Uninsured‐Hospitalizations‐2012.pdf. Accessed March 9, 2016.
- Agency for Healthcare Research and Quality. Mortality measurement: mortality risk adjustment methodology for University HealthSystem Consortium. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Meurer.pdf. Accessed May 10, 2016.
- Department of Health and Human Services. Insurance expansion, hospital uncompensated care, and the Affordable Care Act. Available at: https://aspe.hhs.gov/pdf‐report/insurance‐expansion‐hospital‐uncompensated‐care‐and‐affordable‐care‐act. Accessed May 27, 2016.
- , , , . Our flawed but beneficial Medicaid program. N Engl J Med. 2011;364(16):e31.
- , , , . Changes in self‐reported insurance coverage, access to care, and health under the Affordable Care Act. JAMA. 2015;314(4):366–374.
- , . Early coverage, access, utilization, and health effects associated with the Affordable Care Act Medicaid Expansions: a quasi‐experimental study. Ann Intern Med. 2016;164(12):795–803.
- , . The impact of health care reform on hospital and preventive care: evidence from Massachusetts. J Public Econ. 2012;96(11–12):909–929.
- , , . Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367:1025–1034.
- , , , et al. The Oregon Experiment—effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713–1722.
On January 1, 2014, several major provisions of the Affordable Care Act (ACA) took effect, including introduction of the individual mandate for health insurance coverage, opening of the Health Insurance Marketplace, and expansion of Medicaid eligibility to Americans earning up to 133% of the federal poverty level.[1] Nearly 9 million US adults have enrolled in Medicaid since that time, primarily in the 31 states and Washington, DC that have opted into Medicaid expansion.[2, 3] ACA implementation has also had a significant impact on hospital payer mix, primarily by reducing the volume of uncompensated care in Medicaid‐expansion states.[4, 5]
The differential shift in payer mix in Medicaid‐expansion versus nonexpansion states may be relevant to hospitals beyond reimbursement. Medicaid insurance has historically been associated with longer hospitalizations and higher in‐hospital mortality in diverse patient populations, more so than commercial insurance and often even uninsured payer status.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15] The disparity in outcomes between patients with Medicaid versus other insurance persists even after adjustment for disease severity and baseline comorbidities. Insurance type may influence the delivery of inpatient care through variation in access to invasive procedures and adherence to guideline‐concordant medical therapies.[9, 10, 11, 12] Medicaid patients may be more likely than uninsured patients to remain hospitalized pending postacute care placement rather than be discharged home with family support.[16] Medicaid patients are also less likely to leave against medical advice than uninsured patients.[17]
Currently, little is known about the impact of state Medicaid expansion status on length of stay (LOS) or mortality nationally. It is possible that hospitals in Medicaid‐expansion states have experienced relative worsening in LOS and mortality as their share of Medicaid patients has grown. Determining the impact of ACA implementation on payer mix and patient outcomes is particularly important for academic medical centers (AMCs), as they traditionally care for the greatest percentage of both Medicaid and uninsured patients.[18] We sought to characterize the impact of state Medicaid expansion status on payer mix, LOS, and in‐hospital mortality for general medicine patients at AMCs in the United States.
METHODS
The University HealthSystem Consortium (UHC) is an alliance of 117 AMCs and 310 affiliated hospitals, representing >90% of such institutions in the US. We queried the online UHC Clinical Data Base/Resource Manager (CDB/RM) to obtain hospital‐level insurance, LOS, and mortality data for inpatients discharged from a general medicine service between October 1, 2012 and September 30, 2015. We excluded hospitals that were missing data for any month within the study period. No patient‐level data were accessed.
Our outcomes of interest were the proportion of discharges by primary payer (Medicare, commercial, Medicaid, uninsured, or other [eg, Tri‐Care or Workers' Compensation]), as well as the LOS index and mortality index. Both indices were defined as the ratio of the observed to expected values. To determine the expected LOS and mortality, the UHC 2015 risk adjustment models were applied to all cases, adjusting for variables such as patient demographics, low socioeconomic status, admit source and status, severity of illness, and comorbid conditions, as described by International Classification of Diseases, Ninth Revision codes. These models have been validated and are used for research and quality benchmarking for member institutions.[19]
We next stratified hospitals according to state Medicaid expansion status. We defined Medicaid‐expansion states as those that had expanded Medicaid by the end of the study period: Arizona, Arkansas, California, Colorado, Connecticut, Illinois, Indiana, Iowa, Kentucky, Maryland, Massachusetts, Michigan, Minnesota, Nevada, New Hampshire, New Jersey, New Mexico, New York, Ohio, Oregon, Pennsylvania, Rhode Island, Washington, Washington DC, and West Virginia. Nonexpansion states included Alabama, Florida, Georgia, Kansas, Louisiana, Missouri, Nebraska, North Carolina, South Carolina, Tennessee, Texas, Utah, Virginia, and Wisconsin. We excluded 12 states due to incomplete data: Alaska, Delaware, Hawaii, Idaho, North Dakota, Maine, Mississippi, Montana, Oklahoma, South Dakota, Vermont, and Wyoming.
We then identified our pre‐ and post‐ACA implementation periods. Medicaid coverage expansion took effect in all expansion states on January 1, 2014, with the exception of Michigan (April 1, 2014), New Hampshire (August 15, 2014), Pennsylvania (January 1, 2015), and Indiana (February 1, 2015).[3] We therefore defined October 1, 2012 to December 31, 2013 as the pre‐ACA implementation period and January 1, 2014 to September 30, 2015 as the post‐ACA implementation period for all states except for Michigan, New Hampshire, Pennsylvania, and Indiana. For these 4 states, we customized the pre‐ and post‐ACA implementation periods to their respective dates of Medicaid expansion; for New Hampshire, we designated October 1, 2012 to July 31, 2014 as the pre‐ACA implementation period and September 1, 2014 to September 30, 2015 as the post‐ACA implementation period, as we were unable to distinguish before versus after data in August 2014 based on the midmonth expansion of Medicaid.
After stratifying hospitals into groups based on whether they were located in Medicaid‐expansion or nonexpansion states, the proportion of discharges by payer was compared between pre‐ and post‐ACA implementation periods both graphically by quarter and using linear regression models weighted for the number of cases from each hospital. Next, for both Medicaid‐expansion and nonexpansion hospitals, LOS index and mortality index were compared before and after ACA implementation using linear regression models weighted for the number of cases from each hospital, both overall and by payer. Difference‐in‐differences estimations were then completed to compare the proportion of discharges by payer, LOS index, and mortality index between Medicaid‐expansion and nonexpansion hospitals before and after ACA implementation. Post hoc linear regression analyses were completed to evaluate the effect of clustering by state level strata on payer mix and LOS and mortality indices. A 2‐sided P value of 0.05 was considered statistically significant. Data analyses were performed using Stata 12.0 (StataCorp, College Station, TX).
RESULTS
We identified 4,258,952 discharges among general medicine patients from 211 hospitals in 38 states and Washington, DC between October 1, 2012, and September 30, 2015. This included 3,144,488 discharges from 156 hospitals in 24 Medicaid‐expansion states and Washington, DC and 1,114,464 discharges from 55 hospitals in 14 nonexpansion states.
Figure 1 shows the trends in payer mix over time for hospitals in both Medicaid‐expansion and nonexpansion states. As summarized in Table 1, hospitals in Medicaid‐expansion states experienced a significant 3.7‐percentage point increase in Medicaid discharges (P = 0.013) and 2.9‐percentage point decrease in uninsured discharges (P 0.001) after ACA implementation. This represented an approximately 19% jump and 60% drop in Medicaid and uninsured discharges, respectively. Hospitals in nonexpansion states saw no significant change in the proportion of discharges by payer after ACA implementation. In the difference‐in‐differences analysis, there was a trend toward a greater change in the proportion of Medicaid discharges pre‐ to post‐ACA implementation among hospitals in Medicaid‐expansion states compared to hospitals in nonexpansion states (mean difference‐in‐differences 4.1%, 95% confidence interval [CI]: 0.3%, 8.6%, P = 0.070).
| Medicaid‐expansion n=156 hospitals; 3,144,488 cases | Non‐expansion n=55 hospitals; 1,114,464 cases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐ACA Implementation (1,453,090 Cases) | Post‐ACA Implementation (1,691,398 Cases) | Mean Difference | P Value | Pre‐ACA Implementation (455,440 Cases) | Post‐ACA Implementation (659,024 Cases) | Mean Difference | P Value | Mean Difference‐in‐Differences | P Value | |
| ||||||||||
| Payer mix, % (95% CI) | ||||||||||
| Medicare | 48.6 (46.2, 51.0)* | 48.3 (45.9, 50.7) | 0.3 (3.6, 3.1) | 0.865 | 44.3 (40.7, 47.7)* | 45.3 (41.9, 48.6) | 1.0 (3.8, 5.8) | 0.671 | 1.3 (7.1, 4.5) | 0.655 |
| Commercial | 23.1 (21.4, 24.7) | 23.2 (21.8, 24.6) | 0.2 (2.0, 2.3) | 0.882 | 21.5 (18.5, 24.6) | 22.7 (19.7, 25.8) | 1.2 (3.0, 5.4) | 0.574 | 1.0 (5.7, 3.6) | 0.662 |
| Medicaid | 19.6 (17.6, 21.6) | 23.3 (21.2, 25.5) | 3.7 (0.8, 6.6) | 0.013 | 19.4 (16.9, 21.9) | 19.0 (16.5, 21.4) | 0.4 (3.8, 3.0) | 0.812 | 4.1 (0.3, 8.6) | 0.070 |
| Uninsured | 5.0 (4.0, 5.9) | 2.0 (1.7, 2.3) | 2.9 (3.9, 2.0) | 0.001 | 10.9 (8.1, 13.7) | 9.4 (7.0, 11.7) | 1.5 (5.1, 2.1) | 0.407 | 1.4 (5.1, 2.2) | 0.442 |
| Other | 3.8 (2.6, 4.9) | 3.1 (2.0, 4.3) | 0.7 (2.3, 1.0) | 0.435 | 4.0 (2.9, 5.0) | 3.7 (2.6, 4.7) | 0.3 (1.7, 1.1) | 0.662 | 0.3 (2.5, 1.8) | 0.762 |
| LOS index, mean (95% CI) | ||||||||||
| Overall | 1.017 (0.996, 1.038) | 1.006 (0.981, 1.031) | 0.011 (0.044, 0.021) | 0.488 | 1.008 (0.974, 1.042) | 0.995 (0.961, 1.029) | 0.013 (0.061, 0.034) | 0.574 | 0.002 (0.055, 0.059) | 0.943 |
| Medicare | 1.012 (0.989, 1.035) | 0.999 (0.971, 1.027) | 0.013 (0.049, 0.023) | 0.488 | 0.982 (0.946, 1.017) | 0.979 (0.944, 1.013) | 0.003 (0.052, 0.046) | 0.899 | 0.010 (0.070, 0.051) | 0.754 |
| Commercial | 0.993 (0.974, 1.012) | 0.977 (0.955, 0.998) | 0.016 (0.045, 0.013) | 0.271 | 1.009 (0.978, 1.039) | 0.986 (0.956, 1.016) | 0.022 (0.065, 0.020) | 0.298 | 0.006 (0.044, 0.057) | 0.809 |
| Medicaid | 1.059 (1.036, 1.082) | 1.043 (1.018, 1.067) | 0.016 (0.049, 0.017) | 0.349 | 1.064 (1.020, 1.108) | 1.060 (1.015, 1.106) | 0.004 (0.066, 0.059) | 0.911 | 0.012 (0.082, 0.057) | 0.727 |
| Uninsured | 0.960 (0.933, 0.988) | 0.925 (0.890, 0.961) | 0.035 (0.080, 0.010) | 0.126 | 0.972 (0.935, 1.009) | 0.944 (0.909, 0.979) | 0.028 (0.078, 0.022) | 0.273 | 0.007 (0.074, 0.060) | 0.835 |
| Other | 0.988 (0.960, 1.017) | 0.984 (0.952, 1.015) | 0.005 (0.047, 0.037) | 0.822 | 1.022 (0.973, 1.071) | 0.984 (0.944, 1.024) | 0.038 (0.100, 0.024) | 0.232 | 0.033 (0.042, 0.107) | 0.386 |
| Mortality index, mean (95% CI) | ||||||||||
| Overall | 1.000 (0.955, 1.045) | 0.878 (0.836, 0.921) | 0.122 (0.183, 0.061) | 0.001 | 0.997 (0.931, 1.062) | 0.850 (0.800, 0.900) | 0.147 (0.227, 0.066) | 0.001 | 0.025 (0.076, 0.125) | 0.628 |
| Medicare | 0.990 (0.942, 1.038) | 0.871 (0.826, 0.917) | 0.119 (0.185, 0.053) | 0.001 | 1.000 (0.925, 1.076) | 0.844 (0.788, 0.900) | 0.156 (0.249, 0.064) | 0.001 | 0.038 (0.075, 0.150) | 0.513 |
| Commercial | 1.045 (0.934, 1.155) | 0.908 (0.842, 0.975) | 0.136 (0.264, 0.008) | 0.037 | 1.023 (0.935, 1.111) | 0.820 (0.758, 0.883) | 0.203 (0.309, 0.096) | 0.001 | 0.067 (0.099, 0.232) | 0.430 |
| Medicaid | 0.894 (0.845, 0.942) | 0.786 (0.748, 0.824) | 0.107 (0.168, 0.046) | 0.001 | 0.937 (0.861, 1.013) | 0.789 (0.733, 0.844) | 0.148 (0.242, 0.055) | 0.002 | 0.041 (0.069, 0.151) | 0.464 |
| Uninsured | 1.172 (1.007, 1.337)∥ | 1.136 (0.968, 1.303) | 0.037 (0.271, 0.197) | 0.758 | 0.868 (0.768, 0.968)∥ | 0.850 (0.761, 0.939) | 0.017 (0.149, 0.115) | 0.795 | 0.019 (0.287, 0.248) | 0.887 |
| Other | 1.376 (1.052, 1.700)# | 1.156 (0.910, 1.402) | 0.220 (0.624, 0.184) | 0.285 | 1.009 (0.868, 1.150) # | 0.874 (0.682, 1.066) | 0.135 (0.369, 0.099) | 0.254 | 0.085 (0.555, 0.380) | 0.720 |

Table 1 shows that the overall LOS index remained unchanged pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.017 to 1.006, P = 0.488) and nonexpansion hospitals (1.008 to 0.995, P = 0.574). LOS indices for each payer type also remained unchanged. The overall mortality index significantly improved pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.000 to 0.878, P 0.001) and nonexpansion hospitals (0.997 to 0.850, P = 0.001). Among both Medicaid‐expansion and nonexpansion hospitals, the mortality index significantly improved for Medicare, commercial, and Medicaid discharges but not for uninsured or other discharges. In the difference‐in‐differences analysis, the changes in LOS indices and mortality indices pre‐ to post‐ACA implementation did not differ significantly between hospitals in Medicaid‐expansion versus nonexpansion states.
