User login
Role of imaging in endometriosis
A 32-year-old woman presents with a history of pelvic pain, dysmenorrhea, dyspareunia, dyschezia, and dysuria, with exacerbation of the symptoms during her menstrual cycles. Her menarche occurred at the age of 13 and her menses are regular. She has never undergone surgery and has no relevant pathologic processes. She also reports that for the past 18 months she has been unsuccessfully trying to conceive.
Two months ago, she went to the emergency department because of an acute episode of severe pelvic pain associated with abdominal cramps, vomiting, and dyschezia, occurring at the beginning of her menstrual cycle. At that time, her vital signs were within normal limits, but deep palpation of the right iliac fossa was painful. On that occasion, acute abdomen and bowel obstruction were excluded.
Now, vaginal examination reveals a bluish, painful, bulky induration in the posterior fornix. Digital rectal examination reveals a circular infiltrated area in the anterior rectal wall. Her cancer antigen 125 (CA 125) level is 230 U/mL (normal range 0–35 U/mL).
MENSES-RELATED SYMPTOMS AND THE DIAGNOSIS OF ENDOMETRIOSIS
The diagnosis of endometriosis should be considered in the patient described above. Many of her signs and symptoms can be associated with several diseases. However, the diagnostic hypothesis points strongly toward endometriosis, since her symptoms recur at the beginning of every menstrual cycle.1
Endometriosis is the presence of endometrial tissue outside the uterine cavity. The affected organs usually include the ovaries, fallopian tubes,2 peritoneal surface, vagina, cervix, abdominal wall,3 scar tissue, pouch of Douglas, urinary tract, and bowel. However, any organ can be involved.
So-called deeply infiltrating endometriosis is an endometriotic lesion penetrating into the retroperitoneal space (most often affecting the uterosacral ligaments and the rectovaginal septum) or the pelvic-organ wall to a depth of at least 5 mm and involving structures such as the rectum, vagina, ureters, and bladder.4 Its clinical presentation is highly variable, ranging from no symptoms to severe pain and dysfunction of pelvic organs.
Endometriosis can be diagnosed with certainty only when the endometriotic lesions are observed by laparoscopy or laparotomy and after the histologic examination of surgically resected lesions (Figure 1).1 However, a presumptive diagnosis can be made on the basis of imaging findings, which can be useful in the differential diagnostic process (Table 1).
EXAMINATION AND BLOOD MARKERS PROVIDE LIMITED INFORMATION
Knowing the history of the patient, along with a physical examination that includes speculum and bimanual vaginal and rectal examination, can be helpful in the diagnostic process even if nothing abnormal is found.
Pelvic examination has a poor predictive value, as demonstrated in a study conducted by Nezhat et al5 in 91 patients with surgically confirmed endometriosis, 47% of whom had a normal bimanual examination.
CA 125 is the serologic marker most often used for diagnosing endometriosis. Levels are usually high in the sera of patients with endometriosis, especially in the advanced stages.6 However, levels increase both in the physiologic menstrual cycle and in epithelial ovarian cancers.7 Thus, the diagnostic value of CA 125 is limited in terms of both sensitivity and specificity.
INCLUDE IMAGING IN THE DIAGNOSTIC WORKUP
Surgical treatment is frequently offered to patients who have severe pelvic pain that does not respond to medical treatment, or in cases of infertility. Imaging investigations are mandatory both to ascertain the diagnosis and to assess involvement of internal organs before surgery. Moreover, imaging helps minimize the surgical risks.
The primary aim of the radiologic examination is to describe the precise location, the depth, and the number of pelvic endometriotic lesions. Furthermore, imaging is useful to check for endometriotic foci in pelvic organs such as the bowel, ureters, and bladder, which are often involved in the pathologic process.
Transvaginal ultrasonography and magnetic resonance imaging (MRI) can accurately delineate deeply infiltrating lesions of endometriosis that are not easily accessible laparoscopically.
Transvaginal ultrasonography
Transvaginal ultrasonography is the first-line imaging study when endometriosis is suspected: it is powerful, simple, widely available, and cost-effective. In particular, it is recommended for diagnosing endometriotic ovarian cysts (endometriomas)8,9 and endometriosis of the bladder.10 However, its value for the assessment of superficial peritoneal lesions, ovarian foci, and deeply infiltrating endometriosis is questionable.
Although uncomfortable for the patient, transvaginal ultrasonography should be performed during menses, or when the pain reaches its highest level. In fact, during menstrual bleeding the endometrial implants grow and become easier to detect.
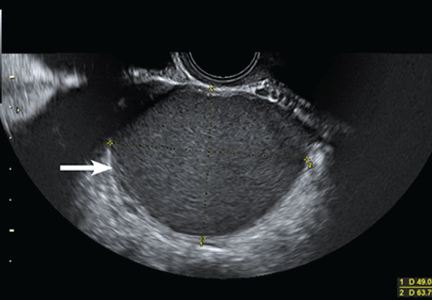
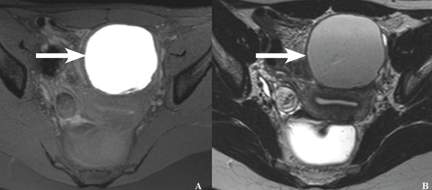
Mais et al8 reported that transvaginal ultrasonography has a sensitivity of 88% in differentiating endometriomas from other ovarian masses, and a specificity of 90% (Figure 2). Furthermore, its specificity is as high as that of MRI.8,9
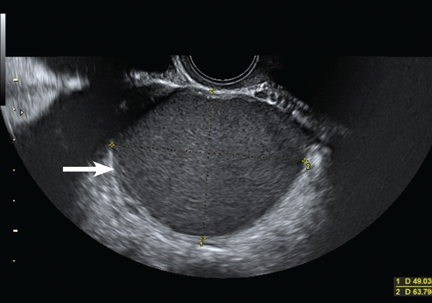
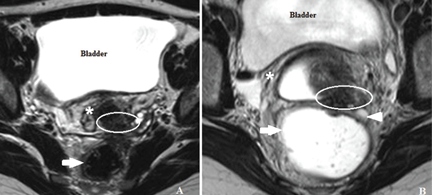
Endometriotic nodules detected in the uterosacral ligaments, rectovaginal septum, vagina, vesicouterine pouch, bladder (Figure 3), and ureters can be signs of deeply infiltrating endometriosis. Pelvic adhesions can be suspected when pelvic organs appear fixed to each other, when hyperechogenic plaques are found between the serosal surfaces of the different organs, and when the pouch of Douglas is partially or completely obliterated.
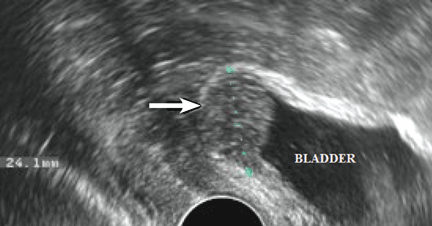
The accuracy of transvaginal ultrasonography strongly depends on the operator’s skill. Furthermore, lesions of the sigmoid colon are impossible to visualize by transvaginal ultrasonography; hence, further diagnostic procedures are required. Transvaginal ultrasonography is the most accurate technique in detecting endometriotic nodules of the bladder wall in patients with urinary symptoms.
Transvaginal ultrasonography combined with color Doppler can also demonstrate the flow of urine through the ureters to the bladder, thereby ascertaining the patency of the ureters and clarifying the anatomic relationship between the ureters and any endometriotic lesions in the detrusors.10 Hydronephrosis can arise from ureteral restriction caused by endometriotic nodules. Thus, transabdominal ultrasonography of the kidneys is always recommended when deeply infiltrating endometriosis is suspected.
Some centers use a bowel-preparation protocol consisting of a laxative taken 24 hours before the procedure, combined with a low-residue diet and an enema 1 hour before the examination to cleanse the rectosigmoid colon of fecal content and gas, which can interfere with the visual examination of the pelvic structures.11
Transabdominal ultrasonography
Transabdominal ultrasonography can be used instead of transvaginal ultrasonography, eg, in young girls and women who have never been sexually active. When transabdominal ultrasonography is selected, the patient should have a full bladder to maximize the visualization of the pelvic structures. However, transvaginal ultrasonography is generally more sensitive than transabdominal in detecting adnexal masses and pelvic nodules.12
Magnetic resonance imaging
MRI has been recently introduced in the diagnosis of endometriosis. MRI is less operator-dependent than transvaginal ultrasonography and is more sensitive for detecting foci of deeply infiltrating endometriosis, because of its ability to completely survey the anterior and posterior compartments of the pelvis. However, its diagnostic value in cases of bladder endometriosis, superficial peritoneal lesions, and ovarian foci is still controversial.13–16
On MRI, lesions of deeply infiltrating endometriosis mainly appear as areas or nodules with regular, irregular, indistinct, or stellate margins. A distortion of the normal pelvic anatomy or the detection of a loculated fluid collection can indirectly signal the presence of adhesions.
MRI has high specificity for the diagnosis of endometriomas as a result of its ability to detect aged hemorrhagic content (Figure 4).17 Despite the many studies that point to the limits of MRI in detecting small endometriotic lesions, recent studies demonstrated that MRI also has good sensitivity for small peritoneal implants and adhesions.18,19 The injection of gadolinium contrast is still a debatable measure, because contrast-enhanced imaging cannot differentiate infiltrating lesions from other normal fibromuscular pelvic anatomic structures.15,20
Bowel preparation can be done with an oral laxative the day before imaging, complemented by a low-residue diet. A single dose of a ready-to-use enema is given 30 minutes before the examination to cleanse the terminal section of the intestinal tract. To avoid motion artifacts caused by bowel peristalsis, images are obtained after intramuscular injections of a myorelaxant are given, if there is no contraindication. Bowel preparation is useful to eliminate fecal residue and gas, thereby allowing proper visualization of lesions of deeply infiltrating endometriosis, but it is not routinely prescribed in all centers.11
In most cases, endometriotic lesions have an MRI signal intensity that comes very close to that of the surrounding fibromuscular structures. In this regard, vaginal and rectal distention and opacification using ultrasonographic gel clearly help to delineate the cervix, vaginal fornices, and vaginal wall, as well as the rectum and wall of the rectosigmoid junction (Figure 5).20
PRESURGICAL IMAGING
Rectal endoscopic ultrasonography
Even though it should not be included in the routine diagnostic workup, rectal endoscopic ultrasonography, using a flexible echoendoscope, is suitable in certain presurgical cases. The aim of this imaging technique is to assess the depth of bowel wall infiltration thanks to the visualization of the different layers.21
Double-contrast barium enema and multislice computed tomography
Double-contrast barium enema is extensively used for the diagnosis of bowel endometriosis, once the decision to perform surgery has been made. It allows evaluation of the degree and length of the bowel occlusion at the level of the sigmoid or high rectosigmoid tract, but it does not permit differentiation of bowel endometriosis from other pathologies.
Multislice computed tomography offers the opportunity to evaluate the depth of the lesions with excellent precision. 22
The most relevant disadvantage of both procedures is the exposure of women of reproductive age to ionizing radiation. In addition, multislice computed tomography requires the administration of an intravenous iodinated contrast medium and a retrograde colonic distention with about 2 L of water.
- Attaran M, Falcone T, Goldberg J. Endometriosis: still tough to diagnose and treat. Cleve Clin J Med 2002; 69:647–653.
- Wenger JM, Soave I, Lo Monte G, Petignat P, Marci R. Tubal endometrioma within a twisted fallopian tube: a clinically complex diagnosis. J Pediatr Adolesc Gynecol 2013; 26:e1–e4.
- Marci R, Lo Monte G, Soave I, Bianchi A, Patella A, Wenger JM. Rectus abdominis muscle endometriotic mass in a woman affected by multiple sclerosis. J Obstet Gynaecol Res 2013; 39:462–465.
- Vercellini P, Frontino G, Pietropaolo G, Gattei U, Daguati R, Crosignani PG. Deep endometriosis: definition, pathogenesis, and clinical management. J Am Assoc Gynecol Laparosc 2004; 11:153–161.
- Nezhat C, Santolaya J, Nezhat FR. Comparison of transvaginal sonography and bimanual pelvic examination in patients with laparoscopically confirmed endometriosis. J Am Assoc Gynecol Laparosc 1994; 1:127–130.
- Barbieri RL, Niloff JM, Bast RC, Scaetzl E, Kistner RW, Knapp RC. Elevated serum concentrations of CA-125 in patients with advanced endometriosis. Fertil Steril 1986; 45:630–634.
- Bon GG, Kenemans P, Dekker JJ, et al. Fluctuations in CA 125 and CA 15-3 serum concentrations during spontaneous ovulatory cycles. Hum Reprod 1999; 14:566–570.
- Mais V, Guerriero S, Ajossa S, Angiolucci M, Paoletti AM, Melis GB. The efficiency of transvaginal ultrasonography in the diagnosis of endometrioma. Fertil Steril 1993; 60:776–780.
- Guerriero S, Mais V, Ajossa S, et al. The role of endovaginal ultrasound in differentiating endometriomas from other ovarian cysts. Clin Exp Obstet Gynecol 1995; 22:20–22.
- Fedele L, Bianchi S, Raffaelli R, Portuese A. Pre-operative assessment of bladder endometriosis. Hum Reprod 1997; 12:2519–2522.
- Chamié LP, Blasbalg R, Pereira RM, Warmbrand G, Serafini PC. Findings of pelvic endometriosis at transvaginal US, MR imaging, and laparoscopy. Radiographics 2011; 31:E77–E100.
- Fleischer AC. Transabdominal and transvaginal sonography of ovarian masses. Clin Obstet Gynecol 1991; 34:433–442.
- Zawin M, McCarthy S, Scoutt L, Comite F. Endometriosis: appearance and detection at MR imaging. Radiology 1989; 171:693–696.
- Togashi K, Nishimura K, Kimura I, et al. Endometrial cysts: diagnosis with MR imaging. Radiology 1991; 180:73–78.
- Balleyguier C, Chapron C, Dubuisson JB, et al. Comparison of magnetic resonance imaging and transvaginal ultrasonography in diagnosing bladder endometriosis. J Am Assoc Gynecol Laparosc 2002; 9:15–23.
- Siegelman ES, Oliver ER. MR imaging of endometriosis: ten imaging pearls. Radiographics 2012; 32:1675–1691.
- Takeuchi M, Matsuzaki K, Kubo H, Nishitani H. Magnetic resonance manifestations of endometrial cysts at 3 T compared with 1.5 T. J Comput Assist Tomogr 2008; 32:369–271.
- Zanardi R, Del Frate C, Zuiani C, Del Frate G, Bazzocchi M. Staging of pelvic endometriosis using magnetic resonance imaging compared with the laparoscopic classification of the American Fertility Society: a prospective study. Radiol Med 2003; 105:326–338.
- Takahashi K, Okada M, Okada S, Kitao M, Imaoka I, Sugimura K. Studies on the detection of small endometrial implants by magnetic resonance imaging using a fat saturation technique. Gynecol Obstet Invest 1996; 41:203–206.
- Loubeyre P, Copercini M, Frossard JL, Wenger JM, Petignat P. Pictorial review: rectosigmoid endometriosis on MRI with gel opacification after rectosigmoid colon cleansing. Clin Imaging 2012; 36:295–300.
- Bahr A, de Parades V, Gadonneix P, et al. Endorectal ultrasonography in predicting rectal wall infiltration in patients with deep pelvic endometriosis: a modern tool for an ancient disease. Dis Colon Rectum 2006; 49:869–875.
- Biscaldi E, Ferrero S, Remorgida V, Rollandi GA. Bowel endometriosis: CT-enteroclysis. Abdom Imaging 2007; 32:441–450.
A 32-year-old woman presents with a history of pelvic pain, dysmenorrhea, dyspareunia, dyschezia, and dysuria, with exacerbation of the symptoms during her menstrual cycles. Her menarche occurred at the age of 13 and her menses are regular. She has never undergone surgery and has no relevant pathologic processes. She also reports that for the past 18 months she has been unsuccessfully trying to conceive.
