User login
Shining a Light to Reduce Hospital Falls
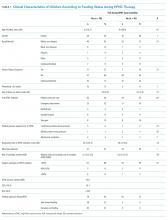
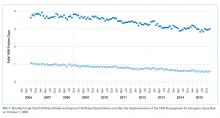
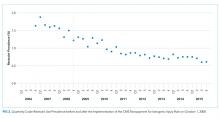
Fall prevention strategies for hospitalized older adults include environmental factors such as adequate room lighting and patient-specific factors such as medications. In 2008, the Centers for Medicare & Medicaid Services (CMS) implemented a regulatory “shining of the light” on hospital-acquired falls by eliminating hospital payment for fall-related injuries. Shorr et al. found that implementation of the CMS Hospital-Acquired Conditions Initiative was associated with only a modest decline in falls and injurious falls over the first seven years, with the greatest reduction occurring in urban, teaching hospitals.1 These disappointing findings were mitigated only by the finding that the prevalence of physical restraints decreased over the seven years of observation from 1.6% to 0.6%, suggesting that the modest reductions in falls did not occur at the expense of further restricting the mobility of hospitalized older adults. Shorr et al. concluded that falls may be largely attributable to individual patient risk and may not be prevented through health system quality and safety programs such as those that have achieved successes in never-events, including wrong-side surgery and catheter-associated blood stream infections.2 The authors expressed concern that hospital leaders remain in the dark regarding proven fall prevention strategies. They question whether hospital-acquired falls are preventable without restricting the mobility of older adults most at risk for falls.
Hoff et al. found in their 2011 literature review of the first three years following implementation of the 2008 CMS hospital payment polices limited evidence-based approaches to address falls as a spotlighted avoidable hospital-acquired condition.3 Swartzell et al. reported that at some level, every patient admitted to an acute care hospital is at risk for falls. “Patients sick enough to be in the hospital have underlying disease, are receiving physiologically altering medications and treatments, and are likely experiencing pain, fatigue, anxiety, sleep disturbance, and other symptoms that interfere with cognitive and physical functioning. The key to preventing falls among hospitalized patients may lie in addressing how the hospital environment creates risk.”4
In 2017, Avanecean et al. published a systematic review of randomized control trials on fall prevention in hospitals.5 Three of five studies demonstrated 20%-30% reductions in fall rates, whereas two studies showed no difference in fall rates among control and intervention groups. In the three studies that demonstrated reduced fall rates, standardized fall risk assessments were used to identify patient-specific risks for falls. Individualized care plans addressed gait and balance disorders, delirium and cognitive deficits, vision and hearing impairments, and toileting needs. For example, physical therapists provided instruction on the safe use of walkers for those with gait and balance disorders. Patients with delirium and cognitive deficits received some form of staff alert of unsupervised transfers out of bed, ranging from bed alarms to customized rubber socks that contained pressure alarms. All three successful intervention studies included patient-centered care plans for toileting.
None of the three studies that measured the secondary outcome of fall-related injuries demonstrated impact of interventions, although the rates of injurious falls were low in both the control and intervention groups (2%-5%).3-5
Since the 2008 CMS policies eliminated hospital payments for complications of falls, patient-centered models of fall risk reduction were widely implemented. The Systems Addressing Frail Elder (SAFE) Care, designed by Ansryan et al. includes nursing, social work, pharmacist, and medical provider assessments.6 Team huddles occur daily to establish individualized care plans, although as Shorr et al. highlight, without report of outcomes.2 Nurses Improving Care for Healthsystem Elders (NICHE) is an New York University-based nursing education and consultation program that has extended to 566 healthcare organizations.7 Factors that promote the adoption of organizational interventions such as NICHE have been identified.8 The findings that NICHE is adopted more in larger, urban healthcare systems are consistent with the findings reported by Shorr et al. that fall rate reductions were greater in such hospital settings. Patient-centered care, although time-consuming, may promote staff satisfaction and is associated with reductions in other hospital-acquired conditions such as delirium.9
Patient-engaged video surveillance systems are recent technological solutions to reduce falls. One staff monitors multiple patients for behaviors that risk falls such as unsupervised transfers out of bed. Staff can speak to a patient through the monitoring system to request the patient to wait for assistance, while the unit staff are alerted to the fall risk. Bedside caregivers can activate virtual privacy screens during personal patient care.
Shorr et al. appropriately call for studies to further illuminate strategies to reduce hospital-acquired falls. A multihospital report of fall rates before and after the implementation of SAFE Care and NICHE would have sufficient scale to address the impact of these patient-centered interventions on injurious falls. Similarly, patient-engaged video surveillance systems need validation from clinical trials.
1. Shorr RI, Staggs VS, Waters TM, et al. Impact of the hospital-acquired conditions initiative on falls and physical restraints: a longitudinal study. J Hosp Med. 2019;14:E31-E36. https://doi.org/10.12788/jhm.3295.
2. Austin JM, Demski R, Callender T, et al. From board to bedside: how the application of financial structures to safety and quality can drive accountability in a large health care system. Jt Comm J Qual Patient Saf. 2017;43(4):166-175. https://doi.org/10.1016/j.jcjq.2017.01.001.
3. Hoff TJ, Soerensen C. No payment for preventable complications: reviewing the early literature for content, guidance, and impressions. Qual Manag Health Care. 2011;20(1):62-75. https://doi.org/10.1097/QMH.0b013e31820311d2.
4. Swartzell KL, Fulton JS, Friesth BM. Relationship between occurrence of falls and fall-risk scores in an acute care setting using the Hendrich II fall risk model. Medsurg Nurs. 2013;22(3):180-187.
5. Avanecean D, Calliste D, Contreras T, Lim Y, Fitzpatrick A. Effectiveness of patient-centered interventions on falls in the acute care setting compared to usual care: a systematic review. JBI Database System Rev Implement Rep. 2017;15(12): 3006-3048. https://doi.org/10.11124/JBISRIR-2016-003331.
6. Ansryan LZ1, Aronow HU, Borenstein JE, et al. Systems addressing frail elder care: description of a successful model. J Nurs Adm. 2018;48(1):11-17. https://doi.org/10.1097/NNA.0000000000000564.
7. Boltz M1, Capezuti E, Bowar-Ferres S, et al. Changes in the geriatric care environment associated with NICHE (Nurses Improving Care for HealthSystem Elders). Geriatr Nurs. 2008;29(3):176-185. https://doi.org/10.1016/j.gerinurse.2008.02.002.
8. Stimpfel AW1, Gilmartin MJ. Factors predicting adoption of the nurses improving care of healthsystem elders program. Nurs Res. 2019;68(1):13-21. https://doi.org/10.1097/NNR.0000000000000327.
9.
Fall prevention strategies for hospitalized older adults include environmental factors such as adequate room lighting and patient-specific factors such as medications. In 2008, the Centers for Medicare & Medicaid Services (CMS) implemented a regulatory “shining of the light” on hospital-acquired falls by eliminating hospital payment for fall-related injuries. Shorr et al. found that implementation of the CMS Hospital-Acquired Conditions Initiative was associated with only a modest decline in falls and injurious falls over the first seven years, with the greatest reduction occurring in urban, teaching hospitals.1 These disappointing findings were mitigated only by the finding that the prevalence of physical restraints decreased over the seven years of observation from 1.6% to 0.6%, suggesting that the modest reductions in falls did not occur at the expense of further restricting the mobility of hospitalized older adults. Shorr et al. concluded that falls may be largely attributable to individual patient risk and may not be prevented through health system quality and safety programs such as those that have achieved successes in never-events, including wrong-side surgery and catheter-associated blood stream infections.2 The authors expressed concern that hospital leaders remain in the dark regarding proven fall prevention strategies. They question whether hospital-acquired falls are preventable without restricting the mobility of older adults most at risk for falls.
Hoff et al. found in their 2011 literature review of the first three years following implementation of the 2008 CMS hospital payment polices limited evidence-based approaches to address falls as a spotlighted avoidable hospital-acquired condition.3 Swartzell et al. reported that at some level, every patient admitted to an acute care hospital is at risk for falls. “Patients sick enough to be in the hospital have underlying disease, are receiving physiologically altering medications and treatments, and are likely experiencing pain, fatigue, anxiety, sleep disturbance, and other symptoms that interfere with cognitive and physical functioning. The key to preventing falls among hospitalized patients may lie in addressing how the hospital environment creates risk.”4
In 2017, Avanecean et al. published a systematic review of randomized control trials on fall prevention in hospitals.5 Three of five studies demonstrated 20%-30% reductions in fall rates, whereas two studies showed no difference in fall rates among control and intervention groups. In the three studies that demonstrated reduced fall rates, standardized fall risk assessments were used to identify patient-specific risks for falls. Individualized care plans addressed gait and balance disorders, delirium and cognitive deficits, vision and hearing impairments, and toileting needs. For example, physical therapists provided instruction on the safe use of walkers for those with gait and balance disorders. Patients with delirium and cognitive deficits received some form of staff alert of unsupervised transfers out of bed, ranging from bed alarms to customized rubber socks that contained pressure alarms. All three successful intervention studies included patient-centered care plans for toileting.
None of the three studies that measured the secondary outcome of fall-related injuries demonstrated impact of interventions, although the rates of injurious falls were low in both the control and intervention groups (2%-5%).3-5
Since the 2008 CMS policies eliminated hospital payments for complications of falls, patient-centered models of fall risk reduction were widely implemented. The Systems Addressing Frail Elder (SAFE) Care, designed by Ansryan et al. includes nursing, social work, pharmacist, and medical provider assessments.6 Team huddles occur daily to establish individualized care plans, although as Shorr et al. highlight, without report of outcomes.2 Nurses Improving Care for Healthsystem Elders (NICHE) is an New York University-based nursing education and consultation program that has extended to 566 healthcare organizations.7 Factors that promote the adoption of organizational interventions such as NICHE have been identified.8 The findings that NICHE is adopted more in larger, urban healthcare systems are consistent with the findings reported by Shorr et al. that fall rate reductions were greater in such hospital settings. Patient-centered care, although time-consuming, may promote staff satisfaction and is associated with reductions in other hospital-acquired conditions such as delirium.9
Patient-engaged video surveillance systems are recent technological solutions to reduce falls. One staff monitors multiple patients for behaviors that risk falls such as unsupervised transfers out of bed. Staff can speak to a patient through the monitoring system to request the patient to wait for assistance, while the unit staff are alerted to the fall risk. Bedside caregivers can activate virtual privacy screens during personal patient care.
Shorr et al. appropriately call for studies to further illuminate strategies to reduce hospital-acquired falls. A multihospital report of fall rates before and after the implementation of SAFE Care and NICHE would have sufficient scale to address the impact of these patient-centered interventions on injurious falls. Similarly, patient-engaged video surveillance systems need validation from clinical trials.
Fall prevention strategies for hospitalized older adults include environmental factors such as adequate room lighting and patient-specific factors such as medications. In 2008, the Centers for Medicare & Medicaid Services (CMS) implemented a regulatory “shining of the light” on hospital-acquired falls by eliminating hospital payment for fall-related injuries. Shorr et al. found that implementation of the CMS Hospital-Acquired Conditions Initiative was associated with only a modest decline in falls and injurious falls over the first seven years, with the greatest reduction occurring in urban, teaching hospitals.1 These disappointing findings were mitigated only by the finding that the prevalence of physical restraints decreased over the seven years of observation from 1.6% to 0.6%, suggesting that the modest reductions in falls did not occur at the expense of further restricting the mobility of hospitalized older adults. Shorr et al. concluded that falls may be largely attributable to individual patient risk and may not be prevented through health system quality and safety programs such as those that have achieved successes in never-events, including wrong-side surgery and catheter-associated blood stream infections.2 The authors expressed concern that hospital leaders remain in the dark regarding proven fall prevention strategies. They question whether hospital-acquired falls are preventable without restricting the mobility of older adults most at risk for falls.
Hoff et al. found in their 2011 literature review of the first three years following implementation of the 2008 CMS hospital payment polices limited evidence-based approaches to address falls as a spotlighted avoidable hospital-acquired condition.3 Swartzell et al. reported that at some level, every patient admitted to an acute care hospital is at risk for falls. “Patients sick enough to be in the hospital have underlying disease, are receiving physiologically altering medications and treatments, and are likely experiencing pain, fatigue, anxiety, sleep disturbance, and other symptoms that interfere with cognitive and physical functioning. The key to preventing falls among hospitalized patients may lie in addressing how the hospital environment creates risk.”4
In 2017, Avanecean et al. published a systematic review of randomized control trials on fall prevention in hospitals.5 Three of five studies demonstrated 20%-30% reductions in fall rates, whereas two studies showed no difference in fall rates among control and intervention groups. In the three studies that demonstrated reduced fall rates, standardized fall risk assessments were used to identify patient-specific risks for falls. Individualized care plans addressed gait and balance disorders, delirium and cognitive deficits, vision and hearing impairments, and toileting needs. For example, physical therapists provided instruction on the safe use of walkers for those with gait and balance disorders. Patients with delirium and cognitive deficits received some form of staff alert of unsupervised transfers out of bed, ranging from bed alarms to customized rubber socks that contained pressure alarms. All three successful intervention studies included patient-centered care plans for toileting.
None of the three studies that measured the secondary outcome of fall-related injuries demonstrated impact of interventions, although the rates of injurious falls were low in both the control and intervention groups (2%-5%).3-5
Since the 2008 CMS policies eliminated hospital payments for complications of falls, patient-centered models of fall risk reduction were widely implemented. The Systems Addressing Frail Elder (SAFE) Care, designed by Ansryan et al. includes nursing, social work, pharmacist, and medical provider assessments.6 Team huddles occur daily to establish individualized care plans, although as Shorr et al. highlight, without report of outcomes.2 Nurses Improving Care for Healthsystem Elders (NICHE) is an New York University-based nursing education and consultation program that has extended to 566 healthcare organizations.7 Factors that promote the adoption of organizational interventions such as NICHE have been identified.8 The findings that NICHE is adopted more in larger, urban healthcare systems are consistent with the findings reported by Shorr et al. that fall rate reductions were greater in such hospital settings. Patient-centered care, although time-consuming, may promote staff satisfaction and is associated with reductions in other hospital-acquired conditions such as delirium.9
Patient-engaged video surveillance systems are recent technological solutions to reduce falls. One staff monitors multiple patients for behaviors that risk falls such as unsupervised transfers out of bed. Staff can speak to a patient through the monitoring system to request the patient to wait for assistance, while the unit staff are alerted to the fall risk. Bedside caregivers can activate virtual privacy screens during personal patient care.
Shorr et al. appropriately call for studies to further illuminate strategies to reduce hospital-acquired falls. A multihospital report of fall rates before and after the implementation of SAFE Care and NICHE would have sufficient scale to address the impact of these patient-centered interventions on injurious falls. Similarly, patient-engaged video surveillance systems need validation from clinical trials.
1. Shorr RI, Staggs VS, Waters TM, et al. Impact of the hospital-acquired conditions initiative on falls and physical restraints: a longitudinal study. J Hosp Med. 2019;14:E31-E36. https://doi.org/10.12788/jhm.3295.
2. Austin JM, Demski R, Callender T, et al. From board to bedside: how the application of financial structures to safety and quality can drive accountability in a large health care system. Jt Comm J Qual Patient Saf. 2017;43(4):166-175. https://doi.org/10.1016/j.jcjq.2017.01.001.
3. Hoff TJ, Soerensen C. No payment for preventable complications: reviewing the early literature for content, guidance, and impressions. Qual Manag Health Care. 2011;20(1):62-75. https://doi.org/10.1097/QMH.0b013e31820311d2.
4. Swartzell KL, Fulton JS, Friesth BM. Relationship between occurrence of falls and fall-risk scores in an acute care setting using the Hendrich II fall risk model. Medsurg Nurs. 2013;22(3):180-187.
5. Avanecean D, Calliste D, Contreras T, Lim Y, Fitzpatrick A. Effectiveness of patient-centered interventions on falls in the acute care setting compared to usual care: a systematic review. JBI Database System Rev Implement Rep. 2017;15(12): 3006-3048. https://doi.org/10.11124/JBISRIR-2016-003331.
6. Ansryan LZ1, Aronow HU, Borenstein JE, et al. Systems addressing frail elder care: description of a successful model. J Nurs Adm. 2018;48(1):11-17. https://doi.org/10.1097/NNA.0000000000000564.
7. Boltz M1, Capezuti E, Bowar-Ferres S, et al. Changes in the geriatric care environment associated with NICHE (Nurses Improving Care for HealthSystem Elders). Geriatr Nurs. 2008;29(3):176-185. https://doi.org/10.1016/j.gerinurse.2008.02.002.
8. Stimpfel AW1, Gilmartin MJ. Factors predicting adoption of the nurses improving care of healthsystem elders program. Nurs Res. 2019;68(1):13-21. https://doi.org/10.1097/NNR.0000000000000327.
9.
1. Shorr RI, Staggs VS, Waters TM, et al. Impact of the hospital-acquired conditions initiative on falls and physical restraints: a longitudinal study. J Hosp Med. 2019;14:E31-E36. https://doi.org/10.12788/jhm.3295.
2. Austin JM, Demski R, Callender T, et al. From board to bedside: how the application of financial structures to safety and quality can drive accountability in a large health care system. Jt Comm J Qual Patient Saf. 2017;43(4):166-175. https://doi.org/10.1016/j.jcjq.2017.01.001.
3. Hoff TJ, Soerensen C. No payment for preventable complications: reviewing the early literature for content, guidance, and impressions. Qual Manag Health Care. 2011;20(1):62-75. https://doi.org/10.1097/QMH.0b013e31820311d2.
4. Swartzell KL, Fulton JS, Friesth BM. Relationship between occurrence of falls and fall-risk scores in an acute care setting using the Hendrich II fall risk model. Medsurg Nurs. 2013;22(3):180-187.
5. Avanecean D, Calliste D, Contreras T, Lim Y, Fitzpatrick A. Effectiveness of patient-centered interventions on falls in the acute care setting compared to usual care: a systematic review. JBI Database System Rev Implement Rep. 2017;15(12): 3006-3048. https://doi.org/10.11124/JBISRIR-2016-003331.
6. Ansryan LZ1, Aronow HU, Borenstein JE, et al. Systems addressing frail elder care: description of a successful model. J Nurs Adm. 2018;48(1):11-17. https://doi.org/10.1097/NNA.0000000000000564.
7. Boltz M1, Capezuti E, Bowar-Ferres S, et al. Changes in the geriatric care environment associated with NICHE (Nurses Improving Care for HealthSystem Elders). Geriatr Nurs. 2008;29(3):176-185. https://doi.org/10.1016/j.gerinurse.2008.02.002.
8. Stimpfel AW1, Gilmartin MJ. Factors predicting adoption of the nurses improving care of healthsystem elders program. Nurs Res. 2019;68(1):13-21. https://doi.org/10.1097/NNR.0000000000000327.
9.
© 2020 Society of Hospital Medicine DOI 10.12788/jhm.3345
Hospital Medicine Update: High-Impact Literature from March 2018 to April 2019
Given the breadth and depth of patients cared for by hospital medicine providers, it is challenging to remain current with the literature. The authors critically appraised the literature from March 2018 to April 2019 for high-quality studies relevant to hospital medicine. Articles were selected based on methodologic rigor and likelihood to impact clinical practice. Thirty articles were selected by the presenting authors for the Hospital Medicine Updates at the 2019 Society of Hospital Medicine (CH, CM) and Society of General Internal Medicine Annual Meetings (BS, AB). After two sequential rounds of voting and group discussion to adjudicate voting discrepancies, the authors selected the 10 most impactful articles for this review. Each article is described below with the key points summarized in the Table.
ESSENTIAL PUBLICATIONS
Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). McDonald LC, et al. Clin Infect Dis. 2018;66(7):e1–e48.1
Background. In the United States, approximately 500,000 Clostridioides difficile infections (CDI) occur annually with 15,000-30,000 deaths. CDI has become a marker of hospital quality and has been placed under numerous “pay for performance” metrics. The Infectious Diseases Society of America/Society of Healthcare Epidemiology of America updated their guidelines from 2010 regarding hospital surveillance, diagnostic testing, treatment, and infection precautions and control.
Findings. The panel included 14 multidisciplinary experts in epidemiology, diagnosis, infection control, and clinical management of adult and pediatric CDI. They used problem intervention comparison-outcome (PICO)-formatted, evidence-based questions. The selection of data and final recommendations were made in accordance with the GRADE criteria. A total of 35 recommendations were made.
Key clinical recommendations for hospitalists caring for adults: (1) Prescribe vancomycin or fidaxomicin over metronidazole for the initial treatment of CDI (strong recommendation, high quality of evidence); (2) Limit testing to the patients with unexplained new onset diarrhea, which is defined as greater than or equal to 3 unformed stools in 24 hours (weak recommendation, very low-quality evidence); (3) Avoid routine repeat testing within seven days, and only test asymptomatic patients for epidemiologic reasons (strong recommendation, moderate-quality evidence); (4) Minimize the frequency and duration of high-risk antibiotic therapy and the number of antibiotic agents prescribed (strong recommendation, moderate quality of evidence); (5) Discontinue therapy with the inciting antibiotic agent as soon as possible (strong recommendation, moderate quality of evidence).
Caveats. As with the clinical application of any guidelines, individual case adjustments may be required.
Implications. Vancomycin or fidaxomicin should be used for the initial episode of CDI instead of metronidazole.
Mortality and Morbidity in Acutely Ill Adults Treated with Liberal versus Conservative Oxygen Therapy (IOTA): a Systematic Review and Meta-analysis. Chu DK, et al. Lancet. 2018;391(10131):1693-1705.2
Background. Supplemental oxygen is often given to acutely ill hospitalized adults, even when they are not hypoxic or dyspneic. The safety and efficacy of this practice is unknown.
Findings. This systematic review and meta-analysis evaluated 25 randomized controlled trials enrolling 16,037 patients. Patients presented with several conditions, including sepsis, critical illness, stroke, myocardial infarction, and emergency surgery. The fraction of inspired oxygen in the liberal arms varied from 30% to 100%. Most patients randomized to the conservative arm received no supplemental oxygen. Delivery of liberal oxygen to acutely ill adults was associated with increased in-hospital mortality (relative risk [RR]: 1.21; 95% CI: 1.03-1.43), 30-day mortality (RR: 1.14; 95% CI: 1.01-1.29), and 90-day mortality (RR: 1.10; 95% CI: 1.00-1.20). The results were believed to be of high quality and were robust across multiple sensitivity analyses. It seemed that the mortality began to increase when supplemental oxygen raised the peripheral oxygen saturation (Sp02) above a range of 94%-96%.
Caveats. Heterogeneity was observed in the study settings and oxygen delivery. In addition, the cause for increased mortality could not be determined.
Implications. In hospitalized acutely ill adults, “liberal” supplemental oxygen was associated with increased in-hospital and longer-term mortality. The study authors postulated that this finding resulted from the direct toxic effects of oxygen or that oxygen delivery may “mask” illness and lead to delays in diagnosis and treatment. A subsequent clinical practice guideline recommends (1) a target SpO2 of less than 96% for patients receiving oxygen therapy; (2) a target SpO2 range of 90%-94% seems appropriate for most hospitalized adults.3
Do Words Matter? Stigmatizing Language and the Transmission of Bias in the Medical Record. P Goddu A, et al. J Gen Intern Med. 2018;33(5):68-91.4
Background. Previous work has shown that clinician bias affects health outcomes, often worsening health disparities. It is unknown whether clinicians’ language in medical records biases other clinicians and whether this affects patients.
Findings. The investigators randomized medical students and residents in internal and emergency medicine at one academic medical center to review one of two vignettes in the format of notes on the same hypothetical patient with sickle cell disease (SCD) admitted with a pain crisis. One vignette contained stigmatizing language, and the other contained neutral language. The trainees exposed to the vignettes with stigmatizing language showed a more negative attitude toward the patient, as measured by a previously validated scale of attitudes toward patients with SCD (20.6 stigmatizing vs 25.6 neutral, with a total score range of 7-35 for the instrument; higher scores indicate more positive attitudes; P < .001). Furthermore, the intensity of pain treatment was assessed in the resident group and was less aggressive when residents were exposed to stigmatizing language (5.56 stigmatizing vs 6.22 neutral on a scale of 2-7, with higher scores indicating more aggressive pain treatment; P = .003).
Cautions. This research was a single-center study of residents and medical students in two departments. Additionally, the study used vignettes on a hypothetical patient so trainees in the study group might have witnessed stronger stigmatizing language than what is typically observed in an actual patients’ notes.
Implications. Stigmatizing language used in medical records possibly contributed to health disparities by negatively impacting other physicians’ biases and prescribing practices toward patients with SCD at an academic medical center. Clinicians should avoid stigmatizing language in medical records.
Catheter Ablation for Atrial Fibrillation with Heart Failure. Marrouche, NF et al. New Engl J Med. 2018;378:417-427.5
Background. Atrial fibrillation (AF) in patients with heart failure is associated with increased mortality and morbidity. Small-scale studies have suggested that ablation of AF may benefit patients with heart failure.
Findings. This multicenter trial included 398 patients with heart failure and symptomatic AF. Patients had New York Heart Association Class II-IV heart failure, an ejection fraction (EF) of 35% or less, and an internal cardiac defibrillator (ICD). Patients were randomized to either ablation or medical therapy. All enrolled patients either refused, failed, or showed poor tolerance to antiarrhythmic therapy for AF. The primary outcome was death from any cause or hospitalization for heart failure.
The composite endpoint occurred in 28.5% of the ablation group versus 44.6% of patients in the medical therapy group (hazard ratio [HR]: 0.62; 95% CI: 0.43-0.87). Fewer patients in the ablation group died (13% vs 25%; HR: 0.53; 95% CI: 0.32-0.86) or were hospitalized for heart failure (21% vs 36%; HR: 0.56; 95% CI: 0.37-0.83). The patients in the ablation group had higher EF increases above baseline and a greater proportion were in sinus rhythm at the 60-month follow-up visit.
Cautions. The trial was terminated early due to slow recruitment and lower than expected events. Over twice as many patients were lost to follow-up in the ablation group versus the medical therapy group, and by 60 months, AF recurred in 50% of patients who underwent ablation. The sample size was small, and the trial was unblinded.
Implications. Ablation should be considered for AF in patients with heart failure. Additional studies to evaluate ablation versus medical therapy for patients with heart failure and AF are underway.
Medication for Opioid Use Disorder after Nonfatal Opioid Overdose and Association with Mortality. Larochelle MR, et al. Ann Intern Med. 2018;169(3):137-145.6
Background. More than 70,000 Americans died of drug overdose in 2017; this number is higher than the deaths resulting from human immunodeficiency virus, car crash, or gun violence at their peaks.7 Methadone, buprenorphine, and naltrexone are approved by the Federal Drug Administration for the treatment of opioid use disorder (OUD). These medications increase treatment retention; methadone and buprenorphine have been associated with significant decreases in all-cause and overdose mortality.8 However, whether receipt of these medications following a nonfatal opioid overdose reduces mortality is unknown.
Findings. This retrospective cohort study included 17,568 opioid overdose survivors from the Massachusetts’s Public Health Dataset between 2012 and 2014. Only three in 10 of these patients received any medications for OUD over 12 months following overdose. All-cause mortality was 4.7 deaths (95% CI: 4.4-5.0 deaths) per 100 person-years. The relative risk for all-cause mortality was 53% lower with methadone (adjusted hazard ratio [aHR]: 0.47; 95% CI: 0.32-0.71) and 37% lower with buprenorphine (aHR: 0.63; 95% CI: 0.46-0.87).
Caveats. This cohort study may have missed confounders explaining why certain patients received medications for OUD. As a result, association cannot be interpreted as causation.
Implications. Methadone and buprenorphine are associated with a reduction in preventable deaths in patients with OUD who have survived an overdose. All patients with OUD should be considered for therapy.
Outcomes Associated with Apixaban Use in Patients with End-Stage Kidney Disease and Atrial Fibrillation in the United States. Siontis, KC, et al. Circulation. 2018;138:1519–1529.9
Background. Patients with end-stage kidney disease (ESKD) have poor outcomes when treated with warfarin for AF. These patients were excluded from clinical trials of direct oral anticoagulants. The goal of this study was to determine the outcomes of the use of apixaban in patients with ESKD and AF.
Findings. This retrospective cohort study included 25,523 Medicare patients with ESKD and AF on anticoagulants. A 3:1 propensity score match was performed between patients on warfarin and apixaban. Time without stroke/systemic embolism, bleeding (major, gastrointestinal, and intracranial), and death were assessed. A total of 2,351 patients were on apixaban, and 23,172 patients were on warfarin. No difference was observed in the risk of stroke/systemic embolism between apixaban and warfarin (HR 0.88; 95% CI: 0.69-1.12). Apixaban was associated with a lower risk of major bleeding (HR: 0.72; 95% CI: 0.59-0.87). Standard-dose apixaban (5 mg twice a day) was associated with lower risks of stroke/systemic embolism and death compared with reduced-dose apixaban (2.5 mg twice a day; n = 1,317; HR: 0.61; 95% CI: 0.37-0.98; P = .04 for stroke/systemic embolism; HR: 0.64; 95% CI: 0.45-0.92; P = .01 for death) or warfarin (HR: 0.64; 95% CI: 0.42-0.97; P = .04 for stroke/systemic embolism; HR: 0.63; 95% CI: 0.46-0.85; P = .003 for death).
Cautions. There may be unique patient factors that led providers to prescribe apixaban to patients with ESKD.
Implications. The use of standard-dose apixaban appears safe and potentially preferable in patients with ESKD and AF due to reductions in major bleeding, thromboembolism, and mortality risk compared with warfarin. Several additional studies are pending to evaluate the use and dose of apixaban in patients with ESKD and AF.
Outcomes Associated with De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Cowley MC, et al. Chest. 2019;155(1):53-59.10
Background. Patients diagnosed with hospital-acquired pneumonia (HAP) are often treated empirically with broad-spectrum antibiotics. In many patients with HAP, cultures remain negative, and providers must decide if antibiotics can safely be narrowed. Specifically, the safety of deciding to “de-escalate” and discontinue the coverage for methicillin-resistant Staphylococcus aureus (MRSA) if cultures remain negative is unclear.
Findings. In this single-center retrospective cohort study, 279 patients who were (1) diagnosed with HAP and (2) had negative sputum cultures were enrolled. The patients in whom MRSA coverage was de-escalated by day four were compared with those with continued anti-MRSA coverage. No difference was observed between the two groups in terms of degree of illness or comorbidities. The patients who were de-escalated received five fewer days of anti-MRSA coverage than patients who were not. No difference was noted in the 28-day mortality between the two groups (de-escalation: 23% vs no de-escalation: 28%; 95% CI: −16.1%-6.5%). The incidence of acute kidney injury (AKI) was significantly lower in the de-escalation group (36% vs 50%; 95% CI: −26.9- 0.04), and the overall length of stay was five days shorter in the de-escalation group (95% CI: 0.1-6.4 days).
Caveats. Given the retrospective nature, unmeasured confounders may have impacted the decision to de-escalate anti-MRSA coverage. The observed lower risk of AKI in the de-escalation group may be due to the simultaneous de-escalation of anti-Pseudomonas antibiotic agents in addition to the de-escalation of anti-MRSA coverage, as opposed to de-escalation of the anti-MRSA coverage alone.
Implications. De-escalation of anti-MRSA coverage in patients with HAP with negative cultures is associated with fewer antibiotic days, less AKI, and possibly shorter length of stay.
Partial Oral versus Intravenous Antibiotic Treatment for Endocarditis (POET). Iversen K et al. New Engl J Med. 2019;380(5):415-424.11
Background. Patients with left-sided infective endocarditis are typically treated with up to six weeks of intravenous (IV) antibiotics. The investigators studied the effectiveness and safety of switching to oral antibiotics after at least 10 days of IV therapy.
Findings. This randomized, multicenter, noninferiority trial at cardiac centers across Denmark included 400 adults with left-sided endocarditis who were clinically stable after at least 10 days of IV antibiotics. Half of the patients were randomized to continue IV therapy, whereas the other half was switched to oral antibiotics to complete the treatment course. Six months after therapy, no significant difference was observed between the two groups in terms of the primary composite outcomes, including all-cause mortality, unplanned cardiac surgery, embolic events, or relapse of bacteremia with the primary pathogen (IV-treated group: 12.1%; orally treated group: 9.0% [between-group difference: 3.1%; P = .40]).
Caveats. A total of 20% of the screened population (1,954 adults) was randomized, and about 1% (5/400) of patients used injection drugs. None of the patients had MRSA. Patients in the oral group were assessed two to three times per week as outpatients, which may not be feasible in most settings.
Implications. Switching to oral antibiotics after at least 10 days of IV therapy appears to be safe and effective in selected patients with left-sided endocarditis. However, this study largely excluded patients with injection drug use and/or MRSA infections.
Oral versus Intravenous Antibiotics for Bone and Joint Infection (OVIVA). Li HK, et al. New Engl J Med. 2019;380(5):425-436.12
Background. Most complex orthopedic infections are treated with several weeks of IV antibiotics. This study sought to determine whether oral antibiotics are noninferior to IV antibiotics for bone and joint infections.
Findings. This randomized, multicenter, noninferiority, open-label trial of 1,054 adults with bone and joint infections in the United Kingdom included patients with prosthetic joints, other indwelling joint hardware, and native joint infections. Within seven days of antibiotic medication or within seven days of surgery (if performed), the patients received either IV or oral antibiotics for six weeks with a primary endpoint of treatment failure one year after the study randomization. The choice and duration of antibiotic treatment were determined by the involved infectious disease physician. A majority (77%) of patients received greater than six weeks of therapy. Treatment failure was defined by clinical, microbiologic, or histologic criteria. Most enrolled patients were infected with Staphylococcus aureus, with 10% having methicillin-resistant S. aureus. Treatment failure was more frequent in the IV group than the oral group (14.6% vs 13.2%), and these findings were consistent across all subgroups. More patients discontinued treatment in the IV group than the oral group.
Cautions. This study included a heterogenous population of patients with bone and joint infections, with or without hardware, and with different species of bacteria. Patients with bacteremia, endocarditis, or another indication for IV therapy were excluded. Limited injection drug use history was available for the enrolled patients. Most patients had lower limb infections. Thus, these findings are less applicable to vertebral osteomyelitis. Additionally, the study offered no comparison of specific antibiotics.
Implications. With appropriate oversight from infectious disease specialists, targeted oral therapy may be appropriate for the treatment of osteomyelitis. This shift in practice likely requires more study before broad implementation.
Prognostic Accuracy of the HEART Score for Prediction of Major Adverse Cardiac Events in Patients Presenting with Chest Pain: A Systematic Review and Meta‐analysis. Fernando S, et al. Acad Emerg Med. 2019;26(2):140-151.13
Background. Chest pain accounts for over eight million emergency department (ED) visits yearly in the United States. Of those presenting with chest pain, 10%-20% will experience acute coronary syndrome (ACS) requiring further medical treatment. Given the fear of missing ACS, many low-risk patients are hospitalized. The American Heart Association has advocated using validated predictive scoring models to identify patients with chest pain who are at low risk for short-term major cardiovascular adverse event (MACE) for potential discharge without further testing. The authors evaluated the prognostic accuracy of higher risk scores to predict MACE in adult ED patients presenting with chest pain.
Findings. This study was a systematic review and meta-analysis of 30 prospective and retrospective studies evaluating the history–electrocardiogram–age–risk factors–troponin (HEART) score through May 1, 2018. Meta-analysis compared the sensitivity, specificity, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios of the HEART score and the Thrombolysis in Myocardial Infarction (TIMI) score when reported. An intermediate HEART score of 4-6 had a sensitivity of 95.9% and a specificity of 44.6%. A high HEART score of greater than or equal to 7 had a sensitivity of 39.5% and a specificity of 95.0%. Similarly, a high TIMI score of great than or equal to 6 had a sensitivity of only 2.8% and a specificity of 99.6%. The authors concluded that a HEART score of greater than or equal to 4 best identifies patients at risk of MACE who need greater consideration for additional testing.
Caveats. This meta-analysis failed to assess the potential adverse effects of false positive downstream testing. Additionally, no study compared the HEART score with the experienced clinician gestalt, which has often been equivalent to decision rules.
Implication. A HEART score greater than or equal to 4 risk stratifies ED patients with chest pain requiring further consideration for evaluation versus those that can be discharged with low risk for short-term MACE.
1. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. https://doi.org/10.1093/cid/cix1085.
2. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
3. Siemieniuk RAC, Chu DK, Kim LH, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/https://doi.org/10.1136/bmj.k4169
4. A PG, O’Conor KJ, Lanzkron S, et al. Do words matter? Stigmatizing language and the transmission of bias in the medical record. J Gen Intern Med. 2018;33(5):685-691. https://doi.org/10.1007/s11606-017-4289-2.
5. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379(5):492. https://doi.org/10.1056/NEJMoa1707855.
6. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. https://doi.org/10.7326/M17-3107.
7. Hedegaard HM, A; Warner, M. Drug Overdose Deaths in the United States, 1999-2017. 2018; https://www.cdc.gov/nchs/products/databriefs/db329.htm. Accessed March 07, 2019.
8. Medications for Opioid Use Disorder Save Lives. 2019; http://www.nationalacademies.org/hmd/Reports/2019/medications-for-opioid-use-disorder-save-lives.aspx. Accessed March 07, 2019.
9. Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138(15):1519-1529. https://doi.org/10.1161/CIRCULATIONAHA.118.035418.
10. Cowley MC, Ritchie DJ, Hampton N, Kollef MH, Micek ST. Outcomes Associated With De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Chest. 2019;155(1):53-59. https://doi.org/10.1016/j.chest.2018.10.014
11. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
12. Li HK, Rombach I, Zambellas R, et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N Engl J Med. 2019;380(5):425-436. https://doi.org/10.1056/NEJMoa1710926
13. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(2):140-151. https://doi.org/10.1111/acem.13649.
Given the breadth and depth of patients cared for by hospital medicine providers, it is challenging to remain current with the literature. The authors critically appraised the literature from March 2018 to April 2019 for high-quality studies relevant to hospital medicine. Articles were selected based on methodologic rigor and likelihood to impact clinical practice. Thirty articles were selected by the presenting authors for the Hospital Medicine Updates at the 2019 Society of Hospital Medicine (CH, CM) and Society of General Internal Medicine Annual Meetings (BS, AB). After two sequential rounds of voting and group discussion to adjudicate voting discrepancies, the authors selected the 10 most impactful articles for this review. Each article is described below with the key points summarized in the Table.
ESSENTIAL PUBLICATIONS
Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). McDonald LC, et al. Clin Infect Dis. 2018;66(7):e1–e48.1
Background. In the United States, approximately 500,000 Clostridioides difficile infections (CDI) occur annually with 15,000-30,000 deaths. CDI has become a marker of hospital quality and has been placed under numerous “pay for performance” metrics. The Infectious Diseases Society of America/Society of Healthcare Epidemiology of America updated their guidelines from 2010 regarding hospital surveillance, diagnostic testing, treatment, and infection precautions and control.
Findings. The panel included 14 multidisciplinary experts in epidemiology, diagnosis, infection control, and clinical management of adult and pediatric CDI. They used problem intervention comparison-outcome (PICO)-formatted, evidence-based questions. The selection of data and final recommendations were made in accordance with the GRADE criteria. A total of 35 recommendations were made.
Key clinical recommendations for hospitalists caring for adults: (1) Prescribe vancomycin or fidaxomicin over metronidazole for the initial treatment of CDI (strong recommendation, high quality of evidence); (2) Limit testing to the patients with unexplained new onset diarrhea, which is defined as greater than or equal to 3 unformed stools in 24 hours (weak recommendation, very low-quality evidence); (3) Avoid routine repeat testing within seven days, and only test asymptomatic patients for epidemiologic reasons (strong recommendation, moderate-quality evidence); (4) Minimize the frequency and duration of high-risk antibiotic therapy and the number of antibiotic agents prescribed (strong recommendation, moderate quality of evidence); (5) Discontinue therapy with the inciting antibiotic agent as soon as possible (strong recommendation, moderate quality of evidence).
Caveats. As with the clinical application of any guidelines, individual case adjustments may be required.
Implications. Vancomycin or fidaxomicin should be used for the initial episode of CDI instead of metronidazole.
Mortality and Morbidity in Acutely Ill Adults Treated with Liberal versus Conservative Oxygen Therapy (IOTA): a Systematic Review and Meta-analysis. Chu DK, et al. Lancet. 2018;391(10131):1693-1705.2
Background. Supplemental oxygen is often given to acutely ill hospitalized adults, even when they are not hypoxic or dyspneic. The safety and efficacy of this practice is unknown.
Findings. This systematic review and meta-analysis evaluated 25 randomized controlled trials enrolling 16,037 patients. Patients presented with several conditions, including sepsis, critical illness, stroke, myocardial infarction, and emergency surgery. The fraction of inspired oxygen in the liberal arms varied from 30% to 100%. Most patients randomized to the conservative arm received no supplemental oxygen. Delivery of liberal oxygen to acutely ill adults was associated with increased in-hospital mortality (relative risk [RR]: 1.21; 95% CI: 1.03-1.43), 30-day mortality (RR: 1.14; 95% CI: 1.01-1.29), and 90-day mortality (RR: 1.10; 95% CI: 1.00-1.20). The results were believed to be of high quality and were robust across multiple sensitivity analyses. It seemed that the mortality began to increase when supplemental oxygen raised the peripheral oxygen saturation (Sp02) above a range of 94%-96%.
Caveats. Heterogeneity was observed in the study settings and oxygen delivery. In addition, the cause for increased mortality could not be determined.
Implications. In hospitalized acutely ill adults, “liberal” supplemental oxygen was associated with increased in-hospital and longer-term mortality. The study authors postulated that this finding resulted from the direct toxic effects of oxygen or that oxygen delivery may “mask” illness and lead to delays in diagnosis and treatment. A subsequent clinical practice guideline recommends (1) a target SpO2 of less than 96% for patients receiving oxygen therapy; (2) a target SpO2 range of 90%-94% seems appropriate for most hospitalized adults.3
Do Words Matter? Stigmatizing Language and the Transmission of Bias in the Medical Record. P Goddu A, et al. J Gen Intern Med. 2018;33(5):68-91.4
Background. Previous work has shown that clinician bias affects health outcomes, often worsening health disparities. It is unknown whether clinicians’ language in medical records biases other clinicians and whether this affects patients.
Findings. The investigators randomized medical students and residents in internal and emergency medicine at one academic medical center to review one of two vignettes in the format of notes on the same hypothetical patient with sickle cell disease (SCD) admitted with a pain crisis. One vignette contained stigmatizing language, and the other contained neutral language. The trainees exposed to the vignettes with stigmatizing language showed a more negative attitude toward the patient, as measured by a previously validated scale of attitudes toward patients with SCD (20.6 stigmatizing vs 25.6 neutral, with a total score range of 7-35 for the instrument; higher scores indicate more positive attitudes; P < .001). Furthermore, the intensity of pain treatment was assessed in the resident group and was less aggressive when residents were exposed to stigmatizing language (5.56 stigmatizing vs 6.22 neutral on a scale of 2-7, with higher scores indicating more aggressive pain treatment; P = .003).
Cautions. This research was a single-center study of residents and medical students in two departments. Additionally, the study used vignettes on a hypothetical patient so trainees in the study group might have witnessed stronger stigmatizing language than what is typically observed in an actual patients’ notes.
Implications. Stigmatizing language used in medical records possibly contributed to health disparities by negatively impacting other physicians’ biases and prescribing practices toward patients with SCD at an academic medical center. Clinicians should avoid stigmatizing language in medical records.
Catheter Ablation for Atrial Fibrillation with Heart Failure. Marrouche, NF et al. New Engl J Med. 2018;378:417-427.5
Background. Atrial fibrillation (AF) in patients with heart failure is associated with increased mortality and morbidity. Small-scale studies have suggested that ablation of AF may benefit patients with heart failure.
Findings. This multicenter trial included 398 patients with heart failure and symptomatic AF. Patients had New York Heart Association Class II-IV heart failure, an ejection fraction (EF) of 35% or less, and an internal cardiac defibrillator (ICD). Patients were randomized to either ablation or medical therapy. All enrolled patients either refused, failed, or showed poor tolerance to antiarrhythmic therapy for AF. The primary outcome was death from any cause or hospitalization for heart failure.
The composite endpoint occurred in 28.5% of the ablation group versus 44.6% of patients in the medical therapy group (hazard ratio [HR]: 0.62; 95% CI: 0.43-0.87). Fewer patients in the ablation group died (13% vs 25%; HR: 0.53; 95% CI: 0.32-0.86) or were hospitalized for heart failure (21% vs 36%; HR: 0.56; 95% CI: 0.37-0.83). The patients in the ablation group had higher EF increases above baseline and a greater proportion were in sinus rhythm at the 60-month follow-up visit.
Cautions. The trial was terminated early due to slow recruitment and lower than expected events. Over twice as many patients were lost to follow-up in the ablation group versus the medical therapy group, and by 60 months, AF recurred in 50% of patients who underwent ablation. The sample size was small, and the trial was unblinded.
Implications. Ablation should be considered for AF in patients with heart failure. Additional studies to evaluate ablation versus medical therapy for patients with heart failure and AF are underway.
Medication for Opioid Use Disorder after Nonfatal Opioid Overdose and Association with Mortality. Larochelle MR, et al. Ann Intern Med. 2018;169(3):137-145.6
Background. More than 70,000 Americans died of drug overdose in 2017; this number is higher than the deaths resulting from human immunodeficiency virus, car crash, or gun violence at their peaks.7 Methadone, buprenorphine, and naltrexone are approved by the Federal Drug Administration for the treatment of opioid use disorder (OUD). These medications increase treatment retention; methadone and buprenorphine have been associated with significant decreases in all-cause and overdose mortality.8 However, whether receipt of these medications following a nonfatal opioid overdose reduces mortality is unknown.
Findings. This retrospective cohort study included 17,568 opioid overdose survivors from the Massachusetts’s Public Health Dataset between 2012 and 2014. Only three in 10 of these patients received any medications for OUD over 12 months following overdose. All-cause mortality was 4.7 deaths (95% CI: 4.4-5.0 deaths) per 100 person-years. The relative risk for all-cause mortality was 53% lower with methadone (adjusted hazard ratio [aHR]: 0.47; 95% CI: 0.32-0.71) and 37% lower with buprenorphine (aHR: 0.63; 95% CI: 0.46-0.87).
Caveats. This cohort study may have missed confounders explaining why certain patients received medications for OUD. As a result, association cannot be interpreted as causation.
Implications. Methadone and buprenorphine are associated with a reduction in preventable deaths in patients with OUD who have survived an overdose. All patients with OUD should be considered for therapy.
Outcomes Associated with Apixaban Use in Patients with End-Stage Kidney Disease and Atrial Fibrillation in the United States. Siontis, KC, et al. Circulation. 2018;138:1519–1529.9
Background. Patients with end-stage kidney disease (ESKD) have poor outcomes when treated with warfarin for AF. These patients were excluded from clinical trials of direct oral anticoagulants. The goal of this study was to determine the outcomes of the use of apixaban in patients with ESKD and AF.
Findings. This retrospective cohort study included 25,523 Medicare patients with ESKD and AF on anticoagulants. A 3:1 propensity score match was performed between patients on warfarin and apixaban. Time without stroke/systemic embolism, bleeding (major, gastrointestinal, and intracranial), and death were assessed. A total of 2,351 patients were on apixaban, and 23,172 patients were on warfarin. No difference was observed in the risk of stroke/systemic embolism between apixaban and warfarin (HR 0.88; 95% CI: 0.69-1.12). Apixaban was associated with a lower risk of major bleeding (HR: 0.72; 95% CI: 0.59-0.87). Standard-dose apixaban (5 mg twice a day) was associated with lower risks of stroke/systemic embolism and death compared with reduced-dose apixaban (2.5 mg twice a day; n = 1,317; HR: 0.61; 95% CI: 0.37-0.98; P = .04 for stroke/systemic embolism; HR: 0.64; 95% CI: 0.45-0.92; P = .01 for death) or warfarin (HR: 0.64; 95% CI: 0.42-0.97; P = .04 for stroke/systemic embolism; HR: 0.63; 95% CI: 0.46-0.85; P = .003 for death).
Cautions. There may be unique patient factors that led providers to prescribe apixaban to patients with ESKD.
Implications. The use of standard-dose apixaban appears safe and potentially preferable in patients with ESKD and AF due to reductions in major bleeding, thromboembolism, and mortality risk compared with warfarin. Several additional studies are pending to evaluate the use and dose of apixaban in patients with ESKD and AF.
Outcomes Associated with De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Cowley MC, et al. Chest. 2019;155(1):53-59.10
Background. Patients diagnosed with hospital-acquired pneumonia (HAP) are often treated empirically with broad-spectrum antibiotics. In many patients with HAP, cultures remain negative, and providers must decide if antibiotics can safely be narrowed. Specifically, the safety of deciding to “de-escalate” and discontinue the coverage for methicillin-resistant Staphylococcus aureus (MRSA) if cultures remain negative is unclear.
Findings. In this single-center retrospective cohort study, 279 patients who were (1) diagnosed with HAP and (2) had negative sputum cultures were enrolled. The patients in whom MRSA coverage was de-escalated by day four were compared with those with continued anti-MRSA coverage. No difference was observed between the two groups in terms of degree of illness or comorbidities. The patients who were de-escalated received five fewer days of anti-MRSA coverage than patients who were not. No difference was noted in the 28-day mortality between the two groups (de-escalation: 23% vs no de-escalation: 28%; 95% CI: −16.1%-6.5%). The incidence of acute kidney injury (AKI) was significantly lower in the de-escalation group (36% vs 50%; 95% CI: −26.9- 0.04), and the overall length of stay was five days shorter in the de-escalation group (95% CI: 0.1-6.4 days).
Caveats. Given the retrospective nature, unmeasured confounders may have impacted the decision to de-escalate anti-MRSA coverage. The observed lower risk of AKI in the de-escalation group may be due to the simultaneous de-escalation of anti-Pseudomonas antibiotic agents in addition to the de-escalation of anti-MRSA coverage, as opposed to de-escalation of the anti-MRSA coverage alone.
Implications. De-escalation of anti-MRSA coverage in patients with HAP with negative cultures is associated with fewer antibiotic days, less AKI, and possibly shorter length of stay.
Partial Oral versus Intravenous Antibiotic Treatment for Endocarditis (POET). Iversen K et al. New Engl J Med. 2019;380(5):415-424.11
Background. Patients with left-sided infective endocarditis are typically treated with up to six weeks of intravenous (IV) antibiotics. The investigators studied the effectiveness and safety of switching to oral antibiotics after at least 10 days of IV therapy.
Findings. This randomized, multicenter, noninferiority trial at cardiac centers across Denmark included 400 adults with left-sided endocarditis who were clinically stable after at least 10 days of IV antibiotics. Half of the patients were randomized to continue IV therapy, whereas the other half was switched to oral antibiotics to complete the treatment course. Six months after therapy, no significant difference was observed between the two groups in terms of the primary composite outcomes, including all-cause mortality, unplanned cardiac surgery, embolic events, or relapse of bacteremia with the primary pathogen (IV-treated group: 12.1%; orally treated group: 9.0% [between-group difference: 3.1%; P = .40]).
Caveats. A total of 20% of the screened population (1,954 adults) was randomized, and about 1% (5/400) of patients used injection drugs. None of the patients had MRSA. Patients in the oral group were assessed two to three times per week as outpatients, which may not be feasible in most settings.
Implications. Switching to oral antibiotics after at least 10 days of IV therapy appears to be safe and effective in selected patients with left-sided endocarditis. However, this study largely excluded patients with injection drug use and/or MRSA infections.
Oral versus Intravenous Antibiotics for Bone and Joint Infection (OVIVA). Li HK, et al. New Engl J Med. 2019;380(5):425-436.12
Background. Most complex orthopedic infections are treated with several weeks of IV antibiotics. This study sought to determine whether oral antibiotics are noninferior to IV antibiotics for bone and joint infections.
Findings. This randomized, multicenter, noninferiority, open-label trial of 1,054 adults with bone and joint infections in the United Kingdom included patients with prosthetic joints, other indwelling joint hardware, and native joint infections. Within seven days of antibiotic medication or within seven days of surgery (if performed), the patients received either IV or oral antibiotics for six weeks with a primary endpoint of treatment failure one year after the study randomization. The choice and duration of antibiotic treatment were determined by the involved infectious disease physician. A majority (77%) of patients received greater than six weeks of therapy. Treatment failure was defined by clinical, microbiologic, or histologic criteria. Most enrolled patients were infected with Staphylococcus aureus, with 10% having methicillin-resistant S. aureus. Treatment failure was more frequent in the IV group than the oral group (14.6% vs 13.2%), and these findings were consistent across all subgroups. More patients discontinued treatment in the IV group than the oral group.
Cautions. This study included a heterogenous population of patients with bone and joint infections, with or without hardware, and with different species of bacteria. Patients with bacteremia, endocarditis, or another indication for IV therapy were excluded. Limited injection drug use history was available for the enrolled patients. Most patients had lower limb infections. Thus, these findings are less applicable to vertebral osteomyelitis. Additionally, the study offered no comparison of specific antibiotics.
Implications. With appropriate oversight from infectious disease specialists, targeted oral therapy may be appropriate for the treatment of osteomyelitis. This shift in practice likely requires more study before broad implementation.
Prognostic Accuracy of the HEART Score for Prediction of Major Adverse Cardiac Events in Patients Presenting with Chest Pain: A Systematic Review and Meta‐analysis. Fernando S, et al. Acad Emerg Med. 2019;26(2):140-151.13
Background. Chest pain accounts for over eight million emergency department (ED) visits yearly in the United States. Of those presenting with chest pain, 10%-20% will experience acute coronary syndrome (ACS) requiring further medical treatment. Given the fear of missing ACS, many low-risk patients are hospitalized. The American Heart Association has advocated using validated predictive scoring models to identify patients with chest pain who are at low risk for short-term major cardiovascular adverse event (MACE) for potential discharge without further testing. The authors evaluated the prognostic accuracy of higher risk scores to predict MACE in adult ED patients presenting with chest pain.
Findings. This study was a systematic review and meta-analysis of 30 prospective and retrospective studies evaluating the history–electrocardiogram–age–risk factors–troponin (HEART) score through May 1, 2018. Meta-analysis compared the sensitivity, specificity, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios of the HEART score and the Thrombolysis in Myocardial Infarction (TIMI) score when reported. An intermediate HEART score of 4-6 had a sensitivity of 95.9% and a specificity of 44.6%. A high HEART score of greater than or equal to 7 had a sensitivity of 39.5% and a specificity of 95.0%. Similarly, a high TIMI score of great than or equal to 6 had a sensitivity of only 2.8% and a specificity of 99.6%. The authors concluded that a HEART score of greater than or equal to 4 best identifies patients at risk of MACE who need greater consideration for additional testing.
Caveats. This meta-analysis failed to assess the potential adverse effects of false positive downstream testing. Additionally, no study compared the HEART score with the experienced clinician gestalt, which has often been equivalent to decision rules.
Implication. A HEART score greater than or equal to 4 risk stratifies ED patients with chest pain requiring further consideration for evaluation versus those that can be discharged with low risk for short-term MACE.
Given the breadth and depth of patients cared for by hospital medicine providers, it is challenging to remain current with the literature. The authors critically appraised the literature from March 2018 to April 2019 for high-quality studies relevant to hospital medicine. Articles were selected based on methodologic rigor and likelihood to impact clinical practice. Thirty articles were selected by the presenting authors for the Hospital Medicine Updates at the 2019 Society of Hospital Medicine (CH, CM) and Society of General Internal Medicine Annual Meetings (BS, AB). After two sequential rounds of voting and group discussion to adjudicate voting discrepancies, the authors selected the 10 most impactful articles for this review. Each article is described below with the key points summarized in the Table.
ESSENTIAL PUBLICATIONS
Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). McDonald LC, et al. Clin Infect Dis. 2018;66(7):e1–e48.1
Background. In the United States, approximately 500,000 Clostridioides difficile infections (CDI) occur annually with 15,000-30,000 deaths. CDI has become a marker of hospital quality and has been placed under numerous “pay for performance” metrics. The Infectious Diseases Society of America/Society of Healthcare Epidemiology of America updated their guidelines from 2010 regarding hospital surveillance, diagnostic testing, treatment, and infection precautions and control.
Findings. The panel included 14 multidisciplinary experts in epidemiology, diagnosis, infection control, and clinical management of adult and pediatric CDI. They used problem intervention comparison-outcome (PICO)-formatted, evidence-based questions. The selection of data and final recommendations were made in accordance with the GRADE criteria. A total of 35 recommendations were made.
Key clinical recommendations for hospitalists caring for adults: (1) Prescribe vancomycin or fidaxomicin over metronidazole for the initial treatment of CDI (strong recommendation, high quality of evidence); (2) Limit testing to the patients with unexplained new onset diarrhea, which is defined as greater than or equal to 3 unformed stools in 24 hours (weak recommendation, very low-quality evidence); (3) Avoid routine repeat testing within seven days, and only test asymptomatic patients for epidemiologic reasons (strong recommendation, moderate-quality evidence); (4) Minimize the frequency and duration of high-risk antibiotic therapy and the number of antibiotic agents prescribed (strong recommendation, moderate quality of evidence); (5) Discontinue therapy with the inciting antibiotic agent as soon as possible (strong recommendation, moderate quality of evidence).
Caveats. As with the clinical application of any guidelines, individual case adjustments may be required.
Implications. Vancomycin or fidaxomicin should be used for the initial episode of CDI instead of metronidazole.
Mortality and Morbidity in Acutely Ill Adults Treated with Liberal versus Conservative Oxygen Therapy (IOTA): a Systematic Review and Meta-analysis. Chu DK, et al. Lancet. 2018;391(10131):1693-1705.2
Background. Supplemental oxygen is often given to acutely ill hospitalized adults, even when they are not hypoxic or dyspneic. The safety and efficacy of this practice is unknown.
Findings. This systematic review and meta-analysis evaluated 25 randomized controlled trials enrolling 16,037 patients. Patients presented with several conditions, including sepsis, critical illness, stroke, myocardial infarction, and emergency surgery. The fraction of inspired oxygen in the liberal arms varied from 30% to 100%. Most patients randomized to the conservative arm received no supplemental oxygen. Delivery of liberal oxygen to acutely ill adults was associated with increased in-hospital mortality (relative risk [RR]: 1.21; 95% CI: 1.03-1.43), 30-day mortality (RR: 1.14; 95% CI: 1.01-1.29), and 90-day mortality (RR: 1.10; 95% CI: 1.00-1.20). The results were believed to be of high quality and were robust across multiple sensitivity analyses. It seemed that the mortality began to increase when supplemental oxygen raised the peripheral oxygen saturation (Sp02) above a range of 94%-96%.
Caveats. Heterogeneity was observed in the study settings and oxygen delivery. In addition, the cause for increased mortality could not be determined.
Implications. In hospitalized acutely ill adults, “liberal” supplemental oxygen was associated with increased in-hospital and longer-term mortality. The study authors postulated that this finding resulted from the direct toxic effects of oxygen or that oxygen delivery may “mask” illness and lead to delays in diagnosis and treatment. A subsequent clinical practice guideline recommends (1) a target SpO2 of less than 96% for patients receiving oxygen therapy; (2) a target SpO2 range of 90%-94% seems appropriate for most hospitalized adults.3
Do Words Matter? Stigmatizing Language and the Transmission of Bias in the Medical Record. P Goddu A, et al. J Gen Intern Med. 2018;33(5):68-91.4
Background. Previous work has shown that clinician bias affects health outcomes, often worsening health disparities. It is unknown whether clinicians’ language in medical records biases other clinicians and whether this affects patients.
Findings. The investigators randomized medical students and residents in internal and emergency medicine at one academic medical center to review one of two vignettes in the format of notes on the same hypothetical patient with sickle cell disease (SCD) admitted with a pain crisis. One vignette contained stigmatizing language, and the other contained neutral language. The trainees exposed to the vignettes with stigmatizing language showed a more negative attitude toward the patient, as measured by a previously validated scale of attitudes toward patients with SCD (20.6 stigmatizing vs 25.6 neutral, with a total score range of 7-35 for the instrument; higher scores indicate more positive attitudes; P < .001). Furthermore, the intensity of pain treatment was assessed in the resident group and was less aggressive when residents were exposed to stigmatizing language (5.56 stigmatizing vs 6.22 neutral on a scale of 2-7, with higher scores indicating more aggressive pain treatment; P = .003).
Cautions. This research was a single-center study of residents and medical students in two departments. Additionally, the study used vignettes on a hypothetical patient so trainees in the study group might have witnessed stronger stigmatizing language than what is typically observed in an actual patients’ notes.
Implications. Stigmatizing language used in medical records possibly contributed to health disparities by negatively impacting other physicians’ biases and prescribing practices toward patients with SCD at an academic medical center. Clinicians should avoid stigmatizing language in medical records.
Catheter Ablation for Atrial Fibrillation with Heart Failure. Marrouche, NF et al. New Engl J Med. 2018;378:417-427.5
Background. Atrial fibrillation (AF) in patients with heart failure is associated with increased mortality and morbidity. Small-scale studies have suggested that ablation of AF may benefit patients with heart failure.
Findings. This multicenter trial included 398 patients with heart failure and symptomatic AF. Patients had New York Heart Association Class II-IV heart failure, an ejection fraction (EF) of 35% or less, and an internal cardiac defibrillator (ICD). Patients were randomized to either ablation or medical therapy. All enrolled patients either refused, failed, or showed poor tolerance to antiarrhythmic therapy for AF. The primary outcome was death from any cause or hospitalization for heart failure.
The composite endpoint occurred in 28.5% of the ablation group versus 44.6% of patients in the medical therapy group (hazard ratio [HR]: 0.62; 95% CI: 0.43-0.87). Fewer patients in the ablation group died (13% vs 25%; HR: 0.53; 95% CI: 0.32-0.86) or were hospitalized for heart failure (21% vs 36%; HR: 0.56; 95% CI: 0.37-0.83). The patients in the ablation group had higher EF increases above baseline and a greater proportion were in sinus rhythm at the 60-month follow-up visit.
Cautions. The trial was terminated early due to slow recruitment and lower than expected events. Over twice as many patients were lost to follow-up in the ablation group versus the medical therapy group, and by 60 months, AF recurred in 50% of patients who underwent ablation. The sample size was small, and the trial was unblinded.
Implications. Ablation should be considered for AF in patients with heart failure. Additional studies to evaluate ablation versus medical therapy for patients with heart failure and AF are underway.
Medication for Opioid Use Disorder after Nonfatal Opioid Overdose and Association with Mortality. Larochelle MR, et al. Ann Intern Med. 2018;169(3):137-145.6
Background. More than 70,000 Americans died of drug overdose in 2017; this number is higher than the deaths resulting from human immunodeficiency virus, car crash, or gun violence at their peaks.7 Methadone, buprenorphine, and naltrexone are approved by the Federal Drug Administration for the treatment of opioid use disorder (OUD). These medications increase treatment retention; methadone and buprenorphine have been associated with significant decreases in all-cause and overdose mortality.8 However, whether receipt of these medications following a nonfatal opioid overdose reduces mortality is unknown.
Findings. This retrospective cohort study included 17,568 opioid overdose survivors from the Massachusetts’s Public Health Dataset between 2012 and 2014. Only three in 10 of these patients received any medications for OUD over 12 months following overdose. All-cause mortality was 4.7 deaths (95% CI: 4.4-5.0 deaths) per 100 person-years. The relative risk for all-cause mortality was 53% lower with methadone (adjusted hazard ratio [aHR]: 0.47; 95% CI: 0.32-0.71) and 37% lower with buprenorphine (aHR: 0.63; 95% CI: 0.46-0.87).
Caveats. This cohort study may have missed confounders explaining why certain patients received medications for OUD. As a result, association cannot be interpreted as causation.
Implications. Methadone and buprenorphine are associated with a reduction in preventable deaths in patients with OUD who have survived an overdose. All patients with OUD should be considered for therapy.
Outcomes Associated with Apixaban Use in Patients with End-Stage Kidney Disease and Atrial Fibrillation in the United States. Siontis, KC, et al. Circulation. 2018;138:1519–1529.9
Background. Patients with end-stage kidney disease (ESKD) have poor outcomes when treated with warfarin for AF. These patients were excluded from clinical trials of direct oral anticoagulants. The goal of this study was to determine the outcomes of the use of apixaban in patients with ESKD and AF.
Findings. This retrospective cohort study included 25,523 Medicare patients with ESKD and AF on anticoagulants. A 3:1 propensity score match was performed between patients on warfarin and apixaban. Time without stroke/systemic embolism, bleeding (major, gastrointestinal, and intracranial), and death were assessed. A total of 2,351 patients were on apixaban, and 23,172 patients were on warfarin. No difference was observed in the risk of stroke/systemic embolism between apixaban and warfarin (HR 0.88; 95% CI: 0.69-1.12). Apixaban was associated with a lower risk of major bleeding (HR: 0.72; 95% CI: 0.59-0.87). Standard-dose apixaban (5 mg twice a day) was associated with lower risks of stroke/systemic embolism and death compared with reduced-dose apixaban (2.5 mg twice a day; n = 1,317; HR: 0.61; 95% CI: 0.37-0.98; P = .04 for stroke/systemic embolism; HR: 0.64; 95% CI: 0.45-0.92; P = .01 for death) or warfarin (HR: 0.64; 95% CI: 0.42-0.97; P = .04 for stroke/systemic embolism; HR: 0.63; 95% CI: 0.46-0.85; P = .003 for death).
Cautions. There may be unique patient factors that led providers to prescribe apixaban to patients with ESKD.
Implications. The use of standard-dose apixaban appears safe and potentially preferable in patients with ESKD and AF due to reductions in major bleeding, thromboembolism, and mortality risk compared with warfarin. Several additional studies are pending to evaluate the use and dose of apixaban in patients with ESKD and AF.
Outcomes Associated with De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Cowley MC, et al. Chest. 2019;155(1):53-59.10
Background. Patients diagnosed with hospital-acquired pneumonia (HAP) are often treated empirically with broad-spectrum antibiotics. In many patients with HAP, cultures remain negative, and providers must decide if antibiotics can safely be narrowed. Specifically, the safety of deciding to “de-escalate” and discontinue the coverage for methicillin-resistant Staphylococcus aureus (MRSA) if cultures remain negative is unclear.
Findings. In this single-center retrospective cohort study, 279 patients who were (1) diagnosed with HAP and (2) had negative sputum cultures were enrolled. The patients in whom MRSA coverage was de-escalated by day four were compared with those with continued anti-MRSA coverage. No difference was observed between the two groups in terms of degree of illness or comorbidities. The patients who were de-escalated received five fewer days of anti-MRSA coverage than patients who were not. No difference was noted in the 28-day mortality between the two groups (de-escalation: 23% vs no de-escalation: 28%; 95% CI: −16.1%-6.5%). The incidence of acute kidney injury (AKI) was significantly lower in the de-escalation group (36% vs 50%; 95% CI: −26.9- 0.04), and the overall length of stay was five days shorter in the de-escalation group (95% CI: 0.1-6.4 days).
Caveats. Given the retrospective nature, unmeasured confounders may have impacted the decision to de-escalate anti-MRSA coverage. The observed lower risk of AKI in the de-escalation group may be due to the simultaneous de-escalation of anti-Pseudomonas antibiotic agents in addition to the de-escalation of anti-MRSA coverage, as opposed to de-escalation of the anti-MRSA coverage alone.
Implications. De-escalation of anti-MRSA coverage in patients with HAP with negative cultures is associated with fewer antibiotic days, less AKI, and possibly shorter length of stay.
Partial Oral versus Intravenous Antibiotic Treatment for Endocarditis (POET). Iversen K et al. New Engl J Med. 2019;380(5):415-424.11
Background. Patients with left-sided infective endocarditis are typically treated with up to six weeks of intravenous (IV) antibiotics. The investigators studied the effectiveness and safety of switching to oral antibiotics after at least 10 days of IV therapy.
Findings. This randomized, multicenter, noninferiority trial at cardiac centers across Denmark included 400 adults with left-sided endocarditis who were clinically stable after at least 10 days of IV antibiotics. Half of the patients were randomized to continue IV therapy, whereas the other half was switched to oral antibiotics to complete the treatment course. Six months after therapy, no significant difference was observed between the two groups in terms of the primary composite outcomes, including all-cause mortality, unplanned cardiac surgery, embolic events, or relapse of bacteremia with the primary pathogen (IV-treated group: 12.1%; orally treated group: 9.0% [between-group difference: 3.1%; P = .40]).
Caveats. A total of 20% of the screened population (1,954 adults) was randomized, and about 1% (5/400) of patients used injection drugs. None of the patients had MRSA. Patients in the oral group were assessed two to three times per week as outpatients, which may not be feasible in most settings.
Implications. Switching to oral antibiotics after at least 10 days of IV therapy appears to be safe and effective in selected patients with left-sided endocarditis. However, this study largely excluded patients with injection drug use and/or MRSA infections.
Oral versus Intravenous Antibiotics for Bone and Joint Infection (OVIVA). Li HK, et al. New Engl J Med. 2019;380(5):425-436.12
Background. Most complex orthopedic infections are treated with several weeks of IV antibiotics. This study sought to determine whether oral antibiotics are noninferior to IV antibiotics for bone and joint infections.
Findings. This randomized, multicenter, noninferiority, open-label trial of 1,054 adults with bone and joint infections in the United Kingdom included patients with prosthetic joints, other indwelling joint hardware, and native joint infections. Within seven days of antibiotic medication or within seven days of surgery (if performed), the patients received either IV or oral antibiotics for six weeks with a primary endpoint of treatment failure one year after the study randomization. The choice and duration of antibiotic treatment were determined by the involved infectious disease physician. A majority (77%) of patients received greater than six weeks of therapy. Treatment failure was defined by clinical, microbiologic, or histologic criteria. Most enrolled patients were infected with Staphylococcus aureus, with 10% having methicillin-resistant S. aureus. Treatment failure was more frequent in the IV group than the oral group (14.6% vs 13.2%), and these findings were consistent across all subgroups. More patients discontinued treatment in the IV group than the oral group.
Cautions. This study included a heterogenous population of patients with bone and joint infections, with or without hardware, and with different species of bacteria. Patients with bacteremia, endocarditis, or another indication for IV therapy were excluded. Limited injection drug use history was available for the enrolled patients. Most patients had lower limb infections. Thus, these findings are less applicable to vertebral osteomyelitis. Additionally, the study offered no comparison of specific antibiotics.
Implications. With appropriate oversight from infectious disease specialists, targeted oral therapy may be appropriate for the treatment of osteomyelitis. This shift in practice likely requires more study before broad implementation.
Prognostic Accuracy of the HEART Score for Prediction of Major Adverse Cardiac Events in Patients Presenting with Chest Pain: A Systematic Review and Meta‐analysis. Fernando S, et al. Acad Emerg Med. 2019;26(2):140-151.13
Background. Chest pain accounts for over eight million emergency department (ED) visits yearly in the United States. Of those presenting with chest pain, 10%-20% will experience acute coronary syndrome (ACS) requiring further medical treatment. Given the fear of missing ACS, many low-risk patients are hospitalized. The American Heart Association has advocated using validated predictive scoring models to identify patients with chest pain who are at low risk for short-term major cardiovascular adverse event (MACE) for potential discharge without further testing. The authors evaluated the prognostic accuracy of higher risk scores to predict MACE in adult ED patients presenting with chest pain.
Findings. This study was a systematic review and meta-analysis of 30 prospective and retrospective studies evaluating the history–electrocardiogram–age–risk factors–troponin (HEART) score through May 1, 2018. Meta-analysis compared the sensitivity, specificity, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios of the HEART score and the Thrombolysis in Myocardial Infarction (TIMI) score when reported. An intermediate HEART score of 4-6 had a sensitivity of 95.9% and a specificity of 44.6%. A high HEART score of greater than or equal to 7 had a sensitivity of 39.5% and a specificity of 95.0%. Similarly, a high TIMI score of great than or equal to 6 had a sensitivity of only 2.8% and a specificity of 99.6%. The authors concluded that a HEART score of greater than or equal to 4 best identifies patients at risk of MACE who need greater consideration for additional testing.
Caveats. This meta-analysis failed to assess the potential adverse effects of false positive downstream testing. Additionally, no study compared the HEART score with the experienced clinician gestalt, which has often been equivalent to decision rules.
Implication. A HEART score greater than or equal to 4 risk stratifies ED patients with chest pain requiring further consideration for evaluation versus those that can be discharged with low risk for short-term MACE.
1. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. https://doi.org/10.1093/cid/cix1085.
2. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
3. Siemieniuk RAC, Chu DK, Kim LH, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/https://doi.org/10.1136/bmj.k4169
4. A PG, O’Conor KJ, Lanzkron S, et al. Do words matter? Stigmatizing language and the transmission of bias in the medical record. J Gen Intern Med. 2018;33(5):685-691. https://doi.org/10.1007/s11606-017-4289-2.
5. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379(5):492. https://doi.org/10.1056/NEJMoa1707855.
6. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. https://doi.org/10.7326/M17-3107.
7. Hedegaard HM, A; Warner, M. Drug Overdose Deaths in the United States, 1999-2017. 2018; https://www.cdc.gov/nchs/products/databriefs/db329.htm. Accessed March 07, 2019.
8. Medications for Opioid Use Disorder Save Lives. 2019; http://www.nationalacademies.org/hmd/Reports/2019/medications-for-opioid-use-disorder-save-lives.aspx. Accessed March 07, 2019.
9. Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138(15):1519-1529. https://doi.org/10.1161/CIRCULATIONAHA.118.035418.
10. Cowley MC, Ritchie DJ, Hampton N, Kollef MH, Micek ST. Outcomes Associated With De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Chest. 2019;155(1):53-59. https://doi.org/10.1016/j.chest.2018.10.014
11. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
12. Li HK, Rombach I, Zambellas R, et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N Engl J Med. 2019;380(5):425-436. https://doi.org/10.1056/NEJMoa1710926
13. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(2):140-151. https://doi.org/10.1111/acem.13649.
1. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. https://doi.org/10.1093/cid/cix1085.
2. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
3. Siemieniuk RAC, Chu DK, Kim LH, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/https://doi.org/10.1136/bmj.k4169
4. A PG, O’Conor KJ, Lanzkron S, et al. Do words matter? Stigmatizing language and the transmission of bias in the medical record. J Gen Intern Med. 2018;33(5):685-691. https://doi.org/10.1007/s11606-017-4289-2.
5. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379(5):492. https://doi.org/10.1056/NEJMoa1707855.
6. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. https://doi.org/10.7326/M17-3107.
7. Hedegaard HM, A; Warner, M. Drug Overdose Deaths in the United States, 1999-2017. 2018; https://www.cdc.gov/nchs/products/databriefs/db329.htm. Accessed March 07, 2019.
8. Medications for Opioid Use Disorder Save Lives. 2019; http://www.nationalacademies.org/hmd/Reports/2019/medications-for-opioid-use-disorder-save-lives.aspx. Accessed March 07, 2019.
9. Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138(15):1519-1529. https://doi.org/10.1161/CIRCULATIONAHA.118.035418.
10. Cowley MC, Ritchie DJ, Hampton N, Kollef MH, Micek ST. Outcomes Associated With De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Chest. 2019;155(1):53-59. https://doi.org/10.1016/j.chest.2018.10.014
11. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312
12. Li HK, Rombach I, Zambellas R, et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N Engl J Med. 2019;380(5):425-436. https://doi.org/10.1056/NEJMoa1710926
13. Fernando SM, Tran A, Cheng W, et al. Prognostic accuracy of the HEART score for prediction of major adverse cardiac events in patients presenting with chest pain: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(2):140-151. https://doi.org/10.1111/acem.13649.
© 2019 Society of Hospital Medicine
Feeding during High-Flow Nasal Cannula for Bronchiolitis: Associations with Time to Discharge
Bronchiolitis is the most common cause of nonbirth hospitalization in children in the United States less than one year of age.1 For children with severe bronchiolitis, high-flow nasal cannula (HFNC) is increasingly used2-4 to reduce work of breathing and prevent the need for further escalation of ventilatory support.5,6 Although previous studies suggest that enteral feeding is recommended in the management of patients hospitalized with bronchiolitis,7-9 limited evidence exists to guide feeding practices for patients receiving HFNC support.5,10,11
Respiratory support with HFNC has been associated with prolonged periods without enteral hydration/nutrition (ie, nil per os [NPO])12 primarily due to anticipation of further escalation of respiratory support or concern for increased risk of aspiration. The majority of patients with bronchiolitis managed with HFNC, however, do not require escalation of care.5,13 When feeding is attempted during HFNC support, it is frequently interrupted.5 Moreover, keeping all children NPO when receiving HFNC may be associated with weight loss and longer length of stay (LOS).12,14 Two small studies found that children admitted to the intensive care unit who received HFNC support for bronchiolitis did not have increased rates of emesis, worsening respiratory distress or aspiration pneumonia when enterally fed.10,11 However, no comparison of adverse events or LOS has been made between patients who were fed and those who were not fed during HFNC therapy, and previous studies have included only patients who have received HFNC in the intensive care setting.
Supporting safe feeding early in hospitalizations for bronchiolitis may facilitate expedited clinical improvement and discharge. As part of an ongoing bronchiolitis quality improvement initiative at our hospital, we sought to characterize feeding practices during HFNC therapy and assess whether feeding exposure was associated with (1) time to discharge after HFNC or (2) feeding-related adverse events. We hypothesized that feeding during HFNC therapy would be associated with a shorter time to discharge after HFNC cessation.
METHODS
Study Design, Setting, Participants
This was a retrospective cohort study of patients aged 1-24 months receiving HFNC support for respiratory failure due to bronchiolitis at an academic children’s hospital between January 1, 2015 and March 1, 2017. Our institution has had a clinical practice guideline, associated order set, and respiratory therapy protocol for general care patients with bronchiolitis since 2009. Patients with bronchiolitis who were weaning HFNC have been cared for in both the intensive and general care settings since 2013. A formal process for initiation of HFNC on general care units was instituted in January of 2017. During the study period, no patients with HFNC support for bronchiolitis had all their care entirely outside the intensive care unit at our institution. However, initiation and subsequent use of HFNC may have occurred in either the intensive care or general care setting. No specific guidance for feeding during HFNC existed during this period.
Patients were identified using the Virtual PICU Systems database, (VPS LLC, myvps.org, Los Angeles, California) and, by definition, all patients received at least some of their care in the intensive care unit. Patients with comorbid conditions of prematurity (<35 weeks) and those with cardiopulmonary, neuromuscular, and genetic diseases were included. Patients with preexisting dysphagia, defined as ongoing outpatient speech therapy for swallowing concerns, an admission diagnosis of aspiration pneumonia or on home respiratory support, were excluded. Children (n = 7) were excluded if they had more than one period of HFNC during admission. This study was determined to be exempt by the University of Wisconsin School of Medicine and Public Health’s Institutional Review Board.
