User login
The impact of patient education on consideration of enrollment in clinical trials
The low rate of participation in clinical trials is partly owing to the lack of awareness of these trials not only among potential participants but the US population as a whole.1 This lack of awareness, however, can be reversed. For example, findings from a single-institution observational study showed that systematically sending letters about clinical trial participation to all new lung cancer patients was associated with increased trial participation.2 More recently, a large, multicenter, randomized experiment showed that attitudes toward clinical trials were improved through preparatory education about clinical trials before a patient’s first oncologic visit.3
Such clinical trial education can be used before any medical diagnosis to increase clinical trial awareness in the general population. It may be advantageous to do so because people tend to process information more effectively during less stressful times.4 Clinical trial awareness in the US population has increased slightly over time, but in 2012, one study reported that 26% of its participants lacked general awareness about clinical trials.5
Comprehensive educational material, such as a multimedia psychoeducational intervention,6 a 28-video library,3 or a 160-page book,7 which have been proposed for oncology patients, may be too intensive for someone who is not immediately deciding whether to participate in a clinical trial. However, a simple, concise form of education might be preferable and appropriate to increase basic knowledge and awareness among the general population, especially among those who are less educated.8
Our aim in the present study was to evaluate whether providing brief educational material about clinical trials would increase patient willingness to participate in these trials.
Methods
This is a single-group, cross-sectional design study in which all participants were administered the questions and the 240-word educational statement in the same order.
Sample
An electronic survey was conducted by Marketing and Planning Systems (the analytics practice of Kantar Millwardbrown) on behalf of the Memorial Sloan Kettering Cancer Center (MSK). The survey included a national sample of 1011 participants and a local sample of 500 participants from the MSK catchment area (22 counties across the 5 boroughs of New York City, Long Island, southern New York State, northern New Jersey, and southwestern Connecticut).
Survey participants were aged 18 to 69 years in the national sample and 25 to 69 years in the local sample, representing 87% and 75% of the adult populations of those areas, respectively. Respondents who were or who had a family member currently working in the fields of news, advertising/marketing, or medical care were not surveyed. Participants were sourced from an online incentivized panel with millions of potential respondents representative of the US adult population.
Questionnaire
The questionnaire collected data on participant demographics and main medical history (including previous participation in a clinical trial), and asked questions about clinical trials, focusing on:
Analyses
Descriptive and bivariate statistical analyses of participants’ characteristics were weighted to ensure national representativeness for gender, age, ethnicity, and income. Mean standard deviation (SD) was computed for every quantitative variable. Categorical variables were expressed as proportions.
Student t tests and analyses of variance (ANOVAs) were used to compare continuous variables, while chi-square tests were used to compare categorical data. Repeated measures ANOVAs were then used to determine the sociodemographic and medical characteristics associated with the impression of and willingness to enroll in a clinical trial before and after reading the educational material. The interaction between education level and time (pre- or postreading assessment) was tested to determine if the changes after reading the brief statement were different depending on education level.
All statistical analyses were 2-tailed and considered statistically significant at P < .05. Analyses were performed using SPSS PAWS Statistics 24 (IBM Inc, Armonk, New York). Effect sizes (standardized mean differences) and their 95% confidence intervals (CIs) were computed using the compute.es package for R 3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
From October 23, 2015, through November 12, 2015, 1511 US participants responded to the survey request, including 1507 respondents (99.7%) who reported their education level and are included in the analyses of this report. The mean age of the respondents was 43.5 years (SD, 4.6). More than half of the respondents (57.8%) reported a current medical condition, mainly cardiovascular (20.0%), arthritis (20.0%), or other type or chronic pain (20.0%), and 9.0% reported a cancer diagnosis (current, 2.9%; previous, 6.1%).
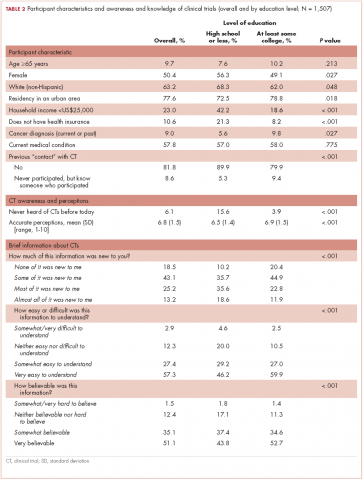
Participants who at most had completed high school (18.9%, including 1.4% who had never even attended high school) were more often white women, lived outside urban areas, had lower household income, and were less likely to have health care insurance (Table 2). They also reported a current or previous cancer diagnosis less often than those of similar age who had attended college.
Previous participation in a clinical trial was reported by 9.6% of participants. Most of the clinical trials (75.0%) were testing a new drug. Previous trial participants were more likely to be older than those who had not participated in trials (46.1 years [SD, 14.8] vs 43.3 [SD, 4.6], repectively; P = .033), have a current health condition (86.2% vs 54.8%; P < .001), and know another trial participant (39.9% vs 9.5%; P < .001).
Education level and baseline impression of and
willingness to enroll in a clinical trial
A lower level of education was associated with a decreased likelihood of previous trial participation or of knowing a trial participant, as well as with less awareness and inaccurate perceptions of clinical trials (Table 2).
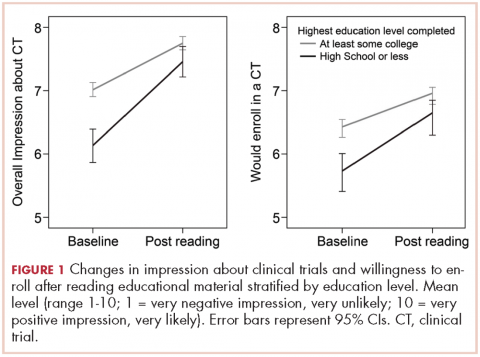
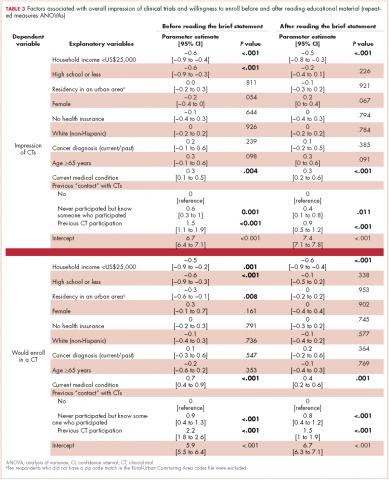
Participants with a high school degree or less were more likely to have a worse impression of and were less likely to enroll in a future hypothetical clinical trial before reading the educational material (Figure 1). Multivariable analyses confirmed that lower education level was associated with lower baseline overall impression, regardless of other personal characteristics (Table 3). Lowest household income was also associated with a more negative impression of trials, whereas participants with a current medical condition and with previous contact with clinical trials had a more positive impression of them. The same effects were observed with likeliness to enroll in a future hypothetical clinical trial (correlated with the overall impression: r, 0.63; P < .001), except that the negative effect of female gender was statistically significant (Table 3).