Two months ago, she went to the emergency department because of an acute episode of severe pelvic pain associated with abdominal cramps, vomiting, and dyschezia, occurring at the beginning of her menstrual cycle. At that time, her vital signs were within normal limits, but deep palpation of the right iliac fossa was painful. On that occasion, acute abdomen and bowel obstruction were excluded.
Now, vaginal examination reveals a bluish, painful, bulky induration in the posterior fornix. Digital rectal examination reveals a circular infiltrated area in the anterior rectal wall. Her cancer antigen 125 (CA 125) level is 230 U/mL (normal range 0–35 U/mL).
MENSES-RELATED SYMPTOMS AND THE DIAGNOSIS OF ENDOMETRIOSIS
The diagnosis of endometriosis should be considered in the patient described above. Many of her signs and symptoms can be associated with several diseases. However, the diagnostic hypothesis points strongly toward endometriosis, since her symptoms recur at the beginning of every menstrual cycle.1
Endometriosis is the presence of endometrial tissue outside the uterine cavity. The affected organs usually include the ovaries, fallopian tubes,2 peritoneal surface, vagina, cervix, abdominal wall,3 scar tissue, pouch of Douglas, urinary tract, and bowel. However, any organ can be involved.
So-called deeply infiltrating endometriosis is an endometriotic lesion penetrating into the retroperitoneal space (most often affecting the uterosacral ligaments and the rectovaginal septum) or the pelvic-organ wall to a depth of at least 5 mm and involving structures such as the rectum, vagina, ureters, and bladder.4 Its clinical presentation is highly variable, ranging from no symptoms to severe pain and dysfunction of pelvic organs.
Endometriosis can be diagnosed with certainty only when the endometriotic lesions are observed by laparoscopy or laparotomy and after the histologic examination of surgically resected lesions (Figure 1).1 However, a presumptive diagnosis can be made on the basis of imaging findings, which can be useful in the differential diagnostic process (Table 1).

EXAMINATION AND BLOOD MARKERS PROVIDE LIMITED INFORMATION
Knowing the history of the patient, along with a physical examination that includes speculum and bimanual vaginal and rectal examination, can be helpful in the diagnostic process even if nothing abnormal is found.
Pelvic examination has a poor predictive value, as demonstrated in a study conducted by Nezhat et al5 in 91 patients with surgically confirmed endometriosis, 47% of whom had a normal bimanual examination.
CA 125 is the serologic marker most often used for diagnosing endometriosis. Levels are usually high in the sera of patients with endometriosis, especially in the advanced stages.6 However, levels increase both in the physiologic menstrual cycle and in epithelial ovarian cancers.7 Thus, the diagnostic value of CA 125 is limited in terms of both sensitivity and specificity.
INCLUDE IMAGING IN THE DIAGNOSTIC WORKUP
Surgical treatment is frequently offered to patients who have severe pelvic pain that does not respond to medical treatment, or in cases of infertility. Imaging investigations are mandatory both to ascertain the diagnosis and to assess involvement of internal organs before surgery. Moreover, imaging helps minimize the surgical risks.
The primary aim of the radiologic examination is to describe the precise location, the depth, and the number of pelvic endometriotic lesions. Furthermore, imaging is useful to check for endometriotic foci in pelvic organs such as the bowel, ureters, and bladder, which are often involved in the pathologic process.
Transvaginal ultrasonography and magnetic resonance imaging (MRI) can accurately delineate deeply infiltrating lesions of endometriosis that are not easily accessible laparoscopically.
Transvaginal ultrasonography
Transvaginal ultrasonography is the first-line imaging study when endometriosis is suspected: it is powerful, simple, widely available, and cost-effective. In particular, it is recommended for diagnosing endometriotic ovarian cysts (endometriomas)8,9 and endometriosis of the bladder.10 However, its value for the assessment of superficial peritoneal lesions, ovarian foci, and deeply infiltrating endometriosis is questionable.
Although uncomfortable for the patient, transvaginal ultrasonography should be performed during menses, or when the pain reaches its highest level. In fact, during menstrual bleeding the endometrial implants grow and become easier to detect.
Mais et al8 reported that transvaginal ultrasonography has a sensitivity of 88% in differentiating endometriomas from other ovarian masses, and a specificity of 90% (Figure 2). Furthermore, its specificity is as high as that of MRI.8,9

Endometriotic nodules detected in the uterosacral ligaments, rectovaginal septum, vagina, vesicouterine pouch, bladder (Figure 3), and ureters can be signs of deeply infiltrating endometriosis. Pelvic adhesions can be suspected when pelvic organs appear fixed to each other, when hyperechogenic plaques are found between the serosal surfaces of the different organs, and when the pouch of Douglas is partially or completely obliterated.

The accuracy of transvaginal ultrasonography strongly depends on the operator’s skill. Furthermore, lesions of the sigmoid colon are impossible to visualize by transvaginal ultrasonography; hence, further diagnostic procedures are required. Transvaginal ultrasonography is the most accurate technique in detecting endometriotic nodules of the bladder wall in patients with urinary symptoms.
Transvaginal ultrasonography combined with color Doppler can also demonstrate the flow of urine through the ureters to the bladder, thereby ascertaining the patency of the ureters and clarifying the anatomic relationship between the ureters and any endometriotic lesions in the detrusors.10 Hydronephrosis can arise from ureteral restriction caused by endometriotic nodules. Thus, transabdominal ultrasonography of the kidneys is always recommended when deeply infiltrating endometriosis is suspected.
Some centers use a bowel-preparation protocol consisting of a laxative taken 24 hours before the procedure, combined with a low-residue diet and an enema 1 hour before the examination to cleanse the rectosigmoid colon of fecal content and gas, which can interfere with the visual examination of the pelvic structures.11
Transabdominal ultrasonography
Transabdominal ultrasonography can be used instead of transvaginal ultrasonography, eg, in young girls and women who have never been sexually active. When transabdominal ultrasonography is selected, the patient should have a full bladder to maximize the visualization of the pelvic structures. However, transvaginal ultrasonography is generally more sensitive than transabdominal in detecting adnexal masses and pelvic nodules.12
Magnetic resonance imaging
MRI has been recently introduced in the diagnosis of endometriosis. MRI is less operator-dependent than transvaginal ultrasonography and is more sensitive for detecting foci of deeply infiltrating endometriosis, because of its ability to completely survey the anterior and posterior compartments of the pelvis. However, its diagnostic value in cases of bladder endometriosis, superficial peritoneal lesions, and ovarian foci is still controversial.13–16
On MRI, lesions of deeply infiltrating endometriosis mainly appear as areas or nodules with regular, irregular, indistinct, or stellate margins. A distortion of the normal pelvic anatomy or the detection of a loculated fluid collection can indirectly signal the presence of adhesions.
MRI has high specificity for the diagnosis of endometriomas as a result of its ability to detect aged hemorrhagic content (Figure 4).17 Despite the many studies that point to the limits of MRI in detecting small endometriotic lesions, recent studies demonstrated that MRI also has good sensitivity for small peritoneal implants and adhesions.18,19 The injection of gadolinium contrast is still a debatable measure, because contrast-enhanced imaging cannot differentiate infiltrating lesions from other normal fibromuscular pelvic anatomic structures.15,20

Bowel preparation can be done with an oral laxative the day before imaging, complemented by a low-residue diet. A single dose of a ready-to-use enema is given 30 minutes before the examination to cleanse the terminal section of the intestinal tract. To avoid motion artifacts caused by bowel peristalsis, images are obtained after intramuscular injections of a myorelaxant are given, if there is no contraindication. Bowel preparation is useful to eliminate fecal residue and gas, thereby allowing proper visualization of lesions of deeply infiltrating endometriosis, but it is not routinely prescribed in all centers.11
In most cases, endometriotic lesions have an MRI signal intensity that comes very close to that of the surrounding fibromuscular structures. In this regard, vaginal and rectal distention and opacification using ultrasonographic gel clearly help to delineate the cervix, vaginal fornices, and vaginal wall, as well as the rectum and wall of the rectosigmoid junction (Figure 5).20

PRESURGICAL IMAGING
Rectal endoscopic ultrasonography
Even though it should not be included in the routine diagnostic workup, rectal endoscopic ultrasonography, using a flexible echoendoscope, is suitable in certain presurgical cases. The aim of this imaging technique is to assess the depth of bowel wall infiltration thanks to the visualization of the different layers.21
Double-contrast barium enema and multislice computed tomography
Double-contrast barium enema is extensively used for the diagnosis of bowel endometriosis, once the decision to perform surgery has been made. It allows evaluation of the degree and length of the bowel occlusion at the level of the sigmoid or high rectosigmoid tract, but it does not permit differentiation of bowel endometriosis from other pathologies.
Multislice computed tomography offers the opportunity to evaluate the depth of the lesions with excellent precision. 22
The most relevant disadvantage of both procedures is the exposure of women of reproductive age to ionizing radiation. In addition, multislice computed tomography requires the administration of an intravenous iodinated contrast medium and a retrograde colonic distention with about 2 L of water.
A 32-year-old woman presents with a history of pelvic pain, dysmenorrhea, dyspareunia, dyschezia, and dysuria, with exacerbation of the symptoms during her menstrual cycles. Her menarche occurred at the age of 13 and her menses are regular. She has never undergone surgery and has no relevant pathologic processes. She also reports that for the past 18 months she has been unsuccessfully trying to conceive.
Two months ago, she went to the emergency department because of an acute episode of severe pelvic pain associated with abdominal cramps, vomiting, and dyschezia, occurring at the beginning of her menstrual cycle. At that time, her vital signs were within normal limits, but deep palpation of the right iliac fossa was painful. On that occasion, acute abdomen and bowel obstruction were excluded.
Now, vaginal examination reveals a bluish, painful, bulky induration in the posterior fornix. Digital rectal examination reveals a circular infiltrated area in the anterior rectal wall. Her cancer antigen 125 (CA 125) level is 230 U/mL (normal range 0–35 U/mL).
MENSES-RELATED SYMPTOMS AND THE DIAGNOSIS OF ENDOMETRIOSIS
The diagnosis of endometriosis should be considered in the patient described above. Many of her signs and symptoms can be associated with several diseases. However, the diagnostic hypothesis points strongly toward endometriosis, since her symptoms recur at the beginning of every menstrual cycle.1
Endometriosis is the presence of endometrial tissue outside the uterine cavity. The affected organs usually include the ovaries, fallopian tubes,2 peritoneal surface, vagina, cervix, abdominal wall,3 scar tissue, pouch of Douglas, urinary tract, and bowel. However, any organ can be involved.
So-called deeply infiltrating endometriosis is an endometriotic lesion penetrating into the retroperitoneal space (most often affecting the uterosacral ligaments and the rectovaginal septum) or the pelvic-organ wall to a depth of at least 5 mm and involving structures such as the rectum, vagina, ureters, and bladder.4 Its clinical presentation is highly variable, ranging from no symptoms to severe pain and dysfunction of pelvic organs.
Endometriosis can be diagnosed with certainty only when the endometriotic lesions are observed by laparoscopy or laparotomy and after the histologic examination of surgically resected lesions (Figure 1).1 However, a presumptive diagnosis can be made on the basis of imaging findings, which can be useful in the differential diagnostic process (Table 1).

EXAMINATION AND BLOOD MARKERS PROVIDE LIMITED INFORMATION
Knowing the history of the patient, along with a physical examination that includes speculum and bimanual vaginal and rectal examination, can be helpful in the diagnostic process even if nothing abnormal is found.
Pelvic examination has a poor predictive value, as demonstrated in a study conducted by Nezhat et al5 in 91 patients with surgically confirmed endometriosis, 47% of whom had a normal bimanual examination.
CA 125 is the serologic marker most often used for diagnosing endometriosis. Levels are usually high in the sera of patients with endometriosis, especially in the advanced stages.6 However, levels increase both in the physiologic menstrual cycle and in epithelial ovarian cancers.7 Thus, the diagnostic value of CA 125 is limited in terms of both sensitivity and specificity.
INCLUDE IMAGING IN THE DIAGNOSTIC WORKUP
Surgical treatment is frequently offered to patients who have severe pelvic pain that does not respond to medical treatment, or in cases of infertility. Imaging investigations are mandatory both to ascertain the diagnosis and to assess involvement of internal organs before surgery. Moreover, imaging helps minimize the surgical risks.
The primary aim of the radiologic examination is to describe the precise location, the depth, and the number of pelvic endometriotic lesions. Furthermore, imaging is useful to check for endometriotic foci in pelvic organs such as the bowel, ureters, and bladder, which are often involved in the pathologic process.
Transvaginal ultrasonography and magnetic resonance imaging (MRI) can accurately delineate deeply infiltrating lesions of endometriosis that are not easily accessible laparoscopically.
Transvaginal ultrasonography
Transvaginal ultrasonography is the first-line imaging study when endometriosis is suspected: it is powerful, simple, widely available, and cost-effective. In particular, it is recommended for diagnosing endometriotic ovarian cysts (endometriomas)8,9 and endometriosis of the bladder.10 However, its value for the assessment of superficial peritoneal lesions, ovarian foci, and deeply infiltrating endometriosis is questionable.
Although uncomfortable for the patient, transvaginal ultrasonography should be performed during menses, or when the pain reaches its highest level. In fact, during menstrual bleeding the endometrial implants grow and become easier to detect.
Mais et al8 reported that transvaginal ultrasonography has a sensitivity of 88% in differentiating endometriomas from other ovarian masses, and a specificity of 90% (Figure 2). Furthermore, its specificity is as high as that of MRI.8,9

Endometriotic nodules detected in the uterosacral ligaments, rectovaginal septum, vagina, vesicouterine pouch, bladder (Figure 3), and ureters can be signs of deeply infiltrating endometriosis. Pelvic adhesions can be suspected when pelvic organs appear fixed to each other, when hyperechogenic plaques are found between the serosal surfaces of the different organs, and when the pouch of Douglas is partially or completely obliterated.

The accuracy of transvaginal ultrasonography strongly depends on the operator’s skill. Furthermore, lesions of the sigmoid colon are impossible to visualize by transvaginal ultrasonography; hence, further diagnostic procedures are required. Transvaginal ultrasonography is the most accurate technique in detecting endometriotic nodules of the bladder wall in patients with urinary symptoms.
Transvaginal ultrasonography combined with color Doppler can also demonstrate the flow of urine through the ureters to the bladder, thereby ascertaining the patency of the ureters and clarifying the anatomic relationship between the ureters and any endometriotic lesions in the detrusors.10 Hydronephrosis can arise from ureteral restriction caused by endometriotic nodules. Thus, transabdominal ultrasonography of the kidneys is always recommended when deeply infiltrating endometriosis is suspected.
Some centers use a bowel-preparation protocol consisting of a laxative taken 24 hours before the procedure, combined with a low-residue diet and an enema 1 hour before the examination to cleanse the rectosigmoid colon of fecal content and gas, which can interfere with the visual examination of the pelvic structures.11
Transabdominal ultrasonography
Transabdominal ultrasonography can be used instead of transvaginal ultrasonography, eg, in young girls and women who have never been sexually active. When transabdominal ultrasonography is selected, the patient should have a full bladder to maximize the visualization of the pelvic structures. However, transvaginal ultrasonography is generally more sensitive than transabdominal in detecting adnexal masses and pelvic nodules.12
Magnetic resonance imaging
MRI has been recently introduced in the diagnosis of endometriosis. MRI is less operator-dependent than transvaginal ultrasonography and is more sensitive for detecting foci of deeply infiltrating endometriosis, because of its ability to completely survey the anterior and posterior compartments of the pelvis. However, its diagnostic value in cases of bladder endometriosis, superficial peritoneal lesions, and ovarian foci is still controversial.13–16
On MRI, lesions of deeply infiltrating endometriosis mainly appear as areas or nodules with regular, irregular, indistinct, or stellate margins. A distortion of the normal pelvic anatomy or the detection of a loculated fluid collection can indirectly signal the presence of adhesions.