Data Collection and Study Variables
The following variables were collected from VPS administrative data: patient gender, age, admission and discharge date and time, type and total hours of respiratory support, intensive care admission, and LOS (in hours). Additional demographic, clinical, and feeding exposure variables were abstracted manually from the electronic medical record (Epic, Verona, Wisconsin) using a structured data collection tool and stored in REDCap (Research Electronic Data Capture)15 including prematurity, race/ethnicity, insurance status, primary language, and passive tobacco smoke exposure. Clinical variables included duration of illness (days) at the time of admission, unit of HFNC initiation (emergency department, general care, intensive care, respiratory rate and oxygen saturation at HFNC initiation (<90%, 91%-92%, or >92%), acquisition of blood gas at HFNC admission, duration of time on HFNC (hours) and need for intubation or noninvasive ventilation prior to HFNC. The Pediatric Index of Mortality 2 Risk of Mortality (PIM 2 ROM)16 was used to estimate the severity of illness. The PIM2ROM uses clinical variables (systolic blood pressure, fixed pupils, measure of hypoxia using PaO2/FiO2 ratio, base excess, mechanical ventilation, elective admission, recovery from surgery, cardiac bypass, high-risk diagnosis, low-risk diagnosis) collected at the time of intensive care admission to generate a score that predicts the risk of mortality for an individual patient.17
Feeding exposures were documented in three-hour intervals from HFNC initiation to completion using a structured protocol. At each interval the following feeding information was abstracted from a review of nursing and physician documentation and relevant clinical flowsheets: presence or absence of feeding during the interval, route of feeding (oral, nasogastric [NG] or nasojejunal [NJ]). Feeding exposure was categorized a priori as fed at any point during HFNC (vs not fed at any point). Fed children were further characterized as (1) mixed feeding consisting of oral and tube feeds (NG or NJ) or (2) exclusive oral feeding throughout HFNC support (Appendix 1).
The primary outcome was the number of hours to discharge from HFNC cessation. Secondary outcomes were time to discharge from HFNC initiation, all-cause readmissions within seven days of discharge, and potential feeding-related adverse events. Potential adverse events included: (1) aspiration, defined as initiation of antibiotic AND either chest radiograph official interpreted as evidence for aspiration and/or documented concern for aspiration from the treating physician, or (2) intubation after feeding during HFNC.
Statistical Analysis
Descriptive statistics evaluated differences in demographics and clinical variables for feeding exposure groups. We used chi-squared tests for differences in proportions and t-tests or Wilcoxon Rank-Sum tests for differences in means or medians for continuous variables, respectively. Associations between feeding exposure during HFNC and time to discharge (measured in hours) after HFNC completion were modeled with Cox proportional hazards regression. Using this approach, hazard ratios (HR)>1 indicate a higher hazard (rate) of discharge for children with a feeding exposure than for children without the exposure. For example, a hazard ratio equal to two indicates that the exposed population is discharged at twice the rate per unit time as the nonexposed population. Death or censoring events did not occur. Feeding exposure was first modeled dichotomously as not fed or fed. To further explore associations between feeding modality and our outcome, we then modeled feeding exposure categorically as not fed (reference), mixed (oral and tube) feeding, or exclusive oral feeding throughout HFNC.
After constructing a set of unadjusted models, we then adjusted the models for variables having independent (bivariate P < .10) associations with time to discharge: age, unit of HFNC initiation, highest respiratory support required before HFNC, and HFNC duration. Finally, to attempt to account for residual confounding from latent constructs, we also created a set of propensity-weighted Cox proportional hazards models. Propensity weights18 reflecting the probability of being fed or never being fed during HFNC were created using logistic regression with predictors we hypothesized a priori that may have influenced the clinical decision to feed during HFNC: age, day of illness on admission, prematurity, PIM2 ROM score, respiratory rate, oxygen saturation and blood gas acquisition at HFNC initiation, and highest respiratory support required before HFNC. All analyses were conducted using STATA 14.0 (StataCorp, College Station, Texas), and adjusted hazard ratios (aHR) with 95% confidence intervals (95% CIs) were reported.
RESULTS
Patients (n = 123) had a mean age of 7.3 months (standard deviation [SD] 7.1) and presented on day of illness 4.8 (SD 2.3). Prior to HFNC, 10% required higher respiratory support (3% mechanical ventilation). Former preterm children were 12% of the overall sample.
During HFNC, 37% of patients were never fed, 41% were exclusively orally fed, and 23% had tube or mixed oral and tube feedings (Table 1 and Appendix 2). Children who were not fed were older, but groups were otherwise similar in terms of gender, race/ethnicity, passive smoke exposure, day of illness, unit of HFNC initiation, respiratory support required prior to HFNC, and respiratory rate at HFNC initiation.
Median time to discharge after HFNC completion was 31.4 hours (interquartile range [IQR] 23.9-52). Median (IQR) time to discharge was 29.5 (IQR 23.5-47.9) hours in children who were fed and 39.8 (26.4-61.5) hours in those who were not fed (unadjusted HR 1.25 [0.86-1.82], aHR 1.83 [95% CI: 1.16-2.88]). Time to discharge was shortest when children were fed exclusively orally (Figure). Compared with children who were not fed, time to discharge following HFNC completion was significantly shorter for those who were exclusively orally fed (aHR 2.13 [95% CI: 1.31-3.45]; Table 2). Results of the propensity-weighted model were similar: time to discharge after completing HFNC was shorter in fed versus not fed children (HR 2.17; 95 % CI: 1.34-3.50). The secondary outcome, time to discharge from HFNC initiation, had a similar relationship, ie, shorter time to discharge with exclusive oral feeding vs not feeding [aHR 1.95 (95% CI: 1.19-3.18)]. Time to discharge after initiation of HFNC was also shorter for fed versus not fed in propensity-weighted analysis (HR 1.97; 95% CI: 1.13-3.43).
DISCUSSION
This observational study found that being fed during HFNC was associated with shorter time to discharge after HFNC support was completed. Exclusive oral feeding was associated with the shortest time to discharge, and these results were consistent across a variety of analytical approaches. Adverse events were rare and occurred in both fed and unfed children.
These findings advance research on relationships between nutrition and bronchiolitis outcomes. Studies of general care patients with bronchiolitis without HFNC have observed associations between poor nutrition and prolonged LOS.19,20 Two previous studies of patients receiving HFNC therapy for bronchiolitis concluded that frequent interruption11 and later initiation of enteral nutrition10 during ICU stay was associated with longer time to discharge.11 To our knowledge, this is the first study of patients with bronchiolitis treated with HFNC in both general care and ICU settings that compared outcomes according to whether children were fed during HFNC therapy. Our results extend previous work demonstrating that delays in feeding may be associated with longer LOS.
Decisions to feed children with respiratory distress due to bronchiolitis are complex and often subjective. Readiness to feed may be based upon the assessment of a child’s work of breathing, trajectory of illness, institutional culture, and individual physician, nurse, respiratory therapist or speech-language pathologist comfort. In the absence of established feeding best practices,21 some institutions have developed guidelines based on local expert opinion; however, often these recommendations remain largely subjective and nonspecific.5,10,22-24 Although decisions to feed may be influenced by concern about a child’s clinical stability and feeding risk, we found few objective clinical differences between children fed (orally or by enteral tube) or not fed. Moreover, our results were consistent even when we used a propensity-weighted model to account for measured factors that may have been associated with the decision to initiate feeding. This suggests the decision to feed could be more arbitrary than we assume and is important to investigate in future research.
Additionally, although a few early studies have aimed to standardize the process of weaning HFNC support in bronchiolitis,25,26 this process is also largely subjective.10,22,23 As such, the weaning process may be influenced by perceptions of the child’s overall health. Orally fed children may be viewed as more comfortable or well and thus, more readily weaned, which ultimately influences the length of HFNC therapy. Our study design attempted to account for this potential bias by measuring time to discharge following HFNC therapy, rather than measuring total LOS. Meeting adequate calorie, weight, or hydration goals prior to discharge may take longer if feeds have been withheld. We speculate that prolonged periods of NPO might also risk transient oral aversion or feeding discoordination that could influence LOS. Previous research involving broad intensive care unit populations has established the importance of providing nutrition to critically ill children as soon as possible as a means of improving outcomes.27-29 Patients receiving HFNC support for bronchiolitis could plausibly experience similar benefits.
This single-center study with a relatively small sample size has important limitations to consider. The observational design limits our ability to draw conclusions about causal relationships between feeding, time to discharge, and adverse events. In particular, feeding exposure did not account for nuances in feeding timing, feeding density, and other elements of feeding exposure. Additionally, adverse events are rare, and this study is inadequately powered to detect differences between exposure groups. Although we included children cared for in general and intensive care units, our findings may not be generalizable to other hospitals with different placement criteria. Despite the creation of adjusted and propensity-weighted models, our results are still subject to possible residual indication bias. We cannot control for all possible confounders, particularly unmeasured factors which might simultaneously motivate decisions whether, when, and how to feed children receiving HFNC therapy and influence time to discharge after HFNC is finished. Although this study observed associations between feeding during HFNC and both our primary (time to discharge after HFNC was complete) and secondary (time to discharge after HFNC was initiated) outcomes, future work should evaluate how feeding strategies might impact total LOS, particularly as management becomes more standardized.
Prospective studies of feeding exposures during HFNC therapy in bronchiolitis, as well as rigorous interventional study designs, are needed to confirm shorter lengths of stay and safety with larger and more diverse samples. Future research should evaluate methods to safely and effectively feed children with severe bronchiolitis, which would inform standardized evidence-based approaches. Given the scale on which children with bronchiolitis are admitted each year, the implications of such work could be substantial.
CONCLUSION
Children fed while receiving HFNC for bronchiolitis may have shorter time to discharge than those who are not fed. Feeding-related adverse events were rare regardless of the feeding method. Controlled prospective studies addressing residual confounding are needed to justify a change in the current practice.
Acknowledgments
The authors would like to acknowledge the valuable feedback on earlier drafts from members of the University of Wisconsin Division of Pediatric Hospital Medicine CREATE writing group.
1. HCUPnet. http s://hcupnet.ahrq.gov/. Accessed February 7, 2019.
2. Beggs S, Wong ZH, Kaul S, Ogden KJ, Walters JA. High-flow nasal cannula therapy for infants with bronchiolitis. Cochrane Database Syst Rev. 2014;1(1):CD009609. https://doi.org/10.1002/14651858.CD009609.pub2.
3. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
4. Hilliard TN, Archer N, Laura H, et al. Pilot study of vapotherm oxygen delivery in moderately severe bronchiolitis. Arch Dis Child. 2012;97(2):182-183. https://doi.org/10.1136/archdischild-2011-301151.
5. Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(12):1121-1131. https://doi.org/10.1056/NEJMoa1714855.
6. McKiernan C, Chua LC, Visintainer PF, Allen H. High flow nasal cannulae therapy in infants with bronchiolitis. J Pediatr. 2010;156(4):634-638. https://doi.org/10.1016/j.jpeds.2009.10.039.
7. Maffey A, Moviglia T, Mirabello C, et al. Swallowing and respiratory distress in hospitalized patients with bronchiolitis. Dysphagia. 2013;28(4):582-587. https://doi.org/10.1007/s00455-013-9470-0.
8. Kugelman A, Raibin K, Dabbah H, et al. Intravenous fluids versus gastric-tube feeding in hospitalized infants with viral bronchiolitis: a randomized, prospective pilot study. J Pediatr. 2013;162(3):640-642.e641. https://doi.org/10.1016/j.jpeds.2012.10.057.
9. Oakley E, Borland M, Neutze J, et al. Nasogastric hydration versus intravenous hydration for infants with bronchiolitis: a randomised trial. Lancet Respir Med. 2013;1(2):113-120. https://doi.org/10.1016/S2213-2600(12)70053-X.
10. Slain KN, Martinez-Schlurmann N, Shein SL, Stormorken A. Nutrition and high-flow nasal cannula respiratory support in children with bronchiolitis. Hosp Pediatr. 2017;7(5):256-262. https://doi.org/10.1542/hpeds.2016-0194.
11. Sochet AA, McGee JA, October TW. Oral nutrition in children with bronchiolitis on high-flow nasal cannula is well tolerated. Hosp Pediatr. 2017;7(5):249-255. https://doi.org/10.1542/hpeds.2016-0131.
12. Canarie MF, Barry S, Carroll CL, et al. Risk factors for delayed enteral nutrition in critically ill children. Pediatr Crit Care Med. 2015;16(8):e283-e289. https://doi.org/10.1097/PCC.0000000000000527.
13. Schibler A, Pham TM, Dunster KR, et al. Reduced intubation rates for infants after introduction of high-flow nasal prong oxygen delivery. Intensive Care Med. 2011;37(5):847-852. https://doi.org/10.1007/s00134-011-2177-5.
14. Hamilton S, McAleer DM, Ariagno K, et al. A stepwise enteral nutrition algorithm for critically ill children helps achieve nutrient delivery goals*. Pediatr Crit Care Med. 2014;15(7):583-589. https://doi.org/10.1097/PCC.0000000000000179.
15. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
16. Slater A, Shann F, Group APS. The suitability of the Pediatric Index of Mortality (PIM), PIM2, the Pediatric Risk of Mortality (PRISM), and PRISM III for monitoring the quality of pediatric intensive care in Australia and New Zealand. Pediatr Crit Care Med. 2004;5(5):447-454. https://doi.org/10.1097/01.PCC.0000138557.31831.65.
17. Slater A, Shann F, Pearson G, Paediatric Index of Mortality Study G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29(2):278-285. https://doi.org/10.1007/s00134-002-1601-2.
18. Lanza ST, Moore JE, Butera NM. Drawing causal inferences using propensity scores: a practical guide for community psychologists. Am J Commun Psychol. 2013;52(3-4):380-392. https://doi.org/10.1007/s10464-013-9604-4.
19. Weisgerber MC, Lye PS, Li SH, et al. Factors predicting prolonged hospital stay for infants with bronchiolitis. J Hosp Med. 2011;6(5):264-270. https://doi.org/10.1002/jhm.903.
20. Halvorson EE, Chandler N, Neiberg R, Ervin SE. Association of NPO status and type of nutritional support on weight and length of stay in infants hospitalized with bronchiolitis. Hosp Pediatr. 2013;3(4):366-370. https://doi.org/10.1542/hpeds.2013-0011.
21. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742.
22. Seattle Children’s Hospital ZS, Beardsley E, Crotwell D, et al. Bronchiolitis Pathway. http:// www.seattlechildrens.org/pdf/bronchiolitis-pathway.pdf. Accessed January 29, 2019.
23. Children’s Hospital of Philidelphia DM, Zorc J, Kreindler, J, et al. Inpatient Pathway for Treatment of the Child with Bronchiolitis. https://www.chop.edu/clinical-pathway/bronchiolitis-inpatient-treatment-clinical-pathway. Accessed January 29, 2019.
24. Children’s Hospital Colorado TA, Topoz I, Freeman J, et al. Pediatric Viral Bronchiolitis. https://www.childrenscolorado.org/globalassets/healthcare-professionals/clinical-pathways/bronchiolitis.pdf. Accessed January 29, 2019.
25. Betters KA, Hebbar KB, McCracken C, et al. A novel weaning protocol for high-flow nasal cannula in the PICU. Pediatr Crit Care Med. 2017;18(7):e274-e280. https://doi.org/10.1097/PCC.0000000000001181.
26. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
Bronchiolitis is the most common cause of nonbirth hospitalization in children in the United States less than one year of age.1 For children with severe bronchiolitis, high-flow nasal cannula (HFNC) is increasingly used2-4 to reduce work of breathing and prevent the need for further escalation of ventilatory support.5,6 Although previous studies suggest that enteral feeding is recommended in the management of patients hospitalized with bronchiolitis,7-9 limited evidence exists to guide feeding practices for patients receiving HFNC support.5,10,11
Respiratory support with HFNC has been associated with prolonged periods without enteral hydration/nutrition (ie, nil per os [NPO])12 primarily due to anticipation of further escalation of respiratory support or concern for increased risk of aspiration. The majority of patients with bronchiolitis managed with HFNC, however, do not require escalation of care.5,13 When feeding is attempted during HFNC support, it is frequently interrupted.5 Moreover, keeping all children NPO when receiving HFNC may be associated with weight loss and longer length of stay (LOS).12,14 Two small studies found that children admitted to the intensive care unit who received HFNC support for bronchiolitis did not have increased rates of emesis, worsening respiratory distress or aspiration pneumonia when enterally fed.10,11 However, no comparison of adverse events or LOS has been made between patients who were fed and those who were not fed during HFNC therapy, and previous studies have included only patients who have received HFNC in the intensive care setting.
Supporting safe feeding early in hospitalizations for bronchiolitis may facilitate expedited clinical improvement and discharge. As part of an ongoing bronchiolitis quality improvement initiative at our hospital, we sought to characterize feeding practices during HFNC therapy and assess whether feeding exposure was associated with (1) time to discharge after HFNC or (2) feeding-related adverse events. We hypothesized that feeding during HFNC therapy would be associated with a shorter time to discharge after HFNC cessation.
METHODS
Study Design, Setting, Participants
This was a retrospective cohort study of patients aged 1-24 months receiving HFNC support for respiratory failure due to bronchiolitis at an academic children’s hospital between January 1, 2015 and March 1, 2017. Our institution has had a clinical practice guideline, associated order set, and respiratory therapy protocol for general care patients with bronchiolitis since 2009. Patients with bronchiolitis who were weaning HFNC have been cared for in both the intensive and general care settings since 2013. A formal process for initiation of HFNC on general care units was instituted in January of 2017. During the study period, no patients with HFNC support for bronchiolitis had all their care entirely outside the intensive care unit at our institution. However, initiation and subsequent use of HFNC may have occurred in either the intensive care or general care setting. No specific guidance for feeding during HFNC existed during this period.
Patients were identified using the Virtual PICU Systems database, (VPS LLC, myvps.org, Los Angeles, California) and, by definition, all patients received at least some of their care in the intensive care unit. Patients with comorbid conditions of prematurity (<35 weeks) and those with cardiopulmonary, neuromuscular, and genetic diseases were included. Patients with preexisting dysphagia, defined as ongoing outpatient speech therapy for swallowing concerns, an admission diagnosis of aspiration pneumonia or on home respiratory support, were excluded. Children (n = 7) were excluded if they had more than one period of HFNC during admission. This study was determined to be exempt by the University of Wisconsin School of Medicine and Public Health’s Institutional Review Board.
Data Collection and Study Variables
The following variables were collected from VPS administrative data: patient gender, age, admission and discharge date and time, type and total hours of respiratory support, intensive care admission, and LOS (in hours). Additional demographic, clinical, and feeding exposure variables were abstracted manually from the electronic medical record (Epic, Verona, Wisconsin) using a structured data collection tool and stored in REDCap (Research Electronic Data Capture)15 including prematurity, race/ethnicity, insurance status, primary language, and passive tobacco smoke exposure. Clinical variables included duration of illness (days) at the time of admission, unit of HFNC initiation (emergency department, general care, intensive care, respiratory rate and oxygen saturation at HFNC initiation (<90%, 91%-92%, or >92%), acquisition of blood gas at HFNC admission, duration of time on HFNC (hours) and need for intubation or noninvasive ventilation prior to HFNC. The Pediatric Index of Mortality 2 Risk of Mortality (PIM 2 ROM)16 was used to estimate the severity of illness. The PIM2ROM uses clinical variables (systolic blood pressure, fixed pupils, measure of hypoxia using PaO2/FiO2 ratio, base excess, mechanical ventilation, elective admission, recovery from surgery, cardiac bypass, high-risk diagnosis, low-risk diagnosis) collected at the time of intensive care admission to generate a score that predicts the risk of mortality for an individual patient.17
Feeding exposures were documented in three-hour intervals from HFNC initiation to completion using a structured protocol. At each interval the following feeding information was abstracted from a review of nursing and physician documentation and relevant clinical flowsheets: presence or absence of feeding during the interval, route of feeding (oral, nasogastric [NG] or nasojejunal [NJ]). Feeding exposure was categorized a priori as fed at any point during HFNC (vs not fed at any point). Fed children were further characterized as (1) mixed feeding consisting of oral and tube feeds (NG or NJ) or (2) exclusive oral feeding throughout HFNC support (Appendix 1).
The primary outcome was the number of hours to discharge from HFNC cessation. Secondary outcomes were time to discharge from HFNC initiation, all-cause readmissions within seven days of discharge, and potential feeding-related adverse events. Potential adverse events included: (1) aspiration, defined as initiation of antibiotic AND either chest radiograph official interpreted as evidence for aspiration and/or documented concern for aspiration from the treating physician, or (2) intubation after feeding during HFNC.
Statistical Analysis
Descriptive statistics evaluated differences in demographics and clinical variables for feeding exposure groups. We used chi-squared tests for differences in proportions and t-tests or Wilcoxon Rank-Sum tests for differences in means or medians for continuous variables, respectively. Associations between feeding exposure during HFNC and time to discharge (measured in hours) after HFNC completion were modeled with Cox proportional hazards regression. Using this approach, hazard ratios (HR)>1 indicate a higher hazard (rate) of discharge for children with a feeding exposure than for children without the exposure. For example, a hazard ratio equal to two indicates that the exposed population is discharged at twice the rate per unit time as the nonexposed population. Death or censoring events did not occur. Feeding exposure was first modeled dichotomously as not fed or fed. To further explore associations between feeding modality and our outcome, we then modeled feeding exposure categorically as not fed (reference), mixed (oral and tube) feeding, or exclusive oral feeding throughout HFNC.
After constructing a set of unadjusted models, we then adjusted the models for variables having independent (bivariate P < .10) associations with time to discharge: age, unit of HFNC initiation, highest respiratory support required before HFNC, and HFNC duration. Finally, to attempt to account for residual confounding from latent constructs, we also created a set of propensity-weighted Cox proportional hazards models. Propensity weights18 reflecting the probability of being fed or never being fed during HFNC were created using logistic regression with predictors we hypothesized a priori that may have influenced the clinical decision to feed during HFNC: age, day of illness on admission, prematurity, PIM2 ROM score, respiratory rate, oxygen saturation and blood gas acquisition at HFNC initiation, and highest respiratory support required before HFNC. All analyses were conducted using STATA 14.0 (StataCorp, College Station, Texas), and adjusted hazard ratios (aHR) with 95% confidence intervals (95% CIs) were reported.
RESULTS
Patients (n = 123) had a mean age of 7.3 months (standard deviation [SD] 7.1) and presented on day of illness 4.8 (SD 2.3). Prior to HFNC, 10% required higher respiratory support (3% mechanical ventilation). Former preterm children were 12% of the overall sample.
During HFNC, 37% of patients were never fed, 41% were exclusively orally fed, and 23% had tube or mixed oral and tube feedings (Table 1 and Appendix 2). Children who were not fed were older, but groups were otherwise similar in terms of gender, race/ethnicity, passive smoke exposure, day of illness, unit of HFNC initiation, respiratory support required prior to HFNC, and respiratory rate at HFNC initiation.
Median time to discharge after HFNC completion was 31.4 hours (interquartile range [IQR] 23.9-52). Median (IQR) time to discharge was 29.5 (IQR 23.5-47.9) hours in children who were fed and 39.8 (26.4-61.5) hours in those who were not fed (unadjusted HR 1.25 [0.86-1.82], aHR 1.83 [95% CI: 1.16-2.88]). Time to discharge was shortest when children were fed exclusively orally (Figure). Compared with children who were not fed, time to discharge following HFNC completion was significantly shorter for those who were exclusively orally fed (aHR 2.13 [95% CI: 1.31-3.45]; Table 2). Results of the propensity-weighted model were similar: time to discharge after completing HFNC was shorter in fed versus not fed children (HR 2.17; 95 % CI: 1.34-3.50). The secondary outcome, time to discharge from HFNC initiation, had a similar relationship, ie, shorter time to discharge with exclusive oral feeding vs not feeding [aHR 1.95 (95% CI: 1.19-3.18)]. Time to discharge after initiation of HFNC was also shorter for fed versus not fed in propensity-weighted analysis (HR 1.97; 95% CI: 1.13-3.43).
DISCUSSION
This observational study found that being fed during HFNC was associated with shorter time to discharge after HFNC support was completed. Exclusive oral feeding was associated with the shortest time to discharge, and these results were consistent across a variety of analytical approaches. Adverse events were rare and occurred in both fed and unfed children.
These findings advance research on relationships between nutrition and bronchiolitis outcomes. Studies of general care patients with bronchiolitis without HFNC have observed associations between poor nutrition and prolonged LOS.19,20 Two previous studies of patients receiving HFNC therapy for bronchiolitis concluded that frequent interruption11 and later initiation of enteral nutrition10 during ICU stay was associated with longer time to discharge.11 To our knowledge, this is the first study of patients with bronchiolitis treated with HFNC in both general care and ICU settings that compared outcomes according to whether children were fed during HFNC therapy. Our results extend previous work demonstrating that delays in feeding may be associated with longer LOS.
Decisions to feed children with respiratory distress due to bronchiolitis are complex and often subjective. Readiness to feed may be based upon the assessment of a child’s work of breathing, trajectory of illness, institutional culture, and individual physician, nurse, respiratory therapist or speech-language pathologist comfort. In the absence of established feeding best practices,21 some institutions have developed guidelines based on local expert opinion; however, often these recommendations remain largely subjective and nonspecific.5,10,22-24 Although decisions to feed may be influenced by concern about a child’s clinical stability and feeding risk, we found few objective clinical differences between children fed (orally or by enteral tube) or not fed. Moreover, our results were consistent even when we used a propensity-weighted model to account for measured factors that may have been associated with the decision to initiate feeding. This suggests the decision to feed could be more arbitrary than we assume and is important to investigate in future research.
Additionally, although a few early studies have aimed to standardize the process of weaning HFNC support in bronchiolitis,25,26 this process is also largely subjective.10,22,23 As such, the weaning process may be influenced by perceptions of the child’s overall health. Orally fed children may be viewed as more comfortable or well and thus, more readily weaned, which ultimately influences the length of HFNC therapy. Our study design attempted to account for this potential bias by measuring time to discharge following HFNC therapy, rather than measuring total LOS. Meeting adequate calorie, weight, or hydration goals prior to discharge may take longer if feeds have been withheld. We speculate that prolonged periods of NPO might also risk transient oral aversion or feeding discoordination that could influence LOS. Previous research involving broad intensive care unit populations has established the importance of providing nutrition to critically ill children as soon as possible as a means of improving outcomes.27-29 Patients receiving HFNC support for bronchiolitis could plausibly experience similar benefits.
This single-center study with a relatively small sample size has important limitations to consider. The observational design limits our ability to draw conclusions about causal relationships between feeding, time to discharge, and adverse events. In particular, feeding exposure did not account for nuances in feeding timing, feeding density, and other elements of feeding exposure. Additionally, adverse events are rare, and this study is inadequately powered to detect differences between exposure groups. Although we included children cared for in general and intensive care units, our findings may not be generalizable to other hospitals with different placement criteria. Despite the creation of adjusted and propensity-weighted models, our results are still subject to possible residual indication bias. We cannot control for all possible confounders, particularly unmeasured factors which might simultaneously motivate decisions whether, when, and how to feed children receiving HFNC therapy and influence time to discharge after HFNC is finished. Although this study observed associations between feeding during HFNC and both our primary (time to discharge after HFNC was complete) and secondary (time to discharge after HFNC was initiated) outcomes, future work should evaluate how feeding strategies might impact total LOS, particularly as management becomes more standardized.
Prospective studies of feeding exposures during HFNC therapy in bronchiolitis, as well as rigorous interventional study designs, are needed to confirm shorter lengths of stay and safety with larger and more diverse samples. Future research should evaluate methods to safely and effectively feed children with severe bronchiolitis, which would inform standardized evidence-based approaches. Given the scale on which children with bronchiolitis are admitted each year, the implications of such work could be substantial.
CONCLUSION
Children fed while receiving HFNC for bronchiolitis may have shorter time to discharge than those who are not fed. Feeding-related adverse events were rare regardless of the feeding method. Controlled prospective studies addressing residual confounding are needed to justify a change in the current practice.
Acknowledgments
The authors would like to acknowledge the valuable feedback on earlier drafts from members of the University of Wisconsin Division of Pediatric Hospital Medicine CREATE writing group.
Bronchiolitis is the most common cause of nonbirth hospitalization in children in the United States less than one year of age.1 For children with severe bronchiolitis, high-flow nasal cannula (HFNC) is increasingly used2-4 to reduce work of breathing and prevent the need for further escalation of ventilatory support.5,6 Although previous studies suggest that enteral feeding is recommended in the management of patients hospitalized with bronchiolitis,7-9 limited evidence exists to guide feeding practices for patients receiving HFNC support.5,10,11
Respiratory support with HFNC has been associated with prolonged periods without enteral hydration/nutrition (ie, nil per os [NPO])12 primarily due to anticipation of further escalation of respiratory support or concern for increased risk of aspiration. The majority of patients with bronchiolitis managed with HFNC, however, do not require escalation of care.5,13 When feeding is attempted during HFNC support, it is frequently interrupted.5 Moreover, keeping all children NPO when receiving HFNC may be associated with weight loss and longer length of stay (LOS).12,14 Two small studies found that children admitted to the intensive care unit who received HFNC support for bronchiolitis did not have increased rates of emesis, worsening respiratory distress or aspiration pneumonia when enterally fed.10,11 However, no comparison of adverse events or LOS has been made between patients who were fed and those who were not fed during HFNC therapy, and previous studies have included only patients who have received HFNC in the intensive care setting.
Supporting safe feeding early in hospitalizations for bronchiolitis may facilitate expedited clinical improvement and discharge. As part of an ongoing bronchiolitis quality improvement initiative at our hospital, we sought to characterize feeding practices during HFNC therapy and assess whether feeding exposure was associated with (1) time to discharge after HFNC or (2) feeding-related adverse events. We hypothesized that feeding during HFNC therapy would be associated with a shorter time to discharge after HFNC cessation.
METHODS
Study Design, Setting, Participants
This was a retrospective cohort study of patients aged 1-24 months receiving HFNC support for respiratory failure due to bronchiolitis at an academic children’s hospital between January 1, 2015 and March 1, 2017. Our institution has had a clinical practice guideline, associated order set, and respiratory therapy protocol for general care patients with bronchiolitis since 2009. Patients with bronchiolitis who were weaning HFNC have been cared for in both the intensive and general care settings since 2013. A formal process for initiation of HFNC on general care units was instituted in January of 2017. During the study period, no patients with HFNC support for bronchiolitis had all their care entirely outside the intensive care unit at our institution. However, initiation and subsequent use of HFNC may have occurred in either the intensive care or general care setting. No specific guidance for feeding during HFNC existed during this period.
Patients were identified using the Virtual PICU Systems database, (VPS LLC, myvps.org, Los Angeles, California) and, by definition, all patients received at least some of their care in the intensive care unit. Patients with comorbid conditions of prematurity (<35 weeks) and those with cardiopulmonary, neuromuscular, and genetic diseases were included. Patients with preexisting dysphagia, defined as ongoing outpatient speech therapy for swallowing concerns, an admission diagnosis of aspiration pneumonia or on home respiratory support, were excluded. Children (n = 7) were excluded if they had more than one period of HFNC during admission. This study was determined to be exempt by the University of Wisconsin School of Medicine and Public Health’s Institutional Review Board.
Data Collection and Study Variables
The following variables were collected from VPS administrative data: patient gender, age, admission and discharge date and time, type and total hours of respiratory support, intensive care admission, and LOS (in hours). Additional demographic, clinical, and feeding exposure variables were abstracted manually from the electronic medical record (Epic, Verona, Wisconsin) using a structured data collection tool and stored in REDCap (Research Electronic Data Capture)15 including prematurity, race/ethnicity, insurance status, primary language, and passive tobacco smoke exposure. Clinical variables included duration of illness (days) at the time of admission, unit of HFNC initiation (emergency department, general care, intensive care, respiratory rate and oxygen saturation at HFNC initiation (<90%, 91%-92%, or >92%), acquisition of blood gas at HFNC admission, duration of time on HFNC (hours) and need for intubation or noninvasive ventilation prior to HFNC. The Pediatric Index of Mortality 2 Risk of Mortality (PIM 2 ROM)16 was used to estimate the severity of illness. The PIM2ROM uses clinical variables (systolic blood pressure, fixed pupils, measure of hypoxia using PaO2/FiO2 ratio, base excess, mechanical ventilation, elective admission, recovery from surgery, cardiac bypass, high-risk diagnosis, low-risk diagnosis) collected at the time of intensive care admission to generate a score that predicts the risk of mortality for an individual patient.17
Feeding exposures were documented in three-hour intervals from HFNC initiation to completion using a structured protocol. At each interval the following feeding information was abstracted from a review of nursing and physician documentation and relevant clinical flowsheets: presence or absence of feeding during the interval, route of feeding (oral, nasogastric [NG] or nasojejunal [NJ]). Feeding exposure was categorized a priori as fed at any point during HFNC (vs not fed at any point). Fed children were further characterized as (1) mixed feeding consisting of oral and tube feeds (NG or NJ) or (2) exclusive oral feeding throughout HFNC support (Appendix 1).
The primary outcome was the number of hours to discharge from HFNC cessation. Secondary outcomes were time to discharge from HFNC initiation, all-cause readmissions within seven days of discharge, and potential feeding-related adverse events. Potential adverse events included: (1) aspiration, defined as initiation of antibiotic AND either chest radiograph official interpreted as evidence for aspiration and/or documented concern for aspiration from the treating physician, or (2) intubation after feeding during HFNC.
Statistical Analysis
Descriptive statistics evaluated differences in demographics and clinical variables for feeding exposure groups. We used chi-squared tests for differences in proportions and t-tests or Wilcoxon Rank-Sum tests for differences in means or medians for continuous variables, respectively. Associations between feeding exposure during HFNC and time to discharge (measured in hours) after HFNC completion were modeled with Cox proportional hazards regression. Using this approach, hazard ratios (HR)>1 indicate a higher hazard (rate) of discharge for children with a feeding exposure than for children without the exposure. For example, a hazard ratio equal to two indicates that the exposed population is discharged at twice the rate per unit time as the nonexposed population. Death or censoring events did not occur. Feeding exposure was first modeled dichotomously as not fed or fed. To further explore associations between feeding modality and our outcome, we then modeled feeding exposure categorically as not fed (reference), mixed (oral and tube) feeding, or exclusive oral feeding throughout HFNC.
After constructing a set of unadjusted models, we then adjusted the models for variables having independent (bivariate P < .10) associations with time to discharge: age, unit of HFNC initiation, highest respiratory support required before HFNC, and HFNC duration. Finally, to attempt to account for residual confounding from latent constructs, we also created a set of propensity-weighted Cox proportional hazards models. Propensity weights18 reflecting the probability of being fed or never being fed during HFNC were created using logistic regression with predictors we hypothesized a priori that may have influenced the clinical decision to feed during HFNC: age, day of illness on admission, prematurity, PIM2 ROM score, respiratory rate, oxygen saturation and blood gas acquisition at HFNC initiation, and highest respiratory support required before HFNC. All analyses were conducted using STATA 14.0 (StataCorp, College Station, Texas), and adjusted hazard ratios (aHR) with 95% confidence intervals (95% CIs) were reported.
RESULTS
Patients (n = 123) had a mean age of 7.3 months (standard deviation [SD] 7.1) and presented on day of illness 4.8 (SD 2.3). Prior to HFNC, 10% required higher respiratory support (3% mechanical ventilation). Former preterm children were 12% of the overall sample.
During HFNC, 37% of patients were never fed, 41% were exclusively orally fed, and 23% had tube or mixed oral and tube feedings (Table 1 and Appendix 2). Children who were not fed were older, but groups were otherwise similar in terms of gender, race/ethnicity, passive smoke exposure, day of illness, unit of HFNC initiation, respiratory support required prior to HFNC, and respiratory rate at HFNC initiation.
Median time to discharge after HFNC completion was 31.4 hours (interquartile range [IQR] 23.9-52). Median (IQR) time to discharge was 29.5 (IQR 23.5-47.9) hours in children who were fed and 39.8 (26.4-61.5) hours in those who were not fed (unadjusted HR 1.25 [0.86-1.82], aHR 1.83 [95% CI: 1.16-2.88]). Time to discharge was shortest when children were fed exclusively orally (Figure). Compared with children who were not fed, time to discharge following HFNC completion was significantly shorter for those who were exclusively orally fed (aHR 2.13 [95% CI: 1.31-3.45]; Table 2). Results of the propensity-weighted model were similar: time to discharge after completing HFNC was shorter in fed versus not fed children (HR 2.17; 95 % CI: 1.34-3.50). The secondary outcome, time to discharge from HFNC initiation, had a similar relationship, ie, shorter time to discharge with exclusive oral feeding vs not feeding [aHR 1.95 (95% CI: 1.19-3.18)]. Time to discharge after initiation of HFNC was also shorter for fed versus not fed in propensity-weighted analysis (HR 1.97; 95% CI: 1.13-3.43).
DISCUSSION
This observational study found that being fed during HFNC was associated with shorter time to discharge after HFNC support was completed. Exclusive oral feeding was associated with the shortest time to discharge, and these results were consistent across a variety of analytical approaches. Adverse events were rare and occurred in both fed and unfed children.
These findings advance research on relationships between nutrition and bronchiolitis outcomes. Studies of general care patients with bronchiolitis without HFNC have observed associations between poor nutrition and prolonged LOS.19,20 Two previous studies of patients receiving HFNC therapy for bronchiolitis concluded that frequent interruption11 and later initiation of enteral nutrition10 during ICU stay was associated with longer time to discharge.11 To our knowledge, this is the first study of patients with bronchiolitis treated with HFNC in both general care and ICU settings that compared outcomes according to whether children were fed during HFNC therapy. Our results extend previous work demonstrating that delays in feeding may be associated with longer LOS.
Decisions to feed children with respiratory distress due to bronchiolitis are complex and often subjective. Readiness to feed may be based upon the assessment of a child’s work of breathing, trajectory of illness, institutional culture, and individual physician, nurse, respiratory therapist or speech-language pathologist comfort. In the absence of established feeding best practices,21 some institutions have developed guidelines based on local expert opinion; however, often these recommendations remain largely subjective and nonspecific.5,10,22-24 Although decisions to feed may be influenced by concern about a child’s clinical stability and feeding risk, we found few objective clinical differences between children fed (orally or by enteral tube) or not fed. Moreover, our results were consistent even when we used a propensity-weighted model to account for measured factors that may have been associated with the decision to initiate feeding. This suggests the decision to feed could be more arbitrary than we assume and is important to investigate in future research.
Additionally, although a few early studies have aimed to standardize the process of weaning HFNC support in bronchiolitis,25,26 this process is also largely subjective.10,22,23 As such, the weaning process may be influenced by perceptions of the child’s overall health. Orally fed children may be viewed as more comfortable or well and thus, more readily weaned, which ultimately influences the length of HFNC therapy. Our study design attempted to account for this potential bias by measuring time to discharge following HFNC therapy, rather than measuring total LOS. Meeting adequate calorie, weight, or hydration goals prior to discharge may take longer if feeds have been withheld. We speculate that prolonged periods of NPO might also risk transient oral aversion or feeding discoordination that could influence LOS. Previous research involving broad intensive care unit populations has established the importance of providing nutrition to critically ill children as soon as possible as a means of improving outcomes.27-29 Patients receiving HFNC support for bronchiolitis could plausibly experience similar benefits.
This single-center study with a relatively small sample size has important limitations to consider. The observational design limits our ability to draw conclusions about causal relationships between feeding, time to discharge, and adverse events. In particular, feeding exposure did not account for nuances in feeding timing, feeding density, and other elements of feeding exposure. Additionally, adverse events are rare, and this study is inadequately powered to detect differences between exposure groups. Although we included children cared for in general and intensive care units, our findings may not be generalizable to other hospitals with different placement criteria. Despite the creation of adjusted and propensity-weighted models, our results are still subject to possible residual indication bias. We cannot control for all possible confounders, particularly unmeasured factors which might simultaneously motivate decisions whether, when, and how to feed children receiving HFNC therapy and influence time to discharge after HFNC is finished. Although this study observed associations between feeding during HFNC and both our primary (time to discharge after HFNC was complete) and secondary (time to discharge after HFNC was initiated) outcomes, future work should evaluate how feeding strategies might impact total LOS, particularly as management becomes more standardized.
Prospective studies of feeding exposures during HFNC therapy in bronchiolitis, as well as rigorous interventional study designs, are needed to confirm shorter lengths of stay and safety with larger and more diverse samples. Future research should evaluate methods to safely and effectively feed children with severe bronchiolitis, which would inform standardized evidence-based approaches. Given the scale on which children with bronchiolitis are admitted each year, the implications of such work could be substantial.
CONCLUSION
Children fed while receiving HFNC for bronchiolitis may have shorter time to discharge than those who are not fed. Feeding-related adverse events were rare regardless of the feeding method. Controlled prospective studies addressing residual confounding are needed to justify a change in the current practice.
Acknowledgments
The authors would like to acknowledge the valuable feedback on earlier drafts from members of the University of Wisconsin Division of Pediatric Hospital Medicine CREATE writing group.
1. HCUPnet. http s://hcupnet.ahrq.gov/. Accessed February 7, 2019.
2. Beggs S, Wong ZH, Kaul S, Ogden KJ, Walters JA. High-flow nasal cannula therapy for infants with bronchiolitis. Cochrane Database Syst Rev. 2014;1(1):CD009609. https://doi.org/10.1002/14651858.CD009609.pub2.
3. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
4. Hilliard TN, Archer N, Laura H, et al. Pilot study of vapotherm oxygen delivery in moderately severe bronchiolitis. Arch Dis Child. 2012;97(2):182-183. https://doi.org/10.1136/archdischild-2011-301151.
5. Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(12):1121-1131. https://doi.org/10.1056/NEJMoa1714855.
6. McKiernan C, Chua LC, Visintainer PF, Allen H. High flow nasal cannulae therapy in infants with bronchiolitis. J Pediatr. 2010;156(4):634-638. https://doi.org/10.1016/j.jpeds.2009.10.039.
7. Maffey A, Moviglia T, Mirabello C, et al. Swallowing and respiratory distress in hospitalized patients with bronchiolitis. Dysphagia. 2013;28(4):582-587. https://doi.org/10.1007/s00455-013-9470-0.
8. Kugelman A, Raibin K, Dabbah H, et al. Intravenous fluids versus gastric-tube feeding in hospitalized infants with viral bronchiolitis: a randomized, prospective pilot study. J Pediatr. 2013;162(3):640-642.e641. https://doi.org/10.1016/j.jpeds.2012.10.057.
9. Oakley E, Borland M, Neutze J, et al. Nasogastric hydration versus intravenous hydration for infants with bronchiolitis: a randomised trial. Lancet Respir Med. 2013;1(2):113-120. https://doi.org/10.1016/S2213-2600(12)70053-X.
10. Slain KN, Martinez-Schlurmann N, Shein SL, Stormorken A. Nutrition and high-flow nasal cannula respiratory support in children with bronchiolitis. Hosp Pediatr. 2017;7(5):256-262. https://doi.org/10.1542/hpeds.2016-0194.
11. Sochet AA, McGee JA, October TW. Oral nutrition in children with bronchiolitis on high-flow nasal cannula is well tolerated. Hosp Pediatr. 2017;7(5):249-255. https://doi.org/10.1542/hpeds.2016-0131.
12. Canarie MF, Barry S, Carroll CL, et al. Risk factors for delayed enteral nutrition in critically ill children. Pediatr Crit Care Med. 2015;16(8):e283-e289. https://doi.org/10.1097/PCC.0000000000000527.
13. Schibler A, Pham TM, Dunster KR, et al. Reduced intubation rates for infants after introduction of high-flow nasal prong oxygen delivery. Intensive Care Med. 2011;37(5):847-852. https://doi.org/10.1007/s00134-011-2177-5.
14. Hamilton S, McAleer DM, Ariagno K, et al. A stepwise enteral nutrition algorithm for critically ill children helps achieve nutrient delivery goals*. Pediatr Crit Care Med. 2014;15(7):583-589. https://doi.org/10.1097/PCC.0000000000000179.
15. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
16. Slater A, Shann F, Group APS. The suitability of the Pediatric Index of Mortality (PIM), PIM2, the Pediatric Risk of Mortality (PRISM), and PRISM III for monitoring the quality of pediatric intensive care in Australia and New Zealand. Pediatr Crit Care Med. 2004;5(5):447-454. https://doi.org/10.1097/01.PCC.0000138557.31831.65.
17. Slater A, Shann F, Pearson G, Paediatric Index of Mortality Study G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29(2):278-285. https://doi.org/10.1007/s00134-002-1601-2.
18. Lanza ST, Moore JE, Butera NM. Drawing causal inferences using propensity scores: a practical guide for community psychologists. Am J Commun Psychol. 2013;52(3-4):380-392. https://doi.org/10.1007/s10464-013-9604-4.
19. Weisgerber MC, Lye PS, Li SH, et al. Factors predicting prolonged hospital stay for infants with bronchiolitis. J Hosp Med. 2011;6(5):264-270. https://doi.org/10.1002/jhm.903.
20. Halvorson EE, Chandler N, Neiberg R, Ervin SE. Association of NPO status and type of nutritional support on weight and length of stay in infants hospitalized with bronchiolitis. Hosp Pediatr. 2013;3(4):366-370. https://doi.org/10.1542/hpeds.2013-0011.
21. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742.
22. Seattle Children’s Hospital ZS, Beardsley E, Crotwell D, et al. Bronchiolitis Pathway. http:// www.seattlechildrens.org/pdf/bronchiolitis-pathway.pdf. Accessed January 29, 2019.
23. Children’s Hospital of Philidelphia DM, Zorc J, Kreindler, J, et al. Inpatient Pathway for Treatment of the Child with Bronchiolitis. https://www.chop.edu/clinical-pathway/bronchiolitis-inpatient-treatment-clinical-pathway. Accessed January 29, 2019.
24. Children’s Hospital Colorado TA, Topoz I, Freeman J, et al. Pediatric Viral Bronchiolitis. https://www.childrenscolorado.org/globalassets/healthcare-professionals/clinical-pathways/bronchiolitis.pdf. Accessed January 29, 2019.
25. Betters KA, Hebbar KB, McCracken C, et al. A novel weaning protocol for high-flow nasal cannula in the PICU. Pediatr Crit Care Med. 2017;18(7):e274-e280. https://doi.org/10.1097/PCC.0000000000001181.
26. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
1. HCUPnet. http s://hcupnet.ahrq.gov/. Accessed February 7, 2019.
2. Beggs S, Wong ZH, Kaul S, Ogden KJ, Walters JA. High-flow nasal cannula therapy for infants with bronchiolitis. Cochrane Database Syst Rev. 2014;1(1):CD009609. https://doi.org/10.1002/14651858.CD009609.pub2.
3. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
4. Hilliard TN, Archer N, Laura H, et al. Pilot study of vapotherm oxygen delivery in moderately severe bronchiolitis. Arch Dis Child. 2012;97(2):182-183. https://doi.org/10.1136/archdischild-2011-301151.
5. Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(12):1121-1131. https://doi.org/10.1056/NEJMoa1714855.
6. McKiernan C, Chua LC, Visintainer PF, Allen H. High flow nasal cannulae therapy in infants with bronchiolitis. J Pediatr. 2010;156(4):634-638. https://doi.org/10.1016/j.jpeds.2009.10.039.
7. Maffey A, Moviglia T, Mirabello C, et al. Swallowing and respiratory distress in hospitalized patients with bronchiolitis. Dysphagia. 2013;28(4):582-587. https://doi.org/10.1007/s00455-013-9470-0.
8. Kugelman A, Raibin K, Dabbah H, et al. Intravenous fluids versus gastric-tube feeding in hospitalized infants with viral bronchiolitis: a randomized, prospective pilot study. J Pediatr. 2013;162(3):640-642.e641. https://doi.org/10.1016/j.jpeds.2012.10.057.
9. Oakley E, Borland M, Neutze J, et al. Nasogastric hydration versus intravenous hydration for infants with bronchiolitis: a randomised trial. Lancet Respir Med. 2013;1(2):113-120. https://doi.org/10.1016/S2213-2600(12)70053-X.
10. Slain KN, Martinez-Schlurmann N, Shein SL, Stormorken A. Nutrition and high-flow nasal cannula respiratory support in children with bronchiolitis. Hosp Pediatr. 2017;7(5):256-262. https://doi.org/10.1542/hpeds.2016-0194.
11. Sochet AA, McGee JA, October TW. Oral nutrition in children with bronchiolitis on high-flow nasal cannula is well tolerated. Hosp Pediatr. 2017;7(5):249-255. https://doi.org/10.1542/hpeds.2016-0131.
12. Canarie MF, Barry S, Carroll CL, et al. Risk factors for delayed enteral nutrition in critically ill children. Pediatr Crit Care Med. 2015;16(8):e283-e289. https://doi.org/10.1097/PCC.0000000000000527.
13. Schibler A, Pham TM, Dunster KR, et al. Reduced intubation rates for infants after introduction of high-flow nasal prong oxygen delivery. Intensive Care Med. 2011;37(5):847-852. https://doi.org/10.1007/s00134-011-2177-5.
14. Hamilton S, McAleer DM, Ariagno K, et al. A stepwise enteral nutrition algorithm for critically ill children helps achieve nutrient delivery goals*. Pediatr Crit Care Med. 2014;15(7):583-589. https://doi.org/10.1097/PCC.0000000000000179.
15. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
16. Slater A, Shann F, Group APS. The suitability of the Pediatric Index of Mortality (PIM), PIM2, the Pediatric Risk of Mortality (PRISM), and PRISM III for monitoring the quality of pediatric intensive care in Australia and New Zealand. Pediatr Crit Care Med. 2004;5(5):447-454. https://doi.org/10.1097/01.PCC.0000138557.31831.65.
17. Slater A, Shann F, Pearson G, Paediatric Index of Mortality Study G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29(2):278-285. https://doi.org/10.1007/s00134-002-1601-2.
18. Lanza ST, Moore JE, Butera NM. Drawing causal inferences using propensity scores: a practical guide for community psychologists. Am J Commun Psychol. 2013;52(3-4):380-392. https://doi.org/10.1007/s10464-013-9604-4.
19. Weisgerber MC, Lye PS, Li SH, et al. Factors predicting prolonged hospital stay for infants with bronchiolitis. J Hosp Med. 2011;6(5):264-270. https://doi.org/10.1002/jhm.903.
20. Halvorson EE, Chandler N, Neiberg R, Ervin SE. Association of NPO status and type of nutritional support on weight and length of stay in infants hospitalized with bronchiolitis. Hosp Pediatr. 2013;3(4):366-370. https://doi.org/10.1542/hpeds.2013-0011.
21. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742.
22. Seattle Children’s Hospital ZS, Beardsley E, Crotwell D, et al. Bronchiolitis Pathway. http:// www.seattlechildrens.org/pdf/bronchiolitis-pathway.pdf. Accessed January 29, 2019.
23. Children’s Hospital of Philidelphia DM, Zorc J, Kreindler, J, et al. Inpatient Pathway for Treatment of the Child with Bronchiolitis. https://www.chop.edu/clinical-pathway/bronchiolitis-inpatient-treatment-clinical-pathway. Accessed January 29, 2019.
24. Children’s Hospital Colorado TA, Topoz I, Freeman J, et al. Pediatric Viral Bronchiolitis. https://www.childrenscolorado.org/globalassets/healthcare-professionals/clinical-pathways/bronchiolitis.pdf. Accessed January 29, 2019.
25. Betters KA, Hebbar KB, McCracken C, et al. A novel weaning protocol for high-flow nasal cannula in the PICU. Pediatr Crit Care Med. 2017;18(7):e274-e280. https://doi.org/10.1097/PCC.0000000000001181.
26. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
© 2019 Society of Hospital Medicine
Does Scheduling a Postdischarge Visit with a Primary Care Physician Increase Rates of Follow-up and Decrease Readmissions?
Under the Hospital Readmission Reduction Program (HRRP), hospitals with higher than expected readmissions for select conditions receive a financial penalty. In 2017, hospitals were penalized a total of $528 million.1,2 In an effort to deter readmissions, hospitals have focused on the transition from inpatient to outpatient care with particular emphasis on timely follow-up with a primary care physician (PCP).3-7 Medicare has also introduced transitional care codes, which reimburse physicians for follow-up care after a hospitalization.
METHODS
Postdischarge Appointment Service
In the fall of 2009, Beth Israel Deaconess introduced a postdischarge appointment intervention to facilitate follow-up with PCPs and specialty physicians after discharge from the hospital. Within the provider order entry system, attending and resident physicians enter a discharge appointment request for specified providers within and outside of the medical center and a specified time period. For example, a physician may enter a request to schedule a PCP appointment within 2-3, 4-8, 9-15, 16-30, or >30 days of discharge.
Study Population
We conducted a retrospective, cohort study at Beth Israel Deaconess Medical Center, a tertiary care hospital, using data derived from electronic health records for all hospitalizations
Outcomes
The primary outcomes of this study were kept PCP follow-up visits within seven days and readmission within 30 days of discharge. We focused on PCP visits within seven days, as this has been the measure used in prior research,5,7 but conducted a sensitivity analysis of PCP follow-up within 14 days. No-shows for the scheduled follow-up PCP appointments were not included. We focused on readmissions within 30 days of discharge, given this is the measure used in the HRRP,16 but conducted a sensitivity analysis of 14 days. Secondary outcomes included ED revisit within the 30 days. Given the data available, we only observed physician visits and hospitalizations that occurred within the Beth Israel Deaconess system.
Analyses
We conducted two analyses to assess whether the implementation of the postdischarge appointment service was associated with an increase in PCP follow-up and a decrease in the readmission rate.
In the first analysis, we focused only on hospitalizations from the medical and cardiology services during the postintervention period between January 2011 and September 2015 (n = 17,582). We compared the PCP follow-up rate and the readmission rate among hospitalizations where the postdischarge appointment service was used versus those where it was not used. We used a multivariable logistic regression, and the covariates included in the model were age, gender, hospital length of stay, and diagnosis-related group (DRG) cost weight. The DRG cost weight captures the average resources used to treat Medicare patients’ hospitalizations within a given DRG category and was used as a surrogate marker for the complexity of hospitalization.17 Instead of presenting odds ratios, we used predictive margins to generate adjusted percentage point estimates of the differences in our outcomes associated with the use of the postdischarge appointment service.18
This instrumental variable exploits the fact that the postdischarge appointment service was only available on weekdays and that physicians are asked to only submit the order for follow-up appointments on the day of discharge. We focused on the day of the week of admission (versus discharge) because of concerns that patients with more complicated hospital courses might be kept in the hospital over the weekend (eg, to facilitate testing available only on weekdays or to consult with regular physicians only available on weekdays). This would create a relationship between the day of discharge and the outcomes (follow-up visits, readmissions). The day of admission is less likely to be impacted by this bias. Given concerns that admissions on different days of the week might be different, our instrument is the day of the week interacted with the time period. Therefore, to create bias, there must be a systematic change in the nature of admissions on a given day of the week during this time period. We provide more details on this analysis, testing of the instrument, and results in the Appendix.
Analyses were conducted in Stata, version 14.2 (StataCorp LP, College Station, Texas). Statistical testing was two-sided, with a significance level of 0.05, and the project was judged exempt by the Committee on Clinical Investigations for Beth Israel Deaconess Medical Center.
RESULTS
Overall, there were 17,582 hospitalizations on the medicine and cardiology services following implementation of the postdischarge appointment service. The use of the postdischarge appointment service rose rapidly after it was introduced (Figure) and then plateaued at roughly 50%.
Multivariable Logistic Regression
In this analysis, we focused on the 17,582 hospitalizations from January 2011 to September 2015 on the general medicine and cardiology services that occurred after the postdischarge appointment service was introduced. Among these hospitalizations, the postdischarge appointment service was used in 51.8% of discharges.
In an unadjusted analysis, patients discharged using the tool had higher rates of seven-day PCP follow-up (60.2% vs 29.2%, P < .001) and lower 30-day readmission rates (14.7% vs 16.7%; P < .001) than those who were not (Table 2). There was no significant difference in 30-day ED revisit between hospitalizations with and without use of the postdischarge appointment service (22.3% vs 23.1%; P = .23).
This was echoed in our multivariable analysis where, controlling for other patient factors, use of the postdischarge appointment service was associated with an increased rate of follow-up with a PCP in seven days (+31.9 percentage points; 95% CI: 30.2, 33.6; P < .01) and a decreased likelihood of readmission within 30 days (−3.8 percentage points; 95% CI: −5.2, −2.4; P < .01) (Table 2).
Instrumental Variable Analysis
In our instrumental variable analysis, we used all hospitalizations both before and after the introduction of the intervention. In this analysis, we estimate that use of the postdischarge appointment service increases the probability of visiting a PCP within seven days by 33.4 percentage points (95% CI: 7.9%, 58.9%; P = .01) (Table 3). The use of the postdischarge appointment was associated with a 2.5 percentage point (95% CI: −22.0%, 17.1%; P = .80) reduction in readmissions and a 4.8 percentage point (95% CI; −27.5%, 17.9%; P = .68) reduction in an ED visit within 30 days (Table 3). Neither of these differences were statistically significant with wide confidence intervals.
In sensitivity analyses, we obtained similar results when we considered PCP visits and readmissions within 14 days.
DISCUSSION
The hospital introduced the postdischarge appointment service to facilitate postdischarge appointments and to deter readmissions. In our analyses the use of the postdischarge appointment service was associated with a substantial 30 percentage point increase in the likelihood of a PCP follow-up visit within seven days after hospital discharge. There was a roughly 2% reduction in 30-day readmissions, but this difference was not consistently statistically significant across our analyses. Together, our evaluation implies that this type of intervention may make it much easier for patients to attend a PCP appointment, but scheduling an appointment alone may have a modest impact on deterring a readmission.
Our findings are inconsistent with prior studies that described a strong association between early PCP follow-up and readmissions. However, our results were consistent with research where follow-up visits were not clearly protective against readmissions.20 One potential explanation of the discrepant findings is that there are unmeasured socioeconomic differences between patients who have a PCP follow-up appointment and those who do not.
Regardless of the impact on readmissions, it is important to acknowledge that early PCP follow-up offers many potential benefits. Continuing to evaluate and treat new diagnoses, adjusting and reconciling medications, reconnecting with outpatient providers, capturing new incidental findings, and ensuring stability through regular follow-up are just a few of the potential benefits. We believe the dramatic increase observed in PCP follow-up reflects the administrative complexity required for a patient to call their PCP’s office and to schedule a follow-up appointment soon after they are discharged from the hospital.
Our study has many limitations. The study was limited to a single academic center, and the intervention was limited to patients cared for by the general medicine and cardiology services.
In summary, we found that the introduction of a postdischarge appointment service resulted in substantially increased rates of early PCP follow-up but less clear benefits in preventing readmissions.
1. Boccutti C, Casillas G. Aiming for Fewer Hospital U-turns: The Medicare Hospital Readmission Reduction Program; March 10, 2017. https://www.kff.org/medicare/issue-brief/aiming-for-fewer-hospital-u-turns-the-medicare-hospital-readmission-reduction-program. Accessed July 22, 2019
2. Centers for Medicare and Medicaid Services. FY 2017 IPPS Final Rule: Hospital Readmissions Reduction Program Su pplemental Data File. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Archived-Supplemental-Data-Files.html. Accessed June 22, 2019
3. Sharma G, Kuo YF, Freeman JL, Zhang DD, Goodwin JS. Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010;170(18):1664-1670. https://doi.org/10.1001/archinternmed.2010.345.
4. Rennke S, Nguyen OK, Shoeb MH, et al. Hospital-initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):433-440. https://doi.org/10.7326/0003-4819-158-5-201303051-00011.
5. Misky GJ, Wald HL, Coleman EA. Post hospitalization transitions: examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5(7):392-397. https://doi.org/10.1002/jhm.666.
6. Hesselink G, Schoonhoven L, Barach P, et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med. 2012;157(6):417-428. https://doi.org/10.7326/0003-4819-157-6-201209180-00006.
7. Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716-1722. https://doi.org/10.1001/jama.2010.533.
8. Muus KJ, Knudson A, Klug MG, et al. Effect of post discharge follow-up care on re-admissions among US veterans with congestive heart failure: a rural-urban comparison. Rural Remote Health. 2010;10(2):1447.
9. Brooke BS, Stone DH, Cronenwett JL, et al. Early primary care provider follow-up and readmission after high-risk surgery. JAMA Surg. 2014;149(8):821-828. https://doi.org/10.1001/jamasurg.2014.157.
10. Leschke J, Panepinto JA, Nimmer M, et al. Outpatient follow-up and rehospitalizations for sickle cell disease patients. Pediatr Blood Cancer. 2012;58(3):406-409. https://doi.org/10.1002/pbc.23140.
11. Field TS, Ogarek J, Garber L, Reed G, Gurwitz JH. Association of early post discharge follow-up by a primary care physician and 30-day rehospitalization among older adults. J Gen Intern Med. 2015;30(5):565-571. https://doi.org/10.1007/s11606-014-3106-4.
12. Kashiwagi DT, Burton MC, Kirkland LL, Cha S, Varkey P. Do timely outpatient follow-up visits decrease hospital readmission rates? Am J Med Qual. 2012;27(1):11-15. https://doi.org/10.1177/1062860611409197.
13. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. https://doi.org/10.7326/0003-4819-155-8-201110180-00008.
14. Ryan J, Kang S, Dolacky S, Ingrassia J, Ganeshan R. Change in readmissions and follow-up visits as part of a heart failure readmission quality improvement initiative. Am J Med. 2013;126(11):989–994.e1. https://doi.org/10.1016/j.amjmed.2013.06.027.
15. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. https://doi.org/10.1001/archinte.166.17.1822.
16. Thomas JW. Should episode-based economic profiles be risk adjusted to account for differences in patients’ health risks? Health Serv Res. 2006;41(2):581-598. https://doi.org/10.1111/j.1475-6773.2005.00499.x.
17. Mendez CM, Harrington DW, Christenson P, Spellberg B. Impact of hospital variables on case mix index as a marker of disease severity. Popul Health Manag. 2014;17(1):28-34. https://doi.org/10.1089/pop.2013.0002.
18. Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43(3):962-970. https://doi.org/10.1093/ije/dyu029.
19. Angrist JD, Krueger AB. Instrumental variables and the search for identification: From supply and demand to natural experiments. J Econ Perspect. 2001;15(4):69-85. https://doi.org/10.1257/jep.15.4.69.
20. Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA. 2014;312(22):2401-2402. https://doi.org/10.1001/jama.2014.16153.
21. Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301(6):603-618. https://doi.org/10.1001/jama.2009.126.
22. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178-187. https://doi.org/10.7326/0003-4819-150-3-200902030-00007.
23. Naylor MD, Brooten DA, Campbell RL, et al. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675-684. https://doi.org/10.1111/j.1532-5415.2004.52202.x.
24. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. https://doi.org/10.1001/jamainternmed.2014.1608.
25. Kripalani S, LeFevre F, Phillips CO, et al. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841. https://doi.org/10.1001/jama.297.8.831.
26. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186-192. https://doi.org/10.1046/j.1525-1497.2002.10741.x.
27. Hoyer EH, Brotman DJ, Apfel A, et al. Improving outcomes after hospitalization: A prospective observational multicenter evaluation of care coordination strategies for reducing 30-day readmissions to Maryland Hospitals. J Gen Intern Med. 2018;33(5):621-627. https://doi.org/10.1007/s11606-017-4218-4.
Under the Hospital Readmission Reduction Program (HRRP), hospitals with higher than expected readmissions for select conditions receive a financial penalty. In 2017, hospitals were penalized a total of $528 million.1,2 In an effort to deter readmissions, hospitals have focused on the transition from inpatient to outpatient care with particular emphasis on timely follow-up with a primary care physician (PCP).3-7 Medicare has also introduced transitional care codes, which reimburse physicians for follow-up care after a hospitalization.
METHODS
Postdischarge Appointment Service
In the fall of 2009, Beth Israel Deaconess introduced a postdischarge appointment intervention to facilitate follow-up with PCPs and specialty physicians after discharge from the hospital. Within the provider order entry system, attending and resident physicians enter a discharge appointment request for specified providers within and outside of the medical center and a specified time period. For example, a physician may enter a request to schedule a PCP appointment within 2-3, 4-8, 9-15, 16-30, or >30 days of discharge.
Study Population
We conducted a retrospective, cohort study at Beth Israel Deaconess Medical Center, a tertiary care hospital, using data derived from electronic health records for all hospitalizations
Outcomes
The primary outcomes of this study were kept PCP follow-up visits within seven days and readmission within 30 days of discharge. We focused on PCP visits within seven days, as this has been the measure used in prior research,5,7 but conducted a sensitivity analysis of PCP follow-up within 14 days. No-shows for the scheduled follow-up PCP appointments were not included. We focused on readmissions within 30 days of discharge, given this is the measure used in the HRRP,16 but conducted a sensitivity analysis of 14 days. Secondary outcomes included ED revisit within the 30 days. Given the data available, we only observed physician visits and hospitalizations that occurred within the Beth Israel Deaconess system.
Analyses
We conducted two analyses to assess whether the implementation of the postdischarge appointment service was associated with an increase in PCP follow-up and a decrease in the readmission rate.
In the first analysis, we focused only on hospitalizations from the medical and cardiology services during the postintervention period between January 2011 and September 2015 (n = 17,582). We compared the PCP follow-up rate and the readmission rate among hospitalizations where the postdischarge appointment service was used versus those where it was not used. We used a multivariable logistic regression, and the covariates included in the model were age, gender, hospital length of stay, and diagnosis-related group (DRG) cost weight. The DRG cost weight captures the average resources used to treat Medicare patients’ hospitalizations within a given DRG category and was used as a surrogate marker for the complexity of hospitalization.17 Instead of presenting odds ratios, we used predictive margins to generate adjusted percentage point estimates of the differences in our outcomes associated with the use of the postdischarge appointment service.18
This instrumental variable exploits the fact that the postdischarge appointment service was only available on weekdays and that physicians are asked to only submit the order for follow-up appointments on the day of discharge. We focused on the day of the week of admission (versus discharge) because of concerns that patients with more complicated hospital courses might be kept in the hospital over the weekend (eg, to facilitate testing available only on weekdays or to consult with regular physicians only available on weekdays). This would create a relationship between the day of discharge and the outcomes (follow-up visits, readmissions). The day of admission is less likely to be impacted by this bias. Given concerns that admissions on different days of the week might be different, our instrument is the day of the week interacted with the time period. Therefore, to create bias, there must be a systematic change in the nature of admissions on a given day of the week during this time period. We provide more details on this analysis, testing of the instrument, and results in the Appendix.
Analyses were conducted in Stata, version 14.2 (StataCorp LP, College Station, Texas). Statistical testing was two-sided, with a significance level of 0.05, and the project was judged exempt by the Committee on Clinical Investigations for Beth Israel Deaconess Medical Center.
RESULTS
Overall, there were 17,582 hospitalizations on the medicine and cardiology services following implementation of the postdischarge appointment service. The use of the postdischarge appointment service rose rapidly after it was introduced (Figure) and then plateaued at roughly 50%.
Multivariable Logistic Regression
In this analysis, we focused on the 17,582 hospitalizations from January 2011 to September 2015 on the general medicine and cardiology services that occurred after the postdischarge appointment service was introduced. Among these hospitalizations, the postdischarge appointment service was used in 51.8% of discharges.
In an unadjusted analysis, patients discharged using the tool had higher rates of seven-day PCP follow-up (60.2% vs 29.2%, P < .001) and lower 30-day readmission rates (14.7% vs 16.7%; P < .001) than those who were not (Table 2). There was no significant difference in 30-day ED revisit between hospitalizations with and without use of the postdischarge appointment service (22.3% vs 23.1%; P = .23).
This was echoed in our multivariable analysis where, controlling for other patient factors, use of the postdischarge appointment service was associated with an increased rate of follow-up with a PCP in seven days (+31.9 percentage points; 95% CI: 30.2, 33.6; P < .01) and a decreased likelihood of readmission within 30 days (−3.8 percentage points; 95% CI: −5.2, −2.4; P < .01) (Table 2).
Instrumental Variable Analysis
In our instrumental variable analysis, we used all hospitalizations both before and after the introduction of the intervention. In this analysis, we estimate that use of the postdischarge appointment service increases the probability of visiting a PCP within seven days by 33.4 percentage points (95% CI: 7.9%, 58.9%; P = .01) (Table 3). The use of the postdischarge appointment was associated with a 2.5 percentage point (95% CI: −22.0%, 17.1%; P = .80) reduction in readmissions and a 4.8 percentage point (95% CI; −27.5%, 17.9%; P = .68) reduction in an ED visit within 30 days (Table 3). Neither of these differences were statistically significant with wide confidence intervals.
In sensitivity analyses, we obtained similar results when we considered PCP visits and readmissions within 14 days.
DISCUSSION
The hospital introduced the postdischarge appointment service to facilitate postdischarge appointments and to deter readmissions. In our analyses the use of the postdischarge appointment service was associated with a substantial 30 percentage point increase in the likelihood of a PCP follow-up visit within seven days after hospital discharge. There was a roughly 2% reduction in 30-day readmissions, but this difference was not consistently statistically significant across our analyses. Together, our evaluation implies that this type of intervention may make it much easier for patients to attend a PCP appointment, but scheduling an appointment alone may have a modest impact on deterring a readmission.
Our findings are inconsistent with prior studies that described a strong association between early PCP follow-up and readmissions. However, our results were consistent with research where follow-up visits were not clearly protective against readmissions.20 One potential explanation of the discrepant findings is that there are unmeasured socioeconomic differences between patients who have a PCP follow-up appointment and those who do not.
Regardless of the impact on readmissions, it is important to acknowledge that early PCP follow-up offers many potential benefits. Continuing to evaluate and treat new diagnoses, adjusting and reconciling medications, reconnecting with outpatient providers, capturing new incidental findings, and ensuring stability through regular follow-up are just a few of the potential benefits. We believe the dramatic increase observed in PCP follow-up reflects the administrative complexity required for a patient to call their PCP’s office and to schedule a follow-up appointment soon after they are discharged from the hospital.
Our study has many limitations. The study was limited to a single academic center, and the intervention was limited to patients cared for by the general medicine and cardiology services.
In summary, we found that the introduction of a postdischarge appointment service resulted in substantially increased rates of early PCP follow-up but less clear benefits in preventing readmissions.
Under the Hospital Readmission Reduction Program (HRRP), hospitals with higher than expected readmissions for select conditions receive a financial penalty. In 2017, hospitals were penalized a total of $528 million.1,2 In an effort to deter readmissions, hospitals have focused on the transition from inpatient to outpatient care with particular emphasis on timely follow-up with a primary care physician (PCP).3-7 Medicare has also introduced transitional care codes, which reimburse physicians for follow-up care after a hospitalization.
METHODS
Postdischarge Appointment Service
In the fall of 2009, Beth Israel Deaconess introduced a postdischarge appointment intervention to facilitate follow-up with PCPs and specialty physicians after discharge from the hospital. Within the provider order entry system, attending and resident physicians enter a discharge appointment request for specified providers within and outside of the medical center and a specified time period. For example, a physician may enter a request to schedule a PCP appointment within 2-3, 4-8, 9-15, 16-30, or >30 days of discharge.
Study Population
We conducted a retrospective, cohort study at Beth Israel Deaconess Medical Center, a tertiary care hospital, using data derived from electronic health records for all hospitalizations
Outcomes
The primary outcomes of this study were kept PCP follow-up visits within seven days and readmission within 30 days of discharge. We focused on PCP visits within seven days, as this has been the measure used in prior research,5,7 but conducted a sensitivity analysis of PCP follow-up within 14 days. No-shows for the scheduled follow-up PCP appointments were not included. We focused on readmissions within 30 days of discharge, given this is the measure used in the HRRP,16 but conducted a sensitivity analysis of 14 days. Secondary outcomes included ED revisit within the 30 days. Given the data available, we only observed physician visits and hospitalizations that occurred within the Beth Israel Deaconess system.
Analyses
We conducted two analyses to assess whether the implementation of the postdischarge appointment service was associated with an increase in PCP follow-up and a decrease in the readmission rate.
In the first analysis, we focused only on hospitalizations from the medical and cardiology services during the postintervention period between January 2011 and September 2015 (n = 17,582). We compared the PCP follow-up rate and the readmission rate among hospitalizations where the postdischarge appointment service was used versus those where it was not used. We used a multivariable logistic regression, and the covariates included in the model were age, gender, hospital length of stay, and diagnosis-related group (DRG) cost weight. The DRG cost weight captures the average resources used to treat Medicare patients’ hospitalizations within a given DRG category and was used as a surrogate marker for the complexity of hospitalization.17 Instead of presenting odds ratios, we used predictive margins to generate adjusted percentage point estimates of the differences in our outcomes associated with the use of the postdischarge appointment service.18
This instrumental variable exploits the fact that the postdischarge appointment service was only available on weekdays and that physicians are asked to only submit the order for follow-up appointments on the day of discharge. We focused on the day of the week of admission (versus discharge) because of concerns that patients with more complicated hospital courses might be kept in the hospital over the weekend (eg, to facilitate testing available only on weekdays or to consult with regular physicians only available on weekdays). This would create a relationship between the day of discharge and the outcomes (follow-up visits, readmissions). The day of admission is less likely to be impacted by this bias. Given concerns that admissions on different days of the week might be different, our instrument is the day of the week interacted with the time period. Therefore, to create bias, there must be a systematic change in the nature of admissions on a given day of the week during this time period. We provide more details on this analysis, testing of the instrument, and results in the Appendix.
Analyses were conducted in Stata, version 14.2 (StataCorp LP, College Station, Texas). Statistical testing was two-sided, with a significance level of 0.05, and the project was judged exempt by the Committee on Clinical Investigations for Beth Israel Deaconess Medical Center.
RESULTS
Overall, there were 17,582 hospitalizations on the medicine and cardiology services following implementation of the postdischarge appointment service. The use of the postdischarge appointment service rose rapidly after it was introduced (Figure) and then plateaued at roughly 50%.
Multivariable Logistic Regression
In this analysis, we focused on the 17,582 hospitalizations from January 2011 to September 2015 on the general medicine and cardiology services that occurred after the postdischarge appointment service was introduced. Among these hospitalizations, the postdischarge appointment service was used in 51.8% of discharges.
In an unadjusted analysis, patients discharged using the tool had higher rates of seven-day PCP follow-up (60.2% vs 29.2%, P < .001) and lower 30-day readmission rates (14.7% vs 16.7%; P < .001) than those who were not (Table 2). There was no significant difference in 30-day ED revisit between hospitalizations with and without use of the postdischarge appointment service (22.3% vs 23.1%; P = .23).
This was echoed in our multivariable analysis where, controlling for other patient factors, use of the postdischarge appointment service was associated with an increased rate of follow-up with a PCP in seven days (+31.9 percentage points; 95% CI: 30.2, 33.6; P < .01) and a decreased likelihood of readmission within 30 days (−3.8 percentage points; 95% CI: −5.2, −2.4; P < .01) (Table 2).
Instrumental Variable Analysis
In our instrumental variable analysis, we used all hospitalizations both before and after the introduction of the intervention. In this analysis, we estimate that use of the postdischarge appointment service increases the probability of visiting a PCP within seven days by 33.4 percentage points (95% CI: 7.9%, 58.9%; P = .01) (Table 3). The use of the postdischarge appointment was associated with a 2.5 percentage point (95% CI: −22.0%, 17.1%; P = .80) reduction in readmissions and a 4.8 percentage point (95% CI; −27.5%, 17.9%; P = .68) reduction in an ED visit within 30 days (Table 3). Neither of these differences were statistically significant with wide confidence intervals.
In sensitivity analyses, we obtained similar results when we considered PCP visits and readmissions within 14 days.
DISCUSSION
The hospital introduced the postdischarge appointment service to facilitate postdischarge appointments and to deter readmissions. In our analyses the use of the postdischarge appointment service was associated with a substantial 30 percentage point increase in the likelihood of a PCP follow-up visit within seven days after hospital discharge. There was a roughly 2% reduction in 30-day readmissions, but this difference was not consistently statistically significant across our analyses. Together, our evaluation implies that this type of intervention may make it much easier for patients to attend a PCP appointment, but scheduling an appointment alone may have a modest impact on deterring a readmission.
Our findings are inconsistent with prior studies that described a strong association between early PCP follow-up and readmissions. However, our results were consistent with research where follow-up visits were not clearly protective against readmissions.20 One potential explanation of the discrepant findings is that there are unmeasured socioeconomic differences between patients who have a PCP follow-up appointment and those who do not.
Regardless of the impact on readmissions, it is important to acknowledge that early PCP follow-up offers many potential benefits. Continuing to evaluate and treat new diagnoses, adjusting and reconciling medications, reconnecting with outpatient providers, capturing new incidental findings, and ensuring stability through regular follow-up are just a few of the potential benefits. We believe the dramatic increase observed in PCP follow-up reflects the administrative complexity required for a patient to call their PCP’s office and to schedule a follow-up appointment soon after they are discharged from the hospital.
Our study has many limitations. The study was limited to a single academic center, and the intervention was limited to patients cared for by the general medicine and cardiology services.
In summary, we found that the introduction of a postdischarge appointment service resulted in substantially increased rates of early PCP follow-up but less clear benefits in preventing readmissions.
1. Boccutti C, Casillas G. Aiming for Fewer Hospital U-turns: The Medicare Hospital Readmission Reduction Program; March 10, 2017. https://www.kff.org/medicare/issue-brief/aiming-for-fewer-hospital-u-turns-the-medicare-hospital-readmission-reduction-program. Accessed July 22, 2019
2. Centers for Medicare and Medicaid Services. FY 2017 IPPS Final Rule: Hospital Readmissions Reduction Program Su pplemental Data File. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Archived-Supplemental-Data-Files.html. Accessed June 22, 2019
3. Sharma G, Kuo YF, Freeman JL, Zhang DD, Goodwin JS. Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010;170(18):1664-1670. https://doi.org/10.1001/archinternmed.2010.345.
4. Rennke S, Nguyen OK, Shoeb MH, et al. Hospital-initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):433-440. https://doi.org/10.7326/0003-4819-158-5-201303051-00011.
5. Misky GJ, Wald HL, Coleman EA. Post hospitalization transitions: examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5(7):392-397. https://doi.org/10.1002/jhm.666.
6. Hesselink G, Schoonhoven L, Barach P, et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med. 2012;157(6):417-428. https://doi.org/10.7326/0003-4819-157-6-201209180-00006.
7. Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716-1722. https://doi.org/10.1001/jama.2010.533.
8. Muus KJ, Knudson A, Klug MG, et al. Effect of post discharge follow-up care on re-admissions among US veterans with congestive heart failure: a rural-urban comparison. Rural Remote Health. 2010;10(2):1447.
9. Brooke BS, Stone DH, Cronenwett JL, et al. Early primary care provider follow-up and readmission after high-risk surgery. JAMA Surg. 2014;149(8):821-828. https://doi.org/10.1001/jamasurg.2014.157.
10. Leschke J, Panepinto JA, Nimmer M, et al. Outpatient follow-up and rehospitalizations for sickle cell disease patients. Pediatr Blood Cancer. 2012;58(3):406-409. https://doi.org/10.1002/pbc.23140.
