User login
Central pain states: a shift in thinking about chronic pain
What do we mean when we talk about pain? Traditionally pain is defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage.1 Pain can result when intense or noxious stimuli activate peripheral nociceptors. It serves as a warning against impending tissue damage and acts reflectively to protect against or minimize that damage.
We have known since the time of Descartes about the existence of an ascending sensory pain pathway that sends “distress” signals from the source of tissue damage to the brain. We also know of the Gate Control theory described by Melzack and Wall in 1965, in which stimulation of the skin evokes responses that transmit signal injury to transmission cells (the “gate”) in the dorsal horn of the spinal cord that continues to the brain, triggering response signals that modulate the activity of inhibitory cells (which close the “gate”), thereby decreasing the intensity of pain.2 But how do we explain pain in the absence of tissue damage, pain that is not triggered in the periphery, that often appears long after the noxious stimulus has stopped exerting its unpleasant effect?
Types of chronic pain
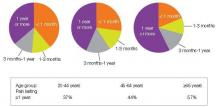
An estimated 116 million American adults suffer from chronic pain, defined as pain that lasts more than 3 months after onset and well into the phase of healing.1,3 According to a 2006 report from the Centers for Disease Control and Prevention with a special focus on pain, almost 57% of adults age 65 or older and 37% of younger adults ages 20–44 reported pain that lasted one year or more [Figure 1].4 Chronic pain exacts a cost of between $560 billion and $635 billion annually in medical treatment and lost productivity.5 There is a tremendous need to understand the molecular and cellular mechanisms of chronic pain in an effort to develop new, more effective treatments for these patients. This understanding may come as a result of our recent advances in visualizing the peripheral and central processes involved in pain. The emerging data suggest that for some individuals central factors play a key role in the maintenance and establishment of certain chronic pain conditions. That is, for some, the problem is really not inthe periphery.
| FIGURE 1: Pain duration by age group, 1999-2002 |
| Source: Centers for Disease Control and Prevention, National Center for Health Statistics, Health, United States, 2006. Data from theNational Health and Nutrition Examination Survey. |
Knee and hip pain. When peripheral tissue damage is unavoidable, the inflamed tissues and those nearby become hypersensitive, a protective response to guard the area during the period of healing. Conditions like chronic low-back pain and knee or hip osteoarthritis classically have been thought to be due to inflammation or damage to tissues in the back, knee, or hip. However, recent studies show that these conditions may have complex factors entailing both the peripheral and central nervous systems.
Analysis of data from the National Health and Nutrition Examination Survey (NHANES I) of patients with radiographic evidence of structural damage to the knee due to osteoarthritis found discordance between the amount of damage visible on x-ray and patients’ self-report of the degree of pain. In 319 patients with radiographic stage 2–4 knee osteoarthritis, only 47% reported knee pain, suggesting that something more than the degree of tissue injury was involved in the perception of pain.6,7 One explanation of these findings is that pain is a complex system incorporating structural changes, peripheral and central pain mechanisms, and subjective factors, including the patient’s history, psychological experience, genetics, and culture.
Diabetic neuropathy and postherpetic neuralgia. Chronic neuropathic pain results when there is actual damage to the nervous system—the peripheral nerve, dorsal root, or central nervous system. Peripheral neuropathic pain occurs after damage or alterations to sensory neurons. Some neuropathic pain disorders, such as diabetic neuropathy and postherpetic neuralgia, are well-defined disorders in which symptoms are unrelated to a stimulus and pain is related to peripheral as well as central processing.8
Stroke. Central poststroke pain, in which pain and hypersensitivity occurs in a body part due to injury to the corresponding part of the brain affected by the cerebrovascular lesion, is also considered a neuropathic pain syndrome. The onset of central poststroke pain typically occurs more than one month after the stroke, and exists with somatosensory abnormalities.9-11 For these types of neuropathies, altered function due to loss or damage of neuronal tissue is likely the cause of the pain condition.
Many of the people suffering from these central chronic pain conditions find it difficult to obtain relief, and probably will not benefit from surgeries or manipulations in the periphery. Instead, they may benefit from a targeted approach that addresses the central nervous system.
Recent studies on fibromyalgia and pain
Fibromyalgia (FM) may be considered the prototypical central pain disorder, in which the pain originates or is maintained in part in the central nervous system. Although new diagnostic criteria are being validated for this disorder, FM classically has been diagnosed by the detection of 11 of 18 tender points and the presence of chronic widespread pain for 3 months or longer.12
FM is a common disorder found to affect between 2% and 4% of the US population.13 It was one of the first disorders shown to have central factors predominant in the pathology, and as a result it has been the focus of numerous studies. Irritable bowel syndrome and chronic fatigue syndrome, often comorbid with FM, are also commonly studied. Until recently, these disorders have largely been considered “wastebasket” terms to categorize the complaints of patients with unexplained symptoms, because there were no objective signs to support their complaints. However with the advent of new imaging techniques to look into the brain and the central nervous system, researchers are finding very real physiological differences. For example, one study using sensory testing with thermal, mechanical, and electrical stimuli showed a correlation between FM patients’ subjective reports of pain and significantly altered cold and heat thresholds when compared with controls.14 Based on such studies it appears that patients with FM perceive stimuli as noxious at lower levels than healthy, pain-free controls.
