User login
Recommendations on the Use of Ultrasound Guidance for Central and Peripheral Vascular Access in Adults: A Position Statement of the Society of Hospital Medicine
Approximately five million central venous catheters (CVCs) are inserted in the United States annually, with over 15 million catheter days documented in intensive care units alone.1 Traditional CVC insertion techniques using landmarks are associated with a high risk of mechanical complications, particularly pneumothorax and arterial puncture, which occur in 5%-19% patients.2,3
Since the 1990s, several randomized controlled studies and meta-analyses have demonstrated that the use of real-time ultrasound guidance for CVC insertion increases procedure success rates and decreases mechanical complications.4,5 Use of real-time ultrasound guidance was recommended by the Agency for Healthcare Research and Quality, the Institute of Medicine, the National Institute for Health and Care Excellence, the Centers for Disease Control and Prevention, and several medical specialty societies in the early 2000s.6-14 Despite these recommendations, ultrasound guidance has not been universally adopted. Currently, an estimated 20%-55% of CVC insertions in the internal jugular vein are performed without ultrasound guidance.15-17
Following the emergence of literature supporting the use of ultrasound guidance for CVC insertion, observational and randomized controlled studies demonstrated improved procedural success rates with the use of ultrasound guidance for the insertion of peripheral intravenous lines (PIVs), arterial catheters, and peripherally inserted central catheters (PICCs).18-23
The purpose of this position statement is to present evidence-based recommendations on the use of ultrasound guidance for the insertion of central and peripheral vascular access catheters in adult patients. This document presents consensus-based recommendations with supporting evidence for clinical outcomes, techniques, and training for the use of ultrasound guidance for vascular access. We have subdivided the recommendations on techniques for central venous access, peripheral venous access, and arterial access individually, as some providers may not perform all types of vascular access procedures.
These recommendations are intended for hospitalists and other healthcare providers that routinely place central and peripheral vascular access catheters in acutely ill patients. However, this position statement does not mandate that all hospitalists should place central or peripheral vascular access catheters given the diverse array of hospitalist practice settings. For training and competency assessments, we recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals, where equipment and staffing for assessments are not available. Recommendations and frameworks for initial and ongoing credentialing of hospitalists in ultrasound-guided bedside procedures have been previously published in an Society of Hospital Medicine (SHM) position statement titled, “Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.”24
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interest (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the vascular access working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. An updated search was conducted in November 2017. The literature search strings are included in Appendix 3. All article abstracts were initially screened for relevance by at least two members of the vascular access working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide vascular access were selected. The following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled studies, and observational studies of ultrasound-guided vascular access were screened and selected (Appendix 3, Figure 1). All full-text articles were shared electronically among the working group members, and final article selection was based on working group consensus. Selected articles were incorporated into the draft recommendations.
These recommendations were developed using the Research and Development (RAND) Appropriateness Method that required panel judgment and consensus.14 The 28 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Using an internet-based electronic data collection tool (REDCap™), panel members participated in two rounds of electronic voting, one in August 2018 and the other in October 2018 (Appendix 4). Voting on appropriateness was conducted using a nine-point Likert scale. The three zones of the nine-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix 1, Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” Disagreement was defined as >30% of panelists voting outside of the zone of the median. A strong recommendation required at least 80% of the votes within one integer of the median per the RAND rules.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording for each recommendation (Table 2). The final version of the consensus-based recommendations underwent internal and external review by members of the SHM POCUS Task Force, the SHM Education Committee, and the SHM Executive Committee. The SHM Executive Committee reviewed and approved this position statement prior to its publication in the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 5,563 references were pooled from an initial search performed by a certified medical librarian in December 2015 (4,668 citations) which was updated in November 2017 (791 citations), and from the personal bibliographies and searches (104 citations) performed by working group members. A total of 514 full-text articles were reviewed. The final selection included 192 articles that were abstracted into a data table and incorporated into the draft recommendations. See Appendix 3 for details of the literature search strategy.
Recommendations
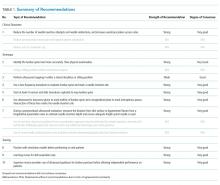
Four domains (technique, clinical outcomes, training, and knowledge gaps) with 31 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation cite supporting evidence. After two rounds of panel voting, 31 recommendations achieved agreement based on the RAND rules. During the peer review process, two of the recommendations were merged with other recommendations. Thus, a total of 29 recommendations received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Twenty-seven statements were approved as strong recommendations, and two were approved as weak/conditional recommendations. The strength of each recommendation and degree of consensus are summarized in Table 3.
Terminology
Central Venous Catheterization
Central venous catheterization refers to insertion of tunneled or nontunneled large bore vascular catheters that are most commonly inserted into the internal jugular, subclavian, or femoral veins with the catheter tip located in a central vein. These vascular access catheters are synonymously referred to as central lines or central venous catheters (CVCs). Nontunneled catheters are designed for short-term use and should be removed promptly when no longer clinically indicated or after a maximum of 14 days.25
Peripherally Inserted Central Catheter (PICC)
Peripherally inserted central catheters, or PICC lines, are inserted most commonly in the basilic or brachial veins in adult patients, and the catheter tip terminates in the distal superior vena cava or cavo-atrial junction. These catheters are designed to remain in place for a duration of several weeks, as long as it is clinically indicated.
Midline Catheterization
Midline catheters are a type of peripheral venous catheter that are an intermediary between a peripheral intravenous catheter and PICC line. Midline catheters are most commonly inserted in the brachial or basilic veins, but unlike PICC lines, the tips of these catheters terminate in the axillary or subclavian vein. Midline catheters are typically 8 cm to 20 cm in length and inserted for a duration <30 days.
Peripheral Intravenous Catheterization
Peripheral intravenous lines (PIV) refer to small bore venous catheters that are most commonly 14G to 24G and inserted into patients for short-term peripheral venous access. Common sites of ultrasound-guided PIV insertion include the superficial and deep veins of the hand, forearm, and arm.
Arterial Catheterization
Arterial catheters are commonly used for reliable blood pressure monitoring, frequent arterial blood
RECOMMENDATIONS
Preprocedure
1. We recommend that providers should be familiar with the operation of their specific ultrasound machine prior to initiation of a vascular access procedure.
Rationale: There is strong consensus that providers must be familiar with the knobs and functions of the specific make and model of ultrasound machine that will be utilized for a vascular access procedure. Minimizing adjustments to the ultrasound machine during the procedure may reduce the risk of contaminating the sterile field.
2. We recommend that providers should use a high-frequency linear transducer with a sterile sheath and sterile gel to perform vascular access procedures.
Rationale: High-frequency linear-array transducers are recommended for the vast majority of vascular access procedures due to their superior resolution compared to other transducer types. Both central and peripheral vascular access procedures, including PIV, PICC, and arterial line placement, should be performed using sterile technique. A sterile transducer cover and sterile gel must be utilized, and providers must be trained in sterile preparation of the ultrasound transducer.13,26,27
The depth of femoral vessels correlates with body mass index (BMI). When accessing these vessels in a morbidly obese patient with a thigh circumference >60 cm and vessel depth >8 cm, a curvilinear transducer may be preferred for its deeper penetration.28 For patients who are poor candidates for bedside insertion of vascular access catheters, such as uncooperative patients, patients with atypical vascular anatomy or poorly visualized target vessels, we recommend consultation with a vascular access specialist prior to attempting the procedure.
3. We recommend that providers should use two-dimensional ultrasound to evaluate for anatomical variations and absence of vascular thrombosis during preprocedural site selection.
Rationale: A thorough ultrasound examination of the target vessel is warranted prior to catheter placement. Anatomical variations that may affect procedural decision-making are easily detected with ultrasound. A focused vascular ultrasound examination is particularly important in patients who have had temporary or tunneled venous catheters, which can cause stenosis or thrombosis of the target vein.
For internal jugular vein (IJV) CVCs, ultrasound is useful for visualizing the relationship between the IJV and common carotid artery (CCA), particularly in terms of vessel overlap. Furthermore, ultrasound allows for immediate revisualization upon changes in head position.29-32 Troianos et al. found >75% overlap of the IJV and CCA in 54% of all patients and in 64% of older patients (age >60 years) whose heads were rotated to the contralateral side.30 In one study of IJV CVC insertion, inadvertent carotid artery punctures were reduced (3% vs 10%) with the use of ultrasound guidance vs landmarks alone.33 In a cohort of 64 high-risk neurosurgical patients, cannulation success was 100% with the use of ultrasound guidance, and there were no injuries to the carotid artery, even though the procedure was performed with a 30-degree head elevation and anomalous IJV anatomy in 39% of patients.34 In a prospective, randomized controlled study of 1,332 patients, ultrasound-guided cannulation in a neutral position was demonstrated to be as safe as the 45-degree rotated position.35
Ultrasound allows for the recognition of anatomical variations which may influence the selection of the vascular access site or technique. Benter et al. found that 36% of patients showed anatomical variations in the IJV and surrounding tissue.36 Similarly Caridi showed the anatomy of the right IJV to be atypical in 29% of patients,37 and Brusasco found that 37% of bariatric patients had anatomical variations of the IJV.38 In a study of 58 patients, there was significant variability in the IJV position and IJV diameter, ranging from 0.5 cm to >2 cm.39 In a study of hemodialysis patients, 75% of patients had sonographic venous abnormalities that led to a change in venous access approach.40
To detect acute or chronic upper extremity deep venous thrombosis or stenosis, two-dimensional visualization with compression should be part of the ultrasound examination prior to central venous catheterization. In a study of patients that had undergone CVC insertion 9-19 weeks earlier, 50% of patients had an IJV thrombosis or stenosis leading to selection of an alternative site. In this study, use of ultrasound for a preprocedural site evaluation reduced unnecessary attempts at catheterizing an occluded vein.41 At least two other studies demonstrated an appreciable likelihood of thrombosis. In a study of bariatric patients, 8% of patients had asymptomatic thrombosis38 and in another study, 9% of patients being evaluated for hemodialysis catheter placement had asymptomatic IJV thrombosis.37
4. We recommend that providers should evaluate the target blood vessel size and depth during a preprocedural ultrasound evaluation.
Rationale: The size, depth, and anatomic location of central veins can vary considerably. These features are easily discernable using ultrasound. Contrary to traditional teaching, the IJV is located 1 cm anterolateral to the CCA in only about two-thirds of patients.37,39,42,43 Furthermore, the diameter of the IJV can vary significantly, ranging from 0.5 cm to >2 cm.39 The laterality of blood vessels may vary considerably as well. A preprocedural ultrasound evaluation of contralateral subclavian and axillary veins showed a significant absolute difference in cross-sectional area of 26.7 mm2 (P < .001).42
Blood vessels can also shift considerably when a patient is in the Trendelenburg position. In one study, the IJV diameter changed from 11.2 (± 1.5) mm to 15.4 (± 1.5) mm in the supine versus the Trendelenburg position at 15 degrees.33 An observational study demonstrated a frog-legged position with reverse Trendelenburg increased the femoral vein size and reduced the common surface area with the common femoral artery compared to a neutral position. Thus, a frog-legged position with reverse Trendelenburg position may be preferred, since overall catheterization success rates are higher in this position.44
Techniques
General Techniques
5. We recommend that providers should avoid using static ultrasound alone to mark the needle insertion site for vascular access procedures.
Rationale: The use of static ultrasound guidance to mark a needle insertion site is not recommended because normal anatomical relationships of vessels vary, and site marking can be inaccurate with minimal changes in patient position, especially of the neck.43,45,46 Benefits of using ultrasound guidance for vascular access are attained when ultrasound is used to track the needle tip in real-time as it is advanced toward the target vessel.
Although continuous-wave Doppler ultrasound without two-dimensional visualization was used in the past, it is no longer recommended for IJV CVC insertion.47 In a study that randomized patients to IJV CVC insertion with continuous-wave Doppler alone vs two-dimensional ultrasound guidance, the use of two-dimensional ultrasound guidance showed significant improvement in first-pass success rates (97% vs 91%, P = .045), particularly in patients with BMI >30 (97% vs 77%, P = .011).48
A randomized study comparing real-time ultrasound-guided, landmark-based, and ultrasound-marked techniques found higher success rates in the real-time ultrasound-guided group than the other two groups (100% vs 74% vs 73%, respectively; P = .01). The total number of mechanical complications was higher in the landmark-based and ultrasound-marked groups than in the real-time ultrasound-guided group (24% and 36% versus 0%, respectively; P = .01).49 Another randomized controlled study found higher success rates with real-time ultrasound guidance (98%) versus an ultrasound-marked (82%) or landmark-based (64%) approach for central line placement.50
6. We recommend that providers should use real-time (dynamic), two-dimensional ultrasound guidance with a high-frequency linear transducer for CVC insertion, regardless of the provider’s level of experience.
7. We suggest using either a transverse (short-axis) or longitudinal (long-axis) approach when performing real-time ultrasound-guided vascular access procedures.
Rationale: In clinical practice, the phrases transverse, short-axis, or out-of-plane approach are synonymous, as are longitudinal, long-axis, and in-plane approach. The short-axis approach involves tracking the needle tip as it approximates the target vessel with the ultrasound beam oriented in a transverse plane perpendicular to the target vessel. The target vessel is seen as a circular structure on the ultrasound screen as the needle tip approaches the target vessel from above. This approach is also called the out-of-plane technique since the needle passes through the ultrasound plane. The advantages of the short-axis approach include better visualization of adjacent vessels or nerves and the relative ease of skill acquisition for novice operators.9 When using the short-axis approach, extra care must be taken to track the needle tip from the point of insertion on the skin to the target vessel. A disadvantage of the short-axis approach is unintended posterior wall puncture of the target vessel.55
In contrast to a short-axis approach, a long-axis approach is performed with the ultrasound beam aligned parallel to the vessel. The vessel appears as a long tubular structure and the entire needle is visualized as it traverses across the ultrasound screen to approach the target vessel. The long-axis approach is also called an in-plane technique because the needle is maintained within the plane of the ultrasound beam. The advantage of a long-axis approach is the ability to visualize the entire needle as it is inserted into the vessel.14 A randomized crossover study with simulation models compared a long-axis versus short-axis approach for both IJV and subclavian vein catheterization. This study showed decreased number of needle redirections (relative risk (RR) 0.5, 95% confidence interval (CI) 0.3 to 0.7), and posterior wall penetrations (OR 0.3, 95% CI 0.1 to 0.9) using a long-axis versus short-axis approach for subclavian vein catheterization.56
A randomized controlled study comparing a long-axis or short-axis approach with ultrasound versus a landmark-based approach for IJV CVC insertion showed higher success rates (100% vs 90%; P < .001), lower insertion time (53 vs 116 seconds; P < .001), and fewer attempts to obtain access (2.5 vs 1.2 attempts, P < .001) with either the long- or short-axis ultrasound approach. The average time to obtain access and number of attempts were comparable between the short-axis and long-axis approaches with ultrasound. The incidence of carotid puncture and hematoma was significantly higher with the landmark-based approach versus either the long- or short-axis ultrasound approach (carotid puncture 17% vs 3%, P = .024; hematoma 23% vs 3%, P = .003).57
High success rates have been reported using a short-axis approach for insertion of PIV lines.58 A prospective, randomized trial compared the short-axis and long-axis approach in patients who had had ≥2 failed PIV insertion attempts. Success rate was 95% (95% CI, 0.85 to 1.00) in the short-axis group compared with 85% (95% CI, 0.69 to 1.00) in the long-axis group. All three subjects with failed PIV placement in the long-axis group had successful rescue placement using a short-axis approach. Furthermore, the short-axis approach was faster than the long-axis approach.59
For radial artery cannulation, limited data exist comparing the short- and long-axis approaches. A randomized controlled study compared a long-axis vs short-axis ultrasound approach for radial artery cannulation. Although the overall procedure success rate was 100% in both groups, the long-axis approach had higher first-pass success rates (1.27 ± 0.4 vs 1.5 ± 0.5, P < .05), shorter cannulation times (24 ± 17 vs 47 ± 34 seconds, P < .05), fewer hematomas (4% vs 43%, P < .05) and fewer posterior wall penetrations (20% vs 56%, P < .05).60
Another technique that has been described for IJV CVC insertion is an oblique-axis approach, a hybrid between the long- and short-axis approaches. In this approach, the transducer is aligned obliquely over the IJV and the needle is inserted using a long-axis or in-plane approach. A prospective randomized trial compared the short-axis, long-axis, and oblique-axis approaches during IJV cannulation. First-pass success rates were 70%, 52%, and 74% with the short-axis, long-axis, and oblique-axis approaches, respectively, and a statistically significant difference was found between the long- and oblique-axis approaches (P = .002). A higher rate of posterior wall puncture was observed with a short-axis approach (15%) compared with the oblique-axis (7%) and long-axis (4%) approaches (P = .047).61
8. We recommend that providers should visualize the needle tip and guidewire in the target vein prior to vessel dilatation.
Rationale: When real-time ultrasound guidance is used, visualization of the needle tip within the vein is the first step to confirm cannulation of the vein and not the artery. After the guidewire is advanced, the provider can use transverse and longitudinal views to reconfirm cannulation of the vein. In a longitudinal view, the guidewire is readily seen positioned within the vein, entering the anterior wall and lying along the posterior wall of the vein. Unintentional perforation of the posterior wall of the vein with entry into the underlying artery can be detected by ultrasound, allowing prompt removal of the needle and guidewire before proceeding with dilation of the vessel. In a prospective observational study that reviewed ultrasound-guided IJV CVC insertions, physicians were able to more readily visualize the guidewire than the needle in the vein.62 A prospective observational study determined that novice operators can visualize intravascular guidewires in simulation models with an overall accuracy of 97%.63
In a retrospective review of CVC insertions where the guidewire position was routinely confirmed in the target vessel prior to dilation, there were no cases of arterial dilation, suggesting confirmation of guidewire position can potentially eliminate the morbidity and mortality associated with arterial dilation during CVC insertion.64
9. To increase the success rate of ultrasound-guided vascular access procedures, we recommend that providers should utilize echogenic needles, plastic needle guides, and/or ultrasound beam steering when available.
Rationale: Echogenic needles have ridged tips that appear brighter on the screen, allowing for better visualization of the needle tip. Plastic needle guides help stabilize the needle alongside the transducer when using either a transverse or longitudinal approach. Although evidence is limited, some studies have reported higher procedural success rates when using echogenic needles, plastic needle guides, and ultrasound beam steering software. In a prospective observational study, Augustides et al. showed significantly higher IJV cannulation rates with versus without use of a needle guide after first (81% vs 69%, P = .0054) and second (93% vs 80%. P = .0001) needle passes.65 A randomized study by Maecken et al. compared subclavian vein CVC insertion with or without use of a needle guide, and found higher procedure success rates within the first and second attempts, reduced time to obtain access (16 seconds vs 30 seconds; P = .0001) and increased needle visibility (86% vs 32%; P < .0001) with the use of a needle guide.66 Another study comparing a short-axis versus long-axis approach with a needle guide showed improved needle visualization using a long-axis approach with a needle guide.67 A randomized study comparing use of a novel, sled-mounted needle guide to a free-hand approach for venous cannulation in simulation models showed the novel, sled-mounted needle guide improved overall success rates and efficiency of cannulation.68
Central Venous Access Techniques
10. We recommend that providers should use a standardized procedure checklist that includes use of real-time ultrasound guidance to reduce the risk of central line-associated bloodstream infection (CLABSI) from CVC insertion.
Rationale: A standardized checklist or protocol should be developed to ensure compliance with all recommendations for insertion of CVCs. Evidence-based protocols address periprocedural issues, such as indications for CVC, and procedural techniques, such as use of maximal sterile barrier precautions to reduce the risk of infection. Protocols and checklists that follow established guidelines for CVC insertion have been shown to decrease CLABSI rates.69,70 Similarly, development of checklists and protocols for maintenance of central venous catheters have been effective in reducing CLABSIs.71 Although no externally-validated checklist has been universally accepted or endorsed by national safety organizations, a few internally-validated checklists are available through peer-reviewed publications.72,73 An observational educational cohort of internal medicine residents who received training using simulation of the entire CVC insertion process was able to demonstrate fewer CLABSIs after the simulator-trained residents rotated in the intensive care unit (ICU) (0.50 vs 3.2 infections per 1,000 catheter days, P = .001).74
11. We recommend that providers should use real-time ultrasound guidance, combined with aseptic technique and maximal sterile barrier precautions, to reduce the incidence of infectious complications from CVC insertion.
Rationale: The use of real-time ultrasound guidance for CVC placement has demonstrated a statistically significant reduction in CLABSIs compared to landmark-based techniques.75 The Centers for Disease Control and Prevention (CDC) guidelines for the prevention of intravascular catheter-related infections recommend the use of ultrasound guidance to reduce the number of cannulation attempts and risk of mechanical complications.69 A prospective, three-arm study comparing ultrasound-guided long-axis, short-axis, and landmark-based approaches showed a CLABSI rate of 20% in the landmark-based group versus 10% in each of the ultrasound groups.57 Another randomized study comparing use of ultrasound guidance to a landmark-based technique for IJV CVC insertion demonstrated significantly lower CLABSI rates with the use of ultrasound (2% vs 10%; P < .05).72
Studies have shown that a systems-based intervention featuring a standardized catheter kit or catheter bundle significantly reduced CLABSI rates.76-78 A complete review of all preventive measures to reduce the risk of CLABSI is beyond the scope of this review, but a few key points will be mentioned. First, aseptic technique includes proper hand hygiene and skin sterilization, which are essential measures to reduce cutaneous colonization of the insertion site and reduce the risk of CLABSIs.79 In a systematic review and meta-analysis of eight studies including over 4,000 catheter insertions, skin antisepsis with chlorhexidine was associated with a 50% reduction in CLABSIs compared with povidone iodine.11 Therefore, a chlorhexidine-containing solution is recommended for skin preparation prior to CVC insertion per guidelines by Healthcare Infection Control Practices Advisory Committee/CDC, Society for Healthcare Epidemiology of America/Infectious Diseases Society of America, and American Society of Anesthesiologists.11,69,80,81 Second, maximal sterile barrier precautions refer to the use of sterile gowns, sterile gloves, caps, masks covering both the mouth and nose, and sterile full-body patient drapes. Use of maximal sterile barrier precautions during CVC insertion has been shown to reduce the incidence of CLABSIs compared to standard precautions.26,79,82-84 Third, catheters containing antimicrobial agents may be considered for hospital units with higher CLABSI rates than institutional goals, despite a comprehensive preventive strategy, and may be considered in specific patient populations at high risk of severe complications from a CLABSI.11,69,80 Finally, providers should use a standardized procedure set-up when inserting CVCs to reduce the risk of CLABSIs. The operator should confirm availability and proper functioning of ultrasound equipment prior to commencing a vascular access procedure. Use of all-inclusive procedure carts or kits with sterile ultrasound probe covers, sterile gel, catheter kits, and other necessary supplies is recommended to minimize interruptions during the procedure, and can ultimately reduce the risk of CLABSIs by ensuring maintenance of a sterile field during the procedure.13
12. We recommend that providers should use real-time ultrasound guidance for internal jugular vein catheterization, which reduces the risk of mechanical and infectious complications, the number of needle passes, and time to cannulation and increases overall procedure success rates.
Rationale: The use of real-time ultrasound guidance for CVC insertion has repeatedly demonstrated better outcomes compared to a landmark-based approach in adults.13 Several randomized controlled studies have demonstrated that real-time ultrasound guidance for IJV cannulation reduces the risk of procedure-related mechanical and infectious complications, and improves first-pass and overall success rates in diverse care settings.27,29,45,50,53,65,75,85-90 Mechanical complications that are reduced with ultrasound guidance include pneumothorax and carotid artery puncture.4,5,45,46,53,62,75,86-93 Currently, several medical societies strongly recommend the use of ultrasound guidance during insertion of IJV CVCs.10-12,14,94-96
A meta-analysis by Hind et al. that included 18 randomized controlled studies demonstrated use of real-time ultrasound guidance reduced failure rates (RR 0.14, 95% CI 0.06 to 0.33; P < .0001), increased first-attempt success rates (RR 0.59, 95% CI 0.39 to 0.88; P = .009), reduced complication rates (RR 0.43, 95% CI 0.22 to 0.87; P = .02) and reduced procedure time (P < .0001), compared to a traditional landmark-based approach when inserting IJV CVCs.5
A Cochrane systematic review compared ultrasound-guided versus landmark-based approaches for IJV CVC insertion and found use of real-time ultrasound guidance reduced total complication rates by 71% (RR 0.29, 95% CI 0.17 to 0.52; P < .0001), arterial puncture rates by 72% (RR 0.28, 95% CI 0.18 to 0.44; P < .00001), and rates of hematoma formation by 73% (RR 0.27, 95% CI 0.13 to 0.55; P = .0004). Furthermore, the number of attempts for successful cannulation was reduced (mean difference -1.19 attempts, 95% CI -1.45 to -0.92; P < .00001), the chance of successful insertion on the first attempt was increased by 57% (RR 1.57, 95% CI 1.36 to 1.82; P < .00001), and overall procedure success rates were modestly increased in all groups by 12% (RR 1.12, 95% CI 1.08 to 1.17; P < .00001).46
An important consideration in performing ultrasound guidance is provider experience. A prospective observational study of patients undergoing elective CVC insertion demonstrated higher complication rates for operators that were inexperienced (25.2%) versus experienced (13.6%).54 A randomized controlled study comparing experts and novices with or without the use of ultrasound guidance for IJV CVC insertion demonstrated higher success rates among expert operators and with the use of ultrasound guidance. Among novice operators, the complication rates were lower with the use of ultrasound guidance.97 One study evaluated the procedural success and complication rates of a two-physician technique with one physician manipulating the transducer and another inserting the needle for IJV CVC insertion. This study concluded that procedural success rates and frequency of complications were directly affected by the experience of the physician manipulating the transducer and not by the experience of the physician inserting the needle.98
The impact of ultrasound guidance on improving procedural success rates and reducing complication rates is greatest in patients that are obese, short necked, hypovolemic, or uncooperative.93 Several studies have demonstrated fewer needle passes and decreased time to cannulation compared to the landmark technique in these populations.46,49,53,86-88,92,93
Ultrasound-guided placement of IJV catheters can safely be performed in patients with disorders of hemostasis and those with multiple previous catheter insertions in the same vein.9 Ultrasound-guided placement of CVCs in patients with disorders of hemostasis is safe with high success and low complication rates. In a case series of liver patients with coagulopathy (mean INR 2.17 ± 1.16, median platelet count 150K), the use of ultrasound guidance for CVC insertion was highly successful with no major bleeding complications.99
A study of renal failure patients found high success rates and low complication rates in the patients with a history of multiple previous catheterizations, poor compliance, skeletal deformities, previous failed cannulations, morbid obesity, and disorders of hemostasis.100 A prospective observational study of 200 ultrasound-guided CVC insertions for apheresis showed a 100% success rate with a 92% first-pass success rate.101
The use of real-time ultrasound guidance for IJV CVC insertion has been shown to be cost effective by reducing procedure-related mechanical complications and improving procedural success rates. A companion cost-effectiveness analysis estimated that for every 1,000 patients, 90 complications would be avoided, with a net cost savings of approximately $3,200 using 2002 prices.102
13. We recommend that providers who routinely insert subclavian vein CVCs should use real-time ultrasound guidance, which has been shown to reduce the risk of mechanical complications and number of needle passes and increase overall procedure success rates compared with landmark-based techniques.
Rationale: In clinical practice, the term ultrasound-guided subclavian vein CVC insertion is commonly used. However, the needle insertion site is often lateral to the first rib and providers are technically inserting the CVC in the axillary vein. The subclavian vein becomes the axillary vein at the lateral border of the first rib where the cephalic vein branches from the subclavian vein. To be consistent with common medical parlance, we use the phrase ultrasound-guided subclavian vein CVC insertion in this document.
Advantages of inserting CVCs in the subclavian vein include reliable surface anatomical landmarks for vein location, patient comfort, and lower risk of infection.103 Several observational studies have demonstrated the technique for ultrasound-guided subclavian vein CVC insertion is feasible and safe.104-107 In a large retrospective observational study of ultrasound-guided central venous access among a complex patient group, the majority of patients were cannulated successfully and safely. The subset of patients undergoing axillary vein CVC insertion (n = 1,923) demonstrated a low rate of complications (0.7%), proving it is a safe and effective alternative to the IJV CVC insertion.107
A Cochrane review of ultrasound-guided subclavian vein cannulation (nine studies, 2,030 participants, 2,049 procedures), demonstrated that real-time two-dimensional ultrasound guidance reduced the risk of inadvertent arterial punctures (three studies, 498 participants, RR 0.21, 95% CI 0.06 to 0.82; P = .02) and hematoma formation (three studies, 498 participants, RR 0.26, 95% CI 0.09 to 0.76; P = .01).46 A systematic review and meta-analysis of 10 randomized controlled studies comparing ultrasound-guided versus landmark-based subclavian vein CVC insertion demonstrated a reduction in the risk of arterial punctures, hematoma formation, pneumothorax, and failed catheterization with the use of ultrasound guidance.105
A randomized controlled study comparing ultrasound-guided vs landmark-based approaches to subclavian vein cannulation found that use of ultrasound guidance had a higher success rate (92% vs 44%, P = .0003), fewer minor complications (1 vs 11, P = .002), fewer attempts (1.4 vs 2.5, P = .007) and fewer catheter kits used (1.0 vs 1.4, P = .0003) per cannulation.108
Fragou et al. randomized patients undergoing subclavian vein CVC insertion to a long-axis approach versus a landmark-based approach and found a significantly higher success rate (100% vs 87.5%, P < .05) and lower rates of mechanical complications: artery puncture (0.5% vs 5.4%), hematoma (1.5% vs 5.4%), hemothorax (0% vs 4.4%), pneumothorax (0% vs 4.9%), brachial plexus injury (0% vs 2.9%), phrenic nerve injury (0% vs 1.5%), and cardiac tamponade (0% vs 0.5%).109 The average time to obtain access and the average number of insertion attempts (1.1 ± 0.3 vs 1.9 ± 0.7, P < .05) were significantly reduced in the ultrasound group compared to the landmark-based group.95
A retrospective review of subclavian vein CVC insertions using a supraclavicular approach found no reported complications with the use of ultrasound guidance vs 23 mechanical complications (8 pneumothorax, 15 arterial punctures) with a landmark-based approach.106 However, it is important to note that a supraclavicular approach is not commonly used in clinical practice.
14. We recommend that providers should use real-time ultrasound guidance for femoral venous access, which has been shown to reduce the risk of arterial punctures and total procedure time and increase overall procedure success rates.
Rationale: Anatomy of the femoral region varies, and close proximity or overlap of the femoral vein and artery is common.51 Early studies showed that ultrasound guidance for femoral vein CVC insertion reduced arterial punctures compared with a landmark-based approach (7% vs 16%), reduced total procedure time (55 ± 19 vs 79 ± 62 seconds), and increased procedure success rates (100% vs 90%).52 A Cochrane review that pooled data from four randomized studies comparing ultrasound-guided vs landmark-based femoral vein CVC insertion found higher first-attempt success rates with the use of ultrasound guidance (RR 1.73, 95% CI 1.34 to 2.22; P < .0001) and a small increase in the overall procedure success rates (RR 1.11, 95% CI 1.00 to 1.23; P = .06). There was no difference in inadvertent arterial punctures or other complications.110
Peripheral Venous Access Techniques
15. We recommend that providers should use real-time ultrasound guidance for the insertion of peripherally inserted central catheters (PICCs), which is associated with higher procedure success rates and may be more cost effective compared with landmark-based techniques.
Rationale: Several studies have demonstrated that providers who use ultrasound guidance vs landmarks for PICC insertion have higher procedural success rates, lower complication rates, and lower total placement costs. A prospective observational report of 350 PICC insertions using ultrasound guidance reported a 99% success rate with an average of 1.2 punctures per insertion and lower total costs.20 A retrospective observational study of 500 PICC insertions by designated specialty nurses revealed an overall success rate of 95%, no evidence of phlebitis, and only one CLABSI among the catheters removed.21 A retrospective observational study comparing several PICC variables found higher success rates (99% vs 77%) and lower thrombosis rates (2% vs 9%) using ultrasound guidance vs landmarks alone.22 A study by Robinson et al. demonstrated that having a dedicated PICC team equipped with ultrasound increased their institutional insertion success rates from 73% to 94%.111
A randomized controlled study comparing ultrasound-guided versus landmark-based PICC insertion found high success rates with both techniques (100% vs 96%). However, there was a reduction in the rate of unplanned catheter removals (4.0% vs 18.7%; P = .02), mechanical phlebitis (0% vs 22.9%; P < .001), and venous thrombosis (0% vs 8.3%; P = .037), but a higher rate of catheter migration (32% vs 2.1%; P < .001). Compared with the landmark-based group, the ultrasound-guided group had significantly lower incidence of severe contact dermatitis (P = .038), and improved comfort and costs up to 3 months after PICC placement (P < .05).112
Routine postprocedure chest x-ray (CXR) is generally considered unnecessary if the PICC is inserted with real-time ultrasound guidance along with use of a newer tracking devices, like the magnetic navigation system with intracardiac electrodes.9 Ultrasound can also be used to detect malpositioning of a PICC immediately after completing the procedure. A randomized controlled study comparing ultrasound versus postprocedure CXR detected one malpositioned PICC in the ultrasound group versus 11 in the control group. This study suggested that ultrasound can detect malpositioning immediately postprocedure and reduce the need for a CXR and the possibility of an additional procedure to reposition a catheter.113
16. We recommend that providers should use real-time ultrasound guidance for the placement of peripheral intravenous lines (PIV) in patients with difficult peripheral venous access to reduce the total procedure time, needle insertion attempts, and needle redirections. Ultrasound-guided PIV insertion is also an effective alternative to CVC insertion in patients with difficult venous access.
Rationale: Difficult venous access refers to patients that have had two unsuccessful attempts at PIV insertion using landmarks or a history of difficult access (i.e. edema, obesity, intravenous drug use, chemotherapy, diabetes, hypovolemia, chronic illness, vasculopathy, multiple prior hospitalizations). A meta-analysis of seven randomized controlled studies concluded that ultrasound guidance increases the likelihood of successful PIV insertion (pooled OR 2.42, 95% CI 1.26 to 4.68; P < .008).18 A second meta-analysis that pooled data from seven studies (six randomized controlled studies) confirmed that ultrasound guidance improves success rates of PIV insertion (OR 3.96, 95% CI 1.75 to 8.94).19 Approximately half of these studies had physician operators while the other half had nurse operators.
In one prospective observational study of emergency department patients with two failed attempts of landmark-based PIV insertion, ultrasound guidance with a modified-Seldinger technique showed a relatively high success rate (96%), fewer needle sticks (mean 1.32 sticks, 95% CI 1.12 to 1.52), and shorter time to obtain access (median time 68 seconds).114 Other prospective observational studies have demonstrated that ultrasound guidance for PIV insertion has a high success rate (87%),115 particularly with brachial or basilic veins PIV insertion, among patients with difficult PIV access, defined as having had ≥2 failed attempts.58
Since insertion of PIVs with ultrasound guidance has a high success rate, there is potential to reduce the reliance on CVC insertion for venous access only. In a study of patients that had had two failed attempts at PIV insertion based on landmarks, a PIV was successfully inserted with ultrasound guidance in 84% of patients, obviating the need for CVC placement for venous access.116 A prospective observational study showed ultrasound-guided PIV insertion was an effective alternative to CVC placement in ED patients with difficult venous access with only 1% of patients requiring a CVC.117 Use of ultrasound by nurses for PIV placement has also been shown to reduce the time to obtain venous access, improve patient satisfaction, and reduce the need for physician intervention.118 In a prospective observational study of patients with difficult access, the majority of patients reported a better experience with ultrasound-guided PIV insertion compared to previous landmark-based attempts with an average satisfaction score of 9.2/10 with 76% of patients rating the experience a 10.119 A strong recommendation has been made for use of ultrasound guidance in patients with difficult PIV placement by la Société Française d’Anesthésie et de Réanimation (The French Society of Anesthesia and Resuscitation).95
17. We suggest using real-time ultrasound guidance to reduce the risk of vascular, infectious, and neurological complications during PIV insertion, particularly in patients with difficult venous access.
Rationale: The incidence of complications from PIV insertion is often underestimated. Vascular complications include arterial puncture, hematoma formation, local infiltration or extravasation of fluid, and superficial or deep venous thrombosis. The most common infectious complications with PIV insertion are phlebitis and cellulitis.120 One observational study reported PIV complications occurring in approximately half of all patients with the most common complications being phlebitis, hematoma formation, and fluid/blood leakage.121
A retrospective review of ICU patients who underwent ultrasound-guided PIV insertion by a single physician showed high success rates (99%) with low rates of phlebitis/cellulitis (0.7%).There was an assumed benefit of risk reduction due to the patients no longer requiring a CVC after successful PIV placement.122 Another study found very low rates of infection with both landmark-based and ultrasound-guided PIV placement performed by emergency department nurses, suggesting that there is no increased risk of infection with the use of ultrasound.123 To reduce the risk of infection from PIV insertion, we recommend the use of sterile gel and sterile transducer cover (See Recommendation 2).
Arterial Access Techniques
18. We recommend that providers should use real-time ultrasound guidance for arterial access, which has been shown to increase first-pass success rates, reduce the time to cannulation, and reduce the risk of hematoma development compared with landmark-based techniques.
Rationale: Several randomized controlled studies have assessed the value of ultrasound in arterial catheter insertion. Shiver et al. randomized 60 patients admitted to a tertiary center emergency department to either palpation or ultrasound-guided arterial cannulation. They demonstrated a first-pass success rate of 87% in the ultrasound group compared with 50% in the landmark technique group. In the same study, the use of ultrasound was also associated with reduced time needed to establish arterial access and a 43% reduction in the development of hematoma at the insertion site.124 Levin et al. demonstrated a first-pass success rate of 62% using ultrasound versus 34% by palpation alone in 69 patients requiring intraoperative invasive hemodynamic monitoring.125 Additional randomized controlled studies have demonstrated that ultrasound guidance increases first-attempt success rates compared to traditional palpation.23,126,127
19. We recommend that providers should use real-time ultrasound guidance for femoral arterial access, which has been shown to increase first-pass success rates and reduce the risk of vascular complications.
Rationale: Although it is a less frequently used site, the femoral artery may be accessed for arterial blood sampling or invasive hemodynamic monitoring, and use of ultrasound guidance has been shown to improve the first-pass success rates of femoral artery cannulation. It is important to note that most of these studies comparing ultrasound-guided vs landmark-based femoral artery cannulation were performed in patients undergoing diagnostic or interventional vascular procedures.
A meta-analysis of randomized controlled studies comparing ultrasound-guided vs landmark-based femoral artery catheterization found use of ultrasound guidance was associated with a 49% reduction in overall complications (RR 0.51, 95% CI 0.28 to 0.91; P > .05) and 42% improvement in first-pass success rates.128 In another study, precise site selection with ultrasound was associated with fewer pseudoaneurysms in patients undergoing femoral artery cannulation by ultrasound guidance vs palpation for cardiac catheterization (3% vs 5%, P < .05).129
A multicenter randomized controlled study comparing ultrasound vs fluoroscopic guidance for femoral artery catheterization demonstrated ultrasound guidance improved rates of common femoral artery (CFA) cannulation in patients with high CFA bifurcations (83% vs 70%, P < .01).130 Furthermore, ultrasound guidance improved first-pass success rates (83% vs 46%, P < .0001), reduced number of attempts (1.3 vs 3.0, P < .0001), reduced risk of venipuncture (2.4% vs 15.8%, P < .0001), and reduced median time to obtain access (136 seconds vs148 seconds, P = .003). Vascular complications occurred in fewer patients in the ultrasound vs fluoroscopy groups (1.4% vs 3.4% P = .04). Reduced risk of hematoma formation with routine use of ultrasound guidance was demonstrated in one retrospective observational study (RR 0.62, 95% CI 0.46 to 0.84; P < .01).131
20. We recommend that providers should use real-time ultrasound guidance for radial arterial access, which has been shown to increase first-pass success rates, reduce the time to successful cannulation, and reduce the risk of complications compared with landmark-based techniques.
Rationale: Ultrasound guidance is particularly useful for radial artery cannulation in patients with altered anatomy, obesity, nonpulsatile blood flow, low perfusion, and previously unsuccessful cannulation attempts using a landmark-guided approach.132
A multicenter randomized controlled study that was not included in the abovementioned meta-analyses showed similar benefits of using ultrasound guidance vs landmarks for radial artery catheterization: a reduction in the number of attempts with ultrasound guidance (1.65 ± 1.2 vs 3.05 ± 3.4, P < .0001) and time to obtain access (88 ± 78 vs 108 ± 112 seconds, P = .006), and increased first-pass success rates (65% vs 44%, P < .0001). The use of ultrasound guidance was found to be particularly useful in patients with difficult access by palpation alone.135
Regarding the level of expertise required to use ultrasound guidance, a prospective observational study demonstrated that physicians with little previous ultrasound experience were able to improve their first-attempt success rates and procedure time for radial artery cannulation compared to historical data of landmark-based insertions.136
Postprocedure
21. We recommend that post-procedure pneumothorax should be ruled out by the detection of bilateral lung sliding using a high-frequency linear transducer before and after insertion of internal jugular and subclavian vein CVCs.
Rationale: Detection of lung sliding with two-dimensional ultrasound rules out pneumothorax, and disappearance of lung sliding in an area where it was previously seen is a strong predictor of postprocedure pneumothorax. In a study of critically ill patients, the disappearance of lung sliding was observed in 100% of patients with pneumothorax vs 8.8% of patients without pneumothorax. For detection of pneumothorax, lung sliding showed a sensitivity of 95%, specificity of 91%, and negative predictive value of 100% (P < .001).137 Another study by the same author showed that the combination of horizontal artifacts (absence of comet-tail artifact) and absence of lung sliding had a sensitivity of 100%, specificity of 96.5%, and negative predictive value of 100% for the detection of pneumothorax.138 A meta-analysis of 10 studies on the diagnostic accuracy of CVC confirmation with bedside ultrasound vs chest radiography reported detection of all 12 pneumothoraces with ultrasound, whereas chest radiography missed two pneumothoraces. The pooled sensitivity and specificity of ultrasound for the detection of pneumothorax was 100%, although an imperfect gold standard bias likely affected the results. An important advantage of bedside ultrasound is the ability to rule out pneumothorax immediately after the procedure while at the bedside. The mean time for confirmation of CVC placement with bedside ultrasound was 6 minutes versus 64 minutes and 143 minutes for completion and interpretation of a chest radiograph, respectively.139
22. We recommend that providers should use ultrasound with rapid infusion of agitated saline to visualize a right atrial swirl sign (RASS) for detecting catheter tip misplacement during CVC insertion. The use of RASS to detect the catheter tip may be considered an advanced skill that requires specific training and expertise.
Rationale: Bedside echocardiography is a reliable tool to detect catheter tip misplacement during CVC insertion. In one study, catheter misplacement was detected by bedside echocardiography with a sensitivity of 96% and specificity of 83% (positive predictive value 98%, negative predictive value 55%) and prevented distal positioning of the catheter tip.140 A prospective observational study assessed for RASS, which is turbulent flow in the right atrium after a rapid saline flush of the distal CVC port, to exclude catheter malposition. In this study with 135 CVC placements, visualization of RASS with ultrasound was able to identify all correct CVC placements and three of four catheter misplacements. Median times to complete the ultrasound exam vs CXR were 1 vs 20 minutes, respectively, with a median difference of 24 minutes (95% CI 19.6 to 29.3, P < .0001) between the two techniques.141
A prospective observational study assessed the ability of bedside transthoracic echocardiography to detect the guidewire, microbubbles, or both, in the right atrium compared to transesophageal echocardiography as the gold standard. Bedside transthoracic echocardiography allowed visualization of the right atrium in 94% of patients, and both microbubbles plus guidewire in 91% of patients.142 Hence, bedside transthoracic echocardiography allows adequate visualization of the right atrium. Another prospective observational study combining ultrasonography and contrast enhanced RASS resulted in 96% sensitivity and 93% specificity for the detection of a misplaced catheter, and the concordance with chest radiography was 96%.143
Training
23. To reduce the risk of mechanical and infectious complications, we recommend that novice providers should complete a systematic training program that includes a combination of simulation-based practice, supervised insertion on patients, and evaluation by an expert operator before attempting ultrasound-guided CVC insertion independently on patients.
Rationale: Cumulative experience has been recognized to not be a proxy for mastery of a clinical skill.144 The National Institute for Clinical Excellence (NICE) has recommended that providers performing ultrasound-guided CVC insertion should receive appropriate training to achieve competence before performing the procedure independently.7 Surveys have demonstrated that lack of training is a commonly reported barrier for not using ultrasound.145,146
Structured training programs on CVC insertion have been shown to reduce the occurrence of infectious and mechanical complications.74,143,147-149 The use of ultrasound and checklists, bundling of supplies, and practice with simulation models, as a part of a structured training program, can improve patient safety related to CVC insertion.9,140,150-154
Simulation-based practice has been used in medical education to provide deliberate practice and foster skill development in a controlled learning environment.155-158 Studies have shown transfer of skills demonstrated in a simulated environment to clinical practice, which can improve CVC insertion practices.159,160 Simulation accelerates learning of all trainees, especially novice trainees, and mitigates risks to patients by allowing trainees to achieve a minimal level of competence before attempting the procedure on real patients.152,161,162 Residents that have been trained using simulation preferentially select the IJV site,147 and more reliably use ultrasound to guide their CVC insertions.160,163
Additionally, simulation-based practice allows exposure to procedures and scenarios that may occur infrequently in clinical practice.
Although there is evidence on efficacy of simulation-based CVC training programs, there is no broadly accepted consensus on timing, duration, and content of CVC training programs for trainees or physicians in practice. The minimum recommended technical skills a trainee must master include the ability to (1) manipulate the ultrasound machine to produce a high-quality image to identify the target vessel, (2) advance the needle under direct visualization to the desired target site and depth, (3) deploy the catheter into the target vessel and confirm catheter placement in the target vessel using ultrasound, and (4) ensure the catheter has not been inadvertently placed in an unintended vessel or structure.153
A variety of simulation models are currently used to practice CVC insertion at the most common sites: the internal jugular, subclavian, basilic, and brachial veins.164,165 Effective simulation models should contain vessels that mimic normal anatomy with muscles, soft tissues, and bones. Animal tissue models, such as turkey or chicken breasts, may be effective for simulated practice of ultrasound-guided CVC insertion.166,167 Ultrasound-guided CVC training using human cadavers has also been shown to be effective.168
24. We recommend that cognitive training in ultrasound-guided CVC insertion should include basic anatomy, ultrasound physics, ultrasound machine knobology, fundamentals of image acquisition and interpretation, detection and management of procedural complications, infection prevention strategies, and pathways to attain competency.
Rationale: After receiving training in ultrasound-guided CVC insertion, physicians report significantly higher comfort with the use of ultrasound compared to those who have not received such training.145 Learners find training sessions worthwhile to increase skill levels,167 and skills learned from simulation-based mastery learning programs have been retained up to one year.158
Several commonalities have been noted across training curricula. Anatomy and physiology didactics should include vessel anatomy (location, size, and course);9 vessel differentiation by ultrasound;9,69 blood flow dynamics;69 Virchow’s triad;69 skin integrity and colonization;150 peripheral nerve identification and distribution;9 respiratory anatomy;9,69 upper and lower extremity, axillary, neck, and chest anatomy.9,69 Vascular anatomy is an essential curricular component that may help avoid preventable CVC insertion complications, such as inadvertent nerve, artery, or lung puncture.150,169 Training curricula should also include ultrasound physics (piezoelectric effect, frequency, resolution, attenuation, echogenicity, Doppler ultrasound, arterial and venous flow characteristics), image acquisition and optimization (imaging mode, focus, dynamic range, probe types), and artifacts (reverberation, mirror, shadowing, enhancement).
CVC-related infections are an important cause of morbidity and mortality in the acute and long-term care environment.69 Infection and thrombosis can both be impacted by the insertion site selection, skin integrity, and catheter–vein ratio.2,3,84 Inexperience generally leads to more insertion attempts that can increase trauma during CVC insertion and potentially increase the risk of infections.170 To reduce the risk of infectious complications, training should include important factors to consider in site selection and maintenance of a sterile environment during CVC insertion, including use of maximal sterile barrier precautions, hand hygiene, and appropriate use of skin antiseptic solutions.
Professional society guidelines have been published with recommendations of appropriate techniques for ultrasound-guided vascular access that include training recommendations.9,154 Training should deconstruct the insertion procedure into readily understood individual steps, and can be aided by demonstration of CVC insertion techniques using video clips. An alternative to face-to-face training is internet-based training that has been shown to be as effective as traditional teaching methods in some medical centers.171 Additional methods to deliver cognitive instruction include textbooks, continuing medical education courses, and digital videos.164,172
25. We recommend that trainees should demonstrate minimal competence before placing ultrasound-guided CVCs independently. A minimum number of CVC insertions may inform this determination, but a proctored assessment of competence is most important.
Rationale: CVC catheter placement carries the risk of serious complications including arterial injury or dissection, pneumothorax, or damage to other local structures; arrhythmias; catheter malposition; infection; and thrombosis. Although there is a lack of consensus and high-quality evidence for the certification of skills to perform ultrasound-guided CVC insertion, recommendations have been published advocating for formal and comprehensive training programs in ultrasound-guided CVC insertion with an emphasis on expert supervision prior to independent practice.9,153,154 Two groups of expert operators have recommended that training should include at least 8-10 supervised ultrasound-guided CVC insertions.154,173,174 A consensus task force from the World Congress of Vascular Access has recommended a minimum of six to eight hours of didactic education, four hours of hands-on training on simulation models, and six hours of hands-on ultrasound training on human volunteers to assess normal anatomy.175 This training should be followed by supervised ultrasound-guided CVC insertions until the learner has demonstrated minimal competence with a low rate of complications.35 There is general consensus that arbitrary numbers should not be the sole determinant of competence, and that the most important determinant of competence should be an evaluation by an expert operator.176
26. We recommend that didactic and hands-on training for trainees should coincide with anticipated times of increased performance of vascular access procedures. Refresher training sessions should be offered periodically.
Rationale: Simulation-based CVC training courses have shown a rapid improvement in skills, but lack of practice leads to deterioration of technical skills.161,162,177,178 Thus, a single immersive training session is insufficient to achieve and maintain mastery of skills, and an important factor to acquire technical expertise is sustained, deliberate practice with feedback.179 Furthermore, an insidious decay in skills may go unrecognized as a learner’s comfort and self-confidence does not always correlate with actual performance, leading to increased risk of errors and potential for procedural complications.147,158,180-183 Given the decay in technical skills over time, simulation-based training sessions are most effective when they occur in close temporal proximity to times when those skills are most likely to be used; for example, a simulation-based training session for trainees may be most effective just before the start of a critical care rotation.152 Regularly scheduled training sessions with monitoring and feedback by expert operators can reinforce procedural skills and prevent decay. Some experts have recommended that a minimum of 10 ultrasound-guided CVC insertions should be performed annually to maintain proficiency.153
27. We recommend that competency assessments should include formal evaluation of knowledge and technical skills using standardized assessment tools.
Rationale: Hospitalists and other healthcare providers that place vascular access catheters should undergo competency assessments proctored by an expert operator to verify that they have the required knowledge and skills.184,185 Knowledge competence can be partially evaluated using a written assessment, such as a multiple-choice test, assessing the provider’s cognitive understanding of the procedure.175 For ultrasound-guided CVC insertion, a written examination should be administered in conjunction with an ultrasound image assessment to test the learner’s recognition of normal vs abnormal vascular anatomy. Minimum passing standards should be established a priori according to local or institutional standards.
The final skills assessment should be objective, and the learner should be required to pass all critical steps of the procedure. Failure of the final skills assessment should lead to continued practice with supervision until the learner can consistently demonstrate correct performance of all critical steps. Checklists are commonly used to rate the technical performance of learners because they provide objective criteria for evaluation, can identify specific skill deficiencies, and can determine a learner’s readiness to perform procedures independently.186,187 The administration of skills assessments and feedback methods should be standardized across faculty. Although passing scores on both knowledge and skills assessments do not guarantee safe performance of a procedure independently, they provide a metric to ensure that a minimum level of competence has been achieved before allowing learners to perform procedures on patients without supervision.188
Competency assessments are a recommended component of intramural and extramural certification of skills in ultrasound-guided procedures. Intramural certification pathways differ by institution and often require additional resources including ultrasound machine(s), simulation equipment, and staff time, particularly when simulation-based assessments are incorporated into certification pathways. We recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals. However, initial and ongoing competency assessments can be performed during routine performance of procedures on patients. For an in-depth review of credentialing pathways for ultrasound-guided bedside procedures, we recommend reviewing the SHM Position Statement on Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.24
28. We recommend that competency assessments should evaluate for proficiency in the following knowledge and skills of CVC insertion:
a. Knowledge of the target vein anatomy, proper vessel identification, and recognition of anatomical variants
b. Demonstration of CVC insertion with no technical errors based on a procedural checklist
c. Recognition and management of acute complications, including emergency management of life-threatening complications
d. Real-time needle tip tracking with ultrasound and cannulation on the first attempt in at least five consecutive simulations.
Rationale: Recommendations have been published with the minimal knowledge and skills learners must demonstrate to perform ultrasound-guided vascular access procedures. These include operation of an ultrasound machine to produce high-quality images of the target vessel, tracking of the needle tip with real-time ultrasound guidance, and recognition and understanding of the management of procedural complications.154,175
First, learners must be able to perform a preprocedural assessment of the target vein, including size and patency of the vein; recognition of adjacent critical structures; and recognition of normal anatomical variants.175,189 Second, learners must be able to demonstrate proficiency in tracking the needle tip penetrating the target vessel, inserting the catheter into the target vessel, and confirming catheter placement in the target vessel with ultrasound.154,175 Third, learners must be able to demonstrate recognition of acute complications, including arterial puncture, hematoma formation, and development of pneumothorax.154,175 Trainees should be familiar with recommended evaluation and management algorithms, including indications for emergent consultation.190
29. We recommend a periodic proficiency assessments of all operators should be conducted to ensure maintenance of competency.
Rationale: Competency extends to periodic assessment and not merely an initial evaluation at the time of training.191 Periodic competency assessments should include assessment of proficiency of all providers that perform a procedure, including instructors and supervisors. Supervising providers should maintain their competency in CVC insertion through routine use of their skills in clinical practice.175 An observational study of emergency medicine residents revealed that lack of faculty comfort with ultrasound hindered the residents’ use of ultrasound.192 Thus, there is a need to examine best practices for procedural supervision of trainees because providers are often supervising procedures that they are not comfortable performing on their own.193
KNOWLEDGE GAPS
The process of producing this position statement revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for central and peripheral venous access and arterial access.
This position statement recommends a preprocedural ultrasound evaluation of blood vessels based on evidence that providers may detect anatomic anomalies, thrombosis, or vessel stenosis. Ultrasound can also reveal unsuspected high-risk structures in near proximity to the procedure site. Although previous studies have shown that providers can accurately assess vessels with ultrasound for these features, further study is needed to evaluate the effect of a standardized preprocedural ultrasound exam on clinical and procedural decision-making, as well as procedural outcomes.
Second, two ultrasound applications that are being increasingly used but have not been widely implemented are the use of ultrasound to evaluate lung sliding postprocedure to exclude pneumothorax and the verification of central line placement using a rapid infusion of agitated saline to visualize the RASS.139-141 Both of these applications have the potential to expedite postprocedure clearance of central lines for usage and decrease patient radiation exposure by obviating the need for postprocedure CXRs. Despite the supporting evidence, both of these applications are not yet widely used, as few providers have been trained in these techniques which may be considered advanced skills.
Third, despite advances in our knowledge of effective training for vascular access procedures, there is limited agreement on how to define procedural competence. Notable advancements in training include improved understanding of systematic training programs, development of techniques for proctoring procedures, definition of elements for hands-on assessments, and definition of minimum experience needed to perform vascular access procedures independently. However, application of these concepts to move learners toward independent practice remains variably interpreted at different institutions, likely due to limited resources, engrained cultures about procedures, and a lack of national standards. The development of hospitalist-based procedure services at major academic medical centers with high training standards, close monitoring for quality assurance, and the use of databases to track clinical outcomes may advance our understanding and delivery of optimal procedural training.
Finally, ultrasound technology is rapidly evolving which will affect training, techniques, and clinical outcomes in coming years. Development of advanced imaging software with artificial intelligence can improve needle visualization and tracking. These technologies have the potential to facilitate provider training in real-time ultrasound-guided procedures and improve the overall safety of procedures. Emergence of affordable, handheld ultrasound devices is improving access to ultrasound technology, but their role in vascular access procedures is yet to be defined. Furthermore, availability of wireless handheld ultrasound technology and multifrequency transducers will create new possibilities for use of ultrasound in vascular access procedures.
CONCLUSION
We have presented several evidence-based recommendations on the use of ultrasound guidance for placement of central and peripheral vascular access catheters that are intended for hospitalists and other healthcare providers who routinely perform vascular access procedures. By allowing direct visualization of the needle tip and target vessel, the use of ultrasound guidance has been shown in randomized studies to reduce needle insertion attempts, reduce needle redirections, and increase overall procedure success rates. The accuracy of ultrasound to identify the target vessel, assess for thrombosis, and detect anatomical anomalies is superior to that of physical examination. Hospitalists can attain competence in performing ultrasound-guided vascular access procedures through systematic training programs that combine didactic and hands-on training, which optimally include patient-based competency assessments.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators of Society of Hospital Medicine Point-of-care Ultrasound Task Force: Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Venkat Kalidindi, Ketino Kobaidze, Joshua Lenchus, Paul Mayo, Satyen Nichani, Vicki Noble, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Gerard Salame, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam J. Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Mathews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Mathews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El-Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Raad I. Intravascular-catheter-related infections. Lancet. 1998;351(9106):893-898. https://doi.org/10.1016/S0140-6736(97)10006-X.
2. Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286(6):700-707. https://doi.org/10.1001/jama.286.6.700.
3. Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986;146(2):259-261. https://doi.org/10.1001/archinte.146.2.259.
4. Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med. 1996;24(12):2053-2058. https://doi.org/10.1097/00003246-199612000-00020.
5. Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327(7411):361. https://doi.org/10.1136/bmj.327.7411.361.
6. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4):S1-S34. https://doi.org/10.1016/j.ajic.2011.01.003.
7. National Institute for Health and Care Excellence (NICE). Guidance on the Use of Ultrasound Locating Devices for Placing Central Venous Catheters; 2002.
8. Shojania KG, Duncan BW, McDonald KM, et al. Making health care safer: A critical analysis of patient safety practices. Evid Rep Technol Assess. Rockville, MD: Agency for Healthcare Research and Quality. 2001;43(43):i–x, 1.
9. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105-1117. https://doi.org/10.1007/s00134-012-2597-x.
10. Frykholm P, Pikwer A, Hammarskjöld F, et al. Clinical guidelines on central venous catheterisation. Swedish Society of Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiol Scand. 2014;58(5):508-524. https://doi.org/10.1111/aas.12295.
11. American Society of Anesthesiologists Task Force on Central Venous Access, Rupp SM, Apfelbaum JL, et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539-573. https://doi.org/10.1097/ALN.0b013e31823c9569.
12. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: General ultrasonography. Crit Care Med. 2015;43(11):2479-2502. https://doi.org/10.1097/CCM.0000000000001216.
13. Shekelle PG, Wachter RM, Pronovost PJ, et al. Making health care safer II: An updated critical analysis of the evidence for patient safety practices. Evid Rep Technol Assess. 2013;211:1-945.
14. Troianos CA, Hartman GS, Glas KE, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2011;24(12):1291-1318. https://doi.org/10.1016/j.echo.2011.09.021.
15. Soni NJ, Reyes LF, Keyt H, et al. Use of ultrasound guidance for central venous catheterization: a national survey of intensivists and hospitalists. J Crit Care. 2016;36:277-283. https://doi.org/10.1016/j.jcrc.2016.07.014.
16. Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220-1229. https://doi.org/10.1056/NEJMoa1500964.
17. Maizel J, Bastide MA, Richecoeur J, et al. Practice of ultrasound-guided central venous catheter technique by the French intensivists: a survey from the BoReal study group. Ann Intensive Care. 2016;6(1):76. https://doi.org/10.1186/s13613-016-0177-x.
18. Egan G, Healy D, O’Neill H, et al. Ultrasound guidance for difficult peripheral venous access: systematic review and meta-analysis. Emerg Med J. 2013;30(7):521-526. https://doi.org/10.1136/emermed-2012-201652.
19. Stolz LA, Stolz U, Howe C, Farrell IJ, Adhikari S. Ultrasound-guided peripheral venous access: a meta-analysis and systematic review. J Vasc Access. 2015;16(4):321-326. https://doi.org/10.5301/jva.5000346.
20. Sofocleous CT, Schur I, Cooper SG, et al. Sonographically guided placement of peripherally inserted central venous catheters: review of 355 procedures. AJR Am J Roentgenol. 1998;170(6):1613-1616. https://doi.org/10.2214/ajr.170.6.9609183.
21. Nichols I, Humphrey JP. The efficacy of upper arm placement of peripherally inserted central catheters using bedside ultrasound and microintroducer technique. J Infus Nurs Off Publ Infus Nurs Soc. 2008;31(3):165-176. https://doi.org/10.1097/01.NAN.0000317703.66395.b8.
22. Stokowski G, Steele D, Wilson D. The use of ultrasound to improve practice and reduce complication rates in peripherally inserted central catheter insertions. J Infus Nurs Off Publ Infus Nurs Soc. 2009;32(3):145-155. https://doi.org/10.1097/NAN.0b013e3181a1a98f.
23. Shiloh AL, Savel RH, Paulin LM, Eisen LA. Ultrasound-guided catheterization of the radial artery: a systematic review and meta-analysis of randomized controlled trials. Chest. 2011;139(3):524-529. https://doi.org/10.1378/chest.10-0919.
24. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: A position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
25. Chopra V, Flanders SA, Saint S, et al. The Michigan appropriateness guide for intravenous catheters (Magic): results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6):S1-S40. https://doi.org/10.7326/M15-0744.
26. Raad II, Hohn DC, Gilbreath BJ, et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994;15(4):231-238.
27. Slama M, Novara A, Safavian A, et al. Improvement of internal jugular vein cannulation using an ultrasound-guided technique. Intensive Care Med. 1997;23(8):916-919. https://doi.org/10.1007/s001340050432.
28. Seyahi N, Kahveci A, Altiparmak MR, Serdengecti K, Erek E. Ultrasound imaging findings of femoral veins in patients with renal failure and its impact on vascular access. Nephrol Dial Transplant. 2005;20(9):1864-1867. https://doi.org/10.1093/ndt/gfh942.
29. Lin BS, Kong CW, Tarng DC, Huang TP, Tang GJ. Anatomical variation of the internal jugular vein and its impact on temporary haemodialysis vascular access: an ultrasonographic survey in uraemic patients. Nephrol Dial Transplant. 1998;13(1):134-138. https://doi.org/10.1093/ndt/13.1.134.
30. Troianos CA, Kuwik RJ, Pasqual JR, Lim AJ, Odasso DP. Internal jugular vein and carotid artery anatomic relation as determined by ultrasonography. Anesthesiology. 1996;85(1):43-48. https://doi.org/10.1097/00000542-199607000-00007.
31. Gordon AC, Wright I, Pugh ND. Duplication of the superficial femoral vein: recognition with duplex ultrasonography. Clin Rad. 1996;51(9):622-624. https://doi.org/10.1016/S0009-9260(96)80055-9.
32. Sulek CA, Gravenstein N, Blackshear RH, Weiss L. Head rotation during internal jugular vein cannulation and the risk of carotid artery puncture. Anesth Analg. 1996;82(1):125-128. https://doi.org/10.1097/00000539-199601000-00022.
33. Shrestha BR, Gautam B. Ultrasound versus the landmark technique: a prospective randomized comparative study of internal jugular vein cannulation in an intensive care unit. JNMA J Nepal Med Assoc. 2011;51(182):56-61. https://doi.org/10.31729/jnma.148.
34. Brederlau J, Greim C, Schwemmer U, et al. Ultrasound-guided cannulation of the internal jugular vein in critically ill patients positioned in 30 degrees dorsal elevation. Eur J Anaesthesiol. 2004;21(9):684-687. https://doi.org/10.1097/00003643-200409000-00003.
35. Lamperti M, Subert M, Cortellazzi P, et al. Is a neutral head position safer than 45-degree neck rotation during ultrasound-guided internal jugular vein cannulation? Results of a randomized controlled clinical trial. Anesth Analg. 2012;114(4):777-784. https://doi.org/10.1213/ANE.0b013e3182459917.
36. Benter T, Teichgräber UK, Klühs L, et al. Anatomical variations in the internal jugular veins of cancer patients affecting central venous access. Anatomical variation of the internal jugular vein. Ultraschall Med. 2001;22(1):23-26. https://doi.org/10.1055/s-2001-11243.
37. Caridi JG, Hawkins IF, Jr, Wiechmann BN, Pevarski DJ, Tonkin JC. Sonographic guidance when using the right internal jugular vein for central vein access. AJR Am J Roentgenol. 1998;171(5):1259-1263. https://doi.org/10.2214/ajr.171.5.9798857.
38. Brusasco C, Corradi F, Zattoni PL, et al. Ultrasound-guided central venous cannulation in bariatric patients. Obes Surg. 2009;19(10):1365-1370. https://doi.org/10.1007/s11695-009-9902-y.
39. Armstrong PJ, Cullen M, Scott DH. The ‘SiteRite’ ultrasound machine--an aid to internal jugular vein cannulation. Anaesthesia. 1993;48(4):319-323. https://doi.org/10.1111/j.1365-2044.1993.tb06953.x.
40. Forauer AR, Glockner JF. Importance of US findings in access planning during jugular vein hemodialysis catheter placements. J Vasc Interv Rad. 2000;11(2 Pt 1):233-238. https://doi.org/10.1016/S1051-0443(07)61471-7.
41. Hassan C, Girishkumar HT, Thatigotla B, et al. Value of ultrasound guidance in placement of hemodialysis access catheters in patients with end-stage renal disease. Am Surg. 2008;74(11):1111-1113.
42. Tan CO, Weinberg L, Peyton P, Story D, McNicol L. Size variation between contralateral infraclavicular axillary veins within individual patients-implications for subclavian venous central line insertion. Crit Care Med. 2013;41(2):457-463. https://doi.org/10.1097/CCM.0b013e31826ab1dd.
43. Wu SY, Ling Q, Cao LH, et al. Real-time two-dimensional ultrasound guidance for central venous cannulation: a meta-analysis. Anesthesiology. 2013;118(2):361-375. https://doi.org/10.1097/ALN.0b013e31827bd172.
44. Kim W, Chung RK, Lee GY, Han JI. The effects of hip abduction with external rotation and reverse Trendelenburg position on the size of the femoral vein; ultrasonographic investigation. Korean J Anesthesiol. 2011;61(3):205-209. https://doi.org/10.4097/kjae.2011.61.3.205.
45. Denys BG, Uretsky BF. Anatomical variations of internal jugular vein location: impact on central venous access. Crit Care Med. 1991;19(12):1516-1519. https://doi.org/10.1097/00003246-199112000-00013.
46. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev. 2015;1:CD006962. https://doi.org/10.1002/14651858.CD006962.pub2.
47. Gilbert TB, Seneff MG, Becker RB. Facilitation of internal jugular venous cannulation using an audio-guided Doppler ultrasound vascular access device: results from a prospective, dual-center, randomized, crossover clinical study. Crit Care Med. 1995;23(1):60-65. https://doi.org/10.1097/00003246-199501000-00012.
48. Schummer W, Schummer C, Tuppatsch H, et al. Ultrasound-guided central venous cannulation: is there a difference between Doppler and B-mode ultrasound? J Clin Anesth. 2006;18(3):167-172. https://doi.org/10.1016/j.jclinane.2005.12.010.
49. Airapetian N, Maizel J, Langelle F, et al. Ultrasound-guided central venous cannulation is superior to quick-look ultrasound and landmark methods among inexperienced operators: a prospective randomized study. Intensive Care Med. 2013;39(11):1938-1944. https://doi.org/10.1007/s00134-013-3072-z.
50. Milling TJ, Jr, Rose J, Briggs WM, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Crit Care Med. 2005;33(8):1764-1769. https://doi.org/10.1097/01.ccm.0000171533.92856.e5.
51. Beaudoin FL, Merchant RC, Lincoln J, et al. Bedside ultrasonography detects significant femoral vessel overlap: implications for central venous cannulation. CJEM. 2011;13(4):245-250. https://doi.org/10.2310/8000.2011.110482.
52. Kwon TH, Kim YL, Cho DK. Ultrasound-guided cannulation of the femoral vein for acute haemodialysis access. Nephrol Dial Transplant. 1997;12(5):1009-1012. https://doi.org/10.1093/ndt/12.5.1009.
53. Rothschild JM. Ultrasound guidance of central vein catheterization. Evid Rep Technol Assess. 2001;43. Chapter 21.: http://archive.ahrq.gov/clinic/ptsafety/chap21.htm.
54. Lennon M, Zaw NN, Pöpping DM, Wenk M. Procedural complications of central venous catheter insertion. Minerva Anestesiol. 2012;78(11):1234-1240.
55. Blaivas M, Adhikari S. An unseen danger: frequency of posterior vessel wall penetration by needles during attempts to place internal jugular vein central catheters using ultrasound guidance. Crit Care Med. 2009;37(8):2345-2349; quiz 2359. https://doi.org/10.1097/CCM.0b013e3181a067d4.
56. Vogel JA, Haukoos JS, Erickson CL, et al. Is long-axis view superior to short-axis view in ultrasound-guided central venous catheterization? Crit Care Med. 2015;43(4):832-839. https://doi.org/10.1097/CCM.0000000000000823.
57. Tammam TF, El-Shafey EM, Tammam HF. Ultrasound-guided internal jugular vein access: comparison between short axis and long axis techniques. Saudi J Kidney Dis Transpl. 2013;24(4):707-713. https://doi.org/10.4103/1319-2442.113861.
58. Keyes LE, Frazee BW, Snoey ER, Simon BC, Christy D. Ultrasound-guided brachial and basilic vein cannulation in emergency department patients with difficult intravenous access. Ann Emerg Med. 1999;34(6):711-714. https://doi.org/10.1016/S0196-0644(99)70095-8.
59. Mahler SA, Wang H, Lester C, et al. Short- vs long-axis approach to ultrasound-guided peripheral intravenous access: a prospective randomized study. Am J Emerg Med. 2011;29(9):1194-1197. https://doi.org/10.1016/j.ajem.2010.07.015.
60. Berk D, Gurkan Y, Kus A, et al. Ultrasound-guided radial arterial cannulation: long axis/in-plane versus short axis/out-of-plane approaches? J Clin Monit Comput. 2013;27(3):319-324. https://doi.org/10.1007/s10877-013-9437-6.
61. Batllori M, Urra M, Uriarte E, et al. Randomized comparison of three transducer orientation approaches for ultrasound guided internal jugular venous cannulation. Br J Anaesth. 2016;116(3):370-376. https://doi.org/10.1093/bja/aev399.
62. Moak JH, Lyons MS, Wright SW, Lindsell CJ. Needle and guidewire visualization in ultrasound-guided internal jugular vein cannulation. Am J Emerg Med. 2011;29(4):432-436. https://doi.org/10.1016/j.ajem.2010.01.004.
63. Moak JH, Rajkumar JS, Woods WA. The wire is really easy to see (WIRES): sonographic visualization of the guidewire by novices. CJEM. 2013;15(1):18-23. https://doi.org/10.2310/8000.2012.120800.
64. Gillman LM, Blaivas M, Lord J, Al-Kadi A, Kirkpatrick AW. Ultrasound confirmation of guidewire position may eliminate accidental arterial dilatation during central venous cannulation. Scand J Trauma Resusc Emerg Med. 2010;18:39. https://doi.org/10.1186/1757-7241-18-39.
65. Augoustides JG, Horak J, Ochroch AE, et al. A randomized controlled clinical trial of real-time needle-guided ultrasound for internal jugular venous cannulation in a large university anesthesia department. J Cardiothorac Vasc Anesth. 2005;19(3):310-315. https://doi.org/10.1053/j.jvca.2005.03.007.
66. Maecken T, Heite L, Wolf B, Zahn PK, Litz RJ. Ultrasound-guided catheterisation of the subclavian vein: freehand vs needle-guided technique. Anaesthesia. 2015;70(11):1242-1249. https://doi.org/10.1111/anae.13187.
67. Stone MB, Nagdev A, Murphy MC, Sisson CA. Ultrasound detection of guidewire position during central venous catheterization. Am J Emerg Med. 2010;28(1):82-84. https://doi.org/10.1016/j.ajem.2008.09.019.
68. Luyet C, Hartwich V, Urwyler N, et al. Evaluation of a novel needle guide for ultrasound-guided phantom vessel cannulation. Anaesthesia. 2011;66(8):715-720. https://doi.org/10.1111/j.1365-2044.2011.06781.x.
69. O’Grady NP, Alexander M, Burns LA, et al. Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis. 2011;52(9):1087-1099. https://doi.org/10.1093/cid/cir138.
70. Southworth SL, Henman LJ, Kinder LA, Sell JL. The journey to zero central catheter-associated bloodstream infections: culture change in an intensive care unit. Crit Care Nurse. 2012;32(2):49-54. https://doi.org/10.4037/ccn2012915.
71. Guerin K, Wagner J, Rains K, Bessesen M. Reduction in central line-associated bloodstream infections by implementation of a postinsertion care bundle. Am J Infect Control. 2010;38(6):430-433. https://doi.org/10.1016/j.ajic.2010.03.007.
72. Hartman N, Wittler M, Askew K, Manthey D. Delphi method validation of a procedural performance checklist for insertion of an ultrasound-guided internal jugular central line. Am J Med Qual Off J Am Coll Med Qual. 2016;31(1):81-85. https://doi.org/10.1177/1062860614549762.
73. Lenchus JD, Carvalho CM, Ferreri K, et al. Filling the void: defining invasive bedside procedural competency for internal medicine residents. J Grad Med Educ. 2013;5(4):605-612. https://doi.org/10.4300/JGME-D-13-00030.1.
74. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. https://doi.org/10.1001/archinternmed.2009.215.
75. Karakitsos D, Labropoulos N, De Groot E, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care. 2006;10(6):R162. https://doi.org/10.1186/cc5101.
76. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. https://doi.org/10.1097/01.ccm.0000142399.70913.2f.
77. Young EM, Commiskey ML, Wilson SJ. Translating evidence into practice to prevent central venous catheter-associated bloodstream infections: a systems-based intervention. Am J Infect Control. 2006;34(8):503-506. https://doi.org/10.1016/j.ajic.2006.03.011.
78. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495; discussion 495. https://doi.org/10.1016/j.surg.2008.06.004.
79. Mermel LA, McCormick RD, Springman SR, Maki DG. The pathogenesis and epidemiology of catheter-related infection with pulmonary artery swan-Ganz catheters: a prospective study utilizing molecular subtyping. Am J Med. 1991;91(3b):197S–205S. https://doi.org/10.1016/0002-9343(91)90369-9.
80. Marschall J, Mermel LA, Classen D, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(1):S22-S30. https://doi.org/10.1086/591059.
81. Marschall J, Mermel LA, Fakih M, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(7):753-771. https://doi.org/10.1086/676533.
82. Garcia-Rodriguez JF, Álvarez-Díaz H, Vilariño-Maneiro L, et al. Epidemiology and impact of a multifaceted approach in controlling central venous catheter associated blood stream infections outside the intensive care unit. BMC Infect Dis. 2013;13:445. https://doi.org/10.1186/1471-2334-13-445.
83. Lee DH, Jung KY, Choi YH. Use of maximal sterile barrier precautions and/or antimicrobial-coated catheters to reduce the risk of central venous catheter-related bloodstream infection. Infect Control Hosp Epidemiol. 2008;29(10):947-950. https://doi.org/10.1086/590356.
84. Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132(5):391-402. https://doi.org/10.7326/0003-4819-132-5-200003070-00009.
85. Troianos CA, Jobes DR, Ellison N. Ultrasound-guided cannulation of the internal jugular vein. A prospective, randomized study. Anesth Analg. 1991;72(6):823-826. https://doi.org/10.1213/00000539-199106000-00020.
86. Troianos CA, Savino JS. Internal jugular vein cannulation guided by echocardiography. Anesthesiology. 1991;74(4):787-789. https://doi.org/10.1097/00000542-199104000-00026.
87. Denys BG, Uretsky BF, Reddy PS, et al. An ultrasound method for safe and rapid central venous access. N Engl J Med. 1991;324(8):566. https://doi.org/10.1056/NEJM199102213240816.
88. Riaz A, Shan Khan RA, Salim F. Ultrasound guided internal jugular venous cannulation: comparison with land-mark technique. J Coll Phys Surg Pak. 2015;25(5):315-319. https://doi.org/05.2015/JCPSP.315319.
89. Gok F, Kilicaslan A, Sarkilar G, Kandemir B, Yosunkaya A. The effect of ultrasound guidance on central venous catheter-associated bloodstream infection in critical care patients. Acta Med Mediterr. 2013;29:677-682.
90. Lamperti M, Cortellazzi P, D’Onofrio G, et al. An outcome study on complications using routine ultrasound assistance for internal jugular vein cannulation. Acta Anaesthesiol Scand. 2007;51(10):1327-1330. https://doi.org/10.1111/j.1399-6576.2007.01442.x.
91. Vezzani A, Manca T, Vercelli A, Braghieri A, Magnacavallo A. Ultrasonography as a guide during vascular access procedures and in the diagnosis of complications. J Ultrasound. 2013;16(4):161-170. https://doi.org/10.1007/s40477-013-0046-5.
92. Palepu GB, Deven J, Subrahmanyam M, Mohan S. Impact of ultrasonography on central venous catheter insertion in intensive care. Indian J Rad Imaging. 2009;19(3):191-198. https://doi.org/10.4103/0971-3026.54877.
93. Agarwal A, Singh DK, Singh AP. Ultrasonography: a novel approach to central venous cannulation. Indian J Crit Care Med. 2009;13(4):213-216. https://doi.org/10.4103/0972-5229.60174.
94. Wong SW, Niazi AU, Chin KJ, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS® needle tracking system: a case report. Can J Anaesth. 2013;60(1):50-53. https://doi.org/10.1007/s12630-012-9809-2.
95. Bouaziz H, Zetlaoui PJ, Pierre S, et al. Guidelines on the use of ultrasound guidance for vascular access. Anaesth, Crit Care Pain Med. 2015;34(1):65-69. https://doi.org/10.1016/j.accpm.2015.01.004.
96. Jenssen C, Brkljacic B, Hocke M, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part VI - Ultrasound-guided vascular interventions. Ultraschall Med. 2016;37(5):473-476. https://doi.org/10.1055/s-0035-1553450.
97. Rando K, Castelli J, Pratt JP, et al. Ultrasound-guided internal jugular vein catheterization: a randomized controlled trial. Heart Lung Vessels. 2014;6(1):13-23.
98. Mey U, Glasmacher A, Hahn C, et al. Evaluation of an ultrasound-guided technique for central venous access via the internal jugular vein in 493 patients. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2003;11(3):148-155. https://doi.org/10.1007/s00520-002-0399-3.
99. Singh SA, Sharma S, Singh A, et al. The safety of ultrasound guided central venous cannulation in patients with liver disease. Saudi J Anaesth. 2015;9(2):155-160. https://doi.org/10.4103/1658-354X.152842.
100. Akoglu H, Piskinpasa S, Yenigun EC, et al. Real-time ultrasound guided placement of temporary internal jugular vein catheters: assessment of technical success and complication rates in nephrology practice. Nephrol (Carlton). 2012;17(7):603-606. https://doi.org/10.1111/j.1440-1797.2012.01637.x.
101. Sadler DJ, Gordon AC, Klassen J, et al. Image-guided central venous catheters for apheresis. Bone Marrow Transplant. 1999;23(2):179-182. https://doi.org/10.1038/sj.bmt.1701545.
102. Calvert N, Hind D, McWilliams R, et al. Ultrasound for central venous cannulation: economic evaluation of cost-effectiveness. Anaesthesia. 2004;59(11):1116-1120. https://doi.org/10.1111/j.1365-2044.2004.03906.x.
103. Goetz AM, Wagener MM, Miller JM, Muder RR. Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infect Control Hosp Epidemiol. 1998;19(11):842-845.
104. Bertini P, Frediani M. Ultrasound guided supraclavicular central vein cannulation in adults: a technical report. J Vasc Access. 2013;14(1):89-93. https://doi.org/10.5301/jva.5000088.
105. Lalu MM, Fayad A, Ahmed O, et al. Ultrasound-guided subclavian vein catheterization: A systematic review and meta-analysis. Crit Care Med. 2015;43(7):1498-1507. https://doi.org/10.1097/CCM.0000000000000973.
106. Milone M, Di Minno G, Di Minno MN, et al. The real effectiveness of ultrasound guidance in subclavian venous access. Ann ital chir. 2010;81(5):331-334.
107. O’Leary R, Ahmed SM, McLure H, et al. Ultrasound-guided infraclavicular axillary vein cannulation: a useful alternative to the internal jugular vein. Br J Anaesth. 2012;109(5):762-768. https://doi.org/10.1093/bja/aes262.
108. Gualtieri E, Deppe SA, Sipperly ME, Thompson DR. Subclavian venous catheterization: greater success rate for less experienced operators using ultrasound guidance. Crit Care Med. 1995;23(4):692-697. https://doi.org/10.1097/00003246-199504000-00018.
109. Fragou M, Gravvanis A, Dimitriou V, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med. 2011;39(7):1607-1612. https://doi.org/10.1097/CCM.0b013e318218a1ae.
110. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015;1:CD011447. https://doi.org/10.1002/14651858.CD011447.
111. Robinson MK, Mogensen KM, Grudinskas GF, Kohler S, Jacobs DO. Improved care and reduced costs for patients requiring peripherally inserted central catheters: the role of bedside ultrasound and a dedicated team. JPEN J Parenter Enter Nutr. 2005;29(5):374-379. https://doi.org/10.1177/0148607105029005374.
112. Li J, Fan YY, Xin MZ, et al. A randomised, controlled trial comparing the long-term effects of peripherally inserted central catheter placement in chemotherapy patients using B-mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94-103. https://doi.org/10.1016/j.ejon.2013.08.003.
113. Schweickert WD, Herlitz J, Pohlman AS, et al. A randomized, controlled trial evaluating postinsertion neck ultrasound in peripherally inserted central catheter procedures. Crit Care Med. 2009;37(4):1217-1221. https://doi.org/10.1097/CCM.0b013e31819cee7f.
114. Mahler SA, Wang H, Lester C, Conrad SA. Ultrasound-guided peripheral intravenous access in the emergency department using a modified Seldinger technique. J Emerg Med. 2010;39(3):325-329. https://doi.org/10.1016/j.jemermed.2009.02.013.
115. Brannam L, Blaivas M, Lyon M, Flake M. Emergency nurses’ utilization of ultrasound guidance for placement of peripheral intravenous lines in difficult-access patients. Acad Emerg Med. 2004;11(12):1361-1363. https://doi.org/10.1197/j.aem.2004.08.027.
116. Au A, Rotte M, Gryzbowski R, Ku B, Fields J. 157 Decrease in central venous catheter placement and complications due to utilization of ultrasound-guided peripheral intravenous catheters. Ann Emerg Med. 2011;58(4):S230. https://doi.org/10.1016/j.annemergmed.2011.06.185.
117. Dargin JM, Rebholz CM, Lowenstein RA, Mitchell PM, Feldman JA. Ultrasonography-guided peripheral intravenous catheter survival in ED patients with difficult access. Am J Emerg Med. 2010;28(1):1-7. https://doi.org/10.1016/j.ajem.2008.09.001.
118. Bauman M, Braude D, Crandall C. Ultrasound-guidance vs standard technique in difficult vascular access patients by ED technicians. Am J Emerg Med. 2009;27(2):135-140. https://doi.org/10.1016/j.ajem.2008.02.005.
119. Schoenfeld E, Shokoohi H, Boniface K. Ultrasound-guided peripheral intravenous access in the emergency department: patient-centered survey. West J Emerg Med. 2011;12(4):475-477. https://doi.org/10.5811/westjem.2011.3.1920.
120. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. https://doi.org/10.7326/0003-4819-114-10-845.
121. Miliani K, Taravella R, Thillard D, et al. Peripheral venous catheter-related adverse events: evaluation from a multicentre epidemiological study in France (the CATHEVAL Project). PLOS ONE. 2017;12(1):e0168637. https://doi.org/10.1371/journal.pone.0168637.
122. Gregg SC, Murthi SB, Sisley AC, Stein DM, Scalea TM. Ultrasound-guided peripheral intravenous access in the intensive care unit. J Crit Care. 2010;25(3):514-519. https://doi.org/10.1016/j.jcrc.2009.09.003.
123. Adhikari S, Blaivas M, Morrison D, Lander L. Comparison of infection rates among ultrasound-guided versus traditionally placed peripheral intravenous lines. J Ultrasound Med. 2010;29(5):741-747. https://doi.org/10.7863/jum.2010.29.5.741.
124. Shiver S, Blaivas M, Lyon M. A prospective comparison of ultrasound-guided and blindly placed radial arterial catheters. Acad Emerg Med. 2006;13(12):1275-1279. https://doi.org/10.1197/j.aem.2006.07.015.
125. Levin PD, Sheinin O, Gozal Y. Use of ultrasound guidance in the insertion of radial artery catheters. Crit Care Med. 2003;31(2):481-484. https://doi.org/10.1097/01.CCM.0000050452.17304.2F.
126. Gu WJ, Tie HT, Liu JC, Zeng XT. Efficacy of ultrasound-guided radial artery catheterization: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2014;18(3):R93. https://doi.org/10.1186/cc13862.
127. Tang L, Wang F, Li Y, et al. Ultrasound guidance for radial artery catheterization: an updated meta-analysis of randomized controlled trials. PLOS ONE. 2014;9(11):e111527. https://doi.org/10.1371/journal.pone.0111527.
128. Sobolev M, Slovut DP, Lee Chang A, Shiloh AL, Eisen LA. Ultrasound-guided catheterization of the femoral artery: A systematic review and meta-analysis of randomized controlled trials. J Invas Cardiol. 2015;27(7):318-323. https://doi.org/10.1378/chest.1991181.
129. Gabriel M, Pawlaczyk K, Waliszewski K, Krasiński Z, Majewski W. Location of femoral artery puncture site and the risk of postcatheterization pseudoaneurysm formation. Int J Cardiol. 2007;120(2):167-171. https://doi.org/10.1016/j.ijcard.2006.09.018.
130. Seto AH, Abu-Fadel MS, Sparling JM, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With ultrasound Trial). JACC Cardiovasc Interv. 2010;3(7):751-758. https://doi.org/10.1016/j.jcin.2010.04.015.
131. Kalish J, Eslami M, Gillespie D, et al. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg. 2015;61(5):1231-1238. https://doi.org/10.1016/j.jvs2014.12.003.
132. Sandhu NS, Patel B. Use of ultrasonography as a rescue technique for failed radial artery cannulation. J Clin Anesth. 2006;18(2):138-141. https://doi.org/10.1016/j.jclinane.2005.06.011.
133. White L, Halpin A, Turner M, Wallace L. Ultrasound-guided radial artery cannulation in adult and paediatric populations: a systematic review and meta-analysis. Br J Anaesth. 2016;116(5):610-617. https://doi.org/10.1093/bja/aew097.
134. Gao YB, Yan JH, Gao FQ, et al. Effects of ultrasound-guided radial artery catheterization: an updated meta-analysis. Am J Emerg Med. 2015;33(1):50-55. https://doi.org/10.1016/j.ajem.2014.10.008.
135. Seto AH, Roberts JS, Abu-Fadel MS, et al. Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery Access with Ultrasound Trial). JACC Cardiovasc Interv. 2015;8(2):283-291. https://doi.org/10.1016/j.jcin.2014.05.036.
136. Roberts J, Manur R. Ultrasound-guided radial artery access by a non-ultrasound trained interventional cardiologist improved first-attempt success rates and shortened time for successful radial artery cannulation. J Invas Cardiol. 2013;25(12):676-679.
137. Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108(5):1345-1348. https://doi.org/10.1378/chest.108.5.1345.
138. Lichtenstein D, Mezière G, Biderman P, Gepner A. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999;25(4):383-388. https://doi.org/10.1007/s001340050862.
139. Ablordeppey EA, Drewry AM, Beyer AB, et al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound Versus chest radiography in critically ill patients: A systematic review and meta-analysis. Crit Care Med. 2017;45(4):715-724. https://doi.org/10.1097/CCM.0000000000002188.
140. Bedel J, Vallée F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013;39(11):1932-1937. https://doi.org/10.1007/s00134-013-3097-3.
141. Weekes AJ, Keller SM, Efune B, Ghali S, Runyon M. Prospective comparison of ultrasound and CXR for confirmation of central vascular catheter placement. Emerg Med J EMJ. 2016;33(3):176-180. https://doi.org/10.1136/emermed-2015-205000.
142. Arellano R, Nurmohamed A, Rumman A, et al. The utility of transthoracic echocardiography to confirm central line placement: an observational study. Can J Anaesth. 2014;61(4):340-346. https://doi.org/10.1007/s12630-014-0111-3.
143. Vezzani A, Brusasco C, Palermo S, et al. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38(2):533-538. https://doi.org/10.1097/CCM.0b013e3181c0328f.
144. Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142(4):260-273. https://doi.org/10.7326/0003-4819-142-4-200502150-00008.
145. Backlund BH, Hopkins E, Kendall JL. Ultrasound guidance for central venous access by emergency physicians in Colorado. West J Emerg Med. 2012;13(4):320-325. https://doi.org/10.5811/westjem.2011.11.6821.
146. Buchanan MS, Backlund B, Liao MM, et al. Use of ultrasound guidance for central venous catheter placement: survey from the American Board of Emergency Medicine Longitudinal Study of Emergency Physicians. Acad Emerg Med. 2014;21(4):416-421. https://doi.org/10.1111/acem.12350.
147. Barsuk JH, McGaghie WC, Cohen ER, O’Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697-2701. https://doi.org/10.1097/00003246-200910000-00003.
148. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. https://doi.org/10.1097/00003246-200201000-00009.
149. Woo MY, Frank J, Lee AC, et al. Effectiveness of a novel training program for emergency medicine residents in ultrasound-guided insertion of central venous catheters. CJEM. 2009;11(4):343-348. https://doi.org/10.1017/S1481803500011398.
150. McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348(12):1123-1133. https://doi.org/10.1056/NEJMra011883.
151. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. https://doi.org/10.1002/jhm.468.
152. Sekiguchi H, Tokita JE, Minami T, et al. A prerotational, simulation-based workshop improves the safety of central venous catheter insertion: results of a successful internal medicine house staff training program. Chest. 2011;140(3):652-658. https://doi.org/10.1378/chest.10-3319.
153. Feller-Kopman D. Ultrasound-guided internal jugular access: a proposed standardized approach and implications for training and practice. Chest. 2007;132(1):302-309. https://doi.org/10.1378/chest.06-2711.
154. Troianos CA, Hartman GS, Glas KE, et al. Special articles: guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg. 2012;114(1):46-72. https://doi.org/10.1213/ANE.0b013e3182407cd8.
155. Issenberg SB, McGaghie WC, Hart IR, et al. Simulation technology for health care professional skills training and assessment. JAMA. 1999;282(9):861-866. https://doi.org/10.1001/jama.282.9.861.
156. Millington SJ, Wong RY, Kassen BO, Roberts JM, Ma IW. Improving internal medicine residents’ performance, knowledge, and confidence in central venous catheterization using simulators. J Hosp Med. 2009;4(7):410-416. https://doi.org/10.1002/jhm.570.
157. Beaulieu Y, Laprise R, Drolet P, et al. Bedside ultrasound training using web-based e-learning and simulation early in the curriculum of residents. Crit Ultrasound J. 2015;7:1. https://doi.org/10.1186/s13089-014-0018-9.
158. Barsuk JH, Cohen ER, McGaghie WC, Wayne DB. Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med. 2010;85(10 Suppl):S9-S12. https://doi.org/10.1097/ACM.0b013e3181ed436c.
159. Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133(1):56-61. https://doi.org/10.1378/chest.07-0131.
160. Evans LV, Dodge KL, Shah TD, et al. Simulation training in central venous catheter insertion: improved performance in clinical practice. Acad Med. 2010;85(9):1462-1469. https://doi.org/10.1097/ACM.0b013e3181eac9a3.
161. Smith CC, Huang GC, Newman LR, et al. Simulation training and its effect on long-term resident performance in central venous catheterization. Simul Healthc J Soc Simul Healthc. 2010;5(3):146-151. https://doi.org/10.1097/SIH.0b013e3181dd9672.
162. Laack TA, Dong Y, Goyal DG, et al. Short-term and long-term impact of the central line workshop on resident clinical performance during simulated central line placement. Simul Healthc J Soc Simul Healthc. 2014;9(4):228-233. https://doi.org/10.1097/SIH.0000000000000015.
163. Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31(10):1519-1526. https://doi.org/10.7863/jum.2012.31.10.1519.
164. Bayci AW, Mangla J, Jenkins CS, Ivascu FA, Robbins JM. Novel educational module for subclavian central venous catheter insertion using real-time ultrasound guidance. J Surg Educ. 2015;72(6):1217-1223. https://doi.org/10.1016/j.jsurg.2015.07.010.
165. Andreatta P, Chen Y, Marsh M, Cho K. Simulation-based training improves applied clinical placement of ultrasound-guided PICCs. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2011;19(4):539-543. https://doi.org/10.1007/s00520-010-0849-2.
166. Rosen BT, Uddin PQ, Harrington AR, Ault BW, Ault MJ. Does personalized vascular access training on a nonhuman tissue model allow for learning and retention of central line placement skills? Phase II of the procedural patient safety initiative (PPSI-II). J Hosp Med. 2009;4(7):423-429. https://doi.org/10.1002/jhm.571.
167. Ault MJ, Rosen BT, Ault B. The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative. J Gen Intern Med. 2006;21(5):514-517. https://doi.org/10.1111/j.1525-1497.2006.00440.x.
168. Varga S, Smith J, Minneti M, et al. Central venous catheterization using a perfused human cadaveric model: application to surgical education. J Surg Educ. 2015;72(1):28-32. https://doi.org/10.1016/j.jsurg.2014.07.005.
169. Sansivero GE. Venous anatomy and physiology. Considerations for vascular access device placement and function. J Intraven Nurs Off Publ Intraven Nurs Soc. 1998;21(5 Suppl):S107-S114.
170. Eisen LA, Narasimhan M, Berger JS, et al. Mechanical complications of central venous catheters. Journal of intensive care medicine. 2006;21(1):40-46. https://doi.org/10.1177/0885066605280884.
171. Chenkin J, Lee S, Huynh T, Bandiera G. Procedures can be learned on the Web: a randomized study of ultrasound-guided vascular access training. Acad Emerg Med. 2008;15(10):949-954. https://doi.org/10.1111/j.1553-2712.2008.00231.x.
172. Abualenain J, Calabrese K, Tansek R, Ranniger C. 319 Comparing standard versus video-based teaching for ultrasound-guided internal jugular central venous catheter access for fourth-year medical students. Ann Emerg Med. 2014;64(4):S113. https://doi.org/10.1016/j.annemergmed.2014.07.347.
173. Pustavoitau A, Blaivas M, Brown SM, et al. Recommendations for achieving and maintaining competence and credentialing in critical care ultrasound with focused cardiac ultrasound and advanced critical care echocardiography. Crit Care Med. 2016.
174. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: A survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
175. Moureau N, Lamperti M, Kelly LJ, et al. Evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training. Br J Anaesth. 2013;110(3):347-356. https://doi.org/10.1093/bja/aes499.
176. Ernst A, Silvestri GA, Johnstone D, American College of Chest Physicians. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123(5):1693-1717. https://doi.org/10.1378/chest.123.5.1693.
177. Thomas SM, Burch W, Kuehnle SE, et al. Simulation training for pediatric residents on central venous catheter placement: a pilot study. Pediatr Crit Care Med J Soc Crit Care Med.. 2013;14(9):e416-e423. https://doi.org/10.1097/PCC.0b013e31829f5eda.
178. Smith KK, Gilcreast D, Pierce K. Evaluation of staff’s retention of ACLS and BLS skills. Resuscitation. 2008;78(1):59-65. https://doi.org/10.1016/j.resuscitation.2008.02.007.
179. Ericsson KA. Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med. 2004;79(10 Suppl):S70-S81. https://doi.org/10.1097/00001888-200410001-00022.
180. Gerard JM, Thomas SM, Germino KW, et al. The effect of simulation training on PALS skills among family medicine residents. Fam Med. 2011;43(6):392-399.
181. Brydges R, Nair P, Ma I, Shanks D, Hatala R. Directed self-regulated learning versus instructor-regulated learning in simulation training. Med Educ. 2012;46(7):648-656. https://doi.org/10.1111/j.1365-2923.2012.04268.x.
182. Wayne DB, Butter J, Siddall VJ, et al. Simulation-based training of internal medicine residents in advanced cardiac life support protocols: a randomized trial. Teach Learn Med. 2005;17(3):210-216. https://doi.org/10.1207/s15328015tlm1703_3.
183. Arthur Jr. W, Bennett Jr. W, Stanush PL, McNelly TL. Factors that influence skill decay and retention: A quantitative review and analysis. Hum Perform. 1998;11(1):57-101. https://doi.org/10.1207/s15327043hup1101_3.
184. Rusche JD, Besuner P, Partusch SK, Berning PA. Competency program development across a merged healthcare network. J Nurs Staff Dev. 2001;17(5):234-240; quiz 241-232. https://doi.org/10.1097/00124645-200109000-00004.
185. O’Hearne Rebholz M. A review of methods to assess competency. J Nurs Staff Dev. 2006;22(5):241-245. https://doi.org/10.1097/00124645-200609000-00007.
186. Reznick RK. Teaching and testing technical skills. Am J Surg. 1993;165(3):358-361. https://doi.org/10.1016/s0002-9610(05)80843-8.
187. Reznick RK, MacRae H. Teaching surgical skills--changes in the wind. N Engl J Med. 2006;355(25):2664-2669. https://doi.org/10.1056/NEJMra054785.
188. Murin S, Stollenwerk NS. Simulation in procedural training: at the tipping point. Chest. 2010;137(5):1009-1011. https://doi.org/10.1378/chest.10-0199.
189. American College of Emergency Physicians. Emergency ultrasound guidelines. Ann Emerg Med. 2009;53(4):550-570. https://doi.org/10.1016/j.annemergmed.2008.12.013.
190. Guilbert MC, Elkouri S, Bracco D, et al. Arterial trauma during central venous catheter insertion: case series, review and proposed algorithm. J Vasc Surg. 2008;48(4):918-925; discussion 925. https://doi.org/10.1016/j.jvs2008.04.046.
191. Dong Y, Suri HS, Cook DA, et al. Simulation-based objective assessment discerns clinical proficiency in central line placement: a construct validation. Chest. 2010;137(5):1050-1056. https://doi.org/10.1378/chest.09-1451.
192. Adhikari S, Theodoro D, Raio C, et al. Central venous catheterization: are we using ultrasound guidance? J Ultrasound Med. 2015;34(11):2065-2070. https://doi.org/10.7863/ultra.15.01027.
193. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors--procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z.
Approximately five million central venous catheters (CVCs) are inserted in the United States annually, with over 15 million catheter days documented in intensive care units alone.1 Traditional CVC insertion techniques using landmarks are associated with a high risk of mechanical complications, particularly pneumothorax and arterial puncture, which occur in 5%-19% patients.2,3
Since the 1990s, several randomized controlled studies and meta-analyses have demonstrated that the use of real-time ultrasound guidance for CVC insertion increases procedure success rates and decreases mechanical complications.4,5 Use of real-time ultrasound guidance was recommended by the Agency for Healthcare Research and Quality, the Institute of Medicine, the National Institute for Health and Care Excellence, the Centers for Disease Control and Prevention, and several medical specialty societies in the early 2000s.6-14 Despite these recommendations, ultrasound guidance has not been universally adopted. Currently, an estimated 20%-55% of CVC insertions in the internal jugular vein are performed without ultrasound guidance.15-17
Following the emergence of literature supporting the use of ultrasound guidance for CVC insertion, observational and randomized controlled studies demonstrated improved procedural success rates with the use of ultrasound guidance for the insertion of peripheral intravenous lines (PIVs), arterial catheters, and peripherally inserted central catheters (PICCs).18-23
The purpose of this position statement is to present evidence-based recommendations on the use of ultrasound guidance for the insertion of central and peripheral vascular access catheters in adult patients. This document presents consensus-based recommendations with supporting evidence for clinical outcomes, techniques, and training for the use of ultrasound guidance for vascular access. We have subdivided the recommendations on techniques for central venous access, peripheral venous access, and arterial access individually, as some providers may not perform all types of vascular access procedures.
These recommendations are intended for hospitalists and other healthcare providers that routinely place central and peripheral vascular access catheters in acutely ill patients. However, this position statement does not mandate that all hospitalists should place central or peripheral vascular access catheters given the diverse array of hospitalist practice settings. For training and competency assessments, we recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals, where equipment and staffing for assessments are not available. Recommendations and frameworks for initial and ongoing credentialing of hospitalists in ultrasound-guided bedside procedures have been previously published in an Society of Hospital Medicine (SHM) position statement titled, “Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.”24
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interest (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the vascular access working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. An updated search was conducted in November 2017. The literature search strings are included in Appendix 3. All article abstracts were initially screened for relevance by at least two members of the vascular access working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide vascular access were selected. The following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled studies, and observational studies of ultrasound-guided vascular access were screened and selected (Appendix 3, Figure 1). All full-text articles were shared electronically among the working group members, and final article selection was based on working group consensus. Selected articles were incorporated into the draft recommendations.
These recommendations were developed using the Research and Development (RAND) Appropriateness Method that required panel judgment and consensus.14 The 28 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Using an internet-based electronic data collection tool (REDCap™), panel members participated in two rounds of electronic voting, one in August 2018 and the other in October 2018 (Appendix 4). Voting on appropriateness was conducted using a nine-point Likert scale. The three zones of the nine-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix 1, Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” Disagreement was defined as >30% of panelists voting outside of the zone of the median. A strong recommendation required at least 80% of the votes within one integer of the median per the RAND rules.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording for each recommendation (Table 2). The final version of the consensus-based recommendations underwent internal and external review by members of the SHM POCUS Task Force, the SHM Education Committee, and the SHM Executive Committee. The SHM Executive Committee reviewed and approved this position statement prior to its publication in the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 5,563 references were pooled from an initial search performed by a certified medical librarian in December 2015 (4,668 citations) which was updated in November 2017 (791 citations), and from the personal bibliographies and searches (104 citations) performed by working group members. A total of 514 full-text articles were reviewed. The final selection included 192 articles that were abstracted into a data table and incorporated into the draft recommendations. See Appendix 3 for details of the literature search strategy.
Recommendations
Four domains (technique, clinical outcomes, training, and knowledge gaps) with 31 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation cite supporting evidence. After two rounds of panel voting, 31 recommendations achieved agreement based on the RAND rules. During the peer review process, two of the recommendations were merged with other recommendations. Thus, a total of 29 recommendations received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Twenty-seven statements were approved as strong recommendations, and two were approved as weak/conditional recommendations. The strength of each recommendation and degree of consensus are summarized in Table 3.
Terminology
Central Venous Catheterization
Central venous catheterization refers to insertion of tunneled or nontunneled large bore vascular catheters that are most commonly inserted into the internal jugular, subclavian, or femoral veins with the catheter tip located in a central vein. These vascular access catheters are synonymously referred to as central lines or central venous catheters (CVCs). Nontunneled catheters are designed for short-term use and should be removed promptly when no longer clinically indicated or after a maximum of 14 days.25
Peripherally Inserted Central Catheter (PICC)
Peripherally inserted central catheters, or PICC lines, are inserted most commonly in the basilic or brachial veins in adult patients, and the catheter tip terminates in the distal superior vena cava or cavo-atrial junction. These catheters are designed to remain in place for a duration of several weeks, as long as it is clinically indicated.
Midline Catheterization
Midline catheters are a type of peripheral venous catheter that are an intermediary between a peripheral intravenous catheter and PICC line. Midline catheters are most commonly inserted in the brachial or basilic veins, but unlike PICC lines, the tips of these catheters terminate in the axillary or subclavian vein. Midline catheters are typically 8 cm to 20 cm in length and inserted for a duration <30 days.
Peripheral Intravenous Catheterization
Peripheral intravenous lines (PIV) refer to small bore venous catheters that are most commonly 14G to 24G and inserted into patients for short-term peripheral venous access. Common sites of ultrasound-guided PIV insertion include the superficial and deep veins of the hand, forearm, and arm.
Arterial Catheterization
Arterial catheters are commonly used for reliable blood pressure monitoring, frequent arterial blood
RECOMMENDATIONS
Preprocedure
1. We recommend that providers should be familiar with the operation of their specific ultrasound machine prior to initiation of a vascular access procedure.
Rationale: There is strong consensus that providers must be familiar with the knobs and functions of the specific make and model of ultrasound machine that will be utilized for a vascular access procedure. Minimizing adjustments to the ultrasound machine during the procedure may reduce the risk of contaminating the sterile field.
2. We recommend that providers should use a high-frequency linear transducer with a sterile sheath and sterile gel to perform vascular access procedures.
Rationale: High-frequency linear-array transducers are recommended for the vast majority of vascular access procedures due to their superior resolution compared to other transducer types. Both central and peripheral vascular access procedures, including PIV, PICC, and arterial line placement, should be performed using sterile technique. A sterile transducer cover and sterile gel must be utilized, and providers must be trained in sterile preparation of the ultrasound transducer.13,26,27
The depth of femoral vessels correlates with body mass index (BMI). When accessing these vessels in a morbidly obese patient with a thigh circumference >60 cm and vessel depth >8 cm, a curvilinear transducer may be preferred for its deeper penetration.28 For patients who are poor candidates for bedside insertion of vascular access catheters, such as uncooperative patients, patients with atypical vascular anatomy or poorly visualized target vessels, we recommend consultation with a vascular access specialist prior to attempting the procedure.
