User login
Point of Care Ultrasound (POCUS) for Small Bowel Obstruction in the ED
Small bowel obstruction (SBO) accounts for 2% of all cases of abdominal pain presenting to the ED and 15% of abdominal pain admissions to surgical units from the ED.1,2 SBO can be a difficult diagnosis; the most common symptoms include nausea, vomiting, abdominal pain, obstipation, and constipation. The symptomatology depends on multiple factors: the area of the blockage, length of obstruction, and degree of the obstruction (either partial or complete).3 An upper gastrointestinal (GI) blockage classically presents with nausea and vomiting, while a lower GI blockage often presents with abdominal pain, constipation, and obstipation. Complications of obstruction range from significant morbidity—such as bowel strangulation (23%) and sepsis (31%)—to mortality (9%).4 ED POCUS allows for rapid and accurate diagnosis of SBO.
CASE
A 60-year-old female with a past medical history of peptic ulcer disease and multiple abdominal surgeries, including umbilical hernia repair, appendectomy, and total abdominal hysterectomy, presented to the ED with an 8-hour history of nausea and vomiting. She reported that her abdomen felt bloated. She had experienced non-bloody, watery stools for the prior 3 weeks. She also reported three to four weeks of epigastric abdominal pain similar to her previous “ulcer pain.” Of note, she was evaluated in GI clinic one day prior to her ED visit for dysphagia, abdominal distention, and diarrhea and was scheduled for an outpatient upper endoscopy. Initial vitals were significant for a heart rate of 100 beats/min. Physical exam was significant for a mildly distended abdomen, tender to palpation at epigastrium without rebound or guarding. Labs showed a white blood cell count of 11.8 K/uL and otherwise unremarkable complete blood count, basic metabolic panel, liver function tests, and lactate measurement. Given the patient’s history of multiple abdominal surgeries and clinical presentation, POCUS was performed to evaluate for SBO. Dilated loops of small bowel were visualized in the lower abdomen gas, suggestive of SBO.
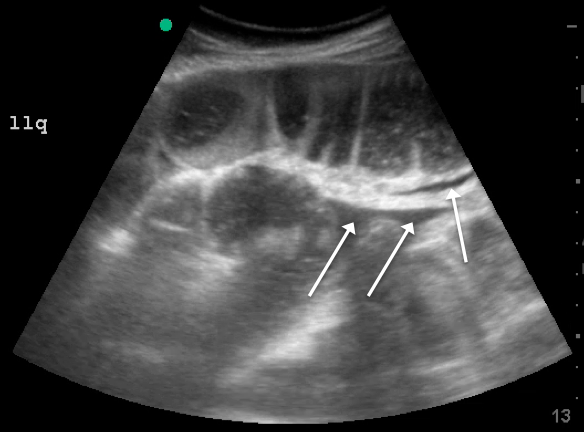
Since the small bowel encompasses a large portion of the abdomen, to fully evaluate for SBO, multiple views are necessary. These include the epigastrium, bilateral colic gutters, and suprapubic regions.5 Use the low-frequency curvilinear transducer to obtain these views, scanning in the transverse and sagittal planes (see Figures 1 and 2). Scan while moving the transducer in columns (ie, “mowing the lawn”), making sure to cover the entire abdomen. To assure that you are evaluating the small bowel, and not the large bowel, look for the characteristic plicae circularis of the small bowel (shown in Figure 3). In children and very slender adults, the high-frequency linear probe may provide enough depth to obtain adequate views.
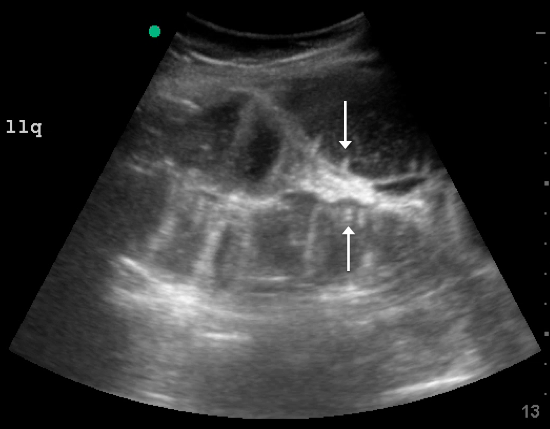
A fluid-filled small intestinal segment >2.5 cm is consistent with a diagnosis of SBO. Measuring the diameter of the small bowel is both the most sensitive and specific sign; a measurement of greater than 2.5 cm is diagnostic, with a sensitivity of 97% and specificity of 91% (see Figure 4).6 This can be somewhat difficult to visualize, as bowel loops are multidirectional and diameters can mistakenly be taken on an indirect cut; to avoid over- or underestimation of bowel diameter, you may want to measure in the short axis using a transverse cross-sectional view of the bowel.
