User login
Glycemic Control in the Hospital
Hyperglycemia is common in hospitalized patients, and hyperglycemia has been associated with poor hospital outcomes. The adverse physiologic effects of acute hyperglycemia are well established1 and several clinical studies have linked hyperglycemia with poor clinical outcomes in certain patient populations.28 Although the optimal target range for inpatient glycemic control has not yet been defined, these studies support the goal of metabolic control for hospital patients. However, there are many barriers to achieving adequate glycemic control in the hospital, and blood glucoses in the hospital are often far from recommended targets.9, 10 One barrier appears to be the low priority given to glycemic control in the hospital. Hyperglycemia in the hospital is often ignored,11 and insulin regimens are often chosen for simplicity as opposed to effectiveness.12 Other barriers to glycemic control in the hospital include the physiologic effects (stress) of acute illness, and the frequent nutritional changes and interruptions that occur.
Most hyperglycemic patients on a general medicine unit are treated with subcutaneous insulin, but the optimal strategy for prescribing insulin in the hospital remains uncertain. A technical review of the literature on the management of diabetes in the hospital setting from 2004 recommends prescribing insulin in a way that mimics physiologic insulin secretion (ie, physiologic or basal‐bolus insulin).1 This approach has been promulgated by experts, but there has been very little research to support these recommendations. One small, randomized trial concluded that a basal‐bolus approach achieved better glycemic control than the use of sliding‐scale insulin alone,13 and 2 quality improvement studies using a before/after design have demonstrated improvements in glycemic control after the implementation of interventions designed to encourage physiologic insulin use.14, 15
In this study we hypothesized that a few simple interventions (education for physicians and nurses, and a standardized insulin order form) would lead to a higher rate of basal‐bolus insulin use and simultaneously improve glycemic control and patient safety.
Methods
Study Design
This study was performed at the University of Michigan Hospital over a 6‐month period, and the protocol was approved by the Institutional Review Board. We performed a quasi‐experimental study comparing 3 patient groups. The intervention group (IG) was subject to all of the interventions discussed below (physician education, nurse education, and the standardized order form). The concurrent control group (CCG) was hospitalized during the same time period as the IG, but was only subject to 1 of the interventions (physician education). These patients were cared for by the same physicians as the IG, but on a different unit where the nurses had not received the education and where the standardized insulin order form was not available. Patients were admitted to the IG unit or the CCG unit via the institution's usual admission process. In addition, we examined an historic control group (HCG) which was hospitalized during the same months of the year, but 2 years prior. The HCG was not subject to any of the interventions.
Interventions
Standardized Subcutaneous Insulin Order Form
This form (Supporting Information Appendix 1) was designed to encourage physicians to prescribe insulin in a physiologic way, providing basal, nutritional, and correctional insulin. The form is based on best practice guidelines,1 and is in agreement with the principles of the inpatient management of diabetes and hyperglycemia endorsed by several professional organizations.16, 17 The form was engineered by a multidisciplinary team, including an endocrinologist, several hospitalists, several nurses, a certified diabetes educator, a pharmacist, and others. It is derived from the extensive experience of the University of Michigan Hospital Intensive Insulin Program (HIIP) in the Division of Endocrinology, and on work done by the Society of Hospital Medicine (SHM) Glycemic Control Task Force.1719 This form was only used in the care of patients in the IG. The form, which was not approved for use on other floors, did not creep to other units. The standardized order form was the only way to order insulin or to modify the insulin regimen on the IG unit. The frequency of review or revision of the insulin orders was left to the discretion of the inpatient physicians.
Physician/Midlevel Provider Education
Physicians and midlevel providers caring for patients in the IG and the CCG were given specific education about the best practice recommendations for the management of diabetes and hyperglycemia in hospitalized patients. This education was based on the principles of anticipatory, physiologic insulin use. On nonhouse staff services, the education was provided to the attending physicians and midlevel providers, and on house staff services, the education was provided to the residents. All physician education was provided by the physician authors (D.W. and R.G.). A summary of the content of the physician education is provided in Supporting Information Appendix 2.
Nurse Education
Nurses caring for patients in the IG were given education similar to that which was provided to the physicians (see above), with an emphasis on practical issues related to delivering physiologic insulin. It included topics such as blood glucose monitoring, and the real‐time manipulation of nutritional insulin doses in accordance with the clinical situation (decision‐making that was specifically delegated to the nursing staff by the order set).
Patients
Patients were eligible for inclusion in the analysis if they met the following inclusion criteria: they were admitted to the inpatient General Internal Medicine Services; subcutaneous insulin was provided to the patient during the hospitalization; they had at least 2 blood glucose values >180 mg/dL; they were discharged from the hospital on a pharmacologic glucose lowering agent (insulin or oral); and their total length‐of‐stay was 3 days to 14 days. Patients were excluded from the analysis if they were admitted with a primary diagnosis of diabetic ketoacidosis, diabetic hyperosmolar state, or hypoglycemia. Up to 10 consecutive days of glucose data were recorded for each patient, and the first day on which blood glucose information was available from the admitting floor was excluded from the analysis. Also, specific patient‐days were not analyzed if there were no bedside glucoses recorded, or if the patient was treated with an IV insulin infusion on that day.
Outcomes
The primary outcome was glycemic control. The primary unit of measure was the patient‐day (ie, all of the information for 1 patient on a single qualifying day). This was done to correct for the phenomenon of frequent repeat testing in response to abnormal values. It also allows for a more clinically relevant description of the actual glycemic control on a given day. Specifically, each patient‐day was categorized as in‐range (70‐180 mg/dL), hyperglycemic (>180 mg/dL), severely hyperglycemic (>250 mg/dL), hypoglycemic (<70 mg/dL), and/or severely hypoglycemic (<50 mg/dL). The primary endpoint was glycemic control in‐range. For a patient‐day to be in‐range, all readings for that particular day were within 70 mg/dL to180 mg/dL. For the readings that were not in the desired range, a minimum of 1 deviant reading in a particular day constituted classification into that category, and a single out‐of‐range patient‐day could be included in 1 or more of the out‐of‐range categories (eg, a patient‐day could be categorized as both severely hyperglycemic and hypoglycemic if it contained glucose readings in both of those ranges).
The day‐weighted mean blood glucose value was also calculated for each of the groups. This calculation utilized the mean blood glucose for each patient‐day, and then averaged these values for each group. These metrics have been endorsed as appropriate measures of glycemic control by the SHM Glycemic Control Task Force.20
Other Data
Several other clinical features were also examined, including the following: primary diagnoses listed in the hospital discharge summary for each patient (3 maximum); possible confounders including patient weight, length‐of‐stay, days receiving tube feeds, days receiving parenteral nutrition, and days during which patients were treated with high‐dose glucocorticoids (>10 mg/day of prednisone, or its equivalent) or oral diabetes medications; and the composition of the insulin regimen on each hospital day. Definitions of insulin regimens are provided in Table 1.
| |
| Any basal insulin day | Any day in which intermediate‐acting or long‐acting, scheduled insulin was given. |
| Basal insulin alone day | A day in which intermediate‐acting or long‐acting insulin was the only scheduled insulin given. |
| Any nutritional insulin day | Any day in which rapid‐acting or short‐acting, scheduled insulin was given. |
| Nutritional insulin alone day | A day in which rapid‐acting or short‐acting insulin was the only scheduled insulin given. |
| Basal plus nutritional day | A day in which both scheduled, intermediate‐acting or long‐acting insulin and scheduled, rapid‐acting or short‐acting insulin were given. |
| Pre‐mixed insulin day | Any day in which a pre‐mixed combination insulin was given. |
| Basal plus nutritional or pre‐mixed insulin day | A composite of the basal plus nutritional day category and the mixed insulin day category described above. This group includes any day in which either a pre‐mixed combination insulin was given OR a day in which both: (a) scheduled, intermediate‐acting or long‐acting and (b) scheduled, rapid‐acting or short‐acting insulin were given. |
| Sliding scale insulin alone day* | Any day when only correctional (as needed) insulin was given. |
Statistical Analysis
Bivariate analyses (chi‐square, and t‐tests) were carried out to compare demographic characteristics of the intervention and control populations. Since there were multiple glucose readings nested within individuals, multilevel mixed‐effects logistic regression was used to evaluate the association between the intervention and outcomes. A 2‐level hierarchical model was developed in which patient‐days were nested within patients; this accounted for the correlation between glycemic control across days for a given patient. Patient‐day was modeled as a random intercept and the log likelihood was estimated using adaptive Gaussian quadrature with 7 integration points. Alpha was set at 0.05, 2‐tailed. The final model was adjusted for gender, age, weight, length‐of‐stay, use of oral diabetes agents, use of sulfonylureas, and use of high‐dose corticosteroids. The use of a nonoral feeding route (eg, tube feeding or parenteral nutrition) was too infrequent to be considered in the adjusted analysis. All analyses were conducted in Stata/IC 10.0 (College Station, TX).