Recent studies of FM have incorporated the use of functional magnetic resonance imaging (fMRI) to look at brain activations in response to painful stimuli. A study that included patients with FM and others with chronic low-back pain used fMRI to visualize the participants’ response to equal amounts of thumbnail pressure. In the FM and groups, 5 areas of neuronal activation within the cortex related to pain were detected, compared with only one activation in controls.15 Another study to evaluate the pattern of cerebral activation in FM patients found that in response to similar thumbnail pressures there were 13 regions of greater activation in the FM group compared with one region in the healthy control group.16 Additionally, mild pressure resulted in subjective pain reports and cerebral responses in the FM group that were similar to responses produced by twice the pressure applied in controls.
Another important area of research in pain processing looks at gray matter in the brain using voxel-based morphometry. A study of patients with FM found significantly less volume of gray matter and an age-associated decrease in gray matter that was 3.3 times greater than healthy controls.17
Using MRI to look at gray matter volume in patients with chronic musculoskeletal pain, significant differences in gray matter volume were found in osteoarthritis patients prior to hip arthroplasty compared with healthy controls. Specifically, areas of the thalamus, understood to play a role in central pain processing, showed decreased gray matter volume in the osteoarthritis group. Significantly, a comparison of gray matter volume 9 months after surgery showed that the levels of reduced thalamic gray matter volume in osteoarthritis patients “reversed” to levels similar to the those of the healthy control group.18
Although the mechanism that drives the loss or degradation of brain tissue in patients with chronic pain remains to be determined, one theory is that pain is associated with certain areas of the brain becoming hyperactive. Imaging studies using fMRI show that a constellation of regions typically are activated in pain processing, including the insula,
cingulate, primary somatosensory and secondary somatosensory cortices, amygdala, and thalamus [Figure 2].19 These regions have been shown to be more active in chronic pain states when patients respond to stimuli such as painful pressure or heat. Indeed, these regions have shown overamplification or augmentation of neural activity.
| FIGURE 2: Neuroanatomy of pain processing. Main brain regions that activate during a painful experience are highlighted as bilaterally active but with more dominant activation on the contralateral hemisphere (red). |
| Source: Tracey I. Br J Anaesth. 2008;101:32-39. |
Since overstimulation of nerve cells can trigger a toxic release of glutamate into surrounding tissues of the brain, this may cause nerve cells to die, ultimately reducing the amount of gray matter visualized in the brains of patients with chronic pain. In addition, some studies of FM have shown elevated levels of glutamate, an excitatory neurotransmitter that is known to cause excitotoxicity.20
Another significant consequence of long-term pain appears to be alterations in the normal connectivity of the brain, including the “default mode network” (DMN) which is noted to be important during the resting state. Recent studies of chronic pain suggest alterations in key DMN regions that may be related to the chronic pain state and existing comorbidities.21
The role of stress and depression in pain
The association among physical and psychosocial stressors, depression, and chronic pain syndromes has been the subject of numerous studies.
Posttraumatic stress disorder (PTSD) has been closely correlated with chronic pain. An example of one such stressor may be deployment to a military conflict. Soldiers and military personnel throughout history have reported a cluster of symptoms such as pain, fatigue, and cognitive impairment that are very similar to FM. From US military conflicts, these syndromes include Gulf War illness, the condition known as “shell shock” in World War I, and “soldier’s heart” during the Civil War.
A review of the literature addressing the association between chronic pain and PTSD by the Department of Veterans Affairs found such a high degree of correlation that the authors suggested clinicians who conduct diagnostic assessments for one disorder should also assess for the other.22 In a study that evaluated patients for FM, chronic fatigue, and psychiatric symptoms, patients with FM who had both tender points and diffuse pain were significantly more likely to have an increased prevalence of lifetime PTSD.23
The relationship between depression and chronic pain has been well documented. Kaiser Permanente surveyed patients seen in primary care and found that a significantly higher proportion of patients with major depressive disorder (MDD) reported chronic pain than did patients without MDD (66% vs 43%, respectively).24 These conditions share common physiologic features and a high degree of comorbidity.
A study of patients with FM and depressive symptoms or MDD looked at neural responses to painful pressure and found no association between the extent of depressive symptoms or MDD and neural activation in the primary and secondary cortices, areas associated with the sensation of pain. However, activation was seen in the amygdala and contralateral anterior insula, areas associated with affective pain processing.25
These findings were supported in a more recent study in which patients who met the criteria for FM were given a series of questionnaires to assess depressive symptoms, anxiety, and catastrophizing, and were tested for painful pressure responses using fMRI. The results established a correlation between this cluster of affective symptoms, but there was no correlation with clinical pain symptoms or responses to painful pressure.26 Rather than suggesting that there is no alignment between the mental and physical aspects of pain, results from both of these studies suggest that 2 independent pain networks exist to process the sensory and affective dimensions of pain, and that these pathways may operate simultaneously.
Pain in the clinical setting
The evidence is strong that many patients experience chronic pain that is not site-specific and arises not merely from the periphery but from intricate neural systems. With a new appreciation for the complexity of pain processing, the clinician is compelled to probe beyond, “Where does it hurt?” [Table].
| TABLE: Clinical diagnosis of central pain
|
When patients complain of widespread or chronic pain, the clinician is well advised to take the time to examine further by inquiring about depression, anxiety, fatigue, sleep disturbances, and cognitive difficulties in order to understand what is driving the patient’s symptoms.13 The results may be revealing. In a study of primary care patients, participants who complained of muscle pain, headache, and stomach pain were found to be 2.5 to 10 times more likely to screen positively for panic disorder, generalized anxiety, or MDD.27
An article in a following issue will discuss practical tools that can be used to assess comorbidities such as anxiety and depression, and interventions that might be helpful for central pain and neurorehabilitation. An approach that acknowledges the patient’s account of pain, recognizes the cluster of symptoms and conditions that can accompany pain, and utilizes a multidisciplinary approach for diagnosis and treatment will have the best chance of yielding positive outcomes.