MRI has high specificity for the diagnosis of endometriomas as a result of its ability to detect aged hemorrhagic content (Figure 4).17 Despite the many studies that point to the limits of MRI in detecting small endometriotic lesions, recent studies demonstrated that MRI also has good sensitivity for small peritoneal implants and adhesions.18,19 The injection of gadolinium contrast is still a debatable measure, because contrast-enhanced imaging cannot differentiate infiltrating lesions from other normal fibromuscular pelvic anatomic structures.15,20

Bowel preparation can be done with an oral laxative the day before imaging, complemented by a low-residue diet. A single dose of a ready-to-use enema is given 30 minutes before the examination to cleanse the terminal section of the intestinal tract. To avoid motion artifacts caused by bowel peristalsis, images are obtained after intramuscular injections of a myorelaxant are given, if there is no contraindication. Bowel preparation is useful to eliminate fecal residue and gas, thereby allowing proper visualization of lesions of deeply infiltrating endometriosis, but it is not routinely prescribed in all centers.11
In most cases, endometriotic lesions have an MRI signal intensity that comes very close to that of the surrounding fibromuscular structures. In this regard, vaginal and rectal distention and opacification using ultrasonographic gel clearly help to delineate the cervix, vaginal fornices, and vaginal wall, as well as the rectum and wall of the rectosigmoid junction (Figure 5).20

PRESURGICAL IMAGING
Rectal endoscopic ultrasonography
Even though it should not be included in the routine diagnostic workup, rectal endoscopic ultrasonography, using a flexible echoendoscope, is suitable in certain presurgical cases. The aim of this imaging technique is to assess the depth of bowel wall infiltration thanks to the visualization of the different layers.21
Double-contrast barium enema and multislice computed tomography
Double-contrast barium enema is extensively used for the diagnosis of bowel endometriosis, once the decision to perform surgery has been made. It allows evaluation of the degree and length of the bowel occlusion at the level of the sigmoid or high rectosigmoid tract, but it does not permit differentiation of bowel endometriosis from other pathologies.
Multislice computed tomography offers the opportunity to evaluate the depth of the lesions with excellent precision. 22
The most relevant disadvantage of both procedures is the exposure of women of reproductive age to ionizing radiation. In addition, multislice computed tomography requires the administration of an intravenous iodinated contrast medium and a retrograde colonic distention with about 2 L of water.
- Attaran M, Falcone T, Goldberg J. Endometriosis: still tough to diagnose and treat. Cleve Clin J Med 2002; 69:647–653.
- Wenger JM, Soave I, Lo Monte G, Petignat P, Marci R. Tubal endometrioma within a twisted fallopian tube: a clinically complex diagnosis. J Pediatr Adolesc Gynecol 2013; 26:e1–e4.
- Marci R, Lo Monte G, Soave I, Bianchi A, Patella A, Wenger JM. Rectus abdominis muscle endometriotic mass in a woman affected by multiple sclerosis. J Obstet Gynaecol Res 2013; 39:462–465.
- Vercellini P, Frontino G, Pietropaolo G, Gattei U, Daguati R, Crosignani PG. Deep endometriosis: definition, pathogenesis, and clinical management. J Am Assoc Gynecol Laparosc 2004; 11:153–161.
- Nezhat C, Santolaya J, Nezhat FR. Comparison of transvaginal sonography and bimanual pelvic examination in patients with laparoscopically confirmed endometriosis. J Am Assoc Gynecol Laparosc 1994; 1:127–130.
- Barbieri RL, Niloff JM, Bast RC, Scaetzl E, Kistner RW, Knapp RC. Elevated serum concentrations of CA-125 in patients with advanced endometriosis. Fertil Steril 1986; 45:630–634.
- Bon GG, Kenemans P, Dekker JJ, et al. Fluctuations in CA 125 and CA 15-3 serum concentrations during spontaneous ovulatory cycles. Hum Reprod 1999; 14:566–570.
- Mais V, Guerriero S, Ajossa S, Angiolucci M, Paoletti AM, Melis GB. The efficiency of transvaginal ultrasonography in the diagnosis of endometrioma. Fertil Steril 1993; 60:776–780.
- Guerriero S, Mais V, Ajossa S, et al. The role of endovaginal ultrasound in differentiating endometriomas from other ovarian cysts. Clin Exp Obstet Gynecol 1995; 22:20–22.
- Fedele L, Bianchi S, Raffaelli R, Portuese A. Pre-operative assessment of bladder endometriosis. Hum Reprod 1997; 12:2519–2522.
- Chamié LP, Blasbalg R, Pereira RM, Warmbrand G, Serafini PC. Findings of pelvic endometriosis at transvaginal US, MR imaging, and laparoscopy. Radiographics 2011; 31:E77–E100.
- Fleischer AC. Transabdominal and transvaginal sonography of ovarian masses. Clin Obstet Gynecol 1991; 34:433–442.
- Zawin M, McCarthy S, Scoutt L, Comite F. Endometriosis: appearance and detection at MR imaging. Radiology 1989; 171:693–696.
- Togashi K, Nishimura K, Kimura I, et al. Endometrial cysts: diagnosis with MR imaging. Radiology 1991; 180:73–78.
- Balleyguier C, Chapron C, Dubuisson JB, et al. Comparison of magnetic resonance imaging and transvaginal ultrasonography in diagnosing bladder endometriosis. J Am Assoc Gynecol Laparosc 2002; 9:15–23.
- Siegelman ES, Oliver ER. MR imaging of endometriosis: ten imaging pearls. Radiographics 2012; 32:1675–1691.
- Takeuchi M, Matsuzaki K, Kubo H, Nishitani H. Magnetic resonance manifestations of endometrial cysts at 3 T compared with 1.5 T. J Comput Assist Tomogr 2008; 32:369–271.
- Zanardi R, Del Frate C, Zuiani C, Del Frate G, Bazzocchi M. Staging of pelvic endometriosis using magnetic resonance imaging compared with the laparoscopic classification of the American Fertility Society: a prospective study. Radiol Med 2003; 105:326–338.
- Takahashi K, Okada M, Okada S, Kitao M, Imaoka I, Sugimura K. Studies on the detection of small endometrial implants by magnetic resonance imaging using a fat saturation technique. Gynecol Obstet Invest 1996; 41:203–206.
- Loubeyre P, Copercini M, Frossard JL, Wenger JM, Petignat P. Pictorial review: rectosigmoid endometriosis on MRI with gel opacification after rectosigmoid colon cleansing. Clin Imaging 2012; 36:295–300.
- Bahr A, de Parades V, Gadonneix P, et al. Endorectal ultrasonography in predicting rectal wall infiltration in patients with deep pelvic endometriosis: a modern tool for an ancient disease. Dis Colon Rectum 2006; 49:869–875.
- Biscaldi E, Ferrero S, Remorgida V, Rollandi GA. Bowel endometriosis: CT-enteroclysis. Abdom Imaging 2007; 32:441–450.
- Attaran M, Falcone T, Goldberg J. Endometriosis: still tough to diagnose and treat. Cleve Clin J Med 2002; 69:647–653.
- Wenger JM, Soave I, Lo Monte G, Petignat P, Marci R. Tubal endometrioma within a twisted fallopian tube: a clinically complex diagnosis. J Pediatr Adolesc Gynecol 2013; 26:e1–e4.
- Marci R, Lo Monte G, Soave I, Bianchi A, Patella A, Wenger JM. Rectus abdominis muscle endometriotic mass in a woman affected by multiple sclerosis. J Obstet Gynaecol Res 2013; 39:462–465.
- Vercellini P, Frontino G, Pietropaolo G, Gattei U, Daguati R, Crosignani PG. Deep endometriosis: definition, pathogenesis, and clinical management. J Am Assoc Gynecol Laparosc 2004; 11:153–161.
- Nezhat C, Santolaya J, Nezhat FR. Comparison of transvaginal sonography and bimanual pelvic examination in patients with laparoscopically confirmed endometriosis. J Am Assoc Gynecol Laparosc 1994; 1:127–130.
- Barbieri RL, Niloff JM, Bast RC, Scaetzl E, Kistner RW, Knapp RC. Elevated serum concentrations of CA-125 in patients with advanced endometriosis. Fertil Steril 1986; 45:630–634.
- Bon GG, Kenemans P, Dekker JJ, et al. Fluctuations in CA 125 and CA 15-3 serum concentrations during spontaneous ovulatory cycles. Hum Reprod 1999; 14:566–570.
- Mais V, Guerriero S, Ajossa S, Angiolucci M, Paoletti AM, Melis GB. The efficiency of transvaginal ultrasonography in the diagnosis of endometrioma. Fertil Steril 1993; 60:776–780.
- Guerriero S, Mais V, Ajossa S, et al. The role of endovaginal ultrasound in differentiating endometriomas from other ovarian cysts. Clin Exp Obstet Gynecol 1995; 22:20–22.
- Fedele L, Bianchi S, Raffaelli R, Portuese A. Pre-operative assessment of bladder endometriosis. Hum Reprod 1997; 12:2519–2522.
- Chamié LP, Blasbalg R, Pereira RM, Warmbrand G, Serafini PC. Findings of pelvic endometriosis at transvaginal US, MR imaging, and laparoscopy. Radiographics 2011; 31:E77–E100.
- Fleischer AC. Transabdominal and transvaginal sonography of ovarian masses. Clin Obstet Gynecol 1991; 34:433–442.
- Zawin M, McCarthy S, Scoutt L, Comite F. Endometriosis: appearance and detection at MR imaging. Radiology 1989; 171:693–696.
- Togashi K, Nishimura K, Kimura I, et al. Endometrial cysts: diagnosis with MR imaging. Radiology 1991; 180:73–78.
- Balleyguier C, Chapron C, Dubuisson JB, et al. Comparison of magnetic resonance imaging and transvaginal ultrasonography in diagnosing bladder endometriosis. J Am Assoc Gynecol Laparosc 2002; 9:15–23.
- Siegelman ES, Oliver ER. MR imaging of endometriosis: ten imaging pearls. Radiographics 2012; 32:1675–1691.
- Takeuchi M, Matsuzaki K, Kubo H, Nishitani H. Magnetic resonance manifestations of endometrial cysts at 3 T compared with 1.5 T. J Comput Assist Tomogr 2008; 32:369–271.
- Zanardi R, Del Frate C, Zuiani C, Del Frate G, Bazzocchi M. Staging of pelvic endometriosis using magnetic resonance imaging compared with the laparoscopic classification of the American Fertility Society: a prospective study. Radiol Med 2003; 105:326–338.
- Takahashi K, Okada M, Okada S, Kitao M, Imaoka I, Sugimura K. Studies on the detection of small endometrial implants by magnetic resonance imaging using a fat saturation technique. Gynecol Obstet Invest 1996; 41:203–206.
- Loubeyre P, Copercini M, Frossard JL, Wenger JM, Petignat P. Pictorial review: rectosigmoid endometriosis on MRI with gel opacification after rectosigmoid colon cleansing. Clin Imaging 2012; 36:295–300.
- Bahr A, de Parades V, Gadonneix P, et al. Endorectal ultrasonography in predicting rectal wall infiltration in patients with deep pelvic endometriosis: a modern tool for an ancient disease. Dis Colon Rectum 2006; 49:869–875.
- Biscaldi E, Ferrero S, Remorgida V, Rollandi GA. Bowel endometriosis: CT-enteroclysis. Abdom Imaging 2007; 32:441–450.
KEY POINTS
- The diagnostic evaluation should always start with transvaginal ultrasonography of the pelvic structures followed by magnetic resonance imaging, especially if deeply infiltrating endometriosis is suspected.
- An inaccurate imaging evaluation may lead to an incomplete excision of lesions if the patient undergoes surgery.
- Transvaginal ultrasonography and magnetic resonance imaging allow the assessment of the size, location, and extent of the lesions.
- Given the multifocal nature of the disease, a thorough evaluation of all pelvic structures, including the bowel, the bladder, and the ureters, is always recommended.
Preserving fertility in female cancer patients: A snapshot of the options
In the last few decades, the survival rates have improved in many of the malignancies that affect young adults. This progress has made fertility preservation and quality of life after cancer treatment important, most of all in survivors of childhood cancers.
Men who are about to undergo cancer treatment can bank their sperm, but as yet no analogous noninvasive option is available for women. The most studied methods are often invasive and require the woman to take large doses of hormones. They may also necessitate a delay in starting cancer treatment.
Which method of fertility preservation a woman should choose depends on several factors, including the type of disease, the treatment required, the age of the patient, whether she has a long-term partner, and whether treatment can be delayed.
Chemotherapy and radiotherapy have well-known deleterious effects on female reproductive function. Many studies have shown that acute loss of growing follicles within the ovary and resultant premature ovarian failure often follow chemotherapy. This ovarian damage has long-term consequences, such as shortened reproductive life span and hormone deficiency.1
Fertility preservation requires a team effort. It should be managed by an oncology center that has built a close collaboration between oncologists, fertility specialists, psychologists, and primary care physicians to allow early discussion and to offer a full range of options to these patients.
The aim of this paper is to discuss the current options for preserving fertility in female cancer patients who have to undergo gonadotoxic cancer treatment.
FEMALE FERTILITY AND ASSESSMENT OF OVARIAN RESERVE
At birth, baby girls have about 1 million primordial ovarian follicles, which is the most they will ever have. By the time they reach menarche, this number has declined to 180,000, and at menopause only about 1,000 remain.2
Throughout a woman’s reproductive life, the number of oocytes remaining—both primordial follicles and the relatively small number of maturing, growing follicles—is called her ovarian reserve. When cancer is diagnosed, we need to assess the patient’s ovarian reserve to direct the discussion about the need for fertility preservation.
In search of markers of ovarian reserve
Fertility experts have been looking for clinical biomarkers that could give us an estimate of the number of nongrowing follicles and, consequently, of the ovarian reserve.
All methods used for the assessment of ovarian reserve actually provide an indirect determination of the remaining pool of oocytes. In clinical practice, a high blood level of follicle-stimulating hormone (FSH) on cycle day 3 (>15 mU/mL) or a low level of anti-Müllerian hormone (AMH) (<1 ng/mL) is generally associated with a low ovarian reserve.
The serum FSH level is the marker most commonly used, but it varies widely at different times in the menstrual cycle.
The FSH test is usually performed on day 3 of the menstrual cycle, when the estrogen level is expected to be low due to negative feedback. The FSH result needs to be combined with the estradiol level, especially in those patients with irregular menstruation or in cases of amenorrhea. A random FSH test is considered valid if the detected estrogen level is low. Women undergoing in vitro fertilization with a day 3 FSH lower than 15 mIU/mL are more likely to conceive than women with a higher FSH level.
AMH and antral follicles. Recently, two other variables have been introduced in clinical practice: the AMH concentration and the antral follicle count (the number of small antral follicles within the ovary) as assessed by transvaginal ultrasonography.
AMH is released by the granulosa cells of small, growing follicles. Because its level is much more stable over the menstrual cycle than the FSH level, it can be measured on any day of the cycle.3 In cancer survivors, AMH is particularly useful in demonstrating the degree of ovarian tissue damage induced by radiotherapy and chemotherapy and in evaluating the ovarian reserve.4
A reduced number of antral follicles causes a diminished AMH production, which becomes undetectable with menopause. The AMH level is strongly associated with the basal antral follicle count. Various threshold values (0.2–1.26 ng/mL) have been used to identify women with low ovarian reserve.
HOW CANCER TREATMENT DAMAGES THE OVARIES
Advances in surgery, radiotherapy, and chemotherapy have significantly improved the prognosis for young cancer patients. However, cancer treatments often result in ovarian dysfunction, and premature menopause and irreversible sterility are the most dramatic outcomes. The resulting low estrogen levels, in addition to their physiologic consequences, also worsen quality of life through psychological effects, which can as well influence the patient’s compliance with treatment.
Chemotherapy: Alkylating agents are the most gonadotoxic
The mechanism by which chemotherapy affects ovarian function is poorly understood. Histologically, chemotherapeutic drugs could lead to ovarian atrophy and stromal fibrosis, to depletion of the primordial follicle stockpile, and to reduced ovarian weight, resulting in ovarian dysfunction.5
The patient's age correlates with the probability of ovarian damage or, inversely, ovarian resistance to chemotherapy. Young women have more primordial oocytes, and after chemotherapy they face a sharp reduction of their ovarian reserve. Still, younger patients show a lower rate of ovarian toxicity than older women.6
The type and the cumulative dose of cytotoxic agents used are other important variables.7 There are six main classes of chemotherapeutic drugs: alkylating agents, platinum derivatives, antibiotics, antimetabolites, plant alkaloids, and taxanes. They all affect ovarian function, but alkylating agents are the most gonadotoxic.