In post hoc linear regression analyses of payer mix and LOS and mortality indices clustered by state‐level strata, point estimates were minimally changed. Although 95% CIs were slightly wider, statistical significance was unchanged from our primary analyses (data not shown).
DISCUSSION
We found that ACA implementation had a significant impact on payer mix for general medicine patients at AMCs in the United States, primarily by increasing the number of Medicaid beneficiaries and by decreasing the number of uninsured patients in Medicaid‐expansion states. State Medicaid expansion status did not appear to influence either LOS or in‐hospital mortality.
Our study offers some of the longest‐term data currently available on the impact of ACA implementation on payer mix trends and encompasses more states than others have previously. Although we uniquely focused on general medicine patients at AMCs, our results are similar to those seen for US hospitals overall. Nikpay and colleagues evaluated payer mix trends for non‐Medicare adult inpatient stays in 16 states through the second quarter of 2014 using the Healthcare Cost and Utilization Project database through the Agency for Healthcare Research and Quality.[4] They found a relative 20% increase and 50% decrease in Medicaid and uninsured discharges in Medicaid‐expansion states, along with nonsignificant changes in nonexpansion states. Hempstead and Cantor assessed payer mix for non‐Medicare discharges using state hospital association data from 21 states through the fourth quarter of 2014 and found a significant increase in Medicaid patients as well as a nearly significant decrease in uninsured patients in expansion states relative to nonexpansion states.[5] The Department of Health and Human Services also reported that uninsured/self‐pay discharges fell substantially (65%73%) in Medicaid‐expansion states by the end of 2014, with slight decreases in nonexpansion states.[20]
In contrast to our hypothesis, the overall LOS and in‐hospital mortality indices were not influenced by state Medicaid expansion status. From a purely mathematical standpoint, the contribution of Medicaid patients to the overall LOS and mortality indices may have been eclipsed by Medicare and commercially insured patients, who represented a higher proportion of total discharges. The lack of impact of state Medicaid expansion status on overall LOS and mortality indices did not appear to occur as a result of indices for Medicaid patients trending toward the mean. As predicted based on observational studies, Medicaid patients in our study tended to have a higher LOS index than those with other insurance types. Medicaid patients actually tended to have a lower mortality index in our analysis; the reason for this latter finding is unclear and in contrast to other published studies.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 21]
To our knowledge, no other studies have evaluated the effect of payer mix changes under the ACA on inpatient outcomes. However, new evidence is emerging on outpatient outcomes. Low‐income adults in Medicaid‐expansion states have reported greater gains in access to primary care services and in the diagnosis of certain chronic health conditions than those in nonexpansion states as a result of ACA implementation.[22, 23] Such improvements in the outpatient setting might be expected to reduce patient acuity on admission. However, they would not necessarily translate to relative improvements in LOS or mortality indices for Medicaid‐expansion hospitals, as the UHC risk adjustment models controlled for disease severity on admission.
Similarly, few studies have assessed the impact of payer mix changes under previous state Medicaid expansions on inpatient outcomes. After Massachusetts expanded Medicaid and enacted near‐universal healthcare coverage in 2006, a minimal LOS reduction of just 0.05 days was observed.[24] New York expanded Medicaid eligibility to nondisabled childless adults with incomes below 100% of the federal poverty level in September 2001, whereas Arizona did so in November 2001 and Maine in October 2002. A study comparing outcomes in these states to 4 neighboring nonexpansion states found a relative reduction in annual all‐cause mortality of 6.1% population wide; however, it did not assess in‐hospital mortality.[25] The Oregon Health Insurance Experiment that randomized low‐income adults to expanded Medicaid coverage or not in 2008 has also reported on outpatient rather than inpatient outcomes.[26]
Our findings have potential implications for health policymakers. That Medicaid expansion status had a neutral effect on both LOS and mortality indices in our analysis should be reassuring for states contemplating Medicaid expansion in the future. Our results also highlight the need for further efforts to reduce disparities in inpatient care based on payer status. For example, although Medicare, commercially insured, and Medicaid patients witnessed significant improvements in mortality indices pre‐ to post‐ACA implementation in hospitals in both Medicaid‐expansion and nonexpansion states, uninsured patients did not.
This study has several limitations. First, our analysis of the impact of ACA implementation on payer mix did not account for concurrent socioeconomic trends that may have influenced insurance coverage across the United States. However, the main goal of this analysis was to demonstrate that changes in payer mix did in fact occur over time, to provide rationale for our subsequent LOS and mortality analyses. Second, we could not control for variation in the design and implementation of Medicaid expansions across states as permitted under the federal Section 1115 waiver process. Third, we only had access to hospital‐level data through the UHC CDB/RM, rather than individual patient data. We attempted to mitigate this limitation by weighting data according to the number of cases per hospital. Lastly, additional patient‐level factors that may influence LOS or mortality may not be included in the UHC risk adjustment models.
In summary, the differential shift in payer mix between Medicaid‐expansion and nonexpansion states did not influence overall LOS or in‐hospital mortality for general medicine patients at AMCs in the United States. Additional research could help to determine the impact of ACA implementation on other patient outcomes that may be dependent on insurance status, such as readmissions or hospital‐acquired complications.
Disclosures: M.E.A. conceived of the study concept and design, assisted with data acquisition, and drafted the manuscript. J.J.G. assisted with study design and made critical revisions to the manuscript. D.A. assisted with study design and made critical revisions to the manuscript. R.P. assisted with study design and made critical revisions to the manuscript. M.L. assisted with study design and data acquisition and made critical revisions to the manuscript. C.D.J. assisted with study design, performed data analyses, and made critical revisions to the manuscript. A modified abstract was presented in poster format at the University HealthSystem Consortium Annual Conference held September 30 to October 2, 2015 in Orlando, Florida, as well as at the Society of Hospital Medicine Research, Innovations, and Vignettes 2016 Annual Meeting held March 69, 2016, in San Diego, California. The authors report no conflicts of interest.
On January 1, 2014, several major provisions of the Affordable Care Act (ACA) took effect, including introduction of the individual mandate for health insurance coverage, opening of the Health Insurance Marketplace, and expansion of Medicaid eligibility to Americans earning up to 133% of the federal poverty level.[1] Nearly 9 million US adults have enrolled in Medicaid since that time, primarily in the 31 states and Washington, DC that have opted into Medicaid expansion.[2, 3] ACA implementation has also had a significant impact on hospital payer mix, primarily by reducing the volume of uncompensated care in Medicaid‐expansion states.[4, 5]
The differential shift in payer mix in Medicaid‐expansion versus nonexpansion states may be relevant to hospitals beyond reimbursement. Medicaid insurance has historically been associated with longer hospitalizations and higher in‐hospital mortality in diverse patient populations, more so than commercial insurance and often even uninsured payer status.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15] The disparity in outcomes between patients with Medicaid versus other insurance persists even after adjustment for disease severity and baseline comorbidities. Insurance type may influence the delivery of inpatient care through variation in access to invasive procedures and adherence to guideline‐concordant medical therapies.[9, 10, 11, 12] Medicaid patients may be more likely than uninsured patients to remain hospitalized pending postacute care placement rather than be discharged home with family support.[16] Medicaid patients are also less likely to leave against medical advice than uninsured patients.[17]
Currently, little is known about the impact of state Medicaid expansion status on length of stay (LOS) or mortality nationally. It is possible that hospitals in Medicaid‐expansion states have experienced relative worsening in LOS and mortality as their share of Medicaid patients has grown. Determining the impact of ACA implementation on payer mix and patient outcomes is particularly important for academic medical centers (AMCs), as they traditionally care for the greatest percentage of both Medicaid and uninsured patients.[18] We sought to characterize the impact of state Medicaid expansion status on payer mix, LOS, and in‐hospital mortality for general medicine patients at AMCs in the United States.
METHODS
The University HealthSystem Consortium (UHC) is an alliance of 117 AMCs and 310 affiliated hospitals, representing >90% of such institutions in the US. We queried the online UHC Clinical Data Base/Resource Manager (CDB/RM) to obtain hospital‐level insurance, LOS, and mortality data for inpatients discharged from a general medicine service between October 1, 2012 and September 30, 2015. We excluded hospitals that were missing data for any month within the study period. No patient‐level data were accessed.
Our outcomes of interest were the proportion of discharges by primary payer (Medicare, commercial, Medicaid, uninsured, or other [eg, Tri‐Care or Workers' Compensation]), as well as the LOS index and mortality index. Both indices were defined as the ratio of the observed to expected values. To determine the expected LOS and mortality, the UHC 2015 risk adjustment models were applied to all cases, adjusting for variables such as patient demographics, low socioeconomic status, admit source and status, severity of illness, and comorbid conditions, as described by International Classification of Diseases, Ninth Revision codes. These models have been validated and are used for research and quality benchmarking for member institutions.[19]
We next stratified hospitals according to state Medicaid expansion status. We defined Medicaid‐expansion states as those that had expanded Medicaid by the end of the study period: Arizona, Arkansas, California, Colorado, Connecticut, Illinois, Indiana, Iowa, Kentucky, Maryland, Massachusetts, Michigan, Minnesota, Nevada, New Hampshire, New Jersey, New Mexico, New York, Ohio, Oregon, Pennsylvania, Rhode Island, Washington, Washington DC, and West Virginia. Nonexpansion states included Alabama, Florida, Georgia, Kansas, Louisiana, Missouri, Nebraska, North Carolina, South Carolina, Tennessee, Texas, Utah, Virginia, and Wisconsin. We excluded 12 states due to incomplete data: Alaska, Delaware, Hawaii, Idaho, North Dakota, Maine, Mississippi, Montana, Oklahoma, South Dakota, Vermont, and Wyoming.
We then identified our pre‐ and post‐ACA implementation periods. Medicaid coverage expansion took effect in all expansion states on January 1, 2014, with the exception of Michigan (April 1, 2014), New Hampshire (August 15, 2014), Pennsylvania (January 1, 2015), and Indiana (February 1, 2015).[3] We therefore defined October 1, 2012 to December 31, 2013 as the pre‐ACA implementation period and January 1, 2014 to September 30, 2015 as the post‐ACA implementation period for all states except for Michigan, New Hampshire, Pennsylvania, and Indiana. For these 4 states, we customized the pre‐ and post‐ACA implementation periods to their respective dates of Medicaid expansion; for New Hampshire, we designated October 1, 2012 to July 31, 2014 as the pre‐ACA implementation period and September 1, 2014 to September 30, 2015 as the post‐ACA implementation period, as we were unable to distinguish before versus after data in August 2014 based on the midmonth expansion of Medicaid.