Acknowledgement—The author wishes to thank Kristen Georgi for her assistance in the research and writing of this article.
REFERENCES
1. International Association for the Study of Pain. IASP taxonomy: pain terms. Pain. Available at: http://www.iasp-pain.org/AM/Template.cfm?Section=Pain_Defi...isplay.cfm&ContentID=1728#Pain.
2. Melzack R, Wall PD. Pain mechanisms. A new theory. Science. 1965;150:971-979.
3. Carr DB. How prevalent is chronic pain? Pain Clinical Updates. 2003;11:1-4. Available at: http://www.iasp-pain.org/AM/AMTemplate.cfm?Section=Home&CONTENTID=7594&TEMPLATE=/CM/ContentDisplay.cfm&SECTION=Home.
4. Washington, DC: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2006. Bernstein AB, Makuc DM, Bilheimer LT. Health, United States, 2006. Available at: http: //www.cdc.gov/nchs/data/hus/hus06.pdf.
5. Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011.
6. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513-1517.
7. Creamer P, Hochberg MC. Why does osteoarthritis of the knee hurt—sometimes? Br J Rheumatol. 1997;36:726-728.
8. Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959-1964.
9. Andersen G, Vestergaard K, Ingeman-Nielsen M, et al. Incidence of central post-stroke pain. Pain. 1995;61:187-193.
10. Bowsher D. Central pain: clinical and physiological characteristics. J Neurol Neurosurg Psychiatry. 1996;61:62-69.
11. Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet. 2009;8:857-868.
12. Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160-172.
13. Wolfe F, Ross K, Anderson J, et al. Aspects of fibromyalgia in the general population: sex, pain threshold, and fibromyalgia symptoms. J Rheumatol. 1995;22:151-156.
14. Desmeules JA, Cedraschi C, Rapiti E, et al. Neurolopshysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48:1420-1429.
15. Giesecke T, Gracely RH, Grant MAB, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613-623.
16. Gracely RH, Petzke F, Wolf JM, et al. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333-1343.
17. Kuchinad A, Schweinhardt P, Seminowicz DA, et al. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27:4004-4007.
18. Gwilym SE, Fillipini N, Douaud G, et al. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty. Arthritis Rheum. 2010;62:2930-2940.
19. Tracey I. Imaging pain. Br J Anaesth. 2008;101:32-39.
20. Harris RE, Sundgren PC, Craig AD, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146-3152.
21. Baliki MN, Geha PY, Apkarian AV, et al. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28:
1398-1403.
22. Otis JD, Keane TM, Kerns RD. An examination of the relationship between chronic pain and posttraumatic stress disorder. J Rehabil Res Dev. 2003;40:397-406.
23. Roy-Byrne P, Smith WR, Goldberg N, et al. Posttraumatic stress disorder among patients with chronic pain and chronic fatigue. Psychol Med. 2004;34:
363-368.
24. Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68:262-268.
25. Giesecke T, Gracely RH, Williams DA, et al. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577-1584.
26. Jensen KB, Petzke F, Carville S, et al. Anxiety and depressive symptoms in fibromyalgia are related to poor perception of health but not to pain sensitivity or cerebral processing of pain. Arthritis Rheum. 2010;62:3488-3495.
27. Means-Christensen AJ, Roy-Byrne PR, Sherbourne CD, et al. Relationships among pain, anxiety, and depression in primary care. Depress Anxiety. 2008;25:
593-600.
What do we mean when we talk about pain? Traditionally pain is defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage.1 Pain can result when intense or noxious stimuli activate peripheral nociceptors. It serves as a warning against impending tissue damage and acts reflectively to protect against or minimize that damage.
We have known since the time of Descartes about the existence of an ascending sensory pain pathway that sends “distress” signals from the source of tissue damage to the brain. We also know of the Gate Control theory described by Melzack and Wall in 1965, in which stimulation of the skin evokes responses that transmit signal injury to transmission cells (the “gate”) in the dorsal horn of the spinal cord that continues to the brain, triggering response signals that modulate the activity of inhibitory cells (which close the “gate”), thereby decreasing the intensity of pain.2 But how do we explain pain in the absence of tissue damage, pain that is not triggered in the periphery, that often appears long after the noxious stimulus has stopped exerting its unpleasant effect?
Types of chronic pain
An estimated 116 million American adults suffer from chronic pain, defined as pain that lasts more than 3 months after onset and well into the phase of healing.1,3 According to a 2006 report from the Centers for Disease Control and Prevention with a special focus on pain, almost 57% of adults age 65 or older and 37% of younger adults ages 20–44 reported pain that lasted one year or more [Figure 1].4 Chronic pain exacts a cost of between $560 billion and $635 billion annually in medical treatment and lost productivity.5 There is a tremendous need to understand the molecular and cellular mechanisms of chronic pain in an effort to develop new, more effective treatments for these patients. This understanding may come as a result of our recent advances in visualizing the peripheral and central processes involved in pain. The emerging data suggest that for some individuals central factors play a key role in the maintenance and establishment of certain chronic pain conditions. That is, for some, the problem is really not inthe periphery.
| FIGURE 1: Pain duration by age group, 1999-2002 |
| Source: Centers for Disease Control and Prevention, National Center for Health Statistics, Health, United States, 2006. Data from theNational Health and Nutrition Examination Survey. |
Knee and hip pain. When peripheral tissue damage is unavoidable, the inflamed tissues and those nearby become hypersensitive, a protective response to guard the area during the period of healing. Conditions like chronic low-back pain and knee or hip osteoarthritis classically have been thought to be due to inflammation or damage to tissues in the back, knee, or hip. However, recent studies show that these conditions may have complex factors entailing both the peripheral and central nervous systems.
