User login
Managing cancer pain: Frequently asked questions
Some 90% of patients with cancer experience pain during their illness.1 The pain usually worsens as the disease progresses, and patients may experience different types of pain.
Persistent pain decreases function, appetite, and sleep, induces fear, causes depression, and generally lowers the quality of life.2 Persistent pain is demoralizing and debilitating for patients and their caregivers.3
Adequate pain control is important to ensure that patients can function productively, maintain social relationships, and improve their quality of life.2 Yet 86% of practicing physicians surveyed believed that most cancer patients with pain were undermedicated,2 and most felt that pain management is unsuccessful in more than half of patients who seek help.3
The critical importance of pain management has been emphasized by the World Health Organization (WHO), by international and national professional organizations, and by government agencies. All practitioners who care for cancer patients need to be well educated in managing cancer pain, a key part of which is to educate patients about the process and what to expect. This results in better pain control.4
Although much has been written on the management of cancer pain in a referral setting, little has been published on how to manage it in primary care. In this article, we discuss common questions faced by generalists. We emphasize the use of opioids, perhaps the most challenging aspect of cancer pain management. We also discuss when consultation with a specialist in pain management or a palliative medicine specialist is especially helpful.
WHAT ARE THE DIFFERENT TYPES OF PAIN SYNDROMES?
Pain is classified in several ways1–6:
Nociceptive vs neuropathic. Nociceptive pain comprises somatic and visceral components and is the result of continued tissue injury.4 Neuropathic pain is due to injury to the peripheral and central nervous systems and occurs within an area of sensory or motor deficit.
Continuous vs intermittent. Continuous pain, even if controlled, can have breakthroughs, ie, flares of pain above the controlled baseline level. Intermittent pain is a pain flare without chronic baseline pain. Intermittent pain is further divided into incident pain (ie, on movement) and end-of-dose failure (ie, pain occurring just before the next scheduled opioid dose).5 Pain specialists continue to debate the meaning and the use of these terms.
Malignant vs nonmalignant. Cancer pain is multifactorial,1 being induced by the disease itself, by the treatment of cancer, and by pain unrelated to cancer or its treatment (eg, osteoarthritis or diabetic neuropathy).2
Familiarity with the causes and the types of pain, including pain related to cancer, is important, as this influences treatment decisions.
HOW IS PAIN ASSESSED?
The assessment of pain is vital in managing it.
Since pain is inherently subjective, the patient’s self-report is the gold standard.4 Characteristics of the pain along with a physical examination, laboratory testing, and imaging studies can define the pathophysiology of the pain and influence the decision to undertake further assessment or specific therapies.
Patients and physicians can use various scales, such as a visual analog scale, a numerical rating scale, a graphic scale, a verbal scale, a word descriptor scale, and a functional pain scale. A verbal scale can be used if the patient is alert, or a nonverbal scale if the patient has impaired cognition or speaks a different language. Intensity is the most common dimension evaluated in cancer pain, primarily via a numerical or visual analog scale. A numerical scale score of 0 to 10 has been found to be as effective as a visual analog scale (0 to 100 mm),7,8 and the numerical rating scale is generally preferred as a measure of pain intensity.9
There are no clear guidelines for selecting one scale over another.7 A clinically meaningful response (ie, meaningful to patients) is at least a two-point decrease on the 10-point numerical scale or a 13-mm decrease on the 100-mm visual analog scale. A decrease in the percentage of the pain relates to global improvement better than an absolute reduction on the numerical scale.
WHAT PROBLEMS ARE ENCOUNTERED IN MANAGING CANCER PAIN?
WHAT ARE THE DIFFERENT WAYS TO MANAGE CANCER PAIN?
Pain should be treated promptly and aggressively, because if untreated it can lead to delays in healing, changes in the central nervous system (eg, sensitization, plasticity), chronic stress, family stress, depression, job loss, and even suicide.12–14
Comprehensive pain management improves outcomes and includes the rational use of opioids and adjuvant analgesics, physical rehabilitation, cognitive behavioral (non-drug) therapies, family counseling, interventional procedures (kyphoplasty, nerve blocks, local injections, spinal analgesia), and complementary therapies such as acupuncture.12 Adjuvant analgesics include antidepressants, anticonvulsants, and local anesthetics.
HOW DO OPIOIDS RELIEVE CANCER PAIN?
Opioids bind to receptors in tissues throughout the body, including in the central and peripheral nervous systems15 and the digestive tract. The binding of an opioid to an opioid receptor—including mu, kappa, and delta receptors and orphan receptor-like ligand-1—initiates a cascade of intracellular reactions. Due to the nature of different interactions of opioids with each of these receptors, individuals vary in their response to opioids.15
WHAT ARE THE CHARACTERISTICS OF COMMON OPIOIDS?
- Step 1. Mild pain calls for a nonopioid analgesic with or without an adjuvant (more about adjuvants below).
- Step 2. Mild or moderate pain that persists or increases calls for a weak opioid such as codeine, tramadol (Ultram), or hydrocodone, with or without a nonopioid and with or without an adjuvant.
- Step 3. Severe pain calls for a strong opioid with or without a nonopioid, and with or without an adjuvant.
Morphine, the prototypical opioid, is well studied and versatile, as it can be given orally, parenterally, rectally, or intraspinally. It is readily available in the United States and Western Europe but not in some parts of the world, such as Asia and Africa. It is also cost-effective.
Hydromorphone (Dilaudid) is similar to morphine in terms of versatility, cost, and effectiveness in pain management. An extended-release form (Exalgo) is now available in the United States.
Oxycodone is readily available in both slow-release (eg, OxyContin) and immediate-release (eg, Oxy-IR) preparations and is also cost-effective. However, there is no parenteral formulation in the United States.
Methadone is inexpensive and can be used as a long-acting or an immediate-release opioid. However, it should be used with caution in patients with a prolonged QTc interval: in general, a QTc interval of 430 to 450 msec is not a contraindication, but there is a risk of torsades de pointes when the QTc is greater than 500 msec. The physician should also look for drug interactions when prescribing methadone, which is metabolized in the liver via the cytochrome P450 3A4 system. Methadone use can also lead to respiratory depression, prolonged QTc interval, and sudden death.
Buprenorphine can be used as a third- or fourth-tier opioid for patients with both kidney and liver failure. It can be given sublingually or parenterally. It may not be readily available, may not be covered by insurance, and is expensive.
Selecting an opioid to try first
The following are some general considerations when selecting an opioid to try first:
- Does the patient have a history of organ failure? Has the patient had a therapeutic response to, or adverse effects from, a particular opioid in the past?
- Which route would best fit the patient’s needs? (Oral is always preferable.)
- How often will breakthrough dosing be required? (In general, the breakthrough dose is administered at the drug’s half-life, but it can be administered between 1 and 4 hours.)
- How much will it cost? (Consider the cost, insurance coverage, and co-pays.)
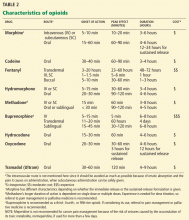
Table 2 shows different characteristics of commonly used opioids, including route of administration, onset of action, peak effect, and duration of action.1
WHAT ARE THE EQUIANALGESIC DOSES OF COMMONLY USED OPIOIDS?
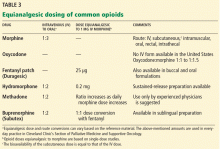
Table 3 lists equianalgesic doses and route conversions of commonly used opioids.18–20
WHAT ARE THE PRINCIPLES BEHIND OPIOID DOSING?
Table 4 shows important strategies for opioid dosing. An in-depth discussion of specific opioid dosing strategies is beyond the scope of this article.5
WHAT ARE THE COMMON ADVERSE EFFECTS OF OPIOIDS?
Adverse effects are among the most common reasons for failure of opioids to relieve pain. If these effects are not anticipated and treated prophylactically, patients may avoid taking their opioid drugs or may complain that they are “allergic” to them. In reality, true allergy to any of the opioids is rare. Patients comply better if they are taught to expect that most adverse effects are either preventable or manageable.21 A simple strategy includes reducing the opioid dose by 25% to 50%, using different opioids (“rotation”), changing the route of administration, and directly treating adverse effects.21,22
WHAT IS OPIOID ROTATION AND HOW IS IT DONE?
Opioid rotation involves changing to a different drug using the same administration route, with the aim of improving the analgesic response or reducing adverse effects.16 It may be useful in widening the therapeutic window, ie, establishing a more advantageous relationship between analgesia and toxicity.16 This strategy applies, for example, to patients who have an adverse reaction to morphine, and who may need rotation to fentanyl or methadone.
The major indication for switching opioids is poorly controlled pain with unacceptable adverse effects due to opioid toxicity, the rapid development of tolerance, refractory pain, or difficult pain syndromes.24 A recent prospective study showed that 42% of patients underwent opioid rotation, and the two most common reasons were inadequate analgesia and severe adverse effects.25 Opioid rotation resulted in relief of confusion (72%), nausea and vomiting (68%), and drowsiness (53%).25
Before trying opioid rotation, review the patient’s pain syndromes and the use of an adjuvant analgesic, and assess for evidence of opioid toxicity or contributing abnormal biochemical factors such as hydration status.24,26 Most opioids are mu-receptor agonists and may exhibit cross-tolerance, a phenomenon in which the alternative drug does not have the expected effects because of similar pharmacologic action of the first drug. Because the degree of cross-tolerance may change as opioid doses are escalated, it is advisable to proceed with caution when switching from one opioid to another in patients who are receiving very high doses. Opioid rotation generally would be ineffective if there is complete analgesic cross-tolerance between opioids.
The common equivalency conversion tables are based either on studies in patients who received low doses of opioids or on single-dose studies.16,24 By substituting opioids and using lower doses than expected according to the equivalency conversion tables (generally a 25% to 30% decrease), it is possible in most cases to reduce or relieve the symptoms of opioid toxicity and to manage patients highly tolerant to previous opioids while improving analgesia.24
Alternatives to opioid rotation are route conversion (oral to parenteral or spinal), addition of an adjuvant analgesic, and opioid dose reduction.
WHAT IS OPIOID TOXICITY AND HOW IS IT MANAGED?
Opioid overdose is commonly the result of an error in pain assessment, opioid prescribing, or dose administration. Opioid overdose classically presents as sedation or respiratory depression. The combination of coma, reduced respiratory rate, and pinpoint pupils is highly suggestive of opioid toxicity, and treatment should be initiated promptly.
This scenario, however, is the extreme example of opioid overdose, and it is rare when a patient is given the correct opioid dose titrated gradually over a period of time. The more common scenario is when a patient’s pain has finally been managed and the patient is resting comfortably with slow respirations. This would not warrant naloxone (Narcan) administration but rather close observation and monitoring of vital signs.
Naloxone has antagonist activity at all of the receptor sites.27 It is important to be alert for acute opioid withdrawal in patients taking high-dose opioids for a long time.27 There are no guidelines as to the route of administration and the dosing of naloxone. Table 6 summarizes the management of opioid overdose using naloxone.5
WHAT IS THE ROLE OF ADJUVANTS?
