User login
Mild cough • wheezing • loud heart sounds • Dx?
THE CASE
A 25-year-old man, who was an active duty US Navy sailor, went to his ship’s medical department complaining of a mild cough that he’d had for 2 days. He denied having any fevers, chills, night sweats, angina, or dyspnea. He said he hadn’t experienced any exertional fatigue or difficulty completing the rigorous physical tasks of his occupation as an engineman on the ship. The patient had no medical or surgical history of significance, and he wasn’t taking any medications or supplements.
On exam, he was not in acute distress and his vital signs were within normal limits. Auscultation revealed mild wheezing throughout the upper lung fields and loud heart sounds throughout his chest that were audible even with gentle contact of the stethoscope diaphragm. He had no discernible murmurs, rubs, or gallops.
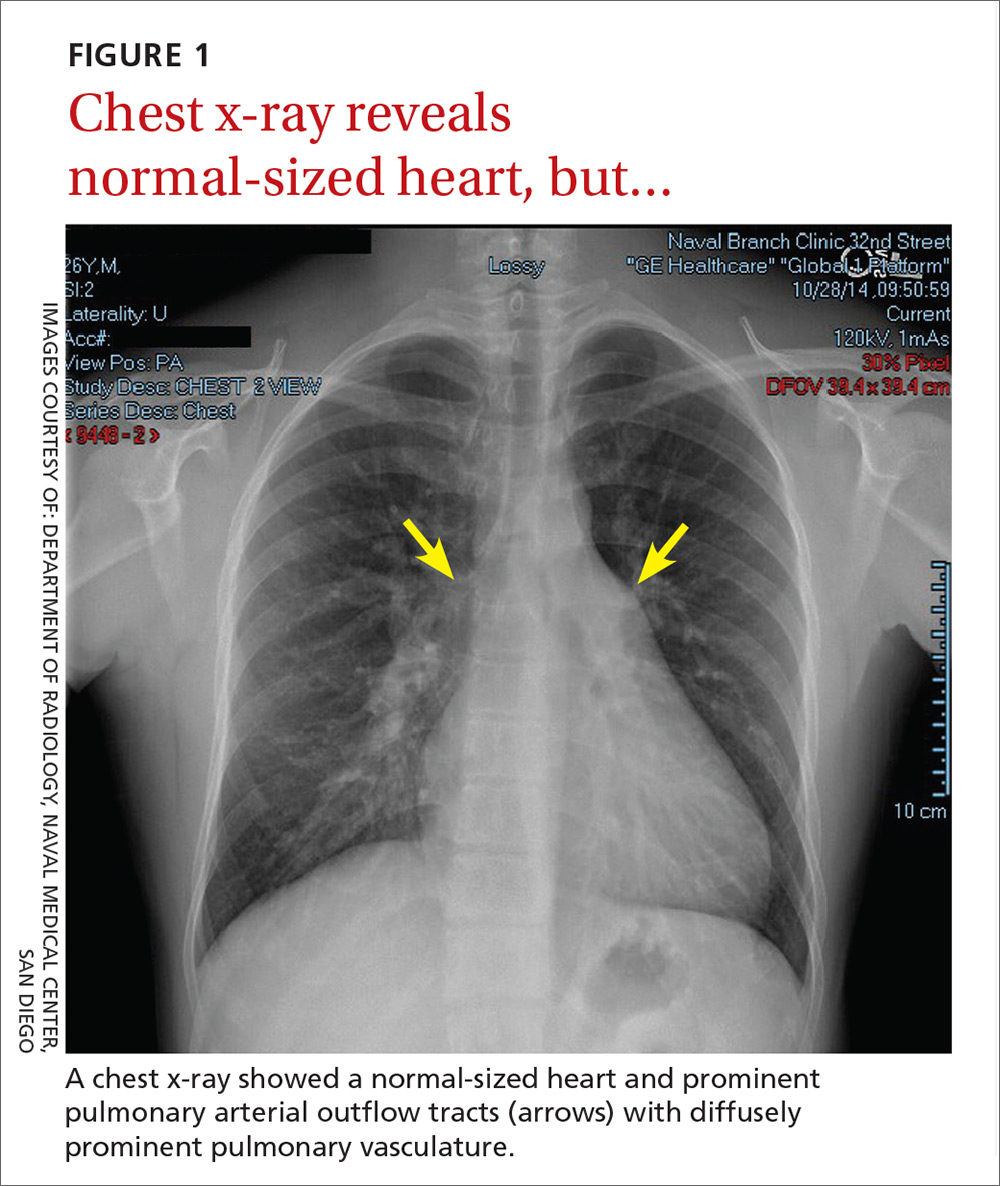
In light of the unusually loud heart sounds heard on exam, we performed an electrocardiogram. The EKG revealed a normal sinus rhythm, slight right axis deviation indicated by tall R-waves in V1 (also suggestive of right ventricular hypertrophy), an incomplete right bundle branch block, and a crochetage sign (a notch in the R-waves of the inferior leads).1 A chest x-ray (FIGURE 1) revealed a normal-sized heart and dilated pulmonary vasculature suggestive of pulmonary hypertension.