3. We recommend that providers should use two-dimensional ultrasound to evaluate for anatomical variations and absence of vascular thrombosis during preprocedural site selection.
Rationale: A thorough ultrasound examination of the target vessel is warranted prior to catheter placement. Anatomical variations that may affect procedural decision-making are easily detected with ultrasound. A focused vascular ultrasound examination is particularly important in patients who have had temporary or tunneled venous catheters, which can cause stenosis or thrombosis of the target vein.
For internal jugular vein (IJV) CVCs, ultrasound is useful for visualizing the relationship between the IJV and common carotid artery (CCA), particularly in terms of vessel overlap. Furthermore, ultrasound allows for immediate revisualization upon changes in head position.29-32 Troianos et al. found >75% overlap of the IJV and CCA in 54% of all patients and in 64% of older patients (age >60 years) whose heads were rotated to the contralateral side.30 In one study of IJV CVC insertion, inadvertent carotid artery punctures were reduced (3% vs 10%) with the use of ultrasound guidance vs landmarks alone.33 In a cohort of 64 high-risk neurosurgical patients, cannulation success was 100% with the use of ultrasound guidance, and there were no injuries to the carotid artery, even though the procedure was performed with a 30-degree head elevation and anomalous IJV anatomy in 39% of patients.34 In a prospective, randomized controlled study of 1,332 patients, ultrasound-guided cannulation in a neutral position was demonstrated to be as safe as the 45-degree rotated position.35
Ultrasound allows for the recognition of anatomical variations which may influence the selection of the vascular access site or technique. Benter et al. found that 36% of patients showed anatomical variations in the IJV and surrounding tissue.36 Similarly Caridi showed the anatomy of the right IJV to be atypical in 29% of patients,37 and Brusasco found that 37% of bariatric patients had anatomical variations of the IJV.38 In a study of 58 patients, there was significant variability in the IJV position and IJV diameter, ranging from 0.5 cm to >2 cm.39 In a study of hemodialysis patients, 75% of patients had sonographic venous abnormalities that led to a change in venous access approach.40
To detect acute or chronic upper extremity deep venous thrombosis or stenosis, two-dimensional visualization with compression should be part of the ultrasound examination prior to central venous catheterization. In a study of patients that had undergone CVC insertion 9-19 weeks earlier, 50% of patients had an IJV thrombosis or stenosis leading to selection of an alternative site. In this study, use of ultrasound for a preprocedural site evaluation reduced unnecessary attempts at catheterizing an occluded vein.41 At least two other studies demonstrated an appreciable likelihood of thrombosis. In a study of bariatric patients, 8% of patients had asymptomatic thrombosis38 and in another study, 9% of patients being evaluated for hemodialysis catheter placement had asymptomatic IJV thrombosis.37
4. We recommend that providers should evaluate the target blood vessel size and depth during a preprocedural ultrasound evaluation.
Rationale: The size, depth, and anatomic location of central veins can vary considerably. These features are easily discernable using ultrasound. Contrary to traditional teaching, the IJV is located 1 cm anterolateral to the CCA in only about two-thirds of patients.37,39,42,43 Furthermore, the diameter of the IJV can vary significantly, ranging from 0.5 cm to >2 cm.39 The laterality of blood vessels may vary considerably as well. A preprocedural ultrasound evaluation of contralateral subclavian and axillary veins showed a significant absolute difference in cross-sectional area of 26.7 mm2 (P < .001).42
Blood vessels can also shift considerably when a patient is in the Trendelenburg position. In one study, the IJV diameter changed from 11.2 (± 1.5) mm to 15.4 (± 1.5) mm in the supine versus the Trendelenburg position at 15 degrees.33 An observational study demonstrated a frog-legged position with reverse Trendelenburg increased the femoral vein size and reduced the common surface area with the common femoral artery compared to a neutral position. Thus, a frog-legged position with reverse Trendelenburg position may be preferred, since overall catheterization success rates are higher in this position.44
Techniques
General Techniques
5. We recommend that providers should avoid using static ultrasound alone to mark the needle insertion site for vascular access procedures.
Rationale: The use of static ultrasound guidance to mark a needle insertion site is not recommended because normal anatomical relationships of vessels vary, and site marking can be inaccurate with minimal changes in patient position, especially of the neck.43,45,46 Benefits of using ultrasound guidance for vascular access are attained when ultrasound is used to track the needle tip in real-time as it is advanced toward the target vessel.
Although continuous-wave Doppler ultrasound without two-dimensional visualization was used in the past, it is no longer recommended for IJV CVC insertion.47 In a study that randomized patients to IJV CVC insertion with continuous-wave Doppler alone vs two-dimensional ultrasound guidance, the use of two-dimensional ultrasound guidance showed significant improvement in first-pass success rates (97% vs 91%, P = .045), particularly in patients with BMI >30 (97% vs 77%, P = .011).48
A randomized study comparing real-time ultrasound-guided, landmark-based, and ultrasound-marked techniques found higher success rates in the real-time ultrasound-guided group than the other two groups (100% vs 74% vs 73%, respectively; P = .01). The total number of mechanical complications was higher in the landmark-based and ultrasound-marked groups than in the real-time ultrasound-guided group (24% and 36% versus 0%, respectively; P = .01).49 Another randomized controlled study found higher success rates with real-time ultrasound guidance (98%) versus an ultrasound-marked (82%) or landmark-based (64%) approach for central line placement.50
6. We recommend that providers should use real-time (dynamic), two-dimensional ultrasound guidance with a high-frequency linear transducer for CVC insertion, regardless of the provider’s level of experience.
7. We suggest using either a transverse (short-axis) or longitudinal (long-axis) approach when performing real-time ultrasound-guided vascular access procedures.
Rationale: In clinical practice, the phrases transverse, short-axis, or out-of-plane approach are synonymous, as are longitudinal, long-axis, and in-plane approach. The short-axis approach involves tracking the needle tip as it approximates the target vessel with the ultrasound beam oriented in a transverse plane perpendicular to the target vessel. The target vessel is seen as a circular structure on the ultrasound screen as the needle tip approaches the target vessel from above. This approach is also called the out-of-plane technique since the needle passes through the ultrasound plane. The advantages of the short-axis approach include better visualization of adjacent vessels or nerves and the relative ease of skill acquisition for novice operators.9 When using the short-axis approach, extra care must be taken to track the needle tip from the point of insertion on the skin to the target vessel. A disadvantage of the short-axis approach is unintended posterior wall puncture of the target vessel.55
In contrast to a short-axis approach, a long-axis approach is performed with the ultrasound beam aligned parallel to the vessel. The vessel appears as a long tubular structure and the entire needle is visualized as it traverses across the ultrasound screen to approach the target vessel. The long-axis approach is also called an in-plane technique because the needle is maintained within the plane of the ultrasound beam. The advantage of a long-axis approach is the ability to visualize the entire needle as it is inserted into the vessel.14 A randomized crossover study with simulation models compared a long-axis versus short-axis approach for both IJV and subclavian vein catheterization. This study showed decreased number of needle redirections (relative risk (RR) 0.5, 95% confidence interval (CI) 0.3 to 0.7), and posterior wall penetrations (OR 0.3, 95% CI 0.1 to 0.9) using a long-axis versus short-axis approach for subclavian vein catheterization.56
A randomized controlled study comparing a long-axis or short-axis approach with ultrasound versus a landmark-based approach for IJV CVC insertion showed higher success rates (100% vs 90%; P < .001), lower insertion time (53 vs 116 seconds; P < .001), and fewer attempts to obtain access (2.5 vs 1.2 attempts, P < .001) with either the long- or short-axis ultrasound approach. The average time to obtain access and number of attempts were comparable between the short-axis and long-axis approaches with ultrasound. The incidence of carotid puncture and hematoma was significantly higher with the landmark-based approach versus either the long- or short-axis ultrasound approach (carotid puncture 17% vs 3%, P = .024; hematoma 23% vs 3%, P = .003).57
High success rates have been reported using a short-axis approach for insertion of PIV lines.58 A prospective, randomized trial compared the short-axis and long-axis approach in patients who had had ≥2 failed PIV insertion attempts. Success rate was 95% (95% CI, 0.85 to 1.00) in the short-axis group compared with 85% (95% CI, 0.69 to 1.00) in the long-axis group. All three subjects with failed PIV placement in the long-axis group had successful rescue placement using a short-axis approach. Furthermore, the short-axis approach was faster than the long-axis approach.59
For radial artery cannulation, limited data exist comparing the short- and long-axis approaches. A randomized controlled study compared a long-axis vs short-axis ultrasound approach for radial artery cannulation. Although the overall procedure success rate was 100% in both groups, the long-axis approach had higher first-pass success rates (1.27 ± 0.4 vs 1.5 ± 0.5, P < .05), shorter cannulation times (24 ± 17 vs 47 ± 34 seconds, P < .05), fewer hematomas (4% vs 43%, P < .05) and fewer posterior wall penetrations (20% vs 56%, P < .05).60
Another technique that has been described for IJV CVC insertion is an oblique-axis approach, a hybrid between the long- and short-axis approaches. In this approach, the transducer is aligned obliquely over the IJV and the needle is inserted using a long-axis or in-plane approach. A prospective randomized trial compared the short-axis, long-axis, and oblique-axis approaches during IJV cannulation. First-pass success rates were 70%, 52%, and 74% with the short-axis, long-axis, and oblique-axis approaches, respectively, and a statistically significant difference was found between the long- and oblique-axis approaches (P = .002). A higher rate of posterior wall puncture was observed with a short-axis approach (15%) compared with the oblique-axis (7%) and long-axis (4%) approaches (P = .047).61
8. We recommend that providers should visualize the needle tip and guidewire in the target vein prior to vessel dilatation.
Rationale: When real-time ultrasound guidance is used, visualization of the needle tip within the vein is the first step to confirm cannulation of the vein and not the artery. After the guidewire is advanced, the provider can use transverse and longitudinal views to reconfirm cannulation of the vein. In a longitudinal view, the guidewire is readily seen positioned within the vein, entering the anterior wall and lying along the posterior wall of the vein. Unintentional perforation of the posterior wall of the vein with entry into the underlying artery can be detected by ultrasound, allowing prompt removal of the needle and guidewire before proceeding with dilation of the vessel. In a prospective observational study that reviewed ultrasound-guided IJV CVC insertions, physicians were able to more readily visualize the guidewire than the needle in the vein.62 A prospective observational study determined that novice operators can visualize intravascular guidewires in simulation models with an overall accuracy of 97%.63
In a retrospective review of CVC insertions where the guidewire position was routinely confirmed in the target vessel prior to dilation, there were no cases of arterial dilation, suggesting confirmation of guidewire position can potentially eliminate the morbidity and mortality associated with arterial dilation during CVC insertion.64
9. To increase the success rate of ultrasound-guided vascular access procedures, we recommend that providers should utilize echogenic needles, plastic needle guides, and/or ultrasound beam steering when available.
Rationale: Echogenic needles have ridged tips that appear brighter on the screen, allowing for better visualization of the needle tip. Plastic needle guides help stabilize the needle alongside the transducer when using either a transverse or longitudinal approach. Although evidence is limited, some studies have reported higher procedural success rates when using echogenic needles, plastic needle guides, and ultrasound beam steering software. In a prospective observational study, Augustides et al. showed significantly higher IJV cannulation rates with versus without use of a needle guide after first (81% vs 69%, P = .0054) and second (93% vs 80%. P = .0001) needle passes.65 A randomized study by Maecken et al. compared subclavian vein CVC insertion with or without use of a needle guide, and found higher procedure success rates within the first and second attempts, reduced time to obtain access (16 seconds vs 30 seconds; P = .0001) and increased needle visibility (86% vs 32%; P < .0001) with the use of a needle guide.66 Another study comparing a short-axis versus long-axis approach with a needle guide showed improved needle visualization using a long-axis approach with a needle guide.67 A randomized study comparing use of a novel, sled-mounted needle guide to a free-hand approach for venous cannulation in simulation models showed the novel, sled-mounted needle guide improved overall success rates and efficiency of cannulation.68
Central Venous Access Techniques
10. We recommend that providers should use a standardized procedure checklist that includes use of real-time ultrasound guidance to reduce the risk of central line-associated bloodstream infection (CLABSI) from CVC insertion.
Rationale: A standardized checklist or protocol should be developed to ensure compliance with all recommendations for insertion of CVCs. Evidence-based protocols address periprocedural issues, such as indications for CVC, and procedural techniques, such as use of maximal sterile barrier precautions to reduce the risk of infection. Protocols and checklists that follow established guidelines for CVC insertion have been shown to decrease CLABSI rates.69,70 Similarly, development of checklists and protocols for maintenance of central venous catheters have been effective in reducing CLABSIs.71 Although no externally-validated checklist has been universally accepted or endorsed by national safety organizations, a few internally-validated checklists are available through peer-reviewed publications.72,73 An observational educational cohort of internal medicine residents who received training using simulation of the entire CVC insertion process was able to demonstrate fewer CLABSIs after the simulator-trained residents rotated in the intensive care unit (ICU) (0.50 vs 3.2 infections per 1,000 catheter days, P = .001).74
11. We recommend that providers should use real-time ultrasound guidance, combined with aseptic technique and maximal sterile barrier precautions, to reduce the incidence of infectious complications from CVC insertion.
Rationale: The use of real-time ultrasound guidance for CVC placement has demonstrated a statistically significant reduction in CLABSIs compared to landmark-based techniques.75 The Centers for Disease Control and Prevention (CDC) guidelines for the prevention of intravascular catheter-related infections recommend the use of ultrasound guidance to reduce the number of cannulation attempts and risk of mechanical complications.69 A prospective, three-arm study comparing ultrasound-guided long-axis, short-axis, and landmark-based approaches showed a CLABSI rate of 20% in the landmark-based group versus 10% in each of the ultrasound groups.57 Another randomized study comparing use of ultrasound guidance to a landmark-based technique for IJV CVC insertion demonstrated significantly lower CLABSI rates with the use of ultrasound (2% vs 10%; P < .05).72
Studies have shown that a systems-based intervention featuring a standardized catheter kit or catheter bundle significantly reduced CLABSI rates.76-78 A complete review of all preventive measures to reduce the risk of CLABSI is beyond the scope of this review, but a few key points will be mentioned. First, aseptic technique includes proper hand hygiene and skin sterilization, which are essential measures to reduce cutaneous colonization of the insertion site and reduce the risk of CLABSIs.79 In a systematic review and meta-analysis of eight studies including over 4,000 catheter insertions, skin antisepsis with chlorhexidine was associated with a 50% reduction in CLABSIs compared with povidone iodine.11 Therefore, a chlorhexidine-containing solution is recommended for skin preparation prior to CVC insertion per guidelines by Healthcare Infection Control Practices Advisory Committee/CDC, Society for Healthcare Epidemiology of America/Infectious Diseases Society of America, and American Society of Anesthesiologists.11,69,80,81 Second, maximal sterile barrier precautions refer to the use of sterile gowns, sterile gloves, caps, masks covering both the mouth and nose, and sterile full-body patient drapes. Use of maximal sterile barrier precautions during CVC insertion has been shown to reduce the incidence of CLABSIs compared to standard precautions.26,79,82-84 Third, catheters containing antimicrobial agents may be considered for hospital units with higher CLABSI rates than institutional goals, despite a comprehensive preventive strategy, and may be considered in specific patient populations at high risk of severe complications from a CLABSI.11,69,80 Finally, providers should use a standardized procedure set-up when inserting CVCs to reduce the risk of CLABSIs. The operator should confirm availability and proper functioning of ultrasound equipment prior to commencing a vascular access procedure. Use of all-inclusive procedure carts or kits with sterile ultrasound probe covers, sterile gel, catheter kits, and other necessary supplies is recommended to minimize interruptions during the procedure, and can ultimately reduce the risk of CLABSIs by ensuring maintenance of a sterile field during the procedure.13
12. We recommend that providers should use real-time ultrasound guidance for internal jugular vein catheterization, which reduces the risk of mechanical and infectious complications, the number of needle passes, and time to cannulation and increases overall procedure success rates.
Rationale: The use of real-time ultrasound guidance for CVC insertion has repeatedly demonstrated better outcomes compared to a landmark-based approach in adults.13 Several randomized controlled studies have demonstrated that real-time ultrasound guidance for IJV cannulation reduces the risk of procedure-related mechanical and infectious complications, and improves first-pass and overall success rates in diverse care settings.27,29,45,50,53,65,75,85-90 Mechanical complications that are reduced with ultrasound guidance include pneumothorax and carotid artery puncture.4,5,45,46,53,62,75,86-93 Currently, several medical societies strongly recommend the use of ultrasound guidance during insertion of IJV CVCs.10-12,14,94-96
A meta-analysis by Hind et al. that included 18 randomized controlled studies demonstrated use of real-time ultrasound guidance reduced failure rates (RR 0.14, 95% CI 0.06 to 0.33; P < .0001), increased first-attempt success rates (RR 0.59, 95% CI 0.39 to 0.88; P = .009), reduced complication rates (RR 0.43, 95% CI 0.22 to 0.87; P = .02) and reduced procedure time (P < .0001), compared to a traditional landmark-based approach when inserting IJV CVCs.5
A Cochrane systematic review compared ultrasound-guided versus landmark-based approaches for IJV CVC insertion and found use of real-time ultrasound guidance reduced total complication rates by 71% (RR 0.29, 95% CI 0.17 to 0.52; P < .0001), arterial puncture rates by 72% (RR 0.28, 95% CI 0.18 to 0.44; P < .00001), and rates of hematoma formation by 73% (RR 0.27, 95% CI 0.13 to 0.55; P = .0004). Furthermore, the number of attempts for successful cannulation was reduced (mean difference -1.19 attempts, 95% CI -1.45 to -0.92; P < .00001), the chance of successful insertion on the first attempt was increased by 57% (RR 1.57, 95% CI 1.36 to 1.82; P < .00001), and overall procedure success rates were modestly increased in all groups by 12% (RR 1.12, 95% CI 1.08 to 1.17; P < .00001).46
An important consideration in performing ultrasound guidance is provider experience. A prospective observational study of patients undergoing elective CVC insertion demonstrated higher complication rates for operators that were inexperienced (25.2%) versus experienced (13.6%).54 A randomized controlled study comparing experts and novices with or without the use of ultrasound guidance for IJV CVC insertion demonstrated higher success rates among expert operators and with the use of ultrasound guidance. Among novice operators, the complication rates were lower with the use of ultrasound guidance.97 One study evaluated the procedural success and complication rates of a two-physician technique with one physician manipulating the transducer and another inserting the needle for IJV CVC insertion. This study concluded that procedural success rates and frequency of complications were directly affected by the experience of the physician manipulating the transducer and not by the experience of the physician inserting the needle.98
The impact of ultrasound guidance on improving procedural success rates and reducing complication rates is greatest in patients that are obese, short necked, hypovolemic, or uncooperative.93 Several studies have demonstrated fewer needle passes and decreased time to cannulation compared to the landmark technique in these populations.46,49,53,86-88,92,93
Ultrasound-guided placement of IJV catheters can safely be performed in patients with disorders of hemostasis and those with multiple previous catheter insertions in the same vein.9 Ultrasound-guided placement of CVCs in patients with disorders of hemostasis is safe with high success and low complication rates. In a case series of liver patients with coagulopathy (mean INR 2.17 ± 1.16, median platelet count 150K), the use of ultrasound guidance for CVC insertion was highly successful with no major bleeding complications.99
A study of renal failure patients found high success rates and low complication rates in the patients with a history of multiple previous catheterizations, poor compliance, skeletal deformities, previous failed cannulations, morbid obesity, and disorders of hemostasis.100 A prospective observational study of 200 ultrasound-guided CVC insertions for apheresis showed a 100% success rate with a 92% first-pass success rate.101
The use of real-time ultrasound guidance for IJV CVC insertion has been shown to be cost effective by reducing procedure-related mechanical complications and improving procedural success rates. A companion cost-effectiveness analysis estimated that for every 1,000 patients, 90 complications would be avoided, with a net cost savings of approximately $3,200 using 2002 prices.102
13. We recommend that providers who routinely insert subclavian vein CVCs should use real-time ultrasound guidance, which has been shown to reduce the risk of mechanical complications and number of needle passes and increase overall procedure success rates compared with landmark-based techniques.
Rationale: In clinical practice, the term ultrasound-guided subclavian vein CVC insertion is commonly used. However, the needle insertion site is often lateral to the first rib and providers are technically inserting the CVC in the axillary vein. The subclavian vein becomes the axillary vein at the lateral border of the first rib where the cephalic vein branches from the subclavian vein. To be consistent with common medical parlance, we use the phrase ultrasound-guided subclavian vein CVC insertion in this document.
Advantages of inserting CVCs in the subclavian vein include reliable surface anatomical landmarks for vein location, patient comfort, and lower risk of infection.103 Several observational studies have demonstrated the technique for ultrasound-guided subclavian vein CVC insertion is feasible and safe.104-107 In a large retrospective observational study of ultrasound-guided central venous access among a complex patient group, the majority of patients were cannulated successfully and safely. The subset of patients undergoing axillary vein CVC insertion (n = 1,923) demonstrated a low rate of complications (0.7%), proving it is a safe and effective alternative to the IJV CVC insertion.107
A Cochrane review of ultrasound-guided subclavian vein cannulation (nine studies, 2,030 participants, 2,049 procedures), demonstrated that real-time two-dimensional ultrasound guidance reduced the risk of inadvertent arterial punctures (three studies, 498 participants, RR 0.21, 95% CI 0.06 to 0.82; P = .02) and hematoma formation (three studies, 498 participants, RR 0.26, 95% CI 0.09 to 0.76; P = .01).46 A systematic review and meta-analysis of 10 randomized controlled studies comparing ultrasound-guided versus landmark-based subclavian vein CVC insertion demonstrated a reduction in the risk of arterial punctures, hematoma formation, pneumothorax, and failed catheterization with the use of ultrasound guidance.105
A randomized controlled study comparing ultrasound-guided vs landmark-based approaches to subclavian vein cannulation found that use of ultrasound guidance had a higher success rate (92% vs 44%, P = .0003), fewer minor complications (1 vs 11, P = .002), fewer attempts (1.4 vs 2.5, P = .007) and fewer catheter kits used (1.0 vs 1.4, P = .0003) per cannulation.108
Fragou et al. randomized patients undergoing subclavian vein CVC insertion to a long-axis approach versus a landmark-based approach and found a significantly higher success rate (100% vs 87.5%, P < .05) and lower rates of mechanical complications: artery puncture (0.5% vs 5.4%), hematoma (1.5% vs 5.4%), hemothorax (0% vs 4.4%), pneumothorax (0% vs 4.9%), brachial plexus injury (0% vs 2.9%), phrenic nerve injury (0% vs 1.5%), and cardiac tamponade (0% vs 0.5%).109 The average time to obtain access and the average number of insertion attempts (1.1 ± 0.3 vs 1.9 ± 0.7, P < .05) were significantly reduced in the ultrasound group compared to the landmark-based group.95
A retrospective review of subclavian vein CVC insertions using a supraclavicular approach found no reported complications with the use of ultrasound guidance vs 23 mechanical complications (8 pneumothorax, 15 arterial punctures) with a landmark-based approach.106 However, it is important to note that a supraclavicular approach is not commonly used in clinical practice.
14. We recommend that providers should use real-time ultrasound guidance for femoral venous access, which has been shown to reduce the risk of arterial punctures and total procedure time and increase overall procedure success rates.
Rationale: Anatomy of the femoral region varies, and close proximity or overlap of the femoral vein and artery is common.51 Early studies showed that ultrasound guidance for femoral vein CVC insertion reduced arterial punctures compared with a landmark-based approach (7% vs 16%), reduced total procedure time (55 ± 19 vs 79 ± 62 seconds), and increased procedure success rates (100% vs 90%).52 A Cochrane review that pooled data from four randomized studies comparing ultrasound-guided vs landmark-based femoral vein CVC insertion found higher first-attempt success rates with the use of ultrasound guidance (RR 1.73, 95% CI 1.34 to 2.22; P < .0001) and a small increase in the overall procedure success rates (RR 1.11, 95% CI 1.00 to 1.23; P = .06). There was no difference in inadvertent arterial punctures or other complications.110
Peripheral Venous Access Techniques
15. We recommend that providers should use real-time ultrasound guidance for the insertion of peripherally inserted central catheters (PICCs), which is associated with higher procedure success rates and may be more cost effective compared with landmark-based techniques.
Rationale: Several studies have demonstrated that providers who use ultrasound guidance vs landmarks for PICC insertion have higher procedural success rates, lower complication rates, and lower total placement costs. A prospective observational report of 350 PICC insertions using ultrasound guidance reported a 99% success rate with an average of 1.2 punctures per insertion and lower total costs.20 A retrospective observational study of 500 PICC insertions by designated specialty nurses revealed an overall success rate of 95%, no evidence of phlebitis, and only one CLABSI among the catheters removed.21 A retrospective observational study comparing several PICC variables found higher success rates (99% vs 77%) and lower thrombosis rates (2% vs 9%) using ultrasound guidance vs landmarks alone.22 A study by Robinson et al. demonstrated that having a dedicated PICC team equipped with ultrasound increased their institutional insertion success rates from 73% to 94%.111
A randomized controlled study comparing ultrasound-guided versus landmark-based PICC insertion found high success rates with both techniques (100% vs 96%). However, there was a reduction in the rate of unplanned catheter removals (4.0% vs 18.7%; P = .02), mechanical phlebitis (0% vs 22.9%; P < .001), and venous thrombosis (0% vs 8.3%; P = .037), but a higher rate of catheter migration (32% vs 2.1%; P < .001). Compared with the landmark-based group, the ultrasound-guided group had significantly lower incidence of severe contact dermatitis (P = .038), and improved comfort and costs up to 3 months after PICC placement (P < .05).112
Routine postprocedure chest x-ray (CXR) is generally considered unnecessary if the PICC is inserted with real-time ultrasound guidance along with use of a newer tracking devices, like the magnetic navigation system with intracardiac electrodes.9 Ultrasound can also be used to detect malpositioning of a PICC immediately after completing the procedure. A randomized controlled study comparing ultrasound versus postprocedure CXR detected one malpositioned PICC in the ultrasound group versus 11 in the control group. This study suggested that ultrasound can detect malpositioning immediately postprocedure and reduce the need for a CXR and the possibility of an additional procedure to reposition a catheter.113
16. We recommend that providers should use real-time ultrasound guidance for the placement of peripheral intravenous lines (PIV) in patients with difficult peripheral venous access to reduce the total procedure time, needle insertion attempts, and needle redirections. Ultrasound-guided PIV insertion is also an effective alternative to CVC insertion in patients with difficult venous access.
Rationale: Difficult venous access refers to patients that have had two unsuccessful attempts at PIV insertion using landmarks or a history of difficult access (i.e. edema, obesity, intravenous drug use, chemotherapy, diabetes, hypovolemia, chronic illness, vasculopathy, multiple prior hospitalizations). A meta-analysis of seven randomized controlled studies concluded that ultrasound guidance increases the likelihood of successful PIV insertion (pooled OR 2.42, 95% CI 1.26 to 4.68; P < .008).18 A second meta-analysis that pooled data from seven studies (six randomized controlled studies) confirmed that ultrasound guidance improves success rates of PIV insertion (OR 3.96, 95% CI 1.75 to 8.94).19 Approximately half of these studies had physician operators while the other half had nurse operators.
In one prospective observational study of emergency department patients with two failed attempts of landmark-based PIV insertion, ultrasound guidance with a modified-Seldinger technique showed a relatively high success rate (96%), fewer needle sticks (mean 1.32 sticks, 95% CI 1.12 to 1.52), and shorter time to obtain access (median time 68 seconds).114 Other prospective observational studies have demonstrated that ultrasound guidance for PIV insertion has a high success rate (87%),115 particularly with brachial or basilic veins PIV insertion, among patients with difficult PIV access, defined as having had ≥2 failed attempts.58
Since insertion of PIVs with ultrasound guidance has a high success rate, there is potential to reduce the reliance on CVC insertion for venous access only. In a study of patients that had had two failed attempts at PIV insertion based on landmarks, a PIV was successfully inserted with ultrasound guidance in 84% of patients, obviating the need for CVC placement for venous access.116 A prospective observational study showed ultrasound-guided PIV insertion was an effective alternative to CVC placement in ED patients with difficult venous access with only 1% of patients requiring a CVC.117 Use of ultrasound by nurses for PIV placement has also been shown to reduce the time to obtain venous access, improve patient satisfaction, and reduce the need for physician intervention.118 In a prospective observational study of patients with difficult access, the majority of patients reported a better experience with ultrasound-guided PIV insertion compared to previous landmark-based attempts with an average satisfaction score of 9.2/10 with 76% of patients rating the experience a 10.119 A strong recommendation has been made for use of ultrasound guidance in patients with difficult PIV placement by la Société Française d’Anesthésie et de Réanimation (The French Society of Anesthesia and Resuscitation).95
17. We suggest using real-time ultrasound guidance to reduce the risk of vascular, infectious, and neurological complications during PIV insertion, particularly in patients with difficult venous access.
Rationale: The incidence of complications from PIV insertion is often underestimated. Vascular complications include arterial puncture, hematoma formation, local infiltration or extravasation of fluid, and superficial or deep venous thrombosis. The most common infectious complications with PIV insertion are phlebitis and cellulitis.120 One observational study reported PIV complications occurring in approximately half of all patients with the most common complications being phlebitis, hematoma formation, and fluid/blood leakage.121
A retrospective review of ICU patients who underwent ultrasound-guided PIV insertion by a single physician showed high success rates (99%) with low rates of phlebitis/cellulitis (0.7%).There was an assumed benefit of risk reduction due to the patients no longer requiring a CVC after successful PIV placement.122 Another study found very low rates of infection with both landmark-based and ultrasound-guided PIV placement performed by emergency department nurses, suggesting that there is no increased risk of infection with the use of ultrasound.123 To reduce the risk of infection from PIV insertion, we recommend the use of sterile gel and sterile transducer cover (See Recommendation 2).
Arterial Access Techniques
18. We recommend that providers should use real-time ultrasound guidance for arterial access, which has been shown to increase first-pass success rates, reduce the time to cannulation, and reduce the risk of hematoma development compared with landmark-based techniques.
Rationale: Several randomized controlled studies have assessed the value of ultrasound in arterial catheter insertion. Shiver et al. randomized 60 patients admitted to a tertiary center emergency department to either palpation or ultrasound-guided arterial cannulation. They demonstrated a first-pass success rate of 87% in the ultrasound group compared with 50% in the landmark technique group. In the same study, the use of ultrasound was also associated with reduced time needed to establish arterial access and a 43% reduction in the development of hematoma at the insertion site.124 Levin et al. demonstrated a first-pass success rate of 62% using ultrasound versus 34% by palpation alone in 69 patients requiring intraoperative invasive hemodynamic monitoring.125 Additional randomized controlled studies have demonstrated that ultrasound guidance increases first-attempt success rates compared to traditional palpation.23,126,127
19. We recommend that providers should use real-time ultrasound guidance for femoral arterial access, which has been shown to increase first-pass success rates and reduce the risk of vascular complications.
Rationale: Although it is a less frequently used site, the femoral artery may be accessed for arterial blood sampling or invasive hemodynamic monitoring, and use of ultrasound guidance has been shown to improve the first-pass success rates of femoral artery cannulation. It is important to note that most of these studies comparing ultrasound-guided vs landmark-based femoral artery cannulation were performed in patients undergoing diagnostic or interventional vascular procedures.
A meta-analysis of randomized controlled studies comparing ultrasound-guided vs landmark-based femoral artery catheterization found use of ultrasound guidance was associated with a 49% reduction in overall complications (RR 0.51, 95% CI 0.28 to 0.91; P > .05) and 42% improvement in first-pass success rates.128 In another study, precise site selection with ultrasound was associated with fewer pseudoaneurysms in patients undergoing femoral artery cannulation by ultrasound guidance vs palpation for cardiac catheterization (3% vs 5%, P < .05).129
A multicenter randomized controlled study comparing ultrasound vs fluoroscopic guidance for femoral artery catheterization demonstrated ultrasound guidance improved rates of common femoral artery (CFA) cannulation in patients with high CFA bifurcations (83% vs 70%, P < .01).130 Furthermore, ultrasound guidance improved first-pass success rates (83% vs 46%, P < .0001), reduced number of attempts (1.3 vs 3.0, P < .0001), reduced risk of venipuncture (2.4% vs 15.8%, P < .0001), and reduced median time to obtain access (136 seconds vs148 seconds, P = .003). Vascular complications occurred in fewer patients in the ultrasound vs fluoroscopy groups (1.4% vs 3.4% P = .04). Reduced risk of hematoma formation with routine use of ultrasound guidance was demonstrated in one retrospective observational study (RR 0.62, 95% CI 0.46 to 0.84; P < .01).131
20. We recommend that providers should use real-time ultrasound guidance for radial arterial access, which has been shown to increase first-pass success rates, reduce the time to successful cannulation, and reduce the risk of complications compared with landmark-based techniques.
Rationale: Ultrasound guidance is particularly useful for radial artery cannulation in patients with altered anatomy, obesity, nonpulsatile blood flow, low perfusion, and previously unsuccessful cannulation attempts using a landmark-guided approach.132
A multicenter randomized controlled study that was not included in the abovementioned meta-analyses showed similar benefits of using ultrasound guidance vs landmarks for radial artery catheterization: a reduction in the number of attempts with ultrasound guidance (1.65 ± 1.2 vs 3.05 ± 3.4, P < .0001) and time to obtain access (88 ± 78 vs 108 ± 112 seconds, P = .006), and increased first-pass success rates (65% vs 44%, P < .0001). The use of ultrasound guidance was found to be particularly useful in patients with difficult access by palpation alone.135
Regarding the level of expertise required to use ultrasound guidance, a prospective observational study demonstrated that physicians with little previous ultrasound experience were able to improve their first-attempt success rates and procedure time for radial artery cannulation compared to historical data of landmark-based insertions.136
Postprocedure
21. We recommend that post-procedure pneumothorax should be ruled out by the detection of bilateral lung sliding using a high-frequency linear transducer before and after insertion of internal jugular and subclavian vein CVCs.
Rationale: Detection of lung sliding with two-dimensional ultrasound rules out pneumothorax, and disappearance of lung sliding in an area where it was previously seen is a strong predictor of postprocedure pneumothorax. In a study of critically ill patients, the disappearance of lung sliding was observed in 100% of patients with pneumothorax vs 8.8% of patients without pneumothorax. For detection of pneumothorax, lung sliding showed a sensitivity of 95%, specificity of 91%, and negative predictive value of 100% (P < .001).137 Another study by the same author showed that the combination of horizontal artifacts (absence of comet-tail artifact) and absence of lung sliding had a sensitivity of 100%, specificity of 96.5%, and negative predictive value of 100% for the detection of pneumothorax.138 A meta-analysis of 10 studies on the diagnostic accuracy of CVC confirmation with bedside ultrasound vs chest radiography reported detection of all 12 pneumothoraces with ultrasound, whereas chest radiography missed two pneumothoraces. The pooled sensitivity and specificity of ultrasound for the detection of pneumothorax was 100%, although an imperfect gold standard bias likely affected the results. An important advantage of bedside ultrasound is the ability to rule out pneumothorax immediately after the procedure while at the bedside. The mean time for confirmation of CVC placement with bedside ultrasound was 6 minutes versus 64 minutes and 143 minutes for completion and interpretation of a chest radiograph, respectively.139
22. We recommend that providers should use ultrasound with rapid infusion of agitated saline to visualize a right atrial swirl sign (RASS) for detecting catheter tip misplacement during CVC insertion. The use of RASS to detect the catheter tip may be considered an advanced skill that requires specific training and expertise.
Rationale: Bedside echocardiography is a reliable tool to detect catheter tip misplacement during CVC insertion. In one study, catheter misplacement was detected by bedside echocardiography with a sensitivity of 96% and specificity of 83% (positive predictive value 98%, negative predictive value 55%) and prevented distal positioning of the catheter tip.140 A prospective observational study assessed for RASS, which is turbulent flow in the right atrium after a rapid saline flush of the distal CVC port, to exclude catheter malposition. In this study with 135 CVC placements, visualization of RASS with ultrasound was able to identify all correct CVC placements and three of four catheter misplacements. Median times to complete the ultrasound exam vs CXR were 1 vs 20 minutes, respectively, with a median difference of 24 minutes (95% CI 19.6 to 29.3, P < .0001) between the two techniques.141
A prospective observational study assessed the ability of bedside transthoracic echocardiography to detect the guidewire, microbubbles, or both, in the right atrium compared to transesophageal echocardiography as the gold standard. Bedside transthoracic echocardiography allowed visualization of the right atrium in 94% of patients, and both microbubbles plus guidewire in 91% of patients.142 Hence, bedside transthoracic echocardiography allows adequate visualization of the right atrium. Another prospective observational study combining ultrasonography and contrast enhanced RASS resulted in 96% sensitivity and 93% specificity for the detection of a misplaced catheter, and the concordance with chest radiography was 96%.143
Training
23. To reduce the risk of mechanical and infectious complications, we recommend that novice providers should complete a systematic training program that includes a combination of simulation-based practice, supervised insertion on patients, and evaluation by an expert operator before attempting ultrasound-guided CVC insertion independently on patients.
Rationale: Cumulative experience has been recognized to not be a proxy for mastery of a clinical skill.144 The National Institute for Clinical Excellence (NICE) has recommended that providers performing ultrasound-guided CVC insertion should receive appropriate training to achieve competence before performing the procedure independently.7 Surveys have demonstrated that lack of training is a commonly reported barrier for not using ultrasound.145,146
Structured training programs on CVC insertion have been shown to reduce the occurrence of infectious and mechanical complications.74,143,147-149 The use of ultrasound and checklists, bundling of supplies, and practice with simulation models, as a part of a structured training program, can improve patient safety related to CVC insertion.9,140,150-154
Simulation-based practice has been used in medical education to provide deliberate practice and foster skill development in a controlled learning environment.155-158 Studies have shown transfer of skills demonstrated in a simulated environment to clinical practice, which can improve CVC insertion practices.159,160 Simulation accelerates learning of all trainees, especially novice trainees, and mitigates risks to patients by allowing trainees to achieve a minimal level of competence before attempting the procedure on real patients.152,161,162 Residents that have been trained using simulation preferentially select the IJV site,147 and more reliably use ultrasound to guide their CVC insertions.160,163
Additionally, simulation-based practice allows exposure to procedures and scenarios that may occur infrequently in clinical practice.
Although there is evidence on efficacy of simulation-based CVC training programs, there is no broadly accepted consensus on timing, duration, and content of CVC training programs for trainees or physicians in practice. The minimum recommended technical skills a trainee must master include the ability to (1) manipulate the ultrasound machine to produce a high-quality image to identify the target vessel, (2) advance the needle under direct visualization to the desired target site and depth, (3) deploy the catheter into the target vessel and confirm catheter placement in the target vessel using ultrasound, and (4) ensure the catheter has not been inadvertently placed in an unintended vessel or structure.153
A variety of simulation models are currently used to practice CVC insertion at the most common sites: the internal jugular, subclavian, basilic, and brachial veins.164,165 Effective simulation models should contain vessels that mimic normal anatomy with muscles, soft tissues, and bones. Animal tissue models, such as turkey or chicken breasts, may be effective for simulated practice of ultrasound-guided CVC insertion.166,167 Ultrasound-guided CVC training using human cadavers has also been shown to be effective.168
24. We recommend that cognitive training in ultrasound-guided CVC insertion should include basic anatomy, ultrasound physics, ultrasound machine knobology, fundamentals of image acquisition and interpretation, detection and management of procedural complications, infection prevention strategies, and pathways to attain competency.
Rationale: After receiving training in ultrasound-guided CVC insertion, physicians report significantly higher comfort with the use of ultrasound compared to those who have not received such training.145 Learners find training sessions worthwhile to increase skill levels,167 and skills learned from simulation-based mastery learning programs have been retained up to one year.158
Several commonalities have been noted across training curricula. Anatomy and physiology didactics should include vessel anatomy (location, size, and course);9 vessel differentiation by ultrasound;9,69 blood flow dynamics;69 Virchow’s triad;69 skin integrity and colonization;150 peripheral nerve identification and distribution;9 respiratory anatomy;9,69 upper and lower extremity, axillary, neck, and chest anatomy.9,69 Vascular anatomy is an essential curricular component that may help avoid preventable CVC insertion complications, such as inadvertent nerve, artery, or lung puncture.150,169 Training curricula should also include ultrasound physics (piezoelectric effect, frequency, resolution, attenuation, echogenicity, Doppler ultrasound, arterial and venous flow characteristics), image acquisition and optimization (imaging mode, focus, dynamic range, probe types), and artifacts (reverberation, mirror, shadowing, enhancement).
CVC-related infections are an important cause of morbidity and mortality in the acute and long-term care environment.69 Infection and thrombosis can both be impacted by the insertion site selection, skin integrity, and catheter–vein ratio.2,3,84 Inexperience generally leads to more insertion attempts that can increase trauma during CVC insertion and potentially increase the risk of infections.170 To reduce the risk of infectious complications, training should include important factors to consider in site selection and maintenance of a sterile environment during CVC insertion, including use of maximal sterile barrier precautions, hand hygiene, and appropriate use of skin antiseptic solutions.
Professional society guidelines have been published with recommendations of appropriate techniques for ultrasound-guided vascular access that include training recommendations.9,154 Training should deconstruct the insertion procedure into readily understood individual steps, and can be aided by demonstration of CVC insertion techniques using video clips. An alternative to face-to-face training is internet-based training that has been shown to be as effective as traditional teaching methods in some medical centers.171 Additional methods to deliver cognitive instruction include textbooks, continuing medical education courses, and digital videos.164,172
25. We recommend that trainees should demonstrate minimal competence before placing ultrasound-guided CVCs independently. A minimum number of CVC insertions may inform this determination, but a proctored assessment of competence is most important.
Rationale: CVC catheter placement carries the risk of serious complications including arterial injury or dissection, pneumothorax, or damage to other local structures; arrhythmias; catheter malposition; infection; and thrombosis. Although there is a lack of consensus and high-quality evidence for the certification of skills to perform ultrasound-guided CVC insertion, recommendations have been published advocating for formal and comprehensive training programs in ultrasound-guided CVC insertion with an emphasis on expert supervision prior to independent practice.9,153,154 Two groups of expert operators have recommended that training should include at least 8-10 supervised ultrasound-guided CVC insertions.154,173,174 A consensus task force from the World Congress of Vascular Access has recommended a minimum of six to eight hours of didactic education, four hours of hands-on training on simulation models, and six hours of hands-on ultrasound training on human volunteers to assess normal anatomy.175 This training should be followed by supervised ultrasound-guided CVC insertions until the learner has demonstrated minimal competence with a low rate of complications.35 There is general consensus that arbitrary numbers should not be the sole determinant of competence, and that the most important determinant of competence should be an evaluation by an expert operator.176
26. We recommend that didactic and hands-on training for trainees should coincide with anticipated times of increased performance of vascular access procedures. Refresher training sessions should be offered periodically.
Rationale: Simulation-based CVC training courses have shown a rapid improvement in skills, but lack of practice leads to deterioration of technical skills.161,162,177,178 Thus, a single immersive training session is insufficient to achieve and maintain mastery of skills, and an important factor to acquire technical expertise is sustained, deliberate practice with feedback.179 Furthermore, an insidious decay in skills may go unrecognized as a learner’s comfort and self-confidence does not always correlate with actual performance, leading to increased risk of errors and potential for procedural complications.147,158,180-183 Given the decay in technical skills over time, simulation-based training sessions are most effective when they occur in close temporal proximity to times when those skills are most likely to be used; for example, a simulation-based training session for trainees may be most effective just before the start of a critical care rotation.152 Regularly scheduled training sessions with monitoring and feedback by expert operators can reinforce procedural skills and prevent decay. Some experts have recommended that a minimum of 10 ultrasound-guided CVC insertions should be performed annually to maintain proficiency.153
27. We recommend that competency assessments should include formal evaluation of knowledge and technical skills using standardized assessment tools.
Rationale: Hospitalists and other healthcare providers that place vascular access catheters should undergo competency assessments proctored by an expert operator to verify that they have the required knowledge and skills.184,185 Knowledge competence can be partially evaluated using a written assessment, such as a multiple-choice test, assessing the provider’s cognitive understanding of the procedure.175 For ultrasound-guided CVC insertion, a written examination should be administered in conjunction with an ultrasound image assessment to test the learner’s recognition of normal vs abnormal vascular anatomy. Minimum passing standards should be established a priori according to local or institutional standards.
The final skills assessment should be objective, and the learner should be required to pass all critical steps of the procedure. Failure of the final skills assessment should lead to continued practice with supervision until the learner can consistently demonstrate correct performance of all critical steps. Checklists are commonly used to rate the technical performance of learners because they provide objective criteria for evaluation, can identify specific skill deficiencies, and can determine a learner’s readiness to perform procedures independently.186,187 The administration of skills assessments and feedback methods should be standardized across faculty. Although passing scores on both knowledge and skills assessments do not guarantee safe performance of a procedure independently, they provide a metric to ensure that a minimum level of competence has been achieved before allowing learners to perform procedures on patients without supervision.188
Competency assessments are a recommended component of intramural and extramural certification of skills in ultrasound-guided procedures. Intramural certification pathways differ by institution and often require additional resources including ultrasound machine(s), simulation equipment, and staff time, particularly when simulation-based assessments are incorporated into certification pathways. We recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals. However, initial and ongoing competency assessments can be performed during routine performance of procedures on patients. For an in-depth review of credentialing pathways for ultrasound-guided bedside procedures, we recommend reviewing the SHM Position Statement on Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.24
28. We recommend that competency assessments should evaluate for proficiency in the following knowledge and skills of CVC insertion:
a. Knowledge of the target vein anatomy, proper vessel identification, and recognition of anatomical variants
b. Demonstration of CVC insertion with no technical errors based on a procedural checklist
c. Recognition and management of acute complications, including emergency management of life-threatening complications
d. Real-time needle tip tracking with ultrasound and cannulation on the first attempt in at least five consecutive simulations.
Rationale: Recommendations have been published with the minimal knowledge and skills learners must demonstrate to perform ultrasound-guided vascular access procedures. These include operation of an ultrasound machine to produce high-quality images of the target vessel, tracking of the needle tip with real-time ultrasound guidance, and recognition and understanding of the management of procedural complications.154,175
First, learners must be able to perform a preprocedural assessment of the target vein, including size and patency of the vein; recognition of adjacent critical structures; and recognition of normal anatomical variants.175,189 Second, learners must be able to demonstrate proficiency in tracking the needle tip penetrating the target vessel, inserting the catheter into the target vessel, and confirming catheter placement in the target vessel with ultrasound.154,175 Third, learners must be able to demonstrate recognition of acute complications, including arterial puncture, hematoma formation, and development of pneumothorax.154,175 Trainees should be familiar with recommended evaluation and management algorithms, including indications for emergent consultation.190
29. We recommend a periodic proficiency assessments of all operators should be conducted to ensure maintenance of competency.
Rationale: Competency extends to periodic assessment and not merely an initial evaluation at the time of training.191 Periodic competency assessments should include assessment of proficiency of all providers that perform a procedure, including instructors and supervisors. Supervising providers should maintain their competency in CVC insertion through routine use of their skills in clinical practice.175 An observational study of emergency medicine residents revealed that lack of faculty comfort with ultrasound hindered the residents’ use of ultrasound.192 Thus, there is a need to examine best practices for procedural supervision of trainees because providers are often supervising procedures that they are not comfortable performing on their own.193
KNOWLEDGE GAPS
The process of producing this position statement revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for central and peripheral venous access and arterial access.
This position statement recommends a preprocedural ultrasound evaluation of blood vessels based on evidence that providers may detect anatomic anomalies, thrombosis, or vessel stenosis. Ultrasound can also reveal unsuspected high-risk structures in near proximity to the procedure site. Although previous studies have shown that providers can accurately assess vessels with ultrasound for these features, further study is needed to evaluate the effect of a standardized preprocedural ultrasound exam on clinical and procedural decision-making, as well as procedural outcomes.
Second, two ultrasound applications that are being increasingly used but have not been widely implemented are the use of ultrasound to evaluate lung sliding postprocedure to exclude pneumothorax and the verification of central line placement using a rapid infusion of agitated saline to visualize the RASS.139-141 Both of these applications have the potential to expedite postprocedure clearance of central lines for usage and decrease patient radiation exposure by obviating the need for postprocedure CXRs. Despite the supporting evidence, both of these applications are not yet widely used, as few providers have been trained in these techniques which may be considered advanced skills.
Third, despite advances in our knowledge of effective training for vascular access procedures, there is limited agreement on how to define procedural competence. Notable advancements in training include improved understanding of systematic training programs, development of techniques for proctoring procedures, definition of elements for hands-on assessments, and definition of minimum experience needed to perform vascular access procedures independently. However, application of these concepts to move learners toward independent practice remains variably interpreted at different institutions, likely due to limited resources, engrained cultures about procedures, and a lack of national standards. The development of hospitalist-based procedure services at major academic medical centers with high training standards, close monitoring for quality assurance, and the use of databases to track clinical outcomes may advance our understanding and delivery of optimal procedural training.
Finally, ultrasound technology is rapidly evolving which will affect training, techniques, and clinical outcomes in coming years. Development of advanced imaging software with artificial intelligence can improve needle visualization and tracking. These technologies have the potential to facilitate provider training in real-time ultrasound-guided procedures and improve the overall safety of procedures. Emergence of affordable, handheld ultrasound devices is improving access to ultrasound technology, but their role in vascular access procedures is yet to be defined. Furthermore, availability of wireless handheld ultrasound technology and multifrequency transducers will create new possibilities for use of ultrasound in vascular access procedures.
CONCLUSION
We have presented several evidence-based recommendations on the use of ultrasound guidance for placement of central and peripheral vascular access catheters that are intended for hospitalists and other healthcare providers who routinely perform vascular access procedures. By allowing direct visualization of the needle tip and target vessel, the use of ultrasound guidance has been shown in randomized studies to reduce needle insertion attempts, reduce needle redirections, and increase overall procedure success rates. The accuracy of ultrasound to identify the target vessel, assess for thrombosis, and detect anatomical anomalies is superior to that of physical examination. Hospitalists can attain competence in performing ultrasound-guided vascular access procedures through systematic training programs that combine didactic and hands-on training, which optimally include patient-based competency assessments.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators of Society of Hospital Medicine Point-of-care Ultrasound Task Force: Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Venkat Kalidindi, Ketino Kobaidze, Joshua Lenchus, Paul Mayo, Satyen Nichani, Vicki Noble, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Gerard Salame, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam J. Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Mathews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Mathews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El-Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Approximately five million central venous catheters (CVCs) are inserted in the United States annually, with over 15 million catheter days documented in intensive care units alone.1 Traditional CVC insertion techniques using landmarks are associated with a high risk of mechanical complications, particularly pneumothorax and arterial puncture, which occur in 5%-19% patients.2,3
Since the 1990s, several randomized controlled studies and meta-analyses have demonstrated that the use of real-time ultrasound guidance for CVC insertion increases procedure success rates and decreases mechanical complications.4,5 Use of real-time ultrasound guidance was recommended by the Agency for Healthcare Research and Quality, the Institute of Medicine, the National Institute for Health and Care Excellence, the Centers for Disease Control and Prevention, and several medical specialty societies in the early 2000s.6-14 Despite these recommendations, ultrasound guidance has not been universally adopted. Currently, an estimated 20%-55% of CVC insertions in the internal jugular vein are performed without ultrasound guidance.15-17
Following the emergence of literature supporting the use of ultrasound guidance for CVC insertion, observational and randomized controlled studies demonstrated improved procedural success rates with the use of ultrasound guidance for the insertion of peripheral intravenous lines (PIVs), arterial catheters, and peripherally inserted central catheters (PICCs).18-23
The purpose of this position statement is to present evidence-based recommendations on the use of ultrasound guidance for the insertion of central and peripheral vascular access catheters in adult patients. This document presents consensus-based recommendations with supporting evidence for clinical outcomes, techniques, and training for the use of ultrasound guidance for vascular access. We have subdivided the recommendations on techniques for central venous access, peripheral venous access, and arterial access individually, as some providers may not perform all types of vascular access procedures.
These recommendations are intended for hospitalists and other healthcare providers that routinely place central and peripheral vascular access catheters in acutely ill patients. However, this position statement does not mandate that all hospitalists should place central or peripheral vascular access catheters given the diverse array of hospitalist practice settings. For training and competency assessments, we recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals, where equipment and staffing for assessments are not available. Recommendations and frameworks for initial and ongoing credentialing of hospitalists in ultrasound-guided bedside procedures have been previously published in an Society of Hospital Medicine (SHM) position statement titled, “Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.”24
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interest (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the vascular access working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. An updated search was conducted in November 2017. The literature search strings are included in Appendix 3. All article abstracts were initially screened for relevance by at least two members of the vascular access working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide vascular access were selected. The following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled studies, and observational studies of ultrasound-guided vascular access were screened and selected (Appendix 3, Figure 1). All full-text articles were shared electronically among the working group members, and final article selection was based on working group consensus. Selected articles were incorporated into the draft recommendations.
These recommendations were developed using the Research and Development (RAND) Appropriateness Method that required panel judgment and consensus.14 The 28 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Using an internet-based electronic data collection tool (REDCap™), panel members participated in two rounds of electronic voting, one in August 2018 and the other in October 2018 (Appendix 4). Voting on appropriateness was conducted using a nine-point Likert scale. The three zones of the nine-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix 1, Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” Disagreement was defined as >30% of panelists voting outside of the zone of the median. A strong recommendation required at least 80% of the votes within one integer of the median per the RAND rules.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording for each recommendation (Table 2). The final version of the consensus-based recommendations underwent internal and external review by members of the SHM POCUS Task Force, the SHM Education Committee, and the SHM Executive Committee. The SHM Executive Committee reviewed and approved this position statement prior to its publication in the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 5,563 references were pooled from an initial search performed by a certified medical librarian in December 2015 (4,668 citations) which was updated in November 2017 (791 citations), and from the personal bibliographies and searches (104 citations) performed by working group members. A total of 514 full-text articles were reviewed. The final selection included 192 articles that were abstracted into a data table and incorporated into the draft recommendations. See Appendix 3 for details of the literature search strategy.
Recommendations
Four domains (technique, clinical outcomes, training, and knowledge gaps) with 31 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation cite supporting evidence. After two rounds of panel voting, 31 recommendations achieved agreement based on the RAND rules. During the peer review process, two of the recommendations were merged with other recommendations. Thus, a total of 29 recommendations received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Twenty-seven statements were approved as strong recommendations, and two were approved as weak/conditional recommendations. The strength of each recommendation and degree of consensus are summarized in Table 3.
Terminology
Central Venous Catheterization
Central venous catheterization refers to insertion of tunneled or nontunneled large bore vascular catheters that are most commonly inserted into the internal jugular, subclavian, or femoral veins with the catheter tip located in a central vein. These vascular access catheters are synonymously referred to as central lines or central venous catheters (CVCs). Nontunneled catheters are designed for short-term use and should be removed promptly when no longer clinically indicated or after a maximum of 14 days.25
Peripherally Inserted Central Catheter (PICC)
Peripherally inserted central catheters, or PICC lines, are inserted most commonly in the basilic or brachial veins in adult patients, and the catheter tip terminates in the distal superior vena cava or cavo-atrial junction. These catheters are designed to remain in place for a duration of several weeks, as long as it is clinically indicated.
Midline Catheterization
Midline catheters are a type of peripheral venous catheter that are an intermediary between a peripheral intravenous catheter and PICC line. Midline catheters are most commonly inserted in the brachial or basilic veins, but unlike PICC lines, the tips of these catheters terminate in the axillary or subclavian vein. Midline catheters are typically 8 cm to 20 cm in length and inserted for a duration <30 days.
Peripheral Intravenous Catheterization
Peripheral intravenous lines (PIV) refer to small bore venous catheters that are most commonly 14G to 24G and inserted into patients for short-term peripheral venous access. Common sites of ultrasound-guided PIV insertion include the superficial and deep veins of the hand, forearm, and arm.
Arterial Catheterization
Arterial catheters are commonly used for reliable blood pressure monitoring, frequent arterial blood
RECOMMENDATIONS
Preprocedure
1. We recommend that providers should be familiar with the operation of their specific ultrasound machine prior to initiation of a vascular access procedure.
Rationale: There is strong consensus that providers must be familiar with the knobs and functions of the specific make and model of ultrasound machine that will be utilized for a vascular access procedure. Minimizing adjustments to the ultrasound machine during the procedure may reduce the risk of contaminating the sterile field.
2. We recommend that providers should use a high-frequency linear transducer with a sterile sheath and sterile gel to perform vascular access procedures.
Rationale: High-frequency linear-array transducers are recommended for the vast majority of vascular access procedures due to their superior resolution compared to other transducer types. Both central and peripheral vascular access procedures, including PIV, PICC, and arterial line placement, should be performed using sterile technique. A sterile transducer cover and sterile gel must be utilized, and providers must be trained in sterile preparation of the ultrasound transducer.13,26,27
The depth of femoral vessels correlates with body mass index (BMI). When accessing these vessels in a morbidly obese patient with a thigh circumference >60 cm and vessel depth >8 cm, a curvilinear transducer may be preferred for its deeper penetration.28 For patients who are poor candidates for bedside insertion of vascular access catheters, such as uncooperative patients, patients with atypical vascular anatomy or poorly visualized target vessels, we recommend consultation with a vascular access specialist prior to attempting the procedure.
3. We recommend that providers should use two-dimensional ultrasound to evaluate for anatomical variations and absence of vascular thrombosis during preprocedural site selection.
Rationale: A thorough ultrasound examination of the target vessel is warranted prior to catheter placement. Anatomical variations that may affect procedural decision-making are easily detected with ultrasound. A focused vascular ultrasound examination is particularly important in patients who have had temporary or tunneled venous catheters, which can cause stenosis or thrombosis of the target vein.
For internal jugular vein (IJV) CVCs, ultrasound is useful for visualizing the relationship between the IJV and common carotid artery (CCA), particularly in terms of vessel overlap. Furthermore, ultrasound allows for immediate revisualization upon changes in head position.29-32 Troianos et al. found >75% overlap of the IJV and CCA in 54% of all patients and in 64% of older patients (age >60 years) whose heads were rotated to the contralateral side.30 In one study of IJV CVC insertion, inadvertent carotid artery punctures were reduced (3% vs 10%) with the use of ultrasound guidance vs landmarks alone.33 In a cohort of 64 high-risk neurosurgical patients, cannulation success was 100% with the use of ultrasound guidance, and there were no injuries to the carotid artery, even though the procedure was performed with a 30-degree head elevation and anomalous IJV anatomy in 39% of patients.34 In a prospective, randomized controlled study of 1,332 patients, ultrasound-guided cannulation in a neutral position was demonstrated to be as safe as the 45-degree rotated position.35
Ultrasound allows for the recognition of anatomical variations which may influence the selection of the vascular access site or technique. Benter et al. found that 36% of patients showed anatomical variations in the IJV and surrounding tissue.36 Similarly Caridi showed the anatomy of the right IJV to be atypical in 29% of patients,37 and Brusasco found that 37% of bariatric patients had anatomical variations of the IJV.38 In a study of 58 patients, there was significant variability in the IJV position and IJV diameter, ranging from 0.5 cm to >2 cm.39 In a study of hemodialysis patients, 75% of patients had sonographic venous abnormalities that led to a change in venous access approach.40
To detect acute or chronic upper extremity deep venous thrombosis or stenosis, two-dimensional visualization with compression should be part of the ultrasound examination prior to central venous catheterization. In a study of patients that had undergone CVC insertion 9-19 weeks earlier, 50% of patients had an IJV thrombosis or stenosis leading to selection of an alternative site. In this study, use of ultrasound for a preprocedural site evaluation reduced unnecessary attempts at catheterizing an occluded vein.41 At least two other studies demonstrated an appreciable likelihood of thrombosis. In a study of bariatric patients, 8% of patients had asymptomatic thrombosis38 and in another study, 9% of patients being evaluated for hemodialysis catheter placement had asymptomatic IJV thrombosis.37
4. We recommend that providers should evaluate the target blood vessel size and depth during a preprocedural ultrasound evaluation.
Rationale: The size, depth, and anatomic location of central veins can vary considerably. These features are easily discernable using ultrasound. Contrary to traditional teaching, the IJV is located 1 cm anterolateral to the CCA in only about two-thirds of patients.37,39,42,43 Furthermore, the diameter of the IJV can vary significantly, ranging from 0.5 cm to >2 cm.39 The laterality of blood vessels may vary considerably as well. A preprocedural ultrasound evaluation of contralateral subclavian and axillary veins showed a significant absolute difference in cross-sectional area of 26.7 mm2 (P < .001).42
Blood vessels can also shift considerably when a patient is in the Trendelenburg position. In one study, the IJV diameter changed from 11.2 (± 1.5) mm to 15.4 (± 1.5) mm in the supine versus the Trendelenburg position at 15 degrees.33 An observational study demonstrated a frog-legged position with reverse Trendelenburg increased the femoral vein size and reduced the common surface area with the common femoral artery compared to a neutral position. Thus, a frog-legged position with reverse Trendelenburg position may be preferred, since overall catheterization success rates are higher in this position.44
Techniques
General Techniques
5. We recommend that providers should avoid using static ultrasound alone to mark the needle insertion site for vascular access procedures.
Rationale: The use of static ultrasound guidance to mark a needle insertion site is not recommended because normal anatomical relationships of vessels vary, and site marking can be inaccurate with minimal changes in patient position, especially of the neck.43,45,46 Benefits of using ultrasound guidance for vascular access are attained when ultrasound is used to track the needle tip in real-time as it is advanced toward the target vessel.
Although continuous-wave Doppler ultrasound without two-dimensional visualization was used in the past, it is no longer recommended for IJV CVC insertion.47 In a study that randomized patients to IJV CVC insertion with continuous-wave Doppler alone vs two-dimensional ultrasound guidance, the use of two-dimensional ultrasound guidance showed significant improvement in first-pass success rates (97% vs 91%, P = .045), particularly in patients with BMI >30 (97% vs 77%, P = .011).48
A randomized study comparing real-time ultrasound-guided, landmark-based, and ultrasound-marked techniques found higher success rates in the real-time ultrasound-guided group than the other two groups (100% vs 74% vs 73%, respectively; P = .01). The total number of mechanical complications was higher in the landmark-based and ultrasound-marked groups than in the real-time ultrasound-guided group (24% and 36% versus 0%, respectively; P = .01).49 Another randomized controlled study found higher success rates with real-time ultrasound guidance (98%) versus an ultrasound-marked (82%) or landmark-based (64%) approach for central line placement.50
6. We recommend that providers should use real-time (dynamic), two-dimensional ultrasound guidance with a high-frequency linear transducer for CVC insertion, regardless of the provider’s level of experience.
7. We suggest using either a transverse (short-axis) or longitudinal (long-axis) approach when performing real-time ultrasound-guided vascular access procedures.
Rationale: In clinical practice, the phrases transverse, short-axis, or out-of-plane approach are synonymous, as are longitudinal, long-axis, and in-plane approach. The short-axis approach involves tracking the needle tip as it approximates the target vessel with the ultrasound beam oriented in a transverse plane perpendicular to the target vessel. The target vessel is seen as a circular structure on the ultrasound screen as the needle tip approaches the target vessel from above. This approach is also called the out-of-plane technique since the needle passes through the ultrasound plane. The advantages of the short-axis approach include better visualization of adjacent vessels or nerves and the relative ease of skill acquisition for novice operators.9 When using the short-axis approach, extra care must be taken to track the needle tip from the point of insertion on the skin to the target vessel. A disadvantage of the short-axis approach is unintended posterior wall puncture of the target vessel.55
In contrast to a short-axis approach, a long-axis approach is performed with the ultrasound beam aligned parallel to the vessel. The vessel appears as a long tubular structure and the entire needle is visualized as it traverses across the ultrasound screen to approach the target vessel. The long-axis approach is also called an in-plane technique because the needle is maintained within the plane of the ultrasound beam. The advantage of a long-axis approach is the ability to visualize the entire needle as it is inserted into the vessel.14 A randomized crossover study with simulation models compared a long-axis versus short-axis approach for both IJV and subclavian vein catheterization. This study showed decreased number of needle redirections (relative risk (RR) 0.5, 95% confidence interval (CI) 0.3 to 0.7), and posterior wall penetrations (OR 0.3, 95% CI 0.1 to 0.9) using a long-axis versus short-axis approach for subclavian vein catheterization.56
A randomized controlled study comparing a long-axis or short-axis approach with ultrasound versus a landmark-based approach for IJV CVC insertion showed higher success rates (100% vs 90%; P < .001), lower insertion time (53 vs 116 seconds; P < .001), and fewer attempts to obtain access (2.5 vs 1.2 attempts, P < .001) with either the long- or short-axis ultrasound approach. The average time to obtain access and number of attempts were comparable between the short-axis and long-axis approaches with ultrasound. The incidence of carotid puncture and hematoma was significantly higher with the landmark-based approach versus either the long- or short-axis ultrasound approach (carotid puncture 17% vs 3%, P = .024; hematoma 23% vs 3%, P = .003).57
High success rates have been reported using a short-axis approach for insertion of PIV lines.58 A prospective, randomized trial compared the short-axis and long-axis approach in patients who had had ≥2 failed PIV insertion attempts. Success rate was 95% (95% CI, 0.85 to 1.00) in the short-axis group compared with 85% (95% CI, 0.69 to 1.00) in the long-axis group. All three subjects with failed PIV placement in the long-axis group had successful rescue placement using a short-axis approach. Furthermore, the short-axis approach was faster than the long-axis approach.59
For radial artery cannulation, limited data exist comparing the short- and long-axis approaches. A randomized controlled study compared a long-axis vs short-axis ultrasound approach for radial artery cannulation. Although the overall procedure success rate was 100% in both groups, the long-axis approach had higher first-pass success rates (1.27 ± 0.4 vs 1.5 ± 0.5, P < .05), shorter cannulation times (24 ± 17 vs 47 ± 34 seconds, P < .05), fewer hematomas (4% vs 43%, P < .05) and fewer posterior wall penetrations (20% vs 56%, P < .05).60
Another technique that has been described for IJV CVC insertion is an oblique-axis approach, a hybrid between the long- and short-axis approaches. In this approach, the transducer is aligned obliquely over the IJV and the needle is inserted using a long-axis or in-plane approach. A prospective randomized trial compared the short-axis, long-axis, and oblique-axis approaches during IJV cannulation. First-pass success rates were 70%, 52%, and 74% with the short-axis, long-axis, and oblique-axis approaches, respectively, and a statistically significant difference was found between the long- and oblique-axis approaches (P = .002). A higher rate of posterior wall puncture was observed with a short-axis approach (15%) compared with the oblique-axis (7%) and long-axis (4%) approaches (P = .047).61
8. We recommend that providers should visualize the needle tip and guidewire in the target vein prior to vessel dilatation.
Rationale: When real-time ultrasound guidance is used, visualization of the needle tip within the vein is the first step to confirm cannulation of the vein and not the artery. After the guidewire is advanced, the provider can use transverse and longitudinal views to reconfirm cannulation of the vein. In a longitudinal view, the guidewire is readily seen positioned within the vein, entering the anterior wall and lying along the posterior wall of the vein. Unintentional perforation of the posterior wall of the vein with entry into the underlying artery can be detected by ultrasound, allowing prompt removal of the needle and guidewire before proceeding with dilation of the vessel. In a prospective observational study that reviewed ultrasound-guided IJV CVC insertions, physicians were able to more readily visualize the guidewire than the needle in the vein.62 A prospective observational study determined that novice operators can visualize intravascular guidewires in simulation models with an overall accuracy of 97%.63
In a retrospective review of CVC insertions where the guidewire position was routinely confirmed in the target vessel prior to dilation, there were no cases of arterial dilation, suggesting confirmation of guidewire position can potentially eliminate the morbidity and mortality associated with arterial dilation during CVC insertion.64
9. To increase the success rate of ultrasound-guided vascular access procedures, we recommend that providers should utilize echogenic needles, plastic needle guides, and/or ultrasound beam steering when available.
Rationale: Echogenic needles have ridged tips that appear brighter on the screen, allowing for better visualization of the needle tip. Plastic needle guides help stabilize the needle alongside the transducer when using either a transverse or longitudinal approach. Although evidence is limited, some studies have reported higher procedural success rates when using echogenic needles, plastic needle guides, and ultrasound beam steering software. In a prospective observational study, Augustides et al. showed significantly higher IJV cannulation rates with versus without use of a needle guide after first (81% vs 69%, P = .0054) and second (93% vs 80%. P = .0001) needle passes.65 A randomized study by Maecken et al. compared subclavian vein CVC insertion with or without use of a needle guide, and found higher procedure success rates within the first and second attempts, reduced time to obtain access (16 seconds vs 30 seconds; P = .0001) and increased needle visibility (86% vs 32%; P < .0001) with the use of a needle guide.66 Another study comparing a short-axis versus long-axis approach with a needle guide showed improved needle visualization using a long-axis approach with a needle guide.67 A randomized study comparing use of a novel, sled-mounted needle guide to a free-hand approach for venous cannulation in simulation models showed the novel, sled-mounted needle guide improved overall success rates and efficiency of cannulation.68
Central Venous Access Techniques
10. We recommend that providers should use a standardized procedure checklist that includes use of real-time ultrasound guidance to reduce the risk of central line-associated bloodstream infection (CLABSI) from CVC insertion.
Rationale: A standardized checklist or protocol should be developed to ensure compliance with all recommendations for insertion of CVCs. Evidence-based protocols address periprocedural issues, such as indications for CVC, and procedural techniques, such as use of maximal sterile barrier precautions to reduce the risk of infection. Protocols and checklists that follow established guidelines for CVC insertion have been shown to decrease CLABSI rates.69,70 Similarly, development of checklists and protocols for maintenance of central venous catheters have been effective in reducing CLABSIs.71 Although no externally-validated checklist has been universally accepted or endorsed by national safety organizations, a few internally-validated checklists are available through peer-reviewed publications.72,73 An observational educational cohort of internal medicine residents who received training using simulation of the entire CVC insertion process was able to demonstrate fewer CLABSIs after the simulator-trained residents rotated in the intensive care unit (ICU) (0.50 vs 3.2 infections per 1,000 catheter days, P = .001).74
11. We recommend that providers should use real-time ultrasound guidance, combined with aseptic technique and maximal sterile barrier precautions, to reduce the incidence of infectious complications from CVC insertion.
Rationale: The use of real-time ultrasound guidance for CVC placement has demonstrated a statistically significant reduction in CLABSIs compared to landmark-based techniques.75 The Centers for Disease Control and Prevention (CDC) guidelines for the prevention of intravascular catheter-related infections recommend the use of ultrasound guidance to reduce the number of cannulation attempts and risk of mechanical complications.69 A prospective, three-arm study comparing ultrasound-guided long-axis, short-axis, and landmark-based approaches showed a CLABSI rate of 20% in the landmark-based group versus 10% in each of the ultrasound groups.57 Another randomized study comparing use of ultrasound guidance to a landmark-based technique for IJV CVC insertion demonstrated significantly lower CLABSI rates with the use of ultrasound (2% vs 10%; P < .05).72
Studies have shown that a systems-based intervention featuring a standardized catheter kit or catheter bundle significantly reduced CLABSI rates.76-78 A complete review of all preventive measures to reduce the risk of CLABSI is beyond the scope of this review, but a few key points will be mentioned. First, aseptic technique includes proper hand hygiene and skin sterilization, which are essential measures to reduce cutaneous colonization of the insertion site and reduce the risk of CLABSIs.79 In a systematic review and meta-analysis of eight studies including over 4,000 catheter insertions, skin antisepsis with chlorhexidine was associated with a 50% reduction in CLABSIs compared with povidone iodine.11 Therefore, a chlorhexidine-containing solution is recommended for skin preparation prior to CVC insertion per guidelines by Healthcare Infection Control Practices Advisory Committee/CDC, Society for Healthcare Epidemiology of America/Infectious Diseases Society of America, and American Society of Anesthesiologists.11,69,80,81 Second, maximal sterile barrier precautions refer to the use of sterile gowns, sterile gloves, caps, masks covering both the mouth and nose, and sterile full-body patient drapes. Use of maximal sterile barrier precautions during CVC insertion has been shown to reduce the incidence of CLABSIs compared to standard precautions.26,79,82-84 Third, catheters containing antimicrobial agents may be considered for hospital units with higher CLABSI rates than institutional goals, despite a comprehensive preventive strategy, and may be considered in specific patient populations at high risk of severe complications from a CLABSI.11,69,80 Finally, providers should use a standardized procedure set-up when inserting CVCs to reduce the risk of CLABSIs. The operator should confirm availability and proper functioning of ultrasound equipment prior to commencing a vascular access procedure. Use of all-inclusive procedure carts or kits with sterile ultrasound probe covers, sterile gel, catheter kits, and other necessary supplies is recommended to minimize interruptions during the procedure, and can ultimately reduce the risk of CLABSIs by ensuring maintenance of a sterile field during the procedure.13
12. We recommend that providers should use real-time ultrasound guidance for internal jugular vein catheterization, which reduces the risk of mechanical and infectious complications, the number of needle passes, and time to cannulation and increases overall procedure success rates.
Rationale: The use of real-time ultrasound guidance for CVC insertion has repeatedly demonstrated better outcomes compared to a landmark-based approach in adults.13 Several randomized controlled studies have demonstrated that real-time ultrasound guidance for IJV cannulation reduces the risk of procedure-related mechanical and infectious complications, and improves first-pass and overall success rates in diverse care settings.27,29,45,50,53,65,75,85-90 Mechanical complications that are reduced with ultrasound guidance include pneumothorax and carotid artery puncture.4,5,45,46,53,62,75,86-93 Currently, several medical societies strongly recommend the use of ultrasound guidance during insertion of IJV CVCs.10-12,14,94-96
A meta-analysis by Hind et al. that included 18 randomized controlled studies demonstrated use of real-time ultrasound guidance reduced failure rates (RR 0.14, 95% CI 0.06 to 0.33; P < .0001), increased first-attempt success rates (RR 0.59, 95% CI 0.39 to 0.88; P = .009), reduced complication rates (RR 0.43, 95% CI 0.22 to 0.87; P = .02) and reduced procedure time (P < .0001), compared to a traditional landmark-based approach when inserting IJV CVCs.5
A Cochrane systematic review compared ultrasound-guided versus landmark-based approaches for IJV CVC insertion and found use of real-time ultrasound guidance reduced total complication rates by 71% (RR 0.29, 95% CI 0.17 to 0.52; P < .0001), arterial puncture rates by 72% (RR 0.28, 95% CI 0.18 to 0.44; P < .00001), and rates of hematoma formation by 73% (RR 0.27, 95% CI 0.13 to 0.55; P = .0004). Furthermore, the number of attempts for successful cannulation was reduced (mean difference -1.19 attempts, 95% CI -1.45 to -0.92; P < .00001), the chance of successful insertion on the first attempt was increased by 57% (RR 1.57, 95% CI 1.36 to 1.82; P < .00001), and overall procedure success rates were modestly increased in all groups by 12% (RR 1.12, 95% CI 1.08 to 1.17; P < .00001).46
An important consideration in performing ultrasound guidance is provider experience. A prospective observational study of patients undergoing elective CVC insertion demonstrated higher complication rates for operators that were inexperienced (25.2%) versus experienced (13.6%).54 A randomized controlled study comparing experts and novices with or without the use of ultrasound guidance for IJV CVC insertion demonstrated higher success rates among expert operators and with the use of ultrasound guidance. Among novice operators, the complication rates were lower with the use of ultrasound guidance.97 One study evaluated the procedural success and complication rates of a two-physician technique with one physician manipulating the transducer and another inserting the needle for IJV CVC insertion. This study concluded that procedural success rates and frequency of complications were directly affected by the experience of the physician manipulating the transducer and not by the experience of the physician inserting the needle.98
The impact of ultrasound guidance on improving procedural success rates and reducing complication rates is greatest in patients that are obese, short necked, hypovolemic, or uncooperative.93 Several studies have demonstrated fewer needle passes and decreased time to cannulation compared to the landmark technique in these populations.46,49,53,86-88,92,93
Ultrasound-guided placement of IJV catheters can safely be performed in patients with disorders of hemostasis and those with multiple previous catheter insertions in the same vein.9 Ultrasound-guided placement of CVCs in patients with disorders of hemostasis is safe with high success and low complication rates. In a case series of liver patients with coagulopathy (mean INR 2.17 ± 1.16, median platelet count 150K), the use of ultrasound guidance for CVC insertion was highly successful with no major bleeding complications.99
A study of renal failure patients found high success rates and low complication rates in the patients with a history of multiple previous catheterizations, poor compliance, skeletal deformities, previous failed cannulations, morbid obesity, and disorders of hemostasis.100 A prospective observational study of 200 ultrasound-guided CVC insertions for apheresis showed a 100% success rate with a 92% first-pass success rate.101
The use of real-time ultrasound guidance for IJV CVC insertion has been shown to be cost effective by reducing procedure-related mechanical complications and improving procedural success rates. A companion cost-effectiveness analysis estimated that for every 1,000 patients, 90 complications would be avoided, with a net cost savings of approximately $3,200 using 2002 prices.102
13. We recommend that providers who routinely insert subclavian vein CVCs should use real-time ultrasound guidance, which has been shown to reduce the risk of mechanical complications and number of needle passes and increase overall procedure success rates compared with landmark-based techniques.
Rationale: In clinical practice, the term ultrasound-guided subclavian vein CVC insertion is commonly used. However, the needle insertion site is often lateral to the first rib and providers are technically inserting the CVC in the axillary vein. The subclavian vein becomes the axillary vein at the lateral border of the first rib where the cephalic vein branches from the subclavian vein. To be consistent with common medical parlance, we use the phrase ultrasound-guided subclavian vein CVC insertion in this document.
Advantages of inserting CVCs in the subclavian vein include reliable surface anatomical landmarks for vein location, patient comfort, and lower risk of infection.103 Several observational studies have demonstrated the technique for ultrasound-guided subclavian vein CVC insertion is feasible and safe.104-107 In a large retrospective observational study of ultrasound-guided central venous access among a complex patient group, the majority of patients were cannulated successfully and safely. The subset of patients undergoing axillary vein CVC insertion (n = 1,923) demonstrated a low rate of complications (0.7%), proving it is a safe and effective alternative to the IJV CVC insertion.107
A Cochrane review of ultrasound-guided subclavian vein cannulation (nine studies, 2,030 participants, 2,049 procedures), demonstrated that real-time two-dimensional ultrasound guidance reduced the risk of inadvertent arterial punctures (three studies, 498 participants, RR 0.21, 95% CI 0.06 to 0.82; P = .02) and hematoma formation (three studies, 498 participants, RR 0.26, 95% CI 0.09 to 0.76; P = .01).46 A systematic review and meta-analysis of 10 randomized controlled studies comparing ultrasound-guided versus landmark-based subclavian vein CVC insertion demonstrated a reduction in the risk of arterial punctures, hematoma formation, pneumothorax, and failed catheterization with the use of ultrasound guidance.105
A randomized controlled study comparing ultrasound-guided vs landmark-based approaches to subclavian vein cannulation found that use of ultrasound guidance had a higher success rate (92% vs 44%, P = .0003), fewer minor complications (1 vs 11, P = .002), fewer attempts (1.4 vs 2.5, P = .007) and fewer catheter kits used (1.0 vs 1.4, P = .0003) per cannulation.108
Fragou et al. randomized patients undergoing subclavian vein CVC insertion to a long-axis approach versus a landmark-based approach and found a significantly higher success rate (100% vs 87.5%, P < .05) and lower rates of mechanical complications: artery puncture (0.5% vs 5.4%), hematoma (1.5% vs 5.4%), hemothorax (0% vs 4.4%), pneumothorax (0% vs 4.9%), brachial plexus injury (0% vs 2.9%), phrenic nerve injury (0% vs 1.5%), and cardiac tamponade (0% vs 0.5%).109 The average time to obtain access and the average number of insertion attempts (1.1 ± 0.3 vs 1.9 ± 0.7, P < .05) were significantly reduced in the ultrasound group compared to the landmark-based group.95
A retrospective review of subclavian vein CVC insertions using a supraclavicular approach found no reported complications with the use of ultrasound guidance vs 23 mechanical complications (8 pneumothorax, 15 arterial punctures) with a landmark-based approach.106 However, it is important to note that a supraclavicular approach is not commonly used in clinical practice.
14. We recommend that providers should use real-time ultrasound guidance for femoral venous access, which has been shown to reduce the risk of arterial punctures and total procedure time and increase overall procedure success rates.
Rationale: Anatomy of the femoral region varies, and close proximity or overlap of the femoral vein and artery is common.51 Early studies showed that ultrasound guidance for femoral vein CVC insertion reduced arterial punctures compared with a landmark-based approach (7% vs 16%), reduced total procedure time (55 ± 19 vs 79 ± 62 seconds), and increased procedure success rates (100% vs 90%).52 A Cochrane review that pooled data from four randomized studies comparing ultrasound-guided vs landmark-based femoral vein CVC insertion found higher first-attempt success rates with the use of ultrasound guidance (RR 1.73, 95% CI 1.34 to 2.22; P < .0001) and a small increase in the overall procedure success rates (RR 1.11, 95% CI 1.00 to 1.23; P = .06). There was no difference in inadvertent arterial punctures or other complications.110
Peripheral Venous Access Techniques
15. We recommend that providers should use real-time ultrasound guidance for the insertion of peripherally inserted central catheters (PICCs), which is associated with higher procedure success rates and may be more cost effective compared with landmark-based techniques.
Rationale: Several studies have demonstrated that providers who use ultrasound guidance vs landmarks for PICC insertion have higher procedural success rates, lower complication rates, and lower total placement costs. A prospective observational report of 350 PICC insertions using ultrasound guidance reported a 99% success rate with an average of 1.2 punctures per insertion and lower total costs.20 A retrospective observational study of 500 PICC insertions by designated specialty nurses revealed an overall success rate of 95%, no evidence of phlebitis, and only one CLABSI among the catheters removed.21 A retrospective observational study comparing several PICC variables found higher success rates (99% vs 77%) and lower thrombosis rates (2% vs 9%) using ultrasound guidance vs landmarks alone.22 A study by Robinson et al. demonstrated that having a dedicated PICC team equipped with ultrasound increased their institutional insertion success rates from 73% to 94%.111
A randomized controlled study comparing ultrasound-guided versus landmark-based PICC insertion found high success rates with both techniques (100% vs 96%). However, there was a reduction in the rate of unplanned catheter removals (4.0% vs 18.7%; P = .02), mechanical phlebitis (0% vs 22.9%; P < .001), and venous thrombosis (0% vs 8.3%; P = .037), but a higher rate of catheter migration (32% vs 2.1%; P < .001). Compared with the landmark-based group, the ultrasound-guided group had significantly lower incidence of severe contact dermatitis (P = .038), and improved comfort and costs up to 3 months after PICC placement (P < .05).112
Routine postprocedure chest x-ray (CXR) is generally considered unnecessary if the PICC is inserted with real-time ultrasound guidance along with use of a newer tracking devices, like the magnetic navigation system with intracardiac electrodes.9 Ultrasound can also be used to detect malpositioning of a PICC immediately after completing the procedure. A randomized controlled study comparing ultrasound versus postprocedure CXR detected one malpositioned PICC in the ultrasound group versus 11 in the control group. This study suggested that ultrasound can detect malpositioning immediately postprocedure and reduce the need for a CXR and the possibility of an additional procedure to reposition a catheter.113
16. We recommend that providers should use real-time ultrasound guidance for the placement of peripheral intravenous lines (PIV) in patients with difficult peripheral venous access to reduce the total procedure time, needle insertion attempts, and needle redirections. Ultrasound-guided PIV insertion is also an effective alternative to CVC insertion in patients with difficult venous access.
Rationale: Difficult venous access refers to patients that have had two unsuccessful attempts at PIV insertion using landmarks or a history of difficult access (i.e. edema, obesity, intravenous drug use, chemotherapy, diabetes, hypovolemia, chronic illness, vasculopathy, multiple prior hospitalizations). A meta-analysis of seven randomized controlled studies concluded that ultrasound guidance increases the likelihood of successful PIV insertion (pooled OR 2.42, 95% CI 1.26 to 4.68; P < .008).18 A second meta-analysis that pooled data from seven studies (six randomized controlled studies) confirmed that ultrasound guidance improves success rates of PIV insertion (OR 3.96, 95% CI 1.75 to 8.94).19 Approximately half of these studies had physician operators while the other half had nurse operators.
In one prospective observational study of emergency department patients with two failed attempts of landmark-based PIV insertion, ultrasound guidance with a modified-Seldinger technique showed a relatively high success rate (96%), fewer needle sticks (mean 1.32 sticks, 95% CI 1.12 to 1.52), and shorter time to obtain access (median time 68 seconds).114 Other prospective observational studies have demonstrated that ultrasound guidance for PIV insertion has a high success rate (87%),115 particularly with brachial or basilic veins PIV insertion, among patients with difficult PIV access, defined as having had ≥2 failed attempts.58
Since insertion of PIVs with ultrasound guidance has a high success rate, there is potential to reduce the reliance on CVC insertion for venous access only. In a study of patients that had had two failed attempts at PIV insertion based on landmarks, a PIV was successfully inserted with ultrasound guidance in 84% of patients, obviating the need for CVC placement for venous access.116 A prospective observational study showed ultrasound-guided PIV insertion was an effective alternative to CVC placement in ED patients with difficult venous access with only 1% of patients requiring a CVC.117 Use of ultrasound by nurses for PIV placement has also been shown to reduce the time to obtain venous access, improve patient satisfaction, and reduce the need for physician intervention.118 In a prospective observational study of patients with difficult access, the majority of patients reported a better experience with ultrasound-guided PIV insertion compared to previous landmark-based attempts with an average satisfaction score of 9.2/10 with 76% of patients rating the experience a 10.119 A strong recommendation has been made for use of ultrasound guidance in patients with difficult PIV placement by la Société Française d’Anesthésie et de Réanimation (The French Society of Anesthesia and Resuscitation).95
17. We suggest using real-time ultrasound guidance to reduce the risk of vascular, infectious, and neurological complications during PIV insertion, particularly in patients with difficult venous access.
Rationale: The incidence of complications from PIV insertion is often underestimated. Vascular complications include arterial puncture, hematoma formation, local infiltration or extravasation of fluid, and superficial or deep venous thrombosis. The most common infectious complications with PIV insertion are phlebitis and cellulitis.120 One observational study reported PIV complications occurring in approximately half of all patients with the most common complications being phlebitis, hematoma formation, and fluid/blood leakage.121
A retrospective review of ICU patients who underwent ultrasound-guided PIV insertion by a single physician showed high success rates (99%) with low rates of phlebitis/cellulitis (0.7%).There was an assumed benefit of risk reduction due to the patients no longer requiring a CVC after successful PIV placement.122 Another study found very low rates of infection with both landmark-based and ultrasound-guided PIV placement performed by emergency department nurses, suggesting that there is no increased risk of infection with the use of ultrasound.123 To reduce the risk of infection from PIV insertion, we recommend the use of sterile gel and sterile transducer cover (See Recommendation 2).
Arterial Access Techniques
18. We recommend that providers should use real-time ultrasound guidance for arterial access, which has been shown to increase first-pass success rates, reduce the time to cannulation, and reduce the risk of hematoma development compared with landmark-based techniques.
Rationale: Several randomized controlled studies have assessed the value of ultrasound in arterial catheter insertion. Shiver et al. randomized 60 patients admitted to a tertiary center emergency department to either palpation or ultrasound-guided arterial cannulation. They demonstrated a first-pass success rate of 87% in the ultrasound group compared with 50% in the landmark technique group. In the same study, the use of ultrasound was also associated with reduced time needed to establish arterial access and a 43% reduction in the development of hematoma at the insertion site.124 Levin et al. demonstrated a first-pass success rate of 62% using ultrasound versus 34% by palpation alone in 69 patients requiring intraoperative invasive hemodynamic monitoring.125 Additional randomized controlled studies have demonstrated that ultrasound guidance increases first-attempt success rates compared to traditional palpation.23,126,127
19. We recommend that providers should use real-time ultrasound guidance for femoral arterial access, which has been shown to increase first-pass success rates and reduce the risk of vascular complications.
Rationale: Although it is a less frequently used site, the femoral artery may be accessed for arterial blood sampling or invasive hemodynamic monitoring, and use of ultrasound guidance has been shown to improve the first-pass success rates of femoral artery cannulation. It is important to note that most of these studies comparing ultrasound-guided vs landmark-based femoral artery cannulation were performed in patients undergoing diagnostic or interventional vascular procedures.
A meta-analysis of randomized controlled studies comparing ultrasound-guided vs landmark-based femoral artery catheterization found use of ultrasound guidance was associated with a 49% reduction in overall complications (RR 0.51, 95% CI 0.28 to 0.91; P > .05) and 42% improvement in first-pass success rates.128 In another study, precise site selection with ultrasound was associated with fewer pseudoaneurysms in patients undergoing femoral artery cannulation by ultrasound guidance vs palpation for cardiac catheterization (3% vs 5%, P < .05).129
A multicenter randomized controlled study comparing ultrasound vs fluoroscopic guidance for femoral artery catheterization demonstrated ultrasound guidance improved rates of common femoral artery (CFA) cannulation in patients with high CFA bifurcations (83% vs 70%, P < .01).130 Furthermore, ultrasound guidance improved first-pass success rates (83% vs 46%, P < .0001), reduced number of attempts (1.3 vs 3.0, P < .0001), reduced risk of venipuncture (2.4% vs 15.8%, P < .0001), and reduced median time to obtain access (136 seconds vs148 seconds, P = .003). Vascular complications occurred in fewer patients in the ultrasound vs fluoroscopy groups (1.4% vs 3.4% P = .04). Reduced risk of hematoma formation with routine use of ultrasound guidance was demonstrated in one retrospective observational study (RR 0.62, 95% CI 0.46 to 0.84; P < .01).131
20. We recommend that providers should use real-time ultrasound guidance for radial arterial access, which has been shown to increase first-pass success rates, reduce the time to successful cannulation, and reduce the risk of complications compared with landmark-based techniques.
Rationale: Ultrasound guidance is particularly useful for radial artery cannulation in patients with altered anatomy, obesity, nonpulsatile blood flow, low perfusion, and previously unsuccessful cannulation attempts using a landmark-guided approach.132
A multicenter randomized controlled study that was not included in the abovementioned meta-analyses showed similar benefits of using ultrasound guidance vs landmarks for radial artery catheterization: a reduction in the number of attempts with ultrasound guidance (1.65 ± 1.2 vs 3.05 ± 3.4, P < .0001) and time to obtain access (88 ± 78 vs 108 ± 112 seconds, P = .006), and increased first-pass success rates (65% vs 44%, P < .0001). The use of ultrasound guidance was found to be particularly useful in patients with difficult access by palpation alone.135
Regarding the level of expertise required to use ultrasound guidance, a prospective observational study demonstrated that physicians with little previous ultrasound experience were able to improve their first-attempt success rates and procedure time for radial artery cannulation compared to historical data of landmark-based insertions.136
Postprocedure
21. We recommend that post-procedure pneumothorax should be ruled out by the detection of bilateral lung sliding using a high-frequency linear transducer before and after insertion of internal jugular and subclavian vein CVCs.
Rationale: Detection of lung sliding with two-dimensional ultrasound rules out pneumothorax, and disappearance of lung sliding in an area where it was previously seen is a strong predictor of postprocedure pneumothorax. In a study of critically ill patients, the disappearance of lung sliding was observed in 100% of patients with pneumothorax vs 8.8% of patients without pneumothorax. For detection of pneumothorax, lung sliding showed a sensitivity of 95%, specificity of 91%, and negative predictive value of 100% (P < .001).137 Another study by the same author showed that the combination of horizontal artifacts (absence of comet-tail artifact) and absence of lung sliding had a sensitivity of 100%, specificity of 96.5%, and negative predictive value of 100% for the detection of pneumothorax.138 A meta-analysis of 10 studies on the diagnostic accuracy of CVC confirmation with bedside ultrasound vs chest radiography reported detection of all 12 pneumothoraces with ultrasound, whereas chest radiography missed two pneumothoraces. The pooled sensitivity and specificity of ultrasound for the detection of pneumothorax was 100%, although an imperfect gold standard bias likely affected the results. An important advantage of bedside ultrasound is the ability to rule out pneumothorax immediately after the procedure while at the bedside. The mean time for confirmation of CVC placement with bedside ultrasound was 6 minutes versus 64 minutes and 143 minutes for completion and interpretation of a chest radiograph, respectively.139
22. We recommend that providers should use ultrasound with rapid infusion of agitated saline to visualize a right atrial swirl sign (RASS) for detecting catheter tip misplacement during CVC insertion. The use of RASS to detect the catheter tip may be considered an advanced skill that requires specific training and expertise.
Rationale: Bedside echocardiography is a reliable tool to detect catheter tip misplacement during CVC insertion. In one study, catheter misplacement was detected by bedside echocardiography with a sensitivity of 96% and specificity of 83% (positive predictive value 98%, negative predictive value 55%) and prevented distal positioning of the catheter tip.140 A prospective observational study assessed for RASS, which is turbulent flow in the right atrium after a rapid saline flush of the distal CVC port, to exclude catheter malposition. In this study with 135 CVC placements, visualization of RASS with ultrasound was able to identify all correct CVC placements and three of four catheter misplacements. Median times to complete the ultrasound exam vs CXR were 1 vs 20 minutes, respectively, with a median difference of 24 minutes (95% CI 19.6 to 29.3, P < .0001) between the two techniques.141
A prospective observational study assessed the ability of bedside transthoracic echocardiography to detect the guidewire, microbubbles, or both, in the right atrium compared to transesophageal echocardiography as the gold standard. Bedside transthoracic echocardiography allowed visualization of the right atrium in 94% of patients, and both microbubbles plus guidewire in 91% of patients.142 Hence, bedside transthoracic echocardiography allows adequate visualization of the right atrium. Another prospective observational study combining ultrasonography and contrast enhanced RASS resulted in 96% sensitivity and 93% specificity for the detection of a misplaced catheter, and the concordance with chest radiography was 96%.143
Training
23. To reduce the risk of mechanical and infectious complications, we recommend that novice providers should complete a systematic training program that includes a combination of simulation-based practice, supervised insertion on patients, and evaluation by an expert operator before attempting ultrasound-guided CVC insertion independently on patients.
Rationale: Cumulative experience has been recognized to not be a proxy for mastery of a clinical skill.144 The National Institute for Clinical Excellence (NICE) has recommended that providers performing ultrasound-guided CVC insertion should receive appropriate training to achieve competence before performing the procedure independently.7 Surveys have demonstrated that lack of training is a commonly reported barrier for not using ultrasound.145,146
Structured training programs on CVC insertion have been shown to reduce the occurrence of infectious and mechanical complications.74,143,147-149 The use of ultrasound and checklists, bundling of supplies, and practice with simulation models, as a part of a structured training program, can improve patient safety related to CVC insertion.9,140,150-154
Simulation-based practice has been used in medical education to provide deliberate practice and foster skill development in a controlled learning environment.155-158 Studies have shown transfer of skills demonstrated in a simulated environment to clinical practice, which can improve CVC insertion practices.159,160 Simulation accelerates learning of all trainees, especially novice trainees, and mitigates risks to patients by allowing trainees to achieve a minimal level of competence before attempting the procedure on real patients.152,161,162 Residents that have been trained using simulation preferentially select the IJV site,147 and more reliably use ultrasound to guide their CVC insertions.160,163
Additionally, simulation-based practice allows exposure to procedures and scenarios that may occur infrequently in clinical practice.
Although there is evidence on efficacy of simulation-based CVC training programs, there is no broadly accepted consensus on timing, duration, and content of CVC training programs for trainees or physicians in practice. The minimum recommended technical skills a trainee must master include the ability to (1) manipulate the ultrasound machine to produce a high-quality image to identify the target vessel, (2) advance the needle under direct visualization to the desired target site and depth, (3) deploy the catheter into the target vessel and confirm catheter placement in the target vessel using ultrasound, and (4) ensure the catheter has not been inadvertently placed in an unintended vessel or structure.153
A variety of simulation models are currently used to practice CVC insertion at the most common sites: the internal jugular, subclavian, basilic, and brachial veins.164,165 Effective simulation models should contain vessels that mimic normal anatomy with muscles, soft tissues, and bones. Animal tissue models, such as turkey or chicken breasts, may be effective for simulated practice of ultrasound-guided CVC insertion.166,167 Ultrasound-guided CVC training using human cadavers has also been shown to be effective.168
24. We recommend that cognitive training in ultrasound-guided CVC insertion should include basic anatomy, ultrasound physics, ultrasound machine knobology, fundamentals of image acquisition and interpretation, detection and management of procedural complications, infection prevention strategies, and pathways to attain competency.
Rationale: After receiving training in ultrasound-guided CVC insertion, physicians report significantly higher comfort with the use of ultrasound compared to those who have not received such training.145 Learners find training sessions worthwhile to increase skill levels,167 and skills learned from simulation-based mastery learning programs have been retained up to one year.158
Several commonalities have been noted across training curricula. Anatomy and physiology didactics should include vessel anatomy (location, size, and course);9 vessel differentiation by ultrasound;9,69 blood flow dynamics;69 Virchow’s triad;69 skin integrity and colonization;150 peripheral nerve identification and distribution;9 respiratory anatomy;9,69 upper and lower extremity, axillary, neck, and chest anatomy.9,69 Vascular anatomy is an essential curricular component that may help avoid preventable CVC insertion complications, such as inadvertent nerve, artery, or lung puncture.150,169 Training curricula should also include ultrasound physics (piezoelectric effect, frequency, resolution, attenuation, echogenicity, Doppler ultrasound, arterial and venous flow characteristics), image acquisition and optimization (imaging mode, focus, dynamic range, probe types), and artifacts (reverberation, mirror, shadowing, enhancement).
CVC-related infections are an important cause of morbidity and mortality in the acute and long-term care environment.69 Infection and thrombosis can both be impacted by the insertion site selection, skin integrity, and catheter–vein ratio.2,3,84 Inexperience generally leads to more insertion attempts that can increase trauma during CVC insertion and potentially increase the risk of infections.170 To reduce the risk of infectious complications, training should include important factors to consider in site selection and maintenance of a sterile environment during CVC insertion, including use of maximal sterile barrier precautions, hand hygiene, and appropriate use of skin antiseptic solutions.
Professional society guidelines have been published with recommendations of appropriate techniques for ultrasound-guided vascular access that include training recommendations.9,154 Training should deconstruct the insertion procedure into readily understood individual steps, and can be aided by demonstration of CVC insertion techniques using video clips. An alternative to face-to-face training is internet-based training that has been shown to be as effective as traditional teaching methods in some medical centers.171 Additional methods to deliver cognitive instruction include textbooks, continuing medical education courses, and digital videos.164,172
25. We recommend that trainees should demonstrate minimal competence before placing ultrasound-guided CVCs independently. A minimum number of CVC insertions may inform this determination, but a proctored assessment of competence is most important.
Rationale: CVC catheter placement carries the risk of serious complications including arterial injury or dissection, pneumothorax, or damage to other local structures; arrhythmias; catheter malposition; infection; and thrombosis. Although there is a lack of consensus and high-quality evidence for the certification of skills to perform ultrasound-guided CVC insertion, recommendations have been published advocating for formal and comprehensive training programs in ultrasound-guided CVC insertion with an emphasis on expert supervision prior to independent practice.9,153,154 Two groups of expert operators have recommended that training should include at least 8-10 supervised ultrasound-guided CVC insertions.154,173,174 A consensus task force from the World Congress of Vascular Access has recommended a minimum of six to eight hours of didactic education, four hours of hands-on training on simulation models, and six hours of hands-on ultrasound training on human volunteers to assess normal anatomy.175 This training should be followed by supervised ultrasound-guided CVC insertions until the learner has demonstrated minimal competence with a low rate of complications.35 There is general consensus that arbitrary numbers should not be the sole determinant of competence, and that the most important determinant of competence should be an evaluation by an expert operator.176
26. We recommend that didactic and hands-on training for trainees should coincide with anticipated times of increased performance of vascular access procedures. Refresher training sessions should be offered periodically.
Rationale: Simulation-based CVC training courses have shown a rapid improvement in skills, but lack of practice leads to deterioration of technical skills.161,162,177,178 Thus, a single immersive training session is insufficient to achieve and maintain mastery of skills, and an important factor to acquire technical expertise is sustained, deliberate practice with feedback.179 Furthermore, an insidious decay in skills may go unrecognized as a learner’s comfort and self-confidence does not always correlate with actual performance, leading to increased risk of errors and potential for procedural complications.147,158,180-183 Given the decay in technical skills over time, simulation-based training sessions are most effective when they occur in close temporal proximity to times when those skills are most likely to be used; for example, a simulation-based training session for trainees may be most effective just before the start of a critical care rotation.152 Regularly scheduled training sessions with monitoring and feedback by expert operators can reinforce procedural skills and prevent decay. Some experts have recommended that a minimum of 10 ultrasound-guided CVC insertions should be performed annually to maintain proficiency.153
27. We recommend that competency assessments should include formal evaluation of knowledge and technical skills using standardized assessment tools.
Rationale: Hospitalists and other healthcare providers that place vascular access catheters should undergo competency assessments proctored by an expert operator to verify that they have the required knowledge and skills.184,185 Knowledge competence can be partially evaluated using a written assessment, such as a multiple-choice test, assessing the provider’s cognitive understanding of the procedure.175 For ultrasound-guided CVC insertion, a written examination should be administered in conjunction with an ultrasound image assessment to test the learner’s recognition of normal vs abnormal vascular anatomy. Minimum passing standards should be established a priori according to local or institutional standards.
The final skills assessment should be objective, and the learner should be required to pass all critical steps of the procedure. Failure of the final skills assessment should lead to continued practice with supervision until the learner can consistently demonstrate correct performance of all critical steps. Checklists are commonly used to rate the technical performance of learners because they provide objective criteria for evaluation, can identify specific skill deficiencies, and can determine a learner’s readiness to perform procedures independently.186,187 The administration of skills assessments and feedback methods should be standardized across faculty. Although passing scores on both knowledge and skills assessments do not guarantee safe performance of a procedure independently, they provide a metric to ensure that a minimum level of competence has been achieved before allowing learners to perform procedures on patients without supervision.188
Competency assessments are a recommended component of intramural and extramural certification of skills in ultrasound-guided procedures. Intramural certification pathways differ by institution and often require additional resources including ultrasound machine(s), simulation equipment, and staff time, particularly when simulation-based assessments are incorporated into certification pathways. We recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals. However, initial and ongoing competency assessments can be performed during routine performance of procedures on patients. For an in-depth review of credentialing pathways for ultrasound-guided bedside procedures, we recommend reviewing the SHM Position Statement on Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.24
28. We recommend that competency assessments should evaluate for proficiency in the following knowledge and skills of CVC insertion:
a. Knowledge of the target vein anatomy, proper vessel identification, and recognition of anatomical variants
b. Demonstration of CVC insertion with no technical errors based on a procedural checklist
c. Recognition and management of acute complications, including emergency management of life-threatening complications
d. Real-time needle tip tracking with ultrasound and cannulation on the first attempt in at least five consecutive simulations.
Rationale: Recommendations have been published with the minimal knowledge and skills learners must demonstrate to perform ultrasound-guided vascular access procedures. These include operation of an ultrasound machine to produce high-quality images of the target vessel, tracking of the needle tip with real-time ultrasound guidance, and recognition and understanding of the management of procedural complications.154,175
First, learners must be able to perform a preprocedural assessment of the target vein, including size and patency of the vein; recognition of adjacent critical structures; and recognition of normal anatomical variants.175,189 Second, learners must be able to demonstrate proficiency in tracking the needle tip penetrating the target vessel, inserting the catheter into the target vessel, and confirming catheter placement in the target vessel with ultrasound.154,175 Third, learners must be able to demonstrate recognition of acute complications, including arterial puncture, hematoma formation, and development of pneumothorax.154,175 Trainees should be familiar with recommended evaluation and management algorithms, including indications for emergent consultation.190
29. We recommend a periodic proficiency assessments of all operators should be conducted to ensure maintenance of competency.
Rationale: Competency extends to periodic assessment and not merely an initial evaluation at the time of training.191 Periodic competency assessments should include assessment of proficiency of all providers that perform a procedure, including instructors and supervisors. Supervising providers should maintain their competency in CVC insertion through routine use of their skills in clinical practice.175 An observational study of emergency medicine residents revealed that lack of faculty comfort with ultrasound hindered the residents’ use of ultrasound.192 Thus, there is a need to examine best practices for procedural supervision of trainees because providers are often supervising procedures that they are not comfortable performing on their own.193
KNOWLEDGE GAPS
The process of producing this position statement revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for central and peripheral venous access and arterial access.
This position statement recommends a preprocedural ultrasound evaluation of blood vessels based on evidence that providers may detect anatomic anomalies, thrombosis, or vessel stenosis. Ultrasound can also reveal unsuspected high-risk structures in near proximity to the procedure site. Although previous studies have shown that providers can accurately assess vessels with ultrasound for these features, further study is needed to evaluate the effect of a standardized preprocedural ultrasound exam on clinical and procedural decision-making, as well as procedural outcomes.
Second, two ultrasound applications that are being increasingly used but have not been widely implemented are the use of ultrasound to evaluate lung sliding postprocedure to exclude pneumothorax and the verification of central line placement using a rapid infusion of agitated saline to visualize the RASS.139-141 Both of these applications have the potential to expedite postprocedure clearance of central lines for usage and decrease patient radiation exposure by obviating the need for postprocedure CXRs. Despite the supporting evidence, both of these applications are not yet widely used, as few providers have been trained in these techniques which may be considered advanced skills.