An adjuvant analgesic is any drug with a primary indication other than pain, but with analgesic properties in some painful conditions. Adjuvants are best used when a patient cannot obtain satisfactory pain relief from an opioid.28 Antidepressants, anticonvulsants, neuroleptics, antiarrhythmics, antihistamines, N-methyl-d-aspartate (NMDA) receptor antagonists, steroids, muscle relaxants, bisphosphonates, and radiopharmaceuticals can be adjuvant agents.29
Adjuvants are generally used to complement the analgesic effects of opioids to achieve optimal pain control with a minimum of adverse effects.28 The following scenarios should prompt the use of adjuvants in clinical practice28:
- The toxic limit of a primary pain medication has been reached.
- The therapeutic benefit of the primary pain medication has reached a plateau.
- The primary analgesic could not be used because of substance-abuse behavior, multiple organ failure, allergy, etc.
- The patient has multiple pain syndromes.
- The patient has additional symptoms unrelated to pain, eg, insomnia or depression.
Table 7 lists adjuvants with specific indications and points to remember when prescribing them.28,29
WHAT IS THE ROLE OF NSAIDs FOR CANCER PAIN?
Nonsteroidal anti-inflammatory drugs (NSAIDs) have a well-established role in treating cancer-related pain, either on their own for mild pain or in combination with opioids for moderate to severe pain, leading to additive analgesia. Using NSAIDs as adjuvants is common practice in certain cancer pain syndromes, such as malignant bone pain, although there is considerable variation in response.31
NSAIDs have long been known to inhibit peripheral prostaglandin synthesis, but recently they have also been suggested to have a central action. The central effect is related to NMDA receptor-induced activation of the nitric oxide system.31
NSAIDs have ceiling effects, and there is no therapeutic advantage to increasing the dose beyond that which is recommended.
Ketorolac (Toradol), indomethacin (Indocin), and diclofenac (Voltaren) have potent analgesic activity, whereas the “oxicam” NSAIDs show predominantly anti-inflammatory effects.30
No NSAID is clearly superior for a particular type of pain. Certain NSAIDs block the NMDA receptor and inhibit cyclo-oxygenase-1 and cyclo-oxygenase-2. There is a poor correlation between the analgesic effects of NSAIDs and cyclo-oxygenase inhibition. There is no evidence to support the use of selective cyclo-oxygenase-2 inhibitors for cancer pain, and these agents have no advantage over nonselective NSAIDs on the basis of limited gastrointestinal toxicity.32
In cancer pain, NSAIDs may delay the development of tolerance and allow lower doses of opioids to be used, with fewer central nervous system side effects.31,32 Despite the extensive use of NSAIDs, relatively few randomized studies have documented their efficacy in cancer pain compared with other chronic pain syndromes. Data on safe and effective doses from studies of nonmalignant pain may not apply to cancer pain, since cancer patients often have several serious conditions and are on multiple medications. In addition, the potential for adverse effects of NSAIDs (gastrointestinal bleeding, renal failure, thrombosis) may be greater in patients with advanced cancer.
In conclusion, NSAIDs may help if used judiciously in somatic pain and visceral pain, and perhaps even in neuropathic pain.31
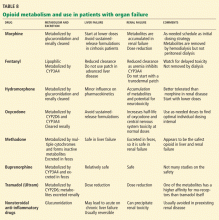
HOW IS CANCER PAIN MANAGED IN PATIENTS WITH ORGAN FAILURE?
- Opioids that can be used in liver failure or cirrhosis: morphine, hydromorphone, methadone, levorphanol, buprenorphine.
- Opioids that can be used in renal failure: methadone, fentanyl, and buprenorphine are safest; oxycodone and hydromorphone are moderately safe; morphine is the least safe.35,36
- Opioids that can be used in both kidney and liver failure: methadone, buprenorphine.
HOW CAN PROBLEMS RELATED TO SUBSTANCE ABUSE BE AVOIDED?
Substance abuse is less a problem in managing cancer pain than in chronic nonmalignant pain. Prescribing opioids safely is challenging, and very little has been published on substance abuse and the management of cancer pain. However, in the absence of practice guidelines, the best approach is to establish a dosing structure, control prescription refills, and monitor the patient.
Abuse is the misuse of an opioid via self-titration or altering the dosing schedule or route of administration. Patients who misuse opioids—ie, take them differently than prescribed—are not necessarily addicted.
Addiction is the abuse of a drug associated with psychological dependence, despite harm.
Diversion can occur without addiction and is done for financial gain, and this is the worst offense as it may harm others.
Pseudoaddiction is abnormal, demanding, often hostile behavior resulting from uncontrolled pain; once the pain is controlled, the behavior resolves.
Behaviors such as forging prescriptions, stealing or borrowing drugs, frequently “losing” prescriptions, and resisting changes to medication despite adverse effects are more predictive of addiction than are behaviors such as aggressive complaining about the need for more drugs, drug-hoarding, and unsanctioned dose escalations or other forms of noncompliance, as the latter three are more likely to indicate poorly controlled pain.37
Predictors of opioid abuse include a family history or a personal history of alcohol or drug abuse (including prescription drugs); a history of psychiatric illness (including anxiety disorder); male sex; nonwhite race; a history of driving under the influence of alcohol or drugs; a record of drug-related convictions; lost or stolen prescriptions; and using supplemental sources to obtain opioids.38 Socioeconomic status and disability level were not found to be significant predictors.38
Different scales are available to predict the risk of aberrant drug behavior in patients on chronic opioid therapy. Of the many available, the Screener and Opioid Assessment for Patients With Pain and the Current Opioid Misuse Measure assess all the key factors.38
After an assessment, the next step is monitoring. Unfortunately, no specific method has been validated. In one study, urine toxicology testing was more effective at identifying problems than monitoring patient behavior alone, and monitoring behavior alone would have resulted in missing about half of the patients with a problem.39 The same study showed that even in the absence of aberrant drug-related behavior based on predictors, a significant number of urine toxicology screens were positive.39
A negative urine screen for the patient’s opioid suggests diversion. The clinician should order a screen for the prescribed opioid because a general screen may not detect nonmorphine opioids. A general screen may detect polysubstance abuse, which is common in individuals with addiction.
Table 9 summarizes a strategy to manage opioid therapy in patients with history of substance abuse.40
WHAT IS THE ROLE OF COMPLEMENTARY AND ALTERNATIVE THERAPIES?
Complementary and alternative medicine therapies are commonly used by cancer patients, with an average prevalence rate of 31%.41–43 As the names suggest, they have been used both as an alternative to and as a complement to conventional medicine. Practitioners of complementary and alternative medicine emphasize its holistic, individualistic, empowering, and educational nature.
Patients do not routinely ask their physicians about these therapies,44 and physicians often have only a limited knowledge of them.45 Surveys of North American physicians showed that they view certain of these therapies as legitimate and effective.46,47
The role of complementary and alternative medicine in cancer pain has been the subject of debate, as relatively little is known about adverse effects and drug interactions. Nevertheless, the American Cancer Society and the National Comprehensive Cancer Network guidelines on cancer pain recommend nonpharmacologic treatment be added for patients who report a pain score of 4 or greater on a 10-point scale after analgesic adjustment.48,49
Most studies of complementary and alternative therapies for cancer pain are of poor quality, with significant shortcomings in methodology and study design and with no clear definition of outcomes.50
Acupuncture is probably the most studied of these therapies, but clinical trials so far have not shown it to be an effective adjunct analgesic for cancer pain.51 A placebo-controlled, blinded randomized trial using auricular acupuncture showed a pain score decrease of 36% from baseline at 2 months compared with controls.52
Studies involving cognitive therapy, supportive psychotherapy, and hypnosis showed modest benefit.53,54 Two trials involving relaxation and imagery reduced cancer pain compared with controls.55,56
Studies of massage therapy have shown mixed results; two studies reported a significant reduction in pain immediately after intervention, and no study found pain relief after 4 weeks.57–60 Studies involving Reiki and touch therapy were inconclusive.60,61
Music therapy has been used to treat patients physically, psychologically, socially, emotionally, and spiritually, with evidence still equivocal. A large prospective observational study involving 200 patients conducted by Gallagher et al62 showed pain was reduced by 30% after music therapy intervention. The same study showed a reduction in depression and anxiety.62 Music therapy could be used as a component of a multimodal approach to pain.
Herbal preparations are often used to treat cancer and symptoms by patients and naturalists. Some herbal medicines are known to cause toxicity in cancer patients. Examples are PC-SPES, mistletoe, and saw palmetto.63
At this juncture, there is some evidence that some complementary and alternative therapies can relieve cancer pain, and the most promising therapy seems to be related to mind-body medicine (eg, biofeedback, relaxation techniques). But before we can legitimately integrate these therapies into the management of cancer pain, we need large randomized controlled trials to determine if they are effective in patients on chronic high-dose opioids and if they decrease the need for opioids.
- Laird B, Colvin L, Fallon M. Management of cancer pain: basic principles and neuropathic cancer pain. Eur J Cancer 2008; 44:1078–1082.
- Chang HM. Cancer pain management. Med Clin North Am 1999; 83:711–736,
- Stannard C, Johnson M. Chronic pain management—can we do better? An interview-based survey in primary care. Curr Med Res Opin 2003; 19:703–706.
- Portenoy RK, Lesage P. Management of cancer pain. Lancet 1999; 353:1695–1700.
- Walsh D, Rivera NI, Davis MP, Lagman R, Legrand SB. Strategies for pain management: Cleveland Clinic Foundation guidelines for opioid dosing for cancer pain. Support Cancer Ther 2004; 1:157–164.
- Foley KM. Acute and chronic pain syndromes. In:Doyle D, Hanks G, Cherny N, Calman K, editors. Oxford Textbook of Palliative Medicine. 3rd ed. Oxford, UK: Oxford University Press; 2005:298–316.
- Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain 2003; 4:2–21.
- Jensen MP, Turner JA, Romano JM. What is the maximum number of levels needed in pain intensity measurement? Pain 1994; 58:387–392.
- Preston CC, Colman AM. Optimal number of response categories in rating scales: reliability, validity, discriminating power, and respondent p. Acta Psychol (Amst) 2000; 104:1–15.
- Peretti-Watel P, Bendiane MK, Obadia Y, Favre R, Lapiana JM, Moatti JP; South-Eastern France Palliative Care Group. The prescription of opioid analgesics to terminal cancer patients: impact of physicians’ general attitudes and contextual factors. Palliat Support Care 2003; 1:345–352.
- Jacobsen R, Liubarskiene Z, Møldrup C, Christrup L, Sjøgren P, Samsanaviciene J. Barriers to cancer pain management: a review of empirical research. Medicina (Kaunas) 2009; 45:427–433.
- Wiedemer NL, Harden PS, Arndt IO, Gallagher RM. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med 2007; 8:573–584.
- Rome HP, Rome JD. Limbically augmented pain syndrome (LAPS): kindling, corticolimbic sensitization, and the convergence of affective and sensory symptoms in chronic pain disorders. Pain Med 2000; 1:7–23.
- Fishbain DA, Rosomoff HL, Rosomoff RS. Drug abuse, dependence, and addiction in chronic pain patients. Clin J Pain 1992; 8:77–85.