11. Field TS, Ogarek J, Garber L, Reed G, Gurwitz JH. Association of early post discharge follow-up by a primary care physician and 30-day rehospitalization among older adults. J Gen Intern Med. 2015;30(5):565-571. https://doi.org/10.1007/s11606-014-3106-4.
12. Kashiwagi DT, Burton MC, Kirkland LL, Cha S, Varkey P. Do timely outpatient follow-up visits decrease hospital readmission rates? Am J Med Qual. 2012;27(1):11-15. https://doi.org/10.1177/1062860611409197.
13. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. https://doi.org/10.7326/0003-4819-155-8-201110180-00008.
14. Ryan J, Kang S, Dolacky S, Ingrassia J, Ganeshan R. Change in readmissions and follow-up visits as part of a heart failure readmission quality improvement initiative. Am J Med. 2013;126(11):989–994.e1. https://doi.org/10.1016/j.amjmed.2013.06.027.
15. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. https://doi.org/10.1001/archinte.166.17.1822.
16. Thomas JW. Should episode-based economic profiles be risk adjusted to account for differences in patients’ health risks? Health Serv Res. 2006;41(2):581-598. https://doi.org/10.1111/j.1475-6773.2005.00499.x.
17. Mendez CM, Harrington DW, Christenson P, Spellberg B. Impact of hospital variables on case mix index as a marker of disease severity. Popul Health Manag. 2014;17(1):28-34. https://doi.org/10.1089/pop.2013.0002.
18. Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43(3):962-970. https://doi.org/10.1093/ije/dyu029.
19. Angrist JD, Krueger AB. Instrumental variables and the search for identification: From supply and demand to natural experiments. J Econ Perspect. 2001;15(4):69-85. https://doi.org/10.1257/jep.15.4.69.
20. Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA. 2014;312(22):2401-2402. https://doi.org/10.1001/jama.2014.16153.
21. Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301(6):603-618. https://doi.org/10.1001/jama.2009.126.
22. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178-187. https://doi.org/10.7326/0003-4819-150-3-200902030-00007.
23. Naylor MD, Brooten DA, Campbell RL, et al. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675-684. https://doi.org/10.1111/j.1532-5415.2004.52202.x.
24. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. https://doi.org/10.1001/jamainternmed.2014.1608.
25. Kripalani S, LeFevre F, Phillips CO, et al. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841. https://doi.org/10.1001/jama.297.8.831.
26. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186-192. https://doi.org/10.1046/j.1525-1497.2002.10741.x.
27. Hoyer EH, Brotman DJ, Apfel A, et al. Improving outcomes after hospitalization: A prospective observational multicenter evaluation of care coordination strategies for reducing 30-day readmissions to Maryland Hospitals. J Gen Intern Med. 2018;33(5):621-627. https://doi.org/10.1007/s11606-017-4218-4.
1. Boccutti C, Casillas G. Aiming for Fewer Hospital U-turns: The Medicare Hospital Readmission Reduction Program; March 10, 2017. https://www.kff.org/medicare/issue-brief/aiming-for-fewer-hospital-u-turns-the-medicare-hospital-readmission-reduction-program. Accessed July 22, 2019
2. Centers for Medicare and Medicaid Services. FY 2017 IPPS Final Rule: Hospital Readmissions Reduction Program Su pplemental Data File. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Archived-Supplemental-Data-Files.html. Accessed June 22, 2019
3. Sharma G, Kuo YF, Freeman JL, Zhang DD, Goodwin JS. Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010;170(18):1664-1670. https://doi.org/10.1001/archinternmed.2010.345.
4. Rennke S, Nguyen OK, Shoeb MH, et al. Hospital-initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):433-440. https://doi.org/10.7326/0003-4819-158-5-201303051-00011.
5. Misky GJ, Wald HL, Coleman EA. Post hospitalization transitions: examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5(7):392-397. https://doi.org/10.1002/jhm.666.
6. Hesselink G, Schoonhoven L, Barach P, et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med. 2012;157(6):417-428. https://doi.org/10.7326/0003-4819-157-6-201209180-00006.
7. Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716-1722. https://doi.org/10.1001/jama.2010.533.
8. Muus KJ, Knudson A, Klug MG, et al. Effect of post discharge follow-up care on re-admissions among US veterans with congestive heart failure: a rural-urban comparison. Rural Remote Health. 2010;10(2):1447.
9. Brooke BS, Stone DH, Cronenwett JL, et al. Early primary care provider follow-up and readmission after high-risk surgery. JAMA Surg. 2014;149(8):821-828. https://doi.org/10.1001/jamasurg.2014.157.
10. Leschke J, Panepinto JA, Nimmer M, et al. Outpatient follow-up and rehospitalizations for sickle cell disease patients. Pediatr Blood Cancer. 2012;58(3):406-409. https://doi.org/10.1002/pbc.23140.
11. Field TS, Ogarek J, Garber L, Reed G, Gurwitz JH. Association of early post discharge follow-up by a primary care physician and 30-day rehospitalization among older adults. J Gen Intern Med. 2015;30(5):565-571. https://doi.org/10.1007/s11606-014-3106-4.
12. Kashiwagi DT, Burton MC, Kirkland LL, Cha S, Varkey P. Do timely outpatient follow-up visits decrease hospital readmission rates? Am J Med Qual. 2012;27(1):11-15. https://doi.org/10.1177/1062860611409197.
13. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. https://doi.org/10.7326/0003-4819-155-8-201110180-00008.
14. Ryan J, Kang S, Dolacky S, Ingrassia J, Ganeshan R. Change in readmissions and follow-up visits as part of a heart failure readmission quality improvement initiative. Am J Med. 2013;126(11):989–994.e1. https://doi.org/10.1016/j.amjmed.2013.06.027.
15. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. https://doi.org/10.1001/archinte.166.17.1822.
16. Thomas JW. Should episode-based economic profiles be risk adjusted to account for differences in patients’ health risks? Health Serv Res. 2006;41(2):581-598. https://doi.org/10.1111/j.1475-6773.2005.00499.x.
17. Mendez CM, Harrington DW, Christenson P, Spellberg B. Impact of hospital variables on case mix index as a marker of disease severity. Popul Health Manag. 2014;17(1):28-34. https://doi.org/10.1089/pop.2013.0002.
18. Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43(3):962-970. https://doi.org/10.1093/ije/dyu029.
19. Angrist JD, Krueger AB. Instrumental variables and the search for identification: From supply and demand to natural experiments. J Econ Perspect. 2001;15(4):69-85. https://doi.org/10.1257/jep.15.4.69.
20. Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA. 2014;312(22):2401-2402. https://doi.org/10.1001/jama.2014.16153.
21. Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301(6):603-618. https://doi.org/10.1001/jama.2009.126.
22. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178-187. https://doi.org/10.7326/0003-4819-150-3-200902030-00007.
23. Naylor MD, Brooten DA, Campbell RL, et al. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675-684. https://doi.org/10.1111/j.1532-5415.2004.52202.x.
24. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. https://doi.org/10.1001/jamainternmed.2014.1608.
25. Kripalani S, LeFevre F, Phillips CO, et al. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841. https://doi.org/10.1001/jama.297.8.831.
26. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186-192. https://doi.org/10.1046/j.1525-1497.2002.10741.x.
27. Hoyer EH, Brotman DJ, Apfel A, et al. Improving outcomes after hospitalization: A prospective observational multicenter evaluation of care coordination strategies for reducing 30-day readmissions to Maryland Hospitals. J Gen Intern Med. 2018;33(5):621-627. https://doi.org/10.1007/s11606-017-4218-4.
© 2019 Society of Hospital Medicine
Impact of the Hospital-Acquired Conditions Initiative on Falls and Physical Restraints: A Longitudinal Study
Accidental falls are among the most common incidents reported in hospitals, complicating approximately 2% of hospital stays.1-3 Approximately 25% of falls in hospitalized patients result in injury, and 2% involve fractures.4 Substantial costs are associated with falls, including patient care costs associated with increased length of stay and liability.5-7
Beginning October 1, 2008, the Centers for Medicare & Medicaid Services (CMS) stopped reimbursing hospitals for the additional care associated with eight hospital-acquired conditions (HACs), including serious fall-related injury, which were believed to be “reasonably preventable.”8,9 Before this change, hospitals recovered the costs of these “never events” by assigning a higher level MS-DRG (Medicare Severity Diagnosis-Related Group) code for patients experiencing such an event. This is no longer allowed under the revised CMS Prospective Payment System rules.
Although the financial penalty for iatrogenic injury was modest, the payment change placed pressure on hospital staff to decrease falls, and some nurses reported changing practice to be more restrictive of patient mobility.10 Increased use of physical restraints is a potential unintended consequence of this rule change.11 Restraints are known to cause agitation, delirium, decubiti, deconditioning, strangulation, and death.12 Not surprisingly, use of restraints is discouraged in hospitals and is a CMS quality of care indicator.13,14 Although there is no evidence that restraint use prevents patients from falling,15,16 there is a perception among both health professionals and patients that restraints reduce the risk of falling, and they are often used as a “last resort” method of fall prevention.17-19
The aim of this longitudinal study was to determine whether this payment change was associated with changes in short-, intermediate-, and long-term rates of falls, injurious falls, and physical restraint use in acute care hospitals. The CMS has included fall-related hip fracture in newer value-based purchasing programs by adding Patient Safety Indictor (PSI) 90 to both the HACs Reduction Program (HACRP)20 and the Hospital Value-Based Purchasing (VBP)21 in FY2015. However, the HACs Initiative remains the only Medicare value program that directly penalizes all injurious inpatient falls.
METHODS
Study Units
As previously described,22 the National Database of Nursing Quality Indicators (NDNQI) is a data collection project initiated by the American Nurses Association (ANA). The NDNQI provides national comparative data at the unit and facility levels on nursing-sensitive indicators endorsed by the National Quality Forum. More than 2,000 hospitals voluntarily participate in the NDNQI, including virtually all ANA Magnet-recognized hospitals, and more than 90% of nursing units participate in the fall measures (NDNQI, personal communication). At the start of study data collection, the project was administered by the School of Nursing at the University of Kansas Medical Center. In 2014, the ownership of the NDNQI was transferred from the ANA to Press Ganey Associates, Inc. In addition to standardized data on unit, facility, and staffing characteristics, the NDNQI member hospitals can elect to submit monthly data on falls and quarterly data on physical restraint use prevalence.
We examined the data collected from adult medical, medical-surgical, and surgical units in United States acute care hospitals that elected to participate in the fall and physical restraint use data collection within the NDNQI for the 27 months before and the 87 months after the implementation of the CMS rule change. Eligible units contributed at least one fall and physical restraint use data point during both the 27 months preceding October 1, 2008, and the 87 months immediately after. The Institutional Review Board at the University of Kansas Medical Center reviewed and approved the study before its implementation.
Endpoints
Fall Events
The NDNQI defines a patient fall as an unplanned descent to the floor, regardless of whether the fall results in injury and regardless of whether the patient was assisted to the floor by a member of the hospital staff. Events in which a patient lands on a surface where one would not expect to find a patient (eg, on a mat next to a low bed) are also counted as falls.
Using internal data sources (eg, medical records, incident reports), participating hospitals report the number of inpatient falls each month to the NDNQI. We analyzed the falls data for the period July 1, 2006, through December 31, 2015. Thus, each unit could contribute 114 months (27 months before the rule change and 87 months after the rule change) of falls data.
Hospitals classify the injury level of each fall as none, minor (resulting in bruise, pain, abrasion, wound cleaning, or limb elevation, or in the use of ice, dressing, or topical medication), moderate (resulting in suturing, splinting, muscle or joint strain, or application of steri-strips or skin glue), major (resulting in surgery, casting, traction, any type of fracture, consultation for neurological or internal injury, or receipt of blood products for patients with coagulopathy), or death (resulting from injuries sustained from falling). For this study, a fall resulting in any injury (including minor) was considered as an injurious fall. The NDNQI data have been validated for falls and fall injury.23,24
Based on patient counts from unit censuses and/or internal data on actual patient hours on the unit, hospitals also report to the NDNQI the monthly number of patient days for each unit for which falls data are reported. The NDNQI uses these data to calculate each unit’s total and injurious fall rate per 1,000 patient days.
Physical Restraint Use
The NDNQI follows the CMS definition of restraint, which is “any manual method, physical or mechanical device, material, or equipment that immobilizes or reduces the ability of a patient to move his or her arms, legs, body, or head freely”.13 The NDNQI restraint use data are collected quarterly. Participating hospitals choose one day each quarter to conduct a restraint use prevalence survey on participating units. On the selected day, designated RNs within these hospitals visually assess each patient on the unit for restraint use. Based on this survey, hospitals report to the NDNQI the total count of patients surveyed and whether each was restrained. For restrained patients, hospitals also report the type of restraint as limb, vest, or other (eg, four side rails, net beds, mitts not attached to the bed).
We analyzed the restraint use data for the period October 1, 2006, through December 31, 2015. Thus, 37 quarters of data (eight pre- and 29 postrule change) were available. For this study, we computed for each unit the quarterly proportion of surveyed patients who were physically restrained by dividing the total count of restrained patients (regardless of the type of restraint) on the day of the survey by the total count of surveyed patients.
Covariates
Unit- and facility-level covariates were included in several model specifications to determine whether patient or facility characteristics affected the results. The unit-level covariates included the type of nursing unit (medical, medical and surgical, or surgical), monthly rates of total nursing hours per patient day, and nursing skill mix (percent registered nurses/total nursing personnel). The three facility-level variables included urban–rural location (defined as metropolitan [located in an area containing an urban core with a population of at least 50,000], micropolitan [located in an area containing an urban core with a population of 10,000-49,999], or neither), bed size (<300 beds or ≥300 beds), and teaching status (academic health center, major teaching hospital, or nonteaching hospital).
Because larger, academically affiliated hospitals are overrepresented in the NDNQI, we conducted stratified analyses of these variables to explore how change in the rates of falls and restraint use in the entire sample might differ between hospitals according to bed size (<300 beds, ≥300 beds) and teaching status (nonteaching versus teaching and academic health center).
Statistical Methods
We compared the mean annual rates of change in falls, injurious falls, and physical restraint use prevalence during the two years before the HACs Initiative went into effect (October 2006-September 2008) with the mean annual rates of change following the implementation of the payment rule. Short-term (one-year) change was the slope from October 2008 to September 2009, intermediate-term (four-year) change was the slope from October 2008 to September 2012, and long-term (seven-year) change was the slope from October 2008 to September 2015.
Monthly rates of falls and injurious falls over the 114-month period were modeled using negative binomial models with a random intercept to account for heterogeneity between units. Each base mean model included the preimplementation intercept and slope (over time), the postimplementation intercept, and slope (both linear and quadratic). We also fit the models that included the terms in the base model and facility-level covariates, unit-level covariates, both individually and combined. All models included terms for seasonality.
Quarterly prevalence rates of restraint use over the 37 quarters were modeled using beta-binomial models with a random intercept to account for heterogeneity between units. Each base mean model included the preimplementation intercept and slope (over time), the postimplementation intercept, and slope (both linear and quadratic). Similar to the one specified for falls, models were also fitted that included facility- and unit-level covariates as described above.
To adjust for multiple comparisons of the three postimplementation slopes, all confidence intervals were Bonferroni corrected.
RESULTS
Nursing Units
We included nursing units with one or more months of falls data and one or more quarters of restraint use data before and after the rule change. Of the 11,117 nursing units that submitted data to the NDNQI, 2,862 units (983 medical, 1,219 medical-surgical, and 660 surgical) with the requisite demographic, falls, and restraint use data were considered for inclusion in the study. The characteristics of the nursing units (ie, the type of unit, total nursing hours per patient day, and nursing skill mix) and hospitals (ie, location, bed size, teaching status) included in the study were similar to those of the overall NDNQI member units.
Baseline Characteristics
In the first study month (July 2006), 1,941 sample nursing units reported 5,101 falls during 1,401,652 patient-days of observation. Of these, 1,502 (29%) resulted in injury (1,281 minor, 144 moderate, 75 major, and two deaths). Across falls, the median (interquartile range [IQR]) patient age was 70 (55-80) years, with males accounting for 51% of falls. Most of the falls, 4,328 (85%), were documented as unassisted. A total of 209 (4%) falls occurred while physical restraints were in use.
In the first quarterly restraint use prevalence survey (October 2006), the 829 participating nursing units surveyed 19,979 patients (median [IQR] = 23 [20-23] patients per nursing unit). The median (IQR) age was 66 (51-78) years, and 54% of them were females. At the time of the survey, restraints were in use for 326 (1.6%) patients. Restrained patients were older than unrestrained patients (median age: 78 vs 65 years) and more likely to be male (56% vs 46%). Limb restraints were used for 139 patients, vest restraints for 66, both limb and vest restraints for 24, and other restraint types were used for 113 patients (including 11 in limb restraints and 5 in limb and vest restraints).
Change in Endpoints after Implementation of the HACs Initiative
Stratified Analysis
At baseline, fall rates and restraint use prevalence were slightly higher, whereas the rate of injurious falls was slightly lower, among teaching and academic medical centers compared to those in nonteaching hospitals. Declines in falls rate and restraint use prevalence were higher in teaching hospitals than in nonteaching hospitals (data not included).
CONCLUSIONS
We examined the rates of falls and fall injuries among 2,862 hospital units before and after the implementation of the HACs Initiative. Implementation of the CMS payment change was associated with a modest improvement in the rate of decrease for falls; a statistically significant effect on the rate of decrease for injurious falls was detectable only at seven years postchange. Fall reductions were the greatest among teaching and larger hospitals. These findings are consistent with our previous analysis of NDNQI data that found no short-term effect of the rule change on the rate of injurious falls.25
We found no evidence indicating that restraint use prevalence increased because of this payment change. Physical -restraint use prevalence showed a rapidly decreasing trend before 2008, and although the rate of decline was attenuated seven years after the rule change, the overall physical restraint use prevalence in these units in 2015 was less than half of that in 2006. Unlike falls, the steepest declines in restraint use prevalence occurred in smaller hospitals.
The CMS decision to include falls with injury among the “reasonably preventable” HACs was controversial.11 Inpatient falls are largely attributable to individual patient risk factors and are unusual among HACs in the extent to which patient behavior plays a role in their occurrence. Although hospital fall prevention guidelines have been published, only a few controlled trials have been conducted, with little evidence supporting the recommendations.1,26 A quantitative review found no evidence of benefit in published hospital fall prevention studies using concurrent controls (internal rate of return = 0.92; 95% CI: 0.65-1.30),26 and a recent, well-executed, cluster randomized trial of multifactorial fall prevention interventions found no change in fall rates compared with controls.27 Current hospital fall prevention guidelines are limited to unproven and time-consuming nursing-level (eg, toileting schedules and use of alarms) or organizational-level strategies (eg, changing staff attitudes regarding the inevitability of falls or “leadership support”).1,28
Despite the large sample size and the use of nurse-reported data that include patient falls from all age groups and not subject to bias due to the regulation itself (eg, ICD coding changes), our findings should be interpreted taking into account several limitations.
First, hospitals participating in the NDNQI self-select to participate and are larger and disproportionately urban compared with nonparticipating hospitals.29 Although our findings were unchanged when hospital-level covariates were included in modeling, analyses stratified by teaching status and bed size demonstrated important differences. Larger teaching hospitals experienced greater fall reductions, whereas restraint use prevalence decreased more rapidly in smaller hospitals.
Second, the absence of a control group prevents us from conclusively attributing changes in falls rate and restraint use prevalence to the 2008 CMS payment change.30 Our findings may have been influenced by other policy changes. For example, in October 2014, the CMS implemented the Hospital-Acquired Condition Reduction Program (HACRP)20 and the Hospital Value-Based Purchasing (VBP)21 Program. Under these programs, falls with hip fractures were an indicator that could alter hospital payment.
Third, we did not ascertain the use of all available fall prevention measures such as companions, bed rails, very low beds, bed alarms, and restricted activity.31 Nor could the study address changes in patient functional status or discharge location. In a before- and after-study of four hospitals in a single hospital system, we found that bed alarm use increased, restraint orders decreased, and the use of room change or sitters remained stable after the implementation of the CMS payment.32
Nevertheless, we believe that these findings are consistent with the hypothesis that the HACs Initiative increased the cost of patient falls to hospitals, and, in response, some hospitals were able to modestly reduce the rate of falls. We found no evidence that physical restraint use prevalence increased.
In summary, our findings suggest modest impact of the HACs Initiative on falls and injurious falls, but no unintended impact on restraint use. These results highlight the importance of ensuring that pay-for-performance initiatives target outcomes where there are evidence-based approaches to prevention. The creation or identification of prevention tools and guidelines does not make an outcome preventable. Despite interval improvement in these self-selected hospital units in fall rates and physical restraint use prevalence, falls remain a difficult patient safety problem for hospitals, and further research is required to develop cost-effective, generalizable strategies for their prevention.
1. Miake-Lye IM, Hempel S, Ganz DA, Shekelle PG. Inpatient fall prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5):390-396. https://doi.org/10.7326/0003-4819-158-5-201303051-00005
2. Healey F, Darowski A. Older patients and falls in hospital. Clin Risk. 2012;18(5):170-176. https://doi.org/10.1258/cr.2012.012020.
3. Oliver D, Healey F, Haines TP. Preventing falls and fall-related injuries in hospitals. Clin Geriatr Med. 2010;26(4):645-692. https://doi.org/10.1016/j.cger.2010.06.005.
4. Currie L. Fall and Injury Prevention. In: Hughes RG, ed. Patient safety and quality: an evidence-based handbook for nurses (Prepared with support from the Robert Wood Johnson Foundation). AHRQ Publication NO.08-0043. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
5. Wong CA, Recktenwald AJ, Jones ML, Waterman BM, Bollini ML, Dunagan WC. The cost of serious fall-related injuries at three Midwestern hospitals. Jt Comm J Qual Patient Saf. 2011;37(2):81-87. https://doi.org/10.1016/S1553-7250(11)37010-9.
6. Bates DW, Pruess K, Souney P, Platt R. Serious falls in hospitalized patients: correlates and resource utilization. Am J Med. 1995;99(2):137-143. https://doi.org/10.1016/s0002-9343(99)80133-8.
7. Fiesta J. Liability for falls. Nurs Manage. 1998;29(3):24-26. https://doi.org/10.1097/00006247-199803000-00007.
8. Rosenthal MB. Nonpayment for performance? Medicare’s new reimbursement rule. N Engl J Med. 2007;357(16):1573-1575. https://doi.org/10.1056/NEJMp078184.
9. Department of Health and Human Services, Centers for Medicare and Medicaid Services. 42 CFR Parts 411, 412, 413, and 489. Medicare program; proposed changes to the hospital inpatient prospective payment systems and fiscal year. 2008 rates; final rule. Federal Register. 2007;72(62):47130-47178.
10. King B, Pecanac K, Krupp A, Liebzeit D, Mahoney J. Impact of fall prevention on nurses and care of fall risk patients. Gerontologist. 2018;58(2):331-340. https://doi.org/10.1093/geront/gnw156.
11. Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med. 2009;360(23):2390-2393. https://doi.org/10.1056/NEJMp0900963.
12. Rakhmatullina M, Taub A, Jacob T. Morbidity and mortality associated with the utilization of restraints : a review of literature. Psychiatr Q. 2013;84(4):499-512. https://doi.org/10.1007/s11126-013-9262-6.
13. State Operations Manual Appendix A - Survey Protocol, Regulations and Interpretive Guidelines for Hospitals. (Revision 116, 06-06-14). http://cms.hhs.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/som107ap_a_hospitals.pdf. Accessed October 26, 2014.
14. Nursing Sensitive Measures. NQF # 0203, Restraint prevalence (vest and limb only). Status: Endorsed on: August 05, 2009; Steward(s): The Joint Commission. Washington, D.C.: National Quality Forum; 2009.
15. Kopke S, Muhlhauser I, Gerlach A, et al. Effect of a guideline-based multicomponent intervention on use of physical restraints in nursing homes: a randomized controlled trial. JAMA. 2012;307(20):2177-2184. https://doi.org/10.1001/jama.2012.4517.
16. Enns E, Rhemtulla R, Ewa V, Fruetel K, Holroyd-Leduc JM. A controlled quality improvement trial to reduce the use of physical restraints in older hospitalized adults. J Am Geriatr Soc. 2014;62(3):541-545. https://doi.org/10.1111/jgs.12710.
17. Heinze C, Dassen T, Grittner U. Use of physical restraints in nursing homes and hospitals and related factors: a cross-sectional study. J Clin Nurs. 2012;21(7-8):1033-1040. https://doi.org/10.1111/j.1365-2702.2011.03931.x.
18. Minnick AF, Fogg L, Mion LC, Catrambone C, Johnson ME. Resource clusters and variation in physical restraint use. J Nurs Scholarsh. 2007;39(4):363-370. https://doi.org/10.1111/j.1547-5069.2007.00194.x.
19. Vassallo M, Wilkinson C, Stockdale R, Malik N, Baker R, Allen S. Attitudes to restraint for the prevention of falls in hospital. Gerontology. 2005;51(1):66-70. https://doi.org/10.1159/000081438.
20. Centers for Medicare & Medicaid Services. Hospital-Acquired Condition Reduction Program (HACRP). https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/HAC-Reduction-Program.html. Accessed September 9. 2018.
21. Centers for Medicare & Medicaid Services. The Hospital Value-Based Purchasing (VBP) Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HVBP/Hospital-Value-Based-Purchasing.html. Accessed September 9, 2018.
22. Dunton NE. Take a cue from the NDNQI. Nurs Manage. 2008;39(4):20, 22-23. https://doi.org/10.1097/01.NUMA.0000316054.35317.bf.
23. Garrard L, Boyle DK, Simon M, Dunton N, Gajewski B. Reliability and Validity of the NDNQI(R) Injury Falls Measure. West J Nurs Res. 2016;38(1):111-128. https://doi.org/10.1177/0193945914542851
24. Garrard L, Boyle DK, Simon M, Dunton N, Gajewski B. Reliability and validity of the NDNQI(R) injury falls measure. West J Nurs Res. 2016;38(1):111-128. https://doi.org/10.1177/0193945914542851.
25. Waters TM, Daniels MJ, Bazzoli GJ, et al. Effect of Medicare’s nonpayment for hospital-acquired conditions: lessons for future policy. JAMA Intern Med. 2015;175(3):347-354.
26. Hempel S, Newberry S, Wang Z, et al. Hospital fall prevention: a systematic review of implementation, components, adherence, and effectiveness. J Am Geriatr Soc. 2013;61(4):483-494. https://doi.org/10.1001/jamainternmed.2014.5486.
27. Barker AL, Morello RT, Wolfe R, et al. 6-PACK programme to decrease fall injuries in acute hospitals: cluster randomised controlled trial. BMJ. 2016;352:h6781. https://doi.org/10.1136/bmj.h6781.
28. Goldsack J, Bergey M, Mascioli S, Cunningham J. Hourly rounding and patient falls: what factors boost success? Nursing. 2015;45(2):25-30. https://doi.org/10.1097/01.NURSE.0000459798.79840.95.
29. Montalvo I. The National Database of Nursing Quality Indicators (NDNQI). Online Journal of Issues in Nursing. 2007;12(3).
30. Soumerai SB, Ceccarelli R, Koppel R. False dichotomies and health policy research designs: randomized trials are not always the answer. J Gen Intern Med. 2017;32(2):204-209. https://doi.org/10.1007/s11606-016-3841-9.
31. Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med. 2017;177(6):759-760. https://doi.org/10.1001/jamainternmed.2017.0840.
32. Fehlberg EA, Lucero RJ, Weaver MT, et al. Impact of the CMS no-pay policy on hospital-acquired fall prevention related practice patterns. Innov Aging. 2017;1(3):igx036-igx036. https://doi.org/10.1093/geroni/igx036.
Accidental falls are among the most common incidents reported in hospitals, complicating approximately 2% of hospital stays.1-3 Approximately 25% of falls in hospitalized patients result in injury, and 2% involve fractures.4 Substantial costs are associated with falls, including patient care costs associated with increased length of stay and liability.5-7
Beginning October 1, 2008, the Centers for Medicare & Medicaid Services (CMS) stopped reimbursing hospitals for the additional care associated with eight hospital-acquired conditions (HACs), including serious fall-related injury, which were believed to be “reasonably preventable.”8,9 Before this change, hospitals recovered the costs of these “never events” by assigning a higher level MS-DRG (Medicare Severity Diagnosis-Related Group) code for patients experiencing such an event. This is no longer allowed under the revised CMS Prospective Payment System rules.
Although the financial penalty for iatrogenic injury was modest, the payment change placed pressure on hospital staff to decrease falls, and some nurses reported changing practice to be more restrictive of patient mobility.10 Increased use of physical restraints is a potential unintended consequence of this rule change.11 Restraints are known to cause agitation, delirium, decubiti, deconditioning, strangulation, and death.12 Not surprisingly, use of restraints is discouraged in hospitals and is a CMS quality of care indicator.13,14 Although there is no evidence that restraint use prevents patients from falling,15,16 there is a perception among both health professionals and patients that restraints reduce the risk of falling, and they are often used as a “last resort” method of fall prevention.17-19
The aim of this longitudinal study was to determine whether this payment change was associated with changes in short-, intermediate-, and long-term rates of falls, injurious falls, and physical restraint use in acute care hospitals. The CMS has included fall-related hip fracture in newer value-based purchasing programs by adding Patient Safety Indictor (PSI) 90 to both the HACs Reduction Program (HACRP)20 and the Hospital Value-Based Purchasing (VBP)21 in FY2015. However, the HACs Initiative remains the only Medicare value program that directly penalizes all injurious inpatient falls.
METHODS
Study Units
As previously described,22 the National Database of Nursing Quality Indicators (NDNQI) is a data collection project initiated by the American Nurses Association (ANA). The NDNQI provides national comparative data at the unit and facility levels on nursing-sensitive indicators endorsed by the National Quality Forum. More than 2,000 hospitals voluntarily participate in the NDNQI, including virtually all ANA Magnet-recognized hospitals, and more than 90% of nursing units participate in the fall measures (NDNQI, personal communication). At the start of study data collection, the project was administered by the School of Nursing at the University of Kansas Medical Center. In 2014, the ownership of the NDNQI was transferred from the ANA to Press Ganey Associates, Inc. In addition to standardized data on unit, facility, and staffing characteristics, the NDNQI member hospitals can elect to submit monthly data on falls and quarterly data on physical restraint use prevalence.
We examined the data collected from adult medical, medical-surgical, and surgical units in United States acute care hospitals that elected to participate in the fall and physical restraint use data collection within the NDNQI for the 27 months before and the 87 months after the implementation of the CMS rule change. Eligible units contributed at least one fall and physical restraint use data point during both the 27 months preceding October 1, 2008, and the 87 months immediately after. The Institutional Review Board at the University of Kansas Medical Center reviewed and approved the study before its implementation.
Endpoints
Fall Events
The NDNQI defines a patient fall as an unplanned descent to the floor, regardless of whether the fall results in injury and regardless of whether the patient was assisted to the floor by a member of the hospital staff. Events in which a patient lands on a surface where one would not expect to find a patient (eg, on a mat next to a low bed) are also counted as falls.
Using internal data sources (eg, medical records, incident reports), participating hospitals report the number of inpatient falls each month to the NDNQI. We analyzed the falls data for the period July 1, 2006, through December 31, 2015. Thus, each unit could contribute 114 months (27 months before the rule change and 87 months after the rule change) of falls data.
Hospitals classify the injury level of each fall as none, minor (resulting in bruise, pain, abrasion, wound cleaning, or limb elevation, or in the use of ice, dressing, or topical medication), moderate (resulting in suturing, splinting, muscle or joint strain, or application of steri-strips or skin glue), major (resulting in surgery, casting, traction, any type of fracture, consultation for neurological or internal injury, or receipt of blood products for patients with coagulopathy), or death (resulting from injuries sustained from falling). For this study, a fall resulting in any injury (including minor) was considered as an injurious fall. The NDNQI data have been validated for falls and fall injury.23,24
Based on patient counts from unit censuses and/or internal data on actual patient hours on the unit, hospitals also report to the NDNQI the monthly number of patient days for each unit for which falls data are reported. The NDNQI uses these data to calculate each unit’s total and injurious fall rate per 1,000 patient days.
Physical Restraint Use
The NDNQI follows the CMS definition of restraint, which is “any manual method, physical or mechanical device, material, or equipment that immobilizes or reduces the ability of a patient to move his or her arms, legs, body, or head freely”.13 The NDNQI restraint use data are collected quarterly. Participating hospitals choose one day each quarter to conduct a restraint use prevalence survey on participating units. On the selected day, designated RNs within these hospitals visually assess each patient on the unit for restraint use. Based on this survey, hospitals report to the NDNQI the total count of patients surveyed and whether each was restrained. For restrained patients, hospitals also report the type of restraint as limb, vest, or other (eg, four side rails, net beds, mitts not attached to the bed).
We analyzed the restraint use data for the period October 1, 2006, through December 31, 2015. Thus, 37 quarters of data (eight pre- and 29 postrule change) were available. For this study, we computed for each unit the quarterly proportion of surveyed patients who were physically restrained by dividing the total count of restrained patients (regardless of the type of restraint) on the day of the survey by the total count of surveyed patients.
Covariates
Unit- and facility-level covariates were included in several model specifications to determine whether patient or facility characteristics affected the results. The unit-level covariates included the type of nursing unit (medical, medical and surgical, or surgical), monthly rates of total nursing hours per patient day, and nursing skill mix (percent registered nurses/total nursing personnel). The three facility-level variables included urban–rural location (defined as metropolitan [located in an area containing an urban core with a population of at least 50,000], micropolitan [located in an area containing an urban core with a population of 10,000-49,999], or neither), bed size (<300 beds or ≥300 beds), and teaching status (academic health center, major teaching hospital, or nonteaching hospital).
Because larger, academically affiliated hospitals are overrepresented in the NDNQI, we conducted stratified analyses of these variables to explore how change in the rates of falls and restraint use in the entire sample might differ between hospitals according to bed size (<300 beds, ≥300 beds) and teaching status (nonteaching versus teaching and academic health center).
Statistical Methods
We compared the mean annual rates of change in falls, injurious falls, and physical restraint use prevalence during the two years before the HACs Initiative went into effect (October 2006-September 2008) with the mean annual rates of change following the implementation of the payment rule. Short-term (one-year) change was the slope from October 2008 to September 2009, intermediate-term (four-year) change was the slope from October 2008 to September 2012, and long-term (seven-year) change was the slope from October 2008 to September 2015.
Monthly rates of falls and injurious falls over the 114-month period were modeled using negative binomial models with a random intercept to account for heterogeneity between units. Each base mean model included the preimplementation intercept and slope (over time), the postimplementation intercept, and slope (both linear and quadratic). We also fit the models that included the terms in the base model and facility-level covariates, unit-level covariates, both individually and combined. All models included terms for seasonality.
Quarterly prevalence rates of restraint use over the 37 quarters were modeled using beta-binomial models with a random intercept to account for heterogeneity between units. Each base mean model included the preimplementation intercept and slope (over time), the postimplementation intercept, and slope (both linear and quadratic). Similar to the one specified for falls, models were also fitted that included facility- and unit-level covariates as described above.
To adjust for multiple comparisons of the three postimplementation slopes, all confidence intervals were Bonferroni corrected.
RESULTS
Nursing Units
We included nursing units with one or more months of falls data and one or more quarters of restraint use data before and after the rule change. Of the 11,117 nursing units that submitted data to the NDNQI, 2,862 units (983 medical, 1,219 medical-surgical, and 660 surgical) with the requisite demographic, falls, and restraint use data were considered for inclusion in the study. The characteristics of the nursing units (ie, the type of unit, total nursing hours per patient day, and nursing skill mix) and hospitals (ie, location, bed size, teaching status) included in the study were similar to those of the overall NDNQI member units.
Baseline Characteristics
In the first study month (July 2006), 1,941 sample nursing units reported 5,101 falls during 1,401,652 patient-days of observation. Of these, 1,502 (29%) resulted in injury (1,281 minor, 144 moderate, 75 major, and two deaths). Across falls, the median (interquartile range [IQR]) patient age was 70 (55-80) years, with males accounting for 51% of falls. Most of the falls, 4,328 (85%), were documented as unassisted. A total of 209 (4%) falls occurred while physical restraints were in use.
In the first quarterly restraint use prevalence survey (October 2006), the 829 participating nursing units surveyed 19,979 patients (median [IQR] = 23 [20-23] patients per nursing unit). The median (IQR) age was 66 (51-78) years, and 54% of them were females. At the time of the survey, restraints were in use for 326 (1.6%) patients. Restrained patients were older than unrestrained patients (median age: 78 vs 65 years) and more likely to be male (56% vs 46%). Limb restraints were used for 139 patients, vest restraints for 66, both limb and vest restraints for 24, and other restraint types were used for 113 patients (including 11 in limb restraints and 5 in limb and vest restraints).
Change in Endpoints after Implementation of the HACs Initiative
Stratified Analysis
At baseline, fall rates and restraint use prevalence were slightly higher, whereas the rate of injurious falls was slightly lower, among teaching and academic medical centers compared to those in nonteaching hospitals. Declines in falls rate and restraint use prevalence were higher in teaching hospitals than in nonteaching hospitals (data not included).
CONCLUSIONS
We examined the rates of falls and fall injuries among 2,862 hospital units before and after the implementation of the HACs Initiative. Implementation of the CMS payment change was associated with a modest improvement in the rate of decrease for falls; a statistically significant effect on the rate of decrease for injurious falls was detectable only at seven years postchange. Fall reductions were the greatest among teaching and larger hospitals. These findings are consistent with our previous analysis of NDNQI data that found no short-term effect of the rule change on the rate of injurious falls.25
We found no evidence indicating that restraint use prevalence increased because of this payment change. Physical -restraint use prevalence showed a rapidly decreasing trend before 2008, and although the rate of decline was attenuated seven years after the rule change, the overall physical restraint use prevalence in these units in 2015 was less than half of that in 2006. Unlike falls, the steepest declines in restraint use prevalence occurred in smaller hospitals.
The CMS decision to include falls with injury among the “reasonably preventable” HACs was controversial.11 Inpatient falls are largely attributable to individual patient risk factors and are unusual among HACs in the extent to which patient behavior plays a role in their occurrence. Although hospital fall prevention guidelines have been published, only a few controlled trials have been conducted, with little evidence supporting the recommendations.1,26 A quantitative review found no evidence of benefit in published hospital fall prevention studies using concurrent controls (internal rate of return = 0.92; 95% CI: 0.65-1.30),26 and a recent, well-executed, cluster randomized trial of multifactorial fall prevention interventions found no change in fall rates compared with controls.27 Current hospital fall prevention guidelines are limited to unproven and time-consuming nursing-level (eg, toileting schedules and use of alarms) or organizational-level strategies (eg, changing staff attitudes regarding the inevitability of falls or “leadership support”).1,28
Despite the large sample size and the use of nurse-reported data that include patient falls from all age groups and not subject to bias due to the regulation itself (eg, ICD coding changes), our findings should be interpreted taking into account several limitations.
First, hospitals participating in the NDNQI self-select to participate and are larger and disproportionately urban compared with nonparticipating hospitals.29 Although our findings were unchanged when hospital-level covariates were included in modeling, analyses stratified by teaching status and bed size demonstrated important differences. Larger teaching hospitals experienced greater fall reductions, whereas restraint use prevalence decreased more rapidly in smaller hospitals.
Second, the absence of a control group prevents us from conclusively attributing changes in falls rate and restraint use prevalence to the 2008 CMS payment change.30 Our findings may have been influenced by other policy changes. For example, in October 2014, the CMS implemented the Hospital-Acquired Condition Reduction Program (HACRP)20 and the Hospital Value-Based Purchasing (VBP)21 Program. Under these programs, falls with hip fractures were an indicator that could alter hospital payment.
Third, we did not ascertain the use of all available fall prevention measures such as companions, bed rails, very low beds, bed alarms, and restricted activity.31 Nor could the study address changes in patient functional status or discharge location. In a before- and after-study of four hospitals in a single hospital system, we found that bed alarm use increased, restraint orders decreased, and the use of room change or sitters remained stable after the implementation of the CMS payment.32
Nevertheless, we believe that these findings are consistent with the hypothesis that the HACs Initiative increased the cost of patient falls to hospitals, and, in response, some hospitals were able to modestly reduce the rate of falls. We found no evidence that physical restraint use prevalence increased.
In summary, our findings suggest modest impact of the HACs Initiative on falls and injurious falls, but no unintended impact on restraint use. These results highlight the importance of ensuring that pay-for-performance initiatives target outcomes where there are evidence-based approaches to prevention. The creation or identification of prevention tools and guidelines does not make an outcome preventable. Despite interval improvement in these self-selected hospital units in fall rates and physical restraint use prevalence, falls remain a difficult patient safety problem for hospitals, and further research is required to develop cost-effective, generalizable strategies for their prevention.
Accidental falls are among the most common incidents reported in hospitals, complicating approximately 2% of hospital stays.1-3 Approximately 25% of falls in hospitalized patients result in injury, and 2% involve fractures.4 Substantial costs are associated with falls, including patient care costs associated with increased length of stay and liability.5-7
Beginning October 1, 2008, the Centers for Medicare & Medicaid Services (CMS) stopped reimbursing hospitals for the additional care associated with eight hospital-acquired conditions (HACs), including serious fall-related injury, which were believed to be “reasonably preventable.”8,9 Before this change, hospitals recovered the costs of these “never events” by assigning a higher level MS-DRG (Medicare Severity Diagnosis-Related Group) code for patients experiencing such an event. This is no longer allowed under the revised CMS Prospective Payment System rules.
Although the financial penalty for iatrogenic injury was modest, the payment change placed pressure on hospital staff to decrease falls, and some nurses reported changing practice to be more restrictive of patient mobility.10 Increased use of physical restraints is a potential unintended consequence of this rule change.11 Restraints are known to cause agitation, delirium, decubiti, deconditioning, strangulation, and death.12 Not surprisingly, use of restraints is discouraged in hospitals and is a CMS quality of care indicator.13,14 Although there is no evidence that restraint use prevents patients from falling,15,16 there is a perception among both health professionals and patients that restraints reduce the risk of falling, and they are often used as a “last resort” method of fall prevention.17-19
The aim of this longitudinal study was to determine whether this payment change was associated with changes in short-, intermediate-, and long-term rates of falls, injurious falls, and physical restraint use in acute care hospitals. The CMS has included fall-related hip fracture in newer value-based purchasing programs by adding Patient Safety Indictor (PSI) 90 to both the HACs Reduction Program (HACRP)20 and the Hospital Value-Based Purchasing (VBP)21 in FY2015. However, the HACs Initiative remains the only Medicare value program that directly penalizes all injurious inpatient falls.
METHODS
Study Units
As previously described,22 the National Database of Nursing Quality Indicators (NDNQI) is a data collection project initiated by the American Nurses Association (ANA). The NDNQI provides national comparative data at the unit and facility levels on nursing-sensitive indicators endorsed by the National Quality Forum. More than 2,000 hospitals voluntarily participate in the NDNQI, including virtually all ANA Magnet-recognized hospitals, and more than 90% of nursing units participate in the fall measures (NDNQI, personal communication). At the start of study data collection, the project was administered by the School of Nursing at the University of Kansas Medical Center. In 2014, the ownership of the NDNQI was transferred from the ANA to Press Ganey Associates, Inc. In addition to standardized data on unit, facility, and staffing characteristics, the NDNQI member hospitals can elect to submit monthly data on falls and quarterly data on physical restraint use prevalence.
We examined the data collected from adult medical, medical-surgical, and surgical units in United States acute care hospitals that elected to participate in the fall and physical restraint use data collection within the NDNQI for the 27 months before and the 87 months after the implementation of the CMS rule change. Eligible units contributed at least one fall and physical restraint use data point during both the 27 months preceding October 1, 2008, and the 87 months immediately after. The Institutional Review Board at the University of Kansas Medical Center reviewed and approved the study before its implementation.
Endpoints
Fall Events
The NDNQI defines a patient fall as an unplanned descent to the floor, regardless of whether the fall results in injury and regardless of whether the patient was assisted to the floor by a member of the hospital staff. Events in which a patient lands on a surface where one would not expect to find a patient (eg, on a mat next to a low bed) are also counted as falls.
Using internal data sources (eg, medical records, incident reports), participating hospitals report the number of inpatient falls each month to the NDNQI. We analyzed the falls data for the period July 1, 2006, through December 31, 2015. Thus, each unit could contribute 114 months (27 months before the rule change and 87 months after the rule change) of falls data.
Hospitals classify the injury level of each fall as none, minor (resulting in bruise, pain, abrasion, wound cleaning, or limb elevation, or in the use of ice, dressing, or topical medication), moderate (resulting in suturing, splinting, muscle or joint strain, or application of steri-strips or skin glue), major (resulting in surgery, casting, traction, any type of fracture, consultation for neurological or internal injury, or receipt of blood products for patients with coagulopathy), or death (resulting from injuries sustained from falling). For this study, a fall resulting in any injury (including minor) was considered as an injurious fall. The NDNQI data have been validated for falls and fall injury.23,24
Based on patient counts from unit censuses and/or internal data on actual patient hours on the unit, hospitals also report to the NDNQI the monthly number of patient days for each unit for which falls data are reported. The NDNQI uses these data to calculate each unit’s total and injurious fall rate per 1,000 patient days.
Physical Restraint Use
The NDNQI follows the CMS definition of restraint, which is “any manual method, physical or mechanical device, material, or equipment that immobilizes or reduces the ability of a patient to move his or her arms, legs, body, or head freely”.13 The NDNQI restraint use data are collected quarterly. Participating hospitals choose one day each quarter to conduct a restraint use prevalence survey on participating units. On the selected day, designated RNs within these hospitals visually assess each patient on the unit for restraint use. Based on this survey, hospitals report to the NDNQI the total count of patients surveyed and whether each was restrained. For restrained patients, hospitals also report the type of restraint as limb, vest, or other (eg, four side rails, net beds, mitts not attached to the bed).
We analyzed the restraint use data for the period October 1, 2006, through December 31, 2015. Thus, 37 quarters of data (eight pre- and 29 postrule change) were available. For this study, we computed for each unit the quarterly proportion of surveyed patients who were physically restrained by dividing the total count of restrained patients (regardless of the type of restraint) on the day of the survey by the total count of surveyed patients.
Covariates
Unit- and facility-level covariates were included in several model specifications to determine whether patient or facility characteristics affected the results. The unit-level covariates included the type of nursing unit (medical, medical and surgical, or surgical), monthly rates of total nursing hours per patient day, and nursing skill mix (percent registered nurses/total nursing personnel). The three facility-level variables included urban–rural location (defined as metropolitan [located in an area containing an urban core with a population of at least 50,000], micropolitan [located in an area containing an urban core with a population of 10,000-49,999], or neither), bed size (<300 beds or ≥300 beds), and teaching status (academic health center, major teaching hospital, or nonteaching hospital).
Because larger, academically affiliated hospitals are overrepresented in the NDNQI, we conducted stratified analyses of these variables to explore how change in the rates of falls and restraint use in the entire sample might differ between hospitals according to bed size (<300 beds, ≥300 beds) and teaching status (nonteaching versus teaching and academic health center).
Statistical Methods
We compared the mean annual rates of change in falls, injurious falls, and physical restraint use prevalence during the two years before the HACs Initiative went into effect (October 2006-September 2008) with the mean annual rates of change following the implementation of the payment rule. Short-term (one-year) change was the slope from October 2008 to September 2009, intermediate-term (four-year) change was the slope from October 2008 to September 2012, and long-term (seven-year) change was the slope from October 2008 to September 2015.
Monthly rates of falls and injurious falls over the 114-month period were modeled using negative binomial models with a random intercept to account for heterogeneity between units. Each base mean model included the preimplementation intercept and slope (over time), the postimplementation intercept, and slope (both linear and quadratic). We also fit the models that included the terms in the base model and facility-level covariates, unit-level covariates, both individually and combined. All models included terms for seasonality.
Quarterly prevalence rates of restraint use over the 37 quarters were modeled using beta-binomial models with a random intercept to account for heterogeneity between units. Each base mean model included the preimplementation intercept and slope (over time), the postimplementation intercept, and slope (both linear and quadratic). Similar to the one specified for falls, models were also fitted that included facility- and unit-level covariates as described above.
To adjust for multiple comparisons of the three postimplementation slopes, all confidence intervals were Bonferroni corrected.
RESULTS
Nursing Units
We included nursing units with one or more months of falls data and one or more quarters of restraint use data before and after the rule change. Of the 11,117 nursing units that submitted data to the NDNQI, 2,862 units (983 medical, 1,219 medical-surgical, and 660 surgical) with the requisite demographic, falls, and restraint use data were considered for inclusion in the study. The characteristics of the nursing units (ie, the type of unit, total nursing hours per patient day, and nursing skill mix) and hospitals (ie, location, bed size, teaching status) included in the study were similar to those of the overall NDNQI member units.
Baseline Characteristics
In the first study month (July 2006), 1,941 sample nursing units reported 5,101 falls during 1,401,652 patient-days of observation. Of these, 1,502 (29%) resulted in injury (1,281 minor, 144 moderate, 75 major, and two deaths). Across falls, the median (interquartile range [IQR]) patient age was 70 (55-80) years, with males accounting for 51% of falls. Most of the falls, 4,328 (85%), were documented as unassisted. A total of 209 (4%) falls occurred while physical restraints were in use.
In the first quarterly restraint use prevalence survey (October 2006), the 829 participating nursing units surveyed 19,979 patients (median [IQR] = 23 [20-23] patients per nursing unit). The median (IQR) age was 66 (51-78) years, and 54% of them were females. At the time of the survey, restraints were in use for 326 (1.6%) patients. Restrained patients were older than unrestrained patients (median age: 78 vs 65 years) and more likely to be male (56% vs 46%). Limb restraints were used for 139 patients, vest restraints for 66, both limb and vest restraints for 24, and other restraint types were used for 113 patients (including 11 in limb restraints and 5 in limb and vest restraints).
Change in Endpoints after Implementation of the HACs Initiative
Stratified Analysis
At baseline, fall rates and restraint use prevalence were slightly higher, whereas the rate of injurious falls was slightly lower, among teaching and academic medical centers compared to those in nonteaching hospitals. Declines in falls rate and restraint use prevalence were higher in teaching hospitals than in nonteaching hospitals (data not included).
CONCLUSIONS
We examined the rates of falls and fall injuries among 2,862 hospital units before and after the implementation of the HACs Initiative. Implementation of the CMS payment change was associated with a modest improvement in the rate of decrease for falls; a statistically significant effect on the rate of decrease for injurious falls was detectable only at seven years postchange. Fall reductions were the greatest among teaching and larger hospitals. These findings are consistent with our previous analysis of NDNQI data that found no short-term effect of the rule change on the rate of injurious falls.25
We found no evidence indicating that restraint use prevalence increased because of this payment change. Physical -restraint use prevalence showed a rapidly decreasing trend before 2008, and although the rate of decline was attenuated seven years after the rule change, the overall physical restraint use prevalence in these units in 2015 was less than half of that in 2006. Unlike falls, the steepest declines in restraint use prevalence occurred in smaller hospitals.
The CMS decision to include falls with injury among the “reasonably preventable” HACs was controversial.11 Inpatient falls are largely attributable to individual patient risk factors and are unusual among HACs in the extent to which patient behavior plays a role in their occurrence. Although hospital fall prevention guidelines have been published, only a few controlled trials have been conducted, with little evidence supporting the recommendations.1,26 A quantitative review found no evidence of benefit in published hospital fall prevention studies using concurrent controls (internal rate of return = 0.92; 95% CI: 0.65-1.30),26 and a recent, well-executed, cluster randomized trial of multifactorial fall prevention interventions found no change in fall rates compared with controls.27 Current hospital fall prevention guidelines are limited to unproven and time-consuming nursing-level (eg, toileting schedules and use of alarms) or organizational-level strategies (eg, changing staff attitudes regarding the inevitability of falls or “leadership support”).1,28
Despite the large sample size and the use of nurse-reported data that include patient falls from all age groups and not subject to bias due to the regulation itself (eg, ICD coding changes), our findings should be interpreted taking into account several limitations.
First, hospitals participating in the NDNQI self-select to participate and are larger and disproportionately urban compared with nonparticipating hospitals.29 Although our findings were unchanged when hospital-level covariates were included in modeling, analyses stratified by teaching status and bed size demonstrated important differences. Larger teaching hospitals experienced greater fall reductions, whereas restraint use prevalence decreased more rapidly in smaller hospitals.
Second, the absence of a control group prevents us from conclusively attributing changes in falls rate and restraint use prevalence to the 2008 CMS payment change.30 Our findings may have been influenced by other policy changes. For example, in October 2014, the CMS implemented the Hospital-Acquired Condition Reduction Program (HACRP)20 and the Hospital Value-Based Purchasing (VBP)21 Program. Under these programs, falls with hip fractures were an indicator that could alter hospital payment.
Third, we did not ascertain the use of all available fall prevention measures such as companions, bed rails, very low beds, bed alarms, and restricted activity.31 Nor could the study address changes in patient functional status or discharge location. In a before- and after-study of four hospitals in a single hospital system, we found that bed alarm use increased, restraint orders decreased, and the use of room change or sitters remained stable after the implementation of the CMS payment.32
Nevertheless, we believe that these findings are consistent with the hypothesis that the HACs Initiative increased the cost of patient falls to hospitals, and, in response, some hospitals were able to modestly reduce the rate of falls. We found no evidence that physical restraint use prevalence increased.
In summary, our findings suggest modest impact of the HACs Initiative on falls and injurious falls, but no unintended impact on restraint use. These results highlight the importance of ensuring that pay-for-performance initiatives target outcomes where there are evidence-based approaches to prevention. The creation or identification of prevention tools and guidelines does not make an outcome preventable. Despite interval improvement in these self-selected hospital units in fall rates and physical restraint use prevalence, falls remain a difficult patient safety problem for hospitals, and further research is required to develop cost-effective, generalizable strategies for their prevention.
1. Miake-Lye IM, Hempel S, Ganz DA, Shekelle PG. Inpatient fall prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5):390-396. https://doi.org/10.7326/0003-4819-158-5-201303051-00005
2. Healey F, Darowski A. Older patients and falls in hospital. Clin Risk. 2012;18(5):170-176. https://doi.org/10.1258/cr.2012.012020.
3. Oliver D, Healey F, Haines TP. Preventing falls and fall-related injuries in hospitals. Clin Geriatr Med. 2010;26(4):645-692. https://doi.org/10.1016/j.cger.2010.06.005.
4. Currie L. Fall and Injury Prevention. In: Hughes RG, ed. Patient safety and quality: an evidence-based handbook for nurses (Prepared with support from the Robert Wood Johnson Foundation). AHRQ Publication NO.08-0043. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
5. Wong CA, Recktenwald AJ, Jones ML, Waterman BM, Bollini ML, Dunagan WC. The cost of serious fall-related injuries at three Midwestern hospitals. Jt Comm J Qual Patient Saf. 2011;37(2):81-87. https://doi.org/10.1016/S1553-7250(11)37010-9.
6. Bates DW, Pruess K, Souney P, Platt R. Serious falls in hospitalized patients: correlates and resource utilization. Am J Med. 1995;99(2):137-143. https://doi.org/10.1016/s0002-9343(99)80133-8.
7. Fiesta J. Liability for falls. Nurs Manage. 1998;29(3):24-26. https://doi.org/10.1097/00006247-199803000-00007.
8. Rosenthal MB. Nonpayment for performance? Medicare’s new reimbursement rule. N Engl J Med. 2007;357(16):1573-1575. https://doi.org/10.1056/NEJMp078184.
9. Department of Health and Human Services, Centers for Medicare and Medicaid Services. 42 CFR Parts 411, 412, 413, and 489. Medicare program; proposed changes to the hospital inpatient prospective payment systems and fiscal year. 2008 rates; final rule. Federal Register. 2007;72(62):47130-47178.
10. King B, Pecanac K, Krupp A, Liebzeit D, Mahoney J. Impact of fall prevention on nurses and care of fall risk patients. Gerontologist. 2018;58(2):331-340. https://doi.org/10.1093/geront/gnw156.
11. Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med. 2009;360(23):2390-2393. https://doi.org/10.1056/NEJMp0900963.
12. Rakhmatullina M, Taub A, Jacob T. Morbidity and mortality associated with the utilization of restraints : a review of literature. Psychiatr Q. 2013;84(4):499-512. https://doi.org/10.1007/s11126-013-9262-6.
13. State Operations Manual Appendix A - Survey Protocol, Regulations and Interpretive Guidelines for Hospitals. (Revision 116, 06-06-14). http://cms.hhs.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/som107ap_a_hospitals.pdf. Accessed October 26, 2014.
14. Nursing Sensitive Measures. NQF # 0203, Restraint prevalence (vest and limb only). Status: Endorsed on: August 05, 2009; Steward(s): The Joint Commission. Washington, D.C.: National Quality Forum; 2009.
15. Kopke S, Muhlhauser I, Gerlach A, et al. Effect of a guideline-based multicomponent intervention on use of physical restraints in nursing homes: a randomized controlled trial. JAMA. 2012;307(20):2177-2184. https://doi.org/10.1001/jama.2012.4517.
16. Enns E, Rhemtulla R, Ewa V, Fruetel K, Holroyd-Leduc JM. A controlled quality improvement trial to reduce the use of physical restraints in older hospitalized adults. J Am Geriatr Soc. 2014;62(3):541-545. https://doi.org/10.1111/jgs.12710.
17. Heinze C, Dassen T, Grittner U. Use of physical restraints in nursing homes and hospitals and related factors: a cross-sectional study. J Clin Nurs. 2012;21(7-8):1033-1040. https://doi.org/10.1111/j.1365-2702.2011.03931.x.
18. Minnick AF, Fogg L, Mion LC, Catrambone C, Johnson ME. Resource clusters and variation in physical restraint use. J Nurs Scholarsh. 2007;39(4):363-370. https://doi.org/10.1111/j.1547-5069.2007.00194.x.
19. Vassallo M, Wilkinson C, Stockdale R, Malik N, Baker R, Allen S. Attitudes to restraint for the prevention of falls in hospital. Gerontology. 2005;51(1):66-70. https://doi.org/10.1159/000081438.
20. Centers for Medicare & Medicaid Services. Hospital-Acquired Condition Reduction Program (HACRP). https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/HAC-Reduction-Program.html. Accessed September 9. 2018.
21. Centers for Medicare & Medicaid Services. The Hospital Value-Based Purchasing (VBP) Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HVBP/Hospital-Value-Based-Purchasing.html. Accessed September 9, 2018.
22. Dunton NE. Take a cue from the NDNQI. Nurs Manage. 2008;39(4):20, 22-23. https://doi.org/10.1097/01.NUMA.0000316054.35317.bf.
23. Garrard L, Boyle DK, Simon M, Dunton N, Gajewski B. Reliability and Validity of the NDNQI(R) Injury Falls Measure. West J Nurs Res. 2016;38(1):111-128. https://doi.org/10.1177/0193945914542851
24. Garrard L, Boyle DK, Simon M, Dunton N, Gajewski B. Reliability and validity of the NDNQI(R) injury falls measure. West J Nurs Res. 2016;38(1):111-128. https://doi.org/10.1177/0193945914542851.
25. Waters TM, Daniels MJ, Bazzoli GJ, et al. Effect of Medicare’s nonpayment for hospital-acquired conditions: lessons for future policy. JAMA Intern Med. 2015;175(3):347-354.
26. Hempel S, Newberry S, Wang Z, et al. Hospital fall prevention: a systematic review of implementation, components, adherence, and effectiveness. J Am Geriatr Soc. 2013;61(4):483-494. https://doi.org/10.1001/jamainternmed.2014.5486.
27. Barker AL, Morello RT, Wolfe R, et al. 6-PACK programme to decrease fall injuries in acute hospitals: cluster randomised controlled trial. BMJ. 2016;352:h6781. https://doi.org/10.1136/bmj.h6781.
28. Goldsack J, Bergey M, Mascioli S, Cunningham J. Hourly rounding and patient falls: what factors boost success? Nursing. 2015;45(2):25-30. https://doi.org/10.1097/01.NURSE.0000459798.79840.95.
29. Montalvo I. The National Database of Nursing Quality Indicators (NDNQI). Online Journal of Issues in Nursing. 2007;12(3).
30. Soumerai SB, Ceccarelli R, Koppel R. False dichotomies and health policy research designs: randomized trials are not always the answer. J Gen Intern Med. 2017;32(2):204-209. https://doi.org/10.1007/s11606-016-3841-9.
31. Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med. 2017;177(6):759-760. https://doi.org/10.1001/jamainternmed.2017.0840.
32. Fehlberg EA, Lucero RJ, Weaver MT, et al. Impact of the CMS no-pay policy on hospital-acquired fall prevention related practice patterns. Innov Aging. 2017;1(3):igx036-igx036. https://doi.org/10.1093/geroni/igx036.
1. Miake-Lye IM, Hempel S, Ganz DA, Shekelle PG. Inpatient fall prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5):390-396. https://doi.org/10.7326/0003-4819-158-5-201303051-00005
2. Healey F, Darowski A. Older patients and falls in hospital. Clin Risk. 2012;18(5):170-176. https://doi.org/10.1258/cr.2012.012020.
3. Oliver D, Healey F, Haines TP. Preventing falls and fall-related injuries in hospitals. Clin Geriatr Med. 2010;26(4):645-692. https://doi.org/10.1016/j.cger.2010.06.005.
4. Currie L. Fall and Injury Prevention. In: Hughes RG, ed. Patient safety and quality: an evidence-based handbook for nurses (Prepared with support from the Robert Wood Johnson Foundation). AHRQ Publication NO.08-0043. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
5. Wong CA, Recktenwald AJ, Jones ML, Waterman BM, Bollini ML, Dunagan WC. The cost of serious fall-related injuries at three Midwestern hospitals. Jt Comm J Qual Patient Saf. 2011;37(2):81-87. https://doi.org/10.1016/S1553-7250(11)37010-9.
6. Bates DW, Pruess K, Souney P, Platt R. Serious falls in hospitalized patients: correlates and resource utilization. Am J Med. 1995;99(2):137-143. https://doi.org/10.1016/s0002-9343(99)80133-8.
7. Fiesta J. Liability for falls. Nurs Manage. 1998;29(3):24-26. https://doi.org/10.1097/00006247-199803000-00007.
8. Rosenthal MB. Nonpayment for performance? Medicare’s new reimbursement rule. N Engl J Med. 2007;357(16):1573-1575. https://doi.org/10.1056/NEJMp078184.
9. Department of Health and Human Services, Centers for Medicare and Medicaid Services. 42 CFR Parts 411, 412, 413, and 489. Medicare program; proposed changes to the hospital inpatient prospective payment systems and fiscal year. 2008 rates; final rule. Federal Register. 2007;72(62):47130-47178.
10. King B, Pecanac K, Krupp A, Liebzeit D, Mahoney J. Impact of fall prevention on nurses and care of fall risk patients. Gerontologist. 2018;58(2):331-340. https://doi.org/10.1093/geront/gnw156.
11. Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med. 2009;360(23):2390-2393. https://doi.org/10.1056/NEJMp0900963.
12. Rakhmatullina M, Taub A, Jacob T. Morbidity and mortality associated with the utilization of restraints : a review of literature. Psychiatr Q. 2013;84(4):499-512. https://doi.org/10.1007/s11126-013-9262-6.
13. State Operations Manual Appendix A - Survey Protocol, Regulations and Interpretive Guidelines for Hospitals. (Revision 116, 06-06-14). http://cms.hhs.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/som107ap_a_hospitals.pdf. Accessed October 26, 2014.
14. Nursing Sensitive Measures. NQF # 0203, Restraint prevalence (vest and limb only). Status: Endorsed on: August 05, 2009; Steward(s): The Joint Commission. Washington, D.C.: National Quality Forum; 2009.
15. Kopke S, Muhlhauser I, Gerlach A, et al. Effect of a guideline-based multicomponent intervention on use of physical restraints in nursing homes: a randomized controlled trial. JAMA. 2012;307(20):2177-2184. https://doi.org/10.1001/jama.2012.4517.
16. Enns E, Rhemtulla R, Ewa V, Fruetel K, Holroyd-Leduc JM. A controlled quality improvement trial to reduce the use of physical restraints in older hospitalized adults. J Am Geriatr Soc. 2014;62(3):541-545. https://doi.org/10.1111/jgs.12710.
17. Heinze C, Dassen T, Grittner U. Use of physical restraints in nursing homes and hospitals and related factors: a cross-sectional study. J Clin Nurs. 2012;21(7-8):1033-1040. https://doi.org/10.1111/j.1365-2702.2011.03931.x.
18. Minnick AF, Fogg L, Mion LC, Catrambone C, Johnson ME. Resource clusters and variation in physical restraint use. J Nurs Scholarsh. 2007;39(4):363-370. https://doi.org/10.1111/j.1547-5069.2007.00194.x.
19. Vassallo M, Wilkinson C, Stockdale R, Malik N, Baker R, Allen S. Attitudes to restraint for the prevention of falls in hospital. Gerontology. 2005;51(1):66-70. https://doi.org/10.1159/000081438.
20. Centers for Medicare & Medicaid Services. Hospital-Acquired Condition Reduction Program (HACRP). https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/HAC-Reduction-Program.html. Accessed September 9. 2018.
21. Centers for Medicare & Medicaid Services. The Hospital Value-Based Purchasing (VBP) Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HVBP/Hospital-Value-Based-Purchasing.html. Accessed September 9, 2018.
22. Dunton NE. Take a cue from the NDNQI. Nurs Manage. 2008;39(4):20, 22-23. https://doi.org/10.1097/01.NUMA.0000316054.35317.bf.
23. Garrard L, Boyle DK, Simon M, Dunton N, Gajewski B. Reliability and Validity of the NDNQI(R) Injury Falls Measure. West J Nurs Res. 2016;38(1):111-128. https://doi.org/10.1177/0193945914542851
24. Garrard L, Boyle DK, Simon M, Dunton N, Gajewski B. Reliability and validity of the NDNQI(R) injury falls measure. West J Nurs Res. 2016;38(1):111-128. https://doi.org/10.1177/0193945914542851.
25. Waters TM, Daniels MJ, Bazzoli GJ, et al. Effect of Medicare’s nonpayment for hospital-acquired conditions: lessons for future policy. JAMA Intern Med. 2015;175(3):347-354.
26. Hempel S, Newberry S, Wang Z, et al. Hospital fall prevention: a systematic review of implementation, components, adherence, and effectiveness. J Am Geriatr Soc. 2013;61(4):483-494. https://doi.org/10.1001/jamainternmed.2014.5486.
27. Barker AL, Morello RT, Wolfe R, et al. 6-PACK programme to decrease fall injuries in acute hospitals: cluster randomised controlled trial. BMJ. 2016;352:h6781. https://doi.org/10.1136/bmj.h6781.
28. Goldsack J, Bergey M, Mascioli S, Cunningham J. Hourly rounding and patient falls: what factors boost success? Nursing. 2015;45(2):25-30. https://doi.org/10.1097/01.NURSE.0000459798.79840.95.
29. Montalvo I. The National Database of Nursing Quality Indicators (NDNQI). Online Journal of Issues in Nursing. 2007;12(3).
30. Soumerai SB, Ceccarelli R, Koppel R. False dichotomies and health policy research designs: randomized trials are not always the answer. J Gen Intern Med. 2017;32(2):204-209. https://doi.org/10.1007/s11606-016-3841-9.
31. Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med. 2017;177(6):759-760. https://doi.org/10.1001/jamainternmed.2017.0840.
32. Fehlberg EA, Lucero RJ, Weaver MT, et al. Impact of the CMS no-pay policy on hospital-acquired fall prevention related practice patterns. Innov Aging. 2017;1(3):igx036-igx036. https://doi.org/10.1093/geroni/igx036.
© 2019 Society of Hospital Medicine
Effect of Hospital Readmission Reduction Program on Hospital Readmissions and Mortality Rates
Chronic obstructive pulmonary disease (COPD) is recognized as the third leading cause of death nationally. Globally, it has been estimated that 10% of the population has COPD; in the United States, approximately 15 million people are affected.1,2 The annual estimated cost of COPD management in the United States is approximately $50 billion, one-third of which is directly related to inpatient hospitalization for COPD exacerbation.3,4,5 The 30-day readmission rate after hospitalization for acute exacerbation of COPD (AECOPD) is approximately 21% with an approximate cost of $13 billion per year.6,7 To reduce the cost and to improve patient outcomes, the Centers for Medicare and Medicaid Services (CMS) has designed several interventions with little effect.8
In October 2012, the Affordable Care Act added section 1886(q) to the Social Security Act and established the Hospital Readmission Reduction Program (HRRP), an initiative to decrease hospitalization costs by penalizing hospitals with high 30-day readmission rates. Under this program, hospitals received up to 3% penalty for excess readmissions after the index hospitalization with acute myocardial infarction (AMI), heart failure (HF), and pneumonia.9-11 Hospitals are penalized if their annual readmission rates are significantly above the average national readmission rate. In 2014, the HRRP was extended to include AECOPD for the FY 2015.
Since the implementation of readmission penalties, data have shown a significant decrease in the 30-day readmission rates for all conditions.12,13 On the other hand, studies have suggested that, at least for some conditions, the decrease in the 30-day readmission rate is associated with higher adverse patients outcomes, including higher mortality.14,15 However, whether a decrease in readmission rates after an AECOPD hospitalization is associated with a concomitant increase in mortality has not been examined. Therefore, our objective was to examine the association of the 30-day risk-adjusted hospital readmission rate with the 30-day risk-adjusted hospital mortality rate for patients discharged with a diagnosis of AECOPD.
METHOD
Data Sources
Publicly available data from three sources were used. The all-cause 30-day risk-standardized readmission rate (RSRR) and the 30-day risk-standardized mortality rate (RSMR) of each hospital for patients with AECOPD were obtained from the Hospital Compare database; a database maintained by the CMS.16,17 In 2014, the CMS started reporting three-year running average of 30-day mortality and readmission rate data on hospitals for AECOPD hospitalizations; the data start date was July 2010.18-22 We examined data from the FY 2010-2013 to 2014-2017 cycles on readmission and mortality reported by the CMS; this included data before and after the implementation of penalties.
Hospital characteristics were also obtained from the CMS website. Hospital ownership was defined as government (owned by Federal or state), for-profit (owned by physicians or another proprietary), or nonprofit (owned by a nonprofit organization such as a church). A hospital was considered as a teaching hospital if it obtained graduate medical education funding from the CMS.
Data on local population characteristics according to ZIP codes were obtained from the 2010 decennial census and the American Community Survey five-year (2009-2013) data files available at the United States Census Bureau website.23 For each ZIP code, we obtained data on the total population, percentage of African Americans in the population, median income, poverty level, and insurance status.
We used Hospital service area (HSA) information obtained from the Dartmouth Atlas of Health Care crosswalk files to link local population characteristics to hospitals. The Dartmouth Atlas defined 3,436 HSAs by assigning the ZIP codes to the hospital area where the greatest proportion of their Medicare residents was hospitalized.24,25
Hospital Compare data and Census Bureau population data were matched to the HSAs from the Dartmouth Atlas of Healthcare data at the ZIP code level. First, the ZIP code-level data from the Census Bureau were pooled by the HSAs obtained from the Dartmouth Atlas of Healthcare, followed by matching these data by the HSAs to the Hospital Compare data. Merging data from these three sources generated a dataset that contained information about readmission and mortality rates from a particular hospital and the population characteristics of the local healthcare market or neighborhood. Our final dataset included hospitals that had readmission and mortality information available at the Hospital Compare website and were included in the crosswalk files of the Dartmouth Atlas of Healthcare.
Statistical Analysis
Data are summarized as mean and standard deviation (SD), median with interquartile range, or frequencies as appropriate. To model the dependence of observations from the same hospital over time, we used mixed linear models with random intercept and slope. A strength of this modeling approach is that it incorporates information from all hospitals even when some hospitals are missing data for some time periods. We reached our final model through stages with increasing model complexity at each stage. In the first stage, we developed an empty model without any covariates to determine the unconditional variance components so that we can partition mortality variance into between- and within-hospital components. In the second stage, we developed an unconditional growth curve model to determine the shape of time trend in mortality over time using linear and quadratic (by including squared time in the model) growth curves. In the third stage, we added baseline readmission rates (from 2010 to 2013) to the model to determine the effect of baseline readmission rate on mortality trends and also examined its interaction with time and squared time. We generated a change in the readmission rate variable by subtracting the last readmission rate from the baseline readmission rate (readmission rate in 2010-2013 − readmission rate in 2014-2017). In the fourth stage, we included this change in readmission rate into the third-stage model to examine how changes in the readmission rate affected the time trends of mortality and also examined its interaction with time and squared time. In the final model, we included the following potential confounding variables to the fourth stage model: African American percentage in the HSA, HSA median income, percentage of people living in poverty in the HSA, median age, ownership of hospital (government, for profit), teaching status (teaching vs nonteaching), and acute care hospital beds in the HSA. Within each stage, the models were compared using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC), and the model with the lowest value of each was moved to the next stage of model development. All analyses were performed in Stata 14.1 for Windows (College Station, Texas).
RESULTS
Of the 3,685 acute care hospitals analyzed in the 2010-2013 data cycle for COPD, the 30-day RSRR was 20.7% (1.28), which decreased to 19.6% (1.11) in 2014-2017 (Table 1). During the same period, the 30-day all-cause RSMR increased from 7.8% (1.03) in 2010-2013 to 8.4% (1.11) in 2014-2017. The partitioning of variance showed that 57% of variation in the mortality rate over the study period was due to between-hospital differences.
The unconditional growth model examining the linear time trend revealed a 0.13% per year (95% CI = 0.12 to 0.14; P < .0001) increase in mortality rate over the five data cycles. When the squared time variable was added to the model to examine a quadratic trend, both time and squared trend were statistically significant (Table 2) and the AIC and BIC were lower for the quadratic model. Thus, the unconditional growth curve model suggested that the mortality trend was nonlinear and the coefficients demonstrated that not only the mortality rate increased, but the rate of change in the mortality rate was also increasing during the study period.
When we added the baseline readmission rate to the abovementioned quadratic growth model, we found an inverse association; each 1% increase in baseline readmission rate was associated with 0.03% (95% CI = −0.05 to −0.005; P = .02) decrease in mortality rate. These findings suggest that hospitals with higher baseline readmission rates also had lower mortality rates. To examine whether the effect of baseline readmission rate on mortality varied over time, we included the interaction term with time in the model and then added the interaction term with squared time. As the AIC and BIC were the lowest for the model with interaction between time and baseline readmission (and not when interaction between squared time and baseline readmission were included), we accepted this model. In this model, although there was no difference in mortality according to readmissions at baseline, each 1% increase in baseline readmission rate was associated with a smaller increase in mortality rate by 0.015% (95% CI = −0.02 to −0.01; P < .0001; Table 2 and Figure 1). These findings suggest that hospitals with higher readmission rates at baseline had a smaller increase in mortality rate during the study period than those with lower readmission rates.
Inclusion of change in the readmissions variable in the model showed that each 1% decrease in readmission rate during the study period was associated with 0.04% (95% CI = 0.01 to 0.06; P = .008) increase in mortality. However, the interaction between change in readmission and time was not significant and the AIC and BIC of the model were higher than the model without interaction. Therefore, we retained the model without the interaction term and included other potential confounding variables to build our final model. Thus, although hospitals with different baseline readmission rates had different rates of change in mortality rate, the change in readmission rate had a consistent effect on the mortality rate. Including potential confounders in the model did not change the results; the mortality rate and the change in the mortality rate increased during the study period, a high baseline readmission rate was associated with a lower yearly increase in mortality, and a larger decrease in readmission rate was associated with a higher mortality rate (Table 2).
DISCUSSION
As efforts to decrease readmission rates continue as a part of the HRRP implementation by the CMS, our study shows that among hospitals that discharged patients with AECOPD during 2010-2017, the all-cause 30-day RSRR was decreased, whereas the all-cause 30-day RSMR was increased. Of particular concern is that the rate of increase in mortality also increased. We also found that hospitals with higher readmission rates in 2010-2013 had a lower rate of increase in mortality than hospitals with lower readmission rates. In addition, hospitals that had a larger decrease in readmission rates during the study period had a larger rate of increase in mortality than hospitals with a smaller decrease in readmission rates. Our findings were robust to potential confounders such as hospital characteristics and local population characteristics in which hospitals operate.
Our study findings raise the question whether the implementation of the HRRP resulted in unintentional patient harm by forcing hospitals to make changes that may affect overall patient care. This question is particularly important as other studies on hospitalized patients with HF have found similar results.13,14 On the other hand, a similar association between readmission and mortality rates has not been observed in patients with pneumonia or AMI.14 Several possible explanations can be given for the observed discrepancy between the diseases and their effect on the relationship between readmission rate and mortality rate. Both COPD and HF are chronic diseases and characterized by exacerbations, whereas AMI and pneumonia are episodic diseases that are treatable. As the number of patients hospitalized with AECOPD and HF is much larger, hospitals may have a greater focus on reducing the 30-day readmission rates and may attempt to game the process, such as by delaying admissions through the emergency department within the 30-day period or by admitting patients for observation. In fact, a study found a 3% reduction in the within-hospital readmission rate with a concurrent 0.8% increase in observation unit use since the implementation of the HRRP.26 Such approaches to patient care may lead to adverse outcomes.
It is possible that readmissions and mortality act as competing risks and hence hospitals with higher mortality rates are left with fewer patients and thus have fewer readmissions, whereas those with lower mortality rates have more patients and a higher readmission rate.27 Such studies are not possible with hospital-level data, and patient-level studies will be required to examine this competing risk hypothesis. Our study results provide some support to the competing risk hypothesis (hospitals with lower baseline readmission rates had a steeper increase in mortality); however, it is not possible to draw any conclusions due to the high risk of ecological fallacy bias.
This study has important potential implications for healthcare policy, public health, and research. We found that an important national intervention aimed at decreasing readmission rates and improving the quality of care for patients with AECOPD may be associated with higher mortality rates in these patients. There may be a need to redefine measures for determining the performance of an institution. Our study supports research into the underlying mechanisms resulting in an inverse association between readmissions and mortality. In particular, health policy researchers may need to examine how incentives and penalties affect the allocation of resources within hospitals.
This study has several strengths and some potential weaknesses. We used a national dataset to examine readmission and mortality rates that include the majority of hospitals in the United States. We also included data from the local population for each hospital, thus allowing us to examine hospital performance within the context of its target population. One potential limitation is that we used hospital-level data and not patient-level data; however, the readmission penalties are designed for hospitals, which justifies our use of hospital-level data. Furthermore, data were not available for shorter time intervals; data from shorter time intervals may be associated with greater variability. Being an observational study, it is difficult to establish a causal relationship; the longitudinal nature of the study does establish temporality, an important factor in establishing causality.
In conclusion, we found that although the readmission rates decreased, there was an increase in the mortality rate within the 30 days of discharge from the hospital in patients with AECOPD. The rate of increase in mortality was higher in hospitals with lower readmission rates than in hospitals with higher readmission rates. Further research for determining the mechanism responsible for this association is needed. Future health policy interventions may need to consider the potential for adverse outcomes.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61(4):1-117.