Analysis of data from the National Health and Nutrition Examination Survey (NHANES I) of patients with radiographic evidence of structural damage to the knee due to osteoarthritis found discordance between the amount of damage visible on x-ray and patients’ self-report of the degree of pain. In 319 patients with radiographic stage 2–4 knee osteoarthritis, only 47% reported knee pain, suggesting that something more than the degree of tissue injury was involved in the perception of pain.6,7 One explanation of these findings is that pain is a complex system incorporating structural changes, peripheral and central pain mechanisms, and subjective factors, including the patient’s history, psychological experience, genetics, and culture.
Diabetic neuropathy and postherpetic neuralgia. Chronic neuropathic pain results when there is actual damage to the nervous system—the peripheral nerve, dorsal root, or central nervous system. Peripheral neuropathic pain occurs after damage or alterations to sensory neurons. Some neuropathic pain disorders, such as diabetic neuropathy and postherpetic neuralgia, are well-defined disorders in which symptoms are unrelated to a stimulus and pain is related to peripheral as well as central processing.8
Stroke. Central poststroke pain, in which pain and hypersensitivity occurs in a body part due to injury to the corresponding part of the brain affected by the cerebrovascular lesion, is also considered a neuropathic pain syndrome. The onset of central poststroke pain typically occurs more than one month after the stroke, and exists with somatosensory abnormalities.9-11 For these types of neuropathies, altered function due to loss or damage of neuronal tissue is likely the cause of the pain condition.
Many of the people suffering from these central chronic pain conditions find it difficult to obtain relief, and probably will not benefit from surgeries or manipulations in the periphery. Instead, they may benefit from a targeted approach that addresses the central nervous system.
Recent studies on fibromyalgia and pain
Fibromyalgia (FM) may be considered the prototypical central pain disorder, in which the pain originates or is maintained in part in the central nervous system. Although new diagnostic criteria are being validated for this disorder, FM classically has been diagnosed by the detection of 11 of 18 tender points and the presence of chronic widespread pain for 3 months or longer.12
FM is a common disorder found to affect between 2% and 4% of the US population.13 It was one of the first disorders shown to have central factors predominant in the pathology, and as a result it has been the focus of numerous studies. Irritable bowel syndrome and chronic fatigue syndrome, often comorbid with FM, are also commonly studied. Until recently, these disorders have largely been considered “wastebasket” terms to categorize the complaints of patients with unexplained symptoms, because there were no objective signs to support their complaints. However with the advent of new imaging techniques to look into the brain and the central nervous system, researchers are finding very real physiological differences. For example, one study using sensory testing with thermal, mechanical, and electrical stimuli showed a correlation between FM patients’ subjective reports of pain and significantly altered cold and heat thresholds when compared with controls.14 Based on such studies it appears that patients with FM perceive stimuli as noxious at lower levels than healthy, pain-free controls.
Recent studies of FM have incorporated the use of functional magnetic resonance imaging (fMRI) to look at brain activations in response to painful stimuli. A study that included patients with FM and others with chronic low-back pain used fMRI to visualize the participants’ response to equal amounts of thumbnail pressure. In the FM and groups, 5 areas of neuronal activation within the cortex related to pain were detected, compared with only one activation in controls.15 Another study to evaluate the pattern of cerebral activation in FM patients found that in response to similar thumbnail pressures there were 13 regions of greater activation in the FM group compared with one region in the healthy control group.16 Additionally, mild pressure resulted in subjective pain reports and cerebral responses in the FM group that were similar to responses produced by twice the pressure applied in controls.
Another important area of research in pain processing looks at gray matter in the brain using voxel-based morphometry. A study of patients with FM found significantly less volume of gray matter and an age-associated decrease in gray matter that was 3.3 times greater than healthy controls.17
Using MRI to look at gray matter volume in patients with chronic musculoskeletal pain, significant differences in gray matter volume were found in osteoarthritis patients prior to hip arthroplasty compared with healthy controls. Specifically, areas of the thalamus, understood to play a role in central pain processing, showed decreased gray matter volume in the osteoarthritis group. Significantly, a comparison of gray matter volume 9 months after surgery showed that the levels of reduced thalamic gray matter volume in osteoarthritis patients “reversed” to levels similar to the those of the healthy control group.18
Although the mechanism that drives the loss or degradation of brain tissue in patients with chronic pain remains to be determined, one theory is that pain is associated with certain areas of the brain becoming hyperactive. Imaging studies using fMRI show that a constellation of regions typically are activated in pain processing, including the insula,
cingulate, primary somatosensory and secondary somatosensory cortices, amygdala, and thalamus [Figure 2].19 These regions have been shown to be more active in chronic pain states when patients respond to stimuli such as painful pressure or heat. Indeed, these regions have shown overamplification or augmentation of neural activity.