- Murányi M, Radák Z. Pain and opioids. Orv Hetil 2008; 149:2363–2370.
- Vadalouca A, Moka E, Argyra E, Sikioti P, Siafaka I. Opioid rotation in patients with cancer: a review of the current literature. J Opioid Manag 2008; 4:213–250.
- Galvagno SM, Correll DJ, Narang S. Safe oral equianalgesic opioid dosing for patients with moderate-to-severe pain. www.hcplive.com/publications/Resident-and-Staff/2007/2007-04/2007-04_06. Accessed May 25, 2011.
- Walsh D. Pharmacological management of cancer pain. Semin Oncol 2000; 27:45–63.
- Shaheen PE, Walsh D, Lasheen W, Davis MP, Lagman RL. Opioid equianalgesic tables: are they all equally dangerous? J Pain Symptom Manage 2009; 38:409–417.
- Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids. a critical review and proposals for long-term dosing. J Pain Symptom Manage 2001; 22:672–687.
- Harris JD. Management of expected and unexpected opioid-related side effects. Clin J Pain 2008; 24(suppl 10):S8–S13.
- Cherny N, Ripamonti C, Pereira J; Expert Working Group of the European Association of Palliative Care Network. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol 2001; 19:2542–2554.
- Harris JD, Kotob F. Management of opioid-related side effects. In:de Leon-Casasola OA, ed. Cancer Pain: Pharmacological, Interventional and Palliative Care. Philadelphia: Elsevier Inc; 2006:207–230.
- Mercadante S. Opioid rotation for cancer pain: rationale and clinical aspects. Cancer 1999; 86:1856–1866.
- Cheema B, Lagman RL, Walsh D, et al. A prospective study of opioid rotation in pain due to advanced cancer. J Cancer Pain & Symp Palliat 2006; 2:39–46.
- Schug SA, Zech D, Grond S, Jung H, Meuser T, Stobbe B. A long-term survey of morphine in cancer pain patients. J Pain Symptom Manage 1992; 7:259–266.
- Clarke SF, Dargan PI, Jones AL. Naloxone in opioid poisoning: walking the tightrope. Emerg Med J 2005; 22:612–616.
- Knotkova H, Pappagallo M. Adjuvant analgesics. Med Clin North Am 2007; 91:113–124.
- Lussier D, Huskey AG, Portenoy RK. Adjuvant analgesics in cancer pain management. Oncologist 2004; 9:571–591.
- Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancerrelated pain. J Pain Symptom Manage 2010; 39:167–179.
- Mercadante S. The use of anti-inflammatory drugs in cancer pain. Cancer Treat Rev 2001; 27:51–61.
- Davis MP, Walsh D, Lagman R, LeGrand SB. Controversies in pharmacotherapy of pain management. Lancet Oncol 2005; 6:696–704.
- Klotz U. Tramadol—the impact of its pharmacokinetic and pharmacodynamic properties on the clinical management of pain. Arzneimittelforschung 2003; 53:681–687.
- Davis MP, Lasheen W, Gamier P. Practical guide to opioids and their complications in managing cancer pain. What oncologists need to know. Oncology (Williston Park) 2007; 21:1229–1238.
- Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage 2004; 28:497–504.
- Davis MP. Buprenorphine in cancer pain. Support Care Cancer 2005; 13:878–887.
- Portenoy RK. Opioid therapy for chronic nonmalignant pain: a review of the critical issues. J Pain Symptom Manage 1996; 11:203–217.
- Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain 2008; 24:497–508.
- Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg 2003; 97:1097–1102,
- Passik SD, Kirsh KL. Managing pain in patients with aberrant drug-taking behaviors. J Support Oncol 2005; 3:83–86.
- Ernst E, Cassileth BR. The prevalence of complementary/alternative medicine in cancer: a systematic review. Cancer 1998; 83:777–782.
- Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA 1998; 280:1569–1575.
- Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol 2000; 18:2505–2514.
- Adler SR, Fosket JR. Disclosing complementary and alternative medicine use in the medical encounter: a qualitative study in women with breast cancer. J Fam Pract 1999; 48:453–458.
- Newell S, Sanson-Fisher RW. Australian oncologists’ self-reported knowledge and attitudes about non-traditional therapies used by cancer patients. Med J Aust 2000; 172:110–113.
- Berman BM, Singh BK, Lao L, Singh BB, Ferentz KS, Hartnoll SM. Physicians’ attitudes toward complementary or alternative medicine: a regional survey. J Am Board Fam Pract 1995; 8:361–366.
- Verhoef MJ, Sutherland LR. General practitioners’ assessment of and interest in alternative medicine in Canada. Soc Sci Med 1995; 41:511–515.
- American Cancer Society: Treatment guidelines for patients. Version 1. http://www.cancer.org/downloads/CRI/NCCN_pain.pdf.
- Benedetti C, Brock C, Cleeland C, et al; National Comprehensive Cancer Network. NCCN Practice Guidelines for Cancer Pain. Oncology (Williston Park) 2000; 14:135–150.
- Bardia A, Barton DL, Prokop LJ, Bauer BA, Moynihan TJ. Efficacy of complementary and alternative medicine therapies in relieving cancer pain: a systematic review. J Clin Oncol 2006; 24:5457–5464.
- Lee H, Schmidt K, Ernst E. Acupuncture for the relief of cancer-related pain—a systematic review. Eur J Pain 2005; 9:437–444.
- Alimi D, Rubino C, Pichard-Léandri E, Fermand-Brulé S, Dubreuil-Lemaire ML, Hill C. Analgesic effect of auricular acupuncture for cancer pain: a randomized, blinded, controlled trial. J Clin Oncol 2003; 21:4120–4126.
- Spiegel D, Bloom JR. Group therapy and hypnosis reduce metastatic breast carcinoma pain. Psychosom Med 1983; 45:333–339.
- Goodwin PJ, Leszcz M, Ennis M, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med 2001; 345:1719–1726.
- Syrjala KL, Donaldson GW, Davis MW, Kippes ME, Carr JE. Relaxation and imagery and cognitive-behavioral training reduce pain during cancer treatment: a controlled clinical trial. Pain 1995; 63:189–198.
- Sloman R, Brown P, Aldana E, Chee E. The use of relaxation for the promotion of comfort and pain relief in persons with advanced cancer. Contemp Nurse 1994; 3:6–12.
- Weinrich SP, Weinrich MC. The effect of massage on pain in cancer patients. Appl Nurs Res 1990; 3:140–145.
- Wilkie DJ, Kampbell J, Cutshall S, et al. Effects of massage on pain intensity, analgesics and quality of life in patients with cancer pain: a pilot study of a randomized clinical trial conducted within hospice care delivery. Hosp J 2000; 15:31–53.
- Soden K, Vincent K, Craske S, Lucas C, Ashley S. A randomized controlled trial of aromatherapy massage in a hospice setting. Palliat Med 2004; 18:87–92.
- Post-White J, Kinney ME, Savik K, Gau JB, Wilcox C, Lerner I. Therapeutic massage and healing touch improve symptoms in cancer. Integr Cancer Ther 2003; 2:332–344.
- Olson K, Hanson J, Michaud M. A phase II trial of Reiki for the management of pain in advanced cancer patients. J Pain Symptom Manage 2003; 26:990–997.
- Gallagher LM, Lagman R, Walsh D, Davis MP, Legrand SB. The clinical effects of music therapy in palliative medicine. Support Care Cancer 2006; 14:859–866.
- Olaku O, White JD. Herbal therapy use by cancer patients: a literature review on case reports. Eur J Cancer 2011; 47:508–514.
Some 90% of patients with cancer experience pain during their illness.1 The pain usually worsens as the disease progresses, and patients may experience different types of pain.
Persistent pain decreases function, appetite, and sleep, induces fear, causes depression, and generally lowers the quality of life.2 Persistent pain is demoralizing and debilitating for patients and their caregivers.3
Adequate pain control is important to ensure that patients can function productively, maintain social relationships, and improve their quality of life.2 Yet 86% of practicing physicians surveyed believed that most cancer patients with pain were undermedicated,2 and most felt that pain management is unsuccessful in more than half of patients who seek help.3
The critical importance of pain management has been emphasized by the World Health Organization (WHO), by international and national professional organizations, and by government agencies. All practitioners who care for cancer patients need to be well educated in managing cancer pain, a key part of which is to educate patients about the process and what to expect. This results in better pain control.4
Although much has been written on the management of cancer pain in a referral setting, little has been published on how to manage it in primary care. In this article, we discuss common questions faced by generalists. We emphasize the use of opioids, perhaps the most challenging aspect of cancer pain management. We also discuss when consultation with a specialist in pain management or a palliative medicine specialist is especially helpful.
WHAT ARE THE DIFFERENT TYPES OF PAIN SYNDROMES?
Pain is classified in several ways1–6:
Nociceptive vs neuropathic. Nociceptive pain comprises somatic and visceral components and is the result of continued tissue injury.4 Neuropathic pain is due to injury to the peripheral and central nervous systems and occurs within an area of sensory or motor deficit.
Continuous vs intermittent. Continuous pain, even if controlled, can have breakthroughs, ie, flares of pain above the controlled baseline level. Intermittent pain is a pain flare without chronic baseline pain. Intermittent pain is further divided into incident pain (ie, on movement) and end-of-dose failure (ie, pain occurring just before the next scheduled opioid dose).5 Pain specialists continue to debate the meaning and the use of these terms.
Malignant vs nonmalignant. Cancer pain is multifactorial,1 being induced by the disease itself, by the treatment of cancer, and by pain unrelated to cancer or its treatment (eg, osteoarthritis or diabetic neuropathy).2
Familiarity with the causes and the types of pain, including pain related to cancer, is important, as this influences treatment decisions.
HOW IS PAIN ASSESSED?
The assessment of pain is vital in managing it.
Since pain is inherently subjective, the patient’s self-report is the gold standard.4 Characteristics of the pain along with a physical examination, laboratory testing, and imaging studies can define the pathophysiology of the pain and influence the decision to undertake further assessment or specific therapies.
Patients and physicians can use various scales, such as a visual analog scale, a numerical rating scale, a graphic scale, a verbal scale, a word descriptor scale, and a functional pain scale. A verbal scale can be used if the patient is alert, or a nonverbal scale if the patient has impaired cognition or speaks a different language. Intensity is the most common dimension evaluated in cancer pain, primarily via a numerical or visual analog scale. A numerical scale score of 0 to 10 has been found to be as effective as a visual analog scale (0 to 100 mm),7,8 and the numerical rating scale is generally preferred as a measure of pain intensity.9
There are no clear guidelines for selecting one scale over another.7 A clinically meaningful response (ie, meaningful to patients) is at least a two-point decrease on the 10-point numerical scale or a 13-mm decrease on the 100-mm visual analog scale. A decrease in the percentage of the pain relates to global improvement better than an absolute reduction on the numerical scale.
WHAT PROBLEMS ARE ENCOUNTERED IN MANAGING CANCER PAIN?
WHAT ARE THE DIFFERENT WAYS TO MANAGE CANCER PAIN?