After stratifying hospitals into groups based on whether they were located in Medicaid‐expansion or nonexpansion states, the proportion of discharges by payer was compared between pre‐ and post‐ACA implementation periods both graphically by quarter and using linear regression models weighted for the number of cases from each hospital. Next, for both Medicaid‐expansion and nonexpansion hospitals, LOS index and mortality index were compared before and after ACA implementation using linear regression models weighted for the number of cases from each hospital, both overall and by payer. Difference‐in‐differences estimations were then completed to compare the proportion of discharges by payer, LOS index, and mortality index between Medicaid‐expansion and nonexpansion hospitals before and after ACA implementation. Post hoc linear regression analyses were completed to evaluate the effect of clustering by state level strata on payer mix and LOS and mortality indices. A 2‐sided P value of 0.05 was considered statistically significant. Data analyses were performed using Stata 12.0 (StataCorp, College Station, TX).
RESULTS
We identified 4,258,952 discharges among general medicine patients from 211 hospitals in 38 states and Washington, DC between October 1, 2012, and September 30, 2015. This included 3,144,488 discharges from 156 hospitals in 24 Medicaid‐expansion states and Washington, DC and 1,114,464 discharges from 55 hospitals in 14 nonexpansion states.
Figure 1 shows the trends in payer mix over time for hospitals in both Medicaid‐expansion and nonexpansion states. As summarized in Table 1, hospitals in Medicaid‐expansion states experienced a significant 3.7‐percentage point increase in Medicaid discharges (P = 0.013) and 2.9‐percentage point decrease in uninsured discharges (P 0.001) after ACA implementation. This represented an approximately 19% jump and 60% drop in Medicaid and uninsured discharges, respectively. Hospitals in nonexpansion states saw no significant change in the proportion of discharges by payer after ACA implementation. In the difference‐in‐differences analysis, there was a trend toward a greater change in the proportion of Medicaid discharges pre‐ to post‐ACA implementation among hospitals in Medicaid‐expansion states compared to hospitals in nonexpansion states (mean difference‐in‐differences 4.1%, 95% confidence interval [CI]: 0.3%, 8.6%, P = 0.070).
| Medicaid‐expansion n=156 hospitals; 3,144,488 cases | Non‐expansion n=55 hospitals; 1,114,464 cases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐ACA Implementation (1,453,090 Cases) | Post‐ACA Implementation (1,691,398 Cases) | Mean Difference | P Value | Pre‐ACA Implementation (455,440 Cases) | Post‐ACA Implementation (659,024 Cases) | Mean Difference | P Value | Mean Difference‐in‐Differences | P Value | |
| ||||||||||
| Payer mix, % (95% CI) | ||||||||||
| Medicare | 48.6 (46.2, 51.0)* | 48.3 (45.9, 50.7) | 0.3 (3.6, 3.1) | 0.865 | 44.3 (40.7, 47.7)* | 45.3 (41.9, 48.6) | 1.0 (3.8, 5.8) | 0.671 | 1.3 (7.1, 4.5) | 0.655 |
| Commercial | 23.1 (21.4, 24.7) | 23.2 (21.8, 24.6) | 0.2 (2.0, 2.3) | 0.882 | 21.5 (18.5, 24.6) | 22.7 (19.7, 25.8) | 1.2 (3.0, 5.4) | 0.574 | 1.0 (5.7, 3.6) | 0.662 |
| Medicaid | 19.6 (17.6, 21.6) | 23.3 (21.2, 25.5) | 3.7 (0.8, 6.6) | 0.013 | 19.4 (16.9, 21.9) | 19.0 (16.5, 21.4) | 0.4 (3.8, 3.0) | 0.812 | 4.1 (0.3, 8.6) | 0.070 |
| Uninsured | 5.0 (4.0, 5.9) | 2.0 (1.7, 2.3) | 2.9 (3.9, 2.0) | 0.001 | 10.9 (8.1, 13.7) | 9.4 (7.0, 11.7) | 1.5 (5.1, 2.1) | 0.407 | 1.4 (5.1, 2.2) | 0.442 |
| Other | 3.8 (2.6, 4.9) | 3.1 (2.0, 4.3) | 0.7 (2.3, 1.0) | 0.435 | 4.0 (2.9, 5.0) | 3.7 (2.6, 4.7) | 0.3 (1.7, 1.1) | 0.662 | 0.3 (2.5, 1.8) | 0.762 |
| LOS index, mean (95% CI) | ||||||||||
| Overall | 1.017 (0.996, 1.038) | 1.006 (0.981, 1.031) | 0.011 (0.044, 0.021) | 0.488 | 1.008 (0.974, 1.042) | 0.995 (0.961, 1.029) | 0.013 (0.061, 0.034) | 0.574 | 0.002 (0.055, 0.059) | 0.943 |
| Medicare | 1.012 (0.989, 1.035) | 0.999 (0.971, 1.027) | 0.013 (0.049, 0.023) | 0.488 | 0.982 (0.946, 1.017) | 0.979 (0.944, 1.013) | 0.003 (0.052, 0.046) | 0.899 | 0.010 (0.070, 0.051) | 0.754 |
| Commercial | 0.993 (0.974, 1.012) | 0.977 (0.955, 0.998) | 0.016 (0.045, 0.013) | 0.271 | 1.009 (0.978, 1.039) | 0.986 (0.956, 1.016) | 0.022 (0.065, 0.020) | 0.298 | 0.006 (0.044, 0.057) | 0.809 |
| Medicaid | 1.059 (1.036, 1.082) | 1.043 (1.018, 1.067) | 0.016 (0.049, 0.017) | 0.349 | 1.064 (1.020, 1.108) | 1.060 (1.015, 1.106) | 0.004 (0.066, 0.059) | 0.911 | 0.012 (0.082, 0.057) | 0.727 |
| Uninsured | 0.960 (0.933, 0.988) | 0.925 (0.890, 0.961) | 0.035 (0.080, 0.010) | 0.126 | 0.972 (0.935, 1.009) | 0.944 (0.909, 0.979) | 0.028 (0.078, 0.022) | 0.273 | 0.007 (0.074, 0.060) | 0.835 |
| Other | 0.988 (0.960, 1.017) | 0.984 (0.952, 1.015) | 0.005 (0.047, 0.037) | 0.822 | 1.022 (0.973, 1.071) | 0.984 (0.944, 1.024) | 0.038 (0.100, 0.024) | 0.232 | 0.033 (0.042, 0.107) | 0.386 |
| Mortality index, mean (95% CI) | ||||||||||
| Overall | 1.000 (0.955, 1.045) | 0.878 (0.836, 0.921) | 0.122 (0.183, 0.061) | 0.001 | 0.997 (0.931, 1.062) | 0.850 (0.800, 0.900) | 0.147 (0.227, 0.066) | 0.001 | 0.025 (0.076, 0.125) | 0.628 |
| Medicare | 0.990 (0.942, 1.038) | 0.871 (0.826, 0.917) | 0.119 (0.185, 0.053) | 0.001 | 1.000 (0.925, 1.076) | 0.844 (0.788, 0.900) | 0.156 (0.249, 0.064) | 0.001 | 0.038 (0.075, 0.150) | 0.513 |
| Commercial | 1.045 (0.934, 1.155) | 0.908 (0.842, 0.975) | 0.136 (0.264, 0.008) | 0.037 | 1.023 (0.935, 1.111) | 0.820 (0.758, 0.883) | 0.203 (0.309, 0.096) | 0.001 | 0.067 (0.099, 0.232) | 0.430 |
| Medicaid | 0.894 (0.845, 0.942) | 0.786 (0.748, 0.824) | 0.107 (0.168, 0.046) | 0.001 | 0.937 (0.861, 1.013) | 0.789 (0.733, 0.844) | 0.148 (0.242, 0.055) | 0.002 | 0.041 (0.069, 0.151) | 0.464 |
| Uninsured | 1.172 (1.007, 1.337)∥ | 1.136 (0.968, 1.303) | 0.037 (0.271, 0.197) | 0.758 | 0.868 (0.768, 0.968)∥ | 0.850 (0.761, 0.939) | 0.017 (0.149, 0.115) | 0.795 | 0.019 (0.287, 0.248) | 0.887 |
| Other | 1.376 (1.052, 1.700)# | 1.156 (0.910, 1.402) | 0.220 (0.624, 0.184) | 0.285 | 1.009 (0.868, 1.150) # | 0.874 (0.682, 1.066) | 0.135 (0.369, 0.099) | 0.254 | 0.085 (0.555, 0.380) | 0.720 |

Table 1 shows that the overall LOS index remained unchanged pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.017 to 1.006, P = 0.488) and nonexpansion hospitals (1.008 to 0.995, P = 0.574). LOS indices for each payer type also remained unchanged. The overall mortality index significantly improved pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.000 to 0.878, P 0.001) and nonexpansion hospitals (0.997 to 0.850, P = 0.001). Among both Medicaid‐expansion and nonexpansion hospitals, the mortality index significantly improved for Medicare, commercial, and Medicaid discharges but not for uninsured or other discharges. In the difference‐in‐differences analysis, the changes in LOS indices and mortality indices pre‐ to post‐ACA implementation did not differ significantly between hospitals in Medicaid‐expansion versus nonexpansion states.
In post hoc linear regression analyses of payer mix and LOS and mortality indices clustered by state‐level strata, point estimates were minimally changed. Although 95% CIs were slightly wider, statistical significance was unchanged from our primary analyses (data not shown).
DISCUSSION
We found that ACA implementation had a significant impact on payer mix for general medicine patients at AMCs in the United States, primarily by increasing the number of Medicaid beneficiaries and by decreasing the number of uninsured patients in Medicaid‐expansion states. State Medicaid expansion status did not appear to influence either LOS or in‐hospital mortality.
Our study offers some of the longest‐term data currently available on the impact of ACA implementation on payer mix trends and encompasses more states than others have previously. Although we uniquely focused on general medicine patients at AMCs, our results are similar to those seen for US hospitals overall. Nikpay and colleagues evaluated payer mix trends for non‐Medicare adult inpatient stays in 16 states through the second quarter of 2014 using the Healthcare Cost and Utilization Project database through the Agency for Healthcare Research and Quality.[4] They found a relative 20% increase and 50% decrease in Medicaid and uninsured discharges in Medicaid‐expansion states, along with nonsignificant changes in nonexpansion states. Hempstead and Cantor assessed payer mix for non‐Medicare discharges using state hospital association data from 21 states through the fourth quarter of 2014 and found a significant increase in Medicaid patients as well as a nearly significant decrease in uninsured patients in expansion states relative to nonexpansion states.[5] The Department of Health and Human Services also reported that uninsured/self‐pay discharges fell substantially (65%73%) in Medicaid‐expansion states by the end of 2014, with slight decreases in nonexpansion states.[20]
In contrast to our hypothesis, the overall LOS and in‐hospital mortality indices were not influenced by state Medicaid expansion status. From a purely mathematical standpoint, the contribution of Medicaid patients to the overall LOS and mortality indices may have been eclipsed by Medicare and commercially insured patients, who represented a higher proportion of total discharges. The lack of impact of state Medicaid expansion status on overall LOS and mortality indices did not appear to occur as a result of indices for Medicaid patients trending toward the mean. As predicted based on observational studies, Medicaid patients in our study tended to have a higher LOS index than those with other insurance types. Medicaid patients actually tended to have a lower mortality index in our analysis; the reason for this latter finding is unclear and in contrast to other published studies.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 21]
To our knowledge, no other studies have evaluated the effect of payer mix changes under the ACA on inpatient outcomes. However, new evidence is emerging on outpatient outcomes. Low‐income adults in Medicaid‐expansion states have reported greater gains in access to primary care services and in the diagnosis of certain chronic health conditions than those in nonexpansion states as a result of ACA implementation.[22, 23] Such improvements in the outpatient setting might be expected to reduce patient acuity on admission. However, they would not necessarily translate to relative improvements in LOS or mortality indices for Medicaid‐expansion hospitals, as the UHC risk adjustment models controlled for disease severity on admission.