| FIGURE 2: Neuroanatomy of pain processing. Main brain regions that activate during a painful experience are highlighted as bilaterally active but with more dominant activation on the contralateral hemisphere (red). |
| Source: Tracey I. Br J Anaesth. 2008;101:32-39. |
Since overstimulation of nerve cells can trigger a toxic release of glutamate into surrounding tissues of the brain, this may cause nerve cells to die, ultimately reducing the amount of gray matter visualized in the brains of patients with chronic pain. In addition, some studies of FM have shown elevated levels of glutamate, an excitatory neurotransmitter that is known to cause excitotoxicity.20
Another significant consequence of long-term pain appears to be alterations in the normal connectivity of the brain, including the “default mode network” (DMN) which is noted to be important during the resting state. Recent studies of chronic pain suggest alterations in key DMN regions that may be related to the chronic pain state and existing comorbidities.21
The role of stress and depression in pain
The association among physical and psychosocial stressors, depression, and chronic pain syndromes has been the subject of numerous studies.
Posttraumatic stress disorder (PTSD) has been closely correlated with chronic pain. An example of one such stressor may be deployment to a military conflict. Soldiers and military personnel throughout history have reported a cluster of symptoms such as pain, fatigue, and cognitive impairment that are very similar to FM. From US military conflicts, these syndromes include Gulf War illness, the condition known as “shell shock” in World War I, and “soldier’s heart” during the Civil War.
A review of the literature addressing the association between chronic pain and PTSD by the Department of Veterans Affairs found such a high degree of correlation that the authors suggested clinicians who conduct diagnostic assessments for one disorder should also assess for the other.22 In a study that evaluated patients for FM, chronic fatigue, and psychiatric symptoms, patients with FM who had both tender points and diffuse pain were significantly more likely to have an increased prevalence of lifetime PTSD.23
The relationship between depression and chronic pain has been well documented. Kaiser Permanente surveyed patients seen in primary care and found that a significantly higher proportion of patients with major depressive disorder (MDD) reported chronic pain than did patients without MDD (66% vs 43%, respectively).24 These conditions share common physiologic features and a high degree of comorbidity.
A study of patients with FM and depressive symptoms or MDD looked at neural responses to painful pressure and found no association between the extent of depressive symptoms or MDD and neural activation in the primary and secondary cortices, areas associated with the sensation of pain. However, activation was seen in the amygdala and contralateral anterior insula, areas associated with affective pain processing.25
These findings were supported in a more recent study in which patients who met the criteria for FM were given a series of questionnaires to assess depressive symptoms, anxiety, and catastrophizing, and were tested for painful pressure responses using fMRI. The results established a correlation between this cluster of affective symptoms, but there was no correlation with clinical pain symptoms or responses to painful pressure.26 Rather than suggesting that there is no alignment between the mental and physical aspects of pain, results from both of these studies suggest that 2 independent pain networks exist to process the sensory and affective dimensions of pain, and that these pathways may operate simultaneously.
Pain in the clinical setting
The evidence is strong that many patients experience chronic pain that is not site-specific and arises not merely from the periphery but from intricate neural systems. With a new appreciation for the complexity of pain processing, the clinician is compelled to probe beyond, “Where does it hurt?” [Table].
| TABLE: Clinical diagnosis of central pain
|
When patients complain of widespread or chronic pain, the clinician is well advised to take the time to examine further by inquiring about depression, anxiety, fatigue, sleep disturbances, and cognitive difficulties in order to understand what is driving the patient’s symptoms.13 The results may be revealing. In a study of primary care patients, participants who complained of muscle pain, headache, and stomach pain were found to be 2.5 to 10 times more likely to screen positively for panic disorder, generalized anxiety, or MDD.27
An article in a following issue will discuss practical tools that can be used to assess comorbidities such as anxiety and depression, and interventions that might be helpful for central pain and neurorehabilitation. An approach that acknowledges the patient’s account of pain, recognizes the cluster of symptoms and conditions that can accompany pain, and utilizes a multidisciplinary approach for diagnosis and treatment will have the best chance of yielding positive outcomes.
Acknowledgement—The author wishes to thank Kristen Georgi for her assistance in the research and writing of this article.
REFERENCES
1. International Association for the Study of Pain. IASP taxonomy: pain terms. Pain. Available at: http://www.iasp-pain.org/AM/Template.cfm?Section=Pain_Defi...isplay.cfm&ContentID=1728#Pain.
2. Melzack R, Wall PD. Pain mechanisms. A new theory. Science. 1965;150:971-979.
3. Carr DB. How prevalent is chronic pain? Pain Clinical Updates. 2003;11:1-4. Available at: http://www.iasp-pain.org/AM/AMTemplate.cfm?Section=Home&CONTENTID=7594&TEMPLATE=/CM/ContentDisplay.cfm&SECTION=Home.
4. Washington, DC: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2006. Bernstein AB, Makuc DM, Bilheimer LT. Health, United States, 2006. Available at: http: //www.cdc.gov/nchs/data/hus/hus06.pdf.
5. Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011.
6. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513-1517.
7. Creamer P, Hochberg MC. Why does osteoarthritis of the knee hurt—sometimes? Br J Rheumatol. 1997;36:726-728.
8. Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959-1964.
9. Andersen G, Vestergaard K, Ingeman-Nielsen M, et al. Incidence of central post-stroke pain. Pain. 1995;61:187-193.
10. Bowsher D. Central pain: clinical and physiological characteristics. J Neurol Neurosurg Psychiatry. 1996;61:62-69.
11. Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet. 2009;8:857-868.
12. Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160-172.