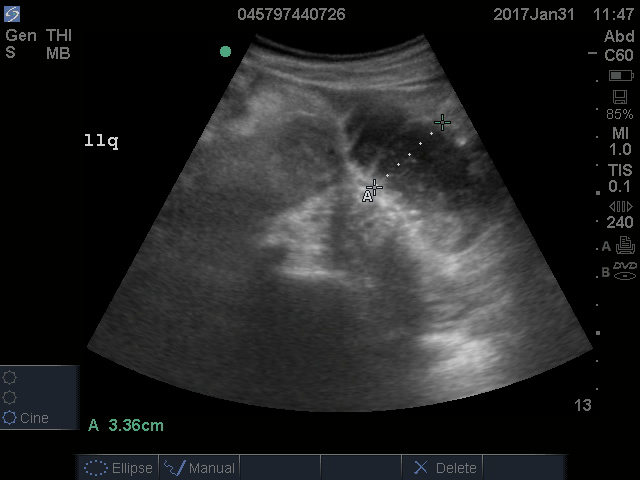
Lack of peristalsis is suggestive of a closed-loop obstruction. However, this finding may be more difficult to visualize, as it requires several continuous minutes of scanning or repeated exams to truly establish absent peristalsis. In prolonged courses of SBO, the bowel wall can measure >3 mm, which suggests necrosis, warranting accelerated surgical intervention. In addition, the detection of extraluminal peritoneal fluid can help determine the severity of the SBO, and small versus large fluid amounts can help determine whether medical or surgical management is warranted (see Figure 5).7
DISCUSSION
Increased time to diagnosis of SBO can lead to prolonged patient suffering and greater complication rates. The gold standard for diagnosing SBO—CT with intravenous and oral contrast—can take hours, requiring patients, who are often nauseated, to ingest and tolerate oral contrast. In the past, an “obstructive series” of x-rays would have been used early in the work-up of possible SBO.6
Recent literature suggests that POCUS is not only faster, more cost effective, and advantageous (involving no ionizing radiation), but also more accurate than x-rays. Specifically, a meta-analysis by Taylor et al showed pooled estimates for obstructive series x-rays have a sensitivity (Sn) of 75%, a specificity (Sp) of 66%, a positive likelihood ratio (+LR) of 1.6, and a negative likelihood ratio (-LR) of 0.43.1 On the other hand, pooled results from ED studies of emergency medicine (EM) residents performing POCUS in patients with signs and symptoms suspicious for SBO showed POCUS had a Sn of 97%, Sp of 90%, +LR of 9.5, and a -LR of 0.04.1,5,8 While detractors point to the operator-dependent nature of POCUS, literature suggests that with EM residents novice to POCUS for SBO (defined as less than 5 previous scans for SBO) were given a 10-minute didactic session and yielded Sn 94%, Sp 81%, +LR 5.0, -LR 0.07.5 Unluer et al trained novice EM residents for 6 hours and found them to yield Sn 98%, Sp 95%, +LR 19.5, and -LR 0.02.8 Thus, while it is no surprise that those with more training attain better results, both studies show it does not take much time for EM providers to surpass the accuracy of x-rays with POCUS.
CASE CONCLUSION
The findings on POCUS highly suggested the diagnosis of an SBO. A CT scan of the abdomen and pelvis with intravenous and oral contrast was ordered to further evaluate obstruction, transition point, and possible complications, including signs of ischemia per surgical request. CT demonstrated dilated loops of small bowel with transition point in the right lower quadrant, with a small amount of mesenteric fluid consistent with SBO with possible early bowel compromise due to ischemia. General surgery admitted the patient; conservative treatment with serial abdominal exams, nasogastric tube, NPO and bowel rest was ordered. The patient’s diet was gradually advanced, and she was discharged on the eleventh day of hospitalization.
SUMMARY
POCUS is a useful non-invasive tool that can accurately diagnose SBO. POCUS has increased sensitivity and specificity when compared to abdominal X-rays. This bedside imaging will not only give the ED provider rapid diagnostic information but also lead to expedited surgical intervention.
- Taylor MR, Lalani N. Adult small bowel obstruction. Acad Emerg Med. 2013;20(6):528-544.
- Hastings RS, Powers RD. Abdominal pain in the ED: a 35-year retrospective. Am J Emerg Med.2011;29:711-716.
- Markogiannakis H, Messaris E, Dardamanis D, et al. Acute mechanical bowel obstruction: clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007;13:432.
- Bickell N, Federman A, Aufses A. Influence of time on risk of bowel resection in complete small bowel obstruction. J Am Coll Surg. 2005;201(6):847-854.
- Jang TB, Chandler D, Kaji AH. Bedside ultrasonography for the detection of small bowel obstruction in the emergency department. Emerg Med J. 2011;28:676-678.
- Carpenter CR, Pines JM. The end of X-rays for suspected small bowel obstruction? Using evidence-based diagnostics to inform best practices in emergency medicine. Acad Emerg Med. 2013;20:618-20.
- Grassi R, Romano S, D’Amario F, et al. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur J Radiol. 2004;50(1):5-14.
- Unlüer E, Yavaşi O, Eroğlu O, Yilmaz C, Akarca F. Ultrasonography by emergency medicine and radiology residents for the diagnosis of small bowel obstruction. Eur J Emerg Med. 2010;17(5):260-264.
Small bowel obstruction (SBO) accounts for 2% of all cases of abdominal pain presenting to the ED and 15% of abdominal pain admissions to surgical units from the ED.1,2 SBO can be a difficult diagnosis; the most common symptoms include nausea, vomiting, abdominal pain, obstipation, and constipation. The symptomatology depends on multiple factors: the area of the blockage, length of obstruction, and degree of the obstruction (either partial or complete).3 An upper gastrointestinal (GI) blockage classically presents with nausea and vomiting, while a lower GI blockage often presents with abdominal pain, constipation, and obstipation. Complications of obstruction range from significant morbidity—such as bowel strangulation (23%) and sepsis (31%)—to mortality (9%).4 ED POCUS allows for rapid and accurate diagnosis of SBO.