Posteducation impression of and willingnes to enroll
willingness to enroll in a clinical trial
The brief educational material was mostly considered believable (86.2%), easy to understand (84.8%), and included information that was new to participants (81.5%; Table 2). Participants with a high school diploma or less more often noted that the material provided them with new information, but they also reported more difficulties in fully understanding and believing the information. Overall, however, few participants found the information difficult to understand (4.6%) or hard to believe (1.8%; Table 2).
Most participants had an improved overall impression of clinical trials (standardized mean difference, 0.42; 95% CI, 0.35-0.50; P < .001) after reading the educational material. This increase was higher among participants with a lower completed level of education (Figure 1; standardized mean difference, 0.62; 95% CI, 0.45-0.79; Pinteraction < .001). The same effects were observed for likeliness to enroll in a future hypothetical clinical trial (P < .001; Figure 1).
After reading the informational statement, education level effect was no longer significantly associated with the overall impression of clinical trials (P = .23) and willingness to enroll in a clinical trial (P = .34), whereas the effects of income, current medical condition, and previous engangement with clinical trials remained statistically significant (Table 3).
Remaining challenges
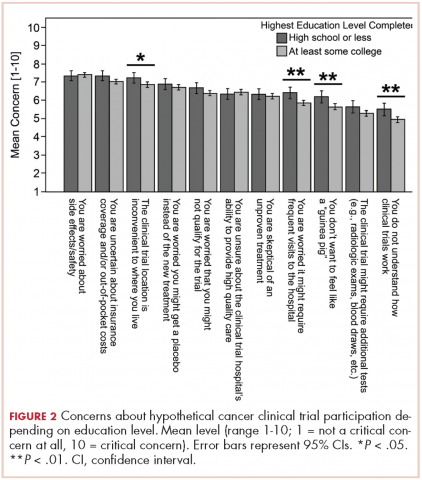
Regarding hypothetical participation in a future clinical trial after a cancer diagnosis, the most critical concerns were related to side effects and the uncertainty of insurance coverage (Figure 2).10 The lack of understanding of clinical trials was the least critical concern; however, it was significantly higher among participants with a lower completed level of education. These participants also expressed more critical concerns about feeling like a “guinea pig,” inconvenient trial location, and the frequent visits needed.
Discussion
Findings from this survey demonstrated that providing brief educational material about cancer clinical trials was associated with a more favorable impression of clinical trials and higher interest in trial participation. Furthermore, as far as we can ascertain, this is the first report showing how a simple intervention such as this may help close the knowledge gap on clinical trials among people of different educational backgrounds. Although most respondents in this interventional survey noted an increased willingness to consider participation in a clinical trial after reading the educational material, those with a lower level of education and knowledge about clinical trials received the most benefit. Previous participation in a clinical trial was also strongly associated with the impression of and willingness to enroll in a trial, both before and after reading the statement.11
Most of the interventions evaluated to date12 have focused on patients who are faced with having to decide whether to participate in a clinical trial2,3 or on very specific populations, such as select ethnic communities.13,14 However, it may be beneficial to provide simple, concise educational information about clinical trials to the general population, especially to those with minimal education. Although level of education concerns the minority of our sample (19%) sourced from an online panel, in the 2015 Current Population Survey, 41% of US participants aged 25 and older had not reached college education.15 Those with a lower level of education have reported a general lack of familiarity with clinical trials and were more likely to have inaccurate perceptions about trials. This is consistent with previous studies that have shown the lack of awareness and knowledge of clinical trials in this population.5,16,17 Our findings suggest that this knowledge gap can be reversed through a simple educational intervention and result in an increased willingness to participate.
The provision of information on clinical trials was positively associated with the 2 outcomes analyzed – improved impression of clinical trials and increased likelihood to enroll in a hypothetical trial. Such improvement might not translate to improved accrual,18 but it is a step toward closing the overall knowledge gap related to clinical trials and increasing the number of people who would consider trial participation. The lack of awareness of clinical trials has been reported as a legitimate explanation for why participation rates are lower in less-educated patient populations.19,20 This brief educational intervention is a simple, technology-sparing way to increase clinical trial awareness in the general population. In a similar survey, most physicians who reviewed an educational statement noted they were likely to use it with patients.21Less-educated patients, those who lived outside of urban areas, and those with lower household incomes were most concerned about trial location and the frequent visits needed when participating in a trial (Figure 2). Living in a nonurban area was not associated with participant impression of clinical trials or willingness to enroll in a trial. However, rural residency may be a barrier to enrollment depending on distance to the hospital22 and out-of-pocket expenses related to travel.23 Some comprehensive cancer centers, such as MSK, have developed alliances with community centers24 as a means of overcoming geographical barriers and increasing clinical trial participation rates.
Another concern shared by most respondents was the uncertainty in insurance coverage and potential out-of-pocket costs related to care. Lower household income, unlike location of residence and lack of insurance, was significantly associated with negative impressions of clinical trials and lower willingness to enroll in a trial, even after adjusting for education level. Cancer patients with higher financial burden have reported more attitudinal barriers, even after accounting for the negative effect of lower education level.25 Recent studies have also discussed the negative impact of lower income on cancer clinical trial participation,19,20,26,27 and new attention has been paid to the negative financial implications or “financial toxicity” of participating in a trial.23,28
White and older survey participants showed similar interest in clinical trial participation after accounting for other characteristics. There is growing evidence that outcome differences attributed to race may in fact be more dependent on socioeconomic status.8 A recent study among breast cancer patients showed that low socioeconomic status, but not race, was associated with decreased participation in clinical trials.29,30 Previous findings have also indicated that interest in clinical trials and barriers to enrollment among older, less-educated patients31 are often related to ineligibility, comorbidity, or communication difficulties.
Among our participants, the fear of side effects also was a common attitudinal barrier to clinical trial participation, as has been reported in previous studies.3,20 However, contrary to one previous study,20 this fear was not significantly increased among our less-educated participants.