2. Halbert RJ, Natoli JL, Gano A, et al. Global burden of copd: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523-532. https://doi.org/10.1183/09031936.06.00124605.
3. Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD. 2010;7(3):214-228. https://doi.org/10.3109/15412555.2010.481697.
4. Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219-1226. https://doi.org/10.1378/chest.14-2181.
5. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/NEJMsa0803563.
6. Stein BD, Charbeneau JT, Lee TA, et al. Hospitalizations for acute exacerbations of chronic obstructive pulmonary disease: how you count matters. COPD. 2010;7(3):164-171. https://doi.org/10.3109/15412555.2010.481696.
7. Stein, B. D., Charbeneau, J. T., Lee, T. A., Schumock, G. T., Lindenauer, P. K., Bautista, A., . . . Krishnan, J. A. (2010). Hospitalizations for acute exacerbations of chronic obstructive pulmonary disease: how you count matters. COPD, 7(3), 164-171. doi:10.3109/15412555.2010.481696
8. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. https://doi.org/10.1161/CIRCULATIONAHA.114.010270.
9. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):179-1803. https://doi.org/10.1161/CIRCULATIONAHA.114.010270.
10. Centers for Medicare and Medicaid Services (CMS), HHS. Medicare Program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and FY 2012 rates; Hospitals’ FTE Resident Caps for Graduate Medical Education Payment. Final Rules. Fed Regist. 2011;76(160):51476-51846.
11. Centers for Medicare and Medicaid Services (CMS). Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and fiscal year 2014 rates; quality reporting requirements for specific providers; hospital conditions of participation; payment policies related to patient status. Final rules. Fed Regist. 2013;78(160):50495-51040.
12. Casillas G. Published: Mar 10 and 2017, “aiming for fewer hospital U-turns: the Medicare Hospital readmission reduction program,” [blog]. https://www.kff.org/medicare/issue-brief/aiming-for-fewer-hospital-u-turns-the-medicare-hospital-readmission-reduction-program/; Accessed March 10, 2017. The Henry J. Kaiser Family Foundation.
13. Desai NR, Ross JS, Kwon JY, et al. Association Between hospital penalty status Under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24): 2647-2656. https://doi.org/10.1001/jama.2016.18533.
14. Gupta A, Allen LA, Bhatt DL, et al. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3(1):44-53. https://doi.org/10.1001/jamacardio.2017.4265.
15. Krumholz HM, Lin Z, Keenan PS, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587-593. https://doi.org/10.1001/jama.2013.333.
16. Medicare Hospital compare overview,” https://www.medicare.gov/hospitalcompare/About/What-Is-HOS.html; Accessed April 17, 2019.
17. Archived datasets. Data.Medicare.Gov. Data.Medicare.Gov. Accessed April 17, 2019. https://data.medicare.gov/data/archives/hospital-compare.
18. Krumholz HM, Lin Z, Drye EE, et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4(2):243-252. https://doi.org/10.1161/CIRCOUTCOMES.110.957498.
19. Bratzler DW, Normand SL, Wang Y, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLOS ONE. 2011;6(4):e17401. https://doi.org/10.1371/journal.pone.0017401.
20. Centers for Medicare, Medicaid Services. Security Boulevard Baltimore, and Md21244 USA, “OutcomeMeasures,”. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures.html 7500; Accessed October 13, 2017.
21. Centers for Medicare and Medicaid Services (CMS), HHS. Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and Fiscal Year 2014 Rates; Quality Reporting Requirements for Specific Providers; Hospital Conditions of Participation; Payment Policies Related to Patient Status. Final Rules.”
22. Feemster LC, Au DH. Penalizing hospitals for chronic obstructive pulmonary disease readmissions. Am J Respir Crit Care Med. 2014;189(6):634-639. https://doi.org/10.1164/rccm.201308-1541PP.
23. United States Census Bureau. Census.Gov. Accessed April 17, 2019. https://www.census.gov/en.html.
24. Dartmouth atlas data,”. https://atlasdata.dartmouth.edu/. Aaccessed April 17, 2019.
25. Home. Dartmouth Atlas Healthc. https://www.dartmouthatlas.org/. Accessed April 17, 2019.
26. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016;374(16):1543-1551. https://doi.org/10.1056/NEJMsa1513024.
27. Gorodeski EZ, Starling RC, Blackstone EH. Are All Readmissions Bad Readmissions?, letter. World. 2010. https://doi.org/10.1056/NEJMc1001882.
Chronic obstructive pulmonary disease (COPD) is recognized as the third leading cause of death nationally. Globally, it has been estimated that 10% of the population has COPD; in the United States, approximately 15 million people are affected.1,2 The annual estimated cost of COPD management in the United States is approximately $50 billion, one-third of which is directly related to inpatient hospitalization for COPD exacerbation.3,4,5 The 30-day readmission rate after hospitalization for acute exacerbation of COPD (AECOPD) is approximately 21% with an approximate cost of $13 billion per year.6,7 To reduce the cost and to improve patient outcomes, the Centers for Medicare and Medicaid Services (CMS) has designed several interventions with little effect.8
In October 2012, the Affordable Care Act added section 1886(q) to the Social Security Act and established the Hospital Readmission Reduction Program (HRRP), an initiative to decrease hospitalization costs by penalizing hospitals with high 30-day readmission rates. Under this program, hospitals received up to 3% penalty for excess readmissions after the index hospitalization with acute myocardial infarction (AMI), heart failure (HF), and pneumonia.9-11 Hospitals are penalized if their annual readmission rates are significantly above the average national readmission rate. In 2014, the HRRP was extended to include AECOPD for the FY 2015.
Since the implementation of readmission penalties, data have shown a significant decrease in the 30-day readmission rates for all conditions.12,13 On the other hand, studies have suggested that, at least for some conditions, the decrease in the 30-day readmission rate is associated with higher adverse patients outcomes, including higher mortality.14,15 However, whether a decrease in readmission rates after an AECOPD hospitalization is associated with a concomitant increase in mortality has not been examined. Therefore, our objective was to examine the association of the 30-day risk-adjusted hospital readmission rate with the 30-day risk-adjusted hospital mortality rate for patients discharged with a diagnosis of AECOPD.
METHOD
Data Sources
Publicly available data from three sources were used. The all-cause 30-day risk-standardized readmission rate (RSRR) and the 30-day risk-standardized mortality rate (RSMR) of each hospital for patients with AECOPD were obtained from the Hospital Compare database; a database maintained by the CMS.16,17 In 2014, the CMS started reporting three-year running average of 30-day mortality and readmission rate data on hospitals for AECOPD hospitalizations; the data start date was July 2010.18-22 We examined data from the FY 2010-2013 to 2014-2017 cycles on readmission and mortality reported by the CMS; this included data before and after the implementation of penalties.
Hospital characteristics were also obtained from the CMS website. Hospital ownership was defined as government (owned by Federal or state), for-profit (owned by physicians or another proprietary), or nonprofit (owned by a nonprofit organization such as a church). A hospital was considered as a teaching hospital if it obtained graduate medical education funding from the CMS.
Data on local population characteristics according to ZIP codes were obtained from the 2010 decennial census and the American Community Survey five-year (2009-2013) data files available at the United States Census Bureau website.23 For each ZIP code, we obtained data on the total population, percentage of African Americans in the population, median income, poverty level, and insurance status.
We used Hospital service area (HSA) information obtained from the Dartmouth Atlas of Health Care crosswalk files to link local population characteristics to hospitals. The Dartmouth Atlas defined 3,436 HSAs by assigning the ZIP codes to the hospital area where the greatest proportion of their Medicare residents was hospitalized.24,25
Hospital Compare data and Census Bureau population data were matched to the HSAs from the Dartmouth Atlas of Healthcare data at the ZIP code level. First, the ZIP code-level data from the Census Bureau were pooled by the HSAs obtained from the Dartmouth Atlas of Healthcare, followed by matching these data by the HSAs to the Hospital Compare data. Merging data from these three sources generated a dataset that contained information about readmission and mortality rates from a particular hospital and the population characteristics of the local healthcare market or neighborhood. Our final dataset included hospitals that had readmission and mortality information available at the Hospital Compare website and were included in the crosswalk files of the Dartmouth Atlas of Healthcare.
Statistical Analysis
Data are summarized as mean and standard deviation (SD), median with interquartile range, or frequencies as appropriate. To model the dependence of observations from the same hospital over time, we used mixed linear models with random intercept and slope. A strength of this modeling approach is that it incorporates information from all hospitals even when some hospitals are missing data for some time periods. We reached our final model through stages with increasing model complexity at each stage. In the first stage, we developed an empty model without any covariates to determine the unconditional variance components so that we can partition mortality variance into between- and within-hospital components. In the second stage, we developed an unconditional growth curve model to determine the shape of time trend in mortality over time using linear and quadratic (by including squared time in the model) growth curves. In the third stage, we added baseline readmission rates (from 2010 to 2013) to the model to determine the effect of baseline readmission rate on mortality trends and also examined its interaction with time and squared time. We generated a change in the readmission rate variable by subtracting the last readmission rate from the baseline readmission rate (readmission rate in 2010-2013 − readmission rate in 2014-2017). In the fourth stage, we included this change in readmission rate into the third-stage model to examine how changes in the readmission rate affected the time trends of mortality and also examined its interaction with time and squared time. In the final model, we included the following potential confounding variables to the fourth stage model: African American percentage in the HSA, HSA median income, percentage of people living in poverty in the HSA, median age, ownership of hospital (government, for profit), teaching status (teaching vs nonteaching), and acute care hospital beds in the HSA. Within each stage, the models were compared using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC), and the model with the lowest value of each was moved to the next stage of model development. All analyses were performed in Stata 14.1 for Windows (College Station, Texas).
RESULTS
Of the 3,685 acute care hospitals analyzed in the 2010-2013 data cycle for COPD, the 30-day RSRR was 20.7% (1.28), which decreased to 19.6% (1.11) in 2014-2017 (Table 1). During the same period, the 30-day all-cause RSMR increased from 7.8% (1.03) in 2010-2013 to 8.4% (1.11) in 2014-2017. The partitioning of variance showed that 57% of variation in the mortality rate over the study period was due to between-hospital differences.
The unconditional growth model examining the linear time trend revealed a 0.13% per year (95% CI = 0.12 to 0.14; P < .0001) increase in mortality rate over the five data cycles. When the squared time variable was added to the model to examine a quadratic trend, both time and squared trend were statistically significant (Table 2) and the AIC and BIC were lower for the quadratic model. Thus, the unconditional growth curve model suggested that the mortality trend was nonlinear and the coefficients demonstrated that not only the mortality rate increased, but the rate of change in the mortality rate was also increasing during the study period.
When we added the baseline readmission rate to the abovementioned quadratic growth model, we found an inverse association; each 1% increase in baseline readmission rate was associated with 0.03% (95% CI = −0.05 to −0.005; P = .02) decrease in mortality rate. These findings suggest that hospitals with higher baseline readmission rates also had lower mortality rates. To examine whether the effect of baseline readmission rate on mortality varied over time, we included the interaction term with time in the model and then added the interaction term with squared time. As the AIC and BIC were the lowest for the model with interaction between time and baseline readmission (and not when interaction between squared time and baseline readmission were included), we accepted this model. In this model, although there was no difference in mortality according to readmissions at baseline, each 1% increase in baseline readmission rate was associated with a smaller increase in mortality rate by 0.015% (95% CI = −0.02 to −0.01; P < .0001; Table 2 and Figure 1). These findings suggest that hospitals with higher readmission rates at baseline had a smaller increase in mortality rate during the study period than those with lower readmission rates.
Inclusion of change in the readmissions variable in the model showed that each 1% decrease in readmission rate during the study period was associated with 0.04% (95% CI = 0.01 to 0.06; P = .008) increase in mortality. However, the interaction between change in readmission and time was not significant and the AIC and BIC of the model were higher than the model without interaction. Therefore, we retained the model without the interaction term and included other potential confounding variables to build our final model. Thus, although hospitals with different baseline readmission rates had different rates of change in mortality rate, the change in readmission rate had a consistent effect on the mortality rate. Including potential confounders in the model did not change the results; the mortality rate and the change in the mortality rate increased during the study period, a high baseline readmission rate was associated with a lower yearly increase in mortality, and a larger decrease in readmission rate was associated with a higher mortality rate (Table 2).
DISCUSSION
As efforts to decrease readmission rates continue as a part of the HRRP implementation by the CMS, our study shows that among hospitals that discharged patients with AECOPD during 2010-2017, the all-cause 30-day RSRR was decreased, whereas the all-cause 30-day RSMR was increased. Of particular concern is that the rate of increase in mortality also increased. We also found that hospitals with higher readmission rates in 2010-2013 had a lower rate of increase in mortality than hospitals with lower readmission rates. In addition, hospitals that had a larger decrease in readmission rates during the study period had a larger rate of increase in mortality than hospitals with a smaller decrease in readmission rates. Our findings were robust to potential confounders such as hospital characteristics and local population characteristics in which hospitals operate.
Our study findings raise the question whether the implementation of the HRRP resulted in unintentional patient harm by forcing hospitals to make changes that may affect overall patient care. This question is particularly important as other studies on hospitalized patients with HF have found similar results.13,14 On the other hand, a similar association between readmission and mortality rates has not been observed in patients with pneumonia or AMI.14 Several possible explanations can be given for the observed discrepancy between the diseases and their effect on the relationship between readmission rate and mortality rate. Both COPD and HF are chronic diseases and characterized by exacerbations, whereas AMI and pneumonia are episodic diseases that are treatable. As the number of patients hospitalized with AECOPD and HF is much larger, hospitals may have a greater focus on reducing the 30-day readmission rates and may attempt to game the process, such as by delaying admissions through the emergency department within the 30-day period or by admitting patients for observation. In fact, a study found a 3% reduction in the within-hospital readmission rate with a concurrent 0.8% increase in observation unit use since the implementation of the HRRP.26 Such approaches to patient care may lead to adverse outcomes.
It is possible that readmissions and mortality act as competing risks and hence hospitals with higher mortality rates are left with fewer patients and thus have fewer readmissions, whereas those with lower mortality rates have more patients and a higher readmission rate.27 Such studies are not possible with hospital-level data, and patient-level studies will be required to examine this competing risk hypothesis. Our study results provide some support to the competing risk hypothesis (hospitals with lower baseline readmission rates had a steeper increase in mortality); however, it is not possible to draw any conclusions due to the high risk of ecological fallacy bias.
This study has important potential implications for healthcare policy, public health, and research. We found that an important national intervention aimed at decreasing readmission rates and improving the quality of care for patients with AECOPD may be associated with higher mortality rates in these patients. There may be a need to redefine measures for determining the performance of an institution. Our study supports research into the underlying mechanisms resulting in an inverse association between readmissions and mortality. In particular, health policy researchers may need to examine how incentives and penalties affect the allocation of resources within hospitals.
This study has several strengths and some potential weaknesses. We used a national dataset to examine readmission and mortality rates that include the majority of hospitals in the United States. We also included data from the local population for each hospital, thus allowing us to examine hospital performance within the context of its target population. One potential limitation is that we used hospital-level data and not patient-level data; however, the readmission penalties are designed for hospitals, which justifies our use of hospital-level data. Furthermore, data were not available for shorter time intervals; data from shorter time intervals may be associated with greater variability. Being an observational study, it is difficult to establish a causal relationship; the longitudinal nature of the study does establish temporality, an important factor in establishing causality.
In conclusion, we found that although the readmission rates decreased, there was an increase in the mortality rate within the 30 days of discharge from the hospital in patients with AECOPD. The rate of increase in mortality was higher in hospitals with lower readmission rates than in hospitals with higher readmission rates. Further research for determining the mechanism responsible for this association is needed. Future health policy interventions may need to consider the potential for adverse outcomes.
Chronic obstructive pulmonary disease (COPD) is recognized as the third leading cause of death nationally. Globally, it has been estimated that 10% of the population has COPD; in the United States, approximately 15 million people are affected.1,2 The annual estimated cost of COPD management in the United States is approximately $50 billion, one-third of which is directly related to inpatient hospitalization for COPD exacerbation.3,4,5 The 30-day readmission rate after hospitalization for acute exacerbation of COPD (AECOPD) is approximately 21% with an approximate cost of $13 billion per year.6,7 To reduce the cost and to improve patient outcomes, the Centers for Medicare and Medicaid Services (CMS) has designed several interventions with little effect.8
In October 2012, the Affordable Care Act added section 1886(q) to the Social Security Act and established the Hospital Readmission Reduction Program (HRRP), an initiative to decrease hospitalization costs by penalizing hospitals with high 30-day readmission rates. Under this program, hospitals received up to 3% penalty for excess readmissions after the index hospitalization with acute myocardial infarction (AMI), heart failure (HF), and pneumonia.9-11 Hospitals are penalized if their annual readmission rates are significantly above the average national readmission rate. In 2014, the HRRP was extended to include AECOPD for the FY 2015.
Since the implementation of readmission penalties, data have shown a significant decrease in the 30-day readmission rates for all conditions.12,13 On the other hand, studies have suggested that, at least for some conditions, the decrease in the 30-day readmission rate is associated with higher adverse patients outcomes, including higher mortality.14,15 However, whether a decrease in readmission rates after an AECOPD hospitalization is associated with a concomitant increase in mortality has not been examined. Therefore, our objective was to examine the association of the 30-day risk-adjusted hospital readmission rate with the 30-day risk-adjusted hospital mortality rate for patients discharged with a diagnosis of AECOPD.
METHOD
Data Sources
Publicly available data from three sources were used. The all-cause 30-day risk-standardized readmission rate (RSRR) and the 30-day risk-standardized mortality rate (RSMR) of each hospital for patients with AECOPD were obtained from the Hospital Compare database; a database maintained by the CMS.16,17 In 2014, the CMS started reporting three-year running average of 30-day mortality and readmission rate data on hospitals for AECOPD hospitalizations; the data start date was July 2010.18-22 We examined data from the FY 2010-2013 to 2014-2017 cycles on readmission and mortality reported by the CMS; this included data before and after the implementation of penalties.
Hospital characteristics were also obtained from the CMS website. Hospital ownership was defined as government (owned by Federal or state), for-profit (owned by physicians or another proprietary), or nonprofit (owned by a nonprofit organization such as a church). A hospital was considered as a teaching hospital if it obtained graduate medical education funding from the CMS.
Data on local population characteristics according to ZIP codes were obtained from the 2010 decennial census and the American Community Survey five-year (2009-2013) data files available at the United States Census Bureau website.23 For each ZIP code, we obtained data on the total population, percentage of African Americans in the population, median income, poverty level, and insurance status.
We used Hospital service area (HSA) information obtained from the Dartmouth Atlas of Health Care crosswalk files to link local population characteristics to hospitals. The Dartmouth Atlas defined 3,436 HSAs by assigning the ZIP codes to the hospital area where the greatest proportion of their Medicare residents was hospitalized.24,25
Hospital Compare data and Census Bureau population data were matched to the HSAs from the Dartmouth Atlas of Healthcare data at the ZIP code level. First, the ZIP code-level data from the Census Bureau were pooled by the HSAs obtained from the Dartmouth Atlas of Healthcare, followed by matching these data by the HSAs to the Hospital Compare data. Merging data from these three sources generated a dataset that contained information about readmission and mortality rates from a particular hospital and the population characteristics of the local healthcare market or neighborhood. Our final dataset included hospitals that had readmission and mortality information available at the Hospital Compare website and were included in the crosswalk files of the Dartmouth Atlas of Healthcare.
Statistical Analysis
Data are summarized as mean and standard deviation (SD), median with interquartile range, or frequencies as appropriate. To model the dependence of observations from the same hospital over time, we used mixed linear models with random intercept and slope. A strength of this modeling approach is that it incorporates information from all hospitals even when some hospitals are missing data for some time periods. We reached our final model through stages with increasing model complexity at each stage. In the first stage, we developed an empty model without any covariates to determine the unconditional variance components so that we can partition mortality variance into between- and within-hospital components. In the second stage, we developed an unconditional growth curve model to determine the shape of time trend in mortality over time using linear and quadratic (by including squared time in the model) growth curves. In the third stage, we added baseline readmission rates (from 2010 to 2013) to the model to determine the effect of baseline readmission rate on mortality trends and also examined its interaction with time and squared time. We generated a change in the readmission rate variable by subtracting the last readmission rate from the baseline readmission rate (readmission rate in 2010-2013 − readmission rate in 2014-2017). In the fourth stage, we included this change in readmission rate into the third-stage model to examine how changes in the readmission rate affected the time trends of mortality and also examined its interaction with time and squared time. In the final model, we included the following potential confounding variables to the fourth stage model: African American percentage in the HSA, HSA median income, percentage of people living in poverty in the HSA, median age, ownership of hospital (government, for profit), teaching status (teaching vs nonteaching), and acute care hospital beds in the HSA. Within each stage, the models were compared using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC), and the model with the lowest value of each was moved to the next stage of model development. All analyses were performed in Stata 14.1 for Windows (College Station, Texas).
RESULTS
Of the 3,685 acute care hospitals analyzed in the 2010-2013 data cycle for COPD, the 30-day RSRR was 20.7% (1.28), which decreased to 19.6% (1.11) in 2014-2017 (Table 1). During the same period, the 30-day all-cause RSMR increased from 7.8% (1.03) in 2010-2013 to 8.4% (1.11) in 2014-2017. The partitioning of variance showed that 57% of variation in the mortality rate over the study period was due to between-hospital differences.
The unconditional growth model examining the linear time trend revealed a 0.13% per year (95% CI = 0.12 to 0.14; P < .0001) increase in mortality rate over the five data cycles. When the squared time variable was added to the model to examine a quadratic trend, both time and squared trend were statistically significant (Table 2) and the AIC and BIC were lower for the quadratic model. Thus, the unconditional growth curve model suggested that the mortality trend was nonlinear and the coefficients demonstrated that not only the mortality rate increased, but the rate of change in the mortality rate was also increasing during the study period.
When we added the baseline readmission rate to the abovementioned quadratic growth model, we found an inverse association; each 1% increase in baseline readmission rate was associated with 0.03% (95% CI = −0.05 to −0.005; P = .02) decrease in mortality rate. These findings suggest that hospitals with higher baseline readmission rates also had lower mortality rates. To examine whether the effect of baseline readmission rate on mortality varied over time, we included the interaction term with time in the model and then added the interaction term with squared time. As the AIC and BIC were the lowest for the model with interaction between time and baseline readmission (and not when interaction between squared time and baseline readmission were included), we accepted this model. In this model, although there was no difference in mortality according to readmissions at baseline, each 1% increase in baseline readmission rate was associated with a smaller increase in mortality rate by 0.015% (95% CI = −0.02 to −0.01; P < .0001; Table 2 and Figure 1). These findings suggest that hospitals with higher readmission rates at baseline had a smaller increase in mortality rate during the study period than those with lower readmission rates.
Inclusion of change in the readmissions variable in the model showed that each 1% decrease in readmission rate during the study period was associated with 0.04% (95% CI = 0.01 to 0.06; P = .008) increase in mortality. However, the interaction between change in readmission and time was not significant and the AIC and BIC of the model were higher than the model without interaction. Therefore, we retained the model without the interaction term and included other potential confounding variables to build our final model. Thus, although hospitals with different baseline readmission rates had different rates of change in mortality rate, the change in readmission rate had a consistent effect on the mortality rate. Including potential confounders in the model did not change the results; the mortality rate and the change in the mortality rate increased during the study period, a high baseline readmission rate was associated with a lower yearly increase in mortality, and a larger decrease in readmission rate was associated with a higher mortality rate (Table 2).
DISCUSSION
As efforts to decrease readmission rates continue as a part of the HRRP implementation by the CMS, our study shows that among hospitals that discharged patients with AECOPD during 2010-2017, the all-cause 30-day RSRR was decreased, whereas the all-cause 30-day RSMR was increased. Of particular concern is that the rate of increase in mortality also increased. We also found that hospitals with higher readmission rates in 2010-2013 had a lower rate of increase in mortality than hospitals with lower readmission rates. In addition, hospitals that had a larger decrease in readmission rates during the study period had a larger rate of increase in mortality than hospitals with a smaller decrease in readmission rates. Our findings were robust to potential confounders such as hospital characteristics and local population characteristics in which hospitals operate.
Our study findings raise the question whether the implementation of the HRRP resulted in unintentional patient harm by forcing hospitals to make changes that may affect overall patient care. This question is particularly important as other studies on hospitalized patients with HF have found similar results.13,14 On the other hand, a similar association between readmission and mortality rates has not been observed in patients with pneumonia or AMI.14 Several possible explanations can be given for the observed discrepancy between the diseases and their effect on the relationship between readmission rate and mortality rate. Both COPD and HF are chronic diseases and characterized by exacerbations, whereas AMI and pneumonia are episodic diseases that are treatable. As the number of patients hospitalized with AECOPD and HF is much larger, hospitals may have a greater focus on reducing the 30-day readmission rates and may attempt to game the process, such as by delaying admissions through the emergency department within the 30-day period or by admitting patients for observation. In fact, a study found a 3% reduction in the within-hospital readmission rate with a concurrent 0.8% increase in observation unit use since the implementation of the HRRP.26 Such approaches to patient care may lead to adverse outcomes.
It is possible that readmissions and mortality act as competing risks and hence hospitals with higher mortality rates are left with fewer patients and thus have fewer readmissions, whereas those with lower mortality rates have more patients and a higher readmission rate.27 Such studies are not possible with hospital-level data, and patient-level studies will be required to examine this competing risk hypothesis. Our study results provide some support to the competing risk hypothesis (hospitals with lower baseline readmission rates had a steeper increase in mortality); however, it is not possible to draw any conclusions due to the high risk of ecological fallacy bias.
This study has important potential implications for healthcare policy, public health, and research. We found that an important national intervention aimed at decreasing readmission rates and improving the quality of care for patients with AECOPD may be associated with higher mortality rates in these patients. There may be a need to redefine measures for determining the performance of an institution. Our study supports research into the underlying mechanisms resulting in an inverse association between readmissions and mortality. In particular, health policy researchers may need to examine how incentives and penalties affect the allocation of resources within hospitals.
This study has several strengths and some potential weaknesses. We used a national dataset to examine readmission and mortality rates that include the majority of hospitals in the United States. We also included data from the local population for each hospital, thus allowing us to examine hospital performance within the context of its target population. One potential limitation is that we used hospital-level data and not patient-level data; however, the readmission penalties are designed for hospitals, which justifies our use of hospital-level data. Furthermore, data were not available for shorter time intervals; data from shorter time intervals may be associated with greater variability. Being an observational study, it is difficult to establish a causal relationship; the longitudinal nature of the study does establish temporality, an important factor in establishing causality.
In conclusion, we found that although the readmission rates decreased, there was an increase in the mortality rate within the 30 days of discharge from the hospital in patients with AECOPD. The rate of increase in mortality was higher in hospitals with lower readmission rates than in hospitals with higher readmission rates. Further research for determining the mechanism responsible for this association is needed. Future health policy interventions may need to consider the potential for adverse outcomes.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61(4):1-117.
2. Halbert RJ, Natoli JL, Gano A, et al. Global burden of copd: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523-532. https://doi.org/10.1183/09031936.06.00124605.
3. Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD. 2010;7(3):214-228. https://doi.org/10.3109/15412555.2010.481697.
4. Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219-1226. https://doi.org/10.1378/chest.14-2181.
5. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/NEJMsa0803563.
6. Stein BD, Charbeneau JT, Lee TA, et al. Hospitalizations for acute exacerbations of chronic obstructive pulmonary disease: how you count matters. COPD. 2010;7(3):164-171. https://doi.org/10.3109/15412555.2010.481696.
7. Stein, B. D., Charbeneau, J. T., Lee, T. A., Schumock, G. T., Lindenauer, P. K., Bautista, A., . . . Krishnan, J. A. (2010). Hospitalizations for acute exacerbations of chronic obstructive pulmonary disease: how you count matters. COPD, 7(3), 164-171. doi:10.3109/15412555.2010.481696
8. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. https://doi.org/10.1161/CIRCULATIONAHA.114.010270.
9. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):179-1803. https://doi.org/10.1161/CIRCULATIONAHA.114.010270.
10. Centers for Medicare and Medicaid Services (CMS), HHS. Medicare Program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and FY 2012 rates; Hospitals’ FTE Resident Caps for Graduate Medical Education Payment. Final Rules. Fed Regist. 2011;76(160):51476-51846.
11. Centers for Medicare and Medicaid Services (CMS). Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and fiscal year 2014 rates; quality reporting requirements for specific providers; hospital conditions of participation; payment policies related to patient status. Final rules. Fed Regist. 2013;78(160):50495-51040.
12. Casillas G. Published: Mar 10 and 2017, “aiming for fewer hospital U-turns: the Medicare Hospital readmission reduction program,” [blog]. https://www.kff.org/medicare/issue-brief/aiming-for-fewer-hospital-u-turns-the-medicare-hospital-readmission-reduction-program/; Accessed March 10, 2017. The Henry J. Kaiser Family Foundation.
13. Desai NR, Ross JS, Kwon JY, et al. Association Between hospital penalty status Under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24): 2647-2656. https://doi.org/10.1001/jama.2016.18533.
14. Gupta A, Allen LA, Bhatt DL, et al. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3(1):44-53. https://doi.org/10.1001/jamacardio.2017.4265.
15. Krumholz HM, Lin Z, Keenan PS, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587-593. https://doi.org/10.1001/jama.2013.333.
16. Medicare Hospital compare overview,” https://www.medicare.gov/hospitalcompare/About/What-Is-HOS.html; Accessed April 17, 2019.
17. Archived datasets. Data.Medicare.Gov. Data.Medicare.Gov. Accessed April 17, 2019. https://data.medicare.gov/data/archives/hospital-compare.
18. Krumholz HM, Lin Z, Drye EE, et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4(2):243-252. https://doi.org/10.1161/CIRCOUTCOMES.110.957498.
19. Bratzler DW, Normand SL, Wang Y, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLOS ONE. 2011;6(4):e17401. https://doi.org/10.1371/journal.pone.0017401.
20. Centers for Medicare, Medicaid Services. Security Boulevard Baltimore, and Md21244 USA, “OutcomeMeasures,”. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures.html 7500; Accessed October 13, 2017.
21. Centers for Medicare and Medicaid Services (CMS), HHS. Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and Fiscal Year 2014 Rates; Quality Reporting Requirements for Specific Providers; Hospital Conditions of Participation; Payment Policies Related to Patient Status. Final Rules.”
22. Feemster LC, Au DH. Penalizing hospitals for chronic obstructive pulmonary disease readmissions. Am J Respir Crit Care Med. 2014;189(6):634-639. https://doi.org/10.1164/rccm.201308-1541PP.
23. United States Census Bureau. Census.Gov. Accessed April 17, 2019. https://www.census.gov/en.html.
24. Dartmouth atlas data,”. https://atlasdata.dartmouth.edu/. Aaccessed April 17, 2019.
25. Home. Dartmouth Atlas Healthc. https://www.dartmouthatlas.org/. Accessed April 17, 2019.
26. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016;374(16):1543-1551. https://doi.org/10.1056/NEJMsa1513024.
27. Gorodeski EZ, Starling RC, Blackstone EH. Are All Readmissions Bad Readmissions?, letter. World. 2010. https://doi.org/10.1056/NEJMc1001882.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61(4):1-117.
2. Halbert RJ, Natoli JL, Gano A, et al. Global burden of copd: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523-532. https://doi.org/10.1183/09031936.06.00124605.
3. Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD. 2010;7(3):214-228. https://doi.org/10.3109/15412555.2010.481697.
4. Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219-1226. https://doi.org/10.1378/chest.14-2181.
5. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/NEJMsa0803563.
6. Stein BD, Charbeneau JT, Lee TA, et al. Hospitalizations for acute exacerbations of chronic obstructive pulmonary disease: how you count matters. COPD. 2010;7(3):164-171. https://doi.org/10.3109/15412555.2010.481696.
7. Stein, B. D., Charbeneau, J. T., Lee, T. A., Schumock, G. T., Lindenauer, P. K., Bautista, A., . . . Krishnan, J. A. (2010). Hospitalizations for acute exacerbations of chronic obstructive pulmonary disease: how you count matters. COPD, 7(3), 164-171. doi:10.3109/15412555.2010.481696
8. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. https://doi.org/10.1161/CIRCULATIONAHA.114.010270.
9. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):179-1803. https://doi.org/10.1161/CIRCULATIONAHA.114.010270.
10. Centers for Medicare and Medicaid Services (CMS), HHS. Medicare Program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and FY 2012 rates; Hospitals’ FTE Resident Caps for Graduate Medical Education Payment. Final Rules. Fed Regist. 2011;76(160):51476-51846.
11. Centers for Medicare and Medicaid Services (CMS). Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and fiscal year 2014 rates; quality reporting requirements for specific providers; hospital conditions of participation; payment policies related to patient status. Final rules. Fed Regist. 2013;78(160):50495-51040.
12. Casillas G. Published: Mar 10 and 2017, “aiming for fewer hospital U-turns: the Medicare Hospital readmission reduction program,” [blog]. https://www.kff.org/medicare/issue-brief/aiming-for-fewer-hospital-u-turns-the-medicare-hospital-readmission-reduction-program/; Accessed March 10, 2017. The Henry J. Kaiser Family Foundation.
13. Desai NR, Ross JS, Kwon JY, et al. Association Between hospital penalty status Under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24): 2647-2656. https://doi.org/10.1001/jama.2016.18533.
14. Gupta A, Allen LA, Bhatt DL, et al. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3(1):44-53. https://doi.org/10.1001/jamacardio.2017.4265.
15. Krumholz HM, Lin Z, Keenan PS, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587-593. https://doi.org/10.1001/jama.2013.333.
16. Medicare Hospital compare overview,” https://www.medicare.gov/hospitalcompare/About/What-Is-HOS.html; Accessed April 17, 2019.
17. Archived datasets. Data.Medicare.Gov. Data.Medicare.Gov. Accessed April 17, 2019. https://data.medicare.gov/data/archives/hospital-compare.
18. Krumholz HM, Lin Z, Drye EE, et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4(2):243-252. https://doi.org/10.1161/CIRCOUTCOMES.110.957498.
19. Bratzler DW, Normand SL, Wang Y, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLOS ONE. 2011;6(4):e17401. https://doi.org/10.1371/journal.pone.0017401.
20. Centers for Medicare, Medicaid Services. Security Boulevard Baltimore, and Md21244 USA, “OutcomeMeasures,”. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures.html 7500; Accessed October 13, 2017.
21. Centers for Medicare and Medicaid Services (CMS), HHS. Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and Fiscal Year 2014 Rates; Quality Reporting Requirements for Specific Providers; Hospital Conditions of Participation; Payment Policies Related to Patient Status. Final Rules.”
22. Feemster LC, Au DH. Penalizing hospitals for chronic obstructive pulmonary disease readmissions. Am J Respir Crit Care Med. 2014;189(6):634-639. https://doi.org/10.1164/rccm.201308-1541PP.
23. United States Census Bureau. Census.Gov. Accessed April 17, 2019. https://www.census.gov/en.html.
24. Dartmouth atlas data,”. https://atlasdata.dartmouth.edu/. Aaccessed April 17, 2019.
25. Home. Dartmouth Atlas Healthc. https://www.dartmouthatlas.org/. Accessed April 17, 2019.
26. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016;374(16):1543-1551. https://doi.org/10.1056/NEJMsa1513024.
27. Gorodeski EZ, Starling RC, Blackstone EH. Are All Readmissions Bad Readmissions?, letter. World. 2010. https://doi.org/10.1056/NEJMc1001882.
© 2019 Society of Hospital Medicine
Recommendations on the Use of Ultrasound Guidance for Central and Peripheral Vascular Access in Adults: A Position Statement of the Society of Hospital Medicine
Approximately five million central venous catheters (CVCs) are inserted in the United States annually, with over 15 million catheter days documented in intensive care units alone.1 Traditional CVC insertion techniques using landmarks are associated with a high risk of mechanical complications, particularly pneumothorax and arterial puncture, which occur in 5%-19% patients.2,3
Since the 1990s, several randomized controlled studies and meta-analyses have demonstrated that the use of real-time ultrasound guidance for CVC insertion increases procedure success rates and decreases mechanical complications.4,5 Use of real-time ultrasound guidance was recommended by the Agency for Healthcare Research and Quality, the Institute of Medicine, the National Institute for Health and Care Excellence, the Centers for Disease Control and Prevention, and several medical specialty societies in the early 2000s.6-14 Despite these recommendations, ultrasound guidance has not been universally adopted. Currently, an estimated 20%-55% of CVC insertions in the internal jugular vein are performed without ultrasound guidance.15-17
Following the emergence of literature supporting the use of ultrasound guidance for CVC insertion, observational and randomized controlled studies demonstrated improved procedural success rates with the use of ultrasound guidance for the insertion of peripheral intravenous lines (PIVs), arterial catheters, and peripherally inserted central catheters (PICCs).18-23
The purpose of this position statement is to present evidence-based recommendations on the use of ultrasound guidance for the insertion of central and peripheral vascular access catheters in adult patients. This document presents consensus-based recommendations with supporting evidence for clinical outcomes, techniques, and training for the use of ultrasound guidance for vascular access. We have subdivided the recommendations on techniques for central venous access, peripheral venous access, and arterial access individually, as some providers may not perform all types of vascular access procedures.
These recommendations are intended for hospitalists and other healthcare providers that routinely place central and peripheral vascular access catheters in acutely ill patients. However, this position statement does not mandate that all hospitalists should place central or peripheral vascular access catheters given the diverse array of hospitalist practice settings. For training and competency assessments, we recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals, where equipment and staffing for assessments are not available. Recommendations and frameworks for initial and ongoing credentialing of hospitalists in ultrasound-guided bedside procedures have been previously published in an Society of Hospital Medicine (SHM) position statement titled, “Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.”24
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interest (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the vascular access working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. An updated search was conducted in November 2017. The literature search strings are included in Appendix 3. All article abstracts were initially screened for relevance by at least two members of the vascular access working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide vascular access were selected. The following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled studies, and observational studies of ultrasound-guided vascular access were screened and selected (Appendix 3, Figure 1). All full-text articles were shared electronically among the working group members, and final article selection was based on working group consensus. Selected articles were incorporated into the draft recommendations.
These recommendations were developed using the Research and Development (RAND) Appropriateness Method that required panel judgment and consensus.14 The 28 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Using an internet-based electronic data collection tool (REDCap™), panel members participated in two rounds of electronic voting, one in August 2018 and the other in October 2018 (Appendix 4). Voting on appropriateness was conducted using a nine-point Likert scale. The three zones of the nine-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix 1, Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” Disagreement was defined as >30% of panelists voting outside of the zone of the median. A strong recommendation required at least 80% of the votes within one integer of the median per the RAND rules.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording for each recommendation (Table 2). The final version of the consensus-based recommendations underwent internal and external review by members of the SHM POCUS Task Force, the SHM Education Committee, and the SHM Executive Committee. The SHM Executive Committee reviewed and approved this position statement prior to its publication in the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 5,563 references were pooled from an initial search performed by a certified medical librarian in December 2015 (4,668 citations) which was updated in November 2017 (791 citations), and from the personal bibliographies and searches (104 citations) performed by working group members. A total of 514 full-text articles were reviewed. The final selection included 192 articles that were abstracted into a data table and incorporated into the draft recommendations. See Appendix 3 for details of the literature search strategy.
Recommendations
Four domains (technique, clinical outcomes, training, and knowledge gaps) with 31 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation cite supporting evidence. After two rounds of panel voting, 31 recommendations achieved agreement based on the RAND rules. During the peer review process, two of the recommendations were merged with other recommendations. Thus, a total of 29 recommendations received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Twenty-seven statements were approved as strong recommendations, and two were approved as weak/conditional recommendations. The strength of each recommendation and degree of consensus are summarized in Table 3.
Terminology
Central Venous Catheterization
Central venous catheterization refers to insertion of tunneled or nontunneled large bore vascular catheters that are most commonly inserted into the internal jugular, subclavian, or femoral veins with the catheter tip located in a central vein. These vascular access catheters are synonymously referred to as central lines or central venous catheters (CVCs). Nontunneled catheters are designed for short-term use and should be removed promptly when no longer clinically indicated or after a maximum of 14 days.25
Peripherally Inserted Central Catheter (PICC)
Peripherally inserted central catheters, or PICC lines, are inserted most commonly in the basilic or brachial veins in adult patients, and the catheter tip terminates in the distal superior vena cava or cavo-atrial junction. These catheters are designed to remain in place for a duration of several weeks, as long as it is clinically indicated.
Midline Catheterization
Midline catheters are a type of peripheral venous catheter that are an intermediary between a peripheral intravenous catheter and PICC line. Midline catheters are most commonly inserted in the brachial or basilic veins, but unlike PICC lines, the tips of these catheters terminate in the axillary or subclavian vein. Midline catheters are typically 8 cm to 20 cm in length and inserted for a duration <30 days.
Peripheral Intravenous Catheterization
Peripheral intravenous lines (PIV) refer to small bore venous catheters that are most commonly 14G to 24G and inserted into patients for short-term peripheral venous access. Common sites of ultrasound-guided PIV insertion include the superficial and deep veins of the hand, forearm, and arm.
Arterial Catheterization
Arterial catheters are commonly used for reliable blood pressure monitoring, frequent arterial blood
RECOMMENDATIONS
Preprocedure
1. We recommend that providers should be familiar with the operation of their specific ultrasound machine prior to initiation of a vascular access procedure.
Rationale: There is strong consensus that providers must be familiar with the knobs and functions of the specific make and model of ultrasound machine that will be utilized for a vascular access procedure. Minimizing adjustments to the ultrasound machine during the procedure may reduce the risk of contaminating the sterile field.
2. We recommend that providers should use a high-frequency linear transducer with a sterile sheath and sterile gel to perform vascular access procedures.
Rationale: High-frequency linear-array transducers are recommended for the vast majority of vascular access procedures due to their superior resolution compared to other transducer types. Both central and peripheral vascular access procedures, including PIV, PICC, and arterial line placement, should be performed using sterile technique. A sterile transducer cover and sterile gel must be utilized, and providers must be trained in sterile preparation of the ultrasound transducer.13,26,27
The depth of femoral vessels correlates with body mass index (BMI). When accessing these vessels in a morbidly obese patient with a thigh circumference >60 cm and vessel depth >8 cm, a curvilinear transducer may be preferred for its deeper penetration.28 For patients who are poor candidates for bedside insertion of vascular access catheters, such as uncooperative patients, patients with atypical vascular anatomy or poorly visualized target vessels, we recommend consultation with a vascular access specialist prior to attempting the procedure.
3. We recommend that providers should use two-dimensional ultrasound to evaluate for anatomical variations and absence of vascular thrombosis during preprocedural site selection.
Rationale: A thorough ultrasound examination of the target vessel is warranted prior to catheter placement. Anatomical variations that may affect procedural decision-making are easily detected with ultrasound. A focused vascular ultrasound examination is particularly important in patients who have had temporary or tunneled venous catheters, which can cause stenosis or thrombosis of the target vein.
For internal jugular vein (IJV) CVCs, ultrasound is useful for visualizing the relationship between the IJV and common carotid artery (CCA), particularly in terms of vessel overlap. Furthermore, ultrasound allows for immediate revisualization upon changes in head position.29-32 Troianos et al. found >75% overlap of the IJV and CCA in 54% of all patients and in 64% of older patients (age >60 years) whose heads were rotated to the contralateral side.30 In one study of IJV CVC insertion, inadvertent carotid artery punctures were reduced (3% vs 10%) with the use of ultrasound guidance vs landmarks alone.33 In a cohort of 64 high-risk neurosurgical patients, cannulation success was 100% with the use of ultrasound guidance, and there were no injuries to the carotid artery, even though the procedure was performed with a 30-degree head elevation and anomalous IJV anatomy in 39% of patients.34 In a prospective, randomized controlled study of 1,332 patients, ultrasound-guided cannulation in a neutral position was demonstrated to be as safe as the 45-degree rotated position.35
Ultrasound allows for the recognition of anatomical variations which may influence the selection of the vascular access site or technique. Benter et al. found that 36% of patients showed anatomical variations in the IJV and surrounding tissue.36 Similarly Caridi showed the anatomy of the right IJV to be atypical in 29% of patients,37 and Brusasco found that 37% of bariatric patients had anatomical variations of the IJV.38 In a study of 58 patients, there was significant variability in the IJV position and IJV diameter, ranging from 0.5 cm to >2 cm.39 In a study of hemodialysis patients, 75% of patients had sonographic venous abnormalities that led to a change in venous access approach.40
To detect acute or chronic upper extremity deep venous thrombosis or stenosis, two-dimensional visualization with compression should be part of the ultrasound examination prior to central venous catheterization. In a study of patients that had undergone CVC insertion 9-19 weeks earlier, 50% of patients had an IJV thrombosis or stenosis leading to selection of an alternative site. In this study, use of ultrasound for a preprocedural site evaluation reduced unnecessary attempts at catheterizing an occluded vein.41 At least two other studies demonstrated an appreciable likelihood of thrombosis. In a study of bariatric patients, 8% of patients had asymptomatic thrombosis38 and in another study, 9% of patients being evaluated for hemodialysis catheter placement had asymptomatic IJV thrombosis.37
4. We recommend that providers should evaluate the target blood vessel size and depth during a preprocedural ultrasound evaluation.
Rationale: The size, depth, and anatomic location of central veins can vary considerably. These features are easily discernable using ultrasound. Contrary to traditional teaching, the IJV is located 1 cm anterolateral to the CCA in only about two-thirds of patients.37,39,42,43 Furthermore, the diameter of the IJV can vary significantly, ranging from 0.5 cm to >2 cm.39 The laterality of blood vessels may vary considerably as well. A preprocedural ultrasound evaluation of contralateral subclavian and axillary veins showed a significant absolute difference in cross-sectional area of 26.7 mm2 (P < .001).42
Blood vessels can also shift considerably when a patient is in the Trendelenburg position. In one study, the IJV diameter changed from 11.2 (± 1.5) mm to 15.4 (± 1.5) mm in the supine versus the Trendelenburg position at 15 degrees.33 An observational study demonstrated a frog-legged position with reverse Trendelenburg increased the femoral vein size and reduced the common surface area with the common femoral artery compared to a neutral position. Thus, a frog-legged position with reverse Trendelenburg position may be preferred, since overall catheterization success rates are higher in this position.44
Techniques
General Techniques
5. We recommend that providers should avoid using static ultrasound alone to mark the needle insertion site for vascular access procedures.
Rationale: The use of static ultrasound guidance to mark a needle insertion site is not recommended because normal anatomical relationships of vessels vary, and site marking can be inaccurate with minimal changes in patient position, especially of the neck.43,45,46 Benefits of using ultrasound guidance for vascular access are attained when ultrasound is used to track the needle tip in real-time as it is advanced toward the target vessel.
Although continuous-wave Doppler ultrasound without two-dimensional visualization was used in the past, it is no longer recommended for IJV CVC insertion.47 In a study that randomized patients to IJV CVC insertion with continuous-wave Doppler alone vs two-dimensional ultrasound guidance, the use of two-dimensional ultrasound guidance showed significant improvement in first-pass success rates (97% vs 91%, P = .045), particularly in patients with BMI >30 (97% vs 77%, P = .011).48
A randomized study comparing real-time ultrasound-guided, landmark-based, and ultrasound-marked techniques found higher success rates in the real-time ultrasound-guided group than the other two groups (100% vs 74% vs 73%, respectively; P = .01). The total number of mechanical complications was higher in the landmark-based and ultrasound-marked groups than in the real-time ultrasound-guided group (24% and 36% versus 0%, respectively; P = .01).49 Another randomized controlled study found higher success rates with real-time ultrasound guidance (98%) versus an ultrasound-marked (82%) or landmark-based (64%) approach for central line placement.50
6. We recommend that providers should use real-time (dynamic), two-dimensional ultrasound guidance with a high-frequency linear transducer for CVC insertion, regardless of the provider’s level of experience.
7. We suggest using either a transverse (short-axis) or longitudinal (long-axis) approach when performing real-time ultrasound-guided vascular access procedures.
Rationale: In clinical practice, the phrases transverse, short-axis, or out-of-plane approach are synonymous, as are longitudinal, long-axis, and in-plane approach. The short-axis approach involves tracking the needle tip as it approximates the target vessel with the ultrasound beam oriented in a transverse plane perpendicular to the target vessel. The target vessel is seen as a circular structure on the ultrasound screen as the needle tip approaches the target vessel from above. This approach is also called the out-of-plane technique since the needle passes through the ultrasound plane. The advantages of the short-axis approach include better visualization of adjacent vessels or nerves and the relative ease of skill acquisition for novice operators.9 When using the short-axis approach, extra care must be taken to track the needle tip from the point of insertion on the skin to the target vessel. A disadvantage of the short-axis approach is unintended posterior wall puncture of the target vessel.55
In contrast to a short-axis approach, a long-axis approach is performed with the ultrasound beam aligned parallel to the vessel. The vessel appears as a long tubular structure and the entire needle is visualized as it traverses across the ultrasound screen to approach the target vessel. The long-axis approach is also called an in-plane technique because the needle is maintained within the plane of the ultrasound beam. The advantage of a long-axis approach is the ability to visualize the entire needle as it is inserted into the vessel.14 A randomized crossover study with simulation models compared a long-axis versus short-axis approach for both IJV and subclavian vein catheterization. This study showed decreased number of needle redirections (relative risk (RR) 0.5, 95% confidence interval (CI) 0.3 to 0.7), and posterior wall penetrations (OR 0.3, 95% CI 0.1 to 0.9) using a long-axis versus short-axis approach for subclavian vein catheterization.56
A randomized controlled study comparing a long-axis or short-axis approach with ultrasound versus a landmark-based approach for IJV CVC insertion showed higher success rates (100% vs 90%; P < .001), lower insertion time (53 vs 116 seconds; P < .001), and fewer attempts to obtain access (2.5 vs 1.2 attempts, P < .001) with either the long- or short-axis ultrasound approach. The average time to obtain access and number of attempts were comparable between the short-axis and long-axis approaches with ultrasound. The incidence of carotid puncture and hematoma was significantly higher with the landmark-based approach versus either the long- or short-axis ultrasound approach (carotid puncture 17% vs 3%, P = .024; hematoma 23% vs 3%, P = .003).57
High success rates have been reported using a short-axis approach for insertion of PIV lines.58 A prospective, randomized trial compared the short-axis and long-axis approach in patients who had had ≥2 failed PIV insertion attempts. Success rate was 95% (95% CI, 0.85 to 1.00) in the short-axis group compared with 85% (95% CI, 0.69 to 1.00) in the long-axis group. All three subjects with failed PIV placement in the long-axis group had successful rescue placement using a short-axis approach. Furthermore, the short-axis approach was faster than the long-axis approach.59
For radial artery cannulation, limited data exist comparing the short- and long-axis approaches. A randomized controlled study compared a long-axis vs short-axis ultrasound approach for radial artery cannulation. Although the overall procedure success rate was 100% in both groups, the long-axis approach had higher first-pass success rates (1.27 ± 0.4 vs 1.5 ± 0.5, P < .05), shorter cannulation times (24 ± 17 vs 47 ± 34 seconds, P < .05), fewer hematomas (4% vs 43%, P < .05) and fewer posterior wall penetrations (20% vs 56%, P < .05).60
Another technique that has been described for IJV CVC insertion is an oblique-axis approach, a hybrid between the long- and short-axis approaches. In this approach, the transducer is aligned obliquely over the IJV and the needle is inserted using a long-axis or in-plane approach. A prospective randomized trial compared the short-axis, long-axis, and oblique-axis approaches during IJV cannulation. First-pass success rates were 70%, 52%, and 74% with the short-axis, long-axis, and oblique-axis approaches, respectively, and a statistically significant difference was found between the long- and oblique-axis approaches (P = .002). A higher rate of posterior wall puncture was observed with a short-axis approach (15%) compared with the oblique-axis (7%) and long-axis (4%) approaches (P = .047).61
8. We recommend that providers should visualize the needle tip and guidewire in the target vein prior to vessel dilatation.
Rationale: When real-time ultrasound guidance is used, visualization of the needle tip within the vein is the first step to confirm cannulation of the vein and not the artery. After the guidewire is advanced, the provider can use transverse and longitudinal views to reconfirm cannulation of the vein. In a longitudinal view, the guidewire is readily seen positioned within the vein, entering the anterior wall and lying along the posterior wall of the vein. Unintentional perforation of the posterior wall of the vein with entry into the underlying artery can be detected by ultrasound, allowing prompt removal of the needle and guidewire before proceeding with dilation of the vessel. In a prospective observational study that reviewed ultrasound-guided IJV CVC insertions, physicians were able to more readily visualize the guidewire than the needle in the vein.62 A prospective observational study determined that novice operators can visualize intravascular guidewires in simulation models with an overall accuracy of 97%.63
In a retrospective review of CVC insertions where the guidewire position was routinely confirmed in the target vessel prior to dilation, there were no cases of arterial dilation, suggesting confirmation of guidewire position can potentially eliminate the morbidity and mortality associated with arterial dilation during CVC insertion.64
9. To increase the success rate of ultrasound-guided vascular access procedures, we recommend that providers should utilize echogenic needles, plastic needle guides, and/or ultrasound beam steering when available.
Rationale: Echogenic needles have ridged tips that appear brighter on the screen, allowing for better visualization of the needle tip. Plastic needle guides help stabilize the needle alongside the transducer when using either a transverse or longitudinal approach. Although evidence is limited, some studies have reported higher procedural success rates when using echogenic needles, plastic needle guides, and ultrasound beam steering software. In a prospective observational study, Augustides et al. showed significantly higher IJV cannulation rates with versus without use of a needle guide after first (81% vs 69%, P = .0054) and second (93% vs 80%. P = .0001) needle passes.65 A randomized study by Maecken et al. compared subclavian vein CVC insertion with or without use of a needle guide, and found higher procedure success rates within the first and second attempts, reduced time to obtain access (16 seconds vs 30 seconds; P = .0001) and increased needle visibility (86% vs 32%; P < .0001) with the use of a needle guide.66 Another study comparing a short-axis versus long-axis approach with a needle guide showed improved needle visualization using a long-axis approach with a needle guide.67 A randomized study comparing use of a novel, sled-mounted needle guide to a free-hand approach for venous cannulation in simulation models showed the novel, sled-mounted needle guide improved overall success rates and efficiency of cannulation.68
Central Venous Access Techniques
10. We recommend that providers should use a standardized procedure checklist that includes use of real-time ultrasound guidance to reduce the risk of central line-associated bloodstream infection (CLABSI) from CVC insertion.
Rationale: A standardized checklist or protocol should be developed to ensure compliance with all recommendations for insertion of CVCs. Evidence-based protocols address periprocedural issues, such as indications for CVC, and procedural techniques, such as use of maximal sterile barrier precautions to reduce the risk of infection. Protocols and checklists that follow established guidelines for CVC insertion have been shown to decrease CLABSI rates.69,70 Similarly, development of checklists and protocols for maintenance of central venous catheters have been effective in reducing CLABSIs.71 Although no externally-validated checklist has been universally accepted or endorsed by national safety organizations, a few internally-validated checklists are available through peer-reviewed publications.72,73 An observational educational cohort of internal medicine residents who received training using simulation of the entire CVC insertion process was able to demonstrate fewer CLABSIs after the simulator-trained residents rotated in the intensive care unit (ICU) (0.50 vs 3.2 infections per 1,000 catheter days, P = .001).74
11. We recommend that providers should use real-time ultrasound guidance, combined with aseptic technique and maximal sterile barrier precautions, to reduce the incidence of infectious complications from CVC insertion.
Rationale: The use of real-time ultrasound guidance for CVC placement has demonstrated a statistically significant reduction in CLABSIs compared to landmark-based techniques.75 The Centers for Disease Control and Prevention (CDC) guidelines for the prevention of intravascular catheter-related infections recommend the use of ultrasound guidance to reduce the number of cannulation attempts and risk of mechanical complications.69 A prospective, three-arm study comparing ultrasound-guided long-axis, short-axis, and landmark-based approaches showed a CLABSI rate of 20% in the landmark-based group versus 10% in each of the ultrasound groups.57 Another randomized study comparing use of ultrasound guidance to a landmark-based technique for IJV CVC insertion demonstrated significantly lower CLABSI rates with the use of ultrasound (2% vs 10%; P < .05).72
Studies have shown that a systems-based intervention featuring a standardized catheter kit or catheter bundle significantly reduced CLABSI rates.76-78 A complete review of all preventive measures to reduce the risk of CLABSI is beyond the scope of this review, but a few key points will be mentioned. First, aseptic technique includes proper hand hygiene and skin sterilization, which are essential measures to reduce cutaneous colonization of the insertion site and reduce the risk of CLABSIs.79 In a systematic review and meta-analysis of eight studies including over 4,000 catheter insertions, skin antisepsis with chlorhexidine was associated with a 50% reduction in CLABSIs compared with povidone iodine.11 Therefore, a chlorhexidine-containing solution is recommended for skin preparation prior to CVC insertion per guidelines by Healthcare Infection Control Practices Advisory Committee/CDC, Society for Healthcare Epidemiology of America/Infectious Diseases Society of America, and American Society of Anesthesiologists.11,69,80,81 Second, maximal sterile barrier precautions refer to the use of sterile gowns, sterile gloves, caps, masks covering both the mouth and nose, and sterile full-body patient drapes. Use of maximal sterile barrier precautions during CVC insertion has been shown to reduce the incidence of CLABSIs compared to standard precautions.26,79,82-84 Third, catheters containing antimicrobial agents may be considered for hospital units with higher CLABSI rates than institutional goals, despite a comprehensive preventive strategy, and may be considered in specific patient populations at high risk of severe complications from a CLABSI.11,69,80 Finally, providers should use a standardized procedure set-up when inserting CVCs to reduce the risk of CLABSIs. The operator should confirm availability and proper functioning of ultrasound equipment prior to commencing a vascular access procedure. Use of all-inclusive procedure carts or kits with sterile ultrasound probe covers, sterile gel, catheter kits, and other necessary supplies is recommended to minimize interruptions during the procedure, and can ultimately reduce the risk of CLABSIs by ensuring maintenance of a sterile field during the procedure.13
12. We recommend that providers should use real-time ultrasound guidance for internal jugular vein catheterization, which reduces the risk of mechanical and infectious complications, the number of needle passes, and time to cannulation and increases overall procedure success rates.
Rationale: The use of real-time ultrasound guidance for CVC insertion has repeatedly demonstrated better outcomes compared to a landmark-based approach in adults.13 Several randomized controlled studies have demonstrated that real-time ultrasound guidance for IJV cannulation reduces the risk of procedure-related mechanical and infectious complications, and improves first-pass and overall success rates in diverse care settings.27,29,45,50,53,65,75,85-90 Mechanical complications that are reduced with ultrasound guidance include pneumothorax and carotid artery puncture.4,5,45,46,53,62,75,86-93 Currently, several medical societies strongly recommend the use of ultrasound guidance during insertion of IJV CVCs.10-12,14,94-96
A meta-analysis by Hind et al. that included 18 randomized controlled studies demonstrated use of real-time ultrasound guidance reduced failure rates (RR 0.14, 95% CI 0.06 to 0.33; P < .0001), increased first-attempt success rates (RR 0.59, 95% CI 0.39 to 0.88; P = .009), reduced complication rates (RR 0.43, 95% CI 0.22 to 0.87; P = .02) and reduced procedure time (P < .0001), compared to a traditional landmark-based approach when inserting IJV CVCs.5
A Cochrane systematic review compared ultrasound-guided versus landmark-based approaches for IJV CVC insertion and found use of real-time ultrasound guidance reduced total complication rates by 71% (RR 0.29, 95% CI 0.17 to 0.52; P < .0001), arterial puncture rates by 72% (RR 0.28, 95% CI 0.18 to 0.44; P < .00001), and rates of hematoma formation by 73% (RR 0.27, 95% CI 0.13 to 0.55; P = .0004). Furthermore, the number of attempts for successful cannulation was reduced (mean difference -1.19 attempts, 95% CI -1.45 to -0.92; P < .00001), the chance of successful insertion on the first attempt was increased by 57% (RR 1.57, 95% CI 1.36 to 1.82; P < .00001), and overall procedure success rates were modestly increased in all groups by 12% (RR 1.12, 95% CI 1.08 to 1.17; P < .00001).46
An important consideration in performing ultrasound guidance is provider experience. A prospective observational study of patients undergoing elective CVC insertion demonstrated higher complication rates for operators that were inexperienced (25.2%) versus experienced (13.6%).54 A randomized controlled study comparing experts and novices with or without the use of ultrasound guidance for IJV CVC insertion demonstrated higher success rates among expert operators and with the use of ultrasound guidance. Among novice operators, the complication rates were lower with the use of ultrasound guidance.97 One study evaluated the procedural success and complication rates of a two-physician technique with one physician manipulating the transducer and another inserting the needle for IJV CVC insertion. This study concluded that procedural success rates and frequency of complications were directly affected by the experience of the physician manipulating the transducer and not by the experience of the physician inserting the needle.98
The impact of ultrasound guidance on improving procedural success rates and reducing complication rates is greatest in patients that are obese, short necked, hypovolemic, or uncooperative.93 Several studies have demonstrated fewer needle passes and decreased time to cannulation compared to the landmark technique in these populations.46,49,53,86-88,92,93
Ultrasound-guided placement of IJV catheters can safely be performed in patients with disorders of hemostasis and those with multiple previous catheter insertions in the same vein.9 Ultrasound-guided placement of CVCs in patients with disorders of hemostasis is safe with high success and low complication rates. In a case series of liver patients with coagulopathy (mean INR 2.17 ± 1.16, median platelet count 150K), the use of ultrasound guidance for CVC insertion was highly successful with no major bleeding complications.99
A study of renal failure patients found high success rates and low complication rates in the patients with a history of multiple previous catheterizations, poor compliance, skeletal deformities, previous failed cannulations, morbid obesity, and disorders of hemostasis.100 A prospective observational study of 200 ultrasound-guided CVC insertions for apheresis showed a 100% success rate with a 92% first-pass success rate.101
The use of real-time ultrasound guidance for IJV CVC insertion has been shown to be cost effective by reducing procedure-related mechanical complications and improving procedural success rates. A companion cost-effectiveness analysis estimated that for every 1,000 patients, 90 complications would be avoided, with a net cost savings of approximately $3,200 using 2002 prices.102
13. We recommend that providers who routinely insert subclavian vein CVCs should use real-time ultrasound guidance, which has been shown to reduce the risk of mechanical complications and number of needle passes and increase overall procedure success rates compared with landmark-based techniques.
Rationale: In clinical practice, the term ultrasound-guided subclavian vein CVC insertion is commonly used. However, the needle insertion site is often lateral to the first rib and providers are technically inserting the CVC in the axillary vein. The subclavian vein becomes the axillary vein at the lateral border of the first rib where the cephalic vein branches from the subclavian vein. To be consistent with common medical parlance, we use the phrase ultrasound-guided subclavian vein CVC insertion in this document.
Advantages of inserting CVCs in the subclavian vein include reliable surface anatomical landmarks for vein location, patient comfort, and lower risk of infection.103 Several observational studies have demonstrated the technique for ultrasound-guided subclavian vein CVC insertion is feasible and safe.104-107 In a large retrospective observational study of ultrasound-guided central venous access among a complex patient group, the majority of patients were cannulated successfully and safely. The subset of patients undergoing axillary vein CVC insertion (n = 1,923) demonstrated a low rate of complications (0.7%), proving it is a safe and effective alternative to the IJV CVC insertion.107
A Cochrane review of ultrasound-guided subclavian vein cannulation (nine studies, 2,030 participants, 2,049 procedures), demonstrated that real-time two-dimensional ultrasound guidance reduced the risk of inadvertent arterial punctures (three studies, 498 participants, RR 0.21, 95% CI 0.06 to 0.82; P = .02) and hematoma formation (three studies, 498 participants, RR 0.26, 95% CI 0.09 to 0.76; P = .01).46 A systematic review and meta-analysis of 10 randomized controlled studies comparing ultrasound-guided versus landmark-based subclavian vein CVC insertion demonstrated a reduction in the risk of arterial punctures, hematoma formation, pneumothorax, and failed catheterization with the use of ultrasound guidance.105
A randomized controlled study comparing ultrasound-guided vs landmark-based approaches to subclavian vein cannulation found that use of ultrasound guidance had a higher success rate (92% vs 44%, P = .0003), fewer minor complications (1 vs 11, P = .002), fewer attempts (1.4 vs 2.5, P = .007) and fewer catheter kits used (1.0 vs 1.4, P = .0003) per cannulation.108
Fragou et al. randomized patients undergoing subclavian vein CVC insertion to a long-axis approach versus a landmark-based approach and found a significantly higher success rate (100% vs 87.5%, P < .05) and lower rates of mechanical complications: artery puncture (0.5% vs 5.4%), hematoma (1.5% vs 5.4%), hemothorax (0% vs 4.4%), pneumothorax (0% vs 4.9%), brachial plexus injury (0% vs 2.9%), phrenic nerve injury (0% vs 1.5%), and cardiac tamponade (0% vs 0.5%).109 The average time to obtain access and the average number of insertion attempts (1.1 ± 0.3 vs 1.9 ± 0.7, P < .05) were significantly reduced in the ultrasound group compared to the landmark-based group.95
A retrospective review of subclavian vein CVC insertions using a supraclavicular approach found no reported complications with the use of ultrasound guidance vs 23 mechanical complications (8 pneumothorax, 15 arterial punctures) with a landmark-based approach.106 However, it is important to note that a supraclavicular approach is not commonly used in clinical practice.
14. We recommend that providers should use real-time ultrasound guidance for femoral venous access, which has been shown to reduce the risk of arterial punctures and total procedure time and increase overall procedure success rates.
Rationale: Anatomy of the femoral region varies, and close proximity or overlap of the femoral vein and artery is common.51 Early studies showed that ultrasound guidance for femoral vein CVC insertion reduced arterial punctures compared with a landmark-based approach (7% vs 16%), reduced total procedure time (55 ± 19 vs 79 ± 62 seconds), and increased procedure success rates (100% vs 90%).52 A Cochrane review that pooled data from four randomized studies comparing ultrasound-guided vs landmark-based femoral vein CVC insertion found higher first-attempt success rates with the use of ultrasound guidance (RR 1.73, 95% CI 1.34 to 2.22; P < .0001) and a small increase in the overall procedure success rates (RR 1.11, 95% CI 1.00 to 1.23; P = .06). There was no difference in inadvertent arterial punctures or other complications.110
Peripheral Venous Access Techniques
15. We recommend that providers should use real-time ultrasound guidance for the insertion of peripherally inserted central catheters (PICCs), which is associated with higher procedure success rates and may be more cost effective compared with landmark-based techniques.
Rationale: Several studies have demonstrated that providers who use ultrasound guidance vs landmarks for PICC insertion have higher procedural success rates, lower complication rates, and lower total placement costs. A prospective observational report of 350 PICC insertions using ultrasound guidance reported a 99% success rate with an average of 1.2 punctures per insertion and lower total costs.20 A retrospective observational study of 500 PICC insertions by designated specialty nurses revealed an overall success rate of 95%, no evidence of phlebitis, and only one CLABSI among the catheters removed.21 A retrospective observational study comparing several PICC variables found higher success rates (99% vs 77%) and lower thrombosis rates (2% vs 9%) using ultrasound guidance vs landmarks alone.22 A study by Robinson et al. demonstrated that having a dedicated PICC team equipped with ultrasound increased their institutional insertion success rates from 73% to 94%.111
A randomized controlled study comparing ultrasound-guided versus landmark-based PICC insertion found high success rates with both techniques (100% vs 96%). However, there was a reduction in the rate of unplanned catheter removals (4.0% vs 18.7%; P = .02), mechanical phlebitis (0% vs 22.9%; P < .001), and venous thrombosis (0% vs 8.3%; P = .037), but a higher rate of catheter migration (32% vs 2.1%; P < .001). Compared with the landmark-based group, the ultrasound-guided group had significantly lower incidence of severe contact dermatitis (P = .038), and improved comfort and costs up to 3 months after PICC placement (P < .05).112
Routine postprocedure chest x-ray (CXR) is generally considered unnecessary if the PICC is inserted with real-time ultrasound guidance along with use of a newer tracking devices, like the magnetic navigation system with intracardiac electrodes.9 Ultrasound can also be used to detect malpositioning of a PICC immediately after completing the procedure. A randomized controlled study comparing ultrasound versus postprocedure CXR detected one malpositioned PICC in the ultrasound group versus 11 in the control group. This study suggested that ultrasound can detect malpositioning immediately postprocedure and reduce the need for a CXR and the possibility of an additional procedure to reposition a catheter.113
16. We recommend that providers should use real-time ultrasound guidance for the placement of peripheral intravenous lines (PIV) in patients with difficult peripheral venous access to reduce the total procedure time, needle insertion attempts, and needle redirections. Ultrasound-guided PIV insertion is also an effective alternative to CVC insertion in patients with difficult venous access.
Rationale: Difficult venous access refers to patients that have had two unsuccessful attempts at PIV insertion using landmarks or a history of difficult access (i.e. edema, obesity, intravenous drug use, chemotherapy, diabetes, hypovolemia, chronic illness, vasculopathy, multiple prior hospitalizations). A meta-analysis of seven randomized controlled studies concluded that ultrasound guidance increases the likelihood of successful PIV insertion (pooled OR 2.42, 95% CI 1.26 to 4.68; P < .008).18 A second meta-analysis that pooled data from seven studies (six randomized controlled studies) confirmed that ultrasound guidance improves success rates of PIV insertion (OR 3.96, 95% CI 1.75 to 8.94).19 Approximately half of these studies had physician operators while the other half had nurse operators.
In one prospective observational study of emergency department patients with two failed attempts of landmark-based PIV insertion, ultrasound guidance with a modified-Seldinger technique showed a relatively high success rate (96%), fewer needle sticks (mean 1.32 sticks, 95% CI 1.12 to 1.52), and shorter time to obtain access (median time 68 seconds).114 Other prospective observational studies have demonstrated that ultrasound guidance for PIV insertion has a high success rate (87%),115 particularly with brachial or basilic veins PIV insertion, among patients with difficult PIV access, defined as having had ≥2 failed attempts.58
Since insertion of PIVs with ultrasound guidance has a high success rate, there is potential to reduce the reliance on CVC insertion for venous access only. In a study of patients that had had two failed attempts at PIV insertion based on landmarks, a PIV was successfully inserted with ultrasound guidance in 84% of patients, obviating the need for CVC placement for venous access.116 A prospective observational study showed ultrasound-guided PIV insertion was an effective alternative to CVC placement in ED patients with difficult venous access with only 1% of patients requiring a CVC.117 Use of ultrasound by nurses for PIV placement has also been shown to reduce the time to obtain venous access, improve patient satisfaction, and reduce the need for physician intervention.118 In a prospective observational study of patients with difficult access, the majority of patients reported a better experience with ultrasound-guided PIV insertion compared to previous landmark-based attempts with an average satisfaction score of 9.2/10 with 76% of patients rating the experience a 10.119 A strong recommendation has been made for use of ultrasound guidance in patients with difficult PIV placement by la Société Française d’Anesthésie et de Réanimation (The French Society of Anesthesia and Resuscitation).95
17. We suggest using real-time ultrasound guidance to reduce the risk of vascular, infectious, and neurological complications during PIV insertion, particularly in patients with difficult venous access.
Rationale: The incidence of complications from PIV insertion is often underestimated. Vascular complications include arterial puncture, hematoma formation, local infiltration or extravasation of fluid, and superficial or deep venous thrombosis. The most common infectious complications with PIV insertion are phlebitis and cellulitis.120 One observational study reported PIV complications occurring in approximately half of all patients with the most common complications being phlebitis, hematoma formation, and fluid/blood leakage.121
A retrospective review of ICU patients who underwent ultrasound-guided PIV insertion by a single physician showed high success rates (99%) with low rates of phlebitis/cellulitis (0.7%).There was an assumed benefit of risk reduction due to the patients no longer requiring a CVC after successful PIV placement.122 Another study found very low rates of infection with both landmark-based and ultrasound-guided PIV placement performed by emergency department nurses, suggesting that there is no increased risk of infection with the use of ultrasound.123 To reduce the risk of infection from PIV insertion, we recommend the use of sterile gel and sterile transducer cover (See Recommendation 2).
Arterial Access Techniques
18. We recommend that providers should use real-time ultrasound guidance for arterial access, which has been shown to increase first-pass success rates, reduce the time to cannulation, and reduce the risk of hematoma development compared with landmark-based techniques.
Rationale: Several randomized controlled studies have assessed the value of ultrasound in arterial catheter insertion. Shiver et al. randomized 60 patients admitted to a tertiary center emergency department to either palpation or ultrasound-guided arterial cannulation. They demonstrated a first-pass success rate of 87% in the ultrasound group compared with 50% in the landmark technique group. In the same study, the use of ultrasound was also associated with reduced time needed to establish arterial access and a 43% reduction in the development of hematoma at the insertion site.124 Levin et al. demonstrated a first-pass success rate of 62% using ultrasound versus 34% by palpation alone in 69 patients requiring intraoperative invasive hemodynamic monitoring.125 Additional randomized controlled studies have demonstrated that ultrasound guidance increases first-attempt success rates compared to traditional palpation.23,126,127
19. We recommend that providers should use real-time ultrasound guidance for femoral arterial access, which has been shown to increase first-pass success rates and reduce the risk of vascular complications.
Rationale: Although it is a less frequently used site, the femoral artery may be accessed for arterial blood sampling or invasive hemodynamic monitoring, and use of ultrasound guidance has been shown to improve the first-pass success rates of femoral artery cannulation. It is important to note that most of these studies comparing ultrasound-guided vs landmark-based femoral artery cannulation were performed in patients undergoing diagnostic or interventional vascular procedures.
A meta-analysis of randomized controlled studies comparing ultrasound-guided vs landmark-based femoral artery catheterization found use of ultrasound guidance was associated with a 49% reduction in overall complications (RR 0.51, 95% CI 0.28 to 0.91; P > .05) and 42% improvement in first-pass success rates.128 In another study, precise site selection with ultrasound was associated with fewer pseudoaneurysms in patients undergoing femoral artery cannulation by ultrasound guidance vs palpation for cardiac catheterization (3% vs 5%, P < .05).129
A multicenter randomized controlled study comparing ultrasound vs fluoroscopic guidance for femoral artery catheterization demonstrated ultrasound guidance improved rates of common femoral artery (CFA) cannulation in patients with high CFA bifurcations (83% vs 70%, P < .01).130 Furthermore, ultrasound guidance improved first-pass success rates (83% vs 46%, P < .0001), reduced number of attempts (1.3 vs 3.0, P < .0001), reduced risk of venipuncture (2.4% vs 15.8%, P < .0001), and reduced median time to obtain access (136 seconds vs148 seconds, P = .003). Vascular complications occurred in fewer patients in the ultrasound vs fluoroscopy groups (1.4% vs 3.4% P = .04). Reduced risk of hematoma formation with routine use of ultrasound guidance was demonstrated in one retrospective observational study (RR 0.62, 95% CI 0.46 to 0.84; P < .01).131
20. We recommend that providers should use real-time ultrasound guidance for radial arterial access, which has been shown to increase first-pass success rates, reduce the time to successful cannulation, and reduce the risk of complications compared with landmark-based techniques.
Rationale: Ultrasound guidance is particularly useful for radial artery cannulation in patients with altered anatomy, obesity, nonpulsatile blood flow, low perfusion, and previously unsuccessful cannulation attempts using a landmark-guided approach.132
A multicenter randomized controlled study that was not included in the abovementioned meta-analyses showed similar benefits of using ultrasound guidance vs landmarks for radial artery catheterization: a reduction in the number of attempts with ultrasound guidance (1.65 ± 1.2 vs 3.05 ± 3.4, P < .0001) and time to obtain access (88 ± 78 vs 108 ± 112 seconds, P = .006), and increased first-pass success rates (65% vs 44%, P < .0001). The use of ultrasound guidance was found to be particularly useful in patients with difficult access by palpation alone.135
Regarding the level of expertise required to use ultrasound guidance, a prospective observational study demonstrated that physicians with little previous ultrasound experience were able to improve their first-attempt success rates and procedure time for radial artery cannulation compared to historical data of landmark-based insertions.136
Postprocedure
21. We recommend that post-procedure pneumothorax should be ruled out by the detection of bilateral lung sliding using a high-frequency linear transducer before and after insertion of internal jugular and subclavian vein CVCs.
Rationale: Detection of lung sliding with two-dimensional ultrasound rules out pneumothorax, and disappearance of lung sliding in an area where it was previously seen is a strong predictor of postprocedure pneumothorax. In a study of critically ill patients, the disappearance of lung sliding was observed in 100% of patients with pneumothorax vs 8.8% of patients without pneumothorax. For detection of pneumothorax, lung sliding showed a sensitivity of 95%, specificity of 91%, and negative predictive value of 100% (P < .001).137 Another study by the same author showed that the combination of horizontal artifacts (absence of comet-tail artifact) and absence of lung sliding had a sensitivity of 100%, specificity of 96.5%, and negative predictive value of 100% for the detection of pneumothorax.138 A meta-analysis of 10 studies on the diagnostic accuracy of CVC confirmation with bedside ultrasound vs chest radiography reported detection of all 12 pneumothoraces with ultrasound, whereas chest radiography missed two pneumothoraces. The pooled sensitivity and specificity of ultrasound for the detection of pneumothorax was 100%, although an imperfect gold standard bias likely affected the results. An important advantage of bedside ultrasound is the ability to rule out pneumothorax immediately after the procedure while at the bedside. The mean time for confirmation of CVC placement with bedside ultrasound was 6 minutes versus 64 minutes and 143 minutes for completion and interpretation of a chest radiograph, respectively.139
22. We recommend that providers should use ultrasound with rapid infusion of agitated saline to visualize a right atrial swirl sign (RASS) for detecting catheter tip misplacement during CVC insertion. The use of RASS to detect the catheter tip may be considered an advanced skill that requires specific training and expertise.
Rationale: Bedside echocardiography is a reliable tool to detect catheter tip misplacement during CVC insertion. In one study, catheter misplacement was detected by bedside echocardiography with a sensitivity of 96% and specificity of 83% (positive predictive value 98%, negative predictive value 55%) and prevented distal positioning of the catheter tip.140 A prospective observational study assessed for RASS, which is turbulent flow in the right atrium after a rapid saline flush of the distal CVC port, to exclude catheter malposition. In this study with 135 CVC placements, visualization of RASS with ultrasound was able to identify all correct CVC placements and three of four catheter misplacements. Median times to complete the ultrasound exam vs CXR were 1 vs 20 minutes, respectively, with a median difference of 24 minutes (95% CI 19.6 to 29.3, P < .0001) between the two techniques.141
A prospective observational study assessed the ability of bedside transthoracic echocardiography to detect the guidewire, microbubbles, or both, in the right atrium compared to transesophageal echocardiography as the gold standard. Bedside transthoracic echocardiography allowed visualization of the right atrium in 94% of patients, and both microbubbles plus guidewire in 91% of patients.142 Hence, bedside transthoracic echocardiography allows adequate visualization of the right atrium. Another prospective observational study combining ultrasonography and contrast enhanced RASS resulted in 96% sensitivity and 93% specificity for the detection of a misplaced catheter, and the concordance with chest radiography was 96%.143
Training
23. To reduce the risk of mechanical and infectious complications, we recommend that novice providers should complete a systematic training program that includes a combination of simulation-based practice, supervised insertion on patients, and evaluation by an expert operator before attempting ultrasound-guided CVC insertion independently on patients.
