User login
Cardiovascular disease in women: Prevention, symptoms, diagnosis, pathogenesis
Although long considered a disease of elderly men, cardiovascular disease is increasingly recognized for its impact on women. In fact, it is now the leading cause of death in women worldwide, and in the United States more women than men die of it.1
Given this epidemic of cardiovascular disease in women, more research is now being dedicated to identifying sex-specific aspects of cardiovascular disease, the better to prevent and treat it.
This review will focus on the most recent information about how prevention, symptoms, and underlying cardiovascular conditions differ in women.
PRIMARY PREVENTION: ONGOING DEBATE
Women who diet, exercise, and abstain from smoking have an 80% lower rate of cardiovascular events than the female population overall.2 However, beyond lifestyle modification and blood pressure control, there is ongoing debate as to the efficacy of our available therapies for preventing cardiovascular disease in women.
Aspirin for primary prevention in women: No benefit?
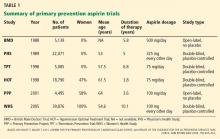
The use of aspirin to prevent cardiovascular disease in women has long been controversial. Several trials showed a lower rate of myocardial infarction in people using aspirin for primary prevention, but most of the patients in the initial trials were men (Table 1).3
The Women’s Health Study4 assigned 39,876 women age 45 and older to receive either aspirin (100 mg on alternate days) or placebo, and monitored them for more than 10 years for major cardiovascular events (non-fatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes).
The results, published in 2005, showed that the rates of myocardial infarction and cardiovascular death were not significantly lower in the aspirin group, although the rate of ischemic stroke was 24% lower. There were more hemorrhagic strokes in the aspirin group (not statistically significant), and there was significantly more gastrointestinal bleeding. The study showed the relative risk (RR) and 95% confidence interval (CI) for several outcomes in aspirin users were:
- Myocardial infarction—RR 1.02, 95% CI 0.84–1.25, P = .83
- Cardiovascular death—RR 0.95, 95% CI 0.74–1.22, P = .68
- Ischemic stroke—RR 0.76, 95% CI 0.63–0.93, P = .009
- Hemorrhagic stroke—RR 1.24, 95% CI 0.82–1.87, P = .31
- Gastrointestinal bleeding—RR 1.4, 95% CI 1.07–1.83, P = .02.
A later analysis indicated that noncompliance had no effect on these results.5
However, a subgroup analysis of women over age 65 found a significant reduction in the rate of myocardial infarction and in the composite end point of myocardial infarction, stroke, and cardiovascular death, although there was a trend toward a higher rate of gastrointestinal bleeding. The numbers in aspirin users in the subgroup over age 65 were as follows:
- Myocardial infarction—RR 0.66, 95% CI 0.44–0.97, P = .04
- Composite end point—RR 0.74, 95% CI 0.59–0.92, P = .008.
Aspirin was taken every other day and at a higher dose than the 81 mg recommended in the United States, although it is unclear how these differences may have affected the results.
United States Preventive Services Task Force (USPSTF) recommendations. Although the USPSTF currently recommends aspirin for men age 45 to 79 to prevent myocardial infarction, it offers no such recommendation for women, largely because of the results of the Women’s Health Initiative study. However, it does recommend aspirin to prevent ischemic stroke in women age 55 to 79.3 Additionally, aspirin can be considered for prevention of myocardial infarction in women who are over age 65 or at high risk.6
This is based on Women’s Health Study data for women over age 65 showing a number needed to treat of 47 to prevent 1 cardiovascular event, whereas the number needed to harm, defined by a major hemorrhagic event, was 128. In contrast, in women younger than age 65, the number needed to treat was 2,001 and the number needed to harm was 196.4
High-risk features, as defined by the guidelines, are a history of coronary artery disease, cerebrovascular disease, peripheral arterial disease, abdominal aortic aneurysm, diabetes, or chronic kidney disease, or a 10-year predicted risk of cardiovascular disease of more than 10%.
Jardine et al7 reported that aspirin was beneficial in patients with chronic kidney disease. The rates of cardiovascular death, death from any cause, and stroke were significantly lower in patients with a glomerular filtration rate (GFR) less than 45 mL/min if they received aspirin. The rates were also lower in aspirin recipients with a GFR between 46 and 60 mL/min, but the difference was not statistically significant.
Comments. Given the risk of significant gastrointestinal bleeding and a trend toward hemorrhagic stroke with aspirin use,4 it is important to weigh the risks and benefits of aspirin for primary prevention in women.
Our understanding of the reasons for sex differences in the clinical benefits of aspirin for primary prevention is limited at this point. Studies have shown a higher prevalence of platelet reactivity and aspirin resistance in women than in men, suggesting that hormonal differences may play a role.8 There has been mention of using higher doses of aspirin in women to achieve the same level of platelet inhibition as in men. However, studies have shown essentially equal platelet inhibition in both men and women after aspirin administration.9 Therefore, more work needs to be done to better understand the observed sex differences in response to aspirin.
Statins for primary prevention in women: Conflicting data
Given suggestions that statins may not be effective in women10 and the fact that women were underrepresented in earlier statin trials, a number of studies have examined this issue in the last several years.
The JUPITER trial (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin)10 enrolled patients who had no history of coronary artery disease and who had a C-reactive protein level equal to or greater than 2 mg/L and a low-density lipoprotein cholesterol level of less than 130 mg/dL. (Of note, these patients would not have met the criteria for receiving a statin for primary prevention according to the current Adult Treatment Panel guidelines.)
The women in the trial who received rosuvastatin had a 46% lower incidence of myocardial infarction, stroke, revascularization, hospitalization for unstable angina, or death from cardiovascular causes. In addition, a meta-analysis performed by the authors showed a one-third reduction of cardiovascular disease end points in women. However, there was no reduction in the mortality rate.
Other statin trials. A later meta-analysis of randomized primary prevention trials found that men and women derived similar benefit from statins in terms of cardiovascular disease end points and all-cause mortality (Figure 1),11 although five of the trials included a small number of secondary prevention patients. In contrast, a meta-analysis of only primary prevention patients showed no benefit of statin therapy in all-cause mortality, although the authors acknowledged that there were insufficient data to look specifically at women in this sample.12
A Cochrane review conducted before the JUPITER data were available concluded that there was insufficient evidence to prescribe statins for primary prevention in patients at low cardiovascular risk.13 However, an updated version that included results of the JUPITER trial concluded that there was a reduction in the rate of all-cause mortality and cardiovascular events in both men and women receiving a statin for primary prevention.14
Given these conflicting results, debate continues as to the benefit of statins for primary prevention, not only in women but in the population as a whole.15,16 The definition of high risk, in terms of comorbidities and lipid profile, also continues to evolve and will likely be an important factor in identifying women who will benefit from statin therapy for primary prevention.
Statin adverse effects. Much of the debate about statins for primary prevention stems from concern about the adverse effects of these drugs. In addition to myopathy, there have been reports of increased risks of new diabetes and cognitive impairment.16 In a post hoc analysis of the Women’s Health Initiative, the adjusted risk of diabetes was 48% higher in women taking a statin for primary prevention than in similar women not taking a statin.17 (This finding should be viewed with caution, since the data are observational.)
There has also been a question of whether women experience more side effects from statin therapy than men do. Although thin or frail women over age 80 are more susceptible to statin side effects, this finding has not been observed in younger women.18
Comment. In view of the data, it appears reasonable to consider statin therapy for primary prevention in women deemed to be at high risk based on the current guidelines. However, as always, one must consider whether the benefits outweigh the risks for the individual patient. More study is needed to better evaluate the utility of statin therapy in primary prevention.
Hormone therapy
Hormone therapy has received enormous attention in both the medical community and the public media. (Hormone therapy is either combined estrogen and progestin or estrogen alone, used to treat symptoms of menopause and to prevent osteoporosis in postmenopausal women. Here, we will discuss hormone therapy and not hormone replacement therapy, which is used specifically to treat premature menopause.)
The safety of estrogen-progestin combination therapy has been the subject of great debate since a Women’s Health Initiative study showed a trend toward a greater risk of cardiovascular disease in estrogen-progestin users.19
Women who received estrogen by itself showed no difference in cardiovascular risk compared with those who received placebo. Unopposed estrogen is rarely prescribed, since it increases the risk of endometrial cancer in women who have not undergone hysterectomy.20
Both unopposed estrogen and combination therapy have also been found to increase the risk of stroke,20 deep vein thrombosis, gallbladder disease, and certain forms of urinary incontinence.
Guidelines on hormone therapy. The USPSTF does not recommend hormone therapy to prevent chronic conditions, basing its decision on the findings from the Women’s Health Initiative.21 The American College of Cardiology and American Heart Association (ACC/AHA) 2007 guidelines advise against continuing hormone therapy in patients who present with acute coronary syndrome, although recommendations need to address a broader scope of primary and secondary prevention patients.
Does timing matter? There is a hypothesis that when hormone therapy is started may affect the cardiovascular risk. A secondary analysis of the Women’s Health Initiative study22 showed a trend towards less cardiovascular disease in women who started hormone therapy within 9 years of menopause, whereas those starting it later had a statistically significantly higher rate of cardiovascular mortality. However, all women had a higher risk of stroke while on hormone therapy, regardless of timing.22
A study of 1,006 healthy women age 45 to 58 whose last menstrual period was 3 to 24 months before enrollment found a statistically significant reduction in the composite end point of death, hospital admission for myocardial infarction, or heart failure with hormone therapy.23 There was no significant increase in breast cancer, deep vein thrombosis, or stroke after 10 years of randomized treatment.
A retrospective analysis of 71,237 postmenopausal women in the California Teachers Study also found a significant reduction in the rate of cardiovascular disease-related deaths with hormone therapy in younger women (ie, younger than age 65), but not in older women.24 The authors concluded that it may not just be the years after menopause but also the baseline age of the woman that may influence outcomes.
In view of these studies, there is increasing recognition that hormone therapy may, in fact, still be beneficial in terms of cardiovascular and all-cause mortality in carefully selected patients. The cardiovascular risk in women, specifically older women who have had a longer duration of menopause, should also be weighed against the potential benefits of therapy in terms of quality of life and symptom relief.
Trials under way include the Kronos Early Estrogen Prevention (KEEP) and Danish Osteoporosis Prevention (DOPS) studies. KEEP is a 4-year, double-blind, randomized controlled trial of hormone therapy in women within 3 years of menopause. DOPS is an open-label trial that includes more than 1,000 women with early menopause. The results of these trials will likely affect future recommendations.