THE DIAGNOSIS
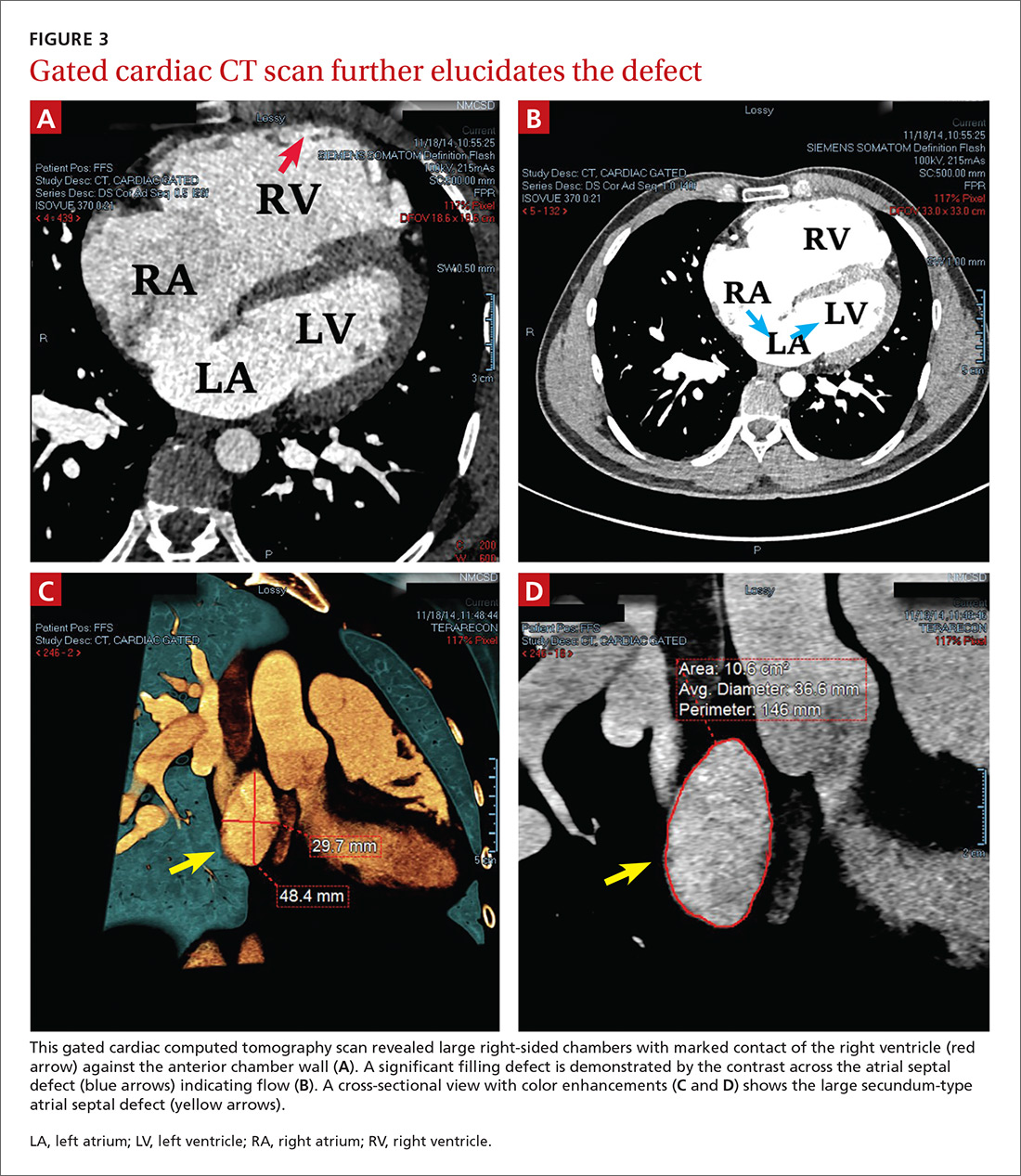
To further evaluate the cardiopulmonary findings, ultrasound studies (transthoracic and transesophageal echocardiography) were performed. These demonstrated a very large secundum-type atrial septal defect (ASD), measuring at its largest point about 30 × 48 mm (FIGURE 2 and FIGURE 3C). Doppler flow analysis and a bubble study (VIDEOS 1 and 2) demonstrated significant shunting across the ASD. Gated cardiac computed tomography (CT) was also used to characterize the ASD (FIGURE 3). It revealed that the superior and posterior rims of the ASD were essentially absent and that the right atrium and ventricle were severely enlarged, while the left chambers were normal in size and function with an ejection fraction >55%. The notching of the R-waves of the inferior leads, seen in our patient’s EKG, is typically seen with large ASDs.1,2
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with color Doppler flow (red) demonstrated significant shunting across a large atrial septal defect (white box). The largest white dot is positioned near the center of the defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with a bubble study showed injected air bubbles traversing the atrial septal defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
DISCUSSION
ASDs are typically uncovered on exam via auscultation of heart sounds, which might reveal a split of the second heart sound (S2) and diastolic murmurs. ASDs are typically classified by size, and their management depends on this factor, along with the patient’s age and symptoms. In children with small defects (<6 mm), treatment usually consists of conservative observation, as more than half of these ASDs will spontaneously close.3 But, as children age, they are more likely to engage in exertional activity (work, recreational sports) and an unrepaired ASD may yield symptoms (angina, dyspnea, fatigue, other cardiopulmonary strain). With such symptoms and when closure is not spontaneously achieved by adolescence or adulthood, an invasive approach is often necessary to correct the defect.