13. Wolfe F, Ross K, Anderson J, et al. Aspects of fibromyalgia in the general population: sex, pain threshold, and fibromyalgia symptoms. J Rheumatol. 1995;22:151-156.
14. Desmeules JA, Cedraschi C, Rapiti E, et al. Neurolopshysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48:1420-1429.
15. Giesecke T, Gracely RH, Grant MAB, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613-623.
16. Gracely RH, Petzke F, Wolf JM, et al. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333-1343.
17. Kuchinad A, Schweinhardt P, Seminowicz DA, et al. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27:4004-4007.
18. Gwilym SE, Fillipini N, Douaud G, et al. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty. Arthritis Rheum. 2010;62:2930-2940.
19. Tracey I. Imaging pain. Br J Anaesth. 2008;101:32-39.
20. Harris RE, Sundgren PC, Craig AD, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146-3152.
21. Baliki MN, Geha PY, Apkarian AV, et al. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28:
1398-1403.
22. Otis JD, Keane TM, Kerns RD. An examination of the relationship between chronic pain and posttraumatic stress disorder. J Rehabil Res Dev. 2003;40:397-406.
23. Roy-Byrne P, Smith WR, Goldberg N, et al. Posttraumatic stress disorder among patients with chronic pain and chronic fatigue. Psychol Med. 2004;34:
363-368.
24. Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68:262-268.
25. Giesecke T, Gracely RH, Williams DA, et al. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577-1584.
26. Jensen KB, Petzke F, Carville S, et al. Anxiety and depressive symptoms in fibromyalgia are related to poor perception of health but not to pain sensitivity or cerebral processing of pain. Arthritis Rheum. 2010;62:3488-3495.
27. Means-Christensen AJ, Roy-Byrne PR, Sherbourne CD, et al. Relationships among pain, anxiety, and depression in primary care. Depress Anxiety. 2008;25:
593-600.
What do we mean when we talk about pain? Traditionally pain is defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage.1 Pain can result when intense or noxious stimuli activate peripheral nociceptors. It serves as a warning against impending tissue damage and acts reflectively to protect against or minimize that damage.
We have known since the time of Descartes about the existence of an ascending sensory pain pathway that sends “distress” signals from the source of tissue damage to the brain. We also know of the Gate Control theory described by Melzack and Wall in 1965, in which stimulation of the skin evokes responses that transmit signal injury to transmission cells (the “gate”) in the dorsal horn of the spinal cord that continues to the brain, triggering response signals that modulate the activity of inhibitory cells (which close the “gate”), thereby decreasing the intensity of pain.2 But how do we explain pain in the absence of tissue damage, pain that is not triggered in the periphery, that often appears long after the noxious stimulus has stopped exerting its unpleasant effect?
Types of chronic pain
An estimated 116 million American adults suffer from chronic pain, defined as pain that lasts more than 3 months after onset and well into the phase of healing.1,3 According to a 2006 report from the Centers for Disease Control and Prevention with a special focus on pain, almost 57% of adults age 65 or older and 37% of younger adults ages 20–44 reported pain that lasted one year or more [Figure 1].4 Chronic pain exacts a cost of between $560 billion and $635 billion annually in medical treatment and lost productivity.5 There is a tremendous need to understand the molecular and cellular mechanisms of chronic pain in an effort to develop new, more effective treatments for these patients. This understanding may come as a result of our recent advances in visualizing the peripheral and central processes involved in pain. The emerging data suggest that for some individuals central factors play a key role in the maintenance and establishment of certain chronic pain conditions. That is, for some, the problem is really not inthe periphery.
| FIGURE 1: Pain duration by age group, 1999-2002 |
| Source: Centers for Disease Control and Prevention, National Center for Health Statistics, Health, United States, 2006. Data from theNational Health and Nutrition Examination Survey. |
Knee and hip pain. When peripheral tissue damage is unavoidable, the inflamed tissues and those nearby become hypersensitive, a protective response to guard the area during the period of healing. Conditions like chronic low-back pain and knee or hip osteoarthritis classically have been thought to be due to inflammation or damage to tissues in the back, knee, or hip. However, recent studies show that these conditions may have complex factors entailing both the peripheral and central nervous systems.
Analysis of data from the National Health and Nutrition Examination Survey (NHANES I) of patients with radiographic evidence of structural damage to the knee due to osteoarthritis found discordance between the amount of damage visible on x-ray and patients’ self-report of the degree of pain. In 319 patients with radiographic stage 2–4 knee osteoarthritis, only 47% reported knee pain, suggesting that something more than the degree of tissue injury was involved in the perception of pain.6,7 One explanation of these findings is that pain is a complex system incorporating structural changes, peripheral and central pain mechanisms, and subjective factors, including the patient’s history, psychological experience, genetics, and culture.
Diabetic neuropathy and postherpetic neuralgia. Chronic neuropathic pain results when there is actual damage to the nervous system—the peripheral nerve, dorsal root, or central nervous system. Peripheral neuropathic pain occurs after damage or alterations to sensory neurons. Some neuropathic pain disorders, such as diabetic neuropathy and postherpetic neuralgia, are well-defined disorders in which symptoms are unrelated to a stimulus and pain is related to peripheral as well as central processing.8
Stroke. Central poststroke pain, in which pain and hypersensitivity occurs in a body part due to injury to the corresponding part of the brain affected by the cerebrovascular lesion, is also considered a neuropathic pain syndrome. The onset of central poststroke pain typically occurs more than one month after the stroke, and exists with somatosensory abnormalities.9-11 For these types of neuropathies, altered function due to loss or damage of neuronal tissue is likely the cause of the pain condition.