Third, despite advances in our knowledge of effective training for vascular access procedures, there is limited agreement on how to define procedural competence. Notable advancements in training include improved understanding of systematic training programs, development of techniques for proctoring procedures, definition of elements for hands-on assessments, and definition of minimum experience needed to perform vascular access procedures independently. However, application of these concepts to move learners toward independent practice remains variably interpreted at different institutions, likely due to limited resources, engrained cultures about procedures, and a lack of national standards. The development of hospitalist-based procedure services at major academic medical centers with high training standards, close monitoring for quality assurance, and the use of databases to track clinical outcomes may advance our understanding and delivery of optimal procedural training.
Finally, ultrasound technology is rapidly evolving which will affect training, techniques, and clinical outcomes in coming years. Development of advanced imaging software with artificial intelligence can improve needle visualization and tracking. These technologies have the potential to facilitate provider training in real-time ultrasound-guided procedures and improve the overall safety of procedures. Emergence of affordable, handheld ultrasound devices is improving access to ultrasound technology, but their role in vascular access procedures is yet to be defined. Furthermore, availability of wireless handheld ultrasound technology and multifrequency transducers will create new possibilities for use of ultrasound in vascular access procedures.
CONCLUSION
We have presented several evidence-based recommendations on the use of ultrasound guidance for placement of central and peripheral vascular access catheters that are intended for hospitalists and other healthcare providers who routinely perform vascular access procedures. By allowing direct visualization of the needle tip and target vessel, the use of ultrasound guidance has been shown in randomized studies to reduce needle insertion attempts, reduce needle redirections, and increase overall procedure success rates. The accuracy of ultrasound to identify the target vessel, assess for thrombosis, and detect anatomical anomalies is superior to that of physical examination. Hospitalists can attain competence in performing ultrasound-guided vascular access procedures through systematic training programs that combine didactic and hands-on training, which optimally include patient-based competency assessments.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators of Society of Hospital Medicine Point-of-care Ultrasound Task Force: Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Venkat Kalidindi, Ketino Kobaidze, Joshua Lenchus, Paul Mayo, Satyen Nichani, Vicki Noble, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Gerard Salame, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam J. Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Mathews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Mathews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El-Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Raad I. Intravascular-catheter-related infections. Lancet. 1998;351(9106):893-898. https://doi.org/10.1016/S0140-6736(97)10006-X.
2. Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286(6):700-707. https://doi.org/10.1001/jama.286.6.700.
3. Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986;146(2):259-261. https://doi.org/10.1001/archinte.146.2.259.
4. Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med. 1996;24(12):2053-2058. https://doi.org/10.1097/00003246-199612000-00020.
5. Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327(7411):361. https://doi.org/10.1136/bmj.327.7411.361.
6. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4):S1-S34. https://doi.org/10.1016/j.ajic.2011.01.003.
7. National Institute for Health and Care Excellence (NICE). Guidance on the Use of Ultrasound Locating Devices for Placing Central Venous Catheters; 2002.
8. Shojania KG, Duncan BW, McDonald KM, et al. Making health care safer: A critical analysis of patient safety practices. Evid Rep Technol Assess. Rockville, MD: Agency for Healthcare Research and Quality. 2001;43(43):i–x, 1.
9. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105-1117. https://doi.org/10.1007/s00134-012-2597-x.
10. Frykholm P, Pikwer A, Hammarskjöld F, et al. Clinical guidelines on central venous catheterisation. Swedish Society of Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiol Scand. 2014;58(5):508-524. https://doi.org/10.1111/aas.12295.
11. American Society of Anesthesiologists Task Force on Central Venous Access, Rupp SM, Apfelbaum JL, et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539-573. https://doi.org/10.1097/ALN.0b013e31823c9569.
12. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: General ultrasonography. Crit Care Med. 2015;43(11):2479-2502. https://doi.org/10.1097/CCM.0000000000001216.
13. Shekelle PG, Wachter RM, Pronovost PJ, et al. Making health care safer II: An updated critical analysis of the evidence for patient safety practices. Evid Rep Technol Assess. 2013;211:1-945.
14. Troianos CA, Hartman GS, Glas KE, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2011;24(12):1291-1318. https://doi.org/10.1016/j.echo.2011.09.021.
15. Soni NJ, Reyes LF, Keyt H, et al. Use of ultrasound guidance for central venous catheterization: a national survey of intensivists and hospitalists. J Crit Care. 2016;36:277-283. https://doi.org/10.1016/j.jcrc.2016.07.014.
16. Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220-1229. https://doi.org/10.1056/NEJMoa1500964.
17. Maizel J, Bastide MA, Richecoeur J, et al. Practice of ultrasound-guided central venous catheter technique by the French intensivists: a survey from the BoReal study group. Ann Intensive Care. 2016;6(1):76. https://doi.org/10.1186/s13613-016-0177-x.
18. Egan G, Healy D, O’Neill H, et al. Ultrasound guidance for difficult peripheral venous access: systematic review and meta-analysis. Emerg Med J. 2013;30(7):521-526. https://doi.org/10.1136/emermed-2012-201652.
19. Stolz LA, Stolz U, Howe C, Farrell IJ, Adhikari S. Ultrasound-guided peripheral venous access: a meta-analysis and systematic review. J Vasc Access. 2015;16(4):321-326. https://doi.org/10.5301/jva.5000346.
20. Sofocleous CT, Schur I, Cooper SG, et al. Sonographically guided placement of peripherally inserted central venous catheters: review of 355 procedures. AJR Am J Roentgenol. 1998;170(6):1613-1616. https://doi.org/10.2214/ajr.170.6.9609183.
21. Nichols I, Humphrey JP. The efficacy of upper arm placement of peripherally inserted central catheters using bedside ultrasound and microintroducer technique. J Infus Nurs Off Publ Infus Nurs Soc. 2008;31(3):165-176. https://doi.org/10.1097/01.NAN.0000317703.66395.b8.
22. Stokowski G, Steele D, Wilson D. The use of ultrasound to improve practice and reduce complication rates in peripherally inserted central catheter insertions. J Infus Nurs Off Publ Infus Nurs Soc. 2009;32(3):145-155. https://doi.org/10.1097/NAN.0b013e3181a1a98f.
23. Shiloh AL, Savel RH, Paulin LM, Eisen LA. Ultrasound-guided catheterization of the radial artery: a systematic review and meta-analysis of randomized controlled trials. Chest. 2011;139(3):524-529. https://doi.org/10.1378/chest.10-0919.
24. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: A position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
25. Chopra V, Flanders SA, Saint S, et al. The Michigan appropriateness guide for intravenous catheters (Magic): results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6):S1-S40. https://doi.org/10.7326/M15-0744.
26. Raad II, Hohn DC, Gilbreath BJ, et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994;15(4):231-238.
27. Slama M, Novara A, Safavian A, et al. Improvement of internal jugular vein cannulation using an ultrasound-guided technique. Intensive Care Med. 1997;23(8):916-919. https://doi.org/10.1007/s001340050432.
28. Seyahi N, Kahveci A, Altiparmak MR, Serdengecti K, Erek E. Ultrasound imaging findings of femoral veins in patients with renal failure and its impact on vascular access. Nephrol Dial Transplant. 2005;20(9):1864-1867. https://doi.org/10.1093/ndt/gfh942.
29. Lin BS, Kong CW, Tarng DC, Huang TP, Tang GJ. Anatomical variation of the internal jugular vein and its impact on temporary haemodialysis vascular access: an ultrasonographic survey in uraemic patients. Nephrol Dial Transplant. 1998;13(1):134-138. https://doi.org/10.1093/ndt/13.1.134.
30. Troianos CA, Kuwik RJ, Pasqual JR, Lim AJ, Odasso DP. Internal jugular vein and carotid artery anatomic relation as determined by ultrasonography. Anesthesiology. 1996;85(1):43-48. https://doi.org/10.1097/00000542-199607000-00007.
31. Gordon AC, Wright I, Pugh ND. Duplication of the superficial femoral vein: recognition with duplex ultrasonography. Clin Rad. 1996;51(9):622-624. https://doi.org/10.1016/S0009-9260(96)80055-9.
32. Sulek CA, Gravenstein N, Blackshear RH, Weiss L. Head rotation during internal jugular vein cannulation and the risk of carotid artery puncture. Anesth Analg. 1996;82(1):125-128. https://doi.org/10.1097/00000539-199601000-00022.
33. Shrestha BR, Gautam B. Ultrasound versus the landmark technique: a prospective randomized comparative study of internal jugular vein cannulation in an intensive care unit. JNMA J Nepal Med Assoc. 2011;51(182):56-61. https://doi.org/10.31729/jnma.148.
34. Brederlau J, Greim C, Schwemmer U, et al. Ultrasound-guided cannulation of the internal jugular vein in critically ill patients positioned in 30 degrees dorsal elevation. Eur J Anaesthesiol. 2004;21(9):684-687. https://doi.org/10.1097/00003643-200409000-00003.
35. Lamperti M, Subert M, Cortellazzi P, et al. Is a neutral head position safer than 45-degree neck rotation during ultrasound-guided internal jugular vein cannulation? Results of a randomized controlled clinical trial. Anesth Analg. 2012;114(4):777-784. https://doi.org/10.1213/ANE.0b013e3182459917.
36. Benter T, Teichgräber UK, Klühs L, et al. Anatomical variations in the internal jugular veins of cancer patients affecting central venous access. Anatomical variation of the internal jugular vein. Ultraschall Med. 2001;22(1):23-26. https://doi.org/10.1055/s-2001-11243.
37. Caridi JG, Hawkins IF, Jr, Wiechmann BN, Pevarski DJ, Tonkin JC. Sonographic guidance when using the right internal jugular vein for central vein access. AJR Am J Roentgenol. 1998;171(5):1259-1263. https://doi.org/10.2214/ajr.171.5.9798857.
38. Brusasco C, Corradi F, Zattoni PL, et al. Ultrasound-guided central venous cannulation in bariatric patients. Obes Surg. 2009;19(10):1365-1370. https://doi.org/10.1007/s11695-009-9902-y.
39. Armstrong PJ, Cullen M, Scott DH. The ‘SiteRite’ ultrasound machine--an aid to internal jugular vein cannulation. Anaesthesia. 1993;48(4):319-323. https://doi.org/10.1111/j.1365-2044.1993.tb06953.x.
40. Forauer AR, Glockner JF. Importance of US findings in access planning during jugular vein hemodialysis catheter placements. J Vasc Interv Rad. 2000;11(2 Pt 1):233-238. https://doi.org/10.1016/S1051-0443(07)61471-7.
41. Hassan C, Girishkumar HT, Thatigotla B, et al. Value of ultrasound guidance in placement of hemodialysis access catheters in patients with end-stage renal disease. Am Surg. 2008;74(11):1111-1113.
42. Tan CO, Weinberg L, Peyton P, Story D, McNicol L. Size variation between contralateral infraclavicular axillary veins within individual patients-implications for subclavian venous central line insertion. Crit Care Med. 2013;41(2):457-463. https://doi.org/10.1097/CCM.0b013e31826ab1dd.
43. Wu SY, Ling Q, Cao LH, et al. Real-time two-dimensional ultrasound guidance for central venous cannulation: a meta-analysis. Anesthesiology. 2013;118(2):361-375. https://doi.org/10.1097/ALN.0b013e31827bd172.
44. Kim W, Chung RK, Lee GY, Han JI. The effects of hip abduction with external rotation and reverse Trendelenburg position on the size of the femoral vein; ultrasonographic investigation. Korean J Anesthesiol. 2011;61(3):205-209. https://doi.org/10.4097/kjae.2011.61.3.205.
45. Denys BG, Uretsky BF. Anatomical variations of internal jugular vein location: impact on central venous access. Crit Care Med. 1991;19(12):1516-1519. https://doi.org/10.1097/00003246-199112000-00013.
46. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev. 2015;1:CD006962. https://doi.org/10.1002/14651858.CD006962.pub2.
47. Gilbert TB, Seneff MG, Becker RB. Facilitation of internal jugular venous cannulation using an audio-guided Doppler ultrasound vascular access device: results from a prospective, dual-center, randomized, crossover clinical study. Crit Care Med. 1995;23(1):60-65. https://doi.org/10.1097/00003246-199501000-00012.
48. Schummer W, Schummer C, Tuppatsch H, et al. Ultrasound-guided central venous cannulation: is there a difference between Doppler and B-mode ultrasound? J Clin Anesth. 2006;18(3):167-172. https://doi.org/10.1016/j.jclinane.2005.12.010.
49. Airapetian N, Maizel J, Langelle F, et al. Ultrasound-guided central venous cannulation is superior to quick-look ultrasound and landmark methods among inexperienced operators: a prospective randomized study. Intensive Care Med. 2013;39(11):1938-1944. https://doi.org/10.1007/s00134-013-3072-z.
50. Milling TJ, Jr, Rose J, Briggs WM, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Crit Care Med. 2005;33(8):1764-1769. https://doi.org/10.1097/01.ccm.0000171533.92856.e5.
51. Beaudoin FL, Merchant RC, Lincoln J, et al. Bedside ultrasonography detects significant femoral vessel overlap: implications for central venous cannulation. CJEM. 2011;13(4):245-250. https://doi.org/10.2310/8000.2011.110482.
52. Kwon TH, Kim YL, Cho DK. Ultrasound-guided cannulation of the femoral vein for acute haemodialysis access. Nephrol Dial Transplant. 1997;12(5):1009-1012. https://doi.org/10.1093/ndt/12.5.1009.
53. Rothschild JM. Ultrasound guidance of central vein catheterization. Evid Rep Technol Assess. 2001;43. Chapter 21.: http://archive.ahrq.gov/clinic/ptsafety/chap21.htm.
54. Lennon M, Zaw NN, Pöpping DM, Wenk M. Procedural complications of central venous catheter insertion. Minerva Anestesiol. 2012;78(11):1234-1240.
55. Blaivas M, Adhikari S. An unseen danger: frequency of posterior vessel wall penetration by needles during attempts to place internal jugular vein central catheters using ultrasound guidance. Crit Care Med. 2009;37(8):2345-2349; quiz 2359. https://doi.org/10.1097/CCM.0b013e3181a067d4.
56. Vogel JA, Haukoos JS, Erickson CL, et al. Is long-axis view superior to short-axis view in ultrasound-guided central venous catheterization? Crit Care Med. 2015;43(4):832-839. https://doi.org/10.1097/CCM.0000000000000823.
57. Tammam TF, El-Shafey EM, Tammam HF. Ultrasound-guided internal jugular vein access: comparison between short axis and long axis techniques. Saudi J Kidney Dis Transpl. 2013;24(4):707-713. https://doi.org/10.4103/1319-2442.113861.
58. Keyes LE, Frazee BW, Snoey ER, Simon BC, Christy D. Ultrasound-guided brachial and basilic vein cannulation in emergency department patients with difficult intravenous access. Ann Emerg Med. 1999;34(6):711-714. https://doi.org/10.1016/S0196-0644(99)70095-8.
59. Mahler SA, Wang H, Lester C, et al. Short- vs long-axis approach to ultrasound-guided peripheral intravenous access: a prospective randomized study. Am J Emerg Med. 2011;29(9):1194-1197. https://doi.org/10.1016/j.ajem.2010.07.015.
60. Berk D, Gurkan Y, Kus A, et al. Ultrasound-guided radial arterial cannulation: long axis/in-plane versus short axis/out-of-plane approaches? J Clin Monit Comput. 2013;27(3):319-324. https://doi.org/10.1007/s10877-013-9437-6.
61. Batllori M, Urra M, Uriarte E, et al. Randomized comparison of three transducer orientation approaches for ultrasound guided internal jugular venous cannulation. Br J Anaesth. 2016;116(3):370-376. https://doi.org/10.1093/bja/aev399.
62. Moak JH, Lyons MS, Wright SW, Lindsell CJ. Needle and guidewire visualization in ultrasound-guided internal jugular vein cannulation. Am J Emerg Med. 2011;29(4):432-436. https://doi.org/10.1016/j.ajem.2010.01.004.
63. Moak JH, Rajkumar JS, Woods WA. The wire is really easy to see (WIRES): sonographic visualization of the guidewire by novices. CJEM. 2013;15(1):18-23. https://doi.org/10.2310/8000.2012.120800.
64. Gillman LM, Blaivas M, Lord J, Al-Kadi A, Kirkpatrick AW. Ultrasound confirmation of guidewire position may eliminate accidental arterial dilatation during central venous cannulation. Scand J Trauma Resusc Emerg Med. 2010;18:39. https://doi.org/10.1186/1757-7241-18-39.
65. Augoustides JG, Horak J, Ochroch AE, et al. A randomized controlled clinical trial of real-time needle-guided ultrasound for internal jugular venous cannulation in a large university anesthesia department. J Cardiothorac Vasc Anesth. 2005;19(3):310-315. https://doi.org/10.1053/j.jvca.2005.03.007.
66. Maecken T, Heite L, Wolf B, Zahn PK, Litz RJ. Ultrasound-guided catheterisation of the subclavian vein: freehand vs needle-guided technique. Anaesthesia. 2015;70(11):1242-1249. https://doi.org/10.1111/anae.13187.
67. Stone MB, Nagdev A, Murphy MC, Sisson CA. Ultrasound detection of guidewire position during central venous catheterization. Am J Emerg Med. 2010;28(1):82-84. https://doi.org/10.1016/j.ajem.2008.09.019.
68. Luyet C, Hartwich V, Urwyler N, et al. Evaluation of a novel needle guide for ultrasound-guided phantom vessel cannulation. Anaesthesia. 2011;66(8):715-720. https://doi.org/10.1111/j.1365-2044.2011.06781.x.
69. O’Grady NP, Alexander M, Burns LA, et al. Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis. 2011;52(9):1087-1099. https://doi.org/10.1093/cid/cir138.
70. Southworth SL, Henman LJ, Kinder LA, Sell JL. The journey to zero central catheter-associated bloodstream infections: culture change in an intensive care unit. Crit Care Nurse. 2012;32(2):49-54. https://doi.org/10.4037/ccn2012915.
71. Guerin K, Wagner J, Rains K, Bessesen M. Reduction in central line-associated bloodstream infections by implementation of a postinsertion care bundle. Am J Infect Control. 2010;38(6):430-433. https://doi.org/10.1016/j.ajic.2010.03.007.
72. Hartman N, Wittler M, Askew K, Manthey D. Delphi method validation of a procedural performance checklist for insertion of an ultrasound-guided internal jugular central line. Am J Med Qual Off J Am Coll Med Qual. 2016;31(1):81-85. https://doi.org/10.1177/1062860614549762.
73. Lenchus JD, Carvalho CM, Ferreri K, et al. Filling the void: defining invasive bedside procedural competency for internal medicine residents. J Grad Med Educ. 2013;5(4):605-612. https://doi.org/10.4300/JGME-D-13-00030.1.
74. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. https://doi.org/10.1001/archinternmed.2009.215.
75. Karakitsos D, Labropoulos N, De Groot E, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care. 2006;10(6):R162. https://doi.org/10.1186/cc5101.
76. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. https://doi.org/10.1097/01.ccm.0000142399.70913.2f.
77. Young EM, Commiskey ML, Wilson SJ. Translating evidence into practice to prevent central venous catheter-associated bloodstream infections: a systems-based intervention. Am J Infect Control. 2006;34(8):503-506. https://doi.org/10.1016/j.ajic.2006.03.011.
78. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495; discussion 495. https://doi.org/10.1016/j.surg.2008.06.004.
79. Mermel LA, McCormick RD, Springman SR, Maki DG. The pathogenesis and epidemiology of catheter-related infection with pulmonary artery swan-Ganz catheters: a prospective study utilizing molecular subtyping. Am J Med. 1991;91(3b):197S–205S. https://doi.org/10.1016/0002-9343(91)90369-9.
80. Marschall J, Mermel LA, Classen D, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(1):S22-S30. https://doi.org/10.1086/591059.
81. Marschall J, Mermel LA, Fakih M, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(7):753-771. https://doi.org/10.1086/676533.
82. Garcia-Rodriguez JF, Álvarez-Díaz H, Vilariño-Maneiro L, et al. Epidemiology and impact of a multifaceted approach in controlling central venous catheter associated blood stream infections outside the intensive care unit. BMC Infect Dis. 2013;13:445. https://doi.org/10.1186/1471-2334-13-445.
83. Lee DH, Jung KY, Choi YH. Use of maximal sterile barrier precautions and/or antimicrobial-coated catheters to reduce the risk of central venous catheter-related bloodstream infection. Infect Control Hosp Epidemiol. 2008;29(10):947-950. https://doi.org/10.1086/590356.
84. Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132(5):391-402. https://doi.org/10.7326/0003-4819-132-5-200003070-00009.
85. Troianos CA, Jobes DR, Ellison N. Ultrasound-guided cannulation of the internal jugular vein. A prospective, randomized study. Anesth Analg. 1991;72(6):823-826. https://doi.org/10.1213/00000539-199106000-00020.
86. Troianos CA, Savino JS. Internal jugular vein cannulation guided by echocardiography. Anesthesiology. 1991;74(4):787-789. https://doi.org/10.1097/00000542-199104000-00026.
87. Denys BG, Uretsky BF, Reddy PS, et al. An ultrasound method for safe and rapid central venous access. N Engl J Med. 1991;324(8):566. https://doi.org/10.1056/NEJM199102213240816.
88. Riaz A, Shan Khan RA, Salim F. Ultrasound guided internal jugular venous cannulation: comparison with land-mark technique. J Coll Phys Surg Pak. 2015;25(5):315-319. https://doi.org/05.2015/JCPSP.315319.
89. Gok F, Kilicaslan A, Sarkilar G, Kandemir B, Yosunkaya A. The effect of ultrasound guidance on central venous catheter-associated bloodstream infection in critical care patients. Acta Med Mediterr. 2013;29:677-682.
90. Lamperti M, Cortellazzi P, D’Onofrio G, et al. An outcome study on complications using routine ultrasound assistance for internal jugular vein cannulation. Acta Anaesthesiol Scand. 2007;51(10):1327-1330. https://doi.org/10.1111/j.1399-6576.2007.01442.x.
91. Vezzani A, Manca T, Vercelli A, Braghieri A, Magnacavallo A. Ultrasonography as a guide during vascular access procedures and in the diagnosis of complications. J Ultrasound. 2013;16(4):161-170. https://doi.org/10.1007/s40477-013-0046-5.
92. Palepu GB, Deven J, Subrahmanyam M, Mohan S. Impact of ultrasonography on central venous catheter insertion in intensive care. Indian J Rad Imaging. 2009;19(3):191-198. https://doi.org/10.4103/0971-3026.54877.
93. Agarwal A, Singh DK, Singh AP. Ultrasonography: a novel approach to central venous cannulation. Indian J Crit Care Med. 2009;13(4):213-216. https://doi.org/10.4103/0972-5229.60174.
94. Wong SW, Niazi AU, Chin KJ, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS® needle tracking system: a case report. Can J Anaesth. 2013;60(1):50-53. https://doi.org/10.1007/s12630-012-9809-2.
95. Bouaziz H, Zetlaoui PJ, Pierre S, et al. Guidelines on the use of ultrasound guidance for vascular access. Anaesth, Crit Care Pain Med. 2015;34(1):65-69. https://doi.org/10.1016/j.accpm.2015.01.004.
96. Jenssen C, Brkljacic B, Hocke M, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part VI - Ultrasound-guided vascular interventions. Ultraschall Med. 2016;37(5):473-476. https://doi.org/10.1055/s-0035-1553450.
97. Rando K, Castelli J, Pratt JP, et al. Ultrasound-guided internal jugular vein catheterization: a randomized controlled trial. Heart Lung Vessels. 2014;6(1):13-23.
98. Mey U, Glasmacher A, Hahn C, et al. Evaluation of an ultrasound-guided technique for central venous access via the internal jugular vein in 493 patients. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2003;11(3):148-155. https://doi.org/10.1007/s00520-002-0399-3.
99. Singh SA, Sharma S, Singh A, et al. The safety of ultrasound guided central venous cannulation in patients with liver disease. Saudi J Anaesth. 2015;9(2):155-160. https://doi.org/10.4103/1658-354X.152842.
100. Akoglu H, Piskinpasa S, Yenigun EC, et al. Real-time ultrasound guided placement of temporary internal jugular vein catheters: assessment of technical success and complication rates in nephrology practice. Nephrol (Carlton). 2012;17(7):603-606. https://doi.org/10.1111/j.1440-1797.2012.01637.x.
101. Sadler DJ, Gordon AC, Klassen J, et al. Image-guided central venous catheters for apheresis. Bone Marrow Transplant. 1999;23(2):179-182. https://doi.org/10.1038/sj.bmt.1701545.
102. Calvert N, Hind D, McWilliams R, et al. Ultrasound for central venous cannulation: economic evaluation of cost-effectiveness. Anaesthesia. 2004;59(11):1116-1120. https://doi.org/10.1111/j.1365-2044.2004.03906.x.
103. Goetz AM, Wagener MM, Miller JM, Muder RR. Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infect Control Hosp Epidemiol. 1998;19(11):842-845.
104. Bertini P, Frediani M. Ultrasound guided supraclavicular central vein cannulation in adults: a technical report. J Vasc Access. 2013;14(1):89-93. https://doi.org/10.5301/jva.5000088.
105. Lalu MM, Fayad A, Ahmed O, et al. Ultrasound-guided subclavian vein catheterization: A systematic review and meta-analysis. Crit Care Med. 2015;43(7):1498-1507. https://doi.org/10.1097/CCM.0000000000000973.
106. Milone M, Di Minno G, Di Minno MN, et al. The real effectiveness of ultrasound guidance in subclavian venous access. Ann ital chir. 2010;81(5):331-334.
107. O’Leary R, Ahmed SM, McLure H, et al. Ultrasound-guided infraclavicular axillary vein cannulation: a useful alternative to the internal jugular vein. Br J Anaesth. 2012;109(5):762-768. https://doi.org/10.1093/bja/aes262.
108. Gualtieri E, Deppe SA, Sipperly ME, Thompson DR. Subclavian venous catheterization: greater success rate for less experienced operators using ultrasound guidance. Crit Care Med. 1995;23(4):692-697. https://doi.org/10.1097/00003246-199504000-00018.
109. Fragou M, Gravvanis A, Dimitriou V, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med. 2011;39(7):1607-1612. https://doi.org/10.1097/CCM.0b013e318218a1ae.
110. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015;1:CD011447. https://doi.org/10.1002/14651858.CD011447.
111. Robinson MK, Mogensen KM, Grudinskas GF, Kohler S, Jacobs DO. Improved care and reduced costs for patients requiring peripherally inserted central catheters: the role of bedside ultrasound and a dedicated team. JPEN J Parenter Enter Nutr. 2005;29(5):374-379. https://doi.org/10.1177/0148607105029005374.
112. Li J, Fan YY, Xin MZ, et al. A randomised, controlled trial comparing the long-term effects of peripherally inserted central catheter placement in chemotherapy patients using B-mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94-103. https://doi.org/10.1016/j.ejon.2013.08.003.
113. Schweickert WD, Herlitz J, Pohlman AS, et al. A randomized, controlled trial evaluating postinsertion neck ultrasound in peripherally inserted central catheter procedures. Crit Care Med. 2009;37(4):1217-1221. https://doi.org/10.1097/CCM.0b013e31819cee7f.
114. Mahler SA, Wang H, Lester C, Conrad SA. Ultrasound-guided peripheral intravenous access in the emergency department using a modified Seldinger technique. J Emerg Med. 2010;39(3):325-329. https://doi.org/10.1016/j.jemermed.2009.02.013.
115. Brannam L, Blaivas M, Lyon M, Flake M. Emergency nurses’ utilization of ultrasound guidance for placement of peripheral intravenous lines in difficult-access patients. Acad Emerg Med. 2004;11(12):1361-1363. https://doi.org/10.1197/j.aem.2004.08.027.
116. Au A, Rotte M, Gryzbowski R, Ku B, Fields J. 157 Decrease in central venous catheter placement and complications due to utilization of ultrasound-guided peripheral intravenous catheters. Ann Emerg Med. 2011;58(4):S230. https://doi.org/10.1016/j.annemergmed.2011.06.185.
117. Dargin JM, Rebholz CM, Lowenstein RA, Mitchell PM, Feldman JA. Ultrasonography-guided peripheral intravenous catheter survival in ED patients with difficult access. Am J Emerg Med. 2010;28(1):1-7. https://doi.org/10.1016/j.ajem.2008.09.001.
118. Bauman M, Braude D, Crandall C. Ultrasound-guidance vs standard technique in difficult vascular access patients by ED technicians. Am J Emerg Med. 2009;27(2):135-140. https://doi.org/10.1016/j.ajem.2008.02.005.
119. Schoenfeld E, Shokoohi H, Boniface K. Ultrasound-guided peripheral intravenous access in the emergency department: patient-centered survey. West J Emerg Med. 2011;12(4):475-477. https://doi.org/10.5811/westjem.2011.3.1920.
120. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. https://doi.org/10.7326/0003-4819-114-10-845.
121. Miliani K, Taravella R, Thillard D, et al. Peripheral venous catheter-related adverse events: evaluation from a multicentre epidemiological study in France (the CATHEVAL Project). PLOS ONE. 2017;12(1):e0168637. https://doi.org/10.1371/journal.pone.0168637.
122. Gregg SC, Murthi SB, Sisley AC, Stein DM, Scalea TM. Ultrasound-guided peripheral intravenous access in the intensive care unit. J Crit Care. 2010;25(3):514-519. https://doi.org/10.1016/j.jcrc.2009.09.003.
123. Adhikari S, Blaivas M, Morrison D, Lander L. Comparison of infection rates among ultrasound-guided versus traditionally placed peripheral intravenous lines. J Ultrasound Med. 2010;29(5):741-747. https://doi.org/10.7863/jum.2010.29.5.741.
124. Shiver S, Blaivas M, Lyon M. A prospective comparison of ultrasound-guided and blindly placed radial arterial catheters. Acad Emerg Med. 2006;13(12):1275-1279. https://doi.org/10.1197/j.aem.2006.07.015.
125. Levin PD, Sheinin O, Gozal Y. Use of ultrasound guidance in the insertion of radial artery catheters. Crit Care Med. 2003;31(2):481-484. https://doi.org/10.1097/01.CCM.0000050452.17304.2F.
126. Gu WJ, Tie HT, Liu JC, Zeng XT. Efficacy of ultrasound-guided radial artery catheterization: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2014;18(3):R93. https://doi.org/10.1186/cc13862.
127. Tang L, Wang F, Li Y, et al. Ultrasound guidance for radial artery catheterization: an updated meta-analysis of randomized controlled trials. PLOS ONE. 2014;9(11):e111527. https://doi.org/10.1371/journal.pone.0111527.
128. Sobolev M, Slovut DP, Lee Chang A, Shiloh AL, Eisen LA. Ultrasound-guided catheterization of the femoral artery: A systematic review and meta-analysis of randomized controlled trials. J Invas Cardiol. 2015;27(7):318-323. https://doi.org/10.1378/chest.1991181.
129. Gabriel M, Pawlaczyk K, Waliszewski K, Krasiński Z, Majewski W. Location of femoral artery puncture site and the risk of postcatheterization pseudoaneurysm formation. Int J Cardiol. 2007;120(2):167-171. https://doi.org/10.1016/j.ijcard.2006.09.018.
130. Seto AH, Abu-Fadel MS, Sparling JM, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With ultrasound Trial). JACC Cardiovasc Interv. 2010;3(7):751-758. https://doi.org/10.1016/j.jcin.2010.04.015.
131. Kalish J, Eslami M, Gillespie D, et al. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg. 2015;61(5):1231-1238. https://doi.org/10.1016/j.jvs2014.12.003.
132. Sandhu NS, Patel B. Use of ultrasonography as a rescue technique for failed radial artery cannulation. J Clin Anesth. 2006;18(2):138-141. https://doi.org/10.1016/j.jclinane.2005.06.011.
133. White L, Halpin A, Turner M, Wallace L. Ultrasound-guided radial artery cannulation in adult and paediatric populations: a systematic review and meta-analysis. Br J Anaesth. 2016;116(5):610-617. https://doi.org/10.1093/bja/aew097.
134. Gao YB, Yan JH, Gao FQ, et al. Effects of ultrasound-guided radial artery catheterization: an updated meta-analysis. Am J Emerg Med. 2015;33(1):50-55. https://doi.org/10.1016/j.ajem.2014.10.008.
135. Seto AH, Roberts JS, Abu-Fadel MS, et al. Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery Access with Ultrasound Trial). JACC Cardiovasc Interv. 2015;8(2):283-291. https://doi.org/10.1016/j.jcin.2014.05.036.
136. Roberts J, Manur R. Ultrasound-guided radial artery access by a non-ultrasound trained interventional cardiologist improved first-attempt success rates and shortened time for successful radial artery cannulation. J Invas Cardiol. 2013;25(12):676-679.
137. Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108(5):1345-1348. https://doi.org/10.1378/chest.108.5.1345.
138. Lichtenstein D, Mezière G, Biderman P, Gepner A. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999;25(4):383-388. https://doi.org/10.1007/s001340050862.
139. Ablordeppey EA, Drewry AM, Beyer AB, et al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound Versus chest radiography in critically ill patients: A systematic review and meta-analysis. Crit Care Med. 2017;45(4):715-724. https://doi.org/10.1097/CCM.0000000000002188.
140. Bedel J, Vallée F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013;39(11):1932-1937. https://doi.org/10.1007/s00134-013-3097-3.
141. Weekes AJ, Keller SM, Efune B, Ghali S, Runyon M. Prospective comparison of ultrasound and CXR for confirmation of central vascular catheter placement. Emerg Med J EMJ. 2016;33(3):176-180. https://doi.org/10.1136/emermed-2015-205000.
142. Arellano R, Nurmohamed A, Rumman A, et al. The utility of transthoracic echocardiography to confirm central line placement: an observational study. Can J Anaesth. 2014;61(4):340-346. https://doi.org/10.1007/s12630-014-0111-3.
143. Vezzani A, Brusasco C, Palermo S, et al. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38(2):533-538. https://doi.org/10.1097/CCM.0b013e3181c0328f.
144. Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142(4):260-273. https://doi.org/10.7326/0003-4819-142-4-200502150-00008.
145. Backlund BH, Hopkins E, Kendall JL. Ultrasound guidance for central venous access by emergency physicians in Colorado. West J Emerg Med. 2012;13(4):320-325. https://doi.org/10.5811/westjem.2011.11.6821.
146. Buchanan MS, Backlund B, Liao MM, et al. Use of ultrasound guidance for central venous catheter placement: survey from the American Board of Emergency Medicine Longitudinal Study of Emergency Physicians. Acad Emerg Med. 2014;21(4):416-421. https://doi.org/10.1111/acem.12350.
147. Barsuk JH, McGaghie WC, Cohen ER, O’Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697-2701. https://doi.org/10.1097/00003246-200910000-00003.
148. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. https://doi.org/10.1097/00003246-200201000-00009.
149. Woo MY, Frank J, Lee AC, et al. Effectiveness of a novel training program for emergency medicine residents in ultrasound-guided insertion of central venous catheters. CJEM. 2009;11(4):343-348. https://doi.org/10.1017/S1481803500011398.
150. McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348(12):1123-1133. https://doi.org/10.1056/NEJMra011883.
151. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. https://doi.org/10.1002/jhm.468.
152. Sekiguchi H, Tokita JE, Minami T, et al. A prerotational, simulation-based workshop improves the safety of central venous catheter insertion: results of a successful internal medicine house staff training program. Chest. 2011;140(3):652-658. https://doi.org/10.1378/chest.10-3319.
153. Feller-Kopman D. Ultrasound-guided internal jugular access: a proposed standardized approach and implications for training and practice. Chest. 2007;132(1):302-309. https://doi.org/10.1378/chest.06-2711.
154. Troianos CA, Hartman GS, Glas KE, et al. Special articles: guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg. 2012;114(1):46-72. https://doi.org/10.1213/ANE.0b013e3182407cd8.
155. Issenberg SB, McGaghie WC, Hart IR, et al. Simulation technology for health care professional skills training and assessment. JAMA. 1999;282(9):861-866. https://doi.org/10.1001/jama.282.9.861.
156. Millington SJ, Wong RY, Kassen BO, Roberts JM, Ma IW. Improving internal medicine residents’ performance, knowledge, and confidence in central venous catheterization using simulators. J Hosp Med. 2009;4(7):410-416. https://doi.org/10.1002/jhm.570.
157. Beaulieu Y, Laprise R, Drolet P, et al. Bedside ultrasound training using web-based e-learning and simulation early in the curriculum of residents. Crit Ultrasound J. 2015;7:1. https://doi.org/10.1186/s13089-014-0018-9.
158. Barsuk JH, Cohen ER, McGaghie WC, Wayne DB. Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med. 2010;85(10 Suppl):S9-S12. https://doi.org/10.1097/ACM.0b013e3181ed436c.
159. Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133(1):56-61. https://doi.org/10.1378/chest.07-0131.
160. Evans LV, Dodge KL, Shah TD, et al. Simulation training in central venous catheter insertion: improved performance in clinical practice. Acad Med. 2010;85(9):1462-1469. https://doi.org/10.1097/ACM.0b013e3181eac9a3.
161. Smith CC, Huang GC, Newman LR, et al. Simulation training and its effect on long-term resident performance in central venous catheterization. Simul Healthc J Soc Simul Healthc. 2010;5(3):146-151. https://doi.org/10.1097/SIH.0b013e3181dd9672.
162. Laack TA, Dong Y, Goyal DG, et al. Short-term and long-term impact of the central line workshop on resident clinical performance during simulated central line placement. Simul Healthc J Soc Simul Healthc. 2014;9(4):228-233. https://doi.org/10.1097/SIH.0000000000000015.
163. Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31(10):1519-1526. https://doi.org/10.7863/jum.2012.31.10.1519.
164. Bayci AW, Mangla J, Jenkins CS, Ivascu FA, Robbins JM. Novel educational module for subclavian central venous catheter insertion using real-time ultrasound guidance. J Surg Educ. 2015;72(6):1217-1223. https://doi.org/10.1016/j.jsurg.2015.07.010.
165. Andreatta P, Chen Y, Marsh M, Cho K. Simulation-based training improves applied clinical placement of ultrasound-guided PICCs. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2011;19(4):539-543. https://doi.org/10.1007/s00520-010-0849-2.
166. Rosen BT, Uddin PQ, Harrington AR, Ault BW, Ault MJ. Does personalized vascular access training on a nonhuman tissue model allow for learning and retention of central line placement skills? Phase II of the procedural patient safety initiative (PPSI-II). J Hosp Med. 2009;4(7):423-429. https://doi.org/10.1002/jhm.571.
167. Ault MJ, Rosen BT, Ault B. The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative. J Gen Intern Med. 2006;21(5):514-517. https://doi.org/10.1111/j.1525-1497.2006.00440.x.
168. Varga S, Smith J, Minneti M, et al. Central venous catheterization using a perfused human cadaveric model: application to surgical education. J Surg Educ. 2015;72(1):28-32. https://doi.org/10.1016/j.jsurg.2014.07.005.
169. Sansivero GE. Venous anatomy and physiology. Considerations for vascular access device placement and function. J Intraven Nurs Off Publ Intraven Nurs Soc. 1998;21(5 Suppl):S107-S114.
170. Eisen LA, Narasimhan M, Berger JS, et al. Mechanical complications of central venous catheters. Journal of intensive care medicine. 2006;21(1):40-46. https://doi.org/10.1177/0885066605280884.
171. Chenkin J, Lee S, Huynh T, Bandiera G. Procedures can be learned on the Web: a randomized study of ultrasound-guided vascular access training. Acad Emerg Med. 2008;15(10):949-954. https://doi.org/10.1111/j.1553-2712.2008.00231.x.
172. Abualenain J, Calabrese K, Tansek R, Ranniger C. 319 Comparing standard versus video-based teaching for ultrasound-guided internal jugular central venous catheter access for fourth-year medical students. Ann Emerg Med. 2014;64(4):S113. https://doi.org/10.1016/j.annemergmed.2014.07.347.
173. Pustavoitau A, Blaivas M, Brown SM, et al. Recommendations for achieving and maintaining competence and credentialing in critical care ultrasound with focused cardiac ultrasound and advanced critical care echocardiography. Crit Care Med. 2016.
174. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: A survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
175. Moureau N, Lamperti M, Kelly LJ, et al. Evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training. Br J Anaesth. 2013;110(3):347-356. https://doi.org/10.1093/bja/aes499.
176. Ernst A, Silvestri GA, Johnstone D, American College of Chest Physicians. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123(5):1693-1717. https://doi.org/10.1378/chest.123.5.1693.
177. Thomas SM, Burch W, Kuehnle SE, et al. Simulation training for pediatric residents on central venous catheter placement: a pilot study. Pediatr Crit Care Med J Soc Crit Care Med.. 2013;14(9):e416-e423. https://doi.org/10.1097/PCC.0b013e31829f5eda.
178. Smith KK, Gilcreast D, Pierce K. Evaluation of staff’s retention of ACLS and BLS skills. Resuscitation. 2008;78(1):59-65. https://doi.org/10.1016/j.resuscitation.2008.02.007.
179. Ericsson KA. Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med. 2004;79(10 Suppl):S70-S81. https://doi.org/10.1097/00001888-200410001-00022.
180. Gerard JM, Thomas SM, Germino KW, et al. The effect of simulation training on PALS skills among family medicine residents. Fam Med. 2011;43(6):392-399.
181. Brydges R, Nair P, Ma I, Shanks D, Hatala R. Directed self-regulated learning versus instructor-regulated learning in simulation training. Med Educ. 2012;46(7):648-656. https://doi.org/10.1111/j.1365-2923.2012.04268.x.
182. Wayne DB, Butter J, Siddall VJ, et al. Simulation-based training of internal medicine residents in advanced cardiac life support protocols: a randomized trial. Teach Learn Med. 2005;17(3):210-216. https://doi.org/10.1207/s15328015tlm1703_3.
183. Arthur Jr. W, Bennett Jr. W, Stanush PL, McNelly TL. Factors that influence skill decay and retention: A quantitative review and analysis. Hum Perform. 1998;11(1):57-101. https://doi.org/10.1207/s15327043hup1101_3.
184. Rusche JD, Besuner P, Partusch SK, Berning PA. Competency program development across a merged healthcare network. J Nurs Staff Dev. 2001;17(5):234-240; quiz 241-232. https://doi.org/10.1097/00124645-200109000-00004.
185. O’Hearne Rebholz M. A review of methods to assess competency. J Nurs Staff Dev. 2006;22(5):241-245. https://doi.org/10.1097/00124645-200609000-00007.
186. Reznick RK. Teaching and testing technical skills. Am J Surg. 1993;165(3):358-361. https://doi.org/10.1016/s0002-9610(05)80843-8.
187. Reznick RK, MacRae H. Teaching surgical skills--changes in the wind. N Engl J Med. 2006;355(25):2664-2669. https://doi.org/10.1056/NEJMra054785.
188. Murin S, Stollenwerk NS. Simulation in procedural training: at the tipping point. Chest. 2010;137(5):1009-1011. https://doi.org/10.1378/chest.10-0199.
189. American College of Emergency Physicians. Emergency ultrasound guidelines. Ann Emerg Med. 2009;53(4):550-570. https://doi.org/10.1016/j.annemergmed.2008.12.013.
190. Guilbert MC, Elkouri S, Bracco D, et al. Arterial trauma during central venous catheter insertion: case series, review and proposed algorithm. J Vasc Surg. 2008;48(4):918-925; discussion 925. https://doi.org/10.1016/j.jvs2008.04.046.
191. Dong Y, Suri HS, Cook DA, et al. Simulation-based objective assessment discerns clinical proficiency in central line placement: a construct validation. Chest. 2010;137(5):1050-1056. https://doi.org/10.1378/chest.09-1451.
192. Adhikari S, Theodoro D, Raio C, et al. Central venous catheterization: are we using ultrasound guidance? J Ultrasound Med. 2015;34(11):2065-2070. https://doi.org/10.7863/ultra.15.01027.
193. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors--procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z.
1. Raad I. Intravascular-catheter-related infections. Lancet. 1998;351(9106):893-898. https://doi.org/10.1016/S0140-6736(97)10006-X.
2. Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286(6):700-707. https://doi.org/10.1001/jama.286.6.700.
3. Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986;146(2):259-261. https://doi.org/10.1001/archinte.146.2.259.
4. Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med. 1996;24(12):2053-2058. https://doi.org/10.1097/00003246-199612000-00020.
5. Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327(7411):361. https://doi.org/10.1136/bmj.327.7411.361.
6. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4):S1-S34. https://doi.org/10.1016/j.ajic.2011.01.003.
7. National Institute for Health and Care Excellence (NICE). Guidance on the Use of Ultrasound Locating Devices for Placing Central Venous Catheters; 2002.
8. Shojania KG, Duncan BW, McDonald KM, et al. Making health care safer: A critical analysis of patient safety practices. Evid Rep Technol Assess. Rockville, MD: Agency for Healthcare Research and Quality. 2001;43(43):i–x, 1.
9. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105-1117. https://doi.org/10.1007/s00134-012-2597-x.
10. Frykholm P, Pikwer A, Hammarskjöld F, et al. Clinical guidelines on central venous catheterisation. Swedish Society of Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiol Scand. 2014;58(5):508-524. https://doi.org/10.1111/aas.12295.
11. American Society of Anesthesiologists Task Force on Central Venous Access, Rupp SM, Apfelbaum JL, et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539-573. https://doi.org/10.1097/ALN.0b013e31823c9569.
12. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: General ultrasonography. Crit Care Med. 2015;43(11):2479-2502. https://doi.org/10.1097/CCM.0000000000001216.
13. Shekelle PG, Wachter RM, Pronovost PJ, et al. Making health care safer II: An updated critical analysis of the evidence for patient safety practices. Evid Rep Technol Assess. 2013;211:1-945.
14. Troianos CA, Hartman GS, Glas KE, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2011;24(12):1291-1318. https://doi.org/10.1016/j.echo.2011.09.021.
15. Soni NJ, Reyes LF, Keyt H, et al. Use of ultrasound guidance for central venous catheterization: a national survey of intensivists and hospitalists. J Crit Care. 2016;36:277-283. https://doi.org/10.1016/j.jcrc.2016.07.014.
16. Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220-1229. https://doi.org/10.1056/NEJMoa1500964.
17. Maizel J, Bastide MA, Richecoeur J, et al. Practice of ultrasound-guided central venous catheter technique by the French intensivists: a survey from the BoReal study group. Ann Intensive Care. 2016;6(1):76. https://doi.org/10.1186/s13613-016-0177-x.
18. Egan G, Healy D, O’Neill H, et al. Ultrasound guidance for difficult peripheral venous access: systematic review and meta-analysis. Emerg Med J. 2013;30(7):521-526. https://doi.org/10.1136/emermed-2012-201652.
19. Stolz LA, Stolz U, Howe C, Farrell IJ, Adhikari S. Ultrasound-guided peripheral venous access: a meta-analysis and systematic review. J Vasc Access. 2015;16(4):321-326. https://doi.org/10.5301/jva.5000346.
20. Sofocleous CT, Schur I, Cooper SG, et al. Sonographically guided placement of peripherally inserted central venous catheters: review of 355 procedures. AJR Am J Roentgenol. 1998;170(6):1613-1616. https://doi.org/10.2214/ajr.170.6.9609183.
21. Nichols I, Humphrey JP. The efficacy of upper arm placement of peripherally inserted central catheters using bedside ultrasound and microintroducer technique. J Infus Nurs Off Publ Infus Nurs Soc. 2008;31(3):165-176. https://doi.org/10.1097/01.NAN.0000317703.66395.b8.
22. Stokowski G, Steele D, Wilson D. The use of ultrasound to improve practice and reduce complication rates in peripherally inserted central catheter insertions. J Infus Nurs Off Publ Infus Nurs Soc. 2009;32(3):145-155. https://doi.org/10.1097/NAN.0b013e3181a1a98f.
23. Shiloh AL, Savel RH, Paulin LM, Eisen LA. Ultrasound-guided catheterization of the radial artery: a systematic review and meta-analysis of randomized controlled trials. Chest. 2011;139(3):524-529. https://doi.org/10.1378/chest.10-0919.
24. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: A position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
25. Chopra V, Flanders SA, Saint S, et al. The Michigan appropriateness guide for intravenous catheters (Magic): results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6):S1-S40. https://doi.org/10.7326/M15-0744.
26. Raad II, Hohn DC, Gilbreath BJ, et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994;15(4):231-238.
27. Slama M, Novara A, Safavian A, et al. Improvement of internal jugular vein cannulation using an ultrasound-guided technique. Intensive Care Med. 1997;23(8):916-919. https://doi.org/10.1007/s001340050432.
28. Seyahi N, Kahveci A, Altiparmak MR, Serdengecti K, Erek E. Ultrasound imaging findings of femoral veins in patients with renal failure and its impact on vascular access. Nephrol Dial Transplant. 2005;20(9):1864-1867. https://doi.org/10.1093/ndt/gfh942.
29. Lin BS, Kong CW, Tarng DC, Huang TP, Tang GJ. Anatomical variation of the internal jugular vein and its impact on temporary haemodialysis vascular access: an ultrasonographic survey in uraemic patients. Nephrol Dial Transplant. 1998;13(1):134-138. https://doi.org/10.1093/ndt/13.1.134.
30. Troianos CA, Kuwik RJ, Pasqual JR, Lim AJ, Odasso DP. Internal jugular vein and carotid artery anatomic relation as determined by ultrasonography. Anesthesiology. 1996;85(1):43-48. https://doi.org/10.1097/00000542-199607000-00007.
31. Gordon AC, Wright I, Pugh ND. Duplication of the superficial femoral vein: recognition with duplex ultrasonography. Clin Rad. 1996;51(9):622-624. https://doi.org/10.1016/S0009-9260(96)80055-9.
32. Sulek CA, Gravenstein N, Blackshear RH, Weiss L. Head rotation during internal jugular vein cannulation and the risk of carotid artery puncture. Anesth Analg. 1996;82(1):125-128. https://doi.org/10.1097/00000539-199601000-00022.
33. Shrestha BR, Gautam B. Ultrasound versus the landmark technique: a prospective randomized comparative study of internal jugular vein cannulation in an intensive care unit. JNMA J Nepal Med Assoc. 2011;51(182):56-61. https://doi.org/10.31729/jnma.148.
34. Brederlau J, Greim C, Schwemmer U, et al. Ultrasound-guided cannulation of the internal jugular vein in critically ill patients positioned in 30 degrees dorsal elevation. Eur J Anaesthesiol. 2004;21(9):684-687. https://doi.org/10.1097/00003643-200409000-00003.
35. Lamperti M, Subert M, Cortellazzi P, et al. Is a neutral head position safer than 45-degree neck rotation during ultrasound-guided internal jugular vein cannulation? Results of a randomized controlled clinical trial. Anesth Analg. 2012;114(4):777-784. https://doi.org/10.1213/ANE.0b013e3182459917.
36. Benter T, Teichgräber UK, Klühs L, et al. Anatomical variations in the internal jugular veins of cancer patients affecting central venous access. Anatomical variation of the internal jugular vein. Ultraschall Med. 2001;22(1):23-26. https://doi.org/10.1055/s-2001-11243.
37. Caridi JG, Hawkins IF, Jr, Wiechmann BN, Pevarski DJ, Tonkin JC. Sonographic guidance when using the right internal jugular vein for central vein access. AJR Am J Roentgenol. 1998;171(5):1259-1263. https://doi.org/10.2214/ajr.171.5.9798857.
38. Brusasco C, Corradi F, Zattoni PL, et al. Ultrasound-guided central venous cannulation in bariatric patients. Obes Surg. 2009;19(10):1365-1370. https://doi.org/10.1007/s11695-009-9902-y.
39. Armstrong PJ, Cullen M, Scott DH. The ‘SiteRite’ ultrasound machine--an aid to internal jugular vein cannulation. Anaesthesia. 1993;48(4):319-323. https://doi.org/10.1111/j.1365-2044.1993.tb06953.x.
40. Forauer AR, Glockner JF. Importance of US findings in access planning during jugular vein hemodialysis catheter placements. J Vasc Interv Rad. 2000;11(2 Pt 1):233-238. https://doi.org/10.1016/S1051-0443(07)61471-7.
41. Hassan C, Girishkumar HT, Thatigotla B, et al. Value of ultrasound guidance in placement of hemodialysis access catheters in patients with end-stage renal disease. Am Surg. 2008;74(11):1111-1113.
42. Tan CO, Weinberg L, Peyton P, Story D, McNicol L. Size variation between contralateral infraclavicular axillary veins within individual patients-implications for subclavian venous central line insertion. Crit Care Med. 2013;41(2):457-463. https://doi.org/10.1097/CCM.0b013e31826ab1dd.
43. Wu SY, Ling Q, Cao LH, et al. Real-time two-dimensional ultrasound guidance for central venous cannulation: a meta-analysis. Anesthesiology. 2013;118(2):361-375. https://doi.org/10.1097/ALN.0b013e31827bd172.
44. Kim W, Chung RK, Lee GY, Han JI. The effects of hip abduction with external rotation and reverse Trendelenburg position on the size of the femoral vein; ultrasonographic investigation. Korean J Anesthesiol. 2011;61(3):205-209. https://doi.org/10.4097/kjae.2011.61.3.205.
45. Denys BG, Uretsky BF. Anatomical variations of internal jugular vein location: impact on central venous access. Crit Care Med. 1991;19(12):1516-1519. https://doi.org/10.1097/00003246-199112000-00013.
46. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev. 2015;1:CD006962. https://doi.org/10.1002/14651858.CD006962.pub2.
47. Gilbert TB, Seneff MG, Becker RB. Facilitation of internal jugular venous cannulation using an audio-guided Doppler ultrasound vascular access device: results from a prospective, dual-center, randomized, crossover clinical study. Crit Care Med. 1995;23(1):60-65. https://doi.org/10.1097/00003246-199501000-00012.
48. Schummer W, Schummer C, Tuppatsch H, et al. Ultrasound-guided central venous cannulation: is there a difference between Doppler and B-mode ultrasound? J Clin Anesth. 2006;18(3):167-172. https://doi.org/10.1016/j.jclinane.2005.12.010.
49. Airapetian N, Maizel J, Langelle F, et al. Ultrasound-guided central venous cannulation is superior to quick-look ultrasound and landmark methods among inexperienced operators: a prospective randomized study. Intensive Care Med. 2013;39(11):1938-1944. https://doi.org/10.1007/s00134-013-3072-z.
50. Milling TJ, Jr, Rose J, Briggs WM, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Crit Care Med. 2005;33(8):1764-1769. https://doi.org/10.1097/01.ccm.0000171533.92856.e5.
51. Beaudoin FL, Merchant RC, Lincoln J, et al. Bedside ultrasonography detects significant femoral vessel overlap: implications for central venous cannulation. CJEM. 2011;13(4):245-250. https://doi.org/10.2310/8000.2011.110482.
52. Kwon TH, Kim YL, Cho DK. Ultrasound-guided cannulation of the femoral vein for acute haemodialysis access. Nephrol Dial Transplant. 1997;12(5):1009-1012. https://doi.org/10.1093/ndt/12.5.1009.
53. Rothschild JM. Ultrasound guidance of central vein catheterization. Evid Rep Technol Assess. 2001;43. Chapter 21.: http://archive.ahrq.gov/clinic/ptsafety/chap21.htm.
54. Lennon M, Zaw NN, Pöpping DM, Wenk M. Procedural complications of central venous catheter insertion. Minerva Anestesiol. 2012;78(11):1234-1240.
55. Blaivas M, Adhikari S. An unseen danger: frequency of posterior vessel wall penetration by needles during attempts to place internal jugular vein central catheters using ultrasound guidance. Crit Care Med. 2009;37(8):2345-2349; quiz 2359. https://doi.org/10.1097/CCM.0b013e3181a067d4.
56. Vogel JA, Haukoos JS, Erickson CL, et al. Is long-axis view superior to short-axis view in ultrasound-guided central venous catheterization? Crit Care Med. 2015;43(4):832-839. https://doi.org/10.1097/CCM.0000000000000823.
57. Tammam TF, El-Shafey EM, Tammam HF. Ultrasound-guided internal jugular vein access: comparison between short axis and long axis techniques. Saudi J Kidney Dis Transpl. 2013;24(4):707-713. https://doi.org/10.4103/1319-2442.113861.
58. Keyes LE, Frazee BW, Snoey ER, Simon BC, Christy D. Ultrasound-guided brachial and basilic vein cannulation in emergency department patients with difficult intravenous access. Ann Emerg Med. 1999;34(6):711-714. https://doi.org/10.1016/S0196-0644(99)70095-8.
59. Mahler SA, Wang H, Lester C, et al. Short- vs long-axis approach to ultrasound-guided peripheral intravenous access: a prospective randomized study. Am J Emerg Med. 2011;29(9):1194-1197. https://doi.org/10.1016/j.ajem.2010.07.015.
60. Berk D, Gurkan Y, Kus A, et al. Ultrasound-guided radial arterial cannulation: long axis/in-plane versus short axis/out-of-plane approaches? J Clin Monit Comput. 2013;27(3):319-324. https://doi.org/10.1007/s10877-013-9437-6.
61. Batllori M, Urra M, Uriarte E, et al. Randomized comparison of three transducer orientation approaches for ultrasound guided internal jugular venous cannulation. Br J Anaesth. 2016;116(3):370-376. https://doi.org/10.1093/bja/aev399.
62. Moak JH, Lyons MS, Wright SW, Lindsell CJ. Needle and guidewire visualization in ultrasound-guided internal jugular vein cannulation. Am J Emerg Med. 2011;29(4):432-436. https://doi.org/10.1016/j.ajem.2010.01.004.
63. Moak JH, Rajkumar JS, Woods WA. The wire is really easy to see (WIRES): sonographic visualization of the guidewire by novices. CJEM. 2013;15(1):18-23. https://doi.org/10.2310/8000.2012.120800.
64. Gillman LM, Blaivas M, Lord J, Al-Kadi A, Kirkpatrick AW. Ultrasound confirmation of guidewire position may eliminate accidental arterial dilatation during central venous cannulation. Scand J Trauma Resusc Emerg Med. 2010;18:39. https://doi.org/10.1186/1757-7241-18-39.
65. Augoustides JG, Horak J, Ochroch AE, et al. A randomized controlled clinical trial of real-time needle-guided ultrasound for internal jugular venous cannulation in a large university anesthesia department. J Cardiothorac Vasc Anesth. 2005;19(3):310-315. https://doi.org/10.1053/j.jvca.2005.03.007.
66. Maecken T, Heite L, Wolf B, Zahn PK, Litz RJ. Ultrasound-guided catheterisation of the subclavian vein: freehand vs needle-guided technique. Anaesthesia. 2015;70(11):1242-1249. https://doi.org/10.1111/anae.13187.
67. Stone MB, Nagdev A, Murphy MC, Sisson CA. Ultrasound detection of guidewire position during central venous catheterization. Am J Emerg Med. 2010;28(1):82-84. https://doi.org/10.1016/j.ajem.2008.09.019.
68. Luyet C, Hartwich V, Urwyler N, et al. Evaluation of a novel needle guide for ultrasound-guided phantom vessel cannulation. Anaesthesia. 2011;66(8):715-720. https://doi.org/10.1111/j.1365-2044.2011.06781.x.
69. O’Grady NP, Alexander M, Burns LA, et al. Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis. 2011;52(9):1087-1099. https://doi.org/10.1093/cid/cir138.
70. Southworth SL, Henman LJ, Kinder LA, Sell JL. The journey to zero central catheter-associated bloodstream infections: culture change in an intensive care unit. Crit Care Nurse. 2012;32(2):49-54. https://doi.org/10.4037/ccn2012915.
71. Guerin K, Wagner J, Rains K, Bessesen M. Reduction in central line-associated bloodstream infections by implementation of a postinsertion care bundle. Am J Infect Control. 2010;38(6):430-433. https://doi.org/10.1016/j.ajic.2010.03.007.
72. Hartman N, Wittler M, Askew K, Manthey D. Delphi method validation of a procedural performance checklist for insertion of an ultrasound-guided internal jugular central line. Am J Med Qual Off J Am Coll Med Qual. 2016;31(1):81-85. https://doi.org/10.1177/1062860614549762.
73. Lenchus JD, Carvalho CM, Ferreri K, et al. Filling the void: defining invasive bedside procedural competency for internal medicine residents. J Grad Med Educ. 2013;5(4):605-612. https://doi.org/10.4300/JGME-D-13-00030.1.
74. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. https://doi.org/10.1001/archinternmed.2009.215.
75. Karakitsos D, Labropoulos N, De Groot E, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care. 2006;10(6):R162. https://doi.org/10.1186/cc5101.
76. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. https://doi.org/10.1097/01.ccm.0000142399.70913.2f.
77. Young EM, Commiskey ML, Wilson SJ. Translating evidence into practice to prevent central venous catheter-associated bloodstream infections: a systems-based intervention. Am J Infect Control. 2006;34(8):503-506. https://doi.org/10.1016/j.ajic.2006.03.011.
78. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495; discussion 495. https://doi.org/10.1016/j.surg.2008.06.004.
79. Mermel LA, McCormick RD, Springman SR, Maki DG. The pathogenesis and epidemiology of catheter-related infection with pulmonary artery swan-Ganz catheters: a prospective study utilizing molecular subtyping. Am J Med. 1991;91(3b):197S–205S. https://doi.org/10.1016/0002-9343(91)90369-9.
80. Marschall J, Mermel LA, Classen D, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(1):S22-S30. https://doi.org/10.1086/591059.
81. Marschall J, Mermel LA, Fakih M, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(7):753-771. https://doi.org/10.1086/676533.
82. Garcia-Rodriguez JF, Álvarez-Díaz H, Vilariño-Maneiro L, et al. Epidemiology and impact of a multifaceted approach in controlling central venous catheter associated blood stream infections outside the intensive care unit. BMC Infect Dis. 2013;13:445. https://doi.org/10.1186/1471-2334-13-445.
83. Lee DH, Jung KY, Choi YH. Use of maximal sterile barrier precautions and/or antimicrobial-coated catheters to reduce the risk of central venous catheter-related bloodstream infection. Infect Control Hosp Epidemiol. 2008;29(10):947-950. https://doi.org/10.1086/590356.
84. Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132(5):391-402. https://doi.org/10.7326/0003-4819-132-5-200003070-00009.
85. Troianos CA, Jobes DR, Ellison N. Ultrasound-guided cannulation of the internal jugular vein. A prospective, randomized study. Anesth Analg. 1991;72(6):823-826. https://doi.org/10.1213/00000539-199106000-00020.
86. Troianos CA, Savino JS. Internal jugular vein cannulation guided by echocardiography. Anesthesiology. 1991;74(4):787-789. https://doi.org/10.1097/00000542-199104000-00026.
87. Denys BG, Uretsky BF, Reddy PS, et al. An ultrasound method for safe and rapid central venous access. N Engl J Med. 1991;324(8):566. https://doi.org/10.1056/NEJM199102213240816.
88. Riaz A, Shan Khan RA, Salim F. Ultrasound guided internal jugular venous cannulation: comparison with land-mark technique. J Coll Phys Surg Pak. 2015;25(5):315-319. https://doi.org/05.2015/JCPSP.315319.
89. Gok F, Kilicaslan A, Sarkilar G, Kandemir B, Yosunkaya A. The effect of ultrasound guidance on central venous catheter-associated bloodstream infection in critical care patients. Acta Med Mediterr. 2013;29:677-682.
90. Lamperti M, Cortellazzi P, D’Onofrio G, et al. An outcome study on complications using routine ultrasound assistance for internal jugular vein cannulation. Acta Anaesthesiol Scand. 2007;51(10):1327-1330. https://doi.org/10.1111/j.1399-6576.2007.01442.x.
91. Vezzani A, Manca T, Vercelli A, Braghieri A, Magnacavallo A. Ultrasonography as a guide during vascular access procedures and in the diagnosis of complications. J Ultrasound. 2013;16(4):161-170. https://doi.org/10.1007/s40477-013-0046-5.
92. Palepu GB, Deven J, Subrahmanyam M, Mohan S. Impact of ultrasonography on central venous catheter insertion in intensive care. Indian J Rad Imaging. 2009;19(3):191-198. https://doi.org/10.4103/0971-3026.54877.
93. Agarwal A, Singh DK, Singh AP. Ultrasonography: a novel approach to central venous cannulation. Indian J Crit Care Med. 2009;13(4):213-216. https://doi.org/10.4103/0972-5229.60174.
94. Wong SW, Niazi AU, Chin KJ, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS® needle tracking system: a case report. Can J Anaesth. 2013;60(1):50-53. https://doi.org/10.1007/s12630-012-9809-2.
95. Bouaziz H, Zetlaoui PJ, Pierre S, et al. Guidelines on the use of ultrasound guidance for vascular access. Anaesth, Crit Care Pain Med. 2015;34(1):65-69. https://doi.org/10.1016/j.accpm.2015.01.004.
96. Jenssen C, Brkljacic B, Hocke M, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part VI - Ultrasound-guided vascular interventions. Ultraschall Med. 2016;37(5):473-476. https://doi.org/10.1055/s-0035-1553450.
97. Rando K, Castelli J, Pratt JP, et al. Ultrasound-guided internal jugular vein catheterization: a randomized controlled trial. Heart Lung Vessels. 2014;6(1):13-23.
98. Mey U, Glasmacher A, Hahn C, et al. Evaluation of an ultrasound-guided technique for central venous access via the internal jugular vein in 493 patients. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2003;11(3):148-155. https://doi.org/10.1007/s00520-002-0399-3.
99. Singh SA, Sharma S, Singh A, et al. The safety of ultrasound guided central venous cannulation in patients with liver disease. Saudi J Anaesth. 2015;9(2):155-160. https://doi.org/10.4103/1658-354X.152842.
100. Akoglu H, Piskinpasa S, Yenigun EC, et al. Real-time ultrasound guided placement of temporary internal jugular vein catheters: assessment of technical success and complication rates in nephrology practice. Nephrol (Carlton). 2012;17(7):603-606. https://doi.org/10.1111/j.1440-1797.2012.01637.x.
101. Sadler DJ, Gordon AC, Klassen J, et al. Image-guided central venous catheters for apheresis. Bone Marrow Transplant. 1999;23(2):179-182. https://doi.org/10.1038/sj.bmt.1701545.
102. Calvert N, Hind D, McWilliams R, et al. Ultrasound for central venous cannulation: economic evaluation of cost-effectiveness. Anaesthesia. 2004;59(11):1116-1120. https://doi.org/10.1111/j.1365-2044.2004.03906.x.
103. Goetz AM, Wagener MM, Miller JM, Muder RR. Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infect Control Hosp Epidemiol. 1998;19(11):842-845.
104. Bertini P, Frediani M. Ultrasound guided supraclavicular central vein cannulation in adults: a technical report. J Vasc Access. 2013;14(1):89-93. https://doi.org/10.5301/jva.5000088.
105. Lalu MM, Fayad A, Ahmed O, et al. Ultrasound-guided subclavian vein catheterization: A systematic review and meta-analysis. Crit Care Med. 2015;43(7):1498-1507. https://doi.org/10.1097/CCM.0000000000000973.
106. Milone M, Di Minno G, Di Minno MN, et al. The real effectiveness of ultrasound guidance in subclavian venous access. Ann ital chir. 2010;81(5):331-334.
107. O’Leary R, Ahmed SM, McLure H, et al. Ultrasound-guided infraclavicular axillary vein cannulation: a useful alternative to the internal jugular vein. Br J Anaesth. 2012;109(5):762-768. https://doi.org/10.1093/bja/aes262.
108. Gualtieri E, Deppe SA, Sipperly ME, Thompson DR. Subclavian venous catheterization: greater success rate for less experienced operators using ultrasound guidance. Crit Care Med. 1995;23(4):692-697. https://doi.org/10.1097/00003246-199504000-00018.
109. Fragou M, Gravvanis A, Dimitriou V, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med. 2011;39(7):1607-1612. https://doi.org/10.1097/CCM.0b013e318218a1ae.
110. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015;1:CD011447. https://doi.org/10.1002/14651858.CD011447.
111. Robinson MK, Mogensen KM, Grudinskas GF, Kohler S, Jacobs DO. Improved care and reduced costs for patients requiring peripherally inserted central catheters: the role of bedside ultrasound and a dedicated team. JPEN J Parenter Enter Nutr. 2005;29(5):374-379. https://doi.org/10.1177/0148607105029005374.
112. Li J, Fan YY, Xin MZ, et al. A randomised, controlled trial comparing the long-term effects of peripherally inserted central catheter placement in chemotherapy patients using B-mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94-103. https://doi.org/10.1016/j.ejon.2013.08.003.
113. Schweickert WD, Herlitz J, Pohlman AS, et al. A randomized, controlled trial evaluating postinsertion neck ultrasound in peripherally inserted central catheter procedures. Crit Care Med. 2009;37(4):1217-1221. https://doi.org/10.1097/CCM.0b013e31819cee7f.
114. Mahler SA, Wang H, Lester C, Conrad SA. Ultrasound-guided peripheral intravenous access in the emergency department using a modified Seldinger technique. J Emerg Med. 2010;39(3):325-329. https://doi.org/10.1016/j.jemermed.2009.02.013.
115. Brannam L, Blaivas M, Lyon M, Flake M. Emergency nurses’ utilization of ultrasound guidance for placement of peripheral intravenous lines in difficult-access patients. Acad Emerg Med. 2004;11(12):1361-1363. https://doi.org/10.1197/j.aem.2004.08.027.
116. Au A, Rotte M, Gryzbowski R, Ku B, Fields J. 157 Decrease in central venous catheter placement and complications due to utilization of ultrasound-guided peripheral intravenous catheters. Ann Emerg Med. 2011;58(4):S230. https://doi.org/10.1016/j.annemergmed.2011.06.185.
117. Dargin JM, Rebholz CM, Lowenstein RA, Mitchell PM, Feldman JA. Ultrasonography-guided peripheral intravenous catheter survival in ED patients with difficult access. Am J Emerg Med. 2010;28(1):1-7. https://doi.org/10.1016/j.ajem.2008.09.001.
118. Bauman M, Braude D, Crandall C. Ultrasound-guidance vs standard technique in difficult vascular access patients by ED technicians. Am J Emerg Med. 2009;27(2):135-140. https://doi.org/10.1016/j.ajem.2008.02.005.
119. Schoenfeld E, Shokoohi H, Boniface K. Ultrasound-guided peripheral intravenous access in the emergency department: patient-centered survey. West J Emerg Med. 2011;12(4):475-477. https://doi.org/10.5811/westjem.2011.3.1920.
120. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. https://doi.org/10.7326/0003-4819-114-10-845.
121. Miliani K, Taravella R, Thillard D, et al. Peripheral venous catheter-related adverse events: evaluation from a multicentre epidemiological study in France (the CATHEVAL Project). PLOS ONE. 2017;12(1):e0168637. https://doi.org/10.1371/journal.pone.0168637.
122. Gregg SC, Murthi SB, Sisley AC, Stein DM, Scalea TM. Ultrasound-guided peripheral intravenous access in the intensive care unit. J Crit Care. 2010;25(3):514-519. https://doi.org/10.1016/j.jcrc.2009.09.003.
123. Adhikari S, Blaivas M, Morrison D, Lander L. Comparison of infection rates among ultrasound-guided versus traditionally placed peripheral intravenous lines. J Ultrasound Med. 2010;29(5):741-747. https://doi.org/10.7863/jum.2010.29.5.741.
124. Shiver S, Blaivas M, Lyon M. A prospective comparison of ultrasound-guided and blindly placed radial arterial catheters. Acad Emerg Med. 2006;13(12):1275-1279. https://doi.org/10.1197/j.aem.2006.07.015.
125. Levin PD, Sheinin O, Gozal Y. Use of ultrasound guidance in the insertion of radial artery catheters. Crit Care Med. 2003;31(2):481-484. https://doi.org/10.1097/01.CCM.0000050452.17304.2F.
126. Gu WJ, Tie HT, Liu JC, Zeng XT. Efficacy of ultrasound-guided radial artery catheterization: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2014;18(3):R93. https://doi.org/10.1186/cc13862.
127. Tang L, Wang F, Li Y, et al. Ultrasound guidance for radial artery catheterization: an updated meta-analysis of randomized controlled trials. PLOS ONE. 2014;9(11):e111527. https://doi.org/10.1371/journal.pone.0111527.
128. Sobolev M, Slovut DP, Lee Chang A, Shiloh AL, Eisen LA. Ultrasound-guided catheterization of the femoral artery: A systematic review and meta-analysis of randomized controlled trials. J Invas Cardiol. 2015;27(7):318-323. https://doi.org/10.1378/chest.1991181.
129. Gabriel M, Pawlaczyk K, Waliszewski K, Krasiński Z, Majewski W. Location of femoral artery puncture site and the risk of postcatheterization pseudoaneurysm formation. Int J Cardiol. 2007;120(2):167-171. https://doi.org/10.1016/j.ijcard.2006.09.018.
130. Seto AH, Abu-Fadel MS, Sparling JM, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With ultrasound Trial). JACC Cardiovasc Interv. 2010;3(7):751-758. https://doi.org/10.1016/j.jcin.2010.04.015.
131. Kalish J, Eslami M, Gillespie D, et al. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg. 2015;61(5):1231-1238. https://doi.org/10.1016/j.jvs2014.12.003.
132. Sandhu NS, Patel B. Use of ultrasonography as a rescue technique for failed radial artery cannulation. J Clin Anesth. 2006;18(2):138-141. https://doi.org/10.1016/j.jclinane.2005.06.011.
133. White L, Halpin A, Turner M, Wallace L. Ultrasound-guided radial artery cannulation in adult and paediatric populations: a systematic review and meta-analysis. Br J Anaesth. 2016;116(5):610-617. https://doi.org/10.1093/bja/aew097.
134. Gao YB, Yan JH, Gao FQ, et al. Effects of ultrasound-guided radial artery catheterization: an updated meta-analysis. Am J Emerg Med. 2015;33(1):50-55. https://doi.org/10.1016/j.ajem.2014.10.008.
135. Seto AH, Roberts JS, Abu-Fadel MS, et al. Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery Access with Ultrasound Trial). JACC Cardiovasc Interv. 2015;8(2):283-291. https://doi.org/10.1016/j.jcin.2014.05.036.
136. Roberts J, Manur R. Ultrasound-guided radial artery access by a non-ultrasound trained interventional cardiologist improved first-attempt success rates and shortened time for successful radial artery cannulation. J Invas Cardiol. 2013;25(12):676-679.
137. Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108(5):1345-1348. https://doi.org/10.1378/chest.108.5.1345.
138. Lichtenstein D, Mezière G, Biderman P, Gepner A. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999;25(4):383-388. https://doi.org/10.1007/s001340050862.
139. Ablordeppey EA, Drewry AM, Beyer AB, et al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound Versus chest radiography in critically ill patients: A systematic review and meta-analysis. Crit Care Med. 2017;45(4):715-724. https://doi.org/10.1097/CCM.0000000000002188.
140. Bedel J, Vallée F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013;39(11):1932-1937. https://doi.org/10.1007/s00134-013-3097-3.
141. Weekes AJ, Keller SM, Efune B, Ghali S, Runyon M. Prospective comparison of ultrasound and CXR for confirmation of central vascular catheter placement. Emerg Med J EMJ. 2016;33(3):176-180. https://doi.org/10.1136/emermed-2015-205000.
142. Arellano R, Nurmohamed A, Rumman A, et al. The utility of transthoracic echocardiography to confirm central line placement: an observational study. Can J Anaesth. 2014;61(4):340-346. https://doi.org/10.1007/s12630-014-0111-3.
143. Vezzani A, Brusasco C, Palermo S, et al. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38(2):533-538. https://doi.org/10.1097/CCM.0b013e3181c0328f.
144. Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142(4):260-273. https://doi.org/10.7326/0003-4819-142-4-200502150-00008.
145. Backlund BH, Hopkins E, Kendall JL. Ultrasound guidance for central venous access by emergency physicians in Colorado. West J Emerg Med. 2012;13(4):320-325. https://doi.org/10.5811/westjem.2011.11.6821.
146. Buchanan MS, Backlund B, Liao MM, et al. Use of ultrasound guidance for central venous catheter placement: survey from the American Board of Emergency Medicine Longitudinal Study of Emergency Physicians. Acad Emerg Med. 2014;21(4):416-421. https://doi.org/10.1111/acem.12350.
147. Barsuk JH, McGaghie WC, Cohen ER, O’Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697-2701. https://doi.org/10.1097/00003246-200910000-00003.
148. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. https://doi.org/10.1097/00003246-200201000-00009.
149. Woo MY, Frank J, Lee AC, et al. Effectiveness of a novel training program for emergency medicine residents in ultrasound-guided insertion of central venous catheters. CJEM. 2009;11(4):343-348. https://doi.org/10.1017/S1481803500011398.
150. McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348(12):1123-1133. https://doi.org/10.1056/NEJMra011883.
151. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. https://doi.org/10.1002/jhm.468.
152. Sekiguchi H, Tokita JE, Minami T, et al. A prerotational, simulation-based workshop improves the safety of central venous catheter insertion: results of a successful internal medicine house staff training program. Chest. 2011;140(3):652-658. https://doi.org/10.1378/chest.10-3319.
153. Feller-Kopman D. Ultrasound-guided internal jugular access: a proposed standardized approach and implications for training and practice. Chest. 2007;132(1):302-309. https://doi.org/10.1378/chest.06-2711.
154. Troianos CA, Hartman GS, Glas KE, et al. Special articles: guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg. 2012;114(1):46-72. https://doi.org/10.1213/ANE.0b013e3182407cd8.
155. Issenberg SB, McGaghie WC, Hart IR, et al. Simulation technology for health care professional skills training and assessment. JAMA. 1999;282(9):861-866. https://doi.org/10.1001/jama.282.9.861.
156. Millington SJ, Wong RY, Kassen BO, Roberts JM, Ma IW. Improving internal medicine residents’ performance, knowledge, and confidence in central venous catheterization using simulators. J Hosp Med. 2009;4(7):410-416. https://doi.org/10.1002/jhm.570.
157. Beaulieu Y, Laprise R, Drolet P, et al. Bedside ultrasound training using web-based e-learning and simulation early in the curriculum of residents. Crit Ultrasound J. 2015;7:1. https://doi.org/10.1186/s13089-014-0018-9.
158. Barsuk JH, Cohen ER, McGaghie WC, Wayne DB. Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med. 2010;85(10 Suppl):S9-S12. https://doi.org/10.1097/ACM.0b013e3181ed436c.
159. Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133(1):56-61. https://doi.org/10.1378/chest.07-0131.
160. Evans LV, Dodge KL, Shah TD, et al. Simulation training in central venous catheter insertion: improved performance in clinical practice. Acad Med. 2010;85(9):1462-1469. https://doi.org/10.1097/ACM.0b013e3181eac9a3.
161. Smith CC, Huang GC, Newman LR, et al. Simulation training and its effect on long-term resident performance in central venous catheterization. Simul Healthc J Soc Simul Healthc. 2010;5(3):146-151. https://doi.org/10.1097/SIH.0b013e3181dd9672.
162. Laack TA, Dong Y, Goyal DG, et al. Short-term and long-term impact of the central line workshop on resident clinical performance during simulated central line placement. Simul Healthc J Soc Simul Healthc. 2014;9(4):228-233. https://doi.org/10.1097/SIH.0000000000000015.
163. Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31(10):1519-1526. https://doi.org/10.7863/jum.2012.31.10.1519.
164. Bayci AW, Mangla J, Jenkins CS, Ivascu FA, Robbins JM. Novel educational module for subclavian central venous catheter insertion using real-time ultrasound guidance. J Surg Educ. 2015;72(6):1217-1223. https://doi.org/10.1016/j.jsurg.2015.07.010.
165. Andreatta P, Chen Y, Marsh M, Cho K. Simulation-based training improves applied clinical placement of ultrasound-guided PICCs. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2011;19(4):539-543. https://doi.org/10.1007/s00520-010-0849-2.
166. Rosen BT, Uddin PQ, Harrington AR, Ault BW, Ault MJ. Does personalized vascular access training on a nonhuman tissue model allow for learning and retention of central line placement skills? Phase II of the procedural patient safety initiative (PPSI-II). J Hosp Med. 2009;4(7):423-429. https://doi.org/10.1002/jhm.571.
167. Ault MJ, Rosen BT, Ault B. The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative. J Gen Intern Med. 2006;21(5):514-517. https://doi.org/10.1111/j.1525-1497.2006.00440.x.
168. Varga S, Smith J, Minneti M, et al. Central venous catheterization using a perfused human cadaveric model: application to surgical education. J Surg Educ. 2015;72(1):28-32. https://doi.org/10.1016/j.jsurg.2014.07.005.
169. Sansivero GE. Venous anatomy and physiology. Considerations for vascular access device placement and function. J Intraven Nurs Off Publ Intraven Nurs Soc. 1998;21(5 Suppl):S107-S114.
170. Eisen LA, Narasimhan M, Berger JS, et al. Mechanical complications of central venous catheters. Journal of intensive care medicine. 2006;21(1):40-46. https://doi.org/10.1177/0885066605280884.
171. Chenkin J, Lee S, Huynh T, Bandiera G. Procedures can be learned on the Web: a randomized study of ultrasound-guided vascular access training. Acad Emerg Med. 2008;15(10):949-954. https://doi.org/10.1111/j.1553-2712.2008.00231.x.
172. Abualenain J, Calabrese K, Tansek R, Ranniger C. 319 Comparing standard versus video-based teaching for ultrasound-guided internal jugular central venous catheter access for fourth-year medical students. Ann Emerg Med. 2014;64(4):S113. https://doi.org/10.1016/j.annemergmed.2014.07.347.
173. Pustavoitau A, Blaivas M, Brown SM, et al. Recommendations for achieving and maintaining competence and credentialing in critical care ultrasound with focused cardiac ultrasound and advanced critical care echocardiography. Crit Care Med. 2016.
174. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: A survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
175. Moureau N, Lamperti M, Kelly LJ, et al. Evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training. Br J Anaesth. 2013;110(3):347-356. https://doi.org/10.1093/bja/aes499.
176. Ernst A, Silvestri GA, Johnstone D, American College of Chest Physicians. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123(5):1693-1717. https://doi.org/10.1378/chest.123.5.1693.
177. Thomas SM, Burch W, Kuehnle SE, et al. Simulation training for pediatric residents on central venous catheter placement: a pilot study. Pediatr Crit Care Med J Soc Crit Care Med.. 2013;14(9):e416-e423. https://doi.org/10.1097/PCC.0b013e31829f5eda.
178. Smith KK, Gilcreast D, Pierce K. Evaluation of staff’s retention of ACLS and BLS skills. Resuscitation. 2008;78(1):59-65. https://doi.org/10.1016/j.resuscitation.2008.02.007.
179. Ericsson KA. Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med. 2004;79(10 Suppl):S70-S81. https://doi.org/10.1097/00001888-200410001-00022.
180. Gerard JM, Thomas SM, Germino KW, et al. The effect of simulation training on PALS skills among family medicine residents. Fam Med. 2011;43(6):392-399.
181. Brydges R, Nair P, Ma I, Shanks D, Hatala R. Directed self-regulated learning versus instructor-regulated learning in simulation training. Med Educ. 2012;46(7):648-656. https://doi.org/10.1111/j.1365-2923.2012.04268.x.
182. Wayne DB, Butter J, Siddall VJ, et al. Simulation-based training of internal medicine residents in advanced cardiac life support protocols: a randomized trial. Teach Learn Med. 2005;17(3):210-216. https://doi.org/10.1207/s15328015tlm1703_3.
183. Arthur Jr. W, Bennett Jr. W, Stanush PL, McNelly TL. Factors that influence skill decay and retention: A quantitative review and analysis. Hum Perform. 1998;11(1):57-101. https://doi.org/10.1207/s15327043hup1101_3.
184. Rusche JD, Besuner P, Partusch SK, Berning PA. Competency program development across a merged healthcare network. J Nurs Staff Dev. 2001;17(5):234-240; quiz 241-232. https://doi.org/10.1097/00124645-200109000-00004.
185. O’Hearne Rebholz M. A review of methods to assess competency. J Nurs Staff Dev. 2006;22(5):241-245. https://doi.org/10.1097/00124645-200609000-00007.
186. Reznick RK. Teaching and testing technical skills. Am J Surg. 1993;165(3):358-361. https://doi.org/10.1016/s0002-9610(05)80843-8.
187. Reznick RK, MacRae H. Teaching surgical skills--changes in the wind. N Engl J Med. 2006;355(25):2664-2669. https://doi.org/10.1056/NEJMra054785.
188. Murin S, Stollenwerk NS. Simulation in procedural training: at the tipping point. Chest. 2010;137(5):1009-1011. https://doi.org/10.1378/chest.10-0199.
189. American College of Emergency Physicians. Emergency ultrasound guidelines. Ann Emerg Med. 2009;53(4):550-570. https://doi.org/10.1016/j.annemergmed.2008.12.013.
190. Guilbert MC, Elkouri S, Bracco D, et al. Arterial trauma during central venous catheter insertion: case series, review and proposed algorithm. J Vasc Surg. 2008;48(4):918-925; discussion 925. https://doi.org/10.1016/j.jvs2008.04.046.
191. Dong Y, Suri HS, Cook DA, et al. Simulation-based objective assessment discerns clinical proficiency in central line placement: a construct validation. Chest. 2010;137(5):1050-1056. https://doi.org/10.1378/chest.09-1451.
192. Adhikari S, Theodoro D, Raio C, et al. Central venous catheterization: are we using ultrasound guidance? J Ultrasound Med. 2015;34(11):2065-2070. https://doi.org/10.7863/ultra.15.01027.
193. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors--procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z.
© 2019 Society of Hospital Medicine
Recommendations on the Use of Ultrasound Guidance for Adult Lumbar Puncture: A Position Statement of the Society of Hospital Medicine
Approximately 400,000 lumbar punctures (LPs) are performed in the United States annually for either diagnostic workup or therapeutic relief.1 Lumbar punctures are increasingly being performed in the United States, with an estimated 97,000 LPs performed on Medicare fee-for-service beneficiaries in 2011 alone, which is an increase of approximately 4,000 LPs in the same population from 1991.2 Approximately 273,612 LPs were performed on hospitalized patients in the United States in 2010,1 and the inpatient hospital setting is the most common site for LPs.2,3
Many LPs are referred to radiologists who have access to imaging guidance to aid with needle insertion.2 However, referrals to radiology delay performance of LPs, and delayed diagnosis of acute bacterial meningitis, the most common yet serious condition for which LPs are performed, is associated with increased morbidity and mortality.4-8 Furthermore, although initiating empiric antibiotic treatment for suspected acute bacterial meningitis is recommended in some cases, doing so routinely can cause false-negative cerebrospinal fluid (CSF) culture results, complicating decisions about de-escalation and duration of antibiotics that could have been safely avoided by promptly performing an LP.9
Delaying the performance of LP has been associated with increased mortality.10 Demonstration of proficiency in performance of lumbar puncture is considered a core competency for hospitalists,11 and with the increasing availability of point-of-care ultrasound, hospitalists can use ultrasound to guide performance of LPs at the bedside.12 However, 30% of patients requiring LP in emergency departments have difficult-to-palpate lumbar spine landmarks,13 and lumbar puncture performed based on palpation of landmarks alone has been reported to fail or be traumatic in 28% of patients.14 Use of ultrasound guidance for lumbar puncture has been shown in randomized controlled trials to improve procedural success rates, while reducing the time to successful LP, needle passes, patient pain scores, and risk of a traumatic LP.15-17
The purpose of this position statement is to review the literature and present consensus-based recommendations on the performance of ultrasound-guided LP in adult patients. This position statement does not mandate that hospitalists use ultrasound guidance for LP, nor does it establish ultrasound guidance as the standard of care for LP. Similar to previously published Society of Hospital Medicine (SHM) position statements,12,18,19 this document presents recommendations with supporting evidence for the clinical outcomes, techniques, and training for using ultrasound guidance for LP. A manuscript describing the technique of ultrasound guidance for LPs has been previously published by some of the authors of this position statement.20
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the six working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. Updated searches were conducted in November 2016, January 2018, and October 2018. The search strings are included in Appendix 3. All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide LP were selected. In addition, the following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. Moreover, studies focusing on the use of ultrasound guidance for spinal nerve root injections, regional anesthesia, and assessment of lumbar spine anatomy alone were excluded. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided LP were screened and selected. Final article selection was based on working group consensus, and the selected literature was incorporated into the draft recommendations.
The Research and Development (RAND) Appropriateness Method that required panel judgment and consensus was used.21 The 27 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering the following five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (REDCap™) in February 2018 and April 2018 (Appendix 4). Voting on appropriateness was conducted using a 9-point Likert scale. The three zones of the 9-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” A strong recommendation required 80% of the votes within one integer of the median, following the RAND rules. Disagreement was defined as >30% of panelists voting outside of the zone of the median.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording of each recommendation (Table 2). The revised consensus-based recommendations underwent internal and external reviews by POCUS experts from different subspecialties. The final review of this position statement was performed by members of the SHM POCUS Task Force, SHM Education Committee, and SHM Executive Committee. The SHM Executive Committee endorsed this position statement in June 2018 before submission to the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 4,389 references were pooled from four different sources: a search by a certified medical librarian in December 2015 (3,212 citations) that was updated in November 2016 (380 citations), January 2018 (282 citations), and October 2018 (274 citations); working group members’ personal bibliographies and searches (31 citations); and a search focusing on ultrasound-guided LP training (210 citations). A total of 232 full-text articles were reviewed, and the final selection included 77 articles that were abstracted into a data table and incorporated into the draft recommendations. Details of the literature search strategy are presented in Appendix 3.
RECOMMENDATIONS
Four domains (clinical outcomes, technique, training, and knowledge gaps) with 16 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation were drafted citing supporting evidence. After two rounds of panel voting, five recommendations did not achieve agreement based on the RAND rules, one recommendation was combined with another recommendation during peer review, and 10 statements received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Nine statements were approved as strong recommendations, and one was approved as a conditional recommendation. Therefore, the final recommendation count was 10. The strength of the recommendation and degree of consensus for each recommendation are summarized in Table 1.
Terminology
LP is a procedure in which a spinal needle is introduced into the subarachnoid space for the purpose of collecting CSF for diagnostic evaluation and/or therapeutic relief.
Throughout this document, the phrases “ultrasound-guided” and “ultrasound guidance” refer to the use of ultrasound to mark a needle insertion site immediately before performing the procedure. This is also known as static ultrasound guidance. Real-time or dynamic ultrasound guidance refers to direct visualization of the needle tip as it traverses through the skin and soft tissues to reach the ligamentum flavum. Any reference to real-time ultrasound guidance is explicitly stated.
Clinical outcomes
1) When ultrasound equipment is available, along with providers who are appropriately trained to use it, we recommend that ultrasound guidance should be used for site selection of LPs to reduce the number of needle insertion attempts and needle redirections and increase the overall procedure success rates, especially in patients who are obese or have difficult-to-palpate landmarks.
Rationale. LPs have historically been performed by selecting a needle insertion site based on palpation of anatomical landmarks. However, an estimated 30% of patients requiring LP in emergency departments have lumbar spine landmarks that are difficult to palpate, most commonly due to obesity.13 Furthermore, lumbar puncture performed based on palpation of landmarks alone has been reported to fail in 28% of patients.14
Ultrasound can be used at the bedside to elucidate the lumbar spine anatomy to guide performance of LP or epidural catheterization. Since the early 2000s, randomized studies comparing the use of ultrasound guidance (ultrasound-guided) versus anatomical landmarks (landmark-guided) to map the lumbar spine for epidural catheterization have emerged. It is important to recognize that the exact same ultrasound technique is used for site marking of LP, epidural catheterization, and spinal anesthesia—the key difference is how deep the needle tip is inserted. Therefore, data from these three ultrasound-guided procedures are often pooled. Currently, at least 33 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP, epidural catheterization, or spinal anesthesia have been published.22-49 We present three meta-analyses below that pooled data primarily from randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP or spinal anesthesia.
In 2013, Shaikh et al. published the first meta-analysis with 14 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP (n = 5) or epidural catheterization (n = 9). The pooled data showed that use of ultrasound guidance decreased the proportion of failed procedures (risk ratio 0.21, 95% CI 0.10-0.43) with an absolute risk reduction of 6.3% (95% CI 4.1%-8.4%) and a number needed to treat of 16 (95% CI 12-25) to prevent one failed procedure. In addition, the use of ultrasound reduced the mean number of attempts by 0.44 (95% CI 0.24-0.64) and reduced the mean number of needle redirections by 1.00 (95% CI 0.75-1.24). The reduction in risk of a failed procedure was similar for LPs (risk ratio 0.19 [95% CI 0.07-0.56]) and epidural catheterizations (risk ratio 0.23 [95% CI 0.09-0.60]).16
A similar meta-analysis published by Perlas et al. in 2016 included a total of 31 studies, both randomized controlled and cohort studies, evaluating the use of ultrasound guidance for LP, spinal anesthesia, and epidural catheterization.50 The goal of this systematic review and meta-analysis was to establish clinical practice recommendations. The authors concluded (1) the data consistently suggest that ultrasound is more accurate than palpation for lumbar interspace identification, (2) ultrasound allows accurate measurement of the needle insertion depth to reach the epidural space with a mean difference of <3 mm compared with the actual needle insertion depth, and (3) ultrasound increases the efficacy of lumbar epidural or spinal anesthesia by decreasing the mean number of needle passes for success by 0.75 (95% CI 0.44-1.07) and reducing the risk of a failed procedure (risk ratio 0.51 [95% CI 0.32-0.80]), both in patients with normal surface anatomy and in those with technically difficult surface anatomy due to obesity, scoliosis, or previous spine surgery.
Compared to the two earlier meta-analyses that included studies of both LP and spinal anesthesia procedures, the meta-analysis conducted by Gottlieb et al. in 2018 pooled data from 12 randomized controlled studies of ultrasound guidance for LPs only. For the primary outcome, pooled data from both adult and pediatric studies demonstrated higher procedural success rates with ultrasound-guided vs landmark-guided LPs (90% vs 81%) with an odds ratio of 2.1 (95% CI 0.66-7.44) in favor of ultrasound; however, there were no statistically significant differences when the adult and pediatric subgroups were analyzed separately, probably due to underpowering. For the secondary outcomes, data from the adult subgroup showed that use of ultrasound guidance was associated with fewer traumatic LPs (OR 0.28, 95% CI 0.14-0.59), shorter time to procedural success (adjusted mean difference –3.03 minutes, 95% CI –3.54 to –2.52), fewer number of needle passes (adjusted mean difference –0.81 passes, 95% CI –1.57 to –0.05), and lower patient pain scores (adjusted mean difference –2.53, 95% CI –3.89 to –1.17).
At least 12 randomized controlled studies have been published comparing the use of ultrasound guidance vs landmarks for the performance of LP or spinal anesthesia in adult patients, which were not included in the abovementioned meta-analyses. These individual studies demonstrated similar benefits of using ultrasound guidance: reduced needle insertion attempts, reduced needle redirections, and increased overall procedural success rates.17,31,37,40,41,43-49
It is important to recognize that four randomized controlled studies did not demonstrate any benefits of ultrasound guidance on the number of attempts or procedural success rates,23,33,41,51 and three of these studies were included in the abovementioned meta-analyses.23,33,51 Limitations of these negative studies include potential selection bias, inadequate sample sizes, and varying levels of operator skills in procedures, ultrasound guidance, or both. One study included emergency medicine residents as operators with varying degrees of ultrasound skills, and more importantly, patient enrollment occurred by convenience sampling, which may have introduced selection bias. Furthermore, most of the patients were not obese (median BMI of 27 kg/m2), and it is unclear why 10 years lapsed from data collection until publication.33 Another study with three experienced anesthesiologists as operators performing spinal anesthesia enrolled only patients who were not obese (mean BMI of 29 kg/m2) and had easily palpable bony landmarks—two patient characteristics associated with the least benefit of using ultrasound guidance in other studies.23 Another negative study had one experienced anesthesiologist marking obstetric patients with ultrasound, but junior residents performing the actual procedure in the absence of the anesthesiologist who had marked the patient.41
In general, the greatest benefit of using ultrasound guidance for LP has been demonstrated in obese patients.24,32,34,35,52,53 Benefits have been shown in specific obese patient populations, including obstetric,31,54,55 orthopedic,24,56,57 and emergency department patients.30
By increasing the procedural success rates with the use of ultrasound at the bedside, fewer patients may be referred to interventional radiology for fluoroscopic-guided LP, decreasing the patient exposure to ionizing radiation. A randomized study (n = 112) that compared site marking with ultrasound guidance versus fluoroscopic guidance for epidural steroid injections found the two techniques to be equivalent with respect to mean procedure time, number of needle insertion attempts, or needle passes.58 Another randomized study found that the performance time of ultrasound guidance was two minutes shorter (P < .05) than fluoroscopic guidance.59
Techniques
2) We recommend that ultrasound should be used to more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients.
Rationale. Traditionally, an imaginary line connecting the iliac crests (intercristal line, Tuffier’s line, or Jacoby’s line) was considered to identify the L4 vertebra or the L4-L5 interspinous space in the midline; however, studies have revealed this traditional landmark to be much less accurate than previously thought. In general, palpating the iliac crests to mark the intercristal line identifies an interspinous space that is one space cephalad (ie, the L2-L3 interspinous space) but can range from L1-L2 to L4-L5.46,60-64 If an LP is inadvertently performed in the L1-L2 interspinous space, the risk of spinal cord injury is higher than that when performed in a more distal interspinous space.
A study by Margarido et al. with 45 patients with a mean BMI of 30 kg/m2 found that the intercristal line was located above the L4-L5 interspinous space in 100% of patients. More importantly, the intercristal line was above L2-L3 in 36% of patients and above L1-L2 in 4% of patients. It is important to note that patients with scoliosis or previous spine surgery were excluded from this study, and all examinations were performed by two experienced anesthesiologists with patients in a sitting position—all factors that would favor accurate palpation and marking of the iliac crests.60
In a study of nonobese patients (mean BMI 28 kg/m2) undergoing spinal anesthesia, Duniec et al. compared the lumbar level identified by palpation versus ultrasound and found discordance between the two techniques in 36% of patients; 18% were one space too cephalad, 16% were one space too caudal, and 2% were off by two interspinous spaces.61 Another study found discordance in 64% of patients (mean BMI 28 kg/m2) when comparing the interspinous level where spinal anesthesia had been performed by palpation versus a post-procedural ultrasound examination. This study revealed that the interspinous space was more cephalad in 50% of patients with 6% of punctures performed in the L1-L2 interspace.62 A similar study compared the accuracy of palpation vs ultrasound to identify the L3-L4 interspinous space in obese (mean BMI 34 kg/m2) versus nonobese (mean BMI 27 kg/m2) patients. This study found marking a space above L3-L4 in 51% of obese and 40% of nonobese patients and marking of the L1-L2 interspace in 7% of obese and 4% of nonobese patients.64
A study comparing palpation vs ultrasound found that 68% of obese patients with a BMI of >30 kg/m2 had difficult-to-palpate lumbar spine landmarks, but with the use of ultrasound, landmarks were identified in 76% of all patients, including obese and nonobese, with difficult-to-palpate landmarks.65
3) We suggest using ultrasound for selecting and marking a needle insertion site just before performing LPs in either a lateral decubitus or sitting position. The patient should remain in the same position after marking the needle insertion site.
Rationale. Ultrasound mapping of the lumbar spine can be performed in either a lateral decubitus or sitting position. Selecting and marking a needle insertion site should be performed at the bedside just before performing the procedure. The patient must remain in the same position in the interim between marking and inserting the needle, as a slight change in position can alter the needle trajectory, lowering the LP success rate. Although performing LPs in a lateral decubitus position has the advantage of accurately measuring the opening pressure, misalignment of the shoulder and pelvic girdles and bowing of the bed in a lateral decubitus position may lower LP success rates.
One randomized study comparing ultrasound-guided spinal anesthesia in a lateral decubitus versus sitting position found no difference in the number of needle insertion attempts or measurement of the skin-dura distance; however, the needle insertion depth was 0.73 cm greater in a lateral decubitus vs sitting position (P = .002).66 Procedural success rates of LP with ultrasound guidance have not been directly compared in a sitting versus lateral decubitus position, although the overall procedural success rates were higher in one study that allowed the operator to choose either sitting or lateral decubitus position when ultrasound was used.32
4) We recommend that a low-frequency transducer, preferably a curvilinear array transducer, should be used to evaluate the lumbar spine and mark a needle insertion site in most patients. A high-frequency linear array transducer may be used in nonobese patients.
Rationale. Low-frequency transducers emit sound waves that penetrate deep tissues, allowing visualization of bones and ligaments of the lumbar spine. A high-frequency linear transducer offers better resolution but shallower penetration to approximately 6-9 cm, limiting its use for site marking in overweight and obese patients. In obese patients, the ligamentum flavum is often deeper than 6 cm, which requires a low-frequency transducer to be visualized.
Most of the randomized controlled studies demonstrating benefits of using ultrasound guidance compared with landmark guidance for performance of LP, epidural anesthesia, or spinal anesthesia have used a low-frequency, curvilinear transducer.22,24,26-28,31,34-36,39,43-45,67 Two randomized controlled trials used a high-frequency linear transducer for site marking of lumbar procedures.30,32,37 Using a high-frequency linear transducer has been described in real-time, ultrasound-guided LPs, the advantage being better needle visualization with a linear transducer.29 Detection of blood vessels by color flow Doppler may be another advantage of using a high-frequency linear transducer, although a study by Grau et al. showed that use of color flow Doppler with a low-frequency curvilinear transducer permitted visualization of interspinous vessels as small as 0.5 mm in size.68
5) We recommend that ultrasound should be used to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad, sequentially identifying the lumbar spine interspaces.Rationale. Although no studies have directly compared different ultrasound scanning protocols to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad to sequentially identify the lumbar interspinous spaces is the most commonly described technique in studies demonstrating improved clinical outcomes with the use of ultrasound.24,31,34,37,39,40,45,56,57,67 Because the sacrum can be easily recognized, identifying it first is most beneficial in patients with few or no palpable landmarks.
All five lumbar spinous processes and interspinous spaces can be mapped from the sacrum using either a midline or a paramedian approach, and the widest interspinous space can be selected. In a midline approach, either a transverse or a longitudinal view is obtained. The transducer is centered on the sacrum and slid cephalad from L5 to L1 to identify each spinous process and interspinous space. In a paramedian approach, longitudinal paramedian views are obtained from the L5–sacrum interspace to the L1–L2 interspace, and each interspinous space is identified as the transducer is slid cephalad. Both these approaches are effective for mapping the lumbar spine. Whether the entire lumbar spine is mapped, and whether a midline or a paramedian approach is utilized, will depend on the operator’s preference.
6) We recommend that ultrasound should be used in a transverse plane to mark the midline of the lumbar spine and a longitudinal plane to mark the interspinous spaces. The intersection of these two lines marks the needle insertion site.
Rationale. The most common technique described in comparative studies of ultrasound vs landmarks includes visualization of the lumbar spine in two planes, a transverse plane to identify the midline and a longitudinal plane to identify the interspinous spaces. The majority of randomized controlled studies that demonstrated a reduction in the number of needle insertion attempts and an increase in the procedural success rates have used this technique (see Clinical Outcomes).22,24,28,32,35-37,43,44 Marking the midline and interspinous space(s) for LP may be performed in any order, starting with either the transverse or longitudinal plane first.
The midline of the spine is marked by placing the transducer in a transverse plane over the lumbar spine, centering over the spinous processes that have a distinct hyperechoic tip and a prominent acoustic shadow deep to the bone, and drawing a line perpendicular to the center of the transducer delineating the midline. The midline should be marked over a minimum of two or three spinous processes.
To identify the interspinous spaces, the transducer is aligned longitudinally over the midline. The transducer is slid along the midline to identify the widest interspinous space. Once the transducer is centered over the widest interspinous space, a line perpendicular to the center of the transducer is drawn to mark the interspinous space. The intersection of the lines marking the spinal midline and the selected interspinous space identifies the needle entry point.
To visualize the ligamentum flavum from a paramedian view, the transducer is oriented longitudinally over the midline, slid approximately 1 cm laterally, and tilted approximately 15 degrees aiming the ultrasound beam toward the midline. The skin–ligamentum flavum distance is most reliably measured from a paramedian view. Alternatively, in some patients, the ligamentum flavum may be visualized in the midline and the depth can be measured.
7) We recommend that ultrasound should be used during a preprocedural evaluation to measure the distance from the skin surface to the ligamentum flavum from a longitudinal paramedian view to estimate the needle insertion depth and ensure that a spinal needle of adequate length is used.
Rationale. The distance from the skin to the ligamentum flavum can be measured using ultrasound during preprocedural planning. Knowing the depth to the ligamentum flavum preprocedurally allows the operator to procure a spinal needle of adequate length, anticipate the insertion depth before CSF can be obtained, determine the depth to which a local anesthetic will need to be injected, and decide whether the anticipated difficulty of the procedure warrants referral to or consultation with another specialist.
The skin–ligamentum flavum distance can be measured from a transverse midline view or a longitudinal paramedian view. A longitudinal paramedian view provides an unobstructed view of the ligamentum flavum due to less shadowing from bony structures compared with a midline view. Several studies have demonstrated a strong correlation between the skin–ligamentum flavum distance measured by ultrasound and the actual needle insertion depth in both midline and paramedian views.28,34,36,53,54,57,69,70
A meta-analysis that included 13 comparative studies evaluating the correlation between ultrasound-measured depth and actual needle insertion depth to reach the epidural or intrathecal space consistently demonstrated a strong correlation between the measured and actual depth.50 A few studies have reported near-perfect Pearson correlation coefficients of 0.98.55,71,72 The pooled correlation was 0.91 (95% CI 0.87-0.94). All studies measured the depth from the skin to the ventral side of the ligamentum flavum or the intrathecal space from either a longitudinal paramedian view (n = 4) or a transverse midline view (n = 9). Eight of the more recent studies evaluated the accuracy of the ultrasound measurements and found the depth measurements by ultrasound to be accurate within 1-13 mm of the actual needle insertion depth, with seven of the eight studies reporting a mean difference of ≤3 mm.50
Measurement of the distance between the skin and the ligamentum flavum generally underestimates the needle insertion depth. One study reported that measurement of the skin–ligamentum flavum distance underestimates the needle insertion depth by 7.6 mm to obtain CSF, whereas measurement of the skin–posterior longitudinal ligament distance overestimates the needle insertion depth by 2.5 mm.57 A well-accepted contributor to underestimation of the depth measurements using ultrasound is compression of the skin and soft tissues by the transducer, and therefore, pressure on the skin must be released before freezing an image and measuring the depth to the subarachnoid space.