Rationale: Cumulative experience has been recognized to not be a proxy for mastery of a clinical skill.144 The National Institute for Clinical Excellence (NICE) has recommended that providers performing ultrasound-guided CVC insertion should receive appropriate training to achieve competence before performing the procedure independently.7 Surveys have demonstrated that lack of training is a commonly reported barrier for not using ultrasound.145,146
Structured training programs on CVC insertion have been shown to reduce the occurrence of infectious and mechanical complications.74,143,147-149 The use of ultrasound and checklists, bundling of supplies, and practice with simulation models, as a part of a structured training program, can improve patient safety related to CVC insertion.9,140,150-154
Simulation-based practice has been used in medical education to provide deliberate practice and foster skill development in a controlled learning environment.155-158 Studies have shown transfer of skills demonstrated in a simulated environment to clinical practice, which can improve CVC insertion practices.159,160 Simulation accelerates learning of all trainees, especially novice trainees, and mitigates risks to patients by allowing trainees to achieve a minimal level of competence before attempting the procedure on real patients.152,161,162 Residents that have been trained using simulation preferentially select the IJV site,147 and more reliably use ultrasound to guide their CVC insertions.160,163
Additionally, simulation-based practice allows exposure to procedures and scenarios that may occur infrequently in clinical practice.
Although there is evidence on efficacy of simulation-based CVC training programs, there is no broadly accepted consensus on timing, duration, and content of CVC training programs for trainees or physicians in practice. The minimum recommended technical skills a trainee must master include the ability to (1) manipulate the ultrasound machine to produce a high-quality image to identify the target vessel, (2) advance the needle under direct visualization to the desired target site and depth, (3) deploy the catheter into the target vessel and confirm catheter placement in the target vessel using ultrasound, and (4) ensure the catheter has not been inadvertently placed in an unintended vessel or structure.153
A variety of simulation models are currently used to practice CVC insertion at the most common sites: the internal jugular, subclavian, basilic, and brachial veins.164,165 Effective simulation models should contain vessels that mimic normal anatomy with muscles, soft tissues, and bones. Animal tissue models, such as turkey or chicken breasts, may be effective for simulated practice of ultrasound-guided CVC insertion.166,167 Ultrasound-guided CVC training using human cadavers has also been shown to be effective.168
24. We recommend that cognitive training in ultrasound-guided CVC insertion should include basic anatomy, ultrasound physics, ultrasound machine knobology, fundamentals of image acquisition and interpretation, detection and management of procedural complications, infection prevention strategies, and pathways to attain competency.
Rationale: After receiving training in ultrasound-guided CVC insertion, physicians report significantly higher comfort with the use of ultrasound compared to those who have not received such training.145 Learners find training sessions worthwhile to increase skill levels,167 and skills learned from simulation-based mastery learning programs have been retained up to one year.158
Several commonalities have been noted across training curricula. Anatomy and physiology didactics should include vessel anatomy (location, size, and course);9 vessel differentiation by ultrasound;9,69 blood flow dynamics;69 Virchow’s triad;69 skin integrity and colonization;150 peripheral nerve identification and distribution;9 respiratory anatomy;9,69 upper and lower extremity, axillary, neck, and chest anatomy.9,69 Vascular anatomy is an essential curricular component that may help avoid preventable CVC insertion complications, such as inadvertent nerve, artery, or lung puncture.150,169 Training curricula should also include ultrasound physics (piezoelectric effect, frequency, resolution, attenuation, echogenicity, Doppler ultrasound, arterial and venous flow characteristics), image acquisition and optimization (imaging mode, focus, dynamic range, probe types), and artifacts (reverberation, mirror, shadowing, enhancement).
CVC-related infections are an important cause of morbidity and mortality in the acute and long-term care environment.69 Infection and thrombosis can both be impacted by the insertion site selection, skin integrity, and catheter–vein ratio.2,3,84 Inexperience generally leads to more insertion attempts that can increase trauma during CVC insertion and potentially increase the risk of infections.170 To reduce the risk of infectious complications, training should include important factors to consider in site selection and maintenance of a sterile environment during CVC insertion, including use of maximal sterile barrier precautions, hand hygiene, and appropriate use of skin antiseptic solutions.
Professional society guidelines have been published with recommendations of appropriate techniques for ultrasound-guided vascular access that include training recommendations.9,154 Training should deconstruct the insertion procedure into readily understood individual steps, and can be aided by demonstration of CVC insertion techniques using video clips. An alternative to face-to-face training is internet-based training that has been shown to be as effective as traditional teaching methods in some medical centers.171 Additional methods to deliver cognitive instruction include textbooks, continuing medical education courses, and digital videos.164,172
25. We recommend that trainees should demonstrate minimal competence before placing ultrasound-guided CVCs independently. A minimum number of CVC insertions may inform this determination, but a proctored assessment of competence is most important.
Rationale: CVC catheter placement carries the risk of serious complications including arterial injury or dissection, pneumothorax, or damage to other local structures; arrhythmias; catheter malposition; infection; and thrombosis. Although there is a lack of consensus and high-quality evidence for the certification of skills to perform ultrasound-guided CVC insertion, recommendations have been published advocating for formal and comprehensive training programs in ultrasound-guided CVC insertion with an emphasis on expert supervision prior to independent practice.9,153,154 Two groups of expert operators have recommended that training should include at least 8-10 supervised ultrasound-guided CVC insertions.154,173,174 A consensus task force from the World Congress of Vascular Access has recommended a minimum of six to eight hours of didactic education, four hours of hands-on training on simulation models, and six hours of hands-on ultrasound training on human volunteers to assess normal anatomy.175 This training should be followed by supervised ultrasound-guided CVC insertions until the learner has demonstrated minimal competence with a low rate of complications.35 There is general consensus that arbitrary numbers should not be the sole determinant of competence, and that the most important determinant of competence should be an evaluation by an expert operator.176
26. We recommend that didactic and hands-on training for trainees should coincide with anticipated times of increased performance of vascular access procedures. Refresher training sessions should be offered periodically.
Rationale: Simulation-based CVC training courses have shown a rapid improvement in skills, but lack of practice leads to deterioration of technical skills.161,162,177,178 Thus, a single immersive training session is insufficient to achieve and maintain mastery of skills, and an important factor to acquire technical expertise is sustained, deliberate practice with feedback.179 Furthermore, an insidious decay in skills may go unrecognized as a learner’s comfort and self-confidence does not always correlate with actual performance, leading to increased risk of errors and potential for procedural complications.147,158,180-183 Given the decay in technical skills over time, simulation-based training sessions are most effective when they occur in close temporal proximity to times when those skills are most likely to be used; for example, a simulation-based training session for trainees may be most effective just before the start of a critical care rotation.152 Regularly scheduled training sessions with monitoring and feedback by expert operators can reinforce procedural skills and prevent decay. Some experts have recommended that a minimum of 10 ultrasound-guided CVC insertions should be performed annually to maintain proficiency.153
27. We recommend that competency assessments should include formal evaluation of knowledge and technical skills using standardized assessment tools.
Rationale: Hospitalists and other healthcare providers that place vascular access catheters should undergo competency assessments proctored by an expert operator to verify that they have the required knowledge and skills.184,185 Knowledge competence can be partially evaluated using a written assessment, such as a multiple-choice test, assessing the provider’s cognitive understanding of the procedure.175 For ultrasound-guided CVC insertion, a written examination should be administered in conjunction with an ultrasound image assessment to test the learner’s recognition of normal vs abnormal vascular anatomy. Minimum passing standards should be established a priori according to local or institutional standards.
The final skills assessment should be objective, and the learner should be required to pass all critical steps of the procedure. Failure of the final skills assessment should lead to continued practice with supervision until the learner can consistently demonstrate correct performance of all critical steps. Checklists are commonly used to rate the technical performance of learners because they provide objective criteria for evaluation, can identify specific skill deficiencies, and can determine a learner’s readiness to perform procedures independently.186,187 The administration of skills assessments and feedback methods should be standardized across faculty. Although passing scores on both knowledge and skills assessments do not guarantee safe performance of a procedure independently, they provide a metric to ensure that a minimum level of competence has been achieved before allowing learners to perform procedures on patients without supervision.188
Competency assessments are a recommended component of intramural and extramural certification of skills in ultrasound-guided procedures. Intramural certification pathways differ by institution and often require additional resources including ultrasound machine(s), simulation equipment, and staff time, particularly when simulation-based assessments are incorporated into certification pathways. We recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals. However, initial and ongoing competency assessments can be performed during routine performance of procedures on patients. For an in-depth review of credentialing pathways for ultrasound-guided bedside procedures, we recommend reviewing the SHM Position Statement on Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.24
28. We recommend that competency assessments should evaluate for proficiency in the following knowledge and skills of CVC insertion:
a. Knowledge of the target vein anatomy, proper vessel identification, and recognition of anatomical variants
b. Demonstration of CVC insertion with no technical errors based on a procedural checklist
c. Recognition and management of acute complications, including emergency management of life-threatening complications
d. Real-time needle tip tracking with ultrasound and cannulation on the first attempt in at least five consecutive simulations.
Rationale: Recommendations have been published with the minimal knowledge and skills learners must demonstrate to perform ultrasound-guided vascular access procedures. These include operation of an ultrasound machine to produce high-quality images of the target vessel, tracking of the needle tip with real-time ultrasound guidance, and recognition and understanding of the management of procedural complications.154,175
First, learners must be able to perform a preprocedural assessment of the target vein, including size and patency of the vein; recognition of adjacent critical structures; and recognition of normal anatomical variants.175,189 Second, learners must be able to demonstrate proficiency in tracking the needle tip penetrating the target vessel, inserting the catheter into the target vessel, and confirming catheter placement in the target vessel with ultrasound.154,175 Third, learners must be able to demonstrate recognition of acute complications, including arterial puncture, hematoma formation, and development of pneumothorax.154,175 Trainees should be familiar with recommended evaluation and management algorithms, including indications for emergent consultation.190
29. We recommend a periodic proficiency assessments of all operators should be conducted to ensure maintenance of competency.
Rationale: Competency extends to periodic assessment and not merely an initial evaluation at the time of training.191 Periodic competency assessments should include assessment of proficiency of all providers that perform a procedure, including instructors and supervisors. Supervising providers should maintain their competency in CVC insertion through routine use of their skills in clinical practice.175 An observational study of emergency medicine residents revealed that lack of faculty comfort with ultrasound hindered the residents’ use of ultrasound.192 Thus, there is a need to examine best practices for procedural supervision of trainees because providers are often supervising procedures that they are not comfortable performing on their own.193
KNOWLEDGE GAPS
The process of producing this position statement revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for central and peripheral venous access and arterial access.
This position statement recommends a preprocedural ultrasound evaluation of blood vessels based on evidence that providers may detect anatomic anomalies, thrombosis, or vessel stenosis. Ultrasound can also reveal unsuspected high-risk structures in near proximity to the procedure site. Although previous studies have shown that providers can accurately assess vessels with ultrasound for these features, further study is needed to evaluate the effect of a standardized preprocedural ultrasound exam on clinical and procedural decision-making, as well as procedural outcomes.
Second, two ultrasound applications that are being increasingly used but have not been widely implemented are the use of ultrasound to evaluate lung sliding postprocedure to exclude pneumothorax and the verification of central line placement using a rapid infusion of agitated saline to visualize the RASS.139-141 Both of these applications have the potential to expedite postprocedure clearance of central lines for usage and decrease patient radiation exposure by obviating the need for postprocedure CXRs. Despite the supporting evidence, both of these applications are not yet widely used, as few providers have been trained in these techniques which may be considered advanced skills.
Third, despite advances in our knowledge of effective training for vascular access procedures, there is limited agreement on how to define procedural competence. Notable advancements in training include improved understanding of systematic training programs, development of techniques for proctoring procedures, definition of elements for hands-on assessments, and definition of minimum experience needed to perform vascular access procedures independently. However, application of these concepts to move learners toward independent practice remains variably interpreted at different institutions, likely due to limited resources, engrained cultures about procedures, and a lack of national standards. The development of hospitalist-based procedure services at major academic medical centers with high training standards, close monitoring for quality assurance, and the use of databases to track clinical outcomes may advance our understanding and delivery of optimal procedural training.
Finally, ultrasound technology is rapidly evolving which will affect training, techniques, and clinical outcomes in coming years. Development of advanced imaging software with artificial intelligence can improve needle visualization and tracking. These technologies have the potential to facilitate provider training in real-time ultrasound-guided procedures and improve the overall safety of procedures. Emergence of affordable, handheld ultrasound devices is improving access to ultrasound technology, but their role in vascular access procedures is yet to be defined. Furthermore, availability of wireless handheld ultrasound technology and multifrequency transducers will create new possibilities for use of ultrasound in vascular access procedures.
CONCLUSION
We have presented several evidence-based recommendations on the use of ultrasound guidance for placement of central and peripheral vascular access catheters that are intended for hospitalists and other healthcare providers who routinely perform vascular access procedures. By allowing direct visualization of the needle tip and target vessel, the use of ultrasound guidance has been shown in randomized studies to reduce needle insertion attempts, reduce needle redirections, and increase overall procedure success rates. The accuracy of ultrasound to identify the target vessel, assess for thrombosis, and detect anatomical anomalies is superior to that of physical examination. Hospitalists can attain competence in performing ultrasound-guided vascular access procedures through systematic training programs that combine didactic and hands-on training, which optimally include patient-based competency assessments.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators of Society of Hospital Medicine Point-of-care Ultrasound Task Force: Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Venkat Kalidindi, Ketino Kobaidze, Joshua Lenchus, Paul Mayo, Satyen Nichani, Vicki Noble, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Gerard Salame, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam J. Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Mathews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Mathews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El-Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Raad I. Intravascular-catheter-related infections. Lancet. 1998;351(9106):893-898. https://doi.org/10.1016/S0140-6736(97)10006-X.
2. Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286(6):700-707. https://doi.org/10.1001/jama.286.6.700.
3. Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986;146(2):259-261. https://doi.org/10.1001/archinte.146.2.259.
4. Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med. 1996;24(12):2053-2058. https://doi.org/10.1097/00003246-199612000-00020.
5. Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327(7411):361. https://doi.org/10.1136/bmj.327.7411.361.
6. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4):S1-S34. https://doi.org/10.1016/j.ajic.2011.01.003.
7. National Institute for Health and Care Excellence (NICE). Guidance on the Use of Ultrasound Locating Devices for Placing Central Venous Catheters; 2002.
8. Shojania KG, Duncan BW, McDonald KM, et al. Making health care safer: A critical analysis of patient safety practices. Evid Rep Technol Assess. Rockville, MD: Agency for Healthcare Research and Quality. 2001;43(43):i–x, 1.
9. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105-1117. https://doi.org/10.1007/s00134-012-2597-x.
10. Frykholm P, Pikwer A, Hammarskjöld F, et al. Clinical guidelines on central venous catheterisation. Swedish Society of Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiol Scand. 2014;58(5):508-524. https://doi.org/10.1111/aas.12295.
11. American Society of Anesthesiologists Task Force on Central Venous Access, Rupp SM, Apfelbaum JL, et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539-573. https://doi.org/10.1097/ALN.0b013e31823c9569.
12. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: General ultrasonography. Crit Care Med. 2015;43(11):2479-2502. https://doi.org/10.1097/CCM.0000000000001216.
13. Shekelle PG, Wachter RM, Pronovost PJ, et al. Making health care safer II: An updated critical analysis of the evidence for patient safety practices. Evid Rep Technol Assess. 2013;211:1-945.
14. Troianos CA, Hartman GS, Glas KE, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2011;24(12):1291-1318. https://doi.org/10.1016/j.echo.2011.09.021.
15. Soni NJ, Reyes LF, Keyt H, et al. Use of ultrasound guidance for central venous catheterization: a national survey of intensivists and hospitalists. J Crit Care. 2016;36:277-283. https://doi.org/10.1016/j.jcrc.2016.07.014.
16. Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220-1229. https://doi.org/10.1056/NEJMoa1500964.
17. Maizel J, Bastide MA, Richecoeur J, et al. Practice of ultrasound-guided central venous catheter technique by the French intensivists: a survey from the BoReal study group. Ann Intensive Care. 2016;6(1):76. https://doi.org/10.1186/s13613-016-0177-x.
18. Egan G, Healy D, O’Neill H, et al. Ultrasound guidance for difficult peripheral venous access: systematic review and meta-analysis. Emerg Med J. 2013;30(7):521-526. https://doi.org/10.1136/emermed-2012-201652.
19. Stolz LA, Stolz U, Howe C, Farrell IJ, Adhikari S. Ultrasound-guided peripheral venous access: a meta-analysis and systematic review. J Vasc Access. 2015;16(4):321-326. https://doi.org/10.5301/jva.5000346.
20. Sofocleous CT, Schur I, Cooper SG, et al. Sonographically guided placement of peripherally inserted central venous catheters: review of 355 procedures. AJR Am J Roentgenol. 1998;170(6):1613-1616. https://doi.org/10.2214/ajr.170.6.9609183.
21. Nichols I, Humphrey JP. The efficacy of upper arm placement of peripherally inserted central catheters using bedside ultrasound and microintroducer technique. J Infus Nurs Off Publ Infus Nurs Soc. 2008;31(3):165-176. https://doi.org/10.1097/01.NAN.0000317703.66395.b8.
22. Stokowski G, Steele D, Wilson D. The use of ultrasound to improve practice and reduce complication rates in peripherally inserted central catheter insertions. J Infus Nurs Off Publ Infus Nurs Soc. 2009;32(3):145-155. https://doi.org/10.1097/NAN.0b013e3181a1a98f.
23. Shiloh AL, Savel RH, Paulin LM, Eisen LA. Ultrasound-guided catheterization of the radial artery: a systematic review and meta-analysis of randomized controlled trials. Chest. 2011;139(3):524-529. https://doi.org/10.1378/chest.10-0919.
24. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: A position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
25. Chopra V, Flanders SA, Saint S, et al. The Michigan appropriateness guide for intravenous catheters (Magic): results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6):S1-S40. https://doi.org/10.7326/M15-0744.
26. Raad II, Hohn DC, Gilbreath BJ, et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994;15(4):231-238.
27. Slama M, Novara A, Safavian A, et al. Improvement of internal jugular vein cannulation using an ultrasound-guided technique. Intensive Care Med. 1997;23(8):916-919. https://doi.org/10.1007/s001340050432.
28. Seyahi N, Kahveci A, Altiparmak MR, Serdengecti K, Erek E. Ultrasound imaging findings of femoral veins in patients with renal failure and its impact on vascular access. Nephrol Dial Transplant. 2005;20(9):1864-1867. https://doi.org/10.1093/ndt/gfh942.
29. Lin BS, Kong CW, Tarng DC, Huang TP, Tang GJ. Anatomical variation of the internal jugular vein and its impact on temporary haemodialysis vascular access: an ultrasonographic survey in uraemic patients. Nephrol Dial Transplant. 1998;13(1):134-138. https://doi.org/10.1093/ndt/13.1.134.
30. Troianos CA, Kuwik RJ, Pasqual JR, Lim AJ, Odasso DP. Internal jugular vein and carotid artery anatomic relation as determined by ultrasonography. Anesthesiology. 1996;85(1):43-48. https://doi.org/10.1097/00000542-199607000-00007.
31. Gordon AC, Wright I, Pugh ND. Duplication of the superficial femoral vein: recognition with duplex ultrasonography. Clin Rad. 1996;51(9):622-624. https://doi.org/10.1016/S0009-9260(96)80055-9.
32. Sulek CA, Gravenstein N, Blackshear RH, Weiss L. Head rotation during internal jugular vein cannulation and the risk of carotid artery puncture. Anesth Analg. 1996;82(1):125-128. https://doi.org/10.1097/00000539-199601000-00022.
33. Shrestha BR, Gautam B. Ultrasound versus the landmark technique: a prospective randomized comparative study of internal jugular vein cannulation in an intensive care unit. JNMA J Nepal Med Assoc. 2011;51(182):56-61. https://doi.org/10.31729/jnma.148.
34. Brederlau J, Greim C, Schwemmer U, et al. Ultrasound-guided cannulation of the internal jugular vein in critically ill patients positioned in 30 degrees dorsal elevation. Eur J Anaesthesiol. 2004;21(9):684-687. https://doi.org/10.1097/00003643-200409000-00003.
35. Lamperti M, Subert M, Cortellazzi P, et al. Is a neutral head position safer than 45-degree neck rotation during ultrasound-guided internal jugular vein cannulation? Results of a randomized controlled clinical trial. Anesth Analg. 2012;114(4):777-784. https://doi.org/10.1213/ANE.0b013e3182459917.
36. Benter T, Teichgräber UK, Klühs L, et al. Anatomical variations in the internal jugular veins of cancer patients affecting central venous access. Anatomical variation of the internal jugular vein. Ultraschall Med. 2001;22(1):23-26. https://doi.org/10.1055/s-2001-11243.
37. Caridi JG, Hawkins IF, Jr, Wiechmann BN, Pevarski DJ, Tonkin JC. Sonographic guidance when using the right internal jugular vein for central vein access. AJR Am J Roentgenol. 1998;171(5):1259-1263. https://doi.org/10.2214/ajr.171.5.9798857.
38. Brusasco C, Corradi F, Zattoni PL, et al. Ultrasound-guided central venous cannulation in bariatric patients. Obes Surg. 2009;19(10):1365-1370. https://doi.org/10.1007/s11695-009-9902-y.
39. Armstrong PJ, Cullen M, Scott DH. The ‘SiteRite’ ultrasound machine--an aid to internal jugular vein cannulation. Anaesthesia. 1993;48(4):319-323. https://doi.org/10.1111/j.1365-2044.1993.tb06953.x.
40. Forauer AR, Glockner JF. Importance of US findings in access planning during jugular vein hemodialysis catheter placements. J Vasc Interv Rad. 2000;11(2 Pt 1):233-238. https://doi.org/10.1016/S1051-0443(07)61471-7.
41. Hassan C, Girishkumar HT, Thatigotla B, et al. Value of ultrasound guidance in placement of hemodialysis access catheters in patients with end-stage renal disease. Am Surg. 2008;74(11):1111-1113.
42. Tan CO, Weinberg L, Peyton P, Story D, McNicol L. Size variation between contralateral infraclavicular axillary veins within individual patients-implications for subclavian venous central line insertion. Crit Care Med. 2013;41(2):457-463. https://doi.org/10.1097/CCM.0b013e31826ab1dd.
43. Wu SY, Ling Q, Cao LH, et al. Real-time two-dimensional ultrasound guidance for central venous cannulation: a meta-analysis. Anesthesiology. 2013;118(2):361-375. https://doi.org/10.1097/ALN.0b013e31827bd172.
44. Kim W, Chung RK, Lee GY, Han JI. The effects of hip abduction with external rotation and reverse Trendelenburg position on the size of the femoral vein; ultrasonographic investigation. Korean J Anesthesiol. 2011;61(3):205-209. https://doi.org/10.4097/kjae.2011.61.3.205.
45. Denys BG, Uretsky BF. Anatomical variations of internal jugular vein location: impact on central venous access. Crit Care Med. 1991;19(12):1516-1519. https://doi.org/10.1097/00003246-199112000-00013.
46. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev. 2015;1:CD006962. https://doi.org/10.1002/14651858.CD006962.pub2.
47. Gilbert TB, Seneff MG, Becker RB. Facilitation of internal jugular venous cannulation using an audio-guided Doppler ultrasound vascular access device: results from a prospective, dual-center, randomized, crossover clinical study. Crit Care Med. 1995;23(1):60-65. https://doi.org/10.1097/00003246-199501000-00012.
48. Schummer W, Schummer C, Tuppatsch H, et al. Ultrasound-guided central venous cannulation: is there a difference between Doppler and B-mode ultrasound? J Clin Anesth. 2006;18(3):167-172. https://doi.org/10.1016/j.jclinane.2005.12.010.
49. Airapetian N, Maizel J, Langelle F, et al. Ultrasound-guided central venous cannulation is superior to quick-look ultrasound and landmark methods among inexperienced operators: a prospective randomized study. Intensive Care Med. 2013;39(11):1938-1944. https://doi.org/10.1007/s00134-013-3072-z.
50. Milling TJ, Jr, Rose J, Briggs WM, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Crit Care Med. 2005;33(8):1764-1769. https://doi.org/10.1097/01.ccm.0000171533.92856.e5.
51. Beaudoin FL, Merchant RC, Lincoln J, et al. Bedside ultrasonography detects significant femoral vessel overlap: implications for central venous cannulation. CJEM. 2011;13(4):245-250. https://doi.org/10.2310/8000.2011.110482.
52. Kwon TH, Kim YL, Cho DK. Ultrasound-guided cannulation of the femoral vein for acute haemodialysis access. Nephrol Dial Transplant. 1997;12(5):1009-1012. https://doi.org/10.1093/ndt/12.5.1009.
53. Rothschild JM. Ultrasound guidance of central vein catheterization. Evid Rep Technol Assess. 2001;43. Chapter 21.: http://archive.ahrq.gov/clinic/ptsafety/chap21.htm.
54. Lennon M, Zaw NN, Pöpping DM, Wenk M. Procedural complications of central venous catheter insertion. Minerva Anestesiol. 2012;78(11):1234-1240.
55. Blaivas M, Adhikari S. An unseen danger: frequency of posterior vessel wall penetration by needles during attempts to place internal jugular vein central catheters using ultrasound guidance. Crit Care Med. 2009;37(8):2345-2349; quiz 2359. https://doi.org/10.1097/CCM.0b013e3181a067d4.
56. Vogel JA, Haukoos JS, Erickson CL, et al. Is long-axis view superior to short-axis view in ultrasound-guided central venous catheterization? Crit Care Med. 2015;43(4):832-839. https://doi.org/10.1097/CCM.0000000000000823.
57. Tammam TF, El-Shafey EM, Tammam HF. Ultrasound-guided internal jugular vein access: comparison between short axis and long axis techniques. Saudi J Kidney Dis Transpl. 2013;24(4):707-713. https://doi.org/10.4103/1319-2442.113861.
58. Keyes LE, Frazee BW, Snoey ER, Simon BC, Christy D. Ultrasound-guided brachial and basilic vein cannulation in emergency department patients with difficult intravenous access. Ann Emerg Med. 1999;34(6):711-714. https://doi.org/10.1016/S0196-0644(99)70095-8.
59. Mahler SA, Wang H, Lester C, et al. Short- vs long-axis approach to ultrasound-guided peripheral intravenous access: a prospective randomized study. Am J Emerg Med. 2011;29(9):1194-1197. https://doi.org/10.1016/j.ajem.2010.07.015.
60. Berk D, Gurkan Y, Kus A, et al. Ultrasound-guided radial arterial cannulation: long axis/in-plane versus short axis/out-of-plane approaches? J Clin Monit Comput. 2013;27(3):319-324. https://doi.org/10.1007/s10877-013-9437-6.
61. Batllori M, Urra M, Uriarte E, et al. Randomized comparison of three transducer orientation approaches for ultrasound guided internal jugular venous cannulation. Br J Anaesth. 2016;116(3):370-376. https://doi.org/10.1093/bja/aev399.
62. Moak JH, Lyons MS, Wright SW, Lindsell CJ. Needle and guidewire visualization in ultrasound-guided internal jugular vein cannulation. Am J Emerg Med. 2011;29(4):432-436. https://doi.org/10.1016/j.ajem.2010.01.004.
63. Moak JH, Rajkumar JS, Woods WA. The wire is really easy to see (WIRES): sonographic visualization of the guidewire by novices. CJEM. 2013;15(1):18-23. https://doi.org/10.2310/8000.2012.120800.
64. Gillman LM, Blaivas M, Lord J, Al-Kadi A, Kirkpatrick AW. Ultrasound confirmation of guidewire position may eliminate accidental arterial dilatation during central venous cannulation. Scand J Trauma Resusc Emerg Med. 2010;18:39. https://doi.org/10.1186/1757-7241-18-39.
65. Augoustides JG, Horak J, Ochroch AE, et al. A randomized controlled clinical trial of real-time needle-guided ultrasound for internal jugular venous cannulation in a large university anesthesia department. J Cardiothorac Vasc Anesth. 2005;19(3):310-315. https://doi.org/10.1053/j.jvca.2005.03.007.
66. Maecken T, Heite L, Wolf B, Zahn PK, Litz RJ. Ultrasound-guided catheterisation of the subclavian vein: freehand vs needle-guided technique. Anaesthesia. 2015;70(11):1242-1249. https://doi.org/10.1111/anae.13187.
67. Stone MB, Nagdev A, Murphy MC, Sisson CA. Ultrasound detection of guidewire position during central venous catheterization. Am J Emerg Med. 2010;28(1):82-84. https://doi.org/10.1016/j.ajem.2008.09.019.
68. Luyet C, Hartwich V, Urwyler N, et al. Evaluation of a novel needle guide for ultrasound-guided phantom vessel cannulation. Anaesthesia. 2011;66(8):715-720. https://doi.org/10.1111/j.1365-2044.2011.06781.x.
69. O’Grady NP, Alexander M, Burns LA, et al. Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis. 2011;52(9):1087-1099. https://doi.org/10.1093/cid/cir138.
70. Southworth SL, Henman LJ, Kinder LA, Sell JL. The journey to zero central catheter-associated bloodstream infections: culture change in an intensive care unit. Crit Care Nurse. 2012;32(2):49-54. https://doi.org/10.4037/ccn2012915.
71. Guerin K, Wagner J, Rains K, Bessesen M. Reduction in central line-associated bloodstream infections by implementation of a postinsertion care bundle. Am J Infect Control. 2010;38(6):430-433. https://doi.org/10.1016/j.ajic.2010.03.007.
72. Hartman N, Wittler M, Askew K, Manthey D. Delphi method validation of a procedural performance checklist for insertion of an ultrasound-guided internal jugular central line. Am J Med Qual Off J Am Coll Med Qual. 2016;31(1):81-85. https://doi.org/10.1177/1062860614549762.
73. Lenchus JD, Carvalho CM, Ferreri K, et al. Filling the void: defining invasive bedside procedural competency for internal medicine residents. J Grad Med Educ. 2013;5(4):605-612. https://doi.org/10.4300/JGME-D-13-00030.1.
74. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. https://doi.org/10.1001/archinternmed.2009.215.
75. Karakitsos D, Labropoulos N, De Groot E, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care. 2006;10(6):R162. https://doi.org/10.1186/cc5101.
76. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. https://doi.org/10.1097/01.ccm.0000142399.70913.2f.
77. Young EM, Commiskey ML, Wilson SJ. Translating evidence into practice to prevent central venous catheter-associated bloodstream infections: a systems-based intervention. Am J Infect Control. 2006;34(8):503-506. https://doi.org/10.1016/j.ajic.2006.03.011.
78. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495; discussion 495. https://doi.org/10.1016/j.surg.2008.06.004.
79. Mermel LA, McCormick RD, Springman SR, Maki DG. The pathogenesis and epidemiology of catheter-related infection with pulmonary artery swan-Ganz catheters: a prospective study utilizing molecular subtyping. Am J Med. 1991;91(3b):197S–205S. https://doi.org/10.1016/0002-9343(91)90369-9.
80. Marschall J, Mermel LA, Classen D, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(1):S22-S30. https://doi.org/10.1086/591059.
81. Marschall J, Mermel LA, Fakih M, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(7):753-771. https://doi.org/10.1086/676533.
82. Garcia-Rodriguez JF, Álvarez-Díaz H, Vilariño-Maneiro L, et al. Epidemiology and impact of a multifaceted approach in controlling central venous catheter associated blood stream infections outside the intensive care unit. BMC Infect Dis. 2013;13:445. https://doi.org/10.1186/1471-2334-13-445.
83. Lee DH, Jung KY, Choi YH. Use of maximal sterile barrier precautions and/or antimicrobial-coated catheters to reduce the risk of central venous catheter-related bloodstream infection. Infect Control Hosp Epidemiol. 2008;29(10):947-950. https://doi.org/10.1086/590356.
84. Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132(5):391-402. https://doi.org/10.7326/0003-4819-132-5-200003070-00009.
85. Troianos CA, Jobes DR, Ellison N. Ultrasound-guided cannulation of the internal jugular vein. A prospective, randomized study. Anesth Analg. 1991;72(6):823-826. https://doi.org/10.1213/00000539-199106000-00020.
86. Troianos CA, Savino JS. Internal jugular vein cannulation guided by echocardiography. Anesthesiology. 1991;74(4):787-789. https://doi.org/10.1097/00000542-199104000-00026.
87. Denys BG, Uretsky BF, Reddy PS, et al. An ultrasound method for safe and rapid central venous access. N Engl J Med. 1991;324(8):566. https://doi.org/10.1056/NEJM199102213240816.
88. Riaz A, Shan Khan RA, Salim F. Ultrasound guided internal jugular venous cannulation: comparison with land-mark technique. J Coll Phys Surg Pak. 2015;25(5):315-319. https://doi.org/05.2015/JCPSP.315319.
89. Gok F, Kilicaslan A, Sarkilar G, Kandemir B, Yosunkaya A. The effect of ultrasound guidance on central venous catheter-associated bloodstream infection in critical care patients. Acta Med Mediterr. 2013;29:677-682.
90. Lamperti M, Cortellazzi P, D’Onofrio G, et al. An outcome study on complications using routine ultrasound assistance for internal jugular vein cannulation. Acta Anaesthesiol Scand. 2007;51(10):1327-1330. https://doi.org/10.1111/j.1399-6576.2007.01442.x.
91. Vezzani A, Manca T, Vercelli A, Braghieri A, Magnacavallo A. Ultrasonography as a guide during vascular access procedures and in the diagnosis of complications. J Ultrasound. 2013;16(4):161-170. https://doi.org/10.1007/s40477-013-0046-5.
92. Palepu GB, Deven J, Subrahmanyam M, Mohan S. Impact of ultrasonography on central venous catheter insertion in intensive care. Indian J Rad Imaging. 2009;19(3):191-198. https://doi.org/10.4103/0971-3026.54877.
93. Agarwal A, Singh DK, Singh AP. Ultrasonography: a novel approach to central venous cannulation. Indian J Crit Care Med. 2009;13(4):213-216. https://doi.org/10.4103/0972-5229.60174.
94. Wong SW, Niazi AU, Chin KJ, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS® needle tracking system: a case report. Can J Anaesth. 2013;60(1):50-53. https://doi.org/10.1007/s12630-012-9809-2.
95. Bouaziz H, Zetlaoui PJ, Pierre S, et al. Guidelines on the use of ultrasound guidance for vascular access. Anaesth, Crit Care Pain Med. 2015;34(1):65-69. https://doi.org/10.1016/j.accpm.2015.01.004.
96. Jenssen C, Brkljacic B, Hocke M, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part VI - Ultrasound-guided vascular interventions. Ultraschall Med. 2016;37(5):473-476. https://doi.org/10.1055/s-0035-1553450.
97. Rando K, Castelli J, Pratt JP, et al. Ultrasound-guided internal jugular vein catheterization: a randomized controlled trial. Heart Lung Vessels. 2014;6(1):13-23.
98. Mey U, Glasmacher A, Hahn C, et al. Evaluation of an ultrasound-guided technique for central venous access via the internal jugular vein in 493 patients. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2003;11(3):148-155. https://doi.org/10.1007/s00520-002-0399-3.
99. Singh SA, Sharma S, Singh A, et al. The safety of ultrasound guided central venous cannulation in patients with liver disease. Saudi J Anaesth. 2015;9(2):155-160. https://doi.org/10.4103/1658-354X.152842.
100. Akoglu H, Piskinpasa S, Yenigun EC, et al. Real-time ultrasound guided placement of temporary internal jugular vein catheters: assessment of technical success and complication rates in nephrology practice. Nephrol (Carlton). 2012;17(7):603-606. https://doi.org/10.1111/j.1440-1797.2012.01637.x.
101. Sadler DJ, Gordon AC, Klassen J, et al. Image-guided central venous catheters for apheresis. Bone Marrow Transplant. 1999;23(2):179-182. https://doi.org/10.1038/sj.bmt.1701545.
102. Calvert N, Hind D, McWilliams R, et al. Ultrasound for central venous cannulation: economic evaluation of cost-effectiveness. Anaesthesia. 2004;59(11):1116-1120. https://doi.org/10.1111/j.1365-2044.2004.03906.x.
103. Goetz AM, Wagener MM, Miller JM, Muder RR. Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infect Control Hosp Epidemiol. 1998;19(11):842-845.
104. Bertini P, Frediani M. Ultrasound guided supraclavicular central vein cannulation in adults: a technical report. J Vasc Access. 2013;14(1):89-93. https://doi.org/10.5301/jva.5000088.
105. Lalu MM, Fayad A, Ahmed O, et al. Ultrasound-guided subclavian vein catheterization: A systematic review and meta-analysis. Crit Care Med. 2015;43(7):1498-1507. https://doi.org/10.1097/CCM.0000000000000973.
106. Milone M, Di Minno G, Di Minno MN, et al. The real effectiveness of ultrasound guidance in subclavian venous access. Ann ital chir. 2010;81(5):331-334.
107. O’Leary R, Ahmed SM, McLure H, et al. Ultrasound-guided infraclavicular axillary vein cannulation: a useful alternative to the internal jugular vein. Br J Anaesth. 2012;109(5):762-768. https://doi.org/10.1093/bja/aes262.
108. Gualtieri E, Deppe SA, Sipperly ME, Thompson DR. Subclavian venous catheterization: greater success rate for less experienced operators using ultrasound guidance. Crit Care Med. 1995;23(4):692-697. https://doi.org/10.1097/00003246-199504000-00018.
109. Fragou M, Gravvanis A, Dimitriou V, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med. 2011;39(7):1607-1612. https://doi.org/10.1097/CCM.0b013e318218a1ae.
110. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015;1:CD011447. https://doi.org/10.1002/14651858.CD011447.
111. Robinson MK, Mogensen KM, Grudinskas GF, Kohler S, Jacobs DO. Improved care and reduced costs for patients requiring peripherally inserted central catheters: the role of bedside ultrasound and a dedicated team. JPEN J Parenter Enter Nutr. 2005;29(5):374-379. https://doi.org/10.1177/0148607105029005374.
112. Li J, Fan YY, Xin MZ, et al. A randomised, controlled trial comparing the long-term effects of peripherally inserted central catheter placement in chemotherapy patients using B-mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94-103. https://doi.org/10.1016/j.ejon.2013.08.003.
113. Schweickert WD, Herlitz J, Pohlman AS, et al. A randomized, controlled trial evaluating postinsertion neck ultrasound in peripherally inserted central catheter procedures. Crit Care Med. 2009;37(4):1217-1221. https://doi.org/10.1097/CCM.0b013e31819cee7f.
114. Mahler SA, Wang H, Lester C, Conrad SA. Ultrasound-guided peripheral intravenous access in the emergency department using a modified Seldinger technique. J Emerg Med. 2010;39(3):325-329. https://doi.org/10.1016/j.jemermed.2009.02.013.
115. Brannam L, Blaivas M, Lyon M, Flake M. Emergency nurses’ utilization of ultrasound guidance for placement of peripheral intravenous lines in difficult-access patients. Acad Emerg Med. 2004;11(12):1361-1363. https://doi.org/10.1197/j.aem.2004.08.027.
116. Au A, Rotte M, Gryzbowski R, Ku B, Fields J. 157 Decrease in central venous catheter placement and complications due to utilization of ultrasound-guided peripheral intravenous catheters. Ann Emerg Med. 2011;58(4):S230. https://doi.org/10.1016/j.annemergmed.2011.06.185.
117. Dargin JM, Rebholz CM, Lowenstein RA, Mitchell PM, Feldman JA. Ultrasonography-guided peripheral intravenous catheter survival in ED patients with difficult access. Am J Emerg Med. 2010;28(1):1-7. https://doi.org/10.1016/j.ajem.2008.09.001.
118. Bauman M, Braude D, Crandall C. Ultrasound-guidance vs standard technique in difficult vascular access patients by ED technicians. Am J Emerg Med. 2009;27(2):135-140. https://doi.org/10.1016/j.ajem.2008.02.005.
119. Schoenfeld E, Shokoohi H, Boniface K. Ultrasound-guided peripheral intravenous access in the emergency department: patient-centered survey. West J Emerg Med. 2011;12(4):475-477. https://doi.org/10.5811/westjem.2011.3.1920.
120. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. https://doi.org/10.7326/0003-4819-114-10-845.
121. Miliani K, Taravella R, Thillard D, et al. Peripheral venous catheter-related adverse events: evaluation from a multicentre epidemiological study in France (the CATHEVAL Project). PLOS ONE. 2017;12(1):e0168637. https://doi.org/10.1371/journal.pone.0168637.
122. Gregg SC, Murthi SB, Sisley AC, Stein DM, Scalea TM. Ultrasound-guided peripheral intravenous access in the intensive care unit. J Crit Care. 2010;25(3):514-519. https://doi.org/10.1016/j.jcrc.2009.09.003.
123. Adhikari S, Blaivas M, Morrison D, Lander L. Comparison of infection rates among ultrasound-guided versus traditionally placed peripheral intravenous lines. J Ultrasound Med. 2010;29(5):741-747. https://doi.org/10.7863/jum.2010.29.5.741.
124. Shiver S, Blaivas M, Lyon M. A prospective comparison of ultrasound-guided and blindly placed radial arterial catheters. Acad Emerg Med. 2006;13(12):1275-1279. https://doi.org/10.1197/j.aem.2006.07.015.
125. Levin PD, Sheinin O, Gozal Y. Use of ultrasound guidance in the insertion of radial artery catheters. Crit Care Med. 2003;31(2):481-484. https://doi.org/10.1097/01.CCM.0000050452.17304.2F.
126. Gu WJ, Tie HT, Liu JC, Zeng XT. Efficacy of ultrasound-guided radial artery catheterization: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2014;18(3):R93. https://doi.org/10.1186/cc13862.
127. Tang L, Wang F, Li Y, et al. Ultrasound guidance for radial artery catheterization: an updated meta-analysis of randomized controlled trials. PLOS ONE. 2014;9(11):e111527. https://doi.org/10.1371/journal.pone.0111527.
128. Sobolev M, Slovut DP, Lee Chang A, Shiloh AL, Eisen LA. Ultrasound-guided catheterization of the femoral artery: A systematic review and meta-analysis of randomized controlled trials. J Invas Cardiol. 2015;27(7):318-323. https://doi.org/10.1378/chest.1991181.
129. Gabriel M, Pawlaczyk K, Waliszewski K, Krasiński Z, Majewski W. Location of femoral artery puncture site and the risk of postcatheterization pseudoaneurysm formation. Int J Cardiol. 2007;120(2):167-171. https://doi.org/10.1016/j.ijcard.2006.09.018.
130. Seto AH, Abu-Fadel MS, Sparling JM, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With ultrasound Trial). JACC Cardiovasc Interv. 2010;3(7):751-758. https://doi.org/10.1016/j.jcin.2010.04.015.
131. Kalish J, Eslami M, Gillespie D, et al. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg. 2015;61(5):1231-1238. https://doi.org/10.1016/j.jvs2014.12.003.
132. Sandhu NS, Patel B. Use of ultrasonography as a rescue technique for failed radial artery cannulation. J Clin Anesth. 2006;18(2):138-141. https://doi.org/10.1016/j.jclinane.2005.06.011.
133. White L, Halpin A, Turner M, Wallace L. Ultrasound-guided radial artery cannulation in adult and paediatric populations: a systematic review and meta-analysis. Br J Anaesth. 2016;116(5):610-617. https://doi.org/10.1093/bja/aew097.
134. Gao YB, Yan JH, Gao FQ, et al. Effects of ultrasound-guided radial artery catheterization: an updated meta-analysis. Am J Emerg Med. 2015;33(1):50-55. https://doi.org/10.1016/j.ajem.2014.10.008.
135. Seto AH, Roberts JS, Abu-Fadel MS, et al. Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery Access with Ultrasound Trial). JACC Cardiovasc Interv. 2015;8(2):283-291. https://doi.org/10.1016/j.jcin.2014.05.036.
136. Roberts J, Manur R. Ultrasound-guided radial artery access by a non-ultrasound trained interventional cardiologist improved first-attempt success rates and shortened time for successful radial artery cannulation. J Invas Cardiol. 2013;25(12):676-679.
137. Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108(5):1345-1348. https://doi.org/10.1378/chest.108.5.1345.
138. Lichtenstein D, Mezière G, Biderman P, Gepner A. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999;25(4):383-388. https://doi.org/10.1007/s001340050862.
139. Ablordeppey EA, Drewry AM, Beyer AB, et al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound Versus chest radiography in critically ill patients: A systematic review and meta-analysis. Crit Care Med. 2017;45(4):715-724. https://doi.org/10.1097/CCM.0000000000002188.
140. Bedel J, Vallée F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013;39(11):1932-1937. https://doi.org/10.1007/s00134-013-3097-3.
141. Weekes AJ, Keller SM, Efune B, Ghali S, Runyon M. Prospective comparison of ultrasound and CXR for confirmation of central vascular catheter placement. Emerg Med J EMJ. 2016;33(3):176-180. https://doi.org/10.1136/emermed-2015-205000.
142. Arellano R, Nurmohamed A, Rumman A, et al. The utility of transthoracic echocardiography to confirm central line placement: an observational study. Can J Anaesth. 2014;61(4):340-346. https://doi.org/10.1007/s12630-014-0111-3.
143. Vezzani A, Brusasco C, Palermo S, et al. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38(2):533-538. https://doi.org/10.1097/CCM.0b013e3181c0328f.
144. Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142(4):260-273. https://doi.org/10.7326/0003-4819-142-4-200502150-00008.
145. Backlund BH, Hopkins E, Kendall JL. Ultrasound guidance for central venous access by emergency physicians in Colorado. West J Emerg Med. 2012;13(4):320-325. https://doi.org/10.5811/westjem.2011.11.6821.
146. Buchanan MS, Backlund B, Liao MM, et al. Use of ultrasound guidance for central venous catheter placement: survey from the American Board of Emergency Medicine Longitudinal Study of Emergency Physicians. Acad Emerg Med. 2014;21(4):416-421. https://doi.org/10.1111/acem.12350.
147. Barsuk JH, McGaghie WC, Cohen ER, O’Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697-2701. https://doi.org/10.1097/00003246-200910000-00003.
148. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. https://doi.org/10.1097/00003246-200201000-00009.
149. Woo MY, Frank J, Lee AC, et al. Effectiveness of a novel training program for emergency medicine residents in ultrasound-guided insertion of central venous catheters. CJEM. 2009;11(4):343-348. https://doi.org/10.1017/S1481803500011398.
150. McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348(12):1123-1133. https://doi.org/10.1056/NEJMra011883.
151. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. https://doi.org/10.1002/jhm.468.
152. Sekiguchi H, Tokita JE, Minami T, et al. A prerotational, simulation-based workshop improves the safety of central venous catheter insertion: results of a successful internal medicine house staff training program. Chest. 2011;140(3):652-658. https://doi.org/10.1378/chest.10-3319.
153. Feller-Kopman D. Ultrasound-guided internal jugular access: a proposed standardized approach and implications for training and practice. Chest. 2007;132(1):302-309. https://doi.org/10.1378/chest.06-2711.
154. Troianos CA, Hartman GS, Glas KE, et al. Special articles: guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg. 2012;114(1):46-72. https://doi.org/10.1213/ANE.0b013e3182407cd8.
155. Issenberg SB, McGaghie WC, Hart IR, et al. Simulation technology for health care professional skills training and assessment. JAMA. 1999;282(9):861-866. https://doi.org/10.1001/jama.282.9.861.
156. Millington SJ, Wong RY, Kassen BO, Roberts JM, Ma IW. Improving internal medicine residents’ performance, knowledge, and confidence in central venous catheterization using simulators. J Hosp Med. 2009;4(7):410-416. https://doi.org/10.1002/jhm.570.
157. Beaulieu Y, Laprise R, Drolet P, et al. Bedside ultrasound training using web-based e-learning and simulation early in the curriculum of residents. Crit Ultrasound J. 2015;7:1. https://doi.org/10.1186/s13089-014-0018-9.
158. Barsuk JH, Cohen ER, McGaghie WC, Wayne DB. Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med. 2010;85(10 Suppl):S9-S12. https://doi.org/10.1097/ACM.0b013e3181ed436c.
159. Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133(1):56-61. https://doi.org/10.1378/chest.07-0131.
160. Evans LV, Dodge KL, Shah TD, et al. Simulation training in central venous catheter insertion: improved performance in clinical practice. Acad Med. 2010;85(9):1462-1469. https://doi.org/10.1097/ACM.0b013e3181eac9a3.
161. Smith CC, Huang GC, Newman LR, et al. Simulation training and its effect on long-term resident performance in central venous catheterization. Simul Healthc J Soc Simul Healthc. 2010;5(3):146-151. https://doi.org/10.1097/SIH.0b013e3181dd9672.
162. Laack TA, Dong Y, Goyal DG, et al. Short-term and long-term impact of the central line workshop on resident clinical performance during simulated central line placement. Simul Healthc J Soc Simul Healthc. 2014;9(4):228-233. https://doi.org/10.1097/SIH.0000000000000015.
163. Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31(10):1519-1526. https://doi.org/10.7863/jum.2012.31.10.1519.
164. Bayci AW, Mangla J, Jenkins CS, Ivascu FA, Robbins JM. Novel educational module for subclavian central venous catheter insertion using real-time ultrasound guidance. J Surg Educ. 2015;72(6):1217-1223. https://doi.org/10.1016/j.jsurg.2015.07.010.
165. Andreatta P, Chen Y, Marsh M, Cho K. Simulation-based training improves applied clinical placement of ultrasound-guided PICCs. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2011;19(4):539-543. https://doi.org/10.1007/s00520-010-0849-2.
166. Rosen BT, Uddin PQ, Harrington AR, Ault BW, Ault MJ. Does personalized vascular access training on a nonhuman tissue model allow for learning and retention of central line placement skills? Phase II of the procedural patient safety initiative (PPSI-II). J Hosp Med. 2009;4(7):423-429. https://doi.org/10.1002/jhm.571.
167. Ault MJ, Rosen BT, Ault B. The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative. J Gen Intern Med. 2006;21(5):514-517. https://doi.org/10.1111/j.1525-1497.2006.00440.x.
168. Varga S, Smith J, Minneti M, et al. Central venous catheterization using a perfused human cadaveric model: application to surgical education. J Surg Educ. 2015;72(1):28-32. https://doi.org/10.1016/j.jsurg.2014.07.005.
169. Sansivero GE. Venous anatomy and physiology. Considerations for vascular access device placement and function. J Intraven Nurs Off Publ Intraven Nurs Soc. 1998;21(5 Suppl):S107-S114.
170. Eisen LA, Narasimhan M, Berger JS, et al. Mechanical complications of central venous catheters. Journal of intensive care medicine. 2006;21(1):40-46. https://doi.org/10.1177/0885066605280884.
171. Chenkin J, Lee S, Huynh T, Bandiera G. Procedures can be learned on the Web: a randomized study of ultrasound-guided vascular access training. Acad Emerg Med. 2008;15(10):949-954. https://doi.org/10.1111/j.1553-2712.2008.00231.x.
172. Abualenain J, Calabrese K, Tansek R, Ranniger C. 319 Comparing standard versus video-based teaching for ultrasound-guided internal jugular central venous catheter access for fourth-year medical students. Ann Emerg Med. 2014;64(4):S113. https://doi.org/10.1016/j.annemergmed.2014.07.347.
173. Pustavoitau A, Blaivas M, Brown SM, et al. Recommendations for achieving and maintaining competence and credentialing in critical care ultrasound with focused cardiac ultrasound and advanced critical care echocardiography. Crit Care Med. 2016.
174. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: A survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
175. Moureau N, Lamperti M, Kelly LJ, et al. Evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training. Br J Anaesth. 2013;110(3):347-356. https://doi.org/10.1093/bja/aes499.
176. Ernst A, Silvestri GA, Johnstone D, American College of Chest Physicians. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123(5):1693-1717. https://doi.org/10.1378/chest.123.5.1693.
177. Thomas SM, Burch W, Kuehnle SE, et al. Simulation training for pediatric residents on central venous catheter placement: a pilot study. Pediatr Crit Care Med J Soc Crit Care Med.. 2013;14(9):e416-e423. https://doi.org/10.1097/PCC.0b013e31829f5eda.
178. Smith KK, Gilcreast D, Pierce K. Evaluation of staff’s retention of ACLS and BLS skills. Resuscitation. 2008;78(1):59-65. https://doi.org/10.1016/j.resuscitation.2008.02.007.
179. Ericsson KA. Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med. 2004;79(10 Suppl):S70-S81. https://doi.org/10.1097/00001888-200410001-00022.
180. Gerard JM, Thomas SM, Germino KW, et al. The effect of simulation training on PALS skills among family medicine residents. Fam Med. 2011;43(6):392-399.
181. Brydges R, Nair P, Ma I, Shanks D, Hatala R. Directed self-regulated learning versus instructor-regulated learning in simulation training. Med Educ. 2012;46(7):648-656. https://doi.org/10.1111/j.1365-2923.2012.04268.x.
182. Wayne DB, Butter J, Siddall VJ, et al. Simulation-based training of internal medicine residents in advanced cardiac life support protocols: a randomized trial. Teach Learn Med. 2005;17(3):210-216. https://doi.org/10.1207/s15328015tlm1703_3.
183. Arthur Jr. W, Bennett Jr. W, Stanush PL, McNelly TL. Factors that influence skill decay and retention: A quantitative review and analysis. Hum Perform. 1998;11(1):57-101. https://doi.org/10.1207/s15327043hup1101_3.
184. Rusche JD, Besuner P, Partusch SK, Berning PA. Competency program development across a merged healthcare network. J Nurs Staff Dev. 2001;17(5):234-240; quiz 241-232. https://doi.org/10.1097/00124645-200109000-00004.
185. O’Hearne Rebholz M. A review of methods to assess competency. J Nurs Staff Dev. 2006;22(5):241-245. https://doi.org/10.1097/00124645-200609000-00007.
186. Reznick RK. Teaching and testing technical skills. Am J Surg. 1993;165(3):358-361. https://doi.org/10.1016/s0002-9610(05)80843-8.
187. Reznick RK, MacRae H. Teaching surgical skills--changes in the wind. N Engl J Med. 2006;355(25):2664-2669. https://doi.org/10.1056/NEJMra054785.
188. Murin S, Stollenwerk NS. Simulation in procedural training: at the tipping point. Chest. 2010;137(5):1009-1011. https://doi.org/10.1378/chest.10-0199.
189. American College of Emergency Physicians. Emergency ultrasound guidelines. Ann Emerg Med. 2009;53(4):550-570. https://doi.org/10.1016/j.annemergmed.2008.12.013.
190. Guilbert MC, Elkouri S, Bracco D, et al. Arterial trauma during central venous catheter insertion: case series, review and proposed algorithm. J Vasc Surg. 2008;48(4):918-925; discussion 925. https://doi.org/10.1016/j.jvs2008.04.046.
191. Dong Y, Suri HS, Cook DA, et al. Simulation-based objective assessment discerns clinical proficiency in central line placement: a construct validation. Chest. 2010;137(5):1050-1056. https://doi.org/10.1378/chest.09-1451.
192. Adhikari S, Theodoro D, Raio C, et al. Central venous catheterization: are we using ultrasound guidance? J Ultrasound Med. 2015;34(11):2065-2070. https://doi.org/10.7863/ultra.15.01027.
193. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors--procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z.
Approximately five million central venous catheters (CVCs) are inserted in the United States annually, with over 15 million catheter days documented in intensive care units alone.1 Traditional CVC insertion techniques using landmarks are associated with a high risk of mechanical complications, particularly pneumothorax and arterial puncture, which occur in 5%-19% patients.2,3
Since the 1990s, several randomized controlled studies and meta-analyses have demonstrated that the use of real-time ultrasound guidance for CVC insertion increases procedure success rates and decreases mechanical complications.4,5 Use of real-time ultrasound guidance was recommended by the Agency for Healthcare Research and Quality, the Institute of Medicine, the National Institute for Health and Care Excellence, the Centers for Disease Control and Prevention, and several medical specialty societies in the early 2000s.6-14 Despite these recommendations, ultrasound guidance has not been universally adopted. Currently, an estimated 20%-55% of CVC insertions in the internal jugular vein are performed without ultrasound guidance.15-17
Following the emergence of literature supporting the use of ultrasound guidance for CVC insertion, observational and randomized controlled studies demonstrated improved procedural success rates with the use of ultrasound guidance for the insertion of peripheral intravenous lines (PIVs), arterial catheters, and peripherally inserted central catheters (PICCs).18-23
The purpose of this position statement is to present evidence-based recommendations on the use of ultrasound guidance for the insertion of central and peripheral vascular access catheters in adult patients. This document presents consensus-based recommendations with supporting evidence for clinical outcomes, techniques, and training for the use of ultrasound guidance for vascular access. We have subdivided the recommendations on techniques for central venous access, peripheral venous access, and arterial access individually, as some providers may not perform all types of vascular access procedures.
These recommendations are intended for hospitalists and other healthcare providers that routinely place central and peripheral vascular access catheters in acutely ill patients. However, this position statement does not mandate that all hospitalists should place central or peripheral vascular access catheters given the diverse array of hospitalist practice settings. For training and competency assessments, we recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals, where equipment and staffing for assessments are not available. Recommendations and frameworks for initial and ongoing credentialing of hospitalists in ultrasound-guided bedside procedures have been previously published in an Society of Hospital Medicine (SHM) position statement titled, “Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.”24
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interest (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the vascular access working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. An updated search was conducted in November 2017. The literature search strings are included in Appendix 3. All article abstracts were initially screened for relevance by at least two members of the vascular access working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide vascular access were selected. The following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled studies, and observational studies of ultrasound-guided vascular access were screened and selected (Appendix 3, Figure 1). All full-text articles were shared electronically among the working group members, and final article selection was based on working group consensus. Selected articles were incorporated into the draft recommendations.
These recommendations were developed using the Research and Development (RAND) Appropriateness Method that required panel judgment and consensus.14 The 28 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Using an internet-based electronic data collection tool (REDCap™), panel members participated in two rounds of electronic voting, one in August 2018 and the other in October 2018 (Appendix 4). Voting on appropriateness was conducted using a nine-point Likert scale. The three zones of the nine-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix 1, Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” Disagreement was defined as >30% of panelists voting outside of the zone of the median. A strong recommendation required at least 80% of the votes within one integer of the median per the RAND rules.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording for each recommendation (Table 2). The final version of the consensus-based recommendations underwent internal and external review by members of the SHM POCUS Task Force, the SHM Education Committee, and the SHM Executive Committee. The SHM Executive Committee reviewed and approved this position statement prior to its publication in the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 5,563 references were pooled from an initial search performed by a certified medical librarian in December 2015 (4,668 citations) which was updated in November 2017 (791 citations), and from the personal bibliographies and searches (104 citations) performed by working group members. A total of 514 full-text articles were reviewed. The final selection included 192 articles that were abstracted into a data table and incorporated into the draft recommendations. See Appendix 3 for details of the literature search strategy.
Recommendations
Four domains (technique, clinical outcomes, training, and knowledge gaps) with 31 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation cite supporting evidence. After two rounds of panel voting, 31 recommendations achieved agreement based on the RAND rules. During the peer review process, two of the recommendations were merged with other recommendations. Thus, a total of 29 recommendations received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Twenty-seven statements were approved as strong recommendations, and two were approved as weak/conditional recommendations. The strength of each recommendation and degree of consensus are summarized in Table 3.
Terminology
Central Venous Catheterization
Central venous catheterization refers to insertion of tunneled or nontunneled large bore vascular catheters that are most commonly inserted into the internal jugular, subclavian, or femoral veins with the catheter tip located in a central vein. These vascular access catheters are synonymously referred to as central lines or central venous catheters (CVCs). Nontunneled catheters are designed for short-term use and should be removed promptly when no longer clinically indicated or after a maximum of 14 days.25
Peripherally Inserted Central Catheter (PICC)
Peripherally inserted central catheters, or PICC lines, are inserted most commonly in the basilic or brachial veins in adult patients, and the catheter tip terminates in the distal superior vena cava or cavo-atrial junction. These catheters are designed to remain in place for a duration of several weeks, as long as it is clinically indicated.
Midline Catheterization
Midline catheters are a type of peripheral venous catheter that are an intermediary between a peripheral intravenous catheter and PICC line. Midline catheters are most commonly inserted in the brachial or basilic veins, but unlike PICC lines, the tips of these catheters terminate in the axillary or subclavian vein. Midline catheters are typically 8 cm to 20 cm in length and inserted for a duration <30 days.
Peripheral Intravenous Catheterization
Peripheral intravenous lines (PIV) refer to small bore venous catheters that are most commonly 14G to 24G and inserted into patients for short-term peripheral venous access. Common sites of ultrasound-guided PIV insertion include the superficial and deep veins of the hand, forearm, and arm.
Arterial Catheterization
Arterial catheters are commonly used for reliable blood pressure monitoring, frequent arterial blood
RECOMMENDATIONS
Preprocedure
1. We recommend that providers should be familiar with the operation of their specific ultrasound machine prior to initiation of a vascular access procedure.
Rationale: There is strong consensus that providers must be familiar with the knobs and functions of the specific make and model of ultrasound machine that will be utilized for a vascular access procedure. Minimizing adjustments to the ultrasound machine during the procedure may reduce the risk of contaminating the sterile field.
2. We recommend that providers should use a high-frequency linear transducer with a sterile sheath and sterile gel to perform vascular access procedures.
Rationale: High-frequency linear-array transducers are recommended for the vast majority of vascular access procedures due to their superior resolution compared to other transducer types. Both central and peripheral vascular access procedures, including PIV, PICC, and arterial line placement, should be performed using sterile technique. A sterile transducer cover and sterile gel must be utilized, and providers must be trained in sterile preparation of the ultrasound transducer.13,26,27
The depth of femoral vessels correlates with body mass index (BMI). When accessing these vessels in a morbidly obese patient with a thigh circumference >60 cm and vessel depth >8 cm, a curvilinear transducer may be preferred for its deeper penetration.28 For patients who are poor candidates for bedside insertion of vascular access catheters, such as uncooperative patients, patients with atypical vascular anatomy or poorly visualized target vessels, we recommend consultation with a vascular access specialist prior to attempting the procedure.
3. We recommend that providers should use two-dimensional ultrasound to evaluate for anatomical variations and absence of vascular thrombosis during preprocedural site selection.
Rationale: A thorough ultrasound examination of the target vessel is warranted prior to catheter placement. Anatomical variations that may affect procedural decision-making are easily detected with ultrasound. A focused vascular ultrasound examination is particularly important in patients who have had temporary or tunneled venous catheters, which can cause stenosis or thrombosis of the target vein.
For internal jugular vein (IJV) CVCs, ultrasound is useful for visualizing the relationship between the IJV and common carotid artery (CCA), particularly in terms of vessel overlap. Furthermore, ultrasound allows for immediate revisualization upon changes in head position.29-32 Troianos et al. found >75% overlap of the IJV and CCA in 54% of all patients and in 64% of older patients (age >60 years) whose heads were rotated to the contralateral side.30 In one study of IJV CVC insertion, inadvertent carotid artery punctures were reduced (3% vs 10%) with the use of ultrasound guidance vs landmarks alone.33 In a cohort of 64 high-risk neurosurgical patients, cannulation success was 100% with the use of ultrasound guidance, and there were no injuries to the carotid artery, even though the procedure was performed with a 30-degree head elevation and anomalous IJV anatomy in 39% of patients.34 In a prospective, randomized controlled study of 1,332 patients, ultrasound-guided cannulation in a neutral position was demonstrated to be as safe as the 45-degree rotated position.35
Ultrasound allows for the recognition of anatomical variations which may influence the selection of the vascular access site or technique. Benter et al. found that 36% of patients showed anatomical variations in the IJV and surrounding tissue.36 Similarly Caridi showed the anatomy of the right IJV to be atypical in 29% of patients,37 and Brusasco found that 37% of bariatric patients had anatomical variations of the IJV.38 In a study of 58 patients, there was significant variability in the IJV position and IJV diameter, ranging from 0.5 cm to >2 cm.39 In a study of hemodialysis patients, 75% of patients had sonographic venous abnormalities that led to a change in venous access approach.40
To detect acute or chronic upper extremity deep venous thrombosis or stenosis, two-dimensional visualization with compression should be part of the ultrasound examination prior to central venous catheterization. In a study of patients that had undergone CVC insertion 9-19 weeks earlier, 50% of patients had an IJV thrombosis or stenosis leading to selection of an alternative site. In this study, use of ultrasound for a preprocedural site evaluation reduced unnecessary attempts at catheterizing an occluded vein.41 At least two other studies demonstrated an appreciable likelihood of thrombosis. In a study of bariatric patients, 8% of patients had asymptomatic thrombosis38 and in another study, 9% of patients being evaluated for hemodialysis catheter placement had asymptomatic IJV thrombosis.37
4. We recommend that providers should evaluate the target blood vessel size and depth during a preprocedural ultrasound evaluation.
Rationale: The size, depth, and anatomic location of central veins can vary considerably. These features are easily discernable using ultrasound. Contrary to traditional teaching, the IJV is located 1 cm anterolateral to the CCA in only about two-thirds of patients.37,39,42,43 Furthermore, the diameter of the IJV can vary significantly, ranging from 0.5 cm to >2 cm.39 The laterality of blood vessels may vary considerably as well. A preprocedural ultrasound evaluation of contralateral subclavian and axillary veins showed a significant absolute difference in cross-sectional area of 26.7 mm2 (P < .001).42
Blood vessels can also shift considerably when a patient is in the Trendelenburg position. In one study, the IJV diameter changed from 11.2 (± 1.5) mm to 15.4 (± 1.5) mm in the supine versus the Trendelenburg position at 15 degrees.33 An observational study demonstrated a frog-legged position with reverse Trendelenburg increased the femoral vein size and reduced the common surface area with the common femoral artery compared to a neutral position. Thus, a frog-legged position with reverse Trendelenburg position may be preferred, since overall catheterization success rates are higher in this position.44
Techniques
General Techniques
5. We recommend that providers should avoid using static ultrasound alone to mark the needle insertion site for vascular access procedures.
Rationale: The use of static ultrasound guidance to mark a needle insertion site is not recommended because normal anatomical relationships of vessels vary, and site marking can be inaccurate with minimal changes in patient position, especially of the neck.43,45,46 Benefits of using ultrasound guidance for vascular access are attained when ultrasound is used to track the needle tip in real-time as it is advanced toward the target vessel.
Although continuous-wave Doppler ultrasound without two-dimensional visualization was used in the past, it is no longer recommended for IJV CVC insertion.47 In a study that randomized patients to IJV CVC insertion with continuous-wave Doppler alone vs two-dimensional ultrasound guidance, the use of two-dimensional ultrasound guidance showed significant improvement in first-pass success rates (97% vs 91%, P = .045), particularly in patients with BMI >30 (97% vs 77%, P = .011).48
A randomized study comparing real-time ultrasound-guided, landmark-based, and ultrasound-marked techniques found higher success rates in the real-time ultrasound-guided group than the other two groups (100% vs 74% vs 73%, respectively; P = .01). The total number of mechanical complications was higher in the landmark-based and ultrasound-marked groups than in the real-time ultrasound-guided group (24% and 36% versus 0%, respectively; P = .01).49 Another randomized controlled study found higher success rates with real-time ultrasound guidance (98%) versus an ultrasound-marked (82%) or landmark-based (64%) approach for central line placement.50
6. We recommend that providers should use real-time (dynamic), two-dimensional ultrasound guidance with a high-frequency linear transducer for CVC insertion, regardless of the provider’s level of experience.
7. We suggest using either a transverse (short-axis) or longitudinal (long-axis) approach when performing real-time ultrasound-guided vascular access procedures.
Rationale: In clinical practice, the phrases transverse, short-axis, or out-of-plane approach are synonymous, as are longitudinal, long-axis, and in-plane approach. The short-axis approach involves tracking the needle tip as it approximates the target vessel with the ultrasound beam oriented in a transverse plane perpendicular to the target vessel. The target vessel is seen as a circular structure on the ultrasound screen as the needle tip approaches the target vessel from above. This approach is also called the out-of-plane technique since the needle passes through the ultrasound plane. The advantages of the short-axis approach include better visualization of adjacent vessels or nerves and the relative ease of skill acquisition for novice operators.9 When using the short-axis approach, extra care must be taken to track the needle tip from the point of insertion on the skin to the target vessel. A disadvantage of the short-axis approach is unintended posterior wall puncture of the target vessel.55
In contrast to a short-axis approach, a long-axis approach is performed with the ultrasound beam aligned parallel to the vessel. The vessel appears as a long tubular structure and the entire needle is visualized as it traverses across the ultrasound screen to approach the target vessel. The long-axis approach is also called an in-plane technique because the needle is maintained within the plane of the ultrasound beam. The advantage of a long-axis approach is the ability to visualize the entire needle as it is inserted into the vessel.14 A randomized crossover study with simulation models compared a long-axis versus short-axis approach for both IJV and subclavian vein catheterization. This study showed decreased number of needle redirections (relative risk (RR) 0.5, 95% confidence interval (CI) 0.3 to 0.7), and posterior wall penetrations (OR 0.3, 95% CI 0.1 to 0.9) using a long-axis versus short-axis approach for subclavian vein catheterization.56
A randomized controlled study comparing a long-axis or short-axis approach with ultrasound versus a landmark-based approach for IJV CVC insertion showed higher success rates (100% vs 90%; P < .001), lower insertion time (53 vs 116 seconds; P < .001), and fewer attempts to obtain access (2.5 vs 1.2 attempts, P < .001) with either the long- or short-axis ultrasound approach. The average time to obtain access and number of attempts were comparable between the short-axis and long-axis approaches with ultrasound. The incidence of carotid puncture and hematoma was significantly higher with the landmark-based approach versus either the long- or short-axis ultrasound approach (carotid puncture 17% vs 3%, P = .024; hematoma 23% vs 3%, P = .003).57
High success rates have been reported using a short-axis approach for insertion of PIV lines.58 A prospective, randomized trial compared the short-axis and long-axis approach in patients who had had ≥2 failed PIV insertion attempts. Success rate was 95% (95% CI, 0.85 to 1.00) in the short-axis group compared with 85% (95% CI, 0.69 to 1.00) in the long-axis group. All three subjects with failed PIV placement in the long-axis group had successful rescue placement using a short-axis approach. Furthermore, the short-axis approach was faster than the long-axis approach.59
For radial artery cannulation, limited data exist comparing the short- and long-axis approaches. A randomized controlled study compared a long-axis vs short-axis ultrasound approach for radial artery cannulation. Although the overall procedure success rate was 100% in both groups, the long-axis approach had higher first-pass success rates (1.27 ± 0.4 vs 1.5 ± 0.5, P < .05), shorter cannulation times (24 ± 17 vs 47 ± 34 seconds, P < .05), fewer hematomas (4% vs 43%, P < .05) and fewer posterior wall penetrations (20% vs 56%, P < .05).60
Another technique that has been described for IJV CVC insertion is an oblique-axis approach, a hybrid between the long- and short-axis approaches. In this approach, the transducer is aligned obliquely over the IJV and the needle is inserted using a long-axis or in-plane approach. A prospective randomized trial compared the short-axis, long-axis, and oblique-axis approaches during IJV cannulation. First-pass success rates were 70%, 52%, and 74% with the short-axis, long-axis, and oblique-axis approaches, respectively, and a statistically significant difference was found between the long- and oblique-axis approaches (P = .002). A higher rate of posterior wall puncture was observed with a short-axis approach (15%) compared with the oblique-axis (7%) and long-axis (4%) approaches (P = .047).61
8. We recommend that providers should visualize the needle tip and guidewire in the target vein prior to vessel dilatation.
Rationale: When real-time ultrasound guidance is used, visualization of the needle tip within the vein is the first step to confirm cannulation of the vein and not the artery. After the guidewire is advanced, the provider can use transverse and longitudinal views to reconfirm cannulation of the vein. In a longitudinal view, the guidewire is readily seen positioned within the vein, entering the anterior wall and lying along the posterior wall of the vein. Unintentional perforation of the posterior wall of the vein with entry into the underlying artery can be detected by ultrasound, allowing prompt removal of the needle and guidewire before proceeding with dilation of the vessel. In a prospective observational study that reviewed ultrasound-guided IJV CVC insertions, physicians were able to more readily visualize the guidewire than the needle in the vein.62 A prospective observational study determined that novice operators can visualize intravascular guidewires in simulation models with an overall accuracy of 97%.63
In a retrospective review of CVC insertions where the guidewire position was routinely confirmed in the target vessel prior to dilation, there were no cases of arterial dilation, suggesting confirmation of guidewire position can potentially eliminate the morbidity and mortality associated with arterial dilation during CVC insertion.64
9. To increase the success rate of ultrasound-guided vascular access procedures, we recommend that providers should utilize echogenic needles, plastic needle guides, and/or ultrasound beam steering when available.
Rationale: Echogenic needles have ridged tips that appear brighter on the screen, allowing for better visualization of the needle tip. Plastic needle guides help stabilize the needle alongside the transducer when using either a transverse or longitudinal approach. Although evidence is limited, some studies have reported higher procedural success rates when using echogenic needles, plastic needle guides, and ultrasound beam steering software. In a prospective observational study, Augustides et al. showed significantly higher IJV cannulation rates with versus without use of a needle guide after first (81% vs 69%, P = .0054) and second (93% vs 80%. P = .0001) needle passes.65 A randomized study by Maecken et al. compared subclavian vein CVC insertion with or without use of a needle guide, and found higher procedure success rates within the first and second attempts, reduced time to obtain access (16 seconds vs 30 seconds; P = .0001) and increased needle visibility (86% vs 32%; P < .0001) with the use of a needle guide.66 Another study comparing a short-axis versus long-axis approach with a needle guide showed improved needle visualization using a long-axis approach with a needle guide.67 A randomized study comparing use of a novel, sled-mounted needle guide to a free-hand approach for venous cannulation in simulation models showed the novel, sled-mounted needle guide improved overall success rates and efficiency of cannulation.68
Central Venous Access Techniques
10. We recommend that providers should use a standardized procedure checklist that includes use of real-time ultrasound guidance to reduce the risk of central line-associated bloodstream infection (CLABSI) from CVC insertion.
Rationale: A standardized checklist or protocol should be developed to ensure compliance with all recommendations for insertion of CVCs. Evidence-based protocols address periprocedural issues, such as indications for CVC, and procedural techniques, such as use of maximal sterile barrier precautions to reduce the risk of infection. Protocols and checklists that follow established guidelines for CVC insertion have been shown to decrease CLABSI rates.69,70 Similarly, development of checklists and protocols for maintenance of central venous catheters have been effective in reducing CLABSIs.71 Although no externally-validated checklist has been universally accepted or endorsed by national safety organizations, a few internally-validated checklists are available through peer-reviewed publications.72,73 An observational educational cohort of internal medicine residents who received training using simulation of the entire CVC insertion process was able to demonstrate fewer CLABSIs after the simulator-trained residents rotated in the intensive care unit (ICU) (0.50 vs 3.2 infections per 1,000 catheter days, P = .001).74
11. We recommend that providers should use real-time ultrasound guidance, combined with aseptic technique and maximal sterile barrier precautions, to reduce the incidence of infectious complications from CVC insertion.
Rationale: The use of real-time ultrasound guidance for CVC placement has demonstrated a statistically significant reduction in CLABSIs compared to landmark-based techniques.75 The Centers for Disease Control and Prevention (CDC) guidelines for the prevention of intravascular catheter-related infections recommend the use of ultrasound guidance to reduce the number of cannulation attempts and risk of mechanical complications.69 A prospective, three-arm study comparing ultrasound-guided long-axis, short-axis, and landmark-based approaches showed a CLABSI rate of 20% in the landmark-based group versus 10% in each of the ultrasound groups.57 Another randomized study comparing use of ultrasound guidance to a landmark-based technique for IJV CVC insertion demonstrated significantly lower CLABSI rates with the use of ultrasound (2% vs 10%; P < .05).72
Studies have shown that a systems-based intervention featuring a standardized catheter kit or catheter bundle significantly reduced CLABSI rates.76-78 A complete review of all preventive measures to reduce the risk of CLABSI is beyond the scope of this review, but a few key points will be mentioned. First, aseptic technique includes proper hand hygiene and skin sterilization, which are essential measures to reduce cutaneous colonization of the insertion site and reduce the risk of CLABSIs.79 In a systematic review and meta-analysis of eight studies including over 4,000 catheter insertions, skin antisepsis with chlorhexidine was associated with a 50% reduction in CLABSIs compared with povidone iodine.11 Therefore, a chlorhexidine-containing solution is recommended for skin preparation prior to CVC insertion per guidelines by Healthcare Infection Control Practices Advisory Committee/CDC, Society for Healthcare Epidemiology of America/Infectious Diseases Society of America, and American Society of Anesthesiologists.11,69,80,81 Second, maximal sterile barrier precautions refer to the use of sterile gowns, sterile gloves, caps, masks covering both the mouth and nose, and sterile full-body patient drapes. Use of maximal sterile barrier precautions during CVC insertion has been shown to reduce the incidence of CLABSIs compared to standard precautions.26,79,82-84 Third, catheters containing antimicrobial agents may be considered for hospital units with higher CLABSI rates than institutional goals, despite a comprehensive preventive strategy, and may be considered in specific patient populations at high risk of severe complications from a CLABSI.11,69,80 Finally, providers should use a standardized procedure set-up when inserting CVCs to reduce the risk of CLABSIs. The operator should confirm availability and proper functioning of ultrasound equipment prior to commencing a vascular access procedure. Use of all-inclusive procedure carts or kits with sterile ultrasound probe covers, sterile gel, catheter kits, and other necessary supplies is recommended to minimize interruptions during the procedure, and can ultimately reduce the risk of CLABSIs by ensuring maintenance of a sterile field during the procedure.13
12. We recommend that providers should use real-time ultrasound guidance for internal jugular vein catheterization, which reduces the risk of mechanical and infectious complications, the number of needle passes, and time to cannulation and increases overall procedure success rates.