WOMEN’S SYMPTOMS: TYPICAL OR ATYPICAL?
Whether the presenting symptoms of acute coronary syndromes differ between men and women has been much debated.
More women than men seem to present with atypical symptoms.25–27 (The term “atypical” refers to symptoms that do not include the three classic components of angina: substernal chest pain or discomfort, provoked by exertion or emotional stress, and relieved by rest or nitroglycerin, or both.28)
However, most women still present with chest pain. In a study by Dey et al,26 92% of the 7,638 women with presumed acute coronary syndrome presented with chest pain. In women who had atypical symptoms, dyspnea, nausea, vomiting, and diaphoresis were the most common symptoms. Women were significantly more likely than men to present with nausea and vomiting (32% vs 23%, P = .001).
Women in the study were also more likely to have angiographically normal coronary arteries (12% vs 6%, P < .001).26 This difference may be largely due to noncardiac chest pain, but it may also represent conditions such as vasospasm, microvascular disease, or stress cardiomyopathy, all of which disproportionately affect women.
An earlier review of 10 major studies found a higher percentage of women presenting with atypical symptoms (37.5% of women vs 27.4% of men).25 However, symptoms were not a focus of these studies, and the findings may therefore be skewed by inaccurate documentation.
Atypical warning signs. Although most women with acute coronary syndrome present acutely with chest pain, women may have different warning signs than men. Only about one-third of women experience angina before presentation.29 Compared with men, women are more likely to complain of shortness of breath, fatigue, and weakness leading up to a diagnosis of a myocardial infarction.29 Therefore, the prodromal symptoms of cardiovascular disease may in fact be significantly more atypical in women than in men, suggesting the need for heightened vigilance in the cardiovascular evaluation of women who have nonanginal symptoms.
THE ROLE OF STRESS TESTING IN WOMEN
Stress testing in various forms continues to be widely used in the diagnosis of heart disease in women, although data are scarce regarding its utility in women.
The ACC/AHA guidelines continue to recommend exercise stress electrocardiography (ECG) for women who have symptoms, are at intermediate risk, and have a normal result on resting ECG.30
Exercise ECG has a higher false-positive rate in women than in men,31 and there appears to be no relationship between exercise-induced ST-segment depression and the rate of cardiovascular mortality or all-cause mortality in women.32,33 On the other hand, exercise ECG yields valuable additional information such as exercise capacity, chronotropic response, heart-rate recovery, and blood pressure response, all of which have important diagnostic and prognostic implications in women.34
For those who have an abnormal resting ECG, the addition of an imaging test, ie, echocardiography or single-photon emission computed tomography (SPECT), is indicated. Both have limitations: SPECT can give false-positive results because of breast attenuation, and echocardiography varies in accuracy depending on the quality of acoustic windows obtained. Both exercise stress SPECT and exercise stress ECG have higher sensitivity and specificity than electrocardiographic exercise stress testing alone,34 and there is evidence that the two imaging tests are comparable in women.35
In those women who have baseline left bundle branch block or who cannot exercise, a pharmacologic stress test should be performed. Of course, this is a less desirable testing method, given the loss of valuable information obtained from exercising the patient.
UNDERLYING CONDITIONS THAT DISPROPORTIONATELY AFFECT WOMEN
Microvascular angina
Perimenopausal and postmenopausal women account for 70% of patients presenting with chest pain and elevated cardiac enzymes but no significant angiographic evidence of coronary artery disease.36 This condition, commonly called syndrome X, is often characterized by lingering, dull chest pain after exertion and is seen more frequently in women younger than those presenting with classic cardiovascular disease.
Because at least some of these patients show evidence of ST-segment depression and reversible perfusion defects on imaging, the condition is thought to be caused by ischemia of the microvascular bed leading to microvascular angina.37
Although this is still an area of research, microvascular dysfunction has recently been proposed as an explanation for these findings. Abnormal vasoconstriction and impaired vasodilation of the microvascular bed, insulin resistance, increased systemic inflammation, and abnormal pain response have all been cited as potentially contributing to microvascular dysfunction.36
Estrogen deficiency is thought to play a central role in the significantly increased burden of microvascular dysfunction seen in women, with some studies suggesting that hormone therapy can relieve symptoms. However, given the concerns about adverse cardiovascular outcomes in women on hormone therapy, there has been little investigation of this treatment for this disorder.
Studies have shown worse cardiovascular outcomes and higher rates of angina-related hospitalization and repeat heart catheterizations in women with microvascular dysfunction.38
Diagnosing microvascular angina must be done indirectly, as there is no safe and minimally invasive technique by which to directly observe the microvasculature. Current coronary angiographic techniques cannot image vessels smaller than 0.5 mm in diameter, and endomyocardial biopsy cannot access the larger periarterioles thought to play a major role in regulating coronary blood flow.39
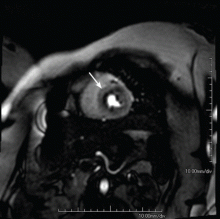
Because the coronary microvasculature controls total coronary resistance and therefore regulates myocardial blood flow, measuring myocardial blood flow at maximum vasodilation, termed coronary flow reserve, can indirectly evaluate the degree of microvascular dysfunction.40 In the absence of obstructive epicardial coronary disease, noninvasive imaging techniques or provocative testing in the coronary catheterization lab can be used for this purpose. In terms of noninvasive imaging, perfusion magnetic resonance imaging (Figure 2) or positron emission tomography is often performed.40
Coronary flow reserve can also be measured by invasive means in the catheterization laboratory after maximum hyperemia is induced by adenosine or other such vasodilatory agents.41 However, measurements obtained in this invasive manner are greatly affected by hemodynamic changes and can have poor reproducibility.40
Proposed therapy for microvascular angina. Once a diagnosis has been made, lifestyle modification, antianginal agents, angiotensin-converting enzyme inhibitors, and statins have been suggested for therapy.39 Pain management techniques are also used, given the increased pain sensitivity observed in women with this condition. However, no therapy to date has proven overwhelmingly effective in these patients, and a disproportionate number of women suffer from chronic symptoms despite these treatments. Currently, researchers are looking for new agents to treat microvascular disease.
Stress cardiomyopathy
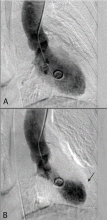
Stress cardiomyopathy, also called takotsubo cardiomyopathy or “broken heart syndrome,” is another condition that disproportionately affects postmenopausal women. It is often associated with sudden emotional or physical stress. Patients present with signs and symptoms of myocardial infarction without demonstrable epicardial coronary artery disease. The hallmark of stress cardiomyopathy is left ventricular dysfunction, often severe, with classic apical ballooning that resembles a Japanese fishing pot (takotsubo) used to trap octopuses, hence the name (Figure 3).
According to a review by Akashi et al42 based on previously reported Mayo Clinic criteria, the diagnosis of stress cardiomyopathy includes each of the following:
- Transient hypokinesis, akinesis, or dyskinesis in the left ventricular midsegments with or without apical involvement; regional wall-motion abnormalities that extend beyond a single epicardial vascular distribution; and frequently, but not always, a stressful trigger
- Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture
- New abnormality on ECG (eg, ST-segment elevation, T-wave inversion) or modest elevation in cardiac troponin
- Absence of pheochromocytoma or myocarditis.
From 80% to 100% of reported cases are in women, with an average age range of 61 to 76.42 It is unclear why there is such an overwhelming postmenopausal female preponderance of the disease. Studies have implicated estrogen deficiency, as it appears to attenuate the levels of cardioprotective substances in the body that in part regulate catecholamine surges and may also increase the level of oxidative stress.42
Several mechanisms for this condition have been proposed. The condition may be caused by multivessel epicardial coronary spasm or spontaneously resolved plaque rupture, resulting in stunned myocardium. However, the regional distribution of wall-motion abnormality is often out of proportion to the level of cardiac enzyme elevation, and in the case of plaque rupture, is frequently not consistent with a single coronary vessel.42 A catecholamine surge causing myocardial and neurogenic stunning has also been proposed, although many of these patients have normal catecholamine levels.42 Finally, microvascular dysfunction has been found in a number of patients with this condition. However, it is difficult to establish a causal relationship, since apical ballooning could result in microvascular dysfunction.42
Treatment of stress cardiomyopathy has not been standardized, in part because the left ventricular dysfunction often resolves spontaneously within several weeks.43,44 Given the proposed catecholaminergic mechanism, some experts believe that beta-blockers are contraindicated because of the resulting unopposed activation of alpha-adrenoreceptors. However, this continues to be a matter of debate. There is also no clear indication for other standard therapies for acute coronary syndrome such as aspirin and heparin, and their use appears to vary in clinical practice.
Although most patients improve with time and recurrence is exceedingly rare, it should be emphasized that they may present acutely with severe hemodynamic instability and cardiogenic shock. Therefore, advanced means of support, such as an intra-aortic balloon pump, may be indicated until the patient recovers from the acute phase of the disease.
Spontaneous coronary artery dissection
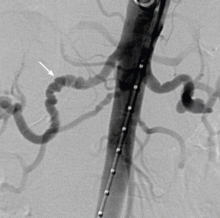
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome resulting from dissection of the coronary intimal or medial layer and associated hematoma formation, leading to coronary occlusion.45,46 In a case series of 87 patients, 49% presented with an ST-segment elevation myocardial infarction, and 23% were found to have multivessel SCAD.46
SCAD occurs predominantly in young, healthy women (mean age 30–45 years). Approximately 70% of cases are in women, 30% of whom are in the peripartum period.45 The reasons for the increased risk during pregnancy have not yet been elucidated, but changing sex hormones, increased cardiac output and shear stress, and an increased inflammatory response have been implicated.45
Diagnosing SCAD. Coronary angiography should be performed with extreme caution in patients suspected of having SCAD, given the risk of further dissection of the artery with forceful injections. In certain cases, it may be difficult to detect SCAD on routine angiography if there is no communication between the true and false lumen.
If the suspicion for SCAD is high, intravascular ultrasonography or optical coherence tomography can be used to better evaluate the vessel.45 Although optical coherence tomography has greater spatial resolution, it is more costly and is not as widely used as intravascular ultrasonography in the clinical setting
Managing SCAD. Although conservative management and coronary artery bypass grafting have been shown to cause minimal in-hospital morbidity, percutaneous coronary intervention has been complicated by technical failure in up to 35% of patients in one series.46
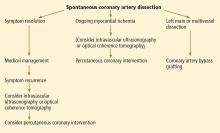
While there is no standardized way to manage these patients, experts currently recommend conservative management with standard therapies for acute coronary syndrome (Figure 4). Although antithrombotic agents can decrease thrombus burden, they must be used with caution, because they also increase the risk of bleeding into the false lumen.