Training
8) We recommend that novices should undergo simulation-based training, where available, before attempting ultrasound-guided LPs on actual patients.
Rationale. Similar to training for other bedside procedures, dedicated training sessions, including didactics, supervised practice on patients, and simulation-based practice, should be considered when teaching novices to perform ultrasound-guided LP. Simulation-based training facilitates acquisition of knowledge and skills to perform invasive bedside procedures, including LP.73 Simulation-based training has been commonly incorporated into procedure training for trainees using an immersive experience, such as a “boot camp,”74-77 or a standardized curriculum,78,79 and has demonstrated improvements in post-course procedural knowledge, technical skills, and operator confidence. Two of these studies included training in the use of ultrasound guidance for LP. These studies showed that simulation-based practice improved skill acquisition and confidence.80,81 Simulation using novel computer software may improve skill acquisition in the use of ultrasound guidance for LP.82
9) We recommend that training in ultrasound-guided LPs should be adapted based on prior ultrasound experience, as learning curves will vary.Rationale. The learning curve to achieve competency in the use of ultrasound guidance for LP has not been well studied. The rate of attaining competency in identifying lumbar spine structures using ultrasound will vary by provider based on prior skills in ultrasound-guided procedures.83 Thus, providers with prior ultrasound experience may require less training than those without such experience to achieve competency. However, extensive experience in performing landmark-guided LPs does not necessarily translate into rapid acquisition of skills to perform the procedure with ultrasound guidance. A study of practicing anesthesiologists with no prior ultrasound experience demonstrated that 20 supervised trials of ultrasound-guided spinal anesthesia were insufficient to achieve competency.84 Although minimums may be a necessary step to gain competence, using them as a sole means to define competence does not account for variable learning curves.12 Based on a national survey of 21 hospitalist procedure experts, the mean current vs suggested minimums for initial and ongoing hospital privileging for LPs were 1.8 vs 6.9 and 2.2 vs 4.6 annually in one report.85
A fundamental question that needs to be answered is how to define competency in the use of ultrasound guidance for LP, including the specific skills and knowledge that must be mastered. At a minimum, providers must be able to identify lumbar spinous processes and distinguish them from the sacrum, identify the lumbar interspinous spaces and their corresponding levels, and estimate the depth from the skin to the ligamentum flavum from the midline and paramedian planes. Novice operators may benefit from practicing lumbar spine mapping of nonobese patients using a high-frequency linear transducer that generates high-resolution images and facilitates recognition of lumbar spine structures.
10) We recommend that novice providers should be supervised when performing ultrasound-guided LPs before performing the procedure independently on patients.
Rationale: Demonstration of competency in the use of ultrasound to identify lumbar spine anatomy should be achieved before routinely performing the procedure independently on patients.18 All providers will require a variable period of supervised practice to demonstrate the appropriate technique, followed by a period of unsupervised practice before competency is achieved. Supervised practice with guidance and feedback has been shown to significantly improve providers’ ability to delineate lumbar spine anatomy.86
KNOWLEDGE GAPS
The process of producing these guidelines revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for LP.
First, it is unclear whether the use of ultrasound guidance for LP reduces postprocedural back pain and whether it improves patient satisfaction. Several studies have evaluated postprocedural back pain28,30,32,33,52 and patient satisfaction28,29,33,51 with the use of ultrasound guidance, but these studies have found inconsistent results. Some of these results were probably due to insufficient statistical power or confounding variables. Furthermore, benefits have been demonstrated in certain subgroups, such as overweight patients or those with anatomical abnormalities, as was found in two studies.52,87 Use of ultrasound guidance for spinal anesthesia has been shown to reduce postprocedural headache28 and improve patient satisfaction51, although similar benefit has not been demonstrated in patients undergoing LP.
Second, the effect of using ultrasound guidance on the frequency of traumatic LPs is an area of uncertainty. A “traumatic tap” is defined as an inadvertent puncture of an epidural vein during passage of the spinal needle through the dura. It remains difficult to discern in these studies whether red blood cells detected in the CSF resulted from puncture of an epidural vein or from needle trauma of the skin and soft tissues. Despite this uncertainty, at least seven randomized controlled studies have assessed the effect of ultrasound guidance on traumatic LPs. The meta-analysis by Shaikh et al. included five randomized controlled studies that assessed the effect of ultrasound guidance on the reporting of traumatic taps. The study found a reduced risk of traumatic taps (risk ratio 0.27 [95% CI 0.11-0.67]), an absolute risk reduction of 5.9% (95% CI 2.3%-9.5%), and a number needed to treat of 17 (95% CI 11-44) to prevent one traumatic tap.16 Similarly, the meta-analysis by Gottlieb et al. showed a lower risk of traumatic taps among adults undergoing LP with ultrasound guidance in five randomized controlled studies with an odds ratio of 0.28 (95% CI 0.14-0.59). The meta-analysis by Gottlieb et al. included two adult studies that were not included by Shaikh et al.
Third, several important questions about the technique of ultrasound-guided LP remain unanswered. In addition to the static technique, a dynamic technique with real-time needle tracking has been described to perform ultrasound-guided LP, epidural catheterization, and spinal anesthesia. A pilot study by Grau et al. found that ultrasound used either statically or dynamically had fewer insertion attempts and needle redirections than use of landmarks alone.29 Three other pilot studies showed successful spinal anesthesia in almost all patients88-90 and one large study demonstrated successful spinal anesthesia with real-time ultrasound guidance in 97 of 100 patients with a median of three needle passes.91 Furthermore, a few industry-sponsored studies with small numbers of patients have described the use of novel needle tracking systems that facilitate needle visualization during real-time ultrasound-guided LP.92,93 However, to our knowledge, no comparative studies of static versus dynamic guidance using novel needle tracking systems in human subjects have been published, and any potential role for these novel needle tracking systems has not yet been defined.
Finally, the effects of using ultrasound guidance on clinical decision-making, timeliness, and cost-effectiveness of LP have not yet been explored but could have important clinical practice implications.
CONCLUSION
Randomized controlled trials have demonstrated that using ultrasound guidance for LPs can reduce the number of needle insertion attempts and needle redirections and increase the overall procedural success rates. Ultrasound can more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients, although the greatest benefit of using ultrasound guidance for LPs has been shown in obese patients.
Ultrasound permits assessment of the interspinous space width and measurement of the ligamentum flavum depth to select an optimal needle insertion site and adequate length spinal needle. Although the use of real-time ultrasound guidance has been described, the use of static ultrasound guidance for LP site marking remains the standard technique.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators from Society of Hospital Medicine Point-of-care Ultrasound Task Force: Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Paul Mayo, Benji Mathews, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Sophia Rodgers, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Matthews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclosures
The authors have nothing to disclose.
Funding
Brian P Lucas: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). Nilam Soni: Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1).
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Wolfe KS, Kress JP. Risk of procedural hemorrhage. Chest. 2016;150(1):237-246. https://doi.org/10.1016/j.chest.2016.01.023.
2. Kroll H, Duszak R, Jr, Nsiah E, et al. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. AJR Am J Roentgenol. 2015;204(1):15-19. https://doi.org/10.2214/AJR.14.12622.
3. Vickers A, Donnelly JP, Moore JX, Wang HE. 263EMF epidemiology of lumbar punctures in hospitalized patients in United States. Ann Emerg Med. 2017;70(4):S104. https://doi.org/10.1016/j.annemergmed.2017.07.241.
4. Køster-Rasmussen R, Korshin A, Meyer CN. Antibiotic treatment delay and outcome in acute bacterial meningitis. J Infect. 2008;57(6):449-454. https://doi.org/10.1016/j.jinf.2008.09.033.
5. Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129(11):862-869. https://doi.org/10.7326/0003-4819-129-11_Part_1-199812010-00004.
6. Lepur D, Barsić B. Community-acquired bacterial meningitis in adults: antibiotic timing in disease course and outcome. Infection. 2007;35(4):225-231. https://doi.org/10.1007/s15010-007-6202-0.
7. Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM. 2005;98(4):291-298. https://doi.org/10.1093/qjmed/hci047.
8. Auburtin M, Wolff M, Charpentier J, et al. Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: the PNEUMOREA prospective multicenter study. Crit Care Med. 2006;34(11):2758-2765. https://doi.org/10.1097/01.CCM.0000239434.26669.65.
9. Michael B, Menezes BF, Cunniffe J, et al. Effect of delayed lumbar punctures on the diagnosis of acute bacterial meningitis in adults. Emerg Med J. 2010;27(6):433-438. https://doi.org/10.1136/emj.2009.075598.
10. Glimåker M, Johansson B, Grindborg Ö, et al. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis. 2015;60(8):1162-1169. https://doi.org/10.1093/cid/civ011.
11. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 Revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715.
12. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079.
13. Shah KH, McGillicuddy D, Spear J, Edlow JA. Predicting difficult and traumatic lumbar punctures. Am J Emerg Med. 2007;25(6):608-611. https://doi.org/10.1016/j.ajem.2006.11.025.
14. Williams P, Tait G, Wijeratne T. Success rate of elective lumbar puncture at a major Melbourne neurology unit. Surg Neurol Int. 2018;9:12. https://doi.org/10.4103/sni.sni_426_17.
15. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2018;26(1). https://doi.org/10.1111/acem.13558.
16. Shaikh F, Brzezinski J, Alexander S, et al. Ultrasound imaging for lumbar punctures and epidural catheterisations: systematic review and meta-analysis. BMJ. 2013;346:f1720. https://doi.org/10.1136/bmj.f1720.
17. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
18. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
19. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
20. Soni NJ, Franco-Sadud R, Schnobrich D, et al. Ultrasound guidance for lumbar puncture. Neurol Clin Pract. 2016;6(4):358-368. https://doi.org/10.1212/CPJ.0000000000000265.
21. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The Rand/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: Rand Corp; 2001.
22. Abdelhamid SA, Mansour MA. Ultrasound-guided intrathecal anesthesia: does scanning help? Egypt J Anaesth. 2013;29(4):389-394. https://doi.org/10.1016/j.egja.2013.06.003.
23. Ansari T, Yousef A, El Gamassy A, Fayez M. Ultrasound-guided spinal anaesthesia in obstetrics: is there an advantage over the landmark technique in patients with easily palpable spines? Int J Obstet Anesth. 2014;23(3):213-216. https://doi.org/10.1016/j.ijoa.2014.03.001.
24. Chin KJ, Perlas A, Chan V, et al. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology. 2011;115(1):94-101. https://doi.org/10.1097/ALN.0b013e31821a8ad4.
25. Cho YC, Koo DH, Oh SK, et al. Comparison of ultrasound-assisted lumbar puncture with lumbar puncture using palpation of landmarks in aged patients in an emergency center. J Korean Soc Emerg Med. 2009;20(3):304.
26. Grau T, Leipold RW, Conradi R, Martin E. Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol Scand. 2001;45(6):766-771. https://doi.org/10.1034/j.1399-6576.2001.045006766.x.
27. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Ultrasound imaging facilitates localization of the epidural space during combined spinal and epidural anesthesia. Reg Anesth Pain Med. 2001;26(1):64-67. https://doi.org/10.1053/rapm.2001.19633.
28. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Efficacy of ultrasound imaging in obstetric epidural anesthesia. J Clin Anesth. 2002;14(3):169-175. https://doi.org/10.1016/S0952-8180(01)00378-6.
29. Grau T, Leipold RW, Fatehi S, Martin E, Motsch J. Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur J Anaesthesiol. 2004;21(1):25-31. https://doi.org/10.1017/S026502150400105X.
30. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
31. Nassar M, Abdelazim IA. Pre-puncture ultrasound guided epidural insertion before vaginal delivery. J Clin Monit Comput. 2015;29(5):573-577. https://doi.org/10.1007/s10877-014-9634-y.
32. Nomura JT, Leech SJ, Shenbagamurthi S, et al. A randomized controlled trial of ultrasound-assisted lumbar puncture. J Ultrasound Med. 2007;26(10):1341-1348. https://doi.org/10.7863/jum.2007.26.10.1341.
33. Peterson MA, Pisupati D, Heyming TW, Abele JA, Lewis RJ. Ultrasound for routine lumbar puncture. Acad Emerg Med. 2014;21(2):130-136. https://doi.org/10.1111/acem.12305.
34. Sahin T, Balaban O, Sahin L, Solak M, Toker K. A randomized controlled trial of preinsertion ultrasound guidance for spinal anaesthesia in pregnancy: outcomes among obese and lean parturients: ultrasound for spinal anesthesia in pregnancy. J Anesth. 2014;28(3):413-419. https://doi.org/10.1007/s00540-013-1726-1.
35. Wang Q, Yin C, Wang TL. Ultrasound facilitates identification of combined spinal-epidural puncture in obese parturients. Chin Med J (Engl). 2012;125(21):3840-3843. PubMed
36. Vallejo MC, Phelps AL, Singh S, Orebaugh SL, Sah N. Ultrasound decreases the failed labor epidural rate in resident trainees. Int J Obstet Anesth. 2010;19(4):373-378. https://doi.org/10.1016/j.ijoa.2010.04.002.
37. Darrieutort-Laffite C, Bart G, Planche L, et al. Usefulness of a pre-procedure ultrasound scanning of the lumbar spine before epidural injection in patients with a presumed difficult puncture: a randomized controlled trial. Joint Bone Spine. 2015;82(5):356-361. https://doi.org/10.1016/j.jbspin.2015.02.001.
38. Vosko MR, Brunner C, Schreiber S. Lumbar puncture with ultrasound study (lupus study)-international prospective randomized multicentre trial. Int J Stroke. 2017;12(1):22. https://doi.org/10.1055/s-0037-1606991.
39. Urfalioğlu A, Bilal B, Öksüz G, et al. Comparison of the landmark and ultrasound methods in cesarean sections performed under spinal anesthesia on obese pregnants. J Matern Fetal Neonatal Med. 2017;30(9):1051-1056. https://doi.org/10.1080/14767058.2016.1199677.
40. Tawfik MM, Atallah MM, Elkharboutly WS, Allakkany NS, Abdelkhalek M. Does preprocedural ultrasound increase the first-pass success rate of epidural catheterization before cesarean delivery? A randomized controlled trial. Anesth Analg. 2017;124(3):851-856. https://doi.org/10.1213/ANE.0000000000001325.
41. Turkstra TP, Marmai KL, Armstrong KP, Kumar K, Singh SI. Preprocedural ultrasound assessment does not improve trainee performance of spinal anesthesia for obstetrical patients: a randomized controlled trial. J Clin Anesth. 2017;37:21-24. https://doi.org/10.1016/j.jclinane.2016.10.034.
42. Chong SE, Mohd Nikman A, Saedah A, et al. Real-time ultrasound-guided paramedian spinal anaesthesia: evaluation of the efficacy and the success rate of single needle pass. Br J Anaesth. 2017;118(5):799-801. https://doi.org/10.1093/bja/aex108.
43. Creaney M, Mullane D, Casby C, Tan T. Ultrasound to identify the lumbar space in women with impalpable bony landmarks presenting for elective caesarean delivery under spinal anaesthesia: a randomised trial. Int J Obstet Anesth. 2016;28:12-16. https://doi.org/10.1016/j.ijoa.2016.07.007.
44. Ekinci M, Alici HA, Ahiskalioglu A, et al. The use of ultrasound in planned cesarean delivery under spinal anesthesia for patients having nonprominent anatomic landmarks. J Clin Anesth. 2017;37:82-85. https://doi.org/10.1016/j.jclinane.2016.10.014.
45. Perna P, Gioia A, Ragazzi R, Volta CA, Innamorato M. Can pre-procedure neuroaxial ultrasound improve the identification of the potential epidural space when compared with anatomical landmarks? A prospective randomized study. Minerva Anestesiol. 2017;83(1):41-49. https://doi.org/10.23736/S0375-9393.16.11399-9.
46. Chin A, Crooke B, Heywood L, et al. A randomised controlled trial comparing needle movements during combined spinal-epidural anaesthesia with and without ultrasound assistance. Anaesthesia. 2018;73(4):466-473. https://doi.org/10.1111/anae.14206.
47. Dhanger S, Vinayagam S, Vaidhyanathan B, Rajesh IJ, Tripathy DK. Comparison of landmark versus pre-procedural ultrasonography-assisted midline approach for identification of subarachnoid space in elective caesarean section: a randomised controlled trial. Indian J Anaesth. 2018;62(4):280-284. https://doi.org/10.4103/ija.IJA_488_17.
48. Evans DP, Tozer J, Joyce M, Vitto MJ. Comparison of ultrasound-guided and landmark-based lumbar punctures in inexperienced resident physicians. J Ultrasound Med. 2019;38(3):613-620. https://doi.org/10.1002/jum.14728.
49. Srinivasan KK, Leo AM, Iohom G, Loughnane F, Lee PJ. Pre-procedure ultrasound-guided paramedian spinal anaesthesia at L5-S1: is this better than landmark-guided midline approach? A randomised controlled trial. Indian J Anaesth. 2018;62(1):53-60. https://doi.org/10.4103/ija.IJA_448_17.
50. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
51. Lim YC, Choo CY, Tan KT. A randomised controlled trial of ultrasound-assisted spinal anaesthesia. Anaesth Intensive Care. 2014;42(2):191-198. https://doi.org/10.1177/0310057X1404200205.
52. Honarbakhsh S, Osman C, Teo JTH, Gabriel C. Ultrasound-guided lumbar puncture as a diagnostic aid to reduce number of attempts and complication rates. Ultrasound. 2013;21(4):170-175. https://doi.org/10.1177/1742271X13504332.
53. Sahota JS, Carvalho JC, Balki M, Fanning N, Arzola C. Ultrasound estimates for midline epidural punctures in the obese parturient: paramedian sagittal oblique is comparable to transverse median plane. Anesth Analg. 2013;116(4):829-835. https://doi.org/10.1213/ANE.0b013e31827f55f0.
54. Balki M, Lee Y, Halpern S, Carvalho JC. Ultrasound imaging of the lumbar spine in the transverse plane: the correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg. 2009;108(6):1876-1881. https://doi.org/10.1213/ane.0b013e3181a323f6.
55. Wallace DH, Currie JM, Gilstrap LC, Santos R. Indirect sonographic guidance for epidural anesthesia in obese pregnant patients. Reg Anesth. 1992;17(4):233-236. PubMed
56. Srinivasan KK, Iohom G, Loughnane F, Lee PJ. Conventional landmark-guided midline versus preprocedure ultrasound-guided paramedian techniques in spinal anesthesia. Anesth Analg. 2015;21(4):1089-1096. https://doi.org/10.1213/ANE.0000000000000911.
57. Chin KJ, Perlas A, Singh M, et al. An ultrasound-assisted approach facilitates spinal anesthesia for total joint arthroplasty. Can J Anaesth. 2009;56(9):643-650. https://doi.org/10.1007/s12630-009-9132-8.
58. Evansa I, Logina I, Vanags I, Borgeat A. Ultrasound versus fluoroscopic-guided epidural steroid injections in patients with degenerative spinal diseases: a randomised study. Eur J Anaesthesiol. 2015;32(4):262-268. https://doi.org/10.1097/EJA.0000000000000103.
59. Park Y, Lee JH, Park KD, et al. Ultrasound-guided vs fluoroscopy-guided caudal epidural steroid injection for the treatment of unilateral lower lumbar radicular pain: a prospective, randomized, single-blind clinical study. Am J Phys Med Rehabil. 2013;92(7):575-586. https://doi.org/10.1097/PHM.0b013e318292356b.
60. Margarido CB, Mikhael R, Arzola C, Balki M, Carvalho JC. The intercristal line determined by palpation is not a reliable anatomical landmark for neuraxial anesthesia. Can J Anaesth. 2011;58(3):262-266. https://doi.org/10.1007/s12630-010-9432-z.
61. Duniec L, Nowakowski P, Kosson D, Łazowski T. Anatomical landmarks based assessment of intravertebral space level for lumbar puncture is misleading in more than 30%. Anaesthesiol Intensive Ther. 2013;45(1):1-6. https://doi.org/10.5603/AIT.2013.0001.
62. Schlotterbeck H, Schaeffer R, Dow WA, et al. Ultrasonographic control of the puncture level for lumbar neuraxial block in obstetric anaesthesia. Br J Anaesth. 2008;100(2):230-234. https://doi.org/10.1093/bja/aem371.
63. Whitty R, Moore M, Macarthur A. Identification of the lumbar interspinous spaces: palpation versus ultrasound. Anesth Analg. 2008;106(2):538-540, table of contents. https://doi.org/10.1213/ane.0b013e31816069d9.
64. Locks Gde F, Almeida MC, Pereira AA. Use of the ultrasound to determine the level of lumbar puncture in pregnant women. Rev Bras Anestesiol. 2010;60(1):13-19. https://doi.org/10.1016/S0034-7094(10)70002-7.
65. Stiffler KA, Jwayyed S, Wilber ST, Robinson A. The use of ultrasound to identify pertinent landmarks for lumbar puncture. Am J Emerg Med. 2007;25(3):331-334. https://doi.org/10.1016/j.ajem.2006.07.010.
66. Gulay U, Meltem T, Nadir SS, Aysin A. Ultrasound-guided evaluation of the lumbar subarachnoid space in lateral and sitting positions in pregnant patients to receive elective cesarean operation. Pak J Med Sci. 2015;31(1):76-81. https://doi.org/10.12669/pjms.311.5647.
67. Kawaguchi R, Yamauchi M, Sugino S, Yamakage M. Ultrasound-aided ipsilateral-dominant epidural block for total hip arthroplasty: a randomised controlled single-blind study. Eur J Anaesthesiol. 2011;28(2):137-140. https://doi.org/10.1097/EJA.0b013e3283423457.
68. Grau T, Leipold RW, Horter J, Martin E, Motsch J. Colour Doppler imaging of the interspinous and epidural space. Eur J Anaesthesiol. 2001;18(11):706-712. https://doi.org/10.1097/00003643-200111000-00002.
69. Arzola C, Davies S, Rofaeel A, Carvalho JC. Ultrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epidurals. Anesth Analg. 2007;104(5):1188-92, tables of contents. https://doi.org/10.1213/01.ane.0000250912.66057.41.
70. Chauhan AK, Bhatia R, Agrawal S. Lumbar epidural depth using transverse ultrasound scan and its correlation with loss of resistance technique: a prospective observational study in Indian population. Saudi J Anaesth. 2018;12(2):279-282. https://doi.org/10.4103/sja.SJA_679_17.
71. Gnaho A, Nguyen V, Villevielle T, et al. Assessing the depth of the subarachnoid space by ultrasound. Rev Bras Anestesiol. 2012;62(4):520-530. https://doi.org/10.1016/S0034-7094(12)70150-2.
72. Cork RC, Kryc JJ, Vaughan RW. Ultrasonic localization of the lumbar epidural space. Anesthesiology. 1980;52(6):513-516. https://doi.org/10.1097/00000542-198006000-00013.
73. Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137. https://doi.org/10.1212/WNL.0b013e31825dd39d.
74. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. https://doi.org/10.3109/0142159X.2010.509412.
75. Wayne DB, Cohen ER, Singer BD, et al. Progress toward improving medical school graduates’ skills via a “boot camp” curriculum. Simul Healthc. 2014;9(1):33-39. https://doi.org/10.1097/SIH.0000000000000001.
76. Cohen ER, Barsuk JH, Moazed F, et al. Making July safer: simulation-based mastery learning during intern boot camp. Acad Med. 2013;88(2):233-239. https://doi.org/10.1097/ACM.0b013e31827bfc0a.
77. Martin R, Gannon D, Riggle J, et al. A comprehensive workshop using simulation to train internal medicine residents in bedside procedures performed by internists. Chest. 2012;142(4):545A. https://doi.org/10.1378/chest.1390093.
78. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
79. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1.
80. Restrepo CG, Baker MD, Pruitt CM, Gullett JP, Pigott DC. Ability of pediatric emergency medicine physicians to identify anatomic landmarks with the assistance of ultrasound prior to lumbar puncture in a simulated obese model. Pediatr Emerg Care. 2015;31(1):15-19. https://doi.org/10.1097/PEC.0000000000000330.
81. VanderWielen BA, Harris R, Galgon RE, VanderWielen LM, Schroeder KM. Teaching sonoanatomy to anesthesia faculty and residents: utility of hands-on gel phantom and instructional video training models. J Clin Anesth. 2015;27(3):188-194. https://doi.org/10.1016/j.jclinane.2014.07.007.
82. Keri Z, Sydor D, Ungi T, et al. Computerized training system for ultrasound-guided lumbar puncture on abnormal spine models: a randomized controlled trial. Can J Anaesth. 2015;62(7):777-784. https://doi.org/10.1007/s12630-015-0367-2.
83. Deacon AJ, Melhuishi NS, Terblanche NC. CUSUM method for construction of trainee spinal ultrasound learning curves following standardised teaching. Anaesth Intensive Care. 2014;42(4):480-486. https://doi.org/10.1177/0310057X1404200409.
84. Margarido CB, Arzola C, Balki M, Carvalho JC. Anesthesiologists’ learning curves for ultrasound assessment of the lumbar spine. Can J Anaesth. 2010;57(2):120-126. https://doi.org/10.1007/s12630-009-9219-2.
85. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
86. Terblanche NC, Arzola C, Wills KE, et al. Standardised training program in spinal ultrasound for epidural insertion: protocol driven versus non-protocol driven teaching approach. Anaesth Intensive Care. 2014;42(4):460-466. https://doi.org/10.1177/0310057X1404200406.
87. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
88. Karmakar MK, Li X, Ho AM, Kwok WH, Chui PT. Real-time ultrasound-guided paramedian epidural access: evaluation of a novel in-plane technique. Br J Anaesth. 2009;102(6):845-854. https://doi.org/10.1093/bja/aep079.
89. Tran D, Kamani AA, Al-Attas E, et al. Single-operator real-time ultrasound-guidance to aim and insert a lumbar epidural needle. Can J Anaesth. 2010;57(4):313-321. https://doi.org/10.1007/s12630-009-9252-1.
90. Liu Y, Qian W, Ke XJ, Mei W. Real-time ultrasound-guided spinal anesthesia using a new paramedian transverse approach. Curr Med Sci. 2018;38(5):910-913. https://doi.org/10.1007/s11596-018-1961-7.
91. Conroy PH, Luyet C, McCartney CJ, McHardy PG. Real-time ultrasound-guided spinal anaesthesia: a prospective observational study of a new approach. Anesthesiol Res Pract. 2013;2013:525818. https://doi.org/10.1155/2013/525818.
92. Brinkmann S, Tang R, Sawka A, Vaghadia H. Single-operator real-time ultrasound-guided spinal injection using SonixGPS™: a case series. Can J Anaesth. 2013;60(9):896-901. https://doi.org/10.1007/s12630-013-9984-9.
93. Niazi AU, Chin KJ, Jin R, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS ultrasound guidance system: a feasibility study. Acta Anaesthesiol Scand. 2014;58(7):875-881. https://doi.org/10.1111/aas.12353.
Approximately 400,000 lumbar punctures (LPs) are performed in the United States annually for either diagnostic workup or therapeutic relief.1 Lumbar punctures are increasingly being performed in the United States, with an estimated 97,000 LPs performed on Medicare fee-for-service beneficiaries in 2011 alone, which is an increase of approximately 4,000 LPs in the same population from 1991.2 Approximately 273,612 LPs were performed on hospitalized patients in the United States in 2010,1 and the inpatient hospital setting is the most common site for LPs.2,3
Many LPs are referred to radiologists who have access to imaging guidance to aid with needle insertion.2 However, referrals to radiology delay performance of LPs, and delayed diagnosis of acute bacterial meningitis, the most common yet serious condition for which LPs are performed, is associated with increased morbidity and mortality.4-8 Furthermore, although initiating empiric antibiotic treatment for suspected acute bacterial meningitis is recommended in some cases, doing so routinely can cause false-negative cerebrospinal fluid (CSF) culture results, complicating decisions about de-escalation and duration of antibiotics that could have been safely avoided by promptly performing an LP.9
Delaying the performance of LP has been associated with increased mortality.10 Demonstration of proficiency in performance of lumbar puncture is considered a core competency for hospitalists,11 and with the increasing availability of point-of-care ultrasound, hospitalists can use ultrasound to guide performance of LPs at the bedside.12 However, 30% of patients requiring LP in emergency departments have difficult-to-palpate lumbar spine landmarks,13 and lumbar puncture performed based on palpation of landmarks alone has been reported to fail or be traumatic in 28% of patients.14 Use of ultrasound guidance for lumbar puncture has been shown in randomized controlled trials to improve procedural success rates, while reducing the time to successful LP, needle passes, patient pain scores, and risk of a traumatic LP.15-17
The purpose of this position statement is to review the literature and present consensus-based recommendations on the performance of ultrasound-guided LP in adult patients. This position statement does not mandate that hospitalists use ultrasound guidance for LP, nor does it establish ultrasound guidance as the standard of care for LP. Similar to previously published Society of Hospital Medicine (SHM) position statements,12,18,19 this document presents recommendations with supporting evidence for the clinical outcomes, techniques, and training for using ultrasound guidance for LP. A manuscript describing the technique of ultrasound guidance for LPs has been previously published by some of the authors of this position statement.20
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the six working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. Updated searches were conducted in November 2016, January 2018, and October 2018. The search strings are included in Appendix 3. All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide LP were selected. In addition, the following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. Moreover, studies focusing on the use of ultrasound guidance for spinal nerve root injections, regional anesthesia, and assessment of lumbar spine anatomy alone were excluded. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided LP were screened and selected. Final article selection was based on working group consensus, and the selected literature was incorporated into the draft recommendations.
The Research and Development (RAND) Appropriateness Method that required panel judgment and consensus was used.21 The 27 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering the following five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (REDCap™) in February 2018 and April 2018 (Appendix 4). Voting on appropriateness was conducted using a 9-point Likert scale. The three zones of the 9-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” A strong recommendation required 80% of the votes within one integer of the median, following the RAND rules. Disagreement was defined as >30% of panelists voting outside of the zone of the median.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording of each recommendation (Table 2). The revised consensus-based recommendations underwent internal and external reviews by POCUS experts from different subspecialties. The final review of this position statement was performed by members of the SHM POCUS Task Force, SHM Education Committee, and SHM Executive Committee. The SHM Executive Committee endorsed this position statement in June 2018 before submission to the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 4,389 references were pooled from four different sources: a search by a certified medical librarian in December 2015 (3,212 citations) that was updated in November 2016 (380 citations), January 2018 (282 citations), and October 2018 (274 citations); working group members’ personal bibliographies and searches (31 citations); and a search focusing on ultrasound-guided LP training (210 citations). A total of 232 full-text articles were reviewed, and the final selection included 77 articles that were abstracted into a data table and incorporated into the draft recommendations. Details of the literature search strategy are presented in Appendix 3.
RECOMMENDATIONS
Four domains (clinical outcomes, technique, training, and knowledge gaps) with 16 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation were drafted citing supporting evidence. After two rounds of panel voting, five recommendations did not achieve agreement based on the RAND rules, one recommendation was combined with another recommendation during peer review, and 10 statements received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Nine statements were approved as strong recommendations, and one was approved as a conditional recommendation. Therefore, the final recommendation count was 10. The strength of the recommendation and degree of consensus for each recommendation are summarized in Table 1.
Terminology
LP is a procedure in which a spinal needle is introduced into the subarachnoid space for the purpose of collecting CSF for diagnostic evaluation and/or therapeutic relief.
Throughout this document, the phrases “ultrasound-guided” and “ultrasound guidance” refer to the use of ultrasound to mark a needle insertion site immediately before performing the procedure. This is also known as static ultrasound guidance. Real-time or dynamic ultrasound guidance refers to direct visualization of the needle tip as it traverses through the skin and soft tissues to reach the ligamentum flavum. Any reference to real-time ultrasound guidance is explicitly stated.
Clinical outcomes
1) When ultrasound equipment is available, along with providers who are appropriately trained to use it, we recommend that ultrasound guidance should be used for site selection of LPs to reduce the number of needle insertion attempts and needle redirections and increase the overall procedure success rates, especially in patients who are obese or have difficult-to-palpate landmarks.
Rationale. LPs have historically been performed by selecting a needle insertion site based on palpation of anatomical landmarks. However, an estimated 30% of patients requiring LP in emergency departments have lumbar spine landmarks that are difficult to palpate, most commonly due to obesity.13 Furthermore, lumbar puncture performed based on palpation of landmarks alone has been reported to fail in 28% of patients.14
Ultrasound can be used at the bedside to elucidate the lumbar spine anatomy to guide performance of LP or epidural catheterization. Since the early 2000s, randomized studies comparing the use of ultrasound guidance (ultrasound-guided) versus anatomical landmarks (landmark-guided) to map the lumbar spine for epidural catheterization have emerged. It is important to recognize that the exact same ultrasound technique is used for site marking of LP, epidural catheterization, and spinal anesthesia—the key difference is how deep the needle tip is inserted. Therefore, data from these three ultrasound-guided procedures are often pooled. Currently, at least 33 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP, epidural catheterization, or spinal anesthesia have been published.22-49 We present three meta-analyses below that pooled data primarily from randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP or spinal anesthesia.
In 2013, Shaikh et al. published the first meta-analysis with 14 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP (n = 5) or epidural catheterization (n = 9). The pooled data showed that use of ultrasound guidance decreased the proportion of failed procedures (risk ratio 0.21, 95% CI 0.10-0.43) with an absolute risk reduction of 6.3% (95% CI 4.1%-8.4%) and a number needed to treat of 16 (95% CI 12-25) to prevent one failed procedure. In addition, the use of ultrasound reduced the mean number of attempts by 0.44 (95% CI 0.24-0.64) and reduced the mean number of needle redirections by 1.00 (95% CI 0.75-1.24). The reduction in risk of a failed procedure was similar for LPs (risk ratio 0.19 [95% CI 0.07-0.56]) and epidural catheterizations (risk ratio 0.23 [95% CI 0.09-0.60]).16
A similar meta-analysis published by Perlas et al. in 2016 included a total of 31 studies, both randomized controlled and cohort studies, evaluating the use of ultrasound guidance for LP, spinal anesthesia, and epidural catheterization.50 The goal of this systematic review and meta-analysis was to establish clinical practice recommendations. The authors concluded (1) the data consistently suggest that ultrasound is more accurate than palpation for lumbar interspace identification, (2) ultrasound allows accurate measurement of the needle insertion depth to reach the epidural space with a mean difference of <3 mm compared with the actual needle insertion depth, and (3) ultrasound increases the efficacy of lumbar epidural or spinal anesthesia by decreasing the mean number of needle passes for success by 0.75 (95% CI 0.44-1.07) and reducing the risk of a failed procedure (risk ratio 0.51 [95% CI 0.32-0.80]), both in patients with normal surface anatomy and in those with technically difficult surface anatomy due to obesity, scoliosis, or previous spine surgery.
Compared to the two earlier meta-analyses that included studies of both LP and spinal anesthesia procedures, the meta-analysis conducted by Gottlieb et al. in 2018 pooled data from 12 randomized controlled studies of ultrasound guidance for LPs only. For the primary outcome, pooled data from both adult and pediatric studies demonstrated higher procedural success rates with ultrasound-guided vs landmark-guided LPs (90% vs 81%) with an odds ratio of 2.1 (95% CI 0.66-7.44) in favor of ultrasound; however, there were no statistically significant differences when the adult and pediatric subgroups were analyzed separately, probably due to underpowering. For the secondary outcomes, data from the adult subgroup showed that use of ultrasound guidance was associated with fewer traumatic LPs (OR 0.28, 95% CI 0.14-0.59), shorter time to procedural success (adjusted mean difference –3.03 minutes, 95% CI –3.54 to –2.52), fewer number of needle passes (adjusted mean difference –0.81 passes, 95% CI –1.57 to –0.05), and lower patient pain scores (adjusted mean difference –2.53, 95% CI –3.89 to –1.17).
At least 12 randomized controlled studies have been published comparing the use of ultrasound guidance vs landmarks for the performance of LP or spinal anesthesia in adult patients, which were not included in the abovementioned meta-analyses. These individual studies demonstrated similar benefits of using ultrasound guidance: reduced needle insertion attempts, reduced needle redirections, and increased overall procedural success rates.17,31,37,40,41,43-49
It is important to recognize that four randomized controlled studies did not demonstrate any benefits of ultrasound guidance on the number of attempts or procedural success rates,23,33,41,51 and three of these studies were included in the abovementioned meta-analyses.23,33,51 Limitations of these negative studies include potential selection bias, inadequate sample sizes, and varying levels of operator skills in procedures, ultrasound guidance, or both. One study included emergency medicine residents as operators with varying degrees of ultrasound skills, and more importantly, patient enrollment occurred by convenience sampling, which may have introduced selection bias. Furthermore, most of the patients were not obese (median BMI of 27 kg/m2), and it is unclear why 10 years lapsed from data collection until publication.33 Another study with three experienced anesthesiologists as operators performing spinal anesthesia enrolled only patients who were not obese (mean BMI of 29 kg/m2) and had easily palpable bony landmarks—two patient characteristics associated with the least benefit of using ultrasound guidance in other studies.23 Another negative study had one experienced anesthesiologist marking obstetric patients with ultrasound, but junior residents performing the actual procedure in the absence of the anesthesiologist who had marked the patient.41
In general, the greatest benefit of using ultrasound guidance for LP has been demonstrated in obese patients.24,32,34,35,52,53 Benefits have been shown in specific obese patient populations, including obstetric,31,54,55 orthopedic,24,56,57 and emergency department patients.30
By increasing the procedural success rates with the use of ultrasound at the bedside, fewer patients may be referred to interventional radiology for fluoroscopic-guided LP, decreasing the patient exposure to ionizing radiation. A randomized study (n = 112) that compared site marking with ultrasound guidance versus fluoroscopic guidance for epidural steroid injections found the two techniques to be equivalent with respect to mean procedure time, number of needle insertion attempts, or needle passes.58 Another randomized study found that the performance time of ultrasound guidance was two minutes shorter (P < .05) than fluoroscopic guidance.59
Techniques
2) We recommend that ultrasound should be used to more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients.
Rationale. Traditionally, an imaginary line connecting the iliac crests (intercristal line, Tuffier’s line, or Jacoby’s line) was considered to identify the L4 vertebra or the L4-L5 interspinous space in the midline; however, studies have revealed this traditional landmark to be much less accurate than previously thought. In general, palpating the iliac crests to mark the intercristal line identifies an interspinous space that is one space cephalad (ie, the L2-L3 interspinous space) but can range from L1-L2 to L4-L5.46,60-64 If an LP is inadvertently performed in the L1-L2 interspinous space, the risk of spinal cord injury is higher than that when performed in a more distal interspinous space.
A study by Margarido et al. with 45 patients with a mean BMI of 30 kg/m2 found that the intercristal line was located above the L4-L5 interspinous space in 100% of patients. More importantly, the intercristal line was above L2-L3 in 36% of patients and above L1-L2 in 4% of patients. It is important to note that patients with scoliosis or previous spine surgery were excluded from this study, and all examinations were performed by two experienced anesthesiologists with patients in a sitting position—all factors that would favor accurate palpation and marking of the iliac crests.60
In a study of nonobese patients (mean BMI 28 kg/m2) undergoing spinal anesthesia, Duniec et al. compared the lumbar level identified by palpation versus ultrasound and found discordance between the two techniques in 36% of patients; 18% were one space too cephalad, 16% were one space too caudal, and 2% were off by two interspinous spaces.61 Another study found discordance in 64% of patients (mean BMI 28 kg/m2) when comparing the interspinous level where spinal anesthesia had been performed by palpation versus a post-procedural ultrasound examination. This study revealed that the interspinous space was more cephalad in 50% of patients with 6% of punctures performed in the L1-L2 interspace.62 A similar study compared the accuracy of palpation vs ultrasound to identify the L3-L4 interspinous space in obese (mean BMI 34 kg/m2) versus nonobese (mean BMI 27 kg/m2) patients. This study found marking a space above L3-L4 in 51% of obese and 40% of nonobese patients and marking of the L1-L2 interspace in 7% of obese and 4% of nonobese patients.64
A study comparing palpation vs ultrasound found that 68% of obese patients with a BMI of >30 kg/m2 had difficult-to-palpate lumbar spine landmarks, but with the use of ultrasound, landmarks were identified in 76% of all patients, including obese and nonobese, with difficult-to-palpate landmarks.65
3) We suggest using ultrasound for selecting and marking a needle insertion site just before performing LPs in either a lateral decubitus or sitting position. The patient should remain in the same position after marking the needle insertion site.
Rationale. Ultrasound mapping of the lumbar spine can be performed in either a lateral decubitus or sitting position. Selecting and marking a needle insertion site should be performed at the bedside just before performing the procedure. The patient must remain in the same position in the interim between marking and inserting the needle, as a slight change in position can alter the needle trajectory, lowering the LP success rate. Although performing LPs in a lateral decubitus position has the advantage of accurately measuring the opening pressure, misalignment of the shoulder and pelvic girdles and bowing of the bed in a lateral decubitus position may lower LP success rates.
One randomized study comparing ultrasound-guided spinal anesthesia in a lateral decubitus versus sitting position found no difference in the number of needle insertion attempts or measurement of the skin-dura distance; however, the needle insertion depth was 0.73 cm greater in a lateral decubitus vs sitting position (P = .002).66 Procedural success rates of LP with ultrasound guidance have not been directly compared in a sitting versus lateral decubitus position, although the overall procedural success rates were higher in one study that allowed the operator to choose either sitting or lateral decubitus position when ultrasound was used.32
4) We recommend that a low-frequency transducer, preferably a curvilinear array transducer, should be used to evaluate the lumbar spine and mark a needle insertion site in most patients. A high-frequency linear array transducer may be used in nonobese patients.
Rationale. Low-frequency transducers emit sound waves that penetrate deep tissues, allowing visualization of bones and ligaments of the lumbar spine. A high-frequency linear transducer offers better resolution but shallower penetration to approximately 6-9 cm, limiting its use for site marking in overweight and obese patients. In obese patients, the ligamentum flavum is often deeper than 6 cm, which requires a low-frequency transducer to be visualized.
Most of the randomized controlled studies demonstrating benefits of using ultrasound guidance compared with landmark guidance for performance of LP, epidural anesthesia, or spinal anesthesia have used a low-frequency, curvilinear transducer.22,24,26-28,31,34-36,39,43-45,67 Two randomized controlled trials used a high-frequency linear transducer for site marking of lumbar procedures.30,32,37 Using a high-frequency linear transducer has been described in real-time, ultrasound-guided LPs, the advantage being better needle visualization with a linear transducer.29 Detection of blood vessels by color flow Doppler may be another advantage of using a high-frequency linear transducer, although a study by Grau et al. showed that use of color flow Doppler with a low-frequency curvilinear transducer permitted visualization of interspinous vessels as small as 0.5 mm in size.68
5) We recommend that ultrasound should be used to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad, sequentially identifying the lumbar spine interspaces.Rationale. Although no studies have directly compared different ultrasound scanning protocols to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad to sequentially identify the lumbar interspinous spaces is the most commonly described technique in studies demonstrating improved clinical outcomes with the use of ultrasound.24,31,34,37,39,40,45,56,57,67 Because the sacrum can be easily recognized, identifying it first is most beneficial in patients with few or no palpable landmarks.
All five lumbar spinous processes and interspinous spaces can be mapped from the sacrum using either a midline or a paramedian approach, and the widest interspinous space can be selected. In a midline approach, either a transverse or a longitudinal view is obtained. The transducer is centered on the sacrum and slid cephalad from L5 to L1 to identify each spinous process and interspinous space. In a paramedian approach, longitudinal paramedian views are obtained from the L5–sacrum interspace to the L1–L2 interspace, and each interspinous space is identified as the transducer is slid cephalad. Both these approaches are effective for mapping the lumbar spine. Whether the entire lumbar spine is mapped, and whether a midline or a paramedian approach is utilized, will depend on the operator’s preference.
6) We recommend that ultrasound should be used in a transverse plane to mark the midline of the lumbar spine and a longitudinal plane to mark the interspinous spaces. The intersection of these two lines marks the needle insertion site.
Rationale. The most common technique described in comparative studies of ultrasound vs landmarks includes visualization of the lumbar spine in two planes, a transverse plane to identify the midline and a longitudinal plane to identify the interspinous spaces. The majority of randomized controlled studies that demonstrated a reduction in the number of needle insertion attempts and an increase in the procedural success rates have used this technique (see Clinical Outcomes).22,24,28,32,35-37,43,44 Marking the midline and interspinous space(s) for LP may be performed in any order, starting with either the transverse or longitudinal plane first.
The midline of the spine is marked by placing the transducer in a transverse plane over the lumbar spine, centering over the spinous processes that have a distinct hyperechoic tip and a prominent acoustic shadow deep to the bone, and drawing a line perpendicular to the center of the transducer delineating the midline. The midline should be marked over a minimum of two or three spinous processes.
To identify the interspinous spaces, the transducer is aligned longitudinally over the midline. The transducer is slid along the midline to identify the widest interspinous space. Once the transducer is centered over the widest interspinous space, a line perpendicular to the center of the transducer is drawn to mark the interspinous space. The intersection of the lines marking the spinal midline and the selected interspinous space identifies the needle entry point.
To visualize the ligamentum flavum from a paramedian view, the transducer is oriented longitudinally over the midline, slid approximately 1 cm laterally, and tilted approximately 15 degrees aiming the ultrasound beam toward the midline. The skin–ligamentum flavum distance is most reliably measured from a paramedian view. Alternatively, in some patients, the ligamentum flavum may be visualized in the midline and the depth can be measured.
7) We recommend that ultrasound should be used during a preprocedural evaluation to measure the distance from the skin surface to the ligamentum flavum from a longitudinal paramedian view to estimate the needle insertion depth and ensure that a spinal needle of adequate length is used.
Rationale. The distance from the skin to the ligamentum flavum can be measured using ultrasound during preprocedural planning. Knowing the depth to the ligamentum flavum preprocedurally allows the operator to procure a spinal needle of adequate length, anticipate the insertion depth before CSF can be obtained, determine the depth to which a local anesthetic will need to be injected, and decide whether the anticipated difficulty of the procedure warrants referral to or consultation with another specialist.
The skin–ligamentum flavum distance can be measured from a transverse midline view or a longitudinal paramedian view. A longitudinal paramedian view provides an unobstructed view of the ligamentum flavum due to less shadowing from bony structures compared with a midline view. Several studies have demonstrated a strong correlation between the skin–ligamentum flavum distance measured by ultrasound and the actual needle insertion depth in both midline and paramedian views.28,34,36,53,54,57,69,70
A meta-analysis that included 13 comparative studies evaluating the correlation between ultrasound-measured depth and actual needle insertion depth to reach the epidural or intrathecal space consistently demonstrated a strong correlation between the measured and actual depth.50 A few studies have reported near-perfect Pearson correlation coefficients of 0.98.55,71,72 The pooled correlation was 0.91 (95% CI 0.87-0.94). All studies measured the depth from the skin to the ventral side of the ligamentum flavum or the intrathecal space from either a longitudinal paramedian view (n = 4) or a transverse midline view (n = 9). Eight of the more recent studies evaluated the accuracy of the ultrasound measurements and found the depth measurements by ultrasound to be accurate within 1-13 mm of the actual needle insertion depth, with seven of the eight studies reporting a mean difference of ≤3 mm.50
Measurement of the distance between the skin and the ligamentum flavum generally underestimates the needle insertion depth. One study reported that measurement of the skin–ligamentum flavum distance underestimates the needle insertion depth by 7.6 mm to obtain CSF, whereas measurement of the skin–posterior longitudinal ligament distance overestimates the needle insertion depth by 2.5 mm.57 A well-accepted contributor to underestimation of the depth measurements using ultrasound is compression of the skin and soft tissues by the transducer, and therefore, pressure on the skin must be released before freezing an image and measuring the depth to the subarachnoid space.
Training
8) We recommend that novices should undergo simulation-based training, where available, before attempting ultrasound-guided LPs on actual patients.
Rationale. Similar to training for other bedside procedures, dedicated training sessions, including didactics, supervised practice on patients, and simulation-based practice, should be considered when teaching novices to perform ultrasound-guided LP. Simulation-based training facilitates acquisition of knowledge and skills to perform invasive bedside procedures, including LP.73 Simulation-based training has been commonly incorporated into procedure training for trainees using an immersive experience, such as a “boot camp,”74-77 or a standardized curriculum,78,79 and has demonstrated improvements in post-course procedural knowledge, technical skills, and operator confidence. Two of these studies included training in the use of ultrasound guidance for LP. These studies showed that simulation-based practice improved skill acquisition and confidence.80,81 Simulation using novel computer software may improve skill acquisition in the use of ultrasound guidance for LP.82
9) We recommend that training in ultrasound-guided LPs should be adapted based on prior ultrasound experience, as learning curves will vary.Rationale. The learning curve to achieve competency in the use of ultrasound guidance for LP has not been well studied. The rate of attaining competency in identifying lumbar spine structures using ultrasound will vary by provider based on prior skills in ultrasound-guided procedures.83 Thus, providers with prior ultrasound experience may require less training than those without such experience to achieve competency. However, extensive experience in performing landmark-guided LPs does not necessarily translate into rapid acquisition of skills to perform the procedure with ultrasound guidance. A study of practicing anesthesiologists with no prior ultrasound experience demonstrated that 20 supervised trials of ultrasound-guided spinal anesthesia were insufficient to achieve competency.84 Although minimums may be a necessary step to gain competence, using them as a sole means to define competence does not account for variable learning curves.12 Based on a national survey of 21 hospitalist procedure experts, the mean current vs suggested minimums for initial and ongoing hospital privileging for LPs were 1.8 vs 6.9 and 2.2 vs 4.6 annually in one report.85
A fundamental question that needs to be answered is how to define competency in the use of ultrasound guidance for LP, including the specific skills and knowledge that must be mastered. At a minimum, providers must be able to identify lumbar spinous processes and distinguish them from the sacrum, identify the lumbar interspinous spaces and their corresponding levels, and estimate the depth from the skin to the ligamentum flavum from the midline and paramedian planes. Novice operators may benefit from practicing lumbar spine mapping of nonobese patients using a high-frequency linear transducer that generates high-resolution images and facilitates recognition of lumbar spine structures.
10) We recommend that novice providers should be supervised when performing ultrasound-guided LPs before performing the procedure independently on patients.
Rationale: Demonstration of competency in the use of ultrasound to identify lumbar spine anatomy should be achieved before routinely performing the procedure independently on patients.18 All providers will require a variable period of supervised practice to demonstrate the appropriate technique, followed by a period of unsupervised practice before competency is achieved. Supervised practice with guidance and feedback has been shown to significantly improve providers’ ability to delineate lumbar spine anatomy.86
KNOWLEDGE GAPS
The process of producing these guidelines revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for LP.
First, it is unclear whether the use of ultrasound guidance for LP reduces postprocedural back pain and whether it improves patient satisfaction. Several studies have evaluated postprocedural back pain28,30,32,33,52 and patient satisfaction28,29,33,51 with the use of ultrasound guidance, but these studies have found inconsistent results. Some of these results were probably due to insufficient statistical power or confounding variables. Furthermore, benefits have been demonstrated in certain subgroups, such as overweight patients or those with anatomical abnormalities, as was found in two studies.52,87 Use of ultrasound guidance for spinal anesthesia has been shown to reduce postprocedural headache28 and improve patient satisfaction51, although similar benefit has not been demonstrated in patients undergoing LP.
Second, the effect of using ultrasound guidance on the frequency of traumatic LPs is an area of uncertainty. A “traumatic tap” is defined as an inadvertent puncture of an epidural vein during passage of the spinal needle through the dura. It remains difficult to discern in these studies whether red blood cells detected in the CSF resulted from puncture of an epidural vein or from needle trauma of the skin and soft tissues. Despite this uncertainty, at least seven randomized controlled studies have assessed the effect of ultrasound guidance on traumatic LPs. The meta-analysis by Shaikh et al. included five randomized controlled studies that assessed the effect of ultrasound guidance on the reporting of traumatic taps. The study found a reduced risk of traumatic taps (risk ratio 0.27 [95% CI 0.11-0.67]), an absolute risk reduction of 5.9% (95% CI 2.3%-9.5%), and a number needed to treat of 17 (95% CI 11-44) to prevent one traumatic tap.16 Similarly, the meta-analysis by Gottlieb et al. showed a lower risk of traumatic taps among adults undergoing LP with ultrasound guidance in five randomized controlled studies with an odds ratio of 0.28 (95% CI 0.14-0.59). The meta-analysis by Gottlieb et al. included two adult studies that were not included by Shaikh et al.
Third, several important questions about the technique of ultrasound-guided LP remain unanswered. In addition to the static technique, a dynamic technique with real-time needle tracking has been described to perform ultrasound-guided LP, epidural catheterization, and spinal anesthesia. A pilot study by Grau et al. found that ultrasound used either statically or dynamically had fewer insertion attempts and needle redirections than use of landmarks alone.29 Three other pilot studies showed successful spinal anesthesia in almost all patients88-90 and one large study demonstrated successful spinal anesthesia with real-time ultrasound guidance in 97 of 100 patients with a median of three needle passes.91 Furthermore, a few industry-sponsored studies with small numbers of patients have described the use of novel needle tracking systems that facilitate needle visualization during real-time ultrasound-guided LP.92,93 However, to our knowledge, no comparative studies of static versus dynamic guidance using novel needle tracking systems in human subjects have been published, and any potential role for these novel needle tracking systems has not yet been defined.
Finally, the effects of using ultrasound guidance on clinical decision-making, timeliness, and cost-effectiveness of LP have not yet been explored but could have important clinical practice implications.
CONCLUSION
Randomized controlled trials have demonstrated that using ultrasound guidance for LPs can reduce the number of needle insertion attempts and needle redirections and increase the overall procedural success rates. Ultrasound can more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients, although the greatest benefit of using ultrasound guidance for LPs has been shown in obese patients.
Ultrasound permits assessment of the interspinous space width and measurement of the ligamentum flavum depth to select an optimal needle insertion site and adequate length spinal needle. Although the use of real-time ultrasound guidance has been described, the use of static ultrasound guidance for LP site marking remains the standard technique.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators from Society of Hospital Medicine Point-of-care Ultrasound Task Force: Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Paul Mayo, Benji Mathews, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Sophia Rodgers, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Matthews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclosures
The authors have nothing to disclose.
Funding
Brian P Lucas: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). Nilam Soni: Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1).
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Approximately 400,000 lumbar punctures (LPs) are performed in the United States annually for either diagnostic workup or therapeutic relief.1 Lumbar punctures are increasingly being performed in the United States, with an estimated 97,000 LPs performed on Medicare fee-for-service beneficiaries in 2011 alone, which is an increase of approximately 4,000 LPs in the same population from 1991.2 Approximately 273,612 LPs were performed on hospitalized patients in the United States in 2010,1 and the inpatient hospital setting is the most common site for LPs.2,3
Many LPs are referred to radiologists who have access to imaging guidance to aid with needle insertion.2 However, referrals to radiology delay performance of LPs, and delayed diagnosis of acute bacterial meningitis, the most common yet serious condition for which LPs are performed, is associated with increased morbidity and mortality.4-8 Furthermore, although initiating empiric antibiotic treatment for suspected acute bacterial meningitis is recommended in some cases, doing so routinely can cause false-negative cerebrospinal fluid (CSF) culture results, complicating decisions about de-escalation and duration of antibiotics that could have been safely avoided by promptly performing an LP.9
Delaying the performance of LP has been associated with increased mortality.10 Demonstration of proficiency in performance of lumbar puncture is considered a core competency for hospitalists,11 and with the increasing availability of point-of-care ultrasound, hospitalists can use ultrasound to guide performance of LPs at the bedside.12 However, 30% of patients requiring LP in emergency departments have difficult-to-palpate lumbar spine landmarks,13 and lumbar puncture performed based on palpation of landmarks alone has been reported to fail or be traumatic in 28% of patients.14 Use of ultrasound guidance for lumbar puncture has been shown in randomized controlled trials to improve procedural success rates, while reducing the time to successful LP, needle passes, patient pain scores, and risk of a traumatic LP.15-17
The purpose of this position statement is to review the literature and present consensus-based recommendations on the performance of ultrasound-guided LP in adult patients. This position statement does not mandate that hospitalists use ultrasound guidance for LP, nor does it establish ultrasound guidance as the standard of care for LP. Similar to previously published Society of Hospital Medicine (SHM) position statements,12,18,19 this document presents recommendations with supporting evidence for the clinical outcomes, techniques, and training for using ultrasound guidance for LP. A manuscript describing the technique of ultrasound guidance for LPs has been previously published by some of the authors of this position statement.20
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the six working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. Updated searches were conducted in November 2016, January 2018, and October 2018. The search strings are included in Appendix 3. All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide LP were selected. In addition, the following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. Moreover, studies focusing on the use of ultrasound guidance for spinal nerve root injections, regional anesthesia, and assessment of lumbar spine anatomy alone were excluded. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided LP were screened and selected. Final article selection was based on working group consensus, and the selected literature was incorporated into the draft recommendations.
The Research and Development (RAND) Appropriateness Method that required panel judgment and consensus was used.21 The 27 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering the following five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (REDCap™) in February 2018 and April 2018 (Appendix 4). Voting on appropriateness was conducted using a 9-point Likert scale. The three zones of the 9-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” A strong recommendation required 80% of the votes within one integer of the median, following the RAND rules. Disagreement was defined as >30% of panelists voting outside of the zone of the median.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording of each recommendation (Table 2). The revised consensus-based recommendations underwent internal and external reviews by POCUS experts from different subspecialties. The final review of this position statement was performed by members of the SHM POCUS Task Force, SHM Education Committee, and SHM Executive Committee. The SHM Executive Committee endorsed this position statement in June 2018 before submission to the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 4,389 references were pooled from four different sources: a search by a certified medical librarian in December 2015 (3,212 citations) that was updated in November 2016 (380 citations), January 2018 (282 citations), and October 2018 (274 citations); working group members’ personal bibliographies and searches (31 citations); and a search focusing on ultrasound-guided LP training (210 citations). A total of 232 full-text articles were reviewed, and the final selection included 77 articles that were abstracted into a data table and incorporated into the draft recommendations. Details of the literature search strategy are presented in Appendix 3.
RECOMMENDATIONS
Four domains (clinical outcomes, technique, training, and knowledge gaps) with 16 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation were drafted citing supporting evidence. After two rounds of panel voting, five recommendations did not achieve agreement based on the RAND rules, one recommendation was combined with another recommendation during peer review, and 10 statements received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Nine statements were approved as strong recommendations, and one was approved as a conditional recommendation. Therefore, the final recommendation count was 10. The strength of the recommendation and degree of consensus for each recommendation are summarized in Table 1.
Terminology
LP is a procedure in which a spinal needle is introduced into the subarachnoid space for the purpose of collecting CSF for diagnostic evaluation and/or therapeutic relief.
Throughout this document, the phrases “ultrasound-guided” and “ultrasound guidance” refer to the use of ultrasound to mark a needle insertion site immediately before performing the procedure. This is also known as static ultrasound guidance. Real-time or dynamic ultrasound guidance refers to direct visualization of the needle tip as it traverses through the skin and soft tissues to reach the ligamentum flavum. Any reference to real-time ultrasound guidance is explicitly stated.
Clinical outcomes
1) When ultrasound equipment is available, along with providers who are appropriately trained to use it, we recommend that ultrasound guidance should be used for site selection of LPs to reduce the number of needle insertion attempts and needle redirections and increase the overall procedure success rates, especially in patients who are obese or have difficult-to-palpate landmarks.
Rationale. LPs have historically been performed by selecting a needle insertion site based on palpation of anatomical landmarks. However, an estimated 30% of patients requiring LP in emergency departments have lumbar spine landmarks that are difficult to palpate, most commonly due to obesity.13 Furthermore, lumbar puncture performed based on palpation of landmarks alone has been reported to fail in 28% of patients.14
Ultrasound can be used at the bedside to elucidate the lumbar spine anatomy to guide performance of LP or epidural catheterization. Since the early 2000s, randomized studies comparing the use of ultrasound guidance (ultrasound-guided) versus anatomical landmarks (landmark-guided) to map the lumbar spine for epidural catheterization have emerged. It is important to recognize that the exact same ultrasound technique is used for site marking of LP, epidural catheterization, and spinal anesthesia—the key difference is how deep the needle tip is inserted. Therefore, data from these three ultrasound-guided procedures are often pooled. Currently, at least 33 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP, epidural catheterization, or spinal anesthesia have been published.22-49 We present three meta-analyses below that pooled data primarily from randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP or spinal anesthesia.
In 2013, Shaikh et al. published the first meta-analysis with 14 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP (n = 5) or epidural catheterization (n = 9). The pooled data showed that use of ultrasound guidance decreased the proportion of failed procedures (risk ratio 0.21, 95% CI 0.10-0.43) with an absolute risk reduction of 6.3% (95% CI 4.1%-8.4%) and a number needed to treat of 16 (95% CI 12-25) to prevent one failed procedure. In addition, the use of ultrasound reduced the mean number of attempts by 0.44 (95% CI 0.24-0.64) and reduced the mean number of needle redirections by 1.00 (95% CI 0.75-1.24). The reduction in risk of a failed procedure was similar for LPs (risk ratio 0.19 [95% CI 0.07-0.56]) and epidural catheterizations (risk ratio 0.23 [95% CI 0.09-0.60]).16
A similar meta-analysis published by Perlas et al. in 2016 included a total of 31 studies, both randomized controlled and cohort studies, evaluating the use of ultrasound guidance for LP, spinal anesthesia, and epidural catheterization.50 The goal of this systematic review and meta-analysis was to establish clinical practice recommendations. The authors concluded (1) the data consistently suggest that ultrasound is more accurate than palpation for lumbar interspace identification, (2) ultrasound allows accurate measurement of the needle insertion depth to reach the epidural space with a mean difference of <3 mm compared with the actual needle insertion depth, and (3) ultrasound increases the efficacy of lumbar epidural or spinal anesthesia by decreasing the mean number of needle passes for success by 0.75 (95% CI 0.44-1.07) and reducing the risk of a failed procedure (risk ratio 0.51 [95% CI 0.32-0.80]), both in patients with normal surface anatomy and in those with technically difficult surface anatomy due to obesity, scoliosis, or previous spine surgery.
Compared to the two earlier meta-analyses that included studies of both LP and spinal anesthesia procedures, the meta-analysis conducted by Gottlieb et al. in 2018 pooled data from 12 randomized controlled studies of ultrasound guidance for LPs only. For the primary outcome, pooled data from both adult and pediatric studies demonstrated higher procedural success rates with ultrasound-guided vs landmark-guided LPs (90% vs 81%) with an odds ratio of 2.1 (95% CI 0.66-7.44) in favor of ultrasound; however, there were no statistically significant differences when the adult and pediatric subgroups were analyzed separately, probably due to underpowering. For the secondary outcomes, data from the adult subgroup showed that use of ultrasound guidance was associated with fewer traumatic LPs (OR 0.28, 95% CI 0.14-0.59), shorter time to procedural success (adjusted mean difference –3.03 minutes, 95% CI –3.54 to –2.52), fewer number of needle passes (adjusted mean difference –0.81 passes, 95% CI –1.57 to –0.05), and lower patient pain scores (adjusted mean difference –2.53, 95% CI –3.89 to –1.17).
At least 12 randomized controlled studies have been published comparing the use of ultrasound guidance vs landmarks for the performance of LP or spinal anesthesia in adult patients, which were not included in the abovementioned meta-analyses. These individual studies demonstrated similar benefits of using ultrasound guidance: reduced needle insertion attempts, reduced needle redirections, and increased overall procedural success rates.17,31,37,40,41,43-49
It is important to recognize that four randomized controlled studies did not demonstrate any benefits of ultrasound guidance on the number of attempts or procedural success rates,23,33,41,51 and three of these studies were included in the abovementioned meta-analyses.23,33,51 Limitations of these negative studies include potential selection bias, inadequate sample sizes, and varying levels of operator skills in procedures, ultrasound guidance, or both. One study included emergency medicine residents as operators with varying degrees of ultrasound skills, and more importantly, patient enrollment occurred by convenience sampling, which may have introduced selection bias. Furthermore, most of the patients were not obese (median BMI of 27 kg/m2), and it is unclear why 10 years lapsed from data collection until publication.33 Another study with three experienced anesthesiologists as operators performing spinal anesthesia enrolled only patients who were not obese (mean BMI of 29 kg/m2) and had easily palpable bony landmarks—two patient characteristics associated with the least benefit of using ultrasound guidance in other studies.23 Another negative study had one experienced anesthesiologist marking obstetric patients with ultrasound, but junior residents performing the actual procedure in the absence of the anesthesiologist who had marked the patient.41
In general, the greatest benefit of using ultrasound guidance for LP has been demonstrated in obese patients.24,32,34,35,52,53 Benefits have been shown in specific obese patient populations, including obstetric,31,54,55 orthopedic,24,56,57 and emergency department patients.30
By increasing the procedural success rates with the use of ultrasound at the bedside, fewer patients may be referred to interventional radiology for fluoroscopic-guided LP, decreasing the patient exposure to ionizing radiation. A randomized study (n = 112) that compared site marking with ultrasound guidance versus fluoroscopic guidance for epidural steroid injections found the two techniques to be equivalent with respect to mean procedure time, number of needle insertion attempts, or needle passes.58 Another randomized study found that the performance time of ultrasound guidance was two minutes shorter (P < .05) than fluoroscopic guidance.59
Techniques
2) We recommend that ultrasound should be used to more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients.
Rationale. Traditionally, an imaginary line connecting the iliac crests (intercristal line, Tuffier’s line, or Jacoby’s line) was considered to identify the L4 vertebra or the L4-L5 interspinous space in the midline; however, studies have revealed this traditional landmark to be much less accurate than previously thought. In general, palpating the iliac crests to mark the intercristal line identifies an interspinous space that is one space cephalad (ie, the L2-L3 interspinous space) but can range from L1-L2 to L4-L5.46,60-64 If an LP is inadvertently performed in the L1-L2 interspinous space, the risk of spinal cord injury is higher than that when performed in a more distal interspinous space.
A study by Margarido et al. with 45 patients with a mean BMI of 30 kg/m2 found that the intercristal line was located above the L4-L5 interspinous space in 100% of patients. More importantly, the intercristal line was above L2-L3 in 36% of patients and above L1-L2 in 4% of patients. It is important to note that patients with scoliosis or previous spine surgery were excluded from this study, and all examinations were performed by two experienced anesthesiologists with patients in a sitting position—all factors that would favor accurate palpation and marking of the iliac crests.60
In a study of nonobese patients (mean BMI 28 kg/m2) undergoing spinal anesthesia, Duniec et al. compared the lumbar level identified by palpation versus ultrasound and found discordance between the two techniques in 36% of patients; 18% were one space too cephalad, 16% were one space too caudal, and 2% were off by two interspinous spaces.61 Another study found discordance in 64% of patients (mean BMI 28 kg/m2) when comparing the interspinous level where spinal anesthesia had been performed by palpation versus a post-procedural ultrasound examination. This study revealed that the interspinous space was more cephalad in 50% of patients with 6% of punctures performed in the L1-L2 interspace.62 A similar study compared the accuracy of palpation vs ultrasound to identify the L3-L4 interspinous space in obese (mean BMI 34 kg/m2) versus nonobese (mean BMI 27 kg/m2) patients. This study found marking a space above L3-L4 in 51% of obese and 40% of nonobese patients and marking of the L1-L2 interspace in 7% of obese and 4% of nonobese patients.64
A study comparing palpation vs ultrasound found that 68% of obese patients with a BMI of >30 kg/m2 had difficult-to-palpate lumbar spine landmarks, but with the use of ultrasound, landmarks were identified in 76% of all patients, including obese and nonobese, with difficult-to-palpate landmarks.65
3) We suggest using ultrasound for selecting and marking a needle insertion site just before performing LPs in either a lateral decubitus or sitting position. The patient should remain in the same position after marking the needle insertion site.
Rationale. Ultrasound mapping of the lumbar spine can be performed in either a lateral decubitus or sitting position. Selecting and marking a needle insertion site should be performed at the bedside just before performing the procedure. The patient must remain in the same position in the interim between marking and inserting the needle, as a slight change in position can alter the needle trajectory, lowering the LP success rate. Although performing LPs in a lateral decubitus position has the advantage of accurately measuring the opening pressure, misalignment of the shoulder and pelvic girdles and bowing of the bed in a lateral decubitus position may lower LP success rates.
One randomized study comparing ultrasound-guided spinal anesthesia in a lateral decubitus versus sitting position found no difference in the number of needle insertion attempts or measurement of the skin-dura distance; however, the needle insertion depth was 0.73 cm greater in a lateral decubitus vs sitting position (P = .002).66 Procedural success rates of LP with ultrasound guidance have not been directly compared in a sitting versus lateral decubitus position, although the overall procedural success rates were higher in one study that allowed the operator to choose either sitting or lateral decubitus position when ultrasound was used.32
4) We recommend that a low-frequency transducer, preferably a curvilinear array transducer, should be used to evaluate the lumbar spine and mark a needle insertion site in most patients. A high-frequency linear array transducer may be used in nonobese patients.
Rationale. Low-frequency transducers emit sound waves that penetrate deep tissues, allowing visualization of bones and ligaments of the lumbar spine. A high-frequency linear transducer offers better resolution but shallower penetration to approximately 6-9 cm, limiting its use for site marking in overweight and obese patients. In obese patients, the ligamentum flavum is often deeper than 6 cm, which requires a low-frequency transducer to be visualized.
Most of the randomized controlled studies demonstrating benefits of using ultrasound guidance compared with landmark guidance for performance of LP, epidural anesthesia, or spinal anesthesia have used a low-frequency, curvilinear transducer.22,24,26-28,31,34-36,39,43-45,67 Two randomized controlled trials used a high-frequency linear transducer for site marking of lumbar procedures.30,32,37 Using a high-frequency linear transducer has been described in real-time, ultrasound-guided LPs, the advantage being better needle visualization with a linear transducer.29 Detection of blood vessels by color flow Doppler may be another advantage of using a high-frequency linear transducer, although a study by Grau et al. showed that use of color flow Doppler with a low-frequency curvilinear transducer permitted visualization of interspinous vessels as small as 0.5 mm in size.68
5) We recommend that ultrasound should be used to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad, sequentially identifying the lumbar spine interspaces.Rationale. Although no studies have directly compared different ultrasound scanning protocols to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad to sequentially identify the lumbar interspinous spaces is the most commonly described technique in studies demonstrating improved clinical outcomes with the use of ultrasound.24,31,34,37,39,40,45,56,57,67 Because the sacrum can be easily recognized, identifying it first is most beneficial in patients with few or no palpable landmarks.
All five lumbar spinous processes and interspinous spaces can be mapped from the sacrum using either a midline or a paramedian approach, and the widest interspinous space can be selected. In a midline approach, either a transverse or a longitudinal view is obtained. The transducer is centered on the sacrum and slid cephalad from L5 to L1 to identify each spinous process and interspinous space. In a paramedian approach, longitudinal paramedian views are obtained from the L5–sacrum interspace to the L1–L2 interspace, and each interspinous space is identified as the transducer is slid cephalad. Both these approaches are effective for mapping the lumbar spine. Whether the entire lumbar spine is mapped, and whether a midline or a paramedian approach is utilized, will depend on the operator’s preference.
6) We recommend that ultrasound should be used in a transverse plane to mark the midline of the lumbar spine and a longitudinal plane to mark the interspinous spaces. The intersection of these two lines marks the needle insertion site.
Rationale. The most common technique described in comparative studies of ultrasound vs landmarks includes visualization of the lumbar spine in two planes, a transverse plane to identify the midline and a longitudinal plane to identify the interspinous spaces. The majority of randomized controlled studies that demonstrated a reduction in the number of needle insertion attempts and an increase in the procedural success rates have used this technique (see Clinical Outcomes).22,24,28,32,35-37,43,44 Marking the midline and interspinous space(s) for LP may be performed in any order, starting with either the transverse or longitudinal plane first.
The midline of the spine is marked by placing the transducer in a transverse plane over the lumbar spine, centering over the spinous processes that have a distinct hyperechoic tip and a prominent acoustic shadow deep to the bone, and drawing a line perpendicular to the center of the transducer delineating the midline. The midline should be marked over a minimum of two or three spinous processes.
To identify the interspinous spaces, the transducer is aligned longitudinally over the midline. The transducer is slid along the midline to identify the widest interspinous space. Once the transducer is centered over the widest interspinous space, a line perpendicular to the center of the transducer is drawn to mark the interspinous space. The intersection of the lines marking the spinal midline and the selected interspinous space identifies the needle entry point.
To visualize the ligamentum flavum from a paramedian view, the transducer is oriented longitudinally over the midline, slid approximately 1 cm laterally, and tilted approximately 15 degrees aiming the ultrasound beam toward the midline. The skin–ligamentum flavum distance is most reliably measured from a paramedian view. Alternatively, in some patients, the ligamentum flavum may be visualized in the midline and the depth can be measured.
7) We recommend that ultrasound should be used during a preprocedural evaluation to measure the distance from the skin surface to the ligamentum flavum from a longitudinal paramedian view to estimate the needle insertion depth and ensure that a spinal needle of adequate length is used.
Rationale. The distance from the skin to the ligamentum flavum can be measured using ultrasound during preprocedural planning. Knowing the depth to the ligamentum flavum preprocedurally allows the operator to procure a spinal needle of adequate length, anticipate the insertion depth before CSF can be obtained, determine the depth to which a local anesthetic will need to be injected, and decide whether the anticipated difficulty of the procedure warrants referral to or consultation with another specialist.
The skin–ligamentum flavum distance can be measured from a transverse midline view or a longitudinal paramedian view. A longitudinal paramedian view provides an unobstructed view of the ligamentum flavum due to less shadowing from bony structures compared with a midline view. Several studies have demonstrated a strong correlation between the skin–ligamentum flavum distance measured by ultrasound and the actual needle insertion depth in both midline and paramedian views.28,34,36,53,54,57,69,70
A meta-analysis that included 13 comparative studies evaluating the correlation between ultrasound-measured depth and actual needle insertion depth to reach the epidural or intrathecal space consistently demonstrated a strong correlation between the measured and actual depth.50 A few studies have reported near-perfect Pearson correlation coefficients of 0.98.55,71,72 The pooled correlation was 0.91 (95% CI 0.87-0.94). All studies measured the depth from the skin to the ventral side of the ligamentum flavum or the intrathecal space from either a longitudinal paramedian view (n = 4) or a transverse midline view (n = 9). Eight of the more recent studies evaluated the accuracy of the ultrasound measurements and found the depth measurements by ultrasound to be accurate within 1-13 mm of the actual needle insertion depth, with seven of the eight studies reporting a mean difference of ≤3 mm.50
Measurement of the distance between the skin and the ligamentum flavum generally underestimates the needle insertion depth. One study reported that measurement of the skin–ligamentum flavum distance underestimates the needle insertion depth by 7.6 mm to obtain CSF, whereas measurement of the skin–posterior longitudinal ligament distance overestimates the needle insertion depth by 2.5 mm.57 A well-accepted contributor to underestimation of the depth measurements using ultrasound is compression of the skin and soft tissues by the transducer, and therefore, pressure on the skin must be released before freezing an image and measuring the depth to the subarachnoid space.
Training
8) We recommend that novices should undergo simulation-based training, where available, before attempting ultrasound-guided LPs on actual patients.
Rationale. Similar to training for other bedside procedures, dedicated training sessions, including didactics, supervised practice on patients, and simulation-based practice, should be considered when teaching novices to perform ultrasound-guided LP. Simulation-based training facilitates acquisition of knowledge and skills to perform invasive bedside procedures, including LP.73 Simulation-based training has been commonly incorporated into procedure training for trainees using an immersive experience, such as a “boot camp,”74-77 or a standardized curriculum,78,79 and has demonstrated improvements in post-course procedural knowledge, technical skills, and operator confidence. Two of these studies included training in the use of ultrasound guidance for LP. These studies showed that simulation-based practice improved skill acquisition and confidence.80,81 Simulation using novel computer software may improve skill acquisition in the use of ultrasound guidance for LP.82
9) We recommend that training in ultrasound-guided LPs should be adapted based on prior ultrasound experience, as learning curves will vary.Rationale. The learning curve to achieve competency in the use of ultrasound guidance for LP has not been well studied. The rate of attaining competency in identifying lumbar spine structures using ultrasound will vary by provider based on prior skills in ultrasound-guided procedures.83 Thus, providers with prior ultrasound experience may require less training than those without such experience to achieve competency. However, extensive experience in performing landmark-guided LPs does not necessarily translate into rapid acquisition of skills to perform the procedure with ultrasound guidance. A study of practicing anesthesiologists with no prior ultrasound experience demonstrated that 20 supervised trials of ultrasound-guided spinal anesthesia were insufficient to achieve competency.84 Although minimums may be a necessary step to gain competence, using them as a sole means to define competence does not account for variable learning curves.12 Based on a national survey of 21 hospitalist procedure experts, the mean current vs suggested minimums for initial and ongoing hospital privileging for LPs were 1.8 vs 6.9 and 2.2 vs 4.6 annually in one report.85
A fundamental question that needs to be answered is how to define competency in the use of ultrasound guidance for LP, including the specific skills and knowledge that must be mastered. At a minimum, providers must be able to identify lumbar spinous processes and distinguish them from the sacrum, identify the lumbar interspinous spaces and their corresponding levels, and estimate the depth from the skin to the ligamentum flavum from the midline and paramedian planes. Novice operators may benefit from practicing lumbar spine mapping of nonobese patients using a high-frequency linear transducer that generates high-resolution images and facilitates recognition of lumbar spine structures.
10) We recommend that novice providers should be supervised when performing ultrasound-guided LPs before performing the procedure independently on patients.
Rationale: Demonstration of competency in the use of ultrasound to identify lumbar spine anatomy should be achieved before routinely performing the procedure independently on patients.18 All providers will require a variable period of supervised practice to demonstrate the appropriate technique, followed by a period of unsupervised practice before competency is achieved. Supervised practice with guidance and feedback has been shown to significantly improve providers’ ability to delineate lumbar spine anatomy.86
KNOWLEDGE GAPS
The process of producing these guidelines revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for LP.
First, it is unclear whether the use of ultrasound guidance for LP reduces postprocedural back pain and whether it improves patient satisfaction. Several studies have evaluated postprocedural back pain28,30,32,33,52 and patient satisfaction28,29,33,51 with the use of ultrasound guidance, but these studies have found inconsistent results. Some of these results were probably due to insufficient statistical power or confounding variables. Furthermore, benefits have been demonstrated in certain subgroups, such as overweight patients or those with anatomical abnormalities, as was found in two studies.52,87 Use of ultrasound guidance for spinal anesthesia has been shown to reduce postprocedural headache28 and improve patient satisfaction51, although similar benefit has not been demonstrated in patients undergoing LP.
Second, the effect of using ultrasound guidance on the frequency of traumatic LPs is an area of uncertainty. A “traumatic tap” is defined as an inadvertent puncture of an epidural vein during passage of the spinal needle through the dura. It remains difficult to discern in these studies whether red blood cells detected in the CSF resulted from puncture of an epidural vein or from needle trauma of the skin and soft tissues. Despite this uncertainty, at least seven randomized controlled studies have assessed the effect of ultrasound guidance on traumatic LPs. The meta-analysis by Shaikh et al. included five randomized controlled studies that assessed the effect of ultrasound guidance on the reporting of traumatic taps. The study found a reduced risk of traumatic taps (risk ratio 0.27 [95% CI 0.11-0.67]), an absolute risk reduction of 5.9% (95% CI 2.3%-9.5%), and a number needed to treat of 17 (95% CI 11-44) to prevent one traumatic tap.16 Similarly, the meta-analysis by Gottlieb et al. showed a lower risk of traumatic taps among adults undergoing LP with ultrasound guidance in five randomized controlled studies with an odds ratio of 0.28 (95% CI 0.14-0.59). The meta-analysis by Gottlieb et al. included two adult studies that were not included by Shaikh et al.
Third, several important questions about the technique of ultrasound-guided LP remain unanswered. In addition to the static technique, a dynamic technique with real-time needle tracking has been described to perform ultrasound-guided LP, epidural catheterization, and spinal anesthesia. A pilot study by Grau et al. found that ultrasound used either statically or dynamically had fewer insertion attempts and needle redirections than use of landmarks alone.29 Three other pilot studies showed successful spinal anesthesia in almost all patients88-90 and one large study demonstrated successful spinal anesthesia with real-time ultrasound guidance in 97 of 100 patients with a median of three needle passes.91 Furthermore, a few industry-sponsored studies with small numbers of patients have described the use of novel needle tracking systems that facilitate needle visualization during real-time ultrasound-guided LP.92,93 However, to our knowledge, no comparative studies of static versus dynamic guidance using novel needle tracking systems in human subjects have been published, and any potential role for these novel needle tracking systems has not yet been defined.
Finally, the effects of using ultrasound guidance on clinical decision-making, timeliness, and cost-effectiveness of LP have not yet been explored but could have important clinical practice implications.
CONCLUSION
Randomized controlled trials have demonstrated that using ultrasound guidance for LPs can reduce the number of needle insertion attempts and needle redirections and increase the overall procedural success rates. Ultrasound can more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients, although the greatest benefit of using ultrasound guidance for LPs has been shown in obese patients.
Ultrasound permits assessment of the interspinous space width and measurement of the ligamentum flavum depth to select an optimal needle insertion site and adequate length spinal needle. Although the use of real-time ultrasound guidance has been described, the use of static ultrasound guidance for LP site marking remains the standard technique.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators from Society of Hospital Medicine Point-of-care Ultrasound Task Force: Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Paul Mayo, Benji Mathews, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Sophia Rodgers, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Matthews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclosures
The authors have nothing to disclose.
Funding
Brian P Lucas: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). Nilam Soni: Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1).
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Wolfe KS, Kress JP. Risk of procedural hemorrhage. Chest. 2016;150(1):237-246. https://doi.org/10.1016/j.chest.2016.01.023.
2. Kroll H, Duszak R, Jr, Nsiah E, et al. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. AJR Am J Roentgenol. 2015;204(1):15-19. https://doi.org/10.2214/AJR.14.12622.
3. Vickers A, Donnelly JP, Moore JX, Wang HE. 263EMF epidemiology of lumbar punctures in hospitalized patients in United States. Ann Emerg Med. 2017;70(4):S104. https://doi.org/10.1016/j.annemergmed.2017.07.241.
4. Køster-Rasmussen R, Korshin A, Meyer CN. Antibiotic treatment delay and outcome in acute bacterial meningitis. J Infect. 2008;57(6):449-454. https://doi.org/10.1016/j.jinf.2008.09.033.
5. Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129(11):862-869. https://doi.org/10.7326/0003-4819-129-11_Part_1-199812010-00004.
6. Lepur D, Barsić B. Community-acquired bacterial meningitis in adults: antibiotic timing in disease course and outcome. Infection. 2007;35(4):225-231. https://doi.org/10.1007/s15010-007-6202-0.
7. Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM. 2005;98(4):291-298. https://doi.org/10.1093/qjmed/hci047.
8. Auburtin M, Wolff M, Charpentier J, et al. Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: the PNEUMOREA prospective multicenter study. Crit Care Med. 2006;34(11):2758-2765. https://doi.org/10.1097/01.CCM.0000239434.26669.65.
9. Michael B, Menezes BF, Cunniffe J, et al. Effect of delayed lumbar punctures on the diagnosis of acute bacterial meningitis in adults. Emerg Med J. 2010;27(6):433-438. https://doi.org/10.1136/emj.2009.075598.
10. Glimåker M, Johansson B, Grindborg Ö, et al. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis. 2015;60(8):1162-1169. https://doi.org/10.1093/cid/civ011.
11. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 Revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715.
12. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079.
13. Shah KH, McGillicuddy D, Spear J, Edlow JA. Predicting difficult and traumatic lumbar punctures. Am J Emerg Med. 2007;25(6):608-611. https://doi.org/10.1016/j.ajem.2006.11.025.
14. Williams P, Tait G, Wijeratne T. Success rate of elective lumbar puncture at a major Melbourne neurology unit. Surg Neurol Int. 2018;9:12. https://doi.org/10.4103/sni.sni_426_17.
15. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2018;26(1). https://doi.org/10.1111/acem.13558.
16. Shaikh F, Brzezinski J, Alexander S, et al. Ultrasound imaging for lumbar punctures and epidural catheterisations: systematic review and meta-analysis. BMJ. 2013;346:f1720. https://doi.org/10.1136/bmj.f1720.
17. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
18. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
19. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
20. Soni NJ, Franco-Sadud R, Schnobrich D, et al. Ultrasound guidance for lumbar puncture. Neurol Clin Pract. 2016;6(4):358-368. https://doi.org/10.1212/CPJ.0000000000000265.
21. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The Rand/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: Rand Corp; 2001.
22. Abdelhamid SA, Mansour MA. Ultrasound-guided intrathecal anesthesia: does scanning help? Egypt J Anaesth. 2013;29(4):389-394. https://doi.org/10.1016/j.egja.2013.06.003.
23. Ansari T, Yousef A, El Gamassy A, Fayez M. Ultrasound-guided spinal anaesthesia in obstetrics: is there an advantage over the landmark technique in patients with easily palpable spines? Int J Obstet Anesth. 2014;23(3):213-216. https://doi.org/10.1016/j.ijoa.2014.03.001.
24. Chin KJ, Perlas A, Chan V, et al. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology. 2011;115(1):94-101. https://doi.org/10.1097/ALN.0b013e31821a8ad4.
25. Cho YC, Koo DH, Oh SK, et al. Comparison of ultrasound-assisted lumbar puncture with lumbar puncture using palpation of landmarks in aged patients in an emergency center. J Korean Soc Emerg Med. 2009;20(3):304.
26. Grau T, Leipold RW, Conradi R, Martin E. Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol Scand. 2001;45(6):766-771. https://doi.org/10.1034/j.1399-6576.2001.045006766.x.
27. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Ultrasound imaging facilitates localization of the epidural space during combined spinal and epidural anesthesia. Reg Anesth Pain Med. 2001;26(1):64-67. https://doi.org/10.1053/rapm.2001.19633.
28. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Efficacy of ultrasound imaging in obstetric epidural anesthesia. J Clin Anesth. 2002;14(3):169-175. https://doi.org/10.1016/S0952-8180(01)00378-6.
29. Grau T, Leipold RW, Fatehi S, Martin E, Motsch J. Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur J Anaesthesiol. 2004;21(1):25-31. https://doi.org/10.1017/S026502150400105X.
30. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
31. Nassar M, Abdelazim IA. Pre-puncture ultrasound guided epidural insertion before vaginal delivery. J Clin Monit Comput. 2015;29(5):573-577. https://doi.org/10.1007/s10877-014-9634-y.
32. Nomura JT, Leech SJ, Shenbagamurthi S, et al. A randomized controlled trial of ultrasound-assisted lumbar puncture. J Ultrasound Med. 2007;26(10):1341-1348. https://doi.org/10.7863/jum.2007.26.10.1341.
33. Peterson MA, Pisupati D, Heyming TW, Abele JA, Lewis RJ. Ultrasound for routine lumbar puncture. Acad Emerg Med. 2014;21(2):130-136. https://doi.org/10.1111/acem.12305.
34. Sahin T, Balaban O, Sahin L, Solak M, Toker K. A randomized controlled trial of preinsertion ultrasound guidance for spinal anaesthesia in pregnancy: outcomes among obese and lean parturients: ultrasound for spinal anesthesia in pregnancy. J Anesth. 2014;28(3):413-419. https://doi.org/10.1007/s00540-013-1726-1.
35. Wang Q, Yin C, Wang TL. Ultrasound facilitates identification of combined spinal-epidural puncture in obese parturients. Chin Med J (Engl). 2012;125(21):3840-3843. PubMed
36. Vallejo MC, Phelps AL, Singh S, Orebaugh SL, Sah N. Ultrasound decreases the failed labor epidural rate in resident trainees. Int J Obstet Anesth. 2010;19(4):373-378. https://doi.org/10.1016/j.ijoa.2010.04.002.
37. Darrieutort-Laffite C, Bart G, Planche L, et al. Usefulness of a pre-procedure ultrasound scanning of the lumbar spine before epidural injection in patients with a presumed difficult puncture: a randomized controlled trial. Joint Bone Spine. 2015;82(5):356-361. https://doi.org/10.1016/j.jbspin.2015.02.001.
38. Vosko MR, Brunner C, Schreiber S. Lumbar puncture with ultrasound study (lupus study)-international prospective randomized multicentre trial. Int J Stroke. 2017;12(1):22. https://doi.org/10.1055/s-0037-1606991.
39. Urfalioğlu A, Bilal B, Öksüz G, et al. Comparison of the landmark and ultrasound methods in cesarean sections performed under spinal anesthesia on obese pregnants. J Matern Fetal Neonatal Med. 2017;30(9):1051-1056. https://doi.org/10.1080/14767058.2016.1199677.
40. Tawfik MM, Atallah MM, Elkharboutly WS, Allakkany NS, Abdelkhalek M. Does preprocedural ultrasound increase the first-pass success rate of epidural catheterization before cesarean delivery? A randomized controlled trial. Anesth Analg. 2017;124(3):851-856. https://doi.org/10.1213/ANE.0000000000001325.
41. Turkstra TP, Marmai KL, Armstrong KP, Kumar K, Singh SI. Preprocedural ultrasound assessment does not improve trainee performance of spinal anesthesia for obstetrical patients: a randomized controlled trial. J Clin Anesth. 2017;37:21-24. https://doi.org/10.1016/j.jclinane.2016.10.034.
42. Chong SE, Mohd Nikman A, Saedah A, et al. Real-time ultrasound-guided paramedian spinal anaesthesia: evaluation of the efficacy and the success rate of single needle pass. Br J Anaesth. 2017;118(5):799-801. https://doi.org/10.1093/bja/aex108.
43. Creaney M, Mullane D, Casby C, Tan T. Ultrasound to identify the lumbar space in women with impalpable bony landmarks presenting for elective caesarean delivery under spinal anaesthesia: a randomised trial. Int J Obstet Anesth. 2016;28:12-16. https://doi.org/10.1016/j.ijoa.2016.07.007.
44. Ekinci M, Alici HA, Ahiskalioglu A, et al. The use of ultrasound in planned cesarean delivery under spinal anesthesia for patients having nonprominent anatomic landmarks. J Clin Anesth. 2017;37:82-85. https://doi.org/10.1016/j.jclinane.2016.10.014.
45. Perna P, Gioia A, Ragazzi R, Volta CA, Innamorato M. Can pre-procedure neuroaxial ultrasound improve the identification of the potential epidural space when compared with anatomical landmarks? A prospective randomized study. Minerva Anestesiol. 2017;83(1):41-49. https://doi.org/10.23736/S0375-9393.16.11399-9.
46. Chin A, Crooke B, Heywood L, et al. A randomised controlled trial comparing needle movements during combined spinal-epidural anaesthesia with and without ultrasound assistance. Anaesthesia. 2018;73(4):466-473. https://doi.org/10.1111/anae.14206.
47. Dhanger S, Vinayagam S, Vaidhyanathan B, Rajesh IJ, Tripathy DK. Comparison of landmark versus pre-procedural ultrasonography-assisted midline approach for identification of subarachnoid space in elective caesarean section: a randomised controlled trial. Indian J Anaesth. 2018;62(4):280-284. https://doi.org/10.4103/ija.IJA_488_17.
48. Evans DP, Tozer J, Joyce M, Vitto MJ. Comparison of ultrasound-guided and landmark-based lumbar punctures in inexperienced resident physicians. J Ultrasound Med. 2019;38(3):613-620. https://doi.org/10.1002/jum.14728.
49. Srinivasan KK, Leo AM, Iohom G, Loughnane F, Lee PJ. Pre-procedure ultrasound-guided paramedian spinal anaesthesia at L5-S1: is this better than landmark-guided midline approach? A randomised controlled trial. Indian J Anaesth. 2018;62(1):53-60. https://doi.org/10.4103/ija.IJA_448_17.
50. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
51. Lim YC, Choo CY, Tan KT. A randomised controlled trial of ultrasound-assisted spinal anaesthesia. Anaesth Intensive Care. 2014;42(2):191-198. https://doi.org/10.1177/0310057X1404200205.
52. Honarbakhsh S, Osman C, Teo JTH, Gabriel C. Ultrasound-guided lumbar puncture as a diagnostic aid to reduce number of attempts and complication rates. Ultrasound. 2013;21(4):170-175. https://doi.org/10.1177/1742271X13504332.
53. Sahota JS, Carvalho JC, Balki M, Fanning N, Arzola C. Ultrasound estimates for midline epidural punctures in the obese parturient: paramedian sagittal oblique is comparable to transverse median plane. Anesth Analg. 2013;116(4):829-835. https://doi.org/10.1213/ANE.0b013e31827f55f0.
54. Balki M, Lee Y, Halpern S, Carvalho JC. Ultrasound imaging of the lumbar spine in the transverse plane: the correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg. 2009;108(6):1876-1881. https://doi.org/10.1213/ane.0b013e3181a323f6.
55. Wallace DH, Currie JM, Gilstrap LC, Santos R. Indirect sonographic guidance for epidural anesthesia in obese pregnant patients. Reg Anesth. 1992;17(4):233-236. PubMed
56. Srinivasan KK, Iohom G, Loughnane F, Lee PJ. Conventional landmark-guided midline versus preprocedure ultrasound-guided paramedian techniques in spinal anesthesia. Anesth Analg. 2015;21(4):1089-1096. https://doi.org/10.1213/ANE.0000000000000911.
57. Chin KJ, Perlas A, Singh M, et al. An ultrasound-assisted approach facilitates spinal anesthesia for total joint arthroplasty. Can J Anaesth. 2009;56(9):643-650. https://doi.org/10.1007/s12630-009-9132-8.
58. Evansa I, Logina I, Vanags I, Borgeat A. Ultrasound versus fluoroscopic-guided epidural steroid injections in patients with degenerative spinal diseases: a randomised study. Eur J Anaesthesiol. 2015;32(4):262-268. https://doi.org/10.1097/EJA.0000000000000103.
59. Park Y, Lee JH, Park KD, et al. Ultrasound-guided vs fluoroscopy-guided caudal epidural steroid injection for the treatment of unilateral lower lumbar radicular pain: a prospective, randomized, single-blind clinical study. Am J Phys Med Rehabil. 2013;92(7):575-586. https://doi.org/10.1097/PHM.0b013e318292356b.
60. Margarido CB, Mikhael R, Arzola C, Balki M, Carvalho JC. The intercristal line determined by palpation is not a reliable anatomical landmark for neuraxial anesthesia. Can J Anaesth. 2011;58(3):262-266. https://doi.org/10.1007/s12630-010-9432-z.
61. Duniec L, Nowakowski P, Kosson D, Łazowski T. Anatomical landmarks based assessment of intravertebral space level for lumbar puncture is misleading in more than 30%. Anaesthesiol Intensive Ther. 2013;45(1):1-6. https://doi.org/10.5603/AIT.2013.0001.
62. Schlotterbeck H, Schaeffer R, Dow WA, et al. Ultrasonographic control of the puncture level for lumbar neuraxial block in obstetric anaesthesia. Br J Anaesth. 2008;100(2):230-234. https://doi.org/10.1093/bja/aem371.
63. Whitty R, Moore M, Macarthur A. Identification of the lumbar interspinous spaces: palpation versus ultrasound. Anesth Analg. 2008;106(2):538-540, table of contents. https://doi.org/10.1213/ane.0b013e31816069d9.
64. Locks Gde F, Almeida MC, Pereira AA. Use of the ultrasound to determine the level of lumbar puncture in pregnant women. Rev Bras Anestesiol. 2010;60(1):13-19. https://doi.org/10.1016/S0034-7094(10)70002-7.
65. Stiffler KA, Jwayyed S, Wilber ST, Robinson A. The use of ultrasound to identify pertinent landmarks for lumbar puncture. Am J Emerg Med. 2007;25(3):331-334. https://doi.org/10.1016/j.ajem.2006.07.010.
66. Gulay U, Meltem T, Nadir SS, Aysin A. Ultrasound-guided evaluation of the lumbar subarachnoid space in lateral and sitting positions in pregnant patients to receive elective cesarean operation. Pak J Med Sci. 2015;31(1):76-81. https://doi.org/10.12669/pjms.311.5647.
67. Kawaguchi R, Yamauchi M, Sugino S, Yamakage M. Ultrasound-aided ipsilateral-dominant epidural block for total hip arthroplasty: a randomised controlled single-blind study. Eur J Anaesthesiol. 2011;28(2):137-140. https://doi.org/10.1097/EJA.0b013e3283423457.
68. Grau T, Leipold RW, Horter J, Martin E, Motsch J. Colour Doppler imaging of the interspinous and epidural space. Eur J Anaesthesiol. 2001;18(11):706-712. https://doi.org/10.1097/00003643-200111000-00002.
69. Arzola C, Davies S, Rofaeel A, Carvalho JC. Ultrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epidurals. Anesth Analg. 2007;104(5):1188-92, tables of contents. https://doi.org/10.1213/01.ane.0000250912.66057.41.
70. Chauhan AK, Bhatia R, Agrawal S. Lumbar epidural depth using transverse ultrasound scan and its correlation with loss of resistance technique: a prospective observational study in Indian population. Saudi J Anaesth. 2018;12(2):279-282. https://doi.org/10.4103/sja.SJA_679_17.
71. Gnaho A, Nguyen V, Villevielle T, et al. Assessing the depth of the subarachnoid space by ultrasound. Rev Bras Anestesiol. 2012;62(4):520-530. https://doi.org/10.1016/S0034-7094(12)70150-2.
72. Cork RC, Kryc JJ, Vaughan RW. Ultrasonic localization of the lumbar epidural space. Anesthesiology. 1980;52(6):513-516. https://doi.org/10.1097/00000542-198006000-00013.
73. Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137. https://doi.org/10.1212/WNL.0b013e31825dd39d.
74. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. https://doi.org/10.3109/0142159X.2010.509412.
75. Wayne DB, Cohen ER, Singer BD, et al. Progress toward improving medical school graduates’ skills via a “boot camp” curriculum. Simul Healthc. 2014;9(1):33-39. https://doi.org/10.1097/SIH.0000000000000001.
76. Cohen ER, Barsuk JH, Moazed F, et al. Making July safer: simulation-based mastery learning during intern boot camp. Acad Med. 2013;88(2):233-239. https://doi.org/10.1097/ACM.0b013e31827bfc0a.
77. Martin R, Gannon D, Riggle J, et al. A comprehensive workshop using simulation to train internal medicine residents in bedside procedures performed by internists. Chest. 2012;142(4):545A. https://doi.org/10.1378/chest.1390093.
78. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
79. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1.
80. Restrepo CG, Baker MD, Pruitt CM, Gullett JP, Pigott DC. Ability of pediatric emergency medicine physicians to identify anatomic landmarks with the assistance of ultrasound prior to lumbar puncture in a simulated obese model. Pediatr Emerg Care. 2015;31(1):15-19. https://doi.org/10.1097/PEC.0000000000000330.
81. VanderWielen BA, Harris R, Galgon RE, VanderWielen LM, Schroeder KM. Teaching sonoanatomy to anesthesia faculty and residents: utility of hands-on gel phantom and instructional video training models. J Clin Anesth. 2015;27(3):188-194. https://doi.org/10.1016/j.jclinane.2014.07.007.
82. Keri Z, Sydor D, Ungi T, et al. Computerized training system for ultrasound-guided lumbar puncture on abnormal spine models: a randomized controlled trial. Can J Anaesth. 2015;62(7):777-784. https://doi.org/10.1007/s12630-015-0367-2.
83. Deacon AJ, Melhuishi NS, Terblanche NC. CUSUM method for construction of trainee spinal ultrasound learning curves following standardised teaching. Anaesth Intensive Care. 2014;42(4):480-486. https://doi.org/10.1177/0310057X1404200409.
84. Margarido CB, Arzola C, Balki M, Carvalho JC. Anesthesiologists’ learning curves for ultrasound assessment of the lumbar spine. Can J Anaesth. 2010;57(2):120-126. https://doi.org/10.1007/s12630-009-9219-2.
85. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
86. Terblanche NC, Arzola C, Wills KE, et al. Standardised training program in spinal ultrasound for epidural insertion: protocol driven versus non-protocol driven teaching approach. Anaesth Intensive Care. 2014;42(4):460-466. https://doi.org/10.1177/0310057X1404200406.
87. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
88. Karmakar MK, Li X, Ho AM, Kwok WH, Chui PT. Real-time ultrasound-guided paramedian epidural access: evaluation of a novel in-plane technique. Br J Anaesth. 2009;102(6):845-854. https://doi.org/10.1093/bja/aep079.
89. Tran D, Kamani AA, Al-Attas E, et al. Single-operator real-time ultrasound-guidance to aim and insert a lumbar epidural needle. Can J Anaesth. 2010;57(4):313-321. https://doi.org/10.1007/s12630-009-9252-1.
90. Liu Y, Qian W, Ke XJ, Mei W. Real-time ultrasound-guided spinal anesthesia using a new paramedian transverse approach. Curr Med Sci. 2018;38(5):910-913. https://doi.org/10.1007/s11596-018-1961-7.
91. Conroy PH, Luyet C, McCartney CJ, McHardy PG. Real-time ultrasound-guided spinal anaesthesia: a prospective observational study of a new approach. Anesthesiol Res Pract. 2013;2013:525818. https://doi.org/10.1155/2013/525818.
92. Brinkmann S, Tang R, Sawka A, Vaghadia H. Single-operator real-time ultrasound-guided spinal injection using SonixGPS™: a case series. Can J Anaesth. 2013;60(9):896-901. https://doi.org/10.1007/s12630-013-9984-9.
93. Niazi AU, Chin KJ, Jin R, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS ultrasound guidance system: a feasibility study. Acta Anaesthesiol Scand. 2014;58(7):875-881. https://doi.org/10.1111/aas.12353.
1. Wolfe KS, Kress JP. Risk of procedural hemorrhage. Chest. 2016;150(1):237-246. https://doi.org/10.1016/j.chest.2016.01.023.
2. Kroll H, Duszak R, Jr, Nsiah E, et al. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. AJR Am J Roentgenol. 2015;204(1):15-19. https://doi.org/10.2214/AJR.14.12622.
3. Vickers A, Donnelly JP, Moore JX, Wang HE. 263EMF epidemiology of lumbar punctures in hospitalized patients in United States. Ann Emerg Med. 2017;70(4):S104. https://doi.org/10.1016/j.annemergmed.2017.07.241.
4. Køster-Rasmussen R, Korshin A, Meyer CN. Antibiotic treatment delay and outcome in acute bacterial meningitis. J Infect. 2008;57(6):449-454. https://doi.org/10.1016/j.jinf.2008.09.033.
5. Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129(11):862-869. https://doi.org/10.7326/0003-4819-129-11_Part_1-199812010-00004.
6. Lepur D, Barsić B. Community-acquired bacterial meningitis in adults: antibiotic timing in disease course and outcome. Infection. 2007;35(4):225-231. https://doi.org/10.1007/s15010-007-6202-0.
7. Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM. 2005;98(4):291-298. https://doi.org/10.1093/qjmed/hci047.
8. Auburtin M, Wolff M, Charpentier J, et al. Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: the PNEUMOREA prospective multicenter study. Crit Care Med. 2006;34(11):2758-2765. https://doi.org/10.1097/01.CCM.0000239434.26669.65.
9. Michael B, Menezes BF, Cunniffe J, et al. Effect of delayed lumbar punctures on the diagnosis of acute bacterial meningitis in adults. Emerg Med J. 2010;27(6):433-438. https://doi.org/10.1136/emj.2009.075598.
10. Glimåker M, Johansson B, Grindborg Ö, et al. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis. 2015;60(8):1162-1169. https://doi.org/10.1093/cid/civ011.
11. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 Revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715.
12. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079.
13. Shah KH, McGillicuddy D, Spear J, Edlow JA. Predicting difficult and traumatic lumbar punctures. Am J Emerg Med. 2007;25(6):608-611. https://doi.org/10.1016/j.ajem.2006.11.025.
14. Williams P, Tait G, Wijeratne T. Success rate of elective lumbar puncture at a major Melbourne neurology unit. Surg Neurol Int. 2018;9:12. https://doi.org/10.4103/sni.sni_426_17.
15. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2018;26(1). https://doi.org/10.1111/acem.13558.
16. Shaikh F, Brzezinski J, Alexander S, et al. Ultrasound imaging for lumbar punctures and epidural catheterisations: systematic review and meta-analysis. BMJ. 2013;346:f1720. https://doi.org/10.1136/bmj.f1720.
17. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
18. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
19. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
20. Soni NJ, Franco-Sadud R, Schnobrich D, et al. Ultrasound guidance for lumbar puncture. Neurol Clin Pract. 2016;6(4):358-368. https://doi.org/10.1212/CPJ.0000000000000265.
21. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The Rand/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: Rand Corp; 2001.
22. Abdelhamid SA, Mansour MA. Ultrasound-guided intrathecal anesthesia: does scanning help? Egypt J Anaesth. 2013;29(4):389-394. https://doi.org/10.1016/j.egja.2013.06.003.
23. Ansari T, Yousef A, El Gamassy A, Fayez M. Ultrasound-guided spinal anaesthesia in obstetrics: is there an advantage over the landmark technique in patients with easily palpable spines? Int J Obstet Anesth. 2014;23(3):213-216. https://doi.org/10.1016/j.ijoa.2014.03.001.