Results
A total of 245 patients provided 1315 patient‐days. Patient demographics are shown in Table 2. The patients' weight, length‐of‐stay, and primary diagnoses were similar across the 3 groups. There was a higher percentage of males in the IG as compared to the HCG.
| IG | CCG | P Value IG vs. CCG | HCG | P Value IG vs. HCG | |
|---|---|---|---|---|---|
| |||||
| Number of patients | 84 | 86 | 75 | ||
| Age, years, mean (SD) | 59.3 (15.3) | 60.4 (15.9) | 0.68 | 59.2 (17.2) | 0.96 |
| Range (n = 245) | 18‐94 | 20‐87 | 24‐92 | ||
| Weight in kg, mean (SD) | 92.2 (29.5) | 89.5 (27.2) | 0.57 | 94.2 (35.4) | 0.69 |
| Range (n = 237) | 40‐198 | 40‐188 | 42‐235 | ||
| Sex, n (%) (n = 245) | 0.17 | 0.04 | |||
| Male | 45 (53.6) | 37 (43.0) | 28 (37.3) | ||
| Female | 39(46.4) | 49 (57.0) | 47 (62.7) | ||
| Length of stay, mean (SD) | 7.6 (3.3) | 7.4 (3.0) | 0.62 | 7.0 (2.5) | 0.14 |
| Range (n = 245) | 4‐15 | 4‐15 | 4‐14 | ||
| Number of diagnoses | 169 | 158 | 160 | ||
| Primary diagnoses, n (%) | 0.56 | 0.10 | |||
| Infections | 40 (23.7) | 45 (28.5) | 49 (30.6) | ||
| Gastrointestinal | 33 (19.5) | 19 (12.0) | 14 (8.8) | ||
| Rheumatologic | 13 (7.7) | 12 (7.6) | 18 (11.2) | ||
| Renal | 14 (8.3) | 10 (6.3) | 16 (10.0) | ||
| Diabetes‐related | 11 (6.5) | 11 (7.0) | 10 (6.2) | ||
| Neurologic | 8 (4.7) | 11 (7.0) | 11 (6.9) | ||
| *Misc/other | 50 (29.6) | 50 (31.6) | 42 (26.3) | ||
Table 3 shows the insulin regimens used in the different groups. The use of basal insulin was similar between groups. Congruent with the goals of the education session and the order set, patients in the IG were more likely to be treated with a combination of basal and nutritional insulin than patients in the other groups. Patients in the HCG were more likely to be treated with a premixed insulin than patients in the other groups. However, even when premixed insulin was categorized as a form of basal plus nutritional insulin and combined into a composite group with the combined basal and nutritional days, this type of regimen remained more common in the IG than in the HCG. The rate of sliding scale insulin use alone (ie, without any scheduled insulin) was similar in the 3 groups.
| Patient‐days on the following | IG (n = 453) | CCG (n = 471) | P Value IG vs. CCG | HCG (n = 391) | P Value IG vs. HCG |
|---|---|---|---|---|---|
| |||||
| Sliding scale alone, n (%) | 105 (23.2) | 130 (27.6) | 0.12 | 89 (22.8) | 0.89 |
| Basal alone, n (%) | 132 (29.1) | 231 (49.0) | <0.01 | 199 (50.9) | <0.01 |
| Nutritional alone, n (%) | 22 (4.9) | 5 (1.1) | <0.01 | 8 (2.0) | 0.03 |
| Basal plus nutritional, n (%) | 166 (36.6) | 71 (15.1) | <0.01 | 14 (3.6) | <0.01 |
| Pre‐mixed insulin included, n (%) | 27 (6.0) | 32 (6.8) | 0.60 | 78 (20.0) | <0.01 |
| No insulin, n (%) | 1 (<1) | 2 (<1) | 0.59 | 3 (<1) | 0.28 |
| Any basal, n (%) | 325 (71.7) | 334 (70.9) | 0.78 | 291 (74.4) | 0.38 |
| Any nutritional, n (%) | 215 (47.5) | 108 (22.9) | <0.01 | 100 (25.6) | <0.01 |
| Basal plus nutritional or pre‐mixed, n (%) | 193 (42.6) | 103 (21.9) | <0.01 | 92 (23.5) | <0.01 |
| Oral diabetes agents, n (%) | 79 (17.4) | 83 (17.6) | 0.94 | 74 (18.9) | 0.58 |
| Sulfonylureas, n (%) | 40 (8.8) | 63 (13.4) | 0.03 | 37 (9.5) | 0.75 |
| Parenteral nutrition/tube feeds, n (%) | 0 (0) | 18 (3.8) | 8 (2.0) | ||
| High dose corticosteroids, n (%) | 66 (14.6) | 93 (19.8) | 0.04 | 51 (13.0) | 0.52 |
Other relevant measures are also shown in Table 3. The use of oral diabetes agents was similar in the 3 groups. The use of a nonoral feeding route (eg, tube feeding or parenteral nutrition) was infrequent.
A comparison of glycemic control in the three groups is shown in Table 4. In contrast to the CCG, patients in the IG experienced more days within the target glucose range (17% vs. 10.6%, P < 0.01), fewer days with severe hyperglycemia (48.3% vs. 59.2%, P < 0.01), and had a lower day‐weighted average blood glucose (195.9 vs. 212.6, P < 0.01). Compared to the HCG, patients in the IG experienced similar rates of hyperglycemia, but fewer hypoglycemic days (5.1% vs. 9.2%, P = 0.02).
| IG (n = 453) | CCG (n = 471) | P Value IG vs. CCG | HCG (n = 391) | P Value IG vs. HCG | |
|---|---|---|---|---|---|
| |||||
| Patient‐days | |||||
| In range, n (%) | 77 (17.0) | 50 (10.6) | <0.01 | 66 (16.9) | 0.98 |
| Out of range, n (%) | 376 (83.0) | 421 (89.4) | <0.01 | 325 (83.1) | 0.98 |
| Hyperglycemic, n (%) | 289 (63.8) | 310 (65.8) | 0.52 | 248 (63.4) | 0.91 |
| Severely hyperglycemic, n (%) | 219 (48.3) | 279 (59.2) | <0.01 | 176 (45.0) | 0.32 |
| Hypoglycemic, n (%) | 23 (5.1) | 36 (7.6) | 0.11 | 36 (9.2) | 0.02 |
| Severely hypoglycemic, n (%) | 13 (2.9) | 10 (2.1) | 0.47 | 15 (3.8) | 0.44 |
| Day weighted average blood glucose (SD) | 195.9 (66.8) | 212.6 (73.4) | <0.01 | 190.5 (63.1) | 0.25 |
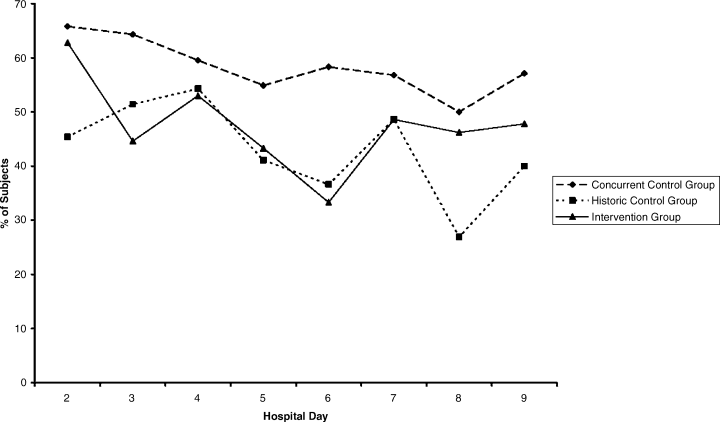
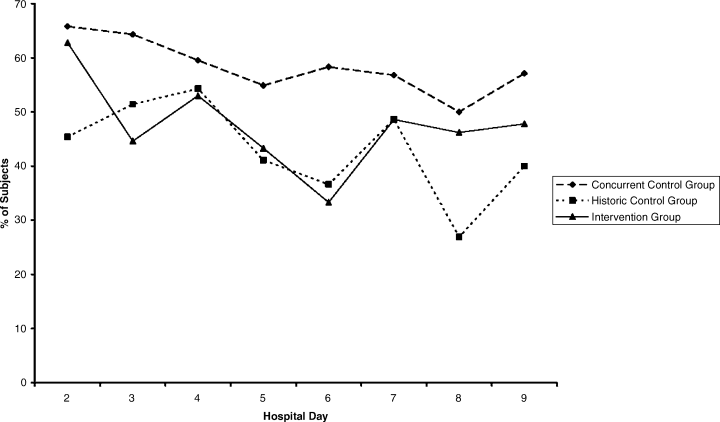
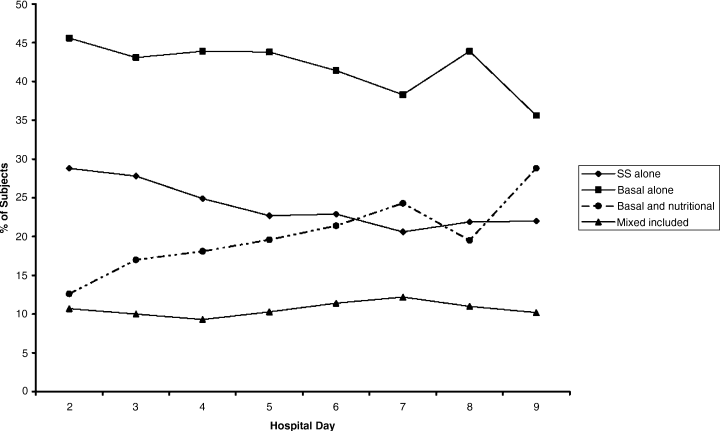
The percentages of patients with severe hyperglycemia in each group are shown in Figure 1 by hospital day. Severe hyperglycemia was common, but there was a trend towards a decrease in the prevalence of severe hyperglycemia with increasing hospital days for all study groups, although it was consistently higher in the CCG than in the IG. Figure 2 shows the types of insulin regimens used by hospital day (composite for all groups). The use of basal plus nutritional insulin (the recommended regimen) increased gradually with increasing hospital days. When taken together, the information in both figures support the hypothesis that the use of the recommended insulin regimen may have contributed to the modest improvements in glycemic control seen in the IG.