CASE
A 60-year-old female with a past medical history of peptic ulcer disease and multiple abdominal surgeries, including umbilical hernia repair, appendectomy, and total abdominal hysterectomy, presented to the ED with an 8-hour history of nausea and vomiting. She reported that her abdomen felt bloated. She had experienced non-bloody, watery stools for the prior 3 weeks. She also reported three to four weeks of epigastric abdominal pain similar to her previous “ulcer pain.” Of note, she was evaluated in GI clinic one day prior to her ED visit for dysphagia, abdominal distention, and diarrhea and was scheduled for an outpatient upper endoscopy. Initial vitals were significant for a heart rate of 100 beats/min. Physical exam was significant for a mildly distended abdomen, tender to palpation at epigastrium without rebound or guarding. Labs showed a white blood cell count of 11.8 K/uL and otherwise unremarkable complete blood count, basic metabolic panel, liver function tests, and lactate measurement. Given the patient’s history of multiple abdominal surgeries and clinical presentation, POCUS was performed to evaluate for SBO. Dilated loops of small bowel were visualized in the lower abdomen gas, suggestive of SBO.
Since the small bowel encompasses a large portion of the abdomen, to fully evaluate for SBO, multiple views are necessary. These include the epigastrium, bilateral colic gutters, and suprapubic regions.5 Use the low-frequency curvilinear transducer to obtain these views, scanning in the transverse and sagittal planes (see Figures 1 and 2). Scan while moving the transducer in columns (ie, “mowing the lawn”), making sure to cover the entire abdomen. To assure that you are evaluating the small bowel, and not the large bowel, look for the characteristic plicae circularis of the small bowel (shown in Figure 3). In children and very slender adults, the high-frequency linear probe may provide enough depth to obtain adequate views.


A fluid-filled small intestinal segment >2.5 cm is consistent with a diagnosis of SBO. Measuring the diameter of the small bowel is both the most sensitive and specific sign; a measurement of greater than 2.5 cm is diagnostic, with a sensitivity of 97% and specificity of 91% (see Figure 4).6 This can be somewhat difficult to visualize, as bowel loops are multidirectional and diameters can mistakenly be taken on an indirect cut; to avoid over- or underestimation of bowel diameter, you may want to measure in the short axis using a transverse cross-sectional view of the bowel.

Lack of peristalsis is suggestive of a closed-loop obstruction. However, this finding may be more difficult to visualize, as it requires several continuous minutes of scanning or repeated exams to truly establish absent peristalsis. In prolonged courses of SBO, the bowel wall can measure >3 mm, which suggests necrosis, warranting accelerated surgical intervention. In addition, the detection of extraluminal peritoneal fluid can help determine the severity of the SBO, and small versus large fluid amounts can help determine whether medical or surgical management is warranted (see Figure 5).7

DISCUSSION
Increased time to diagnosis of SBO can lead to prolonged patient suffering and greater complication rates. The gold standard for diagnosing SBO—CT with intravenous and oral contrast—can take hours, requiring patients, who are often nauseated, to ingest and tolerate oral contrast. In the past, an “obstructive series” of x-rays would have been used early in the work-up of possible SBO.6
Recent literature suggests that POCUS is not only faster, more cost effective, and advantageous (involving no ionizing radiation), but also more accurate than x-rays. Specifically, a meta-analysis by Taylor et al showed pooled estimates for obstructive series x-rays have a sensitivity (Sn) of 75%, a specificity (Sp) of 66%, a positive likelihood ratio (+LR) of 1.6, and a negative likelihood ratio (-LR) of 0.43.1 On the other hand, pooled results from ED studies of emergency medicine (EM) residents performing POCUS in patients with signs and symptoms suspicious for SBO showed POCUS had a Sn of 97%, Sp of 90%, +LR of 9.5, and a -LR of 0.04.1,5,8 While detractors point to the operator-dependent nature of POCUS, literature suggests that with EM residents novice to POCUS for SBO (defined as less than 5 previous scans for SBO) were given a 10-minute didactic session and yielded Sn 94%, Sp 81%, +LR 5.0, -LR 0.07.5 Unluer et al trained novice EM residents for 6 hours and found them to yield Sn 98%, Sp 95%, +LR 19.5, and -LR 0.02.8 Thus, while it is no surprise that those with more training attain better results, both studies show it does not take much time for EM providers to surpass the accuracy of x-rays with POCUS.
CASE CONCLUSION
The findings on POCUS highly suggested the diagnosis of an SBO. A CT scan of the abdomen and pelvis with intravenous and oral contrast was ordered to further evaluate obstruction, transition point, and possible complications, including signs of ischemia per surgical request. CT demonstrated dilated loops of small bowel with transition point in the right lower quadrant, with a small amount of mesenteric fluid consistent with SBO with possible early bowel compromise due to ischemia. General surgery admitted the patient; conservative treatment with serial abdominal exams, nasogastric tube, NPO and bowel rest was ordered. The patient’s diet was gradually advanced, and she was discharged on the eleventh day of hospitalization.
SUMMARY
POCUS is a useful non-invasive tool that can accurately diagnose SBO. POCUS has increased sensitivity and specificity when compared to abdominal X-rays. This bedside imaging will not only give the ED provider rapid diagnostic information but also lead to expedited surgical intervention.