Less-educated participants also reported more difficulties in understanding the information they were provided with, and they remained more concerned about being treated like “guinea pigs.” These concerns are consistent with other results showing that decisional conflict about clinical trial participation among patients with a high school diploma or less remained high even after they had received a National Cancer Institute text as pre-education material.3
Limitations
The lack of randomization makes it difficult to attribute with certainty that the change in acceptability of clinical trial participation is owing to the reading of the educational statement. The survey also sampled only English-speaking and well-educated participants from an online panel (81.1% had at least attended college) despite the use of a weighting procedure to ensure representativeness regarding gender, age, ethnicity, and income. Health literacy level or more specific trial literacy level was not evaluated; however, we were able to show less accurate perceptions of clinical trials among participants with a lower level of education by using agreement toward 8 statements about trials. The responses to hypothetical questions from these participants in the general population may also not be generalizable to a restricted population of patients with cancer. In addition, we measured impression of and willingness to enroll in a clinical trial immediately after providing participants with the educational material. We would have to confirm whether the positive effects of the education persist over time and translate to higher clinical trial participation rates.
Conclusions
Participants were receptive of educational material and expressed greater interest in and likelihood of enrolling in a clinical trial after reading it. The information had a greater effect on those with less education, but increased the willingness of all participants to enroll.
1. Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228-242.
2. Quinn GP, Bell BA, Bell MY, et al. The guinea pig syndrome: improving clinical trial participation among thoracic patients. J Thorac Oncol. 2007;2(3):191-196.
3. Meropol NJ, Wong Y-N, Albrecht T, et al. Randomized trial of a web-based intervention to address barriers to clinical trials. J Clin Oncol. 2016;34(5):469-478.
4. Goldberg RJ. Disclosure of information to adult cancer patients: issues and update. J Clin Oncol. 1984;2(8):948-955.
5. Leiter A, Diefenbach MA, Doucette J, Oh WK, Galsky MD. Clinical trial awareness: changes over time and sociodemographic disparities. Clin Trials. 2015;12(3):215-223.
6. Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30(20):2516-2521.
7. Carney PA, Tucker EK, Newby TA, Beer TM. Feasibility, acceptability and findings from a pilot randomized controlled intervention study on the impact of a book designed to inform patients about cancer clinical trials. J Cancer Educ. 2014;29(1):181-187.
8. Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014;111(9):1684-1687.
9. Regan J, Hickey C, Targett C, Masuda S, Sabbatini P. Framing clinical research and the importance of trial participation: patient and physician perspective. Poster presented at: 8th Annual AACI Clinical Research Initiative Meeting; July 20-21 2016; Chicago, IL.
10. Mancini J, Genre D, Dalenc F, et al. Patients’ regrets after participating in a randomized controlled trials depended on their involvement in the decision making. J Clin Epidemiol. 2012;65(6):635-642
11. Murphy ST, Frank LB, Chatterjee JS, et al. Comparing the relative efficacy of narrative vs nonnarrative health messages in reducing health disparities using a randomized trial. Am J Public Health. 2015;105(10):2117-2123.
12. Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2):e002360. doi:10.1136/bmjopen-2012-002360.
13. Ma GX, Tan Y, Blakeney NC, et al. The impact of a community-based clinical trial educational intervention among underrepresented Chinese Americans. Cancer Epidemiol Biomarkers Prev. 2014;23(3):424-432.
14. Cupertino AP, Molina CSP, de los Rios JB, et al. Knowledge, awareness, and interest in cancer clinical trials among rural latinos following brief education by promotores de salud. J Community Med Health Educ. 2015;5:2161. doi:10.4172/2161-0711.1000358
15. Ryan CL, Bauman K. Educational attainment in the United States: 2015. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf. Published March 2016. Accessed October 18, 2016.
16. Lara PN Jr, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23(36):9282-9289.
17. Dhai A, Etheredge H, Cleaton-Jones P. A pilot study evaluating an intervention designed to raise awareness of clinical trials among potential participants in the developing world. J Med Ethics. 2010;36(4):238-242.
18. Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute–American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9(6):267-276.
19. Davison BJ, So A, Goldenberg SL, Berkowitz J, Gleave ME. Measurement of factors influencing the participation of patients with prostate cancer in clinical trials: a Canadian perspective. BJU Int. 2008;101(8):982-987.
20. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536-542.
21. IRB Advisor. Education may overcome reticence to join trials. Relias website. http://www.ahcmedia.com/articles/138088-education-may-overcome-reticence-to-join-trials. Published July 1, 2016. Accessed July 29, 2016.
22. Vanderpool RC, Kornfeld J, Mills L, Byrne MM. Rural-urban differences in discussions of cancer treatment clinical trials. Patient Educ Couns. 2011;85(2):e69-e74.
23. Meropol NJ. Health policy: overcoming cost barriers to clinical trial participation. Nat Rev Clin Oncol. 2016;13(6):333-334.
24. MSK Cancer Alliance. Memorial Sloan Kettering Cancer Center website. https://www.mskcc.org/about/innovative-collaborations/msk-alliance. Updated March 7, 2018. Accessed August 18, 2016.
25. Manne S, Kashy D, Albrecht T, et al. Attitudinal barriers to participation in oncology clinical trials: factor analysis and correlates of barriers. Eur J Cancer Care (Engl). 2015;24(1):28-38.
26. Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient income level and cancer clinical trial participation: a prospective survey study. JAMA Oncol. 2016;2(1):137-139.
27. Moy B. Clinical trials, disparities, and financial burden: it’s time to intervene. Oncologist. 2015;20(6):571.
28. Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42.
29. Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103(3):483-491.
30. Nickell A, Burke NJ, Cohen E, Caprio M, Joseph G. Educating low-SES and LEP survivors about breast cancer research: pilot test of the Health Research Engagement Intervention. J Cancer Educ. 2014;29(4):746-752.
31. Mancini J, Jansen J, Julian-Reynier C, Bechlian D, Vey N, Chabannon C. Preferences of older adults with cancer for involvement in decision-making about research participation. J Am Geriatr Soc. 2014;62(6):1191-1193.