Many of the people suffering from these central chronic pain conditions find it difficult to obtain relief, and probably will not benefit from surgeries or manipulations in the periphery. Instead, they may benefit from a targeted approach that addresses the central nervous system.
Recent studies on fibromyalgia and pain
Fibromyalgia (FM) may be considered the prototypical central pain disorder, in which the pain originates or is maintained in part in the central nervous system. Although new diagnostic criteria are being validated for this disorder, FM classically has been diagnosed by the detection of 11 of 18 tender points and the presence of chronic widespread pain for 3 months or longer.12
FM is a common disorder found to affect between 2% and 4% of the US population.13 It was one of the first disorders shown to have central factors predominant in the pathology, and as a result it has been the focus of numerous studies. Irritable bowel syndrome and chronic fatigue syndrome, often comorbid with FM, are also commonly studied. Until recently, these disorders have largely been considered “wastebasket” terms to categorize the complaints of patients with unexplained symptoms, because there were no objective signs to support their complaints. However with the advent of new imaging techniques to look into the brain and the central nervous system, researchers are finding very real physiological differences. For example, one study using sensory testing with thermal, mechanical, and electrical stimuli showed a correlation between FM patients’ subjective reports of pain and significantly altered cold and heat thresholds when compared with controls.14 Based on such studies it appears that patients with FM perceive stimuli as noxious at lower levels than healthy, pain-free controls.
Recent studies of FM have incorporated the use of functional magnetic resonance imaging (fMRI) to look at brain activations in response to painful stimuli. A study that included patients with FM and others with chronic low-back pain used fMRI to visualize the participants’ response to equal amounts of thumbnail pressure. In the FM and groups, 5 areas of neuronal activation within the cortex related to pain were detected, compared with only one activation in controls.15 Another study to evaluate the pattern of cerebral activation in FM patients found that in response to similar thumbnail pressures there were 13 regions of greater activation in the FM group compared with one region in the healthy control group.16 Additionally, mild pressure resulted in subjective pain reports and cerebral responses in the FM group that were similar to responses produced by twice the pressure applied in controls.
Another important area of research in pain processing looks at gray matter in the brain using voxel-based morphometry. A study of patients with FM found significantly less volume of gray matter and an age-associated decrease in gray matter that was 3.3 times greater than healthy controls.17
Using MRI to look at gray matter volume in patients with chronic musculoskeletal pain, significant differences in gray matter volume were found in osteoarthritis patients prior to hip arthroplasty compared with healthy controls. Specifically, areas of the thalamus, understood to play a role in central pain processing, showed decreased gray matter volume in the osteoarthritis group. Significantly, a comparison of gray matter volume 9 months after surgery showed that the levels of reduced thalamic gray matter volume in osteoarthritis patients “reversed” to levels similar to the those of the healthy control group.18
Although the mechanism that drives the loss or degradation of brain tissue in patients with chronic pain remains to be determined, one theory is that pain is associated with certain areas of the brain becoming hyperactive. Imaging studies using fMRI show that a constellation of regions typically are activated in pain processing, including the insula,
cingulate, primary somatosensory and secondary somatosensory cortices, amygdala, and thalamus [Figure 2].19 These regions have been shown to be more active in chronic pain states when patients respond to stimuli such as painful pressure or heat. Indeed, these regions have shown overamplification or augmentation of neural activity.
| FIGURE 2: Neuroanatomy of pain processing. Main brain regions that activate during a painful experience are highlighted as bilaterally active but with more dominant activation on the contralateral hemisphere (red). |
| Source: Tracey I. Br J Anaesth. 2008;101:32-39. |
Since overstimulation of nerve cells can trigger a toxic release of glutamate into surrounding tissues of the brain, this may cause nerve cells to die, ultimately reducing the amount of gray matter visualized in the brains of patients with chronic pain. In addition, some studies of FM have shown elevated levels of glutamate, an excitatory neurotransmitter that is known to cause excitotoxicity.20
Another significant consequence of long-term pain appears to be alterations in the normal connectivity of the brain, including the “default mode network” (DMN) which is noted to be important during the resting state. Recent studies of chronic pain suggest alterations in key DMN regions that may be related to the chronic pain state and existing comorbidities.21
The role of stress and depression in pain
The association among physical and psychosocial stressors, depression, and chronic pain syndromes has been the subject of numerous studies.
Posttraumatic stress disorder (PTSD) has been closely correlated with chronic pain. An example of one such stressor may be deployment to a military conflict. Soldiers and military personnel throughout history have reported a cluster of symptoms such as pain, fatigue, and cognitive impairment that are very similar to FM. From US military conflicts, these syndromes include Gulf War illness, the condition known as “shell shock” in World War I, and “soldier’s heart” during the Civil War.
A review of the literature addressing the association between chronic pain and PTSD by the Department of Veterans Affairs found such a high degree of correlation that the authors suggested clinicians who conduct diagnostic assessments for one disorder should also assess for the other.22 In a study that evaluated patients for FM, chronic fatigue, and psychiatric symptoms, patients with FM who had both tender points and diffuse pain were significantly more likely to have an increased prevalence of lifetime PTSD.23
The relationship between depression and chronic pain has been well documented. Kaiser Permanente surveyed patients seen in primary care and found that a significantly higher proportion of patients with major depressive disorder (MDD) reported chronic pain than did patients without MDD (66% vs 43%, respectively).24 These conditions share common physiologic features and a high degree of comorbidity.