Small bowel obstruction (SBO) accounts for 2% of all cases of abdominal pain presenting to the ED and 15% of abdominal pain admissions to surgical units from the ED.1,2 SBO can be a difficult diagnosis; the most common symptoms include nausea, vomiting, abdominal pain, obstipation, and constipation. The symptomatology depends on multiple factors: the area of the blockage, length of obstruction, and degree of the obstruction (either partial or complete).3 An upper gastrointestinal (GI) blockage classically presents with nausea and vomiting, while a lower GI blockage often presents with abdominal pain, constipation, and obstipation. Complications of obstruction range from significant morbidity—such as bowel strangulation (23%) and sepsis (31%)—to mortality (9%).4 ED POCUS allows for rapid and accurate diagnosis of SBO.
CASE
A 60-year-old female with a past medical history of peptic ulcer disease and multiple abdominal surgeries, including umbilical hernia repair, appendectomy, and total abdominal hysterectomy, presented to the ED with an 8-hour history of nausea and vomiting. She reported that her abdomen felt bloated. She had experienced non-bloody, watery stools for the prior 3 weeks. She also reported three to four weeks of epigastric abdominal pain similar to her previous “ulcer pain.” Of note, she was evaluated in GI clinic one day prior to her ED visit for dysphagia, abdominal distention, and diarrhea and was scheduled for an outpatient upper endoscopy. Initial vitals were significant for a heart rate of 100 beats/min. Physical exam was significant for a mildly distended abdomen, tender to palpation at epigastrium without rebound or guarding. Labs showed a white blood cell count of 11.8 K/uL and otherwise unremarkable complete blood count, basic metabolic panel, liver function tests, and lactate measurement. Given the patient’s history of multiple abdominal surgeries and clinical presentation, POCUS was performed to evaluate for SBO. Dilated loops of small bowel were visualized in the lower abdomen gas, suggestive of SBO.
Since the small bowel encompasses a large portion of the abdomen, to fully evaluate for SBO, multiple views are necessary. These include the epigastrium, bilateral colic gutters, and suprapubic regions.5 Use the low-frequency curvilinear transducer to obtain these views, scanning in the transverse and sagittal planes (see Figures 1 and 2). Scan while moving the transducer in columns (ie, “mowing the lawn”), making sure to cover the entire abdomen. To assure that you are evaluating the small bowel, and not the large bowel, look for the characteristic plicae circularis of the small bowel (shown in Figure 3). In children and very slender adults, the high-frequency linear probe may provide enough depth to obtain adequate views.


A fluid-filled small intestinal segment >2.5 cm is consistent with a diagnosis of SBO. Measuring the diameter of the small bowel is both the most sensitive and specific sign; a measurement of greater than 2.5 cm is diagnostic, with a sensitivity of 97% and specificity of 91% (see Figure 4).6 This can be somewhat difficult to visualize, as bowel loops are multidirectional and diameters can mistakenly be taken on an indirect cut; to avoid over- or underestimation of bowel diameter, you may want to measure in the short axis using a transverse cross-sectional view of the bowel.

Lack of peristalsis is suggestive of a closed-loop obstruction. However, this finding may be more difficult to visualize, as it requires several continuous minutes of scanning or repeated exams to truly establish absent peristalsis. In prolonged courses of SBO, the bowel wall can measure >3 mm, which suggests necrosis, warranting accelerated surgical intervention. In addition, the detection of extraluminal peritoneal fluid can help determine the severity of the SBO, and small versus large fluid amounts can help determine whether medical or surgical management is warranted (see Figure 5).7

DISCUSSION
Increased time to diagnosis of SBO can lead to prolonged patient suffering and greater complication rates. The gold standard for diagnosing SBO—CT with intravenous and oral contrast—can take hours, requiring patients, who are often nauseated, to ingest and tolerate oral contrast. In the past, an “obstructive series” of x-rays would have been used early in the work-up of possible SBO.6
Recent literature suggests that POCUS is not only faster, more cost effective, and advantageous (involving no ionizing radiation), but also more accurate than x-rays. Specifically, a meta-analysis by Taylor et al showed pooled estimates for obstructive series x-rays have a sensitivity (Sn) of 75%, a specificity (Sp) of 66%, a positive likelihood ratio (+LR) of 1.6, and a negative likelihood ratio (-LR) of 0.43.1 On the other hand, pooled results from ED studies of emergency medicine (EM) residents performing POCUS in patients with signs and symptoms suspicious for SBO showed POCUS had a Sn of 97%, Sp of 90%, +LR of 9.5, and a -LR of 0.04.1,5,8 While detractors point to the operator-dependent nature of POCUS, literature suggests that with EM residents novice to POCUS for SBO (defined as less than 5 previous scans for SBO) were given a 10-minute didactic session and yielded Sn 94%, Sp 81%, +LR 5.0, -LR 0.07.5 Unluer et al trained novice EM residents for 6 hours and found them to yield Sn 98%, Sp 95%, +LR 19.5, and -LR 0.02.8 Thus, while it is no surprise that those with more training attain better results, both studies show it does not take much time for EM providers to surpass the accuracy of x-rays with POCUS.
CASE CONCLUSION
The findings on POCUS highly suggested the diagnosis of an SBO. A CT scan of the abdomen and pelvis with intravenous and oral contrast was ordered to further evaluate obstruction, transition point, and possible complications, including signs of ischemia per surgical request. CT demonstrated dilated loops of small bowel with transition point in the right lower quadrant, with a small amount of mesenteric fluid consistent with SBO with possible early bowel compromise due to ischemia. General surgery admitted the patient; conservative treatment with serial abdominal exams, nasogastric tube, NPO and bowel rest was ordered. The patient’s diet was gradually advanced, and she was discharged on the eleventh day of hospitalization.