24. Chin KJ, Perlas A, Chan V, et al. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology. 2011;115(1):94-101. https://doi.org/10.1097/ALN.0b013e31821a8ad4.
25. Cho YC, Koo DH, Oh SK, et al. Comparison of ultrasound-assisted lumbar puncture with lumbar puncture using palpation of landmarks in aged patients in an emergency center. J Korean Soc Emerg Med. 2009;20(3):304.
26. Grau T, Leipold RW, Conradi R, Martin E. Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol Scand. 2001;45(6):766-771. https://doi.org/10.1034/j.1399-6576.2001.045006766.x.
27. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Ultrasound imaging facilitates localization of the epidural space during combined spinal and epidural anesthesia. Reg Anesth Pain Med. 2001;26(1):64-67. https://doi.org/10.1053/rapm.2001.19633.
28. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Efficacy of ultrasound imaging in obstetric epidural anesthesia. J Clin Anesth. 2002;14(3):169-175. https://doi.org/10.1016/S0952-8180(01)00378-6.
29. Grau T, Leipold RW, Fatehi S, Martin E, Motsch J. Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur J Anaesthesiol. 2004;21(1):25-31. https://doi.org/10.1017/S026502150400105X.
30. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
31. Nassar M, Abdelazim IA. Pre-puncture ultrasound guided epidural insertion before vaginal delivery. J Clin Monit Comput. 2015;29(5):573-577. https://doi.org/10.1007/s10877-014-9634-y.
32. Nomura JT, Leech SJ, Shenbagamurthi S, et al. A randomized controlled trial of ultrasound-assisted lumbar puncture. J Ultrasound Med. 2007;26(10):1341-1348. https://doi.org/10.7863/jum.2007.26.10.1341.
33. Peterson MA, Pisupati D, Heyming TW, Abele JA, Lewis RJ. Ultrasound for routine lumbar puncture. Acad Emerg Med. 2014;21(2):130-136. https://doi.org/10.1111/acem.12305.
34. Sahin T, Balaban O, Sahin L, Solak M, Toker K. A randomized controlled trial of preinsertion ultrasound guidance for spinal anaesthesia in pregnancy: outcomes among obese and lean parturients: ultrasound for spinal anesthesia in pregnancy. J Anesth. 2014;28(3):413-419. https://doi.org/10.1007/s00540-013-1726-1.
35. Wang Q, Yin C, Wang TL. Ultrasound facilitates identification of combined spinal-epidural puncture in obese parturients. Chin Med J (Engl). 2012;125(21):3840-3843. PubMed
36. Vallejo MC, Phelps AL, Singh S, Orebaugh SL, Sah N. Ultrasound decreases the failed labor epidural rate in resident trainees. Int J Obstet Anesth. 2010;19(4):373-378. https://doi.org/10.1016/j.ijoa.2010.04.002.
37. Darrieutort-Laffite C, Bart G, Planche L, et al. Usefulness of a pre-procedure ultrasound scanning of the lumbar spine before epidural injection in patients with a presumed difficult puncture: a randomized controlled trial. Joint Bone Spine. 2015;82(5):356-361. https://doi.org/10.1016/j.jbspin.2015.02.001.
38. Vosko MR, Brunner C, Schreiber S. Lumbar puncture with ultrasound study (lupus study)-international prospective randomized multicentre trial. Int J Stroke. 2017;12(1):22. https://doi.org/10.1055/s-0037-1606991.
39. Urfalioğlu A, Bilal B, Öksüz G, et al. Comparison of the landmark and ultrasound methods in cesarean sections performed under spinal anesthesia on obese pregnants. J Matern Fetal Neonatal Med. 2017;30(9):1051-1056. https://doi.org/10.1080/14767058.2016.1199677.
40. Tawfik MM, Atallah MM, Elkharboutly WS, Allakkany NS, Abdelkhalek M. Does preprocedural ultrasound increase the first-pass success rate of epidural catheterization before cesarean delivery? A randomized controlled trial. Anesth Analg. 2017;124(3):851-856. https://doi.org/10.1213/ANE.0000000000001325.
41. Turkstra TP, Marmai KL, Armstrong KP, Kumar K, Singh SI. Preprocedural ultrasound assessment does not improve trainee performance of spinal anesthesia for obstetrical patients: a randomized controlled trial. J Clin Anesth. 2017;37:21-24. https://doi.org/10.1016/j.jclinane.2016.10.034.
42. Chong SE, Mohd Nikman A, Saedah A, et al. Real-time ultrasound-guided paramedian spinal anaesthesia: evaluation of the efficacy and the success rate of single needle pass. Br J Anaesth. 2017;118(5):799-801. https://doi.org/10.1093/bja/aex108.
43. Creaney M, Mullane D, Casby C, Tan T. Ultrasound to identify the lumbar space in women with impalpable bony landmarks presenting for elective caesarean delivery under spinal anaesthesia: a randomised trial. Int J Obstet Anesth. 2016;28:12-16. https://doi.org/10.1016/j.ijoa.2016.07.007.
44. Ekinci M, Alici HA, Ahiskalioglu A, et al. The use of ultrasound in planned cesarean delivery under spinal anesthesia for patients having nonprominent anatomic landmarks. J Clin Anesth. 2017;37:82-85. https://doi.org/10.1016/j.jclinane.2016.10.014.
45. Perna P, Gioia A, Ragazzi R, Volta CA, Innamorato M. Can pre-procedure neuroaxial ultrasound improve the identification of the potential epidural space when compared with anatomical landmarks? A prospective randomized study. Minerva Anestesiol. 2017;83(1):41-49. https://doi.org/10.23736/S0375-9393.16.11399-9.
46. Chin A, Crooke B, Heywood L, et al. A randomised controlled trial comparing needle movements during combined spinal-epidural anaesthesia with and without ultrasound assistance. Anaesthesia. 2018;73(4):466-473. https://doi.org/10.1111/anae.14206.
47. Dhanger S, Vinayagam S, Vaidhyanathan B, Rajesh IJ, Tripathy DK. Comparison of landmark versus pre-procedural ultrasonography-assisted midline approach for identification of subarachnoid space in elective caesarean section: a randomised controlled trial. Indian J Anaesth. 2018;62(4):280-284. https://doi.org/10.4103/ija.IJA_488_17.
48. Evans DP, Tozer J, Joyce M, Vitto MJ. Comparison of ultrasound-guided and landmark-based lumbar punctures in inexperienced resident physicians. J Ultrasound Med. 2019;38(3):613-620. https://doi.org/10.1002/jum.14728.
49. Srinivasan KK, Leo AM, Iohom G, Loughnane F, Lee PJ. Pre-procedure ultrasound-guided paramedian spinal anaesthesia at L5-S1: is this better than landmark-guided midline approach? A randomised controlled trial. Indian J Anaesth. 2018;62(1):53-60. https://doi.org/10.4103/ija.IJA_448_17.
50. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
51. Lim YC, Choo CY, Tan KT. A randomised controlled trial of ultrasound-assisted spinal anaesthesia. Anaesth Intensive Care. 2014;42(2):191-198. https://doi.org/10.1177/0310057X1404200205.
52. Honarbakhsh S, Osman C, Teo JTH, Gabriel C. Ultrasound-guided lumbar puncture as a diagnostic aid to reduce number of attempts and complication rates. Ultrasound. 2013;21(4):170-175. https://doi.org/10.1177/1742271X13504332.
53. Sahota JS, Carvalho JC, Balki M, Fanning N, Arzola C. Ultrasound estimates for midline epidural punctures in the obese parturient: paramedian sagittal oblique is comparable to transverse median plane. Anesth Analg. 2013;116(4):829-835. https://doi.org/10.1213/ANE.0b013e31827f55f0.
54. Balki M, Lee Y, Halpern S, Carvalho JC. Ultrasound imaging of the lumbar spine in the transverse plane: the correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg. 2009;108(6):1876-1881. https://doi.org/10.1213/ane.0b013e3181a323f6.
55. Wallace DH, Currie JM, Gilstrap LC, Santos R. Indirect sonographic guidance for epidural anesthesia in obese pregnant patients. Reg Anesth. 1992;17(4):233-236. PubMed
56. Srinivasan KK, Iohom G, Loughnane F, Lee PJ. Conventional landmark-guided midline versus preprocedure ultrasound-guided paramedian techniques in spinal anesthesia. Anesth Analg. 2015;21(4):1089-1096. https://doi.org/10.1213/ANE.0000000000000911.
57. Chin KJ, Perlas A, Singh M, et al. An ultrasound-assisted approach facilitates spinal anesthesia for total joint arthroplasty. Can J Anaesth. 2009;56(9):643-650. https://doi.org/10.1007/s12630-009-9132-8.
58. Evansa I, Logina I, Vanags I, Borgeat A. Ultrasound versus fluoroscopic-guided epidural steroid injections in patients with degenerative spinal diseases: a randomised study. Eur J Anaesthesiol. 2015;32(4):262-268. https://doi.org/10.1097/EJA.0000000000000103.
59. Park Y, Lee JH, Park KD, et al. Ultrasound-guided vs fluoroscopy-guided caudal epidural steroid injection for the treatment of unilateral lower lumbar radicular pain: a prospective, randomized, single-blind clinical study. Am J Phys Med Rehabil. 2013;92(7):575-586. https://doi.org/10.1097/PHM.0b013e318292356b.
60. Margarido CB, Mikhael R, Arzola C, Balki M, Carvalho JC. The intercristal line determined by palpation is not a reliable anatomical landmark for neuraxial anesthesia. Can J Anaesth. 2011;58(3):262-266. https://doi.org/10.1007/s12630-010-9432-z.
61. Duniec L, Nowakowski P, Kosson D, Łazowski T. Anatomical landmarks based assessment of intravertebral space level for lumbar puncture is misleading in more than 30%. Anaesthesiol Intensive Ther. 2013;45(1):1-6. https://doi.org/10.5603/AIT.2013.0001.
62. Schlotterbeck H, Schaeffer R, Dow WA, et al. Ultrasonographic control of the puncture level for lumbar neuraxial block in obstetric anaesthesia. Br J Anaesth. 2008;100(2):230-234. https://doi.org/10.1093/bja/aem371.
63. Whitty R, Moore M, Macarthur A. Identification of the lumbar interspinous spaces: palpation versus ultrasound. Anesth Analg. 2008;106(2):538-540, table of contents. https://doi.org/10.1213/ane.0b013e31816069d9.
64. Locks Gde F, Almeida MC, Pereira AA. Use of the ultrasound to determine the level of lumbar puncture in pregnant women. Rev Bras Anestesiol. 2010;60(1):13-19. https://doi.org/10.1016/S0034-7094(10)70002-7.
65. Stiffler KA, Jwayyed S, Wilber ST, Robinson A. The use of ultrasound to identify pertinent landmarks for lumbar puncture. Am J Emerg Med. 2007;25(3):331-334. https://doi.org/10.1016/j.ajem.2006.07.010.
66. Gulay U, Meltem T, Nadir SS, Aysin A. Ultrasound-guided evaluation of the lumbar subarachnoid space in lateral and sitting positions in pregnant patients to receive elective cesarean operation. Pak J Med Sci. 2015;31(1):76-81. https://doi.org/10.12669/pjms.311.5647.
67. Kawaguchi R, Yamauchi M, Sugino S, Yamakage M. Ultrasound-aided ipsilateral-dominant epidural block for total hip arthroplasty: a randomised controlled single-blind study. Eur J Anaesthesiol. 2011;28(2):137-140. https://doi.org/10.1097/EJA.0b013e3283423457.
68. Grau T, Leipold RW, Horter J, Martin E, Motsch J. Colour Doppler imaging of the interspinous and epidural space. Eur J Anaesthesiol. 2001;18(11):706-712. https://doi.org/10.1097/00003643-200111000-00002.
69. Arzola C, Davies S, Rofaeel A, Carvalho JC. Ultrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epidurals. Anesth Analg. 2007;104(5):1188-92, tables of contents. https://doi.org/10.1213/01.ane.0000250912.66057.41.
70. Chauhan AK, Bhatia R, Agrawal S. Lumbar epidural depth using transverse ultrasound scan and its correlation with loss of resistance technique: a prospective observational study in Indian population. Saudi J Anaesth. 2018;12(2):279-282. https://doi.org/10.4103/sja.SJA_679_17.
71. Gnaho A, Nguyen V, Villevielle T, et al. Assessing the depth of the subarachnoid space by ultrasound. Rev Bras Anestesiol. 2012;62(4):520-530. https://doi.org/10.1016/S0034-7094(12)70150-2.
72. Cork RC, Kryc JJ, Vaughan RW. Ultrasonic localization of the lumbar epidural space. Anesthesiology. 1980;52(6):513-516. https://doi.org/10.1097/00000542-198006000-00013.
73. Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137. https://doi.org/10.1212/WNL.0b013e31825dd39d.
74. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. https://doi.org/10.3109/0142159X.2010.509412.
75. Wayne DB, Cohen ER, Singer BD, et al. Progress toward improving medical school graduates’ skills via a “boot camp” curriculum. Simul Healthc. 2014;9(1):33-39. https://doi.org/10.1097/SIH.0000000000000001.
76. Cohen ER, Barsuk JH, Moazed F, et al. Making July safer: simulation-based mastery learning during intern boot camp. Acad Med. 2013;88(2):233-239. https://doi.org/10.1097/ACM.0b013e31827bfc0a.
77. Martin R, Gannon D, Riggle J, et al. A comprehensive workshop using simulation to train internal medicine residents in bedside procedures performed by internists. Chest. 2012;142(4):545A. https://doi.org/10.1378/chest.1390093.
78. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
79. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1.
80. Restrepo CG, Baker MD, Pruitt CM, Gullett JP, Pigott DC. Ability of pediatric emergency medicine physicians to identify anatomic landmarks with the assistance of ultrasound prior to lumbar puncture in a simulated obese model. Pediatr Emerg Care. 2015;31(1):15-19. https://doi.org/10.1097/PEC.0000000000000330.
81. VanderWielen BA, Harris R, Galgon RE, VanderWielen LM, Schroeder KM. Teaching sonoanatomy to anesthesia faculty and residents: utility of hands-on gel phantom and instructional video training models. J Clin Anesth. 2015;27(3):188-194. https://doi.org/10.1016/j.jclinane.2014.07.007.
82. Keri Z, Sydor D, Ungi T, et al. Computerized training system for ultrasound-guided lumbar puncture on abnormal spine models: a randomized controlled trial. Can J Anaesth. 2015;62(7):777-784. https://doi.org/10.1007/s12630-015-0367-2.
83. Deacon AJ, Melhuishi NS, Terblanche NC. CUSUM method for construction of trainee spinal ultrasound learning curves following standardised teaching. Anaesth Intensive Care. 2014;42(4):480-486. https://doi.org/10.1177/0310057X1404200409.
84. Margarido CB, Arzola C, Balki M, Carvalho JC. Anesthesiologists’ learning curves for ultrasound assessment of the lumbar spine. Can J Anaesth. 2010;57(2):120-126. https://doi.org/10.1007/s12630-009-9219-2.
85. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
86. Terblanche NC, Arzola C, Wills KE, et al. Standardised training program in spinal ultrasound for epidural insertion: protocol driven versus non-protocol driven teaching approach. Anaesth Intensive Care. 2014;42(4):460-466. https://doi.org/10.1177/0310057X1404200406.
87. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
88. Karmakar MK, Li X, Ho AM, Kwok WH, Chui PT. Real-time ultrasound-guided paramedian epidural access: evaluation of a novel in-plane technique. Br J Anaesth. 2009;102(6):845-854. https://doi.org/10.1093/bja/aep079.
89. Tran D, Kamani AA, Al-Attas E, et al. Single-operator real-time ultrasound-guidance to aim and insert a lumbar epidural needle. Can J Anaesth. 2010;57(4):313-321. https://doi.org/10.1007/s12630-009-9252-1.
90. Liu Y, Qian W, Ke XJ, Mei W. Real-time ultrasound-guided spinal anesthesia using a new paramedian transverse approach. Curr Med Sci. 2018;38(5):910-913. https://doi.org/10.1007/s11596-018-1961-7.
91. Conroy PH, Luyet C, McCartney CJ, McHardy PG. Real-time ultrasound-guided spinal anaesthesia: a prospective observational study of a new approach. Anesthesiol Res Pract. 2013;2013:525818. https://doi.org/10.1155/2013/525818.
92. Brinkmann S, Tang R, Sawka A, Vaghadia H. Single-operator real-time ultrasound-guided spinal injection using SonixGPS™: a case series. Can J Anaesth. 2013;60(9):896-901. https://doi.org/10.1007/s12630-013-9984-9.
93. Niazi AU, Chin KJ, Jin R, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS ultrasound guidance system: a feasibility study. Acta Anaesthesiol Scand. 2014;58(7):875-881. https://doi.org/10.1111/aas.12353.
© 2019 Society of Hospital Medicine
Point-of-Care Ultrasound for Hospitalists: A Position Statement of the Society of Hospital Medicine
Many hospitalists incorporate point-of-care ultrasound (POCUS) into their daily practice because it adds value to their bedside evaluation of patients. However, standards for training and assessing hospitalists in POCUS have not yet been established. Other acute care specialties, including emergency medicine and critical care medicine, have already incorporated POCUS into their graduate medical education training programs, but most internal medicine residency programs are only beginning to provide POCUS training.1
Several features distinguish POCUS from comprehensive ultrasound examinations. First, POCUS is designed to answer focused questions, whereas comprehensive ultrasound examinations evaluate all organs in an anatomical region; for example, an abdominal POCUS exam may evaluate only for presence or absence of intraperitoneal free fluid, whereas a comprehensive examination of the right upper quadrant will evaluate the liver, gallbladder, and biliary ducts. Second, POCUS examinations are generally performed by the same clinician who generates the relevant clinical question to answer with POCUS and ultimately integrates the findings into the patient’s care.2 By contrast, comprehensive ultrasound examinations involve multiple providers and steps: a clinician generates a relevant clinical question and requests an ultrasound examination that is acquired by a sonographer, interpreted by a radiologist, and reported back to the requesting clinician. Third, POCUS is often used to evaluate multiple body systems. For example, to evaluate a patient with undifferentiated hypotension, a multisystem POCUS examination of the heart, inferior vena cava, lungs, abdomen, and lower extremity veins is typically performed. Finally, POCUS examinations can be performed serially to investigate changes in clinical status or evaluate response to therapy, such as monitoring the heart, lungs, and inferior vena cava during fluid resuscitation.
The purpose of this position statement is to inform a broad audience about how hospitalists are using diagnostic and procedural applications of POCUS. This position statement does not mandate that hospitalists use POCUS. Rather, it is intended to provide guidance on the safe and effective use of POCUS by the hospitalists who use it and the administrators who oversee its use. We discuss POCUS (1) applications, (2) training, (3) assessments, and (4) program management. This position statement was reviewed and approved by the Society of Hospital Medicine (SHM) Executive Committee in March 2018.
APPLICATIONS
As outlined in our earlier position statements,3,4 ultrasound guidance lowers complication rates and increases success rates of invasive bedside procedures. Diagnostic POCUS can guide clinical decision making prior to bedside procedures. For instance, hospitalists may use POCUS to assess the size and character of a pleural effusion to help determine the most appropriate management strategy: observation, medical treatment, thoracentesis, chest tube placement, or surgical therapy. Furthermore, diagnostic POCUS can be used to rapidly assess for immediate postprocedural complications, such as pneumothorax, or if the patient develops new symptoms.
TRAINING
Basic Knowledge
Basic knowledge includes fundamentals of ultrasound physics; safety;4 anatomy; physiology; and device operation, including maintenance and cleaning. Basic knowledge can be taught by multiple methods, including live or recorded lectures, online modules, or directed readings.
Image Acquisition
Training should occur across multiple types of patients (eg, obese, cachectic, postsurgical) and clinical settings (eg, intensive care unit, general medicine wards, emergency department) when available. Training is largely hands-on because the relevant skills involve integration of 3D anatomy with spatial manipulation, hand-eye coordination, and fine motor movements. Virtual reality ultrasound simulators may accelerate mastery, particularly for cardiac image acquisition, and expose learners to standardized sets of pathologic findings. Real-time bedside feedback on image acquisition is ideal because understanding how ultrasound probe manipulation affects the images acquired is essential to learning.
Image Interpretation
Training in image interpretation relies on visual pattern recognition of normal and abnormal findings. Therefore, the normal to abnormal spectrum should be broad, and learners should maintain a log of what abnormalities have been identified. Giving real-time feedback at the bedside is ideal because of the connection between image acquisition and interpretation. Image interpretation can be taught through didactic sessions, image review sessions, or review of teaching files with annotated images.
Clinical Integration
Learners must interpret and integrate image findings with other clinical data considering the image quality, patient characteristics, and changing physiology. Clinical integration should be taught by instructors that share similar clinical knowledge as learners. Although sonographers are well suited to teach image acquisition, they should not be the sole instructors to teach hospitalists how to integrate ultrasound findings in clinical decision making. Likewise, emphasis should be placed on the appropriate use of POCUS within a provider’s skill set. Learners must appreciate the clinical significance of POCUS findings, including recognition of incidental findings that may require further workup. Supplemental training in clinical integration can occur through didactics that include complex patient scenarios.
Pathways
Clinical competency can be achieved with training adherent to five criteria. First, the training environment should be similar to where the trainee will practice. Second, training and feedback should occur in real time. Third, specific applications should be taught rather than broad training in “hospitalist POCUS.” Each application requires unique skills and knowledge, including image acquisition pitfalls and artifacts. Fourth, clinical competence must be achieved and demonstrated; it is not necessarily gained through experience. Fifth, once competency is achieved, continued education and feedback are necessary to ensure it is maintained.
Residency-based POCUS training pathways can best fulfill these criteria. They may eventually become commonplace, but until then alternative pathways must exist for hospitalist providers who are already in practice. There are three important attributes of such pathways. First, administrators’ expectations about learners’ clinical productivity must be realistically, but only temporarily, relaxed; otherwise, competing demands on time will likely overwhelm learners and subvert training. Second, training should begin through a local or national hands-on training program. The SHM POCUS certificate program consolidates training for common diagnostic POCUS applications for hospitalists.6 Other medical societies offer training for their respective clinical specialties.7 Third, once basic POCUS training has begun, longitudinal training should continue ideally with a local hospitalist POCUS expert.
In some settings, a subgroup of hospitalists may not desire, or be able to achieve, competency in the manual skills of POCUS image acquisition. Nevertheless, hospitalists may still find value in understanding POCUS nomenclature, image pattern recognition, and the evidence and pitfalls behind clinical integration of specific POCUS findings. This subset of POCUS skills allows hospitalists to communicate effectively with and understand the clinical decisions made by their colleagues who are competent in POCUS use.
The minimal skills a hospitalist should possess to serve as a POCUS trainer include proficiency of basic knowledge, image acquisition, image interpretation, and clinical integration of the POCUS applications being taught; effectiveness as a hands-on instructor to teach image acquisition skills; and an in-depth understanding of common POCUS pitfalls and limitations.
ASSESSMENTS
Assessment methods for POCUS can include the following: knowledge-based questions, image acquisition using task-specific checklists on human or simulation models, image interpretation using a series of videos or still images with normal and abnormal findings, clinical integration using “next best step” in a multiple choice format with POCUS images, and simulation-based clinical scenarios. Assessment methods should be aligned with local availability of resources and trainers.
Basic Knowledge
Basic knowledge can be assessed via multiple choice questions assessing knowledge of ultrasound physics, image optimization, relevant anatomy, and limitations of POCUS imaging. Basic knowledge lies primarily in the cognitive domain and does not assess manual skills.
Image Acquisition
Image acquisition can be assessed by observation and rating of image quality. Where resources allow, assessment of image acquisition is likely best done through a combination of developing an image portfolio with a minimum number of high quality images, plus direct observation of image acquisition by an expert. Various programs have utilized minimum numbers of images acquired to help define competence with image acquisition skills.6–8 Although minimums may be a necessary step to gain competence, using them as a sole means to determine competence does not account for variable learning curves.9 As with other manual skills in hospital medicine, such as ultrasound-guided bedside procedures, minimum numbers are best used as a starting point for assessments.3,10 In this regard, portfolio development with meticulous attention to the gain, depth, and proper tomographic plane of images can monitor a hospitalist’s progress toward competence by providing objective assessments and feedback. Simulation may also be used as it allows assessment of image acquisition skills and an opportunity to provide real-time feedback, similar to direct observation but without actual patients.
Image Interpretation
Image interpretation is best assessed by an expert observing the learner at bedside; however, when bedside assessment is not possible, image interpretation skills may be assessed using multiple choice or free text interpretation of archived ultrasound images with normal and abnormal findings. This is often incorporated into the portfolio development portion of a training program, as learners can submit their image interpretation along with the video clip. Both normal and abnormal images can be used to assess anatomic recognition and interpretation. Emphasis should be placed on determining when an image is suboptimal for diagnosis (eg, incomplete exam or poor-quality images). Quality assurance programs should incorporate structured feedback sessions.
Clinical Integration
Assessment of clinical integration can be completed through case scenarios that assess knowledge, interpretation of images, and integration of findings into clinical decision making, which is often delivered via a computer-based assessment. Assessments should combine specific POCUS applications to evaluate common clinical problems in hospital medicine, such as undifferentiated hypotension and dyspnea. High-fidelity simulators can be used to blend clinical case scenarios with image acquisition, image interpretation, and clinical integration. When feasible, comprehensive feedback on how providers acquire, interpret, and apply ultrasound at the bedside is likely the best mechanism to assess clinical integration. This process can be done with a hospitalist’s own patients.
General Assessment
A general assessment that includes a summative knowledge and hands-on skills assessment using task-specific checklists can be performed upon completion of training. A high-fidelity simulator with dynamic or virtual anatomy can provide reproducible standardized assessments with variation in the type and difficulty of cases. When available, we encourage the use of dynamic assessments on actual patients that have both normal and abnormal ultrasound findings because simulated patient scenarios have limitations, even with the use of high-fidelity simulators. Programs are recommended to use formative and summative assessments for evaluation. Quantitative scoring systems using checklists are likely the best framework.11,12
CERTIFICATES AND CERTIFICATION
A certificate of completion is proof of a provider’s participation in an educational activity; it does not equate with competency, though it may be a step toward it. Most POCUS training workshops and short courses provide certificates of completion. Certification of competency is an attestation of a hospitalist’s basic competence within a defined scope of practice (Table 2).13 However, without longitudinal supervision and feedback, skills can decay; therefore, we recommend a longitudinal training program that provides mentored feedback and incorporates periodic competency assessments. At present, no national board certification in POCUS is available to grant external certification of competency for hospitalists.
External Certificate
Certificates of completion can be external through a national organization. An external certificate of completion designed for hospitalists includes the POCUS Certificate of Completion offered by SHM in collaboration with CHEST.6 This certificate program provides regional training options and longitudinal portfolio development. Other external certificates are also available to hospitalists.7,14,15
Most hospitalists are boarded by the American Board of Internal Medicine or the American Board of Family Medicine. These boards do not yet include certification of competency in POCUS. Other specialty boards, such as emergency medicine, include competency in POCUS. For emergency medicine, completion of an accredited residency training program and certification by the national board includes POCUS competency.
Internal Certificate
There are a few examples of successful local institutional programs that have provided internal certificates of competency.12,14 Competency assessments require significant resources including investment by both faculty and learners. Ongoing evaluation of competency should be based on quality assurance processes.
Credentialing and Privileging
The American Medical Association (AMA) House of Delegates in 1999 passed a resolution (AMA HR. 802) recommending hospitals follow specialty-specific guidelines for privileging decisions related to POCUS use.17 The resolution included a statement that, “ultrasound imaging is within the scope of practice of appropriately trained physicians.”
Some institutions have begun to rely on a combination of internal and external certificate programs to grant privileges to hospitalists.10 Although specific privileges for POCUS may not be required in some hospitals, some institutions may require certification of training and assessments prior to granting permission to use POCUS.
Hospitalist programs are encouraged to evaluate ongoing POCUS use by their providers after granting initial permission. If privileging is instituted by a hospital, hospitalists must play a significant role in determining the requirements for privileging and ongoing maintenance of skills.
Maintenance of Skills
All medical skills can decay with disuse, including those associated with POCUS.12,18 Thus, POCUS users should continue using POCUS regularly in clinical practice and participate in POCUS continuing medical education activities, ideally with ongoing assessments. Maintenance of skills may be confirmed through routine participation in a quality assurance program.
PROGRAM MANAGEMENT
Use of POCUS in hospital medicine has unique considerations, and hospitalists should be integrally involved in decision making surrounding institutional POCUS program management. Appointing a dedicated POCUS director can help a program succeed.8
Equipment and Image Archiving
Several factors are important to consider when selecting an ultrasound machine: portability, screen size, and ease of use; integration with the electronic medical record and options for image archiving; manufacturer’s service plan, including technical and clinical support; and compliance with local infection control policies. The ability to easily archive and retrieve images is essential for quality assurance, continuing education, institutional quality improvement, documentation, and reimbursement. In certain scenarios, image archiving may not be possible (such as with personal handheld devices or in emergency situations) or necessary (such as with frequent serial examinations during fluid resuscitation). An image archive is ideally linked to reports, orders, and billing software.10,19 If such linkages are not feasible, parallel external storage that complies with regulatory standards (ie, HIPAA compliance) may be suitable.20
Documentation and Billing
Components of documentation include the indication and type of ultrasound examination performed, date and time of the examination, patient identifying information, name of provider(s) acquiring and interpreting the images, specific scanning protocols used, patient position, probe used, and findings. Documentation can occur through a standalone note or as part of another note, such as a progress note. Whenever possible, documentation should be timely to facilitate communication with other providers.
Billing is supported through the AMA Current Procedural Terminology codes for “focused” or “limited” ultrasound examinations (Appendix 9). The following three criteria must be satisfied for billing. First, images must be permanently stored. Specific requirements vary by insurance policy, though current practice suggests a minimum of one image demonstrating relevant anatomy and pathology for the ultrasound examination coded. For ultrasound-guided procedures that require needle insertion, images should be captured at the point of interest, and a procedure note should reflect that the needle was guided and visualized under ultrasound.21 Second, proper documentation must be entered in the medical record. Third, local institutional privileges for POCUS must be considered. Although privileges are not required to bill, some hospitals or payers may require them.
Quality Assurance
Published guidelines on quality assurance in POCUS are available from different specialty organizations, including emergency medicine, pediatric emergency medicine, critical care, anesthesiology, obstetrics, and cardiology.8,22–28 Quality assurance is aimed at ensuring that physicians maintain basic competency in using POCUS to influence bedside decisions.
Quality assurance should be carried out by an individual or committee with expertise in POCUS. Multidisciplinary QA programs in which hospital medicine providers are working collaboratively with other POCUS providers have been demonstrated to be highly effective.10 Oversight includes ensuring that providers using POCUS are appropriately trained,10,22,28 using the equipment correctly,8,26,28 and documenting properly. Some programs have implemented mechanisms to review and provide feedback on image acquisition, interpretation, and clinical integration.8,10 Other programs have compared POCUS findings with referral studies, such as comprehensive ultrasound examinations.
CONCLUSIONS
Practicing hospitalists must continue to collaborate with their institutions to build POCUS capabilities. In particular, they must work with their local privileging body to determine what credentials are required. The distinction between certificates of completion and certificates of competency, including whether those certificates are internal or external, is important in the credentialing process.
External certificates of competency are currently unavailable for most practicing hospitalists because ABIM certification does not include POCUS-related competencies. As internal medicine residency training programs begin to adopt POCUS training and certification into their educational curricula, we foresee a need to update the ABIM Policies and Procedures for Certification. Until then, we recommend that certificates of competency be defined and granted internally by local hospitalist groups.
Given the many advantages of POCUS over traditional tools, we anticipate its increasing implementation among hospitalists in the future. As with all medical technology, its role in clinical care should be continuously reexamined and redefined through health services research. Such information will be useful in developing practice guidelines, educational curricula, and training standards.
Acknowledgments
The authors would like to thank all members that participated in the discussion and finalization of this position statement during the Point-of-care Ultrasound Faculty Retreat at the 2018 Society of Hospital Medicine Annual Conference: Saaid Abdel-Ghani, Brandon Boesch, Joel Cho, Ria Dancel, Renee Dversdal, Ricardo Franco-Sadud, Benjamin Galen, Trevor P. Jensen, Mohit Jindal, Gordon Johnson, Linda M. Kurian, Gigi Liu, Charles M. LoPresti, Brian P. Lucas, Venkat Kalidindi, Benji Matthews, Anna Maw, Gregory Mints, Kreegan Reierson, Gerard Salame, Richard Schildhouse, Daniel Schnobrich, Nilam Soni, Kirk Spencer, Hiromizu Takahashi, David M. Tierney, Tanping Wong, and Toru Yamada.
1. Schnobrich DJ, Mathews BK, Trappey BE, Muthyala BK, Olson APJ. Entrusting internal medicine residents to use point of care ultrasound: Towards improved assessment and supervision. Med Teach. 2018:1-6. doi:10.1080/0142159X.2018.1457210.
2. Soni NJ, Lucas BP. Diagnostic point-of-care ultrasound for hospitalists. J Hosp Med. 2015;10(2):120-124. doi:10.1002/jhm.2285.
3. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):117-125. doi:10.12788/jhm.2917.
4. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):126-135. doi:10.12788/jhm.2940.
5. National Council on Radiation Protection and Measurements, The Council. Implementation of the Principle of as Low as Reasonably Achievable (ALARA) for Medical and Dental Personnel.; 1990.
6. Society of Hospital Medicine. Point of Care Ultrasound course: https://www.hospitalmedicine.org/clinical-topics/ultrasonography-cert/. Accessed February 6, 2018.
7. Critical Care Ultrasonography Certificate of Completion Program. CHEST. American College of Chest Physicians. http://www.chestnet.org/Education/Advanced-Clinical-Training/Certificate-of-Completion-Program/Critical-Care-Ultrasonography. Accessed February 6, 2018.
8. American College of Emergency Physicians Policy Statement: Emergency Ultrasound Guidelines. 2016. https://www.acep.org/Clinical---Practice-Management/ACEP-Ultrasound-Guidelines/. Accessed February 6, 2018.
9. Blehar DJ, Barton B, Gaspari RJ. Learning curves in emergency ultrasound education. Acad Emerg Med. 2015;22(5):574-582. doi:10.1111/acem.12653.
10. Mathews BK, Zwank M. Hospital medicine point of care ultrasound credentialing: an example protocol. J Hosp Med. 2017;12(9):767-772. doi:10.12788/jhm.2809.
11. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. doi:10.1002/jhm.468.
12. Mathews BK, Reierson K, Vuong K, et al. The design and evaluation of the Comprehensive Hospitalist Assessment and Mentorship with Portfolios (CHAMP) ultrasound program. J Hosp Med. 2018;13(8):544-550. doi:10.12788/jhm.2938.
13. Soni NJ, Tierney DM, Jensen TP, Lucas BP. Certification of point-of-care ultrasound competency. J Hosp Med. 2017;12(9):775-776. doi:10.12788/jhm.2812.
14. Ultrasound Certification for Physicians. Alliance for Physician Certification and Advancement. APCA. https://apca.org/. Accessed February 6, 2018.
15. National Board of Echocardiography, Inc. https://www.echoboards.org/EchoBoards/News/2019_Adult_Critical_Care_Echocardiography_Exam.aspx. Accessed June 18, 2018.
16. Tierney DM. Internal Medicine Bedside Ultrasound Program (IMBUS). Abbott Northwestern. http://imbus.anwresidency.com/index.html. Accessed February 6, 2018.
17. American Medical Association House of Delegates Resolution H-230.960: Privileging for Ultrasound Imaging. Resolution 802. Policy Finder Website. http://search0.ama-assn.org/search/pfonline. Published 1999. Accessed February 18, 2018.
18. Kelm D, Ratelle J, Azeem N, et al. Longitudinal ultrasound curriculum improves long-term retention among internal medicine residents. J Grad Med Educ. 2015;7(3):454-457. doi:10.4300/JGME-14-00284.1.
19. Flannigan MJ, Adhikari S. Point-of-care ultrasound work flow innovation: impact on documentation and billing. J Ultrasound Med. 2017;36(12):2467-2474. doi:10.1002/jum.14284.
20. Emergency Ultrasound: Workflow White Paper. https://www.acep.org/uploadedFiles/ACEP/memberCenter/SectionsofMembership/ultra/Workflow%20White%20Paper.pdf. Published 2013. Accessed February 18, 2018.
21. Ultrasound Coding and Reimbursement Document 2009. Emergency Ultrasound Section. American College of Emergency Physicians. http://emergencyultrasoundteaching.com/assets/2009_coding_update.pdf. Published 2009. Accessed February 18, 2018.
22. Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/La Societe de Reanimation de Langue Francaise statement on competence in critical care ultrasonography. Chest. 2009;135(4):1050-1060. doi:10.1378/chest.08-2305.
23. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: general ultrasonography. Crit Care Med. 2015;43(11):2479-2502. doi:10.1097/ccm.0000000000001216.
24. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part ii: cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-1227. doi:10.1097/ccm.0000000000001847.
25. ACR–ACOG–AIUM–SRU Practice Parameter for the Performance of Obstetrical Ultrasound. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/us-ob.pdf. Published 2013. Accessed February 18, 2018.
26. AIUM practice guideline for documentation of an ultrasound examination. J Ultrasound Med. 2014;33(6):1098-1102. doi:10.7863/ultra.33.6.1098.
27. Marin JR, Lewiss RE. Point-of-care ultrasonography by pediatric emergency medicine physicians. Pediatrics. 2015;135(4):e1113-e1122. doi:10.1542/peds.2015-0343.
28. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(6):567-581. doi:10.1016/j.echo.2013.04.001.
Many hospitalists incorporate point-of-care ultrasound (POCUS) into their daily practice because it adds value to their bedside evaluation of patients. However, standards for training and assessing hospitalists in POCUS have not yet been established. Other acute care specialties, including emergency medicine and critical care medicine, have already incorporated POCUS into their graduate medical education training programs, but most internal medicine residency programs are only beginning to provide POCUS training.1
Several features distinguish POCUS from comprehensive ultrasound examinations. First, POCUS is designed to answer focused questions, whereas comprehensive ultrasound examinations evaluate all organs in an anatomical region; for example, an abdominal POCUS exam may evaluate only for presence or absence of intraperitoneal free fluid, whereas a comprehensive examination of the right upper quadrant will evaluate the liver, gallbladder, and biliary ducts. Second, POCUS examinations are generally performed by the same clinician who generates the relevant clinical question to answer with POCUS and ultimately integrates the findings into the patient’s care.2 By contrast, comprehensive ultrasound examinations involve multiple providers and steps: a clinician generates a relevant clinical question and requests an ultrasound examination that is acquired by a sonographer, interpreted by a radiologist, and reported back to the requesting clinician. Third, POCUS is often used to evaluate multiple body systems. For example, to evaluate a patient with undifferentiated hypotension, a multisystem POCUS examination of the heart, inferior vena cava, lungs, abdomen, and lower extremity veins is typically performed. Finally, POCUS examinations can be performed serially to investigate changes in clinical status or evaluate response to therapy, such as monitoring the heart, lungs, and inferior vena cava during fluid resuscitation.
The purpose of this position statement is to inform a broad audience about how hospitalists are using diagnostic and procedural applications of POCUS. This position statement does not mandate that hospitalists use POCUS. Rather, it is intended to provide guidance on the safe and effective use of POCUS by the hospitalists who use it and the administrators who oversee its use. We discuss POCUS (1) applications, (2) training, (3) assessments, and (4) program management. This position statement was reviewed and approved by the Society of Hospital Medicine (SHM) Executive Committee in March 2018.
APPLICATIONS
As outlined in our earlier position statements,3,4 ultrasound guidance lowers complication rates and increases success rates of invasive bedside procedures. Diagnostic POCUS can guide clinical decision making prior to bedside procedures. For instance, hospitalists may use POCUS to assess the size and character of a pleural effusion to help determine the most appropriate management strategy: observation, medical treatment, thoracentesis, chest tube placement, or surgical therapy. Furthermore, diagnostic POCUS can be used to rapidly assess for immediate postprocedural complications, such as pneumothorax, or if the patient develops new symptoms.
TRAINING
Basic Knowledge
Basic knowledge includes fundamentals of ultrasound physics; safety;4 anatomy; physiology; and device operation, including maintenance and cleaning. Basic knowledge can be taught by multiple methods, including live or recorded lectures, online modules, or directed readings.
Image Acquisition
Training should occur across multiple types of patients (eg, obese, cachectic, postsurgical) and clinical settings (eg, intensive care unit, general medicine wards, emergency department) when available. Training is largely hands-on because the relevant skills involve integration of 3D anatomy with spatial manipulation, hand-eye coordination, and fine motor movements. Virtual reality ultrasound simulators may accelerate mastery, particularly for cardiac image acquisition, and expose learners to standardized sets of pathologic findings. Real-time bedside feedback on image acquisition is ideal because understanding how ultrasound probe manipulation affects the images acquired is essential to learning.
Image Interpretation
Training in image interpretation relies on visual pattern recognition of normal and abnormal findings. Therefore, the normal to abnormal spectrum should be broad, and learners should maintain a log of what abnormalities have been identified. Giving real-time feedback at the bedside is ideal because of the connection between image acquisition and interpretation. Image interpretation can be taught through didactic sessions, image review sessions, or review of teaching files with annotated images.
Clinical Integration
Learners must interpret and integrate image findings with other clinical data considering the image quality, patient characteristics, and changing physiology. Clinical integration should be taught by instructors that share similar clinical knowledge as learners. Although sonographers are well suited to teach image acquisition, they should not be the sole instructors to teach hospitalists how to integrate ultrasound findings in clinical decision making. Likewise, emphasis should be placed on the appropriate use of POCUS within a provider’s skill set. Learners must appreciate the clinical significance of POCUS findings, including recognition of incidental findings that may require further workup. Supplemental training in clinical integration can occur through didactics that include complex patient scenarios.
Pathways
Clinical competency can be achieved with training adherent to five criteria. First, the training environment should be similar to where the trainee will practice. Second, training and feedback should occur in real time. Third, specific applications should be taught rather than broad training in “hospitalist POCUS.” Each application requires unique skills and knowledge, including image acquisition pitfalls and artifacts. Fourth, clinical competence must be achieved and demonstrated; it is not necessarily gained through experience. Fifth, once competency is achieved, continued education and feedback are necessary to ensure it is maintained.
Residency-based POCUS training pathways can best fulfill these criteria. They may eventually become commonplace, but until then alternative pathways must exist for hospitalist providers who are already in practice. There are three important attributes of such pathways. First, administrators’ expectations about learners’ clinical productivity must be realistically, but only temporarily, relaxed; otherwise, competing demands on time will likely overwhelm learners and subvert training. Second, training should begin through a local or national hands-on training program. The SHM POCUS certificate program consolidates training for common diagnostic POCUS applications for hospitalists.6 Other medical societies offer training for their respective clinical specialties.7 Third, once basic POCUS training has begun, longitudinal training should continue ideally with a local hospitalist POCUS expert.
In some settings, a subgroup of hospitalists may not desire, or be able to achieve, competency in the manual skills of POCUS image acquisition. Nevertheless, hospitalists may still find value in understanding POCUS nomenclature, image pattern recognition, and the evidence and pitfalls behind clinical integration of specific POCUS findings. This subset of POCUS skills allows hospitalists to communicate effectively with and understand the clinical decisions made by their colleagues who are competent in POCUS use.
The minimal skills a hospitalist should possess to serve as a POCUS trainer include proficiency of basic knowledge, image acquisition, image interpretation, and clinical integration of the POCUS applications being taught; effectiveness as a hands-on instructor to teach image acquisition skills; and an in-depth understanding of common POCUS pitfalls and limitations.
ASSESSMENTS
Assessment methods for POCUS can include the following: knowledge-based questions, image acquisition using task-specific checklists on human or simulation models, image interpretation using a series of videos or still images with normal and abnormal findings, clinical integration using “next best step” in a multiple choice format with POCUS images, and simulation-based clinical scenarios. Assessment methods should be aligned with local availability of resources and trainers.
Basic Knowledge
Basic knowledge can be assessed via multiple choice questions assessing knowledge of ultrasound physics, image optimization, relevant anatomy, and limitations of POCUS imaging. Basic knowledge lies primarily in the cognitive domain and does not assess manual skills.
Image Acquisition
Image acquisition can be assessed by observation and rating of image quality. Where resources allow, assessment of image acquisition is likely best done through a combination of developing an image portfolio with a minimum number of high quality images, plus direct observation of image acquisition by an expert. Various programs have utilized minimum numbers of images acquired to help define competence with image acquisition skills.6–8 Although minimums may be a necessary step to gain competence, using them as a sole means to determine competence does not account for variable learning curves.9 As with other manual skills in hospital medicine, such as ultrasound-guided bedside procedures, minimum numbers are best used as a starting point for assessments.3,10 In this regard, portfolio development with meticulous attention to the gain, depth, and proper tomographic plane of images can monitor a hospitalist’s progress toward competence by providing objective assessments and feedback. Simulation may also be used as it allows assessment of image acquisition skills and an opportunity to provide real-time feedback, similar to direct observation but without actual patients.
Image Interpretation
Image interpretation is best assessed by an expert observing the learner at bedside; however, when bedside assessment is not possible, image interpretation skills may be assessed using multiple choice or free text interpretation of archived ultrasound images with normal and abnormal findings. This is often incorporated into the portfolio development portion of a training program, as learners can submit their image interpretation along with the video clip. Both normal and abnormal images can be used to assess anatomic recognition and interpretation. Emphasis should be placed on determining when an image is suboptimal for diagnosis (eg, incomplete exam or poor-quality images). Quality assurance programs should incorporate structured feedback sessions.
Clinical Integration
Assessment of clinical integration can be completed through case scenarios that assess knowledge, interpretation of images, and integration of findings into clinical decision making, which is often delivered via a computer-based assessment. Assessments should combine specific POCUS applications to evaluate common clinical problems in hospital medicine, such as undifferentiated hypotension and dyspnea. High-fidelity simulators can be used to blend clinical case scenarios with image acquisition, image interpretation, and clinical integration. When feasible, comprehensive feedback on how providers acquire, interpret, and apply ultrasound at the bedside is likely the best mechanism to assess clinical integration. This process can be done with a hospitalist’s own patients.
General Assessment
A general assessment that includes a summative knowledge and hands-on skills assessment using task-specific checklists can be performed upon completion of training. A high-fidelity simulator with dynamic or virtual anatomy can provide reproducible standardized assessments with variation in the type and difficulty of cases. When available, we encourage the use of dynamic assessments on actual patients that have both normal and abnormal ultrasound findings because simulated patient scenarios have limitations, even with the use of high-fidelity simulators. Programs are recommended to use formative and summative assessments for evaluation. Quantitative scoring systems using checklists are likely the best framework.11,12
CERTIFICATES AND CERTIFICATION
A certificate of completion is proof of a provider’s participation in an educational activity; it does not equate with competency, though it may be a step toward it. Most POCUS training workshops and short courses provide certificates of completion. Certification of competency is an attestation of a hospitalist’s basic competence within a defined scope of practice (Table 2).13 However, without longitudinal supervision and feedback, skills can decay; therefore, we recommend a longitudinal training program that provides mentored feedback and incorporates periodic competency assessments. At present, no national board certification in POCUS is available to grant external certification of competency for hospitalists.
External Certificate
Certificates of completion can be external through a national organization. An external certificate of completion designed for hospitalists includes the POCUS Certificate of Completion offered by SHM in collaboration with CHEST.6 This certificate program provides regional training options and longitudinal portfolio development. Other external certificates are also available to hospitalists.7,14,15
Most hospitalists are boarded by the American Board of Internal Medicine or the American Board of Family Medicine. These boards do not yet include certification of competency in POCUS. Other specialty boards, such as emergency medicine, include competency in POCUS. For emergency medicine, completion of an accredited residency training program and certification by the national board includes POCUS competency.
Internal Certificate
There are a few examples of successful local institutional programs that have provided internal certificates of competency.12,14 Competency assessments require significant resources including investment by both faculty and learners. Ongoing evaluation of competency should be based on quality assurance processes.
Credentialing and Privileging
The American Medical Association (AMA) House of Delegates in 1999 passed a resolution (AMA HR. 802) recommending hospitals follow specialty-specific guidelines for privileging decisions related to POCUS use.17 The resolution included a statement that, “ultrasound imaging is within the scope of practice of appropriately trained physicians.”
Some institutions have begun to rely on a combination of internal and external certificate programs to grant privileges to hospitalists.10 Although specific privileges for POCUS may not be required in some hospitals, some institutions may require certification of training and assessments prior to granting permission to use POCUS.
Hospitalist programs are encouraged to evaluate ongoing POCUS use by their providers after granting initial permission. If privileging is instituted by a hospital, hospitalists must play a significant role in determining the requirements for privileging and ongoing maintenance of skills.
Maintenance of Skills
All medical skills can decay with disuse, including those associated with POCUS.12,18 Thus, POCUS users should continue using POCUS regularly in clinical practice and participate in POCUS continuing medical education activities, ideally with ongoing assessments. Maintenance of skills may be confirmed through routine participation in a quality assurance program.
PROGRAM MANAGEMENT
Use of POCUS in hospital medicine has unique considerations, and hospitalists should be integrally involved in decision making surrounding institutional POCUS program management. Appointing a dedicated POCUS director can help a program succeed.8
Equipment and Image Archiving
Several factors are important to consider when selecting an ultrasound machine: portability, screen size, and ease of use; integration with the electronic medical record and options for image archiving; manufacturer’s service plan, including technical and clinical support; and compliance with local infection control policies. The ability to easily archive and retrieve images is essential for quality assurance, continuing education, institutional quality improvement, documentation, and reimbursement. In certain scenarios, image archiving may not be possible (such as with personal handheld devices or in emergency situations) or necessary (such as with frequent serial examinations during fluid resuscitation). An image archive is ideally linked to reports, orders, and billing software.10,19 If such linkages are not feasible, parallel external storage that complies with regulatory standards (ie, HIPAA compliance) may be suitable.20
Documentation and Billing
Components of documentation include the indication and type of ultrasound examination performed, date and time of the examination, patient identifying information, name of provider(s) acquiring and interpreting the images, specific scanning protocols used, patient position, probe used, and findings. Documentation can occur through a standalone note or as part of another note, such as a progress note. Whenever possible, documentation should be timely to facilitate communication with other providers.
Billing is supported through the AMA Current Procedural Terminology codes for “focused” or “limited” ultrasound examinations (Appendix 9). The following three criteria must be satisfied for billing. First, images must be permanently stored. Specific requirements vary by insurance policy, though current practice suggests a minimum of one image demonstrating relevant anatomy and pathology for the ultrasound examination coded. For ultrasound-guided procedures that require needle insertion, images should be captured at the point of interest, and a procedure note should reflect that the needle was guided and visualized under ultrasound.21 Second, proper documentation must be entered in the medical record. Third, local institutional privileges for POCUS must be considered. Although privileges are not required to bill, some hospitals or payers may require them.
Quality Assurance
Published guidelines on quality assurance in POCUS are available from different specialty organizations, including emergency medicine, pediatric emergency medicine, critical care, anesthesiology, obstetrics, and cardiology.8,22–28 Quality assurance is aimed at ensuring that physicians maintain basic competency in using POCUS to influence bedside decisions.
Quality assurance should be carried out by an individual or committee with expertise in POCUS. Multidisciplinary QA programs in which hospital medicine providers are working collaboratively with other POCUS providers have been demonstrated to be highly effective.10 Oversight includes ensuring that providers using POCUS are appropriately trained,10,22,28 using the equipment correctly,8,26,28 and documenting properly. Some programs have implemented mechanisms to review and provide feedback on image acquisition, interpretation, and clinical integration.8,10 Other programs have compared POCUS findings with referral studies, such as comprehensive ultrasound examinations.
CONCLUSIONS
Practicing hospitalists must continue to collaborate with their institutions to build POCUS capabilities. In particular, they must work with their local privileging body to determine what credentials are required. The distinction between certificates of completion and certificates of competency, including whether those certificates are internal or external, is important in the credentialing process.
External certificates of competency are currently unavailable for most practicing hospitalists because ABIM certification does not include POCUS-related competencies. As internal medicine residency training programs begin to adopt POCUS training and certification into their educational curricula, we foresee a need to update the ABIM Policies and Procedures for Certification. Until then, we recommend that certificates of competency be defined and granted internally by local hospitalist groups.
Given the many advantages of POCUS over traditional tools, we anticipate its increasing implementation among hospitalists in the future. As with all medical technology, its role in clinical care should be continuously reexamined and redefined through health services research. Such information will be useful in developing practice guidelines, educational curricula, and training standards.
Acknowledgments
The authors would like to thank all members that participated in the discussion and finalization of this position statement during the Point-of-care Ultrasound Faculty Retreat at the 2018 Society of Hospital Medicine Annual Conference: Saaid Abdel-Ghani, Brandon Boesch, Joel Cho, Ria Dancel, Renee Dversdal, Ricardo Franco-Sadud, Benjamin Galen, Trevor P. Jensen, Mohit Jindal, Gordon Johnson, Linda M. Kurian, Gigi Liu, Charles M. LoPresti, Brian P. Lucas, Venkat Kalidindi, Benji Matthews, Anna Maw, Gregory Mints, Kreegan Reierson, Gerard Salame, Richard Schildhouse, Daniel Schnobrich, Nilam Soni, Kirk Spencer, Hiromizu Takahashi, David M. Tierney, Tanping Wong, and Toru Yamada.
Many hospitalists incorporate point-of-care ultrasound (POCUS) into their daily practice because it adds value to their bedside evaluation of patients. However, standards for training and assessing hospitalists in POCUS have not yet been established. Other acute care specialties, including emergency medicine and critical care medicine, have already incorporated POCUS into their graduate medical education training programs, but most internal medicine residency programs are only beginning to provide POCUS training.1
Several features distinguish POCUS from comprehensive ultrasound examinations. First, POCUS is designed to answer focused questions, whereas comprehensive ultrasound examinations evaluate all organs in an anatomical region; for example, an abdominal POCUS exam may evaluate only for presence or absence of intraperitoneal free fluid, whereas a comprehensive examination of the right upper quadrant will evaluate the liver, gallbladder, and biliary ducts. Second, POCUS examinations are generally performed by the same clinician who generates the relevant clinical question to answer with POCUS and ultimately integrates the findings into the patient’s care.2 By contrast, comprehensive ultrasound examinations involve multiple providers and steps: a clinician generates a relevant clinical question and requests an ultrasound examination that is acquired by a sonographer, interpreted by a radiologist, and reported back to the requesting clinician. Third, POCUS is often used to evaluate multiple body systems. For example, to evaluate a patient with undifferentiated hypotension, a multisystem POCUS examination of the heart, inferior vena cava, lungs, abdomen, and lower extremity veins is typically performed. Finally, POCUS examinations can be performed serially to investigate changes in clinical status or evaluate response to therapy, such as monitoring the heart, lungs, and inferior vena cava during fluid resuscitation.
The purpose of this position statement is to inform a broad audience about how hospitalists are using diagnostic and procedural applications of POCUS. This position statement does not mandate that hospitalists use POCUS. Rather, it is intended to provide guidance on the safe and effective use of POCUS by the hospitalists who use it and the administrators who oversee its use. We discuss POCUS (1) applications, (2) training, (3) assessments, and (4) program management. This position statement was reviewed and approved by the Society of Hospital Medicine (SHM) Executive Committee in March 2018.
APPLICATIONS
As outlined in our earlier position statements,3,4 ultrasound guidance lowers complication rates and increases success rates of invasive bedside procedures. Diagnostic POCUS can guide clinical decision making prior to bedside procedures. For instance, hospitalists may use POCUS to assess the size and character of a pleural effusion to help determine the most appropriate management strategy: observation, medical treatment, thoracentesis, chest tube placement, or surgical therapy. Furthermore, diagnostic POCUS can be used to rapidly assess for immediate postprocedural complications, such as pneumothorax, or if the patient develops new symptoms.
TRAINING
Basic Knowledge
Basic knowledge includes fundamentals of ultrasound physics; safety;4 anatomy; physiology; and device operation, including maintenance and cleaning. Basic knowledge can be taught by multiple methods, including live or recorded lectures, online modules, or directed readings.
Image Acquisition
Training should occur across multiple types of patients (eg, obese, cachectic, postsurgical) and clinical settings (eg, intensive care unit, general medicine wards, emergency department) when available. Training is largely hands-on because the relevant skills involve integration of 3D anatomy with spatial manipulation, hand-eye coordination, and fine motor movements. Virtual reality ultrasound simulators may accelerate mastery, particularly for cardiac image acquisition, and expose learners to standardized sets of pathologic findings. Real-time bedside feedback on image acquisition is ideal because understanding how ultrasound probe manipulation affects the images acquired is essential to learning.
Image Interpretation
Training in image interpretation relies on visual pattern recognition of normal and abnormal findings. Therefore, the normal to abnormal spectrum should be broad, and learners should maintain a log of what abnormalities have been identified. Giving real-time feedback at the bedside is ideal because of the connection between image acquisition and interpretation. Image interpretation can be taught through didactic sessions, image review sessions, or review of teaching files with annotated images.
Clinical Integration
Learners must interpret and integrate image findings with other clinical data considering the image quality, patient characteristics, and changing physiology. Clinical integration should be taught by instructors that share similar clinical knowledge as learners. Although sonographers are well suited to teach image acquisition, they should not be the sole instructors to teach hospitalists how to integrate ultrasound findings in clinical decision making. Likewise, emphasis should be placed on the appropriate use of POCUS within a provider’s skill set. Learners must appreciate the clinical significance of POCUS findings, including recognition of incidental findings that may require further workup. Supplemental training in clinical integration can occur through didactics that include complex patient scenarios.
Pathways
Clinical competency can be achieved with training adherent to five criteria. First, the training environment should be similar to where the trainee will practice. Second, training and feedback should occur in real time. Third, specific applications should be taught rather than broad training in “hospitalist POCUS.” Each application requires unique skills and knowledge, including image acquisition pitfalls and artifacts. Fourth, clinical competence must be achieved and demonstrated; it is not necessarily gained through experience. Fifth, once competency is achieved, continued education and feedback are necessary to ensure it is maintained.
Residency-based POCUS training pathways can best fulfill these criteria. They may eventually become commonplace, but until then alternative pathways must exist for hospitalist providers who are already in practice. There are three important attributes of such pathways. First, administrators’ expectations about learners’ clinical productivity must be realistically, but only temporarily, relaxed; otherwise, competing demands on time will likely overwhelm learners and subvert training. Second, training should begin through a local or national hands-on training program. The SHM POCUS certificate program consolidates training for common diagnostic POCUS applications for hospitalists.6 Other medical societies offer training for their respective clinical specialties.7 Third, once basic POCUS training has begun, longitudinal training should continue ideally with a local hospitalist POCUS expert.
In some settings, a subgroup of hospitalists may not desire, or be able to achieve, competency in the manual skills of POCUS image acquisition. Nevertheless, hospitalists may still find value in understanding POCUS nomenclature, image pattern recognition, and the evidence and pitfalls behind clinical integration of specific POCUS findings. This subset of POCUS skills allows hospitalists to communicate effectively with and understand the clinical decisions made by their colleagues who are competent in POCUS use.
The minimal skills a hospitalist should possess to serve as a POCUS trainer include proficiency of basic knowledge, image acquisition, image interpretation, and clinical integration of the POCUS applications being taught; effectiveness as a hands-on instructor to teach image acquisition skills; and an in-depth understanding of common POCUS pitfalls and limitations.
ASSESSMENTS
Assessment methods for POCUS can include the following: knowledge-based questions, image acquisition using task-specific checklists on human or simulation models, image interpretation using a series of videos or still images with normal and abnormal findings, clinical integration using “next best step” in a multiple choice format with POCUS images, and simulation-based clinical scenarios. Assessment methods should be aligned with local availability of resources and trainers.
Basic Knowledge
Basic knowledge can be assessed via multiple choice questions assessing knowledge of ultrasound physics, image optimization, relevant anatomy, and limitations of POCUS imaging. Basic knowledge lies primarily in the cognitive domain and does not assess manual skills.
Image Acquisition
Image acquisition can be assessed by observation and rating of image quality. Where resources allow, assessment of image acquisition is likely best done through a combination of developing an image portfolio with a minimum number of high quality images, plus direct observation of image acquisition by an expert. Various programs have utilized minimum numbers of images acquired to help define competence with image acquisition skills.6–8 Although minimums may be a necessary step to gain competence, using them as a sole means to determine competence does not account for variable learning curves.9 As with other manual skills in hospital medicine, such as ultrasound-guided bedside procedures, minimum numbers are best used as a starting point for assessments.3,10 In this regard, portfolio development with meticulous attention to the gain, depth, and proper tomographic plane of images can monitor a hospitalist’s progress toward competence by providing objective assessments and feedback. Simulation may also be used as it allows assessment of image acquisition skills and an opportunity to provide real-time feedback, similar to direct observation but without actual patients.
Image Interpretation
Image interpretation is best assessed by an expert observing the learner at bedside; however, when bedside assessment is not possible, image interpretation skills may be assessed using multiple choice or free text interpretation of archived ultrasound images with normal and abnormal findings. This is often incorporated into the portfolio development portion of a training program, as learners can submit their image interpretation along with the video clip. Both normal and abnormal images can be used to assess anatomic recognition and interpretation. Emphasis should be placed on determining when an image is suboptimal for diagnosis (eg, incomplete exam or poor-quality images). Quality assurance programs should incorporate structured feedback sessions.
Clinical Integration
Assessment of clinical integration can be completed through case scenarios that assess knowledge, interpretation of images, and integration of findings into clinical decision making, which is often delivered via a computer-based assessment. Assessments should combine specific POCUS applications to evaluate common clinical problems in hospital medicine, such as undifferentiated hypotension and dyspnea. High-fidelity simulators can be used to blend clinical case scenarios with image acquisition, image interpretation, and clinical integration. When feasible, comprehensive feedback on how providers acquire, interpret, and apply ultrasound at the bedside is likely the best mechanism to assess clinical integration. This process can be done with a hospitalist’s own patients.
General Assessment
A general assessment that includes a summative knowledge and hands-on skills assessment using task-specific checklists can be performed upon completion of training. A high-fidelity simulator with dynamic or virtual anatomy can provide reproducible standardized assessments with variation in the type and difficulty of cases. When available, we encourage the use of dynamic assessments on actual patients that have both normal and abnormal ultrasound findings because simulated patient scenarios have limitations, even with the use of high-fidelity simulators. Programs are recommended to use formative and summative assessments for evaluation. Quantitative scoring systems using checklists are likely the best framework.11,12
CERTIFICATES AND CERTIFICATION
A certificate of completion is proof of a provider’s participation in an educational activity; it does not equate with competency, though it may be a step toward it. Most POCUS training workshops and short courses provide certificates of completion. Certification of competency is an attestation of a hospitalist’s basic competence within a defined scope of practice (Table 2).13 However, without longitudinal supervision and feedback, skills can decay; therefore, we recommend a longitudinal training program that provides mentored feedback and incorporates periodic competency assessments. At present, no national board certification in POCUS is available to grant external certification of competency for hospitalists.
External Certificate
Certificates of completion can be external through a national organization. An external certificate of completion designed for hospitalists includes the POCUS Certificate of Completion offered by SHM in collaboration with CHEST.6 This certificate program provides regional training options and longitudinal portfolio development. Other external certificates are also available to hospitalists.7,14,15
Most hospitalists are boarded by the American Board of Internal Medicine or the American Board of Family Medicine. These boards do not yet include certification of competency in POCUS. Other specialty boards, such as emergency medicine, include competency in POCUS. For emergency medicine, completion of an accredited residency training program and certification by the national board includes POCUS competency.
Internal Certificate
There are a few examples of successful local institutional programs that have provided internal certificates of competency.12,14 Competency assessments require significant resources including investment by both faculty and learners. Ongoing evaluation of competency should be based on quality assurance processes.
Credentialing and Privileging
The American Medical Association (AMA) House of Delegates in 1999 passed a resolution (AMA HR. 802) recommending hospitals follow specialty-specific guidelines for privileging decisions related to POCUS use.17 The resolution included a statement that, “ultrasound imaging is within the scope of practice of appropriately trained physicians.”
Some institutions have begun to rely on a combination of internal and external certificate programs to grant privileges to hospitalists.10 Although specific privileges for POCUS may not be required in some hospitals, some institutions may require certification of training and assessments prior to granting permission to use POCUS.
Hospitalist programs are encouraged to evaluate ongoing POCUS use by their providers after granting initial permission. If privileging is instituted by a hospital, hospitalists must play a significant role in determining the requirements for privileging and ongoing maintenance of skills.
Maintenance of Skills
All medical skills can decay with disuse, including those associated with POCUS.12,18 Thus, POCUS users should continue using POCUS regularly in clinical practice and participate in POCUS continuing medical education activities, ideally with ongoing assessments. Maintenance of skills may be confirmed through routine participation in a quality assurance program.
PROGRAM MANAGEMENT
Use of POCUS in hospital medicine has unique considerations, and hospitalists should be integrally involved in decision making surrounding institutional POCUS program management. Appointing a dedicated POCUS director can help a program succeed.8
Equipment and Image Archiving
Several factors are important to consider when selecting an ultrasound machine: portability, screen size, and ease of use; integration with the electronic medical record and options for image archiving; manufacturer’s service plan, including technical and clinical support; and compliance with local infection control policies. The ability to easily archive and retrieve images is essential for quality assurance, continuing education, institutional quality improvement, documentation, and reimbursement. In certain scenarios, image archiving may not be possible (such as with personal handheld devices or in emergency situations) or necessary (such as with frequent serial examinations during fluid resuscitation). An image archive is ideally linked to reports, orders, and billing software.10,19 If such linkages are not feasible, parallel external storage that complies with regulatory standards (ie, HIPAA compliance) may be suitable.20
Documentation and Billing
Components of documentation include the indication and type of ultrasound examination performed, date and time of the examination, patient identifying information, name of provider(s) acquiring and interpreting the images, specific scanning protocols used, patient position, probe used, and findings. Documentation can occur through a standalone note or as part of another note, such as a progress note. Whenever possible, documentation should be timely to facilitate communication with other providers.
Billing is supported through the AMA Current Procedural Terminology codes for “focused” or “limited” ultrasound examinations (Appendix 9). The following three criteria must be satisfied for billing. First, images must be permanently stored. Specific requirements vary by insurance policy, though current practice suggests a minimum of one image demonstrating relevant anatomy and pathology for the ultrasound examination coded. For ultrasound-guided procedures that require needle insertion, images should be captured at the point of interest, and a procedure note should reflect that the needle was guided and visualized under ultrasound.21 Second, proper documentation must be entered in the medical record. Third, local institutional privileges for POCUS must be considered. Although privileges are not required to bill, some hospitals or payers may require them.
Quality Assurance
Published guidelines on quality assurance in POCUS are available from different specialty organizations, including emergency medicine, pediatric emergency medicine, critical care, anesthesiology, obstetrics, and cardiology.8,22–28 Quality assurance is aimed at ensuring that physicians maintain basic competency in using POCUS to influence bedside decisions.
Quality assurance should be carried out by an individual or committee with expertise in POCUS. Multidisciplinary QA programs in which hospital medicine providers are working collaboratively with other POCUS providers have been demonstrated to be highly effective.10 Oversight includes ensuring that providers using POCUS are appropriately trained,10,22,28 using the equipment correctly,8,26,28 and documenting properly. Some programs have implemented mechanisms to review and provide feedback on image acquisition, interpretation, and clinical integration.8,10 Other programs have compared POCUS findings with referral studies, such as comprehensive ultrasound examinations.
CONCLUSIONS
Practicing hospitalists must continue to collaborate with their institutions to build POCUS capabilities. In particular, they must work with their local privileging body to determine what credentials are required. The distinction between certificates of completion and certificates of competency, including whether those certificates are internal or external, is important in the credentialing process.
External certificates of competency are currently unavailable for most practicing hospitalists because ABIM certification does not include POCUS-related competencies. As internal medicine residency training programs begin to adopt POCUS training and certification into their educational curricula, we foresee a need to update the ABIM Policies and Procedures for Certification. Until then, we recommend that certificates of competency be defined and granted internally by local hospitalist groups.
Given the many advantages of POCUS over traditional tools, we anticipate its increasing implementation among hospitalists in the future. As with all medical technology, its role in clinical care should be continuously reexamined and redefined through health services research. Such information will be useful in developing practice guidelines, educational curricula, and training standards.
Acknowledgments
The authors would like to thank all members that participated in the discussion and finalization of this position statement during the Point-of-care Ultrasound Faculty Retreat at the 2018 Society of Hospital Medicine Annual Conference: Saaid Abdel-Ghani, Brandon Boesch, Joel Cho, Ria Dancel, Renee Dversdal, Ricardo Franco-Sadud, Benjamin Galen, Trevor P. Jensen, Mohit Jindal, Gordon Johnson, Linda M. Kurian, Gigi Liu, Charles M. LoPresti, Brian P. Lucas, Venkat Kalidindi, Benji Matthews, Anna Maw, Gregory Mints, Kreegan Reierson, Gerard Salame, Richard Schildhouse, Daniel Schnobrich, Nilam Soni, Kirk Spencer, Hiromizu Takahashi, David M. Tierney, Tanping Wong, and Toru Yamada.
1. Schnobrich DJ, Mathews BK, Trappey BE, Muthyala BK, Olson APJ. Entrusting internal medicine residents to use point of care ultrasound: Towards improved assessment and supervision. Med Teach. 2018:1-6. doi:10.1080/0142159X.2018.1457210.
2. Soni NJ, Lucas BP. Diagnostic point-of-care ultrasound for hospitalists. J Hosp Med. 2015;10(2):120-124. doi:10.1002/jhm.2285.
3. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):117-125. doi:10.12788/jhm.2917.
4. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):126-135. doi:10.12788/jhm.2940.
5. National Council on Radiation Protection and Measurements, The Council. Implementation of the Principle of as Low as Reasonably Achievable (ALARA) for Medical and Dental Personnel.; 1990.
6. Society of Hospital Medicine. Point of Care Ultrasound course: https://www.hospitalmedicine.org/clinical-topics/ultrasonography-cert/. Accessed February 6, 2018.
7. Critical Care Ultrasonography Certificate of Completion Program. CHEST. American College of Chest Physicians. http://www.chestnet.org/Education/Advanced-Clinical-Training/Certificate-of-Completion-Program/Critical-Care-Ultrasonography. Accessed February 6, 2018.
8. American College of Emergency Physicians Policy Statement: Emergency Ultrasound Guidelines. 2016. https://www.acep.org/Clinical---Practice-Management/ACEP-Ultrasound-Guidelines/. Accessed February 6, 2018.
9. Blehar DJ, Barton B, Gaspari RJ. Learning curves in emergency ultrasound education. Acad Emerg Med. 2015;22(5):574-582. doi:10.1111/acem.12653.
10. Mathews BK, Zwank M. Hospital medicine point of care ultrasound credentialing: an example protocol. J Hosp Med. 2017;12(9):767-772. doi:10.12788/jhm.2809.
11. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. doi:10.1002/jhm.468.
12. Mathews BK, Reierson K, Vuong K, et al. The design and evaluation of the Comprehensive Hospitalist Assessment and Mentorship with Portfolios (CHAMP) ultrasound program. J Hosp Med. 2018;13(8):544-550. doi:10.12788/jhm.2938.
13. Soni NJ, Tierney DM, Jensen TP, Lucas BP. Certification of point-of-care ultrasound competency. J Hosp Med. 2017;12(9):775-776. doi:10.12788/jhm.2812.
14. Ultrasound Certification for Physicians. Alliance for Physician Certification and Advancement. APCA. https://apca.org/. Accessed February 6, 2018.
15. National Board of Echocardiography, Inc. https://www.echoboards.org/EchoBoards/News/2019_Adult_Critical_Care_Echocardiography_Exam.aspx. Accessed June 18, 2018.
16. Tierney DM. Internal Medicine Bedside Ultrasound Program (IMBUS). Abbott Northwestern. http://imbus.anwresidency.com/index.html. Accessed February 6, 2018.
17. American Medical Association House of Delegates Resolution H-230.960: Privileging for Ultrasound Imaging. Resolution 802. Policy Finder Website. http://search0.ama-assn.org/search/pfonline. Published 1999. Accessed February 18, 2018.
18. Kelm D, Ratelle J, Azeem N, et al. Longitudinal ultrasound curriculum improves long-term retention among internal medicine residents. J Grad Med Educ. 2015;7(3):454-457. doi:10.4300/JGME-14-00284.1.
19. Flannigan MJ, Adhikari S. Point-of-care ultrasound work flow innovation: impact on documentation and billing. J Ultrasound Med. 2017;36(12):2467-2474. doi:10.1002/jum.14284.
20. Emergency Ultrasound: Workflow White Paper. https://www.acep.org/uploadedFiles/ACEP/memberCenter/SectionsofMembership/ultra/Workflow%20White%20Paper.pdf. Published 2013. Accessed February 18, 2018.
21. Ultrasound Coding and Reimbursement Document 2009. Emergency Ultrasound Section. American College of Emergency Physicians. http://emergencyultrasoundteaching.com/assets/2009_coding_update.pdf. Published 2009. Accessed February 18, 2018.
22. Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/La Societe de Reanimation de Langue Francaise statement on competence in critical care ultrasonography. Chest. 2009;135(4):1050-1060. doi:10.1378/chest.08-2305.
23. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: general ultrasonography. Crit Care Med. 2015;43(11):2479-2502. doi:10.1097/ccm.0000000000001216.
24. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part ii: cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-1227. doi:10.1097/ccm.0000000000001847.
25. ACR–ACOG–AIUM–SRU Practice Parameter for the Performance of Obstetrical Ultrasound. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/us-ob.pdf. Published 2013. Accessed February 18, 2018.
26. AIUM practice guideline for documentation of an ultrasound examination. J Ultrasound Med. 2014;33(6):1098-1102. doi:10.7863/ultra.33.6.1098.
27. Marin JR, Lewiss RE. Point-of-care ultrasonography by pediatric emergency medicine physicians. Pediatrics. 2015;135(4):e1113-e1122. doi:10.1542/peds.2015-0343.
28. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(6):567-581. doi:10.1016/j.echo.2013.04.001.
1. Schnobrich DJ, Mathews BK, Trappey BE, Muthyala BK, Olson APJ. Entrusting internal medicine residents to use point of care ultrasound: Towards improved assessment and supervision. Med Teach. 2018:1-6. doi:10.1080/0142159X.2018.1457210.
2. Soni NJ, Lucas BP. Diagnostic point-of-care ultrasound for hospitalists. J Hosp Med. 2015;10(2):120-124. doi:10.1002/jhm.2285.
3. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):117-125. doi:10.12788/jhm.2917.
4. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):126-135. doi:10.12788/jhm.2940.
5. National Council on Radiation Protection and Measurements, The Council. Implementation of the Principle of as Low as Reasonably Achievable (ALARA) for Medical and Dental Personnel.; 1990.
6. Society of Hospital Medicine. Point of Care Ultrasound course: https://www.hospitalmedicine.org/clinical-topics/ultrasonography-cert/. Accessed February 6, 2018.
7. Critical Care Ultrasonography Certificate of Completion Program. CHEST. American College of Chest Physicians. http://www.chestnet.org/Education/Advanced-Clinical-Training/Certificate-of-Completion-Program/Critical-Care-Ultrasonography. Accessed February 6, 2018.
8. American College of Emergency Physicians Policy Statement: Emergency Ultrasound Guidelines. 2016. https://www.acep.org/Clinical---Practice-Management/ACEP-Ultrasound-Guidelines/. Accessed February 6, 2018.
9. Blehar DJ, Barton B, Gaspari RJ. Learning curves in emergency ultrasound education. Acad Emerg Med. 2015;22(5):574-582. doi:10.1111/acem.12653.
10. Mathews BK, Zwank M. Hospital medicine point of care ultrasound credentialing: an example protocol. J Hosp Med. 2017;12(9):767-772. doi:10.12788/jhm.2809.
11. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. doi:10.1002/jhm.468.
12. Mathews BK, Reierson K, Vuong K, et al. The design and evaluation of the Comprehensive Hospitalist Assessment and Mentorship with Portfolios (CHAMP) ultrasound program. J Hosp Med. 2018;13(8):544-550. doi:10.12788/jhm.2938.
13. Soni NJ, Tierney DM, Jensen TP, Lucas BP. Certification of point-of-care ultrasound competency. J Hosp Med. 2017;12(9):775-776. doi:10.12788/jhm.2812.
14. Ultrasound Certification for Physicians. Alliance for Physician Certification and Advancement. APCA. https://apca.org/. Accessed February 6, 2018.
15. National Board of Echocardiography, Inc. https://www.echoboards.org/EchoBoards/News/2019_Adult_Critical_Care_Echocardiography_Exam.aspx. Accessed June 18, 2018.
16. Tierney DM. Internal Medicine Bedside Ultrasound Program (IMBUS). Abbott Northwestern. http://imbus.anwresidency.com/index.html. Accessed February 6, 2018.
17. American Medical Association House of Delegates Resolution H-230.960: Privileging for Ultrasound Imaging. Resolution 802. Policy Finder Website. http://search0.ama-assn.org/search/pfonline. Published 1999. Accessed February 18, 2018.
18. Kelm D, Ratelle J, Azeem N, et al. Longitudinal ultrasound curriculum improves long-term retention among internal medicine residents. J Grad Med Educ. 2015;7(3):454-457. doi:10.4300/JGME-14-00284.1.
19. Flannigan MJ, Adhikari S. Point-of-care ultrasound work flow innovation: impact on documentation and billing. J Ultrasound Med. 2017;36(12):2467-2474. doi:10.1002/jum.14284.
20. Emergency Ultrasound: Workflow White Paper. https://www.acep.org/uploadedFiles/ACEP/memberCenter/SectionsofMembership/ultra/Workflow%20White%20Paper.pdf. Published 2013. Accessed February 18, 2018.
21. Ultrasound Coding and Reimbursement Document 2009. Emergency Ultrasound Section. American College of Emergency Physicians. http://emergencyultrasoundteaching.com/assets/2009_coding_update.pdf. Published 2009. Accessed February 18, 2018.
22. Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/La Societe de Reanimation de Langue Francaise statement on competence in critical care ultrasonography. Chest. 2009;135(4):1050-1060. doi:10.1378/chest.08-2305.
23. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: general ultrasonography. Crit Care Med. 2015;43(11):2479-2502. doi:10.1097/ccm.0000000000001216.
24. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part ii: cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-1227. doi:10.1097/ccm.0000000000001847.
25. ACR–ACOG–AIUM–SRU Practice Parameter for the Performance of Obstetrical Ultrasound. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/us-ob.pdf. Published 2013. Accessed February 18, 2018.
26. AIUM practice guideline for documentation of an ultrasound examination. J Ultrasound Med. 2014;33(6):1098-1102. doi:10.7863/ultra.33.6.1098.
27. Marin JR, Lewiss RE. Point-of-care ultrasonography by pediatric emergency medicine physicians. Pediatrics. 2015;135(4):e1113-e1122. doi:10.1542/peds.2015-0343.
28. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(6):567-581. doi:10.1016/j.echo.2013.04.001.
© 2019 Society of Hospital Medicine
Recommendations on the Use of Ultrasound Guidance for Adult Abdominal Paracentesis: A Position Statement of the Society of Hospital Medicine
Abdominal paracentesis is a common and increasingly performed procedure in the United States. According to Medicare Physician Supplier Procedure Summary Master Files, an estimated 150,000 paracenteses were performed on Medicare fee-for-service beneficiaries in 2008 alone; such a number represents more than a two-fold increase from the same service population in 1993.1 This increasing trend was again noted by the Nationwide Inpatient Sample data, which identified a 10% increase in hospitalized patients with a diagnosis of cirrhosis receiving paracentesis from 2004 (50%) to 2012 (61%; P < .0001).2
Although these data demonstrate that paracentesis is being performed frequently, paracentesis may be underutilized in hospitalized cirrhotics with ascites. In addition, in-hospital mortality of cirrhotics with ascites is higher among those who do not undergo paracentesis than among those who do (9% vs 6%; P = .03).3,4
While complications associated with paracentesis are rare, serious complications, including death, have been documented.5-10 The most common serious complication of paracentesis is bleeding, although puncture of the bowel and other abdominal organs has also been observed. Over the past few decades, ultrasound has been increasingly used with paracentesis due to the ability of ultrasound to improve detection of ascites11,12 and to avoid blood vessels10,13-15 and bowels.16
Three-quarters of all paracenteses are currently performed by interventional radiologists.1 However, paracenteses are often required off-hours,17 when interventional radiologists are less readily available. Weekend admissions have less frequent performance of early paracentesis than weekday admissions, and delaying paracentesis may increase mortality.3,18 High proficiency in ultrasound-guided paracentesis is achievable by nonradiologists19-28 with equal or better patient outcomes after appropriate training.29
The purpose of this guideline is to review the literature and present evidence-based recommendations on the performance of ultrasound-guided paracentesis at the bedside by practicing hospitalists.
METHODS
Detailed methods are described in Appendix 1. The Society of Hospital Medicine (SHM) Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced-practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist, and all Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the five working group members themselves. Key clinical questions and draft recommendations were then prepared, and a systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were initially searched from 1975 to October 2015. Google Scholar was also searched without limiters. An updated search was conducted from November 2015 to November 2017, search strings for which are included in Appendix 3. All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of screened articles were reviewed and articles on ultrasound guidance for paracentesis were selected. The following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided paracentesis were screened and selected. Final article selection was based on working group consensus. The selected literature was incorporated into the draft recommendations.
We used the RAND Appropriateness Method that required panel judgment and consensus to establish recommendations.30 The voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering five transforming factors: (1) problem priority and importance; (2) level of quality of evidence; (3) benefit/harm balance; (4) benefit/burden balance; and (5) certainty/concerns about preferences/equity acceptability/feasibility. Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (Redcap™) during February 2018 and April 2018 (Appendix 4) and voting on appropriateness was conducted using a 9-point Likert scale. The three zones based on the 9-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points), and the degree of consensus was assessed using the RAND algorithm (Appendix 1, Figure 1, and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” A strong recommendation required 80% of the votes within one integer of the median, following RAND rules, and disagreement was defined as >30% of panelists voting outside of the zone of the median.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording for each recommendation (Tables 1 and 2). The revised consensus-based recommendations underwent internal and external review by POCUS experts from different subspecialties, and a final review of the guideline document was performed by members of the SHM POCUS Task Force, SHM Education Committee, and SHM Board of Directors. The SHM Board of Directors endorsed the document prior to submission to the Journal of Hospital Medicine.
RESULTS
Literature search
A total of 794 references were pooled and screened from literature searches conducted by a certified medical librarian in October 2015 (604 citations) and updated in November 2017 (118 citations), and working group members’ personal bibliographies and searches (72 citations; Appendix 3, Figure 2). Final selection included 91 articles that were abstracted into a data table and incorporated into the draft recommendations.
RECOMMENDATIONS
Four domains (terminology, clinical outcomes, technique, and training) with 13 draft recommendations were generated based on the literature review by the paracentesis working group. After two rounds of panel voting, one recommendation did not achieve consensus based on the RAND rules, and 12 statements received final approval. The degree of consensus based on the median score and dispersion of voting around the median are shown in Appendix 5. All 12 statements achieved consensus as strong recommendations. The strength of each recommendation and degree of consensus are summarized in Table 3.
Terminology
Abdominal paracentesis is a procedure in which fluid is aspirated from the intraperitoneal space by percutaneous insertion of a needle with or without a catheter through the abdominal wall. Throughout this document, the term “paracentesis” refers to “abdominal paracentesis.”
In this document, ultrasound-guided paracentesis refers to the use of static ultrasound guidance to mark a needle insertion site immediately prior to performing the procedure. Real-time (dynamic) ultrasound guidance refers to tracking of the needle tip with ultrasound as it traverses the abdominal wall to enter the peritoneal cavity. Landmark-based paracentesis refers to paracentesis based on physical examination alone.
RECOMMENDATIONS
Clinical outcomes
1. We recommend that ultrasound guidance should be used for paracentesis to reduce the risk of serious complications, the most common being bleeding.
Rationale. The occurrence of both minor and serious life-threatening complications from paracentesis has been well described.5-10,31,32 A recent retrospective study that evaluated 515 landmark-guided paracenteses noted that the most common minor complication was persistent ascites leakage (5%) and that the most common serious complication was postprocedural bleeding (1%).8 Studies have shown that abdominal wall hematoma and hemoperitoneum are common hemorrhagic complications of paracentesis, although inferior epigastric artery pseudoaneurysm has also been described.9,33,34
Current literature suggests that ultrasound-guided paracentesis is a safe procedure, even with reduced platelet counts or elevated international normalized ratio.35-42 Most comparative studies have shown that ultrasound guidance reduces the risk of bleeding complications compared with the use of landmarks alone,7,31,32,43-45 although a few studies did not find a significant difference between techniques.20,36,46 One large retrospective observational study that analyzed the administrative data of 69,859 paracenteses from more than 600 hospitals demonstrated that ultrasound guidance reduced the odds of bleeding complications by 68% (OR, 0.32; 95% CI, 0.25–0.41). Bleeding complication rates with and without the use of ultrasound guidance were 0.27% (CI 0.26-0.29) versus 1.25% (CI 1.21-1.29; P < .0001), respectively. More importantly, in this study, paracentesis complicated by bleeding was associated with a higher in-hospital mortality rate
2. We recommend that ultrasound guidance should be used to avoid attempting paracentesis in patients with an insufficient volume of intraperitoneal free fluid to drain.
Rationale. Abdominal physical examination is not a reliable method for determining the presence or volume of intraperitoneal free fluid, as no specific physical examination finding has consistently shown both high sensitivity and specificity for detecting intraperitoneal free fluid.11,12,20,31,47-51 Patient factors limiting the diagnostic accuracy of physical examination include body habitus, abdominal wall edema, and gaseous bowel distention.
In comparative studies, ultrasound has been found to be significantly more sensitive and specific than physical examination in detecting peritoneal free fluid.11,12 Ultrasound can detect as little as 100 mL of peritoneal free fluid,52,53 and larger volumes of fluid have higher diagnostic accuracy.53-55 In one randomized trial of 100 patients suspected of having ascites, patients were randomized to landmark-based and ultrasound-guided paracentesis groups. Of the 56 patients in the ultrasound-guided group, 14 patients suspected of having ascites on physical examination were found to have no or an insufficient volume of ascites to attempt paracentesis.20 Another study with 41 ultrasound examinations on cancer patients suspected of having intraperitoneal free fluid by history and physical examination demonstrated that only 19 (46%) were considered to have a sufficient volume of ascites by ultrasound to attempt paracentesis.38
3. We recommend that ultrasound guidance should be used for paracentesis to improve the success rates of the overall procedure.
Rationale. In addition to avoiding drainage attempts in patients with an insufficient volume of intraperitoneal free fluid, ultrasound can increase the success rate of attempted procedures by localizing the largest fluid collection and guiding selection of an optimal needle insertion site. The success rates of landmark-based paracentesis in patients suspected of having intraperitoneal free fluid by physical examination are not well described in the literature, but multiple studies report success rates of 95%-100% for paracentesis when using ultrasound guidance to select a needle insertion site.20,38,56,57 In one randomized trial comparing ultrasound-guided versus landmark-based paracentesis, ultrasound-guided paracentesis revealed a significantly higher success rate (95% of procedures performed) compared with landmark-based parancentesis (61% of procedures performed). Moreover, 87% of the initial failures in the landmark-based group underwent subsequent successful paracentesis when ultrasound guidance was used. Ultrasound revealed that the rest of the patients (13%) did not have enough fluid to attempt ultrasound-guided paracentesis.20
Technique
4. We recommend that ultrasound should be used to assess the characteristics of intraperitoneal free fluid to guide clinical decision making of where paracentesis can be safely performed.
Rationale. The presence and characteristics of intraperitoneal fluid collections are important determinants of whether paracentesis, another procedure, or no procedure should be performed in a given clinical scenario. One study reported that the overall diagnostic accuracy of physical examination for detecting ascites was only 58%,50 and many providers are unable to detect ascites by physical examination until 1L of fluid has accumulated. One small study showed that at least 500 ml of fluid must accumulate before shifting dullness could be detected.58 By contrast, ultrasound has been shown to reliably detect as little as 100 mL of peritoneal free fluid 52,53 and has been proven to be superior to physical examination in several studies.11,12 Therefore, ultrasound can be used to qualitatively determine whether a sufficient volume of intraperitoneal free fluid is present to safely perform paracentesis.
Studies have shown that ultrasound can also be used to differentiate ascites from other pathologies (eg, matted bowel loops, metastases, abscesses) in patients with suspected ascites on history and physical examination.16 In addition, ultrasound can help to better understand the etiology and distribution of the ascites.59-61 Sonographic measurements allow semiquantitative assessment of the volume of intraperitoneal free fluid, which may correlate with the amount of fluid removed in therapeutic paracentesis procedures.62,63 Furthermore, depth of a fluid collection by ultrasound may be an independent risk factor for the presence of spontaneous bacterial peritonitis (SBP), with one small study showing a higher risk of SBP with larger fluid collections than with small ones.64
5. We recommend that ultrasound should be used to identify a needle insertion site based on size of the fluid collection, thickness of the abdominal wall, and proximity to abdominal organs.
Rationale. When providers perform paracentesis using ultrasound guidance, any fluid collection that is directly visualized and accessible may be considered for drainage. The presence of ascites using ultrasound is best detected using a low-frequency transducer, such as phased array or curvilinear transducer, which provides deep penetration into the abdomen and pelvis to assess peritoneal free fluid.13,14,45,51,65 An optimal needle insertion site should be determined based on a combination of visualization of largest fluid collection, avoidance of underlying abdominal organs, and thickness of abdominal wall.13,31,66,67
6. We recommend the needle insertion site should be evaluated using color flow Doppler ultrasound to identify and avoid abdominal wall blood vessels along the anticipated needle trajectory.
Rationale. The anatomy of the superficial blood vessels of the abdominal wall, especially the lateral branches, varies greatly.68-70 Although uncommon, inadvertent laceration of an inferior epigastric artery or one of its large branches is associated with significant morbidity and mortality.10,15,69,71-73 A review of 126 cases of rectus sheath hematomas, which most likely occur due to laceration of the inferior or superior epigastric artery, at a single institution from 1992 to 2002 showed a mortality rate of 1.6%, even with aggressive intervention.74 Besides the inferior epigastric arteries, several other blood vessels are at risk of injury during paracentesis, including the inferior epigastric veins, thoracoepigastric veins, subcostal artery and vein branches, deep circumflex iliac artery and vein, and recanalized subumbilical vasculature.75-77 Laceration of any of the abdominal wall blood vessels could result in catastrophic bleeding.
Identification of abdominal wall blood vessels is most commonly performed with a high-frequency transducer using color flow Doppler ultrasound.10,13-15 A low-frequency transducer capable of color flow Doppler ultrasound may be utilized in patients with a thick abdominal wall.
Studies suggest that detection of abdominal wall blood vessels with ultrasound may reduce the risk of bleeding complications. One study showed that 43% of patients had a vascular structure present at one or more of the three traditional landmark paracentesis sites.78 Another study directly compared bleeding rates between an approach utilizing a low-frequency transducer to identify the largest collection of fluid only versus a two-transducer approach utilizing both low and high-frequency transducers to identify the largest collection of fluid and evaluate for any superficial blood vessels. In this study, which included 5,777 paracenteses, paracentesis-related minor bleeding rates were similar in both groups, but major bleeding rates were less in the group utilizing color flow Doppler to evaluate for superficial vessels (0.3% vs 0.08%); differences found between groups, however, did not reach statistical significance (P = .07).79
7. We recommend that a needle insertion site should be evaluated in multiple planes to ensure clearance from underlying abdominal organs and detect any abdominal wall blood vessels along the anticipated needle trajectory.
Rationale. Most ultrasound machines have a slice thickness of <4 mm at the focal zone.80 Considering that an ultrasound beam represents a very thin 2-dimentional cross-section of the underlying tissues, visualization in only one plane could lead to inadvertent puncture of nearby critical structures such as loops of bowel or edges of solid organs. Therefore, it is important to evaluate the needle insertion site and surrounding areas in multiple planes by tilting the transducer and rotating the transducer to orthogonal planes.61 Additionally, evaluation with color flow Doppler could be performed in a similar fashion to ensure that no large blood vessels are along the anticipated needle trajectory.
8. We recommend that a needle insertion site should be marked with ultrasound immediately before performing the procedure, and the patient should remain in the same position between marking the site and performing the procedure.
Rationale. Free-flowing peritoneal fluid and abdominal organs, especially loops of small bowel, can easily shift when a patient changes position or takes a deep breath.13,16,53 Therefore, if the patient changes position or there is a delay between marking the needle insertion site and performing the procedure, the patient should be reevaluated with ultrasound to ensure that the marked needle insertion site is still safe for paracentesis.78 After marking the needle insertion site, the skin surface should be wiped completely clean of gel, and the probe should be removed from the area before sterilizing the skin surface.
9. We recommend that using real-time ultrasound guidance for paracentesis should be considered when the fluid collection is small or difficult to access.
Rationale. Use of real-time ultrasound guidance for paracentesis has been described to drain abdominal fluid collections.13,20,62 Several studies have commented that real-time ultrasound guidance for paracentesis may be necessary in obese patients, in patients with small fluid collections, or when performing the procedure near critical structures, such as loops of small bowel, liver, or spleen.57,81 Real-time ultrasound guidance for paracentesis requires additional training in needle tracking techniques and specialized equipment to maintain sterility.
Training
10. We recommend that dedicated training sessions, including didactics, supervised practice on patients, and simulation-based practice, should be used to teach novices how to perform ultrasound-guided paracentesis.
Rationale. Healthcare providers must gain multiple skills to safely perform ultrasound-guided paracentesis. Trainees must learn how to operate the ultrasound machine to identify the most appropriate needle insertion site based on the abdominal wall thickness, fluid collection size, proximity to nearby abdominal organs, and presence of blood vessels. Education regarding the use of ultrasound guidance for paracentesis is both desired 82,83 and being increasingly taught to health care providers who perform paracentesis.20,84-86
Several approaches have shown high uptake of essential skills to perform ultrasound-guided paracentesis after short training sessions. One study showed that first-year medical students can be taught to use POCUS to accurately diagnose ascites after three 30-minute teaching sessions.19 Another study showed that emergency medicine residents can achieve high levels of proficiency in the preprocedural ultrasound evaluation for paracentesis with only one hour of didactic training.20 Other studies also support the concept that adequate proficiency is achievable within brief, focused training sessions.21-28 However, these skills can decay significantly over time without ongoing education.87
11. We recommend that simulation-based practice should be used, when available, to facilitate acquisition of the required knowledge and skills to perform ultrasound-guided paracentesis.
Rationale. Simulation-based practice should be used when available, as it has been shown to increase competence in bedside diagnostic ultrasonography and procedural techniques for ultrasound-guided procedures, including paracentesis.22,25,29,88,89 One study showed that internal medicine residents were able to achieve a high level of proficiency to perform ultrasound-guided paracentesis after a three-hour simulation-based mastery learning session.88 A follow-up study suggested that, after sufficient simulation-based training, a nonradiologist can perform ultrasound-guided paracentesis as safely as an interventional radiologist.29
12. We recommend that competence in performing ultrasound-guided paracentesis should be demonstrated prior to independently performing the procedure on patients.
Rationale. Competence in ultrasound-guided paracentesis requires acquisition of clinical knowledge of paracentesis, skills in basic abdominal ultrasonography, and manual techniques to perform the procedure. Competence in ultrasound-guided paracentesis cannot be assumed for those graduating from internal medicine residency in the United States. While clinical knowledge of paracentesis remains a core competency of graduating internal medicine residents per the American Board of Internal Medicine, demonstration of competence in performing ultrasound-guided or landmark-based paracentesis is not currently mandated.90 A recent national survey of internal medicine residency program directors revealed that the curricula and resources available to train residents in bedside diagnostic ultrasound and ultrasound-guided procedures, including paracentesis, remain quite variable. 83
While it has not been well studied, competence in ultrasound for paracentesis, as with all other skills involved in bedside procedures, is likely best evaluated through direct observation on actual patients.91 As such, individualized systems to evaluate competency in ultrasound-guided paracentesis should be established for each site where it is performed. A list of consensus-derived ultrasound competencies for ultrasound-guided paracentesis has been proposed, and this list may serve as a guide for both training curriculum development and practitioner evaluation.86,91,92
KNOWLEDGE GAPS
In the process of developing these recommendations, we identified several important gaps in the literature regarding the use of ultrasound guidance for paracentesis.
First, while some data suggest that the use of ultrasound guidance for paracentesis may reduce the inpatient length of stay and overall costs, this suggestion has not been studied rigorously. In a retrospective review of 1,297 abdominal paracenteses by Patel et al., ultrasound-guided paracentesis was associated with a lower incidence of adverse events compared with landmark-based paracentesis (1.4% vs 4.7%; P = .01). The adjusted analysis from this study showed significant reductions in adverse events (OR 0.35; 95%CI 0.165-0.739; P = .006) and hospitalization costs ($8,761 ± $5,956 vs $9,848 ± $6,581; P < .001) for paracentesis with ultrasound guidance versus without such guidance. Additionally, the adjusted average length of stay was 0.2 days shorter for paracentesis with ultrasound guidance versus that without guidance (5.6 days vs 5.8 days; P < .0001).44 Similar conclusions were reached by Mercaldi et al., who conducted a retrospective study of 69,859 patients who underwent paracentesis. Fewer bleeding complications occurred when paracentesis was performed with ultrasound guidance (0.27%) versus without ultrasound guidance (1.27%). Hospitalization costs increased by $19,066 (P < .0001) and length of stay increased by 4.3 days (P < .0001) for patients when paracentesis was complicated by bleeding.43 Because both of these studies were retrospective reviews of administrative databases, associations between procedures, complications, and use of ultrasound may be limited by erroneous coding and documentation.
Second, regarding technique, it is unknown whether the use of real-time ultrasound guidance confers additional benefits compared with use of static ultrasound to mark a suitable needle insertion site. In clinical practice, real-time ultrasound guidance is used to sample small fluid collections, particularly when loops of bowel or a solid organ are nearby. It is possible that higher procedural success rates and lower complication rates may be demonstrated in these scenarios in future studies.
Third, the optimal approach to train providers to perform ultrasound-guided paracentesis is unknown. While short training sessions have shown high uptake of essential skills to perform ultrasound-guided paracentesis, data regarding the effectiveness of training using a comprehensive competency assessment are limited. Simulation-based mastery learning as a means to obtain competency for paracentesis has been described in one study,88 but the translation of competency demonstrated by simulation to actual patient outcomes has not been studied. Furthermore, the most effective method to train providers who are proficient in landmark-based paracentesis to achieve competency in ultrasound-guided paracentesis has not been well studied.
Fourth, the optimal technique for identifying blood vessels in the abdominal wall is unknown. We have proposed that color flow Doppler should be used to identify and avoid puncture of superficial vessels, but power Doppler is three times more sensitive at detecting blood vessels, especially at low velocities, such as in veins independent of direction or flow.93 Hence using power Doppler instead of color flow Doppler may further improve the ability to identify and avoid superficial vessels along the needle trajectory.92
Finally, the impact of ultrasound use on patient experience has yet to be studied. Some studies in the literature show high patient satisfaction with use of ultrasound at the bedside,94,95 but patient satisfaction with ultrasound-guided paracentesis has not been compared directly with the landmark-based technique.
CONCLUSIONS
The use of ultrasound guidance for paracentesis has been associated with higher success rates and lower complication rates. Ultrasound is superior to physical examination in assessing the presence and volume of ascites, and determining the optimal needle insertion site to avoid inadvertent injury to abdominal wall blood vessels. Hospitalists can attain competence in ultrasound-guided paracentesis through the use of various training methods, including lectures, simulation-based practice, and hands-on training. Ongoing use and training over time is necessary to maintain competence.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam Soni, Ricardo Franco Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Mathews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen. Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Collaborators of the Society of Hospital Medicine Point-of-care Ultrasound Task Force
Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Michael Blaivas, Dan Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Venkat Kalidindi, Ketino Kobaidze, Josh Lenchus, Paul Mayo, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Sophia Rodgers, Gerard Salame, Daniel Schnobrich, Kirk Spencer, Vivek Tayal, David M. Tierney
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
All 5 appendices are viewable online at https://www.journalofhospitalmedicine.com.
1. Duszak R, Jr., Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859-864. doi: 10.1016/j.jacr.2010.04.013.
2. O’Brien CR, Chang J, Campos RA, et al. Characterizing the safety of paracentesis in hospitalized patients with cirrhosis and ascites from 2004-2012 in the United States. Gastroenterology. 2016;150(4). https:/doi.org /10.1016/S0016-5085(16)32196-5.
3. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. doi: 10.1016/S0016-5085(16)32196-5
4. Orman ES, Hayashi PH, Bataller R, Barritt AS. Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2014;12(3):496-503.e1. doi: 10.1016/j.cgh.2013.08.025.
5. Mallory A, Schaefer JW. Complications of diagnostic paracentesis in patients with liver disease. JAMA. 1978;239(7):628-630. doi: 10.1001/jama.1978.03280340048020.
6. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525-529. doi: 10.1111/j.1365-2036.2005.02387.x.
7. Shekhar C, Ramakrishnan A, Claridge LC. Paracentesis: UK trainees’ practice, experience and attitudes. Gut. 2013;62:A42. doi: 10.1136/gutjnl-2013-304907.096.
8. De Gottardi A, Thevenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7(8):906-909. doi: 10.1016/j.cgh.2009.05.004.
9. Sharzehi K, Jain V, Naveed A, Schreibman I. Hemorrhagic complications of paracentesis: a systematic review of the literature. Gastroenterol Res Pract. 2014;2014:985141. doi: 10.1155/2014/985141.
10. Sekiguchi H, Suzuki J, Daniels CE. Making paracentesis safer: a proposal for the use of bedside abdominal and vascular ultrasonography to prevent a fatal complication. Chest. 2013;143(4):1136-1139. doi: 10.1378/chest.12-0871.
11. Soyuncu S, Cete Y, Bozan H, Kartal M, Akyol AJ. Accuracy of physical and ultrasonographic examinations by emergency physicians for the early diagnosis of intraabdominal haemorrhage in blunt abdominal trauma. Injury. 2007;38(5):564-569. doi: 10.1016/j.injury.2007.01.010.
12. Chongtham DS, Singh MM, Kalantri SP, Pathak S, Jain AP. Accuracy of clinical manoeuvres in detection of minimal ascites. Indian J Med Sci. 1998;52(11):514-520.
13. Ennis J, Schultz G, Perera P, Williams S, Gharahbaghian L, Mandavia D. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293. doi: 10.4236/ijcm.2014.520163.
14. Szabo TL, Lewin PA. Ultrasound transducer selection in clinical imaging practice. J Ultrasound Med. 2013;32(4):573-582. doi: 10.7863/jum.2013.32.4.573.
15. Stone JC, Moak JH. Feasibility of sonographic localization of the inferior epigastric artery before ultrasound-guided paracentesis. Am J Emerg Med. 2015;33(12):1795-1798. doi: 10.1016/j.ajem.2015.06.067.
16. Yeh HC, Wolf BS. Ultrasonography in ascites. Radiology. 1977;124(3):783-790. doi: 10.1148/124.3.783.
17. Lucas BP, Asbury JK, Wang Y, et al. Impact of a bedside procedure service on general medicine inpatients: a firm-based trial. J Hosp Med. 2007;2(3):143-149. doi: 10.1002/jhm.159.
18. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. doi: 10.1038/ajg.2014.212.
19. Arora S, Cheung A, Tarique U, Agarwal A, Firdouse M, Ailon J. First-year medical students use of ultrasound or physical examination to diagnose hepatomegaly and ascites: a randomized controlled trial. J Ultrasound. 2017;20(3):199-204. doi: 10.1007/s40477-017-0261-6.
20. Nazeer SR, Dewbre H, Miller AH. Ultrasound-assisted paracentesis performed by emergency physicians vs the traditional technique: a prospective, randomized study. Am J Emerg Med. 2005;23(3):363-367. doi: 10.1016/j.ajem.2004.11.001.
21. Kotagal M, Quiroga E, Ruffatto BJ, et al. Impact of point-of-care ultrasound training on surgical residents’ confidence. J Surg Educ. 2015;72(4):e82-87. doi: 10.1016/j.jsurg.2015.01.021.
22. Martin R, Gannon D, Riggle J, et al. A comprehensive workshop using simulation to train internal medicine residents in bedside procedures performed by internists. Chest. 2012;142(4):545A. doi: 10.1378/chest.1390093.
23. Quddus A, Minami T, Summerhill E. Impact of a short 3-hour ultrasound training workshop for internal medicine residents. Chest. 2014;146(4): 509A. doi: 10.1378/chest.1989267.
24. Lanoix R, Leak LV, Gaeta T, Gernsheimer JR. A preliminary evaluation of emergency ultrasound in the setting of an emergency medicine training program. Am J Emerg Med. 2000;18(1):41-45. doi: 10.1016/S0735-6757(00)90046-9.
25. Dulohery MM, Eaton J, Tajouri T, Bhagra A. Ultrasound for internal medicine physicians: the future of physical exam. J Ultrasound Med. 2014;33(6):1005-1011. doi: 10.7863/ultra.33.6.1005
26. Lanoix R, Baker WE, Mele JM, Dharmarajan L. Evaluation of an instructional model for emergency ultrasonography. Acad Emerg Med. 1998;5(1):58-63. doi: 10.1111/j.1553-2712.1998.tb02576.x.
27. Terkamp C, Kirchner G, Wedemeyer J, et al. Simulation of abdomen sonography. Evaluation of a new ultrasound simulator. Ultraschall Med. 2003;24(4):239-234. doi: 10.1055/s-2003-41713.
28. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. doi: 10.1136/bmjqs-2013-002665.
29. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. doi: 10.1016/j.amjmed.2012.09.016.