The low rate of participation in clinical trials is partly owing to the lack of awareness of these trials not only among potential participants but the US population as a whole.1 This lack of awareness, however, can be reversed. For example, findings from a single-institution observational study showed that systematically sending letters about clinical trial participation to all new lung cancer patients was associated with increased trial participation.2 More recently, a large, multicenter, randomized experiment showed that attitudes toward clinical trials were improved through preparatory education about clinical trials before a patient’s first oncologic visit.3
Such clinical trial education can be used before any medical diagnosis to increase clinical trial awareness in the general population. It may be advantageous to do so because people tend to process information more effectively during less stressful times.4 Clinical trial awareness in the US population has increased slightly over time, but in 2012, one study reported that 26% of its participants lacked general awareness about clinical trials.5
Comprehensive educational material, such as a multimedia psychoeducational intervention,6 a 28-video library,3 or a 160-page book,7 which have been proposed for oncology patients, may be too intensive for someone who is not immediately deciding whether to participate in a clinical trial. However, a simple, concise form of education might be preferable and appropriate to increase basic knowledge and awareness among the general population, especially among those who are less educated.8
Our aim in the present study was to evaluate whether providing brief educational material about clinical trials would increase patient willingness to participate in these trials.
Methods
This is a single-group, cross-sectional design study in which all participants were administered the questions and the 240-word educational statement in the same order.
Sample
An electronic survey was conducted by Marketing and Planning Systems (the analytics practice of Kantar Millwardbrown) on behalf of the Memorial Sloan Kettering Cancer Center (MSK). The survey included a national sample of 1011 participants and a local sample of 500 participants from the MSK catchment area (22 counties across the 5 boroughs of New York City, Long Island, southern New York State, northern New Jersey, and southwestern Connecticut).
Survey participants were aged 18 to 69 years in the national sample and 25 to 69 years in the local sample, representing 87% and 75% of the adult populations of those areas, respectively. Respondents who were or who had a family member currently working in the fields of news, advertising/marketing, or medical care were not surveyed. Participants were sourced from an online incentivized panel with millions of potential respondents representative of the US adult population.
Questionnaire
The questionnaire collected data on participant demographics and main medical history (including previous participation in a clinical trial), and asked questions about clinical trials, focusing on:
Analyses
Descriptive and bivariate statistical analyses of participants’ characteristics were weighted to ensure national representativeness for gender, age, ethnicity, and income. Mean standard deviation (SD) was computed for every quantitative variable. Categorical variables were expressed as proportions.
Student t tests and analyses of variance (ANOVAs) were used to compare continuous variables, while chi-square tests were used to compare categorical data. Repeated measures ANOVAs were then used to determine the sociodemographic and medical characteristics associated with the impression of and willingness to enroll in a clinical trial before and after reading the educational material. The interaction between education level and time (pre- or postreading assessment) was tested to determine if the changes after reading the brief statement were different depending on education level.
All statistical analyses were 2-tailed and considered statistically significant at P < .05. Analyses were performed using SPSS PAWS Statistics 24 (IBM Inc, Armonk, New York). Effect sizes (standardized mean differences) and their 95% confidence intervals (CIs) were computed using the compute.es package for R 3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
From October 23, 2015, through November 12, 2015, 1511 US participants responded to the survey request, including 1507 respondents (99.7%) who reported their education level and are included in the analyses of this report. The mean age of the respondents was 43.5 years (SD, 4.6). More than half of the respondents (57.8%) reported a current medical condition, mainly cardiovascular (20.0%), arthritis (20.0%), or other type or chronic pain (20.0%), and 9.0% reported a cancer diagnosis (current, 2.9%; previous, 6.1%).
Participants who at most had completed high school (18.9%, including 1.4% who had never even attended high school) were more often white women, lived outside urban areas, had lower household income, and were less likely to have health care insurance (Table 2). They also reported a current or previous cancer diagnosis less often than those of similar age who had attended college.
Previous participation in a clinical trial was reported by 9.6% of participants. Most of the clinical trials (75.0%) were testing a new drug. Previous trial participants were more likely to be older than those who had not participated in trials (46.1 years [SD, 14.8] vs 43.3 [SD, 4.6], repectively; P = .033), have a current health condition (86.2% vs 54.8%; P < .001), and know another trial participant (39.9% vs 9.5%; P < .001).
Education level and baseline impression of and
willingness to enroll in a clinical trial
A lower level of education was associated with a decreased likelihood of previous trial participation or of knowing a trial participant, as well as with less awareness and inaccurate perceptions of clinical trials (Table 2).
Participants with a high school degree or less were more likely to have a worse impression of and were less likely to enroll in a future hypothetical clinical trial before reading the educational material (Figure 1). Multivariable analyses confirmed that lower education level was associated with lower baseline overall impression, regardless of other personal characteristics (Table 3). Lowest household income was also associated with a more negative impression of trials, whereas participants with a current medical condition and with previous contact with clinical trials had a more positive impression of them. The same effects were observed with likeliness to enroll in a future hypothetical clinical trial (correlated with the overall impression: r, 0.63; P < .001), except that the negative effect of female gender was statistically significant (Table 3).
Posteducation impression of and willingnes to enroll
willingness to enroll in a clinical trial
The brief educational material was mostly considered believable (86.2%), easy to understand (84.8%), and included information that was new to participants (81.5%; Table 2). Participants with a high school diploma or less more often noted that the material provided them with new information, but they also reported more difficulties in fully understanding and believing the information. Overall, however, few participants found the information difficult to understand (4.6%) or hard to believe (1.8%; Table 2).
Most participants had an improved overall impression of clinical trials (standardized mean difference, 0.42; 95% CI, 0.35-0.50; P < .001) after reading the educational material. This increase was higher among participants with a lower completed level of education (Figure 1; standardized mean difference, 0.62; 95% CI, 0.45-0.79; Pinteraction < .001). The same effects were observed for likeliness to enroll in a future hypothetical clinical trial (P < .001; Figure 1).
After reading the informational statement, education level effect was no longer significantly associated with the overall impression of clinical trials (P = .23) and willingness to enroll in a clinical trial (P = .34), whereas the effects of income, current medical condition, and previous engangement with clinical trials remained statistically significant (Table 3).
Remaining challenges
Regarding hypothetical participation in a future clinical trial after a cancer diagnosis, the most critical concerns were related to side effects and the uncertainty of insurance coverage (Figure 2).10 The lack of understanding of clinical trials was the least critical concern; however, it was significantly higher among participants with a lower completed level of education. These participants also expressed more critical concerns about feeling like a “guinea pig,” inconvenient trial location, and the frequent visits needed.