SUMMARY
POCUS is a useful non-invasive tool that can accurately diagnose SBO. POCUS has increased sensitivity and specificity when compared to abdominal X-rays. This bedside imaging will not only give the ED provider rapid diagnostic information but also lead to expedited surgical intervention.
- Taylor MR, Lalani N. Adult small bowel obstruction. Acad Emerg Med. 2013;20(6):528-544.
- Hastings RS, Powers RD. Abdominal pain in the ED: a 35-year retrospective. Am J Emerg Med.2011;29:711-716.
- Markogiannakis H, Messaris E, Dardamanis D, et al. Acute mechanical bowel obstruction: clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007;13:432.
- Bickell N, Federman A, Aufses A. Influence of time on risk of bowel resection in complete small bowel obstruction. J Am Coll Surg. 2005;201(6):847-854.
- Jang TB, Chandler D, Kaji AH. Bedside ultrasonography for the detection of small bowel obstruction in the emergency department. Emerg Med J. 2011;28:676-678.
- Carpenter CR, Pines JM. The end of X-rays for suspected small bowel obstruction? Using evidence-based diagnostics to inform best practices in emergency medicine. Acad Emerg Med. 2013;20:618-20.
- Grassi R, Romano S, D’Amario F, et al. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur J Radiol. 2004;50(1):5-14.
- Unlüer E, Yavaşi O, Eroğlu O, Yilmaz C, Akarca F. Ultrasonography by emergency medicine and radiology residents for the diagnosis of small bowel obstruction. Eur J Emerg Med. 2010;17(5):260-264.
- Taylor MR, Lalani N. Adult small bowel obstruction. Acad Emerg Med. 2013;20(6):528-544.
- Hastings RS, Powers RD. Abdominal pain in the ED: a 35-year retrospective. Am J Emerg Med.2011;29:711-716.
- Markogiannakis H, Messaris E, Dardamanis D, et al. Acute mechanical bowel obstruction: clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007;13:432.
- Bickell N, Federman A, Aufses A. Influence of time on risk of bowel resection in complete small bowel obstruction. J Am Coll Surg. 2005;201(6):847-854.
- Jang TB, Chandler D, Kaji AH. Bedside ultrasonography for the detection of small bowel obstruction in the emergency department. Emerg Med J. 2011;28:676-678.
- Carpenter CR, Pines JM. The end of X-rays for suspected small bowel obstruction? Using evidence-based diagnostics to inform best practices in emergency medicine. Acad Emerg Med. 2013;20:618-20.
- Grassi R, Romano S, D’Amario F, et al. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur J Radiol. 2004;50(1):5-14.
- Unlüer E, Yavaşi O, Eroğlu O, Yilmaz C, Akarca F. Ultrasonography by emergency medicine and radiology residents for the diagnosis of small bowel obstruction. Eur J Emerg Med. 2010;17(5):260-264.
Identifying Pediatric Skull Fracture Using Point-of-Care Ultrasound
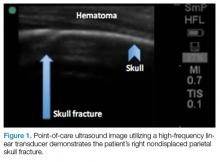
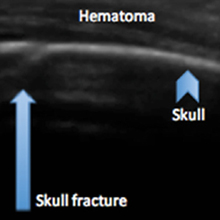
Evaluating pediatric patients presenting to the ED with head trauma can be a challenging task for emergency physicians (EPs). Specifically, identifying a nondisplaced skull fracture is not always possible through physical examination alone.1 However, point-of-care (POC) ultrasound permits rapid identification of skull fractures, which in turn assists the EP to determine if advanced imaging studies such as computed tomography (CT) are necessary.
Case
A previously healthy 10-month-old male infant presented to the ED with his mother for evaluation of rhinorrhea, cough, and fever, the onset of which began 24 hours prior to presentation. The patient’s mother reported that the infant continually tugged at his right ear throughout the previous evening and was increasingly irritable, but not inconsolable.
Initial vital signs at presentation were: blood pressure, 95/54 mm Hg; heart rate, 146 beats/min; respiratory rate, 36 beats/min, and temperature, 101.8°F. Oxygen saturation was 96% on room air. The physical examination was notable for an alert well-appearing infant who had a tender nonecchymotic scalp hematoma superior to the right pinna, clear tympanic membranes, crusted mucous bilaterally at the nares, nonlabored respirations, and wheezing throughout the lung fields.
Imaging Technique
To evaluate for skull fractures using POC ultrasound, the area of localized trauma must first be identified.2,3 Evidence of trauma includes an area of focal tenderness, abrasion, soft-tissue swelling, and hematoma.2,3 The presence of any depressed and open cranial injuries are contraindications to ultrasound. In which case, a physician should consult a neurosurgical specialist and obtain a CT scan of the head.