Rationale: The use of real-time ultrasound guidance for CVC insertion has repeatedly demonstrated better outcomes compared to a landmark-based approach in adults.13 Several randomized controlled studies have demonstrated that real-time ultrasound guidance for IJV cannulation reduces the risk of procedure-related mechanical and infectious complications, and improves first-pass and overall success rates in diverse care settings.27,29,45,50,53,65,75,85-90 Mechanical complications that are reduced with ultrasound guidance include pneumothorax and carotid artery puncture.4,5,45,46,53,62,75,86-93 Currently, several medical societies strongly recommend the use of ultrasound guidance during insertion of IJV CVCs.10-12,14,94-96
A meta-analysis by Hind et al. that included 18 randomized controlled studies demonstrated use of real-time ultrasound guidance reduced failure rates (RR 0.14, 95% CI 0.06 to 0.33; P < .0001), increased first-attempt success rates (RR 0.59, 95% CI 0.39 to 0.88; P = .009), reduced complication rates (RR 0.43, 95% CI 0.22 to 0.87; P = .02) and reduced procedure time (P < .0001), compared to a traditional landmark-based approach when inserting IJV CVCs.5
A Cochrane systematic review compared ultrasound-guided versus landmark-based approaches for IJV CVC insertion and found use of real-time ultrasound guidance reduced total complication rates by 71% (RR 0.29, 95% CI 0.17 to 0.52; P < .0001), arterial puncture rates by 72% (RR 0.28, 95% CI 0.18 to 0.44; P < .00001), and rates of hematoma formation by 73% (RR 0.27, 95% CI 0.13 to 0.55; P = .0004). Furthermore, the number of attempts for successful cannulation was reduced (mean difference -1.19 attempts, 95% CI -1.45 to -0.92; P < .00001), the chance of successful insertion on the first attempt was increased by 57% (RR 1.57, 95% CI 1.36 to 1.82; P < .00001), and overall procedure success rates were modestly increased in all groups by 12% (RR 1.12, 95% CI 1.08 to 1.17; P < .00001).46
An important consideration in performing ultrasound guidance is provider experience. A prospective observational study of patients undergoing elective CVC insertion demonstrated higher complication rates for operators that were inexperienced (25.2%) versus experienced (13.6%).54 A randomized controlled study comparing experts and novices with or without the use of ultrasound guidance for IJV CVC insertion demonstrated higher success rates among expert operators and with the use of ultrasound guidance. Among novice operators, the complication rates were lower with the use of ultrasound guidance.97 One study evaluated the procedural success and complication rates of a two-physician technique with one physician manipulating the transducer and another inserting the needle for IJV CVC insertion. This study concluded that procedural success rates and frequency of complications were directly affected by the experience of the physician manipulating the transducer and not by the experience of the physician inserting the needle.98
The impact of ultrasound guidance on improving procedural success rates and reducing complication rates is greatest in patients that are obese, short necked, hypovolemic, or uncooperative.93 Several studies have demonstrated fewer needle passes and decreased time to cannulation compared to the landmark technique in these populations.46,49,53,86-88,92,93
Ultrasound-guided placement of IJV catheters can safely be performed in patients with disorders of hemostasis and those with multiple previous catheter insertions in the same vein.9 Ultrasound-guided placement of CVCs in patients with disorders of hemostasis is safe with high success and low complication rates. In a case series of liver patients with coagulopathy (mean INR 2.17 ± 1.16, median platelet count 150K), the use of ultrasound guidance for CVC insertion was highly successful with no major bleeding complications.99
A study of renal failure patients found high success rates and low complication rates in the patients with a history of multiple previous catheterizations, poor compliance, skeletal deformities, previous failed cannulations, morbid obesity, and disorders of hemostasis.100 A prospective observational study of 200 ultrasound-guided CVC insertions for apheresis showed a 100% success rate with a 92% first-pass success rate.101
The use of real-time ultrasound guidance for IJV CVC insertion has been shown to be cost effective by reducing procedure-related mechanical complications and improving procedural success rates. A companion cost-effectiveness analysis estimated that for every 1,000 patients, 90 complications would be avoided, with a net cost savings of approximately $3,200 using 2002 prices.102
13. We recommend that providers who routinely insert subclavian vein CVCs should use real-time ultrasound guidance, which has been shown to reduce the risk of mechanical complications and number of needle passes and increase overall procedure success rates compared with landmark-based techniques.
Rationale: In clinical practice, the term ultrasound-guided subclavian vein CVC insertion is commonly used. However, the needle insertion site is often lateral to the first rib and providers are technically inserting the CVC in the axillary vein. The subclavian vein becomes the axillary vein at the lateral border of the first rib where the cephalic vein branches from the subclavian vein. To be consistent with common medical parlance, we use the phrase ultrasound-guided subclavian vein CVC insertion in this document.
Advantages of inserting CVCs in the subclavian vein include reliable surface anatomical landmarks for vein location, patient comfort, and lower risk of infection.103 Several observational studies have demonstrated the technique for ultrasound-guided subclavian vein CVC insertion is feasible and safe.104-107 In a large retrospective observational study of ultrasound-guided central venous access among a complex patient group, the majority of patients were cannulated successfully and safely. The subset of patients undergoing axillary vein CVC insertion (n = 1,923) demonstrated a low rate of complications (0.7%), proving it is a safe and effective alternative to the IJV CVC insertion.107
A Cochrane review of ultrasound-guided subclavian vein cannulation (nine studies, 2,030 participants, 2,049 procedures), demonstrated that real-time two-dimensional ultrasound guidance reduced the risk of inadvertent arterial punctures (three studies, 498 participants, RR 0.21, 95% CI 0.06 to 0.82; P = .02) and hematoma formation (three studies, 498 participants, RR 0.26, 95% CI 0.09 to 0.76; P = .01).46 A systematic review and meta-analysis of 10 randomized controlled studies comparing ultrasound-guided versus landmark-based subclavian vein CVC insertion demonstrated a reduction in the risk of arterial punctures, hematoma formation, pneumothorax, and failed catheterization with the use of ultrasound guidance.105
A randomized controlled study comparing ultrasound-guided vs landmark-based approaches to subclavian vein cannulation found that use of ultrasound guidance had a higher success rate (92% vs 44%, P = .0003), fewer minor complications (1 vs 11, P = .002), fewer attempts (1.4 vs 2.5, P = .007) and fewer catheter kits used (1.0 vs 1.4, P = .0003) per cannulation.108
Fragou et al. randomized patients undergoing subclavian vein CVC insertion to a long-axis approach versus a landmark-based approach and found a significantly higher success rate (100% vs 87.5%, P < .05) and lower rates of mechanical complications: artery puncture (0.5% vs 5.4%), hematoma (1.5% vs 5.4%), hemothorax (0% vs 4.4%), pneumothorax (0% vs 4.9%), brachial plexus injury (0% vs 2.9%), phrenic nerve injury (0% vs 1.5%), and cardiac tamponade (0% vs 0.5%).109 The average time to obtain access and the average number of insertion attempts (1.1 ± 0.3 vs 1.9 ± 0.7, P < .05) were significantly reduced in the ultrasound group compared to the landmark-based group.95
A retrospective review of subclavian vein CVC insertions using a supraclavicular approach found no reported complications with the use of ultrasound guidance vs 23 mechanical complications (8 pneumothorax, 15 arterial punctures) with a landmark-based approach.106 However, it is important to note that a supraclavicular approach is not commonly used in clinical practice.
14. We recommend that providers should use real-time ultrasound guidance for femoral venous access, which has been shown to reduce the risk of arterial punctures and total procedure time and increase overall procedure success rates.
Rationale: Anatomy of the femoral region varies, and close proximity or overlap of the femoral vein and artery is common.51 Early studies showed that ultrasound guidance for femoral vein CVC insertion reduced arterial punctures compared with a landmark-based approach (7% vs 16%), reduced total procedure time (55 ± 19 vs 79 ± 62 seconds), and increased procedure success rates (100% vs 90%).52 A Cochrane review that pooled data from four randomized studies comparing ultrasound-guided vs landmark-based femoral vein CVC insertion found higher first-attempt success rates with the use of ultrasound guidance (RR 1.73, 95% CI 1.34 to 2.22; P < .0001) and a small increase in the overall procedure success rates (RR 1.11, 95% CI 1.00 to 1.23; P = .06). There was no difference in inadvertent arterial punctures or other complications.110
Peripheral Venous Access Techniques
15. We recommend that providers should use real-time ultrasound guidance for the insertion of peripherally inserted central catheters (PICCs), which is associated with higher procedure success rates and may be more cost effective compared with landmark-based techniques.
Rationale: Several studies have demonstrated that providers who use ultrasound guidance vs landmarks for PICC insertion have higher procedural success rates, lower complication rates, and lower total placement costs. A prospective observational report of 350 PICC insertions using ultrasound guidance reported a 99% success rate with an average of 1.2 punctures per insertion and lower total costs.20 A retrospective observational study of 500 PICC insertions by designated specialty nurses revealed an overall success rate of 95%, no evidence of phlebitis, and only one CLABSI among the catheters removed.21 A retrospective observational study comparing several PICC variables found higher success rates (99% vs 77%) and lower thrombosis rates (2% vs 9%) using ultrasound guidance vs landmarks alone.22 A study by Robinson et al. demonstrated that having a dedicated PICC team equipped with ultrasound increased their institutional insertion success rates from 73% to 94%.111
A randomized controlled study comparing ultrasound-guided versus landmark-based PICC insertion found high success rates with both techniques (100% vs 96%). However, there was a reduction in the rate of unplanned catheter removals (4.0% vs 18.7%; P = .02), mechanical phlebitis (0% vs 22.9%; P < .001), and venous thrombosis (0% vs 8.3%; P = .037), but a higher rate of catheter migration (32% vs 2.1%; P < .001). Compared with the landmark-based group, the ultrasound-guided group had significantly lower incidence of severe contact dermatitis (P = .038), and improved comfort and costs up to 3 months after PICC placement (P < .05).112
Routine postprocedure chest x-ray (CXR) is generally considered unnecessary if the PICC is inserted with real-time ultrasound guidance along with use of a newer tracking devices, like the magnetic navigation system with intracardiac electrodes.9 Ultrasound can also be used to detect malpositioning of a PICC immediately after completing the procedure. A randomized controlled study comparing ultrasound versus postprocedure CXR detected one malpositioned PICC in the ultrasound group versus 11 in the control group. This study suggested that ultrasound can detect malpositioning immediately postprocedure and reduce the need for a CXR and the possibility of an additional procedure to reposition a catheter.113
16. We recommend that providers should use real-time ultrasound guidance for the placement of peripheral intravenous lines (PIV) in patients with difficult peripheral venous access to reduce the total procedure time, needle insertion attempts, and needle redirections. Ultrasound-guided PIV insertion is also an effective alternative to CVC insertion in patients with difficult venous access.
Rationale: Difficult venous access refers to patients that have had two unsuccessful attempts at PIV insertion using landmarks or a history of difficult access (i.e. edema, obesity, intravenous drug use, chemotherapy, diabetes, hypovolemia, chronic illness, vasculopathy, multiple prior hospitalizations). A meta-analysis of seven randomized controlled studies concluded that ultrasound guidance increases the likelihood of successful PIV insertion (pooled OR 2.42, 95% CI 1.26 to 4.68; P < .008).18 A second meta-analysis that pooled data from seven studies (six randomized controlled studies) confirmed that ultrasound guidance improves success rates of PIV insertion (OR 3.96, 95% CI 1.75 to 8.94).19 Approximately half of these studies had physician operators while the other half had nurse operators.
In one prospective observational study of emergency department patients with two failed attempts of landmark-based PIV insertion, ultrasound guidance with a modified-Seldinger technique showed a relatively high success rate (96%), fewer needle sticks (mean 1.32 sticks, 95% CI 1.12 to 1.52), and shorter time to obtain access (median time 68 seconds).114 Other prospective observational studies have demonstrated that ultrasound guidance for PIV insertion has a high success rate (87%),115 particularly with brachial or basilic veins PIV insertion, among patients with difficult PIV access, defined as having had ≥2 failed attempts.58
Since insertion of PIVs with ultrasound guidance has a high success rate, there is potential to reduce the reliance on CVC insertion for venous access only. In a study of patients that had had two failed attempts at PIV insertion based on landmarks, a PIV was successfully inserted with ultrasound guidance in 84% of patients, obviating the need for CVC placement for venous access.116 A prospective observational study showed ultrasound-guided PIV insertion was an effective alternative to CVC placement in ED patients with difficult venous access with only 1% of patients requiring a CVC.117 Use of ultrasound by nurses for PIV placement has also been shown to reduce the time to obtain venous access, improve patient satisfaction, and reduce the need for physician intervention.118 In a prospective observational study of patients with difficult access, the majority of patients reported a better experience with ultrasound-guided PIV insertion compared to previous landmark-based attempts with an average satisfaction score of 9.2/10 with 76% of patients rating the experience a 10.119 A strong recommendation has been made for use of ultrasound guidance in patients with difficult PIV placement by la Société Française d’Anesthésie et de Réanimation (The French Society of Anesthesia and Resuscitation).95
17. We suggest using real-time ultrasound guidance to reduce the risk of vascular, infectious, and neurological complications during PIV insertion, particularly in patients with difficult venous access.
Rationale: The incidence of complications from PIV insertion is often underestimated. Vascular complications include arterial puncture, hematoma formation, local infiltration or extravasation of fluid, and superficial or deep venous thrombosis. The most common infectious complications with PIV insertion are phlebitis and cellulitis.120 One observational study reported PIV complications occurring in approximately half of all patients with the most common complications being phlebitis, hematoma formation, and fluid/blood leakage.121
A retrospective review of ICU patients who underwent ultrasound-guided PIV insertion by a single physician showed high success rates (99%) with low rates of phlebitis/cellulitis (0.7%).There was an assumed benefit of risk reduction due to the patients no longer requiring a CVC after successful PIV placement.122 Another study found very low rates of infection with both landmark-based and ultrasound-guided PIV placement performed by emergency department nurses, suggesting that there is no increased risk of infection with the use of ultrasound.123 To reduce the risk of infection from PIV insertion, we recommend the use of sterile gel and sterile transducer cover (See Recommendation 2).
Arterial Access Techniques
18. We recommend that providers should use real-time ultrasound guidance for arterial access, which has been shown to increase first-pass success rates, reduce the time to cannulation, and reduce the risk of hematoma development compared with landmark-based techniques.
Rationale: Several randomized controlled studies have assessed the value of ultrasound in arterial catheter insertion. Shiver et al. randomized 60 patients admitted to a tertiary center emergency department to either palpation or ultrasound-guided arterial cannulation. They demonstrated a first-pass success rate of 87% in the ultrasound group compared with 50% in the landmark technique group. In the same study, the use of ultrasound was also associated with reduced time needed to establish arterial access and a 43% reduction in the development of hematoma at the insertion site.124 Levin et al. demonstrated a first-pass success rate of 62% using ultrasound versus 34% by palpation alone in 69 patients requiring intraoperative invasive hemodynamic monitoring.125 Additional randomized controlled studies have demonstrated that ultrasound guidance increases first-attempt success rates compared to traditional palpation.23,126,127
19. We recommend that providers should use real-time ultrasound guidance for femoral arterial access, which has been shown to increase first-pass success rates and reduce the risk of vascular complications.
Rationale: Although it is a less frequently used site, the femoral artery may be accessed for arterial blood sampling or invasive hemodynamic monitoring, and use of ultrasound guidance has been shown to improve the first-pass success rates of femoral artery cannulation. It is important to note that most of these studies comparing ultrasound-guided vs landmark-based femoral artery cannulation were performed in patients undergoing diagnostic or interventional vascular procedures.
A meta-analysis of randomized controlled studies comparing ultrasound-guided vs landmark-based femoral artery catheterization found use of ultrasound guidance was associated with a 49% reduction in overall complications (RR 0.51, 95% CI 0.28 to 0.91; P > .05) and 42% improvement in first-pass success rates.128 In another study, precise site selection with ultrasound was associated with fewer pseudoaneurysms in patients undergoing femoral artery cannulation by ultrasound guidance vs palpation for cardiac catheterization (3% vs 5%, P < .05).129
A multicenter randomized controlled study comparing ultrasound vs fluoroscopic guidance for femoral artery catheterization demonstrated ultrasound guidance improved rates of common femoral artery (CFA) cannulation in patients with high CFA bifurcations (83% vs 70%, P < .01).130 Furthermore, ultrasound guidance improved first-pass success rates (83% vs 46%, P < .0001), reduced number of attempts (1.3 vs 3.0, P < .0001), reduced risk of venipuncture (2.4% vs 15.8%, P < .0001), and reduced median time to obtain access (136 seconds vs148 seconds, P = .003). Vascular complications occurred in fewer patients in the ultrasound vs fluoroscopy groups (1.4% vs 3.4% P = .04). Reduced risk of hematoma formation with routine use of ultrasound guidance was demonstrated in one retrospective observational study (RR 0.62, 95% CI 0.46 to 0.84; P < .01).131
20. We recommend that providers should use real-time ultrasound guidance for radial arterial access, which has been shown to increase first-pass success rates, reduce the time to successful cannulation, and reduce the risk of complications compared with landmark-based techniques.
Rationale: Ultrasound guidance is particularly useful for radial artery cannulation in patients with altered anatomy, obesity, nonpulsatile blood flow, low perfusion, and previously unsuccessful cannulation attempts using a landmark-guided approach.132
A multicenter randomized controlled study that was not included in the abovementioned meta-analyses showed similar benefits of using ultrasound guidance vs landmarks for radial artery catheterization: a reduction in the number of attempts with ultrasound guidance (1.65 ± 1.2 vs 3.05 ± 3.4, P < .0001) and time to obtain access (88 ± 78 vs 108 ± 112 seconds, P = .006), and increased first-pass success rates (65% vs 44%, P < .0001). The use of ultrasound guidance was found to be particularly useful in patients with difficult access by palpation alone.135
Regarding the level of expertise required to use ultrasound guidance, a prospective observational study demonstrated that physicians with little previous ultrasound experience were able to improve their first-attempt success rates and procedure time for radial artery cannulation compared to historical data of landmark-based insertions.136
Postprocedure
21. We recommend that post-procedure pneumothorax should be ruled out by the detection of bilateral lung sliding using a high-frequency linear transducer before and after insertion of internal jugular and subclavian vein CVCs.
Rationale: Detection of lung sliding with two-dimensional ultrasound rules out pneumothorax, and disappearance of lung sliding in an area where it was previously seen is a strong predictor of postprocedure pneumothorax. In a study of critically ill patients, the disappearance of lung sliding was observed in 100% of patients with pneumothorax vs 8.8% of patients without pneumothorax. For detection of pneumothorax, lung sliding showed a sensitivity of 95%, specificity of 91%, and negative predictive value of 100% (P < .001).137 Another study by the same author showed that the combination of horizontal artifacts (absence of comet-tail artifact) and absence of lung sliding had a sensitivity of 100%, specificity of 96.5%, and negative predictive value of 100% for the detection of pneumothorax.138 A meta-analysis of 10 studies on the diagnostic accuracy of CVC confirmation with bedside ultrasound vs chest radiography reported detection of all 12 pneumothoraces with ultrasound, whereas chest radiography missed two pneumothoraces. The pooled sensitivity and specificity of ultrasound for the detection of pneumothorax was 100%, although an imperfect gold standard bias likely affected the results. An important advantage of bedside ultrasound is the ability to rule out pneumothorax immediately after the procedure while at the bedside. The mean time for confirmation of CVC placement with bedside ultrasound was 6 minutes versus 64 minutes and 143 minutes for completion and interpretation of a chest radiograph, respectively.139
22. We recommend that providers should use ultrasound with rapid infusion of agitated saline to visualize a right atrial swirl sign (RASS) for detecting catheter tip misplacement during CVC insertion. The use of RASS to detect the catheter tip may be considered an advanced skill that requires specific training and expertise.
Rationale: Bedside echocardiography is a reliable tool to detect catheter tip misplacement during CVC insertion. In one study, catheter misplacement was detected by bedside echocardiography with a sensitivity of 96% and specificity of 83% (positive predictive value 98%, negative predictive value 55%) and prevented distal positioning of the catheter tip.140 A prospective observational study assessed for RASS, which is turbulent flow in the right atrium after a rapid saline flush of the distal CVC port, to exclude catheter malposition. In this study with 135 CVC placements, visualization of RASS with ultrasound was able to identify all correct CVC placements and three of four catheter misplacements. Median times to complete the ultrasound exam vs CXR were 1 vs 20 minutes, respectively, with a median difference of 24 minutes (95% CI 19.6 to 29.3, P < .0001) between the two techniques.141
A prospective observational study assessed the ability of bedside transthoracic echocardiography to detect the guidewire, microbubbles, or both, in the right atrium compared to transesophageal echocardiography as the gold standard. Bedside transthoracic echocardiography allowed visualization of the right atrium in 94% of patients, and both microbubbles plus guidewire in 91% of patients.142 Hence, bedside transthoracic echocardiography allows adequate visualization of the right atrium. Another prospective observational study combining ultrasonography and contrast enhanced RASS resulted in 96% sensitivity and 93% specificity for the detection of a misplaced catheter, and the concordance with chest radiography was 96%.143
Training
23. To reduce the risk of mechanical and infectious complications, we recommend that novice providers should complete a systematic training program that includes a combination of simulation-based practice, supervised insertion on patients, and evaluation by an expert operator before attempting ultrasound-guided CVC insertion independently on patients.
Rationale: Cumulative experience has been recognized to not be a proxy for mastery of a clinical skill.144 The National Institute for Clinical Excellence (NICE) has recommended that providers performing ultrasound-guided CVC insertion should receive appropriate training to achieve competence before performing the procedure independently.7 Surveys have demonstrated that lack of training is a commonly reported barrier for not using ultrasound.145,146
Structured training programs on CVC insertion have been shown to reduce the occurrence of infectious and mechanical complications.74,143,147-149 The use of ultrasound and checklists, bundling of supplies, and practice with simulation models, as a part of a structured training program, can improve patient safety related to CVC insertion.9,140,150-154
Simulation-based practice has been used in medical education to provide deliberate practice and foster skill development in a controlled learning environment.155-158 Studies have shown transfer of skills demonstrated in a simulated environment to clinical practice, which can improve CVC insertion practices.159,160 Simulation accelerates learning of all trainees, especially novice trainees, and mitigates risks to patients by allowing trainees to achieve a minimal level of competence before attempting the procedure on real patients.152,161,162 Residents that have been trained using simulation preferentially select the IJV site,147 and more reliably use ultrasound to guide their CVC insertions.160,163
Additionally, simulation-based practice allows exposure to procedures and scenarios that may occur infrequently in clinical practice.
Although there is evidence on efficacy of simulation-based CVC training programs, there is no broadly accepted consensus on timing, duration, and content of CVC training programs for trainees or physicians in practice. The minimum recommended technical skills a trainee must master include the ability to (1) manipulate the ultrasound machine to produce a high-quality image to identify the target vessel, (2) advance the needle under direct visualization to the desired target site and depth, (3) deploy the catheter into the target vessel and confirm catheter placement in the target vessel using ultrasound, and (4) ensure the catheter has not been inadvertently placed in an unintended vessel or structure.153
A variety of simulation models are currently used to practice CVC insertion at the most common sites: the internal jugular, subclavian, basilic, and brachial veins.164,165 Effective simulation models should contain vessels that mimic normal anatomy with muscles, soft tissues, and bones. Animal tissue models, such as turkey or chicken breasts, may be effective for simulated practice of ultrasound-guided CVC insertion.166,167 Ultrasound-guided CVC training using human cadavers has also been shown to be effective.168
24. We recommend that cognitive training in ultrasound-guided CVC insertion should include basic anatomy, ultrasound physics, ultrasound machine knobology, fundamentals of image acquisition and interpretation, detection and management of procedural complications, infection prevention strategies, and pathways to attain competency.
Rationale: After receiving training in ultrasound-guided CVC insertion, physicians report significantly higher comfort with the use of ultrasound compared to those who have not received such training.145 Learners find training sessions worthwhile to increase skill levels,167 and skills learned from simulation-based mastery learning programs have been retained up to one year.158
Several commonalities have been noted across training curricula. Anatomy and physiology didactics should include vessel anatomy (location, size, and course);9 vessel differentiation by ultrasound;9,69 blood flow dynamics;69 Virchow’s triad;69 skin integrity and colonization;150 peripheral nerve identification and distribution;9 respiratory anatomy;9,69 upper and lower extremity, axillary, neck, and chest anatomy.9,69 Vascular anatomy is an essential curricular component that may help avoid preventable CVC insertion complications, such as inadvertent nerve, artery, or lung puncture.150,169 Training curricula should also include ultrasound physics (piezoelectric effect, frequency, resolution, attenuation, echogenicity, Doppler ultrasound, arterial and venous flow characteristics), image acquisition and optimization (imaging mode, focus, dynamic range, probe types), and artifacts (reverberation, mirror, shadowing, enhancement).
CVC-related infections are an important cause of morbidity and mortality in the acute and long-term care environment.69 Infection and thrombosis can both be impacted by the insertion site selection, skin integrity, and catheter–vein ratio.2,3,84 Inexperience generally leads to more insertion attempts that can increase trauma during CVC insertion and potentially increase the risk of infections.170 To reduce the risk of infectious complications, training should include important factors to consider in site selection and maintenance of a sterile environment during CVC insertion, including use of maximal sterile barrier precautions, hand hygiene, and appropriate use of skin antiseptic solutions.
Professional society guidelines have been published with recommendations of appropriate techniques for ultrasound-guided vascular access that include training recommendations.9,154 Training should deconstruct the insertion procedure into readily understood individual steps, and can be aided by demonstration of CVC insertion techniques using video clips. An alternative to face-to-face training is internet-based training that has been shown to be as effective as traditional teaching methods in some medical centers.171 Additional methods to deliver cognitive instruction include textbooks, continuing medical education courses, and digital videos.164,172
25. We recommend that trainees should demonstrate minimal competence before placing ultrasound-guided CVCs independently. A minimum number of CVC insertions may inform this determination, but a proctored assessment of competence is most important.
Rationale: CVC catheter placement carries the risk of serious complications including arterial injury or dissection, pneumothorax, or damage to other local structures; arrhythmias; catheter malposition; infection; and thrombosis. Although there is a lack of consensus and high-quality evidence for the certification of skills to perform ultrasound-guided CVC insertion, recommendations have been published advocating for formal and comprehensive training programs in ultrasound-guided CVC insertion with an emphasis on expert supervision prior to independent practice.9,153,154 Two groups of expert operators have recommended that training should include at least 8-10 supervised ultrasound-guided CVC insertions.154,173,174 A consensus task force from the World Congress of Vascular Access has recommended a minimum of six to eight hours of didactic education, four hours of hands-on training on simulation models, and six hours of hands-on ultrasound training on human volunteers to assess normal anatomy.175 This training should be followed by supervised ultrasound-guided CVC insertions until the learner has demonstrated minimal competence with a low rate of complications.35 There is general consensus that arbitrary numbers should not be the sole determinant of competence, and that the most important determinant of competence should be an evaluation by an expert operator.176
26. We recommend that didactic and hands-on training for trainees should coincide with anticipated times of increased performance of vascular access procedures. Refresher training sessions should be offered periodically.
Rationale: Simulation-based CVC training courses have shown a rapid improvement in skills, but lack of practice leads to deterioration of technical skills.161,162,177,178 Thus, a single immersive training session is insufficient to achieve and maintain mastery of skills, and an important factor to acquire technical expertise is sustained, deliberate practice with feedback.179 Furthermore, an insidious decay in skills may go unrecognized as a learner’s comfort and self-confidence does not always correlate with actual performance, leading to increased risk of errors and potential for procedural complications.147,158,180-183 Given the decay in technical skills over time, simulation-based training sessions are most effective when they occur in close temporal proximity to times when those skills are most likely to be used; for example, a simulation-based training session for trainees may be most effective just before the start of a critical care rotation.152 Regularly scheduled training sessions with monitoring and feedback by expert operators can reinforce procedural skills and prevent decay. Some experts have recommended that a minimum of 10 ultrasound-guided CVC insertions should be performed annually to maintain proficiency.153
27. We recommend that competency assessments should include formal evaluation of knowledge and technical skills using standardized assessment tools.
Rationale: Hospitalists and other healthcare providers that place vascular access catheters should undergo competency assessments proctored by an expert operator to verify that they have the required knowledge and skills.184,185 Knowledge competence can be partially evaluated using a written assessment, such as a multiple-choice test, assessing the provider’s cognitive understanding of the procedure.175 For ultrasound-guided CVC insertion, a written examination should be administered in conjunction with an ultrasound image assessment to test the learner’s recognition of normal vs abnormal vascular anatomy. Minimum passing standards should be established a priori according to local or institutional standards.
The final skills assessment should be objective, and the learner should be required to pass all critical steps of the procedure. Failure of the final skills assessment should lead to continued practice with supervision until the learner can consistently demonstrate correct performance of all critical steps. Checklists are commonly used to rate the technical performance of learners because they provide objective criteria for evaluation, can identify specific skill deficiencies, and can determine a learner’s readiness to perform procedures independently.186,187 The administration of skills assessments and feedback methods should be standardized across faculty. Although passing scores on both knowledge and skills assessments do not guarantee safe performance of a procedure independently, they provide a metric to ensure that a minimum level of competence has been achieved before allowing learners to perform procedures on patients without supervision.188
Competency assessments are a recommended component of intramural and extramural certification of skills in ultrasound-guided procedures. Intramural certification pathways differ by institution and often require additional resources including ultrasound machine(s), simulation equipment, and staff time, particularly when simulation-based assessments are incorporated into certification pathways. We recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals. However, initial and ongoing competency assessments can be performed during routine performance of procedures on patients. For an in-depth review of credentialing pathways for ultrasound-guided bedside procedures, we recommend reviewing the SHM Position Statement on Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.24
28. We recommend that competency assessments should evaluate for proficiency in the following knowledge and skills of CVC insertion:
a. Knowledge of the target vein anatomy, proper vessel identification, and recognition of anatomical variants
b. Demonstration of CVC insertion with no technical errors based on a procedural checklist
c. Recognition and management of acute complications, including emergency management of life-threatening complications
d. Real-time needle tip tracking with ultrasound and cannulation on the first attempt in at least five consecutive simulations.
Rationale: Recommendations have been published with the minimal knowledge and skills learners must demonstrate to perform ultrasound-guided vascular access procedures. These include operation of an ultrasound machine to produce high-quality images of the target vessel, tracking of the needle tip with real-time ultrasound guidance, and recognition and understanding of the management of procedural complications.154,175
First, learners must be able to perform a preprocedural assessment of the target vein, including size and patency of the vein; recognition of adjacent critical structures; and recognition of normal anatomical variants.175,189 Second, learners must be able to demonstrate proficiency in tracking the needle tip penetrating the target vessel, inserting the catheter into the target vessel, and confirming catheter placement in the target vessel with ultrasound.154,175 Third, learners must be able to demonstrate recognition of acute complications, including arterial puncture, hematoma formation, and development of pneumothorax.154,175 Trainees should be familiar with recommended evaluation and management algorithms, including indications for emergent consultation.190
29. We recommend a periodic proficiency assessments of all operators should be conducted to ensure maintenance of competency.
Rationale: Competency extends to periodic assessment and not merely an initial evaluation at the time of training.191 Periodic competency assessments should include assessment of proficiency of all providers that perform a procedure, including instructors and supervisors. Supervising providers should maintain their competency in CVC insertion through routine use of their skills in clinical practice.175 An observational study of emergency medicine residents revealed that lack of faculty comfort with ultrasound hindered the residents’ use of ultrasound.192 Thus, there is a need to examine best practices for procedural supervision of trainees because providers are often supervising procedures that they are not comfortable performing on their own.193
KNOWLEDGE GAPS
The process of producing this position statement revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for central and peripheral venous access and arterial access.
This position statement recommends a preprocedural ultrasound evaluation of blood vessels based on evidence that providers may detect anatomic anomalies, thrombosis, or vessel stenosis. Ultrasound can also reveal unsuspected high-risk structures in near proximity to the procedure site. Although previous studies have shown that providers can accurately assess vessels with ultrasound for these features, further study is needed to evaluate the effect of a standardized preprocedural ultrasound exam on clinical and procedural decision-making, as well as procedural outcomes.
Second, two ultrasound applications that are being increasingly used but have not been widely implemented are the use of ultrasound to evaluate lung sliding postprocedure to exclude pneumothorax and the verification of central line placement using a rapid infusion of agitated saline to visualize the RASS.139-141 Both of these applications have the potential to expedite postprocedure clearance of central lines for usage and decrease patient radiation exposure by obviating the need for postprocedure CXRs. Despite the supporting evidence, both of these applications are not yet widely used, as few providers have been trained in these techniques which may be considered advanced skills.
Third, despite advances in our knowledge of effective training for vascular access procedures, there is limited agreement on how to define procedural competence. Notable advancements in training include improved understanding of systematic training programs, development of techniques for proctoring procedures, definition of elements for hands-on assessments, and definition of minimum experience needed to perform vascular access procedures independently. However, application of these concepts to move learners toward independent practice remains variably interpreted at different institutions, likely due to limited resources, engrained cultures about procedures, and a lack of national standards. The development of hospitalist-based procedure services at major academic medical centers with high training standards, close monitoring for quality assurance, and the use of databases to track clinical outcomes may advance our understanding and delivery of optimal procedural training.
Finally, ultrasound technology is rapidly evolving which will affect training, techniques, and clinical outcomes in coming years. Development of advanced imaging software with artificial intelligence can improve needle visualization and tracking. These technologies have the potential to facilitate provider training in real-time ultrasound-guided procedures and improve the overall safety of procedures. Emergence of affordable, handheld ultrasound devices is improving access to ultrasound technology, but their role in vascular access procedures is yet to be defined. Furthermore, availability of wireless handheld ultrasound technology and multifrequency transducers will create new possibilities for use of ultrasound in vascular access procedures.
CONCLUSION
We have presented several evidence-based recommendations on the use of ultrasound guidance for placement of central and peripheral vascular access catheters that are intended for hospitalists and other healthcare providers who routinely perform vascular access procedures. By allowing direct visualization of the needle tip and target vessel, the use of ultrasound guidance has been shown in randomized studies to reduce needle insertion attempts, reduce needle redirections, and increase overall procedure success rates. The accuracy of ultrasound to identify the target vessel, assess for thrombosis, and detect anatomical anomalies is superior to that of physical examination. Hospitalists can attain competence in performing ultrasound-guided vascular access procedures through systematic training programs that combine didactic and hands-on training, which optimally include patient-based competency assessments.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators of Society of Hospital Medicine Point-of-care Ultrasound Task Force: Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Venkat Kalidindi, Ketino Kobaidze, Joshua Lenchus, Paul Mayo, Satyen Nichani, Vicki Noble, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Gerard Salame, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam J. Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Mathews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Mathews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El-Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Approximately five million central venous catheters (CVCs) are inserted in the United States annually, with over 15 million catheter days documented in intensive care units alone.1 Traditional CVC insertion techniques using landmarks are associated with a high risk of mechanical complications, particularly pneumothorax and arterial puncture, which occur in 5%-19% patients.2,3
Since the 1990s, several randomized controlled studies and meta-analyses have demonstrated that the use of real-time ultrasound guidance for CVC insertion increases procedure success rates and decreases mechanical complications.4,5 Use of real-time ultrasound guidance was recommended by the Agency for Healthcare Research and Quality, the Institute of Medicine, the National Institute for Health and Care Excellence, the Centers for Disease Control and Prevention, and several medical specialty societies in the early 2000s.6-14 Despite these recommendations, ultrasound guidance has not been universally adopted. Currently, an estimated 20%-55% of CVC insertions in the internal jugular vein are performed without ultrasound guidance.15-17
Following the emergence of literature supporting the use of ultrasound guidance for CVC insertion, observational and randomized controlled studies demonstrated improved procedural success rates with the use of ultrasound guidance for the insertion of peripheral intravenous lines (PIVs), arterial catheters, and peripherally inserted central catheters (PICCs).18-23
The purpose of this position statement is to present evidence-based recommendations on the use of ultrasound guidance for the insertion of central and peripheral vascular access catheters in adult patients. This document presents consensus-based recommendations with supporting evidence for clinical outcomes, techniques, and training for the use of ultrasound guidance for vascular access. We have subdivided the recommendations on techniques for central venous access, peripheral venous access, and arterial access individually, as some providers may not perform all types of vascular access procedures.
These recommendations are intended for hospitalists and other healthcare providers that routinely place central and peripheral vascular access catheters in acutely ill patients. However, this position statement does not mandate that all hospitalists should place central or peripheral vascular access catheters given the diverse array of hospitalist practice settings. For training and competency assessments, we recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals, where equipment and staffing for assessments are not available. Recommendations and frameworks for initial and ongoing credentialing of hospitalists in ultrasound-guided bedside procedures have been previously published in an Society of Hospital Medicine (SHM) position statement titled, “Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.”24
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interest (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the vascular access working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. An updated search was conducted in November 2017. The literature search strings are included in Appendix 3. All article abstracts were initially screened for relevance by at least two members of the vascular access working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide vascular access were selected. The following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled studies, and observational studies of ultrasound-guided vascular access were screened and selected (Appendix 3, Figure 1). All full-text articles were shared electronically among the working group members, and final article selection was based on working group consensus. Selected articles were incorporated into the draft recommendations.
These recommendations were developed using the Research and Development (RAND) Appropriateness Method that required panel judgment and consensus.14 The 28 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Using an internet-based electronic data collection tool (REDCap™), panel members participated in two rounds of electronic voting, one in August 2018 and the other in October 2018 (Appendix 4). Voting on appropriateness was conducted using a nine-point Likert scale. The three zones of the nine-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix 1, Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” Disagreement was defined as >30% of panelists voting outside of the zone of the median. A strong recommendation required at least 80% of the votes within one integer of the median per the RAND rules.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording for each recommendation (Table 2). The final version of the consensus-based recommendations underwent internal and external review by members of the SHM POCUS Task Force, the SHM Education Committee, and the SHM Executive Committee. The SHM Executive Committee reviewed and approved this position statement prior to its publication in the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 5,563 references were pooled from an initial search performed by a certified medical librarian in December 2015 (4,668 citations) which was updated in November 2017 (791 citations), and from the personal bibliographies and searches (104 citations) performed by working group members. A total of 514 full-text articles were reviewed. The final selection included 192 articles that were abstracted into a data table and incorporated into the draft recommendations. See Appendix 3 for details of the literature search strategy.
Recommendations
Four domains (technique, clinical outcomes, training, and knowledge gaps) with 31 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation cite supporting evidence. After two rounds of panel voting, 31 recommendations achieved agreement based on the RAND rules. During the peer review process, two of the recommendations were merged with other recommendations. Thus, a total of 29 recommendations received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Twenty-seven statements were approved as strong recommendations, and two were approved as weak/conditional recommendations. The strength of each recommendation and degree of consensus are summarized in Table 3.
Terminology
Central Venous Catheterization
Central venous catheterization refers to insertion of tunneled or nontunneled large bore vascular catheters that are most commonly inserted into the internal jugular, subclavian, or femoral veins with the catheter tip located in a central vein. These vascular access catheters are synonymously referred to as central lines or central venous catheters (CVCs). Nontunneled catheters are designed for short-term use and should be removed promptly when no longer clinically indicated or after a maximum of 14 days.25
Peripherally Inserted Central Catheter (PICC)
Peripherally inserted central catheters, or PICC lines, are inserted most commonly in the basilic or brachial veins in adult patients, and the catheter tip terminates in the distal superior vena cava or cavo-atrial junction. These catheters are designed to remain in place for a duration of several weeks, as long as it is clinically indicated.
Midline Catheterization
Midline catheters are a type of peripheral venous catheter that are an intermediary between a peripheral intravenous catheter and PICC line. Midline catheters are most commonly inserted in the brachial or basilic veins, but unlike PICC lines, the tips of these catheters terminate in the axillary or subclavian vein. Midline catheters are typically 8 cm to 20 cm in length and inserted for a duration <30 days.
Peripheral Intravenous Catheterization
Peripheral intravenous lines (PIV) refer to small bore venous catheters that are most commonly 14G to 24G and inserted into patients for short-term peripheral venous access. Common sites of ultrasound-guided PIV insertion include the superficial and deep veins of the hand, forearm, and arm.
Arterial Catheterization
Arterial catheters are commonly used for reliable blood pressure monitoring, frequent arterial blood
RECOMMENDATIONS
Preprocedure
1. We recommend that providers should be familiar with the operation of their specific ultrasound machine prior to initiation of a vascular access procedure.
Rationale: There is strong consensus that providers must be familiar with the knobs and functions of the specific make and model of ultrasound machine that will be utilized for a vascular access procedure. Minimizing adjustments to the ultrasound machine during the procedure may reduce the risk of contaminating the sterile field.
2. We recommend that providers should use a high-frequency linear transducer with a sterile sheath and sterile gel to perform vascular access procedures.
Rationale: High-frequency linear-array transducers are recommended for the vast majority of vascular access procedures due to their superior resolution compared to other transducer types. Both central and peripheral vascular access procedures, including PIV, PICC, and arterial line placement, should be performed using sterile technique. A sterile transducer cover and sterile gel must be utilized, and providers must be trained in sterile preparation of the ultrasound transducer.13,26,27
The depth of femoral vessels correlates with body mass index (BMI). When accessing these vessels in a morbidly obese patient with a thigh circumference >60 cm and vessel depth >8 cm, a curvilinear transducer may be preferred for its deeper penetration.28 For patients who are poor candidates for bedside insertion of vascular access catheters, such as uncooperative patients, patients with atypical vascular anatomy or poorly visualized target vessels, we recommend consultation with a vascular access specialist prior to attempting the procedure.
3. We recommend that providers should use two-dimensional ultrasound to evaluate for anatomical variations and absence of vascular thrombosis during preprocedural site selection.
Rationale: A thorough ultrasound examination of the target vessel is warranted prior to catheter placement. Anatomical variations that may affect procedural decision-making are easily detected with ultrasound. A focused vascular ultrasound examination is particularly important in patients who have had temporary or tunneled venous catheters, which can cause stenosis or thrombosis of the target vein.
For internal jugular vein (IJV) CVCs, ultrasound is useful for visualizing the relationship between the IJV and common carotid artery (CCA), particularly in terms of vessel overlap. Furthermore, ultrasound allows for immediate revisualization upon changes in head position.29-32 Troianos et al. found >75% overlap of the IJV and CCA in 54% of all patients and in 64% of older patients (age >60 years) whose heads were rotated to the contralateral side.30 In one study of IJV CVC insertion, inadvertent carotid artery punctures were reduced (3% vs 10%) with the use of ultrasound guidance vs landmarks alone.33 In a cohort of 64 high-risk neurosurgical patients, cannulation success was 100% with the use of ultrasound guidance, and there were no injuries to the carotid artery, even though the procedure was performed with a 30-degree head elevation and anomalous IJV anatomy in 39% of patients.34 In a prospective, randomized controlled study of 1,332 patients, ultrasound-guided cannulation in a neutral position was demonstrated to be as safe as the 45-degree rotated position.35
Ultrasound allows for the recognition of anatomical variations which may influence the selection of the vascular access site or technique. Benter et al. found that 36% of patients showed anatomical variations in the IJV and surrounding tissue.36 Similarly Caridi showed the anatomy of the right IJV to be atypical in 29% of patients,37 and Brusasco found that 37% of bariatric patients had anatomical variations of the IJV.38 In a study of 58 patients, there was significant variability in the IJV position and IJV diameter, ranging from 0.5 cm to >2 cm.39 In a study of hemodialysis patients, 75% of patients had sonographic venous abnormalities that led to a change in venous access approach.40
To detect acute or chronic upper extremity deep venous thrombosis or stenosis, two-dimensional visualization with compression should be part of the ultrasound examination prior to central venous catheterization. In a study of patients that had undergone CVC insertion 9-19 weeks earlier, 50% of patients had an IJV thrombosis or stenosis leading to selection of an alternative site. In this study, use of ultrasound for a preprocedural site evaluation reduced unnecessary attempts at catheterizing an occluded vein.41 At least two other studies demonstrated an appreciable likelihood of thrombosis. In a study of bariatric patients, 8% of patients had asymptomatic thrombosis38 and in another study, 9% of patients being evaluated for hemodialysis catheter placement had asymptomatic IJV thrombosis.37
4. We recommend that providers should evaluate the target blood vessel size and depth during a preprocedural ultrasound evaluation.
Rationale: The size, depth, and anatomic location of central veins can vary considerably. These features are easily discernable using ultrasound. Contrary to traditional teaching, the IJV is located 1 cm anterolateral to the CCA in only about two-thirds of patients.37,39,42,43 Furthermore, the diameter of the IJV can vary significantly, ranging from 0.5 cm to >2 cm.39 The laterality of blood vessels may vary considerably as well. A preprocedural ultrasound evaluation of contralateral subclavian and axillary veins showed a significant absolute difference in cross-sectional area of 26.7 mm2 (P < .001).42
Blood vessels can also shift considerably when a patient is in the Trendelenburg position. In one study, the IJV diameter changed from 11.2 (± 1.5) mm to 15.4 (± 1.5) mm in the supine versus the Trendelenburg position at 15 degrees.33 An observational study demonstrated a frog-legged position with reverse Trendelenburg increased the femoral vein size and reduced the common surface area with the common femoral artery compared to a neutral position. Thus, a frog-legged position with reverse Trendelenburg position may be preferred, since overall catheterization success rates are higher in this position.44
Techniques
General Techniques
5. We recommend that providers should avoid using static ultrasound alone to mark the needle insertion site for vascular access procedures.
Rationale: The use of static ultrasound guidance to mark a needle insertion site is not recommended because normal anatomical relationships of vessels vary, and site marking can be inaccurate with minimal changes in patient position, especially of the neck.43,45,46 Benefits of using ultrasound guidance for vascular access are attained when ultrasound is used to track the needle tip in real-time as it is advanced toward the target vessel.
Although continuous-wave Doppler ultrasound without two-dimensional visualization was used in the past, it is no longer recommended for IJV CVC insertion.47 In a study that randomized patients to IJV CVC insertion with continuous-wave Doppler alone vs two-dimensional ultrasound guidance, the use of two-dimensional ultrasound guidance showed significant improvement in first-pass success rates (97% vs 91%, P = .045), particularly in patients with BMI >30 (97% vs 77%, P = .011).48
A randomized study comparing real-time ultrasound-guided, landmark-based, and ultrasound-marked techniques found higher success rates in the real-time ultrasound-guided group than the other two groups (100% vs 74% vs 73%, respectively; P = .01). The total number of mechanical complications was higher in the landmark-based and ultrasound-marked groups than in the real-time ultrasound-guided group (24% and 36% versus 0%, respectively; P = .01).49 Another randomized controlled study found higher success rates with real-time ultrasound guidance (98%) versus an ultrasound-marked (82%) or landmark-based (64%) approach for central line placement.50
6. We recommend that providers should use real-time (dynamic), two-dimensional ultrasound guidance with a high-frequency linear transducer for CVC insertion, regardless of the provider’s level of experience.
7. We suggest using either a transverse (short-axis) or longitudinal (long-axis) approach when performing real-time ultrasound-guided vascular access procedures.
Rationale: In clinical practice, the phrases transverse, short-axis, or out-of-plane approach are synonymous, as are longitudinal, long-axis, and in-plane approach. The short-axis approach involves tracking the needle tip as it approximates the target vessel with the ultrasound beam oriented in a transverse plane perpendicular to the target vessel. The target vessel is seen as a circular structure on the ultrasound screen as the needle tip approaches the target vessel from above. This approach is also called the out-of-plane technique since the needle passes through the ultrasound plane. The advantages of the short-axis approach include better visualization of adjacent vessels or nerves and the relative ease of skill acquisition for novice operators.9 When using the short-axis approach, extra care must be taken to track the needle tip from the point of insertion on the skin to the target vessel. A disadvantage of the short-axis approach is unintended posterior wall puncture of the target vessel.55
In contrast to a short-axis approach, a long-axis approach is performed with the ultrasound beam aligned parallel to the vessel. The vessel appears as a long tubular structure and the entire needle is visualized as it traverses across the ultrasound screen to approach the target vessel. The long-axis approach is also called an in-plane technique because the needle is maintained within the plane of the ultrasound beam. The advantage of a long-axis approach is the ability to visualize the entire needle as it is inserted into the vessel.14 A randomized crossover study with simulation models compared a long-axis versus short-axis approach for both IJV and subclavian vein catheterization. This study showed decreased number of needle redirections (relative risk (RR) 0.5, 95% confidence interval (CI) 0.3 to 0.7), and posterior wall penetrations (OR 0.3, 95% CI 0.1 to 0.9) using a long-axis versus short-axis approach for subclavian vein catheterization.56
A randomized controlled study comparing a long-axis or short-axis approach with ultrasound versus a landmark-based approach for IJV CVC insertion showed higher success rates (100% vs 90%; P < .001), lower insertion time (53 vs 116 seconds; P < .001), and fewer attempts to obtain access (2.5 vs 1.2 attempts, P < .001) with either the long- or short-axis ultrasound approach. The average time to obtain access and number of attempts were comparable between the short-axis and long-axis approaches with ultrasound. The incidence of carotid puncture and hematoma was significantly higher with the landmark-based approach versus either the long- or short-axis ultrasound approach (carotid puncture 17% vs 3%, P = .024; hematoma 23% vs 3%, P = .003).57
High success rates have been reported using a short-axis approach for insertion of PIV lines.58 A prospective, randomized trial compared the short-axis and long-axis approach in patients who had had ≥2 failed PIV insertion attempts. Success rate was 95% (95% CI, 0.85 to 1.00) in the short-axis group compared with 85% (95% CI, 0.69 to 1.00) in the long-axis group. All three subjects with failed PIV placement in the long-axis group had successful rescue placement using a short-axis approach. Furthermore, the short-axis approach was faster than the long-axis approach.59
For radial artery cannulation, limited data exist comparing the short- and long-axis approaches. A randomized controlled study compared a long-axis vs short-axis ultrasound approach for radial artery cannulation. Although the overall procedure success rate was 100% in both groups, the long-axis approach had higher first-pass success rates (1.27 ± 0.4 vs 1.5 ± 0.5, P < .05), shorter cannulation times (24 ± 17 vs 47 ± 34 seconds, P < .05), fewer hematomas (4% vs 43%, P < .05) and fewer posterior wall penetrations (20% vs 56%, P < .05).60
Another technique that has been described for IJV CVC insertion is an oblique-axis approach, a hybrid between the long- and short-axis approaches. In this approach, the transducer is aligned obliquely over the IJV and the needle is inserted using a long-axis or in-plane approach. A prospective randomized trial compared the short-axis, long-axis, and oblique-axis approaches during IJV cannulation. First-pass success rates were 70%, 52%, and 74% with the short-axis, long-axis, and oblique-axis approaches, respectively, and a statistically significant difference was found between the long- and oblique-axis approaches (P = .002). A higher rate of posterior wall puncture was observed with a short-axis approach (15%) compared with the oblique-axis (7%) and long-axis (4%) approaches (P = .047).61
8. We recommend that providers should visualize the needle tip and guidewire in the target vein prior to vessel dilatation.
Rationale: When real-time ultrasound guidance is used, visualization of the needle tip within the vein is the first step to confirm cannulation of the vein and not the artery. After the guidewire is advanced, the provider can use transverse and longitudinal views to reconfirm cannulation of the vein. In a longitudinal view, the guidewire is readily seen positioned within the vein, entering the anterior wall and lying along the posterior wall of the vein. Unintentional perforation of the posterior wall of the vein with entry into the underlying artery can be detected by ultrasound, allowing prompt removal of the needle and guidewire before proceeding with dilation of the vessel. In a prospective observational study that reviewed ultrasound-guided IJV CVC insertions, physicians were able to more readily visualize the guidewire than the needle in the vein.62 A prospective observational study determined that novice operators can visualize intravascular guidewires in simulation models with an overall accuracy of 97%.63
In a retrospective review of CVC insertions where the guidewire position was routinely confirmed in the target vessel prior to dilation, there were no cases of arterial dilation, suggesting confirmation of guidewire position can potentially eliminate the morbidity and mortality associated with arterial dilation during CVC insertion.64
9. To increase the success rate of ultrasound-guided vascular access procedures, we recommend that providers should utilize echogenic needles, plastic needle guides, and/or ultrasound beam steering when available.
Rationale: Echogenic needles have ridged tips that appear brighter on the screen, allowing for better visualization of the needle tip. Plastic needle guides help stabilize the needle alongside the transducer when using either a transverse or longitudinal approach. Although evidence is limited, some studies have reported higher procedural success rates when using echogenic needles, plastic needle guides, and ultrasound beam steering software. In a prospective observational study, Augustides et al. showed significantly higher IJV cannulation rates with versus without use of a needle guide after first (81% vs 69%, P = .0054) and second (93% vs 80%. P = .0001) needle passes.65 A randomized study by Maecken et al. compared subclavian vein CVC insertion with or without use of a needle guide, and found higher procedure success rates within the first and second attempts, reduced time to obtain access (16 seconds vs 30 seconds; P = .0001) and increased needle visibility (86% vs 32%; P < .0001) with the use of a needle guide.66 Another study comparing a short-axis versus long-axis approach with a needle guide showed improved needle visualization using a long-axis approach with a needle guide.67 A randomized study comparing use of a novel, sled-mounted needle guide to a free-hand approach for venous cannulation in simulation models showed the novel, sled-mounted needle guide improved overall success rates and efficiency of cannulation.68
Central Venous Access Techniques
10. We recommend that providers should use a standardized procedure checklist that includes use of real-time ultrasound guidance to reduce the risk of central line-associated bloodstream infection (CLABSI) from CVC insertion.
Rationale: A standardized checklist or protocol should be developed to ensure compliance with all recommendations for insertion of CVCs. Evidence-based protocols address periprocedural issues, such as indications for CVC, and procedural techniques, such as use of maximal sterile barrier precautions to reduce the risk of infection. Protocols and checklists that follow established guidelines for CVC insertion have been shown to decrease CLABSI rates.69,70 Similarly, development of checklists and protocols for maintenance of central venous catheters have been effective in reducing CLABSIs.71 Although no externally-validated checklist has been universally accepted or endorsed by national safety organizations, a few internally-validated checklists are available through peer-reviewed publications.72,73 An observational educational cohort of internal medicine residents who received training using simulation of the entire CVC insertion process was able to demonstrate fewer CLABSIs after the simulator-trained residents rotated in the intensive care unit (ICU) (0.50 vs 3.2 infections per 1,000 catheter days, P = .001).74
11. We recommend that providers should use real-time ultrasound guidance, combined with aseptic technique and maximal sterile barrier precautions, to reduce the incidence of infectious complications from CVC insertion.
Rationale: The use of real-time ultrasound guidance for CVC placement has demonstrated a statistically significant reduction in CLABSIs compared to landmark-based techniques.75 The Centers for Disease Control and Prevention (CDC) guidelines for the prevention of intravascular catheter-related infections recommend the use of ultrasound guidance to reduce the number of cannulation attempts and risk of mechanical complications.69 A prospective, three-arm study comparing ultrasound-guided long-axis, short-axis, and landmark-based approaches showed a CLABSI rate of 20% in the landmark-based group versus 10% in each of the ultrasound groups.57 Another randomized study comparing use of ultrasound guidance to a landmark-based technique for IJV CVC insertion demonstrated significantly lower CLABSI rates with the use of ultrasound (2% vs 10%; P < .05).72
Studies have shown that a systems-based intervention featuring a standardized catheter kit or catheter bundle significantly reduced CLABSI rates.76-78 A complete review of all preventive measures to reduce the risk of CLABSI is beyond the scope of this review, but a few key points will be mentioned. First, aseptic technique includes proper hand hygiene and skin sterilization, which are essential measures to reduce cutaneous colonization of the insertion site and reduce the risk of CLABSIs.79 In a systematic review and meta-analysis of eight studies including over 4,000 catheter insertions, skin antisepsis with chlorhexidine was associated with a 50% reduction in CLABSIs compared with povidone iodine.11 Therefore, a chlorhexidine-containing solution is recommended for skin preparation prior to CVC insertion per guidelines by Healthcare Infection Control Practices Advisory Committee/CDC, Society for Healthcare Epidemiology of America/Infectious Diseases Society of America, and American Society of Anesthesiologists.11,69,80,81 Second, maximal sterile barrier precautions refer to the use of sterile gowns, sterile gloves, caps, masks covering both the mouth and nose, and sterile full-body patient drapes. Use of maximal sterile barrier precautions during CVC insertion has been shown to reduce the incidence of CLABSIs compared to standard precautions.26,79,82-84 Third, catheters containing antimicrobial agents may be considered for hospital units with higher CLABSI rates than institutional goals, despite a comprehensive preventive strategy, and may be considered in specific patient populations at high risk of severe complications from a CLABSI.11,69,80 Finally, providers should use a standardized procedure set-up when inserting CVCs to reduce the risk of CLABSIs. The operator should confirm availability and proper functioning of ultrasound equipment prior to commencing a vascular access procedure. Use of all-inclusive procedure carts or kits with sterile ultrasound probe covers, sterile gel, catheter kits, and other necessary supplies is recommended to minimize interruptions during the procedure, and can ultimately reduce the risk of CLABSIs by ensuring maintenance of a sterile field during the procedure.13
12. We recommend that providers should use real-time ultrasound guidance for internal jugular vein catheterization, which reduces the risk of mechanical and infectious complications, the number of needle passes, and time to cannulation and increases overall procedure success rates.
Rationale: The use of real-time ultrasound guidance for CVC insertion has repeatedly demonstrated better outcomes compared to a landmark-based approach in adults.13 Several randomized controlled studies have demonstrated that real-time ultrasound guidance for IJV cannulation reduces the risk of procedure-related mechanical and infectious complications, and improves first-pass and overall success rates in diverse care settings.27,29,45,50,53,65,75,85-90 Mechanical complications that are reduced with ultrasound guidance include pneumothorax and carotid artery puncture.4,5,45,46,53,62,75,86-93 Currently, several medical societies strongly recommend the use of ultrasound guidance during insertion of IJV CVCs.10-12,14,94-96
A meta-analysis by Hind et al. that included 18 randomized controlled studies demonstrated use of real-time ultrasound guidance reduced failure rates (RR 0.14, 95% CI 0.06 to 0.33; P < .0001), increased first-attempt success rates (RR 0.59, 95% CI 0.39 to 0.88; P = .009), reduced complication rates (RR 0.43, 95% CI 0.22 to 0.87; P = .02) and reduced procedure time (P < .0001), compared to a traditional landmark-based approach when inserting IJV CVCs.5
A Cochrane systematic review compared ultrasound-guided versus landmark-based approaches for IJV CVC insertion and found use of real-time ultrasound guidance reduced total complication rates by 71% (RR 0.29, 95% CI 0.17 to 0.52; P < .0001), arterial puncture rates by 72% (RR 0.28, 95% CI 0.18 to 0.44; P < .00001), and rates of hematoma formation by 73% (RR 0.27, 95% CI 0.13 to 0.55; P = .0004). Furthermore, the number of attempts for successful cannulation was reduced (mean difference -1.19 attempts, 95% CI -1.45 to -0.92; P < .00001), the chance of successful insertion on the first attempt was increased by 57% (RR 1.57, 95% CI 1.36 to 1.82; P < .00001), and overall procedure success rates were modestly increased in all groups by 12% (RR 1.12, 95% CI 1.08 to 1.17; P < .00001).46
An important consideration in performing ultrasound guidance is provider experience. A prospective observational study of patients undergoing elective CVC insertion demonstrated higher complication rates for operators that were inexperienced (25.2%) versus experienced (13.6%).54 A randomized controlled study comparing experts and novices with or without the use of ultrasound guidance for IJV CVC insertion demonstrated higher success rates among expert operators and with the use of ultrasound guidance. Among novice operators, the complication rates were lower with the use of ultrasound guidance.97 One study evaluated the procedural success and complication rates of a two-physician technique with one physician manipulating the transducer and another inserting the needle for IJV CVC insertion. This study concluded that procedural success rates and frequency of complications were directly affected by the experience of the physician manipulating the transducer and not by the experience of the physician inserting the needle.98
The impact of ultrasound guidance on improving procedural success rates and reducing complication rates is greatest in patients that are obese, short necked, hypovolemic, or uncooperative.93 Several studies have demonstrated fewer needle passes and decreased time to cannulation compared to the landmark technique in these populations.46,49,53,86-88,92,93
Ultrasound-guided placement of IJV catheters can safely be performed in patients with disorders of hemostasis and those with multiple previous catheter insertions in the same vein.9 Ultrasound-guided placement of CVCs in patients with disorders of hemostasis is safe with high success and low complication rates. In a case series of liver patients with coagulopathy (mean INR 2.17 ± 1.16, median platelet count 150K), the use of ultrasound guidance for CVC insertion was highly successful with no major bleeding complications.99
A study of renal failure patients found high success rates and low complication rates in the patients with a history of multiple previous catheterizations, poor compliance, skeletal deformities, previous failed cannulations, morbid obesity, and disorders of hemostasis.100 A prospective observational study of 200 ultrasound-guided CVC insertions for apheresis showed a 100% success rate with a 92% first-pass success rate.101
The use of real-time ultrasound guidance for IJV CVC insertion has been shown to be cost effective by reducing procedure-related mechanical complications and improving procedural success rates. A companion cost-effectiveness analysis estimated that for every 1,000 patients, 90 complications would be avoided, with a net cost savings of approximately $3,200 using 2002 prices.102
13. We recommend that providers who routinely insert subclavian vein CVCs should use real-time ultrasound guidance, which has been shown to reduce the risk of mechanical complications and number of needle passes and increase overall procedure success rates compared with landmark-based techniques.
Rationale: In clinical practice, the term ultrasound-guided subclavian vein CVC insertion is commonly used. However, the needle insertion site is often lateral to the first rib and providers are technically inserting the CVC in the axillary vein. The subclavian vein becomes the axillary vein at the lateral border of the first rib where the cephalic vein branches from the subclavian vein. To be consistent with common medical parlance, we use the phrase ultrasound-guided subclavian vein CVC insertion in this document.
Advantages of inserting CVCs in the subclavian vein include reliable surface anatomical landmarks for vein location, patient comfort, and lower risk of infection.103 Several observational studies have demonstrated the technique for ultrasound-guided subclavian vein CVC insertion is feasible and safe.104-107 In a large retrospective observational study of ultrasound-guided central venous access among a complex patient group, the majority of patients were cannulated successfully and safely. The subset of patients undergoing axillary vein CVC insertion (n = 1,923) demonstrated a low rate of complications (0.7%), proving it is a safe and effective alternative to the IJV CVC insertion.107
A Cochrane review of ultrasound-guided subclavian vein cannulation (nine studies, 2,030 participants, 2,049 procedures), demonstrated that real-time two-dimensional ultrasound guidance reduced the risk of inadvertent arterial punctures (three studies, 498 participants, RR 0.21, 95% CI 0.06 to 0.82; P = .02) and hematoma formation (three studies, 498 participants, RR 0.26, 95% CI 0.09 to 0.76; P = .01).46 A systematic review and meta-analysis of 10 randomized controlled studies comparing ultrasound-guided versus landmark-based subclavian vein CVC insertion demonstrated a reduction in the risk of arterial punctures, hematoma formation, pneumothorax, and failed catheterization with the use of ultrasound guidance.105
A randomized controlled study comparing ultrasound-guided vs landmark-based approaches to subclavian vein cannulation found that use of ultrasound guidance had a higher success rate (92% vs 44%, P = .0003), fewer minor complications (1 vs 11, P = .002), fewer attempts (1.4 vs 2.5, P = .007) and fewer catheter kits used (1.0 vs 1.4, P = .0003) per cannulation.108
Fragou et al. randomized patients undergoing subclavian vein CVC insertion to a long-axis approach versus a landmark-based approach and found a significantly higher success rate (100% vs 87.5%, P < .05) and lower rates of mechanical complications: artery puncture (0.5% vs 5.4%), hematoma (1.5% vs 5.4%), hemothorax (0% vs 4.4%), pneumothorax (0% vs 4.9%), brachial plexus injury (0% vs 2.9%), phrenic nerve injury (0% vs 1.5%), and cardiac tamponade (0% vs 0.5%).109 The average time to obtain access and the average number of insertion attempts (1.1 ± 0.3 vs 1.9 ± 0.7, P < .05) were significantly reduced in the ultrasound group compared to the landmark-based group.95
A retrospective review of subclavian vein CVC insertions using a supraclavicular approach found no reported complications with the use of ultrasound guidance vs 23 mechanical complications (8 pneumothorax, 15 arterial punctures) with a landmark-based approach.106 However, it is important to note that a supraclavicular approach is not commonly used in clinical practice.
14. We recommend that providers should use real-time ultrasound guidance for femoral venous access, which has been shown to reduce the risk of arterial punctures and total procedure time and increase overall procedure success rates.
Rationale: Anatomy of the femoral region varies, and close proximity or overlap of the femoral vein and artery is common.51 Early studies showed that ultrasound guidance for femoral vein CVC insertion reduced arterial punctures compared with a landmark-based approach (7% vs 16%), reduced total procedure time (55 ± 19 vs 79 ± 62 seconds), and increased procedure success rates (100% vs 90%).52 A Cochrane review that pooled data from four randomized studies comparing ultrasound-guided vs landmark-based femoral vein CVC insertion found higher first-attempt success rates with the use of ultrasound guidance (RR 1.73, 95% CI 1.34 to 2.22; P < .0001) and a small increase in the overall procedure success rates (RR 1.11, 95% CI 1.00 to 1.23; P = .06). There was no difference in inadvertent arterial punctures or other complications.110
Peripheral Venous Access Techniques
15. We recommend that providers should use real-time ultrasound guidance for the insertion of peripherally inserted central catheters (PICCs), which is associated with higher procedure success rates and may be more cost effective compared with landmark-based techniques.
Rationale: Several studies have demonstrated that providers who use ultrasound guidance vs landmarks for PICC insertion have higher procedural success rates, lower complication rates, and lower total placement costs. A prospective observational report of 350 PICC insertions using ultrasound guidance reported a 99% success rate with an average of 1.2 punctures per insertion and lower total costs.20 A retrospective observational study of 500 PICC insertions by designated specialty nurses revealed an overall success rate of 95%, no evidence of phlebitis, and only one CLABSI among the catheters removed.21 A retrospective observational study comparing several PICC variables found higher success rates (99% vs 77%) and lower thrombosis rates (2% vs 9%) using ultrasound guidance vs landmarks alone.22 A study by Robinson et al. demonstrated that having a dedicated PICC team equipped with ultrasound increased their institutional insertion success rates from 73% to 94%.111
A randomized controlled study comparing ultrasound-guided versus landmark-based PICC insertion found high success rates with both techniques (100% vs 96%). However, there was a reduction in the rate of unplanned catheter removals (4.0% vs 18.7%; P = .02), mechanical phlebitis (0% vs 22.9%; P < .001), and venous thrombosis (0% vs 8.3%; P = .037), but a higher rate of catheter migration (32% vs 2.1%; P < .001). Compared with the landmark-based group, the ultrasound-guided group had significantly lower incidence of severe contact dermatitis (P = .038), and improved comfort and costs up to 3 months after PICC placement (P < .05).112
Routine postprocedure chest x-ray (CXR) is generally considered unnecessary if the PICC is inserted with real-time ultrasound guidance along with use of a newer tracking devices, like the magnetic navigation system with intracardiac electrodes.9 Ultrasound can also be used to detect malpositioning of a PICC immediately after completing the procedure. A randomized controlled study comparing ultrasound versus postprocedure CXR detected one malpositioned PICC in the ultrasound group versus 11 in the control group. This study suggested that ultrasound can detect malpositioning immediately postprocedure and reduce the need for a CXR and the possibility of an additional procedure to reposition a catheter.113
16. We recommend that providers should use real-time ultrasound guidance for the placement of peripheral intravenous lines (PIV) in patients with difficult peripheral venous access to reduce the total procedure time, needle insertion attempts, and needle redirections. Ultrasound-guided PIV insertion is also an effective alternative to CVC insertion in patients with difficult venous access.
Rationale: Difficult venous access refers to patients that have had two unsuccessful attempts at PIV insertion using landmarks or a history of difficult access (i.e. edema, obesity, intravenous drug use, chemotherapy, diabetes, hypovolemia, chronic illness, vasculopathy, multiple prior hospitalizations). A meta-analysis of seven randomized controlled studies concluded that ultrasound guidance increases the likelihood of successful PIV insertion (pooled OR 2.42, 95% CI 1.26 to 4.68; P < .008).18 A second meta-analysis that pooled data from seven studies (six randomized controlled studies) confirmed that ultrasound guidance improves success rates of PIV insertion (OR 3.96, 95% CI 1.75 to 8.94).19 Approximately half of these studies had physician operators while the other half had nurse operators.
In one prospective observational study of emergency department patients with two failed attempts of landmark-based PIV insertion, ultrasound guidance with a modified-Seldinger technique showed a relatively high success rate (96%), fewer needle sticks (mean 1.32 sticks, 95% CI 1.12 to 1.52), and shorter time to obtain access (median time 68 seconds).114 Other prospective observational studies have demonstrated that ultrasound guidance for PIV insertion has a high success rate (87%),115 particularly with brachial or basilic veins PIV insertion, among patients with difficult PIV access, defined as having had ≥2 failed attempts.58
Since insertion of PIVs with ultrasound guidance has a high success rate, there is potential to reduce the reliance on CVC insertion for venous access only. In a study of patients that had had two failed attempts at PIV insertion based on landmarks, a PIV was successfully inserted with ultrasound guidance in 84% of patients, obviating the need for CVC placement for venous access.116 A prospective observational study showed ultrasound-guided PIV insertion was an effective alternative to CVC placement in ED patients with difficult venous access with only 1% of patients requiring a CVC.117 Use of ultrasound by nurses for PIV placement has also been shown to reduce the time to obtain venous access, improve patient satisfaction, and reduce the need for physician intervention.118 In a prospective observational study of patients with difficult access, the majority of patients reported a better experience with ultrasound-guided PIV insertion compared to previous landmark-based attempts with an average satisfaction score of 9.2/10 with 76% of patients rating the experience a 10.119 A strong recommendation has been made for use of ultrasound guidance in patients with difficult PIV placement by la Société Française d’Anesthésie et de Réanimation (The French Society of Anesthesia and Resuscitation).95
17. We suggest using real-time ultrasound guidance to reduce the risk of vascular, infectious, and neurological complications during PIV insertion, particularly in patients with difficult venous access.
Rationale: The incidence of complications from PIV insertion is often underestimated. Vascular complications include arterial puncture, hematoma formation, local infiltration or extravasation of fluid, and superficial or deep venous thrombosis. The most common infectious complications with PIV insertion are phlebitis and cellulitis.120 One observational study reported PIV complications occurring in approximately half of all patients with the most common complications being phlebitis, hematoma formation, and fluid/blood leakage.121
A retrospective review of ICU patients who underwent ultrasound-guided PIV insertion by a single physician showed high success rates (99%) with low rates of phlebitis/cellulitis (0.7%).There was an assumed benefit of risk reduction due to the patients no longer requiring a CVC after successful PIV placement.122 Another study found very low rates of infection with both landmark-based and ultrasound-guided PIV placement performed by emergency department nurses, suggesting that there is no increased risk of infection with the use of ultrasound.123 To reduce the risk of infection from PIV insertion, we recommend the use of sterile gel and sterile transducer cover (See Recommendation 2).
Arterial Access Techniques
18. We recommend that providers should use real-time ultrasound guidance for arterial access, which has been shown to increase first-pass success rates, reduce the time to cannulation, and reduce the risk of hematoma development compared with landmark-based techniques.
Rationale: Several randomized controlled studies have assessed the value of ultrasound in arterial catheter insertion. Shiver et al. randomized 60 patients admitted to a tertiary center emergency department to either palpation or ultrasound-guided arterial cannulation. They demonstrated a first-pass success rate of 87% in the ultrasound group compared with 50% in the landmark technique group. In the same study, the use of ultrasound was also associated with reduced time needed to establish arterial access and a 43% reduction in the development of hematoma at the insertion site.124 Levin et al. demonstrated a first-pass success rate of 62% using ultrasound versus 34% by palpation alone in 69 patients requiring intraoperative invasive hemodynamic monitoring.125 Additional randomized controlled studies have demonstrated that ultrasound guidance increases first-attempt success rates compared to traditional palpation.23,126,127
19. We recommend that providers should use real-time ultrasound guidance for femoral arterial access, which has been shown to increase first-pass success rates and reduce the risk of vascular complications.
Rationale: Although it is a less frequently used site, the femoral artery may be accessed for arterial blood sampling or invasive hemodynamic monitoring, and use of ultrasound guidance has been shown to improve the first-pass success rates of femoral artery cannulation. It is important to note that most of these studies comparing ultrasound-guided vs landmark-based femoral artery cannulation were performed in patients undergoing diagnostic or interventional vascular procedures.
A meta-analysis of randomized controlled studies comparing ultrasound-guided vs landmark-based femoral artery catheterization found use of ultrasound guidance was associated with a 49% reduction in overall complications (RR 0.51, 95% CI 0.28 to 0.91; P > .05) and 42% improvement in first-pass success rates.128 In another study, precise site selection with ultrasound was associated with fewer pseudoaneurysms in patients undergoing femoral artery cannulation by ultrasound guidance vs palpation for cardiac catheterization (3% vs 5%, P < .05).129
A multicenter randomized controlled study comparing ultrasound vs fluoroscopic guidance for femoral artery catheterization demonstrated ultrasound guidance improved rates of common femoral artery (CFA) cannulation in patients with high CFA bifurcations (83% vs 70%, P < .01).130 Furthermore, ultrasound guidance improved first-pass success rates (83% vs 46%, P < .0001), reduced number of attempts (1.3 vs 3.0, P < .0001), reduced risk of venipuncture (2.4% vs 15.8%, P < .0001), and reduced median time to obtain access (136 seconds vs148 seconds, P = .003). Vascular complications occurred in fewer patients in the ultrasound vs fluoroscopy groups (1.4% vs 3.4% P = .04). Reduced risk of hematoma formation with routine use of ultrasound guidance was demonstrated in one retrospective observational study (RR 0.62, 95% CI 0.46 to 0.84; P < .01).131
20. We recommend that providers should use real-time ultrasound guidance for radial arterial access, which has been shown to increase first-pass success rates, reduce the time to successful cannulation, and reduce the risk of complications compared with landmark-based techniques.
Rationale: Ultrasound guidance is particularly useful for radial artery cannulation in patients with altered anatomy, obesity, nonpulsatile blood flow, low perfusion, and previously unsuccessful cannulation attempts using a landmark-guided approach.132
A multicenter randomized controlled study that was not included in the abovementioned meta-analyses showed similar benefits of using ultrasound guidance vs landmarks for radial artery catheterization: a reduction in the number of attempts with ultrasound guidance (1.65 ± 1.2 vs 3.05 ± 3.4, P < .0001) and time to obtain access (88 ± 78 vs 108 ± 112 seconds, P = .006), and increased first-pass success rates (65% vs 44%, P < .0001). The use of ultrasound guidance was found to be particularly useful in patients with difficult access by palpation alone.135
Regarding the level of expertise required to use ultrasound guidance, a prospective observational study demonstrated that physicians with little previous ultrasound experience were able to improve their first-attempt success rates and procedure time for radial artery cannulation compared to historical data of landmark-based insertions.136
Postprocedure
21. We recommend that post-procedure pneumothorax should be ruled out by the detection of bilateral lung sliding using a high-frequency linear transducer before and after insertion of internal jugular and subclavian vein CVCs.
Rationale: Detection of lung sliding with two-dimensional ultrasound rules out pneumothorax, and disappearance of lung sliding in an area where it was previously seen is a strong predictor of postprocedure pneumothorax. In a study of critically ill patients, the disappearance of lung sliding was observed in 100% of patients with pneumothorax vs 8.8% of patients without pneumothorax. For detection of pneumothorax, lung sliding showed a sensitivity of 95%, specificity of 91%, and negative predictive value of 100% (P < .001).137 Another study by the same author showed that the combination of horizontal artifacts (absence of comet-tail artifact) and absence of lung sliding had a sensitivity of 100%, specificity of 96.5%, and negative predictive value of 100% for the detection of pneumothorax.138 A meta-analysis of 10 studies on the diagnostic accuracy of CVC confirmation with bedside ultrasound vs chest radiography reported detection of all 12 pneumothoraces with ultrasound, whereas chest radiography missed two pneumothoraces. The pooled sensitivity and specificity of ultrasound for the detection of pneumothorax was 100%, although an imperfect gold standard bias likely affected the results. An important advantage of bedside ultrasound is the ability to rule out pneumothorax immediately after the procedure while at the bedside. The mean time for confirmation of CVC placement with bedside ultrasound was 6 minutes versus 64 minutes and 143 minutes for completion and interpretation of a chest radiograph, respectively.139
22. We recommend that providers should use ultrasound with rapid infusion of agitated saline to visualize a right atrial swirl sign (RASS) for detecting catheter tip misplacement during CVC insertion. The use of RASS to detect the catheter tip may be considered an advanced skill that requires specific training and expertise.
Rationale: Bedside echocardiography is a reliable tool to detect catheter tip misplacement during CVC insertion. In one study, catheter misplacement was detected by bedside echocardiography with a sensitivity of 96% and specificity of 83% (positive predictive value 98%, negative predictive value 55%) and prevented distal positioning of the catheter tip.140 A prospective observational study assessed for RASS, which is turbulent flow in the right atrium after a rapid saline flush of the distal CVC port, to exclude catheter malposition. In this study with 135 CVC placements, visualization of RASS with ultrasound was able to identify all correct CVC placements and three of four catheter misplacements. Median times to complete the ultrasound exam vs CXR were 1 vs 20 minutes, respectively, with a median difference of 24 minutes (95% CI 19.6 to 29.3, P < .0001) between the two techniques.141
A prospective observational study assessed the ability of bedside transthoracic echocardiography to detect the guidewire, microbubbles, or both, in the right atrium compared to transesophageal echocardiography as the gold standard. Bedside transthoracic echocardiography allowed visualization of the right atrium in 94% of patients, and both microbubbles plus guidewire in 91% of patients.142 Hence, bedside transthoracic echocardiography allows adequate visualization of the right atrium. Another prospective observational study combining ultrasonography and contrast enhanced RASS resulted in 96% sensitivity and 93% specificity for the detection of a misplaced catheter, and the concordance with chest radiography was 96%.143
Training
23. To reduce the risk of mechanical and infectious complications, we recommend that novice providers should complete a systematic training program that includes a combination of simulation-based practice, supervised insertion on patients, and evaluation by an expert operator before attempting ultrasound-guided CVC insertion independently on patients.
Rationale: Cumulative experience has been recognized to not be a proxy for mastery of a clinical skill.144 The National Institute for Clinical Excellence (NICE) has recommended that providers performing ultrasound-guided CVC insertion should receive appropriate training to achieve competence before performing the procedure independently.7 Surveys have demonstrated that lack of training is a commonly reported barrier for not using ultrasound.145,146
Structured training programs on CVC insertion have been shown to reduce the occurrence of infectious and mechanical complications.74,143,147-149 The use of ultrasound and checklists, bundling of supplies, and practice with simulation models, as a part of a structured training program, can improve patient safety related to CVC insertion.9,140,150-154
Simulation-based practice has been used in medical education to provide deliberate practice and foster skill development in a controlled learning environment.155-158 Studies have shown transfer of skills demonstrated in a simulated environment to clinical practice, which can improve CVC insertion practices.159,160 Simulation accelerates learning of all trainees, especially novice trainees, and mitigates risks to patients by allowing trainees to achieve a minimal level of competence before attempting the procedure on real patients.152,161,162 Residents that have been trained using simulation preferentially select the IJV site,147 and more reliably use ultrasound to guide their CVC insertions.160,163
Additionally, simulation-based practice allows exposure to procedures and scenarios that may occur infrequently in clinical practice.