In the final adjusted regression model, the intervention had a positive impact on glycemic control (Table 5). Subjects in the IG had a 72% increase in the odds of being in the target glucose range when compared to subjects in the CCG (P = 0.01). In addition, subjects in the IG had a 35% reduction in the odds of being severely hyperglycemic when compared to those in the CCG (P < 0.01). Finally, the odds ratio (OR) for being hypoglycemic among intervention subjects was 0.59 (P = 0.06) when compared to subjects in the CCG and 0.48 (P = 0.01) when compared to subjects in the HCG.
| Adjusted OR* IG vs. CCG | 95% CI | P value IG vs. CCG | Adjusted OR* IG vs. HCG | 95% CI | P Value IG vs. HCG | |
|---|---|---|---|---|---|---|
| ||||||
| In range | 1.72 | 1.16,2.55 | 0.01 | 1.08 | 0.74,1.58 | 0.68 |
| Hyperglycemic | 0.93 | 0.70,1.22 | 0.58 | 0.95 | 0.71,1.28 | 0.74 |
| Severely Hyperglycemic | 0.65 | 0.49,0.85 | <0.01 | 1.10 | 0.82,1.47 | 0.52 |
| Hypoglycemic | 0.59 | 0.34,1.02 | 0.06 | 0.48 | 0.27,0.85 | 0.01 |
| Severely Hypoglycemic | 1.36 | 0.59,3.14 | 0.47 | 0.97 | 0.29,1.44 | 0.28 |
Discussion
In this study, we investigated the effects of a standardized insulin order set, coupled with physician and nurse education, on glycemic control in hyperglycemic hospitalized patients. These interventions were designed to encourage a standardized approach to the treatment of hyperglycemia in hospitalized patients, based on the principles of physiologic insulin use, as described above. Our data suggest that the interventions did, indeed, alter the way insulin was prescribed, as more patients in the IG received a combination of basal plus nutritional insulin (the recommended regimen) than in the other groups. These interventions were associated with improved glycemic outcomes in the IG as compared to the CCG. The IG experienced a higher percentage of days in the target range and a trend toward fewer hypoglycemic days than the CCG. Although the IG experienced a similar percentage of days in the target range, it had significantly fewer hypoglycemic days than the HCG.
It is useful to consider the results of our study in the context of 2 other similar studies performed by Schnipper et al.14 and Maynard et al.15 Although each of these 3 studies have different study designs, they are similar in intent (to test the effects of simple quality improvement interventions on glycemic control in the hospital) and results (all showed significant improvements in some aspect of glycemic control). In our study, and the study by Maynard et al.,15 the interventions also led to decreases in the rates of hypoglycemia, whereas Schnipper et al.14 observed no difference in hypoglycemia. Of interest, in each of the three studies the interventions were associated with an increase in the use of some type of scheduled insulin. In our study and the study from Schnipper et al.14 the baseline use of basal insulin was quite high, and the interventions were associated with a significant increase in the addition of nutritional insulin. In the Maynard et al.15 study, the baseline use of sliding scale insulin alone was prevalent, and the interventions resulted in an increase in the use of basal insulin. The results of these studies, taken together, prompt us to conclude that the interventions employed in these studies are likely to lead to more frequent prescription of scheduled (anticipatory) insulin, and a modest improvement in glycemic control, without an increase (and perhaps with a decrease) in hypoglycemia.
A few of our study results are unexpected, or difficult to explain. In contrast to the other studies discussed above, our interventions did not affect the frequency of the use of sliding‐scale insulin alone (without any scheduled insulin), which was similar in the 3 groups. Although the reason for this is uncertain, we hypothesize that the high baseline use of basal insulin in our institution, and the lack of a hard stop preventing the use of sliding scale insulin alone explain this finding. Also, it is difficult to explain why measures of hyperglycemia were similar between the IG and the HCG despite the fact that the HCG was less often treated with a combination of basal and nutritional insulin and more often treated with mixed insulin.
There are several different mechanisms by which the interventions might have resulted in improved glycemic control in the IG compared to CCG. Our data clearly shows that insulin was prescribed differently in the IG, and the more frequent use of a combination of scheduled basal and nutritional insulin might have contributed to the differences between the groups. However, the effects of our interventions clearly went beyond physician education into the realm of true process improvement and standardization. The standardized order form was designed to prompt physicians to use a basal‐bolus insulin regimen. The order form also created nursing expectations of how insulin should be ordered, and clarified the roles of the different insulins that were prescribed.
On the medication administration record, each insulin was labeled as basal insulin (to be given even when fasting) or nutritional insulin (to be given along with the meal). The nurses caring for the IG also attended an education program that reinforced the role of the nurse in the bedside management of insulin administration. Specifically, nurses were taught to assess the premeal blood glucose and the patient's nutritional situation before giving the nutritional insulin (ie, Does the patient have food available? Will he tolerate eating the food?). In situations where is was not clear if the patient would be able to tolerate the ordered nutrition, the order set empowered the nurse to give the nutritional insulin after the meal, and to reduce the dose to match the patient's actual intake. These interventions resulted in some fundamental improvements in the nursing process of delivering insulin to the patient, and these changes might have resulted in improvements via mechanisms that are difficult to directly measure. Since the same physicians cared for both the IG and the CCG, interventions other than physician education clearly contributed to the observed improvements in the IG.
This study was not a randomized study, and there could be important undetected differences between the groups. However, all of the patients were admitted to the General Medicine Inpatient Services and the comparison of the general patient demographics and primary diagnoses between the groups do not suggest major differences.
Although the improvements in glycemic control seen in this study were statistically significant, they were quantitatively modest. The rates of hyperglycemia seen in this study, on the other hand, are quite remarkable. Both the American Diabetes Association and the American College of Endocrinology have recommended that blood glucoses in hospitalized patients not exceed a maximum value of 180 mg/dL, but the day‐weighted average blood glucose in this study was above that for each group. Even in the IG, over 80% of all patient‐days included at least 1 blood glucose value outside of the target range. These data suggest that better strategies for achieving metabolic control in hospitalized patients are needed.
It is worth mentioning that our interventions were not aggressively enforced. While the use of the order set was mandatory for the IG, it was flexible enough to allow for substantial practice variation, especially with respect to the dose of insulin prescribed. Although the education sessions discussed the specifics of insulin dosing in hospitalized patients, the order form did not offer dosing guidelines. It is possible that our interventions may have had a larger impact if a starting dose of insulin had been specified on the form. Although the insulin order form prompted physicians to act, there were no forced functions. Also, not all house staff attended the education sessions for physicians, and there was no feedback provided to physicians related to how they might improve their adherence to the recommendations presented in the educational module. Therefore, it is likely that more aggressive interventions could have led to greater changes in physician practice.
In conclusion, this study demonstrates that interventions including physician and nurse education and a standardized insulin order set can lead to improvement in glycemic control and patient safety in hospitalized patients treated with subcutaneous insulin. However, the observed improvements are modest, and poor metabolic control remains common, despite these interventions. These data suggest that standardization of the process of ordering and delivering subcutaneous insulin in the hospital may lead to a reduction in both hyperglycemia and hypoglycemia. However, it is clear that the interventions used in this study were not potent enough to achieve the recommended glycemic targets for the majority of patients. Additional research is needed to determine the best strategy for achieving safe and effective metabolic control in hospitalized, hyperglycemic, noncritically ill patients.
Acknowledgements
The authors thank David Conway for his work in data collection and management.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–360; discussion360–352.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1:141–144.
- ,,, et al.Evaluation of hospital glycemic control at US Academic Medical Centers.J Hosp Med.2009;4:35–44.
- ,,, et al.Diabetes care in the hospital: is there clinical inertia?J Hosp Med.2006;1:151–160.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Effects of a subcutaneous insulin protocol, clinical education, and computerized order set on the quality of inpatient management of hyperglycemia: results of a clinical trial.J Hosp Med.2009;4:16–27.
- ,,,,.Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm.J Hosp Med.2009;4:3–15.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77–82.
- Society of Hospital Medicine. Glycemic Control Resource Room. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section= Quality_Improvement_Resource_Rooms3(5 Suppl):17–28.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(5 Suppl):29–41.
- ,,,,.Society of Hospital Medicine Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(5 Suppl):66–75.
Hyperglycemia is common in hospitalized patients, and hyperglycemia has been associated with poor hospital outcomes. The adverse physiologic effects of acute hyperglycemia are well established1 and several clinical studies have linked hyperglycemia with poor clinical outcomes in certain patient populations.28 Although the optimal target range for inpatient glycemic control has not yet been defined, these studies support the goal of metabolic control for hospital patients. However, there are many barriers to achieving adequate glycemic control in the hospital, and blood glucoses in the hospital are often far from recommended targets.9, 10 One barrier appears to be the low priority given to glycemic control in the hospital. Hyperglycemia in the hospital is often ignored,11 and insulin regimens are often chosen for simplicity as opposed to effectiveness.12 Other barriers to glycemic control in the hospital include the physiologic effects (stress) of acute illness, and the frequent nutritional changes and interruptions that occur.