If patients experience recurrent or ongoing ischemia despite conservative management, then revascularization should be considered. Optical coherence tomography or intravascular ultrasonography is recommended to ensure proper stent alignment and positioning.
Coronary artery bypass grafting could be considered in preference to percutaneous coronary intervention, given that the former appears to be safer,46 although this requires further investigation. Some studies have cautioned against using fibrinolytic therapy, based on anecdotal evidence that it may further propagate the dissection,45 although this therapy has been used in other case studies.46
While mortality rates are relatively low (95% survival at 2 years),45 the estimated risk of recurrent SCAD at 10 years is approximately 30%.46
Associated with fibromuscular dysplasia. Of note, a sizeable number of patients with SCAD have been found to have fibromuscular dysplasia. This is a nonatherosclerotic, noninflammatory vascular condition that can affect any vascular bed in the body, although there is a predilection for the renal and carotid arteries (Figure 5).47 Fibromuscular dysplasia also disproportionately affects women and appears to be a concomitant condition in the majority of patients with SCAD.47 Imaging of the carotid and renal arteries of patients with SCAD has revealed a number of cases of fibromuscular dysplasia.46,48 This noted association will likely allow for ongoing research to better understand the pathophysiology of these two conditions.
- Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol 2007; 49:1230–1250.
- Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000; 343:16–22.
- Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the US Preventive Services Task Force. Ann Intern Med 2009; 150:405–410.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Cook NR, Cole SR, Buring JE. Aspirin in the primary prevention of cardiovascular disease in the Women’s Health Study: effect of non-compliance. Eur J Epidemiol 2012; 27:431–438.
- Mosca L, Benjamin EJ, Berra K, et al; American Heart Association. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011; 57:1404–1423.
- Jardine MJ, Ninomiya T, Perkovic V, et al. Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post-hoc subgroup analysis of a randomized controlled trial. J Am Coll Cardiol 2010; 56:956–965.
- Snoep JD, Roest M, Barendrecht AD, De Groot PG, Rosendaal FR, Van Der Bom JG. High platelet reactivity is associated with myocardial infarction in premenopausal women: a population-based case-control study. J Thromb Haemost 2010; 8:906–913.
- Becker DM, Segal J, Vaidya D, et al. Sex differences in platelet reactivity and response to low-dose aspirin therapy. JAMA 2006; 295:1420–1427.
- Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation 2010; 121:1069–1077.
- Kostis WJ, Cheng JQ, Dobrzynski JM, Cabrera J, Kostis JB. Meta-analysis of statin effects in women versus men. J Am Coll Cardiol 2012; 59:572–582.
- Ray KK, Seshasai SR, Erqou S, et al. Statins and all-cause mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch Intern Med 2010; 170:1024–1031.
- Taylor F, Ward K, Moore TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2011;CD004816.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;CD004816.
- Blaha MJ, Nasir K, Blumenthal RS. Statin therapy for healthy men identified as “increased risk.” JAMA 2012; 307:1489–1490.
- Redberg RF, Katz MH. Healthy men should not take statins. JAMA 2012; 307:1491–1492.
- Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med 2012; 172:144–152.
- Pasternak RC, Smith SC, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Stroke 2002; 33:2337–2341.
- Manson JE, Hsia J, Johnson KC, et al; Women’s Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003; 349:523–534.
- Hsia J, Criqui MH, Herrington DM, et al; Women’s Health Initiative Research Group. Conjugated equine estrogens and peripheral arterial disease risk: The Women’s Health Initiative. Am Heart J 2006; 152:170–176.
- Moyer VAUS Preventive Services Task Force. Menopausal hormone therapy for the primary prevention of chronic conditions: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158:47–54.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 2012; 345:e6409.
- Stram DO, Liu Y, Henderson KD, et al. Age-specific effects of hormone therapy use on overall mortality and ischemic heart disease mortality among women in the California Teachers Study. Menopause 2011; 18:253–261.
- Canto JG, Goldberg RJ, Hand MM, et al. Symptom presentation of women with acute coronary syndromes: myth vs reality. Arch Intern Med 2007; 167:2405–2413.
- Dey S, Flather MD, Devlin G, et al; Global Registry of Acute Coronary Events investigators. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: The Global Registry of Acute Coronary Events. Heart 2009; 95:20–26.
- Canto JG, Shlipak MG, Rogers WJ, et al. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA 2000; 283:3223–3229.
- Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979; 300:1350–1358.
- McSweeney JC, Cody M, O’Sullivan P, Elberson K, Moser DK, Garvin BJ. Women’s early warning symptoms of acute myocardial infarction. Circulation 2003; 108:2619–2623.
- Mieres JH, Shaw LJ, Arai A, et al; Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation 2005; 111:682–696.
- Barolsky SM, Gilbert CA, Faruqui A, Nutter DO, Schlant RC. Differences in electrocardiographic response to exercise of women and men: a non-Bayesian factor. Circulation 1979; 60:1021–1027.
- Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: The St James Women Take Heart Project. Circulation 2003; 108:1554–1559.
- Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the Lipid Research Clinics Prevalence Study. JAMA 2003; 290:1600–1607.
- Kohli P, Gulati M. Exercise stress testing in women: going back to the basics. Circulation 2010; 122:2570–2580.
- Grady D, Chaput L, Kristof M. Diagnosis and treatment of coronary heart disease in women: systematic reviews of evidence on selected topics. Evid Rep Technol Assess (Summ) 2003; 81:1–4.
- Singh M, Singh S, Arora R, Khosla S. Cardiac syndrome X: current concepts. Int J Cardiol 2010; 142:113–119.
- Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007; 356:830–840.
- Johnson BD, Shaw LJ, Buchthal SD, et al; National Institutes of Health-National Heart, Lung, and Blood Institute. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004; 109:2993–2999.
- Beltrame JF, Crea F, Camici P. Advances in coronary microvascular dysfunction. Heart Lung Circ 2009; 18:19–27.
- Leung DY, Leung M. Non-invasive/invasive imaging: significance and assessment of coronary microvascular dysfunction. Heart 2011; 97:587–595.
- Samim A, Nugent L, Mehta PK, Shufelt C, Bairey Merz CN. Treatment of angina and microvascular coronary dysfunction. Curr Treat Options Cardiovasc Med 2010; 12:355–364.
- Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 2008; 118:2754–2762.
- Akashi YJ, Musha H, Kida K, et al. Reversible ventricular dysfunction takotsubo cardiomyopathy. Eur J Heart Fail 2005; 7:1171–1176.
- Regnante RA, Zuzek RW, Weinsier SB, et al. Clinical characteristics and four-year outcomes of patients in the Rhode Island Takotsubo Cardiomyopathy Registry. Am J Cardiol 2009; 103:1015–1019.
- Vrints CJ. Spontaneous coronary artery dissection. Heart 2010; 96:801–808.
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126:579–588.
- Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013; 6:44–52.
- Saw J, Poulter R, Fung A, Wood D, Hamburger J, Buller CE. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series. Circ Cardiovasc Interv 2012; 5:134–137.
Although long considered a disease of elderly men, cardiovascular disease is increasingly recognized for its impact on women. In fact, it is now the leading cause of death in women worldwide, and in the United States more women than men die of it.1
Given this epidemic of cardiovascular disease in women, more research is now being dedicated to identifying sex-specific aspects of cardiovascular disease, the better to prevent and treat it.
This review will focus on the most recent information about how prevention, symptoms, and underlying cardiovascular conditions differ in women.
PRIMARY PREVENTION: ONGOING DEBATE
Women who diet, exercise, and abstain from smoking have an 80% lower rate of cardiovascular events than the female population overall.2 However, beyond lifestyle modification and blood pressure control, there is ongoing debate as to the efficacy of our available therapies for preventing cardiovascular disease in women.
Aspirin for primary prevention in women: No benefit?
The use of aspirin to prevent cardiovascular disease in women has long been controversial. Several trials showed a lower rate of myocardial infarction in people using aspirin for primary prevention, but most of the patients in the initial trials were men (Table 1).3
The Women’s Health Study4 assigned 39,876 women age 45 and older to receive either aspirin (100 mg on alternate days) or placebo, and monitored them for more than 10 years for major cardiovascular events (non-fatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes).
The results, published in 2005, showed that the rates of myocardial infarction and cardiovascular death were not significantly lower in the aspirin group, although the rate of ischemic stroke was 24% lower. There were more hemorrhagic strokes in the aspirin group (not statistically significant), and there was significantly more gastrointestinal bleeding. The study showed the relative risk (RR) and 95% confidence interval (CI) for several outcomes in aspirin users were:
- Myocardial infarction—RR 1.02, 95% CI 0.84–1.25, P = .83
- Cardiovascular death—RR 0.95, 95% CI 0.74–1.22, P = .68
- Ischemic stroke—RR 0.76, 95% CI 0.63–0.93, P = .009
- Hemorrhagic stroke—RR 1.24, 95% CI 0.82–1.87, P = .31
- Gastrointestinal bleeding—RR 1.4, 95% CI 1.07–1.83, P = .02.
A later analysis indicated that noncompliance had no effect on these results.5
However, a subgroup analysis of women over age 65 found a significant reduction in the rate of myocardial infarction and in the composite end point of myocardial infarction, stroke, and cardiovascular death, although there was a trend toward a higher rate of gastrointestinal bleeding. The numbers in aspirin users in the subgroup over age 65 were as follows:
- Myocardial infarction—RR 0.66, 95% CI 0.44–0.97, P = .04
- Composite end point—RR 0.74, 95% CI 0.59–0.92, P = .008.
Aspirin was taken every other day and at a higher dose than the 81 mg recommended in the United States, although it is unclear how these differences may have affected the results.
United States Preventive Services Task Force (USPSTF) recommendations. Although the USPSTF currently recommends aspirin for men age 45 to 79 to prevent myocardial infarction, it offers no such recommendation for women, largely because of the results of the Women’s Health Initiative study. However, it does recommend aspirin to prevent ischemic stroke in women age 55 to 79.3 Additionally, aspirin can be considered for prevention of myocardial infarction in women who are over age 65 or at high risk.6
This is based on Women’s Health Study data for women over age 65 showing a number needed to treat of 47 to prevent 1 cardiovascular event, whereas the number needed to harm, defined by a major hemorrhagic event, was 128. In contrast, in women younger than age 65, the number needed to treat was 2,001 and the number needed to harm was 196.4
High-risk features, as defined by the guidelines, are a history of coronary artery disease, cerebrovascular disease, peripheral arterial disease, abdominal aortic aneurysm, diabetes, or chronic kidney disease, or a 10-year predicted risk of cardiovascular disease of more than 10%.