ASD repair. Traditionally, repair has involved some form of open thoracotomy. More recently, several minimally invasive techniques have been developed. Catheter-based device closure, in which a catheter is percutaneously guided to the defect and a patch is deployed to seal the ASD, is a technique that has been shown to successfully correct large ASDs of up to 40 mm in size.4 Robotic procedures have also been developed to correct ASDs through much smaller incisions.5 Both of these techniques require a significant rim of residual septal tissue around the defect.
Individualized approach. Since our patient had a rather large ASD that did not have sufficient residual septal rim tissue, percutaneous and robotic approaches were not feasible. Instead, he required more invasive cardiothoracic surgery. In cases such as this, the exact technique and type of incision (sternotomy vs access through the lateral chest wall) depend on age, gender, and the presence of other comorbidities.6
Our patient. Because there was concern that any approach other than a median one might not afford enough space to fix an ASD of such considerable size, our patient underwent a median sternotomy by a pediatric cardiothoracic surgeon who specialized in these repairs (in children as well as young adults). During the procedure, the ASD was accessed and confirmed to be as large as predicted by diagnostic imaging. A surgical patch was sutured in place to correct the defect. There were no intra-operative or postop complications.
Four weeks later, the patient had a mild pericardial effusion that was managed medically with daily furosemide and aspirin. At his 8-week postop appointment, the fluid accumulation had resolved, and he was completely asymptomatic. The patient returned to full-time active duty in the US Navy.
Adults with rather large ASDs can present in a relatively asymptomatic manner and report none of the classic complaints (angina, dyspnea, fatigue). They may even engage in heavy exertional activity with no difficulty. The underlying defect may be discovered incidentally on exam by noting a split of the S2 on auscultation. If pulmonary hypertension exists, the clinician may also note a loud S2. An exam that raises suspicion for an ASD can then be followed by tests that solidify the diagnosis. Surgery is usually necessary to correct an ASD in an adult who is symptomatic or exhibits significant cardiopulmonary strain.
1. Heller J, Hagège AA, Besse B, et al. “Crochetage” (notch) on R wave in inferior limb leads: a new independent electrocardiographic sign of atrial septal defect. J Am Coll Cardiol. 1996;27:877-882.