Although there is evidence on efficacy of simulation-based CVC training programs, there is no broadly accepted consensus on timing, duration, and content of CVC training programs for trainees or physicians in practice. The minimum recommended technical skills a trainee must master include the ability to (1) manipulate the ultrasound machine to produce a high-quality image to identify the target vessel, (2) advance the needle under direct visualization to the desired target site and depth, (3) deploy the catheter into the target vessel and confirm catheter placement in the target vessel using ultrasound, and (4) ensure the catheter has not been inadvertently placed in an unintended vessel or structure.153
A variety of simulation models are currently used to practice CVC insertion at the most common sites: the internal jugular, subclavian, basilic, and brachial veins.164,165 Effective simulation models should contain vessels that mimic normal anatomy with muscles, soft tissues, and bones. Animal tissue models, such as turkey or chicken breasts, may be effective for simulated practice of ultrasound-guided CVC insertion.166,167 Ultrasound-guided CVC training using human cadavers has also been shown to be effective.168
24. We recommend that cognitive training in ultrasound-guided CVC insertion should include basic anatomy, ultrasound physics, ultrasound machine knobology, fundamentals of image acquisition and interpretation, detection and management of procedural complications, infection prevention strategies, and pathways to attain competency.
Rationale: After receiving training in ultrasound-guided CVC insertion, physicians report significantly higher comfort with the use of ultrasound compared to those who have not received such training.145 Learners find training sessions worthwhile to increase skill levels,167 and skills learned from simulation-based mastery learning programs have been retained up to one year.158
Several commonalities have been noted across training curricula. Anatomy and physiology didactics should include vessel anatomy (location, size, and course);9 vessel differentiation by ultrasound;9,69 blood flow dynamics;69 Virchow’s triad;69 skin integrity and colonization;150 peripheral nerve identification and distribution;9 respiratory anatomy;9,69 upper and lower extremity, axillary, neck, and chest anatomy.9,69 Vascular anatomy is an essential curricular component that may help avoid preventable CVC insertion complications, such as inadvertent nerve, artery, or lung puncture.150,169 Training curricula should also include ultrasound physics (piezoelectric effect, frequency, resolution, attenuation, echogenicity, Doppler ultrasound, arterial and venous flow characteristics), image acquisition and optimization (imaging mode, focus, dynamic range, probe types), and artifacts (reverberation, mirror, shadowing, enhancement).
CVC-related infections are an important cause of morbidity and mortality in the acute and long-term care environment.69 Infection and thrombosis can both be impacted by the insertion site selection, skin integrity, and catheter–vein ratio.2,3,84 Inexperience generally leads to more insertion attempts that can increase trauma during CVC insertion and potentially increase the risk of infections.170 To reduce the risk of infectious complications, training should include important factors to consider in site selection and maintenance of a sterile environment during CVC insertion, including use of maximal sterile barrier precautions, hand hygiene, and appropriate use of skin antiseptic solutions.
Professional society guidelines have been published with recommendations of appropriate techniques for ultrasound-guided vascular access that include training recommendations.9,154 Training should deconstruct the insertion procedure into readily understood individual steps, and can be aided by demonstration of CVC insertion techniques using video clips. An alternative to face-to-face training is internet-based training that has been shown to be as effective as traditional teaching methods in some medical centers.171 Additional methods to deliver cognitive instruction include textbooks, continuing medical education courses, and digital videos.164,172
25. We recommend that trainees should demonstrate minimal competence before placing ultrasound-guided CVCs independently. A minimum number of CVC insertions may inform this determination, but a proctored assessment of competence is most important.
Rationale: CVC catheter placement carries the risk of serious complications including arterial injury or dissection, pneumothorax, or damage to other local structures; arrhythmias; catheter malposition; infection; and thrombosis. Although there is a lack of consensus and high-quality evidence for the certification of skills to perform ultrasound-guided CVC insertion, recommendations have been published advocating for formal and comprehensive training programs in ultrasound-guided CVC insertion with an emphasis on expert supervision prior to independent practice.9,153,154 Two groups of expert operators have recommended that training should include at least 8-10 supervised ultrasound-guided CVC insertions.154,173,174 A consensus task force from the World Congress of Vascular Access has recommended a minimum of six to eight hours of didactic education, four hours of hands-on training on simulation models, and six hours of hands-on ultrasound training on human volunteers to assess normal anatomy.175 This training should be followed by supervised ultrasound-guided CVC insertions until the learner has demonstrated minimal competence with a low rate of complications.35 There is general consensus that arbitrary numbers should not be the sole determinant of competence, and that the most important determinant of competence should be an evaluation by an expert operator.176
26. We recommend that didactic and hands-on training for trainees should coincide with anticipated times of increased performance of vascular access procedures. Refresher training sessions should be offered periodically.
Rationale: Simulation-based CVC training courses have shown a rapid improvement in skills, but lack of practice leads to deterioration of technical skills.161,162,177,178 Thus, a single immersive training session is insufficient to achieve and maintain mastery of skills, and an important factor to acquire technical expertise is sustained, deliberate practice with feedback.179 Furthermore, an insidious decay in skills may go unrecognized as a learner’s comfort and self-confidence does not always correlate with actual performance, leading to increased risk of errors and potential for procedural complications.147,158,180-183 Given the decay in technical skills over time, simulation-based training sessions are most effective when they occur in close temporal proximity to times when those skills are most likely to be used; for example, a simulation-based training session for trainees may be most effective just before the start of a critical care rotation.152 Regularly scheduled training sessions with monitoring and feedback by expert operators can reinforce procedural skills and prevent decay. Some experts have recommended that a minimum of 10 ultrasound-guided CVC insertions should be performed annually to maintain proficiency.153
27. We recommend that competency assessments should include formal evaluation of knowledge and technical skills using standardized assessment tools.
Rationale: Hospitalists and other healthcare providers that place vascular access catheters should undergo competency assessments proctored by an expert operator to verify that they have the required knowledge and skills.184,185 Knowledge competence can be partially evaluated using a written assessment, such as a multiple-choice test, assessing the provider’s cognitive understanding of the procedure.175 For ultrasound-guided CVC insertion, a written examination should be administered in conjunction with an ultrasound image assessment to test the learner’s recognition of normal vs abnormal vascular anatomy. Minimum passing standards should be established a priori according to local or institutional standards.
The final skills assessment should be objective, and the learner should be required to pass all critical steps of the procedure. Failure of the final skills assessment should lead to continued practice with supervision until the learner can consistently demonstrate correct performance of all critical steps. Checklists are commonly used to rate the technical performance of learners because they provide objective criteria for evaluation, can identify specific skill deficiencies, and can determine a learner’s readiness to perform procedures independently.186,187 The administration of skills assessments and feedback methods should be standardized across faculty. Although passing scores on both knowledge and skills assessments do not guarantee safe performance of a procedure independently, they provide a metric to ensure that a minimum level of competence has been achieved before allowing learners to perform procedures on patients without supervision.188
Competency assessments are a recommended component of intramural and extramural certification of skills in ultrasound-guided procedures. Intramural certification pathways differ by institution and often require additional resources including ultrasound machine(s), simulation equipment, and staff time, particularly when simulation-based assessments are incorporated into certification pathways. We recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals. However, initial and ongoing competency assessments can be performed during routine performance of procedures on patients. For an in-depth review of credentialing pathways for ultrasound-guided bedside procedures, we recommend reviewing the SHM Position Statement on Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.24
28. We recommend that competency assessments should evaluate for proficiency in the following knowledge and skills of CVC insertion:
a. Knowledge of the target vein anatomy, proper vessel identification, and recognition of anatomical variants
b. Demonstration of CVC insertion with no technical errors based on a procedural checklist
c. Recognition and management of acute complications, including emergency management of life-threatening complications
d. Real-time needle tip tracking with ultrasound and cannulation on the first attempt in at least five consecutive simulations.
Rationale: Recommendations have been published with the minimal knowledge and skills learners must demonstrate to perform ultrasound-guided vascular access procedures. These include operation of an ultrasound machine to produce high-quality images of the target vessel, tracking of the needle tip with real-time ultrasound guidance, and recognition and understanding of the management of procedural complications.154,175
First, learners must be able to perform a preprocedural assessment of the target vein, including size and patency of the vein; recognition of adjacent critical structures; and recognition of normal anatomical variants.175,189 Second, learners must be able to demonstrate proficiency in tracking the needle tip penetrating the target vessel, inserting the catheter into the target vessel, and confirming catheter placement in the target vessel with ultrasound.154,175 Third, learners must be able to demonstrate recognition of acute complications, including arterial puncture, hematoma formation, and development of pneumothorax.154,175 Trainees should be familiar with recommended evaluation and management algorithms, including indications for emergent consultation.190
29. We recommend a periodic proficiency assessments of all operators should be conducted to ensure maintenance of competency.
Rationale: Competency extends to periodic assessment and not merely an initial evaluation at the time of training.191 Periodic competency assessments should include assessment of proficiency of all providers that perform a procedure, including instructors and supervisors. Supervising providers should maintain their competency in CVC insertion through routine use of their skills in clinical practice.175 An observational study of emergency medicine residents revealed that lack of faculty comfort with ultrasound hindered the residents’ use of ultrasound.192 Thus, there is a need to examine best practices for procedural supervision of trainees because providers are often supervising procedures that they are not comfortable performing on their own.193
KNOWLEDGE GAPS
The process of producing this position statement revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for central and peripheral venous access and arterial access.
This position statement recommends a preprocedural ultrasound evaluation of blood vessels based on evidence that providers may detect anatomic anomalies, thrombosis, or vessel stenosis. Ultrasound can also reveal unsuspected high-risk structures in near proximity to the procedure site. Although previous studies have shown that providers can accurately assess vessels with ultrasound for these features, further study is needed to evaluate the effect of a standardized preprocedural ultrasound exam on clinical and procedural decision-making, as well as procedural outcomes.
Second, two ultrasound applications that are being increasingly used but have not been widely implemented are the use of ultrasound to evaluate lung sliding postprocedure to exclude pneumothorax and the verification of central line placement using a rapid infusion of agitated saline to visualize the RASS.139-141 Both of these applications have the potential to expedite postprocedure clearance of central lines for usage and decrease patient radiation exposure by obviating the need for postprocedure CXRs. Despite the supporting evidence, both of these applications are not yet widely used, as few providers have been trained in these techniques which may be considered advanced skills.
Third, despite advances in our knowledge of effective training for vascular access procedures, there is limited agreement on how to define procedural competence. Notable advancements in training include improved understanding of systematic training programs, development of techniques for proctoring procedures, definition of elements for hands-on assessments, and definition of minimum experience needed to perform vascular access procedures independently. However, application of these concepts to move learners toward independent practice remains variably interpreted at different institutions, likely due to limited resources, engrained cultures about procedures, and a lack of national standards. The development of hospitalist-based procedure services at major academic medical centers with high training standards, close monitoring for quality assurance, and the use of databases to track clinical outcomes may advance our understanding and delivery of optimal procedural training.
Finally, ultrasound technology is rapidly evolving which will affect training, techniques, and clinical outcomes in coming years. Development of advanced imaging software with artificial intelligence can improve needle visualization and tracking. These technologies have the potential to facilitate provider training in real-time ultrasound-guided procedures and improve the overall safety of procedures. Emergence of affordable, handheld ultrasound devices is improving access to ultrasound technology, but their role in vascular access procedures is yet to be defined. Furthermore, availability of wireless handheld ultrasound technology and multifrequency transducers will create new possibilities for use of ultrasound in vascular access procedures.
CONCLUSION
We have presented several evidence-based recommendations on the use of ultrasound guidance for placement of central and peripheral vascular access catheters that are intended for hospitalists and other healthcare providers who routinely perform vascular access procedures. By allowing direct visualization of the needle tip and target vessel, the use of ultrasound guidance has been shown in randomized studies to reduce needle insertion attempts, reduce needle redirections, and increase overall procedure success rates. The accuracy of ultrasound to identify the target vessel, assess for thrombosis, and detect anatomical anomalies is superior to that of physical examination. Hospitalists can attain competence in performing ultrasound-guided vascular access procedures through systematic training programs that combine didactic and hands-on training, which optimally include patient-based competency assessments.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators of Society of Hospital Medicine Point-of-care Ultrasound Task Force: Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Venkat Kalidindi, Ketino Kobaidze, Joshua Lenchus, Paul Mayo, Satyen Nichani, Vicki Noble, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Gerard Salame, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam J. Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Mathews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Mathews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El-Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Raad I. Intravascular-catheter-related infections. Lancet. 1998;351(9106):893-898. https://doi.org/10.1016/S0140-6736(97)10006-X.
2. Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286(6):700-707. https://doi.org/10.1001/jama.286.6.700.
3. Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986;146(2):259-261. https://doi.org/10.1001/archinte.146.2.259.
4. Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med. 1996;24(12):2053-2058. https://doi.org/10.1097/00003246-199612000-00020.
5. Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327(7411):361. https://doi.org/10.1136/bmj.327.7411.361.
6. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4):S1-S34. https://doi.org/10.1016/j.ajic.2011.01.003.
7. National Institute for Health and Care Excellence (NICE). Guidance on the Use of Ultrasound Locating Devices for Placing Central Venous Catheters; 2002.
8. Shojania KG, Duncan BW, McDonald KM, et al. Making health care safer: A critical analysis of patient safety practices. Evid Rep Technol Assess. Rockville, MD: Agency for Healthcare Research and Quality. 2001;43(43):i–x, 1.
9. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105-1117. https://doi.org/10.1007/s00134-012-2597-x.
10. Frykholm P, Pikwer A, Hammarskjöld F, et al. Clinical guidelines on central venous catheterisation. Swedish Society of Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiol Scand. 2014;58(5):508-524. https://doi.org/10.1111/aas.12295.
11. American Society of Anesthesiologists Task Force on Central Venous Access, Rupp SM, Apfelbaum JL, et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539-573. https://doi.org/10.1097/ALN.0b013e31823c9569.
12. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: General ultrasonography. Crit Care Med. 2015;43(11):2479-2502. https://doi.org/10.1097/CCM.0000000000001216.
13. Shekelle PG, Wachter RM, Pronovost PJ, et al. Making health care safer II: An updated critical analysis of the evidence for patient safety practices. Evid Rep Technol Assess. 2013;211:1-945.
14. Troianos CA, Hartman GS, Glas KE, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2011;24(12):1291-1318. https://doi.org/10.1016/j.echo.2011.09.021.
15. Soni NJ, Reyes LF, Keyt H, et al. Use of ultrasound guidance for central venous catheterization: a national survey of intensivists and hospitalists. J Crit Care. 2016;36:277-283. https://doi.org/10.1016/j.jcrc.2016.07.014.
16. Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220-1229. https://doi.org/10.1056/NEJMoa1500964.
17. Maizel J, Bastide MA, Richecoeur J, et al. Practice of ultrasound-guided central venous catheter technique by the French intensivists: a survey from the BoReal study group. Ann Intensive Care. 2016;6(1):76. https://doi.org/10.1186/s13613-016-0177-x.
18. Egan G, Healy D, O’Neill H, et al. Ultrasound guidance for difficult peripheral venous access: systematic review and meta-analysis. Emerg Med J. 2013;30(7):521-526. https://doi.org/10.1136/emermed-2012-201652.
19. Stolz LA, Stolz U, Howe C, Farrell IJ, Adhikari S. Ultrasound-guided peripheral venous access: a meta-analysis and systematic review. J Vasc Access. 2015;16(4):321-326. https://doi.org/10.5301/jva.5000346.
20. Sofocleous CT, Schur I, Cooper SG, et al. Sonographically guided placement of peripherally inserted central venous catheters: review of 355 procedures. AJR Am J Roentgenol. 1998;170(6):1613-1616. https://doi.org/10.2214/ajr.170.6.9609183.
21. Nichols I, Humphrey JP. The efficacy of upper arm placement of peripherally inserted central catheters using bedside ultrasound and microintroducer technique. J Infus Nurs Off Publ Infus Nurs Soc. 2008;31(3):165-176. https://doi.org/10.1097/01.NAN.0000317703.66395.b8.
22. Stokowski G, Steele D, Wilson D. The use of ultrasound to improve practice and reduce complication rates in peripherally inserted central catheter insertions. J Infus Nurs Off Publ Infus Nurs Soc. 2009;32(3):145-155. https://doi.org/10.1097/NAN.0b013e3181a1a98f.
23. Shiloh AL, Savel RH, Paulin LM, Eisen LA. Ultrasound-guided catheterization of the radial artery: a systematic review and meta-analysis of randomized controlled trials. Chest. 2011;139(3):524-529. https://doi.org/10.1378/chest.10-0919.
24. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: A position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
25. Chopra V, Flanders SA, Saint S, et al. The Michigan appropriateness guide for intravenous catheters (Magic): results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6):S1-S40. https://doi.org/10.7326/M15-0744.
26. Raad II, Hohn DC, Gilbreath BJ, et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994;15(4):231-238.
27. Slama M, Novara A, Safavian A, et al. Improvement of internal jugular vein cannulation using an ultrasound-guided technique. Intensive Care Med. 1997;23(8):916-919. https://doi.org/10.1007/s001340050432.
28. Seyahi N, Kahveci A, Altiparmak MR, Serdengecti K, Erek E. Ultrasound imaging findings of femoral veins in patients with renal failure and its impact on vascular access. Nephrol Dial Transplant. 2005;20(9):1864-1867. https://doi.org/10.1093/ndt/gfh942.
29. Lin BS, Kong CW, Tarng DC, Huang TP, Tang GJ. Anatomical variation of the internal jugular vein and its impact on temporary haemodialysis vascular access: an ultrasonographic survey in uraemic patients. Nephrol Dial Transplant. 1998;13(1):134-138. https://doi.org/10.1093/ndt/13.1.134.
30. Troianos CA, Kuwik RJ, Pasqual JR, Lim AJ, Odasso DP. Internal jugular vein and carotid artery anatomic relation as determined by ultrasonography. Anesthesiology. 1996;85(1):43-48. https://doi.org/10.1097/00000542-199607000-00007.
31. Gordon AC, Wright I, Pugh ND. Duplication of the superficial femoral vein: recognition with duplex ultrasonography. Clin Rad. 1996;51(9):622-624. https://doi.org/10.1016/S0009-9260(96)80055-9.
32. Sulek CA, Gravenstein N, Blackshear RH, Weiss L. Head rotation during internal jugular vein cannulation and the risk of carotid artery puncture. Anesth Analg. 1996;82(1):125-128. https://doi.org/10.1097/00000539-199601000-00022.
33. Shrestha BR, Gautam B. Ultrasound versus the landmark technique: a prospective randomized comparative study of internal jugular vein cannulation in an intensive care unit. JNMA J Nepal Med Assoc. 2011;51(182):56-61. https://doi.org/10.31729/jnma.148.
34. Brederlau J, Greim C, Schwemmer U, et al. Ultrasound-guided cannulation of the internal jugular vein in critically ill patients positioned in 30 degrees dorsal elevation. Eur J Anaesthesiol. 2004;21(9):684-687. https://doi.org/10.1097/00003643-200409000-00003.
35. Lamperti M, Subert M, Cortellazzi P, et al. Is a neutral head position safer than 45-degree neck rotation during ultrasound-guided internal jugular vein cannulation? Results of a randomized controlled clinical trial. Anesth Analg. 2012;114(4):777-784. https://doi.org/10.1213/ANE.0b013e3182459917.
36. Benter T, Teichgräber UK, Klühs L, et al. Anatomical variations in the internal jugular veins of cancer patients affecting central venous access. Anatomical variation of the internal jugular vein. Ultraschall Med. 2001;22(1):23-26. https://doi.org/10.1055/s-2001-11243.
37. Caridi JG, Hawkins IF, Jr, Wiechmann BN, Pevarski DJ, Tonkin JC. Sonographic guidance when using the right internal jugular vein for central vein access. AJR Am J Roentgenol. 1998;171(5):1259-1263. https://doi.org/10.2214/ajr.171.5.9798857.
38. Brusasco C, Corradi F, Zattoni PL, et al. Ultrasound-guided central venous cannulation in bariatric patients. Obes Surg. 2009;19(10):1365-1370. https://doi.org/10.1007/s11695-009-9902-y.
39. Armstrong PJ, Cullen M, Scott DH. The ‘SiteRite’ ultrasound machine--an aid to internal jugular vein cannulation. Anaesthesia. 1993;48(4):319-323. https://doi.org/10.1111/j.1365-2044.1993.tb06953.x.
40. Forauer AR, Glockner JF. Importance of US findings in access planning during jugular vein hemodialysis catheter placements. J Vasc Interv Rad. 2000;11(2 Pt 1):233-238. https://doi.org/10.1016/S1051-0443(07)61471-7.
41. Hassan C, Girishkumar HT, Thatigotla B, et al. Value of ultrasound guidance in placement of hemodialysis access catheters in patients with end-stage renal disease. Am Surg. 2008;74(11):1111-1113.
42. Tan CO, Weinberg L, Peyton P, Story D, McNicol L. Size variation between contralateral infraclavicular axillary veins within individual patients-implications for subclavian venous central line insertion. Crit Care Med. 2013;41(2):457-463. https://doi.org/10.1097/CCM.0b013e31826ab1dd.
43. Wu SY, Ling Q, Cao LH, et al. Real-time two-dimensional ultrasound guidance for central venous cannulation: a meta-analysis. Anesthesiology. 2013;118(2):361-375. https://doi.org/10.1097/ALN.0b013e31827bd172.
44. Kim W, Chung RK, Lee GY, Han JI. The effects of hip abduction with external rotation and reverse Trendelenburg position on the size of the femoral vein; ultrasonographic investigation. Korean J Anesthesiol. 2011;61(3):205-209. https://doi.org/10.4097/kjae.2011.61.3.205.
45. Denys BG, Uretsky BF. Anatomical variations of internal jugular vein location: impact on central venous access. Crit Care Med. 1991;19(12):1516-1519. https://doi.org/10.1097/00003246-199112000-00013.
46. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev. 2015;1:CD006962. https://doi.org/10.1002/14651858.CD006962.pub2.
47. Gilbert TB, Seneff MG, Becker RB. Facilitation of internal jugular venous cannulation using an audio-guided Doppler ultrasound vascular access device: results from a prospective, dual-center, randomized, crossover clinical study. Crit Care Med. 1995;23(1):60-65. https://doi.org/10.1097/00003246-199501000-00012.
48. Schummer W, Schummer C, Tuppatsch H, et al. Ultrasound-guided central venous cannulation: is there a difference between Doppler and B-mode ultrasound? J Clin Anesth. 2006;18(3):167-172. https://doi.org/10.1016/j.jclinane.2005.12.010.
49. Airapetian N, Maizel J, Langelle F, et al. Ultrasound-guided central venous cannulation is superior to quick-look ultrasound and landmark methods among inexperienced operators: a prospective randomized study. Intensive Care Med. 2013;39(11):1938-1944. https://doi.org/10.1007/s00134-013-3072-z.
50. Milling TJ, Jr, Rose J, Briggs WM, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Crit Care Med. 2005;33(8):1764-1769. https://doi.org/10.1097/01.ccm.0000171533.92856.e5.
51. Beaudoin FL, Merchant RC, Lincoln J, et al. Bedside ultrasonography detects significant femoral vessel overlap: implications for central venous cannulation. CJEM. 2011;13(4):245-250. https://doi.org/10.2310/8000.2011.110482.
52. Kwon TH, Kim YL, Cho DK. Ultrasound-guided cannulation of the femoral vein for acute haemodialysis access. Nephrol Dial Transplant. 1997;12(5):1009-1012. https://doi.org/10.1093/ndt/12.5.1009.
53. Rothschild JM. Ultrasound guidance of central vein catheterization. Evid Rep Technol Assess. 2001;43. Chapter 21.: http://archive.ahrq.gov/clinic/ptsafety/chap21.htm.
54. Lennon M, Zaw NN, Pöpping DM, Wenk M. Procedural complications of central venous catheter insertion. Minerva Anestesiol. 2012;78(11):1234-1240.
55. Blaivas M, Adhikari S. An unseen danger: frequency of posterior vessel wall penetration by needles during attempts to place internal jugular vein central catheters using ultrasound guidance. Crit Care Med. 2009;37(8):2345-2349; quiz 2359. https://doi.org/10.1097/CCM.0b013e3181a067d4.
56. Vogel JA, Haukoos JS, Erickson CL, et al. Is long-axis view superior to short-axis view in ultrasound-guided central venous catheterization? Crit Care Med. 2015;43(4):832-839. https://doi.org/10.1097/CCM.0000000000000823.
57. Tammam TF, El-Shafey EM, Tammam HF. Ultrasound-guided internal jugular vein access: comparison between short axis and long axis techniques. Saudi J Kidney Dis Transpl. 2013;24(4):707-713. https://doi.org/10.4103/1319-2442.113861.
58. Keyes LE, Frazee BW, Snoey ER, Simon BC, Christy D. Ultrasound-guided brachial and basilic vein cannulation in emergency department patients with difficult intravenous access. Ann Emerg Med. 1999;34(6):711-714. https://doi.org/10.1016/S0196-0644(99)70095-8.
59. Mahler SA, Wang H, Lester C, et al. Short- vs long-axis approach to ultrasound-guided peripheral intravenous access: a prospective randomized study. Am J Emerg Med. 2011;29(9):1194-1197. https://doi.org/10.1016/j.ajem.2010.07.015.
60. Berk D, Gurkan Y, Kus A, et al. Ultrasound-guided radial arterial cannulation: long axis/in-plane versus short axis/out-of-plane approaches? J Clin Monit Comput. 2013;27(3):319-324. https://doi.org/10.1007/s10877-013-9437-6.
61. Batllori M, Urra M, Uriarte E, et al. Randomized comparison of three transducer orientation approaches for ultrasound guided internal jugular venous cannulation. Br J Anaesth. 2016;116(3):370-376. https://doi.org/10.1093/bja/aev399.
62. Moak JH, Lyons MS, Wright SW, Lindsell CJ. Needle and guidewire visualization in ultrasound-guided internal jugular vein cannulation. Am J Emerg Med. 2011;29(4):432-436. https://doi.org/10.1016/j.ajem.2010.01.004.
63. Moak JH, Rajkumar JS, Woods WA. The wire is really easy to see (WIRES): sonographic visualization of the guidewire by novices. CJEM. 2013;15(1):18-23. https://doi.org/10.2310/8000.2012.120800.
64. Gillman LM, Blaivas M, Lord J, Al-Kadi A, Kirkpatrick AW. Ultrasound confirmation of guidewire position may eliminate accidental arterial dilatation during central venous cannulation. Scand J Trauma Resusc Emerg Med. 2010;18:39. https://doi.org/10.1186/1757-7241-18-39.
65. Augoustides JG, Horak J, Ochroch AE, et al. A randomized controlled clinical trial of real-time needle-guided ultrasound for internal jugular venous cannulation in a large university anesthesia department. J Cardiothorac Vasc Anesth. 2005;19(3):310-315. https://doi.org/10.1053/j.jvca.2005.03.007.
66. Maecken T, Heite L, Wolf B, Zahn PK, Litz RJ. Ultrasound-guided catheterisation of the subclavian vein: freehand vs needle-guided technique. Anaesthesia. 2015;70(11):1242-1249. https://doi.org/10.1111/anae.13187.
67. Stone MB, Nagdev A, Murphy MC, Sisson CA. Ultrasound detection of guidewire position during central venous catheterization. Am J Emerg Med. 2010;28(1):82-84. https://doi.org/10.1016/j.ajem.2008.09.019.
68. Luyet C, Hartwich V, Urwyler N, et al. Evaluation of a novel needle guide for ultrasound-guided phantom vessel cannulation. Anaesthesia. 2011;66(8):715-720. https://doi.org/10.1111/j.1365-2044.2011.06781.x.
69. O’Grady NP, Alexander M, Burns LA, et al. Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis. 2011;52(9):1087-1099. https://doi.org/10.1093/cid/cir138.
70. Southworth SL, Henman LJ, Kinder LA, Sell JL. The journey to zero central catheter-associated bloodstream infections: culture change in an intensive care unit. Crit Care Nurse. 2012;32(2):49-54. https://doi.org/10.4037/ccn2012915.
71. Guerin K, Wagner J, Rains K, Bessesen M. Reduction in central line-associated bloodstream infections by implementation of a postinsertion care bundle. Am J Infect Control. 2010;38(6):430-433. https://doi.org/10.1016/j.ajic.2010.03.007.
72. Hartman N, Wittler M, Askew K, Manthey D. Delphi method validation of a procedural performance checklist for insertion of an ultrasound-guided internal jugular central line. Am J Med Qual Off J Am Coll Med Qual. 2016;31(1):81-85. https://doi.org/10.1177/1062860614549762.
73. Lenchus JD, Carvalho CM, Ferreri K, et al. Filling the void: defining invasive bedside procedural competency for internal medicine residents. J Grad Med Educ. 2013;5(4):605-612. https://doi.org/10.4300/JGME-D-13-00030.1.
74. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. https://doi.org/10.1001/archinternmed.2009.215.
75. Karakitsos D, Labropoulos N, De Groot E, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care. 2006;10(6):R162. https://doi.org/10.1186/cc5101.
76. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. https://doi.org/10.1097/01.ccm.0000142399.70913.2f.
77. Young EM, Commiskey ML, Wilson SJ. Translating evidence into practice to prevent central venous catheter-associated bloodstream infections: a systems-based intervention. Am J Infect Control. 2006;34(8):503-506. https://doi.org/10.1016/j.ajic.2006.03.011.
78. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495; discussion 495. https://doi.org/10.1016/j.surg.2008.06.004.
79. Mermel LA, McCormick RD, Springman SR, Maki DG. The pathogenesis and epidemiology of catheter-related infection with pulmonary artery swan-Ganz catheters: a prospective study utilizing molecular subtyping. Am J Med. 1991;91(3b):197S–205S. https://doi.org/10.1016/0002-9343(91)90369-9.
80. Marschall J, Mermel LA, Classen D, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(1):S22-S30. https://doi.org/10.1086/591059.
81. Marschall J, Mermel LA, Fakih M, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(7):753-771. https://doi.org/10.1086/676533.
82. Garcia-Rodriguez JF, Álvarez-Díaz H, Vilariño-Maneiro L, et al. Epidemiology and impact of a multifaceted approach in controlling central venous catheter associated blood stream infections outside the intensive care unit. BMC Infect Dis. 2013;13:445. https://doi.org/10.1186/1471-2334-13-445.
83. Lee DH, Jung KY, Choi YH. Use of maximal sterile barrier precautions and/or antimicrobial-coated catheters to reduce the risk of central venous catheter-related bloodstream infection. Infect Control Hosp Epidemiol. 2008;29(10):947-950. https://doi.org/10.1086/590356.
84. Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132(5):391-402. https://doi.org/10.7326/0003-4819-132-5-200003070-00009.
85. Troianos CA, Jobes DR, Ellison N. Ultrasound-guided cannulation of the internal jugular vein. A prospective, randomized study. Anesth Analg. 1991;72(6):823-826. https://doi.org/10.1213/00000539-199106000-00020.
86. Troianos CA, Savino JS. Internal jugular vein cannulation guided by echocardiography. Anesthesiology. 1991;74(4):787-789. https://doi.org/10.1097/00000542-199104000-00026.
87. Denys BG, Uretsky BF, Reddy PS, et al. An ultrasound method for safe and rapid central venous access. N Engl J Med. 1991;324(8):566. https://doi.org/10.1056/NEJM199102213240816.
88. Riaz A, Shan Khan RA, Salim F. Ultrasound guided internal jugular venous cannulation: comparison with land-mark technique. J Coll Phys Surg Pak. 2015;25(5):315-319. https://doi.org/05.2015/JCPSP.315319.
89. Gok F, Kilicaslan A, Sarkilar G, Kandemir B, Yosunkaya A. The effect of ultrasound guidance on central venous catheter-associated bloodstream infection in critical care patients. Acta Med Mediterr. 2013;29:677-682.
90. Lamperti M, Cortellazzi P, D’Onofrio G, et al. An outcome study on complications using routine ultrasound assistance for internal jugular vein cannulation. Acta Anaesthesiol Scand. 2007;51(10):1327-1330. https://doi.org/10.1111/j.1399-6576.2007.01442.x.
91. Vezzani A, Manca T, Vercelli A, Braghieri A, Magnacavallo A. Ultrasonography as a guide during vascular access procedures and in the diagnosis of complications. J Ultrasound. 2013;16(4):161-170. https://doi.org/10.1007/s40477-013-0046-5.
92. Palepu GB, Deven J, Subrahmanyam M, Mohan S. Impact of ultrasonography on central venous catheter insertion in intensive care. Indian J Rad Imaging. 2009;19(3):191-198. https://doi.org/10.4103/0971-3026.54877.
93. Agarwal A, Singh DK, Singh AP. Ultrasonography: a novel approach to central venous cannulation. Indian J Crit Care Med. 2009;13(4):213-216. https://doi.org/10.4103/0972-5229.60174.
94. Wong SW, Niazi AU, Chin KJ, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS® needle tracking system: a case report. Can J Anaesth. 2013;60(1):50-53. https://doi.org/10.1007/s12630-012-9809-2.
95. Bouaziz H, Zetlaoui PJ, Pierre S, et al. Guidelines on the use of ultrasound guidance for vascular access. Anaesth, Crit Care Pain Med. 2015;34(1):65-69. https://doi.org/10.1016/j.accpm.2015.01.004.
96. Jenssen C, Brkljacic B, Hocke M, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part VI - Ultrasound-guided vascular interventions. Ultraschall Med. 2016;37(5):473-476. https://doi.org/10.1055/s-0035-1553450.
97. Rando K, Castelli J, Pratt JP, et al. Ultrasound-guided internal jugular vein catheterization: a randomized controlled trial. Heart Lung Vessels. 2014;6(1):13-23.
98. Mey U, Glasmacher A, Hahn C, et al. Evaluation of an ultrasound-guided technique for central venous access via the internal jugular vein in 493 patients. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2003;11(3):148-155. https://doi.org/10.1007/s00520-002-0399-3.
99. Singh SA, Sharma S, Singh A, et al. The safety of ultrasound guided central venous cannulation in patients with liver disease. Saudi J Anaesth. 2015;9(2):155-160. https://doi.org/10.4103/1658-354X.152842.
100. Akoglu H, Piskinpasa S, Yenigun EC, et al. Real-time ultrasound guided placement of temporary internal jugular vein catheters: assessment of technical success and complication rates in nephrology practice. Nephrol (Carlton). 2012;17(7):603-606. https://doi.org/10.1111/j.1440-1797.2012.01637.x.
101. Sadler DJ, Gordon AC, Klassen J, et al. Image-guided central venous catheters for apheresis. Bone Marrow Transplant. 1999;23(2):179-182. https://doi.org/10.1038/sj.bmt.1701545.
102. Calvert N, Hind D, McWilliams R, et al. Ultrasound for central venous cannulation: economic evaluation of cost-effectiveness. Anaesthesia. 2004;59(11):1116-1120. https://doi.org/10.1111/j.1365-2044.2004.03906.x.
103. Goetz AM, Wagener MM, Miller JM, Muder RR. Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infect Control Hosp Epidemiol. 1998;19(11):842-845.
104. Bertini P, Frediani M. Ultrasound guided supraclavicular central vein cannulation in adults: a technical report. J Vasc Access. 2013;14(1):89-93. https://doi.org/10.5301/jva.5000088.
105. Lalu MM, Fayad A, Ahmed O, et al. Ultrasound-guided subclavian vein catheterization: A systematic review and meta-analysis. Crit Care Med. 2015;43(7):1498-1507. https://doi.org/10.1097/CCM.0000000000000973.
106. Milone M, Di Minno G, Di Minno MN, et al. The real effectiveness of ultrasound guidance in subclavian venous access. Ann ital chir. 2010;81(5):331-334.
107. O’Leary R, Ahmed SM, McLure H, et al. Ultrasound-guided infraclavicular axillary vein cannulation: a useful alternative to the internal jugular vein. Br J Anaesth. 2012;109(5):762-768. https://doi.org/10.1093/bja/aes262.
108. Gualtieri E, Deppe SA, Sipperly ME, Thompson DR. Subclavian venous catheterization: greater success rate for less experienced operators using ultrasound guidance. Crit Care Med. 1995;23(4):692-697. https://doi.org/10.1097/00003246-199504000-00018.
109. Fragou M, Gravvanis A, Dimitriou V, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med. 2011;39(7):1607-1612. https://doi.org/10.1097/CCM.0b013e318218a1ae.
110. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015;1:CD011447. https://doi.org/10.1002/14651858.CD011447.
111. Robinson MK, Mogensen KM, Grudinskas GF, Kohler S, Jacobs DO. Improved care and reduced costs for patients requiring peripherally inserted central catheters: the role of bedside ultrasound and a dedicated team. JPEN J Parenter Enter Nutr. 2005;29(5):374-379. https://doi.org/10.1177/0148607105029005374.
112. Li J, Fan YY, Xin MZ, et al. A randomised, controlled trial comparing the long-term effects of peripherally inserted central catheter placement in chemotherapy patients using B-mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94-103. https://doi.org/10.1016/j.ejon.2013.08.003.
113. Schweickert WD, Herlitz J, Pohlman AS, et al. A randomized, controlled trial evaluating postinsertion neck ultrasound in peripherally inserted central catheter procedures. Crit Care Med. 2009;37(4):1217-1221. https://doi.org/10.1097/CCM.0b013e31819cee7f.
114. Mahler SA, Wang H, Lester C, Conrad SA. Ultrasound-guided peripheral intravenous access in the emergency department using a modified Seldinger technique. J Emerg Med. 2010;39(3):325-329. https://doi.org/10.1016/j.jemermed.2009.02.013.
115. Brannam L, Blaivas M, Lyon M, Flake M. Emergency nurses’ utilization of ultrasound guidance for placement of peripheral intravenous lines in difficult-access patients. Acad Emerg Med. 2004;11(12):1361-1363. https://doi.org/10.1197/j.aem.2004.08.027.
116. Au A, Rotte M, Gryzbowski R, Ku B, Fields J. 157 Decrease in central venous catheter placement and complications due to utilization of ultrasound-guided peripheral intravenous catheters. Ann Emerg Med. 2011;58(4):S230. https://doi.org/10.1016/j.annemergmed.2011.06.185.
117. Dargin JM, Rebholz CM, Lowenstein RA, Mitchell PM, Feldman JA. Ultrasonography-guided peripheral intravenous catheter survival in ED patients with difficult access. Am J Emerg Med. 2010;28(1):1-7. https://doi.org/10.1016/j.ajem.2008.09.001.
118. Bauman M, Braude D, Crandall C. Ultrasound-guidance vs standard technique in difficult vascular access patients by ED technicians. Am J Emerg Med. 2009;27(2):135-140. https://doi.org/10.1016/j.ajem.2008.02.005.
119. Schoenfeld E, Shokoohi H, Boniface K. Ultrasound-guided peripheral intravenous access in the emergency department: patient-centered survey. West J Emerg Med. 2011;12(4):475-477. https://doi.org/10.5811/westjem.2011.3.1920.
120. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. https://doi.org/10.7326/0003-4819-114-10-845.
121. Miliani K, Taravella R, Thillard D, et al. Peripheral venous catheter-related adverse events: evaluation from a multicentre epidemiological study in France (the CATHEVAL Project). PLOS ONE. 2017;12(1):e0168637. https://doi.org/10.1371/journal.pone.0168637.
122. Gregg SC, Murthi SB, Sisley AC, Stein DM, Scalea TM. Ultrasound-guided peripheral intravenous access in the intensive care unit. J Crit Care. 2010;25(3):514-519. https://doi.org/10.1016/j.jcrc.2009.09.003.
123. Adhikari S, Blaivas M, Morrison D, Lander L. Comparison of infection rates among ultrasound-guided versus traditionally placed peripheral intravenous lines. J Ultrasound Med. 2010;29(5):741-747. https://doi.org/10.7863/jum.2010.29.5.741.
124. Shiver S, Blaivas M, Lyon M. A prospective comparison of ultrasound-guided and blindly placed radial arterial catheters. Acad Emerg Med. 2006;13(12):1275-1279. https://doi.org/10.1197/j.aem.2006.07.015.
125. Levin PD, Sheinin O, Gozal Y. Use of ultrasound guidance in the insertion of radial artery catheters. Crit Care Med. 2003;31(2):481-484. https://doi.org/10.1097/01.CCM.0000050452.17304.2F.
126. Gu WJ, Tie HT, Liu JC, Zeng XT. Efficacy of ultrasound-guided radial artery catheterization: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2014;18(3):R93. https://doi.org/10.1186/cc13862.
127. Tang L, Wang F, Li Y, et al. Ultrasound guidance for radial artery catheterization: an updated meta-analysis of randomized controlled trials. PLOS ONE. 2014;9(11):e111527. https://doi.org/10.1371/journal.pone.0111527.
128. Sobolev M, Slovut DP, Lee Chang A, Shiloh AL, Eisen LA. Ultrasound-guided catheterization of the femoral artery: A systematic review and meta-analysis of randomized controlled trials. J Invas Cardiol. 2015;27(7):318-323. https://doi.org/10.1378/chest.1991181.
129. Gabriel M, Pawlaczyk K, Waliszewski K, Krasiński Z, Majewski W. Location of femoral artery puncture site and the risk of postcatheterization pseudoaneurysm formation. Int J Cardiol. 2007;120(2):167-171. https://doi.org/10.1016/j.ijcard.2006.09.018.
130. Seto AH, Abu-Fadel MS, Sparling JM, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With ultrasound Trial). JACC Cardiovasc Interv. 2010;3(7):751-758. https://doi.org/10.1016/j.jcin.2010.04.015.
131. Kalish J, Eslami M, Gillespie D, et al. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg. 2015;61(5):1231-1238. https://doi.org/10.1016/j.jvs2014.12.003.
132. Sandhu NS, Patel B. Use of ultrasonography as a rescue technique for failed radial artery cannulation. J Clin Anesth. 2006;18(2):138-141. https://doi.org/10.1016/j.jclinane.2005.06.011.
133. White L, Halpin A, Turner M, Wallace L. Ultrasound-guided radial artery cannulation in adult and paediatric populations: a systematic review and meta-analysis. Br J Anaesth. 2016;116(5):610-617. https://doi.org/10.1093/bja/aew097.
134. Gao YB, Yan JH, Gao FQ, et al. Effects of ultrasound-guided radial artery catheterization: an updated meta-analysis. Am J Emerg Med. 2015;33(1):50-55. https://doi.org/10.1016/j.ajem.2014.10.008.
135. Seto AH, Roberts JS, Abu-Fadel MS, et al. Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery Access with Ultrasound Trial). JACC Cardiovasc Interv. 2015;8(2):283-291. https://doi.org/10.1016/j.jcin.2014.05.036.
136. Roberts J, Manur R. Ultrasound-guided radial artery access by a non-ultrasound trained interventional cardiologist improved first-attempt success rates and shortened time for successful radial artery cannulation. J Invas Cardiol. 2013;25(12):676-679.
137. Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108(5):1345-1348. https://doi.org/10.1378/chest.108.5.1345.
138. Lichtenstein D, Mezière G, Biderman P, Gepner A. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999;25(4):383-388. https://doi.org/10.1007/s001340050862.
139. Ablordeppey EA, Drewry AM, Beyer AB, et al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound Versus chest radiography in critically ill patients: A systematic review and meta-analysis. Crit Care Med. 2017;45(4):715-724. https://doi.org/10.1097/CCM.0000000000002188.
140. Bedel J, Vallée F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013;39(11):1932-1937. https://doi.org/10.1007/s00134-013-3097-3.
141. Weekes AJ, Keller SM, Efune B, Ghali S, Runyon M. Prospective comparison of ultrasound and CXR for confirmation of central vascular catheter placement. Emerg Med J EMJ. 2016;33(3):176-180. https://doi.org/10.1136/emermed-2015-205000.
142. Arellano R, Nurmohamed A, Rumman A, et al. The utility of transthoracic echocardiography to confirm central line placement: an observational study. Can J Anaesth. 2014;61(4):340-346. https://doi.org/10.1007/s12630-014-0111-3.
143. Vezzani A, Brusasco C, Palermo S, et al. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38(2):533-538. https://doi.org/10.1097/CCM.0b013e3181c0328f.
144. Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142(4):260-273. https://doi.org/10.7326/0003-4819-142-4-200502150-00008.
145. Backlund BH, Hopkins E, Kendall JL. Ultrasound guidance for central venous access by emergency physicians in Colorado. West J Emerg Med. 2012;13(4):320-325. https://doi.org/10.5811/westjem.2011.11.6821.
146. Buchanan MS, Backlund B, Liao MM, et al. Use of ultrasound guidance for central venous catheter placement: survey from the American Board of Emergency Medicine Longitudinal Study of Emergency Physicians. Acad Emerg Med. 2014;21(4):416-421. https://doi.org/10.1111/acem.12350.
147. Barsuk JH, McGaghie WC, Cohen ER, O’Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697-2701. https://doi.org/10.1097/00003246-200910000-00003.
148. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. https://doi.org/10.1097/00003246-200201000-00009.
149. Woo MY, Frank J, Lee AC, et al. Effectiveness of a novel training program for emergency medicine residents in ultrasound-guided insertion of central venous catheters. CJEM. 2009;11(4):343-348. https://doi.org/10.1017/S1481803500011398.
150. McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348(12):1123-1133. https://doi.org/10.1056/NEJMra011883.
151. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. https://doi.org/10.1002/jhm.468.
152. Sekiguchi H, Tokita JE, Minami T, et al. A prerotational, simulation-based workshop improves the safety of central venous catheter insertion: results of a successful internal medicine house staff training program. Chest. 2011;140(3):652-658. https://doi.org/10.1378/chest.10-3319.
153. Feller-Kopman D. Ultrasound-guided internal jugular access: a proposed standardized approach and implications for training and practice. Chest. 2007;132(1):302-309. https://doi.org/10.1378/chest.06-2711.
154. Troianos CA, Hartman GS, Glas KE, et al. Special articles: guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg. 2012;114(1):46-72. https://doi.org/10.1213/ANE.0b013e3182407cd8.
155. Issenberg SB, McGaghie WC, Hart IR, et al. Simulation technology for health care professional skills training and assessment. JAMA. 1999;282(9):861-866. https://doi.org/10.1001/jama.282.9.861.
156. Millington SJ, Wong RY, Kassen BO, Roberts JM, Ma IW. Improving internal medicine residents’ performance, knowledge, and confidence in central venous catheterization using simulators. J Hosp Med. 2009;4(7):410-416. https://doi.org/10.1002/jhm.570.
157. Beaulieu Y, Laprise R, Drolet P, et al. Bedside ultrasound training using web-based e-learning and simulation early in the curriculum of residents. Crit Ultrasound J. 2015;7:1. https://doi.org/10.1186/s13089-014-0018-9.
158. Barsuk JH, Cohen ER, McGaghie WC, Wayne DB. Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med. 2010;85(10 Suppl):S9-S12. https://doi.org/10.1097/ACM.0b013e3181ed436c.
159. Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133(1):56-61. https://doi.org/10.1378/chest.07-0131.
160. Evans LV, Dodge KL, Shah TD, et al. Simulation training in central venous catheter insertion: improved performance in clinical practice. Acad Med. 2010;85(9):1462-1469. https://doi.org/10.1097/ACM.0b013e3181eac9a3.
161. Smith CC, Huang GC, Newman LR, et al. Simulation training and its effect on long-term resident performance in central venous catheterization. Simul Healthc J Soc Simul Healthc. 2010;5(3):146-151. https://doi.org/10.1097/SIH.0b013e3181dd9672.
162. Laack TA, Dong Y, Goyal DG, et al. Short-term and long-term impact of the central line workshop on resident clinical performance during simulated central line placement. Simul Healthc J Soc Simul Healthc. 2014;9(4):228-233. https://doi.org/10.1097/SIH.0000000000000015.
163. Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31(10):1519-1526. https://doi.org/10.7863/jum.2012.31.10.1519.
164. Bayci AW, Mangla J, Jenkins CS, Ivascu FA, Robbins JM. Novel educational module for subclavian central venous catheter insertion using real-time ultrasound guidance. J Surg Educ. 2015;72(6):1217-1223. https://doi.org/10.1016/j.jsurg.2015.07.010.
165. Andreatta P, Chen Y, Marsh M, Cho K. Simulation-based training improves applied clinical placement of ultrasound-guided PICCs. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2011;19(4):539-543. https://doi.org/10.1007/s00520-010-0849-2.
166. Rosen BT, Uddin PQ, Harrington AR, Ault BW, Ault MJ. Does personalized vascular access training on a nonhuman tissue model allow for learning and retention of central line placement skills? Phase II of the procedural patient safety initiative (PPSI-II). J Hosp Med. 2009;4(7):423-429. https://doi.org/10.1002/jhm.571.
167. Ault MJ, Rosen BT, Ault B. The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative. J Gen Intern Med. 2006;21(5):514-517. https://doi.org/10.1111/j.1525-1497.2006.00440.x.
168. Varga S, Smith J, Minneti M, et al. Central venous catheterization using a perfused human cadaveric model: application to surgical education. J Surg Educ. 2015;72(1):28-32. https://doi.org/10.1016/j.jsurg.2014.07.005.
169. Sansivero GE. Venous anatomy and physiology. Considerations for vascular access device placement and function. J Intraven Nurs Off Publ Intraven Nurs Soc. 1998;21(5 Suppl):S107-S114.
170. Eisen LA, Narasimhan M, Berger JS, et al. Mechanical complications of central venous catheters. Journal of intensive care medicine. 2006;21(1):40-46. https://doi.org/10.1177/0885066605280884.
171. Chenkin J, Lee S, Huynh T, Bandiera G. Procedures can be learned on the Web: a randomized study of ultrasound-guided vascular access training. Acad Emerg Med. 2008;15(10):949-954. https://doi.org/10.1111/j.1553-2712.2008.00231.x.
172. Abualenain J, Calabrese K, Tansek R, Ranniger C. 319 Comparing standard versus video-based teaching for ultrasound-guided internal jugular central venous catheter access for fourth-year medical students. Ann Emerg Med. 2014;64(4):S113. https://doi.org/10.1016/j.annemergmed.2014.07.347.
173. Pustavoitau A, Blaivas M, Brown SM, et al. Recommendations for achieving and maintaining competence and credentialing in critical care ultrasound with focused cardiac ultrasound and advanced critical care echocardiography. Crit Care Med. 2016.
174. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: A survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
175. Moureau N, Lamperti M, Kelly LJ, et al. Evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training. Br J Anaesth. 2013;110(3):347-356. https://doi.org/10.1093/bja/aes499.
176. Ernst A, Silvestri GA, Johnstone D, American College of Chest Physicians. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123(5):1693-1717. https://doi.org/10.1378/chest.123.5.1693.
177. Thomas SM, Burch W, Kuehnle SE, et al. Simulation training for pediatric residents on central venous catheter placement: a pilot study. Pediatr Crit Care Med J Soc Crit Care Med.. 2013;14(9):e416-e423. https://doi.org/10.1097/PCC.0b013e31829f5eda.
178. Smith KK, Gilcreast D, Pierce K. Evaluation of staff’s retention of ACLS and BLS skills. Resuscitation. 2008;78(1):59-65. https://doi.org/10.1016/j.resuscitation.2008.02.007.
179. Ericsson KA. Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med. 2004;79(10 Suppl):S70-S81. https://doi.org/10.1097/00001888-200410001-00022.
180. Gerard JM, Thomas SM, Germino KW, et al. The effect of simulation training on PALS skills among family medicine residents. Fam Med. 2011;43(6):392-399.
181. Brydges R, Nair P, Ma I, Shanks D, Hatala R. Directed self-regulated learning versus instructor-regulated learning in simulation training. Med Educ. 2012;46(7):648-656. https://doi.org/10.1111/j.1365-2923.2012.04268.x.
182. Wayne DB, Butter J, Siddall VJ, et al. Simulation-based training of internal medicine residents in advanced cardiac life support protocols: a randomized trial. Teach Learn Med. 2005;17(3):210-216. https://doi.org/10.1207/s15328015tlm1703_3.
183. Arthur Jr. W, Bennett Jr. W, Stanush PL, McNelly TL. Factors that influence skill decay and retention: A quantitative review and analysis. Hum Perform. 1998;11(1):57-101. https://doi.org/10.1207/s15327043hup1101_3.
184. Rusche JD, Besuner P, Partusch SK, Berning PA. Competency program development across a merged healthcare network. J Nurs Staff Dev. 2001;17(5):234-240; quiz 241-232. https://doi.org/10.1097/00124645-200109000-00004.
185. O’Hearne Rebholz M. A review of methods to assess competency. J Nurs Staff Dev. 2006;22(5):241-245. https://doi.org/10.1097/00124645-200609000-00007.
186. Reznick RK. Teaching and testing technical skills. Am J Surg. 1993;165(3):358-361. https://doi.org/10.1016/s0002-9610(05)80843-8.
187. Reznick RK, MacRae H. Teaching surgical skills--changes in the wind. N Engl J Med. 2006;355(25):2664-2669. https://doi.org/10.1056/NEJMra054785.
188. Murin S, Stollenwerk NS. Simulation in procedural training: at the tipping point. Chest. 2010;137(5):1009-1011. https://doi.org/10.1378/chest.10-0199.
189. American College of Emergency Physicians. Emergency ultrasound guidelines. Ann Emerg Med. 2009;53(4):550-570. https://doi.org/10.1016/j.annemergmed.2008.12.013.
190. Guilbert MC, Elkouri S, Bracco D, et al. Arterial trauma during central venous catheter insertion: case series, review and proposed algorithm. J Vasc Surg. 2008;48(4):918-925; discussion 925. https://doi.org/10.1016/j.jvs2008.04.046.
191. Dong Y, Suri HS, Cook DA, et al. Simulation-based objective assessment discerns clinical proficiency in central line placement: a construct validation. Chest. 2010;137(5):1050-1056. https://doi.org/10.1378/chest.09-1451.
192. Adhikari S, Theodoro D, Raio C, et al. Central venous catheterization: are we using ultrasound guidance? J Ultrasound Med. 2015;34(11):2065-2070. https://doi.org/10.7863/ultra.15.01027.
193. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors--procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z.
1. Raad I. Intravascular-catheter-related infections. Lancet. 1998;351(9106):893-898. https://doi.org/10.1016/S0140-6736(97)10006-X.
2. Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286(6):700-707. https://doi.org/10.1001/jama.286.6.700.
3. Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986;146(2):259-261. https://doi.org/10.1001/archinte.146.2.259.
4. Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med. 1996;24(12):2053-2058. https://doi.org/10.1097/00003246-199612000-00020.
5. Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327(7411):361. https://doi.org/10.1136/bmj.327.7411.361.
6. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4):S1-S34. https://doi.org/10.1016/j.ajic.2011.01.003.
7. National Institute for Health and Care Excellence (NICE). Guidance on the Use of Ultrasound Locating Devices for Placing Central Venous Catheters; 2002.
8. Shojania KG, Duncan BW, McDonald KM, et al. Making health care safer: A critical analysis of patient safety practices. Evid Rep Technol Assess. Rockville, MD: Agency for Healthcare Research and Quality. 2001;43(43):i–x, 1.
9. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105-1117. https://doi.org/10.1007/s00134-012-2597-x.
10. Frykholm P, Pikwer A, Hammarskjöld F, et al. Clinical guidelines on central venous catheterisation. Swedish Society of Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiol Scand. 2014;58(5):508-524. https://doi.org/10.1111/aas.12295.
11. American Society of Anesthesiologists Task Force on Central Venous Access, Rupp SM, Apfelbaum JL, et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539-573. https://doi.org/10.1097/ALN.0b013e31823c9569.
12. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: General ultrasonography. Crit Care Med. 2015;43(11):2479-2502. https://doi.org/10.1097/CCM.0000000000001216.
13. Shekelle PG, Wachter RM, Pronovost PJ, et al. Making health care safer II: An updated critical analysis of the evidence for patient safety practices. Evid Rep Technol Assess. 2013;211:1-945.
14. Troianos CA, Hartman GS, Glas KE, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2011;24(12):1291-1318. https://doi.org/10.1016/j.echo.2011.09.021.
15. Soni NJ, Reyes LF, Keyt H, et al. Use of ultrasound guidance for central venous catheterization: a national survey of intensivists and hospitalists. J Crit Care. 2016;36:277-283. https://doi.org/10.1016/j.jcrc.2016.07.014.
16. Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220-1229. https://doi.org/10.1056/NEJMoa1500964.
17. Maizel J, Bastide MA, Richecoeur J, et al. Practice of ultrasound-guided central venous catheter technique by the French intensivists: a survey from the BoReal study group. Ann Intensive Care. 2016;6(1):76. https://doi.org/10.1186/s13613-016-0177-x.
18. Egan G, Healy D, O’Neill H, et al. Ultrasound guidance for difficult peripheral venous access: systematic review and meta-analysis. Emerg Med J. 2013;30(7):521-526. https://doi.org/10.1136/emermed-2012-201652.
19. Stolz LA, Stolz U, Howe C, Farrell IJ, Adhikari S. Ultrasound-guided peripheral venous access: a meta-analysis and systematic review. J Vasc Access. 2015;16(4):321-326. https://doi.org/10.5301/jva.5000346.
20. Sofocleous CT, Schur I, Cooper SG, et al. Sonographically guided placement of peripherally inserted central venous catheters: review of 355 procedures. AJR Am J Roentgenol. 1998;170(6):1613-1616. https://doi.org/10.2214/ajr.170.6.9609183.
21. Nichols I, Humphrey JP. The efficacy of upper arm placement of peripherally inserted central catheters using bedside ultrasound and microintroducer technique. J Infus Nurs Off Publ Infus Nurs Soc. 2008;31(3):165-176. https://doi.org/10.1097/01.NAN.0000317703.66395.b8.
22. Stokowski G, Steele D, Wilson D. The use of ultrasound to improve practice and reduce complication rates in peripherally inserted central catheter insertions. J Infus Nurs Off Publ Infus Nurs Soc. 2009;32(3):145-155. https://doi.org/10.1097/NAN.0b013e3181a1a98f.
23. Shiloh AL, Savel RH, Paulin LM, Eisen LA. Ultrasound-guided catheterization of the radial artery: a systematic review and meta-analysis of randomized controlled trials. Chest. 2011;139(3):524-529. https://doi.org/10.1378/chest.10-0919.
24. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: A position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
25. Chopra V, Flanders SA, Saint S, et al. The Michigan appropriateness guide for intravenous catheters (Magic): results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6):S1-S40. https://doi.org/10.7326/M15-0744.
26. Raad II, Hohn DC, Gilbreath BJ, et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994;15(4):231-238.
27. Slama M, Novara A, Safavian A, et al. Improvement of internal jugular vein cannulation using an ultrasound-guided technique. Intensive Care Med. 1997;23(8):916-919. https://doi.org/10.1007/s001340050432.
28. Seyahi N, Kahveci A, Altiparmak MR, Serdengecti K, Erek E. Ultrasound imaging findings of femoral veins in patients with renal failure and its impact on vascular access. Nephrol Dial Transplant. 2005;20(9):1864-1867. https://doi.org/10.1093/ndt/gfh942.
29. Lin BS, Kong CW, Tarng DC, Huang TP, Tang GJ. Anatomical variation of the internal jugular vein and its impact on temporary haemodialysis vascular access: an ultrasonographic survey in uraemic patients. Nephrol Dial Transplant. 1998;13(1):134-138. https://doi.org/10.1093/ndt/13.1.134.
30. Troianos CA, Kuwik RJ, Pasqual JR, Lim AJ, Odasso DP. Internal jugular vein and carotid artery anatomic relation as determined by ultrasonography. Anesthesiology. 1996;85(1):43-48. https://doi.org/10.1097/00000542-199607000-00007.
31. Gordon AC, Wright I, Pugh ND. Duplication of the superficial femoral vein: recognition with duplex ultrasonography. Clin Rad. 1996;51(9):622-624. https://doi.org/10.1016/S0009-9260(96)80055-9.
32. Sulek CA, Gravenstein N, Blackshear RH, Weiss L. Head rotation during internal jugular vein cannulation and the risk of carotid artery puncture. Anesth Analg. 1996;82(1):125-128. https://doi.org/10.1097/00000539-199601000-00022.
33. Shrestha BR, Gautam B. Ultrasound versus the landmark technique: a prospective randomized comparative study of internal jugular vein cannulation in an intensive care unit. JNMA J Nepal Med Assoc. 2011;51(182):56-61. https://doi.org/10.31729/jnma.148.
34. Brederlau J, Greim C, Schwemmer U, et al. Ultrasound-guided cannulation of the internal jugular vein in critically ill patients positioned in 30 degrees dorsal elevation. Eur J Anaesthesiol. 2004;21(9):684-687. https://doi.org/10.1097/00003643-200409000-00003.
35. Lamperti M, Subert M, Cortellazzi P, et al. Is a neutral head position safer than 45-degree neck rotation during ultrasound-guided internal jugular vein cannulation? Results of a randomized controlled clinical trial. Anesth Analg. 2012;114(4):777-784. https://doi.org/10.1213/ANE.0b013e3182459917.
36. Benter T, Teichgräber UK, Klühs L, et al. Anatomical variations in the internal jugular veins of cancer patients affecting central venous access. Anatomical variation of the internal jugular vein. Ultraschall Med. 2001;22(1):23-26. https://doi.org/10.1055/s-2001-11243.
37. Caridi JG, Hawkins IF, Jr, Wiechmann BN, Pevarski DJ, Tonkin JC. Sonographic guidance when using the right internal jugular vein for central vein access. AJR Am J Roentgenol. 1998;171(5):1259-1263. https://doi.org/10.2214/ajr.171.5.9798857.
38. Brusasco C, Corradi F, Zattoni PL, et al. Ultrasound-guided central venous cannulation in bariatric patients. Obes Surg. 2009;19(10):1365-1370. https://doi.org/10.1007/s11695-009-9902-y.
39. Armstrong PJ, Cullen M, Scott DH. The ‘SiteRite’ ultrasound machine--an aid to internal jugular vein cannulation. Anaesthesia. 1993;48(4):319-323. https://doi.org/10.1111/j.1365-2044.1993.tb06953.x.
40. Forauer AR, Glockner JF. Importance of US findings in access planning during jugular vein hemodialysis catheter placements. J Vasc Interv Rad. 2000;11(2 Pt 1):233-238. https://doi.org/10.1016/S1051-0443(07)61471-7.
41. Hassan C, Girishkumar HT, Thatigotla B, et al. Value of ultrasound guidance in placement of hemodialysis access catheters in patients with end-stage renal disease. Am Surg. 2008;74(11):1111-1113.
42. Tan CO, Weinberg L, Peyton P, Story D, McNicol L. Size variation between contralateral infraclavicular axillary veins within individual patients-implications for subclavian venous central line insertion. Crit Care Med. 2013;41(2):457-463. https://doi.org/10.1097/CCM.0b013e31826ab1dd.
43. Wu SY, Ling Q, Cao LH, et al. Real-time two-dimensional ultrasound guidance for central venous cannulation: a meta-analysis. Anesthesiology. 2013;118(2):361-375. https://doi.org/10.1097/ALN.0b013e31827bd172.
44. Kim W, Chung RK, Lee GY, Han JI. The effects of hip abduction with external rotation and reverse Trendelenburg position on the size of the femoral vein; ultrasonographic investigation. Korean J Anesthesiol. 2011;61(3):205-209. https://doi.org/10.4097/kjae.2011.61.3.205.
45. Denys BG, Uretsky BF. Anatomical variations of internal jugular vein location: impact on central venous access. Crit Care Med. 1991;19(12):1516-1519. https://doi.org/10.1097/00003246-199112000-00013.
46. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev. 2015;1:CD006962. https://doi.org/10.1002/14651858.CD006962.pub2.
47. Gilbert TB, Seneff MG, Becker RB. Facilitation of internal jugular venous cannulation using an audio-guided Doppler ultrasound vascular access device: results from a prospective, dual-center, randomized, crossover clinical study. Crit Care Med. 1995;23(1):60-65. https://doi.org/10.1097/00003246-199501000-00012.
48. Schummer W, Schummer C, Tuppatsch H, et al. Ultrasound-guided central venous cannulation: is there a difference between Doppler and B-mode ultrasound? J Clin Anesth. 2006;18(3):167-172. https://doi.org/10.1016/j.jclinane.2005.12.010.
49. Airapetian N, Maizel J, Langelle F, et al. Ultrasound-guided central venous cannulation is superior to quick-look ultrasound and landmark methods among inexperienced operators: a prospective randomized study. Intensive Care Med. 2013;39(11):1938-1944. https://doi.org/10.1007/s00134-013-3072-z.
50. Milling TJ, Jr, Rose J, Briggs WM, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Crit Care Med. 2005;33(8):1764-1769. https://doi.org/10.1097/01.ccm.0000171533.92856.e5.
51. Beaudoin FL, Merchant RC, Lincoln J, et al. Bedside ultrasonography detects significant femoral vessel overlap: implications for central venous cannulation. CJEM. 2011;13(4):245-250. https://doi.org/10.2310/8000.2011.110482.
52. Kwon TH, Kim YL, Cho DK. Ultrasound-guided cannulation of the femoral vein for acute haemodialysis access. Nephrol Dial Transplant. 1997;12(5):1009-1012. https://doi.org/10.1093/ndt/12.5.1009.
53. Rothschild JM. Ultrasound guidance of central vein catheterization. Evid Rep Technol Assess. 2001;43. Chapter 21.: http://archive.ahrq.gov/clinic/ptsafety/chap21.htm.
54. Lennon M, Zaw NN, Pöpping DM, Wenk M. Procedural complications of central venous catheter insertion. Minerva Anestesiol. 2012;78(11):1234-1240.
55. Blaivas M, Adhikari S. An unseen danger: frequency of posterior vessel wall penetration by needles during attempts to place internal jugular vein central catheters using ultrasound guidance. Crit Care Med. 2009;37(8):2345-2349; quiz 2359. https://doi.org/10.1097/CCM.0b013e3181a067d4.
56. Vogel JA, Haukoos JS, Erickson CL, et al. Is long-axis view superior to short-axis view in ultrasound-guided central venous catheterization? Crit Care Med. 2015;43(4):832-839. https://doi.org/10.1097/CCM.0000000000000823.
57. Tammam TF, El-Shafey EM, Tammam HF. Ultrasound-guided internal jugular vein access: comparison between short axis and long axis techniques. Saudi J Kidney Dis Transpl. 2013;24(4):707-713. https://doi.org/10.4103/1319-2442.113861.
58. Keyes LE, Frazee BW, Snoey ER, Simon BC, Christy D. Ultrasound-guided brachial and basilic vein cannulation in emergency department patients with difficult intravenous access. Ann Emerg Med. 1999;34(6):711-714. https://doi.org/10.1016/S0196-0644(99)70095-8.
59. Mahler SA, Wang H, Lester C, et al. Short- vs long-axis approach to ultrasound-guided peripheral intravenous access: a prospective randomized study. Am J Emerg Med. 2011;29(9):1194-1197. https://doi.org/10.1016/j.ajem.2010.07.015.
60. Berk D, Gurkan Y, Kus A, et al. Ultrasound-guided radial arterial cannulation: long axis/in-plane versus short axis/out-of-plane approaches? J Clin Monit Comput. 2013;27(3):319-324. https://doi.org/10.1007/s10877-013-9437-6.
61. Batllori M, Urra M, Uriarte E, et al. Randomized comparison of three transducer orientation approaches for ultrasound guided internal jugular venous cannulation. Br J Anaesth. 2016;116(3):370-376. https://doi.org/10.1093/bja/aev399.
62. Moak JH, Lyons MS, Wright SW, Lindsell CJ. Needle and guidewire visualization in ultrasound-guided internal jugular vein cannulation. Am J Emerg Med. 2011;29(4):432-436. https://doi.org/10.1016/j.ajem.2010.01.004.
63. Moak JH, Rajkumar JS, Woods WA. The wire is really easy to see (WIRES): sonographic visualization of the guidewire by novices. CJEM. 2013;15(1):18-23. https://doi.org/10.2310/8000.2012.120800.
64. Gillman LM, Blaivas M, Lord J, Al-Kadi A, Kirkpatrick AW. Ultrasound confirmation of guidewire position may eliminate accidental arterial dilatation during central venous cannulation. Scand J Trauma Resusc Emerg Med. 2010;18:39. https://doi.org/10.1186/1757-7241-18-39.
65. Augoustides JG, Horak J, Ochroch AE, et al. A randomized controlled clinical trial of real-time needle-guided ultrasound for internal jugular venous cannulation in a large university anesthesia department. J Cardiothorac Vasc Anesth. 2005;19(3):310-315. https://doi.org/10.1053/j.jvca.2005.03.007.
66. Maecken T, Heite L, Wolf B, Zahn PK, Litz RJ. Ultrasound-guided catheterisation of the subclavian vein: freehand vs needle-guided technique. Anaesthesia. 2015;70(11):1242-1249. https://doi.org/10.1111/anae.13187.
67. Stone MB, Nagdev A, Murphy MC, Sisson CA. Ultrasound detection of guidewire position during central venous catheterization. Am J Emerg Med. 2010;28(1):82-84. https://doi.org/10.1016/j.ajem.2008.09.019.
68. Luyet C, Hartwich V, Urwyler N, et al. Evaluation of a novel needle guide for ultrasound-guided phantom vessel cannulation. Anaesthesia. 2011;66(8):715-720. https://doi.org/10.1111/j.1365-2044.2011.06781.x.
69. O’Grady NP, Alexander M, Burns LA, et al. Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis. 2011;52(9):1087-1099. https://doi.org/10.1093/cid/cir138.
70. Southworth SL, Henman LJ, Kinder LA, Sell JL. The journey to zero central catheter-associated bloodstream infections: culture change in an intensive care unit. Crit Care Nurse. 2012;32(2):49-54. https://doi.org/10.4037/ccn2012915.
71. Guerin K, Wagner J, Rains K, Bessesen M. Reduction in central line-associated bloodstream infections by implementation of a postinsertion care bundle. Am J Infect Control. 2010;38(6):430-433. https://doi.org/10.1016/j.ajic.2010.03.007.
72. Hartman N, Wittler M, Askew K, Manthey D. Delphi method validation of a procedural performance checklist for insertion of an ultrasound-guided internal jugular central line. Am J Med Qual Off J Am Coll Med Qual. 2016;31(1):81-85. https://doi.org/10.1177/1062860614549762.
73. Lenchus JD, Carvalho CM, Ferreri K, et al. Filling the void: defining invasive bedside procedural competency for internal medicine residents. J Grad Med Educ. 2013;5(4):605-612. https://doi.org/10.4300/JGME-D-13-00030.1.
74. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. https://doi.org/10.1001/archinternmed.2009.215.
75. Karakitsos D, Labropoulos N, De Groot E, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care. 2006;10(6):R162. https://doi.org/10.1186/cc5101.
76. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. https://doi.org/10.1097/01.ccm.0000142399.70913.2f.
77. Young EM, Commiskey ML, Wilson SJ. Translating evidence into practice to prevent central venous catheter-associated bloodstream infections: a systems-based intervention. Am J Infect Control. 2006;34(8):503-506. https://doi.org/10.1016/j.ajic.2006.03.011.
78. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495; discussion 495. https://doi.org/10.1016/j.surg.2008.06.004.
79. Mermel LA, McCormick RD, Springman SR, Maki DG. The pathogenesis and epidemiology of catheter-related infection with pulmonary artery swan-Ganz catheters: a prospective study utilizing molecular subtyping. Am J Med. 1991;91(3b):197S–205S. https://doi.org/10.1016/0002-9343(91)90369-9.
80. Marschall J, Mermel LA, Classen D, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(1):S22-S30. https://doi.org/10.1086/591059.
81. Marschall J, Mermel LA, Fakih M, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(7):753-771. https://doi.org/10.1086/676533.
82. Garcia-Rodriguez JF, Álvarez-Díaz H, Vilariño-Maneiro L, et al. Epidemiology and impact of a multifaceted approach in controlling central venous catheter associated blood stream infections outside the intensive care unit. BMC Infect Dis. 2013;13:445. https://doi.org/10.1186/1471-2334-13-445.
83. Lee DH, Jung KY, Choi YH. Use of maximal sterile barrier precautions and/or antimicrobial-coated catheters to reduce the risk of central venous catheter-related bloodstream infection. Infect Control Hosp Epidemiol. 2008;29(10):947-950. https://doi.org/10.1086/590356.
84. Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132(5):391-402. https://doi.org/10.7326/0003-4819-132-5-200003070-00009.
85. Troianos CA, Jobes DR, Ellison N. Ultrasound-guided cannulation of the internal jugular vein. A prospective, randomized study. Anesth Analg. 1991;72(6):823-826. https://doi.org/10.1213/00000539-199106000-00020.
86. Troianos CA, Savino JS. Internal jugular vein cannulation guided by echocardiography. Anesthesiology. 1991;74(4):787-789. https://doi.org/10.1097/00000542-199104000-00026.
87. Denys BG, Uretsky BF, Reddy PS, et al. An ultrasound method for safe and rapid central venous access. N Engl J Med. 1991;324(8):566. https://doi.org/10.1056/NEJM199102213240816.
88. Riaz A, Shan Khan RA, Salim F. Ultrasound guided internal jugular venous cannulation: comparison with land-mark technique. J Coll Phys Surg Pak. 2015;25(5):315-319. https://doi.org/05.2015/JCPSP.315319.
89. Gok F, Kilicaslan A, Sarkilar G, Kandemir B, Yosunkaya A. The effect of ultrasound guidance on central venous catheter-associated bloodstream infection in critical care patients. Acta Med Mediterr. 2013;29:677-682.
90. Lamperti M, Cortellazzi P, D’Onofrio G, et al. An outcome study on complications using routine ultrasound assistance for internal jugular vein cannulation. Acta Anaesthesiol Scand. 2007;51(10):1327-1330. https://doi.org/10.1111/j.1399-6576.2007.01442.x.
91. Vezzani A, Manca T, Vercelli A, Braghieri A, Magnacavallo A. Ultrasonography as a guide during vascular access procedures and in the diagnosis of complications. J Ultrasound. 2013;16(4):161-170. https://doi.org/10.1007/s40477-013-0046-5.
92. Palepu GB, Deven J, Subrahmanyam M, Mohan S. Impact of ultrasonography on central venous catheter insertion in intensive care. Indian J Rad Imaging. 2009;19(3):191-198. https://doi.org/10.4103/0971-3026.54877.
93. Agarwal A, Singh DK, Singh AP. Ultrasonography: a novel approach to central venous cannulation. Indian J Crit Care Med. 2009;13(4):213-216. https://doi.org/10.4103/0972-5229.60174.
94. Wong SW, Niazi AU, Chin KJ, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS® needle tracking system: a case report. Can J Anaesth. 2013;60(1):50-53. https://doi.org/10.1007/s12630-012-9809-2.
95. Bouaziz H, Zetlaoui PJ, Pierre S, et al. Guidelines on the use of ultrasound guidance for vascular access. Anaesth, Crit Care Pain Med. 2015;34(1):65-69. https://doi.org/10.1016/j.accpm.2015.01.004.
96. Jenssen C, Brkljacic B, Hocke M, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part VI - Ultrasound-guided vascular interventions. Ultraschall Med. 2016;37(5):473-476. https://doi.org/10.1055/s-0035-1553450.
97. Rando K, Castelli J, Pratt JP, et al. Ultrasound-guided internal jugular vein catheterization: a randomized controlled trial. Heart Lung Vessels. 2014;6(1):13-23.
98. Mey U, Glasmacher A, Hahn C, et al. Evaluation of an ultrasound-guided technique for central venous access via the internal jugular vein in 493 patients. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2003;11(3):148-155. https://doi.org/10.1007/s00520-002-0399-3.
99. Singh SA, Sharma S, Singh A, et al. The safety of ultrasound guided central venous cannulation in patients with liver disease. Saudi J Anaesth. 2015;9(2):155-160. https://doi.org/10.4103/1658-354X.152842.
100. Akoglu H, Piskinpasa S, Yenigun EC, et al. Real-time ultrasound guided placement of temporary internal jugular vein catheters: assessment of technical success and complication rates in nephrology practice. Nephrol (Carlton). 2012;17(7):603-606. https://doi.org/10.1111/j.1440-1797.2012.01637.x.
101. Sadler DJ, Gordon AC, Klassen J, et al. Image-guided central venous catheters for apheresis. Bone Marrow Transplant. 1999;23(2):179-182. https://doi.org/10.1038/sj.bmt.1701545.
102. Calvert N, Hind D, McWilliams R, et al. Ultrasound for central venous cannulation: economic evaluation of cost-effectiveness. Anaesthesia. 2004;59(11):1116-1120. https://doi.org/10.1111/j.1365-2044.2004.03906.x.
103. Goetz AM, Wagener MM, Miller JM, Muder RR. Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infect Control Hosp Epidemiol. 1998;19(11):842-845.
104. Bertini P, Frediani M. Ultrasound guided supraclavicular central vein cannulation in adults: a technical report. J Vasc Access. 2013;14(1):89-93. https://doi.org/10.5301/jva.5000088.
105. Lalu MM, Fayad A, Ahmed O, et al. Ultrasound-guided subclavian vein catheterization: A systematic review and meta-analysis. Crit Care Med. 2015;43(7):1498-1507. https://doi.org/10.1097/CCM.0000000000000973.
106. Milone M, Di Minno G, Di Minno MN, et al. The real effectiveness of ultrasound guidance in subclavian venous access. Ann ital chir. 2010;81(5):331-334.
107. O’Leary R, Ahmed SM, McLure H, et al. Ultrasound-guided infraclavicular axillary vein cannulation: a useful alternative to the internal jugular vein. Br J Anaesth. 2012;109(5):762-768. https://doi.org/10.1093/bja/aes262.
108. Gualtieri E, Deppe SA, Sipperly ME, Thompson DR. Subclavian venous catheterization: greater success rate for less experienced operators using ultrasound guidance. Crit Care Med. 1995;23(4):692-697. https://doi.org/10.1097/00003246-199504000-00018.
109. Fragou M, Gravvanis A, Dimitriou V, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med. 2011;39(7):1607-1612. https://doi.org/10.1097/CCM.0b013e318218a1ae.
110. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015;1:CD011447. https://doi.org/10.1002/14651858.CD011447.
111. Robinson MK, Mogensen KM, Grudinskas GF, Kohler S, Jacobs DO. Improved care and reduced costs for patients requiring peripherally inserted central catheters: the role of bedside ultrasound and a dedicated team. JPEN J Parenter Enter Nutr. 2005;29(5):374-379. https://doi.org/10.1177/0148607105029005374.
112. Li J, Fan YY, Xin MZ, et al. A randomised, controlled trial comparing the long-term effects of peripherally inserted central catheter placement in chemotherapy patients using B-mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94-103. https://doi.org/10.1016/j.ejon.2013.08.003.