Jardine et al7 reported that aspirin was beneficial in patients with chronic kidney disease. The rates of cardiovascular death, death from any cause, and stroke were significantly lower in patients with a glomerular filtration rate (GFR) less than 45 mL/min if they received aspirin. The rates were also lower in aspirin recipients with a GFR between 46 and 60 mL/min, but the difference was not statistically significant.
Comments. Given the risk of significant gastrointestinal bleeding and a trend toward hemorrhagic stroke with aspirin use,4 it is important to weigh the risks and benefits of aspirin for primary prevention in women.
Our understanding of the reasons for sex differences in the clinical benefits of aspirin for primary prevention is limited at this point. Studies have shown a higher prevalence of platelet reactivity and aspirin resistance in women than in men, suggesting that hormonal differences may play a role.8 There has been mention of using higher doses of aspirin in women to achieve the same level of platelet inhibition as in men. However, studies have shown essentially equal platelet inhibition in both men and women after aspirin administration.9 Therefore, more work needs to be done to better understand the observed sex differences in response to aspirin.
Statins for primary prevention in women: Conflicting data
Given suggestions that statins may not be effective in women10 and the fact that women were underrepresented in earlier statin trials, a number of studies have examined this issue in the last several years.
The JUPITER trial (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin)10 enrolled patients who had no history of coronary artery disease and who had a C-reactive protein level equal to or greater than 2 mg/L and a low-density lipoprotein cholesterol level of less than 130 mg/dL. (Of note, these patients would not have met the criteria for receiving a statin for primary prevention according to the current Adult Treatment Panel guidelines.)
The women in the trial who received rosuvastatin had a 46% lower incidence of myocardial infarction, stroke, revascularization, hospitalization for unstable angina, or death from cardiovascular causes. In addition, a meta-analysis performed by the authors showed a one-third reduction of cardiovascular disease end points in women. However, there was no reduction in the mortality rate.
Other statin trials. A later meta-analysis of randomized primary prevention trials found that men and women derived similar benefit from statins in terms of cardiovascular disease end points and all-cause mortality (Figure 1),11 although five of the trials included a small number of secondary prevention patients. In contrast, a meta-analysis of only primary prevention patients showed no benefit of statin therapy in all-cause mortality, although the authors acknowledged that there were insufficient data to look specifically at women in this sample.12
A Cochrane review conducted before the JUPITER data were available concluded that there was insufficient evidence to prescribe statins for primary prevention in patients at low cardiovascular risk.13 However, an updated version that included results of the JUPITER trial concluded that there was a reduction in the rate of all-cause mortality and cardiovascular events in both men and women receiving a statin for primary prevention.14
Given these conflicting results, debate continues as to the benefit of statins for primary prevention, not only in women but in the population as a whole.15,16 The definition of high risk, in terms of comorbidities and lipid profile, also continues to evolve and will likely be an important factor in identifying women who will benefit from statin therapy for primary prevention.
Statin adverse effects. Much of the debate about statins for primary prevention stems from concern about the adverse effects of these drugs. In addition to myopathy, there have been reports of increased risks of new diabetes and cognitive impairment.16 In a post hoc analysis of the Women’s Health Initiative, the adjusted risk of diabetes was 48% higher in women taking a statin for primary prevention than in similar women not taking a statin.17 (This finding should be viewed with caution, since the data are observational.)
There has also been a question of whether women experience more side effects from statin therapy than men do. Although thin or frail women over age 80 are more susceptible to statin side effects, this finding has not been observed in younger women.18
Comment. In view of the data, it appears reasonable to consider statin therapy for primary prevention in women deemed to be at high risk based on the current guidelines. However, as always, one must consider whether the benefits outweigh the risks for the individual patient. More study is needed to better evaluate the utility of statin therapy in primary prevention.
Hormone therapy
Hormone therapy has received enormous attention in both the medical community and the public media. (Hormone therapy is either combined estrogen and progestin or estrogen alone, used to treat symptoms of menopause and to prevent osteoporosis in postmenopausal women. Here, we will discuss hormone therapy and not hormone replacement therapy, which is used specifically to treat premature menopause.)
The safety of estrogen-progestin combination therapy has been the subject of great debate since a Women’s Health Initiative study showed a trend toward a greater risk of cardiovascular disease in estrogen-progestin users.19
Women who received estrogen by itself showed no difference in cardiovascular risk compared with those who received placebo. Unopposed estrogen is rarely prescribed, since it increases the risk of endometrial cancer in women who have not undergone hysterectomy.20
Both unopposed estrogen and combination therapy have also been found to increase the risk of stroke,20 deep vein thrombosis, gallbladder disease, and certain forms of urinary incontinence.
Guidelines on hormone therapy. The USPSTF does not recommend hormone therapy to prevent chronic conditions, basing its decision on the findings from the Women’s Health Initiative.21 The American College of Cardiology and American Heart Association (ACC/AHA) 2007 guidelines advise against continuing hormone therapy in patients who present with acute coronary syndrome, although recommendations need to address a broader scope of primary and secondary prevention patients.
Does timing matter? There is a hypothesis that when hormone therapy is started may affect the cardiovascular risk. A secondary analysis of the Women’s Health Initiative study22 showed a trend towards less cardiovascular disease in women who started hormone therapy within 9 years of menopause, whereas those starting it later had a statistically significantly higher rate of cardiovascular mortality. However, all women had a higher risk of stroke while on hormone therapy, regardless of timing.22
A study of 1,006 healthy women age 45 to 58 whose last menstrual period was 3 to 24 months before enrollment found a statistically significant reduction in the composite end point of death, hospital admission for myocardial infarction, or heart failure with hormone therapy.23 There was no significant increase in breast cancer, deep vein thrombosis, or stroke after 10 years of randomized treatment.
A retrospective analysis of 71,237 postmenopausal women in the California Teachers Study also found a significant reduction in the rate of cardiovascular disease-related deaths with hormone therapy in younger women (ie, younger than age 65), but not in older women.24 The authors concluded that it may not just be the years after menopause but also the baseline age of the woman that may influence outcomes.
In view of these studies, there is increasing recognition that hormone therapy may, in fact, still be beneficial in terms of cardiovascular and all-cause mortality in carefully selected patients. The cardiovascular risk in women, specifically older women who have had a longer duration of menopause, should also be weighed against the potential benefits of therapy in terms of quality of life and symptom relief.
Trials under way include the Kronos Early Estrogen Prevention (KEEP) and Danish Osteoporosis Prevention (DOPS) studies. KEEP is a 4-year, double-blind, randomized controlled trial of hormone therapy in women within 3 years of menopause. DOPS is an open-label trial that includes more than 1,000 women with early menopause. The results of these trials will likely affect future recommendations.
WOMEN’S SYMPTOMS: TYPICAL OR ATYPICAL?
Whether the presenting symptoms of acute coronary syndromes differ between men and women has been much debated.
More women than men seem to present with atypical symptoms.25–27 (The term “atypical” refers to symptoms that do not include the three classic components of angina: substernal chest pain or discomfort, provoked by exertion or emotional stress, and relieved by rest or nitroglycerin, or both.28)
However, most women still present with chest pain. In a study by Dey et al,26 92% of the 7,638 women with presumed acute coronary syndrome presented with chest pain. In women who had atypical symptoms, dyspnea, nausea, vomiting, and diaphoresis were the most common symptoms. Women were significantly more likely than men to present with nausea and vomiting (32% vs 23%, P = .001).
Women in the study were also more likely to have angiographically normal coronary arteries (12% vs 6%, P < .001).26 This difference may be largely due to noncardiac chest pain, but it may also represent conditions such as vasospasm, microvascular disease, or stress cardiomyopathy, all of which disproportionately affect women.
An earlier review of 10 major studies found a higher percentage of women presenting with atypical symptoms (37.5% of women vs 27.4% of men).25 However, symptoms were not a focus of these studies, and the findings may therefore be skewed by inaccurate documentation.
Atypical warning signs. Although most women with acute coronary syndrome present acutely with chest pain, women may have different warning signs than men. Only about one-third of women experience angina before presentation.29 Compared with men, women are more likely to complain of shortness of breath, fatigue, and weakness leading up to a diagnosis of a myocardial infarction.29 Therefore, the prodromal symptoms of cardiovascular disease may in fact be significantly more atypical in women than in men, suggesting the need for heightened vigilance in the cardiovascular evaluation of women who have nonanginal symptoms.
THE ROLE OF STRESS TESTING IN WOMEN
Stress testing in various forms continues to be widely used in the diagnosis of heart disease in women, although data are scarce regarding its utility in women.
The ACC/AHA guidelines continue to recommend exercise stress electrocardiography (ECG) for women who have symptoms, are at intermediate risk, and have a normal result on resting ECG.30
Exercise ECG has a higher false-positive rate in women than in men,31 and there appears to be no relationship between exercise-induced ST-segment depression and the rate of cardiovascular mortality or all-cause mortality in women.32,33 On the other hand, exercise ECG yields valuable additional information such as exercise capacity, chronotropic response, heart-rate recovery, and blood pressure response, all of which have important diagnostic and prognostic implications in women.34
For those who have an abnormal resting ECG, the addition of an imaging test, ie, echocardiography or single-photon emission computed tomography (SPECT), is indicated. Both have limitations: SPECT can give false-positive results because of breast attenuation, and echocardiography varies in accuracy depending on the quality of acoustic windows obtained. Both exercise stress SPECT and exercise stress ECG have higher sensitivity and specificity than electrocardiographic exercise stress testing alone,34 and there is evidence that the two imaging tests are comparable in women.35
In those women who have baseline left bundle branch block or who cannot exercise, a pharmacologic stress test should be performed. Of course, this is a less desirable testing method, given the loss of valuable information obtained from exercising the patient.
UNDERLYING CONDITIONS THAT DISPROPORTIONATELY AFFECT WOMEN
Microvascular angina
Perimenopausal and postmenopausal women account for 70% of patients presenting with chest pain and elevated cardiac enzymes but no significant angiographic evidence of coronary artery disease.36 This condition, commonly called syndrome X, is often characterized by lingering, dull chest pain after exertion and is seen more frequently in women younger than those presenting with classic cardiovascular disease.