Alkylating agents covalently bind an alkyl group to the DNA molecule and inhibit it from replicating. In the ovaries they directly injure primordial oocytes and deplete follicles. 8 They also seriously damage the ovarian vasculature so that the follicles cannot grow.9 Their destructive effect on the primordial follicles is dose-dependent and varies with the age and developmental maturity of the patient at the time of the therapy, with older women more likely to be left infertile afterward.10
Cyclophosphamide is an alkylating agent often used to treat severe manifestations of autoimmune diseases such as systemic lupus erythematosus, BehÇet disease, steroid-resistant glomerulonephritis, inflammatory bowel disease, pemphigus vulgaris, and others. Because it can lead to premature ovarian failure and infertility, women receiving cyclophosphamide for autoimmune conditions may also need treatment to preserve fertility.11
Radiotherapy
Ovarian follicles are remarkably vulnerable to damage from ionizing radiation, which results in ovarian atrophy and reduced follicle stores.1
The risk of radiotherapy-induced infertility is closely related to the patient’s age and developmental maturity at the time of therapy and to dose fractionation and the extent of the irradiation field. Every patient has a different sensitivity to radiation damage that is probably genetically predetermined, but age seems to be the most important variable. Wo and Viswanathan12 used a mathematical model devised by Wallace et al13 to show that the older the patient, the lower the radiation dose necessary to impair ovarian function.
The irradiation field is another aspect to consider. Pelvic radiation can be necessary in rectal cancer, cervical cancer, and lymphoma. In these cases, surgically moving the ovaries to a region outside the radiation field could be an option to minimize radiotherapy-induced ovarian damage.14
Radiotherapy can also damage the uterus. Pelvic irradiation can reduce uterine volume, alter uterine elasticity through myometrial fibrosis, and modify vascularization and endometrial thickness.15,16 These alterations are closely correlated with the total radiation dose, the site of irradiation, and the patient’s age at the time of the treatment, the prepubertal uterus being more susceptible to damage. 15,17 Larsen et al15 found that girls who received uterine irradiation before puberty had lower uterine volumes in adulthood than girls who received chemotherapy alone or radiation to other parts of the body, and that the younger the patient at the time of radiotherapy, the smaller the uterus later. These effects could result in adverse pregnancy outcomes.
Furthermore, radiation could also damage the uterine vasculature. Holm et al16 used ultrasonography to evaluate the effect of total-body irradiation and found that uterine volume and uterine blood flow were both impaired.15
All these possible alterations could lead to a reduced uterine response to cytotrophoblast invasion and to decreased fetoplacental blood flow, which could impair embryonic and fetal growth. In that case, a surrogate pregnancy would be the only method to achieve parenthood using the couple’s gametes.
OPTIONS FOR FERTILITY PRESERVATION
Assisted reproductive technology
Young women diagnosed with an oncologic disease may wish to use assisted reproductive technology (Table 1) to keep open the possibility of having children at a later date. One approach is to harvest oocytes, fertilize them in vitro, and deep-freeze (cryopreserve) the resulting embryos to be thawed and implanted later. Alternatively, oocytes can be frozen directly, although success rates are lower with this method. And another approach is to obtain and cryopreserve ovarian tissue. If none of these is possible, oocytes may be obtained from a donor.
Controlled ovarian stimulation
If oocytes are to be harvested, a number of them should be harvested at one time.
There is not an optimal number of oocytes that should be retrieved, but cryopreservation of a large number of oocytes allows us to perform multiple attempts at in vitro fertilization, improving the chances of pregnancy. The fertilization rate (defined as the total number of zygotes at the 2-pronucleus stage divided by the number of fertilized oocytes) with intracytoplasmic sperm injection is 70% to 80%. On average, for every 10 eggs, 7 to 8 eggs will normally be fertilized. The implantation rate (defined as the total number of pregnancies divided by the total number of embryo transferred) with intracytoplasmic sperm injection is 40% to 50%—ie, only half of the transferred embryos will successfully implant and result in a pregnancy.
So that more than one ripe egg can be obtained at a time, the patient must undergo a regimen of controlled ovarian stimulation to achieve multifollicular growth. Stimulation protocols are based on giving pituitary hormones, ie, analogues of gonadotropin-releasing hormone (GnRH) (both agonists and antagonists), followed by recombinant FSH or human menopausal gonadotropin to promote follicular development. A single dose of human chorionic gonadotropin (hCG) is given to induce ovulation when the lead follicles have reached 18 to 20 mm in size.18
Many oncologists consider controlled ovarian stimulation dangerous for cancer patients because it takes time and thus delays cancer treatment. Furthermore, the regimen boosts the levels of circulating estrogens, which could be harmful in patients with hormone-dependent tumors.19
Oocytes can also be retrieved during unstimulated cycles. This avoids increasing estrogen concentrations above the physiologic level, but no more than a single oocyte is collected per cycle. The patient could undergo multiple oocyte harvestings, one per cycle, but this would delay her cancer treatment even more—by months—which is not recommended.
Serious efforts to minimize estrogen production during controlled ovarian stimulation have been made, although further studies are needed. Research is under way to develop appropriate ovarian stimulation protocols based on drugs with antiestrogenic effects.
Tamoxifen, an antagonist of the estrogen receptor, is widely used in breast cancer treatment. 20 It can also be given during controlled ovarian stimulation because it promotes follicular growth and induces ovulation.21 Oktay et al22 found that the embryo yield was 2.5 times higher in women with breast cancer treated with tamoxifen than in a retrospective control group consisting of breast cancer patients attempting natural-cycle oocyte retrieval.
Letrozole, an aromatase inhibitor, is also commonly used in treating breast and ovarian cancer. Aromatase is an enzyme that catalyzes the conversion of androgenic precursors to estrogens, and it is found in many tissues, including granulosa cells. Several studies report the use of letrozole, alone or in combination with low doses of recombinant FSH, in ovarian stimulation protocols in cancer patients, with positive clinical outcomes.23
Tamoxifen or letrozole, combined with recombinant FSH, is an attractive option in a controlled ovarian stimulation protocol for cancer patients,24 although further investigation is needed.
Cryopreservation methods
Two main protocols are currently used to freeze oocytes, embryos, and ovarian tissue: slow freezing and vitrification.
In the slow-freezing method, cryoprotectant agents are used to draw water out of the cells, raising intracellular viscosity without (or with minimal) intracellular ice crystal formation, while the sample is cooled slowly in a controlled manner. These cryoprotectant chemicals lower the freezing point of the solution, allowing greater cellular dehydration during the slow freezing. They also protect the plasmatic cell membrane by changing its physical state from liquid to partially dry. To avoid excessive deformation that could damage the cytoplasmic structures, cryoprotectant agents are added in successive stages.25
Vitrification is a newer method that uses higher concentrations of cryoprotectants and flash freezing. The instant drop in temperature converts this highly concentrated solution from an aqueous state to a semisolid, amorphous state that does not contain ice crystals, which are the main cause of damage during the freezing process.26 Since most cryoprotectant agents are extremely toxic, it is necessary to minimize the time that oocytes, embryos, and ovarian tissue are exposed to them.
Cryopreservation of embryos
According to the American Society of Clinical Oncology and the American Society of Reproductive Medicine, embryo freezing is the most established method for fertility preservation, with tangible and widely reported success.27 In normal practice, oocytes are retrieved after a controlled ovarian stimulation and then fertilized in vitro. Then they are treated with cryoprotectant agents, frozen, and stored. Upon demand, embryos can be thawed and implanted.
The Society for Assisted Reproductive Technology reports that the current live birth rate per transfer using thawed embryos from nondonor oocytes in US women under age 35 is 38.7%; at age 35 to 37 it is 35.1%.28 In women with cancer, storing as many embryos as possible could help improve embryo survival and the implantation rate. The optimal time to perform embryo cryopreservation is still being discussed,29 but, as in women without cancer, it is commonly done 3 to 5 days after fertilization.
In the past few years, the use of vitrification has greatly increased, as the post-thawing survival rates and pregnancy rates are higher with this method than with slow freezing.30
Despite its success, embryo cryopreservation has important drawbacks. First, the patient must be of reproductive age and have a partner or accept the use of donor sperm. Second, most patients undergo controlled ovarian stimulation before oocyte retrieval, causing a delay in starting cancer treatment, which is not acceptable in many cases. Moreover, the high serum estrogen levels caused by ovarian stimulation may be contraindicated in women with estrogen-sensitive malignancies.19
Oocyte cryopreservation
Cryopreservation of oocytes avoids the need for sperm and, thus, may be offered to more patients than embryo cryopreservation. In addition, it may circumvent ethical or legal considerations associated with embryo freezing, such as ownership of reproductive material.
However, oocytes are more difficult to cryopreserve than embryos. Indeed, ice crystals frequently form inside and outside the cells, damaging the cell membrane and the meiotic spindle. In routine practice, mature oocytes in metaphase II are used for cryopreservation. Metaphase II oocytes are large cells that contain a delicate meiotic spindle. Moreover, their cytoplasm contains a higher proportion of water than other cells, which could affect their viability after freezing and thawing due to ice crystal formation. In addition, cryopreservation could be responsible for hardening of the zona pellucida (through diffuse thickening of the cell membrane), adversely affecting fertilization rates.31
Significant improvements were achieved in fertilization of cryopreserved oocytes with intracytoplasmic sperm injection. Nevertheless, there are still concerns regarding oocyte cryopreservation. Further studies are needed to determine the risk of aneuploidy caused by damage to the meiotic spindle after oocyte cryopreservation. The potentially detrimental effects of high cryopreservant concentrations used in vitrification also need to be investigated.
In vitro maturation
A novel fertility preservation strategy involves collecting immature oocytes from primordial follicles in unstimulated cycles and then letting them mature in vitro.
To date, immature oocytes retrieved at the germinal vesicle stage can be cryopreserved with vitrification followed by in vitro maturation after thawing, but several studies have demonstrated that better results are obtained when vitrification follows the in vitro maturation process.32
Compared with mature oocytes, immature oocytes are less susceptible to damage during cryopreservation and thus have a better chance of surviving freezing and thawing, thanks to some peculiar characteristics: they are small, have few organelles, lack a zona pellucida, have low metabolic activity, and are in a state of relative quiescence.33 Controlled ovarian stimulation is not necessary, so this procedure preserves fertility without delaying the start of cancer treatment.
Patients are usually evaluated with transvaginal ultrasonography in the early follicular phase of the menstrual cycle (between day 2 and day 4) to count and measure the antral follicles. Immature oocytes are collected when the leading follicle has reached 10 to 12 mm in size and 36 hours after a subcutaneous injection of hCG.34 The retrieved oocytes are then incubated for 24 to 48 hours in a special medium supplemented with FSH and luteinizing hormone (LH). Immature oocytes can also be collected in the luteal phase.
This option could be considered when cancer treatment cannot be delayed for conventional follicular-phase retrieval35 or in case of a premature LH surge during ovarian stimulation. 36 It should be offered to patients facing infertility related to cancer treatment only after appropriate counseling and as a part of a clinical study.
Ovarian tissue cryopreservation
Cryopreservation of ovarian tissue is an experimental but highly promising technique for preserving fertility. Like oocyte cryopreservation, it avoids the need for hormonal stimulation and the need to delay cancer treatment. It may also be the only possible option for prepubertal girls, as well as for women who cannot postpone their cancer treatment. Although it is still experimental, it has obtained progressively better results: after having ovarian tissue preserved, thawed, and subsequently reimplanted in the same position or in a different part of the body, some patients transiently resumed having menstrual cycles and endocrine activity and in a very few cases achieved pregnancy.37
Ovarian tissue for cryopreservation is usually taken via laparoscopic surgery, unless the patient has to undergo open laparotomy for another indication. Laparoscopic surgery offers significant advantages, such as the possibility of performing it on short notice without delaying oncologic therapy. Considering that women at age 30 have about 35 primordial follicles per square millimeter of ovarian tissue, 5 cubic fragments 5 mm wide may be sufficient to obtain more than 4,000 primordial follicles.38 In cases in which complete ovariectomy is necessary, it is possible to remove and cryopreserve fragments of normal ovarian tissue located at the margins of the surgical specimen. Ovarian tissue withdrawal can also be performed in pediatric patients and during other surgical procedures.
The most studied method of cryopreserving ovarian tissue is slow freezing, but the use of vitrification is increasing. This technique was initially carried out in order to preserve the largest number of primordial follicles, but in recent years the possibility of cryopreserving the whole ovary with or without its vascular pedicle has also been studied. Martinez-Madrid et al39 described a protocol of cryopreserving the entire ovary with its stem and found it possible to reach a follicular survival rate of 75%, preserving vessels and stromal structure.39
Currently, the most promising approach seems to be the transplantation of the ovarian tissue back into the donor, ie, autotransplantation. This avoids the need for immunosuppression.
The location can be either orthotopic or heterotopic. In orthotopic transplantation the tissue is placed back in its original location. In theory, the patient could then become pregnant in the usual way if the rest of the reproductive system is not damaged.
In heterotopic transplantation the tissue is placed in a different location, usually easily accessible, like the forearm or the subcutaneous abdominal area. Heterotopic transplants have been shown to be able to restore ovarian function, but not to give pregnancies after oocyte collection.40 Indeed, the pregnancies obtained after transplantation came from autografts of ovarian cortex in orthotopic sites like the fossa ovarica or the remnant ovary.
With autotransplantation, there is a high risk of transmission of metastatic cancer cells. Blood-bone cancers such as leukemia and lymphomas are likely to be associated with the highest risk of ovarian metastasis through transplantation of thawed cryopreserved ovarian tissue. Neuroblastoma and breast cancer are associated with a moderate risk of metastasis to the ovaries. Ovarian involvement is extremely rare in Wilms tumor, lymphomas (except for Burkitt lymphoma), osteosarcomas, Ewing sarcoma, and extragenital rhabdomyosarcoma. Squamous cell cervical cancer metastasizes to the ovaries in fewer than 0.2% of cases, even in the most advanced stages. Histologic evaluation of ovarian samples before transplantation has been proposed to prevent cancer transmission, although it is not possible to completely abolish this risk.41,42 This jeopardy could potentially be eliminated by in vitro maturation of immature oocytes collected from cryopreserved ovarian tissue.
Despite significant advances, to date there have been fewer than 20 babies born worldwide through this method.43
Oocyte donation
Assisted reproduction techniques also include in vitro fertilization using a sperm sample from the partner and oocytes from a donor. The embryos obtained are then transferred, saving the woman from ovarian stimulation without any delay in starting the cancer treatment. Although this method has a high success rate, it inevitably raises personal considerations.
OVARIAN TRANSPOSITION
When a woman of childbearing age needs radiation treatment for a pelvic malignancy, transposition of the ovaries above the pelvic brim outside the radiation field (oophoropexy) should be considered before starting therapy. It is indicated in patients diagnosed with malignancies that require pelvic radiation but not the removal of the ovaries. It can be performed during surgical treatment of the tumor or as a separate laparoscopic procedure. The radiation dose that the transposed ovaries receive is considerably less than that in ovaries left in place.
Laparoscopic ovarian transposition is highly effective. However, the risk involved in the surgical procedure should not be underestimated. The most important complications are vascular injury, infarction of the fallopian tube, and ovarian cyst formation.44
PHARMACOLOGIC PROTECTION
Some drugs induce a state of ovarian quiescence similar to menopause. Can they be used during chemotherapy to protect the ovaries, allowing restoration of normal ovarian function and natural fertility after cancer treatment and preventing premature ovarian failure?
GnRH analogues slow the cellular activity of the gonads, in theory making them less sensitive to damage by cytotoxic agents. Initially, the release of gonadotropins is stimulated (flare-up effect), but after 10 to 15 days pituitary GnRH receptors are down-regulated by internalization of receptors. Since chemotherapy affects mainly actively dividing cells such as mature ovarian follicles, the use of the analogues is based on the assumption that by reducing FSH levels, follicles will remain quiescent, decreasing their sensitivity to the gonadotoxic effect of chemotherapy.