Similarly, few studies have assessed the impact of payer mix changes under previous state Medicaid expansions on inpatient outcomes. After Massachusetts expanded Medicaid and enacted near‐universal healthcare coverage in 2006, a minimal LOS reduction of just 0.05 days was observed.[24] New York expanded Medicaid eligibility to nondisabled childless adults with incomes below 100% of the federal poverty level in September 2001, whereas Arizona did so in November 2001 and Maine in October 2002. A study comparing outcomes in these states to 4 neighboring nonexpansion states found a relative reduction in annual all‐cause mortality of 6.1% population wide; however, it did not assess in‐hospital mortality.[25] The Oregon Health Insurance Experiment that randomized low‐income adults to expanded Medicaid coverage or not in 2008 has also reported on outpatient rather than inpatient outcomes.[26]
Our findings have potential implications for health policymakers. That Medicaid expansion status had a neutral effect on both LOS and mortality indices in our analysis should be reassuring for states contemplating Medicaid expansion in the future. Our results also highlight the need for further efforts to reduce disparities in inpatient care based on payer status. For example, although Medicare, commercially insured, and Medicaid patients witnessed significant improvements in mortality indices pre‐ to post‐ACA implementation in hospitals in both Medicaid‐expansion and nonexpansion states, uninsured patients did not.
This study has several limitations. First, our analysis of the impact of ACA implementation on payer mix did not account for concurrent socioeconomic trends that may have influenced insurance coverage across the United States. However, the main goal of this analysis was to demonstrate that changes in payer mix did in fact occur over time, to provide rationale for our subsequent LOS and mortality analyses. Second, we could not control for variation in the design and implementation of Medicaid expansions across states as permitted under the federal Section 1115 waiver process. Third, we only had access to hospital‐level data through the UHC CDB/RM, rather than individual patient data. We attempted to mitigate this limitation by weighting data according to the number of cases per hospital. Lastly, additional patient‐level factors that may influence LOS or mortality may not be included in the UHC risk adjustment models.
In summary, the differential shift in payer mix between Medicaid‐expansion and nonexpansion states did not influence overall LOS or in‐hospital mortality for general medicine patients at AMCs in the United States. Additional research could help to determine the impact of ACA implementation on other patient outcomes that may be dependent on insurance status, such as readmissions or hospital‐acquired complications.
Disclosures: M.E.A. conceived of the study concept and design, assisted with data acquisition, and drafted the manuscript. J.J.G. assisted with study design and made critical revisions to the manuscript. D.A. assisted with study design and made critical revisions to the manuscript. R.P. assisted with study design and made critical revisions to the manuscript. M.L. assisted with study design and data acquisition and made critical revisions to the manuscript. C.D.J. assisted with study design, performed data analyses, and made critical revisions to the manuscript. A modified abstract was presented in poster format at the University HealthSystem Consortium Annual Conference held September 30 to October 2, 2015 in Orlando, Florida, as well as at the Society of Hospital Medicine Research, Innovations, and Vignettes 2016 Annual Meeting held March 69, 2016, in San Diego, California. The authors report no conflicts of interest.
- Department of Health and Human Services. Key features of the Affordable Care Act by year. Available at: http://www.hhs.gov/healthcare/facts‐and‐features/key‐features‐of‐aca‐by‐year/index.html#2014. Accessed April 4, 2016.
- Centers for Medicare and Medicaid Services. Medicaid enrollment data collected through MBES. Available at: https://www.medicaid.gov/medicaid‐chip‐program‐information/program‐information/medicaid‐and‐chip‐enrollment‐data/medicaid‐enrollment‐data‐collected‐through‐mbes.html. Accessed April 4, 2016.
- The Henry J. Kaiser Family Foundation. Status of state action on the Medicaid expansion decision. Available at: http://kff.org/health‐reform/state‐indicator/state‐activity‐around‐expanding‐medicaid‐under‐the‐affordable‐care‐act. Accessed April 4, 2016.
- , , . Affordable Care Act Medicaid expansion reduced uninsured hospital stays in 2014. Health Aff (Millwood). 2016;35(1):106–110.
- , . State Medicaid expansion and changes in hospital volume according to payer. N Engl J Med. 2016;374(2):196–198.
- , , , , , . Understanding predictors of prolonged hospitalizations among general medicine patients: a guide and preliminary analysis. J Hosp Med. 2015;10(9):623–626.
- , , , . Impact of insurance and hospital ownership on hospital length of stay among patients with ambulatory care‐sensitive conditions. Ann Fam Med. 2011;9:489–495.
- , , . Insurance status and hospital care for myocardial infarction, stroke, and pneumonia. J Hosp Med. 2010;5:452–459.
- , , , , , . Payment source, quality of care, and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2011;58(14):1465–1471.
- , , , , . The inpatient experience and predictors of length of stay for patients hospitalized with systolic heart failure: comparison by commercial, Medicaid, and Medicare payer type. J Med Econ. 2013;16(1):43–54.
- , , et al. Insurance coverage and care of patients with non‐ST‐segment elevation acute coronary syndromes. Ann Intern Med. 2006;145:739–748.
- , , , et al. Association of insurance status with inpatient treatment for coronary artery disease: findings from the Get with the Guidelines Program. Am Heart J. 2010;159:1026–1036.
- , , , et al. Primary payer status affects mortality for major surgical operations. Ann Surg. 2010;252:544–551.
- , , . Medicaid payer status is associated with in‐hospital morbidity and resource utilization following primary total joint arthroplasty. J Bone Joint Surg Am. 2014;96(21):e180.
- , , . The quality of care delivered to patients within the same hospital varies by insurance type. Health Aff (Millwood). 2013;32(10):1731–1739.
- , , , , . Effect of insurance status on postacute care among working age stroke survivors. Neurology. 2012;78(20):1590–1595.
- , , , . Hospitalizations in which patients leave the hospital against medical advice (AMA), 2007. HCUP statistical brief #78. August 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2009. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb78.pdf. Accessed May 12, 2016.
- , , , . Characteristics of Medicaid and uninsured hospitalizations, 2012. HCUP statistical brief #182. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb182‐Medicaid‐Uninsured‐Hospitalizations‐2012.pdf. Accessed March 9, 2016.
- Agency for Healthcare Research and Quality. Mortality measurement: mortality risk adjustment methodology for University HealthSystem Consortium. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Meurer.pdf. Accessed May 10, 2016.
- Department of Health and Human Services. Insurance expansion, hospital uncompensated care, and the Affordable Care Act. Available at: https://aspe.hhs.gov/pdf‐report/insurance‐expansion‐hospital‐uncompensated‐care‐and‐affordable‐care‐act. Accessed May 27, 2016.
- , , , . Our flawed but beneficial Medicaid program. N Engl J Med. 2011;364(16):e31.
- , , , . Changes in self‐reported insurance coverage, access to care, and health under the Affordable Care Act. JAMA. 2015;314(4):366–374.
- , . Early coverage, access, utilization, and health effects associated with the Affordable Care Act Medicaid Expansions: a quasi‐experimental study. Ann Intern Med. 2016;164(12):795–803.
- , . The impact of health care reform on hospital and preventive care: evidence from Massachusetts. J Public Econ. 2012;96(11–12):909–929.
- , , . Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367:1025–1034.
- , , , et al. The Oregon Experiment—effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713–1722.
- Department of Health and Human Services. Key features of the Affordable Care Act by year. Available at: http://www.hhs.gov/healthcare/facts‐and‐features/key‐features‐of‐aca‐by‐year/index.html#2014. Accessed April 4, 2016.
- Centers for Medicare and Medicaid Services. Medicaid enrollment data collected through MBES. Available at: https://www.medicaid.gov/medicaid‐chip‐program‐information/program‐information/medicaid‐and‐chip‐enrollment‐data/medicaid‐enrollment‐data‐collected‐through‐mbes.html. Accessed April 4, 2016.
- The Henry J. Kaiser Family Foundation. Status of state action on the Medicaid expansion decision. Available at: http://kff.org/health‐reform/state‐indicator/state‐activity‐around‐expanding‐medicaid‐under‐the‐affordable‐care‐act. Accessed April 4, 2016.
- , , . Affordable Care Act Medicaid expansion reduced uninsured hospital stays in 2014. Health Aff (Millwood). 2016;35(1):106–110.
- , . State Medicaid expansion and changes in hospital volume according to payer. N Engl J Med. 2016;374(2):196–198.
- , , , , , . Understanding predictors of prolonged hospitalizations among general medicine patients: a guide and preliminary analysis. J Hosp Med. 2015;10(9):623–626.
- , , , . Impact of insurance and hospital ownership on hospital length of stay among patients with ambulatory care‐sensitive conditions. Ann Fam Med. 2011;9:489–495.
- , , . Insurance status and hospital care for myocardial infarction, stroke, and pneumonia. J Hosp Med. 2010;5:452–459.
- , , , , , . Payment source, quality of care, and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2011;58(14):1465–1471.
- , , , , . The inpatient experience and predictors of length of stay for patients hospitalized with systolic heart failure: comparison by commercial, Medicaid, and Medicare payer type. J Med Econ. 2013;16(1):43–54.
- , , et al. Insurance coverage and care of patients with non‐ST‐segment elevation acute coronary syndromes. Ann Intern Med. 2006;145:739–748.
- , , , et al. Association of insurance status with inpatient treatment for coronary artery disease: findings from the Get with the Guidelines Program. Am Heart J. 2010;159:1026–1036.
- , , , et al. Primary payer status affects mortality for major surgical operations. Ann Surg. 2010;252:544–551.
- , , . Medicaid payer status is associated with in‐hospital morbidity and resource utilization following primary total joint arthroplasty. J Bone Joint Surg Am. 2014;96(21):e180.
- , , . The quality of care delivered to patients within the same hospital varies by insurance type. Health Aff (Millwood). 2013;32(10):1731–1739.
- , , , , . Effect of insurance status on postacute care among working age stroke survivors. Neurology. 2012;78(20):1590–1595.
- , , , . Hospitalizations in which patients leave the hospital against medical advice (AMA), 2007. HCUP statistical brief #78. August 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2009. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb78.pdf. Accessed May 12, 2016.
- , , , . Characteristics of Medicaid and uninsured hospitalizations, 2012. HCUP statistical brief #182. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb182‐Medicaid‐Uninsured‐Hospitalizations‐2012.pdf. Accessed March 9, 2016.
- Agency for Healthcare Research and Quality. Mortality measurement: mortality risk adjustment methodology for University HealthSystem Consortium. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Meurer.pdf. Accessed May 10, 2016.
- Department of Health and Human Services. Insurance expansion, hospital uncompensated care, and the Affordable Care Act. Available at: https://aspe.hhs.gov/pdf‐report/insurance‐expansion‐hospital‐uncompensated‐care‐and‐affordable‐care‐act. Accessed May 27, 2016.
- , , , . Our flawed but beneficial Medicaid program. N Engl J Med. 2011;364(16):e31.
- , , , . Changes in self‐reported insurance coverage, access to care, and health under the Affordable Care Act. JAMA. 2015;314(4):366–374.
- , . Early coverage, access, utilization, and health effects associated with the Affordable Care Act Medicaid Expansions: a quasi‐experimental study. Ann Intern Med. 2016;164(12):795–803.
- , . The impact of health care reform on hospital and preventive care: evidence from Massachusetts. J Public Econ. 2012;96(11–12):909–929.
- , , . Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367:1025–1034.
- , , , et al. The Oregon Experiment—effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713–1722.
Predictors of Prolonged Hospitalizations
Hospitalizations frequently last longer than warranted by medical necessity alone, due to inefficiencies within the US healthcare system.[1, 2] Discharge delays place patients at risk for hospital‐acquired complications and increase costs. With the growing emphasis on high‐value care, hospital length of stay (LOS) has emerged as a key metric for inpatient care and will remain a central focus of hospital‐based improvement initiatives for the foreseeable future.
Hospitals may find it difficult to identify the primary drivers of inpatient LOS in a dynamic and increasingly complex healthcare system. Multiple recent policy changes have affected inpatient care. The Health Information Technology for Economic and Clinical Health Act of 2009 has led to widespread adoption of electronic health records (EHRs) that have markedly impacted provider workflows.[3] In October 2013, the Centers for Medicare & Medicaid Services implemented the 2‐midnight rule, which reclassified lower acuity inpatients with an expected stay 48 hours to observation status.[4] In January 2014, expansion of insurance coverage under the Affordable Care Act (ACA) altered payer mix for hospitals nationwide.[5] At a local level, hospitals that are rapidly adjusting resource allocation, capital investments, and marketing efforts and making complex operational decisions (eg, to open new units or change admission or referral algorithms) may simultaneously experience shifts in patient volumes, case‐mix index, and staffing ratios with downstream effects on LOS.