Because at least some of these patients show evidence of ST-segment depression and reversible perfusion defects on imaging, the condition is thought to be caused by ischemia of the microvascular bed leading to microvascular angina.37
Although this is still an area of research, microvascular dysfunction has recently been proposed as an explanation for these findings. Abnormal vasoconstriction and impaired vasodilation of the microvascular bed, insulin resistance, increased systemic inflammation, and abnormal pain response have all been cited as potentially contributing to microvascular dysfunction.36
Estrogen deficiency is thought to play a central role in the significantly increased burden of microvascular dysfunction seen in women, with some studies suggesting that hormone therapy can relieve symptoms. However, given the concerns about adverse cardiovascular outcomes in women on hormone therapy, there has been little investigation of this treatment for this disorder.
Studies have shown worse cardiovascular outcomes and higher rates of angina-related hospitalization and repeat heart catheterizations in women with microvascular dysfunction.38
Diagnosing microvascular angina must be done indirectly, as there is no safe and minimally invasive technique by which to directly observe the microvasculature. Current coronary angiographic techniques cannot image vessels smaller than 0.5 mm in diameter, and endomyocardial biopsy cannot access the larger periarterioles thought to play a major role in regulating coronary blood flow.39
Because the coronary microvasculature controls total coronary resistance and therefore regulates myocardial blood flow, measuring myocardial blood flow at maximum vasodilation, termed coronary flow reserve, can indirectly evaluate the degree of microvascular dysfunction.40 In the absence of obstructive epicardial coronary disease, noninvasive imaging techniques or provocative testing in the coronary catheterization lab can be used for this purpose. In terms of noninvasive imaging, perfusion magnetic resonance imaging (Figure 2) or positron emission tomography is often performed.40
Coronary flow reserve can also be measured by invasive means in the catheterization laboratory after maximum hyperemia is induced by adenosine or other such vasodilatory agents.41 However, measurements obtained in this invasive manner are greatly affected by hemodynamic changes and can have poor reproducibility.40
Proposed therapy for microvascular angina. Once a diagnosis has been made, lifestyle modification, antianginal agents, angiotensin-converting enzyme inhibitors, and statins have been suggested for therapy.39 Pain management techniques are also used, given the increased pain sensitivity observed in women with this condition. However, no therapy to date has proven overwhelmingly effective in these patients, and a disproportionate number of women suffer from chronic symptoms despite these treatments. Currently, researchers are looking for new agents to treat microvascular disease.
Stress cardiomyopathy
Stress cardiomyopathy, also called takotsubo cardiomyopathy or “broken heart syndrome,” is another condition that disproportionately affects postmenopausal women. It is often associated with sudden emotional or physical stress. Patients present with signs and symptoms of myocardial infarction without demonstrable epicardial coronary artery disease. The hallmark of stress cardiomyopathy is left ventricular dysfunction, often severe, with classic apical ballooning that resembles a Japanese fishing pot (takotsubo) used to trap octopuses, hence the name (Figure 3).
According to a review by Akashi et al42 based on previously reported Mayo Clinic criteria, the diagnosis of stress cardiomyopathy includes each of the following:
- Transient hypokinesis, akinesis, or dyskinesis in the left ventricular midsegments with or without apical involvement; regional wall-motion abnormalities that extend beyond a single epicardial vascular distribution; and frequently, but not always, a stressful trigger
- Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture
- New abnormality on ECG (eg, ST-segment elevation, T-wave inversion) or modest elevation in cardiac troponin
- Absence of pheochromocytoma or myocarditis.
From 80% to 100% of reported cases are in women, with an average age range of 61 to 76.42 It is unclear why there is such an overwhelming postmenopausal female preponderance of the disease. Studies have implicated estrogen deficiency, as it appears to attenuate the levels of cardioprotective substances in the body that in part regulate catecholamine surges and may also increase the level of oxidative stress.42
Several mechanisms for this condition have been proposed. The condition may be caused by multivessel epicardial coronary spasm or spontaneously resolved plaque rupture, resulting in stunned myocardium. However, the regional distribution of wall-motion abnormality is often out of proportion to the level of cardiac enzyme elevation, and in the case of plaque rupture, is frequently not consistent with a single coronary vessel.42 A catecholamine surge causing myocardial and neurogenic stunning has also been proposed, although many of these patients have normal catecholamine levels.42 Finally, microvascular dysfunction has been found in a number of patients with this condition. However, it is difficult to establish a causal relationship, since apical ballooning could result in microvascular dysfunction.42
Treatment of stress cardiomyopathy has not been standardized, in part because the left ventricular dysfunction often resolves spontaneously within several weeks.43,44 Given the proposed catecholaminergic mechanism, some experts believe that beta-blockers are contraindicated because of the resulting unopposed activation of alpha-adrenoreceptors. However, this continues to be a matter of debate. There is also no clear indication for other standard therapies for acute coronary syndrome such as aspirin and heparin, and their use appears to vary in clinical practice.
Although most patients improve with time and recurrence is exceedingly rare, it should be emphasized that they may present acutely with severe hemodynamic instability and cardiogenic shock. Therefore, advanced means of support, such as an intra-aortic balloon pump, may be indicated until the patient recovers from the acute phase of the disease.
Spontaneous coronary artery dissection
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome resulting from dissection of the coronary intimal or medial layer and associated hematoma formation, leading to coronary occlusion.45,46 In a case series of 87 patients, 49% presented with an ST-segment elevation myocardial infarction, and 23% were found to have multivessel SCAD.46
SCAD occurs predominantly in young, healthy women (mean age 30–45 years). Approximately 70% of cases are in women, 30% of whom are in the peripartum period.45 The reasons for the increased risk during pregnancy have not yet been elucidated, but changing sex hormones, increased cardiac output and shear stress, and an increased inflammatory response have been implicated.45
Diagnosing SCAD. Coronary angiography should be performed with extreme caution in patients suspected of having SCAD, given the risk of further dissection of the artery with forceful injections. In certain cases, it may be difficult to detect SCAD on routine angiography if there is no communication between the true and false lumen.
If the suspicion for SCAD is high, intravascular ultrasonography or optical coherence tomography can be used to better evaluate the vessel.45 Although optical coherence tomography has greater spatial resolution, it is more costly and is not as widely used as intravascular ultrasonography in the clinical setting
Managing SCAD. Although conservative management and coronary artery bypass grafting have been shown to cause minimal in-hospital morbidity, percutaneous coronary intervention has been complicated by technical failure in up to 35% of patients in one series.46
While there is no standardized way to manage these patients, experts currently recommend conservative management with standard therapies for acute coronary syndrome (Figure 4). Although antithrombotic agents can decrease thrombus burden, they must be used with caution, because they also increase the risk of bleeding into the false lumen.
If patients experience recurrent or ongoing ischemia despite conservative management, then revascularization should be considered. Optical coherence tomography or intravascular ultrasonography is recommended to ensure proper stent alignment and positioning.
Coronary artery bypass grafting could be considered in preference to percutaneous coronary intervention, given that the former appears to be safer,46 although this requires further investigation. Some studies have cautioned against using fibrinolytic therapy, based on anecdotal evidence that it may further propagate the dissection,45 although this therapy has been used in other case studies.46
While mortality rates are relatively low (95% survival at 2 years),45 the estimated risk of recurrent SCAD at 10 years is approximately 30%.46
Associated with fibromuscular dysplasia. Of note, a sizeable number of patients with SCAD have been found to have fibromuscular dysplasia. This is a nonatherosclerotic, noninflammatory vascular condition that can affect any vascular bed in the body, although there is a predilection for the renal and carotid arteries (Figure 5).47 Fibromuscular dysplasia also disproportionately affects women and appears to be a concomitant condition in the majority of patients with SCAD.47 Imaging of the carotid and renal arteries of patients with SCAD has revealed a number of cases of fibromuscular dysplasia.46,48 This noted association will likely allow for ongoing research to better understand the pathophysiology of these two conditions.
Although long considered a disease of elderly men, cardiovascular disease is increasingly recognized for its impact on women. In fact, it is now the leading cause of death in women worldwide, and in the United States more women than men die of it.1
Given this epidemic of cardiovascular disease in women, more research is now being dedicated to identifying sex-specific aspects of cardiovascular disease, the better to prevent and treat it.
This review will focus on the most recent information about how prevention, symptoms, and underlying cardiovascular conditions differ in women.
PRIMARY PREVENTION: ONGOING DEBATE
Women who diet, exercise, and abstain from smoking have an 80% lower rate of cardiovascular events than the female population overall.2 However, beyond lifestyle modification and blood pressure control, there is ongoing debate as to the efficacy of our available therapies for preventing cardiovascular disease in women.
Aspirin for primary prevention in women: No benefit?
The use of aspirin to prevent cardiovascular disease in women has long been controversial. Several trials showed a lower rate of myocardial infarction in people using aspirin for primary prevention, but most of the patients in the initial trials were men (Table 1).3
The Women’s Health Study4 assigned 39,876 women age 45 and older to receive either aspirin (100 mg on alternate days) or placebo, and monitored them for more than 10 years for major cardiovascular events (non-fatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes).
The results, published in 2005, showed that the rates of myocardial infarction and cardiovascular death were not significantly lower in the aspirin group, although the rate of ischemic stroke was 24% lower. There were more hemorrhagic strokes in the aspirin group (not statistically significant), and there was significantly more gastrointestinal bleeding. The study showed the relative risk (RR) and 95% confidence interval (CI) for several outcomes in aspirin users were:
- Myocardial infarction—RR 1.02, 95% CI 0.84–1.25, P = .83
- Cardiovascular death—RR 0.95, 95% CI 0.74–1.22, P = .68
- Ischemic stroke—RR 0.76, 95% CI 0.63–0.93, P = .009
- Hemorrhagic stroke—RR 1.24, 95% CI 0.82–1.87, P = .31
- Gastrointestinal bleeding—RR 1.4, 95% CI 1.07–1.83, P = .02.
A later analysis indicated that noncompliance had no effect on these results.5
However, a subgroup analysis of women over age 65 found a significant reduction in the rate of myocardial infarction and in the composite end point of myocardial infarction, stroke, and cardiovascular death, although there was a trend toward a higher rate of gastrointestinal bleeding. The numbers in aspirin users in the subgroup over age 65 were as follows:
- Myocardial infarction—RR 0.66, 95% CI 0.44–0.97, P = .04
- Composite end point—RR 0.74, 95% CI 0.59–0.92, P = .008.