In a randomized study of 281 patients with early breast cancer, Del Mastro et al45 reported a reduction in the occurrence of early menopause in those treated with a GnRH analogue during chemotherapy after 1 year of follow-up. However, the debate regarding the effect of GnRH analogues on the fertility of cancer patients is still open and needs further investigation.
In recent years, research has focused on imatinib, a new, potentially protective drug,46 but a lot of work still needs to be done. To date, the use of GnRH analogues is not recommended outside of clinical studies, and it should be offered only after careful counseling about other options to preserve fertility.
- Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol 2010; 53:727–739.
- Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One 2010; 5:e8772.
- van Disseldorp J, Lambalk CB, Kwee J, et al. Comparison of inter- and intra-cycle variability of anti-Mullerian hormone and antral follicle counts. Hum Reprod 2010; 25:221–227.
- Lie Fong S, Laven JS, Hakvoort-Cammel FG, et al. Assessment of ovarian reserve in adult childhood cancer survivors using anti-Müllerian hormone. Hum Reprod 2009; 24:982–990.
- Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 2007; 110:2222–2229.
- Partridge AH, Ruddy KJ, Gelber S, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril 2010; 94:638–644.
- Lee SJ, Schover LR, Partridge AH, et al; American Society of Clinical Oncology. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006; 24:2917–2931.
- Familiari G, Caggiati A, Nottola SA, Ermini M, Di Benedetto MR, Motta PM. Ultrastructure of human ovarian primordial follicles after combination chemotherapy for Hodgkin’s disease. Hum Reprod 1993; 8:2080–2087.
- Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor Trap R1R2. Endocrinology 2002; 143:2797–2807.
- Aubard Y, Piver P, Pech JC, Galinat S, Teissier MP. Ovarian tissue cryopreservation and gynecologic oncology: a review. Eur J Obstet Gynecol Reprod Biol 2001; 97:5–14.
- Elizur SE, Chian RC, Pineau CA, et al. Fertility preservation treatment for young women with autoimmune diseases facing treatment with gonadotoxic agents. Rheumatology (Oxford) 2008; 47:1506–1509.
- Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys 2009; 73:1304–1312.
- Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys 2005; 62:738–744.
- Gareer W, Gad Z, Gareer H. Needle oophoropexy: a new simple technique for ovarian transposition prior to pelvic irradiation. Surg Endosc 2011; 25:2241–2246.
- Larsen EC, Schmiegelow K, Rechnitzer C, Loft A, Müller J, Andersen AN. Radiotherapy at a young age reduces uterine volume of childhood cancer survivors. Acta Obstet Gynecol Scand 2004; 83:96–102.
- Holm K, Nysom K, Brocks V, Hertz H, Jacobsen N, Müller J. Ultrasound B-mode changes in the uterus and ovaries and Doppler changes in the uterus after total body irradiation and allogeneic bone marrow transplantation in childhood. Bone Marrow Transplant 1999; 23:259–263.
- Critchley HO, Wallace WH, Shalet SM, Mamtora H, Higginson J, Anderson DC. Abdominal irradiation in childhood; the potential for pregnancy. Br J Obstet Gynaecol 1992; 99:392–394.
- Goldberg JM, Falcone T, Attaran M. In vitro fertilization update. Cleve Clin J Med 2007; 74:329–338.
- Prest SJ, May FE, Westley BR. The estrogen-regulated protein, TFF1, stimulates migration of human breast cancer cells. FASEB J 2002; 16:592–594.
- Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Early Breast Cancer Trialists’ Collaborative Group. Lancet 1992; 339:71–85.
- Klopper A, Hall M. New synthetic agent for the induction of ovulation: preliminary trials in women. Br Med J 1971; 1:152–154.
- Oktay K, Buyuk E, Davis O, Yermakova I, Veeck L, Rosenwaks Z. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod 2003; 18:90–95.
- Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol 2005; 23:4347–4353.
- Oktay K. Further evidence on the safety and success of ovarian stimulation with letrozole and tamoxifen in breast cancer patients undergoing in vitro fertilization to cryopreserve their embryos for fertility preservation. J Clin Oncol 2005; 23:3858–3859.
- Gosden R. Cryopreservation: a cold look at technology for fertility preservation. Fertil Steril 2011; 96:264–268.
- Chen SU, Chien CL, Wu MY, et al. Novel direct cover vitrification for cryopreservation of ovarian tissues increases follicle viability and pregnancy capability in mice. Hum Reprod 2006; 21:2794–2800.
- Lee SJ, Schover LR, Partridge AH, et al; American Society of Clinical Oncology. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006; 24:2917–2931.
- Society for Assisted Reproductive Technology (SART). http://www.sart.org. Accessed January 3, 2013.
- Granne I, Child T, Hartshorne G; British Fertility Society. Embryo cryopreservation: evidence for practice. Hum Fertil (Camb) 2008; 11:159–172.
- Loutradi KE, Kolibianakis EM, Venetis CA, et al. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril 2008; 90:186–193.
- Ko CS, Ding DC, Chu TW, et al. Changes to the meiotic spindle and zona pellucida of mature mouse oocytes following different cryopreservation methods. Anim Reprod Sci 2008; 105:272–282.
- Cao Y, Xing Q, Zhang ZG, et al. Cryopreservation of immature and in vitro-matured human oocytes by vitrification. Reprod Biomed Online 2009; 19:369–373.
- Toth TL, Baka SG, Veeck LL, Jones HW, Muasher S, Lanzendorf SE. Fertilization and in vitro development of cryopreserved human prophase I oocytes. Fertil Steril 1994; 61:891–894.
- Chian RC, Gülekli B, Buckett WM, Tan SL. Priming with human chorionic gonadotropin before retrieval of immature oocytes in women with infertility due to the polycystic ovary syndrome. N Engl J Med 1999; 341: 1624,1626.
- Maman E, Meirow D, Brengauz M, Raanani H, Dor J, Hourvitz A. Luteal phase oocyte retrieval and in vitro maturation is an optional procedure for urgent fertility preservation. Fertil Steril 2011; 95:64–67.
- Oktay K, Demirtas E, Son WY, Lostritto K, Chian RC, Tan SL. In vitro maturation of germinal vesicle oocytes recovered after premature luteinizing hormone surge: description of a novel approach to fertility preservation. Fertil Steril 2008; 89:228.e19–e22.
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004; 364:1405–1410.
- Martinez-Madrid B, Dolmans MM, Van Langendonckt A, Defrère S, Donnez J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril 2004; 82:1390–1394.
- Martinez-Madrid B, Camboni A, Dolmans MM, Nottola S, Van Langendonckt A, Donnez J. Apoptosis and ultrastructural assessment after cryopreservation of whole human ovaries with their vascular pedicle. Fertil Steril 2007; 87:1153–1165.
- Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA 2001; 286:1490–1493.
- Practice Committee of American Society for Reproductive Medicine. Ovarian tissue and oocyte cryopreservation. Fertil Steril 2008; 90(suppl 5):S241–S246.
- Oktay K. Ovarian tissue cryopreservation and transplantation: preliminary findings and implications for cancer patients. Hum Reprod Update 2001; 7:526–534.
- Donnez J, Silber S, Andersen CY, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med 2011; 43:437–450.
- Terenziani M, Piva L, Meazza C, Gandola L, Cefalo G, Merola M. Oophoropexy: a relevant role in preservation of ovarian function after pelvic irradiation. Fertil Steril 2009; 91:935.e15–e16.
- Del Mastro L, Boni L, Michelotti A, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA 2011; 306:269–276.
- Gonfloni S, Di Tella L, Caldarola S, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med 2009; 15:1179–1185.
In the last few decades, the survival rates have improved in many of the malignancies that affect young adults. This progress has made fertility preservation and quality of life after cancer treatment important, most of all in survivors of childhood cancers.
Men who are about to undergo cancer treatment can bank their sperm, but as yet no analogous noninvasive option is available for women. The most studied methods are often invasive and require the woman to take large doses of hormones. They may also necessitate a delay in starting cancer treatment.
Which method of fertility preservation a woman should choose depends on several factors, including the type of disease, the treatment required, the age of the patient, whether she has a long-term partner, and whether treatment can be delayed.
Chemotherapy and radiotherapy have well-known deleterious effects on female reproductive function. Many studies have shown that acute loss of growing follicles within the ovary and resultant premature ovarian failure often follow chemotherapy. This ovarian damage has long-term consequences, such as shortened reproductive life span and hormone deficiency.1
Fertility preservation requires a team effort. It should be managed by an oncology center that has built a close collaboration between oncologists, fertility specialists, psychologists, and primary care physicians to allow early discussion and to offer a full range of options to these patients.
The aim of this paper is to discuss the current options for preserving fertility in female cancer patients who have to undergo gonadotoxic cancer treatment.
FEMALE FERTILITY AND ASSESSMENT OF OVARIAN RESERVE
At birth, baby girls have about 1 million primordial ovarian follicles, which is the most they will ever have. By the time they reach menarche, this number has declined to 180,000, and at menopause only about 1,000 remain.2
Throughout a woman’s reproductive life, the number of oocytes remaining—both primordial follicles and the relatively small number of maturing, growing follicles—is called her ovarian reserve. When cancer is diagnosed, we need to assess the patient’s ovarian reserve to direct the discussion about the need for fertility preservation.
In search of markers of ovarian reserve
Fertility experts have been looking for clinical biomarkers that could give us an estimate of the number of nongrowing follicles and, consequently, of the ovarian reserve.
All methods used for the assessment of ovarian reserve actually provide an indirect determination of the remaining pool of oocytes. In clinical practice, a high blood level of follicle-stimulating hormone (FSH) on cycle day 3 (>15 mU/mL) or a low level of anti-Müllerian hormone (AMH) (<1 ng/mL) is generally associated with a low ovarian reserve.
The serum FSH level is the marker most commonly used, but it varies widely at different times in the menstrual cycle.
The FSH test is usually performed on day 3 of the menstrual cycle, when the estrogen level is expected to be low due to negative feedback. The FSH result needs to be combined with the estradiol level, especially in those patients with irregular menstruation or in cases of amenorrhea. A random FSH test is considered valid if the detected estrogen level is low. Women undergoing in vitro fertilization with a day 3 FSH lower than 15 mIU/mL are more likely to conceive than women with a higher FSH level.
AMH and antral follicles. Recently, two other variables have been introduced in clinical practice: the AMH concentration and the antral follicle count (the number of small antral follicles within the ovary) as assessed by transvaginal ultrasonography.
AMH is released by the granulosa cells of small, growing follicles. Because its level is much more stable over the menstrual cycle than the FSH level, it can be measured on any day of the cycle.3 In cancer survivors, AMH is particularly useful in demonstrating the degree of ovarian tissue damage induced by radiotherapy and chemotherapy and in evaluating the ovarian reserve.4
A reduced number of antral follicles causes a diminished AMH production, which becomes undetectable with menopause. The AMH level is strongly associated with the basal antral follicle count. Various threshold values (0.2–1.26 ng/mL) have been used to identify women with low ovarian reserve.
HOW CANCER TREATMENT DAMAGES THE OVARIES
Advances in surgery, radiotherapy, and chemotherapy have significantly improved the prognosis for young cancer patients. However, cancer treatments often result in ovarian dysfunction, and premature menopause and irreversible sterility are the most dramatic outcomes. The resulting low estrogen levels, in addition to their physiologic consequences, also worsen quality of life through psychological effects, which can as well influence the patient’s compliance with treatment.
Chemotherapy: Alkylating agents are the most gonadotoxic
The mechanism by which chemotherapy affects ovarian function is poorly understood. Histologically, chemotherapeutic drugs could lead to ovarian atrophy and stromal fibrosis, to depletion of the primordial follicle stockpile, and to reduced ovarian weight, resulting in ovarian dysfunction.5
The patient's age correlates with the probability of ovarian damage or, inversely, ovarian resistance to chemotherapy. Young women have more primordial oocytes, and after chemotherapy they face a sharp reduction of their ovarian reserve. Still, younger patients show a lower rate of ovarian toxicity than older women.6
The type and the cumulative dose of cytotoxic agents used are other important variables.7 There are six main classes of chemotherapeutic drugs: alkylating agents, platinum derivatives, antibiotics, antimetabolites, plant alkaloids, and taxanes. They all affect ovarian function, but alkylating agents are the most gonadotoxic.
Alkylating agents covalently bind an alkyl group to the DNA molecule and inhibit it from replicating. In the ovaries they directly injure primordial oocytes and deplete follicles. 8 They also seriously damage the ovarian vasculature so that the follicles cannot grow.9 Their destructive effect on the primordial follicles is dose-dependent and varies with the age and developmental maturity of the patient at the time of the therapy, with older women more likely to be left infertile afterward.10
Cyclophosphamide is an alkylating agent often used to treat severe manifestations of autoimmune diseases such as systemic lupus erythematosus, BehÇet disease, steroid-resistant glomerulonephritis, inflammatory bowel disease, pemphigus vulgaris, and others. Because it can lead to premature ovarian failure and infertility, women receiving cyclophosphamide for autoimmune conditions may also need treatment to preserve fertility.11
Radiotherapy
Ovarian follicles are remarkably vulnerable to damage from ionizing radiation, which results in ovarian atrophy and reduced follicle stores.1
The risk of radiotherapy-induced infertility is closely related to the patient’s age and developmental maturity at the time of therapy and to dose fractionation and the extent of the irradiation field. Every patient has a different sensitivity to radiation damage that is probably genetically predetermined, but age seems to be the most important variable. Wo and Viswanathan12 used a mathematical model devised by Wallace et al13 to show that the older the patient, the lower the radiation dose necessary to impair ovarian function.
The irradiation field is another aspect to consider. Pelvic radiation can be necessary in rectal cancer, cervical cancer, and lymphoma. In these cases, surgically moving the ovaries to a region outside the radiation field could be an option to minimize radiotherapy-induced ovarian damage.14
Radiotherapy can also damage the uterus. Pelvic irradiation can reduce uterine volume, alter uterine elasticity through myometrial fibrosis, and modify vascularization and endometrial thickness.15,16 These alterations are closely correlated with the total radiation dose, the site of irradiation, and the patient’s age at the time of the treatment, the prepubertal uterus being more susceptible to damage. 15,17 Larsen et al15 found that girls who received uterine irradiation before puberty had lower uterine volumes in adulthood than girls who received chemotherapy alone or radiation to other parts of the body, and that the younger the patient at the time of radiotherapy, the smaller the uterus later. These effects could result in adverse pregnancy outcomes.
Furthermore, radiation could also damage the uterine vasculature. Holm et al16 used ultrasonography to evaluate the effect of total-body irradiation and found that uterine volume and uterine blood flow were both impaired.15
All these possible alterations could lead to a reduced uterine response to cytotrophoblast invasion and to decreased fetoplacental blood flow, which could impair embryonic and fetal growth. In that case, a surrogate pregnancy would be the only method to achieve parenthood using the couple’s gametes.
OPTIONS FOR FERTILITY PRESERVATION
Assisted reproductive technology
Young women diagnosed with an oncologic disease may wish to use assisted reproductive technology (Table 1) to keep open the possibility of having children at a later date. One approach is to harvest oocytes, fertilize them in vitro, and deep-freeze (cryopreserve) the resulting embryos to be thawed and implanted later. Alternatively, oocytes can be frozen directly, although success rates are lower with this method. And another approach is to obtain and cryopreserve ovarian tissue. If none of these is possible, oocytes may be obtained from a donor.
Controlled ovarian stimulation
If oocytes are to be harvested, a number of them should be harvested at one time.
There is not an optimal number of oocytes that should be retrieved, but cryopreservation of a large number of oocytes allows us to perform multiple attempts at in vitro fertilization, improving the chances of pregnancy. The fertilization rate (defined as the total number of zygotes at the 2-pronucleus stage divided by the number of fertilized oocytes) with intracytoplasmic sperm injection is 70% to 80%. On average, for every 10 eggs, 7 to 8 eggs will normally be fertilized. The implantation rate (defined as the total number of pregnancies divided by the total number of embryo transferred) with intracytoplasmic sperm injection is 40% to 50%—ie, only half of the transferred embryos will successfully implant and result in a pregnancy.