Given the myriad factors influencing inpatient LOS, hospital leaders may encounter real challenges in designing effective LOS reduction strategies. For example, they may expend significant resources on real‐time demand‐capacity management systems to improve hospital‐wide patient flow, but the resultant emphasis on bed placement and early discharges may shave only hours off average LOS.[6, 7] An alternative approach may be to target the small percentage of patients with prolonged hospitalizations who contribute disproportionately to the average LOS, as other initiatives focused on high utilizers have done.[8, 9, 10]
Our institution noted an increase in the average inpatient LOS for general medicine patients from 2012 to 2014, prompting a call to action by hospital leaders. We sought to characterize the predictors of prolonged hospitalizations among medicine patients to guide future efforts aimed at mitigating the contribution of prolonged LOS to overall LOS.
METHODS
Study Design
We performed a retrospective analysis of medicine patients discharged between January 1, 2012 and December 31, 2014, from the University of Colorado Hospital, a 551‐bed urban, quaternary‐care academic medical center in Aurora, Colorado. Patients were included if they were admitted under inpatient status, 18 years of age, and discharged from 1 of our 10 medicine services: 7 services with residents, staffed by hospitalists, general internists, or subspecialists; and 3 services with advanced practice providers, staffed by hospitalists.
Data Collection
We obtained LOS, calendar year of discharge, demographic data, insurance type, discharge disposition, number of medications, consults, intensive care unit (ICU) stays, surgeries (ie, procedures requiring anesthesia), and primary diagnosis by International Classification of Diseases, Ninth Revision codes from an administrative database that had been developed, validated, and maintained by our hospital medicine group. This database was populated with variables from our EHR, which was implemented in September 2011; to minimize variability in data input during the EHR rollout, we excluded data from September 2011 through December 2011. The Colorado Multiple Institutional Review Board reviewed and exempted this database (protocol 13‐2953) as a program evaluation.
Outcomes
We defined a prolonged hospitalization or LOS as >21 days in duration. This represented approximately 2 standard deviations above the mean LOS in our cohort. This cutoff also helped to remove provider‐level variability, as each medicine service was staffed by 2 attendings per month, each working approximately 7 days on and 7 days off. We examined LOS >14 and >30 days in sensitivity analyses to ensure that the selection of >21 days did not impose an arbitrary and invalid limitation on our statistical analysis.
Statistical Analysis
Demographic and clinical data were compared in the group with LOS 21 days versus the group with LOS >21 days with a 2 test for dichotomous variables and Student t test for continuous variables. We then built a multivariable logistic regression model to predict LOS >21 days using the variables that were significantly different between groups in bivariate analyses. A two‐sided P value of 0.05 was considered statistically significant. All data analyses were performed using Stata 12.0 (StataCorp, College Station, TX).
RESULTS
We identified 18,363 inpatient discharges among 12,511 medicine patients between January 1, 2012 and December 31, 2014. Of these discharges, 416 (2.3%) demonstrated prolonged LOS. Prolonged hospitalizations accounted for 18.6% of total inpatient days. The average LOS during the study period was 4.8 days including patients with prolonged LOS and 4.0 days excluding patients with prolonged LOS, a contribution of 0.8 days.
Table 1 compares the characteristics of patients with and without prolonged LOS. Age, insurance, discharge disposition, palliative care consults, ICU stays, and surgeries were among the variables that differed significantly between the 2 groups. Among patients undergoing surgery, those with prolonged LOS were more likely to have surgery >24 hours after admission than those without prolonged LOS (85.7% vs 51.4%, P0.001).
| Variable | LOS 21 Days, N=17,947 | LOS >21 Days, N=416 | P Value |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 56.4 (18.7) | 54.4 (17.1) | 0.030 |
| Female | 9,256 (52%) | 199 (48%) | 0.132 |
| Year of discharge | 0.001 | ||
| 2012 | 5,486 (31%) | 69 (17%) | |
| 2013 | 6,193 (35%) | 162 (39%) | |
| 2014 | 6,268 (35%) | 185 (44%) | |
| Race/ethnicity | 0.003 | ||
| White non‐Hispanic | 9,702 (54%) | 242 (58%) | |
| Black non‐Hispanic | 4,000 (22%) | 68 (16%) | |
| Hispanic | 2,872(16%) | 67 (16%) | |
| Asian | 578 (3%) | 9 (2%) | |
| Other or unknown | 795 (4%) | 30 (7%) | |
| Language preference | 0.795 | ||
| English | 16,049 (89%) | 376 (90%) | |
| Spanish | 1,052 (6%) | 23 (6%) | |
| Other | 846 (5%) | 17 (4%) | |
| Insurance | 0.001 | ||
| Medicare | 5,462 (30%) | 109 (26%) | |
| Medicaid | 3,406 (19%) | 126 (30%) | |
| Dual | 2,815 (16%) | 64 (15%) | |
| Private | 2,714 (15%) | 60 (14%) | |
| Indigent/self‐pay | 2,829 (16%) | 42 (10%) | |
| Other | 721 (4%) | 15 (4%) | |
| Length of stay, d (SD) | 4.0 (3.5) | 39.5 (37.3) | 0.001 |
| Discharge disposition | 0.001 | ||
| Home with self‐care | 13,276 (74%) | 115 (28%) | |
| Home with home health | 1,584 (9%) | 79 (19%) | |
| Hospicehome or inpatient | 369 (2%) | 19 (5%) | |
| Postacute‐care facility or LTAC | 1,761 (10%) | 141 (34%) | |
| Expired | 113 (1%) | 18 (4%) | |
| Other | 844 (5%) | 44 (11%) | |
| No. of admission medications (SD) | 9.7 (7.4) | 10.9 (7.8) | 0.002 |
| Primary diagnosis by ICD‐9 code* | |||
| Sepsis, unspecified | 1,548 (9%) | 55 (13%) | 0.001 |
| Acute respiratory failure | 293 (2%) | 9 (2%) | 0.400 |
| MSSA septicemia | 36 (0.2%) | 8 (2%) | 0.001 |
| MRSA septicemia | 13 (0.1%) | 7 (2%) | 0.001 |
| Alcoholic cirrhosis of the liver | 111 (1%) | 7 (2%) | 0.007 |
| Palliative care consult | 398 (2%) | 64 (15%) | 0.001 |
| ICU stay | 2,030 (11%) | 246 (59%) | 0.001 |
| Surgical procedure | 1,800 (10%) | 182 (44%) | 0.001 |
Unspecified sepsis was the most frequent primary diagnosis, regardless of LOS category (Table 2). However, the second through fifth most frequent diagnoses differed for patients with and without prolonged LOS.
| N | % | |
|---|---|---|
| ||
| LOS 21 days | ||
| 1. Sepsis, unspecified | 1,548 | 8.6% |
| 2. Acute pancreatitis | 435 | 2.4% |
| 3. Pneumonia | 431 | 2.4% |
| 4. Acute kidney failure | 363 | 2.0% |
| 5. COPD exacerbation | 320 | 1.8% |
| LOS >21 days | ||
| 1. Sepsis, unspecified | 55 | 13.0% |
| 2. Acute respiratory failure | 9 | 2.2% |
| 3. Methicillin‐sensitive Staphylococcus aureus septicemia | 8 | 1.9% |
| 4. Methicillin‐resistant Staphylococcus aureus septicemia | 7 | 1.7% |
| 5. Alcoholic cirrhosis of the liver | 7 | 1.7% |
In an adjusted logistic regression model (Table 3), we found lower odds of prolonged LOS for each 10‐year increase in age and higher odds of prolonged LOS for Medicaid insurance, discharge to home with home health, discharge to a postacute‐care or long‐term acute‐care facility, and in‐hospital death. Methicillin‐resistant Staphylococcus aureus (MRSA) septicemia, palliative care consults, ICU stays, and surgeries were all also associated with increased odds of prolonged LOS. We identified a statistically significant interaction between ICU stay and surgical procedure (odds ratio: 2.53, 95% confidence interval: 1.51‐4.26, P0.001).
| Outcome: LOS >21 Days | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| |||
| Age, per 10 years increase in age | 0.80 | 0.73‐0.87 | 0.001 |
| Year of discharge | |||
| 2012 | 0.47 | 0.34‐0.67 | 0.001 |
| 2013 | 1.10 | 0.84‐1.43 | 0.493 |
| 2014 | Ref | ||
| Race/ethnicity | |||
| White non‐Hispanic | Ref | ||
| Black non‐Hispanic | 0.89 | 0.64‐1.22 | 0.454 |
| Hispanic | 1.01 | 0.70‐1.46 | 0.952 |
| Asian | 0.85 | 0.40‐1.83 | 0.679 |
| Other or unknown | 1.29 | 0.73‐2.26 | 0.378 |
| Insurance | |||
| Medicare | Ref | ||
| Medicaid | 1.99 | 1.29‐3.05 | 0.002 |
| Dual | 1.06 | 0.72‐1.57 | 0.765 |
| Private | 1.13 | 0.70‐1.82 | 0.620 |
| Indigent/self‐pay | 1.66 | 0.95‐2.88 | 0.073 |
| Other | 0.96 | 0.47‐1.96 | 0.908 |
| Discharge disposition | |||
| Home with self‐care | Ref | ||
| Home with home health | 4.48 | 3.10‐6.48 | 0.001 |
| Hospicehome or inpatient | 2.11 | 0.98‐4.55 | 0.057 |
| Postacute‐care facility or LTAC | 10.37 | 6.92‐15.56 | 0.001 |
| Expired | 5.38 | 2.27‐12.75 | 0.001 |
| Other | 4.04 | 2.64‐6.18 | 0.001 |
| No. of admission medications | 1.00 | 0.99‐1.02 | 0.775 |
| Primary diagnosis by ICD‐9 code | |||
| Sepsis, unspecified | 1.11 | 0.78‐1.58 | 0.575 |
| MSSA septicemia | 2.44 | 0.68‐8.67 | 0.074 |
| MRSA septicemia | 8.83 | 1.72‐45.36 | 0.009 |
| Alcoholic cirrhosis of the liver | 1.25 | 0.43‐3.65 | 0.687 |
| Palliative care consult | 4.63 | 2.86‐7.49 | 0.001 |
| ICU stay | 6.66 | 5.22‐8.50 | 0.001 |
| Surgical procedure | 5.04 | 3.90‐6.52 | 0.001 |
Sensitivity analyses using LOS >14 and >30 days yielded similar results to LOS >21 days (see Supporting Appendix Table 1 in the online version of this article).
DISCUSSION
We found that a small proportion of medicine patients with prolonged hospitalizations contributed substantially to both total inpatient days and average inpatient LOS. Such disproportionate healthcare utilization is concerning in light of the Institute of Medicine's charge for health systems to deliver timely, efficient, and equitable care.[11]
Few studies in the United States have analyzed patient characteristics that predict prolonged LOS, and to our knowledge, none have evaluated prolonged LOS specifically in general medicine patients.[12, 13, 14] Among selected surgical populations, prolonged hospitalizations are most often related to placement difficulties, operational delays, and payer‐related issues, rather than severity of illness, baseline comorbidities, or in‐hospital complications.[12, 13] In our study, we found that patients with prolonged LOS were more likely to require a palliative care consult, ICU stay, or surgery, all proxies for disease severity. Patients with prolonged LOS were also more likely to undergo surgery >24 hours after admission than those without prolonged LOS, suggesting that the former were either too unstable to proceed directly to surgery or developed complications later during their hospitalization. Even after controlling for palliative care consults, ICU stays, and surgeries, placement at a postacute‐care facility was strongly associated with prolonged LOS. Patients with prolonged LOS were also more likely to have Medicaid compared to other insurance types.
Our findings have several potential implications for efforts aimed at decreasing the number of and length of prolonged hospitalizations. Although demographic and clinical factors such as Medicaid insurance, ICU stays, and surgeries are generally not modifiable, they could, particularly in combination, be used to trigger earlier and more intensive case management involvement. A streamlined insurance approval process for Medicaid pending inpatients could be beneficial, given the recent expansion of Medicaid eligibility under the ACA. Hospital partnerships with postacute‐care facilities could also relieve bottlenecks in placement.[8] Chart review of the patients with MRSA septicemia and prolonged LOS indicated that development of an intensive outpatient parenteral antimicrobial therapy pathway with substance abuse counseling could provide an alternative to extended inpatient treatment for intravenous drug users with complicated infections.[15]
This study has several limitations. First, given the lack of consensus in the literature regarding the definition of a prolonged hospitalization, it is difficult to directly compare our results with existing studies.[8, 12, 13, 14] However, we believe LOS >21 days to be a meaningful cutoff for our cohort. Most demographic and clinical variables that were predictive at >21 days were also predictive at >14 and >30 days, which reassured us that the relationship between variables and prolonged LOS was stable at different thresholds. Second, our database did not allow us to fully adjust for baseline comorbidities or categorize the reasons for discharge delays. Finally, this was a single‐center program evaluation. Although this limits generalization to other institutions, we believe our approach may serve as a guide for others interested in reducing prolonged hospitalizations.