Pain should be treated promptly and aggressively, because if untreated it can lead to delays in healing, changes in the central nervous system (eg, sensitization, plasticity), chronic stress, family stress, depression, job loss, and even suicide.12–14
Comprehensive pain management improves outcomes and includes the rational use of opioids and adjuvant analgesics, physical rehabilitation, cognitive behavioral (non-drug) therapies, family counseling, interventional procedures (kyphoplasty, nerve blocks, local injections, spinal analgesia), and complementary therapies such as acupuncture.12 Adjuvant analgesics include antidepressants, anticonvulsants, and local anesthetics.
HOW DO OPIOIDS RELIEVE CANCER PAIN?
Opioids bind to receptors in tissues throughout the body, including in the central and peripheral nervous systems15 and the digestive tract. The binding of an opioid to an opioid receptor—including mu, kappa, and delta receptors and orphan receptor-like ligand-1—initiates a cascade of intracellular reactions. Due to the nature of different interactions of opioids with each of these receptors, individuals vary in their response to opioids.15
WHAT ARE THE CHARACTERISTICS OF COMMON OPIOIDS?
- Step 1. Mild pain calls for a nonopioid analgesic with or without an adjuvant (more about adjuvants below).
- Step 2. Mild or moderate pain that persists or increases calls for a weak opioid such as codeine, tramadol (Ultram), or hydrocodone, with or without a nonopioid and with or without an adjuvant.
- Step 3. Severe pain calls for a strong opioid with or without a nonopioid, and with or without an adjuvant.
Morphine, the prototypical opioid, is well studied and versatile, as it can be given orally, parenterally, rectally, or intraspinally. It is readily available in the United States and Western Europe but not in some parts of the world, such as Asia and Africa. It is also cost-effective.
Hydromorphone (Dilaudid) is similar to morphine in terms of versatility, cost, and effectiveness in pain management. An extended-release form (Exalgo) is now available in the United States.
Oxycodone is readily available in both slow-release (eg, OxyContin) and immediate-release (eg, Oxy-IR) preparations and is also cost-effective. However, there is no parenteral formulation in the United States.
Methadone is inexpensive and can be used as a long-acting or an immediate-release opioid. However, it should be used with caution in patients with a prolonged QTc interval: in general, a QTc interval of 430 to 450 msec is not a contraindication, but there is a risk of torsades de pointes when the QTc is greater than 500 msec. The physician should also look for drug interactions when prescribing methadone, which is metabolized in the liver via the cytochrome P450 3A4 system. Methadone use can also lead to respiratory depression, prolonged QTc interval, and sudden death.
Buprenorphine can be used as a third- or fourth-tier opioid for patients with both kidney and liver failure. It can be given sublingually or parenterally. It may not be readily available, may not be covered by insurance, and is expensive.
Selecting an opioid to try first
The following are some general considerations when selecting an opioid to try first:
- Does the patient have a history of organ failure? Has the patient had a therapeutic response to, or adverse effects from, a particular opioid in the past?
- Which route would best fit the patient’s needs? (Oral is always preferable.)
- How often will breakthrough dosing be required? (In general, the breakthrough dose is administered at the drug’s half-life, but it can be administered between 1 and 4 hours.)
- How much will it cost? (Consider the cost, insurance coverage, and co-pays.)
Table 2 shows different characteristics of commonly used opioids, including route of administration, onset of action, peak effect, and duration of action.1
WHAT ARE THE EQUIANALGESIC DOSES OF COMMONLY USED OPIOIDS?
Table 3 lists equianalgesic doses and route conversions of commonly used opioids.18–20
WHAT ARE THE PRINCIPLES BEHIND OPIOID DOSING?
Table 4 shows important strategies for opioid dosing. An in-depth discussion of specific opioid dosing strategies is beyond the scope of this article.5
WHAT ARE THE COMMON ADVERSE EFFECTS OF OPIOIDS?
Adverse effects are among the most common reasons for failure of opioids to relieve pain. If these effects are not anticipated and treated prophylactically, patients may avoid taking their opioid drugs or may complain that they are “allergic” to them. In reality, true allergy to any of the opioids is rare. Patients comply better if they are taught to expect that most adverse effects are either preventable or manageable.21 A simple strategy includes reducing the opioid dose by 25% to 50%, using different opioids (“rotation”), changing the route of administration, and directly treating adverse effects.21,22
WHAT IS OPIOID ROTATION AND HOW IS IT DONE?
Opioid rotation involves changing to a different drug using the same administration route, with the aim of improving the analgesic response or reducing adverse effects.16 It may be useful in widening the therapeutic window, ie, establishing a more advantageous relationship between analgesia and toxicity.16 This strategy applies, for example, to patients who have an adverse reaction to morphine, and who may need rotation to fentanyl or methadone.
The major indication for switching opioids is poorly controlled pain with unacceptable adverse effects due to opioid toxicity, the rapid development of tolerance, refractory pain, or difficult pain syndromes.24 A recent prospective study showed that 42% of patients underwent opioid rotation, and the two most common reasons were inadequate analgesia and severe adverse effects.25 Opioid rotation resulted in relief of confusion (72%), nausea and vomiting (68%), and drowsiness (53%).25
Before trying opioid rotation, review the patient’s pain syndromes and the use of an adjuvant analgesic, and assess for evidence of opioid toxicity or contributing abnormal biochemical factors such as hydration status.24,26 Most opioids are mu-receptor agonists and may exhibit cross-tolerance, a phenomenon in which the alternative drug does not have the expected effects because of similar pharmacologic action of the first drug. Because the degree of cross-tolerance may change as opioid doses are escalated, it is advisable to proceed with caution when switching from one opioid to another in patients who are receiving very high doses. Opioid rotation generally would be ineffective if there is complete analgesic cross-tolerance between opioids.
The common equivalency conversion tables are based either on studies in patients who received low doses of opioids or on single-dose studies.16,24 By substituting opioids and using lower doses than expected according to the equivalency conversion tables (generally a 25% to 30% decrease), it is possible in most cases to reduce or relieve the symptoms of opioid toxicity and to manage patients highly tolerant to previous opioids while improving analgesia.24
Alternatives to opioid rotation are route conversion (oral to parenteral or spinal), addition of an adjuvant analgesic, and opioid dose reduction.
WHAT IS OPIOID TOXICITY AND HOW IS IT MANAGED?
Opioid overdose is commonly the result of an error in pain assessment, opioid prescribing, or dose administration. Opioid overdose classically presents as sedation or respiratory depression. The combination of coma, reduced respiratory rate, and pinpoint pupils is highly suggestive of opioid toxicity, and treatment should be initiated promptly.
This scenario, however, is the extreme example of opioid overdose, and it is rare when a patient is given the correct opioid dose titrated gradually over a period of time. The more common scenario is when a patient’s pain has finally been managed and the patient is resting comfortably with slow respirations. This would not warrant naloxone (Narcan) administration but rather close observation and monitoring of vital signs.
Naloxone has antagonist activity at all of the receptor sites.27 It is important to be alert for acute opioid withdrawal in patients taking high-dose opioids for a long time.27 There are no guidelines as to the route of administration and the dosing of naloxone. Table 6 summarizes the management of opioid overdose using naloxone.5
WHAT IS THE ROLE OF ADJUVANTS?
An adjuvant analgesic is any drug with a primary indication other than pain, but with analgesic properties in some painful conditions. Adjuvants are best used when a patient cannot obtain satisfactory pain relief from an opioid.28 Antidepressants, anticonvulsants, neuroleptics, antiarrhythmics, antihistamines, N-methyl-d-aspartate (NMDA) receptor antagonists, steroids, muscle relaxants, bisphosphonates, and radiopharmaceuticals can be adjuvant agents.29
Adjuvants are generally used to complement the analgesic effects of opioids to achieve optimal pain control with a minimum of adverse effects.28 The following scenarios should prompt the use of adjuvants in clinical practice28:
- The toxic limit of a primary pain medication has been reached.
- The therapeutic benefit of the primary pain medication has reached a plateau.
- The primary analgesic could not be used because of substance-abuse behavior, multiple organ failure, allergy, etc.
- The patient has multiple pain syndromes.
- The patient has additional symptoms unrelated to pain, eg, insomnia or depression.
Table 7 lists adjuvants with specific indications and points to remember when prescribing them.28,29
WHAT IS THE ROLE OF NSAIDs FOR CANCER PAIN?
Nonsteroidal anti-inflammatory drugs (NSAIDs) have a well-established role in treating cancer-related pain, either on their own for mild pain or in combination with opioids for moderate to severe pain, leading to additive analgesia. Using NSAIDs as adjuvants is common practice in certain cancer pain syndromes, such as malignant bone pain, although there is considerable variation in response.31
NSAIDs have long been known to inhibit peripheral prostaglandin synthesis, but recently they have also been suggested to have a central action. The central effect is related to NMDA receptor-induced activation of the nitric oxide system.31
NSAIDs have ceiling effects, and there is no therapeutic advantage to increasing the dose beyond that which is recommended.
Ketorolac (Toradol), indomethacin (Indocin), and diclofenac (Voltaren) have potent analgesic activity, whereas the “oxicam” NSAIDs show predominantly anti-inflammatory effects.30
No NSAID is clearly superior for a particular type of pain. Certain NSAIDs block the NMDA receptor and inhibit cyclo-oxygenase-1 and cyclo-oxygenase-2. There is a poor correlation between the analgesic effects of NSAIDs and cyclo-oxygenase inhibition. There is no evidence to support the use of selective cyclo-oxygenase-2 inhibitors for cancer pain, and these agents have no advantage over nonselective NSAIDs on the basis of limited gastrointestinal toxicity.32
In cancer pain, NSAIDs may delay the development of tolerance and allow lower doses of opioids to be used, with fewer central nervous system side effects.31,32 Despite the extensive use of NSAIDs, relatively few randomized studies have documented their efficacy in cancer pain compared with other chronic pain syndromes. Data on safe and effective doses from studies of nonmalignant pain may not apply to cancer pain, since cancer patients often have several serious conditions and are on multiple medications. In addition, the potential for adverse effects of NSAIDs (gastrointestinal bleeding, renal failure, thrombosis) may be greater in patients with advanced cancer.
In conclusion, NSAIDs may help if used judiciously in somatic pain and visceral pain, and perhaps even in neuropathic pain.31
HOW IS CANCER PAIN MANAGED IN PATIENTS WITH ORGAN FAILURE?
- Opioids that can be used in liver failure or cirrhosis: morphine, hydromorphone, methadone, levorphanol, buprenorphine.
- Opioids that can be used in renal failure: methadone, fentanyl, and buprenorphine are safest; oxycodone and hydromorphone are moderately safe; morphine is the least safe.35,36
- Opioids that can be used in both kidney and liver failure: methadone, buprenorphine.
HOW CAN PROBLEMS RELATED TO SUBSTANCE ABUSE BE AVOIDED?
Substance abuse is less a problem in managing cancer pain than in chronic nonmalignant pain. Prescribing opioids safely is challenging, and very little has been published on substance abuse and the management of cancer pain. However, in the absence of practice guidelines, the best approach is to establish a dosing structure, control prescription refills, and monitor the patient.