Aspirin was taken every other day and at a higher dose than the 81 mg recommended in the United States, although it is unclear how these differences may have affected the results.
United States Preventive Services Task Force (USPSTF) recommendations. Although the USPSTF currently recommends aspirin for men age 45 to 79 to prevent myocardial infarction, it offers no such recommendation for women, largely because of the results of the Women’s Health Initiative study. However, it does recommend aspirin to prevent ischemic stroke in women age 55 to 79.3 Additionally, aspirin can be considered for prevention of myocardial infarction in women who are over age 65 or at high risk.6
This is based on Women’s Health Study data for women over age 65 showing a number needed to treat of 47 to prevent 1 cardiovascular event, whereas the number needed to harm, defined by a major hemorrhagic event, was 128. In contrast, in women younger than age 65, the number needed to treat was 2,001 and the number needed to harm was 196.4
High-risk features, as defined by the guidelines, are a history of coronary artery disease, cerebrovascular disease, peripheral arterial disease, abdominal aortic aneurysm, diabetes, or chronic kidney disease, or a 10-year predicted risk of cardiovascular disease of more than 10%.
Jardine et al7 reported that aspirin was beneficial in patients with chronic kidney disease. The rates of cardiovascular death, death from any cause, and stroke were significantly lower in patients with a glomerular filtration rate (GFR) less than 45 mL/min if they received aspirin. The rates were also lower in aspirin recipients with a GFR between 46 and 60 mL/min, but the difference was not statistically significant.
Comments. Given the risk of significant gastrointestinal bleeding and a trend toward hemorrhagic stroke with aspirin use,4 it is important to weigh the risks and benefits of aspirin for primary prevention in women.
Our understanding of the reasons for sex differences in the clinical benefits of aspirin for primary prevention is limited at this point. Studies have shown a higher prevalence of platelet reactivity and aspirin resistance in women than in men, suggesting that hormonal differences may play a role.8 There has been mention of using higher doses of aspirin in women to achieve the same level of platelet inhibition as in men. However, studies have shown essentially equal platelet inhibition in both men and women after aspirin administration.9 Therefore, more work needs to be done to better understand the observed sex differences in response to aspirin.
Statins for primary prevention in women: Conflicting data
Given suggestions that statins may not be effective in women10 and the fact that women were underrepresented in earlier statin trials, a number of studies have examined this issue in the last several years.
The JUPITER trial (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin)10 enrolled patients who had no history of coronary artery disease and who had a C-reactive protein level equal to or greater than 2 mg/L and a low-density lipoprotein cholesterol level of less than 130 mg/dL. (Of note, these patients would not have met the criteria for receiving a statin for primary prevention according to the current Adult Treatment Panel guidelines.)
The women in the trial who received rosuvastatin had a 46% lower incidence of myocardial infarction, stroke, revascularization, hospitalization for unstable angina, or death from cardiovascular causes. In addition, a meta-analysis performed by the authors showed a one-third reduction of cardiovascular disease end points in women. However, there was no reduction in the mortality rate.
Other statin trials. A later meta-analysis of randomized primary prevention trials found that men and women derived similar benefit from statins in terms of cardiovascular disease end points and all-cause mortality (Figure 1),11 although five of the trials included a small number of secondary prevention patients. In contrast, a meta-analysis of only primary prevention patients showed no benefit of statin therapy in all-cause mortality, although the authors acknowledged that there were insufficient data to look specifically at women in this sample.12
A Cochrane review conducted before the JUPITER data were available concluded that there was insufficient evidence to prescribe statins for primary prevention in patients at low cardiovascular risk.13 However, an updated version that included results of the JUPITER trial concluded that there was a reduction in the rate of all-cause mortality and cardiovascular events in both men and women receiving a statin for primary prevention.14
Given these conflicting results, debate continues as to the benefit of statins for primary prevention, not only in women but in the population as a whole.15,16 The definition of high risk, in terms of comorbidities and lipid profile, also continues to evolve and will likely be an important factor in identifying women who will benefit from statin therapy for primary prevention.
Statin adverse effects. Much of the debate about statins for primary prevention stems from concern about the adverse effects of these drugs. In addition to myopathy, there have been reports of increased risks of new diabetes and cognitive impairment.16 In a post hoc analysis of the Women’s Health Initiative, the adjusted risk of diabetes was 48% higher in women taking a statin for primary prevention than in similar women not taking a statin.17 (This finding should be viewed with caution, since the data are observational.)
There has also been a question of whether women experience more side effects from statin therapy than men do. Although thin or frail women over age 80 are more susceptible to statin side effects, this finding has not been observed in younger women.18
Comment. In view of the data, it appears reasonable to consider statin therapy for primary prevention in women deemed to be at high risk based on the current guidelines. However, as always, one must consider whether the benefits outweigh the risks for the individual patient. More study is needed to better evaluate the utility of statin therapy in primary prevention.
Hormone therapy
Hormone therapy has received enormous attention in both the medical community and the public media. (Hormone therapy is either combined estrogen and progestin or estrogen alone, used to treat symptoms of menopause and to prevent osteoporosis in postmenopausal women. Here, we will discuss hormone therapy and not hormone replacement therapy, which is used specifically to treat premature menopause.)
The safety of estrogen-progestin combination therapy has been the subject of great debate since a Women’s Health Initiative study showed a trend toward a greater risk of cardiovascular disease in estrogen-progestin users.19
Women who received estrogen by itself showed no difference in cardiovascular risk compared with those who received placebo. Unopposed estrogen is rarely prescribed, since it increases the risk of endometrial cancer in women who have not undergone hysterectomy.20
Both unopposed estrogen and combination therapy have also been found to increase the risk of stroke,20 deep vein thrombosis, gallbladder disease, and certain forms of urinary incontinence.
Guidelines on hormone therapy. The USPSTF does not recommend hormone therapy to prevent chronic conditions, basing its decision on the findings from the Women’s Health Initiative.21 The American College of Cardiology and American Heart Association (ACC/AHA) 2007 guidelines advise against continuing hormone therapy in patients who present with acute coronary syndrome, although recommendations need to address a broader scope of primary and secondary prevention patients.
Does timing matter? There is a hypothesis that when hormone therapy is started may affect the cardiovascular risk. A secondary analysis of the Women’s Health Initiative study22 showed a trend towards less cardiovascular disease in women who started hormone therapy within 9 years of menopause, whereas those starting it later had a statistically significantly higher rate of cardiovascular mortality. However, all women had a higher risk of stroke while on hormone therapy, regardless of timing.22
A study of 1,006 healthy women age 45 to 58 whose last menstrual period was 3 to 24 months before enrollment found a statistically significant reduction in the composite end point of death, hospital admission for myocardial infarction, or heart failure with hormone therapy.23 There was no significant increase in breast cancer, deep vein thrombosis, or stroke after 10 years of randomized treatment.
A retrospective analysis of 71,237 postmenopausal women in the California Teachers Study also found a significant reduction in the rate of cardiovascular disease-related deaths with hormone therapy in younger women (ie, younger than age 65), but not in older women.24 The authors concluded that it may not just be the years after menopause but also the baseline age of the woman that may influence outcomes.
In view of these studies, there is increasing recognition that hormone therapy may, in fact, still be beneficial in terms of cardiovascular and all-cause mortality in carefully selected patients. The cardiovascular risk in women, specifically older women who have had a longer duration of menopause, should also be weighed against the potential benefits of therapy in terms of quality of life and symptom relief.
Trials under way include the Kronos Early Estrogen Prevention (KEEP) and Danish Osteoporosis Prevention (DOPS) studies. KEEP is a 4-year, double-blind, randomized controlled trial of hormone therapy in women within 3 years of menopause. DOPS is an open-label trial that includes more than 1,000 women with early menopause. The results of these trials will likely affect future recommendations.
WOMEN’S SYMPTOMS: TYPICAL OR ATYPICAL?
Whether the presenting symptoms of acute coronary syndromes differ between men and women has been much debated.
More women than men seem to present with atypical symptoms.25–27 (The term “atypical” refers to symptoms that do not include the three classic components of angina: substernal chest pain or discomfort, provoked by exertion or emotional stress, and relieved by rest or nitroglycerin, or both.28)
However, most women still present with chest pain. In a study by Dey et al,26 92% of the 7,638 women with presumed acute coronary syndrome presented with chest pain. In women who had atypical symptoms, dyspnea, nausea, vomiting, and diaphoresis were the most common symptoms. Women were significantly more likely than men to present with nausea and vomiting (32% vs 23%, P = .001).
Women in the study were also more likely to have angiographically normal coronary arteries (12% vs 6%, P < .001).26 This difference may be largely due to noncardiac chest pain, but it may also represent conditions such as vasospasm, microvascular disease, or stress cardiomyopathy, all of which disproportionately affect women.
An earlier review of 10 major studies found a higher percentage of women presenting with atypical symptoms (37.5% of women vs 27.4% of men).25 However, symptoms were not a focus of these studies, and the findings may therefore be skewed by inaccurate documentation.
Atypical warning signs. Although most women with acute coronary syndrome present acutely with chest pain, women may have different warning signs than men. Only about one-third of women experience angina before presentation.29 Compared with men, women are more likely to complain of shortness of breath, fatigue, and weakness leading up to a diagnosis of a myocardial infarction.29 Therefore, the prodromal symptoms of cardiovascular disease may in fact be significantly more atypical in women than in men, suggesting the need for heightened vigilance in the cardiovascular evaluation of women who have nonanginal symptoms.
THE ROLE OF STRESS TESTING IN WOMEN
Stress testing in various forms continues to be widely used in the diagnosis of heart disease in women, although data are scarce regarding its utility in women.
The ACC/AHA guidelines continue to recommend exercise stress electrocardiography (ECG) for women who have symptoms, are at intermediate risk, and have a normal result on resting ECG.30
Exercise ECG has a higher false-positive rate in women than in men,31 and there appears to be no relationship between exercise-induced ST-segment depression and the rate of cardiovascular mortality or all-cause mortality in women.32,33 On the other hand, exercise ECG yields valuable additional information such as exercise capacity, chronotropic response, heart-rate recovery, and blood pressure response, all of which have important diagnostic and prognostic implications in women.34
For those who have an abnormal resting ECG, the addition of an imaging test, ie, echocardiography or single-photon emission computed tomography (SPECT), is indicated. Both have limitations: SPECT can give false-positive results because of breast attenuation, and echocardiography varies in accuracy depending on the quality of acoustic windows obtained. Both exercise stress SPECT and exercise stress ECG have higher sensitivity and specificity than electrocardiographic exercise stress testing alone,34 and there is evidence that the two imaging tests are comparable in women.35
In those women who have baseline left bundle branch block or who cannot exercise, a pharmacologic stress test should be performed. Of course, this is a less desirable testing method, given the loss of valuable information obtained from exercising the patient.