In summary, prolonged hospitalizations represent a potentially high‐yield target for LOS reduction efforts. Prolonged hospitalizations among medicine patients at our institution particularly affected Medicaid enrollees with complex hospital stays who were not discharged home. Further studies are needed to determine the specific reasons for unnecessary hospital days in this population.
Acknowledgements
The authors thank Essey Yirdaw for her contributions to the building and management of the database that informed this work.
Disclosure: M.E.A. conceived of the study concept and design and drafted the manuscript. J.J.G. assisted with the study design and made critical revisions to the final manuscript. D.A., R.P., and R.C. assisted with the study design and made critical revisions to the manuscript. C.D.J. assisted with the study design, performed data analyses, and made critical revisions to the manuscript. RC has disclosed that her time is funded by a National Institutes of Health grant unrelated to this study. A modified abstract was presented in poster format at the Society of Hospital Medicine Research, Innovations, and Vignettes Competition 2015 Annual Meeting, held March 29 April 1, 2015, in National Harbor, Maryland. The authors have no conflicts of interest to disclose.
- , , , et al. Excess hospitalization days in an academic medical center: perceptions of hospitalists and discharge planners. Am J Manag Care. 2011;17(2):e34–e42.
- , , A prospective study of reasons for prolonged hospitalizations on a general medicine teaching service. J Gen Intern Med. 2005;20(2):108–115.
- , , , et al. Adoption of electronic health records grows rapidly, but fewer than half of U.S. hospitals had at least a basic system in 2012. Health Aff (Millwood). 2013;32(8):1478–1485.
- , , , et al. Observation and inpatient status: clinical impact of the 2‐midnight rule. J Hosp Med. 2014;9(4):203–209.
- , , Impact of insurance expansion on hospital uncompensated care costs in 2014. Department of Health and Human Services Office of the Assistant Secretary for Planning and Evaluation. Available at: http://aspe.hhs.gov/health/reports/2014/uncompensatedcare/ib_uncompensatedcare.pdf. Accessed March 28, 2015.
- , , , Using real‐time demand capacity management to improve hospital‐wide patient flow. Jt Comm J Qual Patient Saf. 2011;37(5):217–227.
- , , , , , Natural history of late discharges from a general medical ward. J Hosp Med. 2009;4(4):226–233.
- , , , Addressing hospital length of stay outlier patients: a community‐wide approach. Adv Biosci Biotechol. 2014;5:188–196.
- , American medical home runs. Health Aff (Millwood). 2009;28(5):1317–1326.
- The hot spotters: can we lower medical costs by giving the neediest patients better care? New Yorker. January 2011:40–51.
- Institute of Medicine. Crossing the Quality Chasm. Washington, DC: National Academies Press; 2001.
- , , , et al. Excessively long hospital stays after trauma are not related to the severity of illness: let's aim to the right target! JAMA Surg. 2013;148(1):956–961.
- , , Extended length of stay after surgery: complications, inefficient practice, or sick patients? JAMA Surg. 2014;149(8):815–820.
- , , , , Nonmedical factors associated with prolonged hospital length of stay in an urban homebound population. J Hosp Med. 2012;7(2):73–78.
- , , , Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65(12):2641–2644.
Hospitalizations frequently last longer than warranted by medical necessity alone, due to inefficiencies within the US healthcare system.[1, 2] Discharge delays place patients at risk for hospital‐acquired complications and increase costs. With the growing emphasis on high‐value care, hospital length of stay (LOS) has emerged as a key metric for inpatient care and will remain a central focus of hospital‐based improvement initiatives for the foreseeable future.
Hospitals may find it difficult to identify the primary drivers of inpatient LOS in a dynamic and increasingly complex healthcare system. Multiple recent policy changes have affected inpatient care. The Health Information Technology for Economic and Clinical Health Act of 2009 has led to widespread adoption of electronic health records (EHRs) that have markedly impacted provider workflows.[3] In October 2013, the Centers for Medicare & Medicaid Services implemented the 2‐midnight rule, which reclassified lower acuity inpatients with an expected stay 48 hours to observation status.[4] In January 2014, expansion of insurance coverage under the Affordable Care Act (ACA) altered payer mix for hospitals nationwide.[5] At a local level, hospitals that are rapidly adjusting resource allocation, capital investments, and marketing efforts and making complex operational decisions (eg, to open new units or change admission or referral algorithms) may simultaneously experience shifts in patient volumes, case‐mix index, and staffing ratios with downstream effects on LOS.
Given the myriad factors influencing inpatient LOS, hospital leaders may encounter real challenges in designing effective LOS reduction strategies. For example, they may expend significant resources on real‐time demand‐capacity management systems to improve hospital‐wide patient flow, but the resultant emphasis on bed placement and early discharges may shave only hours off average LOS.[6, 7] An alternative approach may be to target the small percentage of patients with prolonged hospitalizations who contribute disproportionately to the average LOS, as other initiatives focused on high utilizers have done.[8, 9, 10]
Our institution noted an increase in the average inpatient LOS for general medicine patients from 2012 to 2014, prompting a call to action by hospital leaders. We sought to characterize the predictors of prolonged hospitalizations among medicine patients to guide future efforts aimed at mitigating the contribution of prolonged LOS to overall LOS.
METHODS
Study Design
We performed a retrospective analysis of medicine patients discharged between January 1, 2012 and December 31, 2014, from the University of Colorado Hospital, a 551‐bed urban, quaternary‐care academic medical center in Aurora, Colorado. Patients were included if they were admitted under inpatient status, 18 years of age, and discharged from 1 of our 10 medicine services: 7 services with residents, staffed by hospitalists, general internists, or subspecialists; and 3 services with advanced practice providers, staffed by hospitalists.
Data Collection
We obtained LOS, calendar year of discharge, demographic data, insurance type, discharge disposition, number of medications, consults, intensive care unit (ICU) stays, surgeries (ie, procedures requiring anesthesia), and primary diagnosis by International Classification of Diseases, Ninth Revision codes from an administrative database that had been developed, validated, and maintained by our hospital medicine group. This database was populated with variables from our EHR, which was implemented in September 2011; to minimize variability in data input during the EHR rollout, we excluded data from September 2011 through December 2011. The Colorado Multiple Institutional Review Board reviewed and exempted this database (protocol 13‐2953) as a program evaluation.
Outcomes
We defined a prolonged hospitalization or LOS as >21 days in duration. This represented approximately 2 standard deviations above the mean LOS in our cohort. This cutoff also helped to remove provider‐level variability, as each medicine service was staffed by 2 attendings per month, each working approximately 7 days on and 7 days off. We examined LOS >14 and >30 days in sensitivity analyses to ensure that the selection of >21 days did not impose an arbitrary and invalid limitation on our statistical analysis.
Statistical Analysis
Demographic and clinical data were compared in the group with LOS 21 days versus the group with LOS >21 days with a 2 test for dichotomous variables and Student t test for continuous variables. We then built a multivariable logistic regression model to predict LOS >21 days using the variables that were significantly different between groups in bivariate analyses. A two‐sided P value of 0.05 was considered statistically significant. All data analyses were performed using Stata 12.0 (StataCorp, College Station, TX).
RESULTS
We identified 18,363 inpatient discharges among 12,511 medicine patients between January 1, 2012 and December 31, 2014. Of these discharges, 416 (2.3%) demonstrated prolonged LOS. Prolonged hospitalizations accounted for 18.6% of total inpatient days. The average LOS during the study period was 4.8 days including patients with prolonged LOS and 4.0 days excluding patients with prolonged LOS, a contribution of 0.8 days.
Table 1 compares the characteristics of patients with and without prolonged LOS. Age, insurance, discharge disposition, palliative care consults, ICU stays, and surgeries were among the variables that differed significantly between the 2 groups. Among patients undergoing surgery, those with prolonged LOS were more likely to have surgery >24 hours after admission than those without prolonged LOS (85.7% vs 51.4%, P0.001).
| Variable | LOS 21 Days, N=17,947 | LOS >21 Days, N=416 | P Value |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 56.4 (18.7) | 54.4 (17.1) | 0.030 |
| Female | 9,256 (52%) | 199 (48%) | 0.132 |
| Year of discharge | 0.001 | ||
| 2012 | 5,486 (31%) | 69 (17%) | |
| 2013 | 6,193 (35%) | 162 (39%) | |
| 2014 | 6,268 (35%) | 185 (44%) | |
| Race/ethnicity | 0.003 | ||
| White non‐Hispanic | 9,702 (54%) | 242 (58%) | |
| Black non‐Hispanic | 4,000 (22%) | 68 (16%) | |
| Hispanic | 2,872(16%) | 67 (16%) | |
| Asian | 578 (3%) | 9 (2%) | |
| Other or unknown | 795 (4%) | 30 (7%) | |
| Language preference | 0.795 | ||
| English | 16,049 (89%) | 376 (90%) | |
| Spanish | 1,052 (6%) | 23 (6%) | |
| Other | 846 (5%) | 17 (4%) | |
| Insurance | 0.001 | ||
| Medicare | 5,462 (30%) | 109 (26%) | |
| Medicaid | 3,406 (19%) | 126 (30%) | |
| Dual | 2,815 (16%) | 64 (15%) | |
| Private | 2,714 (15%) | 60 (14%) | |
| Indigent/self‐pay | 2,829 (16%) | 42 (10%) | |
| Other | 721 (4%) | 15 (4%) | |
| Length of stay, d (SD) | 4.0 (3.5) | 39.5 (37.3) | 0.001 |
| Discharge disposition | 0.001 | ||
| Home with self‐care | 13,276 (74%) | 115 (28%) | |
| Home with home health | 1,584 (9%) | 79 (19%) | |
| Hospicehome or inpatient | 369 (2%) | 19 (5%) | |
| Postacute‐care facility or LTAC | 1,761 (10%) | 141 (34%) | |
| Expired | 113 (1%) | 18 (4%) | |
| Other | 844 (5%) | 44 (11%) | |
| No. of admission medications (SD) | 9.7 (7.4) | 10.9 (7.8) | 0.002 |
| Primary diagnosis by ICD‐9 code* | |||
| Sepsis, unspecified | 1,548 (9%) | 55 (13%) | 0.001 |
| Acute respiratory failure | 293 (2%) | 9 (2%) | 0.400 |
| MSSA septicemia | 36 (0.2%) | 8 (2%) | 0.001 |
| MRSA septicemia | 13 (0.1%) | 7 (2%) | 0.001 |
| Alcoholic cirrhosis of the liver | 111 (1%) | 7 (2%) | 0.007 |
| Palliative care consult | 398 (2%) | 64 (15%) | 0.001 |
| ICU stay | 2,030 (11%) | 246 (59%) | 0.001 |
| Surgical procedure | 1,800 (10%) | 182 (44%) | 0.001 |
Unspecified sepsis was the most frequent primary diagnosis, regardless of LOS category (Table 2). However, the second through fifth most frequent diagnoses differed for patients with and without prolonged LOS.
| N | % | |
|---|---|---|
| ||
| LOS 21 days | ||
| 1. Sepsis, unspecified | 1,548 | 8.6% |
| 2. Acute pancreatitis | 435 | 2.4% |
| 3. Pneumonia | 431 | 2.4% |
| 4. Acute kidney failure | 363 | 2.0% |
| 5. COPD exacerbation | 320 | 1.8% |
| LOS >21 days | ||
| 1. Sepsis, unspecified | 55 | 13.0% |
| 2. Acute respiratory failure | 9 | 2.2% |
| 3. Methicillin‐sensitive Staphylococcus aureus septicemia | 8 | 1.9% |
| 4. Methicillin‐resistant Staphylococcus aureus septicemia | 7 | 1.7% |
| 5. Alcoholic cirrhosis of the liver | 7 | 1.7% |
In an adjusted logistic regression model (Table 3), we found lower odds of prolonged LOS for each 10‐year increase in age and higher odds of prolonged LOS for Medicaid insurance, discharge to home with home health, discharge to a postacute‐care or long‐term acute‐care facility, and in‐hospital death. Methicillin‐resistant Staphylococcus aureus (MRSA) septicemia, palliative care consults, ICU stays, and surgeries were all also associated with increased odds of prolonged LOS. We identified a statistically significant interaction between ICU stay and surgical procedure (odds ratio: 2.53, 95% confidence interval: 1.51‐4.26, P0.001).