Most hyperglycemic patients on a general medicine unit are treated with subcutaneous insulin, but the optimal strategy for prescribing insulin in the hospital remains uncertain. A technical review of the literature on the management of diabetes in the hospital setting from 2004 recommends prescribing insulin in a way that mimics physiologic insulin secretion (ie, physiologic or basal‐bolus insulin).1 This approach has been promulgated by experts, but there has been very little research to support these recommendations. One small, randomized trial concluded that a basal‐bolus approach achieved better glycemic control than the use of sliding‐scale insulin alone,13 and 2 quality improvement studies using a before/after design have demonstrated improvements in glycemic control after the implementation of interventions designed to encourage physiologic insulin use.14, 15
In this study we hypothesized that a few simple interventions (education for physicians and nurses, and a standardized insulin order form) would lead to a higher rate of basal‐bolus insulin use and simultaneously improve glycemic control and patient safety.
Methods
Study Design
This study was performed at the University of Michigan Hospital over a 6‐month period, and the protocol was approved by the Institutional Review Board. We performed a quasi‐experimental study comparing 3 patient groups. The intervention group (IG) was subject to all of the interventions discussed below (physician education, nurse education, and the standardized order form). The concurrent control group (CCG) was hospitalized during the same time period as the IG, but was only subject to 1 of the interventions (physician education). These patients were cared for by the same physicians as the IG, but on a different unit where the nurses had not received the education and where the standardized insulin order form was not available. Patients were admitted to the IG unit or the CCG unit via the institution's usual admission process. In addition, we examined an historic control group (HCG) which was hospitalized during the same months of the year, but 2 years prior. The HCG was not subject to any of the interventions.
Interventions
Standardized Subcutaneous Insulin Order Form
This form (Supporting Information Appendix 1) was designed to encourage physicians to prescribe insulin in a physiologic way, providing basal, nutritional, and correctional insulin. The form is based on best practice guidelines,1 and is in agreement with the principles of the inpatient management of diabetes and hyperglycemia endorsed by several professional organizations.16, 17 The form was engineered by a multidisciplinary team, including an endocrinologist, several hospitalists, several nurses, a certified diabetes educator, a pharmacist, and others. It is derived from the extensive experience of the University of Michigan Hospital Intensive Insulin Program (HIIP) in the Division of Endocrinology, and on work done by the Society of Hospital Medicine (SHM) Glycemic Control Task Force.1719 This form was only used in the care of patients in the IG. The form, which was not approved for use on other floors, did not creep to other units. The standardized order form was the only way to order insulin or to modify the insulin regimen on the IG unit. The frequency of review or revision of the insulin orders was left to the discretion of the inpatient physicians.
Physician/Midlevel Provider Education
Physicians and midlevel providers caring for patients in the IG and the CCG were given specific education about the best practice recommendations for the management of diabetes and hyperglycemia in hospitalized patients. This education was based on the principles of anticipatory, physiologic insulin use. On nonhouse staff services, the education was provided to the attending physicians and midlevel providers, and on house staff services, the education was provided to the residents. All physician education was provided by the physician authors (D.W. and R.G.). A summary of the content of the physician education is provided in Supporting Information Appendix 2.
Nurse Education
Nurses caring for patients in the IG were given education similar to that which was provided to the physicians (see above), with an emphasis on practical issues related to delivering physiologic insulin. It included topics such as blood glucose monitoring, and the real‐time manipulation of nutritional insulin doses in accordance with the clinical situation (decision‐making that was specifically delegated to the nursing staff by the order set).
Patients
Patients were eligible for inclusion in the analysis if they met the following inclusion criteria: they were admitted to the inpatient General Internal Medicine Services; subcutaneous insulin was provided to the patient during the hospitalization; they had at least 2 blood glucose values >180 mg/dL; they were discharged from the hospital on a pharmacologic glucose lowering agent (insulin or oral); and their total length‐of‐stay was 3 days to 14 days. Patients were excluded from the analysis if they were admitted with a primary diagnosis of diabetic ketoacidosis, diabetic hyperosmolar state, or hypoglycemia. Up to 10 consecutive days of glucose data were recorded for each patient, and the first day on which blood glucose information was available from the admitting floor was excluded from the analysis. Also, specific patient‐days were not analyzed if there were no bedside glucoses recorded, or if the patient was treated with an IV insulin infusion on that day.
Outcomes
The primary outcome was glycemic control. The primary unit of measure was the patient‐day (ie, all of the information for 1 patient on a single qualifying day). This was done to correct for the phenomenon of frequent repeat testing in response to abnormal values. It also allows for a more clinically relevant description of the actual glycemic control on a given day. Specifically, each patient‐day was categorized as in‐range (70‐180 mg/dL), hyperglycemic (>180 mg/dL), severely hyperglycemic (>250 mg/dL), hypoglycemic (<70 mg/dL), and/or severely hypoglycemic (<50 mg/dL). The primary endpoint was glycemic control in‐range. For a patient‐day to be in‐range, all readings for that particular day were within 70 mg/dL to180 mg/dL. For the readings that were not in the desired range, a minimum of 1 deviant reading in a particular day constituted classification into that category, and a single out‐of‐range patient‐day could be included in 1 or more of the out‐of‐range categories (eg, a patient‐day could be categorized as both severely hyperglycemic and hypoglycemic if it contained glucose readings in both of those ranges).
The day‐weighted mean blood glucose value was also calculated for each of the groups. This calculation utilized the mean blood glucose for each patient‐day, and then averaged these values for each group. These metrics have been endorsed as appropriate measures of glycemic control by the SHM Glycemic Control Task Force.20
Other Data
Several other clinical features were also examined, including the following: primary diagnoses listed in the hospital discharge summary for each patient (3 maximum); possible confounders including patient weight, length‐of‐stay, days receiving tube feeds, days receiving parenteral nutrition, and days during which patients were treated with high‐dose glucocorticoids (>10 mg/day of prednisone, or its equivalent) or oral diabetes medications; and the composition of the insulin regimen on each hospital day. Definitions of insulin regimens are provided in Table 1.
| |
| Any basal insulin day | Any day in which intermediate‐acting or long‐acting, scheduled insulin was given. |
| Basal insulin alone day | A day in which intermediate‐acting or long‐acting insulin was the only scheduled insulin given. |
| Any nutritional insulin day | Any day in which rapid‐acting or short‐acting, scheduled insulin was given. |
| Nutritional insulin alone day | A day in which rapid‐acting or short‐acting insulin was the only scheduled insulin given. |
| Basal plus nutritional day | A day in which both scheduled, intermediate‐acting or long‐acting insulin and scheduled, rapid‐acting or short‐acting insulin were given. |
| Pre‐mixed insulin day | Any day in which a pre‐mixed combination insulin was given. |
| Basal plus nutritional or pre‐mixed insulin day | A composite of the basal plus nutritional day category and the mixed insulin day category described above. This group includes any day in which either a pre‐mixed combination insulin was given OR a day in which both: (a) scheduled, intermediate‐acting or long‐acting and (b) scheduled, rapid‐acting or short‐acting insulin were given. |
| Sliding scale insulin alone day* | Any day when only correctional (as needed) insulin was given. |
Statistical Analysis
Bivariate analyses (chi‐square, and t‐tests) were carried out to compare demographic characteristics of the intervention and control populations. Since there were multiple glucose readings nested within individuals, multilevel mixed‐effects logistic regression was used to evaluate the association between the intervention and outcomes. A 2‐level hierarchical model was developed in which patient‐days were nested within patients; this accounted for the correlation between glycemic control across days for a given patient. Patient‐day was modeled as a random intercept and the log likelihood was estimated using adaptive Gaussian quadrature with 7 integration points. Alpha was set at 0.05, 2‐tailed. The final model was adjusted for gender, age, weight, length‐of‐stay, use of oral diabetes agents, use of sulfonylureas, and use of high‐dose corticosteroids. The use of a nonoral feeding route (eg, tube feeding or parenteral nutrition) was too infrequent to be considered in the adjusted analysis. All analyses were conducted in Stata/IC 10.0 (College Station, TX).
Results
A total of 245 patients provided 1315 patient‐days. Patient demographics are shown in Table 2. The patients' weight, length‐of‐stay, and primary diagnoses were similar across the 3 groups. There was a higher percentage of males in the IG as compared to the HCG.
| IG | CCG | P Value IG vs. CCG | HCG | P Value IG vs. HCG | |
|---|---|---|---|---|---|
| |||||
| Number of patients | 84 | 86 | 75 | ||
| Age, years, mean (SD) | 59.3 (15.3) | 60.4 (15.9) | 0.68 | 59.2 (17.2) | 0.96 |
| Range (n = 245) | 18‐94 | 20‐87 | 24‐92 | ||
| Weight in kg, mean (SD) | 92.2 (29.5) | 89.5 (27.2) | 0.57 | 94.2 (35.4) | 0.69 |
| Range (n = 237) | 40‐198 | 40‐188 | 42‐235 | ||
| Sex, n (%) (n = 245) | 0.17 | 0.04 | |||
| Male | 45 (53.6) | 37 (43.0) | 28 (37.3) | ||
| Female | 39(46.4) | 49 (57.0) | 47 (62.7) | ||
| Length of stay, mean (SD) | 7.6 (3.3) | 7.4 (3.0) | 0.62 | 7.0 (2.5) | 0.14 |
| Range (n = 245) | 4‐15 | 4‐15 | 4‐14 | ||
| Number of diagnoses | 169 | 158 | 160 | ||
| Primary diagnoses, n (%) | 0.56 | 0.10 | |||
| Infections | 40 (23.7) | 45 (28.5) | 49 (30.6) | ||
| Gastrointestinal | 33 (19.5) | 19 (12.0) | 14 (8.8) | ||
| Rheumatologic | 13 (7.7) | 12 (7.6) | 18 (11.2) | ||
| Renal | 14 (8.3) | 10 (6.3) | 16 (10.0) | ||
| Diabetes‐related | 11 (6.5) | 11 (7.0) | 10 (6.2) | ||
| Neurologic | 8 (4.7) | 11 (7.0) | 11 (6.9) | ||
| *Misc/other | 50 (29.6) | 50 (31.6) | 42 (26.3) | ||
Table 3 shows the insulin regimens used in the different groups. The use of basal insulin was similar between groups. Congruent with the goals of the education session and the order set, patients in the IG were more likely to be treated with a combination of basal and nutritional insulin than patients in the other groups. Patients in the HCG were more likely to be treated with a premixed insulin than patients in the other groups. However, even when premixed insulin was categorized as a form of basal plus nutritional insulin and combined into a composite group with the combined basal and nutritional days, this type of regimen remained more common in the IG than in the HCG. The rate of sliding scale insulin use alone (ie, without any scheduled insulin) was similar in the 3 groups.