Discussion
Findings from this survey demonstrated that providing brief educational material about cancer clinical trials was associated with a more favorable impression of clinical trials and higher interest in trial participation. Furthermore, as far as we can ascertain, this is the first report showing how a simple intervention such as this may help close the knowledge gap on clinical trials among people of different educational backgrounds. Although most respondents in this interventional survey noted an increased willingness to consider participation in a clinical trial after reading the educational material, those with a lower level of education and knowledge about clinical trials received the most benefit. Previous participation in a clinical trial was also strongly associated with the impression of and willingness to enroll in a trial, both before and after reading the statement.11
Most of the interventions evaluated to date12 have focused on patients who are faced with having to decide whether to participate in a clinical trial2,3 or on very specific populations, such as select ethnic communities.13,14 However, it may be beneficial to provide simple, concise educational information about clinical trials to the general population, especially to those with minimal education. Although level of education concerns the minority of our sample (19%) sourced from an online panel, in the 2015 Current Population Survey, 41% of US participants aged 25 and older had not reached college education.15 Those with a lower level of education have reported a general lack of familiarity with clinical trials and were more likely to have inaccurate perceptions about trials. This is consistent with previous studies that have shown the lack of awareness and knowledge of clinical trials in this population.5,16,17 Our findings suggest that this knowledge gap can be reversed through a simple educational intervention and result in an increased willingness to participate.
The provision of information on clinical trials was positively associated with the 2 outcomes analyzed – improved impression of clinical trials and increased likelihood to enroll in a hypothetical trial. Such improvement might not translate to improved accrual,18 but it is a step toward closing the overall knowledge gap related to clinical trials and increasing the number of people who would consider trial participation. The lack of awareness of clinical trials has been reported as a legitimate explanation for why participation rates are lower in less-educated patient populations.19,20 This brief educational intervention is a simple, technology-sparing way to increase clinical trial awareness in the general population. In a similar survey, most physicians who reviewed an educational statement noted they were likely to use it with patients.21Less-educated patients, those who lived outside of urban areas, and those with lower household incomes were most concerned about trial location and the frequent visits needed when participating in a trial (Figure 2). Living in a nonurban area was not associated with participant impression of clinical trials or willingness to enroll in a trial. However, rural residency may be a barrier to enrollment depending on distance to the hospital22 and out-of-pocket expenses related to travel.23 Some comprehensive cancer centers, such as MSK, have developed alliances with community centers24 as a means of overcoming geographical barriers and increasing clinical trial participation rates.
Another concern shared by most respondents was the uncertainty in insurance coverage and potential out-of-pocket costs related to care. Lower household income, unlike location of residence and lack of insurance, was significantly associated with negative impressions of clinical trials and lower willingness to enroll in a trial, even after adjusting for education level. Cancer patients with higher financial burden have reported more attitudinal barriers, even after accounting for the negative effect of lower education level.25 Recent studies have also discussed the negative impact of lower income on cancer clinical trial participation,19,20,26,27 and new attention has been paid to the negative financial implications or “financial toxicity” of participating in a trial.23,28
White and older survey participants showed similar interest in clinical trial participation after accounting for other characteristics. There is growing evidence that outcome differences attributed to race may in fact be more dependent on socioeconomic status.8 A recent study among breast cancer patients showed that low socioeconomic status, but not race, was associated with decreased participation in clinical trials.29,30 Previous findings have also indicated that interest in clinical trials and barriers to enrollment among older, less-educated patients31 are often related to ineligibility, comorbidity, or communication difficulties.
Among our participants, the fear of side effects also was a common attitudinal barrier to clinical trial participation, as has been reported in previous studies.3,20 However, contrary to one previous study,20 this fear was not significantly increased among our less-educated participants.
Less-educated participants also reported more difficulties in understanding the information they were provided with, and they remained more concerned about being treated like “guinea pigs.” These concerns are consistent with other results showing that decisional conflict about clinical trial participation among patients with a high school diploma or less remained high even after they had received a National Cancer Institute text as pre-education material.3
Limitations
The lack of randomization makes it difficult to attribute with certainty that the change in acceptability of clinical trial participation is owing to the reading of the educational statement. The survey also sampled only English-speaking and well-educated participants from an online panel (81.1% had at least attended college) despite the use of a weighting procedure to ensure representativeness regarding gender, age, ethnicity, and income. Health literacy level or more specific trial literacy level was not evaluated; however, we were able to show less accurate perceptions of clinical trials among participants with a lower level of education by using agreement toward 8 statements about trials. The responses to hypothetical questions from these participants in the general population may also not be generalizable to a restricted population of patients with cancer. In addition, we measured impression of and willingness to enroll in a clinical trial immediately after providing participants with the educational material. We would have to confirm whether the positive effects of the education persist over time and translate to higher clinical trial participation rates.
Conclusions
Participants were receptive of educational material and expressed greater interest in and likelihood of enrolling in a clinical trial after reading it. The information had a greater effect on those with less education, but increased the willingness of all participants to enroll.
The low rate of participation in clinical trials is partly owing to the lack of awareness of these trials not only among potential participants but the US population as a whole.1 This lack of awareness, however, can be reversed. For example, findings from a single-institution observational study showed that systematically sending letters about clinical trial participation to all new lung cancer patients was associated with increased trial participation.2 More recently, a large, multicenter, randomized experiment showed that attitudes toward clinical trials were improved through preparatory education about clinical trials before a patient’s first oncologic visit.3
Such clinical trial education can be used before any medical diagnosis to increase clinical trial awareness in the general population. It may be advantageous to do so because people tend to process information more effectively during less stressful times.4 Clinical trial awareness in the US population has increased slightly over time, but in 2012, one study reported that 26% of its participants lacked general awareness about clinical trials.5
Comprehensive educational material, such as a multimedia psychoeducational intervention,6 a 28-video library,3 or a 160-page book,7 which have been proposed for oncology patients, may be too intensive for someone who is not immediately deciding whether to participate in a clinical trial. However, a simple, concise form of education might be preferable and appropriate to increase basic knowledge and awareness among the general population, especially among those who are less educated.8
Our aim in the present study was to evaluate whether providing brief educational material about clinical trials would increase patient willingness to participate in these trials.
Methods
This is a single-group, cross-sectional design study in which all participants were administered the questions and the 240-word educational statement in the same order.
Sample
An electronic survey was conducted by Marketing and Planning Systems (the analytics practice of Kantar Millwardbrown) on behalf of the Memorial Sloan Kettering Cancer Center (MSK). The survey included a national sample of 1011 participants and a local sample of 500 participants from the MSK catchment area (22 counties across the 5 boroughs of New York City, Long Island, southern New York State, northern New Jersey, and southwestern Connecticut).