30. Fitch K, Bernstein SJ, Aguilar MD, et al. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: Rand Corp.; 2001.
31. Bard C, Lafortune M, Breton G. Ascites: ultrasound guidance or blind paracentesis? CMAJ. 1986;135(3):209-210. doi: 10.1016/0736-4679(87)90268-X.
32. Sudulagunta SR, Sodalagunta MB, Bangalore Raja SK, Khorram H, Sepehrar M, Noroozpour Z. Clinical profile and complications of paracentesis in refractory ascites patients with cirrhosis. Gastroenterol Res. 2015;8(3-4):228-233. doi: 10.14740/gr661w.
33. Lin S, Wang M, Zhu Y, et al. Hemorrhagic complications following abdominal paracentesis in acute on chronic liver failure: a propensity score analysis. Medicine (Baltimore). 2015;94(49):e2225. doi: 10.1097/MD.0000000000002225.
34. Lam EY, McLafferty RB, Taylor LM, Jr., et al. Inferior epigastric artery pseudoaneurysm: a complication of paracentesis. J Vasc Surg. 1998;28(3):566-569. doi: 10.1016/S0741-5214(98)70147-8.
35. Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound-guided intervention. AJR Am J Roentgenol. 2010;195(4):846-850. doi: 10.2214/AJR.09.3168.
36. Wiese SS, Mortensen C, Bendtsen F. Few complications after paracentesis in patients with cirrhosis and refractory ascites. Dan Med Bull. 2011;58(1):A4212.
37. Jakobson DJ, Shemesh I. Merging ultrasound in the intensive care routine. Isr Med Assoc J. 2013;15(11):688-692.
38. Landers A, Ryan B. The use of bedside ultrasound and community-based paracentesis in a palliative care service. J Prim Health Care. 2014;6(2):148-151.
39. Lin CH, Shih FY, Ma MH, Chiang WC, Yang CW, Ko PC. Should bleeding tendency deter abdominal paracentesis? Dig Liver Dis. 2005;37(12):946-951. doi: 10.1016/j.dld.2005.07.009.
40. Kurup AN, Lekah A, Reardon ST, et al. Bleeding rate for ultrasound-guided paracentesis in thrombocytopenic patients. J Ultrasound Med. 2015;34(10):1833-1838. doi: 10.7863/ultra.14.10034.
41. Reardon S, Atwell TD, Lekah A. Major bleeding complication rate of ultrasound-guided paracentesis in thrombocytopenic patients. J Vasc Interv Radiol. 2013;24(4):S56. doi: 10.1016/j.jvir.2013.01.129.
42. Czul F, Prager M, Lenchus J. Intra-procedural risk of bleeding associated with ultrasound guided paracentesis in patients with abnormal coagulation studies: 1907. Hepatology. 2011;54(4):1259A.
43. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. doi: 10.1378/chest.12-0447.
44. Patel PA, Ernst FR, Gunnarsson CL. Evaluation of hospital complications and costs associated with using ultrasound guidance during abdominal paracentesis procedures. J Med Econ. 2012;15(1):1-7. doi: 10.3111/13696998.2011.628723.
45. Nicolaou S, Talsky A, Khashoggi K, Venu V. Ultrasound-guided interventional radiology in critical care. Crit Care Med. 2007;35(5 Suppl):S186-197. doi: 10.1097/01.CCM.0000260630.68855.DF.
46. Conduit B, Wesley E, Christie J, Thalheimer U. PTU-002 Large volume paracentesis (LVP) can be safely performed by junior doctors without ultrasound guidance. Gut. 2013;62:A42. doi: 10.1136/gutjnl-2013-304907.095.
47. Williams JW, Jr., Simel DL. The rational clinical examination. Does this patient have ascites? How to divine fluid in the abdomen. JAMA. 1992;267(19):2645-2648. doi: 10.1001/jama.1992.03480190087038.
48. Rodriguez A, DuPriest RW, Jr., Shatney CH. Recognition of intra-abdominal injury in blunt trauma victims. A prospective study comparing physical examination with peritoneal lavage. Am Surg. 1982;48(9):457-459.
49. McGibbon A, Chen GI, Peltekian KM, van Zanten SV. An evidence-based manual for abdominal paracentesis. Dig Dis Sci. 2007;52(12):3307-3315. doi: 10.1007/s10620-007-9805-5.
50. Cattau EL, Jr., Benjamin SB, Knuff TE, Castell DO. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247(8):1164-1166. doi: 10.1001/jama.1982.03320330060027.
51. Ali J, Rozycki GS, Campbell JP, Boulanger BR, Waddell JP, Gana TJ. Trauma ultrasound workshop improves physician detection of peritoneal and pericardial fluid. J Surg Res. 1996;63(1):275-279. doi: 10.1006/jsre.1996.0260.
52. Von Kuenssberg Jehle D, Stiller G, Wagner D. Sensitivity in detecting free intraperitoneal fluid with the pelvic views of the FAST exam. Am J Emerg Med. 2003;21(6):476-478. doi: 10.1016/S0735-6757(03)00162-1
53. Goldberg BB, Goodman GA, Clearfield HR. Evaluation of ascites by ultrasound. Radiology. 1970;96(1):15-22. doi: 10.1148/96.1.15.
54. Branney SW, Wolfe RE, Moore EE, et al. Quantitative sensitivity of ultrasound in detecting free intraperitoneal fluid. J Trauma. 1995;39(2):375-380. doi: 10.1016/0736-4679(96)84805-0.
55. Paajanen H, Lahti P, Nordback I. Sensitivity of transabdominal ultrasonography in detection of intraperitoneal fluid in humans. Eur Radiol. 1999;9(7):1423-1425. doi: 10.1007/s003300050861.
56. Prabhakar A, Thabet A, Mueller P, Gee MS. Image-guided peritoneal access for fluid infusion in oncology patients: Indications, technique, and outcomes. J Vasc Interv Radiol. 2014;25(3):S41. doi: 10.1016/j.jvir.2013.12.100.
57. McGahan JP, Anderson MW, Walter JP. Portable real-time sonographic and needle guidance systems for aspiration and drainage. AJR Am J Roentgenol. 1986;147(6):1241-1246. doi: 10.2214/ajr.147.6.1241.
58. Moses WR. Shifting dullness in the abdomen. South Med J. 1946;39(12):985-987.
59. Edell SL, Gefter WB. Ultrasonic differentiation of types of ascitic fluid. AJR Am J Roentgenol. 1979;133(1):111-114. doi: 10.2214/ajr.133.1.111.
60. Doust BD, Thompson R. Ultrasonography of abdominal fluid collections. Gastrointest Radiol. 1978;3(3):273-279. doi: 10.1007/BF01887079.
61. Beaulieu Y, Marik PE. Bedside ultrasonography in the ICU: part 2. Chest. 2005;128(3):1766-1781. doi: 10.1378/chest.128.3.1766.
62. Irshad A, Ackerman SJ, Anis M, Campbell AS, Hashmi A, Baker NL. Can the smallest depth of ascitic fluid on sonograms predict the amount of drainable fluid? J Clin Ultrasound. 2009;37(8):440-444. doi: 10.1002/jcu.20616.
63. Inadomi J, Cello JP, Koch J. Ultrasonographic determination of ascitic volume. Hepatology. 1996;24(3):549-551. doi: 10.1002/hep.510240314.
64. Sideris A, Patel P, Charles HW, Park J, Feldman D, Deipolyi AR. Imaging and clinical predictors of spontaneous bacterial peritonitis diagnosed by ultrasound-guided paracentesis. Proc (Bayl Univ Med Cent). 2017;30(3):262-264. https://doi.org/10.1080/08998280.2017.11929610
65. Hatch N, Wu TS, Barr L, Roque PJ. Advanced ultrasound procedures. Crit Care Clin. 2014;30(2):305-329. doi: 10.1016/j.ccc.2013.10.005.
66. Ross GJ, Kessler HB, Clair MR, Gatenby RA, Hartz WH, Ross LV. Sonographically guided paracentesis for palliation of symptomatic malignant ascites. AJR Am J Roentgenol. 1989;153(6):1309-1311. doi: 10.2214/ajr.153.6.1309.
67. Russell KW, Mone MC, Scaife CL. Umbilical paracentesis for acute hernia reduction in cirrhotic patients. BMJ Case Rep. 2013;2013. doi: 10.1136/bcr-2013-201304.
68. Epstein J, Arora A, Ellis H. Surface anatomy of the inferior epigastric artery in relation to laparoscopic injury. Clin Anat. 2004;17(5):400-408. doi: 10.1002/ca.10192.
69. Suzuki J, Sekiguchi H. Laceration of inferior epigastric artery resulting in abdominal compartment syndrome: a fatal complication of paracentesis. Am J Respir Crit Care Med. 2012;185:A5974. doi: 10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A5974
70. Saber AA, Meslemani AM, Davis R, Pimentel R. Safety zones for anterior abdominal wall entry during laparoscopy: a CT scan mapping of epigastric vessels. Ann Surg. 2004;239(2):182-185. doi: 10.1097/01.sla.0000109151.53296.07.
71. Webster ST, Brown KL, Lucey MR, Nostrant TT. Hemorrhagic complications of large volume abdominal paracentesis. Am J Gastroenterol. 1996;91(2):366-368.
72. Todd AW. Inadvertent puncture of the inferior epigastric artery during needle biopsy with fatal outcome. Clin Radiol. 2001;56(12):989-990. doi: 10.1053/crad.2001.0175.
73. Seidler M, Sayegh K, Roy A, Mesurolle B. A fatal complication of ultrasound-guided abdominal paracentesis. J Clin Ultrasound. 2013;41(7):457-460. doi: 10.1002/jcu.22050.
74. Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore). 2006;85(2):105-110. doi: 10.1097/01.md.0000216818.13067.5a.
75. Oelsner DH, Caldwell SH, Coles M, Driscoll CJ. Subumbilical midline vascularity of the abdominal wall in portal hypertension observed at laparoscopy. Gastrointest Endosc. 1998;47(5):388-390. doi: 10.1016/S0016-5107(98)70224-X.
76. Krupski WC, Sumchai A, Effeney DJ, Ehrenfeld WK. The importance of abdominal wall collateral blood vessels. Planning incisions and obtaining arteriography. Arch Surg. 1984;119(7):854-857. doi: 10.1001/archsurg.1984.01390190092021.
77. Rozen WM, Ashton MW, Taylor GI. Reviewing the vascular supply of the anterior abdominal wall: redefining anatomy for increasingly refined surgery. Clin Anat. 2008;21(2):89-98. doi: 10.1002/ca.20585.
78. Adams A, Roggio A, Wilkerson RG. 368 Sonographic assessment of inadvertent vascular puncture during paracentesis using the traditional landmark approach. Ann Emerg Med. 2015;66:S132-S133. doi: 10.1016/j.annemergmed.2015.07.404
79. Barsuk JH, Rosen BT, Cohen ER, Feinglass J, Ault MJ. Vascular ultrasonography: a novel method to reduce paracentesis related major bleeding. J Hosp Med. 2018;13(1):30-33. doi: 10.12788/jhm.2863.
80. Skolnick ML. Estimation of ultrasound beam width in the elevation (section thickness) plane. Radiology. 1991;180(1):286-288. doi: 10.1148/radiology.180.1.2052713.
81. Keil-Rios D, Terrazas-Solis H, Gonzalez-Garay A, Sanchez-Avila JF, Garcia-Juarez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11(3):461-466. doi: 10.1007/s11739-016-1406-x.
82. Kessler C, Bhandarkar S. Ultrasound training for medical students and internal medicine residents--a needs assessment. J Clin Ultrasound. 2010;38(8):401-408. doi: 10.1002/jcu.20719.
83. Schnobrich DJ, Gladding S, Olson AP, Duran-Nelson A. Point-of-care ultrasound in internal medicine: a national survey of educational leadership. J Grad Med Educ. 2013;5(3):498-502. doi: 10.4300/JGME-D-12-00215.1.
84. Eisen LA, Leung S, Gallagher AE, Kvetan V. Barriers to ultrasound training in critical care medicine fellowships: a survey of program directors. Crit Care Med. 2010;38(10):1978-1983. doi: 10.1097/CCM.0b013e3181eeda53.
85. Neri L, Storti E, Lichtenstein D. Toward an ultrasound curriculum for critical care medicine. Crit Care Med. 2007;35(5 Suppl):S290-304. doi: 10.1097/01.CCM.0000260680.16213.26.
86. Ma I, Arishenkoff S, Wiseman J, et al. Internal medicine point-of-care ultrasound curriculum: consensus recommendations from the Canadian Internal Medicine Ultrasound (CIMUS) Group. J Gen Intern Med. 2017;32(9):1052-1057. doi: 10.1007/s11606-017-4071-5.
87. Kelm D, Ratelle J, Azeem N, et al. Longitudinal ultrasound curriculum improves long-term retention among internal medicine residents. J Grad Med Educ. 2015;7(3):454-457. doi: 10.4300/JGME-14-00284.1.
88. Barsuk JH, Cohen ER, Vozenilek JA, O’Connor LM, McGaghie WC, Wayne DB. Simulation-based education with mastery learning improves paracentesis skills. J Grad Med Educ. 2012;4(1):23-27. doi: 10.4300/JGME-D-11-00161.1.
89. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. doi: 10.7556/jaoa.2010.110.6.340.
90. American Board of Internal Medicine. Policies and Procedures for Certification. Philadelphia, PA: ABIM; 2006.
91. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. doi: 10.12788/jhm.2917.
92. Brown GM, Otremba M, Devine LA, Gray C, Millington SJ, Ma IW. Defining competencies for ultrasound-guided bedside procedures: consensus opinions from Canadian physicians. J Ultrasound Med. 2016;35(1):129-141. doi: 10.7863/ultra.15.01063.
93. Babcock DS, Patriquin H, LaFortune M, Dauzat M. Power doppler sonography: basic principles and clinical applications in children. Pediatr Radiol. 1996;26(2):109-115. doi: 10.1007/BF01372087.
94. Howard ZD, Noble VE, Marill KA, et al. Bedside ultrasound maximizes patient satisfaction. J Emerg Med. 2014;46(1):46-53. doi: 10.1016/j.jemermed.2013.05.044.
95. Lindelius A, Torngren S, Nilsson L, Pettersson H, Adami J. Randomized clinical trial of bedside ultrasound among patients with abdominal pain in the emergency department: impact on patient satisfaction and health care consumption. Scand J Trauma Resusc Emerg Med. 2009;17:60. doi: 10.1186/1757-7241-17-60.
Abdominal paracentesis is a common and increasingly performed procedure in the United States. According to Medicare Physician Supplier Procedure Summary Master Files, an estimated 150,000 paracenteses were performed on Medicare fee-for-service beneficiaries in 2008 alone; such a number represents more than a two-fold increase from the same service population in 1993.1 This increasing trend was again noted by the Nationwide Inpatient Sample data, which identified a 10% increase in hospitalized patients with a diagnosis of cirrhosis receiving paracentesis from 2004 (50%) to 2012 (61%; P < .0001).2
Although these data demonstrate that paracentesis is being performed frequently, paracentesis may be underutilized in hospitalized cirrhotics with ascites. In addition, in-hospital mortality of cirrhotics with ascites is higher among those who do not undergo paracentesis than among those who do (9% vs 6%; P = .03).3,4
While complications associated with paracentesis are rare, serious complications, including death, have been documented.5-10 The most common serious complication of paracentesis is bleeding, although puncture of the bowel and other abdominal organs has also been observed. Over the past few decades, ultrasound has been increasingly used with paracentesis due to the ability of ultrasound to improve detection of ascites11,12 and to avoid blood vessels10,13-15 and bowels.16
Three-quarters of all paracenteses are currently performed by interventional radiologists.1 However, paracenteses are often required off-hours,17 when interventional radiologists are less readily available. Weekend admissions have less frequent performance of early paracentesis than weekday admissions, and delaying paracentesis may increase mortality.3,18 High proficiency in ultrasound-guided paracentesis is achievable by nonradiologists19-28 with equal or better patient outcomes after appropriate training.29
The purpose of this guideline is to review the literature and present evidence-based recommendations on the performance of ultrasound-guided paracentesis at the bedside by practicing hospitalists.
METHODS
Detailed methods are described in Appendix 1. The Society of Hospital Medicine (SHM) Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced-practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist, and all Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the five working group members themselves. Key clinical questions and draft recommendations were then prepared, and a systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were initially searched from 1975 to October 2015. Google Scholar was also searched without limiters. An updated search was conducted from November 2015 to November 2017, search strings for which are included in Appendix 3. All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of screened articles were reviewed and articles on ultrasound guidance for paracentesis were selected. The following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided paracentesis were screened and selected. Final article selection was based on working group consensus. The selected literature was incorporated into the draft recommendations.
We used the RAND Appropriateness Method that required panel judgment and consensus to establish recommendations.30 The voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering five transforming factors: (1) problem priority and importance; (2) level of quality of evidence; (3) benefit/harm balance; (4) benefit/burden balance; and (5) certainty/concerns about preferences/equity acceptability/feasibility. Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (Redcap™) during February 2018 and April 2018 (Appendix 4) and voting on appropriateness was conducted using a 9-point Likert scale. The three zones based on the 9-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points), and the degree of consensus was assessed using the RAND algorithm (Appendix 1, Figure 1, and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” A strong recommendation required 80% of the votes within one integer of the median, following RAND rules, and disagreement was defined as >30% of panelists voting outside of the zone of the median.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording for each recommendation (Tables 1 and 2). The revised consensus-based recommendations underwent internal and external review by POCUS experts from different subspecialties, and a final review of the guideline document was performed by members of the SHM POCUS Task Force, SHM Education Committee, and SHM Board of Directors. The SHM Board of Directors endorsed the document prior to submission to the Journal of Hospital Medicine.
RESULTS
Literature search
A total of 794 references were pooled and screened from literature searches conducted by a certified medical librarian in October 2015 (604 citations) and updated in November 2017 (118 citations), and working group members’ personal bibliographies and searches (72 citations; Appendix 3, Figure 2). Final selection included 91 articles that were abstracted into a data table and incorporated into the draft recommendations.
RECOMMENDATIONS
Four domains (terminology, clinical outcomes, technique, and training) with 13 draft recommendations were generated based on the literature review by the paracentesis working group. After two rounds of panel voting, one recommendation did not achieve consensus based on the RAND rules, and 12 statements received final approval. The degree of consensus based on the median score and dispersion of voting around the median are shown in Appendix 5. All 12 statements achieved consensus as strong recommendations. The strength of each recommendation and degree of consensus are summarized in Table 3.
Terminology
Abdominal paracentesis is a procedure in which fluid is aspirated from the intraperitoneal space by percutaneous insertion of a needle with or without a catheter through the abdominal wall. Throughout this document, the term “paracentesis” refers to “abdominal paracentesis.”
In this document, ultrasound-guided paracentesis refers to the use of static ultrasound guidance to mark a needle insertion site immediately prior to performing the procedure. Real-time (dynamic) ultrasound guidance refers to tracking of the needle tip with ultrasound as it traverses the abdominal wall to enter the peritoneal cavity. Landmark-based paracentesis refers to paracentesis based on physical examination alone.
RECOMMENDATIONS
Clinical outcomes
1. We recommend that ultrasound guidance should be used for paracentesis to reduce the risk of serious complications, the most common being bleeding.
Rationale. The occurrence of both minor and serious life-threatening complications from paracentesis has been well described.5-10,31,32 A recent retrospective study that evaluated 515 landmark-guided paracenteses noted that the most common minor complication was persistent ascites leakage (5%) and that the most common serious complication was postprocedural bleeding (1%).8 Studies have shown that abdominal wall hematoma and hemoperitoneum are common hemorrhagic complications of paracentesis, although inferior epigastric artery pseudoaneurysm has also been described.9,33,34
Current literature suggests that ultrasound-guided paracentesis is a safe procedure, even with reduced platelet counts or elevated international normalized ratio.35-42 Most comparative studies have shown that ultrasound guidance reduces the risk of bleeding complications compared with the use of landmarks alone,7,31,32,43-45 although a few studies did not find a significant difference between techniques.20,36,46 One large retrospective observational study that analyzed the administrative data of 69,859 paracenteses from more than 600 hospitals demonstrated that ultrasound guidance reduced the odds of bleeding complications by 68% (OR, 0.32; 95% CI, 0.25–0.41). Bleeding complication rates with and without the use of ultrasound guidance were 0.27% (CI 0.26-0.29) versus 1.25% (CI 1.21-1.29; P < .0001), respectively. More importantly, in this study, paracentesis complicated by bleeding was associated with a higher in-hospital mortality rate
2. We recommend that ultrasound guidance should be used to avoid attempting paracentesis in patients with an insufficient volume of intraperitoneal free fluid to drain.
Rationale. Abdominal physical examination is not a reliable method for determining the presence or volume of intraperitoneal free fluid, as no specific physical examination finding has consistently shown both high sensitivity and specificity for detecting intraperitoneal free fluid.11,12,20,31,47-51 Patient factors limiting the diagnostic accuracy of physical examination include body habitus, abdominal wall edema, and gaseous bowel distention.
In comparative studies, ultrasound has been found to be significantly more sensitive and specific than physical examination in detecting peritoneal free fluid.11,12 Ultrasound can detect as little as 100 mL of peritoneal free fluid,52,53 and larger volumes of fluid have higher diagnostic accuracy.53-55 In one randomized trial of 100 patients suspected of having ascites, patients were randomized to landmark-based and ultrasound-guided paracentesis groups. Of the 56 patients in the ultrasound-guided group, 14 patients suspected of having ascites on physical examination were found to have no or an insufficient volume of ascites to attempt paracentesis.20 Another study with 41 ultrasound examinations on cancer patients suspected of having intraperitoneal free fluid by history and physical examination demonstrated that only 19 (46%) were considered to have a sufficient volume of ascites by ultrasound to attempt paracentesis.38
3. We recommend that ultrasound guidance should be used for paracentesis to improve the success rates of the overall procedure.
Rationale. In addition to avoiding drainage attempts in patients with an insufficient volume of intraperitoneal free fluid, ultrasound can increase the success rate of attempted procedures by localizing the largest fluid collection and guiding selection of an optimal needle insertion site. The success rates of landmark-based paracentesis in patients suspected of having intraperitoneal free fluid by physical examination are not well described in the literature, but multiple studies report success rates of 95%-100% for paracentesis when using ultrasound guidance to select a needle insertion site.20,38,56,57 In one randomized trial comparing ultrasound-guided versus landmark-based paracentesis, ultrasound-guided paracentesis revealed a significantly higher success rate (95% of procedures performed) compared with landmark-based parancentesis (61% of procedures performed). Moreover, 87% of the initial failures in the landmark-based group underwent subsequent successful paracentesis when ultrasound guidance was used. Ultrasound revealed that the rest of the patients (13%) did not have enough fluid to attempt ultrasound-guided paracentesis.20
Technique
4. We recommend that ultrasound should be used to assess the characteristics of intraperitoneal free fluid to guide clinical decision making of where paracentesis can be safely performed.
Rationale. The presence and characteristics of intraperitoneal fluid collections are important determinants of whether paracentesis, another procedure, or no procedure should be performed in a given clinical scenario. One study reported that the overall diagnostic accuracy of physical examination for detecting ascites was only 58%,50 and many providers are unable to detect ascites by physical examination until 1L of fluid has accumulated. One small study showed that at least 500 ml of fluid must accumulate before shifting dullness could be detected.58 By contrast, ultrasound has been shown to reliably detect as little as 100 mL of peritoneal free fluid 52,53 and has been proven to be superior to physical examination in several studies.11,12 Therefore, ultrasound can be used to qualitatively determine whether a sufficient volume of intraperitoneal free fluid is present to safely perform paracentesis.
Studies have shown that ultrasound can also be used to differentiate ascites from other pathologies (eg, matted bowel loops, metastases, abscesses) in patients with suspected ascites on history and physical examination.16 In addition, ultrasound can help to better understand the etiology and distribution of the ascites.59-61 Sonographic measurements allow semiquantitative assessment of the volume of intraperitoneal free fluid, which may correlate with the amount of fluid removed in therapeutic paracentesis procedures.62,63 Furthermore, depth of a fluid collection by ultrasound may be an independent risk factor for the presence of spontaneous bacterial peritonitis (SBP), with one small study showing a higher risk of SBP with larger fluid collections than with small ones.64
5. We recommend that ultrasound should be used to identify a needle insertion site based on size of the fluid collection, thickness of the abdominal wall, and proximity to abdominal organs.
Rationale. When providers perform paracentesis using ultrasound guidance, any fluid collection that is directly visualized and accessible may be considered for drainage. The presence of ascites using ultrasound is best detected using a low-frequency transducer, such as phased array or curvilinear transducer, which provides deep penetration into the abdomen and pelvis to assess peritoneal free fluid.13,14,45,51,65 An optimal needle insertion site should be determined based on a combination of visualization of largest fluid collection, avoidance of underlying abdominal organs, and thickness of abdominal wall.13,31,66,67
6. We recommend the needle insertion site should be evaluated using color flow Doppler ultrasound to identify and avoid abdominal wall blood vessels along the anticipated needle trajectory.
Rationale. The anatomy of the superficial blood vessels of the abdominal wall, especially the lateral branches, varies greatly.68-70 Although uncommon, inadvertent laceration of an inferior epigastric artery or one of its large branches is associated with significant morbidity and mortality.10,15,69,71-73 A review of 126 cases of rectus sheath hematomas, which most likely occur due to laceration of the inferior or superior epigastric artery, at a single institution from 1992 to 2002 showed a mortality rate of 1.6%, even with aggressive intervention.74 Besides the inferior epigastric arteries, several other blood vessels are at risk of injury during paracentesis, including the inferior epigastric veins, thoracoepigastric veins, subcostal artery and vein branches, deep circumflex iliac artery and vein, and recanalized subumbilical vasculature.75-77 Laceration of any of the abdominal wall blood vessels could result in catastrophic bleeding.
Identification of abdominal wall blood vessels is most commonly performed with a high-frequency transducer using color flow Doppler ultrasound.10,13-15 A low-frequency transducer capable of color flow Doppler ultrasound may be utilized in patients with a thick abdominal wall.
Studies suggest that detection of abdominal wall blood vessels with ultrasound may reduce the risk of bleeding complications. One study showed that 43% of patients had a vascular structure present at one or more of the three traditional landmark paracentesis sites.78 Another study directly compared bleeding rates between an approach utilizing a low-frequency transducer to identify the largest collection of fluid only versus a two-transducer approach utilizing both low and high-frequency transducers to identify the largest collection of fluid and evaluate for any superficial blood vessels. In this study, which included 5,777 paracenteses, paracentesis-related minor bleeding rates were similar in both groups, but major bleeding rates were less in the group utilizing color flow Doppler to evaluate for superficial vessels (0.3% vs 0.08%); differences found between groups, however, did not reach statistical significance (P = .07).79
7. We recommend that a needle insertion site should be evaluated in multiple planes to ensure clearance from underlying abdominal organs and detect any abdominal wall blood vessels along the anticipated needle trajectory.
Rationale. Most ultrasound machines have a slice thickness of <4 mm at the focal zone.80 Considering that an ultrasound beam represents a very thin 2-dimentional cross-section of the underlying tissues, visualization in only one plane could lead to inadvertent puncture of nearby critical structures such as loops of bowel or edges of solid organs. Therefore, it is important to evaluate the needle insertion site and surrounding areas in multiple planes by tilting the transducer and rotating the transducer to orthogonal planes.61 Additionally, evaluation with color flow Doppler could be performed in a similar fashion to ensure that no large blood vessels are along the anticipated needle trajectory.
8. We recommend that a needle insertion site should be marked with ultrasound immediately before performing the procedure, and the patient should remain in the same position between marking the site and performing the procedure.
Rationale. Free-flowing peritoneal fluid and abdominal organs, especially loops of small bowel, can easily shift when a patient changes position or takes a deep breath.13,16,53 Therefore, if the patient changes position or there is a delay between marking the needle insertion site and performing the procedure, the patient should be reevaluated with ultrasound to ensure that the marked needle insertion site is still safe for paracentesis.78 After marking the needle insertion site, the skin surface should be wiped completely clean of gel, and the probe should be removed from the area before sterilizing the skin surface.
9. We recommend that using real-time ultrasound guidance for paracentesis should be considered when the fluid collection is small or difficult to access.
Rationale. Use of real-time ultrasound guidance for paracentesis has been described to drain abdominal fluid collections.13,20,62 Several studies have commented that real-time ultrasound guidance for paracentesis may be necessary in obese patients, in patients with small fluid collections, or when performing the procedure near critical structures, such as loops of small bowel, liver, or spleen.57,81 Real-time ultrasound guidance for paracentesis requires additional training in needle tracking techniques and specialized equipment to maintain sterility.
Training
10. We recommend that dedicated training sessions, including didactics, supervised practice on patients, and simulation-based practice, should be used to teach novices how to perform ultrasound-guided paracentesis.
Rationale. Healthcare providers must gain multiple skills to safely perform ultrasound-guided paracentesis. Trainees must learn how to operate the ultrasound machine to identify the most appropriate needle insertion site based on the abdominal wall thickness, fluid collection size, proximity to nearby abdominal organs, and presence of blood vessels. Education regarding the use of ultrasound guidance for paracentesis is both desired 82,83 and being increasingly taught to health care providers who perform paracentesis.20,84-86
Several approaches have shown high uptake of essential skills to perform ultrasound-guided paracentesis after short training sessions. One study showed that first-year medical students can be taught to use POCUS to accurately diagnose ascites after three 30-minute teaching sessions.19 Another study showed that emergency medicine residents can achieve high levels of proficiency in the preprocedural ultrasound evaluation for paracentesis with only one hour of didactic training.20 Other studies also support the concept that adequate proficiency is achievable within brief, focused training sessions.21-28 However, these skills can decay significantly over time without ongoing education.87
11. We recommend that simulation-based practice should be used, when available, to facilitate acquisition of the required knowledge and skills to perform ultrasound-guided paracentesis.
Rationale. Simulation-based practice should be used when available, as it has been shown to increase competence in bedside diagnostic ultrasonography and procedural techniques for ultrasound-guided procedures, including paracentesis.22,25,29,88,89 One study showed that internal medicine residents were able to achieve a high level of proficiency to perform ultrasound-guided paracentesis after a three-hour simulation-based mastery learning session.88 A follow-up study suggested that, after sufficient simulation-based training, a nonradiologist can perform ultrasound-guided paracentesis as safely as an interventional radiologist.29
12. We recommend that competence in performing ultrasound-guided paracentesis should be demonstrated prior to independently performing the procedure on patients.
Rationale. Competence in ultrasound-guided paracentesis requires acquisition of clinical knowledge of paracentesis, skills in basic abdominal ultrasonography, and manual techniques to perform the procedure. Competence in ultrasound-guided paracentesis cannot be assumed for those graduating from internal medicine residency in the United States. While clinical knowledge of paracentesis remains a core competency of graduating internal medicine residents per the American Board of Internal Medicine, demonstration of competence in performing ultrasound-guided or landmark-based paracentesis is not currently mandated.90 A recent national survey of internal medicine residency program directors revealed that the curricula and resources available to train residents in bedside diagnostic ultrasound and ultrasound-guided procedures, including paracentesis, remain quite variable. 83
While it has not been well studied, competence in ultrasound for paracentesis, as with all other skills involved in bedside procedures, is likely best evaluated through direct observation on actual patients.91 As such, individualized systems to evaluate competency in ultrasound-guided paracentesis should be established for each site where it is performed. A list of consensus-derived ultrasound competencies for ultrasound-guided paracentesis has been proposed, and this list may serve as a guide for both training curriculum development and practitioner evaluation.86,91,92
KNOWLEDGE GAPS
In the process of developing these recommendations, we identified several important gaps in the literature regarding the use of ultrasound guidance for paracentesis.
First, while some data suggest that the use of ultrasound guidance for paracentesis may reduce the inpatient length of stay and overall costs, this suggestion has not been studied rigorously. In a retrospective review of 1,297 abdominal paracenteses by Patel et al., ultrasound-guided paracentesis was associated with a lower incidence of adverse events compared with landmark-based paracentesis (1.4% vs 4.7%; P = .01). The adjusted analysis from this study showed significant reductions in adverse events (OR 0.35; 95%CI 0.165-0.739; P = .006) and hospitalization costs ($8,761 ± $5,956 vs $9,848 ± $6,581; P < .001) for paracentesis with ultrasound guidance versus without such guidance. Additionally, the adjusted average length of stay was 0.2 days shorter for paracentesis with ultrasound guidance versus that without guidance (5.6 days vs 5.8 days; P < .0001).44 Similar conclusions were reached by Mercaldi et al., who conducted a retrospective study of 69,859 patients who underwent paracentesis. Fewer bleeding complications occurred when paracentesis was performed with ultrasound guidance (0.27%) versus without ultrasound guidance (1.27%). Hospitalization costs increased by $19,066 (P < .0001) and length of stay increased by 4.3 days (P < .0001) for patients when paracentesis was complicated by bleeding.43 Because both of these studies were retrospective reviews of administrative databases, associations between procedures, complications, and use of ultrasound may be limited by erroneous coding and documentation.
Second, regarding technique, it is unknown whether the use of real-time ultrasound guidance confers additional benefits compared with use of static ultrasound to mark a suitable needle insertion site. In clinical practice, real-time ultrasound guidance is used to sample small fluid collections, particularly when loops of bowel or a solid organ are nearby. It is possible that higher procedural success rates and lower complication rates may be demonstrated in these scenarios in future studies.
Third, the optimal approach to train providers to perform ultrasound-guided paracentesis is unknown. While short training sessions have shown high uptake of essential skills to perform ultrasound-guided paracentesis, data regarding the effectiveness of training using a comprehensive competency assessment are limited. Simulation-based mastery learning as a means to obtain competency for paracentesis has been described in one study,88 but the translation of competency demonstrated by simulation to actual patient outcomes has not been studied. Furthermore, the most effective method to train providers who are proficient in landmark-based paracentesis to achieve competency in ultrasound-guided paracentesis has not been well studied.
Fourth, the optimal technique for identifying blood vessels in the abdominal wall is unknown. We have proposed that color flow Doppler should be used to identify and avoid puncture of superficial vessels, but power Doppler is three times more sensitive at detecting blood vessels, especially at low velocities, such as in veins independent of direction or flow.93 Hence using power Doppler instead of color flow Doppler may further improve the ability to identify and avoid superficial vessels along the needle trajectory.92
Finally, the impact of ultrasound use on patient experience has yet to be studied. Some studies in the literature show high patient satisfaction with use of ultrasound at the bedside,94,95 but patient satisfaction with ultrasound-guided paracentesis has not been compared directly with the landmark-based technique.
CONCLUSIONS
The use of ultrasound guidance for paracentesis has been associated with higher success rates and lower complication rates. Ultrasound is superior to physical examination in assessing the presence and volume of ascites, and determining the optimal needle insertion site to avoid inadvertent injury to abdominal wall blood vessels. Hospitalists can attain competence in ultrasound-guided paracentesis through the use of various training methods, including lectures, simulation-based practice, and hands-on training. Ongoing use and training over time is necessary to maintain competence.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam Soni, Ricardo Franco Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Mathews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen. Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Collaborators of the Society of Hospital Medicine Point-of-care Ultrasound Task Force
Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Michael Blaivas, Dan Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Venkat Kalidindi, Ketino Kobaidze, Josh Lenchus, Paul Mayo, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Sophia Rodgers, Gerard Salame, Daniel Schnobrich, Kirk Spencer, Vivek Tayal, David M. Tierney
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
All 5 appendices are viewable online at https://www.journalofhospitalmedicine.com.
Abdominal paracentesis is a common and increasingly performed procedure in the United States. According to Medicare Physician Supplier Procedure Summary Master Files, an estimated 150,000 paracenteses were performed on Medicare fee-for-service beneficiaries in 2008 alone; such a number represents more than a two-fold increase from the same service population in 1993.1 This increasing trend was again noted by the Nationwide Inpatient Sample data, which identified a 10% increase in hospitalized patients with a diagnosis of cirrhosis receiving paracentesis from 2004 (50%) to 2012 (61%; P < .0001).2
Although these data demonstrate that paracentesis is being performed frequently, paracentesis may be underutilized in hospitalized cirrhotics with ascites. In addition, in-hospital mortality of cirrhotics with ascites is higher among those who do not undergo paracentesis than among those who do (9% vs 6%; P = .03).3,4
While complications associated with paracentesis are rare, serious complications, including death, have been documented.5-10 The most common serious complication of paracentesis is bleeding, although puncture of the bowel and other abdominal organs has also been observed. Over the past few decades, ultrasound has been increasingly used with paracentesis due to the ability of ultrasound to improve detection of ascites11,12 and to avoid blood vessels10,13-15 and bowels.16
Three-quarters of all paracenteses are currently performed by interventional radiologists.1 However, paracenteses are often required off-hours,17 when interventional radiologists are less readily available. Weekend admissions have less frequent performance of early paracentesis than weekday admissions, and delaying paracentesis may increase mortality.3,18 High proficiency in ultrasound-guided paracentesis is achievable by nonradiologists19-28 with equal or better patient outcomes after appropriate training.29
The purpose of this guideline is to review the literature and present evidence-based recommendations on the performance of ultrasound-guided paracentesis at the bedside by practicing hospitalists.
METHODS
Detailed methods are described in Appendix 1. The Society of Hospital Medicine (SHM) Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced-practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist, and all Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the five working group members themselves. Key clinical questions and draft recommendations were then prepared, and a systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were initially searched from 1975 to October 2015. Google Scholar was also searched without limiters. An updated search was conducted from November 2015 to November 2017, search strings for which are included in Appendix 3. All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of screened articles were reviewed and articles on ultrasound guidance for paracentesis were selected. The following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided paracentesis were screened and selected. Final article selection was based on working group consensus. The selected literature was incorporated into the draft recommendations.
We used the RAND Appropriateness Method that required panel judgment and consensus to establish recommendations.30 The voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering five transforming factors: (1) problem priority and importance; (2) level of quality of evidence; (3) benefit/harm balance; (4) benefit/burden balance; and (5) certainty/concerns about preferences/equity acceptability/feasibility. Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (Redcap™) during February 2018 and April 2018 (Appendix 4) and voting on appropriateness was conducted using a 9-point Likert scale. The three zones based on the 9-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points), and the degree of consensus was assessed using the RAND algorithm (Appendix 1, Figure 1, and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” A strong recommendation required 80% of the votes within one integer of the median, following RAND rules, and disagreement was defined as >30% of panelists voting outside of the zone of the median.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording for each recommendation (Tables 1 and 2). The revised consensus-based recommendations underwent internal and external review by POCUS experts from different subspecialties, and a final review of the guideline document was performed by members of the SHM POCUS Task Force, SHM Education Committee, and SHM Board of Directors. The SHM Board of Directors endorsed the document prior to submission to the Journal of Hospital Medicine.
RESULTS
Literature search
A total of 794 references were pooled and screened from literature searches conducted by a certified medical librarian in October 2015 (604 citations) and updated in November 2017 (118 citations), and working group members’ personal bibliographies and searches (72 citations; Appendix 3, Figure 2). Final selection included 91 articles that were abstracted into a data table and incorporated into the draft recommendations.
RECOMMENDATIONS
Four domains (terminology, clinical outcomes, technique, and training) with 13 draft recommendations were generated based on the literature review by the paracentesis working group. After two rounds of panel voting, one recommendation did not achieve consensus based on the RAND rules, and 12 statements received final approval. The degree of consensus based on the median score and dispersion of voting around the median are shown in Appendix 5. All 12 statements achieved consensus as strong recommendations. The strength of each recommendation and degree of consensus are summarized in Table 3.
Terminology
Abdominal paracentesis is a procedure in which fluid is aspirated from the intraperitoneal space by percutaneous insertion of a needle with or without a catheter through the abdominal wall. Throughout this document, the term “paracentesis” refers to “abdominal paracentesis.”
In this document, ultrasound-guided paracentesis refers to the use of static ultrasound guidance to mark a needle insertion site immediately prior to performing the procedure. Real-time (dynamic) ultrasound guidance refers to tracking of the needle tip with ultrasound as it traverses the abdominal wall to enter the peritoneal cavity. Landmark-based paracentesis refers to paracentesis based on physical examination alone.
RECOMMENDATIONS
Clinical outcomes
1. We recommend that ultrasound guidance should be used for paracentesis to reduce the risk of serious complications, the most common being bleeding.
Rationale. The occurrence of both minor and serious life-threatening complications from paracentesis has been well described.5-10,31,32 A recent retrospective study that evaluated 515 landmark-guided paracenteses noted that the most common minor complication was persistent ascites leakage (5%) and that the most common serious complication was postprocedural bleeding (1%).8 Studies have shown that abdominal wall hematoma and hemoperitoneum are common hemorrhagic complications of paracentesis, although inferior epigastric artery pseudoaneurysm has also been described.9,33,34
Current literature suggests that ultrasound-guided paracentesis is a safe procedure, even with reduced platelet counts or elevated international normalized ratio.35-42 Most comparative studies have shown that ultrasound guidance reduces the risk of bleeding complications compared with the use of landmarks alone,7,31,32,43-45 although a few studies did not find a significant difference between techniques.20,36,46 One large retrospective observational study that analyzed the administrative data of 69,859 paracenteses from more than 600 hospitals demonstrated that ultrasound guidance reduced the odds of bleeding complications by 68% (OR, 0.32; 95% CI, 0.25–0.41). Bleeding complication rates with and without the use of ultrasound guidance were 0.27% (CI 0.26-0.29) versus 1.25% (CI 1.21-1.29; P < .0001), respectively. More importantly, in this study, paracentesis complicated by bleeding was associated with a higher in-hospital mortality rate
2. We recommend that ultrasound guidance should be used to avoid attempting paracentesis in patients with an insufficient volume of intraperitoneal free fluid to drain.
Rationale. Abdominal physical examination is not a reliable method for determining the presence or volume of intraperitoneal free fluid, as no specific physical examination finding has consistently shown both high sensitivity and specificity for detecting intraperitoneal free fluid.11,12,20,31,47-51 Patient factors limiting the diagnostic accuracy of physical examination include body habitus, abdominal wall edema, and gaseous bowel distention.
In comparative studies, ultrasound has been found to be significantly more sensitive and specific than physical examination in detecting peritoneal free fluid.11,12 Ultrasound can detect as little as 100 mL of peritoneal free fluid,52,53 and larger volumes of fluid have higher diagnostic accuracy.53-55 In one randomized trial of 100 patients suspected of having ascites, patients were randomized to landmark-based and ultrasound-guided paracentesis groups. Of the 56 patients in the ultrasound-guided group, 14 patients suspected of having ascites on physical examination were found to have no or an insufficient volume of ascites to attempt paracentesis.20 Another study with 41 ultrasound examinations on cancer patients suspected of having intraperitoneal free fluid by history and physical examination demonstrated that only 19 (46%) were considered to have a sufficient volume of ascites by ultrasound to attempt paracentesis.38
3. We recommend that ultrasound guidance should be used for paracentesis to improve the success rates of the overall procedure.
Rationale. In addition to avoiding drainage attempts in patients with an insufficient volume of intraperitoneal free fluid, ultrasound can increase the success rate of attempted procedures by localizing the largest fluid collection and guiding selection of an optimal needle insertion site. The success rates of landmark-based paracentesis in patients suspected of having intraperitoneal free fluid by physical examination are not well described in the literature, but multiple studies report success rates of 95%-100% for paracentesis when using ultrasound guidance to select a needle insertion site.20,38,56,57 In one randomized trial comparing ultrasound-guided versus landmark-based paracentesis, ultrasound-guided paracentesis revealed a significantly higher success rate (95% of procedures performed) compared with landmark-based parancentesis (61% of procedures performed). Moreover, 87% of the initial failures in the landmark-based group underwent subsequent successful paracentesis when ultrasound guidance was used. Ultrasound revealed that the rest of the patients (13%) did not have enough fluid to attempt ultrasound-guided paracentesis.20
Technique
4. We recommend that ultrasound should be used to assess the characteristics of intraperitoneal free fluid to guide clinical decision making of where paracentesis can be safely performed.
Rationale. The presence and characteristics of intraperitoneal fluid collections are important determinants of whether paracentesis, another procedure, or no procedure should be performed in a given clinical scenario. One study reported that the overall diagnostic accuracy of physical examination for detecting ascites was only 58%,50 and many providers are unable to detect ascites by physical examination until 1L of fluid has accumulated. One small study showed that at least 500 ml of fluid must accumulate before shifting dullness could be detected.58 By contrast, ultrasound has been shown to reliably detect as little as 100 mL of peritoneal free fluid 52,53 and has been proven to be superior to physical examination in several studies.11,12 Therefore, ultrasound can be used to qualitatively determine whether a sufficient volume of intraperitoneal free fluid is present to safely perform paracentesis.
Studies have shown that ultrasound can also be used to differentiate ascites from other pathologies (eg, matted bowel loops, metastases, abscesses) in patients with suspected ascites on history and physical examination.16 In addition, ultrasound can help to better understand the etiology and distribution of the ascites.59-61 Sonographic measurements allow semiquantitative assessment of the volume of intraperitoneal free fluid, which may correlate with the amount of fluid removed in therapeutic paracentesis procedures.62,63 Furthermore, depth of a fluid collection by ultrasound may be an independent risk factor for the presence of spontaneous bacterial peritonitis (SBP), with one small study showing a higher risk of SBP with larger fluid collections than with small ones.64
5. We recommend that ultrasound should be used to identify a needle insertion site based on size of the fluid collection, thickness of the abdominal wall, and proximity to abdominal organs.
Rationale. When providers perform paracentesis using ultrasound guidance, any fluid collection that is directly visualized and accessible may be considered for drainage. The presence of ascites using ultrasound is best detected using a low-frequency transducer, such as phased array or curvilinear transducer, which provides deep penetration into the abdomen and pelvis to assess peritoneal free fluid.13,14,45,51,65 An optimal needle insertion site should be determined based on a combination of visualization of largest fluid collection, avoidance of underlying abdominal organs, and thickness of abdominal wall.13,31,66,67
6. We recommend the needle insertion site should be evaluated using color flow Doppler ultrasound to identify and avoid abdominal wall blood vessels along the anticipated needle trajectory.
Rationale. The anatomy of the superficial blood vessels of the abdominal wall, especially the lateral branches, varies greatly.68-70 Although uncommon, inadvertent laceration of an inferior epigastric artery or one of its large branches is associated with significant morbidity and mortality.10,15,69,71-73 A review of 126 cases of rectus sheath hematomas, which most likely occur due to laceration of the inferior or superior epigastric artery, at a single institution from 1992 to 2002 showed a mortality rate of 1.6%, even with aggressive intervention.74 Besides the inferior epigastric arteries, several other blood vessels are at risk of injury during paracentesis, including the inferior epigastric veins, thoracoepigastric veins, subcostal artery and vein branches, deep circumflex iliac artery and vein, and recanalized subumbilical vasculature.75-77 Laceration of any of the abdominal wall blood vessels could result in catastrophic bleeding.
Identification of abdominal wall blood vessels is most commonly performed with a high-frequency transducer using color flow Doppler ultrasound.10,13-15 A low-frequency transducer capable of color flow Doppler ultrasound may be utilized in patients with a thick abdominal wall.
Studies suggest that detection of abdominal wall blood vessels with ultrasound may reduce the risk of bleeding complications. One study showed that 43% of patients had a vascular structure present at one or more of the three traditional landmark paracentesis sites.78 Another study directly compared bleeding rates between an approach utilizing a low-frequency transducer to identify the largest collection of fluid only versus a two-transducer approach utilizing both low and high-frequency transducers to identify the largest collection of fluid and evaluate for any superficial blood vessels. In this study, which included 5,777 paracenteses, paracentesis-related minor bleeding rates were similar in both groups, but major bleeding rates were less in the group utilizing color flow Doppler to evaluate for superficial vessels (0.3% vs 0.08%); differences found between groups, however, did not reach statistical significance (P = .07).79
7. We recommend that a needle insertion site should be evaluated in multiple planes to ensure clearance from underlying abdominal organs and detect any abdominal wall blood vessels along the anticipated needle trajectory.
Rationale. Most ultrasound machines have a slice thickness of <4 mm at the focal zone.80 Considering that an ultrasound beam represents a very thin 2-dimentional cross-section of the underlying tissues, visualization in only one plane could lead to inadvertent puncture of nearby critical structures such as loops of bowel or edges of solid organs. Therefore, it is important to evaluate the needle insertion site and surrounding areas in multiple planes by tilting the transducer and rotating the transducer to orthogonal planes.61 Additionally, evaluation with color flow Doppler could be performed in a similar fashion to ensure that no large blood vessels are along the anticipated needle trajectory.
8. We recommend that a needle insertion site should be marked with ultrasound immediately before performing the procedure, and the patient should remain in the same position between marking the site and performing the procedure.
Rationale. Free-flowing peritoneal fluid and abdominal organs, especially loops of small bowel, can easily shift when a patient changes position or takes a deep breath.13,16,53 Therefore, if the patient changes position or there is a delay between marking the needle insertion site and performing the procedure, the patient should be reevaluated with ultrasound to ensure that the marked needle insertion site is still safe for paracentesis.78 After marking the needle insertion site, the skin surface should be wiped completely clean of gel, and the probe should be removed from the area before sterilizing the skin surface.
9. We recommend that using real-time ultrasound guidance for paracentesis should be considered when the fluid collection is small or difficult to access.
Rationale. Use of real-time ultrasound guidance for paracentesis has been described to drain abdominal fluid collections.13,20,62 Several studies have commented that real-time ultrasound guidance for paracentesis may be necessary in obese patients, in patients with small fluid collections, or when performing the procedure near critical structures, such as loops of small bowel, liver, or spleen.57,81 Real-time ultrasound guidance for paracentesis requires additional training in needle tracking techniques and specialized equipment to maintain sterility.
Training
10. We recommend that dedicated training sessions, including didactics, supervised practice on patients, and simulation-based practice, should be used to teach novices how to perform ultrasound-guided paracentesis.
Rationale. Healthcare providers must gain multiple skills to safely perform ultrasound-guided paracentesis. Trainees must learn how to operate the ultrasound machine to identify the most appropriate needle insertion site based on the abdominal wall thickness, fluid collection size, proximity to nearby abdominal organs, and presence of blood vessels. Education regarding the use of ultrasound guidance for paracentesis is both desired 82,83 and being increasingly taught to health care providers who perform paracentesis.20,84-86
Several approaches have shown high uptake of essential skills to perform ultrasound-guided paracentesis after short training sessions. One study showed that first-year medical students can be taught to use POCUS to accurately diagnose ascites after three 30-minute teaching sessions.19 Another study showed that emergency medicine residents can achieve high levels of proficiency in the preprocedural ultrasound evaluation for paracentesis with only one hour of didactic training.20 Other studies also support the concept that adequate proficiency is achievable within brief, focused training sessions.21-28 However, these skills can decay significantly over time without ongoing education.87
11. We recommend that simulation-based practice should be used, when available, to facilitate acquisition of the required knowledge and skills to perform ultrasound-guided paracentesis.
Rationale. Simulation-based practice should be used when available, as it has been shown to increase competence in bedside diagnostic ultrasonography and procedural techniques for ultrasound-guided procedures, including paracentesis.22,25,29,88,89 One study showed that internal medicine residents were able to achieve a high level of proficiency to perform ultrasound-guided paracentesis after a three-hour simulation-based mastery learning session.88 A follow-up study suggested that, after sufficient simulation-based training, a nonradiologist can perform ultrasound-guided paracentesis as safely as an interventional radiologist.29
12. We recommend that competence in performing ultrasound-guided paracentesis should be demonstrated prior to independently performing the procedure on patients.
Rationale. Competence in ultrasound-guided paracentesis requires acquisition of clinical knowledge of paracentesis, skills in basic abdominal ultrasonography, and manual techniques to perform the procedure. Competence in ultrasound-guided paracentesis cannot be assumed for those graduating from internal medicine residency in the United States. While clinical knowledge of paracentesis remains a core competency of graduating internal medicine residents per the American Board of Internal Medicine, demonstration of competence in performing ultrasound-guided or landmark-based paracentesis is not currently mandated.90 A recent national survey of internal medicine residency program directors revealed that the curricula and resources available to train residents in bedside diagnostic ultrasound and ultrasound-guided procedures, including paracentesis, remain quite variable. 83
While it has not been well studied, competence in ultrasound for paracentesis, as with all other skills involved in bedside procedures, is likely best evaluated through direct observation on actual patients.91 As such, individualized systems to evaluate competency in ultrasound-guided paracentesis should be established for each site where it is performed. A list of consensus-derived ultrasound competencies for ultrasound-guided paracentesis has been proposed, and this list may serve as a guide for both training curriculum development and practitioner evaluation.86,91,92
KNOWLEDGE GAPS
In the process of developing these recommendations, we identified several important gaps in the literature regarding the use of ultrasound guidance for paracentesis.
First, while some data suggest that the use of ultrasound guidance for paracentesis may reduce the inpatient length of stay and overall costs, this suggestion has not been studied rigorously. In a retrospective review of 1,297 abdominal paracenteses by Patel et al., ultrasound-guided paracentesis was associated with a lower incidence of adverse events compared with landmark-based paracentesis (1.4% vs 4.7%; P = .01). The adjusted analysis from this study showed significant reductions in adverse events (OR 0.35; 95%CI 0.165-0.739; P = .006) and hospitalization costs ($8,761 ± $5,956 vs $9,848 ± $6,581; P < .001) for paracentesis with ultrasound guidance versus without such guidance. Additionally, the adjusted average length of stay was 0.2 days shorter for paracentesis with ultrasound guidance versus that without guidance (5.6 days vs 5.8 days; P < .0001).44 Similar conclusions were reached by Mercaldi et al., who conducted a retrospective study of 69,859 patients who underwent paracentesis. Fewer bleeding complications occurred when paracentesis was performed with ultrasound guidance (0.27%) versus without ultrasound guidance (1.27%). Hospitalization costs increased by $19,066 (P < .0001) and length of stay increased by 4.3 days (P < .0001) for patients when paracentesis was complicated by bleeding.43 Because both of these studies were retrospective reviews of administrative databases, associations between procedures, complications, and use of ultrasound may be limited by erroneous coding and documentation.
Second, regarding technique, it is unknown whether the use of real-time ultrasound guidance confers additional benefits compared with use of static ultrasound to mark a suitable needle insertion site. In clinical practice, real-time ultrasound guidance is used to sample small fluid collections, particularly when loops of bowel or a solid organ are nearby. It is possible that higher procedural success rates and lower complication rates may be demonstrated in these scenarios in future studies.
Third, the optimal approach to train providers to perform ultrasound-guided paracentesis is unknown. While short training sessions have shown high uptake of essential skills to perform ultrasound-guided paracentesis, data regarding the effectiveness of training using a comprehensive competency assessment are limited. Simulation-based mastery learning as a means to obtain competency for paracentesis has been described in one study,88 but the translation of competency demonstrated by simulation to actual patient outcomes has not been studied. Furthermore, the most effective method to train providers who are proficient in landmark-based paracentesis to achieve competency in ultrasound-guided paracentesis has not been well studied.
Fourth, the optimal technique for identifying blood vessels in the abdominal wall is unknown. We have proposed that color flow Doppler should be used to identify and avoid puncture of superficial vessels, but power Doppler is three times more sensitive at detecting blood vessels, especially at low velocities, such as in veins independent of direction or flow.93 Hence using power Doppler instead of color flow Doppler may further improve the ability to identify and avoid superficial vessels along the needle trajectory.92
Finally, the impact of ultrasound use on patient experience has yet to be studied. Some studies in the literature show high patient satisfaction with use of ultrasound at the bedside,94,95 but patient satisfaction with ultrasound-guided paracentesis has not been compared directly with the landmark-based technique.
CONCLUSIONS
The use of ultrasound guidance for paracentesis has been associated with higher success rates and lower complication rates. Ultrasound is superior to physical examination in assessing the presence and volume of ascites, and determining the optimal needle insertion site to avoid inadvertent injury to abdominal wall blood vessels. Hospitalists can attain competence in ultrasound-guided paracentesis through the use of various training methods, including lectures, simulation-based practice, and hands-on training. Ongoing use and training over time is necessary to maintain competence.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam Soni, Ricardo Franco Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Mathews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen. Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Collaborators of the Society of Hospital Medicine Point-of-care Ultrasound Task Force
Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Michael Blaivas, Dan Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Venkat Kalidindi, Ketino Kobaidze, Josh Lenchus, Paul Mayo, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Sophia Rodgers, Gerard Salame, Daniel Schnobrich, Kirk Spencer, Vivek Tayal, David M. Tierney
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
All 5 appendices are viewable online at https://www.journalofhospitalmedicine.com.
1. Duszak R, Jr., Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859-864. doi: 10.1016/j.jacr.2010.04.013.
2. O’Brien CR, Chang J, Campos RA, et al. Characterizing the safety of paracentesis in hospitalized patients with cirrhosis and ascites from 2004-2012 in the United States. Gastroenterology. 2016;150(4). https:/doi.org /10.1016/S0016-5085(16)32196-5.
3. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. doi: 10.1016/S0016-5085(16)32196-5
4. Orman ES, Hayashi PH, Bataller R, Barritt AS. Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2014;12(3):496-503.e1. doi: 10.1016/j.cgh.2013.08.025.
5. Mallory A, Schaefer JW. Complications of diagnostic paracentesis in patients with liver disease. JAMA. 1978;239(7):628-630. doi: 10.1001/jama.1978.03280340048020.
6. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525-529. doi: 10.1111/j.1365-2036.2005.02387.x.
7. Shekhar C, Ramakrishnan A, Claridge LC. Paracentesis: UK trainees’ practice, experience and attitudes. Gut. 2013;62:A42. doi: 10.1136/gutjnl-2013-304907.096.
8. De Gottardi A, Thevenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7(8):906-909. doi: 10.1016/j.cgh.2009.05.004.
9. Sharzehi K, Jain V, Naveed A, Schreibman I. Hemorrhagic complications of paracentesis: a systematic review of the literature. Gastroenterol Res Pract. 2014;2014:985141. doi: 10.1155/2014/985141.
10. Sekiguchi H, Suzuki J, Daniels CE. Making paracentesis safer: a proposal for the use of bedside abdominal and vascular ultrasonography to prevent a fatal complication. Chest. 2013;143(4):1136-1139. doi: 10.1378/chest.12-0871.
11. Soyuncu S, Cete Y, Bozan H, Kartal M, Akyol AJ. Accuracy of physical and ultrasonographic examinations by emergency physicians for the early diagnosis of intraabdominal haemorrhage in blunt abdominal trauma. Injury. 2007;38(5):564-569. doi: 10.1016/j.injury.2007.01.010.
12. Chongtham DS, Singh MM, Kalantri SP, Pathak S, Jain AP. Accuracy of clinical manoeuvres in detection of minimal ascites. Indian J Med Sci. 1998;52(11):514-520.
13. Ennis J, Schultz G, Perera P, Williams S, Gharahbaghian L, Mandavia D. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293. doi: 10.4236/ijcm.2014.520163.
14. Szabo TL, Lewin PA. Ultrasound transducer selection in clinical imaging practice. J Ultrasound Med. 2013;32(4):573-582. doi: 10.7863/jum.2013.32.4.573.
15. Stone JC, Moak JH. Feasibility of sonographic localization of the inferior epigastric artery before ultrasound-guided paracentesis. Am J Emerg Med. 2015;33(12):1795-1798. doi: 10.1016/j.ajem.2015.06.067.
16. Yeh HC, Wolf BS. Ultrasonography in ascites. Radiology. 1977;124(3):783-790. doi: 10.1148/124.3.783.
17. Lucas BP, Asbury JK, Wang Y, et al. Impact of a bedside procedure service on general medicine inpatients: a firm-based trial. J Hosp Med. 2007;2(3):143-149. doi: 10.1002/jhm.159.
18. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. doi: 10.1038/ajg.2014.212.
19. Arora S, Cheung A, Tarique U, Agarwal A, Firdouse M, Ailon J. First-year medical students use of ultrasound or physical examination to diagnose hepatomegaly and ascites: a randomized controlled trial. J Ultrasound. 2017;20(3):199-204. doi: 10.1007/s40477-017-0261-6.
20. Nazeer SR, Dewbre H, Miller AH. Ultrasound-assisted paracentesis performed by emergency physicians vs the traditional technique: a prospective, randomized study. Am J Emerg Med. 2005;23(3):363-367. doi: 10.1016/j.ajem.2004.11.001.
21. Kotagal M, Quiroga E, Ruffatto BJ, et al. Impact of point-of-care ultrasound training on surgical residents’ confidence. J Surg Educ. 2015;72(4):e82-87. doi: 10.1016/j.jsurg.2015.01.021.
22. Martin R, Gannon D, Riggle J, et al. A comprehensive workshop using simulation to train internal medicine residents in bedside procedures performed by internists. Chest. 2012;142(4):545A. doi: 10.1378/chest.1390093.
23. Quddus A, Minami T, Summerhill E. Impact of a short 3-hour ultrasound training workshop for internal medicine residents. Chest. 2014;146(4): 509A. doi: 10.1378/chest.1989267.
24. Lanoix R, Leak LV, Gaeta T, Gernsheimer JR. A preliminary evaluation of emergency ultrasound in the setting of an emergency medicine training program. Am J Emerg Med. 2000;18(1):41-45. doi: 10.1016/S0735-6757(00)90046-9.
25. Dulohery MM, Eaton J, Tajouri T, Bhagra A. Ultrasound for internal medicine physicians: the future of physical exam. J Ultrasound Med. 2014;33(6):1005-1011. doi: 10.7863/ultra.33.6.1005
26. Lanoix R, Baker WE, Mele JM, Dharmarajan L. Evaluation of an instructional model for emergency ultrasonography. Acad Emerg Med. 1998;5(1):58-63. doi: 10.1111/j.1553-2712.1998.tb02576.x.
27. Terkamp C, Kirchner G, Wedemeyer J, et al. Simulation of abdomen sonography. Evaluation of a new ultrasound simulator. Ultraschall Med. 2003;24(4):239-234. doi: 10.1055/s-2003-41713.
28. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. doi: 10.1136/bmjqs-2013-002665.
29. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. doi: 10.1016/j.amjmed.2012.09.016.
30. Fitch K, Bernstein SJ, Aguilar MD, et al. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: Rand Corp.; 2001.
31. Bard C, Lafortune M, Breton G. Ascites: ultrasound guidance or blind paracentesis? CMAJ. 1986;135(3):209-210. doi: 10.1016/0736-4679(87)90268-X.
32. Sudulagunta SR, Sodalagunta MB, Bangalore Raja SK, Khorram H, Sepehrar M, Noroozpour Z. Clinical profile and complications of paracentesis in refractory ascites patients with cirrhosis. Gastroenterol Res. 2015;8(3-4):228-233. doi: 10.14740/gr661w.
33. Lin S, Wang M, Zhu Y, et al. Hemorrhagic complications following abdominal paracentesis in acute on chronic liver failure: a propensity score analysis. Medicine (Baltimore). 2015;94(49):e2225. doi: 10.1097/MD.0000000000002225.
34. Lam EY, McLafferty RB, Taylor LM, Jr., et al. Inferior epigastric artery pseudoaneurysm: a complication of paracentesis. J Vasc Surg. 1998;28(3):566-569. doi: 10.1016/S0741-5214(98)70147-8.
35. Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound-guided intervention. AJR Am J Roentgenol. 2010;195(4):846-850. doi: 10.2214/AJR.09.3168.
36. Wiese SS, Mortensen C, Bendtsen F. Few complications after paracentesis in patients with cirrhosis and refractory ascites. Dan Med Bull. 2011;58(1):A4212.
37. Jakobson DJ, Shemesh I. Merging ultrasound in the intensive care routine. Isr Med Assoc J. 2013;15(11):688-692.
38. Landers A, Ryan B. The use of bedside ultrasound and community-based paracentesis in a palliative care service. J Prim Health Care. 2014;6(2):148-151.
39. Lin CH, Shih FY, Ma MH, Chiang WC, Yang CW, Ko PC. Should bleeding tendency deter abdominal paracentesis? Dig Liver Dis. 2005;37(12):946-951. doi: 10.1016/j.dld.2005.07.009.
40. Kurup AN, Lekah A, Reardon ST, et al. Bleeding rate for ultrasound-guided paracentesis in thrombocytopenic patients. J Ultrasound Med. 2015;34(10):1833-1838. doi: 10.7863/ultra.14.10034.
41. Reardon S, Atwell TD, Lekah A. Major bleeding complication rate of ultrasound-guided paracentesis in thrombocytopenic patients. J Vasc Interv Radiol. 2013;24(4):S56. doi: 10.1016/j.jvir.2013.01.129.
42. Czul F, Prager M, Lenchus J. Intra-procedural risk of bleeding associated with ultrasound guided paracentesis in patients with abnormal coagulation studies: 1907. Hepatology. 2011;54(4):1259A.
43. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. doi: 10.1378/chest.12-0447.
44. Patel PA, Ernst FR, Gunnarsson CL. Evaluation of hospital complications and costs associated with using ultrasound guidance during abdominal paracentesis procedures. J Med Econ. 2012;15(1):1-7. doi: 10.3111/13696998.2011.628723.
45. Nicolaou S, Talsky A, Khashoggi K, Venu V. Ultrasound-guided interventional radiology in critical care. Crit Care Med. 2007;35(5 Suppl):S186-197. doi: 10.1097/01.CCM.0000260630.68855.DF.
46. Conduit B, Wesley E, Christie J, Thalheimer U. PTU-002 Large volume paracentesis (LVP) can be safely performed by junior doctors without ultrasound guidance. Gut. 2013;62:A42. doi: 10.1136/gutjnl-2013-304907.095.
47. Williams JW, Jr., Simel DL. The rational clinical examination. Does this patient have ascites? How to divine fluid in the abdomen. JAMA. 1992;267(19):2645-2648. doi: 10.1001/jama.1992.03480190087038.
48. Rodriguez A, DuPriest RW, Jr., Shatney CH. Recognition of intra-abdominal injury in blunt trauma victims. A prospective study comparing physical examination with peritoneal lavage. Am Surg. 1982;48(9):457-459.
49. McGibbon A, Chen GI, Peltekian KM, van Zanten SV. An evidence-based manual for abdominal paracentesis. Dig Dis Sci. 2007;52(12):3307-3315. doi: 10.1007/s10620-007-9805-5.
50. Cattau EL, Jr., Benjamin SB, Knuff TE, Castell DO. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247(8):1164-1166. doi: 10.1001/jama.1982.03320330060027.
51. Ali J, Rozycki GS, Campbell JP, Boulanger BR, Waddell JP, Gana TJ. Trauma ultrasound workshop improves physician detection of peritoneal and pericardial fluid. J Surg Res. 1996;63(1):275-279. doi: 10.1006/jsre.1996.0260.
52. Von Kuenssberg Jehle D, Stiller G, Wagner D. Sensitivity in detecting free intraperitoneal fluid with the pelvic views of the FAST exam. Am J Emerg Med. 2003;21(6):476-478. doi: 10.1016/S0735-6757(03)00162-1
53. Goldberg BB, Goodman GA, Clearfield HR. Evaluation of ascites by ultrasound. Radiology. 1970;96(1):15-22. doi: 10.1148/96.1.15.
54. Branney SW, Wolfe RE, Moore EE, et al. Quantitative sensitivity of ultrasound in detecting free intraperitoneal fluid. J Trauma. 1995;39(2):375-380. doi: 10.1016/0736-4679(96)84805-0.
55. Paajanen H, Lahti P, Nordback I. Sensitivity of transabdominal ultrasonography in detection of intraperitoneal fluid in humans. Eur Radiol. 1999;9(7):1423-1425. doi: 10.1007/s003300050861.
56. Prabhakar A, Thabet A, Mueller P, Gee MS. Image-guided peritoneal access for fluid infusion in oncology patients: Indications, technique, and outcomes. J Vasc Interv Radiol. 2014;25(3):S41. doi: 10.1016/j.jvir.2013.12.100.
57. McGahan JP, Anderson MW, Walter JP. Portable real-time sonographic and needle guidance systems for aspiration and drainage. AJR Am J Roentgenol. 1986;147(6):1241-1246. doi: 10.2214/ajr.147.6.1241.
58. Moses WR. Shifting dullness in the abdomen. South Med J. 1946;39(12):985-987.
59. Edell SL, Gefter WB. Ultrasonic differentiation of types of ascitic fluid. AJR Am J Roentgenol. 1979;133(1):111-114. doi: 10.2214/ajr.133.1.111.
60. Doust BD, Thompson R. Ultrasonography of abdominal fluid collections. Gastrointest Radiol. 1978;3(3):273-279. doi: 10.1007/BF01887079.
61. Beaulieu Y, Marik PE. Bedside ultrasonography in the ICU: part 2. Chest. 2005;128(3):1766-1781. doi: 10.1378/chest.128.3.1766.
62. Irshad A, Ackerman SJ, Anis M, Campbell AS, Hashmi A, Baker NL. Can the smallest depth of ascitic fluid on sonograms predict the amount of drainable fluid? J Clin Ultrasound. 2009;37(8):440-444. doi: 10.1002/jcu.20616.
63. Inadomi J, Cello JP, Koch J. Ultrasonographic determination of ascitic volume. Hepatology. 1996;24(3):549-551. doi: 10.1002/hep.510240314.
64. Sideris A, Patel P, Charles HW, Park J, Feldman D, Deipolyi AR. Imaging and clinical predictors of spontaneous bacterial peritonitis diagnosed by ultrasound-guided paracentesis. Proc (Bayl Univ Med Cent). 2017;30(3):262-264. https://doi.org/10.1080/08998280.2017.11929610
65. Hatch N, Wu TS, Barr L, Roque PJ. Advanced ultrasound procedures. Crit Care Clin. 2014;30(2):305-329. doi: 10.1016/j.ccc.2013.10.005.
66. Ross GJ, Kessler HB, Clair MR, Gatenby RA, Hartz WH, Ross LV. Sonographically guided paracentesis for palliation of symptomatic malignant ascites. AJR Am J Roentgenol. 1989;153(6):1309-1311. doi: 10.2214/ajr.153.6.1309.
67. Russell KW, Mone MC, Scaife CL. Umbilical paracentesis for acute hernia reduction in cirrhotic patients. BMJ Case Rep. 2013;2013. doi: 10.1136/bcr-2013-201304.
68. Epstein J, Arora A, Ellis H. Surface anatomy of the inferior epigastric artery in relation to laparoscopic injury. Clin Anat. 2004;17(5):400-408. doi: 10.1002/ca.10192.
69. Suzuki J, Sekiguchi H. Laceration of inferior epigastric artery resulting in abdominal compartment syndrome: a fatal complication of paracentesis. Am J Respir Crit Care Med. 2012;185:A5974. doi: 10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A5974
70. Saber AA, Meslemani AM, Davis R, Pimentel R. Safety zones for anterior abdominal wall entry during laparoscopy: a CT scan mapping of epigastric vessels. Ann Surg. 2004;239(2):182-185. doi: 10.1097/01.sla.0000109151.53296.07.
71. Webster ST, Brown KL, Lucey MR, Nostrant TT. Hemorrhagic complications of large volume abdominal paracentesis. Am J Gastroenterol. 1996;91(2):366-368.
72. Todd AW. Inadvertent puncture of the inferior epigastric artery during needle biopsy with fatal outcome. Clin Radiol. 2001;56(12):989-990. doi: 10.1053/crad.2001.0175.
73. Seidler M, Sayegh K, Roy A, Mesurolle B. A fatal complication of ultrasound-guided abdominal paracentesis. J Clin Ultrasound. 2013;41(7):457-460. doi: 10.1002/jcu.22050.
74. Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore). 2006;85(2):105-110. doi: 10.1097/01.md.0000216818.13067.5a.
75. Oelsner DH, Caldwell SH, Coles M, Driscoll CJ. Subumbilical midline vascularity of the abdominal wall in portal hypertension observed at laparoscopy. Gastrointest Endosc. 1998;47(5):388-390. doi: 10.1016/S0016-5107(98)70224-X.
76. Krupski WC, Sumchai A, Effeney DJ, Ehrenfeld WK. The importance of abdominal wall collateral blood vessels. Planning incisions and obtaining arteriography. Arch Surg. 1984;119(7):854-857. doi: 10.1001/archsurg.1984.01390190092021.
77. Rozen WM, Ashton MW, Taylor GI. Reviewing the vascular supply of the anterior abdominal wall: redefining anatomy for increasingly refined surgery. Clin Anat. 2008;21(2):89-98. doi: 10.1002/ca.20585.
78. Adams A, Roggio A, Wilkerson RG. 368 Sonographic assessment of inadvertent vascular puncture during paracentesis using the traditional landmark approach. Ann Emerg Med. 2015;66:S132-S133. doi: 10.1016/j.annemergmed.2015.07.404
79. Barsuk JH, Rosen BT, Cohen ER, Feinglass J, Ault MJ. Vascular ultrasonography: a novel method to reduce paracentesis related major bleeding. J Hosp Med. 2018;13(1):30-33. doi: 10.12788/jhm.2863.
80. Skolnick ML. Estimation of ultrasound beam width in the elevation (section thickness) plane. Radiology. 1991;180(1):286-288. doi: 10.1148/radiology.180.1.2052713.
81. Keil-Rios D, Terrazas-Solis H, Gonzalez-Garay A, Sanchez-Avila JF, Garcia-Juarez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11(3):461-466. doi: 10.1007/s11739-016-1406-x.
82. Kessler C, Bhandarkar S. Ultrasound training for medical students and internal medicine residents--a needs assessment. J Clin Ultrasound. 2010;38(8):401-408. doi: 10.1002/jcu.20719.
83. Schnobrich DJ, Gladding S, Olson AP, Duran-Nelson A. Point-of-care ultrasound in internal medicine: a national survey of educational leadership. J Grad Med Educ. 2013;5(3):498-502. doi: 10.4300/JGME-D-12-00215.1.
84. Eisen LA, Leung S, Gallagher AE, Kvetan V. Barriers to ultrasound training in critical care medicine fellowships: a survey of program directors. Crit Care Med. 2010;38(10):1978-1983. doi: 10.1097/CCM.0b013e3181eeda53.
85. Neri L, Storti E, Lichtenstein D. Toward an ultrasound curriculum for critical care medicine. Crit Care Med. 2007;35(5 Suppl):S290-304. doi: 10.1097/01.CCM.0000260680.16213.26.
86. Ma I, Arishenkoff S, Wiseman J, et al. Internal medicine point-of-care ultrasound curriculum: consensus recommendations from the Canadian Internal Medicine Ultrasound (CIMUS) Group. J Gen Intern Med. 2017;32(9):1052-1057. doi: 10.1007/s11606-017-4071-5.
87. Kelm D, Ratelle J, Azeem N, et al. Longitudinal ultrasound curriculum improves long-term retention among internal medicine residents. J Grad Med Educ. 2015;7(3):454-457. doi: 10.4300/JGME-14-00284.1.
88. Barsuk JH, Cohen ER, Vozenilek JA, O’Connor LM, McGaghie WC, Wayne DB. Simulation-based education with mastery learning improves paracentesis skills. J Grad Med Educ. 2012;4(1):23-27. doi: 10.4300/JGME-D-11-00161.1.
89. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. doi: 10.7556/jaoa.2010.110.6.340.
90. American Board of Internal Medicine. Policies and Procedures for Certification. Philadelphia, PA: ABIM; 2006.
91. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. doi: 10.12788/jhm.2917.
92. Brown GM, Otremba M, Devine LA, Gray C, Millington SJ, Ma IW. Defining competencies for ultrasound-guided bedside procedures: consensus opinions from Canadian physicians. J Ultrasound Med. 2016;35(1):129-141. doi: 10.7863/ultra.15.01063.
93. Babcock DS, Patriquin H, LaFortune M, Dauzat M. Power doppler sonography: basic principles and clinical applications in children. Pediatr Radiol. 1996;26(2):109-115. doi: 10.1007/BF01372087.
94. Howard ZD, Noble VE, Marill KA, et al. Bedside ultrasound maximizes patient satisfaction. J Emerg Med. 2014;46(1):46-53. doi: 10.1016/j.jemermed.2013.05.044.
95. Lindelius A, Torngren S, Nilsson L, Pettersson H, Adami J. Randomized clinical trial of bedside ultrasound among patients with abdominal pain in the emergency department: impact on patient satisfaction and health care consumption. Scand J Trauma Resusc Emerg Med. 2009;17:60. doi: 10.1186/1757-7241-17-60.
1. Duszak R, Jr., Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859-864. doi: 10.1016/j.jacr.2010.04.013.
2. O’Brien CR, Chang J, Campos RA, et al. Characterizing the safety of paracentesis in hospitalized patients with cirrhosis and ascites from 2004-2012 in the United States. Gastroenterology. 2016;150(4). https:/doi.org /10.1016/S0016-5085(16)32196-5.
3. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. doi: 10.1016/S0016-5085(16)32196-5
4. Orman ES, Hayashi PH, Bataller R, Barritt AS. Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2014;12(3):496-503.e1. doi: 10.1016/j.cgh.2013.08.025.
5. Mallory A, Schaefer JW. Complications of diagnostic paracentesis in patients with liver disease. JAMA. 1978;239(7):628-630. doi: 10.1001/jama.1978.03280340048020.
6. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525-529. doi: 10.1111/j.1365-2036.2005.02387.x.
7. Shekhar C, Ramakrishnan A, Claridge LC. Paracentesis: UK trainees’ practice, experience and attitudes. Gut. 2013;62:A42. doi: 10.1136/gutjnl-2013-304907.096.
8. De Gottardi A, Thevenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7(8):906-909. doi: 10.1016/j.cgh.2009.05.004.
9. Sharzehi K, Jain V, Naveed A, Schreibman I. Hemorrhagic complications of paracentesis: a systematic review of the literature. Gastroenterol Res Pract. 2014;2014:985141. doi: 10.1155/2014/985141.
10. Sekiguchi H, Suzuki J, Daniels CE. Making paracentesis safer: a proposal for the use of bedside abdominal and vascular ultrasonography to prevent a fatal complication. Chest. 2013;143(4):1136-1139. doi: 10.1378/chest.12-0871.
11. Soyuncu S, Cete Y, Bozan H, Kartal M, Akyol AJ. Accuracy of physical and ultrasonographic examinations by emergency physicians for the early diagnosis of intraabdominal haemorrhage in blunt abdominal trauma. Injury. 2007;38(5):564-569. doi: 10.1016/j.injury.2007.01.010.
12. Chongtham DS, Singh MM, Kalantri SP, Pathak S, Jain AP. Accuracy of clinical manoeuvres in detection of minimal ascites. Indian J Med Sci. 1998;52(11):514-520.
13. Ennis J, Schultz G, Perera P, Williams S, Gharahbaghian L, Mandavia D. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293. doi: 10.4236/ijcm.2014.520163.
14. Szabo TL, Lewin PA. Ultrasound transducer selection in clinical imaging practice. J Ultrasound Med. 2013;32(4):573-582. doi: 10.7863/jum.2013.32.4.573.
15. Stone JC, Moak JH. Feasibility of sonographic localization of the inferior epigastric artery before ultrasound-guided paracentesis. Am J Emerg Med. 2015;33(12):1795-1798. doi: 10.1016/j.ajem.2015.06.067.
16. Yeh HC, Wolf BS. Ultrasonography in ascites. Radiology. 1977;124(3):783-790. doi: 10.1148/124.3.783.
17. Lucas BP, Asbury JK, Wang Y, et al. Impact of a bedside procedure service on general medicine inpatients: a firm-based trial. J Hosp Med. 2007;2(3):143-149. doi: 10.1002/jhm.159.
18. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. doi: 10.1038/ajg.2014.212.
19. Arora S, Cheung A, Tarique U, Agarwal A, Firdouse M, Ailon J. First-year medical students use of ultrasound or physical examination to diagnose hepatomegaly and ascites: a randomized controlled trial. J Ultrasound. 2017;20(3):199-204. doi: 10.1007/s40477-017-0261-6.
20. Nazeer SR, Dewbre H, Miller AH. Ultrasound-assisted paracentesis performed by emergency physicians vs the traditional technique: a prospective, randomized study. Am J Emerg Med. 2005;23(3):363-367. doi: 10.1016/j.ajem.2004.11.001.
21. Kotagal M, Quiroga E, Ruffatto BJ, et al. Impact of point-of-care ultrasound training on surgical residents’ confidence. J Surg Educ. 2015;72(4):e82-87. doi: 10.1016/j.jsurg.2015.01.021.
22. Martin R, Gannon D, Riggle J, et al. A comprehensive workshop using simulation to train internal medicine residents in bedside procedures performed by internists. Chest. 2012;142(4):545A. doi: 10.1378/chest.1390093.
23. Quddus A, Minami T, Summerhill E. Impact of a short 3-hour ultrasound training workshop for internal medicine residents. Chest. 2014;146(4): 509A. doi: 10.1378/chest.1989267.
24. Lanoix R, Leak LV, Gaeta T, Gernsheimer JR. A preliminary evaluation of emergency ultrasound in the setting of an emergency medicine training program. Am J Emerg Med. 2000;18(1):41-45. doi: 10.1016/S0735-6757(00)90046-9.
25. Dulohery MM, Eaton J, Tajouri T, Bhagra A. Ultrasound for internal medicine physicians: the future of physical exam. J Ultrasound Med. 2014;33(6):1005-1011. doi: 10.7863/ultra.33.6.1005
26. Lanoix R, Baker WE, Mele JM, Dharmarajan L. Evaluation of an instructional model for emergency ultrasonography. Acad Emerg Med. 1998;5(1):58-63. doi: 10.1111/j.1553-2712.1998.tb02576.x.
27. Terkamp C, Kirchner G, Wedemeyer J, et al. Simulation of abdomen sonography. Evaluation of a new ultrasound simulator. Ultraschall Med. 2003;24(4):239-234. doi: 10.1055/s-2003-41713.
28. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. doi: 10.1136/bmjqs-2013-002665.
29. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. doi: 10.1016/j.amjmed.2012.09.016.
30. Fitch K, Bernstein SJ, Aguilar MD, et al. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: Rand Corp.; 2001.
31. Bard C, Lafortune M, Breton G. Ascites: ultrasound guidance or blind paracentesis? CMAJ. 1986;135(3):209-210. doi: 10.1016/0736-4679(87)90268-X.
32. Sudulagunta SR, Sodalagunta MB, Bangalore Raja SK, Khorram H, Sepehrar M, Noroozpour Z. Clinical profile and complications of paracentesis in refractory ascites patients with cirrhosis. Gastroenterol Res. 2015;8(3-4):228-233. doi: 10.14740/gr661w.
33. Lin S, Wang M, Zhu Y, et al. Hemorrhagic complications following abdominal paracentesis in acute on chronic liver failure: a propensity score analysis. Medicine (Baltimore). 2015;94(49):e2225. doi: 10.1097/MD.0000000000002225.
34. Lam EY, McLafferty RB, Taylor LM, Jr., et al. Inferior epigastric artery pseudoaneurysm: a complication of paracentesis. J Vasc Surg. 1998;28(3):566-569. doi: 10.1016/S0741-5214(98)70147-8.
35. Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound-guided intervention. AJR Am J Roentgenol. 2010;195(4):846-850. doi: 10.2214/AJR.09.3168.
36. Wiese SS, Mortensen C, Bendtsen F. Few complications after paracentesis in patients with cirrhosis and refractory ascites. Dan Med Bull. 2011;58(1):A4212.
37. Jakobson DJ, Shemesh I. Merging ultrasound in the intensive care routine. Isr Med Assoc J. 2013;15(11):688-692.
38. Landers A, Ryan B. The use of bedside ultrasound and community-based paracentesis in a palliative care service. J Prim Health Care. 2014;6(2):148-151.
39. Lin CH, Shih FY, Ma MH, Chiang WC, Yang CW, Ko PC. Should bleeding tendency deter abdominal paracentesis? Dig Liver Dis. 2005;37(12):946-951. doi: 10.1016/j.dld.2005.07.009.
40. Kurup AN, Lekah A, Reardon ST, et al. Bleeding rate for ultrasound-guided paracentesis in thrombocytopenic patients. J Ultrasound Med. 2015;34(10):1833-1838. doi: 10.7863/ultra.14.10034.
41. Reardon S, Atwell TD, Lekah A. Major bleeding complication rate of ultrasound-guided paracentesis in thrombocytopenic patients. J Vasc Interv Radiol. 2013;24(4):S56. doi: 10.1016/j.jvir.2013.01.129.
42. Czul F, Prager M, Lenchus J. Intra-procedural risk of bleeding associated with ultrasound guided paracentesis in patients with abnormal coagulation studies: 1907. Hepatology. 2011;54(4):1259A.
43. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. doi: 10.1378/chest.12-0447.
44. Patel PA, Ernst FR, Gunnarsson CL. Evaluation of hospital complications and costs associated with using ultrasound guidance during abdominal paracentesis procedures. J Med Econ. 2012;15(1):1-7. doi: 10.3111/13696998.2011.628723.
45. Nicolaou S, Talsky A, Khashoggi K, Venu V. Ultrasound-guided interventional radiology in critical care. Crit Care Med. 2007;35(5 Suppl):S186-197. doi: 10.1097/01.CCM.0000260630.68855.DF.
46. Conduit B, Wesley E, Christie J, Thalheimer U. PTU-002 Large volume paracentesis (LVP) can be safely performed by junior doctors without ultrasound guidance. Gut. 2013;62:A42. doi: 10.1136/gutjnl-2013-304907.095.
47. Williams JW, Jr., Simel DL. The rational clinical examination. Does this patient have ascites? How to divine fluid in the abdomen. JAMA. 1992;267(19):2645-2648. doi: 10.1001/jama.1992.03480190087038.
48. Rodriguez A, DuPriest RW, Jr., Shatney CH. Recognition of intra-abdominal injury in blunt trauma victims. A prospective study comparing physical examination with peritoneal lavage. Am Surg. 1982;48(9):457-459.
49. McGibbon A, Chen GI, Peltekian KM, van Zanten SV. An evidence-based manual for abdominal paracentesis. Dig Dis Sci. 2007;52(12):3307-3315. doi: 10.1007/s10620-007-9805-5.
50. Cattau EL, Jr., Benjamin SB, Knuff TE, Castell DO. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247(8):1164-1166. doi: 10.1001/jama.1982.03320330060027.
51. Ali J, Rozycki GS, Campbell JP, Boulanger BR, Waddell JP, Gana TJ. Trauma ultrasound workshop improves physician detection of peritoneal and pericardial fluid. J Surg Res. 1996;63(1):275-279. doi: 10.1006/jsre.1996.0260.
52. Von Kuenssberg Jehle D, Stiller G, Wagner D. Sensitivity in detecting free intraperitoneal fluid with the pelvic views of the FAST exam. Am J Emerg Med. 2003;21(6):476-478. doi: 10.1016/S0735-6757(03)00162-1
53. Goldberg BB, Goodman GA, Clearfield HR. Evaluation of ascites by ultrasound. Radiology. 1970;96(1):15-22. doi: 10.1148/96.1.15.
54. Branney SW, Wolfe RE, Moore EE, et al. Quantitative sensitivity of ultrasound in detecting free intraperitoneal fluid. J Trauma. 1995;39(2):375-380. doi: 10.1016/0736-4679(96)84805-0.
55. Paajanen H, Lahti P, Nordback I. Sensitivity of transabdominal ultrasonography in detection of intraperitoneal fluid in humans. Eur Radiol. 1999;9(7):1423-1425. doi: 10.1007/s003300050861.
56. Prabhakar A, Thabet A, Mueller P, Gee MS. Image-guided peritoneal access for fluid infusion in oncology patients: Indications, technique, and outcomes. J Vasc Interv Radiol. 2014;25(3):S41. doi: 10.1016/j.jvir.2013.12.100.
57. McGahan JP, Anderson MW, Walter JP. Portable real-time sonographic and needle guidance systems for aspiration and drainage. AJR Am J Roentgenol. 1986;147(6):1241-1246. doi: 10.2214/ajr.147.6.1241.
58. Moses WR. Shifting dullness in the abdomen. South Med J. 1946;39(12):985-987.
59. Edell SL, Gefter WB. Ultrasonic differentiation of types of ascitic fluid. AJR Am J Roentgenol. 1979;133(1):111-114. doi: 10.2214/ajr.133.1.111.
60. Doust BD, Thompson R. Ultrasonography of abdominal fluid collections. Gastrointest Radiol. 1978;3(3):273-279. doi: 10.1007/BF01887079.
61. Beaulieu Y, Marik PE. Bedside ultrasonography in the ICU: part 2. Chest. 2005;128(3):1766-1781. doi: 10.1378/chest.128.3.1766.
62. Irshad A, Ackerman SJ, Anis M, Campbell AS, Hashmi A, Baker NL. Can the smallest depth of ascitic fluid on sonograms predict the amount of drainable fluid? J Clin Ultrasound. 2009;37(8):440-444. doi: 10.1002/jcu.20616.
63. Inadomi J, Cello JP, Koch J. Ultrasonographic determination of ascitic volume. Hepatology. 1996;24(3):549-551. doi: 10.1002/hep.510240314.
64. Sideris A, Patel P, Charles HW, Park J, Feldman D, Deipolyi AR. Imaging and clinical predictors of spontaneous bacterial peritonitis diagnosed by ultrasound-guided paracentesis. Proc (Bayl Univ Med Cent). 2017;30(3):262-264. https://doi.org/10.1080/08998280.2017.11929610
65. Hatch N, Wu TS, Barr L, Roque PJ. Advanced ultrasound procedures. Crit Care Clin. 2014;30(2):305-329. doi: 10.1016/j.ccc.2013.10.005.
66. Ross GJ, Kessler HB, Clair MR, Gatenby RA, Hartz WH, Ross LV. Sonographically guided paracentesis for palliation of symptomatic malignant ascites. AJR Am J Roentgenol. 1989;153(6):1309-1311. doi: 10.2214/ajr.153.6.1309.
67. Russell KW, Mone MC, Scaife CL. Umbilical paracentesis for acute hernia reduction in cirrhotic patients. BMJ Case Rep. 2013;2013. doi: 10.1136/bcr-2013-201304.
68. Epstein J, Arora A, Ellis H. Surface anatomy of the inferior epigastric artery in relation to laparoscopic injury. Clin Anat. 2004;17(5):400-408. doi: 10.1002/ca.10192.
69. Suzuki J, Sekiguchi H. Laceration of inferior epigastric artery resulting in abdominal compartment syndrome: a fatal complication of paracentesis. Am J Respir Crit Care Med. 2012;185:A5974. doi: 10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A5974
70. Saber AA, Meslemani AM, Davis R, Pimentel R. Safety zones for anterior abdominal wall entry during laparoscopy: a CT scan mapping of epigastric vessels. Ann Surg. 2004;239(2):182-185. doi: 10.1097/01.sla.0000109151.53296.07.
71. Webster ST, Brown KL, Lucey MR, Nostrant TT. Hemorrhagic complications of large volume abdominal paracentesis. Am J Gastroenterol. 1996;91(2):366-368.
72. Todd AW. Inadvertent puncture of the inferior epigastric artery during needle biopsy with fatal outcome. Clin Radiol. 2001;56(12):989-990. doi: 10.1053/crad.2001.0175.
73. Seidler M, Sayegh K, Roy A, Mesurolle B. A fatal complication of ultrasound-guided abdominal paracentesis. J Clin Ultrasound. 2013;41(7):457-460. doi: 10.1002/jcu.22050.
74. Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore). 2006;85(2):105-110. doi: 10.1097/01.md.0000216818.13067.5a.
75. Oelsner DH, Caldwell SH, Coles M, Driscoll CJ. Subumbilical midline vascularity of the abdominal wall in portal hypertension observed at laparoscopy. Gastrointest Endosc. 1998;47(5):388-390. doi: 10.1016/S0016-5107(98)70224-X.
76. Krupski WC, Sumchai A, Effeney DJ, Ehrenfeld WK. The importance of abdominal wall collateral blood vessels. Planning incisions and obtaining arteriography. Arch Surg. 1984;119(7):854-857. doi: 10.1001/archsurg.1984.01390190092021.
77. Rozen WM, Ashton MW, Taylor GI. Reviewing the vascular supply of the anterior abdominal wall: redefining anatomy for increasingly refined surgery. Clin Anat. 2008;21(2):89-98. doi: 10.1002/ca.20585.
78. Adams A, Roggio A, Wilkerson RG. 368 Sonographic assessment of inadvertent vascular puncture during paracentesis using the traditional landmark approach. Ann Emerg Med. 2015;66:S132-S133. doi: 10.1016/j.annemergmed.2015.07.404
79. Barsuk JH, Rosen BT, Cohen ER, Feinglass J, Ault MJ. Vascular ultrasonography: a novel method to reduce paracentesis related major bleeding. J Hosp Med. 2018;13(1):30-33. doi: 10.12788/jhm.2863.
80. Skolnick ML. Estimation of ultrasound beam width in the elevation (section thickness) plane. Radiology. 1991;180(1):286-288. doi: 10.1148/radiology.180.1.2052713.
81. Keil-Rios D, Terrazas-Solis H, Gonzalez-Garay A, Sanchez-Avila JF, Garcia-Juarez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11(3):461-466. doi: 10.1007/s11739-016-1406-x.
82. Kessler C, Bhandarkar S. Ultrasound training for medical students and internal medicine residents--a needs assessment. J Clin Ultrasound. 2010;38(8):401-408. doi: 10.1002/jcu.20719.
83. Schnobrich DJ, Gladding S, Olson AP, Duran-Nelson A. Point-of-care ultrasound in internal medicine: a national survey of educational leadership. J Grad Med Educ. 2013;5(3):498-502. doi: 10.4300/JGME-D-12-00215.1.
84. Eisen LA, Leung S, Gallagher AE, Kvetan V. Barriers to ultrasound training in critical care medicine fellowships: a survey of program directors. Crit Care Med. 2010;38(10):1978-1983. doi: 10.1097/CCM.0b013e3181eeda53.
85. Neri L, Storti E, Lichtenstein D. Toward an ultrasound curriculum for critical care medicine. Crit Care Med. 2007;35(5 Suppl):S290-304. doi: 10.1097/01.CCM.0000260680.16213.26.
86. Ma I, Arishenkoff S, Wiseman J, et al. Internal medicine point-of-care ultrasound curriculum: consensus recommendations from the Canadian Internal Medicine Ultrasound (CIMUS) Group. J Gen Intern Med. 2017;32(9):1052-1057. doi: 10.1007/s11606-017-4071-5.
87. Kelm D, Ratelle J, Azeem N, et al. Longitudinal ultrasound curriculum improves long-term retention among internal medicine residents. J Grad Med Educ. 2015;7(3):454-457. doi: 10.4300/JGME-14-00284.1.
88. Barsuk JH, Cohen ER, Vozenilek JA, O’Connor LM, McGaghie WC, Wayne DB. Simulation-based education with mastery learning improves paracentesis skills. J Grad Med Educ. 2012;4(1):23-27. doi: 10.4300/JGME-D-11-00161.1.
89. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. doi: 10.7556/jaoa.2010.110.6.340.
90. American Board of Internal Medicine. Policies and Procedures for Certification. Philadelphia, PA: ABIM; 2006.
91. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. doi: 10.12788/jhm.2917.
92. Brown GM, Otremba M, Devine LA, Gray C, Millington SJ, Ma IW. Defining competencies for ultrasound-guided bedside procedures: consensus opinions from Canadian physicians. J Ultrasound Med. 2016;35(1):129-141. doi: 10.7863/ultra.15.01063.
93. Babcock DS, Patriquin H, LaFortune M, Dauzat M. Power doppler sonography: basic principles and clinical applications in children. Pediatr Radiol. 1996;26(2):109-115. doi: 10.1007/BF01372087.
94. Howard ZD, Noble VE, Marill KA, et al. Bedside ultrasound maximizes patient satisfaction. J Emerg Med. 2014;46(1):46-53. doi: 10.1016/j.jemermed.2013.05.044.
95. Lindelius A, Torngren S, Nilsson L, Pettersson H, Adami J. Randomized clinical trial of bedside ultrasound among patients with abdominal pain in the emergency department: impact on patient satisfaction and health care consumption. Scand J Trauma Resusc Emerg Med. 2009;17:60. doi: 10.1186/1757-7241-17-60.
© 2019 Society of Hospital Medicine
Recommendations on the Use of Ultrasound Guidance for Adult Thoracentesis: A Position Statement of the Society of Hospital Medicine
Approximately 1.5 million people develop a pleural effusion in the United States annually, and approximately 173,000 people (12%) undergo thoracentesis.1 A recent review of thoracenteses performed at 234 University Health System Consortium hospitals between January 2010 and September 2013 demonstrated that 16% of 132,472 thoracenteses were performed by general internists and hospitalists, 33.1% were performed by interventional radiologists, and 20.3% were performed by pulmonologists.2 The iatrogenic pneumothorax rate was not significantly different between interventional radiologists and internists (2.8% and 2.9% risk, respectively); however, the admissions associated with bedside thoracentesis were less expensive than the admissions associated with thoracentesis performed in radiology suites, even after controlling for clinical covariates.2 In addition, the use of ultrasound guidance has been associated with a reduced risk of complications and cost of thoracentesis.3,4 In most of the early published studies on ultrasound-guided thoracentesis, the procedures were performed by radiologists.5-12 However, in 2010, the British Thoracic Society published guidelines on pleural procedures and thoracic ultrasound geared toward any trained provider.13 The purpose of this guideline is to review the literature and present evidence-based recommendations on the performance of ultrasound-guided thoracentesis at the bedside.
METHODS
Detailed methods are described in Appendix 1. The Society of Hospital Medicine (SHM) Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. The expert panel members were divided into working group members, external peer reviewers, and a methodologist. All the Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the four working group members themselves. Key clinical questions were prepared prior to conducting a systematic literature search by a medical librarian. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to September 2015 initially. Updated searches were conducted in November 2016 and in August 2017 (Appendix 3). All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of the screened articles were reviewed, and the articles focusing on the use of ultrasound to guide thoracentesis were selected. Articles that discussed thoracentesis without ultrasound guidance were excluded. In addition, the following article types were excluded: non-English language, nonhuman, subjects’ age <18 years, meeting abstracts, meeting posters, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided thoracentesis were screened and selected. Final article selection was based on working group consensus, and the selected literature was incorporated into draft recommendations.
We used the RAND Appropriateness Method that required panel judgment and consensus.14 The 30 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering the following five transforming factors: 1) Problem priority and importance, 2) Level of quality of evidence, 3) Benefit/harm balance, 4) Benefit/burden balance, and 5) Certainty/concerns about PEAF (Preferences/Equity Acceptability/Feasibility). Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (Redcap™) in December 2016 and January 2017 (Appendix 4). Voting on appropriateness was conducted using a 9-point Likert scale, and the degree of consensus was assessed using the RAND algorithm. Establishing a recommendation required at least 70% agreement and a strong recommendation required 80% agreement according to the RAND rules (Appendix 1, Figure 1). Disagreement was defined as >30% of panelists voting outside of the zone of the median (appropriate, uncertain, inappropriate).
RESULTS
Literature search
A total of 1,556 references were pooled from the following four different sources: a search by a certified librarian in September 2015 (1066 citations) that was updated in November 2016 (165 citations) and again in August 2017 (9 citations), working group members’ literature searches (47 citations), and a search focused on training (269 citations). The final selection included 94 articles that were abstracted into a data table and incorporated into the draft recommendations. The details of the literature search strategy are given in Appendix 3.
Recommendations
Terminology
- Thoracentesis is a procedure of aspiration of fluid from the pleural space by percutaneous insertion of a needle through the chest wall with or without the insertion of a catheter.
- In this document, ultrasound guidance refers to static guidance and site marking performed at the bedside immediately before the procedure, as opposed to real-time (dynamic) ultrasound guidance or radiology performed site marking. The static method is the most commonly used method of ultrasound guidance and is supported by current evidence.
RECOMMENDATIONS
Clinical Outcomes
1.We recommend that ultrasound should be used to guide thoracentesis to reduce the risk of complications, the most common being pneumothorax.
Rationale: Both static ultrasound guidance and dynamic ultrasound guidance have been reported to be associated with a reduced risk of pneumothorax.4-7,15-18 A meta-analysis of 24 studies that included 6,605 thoracenteses showed a significant decrease in the risk of postprocedure pneumothorax with the use of ultrasound guidance compared to the risk associated with thoracentesis performed based on landmarks alone (OR 0.3, 95% CI 0.2–0.7).3 The meta-analysis included both prospective and retrospective studies conducted using both static and dynamic ultrasound guidance.3 A large retrospective cohort study conducted by Mercaldi et al. comprising more than 61,000 patients who underwent thoracentesis also showed that ultrasound guidance was associated with reduced odds of pneumothorax (OR 0.8 [0.7–0.9]).4 When pneumothorax did occur during that hospitalization, the cost of hospitalization increased by $2800 and the length of stay increased by 1.5 days.4 A 2008 review of 19,339 thoracenteses conducted by Patel et al. also demonstrated an association between ultrasound guidance and reduced odds of pneumothorax (OR 0.8 [0.7–0.96]).18 Although these findings were significant, it is important to note that the studies of both Mercaldi et al. and Patel et al. were reviews of administrative databases conducted using the International Classification of Diseases, 9th Revision (ICD-9) codes for thoracentesis and Current Procedure Terminology–4th edition (CPT) codes for the use of ultrasound.4,18 Patel et al. identified pneumothorax using ICD-9 codes for “pneumothorax–iatrogenic” and “pneumothorax–not specified as due to the procedure.” The association between ultrasound guidance and the reduced odds of pneumothorax was driven by the latter code.18 However, as with most retrospective studies using administrative data, granular data about the patients, procedure, proceduralists, and complications were not available in these reviews and conclusions may be limited by erroneous coding or documentation.4,18 In a third retrospective cohort study, Raptopoulos et al. compared 154 landmark-based thoracenteses performed by “clinical physicians” and 188 ultrasound-guided thoracenteses performed by radiologists and found that ultrasound-guided site selection reduced the rate of pneumothorax from 18% to 3% (P < .0001).6 Finally, one single-center randomized controlled trial of 160 thoracenteses performed by pulmonologists showed that ultrasound guidance reduced the relative risk of pneumothorax by 90% (12.5% vs 1.3%; P =.009) with a number needed to treat of 9.15 It was not possible to blind the operators to the use of ultrasound guidance, but the data analysis was blinded.15 Furthermore, while there was no explicit comparison of the intervention vs. the control groups, randomization would have presumably rendered both groups similar in terms of patient characteristics and effusion characteristics.15 Ultrasound may reduce the risk of pneumothorax through several mechanisms, including identifying patients in whom thoracentesis cannot be safely performed, allowing selection of the safest needle insertion site, and revealing the optimal depth of needle insertion.
2.We recommend that ultrasound guidance should be used to increase the success rate of thoracentesis.
Rationale: Thoracentesis guided by ultrasound has lower rates of failed attempts, or “dry taps,” compared to thoracentesis guided solely by physical examination. In 1977, Ravin described a method of using ultrasound to guide successful drainage of six complex pleural effusions (empyema or loculated effusion) after multiple (5–7) failed attempts by clinicians using physical examination alone.8 In a second study by radiologists, Weingardt et al. demonstrated that 20 of 26 failed landmark-based thoracenteses were due to incorrect site selection by physical examination–15 sites were below the diaphragm and 5 sites were above the pleural effusion or in the consolidated lung–and the use of ultrasound allowed successful sampling in 14 of 16 patients who had a failed landmark-based thoracentesis.9 Diacon et al. asked 30 physicians, ranging from junior housestaff to pulmonologists, to mark 172 potential thoracentesis sites in 67 patients with pleural effusions using physical examination alone. Ultrasound was then used to evaluate the proposed puncture sites. They found that using ultrasound would have avoided puncture on “dry chests” in 2% and avoided potential laceration of a solid organ in 10% of patients compared to site selection by physical examination alone.19 Finally, Perazzo et al. randomized 160 patients to landmark-based thoracentesis and ultrasound-guided thoracentesis and demonstrated that half of the eight dry taps that occurred in the control group could be successfully drained using subsequent ultrasound guidance.15
Technique
3. We recommend that ultrasound-guided thoracentesis should be performed or closely supervised by experienced operators.
Rationale: Current evidence suggests lower complication rates when thoracentesis is performed by experienced healthcare providers. A systematic review of 6,605 thoracenteses showed a significantly lower pneumothorax rate when thoracentesis was performed by pulmonology or radiology faculty versus resident physicians (3.9% vs 8.5%; P =.04), although this finding was not significant in the four studies that directly compared this factor.3 In a quality improvement study performed by Duncan et al., pulmonology and critical care physicians combining multiple quality improvement initiatives to achieve and maintain competency decreased the rate of pneumothorax from 8.6% to 1.1% (P =.0034).20 Interventions included ultrasound training, performance of 10 thoracenteses under expert supervision, and restriction of privileges to proceduralists who perform 10 or more thoracenteses per year.20 Finally, a series of 9,320 ultrasound-guided thoracenteses performed or supervised by a single expert internist over a period of 12 years resulted in a pneumothorax rate of 0.6% and a composite complication rate of 0.98% (pneumothorax, reexpansion pulmonary edema, hemothorax, site bleeding, hematoma, splenic laceration, and vasovagal reaction).21 Notably, pneumothorax rate in resident physician hands was reported to be 8.5% in the meta-analysis performed by Gordon et al., which is similar to the initial rate in the pulmonologists who participated in the study by Duncan et al.3,20 However, after instituting formal ultrasound training and other initiatives aimed at maintaining competency, the pneumothorax rate in the study by Duncan et al. decreased to 1.1%, similar to the rate observed in the series by Ault et al.21 This suggests that training and supervision are necessary to achieve competency and reduce the rate of complications.3,20,21
4. We suggest that ultrasound guidance be used to reduce the risk of complications from thoracentesis in mechanically ventilated patients.