| Patient‐days on the following | IG (n = 453) | CCG (n = 471) | P Value IG vs. CCG | HCG (n = 391) | P Value IG vs. HCG |
|---|---|---|---|---|---|
| |||||
| Sliding scale alone, n (%) | 105 (23.2) | 130 (27.6) | 0.12 | 89 (22.8) | 0.89 |
| Basal alone, n (%) | 132 (29.1) | 231 (49.0) | <0.01 | 199 (50.9) | <0.01 |
| Nutritional alone, n (%) | 22 (4.9) | 5 (1.1) | <0.01 | 8 (2.0) | 0.03 |
| Basal plus nutritional, n (%) | 166 (36.6) | 71 (15.1) | <0.01 | 14 (3.6) | <0.01 |
| Pre‐mixed insulin included, n (%) | 27 (6.0) | 32 (6.8) | 0.60 | 78 (20.0) | <0.01 |
| No insulin, n (%) | 1 (<1) | 2 (<1) | 0.59 | 3 (<1) | 0.28 |
| Any basal, n (%) | 325 (71.7) | 334 (70.9) | 0.78 | 291 (74.4) | 0.38 |
| Any nutritional, n (%) | 215 (47.5) | 108 (22.9) | <0.01 | 100 (25.6) | <0.01 |
| Basal plus nutritional or pre‐mixed, n (%) | 193 (42.6) | 103 (21.9) | <0.01 | 92 (23.5) | <0.01 |
| Oral diabetes agents, n (%) | 79 (17.4) | 83 (17.6) | 0.94 | 74 (18.9) | 0.58 |
| Sulfonylureas, n (%) | 40 (8.8) | 63 (13.4) | 0.03 | 37 (9.5) | 0.75 |
| Parenteral nutrition/tube feeds, n (%) | 0 (0) | 18 (3.8) | 8 (2.0) | ||
| High dose corticosteroids, n (%) | 66 (14.6) | 93 (19.8) | 0.04 | 51 (13.0) | 0.52 |
Other relevant measures are also shown in Table 3. The use of oral diabetes agents was similar in the 3 groups. The use of a nonoral feeding route (eg, tube feeding or parenteral nutrition) was infrequent.
A comparison of glycemic control in the three groups is shown in Table 4. In contrast to the CCG, patients in the IG experienced more days within the target glucose range (17% vs. 10.6%, P < 0.01), fewer days with severe hyperglycemia (48.3% vs. 59.2%, P < 0.01), and had a lower day‐weighted average blood glucose (195.9 vs. 212.6, P < 0.01). Compared to the HCG, patients in the IG experienced similar rates of hyperglycemia, but fewer hypoglycemic days (5.1% vs. 9.2%, P = 0.02).
| IG (n = 453) | CCG (n = 471) | P Value IG vs. CCG | HCG (n = 391) | P Value IG vs. HCG | |
|---|---|---|---|---|---|
| |||||
| Patient‐days | |||||
| In range, n (%) | 77 (17.0) | 50 (10.6) | <0.01 | 66 (16.9) | 0.98 |
| Out of range, n (%) | 376 (83.0) | 421 (89.4) | <0.01 | 325 (83.1) | 0.98 |
| Hyperglycemic, n (%) | 289 (63.8) | 310 (65.8) | 0.52 | 248 (63.4) | 0.91 |
| Severely hyperglycemic, n (%) | 219 (48.3) | 279 (59.2) | <0.01 | 176 (45.0) | 0.32 |
| Hypoglycemic, n (%) | 23 (5.1) | 36 (7.6) | 0.11 | 36 (9.2) | 0.02 |
| Severely hypoglycemic, n (%) | 13 (2.9) | 10 (2.1) | 0.47 | 15 (3.8) | 0.44 |
| Day weighted average blood glucose (SD) | 195.9 (66.8) | 212.6 (73.4) | <0.01 | 190.5 (63.1) | 0.25 |
The percentages of patients with severe hyperglycemia in each group are shown in Figure 1 by hospital day. Severe hyperglycemia was common, but there was a trend towards a decrease in the prevalence of severe hyperglycemia with increasing hospital days for all study groups, although it was consistently higher in the CCG than in the IG. Figure 2 shows the types of insulin regimens used by hospital day (composite for all groups). The use of basal plus nutritional insulin (the recommended regimen) increased gradually with increasing hospital days. When taken together, the information in both figures support the hypothesis that the use of the recommended insulin regimen may have contributed to the modest improvements in glycemic control seen in the IG.


In the final adjusted regression model, the intervention had a positive impact on glycemic control (Table 5). Subjects in the IG had a 72% increase in the odds of being in the target glucose range when compared to subjects in the CCG (P = 0.01). In addition, subjects in the IG had a 35% reduction in the odds of being severely hyperglycemic when compared to those in the CCG (P < 0.01). Finally, the odds ratio (OR) for being hypoglycemic among intervention subjects was 0.59 (P = 0.06) when compared to subjects in the CCG and 0.48 (P = 0.01) when compared to subjects in the HCG.
| Adjusted OR* IG vs. CCG | 95% CI | P value IG vs. CCG | Adjusted OR* IG vs. HCG | 95% CI | P Value IG vs. HCG | |
|---|---|---|---|---|---|---|
| ||||||
| In range | 1.72 | 1.16,2.55 | 0.01 | 1.08 | 0.74,1.58 | 0.68 |
| Hyperglycemic | 0.93 | 0.70,1.22 | 0.58 | 0.95 | 0.71,1.28 | 0.74 |
| Severely Hyperglycemic | 0.65 | 0.49,0.85 | <0.01 | 1.10 | 0.82,1.47 | 0.52 |
| Hypoglycemic | 0.59 | 0.34,1.02 | 0.06 | 0.48 | 0.27,0.85 | 0.01 |
| Severely Hypoglycemic | 1.36 | 0.59,3.14 | 0.47 | 0.97 | 0.29,1.44 | 0.28 |
Discussion
In this study, we investigated the effects of a standardized insulin order set, coupled with physician and nurse education, on glycemic control in hyperglycemic hospitalized patients. These interventions were designed to encourage a standardized approach to the treatment of hyperglycemia in hospitalized patients, based on the principles of physiologic insulin use, as described above. Our data suggest that the interventions did, indeed, alter the way insulin was prescribed, as more patients in the IG received a combination of basal plus nutritional insulin (the recommended regimen) than in the other groups. These interventions were associated with improved glycemic outcomes in the IG as compared to the CCG. The IG experienced a higher percentage of days in the target range and a trend toward fewer hypoglycemic days than the CCG. Although the IG experienced a similar percentage of days in the target range, it had significantly fewer hypoglycemic days than the HCG.
It is useful to consider the results of our study in the context of 2 other similar studies performed by Schnipper et al.14 and Maynard et al.15 Although each of these 3 studies have different study designs, they are similar in intent (to test the effects of simple quality improvement interventions on glycemic control in the hospital) and results (all showed significant improvements in some aspect of glycemic control). In our study, and the study by Maynard et al.,15 the interventions also led to decreases in the rates of hypoglycemia, whereas Schnipper et al.14 observed no difference in hypoglycemia. Of interest, in each of the three studies the interventions were associated with an increase in the use of some type of scheduled insulin. In our study and the study from Schnipper et al.14 the baseline use of basal insulin was quite high, and the interventions were associated with a significant increase in the addition of nutritional insulin. In the Maynard et al.15 study, the baseline use of sliding scale insulin alone was prevalent, and the interventions resulted in an increase in the use of basal insulin. The results of these studies, taken together, prompt us to conclude that the interventions employed in these studies are likely to lead to more frequent prescription of scheduled (anticipatory) insulin, and a modest improvement in glycemic control, without an increase (and perhaps with a decrease) in hypoglycemia.
A few of our study results are unexpected, or difficult to explain. In contrast to the other studies discussed above, our interventions did not affect the frequency of the use of sliding‐scale insulin alone (without any scheduled insulin), which was similar in the 3 groups. Although the reason for this is uncertain, we hypothesize that the high baseline use of basal insulin in our institution, and the lack of a hard stop preventing the use of sliding scale insulin alone explain this finding. Also, it is difficult to explain why measures of hyperglycemia were similar between the IG and the HCG despite the fact that the HCG was less often treated with a combination of basal and nutritional insulin and more often treated with mixed insulin.