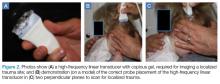
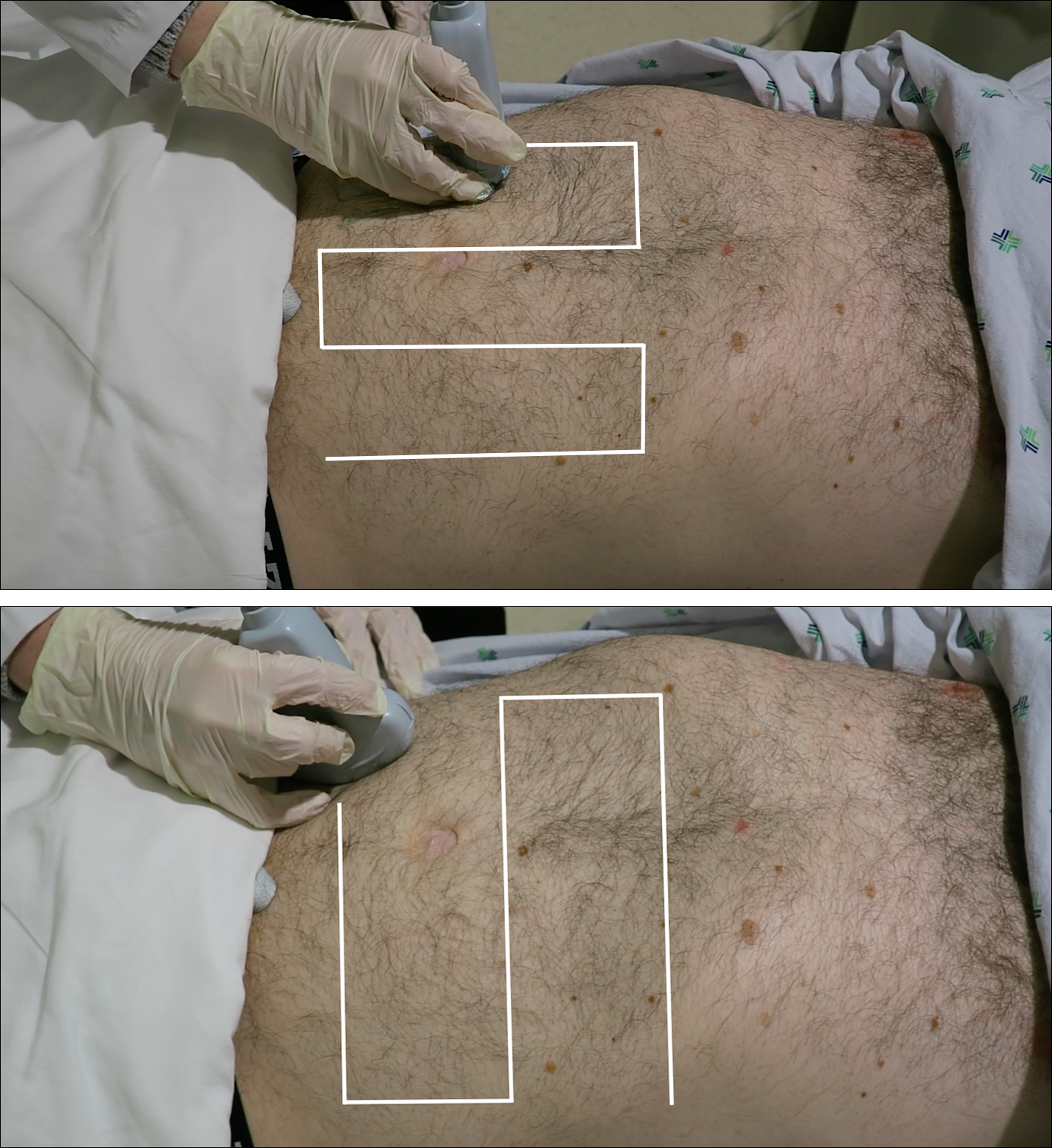
A high-frequency linear probe (5-10 MHz) is used to scan the area of localized trauma; this should be performed in two perpendicular planes using copious gel and light pressure (Figures 2a-2c).
Discussion
Closed head trauma is one of the most common pediatric injuries, accounting for roughly 1.4 million ED visits annually in the United States.5 Four to 12% percent of these minor traumas result in an intracranial injury,2 and the presence of a skull fracture is associated with a 4- to 20-fold increase in risk of underlying intracranial hemorrhage.3
Clinical assessment alone is not always reliable in predicting skull fracture and intracranial injury, especially in children younger than 2 years of age.2,3 Ultrasound is safe, noninvasive, expedient, cost-effective, and well tolerated in the pediatric population for identifying skull fractures,3 and can obviate the need for skull radiographs4 or procedural sedation. Moreover, POC ultrasound can serve as an adjunct to the Pediatric Emergency Care Applied Research Network head injury algorithm for head CT use decision rules if the fracture is not palpable on examination.
Several prospective studies and case reports have demonstrated the usefulness of POC ultrasound in diagnosing pediatric skull fractures in the ED.1-4 Two of the four cases published represented cases in which the EP identified an undisclosed nonaccidental trauma through POC ultrasound. Rabiner et al,3 estimates a combined sensitivity and specificity of 94% and 96%, respectively. It is important to remember that intracranial injury can still occur without an associated skull fracture. As our case demonstrates, POC ultrasound is a useful tool in risk-stratifying minor head trauma in children.
Case Conclusion
The head CT study confirmed a nondisplaced, oblique, and acute-appearing linear fracture of the right parietal bone extending from the squamosal to the lambdoid suture. There was no associated intracranial hemorrhage. The patient was admitted to the hospital for a nonaccidental trauma evaluation. The Department of Children and Family Services was contacted and the patient was discharged in the temporary custody of his maternal grandmother.
Summary
Point-of-care ultrasound is a useful diagnostic tool to rapidly evaluate for, and diagnose skull fractures in pediatric patients. Given its high sensitivity and specificity, ultrasound can help EPs identify occult nondisplaced skull fractures in children.