So that more than one ripe egg can be obtained at a time, the patient must undergo a regimen of controlled ovarian stimulation to achieve multifollicular growth. Stimulation protocols are based on giving pituitary hormones, ie, analogues of gonadotropin-releasing hormone (GnRH) (both agonists and antagonists), followed by recombinant FSH or human menopausal gonadotropin to promote follicular development. A single dose of human chorionic gonadotropin (hCG) is given to induce ovulation when the lead follicles have reached 18 to 20 mm in size.18
Many oncologists consider controlled ovarian stimulation dangerous for cancer patients because it takes time and thus delays cancer treatment. Furthermore, the regimen boosts the levels of circulating estrogens, which could be harmful in patients with hormone-dependent tumors.19
Oocytes can also be retrieved during unstimulated cycles. This avoids increasing estrogen concentrations above the physiologic level, but no more than a single oocyte is collected per cycle. The patient could undergo multiple oocyte harvestings, one per cycle, but this would delay her cancer treatment even more—by months—which is not recommended.
Serious efforts to minimize estrogen production during controlled ovarian stimulation have been made, although further studies are needed. Research is under way to develop appropriate ovarian stimulation protocols based on drugs with antiestrogenic effects.
Tamoxifen, an antagonist of the estrogen receptor, is widely used in breast cancer treatment. 20 It can also be given during controlled ovarian stimulation because it promotes follicular growth and induces ovulation.21 Oktay et al22 found that the embryo yield was 2.5 times higher in women with breast cancer treated with tamoxifen than in a retrospective control group consisting of breast cancer patients attempting natural-cycle oocyte retrieval.
Letrozole, an aromatase inhibitor, is also commonly used in treating breast and ovarian cancer. Aromatase is an enzyme that catalyzes the conversion of androgenic precursors to estrogens, and it is found in many tissues, including granulosa cells. Several studies report the use of letrozole, alone or in combination with low doses of recombinant FSH, in ovarian stimulation protocols in cancer patients, with positive clinical outcomes.23
Tamoxifen or letrozole, combined with recombinant FSH, is an attractive option in a controlled ovarian stimulation protocol for cancer patients,24 although further investigation is needed.
Cryopreservation methods
Two main protocols are currently used to freeze oocytes, embryos, and ovarian tissue: slow freezing and vitrification.
In the slow-freezing method, cryoprotectant agents are used to draw water out of the cells, raising intracellular viscosity without (or with minimal) intracellular ice crystal formation, while the sample is cooled slowly in a controlled manner. These cryoprotectant chemicals lower the freezing point of the solution, allowing greater cellular dehydration during the slow freezing. They also protect the plasmatic cell membrane by changing its physical state from liquid to partially dry. To avoid excessive deformation that could damage the cytoplasmic structures, cryoprotectant agents are added in successive stages.25
Vitrification is a newer method that uses higher concentrations of cryoprotectants and flash freezing. The instant drop in temperature converts this highly concentrated solution from an aqueous state to a semisolid, amorphous state that does not contain ice crystals, which are the main cause of damage during the freezing process.26 Since most cryoprotectant agents are extremely toxic, it is necessary to minimize the time that oocytes, embryos, and ovarian tissue are exposed to them.
Cryopreservation of embryos
According to the American Society of Clinical Oncology and the American Society of Reproductive Medicine, embryo freezing is the most established method for fertility preservation, with tangible and widely reported success.27 In normal practice, oocytes are retrieved after a controlled ovarian stimulation and then fertilized in vitro. Then they are treated with cryoprotectant agents, frozen, and stored. Upon demand, embryos can be thawed and implanted.
The Society for Assisted Reproductive Technology reports that the current live birth rate per transfer using thawed embryos from nondonor oocytes in US women under age 35 is 38.7%; at age 35 to 37 it is 35.1%.28 In women with cancer, storing as many embryos as possible could help improve embryo survival and the implantation rate. The optimal time to perform embryo cryopreservation is still being discussed,29 but, as in women without cancer, it is commonly done 3 to 5 days after fertilization.
In the past few years, the use of vitrification has greatly increased, as the post-thawing survival rates and pregnancy rates are higher with this method than with slow freezing.30
Despite its success, embryo cryopreservation has important drawbacks. First, the patient must be of reproductive age and have a partner or accept the use of donor sperm. Second, most patients undergo controlled ovarian stimulation before oocyte retrieval, causing a delay in starting cancer treatment, which is not acceptable in many cases. Moreover, the high serum estrogen levels caused by ovarian stimulation may be contraindicated in women with estrogen-sensitive malignancies.19
Oocyte cryopreservation
Cryopreservation of oocytes avoids the need for sperm and, thus, may be offered to more patients than embryo cryopreservation. In addition, it may circumvent ethical or legal considerations associated with embryo freezing, such as ownership of reproductive material.
However, oocytes are more difficult to cryopreserve than embryos. Indeed, ice crystals frequently form inside and outside the cells, damaging the cell membrane and the meiotic spindle. In routine practice, mature oocytes in metaphase II are used for cryopreservation. Metaphase II oocytes are large cells that contain a delicate meiotic spindle. Moreover, their cytoplasm contains a higher proportion of water than other cells, which could affect their viability after freezing and thawing due to ice crystal formation. In addition, cryopreservation could be responsible for hardening of the zona pellucida (through diffuse thickening of the cell membrane), adversely affecting fertilization rates.31
Significant improvements were achieved in fertilization of cryopreserved oocytes with intracytoplasmic sperm injection. Nevertheless, there are still concerns regarding oocyte cryopreservation. Further studies are needed to determine the risk of aneuploidy caused by damage to the meiotic spindle after oocyte cryopreservation. The potentially detrimental effects of high cryopreservant concentrations used in vitrification also need to be investigated.
In vitro maturation
A novel fertility preservation strategy involves collecting immature oocytes from primordial follicles in unstimulated cycles and then letting them mature in vitro.
To date, immature oocytes retrieved at the germinal vesicle stage can be cryopreserved with vitrification followed by in vitro maturation after thawing, but several studies have demonstrated that better results are obtained when vitrification follows the in vitro maturation process.32
Compared with mature oocytes, immature oocytes are less susceptible to damage during cryopreservation and thus have a better chance of surviving freezing and thawing, thanks to some peculiar characteristics: they are small, have few organelles, lack a zona pellucida, have low metabolic activity, and are in a state of relative quiescence.33 Controlled ovarian stimulation is not necessary, so this procedure preserves fertility without delaying the start of cancer treatment.
Patients are usually evaluated with transvaginal ultrasonography in the early follicular phase of the menstrual cycle (between day 2 and day 4) to count and measure the antral follicles. Immature oocytes are collected when the leading follicle has reached 10 to 12 mm in size and 36 hours after a subcutaneous injection of hCG.34 The retrieved oocytes are then incubated for 24 to 48 hours in a special medium supplemented with FSH and luteinizing hormone (LH). Immature oocytes can also be collected in the luteal phase.
This option could be considered when cancer treatment cannot be delayed for conventional follicular-phase retrieval35 or in case of a premature LH surge during ovarian stimulation. 36 It should be offered to patients facing infertility related to cancer treatment only after appropriate counseling and as a part of a clinical study.
Ovarian tissue cryopreservation
Cryopreservation of ovarian tissue is an experimental but highly promising technique for preserving fertility. Like oocyte cryopreservation, it avoids the need for hormonal stimulation and the need to delay cancer treatment. It may also be the only possible option for prepubertal girls, as well as for women who cannot postpone their cancer treatment. Although it is still experimental, it has obtained progressively better results: after having ovarian tissue preserved, thawed, and subsequently reimplanted in the same position or in a different part of the body, some patients transiently resumed having menstrual cycles and endocrine activity and in a very few cases achieved pregnancy.37
Ovarian tissue for cryopreservation is usually taken via laparoscopic surgery, unless the patient has to undergo open laparotomy for another indication. Laparoscopic surgery offers significant advantages, such as the possibility of performing it on short notice without delaying oncologic therapy. Considering that women at age 30 have about 35 primordial follicles per square millimeter of ovarian tissue, 5 cubic fragments 5 mm wide may be sufficient to obtain more than 4,000 primordial follicles.38 In cases in which complete ovariectomy is necessary, it is possible to remove and cryopreserve fragments of normal ovarian tissue located at the margins of the surgical specimen. Ovarian tissue withdrawal can also be performed in pediatric patients and during other surgical procedures.
The most studied method of cryopreserving ovarian tissue is slow freezing, but the use of vitrification is increasing. This technique was initially carried out in order to preserve the largest number of primordial follicles, but in recent years the possibility of cryopreserving the whole ovary with or without its vascular pedicle has also been studied. Martinez-Madrid et al39 described a protocol of cryopreserving the entire ovary with its stem and found it possible to reach a follicular survival rate of 75%, preserving vessels and stromal structure.39
Currently, the most promising approach seems to be the transplantation of the ovarian tissue back into the donor, ie, autotransplantation. This avoids the need for immunosuppression.
The location can be either orthotopic or heterotopic. In orthotopic transplantation the tissue is placed back in its original location. In theory, the patient could then become pregnant in the usual way if the rest of the reproductive system is not damaged.
In heterotopic transplantation the tissue is placed in a different location, usually easily accessible, like the forearm or the subcutaneous abdominal area. Heterotopic transplants have been shown to be able to restore ovarian function, but not to give pregnancies after oocyte collection.40 Indeed, the pregnancies obtained after transplantation came from autografts of ovarian cortex in orthotopic sites like the fossa ovarica or the remnant ovary.
With autotransplantation, there is a high risk of transmission of metastatic cancer cells. Blood-bone cancers such as leukemia and lymphomas are likely to be associated with the highest risk of ovarian metastasis through transplantation of thawed cryopreserved ovarian tissue. Neuroblastoma and breast cancer are associated with a moderate risk of metastasis to the ovaries. Ovarian involvement is extremely rare in Wilms tumor, lymphomas (except for Burkitt lymphoma), osteosarcomas, Ewing sarcoma, and extragenital rhabdomyosarcoma. Squamous cell cervical cancer metastasizes to the ovaries in fewer than 0.2% of cases, even in the most advanced stages. Histologic evaluation of ovarian samples before transplantation has been proposed to prevent cancer transmission, although it is not possible to completely abolish this risk.41,42 This jeopardy could potentially be eliminated by in vitro maturation of immature oocytes collected from cryopreserved ovarian tissue.
Despite significant advances, to date there have been fewer than 20 babies born worldwide through this method.43
Oocyte donation
Assisted reproduction techniques also include in vitro fertilization using a sperm sample from the partner and oocytes from a donor. The embryos obtained are then transferred, saving the woman from ovarian stimulation without any delay in starting the cancer treatment. Although this method has a high success rate, it inevitably raises personal considerations.
OVARIAN TRANSPOSITION
When a woman of childbearing age needs radiation treatment for a pelvic malignancy, transposition of the ovaries above the pelvic brim outside the radiation field (oophoropexy) should be considered before starting therapy. It is indicated in patients diagnosed with malignancies that require pelvic radiation but not the removal of the ovaries. It can be performed during surgical treatment of the tumor or as a separate laparoscopic procedure. The radiation dose that the transposed ovaries receive is considerably less than that in ovaries left in place.
Laparoscopic ovarian transposition is highly effective. However, the risk involved in the surgical procedure should not be underestimated. The most important complications are vascular injury, infarction of the fallopian tube, and ovarian cyst formation.44
PHARMACOLOGIC PROTECTION
Some drugs induce a state of ovarian quiescence similar to menopause. Can they be used during chemotherapy to protect the ovaries, allowing restoration of normal ovarian function and natural fertility after cancer treatment and preventing premature ovarian failure?
GnRH analogues slow the cellular activity of the gonads, in theory making them less sensitive to damage by cytotoxic agents. Initially, the release of gonadotropins is stimulated (flare-up effect), but after 10 to 15 days pituitary GnRH receptors are down-regulated by internalization of receptors. Since chemotherapy affects mainly actively dividing cells such as mature ovarian follicles, the use of the analogues is based on the assumption that by reducing FSH levels, follicles will remain quiescent, decreasing their sensitivity to the gonadotoxic effect of chemotherapy.
In a randomized study of 281 patients with early breast cancer, Del Mastro et al45 reported a reduction in the occurrence of early menopause in those treated with a GnRH analogue during chemotherapy after 1 year of follow-up. However, the debate regarding the effect of GnRH analogues on the fertility of cancer patients is still open and needs further investigation.
In recent years, research has focused on imatinib, a new, potentially protective drug,46 but a lot of work still needs to be done. To date, the use of GnRH analogues is not recommended outside of clinical studies, and it should be offered only after careful counseling about other options to preserve fertility.
In the last few decades, the survival rates have improved in many of the malignancies that affect young adults. This progress has made fertility preservation and quality of life after cancer treatment important, most of all in survivors of childhood cancers.
Men who are about to undergo cancer treatment can bank their sperm, but as yet no analogous noninvasive option is available for women. The most studied methods are often invasive and require the woman to take large doses of hormones. They may also necessitate a delay in starting cancer treatment.
Which method of fertility preservation a woman should choose depends on several factors, including the type of disease, the treatment required, the age of the patient, whether she has a long-term partner, and whether treatment can be delayed.
Chemotherapy and radiotherapy have well-known deleterious effects on female reproductive function. Many studies have shown that acute loss of growing follicles within the ovary and resultant premature ovarian failure often follow chemotherapy. This ovarian damage has long-term consequences, such as shortened reproductive life span and hormone deficiency.1
Fertility preservation requires a team effort. It should be managed by an oncology center that has built a close collaboration between oncologists, fertility specialists, psychologists, and primary care physicians to allow early discussion and to offer a full range of options to these patients.
The aim of this paper is to discuss the current options for preserving fertility in female cancer patients who have to undergo gonadotoxic cancer treatment.
FEMALE FERTILITY AND ASSESSMENT OF OVARIAN RESERVE
At birth, baby girls have about 1 million primordial ovarian follicles, which is the most they will ever have. By the time they reach menarche, this number has declined to 180,000, and at menopause only about 1,000 remain.2
Throughout a woman’s reproductive life, the number of oocytes remaining—both primordial follicles and the relatively small number of maturing, growing follicles—is called her ovarian reserve. When cancer is diagnosed, we need to assess the patient’s ovarian reserve to direct the discussion about the need for fertility preservation.
In search of markers of ovarian reserve
Fertility experts have been looking for clinical biomarkers that could give us an estimate of the number of nongrowing follicles and, consequently, of the ovarian reserve.
All methods used for the assessment of ovarian reserve actually provide an indirect determination of the remaining pool of oocytes. In clinical practice, a high blood level of follicle-stimulating hormone (FSH) on cycle day 3 (>15 mU/mL) or a low level of anti-Müllerian hormone (AMH) (<1 ng/mL) is generally associated with a low ovarian reserve.
The serum FSH level is the marker most commonly used, but it varies widely at different times in the menstrual cycle.
The FSH test is usually performed on day 3 of the menstrual cycle, when the estrogen level is expected to be low due to negative feedback. The FSH result needs to be combined with the estradiol level, especially in those patients with irregular menstruation or in cases of amenorrhea. A random FSH test is considered valid if the detected estrogen level is low. Women undergoing in vitro fertilization with a day 3 FSH lower than 15 mIU/mL are more likely to conceive than women with a higher FSH level.
AMH and antral follicles. Recently, two other variables have been introduced in clinical practice: the AMH concentration and the antral follicle count (the number of small antral follicles within the ovary) as assessed by transvaginal ultrasonography.
AMH is released by the granulosa cells of small, growing follicles. Because its level is much more stable over the menstrual cycle than the FSH level, it can be measured on any day of the cycle.3 In cancer survivors, AMH is particularly useful in demonstrating the degree of ovarian tissue damage induced by radiotherapy and chemotherapy and in evaluating the ovarian reserve.4
A reduced number of antral follicles causes a diminished AMH production, which becomes undetectable with menopause. The AMH level is strongly associated with the basal antral follicle count. Various threshold values (0.2–1.26 ng/mL) have been used to identify women with low ovarian reserve.