Survey participants were aged 18 to 69 years in the national sample and 25 to 69 years in the local sample, representing 87% and 75% of the adult populations of those areas, respectively. Respondents who were or who had a family member currently working in the fields of news, advertising/marketing, or medical care were not surveyed. Participants were sourced from an online incentivized panel with millions of potential respondents representative of the US adult population.
Questionnaire
The questionnaire collected data on participant demographics and main medical history (including previous participation in a clinical trial), and asked questions about clinical trials, focusing on:
Analyses
Descriptive and bivariate statistical analyses of participants’ characteristics were weighted to ensure national representativeness for gender, age, ethnicity, and income. Mean standard deviation (SD) was computed for every quantitative variable. Categorical variables were expressed as proportions.
Student t tests and analyses of variance (ANOVAs) were used to compare continuous variables, while chi-square tests were used to compare categorical data. Repeated measures ANOVAs were then used to determine the sociodemographic and medical characteristics associated with the impression of and willingness to enroll in a clinical trial before and after reading the educational material. The interaction between education level and time (pre- or postreading assessment) was tested to determine if the changes after reading the brief statement were different depending on education level.
All statistical analyses were 2-tailed and considered statistically significant at P < .05. Analyses were performed using SPSS PAWS Statistics 24 (IBM Inc, Armonk, New York). Effect sizes (standardized mean differences) and their 95% confidence intervals (CIs) were computed using the compute.es package for R 3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
From October 23, 2015, through November 12, 2015, 1511 US participants responded to the survey request, including 1507 respondents (99.7%) who reported their education level and are included in the analyses of this report. The mean age of the respondents was 43.5 years (SD, 4.6). More than half of the respondents (57.8%) reported a current medical condition, mainly cardiovascular (20.0%), arthritis (20.0%), or other type or chronic pain (20.0%), and 9.0% reported a cancer diagnosis (current, 2.9%; previous, 6.1%).
Participants who at most had completed high school (18.9%, including 1.4% who had never even attended high school) were more often white women, lived outside urban areas, had lower household income, and were less likely to have health care insurance (Table 2). They also reported a current or previous cancer diagnosis less often than those of similar age who had attended college.
Previous participation in a clinical trial was reported by 9.6% of participants. Most of the clinical trials (75.0%) were testing a new drug. Previous trial participants were more likely to be older than those who had not participated in trials (46.1 years [SD, 14.8] vs 43.3 [SD, 4.6], repectively; P = .033), have a current health condition (86.2% vs 54.8%; P < .001), and know another trial participant (39.9% vs 9.5%; P < .001).
Education level and baseline impression of and
willingness to enroll in a clinical trial
A lower level of education was associated with a decreased likelihood of previous trial participation or of knowing a trial participant, as well as with less awareness and inaccurate perceptions of clinical trials (Table 2).
Participants with a high school degree or less were more likely to have a worse impression of and were less likely to enroll in a future hypothetical clinical trial before reading the educational material (Figure 1). Multivariable analyses confirmed that lower education level was associated with lower baseline overall impression, regardless of other personal characteristics (Table 3). Lowest household income was also associated with a more negative impression of trials, whereas participants with a current medical condition and with previous contact with clinical trials had a more positive impression of them. The same effects were observed with likeliness to enroll in a future hypothetical clinical trial (correlated with the overall impression: r, 0.63; P < .001), except that the negative effect of female gender was statistically significant (Table 3).
Posteducation impression of and willingnes to enroll
willingness to enroll in a clinical trial
The brief educational material was mostly considered believable (86.2%), easy to understand (84.8%), and included information that was new to participants (81.5%; Table 2). Participants with a high school diploma or less more often noted that the material provided them with new information, but they also reported more difficulties in fully understanding and believing the information. Overall, however, few participants found the information difficult to understand (4.6%) or hard to believe (1.8%; Table 2).
Most participants had an improved overall impression of clinical trials (standardized mean difference, 0.42; 95% CI, 0.35-0.50; P < .001) after reading the educational material. This increase was higher among participants with a lower completed level of education (Figure 1; standardized mean difference, 0.62; 95% CI, 0.45-0.79; Pinteraction < .001). The same effects were observed for likeliness to enroll in a future hypothetical clinical trial (P < .001; Figure 1).
After reading the informational statement, education level effect was no longer significantly associated with the overall impression of clinical trials (P = .23) and willingness to enroll in a clinical trial (P = .34), whereas the effects of income, current medical condition, and previous engangement with clinical trials remained statistically significant (Table 3).
Remaining challenges
Regarding hypothetical participation in a future clinical trial after a cancer diagnosis, the most critical concerns were related to side effects and the uncertainty of insurance coverage (Figure 2).10 The lack of understanding of clinical trials was the least critical concern; however, it was significantly higher among participants with a lower completed level of education. These participants also expressed more critical concerns about feeling like a “guinea pig,” inconvenient trial location, and the frequent visits needed.
Discussion
Findings from this survey demonstrated that providing brief educational material about cancer clinical trials was associated with a more favorable impression of clinical trials and higher interest in trial participation. Furthermore, as far as we can ascertain, this is the first report showing how a simple intervention such as this may help close the knowledge gap on clinical trials among people of different educational backgrounds. Although most respondents in this interventional survey noted an increased willingness to consider participation in a clinical trial after reading the educational material, those with a lower level of education and knowledge about clinical trials received the most benefit. Previous participation in a clinical trial was also strongly associated with the impression of and willingness to enroll in a trial, both before and after reading the statement.11
Most of the interventions evaluated to date12 have focused on patients who are faced with having to decide whether to participate in a clinical trial2,3 or on very specific populations, such as select ethnic communities.13,14 However, it may be beneficial to provide simple, concise educational information about clinical trials to the general population, especially to those with minimal education. Although level of education concerns the minority of our sample (19%) sourced from an online panel, in the 2015 Current Population Survey, 41% of US participants aged 25 and older had not reached college education.15 Those with a lower level of education have reported a general lack of familiarity with clinical trials and were more likely to have inaccurate perceptions about trials. This is consistent with previous studies that have shown the lack of awareness and knowledge of clinical trials in this population.5,16,17 Our findings suggest that this knowledge gap can be reversed through a simple educational intervention and result in an increased willingness to participate.