2. Kuijpers JM, Mulder BJM, Bouma BJ. Secundum atrial septal defect in adults: a practical review and recent developments. Neth Heart J. 2015;23:205-211.
3. McMahon CJ, Feltes TF, Fraley JK, et al. Natural history of growth of secundum atrial septal defects and implications for transcatheter closure. Heart. 2002;87:256-259.
4. Lopez K, Dalvi BV, Balzer D, et al. Transcatheter closure of large secundum atrial septal defects using the 40 mm amplatzer septal occluder: results of an international registry. Catheter Cardiovasc Interv. 2005;66:580-584.
5. Argenziano M, Oz MC, Kohmoto T, et al. Totally endoscopic atrial septal defect repair with robotic assistance. Circulation. 2003;108 Suppl 1:II191-II194.
6. Hopkins RA, Bert AA, Buchholz B, et al. Surgical patch closure of atrial septal defects. Ann Thorac Surg. 2004;77:2144-2149.
THE CASE
A 25-year-old man, who was an active duty US Navy sailor, went to his ship’s medical department complaining of a mild cough that he’d had for 2 days. He denied having any fevers, chills, night sweats, angina, or dyspnea. He said he hadn’t experienced any exertional fatigue or difficulty completing the rigorous physical tasks of his occupation as an engineman on the ship. The patient had no medical or surgical history of significance, and he wasn’t taking any medications or supplements.
On exam, he was not in acute distress and his vital signs were within normal limits. Auscultation revealed mild wheezing throughout the upper lung fields and loud heart sounds throughout his chest that were audible even with gentle contact of the stethoscope diaphragm. He had no discernible murmurs, rubs, or gallops.

In light of the unusually loud heart sounds heard on exam, we performed an electrocardiogram. The EKG revealed a normal sinus rhythm, slight right axis deviation indicated by tall R-waves in V1 (also suggestive of right ventricular hypertrophy), an incomplete right bundle branch block, and a crochetage sign (a notch in the R-waves of the inferior leads).1 A chest x-ray (FIGURE 1) revealed a normal-sized heart and dilated pulmonary vasculature suggestive of pulmonary hypertension.


THE DIAGNOSIS
To further evaluate the cardiopulmonary findings, ultrasound studies (transthoracic and transesophageal echocardiography) were performed. These demonstrated a very large secundum-type atrial septal defect (ASD), measuring at its largest point about 30 × 48 mm (FIGURE 2 and FIGURE 3C). Doppler flow analysis and a bubble study (VIDEOS 1 and 2) demonstrated significant shunting across the ASD. Gated cardiac computed tomography (CT) was also used to characterize the ASD (FIGURE 3). It revealed that the superior and posterior rims of the ASD were essentially absent and that the right atrium and ventricle were severely enlarged, while the left chambers were normal in size and function with an ejection fraction >55%. The notching of the R-waves of the inferior leads, seen in our patient’s EKG, is typically seen with large ASDs.1,2
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with color Doppler flow (red) demonstrated significant shunting across a large atrial septal defect (white box). The largest white dot is positioned near the center of the defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with a bubble study showed injected air bubbles traversing the atrial septal defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
DISCUSSION
ASDs are typically uncovered on exam via auscultation of heart sounds, which might reveal a split of the second heart sound (S2) and diastolic murmurs. ASDs are typically classified by size, and their management depends on this factor, along with the patient’s age and symptoms. In children with small defects (<6 mm), treatment usually consists of conservative observation, as more than half of these ASDs will spontaneously close.3 But, as children age, they are more likely to engage in exertional activity (work, recreational sports) and an unrepaired ASD may yield symptoms (angina, dyspnea, fatigue, other cardiopulmonary strain). With such symptoms and when closure is not spontaneously achieved by adolescence or adulthood, an invasive approach is often necessary to correct the defect.
ASD repair. Traditionally, repair has involved some form of open thoracotomy. More recently, several minimally invasive techniques have been developed. Catheter-based device closure, in which a catheter is percutaneously guided to the defect and a patch is deployed to seal the ASD, is a technique that has been shown to successfully correct large ASDs of up to 40 mm in size.4 Robotic procedures have also been developed to correct ASDs through much smaller incisions.5 Both of these techniques require a significant rim of residual septal tissue around the defect.