1. Riera A, Chen L. Ultrasound evaluation of skull fractures in children: a feasibility study. Pediatr Emerg Care. 2012;28(5):420-425. doi:10.1097/PEC.0b013e318252da3b.
2. Parri N, Crosby BJ, Glass C, et al. Ability of emergency ultrasonography to detect pediatric skull fractures: a prospective, observational study. J Emerg Med. 2013;44(1)135-141.
3. Rabiner JE, Friedman LM, Khine H, Avner JR, Tsung JW. Accuracy of point-of-care ultrasound for diagnosis of skull fractures in children. Pediatrics. 2013;131(6):e1757-1764. doi:10.1542/peds.2012-3921.
4. Ramirez-Schrempp D, Vinci RJ, Liteplo AS. Bedside ultrasound in the diagnosis of skull fractures in the pediatric emergency department. Pediatr Emerg Care. 2011;27(4):312-314. doi:10.1097/PEC.0b013e3182131579.
5. Coronado VG, Xu L, Basavaraju SV, et al; Centers for Disease Control and Prevention (CDC). Surveillance for traumatic brain injury-related deaths--United States, 1997-2007. MMWR Surveill Summ. 2011;60(5):1-32.
Evaluating pediatric patients presenting to the ED with head trauma can be a challenging task for emergency physicians (EPs). Specifically, identifying a nondisplaced skull fracture is not always possible through physical examination alone.1 However, point-of-care (POC) ultrasound permits rapid identification of skull fractures, which in turn assists the EP to determine if advanced imaging studies such as computed tomography (CT) are necessary.
Case
A previously healthy 10-month-old male infant presented to the ED with his mother for evaluation of rhinorrhea, cough, and fever, the onset of which began 24 hours prior to presentation. The patient’s mother reported that the infant continually tugged at his right ear throughout the previous evening and was increasingly irritable, but not inconsolable.
Initial vital signs at presentation were: blood pressure, 95/54 mm Hg; heart rate, 146 beats/min; respiratory rate, 36 beats/min, and temperature, 101.8°F. Oxygen saturation was 96% on room air. The physical examination was notable for an alert well-appearing infant who had a tender nonecchymotic scalp hematoma superior to the right pinna, clear tympanic membranes, crusted mucous bilaterally at the nares, nonlabored respirations, and wheezing throughout the lung fields.
Imaging Technique
To evaluate for skull fractures using POC ultrasound, the area of localized trauma must first be identified.2,3 Evidence of trauma includes an area of focal tenderness, abrasion, soft-tissue swelling, and hematoma.2,3 The presence of any depressed and open cranial injuries are contraindications to ultrasound. In which case, a physician should consult a neurosurgical specialist and obtain a CT scan of the head.
A high-frequency linear probe (5-10 MHz) is used to scan the area of localized trauma; this should be performed in two perpendicular planes using copious gel and light pressure (Figures 2a-2c).
Discussion
Closed head trauma is one of the most common pediatric injuries, accounting for roughly 1.4 million ED visits annually in the United States.5 Four to 12% percent of these minor traumas result in an intracranial injury,2 and the presence of a skull fracture is associated with a 4- to 20-fold increase in risk of underlying intracranial hemorrhage.3
Clinical assessment alone is not always reliable in predicting skull fracture and intracranial injury, especially in children younger than 2 years of age.2,3 Ultrasound is safe, noninvasive, expedient, cost-effective, and well tolerated in the pediatric population for identifying skull fractures,3 and can obviate the need for skull radiographs4 or procedural sedation. Moreover, POC ultrasound can serve as an adjunct to the Pediatric Emergency Care Applied Research Network head injury algorithm for head CT use decision rules if the fracture is not palpable on examination.
Several prospective studies and case reports have demonstrated the usefulness of POC ultrasound in diagnosing pediatric skull fractures in the ED.1-4 Two of the four cases published represented cases in which the EP identified an undisclosed nonaccidental trauma through POC ultrasound. Rabiner et al,3 estimates a combined sensitivity and specificity of 94% and 96%, respectively. It is important to remember that intracranial injury can still occur without an associated skull fracture. As our case demonstrates, POC ultrasound is a useful tool in risk-stratifying minor head trauma in children.
Case Conclusion
The head CT study confirmed a nondisplaced, oblique, and acute-appearing linear fracture of the right parietal bone extending from the squamosal to the lambdoid suture. There was no associated intracranial hemorrhage. The patient was admitted to the hospital for a nonaccidental trauma evaluation. The Department of Children and Family Services was contacted and the patient was discharged in the temporary custody of his maternal grandmother.
Summary
Point-of-care ultrasound is a useful diagnostic tool to rapidly evaluate for, and diagnose skull fractures in pediatric patients. Given its high sensitivity and specificity, ultrasound can help EPs identify occult nondisplaced skull fractures in children.
Evaluating pediatric patients presenting to the ED with head trauma can be a challenging task for emergency physicians (EPs). Specifically, identifying a nondisplaced skull fracture is not always possible through physical examination alone.1 However, point-of-care (POC) ultrasound permits rapid identification of skull fractures, which in turn assists the EP to determine if advanced imaging studies such as computed tomography (CT) are necessary.
Case
A previously healthy 10-month-old male infant presented to the ED with his mother for evaluation of rhinorrhea, cough, and fever, the onset of which began 24 hours prior to presentation. The patient’s mother reported that the infant continually tugged at his right ear throughout the previous evening and was increasingly irritable, but not inconsolable.