| Outcome: LOS >21 Days | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| |||
| Age, per 10 years increase in age | 0.80 | 0.73‐0.87 | 0.001 |
| Year of discharge | |||
| 2012 | 0.47 | 0.34‐0.67 | 0.001 |
| 2013 | 1.10 | 0.84‐1.43 | 0.493 |
| 2014 | Ref | ||
| Race/ethnicity | |||
| White non‐Hispanic | Ref | ||
| Black non‐Hispanic | 0.89 | 0.64‐1.22 | 0.454 |
| Hispanic | 1.01 | 0.70‐1.46 | 0.952 |
| Asian | 0.85 | 0.40‐1.83 | 0.679 |
| Other or unknown | 1.29 | 0.73‐2.26 | 0.378 |
| Insurance | |||
| Medicare | Ref | ||
| Medicaid | 1.99 | 1.29‐3.05 | 0.002 |
| Dual | 1.06 | 0.72‐1.57 | 0.765 |
| Private | 1.13 | 0.70‐1.82 | 0.620 |
| Indigent/self‐pay | 1.66 | 0.95‐2.88 | 0.073 |
| Other | 0.96 | 0.47‐1.96 | 0.908 |
| Discharge disposition | |||
| Home with self‐care | Ref | ||
| Home with home health | 4.48 | 3.10‐6.48 | 0.001 |
| Hospicehome or inpatient | 2.11 | 0.98‐4.55 | 0.057 |
| Postacute‐care facility or LTAC | 10.37 | 6.92‐15.56 | 0.001 |
| Expired | 5.38 | 2.27‐12.75 | 0.001 |
| Other | 4.04 | 2.64‐6.18 | 0.001 |
| No. of admission medications | 1.00 | 0.99‐1.02 | 0.775 |
| Primary diagnosis by ICD‐9 code | |||
| Sepsis, unspecified | 1.11 | 0.78‐1.58 | 0.575 |
| MSSA septicemia | 2.44 | 0.68‐8.67 | 0.074 |
| MRSA septicemia | 8.83 | 1.72‐45.36 | 0.009 |
| Alcoholic cirrhosis of the liver | 1.25 | 0.43‐3.65 | 0.687 |
| Palliative care consult | 4.63 | 2.86‐7.49 | 0.001 |
| ICU stay | 6.66 | 5.22‐8.50 | 0.001 |
| Surgical procedure | 5.04 | 3.90‐6.52 | 0.001 |
Sensitivity analyses using LOS >14 and >30 days yielded similar results to LOS >21 days (see Supporting Appendix Table 1 in the online version of this article).
DISCUSSION
We found that a small proportion of medicine patients with prolonged hospitalizations contributed substantially to both total inpatient days and average inpatient LOS. Such disproportionate healthcare utilization is concerning in light of the Institute of Medicine's charge for health systems to deliver timely, efficient, and equitable care.[11]
Few studies in the United States have analyzed patient characteristics that predict prolonged LOS, and to our knowledge, none have evaluated prolonged LOS specifically in general medicine patients.[12, 13, 14] Among selected surgical populations, prolonged hospitalizations are most often related to placement difficulties, operational delays, and payer‐related issues, rather than severity of illness, baseline comorbidities, or in‐hospital complications.[12, 13] In our study, we found that patients with prolonged LOS were more likely to require a palliative care consult, ICU stay, or surgery, all proxies for disease severity. Patients with prolonged LOS were also more likely to undergo surgery >24 hours after admission than those without prolonged LOS, suggesting that the former were either too unstable to proceed directly to surgery or developed complications later during their hospitalization. Even after controlling for palliative care consults, ICU stays, and surgeries, placement at a postacute‐care facility was strongly associated with prolonged LOS. Patients with prolonged LOS were also more likely to have Medicaid compared to other insurance types.
Our findings have several potential implications for efforts aimed at decreasing the number of and length of prolonged hospitalizations. Although demographic and clinical factors such as Medicaid insurance, ICU stays, and surgeries are generally not modifiable, they could, particularly in combination, be used to trigger earlier and more intensive case management involvement. A streamlined insurance approval process for Medicaid pending inpatients could be beneficial, given the recent expansion of Medicaid eligibility under the ACA. Hospital partnerships with postacute‐care facilities could also relieve bottlenecks in placement.[8] Chart review of the patients with MRSA septicemia and prolonged LOS indicated that development of an intensive outpatient parenteral antimicrobial therapy pathway with substance abuse counseling could provide an alternative to extended inpatient treatment for intravenous drug users with complicated infections.[15]
This study has several limitations. First, given the lack of consensus in the literature regarding the definition of a prolonged hospitalization, it is difficult to directly compare our results with existing studies.[8, 12, 13, 14] However, we believe LOS >21 days to be a meaningful cutoff for our cohort. Most demographic and clinical variables that were predictive at >21 days were also predictive at >14 and >30 days, which reassured us that the relationship between variables and prolonged LOS was stable at different thresholds. Second, our database did not allow us to fully adjust for baseline comorbidities or categorize the reasons for discharge delays. Finally, this was a single‐center program evaluation. Although this limits generalization to other institutions, we believe our approach may serve as a guide for others interested in reducing prolonged hospitalizations.
In summary, prolonged hospitalizations represent a potentially high‐yield target for LOS reduction efforts. Prolonged hospitalizations among medicine patients at our institution particularly affected Medicaid enrollees with complex hospital stays who were not discharged home. Further studies are needed to determine the specific reasons for unnecessary hospital days in this population.
Acknowledgements
The authors thank Essey Yirdaw for her contributions to the building and management of the database that informed this work.
Disclosure: M.E.A. conceived of the study concept and design and drafted the manuscript. J.J.G. assisted with the study design and made critical revisions to the final manuscript. D.A., R.P., and R.C. assisted with the study design and made critical revisions to the manuscript. C.D.J. assisted with the study design, performed data analyses, and made critical revisions to the manuscript. RC has disclosed that her time is funded by a National Institutes of Health grant unrelated to this study. A modified abstract was presented in poster format at the Society of Hospital Medicine Research, Innovations, and Vignettes Competition 2015 Annual Meeting, held March 29 April 1, 2015, in National Harbor, Maryland. The authors have no conflicts of interest to disclose.
Hospitalizations frequently last longer than warranted by medical necessity alone, due to inefficiencies within the US healthcare system.[1, 2] Discharge delays place patients at risk for hospital‐acquired complications and increase costs. With the growing emphasis on high‐value care, hospital length of stay (LOS) has emerged as a key metric for inpatient care and will remain a central focus of hospital‐based improvement initiatives for the foreseeable future.
Hospitals may find it difficult to identify the primary drivers of inpatient LOS in a dynamic and increasingly complex healthcare system. Multiple recent policy changes have affected inpatient care. The Health Information Technology for Economic and Clinical Health Act of 2009 has led to widespread adoption of electronic health records (EHRs) that have markedly impacted provider workflows.[3] In October 2013, the Centers for Medicare & Medicaid Services implemented the 2‐midnight rule, which reclassified lower acuity inpatients with an expected stay 48 hours to observation status.[4] In January 2014, expansion of insurance coverage under the Affordable Care Act (ACA) altered payer mix for hospitals nationwide.[5] At a local level, hospitals that are rapidly adjusting resource allocation, capital investments, and marketing efforts and making complex operational decisions (eg, to open new units or change admission or referral algorithms) may simultaneously experience shifts in patient volumes, case‐mix index, and staffing ratios with downstream effects on LOS.
Given the myriad factors influencing inpatient LOS, hospital leaders may encounter real challenges in designing effective LOS reduction strategies. For example, they may expend significant resources on real‐time demand‐capacity management systems to improve hospital‐wide patient flow, but the resultant emphasis on bed placement and early discharges may shave only hours off average LOS.[6, 7] An alternative approach may be to target the small percentage of patients with prolonged hospitalizations who contribute disproportionately to the average LOS, as other initiatives focused on high utilizers have done.[8, 9, 10]
Our institution noted an increase in the average inpatient LOS for general medicine patients from 2012 to 2014, prompting a call to action by hospital leaders. We sought to characterize the predictors of prolonged hospitalizations among medicine patients to guide future efforts aimed at mitigating the contribution of prolonged LOS to overall LOS.
METHODS
Study Design
We performed a retrospective analysis of medicine patients discharged between January 1, 2012 and December 31, 2014, from the University of Colorado Hospital, a 551‐bed urban, quaternary‐care academic medical center in Aurora, Colorado. Patients were included if they were admitted under inpatient status, 18 years of age, and discharged from 1 of our 10 medicine services: 7 services with residents, staffed by hospitalists, general internists, or subspecialists; and 3 services with advanced practice providers, staffed by hospitalists.
Data Collection
We obtained LOS, calendar year of discharge, demographic data, insurance type, discharge disposition, number of medications, consults, intensive care unit (ICU) stays, surgeries (ie, procedures requiring anesthesia), and primary diagnosis by International Classification of Diseases, Ninth Revision codes from an administrative database that had been developed, validated, and maintained by our hospital medicine group. This database was populated with variables from our EHR, which was implemented in September 2011; to minimize variability in data input during the EHR rollout, we excluded data from September 2011 through December 2011. The Colorado Multiple Institutional Review Board reviewed and exempted this database (protocol 13‐2953) as a program evaluation.
Outcomes
We defined a prolonged hospitalization or LOS as >21 days in duration. This represented approximately 2 standard deviations above the mean LOS in our cohort. This cutoff also helped to remove provider‐level variability, as each medicine service was staffed by 2 attendings per month, each working approximately 7 days on and 7 days off. We examined LOS >14 and >30 days in sensitivity analyses to ensure that the selection of >21 days did not impose an arbitrary and invalid limitation on our statistical analysis.
Statistical Analysis
Demographic and clinical data were compared in the group with LOS 21 days versus the group with LOS >21 days with a 2 test for dichotomous variables and Student t test for continuous variables. We then built a multivariable logistic regression model to predict LOS >21 days using the variables that were significantly different between groups in bivariate analyses. A two‐sided P value of 0.05 was considered statistically significant. All data analyses were performed using Stata 12.0 (StataCorp, College Station, TX).
RESULTS
We identified 18,363 inpatient discharges among 12,511 medicine patients between January 1, 2012 and December 31, 2014. Of these discharges, 416 (2.3%) demonstrated prolonged LOS. Prolonged hospitalizations accounted for 18.6% of total inpatient days. The average LOS during the study period was 4.8 days including patients with prolonged LOS and 4.0 days excluding patients with prolonged LOS, a contribution of 0.8 days.
Table 1 compares the characteristics of patients with and without prolonged LOS. Age, insurance, discharge disposition, palliative care consults, ICU stays, and surgeries were among the variables that differed significantly between the 2 groups. Among patients undergoing surgery, those with prolonged LOS were more likely to have surgery >24 hours after admission than those without prolonged LOS (85.7% vs 51.4%, P0.001).
| Variable | LOS 21 Days, N=17,947 | LOS >21 Days, N=416 | P Value |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 56.4 (18.7) | 54.4 (17.1) | 0.030 |
| Female | 9,256 (52%) | 199 (48%) | 0.132 |
| Year of discharge | 0.001 | ||
| 2012 | 5,486 (31%) | 69 (17%) | |
| 2013 | 6,193 (35%) | 162 (39%) | |
| 2014 | 6,268 (35%) | 185 (44%) | |
| Race/ethnicity | 0.003 | ||
| White non‐Hispanic | 9,702 (54%) | 242 (58%) | |
| Black non‐Hispanic | 4,000 (22%) | 68 (16%) | |
| Hispanic | 2,872(16%) | 67 (16%) | |
| Asian | 578 (3%) | 9 (2%) | |
| Other or unknown | 795 (4%) | 30 (7%) | |
| Language preference | 0.795 | ||
| English | 16,049 (89%) | 376 (90%) | |
| Spanish | 1,052 (6%) | 23 (6%) | |
| Other | 846 (5%) | 17 (4%) | |
| Insurance | 0.001 | ||
| Medicare | 5,462 (30%) | 109 (26%) | |
| Medicaid | 3,406 (19%) | 126 (30%) | |
| Dual | 2,815 (16%) | 64 (15%) | |
| Private | 2,714 (15%) | 60 (14%) | |
| Indigent/self‐pay | 2,829 (16%) | 42 (10%) | |
| Other | 721 (4%) | 15 (4%) | |
| Length of stay, d (SD) | 4.0 (3.5) | 39.5 (37.3) | 0.001 |
| Discharge disposition | 0.001 | ||
| Home with self‐care | 13,276 (74%) | 115 (28%) | |
| Home with home health | 1,584 (9%) | 79 (19%) | |
| Hospicehome or inpatient | 369 (2%) | 19 (5%) | |
| Postacute‐care facility or LTAC | 1,761 (10%) | 141 (34%) | |
| Expired | 113 (1%) | 18 (4%) | |
| Other | 844 (5%) | 44 (11%) | |
| No. of admission medications (SD) | 9.7 (7.4) | 10.9 (7.8) | 0.002 |
| Primary diagnosis by ICD‐9 code* | |||
| Sepsis, unspecified | 1,548 (9%) | 55 (13%) | 0.001 |
| Acute respiratory failure | 293 (2%) | 9 (2%) | 0.400 |
| MSSA septicemia | 36 (0.2%) | 8 (2%) | 0.001 |
| MRSA septicemia | 13 (0.1%) | 7 (2%) | 0.001 |
| Alcoholic cirrhosis of the liver | 111 (1%) | 7 (2%) | 0.007 |
| Palliative care consult | 398 (2%) | 64 (15%) | 0.001 |
| ICU stay | 2,030 (11%) | 246 (59%) | 0.001 |
| Surgical procedure | 1,800 (10%) | 182 (44%) | 0.001 |
Unspecified sepsis was the most frequent primary diagnosis, regardless of LOS category (Table 2). However, the second through fifth most frequent diagnoses differed for patients with and without prolonged LOS.