There are several different mechanisms by which the interventions might have resulted in improved glycemic control in the IG compared to CCG. Our data clearly shows that insulin was prescribed differently in the IG, and the more frequent use of a combination of scheduled basal and nutritional insulin might have contributed to the differences between the groups. However, the effects of our interventions clearly went beyond physician education into the realm of true process improvement and standardization. The standardized order form was designed to prompt physicians to use a basal‐bolus insulin regimen. The order form also created nursing expectations of how insulin should be ordered, and clarified the roles of the different insulins that were prescribed.
On the medication administration record, each insulin was labeled as basal insulin (to be given even when fasting) or nutritional insulin (to be given along with the meal). The nurses caring for the IG also attended an education program that reinforced the role of the nurse in the bedside management of insulin administration. Specifically, nurses were taught to assess the premeal blood glucose and the patient's nutritional situation before giving the nutritional insulin (ie, Does the patient have food available? Will he tolerate eating the food?). In situations where is was not clear if the patient would be able to tolerate the ordered nutrition, the order set empowered the nurse to give the nutritional insulin after the meal, and to reduce the dose to match the patient's actual intake. These interventions resulted in some fundamental improvements in the nursing process of delivering insulin to the patient, and these changes might have resulted in improvements via mechanisms that are difficult to directly measure. Since the same physicians cared for both the IG and the CCG, interventions other than physician education clearly contributed to the observed improvements in the IG.
This study was not a randomized study, and there could be important undetected differences between the groups. However, all of the patients were admitted to the General Medicine Inpatient Services and the comparison of the general patient demographics and primary diagnoses between the groups do not suggest major differences.
Although the improvements in glycemic control seen in this study were statistically significant, they were quantitatively modest. The rates of hyperglycemia seen in this study, on the other hand, are quite remarkable. Both the American Diabetes Association and the American College of Endocrinology have recommended that blood glucoses in hospitalized patients not exceed a maximum value of 180 mg/dL, but the day‐weighted average blood glucose in this study was above that for each group. Even in the IG, over 80% of all patient‐days included at least 1 blood glucose value outside of the target range. These data suggest that better strategies for achieving metabolic control in hospitalized patients are needed.
It is worth mentioning that our interventions were not aggressively enforced. While the use of the order set was mandatory for the IG, it was flexible enough to allow for substantial practice variation, especially with respect to the dose of insulin prescribed. Although the education sessions discussed the specifics of insulin dosing in hospitalized patients, the order form did not offer dosing guidelines. It is possible that our interventions may have had a larger impact if a starting dose of insulin had been specified on the form. Although the insulin order form prompted physicians to act, there were no forced functions. Also, not all house staff attended the education sessions for physicians, and there was no feedback provided to physicians related to how they might improve their adherence to the recommendations presented in the educational module. Therefore, it is likely that more aggressive interventions could have led to greater changes in physician practice.
In conclusion, this study demonstrates that interventions including physician and nurse education and a standardized insulin order set can lead to improvement in glycemic control and patient safety in hospitalized patients treated with subcutaneous insulin. However, the observed improvements are modest, and poor metabolic control remains common, despite these interventions. These data suggest that standardization of the process of ordering and delivering subcutaneous insulin in the hospital may lead to a reduction in both hyperglycemia and hypoglycemia. However, it is clear that the interventions used in this study were not potent enough to achieve the recommended glycemic targets for the majority of patients. Additional research is needed to determine the best strategy for achieving safe and effective metabolic control in hospitalized, hyperglycemic, noncritically ill patients.
Acknowledgements
The authors thank David Conway for his work in data collection and management.
Hyperglycemia is common in hospitalized patients, and hyperglycemia has been associated with poor hospital outcomes. The adverse physiologic effects of acute hyperglycemia are well established1 and several clinical studies have linked hyperglycemia with poor clinical outcomes in certain patient populations.28 Although the optimal target range for inpatient glycemic control has not yet been defined, these studies support the goal of metabolic control for hospital patients. However, there are many barriers to achieving adequate glycemic control in the hospital, and blood glucoses in the hospital are often far from recommended targets.9, 10 One barrier appears to be the low priority given to glycemic control in the hospital. Hyperglycemia in the hospital is often ignored,11 and insulin regimens are often chosen for simplicity as opposed to effectiveness.12 Other barriers to glycemic control in the hospital include the physiologic effects (stress) of acute illness, and the frequent nutritional changes and interruptions that occur.
Most hyperglycemic patients on a general medicine unit are treated with subcutaneous insulin, but the optimal strategy for prescribing insulin in the hospital remains uncertain. A technical review of the literature on the management of diabetes in the hospital setting from 2004 recommends prescribing insulin in a way that mimics physiologic insulin secretion (ie, physiologic or basal‐bolus insulin).1 This approach has been promulgated by experts, but there has been very little research to support these recommendations. One small, randomized trial concluded that a basal‐bolus approach achieved better glycemic control than the use of sliding‐scale insulin alone,13 and 2 quality improvement studies using a before/after design have demonstrated improvements in glycemic control after the implementation of interventions designed to encourage physiologic insulin use.14, 15
In this study we hypothesized that a few simple interventions (education for physicians and nurses, and a standardized insulin order form) would lead to a higher rate of basal‐bolus insulin use and simultaneously improve glycemic control and patient safety.
Methods
Study Design
This study was performed at the University of Michigan Hospital over a 6‐month period, and the protocol was approved by the Institutional Review Board. We performed a quasi‐experimental study comparing 3 patient groups. The intervention group (IG) was subject to all of the interventions discussed below (physician education, nurse education, and the standardized order form). The concurrent control group (CCG) was hospitalized during the same time period as the IG, but was only subject to 1 of the interventions (physician education). These patients were cared for by the same physicians as the IG, but on a different unit where the nurses had not received the education and where the standardized insulin order form was not available. Patients were admitted to the IG unit or the CCG unit via the institution's usual admission process. In addition, we examined an historic control group (HCG) which was hospitalized during the same months of the year, but 2 years prior. The HCG was not subject to any of the interventions.
Interventions
Standardized Subcutaneous Insulin Order Form
This form (Supporting Information Appendix 1) was designed to encourage physicians to prescribe insulin in a physiologic way, providing basal, nutritional, and correctional insulin. The form is based on best practice guidelines,1 and is in agreement with the principles of the inpatient management of diabetes and hyperglycemia endorsed by several professional organizations.16, 17 The form was engineered by a multidisciplinary team, including an endocrinologist, several hospitalists, several nurses, a certified diabetes educator, a pharmacist, and others. It is derived from the extensive experience of the University of Michigan Hospital Intensive Insulin Program (HIIP) in the Division of Endocrinology, and on work done by the Society of Hospital Medicine (SHM) Glycemic Control Task Force.1719 This form was only used in the care of patients in the IG. The form, which was not approved for use on other floors, did not creep to other units. The standardized order form was the only way to order insulin or to modify the insulin regimen on the IG unit. The frequency of review or revision of the insulin orders was left to the discretion of the inpatient physicians.
Physician/Midlevel Provider Education
Physicians and midlevel providers caring for patients in the IG and the CCG were given specific education about the best practice recommendations for the management of diabetes and hyperglycemia in hospitalized patients. This education was based on the principles of anticipatory, physiologic insulin use. On nonhouse staff services, the education was provided to the attending physicians and midlevel providers, and on house staff services, the education was provided to the residents. All physician education was provided by the physician authors (D.W. and R.G.). A summary of the content of the physician education is provided in Supporting Information Appendix 2.
Nurse Education
Nurses caring for patients in the IG were given education similar to that which was provided to the physicians (see above), with an emphasis on practical issues related to delivering physiologic insulin. It included topics such as blood glucose monitoring, and the real‐time manipulation of nutritional insulin doses in accordance with the clinical situation (decision‐making that was specifically delegated to the nursing staff by the order set).
Patients
Patients were eligible for inclusion in the analysis if they met the following inclusion criteria: they were admitted to the inpatient General Internal Medicine Services; subcutaneous insulin was provided to the patient during the hospitalization; they had at least 2 blood glucose values >180 mg/dL; they were discharged from the hospital on a pharmacologic glucose lowering agent (insulin or oral); and their total length‐of‐stay was 3 days to 14 days. Patients were excluded from the analysis if they were admitted with a primary diagnosis of diabetic ketoacidosis, diabetic hyperosmolar state, or hypoglycemia. Up to 10 consecutive days of glucose data were recorded for each patient, and the first day on which blood glucose information was available from the admitting floor was excluded from the analysis. Also, specific patient‐days were not analyzed if there were no bedside glucoses recorded, or if the patient was treated with an IV insulin infusion on that day.
Outcomes
The primary outcome was glycemic control. The primary unit of measure was the patient‐day (ie, all of the information for 1 patient on a single qualifying day). This was done to correct for the phenomenon of frequent repeat testing in response to abnormal values. It also allows for a more clinically relevant description of the actual glycemic control on a given day. Specifically, each patient‐day was categorized as in‐range (70‐180 mg/dL), hyperglycemic (>180 mg/dL), severely hyperglycemic (>250 mg/dL), hypoglycemic (<70 mg/dL), and/or severely hypoglycemic (<50 mg/dL). The primary endpoint was glycemic control in‐range. For a patient‐day to be in‐range, all readings for that particular day were within 70 mg/dL to180 mg/dL. For the readings that were not in the desired range, a minimum of 1 deviant reading in a particular day constituted classification into that category, and a single out‐of‐range patient‐day could be included in 1 or more of the out‐of‐range categories (eg, a patient‐day could be categorized as both severely hyperglycemic and hypoglycemic if it contained glucose readings in both of those ranges).