HOW CANCER TREATMENT DAMAGES THE OVARIES
Advances in surgery, radiotherapy, and chemotherapy have significantly improved the prognosis for young cancer patients. However, cancer treatments often result in ovarian dysfunction, and premature menopause and irreversible sterility are the most dramatic outcomes. The resulting low estrogen levels, in addition to their physiologic consequences, also worsen quality of life through psychological effects, which can as well influence the patient’s compliance with treatment.
Chemotherapy: Alkylating agents are the most gonadotoxic
The mechanism by which chemotherapy affects ovarian function is poorly understood. Histologically, chemotherapeutic drugs could lead to ovarian atrophy and stromal fibrosis, to depletion of the primordial follicle stockpile, and to reduced ovarian weight, resulting in ovarian dysfunction.5
The patient's age correlates with the probability of ovarian damage or, inversely, ovarian resistance to chemotherapy. Young women have more primordial oocytes, and after chemotherapy they face a sharp reduction of their ovarian reserve. Still, younger patients show a lower rate of ovarian toxicity than older women.6
The type and the cumulative dose of cytotoxic agents used are other important variables.7 There are six main classes of chemotherapeutic drugs: alkylating agents, platinum derivatives, antibiotics, antimetabolites, plant alkaloids, and taxanes. They all affect ovarian function, but alkylating agents are the most gonadotoxic.
Alkylating agents covalently bind an alkyl group to the DNA molecule and inhibit it from replicating. In the ovaries they directly injure primordial oocytes and deplete follicles. 8 They also seriously damage the ovarian vasculature so that the follicles cannot grow.9 Their destructive effect on the primordial follicles is dose-dependent and varies with the age and developmental maturity of the patient at the time of the therapy, with older women more likely to be left infertile afterward.10
Cyclophosphamide is an alkylating agent often used to treat severe manifestations of autoimmune diseases such as systemic lupus erythematosus, BehÇet disease, steroid-resistant glomerulonephritis, inflammatory bowel disease, pemphigus vulgaris, and others. Because it can lead to premature ovarian failure and infertility, women receiving cyclophosphamide for autoimmune conditions may also need treatment to preserve fertility.11
Radiotherapy
Ovarian follicles are remarkably vulnerable to damage from ionizing radiation, which results in ovarian atrophy and reduced follicle stores.1
The risk of radiotherapy-induced infertility is closely related to the patient’s age and developmental maturity at the time of therapy and to dose fractionation and the extent of the irradiation field. Every patient has a different sensitivity to radiation damage that is probably genetically predetermined, but age seems to be the most important variable. Wo and Viswanathan12 used a mathematical model devised by Wallace et al13 to show that the older the patient, the lower the radiation dose necessary to impair ovarian function.
The irradiation field is another aspect to consider. Pelvic radiation can be necessary in rectal cancer, cervical cancer, and lymphoma. In these cases, surgically moving the ovaries to a region outside the radiation field could be an option to minimize radiotherapy-induced ovarian damage.14
Radiotherapy can also damage the uterus. Pelvic irradiation can reduce uterine volume, alter uterine elasticity through myometrial fibrosis, and modify vascularization and endometrial thickness.15,16 These alterations are closely correlated with the total radiation dose, the site of irradiation, and the patient’s age at the time of the treatment, the prepubertal uterus being more susceptible to damage. 15,17 Larsen et al15 found that girls who received uterine irradiation before puberty had lower uterine volumes in adulthood than girls who received chemotherapy alone or radiation to other parts of the body, and that the younger the patient at the time of radiotherapy, the smaller the uterus later. These effects could result in adverse pregnancy outcomes.
Furthermore, radiation could also damage the uterine vasculature. Holm et al16 used ultrasonography to evaluate the effect of total-body irradiation and found that uterine volume and uterine blood flow were both impaired.15
All these possible alterations could lead to a reduced uterine response to cytotrophoblast invasion and to decreased fetoplacental blood flow, which could impair embryonic and fetal growth. In that case, a surrogate pregnancy would be the only method to achieve parenthood using the couple’s gametes.
OPTIONS FOR FERTILITY PRESERVATION
Assisted reproductive technology
Young women diagnosed with an oncologic disease may wish to use assisted reproductive technology (Table 1) to keep open the possibility of having children at a later date. One approach is to harvest oocytes, fertilize them in vitro, and deep-freeze (cryopreserve) the resulting embryos to be thawed and implanted later. Alternatively, oocytes can be frozen directly, although success rates are lower with this method. And another approach is to obtain and cryopreserve ovarian tissue. If none of these is possible, oocytes may be obtained from a donor.
Controlled ovarian stimulation
If oocytes are to be harvested, a number of them should be harvested at one time.
There is not an optimal number of oocytes that should be retrieved, but cryopreservation of a large number of oocytes allows us to perform multiple attempts at in vitro fertilization, improving the chances of pregnancy. The fertilization rate (defined as the total number of zygotes at the 2-pronucleus stage divided by the number of fertilized oocytes) with intracytoplasmic sperm injection is 70% to 80%. On average, for every 10 eggs, 7 to 8 eggs will normally be fertilized. The implantation rate (defined as the total number of pregnancies divided by the total number of embryo transferred) with intracytoplasmic sperm injection is 40% to 50%—ie, only half of the transferred embryos will successfully implant and result in a pregnancy.
So that more than one ripe egg can be obtained at a time, the patient must undergo a regimen of controlled ovarian stimulation to achieve multifollicular growth. Stimulation protocols are based on giving pituitary hormones, ie, analogues of gonadotropin-releasing hormone (GnRH) (both agonists and antagonists), followed by recombinant FSH or human menopausal gonadotropin to promote follicular development. A single dose of human chorionic gonadotropin (hCG) is given to induce ovulation when the lead follicles have reached 18 to 20 mm in size.18
Many oncologists consider controlled ovarian stimulation dangerous for cancer patients because it takes time and thus delays cancer treatment. Furthermore, the regimen boosts the levels of circulating estrogens, which could be harmful in patients with hormone-dependent tumors.19
Oocytes can also be retrieved during unstimulated cycles. This avoids increasing estrogen concentrations above the physiologic level, but no more than a single oocyte is collected per cycle. The patient could undergo multiple oocyte harvestings, one per cycle, but this would delay her cancer treatment even more—by months—which is not recommended.
Serious efforts to minimize estrogen production during controlled ovarian stimulation have been made, although further studies are needed. Research is under way to develop appropriate ovarian stimulation protocols based on drugs with antiestrogenic effects.
Tamoxifen, an antagonist of the estrogen receptor, is widely used in breast cancer treatment. 20 It can also be given during controlled ovarian stimulation because it promotes follicular growth and induces ovulation.21 Oktay et al22 found that the embryo yield was 2.5 times higher in women with breast cancer treated with tamoxifen than in a retrospective control group consisting of breast cancer patients attempting natural-cycle oocyte retrieval.
Letrozole, an aromatase inhibitor, is also commonly used in treating breast and ovarian cancer. Aromatase is an enzyme that catalyzes the conversion of androgenic precursors to estrogens, and it is found in many tissues, including granulosa cells. Several studies report the use of letrozole, alone or in combination with low doses of recombinant FSH, in ovarian stimulation protocols in cancer patients, with positive clinical outcomes.23
Tamoxifen or letrozole, combined with recombinant FSH, is an attractive option in a controlled ovarian stimulation protocol for cancer patients,24 although further investigation is needed.
Cryopreservation methods
Two main protocols are currently used to freeze oocytes, embryos, and ovarian tissue: slow freezing and vitrification.
In the slow-freezing method, cryoprotectant agents are used to draw water out of the cells, raising intracellular viscosity without (or with minimal) intracellular ice crystal formation, while the sample is cooled slowly in a controlled manner. These cryoprotectant chemicals lower the freezing point of the solution, allowing greater cellular dehydration during the slow freezing. They also protect the plasmatic cell membrane by changing its physical state from liquid to partially dry. To avoid excessive deformation that could damage the cytoplasmic structures, cryoprotectant agents are added in successive stages.25
Vitrification is a newer method that uses higher concentrations of cryoprotectants and flash freezing. The instant drop in temperature converts this highly concentrated solution from an aqueous state to a semisolid, amorphous state that does not contain ice crystals, which are the main cause of damage during the freezing process.26 Since most cryoprotectant agents are extremely toxic, it is necessary to minimize the time that oocytes, embryos, and ovarian tissue are exposed to them.
Cryopreservation of embryos
According to the American Society of Clinical Oncology and the American Society of Reproductive Medicine, embryo freezing is the most established method for fertility preservation, with tangible and widely reported success.27 In normal practice, oocytes are retrieved after a controlled ovarian stimulation and then fertilized in vitro. Then they are treated with cryoprotectant agents, frozen, and stored. Upon demand, embryos can be thawed and implanted.
The Society for Assisted Reproductive Technology reports that the current live birth rate per transfer using thawed embryos from nondonor oocytes in US women under age 35 is 38.7%; at age 35 to 37 it is 35.1%.28 In women with cancer, storing as many embryos as possible could help improve embryo survival and the implantation rate. The optimal time to perform embryo cryopreservation is still being discussed,29 but, as in women without cancer, it is commonly done 3 to 5 days after fertilization.
In the past few years, the use of vitrification has greatly increased, as the post-thawing survival rates and pregnancy rates are higher with this method than with slow freezing.30
Despite its success, embryo cryopreservation has important drawbacks. First, the patient must be of reproductive age and have a partner or accept the use of donor sperm. Second, most patients undergo controlled ovarian stimulation before oocyte retrieval, causing a delay in starting cancer treatment, which is not acceptable in many cases. Moreover, the high serum estrogen levels caused by ovarian stimulation may be contraindicated in women with estrogen-sensitive malignancies.19
Oocyte cryopreservation
Cryopreservation of oocytes avoids the need for sperm and, thus, may be offered to more patients than embryo cryopreservation. In addition, it may circumvent ethical or legal considerations associated with embryo freezing, such as ownership of reproductive material.
However, oocytes are more difficult to cryopreserve than embryos. Indeed, ice crystals frequently form inside and outside the cells, damaging the cell membrane and the meiotic spindle. In routine practice, mature oocytes in metaphase II are used for cryopreservation. Metaphase II oocytes are large cells that contain a delicate meiotic spindle. Moreover, their cytoplasm contains a higher proportion of water than other cells, which could affect their viability after freezing and thawing due to ice crystal formation. In addition, cryopreservation could be responsible for hardening of the zona pellucida (through diffuse thickening of the cell membrane), adversely affecting fertilization rates.31
Significant improvements were achieved in fertilization of cryopreserved oocytes with intracytoplasmic sperm injection. Nevertheless, there are still concerns regarding oocyte cryopreservation. Further studies are needed to determine the risk of aneuploidy caused by damage to the meiotic spindle after oocyte cryopreservation. The potentially detrimental effects of high cryopreservant concentrations used in vitrification also need to be investigated.
In vitro maturation
A novel fertility preservation strategy involves collecting immature oocytes from primordial follicles in unstimulated cycles and then letting them mature in vitro.
To date, immature oocytes retrieved at the germinal vesicle stage can be cryopreserved with vitrification followed by in vitro maturation after thawing, but several studies have demonstrated that better results are obtained when vitrification follows the in vitro maturation process.32
Compared with mature oocytes, immature oocytes are less susceptible to damage during cryopreservation and thus have a better chance of surviving freezing and thawing, thanks to some peculiar characteristics: they are small, have few organelles, lack a zona pellucida, have low metabolic activity, and are in a state of relative quiescence.33 Controlled ovarian stimulation is not necessary, so this procedure preserves fertility without delaying the start of cancer treatment.
Patients are usually evaluated with transvaginal ultrasonography in the early follicular phase of the menstrual cycle (between day 2 and day 4) to count and measure the antral follicles. Immature oocytes are collected when the leading follicle has reached 10 to 12 mm in size and 36 hours after a subcutaneous injection of hCG.34 The retrieved oocytes are then incubated for 24 to 48 hours in a special medium supplemented with FSH and luteinizing hormone (LH). Immature oocytes can also be collected in the luteal phase.
This option could be considered when cancer treatment cannot be delayed for conventional follicular-phase retrieval35 or in case of a premature LH surge during ovarian stimulation. 36 It should be offered to patients facing infertility related to cancer treatment only after appropriate counseling and as a part of a clinical study.
Ovarian tissue cryopreservation
Cryopreservation of ovarian tissue is an experimental but highly promising technique for preserving fertility. Like oocyte cryopreservation, it avoids the need for hormonal stimulation and the need to delay cancer treatment. It may also be the only possible option for prepubertal girls, as well as for women who cannot postpone their cancer treatment. Although it is still experimental, it has obtained progressively better results: after having ovarian tissue preserved, thawed, and subsequently reimplanted in the same position or in a different part of the body, some patients transiently resumed having menstrual cycles and endocrine activity and in a very few cases achieved pregnancy.37
Ovarian tissue for cryopreservation is usually taken via laparoscopic surgery, unless the patient has to undergo open laparotomy for another indication. Laparoscopic surgery offers significant advantages, such as the possibility of performing it on short notice without delaying oncologic therapy. Considering that women at age 30 have about 35 primordial follicles per square millimeter of ovarian tissue, 5 cubic fragments 5 mm wide may be sufficient to obtain more than 4,000 primordial follicles.38 In cases in which complete ovariectomy is necessary, it is possible to remove and cryopreserve fragments of normal ovarian tissue located at the margins of the surgical specimen. Ovarian tissue withdrawal can also be performed in pediatric patients and during other surgical procedures.
The most studied method of cryopreserving ovarian tissue is slow freezing, but the use of vitrification is increasing. This technique was initially carried out in order to preserve the largest number of primordial follicles, but in recent years the possibility of cryopreserving the whole ovary with or without its vascular pedicle has also been studied. Martinez-Madrid et al39 described a protocol of cryopreserving the entire ovary with its stem and found it possible to reach a follicular survival rate of 75%, preserving vessels and stromal structure.39
Currently, the most promising approach seems to be the transplantation of the ovarian tissue back into the donor, ie, autotransplantation. This avoids the need for immunosuppression.
The location can be either orthotopic or heterotopic. In orthotopic transplantation the tissue is placed back in its original location. In theory, the patient could then become pregnant in the usual way if the rest of the reproductive system is not damaged.
In heterotopic transplantation the tissue is placed in a different location, usually easily accessible, like the forearm or the subcutaneous abdominal area. Heterotopic transplants have been shown to be able to restore ovarian function, but not to give pregnancies after oocyte collection.40 Indeed, the pregnancies obtained after transplantation came from autografts of ovarian cortex in orthotopic sites like the fossa ovarica or the remnant ovary.
With autotransplantation, there is a high risk of transmission of metastatic cancer cells. Blood-bone cancers such as leukemia and lymphomas are likely to be associated with the highest risk of ovarian metastasis through transplantation of thawed cryopreserved ovarian tissue. Neuroblastoma and breast cancer are associated with a moderate risk of metastasis to the ovaries. Ovarian involvement is extremely rare in Wilms tumor, lymphomas (except for Burkitt lymphoma), osteosarcomas, Ewing sarcoma, and extragenital rhabdomyosarcoma. Squamous cell cervical cancer metastasizes to the ovaries in fewer than 0.2% of cases, even in the most advanced stages. Histologic evaluation of ovarian samples before transplantation has been proposed to prevent cancer transmission, although it is not possible to completely abolish this risk.41,42 This jeopardy could potentially be eliminated by in vitro maturation of immature oocytes collected from cryopreserved ovarian tissue.
Despite significant advances, to date there have been fewer than 20 babies born worldwide through this method.43
Oocyte donation
Assisted reproduction techniques also include in vitro fertilization using a sperm sample from the partner and oocytes from a donor. The embryos obtained are then transferred, saving the woman from ovarian stimulation without any delay in starting the cancer treatment. Although this method has a high success rate, it inevitably raises personal considerations.
OVARIAN TRANSPOSITION
When a woman of childbearing age needs radiation treatment for a pelvic malignancy, transposition of the ovaries above the pelvic brim outside the radiation field (oophoropexy) should be considered before starting therapy. It is indicated in patients diagnosed with malignancies that require pelvic radiation but not the removal of the ovaries. It can be performed during surgical treatment of the tumor or as a separate laparoscopic procedure. The radiation dose that the transposed ovaries receive is considerably less than that in ovaries left in place.