Abuse is the misuse of an opioid via self-titration or altering the dosing schedule or route of administration. Patients who misuse opioids—ie, take them differently than prescribed—are not necessarily addicted.
Addiction is the abuse of a drug associated with psychological dependence, despite harm.
Diversion can occur without addiction and is done for financial gain, and this is the worst offense as it may harm others.
Pseudoaddiction is abnormal, demanding, often hostile behavior resulting from uncontrolled pain; once the pain is controlled, the behavior resolves.
Behaviors such as forging prescriptions, stealing or borrowing drugs, frequently “losing” prescriptions, and resisting changes to medication despite adverse effects are more predictive of addiction than are behaviors such as aggressive complaining about the need for more drugs, drug-hoarding, and unsanctioned dose escalations or other forms of noncompliance, as the latter three are more likely to indicate poorly controlled pain.37
Predictors of opioid abuse include a family history or a personal history of alcohol or drug abuse (including prescription drugs); a history of psychiatric illness (including anxiety disorder); male sex; nonwhite race; a history of driving under the influence of alcohol or drugs; a record of drug-related convictions; lost or stolen prescriptions; and using supplemental sources to obtain opioids.38 Socioeconomic status and disability level were not found to be significant predictors.38
Different scales are available to predict the risk of aberrant drug behavior in patients on chronic opioid therapy. Of the many available, the Screener and Opioid Assessment for Patients With Pain and the Current Opioid Misuse Measure assess all the key factors.38
After an assessment, the next step is monitoring. Unfortunately, no specific method has been validated. In one study, urine toxicology testing was more effective at identifying problems than monitoring patient behavior alone, and monitoring behavior alone would have resulted in missing about half of the patients with a problem.39 The same study showed that even in the absence of aberrant drug-related behavior based on predictors, a significant number of urine toxicology screens were positive.39
A negative urine screen for the patient’s opioid suggests diversion. The clinician should order a screen for the prescribed opioid because a general screen may not detect nonmorphine opioids. A general screen may detect polysubstance abuse, which is common in individuals with addiction.
Table 9 summarizes a strategy to manage opioid therapy in patients with history of substance abuse.40
WHAT IS THE ROLE OF COMPLEMENTARY AND ALTERNATIVE THERAPIES?
Complementary and alternative medicine therapies are commonly used by cancer patients, with an average prevalence rate of 31%.41–43 As the names suggest, they have been used both as an alternative to and as a complement to conventional medicine. Practitioners of complementary and alternative medicine emphasize its holistic, individualistic, empowering, and educational nature.
Patients do not routinely ask their physicians about these therapies,44 and physicians often have only a limited knowledge of them.45 Surveys of North American physicians showed that they view certain of these therapies as legitimate and effective.46,47
The role of complementary and alternative medicine in cancer pain has been the subject of debate, as relatively little is known about adverse effects and drug interactions. Nevertheless, the American Cancer Society and the National Comprehensive Cancer Network guidelines on cancer pain recommend nonpharmacologic treatment be added for patients who report a pain score of 4 or greater on a 10-point scale after analgesic adjustment.48,49
Most studies of complementary and alternative therapies for cancer pain are of poor quality, with significant shortcomings in methodology and study design and with no clear definition of outcomes.50
Acupuncture is probably the most studied of these therapies, but clinical trials so far have not shown it to be an effective adjunct analgesic for cancer pain.51 A placebo-controlled, blinded randomized trial using auricular acupuncture showed a pain score decrease of 36% from baseline at 2 months compared with controls.52
Studies involving cognitive therapy, supportive psychotherapy, and hypnosis showed modest benefit.53,54 Two trials involving relaxation and imagery reduced cancer pain compared with controls.55,56
Studies of massage therapy have shown mixed results; two studies reported a significant reduction in pain immediately after intervention, and no study found pain relief after 4 weeks.57–60 Studies involving Reiki and touch therapy were inconclusive.60,61
Music therapy has been used to treat patients physically, psychologically, socially, emotionally, and spiritually, with evidence still equivocal. A large prospective observational study involving 200 patients conducted by Gallagher et al62 showed pain was reduced by 30% after music therapy intervention. The same study showed a reduction in depression and anxiety.62 Music therapy could be used as a component of a multimodal approach to pain.
Herbal preparations are often used to treat cancer and symptoms by patients and naturalists. Some herbal medicines are known to cause toxicity in cancer patients. Examples are PC-SPES, mistletoe, and saw palmetto.63
At this juncture, there is some evidence that some complementary and alternative therapies can relieve cancer pain, and the most promising therapy seems to be related to mind-body medicine (eg, biofeedback, relaxation techniques). But before we can legitimately integrate these therapies into the management of cancer pain, we need large randomized controlled trials to determine if they are effective in patients on chronic high-dose opioids and if they decrease the need for opioids.
Some 90% of patients with cancer experience pain during their illness.1 The pain usually worsens as the disease progresses, and patients may experience different types of pain.
Persistent pain decreases function, appetite, and sleep, induces fear, causes depression, and generally lowers the quality of life.2 Persistent pain is demoralizing and debilitating for patients and their caregivers.3
Adequate pain control is important to ensure that patients can function productively, maintain social relationships, and improve their quality of life.2 Yet 86% of practicing physicians surveyed believed that most cancer patients with pain were undermedicated,2 and most felt that pain management is unsuccessful in more than half of patients who seek help.3
The critical importance of pain management has been emphasized by the World Health Organization (WHO), by international and national professional organizations, and by government agencies. All practitioners who care for cancer patients need to be well educated in managing cancer pain, a key part of which is to educate patients about the process and what to expect. This results in better pain control.4
Although much has been written on the management of cancer pain in a referral setting, little has been published on how to manage it in primary care. In this article, we discuss common questions faced by generalists. We emphasize the use of opioids, perhaps the most challenging aspect of cancer pain management. We also discuss when consultation with a specialist in pain management or a palliative medicine specialist is especially helpful.
WHAT ARE THE DIFFERENT TYPES OF PAIN SYNDROMES?
Pain is classified in several ways1–6:
Nociceptive vs neuropathic. Nociceptive pain comprises somatic and visceral components and is the result of continued tissue injury.4 Neuropathic pain is due to injury to the peripheral and central nervous systems and occurs within an area of sensory or motor deficit.
Continuous vs intermittent. Continuous pain, even if controlled, can have breakthroughs, ie, flares of pain above the controlled baseline level. Intermittent pain is a pain flare without chronic baseline pain. Intermittent pain is further divided into incident pain (ie, on movement) and end-of-dose failure (ie, pain occurring just before the next scheduled opioid dose).5 Pain specialists continue to debate the meaning and the use of these terms.
Malignant vs nonmalignant. Cancer pain is multifactorial,1 being induced by the disease itself, by the treatment of cancer, and by pain unrelated to cancer or its treatment (eg, osteoarthritis or diabetic neuropathy).2
Familiarity with the causes and the types of pain, including pain related to cancer, is important, as this influences treatment decisions.
HOW IS PAIN ASSESSED?
The assessment of pain is vital in managing it.
Since pain is inherently subjective, the patient’s self-report is the gold standard.4 Characteristics of the pain along with a physical examination, laboratory testing, and imaging studies can define the pathophysiology of the pain and influence the decision to undertake further assessment or specific therapies.
Patients and physicians can use various scales, such as a visual analog scale, a numerical rating scale, a graphic scale, a verbal scale, a word descriptor scale, and a functional pain scale. A verbal scale can be used if the patient is alert, or a nonverbal scale if the patient has impaired cognition or speaks a different language. Intensity is the most common dimension evaluated in cancer pain, primarily via a numerical or visual analog scale. A numerical scale score of 0 to 10 has been found to be as effective as a visual analog scale (0 to 100 mm),7,8 and the numerical rating scale is generally preferred as a measure of pain intensity.9
There are no clear guidelines for selecting one scale over another.7 A clinically meaningful response (ie, meaningful to patients) is at least a two-point decrease on the 10-point numerical scale or a 13-mm decrease on the 100-mm visual analog scale. A decrease in the percentage of the pain relates to global improvement better than an absolute reduction on the numerical scale.
WHAT PROBLEMS ARE ENCOUNTERED IN MANAGING CANCER PAIN?
WHAT ARE THE DIFFERENT WAYS TO MANAGE CANCER PAIN?
Pain should be treated promptly and aggressively, because if untreated it can lead to delays in healing, changes in the central nervous system (eg, sensitization, plasticity), chronic stress, family stress, depression, job loss, and even suicide.12–14
Comprehensive pain management improves outcomes and includes the rational use of opioids and adjuvant analgesics, physical rehabilitation, cognitive behavioral (non-drug) therapies, family counseling, interventional procedures (kyphoplasty, nerve blocks, local injections, spinal analgesia), and complementary therapies such as acupuncture.12 Adjuvant analgesics include antidepressants, anticonvulsants, and local anesthetics.
HOW DO OPIOIDS RELIEVE CANCER PAIN?
Opioids bind to receptors in tissues throughout the body, including in the central and peripheral nervous systems15 and the digestive tract. The binding of an opioid to an opioid receptor—including mu, kappa, and delta receptors and orphan receptor-like ligand-1—initiates a cascade of intracellular reactions. Due to the nature of different interactions of opioids with each of these receptors, individuals vary in their response to opioids.15
WHAT ARE THE CHARACTERISTICS OF COMMON OPIOIDS?
- Step 1. Mild pain calls for a nonopioid analgesic with or without an adjuvant (more about adjuvants below).
- Step 2. Mild or moderate pain that persists or increases calls for a weak opioid such as codeine, tramadol (Ultram), or hydrocodone, with or without a nonopioid and with or without an adjuvant.
- Step 3. Severe pain calls for a strong opioid with or without a nonopioid, and with or without an adjuvant.
Morphine, the prototypical opioid, is well studied and versatile, as it can be given orally, parenterally, rectally, or intraspinally. It is readily available in the United States and Western Europe but not in some parts of the world, such as Asia and Africa. It is also cost-effective.
Hydromorphone (Dilaudid) is similar to morphine in terms of versatility, cost, and effectiveness in pain management. An extended-release form (Exalgo) is now available in the United States.
Oxycodone is readily available in both slow-release (eg, OxyContin) and immediate-release (eg, Oxy-IR) preparations and is also cost-effective. However, there is no parenteral formulation in the United States.
Methadone is inexpensive and can be used as a long-acting or an immediate-release opioid. However, it should be used with caution in patients with a prolonged QTc interval: in general, a QTc interval of 430 to 450 msec is not a contraindication, but there is a risk of torsades de pointes when the QTc is greater than 500 msec. The physician should also look for drug interactions when prescribing methadone, which is metabolized in the liver via the cytochrome P450 3A4 system. Methadone use can also lead to respiratory depression, prolonged QTc interval, and sudden death.
Buprenorphine can be used as a third- or fourth-tier opioid for patients with both kidney and liver failure. It can be given sublingually or parenterally. It may not be readily available, may not be covered by insurance, and is expensive.