Rationale: The rest of this guideline refers to ultrasound-guided thoracentesis performed in spontaneously breathing patients; however, this recommendation is specific to mechanically ventilated patients. Two prospective observational studies have shown no increase in complications when ultrasound-guided thoracentesis is performed on mechanically ventilated patients compared to patients not receiving positive pressure ventilation. A feasibility study of 45 thoracenteses performed on ventilated patients reported no complications,22 whereas another study on 232 patients reported a pneumothorax rate of 1.3%.23 In a larger study conducted by Mayo et al., medicine housestaff performed thoracentesis under the supervision of intensivists who had undergone training in ultrasound prior to performing the procedure.23 In both studies, most of the patients were in a supine position, although positioning and puncture site were at the discretion of the physician, and both studies employed use of static ultrasound guidance.22,23 A large series of 9,320 ultrasound-guided thoracenteses that included 1,377 mechanically ventilated patients did not report a higher rate of pneumothorax (0.8%) compared to that in spontaneously breathing patients (0.61%).21 Finally, a meta-analysis of 19 observational studies comprising 1,124 mechanically ventilated patients who underwent pleural drainage procedures showed a low rate of pneumothorax (3.4%) and hemothorax (1.9%).24 Although the rate of complication was reported to be low in this meta-analysis, ultrasound was not employed in all studies and its use was not associated with a significant reduction in pneumothorax.24 This may be because 8 of the 19 studies used pigtail catheters or large-bore thoracostomy tubes which treat pneumothorax as they occur.24
5. We recommend that ultrasound should be used to identify the chest wall, pleura, diaphragm, lung, and subdiaphragmatic organs throughout the respiratory cycle before selecting a needle insertion site.
Rationale: The use of ultrasound improves the selection of a safe needle insertion site because sites chosen without ultrasound guidance may be below the diaphragm, over solid organs,9,19 or in locations that risk puncture of the lung.9 Visualization of the chest wall, diaphragm, and lung, which define the boundaries of a pleural effusion, allows the clinician to confirm the presence of a drainable pleural effusion and assess for other pathologies, such as ascites and tumor, that may be mistaken for a pleural effusion.22,25,26 Hypoechoic lesions can represent small loculated pleural effusions but also pleural plaques, pleural masses, peripheral lung masses, or abscesses.27,28
6. We recommend that ultrasound should be used to detect the presence or absence of an effusion and approximate the volume of pleural fluid to guide clinical decision-making.
Rationale: The presence and approximate size of pleural fluid collections are important determinants of whether thoracentesis, another procedure, or no procedure should be performed. Ultrasonography has higher sensitivity and specificity for detecting pleural effusions and better differentiates effusions from consolidations compared with chest radiography.29-42 Ultrasound allows semiquantitative estimation of pleural fluid volume to determine whether thoracentesis should be performed.41-45 When using ultrasound to choose a site for thoracentesis, the British Thoracic Society Pleural Disease guidelines recommend ≥10 mm of pleural fluid between the visceral and parietal pleura.13 Pleural effusions of <10–15 mm are considered too small to tap.22,23 In a prospective study of 45 patients, a measurement of >9.9 cm by ultrasound between the chest wall and the “V-point,” the intersection of the diaphragm and the collapsed lung, correlated with a pleural fluid volume of >1 liter.46 Another prospective study of 73 patients showed that a pleural effusion spanning >3 intercostal spaces by ultrasound also correlated with a pleural fluid volume of >1 liter.47 Anticipating the volume of fluid to be removed may aid in preplanning and procurement of larger capacity drainage containers prior to starting the procedure. Lung ultrasound can also change the management if the characteristic of the effusion suggests that an invasive procedure is unsafe or another diagnostic or therapeutic option is more appropriate.39 In a prospective cohort study of 189 mechanically ventilated patients, lung ultrasound guided the management in all patients with suspected effusion, leading to chest tube placement in 7 patients and thoracentesis in 34 patients.48
7. We recommend that ultrasound should be used to detect complex sonographic features, such as septations, to guide clinical decision-making regarding the timing and method of pleural drainage.
Rationale: Pleural effusions can be broadly categorized sonographically as simple or complex. Complex effusions are further categorized as with or without septation. Simple effusions are anechoic and are often, but not invariably, transudative.49-51 The use of sonography and computerized tomography (CT) is complementary, but features of complex pleural effusions (fibrin stranding and septations) may be better visualized by ultrasound than by CT of the thorax.52 Detection of complex features should prompt the consideration of pleural fluid sampling.53,54 Exudative effusions from tuberculosis, malignancy, or other etiologies more often include debris, septations, or other complex features.55,56 Certain features such as a swirling debris, pleural thickening, and nodularity may be more often associated with malignancy,54,56 and advanced ultrasound techniques may be used to detect a trapped lung prior to attempting drainage of a malignant pleural effusion.57 Two studies found complex septated pleural effusions to be invariably exudative50,58 and drainage was unlikely to be successful without the placement of a chest tube.50,58-60 Chest tube placement through fibrinolytic administration or video-assisted thoracoscopic surgery (VATS) may be more appropriate in the management of complex septated pleural effusions,59-61 and expert consultation with a thoracic specialist is recommended in these cases.
8. We suggest that ultrasound can be used to measure the depth from the skin surface to the parietal pleura to help select an appropriate length needle and determine the maximum needle insertion depth.
Rationale: The distance from the skin to the parietal and visceral pleura can be measured by ultrasound to determine whether thoracentesis can be safely performed and to guide selection of an adequate length needle.38 The length of needle required to penetrate the pleural space varies based on the thickness of the chest wall. Percussion of the chest wall is limited when there is more than 6 cm of subcutaneous tissue,62 making physical examination in obese patients unreliable for selecting an appropriate site or needle length for thoracentesis. Ultrasound allows visualization of deep soft tissues, well beyond the limits of percussion, and allows an accurate measurement of the chest wall.63
9. We suggest that ultrasound can be used to evaluate normal lung sliding pre- and postprocedure to rule out pneumothorax.
Rationale: Normal lung sliding indicates normal apposition and movement of visceral and parietal pleura and rules out pneumothorax with a sensitivity that exceeds that of chest radiography, according to a meta-analysis of 20 studies using computed tomography or escape of intrapleural air at the time of drainage as the gold standard.64 In this meta-analysis, the pooled sensitivity of ultrasound was reported to be 88% (85-91%) compared to 52% (49-55%) for radiography, although the analysis also suggests that the test characteristics are dependent on operator skill.64 However, although lung sliding rules out pneumothorax, absence of lung sliding is not specific for pneumothorax and other conditions, including pleural adhesions, pleurodesis, and bronchial obstruction, can cause the absence of lung sliding.64 Detection of a lung point conclusively rules in a pneumothorax.65 Provided that the preprocedure lung ultrasound examination revealed normal lung sliding, a postprocedure examination can be performed to effectively evaluate for pneumothorax. This modality does not use ionizing radiation, is less expensive than computed tomography, can be performed faster than bedside chest radiography, and is more sensitive than supine or upright chest radiography.64,66-71
10. We suggest avoiding delay or interval change in patient position between the time of marking the needle insertion site and performing the thoracentesis.
Rationale: Optimal patient positioning and ultrasound-guided site marking should be performed by the primary operator immediately before beginning an invasive procedure. Remote sonographic localization in which a radiologist marks a needle insertion site using ultrasound and the thoracentesis is performed at a later time by a different provider is an antiquated practice. Two early studies demonstrated that this practice is no safer than landmark-based thoracentesis.6,72 One prospective study of 205 patients performed in 1986 showed no significant decrease in the incidence of complications from thoracentesis performed using remote sonographic localization versus landmark-based drainage.72 Complications in that study included a total of 22 pneumothoraces and 1 hematoma. The rate of complications in the group of patients who had site marking performed by radiology faculty and subsequent thoracentesis by medicine housestaff or attending physicians was 9.7% versus a complication rate of 12.7% in the landmark-based group.72 In addition, Raptopoulos et al. observed no significant difference in the pneumothorax rate between 106 patients with landmark-based thoracenteses and 48 patients who were sonographically marked by radiology faculty and then returned to the ward for completion of the thoracentesis by medicine housestaff (19% vs. 15%, respectively).6 Both groups had significantly higher rates of pneumothorax compared to those who underwent thoracentesis performed using real-time ultrasound guidance by radiology trainees (3%).6 The authors speculated that changing the patient’s position shifted the position of the pleural effusion, ultimately leading to the reliance on physical examination for the tap site.6
11. We recommend against performing routine postprocedure chest radiographs in patients who have undergone thoracentesis successfully with ultrasound guidance and are asymptomatic with normal lung sliding postprocedure.
Rationale: Chest radiography post-thoracentesis is unlikely to add information that changes management, especially if performed routinely, but does add expense, radiation, and inconvenience.73 The most common serious complication of thoracentesis is pneumothorax, which is often accompanied by symptoms, particularly in those patients with pneumothorax large enough to warrant chest tube placement.10,74,75 Pihlajamaa et al. retrospectively studied 264 ultrasound-guided thoracenteses performed by radiologists or radiology residents and noted that of 11 pneumothoraces, only 1 necessitated chest tube placement.10 Aleman et al. prospectively studied 506 ultrasound-guided and physical examination-guided thoracenteses and found that only 1% of asymptomatic patients developed a pneumothorax.74 Eight of the 18 symptomatic patients required chest tube placement as opposed to 1 of the 488 asymptomatic patients.74 A large prospective study of 941 ultrasound-guided thoracentesis reported that only 0.3% of asymptomatic patients with no suspicion of pneumothorax required tube thoracostomy.5 Postprocedure chest radiographs may be considered when thoracentesis is performed on mechanically ventilated patients, particularly when high airway pressures exist. In a study of 434 patients undergoing thoracentesis, only 10 patients had a pneumothorax (2.3%).11 Six of these pneumothoraces occurred in 92 mechanically ventilated patients (6.5%), and 2 of these 6 patients required a chest tube.11 None of the 4 spontaneously breathing patients with pneumothorax required a chest tube.11
Training
12. We recommend that novices who use ultrasound guidance for thoracentesis should receive focused training in lung and pleural ultrasonography and hands-on practice in procedural technique.
Rationale: Healthcare providers have to gain various skills to safely perform ultrasound-guided thoracentesis independently. Trainees should learn how to use ultrasound to identify important structures (chest wall, ribs, lung, pleura, diaphragm, and subdiaphragmatic organs); detect pleural effusions with complex features, such as septations; identify consolidated lung tissue; and rule out a pneumothorax. Prospective studies done with novice learners have shown that focused training combining didactics and hands-on practice using simulation or live models improves skills to assess pleural effusions.76-84 Several additional procedural techniques such as patient positioning and needle insertion are also important but are beyond the scope of these guidelines.
13. We suggest that novices undergo simulation-based training prior to performing ultrasound-guided thoracentesis on patients.
Rationale: Simulation-based training for thoracentesis has been studied in providers with different levels of medical training, ranging from medical students and internal medicine residents to practicing pulmonologists. Studies suggest that training in a zero-risk environment with simulation task trainers leads to increased knowledge and skills without subjecting the patients to inexperienced operators.85-87 One study on simulator-based training in medical students showed skill retention at 6 months and these skills were at least partially transferred to increased competency on live patients.88 Checklists to train providers in ultrasound-guided thoracentesis have been published.89,90 An experiential training program for attending physicians that utilized task trainers, along with standardized equipment and procedural technique, resulted in a reduction in the pneumothorax rate from 8.6% to 1.1%.20
14. Training curves for novices to become competent in lung ultrasound and ultrasound-guided thoracentesis are not completely understood. We recommend that training should be tailored to the skill acquisition of the learner and the resources of the institution.
Rationale: Understanding the rates at which novices progress from performing procedures under direct supervision to performing them independently would be highly desirable to ensure patient safety, guide supervision, and maximize efficiency of training. However, there is limited research describing the rate of progression of learners through these stages, either with regard to time or number of procedures performed. Two studies have shown that with brief training programs, medical students88 and internal medicine residents87 can achieve high levels of proficiency to perform thoracentesis on simulators, which is durable over time; however, whether these findings in a simulated environment translate into clinically significant outcomes is largely unknown, and neither of these studies incorporated the use of ultrasound guidance in their training curricula.87,88 Another study of pulmonary and critical care physicians combined multiple quality improvement initiatives with a half day of ultrasound-guided thoracentesis training, a requirement to perform 10 supervised thoracenteses prior to independent practice, and an additional requirement to perform 10 thoracenteses per year to maintain privileges.20 These interventions resulted in a concentration of competency among a few proceduralists, decreasing the rate of pneumothorax from 8.6% to 1.1%.20 Degradation of skills with disuse may also occur84; thus, procedures performed infrequently should at a minimum be subjected to increased supervision and/or retesting.
KNOWLEDGE GAPS
The process of developing these guidelines revealed important gaps in the literature regarding the use of ultrasound guidance for thoracentesis. First, it is uncertain whether the use of ultrasound reduces the risk of bleeding with thoracentesis. A retrospective cohort study of 19,339 thoracenteses suggests that ultrasound guidance is associated with a 38.7% relative reduction in the odds of hemorrhage, although this reduction did not reach statistical significance (OR 0.6 [0.4–1.04]).18 Ultrasound may reduce the risk of bleeding by reducing the number of attempts and needle passes and potentially avoiding tortuous intercostal vessels, which can be found especially in elderly patients and more cephalad rib spaces.91 In an observational study of 22 patients undergoing thoracentesis, the intercostal artery (ICA) was identified by a high-frequency ultrasound transducer in 74 of 88 intercostal spaces.92 The ICA is more exposed in the intercostal space within the first 6 cm lateral to the spinous processes and can be seen as far lateral as the midaxillary line.92-95 Thus, the ICA will most likely be avoided if a procedure site is selected >6 cm lateral to the spinous processes and the needle is inserted above the rib.
Second, although all three studies conducted using real-time (dynamic) ultrasound guidance reported a pneumothorax rate of <1%, it is uncertain whether real-time ultrasound guidance confers any additional benefit compared to static guidance for site marking as direct comparisons were not made.17,96,97 It is possible that real-time ultrasound guidance may be superior to static guidance in certain situations, such as small pleural effusions of <10–15 mm that have historically been considered too small to tap.13,22,23,96
Third, although one study suggests that general internists can safely perform thoracentesis with low complication rates similar to those of interventional radiologists,2 limited data exists on how to train practicing hospitalists to use ultrasound to guide thoracentesis. The effectiveness of different training protocols to acquire competence in ultrasound-guided thoracentesis has not been compared.
Finally, the impact of ultrasound use on patient experience has yet to be explored.
CONCLUSION
The use of ultrasound guidance for thoracentesis has been associated with increased success rates and decreased complication rates. Ultrasound can be used to estimate the pleural fluid volume, characterize the effusion as simple or complex, identify an optimal needle insertion site, and reduce the need for postprocedural chest radiographs. Training and experience are essential to reap the benefits of using ultrasound for thoracentesis, although our understanding of optimal educational strategies and learning curves is limited. Once training has occurred and competence is achieved, hospitalists can perform ultrasound-guided thoracentesis as safely as radiologists, pulmonologists, and other specialists.
Acknowledgments
Collaborators from the Society of Hospital Medicine Point-of-care Ultrasound Task Force: Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Venkat Kalidindi, Ketino Kobaidze, Joshua Lenchus, Benji Mathews, Paul, Mayo, Satyen Nichani, Vicki Noble, Martin Perez, Aliaksei Pustavoitau, Kreegan Reierson, Sophia Rodgers, Gerard Salame, Kirk Spencer, Vivek Tayal, David M. Tierney.
Disclosures
Ricardo Franco-Sadud reports institutional funds received from the Society of Hospital Medicine Annual Meeting for travel expenses and accommodations outside the submitted work. Nitin Puri reports Payment for lectures including service on speakers bureaus from Fujifilm Sonosite and royalties from Elsevier, both outside the submitted work. All other authors have nothing to disclose.
Funding
Brian P Lucas: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). Nilam Soni: Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1)
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
CHAIRS: Nilam Soni, Ricardo Franco Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Matthews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen. Lumbar puncture Working Group: Nilam Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen. PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Dan Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
1. Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat 13. 1998;139:1-119. PubMed
2. Kozmic SE, Wayne DB, Feinglass J, Hohmann SF, Barsuk JH. Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34-40. PubMed
3. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
4. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
5. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
6. Raptopoulos V, Davis LM, Lee G, Umali C, Lew R, Irwin RS. Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol. 1991;156(5):917-920. PubMed
7. Grogan DR, Irwin RS, Channick R, et al. Complications associated with thoracentesis. A prospective, randomized study comparing three different methods. Arch Intern Med. 1990;150(4):873-877. PubMed
8. Ravin CE. Thoracocentesis of loculated pleural effusions using grey scale ultrasonic guidance. Chest. 1977;71(5):666-668. PubMed
9. Weingardt JP, Guico RR, Nemcek AA, Jr., Li YP, Chiu ST. Ultrasound findings following failed, clinically directed thoracenteses. J Clin Ultrasound. 1994;22(7):419-426. PubMed
10. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
11. Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology. 1997;204(2):503-506. PubMed
12. Boland GW, Gazelle GS, Girard MJ, Mueller PR. Asymptomatic hydropneumothorax after therapeutic thoracentesis for malignant pleural effusions. AJR Am J Roentgenol. 1998;170(4):943-946. PubMed
13. Havelock T, Teoh R, Laws D, Gleeson F. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(2):ii61-76. PubMed
14. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The RAND/UCLA appropriateness method user’s manual. DTIC Document; 2001.
15. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
16. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
17. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
18. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
19. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
20. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
21. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
22. Lichtenstein D, Hulot JS, Rabiller A, Tostivint I, Meziere G. Feasibility and safety of ultrasound-aided thoracentesis in mechanically ventilated patients. Intensive Care Med. 1999;25(9):955-958. PubMed
23. Mayo PH, Goltz HR, Tafreshi M, Doelken P. Safety of ultrasound-guided thoracentesis in patients receiving mechanical ventilation. Chest. 2004;125(3):1059-1062. PubMed
24. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
25. Landay M, Harless W. Ultrasonic differentiation of right pleural effusion from subphrenic fluid on longitudinal scans of the right upper quadrant: importance of recognizing the diaphragm. Radiology. 1977;123(1):155-158. PubMed
26. Mayo PH, Doelken P. Pleural ultrasonography. Clin Chest Med. 2006;27(2):215-227. PubMed
27. Rosenberg ER. Ultrasound in the assessment of pleural densities. Chest. 1983;84(3):283-285. PubMed
28. Gorg C, Restrepo I, Schwerk WB. Sonography of malignant pleural effusion. Eur Radiol. 1997;7(8):1195-1198. PubMed
29. Gryminski J, Krakowka P, Lypacewicz G. The diagnosis of pleural effusion by ultrasonic and radiologic techniques. Chest. 1976;70(1):33-37. PubMed
30. Kalokairinou-Motogna M, Maratou K, Paianid I, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12(1):12-16. PubMed
31. Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9-15. PubMed
32. Grimberg A, Shigueoka DC, Atallah AN, Ajzen S, Iared W. Diagnostic accuracy of sonography for pleural effusion: systematic review. Sao Paulo Med J.. 2010;128(2):90-95. PubMed
33. Kataoka H. Utility of thoracic sonography for follow-up examination of chronic heart failure patients with previous decompensation. Clin Cardiol. 2007;30(7):336-341. PubMed
34. Ma OJ, Mateer JR. Trauma ultrasound examination versus chest radiography in the detection of hemothorax. Ann Emerg Med. 1997;29(3):312-315. PubMed
35. Rocco M, Carbone I, Morelli A, et al. Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand. 2008;52(6):776-784. PubMed
36. Kocijancic I, Vidmar K, Ivanovi-Herceg Z. Chest sonography versus lateral decubitus radiography in the diagnosis of small pleural effusions. J Clin Ultrasound. 2003;31(2):69-74. PubMed
37. Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1. PubMed
38. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
39. Medford AR, Entwisle JJ. Indications for thoracic ultrasound in chest medicine: an observational study. Postgrad Med J. 2010;86(1011):8-11. PubMed
40. Lin MS, Hwang JJ, Chong IW, et al. Ultrasonography of chest diseases: analysis of 154 cases. Gaoxiong Yi Xue Ke Xue Za Zhi . 1992;8(10):525-534. PubMed
41. Eibenberger KL, Dock WI, Ammann ME, Dorffner R, Hormann MF, Grabenwoger F. Quantification of pleural effusions: sonography versus radiography. Radiology. 1994;191(3):681-684. PubMed
42. Vignon P, Chastagner C, Berkane V, et al. Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit Care Med. 2005;33(8):1757-1763. PubMed
43. Usta E, Mustafi M, Ziemer G. Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2010;10(2):204-207. PubMed
44. Remerand F, Dellamonica J, Mao Z, et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010;36(4):656-664.
PubMed
45. Balik M, Plasil P, Waldauf P, et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32(2):318-321. PubMed
46. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracentesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
47. Lisi M, Cameli M, Mondillo S, et al. Incremental value of pocket-sized imaging device for bedside diagnosis of unilateral pleural effusions and ultrasound-guided thoracentesis. Interact Cardiovasc Thorac Surg. 2012;15(4):596-601. PubMed
48. Xirouchaki N, Kondili E, Prinianakis G, Malliotakis P, Georgopoulos D. Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014;40(1):57-65. PubMed
49. Chen HJ, Tu CY, Ling SJ, et al. Sonographic appearances in transudative pleural effusions: not always an anechoic pattern. Ultrasound Med Biol. 2008;34(3):362-369. PubMed
50. Yang PC, Luh KT, Chang DB, Wu HD, Yu CJ, Kuo SH. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol. 1992;159(1):29-33. PubMed
51. Liang SJ, Tu CY, Chen HJ, et al. Application of ultrasound-guided pigtail catheter for drainage of pleural effusions in the ICU. Intensive Care Med. 2009;35(2):350-354. PubMed
52. McLoud TC, Flower CD. Imaging the pleura: sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;156(6):1145-1153. PubMed
53. Tu CY, Hsu WH, Hsia TC, et al. Pleural effusions in febrile medical ICU patients: chest ultrasound study. Chest. 2004;126(4):1274-1280. PubMed
54. Sajadieh H, Afzali F, Sajadieh V, Sajadieh A. Ultrasound as an alternative to aspiration for determining the nature of pleural effusion, especially in older people. Ann N Y Acad Sci. 2004;1019:585-592. PubMed
55. Marcun R, Sustic A. Sonographic evaluation of unexplained pleural exudate: a prospective case series. Wien Klin Wochenschr. 2009;121(9-10):334-338. PubMed
56. Bugalho A, Ferreira D, Dias SS, et al. The diagnostic value of transthoracic ultrasonographic features in predicting malignancy in undiagnosed pleural effusions: a prospective observational study. Respiration. 2014;87(4):270-278. PubMed
57. Salamonsen MR, Lo AK, Ng AC, Bashirzadeh F, Wang WY, Fielding DI. Novel use of pleural ultrasound can identify malignant entrapped lung prior to effusion drainage. Chest. 2014;146(5):1286-1293. PubMed
58. Himelman RB, Callen PW. The prognostic value of loculations in parapneumonic pleural effusions. Chest. 1986;90(6):852-856. PubMed
59. Chen CH, Chen W, Chen HJ, et al. Transthoracic ultrasonography in predicting the outcome of small-bore catheter drainage in empyemas or complicated parapneumonic effusions. Ultrasound Med Biol. 2009;35(9):1468-1474. PubMed
60. Hirsch JH, Rogers JV, Mack LA. Real-time sonography of pleural opacities. AJR Am J Roentgenol. 1981;136(2):297-301. PubMed
61. Chen KY, Liaw YS, Wang HC, Luh KT, Yang PC. Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J Ultrasound Med. 2000;19(12):837-843. PubMed
62. Diaz-Guzman E, Budev MM. Accuracy of the physical examination in evaluating pleural effusion. Cleve Clin J Med. 2008;75(4):297-303. PubMed
63. Rhyne T, Birnholz JC. Simple measurement of chest-wall thickness with ultrasound. Radiology. 1973;108(2):436-438. PubMed
64. Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest. 2011;140(4):859-866. PubMed
65. Lichtenstein D, Meziere G, Biderman P, Gepner A. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26(10):1434-1440. PubMed
66. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
67. Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care. 2013;17(5):R208. PubMed
68. Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest. 2012;141(3):703-708. PubMed
69. Sartori S, Tombesi P, Trevisani L, Nielsen I, Tassinari D, Abbasciano V. Accuracy of transthoracic sonography in detection of pneumothorax after sonographically guided lung biopsy: prospective comparison with chest radiography. AJR Am J Roentgenol. 2007;188(1):37-41. PubMed
70. Blaivas M, Lyon M, Duggal S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med. 2005;12(9):844-849. PubMed
71. Lichtenstein DA, Meziere G, Lascols N, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005;33(6):1231-1238. PubMed
72. Kohan JM, Poe RH, Israel RH, et al. Value of chest ultrasonography versus decubitus roentgenography for thoracentesis. Am Rev Respir Dis. 1986;133(6):1124-1126. PubMed
73. Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc. 1998;73(10):948-950. PubMed
74. Aleman C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med. 1999;107(4):340-343. PubMed
75. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
76. Begot E, Grumann A, Duvoid T, et al. Ultrasonographic identification and semiquantitative assessment of unloculated pleural effusions in critically ill patients by residents after a focused training. Intensive Care Med. 2014;40(10):1475-1480. PubMed
77. Kotagal M, Quiroga E, Ruffatto BJ, et al. Impact of point-of-care ultrasound training on surgical residents’ confidence. J Surg Educ. 2015;72(4):e82-87. PubMed
78. Beaulieu Y, Laprise R, Drolet P, et al. Bedside ultrasound training using web-based e-learning and simulation early in the curriculum of residents. Crit Ultrasound J. 2015;7:1. PubMed
79. Schnobrich DJ, Olson AP, Broccard A, Duran-Nelson A. Feasibility and acceptability of a structured curriculum in teaching procedural and basic diagnostic ultrasound skills to internal medicine residents. J Grad Med Educ. 2013;5(3):493-497. PubMed
80. Chalumeau-Lemoine L, Baudel JL, Das V, et al. Results of short-term training of naive physicians in focused general ultrasonography in an intensive-care unit. Intensive Care Med. 2009;35(10):1767-1771. PubMed
81. Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ. 2011;11:75. PubMed
82. Ramsingh D, Alexander B, Le K, Williams W, Canales C, Cannesson M. Comparison of the didactic lecture with the simulation/model approach for the teaching of a novel perioperative ultrasound curriculum to anesthesiology residents. J Clin Anesth. 2014;26(6):443-454. PubMed
83. Sekiguchi H, Bhagra A, Gajic O, Kashani KB. A general Critical Care Ultrasonography workshop: results of a novel Web-based learning program combined with simulation-based hands-on training. J Crit Care. 2013;28(2):217.e217-212. PubMed
84. Dulohery MM, Stoven S, Kurklinsky AK, Halvorsen A, McDonald FS, Bhagra A. Ultrasound for internal medicine physicians: the future of the physical examination. J Ultrasound Med. 2014;33(6):1005-1011. PubMed
85. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
86. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
87. Wayne DB, Barsuk JH, O’Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
88. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
89. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
90. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
91. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol. 2012;71(4):245-251. PubMed
92. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
93. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
94. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
95. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
96. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
97. Harnsberger HR, Lee TG, Mukuno DH. Rapid, inexpensive real-time directed thoracentesis. Radiology. 1983;146(2):545-546. PubMed
Approximately 1.5 million people develop a pleural effusion in the United States annually, and approximately 173,000 people (12%) undergo thoracentesis.1 A recent review of thoracenteses performed at 234 University Health System Consortium hospitals between January 2010 and September 2013 demonstrated that 16% of 132,472 thoracenteses were performed by general internists and hospitalists, 33.1% were performed by interventional radiologists, and 20.3% were performed by pulmonologists.2 The iatrogenic pneumothorax rate was not significantly different between interventional radiologists and internists (2.8% and 2.9% risk, respectively); however, the admissions associated with bedside thoracentesis were less expensive than the admissions associated with thoracentesis performed in radiology suites, even after controlling for clinical covariates.2 In addition, the use of ultrasound guidance has been associated with a reduced risk of complications and cost of thoracentesis.3,4 In most of the early published studies on ultrasound-guided thoracentesis, the procedures were performed by radiologists.5-12 However, in 2010, the British Thoracic Society published guidelines on pleural procedures and thoracic ultrasound geared toward any trained provider.13 The purpose of this guideline is to review the literature and present evidence-based recommendations on the performance of ultrasound-guided thoracentesis at the bedside.
METHODS
Detailed methods are described in Appendix 1. The Society of Hospital Medicine (SHM) Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. The expert panel members were divided into working group members, external peer reviewers, and a methodologist. All the Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the four working group members themselves. Key clinical questions were prepared prior to conducting a systematic literature search by a medical librarian. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to September 2015 initially. Updated searches were conducted in November 2016 and in August 2017 (Appendix 3). All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of the screened articles were reviewed, and the articles focusing on the use of ultrasound to guide thoracentesis were selected. Articles that discussed thoracentesis without ultrasound guidance were excluded. In addition, the following article types were excluded: non-English language, nonhuman, subjects’ age <18 years, meeting abstracts, meeting posters, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided thoracentesis were screened and selected. Final article selection was based on working group consensus, and the selected literature was incorporated into draft recommendations.
We used the RAND Appropriateness Method that required panel judgment and consensus.14 The 30 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering the following five transforming factors: 1) Problem priority and importance, 2) Level of quality of evidence, 3) Benefit/harm balance, 4) Benefit/burden balance, and 5) Certainty/concerns about PEAF (Preferences/Equity Acceptability/Feasibility). Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (Redcap™) in December 2016 and January 2017 (Appendix 4). Voting on appropriateness was conducted using a 9-point Likert scale, and the degree of consensus was assessed using the RAND algorithm. Establishing a recommendation required at least 70% agreement and a strong recommendation required 80% agreement according to the RAND rules (Appendix 1, Figure 1). Disagreement was defined as >30% of panelists voting outside of the zone of the median (appropriate, uncertain, inappropriate).
RESULTS
Literature search
A total of 1,556 references were pooled from the following four different sources: a search by a certified librarian in September 2015 (1066 citations) that was updated in November 2016 (165 citations) and again in August 2017 (9 citations), working group members’ literature searches (47 citations), and a search focused on training (269 citations). The final selection included 94 articles that were abstracted into a data table and incorporated into the draft recommendations. The details of the literature search strategy are given in Appendix 3.
Recommendations
Terminology
- Thoracentesis is a procedure of aspiration of fluid from the pleural space by percutaneous insertion of a needle through the chest wall with or without the insertion of a catheter.
- In this document, ultrasound guidance refers to static guidance and site marking performed at the bedside immediately before the procedure, as opposed to real-time (dynamic) ultrasound guidance or radiology performed site marking. The static method is the most commonly used method of ultrasound guidance and is supported by current evidence.
RECOMMENDATIONS
Clinical Outcomes
1.We recommend that ultrasound should be used to guide thoracentesis to reduce the risk of complications, the most common being pneumothorax.
Rationale: Both static ultrasound guidance and dynamic ultrasound guidance have been reported to be associated with a reduced risk of pneumothorax.4-7,15-18 A meta-analysis of 24 studies that included 6,605 thoracenteses showed a significant decrease in the risk of postprocedure pneumothorax with the use of ultrasound guidance compared to the risk associated with thoracentesis performed based on landmarks alone (OR 0.3, 95% CI 0.2–0.7).3 The meta-analysis included both prospective and retrospective studies conducted using both static and dynamic ultrasound guidance.3 A large retrospective cohort study conducted by Mercaldi et al. comprising more than 61,000 patients who underwent thoracentesis also showed that ultrasound guidance was associated with reduced odds of pneumothorax (OR 0.8 [0.7–0.9]).4 When pneumothorax did occur during that hospitalization, the cost of hospitalization increased by $2800 and the length of stay increased by 1.5 days.4 A 2008 review of 19,339 thoracenteses conducted by Patel et al. also demonstrated an association between ultrasound guidance and reduced odds of pneumothorax (OR 0.8 [0.7–0.96]).18 Although these findings were significant, it is important to note that the studies of both Mercaldi et al. and Patel et al. were reviews of administrative databases conducted using the International Classification of Diseases, 9th Revision (ICD-9) codes for thoracentesis and Current Procedure Terminology–4th edition (CPT) codes for the use of ultrasound.4,18 Patel et al. identified pneumothorax using ICD-9 codes for “pneumothorax–iatrogenic” and “pneumothorax–not specified as due to the procedure.” The association between ultrasound guidance and the reduced odds of pneumothorax was driven by the latter code.18 However, as with most retrospective studies using administrative data, granular data about the patients, procedure, proceduralists, and complications were not available in these reviews and conclusions may be limited by erroneous coding or documentation.4,18 In a third retrospective cohort study, Raptopoulos et al. compared 154 landmark-based thoracenteses performed by “clinical physicians” and 188 ultrasound-guided thoracenteses performed by radiologists and found that ultrasound-guided site selection reduced the rate of pneumothorax from 18% to 3% (P < .0001).6 Finally, one single-center randomized controlled trial of 160 thoracenteses performed by pulmonologists showed that ultrasound guidance reduced the relative risk of pneumothorax by 90% (12.5% vs 1.3%; P =.009) with a number needed to treat of 9.15 It was not possible to blind the operators to the use of ultrasound guidance, but the data analysis was blinded.15 Furthermore, while there was no explicit comparison of the intervention vs. the control groups, randomization would have presumably rendered both groups similar in terms of patient characteristics and effusion characteristics.15 Ultrasound may reduce the risk of pneumothorax through several mechanisms, including identifying patients in whom thoracentesis cannot be safely performed, allowing selection of the safest needle insertion site, and revealing the optimal depth of needle insertion.
2.We recommend that ultrasound guidance should be used to increase the success rate of thoracentesis.
Rationale: Thoracentesis guided by ultrasound has lower rates of failed attempts, or “dry taps,” compared to thoracentesis guided solely by physical examination. In 1977, Ravin described a method of using ultrasound to guide successful drainage of six complex pleural effusions (empyema or loculated effusion) after multiple (5–7) failed attempts by clinicians using physical examination alone.8 In a second study by radiologists, Weingardt et al. demonstrated that 20 of 26 failed landmark-based thoracenteses were due to incorrect site selection by physical examination–15 sites were below the diaphragm and 5 sites were above the pleural effusion or in the consolidated lung–and the use of ultrasound allowed successful sampling in 14 of 16 patients who had a failed landmark-based thoracentesis.9 Diacon et al. asked 30 physicians, ranging from junior housestaff to pulmonologists, to mark 172 potential thoracentesis sites in 67 patients with pleural effusions using physical examination alone. Ultrasound was then used to evaluate the proposed puncture sites. They found that using ultrasound would have avoided puncture on “dry chests” in 2% and avoided potential laceration of a solid organ in 10% of patients compared to site selection by physical examination alone.19 Finally, Perazzo et al. randomized 160 patients to landmark-based thoracentesis and ultrasound-guided thoracentesis and demonstrated that half of the eight dry taps that occurred in the control group could be successfully drained using subsequent ultrasound guidance.15
Technique
3. We recommend that ultrasound-guided thoracentesis should be performed or closely supervised by experienced operators.
Rationale: Current evidence suggests lower complication rates when thoracentesis is performed by experienced healthcare providers. A systematic review of 6,605 thoracenteses showed a significantly lower pneumothorax rate when thoracentesis was performed by pulmonology or radiology faculty versus resident physicians (3.9% vs 8.5%; P =.04), although this finding was not significant in the four studies that directly compared this factor.3 In a quality improvement study performed by Duncan et al., pulmonology and critical care physicians combining multiple quality improvement initiatives to achieve and maintain competency decreased the rate of pneumothorax from 8.6% to 1.1% (P =.0034).20 Interventions included ultrasound training, performance of 10 thoracenteses under expert supervision, and restriction of privileges to proceduralists who perform 10 or more thoracenteses per year.20 Finally, a series of 9,320 ultrasound-guided thoracenteses performed or supervised by a single expert internist over a period of 12 years resulted in a pneumothorax rate of 0.6% and a composite complication rate of 0.98% (pneumothorax, reexpansion pulmonary edema, hemothorax, site bleeding, hematoma, splenic laceration, and vasovagal reaction).21 Notably, pneumothorax rate in resident physician hands was reported to be 8.5% in the meta-analysis performed by Gordon et al., which is similar to the initial rate in the pulmonologists who participated in the study by Duncan et al.3,20 However, after instituting formal ultrasound training and other initiatives aimed at maintaining competency, the pneumothorax rate in the study by Duncan et al. decreased to 1.1%, similar to the rate observed in the series by Ault et al.21 This suggests that training and supervision are necessary to achieve competency and reduce the rate of complications.3,20,21
4. We suggest that ultrasound guidance be used to reduce the risk of complications from thoracentesis in mechanically ventilated patients.
Rationale: The rest of this guideline refers to ultrasound-guided thoracentesis performed in spontaneously breathing patients; however, this recommendation is specific to mechanically ventilated patients. Two prospective observational studies have shown no increase in complications when ultrasound-guided thoracentesis is performed on mechanically ventilated patients compared to patients not receiving positive pressure ventilation. A feasibility study of 45 thoracenteses performed on ventilated patients reported no complications,22 whereas another study on 232 patients reported a pneumothorax rate of 1.3%.23 In a larger study conducted by Mayo et al., medicine housestaff performed thoracentesis under the supervision of intensivists who had undergone training in ultrasound prior to performing the procedure.23 In both studies, most of the patients were in a supine position, although positioning and puncture site were at the discretion of the physician, and both studies employed use of static ultrasound guidance.22,23 A large series of 9,320 ultrasound-guided thoracenteses that included 1,377 mechanically ventilated patients did not report a higher rate of pneumothorax (0.8%) compared to that in spontaneously breathing patients (0.61%).21 Finally, a meta-analysis of 19 observational studies comprising 1,124 mechanically ventilated patients who underwent pleural drainage procedures showed a low rate of pneumothorax (3.4%) and hemothorax (1.9%).24 Although the rate of complication was reported to be low in this meta-analysis, ultrasound was not employed in all studies and its use was not associated with a significant reduction in pneumothorax.24 This may be because 8 of the 19 studies used pigtail catheters or large-bore thoracostomy tubes which treat pneumothorax as they occur.24
5. We recommend that ultrasound should be used to identify the chest wall, pleura, diaphragm, lung, and subdiaphragmatic organs throughout the respiratory cycle before selecting a needle insertion site.
Rationale: The use of ultrasound improves the selection of a safe needle insertion site because sites chosen without ultrasound guidance may be below the diaphragm, over solid organs,9,19 or in locations that risk puncture of the lung.9 Visualization of the chest wall, diaphragm, and lung, which define the boundaries of a pleural effusion, allows the clinician to confirm the presence of a drainable pleural effusion and assess for other pathologies, such as ascites and tumor, that may be mistaken for a pleural effusion.22,25,26 Hypoechoic lesions can represent small loculated pleural effusions but also pleural plaques, pleural masses, peripheral lung masses, or abscesses.27,28
6. We recommend that ultrasound should be used to detect the presence or absence of an effusion and approximate the volume of pleural fluid to guide clinical decision-making.
Rationale: The presence and approximate size of pleural fluid collections are important determinants of whether thoracentesis, another procedure, or no procedure should be performed. Ultrasonography has higher sensitivity and specificity for detecting pleural effusions and better differentiates effusions from consolidations compared with chest radiography.29-42 Ultrasound allows semiquantitative estimation of pleural fluid volume to determine whether thoracentesis should be performed.41-45 When using ultrasound to choose a site for thoracentesis, the British Thoracic Society Pleural Disease guidelines recommend ≥10 mm of pleural fluid between the visceral and parietal pleura.13 Pleural effusions of <10–15 mm are considered too small to tap.22,23 In a prospective study of 45 patients, a measurement of >9.9 cm by ultrasound between the chest wall and the “V-point,” the intersection of the diaphragm and the collapsed lung, correlated with a pleural fluid volume of >1 liter.46 Another prospective study of 73 patients showed that a pleural effusion spanning >3 intercostal spaces by ultrasound also correlated with a pleural fluid volume of >1 liter.47 Anticipating the volume of fluid to be removed may aid in preplanning and procurement of larger capacity drainage containers prior to starting the procedure. Lung ultrasound can also change the management if the characteristic of the effusion suggests that an invasive procedure is unsafe or another diagnostic or therapeutic option is more appropriate.39 In a prospective cohort study of 189 mechanically ventilated patients, lung ultrasound guided the management in all patients with suspected effusion, leading to chest tube placement in 7 patients and thoracentesis in 34 patients.48
7. We recommend that ultrasound should be used to detect complex sonographic features, such as septations, to guide clinical decision-making regarding the timing and method of pleural drainage.
Rationale: Pleural effusions can be broadly categorized sonographically as simple or complex. Complex effusions are further categorized as with or without septation. Simple effusions are anechoic and are often, but not invariably, transudative.49-51 The use of sonography and computerized tomography (CT) is complementary, but features of complex pleural effusions (fibrin stranding and septations) may be better visualized by ultrasound than by CT of the thorax.52 Detection of complex features should prompt the consideration of pleural fluid sampling.53,54 Exudative effusions from tuberculosis, malignancy, or other etiologies more often include debris, septations, or other complex features.55,56 Certain features such as a swirling debris, pleural thickening, and nodularity may be more often associated with malignancy,54,56 and advanced ultrasound techniques may be used to detect a trapped lung prior to attempting drainage of a malignant pleural effusion.57 Two studies found complex septated pleural effusions to be invariably exudative50,58 and drainage was unlikely to be successful without the placement of a chest tube.50,58-60 Chest tube placement through fibrinolytic administration or video-assisted thoracoscopic surgery (VATS) may be more appropriate in the management of complex septated pleural effusions,59-61 and expert consultation with a thoracic specialist is recommended in these cases.
8. We suggest that ultrasound can be used to measure the depth from the skin surface to the parietal pleura to help select an appropriate length needle and determine the maximum needle insertion depth.
Rationale: The distance from the skin to the parietal and visceral pleura can be measured by ultrasound to determine whether thoracentesis can be safely performed and to guide selection of an adequate length needle.38 The length of needle required to penetrate the pleural space varies based on the thickness of the chest wall. Percussion of the chest wall is limited when there is more than 6 cm of subcutaneous tissue,62 making physical examination in obese patients unreliable for selecting an appropriate site or needle length for thoracentesis. Ultrasound allows visualization of deep soft tissues, well beyond the limits of percussion, and allows an accurate measurement of the chest wall.63
9. We suggest that ultrasound can be used to evaluate normal lung sliding pre- and postprocedure to rule out pneumothorax.
Rationale: Normal lung sliding indicates normal apposition and movement of visceral and parietal pleura and rules out pneumothorax with a sensitivity that exceeds that of chest radiography, according to a meta-analysis of 20 studies using computed tomography or escape of intrapleural air at the time of drainage as the gold standard.64 In this meta-analysis, the pooled sensitivity of ultrasound was reported to be 88% (85-91%) compared to 52% (49-55%) for radiography, although the analysis also suggests that the test characteristics are dependent on operator skill.64 However, although lung sliding rules out pneumothorax, absence of lung sliding is not specific for pneumothorax and other conditions, including pleural adhesions, pleurodesis, and bronchial obstruction, can cause the absence of lung sliding.64 Detection of a lung point conclusively rules in a pneumothorax.65 Provided that the preprocedure lung ultrasound examination revealed normal lung sliding, a postprocedure examination can be performed to effectively evaluate for pneumothorax. This modality does not use ionizing radiation, is less expensive than computed tomography, can be performed faster than bedside chest radiography, and is more sensitive than supine or upright chest radiography.64,66-71
10. We suggest avoiding delay or interval change in patient position between the time of marking the needle insertion site and performing the thoracentesis.
Rationale: Optimal patient positioning and ultrasound-guided site marking should be performed by the primary operator immediately before beginning an invasive procedure. Remote sonographic localization in which a radiologist marks a needle insertion site using ultrasound and the thoracentesis is performed at a later time by a different provider is an antiquated practice. Two early studies demonstrated that this practice is no safer than landmark-based thoracentesis.6,72 One prospective study of 205 patients performed in 1986 showed no significant decrease in the incidence of complications from thoracentesis performed using remote sonographic localization versus landmark-based drainage.72 Complications in that study included a total of 22 pneumothoraces and 1 hematoma. The rate of complications in the group of patients who had site marking performed by radiology faculty and subsequent thoracentesis by medicine housestaff or attending physicians was 9.7% versus a complication rate of 12.7% in the landmark-based group.72 In addition, Raptopoulos et al. observed no significant difference in the pneumothorax rate between 106 patients with landmark-based thoracenteses and 48 patients who were sonographically marked by radiology faculty and then returned to the ward for completion of the thoracentesis by medicine housestaff (19% vs. 15%, respectively).6 Both groups had significantly higher rates of pneumothorax compared to those who underwent thoracentesis performed using real-time ultrasound guidance by radiology trainees (3%).6 The authors speculated that changing the patient’s position shifted the position of the pleural effusion, ultimately leading to the reliance on physical examination for the tap site.6
11. We recommend against performing routine postprocedure chest radiographs in patients who have undergone thoracentesis successfully with ultrasound guidance and are asymptomatic with normal lung sliding postprocedure.
Rationale: Chest radiography post-thoracentesis is unlikely to add information that changes management, especially if performed routinely, but does add expense, radiation, and inconvenience.73 The most common serious complication of thoracentesis is pneumothorax, which is often accompanied by symptoms, particularly in those patients with pneumothorax large enough to warrant chest tube placement.10,74,75 Pihlajamaa et al. retrospectively studied 264 ultrasound-guided thoracenteses performed by radiologists or radiology residents and noted that of 11 pneumothoraces, only 1 necessitated chest tube placement.10 Aleman et al. prospectively studied 506 ultrasound-guided and physical examination-guided thoracenteses and found that only 1% of asymptomatic patients developed a pneumothorax.74 Eight of the 18 symptomatic patients required chest tube placement as opposed to 1 of the 488 asymptomatic patients.74 A large prospective study of 941 ultrasound-guided thoracentesis reported that only 0.3% of asymptomatic patients with no suspicion of pneumothorax required tube thoracostomy.5 Postprocedure chest radiographs may be considered when thoracentesis is performed on mechanically ventilated patients, particularly when high airway pressures exist. In a study of 434 patients undergoing thoracentesis, only 10 patients had a pneumothorax (2.3%).11 Six of these pneumothoraces occurred in 92 mechanically ventilated patients (6.5%), and 2 of these 6 patients required a chest tube.11 None of the 4 spontaneously breathing patients with pneumothorax required a chest tube.11
Training
12. We recommend that novices who use ultrasound guidance for thoracentesis should receive focused training in lung and pleural ultrasonography and hands-on practice in procedural technique.
Rationale: Healthcare providers have to gain various skills to safely perform ultrasound-guided thoracentesis independently. Trainees should learn how to use ultrasound to identify important structures (chest wall, ribs, lung, pleura, diaphragm, and subdiaphragmatic organs); detect pleural effusions with complex features, such as septations; identify consolidated lung tissue; and rule out a pneumothorax. Prospective studies done with novice learners have shown that focused training combining didactics and hands-on practice using simulation or live models improves skills to assess pleural effusions.76-84 Several additional procedural techniques such as patient positioning and needle insertion are also important but are beyond the scope of these guidelines.
13. We suggest that novices undergo simulation-based training prior to performing ultrasound-guided thoracentesis on patients.
Rationale: Simulation-based training for thoracentesis has been studied in providers with different levels of medical training, ranging from medical students and internal medicine residents to practicing pulmonologists. Studies suggest that training in a zero-risk environment with simulation task trainers leads to increased knowledge and skills without subjecting the patients to inexperienced operators.85-87 One study on simulator-based training in medical students showed skill retention at 6 months and these skills were at least partially transferred to increased competency on live patients.88 Checklists to train providers in ultrasound-guided thoracentesis have been published.89,90 An experiential training program for attending physicians that utilized task trainers, along with standardized equipment and procedural technique, resulted in a reduction in the pneumothorax rate from 8.6% to 1.1%.20
14. Training curves for novices to become competent in lung ultrasound and ultrasound-guided thoracentesis are not completely understood. We recommend that training should be tailored to the skill acquisition of the learner and the resources of the institution.
Rationale: Understanding the rates at which novices progress from performing procedures under direct supervision to performing them independently would be highly desirable to ensure patient safety, guide supervision, and maximize efficiency of training. However, there is limited research describing the rate of progression of learners through these stages, either with regard to time or number of procedures performed. Two studies have shown that with brief training programs, medical students88 and internal medicine residents87 can achieve high levels of proficiency to perform thoracentesis on simulators, which is durable over time; however, whether these findings in a simulated environment translate into clinically significant outcomes is largely unknown, and neither of these studies incorporated the use of ultrasound guidance in their training curricula.87,88 Another study of pulmonary and critical care physicians combined multiple quality improvement initiatives with a half day of ultrasound-guided thoracentesis training, a requirement to perform 10 supervised thoracenteses prior to independent practice, and an additional requirement to perform 10 thoracenteses per year to maintain privileges.20 These interventions resulted in a concentration of competency among a few proceduralists, decreasing the rate of pneumothorax from 8.6% to 1.1%.20 Degradation of skills with disuse may also occur84; thus, procedures performed infrequently should at a minimum be subjected to increased supervision and/or retesting.
KNOWLEDGE GAPS
The process of developing these guidelines revealed important gaps in the literature regarding the use of ultrasound guidance for thoracentesis. First, it is uncertain whether the use of ultrasound reduces the risk of bleeding with thoracentesis. A retrospective cohort study of 19,339 thoracenteses suggests that ultrasound guidance is associated with a 38.7% relative reduction in the odds of hemorrhage, although this reduction did not reach statistical significance (OR 0.6 [0.4–1.04]).18 Ultrasound may reduce the risk of bleeding by reducing the number of attempts and needle passes and potentially avoiding tortuous intercostal vessels, which can be found especially in elderly patients and more cephalad rib spaces.91 In an observational study of 22 patients undergoing thoracentesis, the intercostal artery (ICA) was identified by a high-frequency ultrasound transducer in 74 of 88 intercostal spaces.92 The ICA is more exposed in the intercostal space within the first 6 cm lateral to the spinous processes and can be seen as far lateral as the midaxillary line.92-95 Thus, the ICA will most likely be avoided if a procedure site is selected >6 cm lateral to the spinous processes and the needle is inserted above the rib.
Second, although all three studies conducted using real-time (dynamic) ultrasound guidance reported a pneumothorax rate of <1%, it is uncertain whether real-time ultrasound guidance confers any additional benefit compared to static guidance for site marking as direct comparisons were not made.17,96,97 It is possible that real-time ultrasound guidance may be superior to static guidance in certain situations, such as small pleural effusions of <10–15 mm that have historically been considered too small to tap.13,22,23,96
Third, although one study suggests that general internists can safely perform thoracentesis with low complication rates similar to those of interventional radiologists,2 limited data exists on how to train practicing hospitalists to use ultrasound to guide thoracentesis. The effectiveness of different training protocols to acquire competence in ultrasound-guided thoracentesis has not been compared.
Finally, the impact of ultrasound use on patient experience has yet to be explored.
CONCLUSION
The use of ultrasound guidance for thoracentesis has been associated with increased success rates and decreased complication rates. Ultrasound can be used to estimate the pleural fluid volume, characterize the effusion as simple or complex, identify an optimal needle insertion site, and reduce the need for postprocedural chest radiographs. Training and experience are essential to reap the benefits of using ultrasound for thoracentesis, although our understanding of optimal educational strategies and learning curves is limited. Once training has occurred and competence is achieved, hospitalists can perform ultrasound-guided thoracentesis as safely as radiologists, pulmonologists, and other specialists.
Acknowledgments
Collaborators from the Society of Hospital Medicine Point-of-care Ultrasound Task Force: Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Venkat Kalidindi, Ketino Kobaidze, Joshua Lenchus, Benji Mathews, Paul, Mayo, Satyen Nichani, Vicki Noble, Martin Perez, Aliaksei Pustavoitau, Kreegan Reierson, Sophia Rodgers, Gerard Salame, Kirk Spencer, Vivek Tayal, David M. Tierney.
Disclosures
Ricardo Franco-Sadud reports institutional funds received from the Society of Hospital Medicine Annual Meeting for travel expenses and accommodations outside the submitted work. Nitin Puri reports Payment for lectures including service on speakers bureaus from Fujifilm Sonosite and royalties from Elsevier, both outside the submitted work. All other authors have nothing to disclose.
Funding
Brian P Lucas: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). Nilam Soni: Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1)
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
CHAIRS: Nilam Soni, Ricardo Franco Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Matthews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen. Lumbar puncture Working Group: Nilam Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen. PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Dan Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Approximately 1.5 million people develop a pleural effusion in the United States annually, and approximately 173,000 people (12%) undergo thoracentesis.1 A recent review of thoracenteses performed at 234 University Health System Consortium hospitals between January 2010 and September 2013 demonstrated that 16% of 132,472 thoracenteses were performed by general internists and hospitalists, 33.1% were performed by interventional radiologists, and 20.3% were performed by pulmonologists.2 The iatrogenic pneumothorax rate was not significantly different between interventional radiologists and internists (2.8% and 2.9% risk, respectively); however, the admissions associated with bedside thoracentesis were less expensive than the admissions associated with thoracentesis performed in radiology suites, even after controlling for clinical covariates.2 In addition, the use of ultrasound guidance has been associated with a reduced risk of complications and cost of thoracentesis.3,4 In most of the early published studies on ultrasound-guided thoracentesis, the procedures were performed by radiologists.5-12 However, in 2010, the British Thoracic Society published guidelines on pleural procedures and thoracic ultrasound geared toward any trained provider.13 The purpose of this guideline is to review the literature and present evidence-based recommendations on the performance of ultrasound-guided thoracentesis at the bedside.
METHODS
Detailed methods are described in Appendix 1. The Society of Hospital Medicine (SHM) Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. The expert panel members were divided into working group members, external peer reviewers, and a methodologist. All the Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the four working group members themselves. Key clinical questions were prepared prior to conducting a systematic literature search by a medical librarian. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to September 2015 initially. Updated searches were conducted in November 2016 and in August 2017 (Appendix 3). All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of the screened articles were reviewed, and the articles focusing on the use of ultrasound to guide thoracentesis were selected. Articles that discussed thoracentesis without ultrasound guidance were excluded. In addition, the following article types were excluded: non-English language, nonhuman, subjects’ age <18 years, meeting abstracts, meeting posters, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided thoracentesis were screened and selected. Final article selection was based on working group consensus, and the selected literature was incorporated into draft recommendations.
We used the RAND Appropriateness Method that required panel judgment and consensus.14 The 30 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering the following five transforming factors: 1) Problem priority and importance, 2) Level of quality of evidence, 3) Benefit/harm balance, 4) Benefit/burden balance, and 5) Certainty/concerns about PEAF (Preferences/Equity Acceptability/Feasibility). Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (Redcap™) in December 2016 and January 2017 (Appendix 4). Voting on appropriateness was conducted using a 9-point Likert scale, and the degree of consensus was assessed using the RAND algorithm. Establishing a recommendation required at least 70% agreement and a strong recommendation required 80% agreement according to the RAND rules (Appendix 1, Figure 1). Disagreement was defined as >30% of panelists voting outside of the zone of the median (appropriate, uncertain, inappropriate).
RESULTS
Literature search
A total of 1,556 references were pooled from the following four different sources: a search by a certified librarian in September 2015 (1066 citations) that was updated in November 2016 (165 citations) and again in August 2017 (9 citations), working group members’ literature searches (47 citations), and a search focused on training (269 citations). The final selection included 94 articles that were abstracted into a data table and incorporated into the draft recommendations. The details of the literature search strategy are given in Appendix 3.
Recommendations
Terminology
- Thoracentesis is a procedure of aspiration of fluid from the pleural space by percutaneous insertion of a needle through the chest wall with or without the insertion of a catheter.
- In this document, ultrasound guidance refers to static guidance and site marking performed at the bedside immediately before the procedure, as opposed to real-time (dynamic) ultrasound guidance or radiology performed site marking. The static method is the most commonly used method of ultrasound guidance and is supported by current evidence.
RECOMMENDATIONS
Clinical Outcomes
1.We recommend that ultrasound should be used to guide thoracentesis to reduce the risk of complications, the most common being pneumothorax.
Rationale: Both static ultrasound guidance and dynamic ultrasound guidance have been reported to be associated with a reduced risk of pneumothorax.4-7,15-18 A meta-analysis of 24 studies that included 6,605 thoracenteses showed a significant decrease in the risk of postprocedure pneumothorax with the use of ultrasound guidance compared to the risk associated with thoracentesis performed based on landmarks alone (OR 0.3, 95% CI 0.2–0.7).3 The meta-analysis included both prospective and retrospective studies conducted using both static and dynamic ultrasound guidance.3 A large retrospective cohort study conducted by Mercaldi et al. comprising more than 61,000 patients who underwent thoracentesis also showed that ultrasound guidance was associated with reduced odds of pneumothorax (OR 0.8 [0.7–0.9]).4 When pneumothorax did occur during that hospitalization, the cost of hospitalization increased by $2800 and the length of stay increased by 1.5 days.4 A 2008 review of 19,339 thoracenteses conducted by Patel et al. also demonstrated an association between ultrasound guidance and reduced odds of pneumothorax (OR 0.8 [0.7–0.96]).18 Although these findings were significant, it is important to note that the studies of both Mercaldi et al. and Patel et al. were reviews of administrative databases conducted using the International Classification of Diseases, 9th Revision (ICD-9) codes for thoracentesis and Current Procedure Terminology–4th edition (CPT) codes for the use of ultrasound.4,18 Patel et al. identified pneumothorax using ICD-9 codes for “pneumothorax–iatrogenic” and “pneumothorax–not specified as due to the procedure.” The association between ultrasound guidance and the reduced odds of pneumothorax was driven by the latter code.18 However, as with most retrospective studies using administrative data, granular data about the patients, procedure, proceduralists, and complications were not available in these reviews and conclusions may be limited by erroneous coding or documentation.4,18 In a third retrospective cohort study, Raptopoulos et al. compared 154 landmark-based thoracenteses performed by “clinical physicians” and 188 ultrasound-guided thoracenteses performed by radiologists and found that ultrasound-guided site selection reduced the rate of pneumothorax from 18% to 3% (P < .0001).6 Finally, one single-center randomized controlled trial of 160 thoracenteses performed by pulmonologists showed that ultrasound guidance reduced the relative risk of pneumothorax by 90% (12.5% vs 1.3%; P =.009) with a number needed to treat of 9.15 It was not possible to blind the operators to the use of ultrasound guidance, but the data analysis was blinded.15 Furthermore, while there was no explicit comparison of the intervention vs. the control groups, randomization would have presumably rendered both groups similar in terms of patient characteristics and effusion characteristics.15 Ultrasound may reduce the risk of pneumothorax through several mechanisms, including identifying patients in whom thoracentesis cannot be safely performed, allowing selection of the safest needle insertion site, and revealing the optimal depth of needle insertion.
2.We recommend that ultrasound guidance should be used to increase the success rate of thoracentesis.
Rationale: Thoracentesis guided by ultrasound has lower rates of failed attempts, or “dry taps,” compared to thoracentesis guided solely by physical examination. In 1977, Ravin described a method of using ultrasound to guide successful drainage of six complex pleural effusions (empyema or loculated effusion) after multiple (5–7) failed attempts by clinicians using physical examination alone.8 In a second study by radiologists, Weingardt et al. demonstrated that 20 of 26 failed landmark-based thoracenteses were due to incorrect site selection by physical examination–15 sites were below the diaphragm and 5 sites were above the pleural effusion or in the consolidated lung–and the use of ultrasound allowed successful sampling in 14 of 16 patients who had a failed landmark-based thoracentesis.9 Diacon et al. asked 30 physicians, ranging from junior housestaff to pulmonologists, to mark 172 potential thoracentesis sites in 67 patients with pleural effusions using physical examination alone. Ultrasound was then used to evaluate the proposed puncture sites. They found that using ultrasound would have avoided puncture on “dry chests” in 2% and avoided potential laceration of a solid organ in 10% of patients compared to site selection by physical examination alone.19 Finally, Perazzo et al. randomized 160 patients to landmark-based thoracentesis and ultrasound-guided thoracentesis and demonstrated that half of the eight dry taps that occurred in the control group could be successfully drained using subsequent ultrasound guidance.15
Technique
3. We recommend that ultrasound-guided thoracentesis should be performed or closely supervised by experienced operators.
Rationale: Current evidence suggests lower complication rates when thoracentesis is performed by experienced healthcare providers. A systematic review of 6,605 thoracenteses showed a significantly lower pneumothorax rate when thoracentesis was performed by pulmonology or radiology faculty versus resident physicians (3.9% vs 8.5%; P =.04), although this finding was not significant in the four studies that directly compared this factor.3 In a quality improvement study performed by Duncan et al., pulmonology and critical care physicians combining multiple quality improvement initiatives to achieve and maintain competency decreased the rate of pneumothorax from 8.6% to 1.1% (P =.0034).20 Interventions included ultrasound training, performance of 10 thoracenteses under expert supervision, and restriction of privileges to proceduralists who perform 10 or more thoracenteses per year.20 Finally, a series of 9,320 ultrasound-guided thoracenteses performed or supervised by a single expert internist over a period of 12 years resulted in a pneumothorax rate of 0.6% and a composite complication rate of 0.98% (pneumothorax, reexpansion pulmonary edema, hemothorax, site bleeding, hematoma, splenic laceration, and vasovagal reaction).21 Notably, pneumothorax rate in resident physician hands was reported to be 8.5% in the meta-analysis performed by Gordon et al., which is similar to the initial rate in the pulmonologists who participated in the study by Duncan et al.3,20 However, after instituting formal ultrasound training and other initiatives aimed at maintaining competency, the pneumothorax rate in the study by Duncan et al. decreased to 1.1%, similar to the rate observed in the series by Ault et al.21 This suggests that training and supervision are necessary to achieve competency and reduce the rate of complications.3,20,21
4. We suggest that ultrasound guidance be used to reduce the risk of complications from thoracentesis in mechanically ventilated patients.
Rationale: The rest of this guideline refers to ultrasound-guided thoracentesis performed in spontaneously breathing patients; however, this recommendation is specific to mechanically ventilated patients. Two prospective observational studies have shown no increase in complications when ultrasound-guided thoracentesis is performed on mechanically ventilated patients compared to patients not receiving positive pressure ventilation. A feasibility study of 45 thoracenteses performed on ventilated patients reported no complications,22 whereas another study on 232 patients reported a pneumothorax rate of 1.3%.23 In a larger study conducted by Mayo et al., medicine housestaff performed thoracentesis under the supervision of intensivists who had undergone training in ultrasound prior to performing the procedure.23 In both studies, most of the patients were in a supine position, although positioning and puncture site were at the discretion of the physician, and both studies employed use of static ultrasound guidance.22,23 A large series of 9,320 ultrasound-guided thoracenteses that included 1,377 mechanically ventilated patients did not report a higher rate of pneumothorax (0.8%) compared to that in spontaneously breathing patients (0.61%).21 Finally, a meta-analysis of 19 observational studies comprising 1,124 mechanically ventilated patients who underwent pleural drainage procedures showed a low rate of pneumothorax (3.4%) and hemothorax (1.9%).24 Although the rate of complication was reported to be low in this meta-analysis, ultrasound was not employed in all studies and its use was not associated with a significant reduction in pneumothorax.24 This may be because 8 of the 19 studies used pigtail catheters or large-bore thoracostomy tubes which treat pneumothorax as they occur.24
5. We recommend that ultrasound should be used to identify the chest wall, pleura, diaphragm, lung, and subdiaphragmatic organs throughout the respiratory cycle before selecting a needle insertion site.
Rationale: The use of ultrasound improves the selection of a safe needle insertion site because sites chosen without ultrasound guidance may be below the diaphragm, over solid organs,9,19 or in locations that risk puncture of the lung.9 Visualization of the chest wall, diaphragm, and lung, which define the boundaries of a pleural effusion, allows the clinician to confirm the presence of a drainable pleural effusion and assess for other pathologies, such as ascites and tumor, that may be mistaken for a pleural effusion.22,25,26 Hypoechoic lesions can represent small loculated pleural effusions but also pleural plaques, pleural masses, peripheral lung masses, or abscesses.27,28
6. We recommend that ultrasound should be used to detect the presence or absence of an effusion and approximate the volume of pleural fluid to guide clinical decision-making.
Rationale: The presence and approximate size of pleural fluid collections are important determinants of whether thoracentesis, another procedure, or no procedure should be performed. Ultrasonography has higher sensitivity and specificity for detecting pleural effusions and better differentiates effusions from consolidations compared with chest radiography.29-42 Ultrasound allows semiquantitative estimation of pleural fluid volume to determine whether thoracentesis should be performed.41-45 When using ultrasound to choose a site for thoracentesis, the British Thoracic Society Pleural Disease guidelines recommend ≥10 mm of pleural fluid between the visceral and parietal pleura.13 Pleural effusions of <10–15 mm are considered too small to tap.22,23 In a prospective study of 45 patients, a measurement of >9.9 cm by ultrasound between the chest wall and the “V-point,” the intersection of the diaphragm and the collapsed lung, correlated with a pleural fluid volume of >1 liter.46 Another prospective study of 73 patients showed that a pleural effusion spanning >3 intercostal spaces by ultrasound also correlated with a pleural fluid volume of >1 liter.47 Anticipating the volume of fluid to be removed may aid in preplanning and procurement of larger capacity drainage containers prior to starting the procedure. Lung ultrasound can also change the management if the characteristic of the effusion suggests that an invasive procedure is unsafe or another diagnostic or therapeutic option is more appropriate.39 In a prospective cohort study of 189 mechanically ventilated patients, lung ultrasound guided the management in all patients with suspected effusion, leading to chest tube placement in 7 patients and thoracentesis in 34 patients.48
7. We recommend that ultrasound should be used to detect complex sonographic features, such as septations, to guide clinical decision-making regarding the timing and method of pleural drainage.
Rationale: Pleural effusions can be broadly categorized sonographically as simple or complex. Complex effusions are further categorized as with or without septation. Simple effusions are anechoic and are often, but not invariably, transudative.49-51 The use of sonography and computerized tomography (CT) is complementary, but features of complex pleural effusions (fibrin stranding and septations) may be better visualized by ultrasound than by CT of the thorax.52 Detection of complex features should prompt the consideration of pleural fluid sampling.53,54 Exudative effusions from tuberculosis, malignancy, or other etiologies more often include debris, septations, or other complex features.55,56 Certain features such as a swirling debris, pleural thickening, and nodularity may be more often associated with malignancy,54,56 and advanced ultrasound techniques may be used to detect a trapped lung prior to attempting drainage of a malignant pleural effusion.57 Two studies found complex septated pleural effusions to be invariably exudative50,58 and drainage was unlikely to be successful without the placement of a chest tube.50,58-60 Chest tube placement through fibrinolytic administration or video-assisted thoracoscopic surgery (VATS) may be more appropriate in the management of complex septated pleural effusions,59-61 and expert consultation with a thoracic specialist is recommended in these cases.
8. We suggest that ultrasound can be used to measure the depth from the skin surface to the parietal pleura to help select an appropriate length needle and determine the maximum needle insertion depth.
Rationale: The distance from the skin to the parietal and visceral pleura can be measured by ultrasound to determine whether thoracentesis can be safely performed and to guide selection of an adequate length needle.38 The length of needle required to penetrate the pleural space varies based on the thickness of the chest wall. Percussion of the chest wall is limited when there is more than 6 cm of subcutaneous tissue,62 making physical examination in obese patients unreliable for selecting an appropriate site or needle length for thoracentesis. Ultrasound allows visualization of deep soft tissues, well beyond the limits of percussion, and allows an accurate measurement of the chest wall.63
9. We suggest that ultrasound can be used to evaluate normal lung sliding pre- and postprocedure to rule out pneumothorax.
Rationale: Normal lung sliding indicates normal apposition and movement of visceral and parietal pleura and rules out pneumothorax with a sensitivity that exceeds that of chest radiography, according to a meta-analysis of 20 studies using computed tomography or escape of intrapleural air at the time of drainage as the gold standard.64 In this meta-analysis, the pooled sensitivity of ultrasound was reported to be 88% (85-91%) compared to 52% (49-55%) for radiography, although the analysis also suggests that the test characteristics are dependent on operator skill.64 However, although lung sliding rules out pneumothorax, absence of lung sliding is not specific for pneumothorax and other conditions, including pleural adhesions, pleurodesis, and bronchial obstruction, can cause the absence of lung sliding.64 Detection of a lung point conclusively rules in a pneumothorax.65 Provided that the preprocedure lung ultrasound examination revealed normal lung sliding, a postprocedure examination can be performed to effectively evaluate for pneumothorax. This modality does not use ionizing radiation, is less expensive than computed tomography, can be performed faster than bedside chest radiography, and is more sensitive than supine or upright chest radiography.64,66-71
10. We suggest avoiding delay or interval change in patient position between the time of marking the needle insertion site and performing the thoracentesis.
Rationale: Optimal patient positioning and ultrasound-guided site marking should be performed by the primary operator immediately before beginning an invasive procedure. Remote sonographic localization in which a radiologist marks a needle insertion site using ultrasound and the thoracentesis is performed at a later time by a different provider is an antiquated practice. Two early studies demonstrated that this practice is no safer than landmark-based thoracentesis.6,72 One prospective study of 205 patients performed in 1986 showed no significant decrease in the incidence of complications from thoracentesis performed using remote sonographic localization versus landmark-based drainage.72 Complications in that study included a total of 22 pneumothoraces and 1 hematoma. The rate of complications in the group of patients who had site marking performed by radiology faculty and subsequent thoracentesis by medicine housestaff or attending physicians was 9.7% versus a complication rate of 12.7% in the landmark-based group.72 In addition, Raptopoulos et al. observed no significant difference in the pneumothorax rate between 106 patients with landmark-based thoracenteses and 48 patients who were sonographically marked by radiology faculty and then returned to the ward for completion of the thoracentesis by medicine housestaff (19% vs. 15%, respectively).6 Both groups had significantly higher rates of pneumothorax compared to those who underwent thoracentesis performed using real-time ultrasound guidance by radiology trainees (3%).6 The authors speculated that changing the patient’s position shifted the position of the pleural effusion, ultimately leading to the reliance on physical examination for the tap site.6
11. We recommend against performing routine postprocedure chest radiographs in patients who have undergone thoracentesis successfully with ultrasound guidance and are asymptomatic with normal lung sliding postprocedure.
Rationale: Chest radiography post-thoracentesis is unlikely to add information that changes management, especially if performed routinely, but does add expense, radiation, and inconvenience.73 The most common serious complication of thoracentesis is pneumothorax, which is often accompanied by symptoms, particularly in those patients with pneumothorax large enough to warrant chest tube placement.10,74,75 Pihlajamaa et al. retrospectively studied 264 ultrasound-guided thoracenteses performed by radiologists or radiology residents and noted that of 11 pneumothoraces, only 1 necessitated chest tube placement.10 Aleman et al. prospectively studied 506 ultrasound-guided and physical examination-guided thoracenteses and found that only 1% of asymptomatic patients developed a pneumothorax.74 Eight of the 18 symptomatic patients required chest tube placement as opposed to 1 of the 488 asymptomatic patients.74 A large prospective study of 941 ultrasound-guided thoracentesis reported that only 0.3% of asymptomatic patients with no suspicion of pneumothorax required tube thoracostomy.5 Postprocedure chest radiographs may be considered when thoracentesis is performed on mechanically ventilated patients, particularly when high airway pressures exist. In a study of 434 patients undergoing thoracentesis, only 10 patients had a pneumothorax (2.3%).11 Six of these pneumothoraces occurred in 92 mechanically ventilated patients (6.5%), and 2 of these 6 patients required a chest tube.11 None of the 4 spontaneously breathing patients with pneumothorax required a chest tube.11
Training
12. We recommend that novices who use ultrasound guidance for thoracentesis should receive focused training in lung and pleural ultrasonography and hands-on practice in procedural technique.
Rationale: Healthcare providers have to gain various skills to safely perform ultrasound-guided thoracentesis independently. Trainees should learn how to use ultrasound to identify important structures (chest wall, ribs, lung, pleura, diaphragm, and subdiaphragmatic organs); detect pleural effusions with complex features, such as septations; identify consolidated lung tissue; and rule out a pneumothorax. Prospective studies done with novice learners have shown that focused training combining didactics and hands-on practice using simulation or live models improves skills to assess pleural effusions.76-84 Several additional procedural techniques such as patient positioning and needle insertion are also important but are beyond the scope of these guidelines.
13. We suggest that novices undergo simulation-based training prior to performing ultrasound-guided thoracentesis on patients.
Rationale: Simulation-based training for thoracentesis has been studied in providers with different levels of medical training, ranging from medical students and internal medicine residents to practicing pulmonologists. Studies suggest that training in a zero-risk environment with simulation task trainers leads to increased knowledge and skills without subjecting the patients to inexperienced operators.85-87 One study on simulator-based training in medical students showed skill retention at 6 months and these skills were at least partially transferred to increased competency on live patients.88 Checklists to train providers in ultrasound-guided thoracentesis have been published.89,90 An experiential training program for attending physicians that utilized task trainers, along with standardized equipment and procedural technique, resulted in a reduction in the pneumothorax rate from 8.6% to 1.1%.20
14. Training curves for novices to become competent in lung ultrasound and ultrasound-guided thoracentesis are not completely understood. We recommend that training should be tailored to the skill acquisition of the learner and the resources of the institution.
Rationale: Understanding the rates at which novices progress from performing procedures under direct supervision to performing them independently would be highly desirable to ensure patient safety, guide supervision, and maximize efficiency of training. However, there is limited research describing the rate of progression of learners through these stages, either with regard to time or number of procedures performed. Two studies have shown that with brief training programs, medical students88 and internal medicine residents87 can achieve high levels of proficiency to perform thoracentesis on simulators, which is durable over time; however, whether these findings in a simulated environment translate into clinically significant outcomes is largely unknown, and neither of these studies incorporated the use of ultrasound guidance in their training curricula.87,88 Another study of pulmonary and critical care physicians combined multiple quality improvement initiatives with a half day of ultrasound-guided thoracentesis training, a requirement to perform 10 supervised thoracenteses prior to independent practice, and an additional requirement to perform 10 thoracenteses per year to maintain privileges.20 These interventions resulted in a concentration of competency among a few proceduralists, decreasing the rate of pneumothorax from 8.6% to 1.1%.20 Degradation of skills with disuse may also occur84; thus, procedures performed infrequently should at a minimum be subjected to increased supervision and/or retesting.
KNOWLEDGE GAPS
The process of developing these guidelines revealed important gaps in the literature regarding the use of ultrasound guidance for thoracentesis. First, it is uncertain whether the use of ultrasound reduces the risk of bleeding with thoracentesis. A retrospective cohort study of 19,339 thoracenteses suggests that ultrasound guidance is associated with a 38.7% relative reduction in the odds of hemorrhage, although this reduction did not reach statistical significance (OR 0.6 [0.4–1.04]).18 Ultrasound may reduce the risk of bleeding by reducing the number of attempts and needle passes and potentially avoiding tortuous intercostal vessels, which can be found especially in elderly patients and more cephalad rib spaces.91 In an observational study of 22 patients undergoing thoracentesis, the intercostal artery (ICA) was identified by a high-frequency ultrasound transducer in 74 of 88 intercostal spaces.92 The ICA is more exposed in the intercostal space within the first 6 cm lateral to the spinous processes and can be seen as far lateral as the midaxillary line.92-95 Thus, the ICA will most likely be avoided if a procedure site is selected >6 cm lateral to the spinous processes and the needle is inserted above the rib.
Second, although all three studies conducted using real-time (dynamic) ultrasound guidance reported a pneumothorax rate of <1%, it is uncertain whether real-time ultrasound guidance confers any additional benefit compared to static guidance for site marking as direct comparisons were not made.17,96,97 It is possible that real-time ultrasound guidance may be superior to static guidance in certain situations, such as small pleural effusions of <10–15 mm that have historically been considered too small to tap.13,22,23,96
Third, although one study suggests that general internists can safely perform thoracentesis with low complication rates similar to those of interventional radiologists,2 limited data exists on how to train practicing hospitalists to use ultrasound to guide thoracentesis. The effectiveness of different training protocols to acquire competence in ultrasound-guided thoracentesis has not been compared.
Finally, the impact of ultrasound use on patient experience has yet to be explored.
CONCLUSION
The use of ultrasound guidance for thoracentesis has been associated with increased success rates and decreased complication rates. Ultrasound can be used to estimate the pleural fluid volume, characterize the effusion as simple or complex, identify an optimal needle insertion site, and reduce the need for postprocedural chest radiographs. Training and experience are essential to reap the benefits of using ultrasound for thoracentesis, although our understanding of optimal educational strategies and learning curves is limited. Once training has occurred and competence is achieved, hospitalists can perform ultrasound-guided thoracentesis as safely as radiologists, pulmonologists, and other specialists.
Acknowledgments
Collaborators from the Society of Hospital Medicine Point-of-care Ultrasound Task Force: Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Venkat Kalidindi, Ketino Kobaidze, Joshua Lenchus, Benji Mathews, Paul, Mayo, Satyen Nichani, Vicki Noble, Martin Perez, Aliaksei Pustavoitau, Kreegan Reierson, Sophia Rodgers, Gerard Salame, Kirk Spencer, Vivek Tayal, David M. Tierney.
Disclosures
Ricardo Franco-Sadud reports institutional funds received from the Society of Hospital Medicine Annual Meeting for travel expenses and accommodations outside the submitted work. Nitin Puri reports Payment for lectures including service on speakers bureaus from Fujifilm Sonosite and royalties from Elsevier, both outside the submitted work. All other authors have nothing to disclose.
Funding
Brian P Lucas: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). Nilam Soni: Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1)
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
CHAIRS: Nilam Soni, Ricardo Franco Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Matthews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen. Lumbar puncture Working Group: Nilam Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen. PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Dan Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
1. Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat 13. 1998;139:1-119. PubMed
2. Kozmic SE, Wayne DB, Feinglass J, Hohmann SF, Barsuk JH. Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34-40. PubMed
3. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
4. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
5. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
6. Raptopoulos V, Davis LM, Lee G, Umali C, Lew R, Irwin RS. Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol. 1991;156(5):917-920. PubMed
7. Grogan DR, Irwin RS, Channick R, et al. Complications associated with thoracentesis. A prospective, randomized study comparing three different methods. Arch Intern Med. 1990;150(4):873-877. PubMed
8. Ravin CE. Thoracocentesis of loculated pleural effusions using grey scale ultrasonic guidance. Chest. 1977;71(5):666-668. PubMed
9. Weingardt JP, Guico RR, Nemcek AA, Jr., Li YP, Chiu ST. Ultrasound findings following failed, clinically directed thoracenteses. J Clin Ultrasound. 1994;22(7):419-426. PubMed
10. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
11. Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology. 1997;204(2):503-506. PubMed
12. Boland GW, Gazelle GS, Girard MJ, Mueller PR. Asymptomatic hydropneumothorax after therapeutic thoracentesis for malignant pleural effusions. AJR Am J Roentgenol. 1998;170(4):943-946. PubMed
13. Havelock T, Teoh R, Laws D, Gleeson F. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(2):ii61-76. PubMed
14. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The RAND/UCLA appropriateness method user’s manual. DTIC Document; 2001.
15. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
16. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
17. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
18. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
19. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
20. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
21. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
22. Lichtenstein D, Hulot JS, Rabiller A, Tostivint I, Meziere G. Feasibility and safety of ultrasound-aided thoracentesis in mechanically ventilated patients. Intensive Care Med. 1999;25(9):955-958. PubMed
23. Mayo PH, Goltz HR, Tafreshi M, Doelken P. Safety of ultrasound-guided thoracentesis in patients receiving mechanical ventilation. Chest. 2004;125(3):1059-1062. PubMed
24. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
25. Landay M, Harless W. Ultrasonic differentiation of right pleural effusion from subphrenic fluid on longitudinal scans of the right upper quadrant: importance of recognizing the diaphragm. Radiology. 1977;123(1):155-158. PubMed
26. Mayo PH, Doelken P. Pleural ultrasonography. Clin Chest Med. 2006;27(2):215-227. PubMed
27. Rosenberg ER. Ultrasound in the assessment of pleural densities. Chest. 1983;84(3):283-285. PubMed
28. Gorg C, Restrepo I, Schwerk WB. Sonography of malignant pleural effusion. Eur Radiol. 1997;7(8):1195-1198. PubMed
29. Gryminski J, Krakowka P, Lypacewicz G. The diagnosis of pleural effusion by ultrasonic and radiologic techniques. Chest. 1976;70(1):33-37. PubMed
30. Kalokairinou-Motogna M, Maratou K, Paianid I, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12(1):12-16. PubMed
31. Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9-15. PubMed
32. Grimberg A, Shigueoka DC, Atallah AN, Ajzen S, Iared W. Diagnostic accuracy of sonography for pleural effusion: systematic review. Sao Paulo Med J.. 2010;128(2):90-95. PubMed
33. Kataoka H. Utility of thoracic sonography for follow-up examination of chronic heart failure patients with previous decompensation. Clin Cardiol. 2007;30(7):336-341. PubMed
34. Ma OJ, Mateer JR. Trauma ultrasound examination versus chest radiography in the detection of hemothorax. Ann Emerg Med. 1997;29(3):312-315. PubMed
35. Rocco M, Carbone I, Morelli A, et al. Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand. 2008;52(6):776-784. PubMed
36. Kocijancic I, Vidmar K, Ivanovi-Herceg Z. Chest sonography versus lateral decubitus radiography in the diagnosis of small pleural effusions. J Clin Ultrasound. 2003;31(2):69-74. PubMed
37. Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1. PubMed
38. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
39. Medford AR, Entwisle JJ. Indications for thoracic ultrasound in chest medicine: an observational study. Postgrad Med J. 2010;86(1011):8-11. PubMed
40. Lin MS, Hwang JJ, Chong IW, et al. Ultrasonography of chest diseases: analysis of 154 cases. Gaoxiong Yi Xue Ke Xue Za Zhi . 1992;8(10):525-534. PubMed
41. Eibenberger KL, Dock WI, Ammann ME, Dorffner R, Hormann MF, Grabenwoger F. Quantification of pleural effusions: sonography versus radiography. Radiology. 1994;191(3):681-684. PubMed
42. Vignon P, Chastagner C, Berkane V, et al. Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit Care Med. 2005;33(8):1757-1763. PubMed
43. Usta E, Mustafi M, Ziemer G. Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2010;10(2):204-207. PubMed
44. Remerand F, Dellamonica J, Mao Z, et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010;36(4):656-664.
PubMed
45. Balik M, Plasil P, Waldauf P, et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32(2):318-321. PubMed
46. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracentesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
47. Lisi M, Cameli M, Mondillo S, et al. Incremental value of pocket-sized imaging device for bedside diagnosis of unilateral pleural effusions and ultrasound-guided thoracentesis. Interact Cardiovasc Thorac Surg. 2012;15(4):596-601. PubMed
48. Xirouchaki N, Kondili E, Prinianakis G, Malliotakis P, Georgopoulos D. Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014;40(1):57-65. PubMed
49. Chen HJ, Tu CY, Ling SJ, et al. Sonographic appearances in transudative pleural effusions: not always an anechoic pattern. Ultrasound Med Biol. 2008;34(3):362-369. PubMed
50. Yang PC, Luh KT, Chang DB, Wu HD, Yu CJ, Kuo SH. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol. 1992;159(1):29-33. PubMed
51. Liang SJ, Tu CY, Chen HJ, et al. Application of ultrasound-guided pigtail catheter for drainage of pleural effusions in the ICU. Intensive Care Med. 2009;35(2):350-354. PubMed
52. McLoud TC, Flower CD. Imaging the pleura: sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;156(6):1145-1153. PubMed
53. Tu CY, Hsu WH, Hsia TC, et al. Pleural effusions in febrile medical ICU patients: chest ultrasound study. Chest. 2004;126(4):1274-1280. PubMed
54. Sajadieh H, Afzali F, Sajadieh V, Sajadieh A. Ultrasound as an alternative to aspiration for determining the nature of pleural effusion, especially in older people. Ann N Y Acad Sci. 2004;1019:585-592. PubMed
55. Marcun R, Sustic A. Sonographic evaluation of unexplained pleural exudate: a prospective case series. Wien Klin Wochenschr. 2009;121(9-10):334-338. PubMed
56. Bugalho A, Ferreira D, Dias SS, et al. The diagnostic value of transthoracic ultrasonographic features in predicting malignancy in undiagnosed pleural effusions: a prospective observational study. Respiration. 2014;87(4):270-278. PubMed
57. Salamonsen MR, Lo AK, Ng AC, Bashirzadeh F, Wang WY, Fielding DI. Novel use of pleural ultrasound can identify malignant entrapped lung prior to effusion drainage. Chest. 2014;146(5):1286-1293. PubMed
58. Himelman RB, Callen PW. The prognostic value of loculations in parapneumonic pleural effusions. Chest. 1986;90(6):852-856. PubMed
59. Chen CH, Chen W, Chen HJ, et al. Transthoracic ultrasonography in predicting the outcome of small-bore catheter drainage in empyemas or complicated parapneumonic effusions. Ultrasound Med Biol. 2009;35(9):1468-1474. PubMed
60. Hirsch JH, Rogers JV, Mack LA. Real-time sonography of pleural opacities. AJR Am J Roentgenol. 1981;136(2):297-301. PubMed
61. Chen KY, Liaw YS, Wang HC, Luh KT, Yang PC. Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J Ultrasound Med. 2000;19(12):837-843. PubMed
62. Diaz-Guzman E, Budev MM. Accuracy of the physical examination in evaluating pleural effusion. Cleve Clin J Med. 2008;75(4):297-303. PubMed
63. Rhyne T, Birnholz JC. Simple measurement of chest-wall thickness with ultrasound. Radiology. 1973;108(2):436-438. PubMed
64. Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest. 2011;140(4):859-866. PubMed
65. Lichtenstein D, Meziere G, Biderman P, Gepner A. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26(10):1434-1440. PubMed
66. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
67. Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care. 2013;17(5):R208. PubMed
68. Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest. 2012;141(3):703-708. PubMed
69. Sartori S, Tombesi P, Trevisani L, Nielsen I, Tassinari D, Abbasciano V. Accuracy of transthoracic sonography in detection of pneumothorax after sonographically guided lung biopsy: prospective comparison with chest radiography. AJR Am J Roentgenol. 2007;188(1):37-41. PubMed
70. Blaivas M, Lyon M, Duggal S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med. 2005;12(9):844-849. PubMed
71. Lichtenstein DA, Meziere G, Lascols N, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005;33(6):1231-1238. PubMed
72. Kohan JM, Poe RH, Israel RH, et al. Value of chest ultrasonography versus decubitus roentgenography for thoracentesis. Am Rev Respir Dis. 1986;133(6):1124-1126. PubMed
73. Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc. 1998;73(10):948-950. PubMed
74. Aleman C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med. 1999;107(4):340-343. PubMed
75. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
76. Begot E, Grumann A, Duvoid T, et al. Ultrasonographic identification and semiquantitative assessment of unloculated pleural effusions in critically ill patients by residents after a focused training. Intensive Care Med. 2014;40(10):1475-1480. PubMed
77. Kotagal M, Quiroga E, Ruffatto BJ, et al. Impact of point-of-care ultrasound training on surgical residents’ confidence. J Surg Educ. 2015;72(4):e82-87. PubMed
78. Beaulieu Y, Laprise R, Drolet P, et al. Bedside ultrasound training using web-based e-learning and simulation early in the curriculum of residents. Crit Ultrasound J. 2015;7:1. PubMed
79. Schnobrich DJ, Olson AP, Broccard A, Duran-Nelson A. Feasibility and acceptability of a structured curriculum in teaching procedural and basic diagnostic ultrasound skills to internal medicine residents. J Grad Med Educ. 2013;5(3):493-497. PubMed
80. Chalumeau-Lemoine L, Baudel JL, Das V, et al. Results of short-term training of naive physicians in focused general ultrasonography in an intensive-care unit. Intensive Care Med. 2009;35(10):1767-1771. PubMed
81. Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ. 2011;11:75. PubMed
82. Ramsingh D, Alexander B, Le K, Williams W, Canales C, Cannesson M. Comparison of the didactic lecture with the simulation/model approach for the teaching of a novel perioperative ultrasound curriculum to anesthesiology residents. J Clin Anesth. 2014;26(6):443-454. PubMed
83. Sekiguchi H, Bhagra A, Gajic O, Kashani KB. A general Critical Care Ultrasonography workshop: results of a novel Web-based learning program combined with simulation-based hands-on training. J Crit Care. 2013;28(2):217.e217-212. PubMed
84. Dulohery MM, Stoven S, Kurklinsky AK, Halvorsen A, McDonald FS, Bhagra A. Ultrasound for internal medicine physicians: the future of the physical examination. J Ultrasound Med. 2014;33(6):1005-1011. PubMed
85. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
86. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
87. Wayne DB, Barsuk JH, O’Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
88. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
89. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
90. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
91. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol. 2012;71(4):245-251. PubMed
92. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
93. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
94. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
95. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
96. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
97. Harnsberger HR, Lee TG, Mukuno DH. Rapid, inexpensive real-time directed thoracentesis. Radiology. 1983;146(2):545-546. PubMed
1. Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat 13. 1998;139:1-119. PubMed
2. Kozmic SE, Wayne DB, Feinglass J, Hohmann SF, Barsuk JH. Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34-40. PubMed
3. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
4. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
5. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
6. Raptopoulos V, Davis LM, Lee G, Umali C, Lew R, Irwin RS. Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol. 1991;156(5):917-920. PubMed
7. Grogan DR, Irwin RS, Channick R, et al. Complications associated with thoracentesis. A prospective, randomized study comparing three different methods. Arch Intern Med. 1990;150(4):873-877. PubMed
8. Ravin CE. Thoracocentesis of loculated pleural effusions using grey scale ultrasonic guidance. Chest. 1977;71(5):666-668. PubMed
9. Weingardt JP, Guico RR, Nemcek AA, Jr., Li YP, Chiu ST. Ultrasound findings following failed, clinically directed thoracenteses. J Clin Ultrasound. 1994;22(7):419-426. PubMed
10. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
11. Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology. 1997;204(2):503-506. PubMed
12. Boland GW, Gazelle GS, Girard MJ, Mueller PR. Asymptomatic hydropneumothorax after therapeutic thoracentesis for malignant pleural effusions. AJR Am J Roentgenol. 1998;170(4):943-946. PubMed
13. Havelock T, Teoh R, Laws D, Gleeson F. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(2):ii61-76. PubMed
14. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The RAND/UCLA appropriateness method user’s manual. DTIC Document; 2001.
15. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
16. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
17. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
18. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
19. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
20. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
21. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
22. Lichtenstein D, Hulot JS, Rabiller A, Tostivint I, Meziere G. Feasibility and safety of ultrasound-aided thoracentesis in mechanically ventilated patients. Intensive Care Med. 1999;25(9):955-958. PubMed
23. Mayo PH, Goltz HR, Tafreshi M, Doelken P. Safety of ultrasound-guided thoracentesis in patients receiving mechanical ventilation. Chest. 2004;125(3):1059-1062. PubMed
24. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
25. Landay M, Harless W. Ultrasonic differentiation of right pleural effusion from subphrenic fluid on longitudinal scans of the right upper quadrant: importance of recognizing the diaphragm. Radiology. 1977;123(1):155-158. PubMed
26. Mayo PH, Doelken P. Pleural ultrasonography. Clin Chest Med. 2006;27(2):215-227. PubMed
27. Rosenberg ER. Ultrasound in the assessment of pleural densities. Chest. 1983;84(3):283-285. PubMed
28. Gorg C, Restrepo I, Schwerk WB. Sonography of malignant pleural effusion. Eur Radiol. 1997;7(8):1195-1198. PubMed
29. Gryminski J, Krakowka P, Lypacewicz G. The diagnosis of pleural effusion by ultrasonic and radiologic techniques. Chest. 1976;70(1):33-37. PubMed
30. Kalokairinou-Motogna M, Maratou K, Paianid I, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12(1):12-16. PubMed
31. Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9-15. PubMed
32. Grimberg A, Shigueoka DC, Atallah AN, Ajzen S, Iared W. Diagnostic accuracy of sonography for pleural effusion: systematic review. Sao Paulo Med J.. 2010;128(2):90-95. PubMed
33. Kataoka H. Utility of thoracic sonography for follow-up examination of chronic heart failure patients with previous decompensation. Clin Cardiol. 2007;30(7):336-341. PubMed
34. Ma OJ, Mateer JR. Trauma ultrasound examination versus chest radiography in the detection of hemothorax. Ann Emerg Med. 1997;29(3):312-315. PubMed
35. Rocco M, Carbone I, Morelli A, et al. Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand. 2008;52(6):776-784. PubMed
36. Kocijancic I, Vidmar K, Ivanovi-Herceg Z. Chest sonography versus lateral decubitus radiography in the diagnosis of small pleural effusions. J Clin Ultrasound. 2003;31(2):69-74. PubMed
37. Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1. PubMed
38. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
39. Medford AR, Entwisle JJ. Indications for thoracic ultrasound in chest medicine: an observational study. Postgrad Med J. 2010;86(1011):8-11. PubMed
40. Lin MS, Hwang JJ, Chong IW, et al. Ultrasonography of chest diseases: analysis of 154 cases. Gaoxiong Yi Xue Ke Xue Za Zhi . 1992;8(10):525-534. PubMed
41. Eibenberger KL, Dock WI, Ammann ME, Dorffner R, Hormann MF, Grabenwoger F. Quantification of pleural effusions: sonography versus radiography. Radiology. 1994;191(3):681-684. PubMed
42. Vignon P, Chastagner C, Berkane V, et al. Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit Care Med. 2005;33(8):1757-1763. PubMed
43. Usta E, Mustafi M, Ziemer G. Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2010;10(2):204-207. PubMed
44. Remerand F, Dellamonica J, Mao Z, et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010;36(4):656-664.
PubMed
45. Balik M, Plasil P, Waldauf P, et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32(2):318-321. PubMed
46. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracentesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
47. Lisi M, Cameli M, Mondillo S, et al. Incremental value of pocket-sized imaging device for bedside diagnosis of unilateral pleural effusions and ultrasound-guided thoracentesis. Interact Cardiovasc Thorac Surg. 2012;15(4):596-601. PubMed
48. Xirouchaki N, Kondili E, Prinianakis G, Malliotakis P, Georgopoulos D. Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014;40(1):57-65. PubMed
49. Chen HJ, Tu CY, Ling SJ, et al. Sonographic appearances in transudative pleural effusions: not always an anechoic pattern. Ultrasound Med Biol. 2008;34(3):362-369. PubMed
50. Yang PC, Luh KT, Chang DB, Wu HD, Yu CJ, Kuo SH. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol. 1992;159(1):29-33. PubMed
51. Liang SJ, Tu CY, Chen HJ, et al. Application of ultrasound-guided pigtail catheter for drainage of pleural effusions in the ICU. Intensive Care Med. 2009;35(2):350-354. PubMed
52. McLoud TC, Flower CD. Imaging the pleura: sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;156(6):1145-1153. PubMed
53. Tu CY, Hsu WH, Hsia TC, et al. Pleural effusions in febrile medical ICU patients: chest ultrasound study. Chest. 2004;126(4):1274-1280. PubMed
54. Sajadieh H, Afzali F, Sajadieh V, Sajadieh A. Ultrasound as an alternative to aspiration for determining the nature of pleural effusion, especially in older people. Ann N Y Acad Sci. 2004;1019:585-592. PubMed
55. Marcun R, Sustic A. Sonographic evaluation of unexplained pleural exudate: a prospective case series. Wien Klin Wochenschr. 2009;121(9-10):334-338. PubMed
56. Bugalho A, Ferreira D, Dias SS, et al. The diagnostic value of transthoracic ultrasonographic features in predicting malignancy in undiagnosed pleural effusions: a prospective observational study. Respiration. 2014;87(4):270-278. PubMed
57. Salamonsen MR, Lo AK, Ng AC, Bashirzadeh F, Wang WY, Fielding DI. Novel use of pleural ultrasound can identify malignant entrapped lung prior to effusion drainage. Chest. 2014;146(5):1286-1293. PubMed
58. Himelman RB, Callen PW. The prognostic value of loculations in parapneumonic pleural effusions. Chest. 1986;90(6):852-856. PubMed
59. Chen CH, Chen W, Chen HJ, et al. Transthoracic ultrasonography in predicting the outcome of small-bore catheter drainage in empyemas or complicated parapneumonic effusions. Ultrasound Med Biol. 2009;35(9):1468-1474. PubMed
60. Hirsch JH, Rogers JV, Mack LA. Real-time sonography of pleural opacities. AJR Am J Roentgenol. 1981;136(2):297-301. PubMed
61. Chen KY, Liaw YS, Wang HC, Luh KT, Yang PC. Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J Ultrasound Med. 2000;19(12):837-843. PubMed
62. Diaz-Guzman E, Budev MM. Accuracy of the physical examination in evaluating pleural effusion. Cleve Clin J Med. 2008;75(4):297-303. PubMed
63. Rhyne T, Birnholz JC. Simple measurement of chest-wall thickness with ultrasound. Radiology. 1973;108(2):436-438. PubMed
64. Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest. 2011;140(4):859-866. PubMed
65. Lichtenstein D, Meziere G, Biderman P, Gepner A. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26(10):1434-1440. PubMed
66. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
67. Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care. 2013;17(5):R208. PubMed
68. Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest. 2012;141(3):703-708. PubMed
69. Sartori S, Tombesi P, Trevisani L, Nielsen I, Tassinari D, Abbasciano V. Accuracy of transthoracic sonography in detection of pneumothorax after sonographically guided lung biopsy: prospective comparison with chest radiography. AJR Am J Roentgenol. 2007;188(1):37-41. PubMed
70. Blaivas M, Lyon M, Duggal S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med. 2005;12(9):844-849. PubMed
71. Lichtenstein DA, Meziere G, Lascols N, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005;33(6):1231-1238. PubMed
72. Kohan JM, Poe RH, Israel RH, et al. Value of chest ultrasonography versus decubitus roentgenography for thoracentesis. Am Rev Respir Dis. 1986;133(6):1124-1126. PubMed
73. Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc. 1998;73(10):948-950. PubMed
74. Aleman C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med. 1999;107(4):340-343. PubMed
75. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
76. Begot E, Grumann A, Duvoid T, et al. Ultrasonographic identification and semiquantitative assessment of unloculated pleural effusions in critically ill patients by residents after a focused training. Intensive Care Med. 2014;40(10):1475-1480. PubMed
77. Kotagal M, Quiroga E, Ruffatto BJ, et al. Impact of point-of-care ultrasound training on surgical residents’ confidence. J Surg Educ. 2015;72(4):e82-87. PubMed
78. Beaulieu Y, Laprise R, Drolet P, et al. Bedside ultrasound training using web-based e-learning and simulation early in the curriculum of residents. Crit Ultrasound J. 2015;7:1. PubMed
79. Schnobrich DJ, Olson AP, Broccard A, Duran-Nelson A. Feasibility and acceptability of a structured curriculum in teaching procedural and basic diagnostic ultrasound skills to internal medicine residents. J Grad Med Educ. 2013;5(3):493-497. PubMed
80. Chalumeau-Lemoine L, Baudel JL, Das V, et al. Results of short-term training of naive physicians in focused general ultrasonography in an intensive-care unit. Intensive Care Med. 2009;35(10):1767-1771. PubMed
81. Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ. 2011;11:75. PubMed
82. Ramsingh D, Alexander B, Le K, Williams W, Canales C, Cannesson M. Comparison of the didactic lecture with the simulation/model approach for the teaching of a novel perioperative ultrasound curriculum to anesthesiology residents. J Clin Anesth. 2014;26(6):443-454. PubMed
83. Sekiguchi H, Bhagra A, Gajic O, Kashani KB. A general Critical Care Ultrasonography workshop: results of a novel Web-based learning program combined with simulation-based hands-on training. J Crit Care. 2013;28(2):217.e217-212. PubMed
84. Dulohery MM, Stoven S, Kurklinsky AK, Halvorsen A, McDonald FS, Bhagra A. Ultrasound for internal medicine physicians: the future of the physical examination. J Ultrasound Med. 2014;33(6):1005-1011. PubMed
85. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
86. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
87. Wayne DB, Barsuk JH, O’Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
88. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
89. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
90. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
91. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol. 2012;71(4):245-251. PubMed
92. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
93. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
94. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
95. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
96. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
97. Harnsberger HR, Lee TG, Mukuno DH. Rapid, inexpensive real-time directed thoracentesis. Radiology. 1983;146(2):545-546. PubMed
© 2018 Society of Hospital Medicine
Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures: A Position Statement of the Society of Hospital Medicine
The American Board of Internal Medicine (ABIM) changed its certification policy for bedside procedures over a decade ago.
Hospitalists increasingly perform bedside procedures with ultrasound guidance.
Therefore, the Society of Hospital Medicine (SHM) Education Committee convened a group of experts and conducted a systematic literature review in order to provide recommendations for credentialing hospitalist physicians in ultrasound-guided bedside procedures. These recommendations do not include training recommendations, aside from recommendations about remedial training for hospitalists who do not pass certification. Training is a means to competence but does not guarantee it. We believe that training recommendations ought to be considered separately.
METHODS
Working Group Formation
In January 2015, the SHM Board of Directors asked the SHM Education Committee to convene the POCUS Task Force. The purpose of the task force was to develop recommendations on ultrasound guidance for bedside procedures. The SHM Education Committee appointed 3 chairs of the task force: 1 senior member of the SHM Education Committee and 2 POCUS experts. The chairs assembled a task force of 31 members that included 5 working groups, a multispecialty peer review group, and a guideline methodologist (supplemental Appendix 1). Invitation was based on members’ past contributions to SHM POCUS-related activities, up-front commitment, and declared conflicts of interest. Working group members self-identified as “hospitalists,” whereas peer reviewers were nonhospitalists but nationally recognized POCUS physician-leaders specializing in emergency medicine, cardiology, critical care medicine, and anesthesiology. Task force membership was vetted by a chair of the SHM POCUS Task Force and the Director of Education before work began. This position statement was authored by the Credentialing Working Group together with the chairs of the other 4 working groups and a guideline methodologist.
Disclosures
Signed disclosure statements of all task force members were reviewed prior to inclusion on the task force (supplemental Appendix 2); no members received honoraria for participation. Industry representatives did not contribute to the development of the guidelines nor to any conference calls or meetings.
Literature Search Strategy
A literature search was conducted by a biomedical librarian. Records from 1979 to January of 2017 were searched in Medline, Embase, CINAHL, Cochrane, and Google Scholar (supplemental Appendix 3). Search limiters were English language and adults. Articles were manually screened to exclude nonhuman or endoscopic ultrasound applications. Final article selection was based on working group consensus.
Draft Pathways
The Credentialing Working Group drafted initial and ongoing certification pathways (Figure 1 and Figure 2). The other 4 working groups from the task force were surveyed about the elements and overall appropriateness of these draft pathways. This survey and its results have already been published.12 The Credentialing Working Group then revised the certification pathways by using these survey results and codified individual aspects of these pathways into recommendations.
Development of Position Statement
Based on the Grading of Recommendation Assessment Development and Evaluation methodology, all final article selections were initially rated as either low-quality (observational studies) or unclassifiable (expert opinion).16 These initial ratings were downgraded further because of indirectness, because none of the articles involved the intervention of interest (a credentialing pathway) in a population of interest (hospitalists) measuring the outcomes of interest (patient-level outcomes).
First, the Credentialing Working Group drafted an initial position statement composed of recommendations for credentialing pathways and other general aspects of credentialing. All final article selections were incorporated as references in a draft of the position statement and compiled in a full-text compendium. Second, feedback was provided by the other 4 task force working groups, the task force peer reviewers, and the SHM Education Committee. Feedback was incorporated by the authors of this statement who were the Credentialing Working Group, the chairs of the other 4 working groups, and a guideline methodologist. Third, final suggestions from all members of the SHM POCUS Task Force and SHM Education Committee were incorporated before final approval by the SHM Board of Directors in September 2017.
RESULTS
A total of 1438 references were identified in the original search. Manual selection led to 101 articles, which were incorporated into the following 4 domains with 16 recommendations.
General Credentialing Process
Basic Cognitive Competence Can Be Certified with Written or Oral Examinations
The ABIM defines cognitive competence as having 3 abilities: “(1) to explain indications, contraindications, patient preparation methods, sterile techniques, pain management, proper techniques for handling specimens and fluids obtained, and test results; (2) to recognize and manage complications; and, (3) to clearly explain to a patient all facets of the procedure necessary to obtain informed consent.”1 These abilities can be assessed with written or oral examinations that may be integrated into simulation- or patient-based assessments.
Minimum Thresholds of Experience to Trigger the Timing of a Patient-Based Assessment Should Be Determined by Empirical Methods
Learning curves are highly variable22-25 and even plateaus may not herald basic competence.26 Expert opinion
Hospitalists Should Formally Log All of Their Attempted Procedures, Ideally in an Electronic Medical Record
Simple self-reported numbers of procedures performed often misrepresent actual experience33,34 and do not include periprocedural complications.35,36 Thus, hospitalists should report their experience with logs of all attempted procedures, both successful and unsuccessful. Such logs must include information about supervising providers (if applicable) and patient outcomes, including periprocedural adverse events,37 but they must also remain compliant with the Health Insurance Portability and Accountability Act.
Health Information Technology Service Should Routinely Pull Collations of All Attempted Procedures from Comprehensive Electronic Medical Records
Active surveillance may reduce complications by identifying hospitalists who may benefit from further training.38 In order to facilitate active surveillance systems, documentation (such as a procedure note) should be both integrated into an electronic medical record and protocol driven,39 including procedure technique, ultrasound findings, and any safety events (both near misses and adverse events).
Multiple interacting factors, including environment, patients, baseline skills, training, experience, and skills decay, affect manual competence. Certifications that are based solely on reaching minimum thresholds of experience, even when accurate, are not valid reflections of manual competence,15,40-43 and neither are those based on self-perception.44 Patient-based assessments are, thus, necessary to ensure manual competence.45-48
Certification Assessments of Manual Competence Should Combine 2 Types of Structured Instruments: Checklists and Overall Scores
Assessments based on direct observation are more reliable when formally structured.49,50 Though checklists used in observed structured clinical examinations capture many important manual skills,51-56 they do not completely reflect a hospitalist’s manual competence;57 situations may occur in which a hospitalist meets all the individual items on a checklist but cannot perform an entire procedure with basic competence. Therefore, checklists should be paired with overall scores.58-61 Both checklists and overall scores ought to be obtained from reliable and valid instruments.