113. Schweickert WD, Herlitz J, Pohlman AS, et al. A randomized, controlled trial evaluating postinsertion neck ultrasound in peripherally inserted central catheter procedures. Crit Care Med. 2009;37(4):1217-1221. https://doi.org/10.1097/CCM.0b013e31819cee7f.
114. Mahler SA, Wang H, Lester C, Conrad SA. Ultrasound-guided peripheral intravenous access in the emergency department using a modified Seldinger technique. J Emerg Med. 2010;39(3):325-329. https://doi.org/10.1016/j.jemermed.2009.02.013.
115. Brannam L, Blaivas M, Lyon M, Flake M. Emergency nurses’ utilization of ultrasound guidance for placement of peripheral intravenous lines in difficult-access patients. Acad Emerg Med. 2004;11(12):1361-1363. https://doi.org/10.1197/j.aem.2004.08.027.
116. Au A, Rotte M, Gryzbowski R, Ku B, Fields J. 157 Decrease in central venous catheter placement and complications due to utilization of ultrasound-guided peripheral intravenous catheters. Ann Emerg Med. 2011;58(4):S230. https://doi.org/10.1016/j.annemergmed.2011.06.185.
117. Dargin JM, Rebholz CM, Lowenstein RA, Mitchell PM, Feldman JA. Ultrasonography-guided peripheral intravenous catheter survival in ED patients with difficult access. Am J Emerg Med. 2010;28(1):1-7. https://doi.org/10.1016/j.ajem.2008.09.001.
118. Bauman M, Braude D, Crandall C. Ultrasound-guidance vs standard technique in difficult vascular access patients by ED technicians. Am J Emerg Med. 2009;27(2):135-140. https://doi.org/10.1016/j.ajem.2008.02.005.
119. Schoenfeld E, Shokoohi H, Boniface K. Ultrasound-guided peripheral intravenous access in the emergency department: patient-centered survey. West J Emerg Med. 2011;12(4):475-477. https://doi.org/10.5811/westjem.2011.3.1920.
120. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. https://doi.org/10.7326/0003-4819-114-10-845.
121. Miliani K, Taravella R, Thillard D, et al. Peripheral venous catheter-related adverse events: evaluation from a multicentre epidemiological study in France (the CATHEVAL Project). PLOS ONE. 2017;12(1):e0168637. https://doi.org/10.1371/journal.pone.0168637.
122. Gregg SC, Murthi SB, Sisley AC, Stein DM, Scalea TM. Ultrasound-guided peripheral intravenous access in the intensive care unit. J Crit Care. 2010;25(3):514-519. https://doi.org/10.1016/j.jcrc.2009.09.003.
123. Adhikari S, Blaivas M, Morrison D, Lander L. Comparison of infection rates among ultrasound-guided versus traditionally placed peripheral intravenous lines. J Ultrasound Med. 2010;29(5):741-747. https://doi.org/10.7863/jum.2010.29.5.741.
124. Shiver S, Blaivas M, Lyon M. A prospective comparison of ultrasound-guided and blindly placed radial arterial catheters. Acad Emerg Med. 2006;13(12):1275-1279. https://doi.org/10.1197/j.aem.2006.07.015.
125. Levin PD, Sheinin O, Gozal Y. Use of ultrasound guidance in the insertion of radial artery catheters. Crit Care Med. 2003;31(2):481-484. https://doi.org/10.1097/01.CCM.0000050452.17304.2F.
126. Gu WJ, Tie HT, Liu JC, Zeng XT. Efficacy of ultrasound-guided radial artery catheterization: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2014;18(3):R93. https://doi.org/10.1186/cc13862.
127. Tang L, Wang F, Li Y, et al. Ultrasound guidance for radial artery catheterization: an updated meta-analysis of randomized controlled trials. PLOS ONE. 2014;9(11):e111527. https://doi.org/10.1371/journal.pone.0111527.
128. Sobolev M, Slovut DP, Lee Chang A, Shiloh AL, Eisen LA. Ultrasound-guided catheterization of the femoral artery: A systematic review and meta-analysis of randomized controlled trials. J Invas Cardiol. 2015;27(7):318-323. https://doi.org/10.1378/chest.1991181.
129. Gabriel M, Pawlaczyk K, Waliszewski K, Krasiński Z, Majewski W. Location of femoral artery puncture site and the risk of postcatheterization pseudoaneurysm formation. Int J Cardiol. 2007;120(2):167-171. https://doi.org/10.1016/j.ijcard.2006.09.018.
130. Seto AH, Abu-Fadel MS, Sparling JM, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With ultrasound Trial). JACC Cardiovasc Interv. 2010;3(7):751-758. https://doi.org/10.1016/j.jcin.2010.04.015.
131. Kalish J, Eslami M, Gillespie D, et al. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg. 2015;61(5):1231-1238. https://doi.org/10.1016/j.jvs2014.12.003.
132. Sandhu NS, Patel B. Use of ultrasonography as a rescue technique for failed radial artery cannulation. J Clin Anesth. 2006;18(2):138-141. https://doi.org/10.1016/j.jclinane.2005.06.011.
133. White L, Halpin A, Turner M, Wallace L. Ultrasound-guided radial artery cannulation in adult and paediatric populations: a systematic review and meta-analysis. Br J Anaesth. 2016;116(5):610-617. https://doi.org/10.1093/bja/aew097.
134. Gao YB, Yan JH, Gao FQ, et al. Effects of ultrasound-guided radial artery catheterization: an updated meta-analysis. Am J Emerg Med. 2015;33(1):50-55. https://doi.org/10.1016/j.ajem.2014.10.008.
135. Seto AH, Roberts JS, Abu-Fadel MS, et al. Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery Access with Ultrasound Trial). JACC Cardiovasc Interv. 2015;8(2):283-291. https://doi.org/10.1016/j.jcin.2014.05.036.
136. Roberts J, Manur R. Ultrasound-guided radial artery access by a non-ultrasound trained interventional cardiologist improved first-attempt success rates and shortened time for successful radial artery cannulation. J Invas Cardiol. 2013;25(12):676-679.
137. Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108(5):1345-1348. https://doi.org/10.1378/chest.108.5.1345.
138. Lichtenstein D, Mezière G, Biderman P, Gepner A. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999;25(4):383-388. https://doi.org/10.1007/s001340050862.
139. Ablordeppey EA, Drewry AM, Beyer AB, et al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound Versus chest radiography in critically ill patients: A systematic review and meta-analysis. Crit Care Med. 2017;45(4):715-724. https://doi.org/10.1097/CCM.0000000000002188.
140. Bedel J, Vallée F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013;39(11):1932-1937. https://doi.org/10.1007/s00134-013-3097-3.
141. Weekes AJ, Keller SM, Efune B, Ghali S, Runyon M. Prospective comparison of ultrasound and CXR for confirmation of central vascular catheter placement. Emerg Med J EMJ. 2016;33(3):176-180. https://doi.org/10.1136/emermed-2015-205000.
142. Arellano R, Nurmohamed A, Rumman A, et al. The utility of transthoracic echocardiography to confirm central line placement: an observational study. Can J Anaesth. 2014;61(4):340-346. https://doi.org/10.1007/s12630-014-0111-3.
143. Vezzani A, Brusasco C, Palermo S, et al. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38(2):533-538. https://doi.org/10.1097/CCM.0b013e3181c0328f.
144. Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142(4):260-273. https://doi.org/10.7326/0003-4819-142-4-200502150-00008.
145. Backlund BH, Hopkins E, Kendall JL. Ultrasound guidance for central venous access by emergency physicians in Colorado. West J Emerg Med. 2012;13(4):320-325. https://doi.org/10.5811/westjem.2011.11.6821.
146. Buchanan MS, Backlund B, Liao MM, et al. Use of ultrasound guidance for central venous catheter placement: survey from the American Board of Emergency Medicine Longitudinal Study of Emergency Physicians. Acad Emerg Med. 2014;21(4):416-421. https://doi.org/10.1111/acem.12350.
147. Barsuk JH, McGaghie WC, Cohen ER, O’Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697-2701. https://doi.org/10.1097/00003246-200910000-00003.
148. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. https://doi.org/10.1097/00003246-200201000-00009.
149. Woo MY, Frank J, Lee AC, et al. Effectiveness of a novel training program for emergency medicine residents in ultrasound-guided insertion of central venous catheters. CJEM. 2009;11(4):343-348. https://doi.org/10.1017/S1481803500011398.
150. McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348(12):1123-1133. https://doi.org/10.1056/NEJMra011883.
151. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. https://doi.org/10.1002/jhm.468.
152. Sekiguchi H, Tokita JE, Minami T, et al. A prerotational, simulation-based workshop improves the safety of central venous catheter insertion: results of a successful internal medicine house staff training program. Chest. 2011;140(3):652-658. https://doi.org/10.1378/chest.10-3319.
153. Feller-Kopman D. Ultrasound-guided internal jugular access: a proposed standardized approach and implications for training and practice. Chest. 2007;132(1):302-309. https://doi.org/10.1378/chest.06-2711.
154. Troianos CA, Hartman GS, Glas KE, et al. Special articles: guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg. 2012;114(1):46-72. https://doi.org/10.1213/ANE.0b013e3182407cd8.
155. Issenberg SB, McGaghie WC, Hart IR, et al. Simulation technology for health care professional skills training and assessment. JAMA. 1999;282(9):861-866. https://doi.org/10.1001/jama.282.9.861.
156. Millington SJ, Wong RY, Kassen BO, Roberts JM, Ma IW. Improving internal medicine residents’ performance, knowledge, and confidence in central venous catheterization using simulators. J Hosp Med. 2009;4(7):410-416. https://doi.org/10.1002/jhm.570.
157. Beaulieu Y, Laprise R, Drolet P, et al. Bedside ultrasound training using web-based e-learning and simulation early in the curriculum of residents. Crit Ultrasound J. 2015;7:1. https://doi.org/10.1186/s13089-014-0018-9.
158. Barsuk JH, Cohen ER, McGaghie WC, Wayne DB. Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med. 2010;85(10 Suppl):S9-S12. https://doi.org/10.1097/ACM.0b013e3181ed436c.
159. Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133(1):56-61. https://doi.org/10.1378/chest.07-0131.
160. Evans LV, Dodge KL, Shah TD, et al. Simulation training in central venous catheter insertion: improved performance in clinical practice. Acad Med. 2010;85(9):1462-1469. https://doi.org/10.1097/ACM.0b013e3181eac9a3.
161. Smith CC, Huang GC, Newman LR, et al. Simulation training and its effect on long-term resident performance in central venous catheterization. Simul Healthc J Soc Simul Healthc. 2010;5(3):146-151. https://doi.org/10.1097/SIH.0b013e3181dd9672.
162. Laack TA, Dong Y, Goyal DG, et al. Short-term and long-term impact of the central line workshop on resident clinical performance during simulated central line placement. Simul Healthc J Soc Simul Healthc. 2014;9(4):228-233. https://doi.org/10.1097/SIH.0000000000000015.
163. Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31(10):1519-1526. https://doi.org/10.7863/jum.2012.31.10.1519.
164. Bayci AW, Mangla J, Jenkins CS, Ivascu FA, Robbins JM. Novel educational module for subclavian central venous catheter insertion using real-time ultrasound guidance. J Surg Educ. 2015;72(6):1217-1223. https://doi.org/10.1016/j.jsurg.2015.07.010.
165. Andreatta P, Chen Y, Marsh M, Cho K. Simulation-based training improves applied clinical placement of ultrasound-guided PICCs. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2011;19(4):539-543. https://doi.org/10.1007/s00520-010-0849-2.
166. Rosen BT, Uddin PQ, Harrington AR, Ault BW, Ault MJ. Does personalized vascular access training on a nonhuman tissue model allow for learning and retention of central line placement skills? Phase II of the procedural patient safety initiative (PPSI-II). J Hosp Med. 2009;4(7):423-429. https://doi.org/10.1002/jhm.571.
167. Ault MJ, Rosen BT, Ault B. The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative. J Gen Intern Med. 2006;21(5):514-517. https://doi.org/10.1111/j.1525-1497.2006.00440.x.
168. Varga S, Smith J, Minneti M, et al. Central venous catheterization using a perfused human cadaveric model: application to surgical education. J Surg Educ. 2015;72(1):28-32. https://doi.org/10.1016/j.jsurg.2014.07.005.
169. Sansivero GE. Venous anatomy and physiology. Considerations for vascular access device placement and function. J Intraven Nurs Off Publ Intraven Nurs Soc. 1998;21(5 Suppl):S107-S114.
170. Eisen LA, Narasimhan M, Berger JS, et al. Mechanical complications of central venous catheters. Journal of intensive care medicine. 2006;21(1):40-46. https://doi.org/10.1177/0885066605280884.
171. Chenkin J, Lee S, Huynh T, Bandiera G. Procedures can be learned on the Web: a randomized study of ultrasound-guided vascular access training. Acad Emerg Med. 2008;15(10):949-954. https://doi.org/10.1111/j.1553-2712.2008.00231.x.
172. Abualenain J, Calabrese K, Tansek R, Ranniger C. 319 Comparing standard versus video-based teaching for ultrasound-guided internal jugular central venous catheter access for fourth-year medical students. Ann Emerg Med. 2014;64(4):S113. https://doi.org/10.1016/j.annemergmed.2014.07.347.
173. Pustavoitau A, Blaivas M, Brown SM, et al. Recommendations for achieving and maintaining competence and credentialing in critical care ultrasound with focused cardiac ultrasound and advanced critical care echocardiography. Crit Care Med. 2016.
174. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: A survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
175. Moureau N, Lamperti M, Kelly LJ, et al. Evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training. Br J Anaesth. 2013;110(3):347-356. https://doi.org/10.1093/bja/aes499.
176. Ernst A, Silvestri GA, Johnstone D, American College of Chest Physicians. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123(5):1693-1717. https://doi.org/10.1378/chest.123.5.1693.
177. Thomas SM, Burch W, Kuehnle SE, et al. Simulation training for pediatric residents on central venous catheter placement: a pilot study. Pediatr Crit Care Med J Soc Crit Care Med.. 2013;14(9):e416-e423. https://doi.org/10.1097/PCC.0b013e31829f5eda.
178. Smith KK, Gilcreast D, Pierce K. Evaluation of staff’s retention of ACLS and BLS skills. Resuscitation. 2008;78(1):59-65. https://doi.org/10.1016/j.resuscitation.2008.02.007.
179. Ericsson KA. Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med. 2004;79(10 Suppl):S70-S81. https://doi.org/10.1097/00001888-200410001-00022.
180. Gerard JM, Thomas SM, Germino KW, et al. The effect of simulation training on PALS skills among family medicine residents. Fam Med. 2011;43(6):392-399.
181. Brydges R, Nair P, Ma I, Shanks D, Hatala R. Directed self-regulated learning versus instructor-regulated learning in simulation training. Med Educ. 2012;46(7):648-656. https://doi.org/10.1111/j.1365-2923.2012.04268.x.
182. Wayne DB, Butter J, Siddall VJ, et al. Simulation-based training of internal medicine residents in advanced cardiac life support protocols: a randomized trial. Teach Learn Med. 2005;17(3):210-216. https://doi.org/10.1207/s15328015tlm1703_3.
183. Arthur Jr. W, Bennett Jr. W, Stanush PL, McNelly TL. Factors that influence skill decay and retention: A quantitative review and analysis. Hum Perform. 1998;11(1):57-101. https://doi.org/10.1207/s15327043hup1101_3.
184. Rusche JD, Besuner P, Partusch SK, Berning PA. Competency program development across a merged healthcare network. J Nurs Staff Dev. 2001;17(5):234-240; quiz 241-232. https://doi.org/10.1097/00124645-200109000-00004.
185. O’Hearne Rebholz M. A review of methods to assess competency. J Nurs Staff Dev. 2006;22(5):241-245. https://doi.org/10.1097/00124645-200609000-00007.
186. Reznick RK. Teaching and testing technical skills. Am J Surg. 1993;165(3):358-361. https://doi.org/10.1016/s0002-9610(05)80843-8.
187. Reznick RK, MacRae H. Teaching surgical skills--changes in the wind. N Engl J Med. 2006;355(25):2664-2669. https://doi.org/10.1056/NEJMra054785.
188. Murin S, Stollenwerk NS. Simulation in procedural training: at the tipping point. Chest. 2010;137(5):1009-1011. https://doi.org/10.1378/chest.10-0199.
189. American College of Emergency Physicians. Emergency ultrasound guidelines. Ann Emerg Med. 2009;53(4):550-570. https://doi.org/10.1016/j.annemergmed.2008.12.013.
190. Guilbert MC, Elkouri S, Bracco D, et al. Arterial trauma during central venous catheter insertion: case series, review and proposed algorithm. J Vasc Surg. 2008;48(4):918-925; discussion 925. https://doi.org/10.1016/j.jvs2008.04.046.
191. Dong Y, Suri HS, Cook DA, et al. Simulation-based objective assessment discerns clinical proficiency in central line placement: a construct validation. Chest. 2010;137(5):1050-1056. https://doi.org/10.1378/chest.09-1451.
192. Adhikari S, Theodoro D, Raio C, et al. Central venous catheterization: are we using ultrasound guidance? J Ultrasound Med. 2015;34(11):2065-2070. https://doi.org/10.7863/ultra.15.01027.
193. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors--procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z.
© 2019 Society of Hospital Medicine
Update in Hospital Medicine: Practical Lessons from Current Literature
Hospital medicine continues to expand with respect to the number of practitioners as well as the scope of the practice of those practitioners. In addition, the commitment to, and rigor of, scientific inquiry in the field continues to grow. The authors of this article conducted a review of the medical literature, including articles published between March 2017 and March 2018. The key articles reported studies with high methodological quality, clear findings, and a high potential for impact on clinical practice. The literature was independently reviewed by each author, and candidate works were chosen on the basis of relevance to hospital medicine and expected clinical impact. The articles were organized by subject matter, ranked by applicability to the audience, and selected to meet the time constraints of each talk. Twenty-nine articles were presented at the Update in Hospital Medicine at the 2018 Society of Hospital Medicine and Society of General Internal Medicine annual meetings (B Sharpe, A Burger at SGIM and B Slawski, C Cooper at SHM). Nine articles were included in this review through an iterative voting process. Each author ranked their top five articles from one to five. Points were tallied for each article, and the five articles with the highest points were included. A second round of voting identified the remaining four articles for inclusion. Ties were adjudicated by group discussion. Each article is summarized below, and their key points are highlighted in the table.
KEY PUBLICATIONS
Aspirin in Patients with Previous Percutaneous Coronary Intervention Undergoing Noncardiac Surgery. Graham MM et al. Ann Intern Med. 2018;168(4):237-244.1
Background
The Perioperative Ischemic Evaluation 2 (POISE-2) trial found that perioperative aspirin use had no significant effect on the risk of perioperative death and nonfatal myocardial infarction (MI) in patients who are at risk for vascular complications; however, the risk of major bleeding increased with aspirin use in these patients.2 Nevertheless, the POISE-2 trial did not specifically address the role of aspirin in patients who had undergone previous percutaneous coronary intervention (PCI).
Methods
A post hoc subgroup analysis of POISE-2 evaluated 470 PCI patients (234 aspirin-treated and 236 placebo-treated patients) aged >45 years, 90% of whom had stents. The administration of the study drug was initiated within four hours preoperatively and continued postoperatively. Patients who had bare metal stents placed within the six weeks prior to the study or drug-eluting stents placed within the preceding 12 months were excluded.
Findings
The composite endpoint of risk of death and nonfatal MI was 11.5% in the placebo group and 6% in aspirin-treated patients (HR 0.50; CI, 0.26-0.95). Most of the difference in primary outcome was attributed to an increase in nonfatal MI in the placebo group. Major and life-threatening bleeding were not substantially increased in PCI patients but increased in the overall POISE-2 trial (absolute risk increase 0.8% for major bleeding [95% CI, 0.1%-1.6%]; HR 1.22 [95% CI, 1.01-1.48]). Stent type had no effect on death and nonfatal MI.
Cautions
This was a non-prespecified subgroup analysis with a small sample size.
Implications
Perioperative aspirin use in patients with previous PCI appears to provide more benefit than harm, unless a substantial bleeding risk exists.
Association Between Wait Time and 30-Day Mortality in Adults Undergoing Hip Fracture Surgery. Pincus D et al. JAMA. 2017;318(20):1994-2003.3
Background
Wait times to hip fracture surgery have been associated with mortality in previous studies; however, the wait time associated with complications remains controversial.4,5
Methods
This retrospective cohort study of 42,230 adults modeled the probability of complications in accordance with wait time from hospital arrival to hip fracture surgery. It aimed to identify the optimal time window in which to conduct surgery before complications increased. This window to increased complications was used to define early and delayed surgery. The matched cohorts of early and delayed patients were then used to compare outcomes.
Findings
Overall 30-day mortality was 7%. Complication rates increased when wait times reached 24 hours. Comparing the propensity-matched early (<24 hours) and late (>24 hours) surgery patients revealed that late surgery patients had significantly higher 30-day mortality (6.5% vs 5.8%; % absolute RD 0.79; 95% CI, 0.23-1.35) than early surgery patients and the composite outcome of mortality or other medical complications (MI, DVT, PE, and pneumonia; 12.2% vs 10.1%; % absolute RD 2.16; 95% CI, 1.43-2.89).
Cautions
Only 34% of patients in this study had surgery within 24 hours. The observational cohort study design may result in unmeasured confounders, eg, less sick patients go to surgery more quickly than sicker patients.
Implications
A preoperative wait time of 24 hours appears to represent a threshold of increased risk for 30-day perioperative complications and mortality in hip fracture surgery.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review. Hale AJ et al. J Hosp Med. 2018;13(5):328-335.6
Background
Bloodstream infections (BSIs) are significant causes of morbidity and mortality in the United States. Traditionally, clinicians have relied on intravenous antibiotics for treatment. A recent “Choosing Wisely®” initiative recommends that clinicians should use “oral formulations of highly bioavailable antimicrobials wherever possible.”7 Thus, the authors searched for evidence for scenarios wherein BSIs could be safely treated with oral antibiotics.
Methods
A narrative review was conducted given that robust clinical data for an extensive systematic review were insufficient.
Findings
Key decision points on the use of an oral antibiotic for a diagnosed BSI are as follows: (1) Source control must be attained prior to the consideration of oral antibiotics. (2) A highly bioavailable oral option to which the pathogen is sensitive must be available. (3) Patients must be able to comply with the therapy for the full course and not be on interfering medications. Good evidence for use of oral antibiotics against sensitive gram-negative bacilli other than Pseudomonas exists. Evidence for treating Streptococcus pneumoniae with early transition (within three days) to oral antibiotics is robust when treating bacteremia and pneumonia but not for other primary sites of infection. Evidence for the use of oral antibiotics for B-hemolytic streptococcus, including necrotizing fasciitis and Enterococcus, is insufficient. The evidence supports at least two weeks of IV antibiotics for the treatment of Staphylococcus aureus.
Cautions
This is a narrative review due to limited evidence.
Implications
The early use of oral antibiotics in the setting of bacteremia may be appropriate in select clinical situations.
Prevalence of Pulmonary Embolism in Patients with Syncope. Costantino et al. JAMA Intern Med. 2018;178(3):356-362.8
Background
Data on the prevalence of pulmonary embolism in patients presenting with syncope are conflicting.
Methods
This was a retrospective observational study involving five databases in four countries of >1.6 million adults identified through syncope ICD codes. The rates of pulmonary embolism at first evaluation and pulmonary embolism or venous thromboembolism within 90 days were calculated for emergency room patients and a hospitalized subgroup.
Findings
Pulmonary embolism was rare in patients with syncope, eg, less than 3% for hospitalized patients in this database study.
Cautions
The results of this study are based on the use of administrative databases to confirm the diagnosis of syncope. Additionally, the results include hospitalized and nonhospitalized patients. The design of this study differs significantly from those of the PESIT study, which showed a prevalence of 17% in hospitalized patients.9 The PESIT study specifically sought the diagnosis of pulmonary embolism even when other etiologies for syncope existed.
Implications
Ultimately, the clinical impetus to search for pulmonary embolism in hospitalized patients admitted with syncope will depend on individual presentations. The authors argued that pulmonary embolism is rare in syncope and much lower than 17% but should be considered in appropriate patients.
Balanced Crystalloids versus Saline in Noncritically Ill Patients. Self WH et al. N Engl J Med. 2018;378(9):819-828.10
Background
Data on the optimal composition of intravenous fluids (IVF) are limited. Limited experimental evidence suggests that IVF-induced hyperchloremia results in renal vasoconstriction and acute kidney injury.
Methods
This was a single-center, open-label, multiple crossover trial of >13,000 non-ICU hospitalized patients admitted from the Emergency Department. Patients were randomized to receive either only normal saline or a “balanced crystalloid,” eg, either Lactated Ringer’s or Plasmalyte. The primary outcome was hospital-free days. Secondary outcomes were major adverse kidney events (MAKE) at 30 days.
Findings
The study found no difference in the primary outcome of hospital-free days. However, balanced IVF resulted in a lower incidence of hyperchloremia and a slightly reduced incidence of MAKE 30 (4.7% vs 5.6%; adjusted OR 0.82).
Cautions
The incidence of acute kidney injury was low in this single-center ED population. This study, however, did not include hospitalized patients. The long-term effects on renal function could not be ascertained.
Implications
Hospital-free days after inpatient randomization to either normal saline or “balanced IVF” were not significantly different. “Balanced IVF” may be beneficial in select high renal-risk populations.
Speaker Introductions at Internal Medicine Grand Rounds: Forms of Address Reveal Speaker Bias. Files et al. J Womens Health. 2017;26(5):413-419.11
Background
Gender bias is known to contribute to leadership disparities between men and women in several academic medical centers.
Methods
This was a retrospective observational study reviewing video-archived introductions at Internal Medicine Grand Rounds at two connected institutions. All speakers had doctoral degrees. The outcome measured was the use of a speaker’s professional title during his/her introduction as a function of the introducer’s gender.
Findings
Women were more likely than men to introduce speakers of any gender by their professional title in the 321 forms of address analyzed (96% vs 66%, P < .001). When the introducer and speaker were of different genders, women were more likely to introduce male speakers with formal titles than men introducing female speakers (95% vs 49%, P < .001).
Cautions
This study was done at two associated academic institutions and may not reflect the practice or customs of physicians in other departments or institutions.
Implications
Despite the study’s limitations, it supports a theme of prevalent gender bias within academic medical institutions that may affect the outcomes of leadership, promotion, and scholarship.
Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. Raskob GE et al. N Engl J Med. 2018;378(7):615-624.12
Background
Low-molecular-weight heparin (LMWH) is the standard of care for the treatment of venous thromboembolism (VTE) in patients with cancer. Direct oral anticoagulants have not been studied for this indication.
Methods
This open-label, noninferiority trial randomized patients with cancer and acute VTE to either LMWH for a minimum of five days followed by oral edoxaban vs subcutaneous dalteparin.
Findings
A total of 1,046 patients were included in the modified intention-to-treat analysis. Patients received treatment for six to twelve months total. A composite outcome of recurrent VTE or major bleed within 12 months occurred in 67 of 522 (12.8%) of patients in the edoxaban group vs 71 of 524 (13.5%) of patients in the dalteparin group (HR 0.91, 95% CI 0.70-1.36, P = .006 for noninferiority). Recurrent VTE occurred more commonly with dalteparin than with edoxaban (11.3% vs 7.9%), whereas major bleeding was less common with dalteparin than with edoxaban (4% vs 6.9%). The increased bleeding rate with edoxaban was predominantly in patients with an upper gastrointestinal (GI) malignancy.
Cautions
This was an open-label study. Patients in the edoxaban still received five days of LMWH prior to oral edoxaban. More patients in the edoxaban group continued treatment for the entire 12-month period, which contributes to the observed decreased bleeding and increased VTE rates in the dalteparin group.
Implications
Oral edoxaban is noninferior to subcutaneous dalteparin for the primary composite endpoint of VTE and bleeding. Notably, the patients in the edoxaban group experienced a lower rate of recurrent VTE and a higher rate of major bleeding than the patients in the dalteparin group. Additional caution about bleeding risk in those with a GI malignancy is recommended.
Can High-flow Nasal Cannula Reduce the Rate of Endotracheal Intubation in Adult Patients with Acute Respiratory Failure Compared with Conventional Oxygen Therapy and Noninvasive Positive Pressure Ventilation? Ni Y-N et al. Chest. 2017;151(4):764-775.13
Background
High-flow nasal cannula (HFNC) can deliver heated and humidified oxygen at rates of up to 60 L/min. Evidence on the benefits of HFNC over usual oxygen therapy or noninvasive positive pressure ventilation (NIPPV) is conflicting.
Methods
This systematic review and meta-analysis included 18 studies (12 RCTs, four retrospective, and two prospective cohort studies) with 3,881 patients with respiratory failure (medical and surgical causes). The included studies compared HFNC with usual oxygen therapy or NIPPV.
Findings
HFNC was associated with lower rates of endotracheal intubation (OR 0.47, 95% CI 0.27-0.84, P = .01) relative to oxygen therapy. Intubation rates did not differ between HFNC and NIPPV (OR 0.73, 95% CI 0.47-1.13, P = .16). No differences in ICU mortality or ICU length of stay (LOS) were found when HFNC was compared with either usual oxygen therapy or NIPPV.
Cautions
The significant heterogeneity in study design across studies is mainly attributable to varying causes of respiratory failure and differences in flow rate, oxygen concentration, and treatment duration across studies.
Implications
In patients with respiratory failure, HFNC may reduce intubation when compared with usual oxygen therapy and has similar ICU mortality when compared with usual oxygen and NIPPV.
Errors in the Diagnosis of Spinal Epidural Abscesses in the Era of Electronic Health Records. Bhise V et al. Am J Med. 2017;130(8):975-981.14
Background
Diagnostic errors are common in patients with spinal epidural abscess, but the main contributing factors are unclear.15
Methods
All patients who were newly diagnosed with spinal epidural abscess in 2013 were identified from the Veterans Affairs (VA) national database. Charts were reviewed for diagnostic delay and contributing factors, including the presence of “red flag” symptoms (eg, fever and neurological deficits).
Findings
Of the 119 patients with a new diagnosis of spinal epidural abscess, 66 (56%) had a diagnostic error. The median time to diagnosis in those with a diagnostic error was 12 days vs four days in those without error (P < .01). Common missed red flags in error cases included fever (n = 57, 86.4%), focal neurologic deficit (n = 54, 81.8%), and active infection (n = 54, 81.8%). Most errors occurred during the provider–patient encounter (eg, information not gathered during the history or physical). The magnitude of harm was serious for most patients (n = 40, 60.6%) and contributed to death in eight patients (12.1%).
Cautions
The study may not be generalizable because it was limited to the VA health system.
Implications
Diagnostic errors are common in patients with spinal epidural abscesses and can lead to serious harm. Health systems should build mechanisms to support providers in the evaluation of patients with back pain.
1. Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med. 2018;168(4):237-244. doi: 10.7326/M17-2341.
2. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. doi: 10.1056/NEJMoa1401105
3. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994-2003. doi: 10.1001/jama.2017.17606.
4. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616. doi: 10.1503/cmaj.092220.
5. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? ystematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146-154. doi: 10.1007/BF03016088.
6. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018;13(5):328-335. doi: 10.12788/jhm.2949.
7. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. doi: 10.1007/s15010-017-0997-0.
8. Costantino G, Ruwald MH, Quinn J, et al. Prevalence of pulmonary embolism in patients with syncope. JAMA Intern Med. 2018;178(3):356-362. doi: 10.1001/jamainternmed.2017.8175.
9. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016;375(16):1524-1531. doi: 10.1056/NEJMoa1602172
10. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586.
11. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. doi: 10.1089/jwh.2016.6044.
12. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. doi: 10.1056/NEJMoa1711948.
13. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest. 2017;151(4):764-775. doi: 10.1016/j.chest.2017.01.004.
14. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi: 10.1016/j.amjmed.2017.03.009
15. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291. doi: 10.1016/j.jemermed.2003.11.013.
Hospital medicine continues to expand with respect to the number of practitioners as well as the scope of the practice of those practitioners. In addition, the commitment to, and rigor of, scientific inquiry in the field continues to grow. The authors of this article conducted a review of the medical literature, including articles published between March 2017 and March 2018. The key articles reported studies with high methodological quality, clear findings, and a high potential for impact on clinical practice. The literature was independently reviewed by each author, and candidate works were chosen on the basis of relevance to hospital medicine and expected clinical impact. The articles were organized by subject matter, ranked by applicability to the audience, and selected to meet the time constraints of each talk. Twenty-nine articles were presented at the Update in Hospital Medicine at the 2018 Society of Hospital Medicine and Society of General Internal Medicine annual meetings (B Sharpe, A Burger at SGIM and B Slawski, C Cooper at SHM). Nine articles were included in this review through an iterative voting process. Each author ranked their top five articles from one to five. Points were tallied for each article, and the five articles with the highest points were included. A second round of voting identified the remaining four articles for inclusion. Ties were adjudicated by group discussion. Each article is summarized below, and their key points are highlighted in the table.
KEY PUBLICATIONS
Aspirin in Patients with Previous Percutaneous Coronary Intervention Undergoing Noncardiac Surgery. Graham MM et al. Ann Intern Med. 2018;168(4):237-244.1
Background
The Perioperative Ischemic Evaluation 2 (POISE-2) trial found that perioperative aspirin use had no significant effect on the risk of perioperative death and nonfatal myocardial infarction (MI) in patients who are at risk for vascular complications; however, the risk of major bleeding increased with aspirin use in these patients.2 Nevertheless, the POISE-2 trial did not specifically address the role of aspirin in patients who had undergone previous percutaneous coronary intervention (PCI).
Methods
A post hoc subgroup analysis of POISE-2 evaluated 470 PCI patients (234 aspirin-treated and 236 placebo-treated patients) aged >45 years, 90% of whom had stents. The administration of the study drug was initiated within four hours preoperatively and continued postoperatively. Patients who had bare metal stents placed within the six weeks prior to the study or drug-eluting stents placed within the preceding 12 months were excluded.
Findings
The composite endpoint of risk of death and nonfatal MI was 11.5% in the placebo group and 6% in aspirin-treated patients (HR 0.50; CI, 0.26-0.95). Most of the difference in primary outcome was attributed to an increase in nonfatal MI in the placebo group. Major and life-threatening bleeding were not substantially increased in PCI patients but increased in the overall POISE-2 trial (absolute risk increase 0.8% for major bleeding [95% CI, 0.1%-1.6%]; HR 1.22 [95% CI, 1.01-1.48]). Stent type had no effect on death and nonfatal MI.
Cautions
This was a non-prespecified subgroup analysis with a small sample size.
Implications
Perioperative aspirin use in patients with previous PCI appears to provide more benefit than harm, unless a substantial bleeding risk exists.
Association Between Wait Time and 30-Day Mortality in Adults Undergoing Hip Fracture Surgery. Pincus D et al. JAMA. 2017;318(20):1994-2003.3
Background
Wait times to hip fracture surgery have been associated with mortality in previous studies; however, the wait time associated with complications remains controversial.4,5
Methods
This retrospective cohort study of 42,230 adults modeled the probability of complications in accordance with wait time from hospital arrival to hip fracture surgery. It aimed to identify the optimal time window in which to conduct surgery before complications increased. This window to increased complications was used to define early and delayed surgery. The matched cohorts of early and delayed patients were then used to compare outcomes.
Findings
Overall 30-day mortality was 7%. Complication rates increased when wait times reached 24 hours. Comparing the propensity-matched early (<24 hours) and late (>24 hours) surgery patients revealed that late surgery patients had significantly higher 30-day mortality (6.5% vs 5.8%; % absolute RD 0.79; 95% CI, 0.23-1.35) than early surgery patients and the composite outcome of mortality or other medical complications (MI, DVT, PE, and pneumonia; 12.2% vs 10.1%; % absolute RD 2.16; 95% CI, 1.43-2.89).
Cautions
Only 34% of patients in this study had surgery within 24 hours. The observational cohort study design may result in unmeasured confounders, eg, less sick patients go to surgery more quickly than sicker patients.
Implications
A preoperative wait time of 24 hours appears to represent a threshold of increased risk for 30-day perioperative complications and mortality in hip fracture surgery.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review. Hale AJ et al. J Hosp Med. 2018;13(5):328-335.6
Background
Bloodstream infections (BSIs) are significant causes of morbidity and mortality in the United States. Traditionally, clinicians have relied on intravenous antibiotics for treatment. A recent “Choosing Wisely®” initiative recommends that clinicians should use “oral formulations of highly bioavailable antimicrobials wherever possible.”7 Thus, the authors searched for evidence for scenarios wherein BSIs could be safely treated with oral antibiotics.
Methods
A narrative review was conducted given that robust clinical data for an extensive systematic review were insufficient.
Findings
Key decision points on the use of an oral antibiotic for a diagnosed BSI are as follows: (1) Source control must be attained prior to the consideration of oral antibiotics. (2) A highly bioavailable oral option to which the pathogen is sensitive must be available. (3) Patients must be able to comply with the therapy for the full course and not be on interfering medications. Good evidence for use of oral antibiotics against sensitive gram-negative bacilli other than Pseudomonas exists. Evidence for treating Streptococcus pneumoniae with early transition (within three days) to oral antibiotics is robust when treating bacteremia and pneumonia but not for other primary sites of infection. Evidence for the use of oral antibiotics for B-hemolytic streptococcus, including necrotizing fasciitis and Enterococcus, is insufficient. The evidence supports at least two weeks of IV antibiotics for the treatment of Staphylococcus aureus.
Cautions
This is a narrative review due to limited evidence.
Implications
The early use of oral antibiotics in the setting of bacteremia may be appropriate in select clinical situations.
Prevalence of Pulmonary Embolism in Patients with Syncope. Costantino et al. JAMA Intern Med. 2018;178(3):356-362.8
Background
Data on the prevalence of pulmonary embolism in patients presenting with syncope are conflicting.
Methods
This was a retrospective observational study involving five databases in four countries of >1.6 million adults identified through syncope ICD codes. The rates of pulmonary embolism at first evaluation and pulmonary embolism or venous thromboembolism within 90 days were calculated for emergency room patients and a hospitalized subgroup.
Findings
Pulmonary embolism was rare in patients with syncope, eg, less than 3% for hospitalized patients in this database study.
Cautions
The results of this study are based on the use of administrative databases to confirm the diagnosis of syncope. Additionally, the results include hospitalized and nonhospitalized patients. The design of this study differs significantly from those of the PESIT study, which showed a prevalence of 17% in hospitalized patients.9 The PESIT study specifically sought the diagnosis of pulmonary embolism even when other etiologies for syncope existed.
Implications
Ultimately, the clinical impetus to search for pulmonary embolism in hospitalized patients admitted with syncope will depend on individual presentations. The authors argued that pulmonary embolism is rare in syncope and much lower than 17% but should be considered in appropriate patients.
Balanced Crystalloids versus Saline in Noncritically Ill Patients. Self WH et al. N Engl J Med. 2018;378(9):819-828.10
Background
Data on the optimal composition of intravenous fluids (IVF) are limited. Limited experimental evidence suggests that IVF-induced hyperchloremia results in renal vasoconstriction and acute kidney injury.
Methods
This was a single-center, open-label, multiple crossover trial of >13,000 non-ICU hospitalized patients admitted from the Emergency Department. Patients were randomized to receive either only normal saline or a “balanced crystalloid,” eg, either Lactated Ringer’s or Plasmalyte. The primary outcome was hospital-free days. Secondary outcomes were major adverse kidney events (MAKE) at 30 days.
Findings
The study found no difference in the primary outcome of hospital-free days. However, balanced IVF resulted in a lower incidence of hyperchloremia and a slightly reduced incidence of MAKE 30 (4.7% vs 5.6%; adjusted OR 0.82).
Cautions
The incidence of acute kidney injury was low in this single-center ED population. This study, however, did not include hospitalized patients. The long-term effects on renal function could not be ascertained.
Implications
Hospital-free days after inpatient randomization to either normal saline or “balanced IVF” were not significantly different. “Balanced IVF” may be beneficial in select high renal-risk populations.
Speaker Introductions at Internal Medicine Grand Rounds: Forms of Address Reveal Speaker Bias. Files et al. J Womens Health. 2017;26(5):413-419.11
Background
Gender bias is known to contribute to leadership disparities between men and women in several academic medical centers.
Methods
This was a retrospective observational study reviewing video-archived introductions at Internal Medicine Grand Rounds at two connected institutions. All speakers had doctoral degrees. The outcome measured was the use of a speaker’s professional title during his/her introduction as a function of the introducer’s gender.
Findings
Women were more likely than men to introduce speakers of any gender by their professional title in the 321 forms of address analyzed (96% vs 66%, P < .001). When the introducer and speaker were of different genders, women were more likely to introduce male speakers with formal titles than men introducing female speakers (95% vs 49%, P < .001).
Cautions
This study was done at two associated academic institutions and may not reflect the practice or customs of physicians in other departments or institutions.
Implications
Despite the study’s limitations, it supports a theme of prevalent gender bias within academic medical institutions that may affect the outcomes of leadership, promotion, and scholarship.
Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. Raskob GE et al. N Engl J Med. 2018;378(7):615-624.12
Background
Low-molecular-weight heparin (LMWH) is the standard of care for the treatment of venous thromboembolism (VTE) in patients with cancer. Direct oral anticoagulants have not been studied for this indication.
Methods
This open-label, noninferiority trial randomized patients with cancer and acute VTE to either LMWH for a minimum of five days followed by oral edoxaban vs subcutaneous dalteparin.
Findings
A total of 1,046 patients were included in the modified intention-to-treat analysis. Patients received treatment for six to twelve months total. A composite outcome of recurrent VTE or major bleed within 12 months occurred in 67 of 522 (12.8%) of patients in the edoxaban group vs 71 of 524 (13.5%) of patients in the dalteparin group (HR 0.91, 95% CI 0.70-1.36, P = .006 for noninferiority). Recurrent VTE occurred more commonly with dalteparin than with edoxaban (11.3% vs 7.9%), whereas major bleeding was less common with dalteparin than with edoxaban (4% vs 6.9%). The increased bleeding rate with edoxaban was predominantly in patients with an upper gastrointestinal (GI) malignancy.
Cautions
This was an open-label study. Patients in the edoxaban still received five days of LMWH prior to oral edoxaban. More patients in the edoxaban group continued treatment for the entire 12-month period, which contributes to the observed decreased bleeding and increased VTE rates in the dalteparin group.
Implications
Oral edoxaban is noninferior to subcutaneous dalteparin for the primary composite endpoint of VTE and bleeding. Notably, the patients in the edoxaban group experienced a lower rate of recurrent VTE and a higher rate of major bleeding than the patients in the dalteparin group. Additional caution about bleeding risk in those with a GI malignancy is recommended.
Can High-flow Nasal Cannula Reduce the Rate of Endotracheal Intubation in Adult Patients with Acute Respiratory Failure Compared with Conventional Oxygen Therapy and Noninvasive Positive Pressure Ventilation? Ni Y-N et al. Chest. 2017;151(4):764-775.13
Background
High-flow nasal cannula (HFNC) can deliver heated and humidified oxygen at rates of up to 60 L/min. Evidence on the benefits of HFNC over usual oxygen therapy or noninvasive positive pressure ventilation (NIPPV) is conflicting.
Methods
This systematic review and meta-analysis included 18 studies (12 RCTs, four retrospective, and two prospective cohort studies) with 3,881 patients with respiratory failure (medical and surgical causes). The included studies compared HFNC with usual oxygen therapy or NIPPV.
Findings
HFNC was associated with lower rates of endotracheal intubation (OR 0.47, 95% CI 0.27-0.84, P = .01) relative to oxygen therapy. Intubation rates did not differ between HFNC and NIPPV (OR 0.73, 95% CI 0.47-1.13, P = .16). No differences in ICU mortality or ICU length of stay (LOS) were found when HFNC was compared with either usual oxygen therapy or NIPPV.
Cautions
The significant heterogeneity in study design across studies is mainly attributable to varying causes of respiratory failure and differences in flow rate, oxygen concentration, and treatment duration across studies.
Implications
In patients with respiratory failure, HFNC may reduce intubation when compared with usual oxygen therapy and has similar ICU mortality when compared with usual oxygen and NIPPV.
Errors in the Diagnosis of Spinal Epidural Abscesses in the Era of Electronic Health Records. Bhise V et al. Am J Med. 2017;130(8):975-981.14
Background
Diagnostic errors are common in patients with spinal epidural abscess, but the main contributing factors are unclear.15
Methods
All patients who were newly diagnosed with spinal epidural abscess in 2013 were identified from the Veterans Affairs (VA) national database. Charts were reviewed for diagnostic delay and contributing factors, including the presence of “red flag” symptoms (eg, fever and neurological deficits).
Findings
Of the 119 patients with a new diagnosis of spinal epidural abscess, 66 (56%) had a diagnostic error. The median time to diagnosis in those with a diagnostic error was 12 days vs four days in those without error (P < .01). Common missed red flags in error cases included fever (n = 57, 86.4%), focal neurologic deficit (n = 54, 81.8%), and active infection (n = 54, 81.8%). Most errors occurred during the provider–patient encounter (eg, information not gathered during the history or physical). The magnitude of harm was serious for most patients (n = 40, 60.6%) and contributed to death in eight patients (12.1%).
Cautions
The study may not be generalizable because it was limited to the VA health system.
Implications
Diagnostic errors are common in patients with spinal epidural abscesses and can lead to serious harm. Health systems should build mechanisms to support providers in the evaluation of patients with back pain.
Hospital medicine continues to expand with respect to the number of practitioners as well as the scope of the practice of those practitioners. In addition, the commitment to, and rigor of, scientific inquiry in the field continues to grow. The authors of this article conducted a review of the medical literature, including articles published between March 2017 and March 2018. The key articles reported studies with high methodological quality, clear findings, and a high potential for impact on clinical practice. The literature was independently reviewed by each author, and candidate works were chosen on the basis of relevance to hospital medicine and expected clinical impact. The articles were organized by subject matter, ranked by applicability to the audience, and selected to meet the time constraints of each talk. Twenty-nine articles were presented at the Update in Hospital Medicine at the 2018 Society of Hospital Medicine and Society of General Internal Medicine annual meetings (B Sharpe, A Burger at SGIM and B Slawski, C Cooper at SHM). Nine articles were included in this review through an iterative voting process. Each author ranked their top five articles from one to five. Points were tallied for each article, and the five articles with the highest points were included. A second round of voting identified the remaining four articles for inclusion. Ties were adjudicated by group discussion. Each article is summarized below, and their key points are highlighted in the table.
KEY PUBLICATIONS
Aspirin in Patients with Previous Percutaneous Coronary Intervention Undergoing Noncardiac Surgery. Graham MM et al. Ann Intern Med. 2018;168(4):237-244.1
Background
The Perioperative Ischemic Evaluation 2 (POISE-2) trial found that perioperative aspirin use had no significant effect on the risk of perioperative death and nonfatal myocardial infarction (MI) in patients who are at risk for vascular complications; however, the risk of major bleeding increased with aspirin use in these patients.2 Nevertheless, the POISE-2 trial did not specifically address the role of aspirin in patients who had undergone previous percutaneous coronary intervention (PCI).
Methods
A post hoc subgroup analysis of POISE-2 evaluated 470 PCI patients (234 aspirin-treated and 236 placebo-treated patients) aged >45 years, 90% of whom had stents. The administration of the study drug was initiated within four hours preoperatively and continued postoperatively. Patients who had bare metal stents placed within the six weeks prior to the study or drug-eluting stents placed within the preceding 12 months were excluded.
Findings
The composite endpoint of risk of death and nonfatal MI was 11.5% in the placebo group and 6% in aspirin-treated patients (HR 0.50; CI, 0.26-0.95). Most of the difference in primary outcome was attributed to an increase in nonfatal MI in the placebo group. Major and life-threatening bleeding were not substantially increased in PCI patients but increased in the overall POISE-2 trial (absolute risk increase 0.8% for major bleeding [95% CI, 0.1%-1.6%]; HR 1.22 [95% CI, 1.01-1.48]). Stent type had no effect on death and nonfatal MI.
Cautions
This was a non-prespecified subgroup analysis with a small sample size.
Implications
Perioperative aspirin use in patients with previous PCI appears to provide more benefit than harm, unless a substantial bleeding risk exists.
Association Between Wait Time and 30-Day Mortality in Adults Undergoing Hip Fracture Surgery. Pincus D et al. JAMA. 2017;318(20):1994-2003.3
Background
Wait times to hip fracture surgery have been associated with mortality in previous studies; however, the wait time associated with complications remains controversial.4,5
Methods
This retrospective cohort study of 42,230 adults modeled the probability of complications in accordance with wait time from hospital arrival to hip fracture surgery. It aimed to identify the optimal time window in which to conduct surgery before complications increased. This window to increased complications was used to define early and delayed surgery. The matched cohorts of early and delayed patients were then used to compare outcomes.
Findings
Overall 30-day mortality was 7%. Complication rates increased when wait times reached 24 hours. Comparing the propensity-matched early (<24 hours) and late (>24 hours) surgery patients revealed that late surgery patients had significantly higher 30-day mortality (6.5% vs 5.8%; % absolute RD 0.79; 95% CI, 0.23-1.35) than early surgery patients and the composite outcome of mortality or other medical complications (MI, DVT, PE, and pneumonia; 12.2% vs 10.1%; % absolute RD 2.16; 95% CI, 1.43-2.89).
Cautions
Only 34% of patients in this study had surgery within 24 hours. The observational cohort study design may result in unmeasured confounders, eg, less sick patients go to surgery more quickly than sicker patients.
Implications
A preoperative wait time of 24 hours appears to represent a threshold of increased risk for 30-day perioperative complications and mortality in hip fracture surgery.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review. Hale AJ et al. J Hosp Med. 2018;13(5):328-335.6
Background
Bloodstream infections (BSIs) are significant causes of morbidity and mortality in the United States. Traditionally, clinicians have relied on intravenous antibiotics for treatment. A recent “Choosing Wisely®” initiative recommends that clinicians should use “oral formulations of highly bioavailable antimicrobials wherever possible.”7 Thus, the authors searched for evidence for scenarios wherein BSIs could be safely treated with oral antibiotics.
Methods
A narrative review was conducted given that robust clinical data for an extensive systematic review were insufficient.
Findings
Key decision points on the use of an oral antibiotic for a diagnosed BSI are as follows: (1) Source control must be attained prior to the consideration of oral antibiotics. (2) A highly bioavailable oral option to which the pathogen is sensitive must be available. (3) Patients must be able to comply with the therapy for the full course and not be on interfering medications. Good evidence for use of oral antibiotics against sensitive gram-negative bacilli other than Pseudomonas exists. Evidence for treating Streptococcus pneumoniae with early transition (within three days) to oral antibiotics is robust when treating bacteremia and pneumonia but not for other primary sites of infection. Evidence for the use of oral antibiotics for B-hemolytic streptococcus, including necrotizing fasciitis and Enterococcus, is insufficient. The evidence supports at least two weeks of IV antibiotics for the treatment of Staphylococcus aureus.
Cautions
This is a narrative review due to limited evidence.
Implications
The early use of oral antibiotics in the setting of bacteremia may be appropriate in select clinical situations.
Prevalence of Pulmonary Embolism in Patients with Syncope. Costantino et al. JAMA Intern Med. 2018;178(3):356-362.8
Background
Data on the prevalence of pulmonary embolism in patients presenting with syncope are conflicting.
Methods
This was a retrospective observational study involving five databases in four countries of >1.6 million adults identified through syncope ICD codes. The rates of pulmonary embolism at first evaluation and pulmonary embolism or venous thromboembolism within 90 days were calculated for emergency room patients and a hospitalized subgroup.
Findings
Pulmonary embolism was rare in patients with syncope, eg, less than 3% for hospitalized patients in this database study.
Cautions
The results of this study are based on the use of administrative databases to confirm the diagnosis of syncope. Additionally, the results include hospitalized and nonhospitalized patients. The design of this study differs significantly from those of the PESIT study, which showed a prevalence of 17% in hospitalized patients.9 The PESIT study specifically sought the diagnosis of pulmonary embolism even when other etiologies for syncope existed.
Implications
Ultimately, the clinical impetus to search for pulmonary embolism in hospitalized patients admitted with syncope will depend on individual presentations. The authors argued that pulmonary embolism is rare in syncope and much lower than 17% but should be considered in appropriate patients.
Balanced Crystalloids versus Saline in Noncritically Ill Patients. Self WH et al. N Engl J Med. 2018;378(9):819-828.10
Background
Data on the optimal composition of intravenous fluids (IVF) are limited. Limited experimental evidence suggests that IVF-induced hyperchloremia results in renal vasoconstriction and acute kidney injury.
Methods
This was a single-center, open-label, multiple crossover trial of >13,000 non-ICU hospitalized patients admitted from the Emergency Department. Patients were randomized to receive either only normal saline or a “balanced crystalloid,” eg, either Lactated Ringer’s or Plasmalyte. The primary outcome was hospital-free days. Secondary outcomes were major adverse kidney events (MAKE) at 30 days.
Findings
The study found no difference in the primary outcome of hospital-free days. However, balanced IVF resulted in a lower incidence of hyperchloremia and a slightly reduced incidence of MAKE 30 (4.7% vs 5.6%; adjusted OR 0.82).
Cautions
The incidence of acute kidney injury was low in this single-center ED population. This study, however, did not include hospitalized patients. The long-term effects on renal function could not be ascertained.
Implications
Hospital-free days after inpatient randomization to either normal saline or “balanced IVF” were not significantly different. “Balanced IVF” may be beneficial in select high renal-risk populations.
Speaker Introductions at Internal Medicine Grand Rounds: Forms of Address Reveal Speaker Bias. Files et al. J Womens Health. 2017;26(5):413-419.11
Background
Gender bias is known to contribute to leadership disparities between men and women in several academic medical centers.
Methods
This was a retrospective observational study reviewing video-archived introductions at Internal Medicine Grand Rounds at two connected institutions. All speakers had doctoral degrees. The outcome measured was the use of a speaker’s professional title during his/her introduction as a function of the introducer’s gender.
Findings
Women were more likely than men to introduce speakers of any gender by their professional title in the 321 forms of address analyzed (96% vs 66%, P < .001). When the introducer and speaker were of different genders, women were more likely to introduce male speakers with formal titles than men introducing female speakers (95% vs 49%, P < .001).
Cautions
This study was done at two associated academic institutions and may not reflect the practice or customs of physicians in other departments or institutions.
Implications
Despite the study’s limitations, it supports a theme of prevalent gender bias within academic medical institutions that may affect the outcomes of leadership, promotion, and scholarship.
Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. Raskob GE et al. N Engl J Med. 2018;378(7):615-624.12
Background
Low-molecular-weight heparin (LMWH) is the standard of care for the treatment of venous thromboembolism (VTE) in patients with cancer. Direct oral anticoagulants have not been studied for this indication.
Methods
This open-label, noninferiority trial randomized patients with cancer and acute VTE to either LMWH for a minimum of five days followed by oral edoxaban vs subcutaneous dalteparin.
Findings
A total of 1,046 patients were included in the modified intention-to-treat analysis. Patients received treatment for six to twelve months total. A composite outcome of recurrent VTE or major bleed within 12 months occurred in 67 of 522 (12.8%) of patients in the edoxaban group vs 71 of 524 (13.5%) of patients in the dalteparin group (HR 0.91, 95% CI 0.70-1.36, P = .006 for noninferiority). Recurrent VTE occurred more commonly with dalteparin than with edoxaban (11.3% vs 7.9%), whereas major bleeding was less common with dalteparin than with edoxaban (4% vs 6.9%). The increased bleeding rate with edoxaban was predominantly in patients with an upper gastrointestinal (GI) malignancy.
Cautions
This was an open-label study. Patients in the edoxaban still received five days of LMWH prior to oral edoxaban. More patients in the edoxaban group continued treatment for the entire 12-month period, which contributes to the observed decreased bleeding and increased VTE rates in the dalteparin group.
Implications
Oral edoxaban is noninferior to subcutaneous dalteparin for the primary composite endpoint of VTE and bleeding. Notably, the patients in the edoxaban group experienced a lower rate of recurrent VTE and a higher rate of major bleeding than the patients in the dalteparin group. Additional caution about bleeding risk in those with a GI malignancy is recommended.
Can High-flow Nasal Cannula Reduce the Rate of Endotracheal Intubation in Adult Patients with Acute Respiratory Failure Compared with Conventional Oxygen Therapy and Noninvasive Positive Pressure Ventilation? Ni Y-N et al. Chest. 2017;151(4):764-775.13
Background
High-flow nasal cannula (HFNC) can deliver heated and humidified oxygen at rates of up to 60 L/min. Evidence on the benefits of HFNC over usual oxygen therapy or noninvasive positive pressure ventilation (NIPPV) is conflicting.
Methods
This systematic review and meta-analysis included 18 studies (12 RCTs, four retrospective, and two prospective cohort studies) with 3,881 patients with respiratory failure (medical and surgical causes). The included studies compared HFNC with usual oxygen therapy or NIPPV.
Findings
HFNC was associated with lower rates of endotracheal intubation (OR 0.47, 95% CI 0.27-0.84, P = .01) relative to oxygen therapy. Intubation rates did not differ between HFNC and NIPPV (OR 0.73, 95% CI 0.47-1.13, P = .16). No differences in ICU mortality or ICU length of stay (LOS) were found when HFNC was compared with either usual oxygen therapy or NIPPV.
Cautions
The significant heterogeneity in study design across studies is mainly attributable to varying causes of respiratory failure and differences in flow rate, oxygen concentration, and treatment duration across studies.
Implications
In patients with respiratory failure, HFNC may reduce intubation when compared with usual oxygen therapy and has similar ICU mortality when compared with usual oxygen and NIPPV.
Errors in the Diagnosis of Spinal Epidural Abscesses in the Era of Electronic Health Records. Bhise V et al. Am J Med. 2017;130(8):975-981.14
Background
Diagnostic errors are common in patients with spinal epidural abscess, but the main contributing factors are unclear.15
Methods
All patients who were newly diagnosed with spinal epidural abscess in 2013 were identified from the Veterans Affairs (VA) national database. Charts were reviewed for diagnostic delay and contributing factors, including the presence of “red flag” symptoms (eg, fever and neurological deficits).
Findings
Of the 119 patients with a new diagnosis of spinal epidural abscess, 66 (56%) had a diagnostic error. The median time to diagnosis in those with a diagnostic error was 12 days vs four days in those without error (P < .01). Common missed red flags in error cases included fever (n = 57, 86.4%), focal neurologic deficit (n = 54, 81.8%), and active infection (n = 54, 81.8%). Most errors occurred during the provider–patient encounter (eg, information not gathered during the history or physical). The magnitude of harm was serious for most patients (n = 40, 60.6%) and contributed to death in eight patients (12.1%).
Cautions
The study may not be generalizable because it was limited to the VA health system.
Implications
Diagnostic errors are common in patients with spinal epidural abscesses and can lead to serious harm. Health systems should build mechanisms to support providers in the evaluation of patients with back pain.
1. Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med. 2018;168(4):237-244. doi: 10.7326/M17-2341.
2. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. doi: 10.1056/NEJMoa1401105
3. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994-2003. doi: 10.1001/jama.2017.17606.
4. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616. doi: 10.1503/cmaj.092220.
5. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? ystematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146-154. doi: 10.1007/BF03016088.
6. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018;13(5):328-335. doi: 10.12788/jhm.2949.
7. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. doi: 10.1007/s15010-017-0997-0.
8. Costantino G, Ruwald MH, Quinn J, et al. Prevalence of pulmonary embolism in patients with syncope. JAMA Intern Med. 2018;178(3):356-362. doi: 10.1001/jamainternmed.2017.8175.
9. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016;375(16):1524-1531. doi: 10.1056/NEJMoa1602172
10. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586.
11. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. doi: 10.1089/jwh.2016.6044.
12. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. doi: 10.1056/NEJMoa1711948.
13. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest. 2017;151(4):764-775. doi: 10.1016/j.chest.2017.01.004.
14. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi: 10.1016/j.amjmed.2017.03.009
15. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291. doi: 10.1016/j.jemermed.2003.11.013.
1. Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med. 2018;168(4):237-244. doi: 10.7326/M17-2341.
2. Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494-1503. doi: 10.1056/NEJMoa1401105
3. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994-2003. doi: 10.1001/jama.2017.17606.
4. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609-1616. doi: 10.1503/cmaj.092220.
5. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? ystematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146-154. doi: 10.1007/BF03016088.
6. Hale AJ, Snyder GM, Ahern JW, Eliopoulos G, Ricotta D, Alston WK. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018;13(5):328-335. doi: 10.12788/jhm.2949.
7. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. doi: 10.1007/s15010-017-0997-0.
8. Costantino G, Ruwald MH, Quinn J, et al. Prevalence of pulmonary embolism in patients with syncope. JAMA Intern Med. 2018;178(3):356-362. doi: 10.1001/jamainternmed.2017.8175.
9. Prandoni P, Lensing AW, Prins MH, et al. Prevalence of pulmonary embolism among patients hospitalized for syncope. N Engl J Med. 2016;375(16):1524-1531. doi: 10.1056/NEJMoa1602172
10. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586.
11. Files JA, Mayer AP, Ko MG, et al. Speaker introductions at internal medicine grand rounds: forms of address reveal gender bias. J Womens Health (Larchmt). 2017;26(5):413-419. doi: 10.1089/jwh.2016.6044.
12. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. doi: 10.1056/NEJMoa1711948.
13. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest. 2017;151(4):764-775. doi: 10.1016/j.chest.2017.01.004.
14. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. 2017;130(8):975-981. doi: 10.1016/j.amjmed.2017.03.009
15. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285-291. doi: 10.1016/j.jemermed.2003.11.013.
© 2019 Society of Hospital Medicine
Condom Catheters versus Indwelling Urethral Catheters in Men: A Prospective, Observational Study
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
© 2019 Society of Hospital Medicine
In the Hospital: Laura Shea
We spoke with medical social worker Laura Shea, MSW, LICSW on her role at our tertiary care hospital. Laura’s reflections on the struggles and rewards of her job may resonate with those of us who search for balance and meaning in work.
Laura, tell us about yourself. What made you want to be a social worker?
I couldn’t really picture doing anything else. I got a degree in psychology and loved counseling. Social work was a natural fit because of the social justice component and the look into larger systems. I knew I had the skill set for this, and for those most marginalized, to be a supportive person for someone who doesn’t have that.
I also have a family member with major mental illness and chronic suicidality who I supported for a very long time. In many ways, I was a personal social worker advocating on their behalf while growing up. I remember being in high school when they overdosed, and going to the ER in the middle of the night. The next morning, I was back at school. I was a total do-gooder—President of the student council and on top of my grades. I tried dealing with this while keeping up the appearance that everything was ok, even though it wasn’t.
As I got older, there were middle-of-the-night phone calls professing suicidality which were so painful. I learned a lot about compartmentalizing and resiliency. It has given me an incredible amount of empathy for family members of patients. I have learned that it’s not always simple, and decisions aren’t easy, and solutions are complicated and can feel incomplete. We often hear, “Why hasn’t the family stepped in?” Well these issues are hard for families too, I know from firsthand experience.
At the end of the day, as challenging as the work is, I get something from it. I feel honored to bear witness to some of people’s darkest moments and also some of the most beautiful moments—the joys of coming out the other side of their process and journey.
How much of your personal story do you reveal to your patients?
I rarely do. However, to some families that are particularly devastated, I do share some of my family story. I try to affirm their challenge and acknowledge that family and friends can’t always “solve this.”
We have a culture that reveres going above and beyond, however I really honor those family members who can set boundaries. Sometimes caregivers need space, that doesn’t make you a bad person. It’s actually brave and really hard to do. You can’t give from an empty well.
Laura, tell us about your typical day.
Well, it begins with responding to e-mails. Then I meet with patients and obtain collateral to prep for multidisciplinary rounds (with physicians, RNs, case managers). I usually consult on 20-30 patients a day. In the afternoon, it varies -- maybe three patients are leaving that may need my help with things like providing substance use information or shelter resources. Typically, I’ll have a few complicated long-term patients, who may have challenging family dynamics, ongoing goals of care discussions, or behavioral difficulties. These patients keep me just as busy, it’s not quite as time sensitive but I have to keep chipping away at the work.
Seems like a busy day. Do you get a break at all?
When possible, I take a walk in the woods behind the hospital on my lunch break. There’s a beautiful path, it’s an important part of my day -- getting outside and taking a step back. I bring my pager, so I am still connected.
I used to feel like I didn’t have time to take a break, and I would work through lunch. But now I find if I take a break, I am more productive the rest of the day because it makes me more mindful. It quiets me a little, gives me perspective on the stress and stressors of working in the hospital and allows me to better connect to my job and others around me.
What does a successful day look like?
Well, one involved a homeless gentleman and a search for his family. He was in his 40s, though he looked much older, and recently had been assaulted at a shelter. He presented to either the ER or was admitted to various hospitals 14 times over the past month – typically for intoxication and hypothermia. He kept saying “I just need to find my brother” though no one was taking this request too seriously. We spent a lot of time looking for his brother with the Office of Public Guardian’s help, and we actually found him! The patient hadn’t seen his brother in four years and as it turns out was searching for him too. The brother thought the patient had passed away. With his brother’s support, the patient is now housed, going to alcohol treatment, reunited with his family, and taking his medications. His whole life changed. So that was amazing, and a reminder of how rewarding this job can be.
What is most challenging about your work?
The biggest challenge is grappling with the limitations of the system, and discharging someone to the community when the community has limited resources for these patients.
Though it’s not just the limitation of resources, some patients have been through the system so many times that as a coping mechanism and to protect themselves they do everything possible to push you away. They have walls firmly up, because of prior negative experiences with providers. I am not fazed by being yelled at, but it’s hard trying to connect with someone who has learned not to let you in. These are often the patients that need the support the most, and yet I want to respect their ability to have control or to say no. It is a tough balance.
What’s fun about your job?
I love meeting new people. I met a woman a few weeks ago who was talking about being a hippie in the ‘60s in San Francisco, and how great it was and how soft millennials are. She actually put meth in her coffee because she needed a pick-me-up to clean her house. You can’t make this stuff up! It’s just really fascinating how people live their lives, and to have a window into their world and perspective is a privilege.
Do you take work home with you or do you disconnect?
I try to disconnect, however there are days when something sticks with you and you really worry and wonder about a patient. As I mentioned, you can’t give from an empty well—so I try to acknowledge this. I find that trying to have a rich life outside of work is an important part of self-care as well. Social work is a big part of my identity but it’s not entirely who I am. I focus on friends, family, travel, yoga, and things that sustain me. I can’t do my job effectively if I am not taking a step back regularly.
What advice do you have for other providers and for patients?
The hospital is so overwhelming for our patients, more so than some providers realize. I could be in the room with a patient for 45 minutes and six different providers may come in. I try to maintain that this is the patient’s bedroom I’m walking into. It’s a private, and a sacred space for them. That’s where they sleep. This is where they are trying to recover and grapple with what brought them into the hospital.
Laura, thank you so much for telling us about your work. Anything else you’d like to share with us?
Some days I’ll go home completely exhausted and wiped out, and at first, I don’t feel like I did a single solitary thing. Some of the things that I’m trying to help people work through ...it never occurred to me that someone could, for whatever reason, find themselves in such challenging situations. I don’t have a magic wand to provide someone with housing or sobriety, but maybe in that moment I can begin to make a connection. When I just listen, I am beginning to build relationships – which for some patients is something they haven’t had in a long time. It’s in these moments of being present, without an agenda, walking with them in their challenges, that I feel most connected to the work.
Thanks, Laura.
We spoke with medical social worker Laura Shea, MSW, LICSW on her role at our tertiary care hospital. Laura’s reflections on the struggles and rewards of her job may resonate with those of us who search for balance and meaning in work.
Laura, tell us about yourself. What made you want to be a social worker?
I couldn’t really picture doing anything else. I got a degree in psychology and loved counseling. Social work was a natural fit because of the social justice component and the look into larger systems. I knew I had the skill set for this, and for those most marginalized, to be a supportive person for someone who doesn’t have that.
I also have a family member with major mental illness and chronic suicidality who I supported for a very long time. In many ways, I was a personal social worker advocating on their behalf while growing up. I remember being in high school when they overdosed, and going to the ER in the middle of the night. The next morning, I was back at school. I was a total do-gooder—President of the student council and on top of my grades. I tried dealing with this while keeping up the appearance that everything was ok, even though it wasn’t.
As I got older, there were middle-of-the-night phone calls professing suicidality which were so painful. I learned a lot about compartmentalizing and resiliency. It has given me an incredible amount of empathy for family members of patients. I have learned that it’s not always simple, and decisions aren’t easy, and solutions are complicated and can feel incomplete. We often hear, “Why hasn’t the family stepped in?” Well these issues are hard for families too, I know from firsthand experience.
At the end of the day, as challenging as the work is, I get something from it. I feel honored to bear witness to some of people’s darkest moments and also some of the most beautiful moments—the joys of coming out the other side of their process and journey.
How much of your personal story do you reveal to your patients?
I rarely do. However, to some families that are particularly devastated, I do share some of my family story. I try to affirm their challenge and acknowledge that family and friends can’t always “solve this.”
We have a culture that reveres going above and beyond, however I really honor those family members who can set boundaries. Sometimes caregivers need space, that doesn’t make you a bad person. It’s actually brave and really hard to do. You can’t give from an empty well.
Laura, tell us about your typical day.
Well, it begins with responding to e-mails. Then I meet with patients and obtain collateral to prep for multidisciplinary rounds (with physicians, RNs, case managers). I usually consult on 20-30 patients a day. In the afternoon, it varies -- maybe three patients are leaving that may need my help with things like providing substance use information or shelter resources. Typically, I’ll have a few complicated long-term patients, who may have challenging family dynamics, ongoing goals of care discussions, or behavioral difficulties. These patients keep me just as busy, it’s not quite as time sensitive but I have to keep chipping away at the work.
Seems like a busy day. Do you get a break at all?
When possible, I take a walk in the woods behind the hospital on my lunch break. There’s a beautiful path, it’s an important part of my day -- getting outside and taking a step back. I bring my pager, so I am still connected.
I used to feel like I didn’t have time to take a break, and I would work through lunch. But now I find if I take a break, I am more productive the rest of the day because it makes me more mindful. It quiets me a little, gives me perspective on the stress and stressors of working in the hospital and allows me to better connect to my job and others around me.
What does a successful day look like?
Well, one involved a homeless gentleman and a search for his family. He was in his 40s, though he looked much older, and recently had been assaulted at a shelter. He presented to either the ER or was admitted to various hospitals 14 times over the past month – typically for intoxication and hypothermia. He kept saying “I just need to find my brother” though no one was taking this request too seriously. We spent a lot of time looking for his brother with the Office of Public Guardian’s help, and we actually found him! The patient hadn’t seen his brother in four years and as it turns out was searching for him too. The brother thought the patient had passed away. With his brother’s support, the patient is now housed, going to alcohol treatment, reunited with his family, and taking his medications. His whole life changed. So that was amazing, and a reminder of how rewarding this job can be.
What is most challenging about your work?
The biggest challenge is grappling with the limitations of the system, and discharging someone to the community when the community has limited resources for these patients.
Though it’s not just the limitation of resources, some patients have been through the system so many times that as a coping mechanism and to protect themselves they do everything possible to push you away. They have walls firmly up, because of prior negative experiences with providers. I am not fazed by being yelled at, but it’s hard trying to connect with someone who has learned not to let you in. These are often the patients that need the support the most, and yet I want to respect their ability to have control or to say no. It is a tough balance.
What’s fun about your job?
I love meeting new people. I met a woman a few weeks ago who was talking about being a hippie in the ‘60s in San Francisco, and how great it was and how soft millennials are. She actually put meth in her coffee because she needed a pick-me-up to clean her house. You can’t make this stuff up! It’s just really fascinating how people live their lives, and to have a window into their world and perspective is a privilege.
Do you take work home with you or do you disconnect?
I try to disconnect, however there are days when something sticks with you and you really worry and wonder about a patient. As I mentioned, you can’t give from an empty well—so I try to acknowledge this. I find that trying to have a rich life outside of work is an important part of self-care as well. Social work is a big part of my identity but it’s not entirely who I am. I focus on friends, family, travel, yoga, and things that sustain me. I can’t do my job effectively if I am not taking a step back regularly.
What advice do you have for other providers and for patients?
The hospital is so overwhelming for our patients, more so than some providers realize. I could be in the room with a patient for 45 minutes and six different providers may come in. I try to maintain that this is the patient’s bedroom I’m walking into. It’s a private, and a sacred space for them. That’s where they sleep. This is where they are trying to recover and grapple with what brought them into the hospital.
Laura, thank you so much for telling us about your work. Anything else you’d like to share with us?
Some days I’ll go home completely exhausted and wiped out, and at first, I don’t feel like I did a single solitary thing. Some of the things that I’m trying to help people work through ...it never occurred to me that someone could, for whatever reason, find themselves in such challenging situations. I don’t have a magic wand to provide someone with housing or sobriety, but maybe in that moment I can begin to make a connection. When I just listen, I am beginning to build relationships – which for some patients is something they haven’t had in a long time. It’s in these moments of being present, without an agenda, walking with them in their challenges, that I feel most connected to the work.
Thanks, Laura.
We spoke with medical social worker Laura Shea, MSW, LICSW on her role at our tertiary care hospital. Laura’s reflections on the struggles and rewards of her job may resonate with those of us who search for balance and meaning in work.
Laura, tell us about yourself. What made you want to be a social worker?
I couldn’t really picture doing anything else. I got a degree in psychology and loved counseling. Social work was a natural fit because of the social justice component and the look into larger systems. I knew I had the skill set for this, and for those most marginalized, to be a supportive person for someone who doesn’t have that.
I also have a family member with major mental illness and chronic suicidality who I supported for a very long time. In many ways, I was a personal social worker advocating on their behalf while growing up. I remember being in high school when they overdosed, and going to the ER in the middle of the night. The next morning, I was back at school. I was a total do-gooder—President of the student council and on top of my grades. I tried dealing with this while keeping up the appearance that everything was ok, even though it wasn’t.
As I got older, there were middle-of-the-night phone calls professing suicidality which were so painful. I learned a lot about compartmentalizing and resiliency. It has given me an incredible amount of empathy for family members of patients. I have learned that it’s not always simple, and decisions aren’t easy, and solutions are complicated and can feel incomplete. We often hear, “Why hasn’t the family stepped in?” Well these issues are hard for families too, I know from firsthand experience.
At the end of the day, as challenging as the work is, I get something from it. I feel honored to bear witness to some of people’s darkest moments and also some of the most beautiful moments—the joys of coming out the other side of their process and journey.
How much of your personal story do you reveal to your patients?
I rarely do. However, to some families that are particularly devastated, I do share some of my family story. I try to affirm their challenge and acknowledge that family and friends can’t always “solve this.”
We have a culture that reveres going above and beyond, however I really honor those family members who can set boundaries. Sometimes caregivers need space, that doesn’t make you a bad person. It’s actually brave and really hard to do. You can’t give from an empty well.
Laura, tell us about your typical day.
Well, it begins with responding to e-mails. Then I meet with patients and obtain collateral to prep for multidisciplinary rounds (with physicians, RNs, case managers). I usually consult on 20-30 patients a day. In the afternoon, it varies -- maybe three patients are leaving that may need my help with things like providing substance use information or shelter resources. Typically, I’ll have a few complicated long-term patients, who may have challenging family dynamics, ongoing goals of care discussions, or behavioral difficulties. These patients keep me just as busy, it’s not quite as time sensitive but I have to keep chipping away at the work.
Seems like a busy day. Do you get a break at all?
When possible, I take a walk in the woods behind the hospital on my lunch break. There’s a beautiful path, it’s an important part of my day -- getting outside and taking a step back. I bring my pager, so I am still connected.
I used to feel like I didn’t have time to take a break, and I would work through lunch. But now I find if I take a break, I am more productive the rest of the day because it makes me more mindful. It quiets me a little, gives me perspective on the stress and stressors of working in the hospital and allows me to better connect to my job and others around me.
What does a successful day look like?
Well, one involved a homeless gentleman and a search for his family. He was in his 40s, though he looked much older, and recently had been assaulted at a shelter. He presented to either the ER or was admitted to various hospitals 14 times over the past month – typically for intoxication and hypothermia. He kept saying “I just need to find my brother” though no one was taking this request too seriously. We spent a lot of time looking for his brother with the Office of Public Guardian’s help, and we actually found him! The patient hadn’t seen his brother in four years and as it turns out was searching for him too. The brother thought the patient had passed away. With his brother’s support, the patient is now housed, going to alcohol treatment, reunited with his family, and taking his medications. His whole life changed. So that was amazing, and a reminder of how rewarding this job can be.
What is most challenging about your work?
The biggest challenge is grappling with the limitations of the system, and discharging someone to the community when the community has limited resources for these patients.
Though it’s not just the limitation of resources, some patients have been through the system so many times that as a coping mechanism and to protect themselves they do everything possible to push you away. They have walls firmly up, because of prior negative experiences with providers. I am not fazed by being yelled at, but it’s hard trying to connect with someone who has learned not to let you in. These are often the patients that need the support the most, and yet I want to respect their ability to have control or to say no. It is a tough balance.
What’s fun about your job?
I love meeting new people. I met a woman a few weeks ago who was talking about being a hippie in the ‘60s in San Francisco, and how great it was and how soft millennials are. She actually put meth in her coffee because she needed a pick-me-up to clean her house. You can’t make this stuff up! It’s just really fascinating how people live their lives, and to have a window into their world and perspective is a privilege.
Do you take work home with you or do you disconnect?
I try to disconnect, however there are days when something sticks with you and you really worry and wonder about a patient. As I mentioned, you can’t give from an empty well—so I try to acknowledge this. I find that trying to have a rich life outside of work is an important part of self-care as well. Social work is a big part of my identity but it’s not entirely who I am. I focus on friends, family, travel, yoga, and things that sustain me. I can’t do my job effectively if I am not taking a step back regularly.
What advice do you have for other providers and for patients?
The hospital is so overwhelming for our patients, more so than some providers realize. I could be in the room with a patient for 45 minutes and six different providers may come in. I try to maintain that this is the patient’s bedroom I’m walking into. It’s a private, and a sacred space for them. That’s where they sleep. This is where they are trying to recover and grapple with what brought them into the hospital.
Laura, thank you so much for telling us about your work. Anything else you’d like to share with us?
Some days I’ll go home completely exhausted and wiped out, and at first, I don’t feel like I did a single solitary thing. Some of the things that I’m trying to help people work through ...it never occurred to me that someone could, for whatever reason, find themselves in such challenging situations. I don’t have a magic wand to provide someone with housing or sobriety, but maybe in that moment I can begin to make a connection. When I just listen, I am beginning to build relationships – which for some patients is something they haven’t had in a long time. It’s in these moments of being present, without an agenda, walking with them in their challenges, that I feel most connected to the work.
Thanks, Laura.
© 2019 Society of Hospital Medicine