Selecting an opioid to try first
The following are some general considerations when selecting an opioid to try first:
- Does the patient have a history of organ failure? Has the patient had a therapeutic response to, or adverse effects from, a particular opioid in the past?
- Which route would best fit the patient’s needs? (Oral is always preferable.)
- How often will breakthrough dosing be required? (In general, the breakthrough dose is administered at the drug’s half-life, but it can be administered between 1 and 4 hours.)
- How much will it cost? (Consider the cost, insurance coverage, and co-pays.)
Table 2 shows different characteristics of commonly used opioids, including route of administration, onset of action, peak effect, and duration of action.1
WHAT ARE THE EQUIANALGESIC DOSES OF COMMONLY USED OPIOIDS?
Table 3 lists equianalgesic doses and route conversions of commonly used opioids.18–20
WHAT ARE THE PRINCIPLES BEHIND OPIOID DOSING?
Table 4 shows important strategies for opioid dosing. An in-depth discussion of specific opioid dosing strategies is beyond the scope of this article.5
WHAT ARE THE COMMON ADVERSE EFFECTS OF OPIOIDS?
Adverse effects are among the most common reasons for failure of opioids to relieve pain. If these effects are not anticipated and treated prophylactically, patients may avoid taking their opioid drugs or may complain that they are “allergic” to them. In reality, true allergy to any of the opioids is rare. Patients comply better if they are taught to expect that most adverse effects are either preventable or manageable.21 A simple strategy includes reducing the opioid dose by 25% to 50%, using different opioids (“rotation”), changing the route of administration, and directly treating adverse effects.21,22
WHAT IS OPIOID ROTATION AND HOW IS IT DONE?
Opioid rotation involves changing to a different drug using the same administration route, with the aim of improving the analgesic response or reducing adverse effects.16 It may be useful in widening the therapeutic window, ie, establishing a more advantageous relationship between analgesia and toxicity.16 This strategy applies, for example, to patients who have an adverse reaction to morphine, and who may need rotation to fentanyl or methadone.
The major indication for switching opioids is poorly controlled pain with unacceptable adverse effects due to opioid toxicity, the rapid development of tolerance, refractory pain, or difficult pain syndromes.24 A recent prospective study showed that 42% of patients underwent opioid rotation, and the two most common reasons were inadequate analgesia and severe adverse effects.25 Opioid rotation resulted in relief of confusion (72%), nausea and vomiting (68%), and drowsiness (53%).25
Before trying opioid rotation, review the patient’s pain syndromes and the use of an adjuvant analgesic, and assess for evidence of opioid toxicity or contributing abnormal biochemical factors such as hydration status.24,26 Most opioids are mu-receptor agonists and may exhibit cross-tolerance, a phenomenon in which the alternative drug does not have the expected effects because of similar pharmacologic action of the first drug. Because the degree of cross-tolerance may change as opioid doses are escalated, it is advisable to proceed with caution when switching from one opioid to another in patients who are receiving very high doses. Opioid rotation generally would be ineffective if there is complete analgesic cross-tolerance between opioids.
The common equivalency conversion tables are based either on studies in patients who received low doses of opioids or on single-dose studies.16,24 By substituting opioids and using lower doses than expected according to the equivalency conversion tables (generally a 25% to 30% decrease), it is possible in most cases to reduce or relieve the symptoms of opioid toxicity and to manage patients highly tolerant to previous opioids while improving analgesia.24
Alternatives to opioid rotation are route conversion (oral to parenteral or spinal), addition of an adjuvant analgesic, and opioid dose reduction.
WHAT IS OPIOID TOXICITY AND HOW IS IT MANAGED?
Opioid overdose is commonly the result of an error in pain assessment, opioid prescribing, or dose administration. Opioid overdose classically presents as sedation or respiratory depression. The combination of coma, reduced respiratory rate, and pinpoint pupils is highly suggestive of opioid toxicity, and treatment should be initiated promptly.
This scenario, however, is the extreme example of opioid overdose, and it is rare when a patient is given the correct opioid dose titrated gradually over a period of time. The more common scenario is when a patient’s pain has finally been managed and the patient is resting comfortably with slow respirations. This would not warrant naloxone (Narcan) administration but rather close observation and monitoring of vital signs.
Naloxone has antagonist activity at all of the receptor sites.27 It is important to be alert for acute opioid withdrawal in patients taking high-dose opioids for a long time.27 There are no guidelines as to the route of administration and the dosing of naloxone. Table 6 summarizes the management of opioid overdose using naloxone.5
WHAT IS THE ROLE OF ADJUVANTS?
An adjuvant analgesic is any drug with a primary indication other than pain, but with analgesic properties in some painful conditions. Adjuvants are best used when a patient cannot obtain satisfactory pain relief from an opioid.28 Antidepressants, anticonvulsants, neuroleptics, antiarrhythmics, antihistamines, N-methyl-d-aspartate (NMDA) receptor antagonists, steroids, muscle relaxants, bisphosphonates, and radiopharmaceuticals can be adjuvant agents.29
Adjuvants are generally used to complement the analgesic effects of opioids to achieve optimal pain control with a minimum of adverse effects.28 The following scenarios should prompt the use of adjuvants in clinical practice28:
- The toxic limit of a primary pain medication has been reached.
- The therapeutic benefit of the primary pain medication has reached a plateau.
- The primary analgesic could not be used because of substance-abuse behavior, multiple organ failure, allergy, etc.
- The patient has multiple pain syndromes.
- The patient has additional symptoms unrelated to pain, eg, insomnia or depression.
Table 7 lists adjuvants with specific indications and points to remember when prescribing them.28,29
WHAT IS THE ROLE OF NSAIDs FOR CANCER PAIN?
Nonsteroidal anti-inflammatory drugs (NSAIDs) have a well-established role in treating cancer-related pain, either on their own for mild pain or in combination with opioids for moderate to severe pain, leading to additive analgesia. Using NSAIDs as adjuvants is common practice in certain cancer pain syndromes, such as malignant bone pain, although there is considerable variation in response.31
NSAIDs have long been known to inhibit peripheral prostaglandin synthesis, but recently they have also been suggested to have a central action. The central effect is related to NMDA receptor-induced activation of the nitric oxide system.31
NSAIDs have ceiling effects, and there is no therapeutic advantage to increasing the dose beyond that which is recommended.
Ketorolac (Toradol), indomethacin (Indocin), and diclofenac (Voltaren) have potent analgesic activity, whereas the “oxicam” NSAIDs show predominantly anti-inflammatory effects.30
No NSAID is clearly superior for a particular type of pain. Certain NSAIDs block the NMDA receptor and inhibit cyclo-oxygenase-1 and cyclo-oxygenase-2. There is a poor correlation between the analgesic effects of NSAIDs and cyclo-oxygenase inhibition. There is no evidence to support the use of selective cyclo-oxygenase-2 inhibitors for cancer pain, and these agents have no advantage over nonselective NSAIDs on the basis of limited gastrointestinal toxicity.32
In cancer pain, NSAIDs may delay the development of tolerance and allow lower doses of opioids to be used, with fewer central nervous system side effects.31,32 Despite the extensive use of NSAIDs, relatively few randomized studies have documented their efficacy in cancer pain compared with other chronic pain syndromes. Data on safe and effective doses from studies of nonmalignant pain may not apply to cancer pain, since cancer patients often have several serious conditions and are on multiple medications. In addition, the potential for adverse effects of NSAIDs (gastrointestinal bleeding, renal failure, thrombosis) may be greater in patients with advanced cancer.
In conclusion, NSAIDs may help if used judiciously in somatic pain and visceral pain, and perhaps even in neuropathic pain.31
HOW IS CANCER PAIN MANAGED IN PATIENTS WITH ORGAN FAILURE?
- Opioids that can be used in liver failure or cirrhosis: morphine, hydromorphone, methadone, levorphanol, buprenorphine.
- Opioids that can be used in renal failure: methadone, fentanyl, and buprenorphine are safest; oxycodone and hydromorphone are moderately safe; morphine is the least safe.35,36
- Opioids that can be used in both kidney and liver failure: methadone, buprenorphine.
HOW CAN PROBLEMS RELATED TO SUBSTANCE ABUSE BE AVOIDED?
Substance abuse is less a problem in managing cancer pain than in chronic nonmalignant pain. Prescribing opioids safely is challenging, and very little has been published on substance abuse and the management of cancer pain. However, in the absence of practice guidelines, the best approach is to establish a dosing structure, control prescription refills, and monitor the patient.
Abuse is the misuse of an opioid via self-titration or altering the dosing schedule or route of administration. Patients who misuse opioids—ie, take them differently than prescribed—are not necessarily addicted.
Addiction is the abuse of a drug associated with psychological dependence, despite harm.
Diversion can occur without addiction and is done for financial gain, and this is the worst offense as it may harm others.
Pseudoaddiction is abnormal, demanding, often hostile behavior resulting from uncontrolled pain; once the pain is controlled, the behavior resolves.
Behaviors such as forging prescriptions, stealing or borrowing drugs, frequently “losing” prescriptions, and resisting changes to medication despite adverse effects are more predictive of addiction than are behaviors such as aggressive complaining about the need for more drugs, drug-hoarding, and unsanctioned dose escalations or other forms of noncompliance, as the latter three are more likely to indicate poorly controlled pain.37
Predictors of opioid abuse include a family history or a personal history of alcohol or drug abuse (including prescription drugs); a history of psychiatric illness (including anxiety disorder); male sex; nonwhite race; a history of driving under the influence of alcohol or drugs; a record of drug-related convictions; lost or stolen prescriptions; and using supplemental sources to obtain opioids.38 Socioeconomic status and disability level were not found to be significant predictors.38
Different scales are available to predict the risk of aberrant drug behavior in patients on chronic opioid therapy. Of the many available, the Screener and Opioid Assessment for Patients With Pain and the Current Opioid Misuse Measure assess all the key factors.38
After an assessment, the next step is monitoring. Unfortunately, no specific method has been validated. In one study, urine toxicology testing was more effective at identifying problems than monitoring patient behavior alone, and monitoring behavior alone would have resulted in missing about half of the patients with a problem.39 The same study showed that even in the absence of aberrant drug-related behavior based on predictors, a significant number of urine toxicology screens were positive.39
A negative urine screen for the patient’s opioid suggests diversion. The clinician should order a screen for the prescribed opioid because a general screen may not detect nonmorphine opioids. A general screen may detect polysubstance abuse, which is common in individuals with addiction.
Table 9 summarizes a strategy to manage opioid therapy in patients with history of substance abuse.40
WHAT IS THE ROLE OF COMPLEMENTARY AND ALTERNATIVE THERAPIES?
Complementary and alternative medicine therapies are commonly used by cancer patients, with an average prevalence rate of 31%.41–43 As the names suggest, they have been used both as an alternative to and as a complement to conventional medicine. Practitioners of complementary and alternative medicine emphasize its holistic, individualistic, empowering, and educational nature.