UNDERLYING CONDITIONS THAT DISPROPORTIONATELY AFFECT WOMEN
Microvascular angina
Perimenopausal and postmenopausal women account for 70% of patients presenting with chest pain and elevated cardiac enzymes but no significant angiographic evidence of coronary artery disease.36 This condition, commonly called syndrome X, is often characterized by lingering, dull chest pain after exertion and is seen more frequently in women younger than those presenting with classic cardiovascular disease.
Because at least some of these patients show evidence of ST-segment depression and reversible perfusion defects on imaging, the condition is thought to be caused by ischemia of the microvascular bed leading to microvascular angina.37
Although this is still an area of research, microvascular dysfunction has recently been proposed as an explanation for these findings. Abnormal vasoconstriction and impaired vasodilation of the microvascular bed, insulin resistance, increased systemic inflammation, and abnormal pain response have all been cited as potentially contributing to microvascular dysfunction.36
Estrogen deficiency is thought to play a central role in the significantly increased burden of microvascular dysfunction seen in women, with some studies suggesting that hormone therapy can relieve symptoms. However, given the concerns about adverse cardiovascular outcomes in women on hormone therapy, there has been little investigation of this treatment for this disorder.
Studies have shown worse cardiovascular outcomes and higher rates of angina-related hospitalization and repeat heart catheterizations in women with microvascular dysfunction.38
Diagnosing microvascular angina must be done indirectly, as there is no safe and minimally invasive technique by which to directly observe the microvasculature. Current coronary angiographic techniques cannot image vessels smaller than 0.5 mm in diameter, and endomyocardial biopsy cannot access the larger periarterioles thought to play a major role in regulating coronary blood flow.39
Because the coronary microvasculature controls total coronary resistance and therefore regulates myocardial blood flow, measuring myocardial blood flow at maximum vasodilation, termed coronary flow reserve, can indirectly evaluate the degree of microvascular dysfunction.40 In the absence of obstructive epicardial coronary disease, noninvasive imaging techniques or provocative testing in the coronary catheterization lab can be used for this purpose. In terms of noninvasive imaging, perfusion magnetic resonance imaging (Figure 2) or positron emission tomography is often performed.40
Coronary flow reserve can also be measured by invasive means in the catheterization laboratory after maximum hyperemia is induced by adenosine or other such vasodilatory agents.41 However, measurements obtained in this invasive manner are greatly affected by hemodynamic changes and can have poor reproducibility.40
Proposed therapy for microvascular angina. Once a diagnosis has been made, lifestyle modification, antianginal agents, angiotensin-converting enzyme inhibitors, and statins have been suggested for therapy.39 Pain management techniques are also used, given the increased pain sensitivity observed in women with this condition. However, no therapy to date has proven overwhelmingly effective in these patients, and a disproportionate number of women suffer from chronic symptoms despite these treatments. Currently, researchers are looking for new agents to treat microvascular disease.
Stress cardiomyopathy
Stress cardiomyopathy, also called takotsubo cardiomyopathy or “broken heart syndrome,” is another condition that disproportionately affects postmenopausal women. It is often associated with sudden emotional or physical stress. Patients present with signs and symptoms of myocardial infarction without demonstrable epicardial coronary artery disease. The hallmark of stress cardiomyopathy is left ventricular dysfunction, often severe, with classic apical ballooning that resembles a Japanese fishing pot (takotsubo) used to trap octopuses, hence the name (Figure 3).
According to a review by Akashi et al42 based on previously reported Mayo Clinic criteria, the diagnosis of stress cardiomyopathy includes each of the following:
- Transient hypokinesis, akinesis, or dyskinesis in the left ventricular midsegments with or without apical involvement; regional wall-motion abnormalities that extend beyond a single epicardial vascular distribution; and frequently, but not always, a stressful trigger
- Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture
- New abnormality on ECG (eg, ST-segment elevation, T-wave inversion) or modest elevation in cardiac troponin
- Absence of pheochromocytoma or myocarditis.
From 80% to 100% of reported cases are in women, with an average age range of 61 to 76.42 It is unclear why there is such an overwhelming postmenopausal female preponderance of the disease. Studies have implicated estrogen deficiency, as it appears to attenuate the levels of cardioprotective substances in the body that in part regulate catecholamine surges and may also increase the level of oxidative stress.42
Several mechanisms for this condition have been proposed. The condition may be caused by multivessel epicardial coronary spasm or spontaneously resolved plaque rupture, resulting in stunned myocardium. However, the regional distribution of wall-motion abnormality is often out of proportion to the level of cardiac enzyme elevation, and in the case of plaque rupture, is frequently not consistent with a single coronary vessel.42 A catecholamine surge causing myocardial and neurogenic stunning has also been proposed, although many of these patients have normal catecholamine levels.42 Finally, microvascular dysfunction has been found in a number of patients with this condition. However, it is difficult to establish a causal relationship, since apical ballooning could result in microvascular dysfunction.42
Treatment of stress cardiomyopathy has not been standardized, in part because the left ventricular dysfunction often resolves spontaneously within several weeks.43,44 Given the proposed catecholaminergic mechanism, some experts believe that beta-blockers are contraindicated because of the resulting unopposed activation of alpha-adrenoreceptors. However, this continues to be a matter of debate. There is also no clear indication for other standard therapies for acute coronary syndrome such as aspirin and heparin, and their use appears to vary in clinical practice.
Although most patients improve with time and recurrence is exceedingly rare, it should be emphasized that they may present acutely with severe hemodynamic instability and cardiogenic shock. Therefore, advanced means of support, such as an intra-aortic balloon pump, may be indicated until the patient recovers from the acute phase of the disease.
Spontaneous coronary artery dissection
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome resulting from dissection of the coronary intimal or medial layer and associated hematoma formation, leading to coronary occlusion.45,46 In a case series of 87 patients, 49% presented with an ST-segment elevation myocardial infarction, and 23% were found to have multivessel SCAD.46
SCAD occurs predominantly in young, healthy women (mean age 30–45 years). Approximately 70% of cases are in women, 30% of whom are in the peripartum period.45 The reasons for the increased risk during pregnancy have not yet been elucidated, but changing sex hormones, increased cardiac output and shear stress, and an increased inflammatory response have been implicated.45
Diagnosing SCAD. Coronary angiography should be performed with extreme caution in patients suspected of having SCAD, given the risk of further dissection of the artery with forceful injections. In certain cases, it may be difficult to detect SCAD on routine angiography if there is no communication between the true and false lumen.
If the suspicion for SCAD is high, intravascular ultrasonography or optical coherence tomography can be used to better evaluate the vessel.45 Although optical coherence tomography has greater spatial resolution, it is more costly and is not as widely used as intravascular ultrasonography in the clinical setting
Managing SCAD. Although conservative management and coronary artery bypass grafting have been shown to cause minimal in-hospital morbidity, percutaneous coronary intervention has been complicated by technical failure in up to 35% of patients in one series.46
While there is no standardized way to manage these patients, experts currently recommend conservative management with standard therapies for acute coronary syndrome (Figure 4). Although antithrombotic agents can decrease thrombus burden, they must be used with caution, because they also increase the risk of bleeding into the false lumen.
If patients experience recurrent or ongoing ischemia despite conservative management, then revascularization should be considered. Optical coherence tomography or intravascular ultrasonography is recommended to ensure proper stent alignment and positioning.
Coronary artery bypass grafting could be considered in preference to percutaneous coronary intervention, given that the former appears to be safer,46 although this requires further investigation. Some studies have cautioned against using fibrinolytic therapy, based on anecdotal evidence that it may further propagate the dissection,45 although this therapy has been used in other case studies.46
While mortality rates are relatively low (95% survival at 2 years),45 the estimated risk of recurrent SCAD at 10 years is approximately 30%.46
Associated with fibromuscular dysplasia. Of note, a sizeable number of patients with SCAD have been found to have fibromuscular dysplasia. This is a nonatherosclerotic, noninflammatory vascular condition that can affect any vascular bed in the body, although there is a predilection for the renal and carotid arteries (Figure 5).47 Fibromuscular dysplasia also disproportionately affects women and appears to be a concomitant condition in the majority of patients with SCAD.47 Imaging of the carotid and renal arteries of patients with SCAD has revealed a number of cases of fibromuscular dysplasia.46,48 This noted association will likely allow for ongoing research to better understand the pathophysiology of these two conditions.
- Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol 2007; 49:1230–1250.
- Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000; 343:16–22.
- Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the US Preventive Services Task Force. Ann Intern Med 2009; 150:405–410.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Cook NR, Cole SR, Buring JE. Aspirin in the primary prevention of cardiovascular disease in the Women’s Health Study: effect of non-compliance. Eur J Epidemiol 2012; 27:431–438.
- Mosca L, Benjamin EJ, Berra K, et al; American Heart Association. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011; 57:1404–1423.
- Jardine MJ, Ninomiya T, Perkovic V, et al. Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post-hoc subgroup analysis of a randomized controlled trial. J Am Coll Cardiol 2010; 56:956–965.
- Snoep JD, Roest M, Barendrecht AD, De Groot PG, Rosendaal FR, Van Der Bom JG. High platelet reactivity is associated with myocardial infarction in premenopausal women: a population-based case-control study. J Thromb Haemost 2010; 8:906–913.
- Becker DM, Segal J, Vaidya D, et al. Sex differences in platelet reactivity and response to low-dose aspirin therapy. JAMA 2006; 295:1420–1427.
- Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation 2010; 121:1069–1077.
- Kostis WJ, Cheng JQ, Dobrzynski JM, Cabrera J, Kostis JB. Meta-analysis of statin effects in women versus men. J Am Coll Cardiol 2012; 59:572–582.
- Ray KK, Seshasai SR, Erqou S, et al. Statins and all-cause mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch Intern Med 2010; 170:1024–1031.
- Taylor F, Ward K, Moore TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2011;CD004816.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;CD004816.
- Blaha MJ, Nasir K, Blumenthal RS. Statin therapy for healthy men identified as “increased risk.” JAMA 2012; 307:1489–1490.