A study of patients with FM and depressive symptoms or MDD looked at neural responses to painful pressure and found no association between the extent of depressive symptoms or MDD and neural activation in the primary and secondary cortices, areas associated with the sensation of pain. However, activation was seen in the amygdala and contralateral anterior insula, areas associated with affective pain processing.25
These findings were supported in a more recent study in which patients who met the criteria for FM were given a series of questionnaires to assess depressive symptoms, anxiety, and catastrophizing, and were tested for painful pressure responses using fMRI. The results established a correlation between this cluster of affective symptoms, but there was no correlation with clinical pain symptoms or responses to painful pressure.26 Rather than suggesting that there is no alignment between the mental and physical aspects of pain, results from both of these studies suggest that 2 independent pain networks exist to process the sensory and affective dimensions of pain, and that these pathways may operate simultaneously.
Pain in the clinical setting
The evidence is strong that many patients experience chronic pain that is not site-specific and arises not merely from the periphery but from intricate neural systems. With a new appreciation for the complexity of pain processing, the clinician is compelled to probe beyond, “Where does it hurt?” [Table].
| TABLE: Clinical diagnosis of central pain
|
When patients complain of widespread or chronic pain, the clinician is well advised to take the time to examine further by inquiring about depression, anxiety, fatigue, sleep disturbances, and cognitive difficulties in order to understand what is driving the patient’s symptoms.13 The results may be revealing. In a study of primary care patients, participants who complained of muscle pain, headache, and stomach pain were found to be 2.5 to 10 times more likely to screen positively for panic disorder, generalized anxiety, or MDD.27
An article in a following issue will discuss practical tools that can be used to assess comorbidities such as anxiety and depression, and interventions that might be helpful for central pain and neurorehabilitation. An approach that acknowledges the patient’s account of pain, recognizes the cluster of symptoms and conditions that can accompany pain, and utilizes a multidisciplinary approach for diagnosis and treatment will have the best chance of yielding positive outcomes.
Acknowledgement—The author wishes to thank Kristen Georgi for her assistance in the research and writing of this article.
REFERENCES
1. International Association for the Study of Pain. IASP taxonomy: pain terms. Pain. Available at: http://www.iasp-pain.org/AM/Template.cfm?Section=Pain_Defi...isplay.cfm&ContentID=1728#Pain.
2. Melzack R, Wall PD. Pain mechanisms. A new theory. Science. 1965;150:971-979.
3. Carr DB. How prevalent is chronic pain? Pain Clinical Updates. 2003;11:1-4. Available at: http://www.iasp-pain.org/AM/AMTemplate.cfm?Section=Home&CONTENTID=7594&TEMPLATE=/CM/ContentDisplay.cfm&SECTION=Home.
4. Washington, DC: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2006. Bernstein AB, Makuc DM, Bilheimer LT. Health, United States, 2006. Available at: http: //www.cdc.gov/nchs/data/hus/hus06.pdf.
5. Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011.
6. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513-1517.
7. Creamer P, Hochberg MC. Why does osteoarthritis of the knee hurt—sometimes? Br J Rheumatol. 1997;36:726-728.
8. Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959-1964.
9. Andersen G, Vestergaard K, Ingeman-Nielsen M, et al. Incidence of central post-stroke pain. Pain. 1995;61:187-193.
10. Bowsher D. Central pain: clinical and physiological characteristics. J Neurol Neurosurg Psychiatry. 1996;61:62-69.
11. Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet. 2009;8:857-868.
12. Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160-172.
13. Wolfe F, Ross K, Anderson J, et al. Aspects of fibromyalgia in the general population: sex, pain threshold, and fibromyalgia symptoms. J Rheumatol. 1995;22:151-156.
14. Desmeules JA, Cedraschi C, Rapiti E, et al. Neurolopshysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48:1420-1429.
15. Giesecke T, Gracely RH, Grant MAB, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613-623.
16. Gracely RH, Petzke F, Wolf JM, et al. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333-1343.
17. Kuchinad A, Schweinhardt P, Seminowicz DA, et al. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27:4004-4007.
18. Gwilym SE, Fillipini N, Douaud G, et al. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty. Arthritis Rheum. 2010;62:2930-2940.
19. Tracey I. Imaging pain. Br J Anaesth. 2008;101:32-39.
20. Harris RE, Sundgren PC, Craig AD, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146-3152.
21. Baliki MN, Geha PY, Apkarian AV, et al. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28:
1398-1403.
22. Otis JD, Keane TM, Kerns RD. An examination of the relationship between chronic pain and posttraumatic stress disorder. J Rehabil Res Dev. 2003;40:397-406.
23. Roy-Byrne P, Smith WR, Goldberg N, et al. Posttraumatic stress disorder among patients with chronic pain and chronic fatigue. Psychol Med. 2004;34:
363-368.
24. Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68:262-268.
25. Giesecke T, Gracely RH, Williams DA, et al. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577-1584.
26. Jensen KB, Petzke F, Carville S, et al. Anxiety and depressive symptoms in fibromyalgia are related to poor perception of health but not to pain sensitivity or cerebral processing of pain. Arthritis Rheum. 2010;62:3488-3495.
27. Means-Christensen AJ, Roy-Byrne PR, Sherbourne CD, et al. Relationships among pain, anxiety, and depression in primary care. Depress Anxiety. 2008;25:
593-600.