The day‐weighted mean blood glucose value was also calculated for each of the groups. This calculation utilized the mean blood glucose for each patient‐day, and then averaged these values for each group. These metrics have been endorsed as appropriate measures of glycemic control by the SHM Glycemic Control Task Force.20
Other Data
Several other clinical features were also examined, including the following: primary diagnoses listed in the hospital discharge summary for each patient (3 maximum); possible confounders including patient weight, length‐of‐stay, days receiving tube feeds, days receiving parenteral nutrition, and days during which patients were treated with high‐dose glucocorticoids (>10 mg/day of prednisone, or its equivalent) or oral diabetes medications; and the composition of the insulin regimen on each hospital day. Definitions of insulin regimens are provided in Table 1.
| |
| Any basal insulin day | Any day in which intermediate‐acting or long‐acting, scheduled insulin was given. |
| Basal insulin alone day | A day in which intermediate‐acting or long‐acting insulin was the only scheduled insulin given. |
| Any nutritional insulin day | Any day in which rapid‐acting or short‐acting, scheduled insulin was given. |
| Nutritional insulin alone day | A day in which rapid‐acting or short‐acting insulin was the only scheduled insulin given. |
| Basal plus nutritional day | A day in which both scheduled, intermediate‐acting or long‐acting insulin and scheduled, rapid‐acting or short‐acting insulin were given. |
| Pre‐mixed insulin day | Any day in which a pre‐mixed combination insulin was given. |
| Basal plus nutritional or pre‐mixed insulin day | A composite of the basal plus nutritional day category and the mixed insulin day category described above. This group includes any day in which either a pre‐mixed combination insulin was given OR a day in which both: (a) scheduled, intermediate‐acting or long‐acting and (b) scheduled, rapid‐acting or short‐acting insulin were given. |
| Sliding scale insulin alone day* | Any day when only correctional (as needed) insulin was given. |
Statistical Analysis
Bivariate analyses (chi‐square, and t‐tests) were carried out to compare demographic characteristics of the intervention and control populations. Since there were multiple glucose readings nested within individuals, multilevel mixed‐effects logistic regression was used to evaluate the association between the intervention and outcomes. A 2‐level hierarchical model was developed in which patient‐days were nested within patients; this accounted for the correlation between glycemic control across days for a given patient. Patient‐day was modeled as a random intercept and the log likelihood was estimated using adaptive Gaussian quadrature with 7 integration points. Alpha was set at 0.05, 2‐tailed. The final model was adjusted for gender, age, weight, length‐of‐stay, use of oral diabetes agents, use of sulfonylureas, and use of high‐dose corticosteroids. The use of a nonoral feeding route (eg, tube feeding or parenteral nutrition) was too infrequent to be considered in the adjusted analysis. All analyses were conducted in Stata/IC 10.0 (College Station, TX).
Results
A total of 245 patients provided 1315 patient‐days. Patient demographics are shown in Table 2. The patients' weight, length‐of‐stay, and primary diagnoses were similar across the 3 groups. There was a higher percentage of males in the IG as compared to the HCG.
| IG | CCG | P Value IG vs. CCG | HCG | P Value IG vs. HCG | |
|---|---|---|---|---|---|
| |||||
| Number of patients | 84 | 86 | 75 | ||
| Age, years, mean (SD) | 59.3 (15.3) | 60.4 (15.9) | 0.68 | 59.2 (17.2) | 0.96 |
| Range (n = 245) | 18‐94 | 20‐87 | 24‐92 | ||
| Weight in kg, mean (SD) | 92.2 (29.5) | 89.5 (27.2) | 0.57 | 94.2 (35.4) | 0.69 |
| Range (n = 237) | 40‐198 | 40‐188 | 42‐235 | ||
| Sex, n (%) (n = 245) | 0.17 | 0.04 | |||
| Male | 45 (53.6) | 37 (43.0) | 28 (37.3) | ||
| Female | 39(46.4) | 49 (57.0) | 47 (62.7) | ||
| Length of stay, mean (SD) | 7.6 (3.3) | 7.4 (3.0) | 0.62 | 7.0 (2.5) | 0.14 |
| Range (n = 245) | 4‐15 | 4‐15 | 4‐14 | ||
| Number of diagnoses | 169 | 158 | 160 | ||
| Primary diagnoses, n (%) | 0.56 | 0.10 | |||
| Infections | 40 (23.7) | 45 (28.5) | 49 (30.6) | ||
| Gastrointestinal | 33 (19.5) | 19 (12.0) | 14 (8.8) | ||
| Rheumatologic | 13 (7.7) | 12 (7.6) | 18 (11.2) | ||
| Renal | 14 (8.3) | 10 (6.3) | 16 (10.0) | ||
| Diabetes‐related | 11 (6.5) | 11 (7.0) | 10 (6.2) | ||
| Neurologic | 8 (4.7) | 11 (7.0) | 11 (6.9) | ||
| *Misc/other | 50 (29.6) | 50 (31.6) | 42 (26.3) | ||
Table 3 shows the insulin regimens used in the different groups. The use of basal insulin was similar between groups. Congruent with the goals of the education session and the order set, patients in the IG were more likely to be treated with a combination of basal and nutritional insulin than patients in the other groups. Patients in the HCG were more likely to be treated with a premixed insulin than patients in the other groups. However, even when premixed insulin was categorized as a form of basal plus nutritional insulin and combined into a composite group with the combined basal and nutritional days, this type of regimen remained more common in the IG than in the HCG. The rate of sliding scale insulin use alone (ie, without any scheduled insulin) was similar in the 3 groups.
| Patient‐days on the following | IG (n = 453) | CCG (n = 471) | P Value IG vs. CCG | HCG (n = 391) | P Value IG vs. HCG |
|---|---|---|---|---|---|
| |||||
| Sliding scale alone, n (%) | 105 (23.2) | 130 (27.6) | 0.12 | 89 (22.8) | 0.89 |
| Basal alone, n (%) | 132 (29.1) | 231 (49.0) | <0.01 | 199 (50.9) | <0.01 |
| Nutritional alone, n (%) | 22 (4.9) | 5 (1.1) | <0.01 | 8 (2.0) | 0.03 |
| Basal plus nutritional, n (%) | 166 (36.6) | 71 (15.1) | <0.01 | 14 (3.6) | <0.01 |
| Pre‐mixed insulin included, n (%) | 27 (6.0) | 32 (6.8) | 0.60 | 78 (20.0) | <0.01 |
| No insulin, n (%) | 1 (<1) | 2 (<1) | 0.59 | 3 (<1) | 0.28 |
| Any basal, n (%) | 325 (71.7) | 334 (70.9) | 0.78 | 291 (74.4) | 0.38 |
| Any nutritional, n (%) | 215 (47.5) | 108 (22.9) | <0.01 | 100 (25.6) | <0.01 |
| Basal plus nutritional or pre‐mixed, n (%) | 193 (42.6) | 103 (21.9) | <0.01 | 92 (23.5) | <0.01 |
| Oral diabetes agents, n (%) | 79 (17.4) | 83 (17.6) | 0.94 | 74 (18.9) | 0.58 |
| Sulfonylureas, n (%) | 40 (8.8) | 63 (13.4) | 0.03 | 37 (9.5) | 0.75 |
| Parenteral nutrition/tube feeds, n (%) | 0 (0) | 18 (3.8) | 8 (2.0) | ||
| High dose corticosteroids, n (%) | 66 (14.6) | 93 (19.8) | 0.04 | 51 (13.0) | 0.52 |
Other relevant measures are also shown in Table 3. The use of oral diabetes agents was similar in the 3 groups. The use of a nonoral feeding route (eg, tube feeding or parenteral nutrition) was infrequent.
A comparison of glycemic control in the three groups is shown in Table 4. In contrast to the CCG, patients in the IG experienced more days within the target glucose range (17% vs. 10.6%, P < 0.01), fewer days with severe hyperglycemia (48.3% vs. 59.2%, P < 0.01), and had a lower day‐weighted average blood glucose (195.9 vs. 212.6, P < 0.01). Compared to the HCG, patients in the IG experienced similar rates of hyperglycemia, but fewer hypoglycemic days (5.1% vs. 9.2%, P = 0.02).
| IG (n = 453) | CCG (n = 471) | P Value IG vs. CCG | HCG (n = 391) | P Value IG vs. HCG | |
|---|---|---|---|---|---|
| |||||
| Patient‐days | |||||
| In range, n (%) | 77 (17.0) | 50 (10.6) | <0.01 | 66 (16.9) | 0.98 |
| Out of range, n (%) | 376 (83.0) | 421 (89.4) | <0.01 | 325 (83.1) | 0.98 |
| Hyperglycemic, n (%) | 289 (63.8) | 310 (65.8) | 0.52 | 248 (63.4) | 0.91 |
| Severely hyperglycemic, n (%) | 219 (48.3) | 279 (59.2) | <0.01 | 176 (45.0) | 0.32 |
| Hypoglycemic, n (%) | 23 (5.1) | 36 (7.6) | 0.11 | 36 (9.2) | 0.02 |
| Severely hypoglycemic, n (%) | 13 (2.9) | 10 (2.1) | 0.47 | 15 (3.8) | 0.44 |
| Day weighted average blood glucose (SD) | 195.9 (66.8) | 212.6 (73.4) | <0.01 | 190.5 (63.1) | 0.25 |
The percentages of patients with severe hyperglycemia in each group are shown in Figure 1 by hospital day. Severe hyperglycemia was common, but there was a trend towards a decrease in the prevalence of severe hyperglycemia with increasing hospital days for all study groups, although it was consistently higher in the CCG than in the IG. Figure 2 shows the types of insulin regimens used by hospital day (composite for all groups). The use of basal plus nutritional insulin (the recommended regimen) increased gradually with increasing hospital days. When taken together, the information in both figures support the hypothesis that the use of the recommended insulin regimen may have contributed to the modest improvements in glycemic control seen in the IG.