Individualized approach. Since our patient had a rather large ASD that did not have sufficient residual septal rim tissue, percutaneous and robotic approaches were not feasible. Instead, he required more invasive cardiothoracic surgery. In cases such as this, the exact technique and type of incision (sternotomy vs access through the lateral chest wall) depend on age, gender, and the presence of other comorbidities.6
Our patient. Because there was concern that any approach other than a median one might not afford enough space to fix an ASD of such considerable size, our patient underwent a median sternotomy by a pediatric cardiothoracic surgeon who specialized in these repairs (in children as well as young adults). During the procedure, the ASD was accessed and confirmed to be as large as predicted by diagnostic imaging. A surgical patch was sutured in place to correct the defect. There were no intra-operative or postop complications.
Four weeks later, the patient had a mild pericardial effusion that was managed medically with daily furosemide and aspirin. At his 8-week postop appointment, the fluid accumulation had resolved, and he was completely asymptomatic. The patient returned to full-time active duty in the US Navy.
Adults with rather large ASDs can present in a relatively asymptomatic manner and report none of the classic complaints (angina, dyspnea, fatigue). They may even engage in heavy exertional activity with no difficulty. The underlying defect may be discovered incidentally on exam by noting a split of the S2 on auscultation. If pulmonary hypertension exists, the clinician may also note a loud S2. An exam that raises suspicion for an ASD can then be followed by tests that solidify the diagnosis. Surgery is usually necessary to correct an ASD in an adult who is symptomatic or exhibits significant cardiopulmonary strain.
THE CASE
A 25-year-old man, who was an active duty US Navy sailor, went to his ship’s medical department complaining of a mild cough that he’d had for 2 days. He denied having any fevers, chills, night sweats, angina, or dyspnea. He said he hadn’t experienced any exertional fatigue or difficulty completing the rigorous physical tasks of his occupation as an engineman on the ship. The patient had no medical or surgical history of significance, and he wasn’t taking any medications or supplements.
On exam, he was not in acute distress and his vital signs were within normal limits. Auscultation revealed mild wheezing throughout the upper lung fields and loud heart sounds throughout his chest that were audible even with gentle contact of the stethoscope diaphragm. He had no discernible murmurs, rubs, or gallops.

In light of the unusually loud heart sounds heard on exam, we performed an electrocardiogram. The EKG revealed a normal sinus rhythm, slight right axis deviation indicated by tall R-waves in V1 (also suggestive of right ventricular hypertrophy), an incomplete right bundle branch block, and a crochetage sign (a notch in the R-waves of the inferior leads).1 A chest x-ray (FIGURE 1) revealed a normal-sized heart and dilated pulmonary vasculature suggestive of pulmonary hypertension.


THE DIAGNOSIS
To further evaluate the cardiopulmonary findings, ultrasound studies (transthoracic and transesophageal echocardiography) were performed. These demonstrated a very large secundum-type atrial septal defect (ASD), measuring at its largest point about 30 × 48 mm (FIGURE 2 and FIGURE 3C). Doppler flow analysis and a bubble study (VIDEOS 1 and 2) demonstrated significant shunting across the ASD. Gated cardiac computed tomography (CT) was also used to characterize the ASD (FIGURE 3). It revealed that the superior and posterior rims of the ASD were essentially absent and that the right atrium and ventricle were severely enlarged, while the left chambers were normal in size and function with an ejection fraction >55%. The notching of the R-waves of the inferior leads, seen in our patient’s EKG, is typically seen with large ASDs.1,2
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with color Doppler flow (red) demonstrated significant shunting across a large atrial septal defect (white box). The largest white dot is positioned near the center of the defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with a bubble study showed injected air bubbles traversing the atrial septal defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
DISCUSSION
ASDs are typically uncovered on exam via auscultation of heart sounds, which might reveal a split of the second heart sound (S2) and diastolic murmurs. ASDs are typically classified by size, and their management depends on this factor, along with the patient’s age and symptoms. In children with small defects (<6 mm), treatment usually consists of conservative observation, as more than half of these ASDs will spontaneously close.3 But, as children age, they are more likely to engage in exertional activity (work, recreational sports) and an unrepaired ASD may yield symptoms (angina, dyspnea, fatigue, other cardiopulmonary strain). With such symptoms and when closure is not spontaneously achieved by adolescence or adulthood, an invasive approach is often necessary to correct the defect.