The provision of information on clinical trials was positively associated with the 2 outcomes analyzed – improved impression of clinical trials and increased likelihood to enroll in a hypothetical trial. Such improvement might not translate to improved accrual,18 but it is a step toward closing the overall knowledge gap related to clinical trials and increasing the number of people who would consider trial participation. The lack of awareness of clinical trials has been reported as a legitimate explanation for why participation rates are lower in less-educated patient populations.19,20 This brief educational intervention is a simple, technology-sparing way to increase clinical trial awareness in the general population. In a similar survey, most physicians who reviewed an educational statement noted they were likely to use it with patients.21Less-educated patients, those who lived outside of urban areas, and those with lower household incomes were most concerned about trial location and the frequent visits needed when participating in a trial (Figure 2). Living in a nonurban area was not associated with participant impression of clinical trials or willingness to enroll in a trial. However, rural residency may be a barrier to enrollment depending on distance to the hospital22 and out-of-pocket expenses related to travel.23 Some comprehensive cancer centers, such as MSK, have developed alliances with community centers24 as a means of overcoming geographical barriers and increasing clinical trial participation rates.
Another concern shared by most respondents was the uncertainty in insurance coverage and potential out-of-pocket costs related to care. Lower household income, unlike location of residence and lack of insurance, was significantly associated with negative impressions of clinical trials and lower willingness to enroll in a trial, even after adjusting for education level. Cancer patients with higher financial burden have reported more attitudinal barriers, even after accounting for the negative effect of lower education level.25 Recent studies have also discussed the negative impact of lower income on cancer clinical trial participation,19,20,26,27 and new attention has been paid to the negative financial implications or “financial toxicity” of participating in a trial.23,28
White and older survey participants showed similar interest in clinical trial participation after accounting for other characteristics. There is growing evidence that outcome differences attributed to race may in fact be more dependent on socioeconomic status.8 A recent study among breast cancer patients showed that low socioeconomic status, but not race, was associated with decreased participation in clinical trials.29,30 Previous findings have also indicated that interest in clinical trials and barriers to enrollment among older, less-educated patients31 are often related to ineligibility, comorbidity, or communication difficulties.
Among our participants, the fear of side effects also was a common attitudinal barrier to clinical trial participation, as has been reported in previous studies.3,20 However, contrary to one previous study,20 this fear was not significantly increased among our less-educated participants.
Less-educated participants also reported more difficulties in understanding the information they were provided with, and they remained more concerned about being treated like “guinea pigs.” These concerns are consistent with other results showing that decisional conflict about clinical trial participation among patients with a high school diploma or less remained high even after they had received a National Cancer Institute text as pre-education material.3
Limitations
The lack of randomization makes it difficult to attribute with certainty that the change in acceptability of clinical trial participation is owing to the reading of the educational statement. The survey also sampled only English-speaking and well-educated participants from an online panel (81.1% had at least attended college) despite the use of a weighting procedure to ensure representativeness regarding gender, age, ethnicity, and income. Health literacy level or more specific trial literacy level was not evaluated; however, we were able to show less accurate perceptions of clinical trials among participants with a lower level of education by using agreement toward 8 statements about trials. The responses to hypothetical questions from these participants in the general population may also not be generalizable to a restricted population of patients with cancer. In addition, we measured impression of and willingness to enroll in a clinical trial immediately after providing participants with the educational material. We would have to confirm whether the positive effects of the education persist over time and translate to higher clinical trial participation rates.
Conclusions
Participants were receptive of educational material and expressed greater interest in and likelihood of enrolling in a clinical trial after reading it. The information had a greater effect on those with less education, but increased the willingness of all participants to enroll.
1. Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228-242.
2. Quinn GP, Bell BA, Bell MY, et al. The guinea pig syndrome: improving clinical trial participation among thoracic patients. J Thorac Oncol. 2007;2(3):191-196.
3. Meropol NJ, Wong Y-N, Albrecht T, et al. Randomized trial of a web-based intervention to address barriers to clinical trials. J Clin Oncol. 2016;34(5):469-478.
4. Goldberg RJ. Disclosure of information to adult cancer patients: issues and update. J Clin Oncol. 1984;2(8):948-955.
5. Leiter A, Diefenbach MA, Doucette J, Oh WK, Galsky MD. Clinical trial awareness: changes over time and sociodemographic disparities. Clin Trials. 2015;12(3):215-223.
6. Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30(20):2516-2521.
7. Carney PA, Tucker EK, Newby TA, Beer TM. Feasibility, acceptability and findings from a pilot randomized controlled intervention study on the impact of a book designed to inform patients about cancer clinical trials. J Cancer Educ. 2014;29(1):181-187.
8. Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014;111(9):1684-1687.
9. Regan J, Hickey C, Targett C, Masuda S, Sabbatini P. Framing clinical research and the importance of trial participation: patient and physician perspective. Poster presented at: 8th Annual AACI Clinical Research Initiative Meeting; July 20-21 2016; Chicago, IL.
10. Mancini J, Genre D, Dalenc F, et al. Patients’ regrets after participating in a randomized controlled trials depended on their involvement in the decision making. J Clin Epidemiol. 2012;65(6):635-642
11. Murphy ST, Frank LB, Chatterjee JS, et al. Comparing the relative efficacy of narrative vs nonnarrative health messages in reducing health disparities using a randomized trial. Am J Public Health. 2015;105(10):2117-2123.
12. Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2):e002360. doi:10.1136/bmjopen-2012-002360.
13. Ma GX, Tan Y, Blakeney NC, et al. The impact of a community-based clinical trial educational intervention among underrepresented Chinese Americans. Cancer Epidemiol Biomarkers Prev. 2014;23(3):424-432.
14. Cupertino AP, Molina CSP, de los Rios JB, et al. Knowledge, awareness, and interest in cancer clinical trials among rural latinos following brief education by promotores de salud. J Community Med Health Educ. 2015;5:2161. doi:10.4172/2161-0711.1000358
15. Ryan CL, Bauman K. Educational attainment in the United States: 2015. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf. Published March 2016. Accessed October 18, 2016.
16. Lara PN Jr, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23(36):9282-9289.
17. Dhai A, Etheredge H, Cleaton-Jones P. A pilot study evaluating an intervention designed to raise awareness of clinical trials among potential participants in the developing world. J Med Ethics. 2010;36(4):238-242.
18. Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute–American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9(6):267-276.
19. Davison BJ, So A, Goldenberg SL, Berkowitz J, Gleave ME. Measurement of factors influencing the participation of patients with prostate cancer in clinical trials: a Canadian perspective. BJU Int. 2008;101(8):982-987.
20. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536-542.