In the final adjusted regression model, the intervention had a positive impact on glycemic control (Table 5). Subjects in the IG had a 72% increase in the odds of being in the target glucose range when compared to subjects in the CCG (P = 0.01). In addition, subjects in the IG had a 35% reduction in the odds of being severely hyperglycemic when compared to those in the CCG (P < 0.01). Finally, the odds ratio (OR) for being hypoglycemic among intervention subjects was 0.59 (P = 0.06) when compared to subjects in the CCG and 0.48 (P = 0.01) when compared to subjects in the HCG.
| Adjusted OR* IG vs. CCG | 95% CI | P value IG vs. CCG | Adjusted OR* IG vs. HCG | 95% CI | P Value IG vs. HCG | |
|---|---|---|---|---|---|---|
| ||||||
| In range | 1.72 | 1.16,2.55 | 0.01 | 1.08 | 0.74,1.58 | 0.68 |
| Hyperglycemic | 0.93 | 0.70,1.22 | 0.58 | 0.95 | 0.71,1.28 | 0.74 |
| Severely Hyperglycemic | 0.65 | 0.49,0.85 | <0.01 | 1.10 | 0.82,1.47 | 0.52 |
| Hypoglycemic | 0.59 | 0.34,1.02 | 0.06 | 0.48 | 0.27,0.85 | 0.01 |
| Severely Hypoglycemic | 1.36 | 0.59,3.14 | 0.47 | 0.97 | 0.29,1.44 | 0.28 |
Discussion
In this study, we investigated the effects of a standardized insulin order set, coupled with physician and nurse education, on glycemic control in hyperglycemic hospitalized patients. These interventions were designed to encourage a standardized approach to the treatment of hyperglycemia in hospitalized patients, based on the principles of physiologic insulin use, as described above. Our data suggest that the interventions did, indeed, alter the way insulin was prescribed, as more patients in the IG received a combination of basal plus nutritional insulin (the recommended regimen) than in the other groups. These interventions were associated with improved glycemic outcomes in the IG as compared to the CCG. The IG experienced a higher percentage of days in the target range and a trend toward fewer hypoglycemic days than the CCG. Although the IG experienced a similar percentage of days in the target range, it had significantly fewer hypoglycemic days than the HCG.
It is useful to consider the results of our study in the context of 2 other similar studies performed by Schnipper et al.14 and Maynard et al.15 Although each of these 3 studies have different study designs, they are similar in intent (to test the effects of simple quality improvement interventions on glycemic control in the hospital) and results (all showed significant improvements in some aspect of glycemic control). In our study, and the study by Maynard et al.,15 the interventions also led to decreases in the rates of hypoglycemia, whereas Schnipper et al.14 observed no difference in hypoglycemia. Of interest, in each of the three studies the interventions were associated with an increase in the use of some type of scheduled insulin. In our study and the study from Schnipper et al.14 the baseline use of basal insulin was quite high, and the interventions were associated with a significant increase in the addition of nutritional insulin. In the Maynard et al.15 study, the baseline use of sliding scale insulin alone was prevalent, and the interventions resulted in an increase in the use of basal insulin. The results of these studies, taken together, prompt us to conclude that the interventions employed in these studies are likely to lead to more frequent prescription of scheduled (anticipatory) insulin, and a modest improvement in glycemic control, without an increase (and perhaps with a decrease) in hypoglycemia.
A few of our study results are unexpected, or difficult to explain. In contrast to the other studies discussed above, our interventions did not affect the frequency of the use of sliding‐scale insulin alone (without any scheduled insulin), which was similar in the 3 groups. Although the reason for this is uncertain, we hypothesize that the high baseline use of basal insulin in our institution, and the lack of a hard stop preventing the use of sliding scale insulin alone explain this finding. Also, it is difficult to explain why measures of hyperglycemia were similar between the IG and the HCG despite the fact that the HCG was less often treated with a combination of basal and nutritional insulin and more often treated with mixed insulin.
There are several different mechanisms by which the interventions might have resulted in improved glycemic control in the IG compared to CCG. Our data clearly shows that insulin was prescribed differently in the IG, and the more frequent use of a combination of scheduled basal and nutritional insulin might have contributed to the differences between the groups. However, the effects of our interventions clearly went beyond physician education into the realm of true process improvement and standardization. The standardized order form was designed to prompt physicians to use a basal‐bolus insulin regimen. The order form also created nursing expectations of how insulin should be ordered, and clarified the roles of the different insulins that were prescribed.
On the medication administration record, each insulin was labeled as basal insulin (to be given even when fasting) or nutritional insulin (to be given along with the meal). The nurses caring for the IG also attended an education program that reinforced the role of the nurse in the bedside management of insulin administration. Specifically, nurses were taught to assess the premeal blood glucose and the patient's nutritional situation before giving the nutritional insulin (ie, Does the patient have food available? Will he tolerate eating the food?). In situations where is was not clear if the patient would be able to tolerate the ordered nutrition, the order set empowered the nurse to give the nutritional insulin after the meal, and to reduce the dose to match the patient's actual intake. These interventions resulted in some fundamental improvements in the nursing process of delivering insulin to the patient, and these changes might have resulted in improvements via mechanisms that are difficult to directly measure. Since the same physicians cared for both the IG and the CCG, interventions other than physician education clearly contributed to the observed improvements in the IG.
This study was not a randomized study, and there could be important undetected differences between the groups. However, all of the patients were admitted to the General Medicine Inpatient Services and the comparison of the general patient demographics and primary diagnoses between the groups do not suggest major differences.
Although the improvements in glycemic control seen in this study were statistically significant, they were quantitatively modest. The rates of hyperglycemia seen in this study, on the other hand, are quite remarkable. Both the American Diabetes Association and the American College of Endocrinology have recommended that blood glucoses in hospitalized patients not exceed a maximum value of 180 mg/dL, but the day‐weighted average blood glucose in this study was above that for each group. Even in the IG, over 80% of all patient‐days included at least 1 blood glucose value outside of the target range. These data suggest that better strategies for achieving metabolic control in hospitalized patients are needed.
It is worth mentioning that our interventions were not aggressively enforced. While the use of the order set was mandatory for the IG, it was flexible enough to allow for substantial practice variation, especially with respect to the dose of insulin prescribed. Although the education sessions discussed the specifics of insulin dosing in hospitalized patients, the order form did not offer dosing guidelines. It is possible that our interventions may have had a larger impact if a starting dose of insulin had been specified on the form. Although the insulin order form prompted physicians to act, there were no forced functions. Also, not all house staff attended the education sessions for physicians, and there was no feedback provided to physicians related to how they might improve their adherence to the recommendations presented in the educational module. Therefore, it is likely that more aggressive interventions could have led to greater changes in physician practice.
In conclusion, this study demonstrates that interventions including physician and nurse education and a standardized insulin order set can lead to improvement in glycemic control and patient safety in hospitalized patients treated with subcutaneous insulin. However, the observed improvements are modest, and poor metabolic control remains common, despite these interventions. These data suggest that standardization of the process of ordering and delivering subcutaneous insulin in the hospital may lead to a reduction in both hyperglycemia and hypoglycemia. However, it is clear that the interventions used in this study were not potent enough to achieve the recommended glycemic targets for the majority of patients. Additional research is needed to determine the best strategy for achieving safe and effective metabolic control in hospitalized, hyperglycemic, noncritically ill patients.
Acknowledgements
The authors thank David Conway for his work in data collection and management.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–360; discussion360–352.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1:141–144.
- ,,, et al.Evaluation of hospital glycemic control at US Academic Medical Centers.J Hosp Med.2009;4:35–44.
- ,,, et al.Diabetes care in the hospital: is there clinical inertia?J Hosp Med.2006;1:151–160.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Effects of a subcutaneous insulin protocol, clinical education, and computerized order set on the quality of inpatient management of hyperglycemia: results of a clinical trial.J Hosp Med.2009;4:16–27.
- ,,,,.Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm.J Hosp Med.2009;4:3–15.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77–82.
- Society of Hospital Medicine. Glycemic Control Resource Room. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section= Quality_Improvement_Resource_Rooms3(5 Suppl):17–28.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(5 Suppl):29–41.
- ,,,,.Society of Hospital Medicine Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(5 Suppl):66–75.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–360; discussion360–352.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1:141–144.
- ,,, et al.Evaluation of hospital glycemic control at US Academic Medical Centers.J Hosp Med.2009;4:35–44.
- ,,, et al.Diabetes care in the hospital: is there clinical inertia?J Hosp Med.2006;1:151–160.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- ,,,.Effects of a subcutaneous insulin protocol, clinical education, and computerized order set on the quality of inpatient management of hyperglycemia: results of a clinical trial.J Hosp Med.2009;4:16–27.
- ,,,,.Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm.J Hosp Med.2009;4:3–15.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77–82.
- Society of Hospital Medicine. Glycemic Control Resource Room. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section= Quality_Improvement_Resource_Rooms3(5 Suppl):17–28.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(5 Suppl):29–41.
- ,,,,.Society of Hospital Medicine Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(5 Suppl):66–75.
Copyright © 2010 Society of Hospital Medicine