Initial vital signs at presentation were: blood pressure, 95/54 mm Hg; heart rate, 146 beats/min; respiratory rate, 36 beats/min, and temperature, 101.8°F. Oxygen saturation was 96% on room air. The physical examination was notable for an alert well-appearing infant who had a tender nonecchymotic scalp hematoma superior to the right pinna, clear tympanic membranes, crusted mucous bilaterally at the nares, nonlabored respirations, and wheezing throughout the lung fields.
Imaging Technique
To evaluate for skull fractures using POC ultrasound, the area of localized trauma must first be identified.2,3 Evidence of trauma includes an area of focal tenderness, abrasion, soft-tissue swelling, and hematoma.2,3 The presence of any depressed and open cranial injuries are contraindications to ultrasound. In which case, a physician should consult a neurosurgical specialist and obtain a CT scan of the head.
A high-frequency linear probe (5-10 MHz) is used to scan the area of localized trauma; this should be performed in two perpendicular planes using copious gel and light pressure (Figures 2a-2c).
Discussion
Closed head trauma is one of the most common pediatric injuries, accounting for roughly 1.4 million ED visits annually in the United States.5 Four to 12% percent of these minor traumas result in an intracranial injury,2 and the presence of a skull fracture is associated with a 4- to 20-fold increase in risk of underlying intracranial hemorrhage.3
Clinical assessment alone is not always reliable in predicting skull fracture and intracranial injury, especially in children younger than 2 years of age.2,3 Ultrasound is safe, noninvasive, expedient, cost-effective, and well tolerated in the pediatric population for identifying skull fractures,3 and can obviate the need for skull radiographs4 or procedural sedation. Moreover, POC ultrasound can serve as an adjunct to the Pediatric Emergency Care Applied Research Network head injury algorithm for head CT use decision rules if the fracture is not palpable on examination.
Several prospective studies and case reports have demonstrated the usefulness of POC ultrasound in diagnosing pediatric skull fractures in the ED.1-4 Two of the four cases published represented cases in which the EP identified an undisclosed nonaccidental trauma through POC ultrasound. Rabiner et al,3 estimates a combined sensitivity and specificity of 94% and 96%, respectively. It is important to remember that intracranial injury can still occur without an associated skull fracture. As our case demonstrates, POC ultrasound is a useful tool in risk-stratifying minor head trauma in children.
Case Conclusion
The head CT study confirmed a nondisplaced, oblique, and acute-appearing linear fracture of the right parietal bone extending from the squamosal to the lambdoid suture. There was no associated intracranial hemorrhage. The patient was admitted to the hospital for a nonaccidental trauma evaluation. The Department of Children and Family Services was contacted and the patient was discharged in the temporary custody of his maternal grandmother.
Summary
Point-of-care ultrasound is a useful diagnostic tool to rapidly evaluate for, and diagnose skull fractures in pediatric patients. Given its high sensitivity and specificity, ultrasound can help EPs identify occult nondisplaced skull fractures in children.
1. Riera A, Chen L. Ultrasound evaluation of skull fractures in children: a feasibility study. Pediatr Emerg Care. 2012;28(5):420-425. doi:10.1097/PEC.0b013e318252da3b.
2. Parri N, Crosby BJ, Glass C, et al. Ability of emergency ultrasonography to detect pediatric skull fractures: a prospective, observational study. J Emerg Med. 2013;44(1)135-141.
3. Rabiner JE, Friedman LM, Khine H, Avner JR, Tsung JW. Accuracy of point-of-care ultrasound for diagnosis of skull fractures in children. Pediatrics. 2013;131(6):e1757-1764. doi:10.1542/peds.2012-3921.
4. Ramirez-Schrempp D, Vinci RJ, Liteplo AS. Bedside ultrasound in the diagnosis of skull fractures in the pediatric emergency department. Pediatr Emerg Care. 2011;27(4):312-314. doi:10.1097/PEC.0b013e3182131579.
5. Coronado VG, Xu L, Basavaraju SV, et al; Centers for Disease Control and Prevention (CDC). Surveillance for traumatic brain injury-related deaths--United States, 1997-2007. MMWR Surveill Summ. 2011;60(5):1-32.
1. Riera A, Chen L. Ultrasound evaluation of skull fractures in children: a feasibility study. Pediatr Emerg Care. 2012;28(5):420-425. doi:10.1097/PEC.0b013e318252da3b.
2. Parri N, Crosby BJ, Glass C, et al. Ability of emergency ultrasonography to detect pediatric skull fractures: a prospective, observational study. J Emerg Med. 2013;44(1)135-141.
3. Rabiner JE, Friedman LM, Khine H, Avner JR, Tsung JW. Accuracy of point-of-care ultrasound for diagnosis of skull fractures in children. Pediatrics. 2013;131(6):e1757-1764. doi:10.1542/peds.2012-3921.
4. Ramirez-Schrempp D, Vinci RJ, Liteplo AS. Bedside ultrasound in the diagnosis of skull fractures in the pediatric emergency department. Pediatr Emerg Care. 2011;27(4):312-314. doi:10.1097/PEC.0b013e3182131579.
5. Coronado VG, Xu L, Basavaraju SV, et al; Centers for Disease Control and Prevention (CDC). Surveillance for traumatic brain injury-related deaths--United States, 1997-2007. MMWR Surveill Summ. 2011;60(5):1-32.