- Redberg RF, Katz MH. Healthy men should not take statins. JAMA 2012; 307:1491–1492.
- Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med 2012; 172:144–152.
- Pasternak RC, Smith SC, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Stroke 2002; 33:2337–2341.
- Manson JE, Hsia J, Johnson KC, et al; Women’s Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003; 349:523–534.
- Hsia J, Criqui MH, Herrington DM, et al; Women’s Health Initiative Research Group. Conjugated equine estrogens and peripheral arterial disease risk: The Women’s Health Initiative. Am Heart J 2006; 152:170–176.
- Moyer VAUS Preventive Services Task Force. Menopausal hormone therapy for the primary prevention of chronic conditions: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158:47–54.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 2012; 345:e6409.
- Stram DO, Liu Y, Henderson KD, et al. Age-specific effects of hormone therapy use on overall mortality and ischemic heart disease mortality among women in the California Teachers Study. Menopause 2011; 18:253–261.
- Canto JG, Goldberg RJ, Hand MM, et al. Symptom presentation of women with acute coronary syndromes: myth vs reality. Arch Intern Med 2007; 167:2405–2413.
- Dey S, Flather MD, Devlin G, et al; Global Registry of Acute Coronary Events investigators. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: The Global Registry of Acute Coronary Events. Heart 2009; 95:20–26.
- Canto JG, Shlipak MG, Rogers WJ, et al. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA 2000; 283:3223–3229.
- Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979; 300:1350–1358.
- McSweeney JC, Cody M, O’Sullivan P, Elberson K, Moser DK, Garvin BJ. Women’s early warning symptoms of acute myocardial infarction. Circulation 2003; 108:2619–2623.
- Mieres JH, Shaw LJ, Arai A, et al; Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation 2005; 111:682–696.
- Barolsky SM, Gilbert CA, Faruqui A, Nutter DO, Schlant RC. Differences in electrocardiographic response to exercise of women and men: a non-Bayesian factor. Circulation 1979; 60:1021–1027.
- Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: The St James Women Take Heart Project. Circulation 2003; 108:1554–1559.
- Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the Lipid Research Clinics Prevalence Study. JAMA 2003; 290:1600–1607.
- Kohli P, Gulati M. Exercise stress testing in women: going back to the basics. Circulation 2010; 122:2570–2580.
- Grady D, Chaput L, Kristof M. Diagnosis and treatment of coronary heart disease in women: systematic reviews of evidence on selected topics. Evid Rep Technol Assess (Summ) 2003; 81:1–4.
- Singh M, Singh S, Arora R, Khosla S. Cardiac syndrome X: current concepts. Int J Cardiol 2010; 142:113–119.
- Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007; 356:830–840.
- Johnson BD, Shaw LJ, Buchthal SD, et al; National Institutes of Health-National Heart, Lung, and Blood Institute. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004; 109:2993–2999.
- Beltrame JF, Crea F, Camici P. Advances in coronary microvascular dysfunction. Heart Lung Circ 2009; 18:19–27.
- Leung DY, Leung M. Non-invasive/invasive imaging: significance and assessment of coronary microvascular dysfunction. Heart 2011; 97:587–595.
- Samim A, Nugent L, Mehta PK, Shufelt C, Bairey Merz CN. Treatment of angina and microvascular coronary dysfunction. Curr Treat Options Cardiovasc Med 2010; 12:355–364.
- Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 2008; 118:2754–2762.
- Akashi YJ, Musha H, Kida K, et al. Reversible ventricular dysfunction takotsubo cardiomyopathy. Eur J Heart Fail 2005; 7:1171–1176.
- Regnante RA, Zuzek RW, Weinsier SB, et al. Clinical characteristics and four-year outcomes of patients in the Rhode Island Takotsubo Cardiomyopathy Registry. Am J Cardiol 2009; 103:1015–1019.
- Vrints CJ. Spontaneous coronary artery dissection. Heart 2010; 96:801–808.
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126:579–588.
- Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013; 6:44–52.
- Saw J, Poulter R, Fung A, Wood D, Hamburger J, Buller CE. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series. Circ Cardiovasc Interv 2012; 5:134–137.
- Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol 2007; 49:1230–1250.
- Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000; 343:16–22.
- Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the US Preventive Services Task Force. Ann Intern Med 2009; 150:405–410.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Cook NR, Cole SR, Buring JE. Aspirin in the primary prevention of cardiovascular disease in the Women’s Health Study: effect of non-compliance. Eur J Epidemiol 2012; 27:431–438.
- Mosca L, Benjamin EJ, Berra K, et al; American Heart Association. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011; 57:1404–1423.
- Jardine MJ, Ninomiya T, Perkovic V, et al. Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post-hoc subgroup analysis of a randomized controlled trial. J Am Coll Cardiol 2010; 56:956–965.
- Snoep JD, Roest M, Barendrecht AD, De Groot PG, Rosendaal FR, Van Der Bom JG. High platelet reactivity is associated with myocardial infarction in premenopausal women: a population-based case-control study. J Thromb Haemost 2010; 8:906–913.
- Becker DM, Segal J, Vaidya D, et al. Sex differences in platelet reactivity and response to low-dose aspirin therapy. JAMA 2006; 295:1420–1427.
- Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation 2010; 121:1069–1077.
- Kostis WJ, Cheng JQ, Dobrzynski JM, Cabrera J, Kostis JB. Meta-analysis of statin effects in women versus men. J Am Coll Cardiol 2012; 59:572–582.
- Ray KK, Seshasai SR, Erqou S, et al. Statins and all-cause mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch Intern Med 2010; 170:1024–1031.
- Taylor F, Ward K, Moore TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2011;CD004816.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;CD004816.
- Blaha MJ, Nasir K, Blumenthal RS. Statin therapy for healthy men identified as “increased risk.” JAMA 2012; 307:1489–1490.
- Redberg RF, Katz MH. Healthy men should not take statins. JAMA 2012; 307:1491–1492.
- Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med 2012; 172:144–152.
- Pasternak RC, Smith SC, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Stroke 2002; 33:2337–2341.
- Manson JE, Hsia J, Johnson KC, et al; Women’s Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003; 349:523–534.
- Hsia J, Criqui MH, Herrington DM, et al; Women’s Health Initiative Research Group. Conjugated equine estrogens and peripheral arterial disease risk: The Women’s Health Initiative. Am Heart J 2006; 152:170–176.
- Moyer VAUS Preventive Services Task Force. Menopausal hormone therapy for the primary prevention of chronic conditions: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158:47–54.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 2012; 345:e6409.
- Stram DO, Liu Y, Henderson KD, et al. Age-specific effects of hormone therapy use on overall mortality and ischemic heart disease mortality among women in the California Teachers Study. Menopause 2011; 18:253–261.
- Canto JG, Goldberg RJ, Hand MM, et al. Symptom presentation of women with acute coronary syndromes: myth vs reality. Arch Intern Med 2007; 167:2405–2413.
- Dey S, Flather MD, Devlin G, et al; Global Registry of Acute Coronary Events investigators. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: The Global Registry of Acute Coronary Events. Heart 2009; 95:20–26.
- Canto JG, Shlipak MG, Rogers WJ, et al. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA 2000; 283:3223–3229.
- Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979; 300:1350–1358.
- McSweeney JC, Cody M, O’Sullivan P, Elberson K, Moser DK, Garvin BJ. Women’s early warning symptoms of acute myocardial infarction. Circulation 2003; 108:2619–2623.
- Mieres JH, Shaw LJ, Arai A, et al; Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation 2005; 111:682–696.
- Barolsky SM, Gilbert CA, Faruqui A, Nutter DO, Schlant RC. Differences in electrocardiographic response to exercise of women and men: a non-Bayesian factor. Circulation 1979; 60:1021–1027.
- Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: The St James Women Take Heart Project. Circulation 2003; 108:1554–1559.
- Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the Lipid Research Clinics Prevalence Study. JAMA 2003; 290:1600–1607.
- Kohli P, Gulati M. Exercise stress testing in women: going back to the basics. Circulation 2010; 122:2570–2580.
- Grady D, Chaput L, Kristof M. Diagnosis and treatment of coronary heart disease in women: systematic reviews of evidence on selected topics. Evid Rep Technol Assess (Summ) 2003; 81:1–4.
- Singh M, Singh S, Arora R, Khosla S. Cardiac syndrome X: current concepts. Int J Cardiol 2010; 142:113–119.
- Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007; 356:830–840.
- Johnson BD, Shaw LJ, Buchthal SD, et al; National Institutes of Health-National Heart, Lung, and Blood Institute. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004; 109:2993–2999.
- Beltrame JF, Crea F, Camici P. Advances in coronary microvascular dysfunction. Heart Lung Circ 2009; 18:19–27.
- Leung DY, Leung M. Non-invasive/invasive imaging: significance and assessment of coronary microvascular dysfunction. Heart 2011; 97:587–595.
- Samim A, Nugent L, Mehta PK, Shufelt C, Bairey Merz CN. Treatment of angina and microvascular coronary dysfunction. Curr Treat Options Cardiovasc Med 2010; 12:355–364.
- Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 2008; 118:2754–2762.
- Akashi YJ, Musha H, Kida K, et al. Reversible ventricular dysfunction takotsubo cardiomyopathy. Eur J Heart Fail 2005; 7:1171–1176.
- Regnante RA, Zuzek RW, Weinsier SB, et al. Clinical characteristics and four-year outcomes of patients in the Rhode Island Takotsubo Cardiomyopathy Registry. Am J Cardiol 2009; 103:1015–1019.
- Vrints CJ. Spontaneous coronary artery dissection. Heart 2010; 96:801–808.
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126:579–588.
- Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013; 6:44–52.
- Saw J, Poulter R, Fung A, Wood D, Hamburger J, Buller CE. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series. Circ Cardiovasc Interv 2012; 5:134–137.
KEY POINTS
- Aspirin appears to be less beneficial in women than in men in preventing coronary artery disease.
- Debate continues on the benefit of statins for primary prevention, not only in women but in the population as a whole.
- Hormone therapy is not recommended for cardiovascular disease prevention.
- More women than men who present with acute coronary syndromes have atypical symptoms. Nevertheless, most women who have acute coronary syndromes do have typical symptoms such as chest pain.
- Guidelines continue to recommend exercise stress electrocardiography for symptomatic women at intermediate risk who have a normal resting electrocardiogram.
- Conditions that predominantly affect women include microvascular angina, stress cardiomyopathy, and spontaneous coronary artery dissection.