Certification Assessments Should Include Feedback
Assessments without feedback are missed learning opportunities.62 Both simulation-63 and patient-based assessments should provide feedback in real time to reinforce effective behaviors and remedy faulty ones.
If Remedial Training is Needed, Simulator-Based Training Can Supplement but Not Replace Patient-Based Training
Supervised simulator-based training allows hospitalists to master basic components of a procedure64 (including orientation to equipment, sequence of operations, dexterity, ultrasound anatomy, and real-time guidance technique) while improving both cognitive and manual skills.42,43,65-71 In addition to their role in basic training (which is outside the scope of this position statement), simulators can be useful for remedial training. To be sufficient for hospitalists who do not pass their patient-based assessments, however, remedial training that begins with simulation must also include patient-based training and assessment.72-75
Initial Credentialing Process
A Minimum Threshold of Experience Should Be Reached before Patient-Based Assessments are Conducted (Figure 1)
Initial Certification Assessments Should Ideally Begin on Simulators
Simulators allow the assurance of safe manual skills, including proper needle insertion techniques and disposal of sharp objects.3,79 If simulators are not available, however, then patient-based training and assessments can still be performed under direct observation. Safe performance of ultrasound-guided procedures during patient-based assessments (without preceding simulator-based assessments) is sufficient to certify manual competence.
Ongoing Credentialing
Certification to Perform Ultrasound-Guided Procedures Should Be Routinely Re-Evaluated During Ongoing Credentialing (Figure 2)
Observed Patient-Based Assessments Should Occur When a Periprocedural Safety Event Occurs that is Potentially Caused by “Provider Error”
Safety events include both near misses and adverse events. Information about both is ideally “flagged” and “pushed” to hospitalist group leaders by active surveillance and reporting systems. Once reviewed, if a safety event is considered to potentially have been caused by provider error (including knowledge- and skill-based errors),83 then the provider who performed the procedure should undergo an observed patient-based assessment.
Simulation-Based Practice Can Supplement Patient-Based Experience for Ongoing Credentialing
When hospitalists do not achieve a minimum threshold of patient-based experience since the antecedent certification, simulation-based training can supplement their patient-based experience.
Credentialing Infrastructure
Hospitalists Themselves Should Not Bear the Financial Costs of Developing and Maintaining Training and Certification Programs for Ultrasound-Guided Procedures
Equipment and personnel costs
Assessors Should Be Unbiased Expert Providers Who Have Demonstrated Mastery in Performance of the Procedure Being Assessed and Regularly Perform It in a Similar Practice Environment
Assessors should be expert providers who regularly perform the ultrasound-guided procedure in a similar practice environment.9,89-94 For example, providers who are not hospitalists but who are experts in an ultrasound-guided procedure and commonly perform it on the hospital wards would be acceptable assessors. However, a radiologist who only performs that procedure in a fully-staffed interventional radiology suite with fluoroscopy or computed tomography guidance would not be an acceptable assessor. More than 1 assessor may balance idiosyncratic assessments;95 but when assessments are well structured, additional assessors are generally not needed.18
If Intramural Assessors Are Not Available, Extramural Assessors May Be Considered
Intramural assessors are generally preferred because of familiarity with the local practice environment, including the available procedure kits and typical patient characteristics. Nevertheless, extramural assessors27,77,85,96 may theoretically provide even more valid assessments than intramural ones because extramural assessors are neither influenced by relationships with local hospitalists nor biased by local hospitalists’ skills.
DISCUSSION
There are no high-quality randomized trials in support of a single credentialing pathway over any other.94,102 The credentialing pathways at the center of this position statement are based on expert opinion. Our methods can be criticized straightaway, therefore, for reliance on the experience and expertise of our working group and task force. Any position statement written without high-quality supportive evidence would be appropriately subject to the same criticism. Without evidence in support of an overall pathway, we codified specific aspects of the pathways into 16 individual recommendations.
Patient-level outcomes do not back these recommendations. Consider, for example, our recommendation that certification assessments be made from structured instruments and not simply from an assessor’s gestalt. Here, the basis is not improved patient-level outcomes from a trial (such as reduced complications or increased procedural success) but improved psychometric performance from reliability studies. The body of evidence for our recommendations is similarly indirect, mostly because the outcomes studied are more proximate and, thus, less meaningful than patient-level outcomes, which are the outcomes of greatest interest but are woefully understudied for clinical competence.17,97,103
The need for high-quality evidence is most pronounced in distinguishing how recommendations should be modified for various settings. Wide variations in resources and patient-mix will make some recommendations impracticable, meaning that they could not be carried out with available resources. For example, our recommendation that credentialing decisions should ultimately rely on certifications made by assessors during patient-based assessments may not be practicable at small, rural hospitals. Such hospitals may not have access to local assessors, and they may not admit enough patients who need the types of ultrasound-guided procedures for which hospitalists seek certification (especially given the need to coordinate the schedules of patients, procedure-performing hospitalists, and assessors).
Regardless of whether some or all hospitalists at a particular hospital are expected to perform bedside procedures, technology may help to improve the practicability of our recommendations. For example, simulators may evolve to replace actual patient-level experience in achieving minimum thresholds. Certification assessments of manual skills may even someday occur entirely on simulators. Real-time high-definition video streaming enhanced with multiple cameras may allow for remote assessments. Until such advances mature, high-quality patient-level data should be sought through additional research to refine our current recommendations.
We hope that these recommendations will improve how basic competence in ultrasound-guided bedside procedures is assessed. Our ultimate goal is to improve how hospitalists perform these procedures. Patient safety is, therefore, considered paramount to cost. Nevertheless, the hospital administrative leaders and privileging committee members on our Task Force concluded that many hospitals have been seeking guidance on credentialing for bedside procedures, and the likely difficulties of implementing our recommendations (including cost) would not be prohibitive at most hospitals, especially given recognition that these recommendations can be tailored to each setting.
Acknowledgments
Collaborators from SHM POCUS Task Force are Saaid Abdel-Ghani, Michael Blaivas, Dan Brotman, Carolina Candotti, Jagriti Chadha, Joel Cho, Ria Dancel, Ricardo Franco, Richard Hoppmann, Susan Hunt, Venkat Kalidindi, Ketino Kobaidze, Josh Lenchus, Benji Mathews, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Sophia Rodgers, Gerard Salame, Daniel Schnobrich, Kirk Spencer, Vivek Tayal, Jeff Bates, Anjali Bhagra, Kreegan Reierson, Robert Arntfield, Paul Mayo, Loretta Grikis.
Disclosure
Brian P. Lucas received funding from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, and National Center for Translational Science (UL1TR001086). Nilam Soni received funding from the Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (HX002263-01A1). The contents of this publication do not represent the views of the United States Department of Veterans Affairs or the United States Government.
1. American Board of Internal Medicine. Policies and procedures for certification. Philadelphia: American Board of Internal Medicine; 2006.
2. Nichani S, Fitterman N, Lukela M, Crocker J; Society of Hospital Medicine. The Core Competencies in Hospital Medicine 2017 Revision. Section 2: Procedures. J Hosp Med. 2017;12(4 Suppl 1):S44-S54 PubMed
3. Lucas BP, Asbury JK, Franco-Sadud R. Training future hospitalists with simulators: a needed step toward accessible, expertly performed bedside procedures. J Hosp Med. 2009;4(7):395-396. PubMed
4. Schnobrich DJ, Gladding S, Olson APJ, Duran-Nelson A. Point-of-care ultrasound in internal medicine: a national survey of educational leadership. J Grad Med Educ. 2013;5(3):498-502. PubMed
5. Brown GM, Otremba M, Devine LA, Gray C, Millington SJ, Ma IW. Defining competencies for ultrasound-guided bedside procedures: consensus opinions from Canadian physicians. J Ultrasound Med. 2016;35(1):129-141. PubMed
6. Vaisman A, Cram P. Procedural competence among faculty in academic health centers: challenges and future directions. Acad Med. 2017;92(1):31-34. PubMed
7. Kreisman RD. With ED ultrasound, credentialing is at issue. ED Legal Letter. 2010;21:102-103.
8. Goudie AM. Credentialing a new skill: what should the standard be for emergency department ultrasound in Australasia? Emerg Med Australas. 2010;22:263-264. PubMed
9. Maizel J, Guyomarc HL, Henon P, et al. Residents learning ultrasound-guided catheterization are not sufficiently skilled to use landmarks. Crit Care. 2014;18(1):R36. doi:10.1186/cc13741. PubMed
10. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care, and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. PubMed
11. Amini R, Adhikari S, Fiorello A. Ultrasound competency assessment in emergency medicine residency programs. Acad Emerg Med. 2014;21(7):799-801. PubMed
12. Jensen T, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. PubMed
13. Chang W. Is hospitalist proficiency in bedside procedures in decline? The Hospitalist. 2012. http://www.the-hospitalist.org/hospitalist/article/125236/patient-safety/hospitalist-proficiency-bedside-procedures-decline. Accessed September 30, 2017.
14. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties Performing Paracentesis Procedures at University Hospitals: Implications for Training and Certification. J Hosp Med. 2014;9(3):162-168. PubMed
15. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Residents’ Procedural Experience Does Not Ensure Competence: A Research Synthesis. J Grad Med Educ. 2017;9(2):201-208. PubMed
16. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. PubMed
17. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64(12):1303-1310. PubMed
18. Miller GE. The assessment of clinical skills/competence/performance. Acad Med. 1990;65(9 Suppl):S63-S67. PubMed
19. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
20. Millington SJ, Wong RY, Kassen BO, Roberts JM, Ma IWY. Improving internal medicine residents’ performance, knowledge, and confidence in central venous catheterization using simulators. J Hosp Med. 2009;4(7):410-416. PubMed
21. Lenchus JD, Carvalho CM, Ferreri K, et al. Filling the void: defining invasive bedside procedural competency for internal medicine residents. J Grad Med Educ. 2013;5(4):605-612. PubMed
22. Heegeman DJ, Kieke B Jr. Learning curves, credentialing, and the need for ultrasound fellowships. Acad Emerg Med. 2003;10:404-405. PubMed
23. Jang TB, Ruggeri W, Dyne P, Kaji AH. The learning curve of resident physicians using emergency ultrasonography for cholelithaisis and cholecystitis. Acad Emerg Med. 2010;17(11):1247-1252. PubMed
24. Akhtar MI, Hamid M. Ultrasound guided central venous access; a review of literature. Anaesth Pain Intensive Care. 2015;19:317-322.
25. Bahl A, Yunker A. Assessment of the numbers–based model for evaluation of resident competency in emergency ultrasound core applications. J Emerg Med Trauma Acute Care. 2015;2015(5). doi:10.5339/jemtac.2015.5
26. Cazes N, Desmots F, Geffroy Y, Renard A, Leyral J, Chaumoitre K. Emergency ultrasound: a prospective study on sufficient adequate training for military doctors. Diagn Interv Imaging. 2013;94(11):1109-1115. PubMed
27. Arntfield RT, Millington SJ, Ainsworth CD, et al. Canadian recommendations for critical care ultrasound training and competency for the Canadian critical care society. Can Respir J. 2014;21(16):341-345.
28. Bolsin S, Colson M. The use of the Cusum technique in the assessment of trainee competence in new procedures. Int J Qual Health Care. 2000;12(5):433-438. PubMed
29. de Oliveira Filho GR, Helayel PE, da Conceição DB, Garzel IS, Pavei P, Ceccon MS. Learning curves and mathematical models for interventional ultrasound basic skills. Anaesth Analg. 2008;106(2):568-573. PubMed
30. Starkie T, Drake EJ. Assessment of procedural skills training and performance in anesthesia using cumulative sum analysis (cusum). Can J Anaesth. 2013;60(12):1228-1239. PubMed
31. Tierney D. Competency cut-point identification derived from a mastery learning cohort approach: A hybrid model. Ultrasound Med Biol. 2015;41:S19.
32. Rankin JH, Elkhunovich MA, Rangarajan V, Chilstrom M, Mailhot T. Learning Curves for Ultrasound Assessment of Lumbar Puncture Insertion Sites: When is Competency Established? J Emerg Med. 2016;51(1):55-62. PubMed
33. Klasko SK, Cummings RV, Glazerman LR. Resident data collection: Do the numbers add up? Am J Obstet Gynecol. 1995;172(4 Pt 1):1312-1316. PubMed
34. Tierney D. Development & analysis of a mobile POCUS tracking tool. Ultrasound Med Biol. 2015;41(suppl 4):S31.
35. Sethi MV, Zimmer J, Ure B, Lacher M. Prospective assessment of complications on a daily basis is essential to determine morbidity and mortality in routine pediatric surgery. J Pediatr Surg. 2016;51(4):630-633. PubMed
36. Fisher JC, Kuenzler KA, Tomita SS, Sinha P, Shah P, Ginsburg HB. Increased capture of pediatric surgical complications utilizing a novel case-log web application to enhance quality improvement. J Pediatr Surg. 2017;52(1):166-171. PubMed
37. Rethans JJ, Norcini JJ, Barón-Maldonado M, et al. The relationship between competence and performance: implications for assessing practice performance. Med Educ. 2002;36(10):901-909. PubMed
38. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
39. Society of Critical Care Medicine Ultrasound Certification Task Force. Recommendations for achieving and maintaining competence and credentialing in critical care ultrasound with focused cardiac ultrasound and advanced critical care echocardiography. http://journals.lww.com/ccmjournal/Documents/Critical%20Care%20Ultrasound.pdf Published 2013. Accessed February 2, 2017.
40. Carraccio C, Wolfsthal SD, Englander R, Ferentz K, Martin C. Shifting paradigms: from Flexner to competencies. Acad Med. 2002;77(5):361-367. PubMed
41. Clark EG, Paparello JJ, Wayne DB, et al. Use of a national continuing medical education meeting to provide simulation-based training in temporary hemodialysis catheter insertion skills: a pre-test post-test study. Can J Kidney Health Dis. 2014;1:25-31. PubMed
42. Barsuk JH, Cohen ER, Caprio T, McGaghie WC, Simuni T, Wayne DB. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137. PubMed
43. Barsuk JH, McGaghie WC, Cohen ER, O’Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697-2701. PubMed
44. Davis DA, Mazmanian PE, Fordis M, Van Harrison R, Thorpe KE, Perrier L. Accuracy of physician self-assessment compared with observed measures of competence: a systematic review. JAMA. 2006;296(9):1094-1102. PubMed
45. Shah J, Darzi A. Surgical skills assessment: an ongoing debate. BJU Int. 2001;88(7):655-660. PubMed
46. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105-1117. PubMed
47. Tolsgaard MG, Todsen T, Sorensen JL, et al. International multispecialty consensus on how to evaluate ultrasound competence: a Delphi consensus survey. PLOS One. 2013;8(2):e57687. doi:10.1371/journal.pone.0057687 PubMed
48. Moureau N, Laperti M, Kelly LJ, et al. Evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training. Br J Anaesth. 2013;110(3):347-356. PubMed
49. Feldman LS, Hagarty S, Ghitulescu G, Stanbridge D, Fried GM. Relationship between objective assessment of technical skills and subjective in-training evaluations in surgical residents. J Am Coll Surg. 2004;198(1):105-110. PubMed
50. Baker S, Willey B, Mitchell C. The attempt to standardize technical and analytic competence in sonography education. J Diagn Med Sonogr. 2011;27(5):203-211.
51. Tolsgaard MG, Ringsted C, Dreisler E, et al. Reliable and valid assessment of ultrasound operator competence in obstetrics and gynecology. Ultrasound Obstet Gynecol. 2014;43(4):437-443. PubMed
52. Rice J, Crichlow A, Baker M, et al. An assessment tool for the placement of ultrasound-guided peripheral intravenous access. J Grad Med Educ. 2016;8(2):202-207. PubMed
53. Hartman N, Wittler M, Askew K, Hiestand B, Manthey D. Validation of a performance checklist for ultrasound-guided internal jubular central lines for use in procedural instruction and assessment. Postgrad Med J. 2017;93(1096):67-70. PubMed
54. Primdahl SC, Todsen T, Clemmesen L, et al. Rating scale for the assessment of competence in ultrasound-guided peripheral vascular access—a Delphi Consensus Study. J Vasc Access. 2016;17(5):440-445.
55. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
56. Berg K, Riesenberg LA, Berg D, et al. The development of a validated checklist for radial arterial line placement: preliminary results. Am J Med Qual. 2014;29(3):242-246. PubMed
57. Walzak A, Bacchus M, Schaefer MP, et al. Diagnosing technical competence in six bedside procedures: comparing checklists and a global rating scale in the assessment of resident performance. Acad Med. 2015;90(8):1100-1108. PubMed
58. Riesenberg LA, Berg K, Berg D, et al. The development of a validated checklist for femoral venous catheterization: preliminary results. Am J Med Qual. 2014;29(5):445-450. PubMed
59. Riesenberg LA, Berg K, Berg D, et al. The development of a validated checklist for paracentesis: preliminary results. Am J Med Qual. 2013;28(3):227-231. PubMed
60. Huang GC, Newman LR, Schwartzstein RM, et al. Procedural competence in internal medicine residents: validity of a central venous catheter insertion assessment instrument. Acad Med. 2009;84(8):1127-1134. PubMed
61. Salamonsen M, McGrath D, Steiler G, et al. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
62. Boniface K, Yarris LM. Emergency ultrasound: Leveling the training and assessment landscape. Acad Emerg Med. 2014;21(7):803-805. PubMed
63. Boyle E, O’Keeffe D, Naughton P, Hill A, McDonnell C, Moneley D. The importance of expert feedback during endovascular simulator training. J Vasc Surg. 2011;54(1):240-248.e1. PubMed
64. Langhan TS, Rigby IJ, Walker IW, Howes D, Donnon T, Lord JA. Simulation-based training in critical resuscitation procedures improves residents’ competence. CJEM. 2009;11(6):535-539. PubMed
65. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. PubMed
66. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
67. Barsuk JH, Cohen ER, Vozenilek JA, O’Connor LM, McGaghie WC, Wayne DB. Simulation-based education with mastery learning improves paracentesis skills. J Grad Med Educ. 2012;4(1):23-27. PubMed
68. McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706-711. PubMed
69. Ross JG. Simulation and psychomotor skill acquisition: A review of the literature. Clin Simul Nurs. 2012;8(9):e429-e435.
70. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
71. McSparron JI, Michaud GC, Gordan PL, et al. Simulation for skills-based education in pulmonary and critical care medicine. Ann Am Thorac Soc. 2015;12(4):579-586. PubMed
72. Kneebone RL, Scott W, Darzi A, Horrocks M. Simulation and clinical practice: strengthening the relationship. Med Educ. 2004;38(10):1095-1102. PubMed
73. Mema B, Harris I. The barriers and facilitators to transfer of ultrasound-guided central venous line skills from simulation to practice: exploring perceptions of learners and supervisors. Teach Learn Med. 2016;28(2):115-124. PubMed
74. Castanelli DJ. The rise of simulation in technical skills teaching and the implications for training novices in anaestheia. Anaesth Intensive Care. 2009;37(6):903-910. PubMed
75. McGaghie WC, Issenberg SB, Barsuk JH, Wayne DB. A critical review of simulation-based mastery learning with translational outcomes. Med Educ. 2014;48(4):375-385. PubMed
76. Langlois SLP. Focused ultrasound training for clinicians. Crit Care Med. 2007;35(5 suppl):S138-S143.
77. Price S, Via G, Sloth E, et al. Echocardiography practice, training and accreditation in the intesive care: document for the World Interactive Network Focused on Critical Ultrasound (WINFOCUS). Cardiovasc Ultrasound. 2008;6:49-83. PubMed
78. Blehar DJ, Barton B, Gaspari RJ. Learning curves in emergency ultrasound education. Acad Emerg Med. 2015;22(5):574-582. PubMed
79. Ault MJ, Rosen BT, Ault B. The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative. J Gen Intern Med. 2006;21(5):514-517. PubMed
80. Barsuk JH, Cohen ER, McGaghie WC, Wayne DB. Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med. 2010;85(10 Suppl):S9-S12. PubMed
81. Sliman Sean, Amundson S, Shaw D, Phan JN, Waalen J, Kimura B. Recently-acquired cardiac ultrasound skills are rapidly lost when not used: implications for competency in physician imaging. J Amer Coll Cardiol. 2016;67(13S):1569.
82. Kessler CS, Leone KA. The current state of core competency assessment in emergency medicine and a future research agenda: recommendations of the working group on assessment of observable learner performance. Acad Emerg Med. 2012;19(12):1354-1359. PubMed
83. Chang A, Schyve PM, Croteau RJ, O’Leary DS, Loeb JM. The JCAHO patient safety event taxonomy: a standardized terminology and classification schema for near misses and adverse events. Int J Qual Health Care. 2005;17(2):95-105. PubMed
84. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Acad Med. 2015;90(8):1025-1033. PubMed
85. Das D, Kapoor M, Brown C, Ndubuisi A, Gupta S. Current status of emergency department attending physician ultrasound credentialing and quality assurance in the United States. Crit Ultrasound J. 2016;8(1):6-12. PubMed
86. Ndubuisi AK, Gupta S, Brown C, Das D. Current status and future issues in emergency department attending physician ultrasound credentialing. Ann Emerg Med. 2014;64(45):S27-S28.
87. Tandy Tk, Hoffenberg S. Emergency department ultrasound services by emergency physicians: model for gaining hospital approval. Ann Emerg Med. 1997;29(3):367-374. PubMed
88. Lewiss RE, Saul T, Del Rios M. Acquiring credentials in bedside ultrasound: a cross-sectional survey. BMJ Open. 2013;3:e003502. doi:10.1136/bmjopen-2013-003502 PubMed
89. Lanoix R. Credentialing issues in emergency ultrasonography. Emerg Med Clin North Am. 1997;15(4):913-920. PubMed
90. Scalea T, Rodriquez A, Chiu WC, et al. Focused assessment with sonography for trauma (FAST): results from an international consensus conference. J Trauma. 1999;46(3):466-472. PubMed
91. Hertzberg BS, Kliewer MA, Bowie JD, et al. Physician training requirements in sonography: how many cases are needed for competence? AJR. 2000;174(5):1221-1227. PubMed
92. Blaivas M, Theodoro DL, Sierzenski P. Proliferation of ultrasound fellowships in emergency medicine: how do we ensure future experts are expertly trained? Acad Emerg Med. 2002;9(8):863-864. PubMed
93. Bodenham AR. Editorial II: Ultrasound imaging by anaesthetists: training and accreditation issues. Br J Anaesth. 2006;96(4):414-417. PubMed
94. Williamson JP, Twaddell SH, Lee YCG, et al. Thoracic ultrasound recognition of competence: A position paper of the Thoracic Society of Australia and New Zealand. Respirology. 2017;22(2):405-408. PubMed
95. Harrison G. Summative clinical competency assessment: a survey of ultrasound practitioners’ views. Ultrasound. 2015;23(1):11-17. PubMed
96. Evans LV, Morse JL, Hamann CJ, Osborne M, Lin Z, D'Onofrio G. The development of an independent rater system to assess residents' competence in invasive procedures. Acad Med. 2009;84(8):1135-1143. PubMed
97. Wass V, Van der Vleuten C, Shatzer J, Jones R. Assessment of clinical competence. Lancet. 2001;357(9260):945-949. PubMed
98. Arntfield RT. The utility of remote supervision with feedback as a method to deliver high-volume critical care ultrasound training. J Crit Care. 2015;30(2):441.e1-e6. PubMed
99. Akhtar S, Theodoro D, Gaspari R, et al. Resident training in emergency ultrasound: consensus recommendations from the 2008 Council of Emergency Residency Directors Conference. Acad Emerg Med. 2009;16:S32-S36. PubMed
100. Yu E. The assessment of technical skills in a cardiology training program: is the ITER sufficient? Can J Cardiol. 2000;16(4):457-462. PubMed
101. Todsen T, Tolsgaard MG, Olsen BH, et al. Reliable and valid assessment of point-of-care ultrasonography. Ann Surg. 2015;261(2):309-315. PubMed
102. Stein JC, Nobay F. Emergency department ultrasound credentialing: a sample policy and procedure. J Emerg Med. 2009;37(2):153-159. PubMed
103. Chen FM. Burstin H, Huntington J. The importance of clinical outcomes in medical education research. Med Educ. 2005;39(4):350-351. PubMed
104. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1:48-56. PubMed
105. ten Cate O. Nuts and bolts of entrustable professional activities. J Grad Med Educ. 2013;5(1):157-158. PubMed
106. Castillo J, Caruana CJ, Wainwright D. The changing concept of competence and categorisation of learning outcomes in Europe: Implications for the design of higher education radiography curricula at the European level. Radiography. 2011;17(3):230-234.
107. Goldstein SR. Accreditation, certification: why all the confusion? Obstet Gynecol. 2007;110(6):1396-1398. PubMed
108. Moore CL. Credentialing and reimbursement in point-of-care ultrasound. Clin Pediatr Emerg Med. 2011;12(1):73-77. PubMed
109. ten Cate O, Scheele F. Competency-based postgraduate training: can we bridge the gap between theory and clinical practice? Acad Med. 2007;82(6):542-547. PubMed
110. Abuhamad AZ, Benacerraf BR, Woletz P, Burke BL. The accreditation of ultrasound practices: impact on compliance with minimum performance guidelines. J Ultrasound Med. 2004;23(8):1023-1029. PubMed
The American Board of Internal Medicine (ABIM) changed its certification policy for bedside procedures over a decade ago.
Hospitalists increasingly perform bedside procedures with ultrasound guidance.
Therefore, the Society of Hospital Medicine (SHM) Education Committee convened a group of experts and conducted a systematic literature review in order to provide recommendations for credentialing hospitalist physicians in ultrasound-guided bedside procedures. These recommendations do not include training recommendations, aside from recommendations about remedial training for hospitalists who do not pass certification. Training is a means to competence but does not guarantee it. We believe that training recommendations ought to be considered separately.
METHODS
Working Group Formation
In January 2015, the SHM Board of Directors asked the SHM Education Committee to convene the POCUS Task Force. The purpose of the task force was to develop recommendations on ultrasound guidance for bedside procedures. The SHM Education Committee appointed 3 chairs of the task force: 1 senior member of the SHM Education Committee and 2 POCUS experts. The chairs assembled a task force of 31 members that included 5 working groups, a multispecialty peer review group, and a guideline methodologist (supplemental Appendix 1). Invitation was based on members’ past contributions to SHM POCUS-related activities, up-front commitment, and declared conflicts of interest. Working group members self-identified as “hospitalists,” whereas peer reviewers were nonhospitalists but nationally recognized POCUS physician-leaders specializing in emergency medicine, cardiology, critical care medicine, and anesthesiology. Task force membership was vetted by a chair of the SHM POCUS Task Force and the Director of Education before work began. This position statement was authored by the Credentialing Working Group together with the chairs of the other 4 working groups and a guideline methodologist.
Disclosures
Signed disclosure statements of all task force members were reviewed prior to inclusion on the task force (supplemental Appendix 2); no members received honoraria for participation. Industry representatives did not contribute to the development of the guidelines nor to any conference calls or meetings.
Literature Search Strategy
A literature search was conducted by a biomedical librarian. Records from 1979 to January of 2017 were searched in Medline, Embase, CINAHL, Cochrane, and Google Scholar (supplemental Appendix 3). Search limiters were English language and adults. Articles were manually screened to exclude nonhuman or endoscopic ultrasound applications. Final article selection was based on working group consensus.
Draft Pathways
The Credentialing Working Group drafted initial and ongoing certification pathways (Figure 1 and Figure 2). The other 4 working groups from the task force were surveyed about the elements and overall appropriateness of these draft pathways. This survey and its results have already been published.12 The Credentialing Working Group then revised the certification pathways by using these survey results and codified individual aspects of these pathways into recommendations.
Development of Position Statement
Based on the Grading of Recommendation Assessment Development and Evaluation methodology, all final article selections were initially rated as either low-quality (observational studies) or unclassifiable (expert opinion).16 These initial ratings were downgraded further because of indirectness, because none of the articles involved the intervention of interest (a credentialing pathway) in a population of interest (hospitalists) measuring the outcomes of interest (patient-level outcomes).
First, the Credentialing Working Group drafted an initial position statement composed of recommendations for credentialing pathways and other general aspects of credentialing. All final article selections were incorporated as references in a draft of the position statement and compiled in a full-text compendium. Second, feedback was provided by the other 4 task force working groups, the task force peer reviewers, and the SHM Education Committee. Feedback was incorporated by the authors of this statement who were the Credentialing Working Group, the chairs of the other 4 working groups, and a guideline methodologist. Third, final suggestions from all members of the SHM POCUS Task Force and SHM Education Committee were incorporated before final approval by the SHM Board of Directors in September 2017.
RESULTS
A total of 1438 references were identified in the original search. Manual selection led to 101 articles, which were incorporated into the following 4 domains with 16 recommendations.
General Credentialing Process
Basic Cognitive Competence Can Be Certified with Written or Oral Examinations
The ABIM defines cognitive competence as having 3 abilities: “(1) to explain indications, contraindications, patient preparation methods, sterile techniques, pain management, proper techniques for handling specimens and fluids obtained, and test results; (2) to recognize and manage complications; and, (3) to clearly explain to a patient all facets of the procedure necessary to obtain informed consent.”1 These abilities can be assessed with written or oral examinations that may be integrated into simulation- or patient-based assessments.
Minimum Thresholds of Experience to Trigger the Timing of a Patient-Based Assessment Should Be Determined by Empirical Methods
Learning curves are highly variable22-25 and even plateaus may not herald basic competence.26 Expert opinion
Hospitalists Should Formally Log All of Their Attempted Procedures, Ideally in an Electronic Medical Record
Simple self-reported numbers of procedures performed often misrepresent actual experience33,34 and do not include periprocedural complications.35,36 Thus, hospitalists should report their experience with logs of all attempted procedures, both successful and unsuccessful. Such logs must include information about supervising providers (if applicable) and patient outcomes, including periprocedural adverse events,37 but they must also remain compliant with the Health Insurance Portability and Accountability Act.
Health Information Technology Service Should Routinely Pull Collations of All Attempted Procedures from Comprehensive Electronic Medical Records
Active surveillance may reduce complications by identifying hospitalists who may benefit from further training.38 In order to facilitate active surveillance systems, documentation (such as a procedure note) should be both integrated into an electronic medical record and protocol driven,39 including procedure technique, ultrasound findings, and any safety events (both near misses and adverse events).
Multiple interacting factors, including environment, patients, baseline skills, training, experience, and skills decay, affect manual competence. Certifications that are based solely on reaching minimum thresholds of experience, even when accurate, are not valid reflections of manual competence,15,40-43 and neither are those based on self-perception.44 Patient-based assessments are, thus, necessary to ensure manual competence.45-48
Certification Assessments of Manual Competence Should Combine 2 Types of Structured Instruments: Checklists and Overall Scores
Assessments based on direct observation are more reliable when formally structured.49,50 Though checklists used in observed structured clinical examinations capture many important manual skills,51-56 they do not completely reflect a hospitalist’s manual competence;57 situations may occur in which a hospitalist meets all the individual items on a checklist but cannot perform an entire procedure with basic competence. Therefore, checklists should be paired with overall scores.58-61 Both checklists and overall scores ought to be obtained from reliable and valid instruments.
Certification Assessments Should Include Feedback
Assessments without feedback are missed learning opportunities.62 Both simulation-63 and patient-based assessments should provide feedback in real time to reinforce effective behaviors and remedy faulty ones.
If Remedial Training is Needed, Simulator-Based Training Can Supplement but Not Replace Patient-Based Training
Supervised simulator-based training allows hospitalists to master basic components of a procedure64 (including orientation to equipment, sequence of operations, dexterity, ultrasound anatomy, and real-time guidance technique) while improving both cognitive and manual skills.42,43,65-71 In addition to their role in basic training (which is outside the scope of this position statement), simulators can be useful for remedial training. To be sufficient for hospitalists who do not pass their patient-based assessments, however, remedial training that begins with simulation must also include patient-based training and assessment.72-75
Initial Credentialing Process
A Minimum Threshold of Experience Should Be Reached before Patient-Based Assessments are Conducted (Figure 1)
Initial Certification Assessments Should Ideally Begin on Simulators
Simulators allow the assurance of safe manual skills, including proper needle insertion techniques and disposal of sharp objects.3,79 If simulators are not available, however, then patient-based training and assessments can still be performed under direct observation. Safe performance of ultrasound-guided procedures during patient-based assessments (without preceding simulator-based assessments) is sufficient to certify manual competence.
Ongoing Credentialing
Certification to Perform Ultrasound-Guided Procedures Should Be Routinely Re-Evaluated During Ongoing Credentialing (Figure 2)
Observed Patient-Based Assessments Should Occur When a Periprocedural Safety Event Occurs that is Potentially Caused by “Provider Error”
Safety events include both near misses and adverse events. Information about both is ideally “flagged” and “pushed” to hospitalist group leaders by active surveillance and reporting systems. Once reviewed, if a safety event is considered to potentially have been caused by provider error (including knowledge- and skill-based errors),83 then the provider who performed the procedure should undergo an observed patient-based assessment.
Simulation-Based Practice Can Supplement Patient-Based Experience for Ongoing Credentialing
When hospitalists do not achieve a minimum threshold of patient-based experience since the antecedent certification, simulation-based training can supplement their patient-based experience.
Credentialing Infrastructure
Hospitalists Themselves Should Not Bear the Financial Costs of Developing and Maintaining Training and Certification Programs for Ultrasound-Guided Procedures
Equipment and personnel costs
Assessors Should Be Unbiased Expert Providers Who Have Demonstrated Mastery in Performance of the Procedure Being Assessed and Regularly Perform It in a Similar Practice Environment
Assessors should be expert providers who regularly perform the ultrasound-guided procedure in a similar practice environment.9,89-94 For example, providers who are not hospitalists but who are experts in an ultrasound-guided procedure and commonly perform it on the hospital wards would be acceptable assessors. However, a radiologist who only performs that procedure in a fully-staffed interventional radiology suite with fluoroscopy or computed tomography guidance would not be an acceptable assessor. More than 1 assessor may balance idiosyncratic assessments;95 but when assessments are well structured, additional assessors are generally not needed.18
If Intramural Assessors Are Not Available, Extramural Assessors May Be Considered
Intramural assessors are generally preferred because of familiarity with the local practice environment, including the available procedure kits and typical patient characteristics. Nevertheless, extramural assessors27,77,85,96 may theoretically provide even more valid assessments than intramural ones because extramural assessors are neither influenced by relationships with local hospitalists nor biased by local hospitalists’ skills.
DISCUSSION
There are no high-quality randomized trials in support of a single credentialing pathway over any other.94,102 The credentialing pathways at the center of this position statement are based on expert opinion. Our methods can be criticized straightaway, therefore, for reliance on the experience and expertise of our working group and task force. Any position statement written without high-quality supportive evidence would be appropriately subject to the same criticism. Without evidence in support of an overall pathway, we codified specific aspects of the pathways into 16 individual recommendations.
Patient-level outcomes do not back these recommendations. Consider, for example, our recommendation that certification assessments be made from structured instruments and not simply from an assessor’s gestalt. Here, the basis is not improved patient-level outcomes from a trial (such as reduced complications or increased procedural success) but improved psychometric performance from reliability studies. The body of evidence for our recommendations is similarly indirect, mostly because the outcomes studied are more proximate and, thus, less meaningful than patient-level outcomes, which are the outcomes of greatest interest but are woefully understudied for clinical competence.17,97,103
The need for high-quality evidence is most pronounced in distinguishing how recommendations should be modified for various settings. Wide variations in resources and patient-mix will make some recommendations impracticable, meaning that they could not be carried out with available resources. For example, our recommendation that credentialing decisions should ultimately rely on certifications made by assessors during patient-based assessments may not be practicable at small, rural hospitals. Such hospitals may not have access to local assessors, and they may not admit enough patients who need the types of ultrasound-guided procedures for which hospitalists seek certification (especially given the need to coordinate the schedules of patients, procedure-performing hospitalists, and assessors).
Regardless of whether some or all hospitalists at a particular hospital are expected to perform bedside procedures, technology may help to improve the practicability of our recommendations. For example, simulators may evolve to replace actual patient-level experience in achieving minimum thresholds. Certification assessments of manual skills may even someday occur entirely on simulators. Real-time high-definition video streaming enhanced with multiple cameras may allow for remote assessments. Until such advances mature, high-quality patient-level data should be sought through additional research to refine our current recommendations.
We hope that these recommendations will improve how basic competence in ultrasound-guided bedside procedures is assessed. Our ultimate goal is to improve how hospitalists perform these procedures. Patient safety is, therefore, considered paramount to cost. Nevertheless, the hospital administrative leaders and privileging committee members on our Task Force concluded that many hospitals have been seeking guidance on credentialing for bedside procedures, and the likely difficulties of implementing our recommendations (including cost) would not be prohibitive at most hospitals, especially given recognition that these recommendations can be tailored to each setting.
Acknowledgments
Collaborators from SHM POCUS Task Force are Saaid Abdel-Ghani, Michael Blaivas, Dan Brotman, Carolina Candotti, Jagriti Chadha, Joel Cho, Ria Dancel, Ricardo Franco, Richard Hoppmann, Susan Hunt, Venkat Kalidindi, Ketino Kobaidze, Josh Lenchus, Benji Mathews, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Sophia Rodgers, Gerard Salame, Daniel Schnobrich, Kirk Spencer, Vivek Tayal, Jeff Bates, Anjali Bhagra, Kreegan Reierson, Robert Arntfield, Paul Mayo, Loretta Grikis.
Disclosure
Brian P. Lucas received funding from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, and National Center for Translational Science (UL1TR001086). Nilam Soni received funding from the Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (HX002263-01A1). The contents of this publication do not represent the views of the United States Department of Veterans Affairs or the United States Government.
The American Board of Internal Medicine (ABIM) changed its certification policy for bedside procedures over a decade ago.
Hospitalists increasingly perform bedside procedures with ultrasound guidance.
Therefore, the Society of Hospital Medicine (SHM) Education Committee convened a group of experts and conducted a systematic literature review in order to provide recommendations for credentialing hospitalist physicians in ultrasound-guided bedside procedures. These recommendations do not include training recommendations, aside from recommendations about remedial training for hospitalists who do not pass certification. Training is a means to competence but does not guarantee it. We believe that training recommendations ought to be considered separately.
METHODS
Working Group Formation
In January 2015, the SHM Board of Directors asked the SHM Education Committee to convene the POCUS Task Force. The purpose of the task force was to develop recommendations on ultrasound guidance for bedside procedures. The SHM Education Committee appointed 3 chairs of the task force: 1 senior member of the SHM Education Committee and 2 POCUS experts. The chairs assembled a task force of 31 members that included 5 working groups, a multispecialty peer review group, and a guideline methodologist (supplemental Appendix 1). Invitation was based on members’ past contributions to SHM POCUS-related activities, up-front commitment, and declared conflicts of interest. Working group members self-identified as “hospitalists,” whereas peer reviewers were nonhospitalists but nationally recognized POCUS physician-leaders specializing in emergency medicine, cardiology, critical care medicine, and anesthesiology. Task force membership was vetted by a chair of the SHM POCUS Task Force and the Director of Education before work began. This position statement was authored by the Credentialing Working Group together with the chairs of the other 4 working groups and a guideline methodologist.
Disclosures
Signed disclosure statements of all task force members were reviewed prior to inclusion on the task force (supplemental Appendix 2); no members received honoraria for participation. Industry representatives did not contribute to the development of the guidelines nor to any conference calls or meetings.
Literature Search Strategy
A literature search was conducted by a biomedical librarian. Records from 1979 to January of 2017 were searched in Medline, Embase, CINAHL, Cochrane, and Google Scholar (supplemental Appendix 3). Search limiters were English language and adults. Articles were manually screened to exclude nonhuman or endoscopic ultrasound applications. Final article selection was based on working group consensus.
Draft Pathways
The Credentialing Working Group drafted initial and ongoing certification pathways (Figure 1 and Figure 2). The other 4 working groups from the task force were surveyed about the elements and overall appropriateness of these draft pathways. This survey and its results have already been published.12 The Credentialing Working Group then revised the certification pathways by using these survey results and codified individual aspects of these pathways into recommendations.
Development of Position Statement
Based on the Grading of Recommendation Assessment Development and Evaluation methodology, all final article selections were initially rated as either low-quality (observational studies) or unclassifiable (expert opinion).16 These initial ratings were downgraded further because of indirectness, because none of the articles involved the intervention of interest (a credentialing pathway) in a population of interest (hospitalists) measuring the outcomes of interest (patient-level outcomes).
First, the Credentialing Working Group drafted an initial position statement composed of recommendations for credentialing pathways and other general aspects of credentialing. All final article selections were incorporated as references in a draft of the position statement and compiled in a full-text compendium. Second, feedback was provided by the other 4 task force working groups, the task force peer reviewers, and the SHM Education Committee. Feedback was incorporated by the authors of this statement who were the Credentialing Working Group, the chairs of the other 4 working groups, and a guideline methodologist. Third, final suggestions from all members of the SHM POCUS Task Force and SHM Education Committee were incorporated before final approval by the SHM Board of Directors in September 2017.
RESULTS
A total of 1438 references were identified in the original search. Manual selection led to 101 articles, which were incorporated into the following 4 domains with 16 recommendations.
General Credentialing Process
Basic Cognitive Competence Can Be Certified with Written or Oral Examinations
The ABIM defines cognitive competence as having 3 abilities: “(1) to explain indications, contraindications, patient preparation methods, sterile techniques, pain management, proper techniques for handling specimens and fluids obtained, and test results; (2) to recognize and manage complications; and, (3) to clearly explain to a patient all facets of the procedure necessary to obtain informed consent.”1 These abilities can be assessed with written or oral examinations that may be integrated into simulation- or patient-based assessments.
Minimum Thresholds of Experience to Trigger the Timing of a Patient-Based Assessment Should Be Determined by Empirical Methods
Learning curves are highly variable22-25 and even plateaus may not herald basic competence.26 Expert opinion
Hospitalists Should Formally Log All of Their Attempted Procedures, Ideally in an Electronic Medical Record
Simple self-reported numbers of procedures performed often misrepresent actual experience33,34 and do not include periprocedural complications.35,36 Thus, hospitalists should report their experience with logs of all attempted procedures, both successful and unsuccessful. Such logs must include information about supervising providers (if applicable) and patient outcomes, including periprocedural adverse events,37 but they must also remain compliant with the Health Insurance Portability and Accountability Act.
Health Information Technology Service Should Routinely Pull Collations of All Attempted Procedures from Comprehensive Electronic Medical Records
Active surveillance may reduce complications by identifying hospitalists who may benefit from further training.38 In order to facilitate active surveillance systems, documentation (such as a procedure note) should be both integrated into an electronic medical record and protocol driven,39 including procedure technique, ultrasound findings, and any safety events (both near misses and adverse events).
Multiple interacting factors, including environment, patients, baseline skills, training, experience, and skills decay, affect manual competence. Certifications that are based solely on reaching minimum thresholds of experience, even when accurate, are not valid reflections of manual competence,15,40-43 and neither are those based on self-perception.44 Patient-based assessments are, thus, necessary to ensure manual competence.45-48
Certification Assessments of Manual Competence Should Combine 2 Types of Structured Instruments: Checklists and Overall Scores
Assessments based on direct observation are more reliable when formally structured.49,50 Though checklists used in observed structured clinical examinations capture many important manual skills,51-56 they do not completely reflect a hospitalist’s manual competence;57 situations may occur in which a hospitalist meets all the individual items on a checklist but cannot perform an entire procedure with basic competence. Therefore, checklists should be paired with overall scores.58-61 Both checklists and overall scores ought to be obtained from reliable and valid instruments.
Certification Assessments Should Include Feedback
Assessments without feedback are missed learning opportunities.62 Both simulation-63 and patient-based assessments should provide feedback in real time to reinforce effective behaviors and remedy faulty ones.
If Remedial Training is Needed, Simulator-Based Training Can Supplement but Not Replace Patient-Based Training
Supervised simulator-based training allows hospitalists to master basic components of a procedure64 (including orientation to equipment, sequence of operations, dexterity, ultrasound anatomy, and real-time guidance technique) while improving both cognitive and manual skills.42,43,65-71 In addition to their role in basic training (which is outside the scope of this position statement), simulators can be useful for remedial training. To be sufficient for hospitalists who do not pass their patient-based assessments, however, remedial training that begins with simulation must also include patient-based training and assessment.72-75
Initial Credentialing Process
A Minimum Threshold of Experience Should Be Reached before Patient-Based Assessments are Conducted (Figure 1)
Initial Certification Assessments Should Ideally Begin on Simulators
Simulators allow the assurance of safe manual skills, including proper needle insertion techniques and disposal of sharp objects.3,79 If simulators are not available, however, then patient-based training and assessments can still be performed under direct observation. Safe performance of ultrasound-guided procedures during patient-based assessments (without preceding simulator-based assessments) is sufficient to certify manual competence.
Ongoing Credentialing
Certification to Perform Ultrasound-Guided Procedures Should Be Routinely Re-Evaluated During Ongoing Credentialing (Figure 2)
Observed Patient-Based Assessments Should Occur When a Periprocedural Safety Event Occurs that is Potentially Caused by “Provider Error”
Safety events include both near misses and adverse events. Information about both is ideally “flagged” and “pushed” to hospitalist group leaders by active surveillance and reporting systems. Once reviewed, if a safety event is considered to potentially have been caused by provider error (including knowledge- and skill-based errors),83 then the provider who performed the procedure should undergo an observed patient-based assessment.
Simulation-Based Practice Can Supplement Patient-Based Experience for Ongoing Credentialing
When hospitalists do not achieve a minimum threshold of patient-based experience since the antecedent certification, simulation-based training can supplement their patient-based experience.
Credentialing Infrastructure
Hospitalists Themselves Should Not Bear the Financial Costs of Developing and Maintaining Training and Certification Programs for Ultrasound-Guided Procedures
Equipment and personnel costs
Assessors Should Be Unbiased Expert Providers Who Have Demonstrated Mastery in Performance of the Procedure Being Assessed and Regularly Perform It in a Similar Practice Environment
Assessors should be expert providers who regularly perform the ultrasound-guided procedure in a similar practice environment.9,89-94 For example, providers who are not hospitalists but who are experts in an ultrasound-guided procedure and commonly perform it on the hospital wards would be acceptable assessors. However, a radiologist who only performs that procedure in a fully-staffed interventional radiology suite with fluoroscopy or computed tomography guidance would not be an acceptable assessor. More than 1 assessor may balance idiosyncratic assessments;95 but when assessments are well structured, additional assessors are generally not needed.18
If Intramural Assessors Are Not Available, Extramural Assessors May Be Considered
Intramural assessors are generally preferred because of familiarity with the local practice environment, including the available procedure kits and typical patient characteristics. Nevertheless, extramural assessors27,77,85,96 may theoretically provide even more valid assessments than intramural ones because extramural assessors are neither influenced by relationships with local hospitalists nor biased by local hospitalists’ skills.
DISCUSSION
There are no high-quality randomized trials in support of a single credentialing pathway over any other.94,102 The credentialing pathways at the center of this position statement are based on expert opinion. Our methods can be criticized straightaway, therefore, for reliance on the experience and expertise of our working group and task force. Any position statement written without high-quality supportive evidence would be appropriately subject to the same criticism. Without evidence in support of an overall pathway, we codified specific aspects of the pathways into 16 individual recommendations.
Patient-level outcomes do not back these recommendations. Consider, for example, our recommendation that certification assessments be made from structured instruments and not simply from an assessor’s gestalt. Here, the basis is not improved patient-level outcomes from a trial (such as reduced complications or increased procedural success) but improved psychometric performance from reliability studies. The body of evidence for our recommendations is similarly indirect, mostly because the outcomes studied are more proximate and, thus, less meaningful than patient-level outcomes, which are the outcomes of greatest interest but are woefully understudied for clinical competence.17,97,103
The need for high-quality evidence is most pronounced in distinguishing how recommendations should be modified for various settings. Wide variations in resources and patient-mix will make some recommendations impracticable, meaning that they could not be carried out with available resources. For example, our recommendation that credentialing decisions should ultimately rely on certifications made by assessors during patient-based assessments may not be practicable at small, rural hospitals. Such hospitals may not have access to local assessors, and they may not admit enough patients who need the types of ultrasound-guided procedures for which hospitalists seek certification (especially given the need to coordinate the schedules of patients, procedure-performing hospitalists, and assessors).
Regardless of whether some or all hospitalists at a particular hospital are expected to perform bedside procedures, technology may help to improve the practicability of our recommendations. For example, simulators may evolve to replace actual patient-level experience in achieving minimum thresholds. Certification assessments of manual skills may even someday occur entirely on simulators. Real-time high-definition video streaming enhanced with multiple cameras may allow for remote assessments. Until such advances mature, high-quality patient-level data should be sought through additional research to refine our current recommendations.
We hope that these recommendations will improve how basic competence in ultrasound-guided bedside procedures is assessed. Our ultimate goal is to improve how hospitalists perform these procedures. Patient safety is, therefore, considered paramount to cost. Nevertheless, the hospital administrative leaders and privileging committee members on our Task Force concluded that many hospitals have been seeking guidance on credentialing for bedside procedures, and the likely difficulties of implementing our recommendations (including cost) would not be prohibitive at most hospitals, especially given recognition that these recommendations can be tailored to each setting.
Acknowledgments
Collaborators from SHM POCUS Task Force are Saaid Abdel-Ghani, Michael Blaivas, Dan Brotman, Carolina Candotti, Jagriti Chadha, Joel Cho, Ria Dancel, Ricardo Franco, Richard Hoppmann, Susan Hunt, Venkat Kalidindi, Ketino Kobaidze, Josh Lenchus, Benji Mathews, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Sophia Rodgers, Gerard Salame, Daniel Schnobrich, Kirk Spencer, Vivek Tayal, Jeff Bates, Anjali Bhagra, Kreegan Reierson, Robert Arntfield, Paul Mayo, Loretta Grikis.
Disclosure
Brian P. Lucas received funding from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, and National Center for Translational Science (UL1TR001086). Nilam Soni received funding from the Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (HX002263-01A1). The contents of this publication do not represent the views of the United States Department of Veterans Affairs or the United States Government.
1. American Board of Internal Medicine. Policies and procedures for certification. Philadelphia: American Board of Internal Medicine; 2006.
2. Nichani S, Fitterman N, Lukela M, Crocker J; Society of Hospital Medicine. The Core Competencies in Hospital Medicine 2017 Revision. Section 2: Procedures. J Hosp Med. 2017;12(4 Suppl 1):S44-S54 PubMed
3. Lucas BP, Asbury JK, Franco-Sadud R. Training future hospitalists with simulators: a needed step toward accessible, expertly performed bedside procedures. J Hosp Med. 2009;4(7):395-396. PubMed
4. Schnobrich DJ, Gladding S, Olson APJ, Duran-Nelson A. Point-of-care ultrasound in internal medicine: a national survey of educational leadership. J Grad Med Educ. 2013;5(3):498-502. PubMed
5. Brown GM, Otremba M, Devine LA, Gray C, Millington SJ, Ma IW. Defining competencies for ultrasound-guided bedside procedures: consensus opinions from Canadian physicians. J Ultrasound Med. 2016;35(1):129-141. PubMed
6. Vaisman A, Cram P. Procedural competence among faculty in academic health centers: challenges and future directions. Acad Med. 2017;92(1):31-34. PubMed
7. Kreisman RD. With ED ultrasound, credentialing is at issue. ED Legal Letter. 2010;21:102-103.
8. Goudie AM. Credentialing a new skill: what should the standard be for emergency department ultrasound in Australasia? Emerg Med Australas. 2010;22:263-264. PubMed
9. Maizel J, Guyomarc HL, Henon P, et al. Residents learning ultrasound-guided catheterization are not sufficiently skilled to use landmarks. Crit Care. 2014;18(1):R36. doi:10.1186/cc13741. PubMed
10. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care, and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. PubMed
11. Amini R, Adhikari S, Fiorello A. Ultrasound competency assessment in emergency medicine residency programs. Acad Emerg Med. 2014;21(7):799-801. PubMed
12. Jensen T, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. PubMed
13. Chang W. Is hospitalist proficiency in bedside procedures in decline? The Hospitalist. 2012. http://www.the-hospitalist.org/hospitalist/article/125236/patient-safety/hospitalist-proficiency-bedside-procedures-decline. Accessed September 30, 2017.
14. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties Performing Paracentesis Procedures at University Hospitals: Implications for Training and Certification. J Hosp Med. 2014;9(3):162-168. PubMed
15. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Residents’ Procedural Experience Does Not Ensure Competence: A Research Synthesis. J Grad Med Educ. 2017;9(2):201-208. PubMed
16. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. PubMed
17. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64(12):1303-1310. PubMed
18. Miller GE. The assessment of clinical skills/competence/performance. Acad Med. 1990;65(9 Suppl):S63-S67. PubMed
19. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
20. Millington SJ, Wong RY, Kassen BO, Roberts JM, Ma IWY. Improving internal medicine residents’ performance, knowledge, and confidence in central venous catheterization using simulators. J Hosp Med. 2009;4(7):410-416. PubMed
21. Lenchus JD, Carvalho CM, Ferreri K, et al. Filling the void: defining invasive bedside procedural competency for internal medicine residents. J Grad Med Educ. 2013;5(4):605-612. PubMed
22. Heegeman DJ, Kieke B Jr. Learning curves, credentialing, and the need for ultrasound fellowships. Acad Emerg Med. 2003;10:404-405. PubMed
23. Jang TB, Ruggeri W, Dyne P, Kaji AH. The learning curve of resident physicians using emergency ultrasonography for cholelithaisis and cholecystitis. Acad Emerg Med. 2010;17(11):1247-1252. PubMed
24. Akhtar MI, Hamid M. Ultrasound guided central venous access; a review of literature. Anaesth Pain Intensive Care. 2015;19:317-322.
25. Bahl A, Yunker A. Assessment of the numbers–based model for evaluation of resident competency in emergency ultrasound core applications. J Emerg Med Trauma Acute Care. 2015;2015(5). doi:10.5339/jemtac.2015.5
26. Cazes N, Desmots F, Geffroy Y, Renard A, Leyral J, Chaumoitre K. Emergency ultrasound: a prospective study on sufficient adequate training for military doctors. Diagn Interv Imaging. 2013;94(11):1109-1115. PubMed
27. Arntfield RT, Millington SJ, Ainsworth CD, et al. Canadian recommendations for critical care ultrasound training and competency for the Canadian critical care society. Can Respir J. 2014;21(16):341-345.
28. Bolsin S, Colson M. The use of the Cusum technique in the assessment of trainee competence in new procedures. Int J Qual Health Care. 2000;12(5):433-438. PubMed
29. de Oliveira Filho GR, Helayel PE, da Conceição DB, Garzel IS, Pavei P, Ceccon MS. Learning curves and mathematical models for interventional ultrasound basic skills. Anaesth Analg. 2008;106(2):568-573. PubMed
30. Starkie T, Drake EJ. Assessment of procedural skills training and performance in anesthesia using cumulative sum analysis (cusum). Can J Anaesth. 2013;60(12):1228-1239. PubMed
31. Tierney D. Competency cut-point identification derived from a mastery learning cohort approach: A hybrid model. Ultrasound Med Biol. 2015;41:S19.
32. Rankin JH, Elkhunovich MA, Rangarajan V, Chilstrom M, Mailhot T. Learning Curves for Ultrasound Assessment of Lumbar Puncture Insertion Sites: When is Competency Established? J Emerg Med. 2016;51(1):55-62. PubMed
33. Klasko SK, Cummings RV, Glazerman LR. Resident data collection: Do the numbers add up? Am J Obstet Gynecol. 1995;172(4 Pt 1):1312-1316. PubMed
34. Tierney D. Development & analysis of a mobile POCUS tracking tool. Ultrasound Med Biol. 2015;41(suppl 4):S31.
35. Sethi MV, Zimmer J, Ure B, Lacher M. Prospective assessment of complications on a daily basis is essential to determine morbidity and mortality in routine pediatric surgery. J Pediatr Surg. 2016;51(4):630-633. PubMed
36. Fisher JC, Kuenzler KA, Tomita SS, Sinha P, Shah P, Ginsburg HB. Increased capture of pediatric surgical complications utilizing a novel case-log web application to enhance quality improvement. J Pediatr Surg. 2017;52(1):166-171. PubMed
37. Rethans JJ, Norcini JJ, Barón-Maldonado M, et al. The relationship between competence and performance: implications for assessing practice performance. Med Educ. 2002;36(10):901-909. PubMed
38. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
39. Society of Critical Care Medicine Ultrasound Certification Task Force. Recommendations for achieving and maintaining competence and credentialing in critical care ultrasound with focused cardiac ultrasound and advanced critical care echocardiography. http://journals.lww.com/ccmjournal/Documents/Critical%20Care%20Ultrasound.pdf Published 2013. Accessed February 2, 2017.
40. Carraccio C, Wolfsthal SD, Englander R, Ferentz K, Martin C. Shifting paradigms: from Flexner to competencies. Acad Med. 2002;77(5):361-367. PubMed
41. Clark EG, Paparello JJ, Wayne DB, et al. Use of a national continuing medical education meeting to provide simulation-based training in temporary hemodialysis catheter insertion skills: a pre-test post-test study. Can J Kidney Health Dis. 2014;1:25-31. PubMed
42. Barsuk JH, Cohen ER, Caprio T, McGaghie WC, Simuni T, Wayne DB. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137. PubMed
43. Barsuk JH, McGaghie WC, Cohen ER, O’Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697-2701. PubMed
44. Davis DA, Mazmanian PE, Fordis M, Van Harrison R, Thorpe KE, Perrier L. Accuracy of physician self-assessment compared with observed measures of competence: a systematic review. JAMA. 2006;296(9):1094-1102. PubMed
45. Shah J, Darzi A. Surgical skills assessment: an ongoing debate. BJU Int. 2001;88(7):655-660. PubMed
46. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105-1117. PubMed
47. Tolsgaard MG, Todsen T, Sorensen JL, et al. International multispecialty consensus on how to evaluate ultrasound competence: a Delphi consensus survey. PLOS One. 2013;8(2):e57687. doi:10.1371/journal.pone.0057687 PubMed
48. Moureau N, Laperti M, Kelly LJ, et al. Evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training. Br J Anaesth. 2013;110(3):347-356. PubMed
49. Feldman LS, Hagarty S, Ghitulescu G, Stanbridge D, Fried GM. Relationship between objective assessment of technical skills and subjective in-training evaluations in surgical residents. J Am Coll Surg. 2004;198(1):105-110. PubMed
50. Baker S, Willey B, Mitchell C. The attempt to standardize technical and analytic competence in sonography education. J Diagn Med Sonogr. 2011;27(5):203-211.
51. Tolsgaard MG, Ringsted C, Dreisler E, et al. Reliable and valid assessment of ultrasound operator competence in obstetrics and gynecology. Ultrasound Obstet Gynecol. 2014;43(4):437-443. PubMed
52. Rice J, Crichlow A, Baker M, et al. An assessment tool for the placement of ultrasound-guided peripheral intravenous access. J Grad Med Educ. 2016;8(2):202-207. PubMed
53. Hartman N, Wittler M, Askew K, Hiestand B, Manthey D. Validation of a performance checklist for ultrasound-guided internal jubular central lines for use in procedural instruction and assessment. Postgrad Med J. 2017;93(1096):67-70. PubMed
54. Primdahl SC, Todsen T, Clemmesen L, et al. Rating scale for the assessment of competence in ultrasound-guided peripheral vascular access—a Delphi Consensus Study. J Vasc Access. 2016;17(5):440-445.
55. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
56. Berg K, Riesenberg LA, Berg D, et al. The development of a validated checklist for radial arterial line placement: preliminary results. Am J Med Qual. 2014;29(3):242-246. PubMed
57. Walzak A, Bacchus M, Schaefer MP, et al. Diagnosing technical competence in six bedside procedures: comparing checklists and a global rating scale in the assessment of resident performance. Acad Med. 2015;90(8):1100-1108. PubMed
58. Riesenberg LA, Berg K, Berg D, et al. The development of a validated checklist for femoral venous catheterization: preliminary results. Am J Med Qual. 2014;29(5):445-450. PubMed
59. Riesenberg LA, Berg K, Berg D, et al. The development of a validated checklist for paracentesis: preliminary results. Am J Med Qual. 2013;28(3):227-231. PubMed
60. Huang GC, Newman LR, Schwartzstein RM, et al. Procedural competence in internal medicine residents: validity of a central venous catheter insertion assessment instrument. Acad Med. 2009;84(8):1127-1134. PubMed
61. Salamonsen M, McGrath D, Steiler G, et al. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
62. Boniface K, Yarris LM. Emergency ultrasound: Leveling the training and assessment landscape. Acad Emerg Med. 2014;21(7):803-805. PubMed
63. Boyle E, O’Keeffe D, Naughton P, Hill A, McDonnell C, Moneley D. The importance of expert feedback during endovascular simulator training. J Vasc Surg. 2011;54(1):240-248.e1. PubMed
64. Langhan TS, Rigby IJ, Walker IW, Howes D, Donnon T, Lord JA. Simulation-based training in critical resuscitation procedures improves residents’ competence. CJEM. 2009;11(6):535-539. PubMed
65. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. PubMed
66. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
67. Barsuk JH, Cohen ER, Vozenilek JA, O’Connor LM, McGaghie WC, Wayne DB. Simulation-based education with mastery learning improves paracentesis skills. J Grad Med Educ. 2012;4(1):23-27. PubMed
68. McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706-711. PubMed
69. Ross JG. Simulation and psychomotor skill acquisition: A review of the literature. Clin Simul Nurs. 2012;8(9):e429-e435.
70. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
71. McSparron JI, Michaud GC, Gordan PL, et al. Simulation for skills-based education in pulmonary and critical care medicine. Ann Am Thorac Soc. 2015;12(4):579-586. PubMed
72. Kneebone RL, Scott W, Darzi A, Horrocks M. Simulation and clinical practice: strengthening the relationship. Med Educ. 2004;38(10):1095-1102. PubMed
73. Mema B, Harris I. The barriers and facilitators to transfer of ultrasound-guided central venous line skills from simulation to practice: exploring perceptions of learners and supervisors. Teach Learn Med. 2016;28(2):115-124. PubMed
74. Castanelli DJ. The rise of simulation in technical skills teaching and the implications for training novices in anaestheia. Anaesth Intensive Care. 2009;37(6):903-910. PubMed
75. McGaghie WC, Issenberg SB, Barsuk JH, Wayne DB. A critical review of simulation-based mastery learning with translational outcomes. Med Educ. 2014;48(4):375-385. PubMed
76. Langlois SLP. Focused ultrasound training for clinicians. Crit Care Med. 2007;35(5 suppl):S138-S143.
77. Price S, Via G, Sloth E, et al. Echocardiography practice, training and accreditation in the intesive care: document for the World Interactive Network Focused on Critical Ultrasound (WINFOCUS). Cardiovasc Ultrasound. 2008;6:49-83. PubMed
78. Blehar DJ, Barton B, Gaspari RJ. Learning curves in emergency ultrasound education. Acad Emerg Med. 2015;22(5):574-582. PubMed
79. Ault MJ, Rosen BT, Ault B. The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative. J Gen Intern Med. 2006;21(5):514-517. PubMed
80. Barsuk JH, Cohen ER, McGaghie WC, Wayne DB. Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med. 2010;85(10 Suppl):S9-S12. PubMed
81. Sliman Sean, Amundson S, Shaw D, Phan JN, Waalen J, Kimura B. Recently-acquired cardiac ultrasound skills are rapidly lost when not used: implications for competency in physician imaging. J Amer Coll Cardiol. 2016;67(13S):1569.
82. Kessler CS, Leone KA. The current state of core competency assessment in emergency medicine and a future research agenda: recommendations of the working group on assessment of observable learner performance. Acad Emerg Med. 2012;19(12):1354-1359. PubMed
83. Chang A, Schyve PM, Croteau RJ, O’Leary DS, Loeb JM. The JCAHO patient safety event taxonomy: a standardized terminology and classification schema for near misses and adverse events. Int J Qual Health Care. 2005;17(2):95-105. PubMed
84. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Acad Med. 2015;90(8):1025-1033. PubMed
85. Das D, Kapoor M, Brown C, Ndubuisi A, Gupta S. Current status of emergency department attending physician ultrasound credentialing and quality assurance in the United States. Crit Ultrasound J. 2016;8(1):6-12. PubMed
86. Ndubuisi AK, Gupta S, Brown C, Das D. Current status and future issues in emergency department attending physician ultrasound credentialing. Ann Emerg Med. 2014;64(45):S27-S28.
87. Tandy Tk, Hoffenberg S. Emergency department ultrasound services by emergency physicians: model for gaining hospital approval. Ann Emerg Med. 1997;29(3):367-374. PubMed
88. Lewiss RE, Saul T, Del Rios M. Acquiring credentials in bedside ultrasound: a cross-sectional survey. BMJ Open. 2013;3:e003502. doi:10.1136/bmjopen-2013-003502 PubMed
89. Lanoix R. Credentialing issues in emergency ultrasonography. Emerg Med Clin North Am. 1997;15(4):913-920. PubMed
90. Scalea T, Rodriquez A, Chiu WC, et al. Focused assessment with sonography for trauma (FAST): results from an international consensus conference. J Trauma. 1999;46(3):466-472. PubMed
91. Hertzberg BS, Kliewer MA, Bowie JD, et al. Physician training requirements in sonography: how many cases are needed for competence? AJR. 2000;174(5):1221-1227. PubMed
92. Blaivas M, Theodoro DL, Sierzenski P. Proliferation of ultrasound fellowships in emergency medicine: how do we ensure future experts are expertly trained? Acad Emerg Med. 2002;9(8):863-864. PubMed
93. Bodenham AR. Editorial II: Ultrasound imaging by anaesthetists: training and accreditation issues. Br J Anaesth. 2006;96(4):414-417. PubMed
94. Williamson JP, Twaddell SH, Lee YCG, et al. Thoracic ultrasound recognition of competence: A position paper of the Thoracic Society of Australia and New Zealand. Respirology. 2017;22(2):405-408. PubMed
95. Harrison G. Summative clinical competency assessment: a survey of ultrasound practitioners’ views. Ultrasound. 2015;23(1):11-17. PubMed
96. Evans LV, Morse JL, Hamann CJ, Osborne M, Lin Z, D'Onofrio G. The development of an independent rater system to assess residents' competence in invasive procedures. Acad Med. 2009;84(8):1135-1143. PubMed
97. Wass V, Van der Vleuten C, Shatzer J, Jones R. Assessment of clinical competence. Lancet. 2001;357(9260):945-949. PubMed
98. Arntfield RT. The utility of remote supervision with feedback as a method to deliver high-volume critical care ultrasound training. J Crit Care. 2015;30(2):441.e1-e6. PubMed
99. Akhtar S, Theodoro D, Gaspari R, et al. Resident training in emergency ultrasound: consensus recommendations from the 2008 Council of Emergency Residency Directors Conference. Acad Emerg Med. 2009;16:S32-S36. PubMed
100. Yu E. The assessment of technical skills in a cardiology training program: is the ITER sufficient? Can J Cardiol. 2000;16(4):457-462. PubMed
101. Todsen T, Tolsgaard MG, Olsen BH, et al. Reliable and valid assessment of point-of-care ultrasonography. Ann Surg. 2015;261(2):309-315. PubMed
102. Stein JC, Nobay F. Emergency department ultrasound credentialing: a sample policy and procedure. J Emerg Med. 2009;37(2):153-159. PubMed
103. Chen FM. Burstin H, Huntington J. The importance of clinical outcomes in medical education research. Med Educ. 2005;39(4):350-351. PubMed
104. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1:48-56. PubMed
105. ten Cate O. Nuts and bolts of entrustable professional activities. J Grad Med Educ. 2013;5(1):157-158. PubMed
106. Castillo J, Caruana CJ, Wainwright D. The changing concept of competence and categorisation of learning outcomes in Europe: Implications for the design of higher education radiography curricula at the European level. Radiography. 2011;17(3):230-234.
107. Goldstein SR. Accreditation, certification: why all the confusion? Obstet Gynecol. 2007;110(6):1396-1398. PubMed
108. Moore CL. Credentialing and reimbursement in point-of-care ultrasound. Clin Pediatr Emerg Med. 2011;12(1):73-77. PubMed
109. ten Cate O, Scheele F. Competency-based postgraduate training: can we bridge the gap between theory and clinical practice? Acad Med. 2007;82(6):542-547. PubMed
110. Abuhamad AZ, Benacerraf BR, Woletz P, Burke BL. The accreditation of ultrasound practices: impact on compliance with minimum performance guidelines. J Ultrasound Med. 2004;23(8):1023-1029. PubMed
1. American Board of Internal Medicine. Policies and procedures for certification. Philadelphia: American Board of Internal Medicine; 2006.
2. Nichani S, Fitterman N, Lukela M, Crocker J; Society of Hospital Medicine. The Core Competencies in Hospital Medicine 2017 Revision. Section 2: Procedures. J Hosp Med. 2017;12(4 Suppl 1):S44-S54 PubMed
3. Lucas BP, Asbury JK, Franco-Sadud R. Training future hospitalists with simulators: a needed step toward accessible, expertly performed bedside procedures. J Hosp Med. 2009;4(7):395-396. PubMed
4. Schnobrich DJ, Gladding S, Olson APJ, Duran-Nelson A. Point-of-care ultrasound in internal medicine: a national survey of educational leadership. J Grad Med Educ. 2013;5(3):498-502. PubMed
5. Brown GM, Otremba M, Devine LA, Gray C, Millington SJ, Ma IW. Defining competencies for ultrasound-guided bedside procedures: consensus opinions from Canadian physicians. J Ultrasound Med. 2016;35(1):129-141. PubMed
6. Vaisman A, Cram P. Procedural competence among faculty in academic health centers: challenges and future directions. Acad Med. 2017;92(1):31-34. PubMed
7. Kreisman RD. With ED ultrasound, credentialing is at issue. ED Legal Letter. 2010;21:102-103.
8. Goudie AM. Credentialing a new skill: what should the standard be for emergency department ultrasound in Australasia? Emerg Med Australas. 2010;22:263-264. PubMed
9. Maizel J, Guyomarc HL, Henon P, et al. Residents learning ultrasound-guided catheterization are not sufficiently skilled to use landmarks. Crit Care. 2014;18(1):R36. doi:10.1186/cc13741. PubMed
10. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care, and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. PubMed
11. Amini R, Adhikari S, Fiorello A. Ultrasound competency assessment in emergency medicine residency programs. Acad Emerg Med. 2014;21(7):799-801. PubMed
12. Jensen T, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. PubMed
13. Chang W. Is hospitalist proficiency in bedside procedures in decline? The Hospitalist. 2012. http://www.the-hospitalist.org/hospitalist/article/125236/patient-safety/hospitalist-proficiency-bedside-procedures-decline. Accessed September 30, 2017.
14. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties Performing Paracentesis Procedures at University Hospitals: Implications for Training and Certification. J Hosp Med. 2014;9(3):162-168. PubMed
15. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Residents’ Procedural Experience Does Not Ensure Competence: A Research Synthesis. J Grad Med Educ. 2017;9(2):201-208. PubMed
16. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. PubMed
17. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64(12):1303-1310. PubMed
18. Miller GE. The assessment of clinical skills/competence/performance. Acad Med. 1990;65(9 Suppl):S63-S67. PubMed
19. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
20. Millington SJ, Wong RY, Kassen BO, Roberts JM, Ma IWY. Improving internal medicine residents’ performance, knowledge, and confidence in central venous catheterization using simulators. J Hosp Med. 2009;4(7):410-416. PubMed
21. Lenchus JD, Carvalho CM, Ferreri K, et al. Filling the void: defining invasive bedside procedural competency for internal medicine residents. J Grad Med Educ. 2013;5(4):605-612. PubMed
22. Heegeman DJ, Kieke B Jr. Learning curves, credentialing, and the need for ultrasound fellowships. Acad Emerg Med. 2003;10:404-405. PubMed
23. Jang TB, Ruggeri W, Dyne P, Kaji AH. The learning curve of resident physicians using emergency ultrasonography for cholelithaisis and cholecystitis. Acad Emerg Med. 2010;17(11):1247-1252. PubMed
24. Akhtar MI, Hamid M. Ultrasound guided central venous access; a review of literature. Anaesth Pain Intensive Care. 2015;19:317-322.
25. Bahl A, Yunker A. Assessment of the numbers–based model for evaluation of resident competency in emergency ultrasound core applications. J Emerg Med Trauma Acute Care. 2015;2015(5). doi:10.5339/jemtac.2015.5
26. Cazes N, Desmots F, Geffroy Y, Renard A, Leyral J, Chaumoitre K. Emergency ultrasound: a prospective study on sufficient adequate training for military doctors. Diagn Interv Imaging. 2013;94(11):1109-1115. PubMed
27. Arntfield RT, Millington SJ, Ainsworth CD, et al. Canadian recommendations for critical care ultrasound training and competency for the Canadian critical care society. Can Respir J. 2014;21(16):341-345.
28. Bolsin S, Colson M. The use of the Cusum technique in the assessment of trainee competence in new procedures. Int J Qual Health Care. 2000;12(5):433-438. PubMed
29. de Oliveira Filho GR, Helayel PE, da Conceição DB, Garzel IS, Pavei P, Ceccon MS. Learning curves and mathematical models for interventional ultrasound basic skills. Anaesth Analg. 2008;106(2):568-573. PubMed
30. Starkie T, Drake EJ. Assessment of procedural skills training and performance in anesthesia using cumulative sum analysis (cusum). Can J Anaesth. 2013;60(12):1228-1239. PubMed
31. Tierney D. Competency cut-point identification derived from a mastery learning cohort approach: A hybrid model. Ultrasound Med Biol. 2015;41:S19.
32. Rankin JH, Elkhunovich MA, Rangarajan V, Chilstrom M, Mailhot T. Learning Curves for Ultrasound Assessment of Lumbar Puncture Insertion Sites: When is Competency Established? J Emerg Med. 2016;51(1):55-62. PubMed
33. Klasko SK, Cummings RV, Glazerman LR. Resident data collection: Do the numbers add up? Am J Obstet Gynecol. 1995;172(4 Pt 1):1312-1316. PubMed
34. Tierney D. Development & analysis of a mobile POCUS tracking tool. Ultrasound Med Biol. 2015;41(suppl 4):S31.
35. Sethi MV, Zimmer J, Ure B, Lacher M. Prospective assessment of complications on a daily basis is essential to determine morbidity and mortality in routine pediatric surgery. J Pediatr Surg. 2016;51(4):630-633. PubMed
36. Fisher JC, Kuenzler KA, Tomita SS, Sinha P, Shah P, Ginsburg HB. Increased capture of pediatric surgical complications utilizing a novel case-log web application to enhance quality improvement. J Pediatr Surg. 2017;52(1):166-171. PubMed
37. Rethans JJ, Norcini JJ, Barón-Maldonado M, et al. The relationship between competence and performance: implications for assessing practice performance. Med Educ. 2002;36(10):901-909. PubMed
38. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
39. Society of Critical Care Medicine Ultrasound Certification Task Force. Recommendations for achieving and maintaining competence and credentialing in critical care ultrasound with focused cardiac ultrasound and advanced critical care echocardiography. http://journals.lww.com/ccmjournal/Documents/Critical%20Care%20Ultrasound.pdf Published 2013. Accessed February 2, 2017.
40. Carraccio C, Wolfsthal SD, Englander R, Ferentz K, Martin C. Shifting paradigms: from Flexner to competencies. Acad Med. 2002;77(5):361-367. PubMed
41. Clark EG, Paparello JJ, Wayne DB, et al. Use of a national continuing medical education meeting to provide simulation-based training in temporary hemodialysis catheter insertion skills: a pre-test post-test study. Can J Kidney Health Dis. 2014;1:25-31. PubMed
42. Barsuk JH, Cohen ER, Caprio T, McGaghie WC, Simuni T, Wayne DB. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137. PubMed
43. Barsuk JH, McGaghie WC, Cohen ER, O’Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697-2701. PubMed
44. Davis DA, Mazmanian PE, Fordis M, Van Harrison R, Thorpe KE, Perrier L. Accuracy of physician self-assessment compared with observed measures of competence: a systematic review. JAMA. 2006;296(9):1094-1102. PubMed
45. Shah J, Darzi A. Surgical skills assessment: an ongoing debate. BJU Int. 2001;88(7):655-660. PubMed
46. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105-1117. PubMed
47. Tolsgaard MG, Todsen T, Sorensen JL, et al. International multispecialty consensus on how to evaluate ultrasound competence: a Delphi consensus survey. PLOS One. 2013;8(2):e57687. doi:10.1371/journal.pone.0057687 PubMed
48. Moureau N, Laperti M, Kelly LJ, et al. Evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training. Br J Anaesth. 2013;110(3):347-356. PubMed
49. Feldman LS, Hagarty S, Ghitulescu G, Stanbridge D, Fried GM. Relationship between objective assessment of technical skills and subjective in-training evaluations in surgical residents. J Am Coll Surg. 2004;198(1):105-110. PubMed
50. Baker S, Willey B, Mitchell C. The attempt to standardize technical and analytic competence in sonography education. J Diagn Med Sonogr. 2011;27(5):203-211.
51. Tolsgaard MG, Ringsted C, Dreisler E, et al. Reliable and valid assessment of ultrasound operator competence in obstetrics and gynecology. Ultrasound Obstet Gynecol. 2014;43(4):437-443. PubMed
52. Rice J, Crichlow A, Baker M, et al. An assessment tool for the placement of ultrasound-guided peripheral intravenous access. J Grad Med Educ. 2016;8(2):202-207. PubMed
53. Hartman N, Wittler M, Askew K, Hiestand B, Manthey D. Validation of a performance checklist for ultrasound-guided internal jubular central lines for use in procedural instruction and assessment. Postgrad Med J. 2017;93(1096):67-70. PubMed
54. Primdahl SC, Todsen T, Clemmesen L, et al. Rating scale for the assessment of competence in ultrasound-guided peripheral vascular access—a Delphi Consensus Study. J Vasc Access. 2016;17(5):440-445.
55. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
56. Berg K, Riesenberg LA, Berg D, et al. The development of a validated checklist for radial arterial line placement: preliminary results. Am J Med Qual. 2014;29(3):242-246. PubMed
57. Walzak A, Bacchus M, Schaefer MP, et al. Diagnosing technical competence in six bedside procedures: comparing checklists and a global rating scale in the assessment of resident performance. Acad Med. 2015;90(8):1100-1108. PubMed
58. Riesenberg LA, Berg K, Berg D, et al. The development of a validated checklist for femoral venous catheterization: preliminary results. Am J Med Qual. 2014;29(5):445-450. PubMed
59. Riesenberg LA, Berg K, Berg D, et al. The development of a validated checklist for paracentesis: preliminary results. Am J Med Qual. 2013;28(3):227-231. PubMed
60. Huang GC, Newman LR, Schwartzstein RM, et al. Procedural competence in internal medicine residents: validity of a central venous catheter insertion assessment instrument. Acad Med. 2009;84(8):1127-1134. PubMed
61. Salamonsen M, McGrath D, Steiler G, et al. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
62. Boniface K, Yarris LM. Emergency ultrasound: Leveling the training and assessment landscape. Acad Emerg Med. 2014;21(7):803-805. PubMed
63. Boyle E, O’Keeffe D, Naughton P, Hill A, McDonnell C, Moneley D. The importance of expert feedback during endovascular simulator training. J Vasc Surg. 2011;54(1):240-248.e1. PubMed
64. Langhan TS, Rigby IJ, Walker IW, Howes D, Donnon T, Lord JA. Simulation-based training in critical resuscitation procedures improves residents’ competence. CJEM. 2009;11(6):535-539. PubMed
65. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. PubMed
66. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
67. Barsuk JH, Cohen ER, Vozenilek JA, O’Connor LM, McGaghie WC, Wayne DB. Simulation-based education with mastery learning improves paracentesis skills. J Grad Med Educ. 2012;4(1):23-27. PubMed
68. McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706-711. PubMed
69. Ross JG. Simulation and psychomotor skill acquisition: A review of the literature. Clin Simul Nurs. 2012;8(9):e429-e435.
70. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
71. McSparron JI, Michaud GC, Gordan PL, et al. Simulation for skills-based education in pulmonary and critical care medicine. Ann Am Thorac Soc. 2015;12(4):579-586. PubMed
72. Kneebone RL, Scott W, Darzi A, Horrocks M. Simulation and clinical practice: strengthening the relationship. Med Educ. 2004;38(10):1095-1102. PubMed
73. Mema B, Harris I. The barriers and facilitators to transfer of ultrasound-guided central venous line skills from simulation to practice: exploring perceptions of learners and supervisors. Teach Learn Med. 2016;28(2):115-124. PubMed
74. Castanelli DJ. The rise of simulation in technical skills teaching and the implications for training novices in anaestheia. Anaesth Intensive Care. 2009;37(6):903-910. PubMed
75. McGaghie WC, Issenberg SB, Barsuk JH, Wayne DB. A critical review of simulation-based mastery learning with translational outcomes. Med Educ. 2014;48(4):375-385. PubMed
76. Langlois SLP. Focused ultrasound training for clinicians. Crit Care Med. 2007;35(5 suppl):S138-S143.
77. Price S, Via G, Sloth E, et al. Echocardiography practice, training and accreditation in the intesive care: document for the World Interactive Network Focused on Critical Ultrasound (WINFOCUS). Cardiovasc Ultrasound. 2008;6:49-83. PubMed
78. Blehar DJ, Barton B, Gaspari RJ. Learning curves in emergency ultrasound education. Acad Emerg Med. 2015;22(5):574-582. PubMed
79. Ault MJ, Rosen BT, Ault B. The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative. J Gen Intern Med. 2006;21(5):514-517. PubMed
80. Barsuk JH, Cohen ER, McGaghie WC, Wayne DB. Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med. 2010;85(10 Suppl):S9-S12. PubMed
81. Sliman Sean, Amundson S, Shaw D, Phan JN, Waalen J, Kimura B. Recently-acquired cardiac ultrasound skills are rapidly lost when not used: implications for competency in physician imaging. J Amer Coll Cardiol. 2016;67(13S):1569.
82. Kessler CS, Leone KA. The current state of core competency assessment in emergency medicine and a future research agenda: recommendations of the working group on assessment of observable learner performance. Acad Emerg Med. 2012;19(12):1354-1359. PubMed
83. Chang A, Schyve PM, Croteau RJ, O’Leary DS, Loeb JM. The JCAHO patient safety event taxonomy: a standardized terminology and classification schema for near misses and adverse events. Int J Qual Health Care. 2005;17(2):95-105. PubMed
84. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Acad Med. 2015;90(8):1025-1033. PubMed
85. Das D, Kapoor M, Brown C, Ndubuisi A, Gupta S. Current status of emergency department attending physician ultrasound credentialing and quality assurance in the United States. Crit Ultrasound J. 2016;8(1):6-12. PubMed
86. Ndubuisi AK, Gupta S, Brown C, Das D. Current status and future issues in emergency department attending physician ultrasound credentialing. Ann Emerg Med. 2014;64(45):S27-S28.
87. Tandy Tk, Hoffenberg S. Emergency department ultrasound services by emergency physicians: model for gaining hospital approval. Ann Emerg Med. 1997;29(3):367-374. PubMed
88. Lewiss RE, Saul T, Del Rios M. Acquiring credentials in bedside ultrasound: a cross-sectional survey. BMJ Open. 2013;3:e003502. doi:10.1136/bmjopen-2013-003502 PubMed
89. Lanoix R. Credentialing issues in emergency ultrasonography. Emerg Med Clin North Am. 1997;15(4):913-920. PubMed
90. Scalea T, Rodriquez A, Chiu WC, et al. Focused assessment with sonography for trauma (FAST): results from an international consensus conference. J Trauma. 1999;46(3):466-472. PubMed
91. Hertzberg BS, Kliewer MA, Bowie JD, et al. Physician training requirements in sonography: how many cases are needed for competence? AJR. 2000;174(5):1221-1227. PubMed
92. Blaivas M, Theodoro DL, Sierzenski P. Proliferation of ultrasound fellowships in emergency medicine: how do we ensure future experts are expertly trained? Acad Emerg Med. 2002;9(8):863-864. PubMed
93. Bodenham AR. Editorial II: Ultrasound imaging by anaesthetists: training and accreditation issues. Br J Anaesth. 2006;96(4):414-417. PubMed
94. Williamson JP, Twaddell SH, Lee YCG, et al. Thoracic ultrasound recognition of competence: A position paper of the Thoracic Society of Australia and New Zealand. Respirology. 2017;22(2):405-408. PubMed
95. Harrison G. Summative clinical competency assessment: a survey of ultrasound practitioners’ views. Ultrasound. 2015;23(1):11-17. PubMed
96. Evans LV, Morse JL, Hamann CJ, Osborne M, Lin Z, D'Onofrio G. The development of an independent rater system to assess residents' competence in invasive procedures. Acad Med. 2009;84(8):1135-1143. PubMed
97. Wass V, Van der Vleuten C, Shatzer J, Jones R. Assessment of clinical competence. Lancet. 2001;357(9260):945-949. PubMed
98. Arntfield RT. The utility of remote supervision with feedback as a method to deliver high-volume critical care ultrasound training. J Crit Care. 2015;30(2):441.e1-e6. PubMed
99. Akhtar S, Theodoro D, Gaspari R, et al. Resident training in emergency ultrasound: consensus recommendations from the 2008 Council of Emergency Residency Directors Conference. Acad Emerg Med. 2009;16:S32-S36. PubMed
100. Yu E. The assessment of technical skills in a cardiology training program: is the ITER sufficient? Can J Cardiol. 2000;16(4):457-462. PubMed
101. Todsen T, Tolsgaard MG, Olsen BH, et al. Reliable and valid assessment of point-of-care ultrasonography. Ann Surg. 2015;261(2):309-315. PubMed
102. Stein JC, Nobay F. Emergency department ultrasound credentialing: a sample policy and procedure. J Emerg Med. 2009;37(2):153-159. PubMed
103. Chen FM. Burstin H, Huntington J. The importance of clinical outcomes in medical education research. Med Educ. 2005;39(4):350-351. PubMed
104. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1:48-56. PubMed
105. ten Cate O. Nuts and bolts of entrustable professional activities. J Grad Med Educ. 2013;5(1):157-158. PubMed
106. Castillo J, Caruana CJ, Wainwright D. The changing concept of competence and categorisation of learning outcomes in Europe: Implications for the design of higher education radiography curricula at the European level. Radiography. 2011;17(3):230-234.
107. Goldstein SR. Accreditation, certification: why all the confusion? Obstet Gynecol. 2007;110(6):1396-1398. PubMed
108. Moore CL. Credentialing and reimbursement in point-of-care ultrasound. Clin Pediatr Emerg Med. 2011;12(1):73-77. PubMed
109. ten Cate O, Scheele F. Competency-based postgraduate training: can we bridge the gap between theory and clinical practice? Acad Med. 2007;82(6):542-547. PubMed
110. Abuhamad AZ, Benacerraf BR, Woletz P, Burke BL. The accreditation of ultrasound practices: impact on compliance with minimum performance guidelines. J Ultrasound Med. 2004;23(8):1023-1029. PubMed
© 2018 Society of Hospital Medicine
Pulmonary Perspectives® The Sun Should Never Set on an “Un-ultrasound-ed” Pleural Effusion
The adage, “the sun should never set on an untapped pleural effusion,” was instilled in physicians for generations. However, anyone who practices medicine currently knows the sun often rises and sets several times before a pleural effusion is tapped. Why the change in mindset? Since the American Board of Internal Medicine removed the requirement for internal medicine residents to perform a minimum number of bedside procedures for certification, fewer graduating residents feel comfortable performing thoracentesis.
Additionally, the fear of litigation and institutional persecution from a postprocedure complication has caused many frontline clinicians to shy away from performing thoracentesis. Most important, we now appreciate that not all pleural effusions need to be tapped immediately, and the clinical decision making about the timing and technique to drain a pleural effusion is more complex than previously thought.
In recent years, the availability of portable ultrasound for bedside diagnostics and procedural guidance has revolutionized the practice of medicine, including the management of pleural effusions. When confronted with an obscured lower lobe on chest radiograph (Figure, left), clinicians were previously relegated to primitive bedside maneuvers, such as percussion or auscultation, to make critical decisions about the clinical management. Now, clinicians are able to look inside the body with point-of-care ultrasound and visually assess a pleural effusion before making any decisions. Point-of-care ultrasound has shifted the paradigm in the management of pleural effusions in several ways.
Ultrasound allows rapid detection and differentiation of pleural effusions from other pathologic findings.
Chest radiographs cannot accurately differentiate a pleural effusion from other common conditions, such as pneumonia, atelectasis, or an elevated hemidiaphragm. Ultrasound is the only bedside diagnostic modality that can rapidly differentiate these conditions within seconds and may reveal unsuspected findings, such as a mass or pericardial effusion.
For example, the pleural ultrasound exam of a patient in the confirmed the presence of a large, left-sided pleural effusion (Figure, right) but also revealed an unsuspected large pericardial effusion (asterisk) that was causing hemodynamic compromise. The management of this patient shifted focus from the pleural effusion to the pericardial effusion, and urgent pericardiocentesis was performed. The sensitivity of ultrasound to detect a pleural effusion is proportional to the volume of fluid, reaching 100% with as little as 100 mL of fluid (Kalokairinou-Motogna et al. Med Ultra. 2010;12[1]:12). The diagnostic accuracy of ultrasound for detection of pleural effusions is comparable to CT scans of the chest and superior to portable chest radiographs (Lichtenstein et al. Anesthesiology. 2004;100[1]:9).
Ultrasound characterizes pleural effusions to determine the most appropriate management strategy.
Any clinician with basic ultrasonography skills can learn to evaluate pleural effusions and categorize them as simple or complex based on the sonographic appearance. Visualization of fibrinous stranding or loculations increases the probability of pleural fluid being exudative and often drives the decision to drain the fluid. The density and distribution of loculations can guide decisions about the most appropriate type of drainage procedure – thoracentesis versus tube thoracostomy versus surgical intervention. Use of color flow Doppler ultrasound allows clinicians to assess whether or not pleural fluid is free flowing and amenable to drainage, potentially saving the patient from an unnecessary attempt at drainage.
Ultrasound affords frontline clinicians the ability to streamline consultation with the most appropriate specialist based on the type of drainage procedure indicated and potentially prevent duplicate procedures on the same patient from different specialists.
Ultrasound reduces the risk of postprocedure complications from thoracentesis.
The risk of postthoracentesis pneumothorax was reported to be as high as 20%-39% before the routine use of point-of-care ultrasound (Grogan et al. Arch Int Med. 1990;150:873). Ultrasound guidance has been shown to increase procedural success rates and decrease the risk of postprocedure pneumothorax (2.7%), cost of hospitalization, and length of stay (Mercaldi et al. Chest. 2015;143[2]:532).
Regardless of the chest radiograph or CT scan findings, if the ultrasound exam reveals a scant volume of pleural fluid, or densely loculated pleural fluid, clinicians can avoid unnecessary attempts at bedside drainage, which likely partly accounts for the reduction in postprocedure pneumothorax. Use of ultrasound for needle site selection may prevent up to 10% of potential accidental organ punctures and increases accurate site selection by 26%, compared with chest radiograph and physical examination findings combined (Diacon et al. Chest. 2003;123:436).
Ultrasound facilitates patient-centered care.
Point-of-care ultrasound is the only new technology that has taken clinicians back to the bedside to spend more time with patients. Clinicians can simultaneously perform an ultrasound exam and converse with patients to gather a medical history. The ultrasound image serves as a tool to help patients understand their condition and facilitates shared decision making with clinicians at the bedside.
As more specialties have gained expertise in thoracic ultrasonography, the use of ultrasound guidance for thoracentesis has evolved to become the standard of care in many hospitals in the United States. Besides pulmonary specialists, several acute care specialists, including hospitalists, intensivists, and emergency medicine physicians, are routinely using point-of-care ultrasound to guide diagnostic decision making and procedures. Over the past 10 years, nearly a dozen procedure services led by internal medicine-trained hospitalists have been created at academic institutions that are routinely performing ultrasound-guided thoracenteses with low complication rates (Franco-Sadud et al. SGIM Forum. 2016;39[5]:13). More important, ultrasound is being used on the front lines to expeditiously evaluate pleural effusions and perform a diagnostic thoracentesis or consult with the appropriate subspecialist. Even though demonstration of competency in bedside procedures is no longer required for board certification in internal medicine, many internal medicine residency programs have incorporated diagnostic and procedural point-of-care ultrasound training into their education curriculum (Schnobrich et al. JGME. 2013;5[3]:498). Further, approximately 62% of medical schools report integrating ultrasound education in their medical student curriculum, and in coming years, most medical students will likely graduate with a basic skill set in point-of-care ultrasonography (Bahner et al. Academic Med. 2014;89[12]:1681). As point-of-care ultrasound education becomes integrated in training of physicians and other health-care providers, use of ultrasound to guide management of pleural effusions could become universally practiced and accepted as the new standard of care. Thus, it is plausible that a day will come in the near future when the sun will not set on an “un-ultrasound-ed” pleural effusion.
Dr. Franco-Sadud is with the section of hospital medicine/division of general internal medicine, Medical College of Wisconsin, Milwaukee, Wisconsin; Dr. Soni is with the section of hospital medicine and the section of pulmonary and critical care medicine, South Texas Veterans Health Care System and University of Texas Health Science Center, San Antonio.
The adage, “the sun should never set on an untapped pleural effusion,” was instilled in physicians for generations. However, anyone who practices medicine currently knows the sun often rises and sets several times before a pleural effusion is tapped. Why the change in mindset? Since the American Board of Internal Medicine removed the requirement for internal medicine residents to perform a minimum number of bedside procedures for certification, fewer graduating residents feel comfortable performing thoracentesis.
Additionally, the fear of litigation and institutional persecution from a postprocedure complication has caused many frontline clinicians to shy away from performing thoracentesis. Most important, we now appreciate that not all pleural effusions need to be tapped immediately, and the clinical decision making about the timing and technique to drain a pleural effusion is more complex than previously thought.
In recent years, the availability of portable ultrasound for bedside diagnostics and procedural guidance has revolutionized the practice of medicine, including the management of pleural effusions. When confronted with an obscured lower lobe on chest radiograph (Figure, left), clinicians were previously relegated to primitive bedside maneuvers, such as percussion or auscultation, to make critical decisions about the clinical management. Now, clinicians are able to look inside the body with point-of-care ultrasound and visually assess a pleural effusion before making any decisions. Point-of-care ultrasound has shifted the paradigm in the management of pleural effusions in several ways.
Ultrasound allows rapid detection and differentiation of pleural effusions from other pathologic findings.
Chest radiographs cannot accurately differentiate a pleural effusion from other common conditions, such as pneumonia, atelectasis, or an elevated hemidiaphragm. Ultrasound is the only bedside diagnostic modality that can rapidly differentiate these conditions within seconds and may reveal unsuspected findings, such as a mass or pericardial effusion.
For example, the pleural ultrasound exam of a patient in the confirmed the presence of a large, left-sided pleural effusion (Figure, right) but also revealed an unsuspected large pericardial effusion (asterisk) that was causing hemodynamic compromise. The management of this patient shifted focus from the pleural effusion to the pericardial effusion, and urgent pericardiocentesis was performed. The sensitivity of ultrasound to detect a pleural effusion is proportional to the volume of fluid, reaching 100% with as little as 100 mL of fluid (Kalokairinou-Motogna et al. Med Ultra. 2010;12[1]:12). The diagnostic accuracy of ultrasound for detection of pleural effusions is comparable to CT scans of the chest and superior to portable chest radiographs (Lichtenstein et al. Anesthesiology. 2004;100[1]:9).
Ultrasound characterizes pleural effusions to determine the most appropriate management strategy.
Any clinician with basic ultrasonography skills can learn to evaluate pleural effusions and categorize them as simple or complex based on the sonographic appearance. Visualization of fibrinous stranding or loculations increases the probability of pleural fluid being exudative and often drives the decision to drain the fluid. The density and distribution of loculations can guide decisions about the most appropriate type of drainage procedure – thoracentesis versus tube thoracostomy versus surgical intervention. Use of color flow Doppler ultrasound allows clinicians to assess whether or not pleural fluid is free flowing and amenable to drainage, potentially saving the patient from an unnecessary attempt at drainage.
Ultrasound affords frontline clinicians the ability to streamline consultation with the most appropriate specialist based on the type of drainage procedure indicated and potentially prevent duplicate procedures on the same patient from different specialists.
Ultrasound reduces the risk of postprocedure complications from thoracentesis.
The risk of postthoracentesis pneumothorax was reported to be as high as 20%-39% before the routine use of point-of-care ultrasound (Grogan et al. Arch Int Med. 1990;150:873). Ultrasound guidance has been shown to increase procedural success rates and decrease the risk of postprocedure pneumothorax (2.7%), cost of hospitalization, and length of stay (Mercaldi et al. Chest. 2015;143[2]:532).
Regardless of the chest radiograph or CT scan findings, if the ultrasound exam reveals a scant volume of pleural fluid, or densely loculated pleural fluid, clinicians can avoid unnecessary attempts at bedside drainage, which likely partly accounts for the reduction in postprocedure pneumothorax. Use of ultrasound for needle site selection may prevent up to 10% of potential accidental organ punctures and increases accurate site selection by 26%, compared with chest radiograph and physical examination findings combined (Diacon et al. Chest. 2003;123:436).
Ultrasound facilitates patient-centered care.
Point-of-care ultrasound is the only new technology that has taken clinicians back to the bedside to spend more time with patients. Clinicians can simultaneously perform an ultrasound exam and converse with patients to gather a medical history. The ultrasound image serves as a tool to help patients understand their condition and facilitates shared decision making with clinicians at the bedside.
As more specialties have gained expertise in thoracic ultrasonography, the use of ultrasound guidance for thoracentesis has evolved to become the standard of care in many hospitals in the United States. Besides pulmonary specialists, several acute care specialists, including hospitalists, intensivists, and emergency medicine physicians, are routinely using point-of-care ultrasound to guide diagnostic decision making and procedures. Over the past 10 years, nearly a dozen procedure services led by internal medicine-trained hospitalists have been created at academic institutions that are routinely performing ultrasound-guided thoracenteses with low complication rates (Franco-Sadud et al. SGIM Forum. 2016;39[5]:13). More important, ultrasound is being used on the front lines to expeditiously evaluate pleural effusions and perform a diagnostic thoracentesis or consult with the appropriate subspecialist. Even though demonstration of competency in bedside procedures is no longer required for board certification in internal medicine, many internal medicine residency programs have incorporated diagnostic and procedural point-of-care ultrasound training into their education curriculum (Schnobrich et al. JGME. 2013;5[3]:498). Further, approximately 62% of medical schools report integrating ultrasound education in their medical student curriculum, and in coming years, most medical students will likely graduate with a basic skill set in point-of-care ultrasonography (Bahner et al. Academic Med. 2014;89[12]:1681). As point-of-care ultrasound education becomes integrated in training of physicians and other health-care providers, use of ultrasound to guide management of pleural effusions could become universally practiced and accepted as the new standard of care. Thus, it is plausible that a day will come in the near future when the sun will not set on an “un-ultrasound-ed” pleural effusion.
Dr. Franco-Sadud is with the section of hospital medicine/division of general internal medicine, Medical College of Wisconsin, Milwaukee, Wisconsin; Dr. Soni is with the section of hospital medicine and the section of pulmonary and critical care medicine, South Texas Veterans Health Care System and University of Texas Health Science Center, San Antonio.
The adage, “the sun should never set on an untapped pleural effusion,” was instilled in physicians for generations. However, anyone who practices medicine currently knows the sun often rises and sets several times before a pleural effusion is tapped. Why the change in mindset? Since the American Board of Internal Medicine removed the requirement for internal medicine residents to perform a minimum number of bedside procedures for certification, fewer graduating residents feel comfortable performing thoracentesis.
Additionally, the fear of litigation and institutional persecution from a postprocedure complication has caused many frontline clinicians to shy away from performing thoracentesis. Most important, we now appreciate that not all pleural effusions need to be tapped immediately, and the clinical decision making about the timing and technique to drain a pleural effusion is more complex than previously thought.
In recent years, the availability of portable ultrasound for bedside diagnostics and procedural guidance has revolutionized the practice of medicine, including the management of pleural effusions. When confronted with an obscured lower lobe on chest radiograph (Figure, left), clinicians were previously relegated to primitive bedside maneuvers, such as percussion or auscultation, to make critical decisions about the clinical management. Now, clinicians are able to look inside the body with point-of-care ultrasound and visually assess a pleural effusion before making any decisions. Point-of-care ultrasound has shifted the paradigm in the management of pleural effusions in several ways.
Ultrasound allows rapid detection and differentiation of pleural effusions from other pathologic findings.
Chest radiographs cannot accurately differentiate a pleural effusion from other common conditions, such as pneumonia, atelectasis, or an elevated hemidiaphragm. Ultrasound is the only bedside diagnostic modality that can rapidly differentiate these conditions within seconds and may reveal unsuspected findings, such as a mass or pericardial effusion.
For example, the pleural ultrasound exam of a patient in the confirmed the presence of a large, left-sided pleural effusion (Figure, right) but also revealed an unsuspected large pericardial effusion (asterisk) that was causing hemodynamic compromise. The management of this patient shifted focus from the pleural effusion to the pericardial effusion, and urgent pericardiocentesis was performed. The sensitivity of ultrasound to detect a pleural effusion is proportional to the volume of fluid, reaching 100% with as little as 100 mL of fluid (Kalokairinou-Motogna et al. Med Ultra. 2010;12[1]:12). The diagnostic accuracy of ultrasound for detection of pleural effusions is comparable to CT scans of the chest and superior to portable chest radiographs (Lichtenstein et al. Anesthesiology. 2004;100[1]:9).
Ultrasound characterizes pleural effusions to determine the most appropriate management strategy.
Any clinician with basic ultrasonography skills can learn to evaluate pleural effusions and categorize them as simple or complex based on the sonographic appearance. Visualization of fibrinous stranding or loculations increases the probability of pleural fluid being exudative and often drives the decision to drain the fluid. The density and distribution of loculations can guide decisions about the most appropriate type of drainage procedure – thoracentesis versus tube thoracostomy versus surgical intervention. Use of color flow Doppler ultrasound allows clinicians to assess whether or not pleural fluid is free flowing and amenable to drainage, potentially saving the patient from an unnecessary attempt at drainage.
Ultrasound affords frontline clinicians the ability to streamline consultation with the most appropriate specialist based on the type of drainage procedure indicated and potentially prevent duplicate procedures on the same patient from different specialists.
Ultrasound reduces the risk of postprocedure complications from thoracentesis.
The risk of postthoracentesis pneumothorax was reported to be as high as 20%-39% before the routine use of point-of-care ultrasound (Grogan et al. Arch Int Med. 1990;150:873). Ultrasound guidance has been shown to increase procedural success rates and decrease the risk of postprocedure pneumothorax (2.7%), cost of hospitalization, and length of stay (Mercaldi et al. Chest. 2015;143[2]:532).
Regardless of the chest radiograph or CT scan findings, if the ultrasound exam reveals a scant volume of pleural fluid, or densely loculated pleural fluid, clinicians can avoid unnecessary attempts at bedside drainage, which likely partly accounts for the reduction in postprocedure pneumothorax. Use of ultrasound for needle site selection may prevent up to 10% of potential accidental organ punctures and increases accurate site selection by 26%, compared with chest radiograph and physical examination findings combined (Diacon et al. Chest. 2003;123:436).
Ultrasound facilitates patient-centered care.
Point-of-care ultrasound is the only new technology that has taken clinicians back to the bedside to spend more time with patients. Clinicians can simultaneously perform an ultrasound exam and converse with patients to gather a medical history. The ultrasound image serves as a tool to help patients understand their condition and facilitates shared decision making with clinicians at the bedside.
As more specialties have gained expertise in thoracic ultrasonography, the use of ultrasound guidance for thoracentesis has evolved to become the standard of care in many hospitals in the United States. Besides pulmonary specialists, several acute care specialists, including hospitalists, intensivists, and emergency medicine physicians, are routinely using point-of-care ultrasound to guide diagnostic decision making and procedures. Over the past 10 years, nearly a dozen procedure services led by internal medicine-trained hospitalists have been created at academic institutions that are routinely performing ultrasound-guided thoracenteses with low complication rates (Franco-Sadud et al. SGIM Forum. 2016;39[5]:13). More important, ultrasound is being used on the front lines to expeditiously evaluate pleural effusions and perform a diagnostic thoracentesis or consult with the appropriate subspecialist. Even though demonstration of competency in bedside procedures is no longer required for board certification in internal medicine, many internal medicine residency programs have incorporated diagnostic and procedural point-of-care ultrasound training into their education curriculum (Schnobrich et al. JGME. 2013;5[3]:498). Further, approximately 62% of medical schools report integrating ultrasound education in their medical student curriculum, and in coming years, most medical students will likely graduate with a basic skill set in point-of-care ultrasonography (Bahner et al. Academic Med. 2014;89[12]:1681). As point-of-care ultrasound education becomes integrated in training of physicians and other health-care providers, use of ultrasound to guide management of pleural effusions could become universally practiced and accepted as the new standard of care. Thus, it is plausible that a day will come in the near future when the sun will not set on an “un-ultrasound-ed” pleural effusion.
Dr. Franco-Sadud is with the section of hospital medicine/division of general internal medicine, Medical College of Wisconsin, Milwaukee, Wisconsin; Dr. Soni is with the section of hospital medicine and the section of pulmonary and critical care medicine, South Texas Veterans Health Care System and University of Texas Health Science Center, San Antonio.