Patients do not routinely ask their physicians about these therapies,44 and physicians often have only a limited knowledge of them.45 Surveys of North American physicians showed that they view certain of these therapies as legitimate and effective.46,47
The role of complementary and alternative medicine in cancer pain has been the subject of debate, as relatively little is known about adverse effects and drug interactions. Nevertheless, the American Cancer Society and the National Comprehensive Cancer Network guidelines on cancer pain recommend nonpharmacologic treatment be added for patients who report a pain score of 4 or greater on a 10-point scale after analgesic adjustment.48,49
Most studies of complementary and alternative therapies for cancer pain are of poor quality, with significant shortcomings in methodology and study design and with no clear definition of outcomes.50
Acupuncture is probably the most studied of these therapies, but clinical trials so far have not shown it to be an effective adjunct analgesic for cancer pain.51 A placebo-controlled, blinded randomized trial using auricular acupuncture showed a pain score decrease of 36% from baseline at 2 months compared with controls.52
Studies involving cognitive therapy, supportive psychotherapy, and hypnosis showed modest benefit.53,54 Two trials involving relaxation and imagery reduced cancer pain compared with controls.55,56
Studies of massage therapy have shown mixed results; two studies reported a significant reduction in pain immediately after intervention, and no study found pain relief after 4 weeks.57–60 Studies involving Reiki and touch therapy were inconclusive.60,61
Music therapy has been used to treat patients physically, psychologically, socially, emotionally, and spiritually, with evidence still equivocal. A large prospective observational study involving 200 patients conducted by Gallagher et al62 showed pain was reduced by 30% after music therapy intervention. The same study showed a reduction in depression and anxiety.62 Music therapy could be used as a component of a multimodal approach to pain.
Herbal preparations are often used to treat cancer and symptoms by patients and naturalists. Some herbal medicines are known to cause toxicity in cancer patients. Examples are PC-SPES, mistletoe, and saw palmetto.63
At this juncture, there is some evidence that some complementary and alternative therapies can relieve cancer pain, and the most promising therapy seems to be related to mind-body medicine (eg, biofeedback, relaxation techniques). But before we can legitimately integrate these therapies into the management of cancer pain, we need large randomized controlled trials to determine if they are effective in patients on chronic high-dose opioids and if they decrease the need for opioids.
- Laird B, Colvin L, Fallon M. Management of cancer pain: basic principles and neuropathic cancer pain. Eur J Cancer 2008; 44:1078–1082.
- Chang HM. Cancer pain management. Med Clin North Am 1999; 83:711–736,
- Stannard C, Johnson M. Chronic pain management—can we do better? An interview-based survey in primary care. Curr Med Res Opin 2003; 19:703–706.
- Portenoy RK, Lesage P. Management of cancer pain. Lancet 1999; 353:1695–1700.
- Walsh D, Rivera NI, Davis MP, Lagman R, Legrand SB. Strategies for pain management: Cleveland Clinic Foundation guidelines for opioid dosing for cancer pain. Support Cancer Ther 2004; 1:157–164.
- Foley KM. Acute and chronic pain syndromes. In:Doyle D, Hanks G, Cherny N, Calman K, editors. Oxford Textbook of Palliative Medicine. 3rd ed. Oxford, UK: Oxford University Press; 2005:298–316.
- Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain 2003; 4:2–21.
- Jensen MP, Turner JA, Romano JM. What is the maximum number of levels needed in pain intensity measurement? Pain 1994; 58:387–392.
- Preston CC, Colman AM. Optimal number of response categories in rating scales: reliability, validity, discriminating power, and respondent p. Acta Psychol (Amst) 2000; 104:1–15.
- Peretti-Watel P, Bendiane MK, Obadia Y, Favre R, Lapiana JM, Moatti JP; South-Eastern France Palliative Care Group. The prescription of opioid analgesics to terminal cancer patients: impact of physicians’ general attitudes and contextual factors. Palliat Support Care 2003; 1:345–352.
- Jacobsen R, Liubarskiene Z, Møldrup C, Christrup L, Sjøgren P, Samsanaviciene J. Barriers to cancer pain management: a review of empirical research. Medicina (Kaunas) 2009; 45:427–433.
- Wiedemer NL, Harden PS, Arndt IO, Gallagher RM. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med 2007; 8:573–584.
- Rome HP, Rome JD. Limbically augmented pain syndrome (LAPS): kindling, corticolimbic sensitization, and the convergence of affective and sensory symptoms in chronic pain disorders. Pain Med 2000; 1:7–23.
- Fishbain DA, Rosomoff HL, Rosomoff RS. Drug abuse, dependence, and addiction in chronic pain patients. Clin J Pain 1992; 8:77–85.
- Murányi M, Radák Z. Pain and opioids. Orv Hetil 2008; 149:2363–2370.
- Vadalouca A, Moka E, Argyra E, Sikioti P, Siafaka I. Opioid rotation in patients with cancer: a review of the current literature. J Opioid Manag 2008; 4:213–250.
- Galvagno SM, Correll DJ, Narang S. Safe oral equianalgesic opioid dosing for patients with moderate-to-severe pain. www.hcplive.com/publications/Resident-and-Staff/2007/2007-04/2007-04_06. Accessed May 25, 2011.
- Walsh D. Pharmacological management of cancer pain. Semin Oncol 2000; 27:45–63.
- Shaheen PE, Walsh D, Lasheen W, Davis MP, Lagman RL. Opioid equianalgesic tables: are they all equally dangerous? J Pain Symptom Manage 2009; 38:409–417.
- Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids. a critical review and proposals for long-term dosing. J Pain Symptom Manage 2001; 22:672–687.
- Harris JD. Management of expected and unexpected opioid-related side effects. Clin J Pain 2008; 24(suppl 10):S8–S13.
- Cherny N, Ripamonti C, Pereira J; Expert Working Group of the European Association of Palliative Care Network. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol 2001; 19:2542–2554.
- Harris JD, Kotob F. Management of opioid-related side effects. In:de Leon-Casasola OA, ed. Cancer Pain: Pharmacological, Interventional and Palliative Care. Philadelphia: Elsevier Inc; 2006:207–230.
- Mercadante S. Opioid rotation for cancer pain: rationale and clinical aspects. Cancer 1999; 86:1856–1866.
- Cheema B, Lagman RL, Walsh D, et al. A prospective study of opioid rotation in pain due to advanced cancer. J Cancer Pain & Symp Palliat 2006; 2:39–46.
- Schug SA, Zech D, Grond S, Jung H, Meuser T, Stobbe B. A long-term survey of morphine in cancer pain patients. J Pain Symptom Manage 1992; 7:259–266.
- Clarke SF, Dargan PI, Jones AL. Naloxone in opioid poisoning: walking the tightrope. Emerg Med J 2005; 22:612–616.
- Knotkova H, Pappagallo M. Adjuvant analgesics. Med Clin North Am 2007; 91:113–124.
- Lussier D, Huskey AG, Portenoy RK. Adjuvant analgesics in cancer pain management. Oncologist 2004; 9:571–591.
- Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancerrelated pain. J Pain Symptom Manage 2010; 39:167–179.
- Mercadante S. The use of anti-inflammatory drugs in cancer pain. Cancer Treat Rev 2001; 27:51–61.
- Davis MP, Walsh D, Lagman R, LeGrand SB. Controversies in pharmacotherapy of pain management. Lancet Oncol 2005; 6:696–704.
- Klotz U. Tramadol—the impact of its pharmacokinetic and pharmacodynamic properties on the clinical management of pain. Arzneimittelforschung 2003; 53:681–687.
- Davis MP, Lasheen W, Gamier P. Practical guide to opioids and their complications in managing cancer pain. What oncologists need to know. Oncology (Williston Park) 2007; 21:1229–1238.
- Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage 2004; 28:497–504.
- Davis MP. Buprenorphine in cancer pain. Support Care Cancer 2005; 13:878–887.
- Portenoy RK. Opioid therapy for chronic nonmalignant pain: a review of the critical issues. J Pain Symptom Manage 1996; 11:203–217.
- Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain 2008; 24:497–508.
- Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg 2003; 97:1097–1102,
- Passik SD, Kirsh KL. Managing pain in patients with aberrant drug-taking behaviors. J Support Oncol 2005; 3:83–86.
- Ernst E, Cassileth BR. The prevalence of complementary/alternative medicine in cancer: a systematic review. Cancer 1998; 83:777–782.
- Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA 1998; 280:1569–1575.
- Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol 2000; 18:2505–2514.
- Adler SR, Fosket JR. Disclosing complementary and alternative medicine use in the medical encounter: a qualitative study in women with breast cancer. J Fam Pract 1999; 48:453–458.
- Newell S, Sanson-Fisher RW. Australian oncologists’ self-reported knowledge and attitudes about non-traditional therapies used by cancer patients. Med J Aust 2000; 172:110–113.
- Berman BM, Singh BK, Lao L, Singh BB, Ferentz KS, Hartnoll SM. Physicians’ attitudes toward complementary or alternative medicine: a regional survey. J Am Board Fam Pract 1995; 8:361–366.
- Verhoef MJ, Sutherland LR. General practitioners’ assessment of and interest in alternative medicine in Canada. Soc Sci Med 1995; 41:511–515.
- American Cancer Society: Treatment guidelines for patients. Version 1. http://www.cancer.org/downloads/CRI/NCCN_pain.pdf.
- Benedetti C, Brock C, Cleeland C, et al; National Comprehensive Cancer Network. NCCN Practice Guidelines for Cancer Pain. Oncology (Williston Park) 2000; 14:135–150.
- Bardia A, Barton DL, Prokop LJ, Bauer BA, Moynihan TJ. Efficacy of complementary and alternative medicine therapies in relieving cancer pain: a systematic review. J Clin Oncol 2006; 24:5457–5464.
- Lee H, Schmidt K, Ernst E. Acupuncture for the relief of cancer-related pain—a systematic review. Eur J Pain 2005; 9:437–444.
- Alimi D, Rubino C, Pichard-Léandri E, Fermand-Brulé S, Dubreuil-Lemaire ML, Hill C. Analgesic effect of auricular acupuncture for cancer pain: a randomized, blinded, controlled trial. J Clin Oncol 2003; 21:4120–4126.
- Spiegel D, Bloom JR. Group therapy and hypnosis reduce metastatic breast carcinoma pain. Psychosom Med 1983; 45:333–339.
- Goodwin PJ, Leszcz M, Ennis M, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med 2001; 345:1719–1726.
- Syrjala KL, Donaldson GW, Davis MW, Kippes ME, Carr JE. Relaxation and imagery and cognitive-behavioral training reduce pain during cancer treatment: a controlled clinical trial. Pain 1995; 63:189–198.
- Sloman R, Brown P, Aldana E, Chee E. The use of relaxation for the promotion of comfort and pain relief in persons with advanced cancer. Contemp Nurse 1994; 3:6–12.
- Weinrich SP, Weinrich MC. The effect of massage on pain in cancer patients. Appl Nurs Res 1990; 3:140–145.
- Wilkie DJ, Kampbell J, Cutshall S, et al. Effects of massage on pain intensity, analgesics and quality of life in patients with cancer pain: a pilot study of a randomized clinical trial conducted within hospice care delivery. Hosp J 2000; 15:31–53.
- Soden K, Vincent K, Craske S, Lucas C, Ashley S. A randomized controlled trial of aromatherapy massage in a hospice setting. Palliat Med 2004; 18:87–92.