21. IRB Advisor. Education may overcome reticence to join trials. Relias website. http://www.ahcmedia.com/articles/138088-education-may-overcome-reticence-to-join-trials. Published July 1, 2016. Accessed July 29, 2016.
22. Vanderpool RC, Kornfeld J, Mills L, Byrne MM. Rural-urban differences in discussions of cancer treatment clinical trials. Patient Educ Couns. 2011;85(2):e69-e74.
23. Meropol NJ. Health policy: overcoming cost barriers to clinical trial participation. Nat Rev Clin Oncol. 2016;13(6):333-334.
24. MSK Cancer Alliance. Memorial Sloan Kettering Cancer Center website. https://www.mskcc.org/about/innovative-collaborations/msk-alliance. Updated March 7, 2018. Accessed August 18, 2016.
25. Manne S, Kashy D, Albrecht T, et al. Attitudinal barriers to participation in oncology clinical trials: factor analysis and correlates of barriers. Eur J Cancer Care (Engl). 2015;24(1):28-38.
26. Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient income level and cancer clinical trial participation: a prospective survey study. JAMA Oncol. 2016;2(1):137-139.
27. Moy B. Clinical trials, disparities, and financial burden: it’s time to intervene. Oncologist. 2015;20(6):571.
28. Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42.
29. Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103(3):483-491.
30. Nickell A, Burke NJ, Cohen E, Caprio M, Joseph G. Educating low-SES and LEP survivors about breast cancer research: pilot test of the Health Research Engagement Intervention. J Cancer Educ. 2014;29(4):746-752.
31. Mancini J, Jansen J, Julian-Reynier C, Bechlian D, Vey N, Chabannon C. Preferences of older adults with cancer for involvement in decision-making about research participation. J Am Geriatr Soc. 2014;62(6):1191-1193.
1. Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228-242.
2. Quinn GP, Bell BA, Bell MY, et al. The guinea pig syndrome: improving clinical trial participation among thoracic patients. J Thorac Oncol. 2007;2(3):191-196.
3. Meropol NJ, Wong Y-N, Albrecht T, et al. Randomized trial of a web-based intervention to address barriers to clinical trials. J Clin Oncol. 2016;34(5):469-478.
4. Goldberg RJ. Disclosure of information to adult cancer patients: issues and update. J Clin Oncol. 1984;2(8):948-955.
5. Leiter A, Diefenbach MA, Doucette J, Oh WK, Galsky MD. Clinical trial awareness: changes over time and sociodemographic disparities. Clin Trials. 2015;12(3):215-223.
6. Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30(20):2516-2521.
7. Carney PA, Tucker EK, Newby TA, Beer TM. Feasibility, acceptability and findings from a pilot randomized controlled intervention study on the impact of a book designed to inform patients about cancer clinical trials. J Cancer Educ. 2014;29(1):181-187.
8. Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014;111(9):1684-1687.
9. Regan J, Hickey C, Targett C, Masuda S, Sabbatini P. Framing clinical research and the importance of trial participation: patient and physician perspective. Poster presented at: 8th Annual AACI Clinical Research Initiative Meeting; July 20-21 2016; Chicago, IL.
10. Mancini J, Genre D, Dalenc F, et al. Patients’ regrets after participating in a randomized controlled trials depended on their involvement in the decision making. J Clin Epidemiol. 2012;65(6):635-642
11. Murphy ST, Frank LB, Chatterjee JS, et al. Comparing the relative efficacy of narrative vs nonnarrative health messages in reducing health disparities using a randomized trial. Am J Public Health. 2015;105(10):2117-2123.
12. Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2):e002360. doi:10.1136/bmjopen-2012-002360.
13. Ma GX, Tan Y, Blakeney NC, et al. The impact of a community-based clinical trial educational intervention among underrepresented Chinese Americans. Cancer Epidemiol Biomarkers Prev. 2014;23(3):424-432.
14. Cupertino AP, Molina CSP, de los Rios JB, et al. Knowledge, awareness, and interest in cancer clinical trials among rural latinos following brief education by promotores de salud. J Community Med Health Educ. 2015;5:2161. doi:10.4172/2161-0711.1000358
15. Ryan CL, Bauman K. Educational attainment in the United States: 2015. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf. Published March 2016. Accessed October 18, 2016.
16. Lara PN Jr, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23(36):9282-9289.
17. Dhai A, Etheredge H, Cleaton-Jones P. A pilot study evaluating an intervention designed to raise awareness of clinical trials among potential participants in the developing world. J Med Ethics. 2010;36(4):238-242.
18. Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute–American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9(6):267-276.
19. Davison BJ, So A, Goldenberg SL, Berkowitz J, Gleave ME. Measurement of factors influencing the participation of patients with prostate cancer in clinical trials: a Canadian perspective. BJU Int. 2008;101(8):982-987.
20. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536-542.
21. IRB Advisor. Education may overcome reticence to join trials. Relias website. http://www.ahcmedia.com/articles/138088-education-may-overcome-reticence-to-join-trials. Published July 1, 2016. Accessed July 29, 2016.
22. Vanderpool RC, Kornfeld J, Mills L, Byrne MM. Rural-urban differences in discussions of cancer treatment clinical trials. Patient Educ Couns. 2011;85(2):e69-e74.
23. Meropol NJ. Health policy: overcoming cost barriers to clinical trial participation. Nat Rev Clin Oncol. 2016;13(6):333-334.
24. MSK Cancer Alliance. Memorial Sloan Kettering Cancer Center website. https://www.mskcc.org/about/innovative-collaborations/msk-alliance. Updated March 7, 2018. Accessed August 18, 2016.
25. Manne S, Kashy D, Albrecht T, et al. Attitudinal barriers to participation in oncology clinical trials: factor analysis and correlates of barriers. Eur J Cancer Care (Engl). 2015;24(1):28-38.
26. Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient income level and cancer clinical trial participation: a prospective survey study. JAMA Oncol. 2016;2(1):137-139.
27. Moy B. Clinical trials, disparities, and financial burden: it’s time to intervene. Oncologist. 2015;20(6):571.
28. Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42.
29. Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103(3):483-491.
30. Nickell A, Burke NJ, Cohen E, Caprio M, Joseph G. Educating low-SES and LEP survivors about breast cancer research: pilot test of the Health Research Engagement Intervention. J Cancer Educ. 2014;29(4):746-752.
31. Mancini J, Jansen J, Julian-Reynier C, Bechlian D, Vey N, Chabannon C. Preferences of older adults with cancer for involvement in decision-making about research participation. J Am Geriatr Soc. 2014;62(6):1191-1193.