Laparoscopic ovarian transposition is highly effective. However, the risk involved in the surgical procedure should not be underestimated. The most important complications are vascular injury, infarction of the fallopian tube, and ovarian cyst formation.44
PHARMACOLOGIC PROTECTION
Some drugs induce a state of ovarian quiescence similar to menopause. Can they be used during chemotherapy to protect the ovaries, allowing restoration of normal ovarian function and natural fertility after cancer treatment and preventing premature ovarian failure?
GnRH analogues slow the cellular activity of the gonads, in theory making them less sensitive to damage by cytotoxic agents. Initially, the release of gonadotropins is stimulated (flare-up effect), but after 10 to 15 days pituitary GnRH receptors are down-regulated by internalization of receptors. Since chemotherapy affects mainly actively dividing cells such as mature ovarian follicles, the use of the analogues is based on the assumption that by reducing FSH levels, follicles will remain quiescent, decreasing their sensitivity to the gonadotoxic effect of chemotherapy.
In a randomized study of 281 patients with early breast cancer, Del Mastro et al45 reported a reduction in the occurrence of early menopause in those treated with a GnRH analogue during chemotherapy after 1 year of follow-up. However, the debate regarding the effect of GnRH analogues on the fertility of cancer patients is still open and needs further investigation.
In recent years, research has focused on imatinib, a new, potentially protective drug,46 but a lot of work still needs to be done. To date, the use of GnRH analogues is not recommended outside of clinical studies, and it should be offered only after careful counseling about other options to preserve fertility.
- Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol 2010; 53:727–739.
- Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One 2010; 5:e8772.
- van Disseldorp J, Lambalk CB, Kwee J, et al. Comparison of inter- and intra-cycle variability of anti-Mullerian hormone and antral follicle counts. Hum Reprod 2010; 25:221–227.
- Lie Fong S, Laven JS, Hakvoort-Cammel FG, et al. Assessment of ovarian reserve in adult childhood cancer survivors using anti-Müllerian hormone. Hum Reprod 2009; 24:982–990.
- Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 2007; 110:2222–2229.
- Partridge AH, Ruddy KJ, Gelber S, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril 2010; 94:638–644.
- Lee SJ, Schover LR, Partridge AH, et al; American Society of Clinical Oncology. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006; 24:2917–2931.
- Familiari G, Caggiati A, Nottola SA, Ermini M, Di Benedetto MR, Motta PM. Ultrastructure of human ovarian primordial follicles after combination chemotherapy for Hodgkin’s disease. Hum Reprod 1993; 8:2080–2087.
- Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor Trap R1R2. Endocrinology 2002; 143:2797–2807.
- Aubard Y, Piver P, Pech JC, Galinat S, Teissier MP. Ovarian tissue cryopreservation and gynecologic oncology: a review. Eur J Obstet Gynecol Reprod Biol 2001; 97:5–14.
- Elizur SE, Chian RC, Pineau CA, et al. Fertility preservation treatment for young women with autoimmune diseases facing treatment with gonadotoxic agents. Rheumatology (Oxford) 2008; 47:1506–1509.
- Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys 2009; 73:1304–1312.
- Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys 2005; 62:738–744.
- Gareer W, Gad Z, Gareer H. Needle oophoropexy: a new simple technique for ovarian transposition prior to pelvic irradiation. Surg Endosc 2011; 25:2241–2246.
- Larsen EC, Schmiegelow K, Rechnitzer C, Loft A, Müller J, Andersen AN. Radiotherapy at a young age reduces uterine volume of childhood cancer survivors. Acta Obstet Gynecol Scand 2004; 83:96–102.
- Holm K, Nysom K, Brocks V, Hertz H, Jacobsen N, Müller J. Ultrasound B-mode changes in the uterus and ovaries and Doppler changes in the uterus after total body irradiation and allogeneic bone marrow transplantation in childhood. Bone Marrow Transplant 1999; 23:259–263.
- Critchley HO, Wallace WH, Shalet SM, Mamtora H, Higginson J, Anderson DC. Abdominal irradiation in childhood; the potential for pregnancy. Br J Obstet Gynaecol 1992; 99:392–394.
- Goldberg JM, Falcone T, Attaran M. In vitro fertilization update. Cleve Clin J Med 2007; 74:329–338.
- Prest SJ, May FE, Westley BR. The estrogen-regulated protein, TFF1, stimulates migration of human breast cancer cells. FASEB J 2002; 16:592–594.
- Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Early Breast Cancer Trialists’ Collaborative Group. Lancet 1992; 339:71–85.
- Klopper A, Hall M. New synthetic agent for the induction of ovulation: preliminary trials in women. Br Med J 1971; 1:152–154.
- Oktay K, Buyuk E, Davis O, Yermakova I, Veeck L, Rosenwaks Z. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod 2003; 18:90–95.
- Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol 2005; 23:4347–4353.
- Oktay K. Further evidence on the safety and success of ovarian stimulation with letrozole and tamoxifen in breast cancer patients undergoing in vitro fertilization to cryopreserve their embryos for fertility preservation. J Clin Oncol 2005; 23:3858–3859.
- Gosden R. Cryopreservation: a cold look at technology for fertility preservation. Fertil Steril 2011; 96:264–268.
- Chen SU, Chien CL, Wu MY, et al. Novel direct cover vitrification for cryopreservation of ovarian tissues increases follicle viability and pregnancy capability in mice. Hum Reprod 2006; 21:2794–2800.
- Lee SJ, Schover LR, Partridge AH, et al; American Society of Clinical Oncology. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006; 24:2917–2931.
- Society for Assisted Reproductive Technology (SART). http://www.sart.org. Accessed January 3, 2013.
- Granne I, Child T, Hartshorne G; British Fertility Society. Embryo cryopreservation: evidence for practice. Hum Fertil (Camb) 2008; 11:159–172.
- Loutradi KE, Kolibianakis EM, Venetis CA, et al. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril 2008; 90:186–193.
- Ko CS, Ding DC, Chu TW, et al. Changes to the meiotic spindle and zona pellucida of mature mouse oocytes following different cryopreservation methods. Anim Reprod Sci 2008; 105:272–282.
- Cao Y, Xing Q, Zhang ZG, et al. Cryopreservation of immature and in vitro-matured human oocytes by vitrification. Reprod Biomed Online 2009; 19:369–373.
- Toth TL, Baka SG, Veeck LL, Jones HW, Muasher S, Lanzendorf SE. Fertilization and in vitro development of cryopreserved human prophase I oocytes. Fertil Steril 1994; 61:891–894.
- Chian RC, Gülekli B, Buckett WM, Tan SL. Priming with human chorionic gonadotropin before retrieval of immature oocytes in women with infertility due to the polycystic ovary syndrome. N Engl J Med 1999; 341: 1624,1626.
- Maman E, Meirow D, Brengauz M, Raanani H, Dor J, Hourvitz A. Luteal phase oocyte retrieval and in vitro maturation is an optional procedure for urgent fertility preservation. Fertil Steril 2011; 95:64–67.
- Oktay K, Demirtas E, Son WY, Lostritto K, Chian RC, Tan SL. In vitro maturation of germinal vesicle oocytes recovered after premature luteinizing hormone surge: description of a novel approach to fertility preservation. Fertil Steril 2008; 89:228.e19–e22.
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004; 364:1405–1410.
- Martinez-Madrid B, Dolmans MM, Van Langendonckt A, Defrère S, Donnez J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril 2004; 82:1390–1394.
- Martinez-Madrid B, Camboni A, Dolmans MM, Nottola S, Van Langendonckt A, Donnez J. Apoptosis and ultrastructural assessment after cryopreservation of whole human ovaries with their vascular pedicle. Fertil Steril 2007; 87:1153–1165.
- Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA 2001; 286:1490–1493.
- Practice Committee of American Society for Reproductive Medicine. Ovarian tissue and oocyte cryopreservation. Fertil Steril 2008; 90(suppl 5):S241–S246.
- Oktay K. Ovarian tissue cryopreservation and transplantation: preliminary findings and implications for cancer patients. Hum Reprod Update 2001; 7:526–534.
- Donnez J, Silber S, Andersen CY, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med 2011; 43:437–450.
- Terenziani M, Piva L, Meazza C, Gandola L, Cefalo G, Merola M. Oophoropexy: a relevant role in preservation of ovarian function after pelvic irradiation. Fertil Steril 2009; 91:935.e15–e16.
- Del Mastro L, Boni L, Michelotti A, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA 2011; 306:269–276.
- Gonfloni S, Di Tella L, Caldarola S, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med 2009; 15:1179–1185.
- Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol 2010; 53:727–739.
- Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One 2010; 5:e8772.
- van Disseldorp J, Lambalk CB, Kwee J, et al. Comparison of inter- and intra-cycle variability of anti-Mullerian hormone and antral follicle counts. Hum Reprod 2010; 25:221–227.
- Lie Fong S, Laven JS, Hakvoort-Cammel FG, et al. Assessment of ovarian reserve in adult childhood cancer survivors using anti-Müllerian hormone. Hum Reprod 2009; 24:982–990.
- Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 2007; 110:2222–2229.
- Partridge AH, Ruddy KJ, Gelber S, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril 2010; 94:638–644.
- Lee SJ, Schover LR, Partridge AH, et al; American Society of Clinical Oncology. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006; 24:2917–2931.
- Familiari G, Caggiati A, Nottola SA, Ermini M, Di Benedetto MR, Motta PM. Ultrastructure of human ovarian primordial follicles after combination chemotherapy for Hodgkin’s disease. Hum Reprod 1993; 8:2080–2087.
- Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor Trap R1R2. Endocrinology 2002; 143:2797–2807.
- Aubard Y, Piver P, Pech JC, Galinat S, Teissier MP. Ovarian tissue cryopreservation and gynecologic oncology: a review. Eur J Obstet Gynecol Reprod Biol 2001; 97:5–14.
- Elizur SE, Chian RC, Pineau CA, et al. Fertility preservation treatment for young women with autoimmune diseases facing treatment with gonadotoxic agents. Rheumatology (Oxford) 2008; 47:1506–1509.
- Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys 2009; 73:1304–1312.
- Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys 2005; 62:738–744.
- Gareer W, Gad Z, Gareer H. Needle oophoropexy: a new simple technique for ovarian transposition prior to pelvic irradiation. Surg Endosc 2011; 25:2241–2246.
- Larsen EC, Schmiegelow K, Rechnitzer C, Loft A, Müller J, Andersen AN. Radiotherapy at a young age reduces uterine volume of childhood cancer survivors. Acta Obstet Gynecol Scand 2004; 83:96–102.
- Holm K, Nysom K, Brocks V, Hertz H, Jacobsen N, Müller J. Ultrasound B-mode changes in the uterus and ovaries and Doppler changes in the uterus after total body irradiation and allogeneic bone marrow transplantation in childhood. Bone Marrow Transplant 1999; 23:259–263.
- Critchley HO, Wallace WH, Shalet SM, Mamtora H, Higginson J, Anderson DC. Abdominal irradiation in childhood; the potential for pregnancy. Br J Obstet Gynaecol 1992; 99:392–394.
- Goldberg JM, Falcone T, Attaran M. In vitro fertilization update. Cleve Clin J Med 2007; 74:329–338.
- Prest SJ, May FE, Westley BR. The estrogen-regulated protein, TFF1, stimulates migration of human breast cancer cells. FASEB J 2002; 16:592–594.
- Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Early Breast Cancer Trialists’ Collaborative Group. Lancet 1992; 339:71–85.
- Klopper A, Hall M. New synthetic agent for the induction of ovulation: preliminary trials in women. Br Med J 1971; 1:152–154.
- Oktay K, Buyuk E, Davis O, Yermakova I, Veeck L, Rosenwaks Z. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod 2003; 18:90–95.
- Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol 2005; 23:4347–4353.
- Oktay K. Further evidence on the safety and success of ovarian stimulation with letrozole and tamoxifen in breast cancer patients undergoing in vitro fertilization to cryopreserve their embryos for fertility preservation. J Clin Oncol 2005; 23:3858–3859.
- Gosden R. Cryopreservation: a cold look at technology for fertility preservation. Fertil Steril 2011; 96:264–268.
- Chen SU, Chien CL, Wu MY, et al. Novel direct cover vitrification for cryopreservation of ovarian tissues increases follicle viability and pregnancy capability in mice. Hum Reprod 2006; 21:2794–2800.
- Lee SJ, Schover LR, Partridge AH, et al; American Society of Clinical Oncology. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006; 24:2917–2931.
- Society for Assisted Reproductive Technology (SART). http://www.sart.org. Accessed January 3, 2013.
- Granne I, Child T, Hartshorne G; British Fertility Society. Embryo cryopreservation: evidence for practice. Hum Fertil (Camb) 2008; 11:159–172.
- Loutradi KE, Kolibianakis EM, Venetis CA, et al. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril 2008; 90:186–193.
- Ko CS, Ding DC, Chu TW, et al. Changes to the meiotic spindle and zona pellucida of mature mouse oocytes following different cryopreservation methods. Anim Reprod Sci 2008; 105:272–282.
- Cao Y, Xing Q, Zhang ZG, et al. Cryopreservation of immature and in vitro-matured human oocytes by vitrification. Reprod Biomed Online 2009; 19:369–373.
- Toth TL, Baka SG, Veeck LL, Jones HW, Muasher S, Lanzendorf SE. Fertilization and in vitro development of cryopreserved human prophase I oocytes. Fertil Steril 1994; 61:891–894.
- Chian RC, Gülekli B, Buckett WM, Tan SL. Priming with human chorionic gonadotropin before retrieval of immature oocytes in women with infertility due to the polycystic ovary syndrome. N Engl J Med 1999; 341: 1624,1626.
- Maman E, Meirow D, Brengauz M, Raanani H, Dor J, Hourvitz A. Luteal phase oocyte retrieval and in vitro maturation is an optional procedure for urgent fertility preservation. Fertil Steril 2011; 95:64–67.
- Oktay K, Demirtas E, Son WY, Lostritto K, Chian RC, Tan SL. In vitro maturation of germinal vesicle oocytes recovered after premature luteinizing hormone surge: description of a novel approach to fertility preservation. Fertil Steril 2008; 89:228.e19–e22.
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004; 364:1405–1410.
- Martinez-Madrid B, Dolmans MM, Van Langendonckt A, Defrère S, Donnez J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril 2004; 82:1390–1394.
- Martinez-Madrid B, Camboni A, Dolmans MM, Nottola S, Van Langendonckt A, Donnez J. Apoptosis and ultrastructural assessment after cryopreservation of whole human ovaries with their vascular pedicle. Fertil Steril 2007; 87:1153–1165.
- Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA 2001; 286:1490–1493.
- Practice Committee of American Society for Reproductive Medicine. Ovarian tissue and oocyte cryopreservation. Fertil Steril 2008; 90(suppl 5):S241–S246.
- Oktay K. Ovarian tissue cryopreservation and transplantation: preliminary findings and implications for cancer patients. Hum Reprod Update 2001; 7:526–534.
- Donnez J, Silber S, Andersen CY, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med 2011; 43:437–450.
- Terenziani M, Piva L, Meazza C, Gandola L, Cefalo G, Merola M. Oophoropexy: a relevant role in preservation of ovarian function after pelvic irradiation. Fertil Steril 2009; 91:935.e15–e16.
- Del Mastro L, Boni L, Michelotti A, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA 2011; 306:269–276.
- Gonfloni S, Di Tella L, Caldarola S, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med 2009; 15:1179–1185.
KEY POINTS
- Chemotherapy and radiotherapy are toxic to the ovaries, although the damage can be attenuated in some cases.
- The standard option for preserving fertility has been oocyte retrieval after controlled ovarian stimulation, followed by in vitro fertilization and subsequent cryopreservation of the resulting embryos.
- Unfortunately, controlled ovarian stimulation takes time, may delay needed cancer therapy, and may worsen estrogen-dependent cancers. Alternatives are being explored.
- Cryopreservation of unfertilized oocytes is an option for women who do not have a partner, although oocytes are more susceptible to damage during freezing than embryos are.