ASD repair. Traditionally, repair has involved some form of open thoracotomy. More recently, several minimally invasive techniques have been developed. Catheter-based device closure, in which a catheter is percutaneously guided to the defect and a patch is deployed to seal the ASD, is a technique that has been shown to successfully correct large ASDs of up to 40 mm in size.4 Robotic procedures have also been developed to correct ASDs through much smaller incisions.5 Both of these techniques require a significant rim of residual septal tissue around the defect.
Individualized approach. Since our patient had a rather large ASD that did not have sufficient residual septal rim tissue, percutaneous and robotic approaches were not feasible. Instead, he required more invasive cardiothoracic surgery. In cases such as this, the exact technique and type of incision (sternotomy vs access through the lateral chest wall) depend on age, gender, and the presence of other comorbidities.6
Our patient. Because there was concern that any approach other than a median one might not afford enough space to fix an ASD of such considerable size, our patient underwent a median sternotomy by a pediatric cardiothoracic surgeon who specialized in these repairs (in children as well as young adults). During the procedure, the ASD was accessed and confirmed to be as large as predicted by diagnostic imaging. A surgical patch was sutured in place to correct the defect. There were no intra-operative or postop complications.
Four weeks later, the patient had a mild pericardial effusion that was managed medically with daily furosemide and aspirin. At his 8-week postop appointment, the fluid accumulation had resolved, and he was completely asymptomatic. The patient returned to full-time active duty in the US Navy.
Adults with rather large ASDs can present in a relatively asymptomatic manner and report none of the classic complaints (angina, dyspnea, fatigue). They may even engage in heavy exertional activity with no difficulty. The underlying defect may be discovered incidentally on exam by noting a split of the S2 on auscultation. If pulmonary hypertension exists, the clinician may also note a loud S2. An exam that raises suspicion for an ASD can then be followed by tests that solidify the diagnosis. Surgery is usually necessary to correct an ASD in an adult who is symptomatic or exhibits significant cardiopulmonary strain.
1. Heller J, Hagège AA, Besse B, et al. “Crochetage” (notch) on R wave in inferior limb leads: a new independent electrocardiographic sign of atrial septal defect. J Am Coll Cardiol. 1996;27:877-882.
2. Kuijpers JM, Mulder BJM, Bouma BJ. Secundum atrial septal defect in adults: a practical review and recent developments. Neth Heart J. 2015;23:205-211.
3. McMahon CJ, Feltes TF, Fraley JK, et al. Natural history of growth of secundum atrial septal defects and implications for transcatheter closure. Heart. 2002;87:256-259.
4. Lopez K, Dalvi BV, Balzer D, et al. Transcatheter closure of large secundum atrial septal defects using the 40 mm amplatzer septal occluder: results of an international registry. Catheter Cardiovasc Interv. 2005;66:580-584.
5. Argenziano M, Oz MC, Kohmoto T, et al. Totally endoscopic atrial septal defect repair with robotic assistance. Circulation. 2003;108 Suppl 1:II191-II194.
6. Hopkins RA, Bert AA, Buchholz B, et al. Surgical patch closure of atrial septal defects. Ann Thorac Surg. 2004;77:2144-2149.
1. Heller J, Hagège AA, Besse B, et al. “Crochetage” (notch) on R wave in inferior limb leads: a new independent electrocardiographic sign of atrial septal defect. J Am Coll Cardiol. 1996;27:877-882.
2. Kuijpers JM, Mulder BJM, Bouma BJ. Secundum atrial septal defect in adults: a practical review and recent developments. Neth Heart J. 2015;23:205-211.
3. McMahon CJ, Feltes TF, Fraley JK, et al. Natural history of growth of secundum atrial septal defects and implications for transcatheter closure. Heart. 2002;87:256-259.
4. Lopez K, Dalvi BV, Balzer D, et al. Transcatheter closure of large secundum atrial septal defects using the 40 mm amplatzer septal occluder: results of an international registry. Catheter Cardiovasc Interv. 2005;66:580-584.
5. Argenziano M, Oz MC, Kohmoto T, et al. Totally endoscopic atrial septal defect repair with robotic assistance. Circulation. 2003;108 Suppl 1:II191-II194.
6. Hopkins RA, Bert AA, Buchholz B, et al. Surgical patch closure of atrial septal defects. Ann Thorac Surg. 2004;77:2144-2149.