- Post-White J, Kinney ME, Savik K, Gau JB, Wilcox C, Lerner I. Therapeutic massage and healing touch improve symptoms in cancer. Integr Cancer Ther 2003; 2:332–344.
- Olson K, Hanson J, Michaud M. A phase II trial of Reiki for the management of pain in advanced cancer patients. J Pain Symptom Manage 2003; 26:990–997.
- Gallagher LM, Lagman R, Walsh D, Davis MP, Legrand SB. The clinical effects of music therapy in palliative medicine. Support Care Cancer 2006; 14:859–866.
- Olaku O, White JD. Herbal therapy use by cancer patients: a literature review on case reports. Eur J Cancer 2011; 47:508–514.
- Laird B, Colvin L, Fallon M. Management of cancer pain: basic principles and neuropathic cancer pain. Eur J Cancer 2008; 44:1078–1082.
- Chang HM. Cancer pain management. Med Clin North Am 1999; 83:711–736,
- Stannard C, Johnson M. Chronic pain management—can we do better? An interview-based survey in primary care. Curr Med Res Opin 2003; 19:703–706.
- Portenoy RK, Lesage P. Management of cancer pain. Lancet 1999; 353:1695–1700.
- Walsh D, Rivera NI, Davis MP, Lagman R, Legrand SB. Strategies for pain management: Cleveland Clinic Foundation guidelines for opioid dosing for cancer pain. Support Cancer Ther 2004; 1:157–164.
- Foley KM. Acute and chronic pain syndromes. In:Doyle D, Hanks G, Cherny N, Calman K, editors. Oxford Textbook of Palliative Medicine. 3rd ed. Oxford, UK: Oxford University Press; 2005:298–316.
- Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain 2003; 4:2–21.
- Jensen MP, Turner JA, Romano JM. What is the maximum number of levels needed in pain intensity measurement? Pain 1994; 58:387–392.
- Preston CC, Colman AM. Optimal number of response categories in rating scales: reliability, validity, discriminating power, and respondent p. Acta Psychol (Amst) 2000; 104:1–15.
- Peretti-Watel P, Bendiane MK, Obadia Y, Favre R, Lapiana JM, Moatti JP; South-Eastern France Palliative Care Group. The prescription of opioid analgesics to terminal cancer patients: impact of physicians’ general attitudes and contextual factors. Palliat Support Care 2003; 1:345–352.
- Jacobsen R, Liubarskiene Z, Møldrup C, Christrup L, Sjøgren P, Samsanaviciene J. Barriers to cancer pain management: a review of empirical research. Medicina (Kaunas) 2009; 45:427–433.
- Wiedemer NL, Harden PS, Arndt IO, Gallagher RM. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med 2007; 8:573–584.
- Rome HP, Rome JD. Limbically augmented pain syndrome (LAPS): kindling, corticolimbic sensitization, and the convergence of affective and sensory symptoms in chronic pain disorders. Pain Med 2000; 1:7–23.
- Fishbain DA, Rosomoff HL, Rosomoff RS. Drug abuse, dependence, and addiction in chronic pain patients. Clin J Pain 1992; 8:77–85.
- Murányi M, Radák Z. Pain and opioids. Orv Hetil 2008; 149:2363–2370.
- Vadalouca A, Moka E, Argyra E, Sikioti P, Siafaka I. Opioid rotation in patients with cancer: a review of the current literature. J Opioid Manag 2008; 4:213–250.
- Galvagno SM, Correll DJ, Narang S. Safe oral equianalgesic opioid dosing for patients with moderate-to-severe pain. www.hcplive.com/publications/Resident-and-Staff/2007/2007-04/2007-04_06. Accessed May 25, 2011.
- Walsh D. Pharmacological management of cancer pain. Semin Oncol 2000; 27:45–63.
- Shaheen PE, Walsh D, Lasheen W, Davis MP, Lagman RL. Opioid equianalgesic tables: are they all equally dangerous? J Pain Symptom Manage 2009; 38:409–417.
- Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids. a critical review and proposals for long-term dosing. J Pain Symptom Manage 2001; 22:672–687.
- Harris JD. Management of expected and unexpected opioid-related side effects. Clin J Pain 2008; 24(suppl 10):S8–S13.
- Cherny N, Ripamonti C, Pereira J; Expert Working Group of the European Association of Palliative Care Network. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol 2001; 19:2542–2554.
- Harris JD, Kotob F. Management of opioid-related side effects. In:de Leon-Casasola OA, ed. Cancer Pain: Pharmacological, Interventional and Palliative Care. Philadelphia: Elsevier Inc; 2006:207–230.
- Mercadante S. Opioid rotation for cancer pain: rationale and clinical aspects. Cancer 1999; 86:1856–1866.
- Cheema B, Lagman RL, Walsh D, et al. A prospective study of opioid rotation in pain due to advanced cancer. J Cancer Pain & Symp Palliat 2006; 2:39–46.
- Schug SA, Zech D, Grond S, Jung H, Meuser T, Stobbe B. A long-term survey of morphine in cancer pain patients. J Pain Symptom Manage 1992; 7:259–266.
- Clarke SF, Dargan PI, Jones AL. Naloxone in opioid poisoning: walking the tightrope. Emerg Med J 2005; 22:612–616.
- Knotkova H, Pappagallo M. Adjuvant analgesics. Med Clin North Am 2007; 91:113–124.
- Lussier D, Huskey AG, Portenoy RK. Adjuvant analgesics in cancer pain management. Oncologist 2004; 9:571–591.
- Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancerrelated pain. J Pain Symptom Manage 2010; 39:167–179.
- Mercadante S. The use of anti-inflammatory drugs in cancer pain. Cancer Treat Rev 2001; 27:51–61.
- Davis MP, Walsh D, Lagman R, LeGrand SB. Controversies in pharmacotherapy of pain management. Lancet Oncol 2005; 6:696–704.
- Klotz U. Tramadol—the impact of its pharmacokinetic and pharmacodynamic properties on the clinical management of pain. Arzneimittelforschung 2003; 53:681–687.
- Davis MP, Lasheen W, Gamier P. Practical guide to opioids and their complications in managing cancer pain. What oncologists need to know. Oncology (Williston Park) 2007; 21:1229–1238.
- Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage 2004; 28:497–504.
- Davis MP. Buprenorphine in cancer pain. Support Care Cancer 2005; 13:878–887.
- Portenoy RK. Opioid therapy for chronic nonmalignant pain: a review of the critical issues. J Pain Symptom Manage 1996; 11:203–217.
- Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain 2008; 24:497–508.
- Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg 2003; 97:1097–1102,
- Passik SD, Kirsh KL. Managing pain in patients with aberrant drug-taking behaviors. J Support Oncol 2005; 3:83–86.
- Ernst E, Cassileth BR. The prevalence of complementary/alternative medicine in cancer: a systematic review. Cancer 1998; 83:777–782.
- Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA 1998; 280:1569–1575.
- Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol 2000; 18:2505–2514.
- Adler SR, Fosket JR. Disclosing complementary and alternative medicine use in the medical encounter: a qualitative study in women with breast cancer. J Fam Pract 1999; 48:453–458.
- Newell S, Sanson-Fisher RW. Australian oncologists’ self-reported knowledge and attitudes about non-traditional therapies used by cancer patients. Med J Aust 2000; 172:110–113.
- Berman BM, Singh BK, Lao L, Singh BB, Ferentz KS, Hartnoll SM. Physicians’ attitudes toward complementary or alternative medicine: a regional survey. J Am Board Fam Pract 1995; 8:361–366.
- Verhoef MJ, Sutherland LR. General practitioners’ assessment of and interest in alternative medicine in Canada. Soc Sci Med 1995; 41:511–515.
- American Cancer Society: Treatment guidelines for patients. Version 1. http://www.cancer.org/downloads/CRI/NCCN_pain.pdf.
- Benedetti C, Brock C, Cleeland C, et al; National Comprehensive Cancer Network. NCCN Practice Guidelines for Cancer Pain. Oncology (Williston Park) 2000; 14:135–150.
- Bardia A, Barton DL, Prokop LJ, Bauer BA, Moynihan TJ. Efficacy of complementary and alternative medicine therapies in relieving cancer pain: a systematic review. J Clin Oncol 2006; 24:5457–5464.
- Lee H, Schmidt K, Ernst E. Acupuncture for the relief of cancer-related pain—a systematic review. Eur J Pain 2005; 9:437–444.
- Alimi D, Rubino C, Pichard-Léandri E, Fermand-Brulé S, Dubreuil-Lemaire ML, Hill C. Analgesic effect of auricular acupuncture for cancer pain: a randomized, blinded, controlled trial. J Clin Oncol 2003; 21:4120–4126.
- Spiegel D, Bloom JR. Group therapy and hypnosis reduce metastatic breast carcinoma pain. Psychosom Med 1983; 45:333–339.
- Goodwin PJ, Leszcz M, Ennis M, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med 2001; 345:1719–1726.
- Syrjala KL, Donaldson GW, Davis MW, Kippes ME, Carr JE. Relaxation and imagery and cognitive-behavioral training reduce pain during cancer treatment: a controlled clinical trial. Pain 1995; 63:189–198.
- Sloman R, Brown P, Aldana E, Chee E. The use of relaxation for the promotion of comfort and pain relief in persons with advanced cancer. Contemp Nurse 1994; 3:6–12.
- Weinrich SP, Weinrich MC. The effect of massage on pain in cancer patients. Appl Nurs Res 1990; 3:140–145.
- Wilkie DJ, Kampbell J, Cutshall S, et al. Effects of massage on pain intensity, analgesics and quality of life in patients with cancer pain: a pilot study of a randomized clinical trial conducted within hospice care delivery. Hosp J 2000; 15:31–53.
- Soden K, Vincent K, Craske S, Lucas C, Ashley S. A randomized controlled trial of aromatherapy massage in a hospice setting. Palliat Med 2004; 18:87–92.
- Post-White J, Kinney ME, Savik K, Gau JB, Wilcox C, Lerner I. Therapeutic massage and healing touch improve symptoms in cancer. Integr Cancer Ther 2003; 2:332–344.
- Olson K, Hanson J, Michaud M. A phase II trial of Reiki for the management of pain in advanced cancer patients. J Pain Symptom Manage 2003; 26:990–997.
- Gallagher LM, Lagman R, Walsh D, Davis MP, Legrand SB. The clinical effects of music therapy in palliative medicine. Support Care Cancer 2006; 14:859–866.
- Olaku O, White JD. Herbal therapy use by cancer patients: a literature review on case reports. Eur J Cancer 2011; 47:508–514.
KEY POINTS
- Opioids can be used effectively for the management of cancer pain, provided the physician has sufficient knowledge, education, and training.
- Adjuvants, if properly used, can help manage cancer pain more effectively.
- Complementary and alternative therapies look promising, but too little is known about them, so caution is advised when recommending them.
- Patients should be referred to a pain clinic if they have intractable pain or if they have severe side effects from opioid therapy.
- Overall improvement in patient satisfaction and quality of life can be noted when pain is effectively managed.