| N | % | |
|---|---|---|
| ||
| LOS 21 days | ||
| 1. Sepsis, unspecified | 1,548 | 8.6% |
| 2. Acute pancreatitis | 435 | 2.4% |
| 3. Pneumonia | 431 | 2.4% |
| 4. Acute kidney failure | 363 | 2.0% |
| 5. COPD exacerbation | 320 | 1.8% |
| LOS >21 days | ||
| 1. Sepsis, unspecified | 55 | 13.0% |
| 2. Acute respiratory failure | 9 | 2.2% |
| 3. Methicillin‐sensitive Staphylococcus aureus septicemia | 8 | 1.9% |
| 4. Methicillin‐resistant Staphylococcus aureus septicemia | 7 | 1.7% |
| 5. Alcoholic cirrhosis of the liver | 7 | 1.7% |
In an adjusted logistic regression model (Table 3), we found lower odds of prolonged LOS for each 10‐year increase in age and higher odds of prolonged LOS for Medicaid insurance, discharge to home with home health, discharge to a postacute‐care or long‐term acute‐care facility, and in‐hospital death. Methicillin‐resistant Staphylococcus aureus (MRSA) septicemia, palliative care consults, ICU stays, and surgeries were all also associated with increased odds of prolonged LOS. We identified a statistically significant interaction between ICU stay and surgical procedure (odds ratio: 2.53, 95% confidence interval: 1.51‐4.26, P0.001).
| Outcome: LOS >21 Days | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| |||
| Age, per 10 years increase in age | 0.80 | 0.73‐0.87 | 0.001 |
| Year of discharge | |||
| 2012 | 0.47 | 0.34‐0.67 | 0.001 |
| 2013 | 1.10 | 0.84‐1.43 | 0.493 |
| 2014 | Ref | ||
| Race/ethnicity | |||
| White non‐Hispanic | Ref | ||
| Black non‐Hispanic | 0.89 | 0.64‐1.22 | 0.454 |
| Hispanic | 1.01 | 0.70‐1.46 | 0.952 |
| Asian | 0.85 | 0.40‐1.83 | 0.679 |
| Other or unknown | 1.29 | 0.73‐2.26 | 0.378 |
| Insurance | |||
| Medicare | Ref | ||
| Medicaid | 1.99 | 1.29‐3.05 | 0.002 |
| Dual | 1.06 | 0.72‐1.57 | 0.765 |
| Private | 1.13 | 0.70‐1.82 | 0.620 |
| Indigent/self‐pay | 1.66 | 0.95‐2.88 | 0.073 |
| Other | 0.96 | 0.47‐1.96 | 0.908 |
| Discharge disposition | |||
| Home with self‐care | Ref | ||
| Home with home health | 4.48 | 3.10‐6.48 | 0.001 |
| Hospicehome or inpatient | 2.11 | 0.98‐4.55 | 0.057 |
| Postacute‐care facility or LTAC | 10.37 | 6.92‐15.56 | 0.001 |
| Expired | 5.38 | 2.27‐12.75 | 0.001 |
| Other | 4.04 | 2.64‐6.18 | 0.001 |
| No. of admission medications | 1.00 | 0.99‐1.02 | 0.775 |
| Primary diagnosis by ICD‐9 code | |||
| Sepsis, unspecified | 1.11 | 0.78‐1.58 | 0.575 |
| MSSA septicemia | 2.44 | 0.68‐8.67 | 0.074 |
| MRSA septicemia | 8.83 | 1.72‐45.36 | 0.009 |
| Alcoholic cirrhosis of the liver | 1.25 | 0.43‐3.65 | 0.687 |
| Palliative care consult | 4.63 | 2.86‐7.49 | 0.001 |
| ICU stay | 6.66 | 5.22‐8.50 | 0.001 |
| Surgical procedure | 5.04 | 3.90‐6.52 | 0.001 |
Sensitivity analyses using LOS >14 and >30 days yielded similar results to LOS >21 days (see Supporting Appendix Table 1 in the online version of this article).
DISCUSSION
We found that a small proportion of medicine patients with prolonged hospitalizations contributed substantially to both total inpatient days and average inpatient LOS. Such disproportionate healthcare utilization is concerning in light of the Institute of Medicine's charge for health systems to deliver timely, efficient, and equitable care.[11]
Few studies in the United States have analyzed patient characteristics that predict prolonged LOS, and to our knowledge, none have evaluated prolonged LOS specifically in general medicine patients.[12, 13, 14] Among selected surgical populations, prolonged hospitalizations are most often related to placement difficulties, operational delays, and payer‐related issues, rather than severity of illness, baseline comorbidities, or in‐hospital complications.[12, 13] In our study, we found that patients with prolonged LOS were more likely to require a palliative care consult, ICU stay, or surgery, all proxies for disease severity. Patients with prolonged LOS were also more likely to undergo surgery >24 hours after admission than those without prolonged LOS, suggesting that the former were either too unstable to proceed directly to surgery or developed complications later during their hospitalization. Even after controlling for palliative care consults, ICU stays, and surgeries, placement at a postacute‐care facility was strongly associated with prolonged LOS. Patients with prolonged LOS were also more likely to have Medicaid compared to other insurance types.
Our findings have several potential implications for efforts aimed at decreasing the number of and length of prolonged hospitalizations. Although demographic and clinical factors such as Medicaid insurance, ICU stays, and surgeries are generally not modifiable, they could, particularly in combination, be used to trigger earlier and more intensive case management involvement. A streamlined insurance approval process for Medicaid pending inpatients could be beneficial, given the recent expansion of Medicaid eligibility under the ACA. Hospital partnerships with postacute‐care facilities could also relieve bottlenecks in placement.[8] Chart review of the patients with MRSA septicemia and prolonged LOS indicated that development of an intensive outpatient parenteral antimicrobial therapy pathway with substance abuse counseling could provide an alternative to extended inpatient treatment for intravenous drug users with complicated infections.[15]
This study has several limitations. First, given the lack of consensus in the literature regarding the definition of a prolonged hospitalization, it is difficult to directly compare our results with existing studies.[8, 12, 13, 14] However, we believe LOS >21 days to be a meaningful cutoff for our cohort. Most demographic and clinical variables that were predictive at >21 days were also predictive at >14 and >30 days, which reassured us that the relationship between variables and prolonged LOS was stable at different thresholds. Second, our database did not allow us to fully adjust for baseline comorbidities or categorize the reasons for discharge delays. Finally, this was a single‐center program evaluation. Although this limits generalization to other institutions, we believe our approach may serve as a guide for others interested in reducing prolonged hospitalizations.
In summary, prolonged hospitalizations represent a potentially high‐yield target for LOS reduction efforts. Prolonged hospitalizations among medicine patients at our institution particularly affected Medicaid enrollees with complex hospital stays who were not discharged home. Further studies are needed to determine the specific reasons for unnecessary hospital days in this population.
Acknowledgements
The authors thank Essey Yirdaw for her contributions to the building and management of the database that informed this work.
Disclosure: M.E.A. conceived of the study concept and design and drafted the manuscript. J.J.G. assisted with the study design and made critical revisions to the final manuscript. D.A., R.P., and R.C. assisted with the study design and made critical revisions to the manuscript. C.D.J. assisted with the study design, performed data analyses, and made critical revisions to the manuscript. RC has disclosed that her time is funded by a National Institutes of Health grant unrelated to this study. A modified abstract was presented in poster format at the Society of Hospital Medicine Research, Innovations, and Vignettes Competition 2015 Annual Meeting, held March 29 April 1, 2015, in National Harbor, Maryland. The authors have no conflicts of interest to disclose.
- , , , et al. Excess hospitalization days in an academic medical center: perceptions of hospitalists and discharge planners. Am J Manag Care. 2011;17(2):e34–e42.
- , , A prospective study of reasons for prolonged hospitalizations on a general medicine teaching service. J Gen Intern Med. 2005;20(2):108–115.
- , , , et al. Adoption of electronic health records grows rapidly, but fewer than half of U.S. hospitals had at least a basic system in 2012. Health Aff (Millwood). 2013;32(8):1478–1485.
- , , , et al. Observation and inpatient status: clinical impact of the 2‐midnight rule. J Hosp Med. 2014;9(4):203–209.
- , , Impact of insurance expansion on hospital uncompensated care costs in 2014. Department of Health and Human Services Office of the Assistant Secretary for Planning and Evaluation. Available at: http://aspe.hhs.gov/health/reports/2014/uncompensatedcare/ib_uncompensatedcare.pdf. Accessed March 28, 2015.
- , , , Using real‐time demand capacity management to improve hospital‐wide patient flow. Jt Comm J Qual Patient Saf. 2011;37(5):217–227.
- , , , , , Natural history of late discharges from a general medical ward. J Hosp Med. 2009;4(4):226–233.
- , , , Addressing hospital length of stay outlier patients: a community‐wide approach. Adv Biosci Biotechol. 2014;5:188–196.
- , American medical home runs. Health Aff (Millwood). 2009;28(5):1317–1326.
- The hot spotters: can we lower medical costs by giving the neediest patients better care? New Yorker. January 2011:40–51.
- Institute of Medicine. Crossing the Quality Chasm. Washington, DC: National Academies Press; 2001.
- , , , et al. Excessively long hospital stays after trauma are not related to the severity of illness: let's aim to the right target! JAMA Surg. 2013;148(1):956–961.
- , , Extended length of stay after surgery: complications, inefficient practice, or sick patients? JAMA Surg. 2014;149(8):815–820.
- , , , , Nonmedical factors associated with prolonged hospital length of stay in an urban homebound population. J Hosp Med. 2012;7(2):73–78.
- , , , Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65(12):2641–2644.
- , , , et al. Excess hospitalization days in an academic medical center: perceptions of hospitalists and discharge planners. Am J Manag Care. 2011;17(2):e34–e42.
- , , A prospective study of reasons for prolonged hospitalizations on a general medicine teaching service. J Gen Intern Med. 2005;20(2):108–115.
- , , , et al. Adoption of electronic health records grows rapidly, but fewer than half of U.S. hospitals had at least a basic system in 2012. Health Aff (Millwood). 2013;32(8):1478–1485.
- , , , et al. Observation and inpatient status: clinical impact of the 2‐midnight rule. J Hosp Med. 2014;9(4):203–209.
- , , Impact of insurance expansion on hospital uncompensated care costs in 2014. Department of Health and Human Services Office of the Assistant Secretary for Planning and Evaluation. Available at: http://aspe.hhs.gov/health/reports/2014/uncompensatedcare/ib_uncompensatedcare.pdf. Accessed March 28, 2015.
- , , , Using real‐time demand capacity management to improve hospital‐wide patient flow. Jt Comm J Qual Patient Saf. 2011;37(5):217–227.
- , , , , , Natural history of late discharges from a general medical ward. J Hosp Med. 2009;4(4):226–233.
- , , , Addressing hospital length of stay outlier patients: a community‐wide approach. Adv Biosci Biotechol. 2014;5:188–196.
- , American medical home runs. Health Aff (Millwood). 2009;28(5):1317–1326.
- The hot spotters: can we lower medical costs by giving the neediest patients better care? New Yorker. January 2011:40–51.
- Institute of Medicine. Crossing the Quality Chasm. Washington, DC: National Academies Press; 2001.
- , , , et al. Excessively long hospital stays after trauma are not related to the severity of illness: let's aim to the right target! JAMA Surg. 2013;148(1):956–961.
- , , Extended length of stay after surgery: complications, inefficient practice, or sick patients? JAMA Surg. 2014;149(8):815–820.
- , , , , Nonmedical factors associated with prolonged hospital length of stay in an urban homebound population. J Hosp Med. 2012;7(2):73–78.
- , , , Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65(12):2641–2644.